Note: Descriptions are shown in the official language in which they were submitted.
REACTANT FLOW CHANNELS
FOR ELECTROLYZER APPLICATIONS
Field of the Invention
The present invention relates generally to electrolyzers, and in particular to
fluid flow
channels and flow fields for an electrolyzer, and electrolyzers and
electrolyzer stacks
incorporating such fluid flow channels and flow fields.
Background of the Invention
Although many electrolyzers are based on an alkaline (KOH) electrolyte,
another option is to
use a proton exchange membrane (PEM) as the electrolyte. In PEM electrolysis,
water is
supplied to the anode and is split into oxygen, protons and electrons by
applying a DC
voltage. Protons pass through the polymer electrolyte membrane and combine
with electrons
at the cathode to form hydrogen; thus oxygen is produced at the anode, and
hydrogen is
produced at the cathode as illustrated in a schematic diagram in FIG. I. It is
important that the
hydrogen and oxygen, which evolve at the surfaces of the respective
electrodes, are kept
separate and do not mix.
- 1 -
CA 2925051 2020-02-05
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
The electrolysis process is essentially the reverse of the process in a PEM
fuel cell. A PEM
electrolyzer cell can be very similar in structure to a PEM fuel cell, with a
polymer membrane
sandwiched between a pair of porous electrodes and flow field plates. FIG. 2A
shows a
simplified diagram of an electrolyzer unit cell, and FIG. 2B shows a
simplified diagram of a
fuel cell unit cell. The materials used in a PEM electrolyzer are generally
different because
the carbon materials commonly used as catalyst supports, gas diffusion layers
and flow field
plates in fuel cells cannot be used on the oxygen side of a PEM electrolyzer
due to corrosion.
Metallic components (for example, tantalum, niobium, titanium, or stainless
steel plated with
such metals) are often used instead for porous layers and flow field plates in
PEM
electrolyzers. The catalyst is typically platinum or a platinum alloy, and is
designed to operate
in the presence of liquid water.
Multiple electrolyzer cells can be connected either in series or in parallel
(to get the desired
output at a reasonable stack voltage) to form an electrolyzer stack. In
addition to one or more
electrolyzer stacks comprising end plates, bus plates and manifolds, and other
system
components, an electrolyzer system will typically comprise a power supply, a
voltage
regulator, water purification and supply equipment including a circulation
pump, water-gas
separators for hydrogen and optionally oxygen, a thermal management system,
controls and
instrumentation, and equipment for storage and subsequent dispensing of the
product gas(es).
A fuel cell system can be combined with an electrolyzer system, so that a
renewable energy
source can be used to power an electrolyzer to generate hydrogen and oxygen
which can be
stored, and then subsequently used as reactants for a fuel cell to produce
electric power. Such
a combined electrolyzer/fuel cell system is illustrated in FIG. 3A. Efforts
are presently
underway to develop a unitized stack that could serve as both fuel cell and
electrolyzer. Such
a device has been referred to as a "reversible fuel cell" or a "unitized
regenerative fuel cell"
(URFC). A PEM URFC stack delivers power when operated as a fuel cell using
hydrogen as
the fuel, and either air or oxygen as the oxidant, and generates hydrogen and
oxygen when
operated as an electrolysis cell. A URFC system is illustrated in FIG. 3B.
- 2 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
Design of the individual cells and cell components for a URFC should address
the distinctly
different operating conditions occurring during each mode of operation. For
exam'ple, the
oxygen/air electrode potential is quite different in one mode versus the
other. In the
exotheiinic fuel cell mode, humidified, gaseous reactants are generally
required along with
rapid removal of the heat and water produced, while in the electrolysis mode,
liquid water is
required as the reactant at one electrode, with rapid removal of the product
oxygen at the
anode and hydrogen at the cathode. The balance of plant supporting the PEM
URFC is
designed to handle product water in the fuel cell mode, maintain the thermal
balance within
the fuel cell (cooling plates are typically used to remove excess heat when
the fuel cell is
producing power), deliver clean reactants, and produce regulated power.
Balance of plant
issues for a URFC include design of the thermal management system (because
operation in
the electrolysis mode is slightly endothermic), and collection of the product
hydrogen and
optionally oxygen.
In a PEM electrolyzer the issues associated with liquid reactant supply and
gaseous product
removal are somewhat different to those in a PEM fuel cell, where hydrogen and
a gaseous
oxidant (for example, air) are typically supplied to the anode and cathode
respectively, and
water is produced at the cathode. In PEM fuel cells the gaseous reactants are
generally
supplied to the electrodes via channels formed in the flow field plates. A
typical reactant fluid
flow field plate has at least one channel through which a reactant stream
flows. The fluid flow
field is typically integrated with the separator plate by locating a plurality
of open-faced
channels on one or both faces of the separator plate. The open-faced channels
face an
electrode, where the reactants are electrochemically converted. In a single
cell arrangement,
separator plates are provided on each of the anode and cathode sides. In a
stack, bipolar plates
are generally used between adjacent cells; these bipolar plates generally have
flow fields on
both sides of the plate. The plates act as current collectors and provide
structural support for
the electrodes.
The flow field used at both the anode and the cathode can have an important
influence on fuel
cell performance, and much work has been done on the optimization of flow
field designs for
- 3 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
PEM fuel cells. Conventionally the reactant flow channels in fuel cell flow
fields have a
constant cross-section along their length. However, U.S. Patent No. 6,686,082
(which is
hereby incorporated by reference herein in its entirety) describes fuel cell
embodiments in
which the fuel flow channels have a cross-sectional area that decreases
linearly in the flow
direction. For fuel cells operating on air as the oxidant, as the air flows
along the cathode flow
channel(s), the oxygen content in the air stream tends to be depleted and the
air pressure tends
to drop, resulting in reduced performance in the fuel cell. U.S. Patent No.
7,838,169 (which is
hereby incorporated by reference herein in its entirety) describes improved
cathode flow field
channels that can be used to achieve substantially constant oxygen
availability along the
channel.
Less work appears to have been done on studying the effect of flow field
design on the
performance of PEM electrolyzers, although it has been reported that
electrolyzer operation is
generally less sensitive to changes in flow field design than fuel cell
operation.
It has been reported (Hwang, CM, et. al. Abst. #1405 Honolulu PRiME 2012, Me
Electrochemical Society) that in a PEM URFC, a preferred flow field design for
operation in
the fuel cell mode does not work so well in electrolysis mode, particularly at
higher current
densities (where the rate of hydrogen and oxygen production is greater). The
study notes that
serpentine flow fields are popular for PEM fuel cells because gas flow in a
serpentine flow
field has a higher velocity and greater shear force providing efficient
removal of product
water in the channels. Contrary to this, in electrolysis mode the longer
serpentine flow field
channels can be disadvantageous because product gases (hydrogen and oxygen)
can tend to
accumulate in the channels and hinder the supply of water to the electrode,
and limit the rate
of electrochemical oxidation of reactant water.
Although flow fields that are preferred for fuel cells are not necessarily the
same as those that
are preferred for electrolyzers, the Applicants have discovered that flow
fields where the
channel cross-sectional area varies along the length of the channel,
particularly at the oxygen
- 4 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
electrode, can offer advantages in electrolyzers, as well as in URFCs that can
operate in both
fuel cell and electrolyzer modes.
Summary of the Invention
An electrolyzer assembly for generating hydrogen and oxygen from water
comprises one or
more unit cells. The unit cells each comprise a membrane electrode assembly
comprising a
proton exchange membrane interposed between an anode and a cathode; a cathode
flow field
plate adjacent to the cathode; and an anode flow field plate adjacent to the
anode. The cathode
flow field plate optionally has at least one cathode channel formed therein,
for carrying away
hydrogen produced at the cathode during operation of the electrolyzer
assembly. The anode
flow field plate has at least one anode channel formed therein for directing
water in contact
with the anode during operation of the electrolyzer assembly. The at least one
anode channel
has a cross-sectional area that varies along at least a portion of the length
of the anode
channel. For example, anode channel cross-sectional area can be varied by a
variation in at
least one of the channel width, channel depth and channel shape. In some
embodiments, the
channel cross-sectional area varies (such as, by variations in dimensions of
width, depth or
shape) along substantially the entire length of the anode channel.
In some embodiments, the depth of the anode channel is substantially constant,
and the width
of the anode channel decreases along at least a portion of the channel length
in a direction of
reactant (water) flow along the channel. In some embodiments, the depth of the
anode channel
is substantially constant, and the width of the anode channel decreases along
at least a portion
of the channel length in a direction of reactant flow according to a natural
exponential
function. In some embodiments, the depth of the anode channel is substantially
constant, and
the width of the anode channel at a selected lengthwise position along the
channel portion is
proportional to a natural exponential function of the selected lengthwise
position. In some
embodiments, the width of the anode channel is substantially constant for a
portion of the
channel length and the channel width varies along another portion of the
channel length.
- 5 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
In some embodiments, the depth of the anode channel is substantially constant,
and the width
of the anode channel varies as a function of distance along the portion of the
channel length
such that:
sT, ________________________________________ o-ix
STH okH' oit in( )
2
STH20 L
W (x) = Dv e
where W(x) is the anode channel width at lengthwise position x; x is a
selected position along
the channel length; D is the channel depth; v constant flow velocity; STH20 is
water
stoichiometry; kiho is flow rate coefficient for water, id is the total
channel current; and L is
the channel length.
In other embodiments, the width of the anode channel is substantially
constant, and the depth
of the anode channel decreases along at least a portion of the channel length
in a direction of
reactant flow along the channel. The channel depth can, for example, decrease
substantially
linearly along at least a portion of the channel length in a direction of
reactant flow. The
channel depth can, for example, vary as a function of distance along the
portion of the channel
length such that:
STH" x
okH
D(x) = ________________________________ 1 ___
STH,QL
where D(x) is the anode channel depth at lengthwise position x; x is a
selected position along
the channel length; STH70 is water stoichiometry; kii.20 is flow rate
coefficient for water; id is
current density; and L is the channel length.
In the above-described embodiments, the electrolyzer assembly can comprise a
plurality of
the unit cells arranged in a stack.
In some embodiments, the electrolyzer assembly is configured to also operate
as a fuel cell to
generate electric power and water when oxygen and hydrogen are supplied to the
anodes and
cathodes.
- 6 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
A unitized regenerative fuel cell assembly is configured to operate both as an
electrolyzer to
produce hydrogen and oxygen from water, and as a fuel cell to produce electric
power from
hydrogen and oxygen. The unitized regenerative fuel cell comprises one or more
unit cells.
The unit cells each comprise a membrane electrode assembly that comprises a
proton
exchange membrane interposed between a first electrode and a second electrode;
a first flow
field plate adjacent to the first electrode, the flow field plate comprising
at least one oxygen-
side channel for directing a first fluid stream in contact with the adjacent
first electrode. The
at least one oxygen-side channel has a length and a cross-sectional area that
varies along at
least a portion of the channel length (for example, as for the anode channel
in the electrolyzer
assembly embodiments described above). Each unit cell optionally further
comprises a second
flow field plate adjacent to the second electrode, the flow field plate
comprising at least one
hydrogen-side channel. The hydrogen-side channel can, for example, be used for
directing a
second fluid stream in contact with the adjacent second electrode (for
example, delivering
hydrogen to the anode during fuel cell operation) and for carrying away
hydrogen produced at
the second electrode (the cathode, during electrolyzer operation).
The unitized regenerative fuel cell assembly can comprise a plurality of the
unit cells arranged
in a stack.
For operation of the unitized regenerative fuel cell assembly as an
electrolyzer, the at least
one unit cell is connected to a source of electrical power and the at least
one oxygen-side
channel is fluidly connected to a water supply for flowing reactant water
through the at least
one oxygen-side channel. For operation of the unitized regenerative fuel cell
assembly as a
fuel cell, the at least one oxygen-side channel is fluidly connected to
receive an oxygen-
containing reactant stream, the at least one hydrogen-side channel is fluidly
connected to
receive a hydrogen-containing reactant stream, and the at least one unit cell
is connected to an
electrical load, to provide electric current between the first and second
electrodes (anodes and
cathodes).
- 7 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
In some embodiments the depth of the at least one oxygen-side channel is
substantially
constant, and the width of the at least one oxygen-side channel decreases
along at least a
portion of the channel length (in a direction of water reactant flow during
electrolyzer
operation, and in a direction of oxygen-containing reactant stream flow during
fuel cell
operation). The variation in width can be, for example, as described above for
the various
embodiments of an anode channel of an electrolyzer assembly.
Embodiments of the above-described electrolyzer assembly, or unitized
regenerative fuel cell
assembly, can further comprise one or more of the following:
a water supply fluidly coupled, via a valve, to deliver water to the oxygen-
side
channels;
a power supply switchably connected to deliver electrical power to the
electrolyzer assembly or unitized regenerative fuel cell assembly;
a hydrogen containment vessel fluidly coupled, via a valve, to the cathode
flow
field plates to collect hydrogen generated by the electrolyzer assembly or
unitized
regenerative fuel cell assembly;
an oxygen containment vessel fluidly coupled, via a valve, to the cathode flow
field plates to collect oxygen generated by the electrolyzer assembly or
unitized
regenerative fuel cell assembly.
Brief Description of the Drawings
FIG. 1 (Prior Art) is a schematic diagram of an electrolyzer, showing the
reactions occurring
in a water electrolysis process.
FIG. 2A (Prior Art) is a simplified diagram of an electrolyzer unit cell
showing a membrane
electrode assembly sandwiched between a pair of flow field plates
- 8 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 2B (Prior Art) is a simplified diagram of a fuel cell unit cell, showing
a membrane
electrode assembly sandwiched between a pair of flow field plates
FIG. 3A (Prior Art) is a simplified diagram of a combined electrolyzer/fuel
cell system with
separate stacks for the fuel cell and the electrolyzer.
FIG. 3B (Prior Art) is a simplified diagram of a unitized regenerative fuel
cell (URFC)
system.
FIG. 4A is a simplified representation of an electrolyzer flow field plate
comprising a flow
channel that decreases in depth, with constant width, along its length.
FIG. 4B is a simplified representation of an electrolyzer flow field plate
comprising a flow
channel that decreases exponentially in width, with constant depth, along its
length.
FIG. 5 shows a trapezoidal electrolyzer flow field plate comprising multiple
flow channels
that decrease exponentially in width along their length.
FIG. 6A is a graph showing fluid flow velocity along electrolyzer anode flow
channels with
two different profiles, modeled for an electrolyzer operating at a higher
reactant water
stoichiometry than in FIG. 6B.
FIG. 6B is a graph showing fluid flow velocity along electrolyzer anode flow
channels with
two different profiles, modeled for an electrolyzer operating at a lower
reactant water
stoichiometry than in FIG. 6A.
FIG. 7 is a simplified representation showing an example of how a serpentine
flow channel, in
which the channel width varies, can be applied to a rectangular flow field
plate.
FIG. 8A is a simplified representation showing an example of how a wavy flow
channel, in
which the channel width varies, can be applied to a rectangular flow field
plate
- 9 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 8B is a simplified representation showing an example of how multiple wavy
flow
channels can be nested on a rectangular flow field plate.
FIG. 9A (Prior Art) shows a square flow field plate comprising a conventional
serpentine flow
field with 3 flow channels extending between a supply manifold opening and a
discharge
manifold opening.
FIG. 9B shows a similar serpentine flow field to FIG. 9A, but where the width
of each
serpentine flow channel decreases exponentially along its length.
FIG. 10A is a simplified representation of a flow field plate comprising a
flow channel that
decreases exponentially in width for a first portion of the channel length and
is then constant
for a second portion of the channel.
FIG. 10B is a simplified representation of a flow field plate comprising a
flow channel that is
constant in width a first portion of the channel length and decreases
exponentially for a
second portion of the channel length.
FIG. 11 is a simplified representation of a flow field plate comprising a flow
channel that
decreases exponentially in width for a first portion of the channel length,
and then flares with
increasing channel width along a second portion of the channel length.
FIG. 12 is a simplified representation of a flow field plate comprising two
flow channels that
are serpentine with constant width for a first portion of the channel length,
and then the
channel width decreases exponentially for a second portion of the channel
length.
FIG. 13 is a simplified representation of a flow field plate comprising a flow
channel in which
the channel depth is constant along a first portion of the channel length and
then decreases
along second a portion of the channel length.
FIG. 14A (Prior Art) shows a rectangular flow field plate comprising a multi-
channel
serpentine flow field extending between a supply and a discharge manifold
opening.
- 10 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 14B shows a modification to the flow field plate of FIG. 14A, in which
the width of each
channel decreases exponentially along a middle portion of the length of each
channel.
FIG. 15 is a simplified representation of a flow field plate comprising a
substantially
rectangular flow channel having a central rib with exponentially curved side
walls.
FIG. 16A is a simplified representation of a flow field plate comprising a
flow channel that
has a conventional rectangular cross-section at one end and is gradually
filleted to reduce its
cross-section towards the other end, in the reactant flow direction.
FIG. 16B is an alternative view of the flow field plate of FIG. 16A.
FIG. 17 is a simplified representation of a flow field plate comprising a
rectangular flow
channel incorporating rib dots, where density of the rib dots increases in the
reactant flow
direction.
FIG. 18 is a simplified representation of a flow field plate comprising a wavy
flow channel
incorporating rib dots, where density of the rib dots increases in the
reactant flow direction.
FIG. 19 is a simplified representation illustrating an example where the flow
channel width
decreases in a stepwise, non-linear fashion in the reactant flow direction.
FIG. 20 is a simplified representation illustrating another example where the
flow channel
width decreases in a stepwise, non-linear fashion in the reactant flow
direction.
FIG. 21 is a graphical representation illustrating how stepwise or discrete
changes in channel
width can be used to approximate a smooth exponential change in channel width.
FIG. 22 is a block diagram of an electrolyzer/regenerative fuel cell system.
-11-
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
Detailed Description of Preferred Embodiment(s)
An electrolyzer assembly includes a flow field plate comprising at least one
channel, wherein
the cross-sectional area of the channel varies along at least a portion of the
channel length. In
preferred embodiments the channel width decreases in the direction of reactant
flow along at
least a portion of the length of the channel according to a natural
exponential function. The
use of this type of improved flow field channel, particularly at the oxygen
electrode
(electrolyzer anode), can improve performance and/or efficiency of operation
of an
electrolyzer assembly.
Without being bound by theory, the following discussion, equations, and
numerical modeling
can be helpful in explaining at least some of the basis for the benefits that
can be achieved
using embodiments of the invention described herein.
One approach is to design an electrolyzer anode flow channel for substantially
constant water
velocity which maintains a substantially constant availability of water across
the active area.
It is postulated that:
= Water availability is related to cell reaction performance.
= Uniform water availability promotes uniform current density.
In an electrolyzer, water is directed or pumped through a flow field in order
to distribute the
water across the active area of the anode However, as water moves through the
flow field it is
consumed. Furthermore, each mole of water that is consumed is replaced by half
a mole of
oxygen. Several issues arise that can detrimentally affect the efficiency
and/or performance of
the electrolyzer. For example, as the water is consumed the velocity of water
flowing down
the channel will tend to decrease. The result is that the amount of reactant
being delivered per
unit time varies across the active area of the cell. Secondly, the product
oxygen that is evolved
at the anode tends to form bubbles in the flow field. This can impede access
of reactant water
to the anode catalyst sites. Both of these effects can lead to non-uniformity
in the current
distribution. Conventional electrolyzers do not adequately address these
issues.
- 12 -
CA 02925051 2016-03-22
WO 2014/056110
PCT/CA2013/050769
It is believed that maintaining a constant velocity of water in the
electrolyzer anode flow field
channel(s) can address either or both of the above issues and will promote a
more uniform
current density and, as a consequence, improve electrolyzer performance.
To derive the equations and formulae set forth below, various assumptions
including the
following were made:
1. Uniform current density ¨ an objective is to design the electrolyzer
anode flow
channel for substantially uniform current density.
2. Incompressible flow ¨ the water in the system is assumed to remain at a
constant volume and contain a negligible amount of dissolved oxygen, and the
gaseous oxygen produced is equally incompressible.
3. Substantially evenly distributed water concentration, velocity, and mass
flow
across flow section ¨ the model alleviates complexity by neglecting
concentration gradients that may occur in the cross-section of the channel.
4. Above rib activity is not considered ¨ the anode reaction is considered
to be
local to the flow channel only.
5. Water cross-over is not considered ¨ water flow across the electrolyzer
membrane due to concentration gradients, electro-osmotic drag, and/or back-
diffusion of water is not considered as a contributor to anode water
stoi chi ometry.
6. Steady state system ¨ the reaction and flows are assumed to be steady
state, or
unchanging.
The variables used below are defined as follows:
A(v) Cross-sectional flow area [m2]
- 13 -
CA 02925051 2016-03-22
WO 2014/056110
PCT/CA2013/050769
D(x) Depth of channel at position x [m]
Faraday's Constant [A-s/mol]
iciar)jacc(x) Accumulated current up to position x [A]
Current density [A/m2]
id Total channel current [Al
kH2G, Flow rate coefficient for water [m3/s/A]
k02 Flow rate coefficient for oxygen [m3/s/A]
Length of channel [m]
M(H20) Molecular mass of water [kg/mol]
lie Number of moles of electrons per mole of water oxidized
nH20 Assumed to be 1
no, Number of moles of oxygen produced per mole of water oxidized
consumed (x) Volumetric flow rate of water consumed at position x [m3/s]
QH,o(x) Volumetric flow rate of water at position x [m3/s]
Q. Inlet volumetric flow rate of water at position x [m3/s]
292(x) Volumetric flow rate of oxygen at position x [m3/s]
P1120 Density of water [kg/m3]
STH20 Design stoichiometric ratio (stoichiometry) of water
- 14 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
STI/20 Operational stoichiometric ratio (stoichiometry) of water
Constant flow velocity [m/s]
v(x) Flow velocity at position x [m/s]
Vm Molar volume of ideal gas at standard conditions [m3/mo1]
W(x) Width of channel at position x [m]
Position along channel length [m]
Constant Velocity Equation
In order to maintain a constant velocity of water in the electrolyzer anode
flow channel(s)
(ignoring the effect of oxygen production), the channel cross-sectional area
varies with the
decreasing volumetric flow rate of water according to the following equation:
v(x) = Q H20 (x) = const. (1)
A(x)
When the volumetric flow rate of water, QH20(x), is written in terms of the
inlet flow rate of
water Qin and the flow rate of water consumed at a channel position x, then
the constant
velocity, v, becomes:
Qin Q consumed(X)
V = (2)
A(x)
Knowing that Qin is the product of the stoichiometry of water supplied, STH,o,
the water flow
rate coefficient, kH20, and the total current load on the plate, it, and that
the consumed
- 15 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
volumetric flow rate of water is the product of the water flow rate
coefficient and the current
accumulated up to position x along the channel, the velocity can be rewritten
as:
STH20kH2oit ¨ k-n2
Oiacc(X)
V = (3)
A(x)
where:
(4)
W(x)dx
jacc(x) = id
with id being the nominal current density on the plate. The total current can
also be rewritten
as a product of the current density and
the total area, which is the width function integrated over the length of the
channel.
it = id f W(x)dx (5)
Substituting (4) and (5) into equation (3) yields the following expression for
velocity:
STH20kH2oid foL W(x)dx ¨ kH2oid fox W(x)dx (6)
.1) = _____________________________________________
A(x)
The water flow rate coefficient can be calculated as follows:
- 16 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
H2O M(H20)
kH20 = ¨ x ______________________________________________ (7)
neF PH20
where the quantsities of nii3O and tie are constants resulting from the
chemical reactions for
the electrolysis of water, and pi, and Ma/20) are the density and molecular
mass of water,
¨20
respectively.
Channel Profile for Uniform Water Availability
Now that the velocity equation has been developed, an electrolyzer anode
channel profile can
be designed on this basis, to achieve substantially constant or uniform
reactant water
availability. If the channel width is held constant, and the integrals are
appropriately
evaluated, then equation (6) can be rearranged to solve for the channel depth,
D(x):
STH 2 0kH2 0idWL ¨ kH20iciWx
D(x) = (8)
Wv
This can further be reduced to:
STH okH oid L
D(x) = 2 __ 2 1 __ x
STH20L (9))
The result is that the depth profile is a linear function of x.
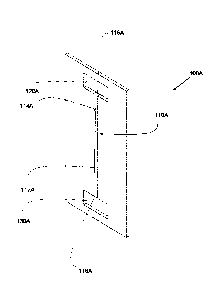
FIG. 4A is a simplified representation of an electrolyzer anode flow field
plate 100A
comprising a flow channel 110A that decreases in depth, with constant width,
along its length.
A channel profile can be defined by solving for D(x) in equation (9) at each
position x along
the length of the channel, given a specified operating reactant water
stoichiometry and
channel length L, and assuming a constant channel width. Referring to FIG. 4A,
the resulting
channel 110A extends between water supply manifold opening 120A and discharge
manifold
- 17 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
opening 130A, and has a linearly decreasing depth floor 112A from inlet 116A
to outlet
118A, with straight (parallel) side walls 114A.
Given the desire to reduce the thickness of the electrolyzer plates, it is
generally desirable to
keep the depth of the channel shallow. Therefore, instead of varying the depth
of the channel,
which would require a sufficiently thick plate to accommodate the deepest part
of the channel,
it can be preferred to keep the channel depth constant (D) and to vary the
width of the channel
to achieve substantially uniform water availability along the length of the
channel.
If the channel depth is held constant, then the channel width can be expressed
as follows:
STH2 okH 2 oid fL W(x)dx ¨ kH2oid fox W(x)dx
W(x) (x) = 0 (10)
V X D
The easiest means of solving this equation is via the guess-and-solve method.
One solution to
guess is a simple exponential of the form:
ox
W(x) = Ae¨E (11)
Two boundary conditions are required to find the particular solution. The
first can be found
by substituting x = 0 into equations (10 and (11):
STH 0 0 it
W(0) = 2 2 = A (12)
D x v
The second can be identified by substituting x = L into equations (10) and
(11):
(STH20 ¨ 1)/4/204 STH2okii2oit
W (L) = B _________________________________________________________ (13)
D x v Dv
- 18-
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
Solving for B, the result is:
(STH20 ¨ 1)
B = ln _____________________________________________________________ (14)
STH-20
Substituting equations (12) and (14) into equation (11) gives:
ST H okH2 ott ln(STH20-1)x
W(x) = 2
e sTH20 (15)
Dv
The resulting channel profile has an exponentially decreasing width.
FIG. 4B is a simplified representation of an electrolyzer anode flow field
plate 100B
comprising a flow channel 110B that decreases in width along its length
according to an
exponential function. A channel profile can be defined by solving for W(x) in
equation (15) at
each position x along the length of the channel, given a specified design
reactant water
stoichiometry, channel length L, total current draw id (or current density and
active area) and
assuming a flat channel floor (constant depth, D). Referring to FIG. 4B, the
resulting channel
110B extends between water supply manifold opening 120B and discharge manifold
opening
130B, and has a constant depth floor 112B with convexly curved side walls 114B
that
converge inwards from inlet to outlet. The walls 114B converge inwards towards
an outlet
end 118B with an inlet 116B having the largest width and the channel profile
delineating at a
diminishing rate. That is, the channel width decreases exponentially along the
length of the
channel from the inlet to the outlet according to the equation (15). It would
be possible for
one of the side walls to be straight and the other to be convexly curved.
Generally, from a practical standpoint, it is preferable to vary the channel
width. Flow field
plates with channels of varying width are generally easier to manufacture than
plates with
channels of varying depth, or channels with a cross-sectional shape that
varies along the
channel length.
- 19 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
Referring to FIG. 5, multiple channels 210 having the channel profile shown in
FIG. 4B can
be applied to an electrolyzer plate 200, to form an electrolyzer anode flow
field 222 extending
between a water supply manifold opening 220 and discharge manifold opening
230. The flow
field 222 is arrayed in a generally trapezoidal geometry to enable separating
ribs 224 to have a
relatively even width along their length.
The separator plate 20 includes partial ribs 26 located at the inlet of each
channel 10. The
partial ribs 26 serve to reduce the distance between channel side walls 14,
and serves as a
bridging structure for the adjacent membrane electrode assembly (not shown).
Embodiments in which the fluid flow channel width varies in an exponential
manner in can in
some circumstances be beneficial in enhancing the localized reactant and/or
product flow
velocity during electrolyzer operation thereby improving performance. Also,
the pressure
drop along the channel can be reduced (relative to a channel of constant cross-
sectional area).
This can lead to reduced parasitic loads improved overall system efficiency.
Furthermore, the
variation in channel width can be designed to adjust or control the localized
residency time of
the gaseous products in the channels, in some circumstances allowing some or
all of the
following:
(a) improved diffusivity of reactants for a more localized homogeneous
concentration, increased access to the catalyst;
(b) more efficient removal of products from the cell;
(c) overall pressure drop and flow friction.
Improved efficiency can be realized through a reduction in electrolyzer power
input, or and
overall improvement in specific output of hydrogen, or a reduction in
stressful environmental
conditions components are subjected to, potentially leading to improved
longevity.
- 20 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
Numerical Model
So far, the solution has focused on compensating for the consumption of water
with a change
in channel cross-sectional area and has ignored the effects of oxygen
production. This can be
expressed as:
00(x) = ko2id f W(x)dx (16)
And k0, can be written a way that is similar to kilo
no
koõ = (17)
neF
Now, it is postulated that an increase in velocity will improve or facilitate
the removal of
gaseous oxygen produced at the electrolyzer anode. Since ko, is approximately
600 times
larger than 4/20, an increase in velocity will occur even if the channel width
and depth are
held constant, assuming incompressible flows. However, a channel mimicking the
profile
outlined in equation (14) will greatly amplify this increase in velocity.
An electrolyzer can be thermally controlled during operation in various ways.
Sometimes the
reactant water supplied to the anode is also used to maintain the temperature
of the
electrolyzer within a desired range. In this situation, it is not uncommon for
the water
stoichiometry to exceed 1000 ( STH20 > 1000). Another mode of operation is
when there are
separate water supplies for the reactant and coolant water. In this case, the
reactant water can
be supplied at significantly lower stoichiometry. One advantage of this
approach is that the
parasitic load required to pump water through the anode flow channel(s) is
greatly reduced.
On the other hand, this potentially reduces the velocity of fluid flow through
the anode flow
channel(s).
-21 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
To exemplify these cases, two channel geometries were modeled: one designed to
compensate
for a consumption of water, and another with no such compensation. This first
channel
follows the profile described in equation (14) and the second channel is a
conventional
channel that has a constant profile along its length (for example, constant
width and constant
depth). Channel dimensions used in the model are summarized in Table 1.
Table 1
Constant profile
Compensated channel
channel
Inlet width (mm) 2.5 2.5
Channel active area (cm2) 1.5 1.5
Channel depth (mm) 1.0 1.0
Channel length (mm) 100 60
Some typical operating parameters were used to model these two scenarios.
These are
summarized in Table 2.
Table 2
Reactant water is used Different reactant and
for cooling coolant water streams
STibo 1000 1.5
id (A/c1112) 0.5 0.5
The graph shown in FIG. 6A illustrates the fluid flow velocities, as generated
by the model,
for an electrolyzer in which the reactant water is also used for thermal
management Plot A
(dashed line) shows the velocity for a channel with a constant profile, and
plot B (solid line)
shows the velocity for a compensated channel. The graph shown in FIG. 6B
illustrates the
fluid flow velocities, as generated by the model, for an electrolyzer in which
the reactant
water stream is separate from the coolant water stream supplied to the
electrolyzer. Plot X
- 22 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
(dashed line) shows the velocity for a channel with a constant profile, and
plot Y (solid line)
shows the velocity for a compensated channel.
In both cases, the compensated channel produces a far larger increase in fluid
flow velocity
down the channel. In the case where the reactant water is also used for
thermal management,
the compensated channel increases velocity 4.86 times the inlet velocity,
whereas the constant
profile channel only achieves 1.62 times the inlet velocity. In the case where
the reactant
water and coolant water streams are separate, the compensated channel
multiplies the inlet
velocity 1,244 times, versus 414 times for the constant profile channel. In
both cases,
however, the advantage in the ratio of inlet velocity to outlet velocity can
be written as:
rout)
vin comp. = 1
(STH20 ¨ 1+ 1)
(Vout)
(18)
vin inon-comp.
In short, the lower the reactant water stoichiometry, the greater the
advantage of the
compensated channel versus the constant profile channel in terms of velocity
multipliers.
When used at the cathode during operation of a URFC in fuel cell mode,
channels with an
exponentially varying width can be used to provide substantially constant
oxygen availability,
significantly improving the uniformity of current density and increasing fuel
cell
performance. They also provide velocity control allowing for more efficient
fuel cell
operation, conventionally achieved in fuel cells through the use of serpentine
flow fields. In
electrolyzer operation serpentine flow fields are not optimum, shorter
channels being
preferred. Use of channels with an exponentially varying width in a PEM URFC
can therefore
provide velocity control for improved fuel cell operation, while achieving a
shorter channel
length that is generally preferred for electrolyzer operation.
- 23 -
CA 02925051 2016-03-22
WO 2014/056110
PCT/CA2013/050769
Typically electrolyzers operating in reverse as fuel cells tend to perform
poorly due to
differences in catalyst layer composition. Electrolyzers are generally
designed to operate with
higher pressure differentials than fuel cells, therefore requiring stronger
and heavier
components for the membranes, gas distribution layers, flow field plates, and
other system
components, but they are therefore a less optimal system for running in
reverse. Cathode flow
field channels similar to those described herein have already been shown to
improve the
performance and efficiency of a fuel cell (see U.S. Patent No. 7,838,169).
With the discovery
that they can offer advantages at an electrolyzer anode (or even if they are
neutral for
electrolysis mode) they can make the URFC design more competitive. This will
allow URFC
to become more commercially viable. For example, a 5.6 kW electrolyzer having
flow field
channels that vary in cross-sectional area as described herein, will provide
about 1.7 kW of
peak power when run in reverse, in fuel cell mode. Comparatively, a standard
flow field can
only provide between 0.8 and 1.35 kW at peak power in fuel cell mode, and can
require 50%
to 28% more active area to produce 1.7 kW, leading to a more expensive URFC
The
improved efficiency as defined by the relationship of peak power consumption
under
electrolyzer mode to peak power output in fuel cell mode for the same stack
with the same
total active area, therefore results in a lower cost URFC.
The channels can be substantially straight from inlet to outlet, or can be
wavy or serpentine.
Generally for electrolysis applications shorter channels are preferred, but
for URFC the
channel profile and path can be a compromise between what is preferred for
fuel cell
operation and what is preferred for electrolyzer operation.
Flow fields based on the equations and description set forth above for the
oxygen electrode
(anode) of an electrolyzer are more likely to be adopted if they can be
accommodated within
conventional flow field plate geometries and into conventional electrochemical
stack
architectures (which typically have rectangular flow field plates). Flow
channels where the
depth profile changes along the length of the channel (such as shown in FIG.
4B) can be
accommodated by using an existing flow field design (pattern) and merely
altering the depth
profile of the channels along their length (keeping the channel width and ribs
the same as in
- 24 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
the original flow field design). However, plates with channels where the depth
profile changes
are generally more challenging to fabricate. They also result in a need for
thicker plates, in
order to accommodate the deepest part of the channel, leading to decreased
stack power
density and higher cost.
FIGS. 7-9 show some examples of ways in which flow fields where the flow
channel width
varies, can be applied to a rectangular electrolyzer flow field plate. FIG. 7
shows a rectangular
electrolyzer flow field plate 300 with a serpentine channel 310 where the
channel width is
decreasing exponentially as it zigzags across the plate between supply
manifold opening 320
and discharge manifold opening 330. FIG. 8A shows a rectangular electrolyzer
reactant flow
field plate 400A with a wavy channel 410A extending between reactant supply
manifold
opening 420A and discharge manifold opening 430A,where the channel width is
decreasing
exponentially along its length. In FIG. 8A the amplitude of the path of the
center-line of the
flow channel 410A increases as the width of the channel decreases, so that the
channel still
occupies most of the width of the plate 400A Making the variable width channel
serpentine
or wavy, rather than straight, allows the channel to occupy a more rectangular
shape making
more efficient use of the surface area of the plate. FIGS. 7 and 8A show a
single flow channel,
however, it is apparent that such channels can be repeated or arrayed across a
rectangular
plate so that a large portion of the plate area can be active area (for
example, so that a large
portion of the plate surface is covered in channels, with a large open channel
area exposed to
the adjacent electrode or MEA). FIG. 8B shows a rectangular electrolyzer flow
field plate
400B with multiple flow channels 410B (like flow channel 410A of FIG. 8A
repeated)
extending between reactant supply manifold opening 420B and discharge manifold
opening
430B, arranged so that the channels nest together.
FIG. 9A shows a square electrolyzer flow field plate 500A comprising a
conventional (Prior
Art) serpentine electrolyzer flow field with three flow channels 510A
extending between
supply manifold opening 520A and discharge manifold opening 530A. FIG. 9B
shows a
similar serpentine electrolyzer flow field plate 500B, but where the width of
each serpentine
- 25 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
channel 510B decreases exponentially along its length as it extends from
supply manifold
opening 520B to discharge manifold opening 530B.
Improvements in electrolyzer performance can be obtained by incorporating a
variation in
channel cross-sectional area along only a portion of the length of the
reactant flow channel.
The performance improvements are not necessarily as great as if the variation
is employed
along the entire channel length, but such flow field designs can in some cases
provide most of
the benefit, and can allow more efficient use of the plate area. FIGS. 10-12
show some
examples where the flow channel width varies along just a portion of the
length of the
channel. FIG. 10A shows a rectangular electrolyzer flow field plate 600A with
a flow channel
610A extending between reactant supply manifold opening 620A and discharge
manifold
opening 630A. Similarly, FIG. 10B shows a rectangular electrolyzer flow field
plate 600B
with a flow channel 610B extending between reactant supply manifold opening
620B and
discharge manifold opening 630B. In FIG. 10A the flow channel width decreases
exponentially for a first portion 625A of the channel length (near the supply
manifold), and is
then constant for a second portion 635A of the channel length (towards the
discharge
manifold). Conversely, in FIG. 10B the flow channel width is constant for a
first portion 625B
and decreases exponentially for a second portion 635B of the channel length.
In some cases it can be beneficial to incorporate a decrease in channel cross-
sectional area (in
accordance with the above equations for constant water availability) along a
first portion of
the length of the channel and then incorporate an increase in channel cross-
sectional area
along a second portion of the length of the channel, in order to help
accommodate the
significant (approximately 600x) change in volume when oxidizing water to
oxygen. FIG. 11
shows a rectangular electrolyzer flow field plate 700 with a flow channel 710
extending
between reactant supply manifold opening 720 and discharge manifold opening
730. The flow
channel width decreases exponentially for a first portion 725 of the channel
length (near the
supply manifold), and is then increases so that the channel is flared for a
second portion 735
of the channel length.
- 26 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 12 shows an electrolyzer flow field plate 800 comprising two flow
channels 810. The
channels are initially serpentine with constant width in portion 825 near the
reactant supply
manifold opening 820, and then, after abruptly increasing, the channel width
decreases
exponentially for a second portion 835 of the channel length (towards
discharge manifold
opening 830).
FIG. 13 shows an example of an electrolyzer flow field plate 900 comprising a
flow channel
910 extending between a reactant supply manifold opening 920 and a discharge
manifold
opening 930. The flow channel depth is constant along a first portion 925 of
the channel
length and then decreases along a second portion 935 of the length of the
channel 910.
In some embodiments, the electrolyzer flow channels can incorporate a
variation in both
width and depth along their entire length, or a portion of their length.
FIGS. 14A and 14B illustrate how an existing flow field design can be readily
modified to
incorporate an exponential variation in channel width along a portion of the
length of the flow
channels. FIG. 14A (Prior Art) shows a rectangular flow field plate 1000A
comprising a fairly
complex serpentine flow field with multiple serpentine channels 1010A
extending between a
supply and a discharge manifold opening. FIG. 14B shows a modification in
which the width
of each channel 1010B decreases exponentially along a middle portion 1025B of
the length of
each channel.
It is also possible to take a conventional flow channel (for example, a
channel with a
rectangular and constant cross-sectional shape and area along its length) and
incorporate a
shaped rib, fillet or other features within the volume of the original channel
to reduce the
channel cross-sectional area in a way that provides at least some of the
desired benefits. FIG.
15 shows an example of an electrolyzer flow field plate 1100 with a single
flow channel 1110
extending between a supply manifold opening 1120 and a discharge manifold
opening 1130.
The channel 1110 comprises a central rib 1140 with exponentially curved side
walls. The rib
splits the flow channel 1110 in two and effectively reduces its width
gradually along most of
- 27 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
its length. FIGS. 16A and 16B show two different views of another example of a
flow field
plate 1200 with a single flow channel 1210 extending between a supply manifold
opening
1220 and a discharge manifold opening 1230. The channel 1210 is of a
conventional
rectangular cross-section at one end 1225, and is gradually filleted to reduce
its cross-section
towards the other end 1235.
In the examples described above, the flow channel dimensions vary along at
least a portion of
the channel length in a smooth and continuous fashion. However, performance
benefits can
also be obtained by using flow channels that incorporate discrete variations.
In other words,
the characteristics of the channel can be varied as a function of distance
along the channel in a
stepwise or discontinuous fashion, but where the overall variation trends the
smooth desired
profile, either in fluctuations about the calculated profile, or in discrete
approximations of the
desired profile. This approach can be used to achieve at least some of the
performance
benefits, and can provide some options for improved flow fields that are
easier to fabricate or
to incorporate into existing plate geometries. In these examples, the outlet,
or region near the
outlet, is smaller or more constricted than the reactant inlet or inlet
region. In some
embodiments, the channels can contain discrete features that obstruct reactant
flow, where the
density and/or size of those features increases in a reactant flow direction.
An example of an
electrolyzer flow field plate 1300 where the flow channels incorporate rib
dots or raised
columns 1350 is shown in FIG. 17. The density of the rib dots 1350 can
increase in the
reactant (water) flow direction (indicated by the arrow) in accordance with
the e flow
equations. Such features can be as high as the channel is deep (so that they
touch the adjacent
electrode) or can obstruct only part of the channel depth. In the example
illustrated in FIG. 17,
the channel is the entire active area and the rib dots (or other such features
that obstruct
reactant flow) are distributed across the active area in a varied density
array approximating an
exponential variation. In other examples, the rib dots or other features can
be incorporated
into one or more separate channels. FIG. 18 is a simplified representation of
an electrolyzer
flow field plate 1400 comprising a wavy flow channel 1410 incorporating rib
dots 1450,
- 28 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
where the density of the rib dots increases in a reactant flow direction
(indicated by the
arrow).
In other examples, the flow channel dimensions (for example, width or depth)
can decrease in
the reactant flow direction in a stepwise fashion. The increments by which the
dimensions
change and the distance between the step-changes are selected so that the
changes in channel
dimensions in the reactant flow direction are consistent with the applicable
equations. In some
embodiments the increments by which the channel dimensions change can be the
same along
the channel length, and in other embodiments it can vary along the channel
length. Similarly,
in some embodiments the distance between (or frequency of) the step-changes in
channel
dimensions can be the same along the channel length, and in other embodiments
it can vary
along the channel length.
FIGS. 19 and 20 illustrate examples where the channel width decreases in a
stepwise, non-
linear fashion in a reactant flow direction in accordance an exponential
function. FIG. 19 is a
simplified representation illustrating an example electrolyzer flow field
plate 1500 where the
width of flow channel 1510 decreases in a stepwise, nonlinear fashion in a
reactant direction
between a reactant supply manifold opening 1520 and a discharge manifold
opening 1530.
FIG. 20 is a simplified representation illustrating another example
electrolyzer flow field plate
1600 where the width of flow channel 1610 decreases in a stepwise, non-linear
fashion in the
reactant flow direction between a supply manifold opening 1620 and a discharge
manifold
opening 1630.
FIG. 21 is a graphical representation 1700 illustrating how stepwise or
discrete changes in
channel width can be used to approximate a smooth exponential change in
channel width. The
solid line 1710 represents changes in channel width and the dashed line 1720
shows a smooth
exponential variation in channel width.
In other examples the porosity of the flow channel varies based the principles
explained
above.
- 29 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 22 is a block diagram illustrating an example of an
electrolyzer/regenerative fuel cell
system 1800 comprising a multi-cell stack 1810. Each unit cell in the stack
can comprise
components and flow channels such as, for example, those described above.
System 1800 also
comprises a power supply 1825, which can be connected by closing switch 1820,
to deliver
electrical power to stack 1810 when stack 1810 is to be operated in
electrolyzer mode to
generate hydrogen and oxygen. Power supply 1825 can comprise, for example, an
electricity
grid, an energy storage device, or a renewable source of electric power such
as a photovoltaic
cell or a wind turbine. When system 1800 is to be operated in electrolyzer
mode, water is
supplied to flow channels within stack 1810 from a water supply 1830 via a
valve system
1840 which can comprise multiple valves for controlling the supply of fluids
(reactants and
products) to and from stack 1810. Water can be supplied as both a reactant and
a coolant to
flow channels adjacent the oxygen-side electrodes (not shown in FIG. 22) in
stack 1810; or
water can be supplied as a reactant to flow channels adjacent the oxygen-side
electrodes, and
optionally to separate cooling channels (not shown in FIG. 22) in stack 1810.
System 1800
also comprises a hydrogen containment vessel 1850 selectively fluidly
coupleable, via valve
system 1840, to collect hydrogen generated during electrolyzer operation of
stack 1810.
System 1800 further comprises an oxygen containment vessel 1860 selectively
fluidly
coupleable, via valve system 1840, to collect oxygen generated during
electrolyzer operation
of stack 1810.
System 1800 can also be configured so that stack 1810 operates as a fuel cell
to generate
electric power which can power electrical load 1870 when switch 1875 is closed
(and switch
1820 is open). In this mode of operation, hydrogen can be supplied to stack
1810 from
hydrogen containment vessel 1850 which is selectively fluidly coupleable to
supply hydrogen
to stack 1810, via valve system 1840. Similarly, oxygen can be supplied to
stack 1810 from
oxygen containment vessel 1850 which is also selectively fluidly coupleable to
supply oxygen
to stack 1810, via valve system 1840. Alternatively air can be supplied to
stack 1810 as the
oxidant, via another oxidant supply subsystem (not shown in FIG. 22). During
fuel cell
operation, water can optionally be supplied as a coolant to cooling channels
(not shown in
- 30 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
FIG. 22) in stack 1810 via valve system 1840. Product water generated during
fuel cell
operation can optionally be directed to water supply 1830 via valve system
1840. A controller
1880 can operate the valve system 1840 to provide reactants and coolant to and
collect
products from stack 1810, as appropriate, during fuel cell and electrolyzer
operation.
Controller 1880 can also close and open switches 1820 and 1875, as
appropriate, for fuel cell
and electrolyzer operation. Controller 1880 can also configure stack 1810 for
operation
alternatively in fuel cell mode and electrolyzer mode.
System 1800 is one embodiment of a system comprising a regenerative fuel
cell/electrolyzer
stack according to various embodiments of the present invention. Other systems
can exclude
some of the components shown in system 1800, or include additional components.
FIGS. 4A, 4B, 7, 8A, 8B, 10A, 10B, 11, 12, 13, 15, 16A, 16B, 17, 18, 19 and 20
are
simplified drawings, in which the size of the flow channel and the manifold
openings, and
variations in channel dimensions and/or characteristics are exaggerated for
the purposes of
clear illustration.
In the above-described embodiments, the dimensions and/or flow characteristics
of the flow
channel vary along at least a portion of the channel length. The variations
can be continuous
or discrete.
Although the focus of the foregoing description has been on the oxygen-side,
flow channels
with variations in cross-sectional area as described herein can be used at
either or both of the
electrodes in an electrolyzer or URFC assembly. However, as described above,
they generally
offer greater benefits when used at the oxygen-side electrode (which is the
anode for an
electrolyzer, and the cathode during fuel cell operation of a URFC). Also they
can be used for
some or all of the unit cells in a particular electrolyzer or URFC stack.
The open channel area versus the rib or landing area on a reactant flow field
plate is generally
selected to give sufficient electrical contact between the plates and the
adjacent MEAs for
efficient current delivery, while providing sufficient water access to the
electrolyzer anode to
-31-
CA 02925051 2016-03-22
WO 2014/056110
PCT/CA2013/050769
support the electrochemical reactions. Using a wider rib area (between flow
channels)
improves electrical connectivity and current delivery in an electrolyzer.
As used herein the "inlet" refers to either the start of the flow channel
where reactant enters
the channel, or the start of a region where the channel characteristics vary
as a function of
channel length as described herein; and "outlet" refers to either the
downstream end of the
channel, or the end of a region over which channel characteristics vary as a
function of
channel length as described herein.
The present invention includes electrolyzer flow field plates comprising any
of the reactant
flow channels or flow field designs described above. Such plates can be made
from any
suitable material or combination of materials, and can be fabricated by any
suitable method.
The present invention also includes other electrolyzer components
incorporating flow
channels or passageways as described herein. For example, such channels could
be
incorporated into the gas diffusion layers, manifolds, or other components of
the unit cell or
stack. Further, the present invention includes electrolyzers and electrolyzer
stacks
incorporating such flow field plates and/or other components The reactant flow
channels and
flow field designs described herein have been found to be particularly
advantageous in PEM
electrolyzer assemblies and URFCs, however they can be applied in other types
of
electrochemical devices.
Where a component is referred to above, unless otherwise indicated, reference
to that
component should be interpreted as including as equivalents of that component
and any
component which perfoims the function of the described component (namely, that
is
functionally equivalent), including components which are not structurally
equivalent to the
disclosed structure but which perform the function in the illustrated
exemplary embodiments.
While particular embodiments and applications of the present invention have
been shown and
described, it will be understood, of course, that the invention is not limited
thereto since
modifications can be made by those skilled in the art without departing from
the scope of the
- 32 -
CA 02925051 2016-03-22
WO 2014/056110 PCT/CA2013/050769
present disclosure, particularly in light of the foregoing teachings For
example, features from
the embodiments described herein can be combined with features of other
embodiments
described herein to provide further embodiments. The changes and alternatives
are considered
within the spirit and scope of the present invention.
- 33 -