Note: Descriptions are shown in the official language in which they were submitted.
WO 2014/176415 PCT/US2014/035279
2,2-DIFLUOROPROPIONAMIDE DERIVATIVES OF BARDOXOLONE
METHYL, POLYMORPHIC FORMS AND METHODS OF USE THEREOF
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates generally to the compound:
N-((4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-2,2,6a,6b,9,9,12a-
heptamethy1-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-
o ctadec ahydropi cen-4a-y1)-2,2-di fl uoroprop anami de,
also referred to herein as RTA 408, 63415, or PP415. The present invention
also relates
to polymorphic forms thereof, methods for preparation and use thereof,
pharmaceutical
compositions thereof, and kits and articles of manufacture thereof.
II. Description of Related Art
The anti-inflammatory and anti-proliferative activity of the naturally
occurring
triterpenoid, oleanolic acid, has been improved by chemical modifications. For
example,
2-cyano-3,12-diooxooleana-1,9(11)-dien-28-oic acid (CDDO) and related
compounds
have been developed. See Honda et al., 1997; Honda et al., 1998; Honda et al.,
1999;
Honda et al., 2000a; Honda et al., 2000b; Honda et al., 2002; Suh et al.,
1998; Suh et al.,
1999; Place et al., 2003; Liby et al., 2005; and U.S. Patents 8,129,429,
7,915,402,
8,124,799, and 7,943,778. The methyl
ester, bardoxolone methyl (CDDO¨Me), has been evaluated in phase IT and III
clinical
trials for the treatment and prevention of diabetic nephropathy and chronic
kidney
disease. See Pergola et al., 2011.
Synthetic triterpenoid analogs of oleanolic acid have also been shown to be
inhibitors of cellular inflammatory processes, such as the induction by IFN-y
of inducible
nitric oxide synthase (iNOS) and of COX-2 in mouse macrophages. See Honda et
al,
(2000a), Honda et al. (2000b), Honda et al. (2002), and U.S. Patents
8,129,429,
7,915,402, 8,124,799, and 7,943,778.
Compounds derived from oleanolic acid have been shown to affect the function
of
multiple protein targets and thereby modulate the activity of several
important cellular
1
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
signaling pathways related to oxidative stress, cell cycle control, and
inflammation (e.g.,
Dinkova-Kostova etal., 2005; Ahmad etal., 2006; Ahmad etal., 2008; Liby et
al., 2007,
and U.S. Patents 8,129,429, 7,915,402, 8,124,799, and 7,943,778).
Given that the biological activity profiles of known triterpenoid derivatives
vary,
and in view of the wide variety of diseases that may be treated or prevented
with
compounds having potent antioxidant and anti-inflammatory effects, and the
high degree
of unmet medical need represented within this variety of diseases, it is
desirable to
synthesize new compounds with different biological activity profiles for the
treatment or
prevention of one or more indications.
SUMMARY OF THE INVENTION
In some aspects of the present invention, there is provided a compound of the
formula (also referred to as RTA 408, 63415, or PP415):
0
0
NC NA)(
F F
0
or a pharmaceutically acceptable salt thereof.
In some embodiments, the compound is in the form of a pharmaceutically
acceptable salt. In some embodiments, the compound is not in the form of a
salt.
In another aspect of the present invention, there are provided polymorphic
forms
of the above compound.
In some embodiments, the polymorphic form is crystalline, having an X-ray
powder diffraction pattern (CuKa) comprising peaks at about 10.601, 11.638,
12.121,
13.021, 13,435, 15.418, 15.760, 17.830, 18.753, and 19.671 028. In some
embodiments,
the X-ray powder diffraction pattern (CuKa) is substantially as shown in FIG.
53.
In some embodiments, the polymorphic form is crystalline, having an X-ray
powder diffraction pattern (CuKa) comprising peaks at about 7.552, 10.339,
11.159,
12.107, 14.729, 15.329, 15.857, 16.824, 17.994, 18.344, 19.444, 19.764,
20.801, and
2
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
22.414 20. In some embodiments, the X-ray diffraction pattern (CuKa) is
substantially
as shown in FIG. 56.
In another aspect of the present invention, there are provided pharmaceutical
compositions comprising an active ingredient consisting of the above compound
or
polymorphic forms thereof (such as, e.g., any one of the polymorphic forms
described
herein above or below), and a pharmaceutically acceptable carrier. In
some
embodiments, the pharmaceutical composition is formulated for administration:
orally,
intraadiposally, intraarterially, intraarticularly,
intracranially, intradermally,
intralesionally, intramuscularly, intranasally, intraocularly,
intrapericardially,
intraperitoneally, intrapleurally, intraprostatically, intrarectally,
intrathecally,
intratracheally, intratumorally, intraumbilically, intravaginally,
intravenously,
intravesicularlly, intravitreally, liposomally, locally, mucosally,
parenterally, rectally,
subconjunctival, subcutaneously, sublingually, topically, transbuccally,
transdermally,
vaginally, in cremes, in lipid compositions, via a catheter, via a lavage, via
continuous
infusion, via infusion, via inhalation, via injection, via local delivery, or
via localized
perfusion. In some embodiments, the pharmaceutical composition is formulated
for oral,
intraarterial, intravenous, or topical administration. In
some embodiments, the
pharmaceutical composition is formulated for oral administration.
In some embodiments, the pharmaceutical composition is formulated as a hard or
soft capsule, a tablet, a syrup, a suspension, a solid dispersion, a wafer, or
an elixir. In
some embodiments, the pharmaceutical composition according to the invention
further
comprises an agent that enhances solubility and dispersibility. In some
embodiments, the
compound or polymorphic form is suspended in sesame oil.
In other embodiments, the pharmaceutical composition is formulated for topical
administration. In other embodiments, the pharmaceutical composition is
formulated as a
lotion, a cream, a gel, an oil, an ointment, a salve, or a suspension. In some
embodiments, the pharmaceutical composition is formulated as a lotion, as a
cream, or as
a gel. In some embodiments, the amount of the active ingredient is from about
0.01% to
about 5% by weight, about 0.01% to about 3% by weight, or 0.01%, 0.1%, 1%, or
3% by
weight.
3
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
In another aspect of the present invention there are provided methods of
treating
or preventing a condition associated with inflammation or oxidative stress in
a patient in
need thereof, comprising administering to the patient a therapeutically
effective amount
of the pharmaceutical composition as described above or below. The invention
likewise
relates to the compound N-((4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-
2,2,6a,6b,9,9,12a-heptamethy1-10,14-dioxo-
1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-octadecahydropicen-4a-y1)-2,2-
difluoropropanamide (or RTA 408, 63415, or PP415) or a pharmaceutically
acceptable
salt thereof, or a polymorphic form of that compound (such as, e.g., any one
of the
polymorphic forms described herein above or below), or a pharmaceutical
composition
comprising any of the aforementioned entities and a pharmaceutically
acceptable carrier
(including, e.g., the pharmaceutical compositions described above), for use in
treating or
preventing a condition associated with inflammation or oxidative stress. The
invention
also relates to the use of the aforementioned compound, polymorphic form or
pharmaceutical composition for the preparation of a medicament for the
treatment or
prevention of a condition associated with inflammation or oxidative stress. In
some
embodiments, the condition is associated with inflammation. In other
embodiments, the
condition is associated with oxidative stress. In some embodiments, the
condition is a
skin disease or disorder, sepsis, dermatitis, osteoarthritis, cancer,
inflammation, an
autoimmune disease, inflammatory bowel disease, a complication from localized
or total-
body exposure to ionizing radiation, mucositis, acute or chronic organ
failure, liver
disease, pancreatitis, an eye disorder, a lung disease or diabetes.
The present invention furthermore relates to the compound N-
((4aS,6aR,6bS,8aR,12 aS,14aR ,14b5)-11-cyano-2,2 ,6a,6b ,9,9,12 a-heptamethyl-
10,14-
dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-octadecahydropicen-4a-
y1)-2,2-
difluoropropanamide (or RTA 408) or a pharmaceutically acceptable salt
thereof, or a
polymorphic form of that compound (such as, e.g., any one of the polymorphic
forms
described herein above or below), or a pharmaceutical composition comprising
any of the
aforementioned entities and a pharmaceutically acceptable carrier (including,
e.g., the
pharmaceutical compositions described above), for use in treating or
preventing a
condition selected from a skin disease or disorder, sepsis, dermatitis,
osteoarthritis,
4
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
cancer, inflammation, an autoimmune disease, inflammatory bowel disease, a
complication from localized or total-body exposure to ionizing radiation,
mucositis, acute
or chronic organ failure, liver disease, pancreatitis, an eye disorder, a lung
disease, or
diabetes. Accordingly, the invention relates to the use of the aforementioned
compound,
polymorphic form or pharmaceutical composition for the preparation of a
medicament for
the treatment or prevention of a condition selected from a skin disease or
disorder, sepsis,
dermatitis, osteoarthritis, cancer, inflammation, an autoimmune disease,
inflammatory
bowel disease, a complication from localized or total-body exposure to
ionizing radiation,
mucositis, acute or chronic organ failure, liver disease, pancreatitis, an eye
disorder, a
lung disease, or diabetes. The invention also relates to a method of treating
or preventing
a condition selected from a skin disease or disorder, sepsis, dermatitis,
osteoarthritis,
cancer, inflammation, an autoimmune disease, inflammatory bowel disease, a
complication from localized or total-body exposure to ionizing radiation,
mucositis, acute
or chronic organ failure, liver disease, pancreatitis, an eye disorder, a lung
disease, or
diabetes in a patient in need thereof, the method comprising administering to
the patient a
therapeutically effective amount of the aforementioned compound, polymorphic
form or
pharmaceutical composition.
In some embodiments, the condition is a skin disease or disorder such as
dermatitis, a thermal or chemical burn, a chronic wound, acne, alopecia, other
disorders
of the hair follicle, epidermolysis bullosa, sunburn, complications of
sunburn, disorders
of skin pigmentation, an aging-related skin condition; a post-surgical wound,
a scar from
a skin injury or burn, psoriasis, a dermatological manifestation of an
autoimmune
diseases or a graft-versus host disease, skin cancer; or a disorder involving
hyperproliferation of skin cells. In some embodiments, the skin disease or
disorder is
dermatitis. In some embodiments, the dermatitis is allergic dermatitis, atopic
dermatitis,
dermatitis due to chemical exposure, or radiation-induced dermatitis. In
other
embodiments, the skin disease or disorder is a chronic wound. In some
embodiments, the
chronic wound is a diabetic ulcer, a pressure sore, or a venous ulcer. In
other
embodiments, the skin disease or disorder is alopecia. In some embodiments,
the
alopecia is selected from baldness or drug-induced alopecia. In other
embodiments, the
skin disease or disorder is a disorder of skin pigmentation. In some
embodiments, the
5
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
disorder of skin pigmentation is vitiligo. In other embodiments, the skin
disease or
disorder is a disorder involving hyperproliferation of skin cells. In some
embodiments,
the disorder involving hyperproliferation of skin cells is hyperkeratosis.
In other embodiments, the condition is an autoimmune disease, such as
rheumatoid arthritis, lupus, Crohn's disease, or psoriasis. In other
embodiments, the
condition is liver disease, such as fatty liver disease or hepatitis.
In other embodiments, the condition is an eye disorder, such as uveitis,
macular
degeneration, glaucoma, diabetic macular edema, blepharitis, diabetic
retinopathy, a
disease or disorder of the corneal endothelium, post-surgical inflammation,
dry eye,
allergic conjunctivitis or a form of conjunctivitis. In some embodiments, the
eye disorder
is macular degeneration. In some embodiments, the macular degeneration is the
dry
form. In other embodiments, the macular degeneration is the wet form. In some
embodiments, the disease or disorder of the corneal endothelium is Fuchs
endothelial
corneal dystrophy.
In other embodiments, the condition is a lung disease, such as pulmonary
inflammation, pulmonary fibrosis, COPD, asthma, cystic fibrosis, or idiopathic
pulmonary fibrosis. In some embodiments, the COPD is induced by cigarette
smoke.
In other embodiments, the condition is sepsis. In other embodiments, the
condition is mucositis resulting from radiation therapy or chemotherapy. In
some
embodiments, the mucositis presents orally. In other embodiments, the
condition is
associated with exposure to radiation. In some embodiments, the radiation
exposure
leads to dermatitis. In some embodiments, the radiation exposure is acute. In
other
embodiments, the radiation exposure is fractionated.
In other embodiments, the condition is cancer. In
some non-limiting
embodiments, the cancer is leukemia, lymphoma, multiple myeloma, or cancer of
the
breast, skin, lung, pancreas, liver, stomach, small intestine, large intestine
or colon, gall
bladder, esophagus, ovary, endometrium, cervix, oral or nasal mucosa, brain,
prostate,
bladder, urogenital tract, testicle, kidney, genitalia, thyroid, or muscle
tissue. In some
embodiments, the cancer is a carcinoma or sarcoma.
In some embodiments, the compound or composition of the invention is
administered before or immediately after a subject is treated with radiation
therapy,
6
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
chemotherapy, or both. In some embodiments, the compound or composition of the
invention is administered both before and after the subject is treated with
radiation
therapy, chemotherapy or both. In some embodiments, the effect of the
composition of
the invention is to reduce side effects of radiation therapy, chemotherapy, or
combined
radio- and chemo- therapy, including mucositis and dermatitis. In some
embodiments,
the effect of the composition of the invention is to enhance the efficacy of
the radiation
therapy, chemotherapy, or combined radio- and chemo- therapy. In some
embodiments,
the effect of the composition of the invention is to reduce the side effects
of, and enhance
the efficacy of, the radiation therapy, chemotherapy, or combined radio- and
chemo-
therapy.
Combination treatment therapy is also contemplated by the present disclosure.
For
example, regarding methods of treating cancer in a subject, comprising
administering to
the subject a pharmaceutically effective amount of a compound of the present
disclosure,
the method may further comprise a treatment selected from the group consisting
of
administering a pharmaceutically effective amount of a second drug,
radiotherapy, gene
therapy, and surgery. Such methods may further comprise (1) contacting a tumor
cell
with the compound prior to contacting the tumor cell with the second drug, (2)
contacting
a tumor cell with the second drug prior to contacting the tumor cell with the
compound,
or (3) contacting a tumor cell with the compound and the second drug at the
same time.
The second drug may, in certain embodiments, be an antibiotic, anti-
inflammatory, anti-
neoplastic, anti-proliferative, anti-viral, immunomodulatory, or
immunosuppressive. The
second drug may be an alkylating agent, androgen receptor modulator,
cytoskeletal
disruptor, estrogen receptor modulator, histone-deacetylase inhibitor, HMG-CoA
reductase inhibitor, prenyl-protein transferase inhibitor, retinoid receptor
modulator,
topoisomerase inhibitor, or tyrosine kinase inhibitor. In certain embodiments,
the second
drug is 5-azacitidine, 5-fluorouracil, 9-cis-retinoic acid, actinomycin D,
alitretinoin, all-
trans-retinoic acid, annamycin, axitinib, belinostat, bevacizumab, bexarotene,
bosutinib,
busulfan, capecitabine, carboplatin, carmustine, CD437, cediranib, cetuximab,
chlorambucil, cisplatin, cyclophosphamide, cytarabine, dacarbazine, dasatinib,
daunorubicin, de citabine, docetaxel, do lastatin-10, doxifluridine,
doxorubicin,
doxorubicin, epirubicin, erlotinib, etoposide, etoposide, gefitinib,
gemcitabine,
7
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
gemtuzumab ozogamicin, hexamethylmelamine, idarubicin, ifosfamide, imatinib,
irinotecan, isotretinoin, ixabepilone, lapatinib, LBH589, lomustine,
mechlorethamine,
melphalan, mercaptopurine, methotrexate, mitomycin, mitoxantrone, MS-275,
neratinib,
nilotinib, nitrosourea, oxaliplatin, paclitaxel, plicamycin, procarbazine,
semaxanib,
semustine, sodium butyrate, sodium phenylacetate, streptozotocin,
suberoylanilide
hydroxamic acid, sunitinib, tamoxifen, teniposide, thiopeta, tioguanine,
topotecan,
TRAIL, trastuzumab, tretinoin, trichostatin A, valproic acid, valrubicin,
vandetanib,
vinblastine, vincristine, vindesine, or vinorelbine.
Methods of treating or preventing a disease with an inflammatory component in
a
subject, comprising administering to the subject a pharmaceutically effective
amount of a
compound of the present disclosure are also contemplated. The disease may be,
for
example, lupus or rheumatoid arthritis. The disease may be an inflammatory
bowel
disease, such as Crohn's disease or ulcerative colitis. The disease with an
inflammatory
component may be a cardiovascular disease. The disease with an inflammatory
component may be diabetes, such as type 1 or type 2 diabetes. RTA 408 may also
be
used to treat complications associated with diabetes. Such complications are
well-known
in the art and include, for example, obesity, hypertension, atherosclerosis,
coronary heart
disease, stroke, peripheral vascular disease, hypertension, nephropathy,
neuropathy,
myonecrosis, retinopathy and metabolic syndrome (syndrome X). The disease with
an
inflammatory component may be a skin disease, such as psoriasis, acne, or
atopic
dermatitis. Administration of a RTA 408 in treatment methods of such skin
diseases may
be, for example, topical or oral.
The disease with an inflammatory component may be metabolic syndrome
(syndrome X). A patient having this syndrome is characterized as having three
or more
symptoms selected from the following group of five symptoms: (1) abdominal
obesity;
(2) hypertriglyceridemia; (3) low high-density lipoprotein cholesterol (HDL);
(4) high
blood pressure; and (5) elevated fasting glucose, which may be in the range
characteristic
of Type 2 diabetes if the patient is also diabetic. Each of these symptoms is
defined in
the Third Report of the National Cholesterol Education Program Expert Panel on
Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult
Treatment Panel III, or ATP III), National Institutes of Health, 2001, NIH
Publication
8
WO 2014/176415 PCT/US2014/035279
No. 01-3670.
Patients with metabolic syndrome,
whether or not they have or develop overt diabetes mellitus, have an increased
risk of
developing the macrovascular and microvascular complications that are listed
above that
occur with type 2 diabetes, such as atherosclerosis and coronary heart
disease.
Another general method of the present disclosure entails a method of treating
or
preventing a cardiovascular disease in a subject, comprising administering to
the subject
a pharmaceutically effective amount of a compound of the present disclosure.
The
cardiovascular disease may be, for example, atherosclerosis, cardiomyopathy,
congenital
heart disease, congestive heart failure, myocarditis, rheumatic heart disease,
valve
disease, coronary artery disease, endocarditis, or myocardial infarction.
Combination
therapy is also contemplated for such methods. For example, such methods may
further
comprise administering a pharmaceutically effective amount of a second drug.
The
second drug may be, for example, a cholesterol lowering drug, an anti-
hyperlipidemic, a
calcium channel blocker, an anti-hypertensive, or an HMG-CoA reductase
inhibitor.
Non-limiting examples of second drugs include amlodipine, aspirin, ezetimibe,
felodipine, lacidipine, lercanidipine, nicardipine, nifedipine, nimodipine,
nisoldipine or
nitrendipine. Other non-limiting examples of second drugs include atenolol,
bucindolol,
carvedilol, clonidine, doxazosin, indoramin, labetalol, methyldopa,
metoprolol, nadolol,
oxprenolol, phenoxybenzamine, phentolamine, pindolol, prazosin, propranolol,
terazosin,
timolol or tolazoline. The second drug may be, for example, a statin, such as
atorvastatin,
cerivastatin, fluvastatin, 1ovastatin, mevastatin, pitavastatin, pravastatin,
rosuvastatin or
simvastatin.
Methods of treating or preventing a neurodegenerative disease in a subject,
comprising administering to the subject a pharmaceutically effective amount of
a
compound of the present disclosure are also contemplated. The
neurodegenerative
disease may, for example, be selected from the group consisting of Parkinson's
disease,
Alzheimer's disease, multiple sclerosis (MS), Huntington's disease and
amyotrophic
lateral sclerosis. In particular embodiments, the neurodegenerative disease is
Alzheimer's
disease In particular embodiments, the neurodegenerative disease is MS, such
as
primary progressive, relapsing-remitting secondary progressive or progressive
relapsing
MS. The subject may be, for example, a primate. The subject may be a human.
9
Date Recue/Date Received 2020-09-03
WO 2014/176415 PCT/US2014/035279
In particular embodiments of methods of treating or preventing a
neurodegenerative disease in a subject, comprising administering to the
subject a
pharmaceutically effective amount of a compound of the present disclosure, the
treatment
suppresses the demyclination of neurons in the subject's brain or spinal cord.
In certain
embodiments, the treatment suppresses inflammatory demyelination. In
certain
embodiments, the treatment suppresses the transection of neuron axons in the
subject's
brain or spinal cord. In certain embodiments, the treatment suppresses the
transection of
neurites in the subject's brain or spinal cord. In certain embodiments, the
treatment
suppresses neuronal apoptosis in the subject's brain or spinal cord. In
certain
embodiments, the treatment stimulates the remyelination of neuron axons in the
subject's
brain or spinal cord. in certain embodiments, the treatment restores lost
function after an
MS attack. In certain embodiments, the treatment prevents a new MS attack. In
certain
embodiments, the treatment prevents a disability resulting from an MS attack.
One general aspect of the present disclosure contemplates a method of treating
or
preventing a disorder characterized by overexpression of iNOS genes in a
subject,
comprising administering to the subject a pharmaceutically effective amount of
a
compound of the present disclosure.
Another general aspect of the present disclosure contemplates a method of
inhibiting IFN-y-induccd nitric oxide production in cells of a subject,
comprising
administering to said subject a pharmaceutically effective amount of a
compound of the
present disclosure.
Yet another general method of the present disclosure contemplates a method of
treating or preventing a disorder characterized by overexpression of COX-2
genes in a
subject, comprising administering to the subject a pharmaceutically effective
amount of
compound of the present disclosure.
Methods of treating renal/kidney disease (RKD) in a subject, comprising
administering to the subject a pharmaceutically effective amount of a compound
of the
present disclosure are also contemplated. See U.S. patent application Ser. No.
12/352,473. The
RKD may
result from, for example, a toxic insult. The toxic insult may result from,
for example, an
imaging agent or a drug. The drug may be a chemotherapeutic, for example. The
RKD
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
may result from ischemia/reperfusion injury, in certain embodiments. In
certain
embodiments, the RKD results from diabetes or hypertension. The RKD may result
from
an autoimmune disease. The RKD may be further defined as chronic RKD, or acute
RKD.
In certain methods of treating renal/kidney disease (RKD) in a subject,
comprising administering to the subject a pharmaceutically effective amount of
a
compound of the present disclosure, the subject has undergone or is undergoing
dialysis.
In certain embodiments, the subject has undergone or is a candidate to undergo
kidney
transplant. The subject may be a primate. The primate may be a human. The
subject in
this or any other method may be, for example, a cow, horse, dog, cat, pig,
mouse, rat or
guinea pig.
Also contemplated by the present disclosure is a method for improving
glomerular filtration rate or creatinine clearance in a subject, comprising
administering to
the subject a pharmaceutically effective amount of a compound of the present
disclosure.
In some embodiments, the pharmaceutical composition is administered in a
single
dose per day. In other embodiments, the pharmaceutical composition is
administered in
more than one dose per day. In some embodiments, the pharmaceutical
composition is
administered in a pharmaceutically effective amount.
In some embodiments, the active ingredient is administered in a dose from
about
1 mg/kg to about 2000 mg/kg. In other embodiments, the dose is from about 3
mg/kg to
about 100 mg/kg. In other embodiments, the dose is about 3, 10, 30, or 100
mg/kg.
In other embodiments, the pharmaceutical composition is administered
topically.
In some embodiments, the topical administration is administered to the skin.
In other
embodiments, the topical administration is administered to the eye.
In other embodiments, the pharmaceutical composition is administered orally.
In
other embodiments, the pharmaceutical composition is administered
intraocularly.
Other objects, features and advantages of the present disclosure will become
apparent from the following detailed description. It should be understood,
however, that
the detailed description and the specific examples, while indicating specific
embodiments
of the invention, are given by way of illustration only, since various changes
and
modifications within the spirit and scope of the invention will become
apparent to those
11
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
skilled in the art from this detailed description. Note that simply because a
particular
compound is ascribed to one particular generic formula does not mean that it
cannot also
belong to another generic formula.
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawings form part of the present specification and are included
to
further demonstrate certain aspects of the present disclosure. The invention
may be better
understood by reference to one of these drawings in combination with the
detailed
description of specific embodiments presented herein.
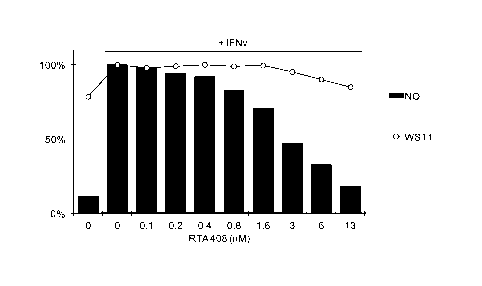
FIG. 1 ¨ Effect of RTA 408 on 1FN7-induced nitric oxide production and cell
viability in RAW264.7 cells.
FIGS. 2a & b ¨ Effect of RTA 408 on antioxidant response element activation:
(a) NQ01-ARE luciferase activity; (b) GSTA2-ARE luciferase activity.
FIGS. 3a¨d ¨ Effect of RTA 408 on Nrf2 target gene expression in HFL1 lung
fibroblasts: (a) NQ01; (b) HMOX1; (c) GCLM; (d) TXNRD1.
FIGS. 4a¨d ¨ Effect of RTA 408 on Nrf2 target gene expression in BEAS-2B
bronchial epithelial cells: (a) NQ01; (b) HMOX1; (c) GCLM; (d) TXNRD1.
FIGS. 5a & b ¨ Effect of RTA 408 on Nrf2 target protein levels: (a) SH-SY5Y
cells; (b) BV2 cells.
FIG. 6 ¨ Effect of RTA 408 on NQ01 enzymatic activity in RAW264.7 cells.
FIG. 7 ¨ Effect of RTA 408 on total glutathione levels in the AML-12
hepatocyte
cell line.
FIG. 8 ¨ Effect of RTA 408 on WST-1 absorbance as a marker of NADPH.
FIGS. 9a¨d ¨ Effect of RTA 408 on expression of genes involved in NADPH
synthesis: (a) H6PD; (b) PGD; (c) TKT; (d) ME1 .
12
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
FIG. 10 ¨ Effect of RTA 408 on TNFa-induced activation of a NF-x13 luciferase
reporter construct.
FIG. 11 ¨ Effect of RTA 408 on TNFa-induced phosphorylation of IxBa.
FIGS. 12a-d ¨ Effect of RTA 408 on transaminase gene expression: (a) ALT1
(GPT1); (b) ALT2 (GPT2); (c) ASTI (GOT1); (d) ASTI (GOT2). Asterisks indicate
a
statistically-significant difference from the control group (*P < 0.05; **P <
0.01).
FIG. 13 ¨ Effect of RTA 408 on pyruvate levels in cultured muscle cells (*P <
0.05).
FIG. 14 ¨ RTA 408 activity in a model of pulmonary LPS-mediated
inflammation (% change in pro-inflammatory cytokines relative to LPS
treatment). RTA
408 was administered QDx3 at Time 0, 24, and 48 hours followed by LPS one hour
after
the last dose of RTA 408 in female BALB/c mice. Animals were sacrificed 20
hours
after LPS administration. BALF was examined for pro-inflammatory cytokine
expression. RTA 408 reduced pro-inflammatory cytokines: Dose-dependent
reductions
were observed, with peak reductions ranging from 50%-80% in TNF, IL-6, and IL-
12.
FIGS. 15a & b ¨ Effect of RTA 408 on LPS-induced pulmonary inflammation in
mice: (a) inflammatory cytokines; (b) Nrf2 targets. Methods: RTA 408
administered to
female BALB/c mice (n = 10) QDx6 at Time 0, 24, 48, 72, 96, and 120 hours
followed
by LPS at 121 hours with animals sacrificed at 141 hours. Pro-inflammatory
cytokine
protein expression assayed in BALF; Nrf2 biomarkers assayed in lung. Asterisks
indicate a statistically significant difference from the saline control group
(*P < 0.05; **P
<0.01; ***P < 0.001).
FIGS. 16a & b ¨ RTA 408 reduces BALF infiltrates in bleomycin-induced
pulmonary inflammation: (a) BAL fluid cell count; (b) body weight. RTA 408 was
administered QDx39 on Days -10 to 28 in C57BL16 mice. Bleomycin was given on
Day 0. Daily weights were measured. BAL fluid cell counts were obtained at
sacrifice.
A notable reduction in inflammatory infiltrate was observed. No
significant
13
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
improvement in chronic inflammation score, interstitial fibrosis, or number of
fibrotic
foci was observed.
FIGS. 17a & b ¨ Effect of RTA 408 on bleomycin-induced pulmonary fibrosis in
rats: (a) PMN; (b) Hydroxyproline. Asterisks indicate a statistically
significant difference
from the bleomycin control group (*P < 0.05).
FIG. 18 ¨ Effect of RTA 408 on Nrf2 target enzymes in lungs from rats with
bleomycin-induced pulmonary fibrosis. Asterisks indicate a statistically
significant
difference from the saline control group (*P< 0.05; **P < 0.01; ***P< 0.001).
FIGS. 19a¨e ¨ Effect of RTA 408 on cigarette smoke-induced COPD in mice: (a)
KC; (b) IL-6; (c) TNF-a; (d) IFN-y; (e) RANTES. RTA 408 (63415) was tested at
dose
levels of 3 mg/kg (low), 10 mg/kg (mid), and 30 mg/kg (high). An AIM analog
(63355)
was tested in the same study for comparison. Asterisks indicate a
statistically significant
difference form the CS control group.
FIG. 20 ¨ Effect of RTA 408 on Nrf2 target enzymes in lungs from mice with
cigarette smoke-induced COPD. Asterisks indicate a statistically significant
difference
from the saline control group (*P < 0.05; **P < 0.01; ***P < 0.001). Daggers
represent
a statistically significant difference from mice expose to cigarette smoke and
administered vehicle (tP < 0.05).
FIGS. 21a¨d ¨ show body weight as a function of time of 63415-treated BALB/c
mice that serves as a model of sepsis. LPS was administered to all animals on
Day 0. (a)
Body Weight: 63415, (b) Body Weight: RTA 405, (c) Systemic LPS: % Survival:
63415,
(d) Systemic LPS: % Survival: RTA 405. Both RTA 408 and 63415 was administered
QDx5 on Days -2 to 2. 63415 improved survival.
FIG. 22 ¨ RTA 408 activity in a model of radiation-induced oral mucositis. RTA
405 or RTA 408 (63415) was administered BIDx20 on Days -5 to -1 and Days 1 to
15 to
male Syrian Golden Hamsters. Radiation occurred on Day 0. Mucositis scores
range
from 0 to 5 based on clinical manifestations (0: completely healthy; 1-2:
light to severe
14
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
erythema; 3-5: varying degrees of ulceration). RTA 408 (63415) meaningfully
improved
mucositis at 30 mg/kg and 100 mg/kg with up to a 36% reduction in ulceration.
FIG. 23 ¨ Nrf2 target gene induction consistent from RTA 408 (63415) 14-day
mouse toxicity study in C57BL/6 mice. mRNA of Nrf2 target genes assessed in
livers of
mice treated PO QDx14. Substantial increases in mRNA expression for multiple
Nrf2
target genes were observed and were consistent with tissue exposure.
FIGS. 24a & b ¨ Induction of Nrf2 target genes in the rat liver by RTA 408
(63415): (a) Target genes; (b) Negative regulators. mRNA of Nrf2 target genes
was
assessed in livers of rats treated PO QDx14.
FIGS. 25a & b ¨ RTA 408 (63415) induces Nrf2 target genes in monkey tissues:
(a) Liver; (b) Lung. mRNAs of Nrf2 target genes were assessed in monkeys
treated PO
QDx14 using Panomics QuantiGene0 2.0 Plex technology.
FIGS. 26a & b ¨ RTA 408 (63415) induces Nrf2 target enzyme activity in the
mouse liver: (a) NQ01 activity; (b) GST activity. Nrf2 target enzyme activity
was
assessed in livers of mice treated PO QDx14. NQ01 and GST enzyme activities
were
induced in a dose dependent manner.
FIGS. 27a & b ¨ Induction of target enzyme activity in the rat liver by RTA
408
(63415): (a) NQ01; (b) GST. Nrf2 target enzyme activity was assessed in livers
of rats
treated PO QDx14. NQ01 and GST enzyme activities were induced dose-
dependently.
FIGS. 28a & b ¨ RTA 408 (63415) induces Nrf2 target enzyme activity in
various tissues of cynomolgus monkeys: (a) NQ01 activity; (b) GSR activity.
FIGS. 29a & b ¨ RTA 408 concentration in mouse liver, lung, and brain, and
NQ01 activity in mouse liver after 14 days of daily oral administration. (a)
Tissue
distribution of RTA 408 in mice after 14 days of daily oral administration.
Data
represent mean SD RTA 408 concentrations in tissue collected 4 hours after
the final
dose of the study. Numbers above the error bars are representative of the
mean. (b)
Correlation of mouse liver RTA 408 content with NQ01 enzyme activity.
Individual
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
mouse liver RTA 408 liver content was plotted against individual enzyme
activity from
this report.
FIGS. 30a & b ¨ RTA 408 concentration in rat plasma, liver, lung, and brain,
and
NQ01 activity in rat liver after 14 days of daily oral administration. (a)
Tissue
distribution of RTA 408 in rats after 14 days of daily oral administration.
Data represent
mean SD RTA 408 concentrations in tissue collected 4 hours after the final
dose of the
study. Numbers above the error bars are representative of the mean. *Two
values were
excluded from the mean calculation due to being outliers, defined as values
causing the
set of data to fail the Shapiro-Wilk normality test. (b) Correlation of rat
liver RTA 408
content with NQ01 enzyme activity. Individual rat liver RTA 408 liver content
was
plotted against individual enzyme activity from this report. The tissues from
the
100 mg/kg RTA 408 dose group were collected on Day 6, and the observed
toxicities in
this group precluded liver NQ01 enzyme activity evaluations.
FIGS. 31a & b ¨ RTA 408 (63415) treatment of monkeys activated Nrf2 in
PBMC cells: (a) PBMC NQ01 vs. Plasma Concentration; (b) Lung NQ01 vs. PBMC
NQ01.
FIG. 32 ¨ Summary of RTA 408 (63415) 14-day monkey toxicity study. All
doses were well-tolerated without adverse clinical signs. Clinical chemistry
data
suggested no obvious toxicity.
FIG. 33 ¨ Plasma concentration of RTA 408 after topical ocular and oral
administrations at different times after dosing. The plasma concentration of
RTA 408
was also measured after 5 days of daily topical ocular administration of RTA
408 and
determined to remain relatively consistent from the measurements taken after
the first
day.
FIGS. 34a & b ¨ Correlation of exposure to RTA 408 in monkey plasma with
NQ01 and SRXN1 mRNA expression in PBMCs: (a) NQ01; (b) SRXN1.
16
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
FIG. 35 ¨ Concentration of RTA 408 in various different tissues or fluids
within
the eye as a function of time after 5 days of topical ocular dosing. RTA 408
concentration in plasma was also measured after topical ocular administration.
FIG. 36 ¨ Effect of RTA 408 on the incidence of grade 3 dermatitis caused by
acute radiation exposure for different concentrations of RTA administered
topically.
FIG. 37 ¨ Effect of RTA 408 on the incidence of grade 2 dermatitis over the
course of 30 days caused by acute radiation exposure for different
concentrations of RTA
administered topically.
FIG. 38 ¨ Effect of RTA 408 on the incidence of grade 3 dermatitis over the
course of 28 days caused by acute radiation exposure for different
concentrations of RTA
administered orally.
FIG. 39a & b ¨ a) An area under the curve analysis of clinical score of the
dermatitis as a function of time for each of the different control groups
including all of
the animals used in the test. b) An area under the curve analysis of the
clinical score of
the dermatitis as a function of the duration of that score for each of the
different control
groups including only animals that completed the entire 30 days in the trial.
FIG. 40 ¨ Average 1st blind score of the acute radiation dermatitis as a
function of
time for untreated, untreated with no radiation exposure, vehicle only and
three oral
amounts of RTA 408 at 3, 10 and 30 mg/kg. The dermatitis score is based upon
the scale
that 0 is completely healthy, 1-2 exhibits mild to moderate erythema with
minimal to
slight desquamation, 3-4 exhibits moderate to severe erythema and
desquamation, and 5
exhibits a frank ulcer.
FIG. 41 ¨ Mean score of the acute radiation dermatitis as a function of time
for
untreated, untreated with no radiation exposure, vehicle only and three oral
amounts of
RTA 408 at 3, 10 and 30 mg/kg measured every other day from day 4 to day 30.
The
dermatitis score is based upon the scale that 0 is completely healthy, 1-2
exhibits mild to
moderate erythema with minimal to slight desquamation, 3-4 exhibits moderate
to severe
erythema and desquamation, and 5 exhibits a frank ulcer.
17
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
FIG. 42¨ Mean score of the acute radiation dermatitis as a function of time
for
untreated, untreated with no radiation exposure, vehicle only and three
topical amounts of
RTA 408 at 0.01, 0.1 and 1% measured every other day from day 4 to day 30. The
dermatitis score is based upon the scale that 0 is completely healthy, 1-2
exhibits mild to
moderate erythema with minimal to slight desquamation, 3-4 exhibits moderate
to severe
erythema and desquamation, and 5 exhibits a frank ulcer.
FIG. 43¨ Clinical scores of fractional radiation dermatitis plotted versus
time and
shows the change in dermatitis score for each testing group. The scale
includes a
dermatitis score from 0 to 5 where 0 is completely healthy, 1-2 indicates mild
to
moderate erythema with minimal to slight desquamation, 3-4 indicates moderate
to
severe erythema and desquamation, and 5 is a frank ulcer.
FIG. 44 ¨ Graph of the AUC analysis showing the dermatitis score (severity *
days) for each of the testing groups over the entire observation period. The
dermatitis
scores were assessed every two days from day 4 to day 30 of the study.
FIG. 45 ¨ Reduction of aqueous humor protein concentrations for different
formulations of RTA 408 (dark bars) compared to literature values for MaxiDex
(0.1%
dexamethasone) and mapracorat (light bars) after induction of paracentesis.
FIG. 46 ¨ RTA 408 (63415) dose-dependently suppresses NO in vivo. CD-1
mice (n = 6) were dosed with dimethyl sulfoxide or AIM by oral gavage. LPS (5
mg/kg)
was administered 24 h later. Twenty-four hours after LPS administration, whole
blood
was collected for NO assay. NO inhibition was determined by Griess Reaction
from
reduced, de-proteinated plasma.
FIG. 47 ¨ RTA 408 (63415) distributes extensively into mouse tissues. Mice
were dosed with 25 mg/kg PO QDx3 of either RTA 408 (63415) or RTA 405. Blood
(plasma and whole blood) and tissues (brain, liver, lung, and kidney) were
collected 6
hours after the last dose. Semi-quantitative analysis of drug content was
performed.
Notable levels were observed in the CNS.
18
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
FIG. 48 ¨ RTA 408 (63415) induces NQ01 activity in mouse liver, lung, and
kidney. Mice were dosed with 25 mg/kg PO QDx3, tissues were collected 6 hours
after
the last dose, and analysis of NQ01 activity was performed. Meaningful
activation of
NQ01 was observed in multiple tissues.
FIG. 49 ¨ Summary of RTA 408 (63415) 14-day mouse toxicity study. C57BL/6
mice were dosed PO QDx14. Endpoints included survival, weight, and clinical
chemistries. All animals survived to day 14. No significant weight changes
occurred
compared to the vehicle group, and there was no evidence of toxicity at any
dose based
on clinical chemistries.
FIG. 50 ¨ Tissue distribution from RTA 408 (63415) 14-day mouse toxicity study
in C57BL/6 mice. Brain, lung, and liver: Collected 4 hours after final dose,
quantified
for RTA 408 (63415) content using sensitive LC/MS/MS method. Exposures at 10
and
100 mg/kg: in lung exceeded in vitro IC50 for NO induction by 55- and 1138-
fold,
respectively, and in brain exceeded in vitro IC50 for NO induction by 29- and
541-fold,
respectively.
FIG. 51 ¨ RTA 408 (63415) tissue distribution in Sprague Dawley rats. RTA 408
(63415) distributes well into target tissues. Tissues were collected four
hours after final
dose on Day 14 or Day 6 (100 mg/kg), extracted, and quantified for RTA 408
(63415)
content using a sensitive LC/MS/MS method. Exposures at 10 mg/kg in lung and
brain
exceed in vitro 1050 for NO inhibition by 294- and 240-fold, respectively.
FIG. 52 ¨ RTA 408 (63415) target tissue distribution in cynomolgus monkeys.
Tissues were collected four hours after final dose on Day 14. RTA 408 (63415)
content
was extracted and quantified using a sensitive LC/MS/MS method.
FIG. 53 - PXRD patterns (2-30 20) of RTA 408 Form A
FIG. 54 - DSC thermogram (25-280 C) of RTA 408 Form A.
FIG. 55 - TGA-MS thermogram (25-200 C) of RTA 408 Form A.
FIG. 56 - PXRD patterns (2-30 '20) of RTA 408 Form B.
19
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
FIG. 57 - DSC thermogram (25-280 C) of RTA 408 Form B.
FIG. 58 - TGA-MS thermogram (25-200 C) of RTA 408 Form B.
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
The present invention provides in one aspect the compound:
N-((4aS,6aR,6bS,8aR,12aS,14aR,141:6)-11-cyano-2,2,6a,6b,9,9,12a-
heptamethy1-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-
octadecahydropicen-4a-y1)-2,2-difluoropropanamide,
which is also referred to herein as RTA 408. In other non-limiting aspects,
the present
invention also provides polymorphic forms thereof, including solvates thereof.
In other
non-limiting aspects, the invention also provides pharmaceutically acceptable
salts
thereof. In other non-limiting aspects, there are also provided methods for
preparation,
pharmaceutical compositions, and kits and articles of manufacture of these
compounds
and polymorphic forms thereof.
I. Definitions
When used in the context of a chemical group: "hydrogen" means ¨H; "hydroxy"
means ¨OH; "oxo" means =0; "carbonyl" means ¨C(=0)¨; "carboxy" means
¨C(=0)0H (also written as ¨COOH or ¨CO2H); "halo" means independently ¨F, ¨Cl,
¨Br or ¨I; "amino" means ¨NH2; "hydroxyamino" means ¨NHOH; "cyano" means ¨CN;
"isocyanatc" means ¨N=C=O; "azido" means ¨N3; in a monovalent context
"phosphate"
means ¨0P(0)(OH)2 or a deprotonated form thereof; in a divalent context
"phosphate"
means ¨0P(0)(OH)0¨ or a deprotonated form thereof; "thio" means =S; and
"sulfonyl"
means ¨S(0)2¨. Any undefined valency on an atom of a structure shown in this
application implicitly represents a hydrogen atom bonded to the atom.
The use of the word "a" or "an," when used in conjunction with the term
"comprising" in the claims and/or the specification may mean "one," but it is
also
consistent with the meaning of "one or more," "at least one," and "one or more
than one."
Throughout this application, the term "about" is used to indicate that a value
includes the inherent variation of error for the device, the method being
employed to
determine the value, or the variation that exists among the study subjects.
When used in
the context of X-ray powder diffraction, the term "about" is used to indicate
a value of
0.2 020 from the reported value, preferably a value of 0.1 020 from the
reported value.
21
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
When used in the context of differential scanning calorimetry or glass
transition
temperatures, the term "about" is used to indicate a value of +10 C relative
to the
maximum of the peak, preferably a value of +2 C relative to the maximum of
the peak.
When used in another context, the term "about" is used to indicate a value of
10% of
the reported value, preferably a value of 5% of the reported value. It is to
be
understood that, whenever the term "about" is used, a specific reference to
the exact
numerical value indicated is also included.
The terms "comprise," "have" and "include" are open-ended linking verbs. Any
forms or tenses of one or more of these verbs, such as "comprises,"
"comprising," "has,"
"having," "includes" and "including," are also open-ended. For example, any
method
that "comprises," "has" or "includes" one or more steps is not limited to
possessing only
those one or more steps and also covers other unlisted steps.
The term "effective," as that term is used in the specification and/or claims,
means adequate to accomplish a desired, expected, or intended result.
"Effective
amount," "Therapeutically effective amount" or "pharmaceutically effective
amount"
when used in the context of treating a patient or subject with a compound
means that
amount of the compound which, when administered to a subject or patient for
treating a
disease, is sufficient to effect such treatment for the disease.
The term "hydrate" when used as a modifier to a compound means that the
compound has less than one (e.g., hemihydrate), one (e.g., monohydrate), or
more than
one (e.g., dihydrate) water molecules associated with each compound molecule,
such as
in solid forms of the compound.
As used herein, the term "IC50" refers to an inhibitory dose which is 50% of
the
maximum response obtained. This quantitative measure indicates how much of a
particular drug or other substance (inhibitor) is needed to inhibit a given
biological,
biochemical or chemical process (or component of a process, i.e. an enzyme,
cell, cell
receptor or microorganism) by half.
An "isomer" of a first compound is a separate compound in which each molecule
contains the same constituent atoms as the first compound, but where the
configuration of
those atoms in three dimensions differs.
22
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
As used herein, the term "patient" or "subject" refers to a living mammalian
organism, such as a human, monkey, cow, sheep, goat, dog, cat, mouse, rat,
guinea pig,
or transgenic species thereof. In certain embodiments, the patient or subject
is a
non-human mammal. In certain embodiments, the patient or subject is a primate.
In
certain embodiments, the patient or subject is a human. Non-limiting examples
of human
subjects are adults, juveniles, infants and fetuses.
As generally used herein "pharmaceutically acceptable" refers to those
compounds, materials, compositions, and/or dosage forms which are, within the
scope of
sound medical judgment, suitable for use in contact with the tissues, organs,
and/or
bodily fluids of human beings and animals without excessive toxicity,
irritation, allergic
response, or other problems or complications commensurate with a reasonable
benefit/risk ratio.
"Pharmaceutically acceptable salts" means salts of compounds of the present
invention which are pharmaceutically acceptable, as defined above, and which
possess
the desired pharmacological activity. Such salts include acid addition salts
formed with
inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid,
nitric acid,
phosphoric acid, and the like; or with organic acids such as 1,2-
ethanedisulfonic acid,
2-hydroxyeth an esulfoni c acid, 2-n aphth al enesulfoni c acid, 3 -ph enyl
propi on i c acid,
4,4'-methylenebis(3-hydroxy-2-ene-1-carboxylic acid), 4-methylbicyclo [2.2 .2]
oct-2 -ene-
1-carboxylic acid, acetic acid, aliphatic mono- and dicarboxylic acids,
aliphatic sulfuric
acids, aromatic sulfuric acids, benzenesulfonic acid, benzoic acid,
camphorsulfonic acid,
carbonic acid, cinnamic acid, citric acid, cyclopentanepropionic acid,
ethanesulfonic acid,
fumaric acid, glucoheptonic acid, gluconic acid, glutamic acid, glycolic acid,
heptanoic
acid, hexanoic acid, hydroxynaphthoic acid, lactic acid, laurylsulfuric acid,
maleic acid,
malic acid, malonic acid, mandelic acid, methanesulfonic acid, muconic acid,
o-(4-hydroxybenzoyl)benzoic acid, oxalic acid, p-chlorobenzenesulfonic acid,
phenyl-
substituted alkanoic acids, propionic acid, p-toluenesulfonic acid, pyruvic
acid, salicylic
acid, stearic acid, succinic acid, tartaric acid, tertiarybutylacetic acid,
trimethylacetic acid,
and the like. Pharmaceutically acceptable salts also include base addition
salts which
may be formed when acidic protons present are capable of reacting with
inorganic or
organic bases. Acceptable inorganic bases include sodium hydroxide, sodium
carbonate,
23
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
potassium hydroxide, aluminum hydroxide and calcium hydroxide. Acceptable
organic
bases include ethanolamine, diethanolamine, triethanolamine, tromethamine,
N-methylglucamine and the like. It should be recognized that the particular
anion or
cation forming a part of any salt of this invention is not critical, so long
as the salt, as a
whole, is pharmacologically acceptable. Additional examples of
pharmaceutically
acceptable salts and their methods of preparation and use are presented in
Handbook of
Pharmaceutical Salts: Properties, and Use (P. H. Stahl & C. G. Wermuth eds.,
Verlag
Helvetica Chimica Acta, 2002).
"Prevention" or "preventing" includes: (1) inhibiting the onset of a disease
in a
subject or patient which may be at risk and/or predisposed to the disease but
does not yet
experience or display any or all of the pathology or symptomatology of the
disease,
and/or (2) slowing the onset of the pathology or symptomatology of a disease
in a subject
or patient which may be at risk and/or predisposed to the disease but does not
yet
experience or display any or all of the pathology or symptomatology of the
disease.
"Prodrug" means a compound that is convertible in vivo metabolically into an
inhibitor according to the present invention. The prodrug itself may or may
not also have
activity with respect to a given target protein. For example, a compound
comprising a
hydroxy group may be administered as an ester that is converted by hydrolysis
in vivo to
the hydroxy compound. Suitable esters that may be converted in vivo into
hydroxy
compounds include acetates, citrates, lactates, phosphates, tartrates,
malonates, oxalates,
salicylates, propionates, succinates, fumarates,
maleates, methylene-
bis- (3-hydroxynaphtho ate, gentisates, isethionates, di-p-
toluoyltartrates,
methanesulfonates, ethanesulfonates, benzenesulfonates, p-toluenesulfonates,
cyclohexylsulfamates, quinates, esters of amino acids, and the like.
Similarly, a
compound comprising an amine group may be administered as an amide that is
converted
by hydrolysis in vivo to the amine compound.
A "stercoisomer" or "optical isomer" is an isomer of a given compound in which
the same atoms are bonded to the same other atoms, but where the configuration
of those
atoms in three dimensions differs. "Enantiomers" are stereoisomers of a given
compound
that are mirror images of each other, like left and right hands.
"Diastereomers" are
stereoisomers of a given compound that are not enantiomers. Chiral molecules
contain a
24
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
chiral center, also referred to as a stereocenter or stereogenic center, which
is any point,
though not necessarily an atom, in a molecule bearing groups such that an
interchanging
of any two groups leads to a stereoisomer. In organic compounds, the chiral
center is
typically a carbon, phosphorus or sulfur atom, though it is also possible for
other atoms to
be stereocenters in organic and inorganic compounds. A molecule can have
multiple
stereocenters, giving it many stereoisomers. In compounds whose
stereoisomerism is due
to tetrahedral stereogenic centers (e.g., tetrahedral carbon), the total
number of
hypothetically possible stereoisomers will not exceed 2n, where n is the
number of
tetrahedral stereocenters. Molecules with symmetry frequently have fewer than
the
maximum possible number of stereoisomers. A 50:50 mixture of enantiomers is
referred
to as a racemic mixture. Alternatively, a mixture of enantiomers can be
enantiomerically
enriched so that one enantiomer is present in an amount greater than 50%.
Typically,
enantiomers and/or diastereomers can be resolved or separated using techniques
known
in the art. It is contemplated that for any stereocenter or axis of chirality
for which
stereochemistry has not been defined, that stereocenter or axis of chirality
can be present
in its R form, S form, or as a mixture of the R and S forms, including racemic
and non-
racemic mixtures. As
used herein, the phrase "substantially free from other
stereoisomers" means that the composition contains < 15%, more preferably <
10%, even
more preferably < 5%, or most preferably < 1% of another stereoisomer(s).
"Treatment" or "treating" includes (1) inhibiting a disease in a subject or
patient
experiencing or displaying the pathology or symptomatology of the disease
(e.g.,
arresting further development of the pathology and/or symptomatology), (2)
ameliorating
a disease in a subject or patient that is experiencing or displaying the
pathology or
symptomatology of the disease (e.g., reversing the pathology and/or
symptomatology),
and/or (3) effecting any measurable decrease in a disease in a subject or
patient that is
experiencing or displaying the pathology or symptomatology of the disease.
In the context of this disclosure, the formulas:
WO 2014/176415 PCT/US2014/035279
0 0
0 0
F
NC N )1X-
NC N
F F F
0 0
and
represent the same structures. When a dot is drawn on a carbon, the dot
indicates that the
hydrogen atom attached to that carbon is coming out of the plane of the page.
The fact that certain terms are defined,
however, should not be considered as indicative that any term that is
undefined is
indefinite. Rather, all terms used are believed to describe the invention in
terms such that
one of ordinary skill can appreciate the scope and practice the present
invention.
RTA 408 and Synthetic Methods
RTA 408 can be prepared according to the methods described in the section
below. These methods can be further modified and optimized using the
principles and
techniques of organic chemistry as applied by a person skilled in the art.
Such principles
and techniques are taught, for example, in March's Advanced Organic Chemistry:
Reactions, Mechanisms, and Structure (2007)
It should be recognized that the particular anion or cation forming a part of
any
salt of this invention is not critical, so long as the salt, as a whole, is
pharmacologically
acceptable. Additional examples of pharmaceutically acceptable salts and their
methods
of preparation and use are presented in Handbook of Pharmaceutical Salts:
Properties,
and Use (2002) .
RTA 408 may also exist in prodrug form. Since prodrugs are known to enhance
numerous desirable qualities of pharmaceuticals, e.g., solubility,
bioavailability,
manufacturing, etc., the compounds employed in some methods of the invention
may, if
desired, be delivered in prodrug form. Thus, the invention contemplates
prodrugs of
compounds of the present invention as well as methods of delivering prodrugs.
Prodrugs
of the compounds employed in the invention may be prepared by modifying
functional
groups present in the compound in such a way that the modifications are
cleaved, either
26
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
in routine manipulation or in vivo, to the parent compound. Accordingly,
prodrugs
include, for example, compounds described herein in which a hydroxy, amino, or
carboxy
group is bonded to any group that, when the prodrug is administered to a
patient, cleaves
to form a hydroxy, amino, or carboxylic acid, respectively.
RTA 408 may contain one or more asymmetrically-substituted carbon or nitrogen
atoms, and may be isolated in optically active or racemic form. Thus, all
chiral,
diastereomeric, racemic form, epimeric form, and all geometric isomeric forms
of a
structure are intended, unless the specific stereochemistry or isomeric form
is specifically
indicated. RTA 408 may occur as racemates and racemic mixtures, single
enantiomers,
diastereomeric mixtures and individual diastereomers. In some embodiments, a
single
diastereomer is obtained. The chiral centers of RTA 408 according to the
present
invention can have the S or the R configuration.
In addition, atoms making up RTA 408 of the present invention are intended to
include all isotopic forms of such atoms. Isotopes, as used herein, include
those atoms
having the same atomic number but different mass numbers. By way of general
example
and without limitation, isotopes of hydrogen include tritium and deuterium,
and isotopes
of carbon include I-3C and "C. Similarly, it is contemplated that one or more
carbon
atom(s) of a compound of the present invention may be replaced by a silicon
atom(s).
Furthermore, it is contemplated that one or more oxygen atom(s) of RTA 408 may
be
replaced by a sulfur or selenium atom(s).
RTA 408 and polymorphic form thereof may also have the advantage that they
may be more efficacious than, be less toxic than, be longer acting than, be
more potent
than, produce fewer side effects than, be more easily absorbed than, and/or
have a better
pharmacokinetic profile (e.g., higher oral bio availability and/or lower
clearance) than,
and/or have other useful pharmacological, physical, or chemical advantages
over,
compounds known in the prior art for use in the indications stated herein.
III. Polymorphic Forms of RTA 408
In some embodiments, the present invention provides different solid forms of
RTA 408, including solvates thereof. A polymorphism study was performed, and
RTA
.. 408 was found in two, essentially solvent-free, crystalline forms (Form A
and Form B).
For a description of the classes, see Table 1 below. Crystalline Form A is
metastable and
27
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
has a melting point at 181.98 C and AH fusion = 42.01 J/g. This form may have
utility
for obtaining amorphous forms of RTA 408 or in extrusion formulations.
Crystalline
Form A may be slightly hygroscopic (mass loss of ¨0.5 wt. % in TGA-MS, FIG.
55).
Crystalline Form B has greater thermodynamic stability than Form A as
indicated by a
higher melting point (250.10 C) and greater enthalpy of fusion (AH fusion =
47.85 J/g).
Greater chemical and physical stability is expected for Form B compared to
Form A both
at ambient and elevated temperatures. A minimal amount of surface water may
exist on
Form B as indicated by TGA-MS (FIG. 58).
The new forms were characterized by PXRD (Table 8 and Table 9).
Table 1. Summary of Solid Forms
Form Melting Point Enthalpy of Fusion
A 181.98 C 42.01 J/g
250.10 C 47.85 J/g
IV. Diseases
Associated with Inflammation and/or Oxidative Stress
Inflammation is a biological process that provides resistance to infectious or
parasitic organisms and the repair of damaged tissue. Inflammation is commonly
characterized by localized vasodilation, redness, swelling, and pain, the
recruitment of
leukocytes to the site of infection or injury, production of inflammatory
cytokines, such
as TNF-a and IL-1, and production of reactive oxygen or nitrogen species, such
as
hydrogen peroxide, superoxide, and peroxynitrite. In later stages of
inflammation, tissue
remodeling, angiogenesis, and scar formation (fibrosis) may occur as part of
the wound
healing process. Under normal circumstances, the inflammatory response is
regulated,
temporary, and is resolved in an orchestrated fashion once the infection or
injury has
been dealt with adequately. However, acute inflammation can become excessive
and
life-threatening if regulatory mechanisms fail. Alternatively, inflammation
can become
chronic and cause cumulative tissue damage or systemic complications. Based at
least on
the evidence presented herein, RTA 408 can be used in the treatment or
prevention of
inflammation or diseases associated with inflammation.
28
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Many serious and intractable human diseases involve dysregulation of
inflammatory processes, including diseases such as cancer, atherosclerosis,
and diabetes,
which were not traditionally viewed as inflammatory conditions. In the case of
cancer,
the inflammatory processes are associated with tumor formation, progression,
metastasis,
and resistance to therapy. Atherosclerosis, long viewed as a disorder of lipid
metabolism,
is now understood to be primarily an inflammatory condition, with activated
macrophages playing an important role in the formation and eventual rupture of
atherosclerotic plaques. Activation of inflammatory signaling pathways has
also been
shown to play a role in the development of insulin resistance, as well as in
the peripheral
tissue damage associated with diabetic hyperglycemia. Excessive production of
reactive
oxygen species and reactive nitrogen species, such as superoxide, hydrogen
peroxide,
nitric oxide, and peroxynitrite, is a hallmark of inflammatory conditions.
Evidence of
dysregulated peroxynitrite production has been reported in a wide variety of
diseases
(Szabo et al., 2007; Schulz et al., 2008; Forstermann, 2006; Pall, 2007).
Autoimmune diseases such as rheumatoid arthritis, lupus, psoriasis, and
multiple
sclerosis involve inappropriate and chronic activation of inflammatory
processes in
affected tissues, arising from dysfunction of self vs. non-self recognition
and response
mechanisms in the immune system. In neurodegenerative diseases such as
Alzheimer's
and Parkinson's diseases, neural damage is correlated with activation of
microglia and
elevated levels of pro-inflammatory proteins, such as inducible nitric oxide
synthase
(iNOS). Chronic organ failure, such as renal failure, heart failure, liver
failure, and
chronic obstructive pulmonary disease, is closely associated with the presence
of chronic
oxidative stress and inflammation, leading to the development of fibrosis and
eventual
loss of organ function. Oxidative stress in vascular endothelial cells, which
line major
and minor blood vessels, can lead to endothelial dysfunction and is believed
to be an
important contributing factor in the development of systemic cardiovascular
disease,
complications of diabetes, chronic kidney disease and other forms of organ
failure, and a
number of other aging-related diseases, including degenerative diseases of the
central
nervous system and the retina.
Many other disorders involve oxidative stress and inflammation in affected
tissues, including inflammatory bowel disease; inflammatory skin diseases;
mucositis and
29
WO 2014/176415 PCT/US2014/035279
dermatitis related to radiation therapy and chemotherapy; eye diseases, such
as uveitis,
glaucoma, macular degeneration, and various forms of retinopathy; transplant
failure and
rejection; ischemia-reperfusion injury; chronic pain; degenerative conditions
of the bones
and joints, including osteoarthritis and osteoporosis; asthma and cystic
fibrosis; seizure
disorders; and neuropsychiatric conditions, including schizophrenia,
depression, bipolar
disorder, post-traumatic stress disorder, attention deficit disorders, autism-
spectrum
disorders, and eating disorders, such as anorexia nervosa. Dysregulation of
inflammatory
signaling pathways is believed to be a major factor in the pathology of muscle
wasting
diseases, including muscular dystrophy and various forms of cachexia.
A variety of life-threatening acute disorders also involve dysregulated
inflammatory signaling, including acute organ failure involving the pancreas,
kidneys,
liver, or lungs, myocardial infarction or acute coronary syndrome, stroke,
septic shock,
trauma, severe bums, and anaphylaxis.
Many complications of infectious diseases also involve dysregulation of
inflammatory responses. Although an inflammatory response can kill invading
pathogens, an excessive inflammatory response can also be quite destructive
and in some
cases can be a primary source of damage in infected tissues. Furthermore, an
excessive
inflammatory response can also lead to systemic complications due to
overproduction of
inflammatory cytokines, such as TNF-a and IL-1. This is believed to be a
factor in
mortality arising from severe influenza, severe acute respiratory syndrome,
and sepsis.
The aberrant or excessive expression of either iNOS or cyclooxygenase-2 (COX-
2) has been implicated in the pathogenesis of many disease processes. For
example, it is
clear that NO is a potent mutagen (Tamir and Tannebaum, 1996), and that nitric
oxide
can also activate COX-2 (Salvemini et al., 1994). Furthermore, there is a
marked
increase in iNOS in rat colon tumors induced by the carcinogen, azoxymethane
(Takahashi et al., 1997). A series of synthetic triterpenoid analogs of
oleanolic acid have
been shown to be powerful inhibitors of cellular inflammatory processes, such
as the
induction by IFN-y of inducible nitric oxide synthase (iNOS) and of COX-2 in
mouse
macrophages. See Honda et al. (2000a), Honda et al. (2000b), and Honda et al.
(2002)
30
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
In one aspect, RTA 408 disclosed herein is in part characterized by its
ability to
inhibit the production of nitric oxide in macrophage-derived RAW 264.7 cells
induced by
exposure to y-interferon. RTA 408 is further characterized by the ability to
induce the
expression of antioxidant proteins, such as NQ01, and reduce the expression of
pro-
inflammatory proteins, such as COX-2 and inducible nitric oxide synthase
(iNOS).
These properties are relevant to the treatment of a wide array of diseases and
disorders
involving oxidative stress and dysregulation of inflammatory processes,
including cancer,
complications from localized or total-body exposure to ionizing radiation,
mucositis and
dermatitis resulting from radiation therapy or chemotherapy, autoimmune
diseases,
cardiovascular diseases, including atherosclerosis, ischemia-reperfusion
injury, acute and
chronic organ failure, including renal failure and heart failure, respiratory
diseases,
diabetes and complications of diabetes, severe allergies, transplant
rejection, graft-versus-
host disease, neurodegenerative diseases, diseases of the eye and retina,
acute and chronic
pain, degenerative bone diseases, including osteoarthritis and osteoporosis,
inflammatory
bowel diseases, dermatitis and other skin diseases, sepsis, burns, seizure
disorders, and
neuropsychiatric disorders.
In another aspect, RTA 408 may be used for treating a subject having a
condition
such as eye diseases. For example, uveitis, macular degeneration (both the dry
form and
wet form), glaucoma, diabetic macular edema, blepharitis, diabetic
retinopathy, diseases
and disorders of the corneal endothelium such as Fuchs endothelial corneal
dystrophy,
post-surgical inflammation, dry eye, allergic conjunctivitis and other forms
of
conjunctivitis are non-limiting examples of eye diseases that could be treated
with RTA
408.
In another aspect, RTA 408 may be used for treating a subject having a
condition
such as skin diseases or disorders. For example, dermatitis, including
allergic dermatitis,
atopic dermatitis, dermatitis due to chemical exposure, and radiation-induced
dermatitis;
thermal or chemical burns; chronic wounds including diabetic ulcers, pressure
sores, and
venous ulcers; acne; alopecia including baldness and drug-induced alopecia;
other
disorders of the hair follicle; epidermolysis bullosa; sunburn and its
complications;
disorders of skin pigmentation including vitiligo; aging-related skin
conditions; post-
surgical wound healing; prevention or reduction of scarring from skin injury,
surgery, or
31
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
burns; psoriasis; dermatological manifestations of autoimmune diseases or
graft-versus
host disease; prevention or treatment of skin cancer; disorders involving
hyperproliferation of skin cells such as hyperkeratosis is a non-limiting
example of skin
diseases that could be treated with RTA 408.
Without being bound by theory, the activation of the antioxidant/anti-
inflammatory Keapl/Nrf2/ARE pathway is believed to be implicated in both the
anti-
inflammatory and anti-carcinogenic properties of the compound disclosed
herein.
In another aspect, RTA 408 may be used for treating a subject having a
condition
caused by elevated levels of oxidative stress in one or more tissues.
Oxidative stress
results from abnormally high or prolonged levels of reactive oxygen species,
such as
superoxide, hydrogen peroxide, nitric oxide, and peroxynitrite (formed by the
reaction of
nitric oxide and superoxide). The oxidative stress may be accompanied by
either acute or
chronic inflammation. The oxidative stress may be caused by mitochondrial
dysfunction,
by activation of immune cells, such as macrophages and neutrophils, by acute
exposure
to an external agent, such as ionizing radiation or a cytotoxic chemotherapy
agent (e.g.,
doxorubicin), by trauma or other acute tissue injury, by ischemia/reperfusion,
by poor
circulation or anemia, by localized or systemic hypoxia or hyperoxia, by
elevated levels
of inflammatory cytokines and other inflammation-related proteins, and/or by
other
abnormal physiological states, such as hyperglycemia or hypoglycemia.
In animal models of many such conditions, stimulating expression of inducible
heme oxygenase (H0-1), a target gene of the Nrf2 pathway, has been shown to
have a
significant therapeutic effect including in models of myocardial infarction,
renal failure,
transplant failure and rejection, stroke, cardiovascular disease, and
autoimmune disease
(e.g., Sacerdoti et al., 2005; Abraham & Kappas, 2005; Bach, 2006; Araujo et
al., 2003;
Liu etal., 2006; Ishikawa etal., 2001; Kruger etal., 2006; Satoh etal., 2006;
Zhou etal.,
2005; Morse and Choi, 2005; Morse and Choi, 2002). This enzyme breaks free
heme
down into iron, carbon monoxide (CO), and biliverdin (which is subsequently
converted
to the potent antioxidant molecule, bilirubin).
In another aspect, RTA 408 may be used in preventing or treating tissue damage
or organ failure, acute and chronic, resulting from oxidative stress
exacerbated by
inflammation. Examples of diseases that fall in this category include heart
failure, liver
32
WO 2014/176415 PCT/US2014/035279
failure, transplant failure and rejection, renal failure, pancreatitis,
fibrotic lung diseases
(cystic fibrosis, COPD, and idiopathic pulmonary fibrosis, among others),
diabetes
(including complications), atherosclerosis, ischemia-reperfusion injury,
glaucoma, stroke,
autoimmune disease, autism, macular degeneration, and muscular dystrophy. For
example, in the case of autism, studies suggest that increased oxidative
stress in the
central nervous system may contribute to the development of the disease
(Chauhan and
Chauhan, 2006).
Evidence also links oxidative stress and inflammation to the development and
pathology of many other disorders of the central nervous system, including
psychiatric
disorders, such as psychosis, major depression, and bipolar disorder; seizure
disorders,
such as epilepsy; pain and sensory syndromes, such as migraine, neuropathic
pain, or
tinnitus; and behavioral syndromes, such as the attention deficit disorders.
See, e.g.,
Dickerson et at., 2007; Hanson et al., 2005; Kendall-Tackett, 2007; Lencz et
al., 2007;
Dudhgaonkar et al., 2006; Lee et al., 2007; Morris et a/., 2002; Ruster et
al., 2005;
McIver et at., 2005; Sarchielli et at., 2006; Kawakami et at., 2006; Ross et
al., 2003.
For example, elevated levels of
inflammatory cytokincs, including TNF, interferon-y, and IL-6, arc associated
with major
mental illness (Dickerson et at., 2007). Microglial activation has also been
linked to
major mental illness. Therefore, downregulating inflammatory cytokines and
inhibiting
excessive activation of microglia could be beneficial in patients with
schizophrenia,
major depression, bipolar disorder, autism-spectrum disorders, and other
neuropsychiatric
disorders.
Accordingly, in pathologies involving oxidative stress alone or oxidative
stress
exacerbated by inflammation, treatment may comprise administering to a subject
a
therapeutically effective amount of a compound of this invention, such as
those described
above or throughout this specification. Treatment may be administered
preventively, in
advance of a predictable state of oxidative stress (e.g., organ
transplantation or the
administration of radiation therapy to a cancer patient), or it may be
administered
therapeutically in settings involving established oxidative stress and
inflammation. In
some instances, such as a cancer patient receiving radiation therapy or
chemotherapy (or
both), the compound of the invention may be administered both before and after
the
33
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
radiation or chemotherapy, or may be administered in combination with the
other
therapies. Depending on the nature of the radiation therapy or chemotherapy,
various
combinations of pre-treatment, post-treatment, or simultaneous administration
of the
compound of the invention may be used. The compound of the invention may
prevent or
reduce the severity of side effects associated with the radiation therapy or
chemotherapy.
Because such side effects may be dose-limiting, their reduction or prevention
may allow
higher or more frequent dosing of the radiation therapy or chemotherapy,
resulting in
greater efficacy. Alternatively, as shown herein, use of the compound of the
invention in
combination with the radiation therapy or chemotherapy may enhance the
efficacy of a
given dose of radiation or chemotherapy. In part, this combinatorial efficacy
may result
from inhibition of the activity of the pro-inflammatory transcription factor
NF-KB by the
compound of the invention. NF-KB is often chronically activated in cancer
cells, and
such activation is associated with resistance to therapy and promotion of
tumor
progression (e.g., Karin M, Nature. 2006 May 25;441(7092):431-6; Aghajan et
al., J
Gastroenterol Hepatol. 2012 Mar;27 Suppl 2:10-4) . Other transcription factors
that
promote inflammation and cancer, such as STAT3 (e.g., He G and Karin M, Cell
Res.
2011 Jan;21(1):159-68; Grivennikov SI and Karin M, Cytokine Growth Factor Rev.
2010
Feb;21(1):11-9), may also be inhibited by the compound of the invention.
RTA 408 may be used to treat or prevent inflammatory conditions, such as
sepsis,
dermatitis, autoimmunc disease, and ostcoarthritis. RTA 408 may also be used
to treat or
prevent inflammatory pain and/or neuropathic pain, for example, by inducing
Nrf2 and/or
inhibiting NF-KB.
RTA 408 may also be used to treat or prevent diseases, such as cancer,
inflammation, Alzheimer's disease, Parkinson's disease, multiple sclerosis,
autism,
amyotrophic lateral sclerosis, Huntington's disease, autoimmune diseases, such
as
rheumatoid arthritis, lupus, Crohn's disease, and psoriasis, inflammatory
bowel disease,
all other diseases whose pathogenesis is believed to involve excessive
production of
either nitric oxide or prostaglandins, and pathologies involving oxidative
stress alone or
oxidative stress exacerbated by inflammation.
Another aspect of inflammation is the production of inflammatory
prostaglandins,
such as prostaglandin E. RTA 408 may be used to promote vasodilation, plasma
34
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
extravasation, localized pain, elevated temperature, and other symptoms of
inflammation.
The inducible form of the enzyme COX-2 is associated with their production,
and high
levels of COX-2 are found in inflamed tissues. Consequently, inhibition of COX-
2 may
relieve many symptoms of inflammation and a number of important anti-
inflammatory
drugs (e.g., ibuprofen and celecoxib) act by inhibiting COX-2 activity. It has
been
demonstrated that a class of cyclopentenone prostaglandins (cyPGs) (e.g., 15-
deoxy
prostaglandin J2, a.k.a. PGJ2) plays a role in stimulating the orchestrated
resolution of
inflammation (e.g., Rajakariar et al., 2007). COX-2 is also associated with
the production
of cyclopentenone prostaglandins. Consequently, inhibition of COX-2 may
interfere with
the full resolution of inflammation, potentially promoting the persistence of
activated
immune cells in tissues and leading to chronic, "smoldering" inflammation.
This effect
may be responsible for the increased incidence of cardiovascular disease in
patients using
selective COX-2 inhibitors for long periods of time.
In one aspect, RTA 408 may be used to control the production of pro-
inflammatory cytokines within the cell by selectively activating regulatory
cysteine
residues (RCRs) on proteins that regulate the activity of redox-sensitive
transcription
factors. Activation of RCRs by cyPGs has been shown to initiate a pro-
resolution program
in which the activity of the antioxidant and cytoprotective transcription
factor Nrf2 is
potently induced and the activities of the pro-oxidant and pro-inflammatory
transcription
factors NF-KB and the STATs are suppressed. In some embodiments, RTA 408 may
be
used to increase the production of antioxidant and reductive molecules (NQ01,
HO-1,
SOD1, y-GCS) and decrease oxidative stress and the production of pro-oxidant
and pro-
inflammatory molecules (iNOS, COX-2, TNF-a). In some embodiments, RTA 408 may
be
used to cause the cells that host the inflammatory event to revert to a non-
inflammatory state
by promoting the resolution of inflammation and limiting excessive tissue
damage to the
host.
A. Cancer
Further, RTA 408 may be used to induce apoptosis in tumor cells, to induce
cell
differentiation, to inhibit cancer cell proliferation, to inhibit an
inflammatory response,
and/or to function in a chemopreventative capacity. For example, RTA 408 has
one or
more of the following properties: (1) an ability to induce apoptosis and
differentiate both
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
malignant and non-malignant cells, (2) an activity at sub-micromolar or
nanomolar levels
as an inhibitor of proliferation of many malignant or premalignant cells, (3)
an ability to
suppress the de novo synthesis of the inflammatory enzyme inducible nitric
oxide
synthase (iNOS), (4) an ability to inhibit NF-KB activation, and (5) an
ability to induce
the expression of heme oxygenase-1 (H0-1).
The levels of iNOS and COX-2 are elevated in certain cancers and have been
implicated in carcinogenesis and COX-2 inhibitors have been shown to reduce
the
incidence of primary colonic adenomas in humans (Rostom et al., 2007; Brown
and
DuBois, 2005; Crowel et al., 2003). iNOS is expressed in myeloid-derived
suppressor
cells (MDSCs) (Angulo et al., 2000) and COX-2 activity in cancer cells has
been shown
to result in the production of prostaglandin E2 (PGE2), which has been shown
to induce
the expression of arginase in MDSCs (Sinha et al., 2007). Arginasc and iNOS
are
enzymes that utilize L-arginine as a substrate and produce L-ornithine and
urea, and L-
citrulline and NO, respectively. The
depletion of argininc from the tumor
microenvironment by MDSCs, combined with the production of NO and
peroxynitrite
has been shown to inhibit proliferation and induce apoptosis of T cells
(Bronte et al.,
2003). Inhibition of COX-2 and iNOS has been shown to reduce the accumulation
of
MDSCs, restore cytotoxic activity of tumor-associated T cells, and delay tumor
growth
(Sinha et al., 2007; Mazzoni et al., 2002; Zhou et al., 2007).
Inhibition of the NF-KB and JAK/STAT signaling pathways has been implicated
as a strategy to inhibit proliferation of cancer epithelial cells and induce
their apoptosis.
Activation of STAT3 and NF-KB has been shown to result in suppression of
apoptosis in
cancer cells, and promotion of proliferation, invasion, and metastasis. Many
of the target
genes involved in these processes have been shown to be transcriptionally
regulated by
both NF-KB and STAT3 (Yu etal., 2007).
In addition to their direct roles in cancer epithelial cells, NF-KB and STAT3
also
have important roles in other cells found within the tumor microenvironment.
Experiments in animal models have demonstrated that NF-KB is required in both
cancer
cells and hematopoeitic cells to propagate the effects of inflammation on
cancer initiation
and progression (Greten et al., 2004). NF-KB inhibition in cancer and myeloid
cells
reduces the number and size, respectively, of the resultant tumors. Activation
of STAT3
36
WO 2014/176415 PCT/US2014/035279
in cancer cells results in the production of several cytokines (IL-6, IL-10)
which suppress
the maturation of tumor-associated dendritic cells (DC). Furthermore, STAT3 is
activated by these cytokines in the dendritic cells themselves. Inhibition of
STAT3 in
mouse models of cancer restores DC maturation, promotes antitumor immunity,
and
inhibits tumor growth (Kortylewski et al., 2005).
B. Treatment of Multiple Sclerosis and Other Neurodegenerative Conditions
The compound and methods of this invention may be used for treating patients
for
multiple sclerosis (MS). MS is known to be an inflammatory condition of the
central
nervous system (Williams et al., 1994; Merrill and Benvenist, 1996; Genain and
Nauser,
1997). Based on several investigations, there is evidence suggesting that
inflammatory,
oxidative, and/or immune mechanisms are involved in the pathogenesis of
Alzheimer's
disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS),
and MS
(Bagasra et al., 1995; McGeer and McGeer, 1995; Simonian and Coyle, 1996;
Kaltschmidt et al., 1997). Both reactive astrocytes and activated microglia
have been
implicated in causation of neurodegenerative disease (NDD) and
neuroinflammatory
disease (NID); there has been a particular emphasis on microglia as cells that
synthesize
both NO and prostaglandins as products of the respective enzymes, iNOS and COX-
2.
De novo formation of these enzymes may be driven by inflammatory cytokines
such as
interferon-y or interleukin-1. In turn, excessive production of NO may lead to
inflammatory cascades and/or oxidative damage in cells and tissues of many
organs,
including neurons and oligodendrocytes of the nervous system, with consequent
manifestations in AD and MS, and possible PD and ALS (Coyle and Puttfarcken,
1993;
Beal, 1996; Merrill and Benvenist, 1996; Simonian and Coyle, 1996; Vodovotz et
al.,
1996). Epidemiologic data indicate that chronic use of NSAID's which block
synthesis
of prostaglandins from arachidonate, markedly lower the risk for development
of AD
(McGeer et al., 1996; Stewart et al., 1997). Thus, agents that block formation
of NO and
prostaglandins, may be used in approaches to prevention and treatment of NDD.
Successful therapeutic candidates for treating such a disease typically
require an ability to
penetrate the blood-brain barrier. See, for example, U.S. Patent
Publication
2009/0060873.
C. Neuroinflammation
37
Date Recue/Date Received 2020-09-03
WO 2014/176415 PCT/US2014/035279
The compound and methods of this invention may be used for treating patients
with neuroinflammation. Neuroinflammation encapsulates the idea that
microglial and
astrocytic responses and actions in the central nervous system have a
fundamentally
inflammation-like character, and that these responses are central to the
pathogenesis and
progression of a wide variety of neurological disorders. This idea originated
in the field
of Alzheimer's disease (Griffin et at., 1989; Rogers et at., 1988), where it
has
revolutionized our understanding of this disease (Akiyama et at., 2000). These
ideas
have been extended to other neurodegenerative diseases (Eikelenboom et at.,
2002;
Ishizawa and Dickson, 2001), to ischemic/toxic diseases (Gehrmann et at.,
1995; Touzani
et at., 1999), to tumor biology (Graeber et at., 2002) and even to normal
brain
development.
Neuroinflammation incorporates a wide spectrum of complex cellular responses
that include activation of microglia and astrocytes and induction of
cytokines,
chemokines, complement proteins, acute phase proteins, oxidative injury, and
related
molecular processes. These events may have detrimental effects on neuronal
function,
leading to neuronal injury, further glial activation, and ultimately
neurodegeneration.
D. Treatment of Renal Failure
The compound and methods of this invention may be used for treating patients
with renal failure. See U.S. patent application Ser. No. 12/352,473 .
Another aspect of the present disclosure concerns new
methods and compounds for the treatment and prevention of renal disease. Renal
failure,
resulting in inadequate clearance of metabolic waste products from the blood
and
abnormal concentrations of electrolytes in the blood, is a significant medical
problem
throughout the world, especially in developed countries. Diabetes and
hypertension are
among the most important causes of chronic renal failure, also known as
chronic kidney
disease (CKD), but it is also associated with other conditions such as lupus.
Acute renal
failure may arise from exposure to certain drugs (e.g., acetaminophen) or
toxic chemicals,
or from ischemia-reperfusion injury associated with shock or surgical
procedures such as
transplantation, and may result in chronic renal failure. In many patients,
renal failure
advances to a stage in which the patient requires regular dialysis or kidney
transplantation
to continue living. Both of these procedures are highly invasive and
associated with
38
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
significant side effects and quality of life issues. Although there are
effective treatments
for some complications of renal failure, such as hyperparathyroidism and
hyperphosphatemia, no available treatment has been shown to halt or reverse
the
underlying progression of renal failure. Thus, agents that can improve
compromised
renal function would represent a significant advance in the treatment of renal
failure.
Inflammation contributes significantly to the pathology of CKD. There is also
a
strong mechanistic link between oxidative stress and renal dysfunction. The NF-
xl3
signaling pathway plays an important role in the progression of CKD as NF-KB
regulates
the transcription of MCP-1, a chemokine that is responsible for the
recruitment of
monocytes/macrophages resulting in an inflammatory response that ultimately
injures the
kidney (Wardle, 2001). The Keapl/Nrf2/ARE pathway controls the transcription
of
several genes encoding antioxidant enzymes, including heme oxygenasc-1 (H0-1).
Ablation of the Nrf2 gene in female mice results in the development of lupus-
like
glomerular nephritis (Yoh et at., 2001). Furthermore, several studies have
demonstrated
that HO-1 expression is induced in response to renal damage and inflammation
and that
this enzyme and its products¨bilirubin and carbon monoxide¨play a protective
role in
the kidney (Nath et at., 2006).
The glomerulus and the surrounding Bowman's capsule constitute the basic
functional unit of the kidney. Glomerular filtration rate (GFR) is the
standard measure of
renal function. Creatinine clearance is commonly used to measure GFR. However,
the
level of serum creatinine is commonly used as a surrogate measure of
creatinine
clearance. For instance, excessive levels of serum creatinine are generally
accepted to
indicate inadequate renal function and reductions in serum creatinine over
time are
accepted as an indication of improved renal function. Normal levels of
creatinine in the
blood are approximately 0.6 to 1.2 milligrams (mg) per deciliter (dl) in adult
males and
0.5 to 1.1 milligrams per deciliter in adult females.
Acute kidney injury (AK1) can occur following ischemia-reperfusion, treatment
with certain pharmacological agents such as cisplatin and rapamycin, and
intravenous
injection of radiocontrast media used in medical imaging. As in CKD,
inflammation and
oxidative stress contribute to the pathology of AKI. The molecular mechanisms
underlying radiocontrast-induced nephropathy (RCN) are not well understood;
however,
39
WO 2014/176415 PCT/US2014/035279
it is likely that a combination of events including prolonged
vasoconstriction, impaired
kidney autoregulation, and direct toxicity of the contrast media all
contribute to renal
failure (Turnlin et al., 2006). Vasoconstriction results in decreased renal
blood flow and
causes ischemia-reperfusion and the production of reactive oxygen species. HO-
1 is
strongly induced under these conditions and has been demonstrated to prevent
ischemia-
reperfusion injury in several different organs, including the kidney (Nath et
al., 2006).
Specifically, induction of HO-1 has been shown to be protective in a rat model
of RCN
(Goodman et al., 2007). Reperfusion also induces an inflammatory response, in
part
though activation of NF-1(13 signaling (Nichols, 2004). Targeting NF-1(13 has
been
proposed as a therapeutic strategy to prevent organ damage (Zingarelli et al.,
2003).
E. Cardiovascular Disease
The compound and methods of this invention may be used for treating patients
with cardiovascular disease. See U.S. patent application Ser. No. 12/352,473 .
Cardiovascular (CV) disease is among
the most important causes of mortality worldwide, and is the leading cause of
death in
many developed nations. The etiology of CV disease is complex, but the
majority of
causes are related to inadequate or completely disrupted supply of blood to a
critical
organ or tissue. Frequently such a condition arises from the rupture of one or
more
atherosclerotic plaques, which leads to the formation of a thrombus that
blocks blood
flow in a critical vessel. Such thrombosis is the principal cause of heart
attacks, in which
one or more of the coronary arteries is blocked and blood flow to the heart
itself is
disrupted. The resulting ischemia is highly damaging to cardiac tissue, both
from lack of
oxygen during the ischemic event and from excessive formation of free radicals
after
blood flow is restored (a phenomenon known as ischemia-reperfusion injury).
Similar
damage occurs in the brain during a thrombotic stroke, when a cerebral artery
or other
major vessel is blocked by thrombosis. Hemorrhagic strokes, in contrast,
involve rupture
of a blood vessel and bleeding into the surrounding brain tissue. This creates
oxidative
stress in the immediate area of the hemorrhage, due to the presence of large
amounts of
free heme and other reactive species, and ischemia in other parts of the brain
due to
compromised blood flow. Subarachnoid hemorrhage, which is frequently
accompanied
by cerebral vasospasm, also causes ischemia/reperfusion injury in the brain.
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Alternatively, atherosclerosis may be so extensive in critical blood vessels
that
stenosis (narrowing of the arteries) develops and blood flow to critical
organs (including
the heart) is chronically insufficient. Such chronic ischemia can lead to end-
organ
damage of many kinds, including the cardiac hypertrophy associated with
congestive
.. heart failure.
Atherosclerosis, the underlying defect leading to many forms of cardiovascular
disease, occurs when a physical defect or injury to the lining (endothelium)
of an artery
triggers an inflammatory response involving the proliferation of vascular
smooth muscle
cells and the infiltration of leukocytes into the affected area. Ultimately, a
complicated
lesion known as an atherosclerotic plaque may form, composed of the above-
mentioned
cells combined with deposits of cholesterol-bearing lipoproteins and other
materials (e.g.,
Hansson et al., 2006).
Pharmaceutical treatments for cardiovascular disease include preventive
treatments, such as the use of drugs intended to lower blood pressure or
circulating levels
of cholesterol and lipoproteins, as well as treatments designed to reduce the
adherent
tendencies of platelets and other blood cells (thereby reducing the rate of
plaque
progression and the risk of thrombus formation). More recently, drugs such as
streptokinase and tissue plasminogen activator have been introduced and are
used to
dissolve the thrombus and restore blood flow. Surgical treatments include
coronary
artery bypass grafting to create an alternative blood supply, balloon
angioplasty to
compress plaque tissue and increase the diameter of the arterial lumen, and
carotid
endarterectomy to remove plaque tissue in the carotid artery. Such treatments,
especially
balloon angioplasty, may be accompanied by the use of stents, expandable mesh
tubes
designed to support the artery walls in the affected area and keep the vessel
open.
Recently, the use of drug-eluting stents has become common in order to prevent
post-
surgical restenosis (renarrowing of the artery) in the affected area. These
devices are
wire stents coated with a biocompatible polymer matrix containing a drug that
inhibits
cell proliferation (e.g., paclitaxel or rapamycin). The polymer allows a slow,
localized
release of the drug in the affected area with minimal exposure of non-target
tissues.
Despite the significant benefits offered by such treatments, mortality from
cardiovascular
41
WO 2014/176415 PCT/US2014/035279
disease remains high and significant unmet needs in the treatment of
cardiovascular
disease remain.
As noted above, induction of HO-1 has been shown to be beneficial in a variety
of
models of cardiovascular disease, and low levels of HO-1 expression have been
clinically
correlated with elevated risk of CV disease. Compounds of the invention,
therefore, may
be used in treating or preventing a variety of cardiovascular disorders
including but not
limited to atherosclerosis, hypertension, myocardial infarction, chronic heart
failure,
stroke, subarachnoid hemorrhage, and restenosis.
F. Diabetes
The compound and methods of this invention may be used for treating patients
with diabetes. See U.S. patent application Ser. No. 12/352,473.
Diabetes is a complex disease characterized by the body's
failure to regulate circulating levels of glucose. This failure may result
from a lack of
insulin, a peptide hormone that regulates both the production and absorption
of glucose in
various tissues. Deficient insulin compromises the ability of muscle, fat, and
other
tissues to absorb glucose properly, leading to hyperglycemia (abnormally high
levels of
glucose in the blood). Most commonly, such insulin deficiency results from
inadequate
production in the islet cells of the pancreas. In the majority of cases this
arises from
autoimmune destruction of these cells, a condition known as type 1 or juvenile-
onset
diabetes, but may also be due to physical trauma or some other cause.
Diabetes may also arise when muscle and fat cells become less responsive to
insulin and do not absorb glucose properly, resulting in hyperglycemia.
This
phenomenon is known as insulin resistance, and the resulting condition is
known as Type
2 diabetes. Type 2 diabetes, the most common type, is highly associated with
obesity and
hypertension. Obesity is associated with an inflammatory state of adipose
tissue that is
thought to play a major role in the development of insulin resistance (e.g.,
Hotamisligil,
2006; Guilherme et al., 2008).
Diabetes is associated with damage to many tissues, largely because
hyperglycemia (and hypoglycemia, which can result from excessive or poorly
timed
doses of insulin) is a significant source of oxidative stress. Chronic kidney
failure,
retinopathy, peripheral neuropathy, peripheral vasculitis, and the development
of dermal
42
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
ulcers that heal slowly or not at all are among the common complications of
diabetes.
Because of their ability to protect against oxidative stress, particularly by
the induction of
HO-1 expression, compounds of the invention may be used in treatments for many
complications of diabetes. As noted above (Cai et al., 2005), chronic
inflammation and
oxidative stress in the liver are suspected to be primary contributing factors
in the
development of Type 2 diabetes.
Furthermore, PPARy agonists such as
thiazolidinediones are capable of reducing insulin resistance and are known to
be
effective treatments for Type 2 diabetes.
The effect of treatment of diabetes may be evaluated as follows. Both the
biological efficacy of the treatment modality as well as the clinical efficacy
are evaluated,
if possible. For example, because the disease manifests itself by increased
blood sugar,
the biological efficacy of the treatment therefore can be evaluated, for
example, by
observation of return of the evaluated blood glucose towards normal.
Measurement of
glycosylated hemoglobin, also called Al c or HbAlc, is another commonly used
parameter of blood glucose control. Measuring a clinical endpoint which can
give an
indication of b-cell regeneration after, for example, a six-month period of
time, can give
an indication of the clinical efficacy of the treatment regimen.
G. Rheumatoid Arthritis
The compound and methods of this invention may be used for treating patients
with RA. Typically the first signs of rheumatoid arthritis (RA) appear in the
synovial
lining layer, with proliferation of synovial fibroblasts and their attachment
to the articular
surface at the joint margin (Lipsky, 1998). Subsequently, macrophages, T cells
and other
inflammatory cells are recruited into the joint, where they produce a number
of
mediators, including the cytokines interleukin-1 (IL-1), which contributes to
the chronic
sequelae leading to bone and cartilage destruction, and tumour necrosis factor
(TNF-a),
which plays a role in inflammation (Dinarello, 1998; Arend and Dayer, 1995;
van den
Berg, 2001). The concentration of IL-1 in plasma is significantly higher in
patients with
RA than in healthy individuals and, notably, plasma IL-1 levels correlate with
RA disease
activity (Eastgate et al., 1988). Moreover, synovial fluid levels of IL-1 are
correlated
with various radiographic and histologic features of RA (Kahle et al., 1992;
Rooney et
al., 1990).
43
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
In normal joints, the effects of these and other proinflammatory cytokines are
balanced by a variety of anti-inflammatory cytokines and regulatory factors
(Burger and
Dayer, 1995). The significance of this cytokine balance is illustrated in
juvenile RA
patients, who have cyclical increases in fever throughout the day (Prieur et
at., 1987).
After each peak in fever, a factor that blocks the effects of IL-1 is found in
serum and
urine. This factor has been isolated, cloned and identified as IL-1 receptor
antagonist
(IL-lra), a member of the IL-1 gene family (Hannum et at., 1990). IL- lra, as
its name
indicates, is a natural receptor antagonist that competes with IL-1 for
binding to type I IL-
1 receptors and, as a result, blocks the effects of IL-1 (Arend et at., 1998).
A 10- to 100-
.. fold excess of IL-lra may be needed to block IL-1 effectively; however,
synovial cells
isolated from patients with RA do not appear to produce enough IL-lra to
counteract the
effects of IL-1 (Firestein et at., 1994; Fujikawa et at., 1995).
H. Psoriatic Arthritis
The compound and methods of this invention may be used for treating patients
with psoriatic arthritis. Psoriasis is an inflammatory and proliferative skin
disorder with a
prevalence of 1.5-3%. Approximately 20% of patients with psoriasis develop a
characteristic form of arthritis that has several patterns (Gladman, 1992;
Jones et at.,
1994; Gladman et at., 1995). Some individuals present with joint symptoms
first but in
the majority, skin psoriasis presents first. About one-third of patients have
simultaneous
exacerbations of their skin and joint disease (Gladman et at., 1987) and there
is a
topographic relationship between nail and distal interphalangeal joint disease
(Jones et
at., 1994; Wright, 1956). Although the inflammatory processes which link skin,
nail and
joint disease remain elusive, an immune-mediated pathology is implicated.
Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy characterized
by
the association of arthritis and psoriasis and was recognized as a clinical
entity distinct
from rheumatoid arthritis (RA) in 1964 (Blumberg et at., 1964). Subsequent
studies have
revealed that PsA shares a number of genetic, pathogenic and clinical features
with other
spondyloarthropathies (SpAs), a group of diseases that comprise ankylosing
spondylitis,
reactive arthritis and enteropathic arthritis (Wright, 1979). The notion that
PsA belongs
to the SpA group has recently gained further support from imaging studies
demonstrating
widespread enthesitis in the, including PsA but not RA (McGonagle et at.,
1999;
44
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
McGonagle et at., 1998). More specifically, enthesitis has been postulated to
be one of
the earliest events occurring in the SpAs, leading to bone remodeling and
ankylosis in the
spine, as well as to articular synovitis when the inflamed entheses are close
to peripheral
joints. However, the link between enthesitis and the clinical manifestations
in PsA
remains largely unclear, as PsA can present with fairly heterogeneous patterns
of joint
involvement with variable degrees of severity (Marsal et at., 1999; Salvarani
et at.,
1998). Thus, other factors must be posited to account for the multifarious
features of
PsA, only a few of which (such as the expression of the HLA-B27 molecule,
which is
strongly associated with axial disease) have been identified. As a
consequence, it
remains difficult to map the disease manifestations to specific pathogenic
mechanisms,
which means that the treatment of this condition remains largely empirical.
Family studies have suggested a genetic contribution to the development of PsA
(Moll and Wright, 1973). Other chronic inflammatory forms of arthritis, such
as
ankylosing spondylitis and rheumatoid arthritis, are thought to have a complex
genetic
basis. However, the genetic component of PsA has been difficult to assess for
several
reasons. There is strong evidence for a genetic predisposition to psoriasis
alone that may
mask the genetic factors that are important for the development of PsA.
Although most
would accept PsA as a distinct disease entity, at times there is a phenotypic
overlap with
rheumatoid arthritis and ankylosing spondylitis. Also, PsA itself is not a
homogeneous
condition and various subgroups have been proposed.
Increased amounts of TNF-a have been reported in both psoriatic skin (Ettehadi
et
at., 1994) and synovial fluid (Partsch et at., 1997). Recent trials have shown
a positive
benefit of anti-TNF treatment in both PsA (Mease et al., 2000) and ankylosing
spondylitis (Brandt et at., 2000).
I. Reactive Arthritis
The compound and methods of this invention may be used for treating patients
with reactive arthritis. In reactive arthritis (ReA) the mechanism of joint
damage is
unclear, but it is likely that cytokines play critical roles. A more prevalent
Thl profile
high levels of interferon gamma (IFN-y) and low levels of interleukin 4 (IL-4)
has been
reported (Lahesmaa et at., 1992; Schlaak et at., 1992; Simon et at., 1993;
Schlaak et at.,
1996; Kotake et at., 1999; Ribbens et at., 2000), but several studies have
shown relative
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
predominance of IL-4 and IL-10 and relative lack of IFN-y and tumor necrosis
factor
alpha (TNF-a) in the synovial membrane (Simon et al., 1994; Yin et al., 1999)
and fluid
(SF) (Yin et al., 1999; Yin et al., 1997) of reactive arthritis patients
compared with
rheumatoid arthritis (RA) patients. A lower level of TNF-a secretion in
reactive arthritis
than in RA patients has also been reported after ex vivo stimulation of
peripheral blood
mononuclear cells (PBMC) (Braun et al., 1999).
It has been argued that clearance of reactive arthritis-associated bacteria
requires
the production of appropriate levels of IFN-y and TNF-a, while IL-10 acts by
suppressing
these responses (Autenrieth et al., 1994; Sieper and Braun, 1995). IL-10 is a
regulatory
cytokine that inhibits the synthesis of IL-12 and TNF-y by activated
macrophages (de
Waal et al., 1991; Hart et al., 1995; Chomarat et al., 1995) and of IFN-y by T
cells
(Macatonia et al., 1993).
J. Enteropathic Arthritis
The compound and methods of this invention may be used for treating patients
with enteropathic arthritis. Typically enteropathic arthritis (EA) occurs in
combination
with inflammatory bowel diseases (IBD) such as Crohn's disease or ulcerative
colitis. It
also can affect the spine and sacroiliac joints. Enteropathic arthritis
involves the
peripheral joints, usually in the lower extremities such as the knees or
ankles. It
commonly involves only a few or a limited number of joints and may closely
follow the
bowel condition. This occurs in approximately 11% of patients with ulcerative
colitis
and 21% of those with Crohn's disease. The synovitis is generally self-limited
and non-
deforming.
Enteropathic arthropathies comprise a collection of rheumatologic conditions
that
share a link to GI pathology. These conditions include reactive (i.e.,
infection-related)
arthritis due to bacteria (e.g., Shigella, Salmonella, Campylobacter, Yersinia
species,
Clostridium difficile), parasites (e.g., Strongyloides stercoralis, Taenia
saginata, Giardia
lamblia, Ascaris lumbricoides, Cryptosporidium species), and
spondyloarthropathies
associated with inflammatory bowel disease (IBD). Other conditions and
disorders
include intestinal bypass (jejunoileal), arthritis, celiac disease, Whipple
disease, and
collagenous colitis.
K. Juvenile Rheumatoid Arthritis
46
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
The compound and methods of this invention may be used for treating patients
with JRA. Juvenile rheumatoid arthritis (JRA), a term for the most prevalent
form of
arthritis in children, is applied to a family of illnesses characterized by
chronic
inflammation and hypertrophy of the synovial membranes. The term overlaps, but
is not
completely synonymous, with the family of illnesses referred to as juvenile
chronic
arthritis and/or juvenile idiopathic arthritis in Europe.
Both innate and adaptive immune systems use multiple cell types, a vast array
of
cell surface and secreted proteins, and interconnected networks of positive
and negative
feedback (Lo et al., 1999). Furthermore, while separable in thought, the
innate and
adaptive wings of the immune system are functionally intersected (Fearon and
Locksley,
1996), and pathologic events occurring at these intersecting points are likely
to be highly
relevant to our understanding of pathogenesis of adult and childhood forms of
chronic
arthritis (Warrington, et al., 2001).
Polyarticular JRA is a distinct clinical subtype characterized by inflammation
and
synovial proliferation in multiple joints (four or more), including the small
joints of the
hands (Jarvis, 2002). This subtype of JRA may be severe, because of both its
multiple
joint involvement and its capacity to progress rapidly over time. Although
clinically
distinct, polyarticular JRA is not homogeneous, and patients vary in disease
manifestations, age of onset, prognosis, and therapeutic response. These
differences very
likely reflect a spectrum of variation in the nature of the immune and
inflammatory attack
that can occur in this disease (Jarvis, 1998).
L. Early Inflammatory Arthritis
The compound and methods of this invention may be used for treating patients
with early inflammatory arthritis. The clinical presentation of different
inflammatory
arthropathies is similar early in the course of disease. As a result, it is
often difficult to
distinguish patients who are at risk of developing the severe and persistent
synovitis that
leads to erosive joint damage from those whose arthritis is more self-limited.
Such
distinction is critical in order to target therapy appropriately, treating
aggressively those
with erosive disease and avoiding unnecessary toxicity in patients with more
self-limited
disease. Current clinical criteria for diagnosing erosive arthropathies such
as rheumatoid
arthritis (RA) are less effective in early disease and traditional markers of
disease activity
47
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
such as joint counts and acute phase response do not adequately identify
patients likely to
have poor outcomes (Harrison et al., 1998). Parameters reflective of the
pathologic
events occurring in the synovium are most likely to be of significant
prognostic value.
Recent efforts to identify predictors of poor outcome in early inflammatory
arthritis have identified the presence of RA specific autoantibodies, in
particular
antibodies towards citrullinated peptides, to be associated with erosive and
persistent
disease in early inflammatory arthritis cohorts. On the basis of this, a
cyclical
citrullinated peptide (CCP) has been developed to assist in the identification
of anti-CCP
antibodies in patient sera. Using this approach, the presence of anti-CCP
antibodies has
been shown to be specific and sensitive for RA, can distinguish RA from other
arthropathies, and can potentially predict persistent, erosive synovitis
before these
outcomes become clinically manifest. Importantly, anti-CCP antibodies are
often
detectable in sera many years prior to clinical symptoms suggesting that they
may be
reflective of subclinical immune events (Nielen et al., 2004; Rantapaa-
Dahlqvist et al.,
2003).
M. Ankylosing Spondylitis
The compound and methods of this invention may be used for treating patients
with ankylosing spondylitis. AS is a disease subset within a broader
disease
classification of spondyloarthropathy. Patients affected with the various
subsets of
spondyloarthropathy have disease etiologies that are often very different,
ranging from
bacterial infections to inheritance. Yet, in all subgroups, the end result of
the disease
process is axial arthritis. Despite the early clinically differences seen in
the various
patient populations, many of them end up nearly identical after a disease
course of ten-to-
twenty years. Recent studies suggest the mean time to clinical diagnosis of
ankylosing
spondylitis from disease onset of disease is 7.5 years (Khan, 1998). These
same studies
suggest that the spondyloarthropathies may have prevalence close to that of
rheumatoid
arthritis (Feldtkeller et al., 2003; Doran et al., 2003).
AS is a chronic systemic inflammatory rheumatic disorder of the axial skeleton
with or without extraskeletal manifestations. Sacroiliac joints and the spine
are primarily
affected, but hip and shoulder joints, and less commonly peripheral joints or
certain
extra-articular structures such as the eye, vasculature, nervous system, and
48
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
gastrointestinal system may also be involved. Its etiology is not yet fully
understood
(Wordsworth, 1995; Calin and Taurog, 1998). It is strongly associated with the
major
histocompatibility class I (MHC I) HLA-B27 allele (Calin and Taurog, 1998). AS
affects
individuals in the prime of their life and is feared because of its potential
to cause chronic
pain and irreversible damage of tendons, ligaments, joints, and bones
(Brewerton et al.,
1973a; Brewerton et al., 1973b; Schlosstein et al., 1973). AS may occur alone
or in
association with another form of spondyloarthropathy such as reactive
arthritis, psoriasis,
psoriatic arthritis, enthesitis, ulcerative colitis, irritable bowel disease,
or Crohn's disease,
in which case it is classified as secondary AS.
Typically, the affected sites include the discovertebral, apophyseal,
costovertebral, and costotransverse joints of the spine, and the paravertebral
ligamentous
structures. Inflammation of the cntheses, which are sites of musculotendinous
and
ligamentous attachment to bones, is also prominent in this disease (Calin and
Taurog,
1998). The site of enthcsitis is known to be infiltrated by plasma cells,
lymphocytes, and
polymorphonuclear cells. The inflammatory process frequently results in
gradual fibrous
and bony ankylosis, (Ball, 1971; Khan, 1990).
Delayed diagnosis is common because symptoms are often attributed to more
common back problems. A dramatic loss of flexibility in the lumbar spine is an
early
sign of AS. Other common symptoms include chronic pain and stiffness in the
lower
back which usually starts where the lower spine is joined to the pelvis, or
hip. Although
most symptoms begin in the lumbar and sacroiliac areas, they may involve the
neck and
upper back as well. Arthritis may also occur in the shoulder, hips and feet.
Some
patients have eye inflammation, and more severe cases must be observed for
heart valve
involvement.
The most frequent presentation is back pain, but disease can begin atypically
in
peripheral joints, especially in children and women, and rarely with acute
iritis (anterior
uveitis). Additional early symptoms and signs are diminished chest expansion
from
diffuse costovertebral involvement, low-grade fever, fatigue, anorexia, weight
loss, and
anemia. Recurrent back pain¨often nocturnal and of varying intensity¨is an
eventual
complaint, as is morning stiffness typically relieved by activity. A flexed or
bent-over
49
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
posture eases back pain and paraspinal muscle spasm; thus, some degree of
kyphosis is
common in untreated patients.
Systemic manifestations occur in 'A of patients. Recurrent, usually self-
limited,
acute iritis (anterior uveitis) rarely is protracted and severe enough to
impair vision.
Neurologic signs can occasionally result from compression radiculitis or
sciatica,
vertebral fracture or subluxation, and cauda equina syndrome (which consists
of
impotence, nocturnal urinary incontinence, diminished bladder and rectal
sensation, and
absence of ankle jerks). Cardiovascular manifestations can include aortic
insufficiency,
angina, pericarditis, and ECG conduction abnormalities. A rare pulmonary
finding is
upper lobe fibrosis, occasionally with cavitation that may be mistaken for TB
and can be
complicated by infection with Aspergillus.
AS is characterized by mild or moderate flares of active spondylitis
alternating
with periods of almost or totally inactive inflammation. Proper treatment in
most patients
results in minimal or no disability and in full, productive lives despite back
stifffiess.
Occasionally, the course is severe and progressive, resulting in pronounced
incapacitating
deformities. The prognosis is bleak for patients with refractory iritis and
for the rare
patient with secondary amyloidosis.
N. Ulcerative Colitis
The compound and methods of this invention may be used for treating patients
with ulcerative colitis. Ulcerative colitis is a disease that causes
inflammation and sores,
called ulcers, in the lining of the large intestine. The inflammation usually
occurs in the
rectum and lower part of the colon, but it may affect the entire colon.
Ulcerative colitis
rarely affects the small intestine except for the end section, called the
terminal ileum.
Ulcerative colitis may also be called colitis or proctitis. The inflammation
makes the
colon empty frequently, causing diarrhea. Ulcers form in places where the
inflammation
has killed the cells lining the colon; the ulcers bleed and produce pus.
Ulcerative colitis is an inflammatory bowel disease (IBD), the general name
for
diseases that cause inflammation in the small intestine and colon. Ulcerative
colitis can
be difficult to diagnose because its symptoms are similar to other intestinal
disorders and
to another type of IBD, Crohn's disease. Crohn's disease differs from
ulcerative colitis
because it causes inflammation deeper within the intestinal wall. Also,
Crohn's disease
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
usually occurs in the small intestine, although it can also occur in the
mouth, esophagus,
stomach, duodenum, large intestine, appendix, and anus.
Ulcerative colitis may occur in people of any age, but most often it starts
between
ages 15 and 30, or less frequently between ages 50 and 70. Children and
adolescents
sometimes develop the disease. Ulcerative colitis affects men and women
equally and
appears to run in some families. Theories about what causes ulcerative colitis
abound,
but none have been proven. The most popular theory is that the body's immune
system
reacts to a virus or a bacterium by causing ongoing inflammation in the
intestinal wall.
People with ulcerative colitis have abnormalities of the immune system, but
doctors do
not know whether these abnormalities are a cause or a result of the disease.
Ulcerative
colitis is not caused by emotional distress or sensitivity to certain foods or
food products,
but these factors may trigger symptoms in some people.
The most common symptoms of ulcerative colitis are abdominal pain and bloody
diarrhea. Patients also may experience fatigue, weight loss, loss of appetite,
rectal
bleeding, and loss of body fluids and nutrients. About half of patients have
mild
symptoms. Others suffer frequent fever, bloody diarrhea, nausea, and severe
abdominal
cramps. Ulcerative colitis may also cause problems such as arthritis,
inflammation of the
eye, liver disease (hepatitis, cirrhosis, and primary sclerosing cholangitis),
osteoporosis,
skin rashes, and anemia. No one knows for sure why problems occur outside the
colon.
Scientists think these complications may occur when the immune system triggers
inflammation in other parts of the body. Some of these problems go away when
the
colitis is treated.
A thorough physical exam and a series of tests may be required to diagnose
ulcerative colitis. Blood tests may be done to check for anemia, which could
indicate
bleeding in the colon or rectum. Blood tests may also uncover a high white
blood cell
count, which is a sign of inflammation somewhere in the body. By testing a
stool
sample, the doctor can detect bleeding or infection in the colon or rectum.
The doctor
may do a colonoscopy or sigmoidoscopy. For either test, the doctor inserts an
endoscope¨a long, flexible, lighted tube connected to a computer and TV
monitor¨into
the anus to see the inside of the colon and rectum. The doctor will be able to
see any
inflammation, bleeding, or ulcers on the colon wall. During the exam, the
doctor may do
51
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
a biopsy, which involves taking a sample of tissue from the lining of the
colon to view
with a microscope. A barium enema x-ray of the colon may also be required.
This
procedure involves filling the colon with barium, a chalky white solution. The
barium
shows up white on x-ray film, allowing the doctor a clear view of the colon,
including
any ulcers or other abnormalities that might be there.
Treatment for ulcerative colitis depends on the seriousness of the disease.
Most
people are treated with medication. In severe cases, a patient may need
surgery to
remove the diseased colon. Surgery is the only cure for ulcerative colitis.
Some people
whose symptoms are triggered by certain foods are able to control the symptoms
by
avoiding foods that upset their intestines, like highly seasoned foods, raw
fruits and
vegetables, or milk sugar (lactose). Each person may experience ulcerative
colitis
differently, so treatment is adjusted for each individual. Emotional and
psychological
support is important. Some people have remissions¨periods when the symptoms go
away¨that last for months or even years. However, most patients' symptoms
eventually
return. This changing pattern of the disease means one cannot always tell when
a
treatment has helped. Some people with ulcerative colitis may need medical
care for
some time, with regular doctor visits to monitor the condition.
0. Crohn's Disease
The compound and methods of this invention may be used for treating patients
with Crohn's disease. Another disorder for which immunosuppression has been
tried is
Crohn's disease. Crohn's disease symptoms include intestinal inflammation and
the
development of intestinal stenosis and fistulas; neuropathy often accompanies
these
symptoms. Anti-inflammatory drugs, such as 5-aminosalicylates (e.g.,
mesalamine) or
corticosteroids, are typically prescribed, but are not always effective
(reviewed in
Botoman et al., 1998). Immunosuppression with cyclosporine is sometimes
beneficial
for patients resistant to or intolerant of corticosteroids (Brynskov et al.,
1989).
Efforts to develop diagnostic and treatment tools against Crohn's disease have
focused on the central role of cytokines (Schreiber, 1998; van Hogezand and
Verspaget,
1998). Cytokines are small secreted proteins or factors (5 to 20 kD) that have
specific
effects on cell-to-cell interactions, intercellular communication, or the
behavior of other
cells. Cytokines are produced by lymphocytes, especially TH1 and TH2
lymphocytes,
52
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
monocytes, intestinal macrophages, granulocytes, epithelial cells, and
fibroblasts
(reviewed in Rogler and Andus, 1998; Galley and Webster, 1996). Some cytokines
are
pro-inflammatory (e.g., TNF-a, IL-1(a and 13), IL-6, IL-8, IL-12, or leukemia
inhibitory
factor [Lin); others are anti-inflammatory (e.g., IL-1 receptor antagonist, IL-
4, IL-10,
IL-11, and TGF-13). However, there may be overlap and functional redundancy in
their
effects under certain inflammatory conditions.
In active cases of Crohn's disease, elevated concentrations of TNF-a and IL-6
are
secreted into the blood circulation, and TNF-a, IL-1, IL-6, and IL-8 are
produced in
excess locally by mucosal cells (id.; Funakoshi et at., 1998). These cytokines
can have
far-ranging effects on physiological systems including bone development,
hematopoiesis,
and liver, thyroid, and neuropsychiatric function. Also, an imbalance of the
IL-113/IL-Ira
ratio, in favor of pro-inflammatory IL-113, has been observed in patients with
Crohn's
disease (Rogler and Andus, 1998; Saiki et at., 1998; Dionne et at., 1998; but
see
Kuboyama, 1998). One study suggested that cytokine profiles in stool samples
could be
a useful diagnostic tool for Crohn's disease (Saiki et at., 1998).
Treatments that have been proposed for Crohn's disease include the use of
various
cytokine antagonists (e.g., IL-Ira), inhibitors (e.g., of IL-1 13 converting
enzyme and
antioxidants) and anti-cytokine antibodies (Rogl er and Andus, 1998; van
Hogezand and
Verspaget, 1998; Reimund et at., 1998; Lugering et at., 1998; McAlindon et
at., 1998).
In particular, monoclonal antibodies against TNF-a have been tried with some
success in
the treatment of Crohn's disease (Targan et at., 1997; Stack et at., 1997; van
Dullemen et
at., 1995). These compounds may be used in combination therapy with compounds
of
the present disclosure.
Another approach to the treatment of Crohn's disease has focused on at least
partially eradicating the bacterial community that may be triggering the
inflammatory
response and replacing it with a non-pathogenic community. For example, U.S.
Pat. No.
5,599,795 discloses a method for the prevention and treatment of Crohn's
disease in
human patients. Their method was directed to sterilizing the intestinal tract
with at least
one antibiotic and at least one anti-fungal agent to kill off the existing
flora and replacing
them with different, select, well-characterized bacteria taken from normal
humans.
Borody taught a method of treating Crohn's disease by at least partial removal
of the
53
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
existing intestinal microflora by lavage and replacement with a new bacterial
community
introduced by fecal inoculum from a disease-screened human donor or by a
composition
comprising Bacteroides and Escherichia coli species. (U.S. Pat. No.
5,443,826).
P. Systemic Lupus Erythematosus
The compound and methods of this invention may be used for treating patients
with SLE. There has also been no known cause for autoimmune diseases such as
systemic lupus erythematosus. Systemic lupus erythematosus (SLE) is an
autoimmune
rheumatic disease characterized by deposition in tissues of autoantibodies and
immune
complexes leading to tissue injury (Kotzin, 1996). In contrast to autoimmune
diseases
such as MS and type 1 diabetes mellitus, SLE potentially involves multiple
organ systems
directly, and its clinical manifestations are diverse and variable (reviewed
by Kotzin and
O'Dell, 1995). For example, some patients may demonstrate primarily skin rash
and joint
pain, show spontaneous remissions, and require little medication. At the other
end of the
spectrum are patients who demonstrate severe and progressive kidney
involvement that
requires therapy with high doses of steroids and cytotoxic drugs such as
cyclophosphamide (Kotzin, 1996).
The serological hallmark of SLE, and the primary diagnostic test available, is
elevated serum levels of IgG antibodies to constituents of the cell nucleus,
such as
double-stranded DNA (dsDNA), single-stranded DNA (ss-DNA), and chromatin.
Among these autoantibodies, IgG anti-dsDNA antibodies play a major role in the
development of lupus glomerulonephritis (G N) (Hahn and Tsao, 1993; Ohnishi et
al.,
1994). Glomerulonephritis is a serious condition in which the capillary walls
of the
kidney's blood purifying glomeruli become thickened by accretions on the
epithelial side
of glomerular basement membranes. The disease is often chronic and progressive
and
may lead to eventual renal failure.
Q. Irritable Bowel Syndrome
The compound and methods of this invention may be used for treating patients
with Irritable bowel syndrome (IBS). IBS is a functional disorder
characterized by
abdominal pain and altered bowel habits. This syndrome may begin in young
adulthood
and can be associated with significant disability. This syndrome is not a
homogeneous
disorder. Rather, subtypes of IBS have been described on the basis of the
predominant
54
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
symptom¨diarrhea, constipation, or pain. In the absence of "alarm" symptoms,
such as
fever, weight loss, and gastrointestinal bleeding, a limited workup is needed.
Once a
diagnosis of IBS is made, an integrated treatment approach can effectively
reduce the
severity of symptoms. IBS is a common disorder, although its prevalence rates
have
varied. In general, IBS affects about 15% of US adults and occurs about three
times
more often in women than in men (Jailwala et al., 2000).
IBS accounts for between 2.4 million and 3.5 million visits to physicians each
year. It not only is the most common condition seen by gastroenterologists but
also is
one of the most common gastrointestinal conditions seen by primary care
physicians
(Everhart et al., 1991; Sandler, 1990).
IBS is also a costly disorder. Compared with persons who do not have bowel
symptoms, persons with IBS miss three times as many workdays and are more
likely to
report being too sick to work (Drossman et al., 1993; Drossman et al., 1997).
Moreover,
those with IBS incur hundreds of dollars more in medical charges than persons
without
bowel disorders (Talley et al., 1995).
No specific abnormality accounts for the exacerbations and remissions of
abdominal pain and altered bowel habits experienced by patients with IBS. The
evolving
theory of IBS suggests dysregulation at multiple levels of the brain-gut axis.
Dysmotility, visceral hypersensitivity, abnormal modulation of the central
nervous
system (CNS), and infection have all been implicated. In addition,
psychosocial factors
play an important modifying role. Abnormal intestinal motility has long been
considered
a factor in the pathogenesis of IBS. Transit time through the small intestine
after a meal
has been shown to be shorter in patients with diarrhea-predominant IBS than in
patients
who have the constipation-predominant or pain-predominant subtype (Cann et
at., 1983).
In studies of the small intestine during fasting, the presence of both
discrete,
clustered contractions and prolonged, propagated contractions has been
reported in
patients with IBS (Kellow and Phillips, 1987). They also experience pain with
irregular
contractions more often than healthy persons (Kellow and Phillips, 1987;
Horwitz and
Fisher, 2001)
These motility findings do not account for the entire symptom complex in
patients
with IBS; in fact, most of these patients do not have demonstrable
abnormalities
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
(Rothstein, 2000). Patients with IBS have increased sensitivity to visceral
pain. Studies
involving balloon distention of the rectosigmoid colon have shown that
patients with IBS
experience pain and bloating at pressures and volumes much lower than control
subjects
(Whitehead et al., 1990). These patients maintain normal perception of somatic
stimuli.
Multiple theories have been proposed to explain this phenomenon. For example,
receptors in the viscera may have increased sensitivity in response to
distention or
intraluminal contents. Neurons in the dorsal horn of the spinal cord may have
increased
excitability. In addition, alteration in CNS processing of sensations may be
involved
(Drossman et al., 1997). Functional magnetic resonance imaging studies have
recently
shown that compared with control subjects, patients with IBS have increased
activation
of the anterior cingulate cortex, an important pain center, in response to a
painful rectal
stimulus (Mertz et al., 2000).
Increasingly, evidence suggests a relationship between infectious enteritis
and
subsequent development of IBS. Inflammatory cytokincs may play a role. In a
survey of
patients with a history of confirmed bacterial gastroenteritis (Neal et al.,
1997), 25%
reported persistent alteration of bowel habits. Persistence of symptoms may be
due to
psychological stress at the time of acute infection (Gwee et al., 1999).
Recent data suggest that bacterial overgrowth in the small intestine may have
a
role in IBS symptoms. In one study (Pimentel et al., 2000), 157 (78%) of 202
IBS
patients referred for hydrogen breath testing had test findings that were
positive for
bacterial overgrowth. Of the 47 subjects who had follow-up testing, 25 (53%)
reported
improvement in symptoms (i.e., abdominal pain and diarrhea) with antibiotic
treatment.
IBS may present with a range of symptoms. However, abdominal pain and
altered bowel habits remain the primary features. Abdominal discomfort is
often
described as crampy in nature and located in the left lower quadrant, although
the
severity and location can differ greatly. Patients may report diarrhea,
constipation, or
alternating episodes of diarrhea and constipation. Diarrheal symptoms are
typically
described as small-volume, loose stools, and stool is sometimes accompanied by
mucus
discharge. Patients also may report bloating, fecal urgency, incomplete
evacuation, and
abdominal distention. Upper gastrointestinal symptoms, such as
gastroesophageal reflux,
dyspepsia, or nausea, may also be present (Lynn and Friedman, 1993).
56
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Persistence of symptoms is not an indication for further testing; it is a
characteristic of IBS and is itself an expected symptom of the syndrome. More
extensive
diagnostic evaluation is indicated in patients whose symptoms are worsening or
changing. Indications for further testing also include presence of alarm
symptoms, onset
of symptoms after age 50, and a family history of colon cancer. Tests may
include
colonoscopy, computed tomography of the abdomen and pelvis, and barium studies
of the
small or large intestine.
R. Sjogren's Syndrome
The compound and methods of this invention may be used for treating patients
with Sjogren's syndrome. Primary Sjogren's syndrome (SS) is a chronic, slowly
progressive, systemic autoimmune disease, which affects predominantly middle-
aged
women (female-to-male ratio 9:1), although it can be seen in all ages
including childhood
(Jonsson et at., 2002). It is characterized by lymphocytic infiltration and
destruction of
the exocrine glands, which are infiltrated by mononuclear cells including
CD4+, CD8+
lymphocytes and B-cells (Jonsson et at., 2002). In addition, extraglandular
(systemic)
manifestations are seen in one-third of patients (Jonsson et at., 2001).
The glandular lymphocytic infiltration is a progressive feature (Jonsson et
at.,
1993), which, when extensive, may replace large portions of the organs.
Interestingly,
the glandular infiltrates in some patients closely resemble ectopic lymphoid
microstructures in the salivary glands (denoted as ectopic germinal centers)
(Salomonsson et al., 2002; Xanthou et at., 2001). In SS, ectopic GCs are
defined as T
and B cell aggregates of proliferating cells with a network of follicular
dendritic cells and
activated endothelial cells. These GC-like structures formed within the target
tissue also
portray functional properties with production of autoantibodies (anti-Ro/SSA
and anti-
La/SSB) (Salomonsson and Jonsson, 2003).
In other systemic autoimmune diseases, such as RA, factors critical for
ectopic
GCs have been identified. Rheumatoid synovial tissues with GCs were shown to
produce
chemokines CXCL13, CCL21 and lymphotoxin (LT)-I3 (detected on follicular
center and
mantle zone B cells). Multivariate regression analysis of these analytes
identified
CXCL13 and LT-I3 as the solitary cytokines predicting GCs in rheumatoid
synovitis
(Weyand and Goronzy, 2003). Recently CXCL13 and CXCR5 in salivary glands has
57
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
been shown to play an essential role in the inflammatory process by recruiting
B and T
cells, therefore contributing to lymphoid neogenesis and ectopic GC formation
in SS
(Salomonsson et al., 2002).
S. Psoriasis
The compound and methods of this invention may be used for treating patients
with psoriasis. Psoriasis is a chronic skin disease of scaling and
inflammation that affects
2 to 2.6 percent of the United States population, or between 5.8 and 7.5
million people.
Although the disease occurs in all age groups, it primarily affects adults. It
appears about
equally in males and females. Psoriasis occurs when skin cells quickly rise
from their
origin below the surface of the skin and pile up on the surface before they
have a chance
to mature. Usually this movement (also called turnover) takes about a month,
but in
psoriasis it may occur in only a few days. In its typical form, psoriasis
results in patches
of thick, red (inflamed) skin covered with silvery scales. These patches,
which are
sometimes referred to as plaques, usually itch or feel sore. They most often
occur on the
elbows, knees, other parts of the legs, scalp, lower back, face, palms, and
soles of the
feet, but they can occur on skin anywhere on the body. The disease may also
affect the
fingernails, the toenails, and the soft tissues of the genitals and inside the
mouth. While it
is not unusual for the skin around affected joints to crack, approximately 1
million people
with psoriasis experience joint inflammation that produces symptoms of
arthritis. This
condition is called psoriatic arthritis.
Psoriasis is a skin disorder driven by the immune system, especially involving
a
type of white blood cell called a T cell. Normally, T cells help protect the
body against
infection and disease. In the case of psoriasis, T cells are put into action
by mistake and
become so active that they trigger other immune responses, which lead to
inflammation
and to rapid turnover of skin cells. In about one-third of the cases, there is
a family
history of psoriasis. Researchers have studied a large number of families
affected by
psoriasis and identified genes linked to the disease. People with psoriasis
may notice that
there are times when their skin worsens, then improves. Conditions that may
cause
flareups include infections, stress, and changes in climate that dry the skin.
Also, certain
medicines, including lithium and beta blockers, which are prescribed for high
blood
pressure, may trigger an outbreak or worsen the disease.
58
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
T. Infectious Diseases
Compound of the present disclosure may be useful in the treatment of
infectious
diseases, including viral and bacterial infections. As noted above, such
infections may be
associated with severe localized or systemic inflammatory responses. For
example,
influenza may cause severe inflammation of the lung and bacterial infection
can cause the
systemic hyperinflammatory response, including the excessive production of
multiple
inflammatory cytokines, that is the hallmark of sepsis. In addition, compounds
of the
invention may be useful in directly inhibiting the replication of viral
pathogens. Previous
studies have demonstrated that related compounds such as CDDO can inhibit the
replication of HIV in macrophages (Vazquez et al., 2005). Other studies have
indicated
that inhibition of NF-kappa B signaling may inhibit influenza virus
replication, and that
cyclopentenone prostaglandins may inhibit viral replication (e.g., Mazur et
al., 2007; Pica
et al., 2000).
The present invention relates to the treatment or prevention of each of the
diseases/disorders/conditions referred to above in section IV using the
compound RTA 408
or a pharmaceutically acceptable salt thereof, or a polymorphic form of that
compound
(such as, e.g., any one of the polymorphic forms described herein above or
below), or a
pharmaceutical composition comprising any of the aforementioned entities and a
pharmaceutically acceptable carrier (including, e.g., the pharmaceutical
compositions
.. described herein above or below).
V. Pharmaceutical Formulations and Routes of Administration
RTA 408 may be administered by a variety of methods, e.g., orally or by
injection
(e.g., subcutaneous, intravenous, intraperitoneal, etc.). Depending on the
route of
administration, the active compounds may be coated in a material to protect
the
compound from the action of acids and other natural conditions which may
inactivate the
compound. They may also be administered by continuous perfusion/infusion of a
disease
or wound site.
To administer RTA 408 by other than parenteral administration, it may be
necessary to coat the compound with, or co-administer the compound with, a
material to
59
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
prevent its inactivation. For example, the therapeutic compound may be
administered to
a patient in an appropriate carrier, for example, liposomes, or a diluent.
Pharmaceutically
acceptable diluents include saline and aqueous buffer solutions. Liposomes
include
water-in-oil-in-water CGF emulsions as well as conventional liposomes (Strejan
et al.,
1984).
RTA 408 may also be administered parenterally, intraperitoneally,
intraspinally,
or intracerebrally. Dispersions can be prepared in glycerol, liquid
polyethylene glycols,
and mixtures thereof and in oils. Under ordinary conditions of storage and
use, these
preparations may contain a preservative to prevent the growth of
microorganisms.
Sterile injectable solutions can be prepared by incorporating RTA 408 in the
required amount in an appropriate solvent with one or a combination of
ingredients
enumerated above, as required, followed by filtered sterilization. Generally,
dispersions
are prepared by incorporating the therapeutic compound into a sterile carrier
that contains
a basic dispersion medium and the required other ingredients from those
enumerated
above. In the case of sterile powders for the preparation of sterile
injectable solutions,
the preferred methods of preparation are vacuum drying and freeze-drying,
which yields
a powder of the active ingredient (i.e., the therapeutic compound) plus any
additional
desired ingredient from a previously sterile-filtered solution thereof.
RTA 408 may be rendered fully amorphous using a direct spray drying procedure.
RTA 408 can be orally administered, for example, with an inert diluent or an
assimilable
edible carrier. The therapeutic compound and other ingredients may also be
enclosed in a
hard or soft shell gelatin capsule, compressed into tablets, or incorporated
directly into
the patient's diet. For oral therapeutic administration, the therapeutic
compound may be
incorporated with excipients and used in the form of ingestible tablets,
buccal tablets,
troches, capsules, elixirs, suspensions, syrups, wafers, and the like. The
percentage of the
therapeutic compound in the compositions and preparations may, of course, be
varied.
The amount of the therapeutic compound in such therapeutically useful
compositions is
such that a suitable dosage will be obtained.
It is especially advantageous to formulate parenteral compositions in dosage
unit
form for ease of administration and uniformity of dosage. Dosage unit form as
used
herein refers to physically discrete units suited as unitary dosages for the
patients to be
CA 02909066 2015-10-07
WO 2014/176415 PCT/1JS2014/035279
treated, each unit containing a predetermined quantity of therapeutic compound
calculated to produce the desired therapeutic effect in association with the
required
pharmaceutical carrier. The specification for the dosage unit forms of the
invention are
dictated by and directly dependent on (a) the unique characteristics of the
therapeutic
compound and the particular therapeutic effect to be achieved, and (b) the
limitations
inherent in the art of compounding such a therapeutic compound for the
treatment of a
selected condition in a patient.
RTA 408 may also be administered topically to the skin, eye, or mucosa.
Alternatively, if local delivery to the lungs is desired the therapeutic
compound may be
administered by inhalation in a dry-powder or aerosol formulation.
RTA 408 will typically be administered at a therapeutically effective dosage
sufficient to treat a condition associated with a given patient. For example,
the efficacy
of a compound can be evaluated in an animal model system that may be
predictive of
efficacy in treating the disease in humans, such as the model systems shown in
the
examples and drawings.
The actual dosage amount of RTA 408 or composition comprising RTA 408
administered to a patient may be determined by physical and physiological
factors, such
as age, sex, body weight, severity of condition, the type of disease being
treated, previous
or concurrent therapeutic interventions, idiopathy of the patient, and the
route of
administration. These factors may be determined by a skilled artisan. The
practitioner
responsible for administration will typically determine the concentration of
active
ingredient(s) in a composition and appropriate dose(s) for the individual
patient. The
dosage may be adjusted by the individual physician in the event of any
complication.
An effective amount typically will vary from about 0.001 mg/kg to about
1000 mg/kg, from about 0.01 mg/kg to about 750 mg/kg, from about 100 mg/kg to
about
500 mg/kg, from about 1.0 mg/kg to about 250 mg/kg, from about 10.0 mg/kg to
about
150 mg/kg in one or more dose administrations daily, for one or several days
(depending
of course of the mode of administration and the factors discussed above).
Other suitable
dose ranges include 1 mg to 10000 mg per day, 100 mg to 10000 mg per day, 500
mg to
10000 mg per day, and 500 mg to 1000 mg per day. In some particular
embodiments, the
amount is less than 10,000 mg per day with a range of 750 mg to 9000 mg per
day.
61
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
The effective amount may be less than 1 mg/kg/day, less than 500 mg/kg/day,
less
than 250 mg/kg/day, less than 100 mg/kg/day, less than 50 mg/kg/day, less than
25 mg/kg/day, or less than 10 mg/kg/day. It may alternatively be in the range
of
1 mg/kg/day to 200 mg/kg/day. In some embodiments, the amount could be 10, 30,
100,
or 150 mg/kg formulated as a suspension in sesame oil. In some embodiments,
the
amount could be 3, 10, 30 or 100 mg/kg administered daily via oral gavage. In
some
embodiments, the amount could be 10, 30, or 100 mg/kg administered orally. For
example, regarding treatment of diabetic patients, the unit dosage may be an
amount that
reduces blood glucose by at least 40% as compared to an untreated patient. In
another
embodiment, the unit dosage is an amount that reduces blood glucose to a level
that is
+10% of the blood glucose level of a non-diabetic patient.
In other non-limiting examples, a dose may also comprise from about 1 micro-
gram/kg/body weight, about 5 microgram/kg/body weight, about 10
microgram/kg/body
weight, about 50 microgram/kg/body weight, about 100 microgram/kg/body weight,
about 200 microgram/kg/body weight, about 350 microgram/kg/body weight, about
500 microgram/kg/body weight, about 1 milligram/kg/body weight, about
5 mil 1 i gram/kg/bo dy weight, about 10
milligram/kg/body weight, about
50 milligram/kg/body weight, about 100 mil 1 i gram/kg/bo dy weight, about
200 milligram/kg/body weight, about 350 milligram/kg/body weight, about
500 milligram/kg/body weight, to about 1000 mg/kg/body weight or more per
administration, and any range derivable therein. In non-limiting examples of a
derivable
range from the numbers listed herein, a range of about 5 mg/kg/body weight to
about
100 mg/kg/body weight, about 5 microgram/kg/body weight to about
500 milligram/kg/body weight, etc., can be administered, based on the numbers
described
above.
In certain embodiments, a pharmaceutical composition of the present disclosure
may comprise, for example, at least about 0.01% of RTA 408. In other
embodiments,
RTA 408 may comprise between about 0.01% to about 75% of the weight of the
unit, or
between about 0.01% to about 5%, for example, and any range derivable therein.
In
some embodiments, RTA 408 may be used in a formulation such as a suspension in
sesame oil of 0.01, 0.1, or 1%.
62
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Single or multiple doses of the agent comprising RTA 408 are contemplated.
Desired time intervals for delivery of multiple doses can be determined by one
of
ordinary skill in the art employing no more than routine experimentation. As
an example,
patients may be administered two doses daily at approximately 12 hour
intervals. In
some embodiments, the agent is administered once a day. The agent(s) may be
administered on a routine schedule. As used herein a routine schedule refers
to a
predetermined designated period of time. The routine schedule may encompass
periods
of time that are identical or that differ in length, as long as the schedule
is predetermined.
For instance, the routine schedule may involve administration twice a day,
every day,
every two days, every three days, every four days, every five days, every six
days, a
weekly basis, a monthly basis, or any set number of days or weeks there-
between.
Alternatively, the predetermined routine schedule may involve administration
on a twice
daily basis for the first week, followed by a daily basis for several months,
etc. In other
embodiments, the invention provides that the agent(s) may be taken orally and
that the
timing of which is or is not dependent upon food intake. Thus, for example,
the agent
can be taken every morning and/or every evening, regardless of when the
patient has
eaten or will eat.
VI. Examples
The following examples are included to demonstrate preferred embodiments of
the invention. It should be appreciated by those of skill in the art that the
techniques
disclosed in the examples which follow represent techniques discovered by the
inventor
to function well in the practice of the invention, and thus can be considered
to constitute
preferred modes for its practice. However, those of skill in the art should,
in light of the
present disclosure, appreciate that many changes can be made in the specific
embodiments which are disclosed and still obtain a like or similar result
without
departing from the spirit and scope of the invention.
63
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
A. Synthesis of RTA 408 (63415)
0
OH N a
E
NC 0
RTA 401 1 2
0 0 0
N)-yCH3
NC NH2 d NC H F F
171
3 RTA 408
Reagents and conditions: (a) (Ph0)2P0N3 (DPPA), triethylamine, toluene, 0 C
for 5
minutes, then ambient temperature overnight, ¨94%; (b) benzene, 80 C for 2
hours; (c)
HC1, CH3CN, ambient temperature for 1 hour; (d) CH3CF2CO2H,
dicyclohexylcarbodiimide, 4-(dimethylamino)pyridine, CH2C12, ambient
temperature
overnight, 73% from RTA 401 (4 steps).
Compound 1: RTA 401 (20.0 g, 40.6 mmol), triethylamine (17.0 nit, 122.0
mmol), and toluene (400 mL) were added into a reactor and cooled to 0 C with
stirring.
Diphenyl phosphoryl azide (DPPA) (13.2 mL, 61.0 mmol) was added with stirring
at 0
C over 5 minutes, and the mixture was continually stirred at room temperature
overnight
(HPLC-MS check shows no RTA 401 left). The reaction mixture was directly
loaded on
a silica gel column and purified by column chromatography (silica gel, 0% to
5% ethyl
acetate in CH2C12) to give compound 1 (19.7 g, ¨94%, partially converted into
compound
2) as a white foam.
Compound 2: Compound 1 (19.7 g, ¨38.1 mmol) and benzene (250 mL) were
added into a reactor and heated to 80 C with stirring for 2 hours (HPLC-MS
check
shows no compound 1 left). The reaction mixture was concentrated at reduced
pressure
to afford crude compound 2 as a solid residue, which was used for the next
step without
purification.
64
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Compound 3: Crude compound 2 (<38.1 mmol) and CH;CN (200 mL) were
added into a reactor and cooled to 0 C with stirring. HC1 (12 N, 90 mL) was
added at
0 C over 1 minute, and the mixture was continually stirred at room
temperature for 1
hour (HPLC-MS check shows no compound 2 left). The reaction mixture was cooled
to
0 C and 10% NaOH (-500 mL) was added with stirring. Then, saturated NaHCO3 (1
L)
was added with stirring. The aqueous phase was extracted by ethyl acetate
(2x500 mL).
The combined organic phase was washed by H20 (200 mL), saturated NaCl (200
mL),
dried over Na2SO4, and concentrated to afford crude compound 3 (16.62 g) as a
light
yellow foam, which was used for the next step without purification.
RTA 408: Crude amine 3 (16.62 g, 35.9 mmol), CH3CF2CO2H (4.7388 g, 43.1
mmol), and CH2C12 (360 mL) were added into a reactor with stirring at room
temperature. Then, dicyclohexylcarbodiimide (DCC) (11.129 g, 53.9 mmol) and 4-
(dimethylamino)pyridine (DMAF') (1.65 g, 13.64 mmol) were added and the
mixture was
continually stirred at room temperature overnight (HPLC-MS check shows no
compound
3 left). The reaction mixture was filtered to remove solid by-products, and
the filtrate
was directly loaded on a silica gel column and purified by column
chromatography (silica
gel, 0% to 20% ethyl acetate in hexanes) twice to give compound RTA 408
(16.347 g,
73% from RTA 401 over 4 steps) as a white foam: 11-1 NMR (400 MHz, CD3C1) 6
ppm
8.04 (s, 1H), 6.00 (s, 1H), 5.94 (s, br, 1H), 3.01 (d, 1H, J= 4.8 Hz), 2.75-
2.82 (m, 1H),
1.92-2.18 (m, 4H), 1.69-1.85 (m, 7H), 1.53-1.64 (m, 1H), 1.60 (s, 3H), 1.50
(s, 3H), 1.42
(s, 3H), 1.11-1.38 (m, 3H), 1.27 (s, 3H), 1.18 (s, 3H), 1.06 (s, 3H), 1.04 (s,
3H), 0.92 (s,
3H); miz 555 (M+1).
B. Pharmacodynamics
A summary of the in vitro and in vivo studies to evaluate the primary
pharmacodynamic effects of RTA 408 is provided below.
1. Effects of RTA 408 on Keapl-Nrf2 and NF-KB in Vitro
Inhibition of IFNy-induced NO production by AIMs is Nrf2-dependent
(Dinkova-Kostova, 2005). RAW264.7 mouse macrophages were pre-treated with
dimethyl sulfoxide (vehicle) or RTA 408 for 2 hours, followed by treatment
with 20
ng/mL of mouse IFNy for 24 hours. Nitrite (NO2-) levels in the media were
measured as
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
a surrogate for nitric oxide using the Griess reagent assay. Cell viability
was assessed
using the WST-1 assay. Treatment with RTA 408 resulted in a dose-dependent
suppression of IFNy-induced NO production, with an average 1050 value of 3.8
1.2 nM.
Results from a representative experiment are shown in FIG. 1. The IC50 value
for RTA
408 was found 45%-65% lower than the IC50 values for compounds 63170 (8 3
nM),
63171 (6.9 0.6 nM), 63179 (11 2 nm), and 63189 (7 2 nM). 63170, 63171,
63179,
and 63189 are compounds of the formulas:
0
0
NC
z
0
63170
0 0
NC 111)1<F
0
63171
0
0 F
N'j<F
NC
0
63179
0 0
N)
NC
0
63189.
66
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
2. Effect of RTA 408 on Nrf2 Target Genes
RTA 408 was tested in two different reporter assays to assess activation of
the
antioxidant response element (ARE). The first reporter tested was controlled
by an ARE
derived from the human NQ01 gene. The HuH-7 human hepatoma cell line was
transiently transfected with an NQ01-ARE luciferase reporter plasmid, and
cells were
treated with RTA 408 for 18 hours. FIG. 2a shows a dose-dependent induction of
luciferase activity by RTA 408 in this cell line. Values represent the average
of three
independent experiments. Twenty percent less RTA 408 (12 nM) than 63189 (14.9
nM)
was required to increase transcription from the NQ01 ARE in HuH-7 cells by 2-
fold.
Likewise, 2.1-2.4 fold less RTA 408 than 63170 (25.2 nM) and 63179 (29.1 nM),
respectively, was required to increase transcription from the NQ01 ARE in HuH-
7 cells
by 2-fold. The effect of RTA 408 on luciferase reporter activation was also
assessed in
the AREc32 reporter cell line. This cell line is derived from human breast
carcinoma
MCF-7 cells and is stably transfected with a luciferase reporter gene under
the
transcriptional control of eight copies of the rat GSTA2 ARE sequence.
Following
treatment with RTA 408 for 18 hours, a similar dose-dependent response was
observed in
the AREc32 reporter cell line (FIG. 2b). An ¨2-fold induction of luciferase
activity was
evident following treatment with 15.6 nM RTA 408 in both reporter assays.
RTA 408 was also shown to increase transcript levels of known Nrf2 target
genes
in the HFL1 human lung fibroblast and BEAS-2B human bronchial epithelial cell
lines.
Treatment of HFL1 lung fibroblasts with RTA 408 for 18 hours resulted in
increased
expression of several Nrf2 target genes, including NQ01, HMOX1, GCLM, and
TXNRD1, as measured by quantitative PCR (FIGS. 3a¨d). For all genes tested,
induction by RTA 408 was dose-dependent and evident at concentrations as low
as
15.6 nM. Treatment of BEAS-2B bronchial epithelial cells with RTA 408 for 18
hours
resulted in a similar dose-dependent increase of all Nrf2 target genes
evaluated
(FIGS. 4a¨d). RTA 408 also increased expression of Nrf2 target genes in normal
human
mesangial cells (nHMC), the mouse BV2 microglial cell line, and the human SH-
SY5Y
neuroblastoma cell line at similar concentrations.
Treatment with RTA 408 also increased NQ01 protein levels in SH-SY5Y cells
in a dose-dependent manner (FIG. 5a). HMOX1 protein was not detected in
untreated or
67
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
RTA 408-treated SH-SY5Y cells. In BV2 cells, treatment with RTA 408 increased
NQ01 and HMOX1 protein levels at concentrations up to 125 nM (FIG. 5b). The
EC's()
value for induction of Nrf2 protein expression in SK-N-SH cells by RTA 408
(56.4 nM)
was 45%-65% lower than the EC50 values for 63171 (122 nM), 63189 (102 nM), and
63179 (126 nM). The same amount of 63170 (54.6 nM) was required.
The EC50 was measured using an in-cell western NQ01 assay where the cells
were incubated with the compound under evaluation for 3 days. After incubation
with
the compound of interest, the cells were reacted with mouse NQ01 antibody and
then the
next day the cells were reacted with IRDye-800CW-anti-mouse IgG antibody. The
target
signals were visualized and then analyzed.
Consistent with induction of Nrf2 target genes and corresponding protein
products, treatment of RAW264.7 mouse macrophage cells for 24 hours increased
NQ01
enzymatic activity in a dose-dependent manner, with increases evident at 7.8
nM
(FIG. 6).
Taken together, these data from multiple cell lines demonstrate that treatment
with RTA 408 increases transcriptional activity controlled by antioxidant
response
elements, increases expression of Nrf2 target genes, and increases the
activity of NQ01,
an Nrf2 target gene product.
3. Effect of RTA 408 on Markers of Cellular Redox Capacity
Glutathione and NADPH are critical factors required for the maintenance of
cellular redox capacity. Several genes involved in the synthesis of
glutathionc (e.g.,
GCLC and GLCM) and NADPH [e.g., hexose-6-phosphate dehydrogenase (H6PD) and
malic enzyme 1 (MEI)] have been demonstrated to be regulated by Nrf2 (Wu,
2011).
The effect of RTA 408 treatment on total glutathione levels was evaluated in
the mouse
AML-12 hepatocyte cell line. Treatment of AML-12 cells for 24 hours with RTA
408
increased total cellular glutathione levels in a dose-dependent manner (FIG.
7). Data
shown are representative of two independent experiments. A >2-fold increase in
total
glutathione was observed at RTA 408 concentrations as low as 15.6 nM. The EC50
value
using a RAW264.7 mouse model for induction of glutathione levels by RTA 408
(9.9 nM) was 22%-57% lower than the EC50 values for 63170 (12.1 nM), 63171
(23.2 nM), and 63189 (16 nM).
68
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
The effect of RTA 408 treatment on the levels of NADPH, as measured by the
absorbance of a redox-sensitive dye, WST-1, was evaluated in HCT-116 cells.
RTA 408
treatment for 24 hours increased WST-1 absorbance in a dose-dependent manner
(FIG.
8), suggesting that NADPH levels were increased.
The effect of RTA 408 on the expression of genes involved in NADPH synthesis
pathways was also evaluated in this study. HCT-116 cells were treated with RTA
408 for
24 hours, and mRNA levels of H6PD, phosphogluconate dehydrogenase (PGD),
transketolase (TKT), and ME1 were measured using quantitative PCR. Treatment
with
RTA 408 resulted in a dose-dependent increase in expression of genes involved
in
NADPH synthesis (FIGS. 9a¨d).
In summary, treatment with RTA 408 increased total glutathione levels in AML-
12 hepatocytes and increased WST-1 absorbance, a marker of NADPH production,
in
HCT-116 cells. This observation correlated with an increase in the expression
of several
key genes encoding enzymes involved in NADPH synthesis.
4. Effect of RTA 408 on TNFa-induced NF-KB Signaling
NF-KB is a transcription factor that plays a central role in the regulation of
many
immune and inflammatory responses. RTA 402 and other AIMs have been shown to
inhibit pro-inflammatory NF-KB signaling in a variety of cell lines
(Shishodia, 2006;
Ahmad, 2006; Yore, 2006). The effect of RTA 408 on TNFa-induced NF-KB
signaling
was evaluated in HeLa/NF-KB-Luc cells, a human cervical adenocarcinoma cell
line
stably transfected with a luciferase reporter construct under the control of
multiple NF-KB
transcriptional response elements. HeLa/NF-KB-Luc cells were pretreated for 1
hour
with RTA 408, followed by treatment with TNFa (10 ng/mL) for an additional 5
hours.
After treatment, luminescence was measured, and the effect of RTA 408
pretreatment on
TNFa-induced luciferase activity was determined. The average results and
standard
deviations from three independent experiments are shown in FIG. 10. RTA 408
dose-
dependently inhibited TNFa-induced NF-KB activation with an IC50 value of
517 83 nM. Similar results were observed in another NF-KB reporter cell line
(A549/NF-KB-Luc) where RTA 408 inhibited TNFa-induced NF-KB activation with an
.. IC50 value of 627 nM (range 614-649 nM). RTA 408 was 1.6-1.8 fold more
efficient at
69
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
reducing expression from the NF-KB promoter reporter in HeLa/NF-KB-Luc cells
than
63189 (854 nM) and 63170 (953 nM), respectively.
The effect of RTA 408 on TNFa-induced phosphorylation of IxBa, a key step in
activation of the NF-KB pathway, was also evaluated in HeLa cells. HeLa cells
were
pretreated with RTA 408 for 6 hours, followed by treatment with TNFa (20
ng/mL) for 5
min. Total and phosphorylated levels of IxBa were evaluated by Western blot.
Consistent with the results from the luciferase reporter assay, RTA 408
inhibited TNFa-
induced phosphorylation of Mk( in a dose-dependent manner (FIG. 11).
RTA 408 has also been demonstrated to inhibit other pro-inflammatory signaling
pathways, such as IL-6-induced signal transducer and activator of
transcription 3
(STAT3) phosphorylation and receptor activator of NF-KB ligand (RANKL)-induced
osteoclastogenesis. In HeLa cells, pretreatment with 1 ,tA4 RTA 408 for 6
hours
inhibited phosphorylation of STAT3 induced by IL-6. Osteoclastogenesis is a
multi-step
differentiation process that results from the binding of RANKL to its
receptor, RANK, on
cells of hematopoietic origin. This results in the activation of NF-KB and
MAPK, which
in turn increase transcription of osteoclast-specific target genes, including
tartrate-
resistant acid phosphatase (TRAP). The effect of RTA 408 on RANKL-induced
osteoclastogenesis was evaluated in the mouse macrophage cell line RAW264.7.
RAW264.7 cells were pretreated for 2 hours with RTA 408 and then treated with
50
ng/mL recombinant mouse RANKL. RTA 408 dose-dependently inhibited RANKL-
induced TRAP activity and the formation of osteoelasts, with an IC50 of ¨5-10
nM.
5. Effect of RTA 408 on Expression of Genes Encoding
Transaminase Enzymes
Transaminase elevations were observed in the 28-day toxicity studies with
RTA 408 in rats and, to a much lower extent, in monkeys. Similar findings have
been
observed following oral administration of a related AIM (bardoxolone methyl)
in humans
(Pergola, 2011). One hypothesis for this effect is that AIMs directly or
indirectly
increase transaminase gene expression in the absence of cellular toxicity. To
assess
whether treatment with RTA 408 affects transaminase mRNA levels, mouse AML-12
hepatocytes were treated with RTA 408 for 18 hours, and the mRNA levels of
genes
encoding transaminases were measured using quantitative PCR. Treatment with
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
RTA 408 increased mRNA levels of alanine transaminase 1 (Altl or Gptl) and
aspartate
transaminase 1 (Astl or Gotl) (FIGS. 12a,c). RTA 408 had no effect on alanine
transaminase 2 (Alt2 or Gpt2) mRNA levels and reduced mRNA levels of aspartate
transaminase 2 (Ast2 or Got2) (FIGS. 12b,d). These results demonstrate that
RTA 408,
at the concentrations tested (250 nM or 500 nM), affects transaminase gene
expression in
vitro in a manner consistent with the effects of other compounds in the AIM
class.
However, it is unclear how the results from this in vitro system at the RTA
408
concentrations tested relate to the potential effects on transaminases at
clinically-relevant
dose levels in humans.
6. Effect of RTA 408 on Levels of Glycolytic Intermediates
Studies in diabetic mice have demonstrated that bardoxolone methyl increases
muscle-specific insulin-stimulated glucose uptake (Saha, 2010). In humans, a
higher
percentage of patients receiving bardoxolone methyl reported experiencing
muscle
cramps compared with patients receiving placebo (Pergola, 2011). Muscle spasms
have
also been reported in diabetic patients following insulin administration,
suggesting a
possible association with muscle glucose metabolism. The effect of RTA 408 on
glycolytic metabolism was evaluated through the assessment of lactate and
pyruvate
levels in cultured rodent C2C12 muscle cells. Similar to treatment with
insulin, treatment
of differentiated C2C12 myotubes with 1 uM or 2 iLtM RTA 408 for 3 hours
significantly
increased intracellular and extracellular lactate levels in a dose-dependent
manner.
Treatment of C2C12 differentiated myotubes with 250 nM or 500 nM RTA 408
for 18 hours also significantly (P < 0.0001, noted by asterisks) increased
intracellular
pyruvate levels in a dose-dependent manner (FIG. 13). Together, these results
demonstrate that RTA 408, at the concentrations tested, can affect muscle
glycolytic
intermediates in vitro; however, it is unclear how the results from this in
vitro system at
the RTA 408 concentrations tested relate to the potential effects on glucose
metabolism at
clinically-relevant dose levels in humans.
7. In Vitro Evaluation of RTA 408 Efflux by MRP-1
The efflux ratio MRP-1 for RTA 408 (1.3) was experimentally determined to be
approximately ten-fold lower than 63170 (10) and 63171 (11.2) and over 40-fold
lower
71
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
than 63189 (57.1). The value determined for RTA 408 indicates that it is not a
substrate
of MRP-1, whereas the other compounds are.
C. Protective Effects of RTA 408 in Animal Models of Lung Disease
RTA 408 was tested in several animal models of pulmonary disease to evaluate
its
potential efficacy in the lung. For all studies, RTA 408 was orally
administered daily in
sesame oil at dose levels in the range of 3 to 150 mg/kg. In most cases, RTA
408 was
administered starting several days prior to the induction of the lung injury
response.
1. LPS-induced Pulmonary Inflammation in Mice
RTA 408 was tested in two studies of LPS-induced pulmonary inflammation in
mice. In the first study, intended to be a preliminary dose-range finder, RTA
408 (30,
100, or 150 mg/kg) was administered once daily for 3 days, followed by LPS
administration 1 hour after the final dose. Bronchoalveolar lavage fluid
(BALF) was
collected 20 hours after LPS administration (21 hours after the final dose of
RTA 408)
and evaluated for levels of pro-inflammatory markers (i.e., IL-6, IL-12p40,
TNF-a, and
RANTES). RTA 408 treatment resulted in a significant reduction in IL-12p40 at
all
doses and in TNFa at the 100 and 150 mg/kg doses (FIG. 14). In the second
study,
RTA 408 (10, 30, or 100 mg/kg) was administered daily for 6 days, followed by
LPS
administration 1 hour after the final dose. In this study, significant
decreases in body
weight were observed at the 100 mg/kg dose level starting on Day 3.
Significant
reductions in TNFa were observed at the 10 mg/kg dose, and significant
reductions in
IL-12p40, 'TNFa, and RANTES were observed at the 30 mg/kg dose (FIGS. 15a).
Further evaluation of lungs from mice in this study revealed meaningful
engagement of
relevant Nrf2 target genes, including significant induction of NQ01 enzyme
activity and
increases in total GSH at 10 and 30 mg/kg (FIG. 15b).
8. Bleomycin-induced Pulmonary Fibrosis
The effect of RTA 408 was also evaluated in models of bleomycin-induced
pulmonary fibrosis in mice and rats. In the first preliminary study, RTA 408
(10, 30, or
100 mg/kg) was administered to mice daily via oral gavage for 39 days, with
bleomycin
challenge (intranasal) on day 10. On the last day of dosing, lung tissue was
collected and
72
CA 02909066 2015-10-07
WO 2014/176415
PCMJS2014/035279
histology was performed to evaluate the extent of inflammation and
interstitial fibrosis.
In this model, no statistically significant effects were observed at the RTA
408 doses
tested (FIGS. 16a & b). Additional evaluation was performed using a rat model
of
pulmonary fibrosis that has been extensively characterized at the Lovelace
Respiratory
Research Institute. In this study, rats were challenged with bleomycin or
saline by
intratracheal administration on day 0. Following the challenge, animals
received
RTA 408 (3, 10, or 30 mg/kg) daily via oral gavage for 28 days. Administration
of the
30-mg/kg dose was stopped on day 14 due to excessive dehydration and diarrhea
in the
animals. For the remaining animals, bronchoalveolar lavage fluid was collected
on
day 28 for assessment of pro-inflammatory infiltrates, and lung tissue was
analyzed for
hydroxyproline levels and histopathology. Challenge with bleomycin sulfate
induced a
substantial release of neutrophils and an increase in soluble collagen in the
BALF, as well
as an increase in hydroxyproline in the lung. Treatment with 3 and 10 mg/kg
RTA 408
significantly suppressed polymorphonuclear (PMN) cell infiltration into the
lungs and
also produced a meaningful reduction (-10%-20%) in hydroxyproline deposition
(FIGS. 17a & b).
Importantly, histopathological evaluation revealed a significant decrease in
collagen deposition, as assessed by trichrsome staining, in rats treated with
RTA 408.
Whereas bleomycin control animals primarily exhibited moderate staining,
animals
.. treated with 10 mg/kg RTA 408 had predominantly minimal to mild staining
(Table 2).
Table 2: Effect of RTA 408 on collagen deposition in rat lung as assessed by
intensity of trichrome staining
RTA 408 RTA 408
(10
Staining Intensity' Bleomycin Control
(3 mg/kg) mg/kg)
Minimal 0 0 3
Mild 1 0 4
Moderate 7 7 1
a Values represent intensity of staining in animals with interstitial
trichrome staining in areas of bleomycin-induced lung alterations.
73
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Further evaluation of lungs from rats in this study also revealed meaningful
engagement of relevant Nrf2 target genes (FIG. 18). RTA 408 significantly and
dose-
dependently increased NQ01, Txnrd, Gsr, and Gst enzyme activity in the lungs
of rats
exposed to bleomycin, demonstrating Nrf2 activation by RTA 408 in this disease
setting.
9. Cigarette Smoke-induced COPD in Mice
RTA 408 was also tested in a mouse model of cigarette smoke-induced COPD.
Mice received RTA 408 (3, 10, or 30 mg/kg) daily via oral gavage for two weeks
and
were exposed to cigarette smoke five days per week during the RTA 408 dosing
period.
At the end of the study, lung tissue and BALF were collected for analysis of
inflammatory infiltrates and cytokines. In this experiment, multiple-dose
administration
of RTA 408 at doses as low as 3 mg/kg RTA 408 resulted in significant
suppression of
pro-inflammatory cytokines, including KC (functional mouse homolog of human IL-
8)
and TNFa. A summary of results from this study is presented in FIGS. 19a-e. An
AIM
analog (63355) was tested in the same study for comparison. 63355 is a
compound of the
formula:
0
OH
NC
0 Fi
Further evaluation of lungs from mice in this study also revealed meaningful
engagement of relevant Nrf2 target genes (FIG. 20). NQ01 enzyme activity in
the lung
was significantly decreased by cigarette smoke exposure; administration of RTA
408
rescued this loss. Txnrd enzyme activity was also induced by the 30 mg/kg dose
of RTA
408. In general Gsr enzyme activity was not altered, and Gst enzyme activity
was
decreased with treatment ¨ both of which were likely the consequence of a
temporal
response for these enzymes.
10. Ovalbumin-induced Asthma in Mice
The potential activity of RTA 408 was also evaluated in a pilot study in a
mouse
model of ovalbumin-induced asthma. Mice were sensitized with an IP injection
of
74
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
ovalbumin and aluminum hydroxide on Day 0 and Day 14 and challenged
intranasally
with ovalbumin in saline on Days 14, 25, 26, and 27. Mice received RTA 408 (3,
10, or
30 mg/kg) daily via oral gavage on Days 1-13 and 15-27. Following
sensitization and
challenge with ovalbumin, vehicle-treated mice had a significant increase in
the total
number of leukocytes compared with positive control (dexamethasone)-treated
mice. An
increase in the number of T cells and B cells was also observed in the vehicle-
treated
mice. Treatment with RTA 408 at 30 mg/kg significantly reduced the number and
percentage of B cells within the airways. RTA 408 (3 and 30 mg/kg) also
significantly
reduced the number of macrophages, but not the mean percentage of macrophages,
.. detected in the airways. These observations are suggestive of potential
efficacy in this
model.
11. Effects of RTA 408 on LPS-induced Sepsis in Mice
Sepsis was induced on Day 0 with an IP injection of LPS (21 mg/kg), and
survival
was followed until Day 4. RTA 408 (10, 30, or 100 mg/kg) was administered
daily via
oral gavage from Day -2 to Day 2. In the vehicle control group, 60% of the
animals
survived until Day 4 (higher than the ¨40% survival rate expected in this
model). In the
RTA 408 treatment groups, 80% of the animals in the 10 mg/kg dose group and
90% of
the animals in the 30 mg/kg dose group survived until Day 4 (FIGS. 21c & d).
For the
100 mg/kg dose group, 90% of the animals survived until Day 4, with only a
single death
.. occurring on Day 4. Although these RTA 408-induced effects are indicative
of profound
efficacy in this model, the relatively high survival rate in the vehicle
control group
precluded a statistically-significant difference between the control and RTA
408-treated
groups. Results obtained using the compound RTA 405 are also presented
(FIGS. 21a & b). RTA 405 is a compound of the formula:
0
NC 0
0
Fi
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
12. Effects of RTA 408 against Radiation-Induced Oral Mucositis
Exposure to acute radiation directed to the buccal cheek pouch of hamsters
produces effects similar to those observed in oral ulcerative mucositis in
humans. These
effects include moderate to severe mucositis characterized by severe erythema
and
vasodilation, erosion of the superficial mucosa, and formation of ulcers. A
single study
was conducted to evaluate the effects of RTA 408 in this model. On Day 0, each
hamster
was given an acute radiation dose of 40 Gy directed to the left buccal cheek
pouch.
RTA 408 (10, 30, or 100 mg/kg) was orally administered twice daily from Day -5
to
Day -1, and Day 1 to Day 15. Beginning on Day 6 and continuing until Day 28 on
alternate days, oral mucositis was evaluated using a standard 6-point scoring
scale. Both
the 30 and 100 mg/kg doses of RTA 408 caused a significant reduction in the
duration of
ulcerative mucositis (FIG. 22). Furthermore, a dose-dependent decrease in
the
percentage of animals with mucositis scores >3 was also observed. However,
administration of RTA 408 at 30 or 100 mg/kg caused significant dose-dependent
reductions in weight gain in irradiated hamsters. Due to weight loss in excess
of 20%,
two out of eight hamsters in the 100 mg/kg dose group were euthanized on Day
2.
13. Effect of RTA 408 on the Induction of Nrf2 Biomarkers in Vivo
As described above, a key molecular target of RTA 408 is Nrf2, a central
transcriptional regulator of antioxidative cellular protection. Activation of
Nrf2 induces
upregulation of a battery of cytoprotective genes, including NQ01, enzymes
involved in
GSH synthesis [i.e., glutamate-cysteine ligase catalytic and modifier subunits
(Gcic and
Gclm)], enzymes involved in detoxification (i.e., glutathione S-transferases
[Gsts]), and
efflux transporters [i.e., multidrug resistance¨associated proteins (Mrps)].
Induction of
these genes results in a coordinated cellular effort to protect against
oxidative insult,
highlighted by increased antioxidative capacity, induction of glutathione
synthesis, and
conjugation and export of potentially harmful molecules from the cell. In
addition to the
efficacy endpoints and Nrf2 target gene expression evaluated in the various
animal
models described above, the ability of RTA 408 to induce expression of Nrf2
target genes
was also assessed using tissues collected from healthy RTA 408-treated mice,
rats, and
monkeys.
76
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
As part of the non-GLP 14-day toxicity studies of RTA 408 in mice, rats, and
monkeys, tissues were collected for the purposes of measuring mRNA and enzyme
activity levels of selected Nrf2 target genes. For mice and rats, liver
samples were
collected 4 hours after the final dose on Day 14. For monkeys, blood (for PBMC
isolation), liver, lung, and brain tissue were collected 24 hours after the
final dose on Day
14. Enzyme activity for NQ01, Gst, and glutathione reductase (Gsr) were
measured in
tissue homogenates. Levels of mRNA were determined using Quantigene Plex 2.0
technology, which involves a hybridization-based assay using xMAP Luminex
magnetic beads for direct quantification of mRNA targets. In addition, RTA 408
concentrations were measured in plasma and tissues by LC/MS/MS methods.
RTA 408 generally increased the expression of various Nrf2 target genes in a
dose-dependent manner at doses of 10, 30, and 100 mg/kg (FIG. 23, FIG. 24a,
FIGS. 25a & b). Transcriptional upregulation of Nrf2 target genes by RTA 408
also
resulted in functional increases in the antioxidant response, as manifested by
dose-
dependent increases in NQ01, Gst, and Gsr enzyme activity in rodent liver, as
well as
monkey liver and lung (FIGS. 26a & b, FIGS. 27a & b, FIGS. 28a & b).
Furthermore, in
rodents liver exposure of RTA 408 correlated with the level of enzyme activity
of NQ01,
the prototypical target gene for Nrf2 (FIG. 29b, FIG. 30b). In monkeys, the
level of
mRNA expression in PBMCs of both NQ01 and sulfiredoxin 1 (SRXN1) correlated
with
plasma exposure to RTA 408 (FIGS. 34a & b). Overall, RTA 408 increased mRNA
levels and activity of Nrf2 targets, and such increases generally correlated
with tissue and
plasma exposures, suggesting Nrf2 targets may serve as feasible biomarkers for
Nrf2
activation (FIGS. 31a & b) and may be useful for assessing pharmacological
activity of
RTA 408 in healthy human subjects.
D. Safety Pharmacology
A GLP-compliant safety pharmacology program was completed using RTA 408.
This included in vitro and in vivo (monkey) studies on the cardiovascular
system, as well
as studies on the respiratory system and central nervous system in rats.
77
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
2. Evaluation of the Effects of RTA 408 on Cloned hERG Channels
Expressed in HEK293 cells
This study was conducted to assess the effects of RTA 408 on the rapidly
activating inward rectifying potassium current (kr) conducted by hERG (human
ether-a-
go-go-related gene) channels stably expressed in the human embryonic kidney
(HEK293)
cell line. The effects of RTA 408 on the hERG-related potassium current were
assessed
using whole-cell patch clamp electrophysiology methods. RTA 408 was determined
to
have IC50 value of 12.4 uM in a hERG QPatch_Kv11.1 assay. This value was 2.5-3
fold
higher than the values for 63170 (4.9 uM) and 63189 (3.8 uM), respectively.
The RTA
408 IC50 value was similar to the 63171 value (15.7 uM).
3. Cardiovascular Evaluation of RTA 408 in the Cynomolgus
Monkey
A single study was conducted to evaluate the potential cardiovascular effects
of
RTA 408 in conscious freely moving cynomolgus monkeys. The same four male and
four female cynomolgus monkeys were administered the vehicle (sesame oil) and
RTA 408 at dose levels of 10, 30, and 100 mg/kg according to a Latin square
design, with
one animal/sex/treatment dosed each week followed by a 14-day washout period
between
administrations, until each animal received all treatments. Vehicle and RTA
408 were
administered to all animals via oral gavage at a dose volume of 5 mL/kg.
Animals were instrumented with telemetry transmitters for measurement of body
temperature, blood pressure, heart rate, and electrocardiogram (ECG)
evaluation. Body
temperature, systolic, diastolic, and mean arterial blood pressure, heart
rate, and ECG
parameters (QRS duration and RR, PR, and QT intervals) were monitored
continuously
from at least 2 hours pre-dose until at least 24 hours post-dose. ECG tracings
were
printed at designated time points from the cardiovascular monitoring data and
were
qualitatively evaluated by a board-certified veterinary cardiologist. Prior to
the first
administration on study, untreated animals were continuously monitored for
cardiovascular endpoints for at least 24 hours, and these data were used in
the calculation
of the corrected QT interval throughout the study.
Observations for morbidity, mortality, injury, and availability of food and
water
were conducted at least twice daily for all animals. Clinical observations
were conducted
78
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
pre-dose, approximately 4 hours post-dose, and following completion of the
cardiovascular monitoring period. Body weights were measured and recorded on
the day
prior to each treatment administration.
RTA 408 at dose levels of 10, 30, and 100 mg/kg did not produce mortality,
adverse clinical signs, or result in meaningful changes in body weight (FIG.
32), body
temperature, blood pressure, or qualitative or quantitative (PR, RR, QRS, QT
intervals)
ECG parameters. In the 100 mg,/kg dose group, a small (1.6% on average) but
statistically significant increase in the corrected QT interval was observed;
however,
individual animal data did not show consistent increases in QTc that would
indicate a test
article related effect. Consequently, due to the small magnitude of change and
lack of a
consistent response in individual animals, these slight increases in QTc were
not
considered to be related to RTA 408 treatment. Therefore, oral administration
of
RTA 408 produced no effects on cardiovascular function in cynomolgus monkeys
at
doses up to and including 100 mg/kg.
4. Neurobehavioral Evaluation of RTA 408 in Rats
The potential acute neurobehavioral toxicity of RTA 408 was evaluated in rats.
Three treatment groups of 10 male and 10 female CD [Crl:CD (SD)] rats
received
RTA 408 at dose levels of 3, 10, or 30 mg/kg. One additional group of 10
animals/sex
served as the control and received vehicle (sesame oil). Vehicle or RTA 408
was
administered to all groups via oral gavage once on Day 1 at a dose volume of
10 mL/kg.
Observations for morbidity, mortality, injury, and availability of food and
water
were conducted twice daily for all animals. Observations for clinical signs
were
conducted prior to dosing on Day 1 and following each functional observational
battery
(FOB) evaluation. FOB evaluations were conducted pre-dose (Day -1) and at
approximately 4 and 24 hours post-dose. Body weights were measured and
recorded pre-
dose on Day 1.
RTA 408 at doses of 3, 10, and 30 mg/kg did not produce mortality, adverse
clinical observations, or effects on any of the neurobehavioral measures
tested. Slight
decreases in body weight gain were observed approximately 24 hours after
dosing in the
30 mg/kg group that may potentially be test article-related. With respect to
the basic
79
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
neurobehavioral endpoints evaluated in this study, RTA 408 did not produce any
adverse
effects in rats at doses up to and including 30 mg/kg.
5. Pulmonary Evaluation of RTA 408 in Rats
The potential effect of RTA 408 on pulmonary function was evaluated in rats.
Three treatment groups of eight male and eight female CD [Crl:CD (SD)] rats
received
RTA 408 at dose levels of 3, 10, or 30 mg/kg. One additional group of eight
animals/sex
served as the control and received vehicle (sesame oil). Vehicle or RTA 408
was
administered to all groups via oral gavage once on Day 1 at a dose volume of
10 mL/kg.
Observations for mortality, morbidity, injury, and availability of food and
water
were conducted twice daily for all animals. Clinical observations were
conducted prior to
dosing, approximately 4 hours post-dose, and following completion of the 8-
hour
pulmonary monitoring period. Body weights were measured and recorded on the
day of
RTA 408 administration. Pulmonary function (respiratory rate, tidal volume,
and minute
volume) was monitored for at least 1 hour prior to dosing to establish a
baseline and for
at least 8 hours post-dose.
RTA 408 at doses of 3, 10, and 30 mg/kg did not produce mortality, adverse
clinical observations, or effects on any of the pulmonary parameters
evaluated.
Therefore, with respect to the basic pulmonary endpoints evaluated in this
study,
RTA 408 did not produce any adverse effects in rats at doses up to and
including
30 mg/kg.
E. Nonclinical Overview
1. Pharmacokinetics
RTA 408 has been investigated both in vitro and in vivo to assess its PK and
metabolism properties. In vitro studies have been conducted to determine RTA
408
plasma protein binding and blood/plasma partitioning, cytochrome P450 (CYP450)
inhibition and induction, and to identify metabolites formed by liver
microsomes of mice,
rats, monkeys, and humans. Data pertaining to the in vivo absorption and
distribution
following repeated administration of RTA 408 has been obtained primarily
through
monitoring of drug levels in plasma and select tissues from toxicology
studies. Sensitive
and selective liquid chromatography-mass spectrometry-based bioanalytical
methods
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
(LC/MS/MS) have been used to measure concentrations of RTA 408 in plasma,
blood,
and tissues with appropriate accuracy and precision.
a. Absorption
The absorption and systemic pharmacokinetic behavior of RTA 408 was studied
in mice, rats, and monkeys following single and repeated (daily) oral
administration.
Following oral administration of a suspension formulation at doses of 10 to
100 mg/kg,
maximal concentrations were observed within 1 to 2 hours in mice, and within 1
to 24
hours in rats and monkeys. Systemic exposure to RTA 408 tended to be highest
in rats,
with lower levels observed in mice and monkeys. Estimates of the apparent
terminal
half-life of RTA 408 observed after oral administration were generally in the
6- to 26-
hour range, though the apparent prolonged absorption phase in some instances
precluded
calculation of a definitive half-life estimate.
Systemic exposure to RTA 408 was generally similar in males and females.
Exposure to RTA 408 following repeated daily oral administration tended to be
slightly
higher (<2-fold) than the exposure observed after a single dose.
Administration of
RTA 408 over a dose range from 3 to 100 mg/kg in a suspension formulation
generally
resulted in dose-proportional increases in systemic exposure. However,
administration of
higher doses (100 to 800 mg/kg in monkeys; 500 to 2000 mg/kg in rats) did not
result in
similar increases in exposure, suggesting saturation of absorption at doses
above
100 mg/kg. Following oral administration of an unoptimized (loose-filled)
capsule
formulation of RTA 408 (3 mg/kg) to monkeys, dose-normalized systemic exposure
tended to be somewhat lower than that observed with a suspension formulation.
The absorption and systemic pharmacokinetic behavior of RTA 408 was studied
in rats using single and repeated topical administration. The administration
of RTA 408
over a range of 0.01 to 3% showed lower plasma concentrations relative to
similar oral
dosing. The systemic exposure to RTA 408 generally increased in a dose
dependent
manner. The topical administration was formulated as a suspension in sesame
oil.
Using rabbits, the ocular absorption and systemic pharmacokinetic behavior of
RTA 408 was evaluated. RTA 408 was administered topically to the eye once per
day
for 5 days. The ocular administration showed lower plasma concentration of RTA
408
relative to when RTA 408 is administered orally (FIG 33). The amount of RTA
408 in
81
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
the plasma even after five consecutive days showed only a small change
compared to the
concentration after the first dose relative to when RTA 408 was administered
orally,
where plasma concentrations were almost 100 fold higher (FIG. 33).
b. Distribution
Plasma protein binding of RTA 408 was evaluated in mouse, rat, rabbit, dog,
minipig, monkey, and human plasma at RTA 408 concentrations of 10-2000 ng/mL
using
ultracentrifugation methodology. RTA 408 was extensively bound to plasma
proteins.
Plasma protein binding in the nonclinical species ranged from 93% (mouse) to
>99%
(minipig), with binding of 95% in the toxicology species (rat and monkey) and
97% in
human. There was no evidence of concentration-dependent protein binding in any
species tested. Results from blood-to-plasma partitioning experiments indicate
that RTA
408 tended to distribute primarily in the plasma fraction of blood in a linear
manner, with
blood :plasma ratios <1.0 for all species and all concentrations tested.
The distribution of RTA 408 into tissues has been investigated after oral
administration to mice, rats, and monkeys. In the 14-day non-GLP toxicity
studies, select
tissues (liver, lung, and brain) were collected at a single time point (4
hours for rat and
mouse; 24 hours for monkey) after the final dose of the study was administered
and were
analyzed for RTA 408 content using LC/MS/MS. RTA 408 readily distributes into
lung,
liver, and brain. In lung, RTA 408 concentrations at 4 hours in mice and rats
were
similar to or slightly higher (<2-fold) than concentrations in plasma, while
at 24 hours in
monkeys, RTA 408 concentrations in lung were 6- to 16-fold higher than plasma
concentrations. A similar pattern was observed for brain. In contrast, RTA 408
concentrations in liver were 5- to 17-fold higher than plasma for mice and
rats at 4 hours,
and 2- to 5-fold higher than plasma at 24 hours in monkeys.
The pharmacodynamic effects of RTA 408 in tissues were assessed in mice, rats,
and monkeys, by monitoring the induction of Nrf2 target genes in the same
tissues
collected for drug exposure from the 14-day toxicity studies. Induction of
Nrf2 target
genes by RTA 408 resulted in increases in the antioxidant response as
manifested by
dose-dependent increases in NQ01, glutathione S-transferase (Gst), and
glutathione
reductase (Gsr) enzyme activity in the examined tissues. Furthermore, in
rodents, RTA
408 liver content correlated with the level of enzyme activity for NQ01, the
prototypical
82
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
target gene for Nrf2. In monkeys, the level of mRNA expression in peripheral
blood
mononuclear cells (PBMCs) for both NQ01 and sulfiredoxin 1 (SRXN1) correlated
with
plasma exposure of RTA 408 (FIGS. 34a & b). Overall, RTA 408 induced
biomarkers of
Nrf2 in rodents and monkeys, and such inductions generally correlated well
with tissue
and plasma exposure to RTA 408.
When RTA 408 was administered to rabbits via ocular topical administration,
the
highest concentrations of the compound were found in the cornea, retina, or
iris while the
vitreous humor, aqueous humor, and plasma showed significantly lower
concentrations of
RTA 408 (FIG. 35).
c. Metabolism
The metabolism of RTA 408 has been investigated after in vitro incubation of
RTA 408 for 60 minutes with liver microsomes from mice, rats, monkeys, and
humans in
the presence of a nicotinamide adenine dinucleotide phosphate (NADPH)-
regenerating
system and a uridine diphosphate glucuronosyltransferase (UGT) reaction
mixture.
Extensive turnover of RTA 408 was observed with primate microsomes, with <10%
of
the parent molecule remaining at the end of the 60-minute incubation in both
monkey and
human microsomes. In contrast, the extent of metabolism was lower in rodent
microsomes, with >65% of the parent molecule remaining at the end of the
incubation.
The lack of available authentic standards for the various potential
metabolites of
RTA 408 precluded quantitative evaluation of the observed metabolites. From a
qualitative perspective, a similar pattern of RTA 408 metabolites was observed
across
species, and included peaks with masses consistent with reduction and
hydroxylation of
RTA 408 as well as glucuronidation of RTA 408 or of its
reduction/hydroxylation
metabolites. No unique human metabolites were observed, with all peaks in the
human
microsome incubations also being observed in one or more of the preclinical
species. In
particular, based on in vitro microsome data, all human metabolites were
present in rat or
monkey, the selected rodent and non-rodent toxicity species.
d. Pharmacokinetic Drug Interactions
The potential for RTA 408 to inhibit cytochrome P450 (CYP450)-mediated
metabolism was evaluated using pooled human liver microsomes and standard
substrates
for specific CYP450 enzymes. RTA 408 directly inhibited CYP2C8 and CYP3A4/5
with
83
CA 02909066 2015-10-07
WO 2014/176415
PCMJS2014/035279
K, values of approximately 0.5 uM for each enzyme. No meaningful inhibition
was
observed for the other enzymes tested (CYP1A2, CYP2B6, CYP2C9, CYP2C19, or
CYP2D6), with inhibition <50% at the highest concentration tested (3 [tIVI).
In addition,
there was little or no evidence of metabolism-dependent inhibition of any of
the enzymes
.. tested. Future studies investigating the potential for CYP3A4/5-mediated
drug-drug
interactions may be warranted based on these data, and the potentially high
concentrations that may be achieved locally in the gastrointestinal (GI) tract
after oral
administration.
The potential for RTA 408 to induce CYP450 enzyme expression was evaluated
.. using cultured human hepatocytes. Under conditions where prototypical
inducers caused
the expected increases in CYP activity, RTA 408 (up to 3 04) was not an
inducer of
CYP IA2, CYP2B6, or CYP3A4 enzyme activity in cultured human hepatocytes.
F. Effects of RTA 408 on Acute Radiation Dermatitis
The effects of RTA 408 as a topical or oral preventative for acute radiation
dermatitis have been examined. Using male BALB/c mice, a 30 Gy dose of
radiation
was administered on day 0 (Table 3). The sesame oil vehicle or RTA 408 was
administered to the rats on day -5 to -1 and days 1 to 30. RTA 408 was
administered
both orally in 3, 10, and 30 mg/kg in sesame oil and topically in percentage
composition
of 0.01, 0.1, and 1% in sesame oil. The dermatitis was blindly evaluated every
other day
.. from day 4 to day 30. On day 12, the typical peak of dermatitis was
observed and 4 mice
were sacrificed 4 hours after administration of the dose. The remaining mice
were
sacrificed on day 30 at 4 hours postdose. Plasma was collected on days 12 and
30 as well
as irradiated skin samples for mRNA and histological examination.
Table 3: Study Design for Acute Radiation Dermatitis Model
Number of Radiation
Group Treatment Treatment
Schedule
Animals (Day 0)
1 9 males Untreated
2 10 males 30 Gy Untreated
Vehicle Control
3 14 males 30 Gy Day -5 to -1 & Day 1 to 30
(sesame oil)
84
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Number of Radiation
Group Treatment Treatment
Schedule
Animals (Day 0)
RTA 408 ¨ 0.01% or
4 14 males 30 Gy Day -5 to -1 & Day 1 to 30
3 mg/kg
RTA 408¨ 0.1% or
14 males 30 Gy Day -5 to -1 & Day 1 to 30
mg/kg
RTA 408 ¨ 1% or
6 14 males 30 Gy Day -5 to -1 & Day 1 to 30
30 mg/kg
In the test groups where the mice were treated with RTA 408, the incidence of
dermatitis appeared to be slightly diminished in severity when RTA 408 was
given in
either an oral or topical administration (FIGS. 36-39). Furthermore, curves
plotting the
average dermatitis clinic score for the test groups as a function of time show
some change
5 with the administration of RTA 408 either in oral or topical form from
the untreated test
groups (FIGS. 40-42) particularly in the case where RTA 408 was given through
an oral
administration. Furthermore, as can be seen in Tables 4 and 5 below, the
percentage of
mice suffering from dermatitis with a clinical score above 3 was significantly
lower for
mice treated with RTA 408 through an oral administration while the percentage
of mice
10 suffering from dermatitis with a clinical score above 2 was slightly
lower for test groups
who were given a topical administration of RTA 408.
o
Table 4 - Percentage of mice per testing group which scored above 2 in their
clinical dermatitis exam and given a topical t..,
=
treatment containing RTA 408
71
,
-4
Day Day Day Day
Day Day Day Day Day Day % animal- % animal-
c,
12 14 16 18 20 22 24 26
28 30 days >=2 days >=3 -,
'../1
1 no radiation, untreated 0.0 0.0 0.0 0.0 0.0 0.0
0.0 0.0 0.0 0.0 0.0 0.0
2 irradiated, untreated 0.0 50.0 83.3 83.3 83.3
100.0 66.7 50.0 50.0 50.0 35.6 0.0
3 irradiated, sesame oil 21.4 45.0 60.0 50.0 40.0
40.0 0.0 0.0 0.0 0.0 16.6 0.0
4 irradiated, RTA 408- 0.01% 0.0 0.0 20.0
50.0 10.0 40.0 40.0 40.0 20.0 10.0 14.4 0.0
irradiated, RTA 408-0.1% 7.1 10.0 20.0 80.0 60.0 40.0
30.0 10.0 0.0 0.0 16.3 0.0
6 irradiated, RTA 408-1.0% 10.7 20.0 10.0 70.0 30.0
10.0 0.0 0.0 0.0 0.0 9.7 0.0 P
2
g;
co
,,
cs, Table 5 - Percentage of mice per testing group which scored above 3 in
their clinical dermatitis exam and given an oral 0
Q.,
,
treatment containing RTA 408
,
,
,
% animal- % Day 16 16 Day 18 Day 20 Day 22 Day 24 Day 26 Day 28
days >=2
days >=3
1 no radiation, untreated 0 , 0
0 0 0 0 0 0.0 0.0
2 irradiated, untreated 20 40 20 20 20 20
20 39.0 8.8
3 irradiated, sesame oil 35 50 40 30
20 0 0 45.6 10.9
-o
4 irradiated, RTA 408-3 mg/kg 10 10 0 0 0
0 0 32.5 1.3 n
5 irradiated, RTA 408-10 mg/kg 10 25 30 0 0
0 0 33.8 4.1 u)
t.4
=
6 irradiated, RTA 408-30 mg/kg 10 20 10 0 0
0 0 28.8 2.5 .
.P
5
-1-
(.4
ful
tV
-.1
CA 02909066 2015-10-07
WO 2014/176415
PCMJS2014/035279
G. Effects of RTA 408 on Fractionated Radiation Dermatitis
Utilizing RTA 408 through topical administration, the effects of RTA 408
towards ameliorating the effects of fractionated radiation dermatitis were
measured.
Using Balb/c mice, RTA 408 in a topical preparation was administered to the
mice daily
from day -5 to day 30 in three doses ranging from 0.01 to 1%. The mice were
irradiated
on days 0-2 and 5-7 with six 10-Gy doses per day. Clinical dermatitis scores
for the mice
were evaluated blindly every two days from day 4 until the end of the study.
In FIG. 43,
the graph shows the change in the average clinical score for each group were
plotted as a
function of time. The graph shows a statistically significant improvement in
the scores
for mice treated with 0.1 to 1% topical formulations of RTA 408. Study and
treatment
parameters can be found in Table 6.
Table 6: Study Conditions for Fractionated Radiation-Induced Dermatitis
Number of Radiation Treatment
Group Treatment
Animals (Days 0-2, 5-7) Schedule
1 9 males Untreated
2 14 males 6x 10 Gy Untreated
Vehicle Control
3 18 males 6x 10 Gy QD Days
-5 to 30
(sesame oil)
4 18 males 6x 10 Gy RTA 408
¨ 0.01% QD Days -5 to 30
5 18 males 6x 10 Gy RTA 408
¨ 0.1% QD Days -5 to 30
6 18 males 6x 10 Gy RTA
408¨ 1% QD Days -5 to 30
By analyzing the average clinical scores that were shown in FIG. 43, an area
under the
curve (AUC) analysis was performed, which yielded the severity of the
dermatitis
relative to how long the dermatitis persisted. This AUC analysis allowed for
direct
comparison between the different groups of mice and the effect of the
different
percentage compositions of RTA 408 (FIG 44 and Table 7). Administration of
topical
RTA 408 formulations reduced Grade 2 and Grade 3 lesions from 60 % and 33 %
when
the mice were only exposed to the vehicle to 21 % and 6% with RTA 408 at 1 %,
87
CA 02909066 2015-10-07
WO 2014/176415
PCMJS2014/035279
concentration respectively. The other RTA composition showed some activity but
was
not as significant as that shown by the 1 % formulation.
Table 7 ¨ Percentage of Dermatitis Score for Each Treatment Group
Group %Days > 2 %Days > 3
No Rad, No Tx 0% 0%
Rad, No Tx 66% 31%
Rad, Sesame Oil 60% 33%
Rad, RTA 408 (0.01%) 54% 29%
Rad, RTA 408 (0.1%) 40% 13%
Rad, RTA 408 (1%) 21% 6%
H. Effects of RTA 408 on a Model of Ocular Inflammation
A study of the effects of RTA 408 on ocular inflammation was carried out using
rabbits of the New Zealand albino strain. The rabbits were divided into 5
groups of 12
rabbits which were given three different concentrations of RTA 408 (0.01, 0.1,
and 1%),
Voltarene collyre at 0.1% and the vehicle (sesame oil). Each rabbit was given
three
instillations within 60 minutes before induction of paracentesis and two
instillations
within 30 minutes after induction of paracentesis. Each instillation was 50 tL
and given
in both eyes. Aqueous humor for 6 animals per time-point was collected 30
minutes and
again 2 hours after induction of paracentesis. The amount of inflammation was
determined by protein concentration in the aqueous humor. As shown in FIG. 45,
RTA
408 showed a reduction in aqueous humor protein similar to that of the highest
concentration of any of the other reference compounds (MaxiDex or mapracorat)
at only
0.01% RTA 408 in the formulation. The effects of increasing concentration of
RTA 408
appeared to be negligible as all concentrations of RTA 408 appeared to show
relatively
similar effects within error in reducing aqueous humor protein concentration.
88
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
I. Polymorphs of RTA 408
RTA 408 Polymorphic Form A
Example 1: 17 g of RTA 408 was dissolved in 68 g of acetone. 620 g of de-
ionized water was added to a 500 mL jacketed reactor and cooled to 2 C. When
the
water was below 7 C, the RTA 408 solution was added to the reactor via an
addition
funnel. A slurry of solids formed. The slurry was stirred in the reactor with
nitrogen
purge. Solids were isolated using vacuum filtration and dried under vacuum at
room
temperature to give Form A.
Example 2: 300 mg of RTA 408 was dissolved in I mL of ethyl acetate. To the
clear solution, 2 mL of heptane was added. Crystallization occurred within 30
minutes.
The slurry was stirred overnight and the solids were isolated by vacuum
filtration and
dried at ambient temperature for 1 hour. The solids were then dried in a
vacuum oven at
50 C overnight to give Form A.
Powder X-ray diffraction (PXRD) pattern and peak listing with relative
intensities
are shown in Figure 53 and Table 8, respectively. Differential scanning
calorimetry
(DSC) and thermogravimetric analysis with mass spectroscopy (TGA-MS) are shown
in
Figures 54 and 55, respectively.
The DSC of Form A indicated an essentially solvent free form with a melting
point of 181.98 C and enthalpy of fusion of 42.01 J/g. The TGA-MS of Form A
shows
the loss of ¨0.5 wt.-% with traces of H20 between 25 and 200 C, predominantly
above
160 C, indicating that RTA 408 Polymorphic Form A may be slightly
hygroscopic.
30
89
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
Table 8: Peak Listing of RTA 408 Form A
Peak Position ( 20) Relative Intensity
10.601 11.0
11.638 7.1
12.121 4.6
13.021 10.9
13.435 100.0
15.418 12.7
15.760 5.9
17.830 19.7
18.753 38.3
19.671 7.5
RTA 408 Polymorphic Form B
Example 3: 1.0 g of RTA 408 was dissolved in 1.5 mL of acetone. In a
scintillation vial, 10 mL of de-ionized water was heated to 50 C and the RTA
408
solution was added to the vial dropwise. Upon stirring for 2 hours, a slurry
of solids
formed. The slurry was then cooled to room temperature. The resulting solids
were
isolated by filtration and dried in a vacuum oven at 50 C overnight to give
Form B.
Example 4: 2.9 g of RTA 408 was dissolved in 20 nit of isopropyl alcohol at
reflux. 20 mL of heptane was added to the solution at reflux. The solution was
cooled to
room temperature and mixed for 1 hour. A slurry of solids formed. The solids
were
isolated by vacuum filtration and dried under vacuum at ambient temperature to
give
Form B.
Powder X-ray diffraction (PXRD) pattern and peak listing with relative
intensities
are shown in Figure 56 and Table 9, respectively. Differential scanning
calorimetry
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
(DSC) and thermogravimetric analysis with mass spectroscopy (TGA-MS) are shown
in
Figures 57 and 58, respectively.
The DSC of Form B indicated an essentially solvent free form with a melting
point of 250.10 C and enthalpy of fusion of 42.01 Eg. The TGA-MS of Form B
shows
the slight loss of ¨0.2 wt.-% with traces of H20 between 25 and 200 C,
indicating that
RTA 408 Polymorphic Form B may be very slightly hygroscopic.
Table 9: Peak Listing of RTA 408 Form B
Peak Position ( 20) Relative Intensity
7.552 4.2
10.339 100.0
11.159 48.4
12.107 80.7
14.729 35.2
15.329 11.4
15.857 8.4
16.824 11.3
17.994 10.9
18.344 4.1
19.444 10.4
19.764 10.2
20.801 5.7
22.414 10.1
J. Instrumental - Typical Measurement Conditions
Powder X-Ray Diffractometry (PXRD)
PXRD data were collected using a G3000 diffractometer (Inel Corp., Artenay,
France) equipped with a curved position sensitive detector and parallel beam
optics. The
diffractometer was operated with a copper anode tube (1.5 kW fine focus) at 40
kV and
30 mA. An incident beam germanium monochromometer provided monochromatic
radiation. The diffractometer was calibrated using the attenuated direct beam
at one-
91
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
degree intervals. Calibration was checked using a silicon powder line position
reference
standard (NIST 640c). The instrument was computer controlled using the
Symphonix
software (Inel Corp., Artenay, France) and the data was analyzed using the
Jade software
(version 9Ø4, Materials Data, Inc., Livermore, CA). The sample was loaded
onto an
aluminum sample holder and leveled with a glass slide.
Thermo Gravimetric Analysis/Mass Spectrometry
The TGA was run with TA instruments, data were collected on a thermal balance
(Q-5000, TA Instruments, New Castle, DE) equipped with a data analyzer
(Universal
Analysis 2000, version 4.5A, TA Instruments, New Castle, DE). During
experiments, the
furnace was purged with nitrogen at 60 mL/minute, while the balance chamber
was
purged at 40 mL/minute. Temperature of the TGA furnace was calibrated using
curie
points of aluminum and nickel. Sample size ranged from 2 to 20 mg, and a
heating rate
of 10 C/minutc was used.
For TGA-MS, the thermogravimetric analysis part was the same as above. The
mass of evolved gas was analyzed with PFEIFFER GSD 301 T3 ThermoStar (PFEIFFER
Vacuum, Asslar, Germany). The instrument was operated and data evaluated with
Software Quadstar 32-bit (V7.01, Inficon, LT-9496 Balzers, Liechtenstein).
Differential Scanning Calorimetery
A DSC (Q-2000, TA Instruments, New Castle, DE) equipped with Universal
Analysis 2000 software (Version 4.5A, TA Instruments, New Castle, DE) was used
to
determine the DSC thermal traces. The temperature axis was calibrated with
biphenyl,
indium, and tin standards. The cell constant was calibrated with indium.
Unless
otherwise stated, the sample (2-5 mg) was encapsulated in a ventilated
aluminum pan,
and heated at a rate of 10 C/minute under a nitrogen gas flow of 50 mL/minute
during
the study.
Abbreviations
Methods:
AUC area under the curve analysis
92
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
DSC differential scanning calorimetry
1H-NMR proton nuclear magnetic resonance spectroscopy
HPLC-MS high-performance liquid chromatography coupled to
mass
spectroscopy
LC/MS/MS liquid chromatography-tandem mass spectrometry
PXRD powder X-ray diffraction
TGA-MS thermogravimetric analysis coupled to mass
spectroscopy
Genes, Proteins, and Biological Parameters:
AIM antioxidant inflammation modulator
ARE antioxidant response element
ALP alkaline phosphatase
ALT alanine transaminase
ARE antioxidant response element
AST asp artate transaminase
AUC area under the curve
BAL bronchoalveolarlavage
BALF bronchoalveolarlavage fluid
COPD chronic obstructive pulmonary disease
COX-2 cyclooxygenase-2
Cr creatine
CYP450 cytochrome P450
Gcic glutamate-cysteine ligase, catalytic subunit
Gclm glutamate-cysteine ligase, modifier subunit
Glu glucose
GOT glutamic-oxaloacetic transaminase
GPT1 glutamic-pyruvate transaminase
GSH glutathione
GSR glutathione reductase
GST glutathione S-transferase
Gy Gray
93
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
H6PD hexose-6-phosphate dehydrogenase
hERG human ether a-go-go-related gene
HMOX1 heme oxygenase (decycling) 1
HO-1 heme oxygenase
IFNy interferon-gamma
IL interleukin
iNOS inducible nitric oxide synthase
IxBa nuclear factor of kappa light polypeptide gene
enhancer in
B-cells inhibitor, alpha
KC mouse IL-8 related protein
Keapl Kelch-like ECH associated protein-1
LPS lipopolysaccharide
ME1 malic enzyme 1
MPCE micronucleated polychromatic erythrocytes
Mrps multidrug resistance-related proteins
NADPH nicotinamide adenine dinucleotide phosphate,
reduced
NF-KB nuclear factor of kappa-light-chain-enhancer of
activated
B cells
NO nitric oxide
NQ01 NAD(P)H quinone oxidoreductase 1
Nrf2 nuclear factor (erythroid-derived)-like 2
p- IKBa phosphorylated IKBa
PBMC peripheral blood mononuclear cell
PCE polychromatic erythrocytes
PGD phosphogluconate dehydrogenase
PMN polymmphonuclear
RANTES regulated and normal T cell expressed and secreted
SOD1 superoxide dismutase 1
S RXN1 sulfiredoxin-1
TG total glycerides
TKT transketolase
94
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
TNFa tumor necrosis factor alpha
TXINRD1 thioredoxin reductase 1
Miscellaneous:
min minute(s)
m.p. melting point
Ph phenyl
temperature
wt.-% weight percent
K. Further Tables
Table 10. Parameters of FIG. 46
Compound NOx Levels (Y0 vs LPS Controls)
13 mg/kg 25 mg/kg 50 mg/kg
RTA 405 * 44% 26% 18%
63415 30% 18% 16%
Table 11. 63415: Primary In Vivo ADMET ¨ Key Primary ADMET Assays and
Endpoints
Assay Key Endpoints
Tolerability, body weight, clinical chemistry
14-day mouse
Tissue distribution
toxicity
Nrf2 target gene mRNA expression & enzyme activation in liver
Tolerability, body weight, clinical chemistry, & limited
histopathology
14-day rat toxicity
Tissue distribution and plasma TK
Nrf2 target gene mRNA expression & enzyme activation in liver
Tolerability, body weight, clinical chemistry, & limited
histopathology
14-day monkey
Tissue distribution and plasma TK
toxicity
Nrf2 target gene mRNA expression and enzyme activation in
multiple tissues & PBMCs
0
Table 12. Parameters of FIG. 49
Vehicle 63415
Dose (mg/kg) 0 10 30
100
ALT (U/L) 100 39 63
91
AST (U/L) 156 98 147
167
ALP (U/L) 120 131 110
98
Tot Bil (mg/dL) <0.2 <0.2 <0.2
<0.2
BUN (mg/dL) 17 15 15
15
Cr (mg/dL) <0.2 <0.2 <0.2
<0.2
G1u (mg/dL) 288 307 285
273
CID
-
(")
-a7
0
Table 13. 63415 is Negative for Genotoxicity in the In Vivo Micronucleus Study
l,1
0
--,
=P
PCE/Total Number of
MPCE/1000 ,--,
Treatment Change from Control
Number of MPCE/PCE -4
Erythrocytes PCE
c.,
.1
(n=5/group) (%)
Scored ,-,
u,
(Mean +/- SD) (Mean +/-
SD)
24-h timepoint
Sesame Oil 0.588 0.04 - 0.2
0.27 2/10000
125 mg/kg 0.543 0.03 -8 0.3
0.27 3/10000
250 mg/kg 0.520 0.06 -12 0.3
0.27 3/10000
500 mg/kg 0.426 0.07 -28 0.0
0.00 0/10000 P
1000 mg/kg 0.498 + 0.05 -15 0.2
0.27 2/10000 2
-a
.
1500 mg/kg 0.499 0.06 -15 0.4
0.22 4/10000 .
0
.,
0,
2000 mg/kg 0.531 0.05 -10 0.2
0.27 2/10000
,r,
,
48-h timepoint
,
0
0
,
Sesame Oil 0.526 0.05 - 0.3
0.27 3/10000
125 mg/kg 0.453 0.03 -14 0.2
0.27 2/10000
250 mg/kg 0.391 0.02 -26 0.2
0.27 2/10000
500 mg/kg 0.339 0.05 -36 0.3
0.45 3/10000
1000 mg/kg 0.344 0.04 -35 0.1
0.22 1/10000
,-d
1500 mg/kg 0.376 0.05 -39 0.4
0.42 4/10000 (-)
2000 mg/kg 0.360 0.03 -32 0.1
0.22 1/10000 5)
.i.-
-a-
,...)
u.
i.)
-.4
,z,
g
o
Table 14. Parameters of FIG. 32
=P
Tot
ALT AST ALP Tot Bil BUN Cr
TG
Prot Albumin Glucose Chol
-4
c.,
Treatment Day
(U/L) (U/L) (U/L) (mg/dL) (mg/dL) (mg/dL)
(g/dL) (g/dL) (mg/dL) (mg/dL) (mg/dL)
.1
I..,
CA
BL 30 29 320 0.15 23 0.63 7.2 4.1 87 124
52
Vehicle Day 37 37 345 0.23 18 0.63 6.9 4.1 63 130 64
14
BL 46 32 351 0.18 35 0.78 7.4 4 74 146
51
mg/kg Day
46 38 382 0.23 27 0.68 7.2
4 39 144 82
P
14
BL 32 32 409 0.18 23 0.7 7.3 4.2 85 125
47 2
0
0
0
0
0
30 mg/kg Day
47 43 416 0.2 20 0.58 7.2
4 53 122 64 0,
co
14
. .
,r,
,
BL 32 35 381 0.15 24 0.7 6.9 4 96 137
37 ,
0
100 mg/kg Day 43 37 390 0.18 24 0.55 6 3.2
32 93 61
14
,-o
n
5
4-
-a-
r.o4
CA
N
--4
0
Table 15. In Vitro Activity of 63415 and 63355
4-
63415 63355
NO IC50 (nM), RAW264.7 4.0 1 0.63 0.06
WST-1 IC50 (nM), RAW264.7 125 150
NQ01-ARE (fold at 62.5 nM in
5.3 1.0 6.5 0.9
HuH7)
Table 16. Parameters of FIG. 47
Compound Plasma Whole Blood Brain Liver
Lung Kidney
RTA 405 (nM) 130 1165 93 1143
1631 2357
63415 (nM) 51 679 1081 985
533 1604
Table 17. Parameters of FIG. 48
Compound Liver Lung
Kidney
RTA 405 1.93 1.48
8.25
63415 10.9 1.75
10.9
CID
(4)
CA 02909066 2015-10-07
WO 2014/176415 PCMJS2014/035279
* * * * * * * * * * * * * * * *
All of the compounds, polymorphs, formulations, and methods disclosed and
claimed herein can be made and executed without undue experimentation in light
of the
present disclosure. While the compounds, polymorphs, formulations, and methods
of this
invention have been described in terms of preferred embodiments, it will be
apparent to
those of skill in the art that variations may be applied to the compounds,
polymorphs,
formulations, and methods, as well as in the steps or in the sequence of steps
of the
method described herein without departing from the concept, spirit, and scope
of the
invention. More specifically, it will be apparent that certain agents which
are both
chemically and physiologically related may be substituted for the agents
described herein
while the same or similar results would be achieved. All such similar
substitutes and
modifications apparent to those skilled in the art arc deemed to be within the
spirit, scope
and concept of the invention as defined by the appended claims.
100
WO 2014/176415
PCT/US2014/035279
REFERENCES
Abraham and Kappas, Free Radical Biol. Med., 39:1-25, 2005.
Ahmad, et. al., Cancer Res., 68:2920-2926, 2008.
Ahmad, et. al., J. Biol. Chem., 281:35764-9, 2006.
Akiyama et al., Alzheimer Dis. Assoc. Disord., 14(1): S47-53, 35
2000.
Angulo et al., Eur. J. Immunol., 30:1263-1271, 2000.
Araujo, et. al., J. Immunol., 171(3):1572-1580, 2003.
Arend and Dayer, Arthritis Rheum., 38:151-160, 1995.
Arend et al., Annu. Rev. Immunol., 16:27-55, 1998.
Autenrieth etal., Infect. Immun., 62:2590-2599, 1994.
Bach, Hum. Immunol., 67(6):430-432, 2006.
Bagasra et al ., Proc. Natl. Acad. Sci. USA, 92:12041-12045, 1995.
Ball, Ann. Rheum. Dis., 30:213-223, 1971.
Beal, Cum Opin. Neurobiol., 6:661-666, 1996.
Blumberg et al., Arthritis Rheum., 7:93-97, 1964.
Botoman et at., Am. Fam. Physician, 57(1):57-68, 1998.
Brandt etal., Arthritis Rheum., 43:1346-1352, 2000.
Braun et at., Arthritis Rheum., 42:2039-2044, 1999.
Brewerton et at., Lancet., 1:904-907, 1973a.
Brewerton et at., Lancet., 1:956-957, 1973b.
Bronte etal., Trends Immunol., 24:302-306, 2003.
Brown and DuBois, J. Clin. Oncol., 23:2840-2855, 2005.
Brynskov etal., N. Engl. J. Med., 321(13):845-850, 1989.
Burger and Dayer, Neurology, 45(6S-6):$39-43, 1995.
Cai etal., Nat. Med., 11(2):183-190, 2005.
101
Date Recue/Date Received 2020-09-03
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Calin and Taurog, In: The Spondylarthritides, Calin et al. (Eds.), Oxford, UK.
Oxford
University Press, 179, 1998.
Cann et al., Gut., 24(12):1135-1140, 1983.
Chauhan and Chauhan, Pathophysiology, 13(3):171-181. 2006.
Chomarat et al.,ArthritisRheum., 38:1046-1054, 1995. 65.
Coyle and Puttfarcken, Science, 262:689-695, 1993.
Crowell et al., Mol. Cancer. Ther., 2:815-823, 2003.
Dickerson, et. al., Prog Neuropsychopharmacol Biol. Psychiatry, March 6, 2007.
de Waal et al., J. Exp. Med., 174:1209-1220, 1991.
Dinarello, Int. Rev. Immunol., 16:457-499, 1998.
Dinkova-Kostova, et. al., Proc. NatL Acad. Sci. USA, 102(12):4584-4589, 2005.
Dionne et al., Clin. Exp. Immunol., 112(3):435-442, 1998.
Doran et al., J. Rheumatol., 30(2):316-320, 2003.
Drossman et al., Dig. Dis. Sci., 38(9):1569-1580, 1993.
Drossman et al., Gastroenterol., 112(6):2120-2137, 1997.
Dudhgaonkar, et al., Eur. J. Pain, 10(7):573-9, 2006.
Eastgatc, et al., Lancet, 2(8613): 706-9, 1988.
Eikelenboom et al., Glia, 40(2):232-239, 2002.
Ettchadi et al., Clin. Exp. Immunol., 96(1):146-151, 1994.
Everhart et al., Gastroenterol., 100(4):998-1005, 1991.
Fcaron and Lockslcy, Science, 272(5258):50-53, 1996.
Feldtkeller et al., Rheumatol. Int., 23(2):61-66, 2003.
Firestein et al., Arthritis Rheum., 37:644-652, 1994.
Forstermann, Biol. Chem., 387:1521, 2006.
Fujikawa et al., Ann. Rheum. Dis., 54:318-320, 1995.
Funakoshi et al., Digestion, 59(1):73-78, 1998.
Galley and Webster, Br. J. Anaesth., 77:11-16, 1996.
Gehrmann et al., Glia, 15(2):141-151, 1995.
Genain and Nauser, J. Mol. Med., 75:187-197, 1997.
Gladman et al., Br. J. Rheumatol., 22:675-679, 1995.
Gladman et al ., J. Med., 62:127-141, 1987.
102
CA 02909066 2015-10-07
WO 2014/176415 PCT/US2014/035279
Gladman, Rheum. Dis. Clin. North Am., 18:247-256, 1992.
Goodman et al., Kidney Int., 72(8):945-953, 2007.
Graeber et al., Glia, 40(2):252-259, 2002.
Greten et al., Cell, 118:285-296, 2004.
Griffin et al., Proc. Natl. Acad. Sci. USA, 86(19):7611-7615, 1989.
Guilherme et al., Nat. Rev. Mol. Cell. Biol., 9(5):367-77, 2008.
Gwee et al., Gut., 44(3):400-406, 1999.
Hahn and Tsao, In: Dubois' Lupus Erythenzatosus, 4th Ed, Wallace and Hahn
(Eds.), Lea
and Febiger, Philadelphia, 195-201, 1993.
Handbook of Pharmaceutical Salts: Properties, and Use, Stahl and Wermuth
Eds.),
Verlag Helvetica Chimica Acta, 2002.
Hannum et al., Nature, 343:336-340, 1990.
Hanson et al., BMC Medical Genetics, 6(7), 2005.
Hansson et al., Annu. Rev. Pathol. Mech. Dis., 1:297-329, 2006.
Harrison et al., J. Rheumatol., 25(12):2324-2330, 1998.
Hart et al., Immunology, 84:536-542, 1995.
Honda, et. al. Bioorg. Med. Chem. Lett., 12:1027-1030, 2002.
Honda, et. al., Bioorg. Med. Chem. Lett., 7:1623-1628, 1997.
Honda, et. al., Bioorg. Med. Chem. Lett., 8(19):2711-2714, 1998.
Honda, et. al., Bioorg. Med. Chem. Lett., 9(24):3429-3434, 1999.
Honda, et. al., J. Med. Chem., 43:4233-4246, 2000a.
Honda, et. al., J. Med. Chem., 43:1866-1877, 2000b.
Horwitz and Fisher, N. Engl. J. Med., 344(24):1846-1850, 2001.
Hotamisligil, Nature, 444(7121):860-7, 2006.
Ishikawa et al., Circulation, 104(15): 1831 - 1836, 2001.
Ishizawa and Dickson, J. Neuropathol. Exp. Neurol., 60(6): 647-657, 2001.
Jacob etal., Proc. Natl. Acad. Sci. USA, 87:1233-1237, 1990.
Jailwa1a etal., Ann. Intern. Med., 133(2):136-147, 2000.
Jarvis, Curr. Opin. Rheumatol., 10(5):459-467, 1998.
Jarvis, Pediatr. Ann., 31(7):437-446, 2002.
Jones etal., Br. J. Rheumatol., 33(9):834-839, 1994.
103
CA 02909066 2015-10-07
WO 2014/176415 PCT/US2014/035279
Jonsson et al., Br. J. Rheumatol., 32(7):578-581 1993.
Jonsson et al., Oral Dis., 8(3): 130-140, 2002.
Jonsson et al., Trends Immunol., 22(12):653-654, 2001.
Kahle et al., Ann. Rheum. Dis., 51:731-734, 1992.
Kaltschmidt et al., Proc. Natl. Acad. Sci. USA, 94:2642-2647, 1997.
Kawakami et al., Brain Dev., 28(4):243-246, 2006.
Kellow and Phillips, Gastroenterol., 92(6): 1885-1893, 1987.
Kendall-Tackett, Trauma Violence Abuse, 8(2):117-126, 2007.
Khan et al., J. Neurochem., 71:78-87, 1998.
Khan et al., Toxicol. AppliedPhannacol., 103:482-490, 1990.
Kortylewski et al ., Nat. Med., 11:1314-1321, 2005.
Kotake et al., Infect. Immun., 67:2682-2686, 1999.
Kotzin and O'Dell, In: Sampler's Immunologic Diseases, 5th Ed., Frank et al.
(Eds.),
Little Brown & Co., Boston, 667-697, 1995.
Kotzin, Cell, 85:303-306, 1996.
Kruger et al., J. Phannacol. Exp. Ther., 319(3): 1144-1152, 2006.
Kuboyama, Kurume Med. J, 45(1):33-37, 1998.
Lahcsmaa et al., J. Immunot, 148:3079-3085, 1992.
Lcc, et. al., Glia., 55(7):712-22, 2007.
Lencz, et. al., Mol. Psychiatry, 12(6):572-80, 2007.
Liby, et. al., Cancer Res., 65(11):4789-4798, 2005.
Liby, et. al., Nat. Rev. Cancer, 7(5):357-356, 2007.
Lipsky, In: Harrison 's Principles of Internal Medicine, Fauci et al. (Eds.),
14th Ed., NY,
McGraw-Hill, 1880-1888, 1998.
Liu, et. al., FASEB J., 20(2):207-216, 2006.
Lo et al., Curr. Dir. Autoimmun., 1:226-246, 1999.
Lugering et al ., Ital. J. Gastroenterol. Hepatol., 30(3):338-344, 1998.
Lynn and Friedman, N. Engl. J. Med., 329(26):1940-1945, 1993.
Macatonia et al., J. Inununol., 150:3755-3765, 1993.
March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure,
2007.
Marsal et al., Rheutnatology, 38:332-337, 1999.
104
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Mazur et al., Cell Microbiol., 9(7): 1683-94, 2007.
Mazzoni et al., J. Immunol., 168:689-695, 2002.
McAlindon et al., Gut, 42(2):214-219, 1998.
McGeer and McGeer, Brain Res. Brain Res. Rev., 21:195-218, 1995.
McGeer et al., Neurology, 19:331-338, 1996.
McGonagle et al., Arthritis Rheum., 41: 694-700, 1998.
McGonagle et al., Curr. Opin. Rheumatol., 11:244-250, 1999.
McIver et al., Pain, 120(1-2):161-9, 2005.
Mease et al., Lancet, 356:385-390, 2000.
Merrill and Benvenist, Trends Neurosci., 19:331-338, 1996.
Mertz et al., Gastroenterol., 118(5):842-848, 2000.
Moll and Wright, Ann. Rheum. Dis., 32:181-201, 1973.
Moll and Wright, Semin. Arthritis Rheum., 3:55-78, 1973.
McIver, et. al., Pain, 120(1-2):161-9, 2005.
Morris, et. al., J. MoL Med., 80(2):96-104, 2002.
Morse and Choi, Am. J. Respir. Crit. Care Med., 172(6):660-670, 2005.
Morse and Choi, Am. J. Respir. Crit. Care Med., 27(1):8-16, 2002.
Nath et al., Neurology, 66(1):149-150, 2006.
Neal etal., BMJ., 314(7083):779-782, 1997.
Nichols, Drug News Perspect., 17(2):99-104, 2004.
Nielen et al., Arthritis Rheum., 50(2):380-386, 2004.
Ohnishi et al., Int. Immunol., 6:817-830, 1994.
Pall, Med. Hypoth., 69:821-825, 2007.
Partsch et al., Br. J. Rhetunatol., 24:518-523, 1997.
Pica et al., AntitnicrobAgents Chemother., 44(0:200-4, 2000.
Pergola, et. al., N Engl J ilfed 365:327-336, 2011.
Pimentel etal., Am. J. Gastroenterol., 95(12):3503-3506, 2000.
Prieur etal., Lancet., 2:1240-1242, 1987.
Place, et. al., Clin. Cancer Res., 9(7):2798-806, 2003.
Rajakariar, et. al., Proc. Arad Acad. Sci. USA, 104(52):20979-84, 2007.
Rantapaa-Dahlqvist et al., Arthritis Rheum., 48(10):2741-2749, 2003.
105
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Reimund et at., Eur. J. Clin. Invest., 28(2):145-150, 1998.
Ribbens et at., Eur. Cytokine Netw., 11:669-676, 2000.
Rogers et al., Neurobiol Aging, 9(4):339-349, 1988.
Rogler and Andus, WorldJ. Surg., 22(4):382-389, 1998.
Rooney et at., Rheumatollnt., 10:217-219, 1990.
Ross, et. at., Am. J. Clin. Pathol., 120(Suppl):S53-71, 2003.
Ross, et. at., Expert Rev. Mol. Diagn., 3(5):573-585, 2003.
Rostom et at., Ann. Intern. Med., 146, 376-389, 2007.
Rothstein, Med. Clin. North Am., 84(5):1247-1257, 2000.
Ruster, et. at., Scand. J. Rheumatol., 34(6):460-3, 2005.
Sacerdoti, et. at., Curr Neurovasc Res. 2(2):103-111, 2005.
Saha, et. al., J Biol Chem 285:40581-92, 2010.
Saiki et at., Scand. J. Gastroenterol., 33(6):616-622, 1998.
Salomonsson and Jonsson, Arthritis Rheum., 48(11):3187-3201, 2003.
Salomonsson et at., Scand. J. Immunol., 55(4):336-342, 2002.
Salvarani et al., CUrr. Opin. Rheumatol. 1998; 10:299-305,25 1998.
Salvemini et al., J. Clin. Invest., 93:1940-1947, 1994.
Sandler, Gastroenterol., 99(2):409-415, 1990.
Salvemini, et. al., J. Clin. Invest., 93(5):1940-1947, 1994.
Sarchielli, et. at., Cephalalgia, 26(9):1071-1079 , 2006.
Satoh, et. at., Proc. Natl. Acad. Sci. USA, 103(3):768-773, 2006.
Schlaak et al., Clin. Exp. Rheumatol., 14:155-162, 1996.
Schlaak et al., Eur. J. Immunol., 22:2771-2776, 1992.
Schlosstein et al., NE J. Medicine, 288:704-706, 1973.
Schreiber, Neth. J. Med., 53(6):S24-31, 1998.
Schulz et al., Antioxid. Redox. Sig., 10:115, 2008.
Shishodia, et. at., Clin Cancer Res 12:1828-38, 2006.
Sieper and Braun, Arthritis Rheum., 38:1 547-1 554, 1995.
Simon et al., Clin. Exp. Immunol., 94:122-126, 1993.
Simon et al ., Proc. Natl. Acad. Sci. USA, 91:8562-85666,40 1994.
Simonian and Coyle, Annu. Rev. Pharmacol. Toxicol., 36:83-106, 1996.
106
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Sinha et al., CancerRes., 67:4507-4513, 2007.
Stack et al., Lancet, 349(9051):521-524, 1997.
Stewart et al., Neurology, 48:626-632, 1997.
Strejan, et. al., J. Neuroimmunol., 7:27, 1984.
Suh, et. al., Cancer Res., 58:717-723, 1998.
Suh, et. al., Cancer Res., 59(2):336-341, 1999.
Szabo, et. al., Nature Rev. Drug Disc., 6:662-680, 2007.
Takahashi, et. al., Cancer Res., 57:1233-1237, 1997.
Talley et al., Gastroenterol., 109(6):1736-1741, 1995.
Tamir and Tannenbaum, Biochim. Biophys. Acta., 1288:F31-F36, 1996.
Targan et al., N. Engl. J. 'Vied., 337(15):1029-1035, 1997.
Touzani et al., J. Neuroimmunol., 100(1-2):203-215, 1999.
Tumlin et al., Am. J. Cardiol., 98(6A): 14K-20K, 2006.
van den Berg, Semin. Arthritis Rheum., 30(5S-2):7-16, 2001.
van Dullemen et al., Gastroenterol., 109(1):129-135, 1995.
van Hogezand and Verspaget, Drugs, 56(3):299-305, 1998.
Vazquez et al., J. Virol., 79(7):4479-91, 2005.
Vodovotz et al., In; Handbook ofExperimental Immunology, Volumes 1-IV, 1996.
Wardle, Nephrol. Dial, Transplant., 16(9):1764-8, 2001.
Warrington et al., Arthritis and Rheumatism, 44:13-20, 2001.
VVeyand and Goronzy, Ann. NY Acad. Sci., 987:140-149, 2003.
Whitehead et al., Gastroenterol., 98(5 Pt 1):1187-1192, 1990.
Williams et al., Clin. Neurosci., 2(3-4):229-245, 1994.
Wordsworth, In: Genes andArthritis, Brit. Medical Bulletin, 51:249-266, 1995.
Wright, Ann. Rheum. Dis., 15:348-356, 1956.
Wright, Clin. Orthop. Related Res., 143:8-14, 1979.
Xanthou et at., Arthritis Rheum., 44(2):408-418, 2001.
Yin et al., Arthritis Rheum., 40:1788-1797, 1997.
Yin et al., Rheumatology, 38:1058-1067, 1999.
Yoh et al., Kidney Mt., 60(4): 1343-1353, 2001.
Yore, et. al., Mol Cancer Ther 5:3232-9, 2006.
107
CA 02909066 2015-10-07
WO 2014/176415
PCT/US2014/035279
Yu et al., Nat. Rev. Immunol., 7:41-51, 2007.
Zhou et al., Am. J. Pathol., 166(1):27-37, 2005.
Zhou et al., Cancer Sc., 98:882-889, 2007.
Zingarelli et al., J. Immunol., 171(12):6827-6837, 2003.
108