Note: Descriptions are shown in the official language in which they were submitted.
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
SINGLE SOLUTION FOR ELECTRO-ELECTROLESS DEPOSITION OF METALS
RELATED APPLICATIONS
This application claims priority and the benefit of 35 USC 119(e) to United
States
Provisional Patent Application No. 61/864135, filed August 9, 2013.
SCOPE OF THE INVENTION
The present invention provides for a single solution for use in a combined
process for
electroplating and electroless deposition of metal coatings as part of a
hybrid deposition
process.
BACKGROUND OF THE INVENTION
The current methods of depositing electroless gold use cyanide for the reason
that
cyanide baths are stable and relatively easy to use in both electroplating and
electroless
plating processes. For example, while Au(I) sulfite has been used in
commercial baths, the
complex is susceptible to disproportionation, forming Au(III) and metallic
gold. This
spontaneous decomposition of the bath has led commercial baths to use
proprietary stabilizing
additives.
Electroless deposition processes have been combined with the deposit of other
non-
conductive and semi-conductive materials such as Teflon, diamond dust and
sulfur
suspended as a powder in the electroless solutions, and which carried with the
metal to the
surface being coated to provide unique properties to the coating process.
SUMMARY OF THE INVENTION
The present invention provides a hybrid deposition process which uses both
electroplating and electroless deposition to co-deposit metals, or deposit
metal coatings or
layers, and most preferably differing metal coatings on layers, on a
substrate.
1
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
More preferably, in one non-limiting embodiment, the process is used to
deposit
metallic gold layers using a sulfite bath without any breakdown, in place of a
cyanide bath.
This is environmentally beneficial, and minimizes cost for waste
treatment/removal.
In another embodiment, simple electro-electroless deposition baths are
provided for
the deposition of different metal layers including, for example, nickel and Ni-
Zn-P coatings,
and Cu and Ni coatings. More preferably, the electro-electroless deposition
baths are used in
a multi metal hybrid electro-electroless coating process to selectively coat
one metal using an
electro-plating process, then turning off the electroplating process and
activating the plating of
a second different metal which then coats the article using the electroless
process.
In another embodiment, the electro-electroless deposition is provided a hybrid
deposition process, whereby multiple layers of thin metal films, or multi-
layers, are deposited
from a single solution by interspacing electroplated layers of a first metal
or alloy, within a
constant electroless deposition of a second different metal or alloy. The
electro-electroless
deposition process may provide a range of possible deposits characterized by
two or more
streams, multi-layers and, optionally, alloying. Most preferably, the process
allows for
control over the degree of alloying of electro-deposited layer, and/or even
electro-doping of
the electroless deposit for alloy creation.
For the formation of electro/electroless multi-layer deposits, it has been
appreciated a
constant slower deposit occurs for the metal or alloy which is to be formed by
way of
electroless deposition, such as nickel phosphorus [Ni-P], while a current or
electric pulses are
only required for the deposition of a second other metal to be formed by
electrodeposition.
Some alloying may further be expected during the electroplating phase. The
degree of
alloying and/or layer formation may be controlled by varying the deposition
rate of the
electroplated layer, compared to the constant electroless deposition rate.
Alloying is
dependent on the overlap, or lack of overlap, of the electroplating windows of
the metal ions.
2
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
If the window is sufficiently narrow, minimal alloying is to be expected, as
only the targeted
species would be deposited.
In cases where a large over-potential would deposit some of the metal to be
electrolessly deposited, it is expected that following criteria from
electroplated multi-layers
would lead to best results. Namely, it is expected the more noble metals, such
as Cu, at a
lower concentration may be electrolessly deposited, while a less noble metal
present at a
higher concentration, such as Ni, is electroplated.
Without being limited to a particular
process or theory, preferably, multi-layer deposition is performed using a
plating bath solution
having an electroless plating ion metal source, and either an anion or
electroplating metal ion
source in solution. Most preferably, the electroplated metal ions are selected
not to interfere
with, and act purely as 'spectators', to the electroless deposition process.
In another possible embodiment, copper/nickel [Cu/Ni] multi-layer metal thin
films
are deposited from a single solution. Cu is present at a lower concentration
and deposited at a
lower potential around 0.17V, whilst Ni is typically present at a higher
concentration, several
thousand times higher, and deposited at the higher potential, around 1.19V. As
such, at 0.17V
Cu alone is deposited, while at 1.19V a small amount of Cu is included in the
Ni deposit. The
higher concentration of the Ni salt is so that during Ni phase of the deposit,
minimal Cu is co-
deposited. Additionally, the Ni phase is deposited faster, using shorter
pulses than the Cu
phase.
In addition to the potential for multi-layer deposition, it has been
appreciated that by
providing a single electroless electrodeposition solution, voltage pulses that
are sufficiently
short and frequent may provide a means to dope the electroless deposit,
creating an alloy. By
providing knowledge of the electroless deposition rate, highly precise alloys
can be created
allowing for precise control over the deposit properties, such as hardness. In
another
preferred embodiment, a hybrid deposit technique further has the possibility
of being further
combined with existing electroplated multi-layers to provide more complex
alloys of multiple,
or even three or more, components.
3
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
The process temperatures, for such hybrid deposition process using aqueous
deposition baths, are primarily those needed for the electroless deposition
bath. In most cases
this will be anywhere from room temperature to just below the boiling point,
generally around
95-98 C. However, in some instances, such as in depositing Ni-Zn-P coatings,
temperatures
below 60 C may be less preferred. In particular, the lower temperatures may
slow or hinder
deposition as the Zn interferes with the deposition (the presence of Zn
contaminates and
slows the deposit) however, in such cases the maximum temperature would remain
the same.
In cases of non-aqueous media, such as ionic liquids, the process temperature
range may tend
to become narrower in the case of electroless deposition. In general, the
deposition
temperature is based on the stability of the electroless process.
As for voltage ranges, it has been recognized that regions of maximum
efficiency may
be ascertained, such as those established with voltammograms. In the case of
electroplating
Ni coatings, the deposit does not typically suffer from over potential, and it
has even been
suggested to use 9V batteries as Ni solutions have a wide potential window for
deposition.
The final chemistry of solution will vary and voltammograms may be undertaken
to determine
the optimum plating window, voltage/current of maximum efficiency, and the
like.
Accordingly, in one aspect, the present resides in a process for the
deposition of
multiple layers of thin metal films on a substrate comprising: preparing a
plating bath
comprising an electroless plating metal component and electroplating metal
component; and
immersing the substrate in a plating bath to effect electroless plating of the
electroless plating
metal component; and with said substrate remaining immersed in said bath,
selectively apply
a selected voltage over a predetermined interval or series of intervals to
effect electroplating
of the electroplating metal component thereon.
The present invention also resides in various further non-limiting aspects,
and which
include:
4
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
1. A process for deposition of a multi-layer metal coating on a metal
substrate to be
plated, the process comprising, at least partially immersing said substrate
into a plating bath,
the plating bath comprising a reducing agent, and a source of metal plating
ions, the plating
ions comprising one or more from the grouping consisting of copper ions, gold
ions, nickel
ions, zinc ions, silver ions, boron ions, cobalt ions and phosphorous ions,
providing a
sacrificial metal anode and cathode in said plating bath, and electrically
connecting said anode
and cathode, selectively supplying power to said anode, wherein said power is
supplied in a
pulsed time-wise manner to alternately effect anode oxidation and the
formation of an electro-
deposition plating layer of metal ions from the anode on the substrate, and
the formation of an
electroless deposition plating layer of the plating ions from the bath
solution.
2. A process for alternatively depositing metal coatings on a substrate,
the process
comprising, immersing a portion of the substrates to be plated in a plating
bath, the plating
bath including a least one source of plating ions selected from the group
consisting of sodium
tetrachloroaurate, nickel sulfate heptahydrate, zinc sulfate heptahydrate,
boric acid, cobalt
chloride heptahydrate, nickel chloride hexahydrate, providing a metal anode
and a cathode in
said plating bath, said anode being in electrical communication with said
cathode, selectively
supplying power to said anode in a pulsed time-wise manner to form on the
portion of the
substrate to be plated, alternating electro-deposited metal layers and
electroless deposited
metal layers.
3. A process for the deposition of multiple layers of thin metal films on a
substrate
comprising: preparing a plating bath comprising an electroless plating metal
component and
electroplating metal component; and immersing the substrate in a plating bath
to effect
electroless plating of the electroless plating metal component; and with said
substrate
remaining immersed in said bath, selectively apply a selected voltage over a
predetermined
interval or series of intervals to effect electroplating of the electroplating
metal component
thereon.
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
4. An aspect according to any of the foregoing 1 to 3, further comprising
maintaining the
plating bath at a temperature of upto 120 C, preferably between about 40 C to
less than
about 99 C, and more preferably from about 95 C to about 98 C.
5. An aspect according to any of the foregoing 1 to 4, wherein said plating
ions are gold
ions, and said anode comprises a metal selected from the group consisting of
nickel, and
cobalt.
6. An aspect according to any of the foregoing 1 to 4, wherein said
substrate comprises
magnesium or a magnesium alloy, said anode comprises zinc, and said plating
ions comprise
nickel, zinc and phosphorous ions in relative amounts selected to form an
electroless Ni-Zn-P
plating layer.
7. An aspect according to any of the foregoing 1 to 6, wherein power is
supplied to said
anode from a power source as alternating current.
8. An aspect according to any of the foregoing 1 to 7, wherein said power
is supplied to
said anode at upto 120 minute intervals, and preferably at 5 to 45 minute
intervals, for a
period of time selected at upto sixty minutes, preferably between about 30 and
600 seconds,
and more preferably between about 120 and 300 seconds.
9. An aspect according to any of the foregoing 1 to 8, wherein said power
is supplied in a
pulsed time-wise manner at a voltage of upto 240 volts, preferably t between
about 1 and 120
volts, and preferably between about 2 and 24 volts.
10. An aspect according to any of the foregoing 1 to 9, wherein the plating
bath solution is
a two part solution comprising, a first part comprising: 1 to 4 grams, and
preferably about 2
grams per litre sodium tetrachloroaurate as an electroless plating metal
component; 5 to 15
grams, and preferably about 10 grams per litre boric acid; and upto 2 grams
and preferably
6
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
about 1 gram per litre sodium hydroxide; and a second part comprising: 7 to 12
grams, and
preferably about 9.5 grams per litre sodium thiosulfate; 2 to 5 grams, and
preferably about
3.75 grams per liter sodium sulfite; and 7.5 to 15 grams, and preferably about
10 grams per
liter of boric acid.
11. An aspect according to any of the foregoing 1 to 10, wherein the
plating bath further
comprises a dopant solution comprising: upto 70 grams and preferably 25 to 50
grams per
litre cobalt chloride heptahydrate; and upto 75 grams, and preferably 25 to 50
grams per litre
nickel chloride hexahydrate.
BRIEF DESCRIPTION OF THE DRAWINGS
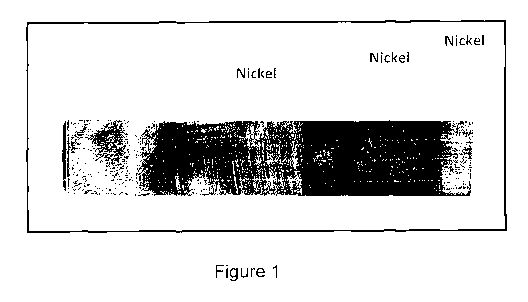
Figures 1, 2a, and 2b show macroscopic photographs showing plating samples
formed
by the electroless gold-electroplated nickel deposition process, illustrating
the alternate
deposition of gold and nickel layers on test substrates;
Figure 3 shows a macroscopic photograph showing the further formation of
sulfur/oxide complexes on the plating samples in accordance with Figures 2a
and 2b;
Figures 4a and 4b show photographs illustrating the anode used in
electrodeposition of
the coatings of Figure 1;
Figures 5a to 5b illustrate photographs of a plating sample and EDS analysis
after
electroless Ni-Zn-P and electro-Zn deposition on a substrate according to the
invention
thereon;
Figures 6a and 6b show macroscopic photographs of an electroless gold and
electroplated cobalt/nickel deposition on sample nickel substrate;
7
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
Figures 7a, 7b and 7c illustrate microscopic (SEM) image and electron
diffraction x-
ray spectroscopy (EDS) results showing the electroless gold deposition on the
nickel substrate
of Figure 5 and the co-deposition of nickel/cobalt at the plating boundary
regions;
Figure 8 shows a macroscopic scanned image of a gold/nickel deposited nickel
substrate sample illustrating the electroless gold and electroplated-Ni
deposition of a four
layer test strip in accordance with the present invention;
Figures 9a, 9b and 9c illustrate SEM microscopic image and electron
diffraction x-ray
spectroscopy (EDS) results showing the electroless gold deposition on the
nickel substrate of
Figure 8 and the co-deposition of nickel at the plating boundary regions;
Figures 10a, 10b and 10c shows SEM microscopic image and electron diffraction
x-
ray spectroscopy (EDS) results showing the electroless gold deposition on a
copper substrate
and the co-deposition of electroplated nickel thereon; and
Figure 11 shows a macroscopic scan illustrating a copper substrate showing an
electroless gold deposited layer thereon as a gold/nickel electroless-electro
deposition.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
An electro-electroless deposition is provided as a hybrid deposition process,
whereby
multiple layers of metal films are deposited from a single plating solution
having both
electroless and electroplating components. An article to be plated is immersed
in the
solution. Electric current is then selectively applied at fixed or variable
voltages. The
current is supplied for predetermined times, and at selected intervals
depending on the desired
electroplated layer thickness. The application of the current effects
electroplating to thereby
form interspaced electroplated layers of a first metal or alloy, and
electroless deposited layers
and/or alternatively with the constant electroless deposition of a second
different metal and
8
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
alloy. The electro-electroless deposition process provides a range of possible
deposits
characterized by two or more streams, multi-layers and, optionally, alloying.
Most preferably, the process allows for control over the degree of alloying of
electro-
deposited layer, and/or even electro-doping of the electroless deposit for
alloy creation. The
solution chemistry and/or plating temperature may be tailored, whereby the
initiation of
electroplating by the application of current effects the electrodeposition of
the electroplating
component to the substantially relative exclusion of the electroless bath
component, and
whereby the bath chemistry allows for electroplating to effectively be turned
on or off.
The applicant has appreciated various potential applications for the use of
electroless/electro hybrid deposition of the present invention. Applications
include without
limitation, the deposition of cobalt [Co] and hardened gold [Au] on suitable
substrates for
electronics. In particular, in one preferred embodiment co-depositing Co with
Au coating
layers may provide increased strength to electronic components by mixing face
centered cubic
(fcc) and hexagonal close packed (hcp) crystal structures of Au and Co,
respectively.
Alternatively, the formation of multilayers, which are traditionally harder
than either
constituent metal, may be used to achieve a comparatively hardened gold
deposit or layer on a
suitable substrate.
In an alternate possible embodiment, the electroless/electro hybrid deposits
may be
used to provide corrosion resistance to Mg alloys or substrates. For example,
by providing an
electroplated layer of Zn from a nickel zinc phosphorus Ni-Zn-P deposition
bath, after the
electroless deposition of a Ni-Zn-P coating layer may provide still enhance
corrosion
resistance in addition to the galvanic corrosion resistance provided by the Ni-
Zn-P coating.
It is envisioned that other electroless/electroplating deposit applications
also remain
possible and will now become apparent.
9
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
Test Sample - Electroless Gold and Electro-plated Nickel Deposition
Figure 1 shows an example test strip showing an electro-electroless deposition
coating
which is formed as a multiple layer structure of alternating gold and nickel
films. The
alternating metal layers illustrated in Figure 1 were formed using a plating
solution providing
a suitable deposit source of gold ions for electroless deposition, such as
sodium
tetrachloroaurate. As will be discussed, the metal, gold and nickel layer were
alternately
deposited as sample strips, and are first shown in Figures 2a and 2b.
The alternating layering shown in Figures 1, 2a and 2b was achieved by raising
the
sample substrate in the plating bath a short distance after each stage, in an
effort to show the
alternating layers provided by the process and deposition bath. Shedding in
the first two
layers is due to rising of the sample to see each layer. The exposure of the
gasses of the bath
and heat of the sample coupled with the smoothness of the polishing and
stresses in the
deposit are believed the cause of the shedding. The last two layers of the
test strips have no
shedding, as the exposure to the environment was minimal. The thick gold
layers were
deposited over 45 minutes each, while the electroplated nickel was deposited
over 5 minutes
using pulse plating. A cross-sectional view is difficult as the gold is soft
and care must be
taken to ensure minimal delamination.
The darker second gold deposits shown are due to pulse plating not being used
in the
deposit. Without pulse plating sulfur/oxide complexes are created and the
affinity of gold for
sulfur incorporates them into the deposit. This phenomenon, when shown,
persists in the
deposition bath once it has occurred (see Figure 3).
Test Sample ¨ Electroless Ni-Zn-P and Electroplated Zn or (Ni)
In a further test sample Zn (or Ni) layers were electrodeposited formation
with
electroless formed layers Ni-Zn-P on Mg/Mg Alloy
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
The application of the present hybrid electro-electroless deposit (HEED)
coating
process allows for improvement of corrosion protecting properties of Ni-Zn-P
coating on Mg
or Mg alloys. In particular, the application of an electroplating step to
deposit Zn or (Ni) after
initial coating formation allows for the deposition of Ni or Zn rich layers on
top of the
electroless deposited Ni-Zn-P layers.
Initial electroless deposition of Ni-Zn-P is performed to provide a continuous
coating
over the substrate surface, accessing recessed areas. The coating of recessed
areas, which is
difficult using standard electroplating techniques, prevents the formation of
a galvanic cell
between uncoated and coated regions of the coated part. Secondary or pulsed
electroplating
then may provide for a reinforcement or outer cladding layers of Ni or Zn. The
electroplated
reinforcing layers thus allow for greater corrosion protection, and in the
case of multi-layers,
greater wear protection of the Mg/Mg alloy substrate.
With the formation of electroless Ni-Zn-P coatings and multi-layers produced
therein,
Zn enrichment of the coatings provides a more anodic layer, relative to the
remainder of the
coating. The arrangement of such layers produces a sacrificial multi-layer
structure which
protects the coating from corrosion by forming corrosion to propagate along
the coating
surface, rather than through the coating to the substrate. The formation of
sacrificial multi-
layer coatings has previously been suggested for Ni/Zn layers, with layers
deposited from
separate electrolytes. Using the current process, electroplating in place of a
two bath system
produces some alloying of the Zn layer, as would be expected when depositing
the less noble
metal of a binary multi-layer electrolyte.
Applying electro-electroless plating techniques to increase the Zn content
deposited
for a Ni-Zn-P electrolyte bath (see for example the following Table) on Mg
alloys, a deposit
darker in colour than the typical Ni-Zn-P coating was obtained.
In experimental examples shown in Figures 5a to 5d, zinc deposition was
effected
using the electro-electroless hybrid deposition process to achieve alternating
zinc and Ni-Zn-P
11
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
layers. An 80 C plating bath with a pH of 11 (at 20 C before use) used in
electro-electroless
deposition was prepared as follows:
Component Chemical Formula g/L
1) Nickel Sulfate
Hexahydrate NiSO4 = 6H20 5.2495 to 6.5715
2) Zinc Sulfate
Heptahydrate ZnSO4 = 7H20 5.7425 to 7.1885
3) Sodium Citrate Tribasic Dihydrate Na3C6H507 = 2H20 23.5 to 25.3
4) Sodium Hypophosphite
Hydrate NaPH202 = H20 17.5
5) Ammonium Hydroxide*
NH4OH 25 to 62.5
*NH4OH in mL/L
The initial results showed a darker color and more metallic finish of the
parts post
electro-Zn deposition, as compared to post-electroless Ni-Zn-P deposition.
Preliminary
testing showed a higher concentration of Zn in the outer layers. This deposit
has been
performed on magnesium [Mg] alloys, and shows promise in providing
developments for
corrosion resistance.
Analysis of the composition using EDS determined that while electroless Ni-Zn-
P
struggles to obtain Zn content within the coating above 24%wt, or 20%at, Zn
content within
the final electrodeposited layer was around 37%wt, or 32%at within the HEED
coating
(Figures 5b to 5d).
Alternating the layers of Ni-Zn-P and Zn is likely to provide increased
corrosion
protection at a level robust enough so that the coating not only resists
galvanic corrosion but
also overall, normal corrosion. This scheme can be adjusted to provide a low
Zn Ni-Zn-P
layer, ¨2-5% Zn, followed by an electroplated-Zn layer essentially making Ni-
P/Zn multilayer
coatings. As Zn is more anodic than Ni and would act as a sacrificial layer,
provided the
coating is not severely damaged, to the point of exposing the Mg substrate.
Test Sample ¨ Electroless Au-Electroplated Co-Ni Deposition
12
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
In a further example, electroless gold-electro-cobalt nickel hybrid deposition
was
achieved using a two-component plating bath, in combination with a dopant
solution as
follows:
Deposition Bath & Conditions:
Bath A
Component Chemical Formula mol/L g/mol g/L
1) Sodium
tetrachloroaurate Na(AuC14) 0.005 397.8 1.9890
2) Boric Acid H3B03 0.16 61.83
9.8928
3) Sodium Hydroxide* NaOH 0.0275 40
1.1000
*pH of Bath A should be adjusted to around 7
Bath B
Component Chemical Formula mol/L g/mol g/L
1) Sodium Thiosulfate Na25203
0.06 158.11 9.4866
2) Sodium Sulfite Na2S03 0.03
126.04 3.7812
3) Boric Acid H3B03 0.16 61.83
9.8928
In preparing the plating bath solution, Bath A was added to B slowly at room
temperature,
mixed, then let stand for 24 hours. After 24 hours, the additives and dopant
metal were added
to the solution, with the creation of a dopant solution as follows.
Additives
Chemical Formula mol/L g/mol g/L
Sodium Hypophosphite* NaH2P02 0.25 87.98 21.995
Sodium Citrate Tribasic Na3C6H507 0.25 294.1
73.525
*Sodium Hypophosphite was not added in the initial experiment but can be
optionally added
to increase the deposition rate of Au.
Dopant Solution
13
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
Dopant Solution Deposition Bath Dopant
g/L
Chemical Formula
g/L g/mL Volume Used [mL] Volume [mL] (for
60mL)
Cobalt Chloride
CoC12 = 7H20 120.0 0.12 60 15
30
Heptahydrate
Nickel Chloride
NiC12 = 6H20 120.0 0.12 60 15
30
Hexahydrate
*Excess liquid volume was removed by evaporation during heating to deposition
temperature.
To ensure best deposition conditions, the pH of the deposition was adjusted
with
NaOH to be above 8. In this example the pH of the 60mL deposition bath was
adjusted with
0.25g of NaOH and 0.8mL of NH4OH to a final pH of 10.23. Both
electrodeposition and
electroless deposition were performed at 60 C. Nickel electrodes measuring
25mm x9Ommx3mm were used as both the anode and cathode.
Stability of the deposition bath in the presence of Co and Ni was found to be
achieved
with the formulation.
The foregoing test samples are not intended to be limiting. The electroless
gold
deposition from the borohydride system may be achieved on a number of noble
metals such as
Pd, Rh, Ag, and Au itself; as well as on substrates comprising active metals
such as Cu, Ni,
Co, Fe, and their alloys. The initial reactions on these two classes of metals
are understood as
different. On noble metals the reaction is catalytic from the very beginning,
whereas the gold
deposition on the active metals is initiated by galvanic displacement, which
results in
accumulation of ions of those metals in the bath. No adverse effect occurs
with copper,
whereas the introduction of ions of Ni, Co, and Fe into the solution may prove
detrimental-
bath as decomposition may set in when these metals are present at a
concentration as low as
10-3M (see Electroless Plating, Chapter 15: Electroless Plating Of Gold and
Gold Alloys,
Yutaka Okinak).
14
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
Figures 6a and 6b shows macroscopic scans of the resulting nickel substrate
test strips
illustrating the formation gold, cobalt-nickel layer deposits thereon. In
Figure 6a, shedding is
believed due to smooth polished surface and excessive deposition time causing
stress in the
coating. It should be noted that the stress may be overcome by the
electroplating indicating
the robustness of the present process.
As shown in Figure 6a, the use of a Ni anode contributed to an increased Ni
signal, as
both Co and Ni were co-deposited. This can be remedied using a Co anode.
Electron
Diffraction X-ray Spectroscopy (EDS) provides a means of analysis that
penetrates the
coating; hence the Au signal remains visible in the electroplated Ni-Co
region. Beam spread
is believed the reason for Cobalt appearing in the upper, electroless Au
region.
Figure 8 shows a scan of nickel substrate illustrating electroless deposition
of gold and
electrodeposition of Ni as deposits thereon. In the illustrated figure,
shedding is due to
prolonged deposition on a smooth surface and resulting stress of the deposit.
Again, in
exemplary testing, four layers were successfully deposited from a single
deposition bath.
As shown in Figures 7a, 7b, and 7c , penetration of the EDS produces a Ni
signal from
the substrate through the gold layer (top analysis); while a small part of the
Ni signal and a
slight Au signal persist in Ni electroplated region (bottom analysis) as shown
in Figures 9a,
9b and 9c.
Figures 10a, 10b and 10c illustrate scans showing the electroless deposition
gold and
electro-Ni deposition on a copper substrate.
In Figure 11 the thin electroless gold appears thin or discontinuous, this
colour is
accentuated by the Cu substrate.
Accordingly it is believed that the present invention provides for a variety
of non-
limiting possible applications in coating and plating processes. In one
possible application, it
CA 02919877 2016-01-29
WO 2015/017913
PCT/CA2014/000472
is envisioned that the hybrid deposition process of the present application
may prove highly
suitable for use in the coating medical implants with biomerit materials. In
particular, in a
non-limiting example, gold and tin may be recognized as metals which are
suitable for
deposition and which are bio-compatible. In addition, other possible alloys
which may be
suitable for possible deposition could include, without restriction, titanium
as well as cobalt
and chromium, depending on the implant composition.
In an alternate embodiment, the hybrid deposition process of the present
invention
could be used to effect the application of conducting coatings on plastics as
either a final layer
or an intermediate layer for subsequent metal plating. In a preferred
application, the coatings
of the present application may be effected in a single deposition bath. In
alternate
embodiments, it is envisioned that certain "additives" may be used to increase
deposition
rates, and which in accordance with preferred aspects would include
nickel/gold deposition
systems, as well as Ni-Zn-P deposition systems.
A further application of the present system may include, without restriction,
the
manufacture of magnets; and most preferably magnets using electroless Ni-Fe-P
deposition
which may contain upto 25% Fe.
In yet another manufacturing method, the hybrid deposition process of the
present
invention may be used in the manufacture of high-wear conductive contact
points, as for
example, are used in electronics and electronic systems. The applicant has
appreciated that
early experimental results have shown the successful formation of multi-layer
nickel- gold
deposits. It is further envisions that the deposition of alloys could equally
be achieved by
slowing the electroplating rate. Further, as the gold bath in questions is
initiated by tandem
electroless deposit and simple displacement reaction, nickel may be
electroplated to achieve
an Au/Ni hardened alloy.
16
CA 02919877 2016-01-29
WO 2015/017913 PCT/CA2014/000472
In addition, the hybrid deposition process of the present invention further
may
advantageously provide enhanced adhesive surfaces for metal adhesive bonding
or welding in
combination or tandem with the deposition of different metals.
While the description describes various preferred embodiment of the invention,
the
invention is not restricted to the specific constructions which are disclosed.
Many
modifications and variations will now occur to persons skilled in the art. For
a definition of
the invention, reference may be made to the appended claims.
17