Note: Descriptions are shown in the official language in which they were submitted.
CA 02923421 2016-03-04
WO 2015/035402
PCT/US2014/054816
METHODS OF DETERMIMNG RESPONSE TO THERAPY
RELATED APPLICATIONS
[0001] This application claims priority to and benefit of provisional
application USSN
61/875,384 filed on September 9, 2013, USSN 14/038258 filed September 26, 2013
and
provisional application USSN 61/991,351 filed May 9, 2014 the contents of
which are each
herein incorporated by reference in their entireties.
FIELD OF THE INVENTION
[0002] The
present invention relates generally to methods of determining the response to
metadoxine therapy for the treatment of Fragile X Syndrome and other cognitive
disorders. The
invention also relates to identifying individuals that will be responsive to
metadoxin.e therapy.
BACKGROUND OF THE INVENTION
100021
Fragile X Syndrome (FXS), as implied by its name, is associated with a fragile
site
expressed as an isochromatid gap in the metaphase chromosome at map position
Xq 27.3. Fragile
X syndrome is a genetic disorder caused by a mutation in the 5'-untranslated
region of the fragile
X. mental retardation 1 (FMR1) gene, located on the X chromosome. The mutation
that causes
FXS is associated with a COG repeat in the fragile X mental retardation gene
FMR1. In most
healthy individuals, the total number of COG repeats ranges from less than 10
to 40, with an
average of about 29. In fragile X syndrome, the CGG sequence is repeated from
200 to more than
1,000 times. When a subject has more than about 200 COG repeats, the fragile
X. gene becomes
hypermethylated, which silences the gene. As a result, fragile X mental
retardation protein
(FMRP) is not produced, or is produced at reduced level, and the subject
displays manifestations
of FXS.
100031 Premutation expansions (55-200 COG repeats) of the FMR1 gene are
frequent in the
general population, with estimated preval.en.ces of 1 per 259 females and 1
per 812 males. Carriers
of the premutation typically have normal IQ, although emotional problems such
as anxiety are
1
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
common. Older male carriers of the premutation (50 years and older) develop
progressive
intention tremor and ataxia. These movement disorders are frequently
accompanied by progressive
cognitive and behavioral difficulties, including memory loss, anxiety, and
deficits of executive
function, reclusive or irritable behavior, and dementia. This disorder has
been designated fragile
X-associated tremor/ataxia syndrome (FXTAS). Magnetic resonance imaging in
subjects with
FXTAS reveals increases in T2-weighted signal intensity in the middle
cerebellar peduncles and
adjacent cerebellar white matter.
[00041 FXS segregates as an X-linked dominant disorder with reduced
penetrance. Either sex
when carrying the fragile X mutation may exhibit intellectual disability,
which is variable in
severity. Children and adults with FXS have varying degrees of intellectual
disability or learning
disabilities and behavioral and emotional problems, including autistic-like
features and tendencies.
Young children with FXS often have delays in developmental milestones, such as
learning how to
sit, walk and talk. Affected children may have frequent tantrums, difficulties
in paying attention,
frequent seizures (e.g., temporal lobe seizures), are often highly anxious,
easily overwhelmed, can
have sensory hyperarousal disorder, gastrointestinal disorders, and may have
speech problems and
unusual behaviors, such as hand flapping and hand biting.
[00051 FXS can be diagnosed by an established genetic test performed on a
sample (e.g.,
blood sample, buccal sample) from. the subject. The test determines whether a
mutation or pre-
mutation is present in the FMR1 gene of the subject based upon the number of
CGG repeats.
[00061 Subjects with FXS can also have autism. About 5% of all children
diagnosed with
autism have a mutation in the FMR.1 gene and also have fragile X syndrome
(FXS). Autism
spectrum disorder (ASD) is seen in approximately 30% of males and 20% of
females with FXS,
and an additional 30% of FXS individuals display autistic symptoms without
having the ASD
diagnosis. Although intellectual disability is a hallmark feature of FXS,
subjects with FXS often
display autistic features ranging from shyness, poor eye contact, and social
anxiety in mild cases
to hand flapping, hand biting and perseverative speech in the severely
affected. Subjects with FXS
display other symptoms associated with autism such as attention deficit and
hyperactivity,
seizures, hypersensitivity to sensory stimuli obsessive-compulsive behavior
and altered
gastrointestinal function. The FMR1 mutation prevents or greatly decreases
expression of a single
2
CA 02923421 2016-03-04
WO 2015/035402
PCT/US2014/054816
protein (FMRP). Brain development in the absence of FMRP is thought to give
rise to the major
symptoms of FXS.
[00071 In
addition to core symptoms, children with FXS frequently have serious
behavioral
disturbances such as irritability, aggression and self-injurious behaviors. In
a recent study of males
with FXS (ages 8-24), self-injurious behavior was reported in 79%, and
aggressive behavior in
75%, of subjects during a two month observation period.
[00081
Currently available treatment regimens for humans with FXS include, for
example,
behavioral modifications and treatment with a range of medications (not
approved by FDA for the
treatment of FXS) including antidepressant and antipsychotic drugs. Cognitive
behavioral therapy
has been used to improve language and socialization in individuals with FXS
and autism. In recent
years, pharmacological treatment with the atypical antipsychotic risperidone
has been commonly
employed to augment non-pharmacological approaches in the treatment of
individuals with
autism. A randomized placebo-controlled trial of risperidone in autistic
children demonstrated
significant improvement on the irritability subscale of the Aberrant Behavior
Checklist and the
Clinical Global Impressions-Improvement (McCracken, J. T., et al., N. Engl. J.
Med. 347:314-321
(2002)). However, adverse events included weight gain, increased appetite,
fatigue, drowsiness,
dizziness, and drooling. Social isolation and communication were not improved
by administration
of risperidone and adverse side effects such as extrapyramidal symptoms and
dyskinesias have
been associated with risperidone use in autistic children.
SUMMARY OF THE INVENTION
100031 The invention provides methods of assessing the effectiveness of a
metadoxine
treatment regimen in a subject having Fragile X Syndrome or other cognitive
disorder who has
received the metadoxine treatment, by measuring the amount of phosphorylated
ERK. and Akt
protein in a sample derived from the subject; measuring the total amount of
ERK and Akt protein
in the sample; calculating a ratio of the amount of ph.osphorylated ERK and
Akt protein to the
total amount of ERK and Akt protein and comparing the calculated ratio to a
calculated ratio
measured from a non-diseased subject. When the calculated ratio of the subject
is similar to the
calculated ratio for a known non-diseased subject, the treatment is effective.
3
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
[0004] Also provided by the invention are methods of determining whether a
subject with
Fragile X Syndrome or other cognitive disorder would derive a benefit from a
metadoxine
treatment regimen by measuring the amount of phosphorylated ERK and Akt
protein in a sample
derived from the subject; measuring the total amount of ERK and Akt protein in
the sample;
calculating a ratio of the amount of phosphorylated ERK and Akt protein to the
total amount of
ERK and Akt protein and comparing the subject calculated ratio to a calculated
ratio measured
from a non-diseased subject. When the subject calculated ratio is higher than
the calculated ratio
of a known non-diseased subject, the subject would derive a benefit from the
metadoxine
treatment regimen.
[0005] Also provided by the invention are methods of monitoring a
metadoxine treatment
regimen in a subject having Fragile X Syndrome or other cognitive disorder, by
measuring the
amount of phosphorylated ERK and Akt proteins in a first sample from the
subject at a first
period of time; measuring the total amount of ERK and Akt protein in the first
sample at the first
period of time; calculating a first ratio of the amount of phosphorylated ERK
and Akt protein to
the total amount of ERK and Akt protein; measuring the amount of
phosphorylated ERK and Akt
protein in a second sample from the subject at a second period of time;
measuring the total
amount of ERK and Akt protein in the second sample at the second period of
time; calculating a
second ratio of the amount of phosphorylated ERK and Akt proteins to the total
amount of ERK.
and Akt proteins and comparing the first ratio to the second ratio. When the
second ratio is
lower than the first ratio, the treatment is effective.
100061 In some aspects the measuring steps comprise an immunoassay. In some
embodiments the sample is whole blood or a fraction thereof. In some
embodiments the sample
is a peripheral blood mononucleated cell (1)BMC). in some embodiments the PMBC
is a
lymphocyte or a monocyte.
[0007] Unless otherwise defined, all technical and scientific terms used
herein have the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
pertains. Although methods and materials similar or equivalent to those
described herein can be
used in the practice of the present invention, suitable methods and materials
are described below.
4
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
All publications, patent applications, patents, and other references mentioned
herein are
expressly incorporated by reference in their entirety. In cases of conflict,
the present
specification, including definitions, will control. in addition, the
materials, methods, and
examples described herein are illustrative only and are not intended to be
limiting.
100081 Other features and advantages of the invention will be apparent from
and
encompassed by the following detailed description and claims.
BRIEF DESCRIPTION OF THE DRAWINGS
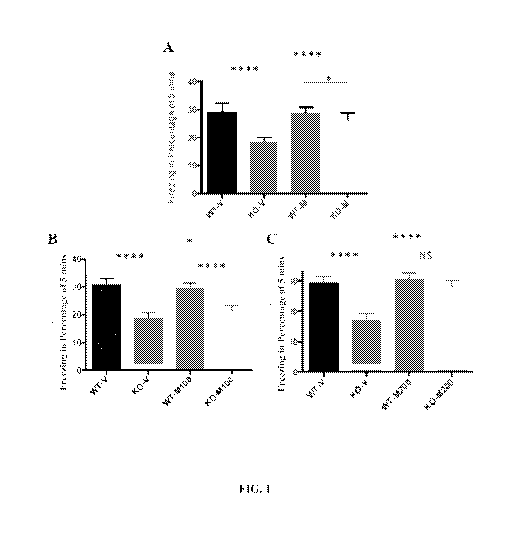
[0009] Fig. 1 shows the effect of seven days of once daily intraperitoneal
(ip) administration
of vehicle (V) or metadoxine (M) (100, 150, or 200 mg/kg) in 2-months oldFmr/
knockout (KO)
or Wild Type (WT) mice on contextual fear conditioning. Specifically, Panel A
shows the effect
of vehicle or 150 mg/kg of m.etadoxine. Panel B shows the effect of vehicle or
100 mg/kg of
metadoxine. Panel C shows the effect of vehicle or 200 mg/kg of metadoxine.
Data shown are
mean + standard error of the mean (sem), N= 10 mice per group. * p<0.05,
****p<0.0001, and
NS = Not Significant.
1000101 Fig. 2 shows the effect of seven days of once daily intraperitoneal
administration of
vehicle (V) or 150 mg/kg metadoxine (M) in 2-months old Finn knockout (1(0) or
Wild Type
(WT) mice on social approach behavior. Data shown are mean + sem, N= 10 mice
per group. *
p<0.05 and ****p<0.0001.
[00011] Fig. 3 shows the effect of seven days of once daily intraperitoneal
administration of
vehicle (V) or 150 mg/kg metadoxine (M) on Y-maze spontaneous alternation
(Panel A), Y-
maze rewarded alternation (Panel B) or Y-maze water maze spatial
discrimination (Panel C) in
2-months old Fmrl knockout (KO) or Wild Type (WT) mice. Data shown are mean +
sem, N=
mice per group. *** p<0.001., ****p<0.0001, and NS = Not Significant.
[000121 Fig. 4 shows the effect of seven days of once daily intraperitoneal
administration of
vehicle (V) or 150 mg/kg metadoxine (M) on T-maze rewarded alternation in 2-
months old Fmrl
knockout (KO) or Wild Type (WT) mice. Data shown are mean + sem., N.= 10 mice
per group.
****p<0.0001.
5
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000131 Fig. 5 shows the effect of seven days of once daily treatment with
vehicle (V) or 150
mg/kg metadoxine (M) on behavior in the successive alleys task in groups of N
=. 10 Wild Type
(WT) or Finn l knockout (K0)2-months old mice. The successive alleys of the
apparatus
presented progressively more anxiogenic environments to explore mice. Movement
down the
alleys therefore assessed anxiety. In addition, overall activity levels could
also be quantitated in
the apparatus.
1000141 Fig. 6 shows the effect of seven days of once daily intraperitoneal
administration of
vehicle (V) or 150 mg/kg metadoxine (M) on whole brain levels of
phosphorylation of ERK
(indicative of ERK activity) (Panel A) and Akt (indicative of Akt activity)
(Panel B) in 2-months
Fmr1 knockout (KO) or Wild Type (WI) mice. Data shown are mean + sem, N= 5
mice per
group. ** p<0.01, *** p<0.001, ****p<0.000I, and NS =Not Significant.
1000151 Fig. 7 shows the effect of once daily ip administration of vehicle
(V) or 150 mg/kg
metadoxine (M) for 7 days in 6 month old Fmr1 knockout (KO) or Wild Type (WI)
mice on
contextual fear conditioning. Data shown are mean + sem, N= 10 mice per group.
****p<0.0001 and ns = Not Significant.
1000161 Fig. 8 shows the effect of once daily ip administration of vehicle
(V) or 150 mg/kg
metadoxine (M) for 7 days in 6 month old Fmrl knockout (KO) or Wild Type (WT)
mice on
social approach (Panels A and C) and social memory (Panels B and D) behavior,
as measured by
number of sniffing bouts or duration of sniffing. Data shown are mean + sem,
N= 10 mice per
group. * p<0.05, ****p<0.0001, and ns = Not Significant.
1000171 Fig. 9 shows the effect of once daily ip administration of vehicle
(V) or 150 mg/kg
metadoxine (M) for 7 days on whole brain levels of phosphorylation of ERK
(Panel A) and Ala
(Panel B) in 6 month old Fmrl knockout (KO) or Wild Type (WT) mice. Data shown
are mean
+ sem, N= 10 mice per group. * p <0.05, ** p<0.01, ****p<0.0001, and ns = Not
Significant.
1000181 Fig. 10 shows the effect of once daily m.etadoxine (M) at 150 mg/kg
ip or oral
administration (po) of vehicle (V) or metadoxine at 150 and 300 mg/kg for 7
days on contextual
fear conditioning in 2 month old Finn 1 knockout (KO) or Wild Type (WT) mice.
Data shown
are mean + sem, N= 10 mice per group. Specifically, Panel A shows ip and oral
treatment with
vehicle in Finn knockout and Wild Type mice. Panel B shows ip and oral
treatment with
6
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
metadoxine in Wild Type mice. Panel C shows ip and oral treatment with
metadoxine in Fmr1
knockout mice. ** p<0.01, ****p<0.0001., and ns = Not Significant.
1000191 Fig. 11 shows the effect of once daily ip or oral administration
(po) of vehicle (V) or
metadoxine (M) at 150 or 300 mg/kg for 7 days on social approach (Panel A) and
social memory
(Panel B) in 2 month old Finn! knockout KO) or Wild Type (WT) mice. Data shown
are mean
+ sem, N= 10 mice per group. ** p<0.01, ****p<0.0001, and ns = Not
Significant.
1000201 Fig. 12 shows the effect of once daily ip or oral administration
(po) of vehicle (V) or
metadoxine (M) at 150 or 300 mg/kg for 7 days on lymphocyte biomarkers as
assessed using
flow cytometry in 2 month old Finn 1 knockout (KO) and Wild Type (WT) mice.
Biomarkers
shown are pAkt (Panel A) and pERK (Panel B) in Find knockout or Wild Type
mice. Data
shown are mean + sem, N= 10 mice per group. ****p<0.0001 and ns = Not
Significant.
1000211 Fig. 13 shows the effect of once daily ip of vehicle (V) or 150
mg/kg metadoxine (M)
for 7 days on pERK levels in brain regions of two month old Wild Type (WT) and
Finri
knockout (KO) mice. The regions analyzed were the hippocampus (Panel A), pre-
frontal cortex
(Panel B), and striatum (Panel C) in Fmr1 knockout or Wild Type mice. Data
shown are mean
+ sem., N= 10 mice per group. ****p<0.0001 and ns = Not Significant.
1000221 Fig. 14 shows the effect of once daily ip of vehicle (V) or 150
mg/kg metadoxine (M)
for 7 days on pAkt levels in brain regions of two month old Wild Type (WT) and
Finn] knockout
(KO) mice. The regions analyzed were the hippocampus (Panel A), pre-frontal
cortex (Panel 13),
and striatum (Panel C) in Fmrl knockout or Wild Type mice. Data shown are mean
+ sem, N=
mice per group. ****p<0.0001 and ns = Not Significant.
1000231 Fig. 15 shows the effect of 5 hour treatment with vehicle (V) or
300 ptM metadoxine
(M) in vitro on filopodia density (Panel A), length (Panel B), and width
(Panel C) in neuronal
hi.ppocampal cultures from Find knockout (KO) or Wild Type (WT) mice. Data
shown are
mean + sem, (Wild Type, N = 20 neurons and Finn 1 knockout mice, N = 20
neurons). **
p<0.01, ***p<0.001, and ns = Not Significant.
1000241 Fig. 16 shows the effect of treatment in vitro with vehicle (V) or
300 AM metadoxine
(M) on basal de novo protein synthesis in 400 j.tM hippocampal slices from.
Finn] knockout (m))
7
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
or Wild Type (WT) mice. Data shown are mean + sem, N= 6 slices per group. *
p<0.00 land
****p<0.0001.
DETAILED DESCRIPTION OF THE INVENTION
1000251 The present invention relates to the identification of biomarkers
associated with the
response to metadoxine therapy for individuals with Fragile .X Syndrome (FXS)
and other
cognitive disorders. Specifically, it was discovered that metadoxine treatment
returns the ratio of
phosphorylated ERK and Akt protein to total ERK and Akt protein in a subject
sample closer to
normal ratios. By normal ratios it is meant the ratio of phosphorylated ERK
and Akt protein to
total ERK and Akt protein found in normal ( i.e, non-diseased) subjects.
Furthermore, it was
unexpectedly discovered that these alterations in phosphorylated ERK and Akt
protein to total
ERK and Akt protein ratio could be detected in the blood.
1000261 Accordingly, the invention provides methods for monitoring subjects
undergoing
metadoxine treatment for FXS or other cognitive disorders by determining the
ratio of
phosphorylated ERK and Akt proteins to total ERK and Akt protein in a subject
sample. The
ratio is compared to a control ratio, such as the ratio obtained from. a
subject not afflicted with
the cognitive disorder. A subject ratio similar to a normal control ratio
indicates that the
treatment is efficacious.
1000271 Additionally the invention provides methods of selecting subjects
who have cognitive
disorders that would derive a benefit from metadoxine treatment, by
determining the ratio of
phosphorylated ERK or Akt protein to total ERK and Akt protein in a subject
sample. The ratio
is compared to a control ratio, such as the ratio obtained from a subject not
afflicted with the
cognitive disorder. A subject ratio greater than a normal control ratio
indicates that the subject
may derive a benefit from metadoxine treatment. Whereas subjects that do not
have a ratio
greater than a normal control ratio may not derive a benefit from metadoxine
treatment
1000281 Although calculation of the ratio is described one way herein, it
is to be understood to
encompass calculating the inverse as is apparent to a person of ordinary
skill. Also, whereas
calculation of ratios as described herein is beneficial in providing useful
comparative numbers,
calculation of absolute differences between phosphorylated ERK and Akt protein
and total ERK
8
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
and Aid proteins levels, and between test subjects and control subjects, could
also be employed
and would be effectively used in practicing the invention.
1000291 Definitions
1000301 "Accuracy" refers to the degree of conformity of a measured or
calculated quantity (a
test reported value) to its actual (or true) value. Clinical accuracy relates
to the proportion of true
outcomes (true positives (TP) or true negatives (TN) versus misclassified
outcomes (false
positives (FP) or false negatives (FN)), and may be stated as a sensitivity,
specificity, positive
predictive values (PPV) or negative predictive values (NPV), or as a
likelihood, odds ratio,
among other measures.
1000311 "Biomarker" in the context of the present invention encompasses,
without limitation,
proteins, nucleic acids, and metabolites, together with their polymorphisms,
mutations, variants,
modifications, subunits, fragments, protein-li.gand complexes, and degradation
products, protein-
ligand complexes, elements, related metabolites, and other analytes or sample-
derived measures.
Biomarkers can also include mutated proteins or mutated nucleic acids.
Biomarkers also
encompass non-blood borne factors or non-analyte physiological markers of
health status, such
as "clinical parameters" defined herein, as well as "traditional laboratory
risk factors", also
defined herein. Biomarkers also include any calculated indices created
mathematically or
combinations of any one or more of the foregoing measurements, including
temporal trends and
differences. Where available, and unless otherwise described herein,
biomarkers which are gene
products are identified based on the official letter abbreviation or gene
symbol assigned by the
international Human Genome Organization Naming Committee (HGNC) and listed at
the date of
this filing at the US National Center for Biotechnology Information (NCBI) web
site.
1000321 A "Clinical indicator" is any physiological datum used alone or in
conjunction with
other data in evaluating the physiological condition of a collection of cells
or of an organism.
This term includes pre-clinical indicators.
1000331 "Clinical parameters" encompasses all non-sample or non-analyte
biomarkers of
subject health status or other characteristics, such as, without limitation,
age (A.ge), ethnicity
(RACE), gender (Sex), or family history (FamHX).
9
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000341 "FN" is false negative, which for a disease state test means
classifying a disease
subject incorrectly as non-disease or normal.
1000351 "FP" is false positive, which for a disease state test means
classifying a normal
subject incorrectly as having disease.
1000361 A "formula," "algorithm," or "model" is any mathematical equation,
algorithmic,
analytical or programmed process, or statistical technique that takes one or
more continuous or
categorical inputs (herein called "parameters") and calculates an output
value, sometim.es
referred to as an "index" or "index value." Non-limiting examples of
"formulas" include sums,
ratios, and regression operators, such as coefficients or exponents, biomarker
value
transformations and normalizations (including, without limitation, those
normalization schemes
based on clinical parameters, such as gender, age, or ethnicity), rules and
guidelines, statistical
classification models, and neural networks trained on historical populations.
In panel and
combination construction, of particular interest are structural and synactic
statistical
classification algorithms, and methods of risk index construction, utilizing
pattern recognition
features, including established techniques such as cross-correlation,
Principal Components
Analysis (PCA), factor rotation, Logistic R.egression (LogReg), Linear
Discriminant Analysis
(LDA), Eigengene Linear Discriminant Analysis (ELDA), Support Vector Machines
(SVM),
Random. Forest (RF), Recursive Partitioning Tree (RPART), as well as other
related decision tree
classification techniques, Shrunken Centroids (SC), StepAIC, Kth-Nearest
Neighbor, Boosting,
Decision Trees, Neural Networks, Bayesian Networks, Support Vector Machines,
and Hidden
Markov Models, among others. Other techniques may be used in survival and time
to event
hazard analysis, including Cox, Weibull, Kaplan-Meier and Greenwood models
well known to
those of skill in the art. Many of these techniques are useful either combined
with a selection
technique, such as forward selection, backwards selection, or stepwise
selection, complete
enumeration of all potential panels of a given size, genetic algorithms, or
they may themselves
include biomarker selection methodologies in their own technique. These may be
coupled with
information criteria, such as Akaike's Information Criterion (AIC) or Bayes
Information.
Criterion (BIC), in order to quantify the tradeoff between additional
biomarkers and model
improvement, and to aid in minimizing overfit. The resulting predictive models
may be validated
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
in other studies, or cross-validated in the study they were originally trained
in, using such
techniques as Bootstrap, Leave-One-Out (LOO) and 10-Fold cross-validation (10-
Fold CV). At
various steps, false discovery rates may be estimated by value permutation
according to
techniques known in the art. A "health economic utility function" is a formula
that is derived
from a combination of the expected probability of a range of clinical outcomes
in an idealized
applicable patient population, both before and after the introduction of a
diagnostic or therapeutic
intervention into the standard of care. It encompasses estimates of the
accuracy, effectiveness
and performance characteristics of such intervention, and a cost and/or value
measurement (a
utility) associated with each outcome, which may be derived from. actual
health system costs of
care (services, supplies, devices and drugs, etc.) and/or as an estimated
acceptable value per
quality adjusted life year (QALY) resulting in each outcome. The sum., across
all predicted
outcomes, of the product of the predicted population size for an outcome
multiplied by the
respective outcome's expected utility is the total health economic utility of
a given standard of
care. The difference between (i) the total health economic utility calculated
for the standard of
care with the intervention versus (ii) the total health economic utility for
the standard of care
without the intervention results in an overall measure of the health economic
cost or value of the
intervention. This may itself be divided amongst the entire patient group
being analyzed (or
solely amongst the intervention group) to arrive at a cost per unit
intervention, and to guide such
decisions as market positioning, pricing, and assumptions of health system
acceptance. Such
health economic utility functions are commonly used to compare the cost-
effectiveness of the
intervention, but may also be transformed to estimate the acceptable value per
(MEV the health
care system is willing to pay, or the acceptable cost-effective clinical
performance characteristics
required of a new intervention.
1000371 For diagnostic (or prognostic) interventions of the invention, as
each outcome (which
in a disease classifying diagnostic test may be a TP, FP, TN, or FN) bears a
different cost, a
health economic utility function may preferentially favor sensitivity over
specificity, or PPV
over NPV based on the clinical situation and individual outcome costs and
value, and thus
provides another measure of health economic performance and value which may be
different
from more direct clinical or analytical performance measures. These different
measurements and
11
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
relative trade-offs generally will converge only in the case of a perfect
test, with zero error rate
(a.k.a., zero predicted subject outcom.e misclassifications or FP and FN),
which all performance
measures will favor over imperfection, but to differing degrees.
1000381 "Measuring" or "measurement," or alternatively "detecting" or
"detection," means
assessing the presence, absence, quantity or amount (which can be an effective
amount) of either
a given substance within a clinical or subject-derived sample, including the
derivation of
qualitative or quantitative concentration levels of such substances, or
otherwise evaluating the
values or categorization of a subject's non-analyte clinical parameters.
1000391 "Negative predictive value" or "NPV" is calculated by TN/(TN-1-FN)
or the true
negative fraction of all negative test results. It also is inherently impacted
by the prevalence of
the disease and pre-test probability of the population intended to be tested.
See, e.g., O'Marcaigh
A S, Jacobson R M, "Estimating The Predictive Value Of A Diagnostic Test, How
To Prevent
Misleading Or Confusing Results," Clin. Ped. 1993, 32(8): 485-491, which
discusses specificity,
sensitivity, and positive and negative predictive values of a test, e.g., a
clinical diagnostic test.
Often, for binary disease state classification approaches using a continuous
diagnostic test
measurement, the sensitivity and specificity is summarized by Receiver
Operating
Characteristics (ROC) curves according to Pepe et al, "Limitations of the Odds
Ratio in Gauging
the Performance of a Diagnostic, Prognostic, or Screening Marker," Am. J.
Epidemiol. 2004, 159
(9): 882-890, and summarized by the Area Under the Curve (AUC) or c-statistic,
an indicator
that allows representation of the sensitivity and specificity of a test,
assay, or method over the
entire range of test (or assay) cut points with just a single value. See also,
e.g., Shultz, "Clinical
Interpretation Of Laboratory Procedures," chapter 14 in Teitz, Fundamentals of
Clinical
Chemistry, Burtis and Ashwood (eds.), 4<sup>th</sup> edition 1996, W.B. Saunders
Company, pages
192-199; and Zweig et al., "ROC Curve Analysis: An Example Showing The
Relationships
Among Serum Lipid And Apolipoprotein. Concentrations In Identifying Subjects
With Coronory
Artery Disease," Clin. Chem., 1992, 38(8): 1425-1428. An alternative approach
using likelihood
functions, odds ratios, information theory, predictive values, calibration
(including goodness-of-
fit), and reclassification measurements is summarized according to Cook, "Use
and Misuse of the
Receiver Operating Characteristic Curve in Risk Prediction," Circulation 2007,
115: 928-935.
12
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
Finally, hazard ratios and absolute and relative risk ratios within subject
cohorts defined by a test
are a further measurement of clinical accuracy and utility. Multiple methods
are frequently used
to defining abnormal or disease values, including reference limits,
discrimination limits, and risk
thresholds.
1000401 "Analytical accuracy" refers to the reproducibility and
predictability of the
measurement process itself, and may be summarized in such measurements as
coefficients of
variation, and tests of concordance and calibration of the same samples or
controls with different
times, users, equipment and/or reagents. These and other considerations in
evaluating new
biomarkers are also summarized in Vasan, 2006.
1000411 "Performance" is a term that relates to the overall usefulness and
quality of a
diagnostic or prognostic test, including, among others, clinical and
analytical accuracy, other
analytical and process characteristics, such as use characteristics (e.g.,
stability, ease of use),
health economic value, and relative costs of components of the test. Any of
these factors may be
the source of superior performance and thus usefulness of the test, and may be
measured by
appropriate "performance metrics," such as AUC, time to result, shelf life,
etc. as relevant.
1000421 "Positive predictive value" or "PPV" is calculated by TP/(TP-1-FP)
or the true positive
fraction of all positive test results. It is inherently impacted by the
prevalence of the disease and
pre-test probability of the population intended to be tested.
1000431 "Risk" in the context of the present invention, relates to the
probability that an event
will occur over a specific time period, as in the responsiveness to treatment,
and can mean a
subject's "absolute" risk or "relative" risk. Absolute risk can be measured
with reference to either
actual observation post-measurement for the relevant time cohort, or with
reference to index
values developed from statistically valid historical cohorts that have been
followed for the
relevant time period. Relative risk refers to the ratio of absolute risks of a
subject compared
either to the absolute risks of low risk cohorts or an average population
risk, which can vary by
how clinical risk factors are assessed. Odds ratios, the proportion of
positive events to negative
events for a given test result, are also commonly used (odds are according to
the formula p/(1-p)
where p is the probability of event and (1-p) is the probability of no event)
to no-conversion.
13
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000441 "Risk evaluation" or "evaluation of risk" in the context of the
present invention
encompasses making a prediction of the probability, odds, or likelihood that
an event or disease
state may occur, the rate of occurrence of the event or conversion from one
disease state. Risk
evaluation can also comprise prediction of future clinical parameters,
traditional laboratory risk
factor values, or other indices of FXS, either in absolute or relative terms
in reference to a
previously measured population. The methods of the present invention may be
used to make
continuous or categorical measurements of the responsiveness to treatment thus
diagnosing and
defining the risk spectrum of a category of subjects defined as being
responders or non-
responders. In the categorical scenario, the invention can be used to
discriminate between normal
and other subject cohorts at higher risk for responding.
1000451 A "sample" in the context of the present invention is a biological
sample isolated
from a subject and can include, by way of exam.ple and not limitation, whole
blood, serum,
plasma, cerebrospinal fluid (CSF), brain cells, or any other secretion,
excretion, or other bodily
fluids. A "sample" may include a single cell or multiple cells or fragments of
cells. The sample is
also a tissue sample. The sample is or contains a brain cell or a lymphocyte.
Preferably the
sample is peripheral blood mononuclear cell such as a lymphocyte or monocyte.
1000461 "Sensitivity" is calculated by TP/(TP+FN) or the true positive
fraction of disease
subjects.
1000471 "Specificity" is calculated by 'TI=1/(TN+FP) or the true negative
fraction of non-
disease or normal subjects.
1000481 By "statistically significant", it is meant that the alteration is
greater than what might
be expected to happen by chance alone (which could be a "false positive").
Statistical
significance can be determined by any method known in the art. Commonly used
measures of
significance include the p-value, which presents the probability of obtaining
a result at least as
extreme as a given data point, assuming the data point was the result of
chance alone. A result is
considered highly significant at a p-value of 0.05 or less. Preferably, the p-
value is 0.04, 0.03,
0.02,0.01, 0.005, 0.001 or less.
1000491 A "subject" in the context of the present invention is preferably a
mammal. The
mammal can be a human, non-human primate, mouse, rat, dog, cat, horse, or cow,
but are not
14
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
limited to these examples. Mammals other than humans can be advantageously
used as subjects
that represent animal models of FXS. .A subject can be male or female. The
subject has or is
suspected of having FXS or other cognitive disorders.
1000501 "TN" is true negative, which for a disease state test means
classifying a non-disease
or normal subject correctly.
1000511 "TP" is true positive, which for a disease state test means
correctly classifying a
disease subject.
1000521 "Traditional laboratory risk factors" correspond to biomarkers
isolated or derived
from subject samples and which are currently evaluated in the clinical
laboratory and used in
traditional global risk assessment algorithms. Other traditional laboratory
risk factors for Fragile
.X known to those skilled in the art.
1000531 Methods of the Invention
1000541 The methods disclosed herein are used with subjects undergoing
metadoxine
treatment and/or therapies for FXS and other cognitive disorders and for
subjects who have been
diagnosed with FXS and other cognitive disorders.
1000551 The methods of the present invention are useful to monitor the
treatment of FX.S and
other cognitive disorders in a subject and to select subjects who would derive
a benefit from
metad.oxine treatment.
1000561 In general, the signs and symptoms of FXS fall into five
categories: intelligence and
learning; physical, social and emotional, speech and language and sensory
disorders commonly
associated or sharing features with Fragile X.. For example, individuals with
FXS have impaired
intellectual functioning, social anxiety, language difficulties and
sensitivity to certain sensations.
1000571 Cognitive disorders include the group of disorders in which a
dysfitnction/
impairment of mental processing constitutes the core symptomatology. Cognitive
disorders
include neurogenetic cognitive disorders or behavioral cognitive disorders
[000581 Cognitive disorders include developmental disorders, attention
deficit hyperactivity
disorder (ADI-ID ), autism spectrum disorders, Alzheimers disease,
schizophrenia and
cerebrovascular disease.
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000591 Autism spectrum disorders and autistic symptoms are commonly
associated with
individuals with Fragile X syndrome. Signs and symptoms of autism include
significant
language delays, social and communication challenges, and unusual behaviors
and interests.
Many people with autistic disorder also have intellectual disability.
1000601 Determination of the ratio of phosphorylated ERK. and Akt protein
to total ERK. and
Akt protein in a subject sample allows for the course of treatment of FXS or
other cognitive
disorder to be monitored. In this method, a biological sample is provided from
a subject
undergoing treatment. If desired, biological samples are obtained from the
subject at various
time points before, during, or after treatment. The ratio of phosphorylated
ERK and Akt protein
to total ERK and Ala protein are then calculated and compared to a control
value. A control
value is a control individual or population whose ratio of phosphorylated ERK
and Akt protein to
total ERK and Akt protein state is known or an index value. The reference
sample or index value
may be taken or derived from one or more individuals who are not diseased
(e.g., not affected
with the FXS or other cognitive disorder.). Alternatively, the reference
sample or index value
may be taken or derived from the subject before treatment. For example,
samples may be
collected from a subject who has not received an initial treatment and after
subsequent treatment
to monitor the progress of the treatment. The reference sample or index value
may be taken or
derived from the subject after the initial treatment. For example, samples may
be collected a
subject who have received initial treatment and subsequent treatment for FXS
to monitor the
progress of the treatment.
1000611 In another embodiment, the reference value is an index value or a
baseline value. An
index value or baseline value is a composite sample of the ratio of
phosphorylated ERK and Akt
protein to total ERK and Akt protein from individuals that do not suffer from
FXS or other
cognitive disorder.
1000621 The effectiveness of treatment can be monitored by determining the
ratio of
phosphorylated ERK and Akt protein to total ERK and Akt protein in a sample
obtained from a
subject over time and comparing the ratios. For example, a first sample can be
obtained prior to
the subject receiving treatment and one or more subsequent samples are taken
after or during
treatment of the subject.
16
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000631 By "efficacious", it is meant that the treatment leads to a ratio
of phosphorylated ERK
and Akt protein to total ERK and Akt protein similar to that from a subject
that does not have
FXS or other cognitive disorder. Efficacy can be determined in association
with any known
method for diagnosing, identifying, or treating FXS.
1000641 Phosphorylated ERK. and Akt and total ERK and Akt protein can be
determined by
any method known in the art, such as immunoassay.
1000651 Performance and Accuracy Measures of the Invention
1000661 The performance and thus absolute and relative clinical usefulness
of the invention
may be assessed in multiple ways as noted above. The accuracy of a diagnostic,
predictive, or
prognostic test, assay, or method concerns the ability of the test, assay, or
method to distinguish
between subjects responsive to metadoxine treatment and those that are not, is
based upon the
ratio of phosphoryl.ated ERK and Akt protein to total ERK and Akt protein. The
difference in
the ratio betwen normal and abnormal is preferably statistically significant.
1000671 Therefore, in assessing the accuracy and usefulness of a proposed
medical test, assay,
or method for assessing a subject's condition, one should always take both
sensitivity and
specificity into account and be mindful of what the cut point is at which the
sensitivity and
specificity are being reported because sensitivity and specificity may vary
significantly over the
range of cut points. Use of statistics such as A.UC, encompassing all
potential cut point values, is
preferred for most categorical risk measures using the invention, while for
continuous risk
measures, statistics of goodness-of-fit and calibration to observed results or
other gold standards,
are preferred.
1000681 Using such statistics, an "acceptable degree of diagnostic
accuracy", is herein defined
as a test or assay in which the AUC (area under the ROC curve for the test or
assay) is at least
0.60, desirably at least 0.65, more desirably at least 0.70, preferably at
least 0.75, more
preferably at least 0.80, and most preferably at least 0.85.
1000691 By a "very high degree of diagnostic accuracy", it is meant a test
or assay in which
the AUC (area under the R.00 curve for the test or assay) is at least 0.80,
desirably at least 0.85,
more desirably at least 0.875, preferably at least 0.90, more preferably at
least 0.925, and most
preferably at least 0.95.
17
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000701 The predictive value of any test depends on the sensitivity and
specificity of the test,
and on the prevalence of the condition in the population being tested. This
notion, based on
Bayes' theorem, provides that the greater the likelihood that the condition
being screened for is
present in an individual or in the population (pre-test probability), the
greater the validity of a
positive test and the greater the likelihood that the result is a true
positive. Thus, the problem
with using a test in any population where there is a low likelihood of the
condition being present
is that a positive result has limited value (i.e., more likely to be a false
positive). Similarly, in
populations at very high risk, a negative test result is more likely to be a
false negative.
1000711 As a result, R.00 and AUC can be misleading as to the clinical
utility of a test in low
disease prevalence tested populations (defined as those with less than 1% rate
of occurrences
(incidence) per annum, or less than 10% cumulative prevalence over a specified
time horizon).
Alternatively, absolute risk and relative risk ratios as defined elsewhere in
this disclosure can be
employed to determine the degree of clinical utility. Populations of subjects
to be tested can also
be categorized into quartiles by the test's measurement values, where the top
quartile (25% of the
population) comprises the group of subjects with the highest relative risk for
therapeutic
unresponsiveness, and the bottom quartile comprising the group of subjects
having the lowest
relative risk for therapeutic unresponsiveness Generally, values derived from
tests or assays
having over 2.5 times the relative risk from. top to bottom quartile in a low
prevalence population
are considered to have a "high degree of diagnostic accuracy," and those with
five to seven times
the relative risk for each quartile are considered to have a "very high degree
of diagnostic
accuracy." Nonetheless, values derived from tests or assays having only 1.2 to
2.5 times the
relative risk for each quartile remain clinically useful are widely used as
risk factors for a
disease; such is the case with total cholesterol and for many inflammatory
biomarkers with
respect to their prediction of future events. Often such lower diagnostic
accuracy tests must be
combined with additional parameters in order to derive meaningful clinical
thresholds for
therapeutic intervention, as is done with the aforementioned global risk
assessment indices.
1000721 A health economic utility function is an yet another means of
measuring the
performance and clinical value of a given test, consisting of weighting the
potential categorical
test outcomes based on actual measures of clinical and economic value for
each. Health
18
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
economic performance is closely related to accuracy, as a health economic
utility function
specifically assigns an economic value for the benefits of correct
classification and the costs of
misclassification of tested subjects. As a performance measure, it is not
unusual to require a test
to achieve a level of performance which results in an increase in health
economic value per test
(prior to testing costs) in excess of the target price of the test.
[000731 Construction of Clinical Algorithms
[000741 Any formula may be used to combine results into indices useful in
the practice of the
invention. As indicated above, and without limitation, such indices may
indicate, among the
various other indications, the probability, likelihood, absolute or relative
chance of responding to
metadoxine . This may be for a specific time period or horizon, or for
remaining lifetime risk, or
simply be provided as an index relative to another reference subject
population.
1000751 Although various preferred formula are described here, several
other model and
formula types beyond those mentioned herein and in the definitions above are
well known to one
skilled in the art. The actual model type or formula used may itself be
selected from the field of
potential models based on the performance and accuracy characteristics of its
results in a training
population. Preferred formulas include the broad class of statistical
classification algorithms, and
in particular the use of discriminant analysis. The goal of discriminant
analysis is to predict class
membership from a previously identified set of features. In the case of linear
discriminant
analysis (LDA), the linear combination of features is identified that
maximizes the separation
among groups by some criteria. Features can be identified for LDA using an
eigengene based
approach with different thresholds (ELDA) or a stepping algorithm based on a
multivariate
analysis of variance (MANOVA). Forward, backward, and stepwise algorithms can
be
performed that minimize the probability of no separation based on the
Hotelling-Lawley statistic.
1000761 Eigengene-based Linear Discriminant Analysis (ELDA) is a feature
selection
technique developed by Shen et at. (2006). The formula selects features (e.g.
biomarkers) in a
multivariate framework using a modified eigen analysis to identify features
associated with the
most important eigenvectors. "Important" is defined as those eigenvectors that
explain the most
variance in the differences among samples that are trying to be classified
relative to some
threshold.
19
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
1000771 A support vector machine (SVM) is a classification formula that
attempts to find a
hyperplane that separates two classes. This hyperplane contains support
vectors, data points that
are exactly the margin distance away from the hyperplane. In the likely event
that no separating
hyperplane exists in the current dimensions of the data, the dimensionality is
expanded greatly
by projecting the data into larger dimensions by taking non-linear functions
of the original
variables (Venables and Ripley, 2002). Although not required, filtering of
features for SVM
often improves prediction. Features (e.g., biomarkers) can be identified for a
support vector
machine using a non-parametric Kruskal-Wallis (KW) test to select the best
univariate features.
A random. forest (RF, Breiman, 2001) or recursive partitioning (RPART, Breiman
et al., 1984)
can also be used separately or in combination to identify biomarker
combinations that are most
important. Both KW and RF require that a number of features be selected from
the total. RPAR.T
creates a single classification tree using a subset of available biomarkers.
1000781 Other formula may be used in order to pre-process the results of
individual
phosphorylation of ERK and/or Akt measurem.ent snto more valuable forms of
information, prior
to their presentation to the predictive formula. Most notably, normalization
of biomarker results,
using either common mathematical transformations such as logarithmic or
logistic functions, as
normal or other distribution positions, in reference to a population's mean
values, etc. are all well
known to those skilled in the art. Of particular interest are a set of
normalizations based on
Clinical Parameters such as age, gender, race, or sex, where specific formula
are used solely on
subjects within a class or continuously combining a Clinical Parameter as an
input. In other
cases, analyte-based biomarkers can be combined into calculated variables
which are
subsequently presented to a formula.
1000791 In addition to the individual parameter values of one subject
potentially being
normalized, an overall predictive formula for all subjects, or any known class
of subjects, may
itself be recalibrated or otherwise adjusted based on adjustment for a
population's expected
prevalence and mean biomarker parameter values, according to the technique
outlined in
D'Agosti.no et al, (2001) JAMA 286:180-187, or other similar normalization and
recalibration.
techniques. Such epidemiological adjustment statistics may be captured,
confirmed, improved
and updated continuously through a registry of past data presented to the
model, which may be
CA 02923421 2016-03-04
WO 2015/035402
PCT/US2014/054816
machine readable or otherwise, or occasionally through the retrospective query
of stored samples
or reference to historical studies of such parameters and statistics.
Additional examples that may
be the subject of formula recalibration or other adjustments include
statistics used in studies by
Pepe, M. S. et al, 2004 on the limitations of odds ratios; Cook, N. R., 2007
relating to ROC
curves. Finally, the numeric result of a classifier formula itself may be
transformed post-
processing by its reference to an actual clinical population and study results
and observed
endpoints, in order to calibrate to absolute risk and provide confidence
intervals for varying
numeric results of the classifier or risk formula. An example of this is the
presentation of
absolute risk, and confidence intervals for that risk, derived using an actual
clinical study, chosen
with reference to the output of the recurrence score formula in the Oncotype
Dx product of
Genomic Health, Inc. (Redwood City, Calif.). A further modification is to
adjust for smaller sub-
populations of the study based on the output of the classifier or risk formula
and defined and
selected by their Clinical Parameters, such as age or sex.
EXAMPLES
1000801 EXAMPLE 1: GENERAL METHODS
1000811 The
examples as described herein were performed using the reagents and methods
generally described below.
Experimental Animals
1000821 Fmr1 knockout mice (1(02) mice (The Dutch-Belgium Fragile X
Consortium, 1994),
initially obtained from the Jackson Laboratory, and wild type (WT) littermates
were generated
on a C57BL/6J background and repeatedly backcrossed onto a C57BL/6J background
for more
than eight generations. The Finn] knockout mice were housed in groups of the
same genotype in
a temperature and humidity controlled room with a 12-h light-dark cycle
(lights on from 7 am to
7 pm; testing was conducted during light phase). Room temperature and humidity
were recorded
continuously in the holding room while food and water were available ad
libitum. Testing was
conducted on healthy Finn] knockout mice and their wild type liftermates (N =
10 mice per
treatment group) at 2 or 6 months of age during the behavioral experiments.
Mice were housed
in commercial plastic cages and experiments were conducted in line with the
requirements of the
21
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
UK Animals (Scientific Procedures) Act, 1986. All experiments were conducted
with
experimenters blind to genotype and drug treatment. Animals were allowed a
minimum
acclimatization period of one week prior to performing any experiment. No
prophylactic or
therapeutic treatment was administered during the acclimatization period.
Drugs
1000831 For Study 1 (Example 2), metadoxine was dissolved in saline and
administered
intraperitoneally at doses of 100, 150, or 200 mg/kg' once daily for 7 days.
For Study 2
(Example 3), in vivo testing, metadoxine was dissolved in saline and
administered at an
intraperitoneal dose of 1.50 mg/kg per day or at an oral dose of 150 or 300
mg/kg/day (in a
volume of 0.1 ml) once daily for seven days. For Study 2, in vitro testing,
metadoxine was
administered at concentration of 300 i.tM for five hours. In all cases, saline
was used as a vehicle
(control).
Behavioral Testing
1000841 Social Interaction and Social Recognition Memory: Mice are a social
species that
engage in easily scored social behaviors including approaching, following,
sniirmg, grooming,
aggressive encounters, sexual interactions, parental behaviors, nesting, and
sleeping in a group
huddle. Social approach in mice was evaluated by sniffing duration directed to
a novel mouse.
1000851 Mice were placed in a test arena/cage of the same order of
magnitude in size as the
adult's home cage (40 x 23 x 12 cm cage, with a Perspex lid to facilitate
viewing the mice) with
fresh wood chippings on the floor. A. background mouse odor was created by
putting in some
non-experimental mice into the apparatus prior to testing. Mice were
transferred to the
experimental room. 10-15 min prior to testing. A. test subject and a juvenile
were placed
simultaneously into the test cage. The total duration and number of bouts of
social investigation,
defined as sniffing and close following (<2 cm from the tail) of the stimulus
juvenile by the
tested mouse, was assessed for 3 min. 30 min later, the test was repeated
using the same
stimulus juvenile. Data parameters collected were the total duration and total
number of bouts of
22
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
sniffing for the acquisition and recognition. A social memory ratio was
derived, defined as trial
2/trial 1+2. Therefore, no memory (e.g. 20/(20+20) =0.5 and memory (e.g.
10/(20+10) = <0.5.
1000861 Y-Maze Alternation: Two tasks were implemented. The first was an
unlearned
assessment of spontaneous alternation between arm entries. The second was a
spatial reference
memory task in which the animal had to learn which of the two arms was baited
with a food
reward. The day prior to the start of the training, mice were allowed to
freely explore the maze
for 5 min. Next, they received two trials, one in which the food was located
on the left arm and
one in which the food was positioned on the right arm. This procedure
prevented the
development of a preference for one of the arms.
1000871 Y-Maze Water Maze: A clear Perspex Y-maze was filled with 2 cm of
water at 20 C.
This motivated the mouse to leave the maze after paddling to an exit tube at
the distal end of one
arm.. The maze was placed in the middle of a room surrounded by prominent
visual cues.
1000881 Rewarded T Maze Alternation: An elevated or enclosed apparatus in the
form of a T
(placed horizontally) was used. Mice were placed at the base of the T and were
allowed to
choose one of the goal arms abutting the other end of the stem. Two trials
were conducted in
quick succession, the second trial required mice to choose the arm not visited
before, reflecting
memory of the first choice (spontaneous alternation). This tendency was
reinforced by making
the animal hungry and rewarding it with a preferred food if it alternated.
Specifically, after a
four day habituation period on the T-maze, mice were trained to alternate arm
choices to receive
sweet condensed milk as reward.
1000891 Successive Alleys: The apparatus consisted of four successive,
linearly arranged,
increasingly anxiogenic alleys (each succeeding alley was painted a lighter
color, had lower
walls and/or was narrower than the previous alley) made of painted wood. Each
section or alley
was 25 cm long. Alley I had 25 cm high walls, was 8.5 cm wide, and was painted
black. A 0.5
cm step down led to alley 2, which was again 8.5 cm wide, but had .1.3 cm high
walls and was
grey. A 1.0 cm step down led to alley 3, which was 3.5 cm wide, had 0.8 cm
high walls, and was
white. A 0.4 cm step led down to alley 4, which was also white, but had 1.2 cm
wide and 0.2 cm
high walls. The apparatus was elevated by anchoring the back of alley 1 to a
stand, 50 cm high.
Padding was provided under arms 3 and 4 in case a mouse fell off. Each mouse
was placed at
23
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
the closed end of alley 1 facing the wall. Timers were started 1) for the
overall length of the test
(5 min) + the latency to enter each arm, and 2) for the time spent in alley 1.
When the mouse
placed all 4 feet on to the next alley, it was considered to have entered the
alley. Total time spent
in each alley (all four feet) was recorded.
1000901 Contextual Fear Conditioning: In the fear conditioning experiment,
mice were placed
into a novel environment (dark chamber) and received pairings of a cue and
electric footshock
(0.2 mA for 1 sec (Study 1) or 0.7 mA for 0.5 sec (Study 2)). Subsequently,
when tested in the
original training context, mice displayed a natural defensive response termed
freezing
(Blanchard, 1969) or contextual fear conditioning. Freezing time was defined
as the time that
the mice spent in immobile behavior, except for respiration. The data was
expressed as the
percentage of the test period. 24 hours after a training session, mice were
tested for 5 min in the
training chamber with no shock presentation and observed for freezing
behavior.
1000911 Statistics: Multivariate analysis of variance was employed to
assess group differences
across data. Repeated measures ANOVA were performed for behavioral data.
Statistically
significant effects in each ANOVA were followed with post hoc comparisons,
using the
Newman-Keuls test (Study 1) or the Tukey test (Study 2). A p value of less
than 0.05 was
considered significant.
Biochemical Testing
1000921 Phosphorylated ERK and Akt: The Ras-Mek-ERK and PI3K-Akt-mToR
signaling
pathways are involved in mediating activity dependent alterations in gene
transcription
underlying changes in synaptic plasticity (Klann and Dever, 2004).
Phosphorylated ERK and Ala
protein expression was measured by western blot analysis as previously
described by Lopez
Verrilli. (Lopez Verrilli et al., 2009). The antibodies employed were anti-
phosphospecific
antibodies against Akt (1/1000) and kinase (ERK) 1/2 (1/2000) (Cell Signaling
Technology,
Danvers, MA, USA). The antibody against phospho-ERK detects phosphorylation at
phospho-
ERK1/2 (Thr202/Tyr204) whereas the antibody against phospho-Akt detects
phosphorylation at
phospho-Akt (Thr308). Total Akt and ERK. 1/2 protein content and
phosphorylated ERK and
Akt were evaluated by blotting membranes with antiphospho-Akt (1/1000) and
antiphospho-
24
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
ERK antibodies (1/2000) (Cell Signaling Technology, Danvers, MA, USA). Akt or
ERK
phosphorylation was normalized to protein content in the same sample and
expressed as % of
change with respect to basal conditions, considering basal levels as 100%.
Protein loading was
evaluated by stripping and re-blotting membranes with 13-actin antibody
(1/1000) (Sigma-
Aldrich, St. Louis, MO, USA). Phosphorylated ERK and Akt protein expression in
blood
lymphocytes was measured by flow cytometty. For lymphocyte biomarker
determinations, a
FACStar plus (Becton Dickinson) was used with the excitation laser tuned at
488 nm. and green
fluorescence from FITC (GST) was collected through a 515-545 nm bandpass
filter. The mean
ETC fluorescence Intensity was calculated in relation to the fluorescence of
reference cells. The
mean cellular fluorescence intensity (MFI) is directly proportional to the
mean number of Ab
molecules bound per cell.
1000931 iVeuronal morphology: Hippocampal cell cultures were prepared from
wild type and
Fmrl KO fetal mice at embryonic day of gestation 17.5 (E 17.5). Mice were
killed by cervical
dislocation and dissociated hippocampal cells were plated in 15 mm multi well
vessels (Falcon
Primaria). After 5d in vitro, green fluorescent protein (GFP) was transfected
to facilitate
monitoring dendritic spine m.orphogenesis after drug treatment (Ethell and
Yam.aguchi, 1999;
Ethell et al., 2001, Henkemeyer et al., 2003). Dendritic spines were formed at
around 16 days in
vitro (DIV). Cultures were treated with metad.oxine at 300 i.tM concentration
at day 17 in vitro
for 5 hrs.
1000941 Filopodia density of GFP transfected neurons was quantified by
performing Sholl
analyses of stacked Zeiss confocal generated images (40xobjective, stack of
20x0.21.tm). With
Metamotph software, concentric equally spaced circles (every 20ttin) were
drawn around the cell
soma of each neuron and subsequently, the amount of filopodia was counted per
circle.
Averages of counts were compared with unpaired two-tailed Student's T-tests.
1000951 Spine maturity of GFP transfected neurons was analyzed with
Metamorph software
(Molecular Devices, Sunnyvale, CA). Two distal dendritic segments of 70 100 pm
were
chosen per neuron for spine morphom.etric analysis. For each spine, the length
and the width
were measured. The length was defined as the distance from the base to the tip
of the protrusion;
whereas width was defined as the maximum distance perpendicular to the long
axis of the spine.
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
Measurements were compared with unpaired two tailed Student's T-tests and
ANOVA
corrected for multiple comparisons.
100096] De novo hippocampal protein synthesis: Transverse hippocampal
slices (400 p,m)
were obtained from 6-week-old Fmr1 knockout and WT mice. A protein synthesis
assay was
performed as previously described using the nonradioactive fluorescence-
activated cell sorting--
based assay, surface sensing of translation (SUnSET) method, which allows the
monitoring and
quantification of global protein synthesis in individual mammalian cells and
in heterogeneous
cell populations. (Hoeffer, 2011). The concentration of metadoxine used in
this study was 300
M.
EXAMPLE 2: THE EFFECT OF METADOXINE (100 to 200 mg/kg) TREATMENT ON
LEARNING AND MEMORY DEFICITS AND BIOCHEMICAL ABNORMALITIES IN
THE Fmr1 KNOCKOUT MOUSE MODEL OF FRAGILE X SYNDROME (Study 1)
Behavioral Analyses
1000971 Contextual Fear Conditioning: An initial experiment tested the
effect of
intraperitoneal administration of vehicle or 150 mg/kg metadoxine once daily
for seven days on
contextual fear conditioning in groups of N = 10 WT and Finn] knockout mice.
Vehicle-treated
Finn knockout mice showed a deficit in learning in the contextual fear
conditioning paradigm as
reflected in a reduction in freezing during the test session (Fig. 1, Panel A
(p <0.0001)).
Metadoxine administration reversed the learning deficit effect in Fnirl
knockout mice, this
reversal being partial such that metadoxine-treated animals differed from the
metadoxine-treated
WT animals (p<0.05). A replication of this experiment investigated the dose-
dependent effects
of intraperitoneal administration of vehicle, 100, or 200 mg/kg metadoxine
once daily for seven
days on contextual fear conditioning in groups of N = 10 WT and Fmrl knockout
mice (Fig. 1,
Panels B and C). In this experiment, vehicle-treated Finn! knockout mice
showed a learning
deficit compared to vehicle-treated WT mice (p<0.0001), replicating the first
experiment. 100
mg/kg metadoxine produced a reversal of the deficit in Fmr1 knockout mice
(P<0.05) but this
was a partial reversal since metadoxine-treated Find knockout mice differed
from the
26
CA 02923421 2016-03-04
WO 2015/035402
PCT/US2014/054816
metadoxine-treated Wild Type mice (p<0.0001). The learning deficit seen in
Finn] knockout
mice was completely reversed following treatment with 200 mg/kg i.p.
metadoxine (treated
Finn mice differed from vehicle-treated Finn knockout mice (P<0.0001) but did
not differ from
metadoxine-treated WT mice). Metadoxine treatment had no effect on WT mice in
either
experiment (Fig. 1, Panels A-C).
1000981 Social Approach: Vehicle-treated Fmrl knockout mice showed less social
approach
as indexed by sniffing bouts (Fig. 2 (p<0.0001)). Once daily intraperitoneal
treatment with 150
mg/kg metadoxine for seven days increased social approach in Finn l knockout
mice (p<0.0001
compared to vehicle treated Fmr.1 knockout mice). Fmr.1 knockout mice treated
with
metadoxine differed from metadoxine-treated WT mice (p<0.05), although there
was a trend
approaching the effect of the WT mice. Metadoxine treatment had no effect on
WI mice.
1000991 Y-
maze Spontaneous Alternation: The effect of seven days of once daily treatment
with vehicle or 150 mg/kg metadoxine on spontaneous alternation in groups of N
= 10 WT or
Fmr.1 knockout mice is shown in Figure 3, Panel A. Vehicle-treated Fmr.1
knockout mice
showed less spontaneous alternation than vehicle treated WT mice (p<0.0001).
Metadoxine
treatment increased spontaneous alternation compared to vehicle treatment in
Fmrl knockout
mice (p<0.0001), although metadoxine-treated Finn] knockout mice showed a
deficit compared
to metadoxine-treated WT mice (p<0.01). Metadoxine therefore produced a
partial reversal of
the deficit seen in Fmr1 knockout mice.
10001001 Y-maze Reference Memory Task: The effect of seven days of once daily
treatment
with vehicle or 150 mg/kg metadoxine on rewarded reference memory learning in
groups of N =
WT or Fmrl knockout mice is shown in Figure 3, Panel B. Vehicle-treated Fmrl
knockout
mice made less appropriate arm entries than vehicle-treated WT mice
(p<0.0001). Metadoxine
treatment reduced this deficit (p<0.0001) compared to vehicle-treated Finn]
knockout mice, such
that metadoxine-treated Fmr1 knockout mice did not differ from metadoxine-
treated WT mice.
Metadoxine treatment had no effect on WT mice.
10001011 Y-niaze Water Maze Left Right Discrimination: The effect of seven
days of once daily
treatment with vehicle or 150 mg/kg metadoxine on aversively motivated spatial
discrimination
learning in groups of N = 10 WT or Find knockout mice is shown in Figure 3,
Panel C.
27
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
Vehicle-treated Fmr1 knockout mice showed a greater number of incorrect arm
entries than
vehicle-treated WT mice. This deficit was reduced by treatment with
metadoxine.
10001021 T-maze Rewarded Alternation Task: The effect of seven days of once
daily treatment
with vehicle or 150 mg/kg metadoxine on rewarded alternation working memory in
groups of N
= 10 WT or Fmrl knockout mice is shown in Figure 4. Vehicle-treated Finn 1
knockout mice
showed a greater latency to reach the correct arm compared to vehicle-treated
WT mice
(p<0.0001). Metadoxin.e treatment reduced this deficit compared to vehicle
treatment in Fmrl
knockout mice (p<0.0001), this reversal being partial since metadoxine-treated
Find knockout
mice responded more slowly than WT mice (p<0.0001).
10001031 Successive Alleys: The effect of seven days of once daily treatment
with vehicle or
150 mg/kg metadoxine on behavior in the successive alleys task in groups of N
= 10 WT or
Finn knockout mice is shown in Figure 5 and further described below.
10001041 The successive alleys test effectively measured anxiety (latency to
enter the Alley 1)
and hyperactivity (Alleys 2 to 4). Progression from Alley 1 through the
successive Alleys 2, 3,
and 4 was associated with exposure to an increasingly brightly colored
environment with
increasingly lower walls and narrower, more exposed open arms. Time spent on,
and entries
into, the open arms indicated anxiety; conversely, increasing time spent in
more open arms
reflected hyperactivity. These factors allowed for a sensitive test bracketing
a range of anxiety-
like behaviors together with hyperactivity.
10001051 Alley 1: The Finn 1 knockout mice showed more anxiety than WT mice
(p<0.001).
Finn knockout mice treated with metadoxine showed an amelioration in anxiety
compared with
the vehicle treated Fmr1 knockout mice (p<0.001), such that complete
normalization occurred.
There was no difference between the metadoxine-treated Finn knockout and
m.etadoxine-treated
wr mice. Also, metadoxine treatment had no effect on WT mice.
10001061 Alley 2: WT mice showed less activity in Alley 2 when compared with
the Fmr1
knockout mice (p<0.0001). Treatment with metadoxine reduced hyperactivity in
the Finn1
knockout mice (p<0.001), although this reversal of hyperactivity was partial
since metadoxine-
treated Fmr1 knockout and WT mice differed (p<0.001). Metadoxine treatment had
no effect on
WT mice.
28
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
10001071 Alley 3: Fmrl knockout mice showed hyperactivity compared to WT mice
(p<0.0001). This hyperactivity was not reversed by metadoxine, since
metadoxine-treated Fmrl
knockout mice did not differ from vehicle-treated Fmr.1 knockout mice.
Metadoxine treatment
had no effect on WT mice.
10001081 Alley 4: Fmr.1 knockout mice showed hyperactivity compared to WI'
mice (p<0.01).
Metadoxine treatment reversed this hyperactivity since metadoxine-treated Fmr1
knockout mice
showed less activity than vehicle-treated Find knockout mice (p<0.01). This
effect reflected a
normalization since metadoxine-treated Fmr1 knockout mice did not differ from
metadoxine-
treated WI mice. Metadoxine treatment had no effect on WT mice.
10001091 Overall, without wishing to be bound by theory, the successive alleys
test showed that
Fmr1 knockout mice had increased anxiety and hyperactivity compared to WT
mice.
Metadoxine treatment reduced this anxiety and hyperactivity in the Pim-1
knockout mice whilst
leaving WT mice unaffected.
Biochemical Analyses
10001101 Phosphorylation of ERK and Akt: The effect of seven days of once
daily
intraperitoneal treatment with either vehicle or 150 mg/kg metadoxine in N = 5
Fmr.1 knockout
or WT mice on whole brain phosphorylation of ERK or Akt in the brain is shown
in Figure 6.
Phosphorylation levels were assessed as the ratio of phosphorylated to total
ERK. An increase in
this ratio indicated activation of ERK. Phosphorylation of ERK was increased
in vehicle-treated
Finr1 knockout mice compared to vehicle controls (p<0.001) ¨ this effect
replicated the aberrant
activation of ERK seen in human subjects with Fragile X Syndrome (Wang et al.,
2012). This
effect was reduced by metadoxine treatment (p<0.01) such that there was no
difference
compared to metadoxine-treated WT mice. Metadoxine had no effect on
phosphorylation of
ERK in WT mice or total ERK levels in any mice. The ratio of phosphorylated
Akt to total AKT
was also increased in vehicle-treated Finn .1 knockout mice compared to
vehicle-treated WT mice
(p<0.0001). Treatment with metadoxine reduced the relative levels of
phosphorylated Ala in
Fmr.1 knockout mice (p<0.01), such that Fmr.1 knockout mice did not differ
from the controls.
Metadoxine treatment had no effect on WT mice, or on the total Akt levels of
any mice.
29
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
EXAMPLE 3: THE EVALUATION OF METADOXINE IN THE Dna KNOCKOUT
FRAGILE X MOUSE MODEL (Study 2)
Behavioral Effects of Metadoxine in 6 Month Old Fmrl Knockout Mice
10001111 Contextual Fear Conditioning: An initial experiment tested the effect
of
intraperitoneal administration of vehicle or 150 mg/kg metadoxine once daily
for seven days on
contextual fear conditioning in groups of N = 10 WT and Fmr1 knockout mice
aged six months.
Vehicle-treated Finn! knockout mice (KO-V) showed a deficit in learning in the
contextual fear
conditioning paradigm when compared with vehicle-treated WT mice (WT-V) as
reflected in a
reduction in freezing during the test session (Fig. 7 (p <0.0001)). Metadoxine
administration
reversed the learning deficit effect in Friar] knockout mice (p<0.0001 KO-M-
150 vs. KO-V).
This was a complete reversal such that metadoxine-treated KO mice did not
differ from
metadoxine-treated WT mice.
10001121 Social Approach and Social Memoty: Social approach data (initial
Trial 1) are shown
in Figure 8, Panel A (number of sniffing bouts) and Panel C (duration of
sniffing). Social
memory data (Trial 2, 24 hour after Trial 1) are shown in Figure 8, Panel B
(number of sniffmg
bouts) and Panel D (duration of sniffing). These results are further discussed
below.
10001131 During Trial 1, Fmrl knockout mice showed an increased number of
sniffing bouts
(p<0.0001) (See Fig. 8, Panel A) and a reduced duration of sniffing (p<0.0001)
(See Fig. 8,
Panel C) compared to WT mice. These social interaction deficits are consistent
with those
reported by other researchers in Fmrl knockout mice (Thomas et al., 2011). For
both number of
bouts and duration of sniffing, treatment with metadoxine produced reversals
of abnormalities in
Fmrl knockout mice (p<0.0001 KO-M-150 vs. KO-V for each), such that metadoxine-
treated
Fmr1 knockout mice did not differ from metadoxine-treated WT mice for the
number of sniffing
bouts measurement. Whilst rescue was shown on the duration of sniffing
measure, this effect
was partial since Fmr1 knockout mice remained different compared to WT mice
after
metadoxine treatment (p<0.05). Metadoxine was without effect on WT mice. These
data
showed that abnormal social approach behaviors in Fmr1 knockout mice were
rescued by
metadoxine.
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
10001141 During Trial 2, Fnirl knockout mice showed both an increase in number
of sniffing
bouts and an increase in duration of sniffing (p<0.0001 for each measure, Fig.
8, Panels B and D,
respectively) compared to Wild Type mice. This reflected a failure of
habituation and therefore,
a social memory deficit. Metadoxine treatment reduced these differences
(p<0.0001 for KO-M-
150 vs. KO-V). The reversal for number of sniffing bouts was partial since a
difference
remained between metadoxine-treated Fmr1 knockout mice and metadoxine-treated
WT mice
(p<0.05). The reversal by m.etadoxine was complete for sniffing duration,
since no difference
was observed between metadoxine-treated Fmr1 knockout and metadoxine-treated
WT mice.
Metadoxine treatment was without effect on WT mice. These data showed that
metadoxine
reduced social memory impairments in Fmr1 knockout mice. This reduction in
social memory
deficit is illustrated below by a calculation of the social memory ratio
(described in Example 1):
100011.51 Social memory ratio was defined as the duration of sniffing bouts:
trial 2/trial 1+2.
Therefore, an example of no memory was (e.g. 20/(20+20) =0.5, while an example
of memory
was (e.g. 10/(20+10) = <0.5.
10001161 The calculated social memory ratios were as follows:
WT-V Trial 2/trial 1-t- Trial 2: 12.4/12.4+ 26.8 = 0.3, <0.5 memory
KO-V Trial 2/trial 1+ Trial 2: 325 / 325 + 24.1 = 0.9, No memory
WT-M Trial 2/trial 1+ Trial 2: 12.5/38.5 + 12.5 = 0.2, <0.5 memory
KO-M Trial 2/trial 1+ Trial 2: 12.7/28.4 + 12.7 = 0.3, <0.5 memory
Biochemical Effects of Metadoxine in 6 Month Old Fmrl Knockout Mice
10001171 The effect of seven days of once daily ip treatment with either
vehicle or 150 mg/kg
metadoxine in N = 10 Finn knockout or WI mice on whole brain pERK (Fig. 9,
Panel A) and
pAkt (Fig. 9, Panel B) in the brain following the behavioral tests described
above is shown in
Figure 9. Specifically, Figure 9, Panel A shows brain levels of pAkt, which
were increased in
Finn 1 knockout mice compared to WT mice as seen in previous experiments
(P<0.0001).
Treatment with m.etadoxine reversed this increase in brain pAkt (p<0.0001 for
KO-M-150 vs.
KO-V) such that metadoxine-treated Finn 1 knockout mice did not differ from
metadoxine-
treated WT mice. Figure 9, Panel B shows brain levels of pERK which were
increased in Finn]
31
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
knockout mice compared to WT mice as seen in previous experiments (p<0.0001
for KO-M-150
vs. KO-V). This increase was reversed by metadoxine treatment (p<0.0001) such
that
metadoxine-treated Fmrl knockout mice did not differ from metadoxine-treated
WT mice.
Effect of Metadoxine Following Intraperitoneal or Oral Administration on the
Behavior of 2
Month Old Mice
(0001181 Figure 10 shows the effect of administration of once daily metadoxine
at doses of 150
mg/kg ip or 150 and 300 mg/kg orally for seven days on contextual fear
conditioning in two
month old Fmr1 knockout and WT mice. Specifically, Figure 10, Panel A shows
contextual fear
conditioning data from Fmr1 knockout and WT mice after ip and oral treatment
with vehicle.
There were no differences related to the route of administration of vehicle.
Fmrl knockout mice
showed a reduction in freezing behavior compared to WT mice after vehicle
treatment via ip and
oral routes (p<0.000I in each case). Figure 10, Panel B shows the effect of
metadoxine
treatment via both routes of administration in WT mice. No effects were seen.
Figure 10, Panel
C shows that ip 150 mg/kg and oral 150 and 300 mg/kg metadoxine treatment in
Finn 1 knockout
mice reversed the decrease in freezing behavior seen in Fmrl knockout mice
(p<0.01, p<0.0001,
and p<0.0001, for KO-M-ip, KO-M-pol50, and KO-M-po 300 vs. KO-V-ip and KO-V
po,
respectively). The effect of administration with 150 mg po metadoxine did not
differ from the
effect of administration of 300 mg/kg po metadoxine. The effect of 150 and 300
mg/kg oral
metadoxine in Fmrl knockout mice did not differ from the effect of 150 mg/kg
ip metadoxine.
In each case, the reversal was complete since metadoxine-treated Fmrl knockout
mice did not
differ from metadoxine-treated WT mice.
[0001191 Figure 11 shows the effect of administration of once daily metadoxine
at doses of 150
mg/kg ip or 150 and 300 mg/kg orally for seven days on social approach and
social memory in
Fmrl knockout and WT mice. Specifically, Figure 11, Panel A shows the effect
of vehicle or
metadoxine at 150 mg/kg ip or 150 and 300 mg/kg orally on social approach
behavior in Fmr 1
knockout or WT mice. After ip or oral treatment with vehicle, the duration of
sniffing behavior
in Finn] knockout mice was reduced compared to WT mice (p<0.0001 for each).
Metadoxine
treatment at any dose was without effect on WT mice. However, metadoxine
treatment at 150
32
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
mg/kg ip, 150 mg/kg, and 300 mg/kg orally produced reversals of the social
approach deficit
seen in Finn knockout mice (p<0.0001 for KO-M-po150 and KO-M-po300 vs. KO-\'
po,
respectively). The effect of oral metadoxine was not dose dependent between
150 and 300
mg/kg. This reversal was complete since metadoxine-treated Fmrl knockout mice
did not differ
from metadoxine-treated WI mice. The effect of 150 mg/kg ip metadoxine in Finn
knockout
mice did not differ from the effect of 150 mg/kg oral or 300 mg/kg oral
metadoxine. Figure 11,
Panel B shows the effect of vehicle or metadoxine at 150 mg/kg ip or 150 and
300 mg/kg orally
on social memory in Fmrl knockout or WT mice. After ip or oral treatment with
vehicle, the
duration of sniffing behavior in Fmr.1 knockout mice was increased compared to
WT mice
(p<0.0001 for each). Metadoxine treatment at any dose was without effect on WT
mice.
However, metadoxine treatment at 150 mg/kg ip, 150 mg/kg orally, and 300 mg/kg
orally
produced reversals of the social approach deficit seen in Fmrl knockout mice
(p<0.0001,
p<0.05, and p<0.01 for KO-M-ip150, KO-M-pol50, and KO-M-po 300 vs. KO-V-ip and
KO-V
po, respectively). This reversal was complete since metadoxine-treated Erni./
knockout mice did
not differ from metadoxine-treated WT mice. The effect of 150 mg/kg ip
metadoxine in Fmrl
knockout mice did not differ from the effect of 150 mg/kg oral or 300 mg/kg
oral metadoxine.
Also, there was no dose dependency for the effects of oral metadoxine
treatment between 150
mg/kg and 300 mg/kg.
Effect of Metadoxine on Biochemical Markers Following Intraperitoneal or Oral
Administration in 2 Month Old Mice
10001201 Peripheral Lymphocytes: Figure 12 shows the effect of administration
of once daily
metadoxine at doses of 150 mg/kg ip or 150 mg/kg and 300 mg/kg orally for 7
days on
lymphocyte pAkt (Fig. 12, Panel A) and pERK (Fig. 12, Panel B) as determined
by flow
cytometry in two month old Fmrl knockout and WT mice. Specifically, Figure 12,
Panel A
shows that vehicle-treated Fmr1 knockout mice exhibited increased
phosphorylation of
lymphocyte Akt (p<0.0001 for both ip and oral administration) compared to WT
mice receiving
equivalent vehicle treatment. Treatment with once daily metadoxine at 150
mg/kg ip or oral
doses of 150 mg/kg or 300 mg/kg for 7 days normalized overactivated Akt, such
that pAlct levels
33
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
did not differ between metadoxine-treated Fmrl knockout mice and WT mice
receiving the same
treatment. Figure 12, Panel B shows that vehicle-treated Fmrl knockout mice
showed increased
phosphorylation of lymphocyte ERK (p<0.0001 for both ip and oral
administration) compared to
WT mice receiving equivalent vehicle treatment. Treatment with once daily
metadoxine at 150
mg/kg ip or oral doses of 150 mg/kg or 300 mg/kg for 7 days normalized
overactivated ERK
such that pERK levels did not differ between metadoxine-treated Fmr1 knockout
mice and WT
mice receiving the same treatment.
10001211 Brain Regions: Figure 13 shows the effect of administration of 150
mg/kg
metadoxine for seven days on pERK levels in hippocampus, pre-frontal cortex,
and striatum.
pERK levels were increased in Finn] knockout mice compared to WT mice in all
three brain
regions (p<0.0001 in all cases). pERK levels were decreased in metadoxine-
treated Finn!
knockout mice compared to vehicle-treated Find knockout mice (p<0.0001 in all
cases). There
were no differences between KO-M and WT-M groups in the hippocampus and
striatum,
showing complete reversal of activation of ERK. The effect in pre-frontal
cortex was partial, the
KO-V and KO-M groups remained different (p<0.05). Metadoxine was without
effect on WT
mice.
10001221 Figure 14 shows the effect of administration of 150 mg/kg metadoxine
for seven days
on pAkt levels in hippocampus, pre-frontal cortex and striatum. pAkt levels
were increased in
Find knockout mice compared to WT mice in all three brain regions (p<0.0001 in
all cases).
pAkt levels were decreased in metadoxine-treated Finn] knockout mice compared
to vehicle-
treated Find knockout mice in all three brain regions (p<0.0001 in all cases).
In all cases, there
were no differences between KO-M and WT-M groups, showing complete reversal of
activation
of Akt. Metadoxine was without effect on WT mice. Reduction in brain and blood
elevated
levels of phosphorylated ERK and Akt correlated with the improved behavioral
outcomes of
Finn! knockout mice, suggesting that the phosphorylation levels are biomarkers
of metadoxine
treatment response
34
CA 02923421 2016-03-04
WO 2015/035402 PCT/US2014/054816
Effects of Metadoxine on .Dendritic Filopodia Density and Menial?lion in
Primary
Hippocampal Neurons from Find Knockout Mice in vitro
10001231 Figure 15 (Panels A-C) shows the effect of treatment for five hours
with 300 p.M
metadoxine. Dendrites were divided into 10 segments of 10 pm, each based on
distance from the
soma (proximal to distal, left to right). Spine density was increased in
neurons from. Fmrl
knockout mice compared to neurons from WT mice in segment 3. Specifically,
Figure 15, Panel
A shows the density of neuronal filopodia. Primary hippocampal neurons from
Fmr1 knockout
mice displayed an increased density of filopodia (p<0.001). Treatment with 300
p,M metadoxine
reduced the aberrant increase in density of neuronal filopodia in Ftnrl
knockout mice (p<0.001).
Neurons from Finn; knockout mice showed filopodia with characteristics of
immaturity, being
longer (Fig. 15, Panel B (p<0.01)) and narrower (Fig. 15, Panel C (p<0.01)).
Treatment with
metadoxine reversed this increase in filopodia length (Fig. 15, Panel B
(p<0.01)) and reversed
the decrease in width (Fig. 15, Panel C (p<0.001)).
Effects of Metadoxine on de novo Hippocampal Protein Synthesis in the Fula
Knockout
Mouse in vitro
10001241 Figure 16 shows the effect of treatment with either vehicle or 300 pM
metadoxine on
basal de novo protein synthesis in 400 AM hippocampal slices from. Fmr1
knockout or WT mice.
Protein synthesis was higher in vehicle-treated hippocampi from Ftnr1 knockout
mice than
vehicle-treated WT control hippocampi (p<0.0001). Metadoxine treatment reduced
protein
synthesis rates in Ftnrl knockout mouse hippocampi. This effect was partial
since hippocampi
from. Fmrl knockout mice retained higher protein synthesis rates than
metadoxine-treated
hippocampi from WT mice (p<0.001).
Table 1: Summary of Effects of Nletadoxine (150 mg/kg ) in the Mouse Model of
Fragile X Syndrome 0
t=-)
o
I¨.
Test
Route I dose 1 Abnormal in fowl KO mice (YIN) [ Reduction of deficit
(YIN) 1 Rklilicatiori of Previous Study tn
--.
Behavioral effects in 6 month old mice
o
ca
en
Contextual fear condition.in i 150 rrig/kg.i,p.
Deficit Y Y 4.
0
Social approach 1 150 mg/kg i.p.Y Y
Y
i
b.)
,
Social memory 150 mg/kg i.p. Y . Y
Not tested
,
Biochemical effects in 6 month old mice
Phosphorylation of brain Alt 150 mg/kg i.p.
Increased in finrl KO mice Y Y
Phosphorylation of brain ERIC 150 mg/kg i.p.
Increased in fmr1 KO mice Y Y
Phosphorylation of brain GSK31.3 150 mg/kg i.p.
Increased infinrl KO mice N Not tested
(tyr219/t3ir279)
Bruin GST levels 150 mg/kg i.p. Decreased infinr1 KO mice Y
Y
Behavioral effects of Metadoxine following intraperitoneal or oral
administration in 2 month old mice
Contextual fear conditioninu 150 mg/kg ip
-------------------------------------------------------------------------------
--------------------------- ¨4 0
Social approach behaviour 150 mg/kg ip Y Y
Y "
Social memory 150 mg/kg ip Y Y
Not tested .
Contextual fear conditioning 150 mg/kg po Y Y
Not tested "
¨
Social approach behaviour -- 150 mg/kg po Y Y
Not tested
_
.
Social memory 150 mg/kg po Y Y
Not tested 0
0
_
.
Contextual tear conditioning 300 mg/kg po Y Y
Not tested 0
0
_
.
Social approach behaviour 300 trig/kg po . Y
Y Not tested _
Social memory 300 mg/kg po Y Y
Not tested
---1
Biochemical effects of Metadoxine following intraperitoneal or oral
administration in 2 month old mice
........4
Lymphocyte pAkt and pERK 150 mg/kg ip Increased
Not tested
¨1
Lymphocyte pGSK3I3(tyr2 I 9Ityr279) 150 mg/kg ip
Increased N Not tested ¨
Lymphocyte GST ----------- 150 mg/kg ip Decreased N
Not tested _
Lymphocyte pAkt and pERK 150 mg/kg po Increased Y
Not tested _
Lymphocyte pGSK311(tyr219/tyr279) 150 mg/kg po
Increased N Not tested 9:1
_
Lymphocyte GS'I7 150 trig/kg po .
Decreased N Not tested A
Lymphocyte pAkt and pERK 300 mg/kg po Increased Y
Not tested
¨I
cil
Lymphocyte pGSK311(tyr219/tyr279) 300 mg/kg po
Increased N Not tested
--i
t=-)
Lymphocyte GST 300 mg/kg po Decreased N
Not tested
¨i o
I¨.
4.
Flippocampal pERK 150 mg/kg ip Increased Y
Not tested --.
. ¨I o
Prefrontal cortex pERK ----- 150 mg/kg ip Increased Y
Not tested tn
Striatum pERK 150 mg/kg ip Increased Y
Not tested H 4.
00
=i
o,
36
'
i
Hippocampal pAkt 150 mg/kg ip Increased ---------------------
Y -------------- Not tested ------- 0
-------------------------------------------------------------------------------
------------------------------- ---1
Prefrontal cortex pAkt 150 mg/kg ip increased
Y Not tested t.)
I o
Striatum pAkt 150 mg/kg ip Increased
Y Not tested
en
-...
0
Test , Route / dose Abnormal in find KO mice (YIN)
Reduction of deficit (Y/N) Replication of Previous Study co)
tn
Hippocatnpal pGSK313(ser9) 150 mg/kg ip Normal levels
NA Not tested 4.
0
Prefrontal cortex pGSK30(ser9) 150 mg/kg ip Decreased
N Not tested i=-)
Striatum pGSK313(ser9) ISO nag/kg ip Decreased
Y Not tested
Hippocampal (1ST 150 mg/kg ip Decreased
N Not tested
Prefrontal cortex (1ST 150 mg/kg ip Decreased
N Not tested
Striatum (1ST 150 tng/kg ip Decreased
Y Not tested
Hippocatnpal pS6K1(ser235/236) 150 mg/kg ip increased
N Not tested
Prefrontal cortex pS6K1(ser235/236) 150 mg/kg ip
Increased N Not tested
Striatum pS6K1(ser235/236) 150 mg/kg ip Increased
N Not tested
Hippocampal pS6K1(ser240/244) 150 mg/kg ip Increased
N Not tested
Prefrontal cortex pS6K1(ser240/244) 150 mg/kg ip
Increased N -------------------- Not tested 0
0
, Striatum pS6K1(ser240/244) 150 mg/kg ip Increased
N Not tested "
Effects of Metadoxine on filopodia density and maturation in neurons from fmrl
knockout mice in vitro .
Neuronal tilopodia density 300 JIM
1Increased N' Not tested .
Neuronal filopodia length 300 n.M Increased --------------------------
Y -------------- Not tested ----------- .
'
.
Neuronal filopodia width 300 IIM I Decreased
Y Not tested 0
.
....
=
Effects of Metadoxine on de novo hippocam )al protein synthesis in fart
knockout mice in vitro . 0
De novo hippocampal protein synthesis rate 300 11M
Increased -st Not tested
V
A
...._
cil
b.)
o
I¨.
4.
s....
0
en
4.
00
=i
01
37