Note: Descriptions are shown in the official language in which they were submitted.
CA 02915917 2015-12-22
4,4100
METHODS FOR REMOVING DISSOLVED ORGANIC MATTER AND OTHER
CONTAMINANTS FROM SAGD WATER
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Patent No.
62/096,313, filed
December 23, 2014; U.S. Provisional Patent Application No. 62/096,310, filed
December 23,
2014; and U.S. Provisional Patent Application No. 62/096,297, filed December
23, 2014.
FIELD OF THE ART
[0002] The embodiments described herein relate to methods for removing
dissolved
organic matter and other contaminants from steam assisted gravity drainage
(SAGD) water.
BACK GROUND
[0003] Steam assisted gravity drainage (SAGD) methods are commonly employed
as
an enhanced oil recovery technique for producing heavy crude oil and bitumen,
especially in
the oil sands projects. In a SAGD method, steam is produced in a boiler or
steam generator
and injected into bitumen bearing deposits. The steam reduces the viscosity of
the bitumen
allowing a mixture of water and bitumen to flow to a production well. After
this mixture is
extracted, most of the oil is removed in an initial primary oil-water
separation step. The
remaining water, often called "produced water," contains various contaminants,
for example,
residual oil, suspended and dissolved solids (organic and inorganic),
dissolved organic matter
and silica.
[0004] Typically, about 80 to 90% of the produced water (PW) is recycled
and treated
to produce boiler feed water (BFW) for use in subsequent SAGD methods. The
bulk of the
residual oil is removed from the PW using gravity based separation methods
like free water
knock out (FW1(0) drums, followed by induced static flotation (1SF). Walnut
shell filters
may then be used to remove traces of oil from the water. Dissolved matter is
conventionally
removed using methods such as warm lime softening (WLS) to remove silica and
hardness,
after filters for suspended solids removal and weak-acid cationic exchange
(WAC) for
removal of calcium (Ca2+) and magnesium (Mg2+) ions. The treated water, known
as BFW,
is then stored in tanks and used in further SAGD methods.
[0005] The boilers used in the SAGD operation are known as "once through
steam
generators" (OTSGs). The OTSGs are capable of handling a feed with high total
dissolved
solids (TDS) content. BFW, when converted to steam in an OTSG, generates a
boiler blow-
down (also known as boiler blown down water) (BBDW) which is about 20% of the
volume
1
CA 02915917 2015-12-22
*4o4,0,
of the boiler feed water. In the current industrial practice, at least a
portion of the BBDW is
recycled back to the WLS process and the rest is earmarked for disposal.
[0006] BFW and BBDW have high levels of dissolved organic matter (DOM) and
total
dissolved solids (IDS). These high levels of DOM have been implicated in
numerous
problems in the industrial processes, such as making highly sticky deposits
with inorganic
salts on boiler surfaces, irreversible fouling on downstream equipment
surfaces, corrosion in
pipelines, and clogging of injection wells.
[0007] Additionally, higher efficiency steam generators, such as drum
boilers, have
lower allowable concentrations of DOM than OTSGs. OTSGs can tolerate a TDS
level as
high as about 8000 ppm, since steam generation is limited to approximately 80%
of the
volume of the boiler feed water. The other 20% of the BFW remains as BBD
water, a brine
stream that contains high levels of DOM, often at fivefold the concentration
of the boiler feed
water.
[0008] As the industry shifts toward drum boilers and evaporative
treatment, the BBD
volume will be substantially reduced, but the evaporator blow down volume will
increase,
which will contain very high levels of TDS and DOM. Thus, the disposal of a
high TDS and
DOM wastewater will remain a problem for the SAGD industry.
BRIEF SUMMARY
[0009] Disclosed herein is a method for removing dissolved organic matter
from an
aqueous stream, the method comprising: adding one or more aluminum-containing
coagulants and/or one or more iron-containing coagulants to the aqueous
stream; optionally
aerating the aqueous stream; and separating the dissolved organic matter from
the aqueous
stream. Also disclosed is a method for removing silica from an aqueous stream,
the method
comprising: adding one or more aluminum-containing coagulants and/or one or
more iron-
containing coagulants to the aqueous stream; optionally aerating the aqueous
stream; and
separating the silica from the aqueous stream.
[0010] Disclosed herein is a method for removing dissolved organic matter
from an
aqueous stream, the method comprising: adding one or more adsorbents and one
or more
aluminum-containing and/or iron-containing coagulants to an aqueous stream
comprising
dissolved organic material, and separating the dissolved organic matter from
the aqueous
stream to provide a treated aqueous stream having a reduced amount of
dissolved organic
matter compared to the untreated aqueous stream. Also, disclosed herein is a
method for
removing silica from an aqueous stream, the method comprising: adding one or
more
adsorbents and one or more coagulants selected from aluminum-containing and/or
iron-
2
containing coagulants to the aqueous stream comprising silica, and separating
the silica from
the aqueous stream, to provide a treated aqueous stream having a reduced
amount of silica
compared to the untreated aqueous stream.
[0011] Disclosed herein is a method for removing dissolved organic
matter from an
aqueous stream, the method comprising: adding one or more aluminum-containing
coagulants and/or one or more iron-containing coagulants to the aqueous stream
comprising
dissolved organic matter, aerating the aqueous stream, and separating the
dissolved organic
matter from the aqueous stream to provide a treated aqueous stream having a
reduced amount
of dissolved organic matter compared to the untreated aqueous stream. Also
disclosed herein
is a method for removing silica from an aqueous stream, the method comprising:
adding one
or more aluminum-containing coagulants and/or one or more iron-containing
coagulants to
the aqueous stream, aerating the aqueous stream, and separating the silica
from the aqueous
stream.
[0012] Also disclosed herein are methods wherein the foregoing methods
further
comprise the addition of one or more divalent ions.
[0012a1 Disclosed herein is a adding one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants to the aqueous stream in an
amount between
0.5 to 15,000 ppm; adding one or more adsorbents to the aqueous stream, the
one or more
adsorbents selected from the group consisting of metakaolin, pumice,
aragonite, recycled
glass powder and combinations thereof; and separating the dissolved organic
matter from the
aqueous stream.
[0012b] Also disclosed is a method for removing silica from an aqueous
stream
comprising: adding one or more aluminum-containing coagulants and/or one or
more iron-
containing coagulants to the aqueous stream in an amount between 0.5 to 15,000
ppm; adding
one or more adsorbents to the aqueous stream, the one or more adsorbents
selected from the
group consisting of metakaolin, pumice, aragonite, recycled glass powder and
combinations
thereof; and separating the silica from the aqueous stream.
[0012c] Also disclosed is a method for removing dissolved organic matter
from an
aqueous stream comprising: adding one or more adsorbents to the aqueous stream
comprising
the dissolved organic matter, wherein the one or more adsorbents are selected
from the group
consisting of metakaolin, pumice, aragonite, recycled glass powder and
combinations thereof;
adding one or more aluminum-containing and/or iron-containing coagulants in an
amount
between 0.5 to 15,000 ppm to the aqueous stream comprising the dissolved
organic matter,
and separating the dissolved organic matter from the aqueous stream to provide
a treated
3
Date Regue/Date Received 2022-11-18
aqueous stream having a reduced amount of dissolved organic matter compared to
an
untreated aqueous stream.
[0012d] Also disclosed is a method for removing silica from an aqueous
stream
comprising: adding one or more adsorbents to the aqueous stream comprising the
silica,
wherein the one or more adsorbents are selected from the group consisting of
metakaolin,
pumice, aragonite, recycled glass powder and combinations thereof;adding one
or more
coagulants selected from aluminum-containing and/or iron-containing coagulants
to the
aqueous stream comprising the silica in an amount between 0.5 to 15,000 ppm,
and
separating the silica from the aqueous stream, to provide a treated aqueous
stream having a
reduced amount of silica compared to an untreated aqueous stream.
[0012e] Also disclosed is a method for removing dissolved organic matter
from an
aqueous stream comprising: adding one or more aluminum-containing coagulants
and/or one
or more iron-containing coagulants to the aqueous stream comprising the
dissolved organic
matter in an amount between 0.5 to 15,000 ppm, adding one or more adsorbents
to the
aqueous stream, the one or more adsorbents selected from the group consisting
of metakaolin,
pumice, aragonite, recycled glass powder and combinations thereof; aerating
the aqueous
stream contemporaneously or after adding the one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants, and separating the dissolved
organic matter
from the aqueous stream to provide a treated aqueous stream having a reduced
amount of
dissolved organic matter compared to an untreated aqueous stream.
[0012f] Also disclosed is a method for removing silica from an aqueous
stream
comprising: adding one or more aluminum-containing coagulants and/or one or
more iron-
containing coagulants to the aqueous stream in an amount between 0.5 to 15,000
ppm, adding
one or more adsorbents to the aqueous stream, the one or more adsorbents
selected from the
group consisting of metakaolin, pumice, aragonite, recycled glass powder and
combinations
thereof; aerating the aqueous stream contemporaneously or after adding the one
or more
aluminum-containing coagulants and/or one or more iron-containing coagulants,
and
separating the silica from the aqueous stream.
3a
Date Regue/Date Received 2022-11-18
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] Figure 1 shows the percentage removal of dissolved organic matter
from the
blow down water at different initial pH treated with an exemplary polyaluminum
chloride
coagulant.
[0014] Figure 2 shows SUVA value of the residual organic matter present
in the blow
down water at different initial pH treated with an exemplary polyaluminum
chloride
coagulant.
[0015] Figure 3 shows the percentage removal of dissolved organic matter
from the
blow down water for different initial pH with and without treatment by an
exemplary
polyaluminwn chloride coagulant.
[0016] Figures 4 and 5 show the percentage removal of dissolved organic
matter from
a blow down water sample at different initial pH values after treatment with
exemplary
ferrous chloride and ferric chloride coagulants.
[0017] Figure 6 shows the SUVA value of the residual organic matter
present in the
blow down water at different initial pH values after treatment with an
exemplary ferric
chloride coagulant.
[0018] Figure 7 shows the percentage removal of dissolved organic matter
from a blow
down water sample at different initial pH values after treatment with an
exemplary poly ferric
sulfate coagulant.
3b
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
*Noe ,NA.0
[0019] Figure 8 shows the SUVA value of the residual organic matter present
in the
blow down water at different initial pfl values after treatment with an
exemplary poly ferric
sulfate coagulant.
[0020] Figure 9 shows the percentage removal of dissolved organic matter
from a
produced water sample at different initial pH values after treatment with an
exemplary ferric
chloride coagulant.
[0021] Figure 10 shows the percentage removal of dissolved organic matter
from a
produced water sample at different initial pH values after treatment with an
exemplary
polyaluminum chloride coagulant.
[0022] Figure II shows the SUVA value of the residual organic matter
present in the
produced water at different initial pH values after treatment with an
exemplary polyaluminum
chloride coagulant.
[0023] Figure 12 shows the percentage removal of dissolved organic matter
from a
produced water sample at different initial pH values after treatment with an
exemplary
aluminum sulfate coagulant.
[0024] Figure 13 shows the SUVA value of the residual organic matter
present in the
produced water at different initial pH values after treatment with an
exemplary aluminum
sulfate coagulant.
[0025] Figure 14 shows the percentage removal of dissolved organic matter
from a
produced water sample at different pH values after treatment with an exemplary
polyaluminum chloride coagulant and a polyacrylamide flocculant.
[0026] Figure 15 shows the SUVA value of the residual organic matter
present in the
produced water at different pH values after treatment with an exemplary
polyaluminum
chloride coagulant and a polyacrylamide flocculant.
[0027] Figure 16 shows the percentage removal of dissolved organic matter
from a
produced water sample at different pH values after treatment with an exemplary
polyaluminum chloride coagulant and a polyacrylamide flocculant.
[0028] Figure 17 shows the SUVA value of the residual organic matter
present in the
produced water at different pH values after treatment with an exemplary
polyaluminum
chloride coagulant and a polyacrylamide flocculant.
[0029] Figure 18 shows the percentage removal of dissolved organic matter
from a
produced water sample at different pH values after treatment with an exemplary
polyaluminum chloride coagulant and a polyacrylamide flocculant.
4
CA 02915917 2015-12-22
'Wipe %woe
[0030] Figure 19 shows the SUVA value of the residual organic matter
present in the
produced water at different pH values after treatment with an exemplary
polyaluminum
chloride coagulant and a polyacrylamide flocculant.
[0031] Figure 20 shows the percentage removal of dissolved organic matter
from a
produced water sample at different pH values after treatment with an exemplary
polyaluminum chloride coagulant and a polyacrylate flocculant.
[0032] Figure 21 shows the SUVA value of the residual organic matter
present in the
produced water at different pH values after treatment with an exemplary
polyaluminum
chloride coagulant and a polyacrylate flocculant.
[0033] Figure 22 shows the residual concentration of silica from a produced
water
sample at pH 10 after treatment with an exemplary aluminum sulfate coagulant.
[0034] Figure 23 shows the residual concentration of silica from a produced
water
sample at pH 10 after treatment with an exemplary ferric chloride coagulant.
[0035] Figure 24 shows the residual concentration of silica from a produced
water
sample at pH 10 after treatment with an exemplary polyaluminum chloride
coagulant.
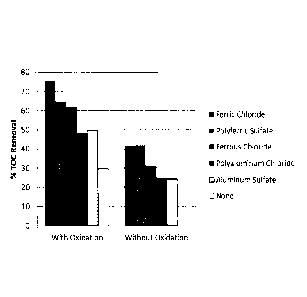
[0036] Figure 25 shows removal of dissolved organic matter from SAGD
produced
water according to the method of the exemplary embodiments, utilizing various
coagulants at
a dosage of 3000 ppm, with and without oxidation.
DETAILED DESCRIPTION
[0037] The exemplary embodiments disclosed herein include methods for
removing
dissolved organic matter (DOM), silica and/or other contaminants from an
aqueous stream,
wherein the aqueous streams are treated with one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants, optionally in combination with
an aeration
step, and/or optionally in combination with adding one or more adsorbents.
Also disclosed
are methods for removing DOM, silica and/or other contaminants from an aqueous
stream,
wherein the aqueous streams are further treated with one or more divalent
ions. Generally, the
methods are suitable for removing DOM, silica and/or other contaminants from
water from a
steam assisted gravity drainage (SAGD) operation.
[0038] Definitions
[0039] The expression "aqueous stream" as used herein refers to any aqueous
liquid
feed, and especially one that contains undesirable amounts of contaminants,
for example
DOM or silica. Exemplary aqueous streams include but are not limited to
produced water
from a SAGD operation, frac water from a hydraulic fracturing operation,
bituminous or oily
water, oilfield produced water, streams from oil processes (e.g., oil
exploration, production,
CA 02915917 2015-12-22
40q;=-=
processing and/or refining), drinking water, ground water, well water, surface
water, such as
waters from lakes, ponds and wetlands, agricultural waters, wastewater,
process make up
water, utility water, tailings impoundment treatment, flotation, and the like.
In exemplary
embodiments, the aqueous stream is produced water from a SAGD operation. In
exemplary
embodiments, the aqueous stream is boiler blow-down (also referred to as
boiler blow-down
water) (BBDW) from a SAGD operation. In exemplary embodiments, the aqueous
stream is
boiler blow down (BBD) produced by an OTSG. In exemplary embodiments, the
aqueous
stream is BBD produced by a conventional drum boiler.in exemplary embodiments,
the
aqueous stream is produced water (PW) from a SAGD operation.
[0040] The phrase "dissolved organic matter" or "DOM" as used herein refers
to an
aqueous mixture of organic compounds which includes one or more types of
organic
compounds. In exemplary embodiments, the organic compounds include
hydrocarbons, for
example residual hydrocarbons derived from bitumen; volatile organic
compounds, for
example acetone and 2-butanone; phenolic compounds; organic acids, for example
naphthenic acids; and polycyclic aromatic hydrocarbons, for example such as
naphthalene
and phenanthrene; and the like. In exemplary embodiments, DOM is measured as
total
organic carbon (TOC).
[0041] The phrase "total dissolved solids" or "TDS" refers to the total
amount of
mobile charged ions, including minerals, salts or metals dissolved in a given
volume of water,
expressed in units of mg per unit volume of water (mg/L), also referred to as
parts per million
(ppm). Total dissolved solids are differentiated from total suspended solids
(TSS), in that the
latter cannot pass through a sieve of two micrometers and yet are indefinitely
suspended in
solution. The two principal methods of measuring total dissolved solids are
gravimetry and
conductivity
[0042] The phrase "Total Organic Carbon" or "TOC" is an indirect measure of
organic
molecules present in water and measured as carbon. As such, TOC represents the
DOM
concentration. Analytical technologies utilized to measure TOC completely
oxidize the
organic molecules in an aliquot of sample water to carbon dioxide (CO2),
measure the
resultant CO2 concentration, and express this response as carbon
concentration.
[0043] In exemplary embodiments, the organic compounds include compounds
derived
from natural organic matter, for example humic substances, humic acids and
fulvic acids.
Humic substances, which are the major organic constituents of soil (humus),
peat, coal, many
upland streams, dystrophic lakes, and ocean water, are produced by
biodegradation of dead
organic matter. Generally, humic substances are a complex mixture of many
different acids
containing carboxyl and phenolate groups. Fulvic acids are humic acids of
lower molecular
6
CA 02915917 2015-12-22
Iwo *00
weight and higher oxygen content than other humic acids. Fulvic acids are poly-
electrolytes
and are unique colloids that diffuse easily through membranes.
[0044] In exemplary embodiments, the organic compounds are hydrophobic
and/or
hydrophilic organic compounds, for example hydrophobic and/or hydrophilic
organic
compounds, such as those derived from SAGD dissolved organic matter.
[0045] In exemplary embodiments, the organic acids are polar organic acids.
In certain
embodiments, the organic acids are low-molecular weight organic acids. In
certain
embodiments, the organic acids are high-molecular weight organic acids. In
certain
embodiments, the organic acids are one or more naphthenic acids of the formula
C(n)}1.(2n+Z)0(x) for combinations of n = 4 to 18, Z = 0 and -2, and X = 2 to
4.
[0046] In exemplary embodiments, the organic compounds are hydrophobic
acids or
hydrophilic neutrals or combinations thereof.
[0047] In exemplary embodiments, between about 10 and about 50% of the DOM
has
a molecular weight of less than about 500 Da.
[0048] In an exemplary embodiment, the aqueous stream is SAGD produced
water
comprising DOM in an amount greater than about 150 ppm, about 300 ppm, about
600 ppm,
about 900 ppm, about 1200 ppm or about 1500 ppm of dissolved organic matter.
[0049] In an exemplary embodiment, the aqueous stream is SAGD produced
water
comprising TDS in an amount greater than about 750 ppm.
[0050] In an exemplary embodiment, the aqueous stream is SAGD produced
water
comprising TOC in an amount greater than about 500 ppm.
[0051] In an exemplary embodiment, the aqueous stream is SAGD boiler blow
down
(BBD) comprising DOM in an amount greater than about 500 ppm.
[0052] In an exemplary embodiment, the aqueous stream is SAGD boiler blow
down
(BBD) comprising TDS in an amount greater than about 1000 ppm.
[0053] In an exemplary embodiment, the aqueous stream is SAGD BBD
comprising
TOC in an amount greater than about 750 ppm.
[0054] The amount of contaminants in the untreated aqueous stream may
depend on a
number of factors, including, for example, various water and/or process
conditions. The
exemplary embodiments are not necessarily limited by the amount of
contaminants in the
untreated aqueous stream. One having ordinary skill in the art, in view of the
description
herein, would understand how to use the exemplary methods herein to treat
various aqueous
streams.
[0055] In an exemplary embodiment, the aqueous stream includes other
contaminants,
such as one or more compounds comprising silicon or a mixture comprising
silicon-based
7
CA 02915917 2015-12-22
%NO
compounds, for example silica. In an exemplary embodiment, the silicon level
is greater than
about 50 ppm. In exemplary embodiments, the method can be used to remove
silica from an
aqueous stream containing greater than about 50 ppm, about 100 ppm, about 150
ppm, about
200 ppm, about 250 ppm, about 300 ppm of silica. In an exemplary embodiment,
the method
may be used to reduce the silicon or silicon-based compounds in the aqueous
stream to a
level of less than about 200 ppm, about 150 ppm, or about 100 ppm. In an
exemplary
embodiment, the levels of silicon or silicon-based compounds in the aqueous
stream are
reduced by about 50 ppm, about 100 ppm or about 150 ppm. In an exemplary
embodiment,
the level of silicon or silicon-based compounds in the aqueous stream is at
least about 150
ppm, about 200 ppm, or about 250 ppm.
[0056] The one or more coagulants may be any suitable coagulant. In
exemplary
embodiments, the coagulant is an aluminum-containing coagulant. In exemplary
embodiments, the one or more aluminum-containing coagulants comprises aluminum
sulfate,
aluminum chloride, sodium aluminate, polyaluminum sulfate, polyaluminum
silicate sulfate,
polyaluminum silica chloride, polyaluminum chloride, polyaluminum sulfate
chloride,
aluminum chlorohydrate, polydiallyldimethylammonium chloride, an aluminum
ferric sulfate
blend, an aluminum ferric chloride blend, a blend of polyaluminum chloride,
aluminum
chlorohydrate, polydiallyldimethylammonium chloride, and an epiamine
coagulant, another
aluminum-containing coagulant, or mixtures thereof. In exemplary embodiments,
the
aluminum sulfate is acidified aluminum sulfate, prehydroxylated aluminum
sulfate or
granulated aluminum sulfate. In certain embodiments, the one or more aluminum-
containing
coagulants is selected from the group consisting of polyaluminum silicate
sulfate,
polyaluminum chloride, aluminum sulfate, and mixtures thereof.
[0057] In exemplary embodiments, the one or more coagulants is an iron-
containing
coagulant. In exemplary embodiments, the one or more iron-containing
coagulants comprises
ferrous chloride, ferric chloride, ferric sulfate, ferrous sulfate, ferric
chloride sulfate, poly
ferric sulfate, ferric salts with polymers, poly ferrous sulfate, mixtures of
iron and aluminum
salts, and mixtures thereof. In certain embodiments, the one or more iron-
containing
coagulants comprises ferrous chloride, ferric chloride, ferric sulfate, and
mixtures thereof.
[0058] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream comprises: adding one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants to the aqueous stream; and
separating the
dissolved organic matter from the aqueous stream. In exemplary embodiments,
the method
further comprises agitating the aqueous stream after adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
aqueous stream.
8
CA 02915917 2015-12-22
+60, ft=o"
In exemplary embodiments, the method further comprises adding one or more
divalent ions
to the aqueous stream. In exemplary embodiments, the method further comprises
adding one
or more divalent ions and one or more flocculants to the aqueous stream. In
exemplary
embodiments, the one or more divalent ions may be added before, concurrently,
or after the
addition of the one or more aluminum-containing coagulants and/or one or more
iron-
containing coagulants to the aqueous stream. In exemplary embodiments, the one
or more
divalent ions may be added sequentially or simultaneously with the one or more
alum inum-
containing coagulants and/or one or more iron-containing coagulants to the
aqueous stream.
In exemplary embodiments, the addition of the one or more divalent ions to the
aqueous
stream enhances the removal of dissolved organic matter from the aqueous
stream. In
exemplary embodiments, the addition of the one or more divalent ions to the
aqueous stream
enhances the removal of dissolved organic matter from the aqueous stream
compared to the
addition of one or more monovalent ions.
[0059] In exemplary embodiments, the one or more divalent ions may be
calcium ions,
magnesium ions, barium ions, or strontium ions. In certain embodiments, the
calcium ions
may be provided in the form of calcium oxide. The one or more divalent ions
may be
provided in the form of a salt, for example calcium salts, magnesium salts,
barium salts, or
strontium salts. In exemplary embodiments, the calcium salt may be calcium
chloride or
calcium nitrate. In exemplary embodiments, the calcium salt is not calcium
hydroxide. In a
particular embodiment, the one or more divalent ions do not include magnesium
oxide.
[0060] In exemplary embodiments, the method further comprises adding one or
more
calcium salts, magnesium salts, barium salts, or strontium salts to the
aqueous stream. In
exemplary embodiments, the one or more calcium salts, magnesium salts, barium
salts, or
strontium salts may be added before, concurrently, or after the addition of
the one or more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the
aqueous stream. In exemplary embodiments, the one or more calcium salts,
magnesium salts,
barium salts, or strontium salts may be added sequentially or simultaneously
with the one or
more aluminum-containing coagulants and/or one or more iron-containing
coagulants to the
aqueous stream. In exemplary embodiments, the addition of the one or more
calcium salts,
magnesium salts, barium salts, or strontium salts to the aqueous stream
enhances the removal
of dissolved organic matter from the aqueous stream. In exemplary embodiments,
the method
further comprises adding calcium chloride or calcium oxide to the aqueous
stream. In
exemplary embodiments, the amount of divalent ions added to the aqueous stream
is any
suitable amount, for example, the amount necessary to achieve a desired level
of removal of
dissolved organic matter.
9
CA 02915917 2015-12-22
[0061] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream comprises: adding one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants to the aqueous stream; adding
one or more
calcium ions to the aqueous stream; and separating the dissolved organic
matter from the
aqueous stream.
[0062] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream comprises: adding one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants to the aqueous stream; adding
one or more
calcium ions to the aqueous stream; adding one or more flocculants to the
aqueous stream;
and separating the dissolved organic matter from the aqueous stream.
[0063] In an exemplary embodiment, a method for removing silica from an
aqueous
stream comprises: adding one or more aluminum-containing coagulants and/or one
or more
iron-containing coagulants to the aqueous stream; and separating the silica
from the aqueous
stream.
[0064] In exemplary embodiments, the method comprises adding one or more
aluminum-containing coagulants to the aqueous stream. In exemplary
embodiments, the
method comprises adding two or more aluminum-containing coagulants. In
exemplary
embodiments, the method comprises adding one or more iron-containing
coagulants. In
exemplary embodiments, the method comprises adding two or more iron-containing
coagulants. In exemplary embodiments, the method comprises adding one or more
aluminum-containing coagulants and one or more iron-containing coagulants.
[0065] In exemplary embodiments, the aqueous stream comprises produced
water from
a steam assisted gravity drainage (SAGD) operation or frac water from a
hydraulic fracturing
operation. In exemplary embodiments, the aqueous stream comprises boiler blow
down water
resulting from conventional treatment of produced water.
[0066] The expression "steam assisted gravity drainage" or "SAGD" is well
known in
the art as an oil recovery technique for producing heavy crude oil and
bitumen, for example
in oil sands projects. Steam assisted gravity drainage (SAGD) is a widely used
process for in-
situ bitumen extraction from oil sands. During SAGD bitumen extraction, steam
is injected
into the production wells which causes a reduction in viscosity of the bitumen
thermally and
facilitates bitumen extraction.
[0067] The water produced during the SAGD extraction of bitumen is known as
SAGD
"produced water." Produced water includes various contaminants including
bitumen and
bitumen derived or associated materials, clay and water. The produced water
may contain, for
CA 02915917 2015-12-22
=====
=
%of 400
example, residual oil, suspended and dissolved solids (organic and inorganic),
dissolved
organic matter and silica.
[0068] In exemplary embodiments, the separation step is accomplished by
gravity
settling, filtration, flotation, reverse osmosis, cyclonic separation devices
or other mechanical
separation methods.
[0069] In exemplary embodiments, the method further comprises adjusting the
pH of
the aqueous stream prior to addition of the one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants. In exemplary embodiments, the
method
further comprises adjusting the pI1 of the aqueous stream after the addition
of the one or more
aluminum-containing coagulants and/or one or more iron-containing coagulants.
In
exemplary embodiments, the pH of the aqueous stream is adjusted by adding one
or more pH
modifying agents, which may be in the form of an aqueous solution, for example
an aqueous
solution comprising a base, an acid, a pH buffer, or any combination thereof.
In exemplary
embodiments, the pH modifying agent is an acid, for example hydrochloric acid,
sulfuric
acid, and aqueous solutions thereof; and carbon dioxide gas. In exemplary
embodiments, the
pH modifying agent is carbon dioxide gas. In exemplary embodiments, the pH
modifying
agent is calcium oxide. In exemplary embodiments, the pH of the aqueous stream
is adjusted
to a pH in the range of about 2 to about 12, about 2 to about 11.8, about 2 to
about 8, about 3
to about 8, about 4 to about 8, about 5 to about 8, about 6 to about 8, about
8 to about 12, or
about 10 to about 12. In exemplary embodiments, the pH of the aqueous stream
is adjusted to
a pH of about 3, about 4, about 5, about 6, about 7, about 8, about 9, about
10, about 11,
about 11.8, or about 12. In exemplary embodiments, the pH of the aqueous
stream is adjusted
to a pH of at least about 3, about 4, about 5, about 6, about 7, about 8,
about 9, about 10,
about 11, about 11.8, or about 12. In exemplary embodiments, the pH of the
aqueous stream
is adjusted to a pH of less than about 8, about 9, about 10, about 11, about
11.8, or about 12.
[0070] In exemplary embodiments, the method further comprises adjusting the
pH to a
pH of about 4 to about 8, about 4 to about 6, about 4 to about 5, about 5 to
about 6, about 6 to
about 7, about 7 to about 8, or about 6 to about 8, of the aqueous stream
prior to, or after,
addition of the one or more aluminum-containing coagulants. In exemplary
embodiments, the
method further comprises adjusting the pH to a pH of about 4 to about 5 of the
aqueous
stream prior to, or after, addition of one or more polyaluminum chloride
coagulants. In
exemplary embodiments, the method further comprises adjusting the pH to a pH
of about 4 to
about 6 of the aqueous stream prior to, or after, addition of one or more
aluminum sulfate
coagulants. In exemplary embodiments, the method further comprises adjusting
the pH to a
11
CA 02915917 2015-12-22
%sr
pH of less than about 4 of the aqueous stream prior to, or after, addition of
one or more
acidified aluminum sulfate coagulants.
[0071] In exemplary embodiments, the method further comprises adjusting the
pH to a
pH of about 4 to about 9, about 7 to about 9, about 6 to about 8, about 6 to
about 7, or about 4
to about 6 of the aqueous stream prior to, or after, addition of the one or
more iron-containing
coagulants. In exemplary embodiments, the method further comprises adjusting
the pH to a
pH of about 4 to about 6 of the aqueous stream prior to, or after, addition of
the one or more
ferrous chloride coagulants. In exemplary embodiments, the method further
comprises
adjusting the pH to a pH of about 4 to about 6 of the aqueous stream prior to,
or after,
addition of the one or more ferric chloride coagulants. In exemplary
embodiments, the
method further comprises adjusting the pH to a pH of about 4 to about 6 of the
aqueous
stream prior to, or after, addition of the one or more poly ferric sulfate
coagulants.
[0072] In exemplary embodiments, the method further comprises adjusting the
pH to a
pH of the aqueous stream prior to, or after, addition of the one or more
coagulants to a pH
which will facilitate about 30 to about 40%, about 40 to about 50%, or greater
than about
50%, removal of the dissolved organic matter.
[0073] In exemplary embodiments, the method can be used to remove dissolved
organic matter from an aqueous stream containing greater than about 500 ppm,
about 1000
ppm, about 1500 ppm, about 2000 ppm, about 2400 ppm or about 2500 ppm of
dissolved
organic matter. In exemplary embodiments, the method can be used to remove
silica from an
aqueous stream containing greater than about 50 ppm, about 100 ppm, about 150
ppm, about
200 ppm, about 250 ppm, about 300 ppm of silica.
[0074] In exemplary embodiments, the one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants can be added in any amount
effective for
agglomerating the dissolved organic matter in the aqueous stream. The
coagulant dosage
depends, at least in part, upon the characteristics of the aqueous stream to
be treated, for
example the pH of the aqueous stream, concentration of dissolved organic
matter in the
aqueous stream or the composition of the dissolved organic matter in the
aqueous stream, as
well as the desired result. In exemplary embodiments, the one or more aluminum-
containing
coagulants and/or one or more iron-containing coagulants are added to the
aqueous stream in
a dosage range of from about 0.5 to about 15,000 ppm, about 10 to about 15,000
ppm, about
100 to about 15,000 ppm, about 500 to about 15,000 ppm, about 1000 to about
15,000 ppm,
about 10 to 5000 ppm, about 100 to 5000 ppm, about 500 to 5000 ppm, about 1000
to 5000
ppm.
12
CA 02915917 2015-12-22
"aft.
orease Nero
[0075] In one embodiment, the dosage of the one or more coagulants is about
1 to
about 15 times the amount of the contaminants by mass. In one embodiment, the
dosage of
the one or more coagulants is less than about 15 times the amount of the
contaminants by
mass.
[0076] In exemplary embodiments, the dose of the one or more coagulants is
about 5,
about 10, about 15, about 20, about 35, about 30, about 35, about 40, about
50, about 55,
about 60, about 65, about 70 or about 75% or less than the dose of coagulant
necessary to
achieve the same result (e.g., removal of DOM, reduction in TOC) in the
absence of the one
or more adsorbents.
[0077] In exemplary embodiments, the amount or dose of the one or more
coagulants
is less than about 5000 ppm, less than about 4500 ppm, less than about 4000
ppm, less than
about 3500 ppm, less than about 3000, or less than about 2500, but in each
case, greater than
zero.
[0078] In exemplary embodiments, the amount or dose of the one or more
coagulants
is about 500 ppm, about 1000 ppm, about 1500 ppm, about 2000 ppm, about 2500
ppm,
about 3000 ppm, about 3500 ppm, about 4000 ppm, or about 4500 ppm.
[0079] The temperature of the aqueous stream may vary. In exemplary
embodiments,
the temperature of the aqueous stream is in the range of about 20 C to about
100 C.
[0080] In exemplary embodiments, the aqueous stream is heated after the
addition of
the one or more aluminum-containing coagulants and/or one or more iron-
containing
coagulants, for example to a temperature of at least about 50 C, about 60 C,
about 70 C,
about 80 C, about 85 C, about 90 C, or about 100 C.
[0081] In exemplary embodiments, the reduction in DOM is evidenced by a
reduction
in TOC.
[0082] In exemplary embodiments, the method reduces the amount of DOM (or
the
TDS or TOC) in the aqueous stream by at least about 10%, at least about 20%,
at least about
30%, at least about 40%, at least about 50% or at least about 60% or more.
[0083] In exemplary embodiments, the method reduces the amount of DOM in
the
SAGD produced water by at least about 10%, at least about 20%, at least about
30%, at least
about 40%, at least about 50%, or at least about 60% or more.
[0084] In exemplary embodiments, the method reduces the TDS in the SAGD
produced water by at least about 10%, at least about 20%, at least about 30%,
at least about
40%, at least about 50%, or at least about 60% or more.
13
CA 02915917 2015-12-22
[0085] In exemplary embodiments, the method reduces the TOC in the SAGD
produced water by at least about 10%, at least about 20%, at least about 30%,
at least about
40%, at least about 50%, or at least about 60% or more.
[0086] In exemplary embodiments, the method results in TOC removal from the
SAGD produced water of at least about 10%, at least about 20%, at least about
30%, at least
about 40%, at least about 50%, at least about 55%, at least about 60% or at
least about 65%
or more.
[0087] In exemplary embodiments, the method reduces the DOM in the BBD by
at
least about 10%, at least about 20%, at least about 30%, at least about 40%,
at least about
50%, at least about 60%, at least about 70% or at least about 80%.
[0088] In exemplary embodiments, the method reduces the TOC in the BBD by
at least
about 10%, at least about 20%, at least about 30%, at least about 40%, at
least about 50%, or
at least about 60% or more.
[0089] In exemplary embodiments, the method results in TOC removal from the
BBD
of at least about 10%, at least about 20%, at least about 30%, at least about
40%, at least
about 50%, at least about 55%, at least about 60% or at least about 65% or
more.
[0090] In a particular embodiment, the dose of the coagulant is less than
5000 ppm and
the method results in TOC removal in the BBD of at least about 50%, at least
about 55%, or
at least about 60% or more.
[0091] In another particular embodiment, the dose of the coagulant is less
than about
4000 ppm and the method results in TOC removal in the BBD of at least about
50%, at least
about 55%, or at least about 60% or more.
[0092] In yet another particular embodiment, the dose of the coagulant is
about 3000
ppm and the method results in TOC removal in the BBD of at least about 50%, at
least about
55%, or at least about 60% or more.
[0093] In a still further embodiment, the dose of the coagulant is about
3000 ppm or
less and the method results in TOC removal in the BBD of at least about 50%,
at least about
55%, or at least about 60% or more.
[0094] In exemplary embodiments, the methods are used to provide water of
suitable
quality to a steam generator, for example a once-through steam generator
(OSTG) or a high
efficiency steam generator. Dissolved organic matter and/or silica in the
water can foul, or
form scales in, a steam generator. The layer of scales can reduce the heat
transfer efficiency
from the fire side of the OTSG to the water side of the OTSG, impeding steam
production
and quality. To maintain steam production rates, more energy is required to
overcome the
insulating effect of the layer of scales to create a constant amount of steam.
The OTSG must
14
CA 02915917 2015-12-22
Nor NW,
be periodically cleaned to remove the build-up. If the OTSG is not cleaned
often enough, the
OTSG may overheat and fail. Heat exchangers are similarly subject to fouling
and must be
cleaned periodically. Further, organic and other contaminants are concentrated
in blowdown
water, which in some cases may impede using a crystallizer to treat the
blowdown. In
exemplary embodiments, the methods are used to provide water of a suitable
quality to an
OTSG.
[0095] In exemplary embodiments, the methods are used to provide water
having a
non-volatile TOC content of less than 600 mg/L non-volatile TOC to an OTSG,
for example,
less than about 200, less than about 300, less than about 400, less than about
500, or less than
about 600 mg/L.
[0096] In exemplary embodiments, the methods are used to provide water of
suitable
quality to a conventional drum boiler. As compared to OTSGs, these boilers
require lower
contaminant concentrations.
[0097] In exemplary embodiments, the methods are used to provide water
having a
non-volatile TOC content of less than about 1 mg/L to a drum boiler, for
example, less than
about 0.90, less than about 0.80, less than about 0.70, less than about 0.60,
less than about
0.50, less than about 0.40, less than about 0.30, less than about 0.20, or
less than about 0.10
mg/Ls.
[0098] In exemplary embodiments, the aqueous stream is first treated with a
water
softening process, such as a lime softening process and/or a cation exchange
softening
process prior to the treatment of the aqueous stream with the one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants.
[0099] In exemplary embodiments, the one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants can be added to, or applied to,
the aqueous
stream in a process that may be a batch process, a continuous process or a
semi-continuous
process. Such processes can include settling or filtering processes. In
exemplary
embodiments, the one or more aluminum-containing coagulants and/or one or more
iron-
containing coagulants can be added as dry materials, liquids, solutions or as
dispersions, for
example dispersions in water.
[00100] In exemplary embodiments, the one or more aluminum-containing
coagulants
and/or one or more iron-containing coagulants are added to an aqueous stream,
for example
in a reactor or mixing tank, and the aqueous stream is stirred or agitated. In
one embodiment,
after adding one or more aluminum-containing coagulants and/or one or more
iron-containing
coagulants, the aqueous stream is stirred or agitated for a period of time
from about 5 minutes
to about 12 hours, or about 1 hour to about 3 hours. In exemplary embodiments,
after adding
CA 02915917 2015-12-22
Nome
the one or more aluminum-containing coagulants and/or one or more iron-
containing
coagulants, the aqueous stream is stirred for at least about 15 minutes, about
30 minutes,
about one hour, about two hours, or about 3 hours. There is no particular
limit on the amount
of time that the aqueous stream may be stirred after adding the one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants.
[00101] In exemplary embodiments, after agitation of the aqueous stream,
the stream
may be transferred to a thickener or settling tank, or may be allowed to
settle where it is. In
certain embodiments, a flocculant may be added to assist in settling.
[00102] In exemplary embodiments, the method may further comprise adding a
flocculant. Any suitable flocculant or mixture of flocculants may be used in
the method
described herein. In certain embodiments, the one or more flocculants added to
the aqueous
stream comprise one or more polymer flocculants. In exemplary embodiments, the
polymer
flocculants may have no charge, low charge, medium charge or high charge.
[00103] In exemplary embodiments, the polymer flocculants may be anionic,
nonionic,
or cationic, for example an acrylamide flocculant. Any polymer flocculants
known in the art
may be used in the processes described herein. Non-limiting examples of
exemplary polymer
flocculants include, for example, flocculant-grade homopolymers, copolymers,
and
terpolymers prepared from monomers such as (meth)acrylic acid,
(meth)acrylamide, 2-
acrylamido-2-methylpropane sulfonic acid, and ethylene oxide. In one
embodiment, the
polymer flocculant is an anionic polymer. In one embodiment, the polymer
flocculant is a
nonionic polymer. In one embodiment, the polymer flocculant is a mixture of an
anionic
polymer and a nonionic polymer. In an exemplary embodiment, the one or more
flocculants
comprise a polyacrylamide flocculant. In exemplary embodiments, the polymer
flocculants
comprise a polyacrylate flocculant.
[00104] As used herein, the terms "polymer," "polymers," "polymeric," and
similar
terms are used in their ordinary sense as understood by one skilled in the
art, and thus may be
used herein to refer to or describe a large molecule (or group of such
molecules) that contains
recurring units. Polymers may be formed in various ways, including by
polymerizing
monomers and/or by chemically modifying one or more recurring units of a
precursor
polymer. A polymer may be a "homopolymer" comprising substantially identical
recurring
units formed by, e.g., polymerizing a particular monomer. A polymer may also
be a
"copolymer" comprising two or more different recurring units formed by, e.g.,
copolymerizing two or more different monomers, and/or by chemically modifying
one or
more recurring units of a precursor polymer. The term "terpolymer" may be used
herein to
refer to polymers containing three or more different recurring units.
16
CA 02915917 2015-12-22
or*.
[00105] In the
exemplary embodiments, the dosage of the one or more flocculants can
be any dosage that will achieve a necessary or desired result. In one
embodiment, the dosage
of the one or more flocculants is about 5 ppm to about 200 ppm; about 25 ppm
to about 150
ppm; about 5 ppm to about 100 ppm; about 10 ppm to about 70 ppm; or about 20
ppm to
about 50 ppm. In one embodiment, the dosage of the one or more flocculants is
less than
about 150 ppm, about 100 ppm, about 70 ppm, about 50 ppm, or about 25 ppm.
[00106] In certain
embodiments, the method may further comprise the step of adding
one or more other coagulants, for example a coagulant that does not contain
aluminum, a
coagulant that does not contain iron, or a coagulant that does not contain
iron or aluminum.
[00107] In an
exemplary embodiment, the method further comprises the step of adding
one or more other coagulants before the addition of the one or more
flocculants. A "other
coagulant" as referred to herein includes, for example, hydrated lime;
magnesium carbonate;
a polymer that contains one or more quaternized ammonium groups or mixtures
thereof.
Polymer coagulants that contain one or more quaternized ammonium groups
include, for
example acryloyloxyethyltrimethylammonium chloride,
methacryloyloxyethyltrimethylammonium chloride,
methacrylamidopropyltrimethylammonium chloride, and
acrylamidopropyltrimethylammonium chloride.
[00108] In the
exemplary embodiments, the dosage of the one or more other coagulants
can be any dosage that will achieve a necessary or desired result. In one
embodiment, the
dosage of the one or more coagulants is about 1 to about 15 times the amount
of the
contaminants by mass (e.g. a ratio of about 1 iron to about 15 arsenic by
mass). In one
embodiment, the dosage of the one or more coagulants is less than about 15
times the amount
of the contaminants by mass.
[00109] In exemplary
embodiments, the dissolved organic matter and contaminants,
once treated according to any of the exemplary methods described herein, may
be handled or
processed in any manner as necessary or desired. In one embodiment, the
dissolved organic
matter and contaminants should be handled in compliance with governmental
regulations. In
some embodiments, the dissolved organic matter and contaminants may be
disposed of, sent
to a landfill, or when solids are a concentrated source of minerals, the
solids may be used as
raw materials or feed to produce compounds for commercial products.
[00110] Exemplary Methods Involving Aeration
[00111] The method
of the present embodiments includes aeration in addition to the
addition of one or more coagulants. Oxidation of the aqueous stream enhances
the removal of
17
CA 02915917 2015-12-22
44.0
dissolved organic matter, while also reducing the residual iron and improving
the clarity of
the treated aqueous stream.
[00112] The phrase "aeration" or "aerating" as used herein refers to a
process by which
air is circulated through, mixed with or dissolved in the aqueous stream. As a
result of
aeration, the oxygen saturation of the aqueous stream is increased. In
exemplary
embodiments, the oxygen saturation of the aqueous stream is increased by at
least about 10%,
at least about 20%, at least about 30%, at least about 40% or at least about
50% or more.
[00113] Aeration methods are riot particularly limited and can be achieved
by any
means known in the art. Any method by which oxygen is added to an aqueous
stream
constitutes aeration. Aeration may be surface aeration or sub-surface
aeration.
[00114] For example, aeration is achieved by: i) passing the liquid through
air by means
of fountains, cascades, paddle-wheels or cones; ii) passing air through the
liquid by means of
the Venturi tube, aeration turbines or compressed air which can be combined
with diffuser(s)
air stone(s), as well as fine bubble diffusers, coarse bubble diffusers or
linear aeration tubing.
Ceramics are suitable for this purpose, often involving dispersion of fine air
or gas bubbles
through the porous ceramic into a liquid. The smaller the bubbles, the more
gas are exposed
to the liquid increasing the gas transfer efficiency. Diffusers or spargers
can also be designed
into the system to cause turbulence or mixing if desired.
[00115] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream is provided, the method comprising: adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
aqueous stream,
aerating the aqueous stream and separating the dissolved organic matter from
the aqueous
stream.
[00116] In exemplary embodiments, the aerating step may occur before,
concurrently
with, or after the addition of the one or more aluminum-containing coagulants
and/or one or
more iron-containing coagulants to the aqueous stream. In exemplary
embodiments, the
aerating step may occur during or after the separating step.
[00117] In exemplary embodiments, a method of removing dissolved organic
matter
from SAGD produced water, comprises: adding one or more coagulants to the SAGD
produced water, wherein the coagulant is selected from the group consisting of
aluminum-
containing coagulants and iron containing coagulants; aerating the aqueous
stream; and
separating the dissolved organic matter from the SAGD produced water. The
result of the
method is a treated SAGD produced water (i.e., BFW) having a reduced amount of
dissolved
organic matter relative to the amount of dissolved organic matter in the SAGD
produced
18
CA 02915917 2015-12-22
%se Now
water prior to treatment. In exemplary embodiments, the reduction in dissolved
organic
matter is evidenced as a reduction in total organic carbon (TOC).
[00118] In an exemplary embodiment, a method of removing dissolved organic
matter
from SAGD produced water, comprises: adding polyaluminum chloride to the SAGD
produced water; aerating the aqueous stream; and separating the dissolved
organic matter
from the SAGD produced water.
[00119] In exemplary embodiments, a method of removing dissolved organic
matter
from boiler blow down, comprises: adding one or more coagulants to the boiler
blow down,
wherein the coagulant is selected from the group consisting of aluminum-
containing
coagulants and iron containing coagulants; aerating the boiler blown down; and
separating
the dissolved organic matter from the boiler blow down. The result of the
exemplary method
is a treated boiler blow down material having a reduced amount of dissolved
organic matter
relative to the boiler blow down prior to treatment. In exemplary embodiments,
the reduction
in dissolved organic matter is evidenced as a reduction in total organic
carbon (TOC).
[00120] In an exemplary embodiment, a method of removing dissolved organic
matter
from boiler blow down, comprises: adding polyaluminum chloride to the boiler
blow down;
aerating the boiler blow down; and separating the dissolved organic matter
from the boiler
blow down.
[00121] The aerating step may occur before, concurrently with, or after the
addition of
the one or more divalent ions to the aqueous stream.
[00122] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream is provided, the method comprising: adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
aqueous stream,
adding one or more divalent ions, aerating the aqueous stream and separating
the dissolved
organic matter from the aqueous stream.
[00123] In an exemplary embodiment, a method for removing dissolved organic
matter
from SAGD produced water is provided, the method comprising: adding one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the SAGD
produced water, adding one or more divalent ions, aerating the SAGD produced
water and
separating the dissolved organic matter from the SAGD produced water. The
result of the
method is a treated SAGD produced water (i.e., BFW) having a reduced amount of
dissolved
organic matter relative to the amount of dissolved organic matter in the SAGD
produced
water prior to treatment. In exemplary embodiments, the reduction in dissolved
organic
matter is evidenced as a reduction in total organic carbon (TOC).
19
CA 02915917 2015-12-22
Nwe =Noit
[00124] In an exemplary embodiment, a method for removing dissolved organic
matter
from boiler blow down is provided, the method comprising: adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
boiler blow
down, adding one or more divalent ions, aerating the boiler blow down and
separating the
dissolved organic matter from the boiler blow down. The result of the
exemplary method is a
treated boiler blow down material having a reduced amount of dissolved organic
matter
relative to the boiler blow down prior to treatment. In exemplary embodiments,
the reduction
in dissolved organic matter is evidenced as a reduction in total organic
carbon (TOC).
[00125] In exemplary embodiments, the method further comprises adding one
or more
calcium salts, magnesium salts, barium salts, or strontium salts to the
aqueous stream. In
exemplary embodiments, the one or more calcium salts, magnesium salts, barium
salts, or
strontium salts may be added before, concurrently, or after the addition of
the one or more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the
aqueous stream. In exemplary embodiments, the one or more calcium salts,
magnesium salts,
barium salts, or strontium salts may be added sequentially or simultaneously
with the one or
more aluminum-containing coagulants and/or one or more iron-containing
coagulants to the
aqueous stream. In exemplary embodiments, the addition of the one or more
calcium salts,
magnesium salts, barium salts, or strontium salts to the aqueous stream
enhances the removal
of dissolved organic matter from the aqueous stream.
[00126] The aerating step may occur before, concurrently with, or after the
addition of
the one or more divalent ions to the aqueous stream.
[00127] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream is provided, the method comprising: adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
aqueous stream,
adding one or more calcium ions, aerating the aqueous stream and separating
the dissolved
organic matter from the aqueous stream.
[00128] In an exemplary embodiment, a method for removing dissolved organic
matter
from SAGD produced water is provided, the method comprising: adding one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the SAGD
produced water, adding one or more calcium ions, aerating the SAGD produced
water and
separating the dissolved organic matter from the SAGD produced water. The
result of the
method is a treated SAGD produced water (i.e., BFW) having a reduced amount of
dissolved
organic matter relative to the amount of dissolved organic matter in the SAGD
produced
water prior to treatment. In exemplary embodiments, the reduction in dissolved
organic
matter is evidenced as a reduction in total organic carbon (TOC).
CA 02915917 2015-12-22
'woo
[00129] In an exemplary embodiment, a method for removing dissolved organic
matter
from boiler blow down is provided, the method comprising: adding one or more
aluminum-
containing coagulants and/or one or more iron-containing coagulants to the
boiler blow
down, adding one or more calcium ions, aerating the boiler blow down and
separating the
dissolved organic matter from the boiler blow down. The result of the
exemplary method is a
treated boiler blow down material having a reduced amount of dissolved organic
matter
relative to the boiler blow down prior to treatment In exemplary embodiments,
the reduction
in dissolved organic matter is evidenced as a reduction in total organic
carbon (TOC).
[00130] In an exemplary embodiment, a method for removing silica from an
aqueous
stream is provided, the method comprising: adding one or more aluminum-
containing
coagulants to the aqueous stream and/or one or more iron-containing
coagulants, aerating the
aqueous stream and separating the silica from the aqueous stream. In certain
embodiments,
aerating the aqueous stream occurs before the separating step. In certain
embodiments,
aerating the aqueous stream occurs after the separating step. The result of
the exemplary
method is a treated aqueous stream having a reduced amount of silica relative
to an aqueous
stream prior to treatment.
[00131] In exemplary embodiments, when used in combination with the
aeration step
the dose of the one or more coagulants necessary to achieve a certain result
(e.g., removal of
DOM) is less than the dosage of the one or more coagulants used alone (without
aeration). In
exemplary embodiments, the dose of the one or more coagulants is about 5%,
about 10%,
about 15%, about 20%, about 35%, about 30%, about 35%, about 40%, about 50%,
about
55%, about 60%, about 65%, about 70% or about 75% less than the dose of
coagulant
necessary to achieve the same result (e.g., removal of DOM, reduction in TOC)
in the
absence of the aeration step. In exemplary embodiments, the aeration step
occurs after the
one or more coagulants are added.
[00132] In exemplary embodiments, the agitation of the aqueous stream after
addition of
the one or more aluminum-containing coagulants and/or one or more iron-
containing
coagulants is achieved by aeration of the aqueous stream. In exemplary
embodiments, the
reactor or mixing tank is fitted with apparatus sufficient to aerate the
aqueous stream held in
the tank.
[00133] Exemplary Methods Involving Adsorbents
[00134] In exemplary embodiments, the method may further comprise the step
of
adding one or more adsorbents. The one or more adsorbents may be any suitable
adsorbent.
In exemplary embodiments, the adsorbent comprises a silica-based compound, for
example,
an inorganic silica-based polymer, a clay-based material, cellulose, alumina-
cased
21
CA 02915917 2015-12-22
+err Near
adsorbents, ferrohydrate adsorbents, carbon, for example carbon black, or a
mixture thereof.
In exemplary embodiments, the adsorbent comprises metakaolin, pumice,
aragonite, recycled
glass powder or combinations thereof.
[00135] In an exemplary embodiment, the method further comprises the step
of adding
one or more additional adsorbents before the addition of the one or more
flocculants.
[00136] In exemplary embodiments, a method of removing dissolved organic
matter
from SAGD produced water, comprises adding one or more adsorbents and one or
more
coagulants to the SAGD produced water, wherein the coagulant is selected from
the group
consisting of aluminum-containing coagulants and iron containing coagulants.
The result of
the method is a treated SAGD produced water (i.e., BFW) having a reduced
amount of
dissolved organic matter relative to the amount of dissolved organic matter in
the SAGD
produced water prior to treatment. In exemplary embodiments, the reduction in
dissolved
organic matter is evidenced as a reduction in total organic carbon (TOC).
[00137] In an exemplary embodiment, a method of removing dissolved organic
matter
from SAGD produced water, comprises adding one or more adsorbents and one or
more
coagulants to the SAGD produced water, wherein (i) the adsorbent is a silica-
based
compound and (ii) the coagulant is selected from the group consisting of
aluminum-
containing coagulants and iron-containing coagulants.
[00138] In an exemplary embodiment, a method of removing dissolved organic
matter
from SAGD produced water, comprises adding one or more adsorbents and one or
more
coagulants to the SAGD produced water, wherein (i) the adsorbent is a silica-
based
compound and (ii) the coagulant is an aluminum-containing coagulant.
[00139] In an exemplary embodiment, a method of removing dissolved organic
matter
from SAGD produced water, comprises: adding one or more adsorbents and one or
more
coagulants to the SAGD produced water, wherein (i) the adsorbent is selected
from the group
consisting of rnetakoalin, pumice, aragonite, recycled glass power and
combinations thereof
and (ii) the coagulant is polyaluminum chloride.
[00140] In exemplary embodiments, a method of removing dissolved organic
matter
from boiler blow down, comprises adding one or more adsorbents and one or more
coagulants to the boiler blow down, wherein the coagulant is selected from the
group
consisting of aluminum-containing coagulants and iron containing coagulants.
The result of
the exemplary method is a treated boiler blow down material having a reduced
amount of
dissolved organic matter relative to the boiler blow down prior to treatment.
In exemplary
embodiments, the reduction in dissolved organic matter is evidenced as a
reduction in total
organic carbon (TOC).
22
CA 02915917 2015-12-22
Noe woe
[00141] In an exemplary embodiment, a method of removing dissolved organic
matter
from boiler blow down, comprises adding one or more adsorbents and one or more
coagulants to the boiler blow down, wherein (i) the adsorbent is a silica-
based compound and
(ii) the coagulant is selected from the group consisting of aluminum-
containing coagulants
and iron containing coagulants.
[00142] In an exemplary embodiment, a method of removing dissolved organic
matter
from boiler blow down, comprises adding one or more adsorbents and one or more
coagulants to the boiler blow down, wherein (i) the adsorbent is a silica-
based compound and
(ii) the coagulant is an aluminum-containing coagulant.
[00143] In an exemplary embodiment, a method of removing dissolved organic
matter
from boiler blow down, comprises adding one or more adsorbents and one or more
coagulants to the boiler blow down, wherein (i) the adsorbent is selected from
the group
consisting of metakoalin, pumice, aragonite, recycled glass power and
combinations thereof
and (ii) the coagulant is polyaluminum chloride.
[00144] In exemplary embodiments, the one or more adsorbents may be added
before,
concurrently, or after the addition of the one or more coagulants to the
aqueous stream.
[00145] The method may optionally comprise one or more additional steps. In
exemplary embodiments, the method further comprises agitating the aqueous
stream after
adding one or more adsorbents and one or more coagulants to the aqueous
stream.
[00146] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream comprises: adding one or more adsorbents and one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the
aqueous stream; adding one or more divalent ions; and separating the dissolved
organic
matter from the aqueous stream.
[00147] In an exemplary embodiment, a method for removing dissolved organic
matter
from SAGD produced water comprises: adding one or more adsorbents and one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the SAGD
produced water; adding one or more divalent ions; and separating the dissolved
organic
matter from the SAGD produced water. The result of the exemplary method is a
treated
SAGD produced water (i.e., BFW) having a reduced amount of dissolved organic
matter
relative to the SAGD produced water prior to treatment. In exemplary
embodiments, the
reduction in dissolved organic matter is evidenced as a reduction in total
organic carbon
(TOC).
[00148] In an exemplary embodiment, a method for removing dissolved organic
matter
from boiler blow down comprises: adding one or more adsorbents and one or more
23
CA 02915917 2015-12-22
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the boiler
blow down; adding one or more divalent ions; and separating the dissolved
organic matter
from the boiler blow down. The result of the exemplary method is a treated
boiler blow down
having a reduced amount dissolved organic matter relative to the boiler blow
down prior to
treatment. In exemplary embodiments, the reduction in dissolved organic matter
is evidenced
by a reduction in total organic carbon (TOC).
[00149] In exemplary embodiments, the method further comprises adding one
or more
calcium salts, magnesium salts, barium salts, or strontium salts to the
aqueous stream. In
exemplary embodiments, the one or more calcium salts, magnesium salts, barium
salts, or
strontium salts may be added before, concurrently, or after the addition of
the one or more
adsorbents and one or more coagulants to the aqueous stream. In exemplary
embodiments,
the one or more calcium salts, magnesium salts, barium salts, or strontium
salts may be added
sequentially or simultaneously with the one or more adsorbents and one or more
coagulants
to the aqueous stream. In exemplary embodiments, the addition of the one or
more calcium
salts, magnesium salts, barium salts, or strontium salts to the aqueous stream
enhances the
removal of dissolved organic matter from the aqueous stream.
[00150] In an exemplary embodiment, a method for removing dissolved organic
matter
from an aqueous stream comprises: adding one or more adsorbents and one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the
aqueous stream; adding one or more calcium ions; and separating the dissolved
organic
matter from the aqueous stream.
[00151] In an exemplary embodiment, a method for removing dissolved organic
matter
from SAGD produced water comprises: adding one or more adsorbents and one or
more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the SAGD
produced water; adding one or more calcium ions; and separating the dissolved
organic
matter from the SAGD produced water. The result of the exemplary method is a
treated
SAGD produced water (i.e., the BFW) having a reduced amount of dissolved
organic matter
relative to the SAGD produced water prior to treatment. In exemplary
embodiments, the
reduction in dissolved organic matter is evidenced by a reduction in total
organic carbon
(TOC).
[00152] In an exemplary embodiment, a method for removing dissolved organic
matter
from boiler blow down comprises: adding one or more adsorbents and one or more
aluminum-containing coagulants and/or one or more iron-containing coagulants
to the boiler
blow down; adding one or more calcium ions; and separating the dissolved
organic matter
from the boiler blow down. The result is a treated boiler blow down having a
reduced amount
24
CA 02915917 2015-12-22
Nene
of dissolved organic matter relative to the boiler blow down prior to
treatment. In exemplary
embodiments, the reduction in dissolved organic matter is evidenced by a
reduction in total
organic carbon (TOC).
[00153] In an exemplary embodiment, a method for removing silica from an
aqueous
stream comprises: adding one or more adsorbents and one or more aluminum-
containing
coagulants to the aqueous stream and/or one or more iron-containing
coagulants; and
separating the silica from the aqueous stream.
[00154] In exemplary embodiments, the method comprises adding three or more
coagulants. In exemplary embodiments, the method comprises adding two or more
aluminum-containing coagulants and one or more iron-containing coagulants. In
exemplary
embodiments, the method comprises adding one or more aluminum-containing
coagulants
and two or more iron-containing coagulants.
[00155] In exemplary embodiments, the method further comprises adjusting
the pH of
the aqueous stream prior to addition of the one or more adsorbents and one or
more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants.
[00156] In exemplary embodiments, the method further comprises adjusting
the pH of
the aqueous stream after the addition of the one or more adsorbents and one or
more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants.
[00157] In exemplary embodiments, the method does not comprise adjusting
the pH of
the aqueous stream prior to, or after, the addition of the one or more
adsorbents and one or
more coagulants, e.g., aluminum-containing and/or iron-containing coagulants.
[00158] In exemplary embodiments, the method does not comprise adding
alkali to the
aqueous stream prior to, or after, the addition of the one or more adsorbents
and one or more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants.
[00159] In exemplary embodiments, the method comprises adding less alkali
to the
aqueous stream prior to, or after, the addition of the one or more adsorbents
and one or more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants than
methods in
which the one or more adsorbents are not added. In certain embodiments, about
10, about 20,
about 30, about 40, about 50, about 60, about 70 or about 80% or less alkali
is added.
[00160] In exemplary embodiments, the one or more adsorbents can be added
to the
aqueous stream in any amount effective for improving the agglomeration of the
dissolved
organic matter in the aqueous stream and improving the removal of DOM. The
adsorbent
dosage depends, at least in part, upon the coagulants used in the method,
characteristics of the
aqueous stream to be treated, for example the pH of the aqueous stream,
concentration of
dissolved organic matter in the aqueous stream or the composition of the
dissolved organic
CA 02915917 2015-12-22
µ11,
matter in the aqueous stream, as well as the desired result. In exemplary
embodiments, the
one or more adsorbents are added to the aqueous stream in a dosage range of
from about 0.5
to about 15,000 ppm, 10 to about 15,000 ppm, 100 to about 15,000 ppm, 500 to
about 15,000
ppm, 1000 to about 15,000 ppm, about 10 to 5000 ppm, about 100 to 5000 ppm,
about 500 to
5000 ppm, about 1000 to 5000 ppm, e.g., about 1000 ppm, about 2000 ppm, about
3000
ppm, about 4000 ppm and about 5000 ppm. In certain exemplary embodiments, the
dosage of
the one or more adsorbents is about 1 to about 10,000 ppm; about 50 to about
5000 ppm; or
about 100 to about 1000 ppm. In one embodiment, the dosage of the one or more
adsorbents
is less than about 10,000 ppm, about 5000 ppm, or about 1000 ppm.
[00161] In exemplary embodiments, the aqueous stream is heated after the
addition of
the one or more adsorbents and one or more coagulants, e.g., aluminum-
containing and/or
iron-containing coagulants, for example to a temperature of at least about 50
C, about 60 C,
about 70 C, about 80 C, about 85 C, about 90 C, or about 100 C.
[00162] In exemplary embodiments, the aqueous stream is first treated with
a water
softening process, such as a lime softening process and/or a cation exchange
softening
process, prior to the treatment of the aqueous stream with the one or more
adsorbents and one
or more coagulants, e.g., aluminum-containing and/or iron-containing
coagulants.
[00163] In exemplary embodiments, the one or more adsorbents and one or
more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants can be
added to, or
applied to, the aqueous stream in a process that may be a batch process, a
continuous process
or a semi-continuous process. Such processes can include settling or filtering
processes. In
exemplary embodiments, the one or more adsorbents and one or more coagulants,
e.g.,
aluminum-containing and/or iron-containing coagulants can be added as dry
materials,
liquids, solutions or as dispersions, for example dispersions in water.
[00164] In exemplary embodiments, the one or more adsorbents and one or
more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants are
added to an
aqueous stream, for example in a reactor or mixing tank, and the aqueous
stream is stirred or
agitated. In one embodiment, after adding one or more adsorbents and one or
more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants, the
aqueous stream
is stirred or agitated for a period of time from about 5 minutes to about 12
hours, or about 1
hour to about 3 hours. In exemplary embodiments, after adding the one or more
adsorbents
and one or more coagulants, e.g., aluminum-containing and/or iron-containing
coagulants, the
aqueous stream is stirred for at least about 15 minutes, about 30 minutes,
about one hour,
about two hours, or about 3 hours. There is no particular limit on the amount
of time that the
26
aqueous stream may be stirred after adding the one or more adsorbents and one
or more
coagulants, e.g., aluminum-containing and/or iron-containing coagulants.
[00165] The following examples are presented for illustrative purposes
only, and are not
intended to be limiting.
EXAMPLES
[00166] Materials and Methods for Examples 1-5.
[00167] Samples of boiler blow down (BBD) water were obtained from a SAGD
process and stored under nitrogen blanket before analysis. All coagulants used
were
commercially available from KemiraTM Oyj.
[00168] Jar Tests: 30 mL of boiler blow down water sample was taken in
autoclavable
centrifuge tubes. The pH of the solution was adjusted to four different pH
values in the range
of 4 to 10. The pH was reduced according to conventional methods, for example,
addition of
acids known in the art including HC1, sulfuric acid or bubbling CO2 gas
through the sample
until the desired pH was attained. The blow down water aliquot was heated to a
temperature
of 85 C. Different dosages of coagulant between 500 to 5000 ppm were added to
the blow
down water samples. The coagulated BBD was then stored in the tubes and placed
in the
oven to maintain a temperature of 85 C. After the coagulated matter had
settled and a
comparatively clear supemata.nt was obtained at the top, the samples were
removed from
oven and analyzed.
[00169] Analytical Techniques:
[00170] The samples were analyzed using at least one of UV-Vis absorbance
spectra
and total organic carbon analyzer.
[00171] The total dissolved organic carbon (DOC) was measured using a TOC
analyzer
(ShimadzuTM, model: TOC-L CPH/CPN) after filtering through a 0.22 pm membrane
filter
(Cellulose acetate, Millipore, USA).
[00172] Absorbance of the samples was measured at a wavelength of 254 am
using a
UV-visible spectrophotometer (Varian, Model: CaryTM 50, USA). Deionized (DI)
water
was used as a blank. The samples were diluted to obtain absorbance in the
range of 0 to 1.2.
[00173] The measurement of the concentration was based on calculation of
total carbon
concentration with reference to potassium hydrogen phthalate standard and
total inorganic
carbon concentration with reference to sodium carbonate and bi-carbonate
mixture.
[00174] A total carbon (TC) standard solution was prepared according to
the following
protocol. A sample of 2.125 g of potassium hydrogen phthalate was accurately
weighed and
taken in a 1 L volumetric flask. DI water was added up to the 1 L mark and the
solution was
27
Date Recue/Date Received 2022-03-31
stirred. The potassium hydrogen phthalate solution prepared has a
concentration equivalent to
1000 ppm total organic carbon concentration. This solution was used as a
standard for total
(TC) analysis. A calibration was performed on the TOC analyzer with four
different
concentrations of the stock standard solution created by in-situ dilution in
the instrument. A
calibration curve was created based on which the TOC calculations were
performed.
[00175] An inorganic carbon (IC) standard solution was prepared according
to the
following protocol. A sample of 3.497 g of reagent grade sodium bicarbonate
and 4.412 g of
sodium carbonate was accurately weighed and taken in a 1 L volumetric flask.
DI water was
added to the flask up to the 1 L mark and the flask was shaken well. In this
manner a liquid
solution equivalent to 1000 ppm carbon concentration was prepared. This
solution was used
as standard for inorganic carbon (IC) analysis.
[00176] The analysis of dissolved organic matter content was carried out
according to
the following protocol. The samples were collected in 40 mL vials for TOC
analysis. The
sample vials were capped in order to prevent the volatilization and
contamination of the TOC
components in the samples. A calibration curve was generated by analyzing
various
concentrations of the TC and IC standard solutions. The TC values of the
samples were
determined by heating the samples at a temperature of 680 C over a high
sensitivity oxidation
catalyst. Samples were injected to the IC reaction vessel equipped with TOC-L
IC reactor kit
to measure the IC content in the sample TOC was measured as the difference
between the
TC and IC analysis values.
[00177] In these examples, the terms TOC concentration and dissolved
organic carbon
(DOC) concentration shall be used interchangeably.
[00178] Example 1. Removal of Dissolved Organic Matter from BBD Water
with a
Polyaluminum Chloride Coagulant
[00179] In this example, several SAGD BBD samples were treated with
polyaluminum
chloride coagulant C, which is a liquid polyaluminum chloride coagulant
containing 9%
elemental aluminum and with a basicity of 42% (available from KemiraTM Oyj).
The pH,
percentage reduction in total organic carbon concentration and specific UV
absorbance of the
treated samples are summarized in Tables 2, 3 and 4, respectively. The pH
listed at the top of
each table is the pH of the sample prior to treatment with the coagulant.
[00180] The percentage organic removal at different pH values is shown in
Figure 1.
From Figure 1 it can be observed that when the initial pH of the BBD water was
reduced to a
value of 6 and 8, more than 50% removal of the organic matter was obtained
with even a
lower dosage of the coagulant, for example 2000 to 3000 ppm.
28
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
-
%ow
[00181] Specific UV absorbance (SUVA) at 254 nm is an indicator of the
chemical
characteristics of the organic matter present in the solution. The graph
depicted in Figure 2
shows the SUVA region for the hydrophobic (aromatic) and hydrophilic
(aliphatic) type of
compounds in the residual organic matter in the supernatants of the treated
BBD water. The
results indicate that with an increase in the dosage of the coagulant and/or
with a decrease in
the starting pH of the BBD water, the SUVA value shifts from the mixed zone
towards the
hydrophilic zone. This indicates that the organic matter in the BBD water and
SAGD
produced water were a mixture of aromatic hydrophobic organics and aliphatic
hydrophilic
organics. Thus, with coagulation the hydrophobic concentration in the blow
down water
could be reduced.
[00182] Figure 2 shows that when the starting pH was 11.8, only with a
dosage of 5000
ppm of polyaluminum chloride coagulant, could the SUVA value of the
supernatant of the
treated blow down be reduced to a value in the hydrophilic zone. However, the
same zone
shift was observed with just 3000 ppm of polyaluminum chloride when starting
pH was 8,
and with even lower coagulant dosages, for example 1000 and 2000 ppm, as the
pH was
lowered to 4 and 6, respectively.
[00183] The data demonstrates that this coagulant was effective for organic
removal
from SAGD BBD water over a broad pH range, including highly alkaline pH (e.g.
p11 of
11.8). Even at pH 11.8 with 5000 ppm dose of this coagulant, it was possible
to remove more
than 65% organic matter and obtain a good supernatant clarity.
[00184] Table 2. pH of the supernatant with different dosages of
polyaluminum chloride
coagulant C
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 4.12 6.07 7.03 10.52
1000 4.01 5.82 6.56 9.26
2000 3.88 4.94 6.18 8.89
3000 3.74 4.34 4.79 8.74
4000 3.59 3.96 4.55 5.67
5000 3.37 3.59 3.99 4.82
[00185] Table 3. Percent reduction in total organic carbon using different
dosages of
polyaluminum chloride coagulant C
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
29
CA 02915917 2015-12-22
500 56.14 27.40 26.97 15.64
1000 60.33 45.46 40.08 16.88
2000 60.60 54.49 47.11 18.67
3000 60.23 63.52 64.12 19.77
4000 61.98 65.23 64.44 37.81
5000 62.44 65.90 64.93 66.28
[00186] Table 4. SUVA254 values of the supernatant at different dosages of
polyaluminum chloride coagulant C
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 2.13 2.87 3.11 " 3.14
1000 2.02 1.99 2.76 3.16
2000 1.83 1.65 2.55 3.10
3000 1.78 1.54 1.59 2.94
4000 1.72 1.57 1.45 2.28
5000 1.62 1.55 1.48 1.25
[00187] The data supports that polyaluminum chloride coagulant C removes
the humic
like hydrophobic fractions in the SAGD BBD even at a pH of 11.8. As the
initial pH of the
sample was reduced, the same amount of organic removal was observed with a
lower
coagulant dosage.
[00188] A comparative experiment was also carried out in which percent TOC
removal
was assessed at various pH values with and without addition of the coagulant.
One set of
SAGD BBD samples were treated with a 20% HC1 solution to reduce the pH by
incremental
amounts. The other set of SAGD samples were treated with coagulant only. The
pH and
percentage reduction in total organic carbon concentration are listed in Table
5 and shown in
Figure 3. Both sample sets started with SAGD BBD at pH 11.8. The results show
that
acidification alone increased the % TOC removal to some extent. However, the
addition of
polyaluminum chloride coagulant C not only resulted in lower pH, it also
produced a higher
% TOC removal at any given pH, as compared to the acidification alone.
[00189] Table 5. % TOC removal for various pH values with and without
addition of
polyaluminum chloride coagulant C
Acid Additions Coagulation Addition % TOC Removal
Final pH
9.5 5.2
8.19 7.33
CA 02915917 2015-12-22
woe
7.48 8.8
6.64 15.5
5.94 22.6
4.9 34.6
3.09 43
2.03 45.7
10.52 15.64
9.26 16.88
8.89 18.67
8.74 19.77
5.67 37.81
4.82 66.28
[00190] Example 2. Removal of Dissolved Organic Matter from BBD water with
Polyaluminum Chloride Coagulant D
[00191] In this example, several SAGD BBD samples were treated with
polyaluminum
chloride coagulant D. The pH, and percentage reduction in total organic carbon
concentration
of the treated samples are summarized in Tables 6, and 7, respectively. The pH
listed at the
top of each table is the pH of the sample prior to treatment with the
coagulant.
[00192] The data demonstrates that this coagulant was effective for organic
removal
from SAGD BBD water, especially in the pH range of 4 to 8.
31
[00193] Table 6. pH of the supematant with different dosages of
polyaluminum chloride
coagulant D
Coagulant pH 4 pH 6 pH 8
Dosage (ppm)
500 4.08 6.20 6.79
1000 4.03 5.83 6.76
2000 4.02 5.39 6.33
3000 3.90 4.86 5.52
4000 3.85 4.42 5.01
5000 3.78 4.23 4.65
[00194] Table 7. Percent reduction in total organic carbon using
different dosages of
polyaluminum chloride coagulant D
Coagulant pH 4 pH 6 pH 8
Dosage (ppm)
500 64.58 44.00 38.38
1000 67.15 51.52 43.41
2000 68.55 59.09 53.03
3000 69.12 66.23 59.49
4000 69.17 68.04 65.79
5000 69.82 69.96 68.36
[00195] Example 3. Removal of Dissolved Organic Matter from BBD water
with
Aluminum Sulfate Coagulant A
[00196] SAGD BBD samples were treated with aluminum sulfate coagulant A,
which is
an acidified aluminum sulfate coagulant with 2.86% aluminum and 10% free acid
(available
from KemiraTM Oyj). The pH, percentage reduction in total organic carbon
concentration and
specific UV absorbance of the treated samples are summarized in Tables 8, 9
and 10,
respectively. The pH listed at the top of each table is the pH of the sample
prior to treatment
with the coagulant.
[00197] The data demonstrates that this coagulant was effective for
organic removal
from SAGD BBD water.
[00198] Table 8. pH of the supernatant with different dosages of aluminum
sulfate
coagulant A
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
32
Date Recue/Date Received 2022-03-31
500 4.02 6.2 7.12 11.2
1000 3.81 5.74 6.7 10.58
2000 3.31 5.02 5.57 9.88
3000 2.99 4.69 4.85 9.02
4000 2.72 3.96 4.22 7.88
5000 ' 2.48 3.71 3.92 7.08
[00199] Table 9. Percent reduction in total organic carbon using
different dosages of
aluminum sulfate coagulant A
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 49.44 23.05 17.94 14.53
1000 49.90 38.86 22.88 15.07
2000 51.57 48.73 45.22 18.13
3000 52.11 52.71 53.74 18.18
4000 52.84 55.87 56.87 18.45
5000 52.98 56.01 63.09 27.51
[00200] Table 10. SUVA254 values of the supernatant at different dosages
of aluminum
sulfate coagulant A
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 2.41 2.84 2.97 3.22
1000 2.24 2.48 2.82 3.25
2000 2.07 2.11 2.10 3.17
3000 2.04 1.92 1.90 3.10
4000 2.14 1.75 1.81 2.87
5000 2.12 1.70 1.64 2.58
[00201] Example 4. Removal of Dissolved Organic Matter from BBD Water
with
an Aluminum Sulfate Coagulant B
[00202] In this example, several SAGD BBD samples were treated with
aluminum
sulfate coagulant B, which is an iron-free liquid aluminum sulfate coagulant
containing about
4.3% aluminum ion (available from KemiraTm Oyj). The pH, percentage reduction
in total
organic carbon concentration and specific UV absorbance of the treated samples
are
33
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
..- .
Iwo war'
summarized in Tables 11, 12 and 13, respectively. The pH listed at the top of
each table is the
pII of the sample prior to treatment with the coagulant.
[00203] The data demonstrates that this coagulant was effective for organic
removal
from SAGD BBD water.
[00204] Table 11. pH of the supernatant with different dosages of aluminum
sulfate
coagulant B
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 4.01 5.91 6.18 11.49
1000 3.77 5.49 6.19 11.48
2000 3.52 4.68 5.31 10.72
3000 3.43 4.18 4.68 9.42
4000 3.38 3.94 4.34 8.19
5000 3.36 3.82 4.03 7.19
[00205] Table 12. Percent reduction in total organic carbon using different
dosages of
aluminum sulfate coagulant B
'Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 62.12 54.06 43.35 9.53
1000 63.47 60.50 49.65 10.99
2000 65.47 67.07 62.44 12.34
3000 65.82 62.82 67.88 14.83
4000 64.90 69.69 69.04 17.31
5000 65.98 69.63 69.20 21.94
[00206] Table 13. SUVA254 values of the supernatant at different dosages of
aluminum
sulfate coagulant B
Coagulant pH 4 pH 6 pH 8 pH 11.8
Dosage (ppm)
500 1.913 2.634 2.681 2.772
1000 - 1.719 2.113 2.584 2.864
2000 1.595 1.534 1.762 2.847
3000 1.606 1.722 1.546 2.811
4000 1.606 1.594 1.545 2.783
5000 1.629 1.616 1.661 2.706
34
CA 02915917 2015-12-22
%woe %Or
[00207] Example 5. Removal of Dissolved Organic Matter from BBD water with
Various Coagulant Combinations
[00208] In this example, BBD water samples from a SAGD process were treated
with
two of various coagulants and the reduction in total organic carbon
concentration was
evaluated via UV spectroscopy (absorbance at 254 nm).
[00209] 30 mL samples of BBD water from a SAGD process containing dissolved
organic matter were taken in autoclavable centrifuge tubes and treated with
sulfuric acid until
a pH of about 7 was obtained. The sample was then heated to a temperature of
85 C in an
oven. A specified dosage of each coagulant in range of 500-5000 ppm was added
to each
sample of the pH-adjusted BBD water. The coagulated BBD was then stored in the
tubes and
placed in oven at a temperature of 85 C. After the coagulated matter settled
and a
comparatively clear supernatant was obtained at the top, the samples were
removed from
oven and analyzed and the pH of the supernatant was recorded. The results are
provided in
Tables 14 and 15. The initial value for the total organics in the untreated
BBD sample was
2219.1 mg/L. The supernatant was also characterized using UV-Vis spectroscopy.
[00210] Table 14. Percent removal of total organics from BBD water
samples treated
with a polyaluminum silicate sulfate coagulant and polyaluminum chloride
coagulant D
pH Polyaluminum Polyaluminum % Removal of TOC
Silicate Chloride Organics from
Supernatant
Sulfate Coagulant D BBD
Coagulant dosage (ppm)
dosage (ppm)
7 1000 1000 59.89 890.01
[00211] Table 15. Percent removal of total organics from BBD water
samples treated
with aluminum sulfate coagulant B and polyaluminum chloride coagulant D
pH Aluminum Sulfate Polyaluminum % Removal of TOC
Coagulant B Chloride Organics from Supernatant
dosage (ppm) Coagulant D BBD
dosage (ppm)
7 2500 2500 70.62 651.86
2500 5000 73.54 586.96
1000 500 64.64 784.63
1000 1000 66.90 734.47
1000 2500 71.97 621.83
2500 1000 68.37 701.69
[00212] Materials and Methods for Examples 6-16.
[00213] The water samples used for this study were SAGD de-oiled produced
water
(SAGDPW) and SAGD boiler blow down water (BBD) samples. The samples were
collected
hot and kept under a nitrogen blanket until they were opened for sample
analysis. Aliquots
from produced water sample were passed through a 0.22 p.m membrane (Cellulose
Acetate,
Millipore, USA) to remove the suspended matter to determine the dissolved
organic carbon
(DOC) concentration in the samples. De-ionized (DI) water (Purelab R Ultra)
was used for
diluting samples, rinsing, resin washing and the preparation of blank samples.
The TOC of
the DI water obtained from the water purification unit was measured to be less
than 1 mg/L.
The pH was adjusted using 20% (w/w) sulfuric acid (Fisher Scientific, USA) and
50% (w/w)
sodium hydroxide (Fisher Scientific) during experiments. Coagulants used in
the coagulation
experiments included: polyaluminum chloride, aluminum sulfate, acidified alum,
poly ferric
sulfate solution, ferrous chloride solution and ferric chloride (all available
from KemiraTM
Oyj). Flocculants used in the flocculation experiments included: non-ionic and
anionic
polymers, for example anionic dry polyacrylamide, high molecular weight, low
charge
anionic dry polyacrylamide, non-anionic dry polyacrylamide and the like (all
available from
KemiraTM
36
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
Oyj).
[00214] Jar tests were conducted to measure the performance of different
coagulants and
flocculants on treating the produced water and boiler blow down water samples.
The
supernatant obtained from the treated produced water and blow down were
filtered through a
0.22 p.-m filter and stored for further analyses.
[00215] Quantitative and qualitative analysis of the supernatant obtained
from treatment
of blow down and produced water were performed mainly through total organic
carbon
(TOC), specific UV absorbance (SUVA) and spectrofluorescence analysis.
Different
analytical techniques used in this study are reported in the following
sections.
[00216] Coagulation of blow down and produced water were performed at 85 C
with
different coagulants and flocculants. The coagulants were obtained as solution
whereas the
flocculants were received dry and flocculant solutions were prepared using DI
water.
[00217] Jar tests with blow down water were carried out according to the
following
protocol. Several 30 mL samples of boiler blow down water were taken in
autoclavable
centrifuge tubes. The pH of each sample solution was adjusted to four
different pH values in
the range of 4 to 10 using sulfuric acid. The blow down water aliquot was
heated to a
temperature of 85 C in an oven. Different dosages of coagulant between 500 to
5000 ppm
were added to the blow down water samples. The coagulated BBD was then stored
in the
tubes and placed in oven at a temperature of 85 C. After the coagulated matter
settled and a
comparatively clear supernatant was obtained at the top, the samples were
removed from the
oven and analyzed and the pH of the supernatant was recorded.
[00218] Jar tests with produced water were carried out according to the
following
protocol. Several 40 mL samples of produced water were taken in autoclavable
centrifuge
tubes and heated to a temperature of 85 C in an oven. The heated samples were
transferred to
a beaker under continuous stirring at 400 rpm. Different dosages of coagulant
between 50 to
500 ppm were added to the produced water samples. On addition of coagulant,
the pH of the
solution was reduced. The final pH was recorded as was the required amount of
sulfuric acid
and caustic soda added to the solutions to adjust the pH of the solution to a
final value of 4, 6,
8 or 10. The coagulated produced water was stored in the tubes and placed in
an oven at a
temperature of 85 C. Settling rates were observed and aggregation of organic
matter were
photographed every 3 minutes until complete settling was observed. Samples
were collected
and filtered through a 0.22 lam filter for further analysis. The coagulant
dosage ranged from
50 ppm to 500 ppm for produced water treatment because no organic
precipitation was
observed beyond 500 ppm of coagulant dosage.
[00219] The sum total of the suspended and dissolved organic carbon present
in a water
37
sample was defined as the total organic carbon (TOC) of the sample. The TOC
concentration
of the filtrate, obtained by filtering a solution through 0.22 gm filter was
earmarked as its
dissolved organic carbon (DOC) concentration (see Danielsson, L.G.; On the use
of filters for
distinguishing between dissolved and particulate fractions in natural waters.
Water Research,
16(2):179 - 182, 1982.). The total dissolved organic carbon (DOC) was measured
using a
TOC analyzer (ShimadzuTM, Model: TOC-L CPH/CPN) after filtering through a 0.22
gm
membrane filter (Cellulose acetate, Millipore, USA). The organic concentration
in blow
down water and produced water was measured as TOC after filtering through a
0.22 gm
filter. Thus, the organic concentration reported in blow down and produced
water was solely
due to dissolved organic compounds.
[00220] Absorbance of the samples was measured at a wavelength of 254 nm
using UV-
visible spectrophotometer (VarianTM, Model: Ca1yTM 50, USA). DI water was used
as a
blank. The samples were diluted to obtain absorbance in the range of 0 to 1.2.
[00221] Example 6. Removal of Dissolved Organic Matter from BBD water
with a
Ferrous Chloride Coagulant
[00222] In this example, several SAGD BBD samples were treated with a
ferrous
chloride coagulant (28-32% aqueous solution of FeCl2, available from KemiraTM
Oyj.) The
total organic carbon analysis and pH of the treated samples were evaluated.
[00223] The raw boiler blow down water sample contains 2219 mg/L of
dissolved
organic carbon.
[00224] Since the samples were filtered through a 0.22 gm membrane filter
the total
organic carbon results were used as dissolved organic carbon (DOC). The
qualitative
characteristics of the residual organic compounds obtained in the supernatant
of the treated
blow down were determined using specific UV absorbance.
[00225] The pH of the blow down samples were adjusted to four initial pH
values of
11.8, 8, 6 and 4. Hydrochloric acid was used as the pH reducing agent. The
amount of
dissolved organic carbon in the supernatant was determined by TOC analysis.
The coagulant
was added to the blow down samples at dosages of 500, 1000, 2000, 3000, 4000
and 5000
ppm. Table 16 and Figure 4 show the results of the coagulant treatment on the
TOC.
[00226] Table 16. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Coagulant Initial pH = 4 Initial pH =6 Initial pH = 8 Initial pH =
11.8
Dosage
(PPm)
TOC Final TOC Final TOC Final TOC Final
(PPm) PH (PP/n) PH (PPm) PH (PPm) PH
0* 1171.26 4.0 1684.11 6.0 1780.59 8.0 2137.71 11.8
38
Date Recue/Date Received 2022-03-31
500 1154.40 3.95 1015.80 5.77 1631.40 6.91 1888.20
10.73
1000 1107.60 3.54 968.40 5.29 1294.20 6.45 1897.20
10.35
2000 1126.20 3.32 939.00 4.51 974.40 5.12 1762.80
9.74
3000 1072.20 3.01 958.80 3.88 875.40 4.24 1559.40
8.72
4000 1063.20 2.86 901.20 3.54 795.00 3.62 1432.20
7.76
5000 1074.60 2.74 880.80 3.32 825.00 3.32 1173.60
7.24
[00227] Initial pH values are pH values before coagulant addition.
[00228] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00229] The data demonstrates that this coagulant was effective for
removing organic
matter from SAGD BBD water over a broad pH range.
[00230] Example 7. Removal of Dissolved Organic Matter from BBD water with
a
Ferric Chloride Coagulant
[00231] In this example, several SAGD BBD samples were treated with a
ferric chloride
coagulant (39-41% aqueous solution of FeCl3, available from KemiraTM Oyj.) The
total
organic carbon analysis, pH, and specific UV absorbance of the treated samples
were
evaluated.
[00232]
[00233] The raw boiler blow down water sample contained 2219 mg/L of
dissolved
organic carbon. Percent removal was calculated based on the initial TOC of the
boiler blow
down water at the pH of 11.8.
[00234] Since the samples were filtered through a 022 urn membrane filter
the total
organic carbon results were used as dissolved organic carbon (DOC). The
qualitative
characteristics of the residual organic compounds obtained in the supernatant
of the treated
blow down were determined using specific UV absorbance.
[00235] The pH of the blow down samples were adjusted to four initial pH
values of
11.8, 8, 6 and 4. Hydrochloric acid was used as the pH reducing agent. The
amount of
dissolved organic carbon in the supernatant was determined by TOC analysis.
The coagulant
was added to the blow down samples at dosages of 500, 1000, 2000, 3000, 4000
and 5000
ppm. Table 17 and Figure 5 show the results of the coagulant treatment on the
TOC.
39
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
[00236] Table 17. Concentration of residual dissolved organic matter in the
supernatant
of the treated sample
Coagulant Initial pH = 4 I Initial pH = 6 Initial pH = 8
Initial pH = 11.8
Dosage
(1)Pm)
TOC Final TOC Final TOC Final TOC Final
(ppm) PH (1)Pm) PH (ppm) PH (PPIn) pH
0* 1171.3 4.0 1684.1 6.0 1780.6 8.0 2137.7 11.8
500 765.0 3.64 872.4 5.46 1074.6 6.3 1928.4 11.36
1000 706.2 2.99 883.8 4.71 774.0 5.01 1869.6 10.74
2000 6912 2.4 621.6 3.41 637.8 3.77 1861.8 9.54
3000 668.4 2.08 627.6 2.47 639.0 2.87 1585.2 7.17 -
4000 668.4 1.9 625.8 2.08 657.6 2.31 899.4 5.18
5000 659.4 1.82 624.6 1.97 656.4 2.12 759.0 4.42
[00237] Initial pH values are pH values before coagulant addition.
[00238] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00239] Specific UV Absorbance
[00240] Figure 6 shows the SUVA value of the supernatant obtained from the
treated
blow down samples plotted as a function of the coagulant dosage. Where the
initial pH was
11.8, the SUVA remained unaffected as the coagulant dosage was increased from
500 to
3000 ppm. As the dosage was increased to 4000 and 5000 ppm the SUVA value
decreased
and reached the interface of the mixed and hydrophilic region, showing a
reduction in the
hydrophobic content in the supernatant.
[00241] For samples having an initial pH of 8, with 500 ppm coagulant
dosage the
SUVA remained between 2 and 4 (2.76) indicating a mixture of both hydrophobic
and
hydrophilic compounds in the treated blow down sample. As the coagulant dosage
was
increased there was a reduction in the SUVA value near or below 2 indicating
removal of
aromatic hydrophobic compounds from the blow down water. For samples having an
initial
pH 4 and 6, the SUVA values were near or below 2 regardless of the coagulant
dosage,
indicating a complete elimination of hydrophobic fractions. However, it was
observed that at
pH 4 with higher dosages of coagulant (e.g., 4000 and 5000 ppm) there was an
increase in the
absorbance at 254 nm resulting in higher SUVA values. This may be attributed
to the residual
iron interference as it appeared from the TOC analysis that majority of the
organic matter was
removed at that pH.
[00242] The TOC analysis indicates that as the starting pH was reduced to
8 or below a
very high removal of organic matter from the blow down was observed.
[00243] The data demonstrates that this coagulant was effective for
organic removal
from SAGD BBD water over a broad pH range.
[00244] Example 8. Removal of Dissolved Organic Matter from BBD water
with a
Poly Ferric Sulfate Coagulant
[00245] In this example, several SAGD BBD samples were treated with a
poly ferric
sulfate coagulant (liquid ferric sulfate coagulant based on trivalent iron,
13% iron, available
from KemiraTM Oyj.) The total organic carbon analysis, pH and specific UV
absorbance of
the treated samples were evaluated.
[00246] The raw boiler blow down water sample contained 2219 mg/L of
dissolved
organic carbon. Percent removal was calculated based on the initial TOC of the
boiler blow
down water at the pH of 11.8.
[00247] Since the samples were filtered through a 0.22 pm membrane filter
the total
organic carbon results were used as dissolved organic carbon (DOC). The
qualitative
characteristics of the residual organic compounds obtained in the supernatant
of the treated
blow down was determined using specific UV absorbance.
[00248] "lbe pH of the blow down samples were adjusted to four initial pH
values of
11.8, 8, 6 and 4. Hydrochloric acid was used as the pH reducing agent. The
amount of
dissolved organic carbon in the supernatant was determined by TOC analysis.
The coagulant
was added to the blow down samples at dosages of 500, 1000, 2000, 3000, 4000
and 5000
ppm. Table 18 and Figure 7 show the results of the coagulant treatment on the
TOC.
41
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
=ft,
[00249] Table 18. Concentration of residual dissolved organic matter in the
supernatant
of the treated sample
Coagulant Initial pH = 4 Initial pH = 6 Initial pH = 8 Initial
pH = 11.8
Dosage
(PM)
TOC Final TOC Final TOC Final TOC Final
(PP111) PH (ppm) PH (PM) PH (ppm) PH
0* 1171.3 4 1684.1 6 1780.6 8 2137.7 11.8
500 741.0 185 969.0 5.42 1276.8 7.01 1921.2 11.63
1000 697.8 3.44 720.0 4.72 964.2 5.98 1899.6 11.24
2000 660.6 2.62 614.4 4.07 738.6 4.82 1887.0 9.8
3000 656.4 2.33 615.6 2.71 655.2 3.63 1876.8 8.78
4000 655.2 2.31 621.0 2.43 669.0 2.82 1009.2 5.58
5000 646.8 2.18 620.4 2.37 688.8 2.66 915.0 4.93
[00250] Initial pH values are pH values before coagulant addition.
[00251] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00252] Specific UV Absorbance
[00253] Figure 8 shows the SUVA value of the supernatant obtained from the
treated
blow down samples plotted as a function of the coagulant dosage. At initial pH
11.8, the
SUVA remained unaffected as the dosage was increased from 500 to 3000 ppm. As
the
dosage was increased to 4000 and 5000 ppm the SUVA value decreases and falls
in the
hydrophilic zone showing removal of hydrophobic content from the blow down. At
initial pH
8, with 500 ppm dosage the SUVA remained between 2 and 4 (2.74) indicating a
mixture of
both hydrophobic and hydrophilic compounds in the treated blow down but as the
dosage was
increased there was a reduction in the SUVA value near or below 2 indicating
removal of
aromatic hydrophobic compounds from the blow down water. At pH 4 and 6, the
SUVA
values were consistently near or below 2 regardless of the dosage. Hence, with
starting pH of
4 and 6 a complete elimination of hydrophobic fractions was noted.
[00254] The TOC analysis indicates that as the starting pH was reduced to 8
or below a
very high removal of organic matter from the blow down was observed.
[00255] Example 9. Removal of Dissolved Organic Matter from Produced Water
with a Ferric Chloride Coagulant
42
[00256] In this example, several SAGD produced water samples were treated
with a
ferric chloride coagulant (39-41% aqueous solution of FeC13, available from
KemiraTM Oyj.)
The total organic carbon analysis and pH of the treated samples were
evaluated.
[00257] The raw de-oiled produced water contained 511.97 mg/L of DOC.
Since the
samples were filtered through a 0.22 gm membrane filter the total organic
carbon results were
used as DOC. The qualitative characteristics of the residual organic compounds
obtained in
the supernatant of the treated sample were determined using specific UV
absorbance.
[00258] The coagulant was added to the produced water samples at six
different
dosages: 50, 100, 200, 300, 400 and 500 ppm. Sulfuric acid and sodium
hydroxide solutions
were used to adjust the p1-1 of the samples to 4, 6, 8 or 10. The amount of
dissolved organic
carbon in the supernatant was determined by TOC analysis. Table 19 and Figure
9 show the
results of the coagulant treatment on the TOC.
[00259] Table 19. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Coagulant Dosage Residual TOC (ppm)
(1)Pm)
pH 4 pH 6 pH 8 pH 10
0* 343.21 455.98 473.6 485.27
50 358.46 438.54 489.72 484.96
100 343.38 446.62 483.42 475.44
200 329.86 415.4 491.86 493.12
300 316.86 399.92 474.2 471.76
400 299.44 400.82 474.82 469.5
500 290.14 405.1 476.22 480.12
[00260] Initial pH values are pH values before coagulant addition.
[00261] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00262] The data demonstrates that this coagulant was effective for
organic removal
from SAGD produced water.
[00263] Example 10. Removal of Dissolved Organic Matter from Produced
Water
with a Polyaluminum Chloride Coagulant
[00264] In this example, several SAGD produced water samples were treated
with
polyaluminum chloride coagulant C, which was a liquid polyaluminum chloride
coagulant
containing 9% elemental aluminum and with a basicity of 42% (available from
KemiraTM
Oyj).
43
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
The total organic carbon analysis, pH, turbidity, and specific UV absorbance
of the treated
samples were evaluated.
[00265] The raw de-oiled produced water contained 511.97 mg,/L of DOC.
Since the
samples were filtered through a 0.22 Lim membrane filter the total organic
carbon results were
used as DOC. The qualitative characteristics of the residual organic compounds
obtained in
the supernatant of the treated sample were determined using specific UV
absorbance.
[00266] The coagulant was added to the produced water samples at six
different
dosages: 50, 100, 200, 300, 400 and 500 ppm. Sulfuric acid and sodium
hydroxide solutions
were used to adjust the pH of the samples to 4, 6, 8 or 10. The amount of
dissolved organic
carbon in the supernatant was determined by TOC analysis. Table 20 and Figure
10 show the
results of the coagulant treatment on the TOC.
[00267] Table 20. Concentration of residual dissolved organic matter in the
supernatant
of the treated sample
Coagulant Dosage Residual TOC (ppm)
(ppm)
pH 4 pH 6 pH 8 p1110
0* 343.21 455.98 473.6 485.27
50 377.24 471 497.26 506.42
100 372.32 460.18 496.24 507.68
200 348 420.76 496 506.64
300 326.92 388.36 492.94 506.12
400 321.14 366.7 490.7 506
500 317.76 360.58 479.06 506.32
[00268] Initial pH values are pH values before coagulant addition.
[00269] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00270] Turbidity
[00271] For samples having a pH of 4, a coagulant dosage of 50 and 100 ppm
resulted
in no changes in the turbidity of the solution. When the coagulant dosage was
200 ppm to 500
ppm, an aggregation of organic matter was observed. It was observed that
settling of the
organic matter began at t = 3 mins and final settling was at t = 15 mins. In
the case of 300
ppm, the final settling time was 12 mins. For both 400 and 500 ppm, the final
settling time
for the aggregated organics was 9 mins. For samples having a pH of 6, with a
coagulant
dosage of 50 and 200 ppm no changes in the turbidity of the solution were
observed. When
44
the dosage was increased to 300 ppm, a slight change in the turbidity of the
solution was
observed. The coagulated organic matter settled completely at t = 15 mins.
Clear supernatants
were obtained with 400 and 500 ppm dosages at pH 6. The settling of organic
matter began at
t = 3 mins and final settling occurred at t = 15 mins.
[00272] Specific UV Absorbance
[00273] Figure 11 shows the SUVA value of the supernatant obtained from
the treated
produced water samples plotted as a function of the coagulant dosage. For
samples having pH
of 8 and 10 no significant changes in SUVA were observed as a result of change
in coagulant
dosage. For samples having a pH of 6, with a coagulant dosage of 50 to 200 ppm
the SUVA
value was in the mixed zone, but with the coagulant dosage of 300ppm and
greater, the
SUVA shifted to the hydrophilic zone. For samples having a pH of 4, the SUVA
was
consistently in the hydrophilic zone, regardless of coagulant dosage, and with
increase in
dosage the SUVA value was reduced indicating that some amount of hydrophilic
organic was
removed from the produced water at pH 4.
[00274] Example 11. Removal of Dissolved Organic Matter from Produced
Water
with an Aluminum Sulfate Coagulant
[00275] In this example, several SAGD produced water samples were treated
with
aluminum sulfate coagulant B, which was an iron-free liquid aluminum sulfate
coagulant
containing about 4.3% aluminum ion (available from KemiraTM Oyj). The total
organic
carbon analysis, pH, turbidity, and specific U V absorbance of the treated
samples were
evaluated.
[00276] The raw de-oiled produced water contained 511.97 mg/L of DOC.
Since the
samples were filtered through a 0.22 gm membrane filter the total organic
carbon results were
used as DOC. The qualitative characteristics of the residual organic compounds
obtained in
the supernatant of the treated sample were determined using specific UV
absorbance.
[00277] The coagulant was added to the produced water sample at six
different dosages:
50, 100, 200, 300, 400 and 500 ppm. Sulfuric acid and sodium hydroxide
solutions were used
to adjust the pH of the samples to 4, 6, 8 or 10. The amount of dissolved
organic carbon in
the supernatant was determined by TOC analysis. Table 21 and Figure 12 show
the results of
the coagulant treatment on the TOC.
[00278] Table 21. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Coagulant Dosage Residual TOC (ppm)
(PPnl)
pH 4 pH 6 pH 8 pH 10
Date Recue/Date Received 2022-03-31
0* 343.21 455.98 473.6 485.27
50 364.8 481.64 484.8 498.26
100 364.6 469.74 500.06 502.48
200 348.48 455.02 479.06 484.38
300 339.6 414.94 490.02 489.46
400 328.46 414.94 490.02 489.46
500 327.08 408.04 484.8 490.34
[00279] Initial pH values are pH values before coagulant addition.
[00280] * = residual dissolved organic matter concentration on acid
addition without
coagulant.
[00281] Turbidity
[00282] For samples having a pH of 4, with a coagulant dosage of 50 and
100 ppm no
changes in the turbidity of the solution were observed. When the coagulant
dosage was
increased 200 ppm to 500 ppm, an aggregation of organic matter was observed.
Clear
supernatants were obtained for a dosage of 200 to 500 ppm of the coagulant at
pH 4. For
samples having a pH of 6, with a dosage of 50 and 500 ppm no changes in the
turbidity of the
solution were observed. For samples having a pH of 8 and 10, no significant
changes in the
turbidity of the solution were observed for various dosages of the coagulant.
[00283] Specific UV Absorbance
[00284] Figure 13 shows the SUVA value of the supernatant obtained from
the treated
produced water samples plotted as a function of the coagulant dosage. For
samples having at
pH 8 and 10 no significant changes in SUVA were observed as coagulant dosage
increased.
For samples having a pH 6, with coagulant dosage of 50 to 200 ppm the SUVA
value was in
the mixed zone, but with the coagulant dosage of 300ppm and greater, the SUVA
shifted to
the hydrophilic zone. For samples having a pH 4, the SUVA was consistently in
the
hydrophilic zone, regardless of coagulant dosage, and with increase in dosage
the SUVA
value was reduced indicating that some amount of hydrophilic organic was
removed from the
produced water at pH 4.
[00285] Example 12. Removal of Dissolved Organic Matter from Produced
Water
with a Polyaluminum Chloride Coagulant and a Polyacrylamide Flocculant
[00286] In this example, several SAGD produced water samples were treated
with
polyaluminum chloride coagulant C, which was a liquid polyabiminurn chloride
coagulant
containing 9% elemental aluminum and with a basicity of 42%, and a high
molecular weight,
non-ionic, dry polyacrylamide flocculant (both available from KemiraTM Oyj) .
The total
46
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
====,
organic carbon analysis, pH, turbidity, and specific UV absorbance of the
treated samples
were evaluated.
[00287] The raw de-oiled produced water contained 511.97 mg/L of DOC. The
qualitative characteristics of the residual organic compounds obtained in the
supernatant of
the treated sample were determined using specific UV absorbance.
[00288] The coagulant was added to the produced water sample at a dosage of
400 ppm.
Sulfuric acid and sodium hydroxide solutions were used to adjust the pH of the
samples to 4,
6 or 8, respectively. The amount of dissolved organic carbon in the
supernatant was
determined by TOC analysis. The DOC concentration in the SAGD produced water
samples
after treatment with the coagulant was: 490.7 at pH 8, 366.7 at pH 6, and
321.14 at pH 4.
[00289] After treatment with the coagulant, the flocculant was added to
each of the
samples at a dosage of 25, 50, 100 or 150 ppm. Table 22 and Figure 14 show the
results of
the coagulant and flocculant treatment on the TOC.
[00290] Table 22. Concentration of residual dissolved organic matter in the
supernatant
of the treated sample
Flocculant Residual TOC (ppm)
Dosage (ppm)
pH 4 pH 6 pH 8
25 256.26 373.98 427.38
50 256.98 383.94 440.82
100 325.74 401.7 437.52
150 309.24 410.52 445.26
[00291] Turbidity
[00292] For samples having a pH of 8, no aggregates were observed in the
treated
samples. The organics which precipitated out in the pH 4 and 6 samples,
resolubilized with
the addition of sodium hydroxide and no aggregation was observed at higher pH
conditions.
There were no changes in the turbidity of the supernatant even with the
addition of flocculant.
For the samples having pH 4 and 6, apart from the organic matter removal
enhancement,
addition of the flocculant also improved the settling time of the organics.
With the coagulant
alone a settling time of 12 ¨ 15 mins was recorded. On addition of flocculants
the settling
time was lowered to 4 mins.
[00293] Specific UV Absorbance
47
[00294] Figure 15 shows the SUVA value of the supernatant obtained from
the treated
produced water samples plotted as a function of the flocculant dosage. At all
pH values, the
addition of flocculant resulted in a reduction in the SUVA value.
[00295] Example 13. Removal of Dissolved Organic Matter from Produced
Water
with a Polyaluminum Chloride Coagulant and a Polyacrylamide Flocculant
[00296] In this example, several SAGD produced water samples were treated
with
pol y al umi num chloride coagulant C, which was a liquid pol y al um n um
chloride coagulant
containing 9% elemental aluminum and with a basicity of 42%, and a high
molecular weight,
low-charge, anionic, dry polyacrylamide flocculant (both available from
KemiraTM Oyj) . The
total organic carbon analysis, pH, turbidity, and specific UV absorbance of
the treated
samples were evaluated.
[00297] The raw de-oiled produced water contained 511.97 mg/L of DOC. The
qualitative characteristics of the residual organic compounds obtained in the
supernatant of
the treated sample were determined using specific UV absorbance.
[00298] The coagulant was added to the produced water sample at a dosage
of 400 ppm.
Sulfuric acid and sodium hydroxide solutions were used to adjust the pH of the
samples to 4,
6, or 8, respectively. The amount of dissolved organic carbon in the
supernatant was
determined by TOC analysis. The DOC concentration in the SAGD produced water
samples
after treatment with the coagulant was: 490.7 at pH 8, 366.7 at pH 6, and
321.14 at pH 4.
[00299] After treatment with the coagulant, the flocculant was added to
the samples at a
dosage of 25, 50, 100 or 150 ppm. Table 23 and Figure 16 show the results of
the coagulant
and flocculant treatment on the TOC.
[00300] Table 23. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Flocculant Residual TOC (ppm)
Dosage (ppm)
pH 4 pH 6 pH 8
25 264.54 352.62 397.44
50 271.86 339.6 414.06
100 272.64 382.86 429.66
150 305.7 400.44 495.36
[00301] Turbidity
[00302] For samples having a pH of 8, no aggregates were observed. There
were no
changes in the turbidity of the supernatant even with the addition of
flocculant. In the samples
having pH 4 and 6, apart from the organic matter removal enhancement, addition
of the
48
Date Recue/Date Received 2022-03-31
flocculant also improved the settling time of the organics. With the coagulant
alone a settling
time of 12 ¨ 15 mins was recorded. On addition of flocculants the settling
time was lowered
to 4 mins.
[00303] Specific UV Absorbance
[00304] Figure 17 shows the SUVA value of the supernatant obtained from
the treated
produced water samples plotted as a function of the flocculant dosage. For
samples having a
pH of 8, as the dosage of the flocculant was increased, the SUVA value of the
solution
decreased. However, the SUVA value was located in the mixed zone even with 150
ppm
dosage of flocculant. For samples having a pH of 6, with 100 ppm or more of
the flocculant,
hydrophobic fractions were eliminated from the water. For samples having a pH
of 4, all of
the residual organic matter obtained after the coagulation-flocculation
treatment was
hydrophilic.
[00305] Example 14. Removal of Dissolved Organic Matter from Produced
Water
with a Polyaluminum Chloride Coagulant and a Polyacrylamide Flocculant
[00306] In this example, several SAGD produced water samples were treated
with
polyalmnimim chloride coagulant C, which was a liquid polyaluminum chloride
coagulant
containing 9% elemental aluminum and with a basicity of 42%, and a high
molecular weight,
medium-charge, dry polyacrylamide flocculant (both available from KemiraTM
Oyj). The
total organic carbon analysis, pH, turbidity, and specific UV absorbance of
the treated
samples were evaluated.
[00307] The raw de-oiled produced water contained 511.97 mg/L of DOC. The
qualitative characteristics of the residual organic compounds obtained in the
supernatant of
the treated sample were determined using specific UV absorbance.
[00308] The coagulant was added to the produced water sample at a dosage
of 400 ppm.
Sulfuric acid and sodium hydroxide solutions were used to adjust the pH of the
samples to 4,
6, or 8, respectively. The amount of dissolved organic carbon in the
supernatant was
determined by TOC analysis. The DOC concentration in the SAGD produced water
samples
after treatment with the coagulant was: 490.7 at pH 8, 366.7 at pH 6, and
321.14 at pH 4.
[00309] After treatment with coagulant, the flocculant was added to the
samples at a
dosage of 25, 50, 100 or 150 ppm. Table 24 and Figure 18 show the results of
the coagulant
and flocculant treatment on the TOC.
[00310] Table 24. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
49
Date Recue/Date Received 2022-03-31
Flocculant Residual TOC (ppm)
Dosage (ppm)
pH 4 pH 6 pH 8
25 270.12 259.5 370.23
50 263.88 345.72 412.2
100 275.64 358.08 435.9
150 259.26 403.5 430.44
[00311] Turbidity
[00312] In the pH 8 samples, no aggregates were observed. There were no
changes in
the turbidity of the supernatant even with the addition of flocculant. For
samples having a pH
of 4 and 6, apart from the organic matter removal enhancement, addition of the
flocculant
also improved the settling time of the organics. With the coagulant alone a
settling time of 12
¨ 15 mins was recorded. On addition of flocculants the settling time was
lowered to 4 mins.
[00313] Specific UV Absorbance
[00314] Figure 19 shows the SUVA value of the supernatant obtained from
the treated
produced water samples plotted as a function of the flocculant dosage. For
samples having a
pH of 8, as the dosage of the flocculant was increased, the SUVA value of the
solution
decreased. At pH 8, the residual organic matter was a mixture of hydrophobic
and
hydrophilic compounds. In the pH 6 samples, with 100 ppm or more of the
flocculant, more
of the hydrophobic fractions were eliminated from the water. For samples
having a pH of 4,
all of the residual organic matter obtained after coagulation-flocculation
treatment was
hydrophilic_
[00315] Example 15. Removal of Dissolved Organic Matter from Produced
Water
with a Polyaluminum Chloride Coagulant and a Polyacrylate Flocculant
[00316] In this example several SAGD produced water samples were treated
with
polyaluminum chloride coagulant C, which was a liquid polyaluminum chloride
coagulant
containing 9% elemental aluminum and with a basicity of 42%, and a high
molecular weight,
high-charge, anionic, dry polyacrylate flocculant (both available from
KemiraTM Oyj) . The
total organic carbon analysis, pH, turbidity, and specific UV absorbance of
the treated
samples were evaluated.
[00317] The raw de-oiled produced water contained 511.97 mg/L of DOC. The
qualitative characteristics of the residual organic compounds obtained in the
supernatant of
the treated sample were determined using specific UV.
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
õ.
'44010
[00318] The coagulant was added to the produced water sample at a dosage of
400 ppm.
Sulfuric acid and sodium hydroxide solutions were used to adjust the pH of the
samples to 4,
6, or 8, respectively. The amount of dissolved organic carbon in the
supernatant was
determined by TOC analysis. The DOC concentration in the SAGD produced water
samples
after treatment with the coagulant was: 490.7 at pH 8, 366.7 at pH 6, and
321.14 at pH 4.
[00319] After treatment with the coagulant, the flocculant was added at a
dosage of 25,
50, 100 or 150 ppm. Table 25 and Figure 20 show the results of the coagulant
and flocculant
treatment on the TOC.
[00320] Table 25. Concentration of residual dissolved organic matter in the
supernatant
of the treated sample
Flocculant Residual TOC (ppm)
Dosage (ppm)
pH 4 pH 6 pH 8
25 277.86 338.28 401.58
50 286.02 350.4 410.28
100 273 374.64 441.84
150 290.34 400.86 434.58
[00321] Turbidity
[00322] In the pH 8 samples, no aggregates were observed. There were no
changes in
the turbidity of the supernatant even with the addition of flocculant. In the
pH 4 and 6
samples, apart from the organic matter removal enhancement, addition of the
flocculant also
improved the settling time of the organics. With the coagulant alone a
settling time of 12 ¨ 15
mins was recorded. On addition of flocculants the settling time was lowered to
4 mins. In the
pH 6 sample, using 25 ppm of the flocculant removed about 33% of the organic
matter (see
Figure 20).
[00323] Specific UV Absorbance
[00324] Figure 21 shows the SUVA value of the supernatant obtained from the
treated
produced water samples plotted as a function of the flocculant dosage. At pH 8
and 6, the
residual organic matter in the samples was a mixture of hydrophobic and
hydrophilic organic
compounds. The corresponding SUVA value for the treated sample was 2. Hence,
the organic
matter removed were mainly hydrophobic organic matter.
[00325] Example 16. Removal of Silica from Produced Water with Exemplary
Coagulants and a Polyacrylate Flocculant
51
[00326] In this example, the efficacy of coagulants for silica removal at
pH 10 was
evaluated to see whether the coagulants were capable of removing silica under
warm lime
softening (WLS) conditions. The silica analysis was perfouned using
silicomolybdate method
in a HachTM DR 6000 spectrophotometer. Produced water silica was measured by
diluting the
sample to different concentrations such that the data was obtained between 1
to 100 ppm,
which was the recommended range for the method. DI water was used for
dilution.
[00327] The concentration of silica in the produced water samples was 247
ppm.
Figures 22, 23 and 24 show the efficacy of aluminum sulfate coagulant, ferric
chloride
coagulant, and polyaluminum chloride coagulant, respectively, in reducing the
silica
concentration in the produced water. As shown in Figure 24, it was observed
that although
polyaluminum chloride was highly effective in removing organic matter, it was
not effective
in removing silica from produced water. In comparison to the polyaluminum
chloride, the
aluminum sulfate and ferric chloride coagulants were more effective in
reducing the silica
concentration in the produced water samples (see Figs. 22, 23). With a dosage
of 500 ppm of
aluminum sulfate or ferric chloride, the residual silica concentration in the
produced water
was reduced from 247 ppm to 148 and 115 ppm, respectively.
[00328] Example 17. Removal of Dissolved Organic Matter from BBD Water
with
a Polyaluminum Chloride Coagulant and Calcium Oxide or Sodium Hydroxide
[00329] In this example, SAGD Boiler blow down (BBD) water samples were
treated
with polyaluminum chloride coagulant C, which was a liquid polyaluminum
chloride
coagulant containing 9% elemental aluminum and with a basicity of 42%
(available from
KemiraTM Oyj), and calcium oxide (available from Fisher Scientific) or sodium
hydroxide
(available from Fisher Scientific). The total organic carbon analysis, pH, and
turbidity of the
treated samples were evaluated.
[00330] The boiler blow down water contained 2219.1 mg/L of TOC. The
coagulant
was added to the BBD sample at a dosage of 2000 ppm. Calcium oxide and sodium
hydroxide solutions were used to adjust the pH up to 11.2 or 11.4, as
indicated. The amount
of dissolved organic carbon in the supernatant was determined by TOC analysis.
[00331] Table 26 shows the TOC results of the samples after treatment
with the
coagulant and calcium oxide or sodium hydroxide.
52
Date Recue/Date Received 2022-03-31
[00332] Table 26. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
pH Adjustment Coagulant dosage Final pH Percent TOC
additive (I)Pm) Removal
NaOH 2000 1L4 16.7
CaO 2000 11.2 43.3
[00333] Turbidity
[00334] It was observed that, after treatment of the BBD sample with the
coagulant and
calcium oxide or sodium hydroxide, the supernatant obtained in the sample
treated with the
coagulant and calcium oxide had substantially better clarity than the sample
treated with the
coagulant and sodium hydroxide. The improvement in clarity indicates that the
sample
treated with coagulant and calcium oxide had a lower percentage of residual
organic matter in
the solution. It was also observed that, after treatment with coagulant and
sodium hydroxide,
the organics that precipitated from the sample redissolved in the water. Such
dissolution of
organics was not observed when calcium oxide was used.
[00335] Example 18. Removal of Dissolved Organic Matter from BBD Water
with
a Polyaluminum Chloride Coagulant and Calcium Oxide and Magnesium Oxide
[00336] In this example, SAGD BBD samples were treated with polyaluminum
chloride
coagulant C, which was a liquid polyaluminum chloride coagulant containing 9%
elemental
aluminum and with a basicity of 42% (available from KemiraTM Oyj), and calcium
oxide
(available from Fisher Scientific). In certain experiments, magnesium oxide
(available from
Fisher Scientific) was also used to treat the samples. The total organic
carbon analysis, pH,
and turbidity of the treated samples were evaluated.
[00337] The boiler blow down water contained 2219.1 mg/L of TOC. The
coagulant
was added to the BBD water sample at a dosage of 500, 1000 or 2000 ppm,
respectively.
Calcium oxide was used to adjust the pH up to 11.1, 11.2 or 11.3, as
indicated. Magnesium
oxide at 100 ppm was also added to some of the samples. The amount of
dissolved organic
carbon in the supernatant was determined by TOC analysis.
[00338] Table 27 shows the TOC results after treatment of the samples
with the
coagulant and calcium oxide, with or without added magnesium oxide.
[00339] Table 27. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Without Magnesium Oxide With Magnesium Oxide
Coagulant pH Residual Percent pH Residual Percent
53
Date Recue/Date Received 2022-03-31
Dosage TOC TOC TOC TOC
(ppm) Concentration Removal Concentration Removal
500 11.6 305.1 40.4 11.3 302.4 40.9
1000 11.6 202.5 42.9 11.2 288.2 43.7
2000 11.6 288.5 43.7 11.1 271.8 46.9
[00340] In separate experiments, it was observed that, at pH 10 and
above, only 5%
reduction in BBD TOC concentration was observed when treated with polyaluminum
chloride coagulant C alone. However, when this coagulant was added along with
calcium
oxide, in the presence or absence of magnesium oxide, the percent TOC
reduction improved
to over 40%. Overall, it seems the addition of magnesium oxide did not further
enhance TOC
removal.
[00341] Additional BBD samples, prepared as described above, were further
treated
with 25 ppm of a high molecular weight, non-ionic dry polyacrylamide
flocculant
(commercially available from KemiraTM Oyj). Table 28, below, shows the TOC
results after
further treatment with the flocculant. The results indicate that the TOC
reduction was further
improved with the addition of the flocculant (see Table 28). Thus, coagulation-
flocculation of
BBD at pH 10 and above, in the presence of calcium oxide, reduced the TOC
concentration
by almost 50%.
[00342] Table 28. Concentration of residual dissolved organic matter in
the supernatant
of the treated sample
Without Magnesium Oxide With Magnesium Oxide
Coagulant Flocculant pH Residual Percent pH Residual Percent
Dosage Dosage TOC TOC TOC TOC
(ppm) (ppm) Concentration Removal
Concentration Removal
500 25 11.4 262.4 48.7 11.3 276.5 46.0
1000 25 11.3 260.1 49.2 11.2 264.2 48.4
2000 25 11.0 259.1 49.4 11.2 259.7 49.3
[00343] Example 19. Removal of Dissolved Organic Matter from BBD Water
with
a Polyaluminum Chloride Coagulant and Calcium Chloride
[00344] Boiler blow down (BBD) samples containing 2219.1 mg/L of TOC were
used
for a baseline of evaluation with calcium chloride. Polyaluminum chloride
coagulant D was
added to the BBD water sample at a dosage of 2500 and 5000 ppm. Calcium
chloride was
added to the samples at 2000 ppm and 3000 ppm in combination with the
coagulant addition.
54
Date Recue/Date Received 2022-03-31
Blank samples (i.e., BBD samples without the addition of either coagulant or
calcium
chloride) were also included for comparison. Sodium hydroxide solutions were
used to adjust
the pH of the samples to 7.0, 8.5 and 11.8, as indicated. The amount of
dissolved organic
carbon in the supematant was determined by TOC analysis, and recorded in Table
29, below.
[00345] Table 29.
pH Calcium Chloride Dosage Coagulant Dosage (ppm)
(PM)
0 2500 5000
8.5 0
7.6 46.5 63.3
2000
37.0 61.0 71.4
3000
36.2 59.2 65.8
7 0
8.8 59.0 65.9
2000
35.2 64.7 61.5
3000
40.0 70.9 72.0
11.8 0
6.6 20.9
1000
22.9 23.9 32.4
2000
29.7 36.5 36.7
3000
33.9 34.7 40.8
[00346] Materials and Methods for Examples 20-21.
[00347] The water samples used for this study were SAGD boiler blow down
water
(BBD) samples. The samples were collected hot and kept under a nitrogen
blanket until they
were opened for sample analysis. Coagulation of BBD was performed at 85 C with
polyalnminum chloride coagulant C, which is a liquid polyaluminum chloride
coagulant
containing 9% elemental aluminum and with a basicity of 42% (available from
KemiraTM
Oyj). Quantitative and qualitative analysis of the supernatant obtained from
treatment of
BBD were performed through total organic carbon (TOC) analysis.
[00348] 30 mL of BBD samples were taken in autoclavable centrifuge tubes.
The pH of
the sample was measured to be 11.8. The BBD samples were heated to 85 C. The
coagulant
and adsorbent were added according to the methods disclosed herein, to the BBD
samples.
The resulting coagulated BBD was then stored in the tubes and placed in an
oven at a
temperature of 85 C. After the coagulated matter settled and a relatively
clear supernatant is
observed at the top the samples, the samples were removed from the oven and
analyzed.
[00349] The TOC concentration of the filtrate, obtained by filtering a
solution through
Date Recue/Date Received 2022-03-31
0.22
filter is earmarked as its dissolved organic carbon (DOC) concentration. The
total
dissolved organic carbon (DOC) was measured using a TOC analyzer (ShimadzuTM,
Model:
TOC-L CPH/CPN) after filtering through a 0.22 p-m membrane filter (Cellulose
acetate,
Millipore, USA). The organic concentration in boiler blow down water was
measured as
TOC after filtering through a 0.22 pm filter. Thus, the organic concentration
reported in blow
down and produced water is solely due to dissolved organic compounds.
[00350] The
measurement of the concentration was based on calculation of total carbon
concentration with reference to potassium hydrogen phthalate standard and
total inorganic
carbon concentration with reference to sodium carbonate and bi-carbonate
mixture.
[00351] A
total carbon (TC) standard solution was prepared according to the following
protocol. 2.125 g of potassium hydrogen phthalate was accurately weighed and
taken in a 1 L
volumetric ask. DI water was added up to the 1 L mark and the solution was
stirred. The
potassium hydrogen phthalate solution prepared has a concentration equivalent
to 1000 ppm
total organic carbon concentration. This solution was used as a standard for
total (TC)
analysis. A calibration was performed on the TOC analyzer with four different
concentrations
of the stock standard solution created by in-situ dilution in the instrument.
A calibration curve
was created based on which the TOC calculations were performed.
[00352] An
inorganic carbon (IC) standard solution was prepared according to the
following protocol. 3.497 g of reagent grade sodium bi-carbonate and 4.412 g
of sodium
carbonate was accurately weighed and taken in a 1 L volumetric ask. DI water
was added to
the ask up to the 1 L mark and the ask was shaken well. In this manner a
liquid solution
equivalent to 1000 ppm carbon concentration was prepared. This solution was
used as
standard for inorganic carbon (IC) analysis.
[00353] The
analysis of dissolved organic matter content was carried out according to
the following protocol. The samples were collected in 40 mL vials for TOC
analysis. The
sample vials were capped in order to prevent the volatilization and
contamination of the TOC
components in the samples. A calibration curve was generated by analyzing
various
concentrations of the TC and IC standard solutions. The TC values of the
samples were
determined by heating the samples at a temperature of 680 C over a high
sensitivity oxidation
catalyst. Samples were injected to the IC reaction vessel equipped with TOC-L
IC reactor kit
to measure the IC content in the sample. TOC is measured as the difference
between the TC
and IC analysis values.
[00354]
Example 20: Illustration of TOC concentration and pH value of aqueous
streams at different stages of produced water treatment (Comparative Example)
56
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
Nivv,
[00355] The TOC concentration and pH value of water streams at different
stages of
produced water treatment process are listed in Table 29. The results indicate
that the organic
matter is not removed during conventional treatment operations. Furthermore,
the produced
water is concentrated in the OTSG and a concentrated boiler blow down water is
obtained.
[00356] In previous results we observed that aluminum-based coagulants were
capable
of removing about 70% of the total organic matter present in the SAGD BBD.
However,
adding coagulants reduces the pH of the system. For example, SAGD waste water
treatment
operations are typically maintained at a pH of 10-12. See Table 29, below. At
high dosages of
coagulant, the pH of the treated water may drop to about as low as 4. The
aluminum-based
coagulants are typically more successful at removing DOM at lower values.
However, the
use of coagulants to treat BBD water may require that the pH be adjusted
upward, e.g., by the
addition of alkali.
[00357] Further enhancement of coagulation has been observed in presence of
divalent
ions. Calcium ions have been shown to improve the coagulation capability of
coagulants
even at the raw BBD pH of 11.8. A detailed characterization of BBD shows that
BBD is
essentially a three to five times concentrated version of the SAGD produced
water.
[00358]
[00359] Table 29: Properties of different streams in SAGD PW treatment
Water I pH TOC
Produced Water 7.0-8.5 458.5
WLS Outlet 11.8 452.8
Boiler feed water (BFW) 11.8 455.0
Boiler blow down (BBD) 11.8 2219.1
[00360] Example 21: Enhanced Removal of Dissolved Organic Matter from BBD
Water with a Polyaluminum Chloride Coagulant in Combination With an Adsorbent
[00361] Coagulant alone:
[00362] To provide a baseline and for the purposes of comparison, SAGD BBD
samples
were treated as described in the materials and methods section, above, with
3000 ppm
polyaluminum chloride coagulant C. The initial pH (before treatment), final pH
and
57
CA 02915917 2015-12-22
4,60, %WOO
percentage reduction in total organic carbon concentration of the treated
samples are
summarized in Table 30.
[00363] Coagulant in combination with different adsorbents:
SAGD BBD samples were treated with 3000 ppm polyaluminum chloride coagulant C.
The
initial pH (before treatment), the type and dosage of adsorbent, the final pH
and percentage
reduction in total organic carbon concentration of the treated samples are
also summarized in
Table 30.
Table 30: Result of treatment of BBD using different adsorbents in combination
with
Polyaluminum Chloride Coagulant C
' initial t ..;. /Coagulant, ................ .;., Dosage' .1Adsorbeiiii
Dosage . ''-'1'W ''''' final I
..-
pH (ppm) (ppm) removal pH !
1 ---
11.8 Polyaluminum Chloride 3000 (none) 49% 7.49
Coagulant C
11.8 Polyaluminum Chloride 3000 Meta kaolin 5000 54%
7.35
Coagulant C
11.8 Po lyal uminum Chloride 3000 Pumice 1000 56% 7.32
Coagulant C
11.8 Polyaluminurn Chloride 3000 Pumice 3000 55% 7.42
Coagulant C
11.8 Polyaluminum Chloride 3000 Pumice 5000 54% 7.48
Coagulant C . . _ _
11.8 Polyaluminum Chloride 3000 Aragonite 3000 53%
7.69
Coagulant C
11.8 Polyaluminum Chloride 3000 Aragonite 5000 53%
7.91
Coagulant C
11.8 Polyaluminum Chloride 3000 Recycled 1000 59%
7.16
Coagulant C glass powder
[00364] The above results show enhancement of TOC removal using different
adsorbents at different dosages. In previous results, we observed that in
order to achieve
about 60% removal of TOC in BBD water (without pre-adjusting the pH - at 11.8
pH) by
treating the water with coagulants alone, a dosage as high as 5000 ppm was
necessary.
However, as shown in Table 2, by treating the BBD with a combination of
coagulant and an
adsorbent, the same effect can be obtained with lower coagulant doses.
[00365] In addition, the results here demonstrate that the use of the
adsorbents can help
to maintain the pH at a range of 7 to 8. In previous results we observed that
treatment of
BBD water with a higher dosage of coagulant reduces the pH of the system,
which requires
additional alkali to maintain the operational pH of a SAGD process. However,
as Table 30
shows, the pH of the system is not affected by the addition of the adsorbent.
This helps in
58
maintaining the operational pH during SAGD water treatment without increasing
the cost of
alkali addition, and yet manages to enhance TOC removal.
[00366] Materials and Methods for Example 23.
[00367] Aspects of the exemplary embodiments are illustrated herein such
as the
oxidation of blow down water (BBDW) by aeration in combination with
coagulation
treatment in order to enhance dissolved organic matter removal from the SAGD
produced
and boiler blow down water. The use of oxidation also reduces the residual
iron and improves
the clarity of the treated water.
[00368] The water samples used for this study were SAGD boiler blow down
water
(BBD) samples. The samples were collected hot and kept under a nitrogen
blanket until they
were opened for sample analysis. Coagulants used in the coagulation
experiments included:
poly aluminum chloride, aluminum sulfate, acidified alum, polyferric sulfate
solution, ferrous
chloride solution and ferric chloride (all available from KemiraTm Oyj).
Quantitative and
qualitative analysis of the supernatant obtained from treatment of BBD were
performed
through total organic carbon (TOC) analysis.
[003691 30 mL of BBD water samples were taken in autoclavable centrifuge
tubes. The
pH of the samples were measured to be 1L8. The BBD water samples were heated
to 85 C.
Two samples were treated in an identical fashion for each of the
coagulant/dosage
combinations listed in Table 32. The respective coagulants were added to the
BBD water
samples according to the methods disclosed herein. All of the resulting
coagulated BBD
samples were then stored in the tubes and placed in an oven at 85 C. Blind
samples were
also placed into the oven but no coagulant was added. After the coagulated
matter settled, a
supernatant was observed at the top the samples. One set of the samples,
corresponding to
each of the coagulant/dosage combinations tested, were removed from oven and
analyzed for
TOC. The "% Removal of TOC Without Aeration" value listed in Table 32,
corresponds to
the percent reduction in TOC of the coagulated sample compared to the
untreated BBD
sample.
[00370] The second set of samples were pulled from the oven and
immediately sparged
using house air utilizing a dispersing tube for the next 30 minutes. The
aerated samples
showed a decrease in temperature over approximately 30 minutes. After aeration
was
complete, the samples were submitted for TOC analysis. The "% Removal of TOC
With
Aeration" value listed in Table 32, corresponds to the percent reduction of
TOC of the
coagulated and aerated sample compared to the untreated BBD sample. The "%
change in
TOC removal by aeration" was calculated for each coagulant/dosage combination.
The
difference in % TOC was calculated by dividing the difference in % TOC values
of the non-
59
Date Recue/Date Received 2022-03-31
aerated and the aerated samples and then dividing this difference by the non-
aerated % TOC.
The difference in the % TOC due to aeration indicates the efficacy of aeration
on the
reduction of residual organic matter in the boiler blow down samples.
[00371] The sum total of the suspended and dissolved organic carbon
present in a water
sample is defined as the total organic carbon (TOC) of the sample. The TOC
concentration of
the filtrate, obtained by filtering a solution through 0.22 gm filter is
earmarked as its
dissolved organic carbon (DOC) concentration. The total dissolved organic
carbon (DOC)
was measured using a TOC analyzer (ShimadzuTM, Model: TOC-L CPH/CPN) after
filtering
through a 0.22 [1,-m membrane filter (Cellulose acetate, Millipore, USA). The
organic
concentration in blow down water is measured as TOC after filtering through a
0.22 um filter.
Thus, the organic concentration reported in blow down and produced water is
solely due to
dissolved organic compounds.
[00372] The measurement of the concentration was based on calculation of
total carbon
concentration with reference to potassium hydrogen phthalate standard and
total inorganic
carbon concentration with reference to sodium carbonate and bi-carbonate
mixture.
[00373] A total carbon (TC) standard solution was prepared according to
the following
protocol. 2.125 g of potassium hydrogen phthalate was accurately weighed and
taken in a 1 L
volumetric ask. DI water was added up to the 1 L mark and the solution was
stirred. The
potassium hydrogen phthalate solution prepared has a concentration equivalent
to 1000 ppm
total organic carbon concentration. This solution was used as a standard for
total (TC)
analysis. A calibration was performed on the TOC analyzer with four different
concentrations
of the stock standard solution created by in-situ dilution in the instrument.
A calibration curve
was created based on which the TOC calculations were performed.
[00374] An inorganic carbon (IC) standard solution was prepared according
to the
following protocol. 3.497 g of reagent grade sodium bi-carbonate and 4.412 g
of sodium
carbonate was accurately weighed and taken in a 1 L volumetric ask. DI water
was added to
the ask up to the 1 L mark and the ask was shaken well. In this manner a
liquid solution
equivalent to 1000 ppm carbon concentration was prepared. This solution was
used as
standard for inorganic carbon (IC) analysis.
[00375] The analysis of dissolved organic matter content was carried out
according to
the following protocol. The samples were collected in 40 mL vials for TOC
analysis. The
sample vials were capped in order to prevent the volatilization and
contamination of the TOC
components in the samples. A calibration curve was generated by analyzing
various
concentrations of the TC and IC standard solutions. The TC values of the
samples were
determined by heating the samples at a temperature of 680 C over a high
sensitivity oxidation
Date Recue/Date Received 2022-03-31
CA 02915917 2015-12-22
4,1540 *Ale
catalyst. Samples were injected to the IC reaction vessel equipped with TOC-L
IC reactor kit
to measure the IC content in the sample. TOC is measured as the difference
between the TC
and IC analysis values.
[00376] In these
examples, the terms TOC concentration and dissolved organic carbon
(DOC) concentration shall be used interchangeably.
[00377] Example 22:
Enhanced Removal of Dissolved Organic Matter from BBD
Water with a Polyaluminum Chloride Coagulant in Combination with Aeration
treatment
[00378] SAGD BBD
samples were treated as described in the materials and methods
section, above, with 1000, 3000 and 5000 ppm of various coagulants including a
Ferric
Chloride, Polyferric Sulfate, Ferrous Chloride, polyaluminum chloride
coagulant C, and
Aluminum Sulfate, as provided in Table 31. The samples were treated with and
without
aeration. The percentage reduction in total organic carbon concentration of
the treated
samples are summarized in Table 31 and are shown in Figure 25.
[00379] An
additional run was also completed with only aeration and no coagulant
added to determine % removal potential.
[00380] Table 31:
Enhancement of dissolved organic matter removal using a
combination of oxidation-coagulation
Coagulant 7Dosage (ppm) ! % Removal % Removal of % change in
of TOC with TOC Without TOC removal by:!
Aeration Aeration .õ, aeration
.7..
1000 44.79 __ 23.81 88.1
Ferric Chloride 3000 75.61 41.41 82.6
5000 79.65 74.46 7.0
1000 46.14 29.59 55.9
Polyferric Sulfate 3000 64.63 41.79 54.7
5000 80.75 ______ 72.31 11.7
1000 46.79 20.54 127.8
Ferrous Chloride 3000 61.85 31.30 97.6
5000 75.73 57.04 32.8
1000 46.33 24.59 88.4
Polyaluminum Chloride 3000 48.28 24.81 94.6
5000 54.90 26.81 104.8
Polyaluminum Chloride
5000 80.77 61.52 31.3
Coagulant C
1000 48.28 23.16 108,5
Aluminum Sulfate 3000 49.57 24.21 104.8
5000 70.29 32.59 115.7
61
CA 02915917 2015-12-22
14,-0
None 29.60
[00381] The data shows that while the iron-based coagulants are more
effective at
reducing TOC compared to aluminum coagulants, dosages of 3000 and 5000 ppm are
typically required to reach values of 50% removal of TOC or greater. Also, the
supernatants
resulting from treatment with iron-based coagulants have a higher level of
dissolved iron
causing turbidity and reduced clarity compared to supernatants resulting from
treatment with
aluminum based coagulants and ultimately results in a less attractive
coagulant.
[00382] BBD samples treated by iron-based coagulants in combination with
aeration
resulted in significant improvements of the % removal of TOC. As can be seen
from the data,
% removal of TOC values of greater than 60%, greater than 70%, greater than
75% and even
greater than 80% (compared to untreated BBD water) were capable of being
achieved with
the combination of aeration and coagulant. Visual inspection of these samples
indicated that
clarity is significantly improved and IC analysis indicated that iron ion
content is
significantly reduced. The data shows that the combination of aluminum based
coagulants
with aeration resulted in significant improvement of TOC reduction, e.g., in
some cases
improving the percent TOC removal by greater than 80%, 90% or 100% compared to
percent
removal TOC by coagulant alone. The data also shows that aeration of water
treated with
aluminum-based coagulants was capable of removing greater than about 45%,
about 50%,
about 55%, about 70% and about 80% TOC, compared to the original TOC in the
BBD water
samples.
[00383] The pH of the BBD water did not change significantly by aeration:
the raw
water had a pH of 11.8 whereas after the aeration the pH was reduced to 11.2.
TOC analysis
of the sample treated with ferrous chloride at a 3000 dose shows that the
aerated sample had
30% less TOC concentration than the non-aerated.
[00384] In the preceding specification, various embodiments have been
described with
reference to the examples. It will, however, be evident that various
modifications and
changes may be made thereto, and additional embodiments may be implemented,
without
departing from the broader scope of the exemplary embodiments as set forth in
the claims
that follow. The specification and drawings are accordingly to be regarded in
an illustrative
rather than restrictive sense.
62