Note: Descriptions are shown in the official language in which they were submitted.
CA 02915485 2015-12-17
SYSTEM AND METHOD FOR PRODUCING BLOCK ICE TREATED WITH
NITROGEN SUBSTITUTION
FIELD OF THE INVENTION
[0001] The
present invention relates to a system and a method for producing
pillar-shaped ice, or block ice, by means of freezing water in which dissolved
oxygen is
substituted with nitrogen.
BACKGROUND ART
[0002]
Japanese Unexamined Patent Application Publication No. 2007-155172 discloses
that the surface of water in a fish hold is covered with nitrogen-gas-filled
ice, which is
produced by freezing water containing nitrogen gas, and that the nitrogen-gas-
filled ice thaws
to reduce the amount of dissolved oxygen in the water in the hold, allowing
for keeping fish
fresh.
[0003]
Japanese Unexamined Patent Application Publication No. 2007-282550 discloses
that nitrogen gas is dissolved in marinade for processing fresh food, which is
then frozen in an
icemaker to become ice for covering the surface of a marinade tank, and that
the ice thaws to
reduce the amount of dissolved oxygen in the water in the tank, allowing for
enhanced
protection of fresh food from oxidizing and spoiling.
[0004]
Meanwhile, the maximum standardized ice commonly available in Japanese
market is block ice weighing 135 kg, which is a pillar-shaped ice 280 mm long,
550 mm wide,
and 1080 mm high.
[0005] Such
large-sized pillar-shaped ice having over dozens of centimeters of each side,
which is described herein as block ice, is produced by filling an ice can
having a
predetermined size with water as material and immersing the ice can in a brine
tank so that
brine surrounds the ice can. The brine is a solution of, for example, calcium
chloride, and
1
CA 02915485 2015-12-17
kept at about ¨8 C to ¨12 C. The water in the ice can is frozen with the
brine. During the
time of freezing, blowing air to stir the water with an air pipe placed in the
water helps
bubbles and impurities existing in the water to rise and to discharge in the
atmosphere as well
as enhancing cooling efficiency. In order to obtain block ice with high
transparency,
freezing is normally performed over 36 to 72 hours.
[0006] It is known, however, that the method with aeration cannot produce
highly
transparent ice. Japanese Unexamined Patent Application Publication No. H6-
101943
discloses a way to freeze material water while applying ultrasonic wave under
negative
pressure for producing transparent block ice. Japanese Unexamined Patent
Application
Publication No. 2011-112579 discloses a way to reduce in steps the number of
revolutions of
a water stirring unit provided in an ice can for producing transparent block
ice.
[0007] Japanese Unexamined Patent Application Publication No. 2007-225127
discloses
a way to freeze water containing micro bubbles of gas such as air, nitrogen,
oxygen, carbon
dioxide, or ozone, for producing gas-containing ice with bubbles being
confined as they are,
by which, however, transparent ice cannot be produced.
SUMMARY OF THE INVENTION
[Problems to be Solved by the Invention]
[0008] Among nitrogen-gas-filled ice currently available are block ice with
a diameter of
dozens of millimeters and small-sized ice crushed from plate ice with a
thickness of dozens of
millimeters. The art is yet to be presented to produce large-sized block ice
with low
concentration of dissolved oxygen while maintaining the same level of
transparency as
conventionally obtained.
[0009] The aeration that has been conventionally used in producing block
ice serves to
discharge bubbles in the water with help of stirring and upward current.
Nonetheless, just
replacing the conventional aeration with injection of nitrogen gas is not
sufficient to produce
block ice that is adequately treated with nitrogen substitution (condition in
which nitrogen in
2
CA 02915485 2015-12-17
exchange for oxygen is dissolved).
[0010] In view of the above problems, the present invention has an object
to provide a
system and a method for producing large-sized pillar-shaped ice that is
adequately treated
with nitrogen substitution.
[Means of Solving the Problems]
[0011] According to a first aspect of the present invention, a system for
producing block
ice that is treated with nitrogen substitution includes (a) a nitrogen gas
supplying unit
provided with a feeder for supplying nitrogen gas under a predetermined
pressure, (b) a
nitrogen-dissolved water producing unit arranged for producing nitrogen-
dissolved water,
which is provided with a water receiving tank for storing material water, a
cooler for cooling
the water stored in the water receiving tank, and a nitrogen gas injector for
injecting nitrogen
gas supplied from the nitrogen gas supplying unit into the water stored in the
water receiving
tank, and (c) a nitrogen-substitution block ice producing unit provided with a
plurality of ice
cans immersed in a brine tank that is kept at a freezable temperature for
water, a filling device
for filling each of the ice cans with the nitrogen-dissolved water supplied
from the
nitrogen-dissolved water producing unit, and a gas injector for injecting
nitrogen gas supplied
from the nitrogen gas supplying unit into each unfrozen portion of the
nitrogen-dissolved
water. The amount of dissolved oxygen in the nitrogen-dissolved water produced
in the
nitrogen-dissolved water producing unit is preferably not more than 0.3 mg/L
at a temperature
in the vicinity of 0 C.
[0012] In the above first aspect, the filling device for filling each of
the ice cans with the
nitrogen-dissolved water has a pouring tank for storing the nitrogen-dissolved
water supplied
from the nitrogen-dissolved water producing unit, and a plurality of pouring
ports formed at
the bottom of the pouring tank in such a way that each one of the ice cans is
filled with the
nitrogen-dissolved water through each one of the pouring ports.
[0013] According to a second aspect of the present invention, a method for
producing
block ice that is treated with nitrogen substitution includes a first step of
producing cooled
3
CA 02915485 2015-12-17
nitrogen-dissolved water that is produced by cooling stored material water
while injecting
nitrogen gas into the water, and a second step wherein an ice can kept at a
freezable
temperature for water is filled with the nitrogen-dissolved water, which is
frozen while
receiving injection of nitrogen gas into its unfrozen portion at least for a
certain frame of time
from start to finish freezing. The amount of dissolved oxygen in the nitrogen-
dissolved
water produced in the first step is preferably not more than 0.3 mg/L at a
temperature in the
vicinity of 0 C.
[0014] In
the above second aspect, injection of nitrogen gas into the unfrozen portion
of
the nitrogen-dissolved water is stopped halfway through freezing the nitrogen-
dissolved
water.
[0015] In
the above second aspect, a duration of time from start to finish freezing the
nitrogen-dissolved water is 48 hours for producing pillar-shaped block ice
measuring 280 mm
long, 550 mm wide, and 1080 mm high.
[Effects of the Invention]
[0016] In
the present invention, material water is cooled while receiving injection of
nitrogen gas to produce cooled nitrogen-dissolved water, which is frozen while
further
receiving the injection. This
makes it possible to produce block ice in which
dissolved-oxygen is contained at a level fully lower than usual.
[0017]
Block ice treated with nitrogen substitution, similar to small-sized nitrogen-
gas-filled ice, is used to store various fresh food and other food and
contributes to preserving
freshness. For example, the block ice is capable of preventing and inhibiting
oxidative
deterioration of fresh food and capable of suppressing propagation of various
bacteria.
Further, large-sized block ice has many use which small-sized ice does not
have. For
example, when kept in an ice chamber, the block ice itself plays a role as
cooling source. In
this case, less dissolved oxygen contributes to reduce adverse effect,
compared with general
ice, on its surrounding environment (e.g. fresh food kept in the ice chamber)
caused by
oxygen coming out when thawing.
4
CA 02915485 2015-12-17
BRIEF DESCRIPTION OF THE DRAWFNGS
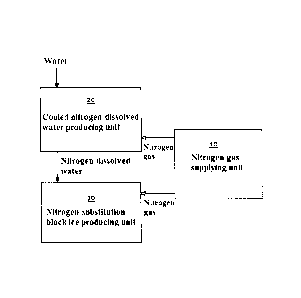
[0018]
Fig.1 is a schematic view showing a system for producing block ice treated
with
nitrogen substitution in accordance with an embodiment of the present
invention.
Fig.2 is a schematic view showing an example of arrangement of the nitrogen
gas supplying
unit shown in Fig.1
Fig.3 is a schematic view showing an example of arrangement of the cooled
nitrogen-dissolved water producing unit shown in Fig.1 .
Fig.4 is a schematic view showing an example of arrangement of the nitrogen-
substitution
block ice producing unit shown in Fig.l.
Fig.5 is a flowchart showing a preferred example of steps of producing block
ice treated with
nitrogen substitution by using the systems shown in Figs. 1 to 4.
Fig.6 is a photo showing a working example of block ice treated with nitrogen
substitution in
accordance with an embodiment of the present invention.
Fig.7 is a photo showing conventional block ice for comparison.
DESCRIPTION OF THE EMBODIMENTS
[0019] A
preferred embodiment of the present invention will be described with reference
to the accompanying drawings. The block ice to which the present invention is
applied is ice
which can be called "ice pillar" in the shape of cuboid including cube. The
maximum
standardized ice commonly available in Japanese market is highly transparent
block ice
weighing 135 kg, which is a pillar-shaped ice 280 mm long, 550 mm wide, and
1080 mm high.
It should be noted that the block ice to which the present invention is
applied is not limited to
the ice having the above size though reference will be made hereinafter taking
the ice with the
CA 02915485 2015-12-17
above size as an example. If the length of each axis of a block ice is within
the range of
20% of that of the above standardized block ice, such block ice is considered
equal to the
standardized block ice. In addition, the present invention is also applied to
the block ice
weighing over about 20 kg.
[0020] Fig.1 is a schematic view showing a system for producing block ice
treated with
nitrogen substitution in accordance with an embodiment of the present
invention, in which
white arrows indicates flow of gas and black arrows indicate flow of liquid
(hereinafter the
same is applied). The system for producing block ice treated with nitrogen
substitution
includes a nitrogen gas supplying unit 10, a cooled nitrogen-dissolved water
producing unit
20, and a nitrogen-substitution block ice producing unit 30. The nitrogen gas
supplying unit
is provided with a feeder for supplying nitrogen gas under a predetermined
pressure. The
cooled nitrogen-dissolved water producing unit 20 is arranged to produce
cooled
nitrogen-dissolved water by using material water and the nitrogen gas supplied
from the
nitrogen gas supplying unit 10. The nitrogen-substitution block ice producing
unit 30 is
arranged to produce block ice having fully-lowered level of dissolved oxygen
by using the
cooled nitrogen-dissolved water supplied from the nitrogen-dissolved water
producing unit 20
and the nitrogen gas supplied from the nitrogen gas supplying unit 10.
[0021] The word "nitrogen substitution" of water as used herein is intended
to define
lowering a normal level of dissolved oxygen in water, which is determined in
accordance with
temperature under atmospheric pressure, and substituting nitrogen for the
amount equivalent
to the reduced dissolved oxygen. Likewise, the word "nitrogen-dissolved water"
is to define
the water having reduced dissolved oxygen and increased dissolved nitrogen
compared with
normal water, or the water in which the dissolved oxygen is substituted with
dissolved
nitrogen. Likewise, the words "nitrogen-substitution ice" and "ice treated
with nitrogen
substitution" are intended to define ice produced by freezing the nitrogen-
dissolved water
while maintaining the lowered level of dissolved oxygen. The lowered level of
dissolved
oxygen is intended to define not more than 0.3 mg/L of dissolved oxygen.
[0022] Fig.2 is a schematic view showing an example of arrangement of the
nitrogen gas
6
CA 02915485 2015-12-17
supplying unit 10 shown in Fig. 1. The nitrogen gas supplying unit 10 includes
an air
compressor 11 for compressing atmosphere, a nitrogen gas generator 12 for
extracting
nitrogen gas from the compressed air, and a nitrogen gas tank 13 for storing
the extracted
nitrogen gas. As to the air compressor 11, for example, Oil Free "BEBICON"
(Registered
Trademark) of Hitachi Industrial Equipment System Co., Ltd., may be used. The
compressor capable of supplying air pressure of 0.5 to 0.9 MPa is used.
[0023] In
the nitrogen gas generator 12, pressed air is taken in through one end of a
pressure vessel provided with a nitrogen separating membrane made of polyimide
hollow
fiber membrane, and oxygen is purged from an opening on the lateral side of
the vessel,
taking out nitrogen from the other end of the vessel. As to the nitrogen gas
generator 12,
which is based on different permeability rate particular to each kind of gas,
for example,
"Ripureru" (Registered Trademark) of KATAYAMA CHEMICAL, INC. may be used.
[0024] The
nitrogen gas tank 13 is for storing nitrogen gas and provided with a regulator
so as to supply the gas under a predetermined pressure. The nitrogen gas tank
13 is capable
of supplying nitrogen gas separately to the cooled nitrogen-dissolved water
producing unit 20
and the nitrogen-substitution block ice producing unit 30. A valve accordingly
provided on
respective nitrogen gas supplying lines 14 and 15 is used to switch on and off
supplying
nitrogen gas.
[0025]
Fig.3 is a schematic view showing an example of arrangement of the
nitrogen-dissolved water producing unit 20 shown in Fig.1 . The nitrogen-
dissolved water
producing unit 20 includes a water receiving tank 21 for storing material
water which is
supplied through a water supplying line 25, a water cooler 22 for cooling
water W stored in
the water receiving tank 21 by means of circulation through a water
circulating line 29 and a
pump 23, and a nitrogen gas injecting pipe 28 for injecting nitrogen gas which
is supplied
from the nitrogen gas supplying unit 10 through a nitrogen gas supplying line
27.
[0026] The
nitrogen gas injecting pipe 28 is, for example, a long pipe which extends
horizontally in the water receiving tank 21 and has a porous wall for jetting
nitrogen gas.
7
CA 02915485 2015-12-17
Nitrogen gas injected into water under high pressure has an effect of raising
dissolved oxygen
and bubbles to release into the air upon dissolving in water. In this way, the
level of
dissolved oxygen in the water W in the water receiving tank 21 is lowered
whereas that of
dissolved nitrogen is increased, producing nitrogen-dissolved water.
[0027] The
water W in the water receiving tank 21 is preferably cooled down to the
vicinity of above 0 C with the water cooler 22, enabling effective start of a
freezing step later
in the nitrogen-substitution block ice producing unit 30. Further, cooling the
water W to the
vicinity of 0 C while injecting nitrogen gas makes it possible to suppress
dissolved oxygen
and to increase dissolved nitrogen compared with a normal condition in which
dissolved
oxygen increases in proportion to decrease in temperature.
[0028] In a
working example, the nitrogen-dissolved water containing 0.3 mg/L of
dissolved oxygen at the vicinity of 0 C was obtained from the material water
containing 99.7
mg/L of dissolved oxygen at room temperature by processing in the water
receiving tank 21.
In another working example, it was confirmed that the nitrogen-dissolved water
containing
0.3 mg/L of dissolved oxygen was obtained. The amount of dissolved oxygen in
normal
water at 0 C is 14.6 mg/L.
[0029] The
cooled nitrogen-dissolved water in the water receiving tank 21 is supplied to
the nitrogen-substitution block ice producing unit 30 through a nitrogen-
dissolved water
supplying line 26 and a pump 24.
[0030]
Fig.4 is a schematic view showing an example of arrangement of the
nitrogen-substitution block ice producing unit 30 shown in Fig. 1. The
nitrogen-substitution
block ice producing unit 30 includes a pouring tank 31 for temporarily storing
the cooled
nitrogen-dissolved water transferred from the nitrogen-dissolved water
producing unit 20
through a nitrogen-dissolved water supplying line 34, a plurality of ice cans
32 for filling with
the cooled nitrogen-dissolved water, and a brine tank 33 for immersing the ice
cans 32.
[0031] The
pouring tank 31 is provided with a plurality of pouring ports 35 at the bottom
8
CA 02915485 2015-12-17
in such a way that each one of the pouring ports is assigned to each one of
the ice cans 32.
Each one of the pouring ports 35 is placed right above an upper opening of
each one of the ice
cans 32. The pouring ports 35 are controlled to close when storing the
nitrogen-dissolved
water in the pouring tank 31 and to open when filling the ice cans 32 with the
nitrogen-dissolved water from the pouring tank 31.
[0032] Each one of the ice cans 32 has a predetermined shape and size so as
to produce
one piece of block of ice and immersed in the brine tank 33. The brine tank 33
is a tank
filled with brine which is, for example, calcium chloride solution. The brine
is cooled to a
predetermined temperature with an external cooler (not shown). The temperature
of brine to
be set is adequate for freezing the nitrogen-dissolved water in the ice cans
32 to produce
high-quality block ice.
[0033] In each of the ice cans 32, an injection pipe 37 is provided for
injecting into the
water the nitrogen gas supplied the nitrogen gas supplying unit 10 through a
supplying line 36.
The injection pipe 37 is, for example, a long pipe which extends vertically
into the ice cans 32
and has porous walls for jetting nitrogen gas. In this way, the amount of
dissolved oxygen is
further decreased whereas the amount of dissolved nitrogen is increased in an
unfrozen
portion of the nitrogen-dissolved water in the ice cans 32 while being frozen.
Injection of
nitrogen gas in the ice cans 32 enables bubbles in the water to be raised and
released into the
air for preventing bubbles and impurities from being taken in block ice,
producing highly
transparent block ice. Opaque portion is often seen in the central portion of
general block
ice that is produced only by means of aeration; however the block ice
according to the present
invention is more transparent than the general one. In addition, effect of
adequate stirring
serves to enhance cooling efficiency.
[0034] Fig.5 is a flowchart showing a preferred example of steps of
producing block ice
treated with nitrogen substitution by using the systems shown in Figs. 1 to 3
or 4. The size
of block ice produced in the example is 280 mm long, 550 mm wide, and 1080 mm
high.
[0035] First, the water receiving tank is filled with material water (Step
S01). After
9
CA 02915485 2015-12-17
filling, the tank may be allowed to stand for several hours to several tens of
hours so as to
decrease bubbles by rising and going into the air by themselves.
[0036]
Second, the water in the tank is cooled to the vicinity of above 0 C while
receiving injection of nitrogen gas (Step S02). The step is carried out taking
time adequate
to the amount of the water for producing cooled nitrogen-dissolved water.
[0037]
Next, the cooled nitrogen-dissolved water in the water receiving tank is
transferred to and filled with the pouring tank (Step S03). After filling the
pouring tank, the
pouring ports of the poring tank are opened to fill the ice cans positioned
thereunder with the
cooled nitrogen-dissolved water (Step SO4). The ice cans immersed in the brine
tank are
already kept at a temperature adequate for cooling, or a freezable temperature
for water, by
the time of filling nitrogen-dissolved water. The freezable temperature for
water is ¨12 C
for example.
[0038]
Subsequently, the nitrogen-dissolved water in the ice cans is cooled while
receiving injection of nitrogen gas into an unfrozen portion of the water. The
brine tank is
kept at a constant temperature until freezing is complete. In the preferred
example, it takes
48 hours from start to finish freezing the nitrogen-dissolved water in the ice
cans. Freezing
with injection of nitrogen gas is advanced up to a certain point of time (Step
S05).
Preferably, the injection is continued until freezing is almost finished. When
the injection is
stopped, the injecting pipe is preferably taken out from the ice cans. After
stopping the
injection, freezing the nitrogen-dissolved water is finished (Step S06),
followed by taking out
of block ice that is treated with nitrogen substitution from the ice cans
(Step S07).
[0039] With
the freezable temperature for water, the overall freezing time, and the time
of
injecting and non-injecting nitrogen gas according to the above preferred
embodiment,
high-quality block ice that is highly transparent and sufficiently treated
with nitrogen
substitution can be produced. The overall freezing time in this case is
shorter than that for
producing conventional block ice produced by means of aeration under the same
conditions in
size and temperature.
CA 02915485 2015-12-17
[0040] The present invention is not limited to the above preferred example
and the
temperature of brine may be accordingly changed. Likewise, the time from start
to finish
freezing, the time of freezing with injection of nitrogen gas, and the time of
freezing without
injection of nitrogen gas may be accordingly changed as necessary. For
example, under the
condition of 48 hours of overall freezing time and brine temperature at ¨10 C,
freezing may
be done with injection of nitrogen gas during a first half (24 hours) of the
overall freezing
time whereas freezing during a latter half (24 hours) may be done without the
injection. In
another example, under the condition of 72 hours of overall freezing time and
brine
temperature at ¨8 C, injection of nitrogen gas is continued from starting
freezing to 60 hours
later, and then, the injection is stopped for last 12 hours.
[0041] Fig.6 is a photo taken from above the block ice treated with
nitrogen substitution
that was produced in the steps of Fig.5. Fig.7 is a photo taken from above a
block ice that
was produced with a conventional method in the same size as the block ice
shown in Fig.6.
The conventional method is to freeze normal water with aeration. Dust is
filtered and removed
from the air used in the aeration in the conventional method. Comparing Fig.6
with Fig.7
reveals that the block ice treated with nitrogen substitution is more
transparent than the
conventional block ice.
Description of the Reference Numeral
[0042]
nitrogen gas supplying unit
11 air compressor
12 nitrogen gas generator
13 nitrogen gas tank
14, 15 nitrogen gas supplying line
nitrogen-dissolved water producing unit
21 water receiving tank
22 water cooler
23, 24 pump
11
CA 02915485 2015-12-17
25 water supplying line
26 nitrogen-dissolved water supplying line
27 nitrogen gas supplying line
28 nitrogen gas injecting pipe
29 water circulating line
30 nitrogen-substitution block ice producing unit
31 pouring tank
32 ice cans
33 brine tank
34 nitrogen-dissolved water supplying line
35 pouring ports
36 nitrogen gas supplying line
37 injection pipe
12