Note: Descriptions are shown in the official language in which they were submitted.
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
APPARATUS AND METHODS FOR
NONINVASIVE MONITORING OF CANCEROUS CELLS
I. Field Of The Invention
[0001] This application relates to apparatus and methods for noninvasive
monitoring of
cancerous cells in intracorporeal fluid accumulations.
II. Background Of The Invention
[0002] Certain cancers can result in pathologic chronic collection of
bodily fluids within
cavities such as the peritoneum, pleura, or pericardial sac. Such cancers may
cause chronic
ascites, pleural effusion, or pericardial effusion, where chronic fluid
collections persist and
result in increased morbidity and mortality.
[0003] In pleural effusion, excess fluid arising from an underlying
pathology, such as
lung cancer, breast cancer, melanoma, leukemia, or lymphoma, accumulates in
the pleural
cavity. If left untreated, the fluid accumulation may interfere with proper
lung function,
significantly increasing morbidity and mortality.
[0004] In pericardial effusion, fluid accumulates in the pericardial sac
and may lead to
increased intrapercardial pressure and reduced cardiac output. The excess
fluid often results
from an underlying cancer, such as cancer that has metastasized to the
pericardium, lung
cancer, breast cancer, melanoma, leukemia, or lymphoma.
[0005] Ascites is a highly debilitating complication associated with many
medical
conditions including liver failure, congestive heart failure, and certain
cancers such as ovarian
cancer, breast cancer, pancreatic cancer, uterine cancer, cancer of the bowels
including colon
cancer, melanoma, leukemia, or lymphoma. Untreated ascites can result in
respiratory
compromise, compression of the inferior vena cava (a vital blood vessel) and
spontaneous
bacterial peritonitis (a life-threatening condition).
[0006] Treatment of cancer typically includes chemotherapy, radiation
therapy, or
medication infused in the area of the cancer. However, an effusion also may be
caused by
cancer treatment, especially chemotherapy or radiation therapy.
- 1 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0007] A patient with cancer and ascites or an effusion, often caused by
the underlying
cancer or cancer treatment, typically requires contemporaneous treatment for
both the cancer
and the ascites/effusion. Conventional treatment for ascites, plural effusion,
or pericardial
effusion involves one of three methods: 1) external drainage, which poses a
risk of infection
and long-term requirement for multiple punctures, 2) drainage to another body
cavity, or 3)
treatment with drugs. Such methods suffer from a variety of drawbacks
including high cost,
continuous visits to a physician, ineffectiveness, and risk of serious
complications.
[0008] During treatment of cancer, it is imperative that a physician
monitor the progress
of cancer cells. Using conventional methods, an invasive biopsy of cancerous
tissue
generally is required to analyze the cancer cells. A biopsy often must be sent
to a remote
laboratory for the analysis, delaying the physician's ability to efficiently
and effectively treat
the cancer.
[0009] In view of the above-noted drawbacks of previously-known systems, it
would be
desirable to provide methods and apparatus for noninvasive monitoring of
cancerous cells
while treating intracorporeal fluid accumulation caused by ascites, pleural
effusion, or
pericardial effusion.
III. Summary Of The Invention
[0010] The present invention overcomes the drawbacks of previously-known
systems for
monitoring cancerous cells by providing a fluid management system that
automatically and
autonomously moves fluid accumulations containing cancerous cells to the
bladder with little
patient involvement. Cancerous cells expelled via urination then are analyzed
to assess
progress of cancer treatment.
[0011] The fluid management system for expulsion of cancerous cells to
permit
noninvasive monitoring of the cancerous cells preferably includes an
implantable pump
having a first controller; an inflow catheter having an inlet end adapted to
be positioned
within a body cavity and an outlet end configured to be coupled to the
implantable pump,
the body cavity having an accumulation of fluid comprising cancerous cells;
and an outflow
catheter having an inlet end configured to be coupled to the implantable pump
and an outlet
end adapted to be positioned in a bladder. The first controller preferably is
programmed to
selectively activate the implantable pump to move the fluid comprising
cancerous cells from
the inlet end of the inflow catheter to the bladder during predetermined time
periods.
- 2 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0012] The inlet end of the inflow catheter may be adapted to be positioned
within the
peritoneal cavity, the pleural cavity, or the pericardial cavity to treat
conditions, such as
ascites, pleural effusion, or pericardial effusion, arising from cancer or
cancer treatment.
[0013] In accordance with one aspect of the present invention, a system for
noninvasive
monitoring of cancerous cells is provided, including a fluid management system
configured
to pump fluid containing cancerous cells from a body cavity to the bladder and
an analysis
station configured to analyze the cancerous cells excreted during urination.
Preferably, the
fluid management system includes an implantable pump having a first
controller; an inflow
catheter having an inlet end adapted to be positioned within a body cavity and
an outlet end
configured to be coupled to the implantable pump; and an outflow catheter
having an inlet
end configured to be coupled to the implantable pump and an outlet end adapted
to be
positioned in a bladder. The first controller is programmed to selectively
activate the
implantable pump to move fluid that includes suspended cancerous cells from
the inlet end
of the inflow catheter to the bladder. The analysis station may be any
conventional system
for analyzing cells to the presence or quantity of cancerous cells, and may be
located
remotely from the fluid management system or it may be located in close
proximity. In one
embodiment, the analysis station is configured to analyze fluid including
cancerous cells
excreted from the bladder during urination to determine a parameter indicative
of the
progress of the cancer or an efficacy of a program to treat to the cancer.
[0014] The fluid management system may include an implantable device (also
referred to
as an implantable pump), a controller, a battery and a transceiver; a charging
and
communication system configured to periodically charge the battery of, and
communicate
with, the implantable device; and monitoring and control software, suitable
for use with a
conventional personal computer, for configuring and controlling operation of
the implantable
device and charging and communication system. Preferably, the monitoring and
control
software is available only to the treating physician, such that the patient
generally interacts
with the implantable device only via the charging and communication system for
purposes of
recharging the implantable device. In accordance with one aspect of the
present invention,
the implantable device is configured to pump fluid in small increments, at
relatively high
flow rates, during predetermined times of the day to achieve a target volume,
and further is
configured to periodically alter the pump position to reduce the risk of
clogging of the
implantable device during non-pumping intervals. The pump also may be
programmed to
- 3 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
perform a rapid sequence of backward and forward movements if a blockage is
detected,
thereby clearing the blockage. Additionally, the fluid management system may
include one
or more sensors configured to detect indicia of the onset of infection, e.g.,
an increase in
temperature, respiratory rate, or the viscosity of ascitic fluid, and one or
more alarms
configured to indicate to the physician a prediction or detection of infection
based on the
output(s) of those sensors.
[0015] In one preferred embodiment, the implantable device includes an
electrically-
driven mechanical gear pump configured for subcutaneous implantation. The pump
has an
inlet port coupled to an inflow catheter and an outlet port coupled to a
bladder catheter. In
accordance with one aspect of the present invention, the pump employs a pair
of floating
gears that function as a positive displacement pump, wherein a driving gear is
coupled to a
splined shaft of an electric motor to minimize power consumption arising due
to
manufacturing variations or shaft eccentricity. The inflow catheter comprises
a tube having a
first end configured to be coupled to the pump inlet and a second end
configured to be
positioned in a selected cavity, e.g., peritoneum, pleura or pericardial sac.
The second end of
the inflow catheter includes a plurality of through-wall apertures that permit
fluid
accumulating to pass into the catheter. The bladder or outflow catheter
comprises a tube
having a first end configured to be coupled to the pump and a second end
configured to be
inserted through the wall of, and fixed within, a patient's bladder. The fluid
circuit further
includes sensors arranged to monitor ambient pressure, pressure at the pump
inlet, pressure at
the pump outlet, pressure in the bladder, and optionally the temperature of
the ascitic fluid
and the respiratory rate of the patient. The inflow and outflow catheters
include connectors
configured to reduce the risk of improper implantation.
[0016] The implantable device further comprises a controller, packaged
together with the
pump, electric motor, battery, charging coil, and radio transceiver within a
low volume sealed
housing. The controller is coupled to the pump motor, battery, transceiver and
a plurality of
sensors to continually monitor pressure, temperature, humidity, charge status,
pump status,
patient movement and other environmental and system related parameters. The
controller
preferably comprises a processor, nonvolatile memory for storing firmware,
implant
identification information, and system and environmental data, and volatile
memory that
serves as a buffer for computations and instructions during execution and
firmware updating.
The pump motor is configured for extended use and low power consumption, and
preferably
- 4 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
includes Hall effect sensors for position sensing and to determine the
direction of rotation
(and correspondingly, flow and fluid viscosity). The battery preferably is a
long-lasting
lithium-ion or lithium polymer battery that is coupled to an inductive
charging circuit,
thereby enabling the battery to be recharged using the external charging and
communication
system. A radio frequency transceiver preferably is employed in the device for
transmitting
system information to, and receiving information from, the external charging
and
communication system, including system performance data, commands, and
firmware
upgrades. All of the foregoing components preferably are disposed within the
housing,
which further includes a filler having a low permeability for water, thereby
reducing
infiltration of moisture into the housing.
[0017] In accordance with one aspect of the present invention, the fluid
management
system includes an external charging and communication system. In a preferred
embodiment, the charging and communication system comprises a housing
containing a
controller, radio transceiver, inductive charging circuit, power source and
quality-of-charging
indicator. The controller is coupled to the inductive charging circuit, power
source, quality-
of-charging indicator, radio transceiver, and memory for storing information
to be transmitted
between the monitoring and control software and implantable device. The
charging and
communication system preferably includes a data port, such as a USB port, or a
wireless port,
such as Bluetooth, Zigbee or GPRS, that permits the charging and communication
system to
be coupled to a conventional computer, such as a personal computer or laptop
computer,
configured to run the monitoring and control software. In one embodiment, the
charging and
communication system may include a cord that enables the system to be directly
coupled to a
conventional power supply, such as 120V AC wall socket. More preferably,
however, the
charging and communication system includes a battery-powered handpiece that
periodically
may be coupled to an AC powered charging base, so that the handpiece may be
separated
from the base to recharge the implantable device without tethering the patient
with a power
cord. In one preferred embodiment, the control circuitry of the charging and
communication
system may be configured to boost power supplied through the inductive
charging circuit to
the motor of the implantable device to unblock potential clogging of the gear
pump.
[0018] The fluid management system further comprises monitoring and control
software
that preferably is accessible only to the patient's physician. The software is
configured to run
on a conventional personal computer or laptop computer, and enables the
physician to
- 5 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
configure and monitor operation of the charging and communication system and
implantable
device. The software may include routines for controlling any of a number of
parameters
associated with the pump operation, such as a target amount of fluid to move
daily or per
motor actuation, and limits for inflow catheter pressure, bladder pressure,
pump pressure, and
implant temperature. The software also may be configured to control operation
of the
implantable device so as not to move fluid during specific periods (e.g., at
night) or to defer
pump actuation if the patient is asleep. The software further may be
configured, for example,
to send immediate commands to the implantable device to start or stop the
pump, or to
operate the pump in reverse or at high power to unblock the pump or its
associated catheters,
such as when the patient is visiting his or her physician. The software may be
configured to
download data collected from the implantable device and stored on the charging
and
communication system, such as during a patient visit to the physician's
office. Optionally,
based on the downloaded information, such as the patient's respiratory rate,
temperature, and
fluid viscosity, the software may be configured to alert the physician of a
prediction or
detection of infection.
[0019] It is contemplated that the system of the present invention may
avoid difficulties
typically associated with the previously-known apparatus and methods for
addressing ascites.
It is expected, for instance, that the system and methods of the present
invention will enable
small quantities of peritoneal fluid to be moved to the bladder without the
inconvenience and
complications generally associated with use of pharmaceuticals or
paracenteses. In
particular, because the apparatus and methods of the present invention avoid
repeated,
periodic removal of large quantities of fluid, as occurs with paracenteses,
the tendency to
generate additional ascites to offset the removed fluid will be reduced. These
effects in turn
are expected to obviate the need to infuse plasma expanders, such as human
albumin, into the
patient following paracentesis, thereby resulting in significant cost savings
to the patient and
health care system. The prediction or detection of infection, particularly at
an early stage of
infection, further may improve patient outcomes and reduce the need for more
expensive
treatments. Finally, the apparatus and methods of the present invention are
expected to
provide improved quality of life for chronic ascites patients, allowing such
patients to pursue
less sedentary lifestyles than would otherwise be possible, and encouraging
better compliance
with medically-directed dietary and exercise regimes.
- 6 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0020] In an alternative embodiment, a fluid management system is provided
generally as
described above, but instead configured for treating pleural or pericardial
effusion arising
from cancer or cancer treatment. Few surgical options are available for
treating these
conditions, and most of those present significant risks for morbidity and
morality. In
particular, the system of the present invention may be configured for treating
pleural or
pericardial effusion, and comprises an implantable device, a charging and
communication
system and software substantially as described above. This embodiment differs
from the
ascites fluid management system of the present invention primarily in that the
pump has an
inflow catheter coupled to a pleural or pericardial cavity and the controller
is configured to
work under negative pressures. More particularly, the inflow catheter has a
first end
configured to be coupled to the pump inlet and a second end configured to be
positioned in
the pleural or pericardial cavity, and includes a plurality of through-wall
apertures that permit
fluid accumulating in the cavity to pass into the catheter without interfering
with proper
functioning of the lungs or heart. As some fluid is required to lubricate
movement of the
organ within these cavities, the implantable device preferably is programmed
not to pump all
of the fluid from the cavity. In addition, the implantable device is
programmed to interpret
and provide drainage that accounts for pressure fluctuations arising in the
cavity during
normal respiration or cardiac activity.
[0021] Methods of implanting and operating the fluid management system of
the present
invention also are provided. The implantable device preferably may be placed
subcutaneously using interventional radiologic techniques including
radiographic imaging or
ultrasound, while the inflow catheter and outflow catheter may be placed using
surgical, or
more preferably, minimally invasive procedures. The inflow catheter, in one
variation, may
be tunneled subcutaneously to the site of drainage and the outflow tubing can
be
subcutaneously channeled to the bladder (or peritoneal cavity). The
implantable device
preferably is programmed using radio frequency coupling of the transceivers in
the
implantable device and charging and communication system, while power is
supplied to the
battery of the implantable device by coupling the inductive charging circuits
of the
implantable device and charging and communication system. Additional details
of methods
of implanting and operating a system in accordance with the present invention
are described
below.
- 7 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0022] In accordance with one aspect of the present invention, a method for
noninvasive
monitoring of cancerous cells is provided. The method includes pumping, via an
implantable
pump, bodily fluid comprising cancerous cells from a body cavity having an
accumulation of
the bodily fluid to the bladder for excretion; and collecting cancerous cells
excreted from the
bladder for analysis. Such preparation may include, for example, receiving a
urination
sample, transferring a urination sample to a physician, transferring a
urination sample to a
local or remote analysis station. The method further may include analyzing the
cancerous
cells to determine a parameter indicative of progress of the cancer or
efficacy of a cancer
treatment program, for example, using the analysis station, to detect the
presence or quantity
of cancerous cells in the patient's urine.
IV. Brief Description Of The Drawings
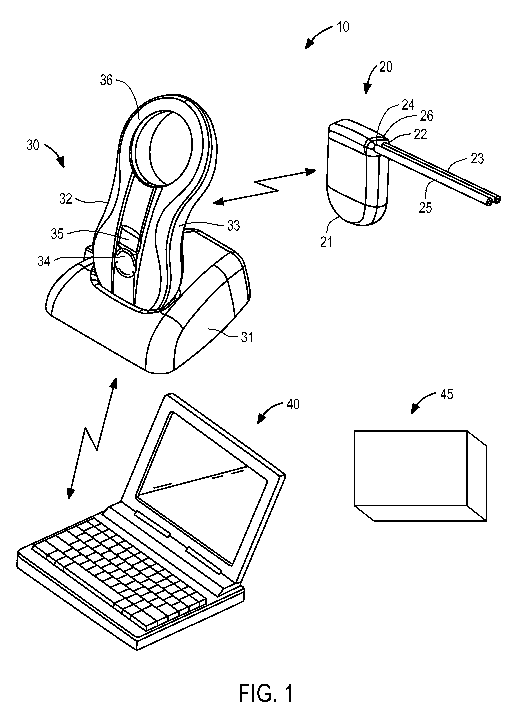
[0023] FIG. 1 is a perspective view of the components of an exemplary fluid
management
system and an exemplary analysis station constructed in accordance with the
principles of the
present invention.
[0024] FIGS. 2A and 2B are, respectively, side view and perspective
detailed views of an
exemplary embodiment of an inflow catheter suitable for use with the system of
the present
invention, in which FIG. 2B corresponds to detail region 2B of FIG. 2A.
[0025] FIGS. 3A and 3B are, respectively, side and perspective views,
respectively, of
first and second embodiments of bladder catheters suitable for use with the
ascites treatment
system of the present invention.
[0026] FIG. 4 is a schematic diagram of the electronic components of an
exemplary
embodiment of the implantable device of the present invention.
[0027] FIGS. 5A and 5B are, respectively, a perspective view of the
implantable device
of the present invention with the housing shown in outline and a perspective
view of the
obverse side of the implantable device with the housing and low water
permeable filler
removed.
[0028] FIGS. 6A, 6B, 6C and 6D are, respectively, an exploded perspective
view of the
drive assembly of the implantable device; front and plan views of the upper
housing; and a
perspective view of the manifold of an exemplary embodiment of the implantable
device.
- 8 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0029] FIGS. 7A and 7B are, respectively, a plan view of the gear pump
housing of the
implantable device of FIG. 5A, and a plan view of a model of the gear pump
constructed in
accordance with the principles of the present invention.
[0030] FIGS. 8A and 8B are, respectively, perspective and top views of the
handpiece
portion of an exemplary charging and communication system of the present
invention;
[0031] FIG. 9 is a schematic diagram of the electronic components of an
exemplary
embodiment of the charging and communication system of the present invention.
[0032] FIG. 10 is a schematic diagram of the functional components of the
monitoring
and control software employed in an exemplary embodiment of the fluid
management system
of the present invention.
[0033] FIGS. 11-15 are exemplary screenshots illustrating various aspects
of the user
interface of the monitoring and control system of the present invention.
V. Detailed Description Of The Invention
[0034] The fluid management system of the present invention comprises
devices for
facilitating removal of fluid from a body region, such as the peritoneum,
pleural cavity or
pericardial sac, where drainage is desired. The devices disclosed herein may
be utilized for
drainage of chronic excess fluid accumulation from one body cavity to a second
body cavity,
preferably the urinary bladder. Such excess fluid may accumulate due to an
underlying
condition, such as cancer. In accordance with the principles of the present
invention, the
fluid management system may be optimized for use in treating chronic ascites
and pleural or
pericardial effusion in patients with cancer, while simultaneously permitting
noninvasive
monitoring of cancerous cells. After the fluid management system moves excess
fluid
containing cancerous cells from the first body cavity to the bladder and the
fluid is excreted
during urination, the cancerous cells may be analyzed to, for example, assess
the progress of
a cancer treatment or the cancer itself
System Overview
[0035] Referring to FIG. 1, an overview of fluid management system 10 of
the present
invention and analysis station 45 of the present invention are provided. In
FIG. 1,
components of the system are not depicted to scale on either a relative or
absolute basis.
- 9 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
Fluid management system 10 comprises implantable device 20, external charging
and
communication system 30, and a software-based monitoring and control system
40. In the
illustrated embodiment, monitoring and control system 40 is installed and run
on a
conventional laptop computer used by the patient's physician. During patient
visits, charging
and communication system 30 may be coupled, either wirelessly or using a
cable, to
monitoring and control system 40 to download for review data stored on
implantable device
20, or to adjust the operational parameters of the implantable device.
Monitoring and control
system 40 also may be configured to upload and store date retrieved from
charging and
communication system 30 to a remote server for later access by the physician
or charging and
communications system 30.
[0036] Implantable device 20 comprises an electromechanical pump having
housing 21
configured for subcutaneous implantation. As described in further detail
below, in an
embodiment suitable for treating ascites and evacuating cancerous cells to
permit noninvasive
monitoring of such cells, implantable device 20 includes an electrically-
driven mechanical
gear pump having inlet port 22 coupled to peritoneal catheter 23 and outlet
port 24 coupled to
bladder catheter 25. Peritoneal catheter 23 comprises a tube having a first
end configured to
be coupled to pump inlet 23 and a second end configured to be positioned in
the peritoneal
cavity. In a patient having ascites arising from cancer, the body generates an
accumulation of
fluid comprising cancerous cells in the peritoneal cavity. Bladder catheter 25
comprises a
tube having a first end configured to be coupled to pump outlet 24 and a
second end
configured to be inserted through the wall of, and fixed within, a patient's
bladder. In a
preferred embodiment, both catheters are made of medical-grade silicone and
include
polyester cuffs at their distal ends (not shown) to maintain the catheters in
position.
Peritoneal catheter 23 and bladder catheter 25 are coupled to pump housing 21
using
connector 26 configured to reduce the risk of improper installation and
inadvertent
disconnection, and may in addition include distinct cross-sections that reduce
the risk of
improper installation.
[0037] Implantable device 20 preferably is configured to move fluid in
short (e.g., 10
second) intervals (e.g., every 10-20 minutes). Such short but frequent
intervals are expected
to overcome the clogging issues common to previously-known ascites shunts, by
preventing
the accumulation of material on the interior lumens of catheters 23 and 25,
and reducing the
risk for tissue ingrowth. For ascites treatment, the fluid circuit of
implantable device 20
- 10 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
preferably is configured to provide an average flow rate of about 60 ml/hour,
although much
higher and lower flow rates are possible if needed. As described in detail
below, the pumping
time and volume, including maximum and minimum limits for daily pumped volume,
may be
programmed by the physician using monitoring and control system 40 as required
for a
specific patient. As further described below, the fluid circuit of implantable
device 20
includes pressure sensors that monitor pressure in both the peritoneal cavity
and the bladder,
such that pumping of fluid into the bladder is disabled until the bladder is
determined to have
sufficient space to accommodate additional fluid. For patient comfort,
implantable device 10
normally is programmed not to pump at night or when an accelerometer included
in the
implantable device indicates that the patient is asleep (and thus unlikely to
be able to void the
bladder). Implantable device 20 preferably includes multiple separate fail-
safe mechanisms,
to ensure that urine cannot pass from the bladder to the peritoneal cavity
through the pump,
thereby reducing the risk of transmitting infection.
[0038] Still referring to FIG. 1, external charging and communication
system 30 in a
preferred form comprises base 31 and handpiece 32. In this embodiment,
handpiece 32
contains a controller, a radio transceiver, an inductive charging circuit, a
battery, a quality-of-
charging indicator and a display, and is removably coupled to base 31 to
recharge its battery.
Base 31 may contain a transformer and circuitry for converting conventional
120V power
service to a suitable DC current to charge handpiece 32 when coupled to base
31. In
alternative embodiments, handpiece 32 may include such circuitry and a
detachable power
cord, thereby permitting the handpiece to be directly plugged into a
convention 120V wall
socket to charge the battery. In a preferred embodiment, each of implantable
device 20 and
handpiece 32 includes a device identifier stored in memory, such that
handpiece 32 provided
to the patient is coded to operate only with that patient's specific
implantable device 20.
[0039] Handpiece 32 preferably includes housing 33 having multi-function
button 34,
display 35, a plurality of light emitting diodes (LEDs, not shown) and
inductive coil portion
36. Multi-function button 34 provides the patient the ability to issue a
limited number of
commands to implantable device 20, while display 35 provides visible
confirmation that a
desired command has been input; it also displays battery status. Inductive
coil portion 36
houses an inductive coil that is used to transfer energy from handpiece 32 to
recharge the
battery of implantable device 20. The LEDs, which are visible through the
material of
housing 33 when lit, may be arranged in three rows of two LEDs each, and are
coupled to the
-11-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
control circuitry and inductive charging circuit contained within handpiece
32. As described
in further detail below, the LEDs may be arranged to light up to reflect the
degree of
inductive coupling achieved between handpiece 32 and implantable device 20
during
recharging of the latter. Alternatively, the LEDs may be omitted and an analog
display
provided on display 35 indicating the quality of inductive coupling.
[0040] As further described in detail below, the control circuitry
contained within
handpiece 32 is coupled to the inductive charging circuit, battery, LEDs and
radio
transceiver, and includes memory for storing information from implantable
device 20.
Handpiece 32 also preferably includes a data port, such as a USB port, that
permits the
handpiece to be coupled to monitoring and control system 40 during visits by
the patient to
the physician's office. Alternatively, handpiece 32 may include a wireless
chip, e.g.,
conforming to the Bluetooth or IEEE 802.11 wireless standards, thereby
enabling the
handpiece to communicate wirelessly with monitoring and control system 40.
[0041] Monitoring and control system 40 is intended primarily for use by
the physician
and comprises software configured to run on a conventional laptop computer.
The software
enables the physician to configure, monitor and control operation of charging
and
communication system 30 and implantable device 20. As described in detail
below, the
software may include routines for configuring and controlling pump operation,
such as a
target amount of fluid to move daily or per motor actuation, intervals between
pump
actuation, and limits on peritoneal cavity pressure, bladder pressure, pump
pressure, and
battery temperature. System 40 also may provide instructions to implantable
device 20 via
charging and control system 30 to control operation of implantable device 20
so as not to
move fluid during specific periods (e.g., at night) or to defer pump actuation
if the patient is
asleep. System 40 further may be configured, for example, to send immediate
commands to
the implantable device to start or stop the pump, or to operate the pump in
reverse or at high
power to unblock the pump or associated catheters. The software of system 40
also may be
configured to download real-time data relating to pump operation, as well as
event logs
stored during operation of implantable device 20. Based on the downloaded
data, e.g., based
on measurements made of the patient's temperature, respiratory rate, and/or
fluid viscosity,
the software of system 40 optionally may be configured to alert the physician
to a prediction
or detection of infection. Finally, system 40 optionally may be configured to
remotely
- 12 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
receive raw or filtered operational data from a patient's handpiece 32 over a
secure Internet
channel.
[0042] Ascites, pleural effusion, or pericardial effusion may arise from
cancer such as
lung cancer, breast cancer, cancer that has metastasized to the pericardium,
ovarian cancer,
pancreatic cancer, uterine cancer, cancer of the bowels including colon
cancer, melanoma,
leukemia, or lymphoma, or from cancer treatment. In such a case, cancerous
cells will be
present in the excess fluid accumulated in the peritoneal cavity, pleural
cavity, or pericardial
cavity resulting from ascites, pleural effusion, or pericardial effusion,
respectively.
[0043] Advantageously, fluid management system 10 of the present invention
permits
noninvasive monitoring of cancerous cells pumped from a body cavity to the
bladder and
excreted during urination. Such an approach enables a patient to comfortably
provide
cancerous cells for analysis without the need of an invasive procedure, such
as a tissue
biopsy. Applicant has discovered that pH levels in the bladder create an
environment that
neutralizes cancerous cells; thereby obviating concerns that delivery of
cancerous cells to the
bladder could cause bladder cancer.
[0044] Analysis station 45 is configured to analyze cancerous cells
excreted from the
patient during urination using techniques known in the art of cancer cell
analysis. Analysis
station 45 receives a urination sample containing fluid comprising cancerous
cells that was
pumped from a first body cavity to the bladder via implantable device 20.
Analysis station
45 may display the analysis results to a user for review or may be coupled to
a computer
configured to receive analysis data containing the results for display.
Analysis station 45
may include communications circuitry configured to wirelessly, or using a
cable, transfer
analysis data to such a computer, and/or to the computer running monitoring
and control
system 40 for physician review. Analysis station 45 may be located in close
proximity to
system 40, e.g., in the same office or hospital, or may be located remotely.
As an added
benefit, a treating physician may review operation of implantable device 20
and obtain a
urine sample for analysis of cancerous cells during a single patient visit. In
an embodiment
where the analysis station is located in close proximity to system 40, the
cancerous cells may
be analyzed more expeditiously than conventional methods, in which a tissue
biopsy is
typically shipped to a remote laboratory for analysis.
- 13 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
Inflow and Outflow Catheters
[0045] Referring to FIGS. 2A and 2B, exemplary inflow catheter 50
constructed in
accordance with the principles of the present invention is described. Inflow
catheter 50 may
be configured for use in the peritoneal cavity (and thus correspond to
peritoneal catheter 23
of FIG. 1) or pleural or pericardial cavity, and preferably comprises tube 51
of medical-grade
silicone including inlet end 52 having a plurality of through-wall holes 53
and outlet end 54.
When configured for placement in the peritoneal cavity, inflow catheter
preferably has length
Li of about 40 cm, with holes 53 extending over length L2 of about 24 cm from
inlet end 52.
Holes 53 preferably are arranged circumferentially offset by about 90 and
longitudinally
offset between about 8 mm to 10 mm, as shown in FIG. 2B. In one preferred
embodiment,
29 holes 53 are arranged in four rows of 7 holes each, extend only through one
wall of the
inflow catheter at each location, and have a size of between 2.0 to 2.5 mm.
Inflow catheter
50 preferably includes solid silicone plug 55 that fills distal end of the
lumen for a distance of
about 7-10 mm to reduce tissue ingrowth, and radiopaque strip 56 disposed on,
or embedded
within, the catheter that extends the entire length of the catheter, that
renders the catheter
visible in fluoroscopic or X-ray images. Inflow catheter 50 may also include a
polyester cuff
in the region away from holes 53, to promote adhesion of the catheter to the
surrounding
tissue, thereby anchoring it in place.
[0046] Alternatively, inlet end 52 of inflow catheter 50 may have a spiral
configuration,
and an atraumatic tip, with holes 53 distributed over a length of the tubing
to reduce the risk
of clogging. As a further alternative, inlet end 52 may include a portion
having an enlarged
diameter, as disclosed in U.S. Patent No. 4,657,530, or a reservoir as
disclosed in FIGS. 9 to
16 of U.S. Patent Application Publication US 2009/0318844, the entire contents
of both of
which are incorporated herein by reference, to further reduce the risk of
clogging. Inlet end
52 also may terminate in a duck-bill valve, as shown for example in U.S.
Patent No.
4,240,434, thereby permitting the catheter to be cleaned in situ by
disconnecting the outlet
end of the catheter from implantable device 20 and extending a rod from the
outlet end of
catheter 50 through the duckbill valve at the inlet end.
[0047] Inlet end 52 also may include a polyester cuff to promote adhesion
of the catheter
to an adjacent tissue wall, thereby ensuring that the inlet end of the
catheter remains in
position. Outlet end 54 also may include a connector for securing the outlet
end of the inflow
catheter to implantable device 20. In one preferred embodiment, the distal end
of the inflow
- 14 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
catheter, up to the ingrowth cuff, may be configured to pass through a
conventional 16 F
peel-away sheath. In addition, the length of the inflow catheter may be
selected to ensure that
it lies along the bottom of the body cavity, and is sufficiently resistant to
torsional motion so
as not to become twisted or kinked during or after implantation.
[0048] In one embodiment, inflow catheter 50 may be shaped, e.g., Y-shaped,
to include
a plurality of inflow segments to permit inflow from more than one body
cavity. For
example, an inlet end of one segment may be positioned in the peritoneal
cavity and the inlet
end of another segment may be positioned in the pleural cavity or the
pericardial cavity. In
an alternative embodiment, more than one inflow catheter may be coupled to
implantable
device 20 to permit inflow from more than one body cavity. For example, an
inlet end of a
first inflow catheter may be positioned in the peritoneal cavity and an inlet
end of second
inflow catheter may be positioned in the pleural cavity or the pericardial
cavity. One or more
valves may be coupled to the multi-inlet catheter, or the multiple inflow
catheters, such that
fluid may be pumped from exclusively one cavity or from more than one cavity
responsive to
commands transmitted by the processor of implantable device 20 to the
valve(s).
[0049] With respect to FIG. 3A, a first embodiment of outflow catheter 60
of the present
invention is described, corresponding to bladder catheter 25 of FIG. 1.
Outflow catheter 60
preferably comprises tube 61 of medical-grade silicone having inlet end 62 and
outlet end 63
including spiral structure 64, and polyester ingrowth cuff 65. Outflow
catheter 60 includes a
single internal lumen that extends from inlet end 62 to a single outlet at the
tip of spiral
structure 64, commonly referred to as a "pigtail" design. Inlet end 62 may
include a
connector for securing the inlet end of the outflow catheter to implantable
device 20, or may
have a length that can be trimmed to fit a particular patient.
[0050] When configured for use as the outflow catheter in an ascites
treatment system,
outflow catheter may have length L3 of about 45 cm, with cuff 65 placed length
L4 of about
to 6 cm from spiral structure 64. Outflow catheter 60 may be loaded onto a
stylet with
spiral structure 64 straightened, and implanted using a minimally invasive
technique in which
outlet end 63 and spiral structure 64 are passed through the wall of a
patient's bladder using
the stylet. When the stylet is removed, spiral structure 64 returns to the
coiled shape shown
in FIG. 3A. Once outlet end 63 of outflow catheter 60 is disposed within the
patient's
bladder, the remainder of the catheter is implanted using a tunneling
technique, such that inlet
end 62 of the catheter may be coupled to implantable device 20. Spiral
structure 64 may
- 15 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
reduce the risk that outlet end 63 accidentally will be pulled out of the
bladder before the
tissue surrounding the bladder heals sufficiently to incorporate ingrowth cuff
65, thereby
anchoring the outflow catheter in place.
[0051] In a preferred embodiment, the outflow catheter is configured to
pass through a
conventional peel-away sheath. Outflow catheter 60 preferably is sufficiently
resistant to
torsional motion so as not to become twisted or kinked during or after
implantation. In a
preferred embodiment, inflow catheter 50 and outflow catheter 60 preferably
are different
colors, have different exterior shapes (e.g., square and round) or have
different connection
characteristics so that they cannot be inadvertently interchanged during
connection to
implantable device 20. Optionally, outflow catheter 60 may include an internal
duckbill
valve positioned midway between inlet 62 and outlet end 63 of the catheter to
insure that
urine does not flow from the bladder into the peritoneal cavity if the outflow
catheter is
accidentally pulled free from the pump outlet of implantable device 20.
[0052] In an alternative embodiment, the inflow and outflow catheters
devices may
incorporate one or several anti-infective agents to inhibit the spread of
infection between
body cavities. Examples of anti-infective agents which may be utilized may
include, e.g.,
bacteriostatic materials, bacteriocidal materials, one or more antibiotic
dispensers, antibiotic
eluting materials, and coatings that prevent bacterial adhesion, and
combinations thereof
[0053] Alternatively, rather than comprising separate catheters, inflow and
outflow
catheters may share a common wall. This arrangement may be utilized ideally
for an ascites
treatment embodiment because the bladder and peritoneal cavity share a common
wall,
thereby facilitating insertion of a single dual-lumen tube. In addition,
either or both of the
inflow or outflow catheters may be reinforced along a portion of its length or
along its entire
length using ribbon or wire braiding or lengths of wire or ribbon embedded or
integrated
within or along the catheters. The braiding or wire may be fabricated from
metals such as
stainless steels, superelastic metals such as nitinol, or from a variety of
suitable polymers.
[0054] With respect to FIG. 3B, a second embodiment of an outflow catheter
of the
present invention is described, in which similar components are identified
with like-primed
numbers. Outflow catheter 60' preferably comprises tube 61' of medical-grade
silicone
having inlet end 62', outlet end 63' and polyester ingrowth cuff 65'. In
accordance with this
embodiment, outlet end 63' includes malecot structure 66, illustratively
comprising four
- 16-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
resilient wings 67 that expand laterally away from the axis of the catheter to
reduce the risk
that outlet end 63' of the catheter will be inadvertently pulled loose after
placement. Inlet
end 62' may include a connector for securing the inlet end of the outflow
catheter to
implantable device 20, or may have a length that can be trimmed to fit a
particular patient.
[0055] Malecot structure 66 preferably is constructed so that wings 67
deform to a
substantially flattened configuration when a stylet is inserted through the
lumen of the
catheter. In this manner, outflow catheter 60' may be loaded onto a stylet,
and using a
minimally invasive technique, outlet end 63' and malecot structure 66 may be
passed through
the wall of a patient's bladder using the stylet. When the stylet is removed,
wings 67 of the
malecot structure return to the expanded shape shown in FIG. 3B. Once outlet
end 63' of
outflow catheter 60' is coupled to the patient's bladder, the remainder of the
catheter is
implanted using a tunneling technique, such that inlet end 62' of the catheter
may be coupled
to implantable device 20. Malecot structure 66 may reduce the risk that outlet
end 63'
accidentally will be pulled out of the bladder before the tissue surrounding
the bladder heals
sufficiently to incorporate ingrowth cuff 65'. As for the embodiment of FIG.
3A, the outflow
catheter of FIG. 3B may be configured to pass through a conventional peel-away
sheath, and
preferably is sufficiently resistant to torsional motion so as not to become
twisted or kinked
during or after implantation.
[0056] As mentioned above, for ascites treatment systems, the outlet end of
the outflow
catheter preferably is configured for placement in the urinary bladder, and
this configuration
also may be employed for pleural effusion and pericardial effusion treatment
systems.
Alternatively, the outflow catheter used for systems designed for treatment of
pleural or
pericardial effusions may be configured so that the outlet end is disposed in
the peritoneal
cavity, such that effusive fluid drained into the peritoneal cavity is
reabsorbed and excreted,
e.g., through the kidneys. For such embodiments, outflow catheter 60 may be
constructed
similar to inflow catheter 50 of FIGS. 2, and may have a plurality of holes to
drain fluid into
the peritoneal cavity. For treatment of ascites, pleural effusion, and/or
pericardial effusion
arising from cancer, the outlet end of the outflow catheter preferably is
configured for
placement in the urinary bladder such that cancerous cells are neutralized by
pH levels in the
bladder, excreted during urination, and analyzed in a noninvasive manner.
- 17 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
The Implantable Device
[0057] Referring now to FIG. 4, a schematic depicting the functional blocks
of
implantable device 20 of the present invention is described. Implantable
device 20 includes
control circuitry, illustratively processor 70 coupled to nonvolatile memory
71, such as flash
memory or electrically erasable programmable read only memory, and volatile
memory 72
via data buses. Processor 70 is electrically coupled to electric motor 73,
battery 74,
inductive circuit 75, radio transceiver 76 and a plurality of sensors,
including humidity sensor
77, a plurality of temperature sensors 78, accelerometer 79, a plurality of
pressure sensors 80,
and respiratory rate sensor 81. Inductive circuit 75 is electrically coupled
to coil 84 to
receive energy transmitted from charging and communication system 30, while
transceiver 76
is coupled to antenna 82, and likewise is configured to communicate with a
transceiver in
charging and communication system 30, as described below. Optionally,
inductive circuit 75
also may be coupled to infrared light emitting diode 83. Motor 73 may include
a dedicated
controller, which interprets and actuates motor 73 responsive to commands from
processor
70. All of the components depicted in FIG. 4 are contained within a low volume
sealed
biocompatible housing, as shown in FIG. 5A.
[0058] Processor 70 executes firmware stored in nonvolatile memory 71 which
controls
operation of motor 73 responsive to signals generated by motor 73, sensors 77-
81 and
commands received from transceiver 76. Processor 70 also controls reception
and
transmission of messages via transceiver 76 and operation of inductive circuit
75 to charge
battery 74. In addition, processor 70 receives signals generated by Hall
Effect sensors
located within motor 73, which are used to compute direction and revolutions
of the gears of
the gear pump, and thus fluid volume pumped and the viscosity of that fluid,
as described
below. Processor 70 preferably includes a low-power mode of operation and
includes an
internal clock, such that the processor can be periodically awakened to handle
pumping,
pump tick mode, or communications and charging functions, and/or awakened to
handle
commands received by transceiver 76 from handpiece 32. In one embodiment,
processor 70
comprises a member of the MSP430 family of microcontroller units available
from Texas
Instruments, Incorporated, Dallas, Texas, and may incorporate the nonvolatile
memory,
volatile memory, and radio transceiver components depicted in FIG. 4. In
addition, the
firmware executed on processor 70 may be configured to respond directly to
commands sent
to implantable device 20 via charging and communication system 30. Processor
70 also is
- 18-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
configured to monitor operation of motor 72 (and any associated motor
controller) and
sensors 78-81, as described below, and to store data reflecting operation of
the implantable
device, including event logs and alarms. Thus, data is reported to the
charging and
communication system when it is next wirelessly coupled to the implantable
device. In a
preferred embodiment, processor 70 generates up to eighty log entries per
second prior to
activating the pump, about eight log entries per second when the implantable
system is
actively pumping and about one log entry per hour when not pumping.
[0059] Nonvolatile memory 71 preferably comprises flash memory or EEPROM,
and
stores a unique device identifier for implantable device 20, firmware to be
executed on
processor 70, configuration set point data relating to operation of the
implantable device, and
optionally, coding to be executed on transceiver 76 and/or inductive circuit
75, and a separate
motor controller, if present. Firmware and set point data stored on
nonvolatile memory 71
may be updated using new instructions provided by control and monitoring
system 40 via
charging and communication system 30. Volatile memory 72 is coupled to and
supports
operation of processor 70, and stores data and event log information gathered
during
operation of implantable device 20. Volatile memory 72 also serves as a buffer
for
communications sent to, and received from, charging and communication system
30.
[0060] Transceiver 76 preferably comprises a radio frequency transceiver
and is
configured for bi-directional communications via antenna 76 with a similar
transceiver circuit
disposed in handpiece 32 of charging and communication system 30. Transceiver
76 also
may include a low power mode of operation, such that it periodically awakens
to listen for
incoming messages and responds only to those messages including the unique
device
identifier assigned to that implantable device. Alternatively, because
transceiver 76
communicates only with the corresponding transceiver in handpiece 32 of its
associated
charging and communication system 30, transceiver 76 may be configured to send
or receive
data only when inductive circuit 75 of the implantable device is active. In
addition,
transceiver 76 may employ an encryption routine to ensure that messages sent
from, or
received by, the implantable device cannot be intercepted or forged.
[0061] Inductive circuit 75 is coupled to coil 84, and is configured to
recharge battery 74
of the implantable device when exposed to a magnetic field supplied by a
corresponding
inductive circuit within handpiece 32 of charging and communication system 30.
In one
embodiment, inductive circuit 75 is coupled to optional infrared LED 83 that
emits an
- 19 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
infrared signal when inductive circuit 75 is active. The infrared signal may
be received by
handpiece 32 of charging and communication system 30 to assist in locating the
handpiece
relative to the implantable device, thereby improving the magnetic coupling
and energy
transmission to the implantable device.
[0062] In accordance with one aspect of the present invention, inductive
circuit 75
optionally may be configured not only to recharge battery 74, but to directly
provide energy
to motor 73 in a "boost" mode or jog/shake mode to unblock the pump. In
particular, if
processor 70 detects that motor 73 is stalled, e.g., due to a block created by
the proteinaceous
ascitic fluid, an alarm may be stored in memory. When implantable device 20
next
communicates with charging and communication system 30, the alarm is reported
to
handpiece 32, and the patient may be given the option of depressing
multifunction button 34
to apply an overvoltage to motor 73 from inductive circuit 75 for a
predetermined time period
to free the pump blockage. Alternatively, depressing the multi-function button
may cause
processor 70 to execute a set of commands by which motor 73 is jogged or
shaken, e.g., by
alternatingly running the motor is reverse and then forward, to disrupt the
blockage. Because
such modes of operation may employ higher energy consumption than expected
during
normal operation, it is advantageous to drive the motor during such procedures
with energy
supplied via inductive circuit 75.
[0063] Battery 74 preferably comprises a lithium ion or lithium polymer
battery capable
of long lasting operation, e.g., up to three years, when implanted in a human,
so as to
minimize the need for re-operations to replace implantable device 20. In one
preferred
embodiment, battery 74 supplies a nominal voltage of 3.6V, a capacity of 150
mAh when
new, and a capacity of about 120 mAh after two years of use. Preferably,
battery 74 is
configured to supply a current of 280 mA to motor 73 when pumping; 25 mA when
the
transceiver is communicating with charging and communication system 30; 8 mA
when
processor 70 and related circuitry is active, but not pumping or
communicating; and 0.3 mA
when the implantable device is in low power mode. More preferably, battery 74
should be
sized to permit a minimum current of at least 450 mAh for a period of 10
seconds and 1 A for
25 milliseconds during each charging cycle.
[0064] Motor 73 preferably is a brushless direct current or electronically
commuted
motor having a splined output shaft that drives a set of floating gears that
operate as a gear
pump, as described below. Motor 73 may include a dedicated motor controller,
separate from
- 20 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
processor 70, for controlling operation of the motor. Motor 73 may include a
plurality of
Hall Effect sensors, preferably two or more, for determining motor position
and direction of
rotation. Due to the high humidity that may be encountered in implantable
device 20,
processor 70 may include programming to operate motor 73, although with
reduced accuracy,
even if some or all of the Hall Effect sensors fail.
[0065] In a preferred embodiment, motor 73 is capable of driving the gear
pump to
generate a nominal flow rate of 150 ml/min and applying a torque of about 1
mNm against a
pressure head of 30 cm water at 3000 RPM. In this embodiment, the motor
preferably is
selected to drive the gears at from 1000 to 5000 RPM, corresponding to flow
rates of from 50
to 260 ml/min, respectively. The motor preferably has a stall torque of at
least 3 mNm at
500 mA at 3 V, and more preferably 6 mNm in order to crush non-solid ascitic
proteinaceous
materials. As discussed above, the motor preferably also supports a boost mode
of
operation, e.g., at 5 V, when powered directly through inductive circuit 75.
Motor 73
preferably also is capable of being driven in reverse as part of a jogging or
shaking procedure
to unblock the gear pump.
[0066] In accordance with one aspect of the present invention, processor 70
may be
programmed to automatically and periodically wake up and enter a pump tick
mode. In this
mode of operation, the gear pump is advanced slightly, e.g., about 120 as
measured by the
Hall Effect sensors, before processor 70 returns to low power mode.
Preferably, this interval
is about every 20 minutes, although it may be adjusted by the physician using
the monitoring
and control system. This pump tick mode is expected to prevent the ascitic
fluid, which has a
high protein content, from partially solidifying, and blocking the gear pump,
and is expected
to be especially advantageous in overcoming the problem of clogging observed
in previously-
known implantable systems designed to treat chronic ascites.
[0067] In addition, processor 70 also may be programmed to enter a jog or
shake mode
when operating on battery power alone, to unblock the gear pump. Similar to
the boost mode
available when charging the implantable device with the handpiece of charging
and
communication system 30, the jog or shake mode causes the motor to rapidly
alternate the
gears between forward and reverse directions to crush or loosen and
proteinaceous buildup in
the gear pump or elsewhere in the fluid path. Specifically, in this mode of
operation, if the
motor does not start to turn within a certain time period after it is
energized (e.g. 1 second),
the direction of the motion is reversed for a short period of time and then
reversed again to let
-21-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
the motor turn in the desired direction. If the motor does still not turn
(e.g., because the gear
pump is jammed) the direction is again reversed for a period of time (e.g.,
another 10 msec).
If the motor still is not able to advance the time interval between reversals
of the motor
direction is reduced to allow for the motor to develop more power, resulting
in a shaking
motion of the gears. If the motor does not turn forward for more than 4
seconds, the jog
mode of operation is stopped, and an alarm is written to the event log. If the
motor was
unable to turn forward, processor 70 will introduce a backwards tick before
the next
scheduled fluid movement. A backward tick is the same as a tick (e.g., about
120 forward
movement of the motor shaft) but in the reverse direction, and is intended to
force the motor
backwards before turning forward, which should allow the motor to gain
momentum.
[0068] Sensors 77-81 continually monitor humidity, temperature,
acceleration, pressure,
and respiratory rate, and provide corresponding signals to processor 70. In
particular,
humidity sensor 77 is arranged to measure humidity within the housing of the
implantable
device, to ensure that the components of implantable device are operated
within expected
operational limits. Humidity sensor 77 preferably is capable of sensing and
reporting
humidity within a range or 20% to 100% with high accuracy. One or more of
temperature
sensors 78 may be disposed within the housing and monitor the temperature of
the
implantable device, and in particular battery 74 to ensure that the battery
does not overheat
during charging, while another one or more of temperature sensors 78 may be
disposed so as
to contact fluid entering at inlet 62 and thus monitor the temperature of the
fluid, e.g., for use
in predicting or detecting infection on the basis of an increase in the
fluid's temperature.
Accelerometer 79 is arranged to measure acceleration of the implant,
preferably along at least
two axes, to detect periods of inactivity, e.g., to determine whether the
patient is sleeping.
This information is provided to processor 70 to ensure that the pump is not
operated when the
patient is indisposed to attend to voiding of the bladder.
[0069] Implantable device 20 preferably includes multiple pressure sensors
80, which are
continually monitored during waking periods of the processor. As described
below with
respect to FIG. 6A, the implantable device of the present invention preferably
includes four
pressure sensors: a sensor to measure the pressure in the source cavity (e.g.,
peritoneal,
pleural or pericardial cavity), a sensor to measure the ambient pressure, a
sensor to measure
the pressure at the outlet of the gear pump, and a sensor to measure the
pressure in the sink
cavity (e.g., bladder, or for pleural or pericardial systems, the peritoneal
cavity). These
- 22 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
sensors preferably are configured to measure absolute pressure between 450
mBar and 1300
mBar while consuming less than 50 mW at 3V. Preferably, the sensors that
measure pressure
at the pump outlet and in the sink are placed across a duckbill valve, which
prevents reverse
flow into the gear pump and also permits computation of flow rate based on the
pressure drop
across the duckbill valve. In an embodiment with multiple inlet ends disposed
in multiple
source cavities, the implantable device of the present invention preferably
includes an
additional sensor to measure the pressure in each additional source cavity
(e.g., peritoneal,
pleural or pericardial cavity).
[0070] Respiratory rate monitor 81 is configured to measure the patient's
respiratory
rate, e.g., for use in predicting or detecting infection based on an increase
in the patient's
respiratory rate. Alternatively, the patient's respiratory rate may be
measured based on the
outputs of one or more of pressure sensors 80, e.g., based on changes in the
ambient pressure
or the pressure in the source cavity (e.g., peritoneal, plural, or pericardial
cavity) caused by
the diaphragm periodically compressing that cavity during breathing.
[0071] In a preferred embodiment, processor 70 is programmed to pump fluid
from the
source cavity to the sink cavity only when the pressure in the source cavity
exceeds a first
predetermined value, and the pressure in the sink cavity is less than a second
predetermined
value. In an embodiment with multiple inlet ends disposed in multiple source
cavities,
processor 70 is programmed to pump fluid from the first source cavity, second
source cavity,
or both to the sink cavity only when the pressure in the first source cavity,
second source
cavity, or both, respectively, exceed a first predetermined value, a second
predetermined
value, or both the first and second predetermined values, respectively, and
the pressure in the
sink cavity is less than a third predetermined value. To account for patient
travel from a
location at sea level to a higher altitude, the ambient pressure measurement
may be used to
calculate a differential value for the peritoneal pressure. In this way, the
predetermined
pressure at which the pump begins operation may be reduced, to account for
lower
atmospheric pressure. Likewise, the ambient pressure may be used to adjust the
predetermined value for bladder pressure. In this way, the threshold pressure
at which the
pumping ceases may be reduced, because the patient may experience bladder
discomfort at a
lower pressure when at a high altitude location.
[0072] Referring now to FIGS. 5A and 5B, further details of an exemplary
embodiment
of implantable device 90 are provided. In FIG. 5A, housing 91 is shown as
transparent,
-23-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
although it should of course be understood that housing 91 comprises opaque
biocompatible
plastic and/or metal alloy materials. In FIG. 5B, the implantable device is
shown with lower
portion 92 of housing 91 removed from upper housing 93 and without a glass
bead/epoxy
filler material that is used to prevent moisture from accumulating in the
device. In FIGS. 5A
and 5B, motor 94 is coupled to gear pump housing 95, which is described in
greater detail
with respect to FIGS. 6 and 7. The electronic components discussed above with
respect to
FIG. 4 are disposed on flexible circuit board substrate 96, which extends
around and is
fastened to support member 97. Coil 98 (corresponding to coil 84 of FIG. 4) is
disposed on
flap 99 of the substrate and is coupled to the electronic components on flap
100 by flexible
cable portion 101. Support member 97 is fastened to upper housing 93 and
provides a cavity
that holds battery 102 (corresponding to battery 74 of FIG. 4). Lower portion
92 of housing
91 includes port 103 for injecting the glass bead/epoxy mixture after upper
portion 93 and
lower portion 92 of housing 91 are fastened together, to reduce space in the
housing in which
moisture can accumulate.
[0073] Housing 91 also may include features designed to reduce movement of
the
implantable pump once implanted within a patient, such as a suture hole to
securely anchor
the implantable device to the surrounding tissue. Housing 91 may in addition
include a
polyester ingrowth patch that facilitates attachment of the implantable device
to the
surrounding tissue following subcutaneous implantation.
[0074] Additionally, the implantable device optionally may incorporate anti-
clogging
agents, such enzyme eluting materials that specifically target the
proteinaceous components
of ascites, enzyme eluting materials that specifically target the
proteinaceous and encrustation
promoting components of urine, chemical eluting surfaces, coatings that
prevent adhesion of
proteinaceous compounds, and combinations thereof Such agents, if provided,
may be
integrated within or coated upon the surfaces of the various components of the
system.
[0075] Referring now to FIGS. 6A to 6D, further details of the gear pump
and fluid path
are described. In FIGS. 6A-6D, like components are identified using the same
reference
numbers from FIGS. 5A and 5B. FIG. 6A is an exploded view showing assembly of
motor
94 with gear pump housing 95 and upper housing 93, as well as the components
of the fluid
path within the implantable device. Upper housing 93 preferably comprises a
high strength
plastic or metal alloy material that can be molded or machined to include
openings and
channels to accommodate inlet nipple 102, outlet nipple 103, pressure sensors
104a-104d,
- 24 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
manifold 105 and screws 106. Nipples 102 and 103 preferably are machined from
a high
strength biocompatible metal alloy, and outlet nipple 103 further includes
channel 107 that
accepts elastomeric duckbill valve 108. Outlet nipple 103 further includes
lateral recess 109
that accepts pressure sensor 104a, which is arranged to measure pressure at
the inlet end of
the outflow catheter, corresponding to pressure in the patient's bladder (or
peritoneal cavity).
[0076] Referring now also to FIG. 6B and 6C, inlet nipple 102 is disposed
within opening
110, which forms a channel in upper housing 93 that includes opening 111 for
pressure
sensor 104b and opening 112 that couples to manifold 105. Pressure sensor 104b
is arranged
to measure the pressure at the outlet end of the inflow catheter,
corresponding to pressure in
the peritoneal (or pleural or pericardial) cavity. Outlet nipple 103,
including duckbill valve
107, is disposed within opening 113 of upper housing 93 so that lateral recess
108 is aligned
with opening 114 to permit access to the electrical contacts of pressure
sensor 104a. Opening
113 forms channel 115 that includes opening 116 for pressure sensor 104c, and
opening 117
that couples to manifold 105. Upper housing 93 preferably further includes
opening 118 that
forms a channel including opening 119 for accepting pressure sensor 104d.
Pressure sensor
104d measures ambient pressure, and the output of this sensor is used to
calculate differential
pressures as described above. Upper housing further includes notch 120 for
accepting
connector 26 (see FIG. 1) for retaining the inflow and outflow catheters
coupled to inlet and
outlet nipples 102 and 103. Upper housing 93 further includes recess 121 to
accept manifold
105, and peg 122, to which support member 97 (see FIG. 5B) is connected.
[0077] As shown in FIGS. 6A and 6D, manifold 105 preferably comprises a
molded
elastomeric component having two separate fluid channels that couple inlet and
outlet flow
paths through upper housing 93 to the gear pump. The first channel includes
inlet 124 and
outlet 125, while the second channel includes inlet 126 and outlet 127. Inlet
124 couples to
opening 112 (see FIG. 6C) of the inflow path and outlet 127 couples to opening
117 of the
outflow path. Manifold 105 is configured to improve manufacturability of the
implantable
device, by simplifying construction of upper housing 93 and obviating the need
to either cast
or machine components with complicated non-linear flow paths.
[0078] Referring now to FIGS. 6A, 7A and 7B, motor 94 is coupled to gear
pump
housing 95 using mating threads 130, such that splined shaft 131 of motor 94
passes through
bearing 132. The gear pump of the present invention comprises intermeshing
gears 133 and
134 enclosed in gear pump housing 95 by 0-ring seal 135 and plate 136. The
gear pump is
- 25 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
self-priming. Plate 136 includes openings 137 and 138 that mate with outlet
125 and inlet
126 of manifold 105, respectively. Splined shaft 131 of motor 94 extends into
opening 139
of gear 133 to provide floating engagement with that gear. Interaction of the
splined shaft
with the gears is described below with respect to FIG. 7B.
[0079] FIG. 7A depicts the obverse side of gear pump housing 95 of FIG. 6A,
and
includes recess 140 that is sized to accept gears 133 and 134, and groove 141
that accepts 0-
ring seal 135. Gears 133 and 134 are seated within recess 140 such that
splined shaft 131
extends through opening 142 and floats within keyed opening 139 of gear 133.
Gears 133
and 134 are dimensioned so as to sit within recess 140 with a close tolerance
(e.g., 0.2 mm) to
wall 143 of the recess, but spin as freely as the viscosity of the fluid
permits. Openings 137
and 138 of plate 136 (see FIG. 6A) are positioned over the juncture of gears
133 and 134
(shown in dotted line in FIG. 7A) so that rotation of gear 133 in a clockwise
direction (when
viewed from above) creates suction that draws fluid into the gear pump housing
through
opening 137, and expels fluid through opening 138. Likewise, if motor 94
drives gear 133 in
a counterclockwise direction (as viewed from above), the gear pump will draw
fluid into the
gear pump housing through opening 138 and expel it through opening 137,
thereby reversing
flow.
[0080] As depicted in the simplified model of FIG. 7B, gear 134 has no
axle, but instead
floats freely within its portion of recess 140. Splined shaft 131 engages
keyed opening 139
of gear 133, so that gear 133 floats on splined shaft 131. Advantageously,
this arrangement
improves pump efficiency and manufacturability, and reduces power consumption
by motor
94 by reducing the effects of manufacturing variations and thermal effects. In
particular,
slight variations in motor shaft eccentricity or straightness, resulting from
manufacturing
tolerances or differential thermal expansion, will not cause the gear to bind
against the
interior of recess 140 or against gear 134. Instead, different portions of the
surfaces of shaft
131 and keyed opening 139 contact one another during revolution of shaft 131
to
continuously transmit rotational torque to gear 133. However, energy-wasting
forces
resulting from shaft eccentricities, variations in manufacturing tolerances or
differential
thermal expansion of the components are reduced. In addition, this floating
arrangement may
reduce the risk that particulate matter causes binding between the gears and
wall 143, since
the gears may move laterally to accommodate such particulate matter.
-26-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
[0081] Gears 133 and 134 include intermeshing lobes 144 that positively
displace fluid as
they engage and disengage, with substantially no bypass flow. In this manner
the volume and
viscosity of fluid transported by gears 133 and 134 may computed by tracking
the number of
motor revolutions sensed by the Hall Effect sensors disposed within motor 94.
As further
shown in FIGS. 7A and 7B, recess 140 of gear pump housing 95 comprises two
interconnected, substantially circular, lobes. This arrangement retains gears
133 and 134 in
proper relation to wall 143 of the recess, as well as relative to one another.
In a preferred
embodiment, cusps 145, formed where the two lobes intersect, are configured to
form
tangents to radii drawn from the centers of the respective lobes.
Advantageously, configuring
the cusps in this manner reduces the potential for gears 133 and 134 to
impinge upon wall
143.
The Charging and Communication System
[0082] Referring to FIGS. 8A, 8B and 9, charging and communication system
150 of the
present invention (corresponding to system 30 of FIG. 1) is now described in
greater detail.
In one preferred embodiment, charging and communication system 150 comprises
handpiece
151 and base 31 (see FIG. 1). Base 31 provides comprises a cradle for
recharging handpiece
151, and preferably contains a transformer and circuitry for converting
conventional 120V
power service to a suitable DC current to charge handpiece 151 when it is
coupled to the
base. Alternatively, handpiece 151 may include circuitry for charging the
handpiece battery,
and a detachable power cord. In this embodiment, handpiece 151 may be directly
plugged
into a convention 120V wall socket for charging, and the power cord removed
when the
handpiece is used to recharge the implantable device.
[0083] As shown in FIG. 9, handpiece 151 contains controller 152,
illustratively the
processor of a micro-controller unit coupled to nonvolatile memory 153 (e.g.,
either
EEPROM or flash memory), volatile memory 154, radio transceiver 155, inductive
circuit
156, battery 157, indicator 158 and display 159. Controller 152, memories 153
and 154, and
radio transceiver 155 may be incorporated into a single microcontroller unit,
such as the
MP5430 family of microprocessors, available from Texas Instruments
Incorporated, Dallas,
Texas. Transceiver 155 is coupled to antenna 160 for sending and receiving
information to
implantable device 20. Battery 157 is coupled to connector 161 that removably
couples with
a connector in base 31 to recharge the battery. Port 162, such as a USB port
or comparable
wireless circuit, is coupled to controller 152 to permit information to be
exchanged between
-27 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
handpiece 151 and the monitoring and control system. Inductive circuit 156 is
coupled to
coil 163. Input device 164, preferably a multi-function button, also is
coupled to controller
152 to enable a patient to input a limited number of commands. Indicator 158
illustratively
comprises a plurality of LEDs that illuminate to indicate the quality of
charge coupling
achieved between the handpiece and implantable device, and therefore assist in
optimizing
the positioning of handpiece 151 relative to the implantable device during
recharging. In one
preferred embodiment, indicator 158 is omitted, and instead a bar indicator
provided on
display 159 that indicates the quality-of-charging resulting from the coupling
of coils 163 and
84.
[0084] In a preferred embodiment, handpiece 151 includes a device
identifier stored in
nonvolatile memory 153 that corresponds to the device identifier stored in
nonvolatile
memory 71 of the implantable device, such that handpiece 151 will communicate
only with
its corresponding implantable device 20. Optionally, a configurable handpiece
for use in a
physician's office may include the ability to interrogate an implantable
device to request that
device's unique device identifier, and then change the device identifier of
the monitoring and
control system 40 to that of the patient's implantable device, so as to mimic
the patient's
handpiece. In this way, a physician may adjust the configuration of the
implantable device if
the patient forgets to bring his handpiece 151 with him during a visit to the
physician's office.
[0085] Controller 152 executes firmware stored in nonvolatile memory 153
that controls
communications and charging of the implantable device. Controller 152 also is
configured to
transfer and store data, such as event logs, uploaded to handpiece 151 from
the implantable
device, for later retransmission to monitoring and control system 40 via port
162, during
physician office visits. Alternatively, handpiece 151 may be configured to
recognize a
designated wireless access point within the physician's office, and to
wirelessly communicate
with monitoring and control system 40 during office visits. As a further
alternative, base 31
may include telephone circuitry for automatically dialing and uploading
information stored
on handpiece 151 to a physician's website via a secure connection, such as
alarm
information.
[0086] Controller 152 preferably includes a low-power mode of operation and
includes
an internal clock, such that the controller periodically awakens to
communicate with the
implantable device to log data or to perform charging functions. Controller
152 preferably is
configured to awaken when placed in proximity to the implantable device to
perform
-28-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
communications and charging functions, and to transmit commands input using
input device
164. Controller 152 further may includes programming for evaluating
information received
from the implantable device, and generating an alarm message on display 159.
Controller
152 also may include firmware for transmitting commands input using input
device 164 to
the implantable device, and monitoring operation of the implantable device
during execution
of such commands, for example, during boost or jogging/shaking operation of
the gear pump
to clear a blockage. In addition, controller 152 controls and monitors various
power
operations of handpiece 151, including operation of inductive circuit 156
during recharging
of the implantable device, displaying the state of charge of battery 74, and
controlling
charging and display of state of charge information for battery 157.
[0087] Nonvolatile memory 153 preferably comprises flash memory or EEPROM,
and
stores the unique device identifier for its associated implantable device,
firmware to be
executed by controller 152, configuration set point, and optionally, coding to
be executed on
transceiver 155 and/or inductive circuit 156. Firmware and set point data
stored on
nonvolatile memory 153 may be updated using information supplied by control
and
monitoring system 40 via port 162. Volatile memory 154 is coupled to and
supports
operation of controller 152, and stores data and event log information
uploaded from
implantable device 20.
[0088] In addition, in a preferred embodiment, nonvolatile memory 153
stores
programming that enables the charging and communication system to perform some
initial
start-up functions without communicating with the monitor and control system.
In particular,
memory 153 may include routines that make it possible to test the implantable
device during
implantation using the charging and communication system alone in a "self-
prime mode" of
operation. In this case, a button may be provided that allows the physician to
manually start
the pump, and display 159 is used to provide feedback whether the pumping
session was
successful or not. Display 159 of the charging and communication system also
may be used
to display error messages designed to assist the physician in adjusting the
position of the
implantable device or inflow or outflow catheters. These functions preferably
are disabled
after the initial implantation of the implantable device.
[0089] Transceiver 155 preferably comprises a radio frequency transceiver,
e.g.,
conforming to the Bluetooth or IEEE 802.11 wireless standards, and is
configured for bi-
directional communications via antenna 160 with transceiver circuit 76
disposed in the
- 29 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
implantable device. Transceiver 155 also may include a low power mode of
operation, such
that it periodically awakens to listen for incoming messages and responds only
to those
messages including the unique device identifier assigned to its associated
implantable device.
Transceiver 155 preferably employs an encryption routine to ensure that
messages sent to, or
received from, the implantable device cannot be intercepted or forged.
[0090] Inductive circuit 156 is coupled to coil 163, and is configured to
inductively
couple with coil 84 of the implantable device to recharge battery 74 of the
implantable
device. In one embodiment, inductive circuit 156 is coupled to indicator 158,
preferably a
plurality of LEDs that light to indicate the extent of magnetic coupling
between coils 163 and
84 (and thus quality of charging), thereby assisting in positioning handpiece
151 relative to
the implantable device. In one preferred embodiment, inductive coils 84 and
163 are capable
of establishing good coupling through a gap of 35 mm, when operating at a
frequency of 315
kHz or less. In an embodiment in which implantable device includes optional
infrared LED
83, charging and communication system 30 may include an optional infrared
sensor (not
shown) which detects that infrared light emitted by LED 83 and further assists
in positioning
handpiece 151 to optimize magnetic coupling between coils 163 and 84, thereby
improving
the energy transmission to the implantable device.
[0091] In accordance with one aspect of the present invention, controller
152 may be
configured to periodically communicate with the implantable device to retrieve
temperature
data generated by temperature sensor 78 and stored in memory 72 during
inductive charging
of battery 74. Controller 152 may include firmware to analyze the battery
temperature, and
to adjust the charging power supplied to inductive circuit 163 to maintain the
temperature of
the implantable device below a predetermined threshold, e.g., less than 2 C
above body
temperature. That threshold may be set to reduce thermal expansion of the
battery and
surrounding electronic and mechanical components, for example, to reduce
thermal
expansion of motor and gear pump components and to reduce the thermal strain
applied to
the seal between lower portion 92 of housing and upper housing 93. In a
preferred
embodiment, power supplied to inductive coil 163 is cycled between high power
(e.g., 120
mA) and low power (e.g., 40 mA) charging intervals responsive to the measured
temperature
within the implantable device.
[0092] As discussed above with respect to inductive circuit 75 of the
implantable device,
inductive circuit 156 optionally may be configured to transfer additional
power to motor 73
- 30 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
of the implantable device, via inductive circuit 75 and battery 74, in a
"boost" mode or
jogging mode to unblock the gear pump. In particular, if an alarm is
transmitted to controller
152 that motor 73 is stalled, e.g., due to a block created by ascitic fluid,
the patient may be
given the option of using input device 164 to apply an overvoltage to motor 73
from
inductive circuit 75 for a predetermined time period to free the blockage.
Alternatively,
activating input device 164 may cause controller 152 to command processor 70
to execute a
routine to jog or shake the gear pump by rapidly operating motor 74 in reverse
and forward
directions to disrupt the blockage. Because such modes of operation may employ
higher
energy consumption than expected during normal operation, inductive circuits
156 and 75
may be configured to supply the additional energy for such motor operation
directly from the
energy stored in battery 157, instead of depleting battery 74 of the
implantable device.
[0093] Battery 157 preferably comprises a lithium ion or lithium polymer
battery capable
of long lasting operation, e.g., up to three years. Battery 157 has sufficient
capacity to supply
power to handpiece 151 to operate controller 152, transceiver 155, inductive
circuit 156 and
the associated electronics while disconnected from base 31 and during charging
of the
implantable device. In a preferred embodiment, battery 157 has sufficient
capacity to fully
recharge battery 74 of the implantable device from a depleted state in a
period of about 2-4
hours. Battery 157 also should be capable of recharging within about 2-4
hours. It is
expected that for daily operation moving 700 ml of fluid, battery 157 and
inductive circuit
156 should be able to transfer sufficient charge to battery 74 via inductive
circuit 75 to
recharge the battery within about 30 minutes. Battery capacity preferably is
supervised by
controller 152 using a charge accumulator algorithm.
[0094] Referring again to FIGS. 8A and 8B, handpiece 151 preferably
includes housing
165 having multi-function button 166 (corresponding to input device 164 of
FIG. 9) and
display 167 (corresponding to display 159 of FIG. 9). Plurality of LEDs 168 is
disposed
beneath a translucent portion of handpiece 151, and corresponds to indicator
158 of FIG. 9.
Port 169 enables the handpiece to be coupled to monitoring and control system
40 (and
corresponds to port 162 of FIG. 9), while connector 170 (corresponding to
connector 161 in
FIG. 9) permits the handpiece to be coupled to base 31 to recharge battery
157. Multi-
function button 166 provides the patient the ability to input a limited number
of commands to
the implantable device. Display 167, preferably an OLED or LCD display,
provides visible
confirmation that a desired command input using multifunction button 166 has
been received.
-31-
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
Display 167 also may display the status and state of charge of battery 74 of
the implantable
device, the status and state of charge of battery 157 of handpiece 151, signal
strength of
wireless communications, quality-of-charging, error and maintenance messages.
Inductive
coil portion 171 of housing 165 houses inductive coil 163.
[0095] LEDs 168 are visible through the material of housing 165 when lit,
and preferably
are arranged in three rows of two LEDs each. During charging, the LEDs light
up to display
the degree of magnetic coupling between inductive coils 163 and 84, e.g., as
determined by
energy loss from inductive circuit 156, and may be used by the patient to
accurately position
handpiece 151 relative to the implantable device. Thus, for example, a low
degree of
coupling may correspond to lighting of only two LEDs, an intermediate degree
of coupling
with lighting of four LEDs, and a preferred degree of coupling being reflected
by lighting of
all six LEDs. Using this information, the patient may adjust the position of
handpiece 151
over the area where implantable device is located to obtain a preferred
position for the
handpiece, resulting in the shortest recharging time. In one preferred
embodiment, LEDs
168 are replaced with an analog bar display on display 167, which indicates
the quality of
charge coupling.
The Monitoring and Control System
[0096] Turning to FIG. 10, the software implementing monitoring and control
system of
FIG. 1 now described. Software 180 comprises a number of functional blocks,
schematically
depicted in FIG. 10, including main block 184, event logging block 182, data
download block
183, configuration setup block 184, user interface block 185, alarm detection
block 186
including infection prediction block 191, sensor calibration block 187,
firmware upgrade
block 188, device identifier block 189 and status information block 190. The
software
preferably is written in C++ and employs an object oriented format. In one
preferred
embodiment, the software is configured to run on top of a Microsoft Windows
(a registered
trademark of Microsoft Corporation, Redmond, Washington) or Unix-based
operating
system, such as are conventionally employed on desktop and laptop computers.
The
computer running monitoring and control system software 180 preferably
includes a data
port, e.g., USB port or comparable wireless connection, that permits handpiece
151 of the
charging and communication system to be coupled via port 169. Alternatively,
as discussed
above, the computer may include a wireless card, e.g., conforming to the IEEE
802.11
standard, thereby enabling handpiece 151 to communicate wirelessly with the
computer
-32 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
running software 180. As a further alternative, the charging and communication
system may
include telephony circuitry that automatically dials and uploads data, such as
alarm data,
from handpiece 151 to a secure website accessible by the patient's physician.
[0097] Main block 184 preferably consists of a main software routine that
executes on the
physician's computer, and controls overall operation of the other functional
blocks. Main
block 184 enables the physician to download event data and alarm information
stored on
handpiece 151 to his office computer, and also permits control and monitoring
software 180
to directly control operation of the implantable device when coupled to
handpiece 151. Main
block also enables the physician to upload firmware updates and configuration
data to the
implantable device.
[0098] Event Log block 182 is a record of operational data downloaded from
the
implantable device via the charging and communication system, and may include,
for
example, pump start and stop times, motor position, sensor data for peritoneal
(or pleural or
pericardial) cavity and sink cavity (e.g. bladder) pressures, patient
temperature, respiratory
rate or fluid temperature, pump outlet pressure, humidity, pump temperature,
battery current,
battery voltage, battery status, and the like. The event log also may include
the occurrence of
events, such as pump blockage, operation in boost or jog modes, alarms or
other abnormal
conditions.
[0099] Data Download block 183 is a routine that handles communication with
handpiece
151 to download data from volatile memory 154 after the handpiece is coupled
to the
computer running monitoring and control software 180. Data Download block 183
may
initiates, either automatically or at the instigation of the physician via
user interface block
185, downloading of data stored in the event log.
[00100] Configuration Setup block 184 is a routine that configures the
parameters stored
within nonvolatile memory 71 that control operation of the implantable device.
The interval
timing parameters may determine, e.g., how long the processor remains in sleep
mode prior
to being awakened to listen for radio communications or to control pump
operation. The
interval timing parameters may control, for example, the duration of pump
operation to move
fluid from the peritoneum (or pleura or pericardial sac) to the sink cavity
and the interval
between periodic tick movements that prevent blockage of the implantable
device and inflow
and outflow catheters. Interval timing settings transmitted to the implantable
device from
-33 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
monitoring and control software 180 also may determine when and how often
event data is
written to nonvolatile memory 71, and to configure timing parameters used by
the firmware
executed by processor 152 of handpiece 151 of the charging and communication
system.
Block 184 also may be used by the physician to configure parameters stored
within
nonvolatile memory 71 relating to limit values on operation of processor 70
and motor 73.
These values may include minimum and maximum pressures at the inflow and
outflow
catheters, the maximum temperature differential during charging, times when
the pump may
and may not operate, etc. The limit values set by block 184 also configure
parameters that
control operation of processor 152 of handpiece 151. Block 184 also may
configure
parameters store within nonvolatile memory 71 of the implantable device
relating to control
of operation of processor 70 and motor 73. These values may include target
daily volumes of
fluid to transport, volume of fluid to be transported per pumping session,
motor speed and
duration per pumping session. Block 184 also may specify the parameters of
operation of
motor 73 during boost mode of operation, when coupled to handpiece 151, and
shake/jog
modes of operation when the implantable device is run using battery 74 alone.
Such
parameters may include motor speed and voltage, duration/number of revolutions
of the
motor shaft when alternating between forward and reverse directions, etc.
[00101] User interface block 185 handles display of information retrieved from
the
monitoring and control system and implantable device via data download block
183, and
presents that information in an intuitive, easily understood format for
physician review. As
described below with respect to FIGS. 11 to 15, such information may include
status of the
implantable device, status of the charging and control system, measured
pressures, volume of
fluid transported per pumping session or per day, etc. User interface block
185 also generates
user interface screens that permit the physician to input information to
configure the interval
timing, limit and pump operation parameters discussed above with respect to
block 184.
[00102] Alarm detection block 186 may include a routine for evaluating the
data retrieved
from the implantable device or charging and communication system, and flagging
abnormal
conditions for the physician's attention. For example, alarm detection block
186 may include
infection prediction block 191, which is configured to predict or detect
infection based on, for
example, one or more of an increase in the patient's temperature above a
predefined
threshold, an increase in the patient's respiratory rate above a predefined
threshold, and/or an
increase in the fluid above a predefined threshold. Such flags may be
communicated to the
- 34 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
physician by changing status indicators presented by user interface block 185,
or by
displaying to the physician specific information about increases in the
patient's temperature,
respiratory rate, or fluid viscosity via user interface block 185.
[00103] Sensor calibration block 187 may include a routines for testing or
measuring drift,
of sensors 70, 78-81 employed in the implantable device, e.g., due to aging or
change in
humidity. Block 187 may then compute offset values for correcting measured
data from the
sensors, and transmit that information to the implantable device for storage
in nonvolatile
memory 71. For example, pressure sensors 104a-104d may experience drift due to
aging or
temperature changes. Block 187 accordingly may compute offset values that are
then
transmitted and stored in the implantable device to account for such drift.
[00104] Firmware upgrade block 188 may comprise a routine for checking the
version
numbers of the processor or motor controller firmware installed on the
implantable device
and/or processor firmware on charging and communication system, and identify
whether
upgraded firmware exists. If so, the routine may notify the physician and
permit the
physician to download revised firmware to the implantable device for storage
in nonvolatile
memory 71 or to download revised firmware to the charging and communication
system for
storage in nonvolatile memory 153.
[00105] Device identifier block 189 consists of a unique identifier for the
implantable
device that is stored in nonvolatile memory 71 and a routine for reading that
data when the
monitoring and control system is coupled to the implantable device via the
charging and
communication system. As described above, the device identifier is used by the
implantable
device to confirm that wireless communications received from a charging and
communication system are intended for that specific implantable device.
Likewise, this
information is employed by handpiece 151 of the charging and communication
system in
determining whether a received message was generated by the implantable device
associated
with that handpiece. Finally, the device identifier information is employed by
monitoring
and control software 180 to confirm that the handpiece and implantable device
constitute a
matched set.
[00106] Status information block 190 comprises a routine for interrogating
implantable
device, when connected via handpiece 151, to retrieve current status date from
the
implantable device, and/or handpiece 151. Such information may include, for
example,
-35 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
battery status, the date and time on the internal clocks of the implantable
device and
handpiece, version control information for the firmware and hardware currently
in use, and
sensor data.
[00107] Referring now to FIGS. 11-15, exemplary screen shots generated by user
interface
block 187 of software 180 are described for an ascites treatment system. FIG.
11 shows main
screen 200 that is displayed to a physician running monitoring and control
software 180.
Main screen 200 includes a status area that displays status information
retrieved from the
implantable device and the charging and communication system by the routine
corresponding
to block 190 of FIG. 10. More particularly, the status area includes status
area 201 for the
charging and communication system (referred to as the "Smart Charger) and
status area 202
for the implantable device (referred to as the "ALFA Pump"). Each status area
includes an
icon showing whether the respective system is operating properly, indicated by
a checkmark,
the device identifier for that system, and whether the system is connected or
active. If a
parameter is evaluated by the alarm detection block 186 to be out of
specification, the icon
may instead include a warning symbol. Menu bar 203 identifies the various
screens that the
physician can move between by highlighting the respective menu item. Workspace
area 204
is provided below the status area, and includes a display that changes
depending upon the
menu item selected. Below workspace area 204, navigation panel 205 is
displayed, which
includes the version number of software 180 and a radio button that enables
the displays in
workspace area 204 to be refreshed.
[00108] In FIG. 11, the menu item "Information" with submenu item "Implant" is
highlighted in menu bar 203. For this menu item selection, workspace area 204
illustratively
shows, for the implantable device, battery status window 204a, measured
pressures window
204b and firmware version control window 204c. Battery status window 204a
includes an
icon representing the charge remaining in battery 74, and may be depicted as
full, three-
quarters, one-half, one-quarter full or show an alarm that the battery is
nearly depleted. The
time component of window 204a indicates the current time as received from the
implantable
device, where the date is expressed in DD/MM/YYYY format and time is expressed
in
HR/MIN/SEC format based on a 24 hour clock. Measured pressures window 204b
displays
the bladder pressure, peritoneal pressure and ambient pressures in mBar
measured by sensors
104a, 104b and 104d respectively (see FIG. 6A). Version control window 204c
indicates the
firmware version for processor 70, for the motor controller, and the hardware
version of the
- 36 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
implantable device. Patient parameters window 204d displays the patient's
temperature,
respiratory rate, and fluid viscosity. Alarm condition window 204e displays
any changes in
parameters that may indicate the possible development of an infection (block
191 in FIG. 10).
For example, as illustrated, alarm condition window 204e may alert the
physician that the
patient's temperature is abnormally high, so that the physician then may
follow up with the
patient regarding the possibility of infection. In some embodiments, based on
information
displayed in windows 204b, 204d, and/or 204e, the physician may adjust the
operating
parameters of the pump, e.g., using the interface described below with
reference to FIG. 14.
[00109] Turning to FIG. 12, screen display 206 corresponding to selection of
the "Smart
Charger" submenu item in FIG. 11 is described. FIG. 12 includes status area
201 for the
charging and communication system, status area 202 for the implantable device,
menu bar
203, workspace area 204, and navigation panel 205 as discussed above with
respect to FIG.
11. Screen display 206 differs from screen display 200 in that the "Smart
Charger" submenu
item is highlighted, and workspace area 204 displays, for the charging and
control system,
battery status window 207a and version control window 207b. Battery status
window 207a
includes an icon representing the charge remaining in battery 157, and may be
depicted as
full, three-quarters, one-half, one-quarter full or show an alarm that the
battery is nearly
depleted. The time component of window 207a indicates the current time as
received from
handpiece 151, where the date is expressed in DD/MM/YYYY format and time is
expressed
in HR/MIN/SEC format based on a 24 hour clock. Version control window 207b
indicates
the firmware version for processor 152, and the hardware version of the
charging and control
system.
[00110] Referring now to FIG. 13, screen display 208 corresponding to
selection of the
"Download" menu item in FIG. 11 and "Log Files" submenu item is described, and
implements the functionality of block 183 of software 180. FIG. 13 includes
status area 201
for the charging and communication system, status area 202 for the implantable
device, menu
bar 203, workspace area 204, and navigation panel 205, all as discussed above.
Screen
display 208 differs from the "Information" screen display in that the "Log
Files" submenu
item is highlighted, and workspace area 204 displays download progress window
209a and
storage path window 209b. Window 209a includes the path for the directory to
which event
logs may be downloaded from the implantable device via the charging and
communication
system. Window 209a also includes an "Open Download Folder" radio button that
allows the
-37 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
physician to choose the directory path to which the event logs are downloaded,
and a progress
bar that is updated to reflect the amount of data downloaded. Window 209b
includes a radio
button that can be activated to download the event log to the path specified
in window 209a,
and also includes an "Abort" radio button to interrupt the download process.
[00111] FIG. 14 is an exemplary depiction of screen display 210, corresponding
to
selection of the "Pump Settings" menu item in FIG. 11 and "Fluid Transport"
submenu item,
and implements the functionality of blocks 184 and 190 of software 180. FIG.
14 includes
status area 201 for the charging and communication system, status area 202 for
the
implantable device, menu bar 203, workspace area 204, and navigation panel
205, all as
discussed above. Screen display 210 differs from the "Information" screen
displays in that
the "Fluid Transport" submenu item is highlighted, and workspace area 204
includes session
volume window 211a, fluid transport program window 211b, minimum daily volume
window
211c, pressure window 211d, and a radio button in navigation panel 205 that
permits values
entered in windows 211a, 211b and 211d to be transmitted and stored in
nonvolatile memory
71 of the implantable device. Session volume window 211a displays the current
setting for
the maximum daily volume to be pumped by the implantable device, the interval
time
between pumping sessions, the times of the day that the pump may be activated,
the total
daily pump time and the session volume per pumping session.
[00112] The maximum daily volume displayed in window 211a corresponds to the
upper
limit of fluid that the pump will transfer to the bladder in a 24-hour period,
although the
actual volume pumped may be lower if the implantable device detects low fluid
conditions.
This value is based on patient general status and daily ascites production,
and may have an
allowed range, e.g., of 20 ml to 4000 ml. The interval time displayed in
window 211a is
used by the configuration setup routine (block 184 of FIG. 10) to compute the
session
volume, which preferably is in a range of 3 ml to 30 ml, and more preferably
in a range of 10
ml to 20 ml. The time segments that the pump may be active, displayed in
window 211a,
define the timeframes during which the implantable device can actively move
fluid to the
bladder; outside of these time segments, the implantable device will not move
fluid but may
implement the pump tick operation described above to turn the gears on a
regular basis to
prevent clogging of the gears. The daily pump time displayed in window 211a is
shown in
read-only format because it is the aggregate of the time segments entered in
the time
- 38 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
segments boxes. Finally, the session volume displayed in window 211a is
computed by block
183 as the amount of fluid transferred to the bladder in a single pumping
session.
[00113] Fluid transport program window 211b displays the status of the program
controlling operation of the pump of the implantable device based on the
parameters set using
block 184 of software 180. In case pump activity must be stopped for any
reason, the fluid
transport program can be stopped by clicking the "Off" button in window 211b,
which will
cause the Pump to stop pumping until it is manually switched back on. In one
embodiment,
the fluid transport program may switched on again by pressing the "On" button
in window
211b. Because the implantable device preferably is implanted with the pump
turned off, the
physician or surgeon may use window 211b to turn on the fluid transport
program after the
implantable device is first implanted.
[00114] Minimum daily volume window 211c displays the expected amount of fluid
to be
pumped to the bladder by the implantable device, and is computed by the
configuration setup
routine as the session volume times the number of sessions per day, based on
the length of the
prescribed time segments and interval timing input in window 211a.
[00115] Pressure window 211d of FIG. 14 permits the physician to input values
of
maximum bladder pressure and minimum peritoneal pressure that are used to
control
operation of the implantable pump. Thus, for example, processor 70 will
command motor 73
to cease a current pumping session, or to skip a planned pumping session
during the time
segments identified in window 211a, if the bladder pressure detected by the
pressure sensors
exceeds the value specified in window 211d. Likewise, processor 70 will
command motor 73
to cease a current pumping session, or to skip a planned pumping session
during the time
segments identified in window 211a, if the peritoneal pressure detected by the
pressure
sensors is less than the value specified in window 211d. If configured to
operate in the
above-described manner, the implantable device will neither cause patient
discomfort by
overfilling the patient's bladder, nor cause the peritoneal, pleural or
pericardial cavity to
become excessively dry.
[00116] Referring now to FIG. 15, an exemplary depiction of screen display
212,
corresponding to selection of the "Test" menu item in FIG. 11 and "Manual Test
Run"
submenu item is described. FIG. 15 includes status area 201 for the charging
and
communication system, status area 202 for the implantable device, menu bar
203, workspace
- 39 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
area 204, and navigation panel 205, all as discussed above. Screen display 212
differs from
the "Information" screen displays in that the "Manual Test Run" submenu item
is
highlighted, and workspace area 204 includes manual pump cycle window 213.
Manual
pump cycle window 213 includes radio button "Start Test" which transmits a
command to the
implantable device via the charging and communication system to cause
processor 70 to
activate the pump for a predetermined period of time, e.g., a few seconds.
Processor 70
receives positional data from the Hall Effect sensors in motor 73 and measured
pressure data
across pressure sensors 104c and 104d. Processor 70 computes a session volume
and relays
that information via the charging and communication system back to software
10, which
compares the measured data to a target session volume and provides a test
result, e.g.,
percentage of session target volume achieved or pass/fail icon. The measured
session
volume, session target volume and test result are displayed in window 213.
[00117] Although the exemplary embodiment described above relates to a fluid
management system for treating chronic ascites, the fluid management system of
the present
invention may be readily adapted for use in treating pleural or pericardial
effusion. In such
embodiments, it would be advantageous to account for fluctuations in the
pressure in the
pleural or pericardial cavities arising due to respiration or normal cardiac
activity, to avoid
draining all fluid from the cavity and interfering with proper lung function
or cardiac activity.
For a fluid management system intended for treatment of pleural effusion, this
may be
accomplished, for example, by programming processor 70 of the implantable
device to
measure pressure in the pleural cavity over the course of the respiratory
cycle. This
information may then be used to compute a mean pressure that is used to
determine when to
cease pumping fluid from the pleural cavity. Likewise, for a fluid management
system of the
present invention intended for treatment of pericardial effusion, processor 70
of the
implantable device may be programmed to measure pressure in the pericardial
cavity over the
course of the cardiac cycle. This information may then be used to compute a
mean pressure
that is used to determine when to cease pumping fluid from the pericardial
sac, so as to
ensure some fluid remains to lubricate heart motion within the pericardial sac
due to normal
cardiac activity.
[00118] While various illustrative embodiments of the invention are described
above, it
will be apparent to one skilled in the art that various changes and
modifications may be made
- 40 -
CA 02934900 2016-06-22
WO 2015/108782
PCT/US2015/010840
therein without departing from the invention. The appended claims are intended
to cover all
such changes and modifications that fall within the true spirit and scope of
the invention.
-41 -