Note: Descriptions are shown in the official language in which they were submitted.
CA 0295 2016--137
1
Device and method for the flexible use of electricity
The present invention relates to a device and a method for
flexible use of power, with which excess electrical energy
can be utilized for production of hydrogen.
The use of renewable energy sources, such as wind energy
and solar energy, is gaining ever-increasing significance
for the generation of electricity. Electrical energy is
typically supplied to a multitude of consumers over long-
ranging, supra-regional and transnationally coupled
electricity supply networks, referred to as electricity
networks for short. Since electrical energy cannot be
stored to a significant extent in the electricity network
itself, the electrical power fed into the electricity
network must be made to match the consumer-side power
demand, known as the load. As is known, the load fluctuates
time-dependently, in particular according to the time of
day, the day of the week or else the time of year. For a
stable and reliable electricity supply, a continuous
balance of electricity generation and electricity
consumption is necessary. Possibly occurring short-term
deviations are balanced out by what is known as positive or
negative control energy or control power. In the case of
regenerative electricity-generating devices, the difficulty
arises that, in the case of certain types, such as wind
energy and solar energy, the energy-generating capacity is
not available at all times and cannot be controlled in a
specific way, but is subject to time-of-day and weather-
dependent fluctuations, which only under some circumstances
are predictable and which generally do not coincide with
the energy demand at the particular time.
The difference between the generating capacity of
fluctuating renewable energy sources and the consumption at
a given time is usually covered by other power plants, such
as, for example, gas, coal and nuclear power plants. With
fluctuating renewable energy sources being increasingly
CA 0295 2016--137
2
extended and covering an increasing share of the
electricity supply, ever greater fluctuations between their
output and the consumption at the particular time must be
balanced out. Thus, even today, not only gas power plants
but increasingly also bituminous coal power plants are
being operated at part load or shut down entirely in order
to balance out the fluctuations. Since this variable
operation of the power plants is associated with
considerable additional costs, the development of
alternative measures has been investigated for some time.
As an alternative or in addition to varying the output of a
power plant in the case of an excess of electrical energy,
a known approach is to utilize excess electrical energy for
production of hydrogen by electrolytic cleavage of water.
This approach has the disadvantage that a separate device
for electrolytic cleavage of water has to be constructed,
which is operated only in the event of an excess of
electrical energy and remains unused for most of the time.
The production of chlorine by chlor-alkali electrolysis of
a sodium chloride solution is one of the industrial
processes with the highest power consumption. For chlor-
alkali electrolysis, plants with a relatively large number
of electrolysis cells operated in parallel are used in
industry. Co-products typically generated in addition to
chlorine are sodium hydroxide solution and hydrogen. In
order to reduce the power consumption of the chlor-alkali
electrolysis, alternative methods have been developed in
which there is no reduction of protons to molecular
hydrogen at the cathode of the electrolysis cell, but
instead reduction of molecular oxygen to water at an
oxygen-consuming electrode. The plants known from the prior
art for chlor-alkali electrolysis with oxygen-consuming
electrodes are not designed for generation of molecular
hydrogen.
CA 0295 2016--107
3
There have already been proposals, for flexible use of
power, to operate a chlor-alkali electrolysis in such a way
that a different number of electrolysis cells is operated
as a function of the power supply. This approach has the
disadvantage that the amount of chlorine produced varies
with the power supply and does not correspond to the
current demand for chlorine, and so a large buffer
reservoir for chlorine becomes necessary for such an
operation of a chlor-alkali electrolysis. However,
intermediate storage of large amounts of chlorine, a
hazardous substance, is undesirable for safety reasons.
It has been found that the disadvantages of the
abovementioned devices and methods can be avoided when, in
an electrolysis cell for chlor-alkali electrolysis, both an
oxygen-consuming electrode as cathode and a second cathode
for generation of hydrogen are arranged in the cathode
half-cell, and the cathode half-cell is equipped with a
conduit for purging of the gas space adjoining the oxygen-
consuming electrode, such that the electrolysis cell can be
operated, as a function of the power supply, either with
generation of hydrogen at the second cathode or with
reduction of oxygen at the oxygen-consuming electrode.
The invention provides a device for flexible use of power,
comprising an electrolysis cell for chlor-alkali
electrolysis having an anode half-cell, a cathode half-cell
and a cation exchange membrane that separates the anode
half-cell and the cathode half-cell from one another, an
anode arranged in the anode half-cell for evolution of
chlorine, an oxygen-consuming electrode arranged in the
cathode half-cell as the cathode, a catholyte space which
is formed between the cation exchange membrane and the
oxygen-consuming electrode, and through which electrolyte
flows, a gas space adjoining the oxygen-consuming electrode
at a surface facing away from the catholyte space, and a
conduit for supply of gaseous oxygen to this gas space,
CA 0295 2016--137
4
characterized in that a second cathode for generation of
hydrogen is arranged within the catholyte space and the
device has at least one conduit for purging of the gas
space with inert gas.
The invention also provides a method for flexible use of
power, in which, in an inventive device, chlorine is
produced by chlor-alkali electrolysis, wherein when power
supply is low, gaseous oxygen is supplied to the oxygen-
consuming electrode and oxygen is reduced at the oxygen-
consuming electrode at a first cell voltage, and when power
supply is high, no oxygen is supplied to the oxygen-
consuming electrode and hydrogen is generated at the second
cathode at a second cell voltage which is higher than the
first cell voltage.
The inventive device comprises an electrolysis cell for
chlor-alkali electrolysis having an anode half-cell, a
cathode half-cell and a cation exchange membrane that
separates the anode half-cell and the cathode half-cell
from one another. This inventive device may comprise a
plurality of such electrolysis cells, which may be
connected to form monopolar or bipolar electrolysers,
preference being given to monopolar electrolysers.
An anode for evolution of chlorine is arranged in the anode
half-cell of the inventive device. Anodes used may be any
of the anodes known from the prior art for chlor-alkali
electrolysis by the membrane method. Preference is given to
using dimensionally stable electrodes having a carrier of
metallic titanium and a coating with a mixed oxide composed
of titanium oxide and ruthenium oxide or iridium oxide.
The anode half-cell and cathode half-cell of the inventive
device are separated from one another by a cation exchange
membrane. Cation exchange membranes used may be any of the
cation exchange membranes known to be suitable for chlor-
alkali electrolysis by the membrane method. Suitable cation
CA 02932955 2016-06-07
exchange membranes are available under the NafionO,
AciplexTM and FlemionTM trade names from Du Pont, Asahi
Kasei and Asahi Glass.
An oxygen-consuming electrode is arranged in the cathode
5 half-cell of the inventive device such that the cathode
half-cell has a catholyte space, through which electrolyte
flows, between the cation exchange membrane and the oxygen-
consuming electrode, and a gas space, which can be supplied
with oxygen via a conduit for supply of gaseous oxygen,
adjoins the oxygen-consuming electrode at a surface facing
away from the catholyte space. The device also has at least
one conduit for purging of the gas space with an inert gas.
The gas space may be continuous over the entire height of
the cathode half-cell or may be divided into a plurality of
gas pockets arranged vertically one on top of another, in
which case the gas pockets each have orifices for pressure
equalization with the electrolyte space. Suitable
embodiments of such gas pockets are known to those skilled
in the art, for example from DE 44 44 114 Al. The conduit
for purging of the gas space with an inert gas may be
separate from the conduit for supply of gaseous oxygen to
the gas space, or it may be connected outside the cathode
half-cell to the conduit for supply of gaseous oxygen, such
that the conduit section between this connection and the
cathode half-cell can be purged with inert gas.
Oxygen-consuming electrodes used may be noble metal-
containing gas diffusion electrodes. Preference is given to
using silver-containing gas diffusion electrodes, more
preferably gas diffusion electrodes having a porous
hydrophobic gas diffusion layer containing metallic silver
and a hydrophobic polymer. The hydrophobic polymer is
preferably a fluorinated polymer, more preferably
polytetrafluoroethylene. More preferably, the gas diffusion
layer consists essentially of polytetrafluoroethylene-
sintered silver particles. The gas diffusion electrode may
CA 02932955 2016-06-07
6
additionally comprise a carrier structure in the form of a
mesh or grid, which is preferably electrically conductive
and more preferably consists of nickel. Particularly
suitable multilayer oxygen-consuming electrodes are known
from EP 2 397 578 A2. The multilayer oxygen-consuming
electrodes known from EP 2 397 578 A2 can be operated with
high pressure differentials and can therefore be used in a
cathode half-cell with a continuous gas space over the
entire height.
Furthermore, a second cathode for generation of hydrogen is
arranged in the cathode half-cell of the inventive device
in the catholyte space. In principle, any of the cathodes
known from the prior art for the generation of hydrogen in
a chlor-alkali electrolysis may be used as second cathode.
The second cathode used is preferably a cathode having a
noble metal-containing coating which preferably contains
platinum or ruthenium as the noble metal. Preferably, the
second cathode is configured in the form of a mesh or grid
and directly abuts the cation exchange membrane, such that
the electrolyte flows through the catholyte space
essentially between the second cathode and the oxygen-
consuming cathode.
The oxygen-consuming electrode and the second cathode are
preferably electrically insulated from one another in the
cathode half-cell and preferably have separate power
connections. This allows reliable prevention of formation
of hydrogen at the second cathode during the operation of
the device with reduction of oxygen at the oxygen-consuming
electrode.
The inventive device preferably comprises a conduit with
which inert gas can be withdrawn from the gas space of the
cathode half-cell, and at which there is arranged a sensor
which can be used to measure the content of oxygen in the
inert gas. The use of such a sensor makes it possible to
monitor, whether the gas space has been sufficiently purged
CA 0295 2016--137
7
with inert gas to avoid formation of an ignitable gas
mixture in the gas space, when changing from operation of
the device with reduction of oxygen at the oxygen-consuming
electrode to operation with formation of hydrogen at the
second cathode.
In a preferred embodiment, the inventive device
additionally comprises at least one conduit for purging the
catholyte space with inert gas. In this case, the device
may comprise a further conduit with which inert gas can be
withdrawn from the catholyte space, and this conduit may be
connected to a gas collector at the upper end of the
catholyte space or may be connected to a separating device
which is arranged outside the cathode half-cell and in
which gas is separated from electrolyte flowing out of the
cathode half-cell. More preferably, the device comprises a
conduit with which inert gas can be removed both from the
gas space and from the catholyte space of the cathode half-
cell, and at which there are arranged one or more sensors
with which the content of oxygen and hydrogen in the inert
gas can be measured.
The gas space adjoining the oxygen-consuming electrode, any
gas pockets present, any gas collector present and the
conduits connected to the cathode half-cell for supply and
withdrawal of gases are preferably configured such that
only low backmixing of gas occurs when purging the gas
space and optionally of the catholyte space with inert gas.
The gas space, any gas pockets present and any gas
collector present are therefore configured with minimum gas
volumes.
In a preferred embodiment, the inventive device comprises a
plurality of electrolysers arranged in parallel. Each of
the electrolysers then comprises a plurality of
electrolysis cells each having a gas space, and a common
conduit for supply of gaseous oxygen to the gas spaces of
the electrolysis cells of the electrolyser and a common
CA 0295 2016--107
8
conduit for purging of the gas spaces of the electrolyser
with inert gas. In addition, the device comprises separate
conduits for supply of oxygen to the electrolysers and
separate conduits for supply of inert gas to the
electrolysers. Such a configuration of the device enables,
with a low level of apparatus complexity, operation of the
device with variability of the proportion of electrolysis
cells in which hydrogen is generated.
The inventive device may additionally have a buffer
reservoir for chlorine generated in the anode half-cell,
which can store an amount of chlorine which can compensate
for the interruption in the generation of chlorine in the
anode half-cell on purging of the cathode half-cell with
inert gas.
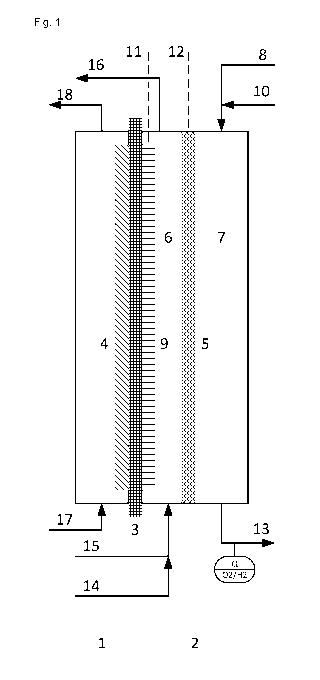
Fig. 1 shows a preferred embodiment of the inventive device
with an electrolysis cell in which the second cathode abuts
the cation exchange membrane. The electrolysis cell
comprises an anode half-cell (1), a cathode half-cell (2)
and a cation exchange membrane (3) that separates the two
half-cells. An anode (4) for evolution of chlorine arranged
in the anode half-cell abuts the cation exchange membrane.
An oxygen-consuming electrode (5) arranged in the cathode
half-cell as the cathode divides the cathode half-cell into
a catholyte space (6), formed between the cation exchange
membrane and the oxygen-consuming electrode, and a gas
space (7). The gas space can be supplied with gaseous
oxygen via a conduit (8). The gas space can be purged with
inert gas via a conduit (10). Inert gas can be withdrawn
from the gas space via a conduit (13), and a sensor is
arranged at the conduit (13) with which the content of
oxygen and hydrogen in the inert gas can be measured. A
second cathode (9) for generation of hydrogen, which abuts
the cation exchange membrane, is arranged in the catholyte
space (6). The oxygen-consuming electrode (5) and the
second cathode (9) have separate power connections (11,
CA 0295 2016--137
9
12). The catholyte space (6) is supplied with a sodium
hydroxide solution via a conduit (15) and an enriched
sodium hydroxide solution is withdrawn via a conduit (16),
optionally together with hydrogen formed, such that the
electrolyte flows through the catholyte space. The
catholyte space can be purged with inert gas via a conduit
(14). The anode half-cell (1) is supplied with a sodium
chloride solution via a conduit (17), and a depleted sodium
chloride solution is withdrawn together with chlorine via a
conduit (18).
In the inventive method for flexible use of power, chlorine
is produced by chlor-alkali electrolysis in a device
according to the invention and at least one electrolysis
cell in the device is operated with different cell voltages
as a function of the power supply. When power supply is
low, the oxygen-consuming electrode of the electrolysis
cell is supplied with gaseous oxygen, and oxygen is reduced
at the oxygen-consuming electrode at a first cell voltage.
When power supply is high, the oxygen-consuming electrode
is not supplied with oxygen, and hydrogen is generated at
the second cathode at a second cell voltage which is higher
than the first cell voltage.
Preferably, in the inventive method, the preferred
embodiment of the device in which the oxygen-consuming
electrode and the second cathode have separate power
connections is used, and durimg operation with the first
cell voltage the cell voltage is applied only to the
oxygen-consuming electrode, and during operation with the
second cell voltage the cell voltage is applied only to the
second cathode.
A high power supply may result from a power surplus, and a
low power supply may result from a power deficit. A power
surplus arises when at some point more power from renewable
energy sources is being provided than the total amount of
power being consumed at this time. A power surplus also
CA 0295 2016--137
arises when large amounts of electrical energy are being
provided from fluctuating renewable energy sources, and the
throttling or shutdown of power plants is associated with
high costs. A power deficit arises when comparatively small
5 amounts of renewable energy sources are available and
inefficient power plants, or power plants associated with
high costs, have to be operated. A power surplus may also
exist when the operator of a power generator, for example
of a windfarm, is producing more power than has been
10 predicted and sold. Analogously, a power deficit may exist
when less power is being produced than predicted. The
distinction between a high power supply and a low power
supply can alternatively also be made on the basis of a
price at a power exchange, in which case a low power price
corresponds to a high power supply and a high power price
to a low power supply. In this case, for the distinction
between a high power supply and a low power supply, it is
possible to use a fixed or a time-variable threshold value
for the power price at a power exchange.
In a preferred embodiment, a threshold value for a power
supply is defined for the inventive method. In that case,
the current power supply is determined at regular or
irregular intervals and the electrolysis cell is operated
with the first cell voltage with supply of gaseous oxygen
to the oxygen-consuming electrode when the power supply is
below the threshold value, and with the second cell voltage
without supply of oxygen to the oxygen-consuming electrode
when the power supply is above the threshold value. The
threshold value for the power supply and the current power
supply can, as described above, be defined or ascertained
on the basis of the difference between power generation and
power consumption, on the basis of the current output of a
power generator, or on the basis of the power price at a
power exchange.
CA 0295 2016--137
11
By changing between two modes of operation with different
cell voltage, it is possible in the inventive method to
match the power consumption of the chlor-alkali
electrolysis flexibly to the power supply, without any need
for alteration of the production output of chlorine and for
intermediate storage of chlorine for that purpose. The
electrical energy consumed additionally as a result of the
higher second cell voltage is used for generation of
hydrogen and enables storage of surplus power in the form
of chemical energy without the construction and operation
of additional installations for power storage. This way,
more hydrogen is generated per additional kWh consumed than
in the case of hydrogen generation by water electrolysis.
Through the use of two different cathodes for the two modes
of operation, which can be optimized to the respective mode
of operation, it is possible in both modes of operation to
work with low overpotentials and to minimize power
consumption in the two modes of operation.
Suitable values for the first cell voltage for reduction of
oxygen at the oxygen-consuming electrode and for the second
cell voltage for production of hydrogen at the second
cathode depend on the design of the oxygen-consuming
electrode used and of the second cathode, and on the
current density envisaged for the chlor-alkali
electrolysis, and can be ascertained in a known manner by
the measurement of current-voltage curves for the two modes
of operation.
The gaseous oxygen can be supplied in the form of
essentially pure oxygen or in the form of oxygen-rich gas,
in which case the oxygen-rich gas contains preferably more
than 50% by volume of oxygen and more preferably more than
80% by volume of oxygen. Preferably, the oxygen-rich gas
consists essentially of oxygen and nitrogen, and may
optionally additionally contain argon. A suitable oxygen-
rich gas can be obtained from air by known methods, for
CA 0295 2016--137
12
example by pressure swing adsorption or a membrane
separation.
Preferably, when changing from hydrogen generation at the
second cell voltage to oxygen reduction at the first cell
voltage, the cell voltage is reduced until essentially no
more current flows, and the gas space is purged with an
inert gas, before gaseous oxygen is supplied to the oxygen-
consuming electrode. Analogously and preferably, when
changing from oxygen reduction at the first cell voltage to
hydrogen generation at the second cell voltage, the cell
voltage is reduced until essentially no more current flows,
and the gas space is purged with an inert gas, before
hydrogen is generated at the second cathode. Suitable inert
gases are all gases which do not form ignitable mixtures
either with oxygen or with hydrogen and which do not react
with aqueous sodium hydroxide solution. The inert gas used
is preferably nitrogen. Preferably, purging with inert gas
and maintenance of a reduced cell voltage is continued
until the content of hydrogen or oxygen in the gas which
leaves the cathode half-cell because of the purging falls
below a defined limit. The limit for hydrogen is preferably
selected such that the mixing of the hydrogen containing
gas with pure oxygen cannot give a flammable mixture, and
the limit for oxygen is preferably selected such that
mixing of the oxygen containing gas with pure hydrogen
cannot give a flammable mixture. Suitable limits can be
taken from known diagrams for the flammability of gas
mixtures, or be ascertained by methods known to those
skilled in the art for determining flammability. The
reduction in the cell voltage and the purging with inert
gas can reliably avoid the formation of flammable gas
mixtures when changing between the two modes of operation
of the inventive method.
When changing from hydrogen generation at the second cell
voltage to oxygen reduction at the first cell voltage, the
CA 0295 2016--137
13
purging with inert gas is preferably additionally followed
by purging with an oxygen containing gas, in order to avoid
mass transfer inhibition in the reduction of oxygen as a
result of a high content of inert gas in the gas diffusion
layer of the oxygen-consuming electrode.
Preferably, a prediction of the expected power supply is
made for the method of the invention, a minimum duration
for operation with the first and with the second cell
voltage is set, and a swichover between operation with the
first cell voltage with supply of gaseous oxygen to
operation with the second cell voltage without supply of
oxygen is performed only when the predicted duration of a
low or high power supply is longer than the minimum
duration set. Through such a mode of operation, it is
possible to avoid losses of production capacity for
chlorine as a result of too many changes of the cell
voltage and associated interruptions in chlorine production
during purging with inert gas.
In a preferred embodiment of the inventive method, after
changing from oxygen reduction at the first cell voltage to
hydrogen generation at the second cell voltage, a gas
mixture comprising hydrogen and inert gas is withdrawn from
the cathode half-cell and hydrogen is separated from this
gas mixture, preferably through a membrane. With such a
separation, essentially all the hydrogen generated can be
obtained in high purity and with constant quality.
Preferably, the method of the invention is performed in a
device having a plurality of electrolysis cells according
to the invention, and the proportion of electrolysis cells
to which no oxygen is supplied and in which hydrogen is
generated at the second cathode is altered as a function of
the power supply. More preferably, for this purpose, the
device described above with a plurality of electrolysers
arranged in parallel is used, and the proportion of the
electrolysers to which no oxygen is supplied and in which
CA 02932955 2016-06-07
14
hydrogen is generated at the second cathode is altered as a
function of the power supply. This allows for adjusting the
power consumption of the chlor-alkali electrolysis within a
wide range with essentially constant chlorine production.
In this embodiment, the inventive method can be used,
without any adverse effects on chlorine production, for
providing negative control energy for the operation of a
power distribution grid.