Note: Descriptions are shown in the official language in which they were submitted.
= CA 02926928 2016-04-11
1
AMINOBENZOIC ACID DERIVATIVES FOR USE AS ANTI-INFLAMMATORY
AGENTS, ANTI-METASTATIC AGENTS AND/OR ANTICANCER AGENTS
FIELD OF THE DISCLOSURE
[0001] This disclosure relates to the field of active agents.
More particularly,
this disclosure relates to anti-inflammatory agents, anti-metastatic agents
and
anticancer agents.
BACKGROUND OF THE DISCLOSURE
[0002] There are several methods used to treat cancer.' The
most common are:
surgery, chemotherapy, radiation therapy, targeted therapy and immunotherapy."
Other procedures are based on stem cell transplant, photodynamic therapy, and
cryogenic therapy. Lasers are nowadays a useful tool during surgery of
localized
cancers. Many of these methods are quite effective. However, most present
important
side effects.7'8 Hence, the need to discover alternative therapeutics and
treatment
modalities. Particularly, compounds and treatment protocols that could
simultaneously attack cancer on diverse fronts (initiation, propagation,
metastasis etc.)
are of great interest.
SUMMARY OF THE DISCLOSURE
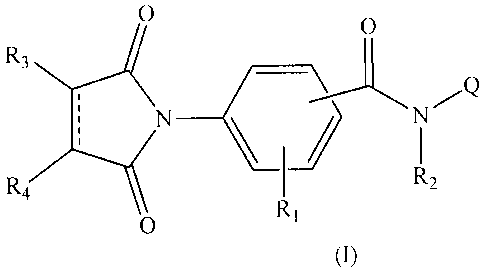
[0003] According to one aspect, there are included compounds of
formula (1) :
CA 02926928 2016-04-11
2
0
0
R3
R4 R2
0
(I)
wherein
R1 is H, alkyl or halogen;
R2 is H, or a substitued or unsubstituted member chosen from acetyl,
propiolyl, butyryl, isobutyryl and benzoyl;
Q is QA or QB;
R5
õN
QA= R6 .
(
QB .
= a single bond or a double bond;
R5 is H, or a substitued or unsubstituted member chosen from acetyl,
propiolyl, butyryl, isobutyryl and benzoyl;
R6 is H, Boc, or a substitued or unsubstituted member chosen from
acetyl, propiolyl, butyryl, isobutyryl and benzoyl;
= CA 02926928 2016-04-11
3
R7 is a substitued or unsubstituted member chosen from C1-C8 alkyl,
C3-C8 cycloalkyl, phenyl, furanyl, thiophenyl, pyridinyl, naphthyl,
quinolyl and isoquinoly1;
R3 and R4 are independently chosen from H, -SR8 and ¨NR9R10,
or R3 and R4 are joined together to form a 5-7 membered ring that
optionally comprises an heteroatom chosen from N, S and 0;
R8 is H, C1-C8 alkyl, -(CH2)õNHBoc, or -(CH2)nNH2 wherein n = 1 to
6;
R9 is H or CI-C8 alkyl;
R10 is H, Cl-C8 alkyl, acetyl, propiolyl, butyryl, isobutyryl, or benzoyl;
wherein R2, R5, R6 and R7, when substituted, are substituted with at
least one substituent chosen from -0R9, -F, -Cl, -Br, -I, acetyl,
propiolyl, butyryl, isobutyryl, benzoyl, -NO2, CI-Cs alkyl,
methoxycarbonyl-, or alkyloxycarbonyl-;
or an enantiomer, diastereoisomer, racemic mixture, pharmaceutically
acceptable
salt, solvate or prodrug thereof.
[0004] According to another aspect, there is included a
composition
comprising a pharmaceutically acceptable carrier and at least one compound of
the
present disclosure.
[0005] According to another aspect, there is included a method
for treating
cancer or at least one cancer chosen from breast cancer, uterine cancer,
ovarian
cancer, prostate cancer, bladder cancer, and melanoma, said method comprising
administering to a subject in need thereof an effective amount of at least one
compound of the present disclosure.
[0006] According to another aspect, there is included a method
for reducing
the risks of developing cancer or for reducing the risk of developing at least
one
cancer in a subject, the cancer being, for example, chosen from breast cancer,
uterine
= CA 02926928 2016-04-11
4
cancer, ovarian cancer, prostate cancer, bladder cancer, and melanoma, said
method
comprising administering to the subject an effective amount of at least one
compound
of the present disclosure.
[0007] According to another aspect, there is included a method
for inhibiting
cancer cell growth, the method comprising administering to a subject in need
thereof
an effective amount of at least one compound of the present disclosure. For
example,
the cancer can be chosen from breast cancer, uterine cancer, ovarian cancer,
prostate
cancer, bladder cancer, and melanoma.
[0008] According to another aspect, there is included the use
of at least one
compound of the present disclosure for treating cancer or for treating at
least one
cancer chosen from breast cancer, uterine cancer, ovarian cancer, prostate
cancer,
bladder cancer, and melanoma
[0009] According to another aspect, there is included the use
of at least one
compound of the present disclosure for reducing the risks of developing cancer
or for
reducing the risks of developing at least one cancer chosen from breast
cancer, uterine
cancer, ovarian cancer, prostate cancer, bladder cancer, and melanoma
[0010] According to another aspect, there is included the use
of at least one
compound of the present disclosure in the manufacture of a medicament for
treating
cancer or for treating at least one cancer chosen from breast cancer, uterine
cancer,
ovarian cancer, prostate cancer, bladder cancer, and melanoma
[0011] According to another aspect, there is included the use
of at least one
compound of the present disclosure in the manufacture of a medicament for
reducing
the risks of developing cancer or for reducing the risks of developing at
least one
cancer chosen from breast cancer, uterine cancer, ovarian cancer, prostate
cancer,
bladder cancer, and melanoma
[0012] According to another aspect, there is included the use
of at least one
compound of the present disclosure for inhibiting cancer cell growth. For
example,
the cancer can be chosen from breast cancer, uterine cancer, ovarian cancer,
prostate
cancer, bladder cancer, and melanoma.
[0013] According to another aspect, there is included the use
of at least one
compound of the present disclosure in the manufacture of a medicament for
inhibiting
= CA 02926928 2016-04-11
cancer cell growth. For example, the cancer can be chosen from breast cancer,
uterine
cancer, ovarian cancer, prostate cancer, bladder cancer, and melanoma.
BRIEF DESCRIPTION OF FIGURES
[0014]
The following drawings represent in a non-limitative manner examples
of specific embodiments in which
Figure 1 represents images (a) and graphical analysis (b) showing Western blot
analysis to determine the expression level of phosphorylated STAT1 and STAT3
in
human macrophages (MO) pretreated for 30 min with vehicle (DMSO) or compounds
1 and 1A, and then washed and recovered immediately (t= 0) or after 30 min of
activation with either 50 U/mL IFNy or 25 ng/mL IL6. The ratio
ofphosphorylated/no
phosphorylated proteins was calculated from densitometric analysis of each
sample to
evaluate the relative activation of pSTAT1 or pSTAT3. * p < 0.05 and ** p <
0.01
denote significant differences between treatments;
Figure 2 represents images (a) and graphical analysis (b) showing flow
cytometry
analysis to determine the expression level of MHC-II and CD40 surface antigens
in
resting and IFNy-activated hMitIs untreated and pretreated with compounds 1
(10 i_tM)
and 1A (25 M). * p < 0.05 and ** p < 0.01 denote significant differences
between
treatments;
Figure 3 represents images (a) and graphical analysis (b) showing scratch
wound
healing assays to determine the motility of hM(13, monolayers cultured for 3 h
with
vehicle (DMSO) or compounds 1 (10 ?AM) and 1A (25 04), and then activated for
48
h with vehicle (PBS) or 25 ng/mL IL6. The images of the scratch were acquired
at t=
O h and t= 48 h by fluorescence microscopy. Five fields were taken randomly
for each
different treatment. All observations were performed at 5x magnification. Cell
motility was expressed as percent (%) of control of motile cells at t= 48 h
relative to
motile cells at t= 0 h. * p < 0.05 and ** p < 0.01 denote significant
differences
between treatments;
CA 02926928 2016-04-11
6
Figure 4 is a graphical representation of NO production in the macrophage-like
J774A.1 cells following a pro-inflammatory stimulation by IFNI, and TNFa after
pretreatment with vehicle (DMSO) and the derivatives 1 and 1A. * p < 0.05 and
** p
< 0.01 denote significant differences between treatments;
Figure 5 is a graphical representation of relative cell viability on the
murine BCa cell
line MB49-I following a pro-inflammatory stimulation by IFNy and TNFa after
pretreatment with vehicle (DMSO) and anti-inflammatory derivatives 1 and 1A at
different concentrations. * p < 0.05 and ** p < 0.01 denote significant
differences
between treatments;
Figure 6 is a graphical representation of NO production by the murine BCa cell
line
MB49-I following a pro-inflammatory stimulation by IFN7 and TNFa after
pretreatment with vehicle (DMSO) and derivatives 1 and 1A and various
precursor
intermediates (3, 4, 6), reagents (2, PABA) and maleic anhydride (MA) as well
as the
left hand (7) and right hand (9) of the main anti-inflammatory compound 1. *p
< 0.05
and ** p < 0.01 denote significant differences between treatments. (See
publication
no.11 for a complete description of the molecules shown in this figure);
Figure 7 refers to episodic intraperitoneal injections of AL-549 in C57B1/6J
mice
(n=4) during 3 weeks have no effect on normal development and viability (A),
as well
as in organs weight (B) and hematocrit (C);
Figure 8 represents images showing histological analysis of liver sections
from
C57B1/6J mice (n=4) after episodic intraperitoneal injections of AL-549 during
3
weeks. Treatment does not induce features of liver dysfunction such as
hepatocellular
injury, inflammation, fibrosis and steatosis (haematoxylin and eosin, 100 x
and 400 x
magnification);
Figure 9 refers to episodic intraperitoneal injections of AL-549 in C57B1/6J
mice
(n=6) affects tumor development of MB49-I cells subcutaneously implanted in
mice.
*p < 0.01 compared to control;
CA 02926928 2016-04-11
7
Figure 10 refers to episodic intraperitoneal injections of AL-549 in C57B1/6J
mice
(n=6) reduce the size of MB49-I tumors subcutaneously implanted in C57B1/6J
mice
(A) and the number of lung metastases (B). *p < 0.01 compared to control;
Figure 11 represents (A) Western blot analysis: AL-549 efficiently inhibited
TNFa/NFKB and IL6/STAT3 signaling pathways in murine UBC MB49-I cells at
lower doses (10 mM). (B) Luciferase assay: AL-549 efficiently inhibited NFkB
activation. MB49-I cells were transfected with a NFKB-responsive luciferase
construct encoding the firefly luciferase reporter gene under the control of
CMV
promoter and tandem repeats of the NFKB transcriptional response element. * p
<
0.01 denote significant difference compared to control;
Figure 12 refers to AL-549 efficiently inhibited (A) Ml-induced human UBC T24
cell invasion (matrigel in Boyden chamber) and 1L6-induced motility (scratch
assay)
in T24 cells (B). * p < 0.05 denote significant difference compared to control
(M1
DMSO);
Figure 13 refers to AL-549 efficiently inhibited MDA-MB-241 breast cancer cell
invasion (matrigel in 3D cultures). * p < 0.01 denote significant difference
compared
to day 0 (DO.);
Figure 14 is a graphical representation of relative cell viability on MB49 and
M1349-I
cell lines after pretreatment (3h) with either vehicle (DMSO) or AL-549 at
different
concentrations. * p < 0.05 denote significant difference compared to control
(without
AL-549);
Figure 15 is a graphical representation of LPS/IFNy-induced NO production in
MB49-I cells after pretreatment with vehicle (DMSO) and the molecules AL-549
(30
iaM) and ML-19 (10, 20, 37.5 and 50 p,M). * p < 0.01 denote significant
difference
compared to positive control (DMSO + LPS /IFNy);
= CA 02926928 2016-04-11
8
Figure 16 is a graphical representation of LPS/IFNy-induced NO production in
MB49-I cells after pretreatment with vehicle (DMSO) and the molecules AL-549
(37.5 M) and ML-28 (25, 37.5 and 50 *
p < 0.01 denote significant difference
compared to positive control (DMSO + LPS /IFNy). ** p < 0.05 denote
significant
difference compared to AL-549 at 37.5 IAM (+ LPS /IFNy);
Figure 17 refers to episodic intraperitoneal injections of ML-28 in C57BI/6J
mice
(n=8) affects tumor development of MB49-I cells subcutaneously implanted in
mice.
*p < 0.05 and **p < 0.01 compared to control;
Figure 18 refers to Non-muscle invasive UBC MB49 cells produced low levels of
iNOS (A) and NO (B) relative to highly invasive MB49-I cells in response
toTNFcc
but not IL6. *p < 0.01 compared to control;
Figure 19 Loss of basal and TNFa-induced iNOS expression (A) and NO production
(B) by shRNA affects MB49-I tumor development (C) subcutaneously implanted in
C57BI/6J mice. *p < 0.01 compared to control; and
Figure 20 refers to episodic intraperitoneal injections of AL-549 in C57B1/6J
mice
(n=6) reduce the size of non-muscle invasive MB49 tumors subcutaneously
implanted
in C57B1/6J mice. *p < 0.001 compared to Ctl (ANOVA, Mann Whitney Test).
[0015] In
figures 1 to 6: Compounds 1 and 1A correspond to 4 (AL-549) and
(AL-361) of the present application.
DETAILED DESCRIPTION OF THE DISCLOSURE
[0016]
The present disclosure concerns the discovery of small aminobenzoic
acid derivatives showing anti-inflammatory, anti-metastatic and anticancer
properties
in vitro and in vivo. It describes the synthetic methodology to make these
derivatives
from readily available ortho-, meta- and para-benzoic acid and their
biological
= CA 02926928 2016-04-11
9
applications for the treatment of a several types of cancers. In addition,
this disclosure
relates to different pharmaceutical compositions comprising these compounds.
The
compounds and the pharmaceutical composition of this disclosure have been
shown to
possess anticancerous activity on various types of cancers. Furthermore, this
disclosure provides novel treatment modalities against cancer. The unique
biological
properties of these compounds may be advantageously used to provide compounds
with anticancer activity against cancers including but not limited to breast,
prostate,
ovarian and bladder cancers.
[0017] The term a "therapeutically effective amount",
"effective amount" or a
"sufficient amount" of a compound of the present disclosure is a quantity
sufficient to,
when administered to the subject, including a mammal, for example a human,
effect
beneficial or desired results, including clinical results, and, as such, an
"effective
amount" or synonym thereto depends upon the context in which it is being
applied.
For example, in the context of treating cancer, for example, it is an amount
of the
compound sufficient to achieve such treatment of the cancer as compared to the
response obtained without administration of the compound. The amount of a
given
compound of the present disclosure that will correspond to an effective amount
will
vary depending upon various factors, such as the given drug or compound, the
pharmaceutical formulation, the route of administration, the type of disease
or
disorder, the identity of the subject or host being treated, and the like, but
can
nevertheless be routinely determined by one skilled in the art. Also, as used
herein, a
"therapeutically effective amount" , "effective amount or a "sufficient amount
of a
compound of the present disclosure is an amount which inhibits, suppresses or
reduces a cancer (e.g., as determined by clinical symptoms or the amount of
cancerous cells) in a subject as compared to a control.
[0018] The term "subject" as used herein includes all members
of the animal
kingdom including human. According to one embodiment, the subject is a human.
[0019] The term "alkyl" as used herein means straight and/or
branched chain,
saturated alkyl groups containing from one to n carbon atoms and includes
(depending
on the identity of n) methyl, ethyl, propyl, isopropyl, n-butyl, s-butyl,
isobutyl, t-
CA 02926928 2016-04-11
butyl, 2,2-dimethylbutyl, n-pentyl, 2-methylpentyl, 3-methylpentyl, 4-
methylpentyl,
n-hexyl and the like, wherein n is the maximum number of carbon atoms in the
group.
[0020] The expression an alkyl component of a naturally occurring amino
acid" as used herein refers to the portion of a naturally occurring amino acid
that is
comprised between the carbon of the carbonyl group of the amino acid and the
nitrogen atom of the amino acid.
[0021] The expression "compound(s) of the present disclosure" as used in
the
present document refers to compounds of formulae I, IA, IB, IC, ID and IE,
presented
in the present disclosure, isomers thereof, such as stereoisomers (for
example,
enantiomers, diastereoisomers, including racemic mixtures) or tautomers, or to
pharmaceutically acceptable salts, solvates, hydrates and/or prodrugs of these
compounds, isomers of these latter compounds, or racemic mixtures of these
latter
compounds. The expression "compound(s) of the present disclosure" also refers
to
mixtures of the various compounds or variants mentioned in the present
paragraph.
[0022] The term "halogen" as used herein comprises fluoro, chloro, bromo
and iodo.
[0023] It is to be clear that the present disclosure includes isomers,
racemic
mixtures, pharmaceutically acceptable salts, solvates, hydrates and prodrugs
of
compounds described therein and mixtures comprising two or more of such
compounds.
[0024] The compounds of the disclosure may have at least one asymmetric
centre. Where the compounds according to the present document possess more
than
one asymmetric centre, they may exist as diastereomers. It is to be understood
that all
such isomers and mixtures thereof in any proportion are encompassed within the
scope of the present disclosure. It is to be understood that while the
stereochemistry of
the compounds of the present disclosure may be as provided for in any given
compound listed herein, such compounds of the disclosure may also contain
certain
amounts (for example less than 30%, less than 20%, less than 10%, or less than
5%)
of compounds of the present disclosure having alternate stereochemistry.
[0025] The term "suitable", as in for example, "suitable counter anion" or
"suitable reaction conditions" means that the selection of the particular
group or
conditions would depend on the specific synthetic manipulation to be performed
and
CA 02926928 2016-04-11
11
the identity of the molecule but the selection would be well within the skill
of a
person trained in the art. All process steps described herein are to be
conducted under
conditions suitable to provide the product shown. A person skilled in the art
would
understand that all reaction conditions, including, for example, reaction
solvent,
reaction time, reaction temperature, reaction pressure, reactant ratio and
whether or
not the reaction should be performed under an anhydrous or inert atmosphere,
can be
varied to optimize the yield of the desired product and it is within their
skill to do so.
[0026] The expression "pharmaceutically acceptable means compatible with
the treatment of subjects such as animals or humans.
[0027] The expression "pharmaceutically acceptable salt means an acid
addition salt or basic addition salt which is suitable for or compatible with
the
treatment of subjects such as animals or humans.
[0028] The expression "pharmaceutically acceptable acid addition salt as
used herein means any non-toxic organic or inorganic salt of any compound of
the
present disclosure, or any of its intermediates. Illustrative inorganic acids
which form
suitable salts include hydrochloric, hydrobromic, sulfuric and phosphoric
acids, as
well as metal salts such as sodium monohydrogen orthophosphate and potassium
hydrogen sulfate. Illustrative organic acids that form suitable salts include
mono-, di-,
and tricarboxylic acids such as glycolic, lactic, pyruvic, malonic, succinic,
glutaric,
fumaric, malic, tartaric, citric, ascorbic, maleic, benzoic, phenylacetic,
cinnamic and
salicylic acids, as well as sulfonic acids such as para-toluene sulfonic and
methanesulfonic acids. Either the mono or di-acid salts can be formed, and
such salts
may exist in either a hydrated, solvated or substantially anhydrous form. In
general,
the acid addition salts of the compounds of the present disclosure are more
soluble in
water and various hydrophilic organic solvents, and generally demonstrate
higher
melting points in comparison to their free base forms. The selection of the
appropriate salt will be known to one skilled in the art. Other non-
pharmaceutically
acceptable salts, e.g. oxalates, may be used, for example, in the isolation of
the
compounds of the present disclosure, for laboratory use, or for subsequent
conversion
to a pharmaceutically acceptable acid addition salt. In embodiments of the
present
disclosure, the pharmaceutically acceptable acid addition salt is the
hydrochloride salt.
CA 02926928 2016-04-11
12
[0029] The term
"pharmaceutically acceptable basic addition salt" as used
herein means any non-toxic organic or inorganic base addition salt of any acid
compound of the disclosure, or any of its intermediates. Acidic compounds of
the
disclosure that may form a basic addition salt include, for example, where R
is CO2H.
Illustrative inorganic bases which form suitable salts include lithium,
sodium,
potassium, calcium, magnesium or barium hydroxide. Illustrative organic bases
which form suitable salts include aliphatic, alicyclic or aromatic organic
amines such
as methylamine, trimethylamine and picoline or ammonia. The selection of the
appropriate salt will be known to a person skilled in the art. Other non-
pharmaceutically acceptable basic addition salts, may be used, for example, in
the
isolation of the compounds of the disclosure, for laboratory use, or for
subsequent
conversion to a pharmaceutically acceptable acid addition salt.
[0030] The formation
of a desired compound salt is achieved using standard
techniques. For example, the neutral compound is treated with an acid or base
in a
suitable solvent and the formed salt is isolated by filtration, extraction or
any other
suitable method.
[0031] The term
"solvate" as used herein means a compound of the present
disclosure, wherein molecules of a suitable solvent are incorporated in the
crystal
lattice. A suitable solvent is physiologically tolerable at the dosage
administered.
Examples of suitable solvents are ethanol, water and the like. When water is
the
solvent, the molecule is referred to as a "hydrate". The formation of solvates
of the
compounds of the present disclosure will vary depending on the compound and
the
solvate. In general,
solvates are formed by dissolving the compound in the
appropriate solvent and isolating the solvate by cooling or using an
antisolvent. The
solvate is typically dried or azeotroped under ambient conditions.
[0032] Compounds of
the present disclosure include prodrugs. In general,
such prodrugs will be functional derivatives of these compounds which are
readily
convertible in vivo into the compound from which it is notionally derived.
Prodrugs
of the compounds of the present disclosure may be conventional esters formed
with
available hydroxy, or amino group. For example, an available OH or nitrogen in
a
compound of the present disclosure may be acylated using an activated acid in
the
presence of a base, and optionally, in inert solvent (e.g. an acid chloride in
pyridine).
Some common esters which have been utilized as prodrugs are phenyl esters,
aliphatic
= CA 02926928 2016-04-11
13
(C8-C24) esters, acyloxymethyl esters, carbamates and amino acid esters. In
certain
instances, the prodrugs of the compounds of the present disclosure are those
in which
one or more of the hydroxy groups in the compounds is masked as groups which
can
be converted to hydroxy groups in vivo. Conventional procedures for the
selection
and preparation of suitable prodrugs are described, for example, in "Design of
Prodrugs" ed. H. Bundgaard, Elsevier, 1985.
[0033] Compounds of the present disclosure include radiolabeled
forms, for
example, compounds labeled by incorporation within the structure 2H, 3H, 14C,
15N,
or a radioactive halogen such as 1251. A radiolabeled compound of the
compounds of
the present disclosure may be prepared using standard methods known in the
art.
[0034] As used herein, and as well understood in the art,
"treatment" or
"treating" is an approach for obtaining beneficial or desired results,
including clinical
results. Beneficial or desired clinical results can include, but are not
limited to,
alleviation or amelioration of one or more symptoms or conditions,
diminishment of
extent of disease, stabilized (i.e. not worsening) state of disease,
preventing spread of
disease, delay or slowing of disease progression, amelioration or palliation
of the
disease state, and remission (whether partial or total), whether detectable or
undetectable. "Treatment" or "treating" can also mean prolonging survival as
compared to expected survival if not receiving treatment.
[0035] "Palliating" a disease or disorder, means that the
extent and/or
undesirable clinical manifestations of a disorder or a disease state are
lessened and/or
time course of the progression is slowed or lengthened, as compared to not
treating
the disorder.
[0036] In understanding the scope of the present disclosure,
the term
"comprising" and its derivatives, as used herein, are intended to be open
ended terms
that specify the presence of the stated features, elements, components,
groups,
integers, and/or steps, but do not exclude the presence of other unstated
features,
elements, components, groups, integers and/or steps. The foregoing also
applies to
words having similar meanings such as the terms, "including", "having" and
their
derivatives. Finally, terms of degree such as "substantially",
"about" and
"approximately" as used herein mean a reasonable amount of deviation of the
modified term such that the end result is not significantly changed. These
terms of
= CA 02926928 2016-04-11
14
degree should be construed as including a deviation of at least 5% of the
modified
term if this deviation would not negate the meaning of the word it modifies.
[0037] In an embodiment of the present disclosure, there are
included
compounds of formula (IA) :
0
0
R3 \R2
R4
0
(IA)
wherein R2, R3, R4 and Q are as previously defined.
[0038] In another embodiment of the present disclosure, there
are included
compounds of formula (IB) :
0
R3
0
N-Q
R4
0 R2
(IB)
wherein R2, R3, R4 and Q are as previously defined.
[0039] In a further embodiment of the present disclosure, there
are included
compounds of formula (IC) :
= CA 02926928 2016-04-11
0
0
N¨NT5
0 R2 R6
(1C)
wherein R2, R5 and R6 are as previously defined.
[0040] In still a further embodiment of the present disclosure,
there are
included compounds of formula (ID) :
0
R3
0
(R4 N¨N
0 R2 R7
(ID)
wherein R2, R3, R4 and R7 are as previously defined.
[0041] In still a further embodiment of the present disclosure,
there are
included compounds of formula (IE) :
= CA 02926928 2016-04-11
16
0
H 0
H
N
111 / ________________________________________________________
7;---------<
(
0 R2 R7
(1E)
wherein R2 and R7 are as previously defined.
[0042] In still a further embodiment of the present disclosure,
R2 is H, unsubstituted member chosen from acetyl and propiolyl;
Q is QA;
R5 is H, unsubstituted member chosen from acetyl and propiolyl; and
R6 is Boc, H, or an unsubstituted member chosen from acetyl and
propiolyl.
[0043] In still a further embodiment of the present disclosure,
R2 is H or unsubstituted member chosen from acetyl and propiolyl; and
R7 is an unsubstituted member chosen from C1-C8 alkyl, C3-C6
cycloalkyl, phenyl, furanyl, thiophenyl, pyridinyl, naphthyl, quinolyl
and isoquinolyl.
[0044] For example, in a further embodiment of the present
disclosure, the
compound of formula I is as previously defined with the proviso that the
compound is
different from
o o o
N . 0
NHNH4 ( NHNH2*HC1 0 and N
NHNH2*CF3CO2H
0
.
[0045] However, accodring to an embodiment the above three
excluded
compounds are not to be excluded from the scope of the various uses and
methods as
previously described.
' CA 02926928 2016-04-11
17
[0046] According to another embodiment, the above three excluded
compounds are to be excluded from the scope of the various uses and methods as
previously described.
[0047] In still a further embodiment of the present disclosure, there are
included compounds of the following formulas:
0
O 9 o CI
NHNH--`< / 0
(1\I * 0
N * p
O CI _____________ 0 __ 0
4a 4b
O 0
( = 0 )0LR --/,
I N =0
NHN NHNBocIr-R ----
O o ).
o R (i31
8 (R = CH3, ML-28) 10 (R = CH3, ML-33B)
O 0
o
ili\I .11 o Boc * *
---- N¨N. NHNHBoc
O R-i >0 o
OR 12 (ML-19)
11 (R = CH3, ML-31E)
0
0
H /c1\1 leo
Boc-NS HN-NH
0 'Boc
13 (ML-21)
[0048] For example, the compound can be chosen from
= CA 02926928 2016-04-11
=
18
0 0
1-11\1 X.0 H3
NHN N N CH3 NHNBoc
N¨NBoc
0 0 /t 0 H3C-
0 H3C O OH3C
T-AN 0
41,
HN-NH N¨NH
0 and 0 H3C--
H3C 0 H3C
[0049] Schemes 1 to 4 represent examples of synthetic
routes used for the
preparation of the compounds of the present disclosure. The reaction
conditions of
each step are presented directly in the schemes.
[0050] Using para-amino benzoic acid (1) as the starting
material derivative 4
was made in a three-step reaction sequence (Scheme 1).911 Para-amino benzoic
acid
(1 or PABA) was first reacted with maleic anhydride (MA) in dry acetone to
give the
diacid (2) with 90% yield. Cyclisation to form the maleimide was accomplished
with
acetic anhydride and sodium acetate to give compound 3 with 89% yield after
hydrolysis of the mixed anhydride intermediate with water. Finally, activation
of acid
3 with /so-butyl chloroformate in the presence of pyridine was done followed
by
treatment with tert-butyl carbazate gave the desired anti-inflammatory
derivative 4
with 54% yield. Deprotection of 4 with hydrochloric acid in ether gave the
hydrochloride salt 5 with 46% yield after recrystallization. Example 1 shows
the
preparation of compounds 4 and 5. This reaction sequence can be used to
produce
other derivatives starting from unsubstituted or substituted ortho-, meta- and
para-
benzoic acid starting materials. Hence, this approach lead to the synthesis of
N'-[3-
(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-chloro-benzoyll-hydrazine carboxylic
acid tert-
butyl ester (4a) and N'44-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-2-chloro-
benzoy1]-
hydrazine carboxylic acid tert-butyl ester (4b) shown in examples 2 and 3,
respectively.
CA 02926928 2016-04-11
19
0 0
H2N= 0
OH
a
(c1\1=
CO2H OH OH
0
1 2 (90%) 3 (88%)
N O lN
NHNH¨µ( ( NHNH2*HC1
4 (AL-549, 54%) 5 (AL-361, 46%)
Reagents and conditions: a) Maleic anhydride (MA), dry acetone, methanol, 22
C, 1 h; b) 1) Ac20,
AcONa, 50 C, 2 h; 2) H20, 70 C, 2 h; c) 1) iso-butyl chloroformate, Et3N,
CH2C12, 0 C, 1 h and 22
C, 1 h; 2) tert-butyl carbazate, CH2C12, 22 C, 12 h; d) HC1, dioxane, 22 C,
5 h.
Scheme 1
[0051] Further
transformations of derivative 4 can lead to novel compounds
with anti-inflammatory, anti-metastatic and anticancer activities (Scheme 2).
However, catalytic hydrogenation of 4 led to compound 12 which lost its anti-
inflammatory activity (see example 4). Otherwise, compound 4 is transformed
into its
trifluoroacetate salt 6 upon treatment with trifluoroacetic acid in
dichloromethane.
The crude material 6 can be reacted either with acetyl chloride or acetic
anhydride (or
any relevant anhydride or acid chloride) to give derivative 8. Of note,
derivatives 7
and 9 were likely produced but not isolated in example 5. Alternatively,
compound 4
can be acylated with a relevant anhydride or acid chloride to yield compound
10 and
11. Interestingly, using acetyl chloride, compound 4 was transformed
efficienly into
the diacetylated derivative 11 (R = CH3, ML-3113) and, with acetic anhydride
the
main product of the reaction was the monoacetylated derivative 10 (R = CH3, ML-
33B). Derivative 10 can be deprotected to give 7 and derivative 11 can lead to
derivative 9. It is possible to produce efficienly compound 7 and 9 via a two-
step
sequence from 4.
' CA 02926928 2016-04-11
o
O o - o
R
-----
O O b ---k - HN-NH
i N * --0- N il ¨4'7 l N .
---\ NHNHBoc NHNH2*CF3CO2H --1 o
O - o - o
4 6 (ML-15) 7
O o +
b 1 N li IN 0 0
(( lik )1.-
R
NHNHBoc O. NHNr.R
0 . 'c
/ 0,..\N
12 (ML-19) % o
o 0
\
O o CDSCN 8 (R =
CH3, ML-28)
N . O + -1---AN * o =Boc c.
O +
NHNBoc
0 0 R¨ 0
N II
R 0 0 R (from 11 to yield 9)
NNH R
O
.'
10 (R = CH3, ML-33B) 11 (R = CH3, ML-31E) 9 0 R
Reagents and conditions: a) I-FA, CH2C12, 22 C, 0.5 h; b) Relevant anhydride
or acid chloride, Et0Ac, 22 C, 30 min to
2 h; c) H2, Pd/C, CH3OH, 22 C, 3 h.
Scheme 2
[0052] Scheme 3 presents the methodology leading to 13 the
alkylhydrazones
or arylhydrazones derivatives following the procedure described by Taha et
a/.12
Accordingly, compound 5 can be treated with a relevant aldehyde (alkyl
aldehydes
(linear or branched), benzaldehyde or substituted benzaldehydes or other
arylaldehydes) under acidic conditions at reflux in butanol (or other solvent)
to give
the desired derivatives of general structure 13.
O 0
0
N a 1\1 le
FINH 7 ¨1.-- HN¨N--=\
O 0 R
5 13
R = alkyl or aromatic (unsubstituted or substituted)
Reagents and conditions: a) RCHO, H+, Butanol, reflux, 1 to 5 h.
Scheme 3
[0053] On Scheme 4, derivative 4 (or any other maleimides
described herein)
can be reacted with an appropriate diene (butadiene (unsubstituted or
substituted),
cyclopentadiene, cyclohexadiene cycloheptadiene, furane, thiophene, pyrrole, N-
= CA 02926928 2016-04-11
21
alkylpyrrole) to give the desired cycloadducts (Diels-Alder products) such as
14, 15
and 16. This reaction can be performed by heating the pure reagents (diene and
dienophile) either neat or in solution, with or without pressure as it is
described in
example 10.
H
= N
H o HN¨Q
a
NH-Q ¨0.- or or N
NH-Q
0 H 0
4 H X = 0, S,
NH or NR
N 15
NH-Q
H 0
n=1,2or3
16
Relevant diene = butadiene (unsubstituted or substituted), cyclopentadiene,
cyclohexadiene, furane, thiophene, pyrrole, N-alkyl
pyrrole.
Reagents and conditions: a) Relevant diene, toluene, 4, 3 h.
Scheme 4
[0054] As it can be appreciated by the skilled artisan, the above synthetic
schemes are not intended to be a comprehensive list of all means by which the
compounds described and claimed in this application may be synthesized.
Further
methods can also potentially be used to prepare the compounds of the present
disclosure.
[0055] The compounds of the present disclosure may be modified by
appending appropriate functionalities to enhance selective biological
properties. Such
modifications are known in the art and include those which increase biological
penetration into a given biological system (e.g., blood, lymphatic system,
central
nervous system), increase oral availability, increase solubility to allow
administration
by injection, alter metabolism and alter rate of excretion.
[0056] The compounds of the present disclosure may contain one or more
asymmetric carbon atoms and thus may occur as racemates and racemic mixtures,
single enantiomer, diastereomeric mixtures and individual diastereoisomers.
All such
CA 02926928 2016-04-11
22
isomeric forms of these compounds are expressly included in the present
disclosure.
Each stereogenic carbon may be of the R or S configuration.
[0057] In the present disclosure, the following abbreviations are used:
Abbreviation Meaning
Ac20 Acetic anhydride
AcONa Sodium acetate
CH2C12 Dichloromethane
Boc t-Butyloxycarbonyl
Et20 Diethyl ether
Et0Ac Ethyl acetate
Et3N Triethyl amine
Hour
HC1 Chlorhydric acid
i.p. Intraperitoneal
Meta
MA Maleic anhydride
Me0H Methanol
min Minute
mmol Millimole
MTT 3-(4,5-Dimethylthiazol-2-y1)-2,5-diphenyltetrazolium
bromide
NMR Nuclear magnetic resonance
o Ortho
Para
Phe Phenyl
s.c. Subcutaneously
TLC Thin layer chromatography
TFA Trifluoroacetic acid
UBC Urothelial bladder cancer
CA 02926928 2016-04-11
23
EXAMPLES
[0058] This section also describes the synthesis of several compounds that
are
presented in this document. These examples are not to be construed as limiting
the
scope of the present disclosure in any way.
Materials and Methods - Chemistry
[0059] Anhydrous reactions were performed under an inert atmosphere, the
set-up assembled and cooled under dry nitrogen. Unless otherwise noted,
starting
material, reactant and solvents were obtained commercially and were used as
such or
purified and dried by standard means.I3 Organic solutions were dried over
magnesium
sulfate, evaporated on a rotatory evaporator and under reduced pressure. All
reactions
were monitored by UV fluorescence, or staining with iodine. Commercial TLC
plates
were Sigma T 6145 (polyester silica gel 60 A, 0.25mm). Flash column
chromatography was performed according to the method of Still and co-workers
on
Merck grade 60 silica gel, 230-400 mesh." All solvents used in chromatography
had
been distilled prior to use.
[0060] The infrared spectra were taken on a Nicolet Impact 420 FT-IR. Mass
spectral assays were obtained using a MS model 6210, Agilent technology
instrument.
The high resolution mass spectra (HRMS) were obtained by TOF (time of flight)
using ESI (electrospray ionization) using the positive mode (ESI+).
(Plateforme
analytique pour molecules organiques de l'Universite du Quebec à Montreal).
[0061] Nuclear magnetic resonance (NMR) spectra were recorded on a Varian
200 MHz NMR apparatus. Samples were dissolved in deuterochloroform (CDC13),
deuteroacetone (acetone-d6) or deuterodimethylsulfoxide (DMSO-d6) for data
acquisition using tetramethylsilane or chloroform as internal standard (TMS, 8
0.0
ppm for 1H-NMR and CDC13 8 77.0 ppm for 13C-NMR). Chemical shifts (8) are
expressed in parts per million (ppm), the coupling constants (J) are expressed
in hertz
(Hz). Multiplicities are described by the following abbreviations: s for
singlet, d for
CA 02926928 2016-04-11
24
doublet, dd for doublet of doublets, t for triplet, dt for doublet of
triplets, q for quartet,
dq for doublet of quartets, m for multiplet, #m for several multiplets, br for
a broad
signal.
[0062] The following compounds were prepared from a relevant
aminobenzoic acid derivative using the procedures summarized in schemes 1, 2,
3 or
4.
Example 1. Preparation of N'44-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-benzoyli-
hydrazine carboxylic acid tert-butyl ester (4 or AL-549), its
hydrochloric acid salt (5 or AL-361) and its trifluoroacetic acid salt
(6 or ML-15)
Step A. Synthesis of 4-(3-carboxy-acryloylamino)-benzoic acid (2)
[0063] 4-Aminobenzoic acid (1, 5.34 g, 38.93 mmol) was dissolved in dry
acetone (12 mL) to which was added methanol (l mL). Maleic anhydride (1.05
eq.)
dissolved in dry acetone was added to the first solution. The reaction mixture
was
stirred for a period of 2 h allowing sufficient time for the complete
precipitation of the
diacid 2. The precipitate was filtered and washed twice with acetone (2 x 2
mL) and
dried in a desiccator overnight. The crude diacid 2 (9.16 g, 90%) was
sufficiently pure
to be use without further purification at the next step. IR (v, cm-1): 3500-
2500
(CO2H), 1686 cm-1 (C=0); I H NMR (DMSO-d6, 6 ppm): 12.79 (br s, 2 H, 2 x
CO21-1), 10.58 (s, 1H, NH), 7.89 and 7.71 (2 x d, J=8.6 Hz, 4H, aromatic),
6.48 and
6.30 (2 x d, J=12.2 Hz, 2H, maleimide); I3C NMR (DMSO-d6, 6 ppm): 167.4,
167.3,
164.1, 143.2, 132.1, 130.9 (2), 130.6, 126.0, 119.2 (2); ESI+ HRMS: (M+H)+
calculated for CiiHi0N05= 236.0553; found = 236.0558.
Step B. Synthesis of 4-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-benzoic acid (3)
[0064] The diacid 2 (2.01 g, 8.54 mmol) was treated with acetic anhydride
(4.0 mL, 36.28 mmol) and anhydrous sodium acetate (350 mg, 4.27 mmol) and the
CA 02926928 2016-04-11
mixture heated at 50 C for 2 h. Afterwards, the solution was evaporated to
dryness
and stirred with water at 70 C for a period of 2 h. The resulting precipitate
was
filtered and dried in a desiccator overnight to yield 1.65 g (89%) of
maleimide 4. The
spectral data of this derivative correspond to those reported in the
literature.91 IR (v,
cm-1): 3475-2600 (CO2H), 1715 (C=0), 1704 (C=0); 1H NMR (acetone-d6, 6 ppm):
8.14 and 7.57 (2 x d, J=8.6 Hz, 4H, aromatic), 7.08 (s, 2H, maleimide); 13C
NMR
(acetone-d6, 6 ppm): 169.3 (2), 166.2, 136.2, 134.7 (2), 130.1 (2), 129.3,
125.8 (2);
ESI+ HRMS: (M+H)+ calculated for CI IH8N04 = 218.0448; found = 218.0447.
Step C. Synthesis of N'44-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-benzoyli-
hydrazine carboxylic acid tert-butyl ester (4 or AL-549)
[0065] Derivative 4 was synthesized using a modified procedure reported by
Willner et al.'s as it is also described by Lau et al. and Hamelin-Morrissette
et al.9-11
A cooled suspension (0 C) of molecule 3 (211 mg, 0.97 mmol) in methylene
chloride
(4.5 mL) was treated with triethylamine (190 pt, 1.36 mmol) and isobutyl
chloroformate (175 IAL, 1.34 mmol). The mixture was stirrred for 1 h at 0 C
and at
room temperature (22 C) for about 1 h. Afterwards, tert-butyl carbazate (128
mg,
0.97 mmol) dissolved in methylene chloride (0.8 mL) was added dropwise to the
mixture and stirred for an additional 12 h at 22 C. The reaction mixture was
diluted
with ethyl acetate (55 mL) and methylene chloride (20 mL) and washed twice
with
saturated NaHCO3 (2 x 50 mL), twice with 0.1 N HCI (2 x 50 mL), twice with
saturated NaC1 (2 x 50 mL), and finally with H20 (50 mL). The organic phase
was
dried (MgSO4) and evaporated to give crude derivative 4. The product was
purified by
flash chromatography, using a mixture of hexanes / acetone (3/2), to yield 173
mg
(54%) of 4. The spectral data of this derivative correspond to those reported
in the
literature.1 IR (v, cm-I): 3360-3240 (NH), 3087 (C=C), 2988 (CH, aliphatic),
1733
(C=0), 1706 (C=0); IH NMR (acetone-d6, 6 ppm): 9.05 (s, 1H, NH), 8.02 and 7.53
(2
x d, J=8.6 Hz, 4H, aromatic), 7.07 (s, 2H, maleimide), 2.84 (br s, 1H, NH),
1.45 (s,
9H, 3 x CH3); 13C NMR (acetone-d6, 6 ppm): 169.3 (2), 166.0, 155.7, 135.1,
134.6
(2), 131.6, 127.9 (2), 125.9 (2), 79.6, 27.5 (3); ESI+ HRMS: (M+Na)+
calculated for
= CA 02926928 2016-04-11
26
C16Hi7N3Na05 = 354.1060; found = 354.1072; (M -2-methylpropene +H)+ calculated
for C12H11 N305 = 276.0620; found = 276.0627.
Step D. Synthesis of 4-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-
benzoic acid
hydrazide hydrochloride (or 4-maleimidbenzoic acid hydrazide hydrochloride)
(5 or AL-361)
[0066] The hydrolysis of 4 was performed using a similar
procedure reported
by Heindel et al. for the cleavage of maleimidoacetic acid (tert-
butyloxycarbonyl)
hydrazide with hydrochloric acid to form maleimidoacetic acid hydrazide
hydrochloride.16 To a solution of 4 (2.41 g, 7.27 mmol) dissolved in dry
dioxane (30
mL) was added a solution of hydrochloric acid (60 mL, 1.0 M in diethyl ether,
60
mmol). The mixture was stirred at room temperature for a period of 5 hours.
Afterwards, 150 mL of hexanes were added to complete the precipitation of the
hydrochloride salt 5. The crude precipitated was filtered, washed with hexanes
and
recrystallized twice with a mixture of methanol / isopropyl alcohol / hexanes
(8 / 3 /
10) to yield 1.7 g (46%) of the desired material. IR (v, cm-I): 3200-2500
(CO2H),
3269 (NH), 1702 (C=0), 1693 (C=0); 1H NMR (DMSO-d6, 6 ppm): 8.06 and 7.52 (2
x d, J=8.8 Hz, 4H, aromatic), 7.21 (s, 2H, maleimide); 13C NMR (DMSO-d6, 6
ppm):
170.0 (2), 165.6, 135.9, 135.4 (2), 129.6, 129.0 (2), 126.8 (2); ES1+ HRMS:
(M+H)+
calculated for CiiHi0N303 = 232.0717; found = 232.0717 and ESI+ HRMS: (M+H)+
calculated for Ci1HiiCIN303 = 268.0483; found = 268.0483.
Step E. Synthesis of 4-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-
benzoic acid
hydrazide trifluoroacetic acid salt (6 or ML-15)
[0067] A solution of 4 (106 mg, 0.32 mmol) dissolved in
trifluoroacetic acid
(0.5 mL) was stirred at 0 C for a period of 30 minutes. Afterwards, the
excess
trifluoroacetic acid was removed under vacum at 22 C to give compound 6
quantitatively. IR (v, cm-I): 3500-2500 (CO2H), 3277 (NH), 1710 (C=0); IH NMR
(DMSO-d6, 6 ppm): 11.62 (br s, 1H, NHNH3+CF3CO2-), 8.63 (br s, NH3), 8.01 and
= CA 02926928 2016-04-11
27
7.56 (2 x d, J=8.5 Hz, 4H, aromatic), 7.24 (s, 2H, maleimide); I3C NMR (DMSO-
d6,
6 ppm): 169.6 (2), 165.4, 135.5, 135.0 (2), 129.4, 128.4 (2), 126.5 (2).
Example 2. Preparation of Nr-13-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-chloro-
benzoy1Fhydrazine carboxylic acid tert-butyl ester (4a)
Following the procedure of Example 1, steps A ¨ C described above using 3-
amino-4-
chloro benzoic acid as the starting material instead of 4-amino benzoic acid
derivative
4a was prepared efficiently.
Step A. Synthesis of 3-(cis-3-carboxy-acryloylamino)-4-chloro
benzoic acid
(2a)
[0068] Spectral data for 2a: IR (v, cm-1): 3500-2500 (CO2H),
3310 (NH),
1696 (C=0); I H NMR (DMSO-d6, 6 ppm): 13.08 (s, 2H, 2 x CO2H), 10.13 (s, 1H,
NH), 8.37 (s, 1H, aromatic) 7.72 and 7.61 (2 x d, 2H, J = 10.0 Hz, aromatic),
6.59 and
6.34 (2 x d, 2H, J = 12.0 Hz, maleimide); I3C MNR (DMSO-d6, 6 ppm): 167.5,
166.7, 164.1, 135.0, 131.6, 131.2 (2), 130.4 (2), 127.2 (2).
Step B. Synthesis of 3-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-
chloro benzoic
acid (3a)
[0069] Spectral data for 3a: IR (v, cm-I): 3300-2500 (CO2H),
3490 (amine),
1674 (C=0). 1H NMR (acetone-d6, 6 ppm): 8.1 (m, 2H, aromatic), 7.76 (d, 1H, J
= 8.0
Hz, aromatic), 7.14 (s, 2H, maleimide) I3C NMR (acetone-d6, 6 ppm): 168.7 (2),
165.0, 143.0, 137.84, 134.9 (2), 132.3, 131.6, 130.6, 130.2.
Step C. Synthesis of N'-13-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-
chloro-
benzoylPhydrazine carboxylic acid tert-butyl ester (4a)
= CA 02926928 2016-04-11
28
[0070] Spectral data for 4a: IR (v, cm-1): 3494 (amine), 3090
(C=C), 2974
(aliphatic), 1717 (C=0). H NMR (acetone-d6, 6 ppm): 9.71 (s, 1H, NH), 8.05
(dd,
1H, J = 8.2 Hz and J = 1.8 Hz, aromatic) 7.95 (d, 1H, J = 1.8 Hz, aromatic),
7.75 (d,
1H, J = 8.6 Hz, aromatic), 7.11 (s, 2H, maleimide), 2.91 (s, 1H, NH), 1.43 (s,
9H, 3 x
CH3); I3C NMR (acetone-d6, 6 ppm): 169.5 (2), 166.0, 156.5, 135.0 (2), 134.0
(2),
130.0, 130.7, 129.0 (2), 80.0, 28.1(3).
Example 3. Preparation of N'44-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-2-chloro-
benzoylphydrazine carboxylic acid tert-butyl ester (4b)
Following the procedure of Example I, steps A ¨ C described above using 4-
amino-2-
chloro benzoic acid as the starting material instead of 4-amino benzoic acid
derivative
4b was prepared efficiently.
Step A. Synthesis of 4-(cis-3-carboxy-acryloylamino)-2-chloro
benzoic acid
(2b)
[0071] Spectral data for 2b : IR (v, cm-I): 3500-2500 (CO2H),
3262 (NH),
1689 (C=0); I H NMR (DMSO-d6, 6 ppm): 13.8 (s, 2H, 2 x CO2H), 10.7 (s, 1H,
NH),
7.85 (m, 2H, aromatic) 7.55 (dd, 1H, J = 10.0 Hz and J = 2.0 Hz, aromatic),
6.45 and
6.31 (2 x d, 2H, J = 12.0 Hz, maleimide); 13C MNR (DMSO-d6, 6 ppm): 168.0,
166.0, 164.0, 143.0, 136.5, 135.0, 132.0, 131.5, 126.0, 121.5, 119.2.
Step B. Synthesis of 3-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-
chloro benzoic
acid (3b)
[0072] Spectral data for 3b : IR (v, cm-I): 3300-2500 (CO2H),
3470 (amine),
1723 (C=0). I H NMR (acetone-d6, 6 ppm): 7.90 (d, 1H, J = 8.6 Hz, aromatic),
7,67
= CA 02926928 2016-04-11
29
(d, 1H, J = 2.2 Hz, aromatic), 7.50 (dd, 1H, J = 8.3 Hz and J = 2.0 Hz,
aromatic) 7.11
(s, 2H, maleimide); 13C NMR (acetone-d6, 6 ppm): 168.0 (2), 165.0, 138.0,
136.0 (2),
135.0, 132.0, 128.0, 124.0, 118Ø
Step C.
Synthesis of N'-13-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-4-chloro-
benzoy1]-hydrazine carboxylic acid tert-butyl ester (4b)
[0073]
Spectral data for 4b : IR (v, cm-1): 3473 (amine), 3090 (C=C), 2984
(aliphatic), 1708 (C=0). I H NMR (acetone-d6, 6 ppm): 9.03 (s, 1H, NH),
7.65(d, 1H,
J = 8.0 Hz, aromatic) 7.59 (d, 1H, J = 1.6 Hz, aromatic), 7.45 (dd, 1H, J =
8.2 and J --
1.8 Hz aromatic), 7.08 (s, 2H, maleimide), 2.96 (s, 1H, NH), 1.47 (s. 9H, 3 x
CH3);
I3C NMR (acetone-d6, 6 ppm): 169.0 (2), 166.0, 156.0, 135.0 (2), 134.6, 133.0,
131.0,
127.9(2), 125.9, 80.0, 27.5 (3).
Example 4. Preparation of N'-
14-(2,5-dioxo-pyrrolidin-1-y1)-benzoy1]-
hydrazine carboxylic acid tert-butyl ester (12 or ML-19)
[0074]
Maleimide 4 (103 mg, 0.31 mmol) was dissolved in methanol (1 mL)
to which was added 5% Pd/C (14 mg). Some hydrogen gas was bubbled during 30
seconds into the mixture. The suspension was stirred vigorously under a
hydrogen
atmosphere for a period of 3 hours. Of note, a longer period of time is
required on a
larger scale. Afterwards, the suspension was filtered on silica with a mixture
of
hexanes / acetone (3/2) as the eluent, to yield 87 mg (84%) of 12. IR (v, cm-
1): 3400-
3100 (NH), 2981 (CH), 1703 (C=0); 1H NMR (acetone-d6, 6 ppm) : 9.54 (br s, 1H,
NH), 8.00 and 7.45 (2 x d, J=8.6 Hz, 4FI, aromatic), 1.45 (s, 9H, 3 x CH3);
13C MNR
(acetone-d6, 6 ppm) 176.2 (2), 165.9, 155.7, 136.1, 132.4, 127.8 (2), 126.7
(2), 79.6,
28.2, 27.5; ESI+ HRMS: (M+H)+ calculated for Ci6H20N305 = 334.1397; found =
334.1391 and ESI+ HRMS: (M+H -C4H9)+ calculated for Cl2F112N305 = 278.0771;
found = 278.0769.
= CA 02926928 2016-04-11
Example 5. Preparation of 4-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-benzoic acid
N'-diacetyl-hydrazide (8, R = CH3 or ML-28)
[0075] To a solution of crude 6 (444 mg, 1.28 mmol) dissolved
in
dichloromethane (10 mL) was added triethylamine (1.07 mL, 780 mg, 7.7 mmol)
and
acetic anhydride (0.61 mL, 659 mg, 6.4 mmol). The mixtured was stirred at 22
C for
about 30 minutes. Afterwards, the organic phase was diluted with ethyl acetate
(75
mL) directly into an extraction funnel. The organic phase was washed
successively
with a 5% sodium bicarbonate aqueous solution (50 mL), with a 10% sodium
chloride
aoous solution (50 mL) and finnally with water (50 mL). The organic phase was
dried
with anhydrous magnesium sulfate, filtered and evaporated to the crude
material (111
mg). The product was purified by flash column chromatography using a mixture
of
hexanes / acetone (7/3) to give 75 mg (18%) of the desired material 8, R =
CH3). Of
note, different anhydrides or alkaloyl chlorides can be used to produce
analogs of this
specific derivative. IR (v, cm-1): 3200 (NH), 1702 (C=0), 1662 (C=0); 1H NMR
(acetone-d6, 6 ppm): 10.15 (br s, 1H, NH), 8.09 and 7.61 (2 x d, J = 8.6 Hz,
4H,
aromatic), 7.10 (s, 2H, maleimide), 2.41 (s, 6H, 2 x CH3); 13C NMR (acetone-
d6,
6 ppm): 171.1 (2), 169.3 (2), 166.0, 135.8, 134.7 (2), 130.8, 128.2 (2), 126.1
(2), 24.2
(2). ESI+ HRMS: (M+H)+ calculated for C15H14N305 = 316.0928; found = 316.0945
and ES1+ HRMS: (M+H -Ac)+ calculated for C13H12N304 =275.08252; found =
275.0856.
Example 6. Preparation of N-acetyl-/V-[4-(2,5-dioxo-2,5-dihydro-pyrrol-1-y1)-
benzoyll-hydrazinecarboxylic acid tert-butyl ester (10, R = CH3 or
ML-33B)
[0076] To a solution of 4 (100 mg, 0.30 mmol) dissolved in
dichloromethane
(3 mL) was added triethylamine (252 tL, 183 mg, 1.81 mmol) and acetic
anhydride
(71 1AL, 76.7 mg, 0.75mmol). The mixtured was stirred at 22 C for about 30
minutes.
Afterwards, the organic phase was diluted with ethyl acetate (25 mL) directly
into an
extraction funnel. The organic phase was washed successively with a 5% sodium
= CA 02926928 2016-04-11
31
bicarbonate aqueous solution (10 mL), with a 10% sodium chloride aoous
solution (10
mL) and finnally with water (20 mL). The organic phase was dried with
anhydrous
magnesium sulfate, filtered and evaporated to the crude material (123 mg). The
product was purified by flash column chromatography using a mixture of hexanes
/
acetone (4/1) to give 22 mg (20%) of the desired material 10, R = CH3). It is
noteworthy, that a longer reaction time (2 hours) lead to higher yield of the
desired
material. However, some diacetylated product (11) is also present as a side
product.
IR (v, cm-I): 3286 (NH), 1753 (C=0), 1711 (C=0), 1652 (C=0); 1H NMR (CDC13,
6 ppm): 8.28 (br s, 1H, NH), 7.90 and 7.50 (2 x d, J = 8.6 Hz, 4H, aromatic),
6.89 (s,
2H, maleimide), 2.61 (s, 31-1, CH3), 1.51 (s, 9H, 3 x CH3); I3C NMR (CDC13, 6
ppm):
170.5, 168.9 (2), 165.2, 151.0, 135.0, 134.4 (2), 130.9, 128.3 (2), 125.7 (2),
84.8, 27.8
(3), 25.5; ESI+ HRMS: (M+Na)+ calculated for Ci8H19N3Na06 = 396.1166; found =
396.1165 and ESI+ HRMS: (M+H -tert-Boc)+ calculated for Ci3Hi2N304 = 274.0822;
found = 274.0824.
Example 7. Preparation of N,N'-diacetyl-N'-14-(2,5-dioxo-2,5-dihydro-pyrrol-
1-y1)-benzoyll-hydrazinecarboxylic acid tert-butyl ester (11, R =
CH3 or ML-31B)
[0077] To
a solution of 4 (100 mg, 0.30 mmol) dissolved in dichloromethane
(3 mL) was added triethylamine (252 ttL, 183 mg, 1.81 mmol) and acetyl
chloride (54
iAL, 60 mg, 0.75 mmol). The mixtured was stirred at 22 C for about 2 hours.
Afterwards, the organic phase was diluted with ethyl acetate (25 mL) directly
into an
extraction funnel. The organic phase was washed successively with a 5% sodium
bicarbonate aqueous solution (10 mL), with a 10% sodium chloride aoous
solution (10
mL) and finally with water (20 mL). The organic phase was dried with anhydrous
magnesium sulfate, filtered and evaporated to the crude material (118 mg). The
product was purified by flash column chromatography using a mixture of hexanes
/
acetone (4/1) to give 100 mg (80%) of the desired material 11, R = CH3). IR
(v, cm-I):
3193 (NH), 1701 (C=0), 1660 (C=0); I H NMR (CDC13, 6 ppm): 7.68 and 7.50 (2 x
d,
J = 8.6 Hz, 4H, aromatic), 6.89 (s, 2H, maleimide), 2.49 and 2.48 (2 x s, 6H,
2 x CH3),
1.50 (s, 9H, 3 x CH3); 13C NMR (CDC13, 6 ppm): 170.2, 169.9 (2), 168.8 (2),
150.2,
= CA 02926928 2016-04-11
32
134.6, 134.4 (2), 132.9, 128.2 (2), 125.0 (2), 85.9, 27.8 (3), 25.4, 24.6;
ESI+ HRMS:
(M+Na)+ calculated for C201-1211\13Na07 = 438.1272; found = 438.1281 and ESI+
HRMS: (M+H -Ac and -tert-Boc)+ calculated for Ci3H12N304 = 274.0822; found =
274.0833.
Example 8. Preparation of N'-
{4-13-(2-tert-butoxycarbonylamino-
ethylsulfany1)-2,5-dioxo-pyrrolidin-1-yli-benzoy1}-
hydrazinecarboxylic acid ter(-butyl ester (13 or ML-21)
[0078] To
a solution of 4 (115 mg, 0.34 mmol) dissolved in methanol (3 mL)
was added 2-(Boc-amino) ethanethiol (70 pt, 73 mg, 0.41 mmol). The mixture was
stirred at 22 'C for 2 hours and at 50 C for 1 hour. The organic phase was
diluted with
ethyl acetate (30 mL) directly into an extraction funnel and washed
successively with
a 5% sodium bicarbonate aqueous solution (2 x 10 mL) and with water (2 x 20
mL).
The organic phase was dried with anhydrous magnesium sulfate, filtered and
evaporated to the crude material (191 mg). The product was purified by flash
column
chromatography using a mixture of hexanes / acetone (3/2) to give 107 mg (61%)
of
the desired material 15. IR (v, cm-1): 3300 (NH), 1707 (C=0), 1680 (C=0); 1H
NMR
(CDCI3, 6 ppm): 8.80 (br s, 1H, NH), 7.86 and 7.34 (2 x d, J = 8.2 Hz, 4H,
aromatic),
6.90 (br s, 1H, NH), 5.07 (br s, 1H, NH), 3.98 (1H, m, -CHS-) 2.6-3.6 (several
m, 6H,
3 x -CH2-), 1.50 and 1.45 (2 x s, 18H, 2 x 3 x CH3); 13C NMR (CDC13, 6 ppm):
175.4, 173.2, 165.8, 155.9 (2), 134.8, 131.7, 128.2 (2), 126.4 (2), 82.1,
79.7, 39.4,
38.9, 36.1, 32.7, 28.4 (3), 28.2 (3); ESI+ HRMS: (M+Na)+ calculated for
C23H32N4Na07S = 531.1884; found = 531.1881.
Example 9. General procedure for the preparation of derivatives of general
structure 13 (see scheme 3)
[0079]
Following the procedure described by Taha et al.12 compound 5 can be
treated with a relevant aldehyde (alkyl aldehydes (linear or branched),
benzaldehyde
= CA 02926928 2016-04-11
33
or substituted benzaldehydes) under acidic conditions at reflux in butanol (or
other
solvent) to give the desired alkylhydrazones or benzoylhydrazones derivatives.
Example 10. General procedure for the preparation of derivatives 14, 15 or 16
(see scheme 4)
[0080] Derivative 4 (or any other maleimides described herein)
can be reacted
with an appropriate diene (butadiene (unsubstituted or substituted),
cyclopentadiene,
cyclohexadiene cycloheptadiene, furane, thiophene, pyrrole, N-alkylpyrrole) to
give
the desired cycloadducts (DieIs-Alder products) such as for example 14, 15 and
16.
This reaction can be performed by heating the pure reagents (diene and
dienophile)
either neat or in solution, with or without pressure.
Materials and Methods - Biology
In vitro studies (for derivatives 4 (A1-549), 5 (AL-361), 12 (ML-19), 8 (ML-
28), etc.)
[0081] Cell culture ¨ general: Biological assays were performed
using the
human monocytic cell line THP1, the murine macrophage-like cell line J774A.1,
and
the murine BCa cell line MB49-I. The cells were maintained in RPMI medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS) and containing
1
mM sodium pyruvate, 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
(HEPES) and 50 lAg/mL gentamycin (referred as 10% FBS RPMI-1640). The cells
were maintained at 37 C in a moisture-saturated atmosphere containing 5% CO2.
THP1 cells and J774A.1 cells are the most widely used cell lines to
investigate
the function and differentiation of monocytes and macrophages (MO) in response
to
various inflammatory mediators.I7'18 Undifferentiated THP1 cells resemble
primary
monocytes/M0 isolated from healthy donors or donors with inflammatory
diseases,
such as diabetes mellitus and atherosclerosis.19 After treatment with phorbol
esters,
THP1 cells differentiate into MOike cells which mimic native monocyte-derived
CA 02926928 2016-04-11
34
Ms in several respects.2 As we previously described, green fluorescent
protein-
expressing (GFP)-THP1 cells were cultured for 18 h in 50 nM phorbol 12-
myristate
13-acetate to induce monocyte-to-M43. differentiation.21-23 The J774A.1 cell
line was
kindly provided by Dr Tatiana Scorza (Universite du Quebec à Montreal,
Canada).
This cell line is a macrophage-like cell model which produce large amount of
NO in
response to IFNy, TNFa, bacterial infection and bacterial products, such as
lipopolysaccharide (LPS),I7 The cell line MB49-I is a highly invasive and
tumorigenic
BCa cell model that was developed by successive in vivo passages of MB49
primary
tumors.24
[0082] Cell signaling studies: THP1-derived hM4>s (750x103 cells/mL) were
pretreated for 30 min with vehicle (DMSO) or compounds 1 and 1A (both at 10
)1M
and 50 WVI), and then washed and recovered immediately (t= 0 min) or after 30
min
of activation with 50 U/mL IFNy and 25 ng/mL IL6. Cell lysates were prepared
and
analyzed by immunoblotting as described.21-23'25 Briefly, protein samples were
resolved by SDS-PAGE under reducing conditions and transferred onto a PVDF
membrane. Blots were first probed with rabbit polyclonal antibodies against
phospho-
STAT1 (pSTAT1) and pSTAT3 (both at 1:2000) overnight at 4 C. Blots were then
incubated with HRP-conjugated goat anti-rabbit IgG Ab (1:3000) for 1 h at room
temperature. The same blots were stripped and then probed with anti-STAT1 and
anti-
STAT3 Abs (both at 1:1000). In both cases, probed molecules were visualized
using
an enhancement chemiluminescence detection kit (Thermo Fisher Scientific).
[0083] Surface antigen expression analysis: To study membrane receptor
expression, THP1-derived hni)s (750x104 cells/mL) were pretreated for 3 h with
DMSO or compounds 1 (10 ilM) and 1A (25 [tM), and then left untreated
(control) or
treated for 48 h with 50 U/mL IFNy. The expression level of MHC-II and CD40
was
evaluated by flow cytometry as described.21-23
[0084] Motility assays: The in vitro scratch wound healing assay was
performed to study the effects of compounds 1 (10 1AM) and 1A (25 1AM) in 1L6-
induced hM,11, cell migration, as described.26 Briefly, THP1-derived hM(I)s
(750x103
CA 02926928 2016-04-11
cells/mL) were seeded into 24-well tissue culture plate to reach ¨70-80%
confluence
as a monolayer. The cell monolayers were scraped in a straight line in one
direction to
create a "scratch" with a p200 pipet tip. To obtain the same field during the
image
acquisition, another straight line was scratched perpendicular to the first
would line to
create a cross in each well. Cell debris were removed and the edges of the
scratch
were smoothed by washing the cells once with 1 mL of Hank's buffer. Cell
monolayers were pretreated for 3 h with vehicle (DMSO) or compounds 1 (10 1AM)
and 1A (25 M), and then left untreated (control) or treated for 48 h with 25
ng/mL
IL6. Using the cross as reference points the plate was placed under an
inverted
fluorescence microscope, and the images of the scratch were acquired at t= 0 h
and t=
48 h. The number of motile cells was determined using Java-based image
processing
program ImageJ (National Institutes of Health) and relative cell motility was
expressed as percent (%) of control of motile cells at t= 48 h relative to
motile cells
within the initial wound (at t= 0 h).
[0085] Evaluation of NO production by the Griess reagent method: The
MB49-I cells and the J774A.1 cells (25x103 cells/well) were grown and
pretreated, as
indicated, with various anti-inflammatory derivatives, precursors and mono-
functional
derivatives for a period of 3 h. Afterwards the cells were washed twice with
10% FBS
RPMI-1640 and then activated to produce NO for a period of 24 h with cytokines
INFy and TNFa. NO production was measured using the Griess reagent method as
previously described.27 This method involves the detection of nitrite ions
(NO2-)
formed by the spontaneous oxidation of NO under physiological conditions.
According the manufacture procedure (Life Technologies; # G-7921), equal
volumes
of sulfanilic acid and N-(1-naphthyl)ethylenediamine are mixed together to
form the
Griess reagent. In the presence of NO2-, sulfanilic acid is converted to a
diazonium
salt, which in turn is coupled to N-(1-naphthyl)ethylenediamine to produce a
pink
coloration that is measured with a spectrophotometer (Biotek, synergy HT) at
548 nm.
[0086] Evaluation of cell proliferation by the MU assay: To evaluate the
anti-
proliferative activity, cell viability/proliferation MTT assays were performed
as
previously described.21-23=25'28 Briefly, MB49-I cells (5x103 cells/well) were
plated in
CA 02926928 2016-04-11
36
96-well plates in 100 viL 10% FBS RPMI-1640 and cultured for 24 h at 37 C and
5%
CO2. Cells were pretreated for a period of 3 h with vehicle (DMSO) or
derivatives 1
and 1A at 0, 10, 25, 37.5, and 50 1AM, and then incubated for 24 h in the
absence or
the presence of INFy and TNFa. At the end of the culture period, 10 tL of 5
mg/mL
methylthiazolyldiphenyl-tetrazolium bromide (MTT) solution was added to each
well.
After a 3-h incubation period with MTT reagent, 100 1., of MTT solubilization
buffer
(10% SDS in 10 mM HC1) was added and plates were placed overnight in the cell
incubator before absorbance measure. The optical density was read at 580 nm
using
the Microplate Reader Manager (from Bio-Rad Laboratories).
[0087] Statistical analyses: For all biological assays, data were
presented as
mean SD from three independent experiments. Data were analyzed by one-way
ANOVA followed by Bonferonni post-test using Prism software, version 3.03
(GraphPad, San Diego, CA). p values of < 0.05 were considered to indicate
statistical
significance.
In vivo studies (for derivatives 4 (A1-549), 8 (ML-28))
[0088] Urothelial bladder cancer (UBC) is the fifth most common malignancy
of all cancers in North America. Although most of detectable tumors are
initially non-
muscle-invasive and are generally curable by means of chirurgical resection,
27-30%
of them exhibit a lethal phenotype characterized by high histological grade
and
muscle invasion. This cancer was selected to test derivatives 4 (A1-549), 8
(ML-28).
[0089] Male C57BL/6J mice (6-8 weeks old), each weighing 15-18 g, were
used for the experiments (supplied by Charles River). The mice were housed
with free
access to food and water on a 12:12 h light:dark cycle with the room
temperature
maintained at 21 C. MB49 cells, MB49-I cells, and iNOS-deficient MB49-I cells
(5 x
104 in 100 vtL PBS) were injected subcutaneously (s.c.) into the right flank
of the
mice. Growth rates of the s.c. tumors were monitored. The size of tumors was
determined every 3 days for 24-28 days using a digital caliper and by
measuring
luciferin luminescence at days 15 and 25 using the IVIS imaging system. A
blinded
= CA 02926928 2016-04-11
37
observer measured tumor length and width. The volume of the tumor was
calculated
from the formula: Length x width2 x 0.52, where length and width were tumor
diameters measured with calipers in mutually perpendicular directions. At a
tumor
size of approximately 10 mm3 the mice were divided into different groups. A
control
group received PBS as treatment. As indicated, other groups were treated at
different
doses (90, 150 or 300 uM) with an intraperitoneal (i.p.) injection of AL-549
or ML-28
every 3-4 days for 18-20 days.24'27
The results from the in vitro and in vivo experiments are presented in figures
1-19.
REFERENCES
1. LaFond, R. In Cancer: The outlaw cell, 3Ird Ed., ACS publication,
Washington,
DC, 384 pages (2012)
2. Alderton GK. The tumor microenvironment drives metastasis, Nat. Rev.
Cancer 16, 199 (2016)
3. Steeg PS. Targeting metastasis, Nat. Rev. Cancer 16, 201-218 (2016)
4. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, Richter
C, Zips D, Bortfeld T. Radiation oncology in the era of precision medicine,
Nat. Rev. Cancer 16, 234-249 (2016)
5. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies
in cancer treatment, Nat. Rev. Cancer 12, 237-251 (2012)
6. Scott AM, Wolchok JD, Old Li. Antibody therapy of cancer. Nat. Rev.
Cancer
12, 278-287 (2012)
7. Phillips AP, Mentha JW. US Patent 3,046,301 (October 29, 1959); Deglin JH,
Vallerand AH. In Guide des medicaments, ERPI, pp. 424-427 (1995)
8. Remers, WA. Antineoplastic agents, In Wilson and Gisvold's Textbook of
organic Medicinal and pharmaceutical chemistry, 9th Ed., Delgado JN,
Remers, WA. J.B. Eds, Lippincott, New York, Chapter 8, 321-322 (1989)
9. Lau A, Berube G, Ford CHJ. Conjugation of doxorubicin to monoclonal anti-
carcinoembryonic antigen antibody via novel thiol-directed cross-linking
reagents, Bioorg. Med. Chem., 3, 1299-1304 (1995)
CA 02926928 2016-04-11
38
10. Lau A, Berube G, Ford CHJ, Gallant M. Novel doxorubicin-monoclonal anti-
carcinoembryonic antigen antibody immunoconjugate activity in vitro, Bioorg.
Med. Chem., 3, 1305-1312 (1995)
11. Hamelin-Morrissette J, Cloutier S, Girouard J, Belgorosky D, Eijan A-M,
Legault J, Reyes-Moreno C, and Berube G. Identification of an anti-
inflammatory derivative with anti-cancer potential: The impact of each of its
structural components on inflammatory responses in macrophages and bladder
cancer cells, Eur. J. Med. Chem., 96, 259-269 (2015)
12. Taha M, Ismail NH Lalani S, Fatmi MQ, Atia-tul-Wahab, Siddiqui S, Khan
KM, Imran S, Choudhary MI. Synthesis of novel inhibitors of a-glucosidase
based on the benzothiazole skeleton containing benzohydrazide moiety and
their molecular docking studies, Eur. J. Med. Chem., 92, 387-400 (2015)
13. Perrin DD, Armarego CF. In Purification of Laboratory Chemicals, 3rd Ed.,
Pergamon Press, Oxford, New York (1988)
14. Still WC, Kahn M, Mitra A. Rapid chromatographic technique for preparative
separations with moderate resolution, i Org. Chem., 43, 2923-2925 (1978)
15. Willner D, Trail PA, Hofstead SJ, King HD, Lasch SJ, Braslawsky GR,
Greenfield RS, Kaneko T, Firestone RA. (6-Maleimidocaproyl)hydrazone of
doxorubicin. A new derivative for the preparation of immunoconjugates of
doxorubicin, Bioconjug. Chem., 4, 521-527 (1993)
16. Heindel ND, Zhao HR, Egolf RA, Chang CH, Schray KJ, Emrich JG,
McLaughlin JP, Woo DV, A novel heterobifunctional linker for formyl to thiol
coupling, Bioconjug. Chem., 2, 427-430 (1991)
17. Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Study of
macrophage functions in murine J774 cells and human activated THP-1 cells
exposed to oritavancin, a lipoglycopeptide with high cellular accumulation,
Antimicrobial agents and chemotherapy, 58 2059-2066 (2014)
18. Auwerx J, The human leukemia cell line, THP-1: A multifacetted model for
the study of monocyte-macrophage differentiation, Experientia, 47, 22-31
(1991)
19. Qin Z, The use of THP-1 cells as a model for mimicking the function and
regulation of monocytes and macrophages in the vasculature, Atherosclerosis,
221,2-11 (2012)
20. Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The
identification of markers of macrophage differentiation in PMA-stimulated
THP-1 cells and monocyte-derived macrophages, PloS one, 5, 0008668
(2010)
= CA 02926928 2016-04-11
39
21. Dufresne M, Dumas G, Asselin E, Carrier C, Pouliot M, Reyes-Moreno C,
Pro-inflammatory type-1 and anti-inflammatory type-2 macrophages
differentially modulate cell survival and invasion of human bladder carcinoma
T24 cells, Mol. Immunol., 48 1556-1567 (2011)
22. Dumas G, Dufresne M, Asselin E, Girouard J, Carrier C, Reyes-Moreno C.
CD40 pathway activation reveals dual function for macrophages in human
endometrial cancer cell survival and invasion, Cancer Immunol. Immunother.,
62, 273-283,(2013)
23. Dallagi A, Girouard 1, Hamelin-Morrissette J, Dadzie R, Laurent L,
Vaillancourt C, Lafond J, Carrier C, Reyes-Moreno C. The activating effect of
IFN-gamma on monocytes/macrophages is regulated by the LIF-trophoblast-
IL-10 axis via Statl inhibition and Stat3 activation, Cell. Mol. Immunol., 12,
326-341 (2015).
24. Fabris VT, Lodillinsky C, Pampena MB, Belgorosky D, Lanari C, Eijan AM.
Cytogenetic characterization of the murine bladder cancer model MB49 and
the derived invasive line MB49-I, Cancer Genet., 205, 168-176 (2012).
25. Leduc, Bourassa V, Asselin E, Leclerc P, Lafond, Reyes-Moreno C. Leukemia
Inhibitory Factor Regulates Differentiation of Trophoblast-Like BeWo Cells
Through the Activation of JAK/STAT and MAPK3/1 MAP Kinase-Signaling
Pathways, Biol. Reprod., 86, 54, 1-10 (2012)
26. Menon MB, Ronkina N, Schwermann J, Kotlyarov A, Gaestel M.
Fluorescence-based quantitative scratch wound healing assay demonstrating
the role of MAPKAPK-2/3 in fibroblast migration, Cell. Motil. Cytoskeleton,
66, 1041-1047(2009)
27. Belgorosky D, Langle Y, Prack Mc Cormick B, Colombo L, Sandes E, Eijan
A.M. Inhibition of nitric oxide is a good therapeutic target for bladder
tumors
that express iNOS, Nitric Oxide, 36, 11-18 (2014)
28. Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchell JD. Evaluation of
a tetrazolium-based semiautmated colorimetric assay : Assessment of
radiosensitivity. Cancer Res., 47, 943-946 (1987)
[0090]
The present disclosure has been described with regard to specific
examples. The description was intended to help the understanding of the
present
disclosure, rather than to limit its scope. It will be apparent to one skilled
in the art
that various modifications may be made to the present disclosure without
departing
from the scope of the present disclosure as described herein, and such
modifications
are intended to be covered by the present document.