Note: Descriptions are shown in the official language in which they were submitted.
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
ANODIZED METAL PRODUCT WITH ANTIMICROBIAL PROPERTIES
AND METHOD FOR PRODUCING THE SAME
FIELD OF THE INVENTION
[0001] The
invention relates to metal products having an anodized surface with
antimicrobial properties. It also concerns methods for producing the same and
articles of
manufacture comprising such metal products.
BACKGROUND OF THE INVENTION
[0002]
Aluminum alloys materials are light, have strength, softness and provide
some durability against corrosion once anodized. Applications of aluminum
alloys
products are numerous and include kitchen wares, household furniture,
appliances, door
knobs, and medical devices. Unfortunately, aluminum does not have
antimicrobial
property of its own and microorganisms can easily stay alive on its surface.
[0003]
Various methods have been developed for treating the surface of aluminum
or aluminum alloy products for improving its antimicrobial properties. The
following
documents are selected examples of such methods: US patent No. 5,753,322
(Yamaduchi et al.); US 6,168,869 (Tomioka et al.); European patent No. 1 207
220
(Takayssy et al.); Japanese patent application No. 11 323 597 (Kazuo et al.);
Japanese
patent publication No. 10 168 597 (Hisao etal.); Japanese patent publication
No. 10 168
598 (Hisao etal.); Japanese patent publication No. 10 168 598 (Hisao etal.);
Chi etal.,
Antibacterial activity of anodized aluminum with deposited silver, Surface and
Coatings
Technology, 157, (2002) 162-165; Chi etal., Antibacterial surface treatment of
aluminum
materials, Chinese J. Chem. Eng., 10(5), (2002), 622-624. However, these
methods and
the products obtained therefrom are less than optimal because antimicrobial
activity is
either weak or limited to few bacterial species and/or because antibacterial
treatment is
not compatible to dying of the aluminum product. Furthermore, these methods
may not
be applicable to antimicrobial treatment of other anodizable metals such as
magnesium,
zinc, niobium, tantalum and titanium.
[0004] There
is thus a need for improved methods for treating the surface of
anodizable metals such as aluminum in order to obtain a colored metal product
having
antimicrobial properties. There is also a need for anodizable metal products
having a
surface which has been coated such that it is effective in killing a broad
spectrum of
microorganism, including Gram-positive microbial pathogens, Gram-negative
microbial
- 1 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
pathogens and yeasts. There is also a need for antimicrobial solutions useful
for
impregnating anodizable metal products and providing metal products with an
antimicrobial coating.
[0005] The
present invention addresses these needs and other needs as it will be
apparent from review of the disclosure, drawings and description of the
features of the
invention hereinafter.
BRIEF SUMMARY OF THE INVENTION
[0006] The
invention relates to metal products having an anodized surface with
antimicrobial properties.
[0007] One
particular aspect of the present invention concerns an antimicrobial
metal product. According to one particular embodiment, the antimicrobial metal
product
comprises a porous surface layer formed by anodization, the porous surface
layer
comprising an electrodeposit of at least one metal and at least one
antimicrobial
compound.
[0008] According to
another embodiment, the antimicrobial metal product comprises
an anodized metal substrate and an antimicrobial surface coating. The
antimicrobial
surface coating comprises a porous surface layer formed by anodization of the
metal
substrate. The porous surface layer comprises an electrodeposit of at least
one metal
and at least one antimicrobial compound in the pores of the surface layer.
[0009] The metal
product may be selected from aluminum, titanium, zinc,
magnesium, niobium, tantalum and anodizable alloys thereof. The
electrodeposited
metal may be selected from silver, gold, copper, nickel, zinc, tin, palladium,
cadmium
and platinum.
[00010] The antimicrobial compound may be selected from antivirals,
antibiotics, and
antifungals. The antimicrobial compound(s) may be selected such that the
antimicrobial
surface coating of the antimicrobial metal product possesses antimicrobial
activity
against Gram-positive bacteria, Gram-negative bacteria and/or yeast. In
preferred
embodiments the antimicrobial compound is not irreversibly captured in the
pores such
that it can diffuse outside the pores.
[00011] In preferred
embodiments, the antimicrobial compound is a quaternary
ammonium such as Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC). More
- 2 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
preferably, the porous surface layer comprises at least two quaternary
ammonium
compounds i.e. Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) and Didecyl
Dimethyl Ammonium Chloride (DDAC).
[00012] The porous surface layer may further comprise a dye and the surface
layer
may also be sealed partially or completely.
[00013] A preferred aspect of the invention concerns an antimicrobial piece of
aluminum. The piece of aluminum comprises a porous surface layer formed by
anodization and the porous surface layer comprises an electrodeposit of silver
and at
least one quaternary ammonium compound. More preferably, at least one
quaternary
ammonium compound is in the pores of the surface layer.
[00014] The
invention also concerns all kinds of articles of manufacture incorporating
an antimicrobial metal product and/or an antimicrobial piece of aluminum as
defined
herein. Particular articles covered by the invention include kitchen wares,
kitchen
countertops, hospital countertops, furniture, appliances, office equipment,
door knobs,
medical devices, laminar flow hoods, laboratory incubators, wall panels, floor
panels,
boat hulls, pipes, etc.
[00015] The
invention further relates to a method for obtaining an antimicrobial metal
product having an antimicrobial surface coating. In one embodiment, the method
comprises the steps of:
- providing an anodized metal product having a porous surface layer formed by
anodization;
- electrodepositing a metal to the porous surface layer; and
- impregnating the porous layer with at least one antimicrobial compound.
[00016] The anodized metal product, the metal electrodeposited and the
antimicrobial
compound may be selected as defined previously. In one particular embodiment,
electrodeposition of silver is carried out in an aqueous solution of sulfuric
acid.
[00017] The method may further comprise a sealing or clogging step which is
carried
out simultaneously or after the impregnation. In particulars embodiments, the
sealing is
carried out by soaking in a solution or by exposing the porous layer to steam
at ambient
pressure or in an autoclave. The method may further comprise a dying step
before
impregnating the porous layer with the at least one antimicrobial compound.
- 3 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00018] A related aspect on the invention concerns an antimicrobial solution
for
impregnating a porous layer of an anodized metal product. In a preferred
embodiment,
the antimicrobial solution comprises at least two quaternary ammonium
compounds
having antimicrobial activity. More preferably, the at least two quaternary
ammonium
compounds consists ADBAC and DDAC.
[00019] The antimicrobial solution may further comprises a metallic salt such
as
AgNO3, Cu(NO3)2, Zn(NO3)2, Ni(NO3)2 and mixtures thereof. The antimicrobial
solution
may also comprise additional antimicrobial agent(s) selected from antivirals,
antibiotics,
and antifungals.
[00020] A related aspect on the invention method for controlling growth of
microorganisms and/or microbial pathogens on a metallic surface, comprising
impregnating the metallic surface with an antimicrobial solution as defined
herein prior to
contacting the metallic surface with microorganism(s) or pathogen(s). The
invention also
encompasses the use of an antimicrobial solution as defined herein for
impregnating a
porous layer of an anodized metal product, thereby creating an antimicrobial
coating on
the surface of the anodized metal product.
[00021] An advantage of the present invention is that it provides simple,
cheap and
relatively fast and efficient means for treating the surface of anodizable
metals for
conferring antimicrobial properties to these metallic surfaces. The methods of
the
invention may be performed on different metals and alloys of different quality
and/or of
different sizes. The invention also provides anodizable metal products having
a surface
effective in killing a broad spectrum of microorganisms, including various
pathogens.
Preferably, the killing action may require only a very short contacting period
(e.g. less
than 5 minutes).
[00022] Additional aspects, advantages and features of the present invention
will
become more apparent upon reading of the following non-restrictive description
of
preferred embodiments which are exemplary and should not be interpreted as
limiting
the scope of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[00023] Figures 1 is bar graph showing antimicrobial activity of paper
disks treated
with different antimicrobial solutions. Solutions 1 to 5 are as defined in
Example 1.
Antimicrobial activity was evaluated using a disk diffusion assay against the
following
- 4 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
bacterial strains: E. coli (EC), P. aeruginosa (PA), S. aureus (SA), B.
subtilis (BA), and
C. albicans (CA).
[00024] Figure
2 is a panel showing pictures of Petri dishes illustrating antimicrobial
activity against Gram positive bacteria, Gram negative bacteria and yeast of
aluminum
disks treated with various antimicrobial solutions: A: benzalkonium chloride
(2,12 % W/V)
+ silver nitrate (1.02 % W/V) or B: Benzalkonium chloride (0,96%) + Didecyl
dimethyl
ammonium chloride (1,44 %) + silver nitrate (1.02 W/V)).
Antimicrobial activity of the
disks against the following microbial strains was assessed: E. coli ATCC 8739;
P. aeruginosa ATCC 9027; S. aureus ATCC 6538; B. subtilis ATCC 6633; and C.
albicans ATCC 10231. For each dish, lanes 1 and 3 shows growth of bacteria for
a pair
of disks treated with the antimicrobial solution whereas lanes 2 and 4 growth
of bacteria
for a pair of untreated disks (negative control).
[00025] Figure 3 is a panel of microscopic pictures made at 500X of
transversal cuts
of aluminum disks anodized for 11 min (#2467), 22 min (#2468), 33 min (#2469)
or 44
minutes (#2469). As expected, the thickness of porous aluminum anodic film
(lined area
at the top of each picture) increases with the duration of the anodization
period (i.e. 4
pm, 8 pm, 13 pm, 19 pm respectively).
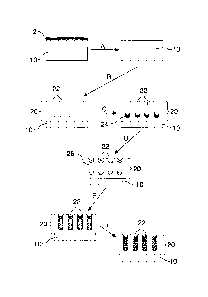
[00026] Figure 4 is a schematization of a process for making an anodized metal
product with antimicrobial properties according to one embodiment of the
invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Antimicrobial metal product and Methods for making same
[00027] One
particular aspect of the present invention relates to an antimicrobial
metal product comprising an anodized metal substrate and an antimicrobial
surface
coating. The invention also concerns methods for obtaining an antimicrobial
metal
product having an antimicrobial surface coating.
[00028] As it is well known, anodization increases the thickness of the
natural oxide
layer on the surface of metal parts. Anodization changes the microscopic
texture of the
surface and changes the crystal structure of the metal near the surface.
Typically, the
end result of the anodization is the formation of a porous surface layer which
is harder,
stronger, more adherent, and more brittle than unanodized products.
[00029] The invention adds to the known advantageous properties on anodized
metals by providing an anodized metal substrate having an antimicrobial
surface coating.
- 5 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
The antimicrobial surface coating comprises a porous surface layer formed by
anodization. In addition, the porous surface layer comprises an electrodeposit
of at least
one metal and it further comprises at least one antimicrobial compound.
[00030] According to an embodiment of the methods of invention, an anodized
metal
product having a porous surface layer formed by anodization is first provided.
Any metal
which can be anodized is suitable according to the invention, including, but
not limited to
aluminum, titanium, zinc, magnesium, niobium, tantalum and anodizable alloys
thereof
(e.g. aluminum alloys and others). Anodization of such metals in well known to
those
skilled in the art and any anodization process resulting in the formation of a
porous
surface layer at the surface of the metal is acceptable according to the
invention. In
various embodiments according to the invention, the porous surface layer
formed by
anodization has a thickness ranging from about 1 pm to about 150 pm,
preferably a
thickness ranging from between 2 pm to about 35 pm, more preferably a
thickness
ranging from between 10 pm to about 20 pm. Typically the pores are nanopores
having a
diameter ranging from 5 nm to about 100 nm.
[00031] Prior to its anodization the metal substrate may be subjected to one
or more
pretreatment steps of such as degreasing, electropolishing, etching, etc.
according to
procedures known in the art. In one particular embodiment, for obtaining an
aluminum
product with a antimicrobial surface coating, the following steps are carried
out:
aluminum is degreased with acetone; etched with 10% weight/vol NaOH for 2 min
at 50-
60 C; neutralized in 35% vol/vol HNO3 for 30 sec. at room temperature; and
submitted to
a P2 etch treatment for 10 min at 50-60 C with 33% v/v sulfuric acid and
ferrite (Russell
and Garnis, 1977, Chromate-Free Method of Preparing Aluminum Surfaces for
Adhesive
Bonding. An Etchant Composition of Low Toxicity, Army Armament Research and
Development Center, Dover NJ, Large Caliber Weapon Systems Lab).
[00032] It is
within the skill of those in the art to anodize various anodizable metals
and alloys. According to particular embodiments, the aluminum substrate is
anodized in
a 15% v/v stirred sulfuric acid solution. A current of 1.5 Amps (13 volts) is
applied to the
aluminum piece for 44 minutes (given the selected size of the anodized piece,
this allows
an anodic/cathodic area ratio of 3/1 (the anode is anodized aluminum with a
surface area
3 time the cathode surface) (the ratio can be to 1/1 to 4/1)). This leads to a
layer of
anodization of approximately 20 pm. The temperature of the solution is
maintained
between 21 C and 23 C.
- 6 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00033]
Electrodeposition (also known as electroplating) is well known to those
skilled
in the art. According to particular embodiments of the invention, once an
anodized metal
product having a porous surface layer is obtained, the porous surface layer
created by
the anodization is subjected to a step of electrodeposition of a metal. The
metal to be
electrodeposited may be selected among silver, copper, gold, nickel, zinc,
tin, palladium,
cadmium, platinum and combinations thereof. The metal to be electrodeposited
can be
selected according to the desired properties of the final product, including
desired
coloration, desired antimicrobial activity, desired durability and/or
combination of these
properties. In preferred embodiments, silver (Ag) is electrodeposited to the
porous
surface layer.
[00034] Any suitable electrodeposition process may be used according to the
invention. In one particular embodiment, silver is electrodeposited onto
aluminum and
electrodeposition is carried out for about 30 sec to about 10 minutes in an
aqueous
solution comprising about 1.5 % vol/vol sulfuric acid and about 5.1 g/I silver
nitrate
(AgNO3) at room temperature, Ac voltage 18 V. The invention also encompasses
additional electrodeposition methods and conditions. In another particular
embodiment,
the method uses 8 g/I stannous sulphate (SnSO4), 20 g/I nickel sulphate
(NiSO4), 17 g/I
sulfuric acid (H2SO4) and 10 g/I tartaric acid (C4H606) at room temperature,
Ac voltage 15
V, for 1 to 10 minutes. In another particular embodiment, electrodeposition is
carried out
with 40 g/I nickel sulphate (NiSO4), 25 g/I boric acid (H3B03), 20 g/I
magnesium sulphate
(MgSO4), 50 g/I ammonium sulphate ((NH4)2SO4) and 5 g/I Tri-Ammonium citrate
(C6H17N307) at room temperature, Ac voltage 15 V, for 1 to 10 minutes. Another
method
is 35 g/L copper sulfate (CuSO4), 10 g/L sulfuric acid (H2SO4), 2 g/I sodium
sulfate
(Na2SO4) at 40 C with a voltage of 30 V for 50 min. Preferably, theses
techniques should
use metals with demonstrated or potential antimicrobial activity. The general
process of
the invention may be applicable to electrodeposition of Ag and additional
metals (e.g. Au,
Cu, Ni, Sn, Zn, Pt, Pd, Cd) with either documented and/or with potential
antimicrobial
activity.
[00035] In some embodiments, electrodeposition is used mainly to color and/or
improve the visual aesthetic properties of the anodized metal product. Various
metals
can be electrodeposited according to the invention for coloration purposes
including, but
not limited to, silver, gold, copper, nickel, zinc, tin, palladium, cadmium,
platinum and
combinations thereof. In preferred embodiments, it is silver (Ag) which is
electrodeposited to the porous surface layer because this metal possesses
combined
aesthetic and antimicrobial properties.
- 7 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00036] According to some embodiments of the invention, after the
electrodeposition
step, the porous surface layer of the anodized metal is next impregnated with
at least
one antimicrobial compound. Suitable antimicrobial agent or compound includes
antivirals, antibiotics, and/or antifungals, and these can consists of an
organic or
inorganic chemicals or salts. Impregnation may be carried out using any
suitable method
known to those skilled in the art. In preferred embodiments, the anodized
metal is
soaked in a product solution (with or without agitation) for about 1 second to
about 12
hours or more at about 0 C to about 200 C. More preferably, the anodized metal
is
soaked at about 97 C for about 30 min. Various antimicrobial chemicals are
compatible
with the invention, including but not limited to quaternary ammonium compound,
antibiotics, sulfamides, detergents, colorant, meal conservators, antifungal
agent,
antivirals, metallic ions (salts), alcohols (e.g. ethyl alcohol, methanol,
isopropyl alcohol),
acids (e.g. hydrochloric acid, phosphoric acid, ethylenediaminetetraacetic
(EDTA)), etc.
Depending of the particular compound(s), the concentration of the
antimicrobial chemical
in the solution may vary from about 0.001% to about 100%.
[00037] In a
preferred embodiment, the porous layer is impregnated with at least one
quaternary ammonium compound. As used herein, the term "quaternary ammonium
compound" refers to any compound having antimicrobial activity and comprising
positively charged polyatomic ions of the structure NR4+ with R being an alkyl
group.
Suitable examples according to the invention include, but are not limited to,
Alkyl
Dimethyl Benzyl Ammonium Chloride (ADBAC), Didecyl Dimethyl Ammonium Chloride
(DDAC), Benzyldimethyl(2-dodecyloxyethyl)-ammonium chloride, Benzyldimethyl(2-
hydroxyethyl)ammonium chloride,
benzyldimethyl
(hexadecylcarbamoylmethyl)ammonium chloride,
benzyldimethyl
(tetradecylcarboamoylmethyl)ammonium chloride, benzyloxycarbonylmethyl-
trimethyl-
ammonium chloride, bis-(2-hydroxyethyl)-ciannamy1(2-dodecyloxymethyl)ammonium
chloride, Benzyltriethylammonium chloride, Tetramethylammonium chloride,
Tetramethylammonium iodide, Tetraethylammonium hydroxide, Tetramethylammonium
hydroxide, Benzyltrimethylammonium hydroxide, Dimethyldioctadecylammonium
chloride, Dodecyltrimethylammonium choride, Trimethylphenylammonium chloride,
Octadecyltrimethyl ammonium bromide, Tetrabutyl ammonium bromide,
Tetramethylammonium nitrate, Tetrabutylammonium hydroxide, Didodecyldimethyl
ammonium bromide, Didodecyldimethylammonium chloride, Dimethyldioctadecyl
ammonium bromide, (2-(Methacryloyloxy)ethyl)trimethylammonium chloride,
Dioctyl
dimethyl ammonium chloride, Tetrapropylammonium chloride,
Didecyldimethylammonium chloride, Bezyldodecyldimethyl ammonium bromide,
Diallyl
- 8 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
dimethyl ammonium chloride, Benzalkonium bromide, Ammonium bromide,
Benzyltributylammonium chloride, Octyldecyl dimethyl ammonium chloride,
Tetrabutylammonium hydrogen sulfate, Tetrabutylammonium
tribromide,
Methyltributylammonium chloride, Bis(hydrogenated tallow)dimethylammonium
chloride,
N-Alkyl dimethyl benzyl ammonium chloride, Tetrabutylammonium fluoride
trihydrate.
[00038] In a preferred embodiment, the porous layer comprises at least one
inorganic
compound. As used herein, the term "inorganic compound" refers to any compound
having antimicrobial activity which is not an organic compound. Suitable
examples
according to the invention include, but are not limited to metallic salts such
as silver
nitrate (AgNO3), silver chloride (AgCI), copper nitrate (Cu(NO3)2), copper
chloride
(CuC12), zinc nitrate (Zn(NO3)2), nickel nitrate (Ni(NO3)2), etc.
[00039] In
selected embodiment, the porous layer comprises at least one antibacterial
agent. As used herein, the term "antibacterial" refers to any compound having
antibacterial activity included but not limited to detergents, meal
conservators, alcohols
(e.g. ethyl alcohol, methanol, isopropyl alcohol), acids (e.g. hydrochloric
acid, phosphoric
acid, ethylenediaminetetraacetic (EDTA)), etc.
[00040] In
selected embodiments, the porous layer comprises at least one antibiotic.
As used herein, the term "antibiotic" refers to any compound having
antibacterial activity
including, but not limited to beta-lactams (ex: penicillin, cephalosporin,
etc.),
aminoglycosides (ex: streptomycin, neomycin, kanamycin, etc.), cyclins (ex:
tetracycline),
amphenicols (ex: chloramphenicol, thiamphenicol, etc.), macrolides
(erythromycin,
clarithromycin, etc), glycopeptides (ex: vancomycin, bleomycin, etc.),
quinolones
(ciprofloxacin, levofloxacin, etc.), polypeptides (actinomycin, bacitracin,
polymyxin B,
etc.), nitrofurans (furazolidone, nitrofurazone, etc.),
[00041] In preferred embodiments, the antimicrobial surface coating may
possess
antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria
and/or
yeasts. In preferred embodiments, the porous layer comprises at least one
antimicrobial.
As used herein, the term "antimicrobial" or "antimicrobial activity" refers to
killing or
inhibiting growth of microbes including, but not limited to, bacteria,
viruses, algae, yeasts
and mold. In preferred embodiments, the antimicrobial surface coating
possesses
antimicrobial activity of at least 90%, preferably at least 99%, and more
preferably of at
least 100%, as measured by bioburden testing (microorganism spike/recovery
experiments). In particular embodiments, the microbe is a Gram-positive
bacteria. In
other embodiments, the microbe is a Gram-negative bacteria.
- 9 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00042]
Examples of Gram-positive bacteria include, but are not limited to, many well-
known genera such as Staphylococcus, Streptococcus, Enterococcus and Bacillus.
Examples of Gram-negative bacteria includes, but are not limited to,
Escherichia coli,
Salmonella, Shigella, and other Enterobacteriaceae, Pseudomonas, Moraxella,
Helicobacter, Stenotrophomonas, Bdellovibrio, acetic acid bacteria, Legionella
and
alpha-proteobacteria as Wolbachia and numerous others. Other notable groups of
Gram-
negative bacteria include the cyanobacteria, spirochaetes, green sulfur and
green non-
sulfur bacteria. Examples of yeasts include, but are not limited to,
Saccharomyces
cerevisiae and pathogenic yeast such as Candida. In preferred embodiments, the
anodized metal products according to the invention have an antimicrobial
activity against
one or more of the following pathogens: Staphylococcus aureus, Bacillus
subtilis,
Escherichia coli, Pseudomonas aeruginosa, and Candida albicans.
[00043] In selected embodiments, the porous layer comprises at least one
antifungal
agent. As used herein, the term "antifungal agent" refers to any compound
having
antifungal activity including, but not limited to polyene (Natamycin,
rimocidin, candicin,
etc.), imidazole, triazole, thiazole (miconazole, fluconazole, abafungin,
etc.), allylamines
(terbinafine, naftifine, etc.), echinocandin (caspofungin, micafungin, etc.),
others
(ciclopirox, griseofulvin, etc.).
[00044] In
selected embodiments, the porous layer comprises at least one antiviral.
As used herein, the term "antiviral" refers to any compound having the
potential of
destruction of a virus or the potential to inhibit the penetration of the
virus into a host cell.
Examples include, but are not limited to, glutaraldehyde, amantadine and
combination
thereof.
[00045] The methods of the invention may further comprise sealing or clogging
the
impregnated metal material in order to ensure an increased durability of the
metal
product and to ensure a longer durability of the antimicrobial activity. The
more the
impregnated metal material is sealed, lower the speed of diffusion of the
antimicrobial
agent(s) is going to be. Accordingly, sealing may be suitable to modulate
(increase or
decrease) speed of diffusion of the antimicrobial agent. In one embodiment,
the sealing
step is used to slow down the diffusion of the product and the impregnated
metal
material is sealed (i.e. at about 1% to about 100%, or about 25% to about
75%). Those
skilled in the art know how to assess sealing using various methods.
[00046] The sealing can be concurrent (i.e. during) or not with the
impregnation. In
some embodiments, sealing is carried simultaneously with impregnation by
heating the
-10-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
metallic material from 50 C to 100 C. In another embodiment, sealing is
achieved by
treating the metal under pressurized vapor (for instance in an autoclave, with
steam at
100 C to 140 C) or with chemical treatments at 30 C to 50 C (with salts). In
one
embodiment, the metal is submitted to pressurized vapor at about 100 C to 130
C.
Various treatment combinations are conceivable for achieving sealing. For
instance, one
can simultaneously impregnate and seal the metal product by soaking the
anodized
metal substrate in the antimicrobial solution. Another possibility is to
impregnate the
anodized metal substrate and then seal it with steam at room temperature or in
an
autoclave. In the alternative, the anodized metal substrate can be impregnated
then
sealed by heating (e.g. about 50 C to about 100 C), sealed by soaking in
water, sealed
with steam (e.g. about 100 C to about 140 C) or sealed with chemical treatment
(e.g.
salts).
[00047]
Depending on the anticipated use for the metal product (e.g. for decorative
purposes), it may be advantageous to color the antimicrobial metal product.
Accordingly,
in some embodiments, the methods of the invention further comprise a dying or
coloring
step. The invention encompasses any dying or coloration procedure compatible
with
obtaining an anodized metal product with an antimicrobial surface coating
according to
the methods of the invention. For instance, it is well known to
electrolytically deposit (i.e.
by electrodeposition) various metals in the pores of an aluminum anodic
coating to
provide lightfast colors (e.g. bronze shades and pale champagne to black).
Alternatively,
the color may be produced integral to the coating during the anodizing process
using
organic acids mixed with a sulfuric electrolyte and a pulsed current. It is
also possible to
dye the unsealed porous surface of the metal in lighter colors and then
splashing darker
color dyes onto the surface. Another approach comprises impregnation of the
metal
substrate in a dying solution.
[00048] In preferred embodiments, manufacture of colored antibacterial metal
products is carried out in two consecutive steps: 1) coloration and 2)
impregnation (i.e.
the dying step precedes impregnation of the porous layer with the
antimicrobial
compound(s)). This order is preferred for ensuring that the desired colored
metal product
possesses acceptable antibacterial properties. in one particular embodiment a
colorant
solution is heated at about 50 C to about 70 C and the metal substrate is
soaked 30 min
with the heated colorant solution, then the metal substrate is impregnated
with the
antimicrobial substance. Specific conditions may also vary depending of the
colorant and
the metal material. For instance, in particular embodiments heating of the
colorant
solution is facultative (ambient temperature is acceptable).
- 11 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00049] An example of method for obtaining an antimicrobial metal product
having an
antimicrobial surface coating according to a preferred embodiment of the
invention is
schematized in Figure 4. Step A: An anodizable metal substrate (10) (e.g. a
sheet of
aluminum) is degreased with acetone to remove impurities (2) from the surface
of the
substrate (10); Step B: The metal substrate (10) is anodized, which results in
the
formation of a porous surface layer (20) comprising nanopores (22); Step C:
Metal ions
(24) (e.g. Ag) are electrodeposited to the porous surface layer (20); Step D:
The porous
surface layer (20) and its pores (22) are impregnated with an antimicrobial
solution (26);
Steps E and F: The pores (22) of the porous surface layer (20) are sealed
either partially
(Step E) or completely (Step F).
Antimicrobial solutions
[00050] An additional aspect of the invention concerns antimicrobial solutions
and
uses thereof for impregnating a porous layer of an anodized metal product in
order to
create an antimicrobial coating on the surface of the anodized metal product.
[00051] In one particular embodiment, the antimicrobial solution comprises
at least
two quaternary ammonium compounds selected among quaternary ammonium
compounds having antimicrobial activity, including but not limited to those
mentioned
hereinbefore. Preferably, the quaternary ammoniums are Alkyl Dimethyl Benzyl
Ammonium Chloride (ADBAC) and Didecyl Dimethyl Ammonium Chloride (DDAC). More
preferably, ADBAC is present at a concentration of about 1 mM to about 1 M and
DDAC
is present at a concentration of about 1 mM to about 1 M. Even more
preferably, ADBAC
is present at a concentration of about 10 mM to about 50 mM and wherein DDAC
is
present at a concentration of about 10 mM to about 100 mM.
[00052] The antimicrobial solution of the invention may further comprises a
metallic
salt, including but not limited to AgNO3, Cu(NO3)2, Zn(NO3)2, Ni(NO3)2 and
mixtures
thereof.
[00053] The antimicrobial solution may further comprise at least one
antimicrobial
agent including, but not limited to, antivirals, antibiotics, and antifungals
as defined
herein.
Controlling growth of microorganisms and pathogens
[00054] An additional aspect of the invention concerns a method for
controlling growth
of microorganisms and/or microbial pathogens on a metallic surface. In one
embodiment,
the method comprises impregnating the metallic surface with an antimicrobial
solution as
-12-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
defined herein prior to contacting the metallic surface with microorganism(s)
or
pathogen(s). In another embodiment, the method comprises providing an
antimicrobial
metal product having an antimicrobial porous surface layer as defined herein
prior to
contacting the antimicrobial porous surface of the metal product with
microorganism(s) or
pathogen(s).
[00055] As
used herein, "controlling" include, but is not limited to, preventing
adhesion of the microorganism(s) to the metallic surface; preventing formation
of a
microbial biofilm on the metallic surface; inhibiting or slowing growth of the
microorganism(s) on the metallic surface; killing, and/or eradicating the
microorganism(s), etc.
[00056] As used herein, the term "microbial pathogen" refers to any
microorganism
susceptible to harm a human being. The term microbial pathogen encompasses
bacteria
(e.g. Gram-positive or Gram-negative bacteria), viruses (orthomyxoviridae,
retroviridae,
adenovirus, papillomavirus, etc.), mold (e.g. Aspergillus, Penicillium,
Fusarium, etc.),
yeasts (e.g. Candida, Saccharomyces, Rhodotorula, etc.) and algae (e.g.
Cyanobacteria,
Euglenophyta, Rhodophyta, etc.). The invention encompasses controlling
microbial
pathogens including, but not limited to Gram-positive bacteria, Gram-negative
bacteria,
viruses and yeast. Accordingly, the invention encompasses controlling
microbial
pathogens which may be harmful to humans. Human pathogens against which the
methods and compositions of the invention may be useful include, but are not
limited to,
Escherichia coli, Staphylococcus aureus, Salmonella species, Listeria species,
Mycobacterium tuberculosis, viruses responsible for humans diseases such as
flu, foot
and mouth disease, swine fever, etc. and yeasts (e.g. Candida).
[00057] The invention encompasses controlling microbial pathogens including,
but not
limited to viruses. Viruses against which the methods and compositions of the
invention
may be useful include, but are not limited to orthomyxoviridae, retroviridae,
adenovirus,
papillomavirus, etc.
Articles of manufacture
[00058] A large variety of articles of manufacture may benefit from
incorporating an
antimicrobial metal product as described herein. These include, but are not
limited to,
kitchen wares, kitchen countertops, hospital countertops, furniture, office
equipment (e.g.
pen, stapler, computers and keyboards, etc), appliances, door knobs, medical
devices,
jewellery, laminar flow hoods, laboratory incubators, wall panels, air
purification systems,
gloves, masks, panels (e.g. walls, floors, ceilings), boat hulls and pipes.
The invention
-13-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
encompasses all these articles of manufacture. Preferably the metal product is
made of
aluminum or aluminum alloy.
[00059]
Conceivably, air filters and filter masks comprising a metal product having an
antimicrobial surface coating according to the invention may be useful for the
removal
and killing of germs. Such product could be used in air systems (circulation,
heating,
climatization) for buildings (e.g. for reducing risk of contamination by
Legionella), for
decontamination (e.g. bioterrorism) or for individual use (e.g. mask).
[00060] The invention may also find applications in buildings and room
constructions.
For instance, antimicrobial metal products entering in the construction of
buildings may
prevent mould contamination (e.g. inside walls, ceilings and floors).
[00061] Similarly, the invention may be helpful to manufacture safer clean
rooms and
sterile rooms (e.g. rooms and laboratories of hospitals and pharmaceutical
companies)
and replace current walls and floors made of stainless steel. Using an
antimicrobial metal
product in the construction of such rooms may help to obtain and maintain a
sterile
environment and reduce the need of cleaning or decontamination.
[00062] Office supplies and hardware (keyboard, mice, pens, etc.) made with
the
metal products or methods of the invention would be desirable since their
antimicrobial
activity could prevent the spread of microorganisms.
[00063] Additional medical applications include manufacture of medical devices
requiring sterilization (e.g. endoscope, scalpels, dentist tools, etc.). Use
of medical
equipment comprising an antimicrobial surface according to the invention may
help
preventing contamination and infections in humans and animals.
[00064] The invention may also be helpful to manufacture jewellery (e.g.
earrings,
piercings, etc.) thereby minimizing risks of infections and/or stimulating
healing
(particularly for new body piercings).
[00065] The
invention may also find sanitary applications, for instance in the
manufacture of toilets (seat, handle, whole toilet, etc.), distributors (hand
soap
distributors, paper towel distributors, etc.) for either personnel use or
public uses (e.g.
public restrooms, planes, buses, etc.).
[00066] The invention may also find naval applications, for instance in the
construction of boats having a hull comprising an antimicrobial metal product
according
to the invention. According to that particular use, the antimicrobial surface
could prevent
- 14-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
the formation of bacterial biofilms and/or prevent attachment of organisms
(e.g. algae,
mussel, etc), which is undesirable since such attachment increases the
friction
coefficient of the hull. A boat comprising an antimicrobial hull according to
the invention
could consume significantly less energy to reach a desired speed. Preventing
attachment of living organisms to the hull may also prevent undesirable
propagation of
undesirable living organisms in different countries.
[00067] Metal
products according to the invention could also be used in pipes (e.g.
purified water systems) to avoid formation of bacterial biofilms in the pipes.
[00068] Those skilled in the art will recognize, or be able to ascertain using
no more
than routine experimentation, numerous equivalents to the specific procedures,
embodiments, claims, and examples described herein. Such equivalents are
considered
to be within the scope of this invention and covered by the claims appended
hereto. The
invention is further illustrated by the following examples, which should not
be construed
as further limiting.
EXAMPLES
EXAMPLE 1: Preparation and testing of antimicrobial solutions
[00069] Before
their application to anodized aluminum surface, various antimicrobial
solutions were tested, alone or in combination, for assessing their potential
antimicrobial
activity:
Solution No Components
1 1.02% w/v AgNO3 (silver nitrate; 60 mM)
2 (25 mM) Benzalkonium chloride (0.96%) + (40 mM) Didecyl dimethyl
ammonium chloride (1.44 %);
3 (25 mM) Benzalkonium chloride (0.96%) + (40 mM) Didecyl dimethyl
ammonium chloride (1.44 %) + (60 mM) silver nitrate (1.02 % WN).
4 2.12% w/v benzalkonium chloride (56 mM)
5 (56 mM) benzalkonium chloride (2,12 % WN) + (60 mM) silver nitrate
(1.02 % WN);
[00070] Antimicrobial activities of the solutions were tested on various
microorganisms, including Escherichia coli (ATCC 8739), Pseudomonas aeruginosa
(ATCC 9027), Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633)
and
Candida albicans (ATCC 10231). All strains were freshly grown at 35 C on agar
plates
prior to the analysis. Microbial colonies were picked and resuspended into MHB
bacterial
growth medium to an 0.D.660 nm of approximately 0.4.
-15-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00071] The
antimicrobial properties of various agents were initially tested using a
disk diffusion method and different microbial strains. Five (5) mm Whatman TM
filter paper
punches were dipped into serial dilutions of antimicrobial solutions and
deposited onto
agar plate microbial lawns prior to incubation overnight at 35 C.
Antimicrobial activities
were compared by measuring in duplicates the diameter of the growth inhibited
zone
(Figure 1).
[00072] As shown in Figure 1, Silver nitrate at 60 mM (solution 1) was more
effective
on Gram-negative bacteria (Escherichia coli (EC), Pseudomonas aeruginosa (PA))
than
on Gram-positive bacteria (Staphylococcus aureus (SA), Bacillus subtilis (BS))
or yeast
(Candida albicans (CA)). However, solutions 2, 3, 4 and 5 were more effective
against
Gram-positive bacteria and yeast than Gram-negative bacteria. Overall,
solutions 3 and
5 were the most effective against all microorganisms types.
EXAMPLE 2: Preparation and testing aluminum with antimicrobial property
Experimental
Anodization of the Aluminum surfaces
[00073] Anodization was used to increase the thickness of the natural oxide
layer on
the surface of aluminum plates and for creating at their surface an anodic
film having
nanopores. Aluminum plates of the 6000 serie were used throughout this study.
Plates of
2.3 mm thickness were cut using a laser into 22.2 mm disks attached in groups
of five by
a 5 mm linker region. The resulting necklaces were degreased with acetone;
etched with
10% weight/vol NaOH for 2 min at 50-60 C; neutralized in 35% vol/vol HNO3 for
30 sec.
at room temperature; and submitted to a P2 etching (33% v/v sulfuric acid and
ferrite)
for 10 min at 50-60 C and electrochemically oxidized at room temperature in
15% vol/vol
sulfuric acid solution at 1.5 amps for 11 min, 22 min, 33 min or 44 minutes
prior to rinsing
with distilled water.
[00074] The standardized time of anodization according to the theoretical
formula was
used to obtain the needed thickness. The theoretical thickness was measured
using the
following equation* :
Oxide thickness (pm) = 0.3 x current density (Ndm2) x anodizing time (min)
*Equation obtained from: Anodizing and coloring of aluminum alloys (2002), S.
Kawai
editor, Publisher: ASM International, p.170.
-16-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00075] Efficiency of the anodization process was estimated by microscopy at
500X
coupled to an image processor (Figure 3) and by impedance using 500-4000
wavelength cm-1 to verify the exactness of the theoretical formula (Table 1
hereinafter).
Impregnation and sealing
[00076] The anodized aluminum necklaces were next immerged into various
antimicrobial solutions at 97 C for 11 minutes while mixing with a magnetic
stir bar. This
immersion allowed impregnation the antimicrobial agents into the nanopores and
a
concurrent partial sealing of the nanopores (through water hydration process)
estimated
to about 25%. Thereafter the anodized and impregnated aluminum disks were
separated
using a metal cutter, rinsed twice with distilled water, sterilized with
ethanol and kept in a
sterile environment until further use.
Electroplating of the anodized aluminum surfaces
[00077] The anodized aluminum surface was electroplated with AgNO3 (30 mM) in
150 mM sulfuric acid for 30 sec and up to 10 min for a constant tension of 18
volts AC at
room temperature.
Measurement of antimicrobial activity
[00078] For semi-quantitative assays, microbial lawns were first prepared by
spreading the microbial dilutions onto agar plates using cotton swabs. The
aluminum
disks were deposited onto the agar surfaces for 5 min, then removed and plates
were
incubated at 35 C until the next day. The inhibition zone (diameter) was
measured for
semi-quantitative results.
[00079] Quantitative analyses were performed by spreading 10 pL of the initial
bacterial suspensions onto the aluminum disks and by incubating the disks at
room
temperature for 5 min. The disks were transferred into 5-10 mL of MHB growth
medium,
vortexed to disperse and to recover the microorganisms, diluted and aliquots
were plated
in triplicates onto agar plates. After overnight incubation, the CFU were
counted and
positive and negative controls were compared. The amounts of CFU/mL for each
of the
initial microbial suspension were determined by serial dilutions followed by
plating 10 pL
and 100 pL aliquots onto MHA plates in triplicates followed by incubation at
35 C for at
least 18 hrs. The average counts were used to determine the amounts of CFU
deposited
onto the aluminum surfaces.
-17-
CA 02909198 2015-10-09
WO 2013/155618 PCT/CA2013/000389
Results
Anodization efficiency
[00080] Efficiency of the anodization process was estimated by using two
different
approaches (by impedance and by microscopy).
[00081] Table 1: Thickness of the anodized aluminum surfaces as measured
using different methods
Anodization Estimated with Quantification by Quantification by
time (min) equation (pm) microscopy (pm) Impedance (pm)
11 5,0 4,0 4,0
22 10,0 8,0 8,0
33 15,0 13,0 12,8
44 20,0 19,0 16,3
[00082] These results indicate that the theoretical thicknesses estimated
using the
equation was highly comparable to those obtained by impedance and microscopy.
As
expected, impedance was less precise at higher thicknesses.
Antimicrobial activity of the treated aluminum
[00083] Antimicrobial solutions for treatment of aluminum were chosen based on
results of antimicrobial-like test (Figure 1). The antimicrobial property of
the chosen
antimicrobial solutions incorporated into the aluminum oxide nanopores was
evaluated
using two different approaches.
[00084] Figure 2 illustrates the qualitative results of the first
approach where the
aluminum disks were laid onto microbial lawns for 5 min. Disks impregnated
with the
benzalkonium chloride (2,12 % WN) + silver nitrate (1.02 % WN) [solution 5,
(A)]
prevented the growth of the Gram-positive strains and yeast C. albicans
(column 1)
whereas the anodized aluminum alone did not (column 2). E. coli showed a
slight
reduced growth whereas P. aeruginosa growth was not inhibited at all. None of
the
anodized disks without antimicrobial solution prevented microbial growth
(columns 2 and
4). However, disks impregnated with the Benzalkonium chloride (0,96%) +
Didecyl
dimethyl ammonium chloride (1,44 %) + silver nitrate (1.02 % WN) [solution 3,
(B)]
prevented the growth of all the strains tested (column 3).
[00085] The antimicrobial activity was maintained for a long period since the
disks
with the benzalkonium chloride (2.12 % WN) + silver nitrate (1.02 % WN)
solution were
still active when tested on S. aureus after 1, 3, 5, 7, 12, 14 and 24 months
of storage at
-18-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
room temperature (data not shown) confirming that antimicrobial metal products
according to the invention can withstand time.
[00086] Table 2A and 2B hereinafter show the results of quantitative analyses
of
anodized aluminum disks incorporating two types of antimicrobial solutions.
Aluminum
disks impregnated with the benzalkonium chloride (2.12% WN) + silver nitrate
(1.02 %
WN) solution (Solution 5, Table 2A) did not prevent the growth of E. coli and
of P.
aeruginosa. However, these disks had strong antimicrobial activity against S.
aureus
25923 and S. aureus 6538 with >99.99% and 100% efficiency, respectively. The
efficiency against B. subtilis and C. albicans strains was also remarkable
since for these
two strains, the bacteria in the original inoculums were completely
eliminated. Aluminum
disks impregnated with the Benzalkonium chloride (0.96%) + Didecyl dimethyl
ammonium chloride (1,44 %) + silver nitrate (1.02 % WN) solution (Solution 3,
Table 2B)
had even a stronger antimicrobial activity since they killed almost completely
all the
strains, including the Gram-negative strains E. coli and P. Aeruginosa.
[00087] Table 2A: Antimicrobial activity of aluminum incorporating the
benzalkonium chloride (2,12 % WN) + silver nitrate (1,02 % WN) solution
[Solution
5]
Strains Input CFU % survival %
killing
E. Coll 8739 4.8 x 108 100 0
P. Aeruginosa 9027 4.4 x 108 88 12
S. Aureus 25923 2.0 x 109 0.0032 99.9968
S. Aureus 6538 1.3x 109 0 100
B. Subtilis 6633 4.7 x 105 0 100
C. Albicans 10231 6.5 x 106 0 100
-19-
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
Table 2B: Antimicrobial activity of aluminum incorporating the Benzalkonium
chloride (0,96%) + Didecyl dimethyl ammonium chloride (1,44 %) + silver
nitrate
(1,02 % W/V) solution [Solution 3]
Strains Input CFU % survival % killing
E. Coll 8739 4.4x 108 0 100
P. Aeruginosa 9027 6.4 x 108 1 99
S. Aureus 25923 1.2x 109 0 100
S. Aureus 6538 1.3x 109 0 100
B. Subtilis 6633 4.7 x 108 0 100
C. Albicans 10231 1.0 x 108 0 100
[00088] As can be appreciated, the quantitative results of Table 2A and 2B
corroborate qualitative results illustrated in Figure 2.
Abrasion test
[00089] An abrasion test was developed to assess the effectiveness against the
wear
of the antimicrobial surface on antimicrobial activity. Briefly, anodized
aluminum disks
impregnated with Solution No 3 was submitted to a controlled to and fro
movement (110
cycles/min) with a puck of sandpaper (caliber 320) for 120 minutes. A
continuous
pressure of 12.8 KPa was applied onto the puck to ensure a constant friction
of the
sandpaper on the aluminum disks. After the abrasion period, residual
antimicrobial
activity anodized aluminum disks was assessed by laying the disks over
microbial lawns
of Staphylococcus aureus ATCC 6538 for 5 min. The results are shown in Table 3
hereinafter:
Table 3: Effect of abrasion on antimicrobial activity
Zone of inhibition Surface (mm2)
(diameter in mm)
Without abrasion 35 x 36 989
With abrasion 24 x 31 584
Untreated disk shown no zone of inhibition (surface = 0 mm2)
- 20 -
CA 02909198 2015-10-09
WO 2013/155618
PCT/CA2013/000389
[00090] As can be appreciated, abrasion had a limited impact on antimicrobial
activity,
confirming that metal products according to the invention can withstand
wearing and
maintain their effectiveness under harsh conditions.
[00091] Altogether these results demonstrate the antimicrobial metal products
according to the invention, including aluminum anodized surfaces, have
interesting
antimicrobial properties. Therefore, metallic articles comprising the same may
have
numerous applications in various household, hospital and industrial
environments.
[00092]
Headings are included herein for reference and to aid in locating certain
sections. These headings are not intended to limit the scope of the concepts
described
therein under, and these concepts may have applicability in other sections
throughout
the entire specification. Thus, the present invention is not intended to be
limited to the
embodiments shown herein but is to be accorded the widest scope consistent
with the
principles and novel features disclosed herein.
[00093] As used herein and in the appended claims, the singular forms "a",
"an", and
"the" include plural referents unless the context clearly indicates otherwise.
[00094] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
reaction conditions, concentrations, properties, and so forth used in the
specification and
claims are to be understood as being modified in all instances by the term
"about". At the
very least, each numerical parameter should at least be construed in light of
the number
of reported significant digits and by applying ordinary rounding techniques.
Accordingly,
unless indicated to the contrary, the numerical parameters set forth in the
present
specification and attached claims are approximations that may vary depending
upon the
properties sought to be obtained. Notwithstanding that the numerical ranges
and
parameters setting forth the broad scope of the embodiments are
approximations, the
numerical values set forth in the specific examples are reported as precisely
as possible.
Any numerical value, however, inherently contain certain errors resulting from
variations
in experiments, testing measurements, statistical analyses and such.
[00095] It is understood that the examples and embodiments described herein
are for
illustrative purposes only and that various modifications or changes in light
thereof will be
suggested to persons skilled in the art.
- 21 -