Note: Descriptions are shown in the official language in which they were submitted.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
COMPOSITIONS FOR ORAL ADMINISTRATION OF ZOLEDRONIC ACID OR RELATED
COMPOUNDS FOR TREATING DISEASE
Inventor: Herriet Tabuteau
BACKGROUND
[001] Bisphosphonate compounds are potent inhibitors of osteoclast
activity, and are
used clinically to treat bone-related conditions such as osteoporosis and
Paget's disease of bone;
and cancer-related conditions including multiple myeloma, and bone metastases
from solid tumors.
They generally have low oral bioavailability.
SUMMARY
[002] It has been discovered that oral dosage forms of bisphosphonate
compounds,
such as zoledronic acid, can be used to treat or alleviate pain or related
conditions.
[003] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the zoledronic acid is present in an amount that results in an area
under the plasma
concentration curve (AUC) of zoledronic acid of about 50 ng=hr/mL to about 700
ng=hr/mL upon
administration of the oral dosage form to the particular species of mammal.
[004] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the zoledronic acid is present in an amount that results in a Cm,x of
zoledronic acid of about
ng/mL to about 300 ng/mL upon administration of the oral dosage form to the
particular species of
mammal.
[005] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that administration of the oral
dosage form to the
particular species of mammal results in a Tmax of zoledronic acid of about 0.4
hr to about 1 hr.
[006] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the zoledronic acid has a
12 hour sustained
plasma level factor of about 12 to about 50 for the particular species of
mammal.
[007] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
1
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
wherein the oral dosage form is configured so that the zoledronic acid has a
24 hour sustained
plasma level factor of about 10 to about 30 for the particular species of
mammal.
[008] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the zoledronic acid has a
36 hour sustained
plasma level factor of about 6 to about 20 for the particular species of
mammal.
[009] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the zoledronic acid has a
48 hour sustained
plasma level factor of about 5 to about 20 for the particular species of
mammal.
[010] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the zoledronic acid has a
72 hour sustained
plasma level factor of about 4 to about 20 for the particular species of
mammal.
[011] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the particular species of
mammal has a plasma
concentration of zoledronic acid at 12 hours that is about 0.5 ng/mL to about
5 ng/mL.
[012] Some embodiments include an oral dosage form comprising zoledronic
acid
having a dose of zoledronic acid and a configuration suitable for a particular
species of mammal,
wherein the oral dosage form is configured so that the elimination half-life
of zoledronic acid in the
particular species of mammal is about 30 hours to about 100 hours.
[013] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the zoledronic acid is present in the oral
dosage form in an
amount that results in an AUC of zoledronic acid of about 50 ng=hr/mL to about
700 ng=hr/mL upon
administration of the oral dosage form to the mammal.
[014] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the zoledronic acid is present in the oral
dosage form in an
amount that results in a Cmõ of zoledronic acid of about 5 ng/mL to about 300
ng/mL upon
administration of the oral dosage form to the mammal.
[015] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
2
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
mammal in need thereof, wherein the oral dosage form is configured so that
administration of the
oral dosage form to the particular species of mammal results in a Tmax of
zoledronic acid of about
0.4 hr to about 1 hr.
[016] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
zoledronic acid has
a 12 hour sustained plasma level factor of about 12 to about 50 for the
mammal.
[017] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
zoledronic acid has
a 24 hour sustained plasma level factor of about 10 to about 30 for the
mammal.
[018] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
zoledronic acid has
a 36 hour sustained plasma level factor of about 6 to about 20 for the mammal.
[019] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
zoledronic acid has
a 48 hour sustained plasma level factor of about 5 to about 20 for the mammal.
[020] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
zoledronic acid has
a 72 hour sustained plasma level factor of about 4 to about 20 for the mammal.
[021] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
particular species
of mammal has a plasma concentration of zoledronic acid at 12 hours that is
about 0.5 ng/mL to
about 5 ng/mL.
[022] Some embodiments include a method of treating a disease or condition
related to
bone, cancer, or pain, comprising administering an oral dosage form comprising
zoledronic acid to a
mammal in need thereof, wherein the oral dosage form is configured so that the
elimination half-life
of zoledronic acid in the particular species of mammal is about 30 hours to
about 100 hours.
3
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[023] Some embodiments include a method of enhancing the oral
bioavailability of
zoledronic acid comprising orally administering a dosage form containing
zoledronic acid in the
disodium salt form.
[024] Some embodiments include a dosage form comprising zoledronic acid in
the
disodium salt form, wherein the bioavailability, in a mammal, of zoledronic
acid in the disodium salt
form is greater than the bioavailability of zoledronic acid in the diacid form
would be in the same
dosage form.
[025] Some embodiments include a dosage form comprising zoledronic acid in
the
disodium salt form, wherein the dosage form contains an amount of zoledronic
acid in the disodium
salt form that provides an area under the plasma concentration curve of
zoledronic acid of about 4
ng=h/mL to about 2000 ng=h/mL to a human being to which the dosage form is
administered.
[026] Some embodiments include a dosage form comprising zoledronic acid in
the
disodium salt form, wherein the disodium salt form is present in a lower molar
amount than would be
present if the zoledronic acid were in the diacid form; and wherein the
zoledronic acid in the
disodium salt form has an improved bioavailability as compared to the
zoledronic acid in the diacid
form to the extent that the lower molar amount of the disodium salt in the
dosage form does not
reduce the amount of zoledronic acid delivered to the plasma of a mammal.
[027] Although an oral dosage form with enhanced bioavailability with
respect to the
bisphosphonate compound can be used, the treatment can also be effective using
an oral dosage
form that includes a bisphosphonate compound, such as zoledronic acid, wherein
the bioavailability
of the bisphosphonate is unenhanced, or is substantially unenhanced.
[028] Some embodiments include a method of relieving inflammatory pain
comprising
administering an oral dosage form containing zoledronic acid to a mammal in
need thereof, wherein
the mammal experiences significant pain relief more than 3 hours after
administration of the dosage
form.
[029] Some embodiments include a method of relieving pain associated with
an
arthritis comprising administering an oral dosage form containing zoledronic
acid to a human being
in need thereof.
[030] Some embodiments include a method of treating complex regional pain
syndrome comprising administering an oral dosage form containing zoledronic
acid to a mammal in
need thereof.
[031] Some embodiments include an oral dosage form comprising zoledronic
acid,
wherein the oral bioavailability of zoledronic acid is substantially
unenhanced. For example, in
some embodiments, the oral bioavailability in the dosage form is about 0.01%
to about 4%.
4
CA 2928350 2017-05-10
81796381
[032] Some embodiments include a pharmaceutical product comprising more
than one unit of an oral dosage form described herein. In some embodiments,
each
unit of the oral dosage form contains about 1 mg to about 50 mg of zoledronic
acid.
[033] Some embodiments include a method of relieving inflammatory pain
comprising administering an oral dosage form containing zoledronic acid to a
mammal in need thereof.
[034] In some embodiments, the mammal receives a total monthly dose of
zoledronic acid that is about 800 mg/m2 or less.
[035] In some embodiments, the dosage form contains about 10 mg/m2 to
about 20 mg/m2 based upon the body surface area of the mammal.
[036] Some embodiments include a method of relieving inflammatory pain
comprising orally administering zoledronic acid to a mammal in need thereof.
[037] In some embodiments, about 300 mg/m2 to about 600 mg/m2 of
zoledronic acid is administered per month, based upon the body surface area of
the
mammal.
[038] In some embodiments, about 50 mg/m2 to about 600 mg/m2 of
zoledronic acid is administered per month, based upon the body surface area of
the
mammal.
[038a] The present invention as claimed relates to:
- an oral dosage form that enhances the oral bioavailability of zoledronic
acid in
a mammal, comprising zoledronic acid in the disodium salt form, wherein the
oral
dosage form is free of any enhancer that is not a sodium salt of zoledronic
acid; and
- use of an oral dosage form of the invention for the treatment of pain in a
human being in need thereof.
5
CA 2928350 2017-05-10
81796381
BRIEF DESCRIPTION OF DRAWINGS
[039] FIG. 1 is a plot of pain compression thresholds in a rat model of
inflammatory pain using three different doses of zoledronic acid. Measurements
were taken at baseline (BL) and at various time points after dosing on the
days
indicated.
[040] FIG. 2A is a graph depicting reversal of arthritis pain for two
different
doses of zoledronic acid in a rat model of arthritis pain.
[041] FIG. 2B is a graph depicting pain thresholds for two different doses of
zoledronic acid in a rat model of arthritis pain.
[042] FIG. 3 is a graph summarizing the results for vehicle and zoledronic
acid treated rats in a rat model of complex regional pain syndrome.
[043] FIG. 4 depicts hindpaw pain thresholds for vehicle and zoledronic acid
treated rats in a rat model of complex regional pain syndrome.
[044] FIG. 5 depicts weight bearing for vehicle and zoledronic acid treated
rats in a rat model of complex regional pain syndrome.
[045] FIG. 6 depicts paw thickness change for vehicle and zoledronic acid
treated rats in a rat model of complex regional pain syndrome.
5a
CA 02928350 2016-10-14
51432-218S0 .
[044] FIG. 5 depicts weight bearing for vehicle and zoledronic acid treated
rats in a rat model of complex regional pain syndrome.
[045] FIG. 6 depicts paw thickness change for vehicle and zoledronic acid
treated rats in a rat model of complex regional pain syndrome.
=
-
=
5b
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[046] FIG. 7 depicts the aqueous solubility of disodium zoledronate
tetrahydrate as
compared to the diacid form of zoledronic acid.
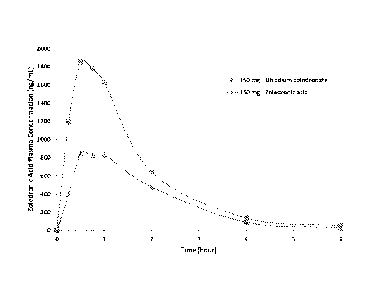
[047] FIG. 8 depicts the plasma concentration of zoledronic acid in dogs
over time after
administration of 150 mg of the disodium salt form of zoledronic acid and the
diacid form of
zoledronic acid.
[048] FIG. 9 depicts the compressibility of dosage forms containing
zoledronic acid in
the disodium salt form as compared to the diacid form.
DETAILED DESCRIPTION
[049] Bisphosphonate compounds such as pamidronate or pamidronic acid,
neridronate or neridronic acid, olpadronate or olpadronic acid, alendronate or
alendronic acid,
incadronate or incadronic acid, ibandronate or ibandronic acid, risedronate or
risedronic acid,
zoledronate or zoledronic acid, etidronate or etidronic acid, clodronate or
clodronic acid, tiludronate
or tiludronic acid, etc., may be used for a number of medical purposes, such
as treatment of
undesirable conditions or diseases, including pain relief. This may be
accomplished in many
instances by administration of oral dosage forms. Generally, an oral dosage
form comprising a
bisphosphonate such as zoledronic acid is administered orally to a mammal,
such as a human
being, at least once, to treat a disease or condition, or to relieve pain.
[050] The term "treating" or "treatment" broadly includes any kind of
treatment activity,
including the diagnosis, cure, mitigation, or prevention of disease in man or
other animals, or any
activity that otherwise affects the structure or any function of the body of
man or other animals.
[051] An oral dosage form of a bisphosphonate such as zoledronic acid may
be used to
treat, or provide relief of, any type of pain including, but not limited to,
inflammatory pain, arthritis
pain, complex regional pain syndrome, lumbosacral pain, musculoskeletal pain,
neuropathic pain,
chronic pain, cancer-related pain, acute pain, postoperative pain, etc. In
some instances, pain
relief may be palliative, or pain relief may be provided independent of
improvement of the disease or
condition or the underlying cause of the disease or condition. For example,
although the underlying
disease may not improve, or may continue to progress, an individual suffering
from the disease may
experience pain relief. In some embodiments, enhanced bioavailability of the
zoledronic acid may
be achieved in treating one of these conditions by administering a dosage form
comprising
zoledronic acid in the form of a disodium salt. This may allow a reduced molar
amount of the
disodium salt to be used as compared to what would be used with the diacid
form.
[052] In some embodiments, the mammal being treated is not suffering from
bone
metastasis. In some embodiments, the mammal being treated is not suffering
from cancer. In
some embodiments, the mammal being treated is not suffering from osteoporosis.
6
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[053] For example, zoledronic acid or another bisphosphonate may be
administered
orally to relieve musculoskeletal pain including low back pain, and pain
associated with rheumatoid
arthritis, juvenile rheumatoid arthritis, osteoarthritis, erosive
osteoarthritis, sero-negative (non-
rheumatoid) arthropathies, non-articular rheumatism, peri-articular disorders,
axial spondyloarthritis
including ankylosing spondylitis, Paget's disease, fibrous dysplasia, SAPHO
syndrome, transient
osteoarthritis of the hip, vertebral crush fractures, osteoporosis, etc. In
some embodiments,
enhanced bioavailability of the zoledronic acid may be achieved in treating
one of these conditions
by administering a dosage form comprising zoledronic acid in the form of a
disodium salt. This may
allow a reduced molar amount of the disodium salt to be used as compared to
what would be used
with the diacid form.
[054] A bisphosphonate, such as zoledronic acid, may also be used to treat
bone
fractures or to enhance the healing of bone fractures.
[055] In some embodiments, zoledronic acid or another bisphosphonate may
also be
administered orally to relieve neuropathic pain, including diabetic peripheral
neuropathy, post-
herpetic neuralgia, trigeminal neuralgia, monoradiculopathies, phantom limb
pain, and central pain.
Other causes of neuropathic pain include cancer-related pain, lumbar nerve
root compression,
spinal cord injury, post-stroke pain, central multiple sclerosis pain, HIV-
associated neuropathy, and
radio-therapy or chemo-therapy associated neuropathy. In
some embodiments, enhanced
bioavailability of the zoledronic acid may be achieved in treating one of
these conditions by
administering a dosage form comprising zoledronic acid in the form of a
disodium salt. This may
allow a reduced molar amount of the disodium salt to be used as compared to
what would be used
with the diacid form.
[056] In some embodiments, zoledronic acid or another bisphosphonate may be
administered orally to relieve inflammatory pain including musculoskeletal
pain, arthritis pain, and
complex regional pain syndrome. In some embodiments, enhanced bioavailability
of the zoledronic
acid may be achieved in treating one of these conditions by administering a
dosage form comprising
zoledronic acid in the form of a disodium salt. This may allow a reduced molar
amount of the
disodium salt to be used as compared to what would be used with the diacid
form.
[057] Examples of musculoskeletal pain include low back pain; and pain
associated
with vertebral crush fractures, fibrous dysplasia, osteogenesis imperfecta,
Paget's disease of bone,
transient osteoporosis, and transient osteoporosis of the hip.
[058] A bisphosphonate, such as zoledronic acid, may also be used to treat
low back
pain, or other musculoskeletal or inflammatory conditions, having a change in
bone that is
detectable by MRI or another medical imaging instrument. For example, a
bisphosphonate, such as
zoledronic acid, may be used to treat low back pain associated Modic changes,
or vertebral
7
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
endplate signal changes (VESC) and bone marrow changes visible using magnetic
resonance
imaging (MRI). Modic changes, can be classified into various types including
type 1 (M1), type 2
(M2), and type 3 (M3) lesions or changes, any of which may be treated using a
bisphosphonate
such as zoledronic acid. VESCs may be found in patients with different types
of low back pain
including but not limited to spondylitis, trauma, spondyloarthropathies
including ankylosing
spondylitis, Schmorl's nodes, fracture, tumor, and spinal cord infarction.
Lesions in ankylosing
spondylitis include osteitis and spondylodiscitis which can be detected using
MRI or another medical
imaging instrument.
[059] A bisphoshosphonate, such as zoledronic acid may also be used to
treat
osteoarthritis of the knee, such as osteoarthritis of the knee associated with
bone marrow lesions
(BML), including BML that may be detected using MRI or another medical imaging
instrument. In
some embodiments, a bisphosphonate, such as zoledronic acid, may be used to
treat osteoarthritis
of the knee associated with bone marrow edema (BME), including BME which may
be detected
using MRI or another medical imaging instrument.
[060] Arthritis refers to inflammatory joint diseases that can be
associated with pain.
Examples of arthritis pain include pain associated with osteoarthritis,
erosive osteoarthritis,
rheumatoid arthritis, juvenile rheumatoid arthritis, sero-negative (non-
rheumatoid) arthropathies,
non-articular rheumatism, peri-articular disorders, neuropathic arthropaties
including Charcot's foot,
axial spondyloarthritis including ankylosing spondylitis, and SAPHO syndrome.
[061] In some embodiments, a human being that is treated for a disease or
condition,
such as an inflammatory condition, e.g. arthritis, by an oral dosage form of
zoledronic acid, has an
age of about 10 years to about 90 years, about 20 years to about 80 years,
about 30 years to about
75 years old, about 40 years to about 70 years, about 1 year to about 16
years, or about 80 years to
about 95 years.
[062] In some embodiments, a human being that is treated for a disease or
condition,
such as an inflammatory condition, e.g. arthritis, by an oral dosage form of
zoledronic acid, has
suffered from the arthritis for at least 1 month, at least 2 months, at least
6 months, or at least 1
year.
[063] In some embodiments, the disease or condition, such as an
inflammatory
condition, e.g. arthritis, affects a knee, an elbow, a finger, a wrist, a
shoulder, or a hip.
[064] In some embodiments, zoledronic acid or another bisphosphonate may be
administered orally to relieve complex regional pain syndrome, such as complex
regional pain
syndrome type I (CRPS-I), complex regional pain syndrome type ll (CRPS-II),
CRPS-NOS, or
8
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
another type of CRPS. CRPS is a type of inflammatory pain. CRPS can also have
a neuropathic
component.
[065] Complex regional pain syndrome is a debilitating pain syndrome. It is
characterized by severe pain in a limb accompanied by edema, and autonomic,
motor and sensory
changes.
[066] With respect to use of oral zoledronic acid for relieving pain
associated with an
inflammatory condition, relief of pain can be short-term, e.g. for a period of
hours after
administration of the dosage form, and/or relief of pain can be long-term,
e.g. lasting for days,
weeks, or even months after oral administration of zoledronic acid. In some
embodiments, a
mammal, such as a human being, experiences significant pain relief at least
about 3 hours, at least
about 6 hours, at least about 12 hours, at least about 24 hours, at least
about 48 hours, at least
about one week, at least about 2 weeks, or at least about 3 weeks after
administration of an oral
dosage form comprising zoledronic acid. In some embodiments, a mammal, such as
a human
being, experiences significant pain relief during at least part of the time
from about 3 hours to about
2 weeks, about 3 hours to about 3 weeks, about 3 hours to about 24 hours,
about 6 hours to about
2 weeks, or about 6 hours to about 24 hours, about 3 days to about 2 weeks,
about 6 days to about
2 weeks, after administration of an oral dosage form comprising zoledronic
acid.
[067] With respect to the treatment of any condition recited herein, in
some
embodiments a first oral dosage form comprising zoledronic acid is
administered and a second oral
dosage form comprising oral zoledronic acid is administered. The timing of the
administration of the
two dosage forms may be such that, with respect to the first oral dosage form,
the second oral
dosage with respect to the first oral dosage form, the second oral dosage form
is administered at 5 x
Tmax or greater (e.g., if Tmax is 1 hour, at 5 hours or later), at least 10 x
Tmax or greater, at least about
15 x Tõõ or greater, at least about 20 x Tõõ or greater, at least about 50 x
Tmax or greater, or at
least about 200 x Tmax or greater, wherein Tmax is the time of maximum plasma
concentration for the
first oral dosage form.
[068] Some embodiments include treatment of a condition recited herein,
such as
inflammatory pain, arthritis, or complex regional pain syndrome, wherein the
treatment comprises
either: administering only one dosage form to a mammal to treat the condition,
or administering a
first dosage form to the mammal, followed by administering a second dosage
form to the mammal.
If two or more dosage forms are administered, the second oral dosage form is
administered before
the maximum pain relieving effect of the first oral dosage form is achieved,
or before a peak in the
pain relieving effect of the first oral dosage form is experienced by a
mammal, receiving the dosage
form. In some embodiments, the second oral dosage form is administered before
an observable
pain relieving effect is achieved. In some embodiments, the second dosage form
is administered
9
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
about 12 hours to about 60 days, about 24 hours to about 28 days, about 24
hours to about 7 days,
about 24 hours to about 14 days, or about 24 hours to about 21 days, after the
first dosage form is
administered.
[069] Some embodiments include treatment of a condition recited herein,
such as
inflammatory pain, arthritis, or complex regional pain syndrome, wherein the
treatment comprises
administering a first dosage form to the mammal, followed by administering a
second dosage form
to the mammal, wherein the second dosage form is administered after the
maximum pain relieving
effect of the first oral dosage form is achieved, and the second oral dosage
form is administered
while the mammal is still experiencing pain relief from the first oral dosage
form, or while the pain
relieving effect from the first oral dosage form is observable. In some
embodiments, the second
dosage form is administered about 12 hours to about 60 days, about 24 hours to
about 28 days,
about 24 hours to about 7 days, about 24 hours to about 14 days, or about 24
hours to about 21
days, after the first dosage form is administered.
[070] Zoledronic acid or another bisphosphonate may also be administered
orally to
relieve cancer-related pain, including pain associated with multiple myeloma
and bone metastases
from solid tumors. In some embodiments, zoledronic acid is used to treat pain
that is not cancer-
related pain. For example, zoledronic acid may be used to treat pain that is
not associated with
multiple myeloma, bone metastasis from solid tumors, hypercalcemia of
malignancy, giant cell
tumor of bone, blood cancers or leukemias, or solid tumors or cancers. In some
embodiments,
enhanced bioavailability of the zoledronic acid may be achieved in treating
one of these conditions
by administering a dosage form comprising zoledronic acid in the form of a
disodium salt. This may
allow a reduced molar amount of the disodium salt to be used as compared to
what would be used
with the diacid form.
[071] In addition to relieving pain, oral administration of zoledronic acid
or another
bisphosphonate may also be useful to treat diseases or conditions that may or
may not include a
pain component. For example, zoledronic acid or another bisphosphonate may be
useful to treat
any of the pain conditions or types of conditions listed above, including
treatment that does not
simply relieve the pain of those conditions, and treatment that is carried out
in such a way that the
condition is treated without pain relief occurring. In
addition to any pain relief zoledronic acid or
another bisphosphonate may or may not provide, zoledronic acid or another
bisphosphonates may
be used to treat a disease or condition such as a metabolic disease or
condition; an inflammatory
disease or condition, including an inflammatory disease or condition that is
not associated with pain;
a cancer disease or condition; a neurological disease or condition; etc. In
some embodiments,
enhanced bioavailability of the zoledronic acid may be achieved in treating
one of these conditions
by administering a dosage form comprising zoledronic acid in the form of a
disodium salt. This may
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
allow a reduced molar amount of the disodium salt to be used as compared to
what would be used
with the diacid form.
[072] In some embodiments, oral administration of zoledronic acid or
another
bisphosphonate may also be useful to treat complex regional pain syndrome,
rheumatoid arthritis,
osteoarthritis, erosive osteoarthritis, axial spondyloarthritis including
ankylosing spondylitis, acute
vertebral crush fracture, fibrous dysplasia, SAPHO syndrome, osteoporosis,
transient osteoporosis,
or transient osteoporosis of the hip. In some embodiments, enhanced
bioavailability of the
zoledronic acid may be achieved in treating one of these conditions by
administering a dosage form
comprising zoledronic acid in the form of a disodium salt. This may allow a
reduced molar amount
of the disodium salt to be used as compared to what would be used with the
diacid form.
[073] In some embodiments, oral administration of zoledronic acid or
another
bisphosphonate may also be useful to treat hypercalcemia of malignancy,
multiple myeloma, bone
metastases from solid tumors, Paget's disease of bone, giant cell tumor of
bone, blood cancers or
leukemias, or solid tumors or cancers. In some embodiments, enhanced
bioavailability of the
zoledronic acid may be achieved in treating one of these conditions by
administering a dosage form
comprising zoledronic acid in the form of a disodium salt. This may allow a
reduced molar amount
of the disodium salt to be used as compared to what would be used with the
diacid form.
[074] In some embodiments, a bisphosphonate such as zoledronic acid may be
used to
treat otosclerosis.
[075] Zoledronic acid has the structure shown below, and is also referred
to as
zoledronate.
P
OH
N
Oki
HO P
ir\ OH
0
Zoledronic acid
[076] Unless otherwise indicated, any reference to a compound herein, such
as
zoledronic acid, by structure, name, or any other means, includes
pharmaceutically acceptable
salts, such as the disodium salt; alternate solid forms, such as polymorphs,
solvates, hydrates, etc.;
11
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
tautomers; or any other chemical species that may rapidly convert to a
compound described herein
under conditions in which the compounds are used as described herein.
[077] In some embodiments, zoledronic acid is administered in a dosage form
comprising a salt form, such as a salt of a dianion of zoledronic acid. In
some embodiments,
zoledronic acid is administered in a dosage form comprising a disodium salt
form of zoledronic acid.
In some embodiments, zoledronic acid is administered in a sodium salt form,
such as a
monosodium salt, a disodium salt, a trisodium salt, etc. In some
circumstances, use of the disodium
salt may be desirable. For example, the disodium salt is much more soluble in
water than the diacid
form. As a result, in some processes, the disodium salt can be easier to work
with than the diacid
form. Additionally, the sodium salt may be more bioavailable and/or more
rapidly absorbed when
taken orally as compared to the diacid form.
[078] Some oral dosage forms comprising zoledronic acid have a dose of
zoledronic
acid and a configuration suitable for a particular species of mammal, e.g.
dog, rat, human, etc.
Such a dosage form may have zoledronic acid present in an amount that results
in a desired range
for an area under the plasma concentration curve (AUG) of zoledronic acid in
that particular species
of mammal. For example the dose of zoledronic acid and a configuration of the
oral dosage form
may result in an AUC of zoledronic acid of about 1 ng=hr/mL to about 700
ng=hr/mL, about 3
ng-hr/mL to about 30 ng=hr/mL, about 3 ng=hr/mL to about 10 ng-hr/mL, about 50
ng-hr/mL to about
700 ng=hr/mL, about 130 ng=hr/mL to about 180 ng=hr/mL, about 300 ng=hr/mL to
about 450
ng=hr/mL, about 300 ng=hr/mL to about 350 ng=hr/mL, about 300 ng=hr/mL to
about 310 ng=hr/mL,
about 340 ng=hr/mL to about 350 ng-hr/mL, about 370 ng-hr/mL to about 420 ng-
hr/mL, about 380
ng=hr/mL to about 390 ng=hr/mL, about 405 ng=hr/mL to about 415 ng=hr/mL,
about 140 ng=hr/mL to
about 160 ng=hr/mL, about 140 ng=hr/mL to about 150 ng=hr/mL, about 150
ng=hr/mL to about 160
ng=hr/mL, about 140 ng=hr/mL, 142 ng=hr/mL, about 155 ng=hr/mL, about 305
ng=hr/mL, 304
ng=hr/mL, about 345 ng=hr/mL, 343 ng=hr/mL, about 385 ng=hr/mL, 384 ng=hr/mL,
about 410
ng=hr/mL, or any AUG in a range bounded by, or between, any of these values,
upon administration
of the oral dosage form to a mammal.
[079] Unless otherwise indicated, the AUG refers to the AUG calculated to
the last
measured concentration (AUC(0_0) and extrapolated to infinity (AUC(o_inf)).
[080] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may have
zoledronic acid present in
an amount that results in a Cmax of zoledronic acid of about 0.2 ng/mL to
about 300 ng/mL, about 0.5
ng/mL to about 5 ng/mL, about 5 ng/mL to about 300 ng/mL, about 5 ng/mL to
about 50 ng/mL,
about 20 ng/mL to about 50 ng/mL, about 30 ng/mL to about 50 ng/mL, about 50
ng/mL to about
200 ng/mL, about 50 ng/mL to about 150 ng/mL, about 80 ng/mL to about 120
ng/mL, about 90
12
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
ng/mL to about 100 ng/mL, about 50 ng/mL to about 200 ng/mL, about 40 ng/mL,
about 95 ng/mL,
about 97 ng/mL, or any Cmax in a range bounded by, or between, any of these
values, upon
administration of the oral dosage form to a mammal.
[081] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that
administration of the oral dosage form to the particular species of mammal
results in a Tõ,, of
zoledronic acid of about 0.4 hr to about 1 hr, about 0.5 hr, or about 0.75 hr,
or any Tõõ in a range
bounded by, or between, any of these values.
[082] The oral bioavailability of zoledronic acid may be enhanced by orally
administering the zoledronic acid in the disodium salt form. For example, the
bioavailability of
zoledronic acid may be improved by at least about 10%, at least about 20%, at
least about 30%, at
least about 50%, and/or up to about 100%, or up to about 200%, as compared to
administration of
zoledronic acid in the diacid form.
[083] Because of the improved bioavailability of the disodium salt a dosage
form may
contain, or a mammal, such as a human being, may receive, on a molar basis,
less of the disodium
salt form of zoledronic acid than would otherwise be administered of the
diacid form of zoledronic
acid. For example, a dosage form may contain, or a mammal may receive, at
least about 10 mole%
less, at least about 20 mole% less, at least about 40 mole% less, at least
about 50 mole% less,
and/or up to about 90 mole% less or 95 mole% less, of the disodium salt form
as compared the
amount of the diacid form of zoledronic acid that would otherwise be
administered, such as a molar
amount that would be administered of zoledronic acid in the diacid form in
order to achieve the
same plasma levels of zoledronic acid.
[084] In some embodiments, a dosage form contains, or a mammal (such as a
human
being) is administered, an amount of the disodium salt form, on a molar basis,
that has a value of
about 0.8nd to about 1.2nd or about 0.9nd to about 1.1nd, wherein:
nd = (ba/ba)(na)
wherein 132 is the bioavailability of the diacid form, bd is the
bioavailability of the disodium salt form,
and na is the number of moles of the diacid that would be administered in a
dosage form containing
the diacid form of zoledronic acid. For example, if the diacid form has a
bioavailability (ba) of 0.01
and the disodium salt form has a bioavailabity (bd) of 0.015, and a dosage
form would normally
contain 0.001 moles of the diacid, nd would be (0.01/0.015)(0.001 moles), or
about 0.00067 moles.
In some embodiments, the disodium salt is administered in an amount that has a
value of about
[085] With respect to oral dosage forms comprising a reduced molar amount
of the
disodium salt of zoledronic acid as compared to the diacid form of zoledronic
acid, in some
13
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
embodiments, the bioavailability of the zoledronic acid in the disodium salt
form is sufficiently high
that, if the drug is administered to a mammal, at least as much zoledronic
acid is present in the
blood of the mammal as would be present if zoledronic acid were administered
in the diacid form.
[086] With respect to oral dosage forms comprising the disodium salt form
of zoledronic
acid, in some embodiments, the disodium salt form is present in a lower molar
amount than would
be present if the zoledronic acid were in the diacid form; and the zoledronic
acid in the disodium salt
form has an improved bioavailability as compared to the zoledronic acid in the
diacid form to the
extent that the lower molar amount of the disodium salt in the dosage form
does not reduce the
amount of zoledronic acid delivered to the plasma of a mammal.
[087] In some embodiments, the zoledronic acid in the disodium salt form is
present in
an amount such that the oral dosage form provides an area under the plasma
concentration curve
(AUC) of zoledronic acid of about 4 ng=h/mL to about 2000 ng=h/mL to the
mammal each time the
zoledronic acid in the disodium salt is administered. In some embodiments, the
zoledronic acid in
the disodium salt form is present in an amount such that the oral dosage form
provides an area
under the plasma concentration curve of zoledronic acid of about 100 ng=h/mL
to about 2000
ng-h/mL, about 100 ng=h/mL to about 1000 ng=h/mL, about 500 ng=h/mL to about
1000 ng=h/mL, or
about 500 ng=h/mL to about 700 ng=h/mL in the mammal to which the dosage form
is administered.
This amount may be suitable for administration of the oral dosage form about
every 3 to 4 weeks.
[088] In some embodiments, the zoledronic acid in the disodium salt form is
present in
an amount such that the oral dosage form provides an area under the plasma
concentration curve
of zoledronic acid of about 20 ng=h/mL to about 700 ng=h/mL, about 50 ng=h/mL
to about 500
ng=h/mL, or about 100 ng=h/mL to about 200 ng=h/mL, in the mammal to which the
dosage form is
administered. This amount may be suitable for weekly administration of the
oral dosage, or for
administration of 3 to 5 individual dosages during a month. The individual
dosages could be given
at regular intervals, given during the first week, or at any other schedule
that provides 3 to 5
dosages during the month.
[089] In some embodiments, the zoledronic acid in the disodium salt form is
present in
an amount such that the oral dosage form provides an area under the plasma
concentration curve
of zoledronic acid of about 4 ng=h/mL to about 100 ng=h/mL, about 10 ng=h/mL
to about 50 ng=h/mL,
or about 10 ng=h/mL to about 30 ng=h/mL, in the mammal to which the dosage
form is administered.
This amount may be suitable for daily administration of the oral dosage form.
[090] Oral administration of zoledronic acid, particularly oral
administration of the
disodium salt form of zoledronic acid, can result in more sustained plasma
levels of the drug as
compared to parenteral modes of administration, such intravenous or
subcutaneous. For example,
the amount of zoledronic acid in the plasma can be significantly higher for
oral administration of the
14
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
disodium salt about 24 hours or 48 hours, or longer, after administration. In
some embodiments,
oral zoledronic acid has a 24 hour sustained plasma level factor of about 1 or
higher, such as about
1 to about 10, about 1 to about 5, about 3 to about 5, or about 3 to about 4.
In some embodiments,
an orally administered dosage form of zoledronic acid has a 24 hour sustained
plasma level factor
or a 48 hour sustained plasma level factor that is higher, such as at least
1.2 times, at least about 2
times, at least about 5 times, about 1.2 times to about 20 times, about 2
times to about 15 times,
about 5 times to about 10 times, or about 8 to about 15 times that of
intravenously administered
zoledronic acid. A "sustained plasma level factor," pf, is determined by the
equation:
pf = 1000 (Ct/Cma.)
wherein Cm2x is the maximum plasma concentration of zoledronic acid after it
is administered and Ct
is the plasma concentration of zoledronic acid at the time of interest, such
as 24 hours. For
parenteral administration, the Cmax can be about the Co, or the concentration
right after injection of
the entire amount of the drug into the body. Sustained plasma level factors
can also be obtained for
other times, such as 48 hours, by using the plasma concentration of zoledronic
acid for Ct in the
equation above. For example, if the maximum plasma level of zoledronic acid
after administration is
1000 ng/mL and the plasma level of zoledronic acid at 24 hours is 1 ng/mL, the
24 hour sustained
plasma level factor is 1.
[091] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
zoledronic acid has a 12 hour sustained plasma level factor of about 12 to
about 50, about 20 to
about 40, about 25 to about 30, about 30 to about 35, about 35 to about 40,
about 33, about 30,
about 35, or any 12 hour sustained plasma level factor in a range bounded by,
or between, any of
these values, for the particular species of mammal.
[092] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
zoledronic acid has a 24 hour sustained plasma level factor of about 10 to
about 30, about 10 to
about 20, about 10 to about 15, about 12 to about 15 or 16, about 15 to about
20, about 14, about
12, about 15, or any 24 hour sustained plasma level factor in a range bounded
by, or between, any
of these values, for the particular species of mammal.
[093] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
zoledronic acid has a 36 hour sustained plasma level factor of about 6 to
about 20, about 8 to about
15, about 9 to about 12 or 13, about 8 to about 10, about 11 to about 13,
about 9, about 13, or any
24 hour sustained plasma level factor in a range bounded by, or between, any
of these values, for
the particular species of mammal.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[094] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
zoledronic acid has a 48 hour sustained plasma level factor of about 5 to
about 20, about 6 to about
15, about 7 or 8 to about 12 or 13, about 8 to about 10, about 11 to about 13,
about 8, about 12, or
any 48 hour sustained plasma level factor in a range bounded by, or between,
any of these values,
for the particular species of mammal.
[095] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
zoledronic acid has a 72 hour sustained plasma level factor of about 4 to
about 20, about 5 to about
10, about 5 or 6 to about 10 or 11, about 5 to about 6, about 9 to about 10,
about 6, about 10, or
any 72 hour sustained plasma level factor in a range bounded by, or between,
any of these values,
for the particular species of mammal.
[096] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
particular species of mammal has a plasma concentration of zoledronic acid at
12 hours that is
about 0.5 ng/mL to about 5 ng/mL, about 1 ng/mL to about 3 ng/mL, about 1
ng/mL to about 2
ng/mL, about 2 ng/mL to about 3 ng/mL, about 3 ng/mL to about 4 ng/mL, about
1.2 ng/mL, about
2.6 ng/mL, about 3.2 ng/mL, or any plasma concentration in a range bounded by,
or between, any
of these values.
[097] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
particular species of mammal has a plasma concentration of zoledronic acid at
24 hours that is
about 0.2 ng/mL to about 2 ng/mL, about 0.5 ng/mL to about 1.5 ng/mL, about
0.5 ng/mL to about 1
ng/mL, about 1 ng/mL to about 1.5 ng/mL, about 0.5 ng/mL, about 1.0 ng/mL,
about 1.4 ng/mL, or
any plasma concentration in a range bounded by, or between, any of these
values.
[098] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
particular species of mammal has a plasma concentration of zoledronic acid at
36 hours that is
about 0.1 ng/mL to about 2 ng/mL, about 0.2 ng/mL to about 1.5 ng/mL, about
0.2 ng/mL to about
0.5 ng/mL, about 0.5 ng/mL to about 1 ng/mL, about 1 ng/mL to about 1.3 ng/mL,
about 0.3 ng/mL,
about 0.8 ng/mL, about 1.1 ng/mL, or any plasma concentration in a range
bounded by, or between,
any of these values.
[099] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
particular species of mammal has a plasma concentration of zoledronic acid at
48 hours that is
16
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
about 0.1 ng/mL to about 2 ng/mL, about 0.2 ng/mL to about 1.5 ng/mL, about
0.2 ng/mL to about
0.5 ng/mL, about 0.5 ng/mL to about 0.9 ng/mL, about 0.9 ng/mL to about 1.3
ng/mL, about 0.3
ng/mL, about 0.7 ng/mL, about 1.1 ng/mL, or any plasma concentration in a
range bounded by, or
between, any of these values.
[0100] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
particular species of mammal has a plasma concentration of zoledronic acid at
72 hours that is
about 0.2 ng/mL to about 1 ng/mL, about 0.2 ng/mL to about 1.5 ng/mL, about
0.1 ng/mL to about
0.3 ng/mL, about 0.3 ng/mL to about 0.6 ng/mL, about 0.6 ng/mL to about 1
ng/mL, about 0.2
ng/mL, about 0.5 ng/mL, about 0.9 ng/mL, or any plasma concentration in a
range bounded by, or
between, any of these values.
[0101] An oral dosage form comprising zoledronic acid having a dose of
zoledronic acid
and a configuration suitable for a particular species of mammal may be
configured so that the
elimination half-life of zoledronic acid in the particular species of mammal
is about 30 hours to about
100 hours, about 40 hours to about 60 hours, about 40 hours to about 50 hours,
about 50 hours to
about 60 hours, about 42 hours, about 51 hours, about 59 hours, or any half-
life in a range bounded
by, or between, any of these values.
[0102] As used herein, the "elimination half-life" refers to the
apparent first-order terminal
plasma elimination half-life, obtained by non-compartmental analysis using Win-
Nonlin. A terminal
plasma elimination half-life is the time required to reduce the plasma
concentration to half after
reaching pseudo-equilibrium, and not the time required to eliminate half the
administered dose. For
orally administered drugs, terminal plasma elimination half-life can be
affected by absorption of the
drug, as well as plasma clearance and extent of distribution.
[0103] In some embodiments, the disodium salt form of zoledronic acid
provides an
enhancement to bioavailability, as compared to the diacid form of zoledronic
acid, which adds to any
enhancement to bioavailability provided by any bioavailability-enhancing
agents in the dosage form.
In some embodiments, the disodium salt form of zoledronic acid provides an
enhancement to
bioavailability, as compared to the diacid form of zoledronic acid, which is
greater than any
enhancement to bioavailability provided by any bioavailability-enhancing
agents in the dosage form.
In some embodiments, the disodium salt form of zoledronic acid may be
administered in a dosage
form that is substantially free of bioavailability-enhancing agents.
[0104] In some embodiments, a dosage form comprising a disodium salt of
zoledronic
acid is a solid.
17
CA 02928350 2016-10-14
51432-218S0
[0105] In some embodiments, a dosage form comprising a disodium salt of
zoledronic
acid is used to treat an inflammatory condition.
[0106] In some embodiments, a dosage form comprising a disodium salt of
zoledronic
acid is used to treat arthritis.
[0107] In some embodiments, a dosage form comprising a disodium salt of
zoledronic
acid is used to treat complex regional pain syndrome.
[0108] In some embodiments, zoledronic acid is in a form that has an
aqueous solubility,
meaning the solubility in water, greater than 1% (w/v), about 5% (w/v) to
about 50% (w/v), about 5%
(w1v) to about 20% (w/v), about 10% (w/v) to about 15% (w/v), or about 12%
(w/v) to about 13%
(w/v).
[0109] The disodium salt form of zoledronic acid can be more
compressible than the
diacid form of zoledronic acid. This ban make it easier for a dosage form to
have a desired
hardness. It can also make it easier to increase the drug load, so that a
smaller tablet can be given
for a given dosage strength. In some embodiments, a solid dosage form of
zoledronic acid, such as
the diacid form of zoledronic acid or the disodium salt form of zoledronic
acid, can have a hardness
of about 5 kPa to about 20 kPa or about 5 kPa to about 14 kPa.
[0110] Zoledronic acid or another bisphosphonate may be combined with a
pharmaceutical carrier selected on the basis of the chosen route of
administration and
standard pharmaceutical practice as described, for example, in Remington's
Pharmaceutical Sciences, 2005. The relative proportions of active ingredient
and
carrier may be determined, for example, by the solubility and chemical nature
of the
compounds, chosen route of administration and standard pharmaceutical
practice.
pm] Zoledronic acid or another bisphosphonate may be administered by
any means
that may result in the contact of the active agent(s) with the desired site or
site(s) of action in the
body of a patient. The compounds may be administered by any conventional means
available for
use in conjunction with pharmaceuticals, either as individual therapeutic
agents or in a combination
of therapeutic agents. For example, they may be administered as the sole
active agents in a
pharmaceutical composition, or they can be used in combination with other
therapeutically, active
ingredients.
[0112] Zoledronic acid or another bisphosphonate may be administered to
a human
patient in a variety of forms adapted to the chosen route of administration,
e.g., orally, rectally, or
parenterally. Parenteral administration in this respect includes, but is not
limited to, administration
by the following routes: pulmonary, intrathecal, intravenous, intramuscular,
subcutaneous,
18 =
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
intraocular, intrasynovial, transepithelial including transdermal, sublingual
and buccal; topically;
nasal inhalation via insufflation; and rectal systemic.
[0113] The effective amount of zoledronic acid or another bisphosphonate
will vary
depending on various factors known to the treating physicians, such as the
severity of the condition
to be treated, route of administration, formulation and dosage forms, physical
characteristics of the
bisphosphonate compound used, and age, weight and response of the individual
patients.
[0114] The amount of zoledronic acid or another bisphosphonate in a
therapeutic
composition may vary. For example, some liquid compositions may comprise about
0.0001% (w/v)
to about 50% (w/v), about 0.01% (w/v) to about 20% (w/v), about 0.01% to about
10% (w/v), about
0.001% (w/v) to about 1% (w/v), about 0.1% (w/v) to about 0.5% (w/v), about 1%
(w/v) to about 3%
(w/v), about 3% (w/v) to about 5% (w/v), about 5% (w/v) to about 7% (w/v),
about 7% (w/v) to about
10% (w/v), about 10% (w/v) to about 15% (w/v), about 15% (w/v) to about 20%
(w/v), about 20%
(w/v) to about 30% (w/v), about 30% (w/v) to about 40% (w/v), or about 40%
(w/v) to about 50%
(w/v) of zoledronic acid.
[0115] Some solid compositions may comprise at least about 5% (w/w), at
least about
10% (w/w), at least about 20% (w/w), at least about 50% (w/w), at least about
70% (w/w), at least
about 80%, about 10% (w/w) to about 30% (w/w), about 10% (w/w) to about 20%
(w/w), about 20%
(w/w) to about 30% (w/w), about 30% (w/w) to about 50% (w/w), about 30% (w/w)
to about 40%
(w/w), about 40% (w/w) to about 50% (w/w), about 50% (w/w) to about 80% (w/w),
about 50% (w/w)
to about 60% (w/w), about 70% (w/w) to about 75% (w/w), about 70% (w/w) to
about 80% (w/w), or
about 80% (w/w) to about 90% (w/w) of zoledronic acid.
[0116] Some solid dosage forms comprising a bisphosphonate, such as
zoledronic acid,
may contain about 1 mg to about 5 mg or 8 mg of the bisphosphonate, and the
total mass of the
dosage form may be less than about 9 mg, about 10 mg, about 11 mg, or about 15
mg.
[0117] Some solid dosage forms comprising a bisphosphonate, such as
zoledronic acid,
may contain about 5 mg to about 10 mg or 15 mg of the bisphosphonate, and the
total mass of the
dosage form may be less than about 15 mg, about 17 mg, about 21 mg, or about
29 mg.
[0118] Some solid dosage forms comprising a bisphosphonate, such as
zoledronic acid,
may contain about 10 mg to about 50 mg or 80 mg of the bisphosphonate, and the
total mass of the
dosage form may be less than about 77 mg, about 85 mg, about 110 mg, or about
150 mg.
[0119] Some solid dosage forms comprising a bisphosphonate, such as
zoledronic acid,
may contain about 100 mg to about 200 mg or 290 mg of the bisphosphonate, and
the total mass of
the dosage form may be less than about 280 mg, about 315 mg, about 360 mg,
about 430 mg, or
about 570 mg.
19
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0120] Some solid dosage forms comprising a bisphosphonate, such as
zoledronic acid,
may contain about 200 mg to about 300 mg or 430 mg of the bisphosphonate, and
the total mass of
the dosage form may be less than about 430 mg, about 500 mg, about 570 mg,
about 640 mg, or
about 710 mg.
[0121] Any suitable amount of zoledronic acid may be used. Some solid or
liquid oral
dosage forms, or units of oral dosage forms (referred to collectively herein
as "oral dosage form(s)")
may contain about 0.005 mg to about 20 mg, about 0.1 mg to about 10 mg, about
0.5 mg to about
mg, about 0.2 mg to about 5 mg, about 1 mg to about 500 mg, about 1 mg to
about 50 mg, about
1 mg to about 75 mg, about 10 mg to about 250 mg, about 100 mg to about 300
mg, about 20 mg to
about 200 mg, about 20 mg to about 150 mg, about 30 mg to about 100 mg, about
30 mg to about
150 mg, about 1 mg to about 1,000 mg, about 10 mg to about 50 mg, about 10 mg
to about 300 mg,
about 10 mg to about 150 mg, about 10 mg to about 100 mg, about 40 mg to about
150 mg, about
40 mg to about 220 mg, about 10 mg to about 600 mg, about 40 mg to about 600
mg, about 40 mg
to about 2000 mg, about 40 mg to about 800 mg, about 25 mg to about 800 mg,
about 30 mg to
about 800 mg, about 10 mg to about 500 mg, about 50 mg to about 150 mg, about
50 mg, about
100 mg, about 50 mg to about 500 mg, about 100 mg to about 2000 mg, about 300
mg to about
1500 mg, about 200 mg to about 1000 mg, about 100 mg to about 500 mg, or about
150 mg of
zoledronic acid, or any amount of zoledronic in a range bounded by, or
between, any of these
values. In some embodiments, the oral zoledronic acid is administered daily,
weekly, monthly,
every two or three months, once a year, or twice a year.
[0122] In some embodiments, an oral dosage form may contain about 10
mg/m2 to
about 20 mg/m2, about 15 mg/m2 to about 20 mg/m2, about 18 mg/m2, about 80
mg/m2 to about 150
mg/m2, about 90 mg/m2 to about 150 mg/m2, about 100 mg/m2 to about 150 mg/m2
of zoledronic
acid, or any amount of zoledronic in a range bounded by, or between, any of
these values. All
dosage ranges or amounts expressed in mg/m2 are based upon the body surface
area of the
mammal.
[0123] In some embodiments the daily oral dose of zoledronic acid is
about 0.005 mg to
about 20 mg, about 0.1 mg to about 10 mg, about 0.5 mg to about 10 mg, about
0.2 mg to about 5
mg, or any amount of zoledronic acid in a range bounded by, or between, any of
these values. In
some embodiments, the daily oral dose of zoledronic acid is less than about 35
mg/m2, less than
about 30 mg/m2; less than about 25 mg/m2, about 1 mg/m2 to about 35 rngim2,
about 1 mg/m2 to
about 30 mgirn2, about 1,5 mg/m2 to about 25 mg/m2, about 1.8 mg/m2 to about
20 mg/m2, about 10
mgiem2 to about 20 rnglrn2, about 10 mg/m2 to about 30 mg/m2, about 15 mg/m2
to about 20 mg/m2,
about 18 mg/m2, or any amount of zoledronic acid in a range bounded by, or
between, any of these
values.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[01241 In some embodiments the weekly oral dose of zoledronic acid is
about 1 mg to
about 1000 mg, about 1 mg to about 500 mg, about 10 mg to about 250 mg, about
100 mg to about
300 mg, about 10 mg to about 100 mg, about 10 rng to about 150 rng, about 10
mg to about 100
mg, about 10 mg to about 300 mg, about 20 mg to about 150 mg, or about 30 mg
to about 100 mg.
In some embodiments, the weeky oral dose of zoledronic acid is less than about
250 mg/m2, less
than about 200 moirn2, less than about 175 mg/m2, about 6 mg/m2 to about 250
mgIrn2, about 10
mg/m2 to about 210 mgirn2, about 10 mg/m2 to about 170 nigirn2, about 4 mg/m2
to about 140
mg/m2. about 100 mg/m2 to about 140 mg/m2, about 126 mg/m2, or any amount of
zoledronic acid in
a range bounded by, or between, any of these
values.
The weekly oral dose may be given as a single dose, given once during the
week, or may be given
in 2, 3, 4, 5, 6, or 7 individual doses during the week.
[0125] In some embodiments, the monthly dose of zoledronic acid, or the
amount of
zoledronic acid that is administered over a period of a month, is about 5000
mg or less, about 4000
mg or less, about 3000 mg or less, about 2000 mg or less, about 1000 mg or
less, about 700 mg or
less, about 600 mg or less, about 1 mg to about 4,000 mg, about 1 mg to about
1,000 mg, about 10
mg to about 1000 mg, about 50 mg to about 1000 mg, about 10 mg to about 600
mg, about 40 mg
to about 600 rng. about 50 mg to about 600 rng, or about 100 mg to about 600
mg, about 40 mg to
about 2000 mg, about 40 mg to about 800 mg, about 50 mg to about 800 mg, or
about 100 mg to
about 800 mg, about 40 rng to about 1000 mg, about 50 rng to about 1000 mg, or
about 100 mg to
about 1000 mg, or any monthly dose in a range bounded by, or between, any of
these values. In
some embodiments, the monthly oral dose of zoledronic acid is less than about
1000 mg/m2, less
than about 800 mg/m2, less than about 600 mg/m2, about 10 mg/m2 to about 1000
mg/m2, about 50
mg/m2 to about 800 mg/m2, about 70 mg/m2 to about 700 mg/m2, about 100 mg/m2
to about 700
mg/m2, about 100 mg/m2 to about 600 mg/m2, about 50 mg/m2 to about 200 mg/m2,
about 300
mg/m2 to about 600 mg/m2, about 450 mg/m2 to about 600 mg/m2, about 300 mg/m2
to about 1000
mg/m2, about 400 mg/m2 to about 1000 mg/m2, about 500 mg/m2 to about 1000
mg/m2, about 400
mg/m2 to about 700 mg/m2, about 500 mg/m2 to about 600 mg/m2, about 540 mg/m2,
or any amount
of zoledronic acid in a range bounded by, or between, any of these values. A
monthly dose may be
given as a single dose, or as two or more individual doses administered during
the month. In some
embodiments, the monthly dose is administered in 2 or 3 weekly doses. In some
embodiments, the
monthly dose is administered in 4 or 5 weekly doses. In some embodiments, the
monthly dose is
administered in 28 to 31 daily doses. In some embodiments, the monthly dose is
administered in 5
to 10 individual doses during the month. The monthly dose may be administered
for only 1 month,
or may be repeatedly administered for 2 or more months.
[0126] In some embodiments, a six week dose of zoledronic acid may be
about 200 mg
to about 500 mg, about 300 mg to about 450 mg, or about 300 mg. In some
embodiments, the six
21
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
week dose of zoledronic acid may be administered only once. In some
embodiments, the six week
dose of zoledronic acid may be administered in six weekly doses, e.g about 35
mg to about 80 mg
or about 50 mg to about 75 mg in each weekly dose.
[0127] The oral zoledronic acid, or disodium salt thereof, may be
administered in
combination with about 0.1 mg to about 10 mg of zoledronic acid, or a salt
thereof, administered
parenterally, such as intravenously. In some embodiments, about 50 mg, about
100 mg, or about
150 mg of the disodium salt of zoledronic acid is administered orally in
combination with 1 mg
parenteral, such as intravenous, zoledronic acid. In some embodiments the
parenteral dose of
zoledronic acid is about 0.25 mg to about 25 mg, about 0.25 mg to about 10 mg,
or about 0.5 mg to
about 7.5 mg.
[0128] With respect to oral administration of zoledronic acid, or
another bisphosphonate,
for the treatment of pain associated with inflammation, arthritis, CRPS, or
any other condition recited
herein, it may helpful if the mammal or human being to which the zoledronic
acid is administered
does not eat food or drink beverage, (other than any water required to swallow
the oral dosage
form) for at least about 1 hour, at least about 2 hours, at least about 4
hours, at least about 6 hours,
at least about 8 hours, at least about 10 hours, or at least about 12 hours
before the zoledronic acid
is administered. It may also be helpful if the mammal or human being to which
the zoledronic acid
is administered does not eat food or drink beverage for at least about 30
minutes, at least about 1
hour, at least about 2 hours, at least about 3 hours, or at least about 4
hours after the zoledronic
acid is administered. In some embodiments, a human being to which the
zoledronic acid is
administered avoids lying down, or remains upright or sits upright, for at
least about 30 minutes or
about 1 hour after receiving a dosage form containing zoledronic acid.
Avoiding food or beverage
before or after oral administration of zoledronic acid can improve the
bioavailability of the zoledronic
acid.
[0129] The oral bioavailability of zoledronic acid in a dosage form can
vary. Some
dosage forms may have ingredients added to enhance the bioavailability.
However, bioavailability
enhancement is not necessary for an oral dosage form to be effective. In some
embodiments, the
dosage form is substantially free of bioavailability-enhancing agents. In some
embodiments, an oral
dosage form may have an oral bioavailability of zoledronic acid of about 0.01%
to about 10%, about
0.1% to about 7%, about 0.1% to about 5%, etc. Without ingredients or other
methods to enhance
bioavailability, zoledronic acid typically has a low bioavailability in an
oral dosage form. In some
embodiments, the oral bioavailability of zoledronic acid is unenhanced or
substantially unenhanced.
For example, the oral bioavailability of zoledronic acid can be about 0.01% to
about 5%, about 1.1%
to about 4%, about 1.1% to about 3.1%, about 1.1% to about 3.5%, about 1.5% to
about 4%, about
1.5% to about 3.1%, about 1.5% to about 3.5%, about 2% to about 3%, about 3%
to about 4%,
22
CA 02928350 2016-04-21
WO 2015/060924 PCT/U S2014/050427
about 0.01% to about 4%, about 0.1% to about 3%, about 0.1% to about 2%, about
0.2% to about
2%, about 0.2% to about 1.5%, about 0.3% to about 1.5%, about 0.3% to about
1%, about 0.1% to
about 0.5%, about 0.3% to about 0.5%, about 0.5% to about 1%, about 0.6% to
about 0.7%, about
0.7% to about 0.8%, about 0.8% to about 0.9%, about 0.9%, about 1% to about
1.1%, about 1.1%
to about 1.2%, about 1.2% to about 1.3%, about 1.3% to about 1.4%, about 1.4%
to about 1.5%,
about 1.5% to about 1.6%, about 1.6% to about 1.8%, or about 1.8% to about 2%.
[0130] One embodiment is a pharmaceutical composition comprising
zoledronic acid
wherein the oral bioavailability of zoledronic acid in the dosage form is from
about 0.01% to about
10%.
[0131] In some embodiments, the oral bioavailability of zoledronic acid
in the dosage
Form is about 0.01% to about 5%.
[0132] in some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0,1% to about 7%.
[0133] In some embodiments, the oral bioavailability of zoledronic acid
in the dosage
Form is about 0.1% to about 5%.
[0134] in some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0,1% to about 3%.
[0135] In some embodiments, the oral bioavailability of zoledronic acid
in the dosage
-form is about 0.1% to about 2%.
[0136] In some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0,2% to about 2%.
[0137] in some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0.2% to about 1.5%.
[0138] In some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0.3% to about 1.5%.
[0139] in some embodiments, the oral bioavailability of zoledronic acid
in the dosage
form is about 0.3% to about 1.0%.
[0140] In some embodiments, an oral dosage form comprises about 10 mg to
about 300
mg of zoledronic acid, and is administered daily for about 2 to about 15
consecutive days. This
regimen may be repeated once monthly, once every two months, once every three
months, once
every four months, once every five months, once every six months, once yearly,
or once every two
years.
23
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0141] ln some embodiments, an oral dosage form comprises about 10 mg to
about 150
mg or about 10 mg to about 100 mg of zoledronic acid, and is administered
daily for about 2 to
about 15 consecutive days. This regimen may be repeated once monthly, once
every two months,
once every three months, once every four months, once every five months, once
every six months,
once yearly, or once every two years.
[0142] In some embodiments, an oral dosage form comprises about 10 mg to
about 150
mg or about 10 mg to about 100 mg of zoledronic acid, and is administered
daily for about 5 to
about 10 consecutive days. This regimen may be repeated once monthly, once
every two months,
once every three months, once every four months, once every five months, once
every six months,
once yearly, or once every two years,
[0143] in some embodiments, an oral dosage form comprises about 40 mg to
about 150
mg of zoledronic acid, and is administered daily for about 5 to about 10
consecutive days. This
regimen may be repeated once monthly, once every two months, once every three
months, once
every four months, once every five months, once every six months, once yearly,
or once every two
years.
[0144] In some embodiments, the oral zoledronic acid may be administered
as one dose
of about 100 mg to about 2000 mg. In some embodiments, the oral zoledronic
acid may be
administered as one dose of about 300 mg to about 1500 mg. In some
embodiments, the oral
zoledronic acid may be administered as one dose of about 200 mg to about 1000
mg. The dose of
zoledronic acid may be administered in a single or divided dose.
[0145] Zoledronic acid may be formulated for oral administration, for
example, with an
inert diluent or with an edible carrier, or it may be enclosed in hard or soft
shell gelatin capsules,
compressed into tablets, or incorporated directly with the food of the diet.
For oral therapeutic
administration, the active compound may be incorporated with an excipient and
used in the form of
ingestible tablets, buccal tablets, coated tablets, troches, capsules,
elixirs, dispersions,
suspensions, solutions, syrups, wafers, patches, and the like.
[0146] Tablets, troches, pills, capsules and the like may also contain
one or more of the
following: a binder such as gum tragacanth, acacia, corn starch or gelatin; an
excipient, such as
dicalcium phosphate; a disintegrating agent such as corn starch, potato
starch, alginic acid and the
like; a lubricant such as magnesium stearate; a sweetening agent such as
sucrose, lactose or
saccharin; or a flavoring agent such as peppermint, oil of wintergreen or
cherry flavoring. When the
unit dosage form is a capsule, it may contain, in addition to materials of the
above type, a liquid
carrier. Various other materials may be present as coating, for instance,
tablets, pills, or capsules
may be coated with shellac, sugar or both. A syrup or elixir may contain the
active compound,
sucrose as a sweetening agent, methyl and propylparabens as preservatives, a
dye and flavoring,
24
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
such as cherry or orange flavor. It may be desirable for material in a dosage
form or pharmaceutical
composition to be pharmaceutically pure and substantially non toxic in the
amounts employed.
[0147] Some compositions or dosage forms may be a liquid, or may
comprise a solid
phase dispersed in a liquid.
[0148] Zoledronic acid may be formulated for parental or intraperitoneal
administration.
Solutions of the active compounds as free acids or pharmacologically
acceptable salts can be
prepared in water suitably mixed with a surfactant, such as
hydroxypropylcellulose. A dispersion
can also have an oil dispersed within, or dispersed in, glycerol, liquid
polyethylene glycols, and
mixtures thereof. Under ordinary conditions of storage and use, these
preparations may contain a
preservative to prevent the growth of microorganisms.
[0149] In some embodiments, an oral dosage form may comprise a
silicified
microcrystalline cellulose such as Prosolv. For example, about 20% (wt/wt) to
about 70% (wt/wt),
about 10% (wt/wt) to about 20% (wt/wt), about 20% (wt/wt) to about 40%
(wt/wt), about 25% (wt/wt)
to about 30% (wt/wt), about 40% (wt/wt) to about 50% (wt/wt), or about 45%
(wt/wt) to about 50%
(wtJwt) silicified nnicrocrystalline cellulose may be present in an oral
dosage form or a unit of an oral
dosage form.
[0150] In some embodiments, an oral dosage form may comprise a
crosslinked
polyvinylpyrrolidone such as crospovidone. For example, about 1% (wt/wt) to
about 10% (wt/wt),
about 1% (wt/wt) to about 5% (wt/wt), or about 1% (wt/wt) to about 3% (wt/wt)
crosslinked
polyvinylpyrrolidone may be present in an oral dosage form or a unit of an
oral dosage form.
[0151] In some embodiments, an oral dosage form may comprise a fumed
silica such as
Aerosil. For example, about 0.1% (wt/wt) to about 10% (wt/wt), about 0.1%
(wt/wt) to about 1%
(wt/wt), or about 0.4% (wt/wt) to about 0.6% (wt/wt) fumed silica may be
present in an oral dosage
form or a unit of an oral dosage form.
[0152] In some embodiments, an oral dosage form may comprise magnesium
stearate.
For example, about 0.1% (wt/wt) to about 10% (wt/wt), about 0.1% (wt/wt) to
about 1% (wt/wt), or
about 0.4% (wt/wt) to about 0.6% (wt/wt) magnesium stearate may be present in
an oral dosage
form or a unit of an oral dosage form.
(01531 An oral dosage form comprising zoledronio acid or another
bisphosphonate may
be included in a pharmaceutical product comprising more than one unit of the
oral dosage form,
[0154] A pharmaceutical product containing oral dosage forms for daily
use can contain
28, 29, 30, or 31 units of the oral dosage form for a monthly supply. An
approximately 6 week daily
supply can contain 40 to 45 units of the oral dosage form. An approximately 3
month daily supply
can contain 85 to 95 units of the or-al dosage form. An approximately six-
month daily supply can
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
contain 170 to 200 units of the oral dosage form. An approximately one year
daily supply can
contain 350 to 380 units of the oral dosage form.
(0155] A pharmaceutical product containing oral dosage forms for weekly
use can
contain 4 or 5 units of the oral dosage form for a monthly supply. An
approximately 2 month weekly
supply can contain 8 or 9 units of the oral dosage form. An approximately 6
week weekly supply
can contain about 6 units of the oral dosage form. An approximately 3 month
weekly supply can
contain 12, 13 or 14 units of the oral dosage form. An approximately six-month
weekly supply can
contain 22 to 30 units of the oral dosage form, An approximately one year
weekly supply can
contain 45 to 60 units of the oral dosage form.
(0156] A pharmaceutical product may accommodate other dosing regimes.
For
example, a pharmaceutical product may comprise 5 to 10 units of the oral
dosage form, wherein
each unit of the oral dosage form contains about 40 mg to about 150 mg of
zoledronic acid. Some
pharmaceutical products may comprise 1 to 10 units of the oral dosage form,
wherein the product
contains about 200 mg to about 2000 mg of zoledronic acid. For such a product,
each unit of the
oral dosage form may be taken daily for 1 to 10 days or 5 to 10 days during a
month, such as at the
beginning of a month.
[0157] Some oral dosage forms comprising zoledronic acid or a salt
thereof may have
enteric coatings or film coatings.
[0158] In the examples below, zoledronic acid was administered in the
disodium salt
form as disodium zoledronate tetrahydrate. No bioavailability enhancing agents
were used in the
test compositions.
Example 1
Effect of Orally Administered Zoledronic Acid in Rat Model of Inflammatory
Pain
Method:
[0159] The effect of orally administered zoledronic acid on inflammatory
pain was
examined using the rat complete Freund's adjuvant (CFA) model. Inflammatory
pain was induced
by injection of 100% CFA in a 75 pL volume into the left hind paws of Sprague-
Dawley rats on day
0, followed by assessments on days 1-3. Animals were orally administered
vehicle (control),
zoledronic acid 18 mg/m2 (or 3 mg/kg), zoledronic acid 120 mg/m2 (or 20
mg/kg), or zoledronic acid
900 mg/m2 (or 150 mg/kg) daily on days 1-3. Drug was dissolved in distilled
water and prepared
fresh daily. Animals were fasted prior to dosing. Under current FDA guidelines
for extrapolating
starting dosages from animals to humans, dosages expressed in mg/m2 are
considered equivalent
between mammalian species. Thus, for example, 18 mg/m2 in a rat is considered
equivalent to 18
mg/m2 in a human being, while 3 mg/kg in a rat may not be equivalent to 3
mg/kg in a human being.
26
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0160] Values for inflammatory pain (mechanical hyperalgesia) in the
vehicle and drug-
treated animals were obtained on day 0 prior to CFA injection, and at baseline
and post-treatment
on days 1-3. Pain was assessed using a digital Randall-Selitto device (dRS;
IITC Life Sciences,
Woodland Hills, CA). Animals were placed in a restraint sling that suspended
the animal, leaving the
hind limbs available for testing. Paw compression threshold was measured by
applying increasing
pressure to the plantar surface of the hind paw with a dome-shaped tip placed
between the 3rd and
4th metatarsus. Pressure was applied gradually over approximately 10 seconds.
Measurements
were taken from the first observed nocifensive behavior of vocalization,
struggle or withdrawal. A
cut-off value of 300 g was used to prevent injury to the animal.
[0161] Reversal of inflammatory pain was calculated according to the
formula:
% reversal = ( Post-treatment ¨ Post-CFA baseline)/(Pre-CFA baseline ¨ Post-
CFA baseline) x
100.
[0162] The experiment was carried out using 9-10 animals per group.
Results:
[0163] Oral administration of zoledronic acid significantly improved
inflammatory pain
thresholds compared to vehicle. Pain threshold measurements taken at various
times are shown in
FIG. 1. Paw compression thresholds in the 18 mg/m2 group were higher than for
vehicle during the
entire measurement period after 30 minutes from the start of treatment. On day
three, paw
compression thresholds for both the 18 mg/m2 and 900 mg/m2 groups were greater
than for vehicle.
An improvement in pain threshold of 49% and 83% from baseline was observed for
the 18 mg/m2
and the 900 mg/m2 groups respectively.
[0164] Orally administered zoledronic acid produced a 29% reversal of
inflammatory
pain at the 18 mg/m2, and a 48% reversal at the 900 mg/m2 dose. This magnitude
of effect is
comparable to that obtained with clinical doses of commercially available
NSAIDs when tested in a
similar model of inflammatory pain. Under current FDA guidelines, the
reference body surface area
of a human adult is 1.62 m2. Thus, a daily dose of 18 mg/m2 corresponds to a
monthly dose of
about 500-560 mg/m2 or a human dose of about 800-900 mg.
[0165] Surprisingly, the two higher doses resulted in thresholds that
were lower than
vehicle on the first two days of dosing. The 120 mg/m2 group was approximately
equal or inferior to
vehicle at all time points during the assessment period. While the 900 mg/m2
group showed
effectiveness on day 3, this result was accompanied by significant toxicity
necessitating
euthanization of all the animals in this group two days after cessation of
dosing.
Example 2
27
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Effect of Orally Administered Zoledronic Acid in Rat Model of Arthritis Pain
Method:
[0166] The effect of orally administered zoledronic acid on arthritis
pain was examined in
the rat complete Freund's adjuvant (CFA) model of arthritis pain. In this
model, injection of 100%
complete Freund's adjuvant (CFA) in a 75 pL volume into the left hind paws is
followed by a 10-14
day period to allow for the development of arthritis pain. Animals were orally
administered vehicle
(control), zoledronic acid 54 mg/m2 (or 9 mg/kg), or zoledronic acid 360 mg/m2
(or 60 mg/kg),
divided in three equal daily doses on the first three days post CFA injection.
Drug was dissolved in
distilled water and prepared fresh daily. Animals were fasted prior to dosing.
[0167] Arthritis pain (mechanical hyperalgesia) in the vehicle and drug-
treated animals
was evaluated on day 14 post CFA injection using a digital Randall-Selitto
device (dRS; IITC Life
Sciences, Woodland Hills, CA). Animals were placed in a restraint sling that
suspended the animal,
leaving the hind limbs available for testing. Paw compression threshold was
measured by applying
increasing pressure to the plantar surface of the hind paw with a dome-shaped
tip placed between
the 3rd and 4th metatarsus. Pressure was applied gradually over approximately
10 seconds.
Measurements were taken from the first observed nocifensive behavior of
vocalization, struggle or
withdrawal. A cut-off value of 300 g was used to prevent injury to the animal.
[0168] Reversal of arthritis pain in the ipsilateral (CFA-injected) paw
was calculated
according to the formula:
% reversal = (ipsilateral drug threshold ¨ ipsilateral vehicle
threshold)/(contralateral vehicle
threshold ¨ ipsilateral vehicle threshold) x 100.
[0169] The experiment was carried out using 7-10 animals per group.
Results:
[0170] Oral administration of zoledronic acid significantly improved
arthritis pain
thresholds compared to vehicle. As shown in FIGS. 2A and 2B, orally
administered zoledronic acid
produced a dose-dependent reversal of arthritis pain. A reversal of 33% was
observed in the 54
mg/m2 group, and reversal of 54% was observed in the 360 mg/m2 group. Under
current FDA
guidelines, the reference body surface area of a human adult is 1.62 m2. Thus,
54 mg/m2 in a rat is
equivalent to an implied human dose of about 87 mg, and 360 mg/m2 in a rat is
equivalent to an
implied human dose of about 583 mg.
Example 3. Treatment of Complex Regional Pain Syndrome with Orally
Administered
Zoledronic Acid,
28
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0171] The effect of orally administered zoledronic acid was examined in
the rat tibia
fracture model of complex regional pain syndrome (CRPS). CRPS was induced in
the rats by
fracturing the right distal tibias of the animals and casting the fractured
hindpaws for 4 weeks, as
described in Guo TZ et al. (Pain. 2004;108:95-107). This animal model has been
shown to replicate
the inciting trauma, natural history, signs, symptoms, and pathologic changes
observed in human
CRPS patients (Kingery WS et al., Pain. 2003;104:75-84).
[0172] Animals were orally administered either vehicle (control) or
zoledronic acid, in a
dosage of 18 mg/m2/day (3 mg/kg/day) for 28 days, starting on the day of
fracture and casting. Drug
was dissolved in distilled water and administered by gavage. Animals were
fasted for 4 hours before
and 2 hours after dosing. At the end of the 28-day period, casts were removed,
and on the following
day, the rats were tested for hindpaw pain, edema, and warmth.
Pain assessments
[0173] Pain was assessed by measuring hyperalgesia, and weight bearing.
[0174] To measure hyperalgesia, an up-down von Frey testing paradigm was
used. Rats
were placed in a clear plastic cylinder (20 cm in diameter) with a wire mesh
bottom and allowed to
acclimate for 15 minutes. The paw was tested with one of a series of eight von
Frey hairs ranging in
stiffness from 0.41 g to 15.14 g. The von Frey hair was applied against the
hindpaw plantar skin at
approximately midsole, taking care to avoid the tori pads. The fiber was
pushed until it slightly
bowed and then it was jiggled in that position for 6 seconds. Stimuli were
presented at an interval of
several seconds. Hindpaw withdrawal from the fiber was considered a positive
response. The initial
fiber presentation was 2.1 g and the fibers were presented according to the up-
down method of
Dixon to generate six responses in the immediate vicinity of the 50%
threshold. Stimuli were
presented at an interval of several seconds.
[0175] An incapacitance device (IITC Inc. Life Science, Woodland, CA,
USA) was used
to measure hindpaw weight bearing, a postural effect of pain. The rats were
manually held in a
vertical position over the apparatus with the hindpaws resting on separate
metal scale plates and
the entire weight of the rat was supported on the hindpaws. The duration of
each measurement was
6 seconds and 10 consecutive measurements were taken at 60-second intervals.
Eight readings
(excluding the highest and lowest ones) were averaged to calculate the
bilateral hindpaw weight-
bearing values. Weight bearing data were analyzed as the ratio between right
(fracture) and left
hindpaw weight bearing values ((2R/(R+L)) x100%).
Edema assessment
[0176] A laser sensor technique was used to determine the dorsal-ventral
thickness of
the hindpaw. Before baseline testing the bilateral hindpaws were tattooed with
a 2 to 3 mm spot on
29
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
the dorsal skin over the midpoint of the third metatarsal. For laser
measurements each rat was
briefly anesthetized with isoflurane and then held vertically so the hindpaw
rested on a table top
below the laser. The paw was gently held flat on the table with a small metal
rod applied to the top
of the ankle joint. Using optical triangulation, a laser with a distance
measuring sensor was used to
determine the distance to the table top and to the top of the hindpaw at the
tattoo site and the
difference was used to calculate the dorsal-ventral paw thickness. The
measurement sensor device
used in these experiments (4381 Precicura, Limab, Goteborg, Sweden) has a
measurement range
of 200 mm with a 0.01 mm resolution.
Hindpaw temperature measurement
[0177] The temperature of the hindpaw was measured using a fine wire
thermocouple
(Omega, Stanford, CT, USA) applied to the paw skin. Six sites were tested per
hindpaw. The six
measurements for each hindpaw were averaged for the mean temperature.
Results
[0178] As illustrated in FIG. 3, treatment with orally administered
zoledronic acid
reversed pain, restored weight bearing, and prevented edema as compared to
vehicle treated
animals.
[0179] As illustrated in FIG. 4, von Frey pain thresholds for the right
(fracture) hindpaw
were reduced by 72% versus the contralateral (normal) hindpaw in vehicle
treated animals.
Zoledronate treatment reversed fracture induced pain by 77% as compared to
vehicle treatment.
[0180] As illustrated in FIG. 5, reduction in weight bearing, a postural
effect of pain, was
significantly higher in the vehicle treated group as compared to the
zoledronic acid treated group.
Weight bearing on the fracture hindlimb was reduced to 55% of normal in the
vehicle treated group.
Zoledronate treatment significantly restored hindlimb weight bearing as
compared to vehicle
treatment (86% of normal).
[0181] As illustrated in FIG. 6, the expected increase in hindpaw
thickness was greater
in the vehicle treated group as compared to the zoledronic acid treated group,
reflecting the
development of edema. Zoledronate treatment reduced hindpaw edema by 60%
versus vehicle
treatment.
[0182] Zoledronic acid reduced hindpaw warmth by 5% versus vehicle
treatment.
[0183] The daily dose in the above experiment was 18 mg/m2/day. Under
current FDA
guidelines, the reference body surface area of a human adult is 1.62 m2. Thus,
a daily dose of 18
mg/m2 corresponds to a monthly dose of about 500-560 mg/m2 or a human dose of
about 800-900
mg.
Example 6. Solubility of Disodium Salt of Zoledronic Acid
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0184] The aqueous solubility of zoledronic acid and disodium
zoledronate tetrahydrate
was determined. One gram of the test compound was measured in to a beaker.
Demineralized
water (pH 5.5) was then added in small increments to the test compound, and
sonification was
applied to the mixture. The procedure was continued until complete dissolution
was achieved. Full
dissolution was determined to have been reached when a clear solution was
present with no visible
material. The volume of water required to reach full dissolution was used to
calculate a solubility
value expressed in grams per 100 mL. The procedure was performed for each
compound.
Results
[0185] As shown in FIG. 7, the aqueous solubility of disodium
zoledronate tetrahydrate
is approximately 50 times that of zoledronic acid. Disodium zoledronate
tetrahydrate has a solubility
of 12.5 g/100 mL compared to only 0.25 g/100 mL for zoledronic acid.
Example 7. Bioayailability of Orally Administered Zoledronic Acid and Disodium
Zoledronate
[0186] Tablets were manufactured containing either pure zoledronic acid
or the disodium
salt of zoledronic acid (disodium zoledronate tetrahydrate). Both types of
tablets contained 50 mg of
zoledronic acid equivalent per tablet. Identical excipients were used in both
types of tablets, with
amounts adjusted to account for the difference in molecular weights between
the acid and the
disodium salt.
[0187] Beagle dogs were orally administered tablets containing 150 mg
zoledronic acid
equivalent either in the form of disodium zoledronate (Group 1) or pure
zoledronic acid (Group 2).
Each animal was given three 50 mg equivalent tablets (150 mg total), which
were administered
together. The animal's oral cavity was wetted with water before placing the
tablets on the back of
the animal's tongue. Animals were fasted before and after dosing. Animals were
6 to 9 months of
age and weighed 6 to 10 kg on the day of dosing. There were three dogs per
group.
[0188] Serial blood samples were collected from each animal by
venipuncture of the
jugular vein at various points after dosing for measurement of plasma
concentrations of zoledronic
acid. Blood samples were collected into chilled tubes containing K2EDTA as the
anticoagulant.
Samples were then centrifuged at approximately 3000 rpm at +4 C for 10 minutes
for plasma
derivation. Plasma concentrations of zoledronic acid were measured using an
LC/MS/MS method.
Results
[0189] The average plasma concentrations of zoledronic acid for each
group of dogs is
summarized in Table 1 and illustrated in Figure 8. Detectable plasma levels of
zoledronic acid were
observed for the entire 48 hours that they were measured.
31
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Table 1
Zoledronic Acid plasma concentrations in beagle
dogs
Plasma
Time concentration
(hour) (ng/mL)
Disodium Zoledronate
Group 1 (N=3) Tablets 0 0.00
(150 mg acid equivalent) 0.25 1193.97
0.5 1852.12
0.75 1776.51
1 1626.56
2 640.57
4 136.93
6 53.11
8 26.97
12 13.74
24 6.78
48 5.39
Group 2 (N=3) Zoledronic Acid Tablets 0 0.00
(150 mg acid equivalent) 0.25 390.92
0.5 846.19
0.75 819.15
1 831.77
2 477.76
4 90.11
6 28.22
8 15.10
12 6.13
24 3.18
48 1.84
[0190] Disodium zoledronate produced significantly higher plasma levels
zoledronic acid
than pure zoledronic acid, indicating improved oral absorption with the salt
form. Measured using
peak plasma concentrations (Cmax), the disodium salt resulted in a 119% actual
and 74% weight-
adjusted increase in bioavailability as compared to pure zoledronic acid.
Measured using area under
the plasma concentration curve (AUC0), bioavailability was 84% and 46% greater
with the
disodium salt than with pure zoledronic acid, on an actual and weight-adjusted
basis respectively.
The average AUCo¨ for the disodium salt was 4073 ng-hr/mL and the average
AUCo¨ for the diacid
was 2217 ng=hr/mL. The AUC0,f was found to be dose proportional. Thus, for
beagle dogs similar
to those tested, about 3 mg to about 4 mg of the disodium salt would be
expected to result in an
32
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
AUCo¨ of about 100 ng=hr/mL, and about 7 mg to about 8 mg of the disodium salt
would be
expected to result in an AUC0¨ of about 200 ng=hr/rnL.
[0191] The elimination half-life for the dogs receiving 150 mg acid
equivalent of the
disodium salt was 20.5 5.2 hr.
Example 8
[0192] Tablets were prepared by blending zoledronic acid, either in the
form of the free
acid or the disodium salt, with identical excipients. For dosage forms with a
greater amount of
active, the amount of the excipients was reduced proportionally to keep the
weight of the tablet at
about 100 mg. After blending, the ingredients were compressed at varying
pressures, followed by
a film coating. The resulting tablets were then tested for hardness using a
Dr. Schleuniger
Pharmatron 8M Tablet Hardness Tester. The results are shown in Table 2 and
FIG. 9.
Table 2
Compression
Force Hardness (kPa)
Disodium Disodium
Diacid Salt Salt
(Psi) 50 mg 50 mg 71 mg
800 4.0 8.7 4.8
1100 6.1 11.2 6.8
1500 7.7 13.7 7.4
2000 8.7 16.3 10.7
2400 8.7 11.3
3000 11.4 14.1
4400 12.5 14.9
5500 12.8 18.2
6100 13.0
[0193] The following embodiments are specifically contemplated:
Embodiment 1. A method of relieving inflammatory pain comprising administering
an
oral dosage form containing zoledronic acid to a mammal in need thereof,
wherein the mammal
receives a total monthly dose of zoledronic acid that is about 800 mg/m2 or
less based upon the
body surface area of the mammal.
Embodiment 2. The method of embodiment 1, wherein the mammal is a human being
that receives a total monthly dose of zoledronic acid that is about 30 mg/m2
to about 700 mg/m2.
Embodiment 3. The method of embodiment 2, wherein the total monthly dose is
administered in 4 or 5 weekly doses.
Embodiment 4. The method of embodiment 2, wherein the total monthly dose is
administered in 28 to 31 daily doses.
33
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 5. The method of embodiment 2, wherein the total monthly dose is
administered in 5 to 10 individual doses during the month.
Embodiment 6. The method of embodiment 1, wherein the mammal is a human being
that receives a total weekly dose of zoledronic acid that is about 10 mg to
about 300 mg.
Embodiment 7. The method of embodiment 6, wherein the total weekly dose is a
single dose, administered once a week.
Embodiment 8. The method of embodiment 6, wherein the total weekly dose is
administered in 2 to 7 individual doses during the week.
Embodiment 9. The method of embodiment 1, wherein the mammal is a human being
that receives a total weekly dose of zoledronic acid that is about 10 mg to
about 150 mg.
Embodiment 10. The method of any preceding embodiment, wherein the mammal
experiences significant pain relief more than 3 hours after administration of
the dosage form.
Embodiment 11. The method of embodiment 10, wherein the mammal experiences
significant pain relief during at least a part of a time from about 3 hours to
about 24 hours after
administration of the dosage form.
Embodiment 12. The method of embodiment 10, wherein the mammal experiences
significant pain relief during at least a part of a time from about 3 hours to
about 3 weeks after
administration of the dosage form.
Embodiment 13. A method of relieving inflammatory pain comprising
administering an
oral dosage form containing zoledronic acid to a mammal in need thereof,
wherein the oral
dosage form contains about 10 mg/m2 to about 20 mg/m2 of zoledronic acid based
upon the
body surface area of the mammal.
Embodiment 14. The method of embodiment 13, wherein the oral dosage form
contains
about 15 mg/m2 to about 20 nng/nri2 of zoledronic acid based upon the body
surface area of the
mammal.
Embodiment 15. A method of relieving inflammatory pain comprising orally
administering to a mammal in need thereof, about 300 mg/m2 to about 600 mg/m2
of zoledronic
acid per month to the mammal, based upon the body surface area of the mammal.
Embodiment 16. The method of embodiment 15, comprising orally administering
about
450 mg/m2 to about 600 mg/m2 of zoledronic acid per month to the mammal, based
upon the
body surface area of the mammal.
Embodiment 17. The method of any preceding embodiment, wherein the mammal is
not suffering from bone metastasis.
Embodiment 18. The method of any preceding embodiment, wherein the mammal is
not suffering from cancer.
34
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 19. The method of any preceding embodiment, wherein the zoledronic
acid is administered as a salt of a dianion of zoledronic acid.
Embodiment 20. A method of relieving pain associated with an arthritis
comprising
administering an oral dosage form containing zoledronic acid to a human being
in need thereof.
Embodiment 21. The method of embodiment 20, wherein the human being receives a
total monthly dose of zoledronic acid that is about 40 mg to about 2000 mg.
Embodiment 22. The method of embodiment 21, wherein the total monthly dose is
administered in 4 or 5 weekly doses.
Embodiment 23. The method of embodiment 21, wherein the total monthly dose is
administered in 28 to 31 daily doses.
Embodiment 24. The method of embodiment 21, wherein the total monthly dose is
administered in 5 to 10 individual doses during the month.
Embodiment 25. The method of embodiment 20, wherein the human being receives a
total weekly dose of zoledronic acid that is about 100 mg to about 300 mg.
Embodiment 26. The method of embodiment 25, wherein the total weekly dose is a
single dose, administered once a week.
Embodiment 27. The method of embodiment 25, wherein the total weekly dose is
administered in 2 to 7 individual doses during the week.
Embodiment 28. The method of embodiment 20, wherein the human being receives a
total weekly dose of zoledronic acid that is about 10 mg to about 100 mg.
Embodiment 29. The method of any of embodiments 20-28, wherein the human being
experiences significant pain relief more than 3 hours after administration of
the dosage form.
Embodiment 30. The method of embodiment 29, wherein the human being
experiences
significant pain relief during at least a part of a time from about 3 hours to
about 24 hours after
administration of the dosage form.
Embodiment 31. The method of embodiment 29, wherein the human being
experiences
significant pain relief during at least a part of a time from about 3 hours to
about 3 weeks after
administration of the dosage form.
Embodiment 32. The method of any of embodiments 20-31, wherein the dosage form
contains about 10 mg/m2 to about 20 mg/m2 of zoledronic acid based upon the
body surface
area of the human being.
Embodiment 33. The method of embodiment 32, wherein the dosage form contains
about 15 mg/m2 to about 20 mg/m2 of zoledronic acid based upon the body
surface area of the
human being.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 34. The method of any of embodiments 20-33, wherein about 50 mg/m2
to
about 200 mg/m2 of zoledronic acid is orally administered per month, based
upon the body
surface area of the human being.
Embodiment 35. The method of any of embodiments 20-31, wherein the dosage form
contains about 80 mg/m2 to about 150 mg/m2 of zoledronic acid based upon the
body surface
area of the human being.
Embodiment 36. The method of embodiment 35, wherein about 300 mg/m2 to about
1000 mg/m2 of zoledronic acid is orally administered per month, based upon the
body surface
area of the human being.
Embodiment 37. The method of any of embodiments 20-36, wherein the human being
is not suffering from bone metastasis.
Embodiment 38. The method of any of embodiments 20-37, wherein the human being
is not suffering from cancer.
Embodiment 39. The method of any preceding embodiment, wherein the zoledronic
acid is in the disodium salt form.
Embodiment 40, An oral dosage form comprising zoledronic acid, wherein the
oral
bioavallability of zoledronic acid in the dosage form is about 0.01% to about
4%.
Embodiment 41. The oral dosage form of embodiment 40, wherein the oral dosage
form contains about 10 mg to about 300 mg of zoledronic acid.
Embodiment 42. The oral dosage form of embodiment 40, wherein the oral dosage
form contains about 10 rng to about 50 rug of zoledronic acid.
Embodiment 43. The oral dosage form of any of embodiments 40-42, wherein the
oral
bioavallability of zoledronic acid in the dosage form is about 0.1% to about
2%.
Embodiment 44. A pharmaceutical product comprising more than one unit of an
oral
dosage form of embodiment 40.
Embodiment 45. The pharmaceutical product of embodiment 44, wherein each unit
of
the oral dosage form contains about 1 mg to about 50 mg of zoledronic acid.
Embodiment 46. The pharmaceutical product of embodiment 45, comprising 28, 29,
30, or 31 units of the oral dosage form, for a total of about 28 mg to about
1600 mg of zoledronic
acid to be administered in about 1 month.
Embodiment 47. The pharmaceutical product of embodiment 45, comprising 85 to
95
units of the oral dosage form, for a total of about 85 mg to about 4800 mg of
zoledronic acid to
be administered in about 3 months.
Embodiment 48. The pharmaceutical product of embodiment 45, comprising 170 to
200
units of the oral dosage form, for a total of about 170 mg to about 10,000 mg
of zoledronic acid
to be administered in about 6 months.
36
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 49. The pharmaceutical product of embodiment 45, comprising 350 to
380
units of the oral dosage form, for a total of about 350 mg to about 19,000 mg
of zoledronic acid
to be administered in about 1 year.
Embodiment 50. The pharmaceutical product of embodiment 44, wherein each unit
of
the oral dosage form contains about 10 mg to about 300 mg.
Embodiment 51, The pharmaceutical product of embodiment 50, comprising 4 or 5
units of the oral dosage form, for a total of about 40 mg to about 1500 mg of
zoledronic acid to
be administered within a period of about 1 month.
Embodiment 52, The pharmaceutical product of embodiment 50, comprising 8 or 9
units of the oral dosage form, for a total of about 80 mg to about 2700 mg of
zoledronic acid to
be administered in about 2 months.
Embodiment 53. The pharmaceutical product of embodiment 50, comprising 12, 13
or
14 units of the oral dosage form, for a total of about 120 mg to about 4200 mg
of zoledronic acid
to be administered in about 3 months.
Embodiment 54. The pharmaceutical product of embodiment 50, comprising 22 to
30
units of the oral dosage form, for a total of about 220 mg to about 9000 mg of
zoledronic acid to
be administered in about 6 months.
Embodiment 55. The pharmaceutical product of embodiment 50, comprising 45 to
60
units of the oral dosage form, for a total of about 450 mg to about 18000 mg
of zoledionic acid
to be administered in about 1 year.
Embodiment 56. The pharmaceutical product of embodiment 44, comprising 1 to 10
units of the oral dosage form, wherein the product contains about 200 mg to
about 2000 mg of
zoledronic acid.
Embodiment 57. The oral dosage form of any preceding embodiment, wherein the
zoledronic acid is in the form of a sodium salt.
Embodiment 58. The oral dosage form of any preceding embodiment, wherein the
zoledronic acid is in a form that has an aqueous solubility greater than 1%
(w/v).
Embodiment 59. The oral dosage form of any preceding embodiment, wherein the
zoledronic acid is in a form that has an aqueous solubility of about 5% (w/v)
to about 50% (w/v).
Embodiment 60. An oral dosage form comprising zoledronic acid and an
excipient,
wherein the zoledronic acid is in a form that has an aqueous solubility
greater than 1% (vviv).
Embodiment 61. The oral dosage form of embodiment 60, wherein the zoledronic
acid
is in a form that has an aqueus solubility of about 5% (w/v) to about 50%
(w/v).
Embodiment 62. A method of treating complex regional pain syndrome comprising
administering an oral dosage form containing zoledronic acid to a mammal in
need thereof.
37
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 63. The method of embodiment 62, wherein the mammal is a human
being that receives an amount of zoledronic acid that is about 30 mg/m2 to
about 700 mg/m2 in a
period of one month or less.
Embodiment 64. The method of embodiment 63, wherein 4 or 5 weekly doses are
administered in a period of one month or less.
Embodiment 65. The method of embodiment 63, wherein 28 to 31 daily doses are
administered in a period of one month or less.
Embodiment 66. The method of embodiment 63, wherein 5 to 10 individual doses
are
administered during a period of one month or less.
Embodiment 67. The method of embodiment 63, wherein about 30 nng/nn2 to about
700
mg/m2 of zoledronic acid is administered during only one month.
Embodiment 68. The method of embodiment 63, wherein about 30 mg/m2 to about
700
mg/m2 of zoledronic acid is administered in a period of one month or less for
2 or more
consecutive months.
Embodiment 69. The method of embodiment 62, wherein the mammal receives about
mg/m2 to about 30 mg/m2 of zoledronic acid daily.
Embodiment 70. The method of embodiment 62, wherein the mammal is a human
being that receives a total weekly dose of zoledronic acid that is about 10 mg
to about 300 mg.
Embodiment 71. The method of embodiment 70, wherein the total weekly dose is a
single dose, administered once a week.
Embodiment 72. The method of embodiment 70, wherein the total weekly dose is
administered in 2 to 7 individual doses during the week.
Embodiment 73. The method of any of embodiments 62-72, wherein the complex
regional pain syndrome is complex regional pain syndrome type I.
Embodiment 74. The method of any of embodiments 62-72, wherein the complex
regional pain syndrome is complex regional pain syndrome type II.
Embodiment 75. The method of any preceding embodiment, wherein the zoledronic
acid is in a salt form.
Embodiment 76. The method of any of embodiments 62-75, wherein the dosage form
contains about 10 mg/m2 to about 20 mg/m2 of zoledronic acid based upon the
body surface
area of the mammal.
Embodiment 77. The method of embodiment 76, wherein the dosage form contains
about 15 mg/m2 to about 20 mg/m2 of zoledronic acid based upon the body
surface area of the
mammal.
Embodiment 78. A method of treating complex regional pain syndrome, comprising
administering pamidronic acid to a human being in need thereof.
38
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 79. A method of treating complex regional pain syndrome, comprising
administering neridronic acid to a human being in need thereof.
Embodiment 80. A method of treating complex regional pain syndrome, comprising
administering olpadronic acid to a human being in need thereof.
Embodiment 81. A method of treating complex regional pain syndrome, comprising
administering alendronic acid to a human being in need thereof.
Embodiment 82. A method of treating complex regional pain syndrome, comprising
administering incadronic acid to a human being in need thereof.
Embodiment 83. A method of treating complex regional pain syndrome, comprising
administering ibandronic acid to a human being in need thereof.
Embodiment 84. A method of treating complex regional pain syndrome, comprising
administering risedronic acid to a human being in need thereof.
Embodiment 85. A method of treating pain, comprising administering pamidronic
acid to
a human being in need thereof.
Embodiment 86. A method of treating pain, comprising administering neridronic
acid to
a human being in need thereof.
Embodiment 87. A method of treating pain, comprising administering olpadronic
acid to
a human being in need thereof.
Embodiment 88. A method of treating pain, comprising administering alendronic
acid to
a human being in need thereof.
Embodiment 89. A method of treating pain, comprising administering incadronic
acid to
a human being in need thereof.
Embodiment 90. A method of treating pain, comprising administering ibandronic
acid to
a human being in need thereof.
Embodiment 91. A method of treating pain, comprising administering risedronic
acid to
a human being in need thereof.
Embodiment 92. A method of treating arthritis pain, comprising administering
pamidronic acid to a human being in need thereof.
Embodiment 93. A method of treating arthritis pain, comprising administering
neridronic
acid to a human being in need thereof.
Embodiment 94. A method of treating arthritis pain, comprising administering
olpadronic acid to a human being in need thereof.
39
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 95. A method of treating arthritis pain, comprising administering
alendronic acid to a human being in need thereof.
Embodiment 96. A method of treating arthritis pain, comprising administering
incadronic
acid to a human being in need thereof.
Embodiment 97. A method of treating arthritis pain, comprising administering
ibandronic acid to a human being in need thereof.
Embodiment 98. A method of treating arthritis pain, comprising administering
risedronic
acid to a human being in need thereof.
Embodiment 99. A method of treating inflammatory pain, comprising
administering
pamidronic acid to a human being in need thereof.
Embodiment 100. A method of treating inflammatory pain, comprising
administering neridronic acid to a human being in need thereof.
Embodiment 101. A method of treating inflammatory pain, comprising
administering olpadronic acid to a human being in need thereof.
Embodiment 102. A method of treating inflammatory pain, comprising
administering alendronic acid to a human being in need thereof.
Embodiment 103. A method of treating inflammatory pain, comprising
administering incadronic acid to a human being in need thereof.
Embodiment 104. A method of treating inflammatory pain, comprising
administering ibandronic acid to a human being in need thereof.
Embodiment 105. A method of treating inflammatory pain, comprising
administering risedronic acid to a human being in need thereof.
Embodiment 106. A method of treating complex regional pain syndrome,
comprising administering etidronic acid to a human being in need thereof.
Embodiment 107. A method of treating pain, comprising administering
etidronic
acid to a human being in need thereof.
Embodiment 108. A method of treating arthritis pain, comprising
administering
etidronic acid to a human being in need thereof.
Embodiment 109. A method of treating inflammatory pain, comprising
administering etidronic acid to a human being in need thereof.
Embodiment 110. A method of treating complex regional pain syndrome,
comprising administering clodronic acid to a human being in need thereof.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 111. A method of treating pain, comprising administering
clodronic
acid to a human being in need thereof.
Embodiment 112. A method of treating arthritis pain, comprising
administering
clodronic acid to a human being in need thereof.
Embodiment 113. A method of treating inflammatory pain, comprising
administering clodronic acid to a human being in need thereof.
Embodiment 114. A method of treating complex regional pain syndrome,
comprising administering tiludronic acid to a human being in need thereof.
Embodiment 115. A method of treating pain, comprising administering
tiludronic
acid to a human being in need thereof.
Embodiment 116. A method of treating arthritis pain, comprising
administering
tiludronic acid to a human being in need thereof.
Embodiment 117. A method of treating inflammatory pain, comprising
administering tiludronic acid to a human being in need thereof.
Embodiment 118. The method of any of embodiments 78-117, wherein the
active
compound is orally administered.
Embodiment 119. The method of any of embodiments 78-117, wherein the
active
compound is parenterally administered.
Embodiment 120. A method of enhancing the oral bioavailability of
zoledronic
acid comprising orally administering a dosage form containing zoledronic acid
in the disodium
salt form.
Embodiment 121. The method of embodiment 120, wherein the zoledronic
acid in
the disodium salt form provides an enhancement to bioavailability, as compared
to zoledronic
acid in the diacid form, which adds to any enhancement to bioavailability
provided by any
bioavailability-enhancing agents in the dosage form.
Embodiment 122. The method of embodiment 120, wherein the dosage form
is
substantially free of bioavailability-enhancing agents.
Embodiment 123. The method of embodiment 120, wherein the zoledronic
acid in
the disodium salt form is administered to a mammal in an amount that provides
an area under
the plasma concentration curve of zoledronic acid of about 4 ng=hirriL to
about 2000 ng-h/nnL to
the mammal each time the zoledronic acid in the disodium salt is administered.
41
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 124. The method of embodiment 123, wherein the zoledronic
acid in
the disodium salt form is administered at an interval of about 3 to about 4
weeks in an amount
that provides an area under the plasma concentration curve of zoledronic acid
of about 100
ng=h/mL to about 2000 ng=h/mL to the mammal each time the zoledronic acid in
the disodium
salt form is administered.
Embodiment 125. The method of embodiment 123, wherein the zoledronic
acid in
the disodium salt form is administered weekly, or 3 to 5 times in a month, in
an amount that
provides an area under the plasma concentration curve of zoledronic acid of
about 20 ng=h/mL
to about 700 ng=h/mL to the mammal each time the zoledronic acid in the
disodium salt form is
administered.
Embodiment 126. The method of embodiment 123, wherein the zoledronic
acid in
the disodium salt form is administered daily in an amount that provides an
area under the
plasma concentration curve of zoledronic acid of about 4 ng=h/mL to about 100
ng=h/mL to the
mammal each time the zoledronic acid in the disodium salt form is
administered.
Embodiment 127. The method of embodiment 120, wherein the dosage form
is a
solid.
Embodiment 128. The method of embodiment 120, 121, 122, 123, 124, 125,
126,
or 127, wherein the bioavailability of zoledronic acid is improved by at least
about 20% as
compared to administration of zoledronic acid in the diacid form.
Embodiment 129. The method of embodiment 120, 121, 122, 123, 124, 125,
126,
127, or 128, further comprising administering, on a molar basis, less of the
zoledronic acid in the
disodium salt form than would be administered of zoledronic acid in the diacid
form in order to
achieve the same plasma levels of zoledronic acid.
Embodiment 130. The method of embodiment 129, wherein at least about 10
mole% less of the disodium salt form is administered as compared the amount of
zoledronic
acid in the diacid form that would be administered in order to achieve the
same plasma levels of
zoledronic acid.
Embodiment 131. The method of embodiment 129, wherein the disodium salt
form is administered in an amount, on a molar basis, that has a value of about
0.8nd to about
1.2nd, wherein:
nd = (batbd)(na)
42
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
wherein ba is the bioavailability of the diacid form, ba is the
bioavailability of the disodium salt form,
and na is the number of moles of zoledronic acid in the diacid form that would
be administered in
order to achieve the same plasma levels of zoledronic acid.
Embodiment 132. The method of embodiment 131, wherein the disodium
salt is
administered in an amount that has a value of about RA.
Embodiment 133. The method of any of embodiments 120-132, wherein
the
zoledronic acid is used to treat an inflammatory condition.
Embodiment 134. The method of embodiment 133, wherein the zoledronic
acid is
used to treat arthritis.
Embodiment 135. The method of embodiment 133, wherein the zoledronic
acid is
used to treat complex regional pain syndrome.
Embodiment 136. The method of any of embodiments 1-39, 62-77, and
120-135,
wherein:
a first oral dosage form is administered; and
a second oral dosage form is administered;
wherein, with respect to the first oral dosage form, the second oral dosage
form is
administered at 10 x Tmax or greater, wherein Tmax is the time of maximum
plasma
concentration for the first oral dosage form.
Embodiment 137. A dosage form comprising zoledronic acid in the
disodium salt
form, wherein the bioavailability, in a mammal, of zoledronic acid in the
disodium salt form is
greater than the bioavailability of zoledronic acid in the diacid form would
be in the same dosage
form.
Embodiment 138. A dosage form comprising zoledronic acid in the
disodium salt
form, wherein the dosage form contains an amount of zoledronic acid in the
disodium salt form
that provides an area under the plasma concentration curve of zoledronic acid
of about 4
ng=h/mL to about 2000 ng=h/mL to a human being to which the dosage form is
administered.
Embodiment 139. The dosage form of embodiment 138, wherein the
dosage
form contains an amount of zoledronic acid in the disodium salt form that
provides an area
under the plasma concentration curve of zoledronic acid of about 100 ng=h/mL
to about 2000
ng=h/mL to a human being to which the dosage form is administered.
Embodiment 140. The dosage form of embodiment 138, wherein the
dosage
form contains an amount of zoledronic acid in the disodium salt form that
provides an area
43
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
under the plasma concentration curve of zoledronic acid of about 20 ng=h/mL to
about 700
ng=h/mL to a human being to which the dosage form is administered.
Embodiment 141. The dosage form of embodiment 138, wherein the dosage
form contains an amount of zoledronic acid in the disodium salt form that
provides an area
under the plasma concentration curve of zoledronic acid of about 4 ng=h/mL to
about 100
ng=h/mL to a human being to which the dosage form is administered.
Embodiment 142. A dosage form comprising zoledronic acid in the
disodium salt
form,
wherein the disodium salt form is present in a lower molar amount than would
be
present if the zoledronic acid were in the diacid form; and
wherein the zoledronic acid in the disodium salt form has an improved
bioavailability
as compared to the zoledronic acid in the diacid form to the extent that the
lower molar
amount of the disodium salt in the dosage form does not reduce the amount of
zoledronic
acid delivered to the plasma of a mammal.
Embodiment 143. The dosage form of embodiment 137, 138, 139, 140, 141,
or
142, wherein the dosage form is a solid.
Embodiment 144. The dosage form of embodiment 142 or 143, wherein the
bioavailability of zoledronic acid in the disodium salt form is improved by at
least about 10% as
compared to an otherwise identical dosage form containing zoledronic acid in
the diacid form.
Embodiment 145. The dosage form of embodiment 142, 143, or 144,
containing
at least about 20 mole% less of the disodium salt form as compared to the
amount of the
zoledronic acid in the diacid form that would be present if the zoledronic
acid were in the diacid
form.
Embodiment 146. The dosage form of embodiment 142, wherein the disodium
salt form is present in an amount, on a molar basis, that has a value of about
0.9nd to about
1.1nd, wherein:
nd = (batbd)(na)
wherein ba is the bioavailability of the diacid form, bd is the
bioavailability of the
disodium salt form, and n, is the number of moles of the diacid form that
would be present if
the zoledronic acid were in the diacid form.
Embodiment 147. The dosage form of embodiment 146, wherein the disodium
salt is administered in an amount that has a value of about ncl.
44
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 148. The method of any of embodiments 1-39, 62-77, and 120-
136,
wherein:
only a single oral dosage form is administered; or
a first oral dosage form is administered, and a second oral dosage form is
administered after the first oral dosage form, wherein the second oral dosage
form is
administered before the maximum pain relieving effect of the first oral dosage
form is
achieved, or the second oral dosage form is administered before an observable
pain
relieving effect is achieved.
Embodiment 149. The method of embodiment 148, wherein the second oral
dosage form is administered before an observable pain relieving effect is
achieved.
Embodiment 150. The method of any of embodiments 1-39, 62-77, and 120-
132,
wherein a first dosage form is administered, followed by administration of a
second dosage
form, wherein the second dosage form is administered after the maximum pain
relieving effect of
the first oral dosage form is achieved, and the second oral dosage form is
administered while a
pain relieving effect from the first oral dosage form is observable.
Embodiment 151. The method of embodiment 148, 149, or 150, wherein the
second oral dosage form is administered about 24 hours to about 28 days after
the first oral
dosage form is administered.
Embodiment 152. The method of any of embodiments 20-39, wherein the
human
being is about 30 years old to about 75 years old.
Embodiment 153. The method of any of embodiments 20-39, wherein the
human
being is about 1 year old to about 16 years old.
Embodiment 154. The method of any of embodiments 20-39, wherein the
human
being is about 80 years old to about 95 years old.
Embodiment 155. The method of any of embodiments 20-39, wherein the
human
being has suffered from the arthritis for at least 2 months.
Embodiment 156. The method of any of embodiments 20-39, wherein the
arthritis
affects, a knee, an elbow, a finger, a wrist, a shoulder, or a hip.
Embodiment 157. The method of any of embodiments 1-44, 62-133, and 144-
156, wherein the mammal or human being to which the zoledronic acid is
administered does not
eat food or drink beverage for at least 1 hour before the zoledronic acid is
administered.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 158. The method of embodiment 157, wherein the mammal or
human being to which the zoledronic acid is administered does not eat food or
drink beverage
for at least 2 hours before the zoledronic acid is administered.
Embodiment 159. The method of embodiment 158, wherein the mammal or
human being to which the zoledronic acid is administered does not eat food or
drink beverage
for at least 4 hours before the zoledronic acid is administered.
Embodiment 160. The method of embodiment 159, wherein the mammal or
human being to which the zoledronic acid is administered does not eat food or
drink beverage
for at least 6 hours before the zoledronic acid is administered.
Embodiment 161. The method of any of embodiments 157-160, wherein the
mammal or human being to which the zoledronic acid is administered does not
eat food or drink
beverage for at least 30 minutes after the zoledronic acid is administered.
Embodiment 162. The method of embodiment 161, wherein the mammal or
human being to which the zoledronic acid is administered does not eat food or
drink beverage
for at least 1 hour after the zoledronic acid is administered.
Embodiment 163. The method of embodiment 161, where in the mammal or
human being to which the zoledronic acid is administered does not eat food or
drink beverage
for at least 2 hours after the zoledronic acid is administered.
Embodiment 164. The method, dosage form, or product, of any preceding
embodiment, wherein the zoledronic acid in the oral dosage form has a 24 hour
sustained
plasma level factor of about 1 or higher.
Embodiment 165. The method, dosage form, or product, of any preceding
embodiment, wherein the zoledronic acid in the oral dosage form has a 24 hour
sustained
plasma level factor that is higher than that of intravenously administered
zoledronic acid.
Embodiment 166. The method, dosage form, or product, of any preceding
embodiment, wherein the oral dosage form is a solid that has a hardness of
about 5 kPa to
about 20 kPa.
Embodiment 167. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the zoledronic acid
is present in an amount that results in an AUC of zoledronic acid of about 50
ng=hr/mL to about
700 ng=hr/mL upon administration of the oral dosage form to the particular
species of mammal.
46
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 168. The method, dosage form, or product of embodiment 167,
wherein the zoledronic acid is present in an amount that results in an AUC of
zoledronic acid of
about 130 ng=hr/mL to about 180 ng=hr/mL upon administration of the oral
dosage form to the
particular species of mammal.
Embodiment 169. The method, dosage form, or product of embodiment 167,
wherein the zoledronic acid is present in an amount that results in an AUG of
zoledronic acid of
about 300 ng-hr/mL to about 450 ng-hr/mL upon administration of the oral
dosage form to the
particular species of mammal.
Embodiment 170. The method, dosage form, or product of embodiment 167,
wherein the zoledronic acid is present in an amount that results in an AUG of
zoledronic acid of
about 300 ng=hr/mL to about 350 ng=hr/mL upon administration of the oral
dosage form to the
particular species of mammal.
Embodiment 171. The method, dosage form, or product of embodiment 167,
wherein the zoledronic acid is present in an amount that results in an AUG of
zoledronic acid of
about 370 ng-hr/mL to about 420 ng-hr/mL upon administration of the oral
dosage form to the
particular species of mammal.
Embodiment 172. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the zoledronic acid
is present in an amount that results in a Cm,x of zoledronic acid of about 5
ng/mL to about 300
ng/mL upon administration of the oral dosage form to the particular species of
mammal.
Embodiment 173. The method, dosage form, or product of embodiment 172,
wherein the zoledronic acid is present in an amount that results in a Cm,x of
zoledronic acid of
about 5 ng/mL to about 50 ng/mL upon administration of the oral dosage form to
the particular
species of mammal.
Embodiment 174. The method, dosage form, or product of embodiment 172,
wherein the zoledronic acid is present in an amount that results in a Cmax of
zoledronic acid of
about 50 ng/mL to about 200 ng/mL upon administration of the oral dosage form
to the particular
species of mammal.
Embodiment 175. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that administration of the oral dosage form to the
particular species of
mammal results in a Tmax of zoledronic acid of about 0.4 hr to about 1 hr.
47
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 176. The method, dosage form, or product of embodiment 175,
wherein the oral dosage form is configured so that administration of the oral
dosage form to the
particular species of mammal results in a Tmõ of zoledronic acid of about 0.5
hr.
Embodiment 177. The method, dosage form, or product of embodiment 175,
wherein the oral dosage form is configured so that administration of the oral
dosage form to the
particular species of mammal results in a Tina), of zoledronic acid of about
0.75 hr.
Embodiment 178. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the zoledronic acid has a 12 hour sustained plasma
level factor of
about 12 to about 50 for the particular species of mammal.
Embodiment 179. The method, dosage form, or product of embodiment 178,
wherein the oral dosage form is configured so that the zoledronic acid has a
12 hour sustained
plasma level factor of about 20 to about 40 for the particular species of
mammal.
Embodiment 180. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the zoledronic acid has a 24 hour sustained plasma
level factor of
about 10 to about 30 for the particular species of mammal.
Embodiment 181. The method, dosage form, or product of embodiment 180,
wherein the oral dosage form is configured so that the zoledronic acid has a
24 hour sustained
plasma level factor of about 10 to about 20 for the particular species of
mammal.
Embodiment 182. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the zoledronic acid has a 36 hour sustained plasma
level factor of
about 6 to about 20 for the particular species of mammal.
Embodiment 183. The method, dosage form, or product of embodiment 182,
wherein the oral dosage form is configured so that the zoledronic acid has a
36 hour sustained
plasma level factor of about 8 to about 15 for the particular species of
mammal.
Embodiment 184. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the zoledronic acid has a 48 hour sustained plasma
level factor of
about 5 to about 20 for the particular species of mammal.
48
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 185. The method, dosage form, or product of embodiment 184,
wherein the oral dosage form is configured so that the zoledronic acid has a
48 hour sustained
plasma level factor of about 6 to about 15 for the particular species of
mammal.
Embodiment 186. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the zoledronic acid has a 72 hour sustained plasma
level factor of
about 4 to about 20 for the particular species of mammal.
Embodiment 187. The method, dosage form, or product of embodiment 186,
wherein the oral dosage form is configured so that the zoledronic acid has a
72 hour sustained
plasma level factor of about 5 to about 10 for the particular species of
mammal.
Embodiment 188. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the particular species of mammal has a plasma
concentration of
zoledronic acid at 12 hours that is about 0.5 ng/mL to about 5 ng/mL.
Embodiment 189. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the particular species of mammal has a plasma
concentration of
zoledronic acid at 24 hours that is about 0.2 ng/mL to about 2 ng/mL.
Embodiment 190. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the particular species of mammal has a plasma
concentration of
zoledronic acid at 36 hours that is about 0.1 ng/mL to about 2 ng/mL.
Embodiment 191. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the particular species of mammal has a plasma
concentration of
zoledronic acid at 48 hours that is about 0.1 ng/mL to about 2 ng/mL.
Embodiment 192. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the particular species of mammal has a plasma
concentration of
zoledronic acid at 72 hours that is about 0.2 ng/mL to about 1 ng/mL.
49
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 193. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the elimination half-life of zoledronic acid in the
particular species of
mammal is about 30 hours to about 100 hours.
Embodiment 194. The method, dosage form, or product of any preceding
embodiment, wherein the oral dosage form comprising zoledronic acid has a dose
of zoledronic
acid and a configuration suitable for a particular species of mammal, wherein
the oral dosage
form is configured so that the elimination half-life of zoledronic acid in the
particular species of
mammal is about 40 hours to about 60 hours.
Embodiment 195. An oral dosage form comprising zoledronic acid having a
dose
of zoledronic acid and a configuration suitable for a particular species of
mammal, wherein the
zoledronic acid is present in an amount that results in an area under the
plasma concentration
curve (AUC) of zoledronic acid of about 50 ng=hr/mL to about 700 ng=hr/mL upon
administration
of the oral dosage form to the particular species of mammal.
Embodiment 196. The oral dosage form of embodiment 195, wherein the
zoledronic acid is present in an amount that results in an AUG of zoledronic
acid of about 130
ng=hr/mL to about 180 ng=hr/mL upon administration of the oral dosage form to
the particular
species of mammal.
Embodiment 197. The oral dosage form of embodiment 195, wherein the
zoledronic acid is present in an amount that results in an AUC of zoledronic
acid of about 300
ng=hr/mL to about 450 ng=hr/mL upon administration of the oral dosage form to
the particular
species of mammal.
Embodiment 198. The oral dosage form of embodiment 195, wherein the
zoledronic acid is present in an amount that results in an AUG of zoledronic
acid of about 300
ng-hr/mL to about 350 ng-hr/mL upon administration of the oral dosage form to
the particular
species of mammal.
Embodiment 199. The oral dosage form of embodiment 195, wherein the
zoledronic acid is present in an amount that results in an AUG of zoledronic
acid of about 370
ng=hr/mL to about 420 ng=hr/mL upon administration of the oral dosage form to
the particular
species of mammal.
Embodiment 200. An oral dosage form comprising zoledronic acid having a
dose
of zoledronic acid and a configuration suitable for a particular species of
mammal, wherein the
zoledronic acid is present in an amount that results in a Cmax of zoledronic
acid of about 5 ng/mL
to about 300 ng/mL upon administration of the oral dosage form to the
particular species of
mammal.
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 201. The oral dosage form of embodiment 200, wherein the
zoledronic acid is present in an amount that results in a Cmax of zoledronic
acid of about 5 ng/mL
to about 50 ng/mL upon administration of the oral dosage form to the
particular species of
mammal.
Embodiment 202. The oral dosage form of embodiment 200, wherein the
zoledronic acid is present in an amount that results in a Cm,õ of zoledronic
acid of about 50
ng/mL to about 200 ng/mL upon administration of the oral dosage form to the
particular species
of mammal.
Embodiment 203. An oral dosage form comprising zoledronic acid having a
dose
of zoledronic acid and a configuration suitable for a particular species of
mammal, wherein the
oral dosage form is configured so that administration of the oral dosage form
to the particular
species of mammal results in a Tmax of zoledronic acid of about 0.4 hr to
about 1 hr.
Embodiment 204. The oral dosage form of embodiment 203, wherein the
oral
dosage form is configured so that administration of the oral dosage form to
the particular
species of mammal results in a Tmax of zoledronic acid of about 0.5 hr.
Embodiment 205. The oral dosage form of embodiment 203, wherein the
oral
dosage form is configured so that administration of the oral dosage form to
the particular
species of mammal results in a Tmax of zoledronic acid of about 0.75 hr.
Embodiment 206. An oral dosage form comprising zoledronic acid having a
dose
of zoledronic acid and a configuration suitable for a particular species of
mammal, wherein the
oral dosage form is configured so that the zoledronic acid has a 12 hour
sustained plasma level
factor of about 12 to about 50 for the particular species of mammal.
Embodiment 207. The oral dosage form of embodiment 206, wherein the
oral
dosage form is configured so that the zoledronic acid has a 12 hour sustained
plasma level
factor of about 20 to about 40 for the particular species of mammal.
Embodiment 208. The oral dosage form of embodiment 206 or 207 wherein
the
oral dosage form is configured so that the zoledronic acid has a 24 hour
sustained plasma level
factor of about 10 to about 30 for the particular species of mammal.
Embodiment 209. The oral dosage form of any of embodiments 206-208,
wherein
the oral dosage form is configured so that the zoledronic acid has a 24 hour
sustained plasma
level factor of about 10 to about 20 for the particular species of mammal.
Embodiment 210. The oral dosage form of any of embodiments 206-209
wherein
the oral dosage form is configured so that the zoledronic acid has a 36 hour
sustained plasma
level factor of about 6 to about 20 for the particular species of mammal.
51
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
Embodiment 211. The oral dosage form of any of embodiments 206-210,
wherein
the oral dosage form is configured so that the zoledronic acid has a 36 hour
sustained plasma
level factor of about 8 to about 15 for the particular species of mammal.
Embodiment 212. The oral dosage form of any of embodiments 206-211,
wherein
the oral dosage form is configured so that the zoledronic acid has a 48 hour
sustained plasma
level factor of about 5 to about 20 for the particular species of mammal.
Embodiment 213. The oral dosage form of any of embodiments 206-212,
wherein
the oral dosage form is configured so that the zoledronic acid has a 48 hour
sustained plasma
level factor of about 6 to about 15 for the particular species of mammal.
Embodiment 214. The oral dosage form of any of embodiments 206-213,
wherein
the oral dosage form is configured so that the zoledronic acid has a 72 hour
sustained plasma
level factor of about 4 to about 20 for the particular species of mammal.
Embodiment 215. The oral dosage form of any of embodiments 206-213,
wherein
the oral dosage form is configured so that the zoledronic acid has a 72 hour
sustained plasma
level factor of about 5 to about 10 for the particular species of mammal.
Embodiment 216. The oral dosage form of any of embodiments 206-215,
wherein
the oral dosage form is configured so that the particular species of mammal
has a plasma
concentration of zoledronic acid at 12 hours that is about 0.5 ng/mL to about
5 ng/mL.
Embodiment 217. The oral dosage form of any of embodiments 206-216,
wherein
the oral dosage form is configured so that the particular species of mammal
has a plasma
concentration of zoledronic acid at 24 hours that is about 0.2 ng/mL to about
2 ng/mL.
Embodiment 218. The oral dosage form of any of embodiments 206-217,
wherein
the oral dosage form is configured so that the particular species of mammal
has a plasma
concentration of zoledronic acid at 36 hours that is about 0.1 ng/mL to about
2 ng/mL.
Embodiment 219. The oral dosage form of any of embodiments 206-218,
wherein
the oral dosage form is configured so that the particular species of mammal
has a plasma
concentration of zoledronic acid at 48 hours that is about 0.1 ng/mL to about
2 ng/mL.
Embodiment 220. The oral dosage form of any of embodiments 206-219,
wherein
the oral dosage form is configured so that the particular species of mammal
has a plasma
concentration of zoledronic acid at 72 hours that is about 0.2 ng/mL to about
1 ng/mL.
Embodiment 221. The oral dosage form of any of embodiments 206-220,
wherein
the oral dosage form is configured so that the elimination half-life of
zoledronic acid in the
particular species of mammal is about 30 hours to about 100 hours.
Embodiment 222. The oral dosage form of any of embodiments 206-221,
wherein
the oral dosage form is configured so that the elimination half-life of
zoledronic acid in the
particular species of mammal is about 40 hours to about 60 hours.
52
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
[0194] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
properties such as molecular weight, reaction conditions, and so forth used in
the specification and
claims are to be understood in all instances as indicating both the exact
values as shown and as
being modified by the term "about." Accordingly, unless indicated to the
contrary, the numerical
parameters set forth in the specification and attached claims are
approximations that may vary
depending upon the desired properties sought to be obtained. At the very
least, and not as an
attempt to limit the application of the doctrine of equivalents to the scope
of the claims, each
numerical parameter should at least be construed in light of the number of
reported significant digits
and by applying ordinary rounding techniques.
[0195] The terms "a," "an," "the" and similar referents used in the
context of describing
the invention (especially in the context of the following claims) are to be
construed to cover both the
singular and the plural, unless otherwise indicated herein or clearly
contradicted by context. All
methods described herein can be performed in any suitable order unless
otherwise indicated herein
or otherwise clearly contradicted by context. The use of any and all examples,
or exemplary
language (e.g., "such as") provided herein is intended merely to better
illuminate the invention and
does not pose a limitation on the scope of any claim. No language in the
specification should be
construed as indicating any non-claimed element essential to the practice of
the invention.
[0196] Groupings of alternative elements or embodiments disclosed herein
are not to be
construed as limitations. Each group member may be referred to and claimed
individually or in any
combination with other members of the group or other elements found herein. It
is anticipated that
one or more members of a group may be included in, or deleted from, a group
for reasons of
convenience and/or patentability. When any such inclusion or deletion occurs,
the specification is
deemed to contain the group as modified thus fulfilling the written
description of all Markush groups
used in the appended claims.
[0197] Certain embodiments are described herein, including the best mode
known to the
inventors for carrying out the invention. Of course, variations on these
described embodiments will
become apparent to those of ordinary skill in the art upon reading the
foregoing description. The
inventor expects skilled artisans to employ such variations as appropriate,
and the inventors intend
for the invention to be practiced otherwise than specifically described
herein. Accordingly, the
claims include all modifications and equivalents of the subject matter recited
in the claims as
permitted by applicable law. Moreover, any combination of the above-described
elements in all
possible variations thereof is contemplated unless otherwise indicated herein
or otherwise clearly
contradicted by context.
[0198] In closing, it is to be understood that the embodiments disclosed
herein are
illustrative of the principles of the claims. Other modifications that may be
employed are within the
53
CA 02928350 2016-04-21
WO 2015/060924 PCT/US2014/050427
scope of the claims. Thus, by way of example, but not of limitation,
alternative embodiments may
be utilized in accordance with the teachings herein. Accordingly, the claims
are not limited to
embodiments precisely as shown and described.
54