Note: Descriptions are shown in the official language in which they were submitted.
CA 02932032 2016-05-27
WO 2015/084417 PCMJS2014/011529
-1-
DEVICES, SYSTEMS, AND METHODS FOR PROCESSING
HETEROGENEOUS MATERIALS
PRIORITY CLAIM
This application claims the benefit of the filing date of United States Patent
Application Serial Number 14/094,236, filed December 2, 2013, for -DEVICES,
SYSTEMS, AND METHODS FOR PROCESSING HETEROGENEOUS
MATERIALS," which is a continuation-in-part application of U.S. Patent
Application No. 13/614,802, filed September 13, 2012, pending, which claims
the
benefit of U.S. Provisional Patent Application Serial No. 61/535,253, filed
September 15, 2011, and entitled "Devices, Systems, and Methods for Processing
Heterogeneous Materials" and U.S. Provisional Patent Application Serial
No. 61/593,741, filed February 1,2012, and entitled "Methods for Processing
Heterogeneous Materials."
TECHNICAL FIELD
The present disclosure relates generally to processing heterogeneous
materials, such as ores or oil-contaminated sands, to separate the materials
into
discrete components.
BACKGROUND
Heterogeneous materials, such as heterogeneous solid materials, occur
naturally and may also be formed by man-made processes. For example, naturally
occurring ores may include volumes containing a material of interest (i.e., a
so-called
"bearing fraction"), such as a metal or a mineral, mixed with volumes not
containing
the material of interest (i.e., a so-called "non-bearing fraction"). Recovery
of the
material of interest generally requires physical or chemical separation of the
bearing
fraction from the non-bearing fraction. Chemical separation may require
reagents
(e.g., cyanide, acids, carbonates), which may be expensive or raise
environmental
challenges.
CA 02932032 2016-05-27
WO 2015/084417
PCMJS2014/011529
-2-
As one example of a heterogeneous material, uranium is typically found in
nature as uranium ore. Low-grade uranium ore may contain any form of
uranium-containing compounds in concentrations up to about 5 lbs of U308
equivalent per ton of ore (about 2.5 kg of U308 equivalent per 1000 kg of ore,
or
about 0.25% uranium oxides by weight), whereas higher grade ore may contain
uranium-containing compounds in concentrations of about 8 lbs of U308
equivalent
per ton of ore (about 4.0 kg of U308 equivalent per 1000 kg of ore, or about
0.4%
uranium oxides by weight), about 30 lbs of U308 equivalent per ton of ore
(about
kg of U308 equivalent per 1000 kg of ore, or about 1.5% uranium oxides by
10 weight) or more.
Uranium deposits may be ft:stilled in sandstone by erosion and redcposition.
For example, an uplift may raise a uranium-bearing source rock and expose the
source rock to the atmosphere. The source rock may then erode, forming
solutions
of uranium and secondary minerals. The solutions may migrate along the surface
of
15 the earth or through permeable subsurface channels into a sandstone
formation,
stopping at a structural or chemical boundary. Uranium minerals may then be
deposited as a patina or coating around or between grains of the formation.
Uranium
may also be present in carbonaceous materials within sandstone. Uranium may be
all or a portion of the cementing material between grains of the formation.
FIG. 1 shows a section photomicrograph of sandstone formations from the
Shirley Basin in central Wyoming. As shown in FIG. 1, uranium-bearing
sandstone 10 may include various constituents. In general, oversize material
12 may
be defined as relatively large particles or fragments, such as homogeneous
particles
of host rock. Oversize material 12 may also be defined as particles larger
than can
be processed in a particular processing system. For example, in some sandstone
10,
oversize material 12 may include cobbles and stones arbitrarily defined as
material
having an average diameter larger than about 0.25 inch (in.) (6.35 mm).
Oversize
materials 12 in sandstone 10 generally do not contain much uranium. Grains 14
may
generally be defined as particles or fragments smaller than oversize material
12.
Grains 14 may include particles having diameters from about 400-mesh (i.e.,
about
0.0015 in. or about 0.037 mm) to about 0.25 in. (6.35 mm), and may include
quartz
CA 02932032 2016-05-27
WO 2015/084417 PCMJS2014/011529
or feldspar. Grains 14 in sandstone 10 do not typically contain much uranium,
but
uranium may be formed around the grains 14 due to deposition. Fines may be
generally defined as particles disposed among the oversize material 12 and the
grains 14, and may include materials also found in the grains 14 and oversized
material 12, such as uranium, quartz, feldspar, etc. Fines may cement the
oversize
material 12 and the gains 14 into a solid mass. Fines in uranium-bearing
sandstone 10 (e.g., particles smaller than about 400-mesh) may include light
fines 16
and heavy fines 18. Light fines 16 generally have a specific gravity up to
about 4.0
with reference to water, whereas heavy fines 18 have a specific gravity
greater than
about 4Ø Uranium compounds are generally components of the heavy fines 18,
but
may also be a part of light fines 16 in the form of deposits on carbonaceous
materials. For example, uraninite has a specific gravity from about 6.5 to
about
10.95, depending on its degree of oxidation, and coffinite has a specific
gravity of
about 5.4. Both light fines 16 and heavy fines 18 may be bound to grains 14 in
the
sandstone 10. In the sandstone 10, the oversize material 12, grains 14, light
fines 16,
and heavy fines 18 may be combined into a single mass.
Uranium may conventionally be recovered through in-situ recovery (ISR),
also known in the art as in-situ leaching (ISL) or solution mining. In ISR, a
leachate
or lixiviant solution is pumped into an ore formation through a well. The
solution
permeates the formation and dissolves a portion of the ore. The solution is
extracted
through another well and processed to recover the uranium. Reagents used to
dissolve uranium of the ore may include an acid or carbonate. ISR may have
various
environmental and operational concerns, such as mobilization of uranium or
heavy
metals into aquifers, footprint of surface operations, interconnection of
wells, etc.
ISR typically requires particular reagents, which must be supplied, recovered,
and
treated. Because ISR relies on the subsurface transport of a solution, ISR
cannot
generally be used in formations that are impermeable or shallow.
Uranium may also conventionally be mined in underground mines or surface
mines (e.g., strip mines, open-pit mines, etc.). During such mining
activities, it may
be necessary to process large quantities of material having a concentration of
uranium too low for economic recovery by conventional processes. Such material
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-4-
(e.g., overburden) may be treated as waste or as a material for use in mine
reclamation. Conventional mining may produce significant amounts of such
low-concentration material, which may require treatment during or subsequent
to
mining operations. It would therefore be advantageous to provide a method of
uranium recovery that minimizes or alleviates these concerns.
DISCLOSURE
In some embodiments, a system for processing a heterogeneous material
includes a conduit for a pressurized fluid and a nozzle assembly in fluid
communication with the conduit. The nozzle assembly includes a plurality of
adjustable nozzles configured such that fluid streams passing through each of
the
plurality of adjustable nozzles intersect at an oblique angle after passing
through the
plurality of adjustable nozzles. At least one of the fluid streams comprises a
heterogeneous material.
In other embodiments, a system includes a conduit for a pressurized fluid, a
nozzle assembly in fluid communication with the conduit, and a separation
system
configured to separate particles of a heterogeneous material into a first
fraction and a
second fraction. The nozzle assembly includes an adjustable nozzle configured
such
that a stream of the heterogeneous material passing through the adjustable
nozzle
contacts a surface approximately perpendicular to the surface after passing
through
the nozzle. The particles of the first fraction have a first average property,
and the
particles of the second fraction have a second average property different from
the
first average property.
In certain embodiments, a method of processing a heterogeneous material
includes entraining heterogeneous particles of a material into at least one
fluid
stream, passing the fluid stream through an adjustable nozzle, impacting the
fluid
stream with another fluid stream at an oblique angle to ablate the
heterogeneous
particles of the material, and classifying the heterogeneous particles.
81797263
-4a-
According to one aspect of the present invention, there is provided a system
for
processing a heterogeneous material, comprising: a conduit for a pressurized
fluid; and a
nozzle assembly in fluid communication with the conduit, the nozzle assembly
comprising a
plurality of adjustable nozzles configured such that fluid streams passing
through each of the
.. plurality of adjustable nozzles intersect at an oblique angle in a range
from about 160 to
179.9 after passing through the plurality of adjustable nozzles, wherein the
at least one of the
fluid streams comprises a heterogeneous material.
According to another aspect of the present invention, there is provided a
method of processing a heterogeneous material, comprising: entraining
heterogeneous
particles of a material into at least one fluid stream; passing the at least
one fluid stream
through an adjustable nozzle; impacting the at least one fluid stream with
another fluid stream
at an oblique angle in a range from about 160 to 179.9 to ablate the
heterogeneous particles
of the material; and classifying the heterogeneous particles.
CA 2932032 2019-01-11
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-5-
BRIEF DESCRIPTION OF THE DRAWINGS
While the specification concludes with claims particularly pointing out and
distinctly claiming what are regarded as embodiments of the present
disclosure,
various features and advantages of embodiments of the present disclosure may
be
more readily ascertained from the following description of some embodiments of
the
present disclosure when read in conjunction with the accompanying drawings, in
which:
FIG. 1 is a photomicrograph of uranium ore in a sandstone formation;
FIG. 2 is a photomicrograph of a carbonaceous material;
FIG. 3 is a simplified schematic illustrating an embodiment of a system for
processing a heterogeneous material;
FIG. 4 is an enlarged cross-sectional view of a nozzle assembly as shown in
the system of FIG. 3;
FIGS. 5 and 6 are enlarged cross-sectional views of nozzle assemblies of
additional embodiments of the present disclosure;
FIG. 7 is a simplified schematic illustrating a portion of the system shown in
FIG. 3;
FIG. 8 is a simplified view of an embodiment of an elutriator;
FIG. 9 is a simplified cross-sectional view of the elutriator of FIG. 8;
FIG. 10 is a simplified view of a cylindrical stage of the elutriator of FIG.
8;
FIG. 11 is a simplified cross-sectional view of the cylindrical stage of
FIG. 10;
FIG. 12 is a graph illustrating the calculated terminal velocity of selected
particles in an clutriator according to an embodiment of the present
disclosure;
FIG. 13 is a side view of an embodiment of a system for processing a
heterogeneous material;
FIG. 14 is a simplified schematic illustrating another embodiment of a
system for processing a heterogeneous material;
FIGS. 15 through 17 are photomicrographs of ore samples from
sandstone-hosted uranium deposits;
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-6-
FIG. 18 is a graph illustrating a particle size distribution for a crushed
sample
of ore from sandstone-hosted uranium deposits;
FIG. 19 is a graph illustrating a particle size distribution and a percentage
of
uranium in each size fraction for a crushed sample of ore from sandstone-
hosted
uranium deposits;
FIG. 20 is a graph illustrating a particle size distribution and a percentage
of
uranium in each size fraction for a crushed sample of ore from sandstone-
hosted
uranium deposits and for a sample of the same material after ablation;
FIGS. 21 and 22 are graphs illustrating concentrations of elements as a
function of ablation time in water used in an ablation process according to an
embodiment of the present disclosure;
FIG. 23 is a photomicrograph of a crushed ore sample from sandstone-hosted
uranium deposits, including a mineral patina;
FIG. 24 is a photomicrograph of an ablated crushed ore sample from
sandstone-hosted uranium deposits;
FIG. 25 is a cross-sectional view of a nozzle assembly of an additional
embodiment of the present disclosure; and
FIG. 26 is a cross-sectional top view of a nozzle assembly of another
embodiment of the present disclosure.
MODE(S) FOR CARRYING OUT THE INVENTION
Devices, systems, and methods for processing heterogeneous materials, such as
heterogeneous solids, are described. In one embodiment, a method includes
entraining
heterogeneous particles into a fluid stream (e.g., air, water, oil, etc.). The
fluid stream
is passed through at least one nozzle of a system, and is impacted to ablate
the
heterogeneous particles via kinetic collisions between particles within the
fluid stream.
As used herein, the term "ablate" means and includes wearing away by flexure,
rebound, and distortion. Ablation may also include wear by friction, chipping,
spalling, or another erosive process. When particles arc ablated, the boundary
between
different materials may become more highly stressed than the bulk materials
themselves. Thus, ablation may be particularly applicable to physical removal
of
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-7-
coatings from an underlying material. Ablation imparts energy to the material
being
ablated to physically dissociate the material into various fractions (e.g., a
solid fraction
and an oil or two solid fractions). The ablated particles may then be
classified to
divide the heterogeneous material into various fractions. Ablation and
separation may
significantly reduce the amount of material to be further processed to recover
the one
or more desired components of the material. A system for the ablation process
may
include a conduit for a pressurized fluid and a nozzle assembly. The nozzle
assembly
may include two or more adjustable nozzles configured such that a stream
passing
through a nozzle intersects another stream passing through another nozzle in
the
nozzle assembly. The method and system may be scalable for operations of any
size.
The system may be portable, and its use may make separation commercially
feasible in
instances wherein conventional separation processes are impractical.
The devices, systems, and methods described herein may be particularly
applicable to ores, such as sandstone, for the recovery of selected minerals,
such as
uranium-containing compounds. Uranium is often a post-depositional material,
carried
into an already established sandstone formation by mineral-bearing solutions.
Without
being bound to any particular theory, it is believed that when these mineral-
bearing
solutions reached a reduction zone, carbon caused the uranium to reduce and
precipitate out of solution to form stable uranium-containing compounds.
Because the
sandstone formation was already in place, these uranium-containing compounds
formed in two very specific locations within the ore¨as a mineral patina
surrounding
grains and in carbonaceous material. Because the grain structure of sandstone
is
relatively impermeable, uranium patinas do not penetrate the grains. Instead,
uranium
patinas form a boundary between the grain and the cementing material in the
sandstone
foimation.
As shown in FIG. 1, the uranium mineral patina includes the heavy fines 18,
and is shown around quartz gains 14. Carbonaceous materials are commonly found
in
sandstone-hosted uranium deposits, such as in the light fines 16 shown in FIG.
1. In
sandstone-hosted uranium deposits, carbonaceous materials generally range in
size
from less than about 1 mm to more than about 25 mm across. Other carbonaceous
materials include partially decomposed trees, coal seams. etc., and vary
widely in size.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-8-
FIG. 2 shows a sample of a carbonaceous material. Carbonaceous materials
generally
have low specific gravities of between about 1.25 and 1.30, and may contain
high
concentrations of uranium or other post-depositional elements deposited by
permeation
of mineral-bearing solutions. However, carbonaceous materials may also have
specific
gravities higher or lower, depending on how the carbonaceous materials formed.
For
example, some carbonaceous materials may have specific gravities less than
about 1Ø
Carbonaceous materials subjected to compressive forces may have specific
gravities
greater than about 1.5. Dissociating and then recovering the light fines 16
from the
oversize material 12, the grains 14, and the heavy fines 18 may therefore
enable
enhanced recovery of certain elements without processing the entire mass of
sandstone
by conventional techniques.
The properties of both the heavy fines 18 (including the mineralized uranium
patina) and the light fines 16 (including the carbonaceous materials) makes
them each
amenable to dissociation and separation from the oversize material 12, which
does not
contain uranium, and grains 14 of sandstone using an ablation process of the
present
disclosure. During ablation, the heavy fines 18 are separated from the
oversize
material 12 and gains 14. Without the structure of the oversize material 12
and
gains 14, the patina has limited structure and forms the heavy fines 18, which
are
smaller than about 400-mesh. That is, the patina forms weak bonds between
particles
such that ablation breaks the patina particles down into particles smaller
than about
400-mesh.
Some illustrations presented herein are not actual views of a particular
system
or process, but are merely idealized representations employed to describe
embodiments
of the present disclosure. Elements common between figures may retain the same
numerical designation.
A system 100 for processing a heterogeneous material 103 is shown
schematically in FIG. 3. To simplify the figures and clarify the present
disclosure, not
every element or component of the system 100 is shown or described herein. The
system 100 may also include appropriate piping, connectors, sensors,
controllers, etc.
(not shown), as will be understood by those of ordinary skill in the art. The
system 100
may include a hopper 101 feeding a tank 102, and a pump 104 in fluid
communication
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-9-
with the tank 102. The pump 104 may transport a mixed heterogeneous material
106
(which may include a mixture of the heterogeneous material 103 from the hopper
101
and an ablated heterogeneous material 124 that is recycled through a portion
of the
system 100, as explained in more detail below) through a continuous-flow
mixing
device 108 and a splitter 110. The mixed heterogeneous material 106 may then
pass
through a nozzle assembly 114, and multiple streams of the mixed heterogeneous
material 106 may impact one another, ablating solid particles therein to form
the
ablated heterogeneous material 124. The ablated heterogeneous material 124
may, in
some embodiments, be recycled through the system 100 by mixing the ablated
heterogeneous material 124 with the unablated heterogeneous material 103 in
the
tank 102. A stream 136 may be drawn off through a pump 138 to a separation
system 140, where it may be separated into two or more components. For
example, in
the system 100, the separation system 140 may separate the stream 136 into
grains 150,
light fines 152, and heavy fines 154. Though shown as a continuous-flow
operation,
the system 100 may also be configured to operate in batch mode, as will be
understood
by a person having ordinary skill in the art. Similarly, the system 100 may
include
multiple pumps, mixing apparatuses, and/or nozzle assemblies operated in
series, such
as with the stream 136 being directed through a second nozzle assembly before
entering the separation system 140. A system 100 having multiple nozzle
assemblies
operating in series may be configured such that each and every particle of the
heterogeneous material 103 necessarily passes through each nozzle assembly at
least
once. In embodiments in which the system 100 includes multiple nozzle
assemblies
operating in series, subsequent nozzle assemblies may operate without
additional
hoppers 101 or separation systems 140.
In some embodiments, the heterogeneous material 103 may be placed into the
hopper 101. The heterogeneous material 103 may include solid particles or a
mixture
of solid particles with a liquid. For example, the heterogeneous material 103
may
include a portion of an ore containing a metal (e.g., uranium, gold, copper,
and/or a
rare-earth element) to be recovered. In some embodiments, the heterogeneous
material 103 may be oil-contaminated sand. The liquid may include water (e.g.,
groundwater, process water, culinary or municipal water, distilled water,
deionized
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-10-
water, etc.), an acid, a base, an organic solvent, a surfactant, a salt, or
any combination
thereof. The liquid may include dissolved materials, such as a carbonate or
oxygen. In
some embodiments, the liquid may be substantially pure water, or water removed
from
a water source (e.g., an underground aquifer) without purification and without
added
components. The composition of the liquid may be selected to balance economic,
environmental, and processing concerns (e.g., mineral solubility or disposal).
The
liquid may be selected to comply with environmental regulations. In one
embodiment,
the liquid may be substantially free of a reagent (e.g., a leachate, an acid,
an alkali,
cyanide, lead nitrate, etc.) that is formulated to chemically react with the
particles in
the heterogeneous material 103. In some embodiments, the liquid may be
omitted.
The hopper 101 may be configured to feed the heterogeneous material 103 into
the
tank 102. For example, the hopper 101 may be placed at a higher elevation than
the
tank 102, such that the heterogeneous material 103 flows by gravity into the
tank 102.
The hopper 101 may include a device to move the heterogeneous material 103 to
the
tank 102, such as an auger, tilt table, etc., which may communicate with or be
controlled by a computer 184, such as a programmable logic controller (PLC).
The
computer 184 may detect operating conditions of the system 100 via one or more
sensors (not shown) and adjust the flow of the heterogeneous material 103
accordingly.
The tank 102 may have an inlet (not shown) configured to receive the
heterogeneous material 103 from the hopper 101. The tank 102 may have one or
more
angled baffles 105 configured to direct the flow of the heterogeneous material
103. In
a continuous-flow system, the heterogeneous material 103 may mix with a mixed
heterogeneous material 106 already in the tank 102. The tank 102 may
optionally have
an input port (not shown) to add liquid to the mixed heterogeneous material
106. The
tank 102 may include a volume that narrows toward the ground, such as a
conical
portion. The narrowed volume may direct solids of the mixed heterogeneous
material 106 into an outlet at the bottom of the tank 102.
The pump 104 may be in fluid communication with the tank 102, and may
draw the mixed heterogeneous material 106 from the outlet of the tank 102. The
pump 104 may be a horizontal centrifugal pump, an axial centrifugal pump, a
vertical
centrifugal pump, or any other pump configured to pressurize and transport the
mixed
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-11-
heterogeneous material 106. The pump 104 may be selected such that solid
particles
of the mixed heterogeneous material 106 may pass through the pump 104 at an
appropriate flow rate without damaging the pump 104. For example, the pump 104
may be selected to pump 30 gallons per minute (gpm) (1.9 liters per second
(Vs) of a
mixed heterogeneous material 106 containing particles up to about 0.25 in.
(6.35 mm)
in diameter at a pressure of 32 pounds per square inch (psi) (221 kilopascals
(kPa)).
For example, the pump 104 may be a 5-horsepower WARMAN Series 1000 pump,
available from Weir Minerals, of Madison, WI. The pump 104 may deliver any
selected pressure and flow rate, and may be selected by a person having
ordinary skill
in the art based on the requirements for a particular application (e.g., a
selected
heterogeneous material 103 feedstock composition and flow rate). The pump 104
may
communicate with or be controlled by the computer 184. The computer 184 may
detect operating conditions of the system 100 (e.g., by sensors (not shown))
and adjust
the operation of the pump 104. In some embodiments, the system 100 may include
multiple pumps 104 (not shown in FIG. 3).
The pump 104 may pressurize and transport the mixed heterogeneous
material 106 through a continuous-flow mixing device 108, such as a pipe
having
mixing vanes inside. The continuous-flow mixing device 108 may promote a
uniform
distribution of the solid particles within the mixed heterogeneous material
106. For
example, mixing vanes may cause larger or more dense particles (which may tend
to
be distributed differently in the mixed heterogeneous material 106 than fines)
to be
remixed throughout the mixed heterogeneous material 106. The mixed
heterogeneous
material 106 may pass through a splitter 110, separating the mixed
heterogeneous
material 106 into a plurality of streams 112 approximately equal in volumetric
flow
and composition. For example, the splitter 110 may produce two, three, four,
or more
streams 112. In some embodiments, a rotor of the pump 104 may be aligned with
respect to the splitter 110 such that each stream 112 includes identical or
nearly
identical amounts of solid particles of each size and/or density. For example,
a plane
of symmetry of the splitter 110 may be perpendicular to an axis of rotation of
the rotor
of the pump 104. In such embodiments, the continuous-flow mixing device 108
may
be omitted, saving energy that would otherwise be used for mixing in the
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-12-
continuous-flow mixing device 108. In embodiments having multiple pumps 104
(not
shown in FIG. 3), the mixed heterogeneous material 106 may be separated into
components without a continuous-flow mixing device 108. Each stream 112 may
pass
through various piping or hoses, and such piping or hoses may be configured to
have
the same dimensions. For example, the length and curvature of the piping for
each
stream 112 may be equivalent and arranged symmetrically, such that each stream
112
experiences equivalent energy loss in the piping.
The streams 112 produced by the splitter 110 or from the multiple pumps 104
(not shown in FIG. 3) may enter a nozzle assembly 114, shown in simplified
cross-sectional view in FIG. 4, through a plurality of inlets 122. At the
point of entry
to the nozzle assembly 114, the streams 112 may each have the same amount of
kinetic
energy. The nozzle assembly 114 may include a body 115 and a plurality of
nozzles 116 arranged and configured such that the streams 112 (not depicted in
FIG. 4)
intersect in an impact zone 118, indicated by a dashed circle in FIG. 4, after
passing
through the nozzles 116. The streams 112 may intersect in an open portion of
the
nozzle assembly 114. The nozzles 116 may faun the streams 112 into coherent,
focused streams. The nozzle assembly 114 may have a plurality of flow
constriction
zones 120 between inlets 122 and the nozzles 116 in which the flow velocity of
the
streams 112 increases. The flow constriction zones 120 may have sizes and
shapes
such that the streams 112 flow through the nozzles 116 without cavitation. The
flow
constriction zones 120 may have a size and shape configured to increase the
flow
velocity of the streams 112 isentropically (i. e. , with little or no increase
in entropy),
such as by a reversible adiabatic compression. The flow constriction zones 120
may
reduce the area through which the streams 112 pass. Each nozzle 116 may have a
plurality of straight sections 121 (e.g., collimating tubes) having one or
more walls
approximately parallel to an axis of symmetry 117 between the flow
constriction
zones 120 and the nozzle exits 119. The straight sections 121 may serve to
collimate
or align the flow of particles and fluid of the streams 112 so that the
particles travel in
directions approximately parallel. Longer straight sections 121 may be more
effective
at aligning the flow than shorter straight sections 121. In some embodiments,
the
cross-sectional area of the straight sections 121 may be approximately the
same as the
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-13-
cross-sectional area of the nozzle exits 119, and may be from about 5% to
about 20%
of the cross-sectional area of the inlets 122. In other embodiments, the cross-
sectional
area of the nozzle exits 119 may be approximately equal to the cross-sectional
area of
the inlets 122, which may, in turn, be approximately equal to the cross
section of an
outlet of the pump(s) 104. The diameter of the nozzle exits 119 may be
selected to be
approximately twice the diameter of the largest particles expected to pass
through the
nozzles 116. The velocity of the streams 112 may vary in proportion to an
inverse of
the cross-sectional area, and the velocity of the streams 112 at the nozzle
exits 119 may
therefore be from about 5 times to about 20 times the velocity of streams 112
at the
inlets 122. The velocity of the streams 112 may be tailored for a specific
application.
For example, the velocity of the streams 112 may be from about 10 feet per
second
(ft/s) (3.0 meters per second (m/s)) to about 1000 ft/s (305 m/s). The
velocity of the
streams 112 may depend on the properties of the heterogeneous material 103
(FIG. 3).
For example, in some applications, the velocity of the streams 112 may be from
about
300 ft/s (91 m/s) to about 500 ti/s (152 m/s), whereas in other applications,
the velocity
of the streams 112 may be from about 40 ft/s (12.2 m/s) to about 60 ft/s (18.3
m/s).
The velocity of the streams 112 may be selected such that solids are carried
along with
liquids in the heterogeneous material 106 and that enough energy is
transferred to
particles to dissociate constituents of the particles without breaking
homogeneous
portions of particles (e.g., to remove a coating without breaking a core over
which a
coating is disposed). In some embodiments, the velocity of the streams may be
selected (i.e., relatively higher) such that enough energy is transferred to
particles to
pulverize homogeneous portions of material into finer particles. Thus, the
ablated
heterogeneous material 124 (FIG. 3) may optionally include particles having a
relatively uniform particle size. Each of the nozzles 116 may have its own
axis of
symmetry 117 in the center thereof The axis of symmetry 117 of one nozzle 116
may
intersect or coincide with the axis of symmetry 117 of another nozzle 116 in
the impact
zone 118. In embodiments in which the nozzle assembly 114 contains two
nozzles 116, the nozzles 116 may share a single axis of symmetry 117.
Furthermore,
the nozzles 116 may be oriented to face one another. That is, two streams 112
may
impact one another traveling in opposite directions (i.e., head-on) through
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-14-
counter-opposing nozzles 116. In such an arrangement, the kinetic energy of
the
streams 112 converted to impact energy may be larger than in nozzle
arrangements in
which the streams impact obliquely or perpendicularly.
FIG. 5 illustrates another embodiment of a nozzle assembly 114'. A
system 100 having nozzle the assembly 114' may not include a splitter 110, but
may
instead be configured such that the entire mixed heterogeneous material 106 is
directed
through a single nozzle 116. The nozzle 116 may be configured to direct the
stream 112 (not depicted in FIG. 5) against a solid object, such as surface
123 of the
impact zone 118. The portion of the surface 123 against which the stream 112
collides
may be the impact zone 118 of the nozzle assembly 114'. In the nozzle assembly
114'
of FIG. 5, the body 115 and nozzle 116 may be a single unitary structure.
FIG. 6 illustrates another embodiment of a nozzle assembly 114". Each
stream 112 (not depicted in FIG. 6) may pass through multiple constriction
zones 120 separated by straight sections 121 before exiting a corresponding
nozzle 116. Two constriction zones 120 are shown for each nozzle 116 in the
nozzle
assembly 114" shown in FIG. 6, but a nozzle assembly 114" may include any
number of constriction zones 120. Multiple constriction zones 120 and multiple
straight sections 121 may contribute to increased collimation and decreased
wear of
the nozzle assembly 114". Thus, additional constriction zones 120 may increase
the
efficiency of the system 100.
The impact zone 118 may be centrally positioned proximate to the
nozzles 116 (e.g., between or among multiple nozzles 116, or on a surface
across a
gap from a single nozzle 116). In embodiments having two nozzles 116, the
impact
zone 118 may be located approximately midway between the two nozzles 116
(i.e.,
if the streams 112 have equivalent mass flow and particle distribution), but
may be
located anywhere between the two nozzles 116 or in any location in which the
streams 112 can intersect. The size of the impact zone 118 may be determined
by
various design parameters, such as the velocity of the mixed heterogeneous
material 106, the size and/or shape of the nozzles 116, the roughness of the
material
of the nozzle assembly 114, the alignment of the nozzles 116, the number of
nozzles 116, the distance between the nozzles 116 (if applicable), the length
and/or
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-15-
number of the straight sections 121, the composition of the streams 112, etc.
The
impact zone 118 may encompass the vena contracta of each stream 112 (i.e., the
point at which the diameter of each stream 112 is at a minimum, and the
velocity of
each stream 112 is at a maximum). The volume or area of the impact zone 118
may
correspond to the concentration of energy of the streams 112. That is, in the
collision of tightly focused streams 112, particles may be more likely to
impact or
collide directly with other particles traveling in an opposite direction than
they are in
streams 112 intersecting in a larger volume. The particles have a greater
probability
of colliding directly if the streams 112 themselves impact directly (e.g., one
stream is
positioned at an angle of about 1800 relative to another, opposing stream) or
nearly
directly (e.g., one stream is positioned at an oblique angle relative to
another,
opposing stream). For example, one stream may be positioned between about 45
and about 180 (e.g., near 180 ) relative to another, opposing stream.
Likewise, in
the collision of a tightly focused stream with a surface 123, particles may be
more
likely to collide with the surface 123 perpendicularly than they are in a
stream 112
tangentially intersecting a larger area of the surface. To control the volume
or area
of the impact zone 118, it may be desirable to limit or prevent flaring of the
streams 112 as the streams 112 leave the nozzles 116. Flaring may be reduced
or
eliminated by, for example, lengthening the straight section 121, precision
machining, reducing surface roughness, including a shielding fluid (e.g., air,
water,
oil, etc.) around the stream 112, etc.
The kinetic energy of the streams 112 may be used to separate materials of
the particles in the streams 112, such as coatings or layers of material
overlying a
core (e.g., a film, patina, varnish, oxide, or crust). For example, if the
mixed
heterogeneous material 106 (and therefore, each of the streams 112) contains
uranium ore, including particles of the sandstone 10 shown in FIG. 1, the
kinetic
energy of the streams 112 may remove the light fines 16 and/or the heavy fines
18
from the grains 14. If the mixed heterogeneous material 106 contains micro-
fine
gold particles having silicate patinas, the kinetic energy may remove the
silicate
from the gold. If the mixed heterogeneous material 106 contains oil-
contaminated
sand, the kinetic energy may remove the oil coating from the grains of sand.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-16-
Separation of materials may be a physical process (e.g., physical
dissociation),
independent of any chemical process (e.g., chemical reaction, dissolution) of
any
materials. Thus, by utilizing embodiments of thc present disclosure, materials
may
be separated without the addition of reagents (e.g., leachates, acids,
alkalis, cyanide,
lead nitrate, etc.), and the system 100 may be used to recover materials that
are
conventionally recovered by environmentally or operationally problematic
techniques. However, reagents may be present in the liquid, such as in the
groundwater, in trace amounts. Thus, embodiments of the present disclosure may
be
used to separate materials from one another even when none of the materials
has
sufficient solubility in the liquid for chemical separation. In other
embodiments,
reagents may nonetheless be added to enhance dissolution of certain species.
For
example, sodium bicarbonate may be added to the streams 112 to promote the
dissolution of uranium in conjunction with the energy input within the system
100.
The nozzle assembly 114 may be customized or tuned for various
applications. For example, the distance from the nozzles 116 to the impact
zone 118
may be varied, such as by moving the nozzles 116 inward or outward in the
nozzle
assembly 114. The nozzles 116 may be adjustable, including threaded fittings
or
other means to adjust the position and/or orientation of the nozzles 116 with
respect
to the impact zone 118 (e.g., to move the vena contracta within the impact
zone 118,
to move the impact zone 118 such that the streams 112 of material leaving the
nozzles 116 do not travel along the same line, etc.). Other properties of the
system 100 that may be adjusted include, for example, nozzle diameter, the
number
of nozzles, the length and/or number of constriction zones 120 and straight
sections 121, the addition of a liquid to the mixed heterogeneous material
106, the
maximum particles size of the heterogeneous material 103 entering the system
100,
etc. Performance may also be adjusted by changing the pressure, velocity,
and/or
composition of the streams 112 exiting the nozzles 116. Some properties may be
made by, for example, adjusting the power output of the pump 104. Such tuning
may be desirable to use the system 100 to process different materials. As
another
example, one fluid may be passed through one nozzle, and another fluid may be
passed through another nozzle. In such embodiments, one or both fluids may
carry
CA 02932032 2016-05-27
WO 2015/084417
PCT/US2014/011529
-17-
particles of the mixed heterogeneous material 106. In some embodiments, tuning
may be performed in the field, such that as changes are encountered in a feed
stream
of heterogeneous material 103, adjustments may be made to maintain or improve
processing efficiency.
In some embodiments, it may be desirable to impact particles with a lower
energy, such as when a bond between two materials to be dissociated is
relatively
low. The impact energy may be lowered by adjusting one or more properties as
described above. The impact energy may also be lowered by colliding the
streams 112 in a configuration other than directly opposing. Two streams 112
may
be aligned such that they intersect at an angle less than 180 , such as in the
shape of
the letter "V." Such an arrangement may also direct the flow of the material
after
impact.
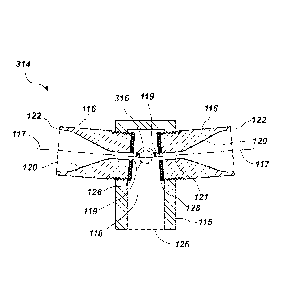
For example, FIG. 25 is a cross-sectional view of a nozzle assembly 314 in
which axes of symmetry 117 intersect at an oblique angle, such that the
streams 112
(not shown in FIG. 25) passing through the nozzles 116 impact at an oblique
angle
(e.g., the axes of symmetry 117 of the nozzles 116 do not fall on the same
line). As
shown in FIG. 25, the nozzle exits 119 and the impact zone 118 may not be
collinear.
For example, the streams 112 may impact at an angle 316 ranging from about 90
to
less than about 180 . It may be beneficial to select the angle 316 to be near
180 , such
that most of the kinetic energy of the streams 112 is converted to impact
energy. For
example, the angle 316 may range from about 160 to about 179.9 , from about
170
to about 179 , or about 175 . Though impacting the streams 112 at an oblique
angle
may result in relatively lower impact energy than a head-on impact, the
oblique angle
may have other benefits. For example, it has been observed that in nozzles 116
oriented directly head-on, such as those shown in FIG. 4, or a nozzle assembly
having
another configuration including an even number of nozzles, small perturbations
in the
flow of the streams 112 may cause the flow through one or more of the nozzles
116 to
stop or clog the nozzles 116, an effect that has not been observed in nozzle
assemblies 314 in which the nozzles 116 arc oriented obliquely to one another.
Without being bound to any particular theory, it is believed that in
embodiments in
which the streams 112 impact each other directly head-on, a perturbation in
the flow of
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-18-
the streams 112 causes a shift in the location of the impact zone 118. Due to
Bernoulli's principle, the pressure in the impact zone 118 is higher than the
pressure in
the streams 112 within the nozzles 116. A change in flow velocity or pressure
of one
stream 112 relative to another stream 112, such that the streams 112 are not
balanced,
may cause the impact zone 118 to shift. If the impact zone 118 shifts near one
of the
nozzles, the flow through that nozzle 116 can stop almost instantaneously,
because the
pressure at the impact zone 118 is greater than the pressure of the stream 112
in the
nozzle 116. When this occurs, flow through the system 100 may be restarted by
stopping and restarting the pump 104. However, in embodiments in which the
streams 112 impact at an oblique angle, such as in the nozzle assembly 314
shown in
FIG. 25, a perturbation in the flow of one of the streams 112 may cause
movement of
the impact zone 118, but does not generally cause flow through any nozzle 116
to stop.
Wear on the exterior surface of the nozzles 116 or on the interior of the
straight
sections 121 (e.g., collimating tubes) may alter the nozzle geometry and
change the
efficiency of the ablation process. In some embodiments, a non-brittle hard
material 128 may be disposed over at least one surface of the nozzles 116 to
protect the
nozzles 116, and particularly the straight sections 121, from wear. For
example, the
non-brittle hard material 128 may be a high-yield-strength metal that is
resistant to
abrasion (e.g., tungsten or hardened steel), a non-brittle ceramic, a
diamond-impregnated ceramic, a hardfacing material, or any other material. The
non-brittle hard material 128 may be in the form of a washer, a surface
coating, a
bonded plate, etc. The non-brittle hard material 128 may be secured to nozzles
116 by
an adhesive, a weld, fasteners (e.g., screws), or any other means or
combination. In
some embodiments, the non-brittle hard material 128 includes a tungsten washer
bonded to the nozzle 116 with epoxy.
FIG. 26 illustrates a cross-sectional top view of another embodiment of a
nozzle assembly 414. The nozzle assembly 414 includes three nozzles 116,
rather
than two. In this nozzle assembly 414, a shift in the location of the impact
zone 118
is unlikely to cause flow through any nozzle 116 to stop because none of the
nozzles 116 directly opposes any other nozzle 116. Some such nozzle
assemblies 414 may include any odd number of nozzles 116, such as five
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
nozzles 116, seven nozzles 116, etc. The splitter 110 (FIG. 3) produces the
same
number of streams 112 as there are nozzles 116. Like the streams 112 that pass
through the nozzles 116 shown in FIG. 25, the streams 112 (not shown in FIG.
26)
that pass through the nozzles 116 in the nozzle assembly 414 shown in FIG. 26
intersect at an oblique angle 416. In the nozzle assembly 414 having three
nozzles 116, the streams 112 intersect at an angle 416 of about 120 . In a
nozzle
assembly having five nozzles 116, streams 112 intersect at an angle of about
72 . In
a nozzle assembly having seven nozzles 116, streams 112 intersect at an angle
of
about 51.4 .
After intersection of the streams 112 of the mixed heterogeneous
material 106 in the impact zone 118, the streams 112 may recombine into a
single
stream of ablated heterogeneous material 124, and may flow through an outlet
126 of
the nozzle assembly 114. The ablated heterogeneous material 124 may contain
more
particles and/or finer particles than the mixed heterogeneous material 106
entering
the nozzle assembly 114. The outlet 126 may have a cross-sectional area larger
than
the combined cross-sectional areas of the nozzles 116, such that the flow of
the
ablated heterogeneous material 124 does not fill the entire outlet 126. Air
may,
therefore, flow freely into or out of the outlet 126 adjacent the impact zone
118. In
some embodiments, the tank 102 (FIG. 3) may be sealed from ambient air, and
may
be filled with a gas. For example, the tank 102 may contain an inert gas. In
such
embodiments, the inert gas may flow freely into or out of the outlet 126. The
outlet 126 may be disposed below the impact zone 118, such that the stream of
ablated heterogeneous material 124 exits the nozzle assembly 114 by the force
of
gravity. For example, if the nozzle assembly 114 (or, alternatively, nozzle
assembly 114" (FIG. 6) or 314 (FIG. 25)) has two nozzles 116, the nozzle
assembly 114 may be shaped like the letter -T," with the two nozzles 116
pointed at
each other, and wherein the outlet 126 is below the impact zone 118 between
the
nozzles 116. In embodiments in which the streams 112 include a slurry, the
nozzle
assembly 114 (or nozzle assembly 114' (FIG. 5), 114" (FIG. 6), 314 (FIG. 25),
or
414 (FIG. 26)) may have air disposed therein, such that the streams 112 flow
through
air after leaving the nozzles 116 and before reaching the impact zone 118.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-20-
Referring again to FIG. 3, the stream of ablated heterogeneous material 124
may pass through the outlet 126 of the nozzle assembly 114 back to the tank
102,
and may mix with the mixed heterogeneous material 106 in the tank 102. A
discharge pump 138 may extract a stream 136 of the mixed heterogeneous
material 106 from the tank 102 and may transfer the stream 136 to a separation
system 140. For example, the stream 136 may be drawn from an outlet located
above one or more baffles 105, and the heterogeneous material 103 may enter
the
tank 102 below one or more of the baffles 105. The baffles 105 may direct the
flow
of the ablated heterogeneous material 124 past the outlet for the stream 136
before
mixing the heterogeneous material 103 from the hopper 101, such that material
of
the stream 136 is drawn from the ablated heterogeneous material 124 that has
been
passed through the nozzle assembly 114 at least once. In some embodiments, and
as
discussed above, the system 100 may include multiple nozzle assemblies 114
operated in series, such that material of the stream 136 passing to the
separation
system 140 has passed through each nozzle assembly 114 at least once. In such
embodiments, the system 100 may include one or more transfer pumps to transfer
material from one nozzle assembly 114 to another. The flow rate of the stream
136
may be varied relative to other flow rates (e.g., the flow rate of the
heterogeneous
material 103 into the tank 102 or the flow rate of the mixed heterogeneous
material 106 through the pump 104) to adjust the average number of times that
particles pass through the system 100. Different heterogeneous materials 103
may
have different bonding properties, and therefore may require different amounts
of
energy to effect dissociation. For example, relatively weaker bonds may be
broken
by relatively less-direct collisions in the impact zone 118 (see FIGS. 4
through 6),
whereas relatively stronger bonds may require more-direct collisions. To
increase
the fraction of particles undergoing direct collision, the particles may be
recycled
through the system 100 (i.e., the flow of the mixed heterogeneous material 106
through the pump 104 may be increased with respect to the flow of the stream
136 to
the separation system 140) and/or passed through more than one ablation system
100
in series.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-21-
In some embodiments, a separation system 140 may be designed to separate
portions of the stream 136 by size, shape, density, magnetic character,
electrostatic
charge, or any other property of particles of the stream 136. For example, in
one
embodiment and as shown in FIG. 7, the separation system 140 may include a
screen 142 (e.g., a rotary screen, an angled screen, etc.) to remove particles
larger
than a selected size. For example, the screen 142 may allow fines 148 (i.e.,
particles
smaller than the mesh size of the screen 142 (e.g., 140 wires per in. (55
wires per
cm))) to pass through the screen 142. Grains 150 (i.e., particles larger than
the mesh
size of the screen 142) may be diverted elsewhere. "f he fines 148, the grains
150, or
both, may be selected for further processing. For example, in a stream 136
containing gold particles, the grains 150 may contain the gold, whereas the
fines 148
may be substantially free of gold. In such embodiments, the fines 148 may be
discarded or returned to the mine as barren waste (i.e., waste substantially
free of a
material of interest). In a stream 136 containing uranium ore, the fines 148
may
contain uranium, whereas the grains 150 may contain barren ore. In such
embodiments, the grains 150 may be returned to a uranium mine as barren waste,
and the fines 148 may be further separated, such as in a gravimetric separator
144.
A portion of the stream 136 (e.g., the fines 148) may pass into a gravimetric
separator 144 for further separation. The particles of the stream 136 in the
gravimetric separator 144 may have approximately unifolin particle sizes,
making
them inseparable by screening, but separable on the basis of density. For
example,
the gravimetric separator 144 may be an elutriation system including a
vertical
column 146. As used herein, the term "elutriation" means and includes a
process of
separating materials based on differences in density. The portion of the
stream 136
to be separated (e.g., the fines 148) may enter the top of the vertical column
146. A
fluid 156 (e.g., water) may be continually introduced into the bottom of the
vertical
column 146 and may flow upward through the vertical column 146. The flow of
fluid 156 through the vertical column 146 may be in either a laminar or a
turbulent
regime. It may be desirable to pass fluid 156 through the vertical column 146
in the
turbulent flow regime because surface roughness and flow perturbations may be
inconsequential for turbulent flow, and control may therefore be simpler. By
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
regulating the rate at which fluid 156 is introduced into the vertical column
146, it
may be possible to control the vertical flow rate within the vertical column
146 so
that light fines 152 (particles having densities below a selected value) exit
the top of
the vertical column 146 with the fluid 156, whereas heavy fines 154 (particles
having densities above the selected value) sink to the bottom of the vertical
column 146. The heavy fines 154 may be continuously extracted from the bottom
of
the vertical column 146, and the volume of the fines removed may be replaced
with
makeup water added at the bottom of the vertical column 146. Alternatively,
the
gravimetric separator 144 may be operated in batch mode, and the heavy fines
154
may be removed between operations.
The light fines 152 may be directed to another apparatus (e.g, a
hydrocyclone, an evaporator, etc.) for separation of the fluid 156 therefrom.
In some
embodiments, the gravimetric separator 144 may include two or more vertical
columns 146 in series, to enhance separation, or in parallel, to increase
volumetric
flow. Separation of the heavy fines 154 from the light fines 152 may decrease
the
amount of material to be processed to recover a target material of interest,
and may
decrease the amount of the target material of interest left in non-bearing
fractions.
Fluids 156 used in the operation of the system may be cleaned by reverse
osmosis,
filtration, ion exchange, or any other method known in the art.
In some embodiments, the gravimetric separator 144 depicted in FIG. 7 may
be an elutriator 200, as shown in FIGS. 8 through 11. A cross section of the
elutriator 200 is shown in FIG. 9. The elutriator 200 includes a column 202
having a
plurality of fluid inputs 204 and a slurry input 206. The column 202 may
include a
generally cylindrical upper portion 208 and a plurality of cylindrical stages
210 (e.g.,
210a, 210b, 210c, 210d, etc.), forming a lower portion 211 having a generally
conical interior. The elutriator 200 may be configured such that the higher-
density
particles settle to the bottom of the column 202, and the lower-density
particles rise
to the top of the column 202. For example, water may enter the column 202 via
the
fluid inputs 204 in the plurality of cylindrical stages 210. The water may be
directed
upward in the column 202 as the water leaves each cylindrical stage 210, such
that
water entering the column 202 from each fluid input 204 flows parallel to
water
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-23-
entering from adjacent fluid inputs 204. The water may flow upward through the
column 202 in a turbulent flow regime (e.g., with a Reynolds number of at
least
about 2,300, at least about 10,000, at least about 50,000, or even at least
about
100,000).
The column 202 may have a geometry selected to minimize or eliminate the
boundary layer between the water and walls of the column 202. For example, the
cylindrical stages 210 may each include a fluid input 204 configured to
deliver a
portion of water. The fluid input 204 in the first stage 210a may provide
water
flowing into a void defined by an inside wall 212b of the second stage 210b at
a
selected velocity. The water flowing into the column 202 through the first
stage 210a fills the entire void defined by an inside wall 212b of the second
stage 210b. The fluid input 204 in the second stage 210b may provide water
such
that the water flows through a void defined by an inside wall 212c of the
third
stage 210c at the same selected velocity. The water flowing into the column
202
through the second stage 210b fills void defined by an inside wall 212c of the
third
stage 210c, which may be significantly smaller than the void defined by the
inside
wall 212b of the second stage 210b. Thus, the flow through the second stage
210b
may be significantly smaller than the flow through the first stage 210a. Thus,
each
fluid input 204 May provide water sufficient to maintain a constant flow
velocity
from the bottom of the column 202 to the top of the column 202.
A top view of a single cylindrical stage 210 is shown in FIG. 10, and a
section view through line A-A is shown in FIG. 11. The stage 210 shown is a
cylindrical body and includes six fluid inputs 204 spaced around the stage
210, but
the stage 210 may be any shape and include any number of fluid inputs 204.
Fluid
enters the stage 210 through the fluid inputs 204, and passes through a
channel 214.
The channel 214 may be a cylindrical void, open along an upper side of the
stage 210. When the stage 210 is stacked in the column 202 (FIGS. 8 and 9),
another stage 210 may provide a boundary of the channel 214 to direct the flow
toward the inside wall 212. The fluid then flows through the channel 214
toward the
center of the stage 210, where a lip 216 deflects the fluid upward. The fluid
then
leaves the stage 210 and flows upward in the column 202.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-74-
The stages 210 may direct the fluid upward within an annular area (e.g., the
area between the lip 216 of the stage 210 and the inside wall 212 of the stage
210
above), and may continuously interrupt the boundary layers at the inside wall
212.
Because the fluid from each stage 210 (starting with second stage 210b) is
directed
upward around flowing fluid from lower stages 210, the volume near the lip 216
in
which the fluid has a low-velocity fluid is relatively small. That is, the
upward-flowing fluid in the center of the column 202 tends to carry fluid that
would
otherwise flow slowly (due to the no-slip boundary condition of fluid
mechanics) at
the lip 216. As the combined fluid flows upward, the fluid entering through
the
stage 210 may tend to mix with the fluid from lower stages 210. The velocity
profile
of the combined fluid may tend to flatten, forming a more uniform flow as the
fluid
rises. In embodiments in which the flow velocity increases slightly from the
bottom
of the column 202 to the top of the column 202, the velocity may be slightly
higher
near the walls of the column 202 than at the center. Such a velocity profile
may tend
to cause heavier particles (e.g., particles having a terminal velocity higher
than the
average velocity of the fluid) to fall downward and toward the center of the
column 202, while lighter particles rise to the top of the column 202.
Particles of material to be separated may enter the elutriator 200 near the
top
of the column 202 via the slurry input 206. Though illustrated as a single
flow into
the center of the column 202, the slurry input 206 may include one or more
nozzles,
a distribution manifold, a spray, or any other means to disperse particles
within the
column 202. Particles of material in the slurry may be separated based on
gravitational forces and forces of the water. Thus, particle mass, particle
surface
area, and fluid flow conditions may each affect the speed and direction of
travel of a
particular particle. In particular, a particle on which the gravitational
force exceeds
the force of the water will fall in the column 202, and a particle on which
the force
of the water exceeds the gravitational force will rise in the column 202.
The movement of particles in the column 202 may be characterized as a flow
of particles in an upward-flowing stream of water. In such a characterization,
calculation of the terminal velocities of particles is instructive, and may
aid in the
design or selection of the elutriator 200. FIG. 12 shows calculated terminal
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-25-
velocities for particles of various geometry and density. FIG. 12 includes
terminal
velocities based on four particle shapes (sphere, cube, tetrahedron, and disk)
and
three densities (p=2.5 g/cm3, p=6.5 g/cm3, and p=10.95 g/cm3). As shown in
FIG. 12, the terminal velocities of smaller particles are influenced less by
the
particles' shapes than the terminal velocities of larger particles. Thus,
terminal
velocities of smaller particles of a selected density are more closely
clustered than
terminal velocities of larger particles of the same density. This makes
classification
of smaller particles by their densities relatively more effective than
classification of
larger particles. For example, in a sample of particles having an effective
diameter
of approximately 0.002 in. (0.051 mm), an upward water flow at a velocity of
between about 0.009 and 0.02 ft/s (between about 0.0027 and 0.0060 m/s) would
effectively separate particles (whether spherical, cubic, tetrahedral, or disk-
shaped)
having a density of 2.5 g/cm3 from particles having a density of 6.5 g/cm3. As
used
herein, the term -effective diameter" of a particle means the diameter of a
hypothetical spherical particle having the same mass as the particle. In a
sample of
particles having an effective diameter of approximately 0.010 in. (0.25 mm), a
water
flow rate of between about 0.13 and 0.16 fl/s (between about 0.040 and 0.049
m/s)
would effectively separate particles (whether spherical, cubic, tetrahedral,
or
disk-shaped) having a density of 2.5 g/cm3 from particles having a density of
6.5 g/cm3. For particles having an effective diameter larger than about 0.015
in.
(0.38 mm), separation of particles having a density of 2.5 g/cm3 from
particles
having a density of 6.5 g/cm3 may not be possible if one or both materials
include
particles of differing geometry. That is, the terminal velocity curve for disk-
shaped
particles having a density of 6.5 g/cm3 crosses the terminal velocity curve
for
spherical particles having a density of 2.5 g/cm3 at a particle diameter of
about
0.015 in. (0.38 mm).
Particles (e.g., lower-density particles) that flow upward in the column 202
may eventually reach an upper outlet 218 (FIGS. 8 and 9), where particles may
be
collected and removed from the elutriator 200 with the fluid. Particles (e.g.,
higher-density particles) that flow downward in the column 202 may eventually
reach a lower outlet 220 (FIG. 9), where particles may be collected and
removed.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-26-
The elutriator 200 may include multiple columns 202 selected and
configured to separate different materials. For example, the particles
collected from
the upper outlet 218 or the lower outlet 220 of the column 202 may be
transferred to
another column 202 having different dimensions or flow rates for subsequent
separation. In some embodiments, the column 202 of the elutriator 200 may
include
additional outlets for withdrawing materials.
The flow of materials into and out of the elutriator 200 may be measured
and/or controlled by flow meters, valves, a computer control system, etc.
(e.g., the
computer 184 shown in FIG. 3).
Referring again to FIG. 7, in embodiments in which the mixed heterogeneous
material 106 (FIG. 3) contains uranium ore, the gravimetric separator 144 may
be
used to separate light fines 152 from heavy fines 154. The light fines 152 may
include barren material and carbonaceous materials, and the heavy fines 154
may
include uranium-bearing minerals, such as uraninite. Processing of uranium ore
in
the system 100 (FIG. 3) including in the separation system 140 may produce a
concentration of less than about 1.0 parts per million (ppm) of uranium in
waste
fractions (e.g., light fines 152, grains 150, and oversize materials). The
system 100
may be used to process uranium left behind in ore previously processed by ISR
techniques.
Though described herein as having a screen 142 followed by a gravimetric
separator 144, other separation equipment and techniques may be used to
separate
portions of the mixed heterogeneous material 106. For example, in some
embodiments, the screen 142 or the gravimetric separator 144 may be used
alone. In
other embodiments, the gravimetric separator 144 may precede the screen 142 in
the
process. Furthermore, the gravimetric separator 144 may include any other
equipment for classifying materials based on specific gravity, such as a
centrifuge, a
shaking table, a spiral separator, etc., instead of or in addition to the
vertical
column 146.
As shown in FIG. 13, the system 100 for processing a heterogeneous material
may be disposed within a single container. For example, the system 100 may be
contained substantially within a frame 180 on a skid or pallet 182 configured
to be
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-27-
carried by a forklift and/or a commercial truck, such that the system 100 may
be
transported and operated without disassembly. In other words, the components
of
the system 100 may be entirely disposed within the frame 180, with the
exception of
portions of piping, wiring, covers, etc. The frame 180 may surround and
protect the
system 100 during transport, but may be open such that the system 100 may be
operated without removing the system 100 from the frame 180. Thus, onsite
setup
requirements and the costs associated with moving the system 100 may be
minimized. The system 100 may include equipment as discussed above and shown
schematically in FIGS. 3 and 7, such as a tank 102, a pump 104, a nozzle
assembly 114, a gravimetric separator 144, etc. Furthermore, the system 100
may
include a computer 184 configured to monitor and/or control operation of the
system 100. In some embodiments, the frame 180 may have a length of from about
2 feet (0.61 m) to about 10 feet (3.0 m), a width of from about 2 feet (0.61
m) to
about 8 feet (2.4 m), and a height of about 2 feet (0.61 m) to about 8 feet
(2.4 m).
The system 100 may have a weight of, for example, from about 100 lbs (45.4 kg)
to
about 4,000 lbs (1814 kg). In some embodiments, the system 100 may be
installed
in a temporary or permanent facility. In other embodiments, the system 100 may
include unitized components configured to be transported by multiple
commercial
vehicles. For example, the system 100 may be transported on five 30-foot
trailers.
The system 100 may also include one or more analytical instruments (not
shown). For example, the system 100 may include instruments configured to test
X-ray fluorescence, gamma radiation (e.g., to determine the concentrations of
various isotopes of a material), turbidity, pH, bicarbonate ion concentration,
particle
size distribution (e.g., by laser particle analysis) etc. The analytical
instruments may
be controlled by the computer 184. The computer 184 may use data from the
analytical instruments to calculate a mass balance in real time. The computed
mass
balance may be used in the control mechanism of the system 100, quality
control,
maintenance, accounting, etc. For example, the computer 184 may track the
amount
of material processed in the system 100 or the amount of a selected material
produced. Thus, an operator of the system 100 may make informed decisions
regarding maintenance intervals, payment of usage fees, etc.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-28-
In some embodiments, the system 100 may be configured to optionally be
used in conjunction with other systems 100. For example, a material (e.g., ore
from
a mining operation) may be processed in a first ablation system. After
ablation in
the first ablation system, ablated material may optionally be processed in a
second
ablation system. In some embodiments, the ablated material leaving the first
ablation system may be tested to determine whether subsequent processing is
necessary or desirable. The material may be processed through as many ablation
systems as necessary to achieve desired material properties. The flow of
material
through ablation systems may be varied during operations. For example, during
a
mining operation, material properties may vary widely within a formation. Some
materials may be profitably processed through a single ablation system,
whereas
other materials may be profitably processed through two or more ablation
systems in
series. The flow of materials through various ablation systems may be varied
during
mining operations in response to changes in materials to be processed.
In some embodiments, and as shown in FIG. 14, system 200 (e.g., a
pneumatic ablation system) may include a pressurized fluid source 107. The
pressurized fluid source 107 may be compressed air from a pump 104 or a
compressor, or may be water, oil, or any other fluid. The pressurized fluid
source 107 may pass through a conduit to a nozzle assembly (e.g., any of
nozzle
assemblies 114, 114', 114", as described previously herein and shown in FIGS.
4
through 6), optionally passing through a splitter 110. The fluid of the
pressurized
fluid source 107 may entrain a heterogeneous material 103, such as from a
hopper 101. An ablated heterogeneous material 124 may pass optionally into a
tank 102 (e.g., a collection bin, a hopper, etc.) and then to a separation
system 140.
A transport apparatus (e.g., a conveyor belt, a chute, etc.) may carry the
ablated
heterogeneous material 124 to the separation system 140. The system 200 may
include a computer 184 for control, data collection, etc.
Heterogeneous materials may be processed with the system 100, 200
described herein. In some embodiments, heterogeneous material is crushed
and/or
screened to remove particles larger than a selected size, such as particles
that are too
large to be effectively processed in the system 100, 200. For example, in some
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-29-
embodiments, particles larger than about 0.25 in. (larger than about 6.35 mm)
may
be removed. In many sandstone-hosted uranium ores, from about 5% to about 30%
or more of the material forms particles larger than about 0.25 in. (larger
than about
6.35 mm) upon crushing. In such materials, particles of ore larger than about
0.25 in. that have been mechanically crushed may contain no uranium compounds.
Therefore, these particles need not be processed by the ablating process
described
herein if the goal is uranium recovery. These particles may instead be
discarded as
barren waste, used to reclaim mines, etc.
In other embodiments, no screening is necessary. For example, some
heterogeneous solid feedstocks may already be entirely within size
requirements of
the system. For example, in the processing of oil-contaminated sand or
silicate-coated gold, grains of material may all be within a range of sizes
that may
pass through the system.
Methods may include mixing the heterogeneous material with a liquid to
form a slurry. For example, the slurry may be formed in a tank 102, as shown
in
FIG. 3. In some embodiments, the heterogeneous material may be mixed with the
liquid before adding the heterogeneous material to the system. For example, in
embodiments in which the heterogeneous material is ore from an underground
formation, the ore may be extracted by borehole mining. In borehole mining,
the ore
is extracted from the formation by high-pressure water jets, and is carried to
the
earth's surface by the water. The mixing of the heterogeneous solid ore with
the
liquid water therefore occurs in the underground foimation. The slurry may
have
any ratio of solids-to-liquids as long as the flow can transport the solids to
an impact
zone. In some embodiments, the slurry may include from about 5% to about 50%
solids by mass, such as between about 10% and about 20% solids by mass.
Methods may further include pumping streams of the slurry through a nozzle
assembly (e.g., any of nozzle assemblies 114, 114', 114", as described
previously
herein and shown in FIGS. 4 through 6) and impacting the streams (and
therefore the
particles therein) to ablate particles of the slurry against one another. The
streams
may, in the process, recombine into a single slurry stream. The heterogeneous
material may separate into discrete fractions in the ablation process. For
example,
CA 02932032 2016-05-27
WO 2015/084417 PCMJS2014/011529
-30-
coatings may be removed from particles of the heterogeneous material in the
ablation process. In some embodiments, all or a portion of the slurry may be
recycled through the system (e.g., returned to the tank 102).
The slurry that has been processed through the nozzle assembly may be
processed to separate particles by size. For example, the slurry may be passed
through a screen to separate particles larger than a mesh size of the screen
from
particles smaller than the mesh size of the screen. For example, the particles
of the
slurry may be separated into grains larger than 0.004 in. (0.10 mm) and fines
smaller
than 0.004 in. (0.10 mm) by appropriately selecting the mesh size of the
screen. In
some embodiments, multiple separations may be performed, such as by passing
portions of the slurry through multiple screens in series. Different size
classifications may be selected by selecting one or more appropriate screens.
Particles having approximately the same size (such that separation by size
classification may be difficult or expensive) may have different compositions.
and
separation of particles with different compositions may be desirable. For
example,
uranium-rich fines may have similar sizes as non-bearing or uranium-depleted
fines
formed from ablation of material from a single formation. Light and heavy
fines
may require different techniques to recover uranium. Therefore, to reduce the
amount of material that must be processed by other means (e.g., chemically) to
extract the uranium, the fines may be separated gravimetrically. For example,
the
fines may be disposed in a vertical column of water, and a fluid may flow
upward
through the column, such as at turbulent flow rates. The fluid may be water,
mineral
oil, an organic solvent, air, etc. Water may be selected based on its flow
properties,
availability, and minimal environmental impact, but other fluids may be used
instead. The fines may be separated in the column by their densities, with
heavier
fines dropping to the bottom, and lighter fines rising to the top. Gravimetric
separation may be performed in one or more stages, with different stages
having
different densities at which the separation occurs. Various parameters may
affect the
separation, such as the type of fluid used, the temperature, the flow rates,
the size of
the column, etc.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-31-
Fluids used in the process, such as in the slurry or in the gravimetric
separation, may be removed from the solids in a dewatering operation. Fluids
may
be processed by filtration, ion exchange, reverse osmosis, etc., to remove
residual
impurities, enabling recycling of the fluids.
The ablation process described herein may be coupled with borehole mining,
the borehole mine providing the heterogeneous material 103 to be processed. In
some embodiments, the heterogeneous material 103 is an ore, such as a
uranium-bearing ore. The use of borehole mining in conjunction with an
ablation
system as described herein may provide operational, environmental, and other
advantages. For example, borehole mining may be used to extract minerals from
unbounded deposits, deposits located above the water table, shallow deposits
with
insufficient hydrologic permeability, deposits in impermeable rock
foiniations, or
small deposits of minerals that may not be economically, technically, or
lawfully
recoverable by conventional ISR. Borehole mining may be perfoimed in
independent wells that do not have to be connected to other wells in the
field. A
single well may be used to penetrate a formation, scour the ore from the
formation,
carry the scoured ore to the surface by a slurry, and return barren fractions
of
processed ore to the formation. This may allow extraction of minerals with a
reduced surface footprint in comparison to conventional methods.
Borehole mining is a technique for extracting mineral deposits from an
underground formation. Typically, a borehole is drilled to a desired depth. A
casing
may be inserted into a portion of the borehole. A borehole mining tool is
inserted
into the borehole, and water is pumped into the tool to produce high-pressure
water
jets. The jets scour ore from the formation, and the mined ore is carried to
the
surface in a sluny of the water. Though borehole mining has been demonstrated
as a
method of mining underground deposits, the method generally requires a nearby
mill, and may require further separation of ore after transport to the
surface.
Borehole mining, a water-only approach, may enable the removal of minerals
that may conventionally (e.g., via ISR) be removed by injecting a leachate or
lixiviant into a foiniation, but without problems associated with the use of
leachates
or lixiviants. In borehole mining, water jets may physically remove formation
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-32-
material without chemically mobilizing or dissolving metals, limiting the risk
of
aquifer contamination. Water jets may operate without modifying foiniation
chemistry and without additional reagent costs. Borehole mining may also be
simpler than conventional ISR. Because material of the formation is extracted,
rather than processed in-situ, borehole mining may begin with less infoimation
known about the formation. Though the boundaries of the formation and
geological
characteristic may still need to be probed, geochemical classification and
peimeability of the formation are not necessary to perform a borehole mining
operation because borehole mining does not rely on chemical reaction or on
permeation.
In some embodiments, borehole mining may be used to scour ore from a
wedge-shaped volume of an underground formation. The extent of the volume may
be tailored by controlling the direction, location, and intensity of the water
jets.
Borehole mining may therefore be used to asymmetrically excavate the
forniation,
roughly following formation boundaries. The ore from the wedge-shaped volume
may be extracted and processed. The wedge may then be refilled, such as with
barren waste or fill and, optionally, a cementing material. Additional volumes
of
material may be extracted in a similar manner. Additional volumes may be
excavated from a well in which volumes have previously been excavated and
refilled. The refilled volumes may provide structural support for later-
excavated
volumes. Reinjection of the barren waste may reduce surface disturbance and
reclamation requirements. When used in conjunction with borehole mining, the
systems described herein may include a surge tank to regulate the flow of
material to
the systems.
95 The ablation process described herein may also be used to process
feedstocks
from other types of mining operations, such as open-pit mining or underground
mining. In such operations, ore may be mined conventionally and processed by
ablation, for example, near the mine. The barren waste may be returned to the
mine,
leaving a small bearing fraction. The bearing fraction may be transported
elsewhere
for further processing. By separating the ore by ablation near the mine,
transportation costs may be greatly reduced.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-33-
In some embodiments, the ablation process described herein may be used to
process material having a concentration of mineral components too low for
economic recovery by conventional processes. For example, waste or overburden
from other mining operations may be processed using ablation. Furthermore,
materials may be treated by ablation to aid in environmental remediation, such
as by
lowering the concentration of chemical species in material previously mined.
For
example, the ablation process may be used for remediation of contaminated land
near mines no longer operating. In such embodiments, the goal may be clean-up
of a
site. The chemical species recovered may be disposed of (the mass containing
the
chemical species being much smaller than the total mass initially
contaminated),
sold, or further processed.
The system and method disclosed herein may be scaled as dictated by
constraints of a particular application (e.g., cost, portability, operating
footprint,
etc.). For example, the system 100, 200 may have a capacity of from about 750
to
about 1,000 lbs per hour (about 340 to about 454 kilograms per hour), and may
fit
within the frame 180, as shown in FIG. 13. Other systems 100, 200 may have a
capacity of about 40,000 lbs per hour (about 20 tons per hour or 18,100
kilograms
per hour) or more. The capacity of the system 100, 200 may be varied by
varying the
capacity of individual components, as known in the art. The capacity of the
nozzle
assembly 114 may be varied by varying the size and/or number of nozzles 116 or
the
particle size distribution of the mixed heterogeneous material 106 entering
the
system 100, 200.
The systems and methods disclosed herein may be used to quickly separate
portions of materials using water, without the addition of chemical reactants.
Water
may provide energy to physically dissociate the portions into discrete
particles that
may be separated based on particle size and density. In materials having
coatings or
patinas, the methods may significantly reduce the amount of material to be
further
processed to recover various components.
For example, in the processing of typical sandstone-hosted uranium ores,
95% or more of the uranium-containing compounds may be concentrated into 10%
of the mass, with the remaining 90% of the mass containing only about 5% or
less of
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-34-
the uranium-containing compounds. For example, the majority of the uranium may
be in particles that pass through a 325-mesh or 400-mesh screen (i.e.,
particles
smaller than about 0.0017 in. (0.044 mm) or 0.0015 in. (0.037 mm) diameter).
In
ores having relatively lower initial concentrations of uranium, the separation
may be
relatively less effective.
Slurry pumps (e.g, slurry pump 104) conventionally have an upper limit on
the size of particles that can be processed in a slurry. Removal of particles
larger
than a selected size (e.g., larger than about 0.25 in. (6.35 mm)) may enable
the use of
a smaller pump 104 than would otherwise be utilized if these larger particles
were
present. However, in the processing of uranium ores, removal of such larger
particles does not significantly affect uranium recovery because this ore
fraction
contains virtually no uranium.
The following examples serve to explain embodiments of the present
disclosure in more detail. These examples are not to be construed as being
exhaustive or exclusive as to the scope of this present disclosure.
EXAMPLES
Example 1: Silicate-plated gold processing
Precious metal ores were extracted from hydrothermal deposits by
conventional mining techniques. The ores contained micro-fine gold particles
having silicate patinas. The silicate patinas interfered with gravity
separation of the
gold-bearing particles from barren material. The silicate chemistry made the
patinas
difficult to remove chemically. The ore was crushed, mixed with water to form
a
slurry, and passed through a pair of opposing nozzles, each having an exit
diameter
of 0.5 in. (12.7 mm), directed to an impact zone 118, as in the nozzle
assembly 114
shown in FIG. 4, at a flow rate of 100 gpm (6.3 1/s) and a pressure of 32 psi
(221 kPa). The collision of the opposing slurry streams imparted enough energy
to
the gold particles to remove the silica patinas after each particle had passed
through
the nozzle assembly 114 an average of 40 times. The process was perfoi wed
in
batch mode, such that an entire batch of ore was continuously recycled through
the
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-35-
nozzle assembly 114 until the patinas were removed from the gold particles.
With
the patinas removed, the gold was recovered by conventional gravity
separation.
Example 2: Oil-contaminated sand processing
A sample of oil-contaminated sand was prepared by mixing a volume of sand
with crude oil. The oil-contaminated sand was mixed with water and a
bio-degradable wood product (available from LB1 Renewable, of Buffalo, WY,
under the trade name DUALZORBR) to form a slurry, and the slurry was passed
through a pair of nozzles, each having an exit diameter of 0.5 in. (12.7 mm),
directed
to an impact zone 118, as in the nozzle assembly 114 shown in FIG. 4, at a
flow rate
of 40 gpm (2.52 Us) and a pressure of 32 psi (221 kPa). The collision of the
opposing slurry streams imparted enough energy to the sand to remove the crude
oil
coating from the sand after each particle of sand had passed through the
nozzle
assembly 114 an average of two times. Upon removal of the oil coating from the
sand, the wood product absorbed the oil. The process was performed in batch
mode,
such that an entire batch of sand was recycled through the nozzle assembly 114
until
the oil was removed from the sand. The cleaned sand was separated from the
oil-soaked wood product and water.
The process may alternatively be performed with a surfactant (e.g., a liquid
surfactant) instead of or in addition to the bio-degradable wood product. The
surfactant may promote the mixture of oil with the water. The surfactant or
the
wood product may prevent the oil from re-coating the sand after the sand
leaves the
impact zone 118.
Example 3: Uranium ore processing
Uranium ores were mechanically extracted from a sandstone formation. The
ores contained oversize materials that contained only minimal amounts of
uranium.
A patina of deposited fine uranium minerals coated non-uranium-bearing grains.
The ores also contained fine deposits of non-uranium-bearing minerals. The ore
was
crushed and screened to remove the oversize materials larger than about 0.25
in.
(6.35 mm). The grains and fines were processed in the system 100 shown in FIG.
3.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-36-
The grains and fines were mixed with water to form a slurry having about 20%
solids by weight. The slun-y was pumped through a pipe having vanes to
increase
uniformity of the slurry, split into two streams, and passed through a pair of
nozzles,
each having an exit diameter of 0.5 in. (12.7 mm) directed toward an impact
zone at
a flow rate of 30 gpm (1.891/s) and a pressure of 32 psi (221 kPa). The nozzle
diameter may be any appropriate size, such as 0.375 in. (9.53 mm). The
collision of
the opposing slurry streams imparted enough energy to the ore particles to
physically
remove the fines from the grains after each particle had passed through the
nozzle
assembly 114 an average of 15 times. With the fines removed, grains were
separated
from fines by screening. The fines were classified by density in a vertical
column,
producing a uranium-rich heavy (i.e., dense) fraction and a barren light
fraction. The
heavy fines were a small portion of the run-of-mine ore and were determined to
be
suitable for further refining (e.g., by conventional chemical means). The
light fines,
grains, and oversize materials were analyzed and it was determined that the
concentration of uranium was low enough that the materials were suitable for
use as
backfill. Water used in the ablation process was found to contain dissolved
uranium
and radium. These elements were recovered from the water via ion exchange and
reverse osmosis.
Comparative Example 4: Particle-size distribution of crushed ore and uranium
distribution as a function of particle size
A sample of uranium-bearing sandstone was mechanically crushed just
enough to break joints between grains, leaving the underlying grain structure
intact.
The crushed ore was segregated by screening to remove particles larger than
0.25 in.
(6.35 mm). The sample included a mixture of ores from multiple sandstone-
hosted
uranium deposits located in the western United States. However, despite being
from
different deposits, each ore exhibited common characteristics, including an
identifiable grain structure of quartz and feldspars, similar pre-ablation
size
distributions, and the presence of carbonaceous materials up to 25.4 mm (1
in.) in
size.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-37-
Like ores from many sandstone-hosted deposits, the ores tested had clearly
identifiable grains ranging in size from less than 1 mm to more than 10 mm. As
shown in FIG. 15, one portion of an ore sample is characterized by relatively
large
grains. As shown in FIG. 16, taken at the same magnification, another portion
of the
same ore has a relatively finer grain structure. A range of grain sizes within
ore from
a single deposit is typical of ore from sandstone-hosted deposits. The
presence of
carbonaceous materials with high post-depositional clement concentrations,
including uranium, is also typical of sandstone-hosted uranium ores.
Carbonaceous
material fragments are visible in FIG. 16 as black material. From the same
ore,
FIG. 17 shows carbonaceous material embedded in the patina surrounding a
grain.
Of the crushed ore that passed through a 0.25-in. (6.35-mm) screen, about
75% of the mass is in particles larger than 60-mesh (about 0.0098 in. (0.25
mm)),
with decreasing percentages present in successively smaller size fractions.
The
average particle-size distribution of the particles smaller than about 0.25
in.
(6.35 mm) is shown in FIG. 18 for the ores tested, including range bars
showing the
variation between the samples analyzed.
The separated particles were tested for uranium content by X-ray
fluorescence (XRF). FIG. 19 shows the percentage of uranium in each size
fraction
smaller than 0.25 in. (6.35 mm). In general, the uranium mass distribution
corresponds to the total mass distribution. FIG. 19 suggests that, in some
sandstone-hosted uranium deposits, removal of a minus 0.25-in, size fraction
by
screening also removes a corresponding percentage of the uranium in the
deposit.
Further, removal of any fraction other than the plus 60-mesh size fraction
would
result in only a marginal reduction in the amount of ore remaining to be
further
processed.
Example 5: Particle-size distribution of ablated crushed ore and uranium
distribution as a function of particle size
A sample of uranium-bearing sandstone was mechanically crushed for
processing by ablation. The crushed sandstone was mixed with water to form a
slurry, and passed through a pair of opposing nozzles, each having an exit
diameter
CA 02932032 2016-05-27
WO 2015/084417
PCT/US2014/011529
-38-
of 0.5 in. (12.7 mm), directed to an impact zone, as in the nozzle assembly
114
shown in FIG. 4, at a flow rate of 30 gpm (1.89 Us) and a pressure of 32 psi
(221 kPa). The collision of the opposing slurry streams imparted enough energy
to
the sandstone particles to remove the patinas and carbonaceous materials after
each
particle had passed through the nozzle assembly 114 an average of 40 times.
The
process was performed in batch mode, such that an entire batch of ore was
continuously recycled through the nozzle assembly 114 until the patinas were
removed from the grains. The fines were separated into light fines and heavy
fines
by elutriation, such as by an elutriator 200 (see FIGS. 8 and 9).
A sample of the light fines was tested for elemental concentrations by XRF.
A sample of the sandstone from which the particles were extracted (i.e., a
sample
that was not processed by ablation) was also tested by XRF. Table 1 lists the
concentration of various elements in parts-per-million (ppm) in the light
fines and in
the sandstone. Carbon is not present in this analysis because the XRF analysis
does
not measure carbon.
Table 1: Concentration of elements in samples tested in Example 5
Element Concentration in light fines
Concentration in Sandstone
(101)m) (f)Pm)
As 25 6.1
Ba 1,468 341
Bi 307 ND
Ca >100,000 2,886
Cl 1,891 ND
Cr 30 14
Cu 67 ND
Fe 11,800 5,974
Hg 12 ND
28,200 28,500
Mn 664 39
21,300 9,270
Sb 1,181 ND
Sr 779 63.8
Ti 1402 840
CA 02932032 2016-05-27
WO 2015/084417
PCT/US2014/011529
-39-
Element Concentration in light fines
Concentration in Sandstone
(ppm) (ppm)
59,300 683
V 411 40
Zn 53 10.3
Zr 105 101
ND = not detected
Example 6: Concentration of uranium in heavy fines as a function of particle
size
A sample of heavy fines was tested from the uranium-bearing sandstone
processed by ablation in Example 5. The sample of heavy fines was screened
through successively finer screens to 600-mesh. After screening, the uranium
concentration in each fraction was measured. The uranium concentration
increased
as the particle diameter decreased, never reaching an inflection point. This
suggests
that ablation of the sandstone forms uranium-containing fines small enough to
pass
through a 600-mesh screen.
Example 7: Concentration of uranium in slurry
Slurry was tested from the sample of uranium-bearing sandstone processed
by ablation in Example 5. The slurry (including heavy fines and light fines)
was
centrifuged at 3,000 rpm for 50 minutes. The supernatant (liquid) was tested
by
inductively coupled plasma optical emission spectroscopy (ICP-OES) with a
spectrometer available from Spectro Analytical Instruments GmbH, or Kleve,
Germany, under the trade name CIROSO VISION, and detemiined to have a
uranium concentration of 16 ppm. This supernatant was then filtered through a
0.45-itm filter. The filtered supernatant was tested by ICP-OES, and the
uranium
concentration was below the lower detection limit (approximately 1 ppm) of the
ICP-OES spectrometer. The removal of uranium by a 0.45-1.tm filter suggests
that
the uranium present in the solution after centrifuging was primarily colloidal
or
near-colloidal in size, rather than dissolved.
In Examples 5 through 7, ablation appears to dissociate carbonaceous
materials from the patinas and cementing minerals, before breaking the
carbonaceous materials down into smaller fragments as light fines. However,
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-40-
because some carbonaceous materials are bonded together independent of
coatings
of grains of larger materials, some carbonaceous materials tend to remain as
particles
larger than minus 400-mesh particles (i.e., particles that pass through a 400-
mesh
screen). The mineralized patina, which appears to have relatively weaker bonds
between particles of the patina, forms relatively smaller particles. After
ablation,
fragments of the carbonaceous material remain within each size fraction
separated by
the screens.
The characteristics of each uranium-bearing fraction of the ore the
pulverized mineral patina and the carbonaceous material¨make both easily
separable from the uranium-barren materials after ablation. Because the
ablated
uranium mineral patina is very fine, it can be separated from the barren
fractions by
simply screening and capturing all the materials smaller than a selected size.
In
contrast, fragments of the carbonaceous materials are present in each size
fraction
after ablation. However, because the carbonaceous materials have relatively
low
specific gravities, they can be separated from barren materials in each post-
ablation
size fraction by elutriation. Because the carbonaceous materials have specific
gravities only slightly higher than that of water, elutriation can efficiently
separate
these particles from the barren grains and cementing minerals. Thus, after
removal
of the fine particles by screening and removal of the light particles by
elutriation, the
remaining material may include virtually no uranium, enabling an almost
complete
recovery of the uranium from the ore by further processing (e.g, by chemical
means)
of only the fines and the light particles.
Example 8: Uranium content of size fractions before and after ablation
A sample of uranium-bearing sandstone was mechanically crushed, as
described in Example 4. The ore was screened to remove materials larger than
0.25-in. (6.35 mm). After screening, the ore was weighed to determine the
volume
of culinary water necessary to perform ablation. For sandstone-hosted uranium
ores,
the ablation system operates at peak efficiency with slurry densities of
between about
10% and about 20% (i.e., when the slurry contains from about 10% to 20% solids
by
mass). With the appropriate volume of water added to the ablation system, the
slurry
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-41-
pump circulated water through a mixing device, a splitter, nozzles, and a
tank. The
ore sample was then added to a hopper feeding the tank, and the resulting
slurry was
circulated through the ablation system at a flow rate of 30 gpm (1.89I/s) and
a
pressure of 32 psi (221 kPa). The ablation system included a pair of opposing
nozzles, each having an exit diameter of 0.5 in. (12.7 mm).
Samples of the slurry were collected after 1, 2, 5, 10, 20, and 50 minutes. At
each time interval, a small amount of the slurry was discharged into a clean 5-
gallon
bucket. Each sample was screened through a 60-mesh stainless steel GILSON
screen and the captured material (the plus 60-mesh fraction) was tested by XRF
to
determine its uranium concentration. The uranium concentration in the plus
60-mesh sample was compared to the uranium concentration in a pre-ablation
plus
60-mesh sample to determine at what point ablation had effectively removed the
mineralized patina from the grains. Ideally, an ablation time may be
determined
during which the mineralized patina is removed, but the grains themselves do
not
break down, maximizing the volume of barren grains that can be separated from
the
pulverized uranium bearing patina by screening.
For these samples, a comparison of the uranium concentrations in the pre-
and post-ablation plus 60 fractions suggested that, after 5 minutes, ablation
had
effectively removed the mineralized patina. Various factors may affect
ablation
time, including the thickness of the patina, the mass distribution of the pre-
ablated
material, and the shape of the underlying grain.
The material removed from the ablation system after five minutes was passed
through a series of GILSON screens ranging from 60-mesh to 325-mesh. The
sample captured on each screen was dried, weighed, and analyzed by XRF to
determine both the mass and uranium balance of each sample. FIG. 20 shows the
percentage of total mass and percentage of uranium mass in each size fraction
smaller than 0.25 in. (6.35 mm), for both the ablated sample (after five
minutes) and
an unablated sample. In addition, the clarified post-ablation water was
analyzed to
determine how much uranium dissolved in the water during ablation.
The difference between the unablated sample and the ablated sample
illustrates how ore from sandstone-hosted uranium deposits behaves during
ablation.
CA 02932032 2016-05-27
WO 2015/084417 PCT/US2014/011529
-42-
When effectively ablated, the mass of particles of sandstone-hosted uranium
ores
showed a minor shift from larger to smaller size fractions, whereas the
uranium was
almost completely concentrated into the minus 325-mesh fraction (see FIG. 20).
Prior to ablation, the plus 60-mesh fraction contained about 74% of the total
mass and 46% of the uranium. After ablation, this fraction contained about 73%
of
the total mass but only 1.8% of the uranium. Before ablation, the minus 325-
mesh
fraction contained about 3% of the total mass and 10.4% of the uranium. After
ablation, this fraction contained about 7% of the total mass and 94.9% of the
uranium. It is believed that the increase in mass in the fines and the almost
complete
transfer of uranium into the minus 325-mesh fraction both occur because,
during
ablation, the mineralized patina around the grain is removed and pulverized
into
particles smaller than 325-mesh. The residual uranium in the plus 325-mesh
fractions appears to be in fragments of carbonaceous material.
Samples of the clarified ablation water collected at 1, 2, 5, 10, 20 and
50 minutes were analyzed using XRF. FIGS. 21 and 22 collectively show the
concentrations of the seven elements detected consistently in the ablation
water (As,
Cl, K, Rb, S, Sr, and U) as a function of ablation time. The uranium
concentration
in the ablation solution was 22 ppm after one minute of ablation, which
represents
27.9% of the uranium in the head ore. The uranium concentration increased to
25 ppm after five minutes of ablation.
The tests performed on sandstone-hosted uranium ores show that, within five
minutes, the ablation process concentrates almost all of the non-solubilized
uranium
into a very small fraction of the original ore. An average of 95% of the
non-solubilized uranium was present in the minus 325-mesh material, which
accounted for between 5% and 7% of the mass of the ablated ore. Therefore,
after
five minutes of ablation, if all materials larger than 325-mesh were removed
from
the post ablation slurry stream, and only the minus 325-mesh post ablated
material
were subsequently processed, a 95% recovery of the uranium would be possible.
Furthermore, subsequent processing could be reduced by between 93% and 95%
(corresponding to the 93%-95% of material that need not be further processed).
Higher mass reductions and recovery rates can be achieved by elutriating and
CA 02932032 2016-05-27
WO 2015/084417
PCT/US2014/011529
-43-
capturing the light carbonaceous materials that remain in each fraction after
ablation.
However, even without elutriation, the ablation-only recovery rates compare
favorably to conventional mining methods because, although 95% is roughly
equivalent to the recovery achieved by leaching, ablation accomplishes this
recovery
in five minutes, using only culinary water, and does so while reducing by 90%
or
more the volume of ore that needs to be processed to recover the uranium.
Another way to gauge the effectiveness of ablation on sandstone-hosted ores
is to visually compare unablated and ablated samples of the same ore. The
pre-ablated sample of Example 8 had clearly identifiable grains, but, because
of the
adhered mineral patina, the underlying grain itself was hidden from view (see
FIG. 23). The patina-coated grains had a grayish appearance. In addition,
identifiable fragments of the carbonaceous materials were visible, often
embedded or
partially coated in the mineralized patina. In comparison, the ablated gains
were
clearly identifiable and free of mineralized patina (see FIG. 24). Ablated
fragments
of carbonaceous materials were interspersed with these grains.
Example 9: Ablation with deionized water
A sample of uranium-bearing sandstone was mechanically crushed and
ablated, as described in Example 8. However, deionized water was used as the
liquid component of the slurry. The ablation slurry had a distinct silvery
appearance
that never settled out of the ablation slurry during centrifugation. This
supernatant
was then filtered through a 0.45-nm filter and analyzed using XRF. No uranium
was
detected in the filtered ablation water. A portion of the supernatant that had
not been
filtered was also analyzed using XRF, and found to contain uranium. This
suggests
that the ablation slurry, before filtering, contained micro-fine uranium
material. The
micro-fine material appears to be small enough to remain in suspension, and
may
include other post-depositional elements that would be dissolved into
untreated
water (e.g., water having dissolved carbonates) if untreated water were used
as the
slurry fluid.
When sandstone-hosted uranium ores are ablated with untreated water (e.g.,
culinary water, ground water, etc.), some of the uranium may dissolve into the
CA 02932032 2016-05-27
WO 2015/084417
PCMJS2014/011529
-44-
ablation fluid. The amount dissolved varies depending on the deposit and the
water
used, but may range from one-tenth to one-third or more of the total uranium
in the
ore. Without being bound to a particular theory, it is believed that naturally
occurring carbonates in the untreated water solubilize some of the uranium
from the
ore during ablation.
While the disclosure is susceptible to various modifications and alternative
forms, specific embodiments have been shown by way of example in the drawings
and have been described in detail herein. However, the disclosure is not
intended to
be limited to the particular forms disclosed. Rather, the disclosure is to
cover all
modifications, equivalents, and alternatives falling within the scope of the
disclosure
as defined by the following appended claims and their legal equivalents. In
addition,
features from one embodiment may be combined with features of another
embodiment while still being encompassed within the scope of the present
disclosure as contemplated by the inventors. Further, embodiments of the
present
disclosure have utility in the processing of various types of heterogeneous
materials.