Note: Descriptions are shown in the official language in which they were submitted.
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
CONVERSION OF SYNTHESIS GAS INTO LIQUID HYDROCARBONS VIA
FISCHER TROPSCH SYNTHESIS
FIELD
[0001]
The present disclosure relates to improvements in the Fischer-Tropsch ("FT")
synthesis and/or process for converting synthesis gas into liquid hydrocarbons
suitable for use
as liquid fuel or as chemical feedstock.
BACKGROUND
[0002] FT-
synthesis is a collection of catalyzed chemical reactions that converts a
mixture of carbon monoxide (CO) and hydrogen (H2) into hydrocarbons and water.
A variety
of catalysts have been used, but the commercially viable catalysts are
comprised of the
transition metals: cobalt, iron, and ruthenium. In addition to the transition
metal, the catalysts
typically contain a number of promoters, including alkali metal oxides and
copper. The
products of FT-synthesis include alkanes (hydrocarbons with a C-C single
straight bond) and
alkenes (also called olefins (hydrocarbons having at least one unsaturated C=C
double bond)),
which are both suitable for diesel fuel. Other FT-synthesis products include
alcohol or other
oxygenated hydrocarbons at times, which may be beneficial in some
circumstances.
[0003] FT-
synthesis has been undergoing development since the 1910's. Examples
include the following: 1) BASF, German Patent DRP 293,787 (1913); 2) A.
Mittasch and C.
Schneider, US Patent 1,201,850 (1916); 3) Fischer and Tropsch, German Patent
484337
(1925); and 4) Kinetics of the Fischer-Tropsch Synthesis on Iron Catalysts
(1964), Anderson,
R.B. et al. Bulletin 614, Bureau of Mines, United States Department of the
Interior.
[0004]
Current market development and new application is directed toward FT-
synthesis using a Cobalt-based catalyst for high FT activity and maximizing
the production of
long chained alkanes.
Companies involved in Cobalt-based FT-synthesis include Shell
(SMDS Bintulu, Pearl GTL Qatar) and Sasol (Oryx Qatar), as well as Velocys, BP-
Davy, and
Axens. When syngas feed is rich in 1-12 and at preferred temperature, the
Cobalt-based FT-
synthesis reaction forms mainly alkanes and water according to the overall
reaction:
1
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
n CO + (2n+1) H2 4 CnH2n+2+ n H20
[0005] The product is mainly paraffinic oil (saturated hydrocarbons).
Consequently the
overall molar H2 to CO consumption ratio is about (2n+1)/n 2. The typical
Cobalt-based FT
reaction temperature is 220 - 230 degrees Celsius.
[0006] Natural gas ("NG") is the most desirable feedstock from the
standpoint of the 1)
chemical H2 demand, 2) the minimal cleaning steps required, and 3) the
economics for
stranded NG resources.
[0007] Liquid and solid based feedstocks have the drawback of shortage of
H2 in the
reaction scheme, as well as process steps required to remove a larger range of
impurities being
present at larger quantities (e.g. sulfur containing compounds, ammonia,
hydrogen cyanide,
metal carbonyls, metals as gas or trapped in compounds/ashes).
[0008] Cobalt-based catalysts' Water Gas Shift ("WGS") activity is too
low to
compensate, for shortage of H2.
[0009] Iron-based catalysts have both FT and WGS activity. The FT
reaction produces
H20 (steam) (same as for the Cobalt-based catalyst), and at shortage of H2,
the WGS reaction
responds as follows:
CO + H20 (steam) - CO2 + H2
[0010] The typical FT reaction temperature currently practiced is 220-240
C (low-
temperature-FT) or 330-360 C (high-temperature-FT).
[0011] With low H2 present and at preferred temperatures, the combined FT
and WGS
reactions form mainly alkenes and CO2 according to the overall reaction:
2n CO + n 112 4 CnH2n n CO2
[0012] The product is mainly olefinic oil (having at least one
unsaturated C=C double
bond). Consequently the overall molar H2 to CO consumption ratio is about n/2n
= 1/2 . By
practice we found that the overall consumption ratio is closer to 0.55 than
0.50
2
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0013] The following is an example of a current process configuration
used in the field:
[0014] A refinery, producing a heavy residual feedstock (vacuum residue,
visbroken
residue, or de-asphalter residue), being in need of 110,000 Sm3/hr pure H2 to
satisfy the Hydro-
cracker H2 demand, and having the application of partial oxidation ("PDX") of
the residual
feedstock, requires the subsequent raw synthesis gas cleanup step at a rate of
19,300 kmol/hr
followed by hydrogen extraction via a pressure swing absorber ("PSA"), with
the PSA-offgas
used as a fuel gas and/or as feed to a combined cycle unit producing
electricity and superheated
steam. The PDX synthesis gas has a molar H2 to CO ratio of 0.9, and the PSA-
offgas has a
molar H2 to CO ratio of 0.35.
[0015] The high value product is pure H2, while the high CO containing
PSA-offgas is
only getting the heating equivalent value of Natural Gas, which in many
regions now is a
fraction of that of liquid fuels.
[0016] The economics for conversion of the CO-containing gas to liquid
hydrocarbons
is very attractive, even with increased consumption of Natural Gas to
compensate for the
alternative use of the CO-containing gas.
[0017] Two currently practised schemes are now discussed below. Both
schemes apply
a Cobalt-based catalyst. This requires an increase of the PDX synthesis gas
molar H2 to CO
ratio from 0.9 to about 1.8.
[0018] In the first scheme the desired ratio is achieved by mixing the H2-
lean PDX
synthesis gas with enough H2-rich steam methane reformer ("SMR") synthesis gas
having a
molar H2 to CO ratio of about six (6). This requires the integration of two
(2) world scale
SMRs. In addition, the hydro-cracker H2 demand is to be satisfied via one
additional world
scale SMR + WGS + PSA combination. The FT hydrocarbon liquid production is
between
21,000 and 29,000 bbl/day, depending on the design efficiency of the process
(e.g. multiple
reactors in series and/or FT-offgas recycle), and in the less efficient case,
an even larger
amount of FT-offgas is routed to the fuelgas pool. Although straightforward,
the complexity
added to the refinery is challenging.
3
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0019] In the second scheme, the desired ratio is achieved by applying
WGS to one
portion of the PDX synthesis gas, removing the CO2 by acid gas absorption, and
mixing this
synthesis gas portion with the other portion of the PDX synthesis gas. The FT
hydrocarbon
liquid production is between 10,000 and 12,000 bbl/day, depending on the
design efficiency of
the process (e.g. multiple reactors in series and/or FT-offgas recycle).
[0020] Unavoidably, the hydro-cracker H2 demand needs to be satisfied,
requiring the
addition of one world scale SMR + WGS + PSA combination.
[0021] The FT reactions are exothermic, releasing some 15% of the
chemical energy
content of the synthesis gas, which typically is transferred via indirect heat
exchange to raise
saturated steam. The steam pressure controls the FT synthesis temperature. For
a Cobalt-based
FT catalyst, the synthesis is controlled below 230 degrees Celsius to prevent
excessive CH4
production. As a consequence, the co-production of saturated steam is at a
pressure of 1.7 to
2.0 MPa only, which is of low value to the refinery.
[0022] There is a need to reduce complexity in existing FT process
technologies.
[0023] There is also a need to generate steam at a more useful (higher)
pressure.
SUMMARY
[0024] In one aspect, there is provided a process for converting H2-lean
syngas into one
or more hydrocarbons, comprising:
supplying a H2-lean syngas, including H2 and CO in a molar ratio of less than
1.0, and
adscititious H20 to the reaction zone such that a reaction mixture becomes
disposed in
sufficient proximity to a catalyst material within the reaction zone, the
catalyst material having
both water gas shift activity and Fischer-Tropsch synthetic activity, such
that the conversion is
effected.
[0025] In some implementations, for example, the H20 of the adscititious
H20 is
adscititious relative to any H20 that is produced during the conversion of the
H2-lean syngas
4
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0026] In some implementations, for example, the effected conversion
includes at least
Fischer-Tropsch synthesis.
[0027] In some implementations, for example, the 1120 of the adscititious
1120 is
adscititious relative to any H20 that is produced during the Fischer-Tropsch
synthesis.
[0028] In some implementations, for example, the reaction mixture is
generated by
admixing of the H2-lean syngas and the adscititious 1120.
[0029] In some implementations, for example, the supplying of the
adscititious 1120 to
the reaction zone is effected independently of the supplying of the H2-lean
syngas to the
reaction zone.
[0030] In some implementations, for example, the H20 of the adscititious
H20 is in the
form of steam.
[0031] In some implementations, for example, the molar H2/C0 ratio is
between 0.25
and 1.0, such as, for example, between 0.25 and 0.6, and such as, for example,
between 0.25
and 0.5.
[0032] In some implementations, for example, the ratio of moles of H2 to
moles of CO
within the supplied H2-lean syngas is less than 0.55, the ratio of moles of
H2O of the supplied
adscititious 1420 to moles of CO of the supplied H2-lean syngas is defined in
accordance with
the following formula:
H20/C0feed = A X (0.55 - 112/CO 1
feed)
or, equivalently:
the ratio of moles of H20 of the supplied adscititious H20 to moles of CO of
the supplied H2-
lean syngas ¨
A x (0.55 ¨ (the ratio of moles of H2 of the H2-lean syngas to moles of CO of
the H2-lean
syngas);
wherein A is between 1.0 and 1.3
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0033] In some implementations, for example, prior to the supplying of
the H2-lean
syngas to the reaction zone, any treatment of the H2-lean syngas feedstock is
such that no H2
enrichment of the H2-lean syngas is effected prior to the reaction zone.
[0034] In some implementations, for example, the catalyst material
includes a Fe-based
catalyst material.
[0035] In some implementations, for example, the catalyst material is
activated.
[0036] In some implementations, for example, the catalyst material
includes at least
one promoter.
[0037] In some implementations, for example, the at least one promoter
includes one or
more metal oxides, and wherein the metal oxide is an oxide of any one of
manganese,
potassium, chromium, and copper.
[0038] In some implementations, for example, the reaction zone is
disposed at a
pressure of between 0.2 to 7 MPa, such as, for example, between 1.5 to 6 MPa,
and such as, for
example, between 1.5 to 3 MPa.
[0039] In some implementations, for example, the reaction zone is
disposed at a
temperature of between 240 degrees Celsius and 320 degrees Celsius, such as,
for example,
between 260 degrees Celsius and 300 degrees Celsius, and such as, for example,
between 270
degrees Celsius and 290 degrees Celsius.
[0040] In some implementations, for example, at least one of the one or
more
hydrocarbons that are produced by the conversion is liquid at standard
temperature and
pressure conditions.
[0041] In some implementations, for example, the H2-lean syngas is
derived from any
one of bitumen, heavy oil, shale oil, heavy hydrocarbon residues from a heavy
oil or bitumen
upgrading process, natural gas, coal, biomass, organic waste.
[0042] In some implementations, for example, the heavy hydrocarbon
residue includes
a residual product from any one of atmospheric distillation, vacuum
distillation, deasphalting,
6
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
coking, visbreaking, thermal cracking, fluid catalytic cracking, resid fluid
catalytic cracking, or
any combination thereof.
[0043] In another aspect, there is provided a process for upgrading a
hydrocarbon feed
comprising:
converting the hydrocarbon feed to a first syngas;
separating the first syngas into at least a H2-rich stream and a H2-lean
syngas, wherein the H2-
lean syngas includes H2 and CO in a molar ratio of less than 1.0; and
supplying the H2-lean syngas and adscititious H20 to the reaction zone such
that a reaction
mixture becomes disposed in sufficient proximity to a catalyst material within
the reaction
zone, the catalyst material having both water gas shift activity and Fischer-
Tropsch synthetic
activity, such that conversion of the H2-lean syngas to one or more
hydrocarbons is effected.
[0044] Relative to the H2-lean syngas, the H2-rich stream includes a
higher
concentration of 142
[0045] In some implementations, for example, the 1120 of the adscititious
1120 is
adscititious relative to any H20 that is produced during the conversion of the
H2-lean syngas.
[0046] In some implementations, for example, the effected conversion
includes at least
Fischer-Tropsch synthesis.
[0047] In some implementations, for example, the H20 of the adscititious
1120 is
adscititious relative to any H20 that is produced during the Fischer-Tropsch
synthesis.
[0048] In some implementations, for example, the reaction mixture is
generated by
admixing of the 112-lean syngas and the adscititious H20.
[0049] In some implementations, for example, the supplying of the
adscititious H20 to
the reaction zone is effected independently of the supplying of the H2-lean
syngas to the
reaction zone.
7
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0050] In some implementations, for example, the H20 of the adscititious
H20 is in the
form of steam.
[0051] In some implementations, for example, the ratio of moles of H2 to
moles of CO
within the H2-lean syngas is between 0.25 and 1.0, such as, for example,
between 0.25 and 0.6,
and such as, for example, between 0.25 and 0.5.
[0052] In some implementations, for example, the ratio of moles of H2 to
moles of CO
within the supplied H2-lean syngas is less than 0.55, the ratio of moles of
H20 of the supplied
adscititious H20 to moles of CO of the supplied Hz-lean syngas is defined in
accordance with
the following formula:
H20/COreed = A x (0.55 - H2/C0feed)
or, equivalently:
the ratio of moles of H20 of the supplied adscititious H20 to moles of CO of
the supplied H2-
lean syngas ¨
A x (0.55 ¨ (the ratio of moles of 112 of the H2-lean syngas to moles of CO of
the H2-lean
syngas);
wherein A is between 1.0 and 1.3
[0053] In some implementations, for example, prior to the supplying of
the H2-lean
syngas to the reaction zone, any treatment of the H2-lean syngas feedstock is
such that no 112
enrichment of the H2-lean syngas is effected prior to the reaction zone.
[0054] In some implementations, for example, the catalyst material
includes a Fe-based
catalyst material.
[0055] In some implementations, for example, the catalyst material is
activated.
[0056] In some implementations, for example, the catalyst material
includes at least
one promoter.
8
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0057] In some implementations, for example, the at least one promoter
includes one or
more metal oxides, and wherein the metal oxide is an oxide of any one of
manganese,
potassium, chromium, and copper.
[0058] In some implementations, for example, the reaction zone is
disposed at a
pressure of between 0.2 to 7 MPa, such as, for example, between 1.5 to 6 MPa,
and such as, for
example, between 1.5 to 3 MPa.
[0059] In some implementations, for example, the reaction zone is
disposed at a
temperature of between 240 degrees Celsius and 320 degrees Celsius, such as,
for example,
between 260 degrees Celsius and 300 degrees Celsius, and such as, for example,
between 270
degrees Celsius and 290 degrees Celsius.
[0060] In some implementations, for example, at least one of the one or
more
hydrocarbons that are produced by the conversion is liquid at standard
temperature and
pressure conditions.
[0061] In some implementations, for example, the heavy hydrocarbon
residue includes
a residual product from any one of atmospheric distillation, vacuum
distillation, deasphalting,
coking, visbreaking, thermal cracking, fluid catalytic cracking, resid fluid
catalytic cracking, or
any combination thereof.
[0062] In some implementations, for example, the converting of
hydrocarbon feed to a
first syngas is effected by any one of gasification, partial oxidation, auto-
thermal reforming,
steam reforming, or any combination thereof
[0063] In some implementations, for example, the process further
comprises effecting
hydrocracking with the H2-rich stream separated from the first syngas.
[0064] In another aspect, there is provided a process for upgrading a
hydrocarbon
residue and producing bitumen (an oil based semi solid substance) via steam-
assisted gravity
drainage ("SAGD") using steam that is generated by at least the heat produced
by the
upgrading, comprising:
converting the heavy hydrocarbon residue to a syngas product;
9
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
converting the syngas product via at least Fischer-Tropsch synthesis, wherein
the conversion
is effected within a reaction zone disposed at a temperature of greater than
260 degrees Celsius;
transferring heat, from the converting, to a steam generator;
with the transferred heat, effecting generation of steam by the steam
generator; and
supplying steam to a hydrocarbon reservoir via a SAGD injection well to effect
mobilization of
bitumen within the hydrocarbon reservoir.
[0065] In some implementations, for example, the transferred heat is
transferred from
the reaction zone.
[0066] In some implementations, for example, the transferred heat is
transferred from
Fischer-Tropsch products generated by the converting.
[0067] In some implementations, for example, the temperature within the
reaction zone
is between 260 degrees Celsius and 300 degrees Celsius, such as, for example,
between 270
degrees Celsius and 290 degrees Celsius.
[0068] In some implementations, for example, the reaction zone is
disposed at a
pressure of between 0.2MPa to 7 MPa, such as, for example, between 1.5 MPa to
6 MPa, and
such as, for example, 1.5 MPa to 3 MPa.
[0069] In some implementations, for example, the conversion is effected
by a catalyst
material.
[0070] In some implementations, for example, the catalyst material is
activated.
[0071] In some implementations, for example, the catalyst material
includes a Fe-based
catalyst material.
[0072] In some implementations, for example, the catalyst material
includes at least
one promoter.
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0073] In some implementations, for example, the at least one promoter
includes one or
more metal oxides, and wherein the metal oxide is an oxide of any one of
manganese,
potassium, chromium, and copper.
[0074] In some implementations, for example, at least one of the one or
more
hydrocarbons that are produced by the conversion by at least Fischer-Tropsch
synthesis are
liquid at standard temperature and pressure conditions.
[0075] In some implementations, for example, the converting of the heavy
hydrocarbon
residue includes:
converting the heavy hydrocarbon to a first syngas; and
separating the first syngas into at least a H2-rich stream and a H2-lean
syngas, wherein the H2-
lean syngas defines the syngas product such that the conversion, by at least
Fischer-Tropsch
synthesis, is of the H2-lean syngas.
[0076] In some implementations, for example, the process further
comprises effecting
hydrocracking with the H2-rich stream separated from the first syngas.
[0077] In some implementations, for example, the H2-lean syngas includes
H2 and CO
in a molar ratio of less than 1.0, and prior to the conversion, by at least
Fischer-Tropsch
synthesis, the process further comprises: supplying the H2-lean syngas and
adscititious H20 to
the reaction zone such that a reaction mixture becomes disposed in sufficient
proximity to a
catalyst material within the reaction zone, the catalyst material having both
water gas shift
activity and Fischer-Tropsch synthetic activity, such that the conversion, by
at least Fischer-
Tropsch synthesis, is effected.
[0078] In some implementations, for example, the H20 of the adscititious
H2O is
adscititious relative to any H20 that is produced during the conversion of the
H2-lean syngas.
[0079] In some implementations, for example, the H20 of the adscititious
H20 is
adscititious relative to any H20 that is produced during the Fischer-Tropsch
synthesis.
11
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0080] In some implementations, for example, the reaction mixture is
generated by
admixing of the H2-lean syngas and the adscititious H20.
[0081] In some implementations, for example, the supplying of the
adscititious H20 to
the reaction zone is effected independently of the supplying of the H2-lean
syngas to the
reaction zone.
[0082] In some implementations, for example, the H2O of the adscititious
H20 is in the
form of steam.
[0083] In some implementations, for example, the ratio of moles of H2 to
moles of CO
within the supplied H2-lean syngas is between 0.25 and 1.0, such as, for
example, between 0.25
and 0.6, and such as, for example, between 0.25 and 0.5.
[0084] In some implementations, for example, when the ratio of moles of
H2 to moles
of CO within the supplied H2-lean syngas is less than 0.55, the ratio of moles
of H2O of the
supplied adscititious H20 to moles of CO of the supplied H2-lean syngas is
defined in
accordance with the following formula:
H20/C0feed = A x (0.55 - H2/C0feed)
or, equivalently:
the ratio of moles of H20 of the supplied adscititious H2O to moles of CO of
the supplied H2-
lean syngas ¨
A x (0.55 ¨ (the ratio of moles of H2 of the H2-lean syngas to moles of CO of
the H2-lean
syngas);
wherein A is between 1 and 1.3.
[0085] In some implementations, for example, prior to the supplying of
the H2-lean
syngas to the reaction zone, any treatment of the H2-lean syngas feedstock is
such that no H2
enrichment of the H2-lean syngas is effected prior to the reaction zone.
[0086] In some implementations, for example, the heavy hydrocarbon
residue includes
a residual product from any one of atmospheric distillation, vacuum
distillation, deasphalting,
12
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
coking, visbreaking, thermal cracking, fluid catalytic cracking, resid fluid
catalytic cracking, or
any combination thereof
BRIEF DESCRIPTION OF THE DRAWINGS
[0087] In the drawings, embodiments of the invention are illustrated by
way of
example. It is to be expressly understood that the description and drawings
are only for the
purpose of illustration and as an aid to understanding, and are not intended
as a definition of
the limits of the invention.
[0088] Embodiments will now be described, by way of example only, with
reference to
the attached figures, wherein:
[0089] Figure 1 is a flow diagram illustrating the processing of a
synthesis gas via a
Fischer-Tropsch synthesis;
[0090] Figure 2 is a flow diagram illustrating the processing of a
synthesis gas,
produced by a heavy oil or bitumen upgrading process, via a Fischer-Tropsch
synthesis; and
[0091] Figure 3 is a flow diagram illustrating the processing of a
synthesis gas via a
Fischer-Tropsch synthesis, and using heat produced within the process for
generating steam for
use in a steam-assisted gravity drainage process.
DETAILED DESCRIPTION
[0092] Referring now to Figure 1, there is provided a configuration used
in processing
synthesis gas, according to one embodiment.
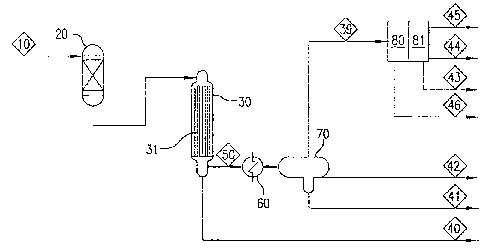
[0093] Synthesis gas (or "syngas") 10 is fed into a guard bed 20, to
remove any catalyst
poisons (such as H2S, COS, NH3, HCN) from the synthesis gas 10, prior to
introduction into a
Fisher-Tropsch ("FT") reactor 30 which includes an Iron-based catalyst 31. The
FT process is
effected within the FT reactor 30. The effluent from FT reactor 30 consists of
two streams, a
first stream being a wax product stream 40, and a second stream 50 that is
cooled via a cooler
60 and further separated via a separator 70, into three streams, an aqueous
product stream 41,
an oil product stream 42 and a tail-gas stream 39. The tail-gas stream 39 is
recovered via
13
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
olefin oligomerization 80, resulting in an oil product stream 46 and a fuel
gas stream that
optionally is separated 81 into a hydrogen gas stream 44, which is sent to an
upgrader, an LPG
product stream 43 for sales, and a residual fuel gas stream 45.
[0094] Table 1 below provides the data when implementing the FT process
using the
system configuration of Figure 1 when the feed 10 is derived from partial
oxidation ("PDX")
synthesis gas. Table 2 below provides data when implementing the FT process
using the
system configuration of Figure 1 when the feed 10 is derived from a H2-lean
syngas resulting
from separating a PDX synthesis gas into at least a H2-rich stream and a H2-
lean syngas, such
as, for example, by way of a separation effected by pressure swing absorption.
Relative to the
H2-lean syngas, the H2-rich stream includes a higher concentration of H2
14
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
TABLE 1 PDX synthesis gas (syngas) processed according Figure 1.
Mass balance Feed Mass balance FT-2 Tailgas
!Olefin Oligo-
calculations ,basismerization
Mol
Coumpounds weight [1] Syngas feed [30] FT feed
[39] Tailgas from FT [43]+[44]+[45] [46] Oil from Oligo
kg/lonol kmol/h kg/h kmol/h kg/h kmol/h kg/h ikmol/h kg/h
Ikmol/h kg/h
Water gas shift
H2 2.02 8630 17396 8630 17396 2714 5470 2714 5470
CO 128.01 19967 279180 19967 279180 1864 52206 11864 52206
CO2 144.01 1585 25740 1586 25784 4204 185014 14204 185014
H2O 118.02 116 281 16 282 64 1155 164 1155
Inert gases ;0 0
;
N2 28.01 42 1176 42 1176 42 1176 1176
142
Ar 39.95 44 1753 44 1753 44 1753 144 1753
Contaminants 0 0
H2S 34.08 1 10 1 0 0
1 1
COS 60.08 1 58 0 0
HCN 27.03 0 1 0 0
NH3 17.03 0 0 0 0
Hydrocarbons 1 0 0
CI-14, methane 16.04 62 987 62 987 1236 3790 236
3790
C2H4, ethylene 28.05 12 326 8 228
C2H6, ethane 30.07 0 0 0 0 33 978 33 978 1
C3H6, propylene 42.08 89 3763 9 376
C3H8, propane ,44.10 i0 0 0 0 ,128 5644 128 5644
C4H8, butenes 56.11 1
' 75 4182 7 418
C4H10, butanes 158.12 0 0 0 0 59 3422 59 3422
C5H10, pentenes 70.13 33 2318 3 232
C51-112, pentanes 172.15 10 0 0 0 47 3362 47 3362
C6H12, hexenes 184.16 0 0 0 0 15
432
C6H14, hexanes ,86.18 0 0 0 0 0
0
C7H14, heptenes 98.19 0 0 0 0 41
4058
C7H16, heptanes 100.20 10 0 0 0 0
0
1
C8H16, octenes 112.21 0 0 0 0 21
2358
C8HI8, octanes 1114.23 10 1 0
0 10 0
C9-C10 olefins 1 0 2019
11
C9-C10 paraffins
1 0 0
CI 1-C14 olefins 1 0 467
CI I -C14 paraffins 1 0 0
C15-C22 olefins 0
CI5-C22 paraffins 0 1
1
C22+ olefins :
, 0
1
C22+ paraffins 0
Oxygenates
C I -C4, alcohols ,
1 0
naphtha alcohols 1 0
distillate alcohols 0 1
1
wax alcohols 0
carboxylic acids
,
1 :
,
1
TOTAL: 1 119346 326574 19346 326559 9642 274560 9462 265225 67 9335
kg/h
[40] Wax product 15993
[41] Aqueous prod. 16716
[42] Oil product 19289
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
TABLE 2 PSA-offgas (syngas) processed according Figure 1.
Mass balance
calculations
Coumpounds Mol weight [1] Syngas [30] FT-1 feed [39] Tailgas
FT [43]+[44]+[45] [46] Oil from Oligo-
feed Tailgas from mserization
Oligomerization
kg/lcmol kmol/ kg/h kmol kg/h kmol/ kg/h kmol/h kg/h kmol/h
kg/h
WGS compounds
H2 2.02 3452 695 3452 6959 1001 2017 1001 2017 .
CO 28.01 9967 279 9967 27918 1515 42423 1515 42423 .
CO2 44.01 585 257 586 25784 5550 244250 5550 244250
H20 18.02 2016 363 2016 36312 58 1051 58 1051
'
Inert gases 0 0
N2 28.01 42 117 42 1176 42 1176 42 1176
Ar 39.95 44 175 44 1753 44 1753 , 44 1753
,
Contaminants 0 0
H2S 34.08 0 1. 0 0
COS 60.08 1 58 . , 0 0
HCN 27.03 0 1. , 0 0
NH3 17.03 0 0, 0 0
Hydrocarbons 0 0
(aliphatic)
CH4, methane 16.04 62 987 62 987 197 3168 197 3168
,
C2H4, ethylene 28.05 9 254 6 177
C2H6, ethane 30.07 0 0 0 - 0 25 761 25 761
C3H6, propylene 42.08 70 2927 7 293
C3H8, propane 44.10 0 0 0 0 100 4390 100 4390
C4H8, butenes 56.11 58 3253 6 325 ,
C4 HIO, butanes 58.12 , 0 0 , 0 0 46 2661 46 2661
C5H10, pentenes 70.13 26 1803 3 180
C5H12, pentanes 72.15 0 0 0 0 36 2615 36 2615
C6H12, hexenes 84.16 0 0 0 0 4 336
,
C6H14, hexanes 86.18 0 0 0 0 0 0
C71-l14, heptenes 98.19 0 0 0 0 32
3156
,
C7H16, heptanes 100.20 0 0 0 0 0 0
C8H I 6, octenes 112.21 0 0 10 0
16 1834
C8H18, octanes 114.23 0 , 0 0 0 0 0
C9-C10 olefins 0 , 0 0 1571
C9-C10 paraffins 0 0 0 0
CII-C14 olefins 0 0 0 363
Cl 1-C14 paraffins 0 0 0 0
C15-C22 olefins 0 - 0 0
C15-C22 paraffins 0 0 0
.
C22+ olefins 0 , 0 0
C22+ paraffins 0 0 0
Oxygenates 0 0
C1-C4, alcohols 0 0 0
naphtha alcohols 0 - 0 0
distillate alcohols 0 0 0
wax alcohols' 0 0 0
carboxylic acids _ 0 0 .
0 0
TOTAL: 1616 352 1616 35215 8776 314500 8635 307240 52 7261
8 166 8 1
kg/h
..
[40] Wax product 1243
-
[41] Aqueous pr 1020
[42] Oil product 1500
16
CA 02911660 2015-11-06
WO 2015/070332
PCT/CA2014/000823
Table 3 below summarizes the two cases.
TABLE 3
PSA-offgas
Case PDX syngas
+ FT Steam
Feed Total syngas & steam 16168 19346 kmol/hr
CO 9967 9967 kmol/hr
H2/C0 0.346 0.866 kmol/kmol
H20/C0 0.202 0.002 kmol/kmol
Total syngas & steam 8452 7838 ton/day
Initial FT COreaction
1.0 1.53 normalized
Rate
Initial FT space velocity 1.0 1.20 normalized
Initial FT COconv rate 1.0 1.28 normalized
Usage,
Consumption
COfeed/C0cons 0.848 0.813 kmol/kmol
H2cons/C0cons 0.290 0.730 kmol/kmol
H2Ocons by reactions 601 -422 ton/day
Output
FT Hydrocarbon Liquid
FT Wax 299 384 ton/day
360 463 ton/day
Olefin 174 224 ton/day
Liquid Hydrocarbon + +
Total Raw 833 1071 ton/day
After Hydro-cracking 6979 8973 bbl/day
Contaminated Water 245 401 ton/day
Offgas post-
Total flow 86359462 kmol/hr
H2/C0 0.661 '
1.456 kmol/kmol
CO2 0.632 0.436 kmol/kmol
Total flow , 7374 6365 ton/day
1-1V 4.40 7.72 MJ/kg
HV 32.48 49.14 TJ/day
17
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[0095] Referring now to Figure 2, there is depicted a process for FT
synthesis from a
synthesis gas in a typical heavy oil or bitumen-processing scenario, according
to another
embodiment.
Step 1
[0096] A hydrocarbon stream 100 is generated from
(a) a residual (reject) product from i) atmospheric distillation, ii)
vacuum distillation, iii) de-
asphalting, iv) visbreaking, v) coking, or
(b) from concentrated biomass, such as via i) torrefaction or ii) liquefaction
(hydrothermal,
pyrolysis).
Step 2
[0097] A raw synthesis gas 120 is generated by partial oxidation
(gasification) 110 of
the hydrocarbon stream 100 obtained in step 1.
[0098] The molar 1-12 to CO ratio of said synthesis gas 120 is optionally
tuned by proper
selection of
(a) the gasification temperature and/or
(b) moderator addition (e.g. water, steam or CO2), and/or
(c) co-firing of a lighter hydrocarbon such as i) natural gas, ii) refinery
gas, iii) FT-offgas,
(d) off-spec or surplus of liquids such as FT-liquids, methanol etc.
18
CA 02911660 2016-03-14
Step 3
[0099] The raw synthesis gas 120 is cleaned and modified in any possible
sequence and
way, such as:
(a) washing or scrubbing with water (not shown)
i) to remove solids, and/or
ii) to remove gaseous component (NH3, RCN, COS, H2S), optionally in
chilled water, and/or
iii) to remove water vapor, optionally with chilled water
(b) catalyst enhanced conversion COS and HCN hydrolysis 130, such as over an
alumina bed,
a ZnO bed or other oxide containing catalyst, prior to acid gas removal
(c) acid gas removal 140 (H2S, CO2, COS, NH3, HCN, iron carbonyl, nickel
carbonyl) using
i) amine liquid (such as MBA, DEA, MDEA or DIPA process);
ii) alcoholic liquid (such as methanol with Rectisoirmprocess); or
iii) glycol liquid (such as dimethyl ethers of polyethylene glycol with
Selexorprocess)
(d) absorption on guard-bed 150 (active carbon, zinc-oxide, zinc-copper) or
sacrificial-bed
160 (catalyst, spent Iron-based FT catalyst)
(e) modified by adding CO2 and/or steam at suitable temperature over
suitable catalyst to
modify the H2 to CO ratio of the synthesis gas (not shown).
(f) modified by extracting 142 170, such as via
i) a PSA process, and/or
ii) via a membrane, having the advantage that the CO rich gas remains
at pressure (so no FT feedgas compressor required),
and with minimum depressurization of the H2 pure gas (not shown).
Step 4
19
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[00100]
The cleaned and modified synthesis gas, of any one of the processes in Step 3,
is
compressed or letdown to a suitable pressure and heated or cooled 180 as
required for FT-
synthesis.
(a) Advantageously with Step 3f, the compression of PSA tailgas results in
a temperature
increase, reducing the need for external heating.
(b) Advantageously with Step 3d, the removal of catalyst poison over a
sacrificial catalyst,
such as an Iron-based FT catalyst, results in a desirable temperature
increase.
Step 5
[00101]
Optionally, if desired, the clean and conditioned synthesis gas of Step 4 may
be
treated for H2 to CO ratio by any of the following:
(a)
addition of H2 190, such as from excess of H2 available (not needed by hydro-
cracker
200), and/or
(b) addition of a Hz-rich gas such as from
i) a steam methane reformer ("SMR") with or without a downstream WGS
Converter,
being dry (majority of the water condensed) or being wet (not shown);
ii) an auto thermal reformer (not shown);
iii) a partial oxidation process (115) using a light feedstock
(such as natural gas, refinery gas, methanol, naphtha), requiring
relatively few and easy cleaning steps;
(c)
addition of steam (adscititious H20) 210, such as by admixing with the
synthesis gas of
Step 4, intended for increasing the H2 content of the synthesis gas (where the
synthesis is a H2-
lean synthesis gas, wherein the Hz-lean synthetis gas includes H2 and CO in a
molar ratio of
less 1.0, such as between 0.25 and 1.0, such as between 0.25 and 0.6, such as
between 0.25 and
0.5) via the in-situ watergas shift ("WGS") occurring at the FT-catalyst;
and/or
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
(d) no additional modification of the synthesis gas.
Step 6
[00102] The synthesis gas of Step 4 or 5 is processed in a FT reactor 220
over a FT-
synthesis active catalyst that is also a WGS active catalyst.
In this embodiment, the FT reactor 220 is selected from:
(a) one single reactor, or multiple reactors in parallel having the inlets
and outlets connected
to a common inlet- and outlet-header respectively; recycling of part of the
product gas stream
from the reactor(s) to the feed synthesis gas stream to the reactor(s) is
optional;
if recycle of product gas is applied, then, optionally, the recycle stream is
treated, and there is
further included, optionally:
i) removing some FT liquid product 230 and/or condensable water 240 by
subsequent cooling, discharging and reheating, and/or
ii) removing FT gases 250 that reduce the conversion rate and/or reaction
selectivity, such as H20 and/or CO2; or
(b) two or more reactors in series, with optional intermediate removal of some
FT liquid
product and/or condensable water by subsequent cooling, discharging and
reheating, and/or
with optional removing FT gases that reduce the conversion rate and/or
reaction selectivity,
such as 1420 and/or CO2.
Each reactor may be constructed from multiple reactors in parallel having the
inlets and outlets
connected to common inlet- and outlet-headers respectively.
[00103] FT-synthesis is an exothermal process, with FT hydrocarbon product
distribution being very sensitive to the process temperature. The higher the
temperature the
more undesirable gases are produced (e.g. CH4) at higher temperatures. The FT
reaction
temperature may be controlled by indirect exchange of the heat of reaction
with water at
boiling conditions, whereby the steam or water pressure is the regulating
factor. For the FT
21
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
catalyst in a fixed bed configuration, the heat can be exchanged via a shell
and tube heat
exchanger with the catalyst at the tube side and the boiling water at the
shell side. The reverse
configuration can also be practiced. For the FT catalyst in a slurry the three
phase (catalyst,
hydrocarbon, gas) bubble column reactor is cooled via a submerged heat
exchanger, such as
cooling coils having boiling water as a coolant on coil side. In some
embodiments, for
example, the temperature within the reaction zone of the FT reactor is between
240 degrees
Celsius and 320 degrees Celsius (such as, for example, between 260 degrees
Celsius and 300
degrees Celsius, such as, for example, between 270 degrees Celsius and 290
degrees Celsius).
By providing such reaction zone temperature, boiling water may be produced at
a pressure,
such as 4.2 MPa which is suitable for application to steam-assisted gravity
drainage ("SAGD"),
without significantly increasing light gas production.
[00104] In some embodiments, for example, the reactor zone of the FT
reactor is
disposed at a pressure of between 0.2 and 7 MPa, such as between 1.5 and 6MPa,
such as
between 1.5 and 3MPa.
[00105] It has been found that the FT synthesis activation energy
increases when
lowering the H2 to CO ratio of the synthesis gas. Thus, for given temperature,
the FT
conversion rate reduces when lowering the H2 to CO ratio of the synthesis gas.
For the
application of synthesis gas with low H2 to CO ratio the increase of the FT-
reaction
temperature from the typical 220-240 degrees Celsius to 270-290 degrees
Celsius range or
above is advantageous, as it increases the conversion rate.
[00106] In comparison to the Cobalt-based catalysts, the Iron-based
catalyst
advantageously supports the higher FT-reactor temperature without producing
relatively
undesirable high quantities of CH4, versus the Cobalt-based catalyst. For the
higher FT-reactor
temperature application, one or more promoters, such as one or more oxides of
the elements
manganese and/or potassium and/or chromium and/or copper are contained in the
Iron-based
catalyst formulation to improve stability and performance.
[00107] The Iron-based catalyst supports the WGS reaction, while the
Cobalt based
catalyst does not.
22
CA 02911660 2015-11-06
WO 2015/070332 PCT/CA2014/000823
[00108] For the Iron-based catalyst, and for a synthesis gas having, at
the FT reactor
inlet, a molar H2 to CO ratio below the overall consumption ratio of 0.55, the
total CO
consumption rate per reactor pass is high, as the CO consumption occurs from
both the FT-
synthesis and the WGS reaction. Effective utilization of the CO and H2 can be
achieved in one
reactor pass without the need for FT-offgas recycling.
[00109] A further benefit of the described process is, that by employing a
low H2 to CO
ratio synthesis gas, the water partial pressure in the FT reactor remains low,
which sustains
long catalytic service. Namely, oxidation of the Iron-based catalyst by water
is a much more
important deactivation mechanism than the deactivation mechanism by CO2.
[00110] Another benefit of the described process is that in employing a
more CO rich
synthesis gas, the H2 availability is lower which suppressing termination of
the hydrocarbon
chain growth (resulting in more heavy waxes) and suppressing methane
formation.
Step 7
[00111] Treatment or refining of the FT-products.
(a) Light olefin recovery 225.
With the Iron-based catalyst and at the higher FT-reactor temperature, some
more light alkenes
(olefins) are produced. In principle this reduces the yield of the liquid
hydrocarbons (C5-C22).
The light products can largely be recovered by the polymerization of the light
(C2-05) olefins
over an appropriate catalyst that is known in the art.
In some embodiments, for example, the conversion is over an acidic catalyst.
In some embodiments, for example, by processing the reagents over a solid
phosphoric acid
catalyst (for example, UOP CatPoly process), a high-octane olefinic motor
gasoline and some
distillate may be yielded.
23
CA 02911660 2016-03-14
In some embodiments, for example, processing the reagents over an acidic MFI-
type zeolite
catalyst (for example, PetroSA Conversion of Olefins to Distillate process),
yielding some
olefinie naphtha and distillate.
In all cases the products can be used as is, separated in appropriate
fractions, and/or further
refined (e.g. by hydro-processing).
(b) Suitable use of FT-offgas 250 as a fuelgas
The remainder of the FT-offgas is made suitable as a fuelgas for firing in
boilers 256 by:
i) condensing of the majority of the water vapor and removal of the water,
ii) condensing and removal of the hydrocarbon (e.g. paraffins, that
may solidify and block feed lines upon further cool down,
iii) optional scrubbing of the offgas to remove CO2 255 (acid gas removal
such as via
amine or SelexolTm), with the objective to increase the fuelgas heating value
and/or for CO2
production and/or purification (e.g. for carbon capture).
(c) Distillation 260 of FT liquid hydrocarbons
Naphtha is a desirable product, as it is in high demand as a diluent in the
bitumen mining. The
boiling range is between 30 and 200 degrees Celsius, with carbon number
between five (5) and
twelve (12). By distillation this fraction is removed, and the remainder is
used as a chemical
feedstock or treated in a hydro-cracker.
(d) Hydro-cracking or Thermal-cracking
The bottom product of the distiller is fed to the hydro-cracker 200, which is
to produce lighter
products by
i) breaking alkane (paraffinic) chains, and/or
24
CA 02911660 2015-11-06
WO 2015/070332
PCT/CA2014/000823
ii) to
saturate the C=C double bond of the alkenes (olefins) and/or to break-down
oxygenates.
Alternatively the bottom product can be co-processed with the de-asphalted oil
in a thermal
cracker.
(e) The hydrocarbon products, oxygenates and water-soluble oxygenates can
be processed in
various other ways as described in the art (e.g. De Klerk, A. Fischer¨Tropsch
refining; Wiley-
VCH: Weinheim, 2011; ISBN 978-3-527-32605-1).
[00112] In some embodiments, for example, heat, at between 260 degrees
Celsius and
300 degrees Celsius (such as, for example, between 270 degrees Celsius and 290
degrees
Celsius), is transferred from the process to effect steam generation at 4.2
MPa. In this respect,
and referring to Figure 3, there is provided a process upgrading a hydrocarbon
residue and
producing bitumen via steam-assisted gravity drainage ("SAGD") using steam
that is generated
by at least the heat produced by the upgrading. The process includes:
(a) converting the heavy hydrocarbon residue 500 (such as, for example,
resulting from
processing of bitumen or heavy oil) to a syngas product 502, such as, for
example, in a gasifier
504;
(b) converting the syngas product 502 to one or more hydrocarbons 508 via
at least Fischer-
Tropsch synthesis within a reactor 506, wherein the conversion is effected
within a reaction
zone disposed at a temperature of greater than 260 degrees Celsius;
(c) transferring heat, from the converting, to a steam generator 510;
(d) with the transferred heat, effecting generation of steam 512 by the
steam generator from
water 511; and
(e) supplying steam to a hydrocarbon reservoir via an injection well 514
(of a SAGD well
pair including the injection well 514 and the production well 516) to effect
mobilization of
bitumen within the hydrocarbon reservoir.
CA 02911660 2016-03-14
[00113]
The appended claims define distinctly and in explicit terms the subject matter
of the
invention for which an exclusive privilege or property is claimed.
26