Note: Descriptions are shown in the official language in which they were submitted.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
COMBINATION SPEAKER AND LIGHT SOURCE RESPONSIVE TO
STATE(S) OF AN ENVIRONMENT BASED ON SENSOR DATA
FIELD
The present invention relates generally to electrical and electronic hardware,
electromechanical and computing devices. More specifically, techniques related
to a
combination speaker and light source responsive to states of an environment
based on sensor
data are described.
BACKGROUND
Conventional devices for lighting typically do not provide audio playback
capabilities,
and conventional devices for audio playback (i.e., speakers) typically do not
provide light.
Although there are conventional speakers equipped with light features for
decoration or as part of
a user interface, such conventional speakers are typically not configured to
provide ambient
lighting or the light an environment. Also, conventional speakers typically
are not configured to
be installed into or powered using a light socket.
Conventional devices for lighting and playing audio also typically lack
capabilities for
responding automatically to a person's state and environment, particularly in
a contextually-
meaningful manner.
Thus, what is needed is a solution for a combination speaker and light source
responsive
to states of an environment based on sensor data without the limitations of
conventional
techniques.
BRIEF DESCRIPTION OF THE DRAWINGS
Various embodiments or examples ("examples") are disclosed in the following
detailed
description and the accompanying drawings:
FIG. lA illustrates an exemplary array of electrodes and a physiological
information
generator disposed in a wearable data-capable band, according to some
embodiments;
FIGs. 1B to 1D illustrate examples of electrode arrays, according to some
embodiments;
FIG. 2 is a functional diagram depicting a physiological information generator
implemented in a wearable device, according to some embodiments;
FIGs. 3A to 3C are cross-sectional views depicting arrays of electrodes
including subsets
of electrodes adjacent an arm of a wearer, according to some embodiments;
FIG. 4 depicts a portion of an array of electrodes disposed within a housing
material of a
wearable device, according to some embodiments;
FIG. 5 depicts an example of a physiological information generator, according
to some
embodiments;
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
FIG. 6 is an example flow diagram for selecting a sensor, according to some
embodiments;
FIG. 7 is an example flow diagram for determining physiological
characteristics using a
wearable device with arrayed electrodes, according to some embodiments;
FIG. 8 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments
FIG. 9 depicts the physiological signal extractor, according to some
embodiments;
FIG. 10 is a flowchart for extracting a physiological signal, according to
some
embodiments;
FIG. 11 is a block diagram depicting an example of a physiological signal
extractor,
according to some embodiments;
FIG. 12 depicts an example of an offset generator, according to some
embodiments;
FIG. 13 is a flowchart depicting example of a flow for decomposing a sensor
signal to
form separate signals, according to some embodiments;
FIGs. 14A to 14C depict various signals used for physiological characteristic
signal
extraction, according to various embodiments;
FIG. 15 depicts recovered signals, according to some embodiments;
FIG. 16 depicts an extracted physiological signal, according to various
embodiments;
FIG. 17 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments;
FIG. 18 is a diagram depicting a physiological state determinator configured
to receive
sensor data originating, for example, at a distal portion of a limb, according
to some
embodiments;
FIG. 19 depicts a sleep manager, according to some embodiments;
FIG. 20A depicts a wearable device including a skin surface microphone
("SSM"),
according to some embodiments;
FIG. 20B depicts an example of data arrangements for physiological
characteristics and
parametric values that can identify a sleep state, according to some
embodiments;
FIG. 21 depicts an anomalous state manager, according to some embodiments;
FIG. 22 depicts an affective state manager configured to receive sensor data
derived from
bioimpedance signals, according to some embodiments;
FIG. 23 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments;
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
FIG. 24 illustrates an exemplary combination speaker and light source powered
using a
light socket;
FIG. 25 illustrates a system for manipulating a combination speaker and light
source
according to a physiological state determined using sensor data; and
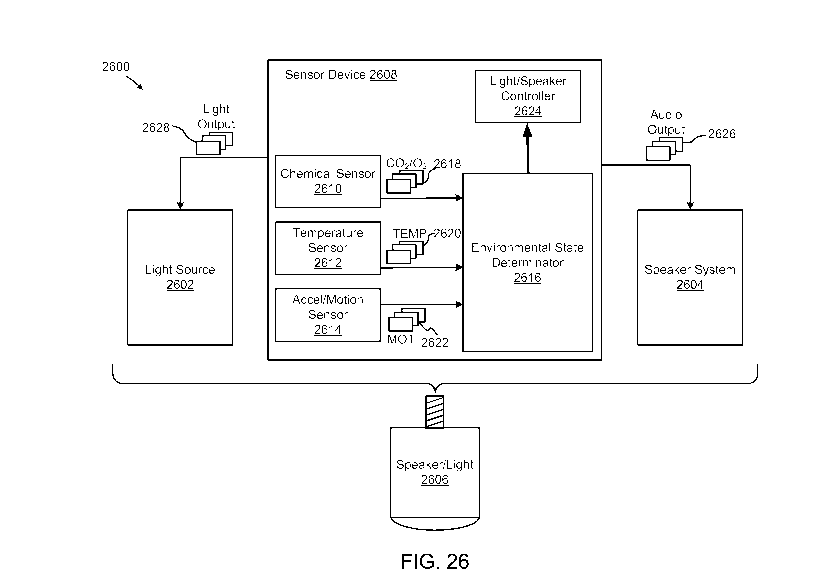
FIG. 26 illustrates a diagram depicting exemplary components in a combination
speaker
and light source including sensor device for determining an environmental
state.
Although the above-described drawings depict various examples of the
invention, the
invention is not limited by the depicted examples. It is to be understood
that, in the drawings,
like reference numerals designate like structural elements. Also, it is
understood that the
drawings are not necessarily to scale.
DETAILED DESCRIPTION
Various embodiments or examples may be implemented in numerous ways, including
as
a system, a process, an apparatus, a device, and a method associated with a
wearable device
structure with enhanced detection by motion sensor. In some embodiments,
motion may be
detected using an accelerometer that responds to an applied force and produces
an output signal
representative of the acceleration (and hence in some cases a velocity or
displacement) produced
by the force. Embodiments may be used to couple or secure a wearable device
onto a body part.
Techniques described are directed to systems, apparatuses, devices, and
methods for using
accelerometers, or other devices capable of detecting motion, to detect the
motion of an element
or part of an overall system. In some examples, the described techniques may
be used to
accurately and reliably detect the motion of a part of the human body or an
element of another
complex system. In general, operations of disclosed processes may be performed
in an arbitrary
order, unless otherwise provided in the claims.
A detailed description of one or more examples is provided below along with
accompanying figures. The detailed description is provided in connection with
such examples,
but is not limited to any particular example. The scope is limited only by the
claims and
numerous alternatives, modifications, and equivalents are encompassed.
Numerous specific
details are set forth in the following description in order to provide a
thorough understanding.
These details are provided for the purpose of example and the described
techniques may be
practiced according to the claims without some or all of these specific
details. For clarity,
technical material that is known in the technical fields related to the
examples has not been
described in detail to avoid unnecessarily obscuring the description.
FIG. lA illustrates an exemplary array of electrodes and a physiological
information
generator disposed in a wearable data-capable band, according to some
embodiments. Diagram
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
100 depicts an array 100 of electrodes 110 coupled to a physiological
information generator 120
that is configured to generate data representing one or more physiological
characteristics
associated with a user that is wearing or carrying array 101. Also shown are
motion sensors 160,
which, for example, can include accelerometers. Motion sensors 160 are not
limited to
accelerometers. Examples of motion sensors 160 can also include gyroscopic
sensors, optical
motion sensors (e.g., laser or LED motion detectors, such as used in optical
mice), magnet-based
motion sensors (e.g., detecting magnetic fields, or changes thereof, to detect
motion),
electromagnetic-based sensors, etc., as well as any sensor configured to
detect or determine
motion, such as motion sensors based on physiological characteristics (e.g.,
using
electromyography ("EMG") to determine existence and/or amounts of motion based
on electrical
signals generated by muscle cells), and the like. Electrodes 110 can include
any suitable
structure for transferring signals and picking up signals, regardless of
whether the signals are
electrical, magnetic, optical, pressure-based, physical, acoustic, etc.,
according to various
embodiments. According to some embodiments, electrodes 110 of array 101 are
configured to
couple capacitively to a target location. In some embodiments, array 101 and
physiological
information generator 120 are disposed in a wearable device, such as a
wearable data-capable
band 170, which may include a housing that encapsulates, or substantially
encapsulates, array
101 of electrodes 110. Examples of a wearable data-capable band are disclosed
in U.S. Patent
Application No. 13/454,040, filed on April 23, 2012, and U.S. Patent
Application No.
13/491,345, filed on June 7, 2012, which are incorporated by reference herein
in their entirety for
all purposes. In some examples, wearable data-capable band 170 may be worn in
various ways
on various parts of a user's body, including a limb (e.g., arm, wrist, leg, or
the like), a torso (e.g.,
as a chest strap, belt, or the like), or other body part, without limitation.
In operations,
physiological information generator 120 can determine the bioelectric
impedance
("bioimpedance") of one or more types of tissues of a wearer to identify,
measure, and monitor
physiological characteristics. For example, a drive signal having a known
amplitude and
frequency can be applied to a user, from which a sink signal is received as
bioimpedance signal.
The bioimpedance signal is a measured signal that includes real and complex
components.
Examples of real components include extra-cellular and intra-cellular spaces
of tissue, among
other things, and examples of complex components include cellular membrane
capacitance,
among other things. Further, the measured bioimpedance signal can include real
and/or complex
components associated with arterial structures (e.g., arterial cells, etc.)
and the presence (or
absence) of blood pulsing through an arterial structure. In some examples, a
heart rate signal, or
other physiological signals, can be determined (i.e., recovered) from the
measured bioimpedance
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
signal by, for example, comparing the measured bioimpedance signal against the
waveform of
the drive signal to determine a phase delay (or shift) of the measured complex
components.
Physiological information generator 120 is shown to include a sensor selector
122, a
motion artifact reduction unit 124, and a physiological characteristic
determinator 126. Sensor
selector 122 is configured to select a subset of electrodes, and is further
configured to use the
selected subset of electrodes to acquire physiological characteristics,
according to some
embodiments. Examples of a subset of electrodes include subset 107, which is
composed of
electrodes 110d and 110e, and subset 105, which is composed of electrodes
110c, 110d and
110e. More or fewer electrodes can be used. Sensor selector 122 is configured
to determine
which one or more subsets of electrodes 110 (out of a number of subsets of
electrodes 110) are
adjacent to a target location. As used herein, the term "target location" can,
for example, refer to
a region in space from which a physiological characteristic can be determined.
A target region
can be adjacent to a source of the physiological characteristic, such as blood
vessel 102, with
which an impedance signal can be captured and analyzed to identify one or more
physiological
characteristics. The target region can reside in two-dimensional space, such
as an area on the
skin of a user adjacent to the source of the physiological characteristic, or
in three-dimensional
space, such as a volume that includes the source of the physiological
characteristic. Sensor
selector 122 operates to either drive a first signal via a selected subset to
a target location, or
receive a second signal from the target location, or both. The second signal
includes data
representing one or more physiological characteristics. For example, sensor
selector 122 can
configure electrode ("D") 110b to operate as a drive electrode that drives a
signal (e.g., an AC
signal) into the target location, such as into the skin of a user, and can
configure electrode ("S")
110a to operate as a sink electrode (i.e., a receiver electrode) to receive a
second signal from the
target location, such as from the skin of the user. In this configuration,
sensor selector 112 can
drive a current signal via electrode ("D") 110b into a target location to
cause a current to pass
through the target location to another electrode ("S") 110a. In various
examples, the target
location can be adjacent to or can include blood vessel 102. Examples of blood
vessel 102
include a radial artery, an ulnar artery, or any other blood vessel. Array 101
is not limited to
being disposed adjacent blood vessel 102 in an arm, but can be disposed on any
portion of a
user's person (e.g., on an ankle, ear lobe, around a finger or on a fingertip,
etc.). Note that each
electrode 110 can be configured as either a driver or a sink electrode. Thus,
electrode 110b is not
limited to being a driver electrode and can be configured as a sink electrode
in some
implementations. As used herein, the term "sensor" can refer, for example, to
a combination of
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
one or more driver electrodes and one or more sink electrodes for determining
one or more
bioimpedance-related values and/or signals, according to some embodiments.
In some embodiments, sensor selector 122 can be configured to determine
(periodically
or aperiodically) whether the subset of electrodes 110a and 110b are optimal
electrodes 110 for
acquiring a sufficient representation of the one or more physiological
characteristics from the
second signal. To illustrate, consider that electrodes 110a and 110b may be
displaced from the
target location when, for instance, wearable device 170 is subject to a
displacement in a plane
substantially perpendicular to blood vessel 102. The displacement of
electrodes 110a and 110b
may increase the impedance (and/or reactance) of a current path between the
electrodes 110a and
110b, or otherwise move those electrodes away from the target location far
enough to degrade or
attenuate the second signals retrieved therefrom. While electrodes 110a and
110b may be
displaced from the target location, other electrodes are displaced to a
position previously
occupied by electrodes 110a and 110b (i.e., adjacent to the target location).
For example,
electrodes 110c and 110d may be displaced to a position adjacent to blood
vessel 102. In this
case, sensor selector 122 operates to determine an optimal subset of
electrodes 110, such as
electrodes 110c and 110d, to acquire the one or more physiological
characteristics. Therefore,
regardless of the displacement of wearable device 170 about blood vessel 102,
sensor selector
122 can repeatedly determine an optimal subset of electrodes for extracting
physiological
characteristic information from adjacent a blood vessel. For example, sensor
selector 122 can
repeatedly test subsets in sequence (or in any other matter) to determine
which one is disposed
adjacent to a target location. For example, sensor selector 122 can select at
least one of subset
109a, subset 109b, subset 109c, and other like subsets, as the subset from
which to acquire
physiological data.
According to some embodiments, array 101 of electrodes can be configured to
acquire
one or more physiological characteristics from multiple sources, such as
multiple blood vessels.
To illustrate, consider that, for example, blood vessel 102 is an ulnar artery
adjacent electrodes
110a and 110b and a radial artery (not shown) is adjacent electrodes 110c and
110d. With
multiple sources of physiological characteristic information being available,
there are thus
multiple target locations. Therefore, sensor selector 122 can select multiple
subsets of electrodes
110, each of which is adjacent to one of a multiple number of target
locations. Physiological
information generator 120 then can use signal data from each of the multiple
sources to confirm
accuracy of data acquired, or to use one subset of electrodes (e.g.,
associated with a radial artery)
when one or more other subsets of electrodes (e.g., associated with an ulnar
artery) are
unavailable.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
Note that the second signal received into electrode 110a can be composed of a
physiological-related signal component and a motion-related signal component,
if array 101 is
subject to motion. The motion-related component includes motion artifacts or
noise induced into
an electrode 110a. Motion artifact reduction unit 124 is configured to receive
motion-related
signals generated at one or more motion sensors 160, and is further configured
to receive at least
the motion-related signal component of the second signal. Motion artifact
reduction unit 124
operates to eliminate the magnitude of the motion-related signal component, or
to reduce the
magnitude of the motion-related signal component relative to the magnitude of
the
physiological-related signal component, thereby yielding as an output the
physiological-related
signal component (or an approximation thereto). Thus, motion artifact
reduction unit 124 can
reduce the magnitude of the motion-related signal component (i.e., the motion
artifact) by an
amount associated with the motion-related signal generated by one or more
accelerometers to
yield the physiological-related signal component.
Physiological characteristic determinator 126 is configured to receive the
physiological-
related signal component of the second signal and is further configured to
process (e.g., digitally)
the signal data including one or more physiological characteristics to derive
physiological
signals, such as either a heart rate ("HR") signal or a respiration signal, or
both. For example,
physiological characteristic determinator 126 is configured to amplify and/or
filter the
physiological-related component signals (e.g., at different frequency ranges)
to extract certain
physiological signals. According to various embodiments, a heart rate signal
can include (or can
be based on) a pulse wave. A pulse wave includes systolic components based on
an initial pulse
wave portion generated by a contracting heart, and diastolic components based
on a reflected
wave portion generated by the reflection of the initial pulse wave portion
from other limbs. In
some examples, an HR signal can include or otherwise relate to an
electrocardiogram ("ECG")
signal. Physiological characteristic determinator 126 is further configured to
calculate other
physiological characteristics based on the acquired one or more physiological
characteristics.
Optionally, physiological characteristic determinator 126 can use other
information to calculate
or derive physiological characteristics. Examples of the other information
include motion-
related data, including the type of activity in which the user is engaged,
such as running or sleep,
location-related data, environmental-related data, such as temperature,
atmospheric pressure,
noise levels, etc., and any other type of sensor data, including stress-
related levels and activity
levels of the wearer.
In some cases, a motion sensor 160 can be disposed adjacent to the target
location (not
shown) to determine a physiological characteristic via motion data indicative
of movement of
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
blood vessel 102 through which blood pulses to identify a heart rate-related
physiological
characteristic. Motion data, therefore, can be used to supplement impedance
determinations of to
obtain the physiological characteristic. Further, one or more motion sensors
160 can also be
used to determine the orientation of wearable device 170, and relative
movement of the same to
determine or predict a target location. By predicting a target location,
sensor selector 122 can
use the predicted target location to begin the selection of optimal subsets of
electrodes 110 in a
manner that reduces the time to identify a target location.
In view of the foregoing, the functions and/or structures of array 101 of
electrodes and
physiological information generator 120, as well as their components, can
facilitate the
acquisition and derivation of physiological characteristics in situ¨during
which a user is
engaged in physical activity that imparts motion on a wearable device, thereby
exposing the
array of electrodes to motion-related artifacts. Physiological information
generator 120 is
configured to dampen or otherwise negate the motion-related artifacts from the
signals received
from the target location, thereby facilitating the provision of heart-related
activity and respiration
activity to the wearer of wearable device 170 in real-time (or near real-
time). As such, the
wearer of wearable device 170 need not be stationary or otherwise interrupt an
activity in which
the wearer is engaged to acquire health-related information. Also, array 101
of electrodes 110
and physiological information generator 120 are configured to accommodate
displacement or
movement of wearable device 170 about, or relative to, one or more target
locations. For
example, if the wearer intentionally rotates wearable device 170 about, for
example, the wrist of
the user, then initial subsets of electrodes 110 adjacent to the target
locations (i.e., before the
rotation) are moved further away from the target location. As another example,
the motion of the
wearer (e.g., impact forces experienced during running) may cause wearable
device 170 to travel
about the wrist. As such, physiological information generator 120 is
configured to determine
repeatedly whether to select other subsets of electrodes 110 as optimal
subsets of electrodes 110
for acquiring physiological characteristics. For example, physiological
information generator
120 can be configured to cycle through multiple combinations of driver
electrodes and sink
electrodes (e.g., subsets 109a, 109b, 109c, etc.) to determine optimal subsets
of electrodes. In
some embodiments, electrodes 110 in array 101 facilitate physiological data
capture irrespective
of the gender of the wearer. For example, electrodes 110 can be disposed in
array 101 to
accommodate data collection of a male or female were irrespective of gender-
specific
physiological dimensions. In at least one embodiment, data representing the
gender of the
wearer can be accessible to assist physiological information generator 120 in
selecting the
optimal subsets of electrodes 110. While electrodes 110 are depicted as being
equally-spaced,
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
array 101 is not so limited. In some embodiments, electrodes 110 can be
clustered more densely
along portions of array 101 at which blood vessels 102 are more likely to be
adjacent. For
example, electrodes 110 may be clustered more densely at approximate portions
172 of wearable
device 170, whereby approximate portions 172 are more likely to be adjacent a
radial or ulnar
artery than other portions. While wearable device 170 is shown to have an
elliptical-like shape,
it is not limited to such a shape and can have any shape.
In some instances, a wearable device 170 can select multiple subsets of
electrodes to
enable data capture using a second subset adjacent to a second target location
when a first subset
adjacent a first target location is unavailable to capture data. For example,
a portion of wearable
device 170 including the first subset of electrodes 110 (initially adjacent to
a first target location)
may be displaced to a position farther away in a radial direction away from a
blood vessel, such
as depicted by a radial distance 392 of FIG. 3C from the skin of the wearer.
That is, subset of
electrodes 310a and 310b are displaced radially be distance 392. Further to
FIG. 3C, the second
subset of electrodes 310f and 310g adjacent to the second target location can
be closer in a radial
direction toward another blood vessel, and, thus, the second subset of
electrodes can acquire
physiological characteristics when the first subset of electrodes cannot.
Referring back to FIG.
1A, array 101 of electrodes 110 facilitates a wearable device 170 that need
not be affixed firmly
to the wearer. That is, wearable device 170 can be attached to a portion of
the wearer in a
manner in which wearable device 170 can be displaced relative to a reference
point affixed to the
wearer and continue to acquire and generate information regarding
physiological characteristics.
In some examples, wearable device 170 can be described as being "loosely
fitting" on or
"floating" about a portion of the wearer, such as a wrist, whereby array 101
has sufficient sensors
points from which to pick up physiological signals.
In addition, accelerometers 160 can be used to replace the implementation of
subsets of
electrodes to detect motion associated with pulsing blood flow, which, in
turn, can be indicative
of whether oxygen-rich blood is present or not present. Or, accelerometers 160
can be used to
supplement the data generated by acquired one or more bioimpedance signals
acquired by array
101. Accelerometers 160 can also be used to determine the orientation of
wearable device 170
and relative movement of the same to determine or predict a target location.
Sensor selector 122
can use the predicted target location to begin the selection of the optimal
subsets of electrodes
110, which likely decreases the time to identify a target location. Electrodes
110 of array 101
can be disposed within a material constituting, for example, a housing,
according to some
embodiments. Therefore, electrodes 110 can be protected from the environment
and, thus, need
not be subject to corrosive elements. In some examples, one or more electrodes
110 can have at
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
least a portion of a surface exposed. As electrodes 110 of array 101 are
configured to couple
capacitively to a target location, electrodes 110 thereby facilitate high
impedance signal coupling
so that the first and second signals can pass through fabric and hair. As
such, electrodes 110
need not be limited to direct contact with the skin of a wearer. Further,
array 101 of electrodes
110 need not circumscribe a limb or source of physiological characteristics.
An array 101 can be
linear in nature, or can configurable to include linear and curvilinear
portions.
In some embodiments, wearable device 170 can be in communication (e.g., wired
or
wirelessly) with a mobile device 180, such as a mobile phone or computing
device. In some
cases, mobile device 180, or any networked computing device (not shown) in
communication
with wearable device 170 or mobile device 180, can provide at least some of
the structures
and/or functions of any of the features described herein. As depicted in FIG.
lA and subsequent
figures, the structures and/or functions of any of the above-described
features can be
implemented in software, hardware, firmware, circuitry, or any combination
thereof Note that
the structures and constituent elements above, as well as their functionality,
may be aggregated
or combined with one or more other structures or elements. Alternatively, the
elements and their
functionality may be subdivided into constituent sub-elements, if any. As
software, at least some
of the above-described techniques may be implemented using various types of
programming or
formatting languages, frameworks, syntax, applications, protocols, objects, or
techniques. For
example, at least one of the elements depicted in FIG. lA (or any subsequent
figure) can
represent one or more algorithms. Or, at least one of the elements can
represent a portion of
logic including a portion of hardware configured to provide constituent
structures and/or
functionalities.
For example, physiological information generator 120 and any of its one or
more
components, such as sensor selector 122, motion artifact reduction unit 124,
and physiological
characteristic determinator 126, can be implemented in one or more computing
devices (i.e., any
mobile computing device, such as a wearable device or mobile phone, whether
worn or carried)
that include one or more processors configured to execute one or more
algorithms in memory.
Thus, at least some of the elements in FIG. lA (or any subsequent figure) can
represent one or
more algorithms. Or, at least one of the elements can represent a portion of
logic including a
portion of hardware configured to provide constituent structures and/or
functionalities. These
can be varied and are not limited to the examples or descriptions provided.
As hardware and/or firmware, the above-described structures and techniques can
be
implemented using various types of programming or integrated circuit design
languages,
including hardware description languages, such as any register transfer
language ("RTL")
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
configured to design field-programmable gate arrays ("FPGAs"), application-
specific integrated
circuits ("ASICs"), multi-chip modules, or any other type of integrated
circuit. For example,
physiological information generator 120, including one or more components,
such as sensor
selector 122, motion artifact reduction unit 124, and physiological
characteristic determinator
126, can be implemented in one or more computing devices that include one or
more circuits.
Thus, at least one of the elements in FIG. lA (or any subsequent figure) can
represent one or
more components of hardware. Or, at least one of the elements can represent a
portion of logic
including a portion of circuit configured to provide constituent structures
and/or functionalities.
According to some embodiments, the term "circuit" can refer, for example, to
any system
including a number of components through which current flows to perform one or
more
functions, the components including discrete and complex components. Examples
of discrete
components include transistors, resistors, capacitors, inductors, diodes, and
the like, and
examples of complex components include memory, processors, analog circuits,
digital circuits,
and the like, including field-programmable gate arrays ("FPGAs"), application-
specific
integrated circuits ("ASICs"). Therefore, a circuit can include a system of
electronic
components and logic components (e.g., logic configured to execute
instructions, such that a
group of executable instructions of an algorithm, for example, and, thus, is a
component of a
circuit). According to some embodiments, the term "module" can refer, for
example, to an
algorithm or a portion thereof, and/or logic implemented in either hardware
circuitry or software,
or a combination thereof (i.e., a module can be implemented as a circuit). In
some embodiments,
algorithms and/or the memory in which the algorithms are stored are
"components" of a circuit.
Thus, the term "circuit" can also refer, for example, to a system of
components, including
algorithms. These can be varied and are not limited to the examples or
descriptions provided.
FIGs. 1B to 1D illustrate examples of electrode arrays, according to some
embodiments.
Diagram 130 of FIG. 1B depicts an array 132 that includes sub-arrays 133a,
133b, and 133c of
electrodes 110 that are configured to generate data that represent one or more
characteristics
associated with a user associated with array 132. In various embodiments,
drive electrodes and
sink electrodes can be disposed in the same sub-array or in different sub-
arrays. Note that
arrangements of sub-arrays 133a, 133b, and 133c can denote physical or spatial
orientations and
need not imply electrical, magnetic, or cooperative relationships among
electrodes 110 within
each sub-array. For example, drive electrode ("D") 110f can be configured in
sub-array 133a as
a drive electrode to drive a signal to sink electrode ("S") 110g in sub-array
133b. As another
example, drive electrode ("D") 110h can be configured in sub-array 133a to
drive a signal to sink
electrode ("S") 110k in sub-array 133c. In some embodiments, distances between
electrodes 110
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
in sub-arrays can vary at different regions, including a region in which the
placement of
electrode group 134 near blood vessel 102 is more probable relative to the
placement of other
electrodes near blood vessel 102. Electrode group 134 can include a higher
density of electrodes
110 than other portions of array 132 as group 134 can be expected to be
disposed adjacent blood
vessel 102 more likely than other groups of electrodes 110. For example, an
elliptical-shaped
array (not shown) can be disposed in device 170 of FIG. 1A. Therefore, group
134 of electrodes
is disposed at a region 172 of FIG. 1A, which is likely adjacent either a
radial artery or an ulna
artery. While three sub-arrays are shown, more or fewer are possible.
Referring to FIG. 1C, diagram 140 depicts an array 142 oriented at any angle
("0") 144 to
an axial line coincident with or parallel to blood vessel 102. Therefore, an
array 142 of
electrodes need not be oriented orthogonally in each implementation; rather
array 142 can be
oriented at angles between 0 and 90 degrees, inclusive thereof In a specific
embodiment, an
array 146 can be disposed parallel (or substantially parallel) to blood vessel
102a (or a portion
thereof).
FIG. 1D is a diagram 150 depicting a wearable device 170a including a
helically-shaped
array 152 of electrodes disposed therein, whereby electrodes 110m and 110n can
be configured
as a pair of drive and sink electrodes. As shown, electrodes 110m and 110n
substantially align in
a direction parallel to an axis 151, which can represent a general direction
of blood flow through
a blood vessel.
FIG. 2 is a functional diagram depicting a physiological information generator
implemented in a wearable device, according to some embodiments. Functional
diagram 200
depicts a user 203 wearing a wearable device 209, which includes a
physiological information
generator 220 configured to generate signals including data representing
physiological
characteristics. As shown, sensor selector 222 is configured to select a
subset 205 of electrodes
or a subset 207 of electrodes. Subset 205 of electrodes includes electrodes
210c, 210d, and 210e,
and subset 207 of electrodes includes electrodes 210d and 210e. For purposes
of illustration,
consider that sensor selector 222 selects electrodes 210d and 210c as a subset
of electrodes with
which to capture physiological characteristics adjacent a target location.
Sensor selector 222
applies an AC signal, as a first signal, into electrodes 210d to generate a
sensor signal ("raw
sensor signal") 225, as a second signal, from electrode 210c. Sensor signal
222 includes a
motion-related signal component and a physiological-related signal component.
A motion
sensor 221 is configured to capture generate a motion artifact signal 223
based on motion data
representing motion experienced by wearable device 209 (or at least the
electrodes). A motion
artifact reduction unit 224 is configured to receive sensor signal 225 and
motion artifact signal
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
223. Motion artifact reduction unit 224 operates to subtract motion artifact
signal 223 from
sensor signal 225 to yield the physiological-related signal component (or an
approximation
thereof) as a raw physiological signal 227. In some examples, raw
physiological signal 227
represents an unamplified, unfiltered signal including data representative of
one or more
physiological characteristics. In some embodiments, motion sensor 221
generates motion
signals, such as accelerometer signals. These signals are provided to motion
artifact reduction
unit 224 (e.g., via dashed lines as shown), which, in turn, is configured to
determine motion
artifact signal 223. In some embodiments, motion artifact signal 223
represents motion included
or embodied within raw sensor signal 225 (e.g., with physiological signal(s)).
Thus, a motion
artifact signal can describe a motion signal, whether sensed by a motion
sensor or integrated with
one or more physiological signals. A physiological characteristic determinator
226 is configured
to receive raw physiological signal 227 to amplify and/or filter different
physiological signal
components from raw physiological signal 227. For example, raw physiological
signal 227 may
include a respiration signal modulated on (or in association with) a heart
rate ("HR") signal.
Regardless, physiological characteristic determinator 226 is configured to
perform digital signal
processing to generate a heart rate ("HR") signal 229a and/or a respiration
signal 229b. Portion
240 of respiration signal 229b represents an impedance signal due to cardiac
activity, at least in
some instances. Further, physiological characteristic determinator 226 is
configured to use either
HR signal 229a or a respiration signal 229b, or both, to derive other
physiological characteristics,
such as blood pressure data ("BP") 229c, a maximal oxygen consumption ("V02
max") 229d, or
any other physiological characteristic.
Physiological characteristic determinator 226 can derive other physiological
characteristics using other data generated or accessible by wearable device
209, such as the type
of activity the wear is engaged, environmental factors, such as temperature,
location, etc.,
whether the wearer is subject to any chronic illnesses or conditions, and any
other health or
wellness-related information. For example, if the wearer is diabetic or has
Parkinson's disease,
motion sensor 221 can be used to detect tremors related to the wearer's
ailment. With the
detection of small, but rapid movements of a wearable device that coincide
with a change in
heart rate (e.g., a change in an HR signal) and/or breathing, physiological
information generator
220 may generate data (e.g., an alarm) indicating that the wearer is
experiencing tremors. For a
diabetic, the wearer may experience shakiness because the blood-sugar level is
extremely low
(e.g., it drops below a range of 38 to 42 mg/di). Below these levels, the
brain may become
unable to control the body. Moreover, if the arms of a wearer shakes with
sufficient motion to
displace a subset of electrodes from being adjacent a target location, the
array of electrodes, as
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
described herein, facilitates continued monitoring of a heart rate by
repeatedly selecting subsets
of electrodes that are positioned optimally (e.g., adjacent a target location)
for receiving robust
and accurate physiological-related signals.
FIGs. 3A to 3C are cross-sectional views depicting arrays of electrodes
including subsets
of electrodes adjacent an arm portion of a wearer, according to some
embodiments. Diagram
300 of FIG. 3A depicts an array of electrodes arranged about, for example, a
wrist of a wearer.
In this cross-sectional view, an array of electrodes includes electrodes 310a,
310b, 310c, 310d,
310e, 310f, 310g, 310h, 310i, 310j, and 310k, among others, arranged about
wrist 303 (or the
forearm). The cross-sectional view of wrist 303 also depicts a radius bone
330, an ulna bone
332, flexor muscles/ligaments 306, a radial artery ("R") 302, and an ulna
artery ("U") 304.
Radial artery 302 is at a distance 301 (regardless of whether linear or
angular) from ulna artery
304. Distance 301 may be different, on average, for different genders, based
on male and female
anatomical structures. Notably, the array of electrodes can obviate specific
placement of
electrodes due to different anatomical structures based on gender, preference
of the wearer,
issues associated with contact (e.g., contact alignment), or any other issue
that affects placement
of electrode that otherwise may not be optimal. To effect appropriate
electrode selection, a
sensor selector, as described herein, can use gender-related information
(e.g., whether the wearer
is male or female) to predict positions of subsets of electrodes such that
they are adjacent (or
substantially adjacent) to one or more target locations 304a and 304b. Target
locations 304a and
304b represent optimal areas (or volumes) at which to measure, monitor and
capture data related
to bioimpedances. In particular, target location 304a represents an optimal
area adjacent radial
artery 302 to pick up bioimpedance signals, whereas target location 304b
represents another
optimal area adjacent ulna artery 304 to pick up other bioimpedance signals.
To illustrate the resiliency of a wearable device to maintain an ability to
monitor
physiological characteristics over one or more displacements of the wearable
device (e.g., around
or along wrist 303), consider that a sensor selector configures initially
electrodes 310b, 310d,
310f, 310h, and 310j as driver electrodes and electrodes 310a, 310c, 310e
310g, 310i, and 310k
as sink electrodes. Further consider that the sensor selector identifies a
first subset of electrodes
that includes electrodes 310b and 310c as a first optimal subset, and also
identifies a second
subset of electrodes that include electrodes 310f and 310g as a second optimal
subset. Note that
electrodes 310b and 310c are adjacent target location 304a and electrodes 310f
and 310g are
adjacent to target location 304b. These subsets are used to periodically (or
aperiodically)
monitor the signals from electrodes 310c and 310g, until the first and second
subsets are no
longer optimal (e.g., when movement of the wearable device displaces the
subsets relative to the
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
target locations). Note that the functionality of driver and sink electrodes
for electrodes 310b,
310c, 310f, and 310g can be reversed (e.g., electrodes 310a and 310g can be
configured as drive
electrodes).
FIG. 3B depicts an array of FIG. 3A being displaced from an initial position,
according to
some examples. In particular, diagram 350 depicts that electrodes 310f and
310g are displaced
to a location adjacent radial artery 302 and electrodes 310j and 310k are
displaced to a location
adjacent ulna artery 304. According to some embodiments, a sensor selector 322
is configured
to test subsets of electrodes to determine at least one subset, such as
electrodes 310f and 310,
being located adjacent to a target location (next to radial artery 302). To
identify electrodes 310f
and 310g as an optimal subset, sensor selector 322 is configured to apply
drive signals to the
drive electrodes to generate a number of data samples, such as data samples
307a, 307b, and
307c. In this example, each data sample represents a portion of a
physiological characteristic,
such as a portion of an HR signal. Sensor selector 322 operates to compare the
data samples
against a profile 309 to determine which of data samples 307a, 307b, and 307c
best fits or is
comparable to a predefined set of data represented by profile data 309.
Profile data 309, in this
example, represents an expected HR portion or thresholds indicating a best
match. Also, profile
data 309 can represent the most robust and accurate HR portion measured during
the sensor
selection mode relative to all other data samples (e.g., data sample 307a is
stored as profile data
309 until, and if, another data sample provides a more robust and/or accurate
data sample). As
shown, data sample 307a substantially matches profile data 309, whereas data
samples 307b and
307c are increasingly attenuated as distances increase away from radial artery
302. Therefore,
sensor selector 322 identifies electrodes 310f and 310g as an optimal subset
and can use this
subset in data capture mode to monitor (e.g., continuously) the physiological
characteristics of
the wearer. Note that the nature of data samples 307a, 307b, and 307c as
portions of an HR
signal is for purposes of explanation and is not intended to be limiting. Data
samples 307a,
307b, and 307c need not be portions of a waveform or signal, and need not be
limited to an HR
signal. Rather, data samples 307a, 307b, and 307c can relate to a respiration
signal, a raw sensor
signal, a raw physiological signal, or any other signal. Data samples 307a,
307b, and 307c can
represent a measured signal attribute, such as magnitude or amplitude, against
which profile data
309 is matched. In some cases, an optimal subset of electrodes can be
associated with a least
amount of impedance and/or reactance (e.g., over a period of time) when
applying a first signal
(e.g., a drive signal) to a target location.
FIG. 3C depicts an array of electrodes of FIG. 3A oriented differently due to
a change in
orientation of a wrist of a wearer, according to some examples. In this
example, the array of
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
electrodes is shown to be disposed in a wearable device 371, which has an
outer surface 374 and
an inner surface 372. In some embodiments, wearable device 371 can be
configured to "loosely
fit" around the wrist, thereby enabling rotation about the wrist. In some
cases, a portion of
wearable devices 371 (and corresponding electrodes 310a and 310b) are subject
to gravity ("G")
390, which pulls the portion away from wrist 303, thereby forming a gap 376.
Gap 376, in turn,
causes inner surface 372 and electrodes 310a and 310b to be displaced radially
by a radial
distance 392 (i.e., in a radial direction away from wrist 303). Gap 376, in
some cases, can be an
air gap. Radial distance 392, at least in some cases, may impact electrodes
310a and 310b and
the ability to receive signals adjacent to radial artery 302. Regardless,
electrodes 310f and 310g
are positioned in another portion of wearable device 371 and can be used to
receive signals
adjacent to ulna artery 304 in cooperation with, or instead of, electrodes
310a and 310b.
Therefore, electrodes 310f and 310g (or any other subset of electrodes) can
provide redundant
data capturing capabilities should other subsets be unavailable.
Next, consider that sensor selector 322 of FIG. 3B is configured to determine
a position
of electrodes 310f and 310g (e.g., on the wearable device 371) relative to a
direction of gravity
390. A motion sensor (not shown) can determine relative movements of the
position of
electrodes 310f and 310g over any number of movements in either a clockwise
direction
("dCW") or a counterclockwise direction ("dCCW"). As wearable device 371 need
not be
affixed firmly to wrist 303, at least in some examples, the position of
electrodes 310f and 310g
may "slip" relative to the position of ulna artery 304. In one embodiment,
sensor selector 322
can be configured to determine whether another subset of electrodes are
optimal, if electrodes
310f and 310g are displaced farther away than a more suitable subset. In
sensor selecting mode,
sensor selector 322 is configured to select another subset, if necessary, by
beginning the capture
of data samples at electrodes 310f and 310g and progressing to other nearby
subsets to either
confirm the initial selection of electrodes 310f and 310g or to select another
subset. In this
manner, the identification of the optimal subset may be determined in less
time than if the
selection process is performed otherwise (e.g., beginning at a specific subset
regardless of the
position of the last known target location).
FIG. 4 depicts a portion of an array of electrodes disposed within a housing
material of a
wearable device, according to some embodiments. Diagram 400 depicts electrodes
410a and
410b disposed in a wearable device 401, which has an outer surface 402 and an
inner surface
404. In some embodiments, wearable device 401 includes a material in which
electrodes 410a
and 410b can be encapsulated in a material to reduce or eliminate exposure to
corrosive elements
in the environment external to wearable device 401. Therefore, material 420 is
disposed between
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
the surfaces of electrodes 410a and 410b and inner surface 404. Driver
electrodes are
capacitively coupled to skin 405 to transmit high impedance signals, such as a
current signal,
over distance ("d") 422 through the material, and, optionally, through fabric
406 or hair into skin
405 of the wearer. Also, the current signal can be driven through an air gap
("AG") 424 between
inner surface 404 and skin 405. Note that in some implementations, electrodes
410a and 410b
can be exposed (or partially exposed) out through inner surface 404. In some
embodiments,
electrodes 410a and 410b can be coupled via conductive materials, such as
conductive polymers
or the like, to the external environment of wearable device 401.
FIG. 5 depicts an example of a physiological information generator, according
to some
embodiments. Diagram 500 depicts an array 501 of electrodes 510 that can be
disposed in a
wearable device. A physiological information generator can include one or more
of a sensor
selector 522, an accelerometer 540 for generating motion data, a motion
artifact reduction unit
524, and a physiological characteristic determinator 526. Sensor selector 522
includes a signal
controller 530, a multiplexer 501 (or equivalent switching mechanism), a
signal driver 532, a
signal receiver 534, a motion determinator 536, and a target location
determinator 538. Sensor
selector 522 is configured to operate in at least two modes. First, sensor
selector 522 can select a
subset of electrodes in a sensor select mode of operation. Second, sensor
selector 522 can use a
selected subset of electrodes to acquire physiological characteristics, such
as in a data capture
mode of operation, according to some embodiments. In sensor select mode,
signal controller 530
is configured to serially (or in parallel) configure subsets of electrodes as
driver electrodes and
sink electrodes, and to cause multiplexer 501 to select subsets of electrodes
510. In this mode,
signal driver 532 applies a drive signal via multiplexer 501 to a selected
subset of electrodes,
from which signal receiver 534 receives via multiplexer 501 a sensor signal.
Signal controller
530 acquires a data sample for the subset under selection, and then selects
another subset of
electrodes 510. Signal controller 530 repeats the capture of data samples, and
is configured to
determine an optimal subset of electrodes for monitoring purposes. Then,
sensor selector 522
can operate in the data capture mode of operation in which sensor selector 522
continuously (or
substantially continuously) captures sensor signal data from at least one
selected subset of
electrodes 501 to identify physiological characteristics in real time (or in
near real-time).
In some embodiments, a target location determinator 538 is configured to
initiate the
above-described sensor selection mode to determine a subset of electrodes 510
adjacent a target
location. Further, target location determinator 538 can also track
displacements of a wearable
device in which array 501 resides based on motion data from accelerometer 540.
For example,
target location determinator 538 can be configured to determine an optimal
subset if the initially-
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
selected electrodes are displaced farther away from the target location. In
sensor selecting mode,
target location determinator 538 can be configured to select another subset,
if necessary, by
beginning the capture of data samples at electrodes for the last known subset
adjacent to the
target location, and progressing to other nearby subsets to either confirm the
initial selection of
electrodes or to select another subset. In some examples, orientation of the
wearable device,
based on accelerometer data (e.g., a direction of gravity), also can be used
to select a subset of
electrodes 501 for evaluation as an optimal subset. Motion determinator 536 is
configured to
detect whether there is an amount of motion associated with a displacement of
the wearable
device. As such, motion determinator 536 can detect motion and generate a
signal to indicate
that the wearable device has been displaced, after which signal controller 530
can determine the
selection of a new subset that is more closely situated near a blood vessel
than other subsets, for
example. Also, motion determinator 536 can cause signal controller 530 to
disable data
capturing during periods of extreme motion (e.g., during which relatively
large amounts of
motion artifacts may be present) and to enable data capturing during moments
when there is less
than an extreme amount of motion (e.g., when a tennis player pauses before
serving). Data
repository 542 can include data representing the gender of the wearer, which
is accessible by
signal controller 530 in determining the electrodes in a subset.
In some embodiments, signal driver 532 may be a constant current source
including an
operational amplifier configured as an amplifier to generate, for example, 100
[LA of alternating
current ("AC") at various frequencies, such as 50 kHz. Note that signal driver
532 can deliver
any magnitude of AC at any frequency or combinations of frequencies (e.g., a
signal composed
of multiple frequencies). For example, signal driver 532 can generate
magnitudes (or
amplitudes), such as between 50 [LA and 200 IAA, as an example. Also, signal
driver 532 can
generate AC signals at frequencies from below 10 kHz to 550 kHz, or greater.
According to
some embodiments, multiple frequencies may be used as drive signals either
individually or
combined into a signal composed of the multiple frequencies. In some
embodiments, signal
receiver 534 may include a differential amplifier and a gain amplifier, both
of which can include
operational amplifiers.
Motion artifact reduction unit 524 is configured to subtract motion artifacts
from a raw
sensor signal received into signal receiver 534 to yield the physiological-
related signal
components for input into physiological characteristic determinator 526.
Physiological
characteristic determinator 526 can include one or more filters to extract one
or more
physiological signals from the raw physiological signal that is output from
motion artifact
reduction unit 524. A first filter can be configured for filtering frequencies
for example, between
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
0.8 Hz and 3 Hz to extract an HR signal, and a second filter can be configured
for filtering
frequencies between 0 Hz and 0.5 Hz to extract a respiration signal from the
physiological-
related signal component. Physiological characteristic determinator 526
includes a
biocharacteristic calculator that is configured to calculate physiological
characteristics 550, such
as V02 max, based on extracted signals from array 501.
FIG. 6 is an example flow diagram for selecting a sensor, according to some
embodiments. At 602, flow 600 provides for the selection of a first subset of
electrodes and the
selection of a second subset of electrodes in a select sensor mode. At 604,
one of the first and
second subset of electrodes is selected as a drive electrode and the other of
the first and second
subset of electrodes is selected as a sink electrode. In particular, the first
subset of electrodes
can, for example, include one or more drive electrodes, and the second subset
of electrodes can
include one or more sink electrodes. At 606, one or more data samples are
captured, the data
samples representing portions of a measured signal (or values thereof). Based
on a
determination that one of the data samples is indicative of a subset of
electrodes adjacent a target
location, the electrodes of the optimal subset are identified at 608. At 610,
the identified
electrodes are selected to capture signals including physiological-relate
components. While there
is no detected motion at 612, flow 600 moves to 616 to capture, for example,
heart and
respiration data continuously. When motion is detected at 612, data capture
may continue. But
flow 600 moves to 614 to determine whether to apply a predicted target
location. In some cases,
a predicted target location is based on the initial target location (e.g.,
relative to the initially-
determined subset of electrodes), with subsequent calculations based on
amounts and directions
of displacement, based on accelerometer data, to predict a new target
location. One or more
motion sensors can be used to determine the orientation of a wearable device,
and relative
movement of the same (e.g., over a period of time or between events), to
determine or predict a
target location. Or, the predicted target location can refer to the last known
target location and/or
subset of electrodes. At 618, electrodes are selected based on the predicted
target location for
confirming whether the previously-selected subset of electrodes are optimal,
or whether a new,
optimal subset is to be determined as flow 600 moves back to 602.
FIG. 7 is an example flow diagram for determining physiological
characteristics using a
wearable device with arrayed electrodes, according to some embodiments. At
702, flow 700
provides for the selection of a sensor in sensor select mode, the sensor
including, for example,
two or more electrodes. At 704, sensor signal data is captured in data capture
mode. At 706,
motion-related artifacts can be reduced or eliminated from the sensor signal
to yield a
physiological-related signal component. One or more physiological
characteristics can be
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
identified at 708, for example, after digitally processing the physiological-
related signal
component. At 710, one or more physiological characteristics can be calculated
based on the
data signals extracted at 708. Examples of calculated physiological
characteristics include
maximal oxygen consumption ("V02 max").
FIG. 8 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments. In some examples, computing platform 800
may be used
to implement computer programs, applications, methods, processes, algorithms,
or other
software to perform the above-described techniques. Computing platform 800
includes a bus
802 or other communication mechanism for communicating information, which
interconnects
subsystems and devices, such as processor 804, system memory 806 (e.g., RAM,
etc.), storage
device 808 (e.g., ROM, etc.), a communication interface 813 (e.g., an Ethernet
or wireless
controller, a Bluetooth controller, etc.) to facilitate communications via a
port on communication
link 821 to communicate, for example, with a computing device, including
mobile computing
and/or communication devices with processors. Processor 804 can be implemented
with one or
more central processing units ("CPUs"), such as those manufactured by Intel
Corporation, or
one or more virtual processors, as well as any combination of CPUs and virtual
processors.
Computing platform 800 exchanges data representing inputs and outputs via
input-and-output
devices 801, including, but not limited to, keyboards, mice, audio inputs
(e.g., speech-to-text
devices), user interfaces, displays, monitors, cursors, touch-sensitive
displays, LCD or LED
displays, and other I/O-related devices.
According to some examples, computing platform 800 performs specific
operations by
processor 804 executing one or more sequences of one or more instructions
stored in system
memory 806, and computing platform 800 can be implemented in a client-server
arrangement,
peer-to-peer arrangement, or as any mobile computing device, including smart
phones and the
like. Such instructions or data may be read into system memory 806 from
another computer
readable medium, such as storage device 808. In some examples, hard-wired
circuitry may be
used in place of or in combination with software instructions for
implementation. Instructions
may be embedded in software or firmware. The term "computer readable medium"
refers to any
tangible medium that participates in providing instructions to processor 804
for execution. Such
a medium may take many forms, including but not limited to, non-volatile media
and volatile
media. Non-volatile media includes, for example, optical or magnetic disks and
the like.
Volatile media includes dynamic memory, such as system memory 806.
Common forms of computer readable media includes, for example, floppy disk,
flexible
disk, hard disk, magnetic tape, any other magnetic medium, CD-ROM, any other
optical
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
medium, punch cards, paper tape, any other physical medium with patterns of
holes, RAM,
PROM, EPROM, FLASH-EPROM, any other memory chip or cartridge, or any other
medium
from which a computer can read. Instructions may further be transmitted or
received using a
transmission medium. The term "transmission medium" may include any tangible
or intangible
medium that is capable of storing, encoding or carrying instructions for
execution by the
machine, and includes digital or analog communications signals or other
intangible medium to
facilitate communication of such instructions. Transmission media includes
coaxial cables,
copper wire, and fiber optics, including wires that comprise bus 802 for
transmitting a computer
data signal.
In some examples, execution of the sequences of instructions may be performed
by
computing platform 800. According to some examples, computing platform 800 can
be coupled
by communication liffl( 821 (e.g., a wired network, such as LAN, PSTN, or any
wireless
network) to any other processor to perform the sequence of instructions in
coordination with (or
asynchronous to) one another. Computing platform 800 may transmit and receive
messages,
data, and instructions, including program code (e.g., application code)
through communication
liffl( 821 and communication interface 813. Received program code may be
executed by
processor 804 as it is received, and/or stored in memory 806 or other non-
volatile storage for
later execution.
In the example shown, system memory 806 can include various modules that
include
executable instructions to implement functionalities described herein. In the
example shown,
system memory 806 includes a physiological information generator module 854
configured to
implement determine physiological information relating to a user that is
wearing a wearable
device. Physiological information generator module 854 can include a sensor
selector module
856, a motion artifact reduction unit module 858, and a physiological
characteristic determinator
859, any of which can be configured to provide one or more functions described
herein.
FIG. 9 depicts the physiological signal extractor, according to some
embodiments.
Diagram 900 depicts a motion artifact reduction unit 924 including a
physiological signal
extractor 936. In some embodiments, motion artifact reduction unit 924 can be
disposed in or
attached to a wearable device 909, which can be configured to attached to or
otherwise be worn
by user 903. As shown, user 903 is running or jogging, whereby movement of the
limbs of user
903 imparts forces that cause wearable device 909 to experience motion. Motion
artifact
reduction unit 924 is configured to receive a sensor signal ("Raw Sensor
Signal") 925, and is
further configured to reduce or negate motion artifacts accompanying, or mixed
with,
physiological signals due to motion-related noise that otherwise affects
sensor signal 925.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
Further to diagram 900, a signal receiver 934 is coupled to a sensor
including, for example, one
or more electrodes. Examples of such electrodes include electrode 910a and
electrode 910b. In
some embodiments, signal receiver 934 includes similar structure and/or
functionality as signal
receiver 534 of FIG. 5. In operation, signal receiver 934 is configured to
receive one or more
AC current signals, such as high impedance signals, as bioimpedance-related
signals. Signal
receiver 934 can include differential amplifiers, gain amplifiers, or any
other operational
amplifier configured to receive, adapt (e.g., amplify), and transmit sensor
signal 925 to motion
artifact reduction unit 924.
In some embodiments, signal receiver 934 is configured to receive electrical
signals
representing acoustic-related information from a microphone 911. An example of
the acoustic-
related information includes data representing a heartbeat or a heart rate as
sensed by
microphone 911, such that sensor signal 925 can be an electrical signal
derived from acoustic
energy associated with a sensed physiological signal, such as a pulse wave or
heartbeat.
Wearable device 909 can include microphone 911 configured to contact (or to be
positioned
adjacent to) the skin of the wearer, whereby microphone 911 is adapted to
receive sound and
acoustic energy generated by the wearer (e.g., the source of sounds associated
with physiological
information). Microphone 911 can also be disposed in wearable device 909.
According to some
embodiments, microphone 911 can be implemented as a skin surface microphone
("SSM"), or a
portion thereof, according to some embodiments. An SSM can be an acoustic
microphone
configured to enable it to respond to acoustic energy originating from human
tissue rather than
airborne acoustic sources. As such, an SSM facilitates relatively accurate
detection of
physiological signals through a medium for which the SSM can be adapted (e.g.,
relative to the
acoustic impedance of human tissue). Examples of SSM structures in which
piezoelectric
sensors can be implemented (e.g., rather than a diaphragm) are described in
U.S. Patent
Application No. 11/199,856, filed on August 8, 2005, and U.S. Patent
Application No.:
13/672,398, filed on November 8, 2012, both of which are incorporated by
reference. As used
herein, the term human tissue can refer to, at least in some examples, as
skin, muscle, blood, or
other tissue. In some embodiments, a piezoelectric sensor can constitute an
SSM. Data
representing sensor signal 925 can include acoustic signal information
received from an SSM or
other microphone, according to some examples.
According to some embodiments, physiological signal extractor 936 is
configured to
receive sensor signal 925 and data representing sensing information 915 from
another, secondary
sensor 913. In some examples, sensor 913 is a motion sensor (e.g., an
accelerometer) configured
to sense accelerations in one or more axes and generates motion signals
indicating an amount of
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
motion and/or acceleration. Note, however, that sensor 913 need not be so
limited and can be
any other sensor. Examples of suitable sensors are disclosed in U.S. Non-
Provisional Patent
Application Serial No. 13/492,857, filed on June 9, 2012, which is
incorporated by reference.
Further, physiological signal extractor 936 is configured to operate to
identify a pattern (e.g., a
motion "signature"), based on motion signal data generated by sensor 913, that
can used to
decompose sensor signal 925 into motion signal components 937a and
physiological signal
components 937b. As shown, motion signal components 937a and physiological
signal
components 937b can correspondingly be used by motion artifact reduction unit
924, or any
other structure and/or function described herein, to form motion data 930 and
one or more
physiological data signals, such as physiological characteristic signals 940,
942, and 944.
Physiological characteristic determinator 926 is configured to receive
physiological signal
components 937b of a raw physiological signal, and to filter different
physiological signal
components to form physiological characteristic signal(s). For example,
physiological
characteristic determinator 926 can be configured to analyze the physiological
signal
components to determine a physiological characteristic, such as a heartbeat,
heart rate, pulse
wave, respiration rate, a Mayer wave, and other like physiological
characteristic. Physiological
characteristic determinator 926 is also configured to generate a physiological
characteristic
signal that includes data representing the physiological characteristic during
one or more
portions of a time interval during which motion is present. Examples of
physiological
characteristic signals include data representing one or more of a heart rate
940, a respiration rate
942, Mayer wave frequencies 944, and any other sensed characteristic, such as
a galvanic skin
response ("GSR") or skin conductance. Note that the term "heart rate" can
refer, at least in some
embodiments, to any heart-related physiological signal, including, but not
limited to, heart beats,
heart beats per minute ("bpm"), pulse, and the like. In some examples, the
term "heart rate" can
refer also to heart rate variability ("HRV"), which describes the variation of
a time interval
between heartbeats. HRV describes a variation in the beat to beat interval and
can be described
in terms of frequency components (e.g., low frequency and high frequency
components), at least
in some cases.
In view of the foregoing, the functions and/or structures of motion artifact
reduction unit
924, as well as its components and/or neighboring components, can facilitate
the extraction and
derivation of physiological characteristics in situ¨during which a user is
engaged in physical
activity that imparts motion on a wearable device, whereby biometric sensors,
such as electrodes,
may receive bioimpedance sensor signals that are exposed to, or include,
motion-related artifacts.
For example, physiological signal extractor 936 can be configured to receive
the sensor signal
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
that includes data representing physical physiological characteristics during
one or more portions
of the time interval in which the wearable devices is in motion. A user 903
need not be required
to remain immobile to determine physiological signal characteristic signals.
Therefore, user 903
can receive heart rate information, respiration information, and other
physiological information
during physical activity or during periods of time in which user 903 is
substantially or relatively
active. Further, according to various embodiments, physiological signal
extractor 936 facilitates
the sensing of physiological characteristic signals at a distal end of a limb
or appendage, such as
at a wrist, of user 903. Therefore, various implementations of motion artifact
reduction unit 924
can enable the detection of physiological signal at the extremities of user
903, with minimal or
reduced effects of motion-related artifacts and their influence on the desired
measured
physiological signal. By facilitating the detection of physiological signals
at the extremities,
wearable device 909 can assist user 903 to detect oncoming ailments or
conditions of the
person's body (e.g., oncoming tremors, states of sleep, etc.) relative to
other portions of the
person's body, such as proximal portions of a limb or appendage.
In accordance with some embodiments, physiological signal extractor 936 can
include an
offset generator, which is not shown. An offset generator can be configured to
determine an
amount of motion that is associated with the motion sensor signal, such as an
accelerometer
signal, and to adjust the dynamic range of operation of an amplifier, where
the amplifier is
configured to receive a sensor signal responsive to the amount of motion. An
example of such
an amplifier is an operational amplifier configured as a front-end amplifier
to enhance, for
example, the signal-to-noise ratio. In situations in which the motion related
artifacts induce a
rapidly-increasing amplitude onto the sensor signal, the amplifier may drive
into saturation,
which, in turn, causes clipping of the output of the amplifier. The offset
generator also is
configured to apply in offset value to an amplifier to modify the dynamic
range of the amplifier
so as to reduce or negate large magnitudes of motion artifacts that may
otherwise influence the
amplitude of the sensor signal. Examples of an offset generator are described
in relation to FIG.
12. In some embodiments, physiological signal extractor 936 can include a
window validator
configured to determine durations (i.e., a valid window of time) in which
sensor signal data can
be predicted to be valid (i.e., durations in which the magnitude of motion-
related artifacts signals
likely do not influence the physiological signals). An example of a window
validator is
described in FIG. 11.
FIG. 10 is a flowchart for extracting a physiological signal, according to
some
embodiments. At 1002, a motion sensor signal is correlated to a sensor signal,
which includes
one or more physiological characteristic signals and one or more motion-
related artifact signals.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
In some examples, correlating motion sensor signals to bioimpedance signals
enables the two
signals to be compared against each other, whereby motion-related artifacts
can be subtracted
from the bioimpedance signals to extract a physiological characteristic
signal. In at least one
embodiment, data correlation at 1002 can be performed to include scaling data
that represents a
motion sensor signal, whereby the scaling facilitates making values for the
data representing
sensor signal equivalent so that they can be compared against each other
(e.g., to facilitate
subtracting one signal from the other). At 1004, a sensor signal is decomposed
to extract one or
more physiological signals and one or more motion sensor signals, thereby
separating
physiological signals from the motion signals. The extracted physiological
signal is analyzed at
1006. In some examples, the frequency of the extracted physiological signal is
analyzed to
identify a dominant frequency component or predominant frequency components.
Also, such an
analysis at 1006 can also determine power spectral densities of the
physiological extract
physiological signal. At 1008, the relevant components of the physiological
signal can be
identified, based on the determination of the predominant frequency
components. At 1010, at
least one physiological signal is generated, such as a heart rate signal, a
respiration signal, or a
Mayer wave signal. These signals each can be associated with one or more
corresponding
dominant frequency component that are used to form the one or more
physiological signals.
FIG. 11 is a block diagram depicting an example of a physiological signal
extractor,
according to some embodiments. Diagram 1100 depicts a physiological signal
extractor 1136
that includes a stream selector 1140, a data correlator 1142, an optional
window validator 1143,
a parameter estimator 1144, and a separation filter 1146. Physiological signal
extractor 1136 can
also include an optional offset generator 1139 to be discussed later. As shown
in FIG. 11,
physiological signal extractor 1136 receives a raw sensor signal from, for
example, a
bioimpedance sensor, and also receives one or more motion sensor signals 1143
from a motion
sensor 1141, which can include one or more accelerometers in some examples.
Multiple data
streams can represent accelerometer data in multiple axes. Stream selector
1140 is configured to
receive, for example, multiple accelerometer signals specifying motion along
one or more
different axes. Further, stream selector 1140 is configured to select an
accelerometer data stream
having a greatest motion component (e.g., the greatest magnitude of
acceleration for an axis). In
some examples, stream selector 1140 is configured to select the axis of
acceleration having the
highest variability in motion, whereby that axis can be used to track motion
or identify a general
direction or plane of motion. Optionally, offset generator 1139 can receive a
magnitude of the
raw sensor signal to modify the dynamic range of an amplifier receiving the
raw sensor signal
prior to that signal entering data correlator 1142.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
Data correlator 1142 is configured to receive the raw sensor signal and the
selected
stream of accelerometer data. Data correlator 1142 operates to correlate the
sensor signal and the
selected motion sensor signal. For example, data correlator 1142 can scale the
magnitudes of the
selected motion sensor signal to an equivalent range for the sensor signal. In
some
embodiments, data correlator 1142 can provide for the transformation of the
signal data between
the bioimpedance sensor signal space and the acceleration data space. Such a
transformation can
be optionally performed to make the motion sensor signals, especially the
selected motion sensor
signal, equivalent to the bioimpedance sensor signal. In some examples, a
cross-correlation
function or an autocorrelation function can be implemented to correlate the
sets of data
representing the motion sensor signal and the sensor signal.
Parameter estimator 1144 is configured to receive the selected motion sensor
signal from
stream selector 1140 and the correlated data signal from data correlator 1142.
In some examples,
parameter estimator 1144 is configured to estimate parameters, such as
coefficients, for filtering
out physiological characteristic signals from motion-related artifact signals.
For example, the
selected motion sensor signal, such as accelerometer signal, generally does
not include biological
derived signal data, and, as such, one or more coefficients for physiological
signal components
can be reduced or effectively determined to be zero. Separation filter 1146 is
configured to
receive the coefficients as well as data correlated by data correlator 1142
and the selected motion
sensor signal from stream selector 1140. In operation, separation filter 1146
is configured to
recover the sources of the signals. For example, separation filter 1146 can
generate a recovered
physiological characteristic signal ("P") 1160 and a recovered motion signal
("M") 1162.
Separation filter 1146, therefore, operates to separate a sensor signal
including both biological
signals and motion-related artifact signals into additive or subtractable
components. Recovered
signals 1160 and 1162 can be used to further determine one or more
physiological characteristics
signals, such as a heart rate, respiration rate, and a Mayer wave.
Window validator 1143 is optional, according to some embodiments. Window
validator
1143 is configured to receive motion sensor signal data to determine a
duration time (i.e., a valid
window of time) in which sensor signal data can be predicted to be valid
(i.e., durations in which
the magnitude of motion-related artifacts signals likely do not affect the
physiological signals).
In some cases, window validator 1143 is configured to predict a saturation
condition for a front-
end amplifier (or any other condition, such as a motion-induced condition),
whereby the sensor
signal data is deemed invalid.
FIG. 12 depicts an example of an offset generator according to some
embodiments.
Diagram 1200 depicts offset generator 1239 including a dynamic range
determinator 1240 and
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
an optional amplifier 1242, which can be disposed within or without offset
generator 1239. In
sensing bioimpedance-related signals, the bioimpedance signals generally are
"small-signal;"
that is, these signals have relatively small amplitudes that can be distorted
by changes in
impedances, such as when the coupling between the electrodes and the skin is
disrupted. Offset
generator 1239 can be configured to determine an amount of motion that is
associated with
motion sensor signal ("M") 1260, such as an accelerometer signal, and to
adjust the dynamic
range of operation of amplifier 1242, which can be an operational amplifier
configured as a
front-end amplifier. Further, offset generate 1239 can also be optionally
configured to receive
sensor signal ("S") 1262 and correlated data ("CD") 1264, either or both of
which can be used to
determine first whether to modify the dynamic range of amplifier 1242, and if
so, to what degree
to which the dynamic range ought to be modified. In some cases, the degree to
which the
dynamic range ought to be modified specified by an offset value. As shown,
amplifier 1242 is
configured to generate an offset sensor signal that is conditioned or
otherwise adapted to avoid or
reduce clipping.
FIG. 13 is a flowchart depicting example of a flow for decomposing a sensor
signal to
form separate signals, according to some embodiments. Flow 1300 can be
implemented in a
variety of different ways using a number of different techniques. In some
examples, flow 1300
and its elements can be implemented by one or more of the components or
elements described
herein, according to various embodiments. In the following example, while not
intended to be
limiting, flow 1300 is described in terms of an analysis for extracting
physiological characteristic
signals in accordance with one or more techniques of performing Independent
Component
Analysis ("ICA"). At 1302, a sensor signal is received, and at 1304 a motion
sensor signal is
selected. When a test subject, or user, is wearing a wearable device and is
physically active, the
received bioimpedance signal can include two signals: 1.) a sensor signal
including one or more
physiological signals such as heart rate, respiration rate, and Mayer waves,
and 2.) motion-
related artifact signals. Further, the one or more physiological signals and
motion sensor signals
(or motion-related artifact signals) may be correlated at 1305. In this
example, a physiological
signal is assumed to be statistically independent (or nearly statistically
independent) of a motion
sensor signal or related artifacts. In some examples, flow 1300 provides for
separating a
multivariate signal into additive or subtractive subcomponents, based on a
presumed mutually-
statistical independence between non-Gaussian source signals. Statistical
independence of
estimated physiological sample components and motion related artifact signal
components can
be maximized based on for example minimizing mutual information, and
maximizing non-
Gaussianity of the source signals.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
Further to flow 1300, consider two statistically independent noun Gaussian
source signals
Si and S2, and two observation points 01 and 02. In some examples, observation
points 01(t)
and 02(t) are time-indexed samples associated with observed samples from the
same sensor, at
different locations. For example, 01(t) and 02(t) can represent observed
samples from a first
bioimpedance sensor (or electrode) and from a second bioimpedance sensor (or
electrode),
respectively. In other examples, 01(t) and 02(t) can represent observed
samples from a first
sensor, such as a bioimpedance sensor, and a second sensor, such as an
accelerometer,
respectively. At 1306, data associated with one or more of the two observation
points 01 and
02 are preprocessed. For example, the data for the observation points can be
centered, whitened,
and/or reduced in dimensions, wherein preprocessing may reduce the complexity
of determining
the source signals and/or reduce the number of parameters or coefficients to
be estimated. An
example of a centering process includes subtracting the meaning of data from a
sample to
translate samples about a center. An example of a whitening process is
eigenvalue
decomposition. In some embodiments, preprocessing at 1306 can be different
from, or similar
to, the correlation of data as described herein, at least in some cases.
Observation points 01(t) and 02(t) can be expressed as follows:
04 = ai15 a12S2 (Eqn11)
= ci2151 a-.252 (Eqn. 2)
where 0 = A xS, which represent matrices, and all, a12, a21, and a22 represent
parameters (or
coefficients) that can be estimated. At 1308, the above equations 1 and 2 can
be used to
determine components for generating two (2) statistically-independent source
signals, whereby A
and S can be extracted from 0. In some examples, A and S can be extracted
iteratively, based on
user-specified error rate and/or maximum number of iterations, among other
things. Further,
coefficients all, a12, a21, and a22 can be modified such that one or more
coefficients for the
physiological characteristic and biological component is set to or near zero,
as the accelerometer
signal generally does not include physiological signals. In at least one
embodiment, parameter
estimator 1144 of FIG. 11 can be configured to determine estimated
coefficients.
In some examples a matrix can be formed based on estimated coefficients, at
1308. At
least some of the coefficients are configured to attenuate values of the
physiological signal
components for the motion sensor signal. An example of the matrix is a mixing
matrix. Further,
the matrix of coefficients can be inverted to form an inverted mixing matrix
(e.g., to form an
"unmixing" matrix). The inverted mixing matrix of coefficients can be applied
(e.g., iteratively)
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
to the samples of observation points 01(t) and 02(t) to recover the source
signals, such as a
recovered physiological characteristic signal and a recovered motion signal
(e.g. a recovered
motion-related artifact signal). In at least one embodiment, separation filter
1146 of FIG. 11 can
be configured to apply an inverted matrix to samples of the physiological
signal components and
the motion signal components to determine the recovered physiological
characteristic signal and
the recovered motion signal (e.g., a recovered muscle movement signal). Note
that various
described functionalities of flow 1300 can be implemented in or distributed
over one or more of
the described structures set forth herein. Note, too, that while flow 1300 is
described in terms of
ICA in the above-mentioned examples, flow 1300 can be implemented using
various techniques
and structures, and the various embodiments are neither restricted nor limited
to the use of ICA.
Other signal separation processes may also be implemented, according to
various embodiments.
FIGs. 14A to 14C depict various signals used for physiological characteristic
signal
extraction, according to various embodiments. FIG. 14A depicts a sensor signal
received as, for
example, a bioimpedance signal in which the magnitude varies about 20 over a
number of
samples. In this example, validation window can be used for heart rate
extraction, whereby the
sensor signal is down-sampled by, for example, a factor of 100 (i.e., the
sensor signal is sampled
at, for example, 15.63 Hz). Also shown in FIG. 14A is an optional window 1402
that indicates a
validation window in which data is deemed valid as determined by, for example,
window
validator 1143 of FIG. 11. Returning back to FIGs. 14A to 14C, FIG. 14B
depicts a first stream
of accelerometer data for a first axis. FIG. 14C and FIG. 14D depict a second
stream of
accelerometer data for a second axis and a third stream of accelerometer data
for a third axis,
respectively. FIGs. 14A to 14C are intended to depict only a few of many
examples and
implementations.
FIG. 15 depicts recovered signals, according to some embodiments. Diagram 1500
depicts the magnitudes of various signals over 160 samples. Signal 1502
represents us
magnitude of the sensor signal, whereas signal 1504 represents the magnitude
of an
accelerometer signal. Signals 1506, 1508, and 1510 represent the magnitudes of
a first of
accelerometer signal, a second accelerometer signal, and a third accelerometer
signal,
respectively.
FIG. 16 depicts an extracted physiological signal, according to various
embodiments.
Diagram 1600 depicts the magnitude, in volts, of an extracted physiological
characteristic signal
using the first accelerometer stream as the selected accelerometer stream. For
this example, a
fast Fourier transform ("FFT") analysis of the data set forth in FIG. 16
yields a heart rate
estimated at, for example, 77.6274 bpm.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
FIG. 17 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments. In some examples, computing platform 1700
may be
used to implement computer programs, applications, methods, processes,
algorithms, or other
software to perform the above-described techniques, and can include similar
structures and/or
functions as set forth in FIG. 8. But in the example shown, system memory 806
can include
various modules that include executable instructions to implement
functionalities described
herein. In the example shown, system memory 806 includes a motion artifact
reduction unit
module 1758 configured to determine physiological information relating to a
user that is wearing
a wearable device. Motion artifact reduction unit module 1758 can include a
stream selector
module 1760, a data correlator module 1762, a coefficient estimator module
1764, and a mix
inversion filter module 1766, any of which can be configured to provide one or
more functions
described herein.
FIG. 18 is a diagram depicting a physiological state determinator configured
to receive
sensor data originating, for example, at a distal portion of a limb, according
to some
embodiments. As shown, diagram 1800 depicts a physiological information
generator 1810 and
a physiological state determinator 1812, which, at least in the example shown,
are configured to
be disposed at, or receive signals from, at a distal portion 1804 of a user
1802. In some
embodiments, physiological information generating 1810 and physiological state
determinator
1812 are disposed in a wearable device (not shown). Physiological information
generator 1810
configured to receive signals and/or data from one or more physiological
sensors and one or
more motion sensors, among other types of sensors. In the example shown,
physiological
information generator 1810 is configured to receive a raw sensor signal 1842,
which can be
similar or substantially similar to other raw sensor signals described herein.
Physiological
information generator 1810 is also configured to receive other sensor signals
including
temperature ("TEMP") 1840, skin conductance (depicted as GSR data signal
1847), pulse waves,
heat rates (e.g., heart beats-per-minute), respiration rates, heart rate
variability, and any other
sensed signal configured to include physiological information or any other
information relating
to the physiology of a person. Examples of other sensors are described in U.S.
Patent
Application No. 13/454,040, filed on April 23, 2012, which is incorporated by
reference.
Physiological information generator 1810 is also configured to receive motion
("MOT") signal
data 1844 from one or more motion sensor(s), such as accelerometers. Note that
raw sensor
signal 1842 can be an electrical signal, such as a bioimpedance signal, or an
acoustic signal, or
any other type of signal. According to some embodiments, physiological
information generator
1810 is configured to extract physiological signals from a raw sensor signal
1842. For example,
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
a heart rate ("HR") signal and/or heart rate variability ("HRV") signal 1845
and respiration rate
("RESP") 1846 can be determined for example, by a motion artifact reduction
unit (not shown).
Physiological information generator 1810 is configured to convey sensed
physiological
characteristics signals or derive physiological characteristic signals (e.g.,
from sensed signals) for
use by physiological state determinator 1812. In some examples, a
physiological characteristic
signal can include electrical impulses of muscles (e.g., as evidenced, in some
cases, by
electromyography ("EMG") to determine the existence and/or amounts of motion
based on
electrical signals generated by muscle cells at rest or in contraction.
As shown, physiological state determinator 1812 includes a sleep manager 1814,
an
anomalous state manager 1816, and an affective state manager 1818.
Physiological state
determinator 1812 is configured to receive various physiological
characteristics signals and to
determine a physiological state of a user, such as user 1802. Physiological
states include, but are
not limited to, states of sleep, wakefulness, a deviation from a normative
physiological state (i.e.,
an anomalous state), an affective state (i.e., mood, feeling, emotion, etc.).
Sleep manager 1814 is
configured to detect a stage of sleep as a physiological state, the stages of
sleep including REM
sleep and non-REM sleep, including as light sleep and deep sleep. Sleep
manager 1814 is also
configured to predict the onset or change into or between different stages of
sleep, even if such
changes are imperceptible to user 1802. Sleep manager 1814 can detect that
user 1802 is
transitioning from a wakefulness state to a sleep state and, for example, can
generate a vibratory
response (i.e., generated by vibration) or any other alert to user 1802. Sleep
manager 1814 also
can predict a sleep stage transition to either alert user 1802 or to disable
such an alert if, for
example, the alert is an alarm (i.e., wake-up time alarm) that coincides with
a state of REM
sleep. By delaying generation of an alarm, the user 1802 is permitted to
complete of a state of
REM sleep to ensure or enhance the quality of sleep. Such an alert can assist
user 1802 to avoid
entering a sleep state from a wakefulness state during critical activities,
such as driving.
Anomalous state manager 1860 is configured to detect a deviation from the
normative
general physiological state in reaction, for example, to various stimuli, such
as stressful
situations, injuries, ailments, conditions, maladies, manifestations of an
illness, and the like.
Anomalous state manager 1860 can be configured to determine the presence of a
tremor that, for
example, can be a manifestation of an ailment or malady. Such a tremor can be
indicative of a
diabetic tremor, an epileptic tremor, a tremor due to Parkinson's disease, or
the like. In some
embodiments, anomalous state manager 1860 is configured to detect the onset of
tremor related
to a malady or condition prior to user 1802 perceiving or otherwise being
aware of such a
tremor. Therefore, anomalous state manager 1860 can predict the onset of a
condition that may
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
be remedied by, for example, medication and can alert user 1802 to the
impending tremor. User
1802 then can take the medication before the intensity of the tremor increases
(e.g., to an
intensity that might impair or otherwise incapacitate user 1802). Further,
anomalous state
manager 1860 can be configured to determine if the physiological state of user
1802 is a pain
state, in which user 1802 is experiencing pain. Upon determining a pain state,
a wearable device
(not shown) can be configured to transmit the presence of pain to a third-
party via a wireless
communication path to alert others of the pain state for resolution.
Affective state manager 1818 is configured to use at least physiological
sensor data to
form affective state data representing an approximate affective state of user
1802. As used
herein, the term "affective state" can refer, at least in some embodiments, to
a feeling, a mood,
and/or an emotional state of a user. In some cases, affective state data can
includes data that
predicts an emotion of user 1802 or an estimated or approximated emotion or
feeling of user
1802 concurrent with and/or in response to the interaction with another
person, environmental
factors, situational factors, and the like. In some embodiments, affective
state manager 1818 is
configured to determine a level of intensity based on sensor derived values
and to determine
whether the level of intensity is associated with a negative affectivity
(e.g., a bad mood) or
positive affectivity (e.g., a good mood). An example of an affective state
manager 1818 is an
affective state prediction unit as described in U.S. Provisional Patent
Application Number
61/705,598 filed on September 25, 2012, which is incorporated by reference
herein for all
purposes. While affective state manager 1818 is configured to receive any
number of
physiological characteristics signals in which to determine of an affective
state of user 1802,
affective state manager 1818 can use sensed and/or derived Mayer waves based
on raw sensor
signal 1842. In some examples, the detected Mayer waves can be used to
determine heart rate
variability ("HRV") as heart rate variability can be correlated to Mayer
waves. Further, affective
state manager 1818 can use, at least in some embodiments, HRV to determine an
affective state
or emotional state of user 1802 as HRV may correlate with an emotion state of
user 1802. Note
that, while physiological information generating 1810 and physiological state
determinator 1812
are described above in reference to distal portion 1804, one or more of these
elements can be
disposed at, or receive signals from, proximal portion 1806, according to some
embodiments.
FIG. 19 depicts a sleep manager, according to some embodiments. As shown, FIG.
19
depicts a sleep manager 912 including a sleep predictor 1914. Sleep manager
1912 is configured
to determine physiological states of sleep, such as a sleep state or a
wakefulness state in which
the user is awake. Sleep manager 1912 is configured to receive physiological
characteristic
signals, such as data representing respiration rates ("RESP") 1901, heart rate
("HR") 1903 (or
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
heart rate variability, HRV), motion-related data 1905, and other
physiological data such as
optional skin conductance ("GSR") 1907 and optional temperature ("TEMP")1909,
among
others. As shown in diagram 1940, a person who is sleeping passes through one
or more sleep
cycles over a duration 1951 between a sleep start time 1950 and sleep end time
1952. There is a
general reduction of motion when a person passes from a wakefulness state 1942
into the stages
of sleep, such as into light sleep 1946 in duration 1954. Motion indicative of
"hypnic jerks" or
involuntary muscle twitching motions typically occur during light sleep state
1946. The person
then passes into a deep sleep state 1948, in which, a person has a decreased
heart rate and body
temperature, with the absence of voluntary muscle motions to confirm or
establish that a user is
in a deep sleep state. Collectively, the light sleep state and the deep sleep
state can be described
as non-REM sleep states. Further to diagram 1940, the sleeping person then
passes into an REM
sleep state 1944 for duration 1953 during which muscles can be immobile.
According to some embodiments, sleep manager 1912 is configured to determine a
stage
of sleep based on at least the heart rate and respiration rate. For example,
sleep manager 1912
can determine the regularity of the heart rate and respiration rate to
determine the person is in a
non-REM sleep state, and, thereby, can generate a signal indicating the stage
of the sleep is a
non-REM sleep states, such as light sleep or deep sleep states. During light
sleep and deep sleep,
a heart rate and/or the respiration rate of the user can be described as
regular or without
significant variability. Thus, the regularity of the heart rate and/or
respiration rate can be used to
determine physiological sleep state of the user. In some examples the
regularity of the heart rate
and/or the respiration rate can include any heart rate or respiration rate
that varies by no more
than 5%. In some other cases, the regularity of the heart rate and/or the
respiration rate can vary
by any amount up to 15%. These percentages are merely examples and are not
intended to be
limiting, and ordinarily skilled artisan will appreciate that the tolerances
for regular heart rates
and respiration rates may be based on user characteristics, such as age, level
of fitness, gender
and the like. Sleep manager 1912 can use motion data 1905 to confirm whether a
user is in a
light sleep state or a deep sleep state by detecting indicative amounts of
motion, such as a portion
of motion that is indicative of involuntary muscle twitching.
As another example, sleep manager 1912 can determine the irregularity (or
variability) of
the heart rate and respiration rate to determine the person is in an REM sleep
state, and, thereby,
can generate a signal indicating the stage of the sleep is an REM sleep
states. During REM
sleep, a heart rate and/or the respiration rate of the user can be described
as irregular or with
sufficient variability to identify that a user is REM sleep. Thus, the
variability of the heart rate
and/or respiration rate can be used to determine physiological sleep state of
the user. In some
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
examples the irregularity of the heart rate and/or the respiration rate can
include any heart rate or
respiration rate that varies by more than 5%. In some other cases, the
variability of the heart rate
and/or the respiration rate can vary by any amounts up from 10% to 15%. These
percentages are
merely examples and are not intended to be limiting, and ordinarily skilled
artisan will appreciate
that the tolerances for variable heart rates and respiration rates may be
based on user
characteristics, such as age, level fitness, gender and the like. Sleep
manager 1912 can use
motion data 1905 to confirm whether a user is in an REM sleep state by
detecting indicative
amounts of motion, such as a portion of motion that includes negligible to no
motion.
Sleep manager 1912 is shown to include sleep predictor 1914, which is
configured to
predict the onset or change into or between different stages of sleep. The
user may not perceive
such changes between sleep states, such as transitioning from a wakefulness
state to a sleep state.
Sleep predictor 1914 can detect this transition from a wakefulness state to a
sleep state, as
depicted as transition 1930. Transition 1930 may be determined by sleep
predictor 1940 based
on the transitions from irregular heart rate and respiration rates during
wakefulness to more
regular heart rates and respiration rates during early sleep stages. Also,
lowered amounts of
motion can also indicate transition 1930. In some embodiments, motion data
1905 includes a
velocity or rate of speed at which a user is traveling, such as an automobile.
Upon detecting an
impending transition from a wakefulness state into a sleep state, sleep
predictor 1914 generates
an alert signal, such as a vibratory initiation signal, configuring to
generate a vibration (or any
other response) to convey to a user that he or she is about to fall asleep. So
if the user is driving,
predictor 914 assists in maintaining a wakefulness state during which the user
can avoid falling
asleep behind the wheel. Sleep predictor 1914 can be configured to also detect
transition 1932
from a light sleep state to a deep sleep state and a transition 1934 from a
deep sleep state to an
REM sleep state. In some embodiments, transitions 1932 in 1934 can be
determined by detected
changes from regular to variable heart rates or respiration rates, in the case
of transition 1934.
Also, transition 1934 can be described by a decreased level of motion to about
zero during the
REM sleep state. Further, sleep predictor 1914 can be configured to predict a
sleep stage
transition to disable an alert, such as wake-up time alarm, that coincides
with a state of REM
sleep. By delaying generation of an alarm, the user is permitted to complete
of a state of REM
sleep to enhance the quality of sleep.
FIG. 20A depicts a wearable device including a skin surface microphone
("SSM"), in
various configurations, according to some embodiments. According to various
embodiments, a
skin surface microphone ("SSM") can be implemented in cooperation with (or
along with) one
or more electrodes for bioimpedance sensors, as described herein. In some
cases, a skin surface
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
microphone ("SSM") can be implemented in lieu of electrodes for bioimpedance
sensors.
Diagram 2000 of FIG. 20 depicts a wearable device 2001, which has an outer
surface 2002 and
an inner surface 2004. In some embodiments, wearable device 2001 includes a
housing 2003
configured to position a sensor 2010a (e.g., an SSM including, for instance, a
piezoelectric
sensor or any other suitable sensor) to receive an acoustic signal originating
from human tissue,
such as skin surface 2005. As shown, at least a portion of sensor 2010a can be
formed external
to surface 2004 of wearable housing 2003. The exposed portion of the sensor
can be configured
to contact skin 2005. In some embodiments, the sensor (e.g., SSM) can be
disposed at position
2010b at a distance ("d") 2022 from inner surface 2004. Material, such as an
encapsulant, can be
used to form wearable housing 2003 to reduce or eliminate exposure to elements
in the
environment external to wearable device 2001. In some embodiments, a portion
of an
encapsulant or any other material can be disposed or otherwise formed at
region 2010a to
facilitate propagation of an acoustic signal to the piezoelectric sensor. The
material and/or
encapsulant can have an acoustic impedance value that matches or substantially
matches the
acoustic impedance of human tissue and/or skin. Values of acoustic impedance
of the material
and/or encapsulant can be described as being substantially similar to the
human tissue and/or
skin when the acoustic impedance of the material and/or encapsulant varies no
more than 60% of
that of human tissue or skin, according to some examples.
Examples of materials having acoustic impedances matching or substantially
matching
the impedance of human tissue can have acoustic impedance values in a range
that includes
1.5x106 Pax s/m (e.g., an approximate acoustic impedance of skin). In some
examples, materials
having acoustic impedances matching or substantially matching the impedance of
human tissue
can provide for a range between 1.0x106 Pax s/m and 1.0x107 Pax s/m. Note that
other values of
acoustic impedance can be implemented to form one or portions of housing 2003.
In some
examples, the material and/or encapsulant can be formed to include at least
one of silicone gel,
dielectric gel, thermoplastic elastomers (TPE), and rubber compounds, but is
not so limited. As
an example, the housing can be formed using Kraiburg TPE products. As another
example,
housing can be formed using Sylgard0 Silicone products. Other materials can
also be used. In
some embodiments, sleep manager 1912 detects increase perspiration via skin
conductance
during an REM sleep state and determines the user is dreaming, whereby in
generates a signal to
store such an event or generate an other action.
Further to FIG. 20A, wearable device 2001 also includes a physiological state
determinator 2024, a sleep manager 1912, a vibratory energy source 2028, and a
transceiver
2026. Physiological state determinator 2024 can be configured to receive
signals originating as
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
acoustic signals either from sensor 2010a or a sensor at location 2010b via
acoustic impedance-
matched material. Upon detecting a sleep state condition (e.g., a sleep state
transition), sleep
manager 1912 can be configured to communicate the condition to physiological
state
determinator 2024, which, in turn, generates a notification signal as a
vibratory activation signal,
thereby causing vibratory energy source 2028 (e.g., mechanical motor as a
vibrator) to impart
vibration through housing 2003 unto a user, responsive to the vibratory
activation signal, to
indicate the presence of the sleep-related condition (e.g., transitioning from
a wakefulness state
to a sleep state). According to some embodiments, sleep manager 1912 can
generate a wake
enable/disable signal 2013 configured to enable or disable the ability of
vibratory energy source
2028 to generate an alarm signal. For example, if sleep manager 1912
determines that the user is
in a REM sleep state, sleep manager 1912 generates a wake disable signal 2013
to prevent
vibratory energy source 2228 from waking the user. But if sleep manager 1912
determines that
the user is in a non-REM sleep state that coincides with a wake alarm time, or
is there shortly
thereafter, sleep manager 1912 will generate enable signal 2013 to permit
vibratory energy
source 2028 to wake up the user. In some cases, a wake enable signal and awake
disable signal
can be the same signal, but at different states. Also, wearable device 2001
can optionally include
a transceiver 2026 configured to transmit signal 2019 as a notification signal
via, for example, an
RF communication signal path. In some examples, transceiver 2026 can be
configured to
transmit signal 2019 to include data representative of the acoustic signal
received from sensor
2010, such as an SSM.
FIG. 20B depicts an example of physiological characteristics and parametric
values that
can identify a sleep state, according to some embodiments. Diagram 2050
depicts a data
arrangement 2060 including data for determining light sleep states, a data
arrangement 2062 that
includes data for determining deep sleep states, and data arrangement 2064
that includes data for
determining REM sleep states, according to various embodiments. Also shown in
FIG. 20B,
sleep manager 1912 and sleep predictor 1914 can use data arrangements 2060,
2062 and 2064 to
determine the various sleep stages of the user. As shown generally, each of
the sleep states can
be defined one or more physiological characteristics, such as heart rate, HRV,
pulse wave,
respiration rate, ranges of motion, types of motion, skin conductance,
temperature, and any other
physiological characteristic or information. As shown, each physiological
characteristic is
associated with a parametric range that may include one or more than one value
associated with
the physical physiological characteristic. For example, should the heart rate
of a user fall within
the range H1-H2, as shown in data arrangement 2064, sleep manager can use this
information in
determining whether the user is in REM sleep. In some cases, the parametric
values that set
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
forth the ranges, maybe based on characteristics of a user, such as age, level
of fitness, gender,
etc. In one example, sleep manager 1912 operates to analyze the various values
of the
physiological characteristics and calculates a best-fit determination of the
parametric values to
identify the corresponding sleep state for the user. The physiological
characteristics and
parametric values, and data arrangements 2062 to 2064 is merely one example
and is not
intended to be limiting.
FIG. 21 depicts an anomalous state manager 2102, according to some
embodiments.
Diagram 2100 depicts that anomalous state manager 2102 includes a tremor
determinator 2110, a
pain/stress analyzer 2114 and a malady determinator 2112. Anomalous state
manager 2102
receives sensor data 2104 and is configured to detect a deviation from the
normative general
physiological state of a user responsive, for example, to various stimuli,
such as stressful
situations, injuries, ailments, conditions, maladies, manifestations of an
illness, symptoms of a
condition, and the like. Also shown in diagram 2100 are repositories
accessible by anomalous
state manager 2102, including motion profile repository 2130, user
characteristic repository 2140
and pain profile repository 2144. Motion profile repository 2130 includes
profile data 2132 that
includes data defining configured to define a tremor, or a portion thereof,
associated with
detected motion. User characteristic repository 2140 includes user-related
data 2142 that
describes the user, for example, in terms of age, fitness level, gender,
diseases, conditions,
ailments, maladies, and any other characteristic that may influence the
determination of the
physiological state of the user. Pain profiles 2144 includes data 2146 that
can define whether the
user is in a pain state. In some embodiments, data 2146 is a data arrangement
that includes
physiological characteristics similar to those shown in FIG. 20B. For example,
physiological
signs of pain may include, for example, an increase in respiration rate, an
increase in the length
of a respiration cycle (e.g., deeper inhalation and exhalation), changes
and/or variations in blood
pressure, changes and/or variations in heart rate, an increase in perspiration
(e.g., increased skin
conductance), an increase in muscle tone (e.g., as determined by physiological
characteristics
indicating increased electrical impulses to or by musculature, and the like).
Based on such
physiological characteristics, pain/stress analyzer 2114 can be configured to
detect that the user
is experiencing pain, and in some cases, the level of pain. Further,
pain/stress analyzer 2114 can
be configured to transmit data representing pain state information to a
communication module
2118 for transmitting of the pain state-related information via wearable
device 2170 or other
mobile devices 2180 to a third-party (or any other entity or computing device)
via
communications path 2182 (e.g., wireless communications path and/or networks).
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
Tremor determinator 2110 is configured to determine the presence of a tremor
that, for
example, can be a manifestation of an ailment or malady. As discussed, such a
tremor can be
indicative of a diabetic tremor, an epileptic tremor, a tremor due to
Parkinson's disease, or the
like. In some embodiments, tremor determinator 2110 is configured to detect
the onset of tremor
related to a malady or condition prior to a user perceiving or otherwise being
aware of such a
tremor. In particular, wearable devices disposed at a distal portion of a limb
may be more likely,
at least in some cases, to detect tremors more readily than when disposed at a
proximal portion.
Therefore, anomalous state manager 2102 can predict the onset of a condition
that may
be remedied by, for example, medication and can alert a user to the impending
tremor. In some
cases, malady determinator 2112 is configured to receive data representing a
tremor and data
2142 representing user characteristics, and is further configured to determine
the malady
afflicting the user. For example, if data 2142 indicates the user is a
diabetic, the tremor data
received from tremor determinator 2110 is likely to indicate a diabetic-
related tremor.
Therefore, malady determinator 2112 can be configured to generate an alert
that, for example,
the user's blood glucose is decreasing to low level amounts that cause such
diabetic tremors.
The alert can be configured to prompt the user to obtaining medication to
treat the impending
anomalous physiological state of the user. In another example, tremor
determinator 2110 in
malady determinator 2112 cooperate to determine that the user is experiencing
and an epileptic
tremor, and generates an alert to enable the user to either take medication or
stop engaging in a
critical activity, such as driving, before the tremors become worse (i.e., to
an intensity that might
impair or otherwise incapacitate the user). Upon detection of tremor and the
corresponding
malady, anomalous state manager 2102 transmits data indicating the presence of
such tremors
via communication module 2118 to wearable device 2170 or mobile computing
device 2180,
which, in turn, transmit via networks 2182 to a third-party or any other
entity. In some
examples, anomalous state manager 2102 is configured to distinguish malady-
related tremors
from movements and/or shaking due to nervousness and or injury.
FIG. 22 depicts an affective state manager configured to receive sensor data
derived from
bioimpedance signals, according to some embodiments. FIG. 22 illustrates an
exemplary
affective state manager 2220 for assessing affective states of a user based on
data derived from,
for example, a wearable computing device, according to some embodiments.
Diagram 2200
depicts a user 2202 including a wearable device 2210, whereby user 2202
experiences one or
more types of stimuli that can changes in physiological states of user 2202,
such as the emotional
state of mind. In some embodiments, wearable device 2210 is a wearable
computing device
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
2210a that includes one or more sensors to detect attributes of the user, the
environment, and
other aspects of the responses from/interaction with stimuli.
Affective state manager 2220 is shown to include a physiological state
analyzer 2222, a
stressor analyzer 2224, and an emotion formation module 2223. According to
some
embodiments, physiological state analyzer 2222 is configured to receive and
analyze the sensor
data, such as bioimpedance-based sensor data 2211, to compute a sensor-derived
value
representative of an intensity of an affective state of user 2202. In some
embodiments, the
sensor-derived value can represent an aggregated value of sensor data (e.g.,
an aggregated an
aggregated value of sensor data value). In some examples, aggregated value of
sensor data can
be derived by, first, assigning a weighting to each of the values (e.g.,
parametric values) sensed
by the sensors associated with one or more physiological characteristics, such
as those shown in
FIG. 20B, and, second, aggregating each of the weightings to form an
aggregated value.
Affective state manager 2220 can also receive activity-related data 2114 from
a number of
activity-related managers (not shown). One or more activity-related managers
(not shown) can
be configured to receive data representing parameters relating to one or more
motion or
movement-related activities of a user and to maintain data representing one or
more activity
profiles. Activity-related parameters describe characteristics, factors or
attributes of motion or
movements in which a user is engaged, and can be established from sensor data
or derived based
on computations. Examples of parameters include motion actions, such as a
step, stride, swim
stroke, rowing stroke, bike pedal stroke, and the like, depending on the
activity in which a user is
participating. As used herein, a motion action is a unit of motion (e.g., a
substantially repetitive
motion) indicative of either a single activity or a subset of activities and
can be detected, for
example, with one or more accelerometers and/or logic configured to determine
an activity
composed of specific motion actions.
According to some examples, the activity-related managers can include a
nutrition
manager, a sleep manager, an activity manager, a sedentary activity manager,
and the like,
examples of which can be found in U.S. Patent Application No. 13/433,204,
filed on March 28,
2012 having Attorney Docket No. ALI-013CIP1; U.S. Patent Application No.
13/433,208, filed
March 28, 2012 having Attorney Docket No. ALI-013CIP2; U.S. Patent Application
No.
13/433,208, filed March 28, 2012 having Attorney Docket No. ALI-013CIP3; U.S.
Patent
Application No. 13/454,040, filed April 23, 2012 having Attorney Docket No.
ALI-
013CIP1CIP1; U.S. Patent Application No. 13/627,997, filed September 26, 2012
having
Attorney Docket No. ALI-100; all of which are incorporated herein by reference
for all purposes.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
In some embodiments, stressor analyzer 2224 is configured to receive activity-
related
data 2114 to determine stress scores that weigh against a positive affective
state in favor of a
negative affective state. For example, if activity-related data 2114 indicates
user 402 has had
little sleep, is hungry, and has just traveled a great distance, then user
2202 is predisposed to
being irritable or in a negative frame of mine (and thus in a relatively "bad"
mood). Also, user
2202 may be predisposed to react negatively to stimuli, especially unwanted or
undesired stimuli
that can be perceived as stress. Therefore, such activity-related data 2114
can be used to
determine whether an intensity derived from physiological state analyzer 2222
is either negative
or positive, as shown.
Emotive formation module 2223 is configured to receive data from physiological
state
analyzer 2222 and stressor analyzer 2224 to predict an emotion in which user
2202 is
experiencing (e.g., as a positive or negative affective state). Affective
state manager 2220 can
transmit affective state data 2230 via network(s) to a third-party, another
person (or a computing
device thereof), or any other entity, as emotive feedback. Note that in some
embodiments,
physiological state analyzer 2222 is sufficient to determine affective state
data 2230. In other
embodiments, stressor analyzer 2224 is sufficient to determine affective state
data 2230. In
various embodiments, physiological state analyzer 2222 and stressor analyzer
2224 can be used
in combination or with other data or functionalities to determine affective
state data 2230.
As shown, aggregated sensor-derived values 2290 can be generated by a
physiological
state analyzer 2222 indicating a level of intensity. Stressor analyzer 2224 is
configured to
determine whether the level of intensity is within a range of negative
affectivity or is within a
range of positive affectivity. For example, an intensity 2240 in a range of
negative affectivity
can represent an emotional state similar to, or approximating, distress,
whereas intensity 2242 in
a range of positive affectivity can represent an emotional state similar to,
or approximating,
happiness. As another example, an intensity 2244 in a range of negative
affectivity can represent
an emotional state similar to, or approximating, depression/sadness, whereas
intensity 2246 in a
range of positive affectivity can represent an emotional state similar to, or
approximating,
relaxation. As shown, intensities 2240 and 2242 are greater than that of
intensities 2244 and
2246. Emotive formulation module 2223 is configured to transmit this
information as affective
state data 230 describing a predicted emotion of a user. An example of
affective state manager
2220 is described as a affective state prediction unit of U.S. Provisional
Patent Application
Number 61/705,598 filed on September 25, 2012, which is incorporated by
reference herein for
all purposes.
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
FIG. 23 illustrates an exemplary computing platform disposed in a wearable
device in
accordance with various embodiments. In some examples, computing platform 2300
may be
used to implement computer programs, applications, methods, processes,
algorithms, or other
software to perform the above-described techniques, and can include similar
structures and/or
functions as set forth in FIG. 8. But in the example shown, system memory 806
can include
various modules that include executable instructions to implement
functionalities described
herein. In the example shown, system memory 806 includes a physiological
information
generator 2358 configured to determine physiological information relating to a
user that is
wearing a wearable device, and a physiological state determinator 2359.
Physiological state
determinator 2359 can include a sleep manager module 2360, anomalous state
manager module
2362, and an affective state manager module 2364, any of which can be
configured to provide
one or more functions described herein.
In at least some examples, the structures and/or functions of any of the above-
described
features can be implemented in software, hardware, firmware, circuitry, or a
combination
thereof Note that the structures and constituent elements above, as well as
their functionality,
may be aggregated with one or more other structures or elements.
Alternatively, the elements
and their functionality may be subdivided into constituent sub-elements, if
any. As software, the
above-described techniques may be implemented using various types of
programming or
formatting languages, frameworks, syntax, applications, protocols, objects, or
techniques. As
hardware and/or firmware, the above-described techniques may be implemented
using various
types of programming or integrated circuit design languages, including
hardware description
languages, such as any register transfer language ("RTL") configured to design
field-
programmable gate arrays ("FPGAs"), application-specific integrated circuits
("ASICs"), or any
other type of integrated circuit. According to some embodiments, the term
"module" can refer,
for example, to an algorithm or a portion thereof, and/or logic implemented in
either hardware
circuitry or software, or a combination thereof. These can be varied and are
not limited to the
examples or descriptions provided.
FIG. 24 illustrates an exemplary combination speaker and light source powered
using a
light socket. Here, combination speaker and light source (hereinafter "speaker
light") 2400
includes housing 2402, parabolic reflector 2404, positioning mechanism 2406,
light socket
connector 2408, passive radiators 2410-2412, light source 2414, circuit board
(PCB) 2416,
speaker 2418, frontplate 2420, backplate 2422 and optical diffuser 2424. In
some examples,
speaker light 2400 may be implemented as a combination speaker and light
source, including a
controllable light source (i.e., light source 2414) and a speaker system
(i.e., speaker 2418). In
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
some examples, light source 2414 may be configured to provide adjustable and
controllable
light, including an on or off state, varying colors, brightness, and
irradiance patterns, without
limitation. In some examples, light source 2414 may be controlled using a
control interface (not
shown) in data communication with light source 2414 (i.e., using a
communication facility
implemented on PCB 2416) using a wired or wireless network (e.g., power line
standards (e.g.,
G.hn, HomePlugAV, HomePlugAV2, IEEE1901, or the like), Ethernet, WiFi (e.g.,
802.11
a/b/g/n/ac, or the like), Bluetooth0, or the like). In some examples, light
source 2414 may be
implemented using one or more light emitting diodes (LEDs) coupled to PCB
2416. In other
examples, light source 2414 may be implemented using a different type of light
source (e.g.,
incandescent, light emitting electrochemical cells, halogen, compact
fluorescent, or the like). In
some examples, PCB 2416 may be bonded to backplate 2422, which may be coupled
to a driver
(not shown) for speaker 2418, to provide a heatsink for light source 2414. In
some examples,
light source 2414 may direct light towards parabolic reflector 2404, as shown.
In some
examples, parabolic reflector 2404 may be configured to direct light from
light source 2414
towards a front of housing 2402 (i.e., towards frontplate 2420 and optical
diffuser 2424), which
may be transparent. In some examples, parabolic reflector 2404 may be movable
(e.g., turned,
shifted, or the like) using positioning mechanism 2406, either manually or
electronically, for
example, using a remote control in data communication with circuitry
implemented in
positioning mechanism 2406. For example, parabolic reflector 2404 may be moved
to change an
output light irradiation pattern. In some examples, parabolic reflector 2404
may be acoustically
transparent such that additional volume within housing 2402 (i.e., around and
outside of
parabolic reflector 2404) may be available for acoustic use with a passive
radiation system (e.g.,
including passive radiators 2410-2412, and the like).
In some examples, light socket connector 2408 may be configured to be coupled
with a
light socket (e.g., standard Edison screw base, as shown, bayonet mount, bi-
post, bi-pin, or the
like) for powering (i.e., electrically) speaker light 2400. In some examples,
light socket
connector 2408 may be coupled to housing 2402 on a side opposite to optical
diffuser 2424
and/or speaker 2418. In some examples, housing 2402 may be configured to house
one or more
of parabolic reflector 2404, positioning mechanism 2406, passive radiators
2410-2412, light
source 2414, PCB 2416, speaker 2418 and frontplate 2420. Electronics (not
shown) configured
to support control, audio playback, light output, and other aspects of speaker
light 2400, may be
mounted anywhere inside or outside of housing 2402. In some examples, light
socket connector
2408 may be configured to receive power from a standard light bulb or power
connector socket
(e.g., E26 or E27 screw style, T12 or GU4 pins style, or the like), using
either or both AC and
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
DC power. In some examples, speaker light 2400 also may be implemented with an
Ethernet
connection.
In some examples, speaker 2418 may be suspended in the center of frontplate
2420,
which may be sealed. In some examples, frontplate 2420 may be transparent and
mounted or
otherwise coupled with one or more passive radiators. In some examples,
speaker 2418 may be
configured to be controlled (e.g., to play audio, to tune volume, or the like)
remotely using a
controller (not shown) in data communication with speaker 2418 using a wired
or wireless
network. In some examples, housing 2402 may be acoustically sealed to provide
a resonant
cavity when combined with passive radiators 2410-2412 (or other passive
radiators (not shown),
for example, disposed on frontplate 2420). In other examples, radiators 2410-
2412 may be
disposed on a different internal surface of housing 2402 than shown. The
combination of an
acoustically sealed housing 2402 with one or more passive radiators (e.g.,
passive radiators
2410-2412) improves low frequency audio signal reproduction, while optical
diffuser 2424 may
be acoustically transparent, thus sound from speaker 2418 may be projected out
of housing 2402
through optical diffuser 2424. In some examples, optical diffuser 2424 may be
configured to be
waterproof (e.g., using a seal, chemical waterproofing material, and the
like). In some examples,
optical diffuser 2424 may be configured to spread light (i.e., reflected using
parabolic reflector
2404) evenly as light exits housing 2402 through a transparent frontplate
2420. In some
examples, optical diffuser 2424 may be configured to be acoustically
transparent in a frequency
selective manner, functioning as an additional acoustic chamber volume (i.e.,
as part of a passive
radiator system including housing 2402, radiators 2410-2412, and other
components of speaker
light 2400). In other examples, the quantity, type, function, structure, and
configuration of the
elements shown may be varied and are not limited to the examples provided.
FIG. 25 illustrates a system for manipulating a combination speaker and light
source
according to a physiological state determined using sensor data. Here, system
2500 includes
wearable device 2502, mobile device 2504, speaker light 2506 and controller
2508. Like-
numbered and named elements may describe the same or substantially similar
elements as those
shown in other descriptions. In some examples, wearable device 2502 may
include sensor array
2502a, physiological state determinator 2502b and communication facility
2502c. As used
herein, "facility" refers to any, some, or all of the features and structures
that are used to
implement a given set of functions. In some examples, communication facility
2502c may be
configured to communicate (i.e., exchange data) with other devices (e.g.,
mobile device 2504,
controller 2508, or the like), for example, using short-range communication
protocols (e.g.,
Bluetooth0, ultra wideband, NFC, or the like) or longer-range communication
protocols (e.g.,
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
satellite, mobile broadband, GPS, WiFi, and the like). In some examples,
physiological state
determinator 2502b may be configured to output data (i.e., state data)
associated with a
physiological state (e.g., states of sleep, wakefulness, a normative
physiological state, a deviation
from a normative physiological state, an affective state, or the like), which
physiological state
determinator 2502b may be configured to generate using sensor data captured
using sensor array
2502a, as described herein. For example, physiological state determinator
2502b may be
configured to generate state data 2520-2522. In some examples, wearable device
2502 may be
configured to communicate state data 2520 to mobile device 2504 using
communication facility
2502c. In some examples, wearable device 2502 may be configured to communicate
state data
2522 to controller 2508 using communication facility 2502c.
In some examples, mobile device 2504 may be configured to run application
2510, which
may be configured to receive and process state data 2520 to generate data
2516. In some
examples, data 2516 may include light data associated with light patterns
congruent with state
data provided by wearable device 2502 (e.g., state data 2520 and the like).
For example, where
state data 2520 indicates a predetermined or designated wake up time,
application 2510 may
generate light data associated with a gradual brightening of a light source
implemented in
speaker light 2506. In another example, where state data 2520 indicates a
sleep or resting state,
application 2510 may generate light data associated with a dimming of a light
source
implemented in speaker light 2506. In still other examples, light data
generated by application
2510 may be associated with a light pattern, a level of light, or the like,
for example, depending
on an activity (e.g., dancing, meditating, exercising, walking, sleeping, or
the like) indicated by
state data 2520. In some examples, data 2516 may include audio data associated
with audio
output congruent with state data provided by wearable device 2502 (e.g., state
data 2520 and the
like). For example, application 2510 may be configured to generate audio data
associated with
playing audio content (e.g., a playlist, an audio file including animal
noises, an audio file
including a voice recording, or the like) associated with an activity (e.g.,
dancing, meditating,
exercising, walking, sleeping, or the like) using a speaker implemented in
speaker light 2506
when state data 2520 indicates said activity is beginning or ongoing. In
another example,
application 2510 may be configured to generate audio data associated with
adjusting white noise
or other ambient noise (e.g., to improve sleep quality, to ease a waking up
process, to match a
mood or activity, or the like) output by a speaker implemented in speaker
light 2506 when state
data 2520 indicates an analogous physiological state. In other examples,
application 2510 may
be implemented directly in controller 2508, for example, using state data
2522, which may
include the same or similar kinds of data associated with physiological states
as described herein
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
in relation to state data 2520. In some examples, controller 2508 may be
configured to generate
one or more control signals, for example, using API 2512, and to send said one
or more control
signals to speaker light 2506 to adjust a light source and/or speaker. For
example, the one or
more control signals may be configured to cause a light source to dim or
brighten. In another
example, the one or more control signals may be configured to cause the light
source to display a
light pattern. In still another example, the one or more control signals may
be configured to
cause a speaker to play audio content. In yet another example, the one or more
control signals
may be configured to cause a speaker to play ambient noise. In other examples,
the quantity,
type, function, structure, and configuration of the elements shown may be
varied and are not
limited to the examples provided.
FIG. 26 illustrates a diagram depicting exemplary components in a combination
speaker
and light source including a sensor device for determining an environmental
state. Here,
diagram 2600 includes speaker light 2606, which includes light source 2602,
speaker system
2604 and sensor device 2608. Like-numbered and named elements may describe the
same or
substantially similar elements as those shown in other descriptions. For
example, light source
2602 may be implemented the same as, or similar to, other light sources
described herein (e.g.,
light source 2414 in FIG. 24, and the like), and speaker system 2604 may
include the same or
similar speaker components, and function the same or similar to, other
speakers described herein
(e.g., speaker 2418 with passive radiators 2410-2412 in FIG. 2, and the like).
In some examples,
sensor device 2608 may include chemical sensor 2610, temperature sensor 2612,
accelerometer/motion sensor (hereinafter "motion sensor") 2614, environmental
state
determinator 2616 and light and speaker controller (hereinafter "controller")
2624. In some
examples, environmental state determinator 2616 may be configured to receive
sensor signals,
including chemical signal 2618 (e.g., data associated with levels of carbon
dioxide, oxygen,
carbon monoxide, an airborne chemical, a toxin, other greenhouse gases, other
pollutants, and
the like) from chemical sensor 2610, temperature signal 2620 from temperature
sensor 2612, and
motion signal 2622 from motion sensor 2614. In other examples, sensor device
2608 may
include other sensors configured to capture data associated with an
environment, for example,
surrounding speaker light 2606. Examples of other sensors are described in
U.S. Patent
Application No. 13/454,040, filed on April 23, 2012, and U.S. Patent
Application No.
13/491,345, filed on June 7, 2012, which are incorporated by reference herein
in their entirety for
all purposes. In some examples, chemical signal 2618, temperature signal 2620
and motion
signal 2622 may comprise an electrical signal. In other examples, sensors
implemented in sensor
device 2608 may provide to environmental state determinator 2616 an acoustic,
or other type of,
CA 02917626 2016-01-06
WO 2014/189862
PCT/US2014/038671
signal. In some examples, environmental state determinator 2616 may be
configured to process
raw sensor data and to derive environmental states (e.g., low oxygen levels,
high carbon dioxide
or carbon monoxide levels, elevated or declining temperature, aberrant motion
(e.g., from an
earthquake, nearby constructions, or the like), increased ambient sound, or
the like) from said
raw sensor data. In some examples, environmental state determinator 2616 may
be configured to
provide environmental state data (not shown) to controller 2624. In some
examples, controller
2624 may be configured to generate a plurality of control signals to cause one
or both of light
source 2602 and speaker system 2604 to output light and audio (i.e., acoustic
output),
respectively. For example, controller 2624 may generate light output signal
2628 configured to
cause light source 2602 to modify light output (e.g., increase light output,
decrease light output,
output a light pattern, or the like) in response to an environmental state
(e.g., elevated or
declining temperature, low oxygen level, high carbon dioxide or carbon
monoxide levels, or the
like). In another example, controller 2624 may generate audio output signal
2626 configured to
cause speaker system 2604 to increase audio output (e.g., in response to
increased ambient
sound, increase in carbon dioxide levels, or the like), decrease audio output
(e.g., in response to
decreased ambient noise, or the like), or to output an audio alarm (e.g., in
response to an
earthquake, low oxygen level, high carbon monoxide level, or the like). In
still another example,
controller 2624 may generate both audio output signal 2626 and light output
signal 2628 to cause
speaker system 2604 to output an audio alarm, and to cause light source 2602
to output a light
pattern (i.e., "visible alarm") simultaneously, for example, to increase the
effectiveness of the
alarm. In other examples, the quantity, type, function, structure, and
configuration of the
elements shown may be varied and are not limited to the examples provided.
Although the foregoing examples have been described in some detail for
purposes of
clarity of understanding, the above-described inventive techniques are not
limited to the details
provided. There are many alternative ways of implementing the above-described
invention
techniques. The disclosed examples are illustrative and not restrictive.