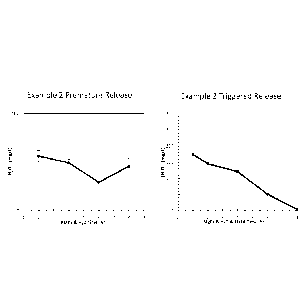Note: Descriptions are shown in the official language in which they were submitted.
CA 02918957 2016-01-20
WO 2015/017633 PCMJS2014/049083
ENCAPSULATED INTERNAL FILTER CAKE BREAKERS WITH IMPROVED RELEASE
PROPERTIES
FIELD Of THE, INVENTION
The present invention relates to a composition and method of use thereof for
an
encapsulated internal filter cake breaker with improved release properties, in
particular lower
premature release. Said encapsulated internal filter cake breaker comprises an
encapsulated
internal breaker, preferably a peroxide source and a film comprising a metal-
crosslinked acrylic.
BACKGROUND OF THE INVENTION
During the creation and subsequent operation of a subterranean well, the
operator may
wish to perform acts that could potentially damage the underground formations
and their ability
to produce desirable formation fluids. For example, the operator may wish to
inject water into
the well. The operator may do this to enhance the productivity of a well or to
dispose of waste
water.
In addition to water, other fluids are routinely used in the operation of a
subterranean
well. Drilling fluids are used to aid in the drilling of a well; both to cool
the drill bit and to
remove drill cuttings from the well. Completion fluids are used when
completion operations are
performed in a producing formation. During these processes, it may be
desirable to seal off
producing formations in order to prevent fluid loss from the well to the
formation and to prevent
possible damage to the formation.
One way of protecting the formation is by forming a filter cake on the surface
of the
subterranean formation. Filter cakes are formed when particles suspended in a
wellbore fluid
coat or plug the pores in the subterranean formation such that fluid is
substantially prevented
from passing between the formation and the wellbore and vice versa. A number
of ways of
forming filter cakes are known in the art, including the use of both clay and
non-clay based
drilling fluids.
In addition to the intentional formation of filter cakes, filter cakes can
also be produced
unintentionally. For instance, when drilling the well, the particles contained
in the drilling mud
can lodge in the pores of a formation that the operator desires to bring into
production.
Whether the formation of the filter cake was unintentional and intentional, it
is desirable
to be able to remove the filter cake when the formation is brought into
production. The
presence of the filter cake can hinder the passage of fluid from the formation
to the wellbore
and thereby retard production rates.
1
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
Various ways have been developed by those skilled in the art to form filter
cakes that
can be easily removed. For example U.S. Pat. No. 5,251,697 discloses the
addition of calcium
carbonate to water being injected into a well. The calcium carbonate particles
either clog the
pores in the subterranean rock formations or collect and build a filter cake.
When the filter cake
is to be removed, the '697 patent directs the operator to circulate an acid
wash, preferably
hydrochloric acid, into the well. The acid wash will dissolve the calcium
carbonate and thereby
destroy the filter cake. At this point the well can be brought into production
or additional work
performed on the well.
In USP 5,607,905 is disclosed a method of forming a filter cake using a fluid
containing
.. polysaccharide polymers, bridging particles and an alkaline earth metal or
zinc peroxide. The
method of the '905 patent seeks to have to particles in the fluid bridge over
the formation pores
rather than plug the holes. The polysaccharide polymers are typically added to
the wellbore
fluid as viscosifiers or fluid loss control additives. An earlier patent, USP
5,238,065, taught that
filter cakes containing polysaccharide polymers could be removed by contacting
the filter cake
.. with a brine fluid containing a zinc or alkaline earth metal peroxide, an
acidic substance such
that the pH of the solution was between 1 and 8 and an activator for the
peroxide. A wash
solution would then be used to remove the filter cake. The degrading substance
incorporated
within the filter cake is generally referred to as an internal breaker. The
use of an internal
breaker was beneficial because it required less peroxide, less loss of wash
fluid to the formation,
.. and gave more complete removal of the filter cake.
However, these systems contain several drawbacks. First, the peroxides used
have a
tendency to emit peroxide prior to activation, resulting in premature
weakening of the filter cake
as well as reducing the effectiveness of the polymers used to control fluid
loss and viscosity.
Additionally, these prior art methods require action on the part of the
operator to remove the
.. filter cake. This results in additional cost and delay. Also, the acid or
other solutions used to
dissolve filter cakes can have a harmful effect on the formation.
Encapsulation of
the peroxide breakers, as disclosed in USP 6,861,394, was introduced to
provide improve
performance regarding delayed release of the peroxide breaker payload.
Peroxide sources were
coated with a polymer such as a non-crosslinked acrylic acid polymer. However,
there remain
several problems related to using encapsulated breakers in hydraulic
fracturing treatments.
Known encapsulation materials are limited to lower down hole temperatures
which leads to
early release of the peroxides. Further, known encapsulation materials are
prone to swelling in
moderate basic conditions which also results in premature release of the
peroxides.
It would be desirable to provide an improved delayed filter cake breaker with
less
premature release for use in removing filter cakes in subterranean wells and
formations wherein
2
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
said delayed filter cake breaker demonstrates improved temperature stability
and lower
permeability and less prone to swelling in moderate basic solutions.
SUMMARY OF THE INVENTION
The present invention is such an improved delayed filter cake breaker with
less
premature release for use in removing filter cakes in subterranean wells and
formations.
One embodiment of the present invention is an encapsulated internal breaker
for
enhancing the removal of a filter cake from a wellbore in a subterranean
formation comprising:
i a filter cake breaker, preferably an acid generating compound, an enzyme, an
oxidizer, a
bromate, an azo compounds, or a combination thereof, and most preferably
magnesium
peroxide, zinc peroxide, or an alkaline earth metal peroxide, ii an anti-
caking agent, preferably
magnesium carbonate, calcium carbonate, colloidal silica micro particles, or
clay particles, iii an
encapsulation film comprising a metal-crosslinked acrylic polymer, preferably
a transition metal
cross-linked acrylic polymer, more preferably a zinc cross-linked acrylic
polymer, said metal
cross-linked polymer which is substantially insoluble in well bore fluids
having a pH value
equal to or greater than 7.5, preferably said film having a Tg greater than 45
C, and iv
optionally a water-soluble polymer as a binder, preferably when present in an
amount of from 1
to 5 weight percent based on the total weight of the encapsulated internal
breaker.
Another embodiment of the present invention is wellbore fluid comprising a
degradable
polymer and the encapsulated internal breaker disclosed herein above.
Another embodiment of the present invention is a process for enhancing the
removal of
a filter cake from a wellbore in a subterranean formation comprising the
steps: a adding one or
more degradable polymer to a wellbore fluid; b adding an encapsulated internal
breaker to a
wellbore fluid; c pumping said wellbore fluids into the wellbore; and d
changing the pH of the
fluid in the wellbore so as to activate the degradable polymer; wherein the
encapsulated internal
breaker is described herein above.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is graphs showing premature and triggered peroxide release for Example
1, not
an encapsulated internal breaker the present invention.
FIG. 2 is graphs showing premature and triggered peroxide release for Example
2, an
encapsulated internal breaker of the present invention.
FIG. 3 is graphs showing premature and triggered peroxide release for Example
3, an
encapsulated internal breaker of the present invention.
3
81794346
FIG. 4 is graphs showing premature and triggered peroxide release for Example
4, an
encapsulated internal breaker of the present invention.
FIG. 5 is graphs showing premature and triggered peroxide release for Example
5, an
encapsulated internal breaker of the present invention.
FIG. 6 is graphs showing premature and triggered peroxide release for Example
6, an
encapsulated internal breaker of the present invention.
FIG. 7 is graphs showing premature and triggered peroxide release for Example
7, an
encapsulated internal breaker of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The processes, methods and compositions described in this disclosure may be
used to
form an easily removed filter cake in a subterranean well. The filter cake may
be formed by
adding various components to a wellbore fluid, pumping the fluid into the
wellbore and
allowing the fluid to contact the desired subterranean formation.
The wellbore fluid may have a variety of compositions, the appropriate
components of
which can be selected by one skilled in the art. Preferably, the wellbore
fluids are alkaline
water based wellbore fluids. The pH of these solutions is preferably between
about 7 to about
12, even more preferably between about 7.5 to about 10.5. The pH can be
adjusted by methods
known to those skilled in the art, including the addition of bases to the
fluid. Such bases include
potassium hydroxide, sodium hydroxide, magnesium oxide, calcium hydroxide and
zinc oxide.
These aqueous fluids are generally brine solutions. Such fluids can be natural
brine solutions,
seawater or brines formed by dissolving suitable salts in water. Suitable
salts include chloride,
bromide, acetate and formate salts of potassium, sodium, calcium, magnesium,
zinc and cesium.
A variety of components may be added to the wellbore fluid to achieve
different
desired properties, as would be known to those skilled in the art. For
example, the wellbore
fluid may include viscosifiers, such as polysaccharides or polysaccharide
derivatives. Some
representative polymers are discussed in USP 4,846,981 and the references
cited therein.
It may also be desirable to add components to the wellbore fluid to help
control fluid
loss. Fluid loss additives keep wellbore fluids from entering the subterranean
formations while
allowing the wellbore fluid to be maintained at a pressure greater than that
of the formation.
This pressure overbalance acts to keep the formation fluids from entering the
wellbore. A
variety of materials have been used to control fluid loss, some of which are
described in USP
5,354,786; 4,387,769 and 4,836,940. In particular, various polymers have been
used to control
fluid loss, including polysaccharides.
4
Date Recue/Date Received 2021-02-12
81794346
Wellbore fluids of the present invention preferably contain polysaccharide
polymers for
incorporation into a filter cake. Preferred polysaccharide polymers include
starch derivatives,
cellulose derivatives, and biopolymers, such as: hydroxypropyl starch,
hydroxyethyl starch,
carboxymethyl starch, and their corresponding lightly crosslinked derivatives;
carboxymethyl
cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, methyl cellulose,
dihydroxypropyl
cellulose, and their corresponding lightly crosslinked derivatives; xanthan
gum, gellan gum,
welan gum and schleroglucan gum.
Various types of solids can be suspended in wellbore fluids to bridge or block
the pores
of a subterranean formation. Such solids include those described USP
4,561,985; 3,872,018;
and 3,785,438. For the purposes of the present invention,
of particular interest are those solids soluble in acid solutions.
Representative acid
soluble bridging solids are calcium carbonate, limestone, marble, dolomite,
iron carbonate and
zinc oxide. However, other solids may be used without departing from the scope
the present
invention. Other representative solids include water soluble and oil soluble
solids as described
in USP 5,783,527.
The wellbore fluids of the present invention include a wellbore fluid that
contains a
filter cake breaker. Filter cake breakers useful in the treatment fluids and
methods of the
present invention may include, but are not limited to, acid generating
compounds, enzymes,
oxidizers, bromates (such as sodium bromate and potassium bromate), azo
compounds, and any
combination thereof. As used herein, the term "acid generating compound"
refers to a
composition that generates acid over time.
Examples of suitable acid generating compounds that may be suitable for use in
the
delayed filter cake breakers useful in the treatment fluids and methods of the
present invention
include, but are not limited to, esters, aliphatic polyesters, ortho esters,
poly (ortho esters), ortho
ethers; poly(ortho ethers); lactides, poly(lactides), glycolides,
poly(glycolides), lactones, e-
caprolactones, poly(e-caprolactones), hydroxybutyrates,
poly(hydroxybutyrates), anhydrides,
poly(anhydrides), polyascorbic acid, aliphatic carbonates, aliphatic
polycarbonates, amino
acids, poly(amino acids), ethylene oxide, poly(ethylene oxide), and
polyphosphazenes, or
copolymers thereof. Derivatives and combinations also may be suitable. Other
suitable acid-
generating compounds may include formate esters, acetate esters, and lactate
esters such as, but
not limited to, ethylene glycol monoformate, ethylene glycol diformate,
diethylene glycol
diformate, glyceryl monoformate, glyceryl diformate, glyceryl triformate,
triethylene glycol
diformate, formate esters of pentaerythritol, glyceryl triacetate, methyl
lactate, butyl lactate, and
derivatives thereof. Other suitable materials may be disclosed in USP
6,877,563 and 7,021,383.
Examples of acid-generating compounds that may
5
Date Recue/Date Received 2021-02-12
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
be suitable in the present invention are commercially available from
Halliburton Energy
Services, Inc., of Duncan, Okla., under the trade names NFC-2, ED-1, and BDF-
325.
In those embodiments where an acid generating compound is used in the
treatment fluid,
the acid generating compound may generate an acid downhole in a delayed
fashion that may
remove at least a portion of a filter cake present in the subterranean
formation. The acid
generating compounds may be reacted with small amounts of reactive materials
such as mineral
acids, organic acids, acidic anhydrides, p-toluenesulfonic acid, and the like
to lower the pH to
accelerate the hydrolysis of the acid generating compound if desired.
Similarly, the hydrolysis
rate may be slowed by the addition of a small amount of a strong base such as
NaOH, Na2CO3,
and Mg(OH)2. The acid generating compound also may generate alcohols downhole
that may be
beneficial to the operation.
Any composition or method known in the art that is capable of producing an
acid may
be used in conjunction with the present invention. Additional examples of such
compositions
and methods include, but are not limited to encapsulated acids, reaction of an
aldehyde group
with an oxidizer such as with reducing sugars, and/or any fermentation process
that produces
acid and oxidation of mineral surfaces.
In some embodiments, an acid generating compound may be present in a treatment
fluid
of the present invention in an amount of about 0.5% to about 40% by weight of
the
composition. In certain embodiments of the present invention, the acid
generating compound
may be provided in a concentrated aqueous solution prior to its combination
with the other
components necessary to form a treatment fluid of the present invention.
The delayed filter cake breakers useful in the treatment fluids and methods of
the
present invention also may comprise an enzyme. In certain embodiments, enzymes
useful in the
delayed filter cake breakers of the treatment fluids and methods of the
present invention may
catalyze the breakdown of an acid-generating compound to generate an acid. In
certain
embodiments, enzymes may be included in the delayed filter cake breakers
useful in the
treatment fluids or methods of the present invention at formation temperatures
below about
160 F. Suitable enzymes may include, but are not limited to, starch specific
enzyme, cellulose
specific enzyme, guar specific enzymes, esterases, amylases, xanthanases,
gluconases,
cellulases, mannanases, and any combination thereof. Examples of suitable
enzymes may
includes, but are not limited to, those available commercially under the trade
names NFC-3Tm
and NFC-41m, available from Halliburton Energy Services, Inc., of Duncan,
Okla., as well as
ARCASOLVETm, available from Cleansorb Limited of Guildford, Surrey, United
Kingdom. In
certain embodiments, the enzyme may be present in an amount of about 0.001% to
about 1% by
weight of the composition.
6
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
The delayed filter cake breakers useful in the treatment fluids and methods of
the
present invention may also comprise an oxidizer. Examples of suitable
oxidizers may include,
but are not limited to, t-butyl hydroperoxide and sodium perborate. In certain
embodiments, the
oxidizer may be present in an amount of about 0.001% to about 5% by weight of
the
composition.
The delayed filter cake breakers useful in the treatment fluids and methods of
the
present invention may also comprise an azo compound. Examples of suitable azo
compounds
may include, but are not limited to, 2,2'-azobis(2-amidinopropane),
dihydrochloride, 2,2'-
azobis-2-methyl-n-(2-hydroxyethyl)propionamide, 4,4'-azobis(4-cyanovaleric
acid). In certain
embodiments, the azo compound may be present in an amount of about 0.001% to
about 1% by
weight of the composition. Other free radical generators may be used as well.
The delayed filter cake breakers useful in the treatment fluids and methods of
the
present invention may also comprise sodium chlorite, hypochlorites,
perborates, persulfates,
and/or peroxides. Preferably the delayed filter cake breaker useful in the
present invention is a
peroxide source. Preferably, the peroxide source is an inorganic peroxide
source such as
peroxide compounds of zinc or alkaline earth metals. Magnesium peroxide is
particularly
preferred. Other peroxide sources known to those skilled in the art can be
used without
departing from the scope of the present invention.
Alkaline earth peroxides and zinc peroxide are known water insoluble
compounds.
Inorganic peroxide compounds are commonly described as compounds whose
structures include the peroxo-group,
Simple peroxide compounds include peroxides in
which the peroxo-group is bonded to a metallic atom via ionic bonding, and
includes
hydroperoxides characterized by the presence of the hydroperoxyl ion (HOD-.
Complex
peroxide compounds include peroxides in which the peroxo-group as such, or in
the form of
11202 and 1102, is bonded to the metallic atom by a covalent bond. Complex
peroxide
compounds also include the addition compounds formed with crystallized
hydrogen peroxide.
The characteristic properties of peroxide compounds both simple and complex,
are: the
formation of hydrogen peroxide upon reaction with dilute acid solution, the
liberation of oxygen
as a result of thermal decomposition, and the liberation of oxygen upon
reaction with water and
other chemical agents. Further characterization is possible by dividing the
simple inorganic
peroxide compounds into four groups: (1) hydroperoxide, characterized by the
(H02)- ion; (2)
peroxides, characterized by the (02)2- ion; (3) superoxides characterized by
the (02)- ion; and
(4) ozonides, characterized by the (03)- ion.
The hydrolysis of peroxides and hydroperoxides proceeds as follows:
7
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
M09 + 2H20 M(OH)2 + H202
and
MOOH + H20 MOH 4 + F202
where M = metal. For this reason, peroxides and hydroperoxides are considered
as being
derived from hydrogen peroxide by replacement of one or both the hydrogen
atoms by metal
atoms.
Complex peroxide compounds include peroxyhydrates, for example, Ca02.2H202,
and
peroxyhydrate hydrates, for example, Ba02.H202.20H20.
Peroxides of calcium, strontium and barium belong to the M2+022- type of
peroxide
compounds, while peroxides of magnesium and zinc, of general formula
M02.xII20, probably
belong to the HO--M--OOH type where the covalent bond between the hydroperoxyl
group and
the metal atom is the same as that in hydrogen peroxide.
Calcium peroxide is generally prepared by industrial processes that provide a
product
containing 60-75 wt. % Ca02. Reaction of Ca02.8H20 with solutions containing
greater than
weight percent of hydrogen peroxide results in the formation of calcium
peroxyhydrate,
Ca02.2H202. Strontium peroxide, industrial grade, generally contains 80-95%
Sr02. Industrial
20 grade barium peroxide generally contains up to about 90% Ba02. Depending
on its method of
production, industrial grade magnesium peroxide contains from about 25% Mg02
to about 50%
Mg02 and generally is a mixture of peroxide, oxide, and magnesium oxide
hydrate. Industrial
grade zinc peroxide contains about 55% Zn02. For all purposes of the present
invention, the
term "peroxide" includes all forms of peroxides, including simple peroxides,
such as
hydroperoxides characterized by the (H02)- ion and peroxides characterized by
the (02)- ion,
and complex peroxides such as peroxyhydrates and peroxyhydrate hydrates.
Preferred peroxides for use in the present invention are zinc oxide; alkaline
earth metal
peroxide, such as magnesium peroxide and calcium peroxide; and organic
peroxides such as t-
butyl hydroperoxide, benzoyl peroxide, ascaridole, and the like.
Optionally, free radical
scavengers or reducing agents may be added to wellbore solution embodiments of
the present
invention. These materials may enhance the stability of the fluid and aid in
avoiding premature
degradation of the polysaccharide or other peroxide degradable polymers in the
wellbore fluid.
Representative reducing agents are water soluble sulfites, bisulfites,
thiosulfates, dithionites,
and mixtures thereof, particularly the alkali metal or ammonium salts thereof,
preferably a water
.. soluble thiosulfate, most preferably sodium thiosulfate. Representative
antioxidants or free
8
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
radical scavengers include water soluble mercaptans, thioethers,
thiocarbonyls, low molecular
weight alcohols and glycols, and mixtures thereof.
The preferred embodiment of the present invention utilizes a peroxide source
that has
been encapsulated. Prior art methods, such as that described in ITSP
5,783,527, suffer
somewhat from premature release of peroxide that can degrade the filter cake
and reduce the
effectiveness of the polysaccharide polymers used for fluid loss and
viscosifing purposes. This
release occurs despite the formation of a coating of magnesium hydroxide that
forms on the
surface of the peroxide when it was added to water. This layer of magnesium
hydroxide
somewhat retards the release of peroxide. However, enough peroxide is still
released to have
negative effects on the filter cake and the properties of the drilling fluid.
It has been found that encapsulating the peroxide source can further aid in
preventing
the early release of peroxide. For the purposes of the present invention, an
encapsulated
peroxide is a peroxide that has a coating sufficient to control the release of
peroxide until a set
of conditions selected by the operator occurs. Some general encapsulating
materials include
natural and synthetic oils, natural and synthetic polymers and enteric
polymers and mixtures
thereof. However, many methods of encapsulating can be used without departing
from the
scope of the present invention. The preferred method of encapsulating the
peroxide sources is
by coating the peroxide with a polymer.
Similarly, many methods could be used to cause the release of the peroxide
upon the
occurrence of specific conditions desired by the operator. For example, the
peroxide could be
caused to be released by a change in temperature, pressure, pH, abrasion or
any number of other
environmental factors. A preferred method of releasing the peroxide for the
purposes of
dissolving a filter cake in a subterranean well is by having the peroxide
release upon a change in
pH in the down hole environment.
Those skilled in the art will appreciate that there are many possible
mechanisms by
which the coating may be removed. The methods discussed below are intended to
illustrate
possible methods by which this release might occur. This discussion is not
intended to in any
way limit the scope of the invention as set forth in the appended claims.
The coating polymer should preferably form a film around the peroxide source.
The
polymer should be chosen such that the coating will remain substantially
intact until the desired
release conditions occur. For the purposes of filter cake removal, it is
preferable that the
coating respond to changes in pH. The preferred polymers of the present
invention arc enteric
polymers, which are defined for the purposes of this invention as polymers
whose solubility
characteristics are pH dependent. Here, this means that peroxide release is
promoted by a
change from conditions of a first predetermined pH value to a second
predetermined pH
condition.
9
81794346
Enteric polymers are commonly used in the pharmaceutical industry for the
controlled
release of drugs and other pharmaceutical agents over time. The use of enteric
polymers allows
for the controlled release of the peroxide source under predetermined
conditions of pH or pH
and temperature. Polymeric materials must meet two criteria to be useful in
this invention.
They must be dissolved or dispersed in water and must contain pendant acid
functionality.
Preferably the polymeric material useful for the present invention is a film-
forming emulsion
(co)polymer composition, most preferably a metal crosslinked film-forming
emulsion
(co)polymer.
Metal crosslinked film-forming emulsion (sometimes referred to as latex)
(co)polymer
compositions are known, see USP 4,517,330; 5,149,745; 5,319,018, and 8,236,903
and US
Publication Nos. 20110118409 and US 20110230612. Suitable metal crosslinked
film-forming emulsion (co)polymers comprise one or
more acid functionalized polymer and optionally one or more comonomer which
are reacted
with a multivalent metal compound at a temperature above or below the Tg of
the acid
functionalized polymer to produce a crosslinked polymer.
The multivalent metal can be added as an aqueous soluble complex as described
in USP
4,517,330, and/or as an aqueous insoluble complex as described in USP
5,319,018 and
5,149,745. If the multivalent metal is added as an insoluble complex, then it
can be added at a
temperature corresponding to or below the calculated Tg of polymer and below
the
decomposition temperature of the polymer.
The multivalent metal compound is maintained in contact with the (co)polymer
for a
time sufficient to allow reaction to occur. The process produces a metal
crosslinked film-
forming emulsion that dries to a crosslinked polymer film. Preferably, the
metal crosslinked
film-forming emulsion (co)polymer is a metal crosslinked acrylic polymer,
preferably
magnesium, calcium, or copper crosslinked acrylic polymer, most preferably
zinc crosslinked
acrylic polymer.
Polymers that contain acid functionality only as terminal or end groups do not
produce
the desired crosslinked polymer film properties. The acid functionality may be
incorporated in
the polymer by known means using an effective amount, preferably from 4 to 90%
by weight of
the total monomers of acidic monomers. Examples of acidic monomers are
ethylenically
unsaturated acid monomers, such as acrylic acid, methacrylic acid, maleic
acid, itaconic acid,
maleic anhydride, vinyl phenol and mixtures thereof.
Other monomers in the polymer preparation are selected to produce the desired
end use
and application properties sought and include the polymerizable comonomers
which form soft
polymers in the presence of free radical catalysts and those that produce hard
polymers in the
presence of free radical catalysts. Examples of comonomers which polymerize to
form soft
Date Recue/Date Received 2021-02-12
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
polymers include primary and secondary alkyl acrylate, with alkyl substituents
up to eighteen or
more carbon atoms, primary or secondary alkyl methacrylates with alkyl
substituents of five to
eighteen or more carbon atoms, or other ethylenically-unsaturated compounds
which are
polymerizable with free radical catalysts to form soft solid polymers,
including vinyl esters of
saturated monocarboxylic acids of more than two carbon atoms. The preferred
ethylenically
unsaturated compounds are the stated acrylates, itaconates, and methacrylates,
and of these the
most preferred esters are those with alkyl groups of not more than 8 carbon
atoms.
The preferred monomers which by themselves yield soft polymers may be
summarized
by the formula:
0
I I
H2C=C¨C-0¨W1
R'
wherein R. is hydrogen or a methyl group and, when R is methyl Rx represents a
primary or
secondary alkyl group of 5 to 18 carbon atoms, and when R' is hydrogen, Rx
represents an alkyl
group of not over 18 carbon atoms, preferably of 2 to 8 carbon atoms and more
preferably 2 to 4
carbon atoms.
Typical compounds coming within the above definition are ethyl acrylate,
propyl
acrylate, isopropyl acrylate, butyl acrylate, isobutyl acrylate, sec-butyl
acrylate, amyl acrylate,
isoamyl acrylate, hexyl acrylate, 2-ethylhexyl acrylate, octyl acrylatc, 3,5,5-
.. trimethylhexylacrylate, decyl acrylate, dodecyl acrylate, cetyl acrylate,
octadecyl acrylate,
octadecenyl acrylate, n-amyl methacrylate, sec-amyl methacrylate, hexyl
methacrylate, 2-
ethylhexyl methacrylate, 2-ethylbutyl methacrylate, octyl methacrylate, 3,5,5-
trimethylhexyl
methacrylate, decyl methacrylate, dodecyl methacrylate, octadecyl
methacrylate, and those with
substituted alkyl groups such as butoxylethyl acrylate or methacrylate.
Another group of monomers which by themselves yield soft polymers are
butadiene,
chloroprene, isobutene, and isoprene. These are monomers commonly used in
rubber latices
along with a hard monomer also useful in this invention, such as
acrylonitrile, styrene, and other
hard monomers as given above. The olefin monomers, particularly ethylene and
propylene, are
also suitable soft monomers.
Examples of polymerizable ethylenically unsaturated monomers which by
themselves
form hard polymers, are alkyl methacrylates having alkyl groups of not more
than four carbon
atoms and alkyl acrylates having alkyl groups of not more than 2 carbon atoms
also tert-amyl
methacrylate, ter-butyl or tert-amyl acrylate, cyclohcxyl, benzyl or isobornyl
acrylate or
11
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
methacrylate, acrylonitrile, or methacrylonitrile, these constituting a
preferred group of the
compounds forming hard polymers. Styrene, vinyl chloride, chloride,
chlorostyrene, vinyl
acetate and a-methylstyrene, which also form hard polymers, may be used.
Preferred monomers, which by themselves form hard polymers, may be summarized
by
the formula:
H2C= C ¨ X
R'
wherein R is hydrogen or a methyl group and wherein X represents one of the
groups -CM,
phenyl, methylphenyl, and ester-forming groups, -COOR", wherein R" is
cyclohexyl or methyl
or ethyl or a tert-alkyl group of four to five carbon atoms, or, when R' is
methyl, an alkyl group
of two to four carbon atoms. Some typical examples of these have already been
named. Other
specific compounds are methyl methacrylate, ethyl methacrylate, propyl
methacrylate, isopropyl
methacrylate, isobutyl methacrylate, n-butyl methacrylate, sec-butyl
methacrylate, and tert-butyl
methacrylate. Acrylamide and methacrylamide may also be used as hardening
components of
the copolymer.
A further class of polymers of this invention is polymers of the esters of
vinyl alcohol
such as vinyl formate, vinyl acetate, vinyl propionate, vinyl butyrate and
vinyl versitate.
Preferred is poly(vinyl acetate) and copolymers of vinyl acetate with one or
more of the
following monomers: vinyl chloride, vinylidene chloride, styrene, vinyl
toluene, acrylonitrile,
methacrylonitrile, acrylate or methacrylate esters, and the functional group
containing
monomers given above.
These polymers can be prepared, for example by emulsion copolymerization of
the
several monomers in the proper proportions. Conventional emulsion
polymerization techniques
are described in USP 2,754,280 and 2,795,564. Thus the monomers may be
emulsified with an
anionic, a cationic, or a nonionic dispersing agent, about 0.5% to 10% thereof
being used on the
weight of total monomers. When water-soluble monomers are used, the dispersing
agent serves
to emulsify the other, less soluble monomers. A polymerization initiator of
the free radical
type, such as ammonium or potassium persulfate, may be used alone or in
conjunction with an
accelerator, such as potassium metabisulfite, or sodium thiosulfate. The
initiator and
accelerator, commonly referred to as catalyst, may be used in proportions of
1/2 to 2% each
based on the weight of monomers to be copolymerized. The polymerization
temperature may be
from room temperature to 90 C or more as is conventional.
12
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
Examples of emulsifiers or soaps suited to this polymerization process include
alkali
metal and ammonium salts of alkyl, aryl, alkaryl, and aralkyl sulfonates,
sulfates, and polyether
sulfates; the corresponding phosphates and phosphonates; and ethoxylated fatty
acids, alcohols,
amines, amides, and alkyl phenols.
Chain transfer agents, including mercaptans, polymercaptans, and polyhalogen
compounds, are often desirable in the polymerization mix.
Staged or sequential copolymers can also be crosslinked according to the
invention.
Particularly useful first stage copolymers are ethylene/ethylacrylate
copolymers and
ethylene/vinyl acetate copolymers containing added hydrophilic monomer.
Unless otherwise indicated, "Tg" is the glass transition temperature and "Tn,"
is the
melting temperature if the metal crosslinked film-forming emulsion
(co)polymer.
All of the transition metals are capable of forming polymeric crosslinks,
though care
must be exercised when considering the use of arsenic, mercury, cobalt,
copper, lead, cadmium,
nickel and chromium for a specific application due to high cost, toxicity, or
the production of a
color in the polymeric film. Certain transition metals such as aluminum,
tungsten, and tin that
could not be used in latent metal salt crosslinking because of their inability
to form a stable
amine complex are useful in the present invention. Combinations of transition
metals may be
effectively used.
The preferred metals, based on criteria of low cost, low toxicity, and low
color in the
crosslinked film, include zinc, aluminum, tin, tungsten and zirconium. Zinc
and aluminum are
particularly preferred. Useful compounds of the transition metals include the
oxide, hydroxide,
carbonate and acetate (usually the basic acetate due to the solubility concern
discussed below).
When used in emulsion or dispersions of acid-containing polymer, the metal
compounds
must be relatively insoluble since even moderately soluble salts (i.e., > 0.4%
in water at 60 C)
can produce excessively high levels of multivalent cations in solution. High
levels of
multivalent cations can cause dispersions or emulsions of acid-containing
polymer to precipitate
or sediment from the dispersion or emulsion because of the polymer's
multivalent cation
instability (the double layer is believed to be disrupted by multivalent
cations). This
requirement for a low solubility transition metal compound does not apply to
acid-containing
polymers in aqueous solution, but only to aqueous dispersions and emulsions of
acid-containing
polymers.
Preferably, the metal crosslinked film-forming emulsion (co)polymer has a Tg
or T.,
equal to or greater than the down hole formation temperature. Preferably, the
metal crosslinked
film-forming emulsion (co)polymer has a Tg equal to or greater than 45 C, more
preferably
equal to or greater than 65 C, more preferably equal to or greater than 90 C,
and more
preferably equal to or greater than 120 C. It is further desirable that the
metal crosslinked film-
13
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
forming emulsion (co)polymer has good compatibility with the surface of the
peroxide it is
coating. Good compatibility means that certain bonding is formed between acid
group in metal
cross-linked emulsion (co)polymer film and the hydroxide group on the surface
of the peroxide.
Films from the metal cross-linked acrylic polymers of the present invention
have very
different properties than films from non- metal cross-linked acrylics, for
example, have higher
tensile strength modulus and durability. Swell ratio gives an indication of
the extent of cross-
linking of the final film ¨ low swell ratio means tightly cross-linked and
high swell ratio less
cross-linked. Films from zinc cross-linked polymers swell less in basic
solution than films
from non-zinc polymers. Not to be held to any particular theory, we believe
that at high pH
(before the peroxide is activated by adding acid) the film of the present
invention swell less and
is less permeable to the filter cake breaker, e.g., magnesium peroxide, and
therefore less likely
to exhibit premature release. The film formed from the metal crosslinked film-
forming
emulsion (co)polymer preferably has a low swelling ratio at pH 10 and a high
swelling ratio at
pH 5. Swell ratio is equal to the mass of swollen polymer film/mass of dry
polymer film.
Preferred the swell ratio at pH 10 is equal to 1 and infinitely large at pH 5.
The application of the metal crosslinked fihn-forming emulsion (co)polymer to
encapsulate the filter cake breaker may be carried out in any suitable manner.
The
microparticles of the filter cake breaker and other coating components are
mixed together to
form an aqueous slurry. Unless otherwise indicated, weight percent of
components is based on
the final dry weight of the encapsulated internal breaker.
A good summary of encapsulation methods can be found in "Recent Development in
Microencapsulation of Food Ingredient" Drying Technology Vol: 23, Page: 1361-
1394, Year:
2005.
Preferably, the metal crosslinked film-forming emulsion (co)polymer is applied
to the
surface of the filter cake breaker by the method of spray drying. Spray drying
is preferably
done with an inlet temperature of from 100 C to 160 C, preferably 120 C to 140
C. Spray
drying is preferably done with an outlet temperature of from 30 C to 90 C,
preferably 60 C to
75 C. Optionally, the sprayed dried material may be heat treated. For example,
the film
encapsulated filter cake breaker may be thermally annealed at a temperature
close to the Tg or
Tm of metal crosslinked film-forming emulsion (co)polymer, typically in the
range of from 90 C
to 125 C for a period of time of from 45 to 90 minutes depending on the Tg or
Tm of the metal
crosslinked film-forming emulsion (co)polymer.
In another embodiment, the metal crosslinked film-forming emulsion (co)polymer
is
applied to the surface of the filter cake breaker by utilizing a fluidized
bed.
In yet another embodiment, the metal crosslinked film-forming emulsion
(co)polymer is
applied to the surface of the filter cake breaker by vaporization plus
grinding.
14
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
In yet another embodiment, the metal crosslinked film-forming emulsion
(co)polymer is
applied to the surface of the filter cake breaker by extrusion coating.
In yet another embodiment, the metal crosslinked film-forming emulsion
(co)polymer is
applied to the surface of the filter cake breaker by coacervation.
In yet another embodiment, the metal crosslinked film-forming emulsion
(co)polymer is
applied to the surface of the filter cake breaker by inclusion complexation.
Preferably, the filter cake breaker is present in the encapsulated internal
breaker in an
amount of from 30 to 90 weight percent, more preferably of from 40 to 90
weight percent, and
even more preferably of form 60 to 90 weight percent based on the total weight
of the
encapsulated internal breaker.
Preferably the encapsulating polymer, such as the metal crosslinked film-
forming
emulsion (co)polymer, is present in the encapsulated internal breaker in an
amount of from 5 to
55 weight percent, more preferably of from 10 to 45 weight percent, and even
more preferably
of from 15 to 25 weight percent based on the weight of the encapsulated
internal breaker.
In a preferred embodiment of the present invention we have found that it is
advantageous to include an anti-caking agent during the encapsulation of the
oxidizing agent.
The anti-caking agent helps to reduce agglomerization and/or facilitates
particle size control of
the final encapsulated oxidizing agent. Preferred anti-caking agents are
insoluble metal
carbonate microparticles such as magnesium carbonate or calcium carbonate;
colloidal silica
micro particles, for example suspensions of fine amorphous, nonporous, and
typically spherical
silica particles in liquid phase; and clay particles such as bentonite, and
the like.
In one embodiment, microparticles of the uncoated filter cake breaker are
spray dried
together with a dispersion consisting of a metal crosslinked film-forming
emulsion (co)polymer
and an anti-caking agent. Preferably, the anti-caking agent is present in an
amount of form 3 to
45 weight percent, more preferably 5 to 20 weight percent, even more
preferably 6 to 10 weight
percent based on the total weight of the encapsulated internal breaker. During
this process, the
metal crosslinked film-forming emulsion (co)polymer particles partially
collapse onto the filter
cake breaker's surface to form a discontinuous film. The anti-caking agent
maintains its
particulate morphology and is embedded into the above discontinuous film.
Afterwards, the
spray-dried encapsulated microparticles are thermally annealed at the Tg or
Tin of the metal
crosslinked film-forming emulsion (co)polymer to promote the formation of
continuous film on
filter cake breaker's surface. The presence of anti-caking particles in the
polymer film prevents
the encapsulated powders from further agglomeration, which is critical for
controlling the
particle size.
Optionally, the encapsulated internal breaker comprises a water-soluble
polymer as a
binder. If present, the water-soluble polymer is present in an amount of from
1 to 5 weight
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
percent based on the weight of the encapsulated internal breaker. The role of
the water-soluble
polymer is to enhance the viscosity of the formulated filter cake breaker
slurry so that uncoated
filter cake breaker microparticles do not readily precipitate out of the
slurry prior to spry drying
or whatever method is used for encapsulation. As the water evaporates from the
encapsulation
process, the water-soluble polymer chains also collapse on the surface of the
oxidizing agent
microparticles to form a minor component in the metal crosslinked emulsion
(co)polymer film.
Examples of suitable water-soluble polymers are polyacrylamide, polyacrylic
acid, poly(N-
isopropylacrylamide), poly(dimethylaminoethyl acrylate), polyvinyl alcohol,
polyethylene
oxide and copolymers of the above mentioned polymers.
In the case where pH alone is used to control peroxide release, a preferred
illustrative
embodiment of the present invention is to select as an encapsulating material
an enteric polymer
that is substantially insoluble at pH values equal to or greater than 7.5 and
that is more soluble
under conditions of decreasing pH. Thus as the pH decreases in value (i.e. the
solution becomes
more acidic) release of peroxide is promoted. In one preferred illustrative
embodiment the
enteric polymer encapsulated peroxide is combined with an alkaline wellbore
fluid of the type
described above. This fluid is then pumped into the wellbore and a filter cake
is formed that
incorporates the encapsulated peroxide. The peroxide source can now be
activated by causing
the down hole environment to become more acidic and thereby degrade the
encapsulating
material. Again, it should be emphasized that this is merely one possible
mechanism by which
the peroxide release may occur in the down hole environment. Those skilled in
the art will
recognize that other factors, or a combination of factors, may in fact result
in the peroxide's
release. The methods discussed here are intended to illustrate possible
mechanisms by which
peroxide release may occur and are not intended to narrow the scope of the
invention, as
defined by the claims herein.
For example, abrasion may contribute to peroxide release. As discussed above,
peroxides, such as magnesium peroxide, may form a protective coating when
added to water. It
is possible that the metal crosslinked film-forming emulsion (co)polymer
enhances this natural
coating, making the peroxide more resistant to abrasion. This abrasion
resistance may result in
the peroxide being more stable in the down hole environment and allowing its
release to be
more precisely controlled.
When the filter cake is no longer needed, it can be easily removed. In order
to remove
the filter cake, the peroxide source should be activated such that peroxide is
released in the
down hole environment. The peroxide will then react with and degrade any
peroxide
degradable polymers in the filter cake. As mentioned above, many different
methods of
activating the peroxide source can be used without departing from the scope of
the present
invention.
16
CA 02918957 2016-01-20
WO 2015/017633
PCT/US2014/049083
In a one illustrative embodiment where a peroxide coated with a metal
crosslinked film-
forming emulsion (co)polymer is used, the method of activating the peroxide is
by changing the
pH value of the down hole environment. Preferably, an alkaline wellbore fluid
is used to
deliver the peroxide and form the filter cake. Subsequently, the down hole
environment is made
more acidic, causing the release of peroxide.
For the general purposes and scope of the present invention, the method of
causing the
down hole environment to become more acidic is not limited to any particular
method.
However, two preferred methods will be discussed. Generally, it has been
discovered that
milder clean up treatments than those reported in the prior art can be
successfully used to
remove peroxide containing filter cakes.
One such method is to add an acid source to the down hole environment from the
surface. According to this method, an acidic soak solution is added to the
well. The acidic soak
solution will activate the peroxide, which will release peroxide, causing the
peroxide degradable
polymers (for example, polysaccharides) to degrade. The acid, depending about
the acid
selected and the strength of the acid, may also decompose the peroxide
degradable polymers to
some extent and may also be used to decompose acid soluble bridging agents
(such as calcium
carbonate). Additionally, the acid soak can be used remove an encapsulating
material from the
peroxide source, such as removing an acid soluble enteric polymer coating.
After decomposing the polysaccharide polymers with the acid solution, it is
preferred
that the filter cake containing the decomposed polymer be washed with a wash
fluid in which
the bridging particles are soluble, such as by contacting or circulating
within the borehole the
washing fluid. In this manner, the major components of the filter cake are
decomposed or
removed, and the filter cake is effectively destroyed and removed from the
sides of the
borehole.
In one non-limiting example of a cleanup solution, it is preferred that the
acidic soak
solution have no appreciable solubilizing effect on the bridging particles
within the filter cake.
This prevents the premature breakthrough of the soak solution through a
portion of the filter
cake and, hence, allows all of the filter cake to be contacted by the soak
solution until the
peroxide has decomposed the polysaccharide polymers substantially. If the
bridging particles
are water soluble, preferably the aqueous liquid in the soak solution will be
saturated with
respect to the bridging particles. Thus if the bridging particles are sized
sodium chloride, the
aqueous liquid will preferably be saturated with respect to sodium chloride.
Additionally, the
soak solution should have a density that is compatible with the density of the
liquid in the
borehole that the soak solution displaces in order to minimize mixing of the
soak solution with
the liquid. Preferably the soak solution contains inorganic water soluble
salts dissolved therein
in amounts up to saturation to provide the desired density. Typical salts are
generally selected
17
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
from the group consisting of sodium chloride, sodium bromide, potassium
chloride, potassium
bromide, calcium chloride, calcium bromide, zinc chloride, zinc bromide,
sodium formate,
potassium formate, cesium formate, and mixtures thereof. Certain of these
salts are acidic, and,
thus, they may also function as acidic substances to provide the required pII.
In this example, it is preferred that the soak solution be solids-free, i.e.,
contain no
appreciable content of suspended solids, thus contains essentially only
dissolved substances.
The soak solution may be filtered using procedures well known in the art of
using clear brine
fluids, if desired.
Although many types of clean up fluids can be used, one example is a soak
solution
that is preferably acidic to solubilize and activate the peroxide in the
filter cake. As mentioned
above, it has been discovered that milder clean up solutions can be used than
those reported in
the prior art. Representative acidic substances include hydrochloric acid,
phosphoric acid,
formic acid, acetic acid, proprionic acid, glycolic acid, sulfamic acid,
citric acid, aluminum
chloride, zinc chloride, zinc bromide, calcium bromide, ferric chloride,
sodium dihydrogen
phosphate, sodium acid pyrophosphate, acidic buffers, and the like. Sulfamic
acid and citric
acid are preferred.
In certain clean up solutions, it may be preferable, as will be appreciated by
those
skilled in the art, that the acidic solution comprise an acid in a saturated
solution of a water
soluble salt in which the bridging particles are not soluble, and that the
bridging particles are
water soluble. It is particularly preferred that the water soluble salt and
the bridging particles are
sodium chloride.
When clean up solutions such as those described above are used, the soak
solution
should contact the filter cake for a period of time that is at least
sufficient to decompose the
polysaccharide polymers in the filter cake to such an extent that the filter
cake becomes a
loosely adherent mass of bridging particles. The greater the acid strength Or
the higher the
formation temperature, and hence, the higher the temperature to which the soak
solution is
heated, the lower the time required to contact the filter cake with the soak
solution. Generally,
the soak solution will contact the filter cake from approximately one-half to
ten hours.
Thereafter the filter cake is preferably contacted with a wash solution to
remove the remaining
filter cake solids, principally the bridging particles and any remaining
polymers and polymer
degradation products.
The wash solution is one in which the bridging particles are at least
partially soluble.
Thus if the bridging solids are water soluble, the wash solution is
undersaturated with respect to
the bridging solids. If the bridging solids are acid soluble, a suitable
acidic solution is used as
the wash solution. If the bridging solids are oil or solvent soluble, a
suitable oil or solvent is
used as the wash solution.
18
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
It is preferred that the wash solution have no appreciable effect on the
permeability of
the hydrocarbon-containing formation. Thus aqueous solutions containing one or
more salts
which inhibit the swelling and/or dispersion of particles within the formation
are preferred.
Representative of such salts are potassium salts, such as potassium chloride
and potassium
.. acetate, ammonium chloride, quaternary ammonium salts of low molecular
weight, such as
tetramethyl ammonium chloride, tetraethylammonium chloride, and in general
tetraalkylammonium salts in which the alkyl groups independently contain from
1 to about 4
carbon atoms, and other salts as is known in the art. Preferably the wash
solution will contain
from about 0.05% to about 10% by weight of the salt, most preferably from
about 0.1% to about
5%. It is particularly preferred that the wash solution contain from about 2%
to about 5% by
weight potassium chloride or ammonium chloride.
A second illustrative method of acidifying the down hole environment such that
a
peroxide source is activated has been discovered in which fluids produced from
the
subterranean formation can be successfully used. It is known that formation
fluids are typically
acidic due to the presence of carbon dioxide, sulfur, mineral acids, oxygen
and organic acids.
Typically, these substances have been problematic due to their corrosive
effects on wellbore
apparatus; see, for example, USP 4,837,323; 5,960,878; and 6,248,700.
The acidic formation fluids can be used to activate peroxide sources in the
down hole
environment, obviating the need for acid solutions to be added to the
wellbore. This is
advantageous because it saves the operator the money otherwise spent on the
acid treatments,
labor costs and lost rig time while administering the acid soak and avoids
possible damage to
the subterranean formations from the acid soak and wash solutions. However,
additional fluids
may be used in addition to the produced fluids without departing from the
scope of the present
invention, so long as a substantial portion of produced fluids are used. For
purposes of the
present invention, a substantial portion of produced fluids means an amount
sufficient to cause a
change in the pH value of a down hole environment.
The formation fluids can be the stimulus that results in the removal of an
encapsulating
material from a protected peroxide source. In particular, the formation fluids
can be used to
lower the pH of a filter cake containing peroxides encapsulated in an enteric
polymer. The
formation fluids may activate peroxides coated with an enteric polymer.
In either method of acidifying the down hole environment, an activator can be
included
in the acidic soak solution to further activate the peroxide and/or to
increase the rate of reaction
between the peroxide and the polysaccharide. Known activators include iron and
copper salts
as well as salts of other transition metals, such as soluble ferric, ferrous,
cuprous, cobaltous,
nickelous, manganous, and the like salts. The activator should be soluble in
the soak solution.
The preferred metallic cation activator is a source of the ferric or ferrous
cation, such as ferrous
19
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
sulfate, ferrous ammonium sulfate, ferrous chloride, ferric ammonium sulfate,
and the like. The
amount of activator, if used, is not critical and need only be sufficient to
provide the desired rate
of reaction. Generally, a metallic cation activator will be present in an
amount from about
0.00025% to about 0.0025% by weight of the soak solution.
The following examples are included to demonstrate certain illustrative
embodiments
the invention. However, those of skill in the art should appreciate that many
changes, consistent
with the present disclosure, can be made in the specific embodiments which are
disclosed and
still obtain a like or similar result without departing from the scope of the
invention.
EXAMPLES
A description of the raw materials used in the Examples is as follows.
Mg02 is a magnesium peroxide with greater than 95
percent of the
particles having a particle size less than 100 micrometers
available from Solvay Chemicals as IXPER 35MTm.
AP is a latex emulsion comprising 48 weight percent
non-
crosslinked acrylic polymer (25% butyl acrylate/47% meth
methacrylate/10% 2-hydroxyethyl methylacrylate/18%
methacrylic acid) with a mean particle size of 230 nanometers in
water.
XAP-1 is a latex emulsion having a pH of 8.5 comprising
50 weight
percent zinc crosslinked (0.58 equivalents) acrylic polymer
(35% butyl acrylate/9% meth methacrylate/40% styrene/16%
methacrylic acid) with a mean particle size of 110 nanometers as
described in USP 4,517,330, 30 weight percent water, and about
20 weight percent solvents and emulsion stabilizers.
XAP-2 is a latex emulsion comprising 33 weight percent
zinc
crosslinked (0.58 equivalents) acrylic polymer (35% butyl
acrylate/9% meth methacrylate/40% styrene/16% methacrylic
acid) with a mean particle size of 100 nanometers as described in
USP 4,517,330, 50 weight percent water, and about 17 weight
percent solvents and emulsion stabilizers.
XAP-3 is a latex emulsion having a pH of 9 comprising 37
weight
percent zinc crosslinked (0.76 equivalents) acrylic polymer
(28% butyl acrylate/52% meth methacrylate/12% styrene/8%
81794346
methacrylic acid) with a mean particle size of 80 nanometers as
described in USP 6,548,596, 42 weight percent water, and about
21 weight percent solvents and emulsion stabilizers.
CaCO3 is an ultrafine ground calcium carbonate available
from Omya
Canada. Inc., as HYDROCARBTm 90.
PAM-PAA is the sodium salt of a water soluble
poly(acrylamide-co-acrylic
acid) copolymer with a molecular weight of 200,000 available
from PolyScience, Inc.
Encapsulation Procedure
Encapsulation is carried out using a Mobile Miner spray dryer equipped with a
two-fluid
nozzle atomizer manufactured by GEA Niro. Nitrogen (N2) pressure to the nozzle
is normally
set at 1 bar with 50% flow rate, equivalent to 6 kg/hr of air flow. The
magnesium peroxide
powder, acrylic encapsulation polymer, anti-caking agent (calcium carbonate),
and water-
soluble polymer binder (poly(acrylamide-acrylic acid sodium salt)) are mixed
and diluted with
deionized water under magnetic stirring for 5 to 10 minutes to make 25 weight
percent slurry.
The slurry is introduced into spray dryer by a rotator pump. The inlet
temperature was set at
125 C and the outlet temperature is controlled at 65 C. Once the atomized
slurry droplet is
sprayed into the chamber, water is rapidly vaporized by contact with hot N2 at
the inlet
temperature. The dried powders are then cooled to the outlet temperature as
they are pulled out
of the chamber by a vacuum fan. Most of the dried powder is separated from the
N2 flow in the
cyclone and recovered in the glass jar attached to the bottom of the cyclone
via an open valve.
Residual powders are filtered before ventilation. The spray-dried encapsulated
magnesium
peroxide samples are thermally treated by annealing at 90 C for 90 mm in an
oven under
ambient atmosphere and allowing it to cool at room temperature.
Particle Size Analyses
The encapsulated magnesium peroxide samples are re-dispersed into deionized
water to
make 1 to2 wt% solutions prior to analysis. Particle size and distribution are
measured by a
TM
Beckman Coulter LS 230 (Brea, CA) laser light scattering particle size
analyzer under standard
procedures and analyzed using a polystyrene latex model preset by the
instrument software (For
details, please see Schmidt, D.; Malotky, D. "Particle Size Distribution of
Aqueous Emulsions
by Laser Diffraction with a Beckman Coulter LS230" DOWN 101979-E03A).
The compositions and properties for Examples 1 to 7 are shown in Table 1.
Compositions are given in weight percent based on the total weight of the
dried encapsulated
magnesium peroxide sample.
21
Date Recue/Date Received 2021-02-12
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
Table 1
Example 1* 2 3 4 5 6 7
Composition, wt%
Mg02 73.6 73.6 49 73.6 49 73.6 49
AP 18.4
XAP-1 18.4 36.7
XAP-2 18.4 36.7
XAP-3 18.4 36.7
CaCO3 6.2 6.2 12.5 6.2 12.5 6.2 12.5
PMA-PAA 1.8 1.8 1.8 1.8 1.8 1.8 1.8
Properties
Mean Particle 10 9 13 10 15 20 45
Size, micrometers
* not an example of the present invention
Peroxide Release Profile
The peroxide release profile is produced using 2 different methods:
Method 1. Quantitative Premature Peroxide Release
1) 0.20 g encapsulated magnesium peroxide sample is mixed with 12.5 ml of
deionized
water in an ACE pressure vessel.
2) The vessel is hot rolled at 20 rpm in a ball-mill oven at 75 C.
3) The vessel is removed from the oven to take a sample aliquot (20 p,1) at
time intervals of
2, 4, 6 and 8 hours.
4) The sample aliquot is diluted 100 times with deionized water.
5) The peroxide level in the above diluted sample aliquot is quantitatively
determined
using a colorimetric hydrogen peroxide assay kit (Catalog Number: STA-343,
Cell
Biolabs, Inc). 20 p,1 diluted sample aliquot is further mixed with 200 p,1
aqueous
working reagent, followed by 30 minutes incubation on a shaker at room
temperature.
The resulting mixture solution is scanned from 540 nm to 600 nm. The peroxide
level
of the diluted sample is determined from the absorbance at 585 nm, from which
the
actual peroxide level in the original sample solution is calculated.
22
CA 02918957 2016-01-20
WO 2015/017633 PCT/US2014/049083
Method 2. Semi-Quantitative Triggered Peroxide Release
1) 0.20 g encapsulated internal breaker sample was mixed with 12.5 ml of
deionized water
in an ACE pressure vessel.
2) The vessel was hot rolled at 20 rpm in a ball-mill oven at 75 C for 16
hours.
3) The ACE vessel was removed from the oven for adding 25 mL of a 5 wt% Na2Ell
LA
solution to reduce the pH from 10 to 5.5. The sample mixture is then returned
to the
oven and further hot rolled at 20 rpm and 75 C.
4) The vessel was removed from the oven to take a sample aliquot (50 ill) at
time intervals
of 1, 2, 4, 6 and 8 hours.
5) The sample aliquot is diluted 40 times with deionized water. The peroxide
level of the
diluted sample is read from the peroxide testing paper (Merck 10011-1: 0-25
mg/L),
which is converted to the actual peroxide level in the original undiluted
sample solution.
The peroxide release profiles for Examples 1 to 7 are shown in FIGs. 1 to 7,
respectively.
23