Note: Descriptions are shown in the official language in which they were submitted.
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
VAPOR PHASE CATALYTIC REACTOR FOR UPGRADE
OF FUELS PRODUCED BY FAST PYROLYSIS OF BIOMASS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This
application claims priority from U.S. Provisional Patent Application No.
61/800,262, filed on March 15, 2013, which is incorporated by reference herein
in its
entirety.
BACKGROUND
[0002] The
extraction of bio-oil from biomass for use as a biofuel is an area of interest
in
the search for reliable alternative energy sources. Bio-oil produced by
traditional fast
pyrolysis processes is typically of relatively low quality due to the bio-
oil's large degree of
oxygenation, significant acidity, storage instability, tendency to polymerize,
and the difficulty
involved in separating the bio-oil from water and polar compounds. The low
quality of the
bio-oil limits its applicability in applications that require high quality
fuels, such as
transportation applications. Biofuels
intended for these applications usually require
additional processing.
[0003] Improved
systems, methods, and apparatuses are needed for processing bio-oil
and making biofuel.
SUMMARY
[0004] In one
embodiment, a biofuel production system is provided, the system may
include: a catalytic vapor phase reactor; a pyrolysis reactor operatively
connected to the
catalytic vapor phase reactor; a quench system operatively connected to the
catalytic vapor
phase reactor; a water gas shift reactor operatively connected to the quench
system; and a
hydrotreatment system operatively connected to the quench system.
[0005] In one
embodiment, a method for catalytic pyrolysis of biomass is provided. The
method may include drying a biomass. The method may include pyrolyzing the
biomass to
- 1 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
create a pyrolysis vapor. The method may include removing at least one of a
char and an ash
from the pyrolysis vapor. The method may include upgrading the pyrolysis vapor
by vapor
phase catalysis to produce an upgraded pyrolysis vapor. The method may include
condensing
a bio-oil from the upgraded pyrolysis vapor.
[0006] In one
embodiment, a method for catalytic pyrolysis of biomass is provided, the
method may include: drying a biomass in a biomass dryer; placing the biomass
in a pyrolysis
reactor and heating the biomass to about 500 C to create a pyrolysis vapor;
directing the
pyrolysis vapor to a char and ash removal system and removing at least one of
a char and an
ash from the pyrolysis vapor; directing the pyrolysis vapor to a catalytic
vapor phase reactor
to upgrade the pyrolysis vapor; directing the pyrolysis vapor to a condenser;
and extracting a
bio-oil from the condenser.
[0007] In one
embodiment, a catalytic vapor phase reactor apparatus is provided, the
apparatus may include: a gas-solid catalytic reactor; a feeding auger; a
return auger; a hot
blower; a first blower; a second blower; a first cyclone; a second cyclone; a
third cyclone; a
split connection; a dip leg pipe; a fluidized bed reactor; a bypass
connection; and a catalyst
feeding vessel; wherein the feeding auger and the return auger are operatively
connected to
the gas-solid catalytic reactor and the fluidized bed reactor; wherein the
first cyclone and the
second cyclone are operatively connected to the gas-solid catalytic reactor;
and wherein the
third cyclone is operatively connected to the fluidized bed reactor.
[0008] In
another embodiment, a catalytic vapor phase reactor apparatus is provided, the
apparatus may include: a housing may include an auger device, a catalyst
inlet, a catalyst
outlet, a pyrolysis vapor inlet, and a pyrolysis vapor outlet; wherein the
auger device is
configured to transport a solid catalyst through at least a portion of the
housing; and wherein
- 2 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
the housing is configured to permit a pyrolysis vapor to flow through at least
a portion of the
housing and come into contact with the solid catalyst.
[0009] In another embodiment, a catalytic vapor phase reactor apparatus is
provided, the
apparatus may include: a housing may include at least one baffle, a catalyst
inlet, a catalyst
outlet, a pyrolysis vapor inlet, and a pyrolysis vapor outlet; wherein the
housing is at least one
of substantially vertical and inclined; wherein the housing is configured to
permit a solid
catalyst to flow through at least a portion of the housing and over at least
one baffle; and
wherein the housing is configured to permit a pyrolysis vapor to flow through
at least a
portion of the housing and come into contact with the solid catalyst.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The accompanying figures, which are incorporated in and constitute a
part of the
specification, illustrate various example apparatuses, systems, and methods,
and are used
merely to illustrate various example embodiments.
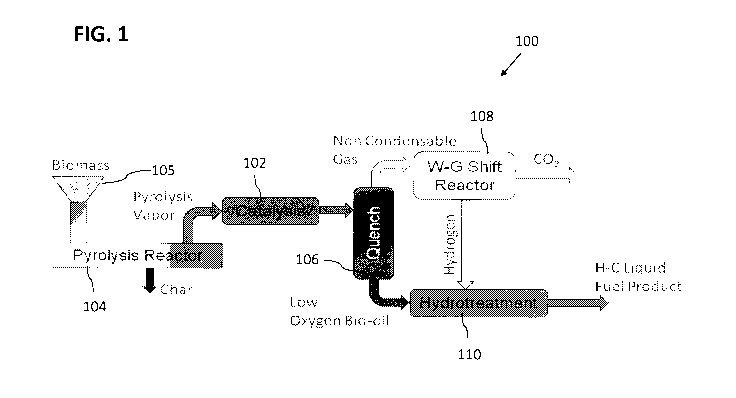
[0011] FIG. 1 illustrates an example arrangement of a biofuels production
system.
[0012] FIG. 2A illustrates an example arrangement of a process for
catalytic fast
pyrolysis of biomass.
[0013] FIG. 2B illustrates an example arrangement of a process for
catalytic fast
pyrolysis of biomass.
[0014] FIG. 3 illustrates an example arrangement of a catalytic vapor phase
reactor for
upgrading bio-oil vapors.
[0015] FIG. 4 illustrates an example arrangement of a catalytic vapor phase
reactor.
[0016] FIG. 5 illustrates an example arrangement of a catalytic vapor phase
reactor.
-3 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
DETAILED DESCRIPTION
[0017] FIG. 1
illustrates an example arrangement of a biofuels production system 100.
Biofuels production system 100 may include a catalytic vapor phase reactor
("VPR") 102
operatively connected to a pyrolysis reactor 104. Pyrolysis reactor 104 may be
configured to
receive a biomass 105. In one embodiment, biomass 105 may include a wood.
[0018] In one
embodiment, biofuels production system 100 may include a conversion
system to produce a hydrocarbon product from biomass 105. The production of
the
hydrocarbon fuel product may include upgrading a bio-oil product. In one
embodiment,
system 100 may be configured to produce at least one of a hydrocarbon fuel
product, a gas, or
a chemical, depending upon the catalyst type and reaction conditions.
[0019] In one
embodiment, biomass 105 enters pyrolysis reactor 104 wherein biomass
105 may be pyrolyzed and converted to a pyrolysis vapor. In one embodiment,
pyrolysis
reactor 104 operates at an elevated temperature. In another embodiment,
pyrolysis reactor
104 operates at a temperature between about 300 C and about 600 C. In
another
embodiment, pyrolysis reactor 104 operates at a temperature between about 350
C and about
550 C. In another embodiment, pyrolysis reactor 104 operates at a temperature
between
about 400 C and about 500 C. In another embodiment, pyrolysis reactor 104
operates at a
temperature capable of converting biomass 105 to a vapor. In one embodiment,
char
produced in the pyrolysis of biomass 105 may be removed from pyrolysis reactor
104. In one
embodiment, pyrolysis reactor 104 may be internally heated. In another
embodiment,
pyrolysis reactor 104 may be externally heated. In another embodiment,
pyrolysis reactor
104 may be heated via microwaves.
- 4 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0020] The
pyrolysis vapor created in pyrolysis reactor 104 may be directed into VPR
102. In one embodiment, VPR 102 may be configured to at least one of
deoxygenate the
pyrolysis vapor and break down higher molecular weight components of the
pyrolysis vapor.
[0021] In one
embodiment, VPR 102 may be an integral component of pyrolysis reactor
104, and catalysis takes place in pyrolysis reactor 104. In another
embodiment, catalysis
occurs in pyrolysis reactor 104 and VPR 102 may be eliminated.
[0022] The
pyrolysis vapor may leave VPR 102 and enter a quench system 106, which
may be operatively connected to VPR 102. Quench system 106 may quench the
pyrolysis
vapor, producing a bio-oil. In one embodiment, the bio-oil may be upgraded to
produce a
hydrocarbon fuel product. The quenching of the pyrolysis vapor in quench
system 106 may
produce at least one of a non-condensable gas and a low oxygen bio-oil.
[0023] In one
embodiment, non-condensable gas may be directed from quench system
106 into a water gas shift reactor 108, which may be operatively connected to
quench system
106. The non-condensable gas may be processed resulting in at least one of
hydrogen and
CO2. Hydrogen for the upgrading may be obtained via a water gas shift of the
CO in the non-
condensable gas exiting quench system 106.
[0024] In one
embodiment, low oxygen bio-oil may be directed from quench system 106
into a hydrotreatment system 110, which may be operatively connected to quench
system
106. In one embodiment, hydrogen from water gas shift reactor 108 may be
directed into
hydrotreatment system 110. In one embodiment, hydrogen may react with the low
oxygen
bio-oil to produce a hydrocarbon fuel product.
[0025] In one
embodiment, biomass 105 may include a wood. Thus, in some
embodiments, the bio-oil produced via pyrolysis may contain a mixture of
water, organic
acids, aldehydes, phenols, and sugar derivatives that require upgrading in
order for the bio-oil
-5 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
to be more readily soluble and usable. In one embodiment, the upgrading may be
achieved
through reaction of the acids, phenols, and sugars with olefins to form esters
and ethers. For
example, in one embodiment, the bio-oil in the vapor phase may be passed
through a catalyst
bed after mixing with an injected amount of isoprene, isobutylene, or
propylene. In one
embodiment, the water may be converted to an alcohol form of the olefin. In
one
embodiment, the alcohols may be etherified. In one embodiment, the carboxylic
acids may
be esterified.
[0026] FIG. 2A
illustrates an example arrangement of a process 201 configured, for
example, for catalytic fast pyrolysis of biomass. Process 201 may generate
high yields of
condensable organics that may be used as liquid fuels. Process 201 may include
250 drying a
biomass. Process 201 may include 252 pyrolyzing the biomass to create a
pyrolysis vapor.
Process 201 may include 254 removing at least one of a char and an ash from
the pyrolysis
vapor. Process 201 may include 256 upgrading the pyrolysis vapor by vapor
phase catalysis
to produce an upgraded pyrolysis vapor. Process 201 may include 258 extracting
a bio-oil
from the upgraded pyrolysis vapor by condensation.
[0027] FIG. 2B
illustrates an example arrangement of a process 200 configured, for
example, for catalytic fast pyrolysis of biomass. Process 200 may be included
within the
scope of process 201. Process 200 may generate high yields of condensable
organics that
may be used as liquid fuels. In one embodiment, process 200 may include
heating of a
biomass to about 500 C for a short period of time, typically on the order of
a second.
Process 200 may include subsequent condensation of organic vapors into a bio-
oil mixed
with water. Process 200 may include the upgrading of bio-oil vapors in a VPR
202.
[0028] Process
200 may include a system including VPR 202, a pyrolysis reactor 204, a
condenser 206, a biomass dryer 212, and a char and ash removal system 214.
Thus, in one
- 6 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
embodiment, process 200 may include: introducing a biomass into biomass dryer
212;
transferring dried biomass into pyrolysis reactor 204; and directing pyrolysis
vapor from
pyrolysis reactor 204 into char and ash removal system 214. Char and ash may
be separated
from the pyrolysis vapor in char and ash removal system 214. Char and ash may
be
discarded from the system.
[0029]
Pyrolysis vapor exiting char and ash removal system 214 may be directed into
VPR 202. In one embodiment, pyrolysis vapor may be upgraded in VPR 202. In one
embodiment, pyrolysis vapor may be contacted with a catalyst after pyrolysis
and char/ash
removal but before vapor condensing. Processing of the pyrolysis vapor between
the
char/ash removal but before vapor condensing may minimize contamination of the
catalyst
with ash and char generated during pyrolysis.
[0030] In one
embodiment, temperatures in VPR 202 and pyrolysis reactor 204 may be
substantially the same, or different. In another embodiment, residence time of
reactants in
VPR 202 and pyrolysis reactor 204 may be substantially the same. In another
embodiment,
residence time of reactants in VPR 202 and pyrolysis reactor 204 may be
different.
[0031] In one
embodiment, VPR 202 utilizes at least one of: a granulated catalyst, a
powdered catalyst, and a catalyst mixture capable of bio-oil vapor or
liquid/vapor upgrade.
In another embodiment, VPR 202 utilizes a fluid catalytic cracking or a
similar granulated
catalyst. In one embodiment, the catalyst may be a granulated catalyst with a
typical granule
size between about 50 [tm and about 100 [tm. The catalyst may have very few
granules
smaller than about 20 [tm. In one embodiment, the catalyst characteristics
provide a desired
combination of rapid external mass transfer in rapid gas-solid reactions
combined with
relatively easy gas-solid separation ability. In one embodiment, the catalyst
may be a fluid
catalytic cracking ("FCC") catalyst. In one embodiment, the catalyst may be at
least one of:
-7 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
fresh FCC, spent FCC, catalyst impregnated on top of fresh FCC, catalyst
impregnated on top
of spent FCC, and catalysts granulated by other means but with the same or
similar particle
size and flow characteristic as FCC. In another embodiment, the catalyst
catalyzes at least
one of the following reactions: deoxygenation, cracking, water-gas shift, and
hydrocarbon
formation.
[0032] Upgraded
pyrolysis gas may be directed to condenser 206, where the upgraded
pyrolysis gas may be separated into at least one of: non-condensable gases,
upgraded bio-oil,
and waste water. Upgraded pyrolysis gas may be separated via condensation.
[0033] FIG. 3
illustrates an example arrangement of a VPR 300 for upgrading bio-oil
vapors. VPR 300 may include a gas-solid catalytic reactor 302, a feeding auger
304, a return
auger 306, a hot blower 308, a first cyclone 310, a second cyclone 312, and a
split connection
314. Split connection 314 may be operatively connected to a dip leg pipe 316,
a fluidized
bed reactor 318, a bypass connection 320, and a catalyst feeding vessel 322.
Fluidized bed
reactor 318 may include a third cyclone 324 operatively connected to a first
blower 326 and a
second blower 328.
[0034] In one
embodiment, VPR 300 may be configured for continuous mode operation.
In another embodiment, VPR 300 may be configured for batch mode operation. In
one
embodiment, VPR 300 may be configured to operate utilizing air at atmospheric
pressure for
catalyst regeneration and does not require the use of gases with reduced
oxygen content,
including, for example, inert gases such as nitrogen, or another gas such as
carbon dioxide.
[0035] In one
embodiment, each of the components of VPR 300, with the exception of
hot blower 308, first blower 326, and second blower 328, are configured to
operate at
relatively high temperatures. In one embodiment, the operation temperature may
be between
about 300 C and about 700 C. In another embodiment, the operation
temperature may be
- 8 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
between about 350 C and about 650 C. In another embodiment, the operation
temperature
may be between about 400 C and about 600 C. In another embodiment, the
operation
temperature may be a temperature as high as may be required to drive a
catalytic upgrade
reaction and a catalyst regeneration (coke oxidation) reaction. In another
embodiment, the
operation temperature may be a temperature as high as may be required to avoid
condensation of pyrolysis gases that can occur on surfaces of components, such
as those
below about 400 C. In one embodiment, operating temperature may be generated
and/or
maintained by placing VPR 300 in a hot box heated by at least one of a gas
burner and an
electric heater.
[0036] In one
embodiment, a catalytic upgrade reaction takes place in gas-solid catalytic
reactor 302. A catalyst (for example, a granulated catalyst) may be introduced
at the top of
gas-solid catalytic reactor 302. In one embodiment, pre-upgrade pyrolysis gas
may be
introduced at the top of gas-solid catalytic reactor 302. In another
embodiment, pre-upgrade
pyrolysis gas may be introduced at one or more of the top, bottom, or side of
gas-solid
catalytic reactor 302.
[0037] In one
embodiment, pre-upgrade pyrolysis gas and a catalyst are allowed to
rapidly mix within gas-solid catalytic reactor 302 to facilitate sufficient
contact with the
catalyst surface. Gas-solid catalytic reactor 302 may include a volume while
the pre-upgrade
pyrolysis gas may include a flow rate, which volume and flow rate may be
adjusted relative
to one another to optimize the contact time between pre-upgrade pyrolysis gas
and a catalyst.
In one embodiment, the pre-upgrade pyrolysis gas has a residence time within
gas-solid
catalytic reactor 302 of about 1 s. In one embodiment, mass transfer
limitations and catalyst
reactivity dictate preferable catalyst-to-pyrolysis gas ratios. In one
embodiment, ratios may
be about 1:1. In another embodiment, higher amounts of catalyst are required.
In one
-9 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
embodiment, VPR 300 may be configured to modulate the catalyst-to-pyrolysis
gas ratio over
more than an order of magnitude.
[0038] Gas-
solid catalytic reactor 302 may include a reaction temperature between about
350 C and about 650 C. In another embodiment, gas-solid catalytic reactor
302 may
include a reaction temperature between about 400 C and about 600 C. In
another
embodiment, gas-solid catalytic reactor 302 may include a reaction temperature
between
about 450 C and about 550 C.
[0039] In one
embodiment, gas-solid catalytic reactor 302 may include a raining bed
reactor ("RBR") haying a series of angled baffles configured to facilitate
contact between
pre-upgrade pyrolysis gas and a catalyst. In another embodiment, gas-solid
catalytic reactor
302 may include any of a variety of reactor designs, including those used in
static mixers,
empty reactor tubes, and other reactors with features to facilitate catalyst
distribution.
[0040]
Following contact with the catalyst in gas-solid catalytic reactor 302,
upgraded
pyrolysis gas exits gas-solid catalytic reactor 302 on at least one of: the
bottom, the top, and
the side of gas-solid catalytic reactor 302. Upgraded pyrolysis gas exiting
gas-solid catalytic
reactor 302 may include catalyst particles picked up in gas-solid catalytic
reactor 302.
Upgraded pyrolysis gas may be at least substantially separated from the
catalyst particles.
[0041] In one
embodiment, at least one of first cyclone 310 and second cyclone 312 are
configured to receive upgraded pyrolysis gas. In another embodiment, first
cyclone 310 and
second cyclone 312 operate in series to receive upgraded pyrolysis gas. In
another
embodiment, additional cyclones may be used in the system. In another
embodiment, at least
one of first cyclone 310 and second cyclone 312 may be replaced with any of a
variety of
alternative solid-gas separation devices, such as baghouses.
- 10 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0042] In one
embodiment, upgraded pyrolysis gas may be introduced into first cyclone
310. First cyclone 310 may process the upgraded pyrolysis gas to separate
catalyst particles
from the upgraded pyrolysis gas, which particles may be at least one of
returned to gas-solid
catalytic reactor 302 or removed from VPR 300. In one embodiment, upgraded
pyrolysis gas
may be directed from first cyclone 310 to second cyclone 312 where second
cyclone 312
processes the upgraded pyrolysis gas to separate remaining catalyst particles
from the
upgraded pyrolysis gas, which particles may be at least one of returned to gas-
solid catalytic
reactor or removed from VPR 300. In one embodiment, catalyst particles
separated in second
cyclone 312 may be purged from VPR 300.
[0043] In one
embodiment, at least one of first cyclone 310 and second cyclone 312
causes a pressure drop within VPR 300. Such pressure drop may be undesirable.
In one
embodiment, pressure within VPR 300 may be maintained using hot blower 308.
Hot blower
308 may force gas into VPR 300 to cause a pressure increase that substantially
balances the
pressure drop experienced at first cyclone 310 and second cyclone 312.
[0044] The
catalytic upgrade reaction may generate a considerable amount of coke. The
coke may deposit on the catalyst, which may block the active sites and pores
of the catalyst,
thus rendering it less effective. As such, in one embodiment the coke should
be removed
from the catalyst. In one embodiment, the coke may be removed by continuous
oxidation in
air at a temperature between about 400 C and about 700 C. In another
embodiment, the
coke may be removed by continuation oxidation in air at a temperature between
about 450 C
and about 650 C. In another embodiment, the coke may be removed by continuous
oxidation in air at a temperature between about 500 C and about 600 C. In
one
embodiment, oxidation of the coke occurs inside fluidized bed reactor 318.
- 11 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0045] In one
embodiment, fluidized bed reactor 318 may be operatively connected to at
least one of a first blower 326 and a second blower 328. At least one of first
blower 326 and
second blower 328 causes an air flow within fluidized bed reactor 318. In
another
embodiment, at least one of first blower 326 and second blower 328 may be
configured to
direct air from fluidized bed reactor 318 into a distributor (not shown). Air
flow and the
volume of fluidized bed reactor 318 may be selected to optimize several design
requirements,
including at least one of: (1) air flow capable of generating appropriate
fluidization
conditions for the catalyst used, (2) air flow sufficient to deliver enough
oxygen for coke
oxidation, and (3) air flow, temperature, and fluidized bed reactor 318 volume
must facilitate
coke oxidation kinetics.
[0046] In one
embodiment, the coke burning reaction may be highly exothermic and heat
generated therein may be dissipated through any of various means, including
heat transfer
from fluidized bed reactor 318 to the gas atmosphere surrounding fluidized bed
reactor 318.
In another embodiment, heat dissipation may be enhanced by using room
temperature air for
fluidization. In another embodiment, heat dissipation may be enhanced by using
another
cooling medium directed into fluidized bed reactor 318.
[0047]
Oxidation of coke may produce byproducts including carbon monoxide, carbon
dioxide, and water. Significant quantities of carbon monoxide generated in
oxidation of coke
may be exhausted through at least one of third cyclone 324 and first blower
326.
[0048] Catalyst
particles from fluidized bed reactor 318 may be entrained by the
fluidizing air and/or exhaust gases. The catalyst particles may be removed
from the
fluidizing air to minimize catalyst losses. Third cyclone 324 may be used to
process the
fluidizing air to at least substantially separate catalyst from the fluidizing
air, which catalyst
may be at least one of returned to fluidized bed reactor 318 or discarded from
VPR 300.
- 12 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
Third cyclone 324 may be contained within fluidized bed reactor 318. In one
embodiment,
additional cyclones may be utilized in series with third cyclone 324. In
another embodiment,
the additional cyclones and/or third cyclone 324 may be located inside or
outside fluidized
bed reactor 318 or a hot box surrounding VPR 300.
[0049] In one
embodiment, at least one of first blower 326 and second blower 328 are
needed to maintain a desired pressure balance between gas-solid catalytic
reactor 302 and
fluidized bed reactor 318. In another embodiment, at least one of first blower
326 and second
blower 328 permit independent control of air flow in fluidized bed reactor 318
and internal
pressure of fluidized bed reactor 318.
[0050] In one
embodiment, feeding of the catalyst into gas-solid catalytic reactor 302 and
fluidized bed reactor 318, and recirculation of catalyst between gas-solid
catalytic reactor 302
and fluidized bed reactor 318 may be effected by feeding auger 304 and return
auger 306.
Feeding auger 304 and return auger 306 may be powered by at least one power
source, for
example an electric motor. The electric motor may be placed outside VPR 300
but coupled
mechanically with feeding auger 304 and return auger 306. Feeding auger 304
and return
auger 306 may include auger screws configured to rotate and advance catalyst
material. Flow
rate of the catalyst can be controlled by adjusting the rotation speed of
feeding auger 304 and
return auger 306. Such adjustment of the rotation speed of feeding auger 304
and return
auger 306 may be utilized to adjust the catalyst to biomass ratio and
effectively control the
catalytic reaction rate. Feeding auger 304 may direct catalyst from catalyst
feeding vessel
322 to gas-solid catalytic reactor 302. Return auger 306 may direct catalyst
from gas-solid
catalytic reactor 302 to split connection 314.
[0051] Split
connection 314 may be configured to preferably direct the catalyst to
fluidized bed reactor 318 for regeneration. Split connection 314 may direct
catalyst material
- 13 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
to dip leg pipe 316 immersed into the catalyst contained in fluidized bed
reactor 318. As a
result of the hydrostatic balance of the catalyst within fluidized bed reactor
318, and the
proper pressure balance between gas-solid catalytic reactor 302 and fluidized
bed reactor 318,
dip leg pipe 316 remains at least partially filled with catalyst. The
partially filled state of dip
leg pipe 316 acts as a gas lock to limit the amount of gas that can pass to
and from fluidized
bed reactor 318. This gas lock at least substantially separates the pyrolysis
gas atmosphere
existing inside gas-solid catalytic reactor 302, feeding auger 304, and return
auger 306, from
the oxygen-rich atmosphere inside fluidized bed reactor 318.
[0052] Bypass
connection 320 allows catalyst to bypass fluidized bed reactor 318 in the
event that catalyst may be not accepted into fluidized bed reactor 318 via dip
leg pipe 316. In
one embodiment, catalyst may be required to bypass fluidized bed reactor 318
due to
insufficient fluidization inside fluidized bed reactor 318, or a loss of air
flow into fluidized
bed reactor 318. Bypass connection 318 may allow for uninterrupted catalytic
upgrade
reaction and will prevent return auger 306 from becoming backed up with
catalyst.
[0053] Catalyst
processed in fluidized bed reactor 318 may be transferred to catalyst
feeding vessel 322 via a transfer connection 330. Upon reaching a sufficient
level in
fluidized bed reactor 318, catalyst may flow from fluidized bed reactor 318 to
catalyst
feeding vessel 322 via transfer connection 330. Similar to dip leg pipe 316,
transfer
connection 330 acts as a gas lock to limit the amount of gas that can pass to
and from
fluidized bed reactor 318.
[0054] Catalyst
transferred through bypass connection 320 into catalyst feeding vessel
322 without passing through fluidized bed reactor 318 may be not processed to
remove coke.
However, a catalyst contaminated with coke may be utilized in gas-solid
catalytic reactor 302
until it may be able to enter fluidized bed reactor 318 for processing.
- 14 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0055] FIG. 4
illustrates an example arrangement of a VPR 400. VPR 400 may include a
housing 402 containing an auger device 404 configured to move a catalyst
material and a
biomass pyrolysis vapor in counter-current directions. VPR 400 may be
configured to bring
the pyrolysis vapor and the catalyst into close contact. Housing 402 and auger
device 404
may include substantially circular cross-sections. In one embodiment, housing
402 may
include an inner diameter that may be substantially the same as or slightly
greater than the
outside diameter of auger device 404.
[0056] In one
embodiment, VPR 400 may be placed in a heated enclosure and coupled to
a catalyst regeneration system, such as that illustrated in FIG. 3. VPR 400
may operate in a
heated enclosure between about 300 C and about 600 C. In another embodiment,
VPR 400
operates in a heated enclosure between about 350 C and about 550 C. In
another
embodiment, VPR 400 operates in a heated enclosure between about 400 C and
about 500
C. In another embodiment, VPR 400 operates at an elevated temperature
configured to
prevent condensation of the pyrolysis vapor.
[0057] In one
embodiment, VPR 400 may include an inclined housing 402 and auger
device 404. A catalyst may enter VPR 400 via a catalyst inlet 406. The
catalyst may be
transported through VPR 400 within housing 402 via rotation of an auger device
404.
Catalyst may exit VPR 400 via a catalyst outlet 408.
[0058] In one
embodiment, pyrolysis vapor enters VPR 400 at a pyrolysis vapor inlet
410. Pyrolysis vapor may move through housing 402 by virtue of a pressure
differential, a
blower, or a pump. Pyrolysis vapor may move through housing 402 in contact
with the
catalyst. In one embodiment, pyrolysis vapor moves spirally about auger device
404. In one
embodiment, pyrolysis vapor exits VPR 400 via a pyrolysis vapor outlet 412. In
one
embodiment, pyrolysis vapor inlet 410 may be near the upper portion of housing
402 and
- 15 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
pyrolysis vapor outlet 412 may be near the lower portion of housing 402. In
another
embodiment, pyrolysis vapor inlet 410 may be near the lower portion of housing
402 and
pyrolysis vapor outlet 412 may be near the upper portion of housing 402. In
one
embodiment, pyrolysis vapor moves counter-current to the flow of the catalyst.
In another
embodiment, pyrolysis vapor moves concurrent to the flow of the catalyst.
[0059] In one
embodiment, pyrolysis vapor flows downward within housing 402 in a
spiral around auger device 404. Limited clearance between the outside diameter
of auger
device 404 and the inside diameter of housing 402 limits the bypass of gas
around the
catalyst, forcing pyrolysis vapor to contact the catalyst.
[0060] The
catalyst may enter and exit VPR 400 through a double block valve system
(such as that illustrated in FIG. 3) so as to substantially prevent pyrolysis
vapor from exiting
VPR 400 with the catalyst.
[0061] FIG. 5
illustrates an example arrangement of a VPR 500. VPR 500 may include a
substantially vertical or inclined housing 502, within which at least one
baffle 504 may be
oriented. A catalyst may enter VPR 500 via a catalyst inlet 506 and may exit
VPR 500 via a
catalyst outlet 508. A pyrolysis vapor may enter VPR 500 via a pyrolysis vapor
inlet 510 and
may exit VPR 500 via a pyrolysis vapor outlet 512.
[0062] In one
embodiment, at least one baffle 504 may be angled downward so as to
cause a catalyst to pour off at least one baffle 504 with pyrolysis vapor
moving through the
stream of catalyst material. In another embodiment, VPR 500 may include a
plurality of
baffles 504 in alternating positions and heights within VPR 500, such that a
catalyst pouring
over baffles 504 forms successive curtains as it falls from a higher baffle
504 to a lower
baffle 504. Pyrolysis vapor passes through the curtains of catalysts, and thus
contacts the
catalyst, as it travels through housing 502.
- 16 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0063] In one
embodiment, pyrolysis vapor inlet 510 may be oriented near the top of
VPR 500 and pyrolysis vapor outlet 512 may be oriented near the bottom of VPR
500. In
this embodiment, pyrolysis vapor flows concurrently with the catalyst. In
another
embodiment, pyrolysis vapor inlet 510 may be oriented near the bottom of VPR
500 and
pyrolysis vapor outlet 512 may be oriented near the top of VPR 500. In this
embodiment,
pyrolysis vapor flows counter-currently with the catalyst.
[0064] In one
embodiment, VPR 500 may be placed in a heated enclosure and coupled to
a catalyst regeneration system, such as that illustrated in FIG. 3. VPR 500
may operate in a
heated enclosure between about 300 C and about 600 C. In another embodiment,
VPR 500
operates in a heated enclosure between about 350 C and about 550 C. In
another
embodiment, VPR 500 operates in a heated enclosure between about 400 C and
about 500
C. In another embodiment, VPR 500 operates at an elevated temperature
configured to
prevent condensation of the pyrolysis vapor.
[0065] The
catalyst may enter and exit VPR 500 through a double block valve system
(such as that illustrated in FIG. 3) so as to substantially prevent pyrolysis
vapor from exiting
VPR 500 with the catalyst.
[0066] In one
embodiment, biomass may be directly added to the top of VPR 400 or VPR
500 to generate pyrolysis vapor while at the same time catalytically upgrading
the pyrolysis
vapor in a one-step process. In this embodiment, a pyrolysis reactor may not
be necessary.
[0067] In
various embodiments, a biofuel production system 100 is provided. The
biofuel production system may include a catalytic vapor phase reactor (VPR)
102. The
biofuel production system may include a pyrolysis reactor 104 operatively
connected to the
catalytic VPR 102. The biofuel production system may include a quench system
106
operatively connected to the catalytic VPR 102. The biofuel production system
may include
- 17 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
a water gas shift reactor 108 operatively connected to the quench system 106.
The biofuel
production system may include a hydrotreatment system 110 operatively
connected to the
quench system 106.
[0068] In some
embodiments, the pyrolysis reactor 104 may be configured to receive a
biomass 105. The pyrolysis reactor 104 may be configured to pyrolyze the
biomass 105 to
produce a pyrolysis vapor. The biomass 105 may include a wood.
[0069] The
pyrolysis reactor 104 may be configured to operate at a temperature capable
of converting at least a portion of biomass 105 to a pyrolysis vapor. The
pyrolysis reactor
may be configured to operate at a temperature in C of about 250, 275, 300,
325, 350, 375,
400, 425, 450, 475, 500, 525, 550, 575, 600, 625, or 650, e.g., about 500 C,
or between
about any two of the preceding values, for example, between about 300 C and
about 600 C,
between about 350 C and about 550 C, between about 400 C and about 500 C,
and the
like. For example, the pyrolysis reactor 104 may be configured to operate at a
temperature
between about 300 C and about 600 C.
[0070] In
several embodiments, the pyrolysis reactor 104 may be configured to pyrolyze
a biomass 105 to produce a pyrolysis vapor and char. The system 100 further
may include a
char removal system (not shown) configured to remove the char from the
pyrolysis reactor
104. The pyrolysis reactor 104 may be configured to pyrolyze a biomass 105 to
produce a
pyrolysis vapor. The pyrolysis vapor may include one or more of: water, an
organic acid, an
aldehyde, a phenol, and a sugar; or one or more derivatives thereof A heater
(not shown)
may be operatively coupled to the pyrolysis reactor 104. The heater may be
configured to at
least one of internally and externally heat pyrolysis reactor 104. The heater
may include one
or more of a resistive heating element, a combustor, a heat exchanger, or a
microwave
generator.
- 18 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0071] In
various embodiments, the catalytic VPR 102 may be configured to receive a
pyrolysis vapor. The catalytic VPR 102 may be configured to modify the
pyrolysis vapor to
produce a modified pyrolysis vapor. The catalytic VPR 102 may be configured to
produce a
modified pyrolysis vapor by deoxygenating a pyrolysis vapor produced by
pyrolyzing a
biomass. The catalytic VPR 102 may be configured to produce a modified
pyrolysis vapor
by cracking or breaking down one or more higher molecular weight components of
a
pyrolysis vapor produced by pyrolyzing a biomass. The catalytic VPR 102 may
include a
catalyst may include one or more of: a granulated catalyst, a powdered
catalyst, and a fluid
catalytic cracking catalyst (FCC). The catalyst may include one or more of:
fresh FCC, spent
FCC, catalyst impregnated on top of the fresh FCC, or catalyst impregnated on
top of the
spent FCC. The catalytic VPR 102 may include the granulated catalyst. The
granulated
catalyst may be characterized by particle size and flow characteristics
substantially similar to
the FCC. The catalytic VPR 102 may include a granulated catalyst characterized
by a
granule size between about 50 nm and about 100 nm. The granulated catalyst may
be
characterized by a size distribution of granules. A substantial fraction of
the size distribution
may be greater than about 20 nm. The catalytic VPR 102 may include a catalyst
configured
to catalyze at least one of: deoxygenation, cracking, water-gas shift, and
hydrocarbon
formation.
[0072] In some
embodiments, the pyrolysis reactor 104 and the catalytic VPR 102 may
be configured together as a single unit. The quench system 106 may be
configured to quench
a pyrolysis vapor to form a liquid bio-oil. The quench system 106 may be
configured to
quench a modified pyrolysis vapor to form a modified bio-oil. The bio-oil may
be a low
oxygen bio-oil. The quench system 106 may be configured to direct a non-
condensable gas
into the water gas shift reactor 108. The water gas shift reactor 108 may be
configured to
process a non-condensable gas including CO to form at least one of hydrogen
and CO2. The
- 19 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
hydrotreatment system 110 may be configured to accept hydrogen from the water
gas shift
reactor 108. The hydrotreatment system 110 may be configured to hydrotreat a
bio-oil, e.g., a
low oxygen bio-oil, with hydrogen to produce a hydrocarbon fuel product.
[0073] The
biofuel production system 100 may include a conversion system (not shown).
The conversion system may be operatively coupled to the catalytic vapor phase
reactor 102.
The conversion system may be operatively coupled to pyrolysis reactor 104. The
conversion
system may be operatively coupled to the hydrotreatment system 110. The
conversion
system may be configured to produce a hydrocarbon product from biomass 105 by
upgrading
a bio-oil. The bio-oil may be produced by one or more of: the catalytic vapor
phase reactor
102, the pyrolysis reactor 104, or the hydrotreatment system 110. The bio-oil
may be a liquid
or vapor bio-oil. The bio-oil may be modified or upgraded, e.g., by
hydrotreating. The
conversion system may be configured to produce at least one of: a hydrocarbon
fuel product,
a gas, or a chemical.
[0074] In
various embodiments, a method 201 for catalytic pyrolysis of biomass is
provided. The method may include 250 drying a biomass. The method may include
252
pyrolyzing the biomass to create a pyrolysis vapor. The method may include 254
removing
at least one of a char and an ash from the pyrolysis vapor. The method may
include 256
upgrading the pyrolysis vapor by vapor phase catalysis to produce an upgraded
pyrolysis
vapor. The method may include 258 condensing a bio-oil from the upgraded
pyrolysis vapor.
[0075] In
various embodiments, pyrolyzing the biomass may be conducted at a
temperature in C of about 250, 275, 300, 325, 350, 375, 400, 425, 450, 475,
500, 525, 550,
575, 600, 625, 650, 675, or 700, for example, about 500 C, or between about
any two of the
preceding values, for example, between about 300 C and about 700 C, between
about 300
C and about 600 C, between about 350 C and about 550 C, between about 400
C and
- 20 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
about 500 C, and the like. Upgrading the pyrolysis vapor may be conducted at
any of the
temperatures or temperature ranges described herein for pyrolyzing the
biomass. Pyrolyzing
the biomass and upgrading the pyrolysis vapor may be conducted at about the
same
temperature, for example, at about substantially the same temperature.
Pyrolyzing the
biomass and upgrading the pyrolysis vapor may be conducted at different
temperatures, for
example, at substantially different temperatures.
[0076] In some
embodiments, pyrolyzing the biomass may be conducted at a biomass
residence time of about 2 seconds or less. Pyrolyzing the biomass may be
conducted at a
biomass residence time of less than about 1 second, for example, about 0.9,
0.8, 0.7, 0.6, 0.5,
0.4, 0.3, 0.2, or 0.1 seconds, or less. Pyrolyzing the biomass and upgrading
the pyrolysis
vapor may be characterized by about the same residence time, for example,
about
substantially the same residence time. Pyrolyzing the biomass and upgrading
the pyrolysis
vapor may be characterized by different residence times, for example,
substantially different
residence times.
[0077] In
various embodiments, upgrading the pyrolysis vapor by vapor phase catalysis
to produce the upgraded pyrolysis vapor may be conducted after pyrolyzing the
biomass to
create the pyrolysis vapor and removing at least one of the char and the ash
from the
pyrolysis vapor, and before condensing the bio-oil from the upgraded pyrolysis
vapor. The
pyrolysis vapor may include one or more of: water, an organic acid, an
aldehyde, a phenol,
and a sugar; or one or more derivatives thereof
[0078] In some
embodiments, pyrolyzing the biomass to create the pyrolysis vapor may
include at least one of internally or externally heating a pyrolysis reactor.
Pyrolyzing the
biomass to create the pyrolysis vapor may include heating by resistive
heating, combustion
heating, heat exchanging, or microwave irradiation. Upgrading the pyrolysis
vapor by vapor
- 21 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
phase catalysis may include deoxygenating the pyrolysis vapor to produce the
upgraded
pyrolysis vapor. Upgrading the pyrolysis vapor by vapor phase catalysis may
include
cracking one or more higher molecular weight components of the pyrolysis vapor
to produce
the upgraded pyrolysis vapor. Upgrading the pyrolysis vapor by vapor phase
catalysis may
include contacting the pyrolysis vapor to one or more of: a granulated
catalyst, a powdered
catalyst, and a fluid catalytic cracking catalyst (FCC). Upgrading the
pyrolysis vapor by
vapor phase catalysis may include contacting the pyrolysis vapor to one or
more of: fresh
FCC, spent FCC, catalyst impregnated on top of the fresh FCC, or catalyst
impregnated on
top of the spent FCC. Upgrading the pyrolysis vapor by vapor phase catalysis
may include
contacting the pyrolysis vapor to the granulated catalyst, the granulated
catalyst characterized
by particle size and flow characteristics substantially similar to the FCC.
Upgrading the
pyrolysis vapor by vapor phase catalysis may include contacting the pyrolysis
vapor to a
granulated catalyst characterized by a granule size between about 50 ilm and
about 100 [tm.
Upgrading the pyrolysis vapor by vapor phase catalysis may include contacting
the pyrolysis
vapor to a granulated catalyst characterized by a size distribution of
granules. A substantial
fraction of the size distribution may be greater than about 20 [tm.
[0079] In
several embodiments, pyrolyzing the biomass and upgrading the pyrolysis
vapor may be conducted in a single pyrolysis-catalytic vapor phase reactor
unit. The bio-oil
may be a low oxygen bio-oil. Pyrolyzing the biomass may include producing a
non-
condensable including CO. The method may include reacting a non-condensable
including
CO in a water gas shift reaction to form at least one of hydrogen and CO2. The
method may
include hydrotreating the bio oil with hydrogen from the water gas shift
reaction to produce a
hydrocarbon fuel product. The method may include hydrotreating the bio oil
with hydrogen
to produce a hydrocarbon fuel product.
- 22 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0080] In some
embodiments, the method may include drying the biomass in a biomass
dryer 212. The method may include placing the biomass in a pyrolysis reactor
204 and
pyrolyzing the biomass at about 500 C to create a pyrolysis vapor. The method
may include
directing the pyrolysis vapor to a char and ash removal system 214 and
removing at least one
of a char and an ash from the pyrolysis vapor. The method may include
directing the
pyrolysis vapor to a catalytic vapor phase reactor 202 and upgrading the
pyrolysis vapor to
form an upgraded pyrolysis vapor. The method may include directing the
upgraded pyrolysis
vapor to a condenser 206. The method may include extracting a bio-oil from the
condenser
206.
[0081] In
various embodiments, a catalytic vapor phase reactor apparatus 300 is
provided. The apparatus may include: a gas-solid catalytic reactor 302; a
feeding auger 304; a
return auger 306; a hot blower 308; a first blower 326; a second blower 328; a
first cyclone
310; a second cyclone 312; a third cyclone 324; a split connection 314; a dip
leg pipe 316
operatively coupled to the split connection; a fluidized bed reactor 318; a
bypass connection
320; and a catalyst feeding vessel 322. The feeding auger 304 and the return
auger 306 may
be operatively connected to the gas-solid catalytic reactor 302 and the
fluidized bed reactor
318. The first cyclone 310 and the second cyclone 312 may be operatively
connected to the
gas-solid catalytic reactor 302. The third cyclone 324 may be operatively
connected to the
fluidized bed reactor 318, the first blower 326, and the second blower 328.
[0082] In some
embodiments, the catalytic vapor phase reactor apparatus may be
configured for continuous mode operation. The apparatus may be configured for
batch mode
operation. The apparatus may be configured to operate using air at atmospheric
pressure for
catalyst regeneration. The apparatus may be configured for catalyst
regeneration without
using gases with reduced oxygen content compared to air.
- 23 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
[0083] In
various embodiments, catalytic vapor phase reactor apparatus 300 may be
configured to operate, at least in part, at a temperature in C of about 250,
275, 300, 325, 350,
375, 400, 425, 450, 475, 500, 525, 550, 575, 600, 625, 650, 675, or 700, for
example, about
500 C, or between about any two of the preceding values, for example, between
about 300
C and about 700 C, between about 300 C and about 600 C, between about 350
C and
about 550 C, between about 400 C and about 500 C, and the like. In some
embodiments,
the components of catalytic vapor phase reactor apparatus 300 may be
configured to operate
at any of the preceding temperatures or temperature ranges, with the exception
of hot blower
308, first blower 326, and second blower 328. The catalytic vapor phase
reactor apparatus
may be configured at least in part to operate at a temperature effective to
drive one or more
of: a catalytic upgrade reaction, a catalyst regeneration reaction, or a coke
oxidation reaction.
The catalytic vapor phase reactor apparatus may be configured at least in part
to operate at a
temperature effective to mitigate condensation of condensable pyrolysis gases.
[0084] In some
embodiments, the catalytic vapor phase reactor apparatus may include a
heater operatively coupled to gas-solid catalytic reactor 302. The heater may
include one or
more of a resistive heating element, a combustor, a heat exchanger, or a
microwave
generator.
[0085] In
several embodiments, the gas-solid catalytic reactor 302 may be configured to
conduct a catalytic upgrade reaction. The gas-solid catalytic reactor 302 may
be configured
to accept introduction of a granulated catalyst. The gas-solid catalytic
reactor 302 may be
configured to accept introduction of a pre-upgrade pyrolysis gas. The gas-
solid catalytic
reactor 302 may be configured to mix the pre-upgrade pyrolysis gas and a
catalyst effective
to contact the catalyst surface with the pre-upgrade pyrolysis gas. The gas-
solid catalytic
reactor 302 may be configured to conduct the pre-upgrade pyrolysis gas with a
residence time
of about 1 second. The gas-solid catalytic reactor 302 may include a raining
bed reactor
- 24 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
configured to contact the pre-upgrade pyrolysis gas and the catalyst. The gas-
solid catalytic
reactor 302 may be configured to remove coke from the catalyst by continuous
oxidation in
air.
[0086] In
various embodiments, at least one of the first cyclone 310 and the second
cyclone 312 may be configured to receive upgraded pyrolysis gas. The first
cyclone 310 and
the second cyclone 312 may be configured to operate in series to receive
upgraded pyrolysis
gas. The first cyclone 310 may be configured to separate an upgraded pyrolysis
gas from at
least a first portion of a plurality of catalyst particles. The first cyclone
310 may be
configured to direct the first portion of the plurality of catalyst particles
separated from the
upgraded pyrolysis gas to one of: the gas-solid catalytic reactor 302; or an
exit of the catalytic
vapor phase reactor apparatus. The first cyclone 310 may be configured to
direct upgraded
pyrolysis gas to the second cyclone 312. The second cyclone 312 may be
configured to
separate the upgraded pyrolysis gas from at least a second portion of the
plurality of catalyst
particles. The second cyclone 312 may be configured to direct the second
portion of the
plurality of catalyst particles separated from the upgraded pyrolysis gas to
one of: the gas-
solid catalytic reactor 302; or an exit of the catalytic vapor phase reactor
apparatus.
[0087] In some
embodiments, bed reactor 318 may be operatively connected to at least
one of the first blower 326 and the second blower 328. Feeding auger 304 and
return auger
306 may be operatively connected for feeding of a catalyst into the gas-solid
catalytic reactor
302 and the fluidized bed reactor 318. Feeding auger 304 and return auger 306
may be
operatively connected for recirculation of catalyst between gas-solid
catalytic reactor 302 and
fluidized bed reactor 318.
[0088] In
several embodiments, split connection 314 may be configured to direct a
catalyst to the fluidized bed reactor 318 for regeneration. Bypass connection
320 may be
- 25 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
configured to cause catalyst to bypass fluidized bed reactor 318. Transfer
connection 330
may be configured to direct a catalyst processed in fluidized bed reactor 318
to catalyst
feeding vessel 322.
[0089] In
various embodiments, a catalytic vapor phase reactor apparatus 400 is
provided. The apparatus may include a housing 402. The housing 402 may include
an auger
device 404, a catalyst inlet 406, a catalyst outlet 408, a pyrolysis vapor
inlet 410, and a
pyrolysis vapor outlet 412. The auger device 404 may be configured to
transport a solid
catalyst through at least a portion of the housing 402. The housing 402 may be
configured to
permit a pyrolysis vapor to flow through at least a portion of the housing 402
and come into
contact with the solid catalyst.
[0090] In some
embodiments, the auger device 404 may be configured to direct the
catalyst material and the biomass pyrolysis vapor in counter-current
directions. The auger
device 404 may be configured to direct the catalyst material and the biomass
pyrolysis vapor
in concurrent directions. The apparatus may be configured to bring the
pyrolysis vapor and
the catalyst into contact. The apparatus may be configured within a heated
enclosure. The
heated enclosure configured to heat to a temperature between about 300 C and
about 700 C,
for example, about any temperature or temperature range described herein. The
apparatus
may be operatively coupled to a catalyst regeneration system, for example as
depicted in
FIG. 3 in apparatus 300.
[0091] In
various embodiments, a catalytic vapor phase reactor apparatus 500 is
provided. The apparatus may include a housing 502. The housing 502 may include
at least
one baffle 504, a catalyst inlet 506, a catalyst outlet 508, a pyrolysis vapor
inlet 510, and a
pyrolysis vapor outlet 512. The housing 502 may be at least one of
substantially vertical and
inclined, for example, with reference to a local direction of gravity. The
housing 502 may be
- 26 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
configured to permit a solid catalyst to flow through at least a portion of
the housing 502 and
over at least one baffle 504. The housing 502 may be configured to permit a
pyrolysis vapor
to flow through at least a portion of the housing 502 and come into contact
with the solid
catalyst. The at least one baffle 504 may be angled effective to cause a
catalyst to pour off
the at least one baffle 504 with the pyrolysis vapor moving through the stream
of the solid
catalyst. The apparatus further may include a plurality of baffles 504
configured effective to
cause a catalyst pouring over the plurality of baffles 504 to form successive
curtains of the
catalyst while falling between each of the plurality of baffles 504. The
pyrolysis vapor inlet
510 and the pyrolysis vapor outlet 512 may be configured to direct a pyrolysis
vapor to flow
concurrently with the solid catalyst. The pyrolysis vapor inlet 510 and the
pyrolysis vapor
outlet 512 may be configured to direct a pyrolysis vapor to flow counter-
currently with the
solid catalyst. The apparatus may be configured within a heated enclosure. The
heated
enclosure may be configured to heat to a temperature between about 300 C and
about 700
C, for example, about any temperature or temperature range described herein.
The apparatus
may be operatively coupled to a catalyst regeneration system, e.g., as
described in FIG. 3 and
apparatus 300.
[0092] To the
extent that the term "includes" or "including" is used in the specification or
the claims, it is intended to be inclusive in a manner similar to the term
"comprising" as that
term is interpreted when employed as a transitional word in a claim.
Furthermore, to the
extent that the term "or" is employed (e.g., A or B) it is intended to mean "A
or B or both."
When the applicants intend to indicate "only A or B but not both" then the
term "only A or B
but not both" will be employed. Thus, use of the term "or" herein is the
inclusive, and not the
exclusive use. See Bryan A. Garner, A Dictionary of Modern Legal Usage 624
(2d. Ed.
1995). Also, to the extent that the terms "in" or "into" are used in the
specification or the
claims, it is intended to additionally mean "on" or "onto." To the extent that
the term
- 27 -
CA 02907106 2015-09-15
WO 2014/145223
PCT/US2014/029946
"selectively" is used in the specification or the claims, it is intended to
refer to a condition of
a component wherein a user of the apparatus may activate or deactivate the
feature or
function of the component as is necessary or desired in use of the apparatus.
To the extent
that the term "operatively connected" is used in the specification or the
claims, it is intended
to mean that the identified components are connected in a way to perform a
designated
function. To the extent that the term "substantially" is used in the
specification or the claims,
it is intended to mean that the identified components have the relation or
qualities indicated
with degree of error as would be acceptable in the subject industry. As used
in the
specification and the claims, the singular forms "a," "an," and "the" include
the plural.
Finally, where the term "about" is used in conjunction with a number, it is
intended to include
10% of the number. In other words, "about 10" may mean from 9 to 11.
[0093] As
stated above, while the present application has been illustrated by the
description of embodiments thereof, and while the embodiments have been
described in
considerable detail, it is not the intention of the applicants to restrict or
in any way limit the
scope of the appended claims to such detail. Additional advantages and
modifications will
readily appear to those skilled in the art, having the benefit of the present
application.
Therefore, the application, in its broader aspects, is not limited to the
specific details,
illustrative examples shown, or any apparatus referred to. Departures may be
made from
such details, examples, and apparatuses without departing from the spirit or
scope of the
general inventive concept.
- 28 -