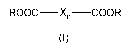Note: Descriptions are shown in the official language in which they were submitted.
CA 02931742 2016-05-26
WO 2015/078966 1 PCT/EP2014/075812
Coating Composition
The present invention relates to coating compositions; in particular to
coating compositions for
use in food and/or beverage containers.
A wide variety of coatings have been used to coat food and/or beverage
containers. The
coating compositions are required to have certain properties such as being
capable of high
speed application, having excellent adhesion to the substrate, being safe for
food contact and
having properties once cured that are suitable for their end use.
Many of the coating compositions currently used for food and beverage
containers contain
epoxy resins. Such epoxy resins are typically formed from polyglycidyl ethers
of bisphenol A
(BPA). BPA is perceived as being harmful to human health and it is therefore
desirable to
eliminate it from coatings for food and/or beverage packaging containers.
Derivatives of BPA
such as diglycidyl ethers of bisphenol A (BADGE), epoxy novolak resins and
polyols prepared
from BPA and bisphenol F (BPF) are also problematic. Therefore there is a
desire to provide
coating compositions for food and beverage containers which are free from BPA,
BADGE
and/or other derivatives, but which retain the required properties as
described above.
Polyester resins produced by the polycondensation reaction of polyols and
polyacids are well
known in the coatings industry. Both linear and branched polyesters have been
widely used in
coating compositions. It is desirable that the polyesters used in coating
compositions for
packaging have a high glass transition temperature (Tg). Typically, high Tg
polyesters have
been synthesised from cyclic, polycyclic and aromatic polyols. However, many
of these
polyesters are not food compact compliant. Alternative polyesters such as
polyethylene
terephthalate (PET) and polyethylene naphthalate (PEN), which are synthesised
from aliphatic
polyols, have been used in a solid form for thermoplastics and films.
It is an object of aspects of the present invention to provide one or more
solutions to the above
mentioned or other problems.
According to a first aspect of the present invention there is provided a
coating composition for
a food and/or beverage container comprising a polyester material, wherein the
polyester
material comprises the reaction product of;
(a) 1,2-propanediol,
(b) terephthalic acid, and
(c) a molecular weight increasing agent,
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
2
characterised in that the polyester material has a number-average molecular
weight (Mn) of at
least 6,100 Da and a glass transition temperature (Tg) of at least 80 C;
wherein the molecular weight increasing agent (c) comprises a polyacid, a
polyol or a
combination thereof;
wherein the polyacid comprises a diacid of general formula (I)
R000¨Xn¨COOR
formula (I)
wherein each R independently represents hydrogen or an alkyl, alkenyl,
alkynyl, or aryl group;
n = 0 or 1; wherein X represents a bridging group selected from: an alkylene
group; an
alkenylene group; an alkynylene group; an arylene group; wherein the bridge
between the ¨
COOR groups is C1 or C2;
and wherein the hydroxyl groups of the polyol are connected by a C1 to C3
alkylene group.
By "molecular weight increasing agent" we mean a substance that increases the
number-
average molecular weight (Mn) of the polyester material.
The molecular weight increasing agent may be any suitable compound capable of
increasing
the Mn of the polyester material. The molecular weight increasing agent
comprises a polyacid,
a polyol or a combination thereof.
In certain embodiments, the molecular weight increasing agent comprises a
polyacid.
"Polyacid" and like terms, as used herein, refers to a compound having two or
more carboxylic
acid groups, such as two, three or four acid groups, and includes an ester of
the polyacid
(wherein one or more of the acid groups is esterified) or an anhydride.
Suitable examples of polyacid molecular weight increasing agents include, but
are not limited
to one or more of the following: oxalic acid; malonic acid; succinic acid;
orthophthalic acid;
isophthalic acid; maleic acid; fumaric acid; itaconic acid; methylmalonic
acid; ethylmalonic
acid; propylmalonic acid; 2-methylsuccinic acid; 2-ethylsuccinic acid; 2-
propylsuccinic acid;
trans-cyclopentane-1,2-dicaboxylic acid; cis- cyclopentane-1,2-dicaboxylic
acid; trans-
cyclohexane-1,2-dicaboxylic acid; cis-cyclohexane-1,2-dicaboxylic acid; 1,4-
cyclohexane
dicarboxylic acid; 2,6-naphthalene dicarboxylic acid; acids and anhydrides of
all the
aforementioned acids and combinations thereof. In certain embodiments, the
polyacid
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
3
comprises maleic anhydride or itaconic acid or a combination thereof.
Suitably, the polyacid
comprises maleic anhydride.
In certain embodiments, the molecular weight increasing agent may comprise a
polyol.
"Polyol" and like terms, as used herein, refers to a compound having two or
more hydroxyl
groups. In certain embodiments, the polyol may have two, three or four
hydroxyl groups.
Suitably, the polyol may comprise a trio!. The hydroxyl groups of the polyol
are connected by
a C1 to C3 alkylene group. The C1 to C3 alkylene group may be substituted or
unsubstituted.
The C1 to C3 alkylene group may be optionally substituted with one or more of
the following:
halo; hydroxyl; nitro; mercapto; amino; alkyl; alkoxy; aryl; sulpho and
sulphoxy groups. The C1
to C3 alkylene group may be linear or branched. The C1 to C3 alkylene group
may be saturated
or unsaturated. There are no more than 3 carbon atoms connecting between the
hydroxyl
groups in the polyol.
Suitable examples of polyol molecular weight increasing agents include, but
are not limited to
one or more of the following: ethylene glycol; neopentyl glycol; 1,3-propane
diol; butane 1.3-
diol; 2-methyl-1,3-propanediol; 2-
ethyl-2-butyl-1,3-propaned iol; trimethylolethane;
trimethylolpropane; glycerol; pentaerythritol and combinations thereof.
Suitably, the polyol
comprises trimethylolpropane, glycerol or a combination thereof.
The term "alk" or "alkyl", as used herein unless otherwise defined, relates to
saturated
hydrocarbon radicals being straight, branched, cyclic or polycyclic moieties
or combinations
thereof and contain 1 to 20 carbon atoms, suitably 1 to 10 carbon atoms, more
suitably 1 to 8
carbon atoms, still more suitably 1 to 6 carbon atoms, yet more suitably 1 to
4 carbon atoms.
These radicals may be optionally substituted with a chloro, bromo, iodo,
cyano, nitro, OR19,
OC(0)R20, c(o)R21, C(0)0R22, NR23R24, C(0)NR29R26, 5-K27,
C(0)5R27, C(S)NR25'-'K26,
aryl or
Het, wherein R19 to R27 each independently represent hydrogen, aryl or alkyl,
and/or be
interrupted by one or more oxygen or sulphur atoms, or by silano or
dialkylsiloxane groups.
Examples of such radicals may be independently selected from methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, 2-methylbutyl, pentyl,
iso-amyl, hexyl,
cyclohexyl, 3-methylpentyl, octyl and the like. The term "alkylene", as used
herein, relates to a
bivalent radical alkyl group as defined above. For example, an alkyl group
such as methyl
which would be represented as ¨CH3, becomes methylene, ¨CH2-, when represented
as an
alkylene. Other alkylene groups should be understood accordingly.
The term "alkenyl", as used herein, relates to hydrocarbon radicals having one
or several,
suitably up to 4, double bonds, being straight, branched, cyclic or polycyclic
moieties or
combinations thereof and containing from 2 to 18 carbon atoms, suitably 2 to
10 carbon atoms,
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
4
more suitably from 2 to 8 carbon atoms, still more suitably 2 to 6 carbon
atoms, yet more
suitably 2 to 4 carbon atoms. These radicals may be optionally substituted
with a hydroxyl,
chloro, bromo, iodo, cyano, nitro, OR19, OC(0)R20, c(0)-21,
C(0)0R22, NR23R24, C(0)NR26R26,
SR27, C(0)SR27, C(S)NR25R26, or aryl, wherein R19 to R27 each independently
represent
hydrogen, aryl or alkyl, and/or be interrupted by one or more oxygen or
sulphur atoms, or by
silano or dialkylsiloxane groups. Examples of such radicals may be
independently selected
from alkenyl groups include vinyl, ally!, isopropenyl, pentenyl, hexenyl,
heptenyl,
cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, 1-propenyl, 2-
butenyl, 2-methyl-2-
butenyl, isoprenyl, farnesyl, geranyl, geranylgeranyl and the like. The term
"alkenylene", as
used herein, relates to a bivalent radical alkenyl group as defined above. For
example, an
alkenyl group such as ethenyl which would be represented as -CH=CH2, becomes
ethenylene, -CH=CH-, when represented as an alkenylene. Other alkenylene
groups should
be understood accordingly.
The term "alkynyl", as used herein, relates to hydrocarbon radicals having one
or several,
suitably up to 4, triple bonds, being straight, branched, cyclic or polycyclic
moieties or
combinations thereof and having from 2 to 18 carbon atoms, suitably 2 to 10
carbon atoms,
more suitably from 2 to 8 carbon atoms, still more suitably from 2 to 6 carbon
atoms, yet more
suitably 2 to 4 carbon atoms. These radicals may be optionally substituted
with a hydroxy,
chloro, bromo, iodo, cyano, nitro, OR19, OC(0)R20, c(0)-21,
C(0)0R22, NR23R24, C(0)NR26R26,
SR27, C(0)SR27, C(S)NR25R26, or aryl, wherein R19 to R27 each independently
represent
hydrogen, aryl or lower alkyl, and/or be interrupted by one or more oxygen or
sulphur atoms,
or by silano or dialkylsiloxane groups. Examples of such radicals may be
independently
selected from alkynyl radicals include ethynyl, propynyl, propargyl, butynyl,
pentynyl, hexynyl
and the like. The term "alkynylene", as used herein, relates to a bivalent
radical alkynyl group
as defined above. For example, an alkynyl group such as ethynyl which would be
represented
as -CECH, becomes ethynylene, -CEC-, when represented as an alkynylene. Other
alkynylene groups should be understood accordingly.
The term "aryl" as used herein, relates to an organic radical derived from an
aromatic
hydrocarbon by removal of one hydrogen, and includes any monocyclic, bicyclic
or polycyclic
carbon ring of up to 7 members in each ring, wherein at least one ring is
aromatic. These
radicals may be optionally substituted with a hydroxy, chloro, bromo, iodo,
cyano, nitro, OR19,
OC(0)R20, c(0)-21,
C(0)0R225 NR23R24,
C(0)NR25R26, SR27, C(0)SR27, C(S)NR25R26, or aryl,
wherein R19 to R27 each independently represent hydrogen, aryl or lower alkyl,
and/or be
interrupted by one or more oxygen or sulphur atoms, or by silano or
dialkylsilcon groups.
Examples of such radicals may be independently selected from phenyl, p-tolyl,
4-
methoxyphenyl, 4-(tert-butoxy)phenyl, 3-methyl-4-methoxyphenyl, 4-
fluorophenyl, 4-
chlorophenyl, 3-nitrophenyl, 3-aminophenyl, 3-acetamidophenyl, 4-
acetamidophenyl, 2-methyl-
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
3-acetamidophenyl, 2-methyl-3-aminophenyl, 3-
methyl-4-am inophenyl, 2-amino-3-
methylphenyl, 2,4-dimethy1-3-aminophenyl, 4-hydroxyphenyl, 3-methyl-4-
hydroxyphenyl, 1-
naphthyl, 2-naphthyl, 3-amino-1-naphthyl, 2-methyl-3-amino-1-naphthyl, 6-amino-
2-naphthyl,
4,6-dimethoxy-2-naphthyl, tetrahydronaphthyl, indanyl, biphenyl, phenanthryl,
anthryl or
5 acenaphthyl and the like. The term "arylene", as used herein, relates to
a bivalent radical aryl
group as defined above. For example, an aryl group such as phenyl which would
be
represented as ¨Ph, becomes phenylene, ¨Ph-, when represented as an arylene.
Other
arylene groups should be understood accordingly.
For the avoidance of doubt, the reference to alkyl, alkenyl, alkynyl, aryl or
aralkyl in composite
groups herein should be interpreted accordingly, for example the reference to
alkyl in
aminoalkyl or alk in alkoxyl should be interpreted as alk or alkyl above etc.
By "terephthalic acid" it is meant terephthalic acid, ester or salt thereof.
The terephthalic acid
(b) may be in any suitable form. It will be well known to a person skilled in
the art that
terephthalic acid is often provided in a form which also contains isophthalic
acid as a
contaminant. However, in one embodiment, the terephthalic acid may be provided
in a form
which is substantially free of isophthalic acid. By "substantially free" we
mean to refer to
terephthalic acid which contains less than about 5 wt% isophthalic acid,
suitably less than
about 2 wt% isophthalic acid, more suitably less than about 0.05 wt%
isophthalic acid. In
certain embodiments the terephthalic acid may contain about 0 wt% isophthalic
acid.
In certain embodiments the terephthalic acid may be in the form of a diester.
Suitable
examples of the diester form of terephthalic acid include, but are not limited
to one or more of
the following: dimethyl terephthalate; diallyl terephthalate; diphenyl
terephthalate and
combinations thereof.
The polyester material may comprise any suitable molar ratio of components
(a):(b) and
(a)+(b):(c). In certain embodiments the ratio of (a):(b) may range from about
5:1 to 1:5, such
as from about 2:1 to 1:2, or even from about 1:1 to 1:2. Suitably, the molar
ratio of (a):(b) in
the polyester material may be about 1:1. In certain embodiments the molar
ratio of (a)+(b):(c)
may range from about 100:1 to 1:1, such as from about 80:1 to 5:1. As a non-
limiting
example, when component (c) is a polyacid the molar ratio of (a)+(b):(c) may
be about 25:1.
As a further non-limiting example, when component (c) is a polyol the molar
ratio of (a)+(b):(c)
may be about 80:1.
In certain embodiments the Tg may be at least about 80 C. In certain
embodiments the Tg
may be up to about 100 C, suitably up to about 120 C, or even up to about
150 C. Suitably,
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
6
the polyester material may have a Tg from about 80 C to 150 C, more suitably
the polyester
material may have a Tg from about 80 C to 120 C.
The Tg of the polyester material may be measured by any suitable method.
Methods to
measure Tg will be well known to a person skilled in the art. Suitably, the Tg
is measured
according to ASTM D6604-00(2013) ("Standard Practice for Glass Transition
Temperatures of
Hydrocarbon Resins by Differential Scanning Calorimetry". Heat-flux
differential scanning
calorimetry (DSC), sample pans: aluminium, reference: blank, calibration:
indium and mercury,
sample weight: 10mg, heating rate: 20 C/min).
In certain embodiments, the polyester material may have an Mn of at least
about 6,100
Da!tons (Da = g/mole), suitably at least about 6,250 Da, more suitably at
least 6,500 Da, such
as at least about 7,000 Da, or even at least about 8,000 Da. In certain
embodiments the
polyester material may have an Mn of up to about 50,000 Da, suitably up to
about 30,000 Da,
or even up to about 20,000 Da. Suitably, the polyester material may have an Mn
from about
6,100 Da to about 50,000 Da, suitably from about 6,250 Da to about 50,000 Da,
such as from
about 6,500 Da to 50,000 Da, such as from about 7,000 Da to 50,000 Da, or even
from about
8,000 Da to 50,000 Da. Suitably, the polyester material may have an Mn from
about 6,100 Da
to about 20,000 Da, suitably from about 6,250 Da to about 30,000 Da, such as
from about
6,500 Da to 30,000 Da, such as from about 7,000 Da to 30,000 Da, or even from
about 8,000
Da to 30,000 Da. Suitably, the polyester material may have an Mn from about
6,100 Da to
about 20,000 Da, suitably from about 6,250 Da to about 20,000 Da, such as from
about 6,500
Da to 20,000 Da, such as from about 7,000 Da to 20,000 Da, or even from about
8,000 Da to
20,000 Da.
It has been surprisingly and advantageously found by the present inventors
that the polyester
material of the present invention has a high Mn, while retaining a higher Tg
than would
normally be expected. This is advantageous in that the coating composition
according to the
present invention has improved film forming properties.
The number-average molecular weight may be measured by any suitable method.
Techniques to measure the number-average molecular weight will be well known
to a person
skilled in the art. Suitably, the Mn may be determined by gel permeation
chromatography
using a polystyrene standard according to ASTM D6579-11("Standard Practice for
Molecular
Weight Averages and Molecular Weight Distribution of Hydrocarbon, Rosin and
Terpene
Resins by Size Exclusion Chromatography". UV detector; 254nm, solvent:
unstabilised THF,
retention time marker: toluene, sample concentration: 2mg/m1).
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
7
A person skilled in the art will appreciated that techniques to measure the
number-average
molecular weight may also be applied to measure the weight-average molecular
weight.
The polyester material may have any suitable weight-average molecular weight
(Mw). In
certain embodiments, the polyester material may have an Mw of at least about
6,100 Da!tons,
suitably at least about 8,000 Da, such as at least about 10,000 Da, or even
about 15,000
Da!tons. In certain embodiments, the polyester material may have an Mw of up
to about
50,000 Da, suitably about 100,000 Da, such as about 150,000 Da, or even up to
about
200,000 Da. Suitably, the polyester material may have an Mw from about 6,100
Da to about
200,000 Da, suitably from about 8,000 Da to about 200,000 Da, such as from
about 10,000 Da
to about 200,000 Da, or even from about 15,000 Da to about 200,000 Da.
Suitably, the
polyester material may have an Mw from about 6,100 Da to about 150,000 Da,
suitably from
about 8,000 Da to about 150,000 Da, such as from about 10,000 Da to about
150,000 Da, or
even from about 15,000 Da to about 150,000 Da. Suitably, the polyester
material may have
an Mw from about 6,100 Da to about 100,000 Da, suitably from about 8,000 Da to
about
100,000 Da, such as from about 10,000 Da to about 100,000 Da, or even from
about 15,000
Da to about 100,000 Da. Suitably, the polyester material may have an Mw from
about 6,100
Da to about 50,000 Da, suitably from about 8,000 Da to about 50,000 Da, such
as from about
10,000 Da to about 50,000 Da, or even from about 15,000 Da to about 50,000 Da.
Suitably, the Mw is higher than the Mn.
Techniques to measure the weight-average molecular weight will be well known
to a person
skilled in the art. Suitably, the Mw may be determined by gel permeation
chromatography
using a polystyrene standard.
The polyester material according to the present invention suitably has a low
degree of
branching. The polyester materials according to the present invention may be
substantially
linear or be slightly branched. For example, the degree of branching of the
polyester material
may be measured by the polydispersity index of the said polyester material.
The
polydispersity index of a polymer is given by the ratio of Mw to Mn (Mw/Mn),
wherein Mw is the
weight-average molecular weight and Mn is the number average molecular weight.
Suitably,
the polydispersity index of the polyester materials of the present is from
about 1 to 20, suitably
from about 2 to 10
In certain embodiments the polyester material may have a molecular weight
above the critical
entanglement molecular weight of said polyester material.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
8
"Critical molecular weight" or "critical entanglement molecular weight" and
like terms, as used
herein, refers to the molecular weight at which the polyester material becomes
large enough to
entangle. For the avoidance of doubt the molecular weight may be the number-
average
molecular weight or the weight-average molecular weight. Critical entanglement
molecular
weight is typically defined as the molecular weight at which the physical
properties, especially
the viscosity of the polymer material, change more rapidly with molecular
weight. It is also
noted that certain rubber-elastic properties of polymers, such as the rubbery
plateau, are only
observed above the critical entanglement molecular weight as described in
"Properties of
Polymer, Their correlation with chemical structure; their numerical estimation
and prediction
from additive group contributions, 4th Edition" by D. W. Van Krevelen and K Te
Nijenhuis,
published by Elsevier, Amsterdam 2009, page 400 and references therein.
Typically, the critical entanglement molecular weight is determined by
plotting the log of the
melt viscosity against the log of the molecular weight of a polymer.
Typically, as the molecular
weight increases, the plot follows a gently upward sloping linear path.
However, once the
critical entanglement molecular weight is reached, the gently sloping linear
path increases to a
more rapidly sloping linear path. This change may occur over a molecular
weight range and
may appear as a curve rather than a distinct point. Hence, the critical
entanglement molecular
weight may be determined as the point on the plot where the slope changes from
gently
sloping to more rapidly sloping; this may require extrapolation of the slopes
before and after
the change to find the point by intersection of the two lines. Examples of
plots of this type
showing the critical entanglement molecular weight and a table giving a
compilation of critical
entanglement molecular weights for a range of polymers are shown in
"Properties of Polymer,
Their correlation with chemical structure; their numerical estimation and
prediction from
additive group contributions, 4th Edition" by D. W. Van Krevelen and K Te
Nijenhuis, published
by Elsevier, Amsterdam 2009, pages 534-536 and references therein.
Techniques to measure the melt viscosity will be well known to a person
skilled in the art.
Suitably, the melt viscosity may be measured at a high shear rate such as that
applied by a
cone and plate rheometer, typical methods are as described in standard methods
such as
ASTM D4287. Films formed from the polyester material according to the present
invention
having a molecular weight above the critical entanglement molecular weight of
the said
polyester material, were found to have superior film forming properties.
The polyester material according to the present invention may have any
suitable gross
hydroxyl value (OHV). In certain embodiments the polyester material may have a
gross OHV
from about 0 to 30 mg KOH/g. The polyester material may have a gross OHV from
about 0 to
20 mg KOH/g, such as from about 5 to 10 mg KOH/g, suitably from about 2 to 5
mg KOH/g.
Suitably, the gross OHV is expressed on solids.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
9
The polyester material of the present invention may have any suitable acid
value (AV). The
polyester material may have an AV from about 0 to 20 mg KOH/g, such as from
about 5 to 10
mg KOH/g, suitably from about 2 to 5 mg KOH/g. Suitably, the AV is expressed
on solids.
The components (a), (b) and (c) may be contacted in any order.
In certain embodiments, the polyester material may be prepared in a one step
process.
Suitably, in a one step process, the components (a), (b) and (c) are all
reacted together at the
same time. Suitably, the polyester material may be prepared in a one step
process where the
molecular weight increasing agent comprises a polyol.
Suitably, in a one step process, components (a), (b) and (c) may be contacted
together at a
first reaction temperature, Ti, wherein Ti may be a temperature from about 90
C to 260 C,
suitably from about 200 C to 250 C, such as from about 200 C to 230 C.
Typically, in a one step process, the reaction is allowed to proceed for a
total period from
about 1 hour to 100 hours, such as from about 2 hours to 80 hours. It will be
appreciated by a
person skilled in the art that the reaction conditions may be varied depending
on the reactants
used.
In certain embodiments the polyester material according to the present
invention may be
prepared in the presence of a catalyst. Suitably, the catalyst may be chosen
to promote the
reaction of components by esterification and trans-esterification. Suitable
examples of
catalysts for use in the preparation of the polyester material include, but
are not limited to one
or more of the following: metal compounds such as stannous octoate; stannous
chloride; butyl
stannoic acid (hydroxy butyl tin oxide); monobutyl tin tris (2-
ethylhexanoate); chloro butyl tin
dihydroxide; tetra-n-propyl titanate; tetra-n-butyl titanate; zinc acetate;
acid compounds such
as phosphoric acid; para-toluene sulphonic acid; dodecyl benzene sulphonic
acid and
combinations thereof. The catalyst, when present, may be used in amounts from
about 0.001
to 1% by weight on total polymer components, suitably from about 0.01 to 0.2%
by weight on
total polymer components.
According to a second aspect of the present invention there is provided a
coating composition
for a food and/or beverage container comprising a polyester material, wherein
the polyester
material comprises the reaction product of a one step process, the one step
process
comprising contacting
(a) 1,2-propanediol,
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
(b) terephthalic acid, and
(c) a polyol molecular weight increasing agent,
characterised in that the polyester material has a number-average molecular
weight (Mn) of at
5 least 6,100 Da and a glass transition temperature (Tg) of at least 80 C;
wherein the molecular weight increasing agent (c) comprises a polyacid, a
polyol or a
combination thereof;
10 wherein the polyacid comprises a diacid of general formula (I)
R000¨Xn¨COOR
formula (I)
wherein each R independently represents hydrogen or an alkyl, alkenyl,
alkynyl, or aryl group;
n = 0 or 1; wherein X represents a bridging group selected from: an alkylene
group; an
alkenylene group; an alkynylene group; an arylene group; wherein the bridge
between the ¨
COOR groups is C1 or C2;
and wherein the hydroxyl groups of the polyol are connected by a C1 to C3
alkylene group.
In certain embodiments, the polyester material may be prepared in a two step
process.
Suitably, in a two step process, two of components (a), (b) and (c) are
contacted together in a
first step under first reaction conditions, then the remaining component (a),
(b) or (c) is
contacted with the products of the first step in a second step under second
reaction conditions.
In one embodiment of such a two step process, components (a) and (b) are
contacted together
in a first step under first reaction conditions, then component (c) is
contacted with the products
of the first step in a second step under second reaction conditions.
Suitably, the polyester material may be prepared in a two step process where
the molecular
weight increasing agent comprises a polyol or a polyacid.
The first reaction conditions may include a temperature from about 90 C to 260
C, suitably at
a temperature from about 150 to 250 C. The temperature from about 90 C and 230
C, suitably
from about 150 to 230 C may be maintained for a time period from about 1 hour
to 100 hours,
such as from 2 hours to 80 hours.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
11
The second reaction conditions may include a temperature from about 90 C to
260 C, suitably
at a temperature from about 150 C to 250 C. The temperature from about 90 C to
230 C,
suitably from about 150 C to 230 C may be maintained for a time period from
about 1 hour to
100 hours, such as from 2 hours to 80 hours.
According to a third aspect of the present invention there is provided a food
and/or beverage
container coating composition comprising a polyester material, wherein the
polyester material
comprises the reaction product of a two step process, the two step process
comprising:
a first step comprising preparing a polyester prepolymer by contacting
(a) 1,2-propanediol,
(b) terephthalic acid, and
a second step comprising contacting the polyester prepolymer with
(c) a molecular weight increasing agent,
characterised in that the polyester material has a number-average molecular
weight (Mn) of at
least 6,100 Da and a glass transition temperature (Tg) of at least 80 C;
wherein the molecular weight increasing agent (c) comprises a polyacid, a
polyol or a
combination thereof;
wherein the polyacid comprises a diacid of general formula (I)
ROOC¨Xn¨COOR
formula (I)
wherein each R independently represents hydrogen or an alkyl, alkenyl,
alkynyl, or aryl group;
n = 0 or 1; wherein X represents a bridging group selected from: an alkylene
group; an
alkenylene group; an alkynylene group; an arylene group; wherein the bridge
between the ¨
COOR groups is C1 or C2;
and wherein the hydroxyl groups of the polyol are connected by a C1 to C3
alkylene group.
The coating composition may further comprise one or more solvent. The coating
composition
may comprise a single solvent or a mixture of solvents. The solvent may
comprise water, an
organic solvent, a mixture of water and an organic solvent or a mixture of
organic solvents.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
12
The organic solvent suitably has sufficient volatility to essentially entirely
evaporate from the
coating composition during the curing process. As a non-limiting example, the
curing process
may be by heating at 130-230 C for 1-15 minutes.
Suitable organic solvents include, but are not limited to one or more of the
following: aliphatic
hydrocarbons such as mineral spirits and high flash point naphtha; aromatic
hydrocarbons
such as benzene; toluene; xylene; solvent naphtha 100, 150, 200; those
available from Exxon-
Mobil Chemical Company under the SOLVESSO trade name; alcohols such as
ethanol; n-
propanol; isopropanol; and n-butanol; ketones such as acetone; cyclohexanone;
methylisobutyl ketone; methyl ethyl ketone; esters such as ethyl acetate;
butyl acetate; n-hexyl
acetate; glycols such as butyl glycol; glycol ethers such as methoxypropanol;
ethylene glycol
monomethyl ether; ethylene glycol monobutyl ether and combinations thereof.
The solvent,
when present, may suitably be used in the coating composition in amounts from
about 10 to 90
wt%, such as from about 20 to 80 wt%, or even from about 30 to 70 wt% based on
the total
solid weight of the coating composition.
The polyester material may be dissolved or dispersed in the said one or more
solvent during
and/or after its formation. It has been advantageously found by the present
inventors that the
polyester materials of the present invention have good solubility in solvents
commonly used in
liquid coatings for packaging.
The coating composition according to the present invention may comprise any
suitable amount
of the polyester material. The coating compositions may comprise from about 1
to 100 wt%,
suitably from about 20 to 90 wt%, such as from about 30 to 80 wt%, or even
from about 50 to
75 wt% of the polyester material based on the total solid weight of the
coating composition.
In certain embodiments the coating compositions may further comprise a
crosslinking agent.
The crosslinking agent may be any suitable crosslinking agent. Suitable
crosslinking agents
will be well known to the person skilled in the art. Suitable crosslinking
agents include, but are
not limited to one or more of the following: phenolic resins (or phenol-
formaldehyde resins);
aminoplast resins (or triazine-formaldehyde resins); amino resins; epoxy
resins; isocyanate
resins; beta-hydroxy (alkyl) amide resins; alkylated carbamate resins;
polyacids; anhydrides;
organometallic acid-functional materials; polyamines; polyamides and
combinations thereof.
In certain embodiments, the crosslinking agent comprises a phenolic resin or
an aminoplast
resin or a combination thereof. Non-limiting examples of phenolic resins are
those formed
from the reaction of a phenol with formaldehyde. Non-limiting examples of
phenols which may
be used to form phenolic resins are phenol, butyl phenol, xylenol and cresol.
General
preparation of phenolic resins is described in "The Chemistry and Application
of Phenolic
Resins or Phenoplasts", Vol V, Part I, edited by Dr Oldring; John Wiley and
Sons/Cita
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
13
Technology Limited, London, 1997. Suitably, the phenolic resins are of the
resol type. By
"resol type" we mean resins formed in the presence of a basic (alkaline)
catalyst and optionally
an excess of formaldehyde. Suitable examples of commercially available
phenolic resins
include, but are not limited to PHENODUR PR285 and BR612 and resins sold
under the
trademark BAKELITE such as BAKELITE 6582 LB. Non-limiting examples of
aminoplast
resins include those which are formed from the reaction of a triazine such as
melamine or
benzoguanamine with formaldehyde. Suitably, the resultant compounds may be
etherified
with an alcohol such as methanol, ethanol, butanol or combinations thereof.
The preparation
and use of aminoplast resins is described in "The Chemistry and Applications
of Amino
Crosslinking Agents or Aminoplast", Vol V, Part II, page 21 if., edited by Dr
Oldring; John Wiley
and Sons/Cita Technology Limited, London, 1998. Suitable examples of
commercially
available aminoplast resins include but are not restricted to those sold under
the trademark
MAPRENAL such as MAPRENAL MF980 and those sold under the trademark CYMEL
such as CYMEL 303 and CYMEL 1128, available from Cytec Industries. Suitably,
the
crosslinking agent comprises a phenolic resin.
In certain embodiments the coating composition may further comprise a
catalyst. Any catalyst
typically used to catalyse crosslinking reactions between polyester materials
and crosslinking
agents, such as for example phenolic resins, may be used. Suitable catalysts
will be well
known to the person skilled in the art. Suitable catalysts include, but are
not limited to one or
more of the following: phosphoric acid; alkyl aryl sulphonic acids such as
dodecyl benzene
sulphonic acid; methane sulphonic acid; para-toluene sulphonic acid; dinonyl
naphthalene
disulphonic acid; phenyl phosphinic acid and combinations thereof. In certain
embodiments
the catalyst may comprise an acid catalyst. Suitably, the catalyst may
comprise phosphoric
acid. The catalyst, when present, may be used in the coating composition in
any suitable
amount. In certain embodiments the catalyst, when present, may be used in
amounts from
about 0.01 to 10 wt%, suitably from about 0.1 to 2 wt% based on the total
solid weight of the
coating composition.
The coating composition according to the present invention may optionally
contain an additive
or combination of additives. The coating composition may optionally contain
any suitable
additive. Suitable additives will be well known to the person skilled in the
art. Examples of
suitable additives include, but are not limited to one or more of the
following: lubricants;
pigments; plasticisers; surfactants; flow control agents; thixotropic agents;
fillers; diluents;
organic solvents and combinations thereof.
Suitable lubricants will be well known to the person skilled in the art.
Suitable examples of
lubricants include, but are not limited to one or more of the following:
carnauba wax and
polyethylene type lubricants. In certain embodiments the lubricant, when
present, may be
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
14
used in the coating composition in amounts of at least 0.01 wt% based on the
total solid weight
of the coating composition.
Suitable pigments will be well known to the person skilled in the art. A
suitable pigment may
be, for example, titanium dioxide. The pigment, when present, may be used in
the coating
composition in any suitable amount. In certain embodiments, the pigment, when
present, may
be used in the coating composition in amounts up to about 90 wt%, such as up
to about 50
wt%, or even up to about 10 wt% based on the total solid weight of the coating
composition.
Surfactants may optionally be added to the coating composition in order to aid
in flow and
wetting of the substrate. Suitable surfactants will be well known to the
person skilled in the art.
Suitably the surfactant, when present, is chosen to be compatible with food
and/or beverage
container applications. Suitable surfactants include, but are not limited to
one or more of the
following: alkyl sulphates (e.g., sodium lauryl sulphate); ether sulphates;
phosphate esters;
sulphonates; and their various alkali, ammonium, amine salts; aliphatic
alcohol ethoxylates;
alkyl phenol ethoxylates (e.g. nonyl phenol polyether); salts and/or
combinations thereof. The
surfactants, when present, may be present in amounts from about 0.01 wt% to 10
wt% based
on the total solid weight of the coating composition.
In certain embodiments, the coating compositions according to the present
invention may be
substantially free, may be essentially free or may be completely free of
bisphenol A (BPA) and
derivatives thereof. Derivatives of bisphenol A include, for example,
bisphenol A diglycidyl
ether (BADGE). In certain embodiments, the coating compositions according to
the present
invention may also be substantially free or completely free of bisphenol F
(BPF) and
derivatives thereof. Derivatives of bisphenol F include, for example,
bisphenol F diglycidyl
ether (BPFG). The compounds or derivatives thereof mentioned above may not be
added to
the composition intentionally but may be present in trace amounts because of
unavoidable
contamination from the environment. By "substantially free" we mean to refer
to coating
compositions containing less than about 1000 parts per million (ppm) of any of
the compounds
or derivatives thereof mentioned above. By "essentially free" we mean to refer
to coating
compositions containing less than about 100 ppm of any of the compounds or
derivatives
thereof mentioned above. By "completely free" we mean to refer to coating
compositions
containing less than about 20 parts per billion (ppb) of any of the compounds
or derivatives
thereof.
In certain embodiments, the coating compositions may be essentially fee or may
be completely
free of dialkyltin compounds, including oxides or other derivatives thereof.
Examples of
dialkyltin compounds include, but are not limited to one or more of the
following:
dibutyltindilaurate (DBTDL); dioctyltindilaurate; dimethyltin oxide;
diethyltin oxide; dipropyltin
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
oxide; dibutyltin oxide (DBTO); dioctyltinoxide (DOTO) or combinations
thereof. By
"substantially free" we mean to refer to coating compositions containing less
than about 1000
parts per million (ppm) of any of the compounds or derivatives thereof
mentioned above. By
"essentially free" we mean to refer to coating compositions containing less
than about 100 ppm
5 of any of the compounds or derivatives thereof mentioned above. By
"completely free" we
mean to refer to coating compositions containing less than about 20 parts per
billion (ppb) of
any of the compounds or derivatives thereof.
The coating compositions according to the present invention may be applied to
any suitable
10 food and/or beverage container or components used to fabricate such
containers. Suitably,
the coating compositions may be applied to food and/or beverage cans. Examples
of cans
include, but are not limited to one or more of the following, two-piece cans,
three-piece cans
and the like. The coating compositions may also be applied to containers for
aerosol
applications such as, but not limited to, deodorant and hair spray containers.
The coating compositions according to the present invention may be applied to
the food and/or
beverage container by any suitable method. Methods of applying said coating
compositions
will be well known to a person skilled in the art. Suitable application
methods include, but are
not limited to one or more of the following, spray coating, roll coating,
dipping and/or
electrocoating. It will be appreciated by a person skilled in the art that for
two-piece cans, one
or more of the coating compositions may typically be applied by spray coating
after the can is
made. It will also be appreciated by the person skilled in the art that for
three-piece cans, a flat
sheet may typically be roll coated with one or more of the present coating
compositions first
and then the can may be formed. However, the application of the coating
compositions is not
limited to these methods. The coating compositions according to the present
information may
be applied to the interior and/or exterior surface or surfaces of the
container. Suitably, all or
part of the surface may be covered.
The coating compositions according to the present invention may be applied to
any suitable
dry film thickness. In certain embodiments the coating compositions may be
applied to a dry
film thickness from about 0.1pm (microns) to 2mm, suitably from about 2pm to
2mm, more
suitably from about 4pm to 2mm, or even from about 4pm to 1mm.
The coating composition according to the present invention may be applied to a
substrate as a
single layer or as part of a multi layer system. In certain embodiments, the
coating
composition may be applied as a single layer. In
certain embodiments, the coating
composition may be applied as the first coat of a multi coat system. Suitably,
the coating
composition may be applied as an undercoat or a primer. The second, third,
fourth etc. coats
may comprise any suitable paint such as those containing, for example, epoxy
resins;
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
16
polyester resins; polyurethane resins; polysiloxane resins; hydrocarbon resins
or combinations
thereof. In certain embodiments, the coating compositions may be applied on
top of another
paint layer as part of a multi layer system. For example, the coating
composition may be
applied on top of a primer. The coating compositions may form an intermediate
layer or a top
coat layer. The coating composition may be applied to a substrate once or
multiple times.
According to a further aspect of the present invention there is provided a
food and/or beverage
container coated on at least a portion thereof with a coating composition of
any of the above
aspects.
All of the features contained herein may be combined with any of the above
aspects and in
any combination.
For a better understanding of the invention, and to show how embodiments of
the same may
be carried into effect, reference will now be made, by way of example, to the
following
experimental data.
Examples
Preparation of Polyesters
Comparative Example 1
1,2-propanediol/terephthalic acid polymer
A polyester material made without a molecular weight increasing agent was
synthesised. The
polymerisation was carried out in a reaction vessel equipped with heating,
cooling, stirring and
a reflux condenser. A sparge of nitrogen was applied to the reactor to provide
an inert
atmosphere. 3165.5g 1,2-propanediol (PD), 6805.5g terephthalic acid (TPA) and
5.06g butyl
stannoic acid (0.05% on charge) were added to the reaction vessel via a packed
column and
heated to 185 C. The reaction vessel was then heated to a maximum temperature
of 230 C
and the contents were held at this temperature until the resin had reached
clarity and had an
acid value (AV) of <10. The reaction vessel was then cooled to 180 C and a
sample was
taken in order to measure the hydroxyl value (OHV). The net OHV was adjusted
to 8.63 with
PD or TPA. The reaction vessel was then reheated to a maximum temperature of
230 C and
held at this temperature until an AV of 3 was achieved. The viscosity was
monitored
throughout using a CAP 2000+ viscometer and GPC. The resin was discharged from
the
reaction vessel at 210-220 C in PTFE trays.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
17
The characteristics of the polyester produced in comparative example 1 were
determined and
are shown in Table 1.
Comparative Example 2
1,2-propanediol/terephthalic acid/isophthalic acid polymer
A polyester material made with terephthalic acid and isophthalic acid was
synthesised. The
polymerisation was carried out in a reaction vessel equipped with heating,
cooling, stirring and
a reflux condenser. A sparge of nitrogen was applied to the reactor to provide
an inert
atmosphere. 3123.0g 1,2-propanediol (PD), 6125.4g terephthalic acid (TPA),
680.6g
isophthalic acid (IPA) and 5.06g butyl stannoic acid (0.05% on charge) were
added to the
reaction vessel via a packed column and heated to 185 C. The reaction vessel
was then
heated to a maximum temperature of 230 C and the contents were held at this
temperature
until the resin had reached clarity and had an acid value (AV) of 20-30. Then
a small amount
of xylene was added to the reaction vessel to convert the process to
azeotropic distillation.
The reaction vessel was then cooled to 180 C and a sample was taken in order
to measure
the hydroxyl value (OHV). The net OHV was adjusted to 4 with PD or TPA. The
reaction
vessel was then reheated to a maximum temperature of 235 C and held at this
temperature
until the in-process viscosity was >2000 Poise at 200 C as measured with a
CAP 2000+
viscometer. The resin was discharged from the reaction vessel at 210-220 C in
PTFE trays.
The characteristics of the polyester produced in comparative example 2 were
determined and
are shown in Table 1.
Comparative Example 3
1,2-propanediol/terephthalic acid/cyclohexane dimethanol/cyclohexane
dicarboxylic
acid polymer
A polyester material made with terephthalic acid, cyclohexane dimethanol and
cyclohexane
dicarboxylic acid was synthesised. The polymerisation was carried out in a
reaction vessel
equipped with heating, cooling, stirring and a reflux condenser. A sparge of
nitrogen was
applied to the reactor to provide an inert atmosphere. 1448.60g 1,2-
propanediol (PD) was
added to the reaction vessel via a packed column followed by 2744.80g 1,4-
cyclohexane
dimethanol (CHDM), which was previously warmed to melt. The contents were
stirred to mix.
5.35g butyl stannoic acid (0.05% on charge), 3271.3g cyclohexane dicarboxylic
acid (CHDA)
and 3157.3g terephthalic acid (TPA) were further added to the reaction vessel
and heated to
160 C. The reaction vessel was then heated to a maximum temperature of 230 C
and the
contents were held at this temperature until the resin had reached clarity and
had an acid
value (AV) of <15. Then a small amount of SOLVESSO 150 ND (available from
Exxon-Mobil
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
18
Chemical Company) was added to the reaction vessel to convert the process to
azeotropic
distillation. The reaction vessel was then cooled to 170 C and a sample was
taken in order to
measure the hydroxyl value (OHV). The net OHV was adjusted 1 with CHDM and
then
reaction vessel was heated to 200 C and held for 2 hours. The OHV was
measured again
and further adjustment with CHDM was made if required. The reaction vessel was
then
reheated to a temperature of 230 C and held at this temperature until the in-
process viscosity
was 1500-1600 Poise at 200 C as measured with a CAP 2000+ viscometer. The
solids
content of the resulting polymer was reduced to 90 wt% by adding SOLVESSO 150
ND to the
reaction vessel. The resin was discharged from the reaction vessel at 210-220
C in PTFE
trays.
The characteristics of the polyester produced in comparative example 3 were
determined and
are shown in Table 1.
Example 1
1,2-propanediol/terephthalic acid/trimethylolpropane (TMP) branched polymer
A polyester material using TMP as the chain increasing agent was synthesised.
The
polymerisation was carried out in a reaction vessel equipped with heating,
cooling, stirring and
a reflux condenser. A sparge of nitrogen was applied to the reactor to provide
an inert
atmosphere. 3010g 1,2-propanediol (PD), 6805.5g terephthalic acid (TPA),
135g
trimethylolpropane (TMP) and 5.06g butyl stannoic acid (0.05% on charge) were
added to the
reaction vessel via a packed column and heated to 185 C. The reaction vessel
was then
heated to a maximum temperature of 230 C and the contents were held at this
temperature
until the resin had reached clarity and had an acid value (AV) of <5. Then
675g xylene was
added to the reaction vessel to convert the process to azeotropic
distillation. The reaction
vessel was then cooled to 180 C and a sample was taken in order to measure
the hydroxyl
value (OHV). The net OHV was adjusted to 1.43 with PD or TPA. The reaction
vessel was
then reheated to a maximum temperature of 235 C and held at this temperature
until the in-
process viscosity was >3000 Poise at 220 C as measured with a CAP 2000+
viscometer.
The resin was discharged from the reaction vessel at 210-220 C in PTFE trays.
The characteristics of the polyester produced in example 1 were determined and
are shown in
Table 1.
Example 2
1,2-propanediol/terephthalic acid/glycerol branched polymer
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
19
A polyester material using glycerol as the chain increasing agent was
synthesised. The
polymerisation was carried out in a reaction vessel equipped with heating,
cooling, stirring and
a reflux condenser. A sparge of nitrogen was applied to the reactor to provide
an inert
atmosphere. 3010g 1,2-propanediol (PD), 6805.5g terephthalic acid (TPA), 93g
glycerol and
5.03g butyl stannoic acid (0.05% on charge) were added to the reaction vessel
via a packed
column and heated to 180 C. The reaction vessel was then heated to a maximum
temperature of 230 C and the contents were held at this temperature until the
resin had
reached clarity and had an acid value (AV) of <5. Then 675g xylene was added
to the reaction
vessel to convert the process to azeotropic distillation. The reaction vessel
was then cooled to
180 C and a sample was taken in order to measure the hydroxyl value (OHV).
The net OHV
was adjusted to 1.44 with PD or TPA. The reaction vessel was then reheated to
a maximum
temperature of 230 C and held at this temperature until the in-process
viscosity was
approximately 2500 Poise at 220 C as measured with a CAP 2000+ viscometer.
The resin
was discharged from the reaction vessel at 200 C in PTFE trays.
The characteristics of the polyester produced in example 2 were determined and
are shown in
Table 1.
Test Methods
Molecular Weight Determination: The number-average molecular weight and weight-
average molecular weight were measured using gel permeation chromatography
(also known
as size exclusion chromatography) according to ASTM D6579-11.
Briefly, a Waters Corporation liquid chromatography system comprising a series
of three size
exclusion columns; 2 x PLgeITM 5pm MIXED-D columns (300mm x 7.5mm from Agilent
Technologies) and 1 x PLgeITM 5pm 50A (300mm x 7.5mm from Agilent
Technologies) and a
UV detector tuned to 254nm was used to conduct the experiments. The columns
were first
calibrated with polystyrene standards of known molecular weight (2348 kDa,
841.7 kDa, 327.3
kDa, 152.8 kDa, 60.45 kDa, 28.77 kDa, 10.44 kDa, 2.94 kDa, and 0.58 kDa where
kDa = 1000
Da = 1000 g/mol). The standards were dissolved in unstabilised THF as mixtures
of 4-6
molecular weight standards per sample. The standards were run under the same
conditions
as were used for the polyester material samples.
Samples were prepared by dissolving 0.01g to 0.05g of the polymer materials
prepared
according to comparative examples 1 and 2 and examples 1 and 2 above in 4 ml
unstabilised
tetrahydrofuran (THF). 20p1 was injected for each run. The experiment was
conducted at a
flow rate of 0.9 ml/min and the system was maintained at a constant
temperature of 22 C
throughout. Data were collected using Turbochrom 4 software from Perkin Elmer.
The data
were then processed using Turbochrom and Turbogel software from Perkin Elmer.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
Glass Transition Temperature: The glass transition temperature of the
polyester materials
were measured according to ASTM D6604-00(2013).
5 Briefly, the polymer samples were dissolved in tetrahydrofuran (THF) and
then vacuum dried.
10mg of the dried samples were placed in an aluminium pan in the differential
scanning
calorimeter along with an empty aluminium pan as the reference sample. A
preliminary
thermal cycle was performed from ambient temperature to 190 C at a heating
rate of 20
C/min. The temperature was then held isothermally at 200 C for 10 minutes
before being
10 crash cooled to -60 C with liquid nitrogen. The temperature was then
held isothermally at this
temperature (-60 C) for 13 minutes. Finally, the temperature of the sample
was increased
from -60 C to 200 C at a rate of 20 C/min and the heating curve was
recorded.
Table 1. Characteristics of Polyester Comparative Examples 1-3 and Examples 1-
2
Comparative Comparative Comparative
Example 1
Example 2
Example 1 Example 2 Example 3
Solids content 93-97% 90.0%
Acid Value (AV) /
7.2 11.2 6.5 9.3 10.3
mg KOH/g
Viscosity / Poise 445 @ 200 C >2000 @ 200 C ¨1600 @ 200 C >3000 @ 200 C
¨2500 @ 200 C
Net hydroxyl value
4.3 -7.7 -3.1 -2.9 -2.57
(OHV) / mg KOH/g
Gross OHV / mg
11.5 3.5 3.4 6.4 7.73
KOH/g
Mn (GPC) / Da 6,000 5,408 11,310 8,358
8,296
Mw (GPC) / Da 14,050 50,300 80,930
58,910
Mw/Mn 2.60 4.45 9.68
7.10
Tg / C 89.5 82.1 67 95.4
95.7
Clear light
Hard pale Hard pale Pale yellow
Pale yellow
Appearance golden brown
yellow solid yellow solid solid solid
solid
15 *Following dilution with SOLVESSO 100 in the reactor
The results show that when the PD/TPA polyester material is made without the
addition of a
molecular weight increasing agent, as in comparative examples 1 and 2, it had
proven difficult
to achieve Mn above 6,000 Da. Further, when isophthalic acid is added as a
monomer
20 component a slight reduction in Tg was observed. Higher Mn polyester
materials can be
prepared by the incorporation of other polyol and polyacid, as in comparative
example 3, but
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
21
resulted in a significant reduction in polymer Tg. However, upon the addition
of a molecular
weight increasing agent according to the invention, it is possible to increase
the Mn to above
about 6,100 Da, while maintaining a high Tg.
Preparation of Coatings
Comparative coating examples 1-6
2750g of the polyester prepared in comparative polyester example 1 was added
to 1687.5g
SOLVESSO 150 ND (available from Exxon-Mobil Chemical Company) and 562.5g 2-
butoxyethanol. The coating compositions were then formulated as outlined in
Table 2.
Comparative coating examples 7-9
2500g of the polyester prepared in comparative polyester example 3 was added
to 2500.0g
SOLVESSO 100 (available from Exxon-Mobil Chemical Company). The coating
compositions
were then formulated as outlined in Table 3.
Coating examples 10-15
2432g of the polyester prepared in polyester example 1 was added to 1743g
SOLVESSO 150
ND (available from Exxon-Mobil Chemical Company) and 825g dibasic ester. The
coating
compositions were then formulated as outlined in Table 4.
Coating examples 16-18
2432g of the polyester prepared in polyester example 2 was added to 1743g
SOLVESSO 150
ND (available from Exxon-Mobil Chemical Company) and 825g dibasic ester. The
coating
compositions were then formulated as outlined in Table 5.
The properties of the coatings were tested via the following methods. Results
are shown in
Tables 2-7. The lactic acid sterilisation test was only performed on
comparative coatings 7-9
and coatings 10-18, which are shown in Tables 6 and 7, respectively.
Test Methods
Test Panel Preparation: The coating samples were applied onto 0.22mm tinplate
using a wire
wound bar coater to give a 5-6g/square metre dried coating weight. The coated
panels were
transferred to a laboratory box oven for 10 minutes at 190 C.
MEK Rub Test: The number of reciprocating rubs required to remove the coating
was
measured using a ball of cotton wool soaked in methyl ethyl ketone (MEK).
Wedge Bend Test: A 10cm x 4cm coated panel was bent on a 6mm steel rod to form
a U-
shaped strip 10cm long and 2cm wide. The U-shaped strip was then placed onto a
metal
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
22
block with a built in tapered recess. A 2kg weight was dropped onto the
recessed block
containing the U-shaped strip from a height of 60cm in order to from a wedge.
The test piece
was then immersed in a copper sulphate (CuSO4) solution acidified with
hydrochloric acid
(HCI) for 2 minutes, followed by rinsing with tap water. The sample was then
carefully dried by
blotting any residual water with tissue paper. The length of coating without
any fracture was
measured. The result was quoted in mm passed. The wedge bends were tested in
triplicate
and the average value was quoted.
Lactic acid sterilisation: This test is used to determine if the coatings are
compatible for use
in food and/or beverage containers. The coated panels were half immersed in a
deionised
water solution comprising 1% lactic acid inside a Kilner jar and sterilised
for 1 hour at 130 C in
an autoclave. After this time, the coated panels were quickly removed whilst
still hot and
rinsed whilst under cold water. The portion of the coated panel immersed in
lactic acid and the
portion exposed to the vapour, which was produced during the sterilisation
process, were
assessed separately for extent of damage. Four aspects were graded;
(A) Coating surface damage (visual assessment; 0 = no damage/defect, 5 =
severe damage/defect)
(B) Extent of blushing wherein the coating turns hazy due to water trapped
in the
coating (visual assessment; 0 = no damage/defect, 5 = severe damage/defect)
(C) Substrate corrosion (visual assessment; 0 = no damage/defect, 5 =
severe
damage/defect)
(D) % coating adhesion loss (assessed by making a cross hatch on the
coating
and taping with Scotch 610 tape; % of coating after taping)
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
23
Table 2. Comparative coating compositions 1-6 and test results
Comparative Comparative Comparative Comparative Comparative Comparative
Coating 1 Coating 2 Coating 3 Coating 4
Coating 5 Coating 6
Comparative
polyester 72.7 64.9 53.5 73.8 66.6
55.8
example 1
Phenolic 1* 9 16 26.3 - - -
Phenolic 2** - - - 7.5 13.5
22.7
Phenolic 3*** - - - - - -
Catalyst* 4.4 4.4 4.4 4.4 4.4
4.4
BYK 310** 0.1 0.1 0.1 0.1 0.1
0.1
SOLVESSO
10.4 11 11.8 14.2 15.3 17
150 ND
2-butoxyethanol 3.5 3.7 3.9 - - -
Propylene
- - - 14.2 15.3 17
carbonate
Total 100 100 100 100 100
100
MEK Rubs 2 2 13 3 5 11
Wedge Bend 0 7 0 74 70 53
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
24
Table 3. Comparative coating compositions 7-9 and test results
Corn parative Corn parative Corn
parative
Coating 7 Coating 8 Coating 9
Comparative polyester
79.2 71.6 60.1
example 3
Phenolic 1* 7.0 12.7 21.4
Phenolic 2** - - -
Phenolic 3*** - - -
Catalyst* 2.4 2.4 2.4
BYK 310** 0.1 0.1 0.1
SOLVESSO 100 11.3 13.2 16.0
2-butoxyethanol - - -
Propylene carbonate - - -
Total 100 100 100
MEK Rubs 1 1 2
Wedge Bend 90 90 85
Table 4. Coating compositions 10-15 and test results
Coating 10 Coating 11 Coating 12 Coating 13 Coating 14 Coating 15
Polyester
82.4 73.9 61.2 77.7 69.6 57.6
example 1
Phenolic 1* 7.7 13.7 22.7
Phenolic 2** - - - - - -
Phenolic 3*** - - - 7.7 13.8 22.9
Catalyst* 3.1 3.1 3.1 3.1 3.1 3.1
BYK 310** 0.1 0.1 0.1 0.1 0.1 0.1
SOLVESSO
6.8 9.2 12.8 11.3 13.3 16.2
150 ND
Total 100 100 100 100 100 100
MEK Rubs 6 15 22 4 170 >200
Wedge Bend 91 92 88 87 79 67
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
Table 5. Coating compositions 16-18 and test results
Coating 16 Coating 17 Coating 18
Polyester
77.3 69.3 57.4
example 2
Phenolic 1* 7.7 13.8 22.8
Phenolic 2**
Phenolic 3***
Catalyst* 3.1 3.1 3.1
BYK 310** 0.1 0.1 0.1
SOLVESSO
150 ND 11.8 13.7 16.6
Total 100 100 100
MEK Rubs 7 18 157
Wedge Bend 90 91 86
*Phenodur PR516/660 from Cytec Industries Inc.
**Bakelite PF 6535LB from Hexion Speciality Chemicals
***BDP2201 from Bit Rez Ltd.
5 *5 weight% of 0-phosphoric acid in 1-methoxy-2-propanol
**From BYK-Chemie
Table 6. Results of lactic acid sterilisation test on comparative coatings 7-9
Comparative Comparative Comparative
Coating 7 Coating 8 Coating 9
A 3.5 3 0
3 1 0
Vapour
0 0 0
5% 5% 5%
A 5.5 5.5 2
4 3 1
Immersed
0 0 0
5% 5% 5%
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
26
Table 7. Results of lactic acid sterilisation tests on coatings 10-18
Coating Coating Coating Coating Coating Coating Coating Coating Coating
11 12 13 14 15 16 17 18
A 3 0 0 3 0 0 3 3
3
B 2 1 1 1 1 1 2
3 2
Vapour
C 0 0 0 0 0 0 0 0
0
D 0% 0% 0% 5% 5% 5% 5% 5% 5%
A 2 2 2 0 0 0 2 2
0
B 4 3 2 4 3 3 5
3 2
Immersed
C 0 0 0 0 0 0 0 0
0
D 5% 5% 5% 5% 5% 5% 5% 5% 5%
The results show that there is more attack on the coating surface by lactic
acid when a
polyester material with a lower Tg, as in comparative coating examples 7 and
8, is used.
5
Solvent rubs is a common method used in the coatings industry to compare the
extent of cure
as well as the chemical resistance of coatings. It can be clearly seen that
the MEK rub
resistance of coatings comprising the polyester materials according to the
present invention
(coating examples 10 to 18) are significantly higher than those made from the
comparative
10 polymer examples (comparative coating examples 1 to 9).
Another important requirement in packaging coatings is the resistance to
acidic medium under
sterilisation conditions. A typical approach is to increase the amount of
crosslinker in the
coating formulation, but this generally leads to a reduction in flexibility.
The lactic acid
sterilisation results clearly show that coatings according to the present
invention (coating
examples 10 to 18) are more resistant with lower amount of crosslinking resins
compared to
the comparative examples (comparative coatings 7 to 9) containing the same
amounts of
crosslinking resin.
Attention is directed to all papers and documents which are filed concurrently
with or previous
to this specification in connection with this application and which are open
to public inspection
with this specification, and the contents of all such papers and documents are
incorporated
herein by reference.
All of the features disclosed in this specification (including any
accompanying claims, abstract
and drawings), and/or all of the steps of any method or process so disclosed,
may be
combined in any combination, except combinations where at least some of such
features
and/or steps are mutually exclusive.
CA 02931742 2016-05-26
WO 2015/078966 PCT/EP2014/075812
27
Each feature disclosed in this specification (including any accompanying
claims, abstract and
drawings) may be replaced by alternative features serving the same, equivalent
or similar
purpose, unless expressly stated otherwise. Thus, unless expressly stated
otherwise, each
feature disclosed is one example only of a generic series of equivalent or
similar features.
The invention is not restricted to the details of the foregoing embodiment(s).
The invention
extends to any novel one, or any novel combination, of the features disclosed
in this
specification (including any accompanying claims, abstract and drawings), or
to any novel one,
or any novel combination, of the steps of any method or process so disclosed.