Note: Descriptions are shown in the official language in which they were submitted.
CA 02916141 2015-12-22
Methods, Systems And Apparatuses For Capturing And Sequestering Carbon
Dioxide Emitted From a Vehicle
Field of the Invention
The present invention relates to methods and apparatuses for capturing carbon
dioxide
and more specifically to methods and apparatuses for capturing carbon dioxide
produced by a combustion engine during operation.
Background
Reducing carbon dioxide (CO2) emissions and lowering the concentration of
greenhouse gases in the atmosphere has quickly become one of the most urgent
environmental issues facing the energy industry and broader society. Since the
beginning of the industrial era, the atmospheric concentration of CO2 has
increased by
over 40%. With fossil fuel combustion accounting for over 85% of the global
energy
demand, it is incumbent upon the industry to leverage new technologies and
find ways
to lessen the environmental footprint associated with the combustion of fossil
fuels from
well-to-wheels [1].
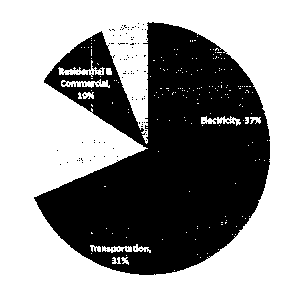
CO2 is a major contributor to the greenhouse effect, accounting for 82% of all
greenhouse gas emissions from human activities in the United States. As shown
in
Figure 1, of these emissions, vehicular exhaust is the second largest source,
accounting
for 31% of the over 5 billion tonnes emitted annually in the United States
[2]. If even a
portion of this CO2 can be captured and sequestered, it would reduce total
emissions
significantly.
Despite their emissions, fossil fuels will continue to supply a large share of
the world's
energy demand for years to come. In terms of energy density, no alternative
fuel comes
close to matching fossil fuels. Even in terms of CO2 emissions on a per energy
basis, so
called "clean fuels" such as ethanol are not much better than fossil fuels as
shown in
Figure 2 [3].
1
CA 02916141 2015-12-22
=
Clearly, some form of carbon capture and sequestration (CCS) will be needed to
offset
the energy demands of a growing global population. CCS is a three step
process,
involving carbon capture (CO2 separation from a mixture of gases),
transportation to a
sequestration site, and permanent sequestration. Most efforts to date on
carbon capture
have focused on large point sources, such as factories or power plants. Mobile
sources
like automobiles have been either neglected or dismissed as unfeasible.
A need therefore exists for providing a method, system or apparatus for
allowing the
capture and sequestering of CO2 produced by the operation of a combustion
engine.
Summary of the Invention
While traditional approaches to carbon capture and sequestration (CCS) have
focused
on large point sources, such as factories and power plants, the present
invention
provides for methods, systems and apparatuses that allow for the possibility
of on-board
vehicular carbon capture. Such a process employs post-combustion capture, as
it can
be retrofitted downstream of the engine. The stored CO2 may then be
transported to a
permanent sequestration site during refueling. The most promising capture
method for
this process is adsorption by metal-organic frameworks (M0F5) or porous
polymer
networks (PPNs). The capture cell is limited mainly by mass, which is
dependent on the
sorbent's uptake capacity towards CO2. With state-of-the-art technology, it
may be
possible to reduce vehicular emissions by 50%.
Brief Description of the Drawings
Figure 1 is a prior art pie chart illustrating CO2 emissions in the US;
Figure 2 is a prior art bar graph illustrating energy density and CO2
emissions from
various common fuels;
Figure 3 is a schematic illustrative of one embodiment of a process for CO2
capture and
sequestering;
Figure 4 is a pie chart illustrating typical composition of a gasoline engine
exhaust gas
stream [9];
2
CA 02916141 2015-12-22
Figure 5 is a chart illustrating capture system mass as a function of
adsorptive surface
CO2 uptake capacity, assuming 100% of CO2 emissions are being captured; and
Figure 6 is a chart illustrating a maximum achievable emissions reduction as a
function
of maximum allowable capture system mass.
Detailed Description
CO2 Separation Techniques
There are three primary CO2 separation techniques for the combustion process:
pre-
combustion separation, oxy-fuel combustion, and post-combustion separation
[4]. Pre-
combustion separation involves partial oxidation of the fuel to produce CO and
H20.
The CO is then reacted with steam to produce CO2 and H2. The CO2 is
selectively
removed from the mixture, leaving only the hydrogen as fuel. While promising,
pre-
combustion capture suffers from high capital costs.
Oxy-fuel combustion requires nearly pure oxygen (instead of air) to be used
for
combustion. Using pure oxygen causes the flue gas to be relatively rich in
CO2, making
CO2 capture easier than with flue gas rich in N2. However, air separation is
an
expensive process, and thus oxy-fuel combustion has high capital costs.
Post-combustion capture involves selectively removing CO2 from flue gas. Post-
combustion capture is the easiest to implement as it can be added downstream
of the
combustion engine. Furthermore, if the CO2 capture system is full or damaged,
the
performance of the engine is not compromised. This is crucial for vehicular
carbon
capture, as there is no guarantee that the user will empty the capture tank
and perform
maintenance at the required frequency. Failure of the capture system should
not render
the car unusable. For these reasons, post-combustion capture is the most
likely
candidate for on-board CO2 capture in vehicles.
Methods of CO2 Capture/Storage
Methods of capturing and storing CO2 fall into two main categories: absorption
and
adsorption. Absorption can be subdivided into physical absorption and chemical
3
CA 02916141 2015-12-22
absorption. There are several types of adsorptive surfaces that can be used,
including
activated carbon, porous polymer networks (PPNS), zeolites, and metal-organic
frameworks (M0Fs).
Physical Absorption
Physical absorption is based on Henry's Law and traditionally occurs at high
pressures
and low temperatures, with desorption occurring at increased temperatures and
reduced pressures. The advantages of physical absorption are low vapour
pressures,
low toxicity, and non-corrosive solvents can be used. Disadvantages include
low CO2
uptake capacity and low volumetric efficiencies [5].
Chemical Absorption
Chemical absorption involves an absorber and a stripper. The gas mixture
containing
CO2 enters the absorber unit and contacts CO2-lean absorbent, typically an
amine
solution such as monoethanolamine (MEA). A chemical reaction occurs, using up
CO2
and creating ammonium carbamate, ammonium carbonate, and ammonium bicarbonate
dissolved in solution. The now CO2-rich absorbent solution flows into the
stripper, where
it is heated to release the CO2 and regenerate the absorbent. The CO2 is
compressed
for subsequent transportation and storage and the absorbent is sent back to
the
absorber to be reused. Chemical absorption is generally cheaper than solid
adsorbents
and is the most commercially-ready technology for CO2 capture. Drawbacks
include low
CO2 loading capacity, low contact area between vapour and liquid, high
equipment
corrosion rate, and low volumetric efficiency. Since new chemical bonds are
being
formed, large amounts of heat are required to break these bonds for desorption
and
absorbent regeneration. Furthermore, amine degradation by contaminants in the
flue
gas is common, requiring high absorbent makeup rates. For these reasons,
chemical
absorption does not appear to be a viable option for on-board vehicular carbon
capture.
Ionic liquids are gaining interest as chemical absorbents due to their low
vapour
pressures, non-toxicity, and good thermal stability; however, they still
suffer from low
volumetric efficiencies and, at this stage, are unlikely to function as on-
board carbon
capture agents [5].
4
CA 02916141 2015-12-22
=
Activated Carbon
Carbon can be processed to contain small pores, increasing the surface area
available
for adsorption. Advantages of activated carbon include wide availability, low
cost, high
thermal stability, and low sensitivity to moisture. However, they have high
pressure
requirements for the gas being treated, high temperature sensitivity, and low
CO2/N2
selectivity, rendering them impractical for vehicular carbon capture [6].
Porous Polymer Networks (PPNS)
Polymers can be synthesized such that all units are joined together in a
polymer
network. These networks can be made porous, increasing surface area for
adsorption.
Advantages of PPNS include low cost, easy processing, high thermal and
chemical
stability, and relatively high surface areas for adsorption and CO2 uptake
capacities [7]
[8].
Zeolites
Zeolites are microporous aluminosilicate minerals that occur naturally and can
be
synthetically produced. They act as molecular sieves, sorting molecules based
on size.
Advantages of zeolites include low cost and low energy requirements for
desorption.
However, their usage is limited due to low CO2 adsorption capacity, low CO2/N2
selectivity (due to zeolites' molecular sieve nature and the similarity of the
kinetic
diameters of CO2 (3.30 A) and N2 (3.64 A)), and instability in the presence of
water due
to their high hydrophilicity [1] [5] [6].
Metal-Organic Frameworks (MOFs)
MOFs are porous, synthetic materials composed of metal cation nodes bridged by
organic ligand linkers. The structures and properties of MOFs can be designed
and
tuned for specific tasks. Functional groups can be added post-synthesis to
further
improve their efficacy [1]. MOFs can be designed with extremely high surface
areas for
adsorption, high uptake capacities for CO2, high selectivity to CO2, and low
energy
requirements for desorption. However, MOFs are expensive and most are unstable
in
the presence of water [5] [6].
5
CA 02916141 2015-12-22
,
Process
One embodiment of a process envisages a few fundamental changes to the way we
use
our vehicles and the refueling system currently in place. As outlined above,
post-
combustion carbon capture will be implemented. After combustion, the exhaust
gas
flows through the capture cell, where the CO2 is selectively removed and
stored. The
exhaust gas may include a small amount of liquid, which may be separated from
the
gas (e.g., utilizing a demister or other known technology) prior to the gas
entering the
capture system. The now CO2-free (or at least CO2-lean) exhaust gas vents to
the
atmosphere. The process is shown schematically in Error! Reference source not
found.
and below in scheme 1. While this may not be an easy to implement change, when
faced with the enormous challenge of reducing emissions, all options must be
considered.
Scheme 1
Gasoline (from tank) ____________
Exhaust gas Capture
Engine
_____________________________________________ >
Air (N2, 02) ____________________ (N2, C0 system 2, H20)
N2, F120 to atmosphere
from atmosphere
The capture system is designed to capture emissions from a single tank of
gasoline. In
one variation of the proposed model, when the user fills their car, hybrid,
bus, or other
vehicle with gasoline, the CO2 is simultaneously desorbed and the sorbent is
regenerated for future use. The CO2 may be stored temporarily at the gas
station using
one of the methods outlined above or may be immediately sent to sequestration.
Infrastructure will be necessary for CO2 transport to permanent sequestration
sites.
Capture Cell Requirements
Based on the composition of exhaust gas, for example as outlined in Figure 4,
and
assuming expulsion at atmospheric pressure, the capture cell ideally will meet
several
requirements, most notably:
6
CA 02916141 2015-12-22
,
1. High CO2 uptake at a partial pressure of 0.14 bar
2. High CO2/N2 selectivity at partial pressures for CO2 and N2 of 0.14 bar and
0.71 bar, respectively
3. High CO2/H20 selectivity at partial pressures for CO2 and H20 of 0.14 bar
and
0.13 bar, respectively
4. Chemical stability in the presence of water
5. Thermal stability
6. Low cost
7. Low energy required for desorption
The suitability of the carbon capture methods outlined above, given the above
ideal
parameters, are summarized in Table 1.
Table 1: Applicability of several carbon capture methods to the proposed
process
Physical Chemical Activated PPNS Zeolites MOFs
Absorbents Absorbents Carbon
High CO2 X X X X
uptake
Low cost V V V X
Low energy X X
required for
desorption
High CO2/N2 X V X I X
selectivity
Stability with V I V I X X
water
High thermal V I V I X X
stability
PPNs currently appear to be the most favourable technology for vehicular
carbon
capture. However, uptake capacity is by far the most crucial parameter when
determining suitability for vehicular carbon capture, and MOFs generally have
higher
uptake capacities than PPNs. MOFs are still a relatively new science, and
future
developments will likely overcome the current obstacles of high cost,
instability in the
presence of water, and low thermal stability. Potentially, a combination of
materials such
as PPNs and MOFs could be implemented to provide an optimized capture
material. By
7
CA 02916141 2015-12-22
compressing a particular MOF powder to form millimeter or nano-sized prills,
the
sorbent may be affixed within the exhaust system of an automobile and CO2
separated
from the flue gas stream. As the exhaust gases flow through the inline capture
vessel
containing MOFs, CO2 will be selectively removed from the gas mixture. The
then
reduced CO2 exhaust would then exit through the original muffler and into the
atmosphere. The capture vessel may utilize a pressure swing regeneration by
means of
an on-board compressor and temporarily store the CO2 in the vehicle until it
can be
discharged at a fueling station. For these reasons, MOFs should not be
discounted as
viable options.
Limiting Factors
The main limiting factors that will be placed on the capture tank are mass and
volume.
Burning one gallon of gasoline (which has a mass of 2.8 kg) releases 8.9 kg of
CO2 [3].
Assuming the total mass of the vehicle system (gasoline tank + carbon capture
tank)
must remain constant as gasoline is consumed, only 31% of emissions can be
captured. To capture all the emissions, the system will gain mass at a rate of
6.1
kg/gallon gasoline burned.
Assuming an average gasoline tank size of 13 gallons, 116 kg of CO2 are
released per
tank [10]. The mass of sorbent required is governed by the sorbent's uptake
capacity
towards CO2 as shown in Figure 5.
Current state-of-the-art carbon capture technology has a CO2 uptake capacity
of around
35% (wt.) at 1 bar total pressure [6]. If, for illustrative purposes, we
assume this capacity
will remain the same at 0.15 bar and further assume a maximum allowable
sorbent
mass of 100 kg, this could reduce emissions by nearly 50% as illustrated in
Figure 6.
This reduction would be a huge step towards an emissions-free future, and will
likely be
within reach in the near future.
Conclusions
Three different CO2 separation techniques have been provided, namely pre-
combustion
capture, oxy-fuel combustion, and post-combustion chapter. Several methods of
8
CA 02916141 2015-12-22
capturing CO2 were analyzed, including physical absorption, chemical
absorption, and
solid adsorption. A process was provided for capturing and storing CO2
emissions in
vehicles, with the CO2 being optionally transported to a permanent
sequestration site
during refueling. It is important to consider all possibilities when faced
with the
enormous challenge of reducing CO2 emissions. Proactive thinking is crucial to
ensuring
quick implementation when the necessary capture agents have been developed.
The
capture cell can be made modular, such that as new capture technologies
emerge, they
can replace the current sorbent in the tank.
Various modifications, adaptations and evolutions of the processes, systems
and
apparatuses will be apparent to those of skill in the art and it is the
inventors intent that
such modifications, adaptations and evolutions are within the scope and spirit
of the
invention.
9
CA 02916141 2015-12-22
=
References
[1] J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B.
Balbuena
and H.-C. Zhou, "Carbon dioxide capture-related gas adsorption and separation
in
metal-organic frameworks," Coordination Chemistry Reviews, vol. 255, pp. 1791-
1823,
2011.
[2] United States Environmental Protection Agency, "Overview of Greenhouse
Gases," 2015. [Online].
Available:
http://www.epa.gov/climatechange/ghgemissions/gases/co2.html. [Accessed 17
June
2015].
[3]
Oak Ridge National Laboratory, "Appendix A: Lower and Higher Heating Values
of Gas, Liquid and Solid Fuels," in Biomass Energy Data Book, Oak Ridge, U.S
Department of Energy, 2011, p. 201.
[4] J. M. Sullivan and M. Sivak, "Carbon Capture in Vehicles: A Review of
General
Support, Available Mechanisms, and Consumer-Acceptance Issues," University of
Michigam Transportation Research Institute, Ann Arbor, 2012.
[5] C.-H. Yu, C.-H. Huang and C.-S. Tan, "A Review of CO2 Capture by
Absorption
and Adsorption," Aerosol and Air Quality Research, vol. 12, pp. 745-769, 2012.
[6] Y. Liu, Z. U. Wang and H.-C. Zhou, "Recent advances in carbon dioxide
capture
with metal-organic frameworks," Greenhouse Gases: Science and Technology, vol.
2,
pp. 239-259, 2012.
[7] W. Lu, D. Yuan, D. Zhao, C. I. Schilling, 0. Plietzsch, T. Muller, S.
Brase, J.
Guenther, J. Blumel, r. Krishna, Z. Li and H.-C. Zhou, "Porous Polymer
Networks:
Synthesis, Porosity, and Applications in Gas Storage/Separation," Chemistry of
Materials, vol. 22, pp. 5964-5972, 2010.
[8] J.
P. Sculley, J.-R. Li, J. Park, W. Lu and H.-C. J. Zhou, "Metal-organic
frameworks and porous polymer networks for carbon capture," Sustainable
Technologies, Systems and Policies, 2012.
CA 02916141 2015-12-22
, r
[9] Audi of America, Inc., "Motor Vehicle Exhaust Emissions," Auburn Hills,
2001.
[10] United States Environmental Protection Agency, "Calculations and
References,"
2015. [Online]. Available: http://www.epa.govicleanenergy/energy-
resources/refs.html.
[Accessed 17 June 2015].
[11] R. Siriwardane, M. Shen, E. Fisher and J. Losch, "CO2 Capture Utilizing
Solid
Sorbents," U.S Department of Energy, Morgantown.
[12] E. Mangano, S. Brandani, M.-C. Ferrari, H. Ahn, M. Lozinska, M. Lozinska,
P.
Wright, J. Kahr, R. Morris, M. Croad, N. McKeown, H. Shamsipour and P. Budd,
"Efficient and Rapid Screening of Novel Adsorbents for Carbon Capture in the
UK
IGSCC Project," Energy Procedia, 2013.
[13] A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and
0. M.
Yaghi, "Synthesis, Structure, and Carbon Dioxide Capture Properties of
Zeolitic
Imidazolate Frameworks," Accounts of Chemical Research, vol. 43, no. 1, pp. 58-
67,
2009.
[14] A. Samanta, A. Zhao, G. K. Shimizu, P. Sarkar and R. Gupta, "Post-
Combustion
CO2 Capture Using Solid Sorbents: A Review," Industrial & Engineering
Chemistry
Research, vol. 51, pp. 1438-1463, 2011.
[15] F. A. Mumpton, "La roca magica: Uses of natural zeolites in agriculture
and
industry," Proceedings of the National Academy of Sciences, vol. 96, pp. 3463-
3470,
1999.
[16] Z. Liang, M. Marshall and A. L. Chaffee, "Comparison of Cu-BTC and
zeolite 13X
for adsorbent based CO2 separation," Energy Procedia, vol. 1, pp. 1266-1271,
2009.
[17] S. Hardie, M. Garnett, A. Fallick, A. Rowland and N. Ostle, "Carbon
Dioxide
Capture Using a Zeolite Molecular Sieve Sampling System for Isotopic Studies
(C13
and C14) of Respiration," Radiocarbon, vol. 47, pp. 441-451, 2005.
11
CA 02916141 2015-12-22
[18] C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, "Comparative Study of CO2
Capture by Carbon Nanotubes, Activated Carbons, and Zeolites," Energy & Fuels,
vol.
22, pp. 3050-3056, 2008.
[19] D. L. Damm and A. G. Fedorov, "Conceptual study of distributed CO2
capture
and the sustainable carbon economy," Energy Conversion and Management, vol.
49,
pp. 1674-1683, 2008.
[20] R. J. Kuppler, D. J. Timmon, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D.
Young, D.
Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, "Potential applications of metal-
organic
frameworks," Coordination Chemistry Reviews, vol. 253, pp. 3042-3066, 2009.
[21] T. Borjigin, F. Sun, J. Zhang, K. Cai, H. Ren and G. Zhu, "A microporous
metal-
organic framework with high stability for GC separation of alcohols from
water,"
Chemical Communications, vol. 48, pp. 7613-7615, 2012.
[22] U.S. Department of Transportation, "Annual Vehicle Distance Traveled in
Miles
and Related Data," 2011. [Online].
Available:
http://www.fhwa.dot.gov/policyinformationistatistics/2011/vm1.cfm. [Accessed
17 June
2015].
[23] W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H.-C. Zhou,
"Sulfonate-
Grafted Porous Polymer Networks for Preferential CO2 Adsorption at Low
Pressure,"
Journal of the American Chemical Society, vol. 133, pp. 18126-18129, 2011.
[24] D. Yuan, W. Lu, D. Zhao and H.-C. Zhou, "Highly Stable Porous Polymer
Networks with Exceptionally High Gas-Uptake Capacities," Advanced Materials,
vol. 23,
pp. 3723-3725, 2011.
[25] H.-S. Choi and M. P. Suh, "Highly Selective CO2 Capture in Flexible 3D
Coordination Polymer Networks," Angewandte Chemie International Edition, vol.
48, pp.
6865-6869, 2009.
[26] T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V.
CroceIla, F. Giordanino, S. 0. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L.
Dzubak, R.
12
CA 02916141 2015-12-22
Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D.
Prendergast, J. B.
Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and
J. R. Long,
"Cooperative insertion of CO2 in diamine-appended metal-organic frameworks,"
Nature,
2015.
[27] A. C. Kizzie, A. G. Wong-Foy and A. J. Matzger, "Effect of Humidity on
the
Performance of Microporous Coordination Polymers as Adsorbents for CO2
Capture,"
Langmuir, vol. 27, pp. 6368-6373, 2011.
13