Note: Descriptions are shown in the official language in which they were submitted.
. CA 02910289 2015-10-26
Reduced Fossil Fuel in an Oxidizer Downstream of a Biomass
Furnace
FIELD
The embodiments disclosed herein are directed
generally to processes that utilize a biomass burner
(referred to as a furnace) and operate an oxidizer.
More
particularly, the embodiments are aimed at reducing fossil
fuels consumed in the oxidizer typically used to clean
gases generated from a process by utilizing or extracting
combustible gases from the furnace and introducing them to
the oxidizer.
The gases preferably are conditioned prior
to their introduction into the oxidizer, making them more
practical for use in supplementing the oxidizer energy
requirements.
BACKGROUND
Thermal oxidizers, and in particular, regenerative
thermal oxidizers, have been used downstream of drying
systems to remove Volatile Organic Compounds (VOC's) and
carbon monoxide (CO) emissions.
These drying systems may
be drying any of a myriad of materials, such as green wood,
wood fiber, coffee beans, agricultural products and other
materials to lower the moisture content of the raw material
so it can be turned into a final product.
Furthermore,
these drying systems often use a biomass fuel furnace to
provide heat to the dryer.
The abatement system often
utilized to meet stringent air quality standards typically
1
CA 02910289 2015-26
includes a device or devices to remove particulate and a
device to remove gaseous organic compounds. For
example,
particulate removal can be accomplished via cyclones,
baghouses, scrubbers and more typically dry or wet
electrostatic precipitators. These particulate devices may
operate by themselves, but when VOC, CO and other gaseous
organic compounds must be removed, they are utilized as a
pre-filter for an oxidizer. Additional manufacturing steps
such as in the pressing process also release VOC's that can
be treated in additional oxidizers. The
most common type
of oxidizer is a regenerative thermal oxidizer (known as an
RTO) which can have up to about 98 percent energy recovery
of the oxidized gas. Oxidizers have been employed to abate
volatile organic compounds (VOC's) from industrial
processes and this practice is well known.
In some industries, the amount of VOC's exhausted
contain a high enough caloric value to equal the thermal
energy requirement of the oxidizer, so once operating
temperature is achieved by the oxidizer burner, the
oxidizer burner turns off or goes to low fire with the
balance of the energy necessary for combustion coming from
the combustion of the VOC's in the process gas. This is
typically not the case in other industries such as panel
board manufacturing. As a
result, since the mid 1990's,
2
CA 02910289 2015-10-26
companies have searched for alternate ways to operate the
oxidizer other than by consuming fossil fuels (such as
natural gas or propane). One
such proposal is to build a
biomass gasifier and fire the oxidizer burner with the
"producer" or "syngas", as it is referred to, in the RTO
burner (hereinafter "syngas"). This
idea has been
impractical for several reasons. The typical syngas has a
much lower heating value then natural gas, typically one
tenth thereof, and therefore does not operate in a
conventional burner very well. The
heating value of the
gas also varies over time. For
this reason, the large
volume of syngas, if injected directly into the burner
chamber, would affect the mass balance of the oxidizer,
resulting in a drop of thermal efficiency. The
most
important reason that this idea has been impractical is
that a stand-alone biomass gasifier fitted with the
required equipment to condition the syngas prior to
delivery to the oxidizer is very expensive and has a very
long return on investment.
Continuous efforts have been undertaken by the present
inventors to devise a more practical and economical way to
supplement or replace the fossil fuel used in an oxidizer.
It was observed that many of these types of manufacturing
facilities, when constructed new in recent years, were
3
CA 02910289 2015-10-26
installing biomass furnaces to heat the dryers and hot oil
systems. These
furnaces were replacing older technology
such as suspension burners that require the use of dry and
fine small particles of wood to create a fire for heating.
Instead, these furnaces can combust wet (typically 25% to
50% moisture) scrap material such as bark, pine needles and
hogged stumps. Many older facilities have retrofitted this
type of furnace to lower the cost of the biomass (scrap)
that is used to heat the process. It was further observed
that these furnaces typically have a two step combustion
process. Step one involves a pile or mound of the material
burning at the bottom of the furnace with minimal
combustion air added from the side or below the pile. This
slow combustion and minimal air causes a low temperature
and a reduced (low oxygen) environment around the fuel.
The gas coming off has similar properties including caloric
value to those produced in gasifier systems. This gas then
travels a distance within the furnace until secondary air
is added in step 2, completing combustion and producing
high grade heat for the drying process.
It would be desirable to provide a process of
effectively and efficiently using the syngas produced in a
gasifier to operate a downstream RTO, such as by
4
CA 02910289 2015-10-26
maintaining the RTO at operating temperature while
minimizing or eliminating the use of fossil fuel.
SUMMARY
The embodiments disclosed herein include a method of
successfully extracting syngas between the zone in the
furnace where the oxygen-starved combustion of biomass
occurs and the zone in the furnace where secondary air is
added to complete combustion, conditioning and cleaning the
extracted syngas, and delivering it in a metered amount to
the oxidizer or upstream of the oxidizer to reduce or
eliminate the need for additional fossil fuels once the
oxidizer has achieved its operating temperature. The
conditioning process allows for transport of the extracted
syngas across a manufacturing facility so that it may be
used to operate even remote oxidizers or perhaps other
devices that typically use fossil fuels.
If a manufacturing facility has a biomass gasifier,
the conditioning methods presented herein can be utilized
to make transportation of the fuel gas and utilization for
the oxidizer practical.
Existing subsystems within these
facilities can be further utilized to reduce the costs of
the gas conditioning system. Accordingly, in certain
embodiments, the gasifier or furnace burns solid waste and
CA 02910289 2015-26
produces a syngas. The syngas, containing relatively high
levels of CO, is extracted from the furnace, conditioned,
and introduced into a regenerative thermal oxidizer as a
fuel source for the oxidizer, which combusts polluted
process gas and vents the clean (e.g., 98-99% clean) gas to
atmosphere.
In certain embodiments, no extraction of syngas from
the furnace takes place.
Instead, the furnace conditions
are appropriately manipulated so that normally undesirable
levels of CO and other VOC's remain in the process stream.
The heat from the furnace is used as intended (e.g., to
heat a dryer), the stream is conditioned, and ultimately
proceeds to a downstream RTO. Since the gas stream remains
rich in CO and VOC's, its fuel value in the RTO is
substantially higher than otherwise would be the case were
the biomass furnace not so manipulated.
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is a schematic diagram of a manufacturing
facility in including a furnace and a regenerative thermal
oxidizer in accordance with certain embodiments;
FIG. 2 is a cross-sectional view of the syngas
extraction point within the furnace of FIG. 1;
6
. CA 02910289 2015-10-26
FIG. 3 is a cross-section view of a venturi scrubber
than can be used to condition the syngas extracted from the
gasifier in accordance with certain embodiments; and
FIG. 4 is a schematic diagram of a particle separator
shown upstream of a regenerative thermal oxidizer in
accordance with certain embodiments.
DETAILED DESCRIPTION
The embodiments disclosed herein relate to methods of
reducing or eliminating the need for auxiliary fuel in the
oxidizer (referred to as the RTO) in a manufacturing
facility that includes a biomass furnace or gasifier and a
dryer. The furnace can be operated A) so excessive CO and
VOC's are released from the furnace, conditioned by the
particulate removal devices, and are directed to the
combustion chamber of an oxidizer where these gases are
combusted to form CO2 and H20 while providing some or all of
the energy to sustain the oxidizer operation, or B) so
syngas formed within the furnace can be extracted,
conditioned and delivered to the oxidizer where these gases
are combusted to form CO2 and H20 while providing some or
all of the energy to sustain the oxidizer operation. A
method of conditioning the gas is also disclosed, which
7
= CA 02910289 2015-10-26
enables the transporting and utilizing of this gas as
energy for use in the oxidizer system.
Even with the high thermal efficiency of the RTO, the
large air volumes exhausted from typical manufacturing
facility dryers (typically 50,000 to 500,000 cubic feet per
minute) and very low VOC concentration (low caloric value),
require substantial amounts of fossil fuel (typically
natural gas or propane) to operate the oxidizer, and
therefore result in on-going operating costs to the owner.
Some of these facilities also have additional oxidizers to
treat volatile organic compounds (VOC) as is the case with
a wood press used to make wood panels such as oriented
strand board, particle board or medium density board.
These oxidizers also would benefit from supplemental energy
transported from the biomass furnace as shown in FIG 1.
Syngas formed within the furnace (bio gas) will
typically have low oxygen levels due to formation in a
reduced atmosphere within the furnace.
Accordingly, in
accordance with certain embodiments, the biogas is blended
with the much larger volume of the process gas prior to
entering the oxidizer and therefore has sufficient oxygen
to combust in the oxidizer (RTC).
In accordance with certain embodiments, the reduction
of fossil fuel use in the oxidizer is accomplished by
8
CA 02910289 2015-10-26
controlling the operation of the furnace so excessive CO
and VOC's are released from the furnace. This
can be
achieved by manipulating biomass fuel feed rate, moisture
and over-fire or secondary combustion air. Another method
is to block one port in the over-fire or secondary
combustion air to purposely create a zone in the furnace
that does not complete combustion and therefore allows some
of the syngas/producer gas to travel through the furnace
and likely a higher amount of CO than typically would be
desirable as well.
Another method is to use a lance to
inject water in a section of the furnace. The
resulting
water vapor will suppress combustion similar to blocking a
portion of the combustion air. This
method requires
additional equipment but may provide a wider range of
control.
Allowing products of incomplete combustion to
pass through the furnace achieves a higher caloric value of
the gas stream ultimately being treated by the oxidizer.
Higher caloric value in the furnace exhaust can be achieved
by manipulating the control of the fuel feed, under fire
air feed and over air feed to produce high CO levels and
other products of partial combustion out of the furnace.
This embodiment is the simplest method in that minimal
changes to existing facilities are required, or the need
for additional equipment in existing facilities is not
9
CA 02910289 2015-10-26
necessary.
However, this method is somewhat limited in
that it will only reduce the fossil fuel requirement of
oxidizer(s) directly downstream of the furnace and does not
provide transport of energy to other oxidizers within the
facility that are not in fluid communication with the
furnace. Another limitation is it may prove difficult to
control the fuel value as it relates to more then one
downstream oxidizer, i.e., it cannot change the fuel value
independently to two or more oxidizers that are treating
the process gas. The higher caloric value of the furnace
exhaust is subject to conditioning by the existing
particulate removal devices (typically a scrubber or
electrostatic precipitator, e.g., FIG. 4) upstream of the
oxidizer as the process gas and thus further results in
clean fuel gas with minimal equipment required. By
reducing the amount of secondary combustion air, and
therefore oxygen in the second step of the combustion
process, excess CO will be produced as a byproduct of
incomplete combustion. This is typically in opposition to
the goal of the furnace operator or supplier in that CO is
typically considered a pollutant to be avoided. It would be
undesirable to vent high concentrations of CO to the
atmosphere if it were not for the position of the oxidizer
between the source of CO and the exhaust stack, downstream
CA 02910289 2015-10-26
of the oxidizer. The high temperatures within the oxidizer
complete the conversion of CO into 002 and in the process
benefit from the exothermic reaction, lowering the
requirement for fossil fuel.
In accordance with certain embodiments, a second
method provides more reliable control of syngas and can be
used to supplement energy to oxidizers located more remote
from the furnace, particularly oxidizers that are not in
fluid communication with the process stream. Syngas formed
within the furnace is extracted, conditioned and delivered
to the oxidizer to which these gases are combusted to form
CO2 and H20 while providing some or all of the energy to
sustain the oxidizer operation. This
method does not
require a change furnace operation or balance to ensure
incomplete combustion as does the first embodiment, as it
merely extracts a relatively small portion (e.g., 5-6%) of
the syngas before that small portion has been completely
combusted.
Furthermore the extracted gas can be divided
after the conditioning process and can be both supplied to
multiple oxidizers or process equipment and the gas rate
can be controlled for the fuel needs of each system
independently, as shown in FIG.1.
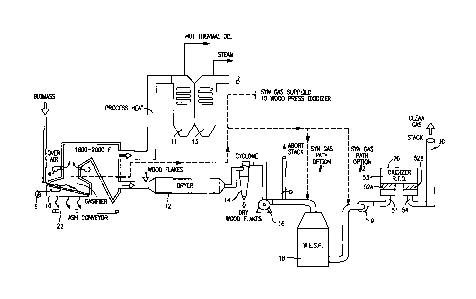
Turning now to FIG. 1, there is shown schematically a
manufacturing facility, such as a facility that produces
11
= CA 02910289 2015-10-26
wood panels. Those skilled in the art will appreciate that
the product being manufactured is not particularly limited;
the common factor being that the production thereof results
in a source of polluted air that includes VOC's that
require destruction prior to venting to atmosphere. In the
embodiment shown, the facility includes a furnace or
gasifier 10, a dryer 12, a particulate removal device 14,
fan 16, a second particulate removal device 18, and an
oxidizer 20. Those skilled in the art will appreciate that
the scheme shown is for illustrative purposes only; not all
components are necessarily essential to the various
embodiments disclosed herein.
Biomass is introduced into
the furnace or gasifier 10 by any suitable means.
Any
suitable gasifier can be used, such as a walking or
reciprocating grate furnace shown in greater detail in FIG.
2, to which under fire air is supplied such as with fan 5,
and over-air is supplied such as with fan 6, and an
associated ash removal device 22 such as a conveyer is
provided.
Heat from the gasifier 10 (combustion chamber
temperatures in the range of about 1600-2000 F are
achieved) can be directed to a thermal oil heater 11 or a
steam boiler 13 for steam turbines and generators to
produce electricity as shown. As seen in FIG. 2, a typical
furnace or gasifier 10 includes a lower gasification
12
CA 02910289 2015-10-26
chamber and an upper combustion chamber 17, which allows
for low emissions with good control of the combustion
process. The
furnace or gasifier 10 has means (typically
at a plurality of locations within the furnace) for the
introduction of secondary combustion air or "over-air" at
23, and additional combustion air at 24 such as with fan 7.
High CO can be produced on furnace gas by restricting a
portion of the secondary combustion air. This
can be a
fixed percentage of the furnace chamber such as blocking 5
percent of total secondary air , or can be actively
controlled by monitoring CO and 02 at the furnace discharge
and adjusting the introduction of secondary air
accordingly, such as by controlling fan 6.
The hot gas product of the gasifier can be used to dry
products such as wood flakes and the like in a dryer 12
where they are dried in a conventional manner. The
separated gases are introduced into a particulate removal
device 14 such as a cyclone, from which particulates are
extracted from the bottom, and the gas stream exits the top
of the cyclone. The separated gas leaving the cyclone can
be further cleaned in a second particulate removal device
18, such as a wet electrostatic precipitator 18 (FIGS. 1
and 4), following which it is sent to a regenerative
thermal oxidizer 20 such as with fan or pump 9. The
13
CA 02910289 2015-10-26
oxidizer incinerates volatile organic components, and
exhausts clean gas to atmosphere via exhaust stack 30.
Because CO and/or VOC's were intentionally left in the gas
existing the furnace 10, the gas entering the RTO has a
high energy value sufficient to maintain operation of the
RTO at optimal temperature without the introduction of
auxiliary fuel such as natural gas.
In accordance with an alternative embodiment, syngas
produced in the gasifier is extracted, preferably at a
location upstream of the combustion chamber 17. As seen in
FIG. 3, this can be accomplished with a syngas extraction
pipe 40 or the like, which penetrates a wall 41 of the
furnace 10. The extracted syngas is preferably conditioned
(e.g., syngas path option #1 shown in FIG. 1), and then
introduced into the regenerative thermal oxidizer 20, such
as into the inlet 51 of one of the heat exchange beds of
the oxidizer 20, or into the combustion zone 53 of the
oxidizer 20. Since
the syngas is extracted from the
furnace 10 prior to complete combustion, it is laden with
CO and VOC's and thus has a relatively high energy value.
It is thus suitable for use in an RTO (e.g., an inline,
downstream RTO, and/or an RTO at the facility that is not
inline) as a fuel for oxidation of process gas VOC's.
14
CA 02910289 2015-10-26
To achieve high particulate removal with the syngas,
especially of the fly ash contained within the syngas, a
high pressure drop venturi scrubber 45, a high temperature
baghouse or a precipitator 18 is preferably used. Normally
a high pressure drop venturi scrubber is not desirable for
fine particulate removal for large gas streams as this
leads to very high electrical demand by the blower, but
where the volume is small this is not a concern. If the
flue gas is maintained above 500 F, a dry electrostatic
precipitator can be used, but this may be impractical for
transporting the syngas any distance over 50 feet because
cooling of the ductwork will cause dropout of the heavy
organics unless the entire pipe work is heat traced and
insulated. While
dry filtration is contemplated in one
embodiment, the lower installed cost at of the wet scrubber
45 (FIG. 3) is preferred, particularly in view of its
ability to prevent fires or spread of fires from the
furnace to the process ductwork. As shown in FIG. 3, the
continuous supply of water as well as the high velocity in
the venturi section both provide efficient flame barriers.
The speed in the venturi is typically 100 to 250 mph, well
over flame propagation speed. To
further enhance the
removal efficiency of heavy organics, the make-up water to
the scrubber can be cooled through a heat exchanger (not
CA 02910289 2015-10-26
shown), thus leading to further reduction of heavy organics
through condensation. A high
pressure wet scrubber does
not have filters or other media to replace and has few
moving parts. Water remaining in the venture scrubber can
be routed to the wet electrostatic precipitator 18 for use
therein. A variable throat venturi is preferred as it
maintains removal efficiency over a wider range of flows.
Both the demand for supplemental energy by the oxidizer,
and the caloric value of the syngas will vary over time so
it is important to build a system that can deliver a
variable amount of syngas to the oxidizer.
Often these facilities have existing water treatment
systems as is the case for an RTO with a wet electrostatic
precipitator (WESP) upstream.
Capital and operating costs
can be reduced by treating the discharge water from the
syngas scrubber 45 by piping it to the water treatment
system for the process gas prefilter. Such
systems
typically contain a rotating screen or centrifuge for
removing solids from the water.
Utilizing existing water
storage tanks and water treatment will save substantial
cost. If a
facility has a biomass gasifier the
conditioning methods presented within can be utilized to
make transportation of the fuel gas and utilization for the
oxidizer practical.
16
CA 02910289 2015-10-26
The cleaned syngas can be introduced into the inlet 51
of the RTO 20, or directly into the RTO combustion chamber.
Such oxidizers typically require high oxidation temperatures
in order to achieve high VOC destruction. To
achieve high
heat recovery efficiency, the "dirty" process gas which is to
be treated is preheated before oxidation. A heat exchanger
column 52A is typically provided to preheat these gases. The
column is usually packed with a heat exchange material having
good thermal and mechanical stability and sufficient thermal
mass. In
operation, the process gas is fed through a
previously heated heat exchanger column, which, in turn,
heats the process gas to a temperature approaching or
attaining its VOC oxidation temperature. This
pre-heated
process gas is then directed into a combustion zone 53 where
any incomplete VOC oxidation is usually completed. The
treated now "clean" gas is then directed out of the
combustion zone 53 and back through the heat exchanger
column, or through a second heat exchange column 52B. As the
hot oxidized gas continues through this column, the gas
transfers its heat to the heat exchange media in that column,
cooling the gas and pre-heating the heat exchange media so
that another batch of process gas may be preheated prior to
the oxidation treatment.
Usually, a regenerative thermal
oxidizer has at least two heat exchanger columns 52A, 52B
17
CA 02910289 2015-10-26
which alternately receive process and treated gases. This
process is continuously carried out via the switch of
suitable valving, allowing a large volume of process gas to
be efficiently treated. Cleaned gas exiting the outlet 54 of
the second column 528 is exhausted via stack 30.
Example 1
A process gas flow of 100,000 wet standard cubic feet
per minute (wscfm) with an inlet temperature of 160 F
containing 100 pounds per hour of VOC is being treated by a
nominal 95% thermally efficient RTO. The
higher heating
value (HHV) of the VOC is 14,000 btu/lb. This
system will
require approximately 9,500,000 btu per hour of additional
energy. The gasses extracted from the furnace between the
two steps of combustion will typically have a heating value
of 80 to 100 btu per standard cubic foot.
Therefore, to
fully supplement the fossil fuel to the RTO, a gas volume
of 1500 to 2000 standard cubic feet must be extracted,
purified and transported to the RTO inlet. The gas in this
part of the furnace is typically 400 to 800 F and contains
fly ash (particulate) nitrogen, carbon monoxide (CO)
hydrogen (H2) water vapor, methane (CH4), water vapor (H20)
and various high molecular weight organics such as turpene.
This poses a problem to transfer in traditional piping due
18
CA 02910289 2015-10-26
to the tendency for particulate and heavy organics to plate
out in the pipe work and valves and is not a clean fuel for
the RTO, which leads to equipment fouling. The
volume of
gas is relatively small (2000 scfm fuel gas compared with
100,000 scfm process gas being treated by the oxidizer);
therefore a high pressure drop scrubber, which is
relatively small in size, is positioned close or adjacent
to the furnace connection. The
syngas immediately passes
through the scrubber to remove harmful particulate, heavy
condensable and other elements not desirable in a fuel gas.
A dedicated blower 46 of approximately 30 to 50 inches
water column pressure is used to draw the syngas from the
furnace and through the scrubber, as well as to provide the
pressure to transport the fuel gas to the RTO. The rate of
syngas withdrawal from the furnace is preferably measured
or monitored as part of the safety system for the fuel
train and the pressure drop of all or part of the scrubber
can be used for this. Placing the blower 46 downstream of
the scrubber 45 saves on operating costs as brake
horsepower is reduced by handling the cooler scrubber
discharge then the hotter furnace gas. If
additional
pressure is required, two blowers can be used in series.
Flow control can be achieved by modulating the fan blower
speed or with modulating dampers.
19
CA 02910289 2015-10-26
A further requirement of the syngas system is to
design the system to either ensure that the gas remains
outside of the flammable range (by eliminating the presence
of an oxidant) or by designing the system to safely handle
and transport a potentially flammable gas. The reason for
this requirement is that although the syngas being
extracted from the furnace is expected to be low in oxygen,
it is difficult to ensure that this is the case. In the
standard furnace operation this is not an issue because the
combustion is quickly completed in the secondary combustion
zone of the furnace.
However, because the combustion
reactions can be quenched in the scrubber while the gas
remains in the flammable range, this is not necessarily
true in the syn-gas extraction system. While
the gases
remain in the presence of water in the scrubber the system
is protected by the extinguishing effect of the water.
However, once the water is removed, the potential of the
gas to be ignited in the presence of a source of ignition
must be considered.
There are four methods that can be used to safely
design the system for this hazard.
CA 02910289 2015-10-26
The first and preferred method of minimizing the
potential for a deflagration or detonation is by
maintaining liquid water in the system at all points.
Liquid water will prevent or immediately extinguish any
spark provided that it is present in the immediate vicinity
of the ignition source. This method is preferred because
of the relatively low cost of maintaining liquid in contact
with the gas and because the small volume of liquid would
not be an operational concern to the remainder of the
system. In
order to ensure that water is present at all
times, flow sensors can be added on any water addition
device whose failure would allow part of the system to lose
water suppression. Additionally this method would not be
viable with any of the embodiments using a dry method of
particulate control (e.g. high temperature baghouse or dry
electrostatic precipitator).
The second method is to design the system to contain,
suppress, or vent any potential fire or explosion. These
are the three safety methods prescribed in the
recommendations of the NFPA, if the conditions to prevent
the event from occurring cannot be met.
21
CA 02910289 2015-10-26
This method is less desirable because of the
difficulty and expense involved in certifying that the
system is safely designed. In
addition, such a system
would require deflagration or detonation arrestors which
are prone to plugging.
The third method is to continuously analyze the gas
for oxygen to ensure that the level is lower than that
required to start a deflagration. If
this path is
followed the reliability and speed of the analysis must be
considered.
While engineering the system to provide a reliable
oxygen reading is readily achievable, it will likely
require gas conditioning in any sampling method which
increases the response time of the analyzer.
Alternatively, a laser type oxygen measurement could be
used to obtain a direct measurement, but at a substantial
addition cost.
Standard zirconia type sensors are not
viable due to the high temperature of the sensor being a
potential ignition source which cannot only potentially
ignite the explosion that is desired to be prevented, it
will also likely provide a falsely low 02 reading due to the
22
CA 02910289 2015-10-26
consumption of 02 by the fuel present at the high
temperatures of the sensor.
Response time of the 02 sensor is critical in that all
components downstream of the 02 sensor that the gas could
reach before a high oxygen level, in the range of 5-7% is
detected and diverted, must be protected by containment,
suppression or venting systems.
Because of the high particulate content of the furnace
gas, oxygen sensing downstream of the gas cleaning device
(i.e. venturi scrubber, Dry ESP, Wet ESP, etc.)is
preferred.
If the method is chosen, an inert gas would be
required for purging the system of oxygen prior to allowing
syngas into the system. The inert gas could also be used
to control the 02 content as necessary.
This method is less desirable because in addition to
the cost of the oxygen sensing and diverting system it
would not allow the system to operate if substantial oxygen
is present and it may be desirable to continue the system
operation in this condition.
The fourth method is control the biomass furnace such
that the 02 level cannot become high enough for the gas to
23
CA 02910289 2015-10-26
be explosive. This method is less desirable because the
widely varying conditions in the furnace will make it very
difficult to consistently control the gas conditions at the
point where syngas is removed.
Additionally to safely add Syngas to the oxidizer as a
fuel source upstream of the RTO it must be shown that the
combined LEL of the syngas and the process gas can be
controlled so that under all operating conditions the Lower
Explosive Limit (LEL) of the combined streams will be under
25% as required by government regulations (e.g., NFPA 86).
There are three methods of accomplishing this. The
first and preferred method is to control syngas injection
so that the combined stream cannot exceed 25% LEL under any
operating condition. To accomplish this the following four
variables must be measured or calculated:
= The minimum flow of process gas (F) in SCFM
= The maximum LEL of the process gas (LEL)
= The maximum flow of syngas (Fs) in SCFM
= The maximum LEL of the syngas (LELJ
24
CA 02910289 2015-10-26
The maximum LEL of the combined stream is defined by
the equation:
LELms, = (FpLELp + FsLELs)/(Fp + Fs)
For those skilled in the art, the maximum LEL of each
stream can be calculated based on process data and
historical information on these processes. The
flow of
process gas must be measured and instrumented to provide a
reliable signal should the process gas flow fall below this
minimum flow.
Similarly, the flow of syngas must be
measured and instrumented to provide a reliable signal
should the syngas flow exceed this maximum flow. Either of
these signals can be used to shut down the syngas injection
system to prevent a process gas concentration exceeding 25%
LEL from entering the oxidizer. Flow
measuring devices
must be selected to work in saturated streams and
particulate laden environments (FIG. 4).
The second method is measure the Energy content of the
syngas with a BTU analyzer (FIG. 3) and use this analyzer
to adjust the maximum amount of syngas flow allowed. This
method is less desirable due to the particulate load and
CA 02910289 2015-10-26
saturated nature of the stream, which could likely require
conditioning the sample before the analyzer, causing a long
response time and the need for additional safety devices to
allow for multiple maximum syngas flow settings.
The third method is to monitor the LEL of the gas
stream, such as by sensing the LEL with an LEL monitor, to
ensure it stays below 25% in all cases.
This method is less desirable because it requires that
the system be able to vent the gases or otherwise prevent
them from entering the oxidizer if 25% LEL is reached.
This requires the response time of the sensor and the
control system to be considered to ensure that the process
gas can be prevented from entering the oxidizer, or other
ignition source, in time.
Furthermore, the particulate
load and saturated nature of the stream could likely
require conditioning the sample before the analyzer,
thereby increasing the response time.
In the embodiment where the furnace control is
manipulated to produce a higher CO and VOC concentrations
exiting the furnace, an LEL or CO monitor would likely be
required (FIG. 2) to ensure that concentration of gas
exiting the furnace does not exceed 25% LEL. These
26
CA 02910289 2015-10-26
analyzers would have similar sample conditioning issues to
those mentioned above.
Having now describing the invention in detail, those
skilled in the art will recognize modifications and
substitutions to the embodiments identified in the
specification which can be used to meet specific
operational requirements. Such
modifications and
substitutions are intended to be within the scope of the
invention as defined by the following claims.
27