Note: Descriptions are shown in the official language in which they were submitted.
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
1
A METHOD OF TREATING OILY SOLID PARTICLES
Field Of Invention
The present invention relates to a method of treating oily solid particles
entrapping
particularly heavy crude oil and the like. Specifically, the disclosed method
conditions the
oily solid particles to a preferred state and reacts the oily solid particles
with a specially
formulated emulsified composition to remove the entrapped crude oil from the
surface of
the solids.
Background Of The Invention
Treatment of oily waste material or oily solids derived majorly from oil
drilling and
refinery activities remain one of the biggest challenges in the oil and gas
industry. These
oily solid or oily waste materials can refer to oil sludge, drill cuttings,
contaminated soils,
contaminated sands/minerals and the like generated from various areas in the
industry. It is
a mixture majorly composed of sands, clay, minerals, and oil (crude oil or
base oil from
drilling fluid) presented in the mixture. It is crucial to at least remove or
recover the crude
oil from the solids prior to disposing the waste material to reduce its impact
towards the
environment. Presence of complex elements in the oily solids poses great
hurdles towards
the attempt to effectively separate the oil fragments from the solid phase and
various
approaches have been proposed along the years to tackle the problems. For
example, in
early year, sole water extraction using water jet to de-oil the oily solids is
described in the
United States patent application no. 3764008. While solvent extraction method
in
conjunction with special pre-treatment on the oil waste can be found in United
States patent
application no. 4260489, 4931161, 5347069 and 2009078612 respectively. Gary et
al.
describes another approach in United States patent application no. 4775457
which mix the
oily solid waste with perlite following by burning the mixture to rid the oil
fragment. With
the advance in surfactant technologies, oil separation methods based on the
use of
emulsified composition and surfactants are developed to yield better
separation of the oil
from the solid particles. Donald offers a method of cleaning oil contaminated
substrates by
using two different surfactants with different HLB value and the difference in
between the
HLB value is at least 3. Cordova claims another method of treating oil-
contaminated
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
2
substrate by pre-treating the substrate with an emulsion breaker with
subsequent steam
distillation treatment to recover the oil phase from the substrate. Further,
French patent
application no. 2814385 provides a method to wash off the oil covered-
particles using a
lighter oil phase containing non-ionic surfactant and further separating the
reactants into at
least three different layers through decantation. Use of surfactant and
aqueous polymer
mixture for de-oiling oil contaminated substrate can be found in W02005033469.
Summary Of The Invention
The present invention aims to disclose a method of treating oily solid waste
or material. The
oily solid particles preferably entrap high content of crude oil and the like
in between the
surface of the solid particles.
Another object of the present invention is to offer a method of treating oily
solid particles
using a specially formulated emulsified composition to effectively repel the
oil phase off
the surface of the oily solid particles.
Further object of the present invention is to offer an effective method of
treating oily solid
particles by preparing or pre-treating the oily solid particles to a suitable
condition to be
readily reacted with the emulsified composition.
At least one of the preceding objects is met, in whole or in part, by the
present invention, in
which one of the embodiments of the present invention is a method of cleaning
oily mass
having heavy oil adsorbed onto solid particles comprising the steps of
creating electrostatic
repulsion in between the surface of the solid particles and the adsorbed heavy
oil using a
basic solution; reacting the oily mass with an emulsified composition
containing light
hydrocarbon in the presence of the basic solution to displace the adsorbed
heavy oil from
the surface of the solid particles using the light hydrocarbon; separating the
reacted oily
mass into a liquid phase and a solid phase; and removing residues of the
emulsified
composition from solid phase, wherein the emulsified composition comprises a
surfactant
in 2 to 40% by weight of total composition selected from alkyl polyglycosides,
glyceryl-
based surfactant, polyglyceryl-based surfactant, sucrose-based surfactant,
sorbitol fatty acid
esters, sulfofatty acid methyl esters, acylated aminoacids, acyl glutamates,
acyl glycinates,
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
3
acyl alaninates, lauroyl sarcosinate, nopol alkoxylates; a co-surfactant in 1
to 30% by
weight of total composition selected from the group consisting of C3 to C18
alcohols, C3 to
C18 alkyl lactates, lecithins, C3 to C18 fatty acids, diols, amino acids and
any mixtures
thereof; an oil phase in 15 to 90% by weight of total composition; and an
aqueous phase in
0.5 to 20% by weight of total composition. Preferably, the disclosed method
includes
additional steps of recovering oil from the liquid phase.
In one aspect, the disclosed method has the oily mass dispersed within the
basic solution in
the creating and reacting steps to assist dissociation of the oil phase and
increase surface
area of the oily mass to be reacted with the basic solution of the emulsified
composition.
Preferably, the emulsified composition constitutes 0.01 to 15% wt of the basic
solution.
In one aspect, the removing step is washing the solid phase with a solvent
mixture of an
aqueous solution containing a co-solvent at least one time followed by
separating the
washed solid phase from the solvent mixture that the co-solvent is ethylene
glycol,
diethylene glycol, triethylene glycol, propylene glycol, diprolylene glycol,
methanol,
ethanol, propanols, butanols, butoxyethanol or any combination derived
thereof. Apart from
that, aqueous solution is employed as the sole washing agent without any co-
solvent in
another embodiment of the disclosed invention.
To further reduce oil content of the acquired solid phase, the removing step
also includes as
well a step of vaporizing the remained light hydrocarbon off the solid phase.
This can be
attained with heating or under an environment with reduced pressure. More
preferably, the
removing step is vaporizing the remained light hydrocarbon off the solid phase
through
heating the solid phase at a temperature below the flash point of the light
hydrocarbon.
To enhance efficiency of the disclosed method, the emulsified composition
preferably
further comprises a chelating agent selected from the group consisting of
ethylenediamine
tetraacetic acid, hydroxyethyl en edi amin e triacetic acid, nitriolotriacetic
acid, citric acid,
acetylacetone, porphyrin, catechol , dithiolene phosphonic acids and their
salts,
polyphosphates, phosphate esters, nonpolymeric phosphonates,
aminophosphonates,
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
4
polyphosphonates phosphino polymers, polyphosphinates, polycarboxylates,
polysulfonates
or any combination derived thereof in another embodiment of the disclosed
invention.
In another embodiment, the disclosed method of cleaning oily solid particles
entrapping
heavy crude oil comprises the steps of reacting the oily solid particles with
an emulsified
composition containing light hydrocarbon in the presence of a basic solution
to displace the
entrapped oil from the surface of the oily solid particles using the light
hydrocarbon;
separating the reacted oily solid particles into a liquid phase and a solid
phase; and
removing residues of the emulsified composition from solid phase, wherein the
emulsified
composition comprises a surfactant in 2 to 40% by weight of total composition
selected
from alkyl polyglycosides, glyceryl-based surfactant, polyglyceryl-based
surfactant,
sucrose-based surfactant, sorbitol fatty acid esters, sulfofatty acid methyl
esters, acylated
aminoacids, acyl glutamates, acyl glycinates, acyl alaninates, lauroyl
sarcosinate, nopol
alkoxylates; a co-surfactant in 1 to 30% by weight of total composition
selected from the
group consisting of C3 to C18 alcohols, C3 to C18 alkyl lactates, lecithins,
C3 to C18 fatty
acids, amino acids, diols, and any mixtures thereof; an oil phase in 15 to 90%
by weight of
total composition; and an aqueous phase in 0.5 to 20% by weight of total
composition.
Brief Description Of The Drawings
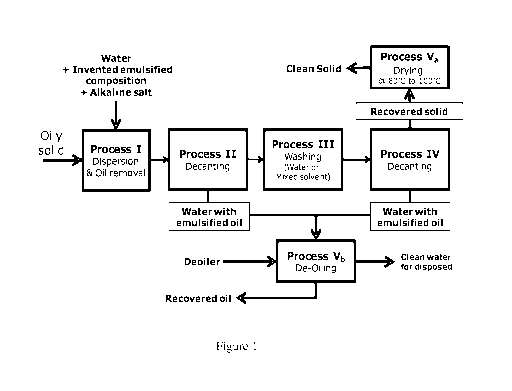
Figure 1 is flowchart showing one embodiment of the invented method.
Detailed Description Of The Invention
It is to be understood that the present invention may be embodied in other
specific forms
and is not limited to the sole embodiment described herein. However
modification and
equivalents of the disclosed concepts such as those which readily occur to one
skilled in the
art are intended to be included within the scope of the claims which are
appended thereto.
It is important to be noted herein that the term "oily mass" used herein
throughout the
specification refers to mixture of solid particles and heavy oil such as oil
sludge, sludge
from oil production well, drilling fluids containing barite and/or bentonite
and/or clay, or
hydrocarbon contaminated-soil, sand, clay and inorganic minerals from oil &
gas
production except mentioned otherwise.
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
The term "heavy oil" used herein shall refer to residual fuel oil or heavy
crude oil which has
density and/or specific gravity higher than light crude oil.
5 The present invention involves a method of cleaning oily mass having
heavy oil adsorbed
onto solid particles comprising the steps of creating electrostatic repulsion
in between the
surfaces of the solid particles and the adsorbed heavy oil using a basic
solution; reacting the
oily mass with an emulsified composition containing light hydrocarbon in the
presence of
the basic solution to displace the adsorbed heavy oil from the surface of the
solid particles
using the light hydrocarbon; separating the reacted oily mass into a liquid
phase and a solid
phase; and removing residues of the emulsified composition from solid phase,
wherein the
emulsified composition comprises a surfactant in 2 to 40% by weight of total
composition
selected from alkyl polyglycosides, glyceryl-based surfactant, polyglyceryl-
based
surfactant, sucrose-based surfactant, sorbitol fatty acid esters, sulfofatty
acid methyl esters,
acylated aminoacids, acyl glutamates, acyl glycinates, acyl alaninates,
lauroyl sarcosinate,
nopol alkoxylates; a co-surfactant in 1 to 30% by weight of total composition
selected from
the group consisting of C3 to C18 alcohols, C3 to C18 alkyl lactates,
lecithins, C3 to C18
fatty acids, diols, amino acids and any mixtures thereof; an oil phase in 15
to 90% by
weight of total composition; and an aqueous phase in 0.5 to 20% by weight of
total
composition. Preferably, the surfactant is biodegradable and substantially low
in toxicity.
Preferably, in one embodiment, the reacting step further comprises the steps
of dispersing
the oily mass in the basic solution. The dispersion can be carried out using
any known
apparatus in the art to stir or apply physical force to break the oily mass
into smaller
portions to increase overall surface area of the oily mass to be reacted with
the basic
solution and the emulsified composition. In the dispersion process, portion of
the oil or
heavy crude oil is dissociated from the solid particles in the form of small
droplets
distributed in the basic solution due to the brute force applied. With
hydrophobic heavy
crude oil coated the surface of the solid particles, these small droplets may
again dissolve
and accumulate onto oil-coating surface of the solid particles. Nevertheless,
it was found by
the inventors of the present invention that presence of the basic solution
changes the surface
charge of the solid particles and the dissociated small oil droplets to be
negative or stronger
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
6
negative forming strong electrostatic forming a charge barrier thereof to
prevent clumping
(re-deposition) of these small droplets onto the hydrophobic surface of the
solid particles
again. Further, the basic solution also builds up negative charges on surfaces
of the solid
particles and the adsorbed heavy oil that an electrostatic repulsive force is
generated there
between to assist the dissociation of the adsorbed heavy oil from the solid
particles. To
effectively change the surface charges of the heavy oil and the solid
particles into negative
charges, the basic solution preferably has a pH value ranged in between 8 to
12. Preferably,
the basic solution is prepared from water soluble alkaline salts of hydroxide,
carbonate,
phosphate or any combination derived thereof.
In the case where the electrostatic repulsive force is insufficient to repel
off the adsorbed
crude oil, the disclosed method further enhance the dissociation using the
emulsified
composition which infiltrates gaps existing in between the surface of the
solid particles and
the adsorbed heavy oil to subsequently repel the adsorbed heavy oil off. More
specifically,
the coated negative charges reduce the interfacial tension between the solid-
oil interface
allowing subsequent infiltration of aqueous phase or emulsified composition
thereof In
conjunction with the basic pH in the reaction environment, the emulsified
composition of
the disclosed method is developed to the deliver the contained surfactant to
adsorb onto the
solid surface thus squeezing off the adsorbed heavy oil. Through
adsolubilizing of the
surfactant, the adsorbed surfactant promotes adsorption of the light
hydrocarbon to displace
the heavy oil from the surface of the solid particles and avoid re-adsorption
of the displaced
heavy oil. In a more preferable embodiment, the light hydrocarbon is any one
or
combination of paraffin, kerosene, arene, mineral oil, triglycerides, esters,
ethers, ketones,
fatty alcohols, and light crude oil.
As in setting forth, the emulsified composition preferably comprises a
surfactant in 2 to
40% by weight of total composition selected from alkyl polyglycosides,
glyceryl-based
surfactant, polyglyceryl-based surfactant, sucrose-based surfactant, sorbitol
fatty acid
esters, sulfofatty acid methyl esters, acylated aminoacids, acyl glutamates,
acyl glycinates,
acyl alaninates, lauroyl sarcosinate, nopol alkoxylates, a co-surfactant in 1
to 30% by
weight of total composition selected from the group consisting of C3 to C18
alcohols, C3 to
C18 alkyl lactates, lecithins, C3 to C18 fatty acids, diols, amino acids and
any mixtures
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
7
thereof; an oil phase in 15 to 90% by weight of total composition; and an
aqueous phase in
0.5 to 20% by weight of total composition. Chelating agents such as
ethylenediamine
tetraacetic acid, hydroxyethyl en edi amin e triacetic acid, nitriolotriacetic
acid, citric acid,
acetylacetone, porphyrin, catechol , dithiolene phosphonic acids and their
salts,
polyphosphates, phosphate esters, nonpolymeric phosphonates,
aminophosphonates,
polyphosphonates phosphino polymers, polyphosphinates, polycarboxylates,
polysulfonates
or any combination thereof can be incorporated into emulsified composition
used in the
disclosed method. The chelating agent preferably has a concentration of 1 to
10% by
weight of total composition to remove metal ion such as calcium, magnesium,
barium,
strontium, ferum, vanadium, nickel and cuprum suspended in the sludge and oil
residue.
According to the preferred embodiment, the oil phase of the emulsified
composition is any
one or combination of terpenes, aromatic hydrocarbons, mineral oil, paraffin
oil, glycols,
esters, fatty acid ester, fatty ester, glycol ethers, palm oil and other oil
from plant source,
diesel, and petroleum distillates. To impart better biodegradability and to be
ecological
friendly, oil from plant source, terpene from plant extraction or chemically
synthesized,
glycol, esters, fatty acid ester, or fatty ester is preferably used to
constitute the oil phase of
the present invention. Relying upon the types and the heavy oil content of
oily mass, the
emulsified composition is of 0.01 to 15% wt of the basic solution.
As in foregoing, the disclosed method further separates the reacted oily mass
into the liquid
phase and the solid phase. The separation of the liquid phase and the solid
phase can be
conducted through decantation with or without centrifugation. Upon complete of
the
decantation, the disclosed method has the liquid phase channel for a de-oiling
process and
the solid phase subjected to washing to further reduce oil content and remove
residues of
the emulsified composition in the solid phase. Specifically, the disclosed
method has
additional step of recovering the heavy oil from the liquid phase. In the oil
recovery step,
deoiler of a concentration of 20 to 200ppm is mixed into the liquid phase to
promote the
aqueous-oil separation. Preferably, the deoiler can be any one or combination
of highly
valent metal salt or polymeric flocculants. Highly valent metal salt is
selected from but not
limit to Iron(III) salts, Zinc(II) salts, and Aluminum(III) salts and mixtures
thereof.
Polymeric flocculants includes but not limit diallyldimethylammonium chloride
polymers,
acrylamide-based polymers, acrylate-based polymers,
polyalkyleneimines,
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
8
polyalkanoamines, polyvinylammonium chloride, polyallylammonium chloride,
branched
polyvinylimidazoline acid salts, polysaccharides, chitosan, condensed tannins,
dithiocarbamates, hydrolyzed polyacrylamide-grafted xanthan gum, poly-y-
glutamic acid,
polyaspartic acid and mixtures thereof. The liquid phase substantially free of
oil is then
discharged or being recycled to reuse in the disclosed method again.
Pursuant to another preferred embodiment, the steps of removing the residues
of emulsified
composition and the remaining heavy oil in the solid phase is washing or
flushing the solid
phase with an aqueous solution at least one time followed by separating the
washed solid
phase from the solution. The aqueous phase can be water or water mixed with co-
solvent
such as ethylene glycol, diethylene glycol, triethylene glycol, propylene
glycol, diprolylene
glycol, methanol, ethanol, propanols, butanols, butoxyethanol or any
combination derived
thereof The aqueous phase dissolves most of the remaining residues of the
emulsified
composition including the surfactant adsorbed onto the surface of the solid
particles in the
solid phase and small amount of the adsorbed heavy oil as well as light
hydrocarbon. Upon
finishing the washing or flushing, the solid phase is separated from the
solution which is
subsequently subjected to decantation to remove the heavy oil and the light
hydrocarbon.
Accordingly, in another embodiment, the removing step is washing or flushing
the solid
phase with a solvent mixture of an aqueous solution mixed with a co-solvent at
least one
time followed by separating the washed solid phase from the solution that the
co-solvent is
ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol,
diprolylene glycol,
methanol, ethanol, propanols, butanols, 2-butoxyethanol or any combination
derived
thereof Particularly, the use of co-solvent in this embodiment improves
efficiency to
extract the remaining hydrophobic compounds on the solid phase especially the
heavy oil
and light hydrocarbon while the aqueous phase dissolves most of the remaining
hydrophilic
residues of the emulsified composition. As in the foregoing, the washed or
flushed solid
phase is separated from the solvent mixture which is subsequently subjected to
decantation
to remove the heavy oil and the light hydrocarbon dissolved.
Similarly, the decantation of the solution or solvent mixture may include as
well recovery
of the oil in the solution using deoiler of a concentration of 20 to 200 ppm
mixed into the
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
9
used solution or solvent mixture to promote the aqueous-oil separation.
Likewise, the
deoiler can be any one or combination of highly valent metal salt or polymeric
flocculants.
Highly valent metal salt is selected from but not limit to Iron(III) salts,
Zinc(II) salts, and
Aluminum(III) salts and mixtures thereof Polymeric flocculants includes but
not limit
diallyldimethylammonium chloride polymers, acrylamide-based polymers, acrylate-
based
polymers, polyalkyleneimines, polyalkanoamines, polyvinylammonium chloride,
polyallylammonium chloride, branched polyvinylimidazoline acid salts,
polysaccharides,
chitosan, condensed tannins, dithiocarbamates, hydrolyzed polyacrylamide-
grafted xanthan
gum, poly-y-glutamic acid, polyaspartic acid and mixtures thereof The solution
or solvent
mixture substantially free of oil is then discharged or being recycled to
reuse in the
disclosed method again.
In the preferred embodiment, the removing step involves also vaporizing the
remained light
hydrocarbon off the solid phase. It is important to be noted herein that the
vaporizing step
1 5 can be conducted with or without having the washing step performed
beforehand though it
is more preferred to have the residue of emulsified composition to be washed
off first. The
evaporation of the light hydrocarbon is conducted in a pressure reduced
environment or
through heating or combination of both. To vaporize the light hydrocarbon off
the solid
phase, it is important to control the temperature of the heating below the
flash point to avoid
ignition of the light hydrocarbon. Specifically, the removing step is
vaporizing the remained
light hydrocarbon off the solid phase through heating the solid phase at a
temperature below
the flash point of the light hydrocarbon.
Figure 1 shows an example of process flow related to the present invention
involving
different steps as listed below.
Process I: Dispersion and oil removal; breaking down the oily solid cluster,
dispersing the
solid and oil removing/degreasing simultaneously. The invented emulsified
composition
and/or alkaline salt are added into this process to treat the oily solid.
Process II: Decantation to separate liquid (water with emulsified/removed oil)
from solid.
Process III: Washing with water or mixed co-solvent with water. This washing
step aims to
remove the remaining composition of the cleaning chemical solution and also
eliminate the
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
effect of adsolubilization (oil solubilized by surfactant admicelles on solid
surface) by
dilution and surfactant desorption.
Process IV: Decantation to separate liquid (water with emulsified/removed oil)
from clean
solid;
5 Process V: Disposal treatment
Process Va: Drying; at the temperature around 80-100 C aiming to remove
moisture and
remaining light oil replacing the heavy oil in the sample during the cleaning
process; and
Process Vb: De-Oiling water; water treatment with de-oiler (< 1000 ppm).
10 One skilled in the art will readily appreciate that the present
invention is well adapted to
carry out the objects and obtain the ends and advantages mentioned, as well as
those
inherent therein. The embodiment describes above is not intended as
limitations on the
scope of the invention.
EXAMPLE 1
The invented emulsified composition presented in following examples are water-
in-oil
nanoemulsion with oil-water interfacial tension lower than 0.01 mN/m (measured
at 25 C
by KRUSS spinning drop tensiometer; model SITE100). The mean particle size of
the
nanoemulsion is smaller than 100 nm (measured at 25 C by particle size
analyzer; Malvern
Zetasizer Nano ZS). The oily solid samples listed in TABLE 1 are the samples
used in
testing various embodiments of the present invention. The properties of each
oily solid
sample were determined by using the retort analysis. All samples are actual
specimens from
Oil & Gas industrial processes. Treatment on these oily solid samples aims to
reduce the oil
content in the disposed solid to < 1 w/w%. The evaluation of the effectiveness
of the
invented emulsified composition, treatment process and other related cleaning
chemicals
are demonstrated in the next EXAMPLEs.
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
11
TABLE 1: Composition of oily solid samples from retort analysis
% Total % Wax (hear oil)
hydrocarbon that % Light oil that that can be
can be extracted can be extracted extracted up to
up to 950 F up to 950 F 950 F %
Water Solids
Sample No. General name Form (w/W/0) (w/W/0) (w/W/0)
(w/W/0) (w/W/0)
A Drill cuttings Solid 13 4 9 0.3
86.7
Sand produced from
B production well Solid 12 5 7 7 81
Solid sediment from the
C used oil-based drilling fluid Solid 13 5
8 8 79
Crude oil sludge solid
D sediment Paste 29 12 17 6
65
Note: Weight of w ax and light oil w ere calculated based on an assumed
densrty of 0.82 g/mL.
EXAMPLE 2
The efficiency of the invented oily solid treatment method and cleaning
chemical
composition was evaluated with different process steps and cleaning chemicals
used. The
efficiency of the process was referred to the total oil content in the solid
after final
treatment. Oil content was determined by the retort analysis and/or Soxhlet
solvent
extraction (if the oil content was expected to be ( 1 w/w%). The typical
target of the oil
content in the solid after treatment is < 1 w/w%. Sample A, which is drill
cuttings had been
used for the experiment in this EXAMPLE. Sodium carbonate, sodium
tripolyphosphate
and potassium hydroxide had been used as the alkaline salt to adjust the pH of
the cleaning
solution to 8-12. The cleaning solution/sample ratio is 1/1 by weight. The
washing solvent
was water or water mixed with co-solvent (ethylene glycol, isopropanol, or
butoxyethanol).
Water was the solvent of cleaning solution for the first process step. The %
concentration of
cleaning solution is the % compared with the treated sample weight otherwise
stated.
Cleaning solution/sample ratio was 1/1 by weight. This experiment also
compares the
efficiency between the invented method and cleaning composition (Experiment A4-
A15)
and the conventional surfactant cleaning method (Experiment A1-A3). All
cleaning
methods were conducted at room temperature, except the drying process in the
final
treatment step.
Conventional surfactant cleaning method
For the conventional surfactant cleaning method using surfactant aqueous
solution to wash
out the oil from the solid samples (A1-A3), according to TABLE 1, conventional
non-ionic
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
12
surfactant, which is insensitive to ions, has been used for this process.
Conventional anionic
surfactants were avoided to be used in treating these samples as precipitation
of surfactants
occurs in the presence of hard ions such as Ca' in the samples.
The oil content was reduced from 13 w/w% to 10 w/w% although the used
surfactant
concentration was very high (20 w/w%). No significant improvement of the
cleaning
efficiency of the surfactant solution to extract the oil from the solid was
observed when the
surfactant concentration was increased from 5 to 20 w/w%. This implies that
the surfactant
cleaning power has limitation, potentially because the surface tension (which
directly refers
to wettability and emulsification efficiency) of the surfactant solution
become constant after
its critical micelle concentration.
In addition, this conventional surfactant was not effective possibly because
of another two
reasons (1) oil/water interfacial tension induced by this surfactant might not
be sufficiently
low and (2) the wax or heavy oil strongly adsorb on the solid surface, as well
as, the heavy
oil molecularly blended with the trapped light oil helping light oil to be
trapped together
with heavy oil and difficult to be emulsified.
Invented treatment method and cleaning chemical compositions
The invented emulsified composition with and without sodium carbonate
(alkaline salt)
were mixed in water to produce treating solution to clean the solid Sample A
(drill cuttings)
with the original oil content of 13 w/w% (Experiment A4-A15).
The treatment without sodium carbonate was shown in experiment A4 and A5.
Experiment
A4 indicated that the 1 w/w% invented emulsified composition was not
sufficient to reduce
the oil content in the solid from 13 w/w% to < 1 w/w% even though the drying
process in
the final process was applied. Increasing concentration of the invented
emulsified
composition to 15 w/w% was showing the significantly increased oil removal
efficiency;
reducing oil content of the solid to < 1 w/w%. Improving cleaning efficiency
using sodium
carbonate was demonstrated in the next experiments.
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
13
Experiment A6 shows that if Sample A was treated with cleaning solution
consisting of 1
w/w% of the invented emulsified composition and 5 w/w% sodium carbonate and
subsequently washed with water without drying process in the last process
step, the oil
content in the solid after treatment was about 3.6 w/w% without drying process
in the final
process step, possibly because the emulsified oil and wax were still trapped
in the void
space between the solid particles, which was promoted by adsolubilization
phenomena (oil
solubilized by surfactant admicelles on solid surface). The oil removal
efficiency of this
process was slightly improved when the concentration of the invented
emulsified
composition was increased from 1 to 5 w/w% without drying process in the final
step.
However, with the drying process as the final cleaning process step, the oil
content was
reduced to 0.6 w/w% (< 1 w/w%). This efficiency is comparable with the
treatment with 15
w/w% invented emulsified composition without sodium carbonate in experiment
A5.
Experiment A9 indicated that increasing concentration of sodium carbonate
higher than 5
w/w% was not effective way to improve the cleaning efficiency of the whole
cleaning
process for this sample, since the surface charge density on the solid surface
might be
already reach the maximum and dispersion of the solid particles were
maximized. Sodium
carbonate is the key component for improving particle dispersion and
degreasing effect.
The different kinds of alkaline salts for the invented cleaning method were
tested in
experiment A10 for sodium tripolyphosphate and All for potassium hydroxide.
The results
showed that these phosphate and hydroxide forms of alkaline salts also worked
very well to
improve oily solid cleaning efficiency as good as the carbonate form. The oil
contents in the
solid after treatment were below 1 w/w% for both cases.
Impact of the co-solvent was investigated in the experiment Al2-15, which used
water
mixed with ethylene glycol, isopropanol or butoxyethanol as the mixed solvent
respectively, for the washing process step. The water/co-solvent ratio is 9/1
by volume. The
results shows that the co-solvent assists in reducing the oil content in the
solid after drying
process in final cleaning process step to < 0.5 w/w%, consistently.
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
14
However, using co-solvent without drying process in the final process step did
not provide
acceptable cleaning efficiency of the invented oily solid cleaning method as
observed the
oil content in the solid after final treatment > 2 w/w%. (target is < 1 w/w%).
TABLE 2: Efficiency of the invented oily solid treatment method and chemical
cleaning
compositions
Process V
Total oil content in Total oil
Experiment Thermal original oily
solid content in
No. Process I Process III treatment
sample solid after
Cleaning solution Cleaning solution
(Vaporization) (before treatment) treatment
Sample A
Al 5% Surfactant aqueous solution Water No 13%
11%
A2 20% Surfactant aqueous solution Water No
13% 10%
A3 20% Surfactant aqueous solution Water Yes (90 C)
13% 9%
A4 1% Invented emulsified composition Water Yes
13% 6%
A5 15% Imented emulsified composition Water Yes
13% 0.8%
1% Invented emulsified composition +
A6 Water No 13% 3.6%
5% sodium carbonate
5% Invented emulsified composition +
A7 Water No 13% 3%
5% sodium carbonate
1% Invented emulsified composition +
A8 Water Yes 13% 0.6%
5% sodium carbonate
1% Invented emulsified composition +
A9 Water Yes 13% 0.6%
10% sodium carbonate
1% Invented emulsified composition +
Al 0 Water Yes 13% 0.8%
10% sodium tripolyphosphate
1% Invented emulsified composition +
All Water Yes 13% 0.7%
10% pottasium hydroxide
1% Invented emulsified composition + Water/Ethylene glycol
Al2 Yes 13%
0.2%
10% sodium carbonate (9/1 v/v)
1% Invented emulsified composition + Water/lsopropanol
A13 Yes 13%
0.1%
10% sodium carbonate (9/1 v/v)
1% Invented emulsified composition + Water/Butoxyethanol
A14 Yes 13%
0.1%
10% sodium carbonate (9/1 v/v)
1% Invented emulsified composition + Water/Butoxyethanol
A15 No 13%
2.7%
10% sodium carbonate (9/1 v/v)
Note: % Concentration of cleaning solution is the % compared with the treated
sample.
EXAMPLE 3
The treatment of different kinds of oily solid samples was evaluated in this
example. There
are 4 kinds of samples shown in TABLE 1 which are drill cuttings (experiment
A8), sand
produced from oil production well (experiment B1), solid sediment from the
used oil-based
drilling fluid (experiment C1), and crude oil sludge solid sediment
(experiment D1, D2 &
D3). The cleaning solution in the first process step contains 1 w/w% of the
invented
emulsified composition and 5 w/w% sodium carbonate in water. Water was used as
the
washing agent in the washing step. All experiments used drying process as the
final
treatment step. It was indicated that the oil could be efficiently removed
from the solid for
CA 02932309 2016-05-31
WO 2015/093934
PCT/MY2014/050015
sample A, B and C (experiment A6, B1 and C1, respectively) effectively,
reducing the oil
content in the solid samples from 12-13 w/w% to < 1 w/w%.
However, the same cleaning solution composition used to treat these 3 samples
was not
5 very effective for sample D (experiment D1) since the oil content is
possibly too high. The
invented emulsified composition might not be sufficient to emulsify, disperse
and replace
the oil on the solid surface (in the sample). Once this issue occurs, sodium
carbonate would
not be able to change the surface charge of the solid surface in the sample
easily, since the
thick adsorbed oil layer on solid surface was blocking.
Another experiment using higher dosage of the invented emulsified composition
and
sodium carbonate was conducted in experiment D2 to improve the cleaning
efficiency.
However, the oil content in the solid after final process step was still
higher than 1 w/w%.
Improvement by using water/co-solvent (butoxyethanol) mixture for washing step
was
exhibited in experiment D3. The oil content in the solid after final treatment
could be
successfully reduced to < 1 w/w%.
TABLE 3: Effectiveness of the invented oily solid cleaning method on different
kinds of
oily solids
Total oil content in
Process V original oily
solid Total oil content in
Experiment Process I Process III Thermal treatment sample
solid after
No. Cleaning solution Cleaning solution (Vaporization)
(before treatment) treatment
1% Imented emulsified composition
A8 Water Yes 13% < 1%
5% sodium carbonate
1% Imented emulsified composition
B1Water Yes 12% < 1%
5% sodium carbonate
1% Imented emulsified composition
C1 Water Yes 13% < 1%
5% sodium carbonate
1% Imented emulsified composition
01 Water Yes 29% 9.0%
5% sodium carbonate
5% Imented emulsified composition
D2 Water Yes 29% 3. 3%
10% sodium carbonate
5% Imented emulsified composition VVater/Butoxyethanol
D3 Yes 29% <1%
10% sodium carbonate (9/1 v/v)
Note: % Concentration of cleaning solution is the % compared with the treated
sample.