Note: Descriptions are shown in the official language in which they were submitted.
CA 02909115 2015-10-08
1
Transmission oil formulation for reducing fuel consumption
The present invention relates to a transmission oil formulation having
advantageous viscosity
properties, by means of which it is possible to lower the fuel consumption of
motor vehicles.
The reduction of fuel consumption of motor vehicles is becoming ever more
important for various
reasons. As well as many improvements to the construction of the motor
vehicles themselves,
efforts are also being made to minimize churning losses caused by the
lubricants, for example by
motor and transmission oils.
In order to achieve this aim, the fresh oil viscosity of the lubricant is
typically lowered. For automatic
transmission oils, this is the reason why the DEXRON-VI specification from
General Motors, for
example, requires that the kinematic viscosity of fresh oil at 100 C be not
more than 6.4 mm2/s.
However, there are limits to lowering of the fresh oil viscosity, since a
reduction in the viscosity also
leads to a reduction in the lubricant film thickness. An insufficient
lubricant film thickness leads to
increased wear and a shorter lifetime of the machine parts to be lubricated.
In the worst case, the
lubricant film thickness is so low that the surface roughness of the materials
is greater than the
lubricant film thickness. Under these conditions, the machine parts come into
point contact, which
leads to pressure and load spikes in underlying material layers and ultimately
to material fatigue.
Prolonged mechanical stress typically leads to a reduction in the viscosity of
the lubricant, since the
mechanical stress, for example, reduces the size of polymeric constituents of
the lubricant. This
effect is also referred to as permanent shear loss. Since permanent shear loss
leads to lasting
reduction in lubricant film thickness, lubricants are required to not go below
a particular minimum
viscosity even after a long period of stress. A recognized method for
characterizing the permanent
shear loss of a lubricant is the tapered roller bearing test to CEC-L-45-A-99.
For automatic
transmission oils, for example, the DEXRON-VI specification specifies a
kinematic minimum
viscosity at 100 C of 5.5 mm2/s after a 20 h tapered roller bearing test.
In order to lower fuel consumption while complying with the minimum viscosity,
it is customary to
increase the viscosity index of the lubricant. The viscosity index (VI)
describes the temperature
dependence of the viscosity of a lubricant. Lubricants having a low viscosity
index exhibit a greater
temperature dependence of the change in viscosity than those having a high
viscosity index. An
increase in the viscosity index at constant viscosity at a particular
temperature means that the
viscosity is less at lower temperatures than for a comparable lubricant with a
lower viscosity index.
Reduced viscosity at lower temperatures, in turn, reduces churning losses and
hence fuel
consumption. However, this advantage is restricted to the warm-up phase of a
motor vehicle.
There is therefore an interest in providing a lubricant through which fuel
consumption can be
reduced at higher operating temperatures as well.
CA 02909115 2015-10-08
2
Lubricant film thickness is not determined exclusively by the viscosity of the
lubricant, but is a
function of viscosity and the relative sliding or rolling speed of the machine
parts moving relative to
one another. At the same viscosity, the lubricant film thickness rises with
speed. At high speed, a
relatively low viscosity of lubricant would accordingly be sufficient to
assure an adequate lubricant
film thickness. A low viscosity would be advantageous at this operating point,
since the reduced
fluid friction would result in less energy being consumed, which leads to a
fuel saving.
At high speeds, the shear forces that occur typically result automatically in
a reduction in the
viscosity of the lubricant. This so-called shear loss may be permanent, as
occurs, for example, in
the event of lasting mechanical stress on lubricants. However, shear loss may
also be temporary,
such that the original viscosity is re-established in the event that the speed
is reduced and the
associated shear forces are lower. Temporary shear loss as a result of shear
forces is also referred
to as shear dilution.
An ideal lubricant would accordingly have maximum shear dilution in order to
reduce fuel
consumption at high sliding or rolling speed. At the same time, an ideal
lubricant would have only a
low permanent shear loss in order to maximize the lifetime of the machine
parts.
Lubricant properties are typically improved by addition of additives to
lubricant oils.
For example, US 5,565,130 and US 5,597,871 disclose using comb polymers based,
for example,
on polybutadiene as viscosity index improvers. However, no satisfactory
improvement in fuel
consumption is disclosed therein.
WO 2007/003238 Al describes oil-soluble comb polymers based on polyolefin-
based
macromonomers, especially polybutadiene-based methacrylic esters, and Cl to
C10 alkyl
methacrylates. The comb polymers can be used as an additive for lubricant
oils, in order to improve
the viscosity index and shear stability. However, no improvement in
coefficient of traction and fuel
consumption is disclosed.
WO 2009/007147 Al discloses the use of comb polymers based on polyolefin-based
macromonomers, especially polybutadiene-based methacrylic esters, and Cl to
C10 alkyl
methacrylates for improving the fuel consumption of motor vehicles. However,
the comb polymers
are disclosed only as additives for motor oil.
WO 2010/102903 Al discloses the use of comb polymers as antifatigue additives
for transmission,
motor and hydraulic oils. However, no reduction in fuel consumption is
described.
DE 10 2009 001 447 Al describes the use of comb polymers for improving the
load-bearing
capacity of hydraulic oils having a high viscosity index. A high viscosity
index and associated
higher viscosities of the formulation at operating temperatures of about 80 C,
for a given ISO
grade, enable the reduction of fuel consumption in hydraulic systems. Of
particular significance in
CA 02909115 2015-10-08
3
this context is the improvement of the volumetric efficiency of the hydraulic
systems. This is
favorably influenced by higher lubricant viscosities, since leakage flows in
the hydraulic pump are
minimized as a result. However, improvement in the volumetric efficiency is
accompanied by
reduced mechanical efficiency. In the case of transmission oils, however,
mechanical efficiency is
crucial, which is the reason why low viscosities and, in particular, low
coefficients of traction of the
lubricant oil are required in the transmission oil sector. Therefore, the
development of energy-
efficient hydraulic oils does not permit any conclusions about the development
of transmission oils.
WO 2012/025901 Al discloses the use of comb polymers in lubricants in
combination with
particular friction modifiers. There is no disclosure of combinations of comb
polymers and base oils
specifically matched to the demands of transmission oils.
Since the properties of the lubricants disclosed in the prior art are still
unsatisfactory in relation to
use as a transmission oil and in respect of the reduction in fuel consumption,
it is the aim of the
present invention to provide a transmission oil formulation having a kinematic
minimum viscosity at
100 C of 5.5 mm2/s to ASTM D445 after a 20 h tapered roller bearing test CEC-L-
45-A-99, and
simultaneously high shear dilution. In addition, the kinematic fresh oil
viscosity at 100 C should be
not more than 6.4 mm2/s to ASTM D445 and preferably about 6.0 mm2/s. The
transmission oil
formulation should also have a high viscosity index, preferably a viscosity
index of greater than 180
to ASTM 02270, more preferably greater than 190.
In addition, the transmission oil formulation should have a low coefficient of
traction. The coefficient
of traction is the force required to move a load, divided by the load. The
numerical value of the
coefficient expresses how easy it is to shear a lubricant film. Transmission
oils ideally have a low
coefficient of traction because, when the coefficient of traction is low, less
energy is consumed
because of lubricant shearing.
This object is achieved by a transmission oil formulation comprising
(i) a base oil having a kinematic viscosity at 100 C of at least 1.5 mm2/s to
ASTM D445 and an
aromatics content of less than 15% by weight to ASTM D 2007; and
(ii) a copolymer obtained by free-radical polymerization from a monomer
composition, said
monomer composition comprising the following monomers:
(A) 30% to 50% by weight of an ester of (meth)acrylic acid and a hydroxylated
hydrogenated
polybutadiene, where the hydroxylated hydrogenated polybutadiene has a number-
average molar
mass Mn to DIN 55672-1 of 4000 to 6000 g/mol;
(B1) 0.2% to 50% by weight of methyl (meth)acrylate;
(B2) 0.2% to 50% by weight of butyl (meth)acrylate;
(B3) 0.2% to 5% by weight of C5-C30 alkyl (meth)acrylates;
(C) 10% to 50% by weight of styrene monomers having 8 to 17 carbon atoms; and
(D) 0% to 5% by weight of further free-radically polymerizable comonomers,
where the sum total of the proportions by weight of monomers (B1), (B2) and
(B3) is at least 10%
by weight.
CA 02909115 2015-10-08
4
The specified proportions by weight of components (A) to (D) are based on the
total weight of the
monomer composition.
In a preferred embodiment, the proportions of components (A) to (D) add up to
100% by weight.
More preferably, the sum total of the proportions by weight of monomers (61)
to (33) is at least
15% by weight, most preferably 15% to 45% by weight.
The transmission oil formulation of the invention meets the prerequisite
mentioned in terms of fresh
oil viscosity, minimum viscosity by the tapered roller bearing test and
viscosity index. In addition,
the transmission oil formulation of the invention has an advantageous
temporary shear loss as a
result of shear forces.
It has also been found that, surprisingly, the inventive combination of a base
oil having a low
aromatics content with the copolymer described leads to a reduction in the
coefficient of traction of
the transmission oil formulation.
The use of the transmission oil formulation of the invention additionally
leads to a decrease in fuel
consumption in a motor vehicle rolling test bed test, this effect being
observed not just during the
cold start phase but also after the transmission oil formulation has heated
up.
The copolymer for use in accordance with the invention as component (ii) of
the transmission oil
formulation can be obtained via free-radical polymerization of the monomers
mentioned. It is
preferable here for the double bonds of the ethylenically unsaturated groups
and vinyl groups of the
monomers mentioned to be opened with formation of covalent bonds between the
monomers. The
copolymer that forms is a comb polymer.
A comb polymer in the context of this invention comprises a first polymer,
which is also referred to
as backbone or main chain, and a multitude of further polymers which are
referred to as side
chains and are bonded covalently to the backbone. In the present case, the
backbone of the comb
polymer is formed by the interlinked unsaturated groups of the monomers
mentioned. The ester
groups of the (meth)acrylic esters, the phenyl radicals of the styrene
monomers and the
substituents of the further free-radically polymerizable comonomers form the
side chains of the
comb polymer.
The styrene monomers having 8 to 17 carbon atoms for use in accordance with
the invention are
styrene and substituted styrene. Examples of styrene monomers having 8 to 17
carbon atoms are
styrene, substituted styrenes having an alkyl substituent in the side chain,
for example a-
methylstyrene and a-ethylstyrene, substituted styrenes having an alkyl
substituent on the ring, such
as vinyltoluene and p-methylstyrene, halogenated styrenes, for example
monochlorostyrenes,
CA 02909115 2015-10-08
dichlorostyrenes, tribromostyrenes and tetrabromostyrenes. Particular
preference is given to
unsubstituted styrene.
Preferably, the monomer composition comprises 10%-45% by weight of styrene
monomers having
5 8 to 17 carbon atoms.
The term "(meth)acrylic acid" refers to acrylic acid, methacrylic acid and
mixtures of acrylic acid
and methacrylic acid. The term "(meth)acrylate" refers to esters of acrylic
acid, esters of
methacrylic acid or mixtures of esters of acrylic acid and methacrylic acid.
Preferably, the monomer composition comprises, as monomer (B1), 0.2% to 45% by
weight of
methyl methacrylate. Likewise preferably, the monomer composition comprises,
as monomer (B2),
0.2% to 45% by weight of butyl methacrylate and/or butyl acrylate, more
preferably n-butyl
methacrylate and/or n-butyl acrylate.
The C5 to C30 alkyl (meth)acrylates for use in accordance with the invention
are esters of
(meth)acrylic acid and alcohols having 5 to 30 carbon atoms. The term "C5 to
C30 alkyl
(meth)acrylates" encompasses individual (meth)acrylic esters with an alcohol
of a particular length,
and likewise mixtures of (meth)acrylic esters with alcohols of different
lengths.
The suitable C5 to C30 alkyl (meth)acrylates include, for example, pentyl
(meth)acrylate, hexyl
(meth)acrylate, 2-ethylhexyl (meth)acrylate, heptyl (meth)acrylate, 2-tert-
butylheptyl (meth)acrylate,
octyl (meth)acrylate, 3-isopropylheptyl (meth)acrylate, nonyl (meth)acrylate,
decyl (meth)acrylate,
undecyl (meth)acrylate, 5-methylundecyl (meth)acrylate, dodecyl
(meth)acrylate, 2-methyldodecyl
(meth)acrylate, tridecyl (meth)acrylate, 5-methyltridecyl (meth)acrylate,
tetradecyl (meth)acrylate,
pentadecyl (meth)acrylate, hexadecyl (meth)acrylate, 2-methylhexadecyl
(meth)acrylate,
heptadecyl (meth)acrylate, 5-isopropylheptadecyl (meth)acrylate, 4-tert-
butyloctadecyl
(meth)acrylate, 5-ethyloctadecyl (meth)acrylate, 3-isopropyloctadecyl
(meth)acrylate, octadecyl
(meth)acrylate, nonadecyl (meth)acrylate, eicosyl (meth)acrylate, cetyleicosyl
(meth)acrylate,
stearyleicosyl (meth)acrylate, docosyl (meth)acrylate and/or
eicosyltetratriacontyl (meth)acrylate.
Particularly preferred C5 to 030 alkyl (meth)acrylates are methacrylic esters
of a linear 012 to C14
alcohol mixture (012 to C14 alkyl methacrylate).
The hydroxylated hydrogenated polybutadiene for use in accordance with the
invention has a
number-average molar mass M, of 4000 to 6000 g/mol, preferably 4000 to 5000
g/mol. Because of
their high molar mass, the hydroxylated hydrogenated polybutadienes can also
be referred to as
macroalcohols in the context of this invention.
The number-average molar mass Mn is determined by size exclusion
chromatography using
commercially available polybutadiene standards. The determination is effected
to DIN 55672-1 by
gel permeation chromatography with THF as eluent.
CA 02909115 2015-10-08
6
Preferably, the hydroxylated hydrogenated polybutadiene has a hydrogenation
level of at least
99%. An alternative measure of the hydrogenation level which can be determined
on the copolymer
of the invention is the iodine number. The iodine number refers to the number
of grams of iodine
which can be added onto 100 g of copolymer. Preferably, the copolymer of the
invention has an
iodine number of not more than 5 g of iodine per 100 g of copolymer. The
iodine number is
determined by the Wijs method according to DIN 53241-1:1995-05.
Preferred hydroxylated hydrogenated polybutadienes can be obtained according
to GB 2270317.
Some hydroxylated hydrogenated polybutadienes are also commercially available.
The
commercially hydroxylated hydrogenated polybutadienes include, for example,
Kraton Liquid L-
1203, a hydrogenated polybutadiene OH-functionalized to an extent of about 98%
by weight (also
called olefin copolymer OCP) having about 50% each of 1,2 repeat units and 1,4
repeat units, of
Mr, = 4200 g/mol, from Kraton Polymers GmbH (Eschborn, Germany). A further
supplier of suitable
alcohols based on hydrogenated polybutadiene is Cray Valley (Paris), a
daughter company of Total
(Paris), or the Sartomer Company (Exton/PA/USA).
Preference is given to monohydroxylated hydrogenated polybutadienes. More
preferably, the
hydroxylated hydrogenated polybutadiene is a hydroxyethyl- or hydroxypropyl-
terminated
hydrogenated polybutadiene. Particular preference is given to hydroxypropyl-
terminated
polybutadienes.
These monohydroxylated hydrogenated polybutadienes can be prepared by first
converting
butadiene monomers by anionic polymerization to polybutadiene. Subsequently,
by reaction of the
polybutadiene monomers with ethylene oxide or propylene oxide, a hydroxy-
functionalized
polybutadiene can be prepared. This hydroxylated polybutadiene can be
hydrogenated in the
presence of a suitable transition metal catalyst.
The esters of (meth)acrylic acid for use in accordance with the invention and
a hydroxylated
hydrogenated polybutadiene described are also referred to as macromonomers in
the context of
this invention because of their high molar mass.
The macromonomers for use in accordance with the invention can be prepared by
transesterification of alkyl (meth)acrylates. Reaction of the alkyl
(meth)acrylate with the
hydroxylated hydrogenated polybutadiene forms the ester of the invention.
Preference is given to
using methyl (meth)acrylate or ethyl (meth)acrylate as reactant.
This transesterification is widely known. For example, it is possible for this
purpose to use a
heterogeneous catalyst system, such as lithium hydroxide/calcium oxide mixture
(Li0H/Ca0), pure
lithium hydroxide (Li0H), lithium methoxide (Li0Me) or sodium methoxide
(Na0Me) or a
homogeneous catalyst system such as isopropyl titanate (Ti(Oilpr)4) or
dioctyltin oxide (Sn(OCt)20).
CA 02909115 2015-10-08
7
The reaction is an equilibrium reaction. Therefore, the low molecular weight
alcohol released is
typically removed, for example by distillation.
In addition, the macromonomers can be obtained by a direct esterification
proceeding, for example,
from (meth)acrylic acid or (meth)acrylic anhydride, preferably under acidic
catalysis by p-
toluenesulfonic acid or methanesulfonic acid, or from free methacrylic acid by
the DCC method
(dicyclohexylcarbodiimide).
Furthermore, the present hydroxylated hydrogenated polybutadiene can be
converted to an ester
by reaction with an acid chloride such as (meth)acryloyl chloride.
Preferably, in the above-detailed preparations of the
esters of the invention, polymerization inhibitors are used, for example the 4-
hydroxy-2,2,6,6-
tetramethylpiperidinooxyl radical and/or hydroquinone monomethyl ether.
Some of the macromonomers for use in accordance with the invention are also
commercially
available, for example Kraton Liquid L-1253 which is produced from Kraton
Liquid L-1203 and is
a hydrogenated polybutadiene methacrylate-functionalized to an extent of about
96% by weight,
having about 50% each of 1,2 repeat units and 1,4 repeat units, from Kraton
Polymers GmbH
(Eschborn, Germany). Kraton L-1253 is likewise synthesized according to GB
2270317.
The monomer composition of the invention may comprise, as monomer (D), up to
5% by weight of
further free-radically polymerizable comonomers. Preferably, the monomer
composition comprises,
as component (D), 0.2% to 5% by weight of further free-radically polymerizable
comonomers.
Monomer (D) does not include the compounds already described as monomers (A)
to (C).
The further comonomers which are suitable as comonomers for preparation of
copolymers by free-
radical polymerization are known to those skilled in the art. Suitable
monomers are described, for
example, in WO 2010/102903 or in Mortier, Roy M., Malcolm F. Fox, and Stefan
T. Orszulik,
"Chemistry and technology of lubricants" (Springer Science+ Business Media,
2010).
In a preferred embodiment, the further free-radically polymerizable comonomers
are selected from
the group consisting of maleic anhydride, (di)alkyl fumarates, (di)alkyl
maleates, aminoalkyl
(meth)acrylates, aminoalkyl(meth)acrylamides, hydroxyalkyl (meth)acrylates,
carbonyl-containing
(meth)acrylates, heterocyclic (meth)acrylates, heterocyclic vinyl compounds
and mixtures thereof.
In this context, the use of aminoalkyl(meth)acrylamides in particular is
advantageous.
If maleic anhydride is used as monomer, it can be reacted with primary or
secondary amines after
polymerization. Such processes are described, for example, in WO 2007/070845
and DE 10 2007
031 247. Particular preference is given to primary amines. Suitable amines for
this purpose are, for
CA 02909115 2015-10-08
8
example, N,N-dimethylaminopropylamine, N-morpholinopropylamine and N-pheny1-
1,4-
phenylenediamine.
The notation "(di)alkyl fumarate" or "(di)alkyl maleate" means that it is
possible to use monoesters,
5 diesters and mixtures of esters of fumaric acid or of maleic acid.
Suitable (di)alkyl fumarates include monomethyl fumarate, dimethyl fumarate,
monoethyl fumarate,
diethyl fumarate, methyl ethyl fumarate, monobutyl fumarate, dibutyl fumarate,
dipentyl fumarate
and dihexyl fumarate. Preferred (di)alkyl fumarates comprise 1 to 10,
preferably 1 to 8 and more
10 preferably 1 to 4 carbon atoms in each of the alcohol groups. The
alcohol groups here may be
linear or branched.
Suitable (di)alkyl maleates include monomethyl maleate, dimethyl maleate,
monoethyl maleate,
diethyl maleate, methyl ethyl maleate, monobutyl maleate, dibutyl maleate.
Preferred (di)alkyl
15 maleates comprise 1 to 10, preferably 1 to 8 and more preferably 1 to 4
carbon atoms in each of
the alcohol groups. The alcohol groups here may be linear or branched.
Suitable aminoalkyl (meth)acrylates are, for example, N,N-dimethylaminoethyl
(meth)acrylate, N,N-
dimethylaminopropyl (meth)acrylate, N,N-diethylaminopentyl (meth)acrylate and
N,N-
20 dibutylaminohexadecyl (meth)acrylate.
An example of a suitable aminoalkyl(meth)acrylamide is N,N-
dimethylaminopropylmethacrylamide.
Suitable hydroxyalkyl (meth)acrylates include 2-hydroxypropyl (meth)acrylate,
3,4-dihydroxybutyl
25 (meth)acrylate, 2-hydroxyethyl (meth)acrylate, 3-hydroxypropyl
(meth)acrylate, 2,5-dimethy1-1,6-
hexanediol (meth)acrylate and 1,10-decanediol (meth)acrylate.
Suitable carbonyl-containing (meth)acrylates are, for example, 2-carboxyethyl
(meth)acrylate,
carboxymethyl (meth)acrylate, oxazolidinylethyl (meth)acrylate, N-
(methacryloyloxy)formamide,
30 acetonyl (meth)acrylate, mono-2-(meth)acryloyloxyethyl succinate, N-
(meth)acryloylmorpholine, N-
(meth)acryloy1-2-pyrrolidinone, N-(2-(meth)acryloyloxyethyl)-2-pyrrolidinone,
N-(3-
(meth)acryloyloxypropy1)-2-pyrrolidinone, N-(2-(meth)acryloyloxypentadecyI)-2-
pyrrolidinone, N-(3-
(meth)acryloyloxyheptadecyI)-2-pyrrolidinone, N-(2-
(meth)acryloyloxyethyl)ethyleneurea and 2-
acetoacetoxyethyl (meth)acrylate.
Suitable heterocyclic (meth)acrylates include 2-(1-imidazolyl)ethyl
(meth)acrylate, 2-(4-
morpholinyl)ethyl (meth)acrylate, 1-(2-methacryloyloxyethyl)-2-pyrrolidone, N-
methacryloylmorpholine, N-methacryloy1-2-pyrrolidinone, N-(2-
methacryloyloxyethyl)-2-
pyrrolidinone and N-(3-methacryloyloxypropy1)-2-pyrrolidinone.
Suitable heterocyclic vinyl compounds are, for example, 2-vinylpyridine, 4-
vinylpyridine, 2-methy1-5-
vinylpyridine, 3-ethyl-4-vinylpyridine, 2,3-dimethyl-5-vinylpyridine,
vinylpyrimidine, vinylpiperidine,
1
CA 02909115 2015-10-08
9
9-vinylcarbazole, 3-vinylcarbazole, 4-vinylcarbazole, 1-vinylimidazole, 2-
methyl-l-vinylimidazole, N-
vinylpyrrolidone, N-vinylpyrrolidine, 3-vinylpyrrolidine, N-vinylcaprolactam,
N-vinylbutyrolactam,
vinyloxolane, vinylfuran, vinyloxazoles and hydrogenated vinyloxazoles.
The copolymer for use in accordance with the invention can be characterized on
the basis of its
molar branching level ('f-branch). The molar branching level refers to the
percentage in mol% of
macromonomers (component (A)) used, based on the total molar amount of all the
monomers in
the monomer composition. The molar amount of the macromonomers used is
calculated on the
basis of the number-average molar mass M0 of the macromonomers. The
calculation of the
branching level is described in detail in WO 2007/003238 Al, especially on
pages 13 and 14, to
which reference is made here explicitly.
The copolymer for use in accordance with the invention preferably has a molar
branching level of
1.0 to 3.1 mol%, more preferably 1.2 to 2.8 mol% and most preferably 1.4 to
1.8 mol%.
The copolymer for use in accordance with the invention can be prepared by free-
radical
polymerization and by related methods of controlled free-radical
polymerization, for example ATRP
(= atom transfer radical polymerization) or RAFT (= reversible addition
fragmentation chain
transfer).
Standard free-radical polymerization is detailed, inter alia, in Ullmann's
Encyclopedia of Industrial
Chemistry, Sixth Edition. In general, a polymerization initiator and
optionally a chain transfer agent
are used for this purpose.
The usable initiators include azo initiators widely known in the technical
field, such as Al BN and
1,1-azobiscyclohexanecarbonitrile, and also peroxy compounds such as methyl
ethyl ketone
peroxide, acetylacetone peroxide, dilauryl peroxide, tert-butyl per-2-
ethylhexanoate, ketone
peroxide, tert-butyl peroctoate, methyl isobutyl ketone peroxide,
cyclohexanone peroxide,
dibenzoyl peroxide, tert-butyl peroxybenzoate, tert-butyl
peroxyisopropylcarbonate, 2,5-bis(2-
ethylhexanoylperoxy)-2,5-dimethylhexane, tert-butyl peroxy-2-ethylhexanoate,
tert-butyl peroxy-
3,5,5-trimethylhexanoate, dicumyl peroxide, 1,1-bis(tert-
butylperoxy)cyclohexane, 1,1-bis(tert-
butylperoxy)-3,3,5-trimethylcyclohexane, cumyl hydroperoxide, tert-butyl
hydroperoxide, bis(4-tert-
butylcyclohexyl) peroxydicarbonate, mixtures of two or more of the
aforementioned compounds
with one another, and mixtures of the aforementioned compounds with
unspecified compounds
which can likewise form free radicals. Suitable chain transfer agents are
especially oil-soluble
mercaptans, for example n-dodecyl mercaptan or 2-mercaptoethanol, or else
chain transfer agents
from the class of the terpenes, for example terpinolene.
The ATRP method is known per se. It is assumed that this is a "living" free-
radical polymerization,
but no restriction is intended by the description of the mechanism. In these
processes, a transition
metal compound is reacted with a compound having a transferable atom group.
This involves
transfer of the transferable atom group to the transition metal compound, as a
result of which the
CA 02909115 2015-10-08
metal is oxidized. This reaction forms a free radical which adds onto
ethylenic groups. However,
the transfer of the atom group to the transition metal compound is reversible,
and so the atom
group is transferred back to the growing polymer chain, which results in
formation of a controlled
polymerization system. It is accordingly possible to control the formation of
the polymer, the
5 molecular weight and the molecular weight distribution.
This reaction regime is described, for example, by J.-S. Wang, et al., J. Am.
Chem. Soc, vol. 117,
p. 5614-5615 (1995), by Matyjaszewski, Macromolecules, vol. 28, p. 7901-7910
(1995). In addition,
patent applications WO 96/30421, WO 97/47661, WO 97/18247, WO 98/40415 and WO
99/10387
10 disclose variants of the above-elucidated ATRP. In addition, the
polymers of the invention can also
be obtained via RAFT methods, for example. This method is described in detail,
for example, in
WO 98/01478 and WO 2004/083169.
The polymerization can be conducted under standard pressure, reduced pressure
or elevated
pressure. The polymerization temperature is also uncritical. In general,
however, it is in the range
from -20 to 200 C, preferably 50 to 150 C and more preferably 80 to 130 C.
The polymerization can be conducted with or without solvent. The term
"solvent" should be
understood here in a broad sense. The solvent is selected according to the
polarity of the
monomers used, it being possible with preference to use 100N oil,
comparatively light gas oil
and/or aromatic hydrocarbons, for example toluene or xylene.
As well as an above-detailed free-radical copolymerization of the monomers
described, the
inventive copolymers can also be obtained by polymer-analogous reactions.
In this case, a polymer is first prepared from low molecular weight monomers
in a known manner,
and is then converted. In this case, the backbone of the copolymer may be
synthesized from a
reactive monomer such as maleic anhydride, methacrylic acid or else glycidyl
methacrylate and
other unreactive short-chain backbone monomers. In this case, the above-
detailed initiator systems
such as t-butyl perbenzoate or t-butyl per-2-ethylhexanoate and chain transfer
agents such as n-
dodecyl mercaptan may be used.
In a further step, it is possible, for example in an alcoholysis or
aminolysis, to generate the side
chains, which are also referred to as arms. In this case, it is possible to
use the above-detailed
hydroxylated hydrogenated polybutadienes.
The reaction of the backbone polymer formed at first with macroalcohols
corresponds essentially to
the reactions of the macroalcohols with low molecular weight compounds
detailed above in
connection with the synthesis of the macromonomers.
Thus, the macroalcohols can be joined to the present maleic anhydride or
methacrylic acid
functionalities in the backbone polymer with catalysis, for example, by p-
toluenesulfonic acid or
CA 02909115 2015-10-08
11
methanesulfonic acid. By addition of low molecular weight alcohols and/or
amines such as n-
butanol or N-(3-aminopropyl)morpholine, this polymer-analogous reaction is
conducted to complete
conversions, especially in the case of maleic anhydride backbones.
In the case of glycidyl functionalities in the backbone, an addition of the
macroalcohol can be
conducted, so as to form comb polymers.
In addition, the macroalcohols can be reacted with a backbone containing short-
chain ester
functionalities by a polymer-analogous alcoholysis in order to generate comb
polymers.
As well as the reaction of the backbone polymer with macromolecular compounds,
it is possible to
react suitably functionalized polymers, which have been obtained by conversion
of low molecular
weight monomers, with further low molecular weight polymers to form comb
polymers. In this case,
the backbone polymer prepared at first has several functionalities which serve
as initiators of
multiple graft polymerizations.
Thus, it is possible to initiate a multiple cationic polymerization of
isobutene, which leads to comb
polymers having polyolefin side arms. Suitable methods for graft
copolymerizations of this kind
include the above-detailed ATRP and/or RAFT methods, in order to obtain comb
polymers having a
defined architecture.
The transmission oil formulation of the invention comprises a base oil as
component (i). This base
oil has a kinematic viscosity at 100 C of at least 1.5 mm2/s to ASTM D445 and
an aromatics
content of less than 15% by weight to ASTM D 2007.
The kinematic viscosity at 100 C preferably 2 mm2/s, more preferably 3 mm2/s,
to ASTM 0445.
The aromatics content of the base oil refers to the proportion in % by weight,
based on the weight
of oil, of compounds having at least one aromatic structural element, and is
determined to ASTM D
2007 by gel absorption chromatography. Preferably, the aromatics content to
ASTM D 2007 is less
than 10% by weight, preferably less than 5% by weight.
In a preferred embodiment, the base oil is additionally characterized by a
small proportion of
aromatic carbon atoms of not more than 2%, preferably not more than 0.5%, more
preferably not
more than 0.1%.
The proportion of aromatic carbon atoms is determined in the context of the
present invention by
infrared spectroscopy by the method of G. Brandes. This method was described
in detail by G.
Brandes in "Die Strukturgruppen von Erdolfraktionen I. Mitteilung: Die
Strukturgruppenanalyse mit
Hilfe der Ultrarotspektroskopie", Brennstoff-Chemie 37 (17/18), 263 (1956). In
this method, the
amount of aromatic carbon atoms CA is determined on the basis of the
absorption band at 1610
cm-1, and the amount of paraffinic carbon atoms Cp on the basis of the band at
720 cm-1. For
CA 02909115 2015-10-08
12
calibration, several oils having different aromatics and paraffin contents are
analyzed, and CA and
Cp are determined by the Brandes method. The amount of naphthenic carbon atoms
CN is found
from the difference from 100%, since no characteristic absorption can be
assigned to naphthenes.
It has been found here, surprisingly, that particularly the combination of the
copolymer for use in
accordance with the invention with a base oil having a low proportion of
aromatic carbon atoms or
a low aromatics content leads to a reduction in the coefficient of traction.
A base oil is typically defined as an oil having a boiling point between 260
and 566 C (500 and
1050 F), consisting of hydrocarbons having 18 to 40 carbon atoms. The base oil
for use in
accordance with the invention may be a mineral oil, a synthetic oil or a
natural oil. It is likewise
possible to use mixtures of various base oils. These oils are common
knowledge.
Mineral oils are known per se and are commercially available. They are
generally obtained from
mineral oil or crude oil by distillation and/or refining and optionally
further cleaning and finishing
processes, the term "mineral oil" especially including the higher-boiling
components of crude oil or
mineral oil. In general, the boiling point of mineral oil is higher than 200
C, preferably higher than
300 C, at 5000 Pa. Production by low-temperature carbonization of shale oil,
coking of hard coal,
distillation of brown coal with exclusion of air, and hydrogenation of hard
coal or brown coal is
likewise possible. Accordingly, mineral oils, depending on their origin, have
different proportions of
aromatic, cyclic, branched and linear hydrocarbons.
A reduction in the aromatics content of mineral oils can be achieved by
hydrogen treatment of the
mineral oils. In this case, aromatic components are reduced in by
hydrogenation and naphthenic
components are built up.
Synthetic oils include organic esters, for example diesters and polyesters,
polyalkylene glycols,
polyethers, synthetic hydrocarbons, especially polyolefins, among which
polyalphaolefins (FAQ)
are preferred, silicone oils and perfluoroalkyl ethers. In addition, it is
possible to use synthetic base
oils originating from gas to liquid (GTL), coal to liquid (CTL) or biomass to
liquid (BTL) processes.
They are usually somewhat more costly than the mineral oils, but have
advantages in terms of their
performance.
Natural oils are animal or vegetable oils, for example neatsfoot oils or
jojoba oils.
Base oils for lubricant oil formulations are divided into groups according to
the American Petroleum
Institute (API) as a function of saturation level, sulfur content and
viscosity index (API 1509, Annex
E - API Base Oil Interchangeability Guidelines for Passenger Car Motor Oils
and Diesel Engine
Oils, September 2011). Mineral oils are subdivided into group I (non-hydrogen-
treated) and,
depending on the saturation level, sulfur content and viscosity index, into
groups ll and ill (both
hydrogen-treated). PAOs correspond to group IV. All other base oils are
encompassed in group V.
CA 02909115 2015-10-08
13
More preferably, the base oil for use in accordance with the invention is a
group III oil as defined by
the American Petroleum Institute, since the combination of the copolymer of
the invention with a
group III oil leads to an exceptional reduction in the coefficient of traction
of the transmission oil
formulation. A group III oil has a viscosity index to ASTM D2270 of at least
120, a proportion of
saturated compounds to ASTM D 2007 of at least 90%, an aromatics content to
ASTM D 2007 of
less than 10% by weight and a sulfur content to one of the standards ASTM
D1552, D2622, D3120,
D4294 and D4927 of not more than 0.03% (API 1509, Annex E - API Base Oil
Interchangeability
Guidelines for Passenger Car Motor Oils and Diesel Engine Oils, September
2011). Group III oils
for use in accordance with the invention additionally have the abovementioned
kinematic viscosity.
The transmission oil formulation of the invention comprises preferably 60% to
99.9% by weight of
base oil (component (i)) based on the total weight of the transmission oil
formulation, preferably
60% to 90% by weight, more preferably 70% to 80% by weight, based on the total
weight of the
transmission oil formulation.
The concentration of the copolymer (component (ii)) in the transmission oil
formulation is preferably
in the range from 0.1% to 40% by weight, based on the total weight of the
transmission oil
formulation, more preferably in the range of 0.2%-20% by weight and most
preferably in the range
of 0.5%-10% by weight, based on the total weight of the transmission oil
formulation.
In a particular embodiment, the proportions of components (i) and (ii) add up
to 100% by weight.
The transmission oil formulation of the invention may also comprise, as
component (iii), a second
polymer selected from the group of the hydrogenated polybutadienes,
hydroxylated hydrogenated
polybutadienes or (meth)acrylic esters thereof, polyalkyl (meth)acrylates and
mixtures thereof.
Preferably, the transmission oil formulation comprises 0% to 3% by weight of
component (iii) based
on the total weight of the transmission oil formulation, preferably 0.005% to
2% by weight, based
on the total weight of the transmission oil formulation.
The hydrogenated polybutadienes and the hydroxylated hydrogenated
polybutadienes or the
methacrylic esters thereof preferably have a number-average molar mass M5 of
4000 to 6000
g/mol. The hydroxylated hydrogenated polybutadienes may, for example, be the
hydroxylated
hydrogenated polybutadienes described, especially hydroxyethyl- or
hydroxypropyl-terminated
hydrogenated polybutadiene or (meth)acrylic esters thereof. The polyalkyl
(meth)acrylates may, for
example, be polymerization products of the (meth)acrylic esters described.
If the transmission oil formulation contains component (iii), the proportions
by weight of
components (i), (ii) and (iii) may add up to 100% by weight.
The transmission oil formulation of the invention may also contain, as
component (iv), further
additives selected from the group consisting of dispersants, defoamers,
detergents, antioxidants,
CA 02909115 2015-10-08
14
antiwear additives, extreme pressure additives, friction modifiers,
anticorrosion additives, dyes and
mixtures thereof.
Preferably, the total concentration of additives is up to 20% by weight, more
preferably 0.05% to
15% by weight, more preferably 5% to 15% by weight, based on the total weight
of the
transmission oil formulation. Dispersants (including borated dispersants) are
preferably used in a
concentration of 0% to 5% by weight, detergents in a concentration of 0.05% to
3% by weight,
anticorrosion additives in a concentration of 0.05% to 2% by weight, friction
modifiers in a
concentration of 0.05% to 5% by weight, antiwear and extreme pressure
additives each in a
concentration of 0.1% to 3% by weight, antioxidants in a concentration of 0.5%
to 1.5% by weight,
defoamers in a concentration of 10 to 2500 ppm and dyes in a concentration of
0.01% to 1% by
weight. The concentration is based in each case on the total weight of the
transmission oil
formulation.
According to the composition, the proportions by weight of components (i),
(ii), (iii) and (iv) or (i), (ii)
and (iv) may add up to 100% by weight.
Appropriate dispersants include poly(isobutylene) derivatives, for example
poly(isobutylene)succinimides (PIBSIs), including borated PIBSIs; ethylene-
propylene oligomers
having N/O functionalities.
The preferred detergents include metal-containing compounds, for example
phenoxides;
salicylates; thiophosphonates, especially thiopyrophosphonates,
thiophosphonates and
phosphonates; sulfonates and carbonates. As metal, these compounds may contain
especially
calcium, magnesium and barium. These compounds may preferably be used in
neutral or
overbased form.
The suitable antioxidants include, for example, phenols, for example 2,6-di-
tert-butylphenol (2,6-
DTB), butylated hydroxytoluene (BHT), 2,6-di-tert-butyl-4-methylphenol, 4,4'-
methylenebis(2,6-di-
tert-butylphenol); aromatic amines, especially alkylated diphenylamines, N-
phenyl-1-naphthylamine
(PNA), polymeric 2,2,4-trimethyldihydroquinone (TMQ); compounds containing
sulfur and
phosphorus, for example metal dithiophosphates, for example zinc
dithiophosphates (ZnDTPs),
"OOS triesters" = reaction products of dithiophosphoric acid with activated
double bonds from
olefins, cyclopentadiene, norbornadiene, a-pinene, polybutene, acrylic esters,
maleic esters
(ashless on combustion); organosulfur compounds, for example dialkyl sulfides,
diaryl sulfides,
polysulfides, modified thiols, thiophene derivatives, xanthates, thioglycols,
thioaldehydes, sulfur-
containing carboxylic acids; heterocyclic sulfur/nitrogen compounds,
especially
dialkyldimercaptothiadiazoles, 2-mercaptobenzimidazoles; zinc
bis(dialkyldithiocarbamate) and
methylene bis(dialkyldithiocarbamate); organophosphorus compounds, for example
triaryl and
trialkyl phosphites; organocopper compounds and overbased calcium- and
magnesium-based
phenoxides and salicylates.
CA 02909115 2015-10-08
The preferred antiwear and extreme pressure additives include phosphorus
compounds, for
example trialkyl phosphates, triaryl phosphates, e.g. tricresyl phosphate,
amine-neutralized mono-
and dialkyl phosphates, ethoxylated mono- and dialkyl phosphates, phosphites,
phosphonates,
phosphines; compounds having sulfur and phosphorus, for example metal
dithiophosphates, e.g.
5 zinc di-C3_12-alkyldithiophosphates (ZnDTPs), ammonium
dialkyldithiophosphates, antimony
dialkyldithiophosphates, molybdenum dialkyldithiophosphates, lead
dialkyldithiophosphates, "OOS
triesters" = reaction products of dithiophosphoric acid with activated double
bonds from olefins,
cyclopentadiene, norbornadiene, a-pinene, polybutene, acrylic esters, maleic
esters, triphenyl
phosphorothionate (TPPT); compounds having sulfur and nitrogen, for example
zinc
10 bis(amyldithiocarbamate) or methylenebis(di-n-butyl dithiocarbamate);
sulfur compounds with
elemental sulfur and H2S sulfurized hydrocarbons (diisobutylene, terpene);
sulfurized glycerides
and fatty acid esters; overbased sulfonates; chlorine compounds or solids,
such as graphite or
molybdenum disulfide.
15 Friction modifiers used may include mechanically active compounds, for
example molybdenum
disulfide, graphite (including fluorinated graphite), poly(trifluoroethylene),
polyamide, polyimide;
compounds that form adsorption layers, for example long-chain carboxylic
acids, fatty acid esters,
ethers, alcohols, amines, amides, imides; compounds which form layers through
tribochennical
reactions, for example saturated fatty acids, phosphoric acid and
thiophosphoric esters,
xanthogenates, sulfurized fatty acids; compounds that form polymer-like
layers, for example
ethoxylated dicarboxylic partial esters, dialkyl phthalates, methacrylates,
unsaturated fatty acids,
sulfurized olefins or organometallic compounds, for example molybdenum
compounds
(molybdenum dithiophosphates and molybdenum dithiocarbamates MoDTCs) and
combinations
thereof with ZnDTPs, copper-containing organic compounds.
Some of the compounds listed above may fulfill multiple functions. ZnDTP, for
example, is primarily
an antiwear additive and extreme pressure additive, but also has the character
of an antioxidant
and corrosion inhibitor (here: metal passivator/deactivator).
The above-detailed additives are described in detail, inter alia, in T. Mang,
W. Dresel (eds.):
"Lubricants and Lubrication", Wiley-VCH, Weinheim 2001; R. M. Mortier, S. T.
Orszulik (eds.):
"Chemistry and Technology of Lubricants".
The present invention also relates to the use of the above-described
transmission oil formulation as
transmission oil for reducing the fuel consumption of motor vehicles.
It is possible to use this transmission oil formulation in manual, automated
manual, double clutch or
DSG, automatic and continuous variable (CVC) transmissions. Particular
preference is given to
using the transmission oil formulation as transmission oil for automatic
transmissions. In addition,
the transmission oil formulation described can be used in transfer cases and
axles or differentials.
CA 02909115 2015-10-08
16
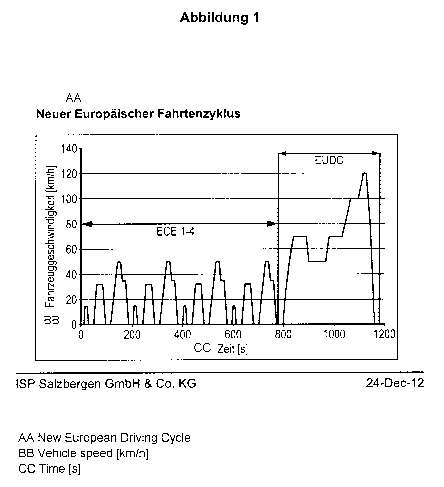
Description of illustrations
Figure 1 shows the speed profile of the New European Driving Cycle (NEDC) for
determination of
the fuel consumption of motor vehicles.
Examples
In the examples which follow, the following abbreviations are used:
MM1 methacrylic ester of a hydroxypropyl-terminated hydrogenated
polybutadiene having
Mr, = 4750 g/mol
AMA1 methacrylic ester of a synthetic iso-C11-C15 alcohol mixture, iso
content about 60%
AMA2 methacrylic ester of a synthetic C10-C15 alcohol mixture, iso
content about 15%
AMA3 methacrylic ester of a linear C12-C14 alcohol mixture
AMA4 mixture of a methacrylic ester of a synthetic C10-C15 alcohol
mixture, iso content
about 15%, with a methacrylic ester of a linear C16-C18 alcohol mixture
BMA n-butyl methacrylate
MMA methyl methacrylate
Sty styrene
BA n-butyl acrylate
t-DDM tert-dodecyl mercaptan
DMAPMAm N,N-dimethylaminomethacrylamide
BDtBPB 2,2-bis(tert-butylperoxy)butane
n-DDM dodecyl mercaptan
tBP0 tert-butyl peroctoate
Synthesis of a hydroxylated hydrogenated polybutadiene
The macroalcohol prepared was a hydroxypropyl-terminated hydrogenated
polybutadiene having
mean molar mass Mr, = 4750 g/mol.
The macroalcohol was synthesized by an anionic polymerization of 1,3-butadiene
with butyllithium
at 20-45 C. On attainment of the desired degree of polymerization, the
reaction was stopped by
adding propylene oxide and lithium was removed by precipitation with methanol.
Subsequently, the
polymer was hydrogenated under a hydrogen atmosphere in the presence of a
noble metal catalyst
at up to 140 C and pressure 200 bar. After the hydrogenation had ended, the
noble metal catalyst
was removed and organic solvent was drawn off under reduced pressure. Finally,
the base oil
Nexbase 3020 (base oil of API group II, kinematic viscosity to ASTM D 445 at
100 C of 2.1 to 2.3
mm2/s) was used for dilution to a polymer content of 70% by weight.
CA 02909115 2015-10-08
17
The vinyl content of the macroalcohol was 61%, the hydrogenation level > 99%
and the OH
functionality > 98%. These values were determined by H NMR (nuclear resonance
spectroscopy).
Synthesis of macromonomer MM1
In a 2 L stirred apparatus equipped with saber stirrer, air inlet tube,
thermocouple with controller,
heating mantle, column having a random packing of 3 mm wire spirals, vapor
divider, top
thermometer, reflux condenser and substrate cooler, 1000 g of the above-
described macroalcohol
are dissolved in 450 g of methyl methacrylate (MMA) by stirring at 60 C. Added
to the solution are
20 ppm of 2,2,6,6-tetramethylpiperidin-1-oxyl radical and 200 ppm of
hydroquinone monomethyl
ether. After heating to MMA reflux (bottom temperature about 110 C) while
passing air through for
stabilization, about 20 g of MMA are distilled off for azeotropic drying.
After cooling to 95 C, 0.30 g
of LiOCH3 is added and the mixture is heated back to reflux. After the
reaction time of about 1 hour,
the top temperature has fallen to ¨64 C because of methanol formation. The
methanol/MMA
azeotrope formed is distilled off constantly until a constant top temperature
of about 100 C is
established again. At this temperature, the mixture is left to react for a
further hour. For further
workup, the bulk of MMA is drawn off under reduced pressure. Insoluble
catalyst residues are
removed by pressure filtration (Seitz T1000 depth filter). The content of
Nexbase 3020 "entrained"
into the copolymer syntheses described further down was taken into account
accordingly.
Synthesis of copolymers
Copolymer 1
In a beaker, the following reaction mixture was made up: 87.9 g of 70%
macromonomer solution in
oil, 3.9 g of AMA3, 27.3 g of BMA, 51.9 g of Sty, 0.3 g of MMA, 5.1 g of
DMAPMAm, 65.0 g of
Shell Risella 907 (light naphthenic/paraffinic base oil) and 8.6 g of Nexbase
3020. A 500 mL 4-neck
round-bottom flask with saber stirrer, nitrogen blanketing, thermometer,
regulated oil bath and
reflux condenser was initially charged with 75 g of the reaction mixture and
heated to 120 C while
stirring. During the heating phase, nitrogen was passed through the reaction
flask for inertization.
On attainment of 120 C, 0.09 g of BDtBPB was added to the reaction flask; at
the same time, the
feed consisting of the rest of the reaction mixture and 0.21 g of BDtBPB was
started. The feed time
was 3 hours; the reaction temperature was kept constant at 120 C. 2 and 5
hours after feeding had
ended, another 0.30 g each time of BDtBPB was added, and the contents of the
flask were diluted
the next day by addition of 102.9 g of Nexbase 3020. A clear, highly viscous
solution was obtained.
Copolymer 2
As copolymer 1, but with the following reaction mixture: 90.0 g of 70%
macromonomer solution in
oil, 0.3 g of AMA3, 19.2 g of BMA, 59.7 g of Sty, 0.3 g of MMA, 7.5 g of BA,
65.0 g of Shell Risella
907 (light naphthenic/paraffinic base oil) and 8.0 g of Nexbase 3020.
CA 02909115 2015-10-08
18
Copolymer 3
As copolymer 1, but with the following reaction mixture: 82.5 g of 70%
macromonomer solution in
oil, 7.4 g of AMA3, 63.0 g of BMA, 16.5 g of Sty, 0.3 g of MMA, 5.1 g of
DMAPMAm, 65.0 g of
Shell RiseIla 907 (light naphthenic/paraffinic base oil) and 10.3 g of Nexbase
3020.
Copolymer 4
A 500 mL 4-neck round-bottom flask with saber stirrer, nitrogen blanketing,
thermometer, regulated
oil bath and reflux condenser was initially charged with 90.0 g of 70%
macromonomer solution in
oil, 0.3 g of AMA3, 0.3 g of BMA, 26.7 g of Sty, 59.7 g of MMA and 73 g of o-
xylene, and heated to
120 C while stirring. During the heating phase, nitrogen was passed through
the reaction flask for
inertization. On attainment of 120 C, 0.30 g of BDtBPB was added to the
reaction flask; the
reaction temperature was kept constant at 120 C. 2 and 5 hours after the first
BDtBPB addition,
another 0.30 g each time of BDtBPB was added, and the contents of the flask
were diluted the next
day by addition of 110.9 g of Nexbase 3020 and Shell RiseIla 907. The o-xylene
was subsequently
drawn off by applying reduced pressure. A highly viscous solution with whitish
turbidity was
obtained.
Copolymer 5
As copolymer 1, but with the following reaction mixture: 90.0 g of 70%
macromonomer solution in
oil, 0.3 g of AMA3, 26.7 g of BMA, 59.7 g of Sty, 0.3 g of MMA, 65.0 g of
Shell RiseIla 907 (light
naphthenic/paraffinic base oil) and 8.0 g of Nexbase 3020.
Copolymer 6 (comparative polymer)
In a beaker, the following reaction mixture was made up: 126.4 g of AMA2,
129.4 g of AMA1, 1.5 g
of AMA4, 29.8 g of MMA, 5.1 g of DMAPMAm and 4.1 g of n-DDM. A 500 mL 4-neck
round-bottom
flask with saber stirrer, nitrogen blanketing, thermometer, regulated oil bath
and reflux condenser
was initially charged with 108 g of 100N oil and 12 g of the reaction mixture,
and heated to 100 C
while stirring. During the heating phase, nitrogen was passed through the
reaction flask for
inertization. On attainment of 100 C, 0.13 g of tBP0 was added to the reaction
flask; at the same
time, the feed consisting of the rest of the reaction mixture and 0.70 g of
tBP0 was started. The
feed time was 3.5 hours; the reaction temperature was kept constant at 100 C.
2 hours after the
feed had ended, another 0.58 g of tBP0 was added and the mixture was stirred
at 100 C
overnight. A clear, viscous solution was obtained.
CA 02909115 2015-10-08
19
Copolymer 7 (comparative polymer)
A 500 mL 4-neck round-bottom flask with saber stirrer, nitrogen blanketing,
thermometer, regulated
oil bath and reflux condenser was initially charged with 241.1 g of AMA, 33.8
g of MMA, 114.6 g of
150N oil, 4.1 g of n-DDM and 3.0 g of t-DDM. The mixture was heated to 110 C
while stirring.
During the heating phase, nitrogen was passed through the reaction flask for
inertization. On
attainment of 110 C, a mixture of 0.69 g of tBP0 and 2.06 g of 150N oil was
metered in by means
of a syringe pump within 3 hours. 1 hour after the feed had ended, another
0.55 g of tBP0 was
added and the mixture was left to continue to react at 110 C overnight. A
clear, viscous solution
was obtained.
Copolymer 8 (comparative polymer)
A 500 mL 4-neck round-bottom flask with saber stirrer, nitrogen blanketing,
thermometer, regulated
oil bath and reflux condenser was initially charged with 34.3 g of 70%
macromonomer solution, 7.5
g of AMA3, 100.5 g of BMA, 18.0 g of Sty, 65.0 g of Shell RiseIla 907 (light
naphthenic/paraffinic
base oil) and 24.7 g of 100N oil. The mixture was heated to 120 C while
stirring. During the heating
phase, nitrogen was passed through the reaction flask for inertization. On
attainment of 120 C, 0.3
g of BDtBPB was added. 3 and 5 hours after the first BDtBPB addition, another
0.3 g each time of
BDtBPB was added and the mixture was left to continue to react at 120 C
overnight. The next day,
the contents of the flask were diluted by addition of 125 g of 150N oil. A
clear, viscous solution was
obtained.
Copolymer 9 (comparative polymer)
As copolymer 8, except that the initial charge was 25.7 g of 70% macromonomer
solution, 7.5 g of
AMA3, 106.5 g of BMA, 18.0 g of Sty, 65.0 g of Shell RiseIla 907 (light
naphthenic/paraffinic base
oil) and 27.3 g of 100N oil.
Copolymer 10 (comparative polymer)
As copolymer 8, except that the initial charge was 25.7 g of 70% macromonomer
solution, 7.5 g of
AMA3, 124.5 g of BMA, 65.0 g of Shell RiseIla 907 (light naphthenic/paraffinic
base oil) and 27.3 g
of 100N oil.
Table 1 shows an overview of the monomer compositions for the copolymer
syntheses.
Copolymers 1, 2, 4 and 5 are copolymers of the invention.
Table 1: Monomer compositions for the copolymer syntheses and branching level
"f-
branch" of the copolymers. The proportions by weight of the individual
monomers are each
CA 02909115 2015-10-08
reported in % by weight based on the total mass of all the monomers.
Copolymers of the
invention are identified by "Inv.", comparative copolymers by "Comp.".
Copolymer 1 2 3 4 5 6 7 8 9 10
Inv. Inv. Inv. Inv. Inv. Comp. Comp. Comp. Comp. Comp.
MM1 41.0 42.0 38.5 42.0 42.0 16.0 12.0 12.0
AMA1 44.4 87.7
AMA2 43.4
AMA3 2.6 0.2 4.9 0.2 0.2 5.0 5.0
5.0
AMA4 0.5
BMA 18.2 12.8 42.0 0.2 17.8 67.0 71.0 83.0
BA 5.0
Sty 34.6 39.8 11.0 17.8 39.8 12.0 12.0
MMA 0.2 0.2 0.2 39.8 0.2 9.9 12.3
DMAPMAm 3.4 3.4 1.8
f-branch 1.62 1.59 1.69 1.43 1.6 0.00 0.00 0.47 0.33 0.35
5 The calculations of the molar branching level ("f-branch) are based on a
macromonomer
conversion of 94%, meaning that all the copolymers still contain residual
macromonomer and are
thus a polymer mixture of copolymer and hydroxypropyl-terminated hydrogenated
polybutadiene.
These polymers are not separated. The transmission oil formulations formulated
from these
copolymer additives thus still contain a polymer mixture.
Transmission oil formulations
The abovementioned copolymers were used to produce transmission oil
formulations (Table 2).
Transmission oil formulations El to E5 are formulations of the invention.
Formulation CE6 is a
comparative formulation known from the prior art.
The base oil used was Nexbase 3030 (available from Neste Oil N.V., Belgium).
Nexbase 3030 is
an API group III base oil having a proportion of aromatic carbon atoms (% CA)
below the detection
limit of the IR method (<0.1%).
The pour point depressant used is a copolymer of C12 to C18 methacrylates from
Evonik Oil
Additives. The DI package is a DEXRON VI-compatible DI package without
viscosity improver.
The base oil viscosity of the formulations El to E5 and CE6 was 3.8 mm2/s at
100 C.
CA 02909115 2015-10-08
21
Table 2: Composition of transmission oil formulations. The proportions by
weight of the
individual formulation components are reported in % by weight based on the
total weight of
the transmission oil formulation.
Transmission oil formulation El E2 E3 E4 E5 CE 6
Copolymer 1 5.2
Copolymer 2 5.4
Copolymer 3 5.4
Copolymer 4 10.7
Copolymer 5 5.7
Copolymer 6 8.5
DI package 15.0 15.0 15.0 15.0 15.0 15.0
Pour point 0.3 0.3 0.3 0.3 0.3 0.3
depressant
Nexbase 3030 79.5 79.3 79.3 74.0 79.0 76.2
Also produced were comparative oil formulations according to Table 3 based on
a 150 N
formulation oil. The base oil viscosity of these formulations was 5.4 mm2/s at
100 C. These
formulations were used to examine the lack of stability of copolymers 6 to 10
(see below).
Table 3: Composition of comparative oil formulations. The proportions by
weight of the
individual formulation components are reported in % by weight based on the
total weight of
the comparative oil formulation.
Comparative oil formulation CE1 CE2 CE3 CE4 CE5
Copolymer 6 24.6
Copolymer 7 29.5
Copolymer 8 15.0
Copolymer 9 15.0
Copolymer 10 15.0
DI package 0.6 0.6 0.6 0.6 0.6
150N 74.8 69.9 84.4 84.4 84.4
formulation oil
Viscometric evaluation of the transmission oil formulations
The viscometric evaluation of the transmission oil formulations was made by
determining the
kinematic viscosity at 40 C (KV40) and 100 C (KV100) to ASTM D445, the
viscosity index (VI) to
ASTM D2270 and the dynamic viscosity at high temperature and high shear at 80
C (HTHS 80 C)
and 100 C (HTHS 100 C) to ASTM 4683.
CA 02909115 2015-10-08
22
The dynamic viscosity (DV) at 100 C under low shear was calculated from the
product of density
and kinematic viscosity at 100 C (KV100).
In addition, the shear stability was determined on the basis of the kinematic
viscosity at 100 C after
a 20-hour tapered roller bearing test CEC-L-45-A-99 (KV100 after TRB20h).
The viscosity values before and after shear were used to calculate the
permanent shear stability
index (PSSI) as follows:
PSSI = (KV100before shear ¨ KV100after shear)/(KV100before shear ¨ base oil
viscosity)*100
The PSSI indicates the percentage loss of viscosity which has been introduced
into the formulation
via the polymer only. The PSSI is accordingly a characteristic feature of a
polymer and is therefore
very substantially independent of the amount added or of other formulation
components (oil and
other additives). This becomes clear from the comparison of the PSSI values of
formulations CE1
and 0E6, which contain the same polymer but different base oils and additives
(see table 4 and
table 5).
The temporary shear stability index (TSSI) was calculated from the dynamic
viscosity under high
shear and low shear as follows:
TSSI = (DV100low shear ¨ DV100high shear)/(DV100low shear ¨ DVbase earl 00
The DV100 of the base oil of the transmission oil formulations (Nexbase 3030)
was 2.45 mPas.
According to the DEXRON-VI specification, a transmission oil formulation must
have a KV100 of
not more than 6.4 mm2/s and a KV100 after shear of at least 5.5 mm2/s. It can
be inferred from
these requirements and a base oil viscosity of 3.8 mm2/s (Nexbase 3030) that a
suitable
transmission oil formulation must have a PSSI of not more than 34.
Of the comparative examples listed in table 4, only formulations CE1 and CE2
and hence
copolymers 6 and 7 meet this requirement. The PSSI of formulations CE3 to CE5,
containing
copolymers 8 to 10, in contrast, is too high. The copolymers used in
formulations CE3 to CE5 are
thus not of adequate shear stability to serve as additives in transmission oil
formulations.
This shows that the exact monomer composition of the inventive copolymers is
crucial for the
suitability thereof as a transmission oil additive.
Since the polymer in CE1 (copolymer 6), with comparable shear stability,
achieves a much higher
viscosity index than the polymer in CE2 (copolymer 7), further viscometric
characterizations were
restricted to copolymer 6.
CA 02909115 2015-10-08
23
Table 4: Viscometric evaluation of comparative oil formulations CE1 to CE5.
Comparative oil CE1 CE2 CE3 CE4 CE5
formulation
KV40 (mm2/s) 92.47 95.62 44.02 41.97
43.13
KV100 (mm2/s) 14.22 13.97 14.12 13.95
15.97
vi. 159 149 336 347 385
KV100 after 12.85 12.87 10.63 not
determinable 10.50
TRB2Oh (mm2/s)
PSSI 15.6 12.9 40.1 insoluble constituents 51.8
The measured data for the transmission oil formulations El to E5 and CE6 are
summarized in
Table 5. All these transmission oil formulations have the fresh oil viscosity
KV100 required by the
DEXRON-VI specification of not more than 6.4 mm2/s.
However, it is found that the comparative formulation CE6 has a much lower
viscosity index than
the inventive formulations El to E5. In addition, comparative formulation CE6
has a lower
temporary shear loss than the formulations of the invention. This is shown by
the higher TSSI
values of formulations El to E6.
The temporary shear loss can additionally be calculated using the difference
between the viscosity
at 100 C and high shear (HTHS 100 C) and the dynamic viscosity at 100 C and
low shear. This
difference is much higher for formulations El to E4 than for formulation CE6.
Table 5: Viscometric evaluation of transmission oil formulations El to E5 and
CE6:
Transmission oil El E2 E3 E4 E5 CE6
formulation
KV40 (mm2/s) 24.30 24.36 21.60 25.85 24.68 28.43
KV100 (mm2/s) 6.02 6.02 6.12 6.02 6.02 6.03
VI 211 211 260 192 210 166
KV100 after 5.63 5.6 5.429 5.67 5.70 5.71
TRB2Oh (mm2/s)
PSSI 17.6 18.9 29.8 15.8 14.6 14.3
TSSI 15.5 12.7 28.0 17.3 13.0 1.1
PSSI/TSSI 1.1 1.5 1.1 0.9 1.1 13.0
HTHS 80 C (mPas) 6.61 6.62 5.92 6.61 6.63 7.18
HTHS 100 C (mPas) 4.43 4.49 4.17 4.39 4.49 4.78
Dynamic viscosity 4.79 4.79 4.84 4.80 4.79 4.81
at 100 C (low shear) (mPas)
Density 100 C (g/cm3) 0.7962 0.7952 0.7952 0.7968 0.7965 0.7969
CA 02909115 2015-10-08
24
Determination of the coefficient of traction of transmission oil formulations
Traction measurements were conducted on a mini-traction machine (MTM 2). The
measurement
parameters and test specimens which follow were used for the measurements. For
each
measurement, a new set of test specimens was utilized.
Test rig MTM 2 from PCS Instruments
Disk Steel, AISI 52100, diameter = 46 mm
RMS = 25-30 nm, Rockwell C hardness = 63
Modulus of elasticity = 207 GPa
Ball Steel, AISI 52100, diameter = 19.05 mm
RMS = 10-13 nm, Rockwell C hardness = 58-65
Modulus of elasticity = 207 GPa
Speed 2000 mm/s
Temperature 100 C
Load 30-75 N
Sliding/rolling ratio 50%
First of all, the traction behavior of transmission oil formulations based on
aromatics-containing
base oils was examined. In terms of their composition, these formulations
correspond to hydraulic
oils as described, for example, in DE 10 2009 001 447 Al. For this purpose,
copolymer 5 or
copolymer 7 was dissolved in a 100N oil of API group I (KV100 = 3.8 mm2/s; %-
CA = 2.1 by IR
method, aromatics content 17%), and the coefficient of traction at 100 C was
measured as
described above. The results are summarized in Table 6.
Table 6: Coefficient of traction of transmission oil formulations based on
aromatics-
containing formulation oil. The proportions by weight of the individual
formulation
components are reported in % by weight based on the total weight of the
transmission oil
formulation.
Transmission oil formulation E6 CE8
Copolymer 5 9.4
Copolymer 7 18.6
100N formulation oil 90.6 81.4
KV40 C (mm2/s) 30.31 40.86
KV100 C (mm2/s) 7.51 7.50
VI 231 152
1Coefficient of traction 0.033 0.034
The deviation of the coefficients of traction of formulations E6 and CE8 is
within the measurement
error, and should not be regarded as significant. It can therefore be
concluded from the analysis
CA 02909115 2015-10-08
that there is no significant difference in the traction behavior of the
copolymers of the invention and
of a known comparative polymer in aromatics-containing oils.
In addition, the traction behavior of transmission oil formulations based on
base oils having a low
5 aromatics content was examined. For this purpose, the coefficient of
traction of formulations El to
E5 and CE6 was determined. The results are shown in table 7.
Table 7: Coefficient of traction of transmission oil formulations based on
formulation oil
with low aromatics content. The percentage reduction in the coefficient of
traction was
10 calculated on the basis of the difference from CE6.
Reduction in coefficient
Transmission oil formulation Coefficient of traction of traction in %
El 0.020 33.3
E2 0.024 20.0
E4 0.023 23.3
E5 0.021 30.0
E3 0.018 40.0
CE6 0.030 0.0
Surprisingly, the transmission oil formulations of the invention have a much
lower coefficient of
traction than the comparative formulation CE6. This demonstrates a synergistic
effect with respect
to the coefficient of traction, which is achieved by the inventive combination
of a copolymer of the
15 invention with a base oil having a low aromatics content, but not by the
combination of a
comparative copolymer with a base oil having a low aromatics content. This
synergistic effect is an
important prerequisite for formulation of fuel-saving transmission oils.
Especially compared with the transmission oil formulations based on aromatics-
containing
20 formulation oil in which the copolymers of the invention do not lead to
any improvement over the
comparative polymers, it was surprising that the coefficient of traction in a
base oil having a low
aromatics content can be distinctly reduced by a copolymer of the invention.
Measurement of fuel consumption
Fuel consumption measurements were conducted on a certified rolling test bed
(ISP Salzbergen).
The motor vehicle used for this purpose was a Hyundai ix35 (gasoline engine,
max. power 120 kW
at 6200 rpm; maximum torque 194 Nm at 4600 rpm) with a six-speed step-change
automatic
transmission system. The motor vehicle was conditioned appropriately before
each test. Before
and after the candidate oils, a reference oil was run (factory-fill oil), in
order to rule out unwanted
drift of the measurement results. Each test oil (including the reference oil)
was analyzed four times
on successive days. Reported results are the mean value from four individual
measurements in
each case. Before the analysis of a new test oil, the automatic transmission
system and torque
CA 02909115 2015-10-08
26
converter were flushed five times with the new test oil to be analyzed, in
order to avoid any carry-
over effects. The consumption of fuel was calculated from the respective CO2
emissions. For all
tests, appropriate reference fuel (RF-02-08 E5, CEC E45) was used. The data
for the test system
used are summarized in the following tables:
2.1 Testing Facility - Chassis Dynamometer
suPPlier NIA1-1A-AIP GmbH 8, Co KG
Type designation ECCM 4.L 4x4
IAccuracy on vehicle load within 0.5)0
simufation (coast-down)
Accuracy on vehicie speed - IN3 km h
contfoi
2.2 Testing Facility - Gas Sampling and Analyzser System
System Horta MEXA-7400HLE Honba CVS-7400S
COI CO2Analyzser MEXA AIA-72 IA MEXA AIA-722
NOx Analyzser MEXA CLA-750LE
HC Analyzser MEXA F1A-725LE
Accuracy on gas within le.* of measuring scale
measurement
The results of the fuel analysis are presented as follows: Fuel consumption in
L/100 km for the
ECE (European City Cycle) 1+2 (cold start) driving cycles, fuel consumption in
L/100 km for the
ECE 3+4 driving cycles (moderate oil temperature), fuel consumption in U100 km
for the EUDC
(Ex-Urban Driving Cycle, "warm" operating range ¨ oil temperature at the end
of the test ¨65 C),
and the NEDC (New European Driving Cycle) averaged from all the cycles,
likewise in L/100 km.
The speed profile of the NEDC is shown in figure 1.
Fuel consumption measurements were conducted with transmission oil
formulations El, E4 and
CE6. The results are shown in table 8.
Table 8: Fuel consumption of transmission oil formulations El, E4 and CE6 in
liters per 100
km as a function of analysis cycle.
% variation
Analysis cycle Reference 1 El E4 CE6 Reference 2
reference
ECE 1+2 12.73 12.83 12.87
ECE 3+4 10.21 10.19 10.29
EUDC 6.82 6.78 6.88
NEDC 8.57 8.53 8.52 8.62 8.54 0.35
CA 02909115 2015-10-08
27
It is found that a lower fuel consumption is achieved with the transmission
oil formulations of the
invention than with the comparative formulation CE6. The percentage fuel
saving of the
transmission oil formulations of the invention compared to the comparative
transmission oil
formulation CE6 is shown in table 9. What is noticeable here is that the fuel
saving is observed not
just in the cold start phase (ECE 1+2). Instead, it becomes clear that a
distinct advantage is still
observed for the formulations of the invention even in the cycles in which the
transmission oil has
warmed up distinctly as a result of operation (EUDC in particular).
This effect cannot be explained by an increased viscosity index alone, since
lubricants El, E4 and
CE6 are each set to the same KV100, and the viscosities of lubricants
therefore increasingly match
one another at higher operating temperature.
Table 9: Percentage fuel saving of the transmission oil formulations of the
invention
compared to the comparative transmission oil formulation CE6 as a function of
analysis
cycle.
Transmission oil formulation El E4
ECE 1+2 1.09 0.31
ECE 3+4 0.78 0.97
EUDC 0.87 1.45
NEDC 1.04 1.16