Note: Descriptions are shown in the official language in which they were submitted.
= PROCESS TO SEPARATE ALKALI METAL SALTS
FROM ALKALI METAL REACTED HYDROCARBONS
FIELD OF THE INVENTION
[0002] The present invention relates to a process for removing
nitrogen, sulfur, and heavy
metals from sulfur-, nitrogen-, and metal-bearing shale oil, bitumen, heavy
oil, or refinery
streams using an alkali metal. More particularly, the invention relates to a
process to facilitate
separation of alkali metal compounds and reduced heavy metals from alkali
metal reacted
hydrocarbons.
BACKGROUND OF THE INVENTION
[0003] U.S. Patent Application Serial No. 13/753,918 has been
published as United States
Patent Application Publication No. 2013/0140217. The reader is presumed to be
familiar with
the disclosure of this published application. This published application will
be referred to herein
as the '918 application."
[0004] U.S. Patent Application Serial No. 12/916,984 has been
published as United States
Patent Application Publication No. 2011/0100874. The reader is presumed to be
familiar with
the disclosure of this published application. This published application will
be referred to herein
as the '984 application."
[0005] U.S. Patent No. 8,088,270 relates to a "Process For Recovering
Alkali Metals And
Sulfur From Alkali Metal Sulfides And PolySulfides." The reader is presumed to
be familiar
1
CA 2909443 2019-04-10
with the disclosure of this published patent. This published patent will be
referred to herein as
the "270 patent."
[0006] The demand for energy and the hydrocarbons from which that energy is
derived is
continually rising. The hydrocarbon raw materials used to provide this energy,
however, can
contain difficult to remove sulfur and metals that hinder their usage. Sulfur
can cause air
pollution, and can poison catalysts designed to remove hydrocarbons and
nitrogen oxide from
motor vehicle exhaust. Similarly, heavy metals contained in the hydrocarbon
stream can poison
catalysts typically utilized for removal of sulfur.
[0007] Extensive reserves of shale oil exist in the U.S. that will
increasingly play a role in
meeting U.S. energy needs. Over 1 trillion barrel reserves lay in a relatively
small area known as
the Green River Formation located in Colorado, Utah, and Wyoming. As the price
of crude oil
rises, these shale oil resources become more attractive. However, technical
issues surrounding
this shale oil remain to be solved. For example, this shale oil has a
relatively high amount of
nitrogen contained therein (in addition to high levels of heavy metals and
sulfur). Shale oil
characteristically is high in nitrogen, sulfur, and heavy metals which makes
subsequent
hydrotreating difficult. It is known that nitrogen is typically around 2% and
sulfur around 1% in
most samples of shale oil. Heavy metals may also present. Heavy metals
contained in shale oil
pose a large problem to upgraders trying to upgrade this shale oil for
commercial use. For
example, sulfur and nitrogen typically are removed from the shale oil via
hydrotreating at
elevated temperatures and pressures using catalysts such as Co¨Mo/A1203 or
Ni¨Mo/A1203.
However, such catalysts are deactivated (poisoned) by the presence of heavy
metals as the heavy
metals operate to mask the catalysts.
[0008] Another example of a source of hydrocarbon fuel where the removal of
sulfur poses a
problem is in bitumen existing in ample quantities in Alberta, Canada and
heavy oils such as in
Venezuela. In order to remove sufficient sulfur from the bitumen for it to be
useful as an energy
resource, excessive hydrogen must be introduced under extreme conditions,
which creates an
inefficient and economically undesirable process.
[0009] Over the last several years, sodium has been recognized as being
effective for the
treatment of high-sulfur petroleum oil distillate, crude, heavy oil, bitumen,
and shale oil. Sodium
is capable of reacting with the oil and its contaminants to dramatically
reduce the sulfur,
nitrogen, and metal content through the formation of sodium sulfide compounds
2
CA 2909443 2019-04-10
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
(sulfide, polysulfide and hydrosulfide). Examples of the processes can be seen
in U.S. Patent
numbers 3,785,965; 3,787,315; 3,788,978; 4,076,613; 5,695,632; 5,935,421; and
6,210,564.
[0010] When shale oil, heavy oil, bitumen or other oil feedstock is reacted
with the alkali
metal, this reaction occurs generally at a temperature between 150 - 450 C.
This reaction is
also performed at a pressure that is anywhere between atmospheric pressure and
2000 psi.
For example, 2 moles alkali metal and 1 mole hydrogen (H2) may be needed per
mole sulfur
according to the following initial reaction:
[0011] R¨S¨R' + 2M + H2 -> R¨H + R'¨H + M2S,
[0012] Where M is an alkali metal such as sodium or lithium.
[0013] By further example, 3 moles alkali metal and 1.5 moles hydrogen (H2)
may be
needed per mole nitrogen according to the following initial reaction:
[0014] R,RI,R"¨N + 3M + 1.5H2 R¨H + R'¨H + R"¨H + M3N
[0015] Where R, R', R" represent portions of organic molecules or organic
rings.
[0016] Alternatively, a method of upgrading an oil feedstock (such as heavy
oil, shale oil,
bitumen, etc.) may be used by combining the oil feedstock with an alkali metal
and an
upgradant hydrocarbon material, as disclosed in the '984 Application. This
reaction operates
to remove the sulfur, nitrogen and/or heavy metals contained within the oil
feedstock.
[0017] It should also be noted that heavy metals contained in the oil
feedstock may also be
removed via the use of alkali metals such as sodium. Heavy metals contained in
organometallic molecules such as complex porphyrins are reduced to the
metallic state by the
alkali metal. Once the heavy metals have been reduced, they can be separated
from the oil
because they no longer are chemically bonded to the organic structure. In
addition, once the
metals are removed from the porphyrin structure, the nitrogen heteroatoms in
the structure are
exposed for further denitrogenation.
[0018] The following is a non-limiting description of the foregoing process
of using alkali
metals to treat the shale oil, bitumen and/or other oil hydrocarbons. Liquid
phase alkali metal
is brought into contact with the organic molecules containing heteroatoms and
metals in the
presence of hydrogen, methane, and also gases such as nitrogen (or inert gases
such as
helium, neon, argon, krypton, xenon and radon). The free energy of reaction
with organic
sulfur, organic nitrogen and organic heavy metals is stronger with alkali
metals than with
hydrogen, so the reaction more readily occurs without full saturation of the
organics with
hydrogen. (Hydrogen is generally used in the reaction to cap broken bonds
previously
attached to heteroatoms and metals, prevent carbon-carbon bonds from forming
or coking.)
Once the alkali metal compounds are formed and heavy metals are reduced to
their metallic
3
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
states, it is necessary to separate these products from the hydrocarbon
materials. In many
cases, a gravimetric separation, such as centrifugation or filtering, can
separate the organic,
upgraded oil, from the salt phase, metallic phase, and organic solids which
may be formed.
[0019] Once the alkali metal compounds are separated from the hydrocarbon
feedstock,
sulfur and metals are substantially removed, and nitrogen is moderately
removed. Also, both
viscosity and density are reduced (API gravity is increased). Bitumen or heavy
oil would be
considered synthetic crude oil (SCO) and can be shipped via pipeline for
further refining.
Similarly, shale oil will have been considerably upgraded after such
processing. Subsequent
refining will be easier since the troublesome metals have been removed.
[0020] However, for some hydrocarbon feedstocks, gravimetric separation it
is not
possible without further processing. For example, the inventors have observed
that certain
hydrocarbon feedstocks that contain a heavy fraction of hydrocarbons, upon
processing with
an alkali metal produce a mixture of alkali metal compounds and reduced heavy
metals that
could not be separated from the upgraded hydrocarbon materials through
conventional
gravimetric separation.
[0021] It is an object of the present invention to provide a process to
facilitate separation
of alkali metal compounds, such as alkali metal sulfides and nitrides, and
reduced heavy
metals from alkali metal reacted hydrocarbons.
BRIEF SUMMARY OF THE INVENTION
[0022] The present invention provides a process to facilitate separation of
alkali metal
compounds and reduced heavy metals from alkali metal reacted hydrocarbons.
[0023] Reference throughout this specification to features, advantages, or
similar language
does not imply that all of the features and advantages that may be realized
with the present
invention should be or are in any single embodiment of the invention. Rather,
language
referring to the features and advantages is understood to mean that a specific
feature,
advantage, or characteristic described in connection with an embodiment is
included in at
least one embodiment of the present invention. Thus, discussion of the
features and
advantages, and similar language, throughout this specification may, but do
not necessarily,
refer to the same embodiment, but may refer to every embodiment.
[0024] Furthermore, the described features, advantages, and characteristics
of the
invention may be combined in any suitable manner in one or more embodiments.
One skilled
in the relevant art will recognize that the invention may be practiced without
one or more of
the specific features or advantages of a particular embodiment. In other
instances, additional
4
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
features and advantages may be recognized in certain embodiments that may not
be present in
all embodiments of the invention.
[0025] The disclosed process facilitates gravimetric separation of alkali
metal salts from
alkali metal reacted hydrocarbons. The process includes heating a mixture
resulting from a
reaction of an alkali metal and a quantity of a hydrocarbon feedstock having
at least one
heavy fraction and mechanically mixing the mixture during the heating step.
The mixture
includes alkali metal salts and alkali metal reacted hydrocarbon feedstock.
The mixture is
heated to a temperature in the range from about 350 C to 400 C. The mixture
may be heated
to a temperature of about 375 C 10 C. The alkali metal may be sodium or
lithium. The
alkali metal salts comprise sodium sulfide and/or sodium polysulfide.
[0026] In one embodiment, the mixture is heated and mechanically mixed for
a time
period of over 15 minutes. In another embodiment, the mixture is heated and
mechanically
mixed for a time period of over 30 minutes. In yet another embodiment, the
mixture is
heated and mechanically mixed for a time period of at least 1 hour. In a
further embodiment,
the mixture is heated and mechanically mixed for a time period between about 1
and 2 hours.
[0027] The quantity of a hydrocarbon feedstock may be a sulfur-, nitrogen-,
and metal-
bearing shale oil, bitumen, heavy oil, or refinery stream that contains a
heavy fraction. In one
embodiment, the hydrocarbon feedstock comprises bitumen.
[0028] The process may further comprise the step of separating
gravimettically the alkali
metal salts from the alkali metal reacted hydrocarbons.
[0029] In one embodiment, the process includes the step of adding a portion
of the
separated alkali metal salts to the mixture of alkali metal salts and alkali
metal reacted
hydrocarbons prior to heating. In another embodiment, the process includes the
step of
adding a portion of the separated alkali metal salts to the hydrocarbon
feedstock prior to
reacting with the alkali metal.
[0030] The disclosed process may be part of a broader process of upgrading
a
hydrocarbon or oil feedstock. The process is most useful with hydrocarbon
feedstocks
having at least one heavy fraction, such as bitumen. The hydrocarbon feedstock
typically
includes at least one carbon atom and a sulfur heteroatom and/or one or more
heavy metals.
In the process, the quantity of hydrocarbon feedstock is reacted with an
alkali metal and an
upgradant hydrocarbon, wherein the upgradant hydrocarbon includes at least one
carbon atom
and at least one hydrogen atom or with hydrogen gas or liquid with hydrogen
dissolved
within. The alkali metal reacts with the sulfur heteroatom and/or the one or
more heavy
metals to form one or more inorganic products comprising alkali metal sulfide
or alkali metal
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
polysulfides. The upgradant hydrocarbon or hydrogen reacts with the
hydrocarbon feedstock
to produce an upgraded hydrocarbon feedstock, wherein the number of carbon
atoms in the
upgraded hydrocarbon feedstock may be greater than the number of carbon atoms
in the
hydrocarbon feedstock.
[0031] The mixture of inorganic products and the upgradant hydrocarbon is
heated to a
temperature in the range from about 350 C to 400 C, while undergoing
mechanical mixing of
the mixture. Thereafter, the inorganic products are gravimetrically separated
from the
upgraded hydrocarbon feedstock.
[0032] The process may include the optional step of adding a portion of the
separated
inorganic products to the mixture of inorganic products the upgradant
hydrocarbon prior to
heating. The process may optionally include the step of adding a portion of
the separated
inorganic products to the hydrocarbon feedstock prior to reacting with the
alkali metal.
[0033] These features and advantages of the present invention will become
more fully
apparent from the following description and appended claims, or may be learned
by the
practice of the invention as set forth hereinafter.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0034] In order that the manner in which the above-recited and other
features and
advantages of the invention are obtained will be readily understood, a more
particular
description of the invention briefly described above will be rendered by
reference to specific
embodiments thereof that are illustrated in the appended drawings.
Understanding that these
drawings depict only typical embodiments of the invention and are not
therefore to be
considered to be limiting of its scope, the invention will be described and
explained with
additional specificity and detail through the use of the accompanying drawings
in which:
[0035] Fig. 1 is flow diagram showing one embodiment of a process of
upgrading an oil
feedstock.
[0036] Fig. 2 is a flow diagram showing one embodiment of a process of
upgrading an oil
feedstock with an alkali metal and for separating inorganic products from the
upgraded oil
feedstock.
DETAILED DESCRIPTION OF THE INVENTION
[0037] The present embodiments of the present invention will be best
understood by
reference to the drawings, wherein like parts are designated by like numerals
throughout. It
will be readily understood that the components of the present invention, as
generally
described and illustrated in the figures herein, could be arranged and
designed in a wide
variety of different configurations. Thus, the following more detailed
description of the
6
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
embodiments of the methods and cells of the present invention, as represented
in Figs. 1 and
2, is not intended to limit the scope of the invention, as claimed, but is
merely representative
of present embodiments of the invention.
[0038] The present embodiments relate to a method of upgrading a
hydrocarbon or oil
feedstock (such as heavy oil, shale oil, bitumen, etc.) by combining the oil
feedstock with an
alkali metal and an upgradant hydrocarbon material or hydrogen, as disclosed
in the '984
Application, to cap broken bonds previously attached to heteroatoms and
metals. This
reaction operates to remove the sulfur, nitrogen and/or heavy metals contained
within the oil
feedstock.
[0039] The upgradant hydrocarbon used in this process, may be hydrogen gas
(H2), or
may be a hydrocarbon. Examples of the hydrocarbons that may be used include
methane,
ethane, propane, butane, pentane, hexane, ethene, propene, butane, pentene,
dienes, and their
isomers. Other hydrocarbons (such as octane, or other carbon containing
compounds
containing one or more carbon atoms) may also be used. The hydrocarbon gas may
also be
comprised of a mixture of hydrocarbon gases (such as natural gas, or shale gas
¨ the gas
produced by retorting oil shale). In many embodiments, the hydrocarbon gas may
be
methane from natural gas because this component is inexpensive and readily
available.
[0040] In one embodiment, the hydrocarbon has at least one carbon atom and
at least one
hydrogen atom. The hydrogen atom should be such that it can be pulled off from
the carbon
atom to form a bond with the organic molecules of the feedstock. The
hydrocarbon atom
may include hydrogen atoms bonded therein, but the hydrocarbon molecule must
include at
least one carbon atom (and thus carmot comprise H2 gas). The hydrocarbon may
be selected
such that it will increase the ratio of hydrogen to carbon in the organic
product. This occurs
by selecting the hydrocarbon such that the hydrocarbon has a greater hydrogen-
to-carbon
ratio than the starting feedstock. Of course, a lower hydrogen-to-carbon ratio
in the
hydrocarbon can still provide upgrading benefits if the heteroatom content is
reduced.
[0041] In another embodiment rather than utilizing an upgradant
hydrocarbon, hydrogen is
utilized or a gas mixture comprising hydrogen.
[0042] The hydrocarbon or oil feedstock is combined with the hydrocarbon
(such as
methane) or hydrogen and the alkali metal (such as sodium) in a reactor vessel
and allowed to
react for a period of time. The reaction may, in some embodiments, be
conducted at a
temperature less than about 450 C. In one embodiment, the reaction is
conducted at a
temperature higher than 150 C. The reaction may be conducted at a pressure
higher than
7
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
about 250 psi. In one embodiment, the reaction is conducted at a pressure
below about 2500.
Other embodiments may be done at lower temperatures and/or lower pressures.
[0043] This process may, in some embodiments, occur in the presence of a
catalyst to help
promote the chemical reactions. The catalysts may include by way of non-
limiting example,
molybdenum, nickel, cobalt or alloys of molybdenum, alloys of nickel, alloys
of cobalt,
alloys of molybdenum containing nickel and/or cobalt, alloys of nickel
containing cobalt
and/or molybdenum, molybdenum oxide, nickel oxide or cobalt oxides, iron or
iron oxide and
combinations thereof. Any alkali metal could be used in the process including,
but not
limited to, mixtures and/or alloys of alkali metals. In some embodiments,
potassium, sodium,
lithium and/or alloys thereof, may be used.
[0044] During this reaction, sulfur and nitrogen atoms separate from the
organic
molecules in the oil feedstock and combine with the alkali metal (sodium or
lithium) to form
sulfides and nitrides. These alkali metal sulfides/nitrides are inorganic
compounds that
separate into an inorganic phase that is distinct from the organic phase
housing the organic
compounds. A portion of the heavy metals originally contained in the organic
materials, such
as iron, arsenic and vanadium, are reduced and can also be separated into the
inorganic phase
as well. The resulting organic compounds are in the organic phase and react
with the
upgradant hydrocarbon, such as methane or with hydrogen. Because the
heteroatoms react
with the alkali metal, the resulting product has a lower heteroatom to carbon
ratio than the
original oil feedstock.
[0045] The alkali metal may be added to the reaction vessel because the
free energy of
formation of the alkali metal sulfide is greater than the free energy of
formation of H2S. In
one embodiment, the reaction proceeds more readily with the introduction of
the alkali metal.
In one embodiment, the alkali metal may include sodium, lithium, or the like.
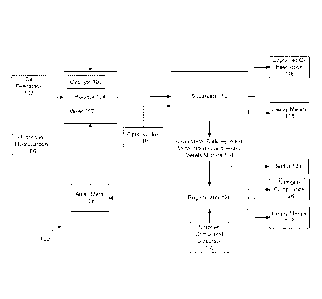
[0046] Referring now to Fig. 1, a schematic method 100 of an embodiment for
upgrading
an oil feedstock is disclosed. As can be seen from Fig. 1, a quantity of oil
feedstock 102 is
obtained. This oil feedstock 102 may comprise bitumen, shale oil, heavy oil,
or other
materials described herein. The oil feedstock 102 may be obtained via mining
or other
processes. The oil feedstock 102 is added to a reaction vessel 104 (which is
referred to herein
as reactor 104). The reactor 104 may include a mixer 107 that is designed to
mix (stir) the
chemicals added therein in order to facilitate a reaction. A catalyst 105 of
the type described
above may also be added to the reactor 104 to foster the reaction.
8
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
[0047] Also added to the reactor 104 is a quantity of an alkali metal 108.
This alkali metal
108 may be any alkali metal 108 and may include mixtures of alkali metals 108.
In some
embodiments, sodium or lithium may be used.
[0048] A quantity of an upgradant hydrocarbon 106 may also be used and added
to the
reactor 104 or in place of upgradant hydrocarbon hydrogen may be used. As
noted above,
this upgradant hydrocarbon 106 may be methane, ethane, propane, etc. or any
other
hydrocarbon (or even mixtures thereof). However, because of its relative
inexpensive nature,
natural gas or shale oil gas (which generally contains methane CH4) may be
used.
[0049] As noted herein, the reactor 104 may cause the reaction to occur at
a certain
temperature or pressure. In some embodiments, the temperature used for the
reaction may be
elevated up to about 450 C. One exemplary temperature may be 350 C. In other
embodiments, the temperature may be such that the alkali metal 108 is in a
molten state. It
will be appreciated by those of skill in the art that sodium becomes molten at
about 98 C
whereas lithium becomes molten at about 180 C. Thus, embodiments may be
designed in
which the temperature of the reactor 104 is at a temperature above the melting
temperature of
the alkali metal 108. The pressure of the reaction may be anywhere from
atmospheric
pressure and above. Some exemplary embodiments are performed at a pressure
that is above
about 250 psi. Other embodiment may be performed at a pressure that is below
about 2500
psi.
[0050] When the temperature is elevated, the alkali metal 108 may be molten
to facilitate
the mixing of this chemical with the other chemicals. However, other
embodiments may be
designed in which a powdered or other solid quantity of the alkali metal 108
is blown into, or
otherwise introduced, into the reactor 104 so that it reacts with the other
chemicals.
[0051] In a reaction that occurs in the reactor 104, the heteroatoms (such
as sulfur and
nitrogen) and other heavy metals are removed from the oil feedstock 102. The
products from
the reactor 104 are then sent to a separator 112. The separator 112 may
include a variety of
devices/processes that are designed to separate the upgraded oil feedstock 116
from the other
reaction products. The separator 112 may include filters, centrifuges and the
like. The
separator 112 may also receive, depending upon the embodiment, an influx of a
flux 119.
This flux material 119 may be hydrogen sulfide H2S or water or other
chemical(s) that
facilitate the separation. Mixing the treated feedstock with hydrogen sulfide
to form an alkali
hydrosulfide can form a separate phase from the organic phase (oil feedstock).
This reaction
is shown below, in which sodium (Na) is the alkali metal, although other
alkali metals may
also be used:
9
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
[0052] Na2S + H2S ¨> 2NaHS (which is a liquid at 375 C)
[0053] Na3N + 3H25 ¨> 3NaHS + NH3
[0054] The nitrogen product is removed in the form of ammonia gas (NH3) which
may be
vented and recovered, whereas the sulfur product is removed in the form of an
alkali hydro
sulfide, NaHS, which is separated for further processing. Any reduced heavy
metals will also
be separated out from the organic hydrocarbons by gravimetric separation
techniques.
[0055] The flux may be ammonia utilized to scavenge unreacted alkali metal.
Then the
alkali metal laden ammonia is separated from the oil, flashed off and the
alkali metal may be
sent back to the reactor for further processing.
[0056] Some heavy metals 118 which were reduced from the feedstock 102 may
separate
here and be extracted as heavy metals 118. The separation also produces the
organic product,
which is the upgraded oil feedstock 116. This upgraded oil feedstock 116 may
be shipped to
a refinery for further processing, as needed, to make this material a suitable
hydrocarbon fuel.
Another output of the separator 112 is a mixture 114 (stream) of alkali metal
sulfides, alkali
metal nitrides, and heavy metals 118. This mixture 114 may be further
processed as
described below. Alternatively or additionally, any nitrogen containing
products (such as via
ammonia gas (NH3) that is vented off and collected) may also be removed from
this stage
depending on the type of the process employed.
10057] The mixture 114 of alkali metal sulfides, alkali metal nitrides, and
heavy metals
118 may be thermally processed as described in the 217 application where the
mixture is
heated to elevated temperature in a non-oxidizing and dry atmosphere then may
be sent to a
regenerator 120. The purpose of the regenerator 120 is to regenerate the
alkali metal 108 so
that it may be reused in further processing at the reactor 104. Thus, one of
the outputs of the
regenerator 120 is a quantity of the alkali metal 108. In many embodiments,
the regeneration
step involves an electrolytic reaction (electrolysis) of an alkali metal
sulfide and/or
polysulfide using an alkali metal ion conductive ceramic membrane (such as,
for example, a
NaSiCON or LiSiCON membrane that is commercially available from Ceramatec,
Inc. of
Salt Lake City, Utah). Non-limiting examples of such processes are found in
U.S. Patent No.
8,088,270, the '918 application, and the '984 application. The result of this
electrolysis
process is that sulfur 124 will be captured. Further, heavy metals 132 may be
separated from
the mixture 114, via the electrolysis process or other processes. In further
embodiments, the
nitrogen containing compounds 128 may also be collected at the regenerator
120. As noted
above, such nitrogen compounds 128 may be ammonia gas that is vented off or
collected. In
other embodiments, nitrogen compound precursors 130 are added to the
regenerator 120 to
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
capture/react with the nitrogen containing compounds in the mixture 114 and
produce the
compounds 128. Those skilled in the art will appreciate the various chemicals
and processes
that may be used to capture the nitrogen compounds 128 (or to otherwise
process the nitrogen
obtained from the reaction).
[0058] The method 100 of Fig. 1 may be run as a batch process or may be a
continuous
process, depending upon the embodiment. Specifically, if it is a continuous
process, the
reactants would be continuously added to the reactor 104 and the products
continuously
removed, separated, etc. Further, the reaction in the reactor 104 may be
performed as a single
step (e.g., placing all of the chemicals into a single reactor 104) or
potentially done as a series
of steps or reactions.
[0059] Referring now to Fig. 2, a schematic method 200 of an embodiment for
upgrading
an oil feedstock is disclosed. The disclosed method is based upon the method
of Fig. 1.
Even though some features shown in Fig. 1 are not reproduced in relation to
Fig. 2, it is to be
understood that Fig. 2 can include the features discussed above. As can be
seen from Fig. 2,
a quantity of oil feedstock 202 is obtained. This oil feedstock 202 may
comprise bitumen,
shale oil, heavy oil, or other materials described herein that contains a
heavy fraction. As
used herein, the term "heavy fraction" refers to one or more fractions that
have a boiling
point above 524 C. Bitumen is known to contain a heavy fraction. The oil
feedstock 202
may be obtained via mining or other processes. The oil feedstock 202 is added
to a reaction
vessel 204 (which is referred to herein as reactor 204). The reactor 204 may
include a mixer
207 that is designed to mix (stir) the chemicals added therein in order to
facilitate a reaction.
The reactor 204 may also include a heater 209 to heat the reactants to a
predetermined
temperature.
[0060] Also added to the reactor 204 is a quantity of an alkali metal 208.
This alkali metal
208 may be any alkali metal 208 and may include mixtures of alkali metals 208.
In some
embodiments, sodium or lithium may be used.
[0061] A quantity of an upgradant hydrocarbon 206 or hydrogen may also be used
and
added to the reactor 204. As noted above, this upgradant hydrocarbon 206 may
be methane,
ethane, propane, etc. or any other hydrocarbon (or even mixtures thereof).
However, because
of its relative inexpensive nature, natural gas or shale oil gas (which
generally contains
methane CH4) may be used but alternatively hydrogen may be used or a mixture
thereof.
[0062] As noted herein, the reactor 204 may cause the reaction to occur at
a certain
temperature or pressure. In some embodiments, the temperature used for the
reaction may be
elevated up to about 450 C. One exemplary temperature may be 350 C. In some
11
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
embodiments, the temperature may be such that the alkali metal 208 is in a
molten state. It
will be appreciated by those of skill in the art that sodium becomes molten at
about 98 C
whereas lithium becomes molten at about 180 C. Thus, embodiments may be
designed in
which the temperature of the reactor 204 is at a temperature above the melting
temperature of
the alkali metal 208. The pressure of the reaction may be anywhere from
atmospheric
pressure and above. Some exemplary embodiments are performed at a pressure
that is above
about 250 psi. Other embodiment may be performed at a pressure that is below
about 2500
psi.
[0063] In a reaction that occurs in the reactor 204, the heteroatoms (such
as sulfur and
nitrogen) and other heavy metals are converted into a mixture of alkali metal
sulfides or
polysulfides, alkali metal nitrides, and heavy metals, collectively referred
to as inorganic
products and the upgraded oil feedstock. It has been observed that when the
oil feedstock
202 contains a heavy fraction, the mixture of inorganic products and upgraded
oil feedstock
cannot be effectively separated without further processing.
[0064] As shown in Fig. 2, the inorganic products and upgraded oil
feedstock are
introduced into a holding vessel 210 that also contains a mixer 207 and a
heater 209. The
holding vessel 210 is shown in dashed lines because it can be a vessel
separate from the
reactor 204 or it can be the reactor 204 itself The mixture of inorganic
products and
upgraded oil feedstock are heated to a temperature in the range from about 350
C to 400 C,
and the mixture is mechanically mixed during the heating step. The mixture may
be heated
to a temperature of about 375 C 10 C.
[0065] In one embodiment, the mixture is heated and mechanically mixed for
a time
period of over 15 minutes. In another embodiment, the mixture is heated and
mechanically
mixed for a time period of over 30 minutes. In yet another embodiment, the
mixture is
heated and mechanically mixed for a time period of at least 1 hour. In a
further embodiment,
the mixture is heated and mechanically mixed for a time period between about 1
and 2 hours.
[0066] The mixture of alkali metal reaction products from the reactor 204
are then sent to
a separator 212. The separator 212 may include a variety of devices/processes
that are
designed to separate the upgraded oil feedstock 216 from the other reaction
products. The
separator 212 may include filters, centrifuges and the like.
[0067] The organic product, which is the upgraded oil feedstock 216. This
upgraded oil
feedstock 216 may be shipped to a refinery for further processing, as needed,
to make this
material a suitable hydrocarbon fuel. Another output of the separator 212 is a
mixture 214
(stream) of alkali metal sulfides, alkali metal nitrides, and heavy metals.
This mixture 214
12
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
may be further processed as described below. Alternatively or additionally,
any nitrogen
containing products (such as via ammonia gas (NH3) that is vented off and
collected) may
also be removed from this stage depending on the type of the process employed.
[0068] The inorganic products 214, which contain a mixture of alkali metal
salts, such as
alkali metal sulfides, alkali metal nitrides, and/or heavy metals, may be
thermally processed
as described in the 217 application where the mixture is heated to elevated
temperature in a
non-oxidizing and dry atmosphere then may be sent to a regenerator 220. The
purpose of the
regenerator 220 is to regenerate the alkali metal 208 so that it may be reused
in further
processing at the reactor 204. Thus, one of the outputs of the regenerator 220
is a quantity of
the alkali metal 208.
[0069] In one embodiment, the process shown in Fig. 2 includes the ability
to add a
portion of the separated inorganic products, such as the alkali metal sulfides
or polysulfides,
to the mixture of inorganic products and upgraded oil feedstock prior to
heating in the
holding vessel 210. Without being bound by theory, it is currently believed
that the separated
inorganic products may provide a "seed" to facilitate the agglomeration of
fine alkali metal
sulfide particles within the mixture, which ultimately facilitates the
separation process. Thus,
a recycle stream 224 is provided in which a portion of the separated inorganic
products may
be fed to the holding vessel 210.
[0070] In another related embodiment, the process shown in Fig. 2 includes
the ability to
add a portion of the separated inorganic products to the oil feedstock prior
to reacting with
the alkali metal. Without being bound by theory, it is currently believed that
the separated
inorganic products may provide a "seed" to facilitate the agglomeration of
fine alkali metal
sulfide particles within the mixture, which ultimately facilitates the
separation process. Thus,
a recycle stream 226 is provided in which a portion of the separated inorganic
products may
be fed to the reactor 204. The seeding process may occur before alkali metal
addition or after
alkali metal addition.
[0071] It has been observed that by taking a mixture of alkali metal
compounds and
reduced heavy metals from alkali metal reacted hydrocarbons to an elevated
temperature, for
example to 375 C 10 C, a temperature where negligible thermal cracking is
expected to
occur, and mixing the constituents for a period of time, for example 1 ¨ 2
hours, and then
cooling the mixture, the solids and liquids could easily be separated by
centrifugation or other
gravimetrical separation methods.
[0072] Without being bound by theory, when sodium reacts with the organic
sulfur in the
oil feedstock, sodium sulfide forms at the molecular level. Initially, the
sodium sulfide
13
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
particles are so fine they will not separate easily even though they have
higher specific
gravity than the oil feedstock. However, by mixing the mixture of alkali metal
compounds,
reduced heavy metals, and alkali metal reacted hydrocarbon feedstock for a
period of time at
elevated temperature, the fine particles are believed to agglomerate or form
clusters to a size
large enough where they can separate gravimetrically. Recycling inorganic has
shown to be
very effective in enabling the reduction of the amount of time necessary to
conduct the
mixing at elevated temperature after reaction and still separate the inorganic
material from
the organic.
[0073] Experiments showed that mixing at too low of a temperature, for
example at 300
C, for 2 hours the fine particles still would not separate using conventional
methods but
mixing at a higher temperature, 380 C for 1 hour resulted in a mixture that
would mostly
separate. For example about 90% of the sodium sulfide formed would separate
easily by
centrifugation. By stirring at 2 hours at 380 C, 99% of the sodium sulfide
formed would
separate by centrifugation.
[0074] In the experiments conducted, it was evident when the alkali metal
sulfide particles
agglomerated to an extent because the viscosity slowly dropped over time then
eventually
stabilized with time. When power was applied to the agitator in the mixing
vessel, the speed
of the agitator increased as the viscosity declined then eventually
stabilized. It is understood
that the time required for the process to occur will vary depending on many
parameters such
as mixing efficiency, starting viscosity, and temperature.
[0075] It is understood that the oil feedstock in this invention may
originate from many
sources such as petroleum, heavy oil, retorted oil shale, bitumen, and oil
refinery streams
where the oil originally comprised organic sulfur. The disclosed process is
most applicable
to oil feedstocks that contain a heavy fraction.
[0076] In another experiment, using an Alberta bitumen feedstock with
approximately
50% resid fraction which boiled above 524 C and with 53% starting sulfur
concentration.
104g of recycled inorganic material from a previous batch reactor run was
added to 650g
fresh bitumen. The recycle material was intended to serve as "seed" for
agglomeration of the
inorganic material as it was formed during the course of reaction with 48.5g
sodium metal
where the reaction was conducted at 357 C and 1500 psi with hydrogen
atmosphere mixed in
during the reaction. There was no further mixing after the initial reaction
with sodium. When
"seed" was utilized the mixture leaving the reactor was centrifuged and the
product sulfur
concentration was 1.32%. When the "seed" was not used the sulfur concentration
remained
14
CA 02909443 2015-10-13
WO 2014/172361 PCT/US2014/034183
nearly the same in the oil because the sodium sulfide that was produced
remained in the oil
and could not be separated through centrifugation.
[0077] In another related embodiment, a process to facilitate separation of
alkali metal
salts from alkali metal reacted hydrocarbons includes adding a portion of the
separated alkali
metal salts to the mixture of alkali metal hydrocarbon feedstock having at
least one heavy
fraction. The mixture may include alkali metal salts and alkali metal reacted
hydrocarbons.
The process may also include adding a portion of the separated alkali metals
salts to the
mixture of alkali metal hydrocarbon feedstock prior to addition of the alkali
metal. In a
related process for removal of residual alkali metal from alkali metal reacted
hydrocarbons, a
flux is mixed with the hydrocarbon to dissolve the alkali metal. In one
embodiment, the flux
is ammonia. The ammonia with dissolved alkali metal may be flashed off to
yield the alkali
metal.
[0078] While specific embodiments of the present invention have been
illustrated and
described, numerous modifications come to mind without significantly departing
from the
spirit of the invention, and the scope of protection is only limited by the
scope of the
accompanying claims.