Note: Descriptions are shown in the official language in which they were submitted.
CA 02909033 2015-10-13
ELECTRONIC MEDICATION COMPLIANCE MONITORING SYSTEM AND
ASSOCIATED METHODS
FIELD OF THE INVENTION
The invention relates to electronic systems and methods for monitoring
medication compliance.
BACKGROUND
Non-compliance of patients to drug regimens prescribed by physicians can cause
a multiplicity of problems, including negative patient outcomes, higher
healthcare costs
and an increased risk of the spread of communicable diseases. Compliance
monitoring is
also critical in, for example, pharmaceutical clinical trials, geriatrics and
mental
health/addiction medicine. Poor medication compliance has a significant
negative impact
on patients, pharmaceutical manufacturers and the healthcare system in
general. Non-
compliant patients suffer from increased mortality, increased recurrence of
chronic
conditions and increased hospital and nursing home admissions. By some
estimates, as
much as 25% of all healthcare costs could be avoided if patients reliably took
their
prescribed medications.
Annual drug development spending has increased more than twelve times in
inflation-adjusted dollars over the past three decades. Clinical trials
consume a major
portion of the development time and costs of introducing a new drug into the
market.
Knowing with certainty a patient's adherence significantly improves the
understanding of
the results from a clinical trial in terms of safety, efficacy, dose response
relationship,
pharmacodynamics, side effects and other results. For instance, in a beta-
blocker heart
attack trial the death rate was reported at 13.6% in subjects whose compliance
was less
than 75% compared to 5.6% in subjects whose compliance was over 75%. None of
the
1
CA 02909033 2015-10-13
existing methods of measuring adherence offer both a qualitative and a
quantitative
measure with proof-positive detection of ingestion of the medication.
Accordingly,
measuring medication regimen compliance continues to be a major problem. The
only
statistical recourse is to enroll large numbers of patients, which
dramatically increases the
-- cost of clinical drug trials that in turn increases the cost of the final
marketed medication.
Compliance monitoring also provides significant benefits in market areas where
patient adherence to a drug therapy protocol is vital to preventing or
avoiding high-cost
consequences for the patient or community. Strict regimen adherence is
important for
preventing emergence of drug-resistant strains of infectious diseases that can
occur when
-- proper dosing schedules are not followed. Such resistant strains result in
increased
transmission, morbidity and mortality and are more expensive to treat or cure,
often by
one or two orders of magnitude.
A traditional method of increasing compliance is direct observance, but this
is
obviously difficult to administer and impractical on a large scale. Other
techniques
-- include blood sampling, urine sampling, biological marker detection, self-
reporting, pill
counting, electronic monitoring and prescription record review. These
techniques are
either invasive or prone to tampering.
In vivo biotelemetry and monitoring have been used for monitoring embedded
oxygen, sensing glucose levels, fetal monitoring and hormone measuring.
Passive radio-
-- frequency identification (RFID) techniques have been suggested to provide
biotelemetry
by including external sensors into existing commercial systems. However, RFID
was not
designed to operate in vivo, and the transmission of electromagnetic signals
from
embedded or internal sensors is hampered by attenuation and reflections from
the body.
Therefore, it would be beneficial to provide an active electronic device,
system
-- and method for non-invasively monitoring drug compliance in a facile
manner.
SUMMARY
Our new medication compliance systems and methods address the drawbacks
associated with transmitting RF signals through the body and/or with
tampering.
2
CA 02909033 2015-10-13
A method of monitoring a patient's compliance with a medication program
includes transmitting a first signal from an electronic ingestible medication
delivery
device located within a patient's body, the signal including information about
a
physiological parameter of the patient; detecting the signal with an
electronic reader
located outside the patient's body; and providing a second signal to the
reader from
different source than the delivery device, the second signal including
information about
the same physiological parameter of the patient.
A first system for monitoring a patient's compliance with a medication
delivery
program includes an electronic ingestible medication delivery device that,
after ingestion
by a patient, makes a first measurement of a physiological parameter of the
patient and
transmits a signal from within the patient's body, the signal including
information from
the first measurement. An electronic reader outside the patient's body reads
the signal and
makes a second measurement of the same physiological parameter of the patient.
This
system can help determine whether the delivery device and reader are operating
on the
same patient.
A second system for monitoring a patient's compliance with a medication
program includes an ingestible medication delivery device carrying an
electronic circuit
that transmits an electromagnetic signal from within a patient's body to
outside the
patient's body. An electronic reader positioned outside the patient's body
detects the
electromagnetic signal. Here, transmission and detection of the
electromagnetic signal
are synchronized according to a physiological parameter measured from the
patient. This
synchronization provides better sensitivity for the transmitted signal.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic diagram of the medication compliance system of the
present
invention.
FIG. 2A is one embodiment of a capsule having a planar substrate with an
antenna and electronics wrapped about a portion of the capsule. FIG. 2B
illustrates a
capsule having a single-coil antenna wrapped about a portion of its periphery.
3
I
CA 02909033 2015-10-13
FIG. 2C illustrates a capsule having a digestible coating on its outer
surface.
FIG. 3 is a schematic chart that illustrates the use of an electronic capsule
with a
wristband reader that is in communication with a cell phone, and which in turn
can be
used to forward information to a data collection center.
FIG. 4 is a block diagram illustrating the tag electronics and antenna fitted
with an
electronic pill or capsule like that shown in FIGS. 1, 2A-2C and 3.
FIGS. 5A and 5B illustrate techniques used to mate an electronic tag shown in
FIGS. 1 and 3 with the inside surface of a capsule (FIG. 5A) or the outer
surface (FIG.
5B).
FIGS. 6A, 6B and 6C are schematic side views illustrating the layers used to
fabricate an electronic tag in accordance with different embodiments of this
invention.
FIG. 7 is a circuit diagram for an exemplary activation switch circuit using a
conductive sensor with a NMOSFET transistor.
FIG. 8 is a circuit diagram for an exemplary deactivation switch circuit using
a
MOSFET transistor.
FIG. 9 is a circuit diagram for an exemplary MOSFET sensor-based bio-switch.
FIG. 10A is a top perspective view of an ingestible switch in accordance with
the
present invention utilizing a hydrogel circuit breaker.
FIG. 10B depicts the switch of FIG. 10A, with a swollen hydrogel circuit
breaker
after exposure to gastrointestinal fluids.
FIG. 11 is a schematic side view of a galvanic gastric sensor for utilization
with
the electronic tag shown in FIGS. 1 and 3.
FIG. 12A is a summary of the results of testing of several phosphate
electrodes at
different modes.
FIGS. 12B and 12C are coded charts depicting the results set forth in FIG. 12A
for a 20K Ohm load and a 1K Ohm, respectively.
FIG. 13 is a block diagram illustrating the electronics associated with the
tag
integrated circuit.
FIG. 14 is a block diagram illustrating the overall in-link and out-link
communications between the electronic tag taken internally by a patient, and
the external
4
CA 02909033 2015-10-13
reader utilized to communicate with the tag illustrating a specific example of
conductive
transmissions from the reader to the tag at 4 MHz and radiative out-link
transmissions
from the tag to the reader at 400 MHz by way of example.
FIG. 15 is a timing chart illustrating the periodic transmissions from the
reader to
the tag and the data bursts from the tag to the reader.
FIG. 16 is a chart depicting the content of the in-link transmission from the
reader
to the tag.
FIG. 17 is a chart depicting the content of information contained in the data
bursts
from the tag to the reader.
FIG. 18 depicts a timing chart for out-link transmissions from multiple tags
to the
reader.
FIG. 19 is another timing diagram illustrating the operation of the system of
the
present invention.
FIG. 20 is a block diagram illustrating further aspects of the operation of
the
system.
FIGS. 21A and 21B are side views illustrating alternate constructions for the
electronic capsule to permit a portion of the electronics to be carried within
the capsule.
FIG. 22 depicts an alternate construction with the tag partially within the
capsule
and partially exposed.
FIGS. 23, FIGS. 24A and 24B depict exemplary constructions of a capsule with a
tag in accordance with this invention.
FIG. 25 is an exploded illustration depicting, from left to right, the
attachment of
an integrated circuit chip to the tag connected to the low frequency and high
frequency
antenna areas, and the wrapping of the tag about a capsule (right hand side).
FIG. 26 is a cross-sectional side view of the tag, illustrating the various
elements
depicted in schematic form in FIGS. 6A and 6B.
FIG. 27 sets forth representative dimensions of the capsule and tag.
FIG. 28 is a top view of a wrist band reader feature shown in FIG. 3.
FIG. 29 depicts a patch reader wearable by a patient.
FIG. 30 depicts a pill container having an on-board reader.
5
,
CA 02909033 2015-10-13
FIG. 31 is a block diagram of the reader 19 in FIG. 1.
FIGS. 32 and 33 illustrate a biometric identification system using an
electronic
pill and an external reader worn by the patient to analyze a physiologic
signal from the
patient.
FIG. 34 is a block diagram of an external reader architecture for receiving
and
processing signals from the system of FIGS. 32 and 33.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the Summary and in the Detailed Description of The Preferred Embodiments,
reference is made to particular features. Where a particular feature is
disclosed in the
context of a particular aspect or embodiment, that feature can also be used,
to the extent
possible, in combination with and/or in the context of other aspects and
embodiments.
In this section, embodiments will be described more fully. These embodiments
may, however, take many different forms and should not be construed as limited
to those
set forth herein.
A description of the preferred embodiments of the present invention will now
be
presented with reference to FIGS. 1-34.
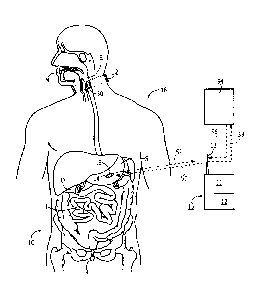
Noting FIG. 1, a system 10 for monitoring medication compliance in a patient
16
comprises an electronic sensor, preferably in the form of an external wireless
monitor or
reader 11 that includes an RF transceiver 12 and one or more antennas 13. The
antenna
13 can be external or internal to the reader 11 and can be implemented in a
variety of
ways as known in the art, including an on-chip antenna or simple pads or
electrical
contacts that function as an antenna. The reader 11 detects the presence of an
electronic
pill 14 in, for example, the gastrointestinal (GI) tract of the patient 16. As
shown the
electronic pill 14 has a tag 15 attached to or part of the pill 14. For
purposes of this
disclosure, the term "pill" can include a capsule or other form of medication
administration or testing. The system 10 is designed to detect the pill 14
when located in
6
1
,
CA 02909033 2015-10-13
the patient's mouth M, esophagus E, stomach S, duodenum D, intestines
(including the
colon) I or rectum R.
With continued reference to FIG.1, the system 10 includes a tag 15 fixed with
the
pill 14, either internally or along the outer surface, or both. After
ingestion of the pill 14,
the tag 15 is made electronically active and begins communication with the
external
reader 11. The external reader 11 in one embodiment is in a housing 19 worn by
or
attached with the patient 16 so as to be comfortable and easy to wear
continuously to
ensure it is always with the patient.
The electronic pill 14 comprises an orally ingestible and biocompatible drug-
transporting device with embedded or attached electronic circuits that
communicates with
the external wireless reader 16. As described in greater detail below, the
electronic pill
14 uses, for example, a silicon-based integrated circuit and/or other passive
components
such as coil antennae and capacitors. The circuit can incorporate millions of
transistors,
patterned through various semiconductor processing steps, to provide an
enormous
amount of intelligence. For instance, the electronic pill 14 can store a
patient's medical
history in addition to detailed information about a drug being administered,
provide a
unique identification number, and implement advanced communication circuits
and
protocols to reliably transmit data to the external wireless reader 16.
Turning now to FIG. 2A, the electronic pill 14 preferably comprises a drug-
transporting device, such as a capsule 17 that has associated therewith the
electronic tag
15. Noting FIG. 1, a signal 18 received by the reader 11 from the electronic
circuit 30
after ingestion of the capsule 17 is thus indicative of medication compliance.
The term
"drug-transporting device" is not intended as a limitation, and other
compositions and
devices for delivering medication are intended to be subsumed hereinto as
known in the
art. Alternatively, the capsule 17 may be devoid of medication to serve as a
placebo
during a drug trial.
Referring again to FIG. 2A, the electronic components of the electronic pill
14 are
capable of wireless transmission and reception over short distances (i.e., in
the range of
20-30 cm). The electronic components in the circuit 47 (i.e., antenna 21,
power source
22, and silicon chip 23), once hermetically sealed and packaged, is small
enough to fit
7
1
CA 02909033 2015-10-13
either to the inside wall or on the outside wall of the capsule 17. This level
of
miniaturization is feasible owing to integration and circuit scaling trends
associated with
standard CMOS technologies.
With continued reference to FIG. 2A, a small chip or other electronics 47 is
attached to the substrate 45 as well. This chip 47 provides a great deal of
functionality
including but not limited to two way communication and complex protocols,
energy
harvesting (mechanical, electrical, etc), sensing of conditions such as
location of the tag,
pH, chlorine content, encryption, and identification code storage and
transmission.
Referring now to Figure 3, the complete system is principally composed of a
data
reader 111 and multiple tags 15 attached to medication 14. Bidirectional data
50/52 is
exchanged between the reader 111 and the tag 15. The reader 111 probes the one
or more
tags 15 inside the body 10 and coordinates the communication to allow multiple
ingested
tags to communicate simultaneously, sequentially, or in other ways to permit
multiple
communication pathways. The tags 15 communicate their unique identification
data and
whether they are in the GI tract. The reader 111 then provides output data 58
to a user
interface 54 such as a laptop or smartphone enabling real-time upload 59 of
medication
events to a remote database 60 or other location. The reader 111 receives
information
from the user interface 54 via the channel 56 indicating medication regimen
status such
as the time of the next scheduled medication event, confirmation of the event
from the
main database, or other information from the user interface 54 or the remote
database or
trial coordination center 60 via the wide area network (cell or wifi network)
channel 59.
The data link from the reader 111 to tag 15 is defined as the "in-link" path
50.
Preferentially, in-link data to the tag includes synchronization, signaling,
address, and tag
configuration information. The reader 111 preferentially transmits information
by way of
differential metallic skin contacts. The in-link signal 50 passes through the
body 10 and is
sensed by the tag 15 through a differential probe network.
The data link from the tag 15 to the reader 111 is defined as the "out-link"
path
52. Preferentially, the out-link data to the reader includes GI sensing,
pharmaceutical,
adherence, signal level, and address information. The out-link channel 52 is a
radio
frequency signal traveling through both the body 10 and the free space between
the body
8
1
CA 02909033 2015-10-13
and the antenna of the reader 111. A small antenna 21 on the tag 15 radiates
the out-link
signal 52 which is received at the reader 111. The reader 111 is capable of
receiving
signals 52 from multiple tags 15 simultaneously.
All of these components work together to complete a system that can accurately
detect a medication event, including the time of ingestion, the dosage, and
specific
identification of the medication. This information is then used to verify
critical
compliance with drug therapy. This data can also be used in combination with
other
patient data to improve adherence and treatment outcomes.
TAG DETAIL AND MANUFACTURING
The following sections describe the detailed construction of the tag 15.
Referring
to FIG 4, the tag 15 comprises a body interface and antenna 203 that allows
for the in-
link 50 and out-link 52 communication. The ingestion detection subsystem 208
utilizes
the body interface and antenna system 203 to determine when the medication
actually
resides in the body 10 and in particular in the digestive tract M,E,S,D,I,R,
and
specifically which portion of the digestive tract. A receive subsystem 204
implements the
in-link 50 communication and interfaces between the body interface and antenna
subsystem 203 and a control subsystem 209. A transmission subsystem 206
implements
the out-link 52 communication and interface between the control subsystem 209
and the
body interface and antenna subsystem 203. An energy harvesting subsystem 205
captures
energy from either the body interface 203 or from the environment that the tag
15 resides
in, for example the motion or temperature of the device. The energy harvesting
subsystem 205 provides energy which is stored in an energy storage system 207
and to
the tag 15 in general to operate the components and provide sufficient power
for out-link
52 transmission. The control subsystem 209 coordinates and controls the
differing
components of the tag 15 and implements any communication protocol, sensor
measurements, maintains tag memory for various identification information, and
implements any other functionality required by the tag 15. The tag 15 can be
attached to a
pill 14 or capsule 17 in a variety of ways, either by being built into the
medication, build
onto the medication, being printed onto the medication, or by being attached
onto the
9
CA 02909033 2015-10-13
outside or inside of the medication carrier (i.e. a capsule). A preferred
embodiment is to
build the tag 15 on a biocompatible substrate 45 that can be built in high
quantities and
then later attached or built into a capsule 17, for example.
SUBSTRATE (PRINTING AND TAG)
Referring now to FIGS.5A and 5B, in another embodiment of the tag 43, the
antenna 44 are printed on a flexible substrate 45 that is biodegradable and
digestible, such
as a flat sheet-like material, and includes an electronic chip 47 mounted on
the substrate.
This substrate 45 is then placed on or wrapped around the capsule 46. The
antenna 44 is
preferably printed in a way that when the material 45 is wrapped around a
capsule 46,
connections can be made from one end of the sheet 45 to the other, thus
forming the
antenna 44 as a continuous loop. Printing on both sides of the substrate 45
simplifies this
process by using a technique similar to circuit board manufacturing with
through-holes.
Preferably, the substrate 45 should have sufficient rigidity for manufacturing
and
attachment, be easily and safely digested, be flexible for wrapping around a
pill or
capsule, and withstand temperatures required for manufacturing and
sterilization.
There are relatively few materials for substrates 45 that are both easily and
safely
digested and can also withstand temperatures required for bonding the chips 47
to the
antennae 44 (up to 190 C) or the sintering of metallic inks. In one
embodiment, an enteric
coating commonly used in colonic-targeted drug release, is utilized to create
a flat and
flexible substrate that meets these requirements and has been used in
prototype tags.
Enteric coatings are commercial materials with good flexibility and proven
biocompatibility. They are currently used in aspirin, acetomenophin and other
drugs that
upset the stomach, as they resist disintegration at low pH. Enteric coatings
usually begin
to disintegrate at a pH above 5.5 or higher, which is the typical pH of the
duodenum and
small intestine. Enteric coatings include but are not limited to
polymethacrylate-
polymethylmethacrylate (PMA-PMMA) copolymers and cellulose acetate phthalate
(CAP), which are commercial coatings under names of "Eudragit" and "Aquacoat
CPD"
that are readily available as pre-mixed solutions.
Referring now to FIG 6A, in another embodiment, the substrate 45 of FIGS. 5A,
B can be manufactured with a coated paper to create a biocompatible coated
paper
I
CA 02909033 2015-10-13
substrate system 260 to provide advantageous properties. A paper substrate 82
coated
with a coating 254 provides improved mechanical properties, increased printing
strength,
reduced dissolution time, and allows for alternative printing methods, while
maintaining
biocompatibility. Enteric coated paper provides a smooth texture 256 for the
paper 82 and
allows for antenna patterns 266 to be easily transferred onto it. The paper 82
also breaks
up rapidly once the coating 254 dissolves. Preferentially, the paper 82 is
coated on all
sides, but in some implementations coating only the top of the paper is
required. In
addition, a biocompatible adhesive 252 is applied to the bottom of the paper
when the tag
will be applied to the outside of the medication. When adhesive 252 is
included, a
10 protective backing 258 is used to simplify handling and attachment to
the medication.
The biocompatible coated paper system 260 of FIG. 6A addresses manufacturing
problems of electroplating/electroetching on biodegradable substrates.
Preferentially,
biocompatible paper 82 is a mixture of biodegradable materials distributed in
impregnated and/or on coated the paper in a number of ways. Paper 82 can be
any
15 substance that can be used as a flexible and strong biocompatible
substrate in the dry
state but weakens in the wet state. Rice papers, pulp papers, plant-based
fiber papers
including linen papers are all substrates that can be used with a
biocompatible material
coating or impregnation. The paper 82 itself is also biocompatible. The
biocompatible
substances 254 used for coating or impregnating the paper alter the
dissolution properties,
thermal properties, mechanical properties, biodegradation rates and other
properties of
the paper 82. The biodegradable materials included in the construction of the
biodegradable substrate 260 allow for digestion of the substrates 45 and
prevents
untoward effects such as lodging in the stomach of the substrate or pill
itself, as may
occur with non-degradable and smoothly-surfaced polymeric substrates.
The substrates 45 allow for the placement of a metallic trace for antennae
266,
chips 47 or other electronics via electro-plate, bonding, gluing, adhesive or
printing. The
antenna 266 may be covered in a protective coating 268 to prevent digestion,
protect the
antenna from handling, and dielectrically isolate the antenna from the
environment. The
paper 82 is superficially coated with the biodegradable substance such as
polymethyl
11
1
,
CA 02909033 2015-10-13
methacrylate-polymethacryalte, cellulose acetate phthalate, poly lactic acid,
poly glycolic
acid, various sugars, oils, waxes or proteins.
The biocompatible materials 254 added to the paper also allow for increased
stability of paper materials in more extreme environments, including those of
very high
temperature and humidity, preventing the tag 15 from warping or deforming (and
possibly fracturing the antenna 266). Referring to FIG 6B, the substrate 45 is
coated in a
multi-layer or patterned method with multiple layers of coatings 254, 264 such
that
various portions of the tag can be exposed or broken down in the body 16 at
varying
times or locations. At different times or locations in the body, different
coatings may
dissolve exposing different types or portions of the antenna 266 or sensors to
the body 16.
These varying conditions provide information to the system allowing for
determination of
ingestion time or tag 15 location. Referring to FIG 6.C, a cross-sectional
side view of an
alternate form of the system 260A, the coatings can be patterned such that
different
portions of the paper 82 are coated with different coatings. This multi-layer
or multi-
coated system 260A tracks the progress of the substrates as they pass through
the
digestive tract M,E,S,D,I,R or encounter different solutions in the body 16.
In a preferred
embodiment, each coating 254, 264 is an Enteric coating that is formulated to
dissolve in
specific areas of the human body 16. In combination with a multi-level
electronic sensor
and in the form of an electronic pill, the location of the medication is
tracked through the
human body. As each layer of the substrate 254,264 dissolves in its pH or
chemically
sensitive environment, a new electronic sensor, which by way of example can be
a
galvanic cell is exposed. In addition to exposing different sensors or probes
in different
portions of the body 16, the selective dissolution of the coatings in
different parts of the
body 16 alters the transmission properties of the antenna 266 or system 260A
in general,
thus making the location of the tag 15 in the body detectable without separate
sensors.
Various bodily chemicals and even organisms (and their respective chemicals)
can cause the degradation of the materials used with the systems 260 and 260A.
Enzymes, hormones, cells (blood cells), proteins, acids, ions, bacteria, and
so forth can
contribute to the degradation of the substrates or any of the substrate
layers.
12
i
CA 02909033 2015-10-13
Furthermore, in another embodiment, the system 10 is triggered to dissolve in
the
patient's body 16 in the presence of both a bodily chemical and an external
impulse for
additional control. For example, the sensitivity of chemical breakdown is
enhanced by the
application of RF energy to the substrates 260,260A (producing heat or
otherwise) from
an outside RF source.
The system 10 may also be loaded with various degradation control chemicals
that can delay or hasten degradation rates. This is useful if the processing
of substrate
layers that require extra-thick amounts of a certain layer to be mechanically
stable or if a
layer requires another chemical in addition to the ones found in the human
body 16 to
begin degrading appreciably.
ANTENNAS AND COATINGS
Pills 14 or capsules 17 are typically printed with edible inks of
pharmaceutical
grade to uniquely identify the product and provide additional information such
as
company logo, brand name, and dosage information. In accordance with this
invention,
these edible inks are replaced with conductive and biocompatible silver inks
to pattern
small antennas 266 (FIG. 6A), coils 41 (FIG. 2C) or deposited conductive
pattern 44
(FIGS. 5A, 5B) directly on the capsule 14, 17. Other compositions known in the
art are
also contemplated such as but not limited to carbon black, iron, gold, copper,
zinc, and
conducting polymers.
Thus, by way of printing, etching, or electroplating, miniature antennae 266
are
made of silver, carbon black, copper, or other biocompatible coatings. Silver,
copper,
zinc and other metals are substantially biologically inert, and when ingested
in small
amounts, are nontoxic to humans and pass through the body without being
absorbed into
tissues. Furthermore, the conductivity of most of these metals is very high,
making them
excellent conductors. Therefore, the antenna 266 performance not only depends
on
physical size constraints, but also on the total usable concentration of
conductive
material.
Coatings. Referring again to FIGS. 6A and 6B, it is preferred to encapsulate
the
components of the tag with a coating 268 for multiple reasons, including but
not limited
to: electrical isolation from the conductive fluids of the GI-tract
M,E,S,D,I,R, prevention
13
CA 02909033 2015-10-13
of dissolution or exposure to the body for safety, and selective dissolution
at different
locations in the body 16. For example, the coating 268 prevents contact with
the body,
limiting exposure of the materials to the patient 16. Certain coatings can be
utilized that
are pH or otherwise selectively dissolved in different portions of the GI
tract. In a
preferred embodiment, enteric coatings that do not dissolve until they reach
the intestine I
or other locations, where very little digestion occurs. This allows the
material to break up
before excretion but not leach materials into the blood stream. It is also
desirable to
activate the tagging system only after the patient has ingested the pill 14.
Once ingested,
the pill 14 or capsules 17, 46 are exposed to stomach acids that eat away
coatings 268 on
the surface of the capsule 17, 46. Coatings 52 (or 268) may be applied or
selectively
applied to cover parts of the tag or capsule/pill. In one embodiment, these
coatings 268
are applied selectively to cover certain portions of the tag and capsule but
allow other
portions of the tag and capsule to break down. This allows the tag to change
shape or
break down in such a way that it easily passes through the digestive tract
while still
protecting sensitive materials from digestion.
In another embodiment, the conductive layers of the antenna 266 on the tag 15
are
made by incorporating a metal that can dissolve, such as iron filings under a
temporary
protective layer 268 such as polyglycolic acid or by incorporating particles
that are
nontoxic by virtue of being non-absorbable (e.g., silver or carbon).
Degradation of the
matrix releases particles that move through the digestive system of the
patient 16 without
absorption. Such particles are present above a percolation threshold for
conductive
"contact" (within 1000 A), and reside in a degradable matrix such as
polylactic/glycolic
acid or starch. The degree of conductivity is adjusted by the degree of close
contact and
by the number of contact points (volume fraction). Particles that are not
spherical can be
added at lower levels to get good conduction. Hence, graphitic carbon plates
can reach a
percolation threshold at lower levels, and silver can be used as planar
particles as well.
In a preferred embodiment, the integrated circuit 47 is encapsulated or coated
in a
protective coating such that it is not exposed to the body 16 and its
digestive process.
Packaging preferably does not interfere with the RF communication but provides
enough
14
I
CA 02909033 2015-10-13
safety for human studies. Methods of use allow access to the aqueous
environment for
sensors while still ensuring safety.
ENERGY HARVESTING
Power sources for body-powered electronic pills must be biocompatible, small
in
size with the appropriate form factor, capable of delivering high power with
good
maximum discharge current characteristics and low self-discharge, and provide
long
calendar life. Referring again to FIG 4, many techniques exist for harvesting
power 205
and storing the power 207 within the circuit 47 for use with the tag 15. For
example, a
capacitor can be used to store the energy at block 207 owing to the short
duration of the
active nature of these devices (less than 1 minute). In one embodiment, the
capacitor is
embedded into the pill electronics and charged from block 205 by a handheld
device via a
magnetic field or other mechanism before being swallowed by the patient 16.
The
capacitor holds this charge until activated by a triggering mechanism, such as
the
dissolving of a specially coated switch by stomach acid.
In another embodiment of the energy harvesting block 205 in FIG. 4, the
chemical
energy of the stomach contents is converted into electrical energy. For
instance, the
chemical reaction between the stomach acid and a zinc electrode oxidizes the
zinc,
creating an electric current via a metal electrode making the return path. In
another
embodiment, the system converts mechanical motion (e.g., peristaltic and other
motion
common in the digestive tract) into electrical energy.
In yet another embodiment, the energy harvesting system 205 of FIG. 4,
harvests
the energy of the in-link channel 50, stores the energy in block 207 and uses
this energy
to power the tag 15 and transmit data via the radiative out-link 52.
Harvesting of the
energy from the reader's in-link 50 is sufficient to power the tag 15 and its
transmission
of the out-link channel 52. In all of these embodiments, the tag 15 harvests
the energy
until it obtains sufficient energy to transmit a signal to the external reader
11 along the
out-link channel 52. As illustrated in FIG. 15, this harvesting process
typically is
substantially longer than the duration of the burst information sent out by
the tag 15, thus
allowing for amplification of the out-link channel 52 with respect to the
instantaneous
power harvested, for instance from the in-link channel 50. For example, if the
in-link
i
CA 02909033 2015-10-13
channel 50 is harvested for 100ms and the out-link burst of information is 1
ms in
duration, the out-link power transmission 52 may be 100 times larger than the
instantaneous power harvested by the pill 14 or capsule 17 from the in-link
signal 50.
INGESTION DETECTION
An important aspect in successful detection of the ingested electronic pills
14 or
capsules 17, 46 is to positively identify the origin of the transmission, that
is, whether the
pill or capsule is transmitting from inside the patient's body 16. Knowledge
of
transmission origin is necessary to detect a patient who might intentionally
spoof the
system into registering a positive compliance.
Multiple methodologies can be
implemented for ingestion detection with element 208 (FIG. 4). Noting FIG. 17,
multiple
techniques exist for "triggering" the system to respond only after reaching
the stomach.
The trigger can be activated by the dissolving of material that opens (or
closes) a switch.
The trigger can be based on electrical, chemical, or mechanical detection of
stomach or
GI tract contents (e.g., pH sensor, ISFET, temperature sensor, three electrode
electrochemical cell, microelectro-mechanical systems (MEMS), microfluidics,
miniaturized or nanoscale lab-on-a-chip, biomarker targeting, biosensors,
optical sensor,
sound transducers, bio- or chemi-luminescent sensor, or the like). When
spoofing is not
an issue, the trigger can be activated just before ingestion or by the reader
11.
Additionally, one also simultaneously measures changes in material properties
such as
physical size (swelling), magnetism, polarizability/polarization, phase (solid-
solid, solid-
liquid, liquid-liquid, etc.), viscosity, chemical/molecular makeup, optical
clarity, thermal
conduction, state of charge and so forth. For example, the sensor may sense
changes in
the outer walls of a capsule, such as temperature or conductivity before it
comes in
contact with the outer environment.
The ingestion detection system using the galvanic gastric sensor 284 (FIG. 11)
with sensor 208 communicates with the control subsystem 209 of FIG. 4. The
control
subsystem 209 is then either programmed to process the data it receives to
determine if
the tag 15 is in the proper location, or passes the data through the
transmission subsystem
206 for analysis by the external components, for example the reader 11 (FIG.
1) or 111,
external device 54, or central database system or healthcare provider 60 (FIG.
3). In
16
CA 02909033 2015-10-13
another embodiment, the tag 15 does not respond to queries from the reader 111
or 11
until the ingestion detection system 208 indicates that the tag 15 is in the
proper location,
such as the stomach S.
Additionally, the presence of specific features in the received signal from
inside
the body may be sufficient to determine the transmission origin. For instance,
signal
strength in out-link 52 coming from inside the body 16 is lower compared with
that from
outside the body due to attenuation from tissue, blood, and bones. It is also
reasonable to
expect a shift in the resonant frequency or a unique characteristic of the
frequency spread
or content when a signal propagates through tissue, which is absent when the
transmission is outside the body 16.
Additional techniques for detecting the origin of the transmission of tag 15
include the following examples. The dynamics of pill motion in the esophagus E
(e.g.,
speed of pill travel, orientation of magnet, and path of travel) and or
stomach S may
provide subtle discriminating differences between the in-link signal 52
received from tag
15 inside the body 16 and a tag 15 that is outside the body 16. The
peristaltic motion of
the esophagus and the tossing and turning in the stomach may produce pill
motion that
affects the signals received due to the natural or purposely modified
directionality of the
fields generated by tag transmission. Additionally, there is a normal
progression from
mouth M to esophagus E to stomach S that will produce a difference in motions
that must
be obtained sequentially to validate the location of the pill 14.
Transmission of unique codes based on a variety of potential sensors attached
to
the tag 15. In one embodiment, body temperature and/or pH sensors are included
in the
ingestion detection system 208 and either processed or relayed by the control
system 209
to the transmission subsystem 206. The control subsystem 209 can transmit
either raw
sensor back to the reader 11 or 111 for analysis of the patterns, or process
the data itself
and transmit back to the reader 11 (FIG. 1) or 111 (FIG. 3) an indication of
its location.
Ensuring that the tag 15 is only active inside the patient's body 16. For
instance,
the tag 15 is inert when dispensed and is activated upon contact with saliva
and/or other
bodily agent.
17
I
,
CA 02909033 2015-10-13
Alternatively, the tag 15 is activated outside the body 16, prior to
ingestion, and
deactivated inside the body 16 after coming in contact with bodily fluid.
The activation/deactivation process can be carried out using, for example:
selectively coated sensors that exhibit change in properties in the presence
of specific
chemical compounds, biodegradable switches based on proteins that are broken
down
when exposed to digestive enzymes in the stomach, and/or unique GI fluid
sensors based
on the properties of the GI fluid.
Conductive sensors. One implementation strategy of a bio-switch is to
interface
the conductive sensor with a transistor (e.g., MOSFET, PET, BJT, etc.), as
shown in FIG.
7. A conducting sensor in series with resistor R1 acts as a simple resistor
divider and
provides the necessary biasing voltage to the transistor gate. The output of
the bio-switch
is taken from the transistor drain and fed to the enable port of the RF
transmitter (an RF
transmitter is used as an example, but can be any electronic device that
requires
activation). A power source provides necessary power to drive the transistor
and
activation voltage. When the bio-switch is outside the body, the resistance of
the
conductive sensor is small compared to R1; thus the activation voltage (VA)
will be
below the gate threshold voltage. When the gate voltage is below the
threshold, the
transistor is turned off and the voltage at output equals zero. When the bio-
switch comes
in contact with a bodily fluid, select chemical molecules will bind to the
conductive
sensor and increase its resistance, thereby also increasing the activation
voltage. When
the activation voltage increases beyond the gate threshold voltage, the
transistor turns on,
and voltage at output equals that of the battery. A large voltage at the
output in turn
enables the RF transmitter and readies it for transmission.
An alternative implementation is shown in FIG. 8, where the circuit
configuration
yields a deactivation circuit. When the bio-switch is outside the body, the
voltage at
output equals that of the battery; that is, the RF transmitter is enabled.
When the
conductive sensor comes in contact with a bodily fluid, the transistor is
turned on and
voltage at output equals zero, thereby disabling the RF transmitter.
A bio-switch implementation using a conductive sensor is not limited to the
above
examples. Variations in transistor type, substrate type, biasing schemes,
selection of
18
1
CA 02909033 2015-10-13
power, etc., can yield several different implementation options. An exemplary
concept
here is the utilization of a conductive sensor to drive a switching mechanism.
In the
above examples a battery was used to drive the transistor and circuitry, but
instead a
charged capacitor can easily replace the battery. The switch can also be used
to modify
the frequency of the signal transmitted or detected externally (e.g., changing
the
frequency response of the pill/antenna). The capacitor can be charged before a
pill is
dispensed or can be charged by RF induction as is done in RFID techniques. One
positive aspect of using a capacitor is that over time the capacitor will
discharge and the
entire system will become inert, meaning the subject must take the pill within
a given
time frame, thereby increasing the robustness of the system to spoofing.
MOSFET sensor. Another implementation of the bio-switch is to use a MOSFET
e-nose sensor instead of a conductive sensor. A bio-switch with a MOSFET e-
nose
sensor can be implemented with much simpler supporting circuitry since the
transistor
does both sensing and switching. An example is shown in FIG. 9. The resistor
divider of
R1 and R2 provides a fixed activation voltage to the transistor gate. When the
bio-switch
is outside the body, the activation voltage is just below the gate threshold
voltage;
therefore, the transistor is off. When the MOSFET sensor comes in contact with
a body
fluid, a catalytic reaction takes place at the transistor gate and changes the
channel
conductivity; i.e., the gate threshold voltage is lowered so that the
activation voltage is
now above the threshold voltage. Therefore, the transistor turns on, and the
voltage at
output equals that of the battery. Again, this illustration is just one
possible
implementation scheme, and variations can be constructed with different types
of
substrate (e.g., n-type and p-type) and supporting circuitry.
An additional embodiment utilizes biodegradable switches that undergo
significant changes in conductivity when exposed to the digestive enzymes of
the
stomach. One can mix a conductive substance (e.g., carbon) with a non-
conductive
substance (e.g., protein). A conductive substance doped with a non-conductive
substance
will tend to have lower conductivity (high resistance) than a pure or even
semi-doped
material. When the doped material comes in contact with digestive enzymes, the
non-
conducting material is broken down or dissolved by the enzymes, leaving behind
just the
19
I
CA 02909033 2015-10-13
conductive material. One possible switch implementation can be based on a
composition
of carbon and albumin. The albumin protein is broken down by pepsin, an enzyme
that is
naturally present in the stomach. When the switch composition is devoid of
albumin, the
conductivity of the switch increases and bridges a gap in the circuit to
complete the
circuit. A number of possibilities exist in selecting a conducting material
and a protein.
Furthermore, it is also possible to incorporate multiple non-conducting
materials to yield
switches that are extremely selective to activation.
In the embodiment shown in FIG. 4, the in-link 50 signal is used for energy
harvesting 205 and is sufficiently low in frequency such that most of the
energy is
transferred conductively through the body of the patient 11. Thus, the tag 15
is only
easily powered when the tag 15 is in contact with the body of the patient 11
since the in-
link signal is significantly attenuated in the air. As such, the tag 15 will
not respond to
queries from the reader 11 until such time as the pill is being touched or
ingested by the
patient 11. This provides a level of spoofing prevention with virtually no
additional
complexity added to the system.
Noting FIGS. 10A and 10B, in order to ensure that the pill is ingested, the
antenna
system can be disabled by electrically "shorting" the antenna through the use
of an
ingestible switch 90 that contains a circuit breaker 92 that becomes modified
in the
presence of gastric juice. An embodiment of this concept includes a method of
breaking a
circuit via the swelling of the breaker material in the presence of stomach
fluid. The
switch 90 comprises a thin layer of hydrogel 92 partially coated with a
conductive trace
94 such as metal flake or micro-thin metal foil. When the hydrogel is exposed
to low pH
liquid, it swells to about sixteen times its normal size and breaks the
conductive trace 94.
This mixture is then coated with an albumin-based layer that will prevent
exposure of the
hydrogel to fluids until the albumin is selectively broken down in the GI
tract by either
pepsin or typsin. The preferred mechanism for deactivating the system using an
ingestible switch 90 is to electrically connect the two pads of an antenna 13,
creating an
electrical "short". The lower the resistance, the more power is diverted from
the signaling
antenna. By way of example, a five ohm resistance is sufficient to reduce the
input power
from the antenna by 95+%. A resistance of 10K ohms will reduce the input power
to the
I
CA 02909033 2015-10-13
signaling chip by only 10% or less. In the case where the in-link 50 is
separate from the
out-link 52, either antenna can be shorted by this technique, essentially
crippling the tag
15. The preferred embodiment is to short the in-link antenna in a system that
utilizes the
in-link to power the system.
Sensor such as pH sensors and other chemical sensors are fairly complex
devices.
Noting FIG. 11, to avoid a requirement to embed such a complex device into the
tag 15
or integrated circuit 20, the preferred embodiment of a gastric sensor 208
utilizes a
galvanic couple 208 that is placed in various bodily fluids (stomach fluid,
esophageal
fluid, GI fluid, etc.) to create a measurable change in electrical properties
such as current,
voltage, and/or resistance and allow digestible electronics to evaluate the
location of a tag
in the human body 16. In one embodiment, the sensor 208 senses changes in the
outer
walls of a pill 14, such as temperature or conductivity before it comes in
contact with the
outer environment. In the preferred embodiment, the galvanic couple can
provide
discrimination of location as well as providing electrical power to the
system. The
15
ingestion detection system 208 on the tag 15 works either outside or inside a
pill 14.
Inside the pill, the detection system 208 operates when the GI fluids permeate
the pill or
dissolve any external layers. The tag 15 begins generating power and voltage
as soon as it
is wetted by ingestion and allows the tag 15 to begin communicating with the
reader 111.
As the tag moves through the GI tract M,E,S,D,I,R, the sensor or voltage
information is
communicated or processed by the control logic 209 such that location
information can
be determined by the reader Ill or other external system. FIG 11 shows the GI
sensor /
energy cell connected to control logic 208. The GI sensor requires two
electrodes for
operation and one or both of these electrodes may also function as antennas.
In the
preferred embodiment, one of the electrodes for the GI sensor comprises a
small strip of
metallic zinc while the second electrode consists of a specially coated silver
electrode
that is shared with the in-link antenna.
In one embodiment, the galvanic couple 284 is constructed of two differing
metals 280, 282 or compounds that, when placed in a bath of any number of
solutions,
produces an electric voltage and subsequent current and is measured by the
control
system 208. Metals used for a galvanic gastric sensor 284 are transformed by a
number of
21
,
CA 02909033 2015-10-13
chemical reactions to produce a new chemical compound. The new compound
changes
the differential voltage. Upon immersion in a target fluid, the compound
transforms into a
different compound and accompanying the transformation is a change in voltage.
Other
changes in the galvanic cell materials can be phase transitions, state
transitions, amount
of the chemical compound (causing a natural change in differential voltage
based on the
degree of material available to sustain such a voltage), and other materials
transitions that
cause a change in the electrical output of the galvanic cell. In the preferred
embodiment,
the GI sensor creates varying degrees of current or voltage depending on the
nature of the
fluids in which it is immersed. Thus, the GI sensor not only gives true/false
data about the
environment, but may also senses the chemical/electrical/thermal/etc. makeup
of the
environment and give a signal corresponding to the state.
The metals or compounds are connected to a measurement means in the control
logic 208 (FIG. 11) and can be a voltmeter, potentiostat/galvanostat,
electronic switch or
other means to measure or gauge the voltage and report if and/or when the
target
electronic reading is reached in the target solution. If the sensor 284 is not
in the target
solution, the voltage will not change appreciably as no chemical
transformation will
proceed. Thus, the voltages or an indicator of location can be transmitted via
the tag 15 to
the reader 11 or 111 to confirm the location of the pill 14 or capsule 17, 46.
Such
chemicals transformations include silver phosphate and other silver compounds,
including silver chloride, silver sulfate, silver carbonate, or even silver
metal itself.
Transformation can occur on the anode or cathode of the galvanic cell. In the
preferred
embodiment of FIG. 11, a silver phosphate electrode 280 is attached to a zinc
electrode
282, and the silver phosphate transforms to silver chloride and silver metal
while the zinc
oxidizes to zinc metal or forms a zinc compound with anions in solution.
Accompanying
the silver materials transformation is a voltage differential (from high-to-
low or low-to-
high state). The voltage/current differential is affected by the selection of
the two metals
or compounds (or mix thereof), whereby a silver phosphate-zinc system differs
in output
voltage/current from that of a silver-phosphate-copper or silver sulfate-zinc
system.
Furthermore, a dissolvable or protective coating 281, 283 may be applied to
the
electrodes 280,282 such that the electrodes are not exposed to the fluid until
a certain
22
1
CA 02909033 2015-10-13
external condition exists, such as when the tag 15 is exposed to the high
chlorine content
of the stomach. Coatings on each electrode 281, 283 can be the same or
different,
providing flexibility in the voltages and currents produced in different
transit times and
locations in the body 16.
FIG. 12A is a chart describing the voltages measured from the silver phosphate
electrode 280 of FIG. 11 formed by applying 9V to a silver chloride electrode
in
KH2PO4 solution with a silver electrode return path 282. The silver phosphate
electrode
280 was tested at loads of 20kOhm and 1 kOhm. The charts in FIGS. 12A, 12B and
12C
show the time course of voltages of the electrodes with differing amounts of
phosphate
(exposure time to the KH2PO4 solution), differing loads, and differing
solutions. The
gastric sensor 284 has a substantially different voltage in HC1, the primary
component of
stomach fluid, versus sulfuric acid solution.
In alternate embodiments, the tag 15 is modified to include sensors to measure
various attributes of the pill's surroundings. For instance, the tag 15 can
have a pH
monitor, temperature probe, or other sensors 42 (FIG. 2C) to verify
compliance. In
addition, it may be beneficial to use a system that can also provide a readout
of the in-link
signal strength. This signal strength is beneficial for optimizing the
communication
protocol dynamically as well as providing potentially discriminating
information relating
to the location of the tag 15.
BIOMETRICS
In addition to determining when the pill 14 or capsule 17, 46 is ingested and
where it resides, it is important to detect that the pill is ingested by the
appropriate
person. As such, a variety of biometrics are utilized to detect that the
reader 11 or 111
and pill 14 or capsule 17, 46 are located on or in the correct person 16.
In one embodiment, an electronic pill monitors physiologic signals inside the
body 16 that are typically difficult to mimic outside the body. For example,
the patient's
electrocardiogram (ECG) is detected and measured by the electrical contacts or
antenna
of the tag 15. The tag 15 either processes the signal or passes the signal via
the out-link
52 to the reader 11 for further processing. Detecting the presence of a valid
ECG signal
indicates that the tag 15 is inside the body 11. Detection of a periodic pulse
between 30
23
,
CA 02909033 2015-10-13
and 120 BPM is sufficient to detect that the tag 15 is inside the body.
Furthermore, a
wide variety of parameters can be extracted from the physiologic signals
detectable inside
the body 16. In a preferred embodiment, the processing system (either in the
tag 15,
reader 11, or elsewhere downstream) detects parameters of the electrical
characteristics of
signals received inside the body 16 including but not limited to: periodicity,
amplitude,
signal shape (including peak geometry, relative height), and signal to noise
ratio. In
addition, the signal detected inside the body can be transmitted to the reader
11 and
verified against a preloaded signature of the ECG or other physiologic signal
recorded
earlier, for example, during the initial administration of the system 10.
Additionally, the
reader 11 can record the same signal outside the body 16 and ensure that the
tag 15 is in
the same person 11 that is wearing the reader and also checked against the
stored
signature. These features of the ECG and other physiologic signals measured at
the tag 15
or reader 11 are also capable of biometric identification.
These physiologic features and their dynamic features (changes in the signals
over
time) are useful to identify the patient 16, ensure ingestion, or determine
the location of
the tag 15 in the digestive tract M,E,S,D,I,R. The dynamic features include
but are not
limited to heart rate variability, changes in signal strength as the tag moves
through the
body 16 and muscle activity in different parts of the body. For example, the
ECG will be
quite strong as the pill passes the heart in the esophagus E and then
gradually get weaker
as it moves farther from the heart in the GI tract S,D,I,R.
In an embodiment, the external reader 11 is used to monitor and assess a given
patient's 16 ECG output (periodicity, peak geometry). This information is used
for a first
calibration step and recorded as the baseline ECG output. The later
measurement of the
ECG by the reader 11 validates that the same person is using the reader.
Additional, the
measurement by the tag 15 can be checked against this calibration to ensure
that the
proper person is taking the medication. In this case, if either the medication
or the
external reader is switched to a different person 16, the results can be
checked against the
calibration data. Calibration can take place in the presence of proper
supervising
personnel, including doctors, nurses, etc., and the calibration can be locked
to those who
either have calibration codes or calibration devices.
24
1
CA 02909033 2015-10-13
These biometric capabilities can also be used to help guard against improper
medication (type or dose) being taken. Each tag 15 can be programmed to be
taken by a
specific patient. The patient specificity will be recorded by certain
physiologic signals
that can identify individual patients, such as parameters of the ECG. The
reader 11 or
interface 54 can first be programmed to register the kind and frequency of
medication to
be taken for a given patient ECG. The reader 11 or interface 54 then alerts
the patient or
proper personnel if the medication taken was given to the wrong person (pill
ECG does
not match reader ECG) or if the medication was taken at improper time
intervals (over-
or under-medication).
CONTROL LOGIC/IC DESIGN
Maximizing power efficiency is of utmost importance to maximize the reading
distance between the reader 11 and the tag 15 as well as power output and
detectability.
Advanced low-voltage and low-power circuit design topologies and a suitable
process
technology are required to achieve operation with small input power levels
from radiated
electromagnetic fields.
Referring to FIG 13, the preferred embodiment of the integrated circuit 20 for
the
tag 15 includes: protocol logic 306 having a random bit generator for robust
two-way
communication and control, data acquisition subsystem 304 and sensor 314 to
determine
the strength of the in-link signal 50, in-link subsystem 302, out-link
subsystem 308, and
energy harvesting and storage system 310. The IC 20 is designed with fallback
operating
modes, including a chirp mode and a beacon mode. In the chirp mode, the
protocol is
suspended and the IC 20 transmits data whenever it has sufficient power from
the energy
harvesting and storage system 310. In beacon mode, the IC 20 transmits a
periodic burst
pattern with no data (bypassing all digital logic). In one embodiment, the
energy
harvesting and storage system 310 extracts power from induced currents
generated from
the external reader 11 via the in-link subsystem 302 or directly via the in-
link antennas
50. In a second embodiment, a galvanic cell, GI sensor, thermocouple, or other
method of
generating power from the digestive tract M,E,S,D,I,R or motion through it
provides
primary or supplemental power via the energy harvesting subsystem 310 to
enable the IC
20. The out-link subsystem 308 drives the out-link signal 52 under control of
the protocol
I
CA 02909033 2015-10-13
logic and control subsystem 306. The protocol and control system 306 contains
all the
logic to control the IC, the communications protocol, the data acquisition,
synchronization, and data storage and output, including the preprogrammed
information
about the medication, patient, study and other information. Minimizing the
power usage
of all these subsystems and in particular the out-link subsystem 308 is of
utmost
importance.
Preferably, the IC 20 is fabricated using industry standard CMOS manufacturing
processes in class 10 or better clean rooms. The physical dimensions of the IC
20 are
expected to be very small, less than 1 mm x 1 mm x 0.1 mm. When affixed to the
tag 15,
the IC 20 is encapsulated in biocompatible epoxy to cover any hard edges and
to prevent
interaction between the IC 20 and the patient's body 16. The preferred IC 20
is a custom
designed microchip that stores the medication information, reads the GI
sensor, and
implements the signaling and communications protocol. The IC is designed to
operate
with extremely low power and to provide reliable deep in vivo communications.
In addition to integrated circuit implementations for the various logic and
systems
of the tag 15, another embodiment includes printed electronic circuits created
with
various inks including metallic, dielectric, and organic materials. The
creation of complex
printed electronics requires the creation of multilayer electronic devices
such as
transistors and capacitors, silver conductive ink and dielectric materials are
typically
loaded into separate ink cartridges. For example, In the case of capacitors,
fabrication can
be achieved by first printing a single line of nanoparticles onto a substrate
that is heated
until the inks are metalized. Next, a dielectric of polymer is printed
directly over the line.
Finally, a second conductive line is printed perpendicular to the original
conductive line.
In this way, the overlapping cross-section of the two conductive lines¨with a
dielectric
between them¨creates a capacitor whose capacitance is defined by the overlap
area and
the dielectric material and thickness Thus, if an antenna is printed
simultaneously and
attached to each conductive line, a simple 3-step inking procedure is enough
to begin
creating simple inductor-capacitor antennas that can resonate at a tuned
frequency.
26
CA 02909033 2015-10-13
Many generally recognized as safe (GRAS) materials are available for use as
dielectrics, for example polytetrafluoroethylene (PTFE), polyimide (from
precursors) and
PVP. In the preferred embodiment, enteric coatings are used as a dielectric
material.
COMMUNICATION LINKS AND PROTOCOL
Transponder Antenna Size and Efficiency. The radiation efficiency of a typical
loop antenna increases with loop area and is inversely proportional to the
excitation
signal wavelength. Since the loop area is limited, it is desirable to operate
at higher
frequencies to improve the antenna efficiency. In typical RF applications,
operating at
higher frequencies can improve the aperture efficiencies of small antennae to
maximize
the received power. However, in biological systems, the operating frequency is
a tradeoff
between increased path loss in tissue and antenna efficiency. Indeed, RF
signal
attenuation behavior of the in-link 50 and out-link 52 in bodily fluids and
body tissue to
and from an ingested tag 15 is complex and difficult to model.
The tag 15 may be coded with a variety of information including but not
limited
to data about medication, the patient 16, the reader 11, or the drug trial the
patient is
participating in. Additionally, the tag 15 can have a unique ID that is
utilized with a
database of other information tagged to each tag ID to obtain similar
information without
storing it on the tag 15. Upon detecting the tag 15, any of the readers 11,
111, 211 or 311
can store a time-stamped reading of a medication event. If the tag 15 is not
detected,
failed compliance can be signaled, for example, to the patient 16 and/or to a
second party
such as a health care provider or other agency 54 via input and output signals
56, 58.
It is preferred that the communication between each reader 11, 111, 211 or 311
and the tag 15 provides two way communication, with the communication from the
reader to the tag 15 being preferably through a conductive in-link channel 50
and the
communication from the tag 15 to the reader being preferably through a
radiative out-link
channel 52. The in-link channel 50 is preferentially in the range of 1-20 MHz;
this
frequency range produces efficient data transfer from outside the body to deep
inside the
body 16 and can travel through the body conductively, requiring very small
antennas or
pads only to receive the signal. Because the in-link transmissions 50 travel
conductively,
the signaling attenuates very rapidly outside the body 16 thus providing for
increased
27
CA 02909033 2015-10-13
privacy for the in-link channel 50. Skin surface contacts with the reader, as
readers 111,
211 and 311, maximizes the efficiency of the in-link transmission 50 from the
reader.
The in-link channel 50 communicates a variety of information to the tag 15,
including but
not limited to querying for the presence of the tag 15, turning the tag's
transmitter on or
off, collision avoidance, and various other protocol-based communications. The
in-link
channel 50 also provides synchronization signals between the reader and tag
15.
Synchronization between the reader 11 and tag 15 are particularly important
when the
out-link signal 52 is very small (as is expected when coming from inside the
body) and/or
when the out-link signal is transmitted in very short bursts for better energy
efficiency.
In an embodiment, the tag 15 is powered using the RF energy received by its
coil
or antenna or in another embodiment where power is generated by energy
harvesting
means. As described previously, this power can be stored temporarily and then
used to
transmit a pulse or signal to the reader 11. Storing the energy internally in
the tag 15
helps alleviate two distinct problems. First, it allows for the storage and
amplification of
the instantaneous power received from the in-link 50 or energy input 312 to
create higher
powered but shorter bursts of out-link transmissions 52. Second, when
transmitting into
the body 16, the external powering signal (in-link 50) creates significant
noise that may
make detection of the out-link signal 52 from the tag 15 very difficult.
One method to create more detectable signals for out-link signal 52 is to
utilize
different frequencies for power transmission and data signaling. This allows
the external
receiver 12 of the reader 11 to be frequency-isolated from its transmitter. A
frequency
selective filter may then remove the noise from the transmitter to allow for
high quality
reception of the data signal. Lower frequency signals typically have lower
losses in the
human body. As such, the power transmission signal 50 may necessarily be lower
in
frequency than the data transmission signal 52 which can be much lower in
power.
Another method involves the use of dual antennas on one or both of the tag 15
and receiver 12; that is one antenna or set of probes/contacts for the
transmission/reception of the in-link or power transmission, the other for
transmission/reception of the out-link signal.
28
I
=
CA 02909033 2015-10-13
As discussed in greater detail below with reference to FIGS. 15-18, another
method involves the time multiplexing of the signals such that the power
transmission
ceases during predefined time periods to allow tag 15 to start transmitting
data. The
circuitry of tag 15 can be designed to utilize this cessation of power
transmission as a
marker to determine when to start transmitting data. Additionally, that
circuitry may store
the power during the "power cycle" for a period of time typically longer than
the
transmission cycle to provide a power multiplication to improve the signal
strength of the
data transmission.
It is preferred that the communications to and from the tag 15 and to and from
the
readers 11,111,... are protected, encrypted, encoded, or made secure in a way
to prevent
interpretation by other devices and have software and/or hardware required to
protect the
data and support privacy or data security requirements of the communication
system.
Many of the tag 15 embodiments support IDs and other stored data that can be
transmitted back to the reader 11 via the out-link channel 52. IDs and other
data can be
transmitted via pulsatile signals (information in the pulse duration, pulse
spacing, pulse
frequency, etc.) or via digital encoding. To increase signal-to-noise ratio,
it is preferable
to have a transmit/receive event wherein the tag 15 responds to a request from
the reader
11 with a predetermined signal. This signal is repeated and then synchronously
averaged
over multiple transmit/receive events to produce a better signal-to-noise
ratio.
Synchronization of the transmissions from and to the reader 11 and tag 15 also
improves
the ability of the reader 11 to detect faint signals 52 from the tag 15 in the
body 16.
An embodiment of an efficient communication and protocol approach is
demonstrated in FIG 14. The approach is based on a unique communication path
between
the tag 15 and the associated reader 11. The reader 11 transmits data to the
tag 15 by way
of the conductive in-link 50 communication channel. The tag 15 transmits data
to the
reader 11 through the out-link radiative channel 52. An electromagnetic
transmitter
block 320 provides the interface to the radiative channel 52 at the tag 15 and
a
corresponding reader RX 322 extracts the signal at the reader 11. A tag 15 for
a patient
16 is linked directly to the patient reader 11 similar to a key and lock. Only
data with the
proper key or data word are recognized by the reader 11 as valid patient data.
As an extra
29
CA 02909033 2015-10-13
measure of protection, the out-link TX carrier 320 is phase locked to the
reader in-link
signal 50 to provide means for synchronization of data. Phase lock also allows
coherent
detection of the tag data at the reader 11, thereby enabling use of phase
modulation and
reduced error rates. Since the in-link signal 50 is propagated by direct body
contact (in-
link conductive channel), only the reader 11 attached to the patient's body 16
can
properly demodulate the return tag out-link 52 data. This feature makes
external
eavesdropping of the data very difficult. Hence, this approach enhances
security of the
data. As will be appreciated, this communication protocol also allows several
tags to be
consumed and data read without the need for individual tag identification
bits, thus
reducing significantly the amount of data that needs to be stored.
The protocol is composed of the communication link timing and the associated
in-
link 50 and out-link 52 data fields. FIG 15 depicts the relative communication
link timing
of the data for the in-link 50 and out-link 52. The process begins with the
reader 11
sending an FM modulated signal, or "in-link header" 330 to the tag 15 with
information
necessary for proper tag operation. The header is sent periodically every T
seconds. On
ingestion, a random bit generator in the circuit 20 for the tag 15 begins
operation. On
completion of the in-link header field 330, the value of the random bit
generator is
latched. This latched value is used to set the time, to at which the tag 15
responds to the
reader 11 by sending a data burst 332. The data burst 332 contains a subset of
the data
stored on the tag 15 to be sent to the reader 11. Several bursts 332 in
sequence make up
the full data transmission through out-link 52. The latched random generator
value
becomes the pill ID or address for this specific tag. Since the ID is random,
each tag
swallowed will have a unique ID. As will be appreciated by those skilled in
the art, the
algorithm may be as simple or complex as necessary to assure no two tags
randomly end
up with the same address. Each tag swallowed will send a pulse 332 delayed in
time
from the end of the header 330 proportional to the value of the random
address. In this
fashion, no two tags 15 can transmit at the same time, thus preventing
interference but
also allowing multiple ingestion of tags. Also, since the tag address is set
randomly, there
is less need for special tag identification bits to be stored on each tag,
thus reducing
i
CA 02909033 2015-10-13
significantly the number of bits of memory required on the tag 15. This
reduces cost and
complexity of the tag 15 significantly.
Referring to FIG. 16, a representative efficient in-link data field 342 and
corresponding definitions are shown. Field selection can be used to improve
the
robustness of the communications between the reader 11 and the tag 15. For
example, the
field N defines the number of data bursts 332 that the tag 15 uses to define a
single tag
information bit. Thus, it is appropriate to use a mechanism by which the
system 10
assigns more data bursts 332 per bit for situations where the signal to noise
ratio may be
poor (very large patients for example). This allows for more integration time
and
improved reliability. Such a system is adaptable for broader utility.
Referring to FIG. 17, a representative efficient out-link data field 352 is
configured to allot two data bursts 332 for a single bit 354. Depending on the
total
number of bits J of information transmitted, NxJ data bursts are generated.
For example,
if the total number of bits 354 is J=16 and N=2, then 32 data bursts 332 are
transmitted.
As a further example of the utility of this protocol, the reader 11 may be
preprogrammed
to only accept out-link data with the proper patient ID. This along with the
fact that the
data is coherently linked to the reader 11 essentially reduces the likelihood
of data not
associated with this patient 16 being received as valid data.
FIG. 18 shows the timing for the case of three tags taken together to further
illustrate the efficiency of the protocol. Tag 1 362 transmits at a random
slot after the in-
link header 330 and transmits multiple bursts 332 per bit 354. Similarly, Tag
2 364 and
Tag 3 366 transmit their multiburst per bit transmission in different time
slots after the in-
link header 330.
As has been previously discussed, a communication network utilizing a phase
based modulation scheme is known to have advantages of reduced bit error rate
(BER)
for the same transmission power compared to simple modulation schemes such as
Amplitude Shift Keying (ASK). Since power is extremely limited in in-
vivo
communication systems, minimizing BER is a challenge. Implementing a phase-
based
modulation scheme requires that the reader 11 be able to coherently demodulate
the
signal 52 for each tag 15. This normally requires that the frequency stability
of each tag
31
1
CA 02909033 2015-10-13
15 be within a tight tolerance (20-40ppm) to permit the reader 11 to phase
lock and
demodulate the received signal. Such frequency tolerance requires that the tag
15
transmit frequency be based on a crystal reference. Size and safety
constraints prohibit
the use of crystals to generate the transmit signal for the tag 15. Further,
since the tag
transmission burst is short in duration, there may be issues in proper
settling which may
cause demodulation errors. Another solution is for the reader 11 to have an
independent
receiver for each tag 15 and preferably use a phase lock loop based approach
to lock to
the incoming signal. There are issues with this approach as well. First, the
burst durations
are short making the design of such a receiver extremely difficult. Second,
the tag 15 still
requires some measure of frequency tolerance to assure regulatory or system
specifications are achieved. This may require the need for tag frequency
trimming which
adds to the manufacturing and test costs of each tag 15. Hence, a method is
required to
eliminate the need for a tight frequency tolerance on the tag 15 as well as a
complex
reader 11 receiver design.
A preferred approach takes advantage of the fact that the reader 11 is
connected
via the conductive in-link 50 communication channel to each tag 15. Hence, by
using the
reader 11 as the initial frequency reference and locking each tag 15 to the
reference signal
for reader 11, a self-synchronized coherent communication system is realized.
FIG. 14
illustrates this concept in detail. First, the reader 11 generates a reference
signal (shown
here for example with frequency of 4MHz). This signal is passed to the
conductive
channel 50 through an interface circuit 372 and propagated to any tag 15
within the
channel. At the same time, the 4MHz reference 376 is frequency multiplied
within the
reader 11 to the tag 15 burst frequency (400MHz by way of example). This
signal is
ultimately used to coherently demodulate data from any tag 15. In the tag 15,
the 4MHz
reference frequency is extracted, amplified and passed to the input of a PLL
demodulator
and TX carrier generation circuit (Tag PLL) 374. This circuit has several
modes of
operation including the tag burst mode. During the tag burst mode, the signal
is frequency
multiplied to the TX frequency of 400MHz. This signal is subsequently passed
through
the out-link 52 channel where it is extracted at the reader 11. The reader
local oscillator
378 derived from the original 4MHz reference is used to demodulate the
received tag
32
I
CA 02909033 2015-10-13
signal 52. The system is self coherent. Thus, the tag 15 achieves a tight
transmission
frequency tolerance by virtue of the phase lock loop 374 and does not require
any
internal crystal reference.
Other advantages of this embodiment may be seen by referring to FIG. 19. This
figure shows the timing relationship between the in-link 50 and out-link 52
burst signal
and defines the three modes of operation of the tag PLL 374 network. The in-
link data is
sent at a periodic rate of T seconds, typically on the order of 1 ms. As
described earlier,
the tag 15 responds some time later (less than lms) with a TX burst. The TX
burst also
repeats at the same 1 ms interval. A single period is expanded in FIG 20 to
further
highlight the modes of operation of the tag PLL. The tag PLL operates
continuously.
During mode 0 382, the reader sends a fixed reference signal of frequency Fref
(4MHz).
The tag PLL locks to this frequency and remains locked until progressing to
mode 1 384.
During mode 1, the tag PLL remains locked; also during mode 1, the reader 11
frequency
modulates the 4MHz reference signal with any required information or
configuration data
for the tag 15. Since the tag PLL is still locked to the reader signal, the
modulated data
can directly be extracted from the VCO control voltage 375 on the tag PLL.
Hence,
during mode 1 384, the tag PLL is acting as a demodulation block. The tag PLL
then
returns to mode 0 382 and stabilizes. Finally, during mode 2 386, the PLL is
given the
command to frequency multiply the 4MHz reference signal, generating the 400MHz
TX
burst signal. This is an efficient realization using the same circuitry for
both in-link
demodulation and TX carrier generation.
FIG. 20 shows more detail of the tag PLL 374. One key to its operation is the
dual
frequency VCO 392. During modes 0 and 1, the VCO 392 operates at 4MHz. During
mode 2 the VCO 392 is switched to 400MHz (with VCO gain parameters changed
accordingly) at the same time a divide by N (100 for this example) is enabled
within the
loop. The frequency of the phase detector 394 remains unchanged and the loop
dynamics
remain the same. As a result the loop quickly settles to a precise 400MHz and
the TX
burst is transmitted. Once the TX burst 332 is sent, the PLL 374 is returned
to mode 0
and the process repeats.
33
CA 02909033 2015-10-13
It will be appreciated by those skilled in the art that this implementation
has
several advantages. Using fine lithography integrated circuit technology, the
power
requirements for the VCO 392 during mode 0 and 1 are very minimal. Simple ring
oscillator approaches may be used for the VCO 392 requiring just a few micro-
amps of
current. This allows the tag PLL 374 to operate continuously which then
permits a very
frequency tolerant transmission burst. The tag PLL 374 will stabilize with
each
subsequent burst. The same circuitry is used for both transmit and receive and
area
requirements are very small leading to a low cost solution.
ATTACHMENT
Wrapped tag embodiments are shown in FIGS. 5A and 5B. Noting FIG. 5B, the
wrapping process is typically partially around or completely around the outer
surface of
capsule 46, soft gelcap, or other medication carrying device as shown in FIG.
5B. The
recent invention includes the method of attaching the tag 43 to the inside
surface of the
capsule 46 as shown in FIG.5A.
Referring again to FIG. 5B, to avoid the accidental or purposeful removal of
the
tag 43 from the outside of the capsule 46, prevent tampering, avoid damage to
the tag 43,
substrate 45, chip 47, or antennas 44 from handling and environmental issues
and to
increase the aesthetic appeal of capsules (and minimize patient hesitation in
taking an
electronically-tagged medication), it is prudent to conceal and protect all
electronic
devices, including antenna 44 and chip 47, under a protective coating 48. The
protective
coating 48 can be colored to match the capsule 46 or an additional layer 42
can be
included to cover the tag 43 or the entire capsule 46 to further obscure the
presence of the
tag.
Now noting FIG. 5A, placing the tag 43 inside the capsule 46 obscures the tag
from view, prevents tampering, and also provides protection to the tag as the
capsule 46
must dissolve or be disassembled before the tag is exposed. Placing the tag 43
inside the
capsule 46 also maintains a minimal change in capsule volume, and simplifies
tag
attachment and functionality. When the pill 14 is in the form of capsule 46
and the tag is
inserted into the capsule 46, the tag 43 operates similar to when the tag 43
is placed on
the outside of the pill. As the capsule 46 begins to absorb fluids or the
capsule begins to
34
I
1
CA 02909033 2015-10-13
dissolve, the tag 43 will come in contact with the GI fluids and begin to
operate normally.
A tag 43 using leads that make contact with the interior wall of the gelatin
capsule 46 and
activates upon wetting of the capsule can react with the environment to
identify location,
chemistry, or otherwise. The tag 43 can then use the gelatin capsule itself as
a protective
system in non-aqueous environments. The diffusion time of fluid through the
capsule
wall is the limiting factor for detection of body fluids or
transmission/reception of
conductive signaling within the body itself. To make contact with the outside
environment in an expedient manner, however, the receiving pads must be in
direct
contact with the capsule as some pills swell outwardly when exposed to fluid.
Thus, for
this approach an adhesive for attaching the tag 43 to the inside wall of the
capsule 46 is
required.
In many cases, placing the tag 43 inside the capsule 46 works well. In some
cases,
the delay between ingestion and activation of the sensor system on the tag 43
may be
problematic. Fitting the tag 43 with external exposed sensor or pads would be
advantageous for quick analysis of the body environment and for advanced
location
discernment. Transit from mouth to stomach typically takes place in less than
8 seconds,
which is faster than most gelatin capsules can absorb fluid and begin to break
down.
Having an external lead or sensor minimizes this delay in sensing time for an
internally-
placed tag 43. The number of pads that need to be exposed can be as few as
one,
depending on configuration.
Referring to FIGS. 21A and B, a trace or foil 49 that runs from inside the
gelatin
capsule to its exterior allows for electronic transmission from within a
capsule or power
harvesting from outside the capsule. Certain portions of the antennas or leads
remain
exposed 79, 44 on the tag 43 allowing for electrical contact between the tag
on inside of
the capsule and the interior-exterior lead 49 on the capsule 43. The interior-
exterior lead
49 is preferably constructed such that an elongated pad 86,87 is included to
make contact
with the antennas or leads 79,44,81 of the tag 43. The tags 43 are constructed
with
coatings such that only the leads or antennas that need to be connected to the
interior-
exterior leads are exposed while all other electrical components and antennas
are coated
in a protective and/or dielectric substance. The elongated pads 79,87 and the
design of the
I
,
CA 02909033 2015-10-13
exposed areas of the antennas or leads 44,79 allow for easy alignment of the
tag 43 and
the internal/external leads 49. For example, in FIGS. 21A and 21B each
elongated pad
86,87 and exposed pad 44, 79 has a distinct depth inside the capsule which
provides for
very simple alignment of the tag 43 and leads 49. To make the interior-
exterior
connection, the metal film 49 is thin enough to allow a 2-part gelatin capsule
to still snap
together. The tag 43 itself, being composed only of thin components (antenna,
chip,
substrate, etc.), takes up a minimal amount of volume within the capsule 46
and should
not impair drug loading amounts.
Referring to FIG. 22, in one embodiment, the tag 43 is inserted partially into
the
cap 110 of the capsule 46 and is partially exposed as the capsule body 112 is
inserted into
the cap 110 but under the tag 43. The leads 44, 79 are thus exposed to the
outside of the
capsule and can be made to appear innocuous. Likewise, the tag 43 can be
attached to the
outside of the body 112 and partially covered once the cap 110 is placed on
top of the
body/tag combination. Again, leaving leads 44,79 exposed for proper sensing
and/or
power generation. Referring to the top portion of FIG 23, the tag 43 is
inserted into the
cap 110 and external leads are printed, built into, or attached via a second
printed antenna
substrate on the capsule body 112. When the cap 110 and tag 43 combination are
inserted
on top of the body 112 with external antennas, 44,79, electrical contact is
made between
the internal tag 43 and the external leads 44,79. This embodiment also allows
for "hot-
swapping" of internal and external components of the tag 70. Different
geometric designs
of an external antenna can be accommodated by a single or few internal tags by
creating a
system that simply needs alignment of antennas with external traces. This way,
a number
of antennas/sensor probes can be produced that have specific applications
(frequency,
power transmission properties, size, complexity) for a specific drug, creating
a modular
design that can have certain unique or complex components placed on the
exterior of the
capsule and maintain a communication pathway. This embodiment also makes the
process of creating multi-metal antennas or sensors simpler as only small
strips of
material need be placed on the outside of the capsule 70.
Referring to the bottom portion of FIG 23, the tag 43 is inserted into the
body of
the capsule 112 and the external leads 44, 79 are attached, built-into, or
printed on the
36
I
,
CA 02909033 2015-10-13
outside of the capsule body 112. Vias, 87 or other methods are then used to
connect the
tag 43 to the external leads 44,79.
FIGS. 24A and B illustrate an alternative embodiment where the tag 43
substrate
is built with elongated portions that contain external antenna or lead
components 44,79
such that when the tag 43 is inserted into one end of the capsule 46 the
elongated portions
can be folded back and adhered to the outside of the capsule 46.
TAG CONSTRUCTION
Referring to FIG. 25, in the preferred embodiment, the main components of the
tag 15 are a very small integrated circuit (IC) 47, a metal antenna 44, a
gastrointestinal
(GI) sensor / energy cell composed of a specially coated GI sensor pad that
doubles as an
in-link antenna 79 and a second metalic GI sensor pad 81, and a substrate 45.
The substrate 45 is composed of a specially coated paper. Non-whitened, low-
weight papers are non-toxic and become softened in the GI tract M,E,S,D,I,R to
allow for
easy passage without risk of lodging as it passes. These papers will then be
coated with a
pharmaceutical enteric coating known as "Eudragit", which provides a smooth
surface to
allow printing of antennas. Eudragit is also a pH-sensitive material that will
dissolve in
the colon I, allowing the tag 15 to remain active long enough to be detected
before
disintegrating.
The biocompatible antennas 44,79 are printed on the substrate and
preferentially
coated with Eudragit as described above to protect the antenna and prevent
interaction
with the antenna materials until the tag passes into the colon I where it
begins to
disintegrate.
The GI sensor / energy cell includes the use of a zinc electrode 81 and silver
electrode 79 with special coatings as described previously. The GI sensor is
designed to
restrict the bioavailability of the materials to levels far below FDA, EPA,
and/or
recommended daily intakes. The simple GI sensor produces induced voltages from
the
voltaic battery when different metals interact with the acidic GI fluids. Zinc
foil is
preferably used for small scale production and is bonded to the tag 15 using a
conductive
adhesive. An analog to digital converter within data acquisition block 304 in
the chip 20
is used to uniquely detect the sensor's response to GI fluid.
37
I
CA 02909033 2015-10-13
FIGS. 25 and 26 show the preferred embodiment of the tag, its size, and its
approximate location of features. The tag 15 consists of four logical
components: the in-
link antenna system 79, the GI sensor 85, the tag integrated circuit (IC) 47,
and the out-
link TX antenna 44. The in-link antenna system 79 includes two in-link 50 pads
(body
contact pads) that are 1-2mm by 4mm. The GI sensor includes a pad 81 that is
approximately 1 mm x 5mm. The out-link antenna 44 utilizes the rest of the
available
space. The tag IC 47 is shown in the inlay of the figure. On the right of FIG
23 is a
diagram of the tag 15 after it is wrapped around a cylindrical object. The in-
link antenna
pads are separated by the maximum available distance, which is 180 degrees
across the
capsule once the tag is wrapped around it. The out-link antenna is optimized
for the
required three dimensional geometry of the capsule (or pill) after attachment.
The tag 15
materials and construction conforms to all safety, regulatory, and
manufacturing
requirements. The physical structure of the tag 15 and its relationship to a
medication
capsule is shown in FIG 27. Target Tag sizes are shown such as to conform to a
size 0
capsule. Future generations are expected to support smaller capsule geometries
and
tablets.
READER
To minimize the size and power requirements of the external reader 11, in one
embodiment it may not include the capabilities to transmit information via a
cell system,
wi-fl, or other wireless network. However, in another embodiment, the reader
11 can
transmit data to a standard cell phone, pager, or other device as shown in FIG
1 and as
described below with reference to FIG 3, to allow for real-time updating of
patient
compliance and monitoring. Using a two part reader system allows for a
miniaturized on-
body receiver 19 and a more powerful mobile device 54 with a more
sophisticated user
interface for messaging and transmission to a global database. In such a two-
part reader
system, an on-body reader 11 has two communication systems, one 50, 52 to
communicate with the tag 15 and one 56, 58 to communicate with the mobile
device (for
example a bluetooth; see FIGS. 3 and 30 and discussion below). In such a
system, the
mobile device only requires special software to operate as both a standard
mobile device
and a front-end user interface and wide area network (WAN) transmission
interface.
38
i
CA 02909033 2015-10-13
The external reader 11 can be embodied in several forms. For example, the
reader
can be the wristband 111 of FIG. 28, or the patch 211 of FIG. 29 that can be
adhered to
the skin like a bandage, an arm band, a handheld device or the like. In some
embodiments, it is advantageous for the reader to have contact with the skin
during a
medication event. It is also advantageous to design the reader to be readily
available
and/or worn to ensure it is present with the patient 16 for all medication
events. Another
form is the pill container 311 shown in FIG. 30 with contacts 313 on the
bottle holder 311
for skin contact during the ingestion of the medication. A minimal user
interface exists on
the bottle holder 311 with a button to indicate ingestion has taken place 312
and an
indicator 313 to determine when the pill was detected. The patient 16 removes
the
medication from the container 311, ingests it, presses button 312 and holds
the pill
container 311 against the skin until the ingestion event is detected at the
contacts 313 and
the indicator 314 confirms that the tag 15 was detected in the body 16. In
other forms, the
reader is also built into a mobile device such as a cell phone, PDA, wrist
watch, or into a
memory card, dongle, or other add-on device that can be attached or inserted
into a
mobile unit.
Noting FIG. 30, each reader 11, 111, 211 and 311 has a small user interface
227
that presents indicators of ingested medication being detected and/or the
capabilities to
indicate when medication should be ingested. The readers are disposable or
reusable, or
contains portions that are disposable and reusable. The readers also
preferably contain
means for storing the recorded data for downloading via USB or other means
directly to a
PC or other computing device. The readers are also preferentially
rechargeable. In
addition, for those applications where the readers do not need to be mobile,
they may be
built into a dongle or other means into a standard computer or laptop.
Continuing with FIG. 31, the reader comprises several RF/analog front-end
components interconnected with a digital processing core to handle the
communication
protocols. The body interface or antenna subsystem 220 interfaces with the
body 16 or
media surrounding the body (e.g. air). It contains the antenna and or contact
points to
transmit the in-link 50 data to the tag 15 and receive the out-link 52 data
from the tag 15.
In addition, the body interface subsystem 220 includes the sensors, contacts,
or antennas
39
,
CA 02909033 2015-10-13
necessary to acquire physiologic or biometric data required to ensure the
reader 11 is on
the right patient 16 and the tag 15 has been ingested by the right patient.
The receive
subsystem 221 and transmission subsystem 222 contains the electronics to drive
the
antennas and/or receive data from the body interface and antenna subsystem
220. The
uplink receive 225 and uplink transmit 226 subsystems transmit data to and
from an
either a mobile device for wide area communication or directly to a wide area
communication system such as cell phone, wifi, or paging networks. The
protocol and
control subsystem 224 manages the communications of the out-link 52, in-link
50, uplink
56, and downlink 58 transmissions, controls the user interface 227 and
processes all data
coming in and out of the reader 11. The user interface system 227 provides
information
to the patient about when a tag 15 has been detected, allows the patient 16 to
initiate a
manual detection, provides indicators of when the pill 14 should be taken, and
provides
other information to the patient.
The transmission subsystem 220 consists of a multiple modules. The first
module
contains a high voltage modulator stage with a programmable low frequency
carrier to
conductively couple RF signals into the body 16. The supply voltage of the
modulator
can be dynamically varied to superimpose in-link telemetry data to communicate
with the
tag on the pill. Digital input signals will be derived from the protocol and
control
subsystem 224 tasked to handle communication protocols to and from the tag and
also to
and from a uplink/downlink transceiver 225,226 that wirelessly interconnects
mobile
devices to the reader 11. The second module is a UHF receiver chipset used to
demodulate out-link 52 data from the tag 15. The receiver is used to
downconvert the
detected out-link RF signals 52 for data extraction by the baseband processor.
Preferably,
all communication protocols between reader 11, tag 15 and mobile devices 54
are be
synchronized to a master clock generation module to ensure proper timing
control.
SOFTWARE SYSTEM
True adherence improvement is likely to only be achieved when the patient 16
is
motivated to follow the prescribed regimen. By connecting the patient with the
medication, the pharmacodynamics (PD) and pharmacokinetics (PK), dose/response
data,
and their own reaction to the medications, patients become more interested in
their
I
CA 02909033 2015-10-13
regimen and become more adherent. The software system is preferentially
implemented
in a smart phone application that is linked to the reader 11 and the
information provided
by the uplink/downlink data. In a preferred embodiment, the software shows
estimated
blood levels of the drug of interest based on the known patient information
and medical
information stored in the system, as well as the exact timing and doses of the
medication
taken by the patient. The software shows the patients how missing doses or
improperly
taking their medication affects their simulated blood levels, drug
effectiveness, and how
it changes their physical responses to the medication.
Other embodiments include personalized calendars that a list each medication
and
dosage listed under the following four time periods: Morning, Noon, Evening,
and
Bedtime. If a patient does not take their medicine, they are asked to write
the reason. It
also lists any special instructions to help prevent adverse effects resulting
in decreased
medication adherence. The software also contains a list of abbreviated
instructions on
how to use and monitor each drug so that the patient understands the benefit
and risk of
each drug. The software also allows the pharmacist to enter how many days late
the
patient comes to the pharmacy for a refill of chronically taken medication. If
the
adherence rate is unsatisfactory, the pharmacist is presented with various
options on how
to enhance adherence through patient education programs designed from well
documented motivational interview techniques.
The Personalized Medication Adherence Registry (PMAR) is a mobile software
system that receives medication adherence data from the patient and device
links, then
presents it to the patient and healthcare providers in an extremely quick and
easy to
understand format. The largest group that will benefit from PMAR are those
patients
taking multiple chronically administered medications that are essential to
wellness.
Another important population are patients who are receiving medications that
create
frequent or severe adverse drug reactions (ADRs). Typical examples of
healthcare
providers are all physicians, pharmacists, nurse practitioners, physician
assistants, clinical
trial personnel, and any other health related professions who advise, monitor
or treat
patients with medications.
41
I
CA 02909033 2015-10-13
When patients visit their physician or other health care providers, they are
usually
asked to produce a comprehensive, up-to-date, and accurate list of all their
medications
including the name of the drug, the dosage strength, and the directions for
use. This list
can become extremely complex very quickly and difficult to recall. This list
is
immensely valuable when a patient is traveling and in an accident. It may be
life saving
if this medication list can be produced as quickly as possible with all the
required details.
Having an electronic copy immediately available can save the patient time and
money
while improving their health and possibly preventing an inappropriate drug
related
catastrophe. A common example is when one of over 20 million Americans with
diabetes becomes extremely weak in a public place. If he/she has recently
taken his
blood sugar lowering agent, a liquid with concentrated sugar e.g. a soft drink
or orange
drink may save their life. The patient must provide their username and
password to allow
others access to this encrypted information. Since this protected information
resides on
their cell phone, access to cellular service is not required. A back-up of all
this protected
information can also be accessed by the patient and any healthcare provider,
family
member or close friend who has access.
If the patient has access to the Internet via their cell phone or personal
computer,
they will be able to click a drug from their drug list and be linked to drug-
specific
information in Wikipedia. They will be reminded to print the information and
have it
validated for its accuracy and personalized application to their situation
based upon
various factors that are relevant, e.g., all their existing disease stated,
medication list, age,
sex, weight, diet, and exercise program.
MEDICATION AND REFILL REMINDERS
The Reminder feature of PMAR provides a timely visual and auditory notice to
the patient via their mobile phone allowing the customer to be alerted for
each of their
scheduled medications. PMAR is easily customizable as to each patient's
preference as
to how they are to be reminded and the sound/vibrate/visual notification rules
governing
the reminder system. They can reminded to take all their prescription
medications
(Rx's), OTCs, herbal medications, and nutritional supplements. This
information is
stored in their cell phone calendar and also does not require access to a
cellular network.
42
i
CA 02909033 2015-10-13
PMAR will also remind the patient several days prior to completing their
medication that it is time to obtain a new refill or if they are probably
almost out of their
OTC, herbal, and nutrition supplement. This prevents one of the leading causes
of proper
medication adherence. These reminders are based upon the date of the last
refill and
whether they received a weekly, monthly, or quarterly refill.
Although reminder systems are not uncommon, when coupled to compliance
monitoring systems, additional features become possible. For example, if
medications
are not ingested when requested, a series of reminders or alerts can be sent
starting with
the patient and following up with family, care givers, doctors, pharmacists,
drug trial
monitors and administrators. If necessary, the system or support personnel can
call or
visit the patient to ensure that there are no problems and that the patient is
taking the
medication regularly.
In addition, various advantageous patient reward systems can be included in
PMAR since medication adherence is positively recorded by the system. For
example,
when patient's take their medication according to their regimen, they may be
provided
with coupons for free or discounted services or products. These coupons could
be funded
by any number of parties with a vested interest in ensuring medication
adherence,
including the pharmacy, the pharmaceutical company, the insurance company or
government agency. For children and young adults, gaming coupons or online
money or
points for online games, music downloads, etc. can be provided for good or
improving
adherence.
ADVERSE DRUG REACTION REPORT (ADR)
When the patient is reminded to take their medication, one of their options
will be
to choose from a list of common side effects and ADRs to document if they have
experienced a recent ADR and if they stopped taking their medication secondary
to the
ADR. This often happens without the pharmacist or physician being aware. This
will
assist healthcare providers in determining the cause of patient non-adherence
and prompt
them to possibly decrease the dose or select an alternative medication. This
feature alone
can help decrease many avoidable hospitalizations.
NECK SENSOR
43
,
CA 02909033 2015-10-13
Referring again to FIG. 1, as an alternate to detection of the pill in the
gastrointestinal system, it is also possible to detect a pill 30 as it passes
through the
esophagus E using a sensor 32 designed to fit around the neck 33. Preferably,
this sensor
32 takes the form of a complete circle around the neck 33, a partial,
horseshoe-like
enclosure, or a simple device held against the neck 33. The sensor 32 detects
all
embodiments of the pill 30 described elsewhere as it passes through the
esophagus E into
the stomach S. The embodiments in which the sensor 32 forms a semi- or full
circle
around the neck 33 also improve the signal-to-noise ratio over a sensor that
is simply held
in front of the patient. There is also less dependence on digestive
mechanisms, providing
less design restrictions on the pill itself
The neck sensor operates in all the same ways as the gastrointestinal reader
11,
but also allows for other, possibly advantageous, protocols. For the case in
which
multiple pills must be detected, a protocol in which the patient takes one
pill at a time can
be employed. In this approach, only one pill will occupy the esophagus E at
any time,
which improves the sensor's capability to identify and tally dosage.
BIOMETRIC IDENTIFICATION AND COMPLIANCE MONITORING WITH
PHYSIOLOGICAL SIGNAL
A biometric identification aspect of the invention is now described with
reference
to FIGS. 31-33. In some instances, it is important to know that the electronic
pill 14 is
inside the body of the person wearing the reader device 111. In an embodiment,
this is
accomplished by both the pill 14 and reader 111 measuring a known physiologic
signal
and communicating attributes of this signal between them. The knowledge that
the reader
111 and pill 14 devices are measuring the same physiologic signal indicates
that the pill
14 is inside the same person that the reader 111 is attached to.
The pill 14 includes a compliance monitoring device 404 attached or inside.
This
compliance monitoring device 404 measures a biometric signal such as the
patient's ECG
405 after being ingested and sends a signal corresponding to the ECG signal
405 to the
reader 111 attached to the patient. The reader 111 also measures the ECG
signal 405 of
the patient and can compare its signal to that measured by the compliance
monitoring
44
I
CA 02909033 2015-10-13
device 404. If the two signals match, the pill 14 is confirmed to have been
ingested by
the person wearing the reader 111.
In a preferred embodiment, in order to reduce the amount of information that
needs to be transmitted, certain characteristics of the ECG signal are
calculated and
transmitted between the pill 14 and reader 111. Many characteristics are
possible, but a
preferred characteristic is the timing of the peaks in the ECG signal 405.
This timing,
sometimes called the R-R time because the peak of the ECG 405 is often
described by the
letters QRS, can be easily calculated at both the pill 14 and reader 111 and
compared,
without the need to transmit the entire ECG signal 405. Other characteristics
of the ECG
that can be measured include: heart rate, ratio of P to QRS or T to QRS
amplitudes,
duration P-R or R-T timing, QRS duration, and others that are known.
In addition to ECG, other physiologic signals may be used, including but not
limited to breath rate, muscle activity, acoustic information, and blood flow
measurements such as pulse oximetry, or body movements read with an
accelerometer or
similar sensors.
In certain communication schemes, synchronizing the transmitter and receiver
provides advantages including but not limited to higher signal to noise
ratios, lower
power transmission, and better reception. The use of the physiologic signal to
synchronize the in vivo and ex vivo transmitter and receiver provides both a
mechanism
for synchronization and also ensuring that only pills ingested by the person
wearing the
reader 111 are detected. If a person other than the person wearing the reader
111 ingests
the pill 14, the physiologic synchronization cannot take place since each
device will have
a different synchronization signal. Only if the same person who is wearing the
ex vivo
reader 111 also ingests the pill 14 can the two devices properly synchronize.
In addition
to this advantage, synchronizing to the physiologic signal prevents the need
for either
device to broadcast a synchronization signal, thus saving power in the
communication
system.
Another use of monitoring the physiologic signal is biometric identification.
By
measuring attributes of the ECG or other physiologic signal of the patient,
identification
of the person actually wearing the watch can be done. In a preferred
embodiment, the
CA 02909033 2015-10-13
physiologic signal is measured by the compliance monitoring device 404 or
reader 111
when the pill 14 is first provisioned. Thus, the device records the important
attributes of
the physiologic signal and then continuously compares the attributes of the
physiologic
signal throughout the use of the devices.
If the attributes change significantly, this indicates that the person wearing
the
reader 111 is not the same as the person who was originally provisioned the
reader 111.
This prevents people from swapping readers 111 or having other people take
their pills 14
for them.
Principal component analysis (PCA) is used for feature extraction from the
vector
of data points representing a single pulse of ECG. For this algorithm, the
fiduciary points
are projected with PCA into a subset of features. For the classifier portion
of the
biometric, a linear model is used to match the PCA features to each person. In
our
experimental trials, this method achieved an identification rate of 93% with
ECG data
sets from sixty different people.
A new algorithm was developed that averages the individual pulses and then
applies a similarity measure that compares the vector of PCA features from one
subject to
the vector of PCA features of other subjects. The similarity metric (for
example mean
squared error or Mahalanobis distance) calculates the distance between the
vector under
study and the average vectors from each subject in the database. For example,
individual
beats are aggregated and pruned for training the model. Pruning is determined
by the
similarity between beats. The similarity is calculated as follows:
n E xiyi¨Zxiyi
i2 ____________________________________________________________
n E x? - (E xi ) \In E Yi ¨ (E yi)2
This similarity measure is also used to determine patient X's distance with
respect
to either patient Y or a model that represents the world (averaged across all
other
patients) i.e. a cohort vs World model. In experimental trials, this model was
able to
correctly identify 98.67% of the patients (including the multi-day
recordings).
The use of continuous data available on a reader 111 that is worn for extended
periods of time provides a methodology for a much more robust biometric
identification.
46
I
CA 02909033 2015-10-13
Methods to remove noisy data during motion or other artifacts while still
matching only
the clean signals provides great flexibility in the identification system. In
addition, the
ability to detect when the signals are lost can be useful in determining when
the reader
111 is removed and replaced on the body. As long as the signal does not change
dramatically or cease to be collected, it can be assumed that the same person
is wearing
the reader 111.
When multiple pills 14 are ingested it is desirable for the reader 111 to be
able to
detect each device independently. In cases where the pills 14 are not in
communication or
synchronized by some other means, it is desirable to have a communication
telemetry
system that may operate asynchronously. Pills 14 provide telemetry data by
sending
amplitude based bursts of radio frequency (RF) energy at a frequency of fc Hz.
These
bursts are separated by a period of TB seconds. For multiple devices the
frequency fc
may be different for each device, either by design or due to process
variations. Similarly,
the period of bursts will be different for each device.
Asynchronous operation may be used to assure a relationship between the burst
separation period and the burst frequency. The reader 111 may then perform
frequency
analysis on the incoming pulse stream and sift out the individual pieces of
telemetry from
each communication device.
By way of example, consider FIG. 33. Here, an individual communication device
generates a burst separation and carrier frequency from a common clock
generator. By
determining the burst separation period at the reader 111, the burst frequency
may be
easily calculated.
FIG. 34 shows a reader 111 receive architecture which accomplishes this. The
input signal to the receiver includes bursts from up to N individual
communication
devices. The bursts contain information from each device 1 through N
represented by
signals Si, S2,
SN. The ensemble burst telemetry is down-converted and filtered to a
first Intermediate frequency, IF1. This signal is digitized by the analog to
digital
converter (ADC). A Fast Fourier Transform (FFT) is carried out which finds the
periodicity of the pulse period for each communication device. Because the
pulse period
is directly related to the carrier frequency, this information is binned and
used to digitally
47
1
CA 02909033 2015-10-13
control oscillator blocks that generate local down conversion signals to bring
each
individual in-vivo signal to baseband. The converted signal is optimally
filtered and
passed to a signal processing block that extracts the information signals Si
....SN.
An alternative approach is to use fixed oscillator signals for c 1 ....cN and
down-
convert directly.
POWER SOURCE ON THE BACK SIDE OF A DIE
Two forms of creating one half of a galvanic cell on the backside of a
semiconductor die are now described. These metallizations are used as a power
source for
the die upon immersion of the device in a fluid and when paired with an
appropriate
cathode/anode.
In each case, a metallization is formed to connect the front side of the die
where
the bond pads have been created to the typically bare backside of the die
which is
metallized with the appropriate cathode/anode material.
An alternative embodiment includes both the cathode and anode material
deposited on the backside with a physical space between them that may or may
not be
filled with a solid material.
Backside metallizations may be sputtered, vapor deposited, laser deposited,
bombarded, condensed, or physically attached/adhered/secured/melted.
Various modifications of the embodiments described here can be made without
departing from the spirit and scope of the invention as described above.
48
1