Note: Descriptions are shown in the official language in which they were submitted.
WO 2014/205161 PCT/US2014/043084
GRANULES WITH SMALL SMOOTH CORES
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims benefit of priority from US provisional application
USSN
61/837,122, filed 19 June 2013.
FIELD OF THE INVENTION
This disclosure is directed towards improved populations of granules
containing active
agents and methods of making and using.
BACKGROUND OF THE INVENTION
The use of active agents, such as enzymes, in dry product formulations in
industrial and
consumer applications, such as detergents, textile compositions, baking, foods
and animal feed
has become a common practice. Enzymes are known to break down stains, modify
fabric colors
and textures, modify the viscosity of dough and foods, and improve
digestibility of food and
animal feed, by improving the availability of nutrients such as soluble
phosphate and reducing
anti-nutritional factors such as phytic acid in food and animal feed, thereby
improving animal
productivity.
Inactivation of enzymes can occur during storage in dry product formulations
such as
powdered laundry and dish detergents, textile processing blends, baking
flours, and animal feeds
composed of ingredients such as grains (such as corn or soy), vitamins,
minerals and nutrients,
such choline chloride, and additionally during industrial processing, (such as
steam pelleting of
animal feed) by exposure to high temperatures, steam or high humidity,
compression or shear
stress, and chemicals (such as acids, bases, surfactants, bleaches, or organic
solvents). The
inactivation is at least partially reversible if the enzyme reactivates after
processing, for
example, upon cooling after steam treatment and pelleting; however, the
inactivation is
frequently irreversible, such that the catalytic activity of the enzyme does
not fully recover after
processing, for example, upon cooling after steam treatment and pelleting. The
irreversible
1
Date Recue/Date Received 2021-05-04
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
inactivation and reduced activity of an enzyme is generally not desirable in
processes such as
steam pelleting.
When compared with dry feed mixes, feed pellets have properties that are
favored by the
industry, such as improved feed quality, decreased pathogens, lower dust
levels during
manufacture, ease of handling, and more uniform ingredient dosing. Preferred
industry pelleting
processes utilize steam injection, in a process known as conditioning, which
adds moisture and
elevates the temperature prior to the pelleting step which forces the steam
heated feed
ingredients, or conditioned mash, through a die. The pelleting process
temperatures may be from
about 70 C to 95 C, or higher.
Because of the steam, temperatures, compression forces and chemicals used in
pelleting
processes, the activity or potency of enzymes are often significantly reduced
during processing
and subsequent storage. In fact, feed enzymes are often provided to the
industry as stabilized
liquid products that are sprayed onto feed pellets after the pelleting process
to avoid enzyme
inactivation. However, homogeneous dosing is difficult to achieve when the
enzyme is applied
post pelleting, for instance, by spraying the enzyme onto the pellets, and the
cost of the
equipment to add enzyme post-pelleting is high. Alternatively, liquid enzyme
formulations, or
dry mix enzyme formulations, may be added to the mixer prior to pelleting. In
certain instances,
higher levels of enzymes than otherwise needed may be added in order to
compensate for losses
during pelleting.
There is a need in the detergent, textile, baking, food and feed industries
for stable,
durable enzyme granules to serve as components in product and premix
formulations that are
stored for up to several months or years, or subjected to industrial
processing operations, such as
mixing or steam treatment pelleting processes, without appreciable loss of
enzyme activity.
Approaches to avoid the problem of irreversibly inactivating enzymes or
reducing the
activity of the enzyme in industrial processes include identifying new sources
of an enzyme (e.g.
the identification of a known enzyme in an extreme therrnophile microorganism)
or identifying
means to stabilize known enzymes. Klibanov, 1983. (Stabilization of Enzymes
against Thermal
Inactivation, Advances in Applied Microbiology. volume 29, page 1-28)
discloses that there are
three basic means for stabilizing enzymes: (1) immobilization, (2) chemical
modification and (3)
inclusion of additives. However. Klibanov (1983) further discloses that any
one of these
methods could lead to stabilization or destabilization, or have no effect at
all. While previous
formulation approaches have made some progress in this area (see for example
W097/23606,
W09854980, W09739116, W02007044968, EP1224273B1, W02009/102770, US Patent
2
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
6,602,841, EP1804592B1, EP0098631B1, and EP1996028) the present teachings make
an
additional advance in overcoming some of these problems by use of an improved
granule
structure.
It is often desirable to provide a solid formulation with a high concentration
of active
.. enzyme in a granular or particulate form. The particulate granule is
formulated with coatings,
barrier coatings, and additives or excipients designed to provide protection
of the enzyme
against inactivation. To minimize the cost of these protective coatings,
additives and excipients,
and to minimize the impact of product transportation costs, it is desirable to
formulate the
granular enzyme with the highest feasible ratio of active enzyme to inactive
ingredients.
However, if the particle size of granules is held constant as the enzyme
concentration or payload
is increased, the number of particles for a given amount of enzyme dosed into
the final product
or application decreases. Too great a reduction in the number of particles per
dose, however,
can lead to high variability between individual doses of product. For example,
a low average
number of enzyme granules in a scoop of detergent, textile treatment blend,
baking dough. or
serving of animal feed, may lead to inhomogeneous distribution of enzyme
concentration
between individual doses or servings, and thus variability in efficacy of the
enzyme treatment
from dose to dose.
To achieve the economic benefits of increased enzyme payload, while avoiding
the
problems of variability resulting from a dearth of particles per dose, one can
attempt to reduce
the average particle diameter of the enzyme granule in order to maintain a
sufficient number of
particles per dose. However, it is difficult to apply enzyme coatings and
protective barrier
coatings to smaller core particles in coating processes such as fluid bed
spray-coating, pan
coating, drum coating and the like. As the diameter of core particles becomes
smaller,
particularly less than about 250, less than 200, less than 175, less than 150
microns, the tendency
of particles to agglomerate increases. In addition, the application of a given
weight percentage
of coating material to a smaller core results in a thinner coating, hence a
less protective coating.
The problem of applying uniform coatings to small cores is challenging
particularly when the
particles are irregular in shape. Applying a coating to cores with pointed or
angular corners and
edges on the surface can result in regions of thin or discontinuous coating.
Coatings applied to
irregular coatings also may not adhere as well, or may result in agglomerates
where asperities or
surface protrusions on neighboring core particles come into contact with each
other.
Compensation for this by applying thicker coatings, if it can be done without
agglomeration,
will tend to add cost.
3
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Thus, there is a need for improved methods of applying enzymes coatings to
cores
without incurring significant agglomeration while ensuring effective,
continuous protective
coatings. Achieving such high quality coatings on smaller particles will
provide enhanced
stability of the enzymes during processing and storage, while providing higher
payloads, and
while maintaining adequate homogeneity of the mixture in order not to increase
dose-to-dose
variability.
BRIEF SUMMARY OF THE INVENTION
An object of the invention is to ensure or improve stability of an enzyme
during storage
alone, industrial processing, or in mixtures with other ingredients, while at
the same time
improving the ability to apply a continuous coating of consistent minimum
thickness to the
particle, that is, a coating with a reduced number of defects or thin spots in
relationship to the
weight percentage of coating material applied to the core or enzyme-containing
core.
We have surprisingly found that providing cores that have a smoother, more
regular
surface, and a narrow particle size distribution allows for the application of
improved coatings,
with consistent minimum thickness, to smaller particles. In some embodiments,
the improved
coated enzyme granules are achieved by starting with a population of cores
with a smoothness
index less than 2.5, a weight average mean particle diameter of less than 300
microns, and a
particle size dispersity index (PSDI) of less than 2.0, and coating the enzyme-
containing cores
with a further coating that comprises a polymer comprising at least 7% w/w of
the final granule.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 depicts illustrative small smooth core particles according to the
present
teachings, compared to other core particles that are small but irregular.
Figure 2 depicts some illustrative data according to the present teachings.
Figure 3 depicts some illustrative data according to the present teachings.
4
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
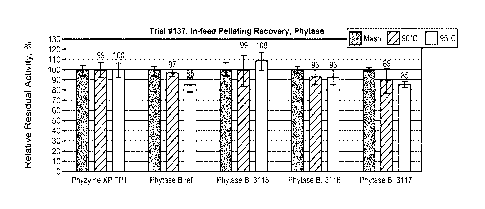
Figure 4 is a graph depicting the pelleting results of three representative
granule
batches. The black bars represent the mash activities, while dark gray and
light grey bars
represent recovery yields in 90 C and 95 C respectively.
Figure 5 is a graph depicting the pelleting results of three representative
phytase
standard TPT batches. The black bars represent the mash activities, while dark
gray and light
grey bars represent recovery yields in 90 C and 95 C respectively.
DETAILED DESCRIPTION
The practice of the present teachings will employ, unless otherwise indicated,
conventional techniques of molecular biology (including recombinant
techniques),
microbiology, cell biology, biochemistry, granulation, and animal feed
pelleting, which are
within the skill of the art. Such techniques are explained fully in the
literature, for example,
Molecular Cloning: A Laboratory Manual, second edition (Sambrook et al.,
1989);
Oligonucleotide Synthesis (M. J. Gait, ed., 1984; Current Protocols in
Molecular Biology (F. M.
Ausubel et al., eds., 1994); PCR: The Polymerase Chain Reaction (Mullis et
al., eds., 1994);
Gene Transfer and Expression: A Laboratory Manual (Kriegler, 1990),
Granulation Technology
for Bioproducts (Kadam, 1990), and Fairfield, D. 1994. Chapter 10, Pelleting
Cost Center. In
Feed Manufacturing Technology IV. (McEllhiney, editor), American Feed Industry
Association,
Arlington, Va., pp. 110-139.
Unless defined otherwise herein, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which the present
teachings belong. Singleton, et al., Dictionary of Microbiology and Molecular
Biology, second
ed., John Wiley and Sons, New York (1994), and Hale & Markham, The Harper
Collins
Dictionary of Biology, Harper Perennial, NY (1991) provide one of skill with a
general
dictionary of many of the terms used in this invention. Any methods and
materials similar or
equivalent to those described herein can be used in the practice or testing of
the present
teachings.
For ease of reference we have described elements of the present teachings
under one or
more headings. It is to be noted that the teachings under each of the headings
also apply to the
teachings under the other headings. For example, each of the stated
embodiments and aspects
concerning the use of the present teachings is equally an embodiment or aspect
concerning the
5
WO 2014/205161 PCT/US2014/043084
method of the present teachings or the composition of the present teachings.
Likewise, each of
the stated embodiments and aspects concerning the method or use of the present
teachings is
equally an embodiment or aspect concerning the composition of the present
teachings. In this
application, the use of the singular includes the plural unless specifically
stated otherwise.
Numeric ranges provided herein are inclusive of the numbers defining the
range.
Definitions
As used herein, the term -granule" refers to a particle which contains a core
(typically a
small smooth core particle), an active agent (typically an enzyme), and
optionally at least one
additional coating.
As used herein, the term "core" refers to the inner nucleus of a granule, and
typically
comprises a "small smooth core particle". The cores of the present teachings
may be produced
by a variety of fabrication techniques including: rotary atomization, wet
granulation, dry
granulation, spray drying, disc granulation, extrusion, pan coating,
spheronization, drum
granulation, fluid-bed agglomeration, high-shear granulation, fluid-bed spray
coating,
crystallization, precipitation, emulsion gelation, spinning disc atomization
and other casting
approaches, and prill processes. Such processes are known in the art and are
described in US
Pat. No. 4689297 and US Pat. No. 5324649 (fluid bed processing); EP656058B1
and US Pat.
No. 454332 (extrusion process); US Pat. No. 6248706 (granulation, high-shear);
and
EP804532B1 and US Pat. No. 6534466 (combination processes utilizing a fluid
bed core and
mixer coating).
The active agent is typically coated around the core. Suitable cores for use
in the present
teachings are can be any material meeting the smoothness index, mass median
diameter, and
particle size disparity definitions as provided herein. In one embodiment of
the present teachings
the core comprises a sodium chloride or sodium sulfate crystal. In another
embodiment of the
present teachings, the core comprises a sucrose crystal seed. Particles
composed of inorganic
salts and/or sugars and/or small organic molecules may be used as the cores of
the present
teachings. Suitable water soluble ingredients for incorporation into cores
include: inorganic salts
such as sodium chloride, ammonium sulfate, sodium sulfate, magnesium sulfate,
zinc sulfate; or
urea, citric acid, sugars such as sucrose, lactose and the like. In some
embodiments, the core
6
Date Recue/Date Received 2021-05-04
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
particles are crystalline inorganic salts. In some embodiments, the core
particles are crystalline
sodium sulfate.
The term "small smooth core particle" as used herein refers to a particle,
typically the
inner particle of a granule, that exhibits certain smoothness index, mass
median diameter, and
particle size dispersity index measures, as provided herein.
The term "smoothness index" as used herein refers to the ratio of the
empirical specific
surface area to the envelope specific surface area calculated for a
representative sample of
cores.
As used herein, "representative sample of cores" refers to a random sample of
at least 15
core particles.
The term "empirical specific surface area" as used herein refers to the
empirically
measured surface area per gram (measured in square meters per gram) of a
representative
sample of cores as determined, using the BET (Brunauer-Emmett-Teller) gas
adsorption method
(S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60,
309. doi:10.1021/ja01269a023). The specific details of the BET method are
described in the
Methods section below.
The term "envelope specific surface area" as used herein refers to the
calculated specific
surface area (in units of square meters per gram) based upon the measured
particle size
distribution and average true density of a representative sample of cores,
using the idealized
assumption that all core particles are perfect spheres. An algorithm for
calculating the envelope
specific surface area is described in the Methods section below.
The term "particle size distribution" (abbreviated PSD) as used herein, refers
to relative
amount by mass percent of small smooth core particles present in each of
several diameter size
intervals. The PSD of the core sample is measured by laser light scattering
(see for example T.
Allen, Particle Size Measurement, Vol. 1 (Chapman and Hall, 1997). In the
present application,
the PSD is characterized by three mass percentile diameters, which can be
determined from a
log-normal plot of the PSD.
D50 or MMD (mass median diameter) The log-normal distribution mass median
diameter. The MMD is considered to be the mass average particle diameter,
below which 50%
w/w of the particles a representative sample have a smaller diameter.
D10: Tenth percentile diameter (TPD), the diameter at which 10% w/w of the
particles in
a representative sample have a smaller diameter.
7
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
D90: Ninetieth percentile diameter (NPD) the diameter at which 90% w/w of the
particles
in a representative sample have a smaller diameter.
Typically, the D10, D50 and D90 are expressed in microns.
The "particle size dispersity index" (abbreviated PSDI) of a core sample is
the D90 / D10
ratio of a representative sample.
The term "coating" as used herein refers to a layer of material surrounding an
underlying
material. The first coating layer generally encapsulates the core in order to
form a substantially
continuous layer so that the core surface has few or no uncoated areas, and is
typically an active
agent coating. Subsequent additional coating layers can encapsulate the
growing granule to
form one or more additional substantially continuous layer(s) ("additional
coatings"). The
materials (e.g. the active agents and components detailed herein) used in the
granule can, but
need not be, suitable for the use in foods and/or animal feeds, and
accordingly can be food grade
or feed grade.
The coatings of the present teachings may further comprise one or more of the
following:
inorganic salts, salts of organic acids, sugars, sugar alcohols, starches
(native, pre-gelatinized,
hydrolyzed, or chemically modified) and other polysaccharides, gums,
additional active agents,
feed or food grade polymers, pigments, clays, plasticizers, surfactants,
fibrous materials, anti-
tack agents, fillers, extenders and other compounds known to be used in
coatings. Suitable
.. polymers include polyvinyl alcohol (PVA), including partially and fully
hydrolyzed PVA,
polyethylene glycol, polyethylene oxide, polyvinyl pyrrolidine, and
carbohydrate polymers
(such as starch, amylose, amylopectin, alpha and beta-glucans, pectin,
glycogen), including
mixtures and derivatives thereof. Suitable fillers useful in the coatings
include inert materials
used to add bulk and reduce cost, or used for the purpose of adjusting the
intended enzyme
activity in the finished granule. Examples of such fillers include, but are
not limited to, water
soluble agents such as salts, sugars and water dispersible agents such as
clays, talc, silicates,
cellulose and starches, and cellulose and starch derivatives. Suitable
plasticizers useful in the
coatings of the present teachings are low molecular weight organic compounds
and are highly
specific to the polymer being plasticized. Examples include, but are not
limited to, sugars (such
as, glucose, fructose and sucrose), sugar alcohols (such as, sorbitol, xylitol
and maltitol and
other glycols), polar low molecular weight organic compounds, such as urea, or
other known
plasticizers such as water or feed grade plasticizers. Suitable fibrous
materials useful in the
coatings of the present teachings include, but are not limited to: cellulose,
and cellulose
8
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
derivatives such as HPMC (hydroxy-propyl-methyl cellulose), CMC (carboxy-
methyl cellulose),
HEC (hydroxy-ethyl cellulose). In one embodiment, particularly for feed
applications, of the
present teachings, a coating comprises a water-soluble or dispersible corn cob
material or sugar
or salt crystal. In another embodiment particularly suitable for household
cleaning applications,
the coating comprises a water-soluble or dispersible sugar or salt crystal or
a non pareil. Those
skilled in the art will recognize that, for feed and food applications, the
coatings (and any
polymers, fillers, plasticizers, fibrous materials, and extenders), are
acceptable for food and/or
feed applications. For household cleaning applications, such a restriction
need not apply.
The term "active agent coating" as used herein refers to the coating that
contains the
active agent (typically an enzyme). Generally, the active agent coating will
be applied to the
small smooth core particle, and thereafter additional coatings may optionally
be applied.
The term "additional coating" as used herein refers to one or more coatings
that are
optionally applied to a small smooth core particle. Typically, the additional
coating will be
applied to a nascent granule that contains a small smooth core particle and an
active agent
coating. Non-limiting examples of additional coatings include moisture barrier
coatings and
moisture hydrating coatings.
The term -moisture barrier coating" as used herein refers to a coating that
comprises a
moisture barrier material.
The term "moisture hydrating coating" as used herein refers to a coating that
comprises a
moisture hydrating material.
The term "moisture barrier material" refers to materials that exclude, prevent
or
substantially retard water uptake. These materials typically are hydrophobic
or amphiphilic,
provide insulation against water and do not inherently absorb and/or bind
water and include, but
are not limited to, film-forming materials. Examples of moisture barrier
materials include barrier
polymers, proteins, lipids, fats and oils, fatty acids and gums. Examples of
film forming
moisture barrier materials are natural and modified barrier polymers, such as
gum arabic, whey,
whey protein concentrate, PVA, including modified PVA and fully hydrolyzed
PVA, and
synthetic polymers such as latex, HPMC, and acid-thinned hydroxypropyl starch,
for example,
PureCoteTM, oxidized starch, and modified starch. Non-film forming moisture
barrier materials
include, for instance, waxes, fats, oils and lipids, and lecithin. Selected
moisture barrier
materials that do not readily oxidize are, for example, latex polymer and
barrier polymers such
as gum arabic.
The term "moisture hydrating material" refers to materials that take up
aqueous liquids,
such as water, by one several mechanisms. In a first non-limiting mechanism,
the materials
9
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
absorb free water. In a second non-limiting mechanism, the materials take up
bound water that
generally is present as crystalline waters of hydration. Accordingly, the
materials may be
provided as partially or fully hydrated materials or as non-hydrated materials
that will absorb or
bind aqueous liquids and retard or reduce the rate or extent of migration of
such liquids to the
.. active agent. In a third non-limiting mechanism, moisture hydrating
materials thermally insulate
the active agent by retarding heat transfer to the active agent within the
granule and by
maintaining the active agent at a lower temperature than the temperature at
the exterior surface
of the granule. Moisture hydrating materials include carbohydrates and
inorganic salts,
including hydrated salts, such as magnesium sulfate, sodium sulfate, and
ammonium sulfate;
maltodextrin; sugars, for example, sucrose; starch, including cornstarch.
As used herein, the terms "pellets" and "pelleting" refer to solid, rounded,
spherical and
cylindrical tablets or pellets and the processes for forming such solid
shapes, particularly feed
pellets and solid, extruded animal feed. Known food and animal feed pelleting
manufacturing
processes generally include admixing together food or feed ingredients for
about 1 to about 5
minutes at room temperature, transferring the resulting admixture to a surge
bin, conveying the
admixture to a steam conditioner, optionally transferring the steam
conditioned admixture to an
expander, transferring the admixture to the pellet mill or extruder, and
finally transferring the
pellets into a pellet cooler. Fairfield, D. 1994. Chapter 10, Pelleting Cost
Center. In Feed
Manufacturing Technology IV. (McEllhiney, editor), American Feed Industry
Association,
Arlington, Va.. pp. 110-139.
As used herein, the term "unpelleted mixtures" refers to premixes or
precursors, base
mixes, mash, and diluents. Premixes typically contain vitamins and trace
minerals. Base mixes
typically contain food and feed ingredients such as dicalcium phosphate,
limestone, salt and a
vitamin and mineral premix, but not grains and protein ingredients. Diluents
include, but are not
limited to grains (for example wheat middlings and rice bran) and clays, such
as phyllosilicates
(the magnesium silicate sepiolite, bentonite, kaolin, montmorillonite,
hectorite, saponite,
beidellite, attapulgite, and stevensite). Clays also function as carriers and
fluidizing agent, or
diluents, for feed premixes. Mash typically comprises a complete animal diet.
As used herein, the term "recovered activity" refers to the ratio of (i) the
activity of an
active agent after a treatment involving one or more of the following
stressors: heating,
increased pressure, increased pH, decreased pH, storage, drying, exposure to
surfactant(s),
exposure to solvent(s) (including water/moisture), and mechanical stress) to
(ii) the activity of
the phytase before the treatment. The recovered activity may be expressed as a
percentage.
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
The percent recovered activity is calculated as follows:
( activity after treatment
% recovered activity = x 100 %
activity before treatment )
In the context of pelleting experiments, the "activity before treatment" can
be
approximated by measuring the active agent activity present in the mash that
does not undergo
treatment in a manner that is otherwise matched to the active agent that does
undergo treatment.
For example, the active agent in the untreated mash is handled and stored for
a similar
time and under similar conditions as the active agent in the treated (e.g,
pelleted) mash, to
control for possible interactions or other effects outside of the specified
treatment per se.
As used herein, the term "active agent" may be any material that is to be
added to a
granule to provide the intended functionality for a given use. The active
agent may be a
biologically viable material, a food or feed ingredient, an antimicrobial
agent, an antibiotic
replacement agent, a prebiotic, a probiotic, an agrochemical ingredient, such
as a pesticide,
fertilizer or herbicide; a pharmaceutical ingredient or a household care
active ingredient, or
combinations thereof. In a preferred embodiment, the active ingredient is a
protein, enzyme,
peptide, polypeptide, amino acid, carbohydrate, lipid or oil, vitamin, co-
vitamin, hormone, or
combinations thereof. In another embodiment, the active ingredient is an
enzyme, bleach, bleach
activator, perfume, or other biologically active ingredient. Inherently
thermostable active agents
are encompassed by the present teachings and can exhibit enhanced
thermostability in the
granules. Most preferred active ingredients for food and feed applications are
enzymes, peptides
and polypeptides, amino acids, antimicrobials, gut health promoting agents,
vitamins, and
combinations thereof. Any enzyme may be used, and a nonlimiting list of
enzymes include
phytases, xylanases,I3-glucanases, phosphatases, proteases, amylases (alpha or
beta or
glucoamylases) cellulases, lipases, cutinases, oxidases, transferases,
reductases, hemicellulases,
mannanases, esterases, isomerases, pectinases, lactases, peroxidases,
laccases, other redox
enzymes and mixtures thereof. Particularly preferred enzymes include a
xylanase from
Trichoderma reesei and a variant xylanase from Trichoderma reesei, both
available from
DuPont Industrial Biosciences or the inherently thermostable xylanase
described in
EP1222256B1 . as well as other xylanases from Aspergillus niger, Aspergillus
kawachii,
Aspergillus tubigensis, Aspergillus clavatus, Bacillus circulans, Bacillus
pumilus, Bacillus
subtilis, Fusari urn. verticilloides, Fusarium oxyspo rum, Neocallimastix
patriciarum, Penicillium
11
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
species, Streptomyces lividans, Strepromyces ihermoviolaceus, Thermomonospora
fitsca,
Trichoderrna harzian urn, Trichoderma reesei, Trichoderma viride. Additional
particularly
preferred enzymes include phytases, such as for example Finase L , a phytase
from Aspergillus
sp., available from AB Enzymes, Darmstadt, Germany; PhyzymeTM XP, a phytase
from E. Coli,
and Axtra Phy, a phytase from Buttiauxella, both available from Danisco Animal
Nutrition,
DuPont. and other phytases from, for example, the following organisms:
Trichoderma,
Penicillium, Fusarium, Buttiauxella, Citrobacter, Enterobacter, Penicillium,
Humicola, Hafnia,
Bacillus, and Peniophora, as well as those phytases described in US patent
applications
61/595,923 and 61/595.941, both filed February 12, 2012. An example of a
cellulase is
Multifect BGL, a cellulase (beta glucanase), available from Danisco Animal
Nutrition, DuPont
and other cellulases from species such as Aspergillus, Trichoderma,
Penicillium, Humicola,
Bacillus, Cellulomonas, Penicillium, Thermomonospore, Clostridium, and
Hypocrea. The
cellulases and endoglucanases described in US20060193897A1 also may be used.
Amylases
may be, for example, from species such as Aspergillus, Trichoderma,
Penicillium, Bacillus, for
instance, B. subtilis, B. stearothermophilus, B. lentus, B. licheniformis. B.
coagulans, and B.
amyloliquefaciens. Suitable fungal amylases are derived from Aspergillus, such
as A. oryzae
and A. niger. Proteases may be from Bacillus amyloliquefaciens, Bacillus
lentus , Bacillus
subtilis, Bacillus licheniformis, and Aspergillus and Trichoderma species.
Phytases, xylanases,
phosphatases, proteases, amylases, esterases, redox enzymes, lipases,
transferases, cellulases,
and f3-glucanases are enzymes frequently used for inclusion in animal feed.
Enzymes suitable for
inclusion into tablets for household care applications are similar,
particularly proteases,
amylases, lipases, pectate lyases, mannanases, hemicellulases, redox enzymes,
peroxidases,
transferases, and cellulases. In particularly preferred aspects of the present
teachings, the
enzymes are selected from phytases, xylanases, beta glucanases, amylases,
proteases, lipases,
esterases, and mixtures thereof. In one embodiment of the present invention,
two enzymes are
provided in the granule, a xylanase and a beta-glucanase. In another
embodiment of the present
invention, two enzymes provided in the granule are a protease and amylase. The
enzymes may
be mixed together or applied to the granule separately. In another embodiment,
three enzymes
are provided in the granule, namely beta-glucanase, xylanase and phytase. The
above enzyme
lists are examples only and are not meant to be exclusive. Any enzyme may be
used in the
granules of the present teachings, including wild type, recombinant and
variant enzymes of
bacterial. fungal, yeast, plant, insect and animal sources, and acid, neutral
or alkaline enzymes. It
will be recognized by those skilled in the art that the amount of enzyme used
will depend, at
least in part, upon the type and property of the selected enzyme and the
intended use.
12
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Methods
BET specific surface area measurement.
To measure the specific surface area of core particles, the BET method was
used to evaluate
pore distribution of the cores by krypton gas adsorption / desorption, using a
Micromeritics
model ASAP 2420 Accelerated Porosimetry and Surface Area System. The BJH
method (Elliott
P. Barrett, Leslie G. Joyner , Paul P. Halenda, J. Am. Chem. Soc., 1951, 73
(1), pp 373-380.
DO!: 10.1021/ja01145a126) was used to determine pore volume distribution in
the range 20 ¨
1000 A. Samples, approximately 4-6 grams in mass, were outgassed in vacuo at
150 C
temperature overnight to remove adsorbed water and / or volatile contaminants.
They were re-
weighed and transferred to the porosimeter, placed under vacuum and cooled to
77K at which
point gas ( Kr) adsorption isotherms were recorded via pressure measurements.
Surface area
coverage is automatically computed by the ASAP 2420 V2.07J software package
provided by
Micromeritics.
Envelope specific surface area calculation.
The envelope specific surface area represents the specific surface area of an
equivalent idealized
sample of perfectly smooth, spherical particles whose PSD matches that of the
actual sample of
cores. The particle size distribution (PSD) of the cores is determined via
laser diffraction
(reference ISO 13320-1:1999) using a Malvern Mastersizer 2000 with hexane as
the background
fluid in the recirculation loop. An amount of the sample of cores is added of
sufficient mass to
reach a laser obscuration of approximately 10%. The PSD is automatically
calculated from the
__ diffraction pattern using Mie theory. In the case of sodium sulfate, the
complex refractive index
of the particle is taken as 1.468 + 0.01i, and the refractive index of the
fluid as 1.375. The
generated PSD is a differential mass fraction showing the percentage of
particles in each of 81
logarithmically-spaced size bins spanning 0.01 microns to 10,000 microns. If
necessary, the
PSD should be corrected and renormalized to exclude artifacts such as dust
fines or
agglomerates, specifically any particles finer than 60 microns or larger than
500 microns.
The geometric mean diameter of the upper and lower size demarcations of each
size bin is
calculated by taking the square root of the product of the upper and lower
size demarcation. The
envelope specific surface area in square meters per gram for a given bin is
given by 6/(d*p),
where d is particle diameter (in cm), p is the true density (density excluding
voids) of the
13
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
particulate solid (in g/cm3). In the case of sodium sulfate, p = 2.664 g/cm3.
Since the PSD is a
mass-weighted distribution, the total envelope surface area is calculated by
multiplying the
differential mass fraction in each size bin by the specific surface area for
that size bin and
summing over all size bins.
Exemplary Embodiments
In some embodiments, the present teachings provide a granule composition which
surprisingly possesses equivalent stability compared to granules containing
non-small smooth
cores, thus allowing for savings in material cost. For example, the granule
composition of the
present teachings can possess surprisingly equivalent storage stability. The
desirable storage
stability can reside in any of a variety of contexts, including dish
detergent, laundry detergent,
animal feed, textiles, and human food. The granules of the present teachings
can demonstrate
improved stability under these various conditions as compared to identically
stored granules
lacking the small smooth core particle of the present teachings. In some
embodiments, the
present teachings are believed to provide improved stability compared to
granules containing
larger and/or non-smooth cores.
In some embodiments, the present teachings provide a granule composition with
equivalent resistance to dust generation as compared to granules containing
non small smooth
cores as measured by the Heubach dust test.
In some embodiments, the present teachings are believed to provide a granule
composition with improved resistance to dust generation relative to granules
containing a non
small smooth core.
In some embodiments, the present teachings provide for a granule composition
of
equivalent resistance to steam-pelleting compared to granules containing a non
small smooth
core. For example, the degree of "recovered activity" as defined supra can be
determined for the
granules of the present teachings, and compared to identically-treated
granules containing a
different core from the small smooth core particle of the present teachings.
In some
embodiments, the present teachings are believed to provide for a granule
composition of
improved resistance to steam-pelleting compared to granules containing a small
smooth core.
Additional applications and methods employing the granules of the present
teachings are
described in the below non-limiting sections.
14
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Compositions and Methods for Baking and Food Preparation
The present teachings also relate to a "food composition," including but not
limited
to a food product, animal feed and/or food/feed additives, comprising the
present granule
composition, and methods for preparing such a food composition comprising
mixing the present
granule composition with one or more food ingredients, or uses thereof.
The present granule composition can be used in the preparation of a food
composition, wherein the food composition is baked subsequent to the addition
of the present
granule composition. As used herein the term "baking composition" means any
composition
and/or additive prepared in the process of providing a baked food product,
including but not
limited to bakers flour, a dough, a baking additive and/or a baked product.
The food
composition or additive may be liquid or solid.
As used herein, the term "flour" means milled or ground cereal grain. The term
"flour" also may mean Sago or tuber products that have been ground or mashed.
In some
embodiments, flour may also contain components in addition to the milled or
mashed cereal or
plant matter. An example of an additional component, although not intended to
be limiting, is a
leavening agent. Cereal grains include wheat, oat, rye, and barley. Tuber
products include
tapioca flour, cassava flour, and custard powder. The term "flour" also
includes ground corn
flour, maize-meal, rice flour, whole-meal flour, self-rising flour, tapioca
flour, cassava flour,
ground rice, enriched flower, and custard powder.
For the commercial and home use of flour for baking and food production, it is
important to maintain an appropriate level of enzyme activity in the flour. A
level of activity
that is too high may result in a product that is sticky and/or doughy and
therefore unmarketable.
Flour with insufficient enzyme activity may not contain enough sugar for
proper yeast function,
resulting in dry, crumbly bread, or baked products. Accordingly, the present
granule
composition in combination with an a-amylase(s), may be added to the flour to
augment the
level of endogenous enzyme activity in flour.
An amylase in the present granule composition can be added alone or in a
combination with other amylases to prevent or retard staling, i.e., crumb
firming of baked
products. The amount of anti-staling amylase will typically be in the range of
0.01-10 mg of
enzyme protein per kg of flour, e.g., 0.5 mg/kg ds. Additional anti-staling
amylases that can be
used and include an endo-amylase, e.g., a bacterial endo-amylase from
Bacillus. The additional
amylase can be another maltogenic a-amylase (EC 3.2.1.133), e.g., from
Bacillus. Novamy10 is
an exemplary maltogenic a-amylase from B. stearothermophilus strain NCIB 11837
and is
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
described, for example, in Christophersen et al. (1997) Starch 50: 39-45.
Other examples of
anti-staling endo-amylases include bacterial a-amylases derived from Bacillus,
such as B.
lichenifonnis or B. amyloliquefaciens. The anti-staling amylase may be an exo-
amylase, such as
13-amylase, e.g., from plant sources, such as soybean, or from microbial
sources, such as
Bacillus.
The baking composition comprising the present granule composition further can
comprise a phospholipase or enzyme with phospholipase activity. An enzyme with
phospholipase activity has an activity that can be measured in Lipase Units
(LU). The
phospholipase may have A1 or A2 activity to remove fatty acid from the
phospholipids, forming
a lysophospholipid. It may or may not have lipase activity, i.e., activity on
triglyceride
substrates. The phospholipase typically has a temperature optimum in the range
of 30-90 C.,
e.g., 30-70 C. The added phospholipases can be of animal origin, for example,
from pancreas,
e.g., bovine or porcine pancreas, snake venom, or bee venom. Alternatively,
the phospholipase
may be of microbial origin, e.g., from filamentous fungi, yeast or bacteria,
for example.
The phospholipase is added in an amount that improves the softness of the
bread
during the initial period after baking, particularly the first 24 hours. The
amount of
phospholipase will typically be in the range of 0.01-10 mg of enzyme protein
per kg of flour.
e.g., 0.1-5 mg/kg. That is, phospholipase activity generally will be in the
range of 20-1000
LU/kg of flour, where a Lipase Unit is defined as the amount of enzyme
required to release 1
Rmol butyric acid per minute at 30 C, pH 7.0, with gum arabic as emulsifier
and tributyrin as
substrate.
Compositions of dough generally comprise wheat meal or wheat flour and/or
other
types of meal, flour or starch such as corn flour, cornstarch, rye meal, rye
flour, oat flour,
oatmeal, soy flour, sorghum meal, sorghum flour, potato meal, potato flour or
potato starch. The
dough may be fresh, frozen, or par-baked. The dough can be a leavened dough or
a dough to be
subjected to leavening. The dough may be leavened in various ways, such as by
adding
chemical leavening agents, e.g., sodium bicarbonate or by adding a leaven,
i.e., fermenting
dough. Dough also may be leavened by adding a suitable yeast culture, such as
a culture of
Saccharomyces cerevisiae (baker's yeast), e.g., a commercially available
strain of S. cerevisiae.
The dough may also comprise other conventional dough ingredients, e.g.,
proteins,
such as milk powder, gluten, and soy; eggs (e.g., whole eggs, egg yolks or egg
whites); an
oxidant, such as ascorbic acid, potassium bromate, potassium iodate,
azodicarbonamide (ADA)
or ammonium persulfate; an amino acid such as L-cysteine; a sugar; or a salt,
such as sodium
16
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
chloride, calcium acetate, sodium sulfate, or calcium sulfate. The dough
further may comprise
fat, e.g., triglyceride, such as granulated fat or shortening. The dough
further may comprise an
emulsifier such as mono- or diglycerides, diacetyl tartaric acid esters of
mono- or diglycerides,
sugar esters of fatty acids, polyglycerol esters of fatty acids, lactic acid
esters of monoglycerides,
acetic acid esters of monoglycerides, polyoxyethylene stearates, or
lysolecithin. For example,
the dough can be made without addition of emulsifiers.
The dough product may be any processed dough product, including fried, deep
fried,
roasted, baked, steamed and boiled doughs, such as steamed bread and rice
cakes. In one
embodiment, the food product is a bakery product. Typical bakery (baked)
products include
bread - such as loaves, rolls, buns, bagels, pizza bases etc. pastry,
pretzels, tortillas, cakes,
cookies, biscuits, crackers etc.
Optionally, an additional enzyme may be used together with the anti-staling
amylase
and the phospholipase. The additional enzyme may be a second amylase, such as
an
amyloglucosidase, a 0-amylase, a cyclodextrin glucanotransferase, or the
additional enzyme may
be a peptidase, in particular an exopeptidase, a transglutaminase, a lipase, a
cellulase, a
xylanase, a protease, a protein disulfide isomerase, e.g., a protein disulfide
isomerase as
disclosed in WO 95/00636, for example, a glycosyltransferase, a branching
enzyme (1,4-a-
glucan branching enzyme), a 4-a-glucanotransferase (dextrin
glycosyltransferase) or an
oxidoreductase, e.g., a peroxidase, a laccase, a glucose oxidase, a pyranose
oxidase, a
lipooxygenase, an L-amino acid oxidase or a carbohydrate oxidase. The
additional enzyme(s)
may be of any origin, including mammalian and plant, and particularly of
microbial (bacterial,
yeast or fungal) origin and may be obtained by techniques conventionally used
in the art.
The xylanase is typically of microbial origin, e.g., derived from a bacterium
or
fungus, such as a strain of Aspergillus. Xylanases include Pentopan@ and
Novozym 384@, for
example, which are commercially available xylanase preparations produced from
Trichodenna
reesei. The amyloglucosidase may be an A. niger amyloglucosidase (such as
AMG@). Other
useful amylase products include Grindamyl@ A 1000 or A 5000 (Grindsted
Products, Denmark)
and Amylase H or Amylase P (DSM). The glucose oxidase may be a fungal
glucose
oxidase, in particular an Aspergillus niger glucose oxidase (such as
Gluzyme@). An exemplary
protease is Neutrase .
The process may be used for any kind of baked product prepared from dough,
either
of a soft or a crisp character, either of a white, light or dark type.
Examples are bread,
particularly white, whole-meal or rye bread, typically in the form of loaves
or rolls, such as, but
17
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
not limited to, French baguette-type bread, pita bread, tortillas, cakes,
pancakes, biscuits,
cookies, piecrusts, crisp bread, steamed bread, pizza and the like.
The present granule composition may be used in a pre-mix, comprising flour
together
with an anti-staling amylase, a phospholipase, and/or a phospholipid. The pre-
mix may contain
other dough-improving and/or bread-improving additives, e.g., any of the
additives, including
enzymes, mentioned above. The present granule composition can be a component
of an enzyme
preparation comprising an anti-staling amylase and a phospholipase, for use as
a baking
additive.
The storing, handling and incorporation of the loaded delivery vehicle can be
accomplished by means of a packaged mix. The packaged mix can comprise the
present granule
composition. However, the packaged mix may further contain additional
ingredients as required
by the manufacturer or baker. After the present granule composition has been
incorporated into
the dough, the baker continues through the normal production process for that
product.
A food composition is contemplated where the food is an oil, meat, lard.
composition
comprising the present granule composition. In this context the term
"[oil/meat/lard]
composition" means any composition, based on, made from and/or containing oil,
meat or lard,
respectively. A method is contemplated for preparing an oil or meat or lard
composition and/or
additive comprising the present granule composition, comprising mixing the
present granule
composition with a oil/meat/lard composition and/or additive ingredients.
The food composition can be an animal feed composition, animal feed additive,
and/or pet food comprising the present granule composition. A method is
contemplated for
preparing such an animal feed composition, animal feed additive composition
and/or pet food
comprising mixing the present granule composition thereof with one or more
animal feed
ingredients and/or animal feed additive ingredients and/or pet food
ingredients. The present
granule composition can be used in the preparation of an animal feed
composition and/or animal
feed additive composition and/or pet food.
The term "animal" includes all non-ruminant and ruminant animals. In a
particular
embodiment, the animal is a non-ruminant animal, such as a horse and a mono-
gastric animal.
Examples of mono-gastric animals include, but are not limited to, pigs and
swine, such as
piglets, growing pigs, sows; poultry such as turkeys, ducks, chicken, broiler
chicks, layers; fish
such as salmon, trout, tilapia, catfish and carps; and crustaceans such as
shrimps and prawns. In
a further embodiment the animal is a ruminant animal including, but not
limited to, cattle, young
18
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
calves, goats, sheep, giraffes, bison, moose, elk, yaks, water buffalo, deer,
camels, alpacas,
llamas, antelope, pronghorn and nilgai.
In the present context, it is intended that the term "pet food" is understood
to mean a
food for a household animal such as, but not limited to dogs, cats, gerbils,
hamsters, chinchillas,
fancy rats, guinea pigs; avian pets, such as canaries, parakeets, and parrots;
reptile pets, such as
turtles, lizards and snakes; and aquatic pets, such as tropical fish and
frogs.
The terms "animal feed composition," "feedstuff" and "fodder" are used
interchangeably and may comprise one or more feed materials selected from the
group
comprising a) cereals, such as small grains (e.g., wheat, barley. rye, oats
and combinations
thereof) and/or large grains such as maize or sorghum; b) by products from
cereals, such as corn
gluten meal, Distillers Dried Grain Solubles (DDGS) (particularly corn based
Distillers Dried
Grain Solubles (cDDGS), wheat bran, wheat middlings, wheat shorts, rice bran,
rice hulls, oat
hulls, palm kernel, and citrus pulp; c) protein obtained from sources such as
soya, sunflower,
peanut, lupin, peas, fava beans, cotton, canola, fish meal, dried plasma
protein, meat and bone
meal, potato protein, whey, copra, sesame; d) oils and fats obtained from
vegetable and animal
sources; e) minerals and vitamins.
Textile Desizing Compositions and Use
Also contemplated are compositions and methods of treating fabrics (e.g., to
desize a
textile) using the present granule composition. Fabric-treating methods are
well known in the
art (see, e.g., U.S. Patent No. 6,077,316). For example, the feel and
appearance of a fabric can
be improved by a method comprising contacting the fabric with the present
granule composition
in a solution. The fabric can be treated with the solution under pressure.
The present granule composition can be applied during or after the weaving of
a
textile, or during the desizing stage, or one or more additional fabric
processing steps. During
the weaving of textiles, the threads are exposed to considerable mechanical
strain. Prior to
weaving on mechanical looms, warp yarns are often coated with sizing starch or
starch
derivatives to increase their tensile strength and to prevent breaking. The
present granule
composition can be applied during or after the weaving to remove these sizing
starches or starch
derivatives. After weaving, a present granule composition can be used to
remove the size
coating before further processing the fabric to ensure a homogeneous and wash-
proof result.
19
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
The present granule composition can be used alone or with other desizing
chemical
reagents and/or desizing enzymes to desize fabrics, including cotton-
containing fabrics, as
detergent additives, e.g., in aqueous compositions. The present granule
composition also can be
used in compositions and methods for producing a stonewashed look on indigo-
dyed denim
fabric and garments. For the manufacture of clothes, the fabric can be cut and
sewn into clothes
or garments, which are afterwards finished. In particular, for the manufacture
of denim jeans,
different enzymatic finishing methods have been developed. The finishing of
denim garment
normally is initiated with an enzymatic desizing step, during which garments
are subjected to the
action of amylolytic enzymes to provide softness to the fabric and make the
cotton more
accessible to the subsequent enzymatic finishing steps. The present granule
composition can be
used in methods of finishing denim garments (e.g., a "bio-stoning process"),
enzymatic desizing
and providing softness to fabrics, and/or finishing process.
Cleaning Compositions
An aspect of the present compositions and methods is a cleaning composition
that
includes the present granule composition as a component. A protease
polypeptide, or other
relevant enzyme, can be used as a component in detergent compositions for hand
washing,
laundry washing, dishwashing, and other hard-surface cleaning.
Preferably, the present granule composition is incorporated into detergents at
or near
a concentration conventionally used for amylase in detergents. For example,
protease
polypeptide may be added in amount corresponding to 0.00001 ¨ 1 mg (calculated
as pure
enzyme protein) of amylase per liter of wash/dishwash liquor. Exemplary
formulations are
provided herein, as exemplified by the following:
A protease polypeptide may be a component of a detergent composition, as the
only
enzyme or with other enzymes including other amylolytic enzymes. As such, it
may be included
in the detergent composition in the form of the present granule composition.
When employed in
this context, non-limiting examples of waxy coating materials are
poly(ethylene oxide) products
(polyethyleneglycol, PEG) with mean molar weights of 1.000 to 20,000;
ethoxylated
nonylphenols having from 16 to 50 ethylene oxide units; ethoxylated fatty
alcohols in which the
alcohol contains from 12 to 20 carbon atoms and in which there are 15 to 80
ethylene oxide
units; fatty alcohols; fatty acids; and mono- and di- and triglycerides of
fatty acids. Examples of
film-forming coating materials suitable for application by fluid bed
techniques are given in, for
example, GB 1483591.
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
The detergent composition can comprise one or more surfactants, each of which
may
be anionic, nonionic, cationic, or zwitterionic. The detergent will usually
contain 0% to about
50% of anionic surfactant, such as linear alkylbenzenesulfonate (LAS); a-
olefinsulfonate
(AOS); alkyl sulfate (fatty alcohol sulfate) (AS); alcohol ethoxysulfate (AEOS
or AES);
secondary alkanesulfonates (SAS); a-sulfo fatty acid methyl esters; alkyl- or
alkenylsuccinic
acid; or soap. The composition may also contain 0% to about 40% of nonionic
surfactant such
as alcohol ethoxylate (AEO or AE), carboxylated alcohol ethoxylates,
nonylphenol ethoxylate,
alkylpolyglycoside, alkyldimethylamineoxide, ethoxylated fatty acid
monoethanolamide, fatty
acid monoethanolamide, or polyhydroxy alkyl fatty acid amide (as described for
example in WO
92/06154).
The detergent composition may additionally comprise one or more other enzymes,
such as another protease, another amylolytic enzyme, cutinase, lipase,
cellulase, pectate lyase,
perhydrolase, mannanase, xylanase, peroxidase, and/or laccase in any
combination.
The detergent may contain about 1% to about 65% of a detergent builder or
complexing agent such as zeolite, diphosphate, triphosphate, phosphonate,
citrate, nitrilotriacetic
acid (NTA), ethylenediaminetetraacetic acid (EDTA),
diethylenetriaminepentaacetic acid
(DTMPA), alkyl- or alkenylsuccinic acid, soluble silicates or layered
silicates (e.g., SKS-6 from
Hoechst). The detergent may also be unbuilt, i.e. essentially free of
detergent builder. Enzymes,
and specifically amylases, either with or without starch binding domains, can
be used in a
variety of compositions including laundry and dishwashing applications,
surface cleaners, as
well as in compositions for ethanol production from starch or biomass.
The detergent may comprise one or more polymers. Examples include
carboxymethylcellulose (CMC), poly(vinylpyrrolidone) (PVP), polyethyleneglycol
(PEG),
poly(vinyl alcohol) (PVA), polycarboxylates such as polyacrylates,
maleic/acrylic acid
copolymers and lauryl methacrylate/acrylic acid copolymers.
The detergent may contain a bleaching system, which may comprise a H202 source
such as perborate or percarbonate, which may be combined with a peracid-
forming bleach
activator such as tetraacetylethylenediamine (TAED) or
nonanoyloxybenzenesulfonate (NOBS).
Alternatively, the bleaching system may comprise peroxyacids (e.g., the amide,
imide, or
sulfone type peroxyacids). The bleaching system can also be an enzymatic
bleaching system,
for example, perhydrolase, such as that described in International PCT
Application WO
2005/056783.
21
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
The enzymes of the detergent composition may be stabilized using conventional
stabilizing agents, e.g., a polyol such as propylene glycol or glycerol; a
sugar or sugar alcohol;
lactic acid; boric acid or a boric acid derivative such as, e.g., an aromatic
borate ester; and the
composition may be formulated as described in, e.g., WO 92/19709 and WO
92/19708.
The detergent may also contain other conventional detergent ingredients such
as e.g.,
fabric conditioners including clays, foam boosters, suds suppressors, anti-
corrosion agents, soil-
suspending agents, anti-soil redeposition agents, dyes, bactericides, tarnish
inhibiters, optical
brighteners, or perfumes.
The pH (measured in aqueous solution at use concentration) is usually neutral
or
alkaline, e.g., pH about 7.0 to about 11Ø
Particular forms of detergent compositions for inclusion of the present
teachings are
described, below.
Heavy Duty Dry/Solid (HDD) laundry detergent composition
Exemplary HDD laundry detergent compositions includes a detersive surfactant,
including anionic detersive surfactants (e.g., linear or branched or random
chain, substituted or
unsubstituted alkyl sulphates, alkyl sulphonates, alkyl alkoxylated sulphate,
alkyl phosphates,
alkyl phosphonates, alkyl carboxylates and/or mixtures thereof), non-ionic
detersive surfactant
(e.g., linear or branched or random chain, substituted or unsubstituted C8-C18
alkyl ethoxylates,
and/or C6-C12 alkyl phenol alkoxylates), cationic detersive surfactants (e.g.,
alkyl pyridinium
compounds, alkyl quaternary ammonium compounds, alkyl quaternary phosphonium
compounds, alkyl ternary sulphonium compounds, and mixtures thereof),
zwitterionic and/or
amphoteric detersive surfactants (e.g., alkanolamine sulpho-betaines),
ampholytic surfactants,
semi-polar non-ionic surfactants, and mixtures thereof; builders including
phosphate free
builders (for example zeolite builders examples which include zeolite A,
zeolite X, zeolite P and
zeolite MAP in the range of Owt% to less than l Owt%), phosphate builders (for
example sodium
tri-polyphosphate in the range of Owt% to less than lOwt%), citric acid,
citrate salts and
nitrilotriacetic acid, silicate salt (e.g., sodium or potassium silicate or
sodium meta-silicate in the
range of Owt% to less than lOwt%, or layered silicate (SKS-6)); carbonate salt
(e.g., sodium
carbonate and/or sodium bicarbonate in the range of 0 wt% to less than 80
wt%); and bleaching
agents including photobleaches (e.g., sulfonated zinc phthalocyanines,
sulfonated aluminum
phthalocyanines, xanthenes dyes, and mixtures thereof) hydrophobic or
hydrophilic bleach
activators (e.g., dodecanoyl oxybenzene sulfonate, decanoyl oxybenzene
sulfonate, decanoyl
22
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
oxybenzoic acid or salts thereof, 3.5,5-trimethy hexanoyl oxybenzene
sulfonate, tetraacetyl
ethylene diamine-TAED, nonanoyloxybenzene sulfonate-NOBS, nitrile quats, and
mixtures
thereof), sources of hydrogen peroxide (e.g., inorganic perhydrate salts
examples of which
include mono or tetra hydrate sodium salt of perborate, percarbonate,
persulfate, perphosphate,
or persilicate), preformed hydrophilic and/or hydrophobic peracids (e.g.,
percarboxylic acids and
salts, percarbonic acids and salts, perimidic acids and salts,
peroxymonosulfuric acids and salts,
and mixtures thereof), and/or bleach catalysts (e.g., imine bleach boosters
(examples of which
include iminium cations and polyions), iminium zwitterions, modified amines,
modified amine
oxides, N-sulphonyl imines, N-phosphonyl imines, N-acyl imines, thiadiazole
dioxides,
perfluoroimines, cyclic sugar ketones, and mixtures thereof, and metal-
containing bleach
catalysts (e.g., copper, iron, titanium, ruthenium, tungsten, molybdenum, or
manganese cations
along with an auxiliary metal cations such as zinc or aluminum and a
sequestrate such as
ethylenediaminetetraacetic acid, ethylenediaminetetra(methylenephosphonic
acid), and water-
soluble salts thereof).
The composition preferably includes enzymes, e.g., proteases, amylases,
lipases,
cellulases, choline oxidases, peroxidases/oxidases, pectate lyases,
mannanases, cutinases,
laccases, phospholipases, lysophospholipases, acyltransferase, perhydrolase,
arylesterase, and
any mixture thereof.
The composition may optionally include additional detergent ingredients
including
perfume microcapsules, starch encapsulated perfume accord, hueing agents,
additional
polymers, including fabric integrity and cationic polymers, dye-lock
ingredients, fabric-
softening agents, brighteners (for example C.I. Fluorescent brighteners),
flocculating agents,
chelating agents, alkoxylated polyamines, fabric deposition aids, and/or
cyclodextrin.
Automatic dishwashing (ADW) detergent composition
Exemplary ADW detergent composition includes non-ionic surfactants, including
ethoxylated non-ionic surfactants, alcohol alkoxylated surfactants, epoxy-
capped
poly(oxyalkylated) alcohols, or amine oxide surfactants present in amounts
from 0 to 10% by
weight; builders in the range of 5-60% including phosphate builders (e.g.,
mono-phosphates, di-
phosphates, tri-polyphosphates, other oligomeric-poylphosphates, sodium
tripolyphosphate-
STPP) and phosphate-free builders (e.g., amino acid-based compounds including
methyl-
glycine-diacetic acid (MGDA) and salts and derivatives thereof, glutamic-N,N-
diacetic acid
(GLDA) and salts and derivatives thereof, iminodisuccinic acid (IDS) and salts
and derivatives
23
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
thereof, carboxy methyl inulin and salts and derivatives thereof,
nitrilotriacetic acid (NTA),
diethylene triamine penta acetic acid (DTPA), B-alaninediacetic acid (B-ADA)
and their salts,
homopolymers and copolymers of poly-carboxylic acids and their partially or
completely
neutralized salts, monomeric polycarboxylic acids and hydroxycarboxylic acids
and their salts
in the range of 0.5% to 50% by weight; sulfonated/carboxylated polymers in the
range of about
0.1 % to about 50% by weight to to provide dimensional stability; drying aids
in the range of
about 0.1 % to about 10% by weight (e.g., polyesters, especially anionic
polyesters, optionally
together with further monomers with 3 to 6 functionalities - typically acid,
alcohol or ester
functionalities which are conducive to polycondensation. polycarbonate-,
polyurethane- and/or
polyurea-polyorganosiloxane compounds or precursor compounds, thereof,
particularly of the
reactive cyclic carbonate and urea type); silicates in the range from about 1
% to about 20% by
weight (including sodium or potassium silicates for example sodium disilicate,
sodium meta-
silicate and crystalline phyllosilicates); inorganic bleach (e.g., perhydrate
salts such as perborate,
percarbonate, perphosphate, persulfate and persilicate salts) and organic
bleach (e.2., organic
peroxyacids, including diacyl and tetraacylperoxides, such as
diperoxydodecanedioc acid,
diperoxytetradecanedioc acid, and diperoxyhexadecanedioc acid); bleach
activators (i.e., organic
peracid precursors in the range from about 0.1 % to about 10% by weight);
bleach catalysts
(e.g., manganese triazacyclononane and related complexes, Co, Cu, Mn, and Fe
bispyridylamine
and related complexes, and pentamine acetate cobalt(III) and related
complexes); metal care
.. agents in the range from about 0.1% to 5% by weight (e.g., benzatriazoles,
metal salts and
complexes, and/or silicates); enzymes in the range from about 0.01 to 5.0 mg
of active enzyme
per gram of automatic dishwashing detergent composition (e.g., proteases,
amylases, lipases,
cellulases, choline oxidases, peroxidases/oxidases, pectate lyases,
mannanases, cutinases,
laccases, phospholipases, lysophospholipases, acyltransferase, perhydrolase,
arylesterase, and
mixtures thereof); and enzyme stabilizer components (e.g., oligosaccharides,
polysaccharides,
and inorganic divalent metal salts).
Additional detergent compositions
Additional exemplary detergent formulations to which any of a variety of
relevant
enzymes in the present granule compositions can be added are described, below,
in the
numbered paragraphs.
1) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising linear alkylbenzenesulfonate (calculated as acid) about 7% to about
12%; alcohol
24
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
ethoxysulfate (e.g., C12_18 alcohol, 1-2 ethylene oxide (E0)) or alkyl sulfate
(e.g., C16_18) about
1% to about 4%; alcohol ethoxylate (e.g., C14_15 alcohol, 7 EO) about 5% to
about 9%; sodium
carbonate (e.g., Na2CO3) about 14% to about 20%; soluble silicate (e.g., Na2O,
2SiO)) about 2
to about 6%; zeolite (e.g., NaA1SiO4) about 15% to about 22%; sodium sulfate
(e.g., Na2SO4)
0% to about 6%; sodium citrate/citric acid (e.g., C6HcNa307/C6H807) about 0%
to about 15%;
sodium perborate (e.g., NaBO3H20) about 11% to about 18%; TAED about 2% to
about 6%;
carboxymethylcellulose (CMC) and 0% to about 2%; polymers (e.g.,
maleic/acrylic acid,
copolymer, PVP, PEG) 0-3%; enzymes (calculated as pure enzyme) 0.0001-0.1%
protein; and
minor ingredients (e.g., suds suppressors, perfumes, optical brightener,
photobleach) 0-5%.
2) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising linear alkylbenzenesulfonate (calculated as acid) about 6% to about
11%; alcohol
ethoxysulfate (e.g., C12-18 alcohol, 1-2 EO) or alkyl sulfate (e.g., C16-18)
about 1% to about 3%;
alcohol ethoxylate (e.g., C14-15 alcohol, 7 EO) about 5% to about 9%; sodium
carbonate (e.g.,
Na2CO3) about 15% to about 21%; soluble silicate (e.g., Na20, 2Si02) about 1%
to about 4%;
zeolite (e.g., NaA1SiO4) about 24% to about 34%; sodium sulfate (e.g,. Na2SO4)
about 4% to
about 10%; sodium citrate/citric acid (e.g., C6H5Na307/ C6H807) 0% to about
15%;
carboxymethylcellulose (CMC) 0% to about 2%; polymers (e.g., maleic/acrylic
acid copolymer,
PVP, PEG) 1-6%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; minor
ingredients
(e.g., suds suppressors, perfume) 0-5%.
3) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising linear alkylbenzenesulfonate (calculated as acid) about 5% to about
9%; alcohol
ethoxylate (e.g., C12-15 alcohol, 7 EO) about 7% to about 14%; Soap as fatty
acid (e.g., C16_99
fatty acid) about 1 to about 3%; sodium carbonate (as Na2C0 about 10% to about
17%; soluble
silicate (e.g., Na2O, 2Si02) about 3% to about 9%; zeolite (as NaA1SiO4) about
23% to about
33%; sodium sulfate (e.g.,Na2SO4) 0% to about 4%; sodium perborate (e.g.,
NaBO3H20) about
8% to about 16%; TAED about 2% to about 8%; phosphonate (e.g., EDTMPA) 0% to
about 1%;
carboxymethylcellulose (CMC) 0% to about 2%; polymers (e.g., maleic/acrylic
acid copolymer,
PVP, PEG) 0-3%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; minor
ingredients
(e.g., suds suppressors, perfume, optical brightener) 0-5%.
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
4) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising linear alkylbenzenesulfonate (calculated as acid) about 8% to about
12%; alcohol
ethoxylate (e.g., C12-15 alcohol, 7 EO) about 10% to about 25%; sodium
carbonate (as Na.2CO3)
about 14% to about 22%; soluble silicate (e.g., Na2O, 2Si02) about 1% to about
5%; zeolite
.. (e.g., NaA1SiO4) about 25% to about 35%; sodium sulfate (e.g., Na7SO4) 0%
to about 10%;
carboxymethylcellulose (CMC) 0% to about 2%; polymers (e.g., maleic/acrylic
acid copolymer,
PVP, PEG) 1-3%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; and
minor
ingredients (e.g., suds suppressors, perfume) 0-5%.
5) An aqueous liquid detergent composition comprising linear
alkylbenzenesulfonate
(calculated as acid) about 15% to about 21%; alcohol ethoxylate (e.g., C12-15
alcohol, 7 EO or
C12_15 alcohol, 5 EO) about 12% to about 18%; soap as fatty acid (e.g., oleic
acid) about 3% to
about 13%; alkenylsuccinic acid (C12-14) 0% to about 13%; aminoethanol about
8% to about
18%; citric acid about 2% to about 8%; phosphonate 0% to about 3%; polymers
(e.g., PVP,
PEG) 0% to about 3%; borate (e.g., B407) 0% to about 2%; ethanol 0% to about
3%; propylene
glycol about 8% to about 14%; enzymes (calculated as pure enzyme protein)
0.0001-0.1%; and
minor ingredients (e.g., dispersants, suds suppressors, perfume, optical
brightener) 0-5%.
6) An aqueous structured liquid detergent composition comprising linear
alkylbenzenesulfonate
(calculated as acid) about 15% to about 21%; alcohol ethoxylate (e.g., C17_15
alcohol, 7 EO, or
C12_15 alcohol, 5 EO) 3-9%; soap as fatty acid (e.g., oleic acid) about 3% to
about 10%; zeolite
(as NaA1SiO4) about 14% to about 22%; potassium citrate about 9% to about 18%;
borate (e.g.,
B407) 0% to about 2%; carboxymethylcellulose (CMC) 0% to about 2%; polymers
(e.g., PEG,
PVP) 0% to about 3%; anchoring polymers such as, e.g., lauryl
methacrylate/acrylic acid
copolymer; molar ratio 25:1, MW 3800) 0% to about 3%; glycerol 0% to about 5%;
enzymes
(calculated as pure enzyme protein) 0.0001-0.1%; and minor ingredients (e.g.,
dispersants, suds
suppressors, perfume, optical brighteners) 0-5%.
7) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising fatty alcohol sulfate about 5% to about 10%; ethoxylated fatty acid
monoethanolamide about 3% to about 9%; soap as fatty acid 0-3%; sodium
carbonate (e.g.,
Na2CO3) about 5% to about 10%; Soluble silicate (e.g., Na2O, 2Si02) about 1%
to about 4%;
zeolite (e.g., NaA1SiO4) about 20% to about 40%; Sodium sulfate (e.g., Na2SO4)
about 2% to
26
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
about 8%; sodium perborate (e.g., NaB031-120) about 12% to about 18%; TAED
about 2% to
about 7%; polymers (e.g., maleic/acrylic acid copolymer, PEG) about 1% to
about 5%; enzymes
(calculated as pure enzyme protein) 0.0001-0.1%; and minor ingredients (e.g.,
optical
brightener, suds suppressors, perfume) 0-5%.
8) A detergent composition formulated as a granulate comprising linear
alkylbenzenesulfonate
(calculated as acid) about 8% to about 14%; ethoxylated fatty acid
monoethanolamide about 5%
to about 11%; soap as fatty acid 0% to about 3%; sodium carbonate (e.g..
Na2CO3) about 4% to
about 10%; soluble silicate (Na2O, 2Si02) about 1% to about 4%; zeolite (e.g.,
NaA1SiO4) about
30% to about 50%; sodium sulfate (e.g., Na7SO4) about 3% to about 11%; sodium
citrate (e.g.,
C6H5Na307) about 5% to about 12%; polymers (e.g.. PVP, maleic/acrylic acid
copolymer, PEG)
about 1% to about 5%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%;
and minor
ingredients (e.g., suds suppressors, perfume) 0-5%.
9) A detergent composition formulated as a granulate comprising linear
alkylbenzenesulfonate
(calculated as acid) about 6% to about 12%; nonionic surfactant about 1% to
about 4%; soap as
fatty acid about 2% to about 6%; sodium carbonate (e.g., Na2CO3) about 14% to
about 22%;
zeolite (e.g., NaA1SiO4) about 18% to about 32%; sodium sulfate (e.g., Na2SO4)
about 5% to
about 20%; sodium citrate (e.g., C6H5Na307) about 3% to about 8%; sodium
perborate (e.g.,
NaBO3H20) about 4% to about 9%; bleach activator (e.g., NOBS or TAED) about 1%
to about
5%; carboxymethylcellulose (CMC) 0% to about 2%; polymers (e.g.,
polycarboxylate or PEG)
about 1% to about 5%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%;
and minor
ingredients (e.g., optical brightener, perfume) 0-5%.
10) An aqueous liquid detergent composition comprising linear
alkylbenzenesulfonate
(calculated as acid) about 15% to about 23%; alcohol ethoxysulfate (e.g., C p
15 alcohol, 2-3 E0)
about 8% to about 15%; alcohol ethoxylate (e.g., C1245 alcohol, 7 EO, or C1245
alcohol, 5 EO)
about 3% to about 9%; soap as fatty acid (e.g., lauric acid) 0% to about 3%;
aminoethanol about
1% to about 5%; sodium citrate about 5% to about 10%; hydrotrope (e.g., sodium
toluensulfonate) about 2% to about 6%; borate (e.g., B407) 0% to about 2%;
carboxymethylcellulose 0% to about 1%; ethanol about 1% to about 3%; propylene
glycol about
2% to about 5%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; and
minor
ingredients (e.g., polymers, dispersants, perfume, optical brighteners) 0-5%.
27
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
11) An aqueous liquid detergent composition comprising linear
alkylbenzenesulfonate
(calculated as acid) about 20% to about 32%; alcohol ethoxylate (e.g., C12_15
alcohol, 7 Ea or
C12-15 alcohol, 5 E0) 6-12%; aminoethanol about 2% to about 6%; citric acid
about 8% to about
.. 14%; borate (e.g., B407) about 1% to about 3%; polymer (e.g.,
maleic/acrylic acid copolymer,
anchoring polymer such as, e.g., lauryl methacrylate/acrylic acid copolymer)
0% to about 3%;
glycerol about 3% to about 8%; enzymes (calculated as pure enzyme protein)
0.0001-0.1%; and
minor ingredients (e.g., hydrotropes, dispersants, perfume, optical
brighteners) 0-5%.
12) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising anionic surfactant (linear alkylbenzenesulfonate, alkyl sulfate, ii-
olefinsulfonate,
sulfo fatty acid methyl esters, alkanesulfonates, soap) about 25% to about
40%; nonionic
surfactant (e.g., alcohol ethoxylate) about 1% to about 10%; sodium carbonate
(e.g., Na2CO3)
about 8% to about 25%; soluble silicates (e.g., Na2O, 2Si02) about 5% to about
15%; sodium
sulfate (e.g., Na2SO4) 0% to about 5%; zeolite (NaA1SiO4) about 15% to about
28%; sodium
perborate (e.g., NaB03.4H20) 0% to about 20%; bleach activator (TAED or NOBS)
about 0% to
about 5%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; minor
ingredients (e.g.,
perfume, optical brighteners) 0-3%.
13) Detergent compositions as described in compositions 1)-12) supra, wherein
all or part of
the linear alkylbenzenesulfonate is replaced by (C12-C18) alkyl sulfate.
14) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising (C12-C18) alkyl sulfate about 9% to about 15%; alcohol ethoxylate
about 3% to about
6%; polyhydroxy alkyl fatty acid amide about 1% to about 5%; zeolite (e.g.,
NaA1SiO4) about
10% to about 20%; layered disilicate (e.g.. SK56 from Hoechst) about 10% to
about 20%;
sodium carbonate (e.g., Na2CO3) about 3% to about 12%; soluble silicate (e.g.,
Na2O, 2Si02)
0% to about 6%; sodium citrate about 4% to about 8%; sodium percarbonate about
13% to about
22%; TAED about 3% to about 8%; polymers (e.g., polycarboxylates and PVP) 0%
to about
5%; enzymes (calculated as pure enzyme protein) 0.0001-0.1%; and minor
ingredients (e.g.,
optical brightener, photobleach, perfume, suds suppressors) 0-5%.
28
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
15) A detergent composition formulated as a granulate having a bulk density of
at least 600 g/L
comprising (C12-C18) alkyl sulfate about 4% to about 8%; alcohol ethoxylate
about 11% to about
15%; soap about 1% to about 4%; zeolite MAP or zeolite A about 35% to about
45%; sodium
carbonate (as Na2CO3) about 2% to about 8%; soluble silicate (e.g., Na2O,
2Si02) 0% to about
4%; sodium percarbonate about 13% to about 22%; TAED 1-8%;
carboxymethylcellulose
(CMC) 0% to about 3%; polymers (e.g., polycarboxylates and PVP) 0% to about
3%; enzymes
(calculated as pure enzyme protein) 0.0001-0.1%; and minor ingredients (e.g.,
optical
brightener, phosphonate, perfume) 0-3%.
16) Detergent formulations as described in 1)-15) supra, which contain a
stabilized or
encapsulated peracid, either as an additional component or as a substitute for
already specified
bleach systems.
17) Detergent compositions as described supra in 1), 3), 7), 9), and 12),
wherein perborate is
replaced by percarbonate.
18) Detergent compositions as described supra in 1), 3), 7), 9), 12), 14), and
15), which
additionally contain a manganese catalyst. The manganese catalyst for example
is one of the
compounds described in "Efficient manganese catalysts for low-temperature
bleaching," Nature
369: 637-639 (1994).
19) Detergent composition formulated as a non-aqueous detergent liquid
comprising a liquid
nonionic surfactant such as, e.g., linear alkoxylated primary alcohol, a
builder system (e.g.,
phosphate), an enzyme(s), and alkali. The detergent may also comprise anionic
surfactant
and/or a bleach system.
As above, the present enzyme may be incorporated at a concentration
conventionally
employed in detergents. It is at present contemplated that, in the detergent
composition, the
enzyme may be added in an amount corresponding to 0.00001-1.0 mg (calculated
as pure
enzyme protein) of enzyme polypeptide per liter of wash liquor.
29
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
The detergent composition may also contain other conventional detergent
ingredients, e.g., deflocculant material, filler material, foam depressors,
anti-corrosion agents,
soil-suspending agents, sequestering agents, anti-soil redeposition agents,
dehydrating agents,
dyes, bactericides, fluorescers, thickeners, and perfumes.
The detergent composition may be formulated as a hand (manual) or machine
(automatic) laundry detergent composition, including a laundry additive
composition suitable for
pre-treatment of stained fabrics and a rinse added fabric softener
composition, or be formulated
as a detergent composition for use in general household hard surface cleaning
operations, or be
formulated for manual or automatic dishwashing operations.
Any of the cleaning compositions described, herein, may include any number of
additional enzymes. In general the enzyme(s) should be compatible with the
selected detergent,
(e.g., with respect to pH-optimum, compatibility with other enzymatic and non-
enzymatic
ingredients, and the like), and the enzyme(s) should be present in effective
amounts. The
following enzymes are provided as examples.
Proteases: Suitable proteases include those of animal, vegetable or microbial
origin.
Chemically modified or protein engineered mutants are included, as well as
naturally processed
proteins. The protease may be a serine protease or a metalloprotease, an
alkaline microbial
protease, a trypsin-like protease, or a chymotrypsin-like protease. Examples
of alkaline
.. proteases are subtilisins, for example those derived from Bacillus, e.g.,
subtilisin Novo,
subtilisin Carlsberg, subtilisin 309, subtilisin 147, and subtilisin 168 (see,
e.g., WO 89/06279).
Examples of trypsin-like proteases are trypsin (e.g., of porcine or bovine
origin), and Fusarium
proteases (see, e.g., WO 89/06270 and WO 94/25583). Examples of useful
proteases also
include but are not limited to the variants described in WO 92/19729, WO
98/20115, WO
98/20116. and WO 98/34946. Commercially available protease enzymes include but
are not
limited to: ALCALASEO, SAVINASEO, PRI1VIASETm, DURALASETM, ESPERASEO,
KANNASETM, and BLAZETM (Novo Nordisk A/S and Novozymes A/S); MAXATASEO,
MAXACALTM, MAXAPEMTm, PROPERASEO, PURAFECTO, PURAFECT OXPTM, FN2Tm,
and FN3TM (Danisco US Inc.). Other exemplary proteases include NprE from
Bacillus
amyloliquifctciens and ASP from Cellulomonas sp. strain 69B4.
Lipases: Suitable lipases include those of bacterial or fungal origin.
Chemically modified,
proteolytically modified, or protein engineered mutants are included. Examples
of useful lipases
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
include but are not limited to lipases from Humicola (synonym Thermomyces),
e.g., from H.
lanuginosa (T. lanuginosus) (see e.g., EP 258068 and EP 305216), from H.
insolens (see e.g.,
WO 96/13580); a Pseudomonas lipase (e.g., from P. alcaligenes or P.
pseudoalcaligenes; see,
e.g., EP 218 272), P. cepacia (see e.g., EP 331 376), P. stutzeri (see e.g.,
GB 1,372.034), P.
fluorescens, Pseudomonas sp. strain SD 705 (see e.g., WO 95/06720 and WO
96/27002), P.
wisconsinensis (see e.g., WO 96/12012); a Bacillus lipase (e.g., from B.
subtilis; see e.g., Dartois
etal. Biochemica et Biophysica Acta, 1131: 253-360 (1993)), B.
stearothermophilus (see e.g.,
JP 64/744992). or B. pumilus (see e.g., WO 91/16422). Additional lipase
variants contemplated
for use in the formulations include those described for example in: WO
92/05249, WO
94/01541. WO 95/35381, WO 96/00292, WO 95/30744, WO 94/25578, WO 95/14783, WO
95/22615. WO 97/04079, WO 97/07202, EP 407225, and EP 260105. Some
commercially
available lipase enzymes include LIPOLASEO and LIPOLASE ULTRATm (Novo Nordisk
A/S
and Novozymes A/S).
Polyesterases: Suitable polyesterases can be included in the composition, such
as those
described in, for example, WO 01/34899, WO 01/14629. and US6933140.
Amylases: The compositions can be combined with amylases, such as non-
production enhanced
amylase. These can include commercially available amylases, such as but not
limited to
STAINZYMEO, NATALASEO, DURAMYLO, TERMAMYLO, FUNGAMYLO and BANTM
(Novo Nordisk A/S and Novozymes A/S); RAPIDASE , POWERASEO, and PURASTARO
(from Danisco US Inc.).
Cellulases: Cellulases can be added to the compositions. Suitable cellulases
include those of
bacterial or fungal origin. Chemically modified or protein engineered mutants
are included.
Suitable cellulases include cellulases from the genera Bacillus, Pseudomonas,
Humicola,
Fusarium, Thielavia, Acremonium, e.g., the fungal cellulases produced from
Hurnicola insolens,
Myceliophthora thermophila and Fusarium oxysporum disclosed for example in
U.S. Patent
Nos. 4,435,307; 5,648,263; 5,691,178; 5,776,757; and WO 89/09259. Exemplary
cellulases
contemplated for use are those having color care benefit for the textile.
Examples of such
cellulases are cellulases described in for example EP 0495257, EP 0531372, WO
96/11262, WO
96/29397. and WO 98/08940. Other examples are cellulase variants, such as
those described in
WO 94/07998; WO 98/12307; WO 95/24471; PCT/DK98/00299; EP 531315; U.S. Patent
Nos.
31
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
5,457,046; 5,686,593; and 5,763,254. Commercially available cellulases include
CELLUZYME and CAREZYME (Novo Nordisk A/S and Novozymes A/S); and
PURADAX HA (Danisco US Inc.); and KAC-500(B)TM (Kao Corporation).
Peroxidases/Oxidases: Suitable peroxidases/oxidases contemplated for use in
the compositions
include those of plant, bacterial or fungal origin. Chemically modified or
protein engineered
mutants are included. Examples of useful peroxidases include peroxidases from
Coprinus, e.g.,
from C. cinereus, and variants thereof as those described in WO 93/24618, WO
95/10602, and
WO 98/15257. Commercially available peroxidases include for example
GUARDZYMETm
.. (Novo Nordisk A/S and Novozymes A/S).
The detergent composition can also comprise 2,6-13-D-fructan hydrolase, which
is
effective for removal/cleaning of biofilm present on household and/or
industrial textile/laundry.
The detergent enzyme(s) may be included in a detergent composition by adding
separate additives containing one or more enzymes, or by adding a combined
additive
comprising all of these enzymes. A detergent additive, i.e. a separate
additive or a combined
additive, can be formulated e.g., as a granulate, a liquid, a slurry, and the
like. Exemplary
detergent additive formulations include but are not limited to granulates, in
particular non-
dusting granulates, liquids, in particular stabilized liquids or slurries.
Reduced-dusting granulates may be produced, and added with the present granule
compositions, e.g., as disclosed in U.S. Patent Nos. 4,106,991 and 4,661,452
and may optionally
be coated by methods known in the art. Examples of waxy coating materials are
poly(ethylene
oxide) products (e.g., polyethyleneglycol, PEG) with mean molar weights of
1,000 to 20,000;
ethoxylated nonylphenols having from 16 to 50 ethylene oxide units;
ethoxylated fatty alcohols
in which the alcohol contains from 12 to 20 carbon atoms and in which there
are 15 to 80
ethylene oxide units; fatty alcohols; fatty acids; and mono- and di- and
triglycerides of fatty
acids. Examples of film-forming coating materials suitable for application by
fluid bed
techniques are given in, for example, GB 1483591. Liquid enzyme preparations
may, for
instance, be stabilized by adding a polyol such as propylene glycol, a sugar
or sugar alcohol,
lactic acid or boric acid according to established methods.
The detergent composition may be in any convenient form, e.g., a bar, a
tablet, a
powder, a granule, a paste, or a liquid. A liquid detergent may be aqueous,
typically containing
32
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
up to about 70% water, and 0% to about 30% organic solvent. Compact detergent
gels
containing about 30% or less water are also contemplated. The detergent
composition can
optionally comprise one or more surfactants, which may be non-ionic, including
semi-polar
and/or anionic and/or cationic and/or zwitterionic. The surfactants can be
present in a wide
range, from about 0.1% to about 60% by weight.
When included therein the detergent will typically contain from about 1% to
about
40% of an anionic surfactant, such as linear alkylbenzenesulfonate, a-
olefinsulfonate, alkyl
sulfate (fatty alcohol sulfate), alcohol ethoxysulfate, secondary
alkanesulfonate, a-sulfo fatty
acid methyl ester, alkyl- or alkenylsuccinic acid, or soap.
When included therein, the detergent will usually contain from about 0.2% to
about
40% of a non-ionic surfactant such as alcohol ethoxylate, nonylphenol
ethoxylate,
alkylpolyglycoside, alkyldimethylamineoxide, ethoxylated fatty acid
monoethanolamide, fatty
acid monoethanolamide, polyhydroxy alkyl fatty acid amide, or N-acyl-N-alkyl
derivatives of
glucosamine ("glucamides").
The detergent may contain 0% to about 65% of a detergent builder or complexing
agent such as zeolite, diphosphate, triphosphate, phosphonate, carbonate,
citrate, nitrilotriacetic
acid, ethylenediaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic
acid, alkyl- or
alkenylsuccinic acid, soluble silicates or layered silicates (e.g.,SKS-6 from
Hoechst).
The detergent may comprise one or more polymers. Exemplary polymers include
carboxymethylcellulose (CMC), poly(vinylpyrrolidone) (PVP), poly(ethylene
glycol) (PEG),
poly(vinyl alcohol) (PVA), poly(vinylpyridine-N-oxide), poly(vinylimidazole),
polycarboxylates e.g., polyacrylates, maleic/acrylic acid copolymers), and
lauryl
methacrylate/acrylic acid copolymers.
The enzyme(s) of the detergent composition may be stabilized using
conventional
stabilizing agents, e.g., as polyol (e.g., propylene glycol or glycerol), a
sugar or sugar alcohol,
lactic acid, boric acid, or a boric acid derivative (e.g., an aromatic borate
ester), or a phenyl
boronic acid derivative (e.g., 4-formylphenyl boronic acid). The composition
may be
formulated as described in WO 92/19709 and WO 92/19708.
It is contemplated that in the enzyme-containing detergent compositions, it
may be
added in an amount corresponding to about 0.01 to about 100 mg of enzyme
protein per liter of
wash liquor (e.g., about 0.05 to about 5.0 mg of enzyme protein per liter of
wash liquor or 0.1 to
about 1.0 mg of enzyme protein per liter of wash liquor).
33
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Although the present compositions and methods have been described with
reference
to the details below, it would be understood that various modifications can be
made.
Numerous enzyme cleaning assays are known in the art, including swatch and
micro-
swatch assays.
Brewing Compositions
The present granule composition may be a component of a brewing composition
used
in a process of providing a fermented beverage, such as brewing. It is
believed that non-
fermentable carbohydrates form the majority of the dissolved solids in the
final beer. This
residue remains because of the inability of malt amylases to hydrolyze the
alpha-1,6-linkages of
the starch. The non-fermentable carbohydrates contribute about 50 calories per
12 ounces
(about 340 grams) of beer. The present granule composition, usually in
combination with a
glucoamylase and optionally a pullulanase and/or isoamylase, assist in
converting the starch into
dextrins and fermentable sugars, lowering the residual non-fermentable
carbohydrates in the
final beer.
The principal raw materials used in making these beverages are water, hops and
malt.
In addition, but also exclusively, adjuncts such as common corn grits, refined
corn grits,
brewer's milled yeast, rice, sorghum, refined corn starch, barley, barley
starch, dehusked barley,
wheat, wheat starch, torrified cereal, cereal flakes, rye, oats, potato,
tapioca, and syrups, such as
corn syrup, sugar cane syrup, inverted sugar syrup, barley and/or wheat
syrups, and the like may
be used as a source of starch.
For a number of reasons, the malt, which is produced principally from selected
varieties of barley, has an important effect on the overall character and
quality of the beer. First,
the malt is the primary flavoring agent in beer. Second, the malt provides the
major portion of
the fermentable sugar. Third, the malt provides the proteins, which will
contribute to the body
and foam character of the beer. Fourth, the malt provides the necessary
enzymatic activity
during mashing. Hops also contribute significantly to beer quality, including
flavoring. In
particular, hops (or hops constituents) add desirable bittering substances to
the beer. In addition,
the hops can act as protein precipitants, establish preservative agents and
aid in foam formation
and stabilization.
Cereals (grains), such as barley, oats, wheat, but also corn and rice are
often used for
brewing, both in industry and for home brewing, but also other plant
components, such as hops
34
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
are often added. The components used in brewing may be unmalted or may be
malted, i.e.,
partially germinated, resulting in an increase in the levels of enzymes,
including a-amylase. For
successful brewing, adequate levels of a-amylase enzyme activity are necessary
to ensure the
appropriate levels of sugars for fermentation. The present granule composition
may also be
added to the components used for brewing.
As used herein, the term -stock" means grains and plant components that are
crushed
or broken. For example, barley used in beer production is a grain that has
been coarsely ground
or crushed to yield a consistency appropriate for producing a mash for
fermentation. As used
herein, the term "stock" includes any of the aforementioned types of plants
and grains in crushed
or coarsely ground forms. The methods described herein may be used to
determine a-amylase
activity levels in both flours and stock.
Processes for making beer are well known in the art. See, e.g., Wolfgang Kunze
(2004) "Technology Brewing and Malting," Research and Teaching Institute of
Brewing, Berlin
(VLB), 3rd edition. Briefly, the process involves: (a) preparing a mash, (b)
filtering the mash to
prepare a wort, and (c) fermenting the wort to obtain a fermented beverage,
such as beer.
Typically, milled or crushed malt, malt and adjunct, or adjunct is mixed with
water and held for
a period of time under controlled temperatures to permit the enzymes present
in the malt and/or
adjunct to convert the starch present in the malt into fermentable sugars. The
mash is then
transferred to a mash filter where the liquid is separated from the grain
residue. This sweet
liquid is called "wort," and the left over grain residue is called "spent
grain." The mash is
typically subjected to an extraction, which involves adding water to the mash
in order to recover
the residual soluble extract from the spent grain. The wort is then boiled
vigorously to sterilizes
the wort and help develop the color, flavor and odor. Hops are added at some
point during the
boiling. The wort is cooled and transferred to a fermentor.
The wort is then contacted in a fermentor with yeast. The fermentor may be
chilled
to stop fermentation. The yeast that may flocculate js removed. Finally, the
beer is cooled and
stored for a period of time, during which the beer clarifies and its flavor
develops, and any
material that might impair the appearance, flavor, and shelf life of the beer
settles out. The beer
usually contains from about 2% to about 10% v/v alcohol, although beer with a
higher alcohol
content, e.g., 18% v/v, may be obtained. Prior to packaging, the beer is
carbonated and,
optionally, filtered, and pasteurized.
The brewing composition comprising an alpha-amylase, often, but not
necessarily in
combination with one or more exogenous enzymes, such as glucoamylase(s),
pullulanase(s)
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
and/or isoamylase(s), and any combination thereof, may be added to the mash of
step (a) above,
i.e., during the preparation of the mash. Alternatively, or in addition, the
brewing composition
may be added to the mash of step (b) above, such as during the filtration of
the mash.
Alternatively, or in addition, the brewing composition may be added to the
wort of step (c)
above, such as during the fermenting of the wort.
Particular embodiments pertains to any of the above uses, methods or fermented
beverages, wherein said fermented beverage is a beer, such as full malted
beer, beer brewed
under the "Reinheitsgebot," ale, IPA, lager, bitter, Happoshu (second beer),
third beer, dry beer,
near beer, light beer, low alcohol beer, low calorie beer, porter. bock beer,
stout, malt liquor,
.. non-alcoholic beer, non-alcoholic malt liquor and the like, but also
alternative cereal and malt
beverages such as fruit flavoured malt beverages, e.g., citrus flavoured, such
as lemon-, orange-,
lime-, or berry-flavoured malt beverages, liquor flavoured malt beverages,
e.g., vodka-, rum-, or
tequila-flavoured malt liquor, or coffee flavoured malt beverages, such as
caffeine-flavoured
malt liquor, and the like.
Additional Animal Feed Embodiments
In some embodiments, especially in the context of animal feed applications,
the
granules of the present teachings can be used with any of a variety of at
least one of the
following enzymes:
XYLANASES
Commercial
Name Company Xylanase type Xylanase source
Allzyme PT Al'tech endo-1,4-f3-xylanase Aspergillus Niger
Andres Pintaluba
Amylofeed S.A endo-1,4-3-xylanase Aspergillus Niger
(phoenicis)
Avemix 02 CS Aveve endo-1,4-P-xylanase Trichodenna reesei
AveMix XG 10 Aveve, NL endo-1,4-13-xylanase Trichodenna reesei
Trichodenna
Avizyme 1100 Danisco endo-1,4-3-xylanase longibrachiaium
Trichoderma
Avizyme 1110 Danisco endo-1,4-P-xyl an ase longibrachiatum
Trichoderma
Avizyme 1202 Danisco endo-1,4-P-xylanase longibrachiatum
Trichoderma
Avizyme 1210 Danisco endo-1,4-13-xylanase longibrachiatum
Trichoderma
Avizyme 1302 Danisco endo-1,4-P-xylanase longibrachiatum
Trichoderma
Avizyme 1500 Danisco endo-1,4-P-xylanase longibrachiatum
36
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
Trichoderma
Avizyme 1505 Danisco endo-1,4-13-xylanase longibrachiatum
Trichoderma
Avizyme SX Danisco endo-1,4-13-xylanase longibrachiatum
Belfeed MP 1 00 Beldem endo-1,4-13-xy1anase Bacillus subtilis
Biofeed Plus DSM endo- 1 ,4- 13-xylanase Humicola insolens
Bio Feed Novozymes
Wheat endo-L4-13-xylanase
Danisco
Glycosidase Danisco Animal
(TPT/L) Nutrition endo- 1 ,4- I3-xylanase Trichoderma reesei
Danisco
Xylanase Danisco endo-1,4-13-xylanase Trichoderma reesei
Trichodenna
longibrachiatum
Dyadic endo-L4-13-D- (formerly Trichoderma
Xylanase PLUS Dyadic xylanase reesei)
Econase XT ABVista endo-1,4-13-xylanase Trichoderma reesei
Andres Pintaluba
Endofeed DC S.A. endo-1,4-P-xylanase Aspergillus Niger
Trichoderma
Feedl yve AXL Lyven endo- 4-P-xylanase longibrachiatum
Grindazym GP Danisco endo- I3-xylanase Aspergillus Niger
Grindazym GV Danisco endo-1,4-13-xylanase Aspergillus Niger
Trichoderma
Hostazym X Huvepharma endo-1,4-f3-xylanase longibrachiatum
Kemzyme Plus
Dry Kemin endo-1,4-13-xylanase Trichoderma viride
Kemzyme Plus
Liquid Kemin endo- 1 ,4- 13-xylanase Trichoderma viride
Kemzyme W dry Kemin endo-1,4-13-xylanase Trichodenna viride
Kemzyme W
liquid Kemin endo-1,4-13-xylanase Trichoderma viride
Trichoderma
Natugrain BASF endo-L4-13-xylanase longibrachiatum
Natugrain TS
Plus BASF endo-1,4-13-xylanase Aspergillus Niger
Natugrain Wheat BASF endo-1,4-13-xylanase Aspergillus Niger
Natugrain TS/L BASF endo-1,4-f3-xylanase Aspergillus Niger
Trichoderma
longibrachiatum
Natuzyme Bioproton endo-1,4-13-xylanase /Trichoderma reesei
Trichoderma
Porzyme 8100 Danisco endo- 1,4- 13-xyl an ase longibrachiatum
Trichoderma
Porzyme 8300 Danisco endo-L4-13-xylanase longibrachiatum
37
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Trichoderma
Porzyme 9102 Danisco endo-1,4-13-xylanase longibrachiatum
Porzyme
9310/Avizyme Trichoderma
1310 Danisco endo-1,4-13-xylanase longibrachiatum
Trichoderma
Porzyme tp100 Danisco endo-1,4-13-xylanase longibrachiatum
The rmomyces lanuginosus
gene
expressed in Aspergillus
Ronozyme AX DSM endo-1,4-13-xy1anase oryzae
Thermomyces lanuginosus
gene
expressed in Aspergillus
Ronozyme WX DSM/Novozymes endo-1,4-13-xylanase oryzae
Rovabio Excel Adisseo endo-1,4-P-xylanase Penicillium funiculosum
Trichoderma
Roxazyme G2 DSM/Novozymes endo-1,4-13-xylanase longibrachiatum
Trichodenna
Safizym X Le Saffre endo-1,4-13-xy1anase longibrachiatum
Trichodenna
Xylanase Lyven endo-1,4-13-xylanase longibrachiatum
Amylase
Commercial
product Company Amylase type Amylase source
AlphaStar PLUS Dyadic alpha amylase Bacillus subtilis
GlucoStar PLUS Dyadic gluco-amylase Aspergillus niger
Andres Pintaluba
Amylofeed S.A alpha amylase Aspergillus
oryzae
Avizyme 1500 Danisco alpha amylase Bacillus
amyloliquefaciens
Avizyme 1505 Danisco alpha amylase Bacillus
amyloliquefaciens
Kemzyme Plus
Dry Kemin alpha-amylase Bacillus
amyloliquefaciens
Kemzyme Plus
Liquid Kemin alpha-amylase Bacillus
amyloliquefaciens
Kemzyme W dry Kemin alpha-amylase Bacillus
amyloliquefaciens
Kemzyme W
liquid Kemin alpha-amylase Bacillus
amyloliquefaciens
Trichoderma longibrachiatum
Natuzyme Bioproton alpha-amylase /Trichoderma
reesei
Porzyme 8100 Danisco alpha-amylase Bacillus
amyloliquefaciens
Porzyme tp100 Danisco alpha-amylase Bacillus
amyloliquefaciens
Ronozyme A DSM/Novozymes alpha-amylase
Bacillus amyloliquefaciens
Ronozyme AX DSM alpha-amylase Bacillus
amyloliquefaciens
Bacillus stearothennophilus
Ronozyme expressed in Bacillus
RumiStar (L/CT) DSM/Novozymes alpha-amylase lichenifonnis
38
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Protease
Commercial product Company Protease type Protease source
Avizyme 1100 Danisco A/S Subtilisin Bacillus subtilis
Avizyme 1202 Danisco A/S Subtilisin Bacillus subtilis
Avizyme 1302 Danisco A/S Subtilisin Bacillus subtilis
Avizyme 1500 Danisco A/S Subtilisin Bacillus subtilis
Avizyme 1505 Danisco A/S Subtilisin Bacillus subtilis
Bacillus
Kemzyme Plus Dry Kemin Bacillolysin amyloliquefaci ens
Bacillus
Kemzyme W dry Kemin Bacillolysin amyloliquefaci ens
Trichoderma
longibrachiatum
/Trichodenna
Natuzyme Bioproton Protease reesei
Porzyme 8300 Danisco Subtilisin Bacillus subtilis
Neocardiopsis
prasina gene
expressed in
Alkaline serine Bacillus
Ronozyme ProAct DSM/Novozymes protease lichemfonnis
Bacillus
Cibenza DP100 Novus Keratinase licheniformis
CIBENZA IND900 Novus
Bacillus
Protease PLUS Dyadic alkaline protease licheniformis
Bacillus
Protease AP CONC Dyadic alkaline protease licheniformis
Phytase
Commercial
product Company Phytase type Phytase source
Axtra Phy Danisco 6-phytase Buttiauxella sp.
Bio-feed Phytase Novozymes.
CIBENZAO PHOS Novus
Finase ABVista 3-phytase Trichoderma reesei
E. coli gene expressed in
Finase EC ABVista 6-phytase Trichoderma reesei
MICROTECH 5000
plus VTR
Natuphos BASF 3-phytase Aspergillus Niger
Trichoderma
phytase (type longibrachiatum
Natuzyme Bioproton not specified) /Trichoderma reesei
E. coli gene expressed in
OPTIPHOSO Huvepharma AD 6-phytase Pichia pastoris
Phytase sp1002 DSM 3-phytase Hansenula polymorpha
E. coli gene expressed in
Phyzyme XP Danisco 6-phytase Schizosaccahomyces pombe
39
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Quantum 2500D, E. coli gene expressed in
5000L ABVista 6-phytase Pichia pastoris
Quantum Blue ABVista
Quantum XT ABVista
Ronozyme Hi-Phos
(M/L) DSM/Novozymes 6-phytase Citrobacter braakii
Ronozyme NP DSM/Novozymes 6-phytase Aspergillus oryzae
Ronozyme P DSM/Novozymes 6-phytase Aspergillus oryzae
Rovabio PHY Adis seo 3-phytase Penicilliumfuniculosum
Cellulases
Commercial
product Company Enzyme type Source
Danisco Animal
Multifect@ BGL Nutrition
Natugrain@ TS BASF
Econase Barley ABVista
Dyadic Cellulase
PLUS Dyadic acid cellulase
Dyadic Beta beta-1, 3-1,4- Trichoderma
Glucanase BP CONC Dyadic glucanase/cellulase longibrachiatum
Dyadic Cellulase CP
CONC Dyadic acid cellulase
HOSTAZYM Huvepharma
Hostazym@ suis Huvepharma
Other
Commercial
product Company Enzyme type Source
CIBENZA DE200 Novus Mannanase
amylase and
RONOZYME@ A DSM beta-glucanase
beta-glucanases,
cellulases and
ROXAZYME@ G2 DSM xylanases
hemicellulases
RONOZYME@ VP DSM and pectinases
xylanase, a-
Ralactosidase
CIBENZA@ CSM Novus and [3-glucanase
By way of example only a feedstuff for chickens, e.g. broiler chickens may be
comprises of one
or more of the ingredients listed in the table below, for example in the %
ages given in the table
below:
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Ingredients Starter (%) Finisher (%)
Maize 46.2 46.7
Wheat Middlings 6.7 10.0
Maize DDGS 7.0 7.0
Soyabean Meal 48%CP 32.8 26.2
An/Veg Fat blend 3.0 5.8
L-Lysine HC1 0.3 0.3
DL-methionine 0.3 0.3
L-threonine 0.1 0.1
Salt 0.3 0.4
Limestone 1.1 1.1
Dicalcium Phosphate 1.2 1.2
Poultry Vitamins and Micro-
0.3 0.3
minerals
By way of example only the diet specification for chickens, such as broiler
chickens, may be as
set out in the Table below:
Diet specification
Crude Protein (%) 23.00 20.40
Metabolizable Energy Poultry
2950 3100
(kcal/kg)
Calcium (%) 0.85 0.85
Available Phosphorus (%) 0.38 0.38
Sodium (%) 0.18 0.19
Dig. Lysine (%) 1.21 1.07
Dig. Methionine (%) 0.62 0.57
Dig. Methionine + Cysteine (%) 0.86 0.78
Dig. Threonine (%) 0.76 0.68
By way of example only a feedstuff laying hens may be comprises of one or more
of the
ingredients listed in the table below, for example in the %ages given in the
table below:
Ingredient Laying phase (%)
Maize 10.0
Wheat 53.6
Maize DDGS 5.0
Soybean Meal 48%CP 14.9
Wheat Middlings 3.0
Soybean Oil 1.8
L-Lysine HC1 0.2
DL-methionine 0.2
L-threonine 0.1
Salt 0.3
Dicalcium Phosphate 1.6
Limestone 8.9
Poultry Vitamins and Micro-
0.6
minerals
41
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
By way of example only the diet specification for laying hens may be as set
out in the Table
below:
Diet specification
Crude Protein (%) 16.10
Metabolizable Energy Poultry
2700
(kcal/kg)
Lysine (%) 0.85
Methionine (%) 0.42
Methionine + Cysteine (%) 0.71
Threonine (%) 0.60
Calcium (%) 3.85
Available Phosphorus (%) 0.42
Sodium (%) 0.16
By way of example only a feedstuff for turkeys may be comprises of one or more
of the
ingredients listed in the table below, for example in the % ages given in the
table below:
Ingredient Phase 1 (%) Phase 2 (%) Phase 3 (%) Phase 4 (%)
Wheat 33.6 42.3 52.4 61.6
Maize DDGS 7.0 7.0 7.0 7.0
Soyabean Meal 48%CP 44.6 36.6 27.2 19.2
Rapeseed Meal 4.0 4.0 4.0 4.0
Soyabean Oil 4.4 4.2 3.9 3.6
L-Lysine HC1 0.5 0.5 0.4 0.4
DL-methionine 0.4 0.4 0.3 0.2
L-threonine 0.2 0.2 0.1 0.1
Salt 0.3 0.3 0.3 0.3
Limestone 1.0 1.1 1.1 1.0
Dicalcium Phosphate 3.5 3.0 2.7 2.0
Poultry Vitamins and Micro-
0.4 0.4 0.4 0.4
minerals
By way of example only the diet specification for turkeys may be as set out in
the Table below:
Diet specification
Crude Protein (%) 29.35 26.37 22.93 20.00
Metabolizable Energy Poultry
2.850 2.900 2.950 3.001
(kcal/kg)
Calcium (%) 1.43 1.33 1.22 1.02
Available Phosphorus (%) 0.80 0.71 0.65 0.53
Sodium (%) 0.16 0.17 0.17 0.17
Dig. Lysine (%) 1.77 1.53 1.27 1.04
Dig. Methionine (%) 0.79 0.71 0.62 0.48
Dig. Methionine + Cysteine (%) 1.12 1.02 0.90 0.74
Dig. Threonine (%) 1.03 0.89 0.73 0.59
42
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
By way of example only a feedstuff for piglets may be comprises of one or more
of the
ingredients listed in the table below, for example in the % ages given in the
table below:
Ingredient Phase 1 (%) Phase 2 (%)
Maize 20.0 7.0
Wheat 25.9 46.6
Rye 4.0 10.0
Wheat middlings 4.0 4.0
Maize DDGS 6.0 8.0
Soyabean Meal 48% CP 25.7 19.9
Dried Whey 10.0 0.0
Soyabean Oil 1.0 0.7
L-Lysine HC1 0.4 0.5
DL-methionine 0.2 0.2
L-threonine 0.1 0.2
L-tryptophan 0.03 0.04
Limestone 0.6 0.7
Dicalcium Phosphate 1.6 1.6
Swine Vitamins and Micro-
0.2 0.2
minerals
Salt 0.2 0.4
By way of example only the diet specification for piglets may be as set out in
the Table below:
Diet specification
Crude Protein (%) 21.50 20.00
Swine Digestible Energy
3380 3320
(kcal/kg)
Swine Net Energy (kcal/kg) 2270 2230
Calcium (%) 0.80 0.75
Digestible Phosphorus (%) 0.40 0.35
Sodium (%) 0.20 0.20
Dig. Lysine (%) 1.23 1.14
Dig. Methionine (%) 0.49 0.44
Dig. Methionine + Cysteine (%) 0.74 0.68
Dig. Threonine (%) 0.80 0.74
By way of example only a feedstuff for grower/finisher pigs may be comprises
of one or more of
the ingredients listed in the table below, for example in the % ages given in
the table below:
43
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Ingredient Grower/ Finisher (%)
Maize 27.5
Soyabean Meal 48% CP 15.4
Maize DDGS 20.0
Wheat bran 11.1
Rice bran 12.0
Canola seed meal 10.0
Limestone 1.6
Dicalcium phosphate 0.01
Salt 0.4
Swine Vitamins and Micro-minerals 0.3
Lysine-HC1 0.2
Vegetable oil 0.5
By way of example only the diet specification for grower/finisher pigs may be
as set out in the
Table below:
Diet specification
Crude Protein (%) 22.60
Swine Metabolizable Energy
3030
(kcal/kg)
Calcium (%) 0.75
Available Phosphorus (%) 0.29
Digestible Lysine (%) 1.01
Dig. Methionine + Cysteine (%) 0.73
Digestible Threonine (%) 0.66
Feed Dosing
The feed additive composition according to the present invention may be
designed for
one-time dosing or may be designed for feeding on a daily basis.
The optimum amount of the composition (and each component therein) to be used
in the
combination of the present invention will depend on the product to be treated
and/or the method
of contacting the product with the composition and/or the intended use for the
same.
The amount of enzymes used in the compositions should be a sufficient amount
to be
effective and to remain sufficiently effective in improving the performance of
the animal fed
feed products containing said composition. This length of time for
effectiveness should extend
up to at least the time of utilisation of the product (e.g. feed additive
composition or feed
containing same).
44
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Combination with Other Components in Feed Applications
The enzyme(s) described herein may be used in combination with other
components.
The components may be prebiotics. Prebiotics are typically non-digestible
carbohydrate
(oligo- or polysaccharides) or a sugar alcohol which is not degraded or
absorbed in the upper
digestive tract. Known prebiotics used in commercial products and useful in
accordance with
the present invention include inulin (fructo-oligosaccharide, or FOS) and
transgalacto-
oligosaccharides (GOS or TOS). Suitable prebiotics include
palatinoseoligosaccharide, soybean
oligosaccharide, alginate. xanthan, pectin, locust bean gum (LBG), inulin,
guar gum, galacto-
oligosaccharide (GOS), fructo-oligosaccharide (FOS), non-degradable starch.
lactosaccharose,
lactulose, lactitol, maltitol, maltodextrin, polydextrose (i.e. Litesse ),
lactitol, lactosucrose,
soybean oligosaccharides, palatinose, isomalto-oligosaccharides, gluco-
oligosaccharides and
xylo-oligosaccharides.
The prebiotic may be administered simultaneously with (e.g. in admixture
together with
or delivered simultaneously by the same or different routes) or sequentially
to (e.g. by the same
or different routes) the feed additive composition (or constituents thereof)
according to the
present invention.
Other components of the combinations of the present invention include
polydextrose,
such as Litesse , and/or a maltodextrin and/or lactitol. These other
components may be
optionally added to the feed additive composition to assist the drying
process.
Further examples of other suitable components include one or more of:
thickeners,
gelling agents, emulsifiers, binders, crystal modifiers, sweeteners (including
artificial
sweeteners), rheology modifiers, stabilisers, anti-oxidants, dyes, enzymes,
carriers, vehicles,
excipients, diluents, lubricating agents, flavouring agents, colouring matter,
suspending agents,
disintegrants, granulation binders etc. These other components may be natural.
These other
components may be prepared by use of chemical and/or enzymatic techniques.
In one preferred embodiment the enzymes for use in the present invention may
be used
in combination with one or more lipids.
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
For example, the enzymes for use in the present invention may be used in
combination
with one or more lipid micelles. The lipid micelle may be a simple lipid
micelle or a complex
lipid micelle.
The lipid micelle may be an aggregate of orientated molecules of amphipathic
substances, such as a lipid and/or an oil.
The present teachings further provide a method of increasing weight gain in a
subject,
e.g. poultry or swine, comprising feeding said subject a feedstuff comprising
a feed additive
composition according to the present invention.
The invention can be further understood by reference to the following
Examples, which
are provided by way of illustration and not meant to be limiting.
EXAMPLES:
Example 1
Particle size distribution and smoothness index of sodium sulfate cores.
Seven samples of anhydrous crystalline sodium sulfate core particles were
obtained
from two suppliers: Minera de Santa Marta (Villarubio, Spain) and Saltex
(Forth Worth, Texas),
Particle size distributions of the samples were obtained by laser light
scattering using a
Malvern Mastersizer 2000 laser diffraction particle size analyzer. Envelope
specific surface
areas for these particle size distributions were calculated using an algorithm
as described in the
Methods section, using the true density of anhydrous sodium sulfate (2.664
g/cm3). BET specific
surface area was determined using a Micromeritics model ASAP 2420 Accelerated
Porosimetry
and Surface Area System, as described in the Methods section. The smoothness
index for each
sample is calculated as the ratio of these two specific suiface areas.
Representative images for
two of the lots of cores are shown in Figure 1.
46
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
Table 1
Particle
Envelope BET Size
Specific
Specific Smoothness Dispersity
Surface Surface Index Index
D10 D50 D90 Area Area (BET/Env (PDSI)
Core Lot (urn) (um) (urn) (m2/g) M2
ig Area Ratio) D90/D10
MSM #1 257 353 480 0.00659 0.0145 2.2 1.87
MSM #2 225 309 424 0.00751 0.0153 2.0 1.88
MSM #3 182 258 362 0.00907 0.0096 1.1 1.99
MSM #4 234 320 431 0.00726 0.0134 1.8 1.84
MSM #5 164 240 345 0.00981 0.0194 2.0 2.10
MSM #6 138 204 300 0.01160 0.0316 2.7 2.17
Saltex #1 171 232 316 0.00843 0.0330 3.9 1.85
Example 2
Production of a sample of detergent enzyme granules using sodium sulfate
cores.
79.8 kg of sodium sulfate cores from Minera de Santa Marta (MSM Lot #3 in
Table 1
of Example 1) were charged into a 150 kg batch size pilot scale fluid bed
coater. By light
scattering, the cores had a mass median diameter (D50) of 258 microns, a D10
of 182 microns
and a D90 of 358 microns; by sieve screening, a D50 of 210 microns. Based upon
this, MSM
Lot #3 had a PSDI of 362/182 = 1.99.
According to Table 1 of Example 1, the smoothness index of the MSM Lot #3
cores
was 1.1, very close to the 1.0 smoothness index of perfectly smooth particles.
Optical and
scanning electron micrographs of the cores are shown in Figure 1. It can be
perceived in the
same figure that these cores are much smoother than a comparative sample of
cores from Saltex.
Table 1 shows that Saltex Lot #1 had a smoothness index of 3.9, indicating a
significant
deviation from a perfect smoothness index of 1Ø
The cores were fluidized at a bed temperature of 41 degrees C, and an aqueous
protease enzyme solution (Purafast) at a concentration of 20% w/w solids (3450
PU/g) ,
containing 1% w/w partially hydrolyzed polyvinyl alcohol (final solids % w/w
loading of the
enzyme/PVA of 16%)and 0.5% w/w antifoam (Polyglycol EP 436E (lot 1L05091501)
was
sprayed onto the cores at an atomization air pressure ramping from 3.5
(initial) to 3.9 (final) bar.
47
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
After the enzyme spray was complete, a solution containing a mixture of sodium
sulfate and magnesium sulfate (MgSO4 7H20, 30.5%, Na2SO4, 15%) was sprayed
onto the
cores to deliver a net 20% w/w ¨ on the basis of the final granule weight --
onto the cores. The
salt solution was sprayed at a bed temperature of 45 degrees C, with an
atomization air pressure
of 3.5 bar.
A final coating solution composed of partially hydrolyzed polyvinyl alcohol
(Celvol 5-
88), titanium dioxide and Lutensol non-ionic surfactant, was sprayed onto the
salt-coated
enzyme cores, to deliver a net 11% w/w total coating weight. The coating
solution was sprayed
at a bed temperature of 55 degrees C, with an initial atomization air pressure
of 5.3 bar, ramping
down to 4.8 bar.
After cooling down the batch of coated granules, 150 kg of product was
harvested from
the fluid bed coater. The final granules had a D50 of 308 um (by sieve
analysis) and a bulk
density of 1.18 g/cm3. The granules had a Hunter whiteness L-value of 73, and
a Heubach
enzyme total dust of 3.2 mg/pad
Example 3
Four additional batches of Purafast granules were prepared, following the
recipe and
process described in Example 2, altering only the amount of Purafast enzyme
concentration
solution coated onto the cores, the core particle size, and adjusting the core
charge as necessary
to accommodate the different levels of enzyme solids. Additional coatings and
process
parameters were not altered.
In Table 2 the granule of Example 2 is
shown as Batch B2. Table 2. Composition
of Granule Batches
Enzyme
layer Core Core size
Example Granule solids solids Core Core D50 (um)
by by
Number Batch (% w/w) (%w/w) Batch Type sieve laser
3 Al 8 61 standard 298 284
3 B1 16 53 standard 298 284
2 B2 16 53 MSM#3 Small 210 258
3 Cl 20 49 standard 298 284
3 C2 20 49 MSM#3 Small 210 258
48
CA 02915538 2015-12-15
WO 2014/205161
PCMJS2014/043084
The below enzyme samples were storage tested in a Laundry Powder Detergent
under
different 'stressed' storage conditions. Stability was assessed via
performance and biochemical
analyses.
Storage testing: sample preparation
Corresponding amount of enzyme (see below) were added to 10 g of a Laundry
detergent
and put in plastic cups (150 ml), which were stored, open, at 70% Relative
Humidity in
acclimatized rooms at 37, 45 and 60 C.
A. 10 g Laundry Powder Detergent + Sample Al
B. 10 g Laundry Powder Detergent + Sample B1
C. 10 g Laundry Powder Detergent + Sample B2
D. 10 g Laundry Powder Detergent + Sample Cl
E. 10 g Laundry Powder Detergent + Sample C2
Samples were analyzed fresh and after storage of 1 and 2 hours, and 3 days (at
60 C); and
after 1, 3 and 8 days (at 37 and 45 C)
Performance assessment.
Wash Testing was carried out in a so-called Laundr-O-meter at 30 C, 25 FH and
15
minutes.
The 10 g stored sample was dissolved in 2.5 liters of 25 FH water (see below)
in a
stirred beaker for 2 minutes. 300 ml of this solution is put into a LOM beaker
and below stains
were added to eve a Liquor to cloth ratio L/C ¨ 90 (without steel balls).
To make 25 1 of 25 FH = 250 ppm Ca+2/Mg+2=2/1, dilute 416.75 ml of a 15.000
ppm
stock solution with 24583.25 ml de-ionized water (to make 25 liter) Stock
solution 15.000ppm:
14.70 gram CaC12.2H20 + 10.165 gram MgC12.6H20 in 1 liter DI water.
Stains per beaker (initial reflectance [L*a*b, D65 illuminant] of the swatches
previously
measured with the reflectometer):
49
WO 2014/205161 PCT/US2014/043084
Stain Batch Size (cm) Weigh Per
(g) beaker
E117 Blood/Milk/Ink on 12-18 6 1/2 X 6 1/2 0.7 2
poly cotton
E116 Blood/Milk/Ink on 20-15 6 1/2 X 6 1/2 0.7 2
cotton
CS08 Grass on cotton 83 6 1/2 X 6 1/2 0.9 2
Total 4.6 6
After washing stains were rinsed for 3 minutes at running tap water (17 FH,
Ca/Mg=4/1),
spin dried and air dried at ambient conditions overnight (covered with dark
clothes).
Triple measurement of the final reflectance [L,*a*b, D65 illuminant] of the
swatches were
done by using the reflectometer. % Delta Soil Removal(% dE) is calculated
according to:
Soil Removal -\/(Lattõ ¨ Lbefoõ) 2 + (aatter ¨ abefore)2 (bafter
bbefore) * 100%
% dE = Initial Soil = (Lief ¨ Lbefõ,e) (n
2 = \--ref ¨ abefore)2 (bref bbefoie)
Where I-wref = 96.0
aref = 0.55
bref = 1.95
Biochemical assessment
This method was used to determine proteolytic activity on fermentation,
recovery, and
final product samples (liquids and granules) of Properase. The assay is
colorimetric and
monitors the rate of degradation of N-succinyl-ala-ala-pro-phe-p-nitroanalide
substrate. The
release of the substrate's p-nitroanalide was measured at 405nm on a Konelab
analyzer. The
assay is calibrated against an assigned standard.
Samples preparation:
- 100 g of Tris Buffer (0.1M TRIS, 0.01M CaCL2, 0.005% Triton TM X-100, pH
8.6) were
added to the lOg of detergent samples and stirred for 30 min. After that 2m1
of the
previous dissolution was taken in an eppendorf tube and centrifuge for 10 min
at 14000
rpm.
- A second dissolution (10X) was made using the HAMILTON diluter machine
and Tris
Buffer.
- Vortexed the samples after diluting, and poured them in the corresponding
cuvettes for
the Konelab. Included also the controls (in these case PU/g). Placed the
cuvettes on
sample rack(s). Insert rack(s) on instrument.
Date Recue/Date Received 2021-05-04
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
To run the Konelab assay:
- Prepared working substrate solution [N-succinyl-ala-ala-pro-phe-p-
nitroanalide (AAPF)
(Sigma # S7388)] and poured into reagent vessel and place onto reagent wheel
(substrate =
20m1 vessel, code = AAPF).
Reagent Preparation
1. Stock substrate solution (100mg/m1): Dissolve 500mg AAPF in 5m1DMSO.
Stored at room temperature protected from light. Discard is solution turns
yellow. Stable for 1 month.
2. Intermediate substrate solution (20mg/m1): Add 800u1 stock substrate
solution to
3.2ml DMSO and mix. Stored at room temperature protected from light.
Discard if solution turns yellow. Stable forl month.
3. Working substrate solution (1mg/m1):Add 250u1 intermediate substrate
solution
to 4.75m1 assay buffer and mix. Prepared as needed. Typically stable for 1
hour
if stored on ice. Discarded if solution turns yellow.
- Labeled the sample cups in the software and define the test to be run in the
Konelab (six
replicates). Run and check the calibration. To calculate the activity of the
granular samples
(This equation assumes that lg = lml as the specific gravity of the
dissolution mixture):
PU/g granule = RPU/m1)*(working dilution)]*[weight of dissolution buffer +
granules(m1)/weight of
granules(g)]
- Calculated the mean value for the replicates of each sample. Calculated the
%CV (coefficient of
variance).
In this Example, the "Mini" granules are those that contain small smooth cores
according to the pres
teachings.
Data are shown in Figure 2.
51
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Example 4
Production of three representative batches of phytase
45 kg of sodium sulfate cores from Minera de Santa Marta (MSM Lot 41418.B)
were
charged into 150kg batch size pilot scale fluid bed coater. By light
scattering, the cores had a
mass median diameter 199 microns.
The cores were fluidized and an aqueous phytase enzyme solution (batch
488115613)
at a concentration of 17,5% w/w solids, containing 2% w/w partially hydrolyzed
polyvinyl
alcohol (Celvol 5-88) and 0,5% w/w sodium phytate solution (Dr. Straetmans;
Dermofeel PA-3;
.. Lot NA2062) was sprayed onto the cores.
After the enzyme spray, a solution containing sodium sulfate (MSM, lot
13130405)
was sprayed onto the cores to deliver a net 50% w/w ¨ on the basis of the
final granule weight.
A final coating solution composed of partially hydrolyzed polyvinyl alcohol
(Celvol
5-88) and talc (Microtalc FC CG-AW, lot 13/00328), was sprayed onto the salt-
coated enzyme
cores to deliver a net 9% w/w total coating weight.
After cooling down each batch of coated granules, 136kg, 140kg and 134kg of
product was harvested from the fluid bed coater. By light scattering, the
final granules had a
D50 of 362 pm on the average and bulk density of 1,27g/cm3.
Figure 4 shows the pelleting results of 3 representative granule batches 3115,
3116
and 3117. Felleting trials were carried out at the Danish Teknological
Institute.
Example 5
Production of three representative batches of phytase standard TPT granules in
pilot scale
from the same concentrate batch
65 kg of sodium sulfate cores from Minera de Santa Marta (MSM Na- G. III-EE,
Lot
41235,12) were charged into 150kg batch size pilot scale fluid bed coater. By
light scattering,
the cores typically have a mass median diameter 290 microns.
52
CA 02915538 2015-12-15
WO 2014/205161 PCMJS2014/043084
Table 3: Part of Product Specification of MSM Na-G. III EE.
GRANULOMETRY
Above to 1000 ium 0%
Above to 355 um 20% maximum
Between 250 and 355 60% minimum IT19B-PA05
tm
Below to 212 pm 10% maximum
Mean size 365 40 microns IT20-PA05
D.F.R 150 40 ml/s
The cores were fluidized and an aqueous (batch 488115613) phytase enzyme
solution
at a concentration of 17,5% w/w solids, containing 2% w/w partially hydrolyzed
polyvinyl
alcohol (Celvol 5-88) and 0,5% w/w sodium phytate solution (Dr. Straetmans;
Dermofeel PA-3;
Lot NA2062) was sprayed onto the cores.
After the enzyme spray, a solution containing sodium sulfate (MSM, lot
13130405)
was sprayed onto the cores to deliver a net 40% w/w ¨ on the basis of the
final granule weight.
A final coating solution composed of partially hydrolyzed polyvinyl alcohol
(Celvol
5-88) and talc (Microtalc FC CG-AW, lot 13/00328), was sprayed onto the salt-
coated enzyme
cores to deliver a net 9% w/w total coating weight.
After cooling down each batch of coated granules, 151kg, 151kg and 150kg of
product was harvested from the fluid bed coater. By light scattering the final
granules had a D50
of 388 pm on the average and bulk density of 1,39g/cm3.
The pelleting of the three representative phytase standard TPT batches are
shown in
Figure 5.
53