Note: Descriptions are shown in the official language in which they were submitted.
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
INTRA-ABDOMINAL PRESSURE TO PROMOTE HEMOSTASIS AND SURVIVAL
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of priority to U.S. Provisional
Patent Application No.
61/864,368 by Adam Rago et al. entitled "INTRA-ABDOMINAL PRESSURE TO PROMOTE
HEMOSTASIS
AND SURVIVAL." This application is also a Continuation-In-Part of United
States Patent Application
No. 13/209,020 entitled In Situ Forming Hemostatic Foam Implants," filed
August 12, 2011("Sharma
I") and claiming priority to United States Patent Application No. 12/862,362
entitled "Systems and
Methods Relating to Polymer Foams," filed August 24, 2010 ("Zugates II"),
which in turn claims priority
to United States Provisional Patent Application No. 61/263,314, also entitled
"Systems and Methods
Relating to Polymer Foams" and filed July 27, 2010 ("Zugates I"). This
application makes reference to
United States Provisional Patent Application No. 61/852,051, filed March 15,
2013 ("Sharma II"). The
entire disclosure of each of the foregoing references is hereby incorporated
by reference for all
purposes.
TECHNICAL FIELD
[0002] Systems and methods relating to polymer foams are generally described.
BACKGROUND
[0003] In situ forming polymer foams, such as the Arsenal Foam Technology
commercialized by
Arsenal Medical (Watertown, MA), have a number of important biomedical
applications including the
prevention or treatment of hemorrhage, particularly from noncompressible or
difficult-to-visualize
wounds, vascular embolization, arteriovenous malformation, AV fistulas,
abdominal aortic aneurysm,
space filling and bulking (e.g. following surgical resection, or for cosmetic
purposes), prevention of
1
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
tissue adhesion, hernia repair, prevention or treatment of reflux, and
temporary or permanent
occlusion of body lumens for a variety of applications including
sterilization, prevention of calculus
migration during lithotripsy, and other applications. The diversity of
applications for in situ forming
foams reflects significant advantages possessed by such foams relative to
existing technology,
including, without limitation their incorporation of well characterized,
biocompatible materials; the
ability to deliver in situ forming foams to closed cavities, for example
intravascularly; the ability to
deliver in situ forming foams to difficult-to-access body sites; the ability
of in situ forming foams to
expand into empty space, potential space, or into space filled with blood,
support surrounding tissues,
and the ability of the foam to fill a body cavity.
[0004] Foams are typically generated in situ by delivering and mixing multiple
liquid-phase
components (such as a polyol component and an isocyanate component, which form
a polyurethane
foam). Pores within the foam may be formed by a blowing reaction and/or by the
entrainment of gas
before or during foam formation, and the foam may harden through the formation
of cross-links
between prepolymers and/or cross-linking agents. When deployed into a body
cavity, the liquid
components react, driving the expansion and hardening of the foam. The foam
applies pressure to the
boundaries of the cavity in a dose dependent and time-dependent manner, for
example as shown in
the pressure curves of Fig. 1. The shapes of these curves are determined by,
among other things, the
composition and quantity of liquid phase components applied to the body
cavity, which govern the
rates of the blowing and cross-linking reactions and foam properties (e.g.,
density or volume
expansion, stiffness, pore size, hydrophilicity, absorption capacity).
[0005] In situ forming foams are particularly well suited to treating
noncompressible hemorrhages in
challenging settings, including the battlefield and rural or wildnerness
settings far from hospital
trauma centers. However, in spite of their advantages, in situ forming foams
have not been widely
used because of the technical challenges associated with developing suitable
in situ foaming
formulations for different applications and delivering of these formulations
to body cavities in
2
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
quantities sufficient to arrest hemorrhages without causing undesirable side
effects of excessive
pressure such as compartment syndrome. Additionally, to maximize their
efficacy in challenging or
remote settings, in situ forming foams should extend patient survival times
for a period sufficient to
permit evacuation of patients to stations or centers where hemorrhages can be
surgically treated.
SUMMARY OF THE INVENTION
[0006] Embodiments of the current invention address the challenges described
above by providing,
in one aspect, a method for treating hemorrhage within a body cavity or
potential space that includes
applying pressure to an interior boundary of the cavity, including pressure to
the injury itself, which
pressure is characterized by a transient peak value and by at least one steady
state value. In various
embodiments, the transient peak value is between 22 mmHg and about 86 mmHg
(e.g. 20, 51 and 84
mmHg). The steady state value is, in various embodiments, between about 14
mmHg and about 28
mmHg (e.g. 14 or 28 mmHg). Pressure may be applied, in certain embodiments, by
an article placed
into the closed cavity, and the article can be a foam which is formed inside
of the cavity by applying a
formulation that includes one or more liquid phases into the cavity. In some
cases, the steady state
pressure occurs within three minutes and is followed by the steady state
value. The steady state value
may be, in some cases, 30%, 50% or 90% of the transient peak value.
[0007] In another aspect, the invention relates to a kit for treating
hemorrhage in closed cavities that
includes (i) a formulation for forming a foam when disposed into a body, which
formulation includes
at least one liquid phase, and (ii) instructions for performing the method set
forth above.
DRAWINGS
[0008] In the drawings, like reference characters refer to like features
through the different views.
The drawings are not necessarily to scale, with emphasis being placed on
illustration of the principles
of the invention.
3
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
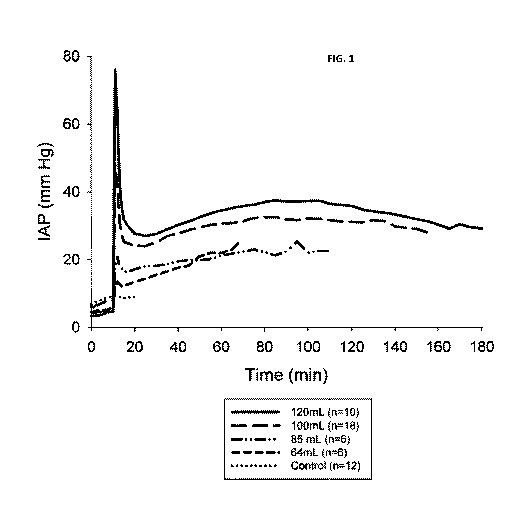
[0009] Fig. 1 plots intra-abdominal pressure over time for four doses of an in
situ forming foam in a
severe porcine liver hemorrhage model compared with an untreated control.
[0010] Fig. 2 plots survival curves for porcine treated with four doses of an
in situ forming foam in a
severe liver hemorrhage model compared with an untreated control.
[0011] Fig. 3 plots the survival times of 81 animals treated either with in
situ forming foams or no
foam treatment controls against the peak intra-abdominal pressure measured in
each animal in a
severe liver hemorrhage model.
[0012] Fig. 4 plots the survival times of 81 animals treated either with in
situ forming foams or no
foam treatment control animals against the intra-abdominal pressure measured
at 30-minutes post
injury in each animal in a severe liver hemorrhage model.
[0013] Fig. 5 plots intra-abdominal pressure over time for two doses of an in
situ forming foam in a
severe arterial hemorrhage model.
[0014] Fig. 6 plots survival curves for animals treated with two doses of an
in situ forming foam in a
severe arterial hemorrhage model.
[0015] Fig. 7 plots intra-abdominal pressure over time for animals given in
situ forming foam, gas
insufflation, or no treatment.
[0016] Fig. 8 plots intra-abdominal pressure over time in recently deceased
cadaver samples
following delivery of an in situ forming foam formulation.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
ANIMAL MODELS
[0017] In situ forming polymer foams were deployed at varying dosages in two
animal models of
hemorrhage: First, a lethal grade V hepatic and portal vein injury in swine,
as described in Duggan MJ,
et al. "Development of a lethal, closed-abdomen grade V hepatoportal injury
model in non-
4
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
coagulopathic swine." J. Surg. Res. 2013 Jun 1; 182(1): 101, 107 ("Duggan 1"),
which is hereby
incorporated by reference in its entirety and for all purposes. In this model,
wires were placed
strategically around the hepatic vasculature and externalized percutaneously.
After closure of a
midline incision, the wires were pulled, resulting in a severe, uncontrolled,
closed-cavity injury and
sudden, massive hypotension. In the absence of intervention the injury was
over 90% lethal at one
hour.
[0018] The second model was a lethal arterial injury, in which a wire was
placed around the external
iliac artery and externalized percutaneously as above as described in Duggan
MJ, et al. "Development
of a lethal, closed-abdomen, arterial hemorrhage model in non-coagulopathic
swine." J. Surg. Res.
2014; 187: 536-541 ("Duggan 2"), which is hereby incorporated by reference in
its entirety and for all
purposes. Also as above, following closure of a midline incision, the wire was
pulled, resulting again
in a severe, uncontrolled, closed cavity injury, and massive hypotension which
was over 80% lethal at
one hour in the absence of intervention.
[0019] These models were selected to approximate certain conditions in which
the systems and
methods of the current invention are used, namely intra-cavity and/or
noncompressible hemorrhages,
or hemorrhages which result in sudden and severe hypotension and/or which are
lethal in the absence
of an intervention.
[0020] In both models, the hemorrhage was generated in, and foams were
deployed into, the
abdominal cavity. The pressure within the abdomen (the "intra-abdominal
pressure" or "IAP") was
measured in all animals by continually monitoring bladder pressure through an
intraabdominal
pressure device (Abviser), consistent with clinical practice in measurement of
IAP.
IN SITU FORMING FOAMS
[0021] According to various embodiments of the invention, a patient suffering
from a closed-cavity
or non-compressible hemorrhage is treated by administering an in situ forming
foam as described in
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
Zugates I and II and Sharma I and II. In preferred embodiments, the liquid
phases include (a) an
isocyanate to generate gas and crosslink and (b) a polyol to control the foam
properties upon reaction
with the isocya nate. The formulation reacts to generate a foam within two
minutes of its deployment
into the body, and is characterized by the parameters shown in Table 1, below.
VOLUME EXPANSION: 26.2 - 85.3 fold
COMPRESSION FORCE DEFLECTION (CFD) 2.2-10.1 kPa
AT 50% DEFLECTION:
WATER UPTAKE: 2.7 - 7.7 gig
RISE TIME: 47.4-80.4 s
TABLE 1: PHYSICAL CHARACTERISTICS OF FOAMS USED
[0022] In preferred embodiments, administration of foams of the invention
cause a rapid, transient
increase or spike in pressure within the cavity or within a portion of a
boundary of a cavity containing
a hemorrhage. Following the spike the pressure preferably remains elevated at
a steady-state value
or within a steady-state range that is less than the peak pressure for an
extended period of time.
Exemplary temporal pressure profiles which include spikes and steady-state
pressure ranges are
shown for various doses of foam in Fig. 1. The steady-state pressure may
persist for a suitable interval
which permits evacuation of the patient to a site where medical treatment is
available, for instance
about 60, 90, 120, 150, 180, 210 or 240 minutes, or longer. The magnitude of
the pressure spike may
vary depending on the formulation and dose used, so that different device
formulations and/or doses
might be selected to suit different applications and different patient body
sizes or types. Additionally,
the magnitude of the steady state pressure may be dose dependent, and may be
affected by
interventions which affect the rate of foaming, cross-linking, or degradation
of the foam. For instance,
in some embodiments, a user may decrease a steady-state pressure by applying a
material which
tends to degrade the foam in a quantity selected to rapidly degrade a portion
of the foam. In other
embodiments, the delivery system may modulate peak or steady state pressure
through a dose
modulation feature, a relief valve, or a similar feature. Exemplary materials
are described in Sharma I
6
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
and in Zugates I and II. Delivery systems and methods suitable for use in
connection with methods of
the present invention are set forth in Sharma II.
[0023] The pressure spike ensures that sufficient force is applied to the
boundaries of the body cavity
and/or the injury site to effectively close hemorrhaging. Studies by others
have generally shown that
sustained, elevated pressure within body cavities, and particularly elevated
IAP above 20 mmHg can
have adverse effects on patients. For example, Vivier et al. ("Effects of
increased intra-abdominal
pressure on central circulation", Br. J. Anaesthesia 96(6): 701-7 (2006),
which is hereby incorporated
by reference for all purposes), found vascular changes including increases in
left ventricular end-
diastolic area and pressure (markers of increased cardiac preload) were
significantly increased at
elevated IAP values.
[0024] Sustained IAP values of 20 mmHg and concomitant organ system failure
are classified as
"compartment syndrome," a condition characterized by pain, paralysis,
paresthesia, and other
undesirable effects including, in some instances, lethality. In compartment
syndrome, increased
compartmental pressure limits blood supply to muscles and nerves within the
compartment. In an
effort to avoid undesirable changes in circulation and/or compartment
syndrome, prior art systems
and methods have been designed to limit applied compartmental pressures to
values below about 20
mmHg or about 30 mmHg.
[0025] In spite of the risks associated with sustained, elevated IAP, some
studies have suggested that
elevated IAP may be useful in reducing hemorrhage in some animal models. For
example, Sava, etal.,
"Abdominal insufflation for prevention of exsanguination," J. Trauma 2003,
Mar; 54(3): 590-4 found
that, in a swine model in which a 2.7 mm hole was made in the inferior vena
cava in which animals
were euthanized after 15 minutes of monitoring, CO2 insufflation resulted in
significantly lower blood
loss and significantly higher arterial pressure relative to control. Velmahos,
et al. "Abdominal
insufflation decreases blood loss without worsening the inflammatory response:
implications for
prehospital control of internal bleeding" found that, in a swine model
involving a lacerated spleen,
7
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
CO2 insufflation reduced blood loss relative to control. Finally, Jaskille, et
al. "Abdominal insufflation
decreases blood loss and mortality after porcine liver injury." J. Trauma 2005
Dec; 59(6)1305-8;
discussion 1308, found that CO2 insufflation at 20 cmH20 in a swine model of
blunt liver trauma
reduced blood loss by 69%. (Each of the foregoing references is hereby
incorporated by reference for
all purposes.) However, these studies have not found elevated IAP to be
completely effective in
reducing hemorrhage or improving survival of lethal abdominal hemorrhages, and
they have not
demonstrated extended survival without significant adverse effects.
[0026] The inventors have found that pressure profiles such as those shown in
Fig. 1 are effective in
treating life-threatening non-compressible hemorrhage in swine (as discussed
in greater detail
below), while avoiding complications associated with compartment syndrome.
Without wishing to
be bound to any theory, it is believed that a transient application of high-
pressure within a cavity
rapidly prevents ongoing hemorrhage from injured vasculature and permits clots
formation in the
absence of robust ongoing flow. The application of a lower, but still elevated
steady-state pressure
maintains hemostasis.
[0027] The inventors have also found that, while pressure alone is sufficient
to achieve some
reduction in hemorrhage, and to improve survivability from non-compressible
hemorrhage, the
application of pressure by a foam such as an in situ forming foam or another
material having a solid
or partially solid surface improves the efficacy of the systems and methods of
the invention. While
pressures may be applied by a variety of means, including gas or liquid
insufflation, the use of foams
to treat hemorrhages in cavities may be particularly advantageous. Though not
wishing to be bound
to any theory, it is believed that three characteristics of the foams
described above and in Sharma I
and Zugates I and II are particularly useful in treating hemorrhages: first,
the in situ reaction of the
foam device spontaneously creates a transient increase in pressure, followed
by a return to steady
state. The foam system does not require monitoring or feedback systems.
Second, the foam system is
portable and easily administered. Third, the material provides a solid, steady
source of intraabdominal
8
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
pressure. It does not readily leak, nor is it generally absorbed by the body.
Therefore, the pressure
does not need to be maintained by the addition of materials.
[0028] In spite of the advantages of in situ forming foams for treatment of
hemorrhages in cavities,
other treatment systems and methods are within the scope of the invention
insofar as it is possible to
achieve the pressure profiles discussed above using these systems and methods.
As non-limiting
examples, gels, elastomeric solids, pre-formed foams, or combinations of foams
and liquids or foams
and gasses can be utilized to apply pressure to the cavity or a portion of the
boundary of the cavity
(including injured tissue and tissues adjacent to injured tissue).
Additionally, materials which undergo
phase changes within cavities, for example reverse thermosensitive materials
including poloxamers,
and electromechanical or hydraulic devices can be used to deliver pressure to
hemorrhages within
cavities. And pressure may be delivered via materials, systems and devices
deployed either within the
cavity or outside of the cavity (e.g. adjacent to the cavity). In some
embodiments, the cavity is
constricted from the outside, for example by constricting the abdomen from the
exterior.
[0029] Pressure can be generated in certain models through the delivery and/or
removal of material
(e.g. liquid, gas, gel, foam) to apply pressure to the cavity, and the
pressure applied may be controlled
manually by an end user, mechanically by a governing device such as a valve
(e.g. a check valve), or by
computerized means.
[0030] The principles of the invention in its various embodiments are further
illustrated by the
following non-limiting examples:
Example 1: Dose-dependent changes in spike and steady-state pressure values
and survival in a hepatic
hemorrhage model.
[0031] In situ forming foams were delivered in the grade V hepatic-portal
model described above.
Animals were given 64 ml, 85 ml, 100 ml or 120 ml of liquid-phase in situ
forming foam formulations,
or sham treatment in the case of control animals, and IAP was measured over an
interval of 3 hours.
9
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
As shown in Fig. 1, control animals showed only a minor increase in IAP over
the first 20 minutes of
the experiment, while foam-treated animals displayed dose-dependent changes in
spike pressures
and steady-state pressures. In addition, survival was quantitated for animals
in each treatment
category.
[0032] Foam dosages in the swine models used herein are likely to be larger
than the volumes used
in humans, owing to differences in the volume of the abdominal cavity.
[0033] The control group was lethal in 11 of 12 animals (8.3% survival), with
a median survival time
of 23 minutes (quartile 20- 38). Survival rate with hepato-portal injury was
highest at the 120m1 dose
(90%; p=0.0002) and decreased in a dose-dependent fashion: 100m1 (72.2%;
p=0.0007), 85m1 (33.3%;
p=0.22), and 64m1 (16.7%; p=0.47). The Kaplan-Meier graph of all groups is
shown in Fig. 2. Median
survival time was 180 (180 - 180) minutes at 120mL, 180 (161-180) minutes at
100mL, 89 (81-158)
minutes at 85mL, and 55 (40-68) at 64mL. Relative to controls, survival time
as measured by the log-
rank test was significantly improved in all groups. Hemorrhage rate was also
reduced in all groups, but
lowest in the 120mL dose group vs. control group (0.34 0.052 vs. 3.0 1.3
ml/kg/min, p=0.001).
[0034] A relationship was also identified between peak (spike) pressure and
survival time in swine.
Fig. 3 plots survival time against peak pressure values observed in 69 pooled
experiments. A significant
survival benefit was observed for higher peak pressures as described in the
Table 2:
Peak IAP (mmHg) Mean survival time (min)
<20 46
20 to 69 141
>69 170
TABLE 2: MEAN SURVIVAL TIME AS A FUNCTION OF PEAK IAP.
[0035] A relationship was also identified between steady-state pressure and
survival time in swine.
Fig. 4 plots the IAP measured 30 minutes after injury against survival time,
and indicates that survival
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
improved at steady-state pressures at or above 18 mmHg, and improved further
at steady state
pressures at or above 28 mmHg (see Table 3):
IAP at 30 minutes post-injury Mean survival time (min)
(mmHg)
>28 55
14 to 28 149
>28 165
TABLE 3: MEAN SURVIVAL TIME AS A FUNCTION OF IAP AT 30 MINUTES POST-INJURY.
Example 2: Dose-dependent changes in spike and steady-state pressure values
and survival in an
arterial hemorrhage model.
[0036] Similar experiments were conducted in the arterial hemorrhage model
described above. As
shown in Figs. 5 and 6, dose dependent effects on IAP and survival were
observed in this model as
well. There was a noticeable increase in percent survival at 1 hour for the
120 ml and 100 ml test
groups relative to the control group (84% and 82%, respectively, vs. 14%;
p<0.001). Hemorrhage rate
at the experimental endpoint was significantly lower in both foam groups
relative to the control.
[0037] By way of summary of the foregoing examples, Table 4 below depicts six
exemplary
experiments in which varying quantities of an in situ foaming formulation was
administered to porcine
in a liver hemorrhage and an iliac hemorrhage model.
Liver Hemorrhage Model Iliac Artery Hemorrhage Model
Swine Foam Peak Steady State Peak Steady State
Dose Pressure Pressure Pressure Pressure
(mmHg) (mmHg) (mmHg) (mmHg)
120 mL 86 28 44 16
100mL 51 25 22 12
85 mL 21 18 -- --
64 mL 14 14 -- --
TABLE 4: TRANSIENT PEAK AND STEADY STATE PRESSURES OBSERVED IN VARIOUS
EXPERIMENTS.
[0038] All doses tested here demonstrated safety and a survival benefit. As
the table illustrates, the
peak pressures observed range from 14 to 86 mmHg in the liver hemorrhage model
and from 22 to 44
11
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
mmHg in the iliac artery across both hemorrhage models. Steady state values
ranged from 12 to 20
mmHg in both injury models.
Example 3: Approximation of survival effects by qas insufflation with foam-
like pressure kinetics.
[0039] While not wishing to be bound to any theory, it is believed that the
foams disclosed above
and used in the previous examples generated pressure spikes by one or more of
a rapid increase in
the volume of the foam, gas generated from the blowing reaction, gas generated
and not fully
contained by the foam, etc. The reduction in pressure to the steady state
values, in turn, may have
been due to one or more of a viscoelastic response of the tissues in the
abdominal cavity, a change in
fundamental abdominal volume, possibly caused by the relaxation of tissues in
the abdomen, a
reduction in the volume of the foam, gas transfer, etc.
[0040] In the models examined, larger doses of foam were associated with
larger peak pressures, as
shown in Fig. 1, and with improved survival, as shown in Fig. 2. To examine
the relationship between
the survival effect and the IAP profiles achieved, controls and animals
treated with 100 ml of foam
formulation were compared with animals treated by a CO2 gas insufflation
protocol which
approximated the pressure profile observed in foam treated animals. The
various IAP measurements
from the three groups are presented in Fig. 7. In gas treated animals, 1-hour
survival was about 75%,
while three hour survival was about 50%. Although these values did not match
those seen in the
highest-dose cohorts of foam-treated animals, they did suggest that employing
gas insufflation or
another pressure-generating technique to achieve a pressure profile that
includes a transient spike or
peak followed by a steady state pressure is a potentially effective treatment
for hemorrhage.
Example 4: Observation of spike-and-steady state pressure profiles in recently
deceased human
subjects.
[0041] To understand performance in representative human anatomy, the Arsenal
Foam System was
deployed in recently deceased human subjects. Subjects with no abdominal
pathology or prior surgery
12
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
were identified and informed consent was obtained from family members post-
mortem. Within three
hours of death, the abdomen was accessed and 1500mL fluid was added to
simulate severe
hemorrhage. Self-expanding polyurethane foam was administered at multiple
doses using a
prototype delivery system. Intraabdominal pressure was monitored as a function
of time for 15
minutes, after which the foam was removed and contact with abdominal tissues
was evaluated.
[0042] The Arsenal Foam System was successfully deployment in 10 subjects
within three hours post-
mortem. Intraabdominal pressure was recorded, as shown for a representative
sample in Fig. 8
[0043] Foam administration resulted in a rapid, transient, and dose-dependent
peak in intraabominal
pressure following deployment in humans. Notably, the shape of the curves was
similar to that
observed in animal models, suggesting that the foam system results in a
characteristic intraabdominal
pressure following deployment.
[0044] Because subjects in this study were not alive, safety and efficacy of
the foam at varying doses
could not be measured directly. However, pressure can be used as a surrogate
endpoint to link results
in swine to results in recently deceased humans. These results suggest that
the intraabdominal
pressure profiles observed in swine can be matched in humans. While not
wishing to be bound by any
theory, it is believed that human doses which result in the intraabdominal
pressure profiles observed
to be effective in swine models will be safe and effective in humans.
CONCLUSION
[0045] The phrase "and/or," as used herein should be understood to mean
"either or both" of the
elements so conjoined, i.e., elements that are conjunctively present in some
cases and disjunctively
present in other cases. Other elements may optionally be present other than
the elements specifically
identified by the "and/or" clause, whether related or unrelated to those
elements specifically
identified unless clearly indicated to the contrary. Thus, as a nonlimiting
example, a reference to "A
and/or B," when used in conjunction with open-ended language such as
"comprising" can refer, in one
embodiment, to A without B (optionally including elements other than B); in
another embodiment, to
13
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
B without A (optionally including elements other than A); in yet another
embodiment, to both A and
B (optionally including other elements); etc.
[0046] The term "cavity' as used herein means closed body compartments such as
the abdominal
cavity, the thoracic cavity, etc., as well as cavities that are open, such as
junctional wounds, and
"pseudocavities" in which at least one boundary of the cavity is defined by a
structure other than an
organ or a tissue, (e.g. a bandage).
[0047] The term "consists essentially of" means excluding other materials that
contribute to function,
unless otherwise defined herein. Nonetheless, such other materials may be
present, collectively or
individually, in trace amounts.
[0048] As used in this specification, the term "substantially,"
"approximately" or "about" means plus
or minus 10% (e.g., by weight or by volume), and in some embodiments, plus or
minus 5%. Reference
throughout this specification to "one example," "an example," "one
embodiment," or "an
embodiment" means that a particular feature, structure, or characteristic
described in connection
with the example is included in at least one example of the present
technology. Thus, the occurrences
of the phrases "in one example," "in an example," "one embodiment," or "an
embodiment" in various
places throughout this specification are not necessarily all referring to the
same example.
Furthermore, the particular features, structures, routines, steps, or
characteristics may be combined
in any suitable manner in one or more examples of the technology. The headings
provided herein are
for convenience only and are not intended to limit or interpret the scope or
meaning of the claimed
technology.
[0049] Certain embodiments of the present invention have described above. It
is, however, expressly
noted that the present invention is not limited to those embodiments, but
rather the intention is that
additions and modifications to what was expressly described herein are also
included within the scope
of the invention. Moreover, it is to be understood that the features of the
various embodiments
described herein were not mutually exclusive and can exist in various
combinations and permutations,
14
CA 02919856 2016-01-28
WO 2015/021375
PCT/US2014/050332
even if such combinations or permutations were not made express herein,
without departing from
the spirit and scope of the invention. In fact, variations, modifications, and
other implementations of
what was described herein will occur to those of ordinary skill in the art
without departing from the
spirit and the scope of the invention. As such, the invention is not to be
defined only by the preceding
illustrative description.