Note: Descriptions are shown in the official language in which they were submitted.
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
1
PROCESS FOR PRODUCING REFINED NICKEL AND OTHER PRODUCTS FROM A
MIXED HYDROXIDE INTERMEDIATE
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to a process for the refining of an impure nickel
containing
intermediate, to produce a refined nickel metal, preferably a solid metallic
nickel powder.
The process may also involve the recovery of a nickel and cobalt containing
mixed sulphide
precipitate, a copper precipitate and/or a zinc precipitate, whilst rejecting
manganese and
magnesium into the leach residue. In a preferred process sulphur is recovered
as
ammonium sulphate.
In particular, the process relates to the processing of a mixed hydroxide
intermediate,
preferably a nickel-cobalt-manganese-magnesium hydroxyl-sulphate intermediate,
which
may also contain zinc and copper. Such intermediates are also known as Mixed
Hydroxide
Product (or Precipitate) or "MHP" or as referred to herein as a "mixed
hydroxide
intermediate".
Description of the Related Art
Recovery of nickel as an intermediate product for subsequent refining to a
metallic product is
well established, with mixed sulphide mattes from smelting operations and
mixed sulphide
precipitates from leaching operations being common examples.
Precipitation of intermediates from sulphuric acid leaching liquors as
hydroxides and
hydroxy-sulphates has been under consideration over an extended time period,
as
discussed for example in U.S. Patents 1,091,545, USP 2,899,300 and USP
3,466,144 and
Canadian Patent 618,826.
Nickel containing intermediates produced by addition of an alkali reagent are
commonly
referred to as mixed hydroxide precipitates (or products) or MHP, particularly
when they
contain significant quantities of cobalt and manganese and minor quantities of
zinc and
cobalt, as when they are derived from nickel laterite acid leach solutions or
nickel sulphide
acidic pressure oxidation leach solutions. In addition, if using magnesia as
the precipitating
agent, they typically contain some unreacted magnesium. Such
mixed hydroxide
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
2
intermediates typically contain 3% to 5% sulphur in the form of sulphate (9 to
15% sulphate),
so can more properly be termed a mixed hydroxy-sulphate or basic sulphate.
U.S Patent 1,091,545 describes a process for producing a nickel hydrate
product with
magnesia, from a purified solution produced from acid leaching of nickel
silicate ore.
Canadian Patent 618,826 describes a process that includes producing a nickel
and cobalt
intermediate product using an alkaline reagent, from a purified solution
produced from
pressure acid leaching of a lateritic nickel ore. The nickel and cobalt
containing intermediate
product may be re-leached in ammonia compounds.
U.S. Patent 2,899,300 includes a description for producing a mixed
nickel/cobalt
intermediate, using magnesia and provides an analysis, but does not give an
indication of a
suitable refining route.
U.S. Patent 3,466,144 describes a process which includes producing a nickel-
cobalt-
manganese containing intermediate product using an alkaline reagent, from a
purified
solution produced from pressure acid leaching of a lateritic nickel ore,
followed by refining of
the intermediate by leaching in ammonium sulphate with ammonia added. No
information is
given on the leaching conditions that may be employed.
Patent Application WO 01/6298 in the name of Anaconda Nickel Limited describes
a process
which includes leaching a MHP in ammoniacal ammonium sulphate solutions of
approximately 500 to 650 g/L ammonium sulphate concentration. The nickel in
solution is
reasonably dilute, such that its concentration is increased by evaporation of
part of the water
from the solution. The solution then undergoes a nickel-cobalt separation by
solvent
extraction, before hydrogen reduction of separate nickel and cobalt solutions.
A bleed of the
nickel-free ammonium sulphate solution is taken and treated with lime, to form
gypsum and
magnesium sulphate to enable the ammonia to be recovered by distillation.
Magnesium
sulphate is removed from the leach circuit in this manner, as indicated by
Figure 4 of the
Anaconda specification. The process described suffers the disadvantages of a
low nickel
tenor, requiring steam for concentration. There is also the need to recover
ammonia by a
two-stage process, requiring lime and steam, so that magnesium can be bled
from the leach
circuit. Subsequently it has the disadvantage of being costly to operate.
U.S. Patents 5,855,858 and 6,383,460 in the name of Cominco Engineering
Services Ltd.
both describe processes that include leaching a nickel/cobalt hydroxide that
also contains
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
3
magnesium, copper and zinc having been removed in upstream process steps. The
leaching of the mixed hydroxide is carried out in ammonium sulphate solution
with no free
ammonia and produces a leach solution with a recommended maximum nickel
concentration
g/L, although a wider range "of about 3 to 25 g/L" is claimed. It is then
followed by three
solvent extraction (SX) circuits. In the first SX circuit cobalt is extracted.
In the second SX
circuit magnesium is extracted. In the third SX circuit, nickel is extracted,
then stripped back
off the organic reagent into sulphuric acid so that it may be concentrated
into a form suitable
for electrowinning.
U.S. Patent 6,171,564, also in the name of Cominco Engineering Services Ltd
also
describes a process which includes leaching a nickel/cobalt hydroxide, also
including a
second ammonia leaching stage, where the nickel/cobalt hydroxide is derived
from
processing a laterite ore and contains manganese.
Due to the dilution of the ammonium sulphate leach liquor with various wash
water streams,
water must be evaporated to increase the ammonium sulphate strength, so that
it is suitable
for recycling as fresh leach liquor.
This process clearly has a number of disadvantages. Separation of cobalt and
magnesium
from nickel requires two solvent extraction processes. Nickel must be
concentrated to a
level suitable for metal recovery by a third solvent extraction process. Water
must be
evaporated from the leach liquor so that it is at a suitable concentration for
recycling to the
nickel/cobalt hydroxide leach.
U.S. Patent 5,976,218 in the name of Henkel Corporation describes a process
that includes
leaching a nickel/cobalt hydroxide in an ammonia - ammonium carbonate
solution, with a
titratable ammonia level of between 40 and 100 g/L. The nickel tenor of this
solution is
around 10 g/L. It is followed by an oxidation step to precipitate manganese
and ensure
cobalt is in the three plus (3+) oxidation state, then a nickel solvent
extraction step to
separate nickel and cobalt and concentrate the nickel for subsequent
electrowinning. Cobalt
is recovered by selective precipitation or solvent extraction. Like the above
described
patents this process suffers disadvantages of having low nickel concentration,
and requiring
a nickel-cobalt separation by solvent extraction. Water balance is maintained
by steam
stripping to recover ammonia and carbon dioxide from a bleed stream.
Another variant of this process is described in U.S. Patent 6,701,314. In this
variant, a
reducing agent in the form of a mixed nickel-cobalt sulphide or hydroxylamine
sulphate is
4
added to an ammonia - ammonium carbonate leach solution to enhance dissolution
of nickel and cobalt
from a mixed hydroxide. Leach solution concentrations of up to 16 wt% ammonia,
12 wt% carbon dioxide
and 1.5 wt% nickel (approximately 160 g/L ammonia, 120 g/L carbon dioxide and
15 g/L nickel) are claimed.
Descriptions of similar processes have been provided in the literature related
to commercial adaptations of
this flowsheet, including three by the same authors as U.S. Patent 5,976,218.
A 2007 paper by Fittock,
one of the inventors listed in U.S. Patent 6,701,314, in J.E. Fittock, Nickel
and Cobalt Refining by QNI Pty
Ltd, Yabulu, Qld, "Mawby" - AUSIMM Monograph 19 Volume Model Paper, Ausl MM,
file date: 2 April 2007,
describes the industrial application of this process variation, which includes
construction of new refining
capacity, nickel solution concentration of 23 g/L after MHP leaching, a nickel
solvent extraction step to
separate nickel and cobalt and concentrate the nickel, and subsequent nickel
recovery by steam distillation
to form basic nickel carbonate, which is then calcined and sintered to produce
nickel oxide and nickel metal
products.
Whilst these processes have been successful in producing refined nickel from
mixed hydroxide
intermediates, they suffer from a number of disadvantages in addition to those
described above, including
sulphate contamination of the leaching solution, and the requirement to build
new refining capacity at high
capital cost.
A number of published papers have included descriptions of leaching
nickel/cobalt hydroxide in ammonia-
ammonium sulphate solutions. A paper by Mason and Hawker presented at the ALTA
1998 Nickel Cobalt
Conference, in P.G. Mason, M. Hawker, Ramu Nickel Process Piloting, ALTA 1998
Nickel/Cobalt Pressure
Leaching & Hydrometallurgy Forum, Perth, 25-27 May 1998, provides a detailed
description of pilot plant
work on the Ramu Nickel / Cobalt project. The nickel/cobalt hydroxide was
produced using lime, so is
heavily contaminated with calcium sulphate (gypsum). The leach solution was
described as containing 45
to 65 g/L free ammonia and 135 g/L ammonium sulphate. After leaching the
solution contained 12 to 14
g/L nickel, with initially 1000 mg/L manganese, reducing to less than15 mg/L
with aeration (oxidation with
air). Similar to U.S. Patent 5,976,218, downstream process steps included an
oxidation step to precipitate
the remaining manganese and oxidise cobalt to the three plus (3+) oxidation
state, nickel solvent extraction
to separate nickel and cobalt and concentrate the nickel for subsequent
electrowinning and cobalt and zinc
recovery by precipitation. Extraction of nickel in the ammonia-ammonium
sulphate leach was relatively
modest at around 90%, requiring a further acid leaching treatment to increase
recovery. Ammonia was
recovered by a lime boil, a two stage process, requiring lime and steam, so
that water could be bled from
the leach circuit. This process has the disadvantages of the processes
described previously and in addition
requires an additional acid leaching step to achieve high nickel recovery.
A paper by Steemson at the ALTA 1999 Nickel Cobalt Conference in M.L.
Steemson, "The Selection of a
Hydroxide Precipitation / Ammoniacal Releach Circuit for Metal Recovery from
Acid Pressure Leach
Liquors", ALTA 1999 Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum,
Perth, 11-12 May
Date Recue/Date Received 2021-06-09
5
1999, ALTA Metallurgical Services, Melbourne, Australia, 1999, gives a
comparison of various aspects of
processing mixed hydroxide precipitates, with particular reference to the Ramu
project discussed by Mason
and Hawker. This paper gives typical mixed hydroxide assays based on using
either a high quality
commercial magnesia (MgO) or lime (either CaO or Ca(OH)2), as follows:
Assay, weight %, dry basis
Precipitant Nickel Cobalt Magnesium Manganese
Magnesia 38 1.4 4 2.5
Lime 19 0.8 0.7 2.1
During the re-leach of the lime based mixed hydroxide precipitate in ammonia-
ammonium sulphate solution,
it was noted that 50 to 70% of the magnesium extracted and calcium reached
saturation concentration in
solution of 500 to 700 mg/L. In particular, the paper states that, "It is
clear that magnesium buildup would
be excessive if an ammonia / ammonium sulphate leachate were treating an MgO
based nickel / cobalt
hydroxide." This is a distinct disadvantage of this combination and process.
It is apparent that the above described processes each suffer from more than
one of the following
disadvantages:
1. Nickel concentrations in the leach solution are less than 30 g/L, meaning
further concentration
steps are required for economic recovery of a final nickel product;
2. Initial manganese concentrations in solution are elevated, requiring
further treatment to reduce
them to manageable levels;
3. Magnesium is soluble in ammonium sulphate solution, which would lead to
contamination of co-
product ammonium sulphate crystal, resulting in the need to recover ammonia by
other means;
4. Cobalt must be separated from nickel by a solvent extraction step; and
5. They are new flowsheets, requiring significant capital expenditure for new
plant and equipment.
An ammonia based nickel refining process which has been used on nickel
sulphide concentrates and nickel
mattes is the Sherritt Gordon pressure leach ¨ pressure reduction process.
This process has been
described in detail in a number of papers and in the book "The Winning of
Nickel", J.R. Boldt, P.E. Queneau,
Pressure Leaching with Ammonia ¨ Sherritt Gordon, in, The Winning of Nickel:
Its Geology, Mining, and
Extractive Metallurgy, Methuen, 1967, 299-314. In this process, nickel
sulphide feed, containing nickel,
cobalt,
Date Recue/Date Received 2021-06-09
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
6
copper, zinc, iron and sulphur is oxidatively leached in an ammonia - ammonium
sulphate
solution at temperatures up to 100 C and pressures up to 1000 kPag using
ammonia, to
form metal ammine complexes in an ammonium sulphate solution. Copper removal
is
combined with an ammonia removal step known as "copper boil". This adjusts the
solution
chemistry, so that cobalt does not substantially contaminate nickel produced
in pressure
reduction. Zinc does not reduce in pressure reduction, so is left in solution
to be recovered
along with cobalt and residual nickel as part of a mixed cobalt-nickel-zinc
sulphide product.
In one variant of this process, as described for instance in U.S Patents U.S.
5,468,281, U.S.
6,264,904, and U.S. 6,267,800, as well as a number of publications, mixed
nickel-cobalt
sulphides, undergo a conventional ammonia - ammonium sulphate leach, as
described
above, followed by nickel-cobalt separation by fractional crystallisation to
produce a
cobalt(III) hexamine salt and a nickel solution. Nickel is then recovered via
the copper boil,
and subsequent conventional oxydrolysis and hydrogen pressure reduction steps.
Cobalt is
re-dissolved then recovered by hydrogen pressure reduction.
In another variant, this process has been modified to produce a leach solution
with a nickel
concentration feeding reduction in excess of 100 g/L.
To date the variants of the Sherritt Gordon process have not treated feeds
high in
manganese and magnesium, due to potential for contamination of refinery
products, in
particular manganese in nickel metal and magnesium in ammonium sulphate
crystal.
U.S. Patent 3,816,098 (G.B. Patent 1,439,380) in the name of Sherritt Gordon
Mines Limited
describes a process to treat a partially refined basic nickel carbonate feed
from which cobalt
and copper have been removed prior to precipitation and containing 0.1 to 0.2%
magnesium
and 0.1 to 0.2% manganese. After leaching in ammonia - ammonium sulphate
solution,
92.7% of the magnesium and 60% of the manganese were extracted. After solid-
liquid
separation and oxidation at >200 C, the resulting solution contained 44.2 g/L
nickel, 2.4 g/L
magnesium and 2.0 g/L manganese. Nickel was reduced by hydrogen under
pressure, to
produce nickel metal powder containing 11 g/t magnesium and 16 g/t manganese.
The
build-up of magnesium and manganese was controlled by bleeding contaminated
ammonium sulphate solution from the process. This patent is silent on methods
by which
the contaminated bleed solution is treated to dispose of the magnesium and
manganese and
recover the ammonium sulphate. Thus, this process too suffers from a number of
disadvantages, including:
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
7
1. The requirement to substantially remove magnesium, manganese, cobalt and
copper ahead of the leaching process;
2. Substantial dissolution of magnesium and manganese in the leach;
3. Nickel metal powder contamination with magnesium and manganese
substantially
higher than typical in hydrogen pressure reduction;
4. The requirement to bleed magnesium and manganese contaminated ammonium
sulphate solution from the process.
The present invention provides a process for producing refined nickel metal
free of
manganese impurities from a mixed hydroxide intermediate that may generally
contain
significant quantities of magnesium and manganese. It also provides a process
for
producing copper sulphide, mixed cobalt-nickel-zinc sulphide and ammonium
sulphate
products, free from unacceptable levels of magnesium and manganese
contamination.
The present invention aims to overcome one or more of the difficulties or
disadvantages
identified in the prior art documents.
A reference herein to a patent document or other matter which is given as
prior art is not to
be taken as an admission that that document or matter was known or that the
information it
contains was part of the common general knowledge as at the priority date of
any of the
claims.
SUMMARY OF THE INVENTION
According to the present invention, there is provided a process to produce a
refined nickel
metal product from a mixed hydroxide intermediate. The process involves
ammonia
leaching a slurry containing the nickel and recovering the refined nickel
product by direct
hydrogen reduction of the resultant leach solution under pressure. In a
preferred
embodiment, other products may also be recovered from the leach solution, for
example
cobalt, zinc, copper and other nickel products from the residual nickel
remaining in the leach
solution, while also recovering ammonia as ammonium sulphate for reuse in the
leach
process, and/or as a saleable product. The process has the advantage of
rejecting
magnesium and manganese impurities from the liquor stream prior to the
recovery of the
metal values.
The mixed hydroxide intermediate product may be produced as an intermediate
product in
the processing of both nickel laterite and nickel sulphide materials including
concentrates. It
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
8
is also commonly referred to as a mixed hydroxide precipitate (or product), or
MHP, but
herein is referred to as a "mixed hydroxide intermediate".
The mixed hydroxide intermediate may be recovered from a sulphuric acid
leaching liquor
during the processing of a nickel laterite ore. For example, such intermediate
products are
produced when an alkali reagent such as magnesia or lime is used to raise the
pH of an
acidic sulphate solution to precipitate a nickel product as a mixed hydroxide
intermediate.
Such intermediates are also recovered from nickel sulphide acidic pressure
oxidation leach
solutions by the addition of a comparable alkali material.
Typically, a mixed hydroxide intermediate produced from processing a nickel
laterite or
nickel sulphide ore, contains the main element of interest, namely nickel,
together with
variable quantities of metals such as cobalt, zinc and copper as well as
sulphur in the form of
sulphates, and impurities such as manganese, magnesium, iron and calcium.
Sodium and
chlorine, in the form of chloride, may also be present.
The process of the present invention provides a process where a refined nickel
metal
product may be recovered from the mixed hydroxide intermediate by mixing the
mixed
hydroxide intermediate with a sulphide containing material having reductant
properties, most
preferably as ground nickel matte, or nickel containing sulphide or
concentrate, which in
general are impure nickel containing sulphide intermediate products from
smelting, selective
precipitation or concentration processes.
In the process of the present application, the combined sulphide containing
reductant
material / mixed hydroxide intermediate is formed into a slurry and leached
under pressure
with ammonia to dissolve any nickel, cobalt, copper and zinc while rejecting
the majority of
the manganese and magnesium. Ultimately, the nickel in the solution is
treated, preferably
with the addition of hydrogen at elevated temperature and pressure to reduce
the nickel to a
metallic nickel product, most preferably a metallic nickel powder. The copper,
cobalt and
zinc may also be recovered in additional sulphiding recovery stages.
Accordingly, in a first embodiment of the present invention there is provided
a process for
the recovery of a refined nickel metal product from a mixed hydroxide
intermediate that has
been produced from the processing of a nickel laterite or sulphide ore, the
process including
the steps of:
a) providing a mixed hydroxide intermediate and blending with a solution to
form an
intermediate slurry;
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
9
b) mixing the mixed hydroxide intermediate slurry with a sulphide containing
material,
preferably ground nickel matte and/or nickel containing mixed sulphide, to
form a
combined slurry;
c) leaching the combined slurry with ammonia in an ammonia pressure leach step
to
dissolve the contained nickel, cobalt, zinc and any copper present into a
leach
solution, while maintaining the majority of the manganese and magnesium in a
leach
residue;
d) adding a reducing agent preferably hydrogen, to the leach solution under
pressure to
reduce the nickel to metallic nickel product; and
e) recovering the solid metallic nickel from the resultant slurry.
Preferably, the metallic nickel product is a metallic nickel powder.
In a preferred embodiment of the process, the mixed hydroxide intermediate is
washed with
water, preferably desalinated water, or water containing low levels of
dissolved salts, prior to
forming the intermediate slurry, so as to substantially or partially remove
any calcium,
sodium and chloride that may be present. The washing particularly has the
advantage of
partially removing some magnesium and sulphate sulphur while converting the
remaining
magnesium to a less reactive hydroxide form.
The washed mixed hydroxide intermediate is then formed into an aqueous
intermediate
slurry. In a preferred embodiment, this step is achieved by the addition of
ammonium
sulphate at atmospheric pressure, either in the form of a solution or slurry
dosed with
ammonium sulphate crystals and preferably containing thiosulphates and
polythionates,
which enables partial reductive leaching of oxidised manganese, cobalt and
nickel.
Preferably, the ammonium sulphate or at least a part of the ammonium sulphate
used is
recycled from the barren solution following the ammonia pressure leach step,
while the
ammonium sulphate crystals are recycled from a cobalt and zinc sulphiding
recovery stage.
The ammonium sulphate solution or slurry preferably has low titratable
ammonia, in the
range of 0 to 20g/L before addition of the mixed hydroxide intermediate.
The intermediate slurry is then mixed with a sulphide containing material,
such as ground
nickel matte or nickel containing mixed sulphide ahead the ammonia pressure
leach step, or
it may be mixed during the ammonia pressure leach step. The preferred ratio of
mixed
hydroxide to matte can vary over a wide range, depending largely on
availability of
feedstocks and economic considerations. Ratios of up to 1 part nickel in mixed
hydroxide
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
intermediate to 1 part nickel in matte or mixed sulphide are preferred, to
avoid having to add
external reductants, which can be expensive and make process chemistry more
difficult to
control.
The ammonia used in the ammonia pressure step is preferably in the form of a
gas, liquid or
aqueous solution. The pressure leach step is preferably conducted at a
temperature of from
70 to 120 C and a pressure of from 600 to 1000 kPag in the presence of oxygen
and carbon
dioxide. This will produce a high concentration of nickel in the leach
solution, typically 70 to
115 g/I. The manganese is oxidised to form an insoluble manganese containing
residue.
The carbon dioxide is absorbed into the ammonia solution and reacts with
magnesium to
form an insoluble basic magnesium carbonate compound, and to enhance magnesium
and
manganese precipitation. Preferably, reductants such as metal sulphides,
thiosulphates or
polythionates are also included into the ammonia pressure leach solution so as
to maximise
leaching of nickel and cobalt from the mixed hydroxide intermediate.
In a preferred embodiment, the leach slurry will undergo a solid/liquid
separation step after
this first stage of ammonia pressure leaching, where the leach solution is
sent for
downstream processing, while the partially leached solids are subjected to a
second stage
ammonia pressure leach. Air is preferably added to this second stage leach.
After a further
solid/liquid separation, the first and second stage leach solutions are
combined for further
processing.
Preferably a third stage ammonia pressure leach of the remaining solids is
included with the
leach solution added to that of the final and second stage leach.
The barren ammonia liquor from this stage may be recycled to the atmospheric
leach stage
in forming the intermediate slurry, or to the ammonia pressure leach step.
It is further preferred that the combined leach solutions containing nickel,
cobalt, copper and
zinc is first treated to remove copper from the solution before recovery of
the nickel, cobalt
and zinc. The copper may be separately precipitated from the solution in a
copper boil
process. In this process it is also preferred to adjust the titratable ammonia
to nickel molar
ratio in the leach solution to the range of 1.8 to 2.2 : 1, most preferably
around 2 : 1, so as to
ensure a minimal amount of cobalt is recovered as metal in the downstream
nickel reduction
step. Copper may also be removed from solution by other techniques such as
precipitation
with a suitable sulphiding reagent, such as hydrogen sulphide.
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
11
Following the copper removal step, the leach solution preferably undergoes
oxydrolysis
where it is oxidised with air and heated to remove any remaining thiosulphate,
polythionate
and sulphamate compounds to avoid potential contamination of nickel and
ammonium
sulphate products. The leach solution then flows to the hydrogen reduction
step where
hydrogen is added to the solution under pressure at elevated temperature, so
as to reduce
the nickel to a nickel metal powder. Nickel may be recovered by
powder/solution separation
techniques and the nickel powder formed into other forms such as briquettes.
The process lends itself to the further benefit in that cobalt and zinc, in
particular, may be
recovered from the leach solution together with any residual nickel that may
remain in the
leach solution. In a preferred embodiment, a suitable sulphiding agent such as
hydrogen
sulphide, sodium hydrosulphide, sodium sulphide or ammonium sulphide is added
to the
leach solution following the step of the metallic nickel powder recovery, to
recover the cobalt,
zinc and any residual nickel as a mixed cobalt/nickel/zinc sulphide product.
Alternatively, the
zinc may advantageously be recovered in a two-stage sulphiding process where
the zinc is
recovered first in the sulphiding process as a zinc sulphide product, and then
the cobalt and
any residual nickel recovered in a second stage of the sulphiding process as a
mixed
nickel/cobalt sulphide product.
The process also has the advantage that any excess ammonium sulphate formed
during this
process, may be recovered by crystallisation of ammonium sulphate from the
solution.
Preferably, steam is used to evaporate the water to enable crystallisation. At
least a portion
of the recovered ammonium sulphate crystals may then be recycled, for use in
the
atmospheric leach step while forming the intermediate slurry, or the ammonia
pressure leach
step.
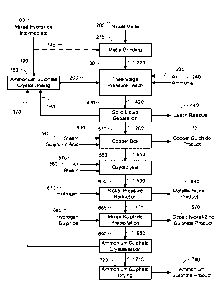
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic flowsheet of the overall process of a preferred
embodiment of the
invention.
Figure 2 is a schematic flowsheet of the leaching phase of the process
described in Figure 1.
Figure 3 is a schematic flowsheet of the product recovery phase of the process
described in
Figure 1.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Mixed hydroxide intermediate 100 is water washed 110 using desalinated water
120, to
remove or partially remove contaminants. These include magnesium, calcium,
sulphate,
chloride, and sodium components. The water wash can consist of the washing of
a filter
12
cake on a filter, re-pulping of the mixed hydroxide intermediate in water to
give a "water leach", a
combination of these, or other techniques such as continuous counter-current
decantation. Multiple stages
of water washing have been found to be beneficial as has extended leaching
times of up to several days.
Multiple contacts are especially advantageous for calcium removal, as calcium
will continue to leach out of
the mixed hydroxide intermediate, constrained only by its solubility limit. An
additional benefit of the water
wash has been found to be the "fixing" of most of the remaining magnesium in
the mixed hydroxide
intermediate, so that it does not substantially dissolve in downstream ammonia
leaching operations. The
waste wash liquor 130 must be disposed of separately to other refinery
liquors, so the water washing step
is preferably carried out at the location where the mixed hydroxide
intermediate is produced.
The washed mixed hydroxide intermediate 140 is initially leached 150 in an
ammonium sulphate containing
solution consisting of liquor recycled from the third stage pressure leach
160, dosed with ammonium
sulphate crystals 170. This leach liquor 190 typically contains 0 to 20 g/L of
titratable ammonia, 500 to 600
g/L of ammonium sulphate, and 3 to 6 g/L of thiosulphate and polythionates,
enabling reductive leaching of
oxidised manganese and cobalt. The ammonium sulphate crystal dosing 180 and
mixed hydroxide
intermediate leaching 150 may be carried out in a combined step. In addition
to nickel and cobalt, a
significant portion of the manganese goes into solution, while most of the
magnesium does not dissolve.
The resultant mixed hydroxide intermediate leach slurry 200 is forwarded to
the first stage ammonia
pressure leach 210 where it is mixed with ground nickel matte 220.
Alternatively washed mixed hydroxide
intermediate 145 may be directly blended with ground nickel matte 220 (Figure
2), or the nickel matte 205 in
matte grinding step 215 as shown in Figure 1, ahead of first stage ammonia
pressure leach. Air 230 and
ammonia 240 are added to leach the nickel matte - mixed hydroxide intermediate
blend in a three stage
pressure leach 30 to produce a high nickel concentration in solution -
typically 70 to 115 g/L, and oxidise
manganese to form an insoluble manganese containing leach residue. Carbon
dioxide, which is naturally
in air at a concentration of 0.04% by volume or 0.06% by mass, is absorbed
into the ammonia solution and
reacts with magnesium to form an insoluble basic magnesium carbonate compound.
Carbon dioxide can
also be added to the leach to enhance magnesium precipitation.
After the first stage leach, the leach slurry 250 undergoes solid-liquid
separation 260. The first stage leach
liquor 270 is forwarded to downstream processes for further purification and
nickel recovery 280. The
partially leached solids 290 are subjected to a second stage ammonia pressure
leach 300. Air 310 and
ammonia 320 are again added to leach the partially leached solids to produce a
high concentration nickel
solution. If necessary, mixed
Date Recue/Date Received 2021-06-09
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
13
hydroxide intermediate leach slurry 330 is added to increase the ammonium
sulphate
concentration to control the leach chemistry. After the second stage leach,
the leach slurry
340 undergoes solid-liquid separation 350. The second stage leach liquor 360
is mixed with
the first stage leach liquor for downstream processing 280.
The partially leached solids 370 are subjected to a third stage ammonia
pressure leach 380.
Air 390 and ammonia 400 are again added to leach any remaining nickel. Mixed
hydroxide
intermediate leach slurry 410 may again be added to increase the ammonium
sulphate
concentration. The third stage leach slurry 420 undergoes solid-liquid
separation 430. The
leach residue solids 440 may be further treated by filtration and washing to
produce a final
filter cake suitable for either disposal or further treatment for metals
recovery. The third
stage leach liquor 160 is forwarded to the ammonium sulphate crystal dosing
tank 180.
Air may be added as separate streams to each pressure leaching stage as shown
in Figure
2, or it may be added in a counter-current manner, such that the exhaust air
from the Stage
3 leach becomes the feed air to Stage 2 leach 310 and the exhaust air from
Stage 2 leach
becomes the feed air for Stage 1 leach 230.
The combined first and second stage leach liquors 280 are forwarded to Copper
Boil 510.
Here copper is precipitated from solution as copper sulphide product 520, and
titratable
ammonia levels are adjusted, by the addition of steam 530 and sulphuric acid
540, to a ratio
of around 2 moles of ammonia per mole of nickel, in preparation for nickel
reduction. The
reduction feed solution 550 is forwarded to Oxydrolysis 560 where it is
oxidised with air 570
and heated with steam 580 to remove any remaining thiosulphate, polythionate
and
sulphamate compounds which would otherwise cause product contamination. The
solution
590 then flows into Nickel Pressure Reduction 600, where hydrogen 610 is added
in a
batchwise manner to an agitated Autoclave to produce nickel metal powder.
Nickel pressure
reduction discharge 620 undergoes powder ¨ solution separation 630. Metallic
nickel powder
product 640 may be transformed into other forms such as briquettes by drying
and
compacting in a conventional manner. The reduction end solution 650 is then
treated with a
sulphiding reagent such as hydrogen sulphide 660 in a mixed sulphide
precipitation step 665
to produce a mixed cobalt-nickel-zinc sulphide product 670. The barren
ammonium sulphate
solution 680 passes to ammonium sulphate crystallisation 690. Steam 700 is
used to
evaporate the water for crystallisation. Crystalliser configurations can
include multiple effect
evaporators, vapour recompression or a combination of both. Ammonium sulphate
crystal
slurry 710 undergoes crystal ¨ liquor separation or drying 720, with the
liquor 730 returned to
crystallisation. Ammonium sulphate crystal is directed to both product 740 and
Ammonium
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
14
Sulphate Crystal Dosing 750 of Third Stage Leach liquor ahead of mixed
hydroxide
intermediate leaching.
Whilst the steps from Copper Boil through to mixed sulphide precipitation are
carried out in a
conventional manner, a surprising aspect of this flowsheet is that the small
quantity of
manganese remaining in solution after the three stage pressure leach, remains
in solution
throughout the remaining process steps so does not contaminate copper
sulphide, nickel
metal or mixed cobalt-nickel-zinc sulphide products.
Examples
Example 1
Typical assays of the mixed hydroxide intermediate and nickel matte refinery
feeds are
shown in the table below. In addition, a typical precipitated nickel sulphide
analysis is
provided, as this is a feed that may partially or totally substitute the
nickel matte.
Refinery Feeds Analysis, wt% dry basis
Ni Co Mn Mg Fe Ca S Zn Cu
Typical Mixed Hydroxide
47 1.7 2.0 1.5 0.13 0.09 4.4 0.31 0.03
Intermediate
Typical Nickel Matte 70.5 0.72 - - 3.75 - 20 - 3.1
Typical Precipitated
55 5 - 1 - 35 1 0.1
Nickel Sulphide
Those skilled in the art would understand that the compositions of the feeds
can vary over a
significant range from those presented above.
Mixed hydroxide intermediates can be highly variable in composition. Iron,
aluminium and
silicon levels vary depending on the efficiency of upstream impurity removal
processes.
Manganese and cobalt levels vary depending on their concentration relative to
nickel in the
CA 02931118 2016-05-19
WO 2015/081368
PCT/AU2014/001087
feed solution to the hydroxide precipitation step, which ultimately reflects
on the ore or
concentrate feed composition to the leaching process upstream of the mixed
hydroxide
precipitation step.
Nickel matte may have differing levels of iron content, depending on the
extent to which iron
is removed in the smelter converting process. Copper and cobalt levels can
also vary
significantly, depending on the concentrate feed to the smelter and the
converting process
used.
Mixed sulphide precipitates will have varying levels of cobalt, also depending
on the on the
ore or concentrate feed composition to the leaching process upstream of the
mixed sulphide
precipitation step.
Example 2
Typical analyses of the leach residue with nickel matte only and with combined
feeds are
shown in the following table. From the assays it is clear that manganese and
magnesium
from the mixed hydroxide intermediate are reporting to the leach residue.
Third Stage (Final) Leach Residue Analysis, dry basis
Ni, Co, Mn, Mg, Fe, Zn, Cu, Ca, S,
wt% wt% wt% wt%
g/t wt% wt% wt%
Nickel Matte Only Feed 5.3 1.0 60 g/t 0.1 51 60 0.7 0.2
3.2
Combined Nickel Matte plus Mixed
5.1 1.0 2 wt% 1.0 49 200 0.7 0.2
3.5
Hydroxide Intermediate
Example 3
Typical analyses of the copper sulphide product with nickel matte only and
with combined
feeds are shown in the following table. From the assays it is clear that
manganese and
magnesium from the mixed hydroxide intermediate are not reporting to the
copper sulphide
product.
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
16
Copper Sulphide Product Analysis, dry basis
Cu, S, Mn, Mg,
wt% wt% g/t g/t
Nickel Matte Only Feed 60 35 <1 <2
Combined Nickel Matte plus Mixed Hydroxide Intermediate 60 35 <1 <2
Example 4
Typical analyses of the nickel pressure reduction feed liquor with nickel
matte only and with
combined feeds are shown in the following table. Manganese has been
substantially
removed from solution at this point, while reporting to leach residue and not
contaminating
copper sulphide product. Magnesium has increased, but remains at least an
order of
magnitude below which it would dilute the nitrogen content of the ammonium
sulphate
product by any significant amount.
Nickel Pressure Reduction Feed Liquor Analysis
Ni, Co, Mn, Mg, Zn, S,
g/L g/L mg/L mg/L mg/L g/L
Nickel Matte Only Feed 110 1.3 0 14 1 142
Combined Nickel Matte plus Mixed Hydroxide
120 1.5 3 35 5 145
Intermediate
Example 5
Typical analyses of the nickel metal product with nickel matte only and with
combined feeds
are shown in the following table. From the assays it is clear that manganese
and
magnesium from the mixed hydroxide intermediate are not reporting to the
nickel metal
product.
Nickel Metal Product Analysis
Ni, Co, Mn, Mg, Fe, Zn, Cu, Ca, S,
wt% g/t g/t g/t g/t g/t g/t g/t g/t
Nickel Matte Only Feed >99.8 650 <1 <2 50 2 15 10 160
Combined Nickel Matte plus Mixed
>99.8 650 <1 <2 50 2 15 10 160
Hydroxide Intermediate
Example 6
Typical analyses of the mixed nickel-cobalt-zinc sulphide product with nickel
matte only and
with combined feeds are shown in the following table. From the assays it is
clear that zinc
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
17
reports to this product, while that manganese and magnesium from the mixed
hydroxide
intermediate are not reporting to the mixed nickel-cobalt-zinc sulphide
product.
Mixed Nickel-Cobalt-Zinc Sulphide Product Analysis, dry basis
Ni, Co, Zn, S, Mn, Mg,
wt% wt% wt% wt% g/t g/t
Nickel Matte Only Feed 28 28 0.1 35 <1 <2
Combined Nickel Matte plus Mixed Hydroxide
28 28 0.5 35 <1 <2
Intermediate
Example 7
Typical analyses of the ammonium sulphate product with nickel matte only and
with
combined feeds are shown in the following table. From the assays it is clear
that
manganese and magnesium from the mixed hydroxide intermediate is not reporting
to this
product in excessive quantities.
Ammonium Sulphate Analysis, dry basis
N, S, Mn, Mg,
wt% wt% g/t g/t
Nickel Matte Only Feed 21 24 <1 39
Combined Nickel Matte plus Mixed Hydroxide Intermediate 21 24 <1 60
The limits of mixed hydroxide intermediate addition depend primarily on
economic drivers.
As the level of cobalt in refinery feed increases, an equivalent mass of
nickel is typically not
reduced and reports to mixed sulphide. As a saleable product, the revenue
received for the
contained nickel in this stream is less than if sold as nickel metal. One
variant to overcome
this limitation is to add the cobalt hexamine precipitation process, as
discussed for instance
in U.S. Patent 5,468,281, into the flowsheet. Zinc may reach an economic
limit, as refiners
of cobalt-nickel-zinc mixed sulphide typically have limits on zinc levels
and/or apply penalties
for high zinc feeds. This limitation may be overcome by producing separate
zinc sulphide
and mixed sulphide products by a two stage sulphiding process.
CA 02931118 2016-05-19
WO 2015/081368 PCT/AU2014/001087
18
The relative quantities of thiosulphates and polythionates that aid manganese
and cobalt
dissolution will reduce as the proportion of nickel feed from the mixed
hydroxide intermediate
increases, but this can be compensated for by the addition of other reductant
species.
As the relative quantity of mixed hydroxide intermediate increases, the
ability of the leach
process to produce a nickel leach solution of high nickel tenor may become a
limitation. This
again is an economic rather than a technical limitation, with a wide range of
nickel
concentrations contemplated, for instance, US Patent 6,949,232 stating, "The
leach solution
produced will typically contain from 40 to 110 g/L nickel..."
Thus, within the constraints described above, there is no particular
limitation to the relative
quantity of mixed hydroxide that may be processed.
Advantages of processing the mixed hydroxide intermediate in this manner are:
1. Nickel pressure reduction capacity is not compromised because the nickel
concentration is maintained, due to the co-processing of feedstocks.
2. Capacity increases in the ammonia pressure leach are possible due to the
lower air
requirements for oxidising manganese in mixed hydroxide intermediate, relative
to
sulphur and iron in nickel sulphide matte. This also results in lower
operating costs.
3. Refining costs are lowered due to lower sulphur levels in mixed hydroxide
intermediate, relative to nickel matte. The typical sulphur to nickel mass
ratio in
mixed hydroxide intermediate is 1 to 12, whereas in nickel matte it is 1 to 3.
This
results in reduced ammonia make up requirements for producing ammonium
sulphate. It also results in lower steam usage for crystallising and drying
the
ammonium sulphate so produced.
4. Copper & zinc do not have to be removed from the mixed hydroxide
intermediate
feedstock.
5. It is also contemplated that the manganese from the mixed hydroxide
intermediate
sequesters arsenic and selenium which may be present in the nickel sulphide
matte,
CA 02931118 2016-05-19
WO 2015/081368
PCT/AU2014/001087
19
allowing reduced quantities of iron to be included in the refinery feed for
sequestering
these elements.
The invention described herein is susceptible to variations, modification
and/or additions
other than those specifically described and it is to be understood that the
invention includes
all such variations, modifications and/or additions which fall within the
spirit and scope of the
above description.
Further patent applications may be filed in Australia or overseas on the basis
of, or claiming
priority from the present application. It is to be understood that the
following provisionial
claims are provided by way of example only and are not intended to limit the
scope of what
may be claimed in any such future application.
Features may be added to or omitted from the provisional claims at a later
date so as to
further define or re-define the invention or inventions.