Note: Descriptions are shown in the official language in which they were submitted.
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
COMPOSITIONS AND METHODS FOR ADMINISTRATION OF VACCINES
AGAINST DENGUE VIRUS
PRIORITY
[0001] This PCT application claims priority to a continuation-in-part
application, U.S. Patent
Application 13/492,884 filed June 10, 2012, which claims the benefit under 35
USC 120 of
U.S Non-Provisional Application Serial No. 12/790,511 filed May 28, 2010,
which claims
priority under 35 USC 119(e) to U.S. Provisional Application Serial No.
61/183,020 filed
on June 01, 2009. All prior applications are incorporated herein by reference
in their entirety
for all purposes.
FIELD
[0002] Embodiments of the present invention report compositions and methods
for
administering a vaccine to a subject against all dengue virus strains. In some
embodiments,
vaccine compositions may be administered by subcutaneous, intradermal,
intramuscular or
other injection or introduction methods. In certain embodiments, injection in
a subject of a
vaccine against all dengue virus types includes multiple anatomical sites at
day 0. Other
embodiments include follow-on injections from within days of the first
treatment to up to 12
months after initial injection(s). In other embodiments, no additional
injections are needed
other than the day 0 treatment. In certain embodiments, subcutaneous,
intradermal,
intramuscular or other modes of introducing to a subject, a vaccine
composition against
dengue virus to provide protection against three or more of the dengue
serotypes DEN-1,
DEN-2, DEN-3 and DEN-4 upon administration at day 0.
BACKGROUND
[0003] Vaccines for protection against viral infections have been effectively
used to reduce
the incidence of human disease. One of the most successful technologies for
viral vaccines is
to immunize animals or humans with a weakened or attenuated strain of the
virus (a "live,
attenuated virus"). Due to limited replication after immunization, the
attenuated strain does
not cause disease. However, the limited viral replication is sufficient to
express the full
repertoire of viral antigens and can generate potent and long-lasting immune
responses to the
virus. Thus, upon subsequent exposure to a pathogenic strain of the virus, the
immunized
individual is protected from disease. These live, attenuated viral vaccines
are among the most
successful vaccines used in public health.
1
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
SUMMARY
[0004] Embodiments of the present invention generally relate to methods and
compositions
for inducing protection in a subject against multiple dengue viruses by, for
example,
administering a multivalent dengue vaccine to a subject. Some embodiments can
include
introducing a vaccine composition to a subject via intradermal (ID) injection.
In accordance
with these embodiments, the vaccine composition can be introduced to a subject
intradermally to, for example, to induce neutralizing antibodies against three
or more dengue
virus serotypes. In certain embodiments, a vaccine composition can include,
but is not
limited to, a single dose of one formulation of a multivalent dengue serotype
vaccine having a
predetermined ratio administered to a subject. In other embodiments, a vaccine
composition
may include, but is not limited to; an initial dose of one formulation of
dengue vaccine (e.g.
tetravalent formulations such as DENVaxTM) and then one or more boosts of the
same, or a
different formulation can be administered to a subject.
[0005] Other aspects herein can concern inducing a humoral or cellular immune
response in a
subject by, for example, introducing a vaccine composition to a subject via an
intradermal
route wherein the vaccine composition includes, but is not limited to, a
dengue virus vaccine.
In accordance with these embodiments, compositions disclosed can be
administered
intradermally to a subject for modulating neutralizing antibody production in
the subject
against three or more dengue virus serotypes. Some aspects concern
predetermined
composition ratios (e.g. 1:1:1, 10:1 1:2:2, 1:10; 10:1, 3:4:3:3, 1:4:1;
5:5:4:5; or any ratio of
three or more serotypes is contemplated) of the various serotypes of dengue
virus or
fragments thereof or attenuated compositions thereof in a single vaccine
composition in order
to increase cross protection and levels of neutralizing antibodies in a
subject against at least
three dengue virus serotypes when the subject is administered the single
vaccine composition.
[0006] In certain embodiments, some advantages of using intradermal
introduction of a
vaccine against dengue virus can include, but are not limited to, multiple
protection (cross
protection) against some or all dengue virus serotypes in a subject, reduced
cost by using
reduced volumes of vaccine doses compared to subcutaneous injection,
modulation of
antibodies produced against some or all dengue virus serotypes in a subject
and reduced pain
at a site of administration in a subject administered a composition of vaccine
against dengue
virus.
[0007] In some embodiments, a single dose vaccine against dengue virus can
include one or
more dengue virus serotype(s). In addition, certain embodiments concern
treating a subject
2
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
with at least one additional injection(s) of a vaccine containing multiple
dengue viruses
administered at a separate site from the first injection, for example, in
close proximity to the
initial injection or in a distant anatomical site on the subject. In addition,
at least one
additional intradermal injection(s) may be performed less than 30 days after
the first
administration to the subject while others are performed 30 days and up to 12
months after
the first administration of the vaccine.
[0008] Other embodiments disclosed herein relate to methods and compositions
for inducing
protection in a subject against all dengue virus serotypes by, for example,
administering a
vaccine to a subject against all dengue virus serotypes in two or more doses
on one or more
than one anatomical location consecutively within a short interval of time.
Some
embodiments can include introducing a vaccine composition to a subject via
intradermal
(ID), subcutaneous (SC), or intramuscular (IM) injection in one location and
consecutively in
another anatomical location by ID, SC, IM or by other introduction method at a
second
different anatomical location. Other embodiments include using any combination
of modes
of administration for introducing a dengue virus vaccine of all dengue virus
serotypes to a
subject where administration of the vaccine occurs at two or more anatomical
sites or by two
or more different routes consecutively on the same day to the subject.
[0009] Some embodiments include treating a subject in need of dengue virus
tetravalent
vaccinations consecutively at two or more anatomical locations. In certain
embodiments, a
subject may need two consecutive administrations in a single day to induce
adequate levels of
neutralizing antibodies which will protect against dengue infection. In other
embodiments, a
subject may be administered dengue virus multivalent vaccinations
consecutively at two or
more anatomical locations, then the subject can be administered at least a
third vaccine within
30 days such as about 7, about 14, about 21 or about 28 days later with a
composition
comprising dengue virus serotypes which may or may not have all serotypes. In
other
embodiments, a subject may be administered dengue virus tetravalent
vaccinations
consecutively at two or more anatomical locations on day 0, then the subject
can be
administered at least a third vaccine within 30 days such as about 7, about
14, about 21 or
about 28 days later with a composition comprising dengue virus serotypes which
may or may
not have all serotypes. Vaccine compositions of these and other embodiments
disclosed
herein may include two or more dengue virus serotypes at a predetermined ratio
for the
subsequent administrations beyond the initial dual vaccination.
These subsequent
vaccinations may depend on personalized titers of antibodies post dual
injection or other
3
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
criteria such as results of test populations. In certain embodiments, a
subsequent vaccination
may only include a single dengue serotype (e.g. DEN-4).
[00010] In certain embodiments, the composition introduced to the subject
comprises
vaccines against all dengue virus serotypes, for example tetravalent DENVaxTM
or another
similar formulation. DENVaxTM comprises a tetravalent dengue vaccine of
predetermined
ratio where the vaccine is made up of constructs on an attenuated DEN-2
backbone (see for
example, PCT Application Number PCT/US01/05142 filed on February 16, 2001
incorporated herein by reference in its entirety for all purposes). In other
compositions, all
dengue vaccine virus serotypes are in equal proportions in the composition. In
yet other
compositions, each dengue vaccine virus serotype may be in a particular ratio
to one another
such that introduction of the composition induces sufficient levels of
neutralizing antibodies
which would provide the subject with sufficient protection against infection
with three or
more dengue viruses (e.g. DEN-1, DEN-2, DEN-3 and/or DEN-4). For example, if a
subject,
after receiving two or more compositions consecutively at two or more
anatomical locations
and the subject has lower protection to one or more particular dengue virus
serotypes, then a
booster for that subject can contain a multiple (more than two) vaccine
components or a
single vaccine component to improve immune responses to all four dengue
viruses in the
subject. In accordance with these embodiments, samples from a subject may be
analyzed for
resistance to dengue infection using standard means known in the art.
[00011] In certain embodiments, the vaccine composition can be introduced
to a
subject by any route in multiple anatomical locations to, for example, protect
against three or
more dengue serotypes after consecutive administrations. In certain
embodiments, a vaccine
composition can include, but is not limited to, a single dose of a formulation
containing all
serotypes of dengue virus (e.g. DENVaxTM) administered to a subject capable of
providing
protection against at least three dengue virus serotypes. In other
embodiments, a vaccine
composition can include attenuated dengue virus serotypes in combination with
other anti-
pathogenic compositions (e.g. Japanese encephalitis, yellow fever, West Nile,
influenza,
Chikungunya or other). Compositions contemplated herein can be administered by
any
method known in the art including, but not limited to, intradermal,
subcutaneous,
intramuscular, intranasal, inhalation, vaginal, intravenous, ingested, and any
other method.
Introduction in two or more anatomical sites can include any combination
administration
including by the same mode in two or more anatomical sites or by two or more
different
modes that include two or more separate anatomical sites. In accordance with
these
4
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
embodiments, two or more anatomical sites can include different limbs. In
other
embodiments, vaccinations can be delivered to a subject using any device known
in the art
including, but not limited to, a needle and syringe, jet injection,
microneedle injection, patch
delivery (e.g. skin), intradermal delivery devices, inhalation device,
intranasal device, slow
release microparticles, and any other acceptable vaccine-delivery device.
[00012] In certain embodiments, a vaccine composition for dual
administration of
dengue virus vaccines can include a composition comprising more than one
chimeric dengue
viruses in a single composition. In certain compositions, the chimeric
constructs used in such
a composition are made up of dengue-dengue serotypes such as a dengue-1,
dengue-3, and/or
dengue-4 on a dengue-2 backbone. In accordance with these embodiments, a
single vaccine
composition can include live, attenuated dengue viruses where an immune
response is
induced in a subject receiving such a compositions to at least three and up to
all four dengue
virus serotypes. Constructs contemplated herein include live, attenuated
dengue viruses
comprising one or more live, attenuated dengue viruses and one or more dengue-
dengue
chimeric viruses further comprising capsid and non-structural proteins of the
attenuated
dengue virus and pre-membrane and envelope proteins of at least a second
dengue virus in a
single construct. In certain embodiments, the capsid and non-structural
proteins are from an
attenuated dengue-1, dengue-2, dengue-3 or dengue-4 virus. In other
embodiments, pre-
membrane and envelope proteins of at least a second dengue virus are dengue-2,
dengue-3 or
dengue-4 when the attenuated dengue virus is dengue-1; or dengue-1, dengue-3
or dengue-4
when the attenuated dengue virus is dengue-2; or dengue-1, dengue-2 or dengue-
4 when the
attenuated dengue virus is dengue-3; or dengue-1, dengue-2 or dengue-3 when
the attenuated
dengue virus is dengue-4. Further, dengue-dengue chimeric viruses can include
the capsid
and non-structural proteins of an attenuated dengue-2 virus and the pre-
membrane and
envelope proteins are dengue-1, dengue-3 or dengue-4.
[00013] Other embodiments include live, attenuated viruses where the
backbone of the
live attenuated virus is dengue-2. Further, dengue-2 can include any dengue-2
strain. In
certain live attenuated dengue-2 viruses, dengue-2 comprises PDK-53 strain. In
another
embodiment, a chimera is a nucleic acid chimera including a first nucleotide
sequence
encoding nonstructural proteins from an attenuated dengue-2 virus, and a
second nucleotide
sequence encoding a structural protein from a second flavivirus. In another
embodiment, the
structural protein can be the C, prM or E protein of a flavivirus. Examples of
flaviviruses
from which the structural protein may be selected include, but are not limited
to, dengue-1
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
virus, dengue-2 virus, dengue-3 virus, dengue-4 virus, West Nile virus,
Japanese encephalitis
virus, St. Louis encephalitis virus, yellow fever virus and tick-borne
encephalitis virus. In a
further embodiment, the structural protein may be selected from non-flavivirus
species that
are closely related to the flaviviruses, such as hepatitis C virus.
[00014] In certain embodiments, amino acid substitution mutations in the
nonstructural
proteins and a nucleotide substitution mutation in the 5' noncoding region can
be present.
This nucleotide substitution mutation occurs in the stem of a stem-loop
structure that is
conserved in all four dengue serotypes. In particular, a single mutation at
NS1-53, a double
mutation at NS1-53 and at 5NC-57, a double mutation at NS1-53 and at N53-250,
and a
triple mutation at NS1-53, at 5NC-57 and at N53-250, can provide the
attenuated DEN-2
virus disclosed herein
[00015] It is contemplated that the genome of any dengue-2 virus
containing non-
conservative amino acid substitutions at these loci can be used as the
backbone in the
avirulent chimeras described herein. Furthermore, other flavivirus genomes
containing
analogous mutations at the same loci, after amino acid sequence or nucleotide
sequence
alignment and stem structure analysis can also be used as the backbone
structure and are
defined herein as being equivalent to attenuating mutations of the dengue-2
PDK-53 genome.
The backbone, that region of the chimera that includes 5' and 3' noncoding
regions and the
region encoding the nonstructural proteins, can also contain further mutations
to maintain
stability of the avirulent phenotype and to reduce the possibility that the
avirulent virus or
chimera might revert back to the virulent wild-type virus. For example, a
second mutation in
the stem of the stem/loop structure in the 5' non-coding region can provide
additional
stability, if desired.
[00016] In other embodiments, chimeric viruses can include nucleotide and
amino acid
substitutions, deletions or insertions in their structural and nonstructural
proteins in addition
to those specifically described herein. Structural and nonstructural proteins
disclosed herein
are to be understood to include any protein including or any gene encoding the
sequence of
the complete protein, an epitope of the protein, or any fragment comprising,
for example, two
or more amino acid residues thereof. Embodiments disclosed herein provide a
method for
making chimeric viruses of embodiments described herein using recombinant
techniques, by
inserting the required substitutions into the appropriate backbone genome.
[00017] In other embodiments, compositions can include a pharmaceutically
acceptable carrier and attenuated chimeric viruses which contain amino acid
sequences
6
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
derived from other dengue virus serotypes, other flavivirus species or other
closely related
species, such as hepatitis C virus, proteins or polypeptides comprising the
amino acid
sequences derived from other dengue virus serotypes, other flavivirus species
or other
closely-related species, can act as immunogens and, thus, be used to induce an
immunogenic
response against other dengue virus serotypes, other flavivirus species or
other closely related
species.
[00018] In one embodiment, nucleic acid chimeras including nucleotide
sequence from
an attenuated dengue-2 virus and nucleotide sequence from a second dengue
virus (or other
flavivirus), wherein the nucleotide sequence from the second flavivirus
directs the synthesis
of flavivirus antigens are contemplated of use for dual administration at day
0. In another
aspect of the invention compositions for vaccines comprising three or more
dengue virus
serotypes is contemplated.
[00019] In another aspect, methods for making immunogenic or vaccine
compositions
using recombinant techniques by inserting the required substitutions into an
appropriate
flavivirus genome. Another object of the invention is to provide compositions
and methods
for imparting immunity against three or more dengue virus serotypes
simultaneously using
dual administration in different anatomical areas to induce other lymph nodes
of a subject
receiving such a regimen.
[00020] Another object of the invention is to provide nucleic acid probes
and primers
for use in any of a number of rapid genetic tests that are diagnostic for each
of the vaccine
viruses of the current invention. This object of the invention may be embodied
in polymerase
chain reaction assays, hybridization assays or other nucleic acid sequence
detection
techniques known to the art. One embodiment includes using an automated PCR-
based
nucleic acid detection system.
[00021] In other embodiments, various mutations can be introduced to the
chimeric
dengue viruses in order to further attenuate the chimeric virus or improve
immunogenicity. In
certain embodiments, a composition can include chimeric dengue viruses capable
of eliciting
an immune response to all four dengue virus serotypes wherein a single
composition is
introduced in two anatomical locations of a subject. Certain embodiments
concern targeting
populations of people visiting dengue endemic countries for short periods of
time such as
tourists.
7
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
Brief Description of the Drawings
[00022] The following drawings form part of the present specification and
are
included to further demonstrate certain embodiments. Some embodiments may be
better
understood by reference to one or more of these drawings alone or in
combination with the
detailed description of specific embodiments presented.
[00023] Fig. 1 represents an example of an intradermal injection device
currently
available.
[00024] Fig. 2 represents examples of injection sites in a non-human
primate subject
having intradermal introduction of a vaccine against dengue virus.
[00025] Fig. 3 represents a bar graph comparison of neutralizing antibody
titer
produced against different ratios of dengue virus serotypes after a one
(primary)
administration via the subcutaneous (SC) versus intradermal (ID) route of
injection of a
vaccine against dengue virus.
[00026] Fig. 4 represents a bar graph comparison of neutralizing antibody
titer
produced against different dengue virus serotypes after a second, boosting
administration
via the subcutaneous (SC) versus intradermal (ID) injection of a vaccine
against dengue
virus.
[00027] Fig. 5 represents a histogram plot of neutralizing antibody titers
after
subcutaneous and intradermal immunizations with a vaccine against a dengue
virus
serotype-4 in mice.
[00028] Figs. 6A and 6B represent graphic depictions of mouse survival
after
vaccination with a dengue vaccine followed by a challenge with wild-type
dengue virus.
Mice were vaccinated by SC or ID route of infection with a dengue vaccine
(e.g. DENVax-
4) or a buffer/placebo (e.g. TFA).
[00029] Fig. 7 represents neutralizing antibody titers for DEN-1, DEN-2,
DEN-3 and
DEN-4 at day 28 and day 56 after two day-0; or 1 day-0 and 1 day-42 injections
(e.g.
DENVaxTM; 4:3:4:5 ratio).
[00030] Fig. 8 represents neutralizing antibody titers for DEN-1, DEN-2,
DEN-3 and
DEN-4 at day 28 and day 56 after two day-0; or 1 day-0 and 1 day-42 injections
(e.g.
DENVaxTM; 3:3:3:3, approximately equivalent amounts used).
[00031] Figs. 9A-9D represent graphs comparing neutralizing antibody
titers
achieved in non-human primates after SC immunization with tetravalent dengue
virus
vaccines. Two groups were vaccinated with the needle-free device via the
subcutaneous
route either twice on the same day (0,0) or once on day 0 and again on day 60
(0,60).
8
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
[00032] Figs. 10A-10B represent data obtained from a human clinical trial.
Seronegative humans (humans demonstrating little to no antibodies to dengue
virus
serotypes at the onset of the trial) were given two doses of a tetravalent
serotype
formulation of dengue vaccine either subcutaneously or intradermally (day 0
and day 90).
Antibody levels against each of the dengue serotypes were analyzed on days 0,
30, 60, 90
and 120.
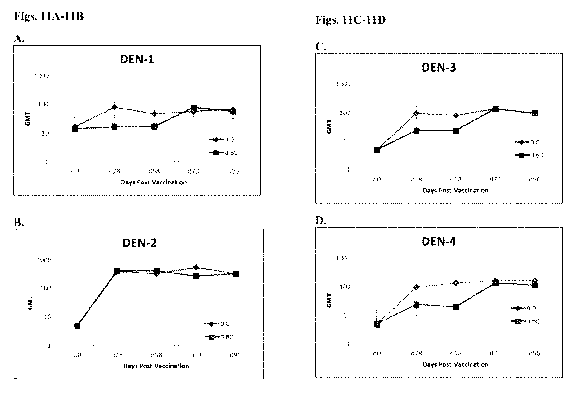
[00033] Figs. 11A-11D represent a graph comparing neutralizing antibody
titers
achieved in non-human primates after subcutaneous immunization with a
tetravalent
serotype dengue vaccine. Two groups were vaccinated either twice on the same
day (0,0) or
once on day 0 and again on day 60 (0,60). Serum was analyzed for presence of
antibodies
on days 0, 28, 58, 73 and 90, and the detection of antibodies against all four
dengue
serotypes were analyzed (DEN-1, DEN-2, DEN-3, DEN-4).
[00034] Fig. 12 represents gene expression levels in samples obtained from
a subject
having single administration or dual (double) administration at separate
anatomical sites of
dengue virus vaccines. This data represents gene cluster variability where
changes in levels
of various gene transcripts in a subject are analyzed after exposure to a
composition using
certain regimens disclosed herein.
[00035] Figs. 13A-13F represent levels of certain genes after single or
dual (double)
administration. The genes represented in cluster 2 Fig. 12 and A-F are
associated with
innate immunity.
Definitions
[00036] As used herein, "a" or "an" may mean one or more than one of an
item.
[00037] As used herein, vessel can include, but is not limited to, test
tube, mini- or
micro-fuge tube, channel, vial, microtiter plate or container.
[00038] As used herein the specification, "subject" or "subjects" may
include but are
not limited mammals such as humans or mammals, domesticated or wild, for
example dogs,
cats, other household pets (e.g., hamster, guinea pig, mouse, rat), ferrets,
rabbits, pigs,
horses, cattle, prairie dogs, or zoo animals.
[00039] As used herein, "about" or "approximately" can mean plus or minus
ten
percent.
[00040] As used herein, "attenuated virus" can mean a virus that
demonstrates
reduced or no clinical signs of disease when administered to a subject such as
a mammal
(e.g., human or an animal).
9
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
[00041] As used herein, "consecutively" can mean in close temporal
proximity,
usually within a single patient visit and within 24 hours.
[00042] As used herein, "administration" can mean delivery of a vaccine or
therapy
to an individual animal or human by any one of many methods such as
intradermal,
subcutaneous, intramuscular, intranasal, inhalation, vaginal, intravenous,
oral, buccal, by
inhalation, intranasally, or any others known in the art.
DESCRIPTION
[00043] In the following sections, various exemplary compositions and
methods are
described in order to detail various embodiments. It will be obvious to one
skilled in the art
that practicing the various embodiments does not require the employment of all
or even
some of the details outlined herein, but rather that concentrations, times and
other details
may be modified through routine experimentation. In some cases, well-known
methods or
components have not been included in the description.
[00044] Certain aspects of the present invention include, but are not
limited to,
administration of vaccine compositions against dengue virus.
[00045] Embodiments of the present invention generally relate to methods
and
compositions for inducing protective neutralizing antibodies in a subject
against three or
more dengue virus serotypes. Other embodiments can include introducing a
vaccine
composition to a subject via any method known in the art including, but not
limited to,
intradermal, subcutaneous, intramuscular, intranasal, inhalation, orally,
intranasally,
vaginal, intravenous, ingested, and any other method wherein the vaccine
composition so
introduced induces neutralizing antibodies against three or more dengue virus
serotypes. In
certain embodiments, the vaccine composition comprises a dose of a vaccine
against three
or more dengue virus serotypes administered to a subject. In other
embodiments, the
vaccine composition comprises an initial dose against all four dengue
serotypes then, one or
more other vaccine compositions administered to a subject.
[00046] Other aspects of the present invention include modulating an
immune
response to a vaccine against dengue virus administered intradermally compared
to
subcutaneously to a subject. Vaccines against dengue virus may include a
composition
comprising predetermined ratios of all four live, attenuated dengue vaccine
viruses,
recombinant dengue vaccine viruses, chimeric viruses or mutants thereof . The
ratios of
various dengue serotypes may be equivalent or nearly equal in representation
or certain
serotypes may be represented at higher concentrations than others depending on
need or
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
ability to induce a balanced neutralizing antibody response in the subject. In
accordance
with these embodiments, ratios of different dengue vaccines may differ by 2 to
100,000 fold
(e.g. plaque forming units) between any two serotypes. This can depend on, for
example,
number of serotypes represented in the formulation, predetermined response and
desired
effect. It is contemplated that any dengue vaccine virus serotype formulation
may be used
to generate a vaccine (e.g. attenuated virus etc.) of use in consecutive
administration to a
subject in need thereof where the composition includes, but is not limited to,
three or more
dengue virus serotypes.
[00047] In other embodiments, compositions of dengue virus vaccine
formulations
may be introduced to a subject prior to, during or after exposure to dengue
virus by the
subject. In accordance with these embodiments, a subject may receive more than
one
administration consecutively or more than one administration comprising a
dengue virus
formulation, optionally, followed by one or more additional administrations at
a later time.
Intradermal, subcutaneous, intramuscular, intranasal, inhalation, vaginal,
intravenous, oral,
and any other method of applications of formulations described herein may be
combined
with any other anti-viral treatment. In some embodiments, it is contemplated
that
intradermal, subcutaneous, intramuscular introduction of a formulation
contemplated herein
may be administered to any appropriate region of a subject's body (e.g. arm,
shoulder, hip,
intranasally etc). In addition, parenteral administration of vaccine
formulations may be
combined with other modes of administration such as intranasal, pulmonary,
oral, buccal, or
vaginal in consecutive administrations. In some embodiments, it is
contemplated that, after
consecutive administrations as described herein primary or booster
administrations may
occur consecutively on the same day, consecutive days, weekly, monthly, bi-
monthly or
other appropriate treatment regimen.
[00048] Dengue is endemic in Asia, Central and South America including
Colombia,
the Caribbean, the Pacific Islands, and parts of Africa and Australia. It is
estimated that 3.6
billion people (55% of the world's population) live in areas at risk of dengue
virus
transmission (DVI). Infection with a dengue virus can result in a range of
symptoms, from
subclinical disease to debilitating but transient dengue fever to life-
threatening dengue
hemorrhagic fever (DHF) or dengue shock syndrome (DSS). Currently, there is no
therapeutic treatment or prophylactic vaccine for dengue fever. Given the
impact of dengue
on populations in endemic countries and on travelers to those regions, a
vaccine to prevent
dengue is needed.
11
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
[00049] Dengue is a mosquito borne viral disease, transmitted from human
to human
primarily by the mosquito, Aedes aegypti. Dengue viruses (DEN) contain a
single-stranded,
positive-sense RNA genome of approximately 11 kb. The genome consists of three
structural
proteins, capsid (C), premembrane (prM), and envelope (E), and seven
nonstructural proteins,
NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. There are four different serotypes
of
dengue viruses, DEN-1, DEN-2, DEN-3 and DEN-4. Primary infection with a given
serotype
induces lifelong serotype specific immunity. However, there is no long-term
cross-protective
immunity against the other three dengue virus serotypes, and subsequent
infection with an
alternate serotype leads to increased probability of more severe disease, such
as DHF or DSS.
[00050] Due to the disease enhancement associated with secondary DENV
infections,
a multivalent (e.g. tetravalent) vaccine that stimulates immunity against more
than one and up
to all four serotypes of DENV is needed. Several DENV vaccine candidates
attenuated by
classical serial passage in cell culture have proven unsafe or poorly
immunogenic. Chimeric
live-attenuated, recombinant DENV vaccines candidates, including viruses based
on the
attenuated genetic background of yellow fever 17D (YF-17D)vaccine virus, DENV-
2 PDK-
53 vaccine virus, or DENV-4 containing a 30-nucleotide 3' non-coding region
(NCR)
deletion are known in the art.
[00051] A challenging issue in the development of an effective live-
attenuated dengue
virus (DENV) vaccine is the interference between the four dengue vaccine
viruses when
administered as a tetravalent formulation. Interference is manifest when one
or more
components of a multivalent mixture will induce lower immune responses than
those elicited
by each individual monovalent vaccine. Interference has been observed with
vaccines for
diseases with multiple pathogenic serotypes, such as polio, dengue or others.
Due in part to
this interference, it was previously discovered that three dose regimen of
oral polio vaccine is
required to induce adequate immune responses to the three key serotypes.
Historically
studies with live attenuated tetravalent dengue vaccines have shown that the
DENV serotype
that elicits the strongest neutralizing antibody response when administered
alone tends to
dominate immune responses when administered in the context of a multivalent
formulation
containing other serotypes. As an example, tetravalent mixtures of four
different live,
attenuated dengue vaccines showed dominant responses to the DEN-3 component
and
reduced immune responses to DEN-1, -2 and -4 (see for example, Sabchareon, et
al., 2002,
Kitchener, et al. 2006). As a result of this dominance, clinical development
of the tetravalent
mixtures was suspended. Interference has been seen with recombinant, live
attenuated
viruses as well. Interference was documented in tetravalent mixtures of
dengue/yellow fever
12
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
chimeras (Guy, et al. 2009. Evaluation of Interferences between Dengue Vaccine
Serotypes
in a Monkey Model. Am. J. Trop Med. Hyg. 80: 3012-311). In these studies, two
serotypes
were found to dominate the responses in tetravalent formulations of ChimeriVax
vaccine
strains. Interference could be overcome by administering two bivalent vaccine
formulations,
either in separate anatomical locations or sequentially in time, or by a third
administration of
the tetravalent formulation after one year. Similarly, it was demonstrated
that improved
multivalent responses with tetravalent recombinant vaccine strains (in this
case, formulations
containing DENY or chimeric DENY with deletions in the 3' non-coding region)
could be
obtained only with a prolonged four month internal between the first and
second
administration. (Blaney, et al., 2005. Recombinant, Live-Attenuated
Tetravalent Dengue
Virus Vaccine Formulations Induce a Balanced, Broad, and Protective
Neutralizing Antibody
Response against Each of the Four Serotypes in Rhesus Monkeys. J. Virology 79:
5516-
5528).
[00052] Successful vaccination often requires vaccine delivery to closely
mimic
natural infection. To date, all clinical trials of dengue candidate vaccines
have utilized the SC
route using needle and syringe. The natural route of dengue infection is
through mosquito
transmission in the dermis. The skin is thought to be an immuno-competent
organ functioning
as an immune barrier to infections A highly dense network of specialized
antigen-presenting
cells (APCs, such as Langerhan's cells and dendritic cells) are present in the
epidermis and
serve to protect the host against infectious pathogens through efficient
uptake and
presentation of antigens to the regional lymph nodes. Both these subsets of
APCs together
with resident macrophages have been shown to be natural targets of dengue
virus infection.
Given the fact that the epidermis is rich in immunocompetent cells, it was
contemplated
herein that the use of intradermal route for dengue virus vaccine delivery
will favor the
induction of more potent and balanced immune responses to all four dengue
virus serotypes.
Specifically, the presence of an increased number of natural host cells in the
skin for virus
replication may reduce interference and permit replication of the less
dominant viruses in
tetravalent formulations. In certain embodiments, intradermal immunization of
multivalent,
live, attenuated dengue vaccines can be used to induce more balanced immune
responses to
dengue virus exposure in a subject.
[00053] Certain embodiments disclosed herein concern DENVaxTM. DENVaxTM is
a
dengue vaccine that consists of a mixture of four recombinant dengue virus
strains designed
to generate immune responses to the four dengue serotypes (DEN-1, DEN-2, DEN-3
and
DEN-4). Not to be bound by any limitations to a particular tetravalent
formulation,
13
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
DENVaxTM, the dengue serotype 2 vaccine component (DENVax-2) corresponds to an
attenuated DEN-2 PDK-53 strain. This construct has already been investigated
in many
clinical studies. The other dengue vaccine strains (DENVax-1, DENVax-3 and
DENVax-4)
are chimeras consisting of the DEN-1, DEN-3 or DEN-4 structural pre-membrane
(prM) and
envelope (E) protein genes cloned into a DEN-2 PDK-53 non-structural gene
backbone.
These recombinant viruses express the surface antigens of DEN-1, DEN-3 or DEN-
4 and
retain the genetic alterations responsible for the attenuation of the DEN-2
PDK-53 strain. In
certain embodiments, DENVaxTM can be used as an example of a multivalent live,
attenuated
dengue vaccine having all four dengue virus serotypes represented in one
vaccine
composition at various ratios. Other embodiments relate to optimizing
tetravalent vaccine
administrations. Yet other embodiments relate to DENVaxTM immunization
methods.
[00054] During the course of exploring intradermal delivery of multivalent
dengue
vaccines, it was discovered that administration of more than one dose of a
multivalent
vaccine in at least two separate anatomical sites induced neutralizing
antibody responses that
were approximately equivalent or superior to administering multiple doses
separated by time.
Further, it was discovered that the benefit of multiple site administration
was independent of
the route of immunization.
[00055] This finding was unexpected. Information was previously disclosed
regarding
multiple subcutaneous administrations of a tetravalent vaccine based on
deleted, attenuated
and/or recombinant viruses. It was reported that a second administration of a
tetravalent
administration 30 days after the first administration failed to increase
neutralizing antibody
titers. In contrast, a second administration 120 days after the first,
improved neutralizing
antibody titers to all four dengue serotypes. Similar information was reported
in clinical
trials of yellow fever/dengue recombinant vaccines (Poo, et at. 2011 Ped. Inf.
Dis J. 30: 1-9)
It was also suggested that a three month interval between administrations was
suboptimal for
generating neutralizing antibody response against multiple dengue viruses.
(Capeding et al.
2011 Vaccine 29: 3863-3872) These reports regarding two clinical studies
suggested that
longer intervals such as 6 - 9 months are required to generate better
multivalent immune
responses. Lastly, in early human challenge studies, it was reported that wild
type dengue
viruses elicit broadly cross-reactive antibodies that persist for up to 6
months after initial
infection. These data support the concept that a short immunization regimen
are suboptimal
for live attenuated vaccines: the transient cross-reactive antibodies
previously observed
would effectively neutralize any of the live, attenuated vaccine components in
a multivalent
14
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
formulation. Until the instant disclosure, immunization regimens with
multivalent, live
attenuated vaccines at shorter intervals in more than one anatomical site were
not considered
a viable option for treating a subject in need of such a treatment. It is
contemplated herein
that multiple site administration, by accessing larger numbers of antigen
presenting cells
and/or more than one draining lymph node, permits immune responses to less
dominant
components of a multivalent, live attenuated vaccine and effectively reduces
vaccine
interference.
[00056] In certain embodiments, the composition introduced to the subject
comprises
vaccines against all dengue virus serotypes (DEN-1, DEN-2, DEN-3, DEN-4). In
other
embodiments, a composition contemplated herein can include DENVaxTM or other
similar
formulation. In some compositions, vaccine viruses against all dengue
serotypes are in
equal proportions in the composition. In yet other compositions, each dengue
vaccine virus
serotype may be in a particular ratio to one another such that introduction of
the
composition provides the subject with sufficient levels of neutralizing
antibodies against all
dengue viruses (e.g. DEN-1, DEN-2, DEN-3, DEN-4).
[00057] In certain embodiments, a vaccine composition for dual
administration of
dengue virus vaccines can include a composition comprising more than one
chimeric dengue
viruses in a single composition. In certain compositions, the chimeric
constructs used in such
a composition are made up of dengue-dengue serotypes such as a dengue-1,
dengue-3, and/or
dengue-4 on a dengue-2 backbone. In accordance with these embodiments, a
single vaccine
composition can include live, attenuated dengue viruses where an immune
response is
induced in a subject receiving such a compositions to at least three and up to
all four dengue
virus serotypes. Constructs contemplated herein include live, attenuated
dengue viruses
comprising one or more live, attenuated dengue viruses and one or more dengue-
dengue
chimeric viruses further comprising capsid and non-structural proteins of the
attenuated
dengue virus and pre-membrane and envelope proteins of at least a second
dengue virus in a
single construct. In certain embodiments, the capsid and non-structural
proteins are from an
attenuated dengue-1, dengue-2, dengue-3 or dengue-4 virus. In other
embodiments, pre-
membrane and envelope proteins of at least a second dengue virus are dengue-2,
dengue-3 or
dengue-4 when the attenuated dengue virus is dengue-1; or dengue-1, dengue-3
or dengue-4
when the attenuated dengue virus is dengue-2; or dengue-1, dengue-2 or dengue-
4 when the
attenuated dengue virus is dengue-3; or dengue-1, dengue-2 or dengue-3 when
the attenuated
dengue virus is dengue-4. Further, dengue-dengue chimeric viruses can include
the capsid
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
and non-structural proteins of an attenuated dengue-2 virus and the pre-
membrane and
envelope proteins are dengue-1, dengue-3 or dengue-4.
[00058] Other embodiments include live, attenuated viruses where the
backbone of the
live attenuated virus is dengue-2. Further, dengue-2 can include any dengue-2
strain. In
certain live attenuated dengue-2 viruses, dengue-2 comprises PDK-53 strain. In
another
embodiment, a chimera is a nucleic acid chimera including a first nucleotide
sequence
encoding nonstructural proteins from an attenuated dengue-2 virus, and a
second nucleotide
sequence encoding a structural protein from a second flavivirus. In another
embodiment, the
structural protein can be the C, prM or E protein of a flavivirus. Examples of
flaviviruses
from which the structural protein may be selected include, but are not limited
to, dengue-1
virus, dengue-2 virus, dengue-3 virus, dengue-4 virus, West Nile virus,
Japanese encephalitis
virus, St. Louis encephalitis virus, yellow fever virus and tick-borne
encephalitis virus. In a
further embodiment, the structural protein may be selected from non-flavivirus
species that
are closely related to the flaviviruses, such as hepatitis C virus.
[00059] In certain embodiments, amino acid substitution mutations in the
nonstructural
proteins and a nucleotide substitution mutation in the 5' noncoding region can
be present.
This nucleotide substitution mutation occurs in the stem of a stem-loop
structure that is
conserved in all four dengue serotypes. In particular, a single mutation at
NS1-53, a double
mutation at NS1-53 and at 5NC-57, a double mutation at NS1-53 and at N53-250,
and a
triple mutation at NS1-53, at 5NC-57 and at N53-250, can provide the
attenuated DEN-2
virus disclosed herein
[00060] It is contemplated that the genome of any dengue-2 virus
containing non-
conservative amino acid substitutions at these loci can be used as the
backbone in the
avirulent chimeras described herein. Furthermore, other flavivirus genomes
containing
analogous mutations at the same loci, after amino acid sequence or nucleotide
sequence
alignment and stem structure analysis can also be used as the backbone
structure and are
defined herein as being equivalent to attenuating mutations of the dengue-2
PDK-53 genome.
The backbone, that region of the chimera that includes 5' and 3' noncoding
regions and the
region encoding the nonstructural proteins, can also contain further mutations
to maintain
stability of the avirulent phenotype and to reduce the possibility that the
avirulent virus or
chimera might revert back to the virulent wild-type virus. For example, a
second mutation in
the stem of the stem/loop structure in the 5' non-coding region can provide
additional
stability, if desired.
16
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
[00061] In other embodiments, chimeric viruses can include nucleotide and
amino acid
substitutions, deletions or insertions in their structural and nonstructural
proteins in addition
to those specifically described herein. Structural and nonstructural proteins
disclosed herein
are to be understood to include any protein including or any gene encoding the
sequence of
the complete protein, an epitope of the protein, or any fragment comprising,
for example, two
or more amino acid residues thereof. Embodiments disclosed herein provide a
method for
making chimeric viruses of embodiments described herein using recombinant
techniques, by
inserting the required substitutions into the appropriate backbone genome.
[00062] In other embodiments, compositions can include a pharmaceutically
acceptable carrier and attenuated chimeric viruses which contain amino acid
sequences
derived from other dengue virus serotypes, other flavivirus species or other
closely related
species, such as hepatitis C virus, proteins or polypeptides comprising the
amino acid
sequences derived from other dengue virus serotypes, other flavivirus species
or other
closely-related species, can act as immunogens and, thus, be used to induce an
immunogenic
response against other dengue virus serotypes, other flavivirus species or
other closely related
species.
[00063] In one embodiment, nucleic acid chimeras including nucleotide
sequence from
an attenuated dengue-2 virus and nucleotide sequence from a second dengue
virus (or other
flavivirus), wherein the nucleotide sequence from the second flavivirus
directs the synthesis
of flavivirus antigens are contemplated of use for dual administration at day
0. In another
aspect of the invention compositions for vaccines comprising three or more
dengue virus
serotypes is contemplated.
[00064] In another aspect, methods for making immunogenic or vaccine
compositions
using recombinant techniques by inserting the required substitutions into an
appropriate
flavivirus genome. Another object of the invention is to provide compositions
and methods
for imparting immunity against three or more dengue virus serotypes
simultaneously using
dual administration in different anatomical areas to induce other lymph nodes
of a subject
receiving such a regimen.
[00065] Another object of the invention is to provide nucleic acid probes
and primers
for use in any of a number of rapid genetic tests that are diagnostic for each
of the vaccine
viruses of the current invention. This object of the invention may be embodied
in polymerase
chain reaction assays, hybridization assays or other nucleic acid sequence
detection
17
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
techniques known to the art. One embodiment includes using an automated PCR-
based
nucleic acid detection system.
[00066] In other embodiments, various mutations can be introduced to the
chimeric
dengue viruses in order to further attenuate the chimeric virus or improve
immunogenicity. In
certain embodiments, a composition can include chimeric dengue viruses capable
of eliciting
an immune response to all four dengue virus serotypes wherein a single
composition is
introduced in two anatomical locations of a subject. Certain embodiments
concern targeting
populations of people visiting dengue endemic countries for short periods of
time such as
tourists.
[00067] Certain embodiments disclosed herein relate to methods and
compositions for
a rapid induction of protection in a subject against all dengue virus
serotypes by, for example,
administering a vaccine to a subject against all dengue virus serotypes in
more than one
anatomical location consecutively on the same day. Some embodiments can
include
introducing a vaccine composition to a subject via intradermal (ID) or
subcutaneous (SC)
injection or other administration mode in one anatomical location then
introducing at least a
second vaccine composition at another anatomical location by ID, SC or other
administration
mode. Some embodiments include using any combination of modes of
administration for
introducing a dengue virus vaccine of all dengue virus serotypes to a subject
where
administration of the vaccine occurs at two or more anatomical sites or by two
or more
different routes on day 0 to the subject. Some embodiments include using the
same mode of
administration but at different anatomical locations.
[00068] Some dengue virus vaccine compositions described herein range in
dosage
from from 102 to 5 x 106 PFU for each serotype in a composition. Other
compositions (e.g.
follow-on vaccinations) contemplated herein include compositions that have
dosages less
than or more than this range based on immune response in the subject after
primary
immunization. In certain embodiments, ratios can vary for the various Dengue
vaccine virus
serotypes depending on need and immune response in a subject.
[00069] In certain embodiments, compositions introduced on the first
vaccination or in
any follow-on vaccination contemplated herein may include one tetravalent
dengue virus
composition. In accordance with these embodiments, the composition can include
DENVaxTM or other similar tetravalent formulation of equal or equivalent
ratios or at
predetermined serotype ratios. Other embodiments, can include using different
formulations
18
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
(e.g. serotype ratios) for each of the vaccine compositions administered at
the primary
vaccination or any follow-on vaccinations (e.g. less than 30 days later).
[00070] Some embodiments herein include treating a subject in need of such
a vaccine,
on day 0 at two or more anatomical locations then administering at least a
second vaccine
within 30 days such as about 7, about 14, about 21 or about 28 days later with
a composition
comprising dengue virus serotypes which may or may not have all serotypes. In
certain
embodiments, each vaccination has all dengue virus serotypes represented in
the vaccine
formulation. Vaccine compositions of follow-on administration disclosed herein
may include
two or more dengue virus serotypes at a predetermined ratio for the subsequent
administration(s).
[00071] In certain embodiments, the composition introduced to the subject
comprises
all dengue virus serotypes. In some embodiments, vaccine compositions comprise
various
formulations of DENVaxTM or other similar formulation. In certain vaccine
compositions,
the ratio of DEN-1:DEN-2:DEN-3:DEN-4 can be 3:3:3:3, 4:3:4:5, 5:4:5:5,
5:4:5:5, 5:5:5:5,
5:5:5;10, 10:1:10:100 or other ratio where the ratio between 2 serotypes can
be about 2 to
about 100,000 fold difference (e.g. DENVax 4:3:4:5TM etc.) in a single
composition. In certain
embodiments a dengue serotype ratio can be DEN-1 at 2 x104: DEN-2 at 5 x104:
DEN-3 at 1
x105: DEN-4 at 3 x105 PFUs or DEN-1 at 8 x103: DEN-2 at 5 x103: DEN-3 at 1
x104: DEN-4
at 2 x105 PFUs. In some compositions, all dengue vaccine virus serotypes are
in equal
proportions in the composition. In yet other compositions, each dengue vaccine
virus
serotype may be in a particular ratio to another serotype such that
introduction of the
composition provides the subject with adequate or more than adequate levels of
neutralizing
antibodies which confer protection against all dengue viruses (e.g. Dengue 1,
2, 3 and 4). For
example, if after receiving two or more consecutive vaccinations on day 0 at
two or more
anatomical locations, the subject has lower protection to one or more
particular dengue virus
serotypes, then a booster for that subject can contain an increased
concentration of the one or
more dengue vaccine virus serotype (that demonstrated lower neutralizing
antibodies) to
provide better protection against all dengue virus types. In accordance with
these
embodiments, samples from a subject may be analyzed for an immune response to
dengue
serotype infection (e.g. Dengue-1, -2, -3, -4) using standard means known in
the art.
[00072] In certain embodiments, the vaccine composition can be
simultaneously or
consecutively introduced to a subject intradermally in multiple anatomical
locations to, for
example, protect against all dengue serotypes (e.g. cross protection). In
certain embodiments,
19
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
a vaccine composition can include, but is not limited to, a single formulation
of all dengue
vaccine virus serotypes (e.g. DENVaxTM) administered to a subject capable of
providing full
protection against infection by all dengue virus serotypes. In other
embodiments, a vaccine
composition can include attenuated dengue virus serotypes in combination with
other anti-
pathogenic compositions (e.g. Japanese encephalitis, West Nile, influenza
etc.).
Compositions contemplated herein can be administered by any method known in
the art
including, but not limited to, intradermal, subcutaneous, intramuscular,
intranasal, inhalation,
vaginal, intravenous, ingested, and any other method. Introduction in two or
more
anatomical sites can include any combination administration including by the
same mode in
two or more anatomical sites or by two different modes that include two
separate anatomical
sites. In accordance with these embodiments, two or more anatomical sites can
include
different limbs.
[00073] For example, if a subject, after receiving two or more consecutive
vaccinations
on day 0 at two or more anatomical locations and the subject does not induce
poor levels of
neutralizing antibodies to one or more particular dengue virus serotypes, then
a booster
vaccination for that subject can contain an increased concentration of the one
or more dengue
vaccine virus serotype (that demonstrated lower levels of neutralizing
antibodies) to provide
complete protection against infection by all dengue virus types. In accordance
with these
embodiments, samples from a subject may be analyzed for resistance to dengue
infection
using standard means known in the art.
[00074] In certain embodiments, doses of the vaccine composition can be
consecutively introduced to a subject in multiple anatomical locations to, for
example, to
protect against all dengue serotypes (e.g. cross protection) at day 0. In
certain embodiments,
a vaccine composition can include, but is not limited to, a single composition
of three or four
dengue virus serotypes (e.g. DENVaxTM) administered to a subject capable of
inducing
neutralizing antibodies to levels which would provide full protection against
infection by all
dengue virus serotypes. Thus, a particular subject may need to visit a clinic
only one time to
receive enough protection to visit or remain in a region having dengue virus
for a
predetermined period of time (e.g. 30 days). In other embodiments, a vaccine
composition
can include attenuated dengue virus serotypes in combination with vaccine
compositions
against other pathogens (e.g. flaviviruses such as Japanese encephalitis, West
Nile, or other
viruses such as influenza etc.). Compositions contemplated herein can be
administered by
any method known in the art including, but not limited to, intradermal,
subcutaneous,
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
intramuscular, intranasal, inhalation, vaginal, intravenous, ingested, and any
other method.
Introduction in two or more anatomical sites can include any combination
administration
including by the same mode in two or more anatomical sites or by two or more
different
modes that include two or more separate anatomical sites. In accordance with
these
embodiments, two or more anatomical sites can include different limbs,
different tissues,
intranasally, as drops (e.g. for the eye), intramuscular in two or more
locations.
[00075] In certain embodiments, vaccine compositions disclosed herein can
be
chimeric constructs that can include a mixture of constructs that make up at
least 3 dengue
serotypes in a vaccine composition for administration to a subject. In other
embodiments,
dengue virus vaccines can include constructs having an attenuated flavivirus
backbone with
various dengue serotype substitutions representing each of the four serotypes
where the
constructs can be mixed in a composition for administration as a vaccine.
[00076] Chimeras contemplated and described herein can be produced by
splicing one
or more of the structural protein genes of the flavivirus against which
immunity is desired
into a a dengue virus genome backbone (e.g. PDK-53), or the equivalent thereof
as described
above, using recombinant engineering techniques well known to those skilled in
the art to
remove the corresponding structural genes and replace it with the desired
structural gene.
Alternatively, using the sequences provided in the sequence listing, the
nucleic acid
molecules encoding the flavivirus proteins may be synthesized using known
nucleic acid
synthesis techniques and inserted into an appropriate vector. Avirulent,
immunogenic virus is
therefore produced using recombinant engineering techniques known to those
skilled in the
art.
[00077] As mentioned above, the gene to be inserted into the backbone
encodes a
flavivirus (e.g. other dengue virus serotype) structural protein. Preferably
the flavivirus gene
to be inserted is a gene encoding a C protein, a PrM protein and/or an E
protein. The
sequence inserted into the dengue-2 backbone can encode both the PrM and E
structural
proteins. The sequence inserted into the dengue-2 backbone can encode the C,
prM and E
structural proteins. The dengue virus backbone is the PDK-53 dengue-2 virus
genome and
includes either the spliced genes that encode the C, PrM and/or E structural
proteins of
dengue-1 (DEN-2/1), the spliced genes that encode the PrM and/or E structural
proteins of
dengue-3 (DEN-2/3), or the spliced genes encode the PrM and/or E structural
proteins of
dengue-4 (DEN-2/4). In one embodiment, the spliced gene that encodes the
structural protein
21
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
of dengue-3 virus directs the synthesis of an E protein that contains a
leucine at amino acid
position 345.
[00078] In another embodiment, a chimera of encodes the C structural
protein of
dengue-2 virus and directs the synthesis of a C protein that contains a serine
at amino acid
position 100 and comprises a spliced gene encoding the structural proteins of
dengue-4 which
directs the synthesis of an E protein that contains a leucine at amino acid
position 447.
[00079] In yet other embodiments, a chimera can encode the C structural
protein of
dengue-2 virus and directs the synthesis of a C protein that contains a serine
at amino acid
position 100 and comprises a spliced gene encoding the structural proteins of
dengue-4 which
directs the synthesis of an E protein that contains a leucine at amino acid
position 447 and a
valine at amino acid position 364. The structural proteins described herein
can be present as
the only flavivirus structural protein or in any combination of flavivirus
structural proteins in
a viral chimera of this invention.
[00080] Chimeras can be engineered by recombination of full genome-length
cDNA
clones derived from both DEN-2 16681 wild type virus and either of the PDK-53
dengue-2
virus variants. Uncloned PDK-53 vaccine contains a mixture of two genotypic
variants,
designated herein as PDK53-E and PDK53-V. The PDK53-V variant contains all
nine PDK-
53 vaccine-specific nucleotide mutations, including the Glu-to-Val mutation at
amino acid
position NS3-250. The PDK53-E variant contains eight of the nine mutations of
the PDK-53
vaccine and the NS3-250-Glu of the parental 16681 virus. Infectious cDNA
clones are
constructed for both variants, and viruses derived from both clones are
attenuated in mice.
The phenotypic markers of attenuation of DEN-2 PDK-53 virus include small
plaque size,
temperature sensitivity (particularly in LLC-MK<sub>2</sub> cells), limited
replication (particularly
in C6/36 cells), attenuation for newborn mice (specifically loss of
neurovirulence for suckling
mice) and decreased incidence of viremia in monkeys. The chimeras that are
useful as
vaccine candidates are constructed in the genetic backgrounds of the two DEN-2
PDK-53
variants which all contain mutations in nonstructural regions of the genome,
including 5NC-
57 C-to-T (16681-to-PDK-53) in the 5' noncoding region, as well as mutations
in the amino
acid sequence of the nonstructural proteins, such as, for example, NS1-53 Gly-
to-Asp and
NS3-250 Glu-to-Val.
[00081] Suitable chimeric viruses or nucleic acid chimeras containing
nucleotide
sequences encoding structural proteins of other flaviviruses or dengue virus
serotypes can be
evaluated for usefulness as vaccines by screening them for the foregoing
phenotypic markers
22
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
of attenuation that indicate avirulence and by screening them for
immunogenicity.
Antigenicity and immunogenicity can be evaluated using in vitro or in vivo
reactivity with
flavivirus antibodies or immunoreactive serum using routine screening
procedures known to
those skilled in the art.
Flavivirus Vaccines
[00082] In certain embodiments, chimeric viruses and nucleic acid chimeras
provide
live, attenuated viruses useful as immunogens or vaccines. These chimeras
exhibit high
immunogenicity while at the same time producing no dangerous pathogenic or
lethal effects.
[00083] Effective vaccination against all strains of dengue virus has been
difficult. To
prevent the possible occurrence of DHF/DSS in patients vaccinated against only
one serotype
of dengue virus, rapid immunization using a trivalent or tetravalent dengue
virus vaccine is
needed to provide simultaneous immunity for all four serotypes of the virus.
One tetravalent
vaccine is produced by combining dengue-2 PDK-53 with the dengue-2/1, dengue-
2/3, and
dengue-2/4 chimeras described above in a suitable pharmaceutical carrier for
administration
as a multivalent vaccine.
[00084] The chimeric viruses or nucleic acid chimeras can include
structural genes of
either wild-type or attenuated virus in a virulent or an attenuated DEN-2
virus backbone. For
example, the chimera may express the structural protein genes of wild-type DEN-
1 16007
virus or its candidate PDK-13 vaccine derivative in either of the DEN-2 PDK-53
backgrounds.
[00085] Tetravalent formulations, e.g. DENVaxTm, can be prepared by mixing
predetermined amounts of each monovalent vaccine component or chimeric
constructs.
Based on input titer of each vaccine component, a defined volume of monovalent
vaccines
can be added to a final volume of either 0.1mL (e.g. for intradermal) or 0.5mL
(e.g. for
subcutaneous) vaccine formulation. The remaining volume of the tetravalent
DENVaxTM
vaccine can be composed of diluent containing Trehalose (15%) F127 (1%) and
human
serum albumin (0.1%) in a saline buffer to stabilize the live, attenuated
vaccine formulation.
In certain embodiments, a predetermined ratio of at least three dengue virus
serotypes can be
represented in a single composition. For example, dengue-1 thru dengue-4
constructs may be
represented in a single composition where more of one serotype of a live,
attenuated virus can
be present compared to the other constructs. For example, dengue-4 can be
several fold pfu
higher than other dengue viruses because it can demonstrate a reduced
response.
23
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
Methods
Nucleic Acid Amplification
[00086] Nucleic acids may be used in any formulation or used to generate
any
formulation contemplated herein. Nucleic acid sequences used as a template for
amplification can be isolated viruses (e.g. dengue viruses), according to
standard
methodologies. A nucleic acid sequence may be genomic DNA or fractionated or
whole
cell RNA. Where RNA is used, it may be desired to convert the RNA to a
complementary
cDNA. In some embodiments, the RNA is whole cell RNA and is used directly as
the
template for amplification. Any method known in the art for amplifying nucleic
acid
molecules is contemplated (e.g., PCR, LCR, Qbeta Replicase, etc).
Expressed Proteins or Peptides
[00087] Genes can be expressed in any number of different recombinant DNA
expression systems to generate large amounts of the polypeptide product, which
can then be
purified and used in methods and compositions reported herein. Any method
known in the
art for generating and using constructs is contemplated. In certain
embodiments, genes or
gene fragments encoding one or more polypeptide may be inserted into an
expression vector
by standard cloning or subcloning techniques known in the art.
[00088] Proteins, peptides and/or antibodies or fragments thereof may be
detected or
analyzed by any means known in the art. In certain embodiments, methods for
separating
and analyzing molecules may be used such as gel electrophoresis or column
chromatography methods.
Electrophoresis
[00089] Electrophoresis may be used to separate molecules (e.g., large
molecules
such as proteins or nucleic acids) based on their size and electrical charge.
There are many
variations of electrophoresis known in the art. A solution through which the
molecules
move may be free, usually in capillary tubes or it may be embedded in a matrix
or other
material known in the art. Common matrices can include, but are not limited
to,
polyacrylamide gels, agarose gels, mass spec, blotting and filter paper.
[00090] Some embodiments, using a gene or gene fragment encoding a
polypeptide
may be inserted into an expression vector by standard subcloning techniques.
An
expression vector may be used which produces the recombinant polypeptide as a
fusion
protein, allowing rapid affinity purification of a peptide or protein.
Examples of such
fusion protein expression systems are the glutathione S-transferase system
(Pharmacia,
24
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
Piscataway, NJ), the maltose binding protein system (NEB, Beverley, MA), the
FLAG
system (IBI, New Haven, CT), and the 6xHis system (Qiagen, Chatsworth, CA).
Pharmaceutical Formulations
[00091] Any pharmaceutical formulation known in the art for a vaccine is
contemplated herein. In certain embodiments, a formulation can contain one or
more
dengue virus serotype in various ratios in a single vaccine. It is
contemplated that
formulations can contain other agents of use in vaccination of a subject
including, but not
limited to other active or inactive ingredients or compositions known to one
skilled in the
art.
[00092] All contemplated vaccinal viruses herein can be administered in
the form of
vaccinal compositions which can be prepared by any method known to one skilled
in the
art. In certain embodiments, the virus compositions are lyophilized and are
mixed with a
pharmaceutically acceptable excipient (e.g. water, phosphate buffered saline
(PBS), wetting
agents etc.) In other embodiments, vaccine compositions can include
stabilizers that are
known to reduce degradation of the formulation and prolong shelf-life of the
compositions.
[00093] In other embodiments, an adjuvant may be added to the composition
to
induce, increase, stimulate or strengthen a cellular or humoral immune
response to
administration of a vaccination described herein. Any adjuvant known in the
art that is
compatible with compositions disclosed herein is contemplated.
[00094] Some embodiments herein concern amounts or doses or volumes of
administration of a tetravalent dengue virus composition and the amount or
dose can depend
on route of administration and other specifications such as the subject
getting the vaccine
(e.g. age, health condition, weight etc.).
[00095] It is contemplated herein that compositions described can be
administered to
a subject living in an area having dengue virus, a subject traveling to an
area having dengue
virus or other subject such as any human or animal capable of getting dengue
fever or other
dengue virus condition. In certain embodiments, it may be recommended that a
subject
traveling to an area having dengue virus is administered one or more vaccine
compositions
(e.g. two or more on Day 0) about 1 to about 3 months prior to dengue virus
exposure.
Vaccines herein can be administered as a prophylactic treatment to prevent
infection in
adults and children. A subject can be naïve or non-naïve subject with respect
to exposure to
dengue virus and vaccine regimens disclosed herein.
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
Kits
[00096] Other embodiments concern kits of use with the methods (e.g.
methods of
application or administration of a vaccine) and compositions described herein.
Some
embodiments concern kits having vaccine compositions of use to prevent or
treat subjects
having been exposed or suspected of being exposed to one or more dengue
viruses. In
certain embodiments, a kit may contain one or more than one formulation of
dengue virus
serotype(s) (e.g. attenuated vaccines, trivalent or tetravalent formulations,
DENVaxTM) at
predetermined ratios. Kits can be portable, for example, able to be
transported and used in
remote areas such as military installations or remote villages in dengue
endemic areas.
Other kits may be of use in a health facility to treat a subject having been
exposed to one or
more dengue viruses or suspected of being at risk of exposure to dengue virus.
[00097] Kits can also include a suitable container, for example, a vessel,
vials, tubes,
mini- or micro fuge tubes, test tube, flask, bottle, syringe or other
container. Where an
additional component or agent is provided, the kit can contain one or more
additional
containers into which this agent or component may be placed. Kits herein will
also
typically include a means for containing the agent (e.g. a vessel),
composition and any other
reagent containers in close confinement for commercial sale. Such containers
may include
injection or blow-molded plastic containers into which the desired vials are
retained.
Optionally, one or more additional agents such as immunogenic agents or other
anti-viral
agents, anti-fungal or anti-bacterial agents may be needed for compositions
described, for
example, for compositions of use as a vaccine against one or more additional
microorganisms.
[00098] In other embodiments, kits can include devices for administering
one or
more vaccination to a subject such as an ID, SQ, IM, an inhaler, intranasal
applicator or
other device for administering a vaccine composition disclosed herein.
[00099] In other embodiments, a single vaccine composition of at least
three serotypes
of live, attenuated dengue virus, or fragments thereof for use in rapidly
inducing an immune
response in a subject against at least three dengue virus serotypes, wherein
at least two doses
of the single vaccine composition are to be administered in two or more
anatomical locations
on the same day of a subject in need thereof, inducing neutralizing antibodies
in the subject
against at least three dengue virus serotypes. In certain embodiments, the
single vaccine
composition can contain at least one additional booster administration of a
formulation of a
live, attenuated dengue vaccine is to be administered 1 to 180 days after the
simultaneous
administrations. In other embodiments, the single vaccine composition can
include a
26
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
predetermined ratio of monovalent vaccines for the three or more dengue virus
serotypes in
the single vaccine composition. In a single vaccine composition, wherein the
single vaccine
composition comprises equivalent ratios of monovalent vaccines for three or
more dengue
virus serotypes in the single vaccine composition.
[000100] A single vaccine composition can include the formulation of the
live,
attenuated dengue vaccine for the at least one additional booster
administration can be the
same or a different formulation as the first formulation. If different than
the first formulation,
a vaccine composition can include a pre-determined concentration of one or
more
monovalent vaccines for the dengue virus serotypes. Further, concentration of
dengue virus
serotypes can include a higher concentration of one or more dengue virus
serotype than the
formulation used for the same day administrations, wherein the higher
concentration is 2 to
100,000 fold greater concentrations than that used in the single formulation
first
administered. In accordance with these embodiments, two or more anatomical
locations
comprise different anatomical locations using the same mode of administration.
Two or more
anatomical sites can include different anatomical locations using different
modes of
administration. A composition can include a tetravalent single vaccine
composition that
represents all four dengue virus serotypes. In accordance with these
embodiments, a single
vaccine composition can include all four dengue virus serotype(s) at a
predetermined ratio.
The live, attenuated dengue viruses can include one or more dengue-dengue
chimeric viruses
further comprising capsid and non-structural proteins of the attenuated dengue
virus and pre-
membrane and envelope proteins of at least a second dengue virus. The capsid
and non-
structural proteins are from an attenuated dengue-1, dengue-2, dengue-3 or
dengue-4 virus.
[000101] Some embodiments include a kit of one or more of the above
referenced
compositions and one or more device for administration by any mode
contemplated herein.
[000102] The following examples are included to demonstrate certain
embodiments
presented herein. It should be appreciated by those of skill in the art that
the techniques
disclosed in the examples that follow represent techniques discovered to
function well in the
practices disclosed herein. However, those of skill in the art should, in
light of the present
disclosure, appreciate that many changes can be made in the certain
embodiments which are
disclosed and still obtain a like or similar result without departing from the
spirit and scope
herein.
27
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
EXAMPLES
Example 1
[000103] Previous studies revealed that natural infection with each DENV
(dengue
virus) serotype leads to long-lived protection against dengue fever caused by
the
homologous serotype. In certain embodiments, administration of an effective
dengue
vaccine closely mimics natural infection and can serve as a mode for
administering vaccines
against Dengue virus. Embodiments reported herein can concern a natural
infection route of
dengue virus (DENV) infection, similar to intradermal delivery by the
transporting host, a
mosquito bite. In certain embodiments, intradermal injection to deposit the
vaccine viruses
into the same tissue can be used. Skin is a highly accessible organ and
represents an
effective immune barrier, mainly attributed to the presence of Langerhans
cells (LCs)
residing in the epidermis. Skin immunization elicits a broad range of immune
responses,
including humoral, cellular, and mucosal and has the potential to bypass the
effect of pre-
existing immunity on the immunogenicity of administered vaccines.
[000104] Some embodiments for intradermal (ID) administration of the
tetravalent
dengue vaccines in a subject in need of such a treatment are reported. One
exemplary
method of intradermal administration was performed on four Cynomologous
macaques
administered a DENVaxTM ((DENVax-1: 1x105PFU, DENVax-2; 1x105PFU, DENVax3:
1x105PFU, DENVax4: 1 x105PFU) Dengue virus vaccine) by intradermal
administration.
To achieve an equivalent dose of virus, 0.15 ml of vaccine was deposited ID in
three closely
spaced sites using a needle-free jet injector (see Figs. 1 and 2, below). Fig.
1 represents an
intradermal inject (e.g., PharmaJet0 or other intradermal device) device used
for
intradermal inoculations.
[000105] Fig. 2 illustrates inoculation sites on Cynomolgus macaques post
vaccination
with PharmaJet device. Animals were boosted 60 days later with the same
formulation by
the same route. Serum samples were collected at predetermined intervals, days
15, 30, 58,
74, and 91 and were tested for the presence of neutralizing antibodies
directed against the
four Dengue serotypes. PRINT (plaque reduction neutralization test, known in
the art for
quantifying levels of anti-DEN neutralizing antibodies) were performed on the
sera
samples.
[000106] It was demonstrated that the neutralizing antibody titers are
significantly
higher after ID administration as compared to SC administration (p<0.05 for
DENV-1 and
DENV-2) after a primary administration (see Fig.3) or after a secondary
administration
(p<0.05 for all DENV serotypes) (See Fig. 4). Since the sites were closely
spaced in the
28
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
same area, and each innoculum consists of all four viruses, this mode of
vaccine delivery
closely resembles a single administration of DENVaxTM. Fig 3 illustrates
50%PRNT
(plaque reduction neutralization titers) Geometric Mean Titers at Day 58 (58
days after the
primary administration). Fig. 4 illustrates 50%PRNT Geometric Mean Titers at
Day 74 (14
days after the secondary administration on Day 60). As can be seen in the
figures, the
neutralizing antibody titers to all four dengue viruses were higher after
intradermal versus
subcutaneous administration. In addition, the number of animals that
demonstrated
neutralizing antibody responses ("seroconversion" defined as PRNT > 10) was
greater after
the first dose of vaccine (see Table 1, the percentage of animals that sero
converted to each
of the four Dengue serotypes is shown after primary and secondary
immunization).
[000107] Table 1: Seroconversion of non-human primates after dengue
immunization
% S e roc nve rs ion
DEN Vax DEN-1 DEN-2 DEN-3 DEN-4
Formulation Prime Boost Fri m:e Boost Prime Boost Prime
Boost
5.:65: 6 SC 87.5%
100.0% 100.0% 10Ø0% 76.0% 100.0% 50.0% 100.0%
5:5:5:5 ID
10Ø0% 100.0% 100.0% 10Ø0% 100.0% 100.0% 10Ø0% 100.0%
[000108] The immunized animals were tested for protection against challenge
with
wild type dengue viruses. In cynomolgus macaques, wild type dengue virus
infection leads
to virus replication and viremia, but no clinical signs. At day 91, two
monkeys were
challenged with DENV-1 (Dengue virus serotype 1) and two monkeys challenged
with
DEN-2 (Dengue virus serotype 2). Serum samples were collected daily for 11
days after
challenge. Levels of dengue virus RNA were measured in the samples by
quantitative real-
time polymerase chain reaction technology (q-rtPCR) and titers of viable virus
were
measured by virus isolation and plaque formation on Vero cells. The results
are shown in
Tables 2 and 3. Neutralizing antibodies against DEN-1 at Day 91, just prior to
challenge
("Pre-Challenge") and Day 105, 14 days after challenge ("Post"). Viremia is
given as the
number of days that live DEN-1 virus could be isolated from blood samples
("Duration")
and the log10 of the peak titer isolated from each animal. Viral RNA is given
as the
number of days viral RNA could be detected in the serum samples ("Duration")
and peak
viral RNA levels in each monkey, expressed as the log10 of the number of viral
RNA
genomes detected.
29
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
Table 2: Responses after challenge with DEN-1
DEN-1 PRN7 Vire:mia Viral RNA
Monkey Formulation Pre-Chailenge Post Duration Peak Duration Peak
0Y0174 55.E.5 SC 240 240 0 0 0 0
.CY0181 51.5:.55 SC 640 81440 0 0 5 Tie
CY0192 5.5:E3 1920 1280 0 0 o 0
.CY01.94 55:5,5 0 7680 1920 0 0 0 0
CY0.06.1 .Controls I 2560 6 2,0 9 5,7
.CY0193 .C-oatrols 25. 756D 3 2.7 7
.CY0052 Controts 1 640 5 2.9 7 5.5
0Y0073 .Controls I 1280 5 3,6 10 6,2
Table 3: Responses after challenge with DEN-2
DEN-2 PRNT Viremia Viral RNA
Monkey Formulation Pre-Chalienge Post Duration Peak Dti ration Peak
CY0172 5:5:5:5 SC 3413 3.413 0 0 1 3.9
CM 77 51515:5 SC 253 533. 0 0 0 0
CY0192 5:5,515 D 24C.I 320 CI 0 0 0
CY0201 55.5.5D 1920 1600 0 0 0 0,
cynaso controls $ 1 02,4o 6 2.3 2 :5.1
CY0199 Contro18. 1 am 5 1.3 9 4.7
CYO-05 Controls 1 10240 5 2,9 8 5.8
CY0104 Controls- 1 10240 4 2.4 8 5.7
[000109] After challenge, the SC and ID immunized animals were completely
protected from DEN-1 or DEN-2 induced viremia (compared to the control animals
that
demonstrated significant viremia of long duration). In all of the ID immunized
animals, but
not all of the SC immunized animals, there was also an absence of viral RNA
replication
and a lack of an increase in antibody titer after challenge (compare the ID
animals to SC
injected CY0181, CY0172 or the control animals). These data suggest that
protection is
"sterilizing" and prevents any virus replication after challenge.
Example 2
[000110] In another example, an optimized DENVaxTM formulation delivered in
different locations and with different timings will be tested in non-human
primates. Groups
of eight Cynomolgus macaques will be immunized with a DENVaxTM formulation
containing 1 x 105 plaque forming units (pfu), 1 x 104 pfu, 1 x 105 pfu and 1
x 105 pfu of
DENVaxTm-1, DENVaxTm-2, DENVaxTm-3 and DENVaxTm-4, respectively (abbreviated
5:4:5:5). Two doses will be administered in 0.1 ml ID. Groups will be
immunized with
either one dose in each arm at Day 0, one dose in one arm at Day 0 and one
dose in the
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
other arm at Day 7, or one dose in one arm at Day 0 and one dose in the other
arm at Day
60. These groups will be compared to a group that receives the same dose
(5:4:5:5) in three
sites in the same are on Day 0 and three sites in the other arm on Day 60 as
well as a group
that receives the same dose in a single 0.5 ml SC immunization in one arm at
Day 0 and in
the other arm at Day 60. A control group will be immunized with vaccine
excipients only
(no vaccine viruses). Following immunization, blood samples will be collected
on days 0,
7 (for peak viremia), 15, 30, 60, and 90 to test the neutralizing antibodies
against the four
Dengue virus serotypes by PRNT50. PBMCs collected on days 30, 60, 90 will be
also
monitored for IFN-y secretion by an ELISPOT assay. On day 90, two animals from
each
group will be challenged with wild type of DEN-1, DEN-2, DEN-3, or DEN-4
viruses.
Challenged animals will be monitored for clinical signs and temperature (twice
daily),
changes in food consumption (once daily) and body weight (weekly). In
addition, all
animals will be bled daily for 11 days post-challenge to monitor viremia and
hematological
parameters. Again, the speed and duration of PRNT responses to all four DEN
viruses and
protection after day 90 challenge will be assessed. It is believed that
intradermal
administration in multiple sites and in distinct anatomical locations may be
more effective
than subcutaneous administration as a single bolus. Multiple sites can provide
exposure of
the vaccine to more antigen presenting cells. Distinct anatomical locations
can permit
vaccine access to multiple lymph nodes. In addition, booster immunizations of
Dengue
vaccines have only been administered after the development of antibody
responses in mice,
primates and human clinical trials, thirty days or longer. At this time,
neutralizing
antibodies inhibit the response to the live viral vaccines. It was previously
shown that
boosting primates one month after primary immunization was less effective than
dosing
four months after primary immunization. It was speculated that high levels of
homologous
and heterologous antibodies that circulate after the initial immunization can
inhibit viral
replication in a second dose. While prolonged (two months or longer)
immunization may
circumvent this inhibition, it has not been tested whether accelerated
immunization regimen
with shorter immunization intervals, before the development of potent
neutralizing antibody
responses may be advantageous. Such a shortened regimen may be an advantage in
endemic countries or for travelers, where exposure to Dengue viruses in
between the
immunizations may put them at risk of disease.
Example 3
[000111] In another example, a human clinical trial has been initiated,
studying the
safety and immunogenicity of two DENVaxTM formulations, administered in 0.1 ml
either
31
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
by ID or SC injection. Groups of 12 individuals will be immunized with for
example, a low
dose DENVaxTM formulation (8 x 103 pfu, 5 x 103 pfu, 1 x 104 pfu and 2 x105
pfu of
DENVaxTm-1, -2, -3 and -4, respectively) or a high dose (2 x 104 pfu, 5 x 104
pfu, 1 x 105
pfu and 3 x105 pfu of DENVaxTm-1, -2, -3 and -4, respectively) of DENVaxTM ID
or SC on
Days 0 and 90. Two control groups will be injected SC or ID with phosphate-
buffered
saline. Patients will be monitored for any adverse events, and for any
significant changes in
hematological or blood chemistry parameters. Serum samples will be collected
to measure
vaccine virus replication and neutralizing antibody responses at periodic
intervals.
Example 4
[000112] Immunogenicity and efficacy of DENVaxTM administered intradermally
in
AG129 mice. In another example, two studies were performed to compare the
effect of
route of administration on immunogenicity and efficacy of DENVaxTM in AG129
mice. In
one example, the immunogenicity of monovalent DENVaxTm-4 (e.g. vaccine against
one
Dengue virus serotype) was compared in AG129 mice by measuring the
neutralizing
antibody responses following SC injection under the skin on the back or ID
injection into
the foot pad using a needle and syringe. Groups of 8 AG129 mice were injected
ID or SC
with 105 PFU/dose of chimeric DENVaxTm-4 vaccine in 50 1 and 100 1 final
volume,
respectively. Six weeks after priming, animals from each treatment group were
boosted via
the corresponding ID or SC route with 105 PFU of DENVaxTm-4 or TFA. Mice were
bled
on Day 31 and 58 and collected sera were pooled to measure neutralizing
antibody
responses.
[000113] Immunization of DENVaxTm-4 via the ID route elicited a 5-fold
higher
neutralizing antibody response to DEN-4 after the boost compared to the
response induced
via the SC route (see for example, Fig. 4). The anti-DEN-4 response elicited
by either route
of immunization had a marked cross-neutralizing activity against DEN-3 but not
against
DEN-1 or DEN-2 serotypes. Fig. 4 represents neutralizing antibody responses
following
primary and secondary immunization of AG129 mice with chimeric DENVaxTm-4.
Mice
were bled on Day 31 and 58 and collected sera were pooled to measure
neutralizing
antibody responses using the plaque reduction assay (PRINTS 0).
[000114] Two weeks after the boost animals from each group were split in to
two
groups and challenged with 106PFU of DEN-1 (Mochizuki virus strain) or DEN-2
(New
Guinea C strain) viruses. Challenged animals were monitored for clinical signs
of disease
and survival rates were recorded over a period of 5 weeks. Mice immunized via
the ID route
showed no signs of disease after DEN-1 challenge (Fig. 5A). In the SC
immunized group
32
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
only one mouse succumbed to infection while the rest of animals had no any
apparent signs
of infection (Fig. 5B). In contrast, all control animals succumbed to
infection by day 13
after DEN-1 challenge (Fig. 5A). Following DEN-2 challenge, all animals
immunized with
only DENVaxTm-4 via the SC route succumbed to infection by day 25 with mean
survival
time (MST) of 19.5 days as compared to the control (FTA) mice that all
succumbed by day
17 (MST=12.5 days) post-challenge (Fig. 5B). In contrast, fifty percent of ID
DENVaxTm-4
immunized mice survived the infection until the end of the 5 week monitoring
period (Fig.
5B). Figs. 5A and 5B represent survivals of DENVaxTm-4 immune AG129 mice
following
challenge with DEN-1 (a) or DEN-2 (b) viruses. Challenged animals were
monitored for
clinical signs of disease and survival rates were recorded over a period of 5
weeks.
[000115] In a second study, immunogenicity of tetravalent DENVaxTM vaccine
administered Sc or ID in mice (e.g. AG129) was tested. Groups of AG129 mice,
six per
group were injected SC or ID with the DENVaxTm in 100 1 or 50 1(fmal volume),
respectively. Mice were immunized with DENVaxTM at a 5:4:5:5 (105 PFU of
DENVaxTm-
1,-3 and -4 and 104 PFU of DENVaxTm-2) dose level of composite chimeric
vaccines. All
immunized animals received a booster injection of 5:4:5:5 DENVaxTM (105 PFU of
DENVaxTm-1,-3 and -4 and 104 PFU of DENVaxTm-2) 42 days' post-primary
inoculation.
Blood samples were collected on days 42 and 56 to measure neutralizing
antibody
responses to each DEN virus serotype.
[000116] As represented in Table 4, both primary and secondary neutralizing
antibody
responses to all four DEN serotypes were induced. Following the boost, the
neutralizing
anti-DEN-1, DEN-3 and DEN-4 antibody titers were increased by 2, 5 and 2 fold,
respectively in the group of mice injected ID as compared to the SC immunized
animals.
Neutralizing responses to DEN-2 virus were comparable in both groups.
Immunization via
the SC route resulted in a profile of dominant neutralizing antibody responses
against DEN-
1>DEN-2>DEN-3>DEN-4, with neutralizing titers 5120, 1280, 640 and 80,
respectively.
The hierarchy of neutralizing antibody responses after ID administration had
shifted as
follows; DEN-1>DEN-3>DEN-2>DEN-4 with neutralizing antibody titers 10240,
3840,
1280 and 160, respectively.
[000117] Table 4: Comparison of the immunogenicity of tetravalent DENVaxTM
bearing the ratio 5:4:5:5 PFU of each composite chimeric virus (105 PFU of
DENVaxTm-1,-
3 and -4 and 104 PFU of DENVaxTm-2) after SC or ID immunization of mice. Blood
samples were collected on days 42 and 56 to measure neutralizing antibody
responses to
each DEN virus serotype.
33
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
Neutralizing Antibody Titers (GMT)
DENVaxTM DEN-1 DEN-2 DEN-3 DEN-4
Formulation Prime Boost Prime Boost Prime Boost Prime Boost
5:4:5:5/SC 1920 5120 3200 1280 1280 640 80 80
N.05A5Manigini.256Ctioing40.240eini4280eigiinit280iniinit60.0ami3840miminOwn160
iiiii]iii
Materials and Methods
[000118] Mice: AG129 mice have an "intact" immune system; deficient for the
interferon (IFN)¨a/13 and ¨y receptors. Dengue infection has been described
for this model.
Other studies: pathogenesis, cell tropism, and ADE have also been examined.
This model
permits challenge with DEN-1 and DEN-2.
[000119] Nonhuman primates: Cynomolgus, rhesus macaques carry virus
(viremia),
but no disease manifests.
Rapid Dosing Study
Example 5
[000120] In one exemplary study, immune responses to tetravalent Dengue
vaccines
were evaluated for different routes of administration and dosing regimens in
the non-human
primate model comparing vaccine delivery by conventional needle injection to
needle-free
administration. The quantifiable endpoints for the nonhuman primate study are
i) the route
for greatest geometric mean neutralizing antibody titer against each of the
four dengue
serotypes in non-human primates and ii) the protection from challenge with two
of the
dengue serotypes.
[000121] Two dosing schedules were evaluated in this study ¨ two
consecutive doses
on Day 0 (at different anatomical sites) were compared to administration of
two doses given
60 days apart (0.60). The high dose formulation of the tetravalent formulation
(e.g.
DENVaxTM) was used for immunization in this study. This vaccine lot is the
same material
used for two Phase 1 studies being conducted. The high dose tetravalent
formulation
vaccine consists of 2x104 pfu of DEN-1, 5x104 pfu of DEN-2, lx105 pfu DEN-3
and 3x105
pfu DEN-4. The study design for the nonhuman primate study is shown in Table
5.
[000122] Table 5. Nonhuman Primate Study
Groupl Treatment Immunizations No. of Challenge on Day 90, Route/
Site(s) for SC route Method of
Dosing
Administration
1 High dose Day 0 Two 3 animals
with wt DENV-2, ID
DENVax (both arms) 3 animals with wt DENV-4 PharmaJet
Injector
34
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
2 High dose Day 0, Day 60 One 3 animals
with wt DENV-2, ID
DENVax (alternate 3 animals with wt
DENV-4 PharmaJet
arms) Injector
3 High dose Day 0, Day 60 One 3 animals
with wt DENV-2, ID
DENVax (alternate 3
animals with wt DENV-4 Needle/Syringe
arms)
4 High dose Day 0 Two 3 animals with wt DENV-
2, SC
DENVax (both arms) 3
animals with wt DENV-4 PharmaJet
Injector
High dose Day 0, Day 60 One 3 animals with wt
DENV-2, SC
DENVax (alternate 3 animals with wt
DENV-4 PharmaJet
arms) Injector
6 High dose Day 0, Day 60 One 3 animals
with wt DENV-2, SC
DENVax (alternate 3
animals with wt DENV-4 Needle/Syringe
arms)
7 PBS Day 0, Day 60 One 3 animals with wt DENV-
2, ID
(alternate 3 animals with wt
DENV-4 PharmaJet
arms) Injector
[000123] Serum
samples were collected after each vaccination and wild type dengue
virus challenge on Days 0, 3, 5, 7, 10, 12, 14, 53, 64, 67, 88, 91, 93, 95,
97, 99, 101, 102
and 104 to analyze the samples for dengue viremia. Serum samples were also
collected on
Days 0, 30, 53, 75, 88 and 104 to determine the levels of neutralizing
antibodies induced by
the tetravalent formulation administered by needle/syringe or the ID injector.
[000124] Serum
samples were collected at specified intervals during the course of the
study. Sera collected on Days 0, Day 30 and Day 88 (pre-boost) have been
assayed for
neutralizing antibodies to Dengue-1, Dengue-2, Dengue-3 and Dengue-4. The GMT
antibody titers are shown below in Table 6.
[000125] Table 6.
Neutralizing antibody titers to all four dengue serotypes for
Days 30, 53, 75 and 88 after one or two immunizations with DENVax.
Dose Schedule Day 30 Post-Dose 1, Day 53 Post-Dose 1,
Group /Route/ Method of Reciprocal GMTs
Reciprocal GMTs
Administration/
DEN-1 DEN-2 DEN-3 DEN-4 DEN-1 DEN-2 DEN-3 DEN-4
Treatment
2 doses (Day 0),
1 80 1280 127 36 63 1280 40 13
PJ ID, DENVax
2 doses (Day 0, 60),
2 18 14 101 11 32 14 22 10
PJ ID, DENVax
2 doses (Day 0, 60),
3 160 36 64 9 45 40 10 5
N/S ID, DENVax
2 doses (Day 0),
4 1016 1016 403 80 640 1280 127 45
PJ SC, DENVax
2 doses (Day 0, 60),
5 154 1816 226 64 285 1280 57 22
PJ SC, DENVax
2 doses (Day 0, 60),
6 80 1140 113 11 80 806 20 28
N/S SC, DENVax
2 doses (Day 0, 60),
7 10 5 6 5 7 5 5 5
PJ ID, PBS
Neutralizing antibody titers of <10 are reported as "5". Serum dilutions
started at 1:10
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
Seroconversion (values in parenthesis) is defined as titer >10 over Day 0<10
baseline titer or a >4-fold rise in
titer if baseline titer on Day 0 was >10.
Dose Schedule Day 75 Post-Dose 1, Day 88 Post-Dose 1,
Group /Route/ Method of Reciprocal GMTs Reciprocal GMTs
Administration/
DEN-1 DEN-2 DEN-3 DEN-4 DEN-1 DEN-2 DEN-3 DEN-4
Treatment
1 2 doses (Day 0),
40 806 25 25 57 1016 25 25
PJ ID, DENVax
2 2 doses (Day 0, 60),
113 28 63 71 160 90 80 45
PJ ID, DENVax
3 2 doses (Day 0, 60),
160 40 57 36 90 101 57 36
N/S ID, DENVax
4 2 doses (Day 0),
403 718 90 57 254 640 90 64
PJ SC, DENVax
2 doses (Day 0, 60),
508 1810 226 113 403 1280 127 80
PJ SC, DENVax
6 2 doses (Day 0, 60),
143 806 71 57 113 4064 32 40
N/S SC, DENVax
7 2 doses (Day 0, 60),
5 5 5 5 5 5 5 5
PJ ID, PBS
Neutralizing antibody titers of <10 are reported as a value of "5".
Serum dilutions for analysis started at 1:10.
Results were generated from duplicates or triplicates.
PJ = exemplary PharmaJet needle-free injector, N/S = needle/syringe;
GMT = Geometric mean titer; ID = intradermal; SC = subcutaneous
Data are presented as geometric mean titer (GMT) standard error (SE)
[000126] All
42 animals in the study were seronegative at the start of the study and
displayed no neutralizing antibody titers to any of the four dengue serotypes
on Day 0. The
results on Day 30 after priming the animals with DENVaxTM showed that animals
receiving
two doses of DENVaxTM on Day 0 (one dose in each arm) by either the ID or SC
route of
administration, displayed a high neutralizing antibody titer to Dengue-1,
Dengue-2 and
Dengue-4 (Groups 1 and 4). Seroconversion rates by day 30 were 100% for both
groups as
compared to groups 2 and 3. Both groups maintained high levels of neutralizing
antibody
responses up to day 88 just prior to virus challenge.
[000127] For
live attenuated vaccines, vaccine virus replication after immunization is
an important measure of vaccine uptake and vaccine safety. Vaccine virus
replication in the
nonhuman primates was evaluated after the first and second immunization with a
live
attenuated tetravalent formulation vaccine (DENVaxTm). Serum samples collected
on Days
0, 3, 5, 7, 10, 12, 14 after the first immunization were tested for the
presence of viral RNA
from the vaccine strains using a qRT-PCR assay (see Table 7).
36
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
[000128] Table 7.
DENVax-2 RNA detected in the serum after primary
immunization with DENVax.
No. Animals Positive for Viral RNA, DENVax-2 Viral
Group Dosing Schedule RNA, Logio GE/mL
Day 5 Day 7 Day 10 Day 12 Day 14
1 2 doses (Day 0), 3/6 4/6 5/6 3/6
PJ ID (3.9-4.8) (4.5-5.3) (3.9-5.2)
(3.8-5.1)
2 2 doses (Day 0, 60),
PJ ID
3 2 doses (Day 0, 60), 1/6 1/6 1/6
N/S ID (3.8) (3.8) (3.8)
4 2 doses (Day 0), 1/6 (3.8) 5/6 5/6 1/6
PJ SC (3.8-5.0) (3.7-5.3) (4.0)
2 doses (Day 0, 60), 1/6 (3.8) 5/6 5/6 5/6 3/6
PJ SC (4.5-5.4) (3.8-5.4) (3.2-4.8)
(3.7-5.0)
6 2 doses (Day 0, 60), 3/6 4/6 3/6 2/6
N/S SC (3.9-5.6) (3.8-5.4) (4.3-5.0)
(3.7-4.2)
7 2 doses of PBS (Day
0, 60), PJ ID
Results are averages from duplicate or triplicate data.
Samples with titers <logio 3.6 were considered negative.
GE/mL = genome equivalents/mL
N/S = needle/syringe; PJ = PharmaJet needle-free injector
[000129] Viral RNA was not detected on Day 0 (pre-vaccination) and Day 3
(post-
immunization). For all groups, viral RNA was detected only for the Dengue-2
serotype
from day 5 to day 14 post-vaccination after the first immunization. For Groups
1, 3, 5 and 6
endpoint titers were not observed by 14 days post-immunization. Peak titers
were observed
on Day 10 for Groups 1 and 4, and on Days 7 and 10 for Groups 5 and 6 (Table
7). Viral
RNA was not detected for any of the groups after the second immunization
evaluated on
Days 64 and 67 (4 and 7 days post-dose 2).
[000130] On Day 90, three animals from each group were challenged with
either wild-
type Dengue-2 or Dengue-4 to demonstrate efficacy upon immunization with the
tetravalent
formulation. Protected animals should exhibit a lack of wild-type Dengue virus
infection
and replication. Wild-type challenge virus (Dengue-2 and Dengue-4) replication
was
analyzed for all of the groups after challenge with 106 PFU wild-type Dengue 2
(New
Guinea C strain) and Dengue 4 (814669 strain) viruses on Days 91, 93, 95, 97,
99, 101, 102
and 104 (Table 8).
37
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
Dengue vaccine (e.g. DENVaxTM.
Table 8. Protection of DENVax-immunized NHPs from wt DENV challenge.
Pre-Challenge Post-Challenge
Vaccination & Antibody Titers (GMT), Day 88 Viremia (logio GE/mL)
Challenge Regimen DEN- DEN- DEN- DEN-
1 2 3
Day 3 Day 5 Day7
4
2 doses on Day 0, DENV-2 -
57 1016 25 25
PJ ID, DENVax DENV-4 - - -
2 doses on Day 0, DENV-2 - - -
160 90 80 45
60, PJ ID, DENVax DENV-4 - - -
2 doses on Day 0, 60, DENV-2 - - -
90 101 57 36
N/S ID, DENVax DENV-4 - - -
2 doses on Day 0, DENV-2 - - -
254 640 90 64
PJ SC, DENVax DENV-4 - - -
2 doses on Day 0, 60, DENV-2 - - -
403 1280 127 80
PJ SC, DENVax DENV-4 - - -
2 doses on Day 0, 60, DENV-2 - - -
113 4064 32 40
N/S SC, DENVax DENV-4 - - -
[000131] Viral RNA of the wild-type challenge viruses was detected only in
Group 7
that had received PBS. For Dengue-2, viral RNA was detected in 3 of 3 animals
on Days 93
to 97. For Dengue-4, viral RNA was detected in only 1 of 3 animals on Day 95.
One
important observation of the groups that were immunized with the tetravalent
formulation is
that no viral RNA for either the Dengue-2 or the Dengue-4 challenge viruses
was observed.
These results suggested that the tetravalent formulations immunization by any
of the dosing
schedules tested conferred immune protection against challenge of both Dengue-
2 and
Dengue-4 wild-type viruses.
[000132] Overall, this nonhuman primate study clearly showed that the novel
dosing
schedule of administering two doses of a tetravalent formulation on Day 0 at
two distinct
sites (e.g. different arms) induced levels of neutralizing antibodies that
were equivalent or
higher than those observed for more traditional dosing schedules of delivering
the prime
and boost immunization 2 to 3 months apart. The onset of the immune responses
was more
rapid for the groups that received two doses on Day 0 and long lasting. The
application of
the needle-free ID or SC injector enhanced the immune responses such that
higher titers
were observed.
Example 6
AG129 mouse studies on rapid immunization
[000133] In another exemplary study, novel dosing schedules were designed
that
explore either administration of two vaccine doses at two distinct sites on a
single occasion
or shorter dosing intervals between two doses of vaccine which will enhance
compliance of
38
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
vaccinated subjects to return for the second immunization. Standard Dengue
vaccines
developed previously typically require three doses over the course of a year
to achieve
robust multivalent Dengue immune responses. With respect to vaccination
schedules
presented herein, response was evaluated for immunization occurring in at
least two
anatomical two sites, and administering, in certain embodiments, a full dose
(see Table 9) at
each site intradermally. This protocol was performed in part to activate
immune cells and
antigen presenting cells in two different lymph nodes on Day 0 to induce
higher levels and
more robust dengue-specific immune responses compared to administering two
doses
intradermally 7, 14 or 42 days apart. In one study two routes of
administration were
compared, SC and ID routes using a conventional 42-day interval between
vaccinations.
The mice were immunized with a low dose formulation of a tetravalent
formulation
(DENVaxTM; 3:3:3:3 ratio of each of the serotypes) which consisted of 103 PFU
of each
Dengue-1, -2, -3, and -4 (e.g. DENVaxTM-1,-2,-3 and -4) in a 0.05 mL volume
given via the
intradermal route (in the foot pad). The in live portion of this study was
conducted prior to
initiation of this contract. The study design is shown in Table 9 below.
Table 9. Study design for AG129 mouse study DEN-012.
Groups Dose Number Qf Imniurnzatwns/Route
vmomogNOMEN.--MMaggEMEggnoggggggggEgNiiiiiibee6r
A DENVaxTM 1 (day 0)/ID 6
(3:3:3:3)
B DENVaxTM 2 (day 0)/ID, giving a full dose into each of two
6
(3:3:3:3) footpads
C DENVaxTM 2 (7 days apart)/ID 6
(3:3:3:3)
D DENVaxTM 2 (14 days apart)/ID 6
(3:3:3:3)
E DENVaxTM 2 (42 days apart)/ID 6
(3:3:3:3)
F FTA (negative 2 (14 days apart)/ID 6
control group)
G DENVaxTM 2 (42 days apart)/SC 6
(3:3:3:3)
[000134] The neutralizing antibody titers to Dengue 1-4 present in the
collected mouse
sera were determined by a microneutralization assay. Sera were collected at
specified time
points throughout the study and the longevity of the immune responses was
studied by
maintaining the study groups until Day 160 (longer than 5 months after study
start). The
results obtained from sera collected on Days 28 and 56 post-immunization are
illustrated in
Table 10.
39
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
Table_.10.,Neutralizing_antibodytiters_(GMTs) to DEN-1, -2, -3 and -4
GMT Day 28
Group Treatment Groups DEN- DEN- DEN- DEN-
A 1 (day 0)/ID 400 100 200 40
2 (day 0)/ID, giving
B a full dose into each 800 200 800 160
of two footpads
C 2(42 days apart)/ID 400 100 200 40
2 (14 days apart)/ID
<20 <20 <20 <20
(negative control)
t ig-DRDEMMDMDEN
:mommmmn=--mn=
K=n no ON-4m u-Niilo N-3 N-4
A 800 200 400 40
3200 400 1600 160
1600 200 800 40
<20 <20 <20 <20
[000135] In previous studies, a conventional dosing schedule of priming
animals was
used on Day 0 and then administering a booster vaccination on Day 42 to
evaluate the
immune responses for the tetravalent dengue vaccine. Both prime and boost
vaccinations
were administered by the subcutaneous (SC) route. This dosing schedule was
included in the
study for comparison to the novel dosing schedules. Initially, one study
(represented in Table
10) compared the SC and ID routes of administration using the conventional
dosing interval
of giving two doses 42 days apart. The results indicate that there is no
significant difference
between the SC and ID routes with respect to neutralizing antibodies induced
in this mouse
model. This study further explored whether two doses administered on Day 0 at
two
anatomical sites (one dose into each of two foot pads) could induce
neutralizing antibody
levels similar to the standard dosing schedule (2 doses 42 days apart)
described above. The
results show that immunization on Day 0 at two sites each, with a full dose of
a tetravalent
formulation of DENVaxTM, via the ID route induced neutralizing antibody levels
to all four
dengue serotypes that are equivalent in magnitude to the conventional dosing
schedule. The
effect of a single vaccine dose administered by the ID route was also studied
(Group A).
Administration of a single dose of DENVaxTM on Day 0 resulted in antibody
responses that
trended slightly lower compared to two doses on Day 0 (compare Groups A and
B).
Increasing the interval between the two doses from 7 to 42 days did increase
antibody
responses beyond the levels observed. Evaluation of the longevity of the
Dengue immune
response revealed that neutralizing antibody titers to all four dengue
serotypes remained at
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
high levels at Day 160 post-immunization independent of route of
administration and dosing
schedule (data not shown).
[000136] Overall, the results suggested that the intradermal route of
administration
induces neutralizing antibody levels equivalent to those observed for the
subcutaneous route.
Further, the administration of two doses on Day 0 at two different sites by
the ID route
induced a robust neutralizing antibody response equivalent to conventional
dosing schedules.
The antibody responses induced were long lasting and decreased only slightly.
The animals
did not display increased morbidity and mortality. This study demonstrated
that
administration of two vaccine doses at two distinct sites is a viable option
for immunization
as the resulting antibody titers and duration of immune responses are
equivalent in magnitude
to those resulting from two doses given 42 days apart. These dosing regimens
will be
beneficial for travelers to dengue endemic regions and others in need of fast
protection from
dengue virus exposure.
Example 7
Another rapid immunization study in AG129 mice
[000137] The objective of this study was to determine whether administering
two
doses at two sites ID on Day 0 will induce higher levels and more robust
Dengue-specific
immune responses compared to administering two doses ID 42 days apart. The
hypothesis
to be tested was whether administration of a full vaccine dose to each of two
sites
intradermally will activate immune cells and antigen presenting cells that
traffic to two
different lymph nodes, thereby reducing interference between the four DENVaxTM
vaccine
components. The design for this AG129 mouse study is shown below in Table 11.
Table 11. Design of Example 7 AG129 mouse study
Mgmng OggggR.-EggggM.-MggNMOMMNtitilbeetir
troup Dose Number of Immurnzations/Route
1 DENVaxIm 2 doses (day 0)/ID using both footpads 8
3:3:3:3
2 DENVaxIm 2 doses (day 0, day 42)/ID using both footpads 8
3:3:3:3
3 DENVaxTM 2 doses (day 0)/ID using both footpads 8
4:3:4:5
4 DENVaxIm 2 doses (day 0, day 42)/ID using both footpads 8
4:3:4:5
FTA (negative 2 doses (day 0)/ID using both footpads 8
control group)
[000138] In this exemplary method, two different vaccine dose levels (low
and medium
dose), were used for immunization using the novel dosing schedule of
administering two
doses on Day 0 compared to two doses 42 days apart. The mice were dosed with
either a low
41
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
dose formulation of DENVaxTM (3:3:3:3) which consisted of 103 PFU of each of
DENVaxTm-
1, -2 , -3, and 4 in a 0.05 mL volume given via the intradermal route (in the
foot pad) or a
medium dose formulation of DENVaxTM (4:3:4:5) which contained 104 PFU of
DENVaxTm-
1, 103 PFU of DENVaxTm-2, 104 PFU of DENVaxTm-3, and 105 PFU of DENVaxTm-4 in
a
0.05 mL volume. On Day 0 all mice were immunized and Groups 2 and 4 were
boosted on
Day 42. Sera for antibody analysis were collected on Days 14, 41 and 56 post-
primary
vaccination and analyzed using a plaque reduction microneutralization assay to
determine the
neutralizing antibody levels to all four dengue serotypes. Immunogenicity
results obtained
from pooled mouse serum samples are shown in Table 12.
Table 12. Neutralizing antibody titers for AG129 mouse study DEN-013
...................... .............................................. .......
oeal .................. ....................... g Antibody Titers (PRNTSO)
.......
...............................................................................
.............................
ii4biiiiite1111111111111111111111111111111111111111111111111111111111111141.fii
iiiiiiiii1111111111111111111111111111111111111111111111111111111111111111111111
1150filiiiiii111111111111111111111111111111111111
iig000p:
un]]mm
1 320 160 80 20 640 640 640 80 800 1600 800 40
2 320 320 40 20 640 640 320 80 800 800 400 40
3 640 80 80 40 2560 640 1280 160 3200 800 800 40
4 320 80 40 20 320 160 640 80 1600 800 800 80
20 20 20 20 20 20 20 20 20 20 20 20
¨ post-infection
[000139] In this example, immunization with either the low or medium dose
tetravalent vaccine (e.g. DENVaxTM) formulation induced neutralizing
antibodies to all four
dengue serotypes at the early check on Day 14 post-vaccination, independent of
administration of one vs. two doses on Day 0. The medium dose DENVaxTM
formulation
induced slightly higher neutralizing antibody titers by Day 28 for Groups 1
and 3
particularly for DEN-1 and DEN-3, that received two doses on Day 0 compared to
groups
that received only a single dose on Day 0 (Groups 2 and 4). The antibody
titers obtained
from sera collected on Day 56 indicate that the neutralizing antibody
responses persisted
and did not wane regardless of whether the animals were boosted on Day 42 or
received
vaccine only on Day 0. The results obtained in this study further support the
application of
the novel dosing schedule of administering two doses on Day 0 at two distinct
sites (e.g.
immunologically).
42
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
Table 13. Neutralizing antibody titers for AG129 mouse study DEN-013.
DEN-1. DEN-2 DEN-3 DEN-4
Day 28 Day 56 Day 28 Day 56 Day
28 Day 56 Day 28 Day 56
2d0) 640 800 320 1600 160 800 20
40
3131313
2{O42) _', 20 8t_:.0 320 800 160 400 40
40
2{d0) 1280 3200 320 800 640 800 80 40
St ud yl 4343
2 {d0,42) 320 1600 1600 80 800 40 so
FTA 2:1O) 40 20 20 20 20 20 20 20
NMS 20 20 20 20 20 20 ==20 20
2 (di)} 800 3200 200 400 8 (i) 1600 160
160
3:3:3:3
2 (d0,42) 400 1600 100 200 200 KnO 40 40
Study 2 _______________________________________________________________
FTA 2 {d0,14) 20 <20 20 .20 20 20 20 .20
NMS 20 20 40 20 20 20 20 20
ELISPOT dengue virus neutralizing titers calculated using 50% NMS cutoff at a
starting
dilution of 1:20. Serum from individual animals within a group were pooled and
tested in
triplicate.
Example 8
[000140] Figs.
9A-9D represent graphs comparing neutralizing antibody titers
achieved in non-human primates after immunization with tetravalent DENVax
containing
DENVax-1 (1x105pfu); DENVax-2 (1x104pfu); DENVax-3 (1x105pfu); DENVax-4
(1x106pfu). Two groups were vaccinated with the needle-free PharmaJet device
via the
subcutaneous route either twice on the same day (0,0) or once on day 0 and
again on day 60
(0,60). Serum was analyzed for presence of antibodies on days 0, 30, 53, 75
and 88, and the
detection of antibodies against four dengue serotypes were analyzed (DEN-1,
DEN-2,
DEN-3, DEN-4).
[000141] In
another example, seronegative human subjects were immunized with two
doses of a tetravalent formulation of DENVax containing DENVax-1 (1x104pfu);
DENVax-
22 (1x103pfu); DENVax3 (1x104pfu); DENVax-4 (1x105pfu). The route of
immunization
was subcutaneous or intradermal, and the vaccinations were given 90 days
apart. Antibody
levels against each of the dengue serotypes were analyzed on days 0, 30, 60,
90 and 120.
The vaccine induced neutralizing antibodies to all four serotypes. However,
the levels of
seroconversion were different when comparing the routes of immunization.
Overall, the
intradermal route of immunization produced appeared to be more "balanced"
immune
43
CA 02915027 2015-12-10
WO 2013/188315 PCT/US2013/045041
responses in this study, with the levels of antibodies being more equivalent
as compared to
the subcutaneous route.
[000142] Fig. 10 represents the data obtained from a human clinical trial
in Colombia.
Seronegative humans were given two doses of a tetravalent formulation of
DENVax
containing DENVax-1 (1x104pfu); DENVax-2 (1x103pfu); DENVax-3 (1x104pfu);
DENVax-4 (1x105pfu) subcutaneously or intradermally. Antibody levels against
each of
the dengue serotypes were analyzed on days 0, 30, 60, 90 and 120.
[000143] In this exemplary method, non-human primates were immunized with
two
doses of a tetravalent vaccine (e.g. DENVaxTM DENVax-1: 2x104pfu, DENVax-2:
5x104pfu, DENVax-3: lx105pfu, DENVax-4: 3x106pfu) either simultaneously on Day
0, or
two separate doses on days 0 and 60. The vaccine induced neutralizing
antibodies to all
four Dengue serotypes. By day 90 post vaccination, the neutralizing antibody
titers of the
two groups were relatively equal (Fig. 11). However, the kinetics of the
immune response
was more rapid in the group which received two immunizations on day 0. The
results
obtained in this study further support the application of the novel dosing
schedule of
administering two doses on Day 0 at two immunologically distinct sites.
[000144] Fig. 11 represents a graph comparing neutralizing antibody titers
achieved in
non-human primates after subcutaneous immunization with tetravalent DENVax
containing
DENVax-1 (1x105pfu); DENVax-2 (1x104pfu); DENVax-3 (1x105pfu); DENVax-4
(1x106pfu). Two groups were vaccinated either twice on the same day (0,0) or
once on day
0 and again on day 60 (0,60). Serum was analyzed for presence of antibodies on
days 0, 28,
58, 73 and 90, and the detection of antibodies against four dengue serotypes
were analyzed
(DEN-1, DEN-2, DEN-3, DEN-4).
Example 9
[000145] Fig. 12 represents analysis of single dose administration versus
dual
administration in separate anatomical locations of dengue virus vaccines. This
data
represents changes in levels of various gene transcripts in a subject after
0,0 or single
injection where the magnitude of change in gene expression is greater for dual
administration (double dose). The genes included in the cluster analysis,
represented in
Cluster 2 include several genes that change in relation to induction of innate
immunity.
Thus, induction of innate immunity and related genes can be greater in a
subject having
dual administration at separate anatomical locations of compositions disclosed
herein. It is
likely that the composition induces a response in multiple anatomical regions
(e.g. lymph
44
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
nodes etc.) reducing interference and affecting multiple genes that
participate in innate
immune responses.
[000146] The four dengue virus serotypes (DENV-1-4) are responsible for the
most
prevalent mosquito-borne viral illnesses in humans worldwide. Tetravalent
vaccines are
under development, but up to the instant application require multiple
immunizations over a
period of 6 months to one year. A rapid immunization strategy (RIS) that
elicits an immune
response to all four DENV serotypes and requires fewer visits to a health
provider, thus
increasing vaccine compliance and inducing rapid seroconversion, would
increase safety for
people in endemic countries as well as protect travelers and military
personnel from
dengue. RIS consisting of two full vaccine doses being administered on the
initial
vaccination visit (day 0) at two different anatomical locations was
investigated using
various tetravalent formulations having predetermined ratios. This vaccination
strategy
resulted in efficient priming and induction of potent neutralizing antibody
responses to all
four dengue virus serotypes of long duration (3 months) as compared to the
traditional
prime and subsequent 2nd dose (boost) several weeks or months later. In
addition, analysis
of innate immune responses following primary immunization support the view
that the
priming efficiency afforded by RIS is a result of a higher magnitude of immune
signatures
stimulated by this immunization protocol as compared to the single dose
immunization.
[000147] Compositions disclosed herein include chimeric dengue virus
compositions
where a backbone of one dengue virus can accommodate one or more of the other
dengue
virus components. Mixtures of these chimeric compositions can be used to
generate
trivalent or tetravalent formulations of use in methods disclosed herein.
[000148] Figs. 13A-13F represent expression levels over time of various
genes
associated with innate immunity in response to single or dual administration
of dengue virus
vaccines. This data illustrates that expression of certain genes are increased
in response to a
dual administration (in separate anatomical locations such as each arm) versus
single
administration of a vaccine composition disclosed herein (e.g. tetravalent
dengue virus
composition). Various genes illustrated include A. interferon-induced 17 kDa
protein
(ISG15), a 15-kDa protein of unique primary amino acid sequence; B. IF144
(interferon-
induced protein 44); C. XAF1, XAF1 antagonizes the anticaspase activity of
XAP1; D.
OASL (The human 2',5'-oligoadenylate synthetase-like gene (OASL) encoding the
interferon-induced 56-kDa protein); E. MX1 is interferon-induced GTP-binding
protein
Mxl is a protein that in humans is encoded by the MX1 gene and F. IFIT1, IFN
induced
protein with tetratricopeptide repeat 1. Therefore, this method of
administration could be
CA 02915027 2015-12-10
WO 2013/188315
PCT/US2013/045041
used to rapidly induce innate immune response in a subject in need thereof
such as a tourist
visiting a dengue endemic country.
[000149] Table 14
Rank Gene Gene name Fold
symbol
change
1 IF144 IFN-induced protein 44 2.96
2 DDX58 DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 1.95
3 IFIT1 IFN-induced protein with tetratricopeptide repeat 1
1.90
4 OASL 2'-5'-oligoadenylate synthetase-like 1.79
MX] Myxovirus (influenza virus) resistance 1, IFN- 1.77
inducible
6 ISG15 ISG15 ubiquitin-like modifier 1.76
7 XAF 1 XIAP-associated factor 1 1.72
8 SECTM1 Secreted and transmembrane 1 1.65
9 1F127 Interferon, alpha-inducible protein 27 1.62
EIF2AK2 Eukaryotic translation initiation factor 2-alpha kinase 2 1.59
11 OAS2 2' ,5' -oligoadenylate synthetase 1.59
12 IFI6 Interferon, alpha-inducible protein 6 1.47
13 RNF213 Ring finger protein 213 1.45
****************************
All of the COMPOSITIONS and METHODS disclosed and claimed herein can be made
and
executed without undue experimentation in light of the present disclosure.
While the
compositions and methods have been described in terms of preferred
embodiments, it is
apparent to those of skill in the art that variations maybe applied to the
COMPOSITIONS and
METHODS and in the steps or in the sequence of steps of the methods described
herein
without departing from the concept, spirit and scope herein. More
specifically, certain agents
that are both chemically and physiologically related may be substituted for
the agents
described herein while the same or similar results would be achieved. All such
similar
substitutes and modifications apparent to those skilled in the art are deemed
to be within the
spirit, scope and concept as defined by the appended claims.
46