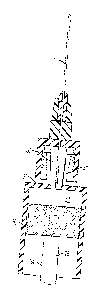Note: Descriptions are shown in the official language in which they were submitted.
CA 02908822 2015-10-05
LOW WASTE SYRINGE AND NEEDLE ASSEMBLAGE
FIELD OF THE INVENTION
[0001] Thc field of this invention relates generally to needles and
syringes for injecting
a selected fluid into a patient, and more particularly to a low-waste syringe
with at least one
interchangeable needle. With more particularity, to a low-waste syringe with
at least one
interchangeable needle for injection of a desired neurotoxin therein a
patient.
BACKGROUND OF THE INVENTION
[0002] Neurotoxins such as Allergan's Botox (Onabotulinumtoxin Type A),
Medicis'
Dysport (Aboborulinumtoxin Type A) and Merz's Xeomin (Incoborulinumtoxin
Type A)
have entered the medical aesthetic marketplace over the last 10-15 years and
arc currently
used worldwide culminating in a global multi-billion dollar industry.
[0003] Neurotoxins are currently used for both medical and aesthetic
purposes. The
U.S. Food and Drug Administration (FDA) has approved Botox for glabellar
wrinkle
reduction, Dysport for cervical dystonia and glabellar creases and Xeomin
for cervical
dystonia, blepharospasm and glabellar frown lines. Although these three drugs
have FDA
approval in the United States for "on label" use of their neurotoxin type A
for the treatment
of glabellar crease lines only, all three are used by most physician and nurse
injectors "off
label" for dynamic wrinkle reduction throughout the head and neck region.
[0004] The predominant company in the global aesthetic marketplace is
Allergan which
manufactures and distributes Botox and Botox Cosmetic globally (referred to
hereinafter
together as Botox ). Botox is dispensed in 50 unit and 100 unit freeze dried
vials and has
to be reconstituted by the physician and/or nurse injectors (the "user") with
preservative free,
sterile injectable saline or the less painful, neutral pH bacteriostatic
saline just before use. As
one skilled in the art will appreciate, conventional neurotoxin vials are both
multi and single
dose and have an elastomeric top that can be pierced sterilely with a needle.
Dysport is
dispensed in 300 unit vials and Xeomin is dispensed in 100 unit vials similar
to Botox .
[0005] According to the FDA approval for Botox and the package insert, the
100 unit
vial should be reconstituted with 2.5 ml of 0.9% sterile, unpreserved saline.
Most users,
however, use bacteriostatic saline for the diminished discomfort and longer
shelf-life it offers.
Since its FDA approval, the clinical use of Botox and the amount of diluent
used for
reconstitution has greatly evolved with most users and there is currently no
standard dilution.
The trend, however, has been toward lesser diluent volumes and more
concentrated
1
CA 02908822 2015-10-05
neurotoxin solutions. For example, the most common diluent volumes used today
are 1 ml
per vial (10 units/0.1 ml), 2 ml per vial (5 units/0.1 ml), 2.5 ml per vial
(on-label for Botox
at 4 units/0.1 ml) and 4 ml per vial (2.5 units/0.1 ml). The reason for this
trend is better
placement control, better potential site efficacy at higher concentrations,
less fluid volume
injected with less pain and swelling and less chance of neurotoxin diffusion
from the site of
injection causing an adverse effect such as ptosis (drooping) of the upper
eyelid or ptosis of
the brow and eyebrow. There is no standard neurotoxin dilution for Botox
(other than the
FDA approved 2.5 ml) and no standard delivery system based upon the
practitioner's chosen
amount of diluent and concentration of neurotoxin.
[0006] The average patient receiving Botox for the treatment of dynamic
glabellar
frown lines will receive approximately 25 units of neurotoxin distributed over
approximately
7 injections. Additional areas to be injected will require more neurotoxin and
more
injections. Each injection delivers approximately 1-4 units per injection
depending upon the
site and injector. If the ncurotoxin is diluted with 1 ml of diluent this will
produce a
concentration of 10 units/0.1 ml or 1 unit/.01 ml. In the above example in
which a patient
receives 25 units, a total volume of 0.25 ml will be injected into the
patient. A conventional
1 ml syringe can be too large to accurately dispense these small volumes and
the gradations
on the syringe can be too difficult to read therefore not allowing for
accurate dispensing of
the neurotoxin. Additionally, 1 ml syringes are graduated in 1 ml increments
and not units.
[0007] Additionally, most 1 ml neurotoxin syringes are not low-waste
syringes, and a
significant amount of neurotoxin can be wasted with each use of a multi-use
vial. With each
use of a syringe, costly neurotoxin will be lost in dead spaces of the syringe
tip, the hub of the
needle and/or the needle lumen. For a 1 ml syringe this has been measured to
be at least
approximately 0.08 ml and the waste is worsened if the needle is exchanged
during the series
of injections.
[0008] Also, conventional neurotoxin syringes are not graduated in unit
dosing which is
how users are trained to inject neurotoxin. Further, if conventional
neurotoxin syringes do
not have Luer lock connections, the needle can come dislodged from the syringe
during an
injection and can cause an injury to the patient and waste the costly
neurotoxin.
[0009] Therefore, many practitioners have circumvented these problems by
using
insulin syringes that are either 30 unit or 50 unit syringes that can
accommodate the 10
unit/0.1 ml concentration to get a true 1:1 injection ratio. Insulin syringes
can only be used
2
CA 02908822 2015-10-05
for unit dosing at the 1 ml dilution of neurotoxin because the resulting
concentration is the
same as subcutaneous insulin at 1 unit/MI ml. In all other concentrations of
neurotoxin that
are injected with insulin syringes, each gradation no longer represents 1 unit
of neurotoxin.
[0010] Most of the insulin syringes are low-waste and have permanent, non-
removable
needles. Insulin syringes are designed for single-injection only in the
subcutaneous tissue
plane and are not engineered for the multiple percutaneous punctures required
of neurotoxin
injections. Insulin needles are not sufficiently engineered to withstand
multiple percutancous
punctures. Insulin syringes are small and it is very difficult to read the
gradations and they
are awkward for larger hands. The needles dull quickly and cannot be changed
for a sharper
needle. The length of the needle is designed for subcutaneous injection and
not intramuscular
injection (indicated for neurotoxin) and it is too short for many patients
thus producing a poor
result. Multiple syringes would be necessary if greater than 30 or 50 units
were to be injected
losing time exchanging syringes and the cost of additional syringes.
[0011] Another problem with insulin syringes with permanent needles is that
if the
neurotoxin is aspirated from the vial through the elastomeric top, the needle
will be dulled
even further for multiple injections. Many practitioners remove the metal seal
and
elastomeric stopper of the vial and insert the entire clean, but not sterile,
syringe into the vial
to aspirate the desired amount of neurotoxin and then replace the elastomeric
stopper. This
procedure contaminates the vial each time it is done (which is typically three
times or more
per vial). This is not standard protocol for a sterile, multi-use vial and
greatly increases the
risk of injection site infection and bacterial contamination of the neurotoxin
left in the vial.
Another problem with insulin syringes with permanent needles is that if the
needle gets dull
and painful during the series of injections there is no way to exchange the
needle for a
sharper one or easily transfer the neurotoxin to another syringe so the costly
neurotoxin is not
wasted. Unfortunately, the injections often continue at the expense and
discomfort of the
patient until the syringe is empty.
[0012] Non-low-waste syringes in this volume range include 1 ml syringes
and some
insulin syringes. The measured waste in the conventional non-low-waste syringe
includes
approximately 0.04 ml in the tip and approximately 0.04 ml in the needle hub.
Thus, at the
end of a series of injections approximately 0.08 ml of fluid is left in the
syringe tip and needle
hub. This translates into a significant loss of neurotoxin and cost to the
practitioner
depending upon the amount of diluent used and the resultant concentration of
the neurotoxin
injected. In some estimates, the lost or wasted neurotoxin can be in the tens
of thousands of
3
CA 02908822 2015-10-05
dollar per user per year. Further, although 1 ml low-waste syringes are
available, they are not
gradated for unit injection, which is how most users arc trained to inject.
Additionally,
conventional low-waste 1 ml syringes only prevent neurotoxin waste in the
syringe tip, not
the needle hub, and thus, costly neurotoxin is still wasted.
[0013] In view of the preceding, there is a need in the art for a low-waste
neurotoxin
syringe and needle that can indicate unit dosage at a plurality of neurotoxin
concentration
levels.
SUMMARY OF THE INVENTION
[0014] Described herein is a needle and syringe assembly for injecting a
fluid into a
patient and more particularly to a low-waste syringe with at least one
interchangeable needle.
[0015] In one aspect, the syringe can comprise a hollow body having an
inner diameter
and an end wall closing a forward end of the body. In another aspect, a rear
end of the body
can be open and a piston means in reciprocable sealing engagement with an
inner wall of the
body can define a fluid chamber in the body. The fluid chamber can be
configured for
selectively containing a medication, such as for example and without
limitation, a neurotoxin,
within the fluid chamber.
[0016] At least a portion of the syringe can be formed from a clear
polymeric material,
according to one aspect. In another aspect, an outer wall of the body can be
marked and/or
labeled to indicate the type of fluid contained in the chamber. For example,
if the fluid is a
neurotoxin, the outer wall of the body can be marked and/or labeled to
indicate the type of
neurotoxin and/or the amount of diluent used in reconstituting the neurotoxin.
In one aspect,
hatch marks can be marked and/or labeled on the outer wall of the body to
indicate the
amount of fluid and/or the concentration of the fluid contained in the
chamber. For example,
the hatch marks can be color coded such that different colored hatch mark can
indicate
dosage amounts based on different concentrations. The clear body allows the
user to
compare the fluid level in the chamber to the hatch mark on the body.
[0017] In one aspect, a syringe tip can be mounted and/or formed on the end
wall of the
syringe to define an interior void. An aperture in the end wall of the body
can place the
interior void of the syringe tip in sealed fluid communication with the fluid
chamber of the
body. In another aspect, the syringe tip can be configured to matingly engage
and secure a
needle assembly to the syringe. In a further aspect, the syringe tip can form
at least a portion
4
of an inverted cone, In this aspect, at least a portion of the syringe tip can
be substantially
frusto-conical in shape defining a frusto-conical interior void.
[0018] The piston means can comprise a plunger and a piston cap. In one
aspect, the
plunger can be formed from a substantially cylindrical shaft and the piston
cap can be securedly
attached to an end of the shaft. In another aspect, the piston cap can be
formed from a clastorner
wherein at least a portion of the piston cap has an outer diameter
substantially equal to the inner
diameter of the body of the syringe, However, in a further aspect, at least a
portion of the piston
cap can have an outer diameter slightly greater than the inner diameter of the
body of the syringe.
In yet another aspect, a distal end of the piston cap can be configured to
complementary engage
the end wall of the body of the syringe. That is, the distal end of the piston
cap can be sized and
shaped so that when in use, the distal end of the piston cap contacts the end
wall. In this aspect,
when in use and the piston cap contacts the end wall of the body, there are
substantially no gaps or
"dead spaces" formed between the end wall and the distal end of the piston
cap. This substantially
allows the fluid contained in the fluid chamber to be ejected from the chamber
through the syringe
tip.
100191 In one aspect, the at least one needle assembly can comprise an
elongate needle and
a polymeric needle hub configured to support the needle and couple the needle
to the syringe so
that an interior lumen of the needle is in fluid communication with the fluid
chamber of the
syringe. In another aspect, the needle can be a conventional needle, such as a
25G needle, a 32G
needle and the like. The needle hub can comprise a substantially cylindrical
hollow needle base
having internal threads configured to matingly engage with flanges on the
syringe as in a
conventional Luer-lock engagement. 'none aspect, a frusto- conical member can
be formed
and/or positioned in the substantially cylindrical hollow needle base of the
needle hub. In this
aspect, the frusto-conical member can be configured to matingly engage the
frusto-conical void
defined in the syringe tip. When the threads of the needle hub engage the
syringe, the frusto-
conical member of the needle hub can create a fluid-tight seal with the frusto-
conical void of the
tip of the syringe, so that when in use, there are substantially no gaps or
"dead spaces" formed
between the needle hub and the syringe.
100201 Additional advantages of the invention will be set forth in part
in the description
which follows, and in part will be obvious from the description, or may be
learned by practice of
the invention. The advantages of the invention will be realized and attained
by means of the
elements and combinations particularly pointed out in the description. It is
lobe
WSLEGA L1074855 00004 \18072108v1
CA 2908822 2017-06-05
CA 02908822 2015-10-05
understood that both the foregoing general description and the following
detailed description
are exemplary and explanatory only and are not restrictive of the invention,
as claimed.
BRIEF DESCRIPTION OF THE FIGURES
[0021] These and other features of the preferred embodiments of the
invention will
become more apparent in the detailed description in which reference is made to
the appended
drawings wherein:
[0022] Figures IA and 1B are elevational views of embodiments of a needle
assembly
and syringe for injecting a fluid contained in the syringe into a patient.
[0023] Figure 2 is an elevational view of the syringe of Figure 1,
according to one
aspect.
[0024] Figure 3 is a cross-sectional view of the syringe of Figure 2, taken
along line 3-
3.
[0025] Figure 4 is an elevational view of a portion of the syringe of
Figure 2, according
to one aspect.
[0026] Figure 5 is an cicvational view of the needle assembly of Figure I
coupled to the
syringe, according to one aspect.
[0027] Figure 6 is an elevational view of the needle assembly coupled to
the syringe,
according to an alternative aspect.
[0028] Figure 7 is an elevational view of a plunger of the syringe of
Figure 1, according
to one aspect.
[0029] Figure 8 is an elevational view of a plunger of the syringe of
Figure 1, in which
the broken lines indicate a head of the plunger, according to one aspect.
[0030] Figure 9 is an elevational view of a protective cover of the syringe
of Figure 1,
according to one aspect.
[0031] Figure 10 is a perspective view of the needle assembly of Figure 1,
according to
one aspect.
[0032] Figure 11 is a cross-sectional view of the needle assembly of Figure
10,
according to one aspect.
6
100331 Figure 12 is a top plan view of needle assembly of Figure 10,
according to one
aspect.
10034] Figure 13 is a cross-sectional view of the needle assembly of
Figure I 0, showing
a needle guard positioned over the needle, according to one aspect.
[0035] Figure 14 is an exploded cross-sectional view of the needle
assembly of Figure
10, showing a needle guard positioned over the needle and a base guard aligned
to overlap at
least a portion of the a needle base, according to one aspect.
[0036] Figure 15 is an elevational view of the needle guard and the base
guard of
Figure 14, showing the guards is a closed position, according to one aspect.
DETAILED DESCRIPTION OF THE INVENTION
100371 The present invention can be understood more readily by reference
to the
following detailed description, examples, drawing, and their previous and
following
description, however, before the present devices, systems, and/or methods are
disclosed and
described, it is to be understood that this invention is not limited to the
specific devices,
systems, and/or methods disclosed unless otherwise specified, as such can, of
course, vary. It
is also to be understood that the terminology used herein is for the purpose
of describing
particular aspects only and is not intended to be limiting.
100381 The following description of the invention is provided as an
enabling teaching
of the invention in its best, currently known embodiment. To this end, those
skilled in the
relevant art will recognize and appreciate that many changes can be made to
the various
aspects of the invention described herein, while still obtaining the
beneficial results of the
present invention. Itvvill also be apparent that some of the desired benefits
of the present
invention can be obtained by selecting some of the features of the present
invention without
utilizing other features. Accordingly, those who work in the art will
recognize that many
modifications and adaptations to the present invention are possible and can
even be desirable
in certain circumstances and are a part of the present invention. Thus, the
following
description is provided as illustrative of the principles of the present
invention and not in
limitation thereof. AS used throughout, the singular forms "a," "an" and "the"
include plural
referents unless the context clearly dictates otherwise. Thus, for example,
reference to "a
needle" can include two or more such needles unless the context indicates
otherwise,
[0039] Ranges can be expressed herein as from "about" one particular
value, and/or to
"about" another particular value. When such a range is expressed, another
aspect includes
7
WS LEGA L 074855 \ 00004 `, 18072108v I
CA 2908822 2017-06-05
CA 02908822 2015-10-05
from the one particular value and/or to the other particular value. Similarly,
when values are
expressed as approximations, by use of the antecedent "about," it will be
understood that the
particular value forms another aspect. It will be further understood that the
endpoints of each
of the ranges are significant both in relation to the other endpoint, and
independently of the
other endpoint.
[0040] As used herein, the terms "optional" or "optionally" mean that the
subsequently
described event or circumstance may or may not occur, and that the description
includes
instances where said event or circumstance occurs and instances where it does
not.
[0041] As used herein, the term "fluid" can refer to any medication such as
a
neurotoxin, insulin, tuberculin and the like. Additionally, the term "fluid"
can refer to a
solution containing a diluent and any medication such as a neurotoxin,
insulin, tuberculin and
the like.
[0042] A needle and syringe assemblage 10 for injecting a fluid into a
patient is
provided, according to various aspects and as illustrated in Figures lA and
1B. In one aspect,
the needle and syringe assemblage comprises a syringe 12 and at least one
needle assembly
14. In another aspect, the needle and syringe assemblage 10 comprises a low-
waste syringe
and at least one interchangeable needle. In a further aspect, the syringe 12
can be a low-
waste, single-use syringe with at least one interchangeable needle assembly
14.
[0043] Referring now to Figures 2 and 3, in one aspect, the syringe 12 can
comprise a
hollow body 16 having an inner diameter 18 and an end wall 20 that can close
the body at a
forward end 22 of the body. In another aspect, a rear end 24 of the body 16
can be open and
a piston means 26 in reciprocable sealing engagement with an inner wall 28 of
the body can
define a chamber 30 in the body. The chamber can be configured for selectively
containing a
fluid, such as for example and without limitation, medication, within the
chamber 30. The
syringe 12 can further comprise at least one finger flange 32 formed or
positioned adjacent
the open rear end of the body 16, according to another aspect.
[0044] In one aspect, the body 16 of the syringe 12 can have a length of
less than about
7.0 cm, about 7.0 cm, about 7.15 cm, about 7.25 cm, about 7.4 cm, about 7.5
cm, about 7.65
cm, about 7.75 cm, about 7.85 cm, about 8.0 cm, about 8.15 cm, about 8.25 cm,
about 8.4
cm, about 8.5 cm, about 8.65 cm, about 8.75 cm, about 8.85 cm, about 9.0 cm,
about 9.15
cm, about 9.25 cm, about 9.4 cm, about 9.5 cm, about 9.65 cm, about 9.75 cm,
about 9.85
8
CA 02908822 2015-10-05
=
cm, about 10.0 cm, or greater than about 10.0 cm. In another aspect, the body
16 of the
syringe has a length between about 7.0 cm to a about10.0 cm.
[0045] In another aspect, an outer diameter 34 of the body 16 can be
less than about
0.25 cm, about 0.25 cm, about 0.3 cm, about 0.35 cm, about 0.4 cm, about 0.45
cm, about 0.5
cm, about 0.55 cm, about 0.6 cm, about 0.65 cm, about 0.7 cm, about 0.75 cm,
about 0.8 cm,
about 0.85 cm, about 0.9 cm, about 0.95 cm, about 1.0 cm, about 1.05 cm, about
1.1 cm,
about 1.15 cm, about 1.2 cm, about 1.25 cm, about 1.3 cm, about 1.35 cm, about
1.4 cm,
about 1.45 cm, about 1.5 cm, about 1.55 cm, about 1.6 cm, about 1.65 cm, about
1.7 cm,
about 1.75 cm, about 1.8 cm, about 1.85 cm, about 1.9 cm, about 1.95 cm, about
2.0 cm or
greater than about 2.0 cm. In this aspect, it is contemplated that the outer
diameter of the
body 16 can be a substantially constant diameter, and the inner diameter 18 of
the body can
be varied to change the volume capacity of the chamber 30 of the body 16. For
example, the
inner diameter can be a predetermined diameter so that the volume capacity of
the body is a
predetermined level. Thus, two syringes having the same body size can contain
a different
amount of fluid based on the volume capacity of the chamber.
[0046] In one aspect, at least a portion of the syringe 12 can be formed
from a clear
polymeric material. In another aspect, the body 16 of the syringe can be
molded from a hard,
clear plastic. An exterior surface or outer wall 36 of the body can be
printed, marked and/or
labeled to indicate the type of fluid contained in the chamber. For example,
if the fluid is a
neurotoxin, the outer wall of the body 16 can be marked and/or labeled to
indicate the type of
neurotoxin and/or the amount of diluent used in reconstituting the neurotoxin.
In a further
aspect, hatch marks 38 can be printed, marked and/or labeled on the outer wall
36 of the body
to indicate the amount of fluid contained in the chamber 30. In yet another
aspect, the hatch
marks can be positioned or printed on either side of a centerline of the body
16 so that both
left handed and right handed users of the syringe can easily see the hatch
marks 38. In this
aspect, the hatch marks can be color coded such that different colored hatch
mark 38 can
indicate different fluid concentrations.
[0047] In one aspect, the hatch marks 38 on the exterior surface or
outer wall 36 of the
syringe 12 can indicate a concentration marking scale. That is, hatch marks
can be printed or
marked on the syringe to refer to a concentration of fluid contained in the
chamber 30 of the
syringe. For example, each hatch mark can refer to a volume of medication per
volume of
diluent. In another aspect, the hatch marks 38 on the outer wall of the
syringe can be
indicative of the relative units of medication per volume of diluent. In an
example and with
9
CA 02908822 2015-10-05
reference to Figure 2, the "10u", "20u",,. markings can indicate the units of
neurotoxin per
volume of diluent. This allows the user of the syringe to easily "unit dose"
the patient as
users have conventionally been trained. As can be appreciated, different
syringes can be
provided to a user based on the user's desired medication concentration level.
[0048] For example, if 100 units of neurotoxin were diluted with 1 ml of
diluent, the
solution would have a concentration of 10 units per 0.1 ml or 1 unit per 0.01
ml. The syringe
12 could have a chamber 30 sized to hold 60 units and a total volume of 0.6m1.
The hatch
marks 38 on the body 16 of the syringe 12 could be unit marked at 1 or 2 unit
increments and
each unit increment could correspond to 0.01 ml of the solution. In another
example, if 100
units of neurotoxin were diluted with 2 ml of diluent, this would create a
solution having a
concentration of 5 units per 0.1 ml or 1 unit per 0.02 ml. In this example,
the syringe could
have a chamber sized to hold 60 units and a total volume of 1.2 ml. That is,
the inner
diameter 18 of the body could be sized so that the chamber 30 could contain
1.2 ml of
medication. In this aspect, the hatch marks on the body 16 of the syringe 12
could be unit
marked at 1 or 2 unit increments and each unit increment could correspond to
.02 ml of the
solution. In another example, if 100 units of neurotoxin were diluted with 2.5
ml of diluent
(such as, for example, on label for Botox ) this would create a concentration
of 4 units per
0.1 ml or 1 unit per 0.025 ml. In this example, the chamber 30 of the body
could hold 50
units and a total volume of 1.25 ml. The hatch marks 38 on the syringe 12
could be unit
marked at 1 or 2 unit increments and each unit increment could correspond to
.025 ml. In
still another example, if 100 units of neurotoxin were diluted with 4 ml of
diluent, this
solution created would have a concentration of 2.5 units per 0.1 ml or 1 unit
per 0.04 ml. The
chamber 30 of the syringe could be sized to hold 30 units and a total volume
of 1.2 ml. The
hatch marks on the syringe 12 could be unit marked at 1 or 2 unit increments
and each unit
increment would correspond to 0.04 ml. It is of course contemplated that
syringes could be
sized and marked according to any predetermined volume and/or dilution amount.
[0049] With reference to Figures 2, 4, 5 and 6 in one aspect, a syringe tip
40 can be
mounted and/or formed on the end wall 20 of the syringe 12 so that the syringe
tip extends
longitudinally away from the body 16 of the syringe. In this aspect, an
interior void 42 can
be defined in the syringe tip, and an aperture 44 in the end wall can place
the interior void of
the syringe tip in sealed fluid communication with the chamber 30 of the body
16, The
syringe tip 40 can be configured to matingly engage and secure a needle
assembly 14 to the
syringe.
CA 02908822 2015-10-05
=
[0050] The syringe tip 40 can be at least a portion of an inverted cone,
according to one
aspect. That is, at least a portion of the syringe tip can be substantially
frusto-conical in
shape. In another aspect, a first end 46 of the syringe tip having a first
diameter can be
coupled to the end wall 20 of the body 16, and a second end 48 of the syringe
tip 40 can be
positioned a predetermined distance from the end wall and having a second
diameter that is
greater than the first diameter. In yet another aspect, the interior void 42
defined in the
syringe tip can define a substantially frusto-conical void that is configured
to receive a frusto-
conical member 50 of a needle assembly 14 (described more fully below). In a
further
aspect, two flanges 52 can project radially away from the second end 48 of the
syringe tip.
The flanges can be configured to selectively engage the Luer-lock mechanism of
a needle
assembly, as known in the art.
[0051] As shown optionally in Figure 6, the syringe tip 40 can have a
substantially
cylindrical outer surface shape. In this aspect, it is contemplated that the
outer surface of the
syringe tip can have a conventional helical threaded surface defined thereon
that can
cooperatively receive a complementarily threaded base 86 of the needle hub SO,
as known in
the art. In yet another aspect, the interior void 42 defined in the syringe
tip can define a
substantially fi-usto-conical void that is configured to receive a frusto-
conical member 50 of a
needle assembly 14 (described more fully below).
[0052] As illustrated in Figures 5-8, the piston means 26 can comprise a
plunger 54 and
a piston cap 56. In one aspect, the plunger can be formed from a substantially
cylindrical
molded shaft 58. The shaft can have an outer diameter smaller than the inner
diameter 18 of
the body 16 of the syringe 12 so that the plunger 54 can move within the
chamber 30 of the
body. A thumb surface 60 can be formed on a proximal end 62 of the shaft
configured to
provide a flat surface for the user of the syringe to press and move the
plunger 54 (and the
piston cap 56) within the chamber. In another aspect, a portion of a distal
end 64 of the
plunger can have an outer diameter less than the outer diameter of the shaft,
forming a
plunger neck 66. In this aspect, the neck can be configured for attachment of
the piston cap
56 to the shaft. A plunger head 68 can be positioned adjacent to the neck. In
one aspect, the
plunger head can be substantially cylindrical having an outer diameter
substantially the same
as the plunger shaft 58, as illustrated in Figure 7. Alternatively, in another
aspect, the plunger
head 68 can be substantially frusto-conical (as illustrated in Figure 8), in
which a portion of
the head has an outer diameter substantially the same as the plunger shaft 58.
11
CA 02908822 2015-10-05
[0053] The piston cap 56 can be formed from a molded elastomer having a
proximal
end 70 and a distal end 72. In one aspect, the piston cap can have an outer
diameter
substantially equal to the inner diameter of the body 16 of the syringe 12. In
a further aspect,
at least a portion of the piston cap 56 can have an outer diameter slightly
greater than the
inner diameter 18 of the body of the syringe. In still another aspect, the
proximal end 70 of
the piston cap can have an outer diameter slightly greater than the outer
diameter of the distal
end 72 of the piston cap 56. In yet another aspect, a central portion 74 of
the piston cap can
have an outer diameter less than either or both the outer diameter of the
distal end and the
proximal end of the piston cap 56.
[0054] According to one aspect, the distal end 72 of the piston cap 56 can
be configured
to complementary engage the end wall 20 of the body 16 of the syringe 12. That
is, the distal
end of the piston cap can be sized and shaped so that when in use, the distal
end 72 of the
piston cap 56 contacts the end wall 20, and that that when contacting each
other, there are
substantially no gaps or "dead spaces" formed between the end wall and the
distal end of the
piston cap. For example, if the end wall 20 of the body 16 is substantially
planar or flat, the
distal end 72 of the piston cap 56 can be substantially planar or flat so that
substantially all
the fluid contained in the chamber 30 is ejected from the chamber through the
needle 78, as
described more fully below.
[0055] In one aspect, an inner bore can be defined in the piston cap 56
configured to
matingly engage the plunger head 68 and/or the plunger neck 66 of the plunger
shaft 58.
That is, due to the elastic nature of the piston cap 56, the inner bore of the
piston cap can be
positioned on and "snap" to the head and/or neck of the piston shaft. For
example and with
reference to Figure 7, the piston cap 56 can snap onto the plunger shaft 58
and can be secured
in position by its elastic properties.
[0056] In a further optional aspect, and as shown in Figure 6, portions of
the walls
defining the distal end of the chamber 30 of the body 16 of the syringe can be
tapered distally
and inwardly toward the aperture 44 in the end wall. In this aspect, it is
contemplated that the
distal end of the piston cap of the plunger will be complementarily shaped
such that, when
the plunder is fully depressed distally toward the end wall, the distal end of
the piston cap of
the plunger is in flush contact with the formed end wall of the chamber to
reduce or eliminate
any dead space within the chamber of the body in this depressed position.
12
CA 02908822 2015-10-05
[0057] In use, described more fully below, the outer diameter of at least a
portion of the
piston cap 56 can tightly engage the inner diameter 18 of the body 16 of the
syringe 12,
forming a fluid-tight seal. Furthermore, the outer diameter of the proximal
end 70 and/or the
distal end 72 of the piston cap 56 can provide stability to the plunger 54 by
preventing or
restricting rotational movement between the plunger and the body. In another
aspect, the seal
formed between the piston cap and the inner diameter of the body 16 can
provide desirable
injection resistance to help control the injection of small amounts of fluid
from the syringe
12.
[0058] Optionally, and as shown in Figure 9, the syringe 12 can further
comprise a
protective cover 76. In one aspect, the protective cover can have inner
threads similar to
conventional Luer lock threads to selectively couple the protective cover to
the forward end
22 and/or the syringe tip 40 of the syringe. When coupled to the syringe 12,
the protective
cover 76 can protect the syringe tip 40 and maintain the sterility of the
chamber 30 of the
syringe itself. It is contemplated that the protective cover can be color
coded for safety
depending upon, for example and without limitation, the amount of diluent used
and the
resulting concentration of fluid to be injected.
[0059] With reference to Figures 10-12, the at least one needle assembly 14
can
comprise at least one of the needle 78 itself and a polymeric needle hub 80
configured to
support the needle and attach the needle to the syringe.
[0060] In one aspect, the needle 78 can be at least one of an approximately
250 Y2"
length needle and a 320 1/2" length needle. For example, a needle 78 for
aspiration can be
the 25G needle to allow for minimal waste of medication while still having
sufficient flow
characteristics so as to not impede filling of the syringe 12. In another
example, a needle
designed for injection can be a 320 needle 78 having excellent flow
characteristics and long
enough for intramuscular injections. As known to one of skill in the art, a
32G needle does
not easily bend and can remain sharp after multiple percutaneous punctures.
The 32G needle
can be injected relatively pain free and can leave negligible medication waste
in the syringe.
In one aspect, and as shown in Figure 11, a proximal end 82 of the needle can
be blunt and a
distal end 84 of the needle can be beveled or blunt. It is contemplated that
the needle can be
color coded to correspond to existing needle gauge convention.
[0061] In one aspect, the needle 78 can be an elongate needle that passes
through the
needle hub 80. In another aspect, the needle hub can comprise a substantially
cylindrical
13
CA 02908822 2015-10-05
hollow needle base 86 having internal threads 88. In this aspect, the internal
threads can be
configured to matingly engage with the flanges 52 of the syringe as in a
conventional Luer-
lock engagement. For example, the internal threads 88 of the base 86 of the
needle hub 80
can be configured so that approximately a 180 degree turn of the needle hub
relative to the
body 16 of the syringe can fully engage and secure the needle 78 into position
on the syringe
12. As can be appreciated, the Luer-lock mechanism can keep the needle-syringe
assemblage
stable so that the needle 78 will not dislodge during injection causing
possible injury and
loss of expensive medication. Furthermore, the Luer-lock mechanism of the
needle hub 80
and the flange of the syringe can allow for rapid, multiple needle changes as
desired.
[0062] As shown in Figure 10, in one aspect, the base 86 of the needle hub
80 can
comprise at least one outer longitudinal groove 90 configured to aid in
handling and securely
fastening the needle hub to the syringe tip 40. In another aspect, the base
can further
comprise an alignment mark 92 so that when the needle base is securedly
attached to the
syringe 12, the distal end 84 of the needle 78 can be rotated to a desired
position (i.e., if the
distal end is beveled, the bevel is in a desired orientation relative to the
syringe) and in line
with the syringe markings when holding the syringe 12 for injection. That is,
in this aspect,
the alignment mark 92 on the needle base can be in line with the needle bevel
and in "front"
of the syringe after the needle hub 80 is fully engaged and rotated into
position on the syringe
tip.
[0063] In one aspect, the frusto-conical member 50 of the needle assembly
14 can be
formed andlor positioned in the substantially cylindrical hollow needle base
86 of the needle
hub 80. In another aspect, the frusto-conical member can comprise a distal end
96 having a
first diameter coupled to an end wall 98 of the base and a proximal end 100
having a second
diameter extending into the hollow cylinder 102 of the base a predetermined
distance. In this
aspect, the second diameter can be less than the first diameter. In a further
aspect, the
proximal end 100 of the frusto-conical member 50 of the needle hub can extend
longitudinally beyond the hollow cylinder of the base (as illustrated in
Figures 10 and 11).
[0064] In one aspect, the frusto-conical member 50 of the needle hub 80 can
be sized
and shaped to matingly engage the substantially frusto-conical void 42 of the
syringe tip 40.
That is, the frusto-conical member of the needle hub can be configured to
slide into the
frusto-conical void of the tip of the syringe 12. When the Luer-lock mechanism
of the needle
hub 80 engages the syringe, the frusto-conical member 50 of the needle hub can
create a
fluid-tight seal with the frusto-conical void 42 of the tip 40 of the syringe.
14
CA 02908822 2015-10-05
=
[0065] In one aspect, the needle hub 80 can further comprise at least two
progressively
smaller cylinders 104, 106 coupled to the needle base 86. In one aspect, these
progressively
diminishing cylinders can allow for better visualization of the puncture site
and can provide
axial stability for the needle 78 itself. In another aspect, a plurality of
flanges 108 can be
spaced from each other and positioned adjacent the smallest cylinder. In this
aspect, the
flanges can also provide axial stability for the needle.
[0066] In one aspect, a central bore 110 can be defined in and extend
through the
cylinders 104, 106, the end wall 98 of the needle base 86, and the frusto-
conical member 50
of the needle base. The central bore can be sized to allow a needle 78 to be
positioned
therein. In another aspect, the needle can be positioned in the central bore
such that a
proximal end 82 of the needle can be substantially aligned with the proximal
end 100 of the
frusto-conical member of the needle base. Optionally, however, the proximal
end of the
needle 78 can extend beyond the proximal end 100 of the frusto-conical member
50, or the
proximal end of the frusto-conical member can extend beyond the proximal end
82 of the
needle. In a further aspect, the distal end 84 of the elongate needle can
protrude from the
needle hub 80. For example, the distal end of the needle 78 can protrude from
the needle hub
less than about 0.25 inches, about 0.25 inches, about 0.30 inches, about 0.35
inches, about .40
inches, about 0.45 inches, about 0.50 inches, about 0.55 inches, about 0.60
inches, about 0.65
inches, about .70 inches, about 0.75 inches, about 0.80 inches, about 0.85
inches, about 0.90
inches, about 0.95 inches, about 1 inch or greater than about 1 inch. In a
further aspect, the
needle 78 can be secured to the needle hub 80 by any of multiple manufacturing
means such
as, for example and without limitation, glue, other adhesive, or the needle
hub can be molded
around the needle that can have laser etched or manufactured "stops" to
prevent needle
slippage through the needle hub 80.
[0067] As shown in Figures 13-15, the needle assembly 14 can further
comprise at least
one of a selectively removable needle guard 112 and a selectively removable
base guard 114,
according to one aspect. In another aspect, the needle guard 112 can be sized
and shaped to
cover the needle 78 and to overlap at least a portion of the needle base 86 of
the needle hub
80. In a further aspect, the base guard 114 can be sized and shaped to cover
the frusto-
conical member 50 of the needle hub and to overlap at least a portion of the
needle base. The
needle guard and the base guard can be removably coupled to the needle
assembly 14 by
snapping to the needle assembly and/or screwing to the needle assembly 14. In
still another
aspect, the needle guard 112 and the base guard 114 can be sized and shaped to
be
CA 02908822 2015-10-05
substantially flush with each other when both guards are installed on the
needle assembly.
Optionally, the needle guard and/or the base guard can be secured to each
other by a twist
breakable label 116 that can have manufacturing and expiration date
information and the like.
When both the needle guard 112 and the base guard 114 are installed and
positioned around
the needle assembly (i.e., in a closed position) the needle guard and the base
guard can
cooperate to maintain the sterility of the needle 78 during packaging,
shipment and storage.
[0068] To use the needle 78 and syringe 12 of the current application, the
base guard
114 can be removed from the needle hub 80 holding the desired needle 78. For
example, if
medication is to be aspirated from a container, a needle hub having a 250
needle can be
selected. The fi-usto-conical member 50 of the needle hub can be inserted into
the frusto-
conical void 42 of the syringe tip 40, and the needle hub 80 can be rotated
approximately 180
degrees so that the flanges 52 of the syringe 12 engage the threads 88 of the
needle base,
thereby securing the needle hub 80 to the syringe. That is, the needle hub and
the syringe can
be oppositely rotated into and relative to one another. The needle hub 80 can
be engaged and
securely and tightly drawn into the syringe tip 40 thus removing most or all
of the dead space
in the interior void 42 of the syringe tip.
[0069] The user can then insert the tip of the needle 78 into a vial
containing the desired
fluid, and withdraw the plunger 54 to suck the fluid through the lumen 118 of
the needle and
into the chamber 30 of the syringe 12. The needle 78 can be changed, if
desired, by reversing
the rotation of the needle hub SO relative to the syringe 12 to disengage the
first needle from
the syringe, and a new needle can be attached to the syringe as before. To
eject the fluid
from the chamber 30, the user can depress the plunger to urge the desired
amount of fluid
from the chamber of the syringe 12, through the aperture 44 in the end wall 20
and into the
lumen 118 of the needle. If the fluid is to be ejected into a patient, the
distal end 84 of the
needle can pierce the skin of the patient prior to depressing the plunger 54.
[0070] As can be appreciated, the body 16 of the syringe can be marked as
appropriate
for the dilution level of medication in the syringe 12. As can also be
appreciated, the flat
surface of the distal end 72 of the piston cap 56 can be urged into contact
with the end wall
20 of the body 16 of the syringe (as illustrated in Figures 2 and 13), thereby
removing most
or all of the dead space in the chamber 30 of the syringe. Furthermore, the
removal of this
dead space (and removal of the dead space between the interior void 42 of the
syringe tip 40
and the frusto-conical member 50 of the needle hub) can remove areas in which
medication
could remain during and after injection, making this needle and syringe
assemblage 10
16
efficient for a low-waste syringe 12 with interchangeable needles. The only
waste with the
syringe and needle of this application can be inside the lumen 118 of the
needles themselves.
100711 Although several embodiments of the invention have been disclosed
in the
foregoing specification, it is understood by those skilled in the art that
many modifications
and other embodiments of the invention will come to mind to which the
invention pertains,
having the benefit of the teaching presented in the tbregoing description and
associated
drawings. ills thus understood that the invention is not limited to the
specific embodiments
disclosed hereinabove, and that many modifications and other embodiments are
intended to
be included. Moreover, although specific terms are employed herein, they are
used only in a
generic and descriptive sense, and not for the purposes of limiting the
described invention,
17
WSLEGAL 074855 \ 00004 \ I 8072108v1
CA 2908822 2017-06-05