Note: Descriptions are shown in the official language in which they were submitted.
CA 02934983 2016-06-30
METHODS AND DELIVERY DEVICES USING HERBAL EXTRACTS
TECHNICAL FIELD
The present application relates to methods and devices using herbal extracts,
and
more particularly, to methods of purifying herbal extract(s) from herbaceous
plants with
naturopathic and/or medicinal properties to create delivery products
containing herbal
extracts useful for naturopathic and/or human beneficial purposes.
BACKGROUND
Herbal extracts, otherwise known as botanical medicines, are derived from
naturally
occurring herbaceous plants by extraction from the seeds, berries, stems,
branches, leaves,
bark, roots or flowers or other parts of the plants. Herbal extracts are well
known for
medicinal purposes dating back to ancient Chinese and Egyptian writings.
Herbaceous plants
also constitute a source for development of modern pharmaceutical medicines
and herbal
extract(s) ranging from the development in the late 1800's of aspirin, a
derivative of the silver
willow bark, to the development in the 1980's of paclitaxel, a terpene
derivative of the yew
bush. When used as medicinals, herbal extracts can provide numerous benefits
and can be
used, for example, to treat pain, cancer, muscle spasm, depression, viral and
bacterial
infection, nausea, cardiovascular problems, lung problems, joint and
osteoporosis problems,
blood clots and other physiological problems.
Herbal extracts traditionally are administered by oral, topical, inhalation
and/or
injection methods. Inhalation of vaporized herbal extracts is a common form of
administration. However, the traditional methods do not control dose or timing
of the
delivery. Moreover, traditional methods do not utilize purified extracts so
that the herbal
extracts typically contain plant side products, carcinogenic substances and
other deleterious
plant substances. Furthermore, in some circumstances, the vapors of the
medicinal herb plant
material are inhaled by burning the plant material, in other words by smoking.
The
combustion of the plant material can also release many toxic substances such
as ammonia and
hydrogen cyanide that can cause tissue damage if ingested. Ingestion of foods
laced with
herbal extracts material can also deliver herbal extract(s) to the body.
However, undesirable
materials in the herbal extracts are also ingested and the dosages of the
ingested herbal
extract(s) can be inconsistent and hard to determine.
1
CA 02934983 2016-06-30
Isolation and purification of herbal extract(s) from herbaceous plants can be
of great
interest and benefit to the medical community. A way to purify herbal
extract(s) from
herbaceous plants and convert the purified forms into an easily-ingestible
form and/or to
administer such purified forms or derivatives thereof is desired.
GOALS OF THE INVENTION
There is an opportunity for an herbal extract(s) delivery product that allows
for
inhalation of herbal extract(s) without inhaling other undesirable components
found in raw
herbaceous plants or created by burning the raw plant material. The amount and
purity of
herbal extract(s) in the delivery product can be controlled for dosage. The
delivery product
can be formed using a separation and coating process, as described herein,
that facilitates
controlled deposition of herbal extract(s) onto a substrate to form the
delivery product.
SUMMARY OF THE INVENTION
The present invention is directed to methods for purifying herbal extract(s)
from
herbaceous plant material; providing substrates containing or incorporating
the purified
herbal extract(s); and providing apparatuses for delivery of herbal extract(s)
to patients and
consumers.
In a first aspect of the invention, the method is directed to controlled
volatilization or
wet extraction of the herbal extract(s) from herbaceous plant material, that
is preferably
comminuted, and absorption, adsorption, deposition or otherwise combining the
volatilized or
extracted herbal extract(s) with a substrate. When an individual herbal
extract is obtained by
volatilization, the substrate is held at a temperature to assure capture of
the volatilized herbal
extract by its condensation on the substrate (preferably cooled). When an
individual herbal
extract is obtained by wet extraction, a concentrate of the herbal extract in
solvent is
deposited onto the substrate with evaporation to form a dried layer on the
substrate.
A second aspect of the invention is directed to the substrate with deposited
herbal
extracts. The substrate with herbal extract(s) is constructed and configured
to enable release
of the herbal extract(s) upon controlled heating. This aspect can include
controlled release of
2
CA 02934983 2016-06-30
the herbal extract(s) so as to provide regulated, controlled, limited doses of
herbal extract(s)
over time.
In a third aspect of the invention, the substrate with deposited herbal
extract(s) is
converted into a delivery cartridge. The delivery cartridge can be used with a
controllable
heating element to volatilize or entrain as a vapor or aerosol the herbal
extract(s) and inhale
the vapor or aerosol.
A fourth aspect of the invention is directed to a delivery system which can
include a
delivery cartridge described above. In an example, the delivery cartridge can
include a
cylindrical structure extending in a longitudinal direction and formed from an
electrically
conductive material. The cylindrical structure can include multiple electrodes
extending
laterally across the substrate at respective longitudinal locations. Each of
the electrodes has
an electrical resistance small enough to conduct current laterally along the
substrate without
heating the cylindrical structure. The cylindrical structure can include at
least one substrate
portion extending longitudinally between a respective pair of electrodes. Each
substrate
portion can have an electrical resistance high enough to conduct current
longitudinally
between the electrodes and resistively heat the respective substrate portion
in response to the
current conducted there through. A dose of an herbal extract(s) can be
disposed on each
substrate portion and configured to volatilize into a gas or vapor or entrain
into an aerosol in
response to the resistive heating of the respective substrate portion.
A fifth aspect of the invention is directed to the configuration and
construction of the
herbal extract(s) on the substrate. If multiple extracts are present, they may
be arranged as
overlapping layers or as segregated layers on the substrate. If the layers are
overlapping, they
may be arranged in any order and preferably are arranged with the herbal
extract having the
lowest volatilization or entrainment temperature as the top layer and the
herbal extract having
the highest volatilization or entrainment temperature being the bottom layer
next to the
substrate. With the overlapping arrangement, volatilization or entrainment is
accomplished
by controlling the temperature in increasing stages. With the segregated
arrangement,
volatilization or entrainment is accomplished by a series of heating elements,
each of which
is controlled to produce the appropriate volatilization or entrainment
temperature for the
corresponding herbal extract of the segment.
A sixth aspect is directed to an apparatus and method for producing herbal
extract(s)
from the raw herbaceous plant material and depositing the herbal extract(s) on
the substrate.
The apparatus includes a component for comminution of the herbaceous plant
material, a
3
CA 02934983 2016-06-30
component for controlled heating of the comminuted plant material to
volatilize the herbal
extract, and a cooled substrate on which the volatilized herbal extract is
condensed and
deposited.
A supplement of this aspect includes a cooled transport belt in place of the
substrate.
The volatilized herbal extract is condensed and deposited on the transport
belt. A knife
scraper or other remover apparatus is positioned to remove the herbal extract
from the belt
which is preferably heated so as to place the herbal extract to a liquid
state. A transport
mechanism, preferably heated, deposits the herbal extract appropriately on the
substrate at a
relatively close location. The appropriate deposit of the herbal extract is
preferably
controlled so as to deposit a unit dose of the herbal extract on the
substrate, the apparatus
being capable of continuously preparing substrate pieces with purified extract
having the
dimensions suitable for use in the delivery system.
A seventh aspect is directed to a method for wet extraction of the herbal
extract(s)
from the herbaceous plant material. The herbaceous plant material is
comminuted to provide
very small particles and the particles optionally dried in air to remove water
within the plant
material. The dried particles are combined with a solvent in which the herbal
extract is
soluble and agitated or otherwise mixed to extract into the solvent the herbal
extract and
produce a solution. The solution is filtered, optionally treated with
activated charcoal and
optionally chromatographed or fractionally distilled or optionally
crystallized to further
purify the herbal extract. Either following the optional purification steps or
without use of
these steps, the herbal extract in solvent is concentrated to produce a
concentrate. The
concentrate may be deposited with evaporation on the substrate to form a dried
layer or
coating of herbal extract on the substrate. Optionally the concentrate may be
treated with a
non-solvent for the herbal extract to crystallize the herbal extract or "oil
out" the herbal
extract as an amorphous solid. The solid may be filtered, dried and may be
directly deposited
or otherwise cast on the substrate. The substrate with solid may be optionally
heated to
liquefy or otherwise convert the solid into a contiguous layer of herbal
extract on the
substrate. The so-coated substrate is used as described above to form a
delivery cartridge and
subsequently a delivery system.
This Summary is intended to provide an overview of subject matter of the
present
patent application. It is not intended to provide an exclusive or exhaustive
explanation of the
invention. The Detailed Description is included to provide further information
about the
present patent application.
4
CA 02934983 2016-06-30
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings, which are not necessarily drawn to scale, like numerals may
describe
similar components in different views. Like numerals having different letter
suffixes may
represent different instances of similar components. The drawings illustrate
generally, by
way of example, but not by way of limitation, various embodiments discussed in
the present
document.
FIG. 1A is a side view of an example of a single herbal extract coated
substrate in
accordance with the present invention.
FIG. 1B is a top view of a substrate coated with herbal extract(s) of FIG. 1A.
FIG. IC is a side view of an example of a multilayer herbal extract coating on
a
substrate in accordance with the present invention.
FIG. 1D is a side view of an example of a substrate with a segregated multi-
coating
herbal extract.
FIG. 2 is a block diagram of an example of a process for making a delivery
cartridge
in accordance with the present invention.
FIG. 3 is an example of a heating chamber having a batch substrate coating
process
for creating a coated substrate in accordance with the present invention.
FIG. 4 is an example of a heating chamber having a continuous substrate
coating
process in accordance with the present invention.
FIGS. 5A, B and C are examples of a series of heating chambers for producing a
multi-layered substrate coating process in accordance with the present
invention.
FIG 5D is an example of a substrate with multiple layers of herbal extract(s)
FIGS. 6A and 6B present an example of a heating chamber with transfer cooling
belt,
knife scraper and substrate deposit; and a storage and deposit system for
processing one of
more batches of herbaceous plant material in accordance with the present
invention.
FIG. 7A is an example of a penultimate form of a delivery cartridge formed of
a
substrate with a multilayer coating of herbal extracts and separation spacers
in accordance
with the present invention.
FIG. 7B is an example of a penultimate form of a delivery cartridge with a
segregated
multi segment substrate in accordance with the present invention.
FIG. 7C is an example of an end view of the delivery cartridge formed from the
penultimate cartridge form of FIG 7A.
CA 02934983 2016-06-30
FIG. 8 is a block diagram of an example of a process to construct a delivery
cartridge
having a spirally wound cylindrical shape, in accordance with the present
invention.
FIG. 9 is an example of a delivery cartridge in accordance with the present
invention.
FIG. 10 is an example of a delivery cartridge having multiple overlapping
layers of
coated substrates, in accordance with the present invention.
FIG. 11 is an example of a delivery cartridge having multiple segregated
layers of
coated substrates, in accordance with the present invention.
FIG. 12 is a block diagram of an example of a process to construct a delivery
cartridge in accordance with the present invention.
FIG. 13A is a top view of an example of a polygonal delivery cartridge in
accordance
with the present invention.
FIG. 13B is a perspective view of the polygonal delivery cartridge of FIG.
13A.
FIG. 13C is a side view of the coated substrate of the delivery cartridge of
FIGS. 13A
and 13B prior to forming the polygonal shape.
FIG. 13D is an end view of the, coated substrate of FIG 13C formed into a
polygonal
shape.
FIG. 14 is a block diagram of an example of a process to construct a polygonal
delivery cartridge in accordance with the present invention.
FIG. 15 is an exploded cross-section view of an example of a multi-layer
substrate in
accordance with the present invention.
FIG. 16 is a block diagram of an example of a process used to make a delivery
cartridge having two or more layers, in accordance with the present invention.
FIG. 17 is a perspective view of an example of a delivery cartridge in
combination
with a delivery device, in accordance with the present invention.
FIG. 18 shows an example of a cylindrically rolled sheet, which can be
suitable for
use with a delivery system.
FIG. 19 shows a cross-section of the rolled sheet of FIG. 18.
FIG. 20 shows the cross-section of the rolled sheet from FIG. 19, with the
addition of
an optional plurality of electrically insulating spacers positioned to space
apart adjacent rolls
of the rolled sheet.
FIG. 21 shows another example of a cylindrically rolled sheet.
FIGS. 22 and 23 show examples of a delivery system with control circuit for
providing multi-temperature staged heating of coated substrate.
6
CA 02934983 2016-06-30
FIG. 24 is a side-view schematic drawing of another example of an herbal
extract(s)
delivery system.
FIG. 25 is a schematic drawing of an example of an interface connector for use
with a
vaporizer nebulizer and controller.
FIGS 26A and 26B are schematic drawings of a wet extraction process and device
and
remote multi-storage and deposit unit.
DEFINITIONS
The following terms as used herein according to the invention have the
following
meanings.
The terms "herbs" and "herbaceous plants" in the singular and plural are
understood
to mean all kinds of plants, funguses and algae that can contain or can
produce substances
that have a pharmacological, physiological, beneficial, sensory or other
perceived or un-
noticed but measurable effect on humans. The term herbaceous plant includes
the stems,
seeds, buds, roots, leaves, branches, bark, flowers fruit and all other parts
of a plant.
Preferably, these parts may be selected to provide only the plant part
containing the desired
herbal extract if appropriate. The term "herbaceous plant material" is
understood to mean
comminuted herbaceous plant material unless in context this term describes a
whole plant.
As used herein pursuant to the invention, an "herbal extract" and/or "extract"
are
understood to mean a substance or derivative thereof obtained directly from an
herbaceous
plant or indirectly through synthetic methods applied to such plants and/or
substances. An
herbal extract can be a solid, oil or liquid and can have a pharmacological,
physiological,
beneficial, sensory or other perceived or unnoticed but measurable effect on
humans ( e.g., an
unnoticed but measurable effect may be, but is not limited to, lowering of
blood pressure). In
addition to the popular understanding that an herbal extract is a flavor,
taste and odiferous
substance for use in foods, the term herbal extract(s) and related terms used
herein include
medicinal agents and substances, pharmacological agents and substances, and
chemical
agent, substances and compounds known or derived from any kind of plant,
fungus or algae.
Included also are semi-synthetic derivatives of such substances. The term
"herbal extract(s)"
includes any of the phrases "one or more herbal extracts" an "herbal extract
or extracts", and
herbal extract, in other words, the singular herbal extract and the plural
herbal extracts, i.e.,
multiple herbal extracts
As used herein according to the invention, the terms "volatilize" and/or
"volatilization" are understood to mean vaporization of an herbal extract from
an herbaceous
7
CA 02934983 2016-06-30
plant, which is either a liquid or a solid and is vaporized to a gas or vapor
phase. In an
example, one or more herbal extracts described herein may start as a solid or
an oil and be
heated such that the one or more herbal extracts vaporize. The one or more
herbal extracts
may transition directly from the solid to the gas phase, a sublimation
process, or the one or
more herbal extracts may become a liquid and then vaporize to a gas. In an
example, the one
or more herbal extracts described herein may be in a liquid or solid form
prior to heating.
As used herein according to the invention, the terms "entrain", "entraining"
and/or
"entrainment" are understood to mean formation of a solid-gas mixture such as
a solid-gas
aerosol with air in which a solid, oil or liquid herbal extract is heated to
an extent that it forms
microparticles or micro-droplets of liquid dispersed and/or mixed in a gas
such as air. The
common form of such a dispersion is a particulate-gas aerosol or a liquid
droplet-gas aerosol.
The entrainment does not require the herbal extract to vaporize into a gaseous
state but
instead to form an aerosol.
Miscellaneous Characterizations
The foregoing detailed description includes references to the accompanying
drawings,
which form a part of the detailed description. The drawings show, by way of
illustration,
specific embodiments in which the invention can be practiced. These
embodiments are also
referred to herein as "examples." Such examples can include elements in
addition to those
shown or described. However, the present inventor also contemplates examples
in which
only those elements shown or described are provided. Moreover, the present
inventor also
contemplates examples using any combination or permutation of those elements
shown or
described (or one or more aspects thereof), either with respect to a
particular example (or one
or more aspects thereof), or with respect to other examples (or one or more
aspects thereof)
shown or described herein.
In this document, the terms "a" or "an" are used, as is common in patent
documents,
to include one or more than one, independent of any other instances or usages
of "at least
one" or "one or more." In this document, the term "or" is used to refer to a
nonexclusive or,
such that "A or B" includes "A but not B," "B but not A," and "A and B,"
unless otherwise
indicated. In this document, the terms "including" and "in which" are used as
the plain-
English equivalents of the respective terms "comprising" and "wherein." Also,
in the
following claims, the terms "including" and "comprising" are open-ended, that
is, a system,
device, article, or process that includes elements in addition to those listed
after such a term
in a claim are still deemed to fall within the scope of that claim. Moreover,
in the following
8
CA 02934983 2016-06-30
claims, the terms "first," "second," and "third," etc. are used merely as
labels, and are not
intended to impose numerical requirements on their objects.
The foregoing description is intended to be illustrative, and not restrictive.
For
example, the above-described examples, statements and the embodiments (or one
or more
aspects thereof) may be used in combination with each other. Other embodiments
can be
used, such as by one of ordinary skill in the art upon reviewing the above
description. The
Abstract is provided to allow the reader to quickly ascertain the nature of
the technical
disclosure. It is submitted with the understanding that it will not be used to
interpret or limit
the scope or meaning of the claims. Also, in the above Detailed Description,
various features
may be grouped together to streamline the disclosure. This should not be
interpreted as
intending that an unclaimed disclosed feature is essential to any claim.
Rather, inventive
subject matter may lie in less than all features of a particular disclosed
embodiment. Thus,
the following claims are hereby incorporated into the Detailed Description,
with each claim
standing on its own as a separate embodiment, and it is contemplated that such
embodiments
can be combined with each other in various combinations or permutations. The
scope of the
invention should be determined with reference to the appended claims, along
with the full
scope of equivalents to which such claims are entitled.
DETAILED DESCRIPTION
The present application relates to methods of purifying the herbal extract(s)
from
herbaceous plant material by heating the herbaceous plant material to vaporize
the herbal
extract(s) and condensing the vapor onto a substrate to form a substrate
coated with herbal
extract(s). Alternatively, the herbaceous plant material can be wet extracted
with an
appropriate solvent to produce solution of herbal extract in solvent. The
solution can be
concentrated to produce a concentrate and the concentrate can be deposited on
a substrate and
dried to produce a substrate coated with herbal extract. With either
technique, multiple
overlapping or segregated layers of one or more herbal extracts can be
deposited on the
substrate.
The coated substrates can be converted into various three-dimensional
structures
configured for use as a delivery cartridge. The delivery cartridge can be
heated and air or
inert gas can be passed through the cartridge, thus volatilizing as a vapor or
entraining as an
aerosol the herbal extract(s) in the delivery cartridge such that the user can
inhale the herbal
9
CA 02934983 2016-06-30
extract(s) for a medicinal effect and/or therapeutic effect and/or beneficial
effect. The purity
and ratios of herbal extract(s) in the delivery cartridge can be controlled
based on the desired
composition, and the quantities of herbal extract(s) can be controlled based
on the desired
dosage. Based on the processes used to form the coated substrates, undesirable
components
in the herbaceous plant material are not included in the delivery cartridge.
The delivery
cartridges described herein can be used with various types of delivery devices
to aid in
inhalation of the herbal extract(s)
The delivery cartridge can be a cylindrical structure extending in a
longitudinal
direction and formed from a substrate of an electrically conductive material.
Electrodes can
extend laterally across the substrate at respective longitudinal locations.
The electrodes can
each have an electrical resistance small enough to conduct current laterally
along the
substrate without heating the cylindrical structure. One or more substrate
portions can have
an electrical resistance sufficient to conduct current longitudinally between
the electrodes and
resistively heat the substrate portions. Herbal extract(s) can be disposed on
the substrate
portions and configured to volatilize or aerosolize or entrain in response to
the resistive
heating of the substrate portions. The cylindrical structure or other type of
delivery cartridge
can be used in various types of delivery systems.
With reference to the figures, details of examples and aspects of embodiments
of the
invention are described. The descriptions of the examples and aspects do not
limit the scope
of the invention.
FIGS. 1A and 1B show side and top views of an example of a coated substrate
100 of
the present disclosure. The coated substrate 100 can include a substrate
component 110 onto
which an herbal extract(s) component 120 can be deposited. The coated
substrate 100 can be
exposed to heated air 130, and the herbal extract(s) component 120 can be
volatilized and/or
entrained in the heated air 130 to form a vapor, an aerosol or a gas-
particulate mixture in
which the herbal extract(s) 140 is present. The vapor or aerosol 140 can then
be ingested by
a user to induce a medicinal or therapeutic effect on the user.
FIGS. 1C and 1D respectively show the side views of an example of a substrate
with a
multi-layer overlapping herbal extract coating and an example of a substrate
with a multi-
layer segregated multi-layer herbal extract coating. Substrate 110 is coated
with overlapping
layers (120A, B and C) or segregated layers (120D, E, F and G) respectively.
The layers can
be exposed to air heated in stages to increase the air temperature or can be
electrically heated
CA 02934983 2016-06-30
in stages to raise the temperature of the layers and volatilize or entrain
simultaneously or
sequentially the multiple herbal extracts.
The substrate component 110 can be constructed from any naturally-occurring
material or any man-made material, such as an FDA-approved polymer for the
delivery of
one or more herbal extracts, or any combination of naturally-occurring and/or
man-made
materials. The material selected for the substrate component 110 is inert at
the heating
temperatures described below for forming the coating on the substrate and the
heating
temperatures for later imbibing, inhaling, ingesting or otherwise
administering the one or
more herbal extract(s)components from the coated substrate. In an example, the
substrate
component 110, can include, but is not limited to, materials where the
substrate component
110 can be elastic, flexible, resilient, permanently deformable or plastically
deformable.
For examples including resistive heating of the substrate, the substrate may
be
electrically conductive such as a metal including aluminum or an electrically
conductive
organic polymer such as high temperature polyethylene, polypropylene or
polycarbonate or
polyacrylate or similar polymers preferably doped to make the polymer(s)
electrically
conductive, as well as inorganic (e.g. silicone) polymers. The resistive
potential of the
substrate will be sufficient to generate heat and volatilize or cause
entrainment of the herbal
extract(s).
In an example, the substrate component 110 can assume the form of any three
dimensional structure, including, but not limited to, a sheet, a mesh, or any
combination of
three dimensional structures. Other types of structures can be employed
without departing
from the present subject matter of the invention. In an example, the substrate
component 110
can be a sheet of polymer material. In an example, the substrate component 110
can be a
sheet of aluminum mesh, a sheet of solid aluminum or a combination of both
aluminum mesh
and aluminum sheet. As used herein, the term aluminum can include all grades
of aluminum
and aluminum alloys. Materials suitable for use as the substrate component 110
are also
described below in reference to FIG. 3.
As described further below, the substrate component 110 can be formed into a
variety
of three-dimensional shapes to form a deliver cartridge with herbal
extract(s). In an example,
the delivery cartridge can be designed to maximize the surface area of the
herbal extract(s)
component 120 exposed to the flow of heated air 130. In an example, the
substrate
component 110 can be shaped into forms including, but not limited to, a cone,
a tube or
tubular structure. As used here, a tubular structure can include any structure
with an open
11
CA 02934983 2016-06-30
cross-sectional area shape, a closed cross-sectional area shape, or a
combination of open and
closed cross-sectional area shapes. In an example, the cross-sectional area
shapes can
include, but are not limited to, circles, ovals, ellipses, squares, rectangles
or other polygonal
shapes. In an example, the cross-sectional area shapes can be open or closed
shapes. Other
types of structures can be employed without departing from the present subject
matter.
The herbal extract(s) component 120 can include any substance or agent having
a
pharmaceutical, physiological, medical, beneficial, sensory, perceived or
unperceived but
measurable effect upon a human. The substance or agent may be present in an
herbaceous
plant material or in a semi-synthetic derivative of plant material. In an
example, the herbal
extract component 120 can include one or more active components for medicinal
purposes,
physiological action or therapeutic effect. In an example, the herbal extract
component 120
can include one or more extracts found in herbaceous plant material, including
one or more of
the plant materials such as herbal extracts of herbaceous plants. As discussed
above,
herbaceous plants in the context of the invention include spice and flavor
producing plants,
flowering plants, trees, bushes fungus, algae, medicinal agent plants,
alkaloid producing
plants, complex hydrocarbon producing plants and any kind of plant that has
been found to
contain or produce an organic compound that has a pharmacological,
physiological, beneficial,
naturopathic, sensory or other desired effect on a human. The herbal extracts
may be
polycyclic hydrocarbons, heterocycle compounds, saturated and unsaturated poly-
hydrocarbon
acids and esters, purines, pyrimidines, alkaloids, terpenes, steroidal
compounds such as
budesonide, mometasone or fluticasone, macrocycles, anti-infectives, naturally
occurring
esters, naturally occurring acids, naturally occurring amines, naturally
occurring amides,
naturally occurring Schiff bases and combinations and semi-synthetic
derivatives thereof
These extracts may exhibit such therapeutic or physiologic effects as
bronchodilator,
cardiovascular, antibacterial, anti-infective, anti-viral, mucolytic,
psychological, endocrine,
gastrointestinal, digestive, anti-asthmatic, cardiopulmonary, renal,
urogenital, reproductive,
anti-conceptive, central nervous system, sympathetic and parasympathetic
nervous system
effects, skin, cranial-sinus, and other pharmacological effects. These
therapeutic,
pharmacological and/or physiologic effects are known attributes of the herbal
extracts.
Administration of herbal extracts that are controlled substances such as
opiates and/or have
significant pharmacological and/or physiological effects should be
accomplished only under
the guidance and wisdom of a registered M.D. or D.O. physician, nurse
practitioner or
physician's assistant who is qualified and licensed to prescribe such
substances. In addition,
12
CA 02934983 2016-06-30
purchase and use of such controlled substances made pursuant to aspects of
this invention
should only be made under the supervision and licensure of qualified
pharmacists.
As an example, the herbaceous plants may be selected from the group consisting
of
damiana, blue lotus, mullein, lobelia, peppermint, spearmint, catnip, thyme,
sage, wild dagga,
lavender, rosemary, salvia divinorum, basil, lemon balm, hops, yerba mate,
calea zacatechichi,
chamomile, ashwagandha eucalyptus, passion flower, St John's wart, valerian,
astragalus,
avena sativa, kinnikinnick, cacao , chago, cinnamon, nutmeg, mace, cordyceps,
Don Quai,
Gotu Kola, ginger root, ginseng, green tea, kava, maca, moringa leaf, mullein,
sacred pink
lotus, red raspberry, rhodiola, rooibos, tong kat ali, vanilla, yohimbine,
garlic, turmeric,
nutmeg, capsaicin, rosemary, cannabis, coniferous trees, yew bush, willow
tree, aspen tree,
blood root, opium poppy, atropa belladonna, strychnine, vinca rosea, coffee
plant, cacao tree
and beans (chocolate), coca plant (cocaine), nicotinaa tabacum, camelia
sinensis, monkshood,
castor oil, henbane, calabar bean, digitalis sp, autumn crocus, peyote,
amanita, orange, lemon,
and similar known herbaceous plants in which useful herbal extracts are known
to be present.
Some of these herbal extracts can be obtained commercially as they have
previously been
extracted for the herbaceous plant materials. Still others have been
synthetically derivatized to
form semi-synthetic compounds. The most useful forms of such herbal extracts
and semi-
synthetic compounds are the free base or free acid forms or neutral,
uncomplexed forms.
These forms lend themselves to volatilization and/or molecular entrainment as
vapors and/or
aerosols. The salt forms of bases and acids as well as complexed forms of
neutral compounds
can preferably be converted into the non-salt and/or non-complexed forms for
use according to
the invention.
The extracts of herbal plant material can exist in several parts of the plant
including,
but not limited to, leaves, stem, roots, branches, bark, flower, flower buds
and/or fruit or
seeds. In an example, the herbaceous plant material can include components
such as all of
the foregoing parts of a plant. As used herein, herbaceous plants can refer to
plant material
that has been harvested but is otherwise unprocessed. In an example, the plant
material such
as leaves, stems, bark, branches, seeds, roots, flowers and/or fruit can be
shredded, chopped
or otherwise comminuted to increase the surface area of the material in
preparation for
purification. In an example, the desired herbaceous material can include small
particles
produced by comminution. In an example, the herbal extract can be obtained by
solvent
extraction treatments or volatilization treatments or fractional distillation
treatments of the
comminuted herbaceous material.
13
CA 02934983 2016-06-30
Multiple references are made herein to a starting material of herbaceous plant
material. It is recognized that any herbaceous plant composition can
alternatively be used in
the descriptions and examples below. Some of the processing steps, such as the
separation or
purification step, may vary depending on whether herbaceous plant material or
an alternative
form of an herbal plant material composition is used.
FIG. 2 shows an example of a process 200 that can be used to form a delivery
product
containing herbal extract(s), also referred to herein as a delivery cartridge.
In an example, the
delivery product includes herbal extract(s). In the process 200, a pre-
processing step 210 can
include receiving source material, such as, for example, raw herbaceous plant
material. In an
example, the pre-processing step 210 can include collection of raw herbaceous
material from
growers for use as source material and removal of undesirable organic and
inorganic
components from the source material. In an example, the source material can be
a whole
plant as discussed above or appropriate parts of the plant known to contain
the desired herbal
extract(s).
A first inspection step 220 can include examination of the source material for
general
suitability in the process 200. In an example, source material that is
diseased or not otherwise
of a specified quality can be removed from the source material before further
processing.
A source material preparation step 230 can further prepare the source material
for
later steps in the process 200. In an example, the source material preparation
step 230 can
include the use of equipment and methods to increase the surface area of the
source material,
such as by shredding, chopping or otherwise comminuting, to aid in a
purification process.
A second inspection step 240 can include examination of source material to
ensure
that the source material has been suitably processed. In an example, source
material that has
been improperly shredded or chopped may be rejected or redirected for further
processing.
A purification and coating step 250 can include a process for separating the
herbal
extracts component 120 of FIG. 1 from the herbaceous plants. The purification
in step 250
can include heating the pre-processed plant material to volatilize the herbal
extract(s).
Specific steps can depend on the form of the herbaceous plant material. Under
step 250, the
volatilized herbal extract(s) can then be condensed onto a carrier material to
form a substrate
coated with the herbal extract(s). In an example, the condensation of
volatilized herbal
extract(s) on a carrier material such as a substrate or on a cooled transport
device can be
accomplished through cooled absorption or cooled adsorption of the volatilized
herbal
extract. The purification and coating step can also be practiced multiple
times or
14
CA 02934983 2016-06-30
simultaneously practiced with multiple apparatuses to produce multiple herbal
extracts
deposited as overlapping layers or segregated layers on the substrate.
Alternatively, the purification and coating step 250 can include an extractive
process
for separating the herbal extract from the herbaceous plants. The appropriate
plant material,
e.g., the whole plant or selected plant parts such as flowers, seeds, buds,
leaves, stems,
branches, bark and/or roots, may be comminuted into very small particles. The
small plant
particles may be extracted with a solvent in which the desired herb extract is
soluble to form
an extract solution. In some examples, the solvent may be water while in
others it may be
ethyl alcohol, chloroform, supercritical carbon dioxide or a hydrocarbon. The
extract
solution may be decolorized with activated charcoal and/or further purified by
column
chromatography on diatomaceous earth or silica gel or other suitable known
chromatographic
support material, for example. The purified extract solution may be
concentrated by
substantial but not complete evaporation of the solvent to form a concentrate.
The
concentrate may be parsed onto the substrate and the remaining solvent
evaporated to deposit
the purified herbal extract on the substrate. Also, if the herbal extract is
commercially
available, it may be purchased in purified form and formulated in a minimum
amount of
appropriate solvent to form a concentrate as discussed above. The subsequent
steps to form
the purified herbal extract on the substrate may be carried out as described
above. If multiple
overlain layers of herbal extract are to be formed, subsequent layers may be
deposited on top
of previous layers by flash evaporation. As the subsequent concentrate is laid
down over a
previous layer, a flow of air or inert gas at a temperature to instantly
evaporate the solvent is
applied. The result is deposition of dry herbal extract and avoidance of
comingling of the
various layers that might result from solvent dissolution.
A third inspection step 260 can include examination of substrate coated with
the
herbal extract(s) for coating uniformity or other predetermined parameters.
A first post-processing step 270 can include identification and handling of
the
substrate coated with one or more herbal extracts. In an example, the coated
substrate with
herbal extract(s) can be marked or labeled for quality assurance and material
handling
purposes, such as delivery to inventory of the substrate coated with one or
more herbal
extracts. In an example, steps 260 and 270 can be skipped and the coated
substrate from step
250 can go directly to step 280 for converting.
A conversion step 280 can include transforming the coated substrate with
herbal
extract(s) into forms convenient for consumption by an individual user. In an
example, the
CA 02934983 2016-06-30
conversion step 280 can include converting the substrate coated with herbal
extract(s) into
segments and forming the segments into delivery products or cartridges. In an
example, the
cartridge is constructed to maximize the volatilization or entrainment surface
area of the
coated substrate while minimizing packaging volume of the cartridge. In an
example, the
cartridge can be of a generally tubular form and assume any cross-sectional
shape without
altering the effect of the cartridge. In an example, the cross-section shape
can include, but is
not limited to, a circle, a square, a hexagon, a polygon or any symmetric or
non-symmetric
cross-sectional profile. Other types of shapes can be employed without
departing from the
present subject matter.
A fourth inspection step 285 can include examination of the cartridges to
ensure that
the cartridges have been suitably processed. In an example, the fourth
inspection step 285
can include examination of the user shapes for visual uniformity or other
parameters.
A second post-processing step 290 can include packaging and labeling of the
cartridges. In an example, each cartridge can be wrapped as an individual
unit. In an
example, individual units can be labeled for quality assurance and
governmental tax
purposes.
In an example, all the aforementioned steps of the process 200 can be subject
to
standard manufacturing control techniques.
FIG. 3 shows an example of a heating chamber 300 of the present disclosure for
use
in a single sheet substrate coating process when the herbal extract can be
volatilized from the
herbaceous plant material. The heating chamber 300 can include a container box
310 and a
container cover 320 that can be removably attached to the container box 310.
The container
box 310 can include an interior surface 312, an exterior surface 314 and a
controlled heat
source 316 located along an interior surface 312 of the container box 310. A
removable tray
330 to contain a source material (plant material) 332 can be located against
an interior surface
312 of the container box 310. A removable screen 318 can be located in the
container box
310 between the removable tray 330 and the container cover 320 to contain
source material
332.
The container cover 320 can include a hinge 326 to attach the container cover
320 to
the container box 310 and a cooling bar 322 to which a substrate 324 can be
located in close
proximity or removably attached. In an example, the substrate 324 can be
removably
attached to the cooling bar 322 with clips or similar attachment aids.
16
CA 02934983 2016-06-30
The substrate 324 can be covered with a coating 328 of herbal extract(s)
using, for
example, a heating process. In an example, herbal extract(s) can be deposited
on the
substrate by sequential processing of the appropriate herbaceous plant
materials. The
depositions may overlay the respective multiple extract layers on top of each
other or may
segregate the layers on the substrate. The controlled heat source 316 can be
initiated to heat
the source material 332 to a selected temperature. Depending on the selected
temperature,
herbal extract(s) can volatilize from the source material 332. The substrate
324 can be cooled
through conduction (when in contact with the cooling bar 322) or radiation
(when located in
close proximity to the cooling bar 322) and the vapors generated during the
heating process
can condense onto the substrate 324 to form a coating 328 on the substrate
324. In an
example, the herbal extract(s) can be absorbed and/or adsorbed within and/or
on the substrate
324. In an example, the herbal extract(s) can be adsorbed onto the surface of
the substrate
324 so as to produce a substrate coated with herbal extract(s). As used
herein, a coated
substrate 334 can refer to a combination of the substrate 324 and the coating
328 formed
thereon.
In an example, the heating chamber 300 can be used to volatilize herbal
extract(s) in
the herbaceous plant material. Using the steps above, the desirable
components, i.e., one or
more herbal extracts, can be sequentially extracted and purified from the
herbaceous plant
material by controlling the temperature in the heating chamber and
sequentially adding
appropriate herbaceous plant material containing the desired herbal extracts.
As described
further below, various one or more substrates coated with herbal extracts can
be formed that
have one or more herbal extracts, in purified form, and contain minimal to no
undesirable
components.
The volatilization of the herbal extract in the heating chamber 300 can be
based on the
known volatilization temperature of particular herbal extract desired.
Depending on a
temperature that the herbaceous plant material is heated to, herbal extract(s)
can be
volatilized if more than one is present in the composition. Typically, the
temperature of the
heating chamber may approach the known volatilization temperature of the
desired herbal
extract. However, maintaining a slightly, to somewhat, lower chamber
temperature can be
utilized to assure primary production of the desired herbal extract in
substantially purified
form. Use of the partial vapor pressure of the herbal extract at a temperature
below its
volatilization temperature can be practiced to assure at least in part the
production of
substantially purified herbal extract.
17
CA 02934983 2016-06-30
Care is practiced to avoid combustion of the cellulosic and other materials of
the
herbaceous plant composition. While not a required condition for
volatilization and
deposition, applying a vacuum to the heating chamber can facilitate
volatilization of the
herbal extract at lower temperatures while not also producing undesirable
substances or
causing combustion. Preferably, the heating with or without partial vacuum is
conducted
under an inert atmosphere, such as a nitrogen or argon atmosphere. This aspect
also is
helpful for avoidance of plant combustion.
Water is almost always present in such plant material. Consequently, the
herbaceous
plant material can be pre-dried at ambient to slightly elevated temperatures
to remove water.
In general, the temperatures at which each of herbal extract(s) can volatilize
relative
to their known volatilization temperatures in isolated, pure states are not
necessarily precisely
known and can depend, for example, on the surrounding conditions, the degree
of
comminution, the pre-drying removal of water and the particular plant part
containing the
herbal extract. The heating chambers described above can be used to heat the
herbal extracts-
containing composition to any given temperature. The particular temperature or
temperature
range selected can depend on multiple factors, including, for example, a
particular
composition of the raw herbal extracts or the desired composition of the
coated substrate.
Samples of the deposited or cooled vapors can be collected, at all or some of
the temperature
intervals, to analyze the fractions and determine the composition of the
coating. Based on the
results, the temperature range sufficient for volatilization can be determined
or adjusted based
on the desired composition of the coating. It is recognized that the
temperature range can
depend on the starting material and how tightly the composition of the coating
is to be
controlled. The composition of the starting material can vary from batch to
batch and can
depend, for example, on where and how the raw herbaceous plants are grown, and
cleaning of
the raw herbaceous plants or other preparation steps, prior to processing.
Practice and variation of these parameters are well within the ordinary and
routine
skill of a chemical technician to achieve the desired volatilization. The
following discussion
examines these parameters.
A composition of the coated substrate 334, including a purity of the herbal
extract(s)
can be a function of the source material used in the heating process. In an
example, the grade
of herbaceous plant used as the source material, such as the species and
source of supply, can
influence the composition of the coated substrate 334, including varying
levels of one or
more herbal extracts. In an example, the pre-processing of the source
material, such as the
18
CA 02934983 2016-06-30
size of particle resulting from shredding and chopping of the source material,
can influence
the composition of the coated substrate 334. In an example, sampling can be
performed on
the source material to determine a composition of the source material.
Specification
parameters and standard processing control can be implemented for monitoring
and
controlling the composition of the source material and the coated substrate
334.
The composition of the coated substrate 334 can be a function of the control
parameters used in the heating process. In an example, the temperature and
partial pressure
(i.e., partial vacuum) of the chamber, the total time the source material is
exposed to the
temperature of the chamber and the temperature of the cooling bar 324 can
influence the
coated substrate 334. In an example, these and other process parameters can be
under
standard processing control.
The substrate 324 can be constructed from any naturally-occurring material or
any
man-made material, such as an FDA-approved polymer for the delivery of one or
more herbal
extracts, or any combination of naturally-occurring or man-made materials.
The substrate 324 can be a pharmaceutically acceptable material or combination
of
materials, including natural and/or synthetic materials, which can capture the
one or more
herbal extracts. In an example, pharmaceutically acceptable materials for the
substrate can
include, but are not limited to, cellulosic materials, synthetically altered
cellulosic materials,
synthetic polymers, natural polymers or any material approved for
pharmaceutical use by the
United States Food and Drug Administration (FDA). In an example, the materials
can be
porous, micro-porous, adsorptive or absorptive or include a combination of
adsorptive and
absorptive properties. In an example, the substrate can be stable and non-
degrading at
temperatures well above the volatilization temperatures of one or more herbal
extracts. In an
example, the substrate 324 can comprise an aluminum or aluminum alloy. If a
substrate is to
be designed as an electrically conductive synthetic or natural organic or
inorganic polymer, it
will include a feature providing the ability to conduct electricity. Such
electrically
conductive polymers are well-known.
FIG. 4 shows an example of a heating chamber 400 of the present disclosure for
use
in a continuous sheet substrate coating process. The heating chamber 400 can
include many
of the same elements as the heating chamber 300 of FIG. 3, but instead of
being a batch
process, it can include additional features to enable a continuous process.
The container
cover 420 can include a roller take-up mechanism 424. In an example, the
roller take-up
mechanism 424 can include a source spool mechanism 425, a receiving spool
mechanism 426
19
CA 02934983 2016-06-30
and a flexible substrate 427 extending from the source spool mechanism 425 to
the receiving
spool mechanism 426 and located in close proximity to the cooling bar 422. In
an example,
the source spool mechanism 425 can include a spindle and bearings to support
the source
spool and a motor attached to the source spool for tensioning of the flexible
substrate 427. In
an example, the receiving spool mechanism 426 can include a spindle and
bearings to support
the receiving spool and a motor attached to the receiving spool to draw the
flexible substrate
427 across the cooling bar 422. During the heating process, the receiving
spool mechanism
426 can draw the flexible substrate 427 across the cooling bar 422 so that the
herbal extract(s)
condense on one side of the flexible substrate 427 to form a continuous
coating 432 on the
flexible substrate 427.
In an example, the roller take-up mechanism 424 can be controlled to perform
continuous deposition processing of the flexible substrate 427. In an example,
the roller take-
up mechanism 424 can be controlled to perform multi-batch deposition
processing of the
flexible substrate 427. Other designs can be used as an alternative to or in
addition to the
mechanisms 424 and 426 for enabling a continuous process.
FIGS. 5A, 5B and 5C show an exemplary series of heating chambers 500, 501 and
502 of the present disclosure for use in preparation of a substrate with a
multiple layer
coating of different herbal extracts. The heating chambers 500, 501 and 502
can be separate
chambers arranged in a tandem order or can be a single chamber which is
replenished with
different herbaceous materials as feed stocks and which serve to convert
chamber 500 to
chamber 501 and hence into chamber 502. Heating chambers 500, 501 and 502 can
include
many of the same elements as the heating chambers 300 and 400 of FIGS. 3 and
4,
respectively. When operated in tandem, the substrate 527 can be arranged on a
continuous
belt and roller as depicted in FIG 4. The continuous belt and roller will
extend through
chambers 500, 501 and 502 with the outtake roller being positioned before
chamber 500 and
the uptake roller being positioned after chamber 502. Alternatively, a single
chamber 500
can be employed and the herbaceous plant material changed to provide an
operational
configuration of chambers 501 and 502. In an example, flexible substrate 527
is coated in
either a multi-batch or continuous deposition process with a first coat 531 of
herbal extract
from volatilization of herbaceous material 530 as shown in FIG 5A. The chamber
500 is
refilled with herbaceous material 540 so as to provide chamber 501.
Alternatively, a second,
separate chamber 501 is employed in this step as shown by FIG 5B. A second
coating of
herbal extract 541 is applied to the substrate with coating 531 by
volatilization and cooling as
CA 02934983 2016-06-30
depicted in FIG 5B. The result is a substrate with coatings 531 and 541. In a
third step as
shown in FIG 5C the original chamber is replenished with herbaceous material
550 so as to
provide chamber 502, or a third separate chamber 502 is used. Through
volatilization and
cooling, a third coating of herbal extract 551 is applied so as to produce a
substrate with
multiple coatings 531, 541 and 551. The substrate coated with three herbaceous
extracts 531,
541 and 551 is shown in FIG 5D.
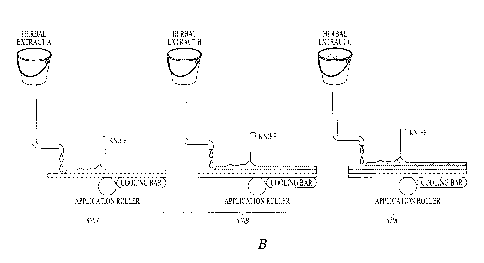
FIG. 6A shows an exemplary heating chamber 600 of the present disclosure for
use in
a continuous substrate coating process with a continuous source material feed
system and a
heated, remote storage and coating subsystem. The remote coating subsystem
enables the use
of a single heating chamber and a multiple number of storage and coating
subsystems. The
overall system enables large runs of herbaceous plant material and multiple
coatings of batch
substrates or continuous substrates. In an example, a screw conveyor 660 can
move
herbaceous plant material 634 into the feed hopper 610 for heating and
volatilization. A
screw conveyor 660 moves the herbaceous material 634 into the heating chamber
600. The
screw conveyer can also be used to remove spent herbaceous plant material from
chamber
600 and dispose it into a waste hopper (not shown). Multiple feed hoppers can
be connected
to a single delivery chute leading to the screw conveyer. Each hopper can
contain a different
herbaceous plant material and the release of each into the screw conveyer can
be controlled
by hopper outlet valves. In this fashion multiple herbaceous materials can be
delivered to the
single heating chamber so as to produce multiple herbal extracts. Each
volatilized herbal
extract is collected on a continuous belt 641 cooled by cooling bar 640. The
belt with
solidified herbal extract moves out of the heating chamber 600 and is
subsequently warmed
by heat source 642. Stripper 643 or a similar device causes the warmed herbal
extract to pass
into storage hopper 650. Storage hopper can be cooled or heated by
heater/cooler 652
depending on whether the herbal extract is to be stored in hopper 650 or is to
be transported
through tube 651 to the remote coating subsystem 670. As shown in FIG 6B, a
remote series
of coating subsystems 670a, 670b and 670c can be employed to provide
substrates with
multiple layers of herbal extracts or a series of substrates each with a
single different herbal
extract. The coating subsystem includes storage hoppers 650 with transport
tubes 651 and
heater coolers 652. The system can be arranged on a rotating platform
synchronized with the
continuous belt 641 and stripper 643 so that a series of herbal extracts can
be collected, stored
and sequentially applied to a substrate. To form a substrate with one or more
layers of herbal
extract, storage hopper 650 is heated and the liquid herbal extract caused to
flow through tube
21
CA 02934983 2016-06-30
651 to the remote coating subsystem 670. The flowable herbal extract is
deposited on
substrate 671, is leveled by a leveling means such as a knife edge or a
curtain of air or by
gravity optionally in combination with movement of substrate 671... The
leveled herbal
extract layer is cooled by direct or indirect contact with a cooler or by cold
air. A deposition
of subsequent layers of different herbal extracts contained in additional
storage hoppers 650
can be accomplished by the same process to produce a multi-coated substrate.
The same
process and coating subsystem can be employed to produce and store multiple
herbal extracts
and coat substrates with single coatings of different herbal extracts.
In an example, any of the heating chambers described above can be part of a
mobile
process such that the purification and coating processes can be done at or
near the origin of
the source material. In an example in which the source material is raw
herbaceous plant
material, the purification and coating processes can be contained or stored
within a
transportation device such that these steps can be performed at or near where
the raw herbal
extracts is grown.
In an example, a batch process similar to the heating chamber 300 of FIG. 3
can be
used to sample source material and determine its composition, to determine,
for example,
levels of herbal extract(s) in the source material.
The heating chambers and processes described above in reference to FIGS. 3-6
are an
example of a separation process for separating one or more components from the
herbaceous
plant composition. Other known processes may be used, such as, for example, a
wet
extraction process or a fractional distillation process. The particular
process used for
separating the desired components from the source material can depend, in
part, on the
composition and form (solid, liquid, etc.) of the source material, the nature
of the herbal
extract(s) desired, the volume of coated substrate to be produced, the time
for production,
technical expertise of the users, equipment availability and budget, and the
cost of
implementation.
Alternatively, a wet extraction method can be used to obtain the herbal
extract(s). In
an example, an herbaceous plant material containing an herbal extract, such as
but not limited
to an alkaloid such as sanguinarine (blood root) or digitalis (digitalin and
digitoxin from
foxglove) can be comminuted to small particles and dried to remove water.
Combining the
dried herbaceous plant material with an appropriate solvent such as ethyl
alcohol or
chloroform or water extracts the herbal extract from the dried plant material
and produces an
herbal extract solution.
22
CA 02934983 2016-06-30
Optionally, treating the herbal extract solution with activated charcoal to
decolorize,
and optionally recrystallizing by addition of water followed by filtration,
can be employed to
produce purified herbal extract as an oil or solid.
Either the herbal extract solution can be concentrated by vacuum evaporation
of a
substantial amount of solvent to produce a concentrate or the purified herbal
extract can be
redissolved in a minimum amount solvent to produce a concentrate. The
concentrate can be
deposited as a layer of concentrate on a substrate.
The deposit can be accomplished by a batchwise technique involving placing the
substrate in a catch pan with sides slightly higher than the side edges of the
substrate. The
catch pan and substrate can be sized to enable subsequent division of the
coated substrate into
dose calculated strips. The concentrate can be deposited onto the substrate in
the catch pan
that holds the concentrate at appropriate depth on top of the substrate. The
concentrate can
be flash evaporated by placing the catch pan with concentrate and substrate
into a vacuum
chamber and applying a vacuum with slightly elevated temperature. This
technique will flash
off the solvent while not volatilizing or subliming the herbal extract to any
detectable degree.
The coated substrate can be cut or otherwise divided along predetermined lines
to
produce the unit dosage forms of substrate coated with herbal extract. As
explained above,
the coated substrate can be configured for use in a hot air delivery cartridge
or configured
with electric heating elements and flowing ambient air to entrain the herbal
extract molecules
in a micro-aerosol or solid particulate-gas mixture or volatize the molecules
into a vapor for
inhalation delivery to the lungs.
Administration of herbal extracts such as for example medicinal alkaloids such
as
sanguinarine (anti-bacterial) or digitalis (cardiovascular) and other
medicinal herbal extracts
by inhalation into the lungs is an effective method for rapid and precise
delivery of the
medicinal to the organ or organs in need. This technique avoids transport of
the medicinal
through the gut-blood barrier and its passage through the hepatic route, both
of which can
cause degradation of the medicinal.
In an example, the purification and coating processes described above can
include re-
processing the coated substrate or transport by heating the coated substrate
such that the
herbal extract(s) on the coated substrate or transport belt are re-vaporized
and then condensed
onto a new substrate. This can be used to further purify the herbal extract(s)
in the coated
substrate and can be repeated until a desired purity of the at least one of
herbal extract(s) is
achieved.
23
CA 02934983 2016-06-30
An amount of the herbal extract(s) components in or on the coated substrates
can be
determined as part of the process for forming the coated substrate and the
delivery cartridges
described below. As described above, process control methods can be
implemented to
control, for example, a thickness of the coating on the substrate. Based on
sampling of the
source material, a composition of the coating on the substrate can also be
determined. Other
known techniques can be used to determine a composition of the coating on the
substrate. As
such, an amount of the herbal extract(s) component can be determined per unit
area of the
coated substrate. This can be used to determine an appropriate surface area of
the delivery
cartridge if there is a specified amount of the herbal extract(s) in the
delivery cartridge.
Similarly, if the surface area of the delivery cartridge is specified, the
thickness of the coating
on the substrate can be adjusted in order to meet a specified amount of the
herbal extract(s) in
the delivery cartridge. The methods described herein for forming the coated
substrates and
the delivery cartridges can be used to effectively and accurately determine a
composition and
amount of the herbal extract(s) which can be used for dosage control.
Coated substrates as described herein containing herbal extract(s) can be used
to form
a three-dimensional structure configured for use as a delivery product. In an
example, a
coated substrate can be used as a delivery cartridge in a delivery device. As
used herein, a
delivery cartridge can refer to a replaceable element in a delivery system
that is slowly
depleted of herbal extract(s) as a consequence of continued use or intervals
of use. The
delivery cartridge can be replaced for continued use of the delivery system.
In an example,
delivery cartridges can be designed to maximize surface area exposed to an air
flow while
minimizing package volume.
Coated substrates can take many structural forms. In an example, coated
substrates
can include, but are not limited to, cubes, cones, parallelenebulizerds, or
other three-
dimensional shapes. In an example, a coated substrate can be in the form of a
sheet. As used
herein, a sheet can be any three-dimensional structure defined by a first
dimension, a second
dimension and a third dimension where the first dimension is much smaller than
the second
and third dimensions. In an example, a sheet can be generally rectangular in
shape with a
first end and a second end opposite the first end.
FIG. 7A shows an example of a coated substrate 700 of the present disclosure
which
can be formed using the techniques described above or generally known in the
art, examples
of which are described above for extracting and purifying herbal extract(s)
and coating the
herbal extract(s) on a substrate. The substrate coated with herbal extract(s)
700 can include a
24
CA 02934983 2016-06-30
substrate component 710, and herbal extract(s) 720 coated on the substrate
component 710
and spacers 722 located on the substrate component 710 or the herbal
extract(s) component
720. In an example, the spacers 722 can be located on the substrate component
710 before
the substrate component 710 is coated. In an example, the spacers 722 can be
located on the
herbal extract(s) component 720 after the substrate component 710 is coated.
FIG. 7B shows an example where the substrate coated with herbal extract(s) 700
can
be converted into a three-dimensional structure configured for use as a
delivery cartridge 702.
In an example, the substrate coated with herbal extract(s) 700 can be rolled
into a spirally
wound cylindrical shape to form the delivery cartridge 702. In an example, the
plurality of
spacers 722 can be used as a structural element to maintain channel(s) 724
between layers of
the delivery cartridge 702 to allow for the passage of heated air. The
delivery cartridge 702
can include any number of layers.
The delivery cartridge 702 can be used with a delivery device, an example of
which is
described below and shown in FIG. 17. In an example, the delivery device can
include, but is
not limited to a vaporizer, an e-cigarette device, a nebulizer or an aerosol
device.
Alternatively, the delivery cartridge 702 can be used by directly applying
heated air to the
delivery cartridge 702 to volatilize or entrain molecules of the herbal
extract(s) from the
delivery cartridge 702. In an example, heated air can be directly applied to
the delivery
cartridge 702 by any heating process or heating device that can include, but
is not limited to,
a vaporizer, an e-cigarette device, a nebulizer or an aerosol device In an
example, heated air
can be directed through the channel 724 to volatilize, and/or entrain as a
solid-gas aerosol,
molecules of the herbal extract(s) from the delivery cartridge 702.
FIG. 8 shows a flow chart of an exemplary process to construct a spirally
wound
cylindrical shape, similar to the cartridge 702 of FIG. 7B. In an example,
step 810 can
include providing a supply of herbaceous plant material; step 820 can include
heating the
herbaceous plant material to a first temperature to release a first vapor or
wet extraction to
produce a solution; step 830 can include condensing the first vapor or
depositing and
evaporating the solution onto a substrate to create a coated substrate; step
840 can include
placing spacers on the coated substrate to allow for airflow through the
cartridge; step 850
can include rolling the coated substrate to form a spirally-wound cylindrical
shape configured
for use as a delivery cartridge.
FIG. 9 shows an example of a coated substrate shaped in a saw-tooth, zig-zag,
or
accordion configuration. In an example, the saw-tooth coated substrate 900
includes a first
CA 02934983 2016-06-30
coating 910 where the first coating 910 can be one of one or more herbal
extracts. In an
example, the saw-tooth coated substrate 900 includes a second coating 920
where the coating
920 can be another of the herbal extract(s)
FIG. 10 shows an example of a two-substrate assembly 1070 where a first saw-
tooth
coated substrate 1035 and a second saw-tooth coated substrate 1045 can be
stacked for use as
delivery cartridge. In an example, a plurality of spacers 1022 can be used as
structural
elements to maintain a plurality of channels 1024 between the first saw-tooth
coated substrate
1035 and the second saw-tooth coated substrate 1045 to allow for the passage
of heated air.
In an example, the two-substrate assembly 1070 can be stacked so that the
first coating 1010
of the first saw-tooth coated substrate 1035 can face the second coating 1020
of the second
saw-tooth coated substrate 1045. In an example, a plurality of two substrate
assembly 1070
can be stacked for use as a delivery cartridge.
FIG. 11 shows an example of a two-substrate assembly 1170 where the first
coating
1110 of a first saw-tooth coated substrate 1135 can face the first coating
1110 of a second
saw-tooth coated substrate 1145. In an example, a plurality of two-substrate
assembly 1170
can be stacked for use as a delivery cartridge.
FIG. 12 shows an example of a process to construct a saw-toothed delivery
cartridge.
In an example, step 1210 can include providing a supply of herbaceous plant
material; step
1220 can include heating the herbaceous plant material to a first temperature
to release a first
vapor or wet extracting a first extract to form a solution; step 1230 can
include condensing
the first vapor or depositing and evaporating the solution onto a first side
of a substrate; step
1240 can include heating a second herbaceous plant material to a second
temperature to
release a second vapor or performing a second wet extraction step; step 1250
can include
condensing the second vapor or depositing and evaporating the second solution
onto a second
side of the substrate; step 1260 can include creating a plurality of notches
in the coated
substrate; step 1270 can include articulating the segments to form a saw-tooth
pattern and
step 1280 can include stacking the substrate for use as a delivery cartridge.
The process of
FIG. 12 can be modified to incorporate the multiple substrate assemblies shown
in FIGS. 10
and 11.
FIGS. 13A and 13B show top and side views, respectively, of an example of a
polygonal delivery cartridge 1300. In an example, the cross-sectional shape of
the polygonal
delivery cartridge can include, but is not limited to, a three-side cross-
section, a four-sided
26
CA 02934983 2016-06-30
cross-section or an "n"-sided cross-section where "n" can be any number equal
to or greater
than 3.
FIG. 13C shows notches 1370 formed in the substrate 1310 and the coating 1320
that
can allow a segment 1375 to articulate with respect to an adjacent segment
1375. As used
herein, a segment 1375 is the portion of the substrate 1310 and coating 1320
located between
two notches 1370.
FIG. 14 shows an example of a process to construct a closed polygonal shaped
delivery cartridge similar to the star-shaped cartridge 1300 of FIG. 13. In an
example, step
1410 can include providing a supply of herbaceous plant material; step 1420
can include
heating the herbaceous plant material to a first temperature to release a
first vapor or wet
extraction to produce a first solution; step 1430 can include condensing the
first vapor or
depositing and evaporating the first solution onto a substrate to create a
coated substrate; step
1440 can include creating a plurality of notches and step 1450 can include
articulating the
segments to form a saw-tooth pattern; and step 1460 can include connecting the
first end to
the second end to form a polygonal shape. In an example, step 1460 can include
manipulating the segments to align the segments in a desired orientation
relative to one
another.
Other shapes can be used for a delivery cartridge. Any of the examples
described and
shown in FIGS. 7, 9, 10, 11 and 13A-13C can include additional layers of
substrate and each
layer of substrate can include one or more coating layers. As stated above in
reference to
FIG. 7B, the delivery cartridges described herein can be used alone or in
combination with a
delivery device. Each delivery cartridge can be designed such that heated air
can be passed
through the cartridge and one or more herbal extract(s) can be volatilized or
entrained and
inhaled by a user.
Dimensions of any of the delivery cartridges described herein can depend, in
part, on
whether a delivery device is intended to be used with the cartridge and a
particular design of
the delivery device. These dimensions can include a length, width and overall
shape of the
delivery cartridge and can depend on the length and width of the coated
substrate used to
form the delivery cartridge. The dimensions of the delivery cartridge can also
depend, in
part, on an amount of the herbal extract(s) in the delivery cartridge and an
intended dosage of
the herbal extract(s).
FIG 15 shows an exploded view of an example of an assembly 1500 comprising
multiple layers of coated substrates. In an example, an herbal extract layer
1550 can include
27
CA 02934983 2016-06-30
a substrate 1552 with a first surface and a second surface where a first
herbal extract coating
1556 can be applied to the first surface and a second herbal extract coating
1557 can be
applied to the second surface. In an example, a taste layer 1560 can include a
substrate 1562
having a taste coating 1566 applied to the substrate 1562 to enhance or mask
the user
sensation as some but not all herbal extracts have bitter and/or unpleasing
tastes and/or odors.
In an example, the taste coating 1566 can include a flavoring that can
include, but is not
limited to, fresh mint and/or a volatile sweetening agent such as sorbitol. In
an example, an
adjuvant layer 1570 can include a substrate 1572 having an adjuvant coating
1576 applied to
the substrate 1572 where the adjuvant coating 1576 can include at least a
second compound
that can augment the therapeutic effect of the herbal extract. In an example,
the second
compound can include, for example, a volatizable adjuvant for medicinal
agents, the adjuvant
being, for example, paraffin oil or squalene. In an example, an amelioration
layer 1580 can
include a substrate 1582 having an amelioration coating 1586 applied to the
substrate 1582
where the amelioration coating 1586 can include at least a third compound that
can minimize
any undesirable side effects of herbal extracts, if applicable. In an example,
the active herbal
extracts layer 1550, the taste layer 1560, the adjuvant layer 1570 and the
amelioration layer
1580 can be assembled together or in any permutation. In a further example
incorporating
any or all of the foregoing features of the coatings applied to the substrate,
an herbal extract
applied as first or second coating 1556 or 1557 respectively to the first or
second surfaces of
substrate 1552 may be one or more cannabis extracts such as THC and/or CBD or
may be
one of the herbal extracts delineated in the foregoing list of extracts.
Preferably, the coatings
may be a combination of cannabis extracts with one of the other herbal
extracts. More
preferably, the coatings may be a combination of cannabis extracts and an
adjuvant or
flavoring. In an example, the assembly 1500 can be converted into a three-
dimensional
structure for use as a delivery cartridge as described above. In other
examples, an assembly
can include any number and combination of layers depending on desired
properties of the
assembly. In an example, spacers similar to the spacers 722 shown in FIGS. 7A
and 7B can
be placed between each layer prior to forming the three-dimensional structure
to allow for the
passage of air between the layers.
FIG. 16 shows an example of a process used to make a delivery cartridge where
the
coated substrate includes two or more layers where at least one provides
flavor or adjuvant.
In an example, step 1610 can include providing a supply of herbaceous plant
material; step
1620 can include heating or wet extracting the herbaceous plant material to
release a first
28
CA 02934983 2016-06-30
vapor or produce a first solution; step 1630 can include condensing the first
vapor or
depositing and drying the first solution onto a substrate to create a coated
substrate; step 1640
can include attaching one or more layers to the coated substrate where the one
or more layers
provide at least one of flavor or adjuvant of the at least one of one or more
herbal extracts,
and step 1650 can include converting the substrate into a three-dimensional
structure for use
as a delivery cartridge. In an example, an additional step can be performed
between steps
1630 and 1640 which can include heating or wet extracting the second,
different herbaceous
plant material to release a second vapor or to produce a second solution and
subsequently
condensing the second vapor or depositing and drying the second solution onto
the substrate,
thus creating a second coating on the coated substrate, as described above.
As described above in reference to the coated substrates, a composition and
amount of
the herbal extract(s) in the delivery cartridge can be determined and
controlled, which can be
used for dosage control of the herbal extract(s). In an example, the delivery
cartridges can
contain a predetermined quantity of herbal extract(s) and can be designed as
single dosage or
multi-dosage cartridges. Using the control parameters described above, a
quantity of herbal
extract(s) in the delivery cartridge can vary depending, for example, on the
intended use of
the herbal extracts.
A delivery cartridge can cooperate with a delivery device that supplies a
volatilizing
heat source to deliver the herbal extract(s) in the delivery cartridge to a
user. In an example,
the delivery device can include, but is not limited to, an e-cigarette, a
bong, a water nebulizer
and a vaporizer.
FIG. 17 shows a delivery cartridge 1750 in combination with an example of a
delivery
device, an electronic nebulizer 1700. In an example, the electronic nebulizer
1700 and the
delivery cartridge 1750 form a delivery system. The electronic nebulizer 1700
can include a
heating element 1710 with an opening 1715 sized and shaped to receive the
delivery cartridge
1750, a power unit 1717, an air intake 1720, a moisturizing and cooling
chamber 1730, a
mouthpiece 1740, a cover 1760, a power switch 1762 and a digital readout 1764.
The heating element 1710 can heat the delivery cartridge 1750 to a specified
temperature. In an example, the heating element 1710 can pre-heat the delivery
cartridge
1750 to a temperature less than a volatizing or entraining temperature of the
herbal extracts
so that the delivery cartridge 1750 can readily volatize or entrain the
molecules of the herbal
extract on user demand. In an example, the heating element 1710 can heat the
delivery
cartridge 1750 to a temperature greater than or equal to a volatizing or
entraining temperature
29
CA 02934983 2016-06-30
of the herbal extract(s) to volatize or entrain the herbal extract(s) for
delivery of the volatized
herbal extracts on user demand.
The air intake 1720 provides makeup air to the electronic nebulizer 1700. In
an
example, the air intake 1720 can be a hole located in the electronic nebulizer
1700 in
communication with the opening 1715, the moisturizing and cooling chamber 1730
and the
mouthpiece 1740. In an example, the air intake 1720 can allow makeup air to
flow into the
electronic nebulizer 1700 when a user induces a negative pressure (or suction)
action at the
mouthpiece 1740.
The cover 1760 can prevent users from contacting the heating element 1710
during
operation of the electronic nebulizer 1700. In an example, the cover 1760
removably attaches
to the electronic nebulizer 1700 to prevent loss of the delivery cartridge
1750 during use.
The power switch 1762 controls the flow of electrical power from a power unit
1717
to the heating element 1710. In an example, electrical power can flow from the
power unit
1717 to the heating element 1710 when the power switch 1762 is in an 'on'
position. In an
example, electrical power can be prevented from flowing from the power unit
1717 to the
heating element 1710 when the power switch 1762 is in an 'off' position.
The delivery cartridge 1750 can be used with the electronic nebulizer 1700 to
deliver
a predetermined and accurate quantity of volatized or entrained herbal
extract(s) to a user. As
described above, the amount of the herbal extract(s) in the cartridge 1750 can
be controlled
and thus known. The cartridge 1750 can be a single dose cartridge or intended
for use over
multiple doses. In an example, a user can remove the cover 1760 from the
electronic
nebulizer 1700 and insert a delivery cartridge 1750 into the opening 1715. In
an example, the
user can removably attach the cover 1760 to the electronic nebulizer 1700
before adjusting
the power switch 1862 to the 'on' position in order to preheat the delivery
cartridge 1750. In
an example, the user can monitor the digital display 1764 for a visual cue
that indicates that
the electronic nebulizer 1700 is ready for use.
A delivery device can be configured to control the dosage of the herbal
extract(s) to
the user such that a multi-dose cartridge can be used with the delivery
device, while still
maintaining dosage control. For example, a delivery device similar to the
electronic
nebulizer 1700 can be configured to deliver a predetermined amount of herbal
extract(s) per
inhalation.
The delivery device can control how much air passes through the delivery
cartridge
and how much air is delivered to the user. In an example, a valve device
inserted into the air
CA 02934983 2016-06-30
flow of the delivery device can be used to control the volume of air available
to the user. For
example, the valve device can be located in the mouthpiece of a delivery
device to throttle the
volume of air flowing through the mouthpiece. In an example, the valve device
can include,
but is not limited to, a flapper valve, a ball valve, a gate valve, a
butterfly valve, a duckbill
valve or an adjustable orifice.
In an example, the valve device can include a timer device that can cause the
valve
device to open or close after an interval of time to regulate air flow through
the delivery
device. For example, the valve device can include an open-loop timer device
utilizing
mechanisms such as a spring or a mechanical linkage to open or close the valve
device. In
another example, the valve device can include a closed-loop timer device using
an actuator,
an electrical control circuit and one or more feedback sensors to implement a
control
algorithm to open and close the valve.
The delivery device can also control other parameters that impact the amount
herbal
extracts(s) delivered to the user, including, for example, a temperature that
the cartridge is
heated to and the rate of airflow. Because the delivery cartridge only
contains the desired
componentsõ which have already been separated from the undesirable components
in the
source material, sufficient heat can be applied to the delivery cartridge to
quickly vaporize or
entrain the herbal extract(s) without worrying about the undesirable
components also being
vaporized.
The delivery cartridge can be configured to control the amount or dose of
herbal
extract(s) delivered. In an example, the delivery cartridge can be coated with
a micro porous
film to control the flow of herbal extract(s) vapor or entrained
microparticles from the
delivery cartridge. For example, the diameter of the pores in the micro porous
film applied to
the coated substrate can be sized to control the dose of herbal extract(s)
delivered. In an
example, the coated substrate used to form a delivery cartridge can be coated
with a micro
porous film to control the flow of herbal extract(s) vapor or entrained herbal
microparticles
from the coated substrate and thereafter formed into a delivery cartridge.
In an example, the delivery cartridge can be constructed from a coated
substrate
comprising a conductive material. In an example, the conductive material can
include, but is
not limited to, aluminum. In an example, an electrical power circuit can be
connected to the
conductive material to resistively heat the conductive material to a
temperature sufficient to
volatilize or entrain the herbal extract(s) on the coated substrate. In an
example, the electrical
power circuit can include an electrical control circuit and one or more
feedback sensors to
31
CA 02934983 2016-06-30
resistively heat the conductive material to a sufficient temperature and
thereafter accurately
maintain the temperature over a period of time.
In an example, the delivery cartridges described herein can be used with a
vaporizer
or entrainer. The vaporizer or entrainer can be configured to include a
chamber or receptacle
into which the delivery cartridge can be placed. The delivery cartridge can be
configured as a
single dose or multi-dose cartridge. Given the control parameters that can be
used in the
process of making the delivery cartridge, the delivery cartridge can include a
known quantity
of the herbal extract(s). As similarly stated above, a heating temperature of
the vaporizer or
entrainer is not a significant concern because the delivery cartridge only
includes the desired
herbal extract(s) and the substrate used in forming delivery cartridge can be
inert at these
operating temperatures.
FIG. 18 shows an example of a cylindrically rolled sheet 1802, which can be
suitable
for use as a delivery cartridge with a delivery system. The term cylindrical,
as used herein, is
intended to mean that the cross-sectional shape of the rolled sheet is the
same at each
longitudinal location along the rolled sheet 1802. For instance, the cross-
section itself can be
a circle, a spiral, a curve that lacks sharp corners, a curve that includes at
least one sharp
corner, a combination of curved and straight portions, a polygon, a square, a
star shape, and
other suitable shapes. In some examples, the cylindrically rolled sheet can
form a tunnel
structure that can support air flow there through. The rolled sheet 1802 of
FIG. 18 is but one
example of a cylindrical structure for use as a delivery cartridge. As
described below, a
cylindrical closed-end structure, such as a tube or a star can alternatively
be used.
As described above and shown in the figures, any suitable shape can be used
for the
delivery cartridge, and the shape and design is not limited to the examples
described and
shown herein. As described above, the delivery cartridge can be cylindrical
such that the
cross-sectional shape is the same at each longitudinal location. In other
examples, non-
cylindrical designs can be used in which the cross-sectional shape varies
longitudinally. In
other examples, the delivery cartridge can be further converted to have a
shape configured for
use with different delivery systems. Further converting can include, for
example, shaping a
cylindrical structure into a J or an S for use in a nebulizer.
Referring back to FIG. 18, the rolled sheet 1802 includes a substrate 1803,
which can
be formed from an electrically conductive material, such as aluminum, copper,
or another
suitable metal or metal alloy. The rolled sheet 1802 is shaped to allow a
gaseous flow in its
interior, along the longitudinal direction (Z), from a first longitudinal end
1826 to a second
32
CA 02934983 2016-06-30
longitudinal end 1828 opposite the first longitudinal end 1826. As further
described below,
all or a portion of the substrate 1803 can be covered with a coating of herbal
extract(s). As
described above, in some examples, the herbal extract(s) component can include
one or more
herbal extracts on one or both sides of the substrate 1803.
The rolled sheet 1802 can include a first electrode 1804 extending laterally
(X) across
the substrate 1803 at a first longitudinal location 1806. In some examples,
the first electrode
1804 can be formed integral to the substrate 1803 to form the rolled sheet
1802, for example,
by extruding the electrode 1804 onto the substrate 1803. In those examples,
the first
electrode 1804 can be thicker relative to the substrate 1803. In some
examples, the first
electrode 1804 can be originally separate from the substrate 1803 and attached
to the
substrate 1803, so that the first electrode 1804 is electrically coupled to
the substrate 1803 to
form the rolled sheet 1802. This is described further below. In some examples,
the first
electrode 1804 can extend outward from the rolled sheet 1802, toward an
exterior of the
rolled sheet 1802. In other examples, the first electrode 1804 can extend
inward from the
rolled sheet 1802, toward an interior of the rolled sheet 1802. In still other
examples, the first
electrode 104 can extend both outward and inward from the rolled sheet 1802.
The first electrode 1804 can be formed from an electrically conductive
material and
can be formed from the same or a different material than the substrate 1803.
Example
materials include, but are not limited to, aluminum, copper, or another
suitable metal or metal
alloy. The particular material selected can depend in part on whether the
first electrode 1804
is integral to or separate from the substrate 1803. The first electrode 1804
can act a contact
portion for use within a housing of an herbal extract(s) delivery system
having corresponding
electrodes, as described further below.
In an example in which the first electrode 1804 is separate from the substrate
1803,
the first electrode 1804 can be made of steel and welded to the substrate 1803
to form the
rolled sheet 1802. In such an example, the steel material can optionally be
formed or
provided as a coiled spring which can be straightened out to weld the material
to the substrate
and then the material can coil back up as the substrate 1803 is rolled to form
the rolled sheet
1802. Other materials and other assembly methods can be used to form the
rolled sheet 1802
out of the substrate 1803 and first electrode 1804.
The rolled sheet 1802 can also include a second electrode 1808 extending
laterally
(X) across the rolled sheet 1802 at a second longitudinal location 1810. The
second electrode
1808 can be similar to the first electrode 1804 and have the properties
described above. The
33
CA 02934983 2016-06-30
first and second electrodes 1804, 1808 can each have an electrical resistance
small enough to
conduct current laterally (X direction) along the rolled sheet 1802 without
heating the rolled
sheet 1802. The second electrode 1808 can also be formed as a thick portion of
the rolled
sheet 1802, or formed separately from the rolled sheet 1802 and attached to
the rolled sheet
1802, as described above with reference to the first electrode 1804.
The rolled sheet 1802 can include a first substrate portion 1812 extending
longitudinally (Z direction) between the first and second electrodes 1804,
1808. The first
substrate portion 1812 can have an electrical resistance high enough to
conduct current
longitudinally (Z) between the first and second electrodes 1804, 1808 and
resistively heat the
first substrate portion 1812 in response to the current conducted there
through.
A first dose 1814 of an herbal extract(s) can be disposed on the first
substrate portion
1812 of the substrate 1803 and configured to volatilize or entrain into vapor
or aerosol in
response to the resistive heating of the first substrate portion 1812. In some
examples, the
first dose 1814 of the herbal extract(s) can be uniformly coated on the first
substrate portion
1812. In other examples, the first dose 1814 of the herbal extract(s) can
include discrete
pieces of multiple herbal extract(s) adhered to the first substrate portion
1812. In some
examples, the herbal extract(s) can be coated on an exterior side of the
substrate 1803 in the
area identified as the first portion 1812. In some examples, the herbal
extract(s) can be
coated on an interior side of the substrate 1803 in the area identified as the
first portion 1812.
In some examples, the herbal extract(s) can be coated on both the interior and
exterior sides
of the substrate 1803. In some examples, different herbal extract(s) or
combinations of herbal
extract(s) can be coated on the interior and exterior sides of the substrate
1803.
In some examples, the rolled sheet 1802 can further include a third electrode
1816
extending laterally (X) across the rolled sheet 1802 at a third longitudinal
location 1818, so
that the second electrode 1808 is positioned longitudinally between the first
and third
electrodes 1804, 1816. The third electrode 1816 can have an electrical
resistance small
enough to conduct current laterally (X) along the rolled sheet 1802 without
heating the rolled
sheet 1802. The third electrode 1816 can also be formed as a thick portion of
the rolled sheet
1802, or formed separately from the rolled sheet 1802 and attached to the
rolled sheet 1802.
In some examples, the rolled sheet 1802 can further include a second substrate
portion
1820 extending longitudinally (Z) between the second and third electrodes
1808, 1816. The
second substrate portion 1820 can have an electrical resistance high enough to
conduct
34
CA 02934983 2016-06-30
current longitudinally (Z) between the second and third electrodes 1808, 1816
and resistively
heat the second substrate portion 1820 in response to the current conducted
there through.
A second dose 1822 of the herbal extract(s) can be disposed on the second
substrate
portion 1820 and configured to volatilize or entrain into a vapor or aerosol
or in response to
the resistive heating of the second substrate portion 1820. In some examples,
the first and
second doses 1814, 1822 include doses of the same herbal extract(s). In other
examples, the
first and second doses 1814, 1822 include doses of different herbal
extract(s).
In some examples, the rolled sheet can include more than three electrodes,
with a
corresponding substrate portion between each pair of adjacent electrodes, and
an herbal
extract(s) dose disposed on each substrate portion of the substrate 1803. As
described below
in reference to FIGS. 22 and 23, a controller can be used to regulate how and
when the herbal
extract(s) doses are delivered to an individual.
FIG. 19 shows a cross-section of the rolled sheet 1802 of FIG. 18. In this
example,
the substrate 1803 is rolled to form a cylindrical structure having a spiral
cross-section, when
viewed from the first longitudinal end 1826 (FIG. 18) of the rolled sheet
1802. The first,
second, and third electrodes are omitted from FIG. 19 for clarity.
FIG. 20 shows the cross-section of the rolled sheet 1802 from FIG. 19, with
the
addition of an optional plurality of electrically insulating spacers 2024
positioned to space
apart adjacent layers of the substrate 1803. The spacers 2024 can be similar
to the spacers
described above in reference to FIGS. 7A and 7B. The electrically insulating
spacers 2024
can be positioned and spaced apart to allow a gaseous flow in the interior of
the rolled sheet
1802, along the longitudinal direction, from the first longitudinal end 1826
(FIG. 18) to the
second longitudinal end 1828 (FIG. 18). The spacers 2024 can be added to the
substrate 1803
prior to forming the rolled sheet 1802 or after the rolled sheet 1802 is
assembled.
In the examples of FIGS. 18-20, the substrate 1803 is rolled in an open-ended
manner
to form the rolled sheet 1802, so that one of its lateral edges 2026 is
disposed at the center of
the rolled sheet 1802 and the opposite lateral edge 2028 is disposed at the
exterior of the
rolled sheet 1802. In other examples, the substrate 1803 can be assembled in a
closed-ended
manner, so that for some methods of assembly, its lateral edges can be joined
during
assembly to form a tube or other cylindrical structure.
FIG. 21 shows an example of a tube 2102, suitable for use as a delivery
cartridge in
delivery system. In the example of FIG. 21, the tube 2102 has a circular cross-
section, when
viewed from a longitudinal end 2126 of the tube 2102. The tube 2102 is formed
of a
CA 02934983 2016-06-30
substrate 2103, and as described above, all or a portion of the substrate 2103
can be coated
with herbal extract(s). The tube 2102 includes a first electrode 2104 at a
first longitudinal
location 2106, a second electrode 2108 at a second longitudinal location 2110,
a first
substrate portion 2112 extending longitudinally (Z) between the first and
second electrodes
2104, 2108, a first dose 2114 of an herbal extract disposed on the first
substrate portion 2112,
a third electrode 2116 disposed at a third longitudinal location 2118, a
second substrate
portion 2120 extending longitudinally (Z) between the second and third
electrodes 2108,
2116, and a second dose 2122 of an herbal extract disposed on the second
substrate portion
2120. In some examples, only one side of the substrate 2103 is coated with the
herbal
extract(s) such that the herbal extract doses are disposed on the exterior of
the tube 2102 or
the interior of the tube 2102. In some examples, both sides of the substrate
2103 are coated
with the herbal extract(s) such that the herbal extract(s) doses are disposed
on the interior and
exterior of the tube 2102.
In an example in which the cylindrical structure is a tube, like the tube
2102, the tube
2102 can be formed in at least the two ways described herein. Other processes
can
alternatively or additionally be used to form the cylindrical structure. In a
first process, the
first electrode 2104 can be open and have a lateral dimension generally equal
to a lateral
dimension of the substrate 2103. The first electrode 2104 can include a hinge,
which can be
generally located at a lateral mid-point on the first electrode 2104. It is
recognized that the
hinge can be at other lateral locations on the first electrode 2104, and more
than one hinge
can be used. The first electrode 404 and the substrate 2103 can be brought
together such that
the first and second lateral ends of each of the substrate 2103 and the
electrode 2104 are
generally aligned. The first and second lateral ends of the substrate 2103 and
the electrode
2104 can then be connected together to form a closed, tubular structure, with
the electrode
2104 connected to an exterior circumference of the substrate 2103. Additional
electrodes can
similarly be attached to the substrate 2103 to form a tube having multiple
electrodes at
various longitudinal locations on the substrate 2103.
In a second process, the first electrode 2104 can be a closed-end structure,
having a
generally circular shape; the substrate 2103 can be converted into a tube by
joining the first
and second longitudinal ends of the substrate 2103. The converted substrate
2103 can then be
inserted into the circular electrode 2104 such that the electrode 2104 is
connected to an
exterior circumference of the substrate 2103. If the tube 2102 is intended to
have multiple
electrodes, the converted substrate 2103 can be separately inserted into each
electrode, or the
36
CA 02934983 2016-06-30
multiple electrodes can be longitudinally spaced from one another and the
converted substrate
2103 can be inserted into the multiple electrodes in one step. In some
examples, a support
structure can be used to support the one or more electrodes as the converted
substrate 2103 is
inserted into the one or more electrodes.
One of ordinary skill in the art will appreciate that the delivery cartridge
can have any
suitable cross-section, such as spiral (FIGS. 17-20), circular (FIG. 21),
elliptical, rounded and
elongated, square, star-shaped, regular and irregular polygonal, and so forth.
FIGS. 22 and 23 show an example of a delivery system 2200. The delivery system
2200 can include a delivery cartridge 2202, which can be similar to the rolled
sheet 1802
(FIGS. 17-20) or alternatively can be a tube such as the tube 2102 (FIG. 21).
The delivery
system 2200 can further include a housing 2230. FIG. 5 shows the rolled sheet
2202 separate
from the housing 2230, which is how the delivery system 2200 can be arranged
as sold or
during storage. FIG. 23 shows the rolled sheet 2202 inserted into the housing
2230, which is
how the delivery system 2200 can be arranged during use.
In some examples, the housing 2230 can be configured to be reusable, and the
rolled
sheet 2202 can be configured to be disposable or recyclable after the herbal
extract dosages
have been delivered. In some of these examples, the rolled sheet 2202 can be
packaged as a
replaceable cartridge. In other examples, the housing 2230 and rolled sheet
2202 can be
packaged together, with one or both being configured to be disposable or
recyclable after the
herbal extracts dosages have been delivered. In some examples, the housing
2230 can be
elongated and can include a first longitudinal end configured to deliver the
volatilized gas
into a user's mouth.
The housing 2230 can be configured to receive the rolled sheet 2202 within a
cylindrical cavity 2232. The cylindrical cavity 2232 can be accessed through
an opening
2234 in the housing 2230. In some examples, such as the example of FIG. 22,
the opening
2234 can face a user, during use. In some of these examples, the opening 2234
is configured
to deliver the aerosol or vapor into a user's mouth. For these examples, the
housing 2230 can
include an air filter 2236, attached to or made integral with the housing
2230, positioned on
an opposite side of the cylindrical cavity 2232 as the opening 2234, and
configured to filter
air intake as air flows from outside the housing 2230, through air filter
2236, toward the
cylindrical cavity 2232. In other examples, the opening 2234 can face away
from a user,
during use. In these examples, the rolled sheet 2202 can optionally include an
air filter. In
some examples, the cylindrical cavity 2232 and the rolled sheet 2202 can be
keyed, or can
37
CA 02934983 2016-06-30
include one or more locating features that can ensure that the rolled sheet
2202 is inserted
into the cylindrical cavity 2232 with a specified rotational orientation. The
housing 2230 can
be designed to receive delivery cartridges having alternative shapes to the
cylindrical design
of the delivery cartridge 2200 by having the cavity 2232 in the housing 530 be
sized and
shaped to correspond to the size and shape of the delivery cartridge.
The housing 2230 can include a first housing electrode 2238 around a
circumference
of the cylindrical cavity 2232 and facing inward toward the cylindrical cavity
2232. The first
housing electrode 2238 can be positioned longitudinally to respectively
contact the first
electrode 2204 of the rolled sheet 2202 when the rolled sheet 2202 is inserted
into the
housing 2230. The first housing electrode 2238, as well as additional housing
electrodes, can
be formed from stainless steel, aluminum, copper, or other suitable conductive
materials.
The housing 2230 can include a second housing electrode 2240 around a
circumference of the cylindrical cavity 2232 and facing inward toward the
cylindrical cavity
2232. The second housing electrode 2240 can be positioned longitudinally to
respectively
contact the second electrode 2208 of the rolled sheet 2202 when the rolled
sheet 2202 is
inserted into the housing 2230. The first and second housing electrodes 2238,
2240 can be
configured to deliver current between the first and second electrodes 2204,
2208 of the rolled
sheet 2202. The first and second housing electrodes 2238, 2240 can be part of
a heating
element to deliver current between the first and second electrodes 2204, 2208
of the rolled
sheet 2202 such that a portion of the rolled sheet 2202 can be resistively
heated, as an
alternative to using heated air.
The housing 2230 can optionally include a third housing electrode 2242 around
a
circumference of the cylindrical cavity 2232 and facing inward toward the
cylindrical cavity
2232. The third housing electrode 2242 can be positioned longitudinally to
respectively
contact the third electrode 2216 of the rolled sheet 2202 when the rolled
sheet 2202 is
inserted into the housing 2230. The second and third housing electrodes 2240,
2242 can be
configured to deliver current between the second and third electrodes 2208,
2216 of the rolled
sheet 2202.
In some examples, the rolled sheet 2202 and housing 2230 can include more than
three electrodes and housing electrodes, respectively. For these examples,
each pair of
adjacent housing electrodes can be configured to deliver current between a
corresponding
pair of adjacent electrodes of the rolled sheet.
38
CA 02934983 2016-06-30
In some examples, a controller 2244 can be positioned in the housing 2230. The
controller 2244 can be configured to deliver current to the housing electrodes
2238, 2240 and
2242. In some examples, the controller can deliver current between the first
and second
housing electrodes 2238, 2240 at a first time to provide a first dose of an
herbal extract to a
user. In some examples, the controller 2244 can be further configured to
deliver current
between the second and third housing electrodes 2240, 2242 at a second time,
different from
the first time, to provide a second dose of the herbal extract to the user.
For delivery
cartridges that include multiple doses, the controller 2244 can be configured
to deliver current
between adjacent pairs of housing electrodes at sequential times to provide a
dose of herbal
extract to a user at each sequential time. In some examples, the controller
2244 can deliver
current to multiple pairs of housing electrodes at the same time to deliver
multiple doses to
the user with a single inhalation. By using a conductive substrate and
delivering current to
the electrodes, the herbal extract(s) can be volatilized or entrained and
inhaled by the user
using room temperature air instead of heated air.
In some examples, the controller 2244 can include one or more batteries. In
some
examples, the controller 2244 can be rechargeable. In some examples, the
controller 2244
can communicate with other electronic devices, such as through short-range
wireless
communication. In some examples, the controller 2244 can communicate with the
Internet.
In some of these examples, the controller 2244 can record a user's dosage
history through
wireless communication with another electronic device or through a web-based
application.
The controller 2244 can be triggered through a button on the housing 2230,
through a touch-
sensitive area on the housing configured to activate the controller 2244 when
the 2230
housing contacts a user's mouth, or through another suitable trigger.
During use, as a user inhales, such as through opening 2234, the user can draw
in air
from the surroundings through the air filter 2236. The air from the
surroundings can combine
with the dose of the herbal extract released from the rolled sheet 2202 in an
optional
expansion/mixing chamber 2246. In some examples, the expansion/mixing chamber
2246
can be positioned between the rolled sheet 2202 and the user's mouth, during
use.
After use, once the doses of the herbal extract(s) on the rolled sheet 2202
have been
dispensed, the housing 2230 can eject or release the expended rolled sheet
2202. The
expended rolled sheet 2202 can then be thrown away or recycled. In some
examples, the
housing 2230 can optionally include storage for one or more additional rolled
sheets 2202.
39
CA 02934983 2016-06-30
FIG. 24 is a side-view schematic drawing of another example of a delivery
system or
nebulizer 2400. The example of FIG. 24 is sized and shaped for ease of use by
a user. The
delivery system 2400 can include a housing 2402.
An air intake nozzle 2404 can receive air flow from the surroundings and can
optionally restrict the air flow into the housing 2402. In some examples, the
air intake nozzle
2404 can be adjustable. In some examples, the air intake nozzle 2404 can allow
a user to
control the rate at which the surrounding air is taken into the housing 2402.
In some
examples, the air intake nozzle 2404 can control a duration of an inhalation.
In some
examples, the air intake nozzle 2404 can produce an internal nebulizer
pressure when the user
inhales.
Air passing through the air intake nozzle 2404 can pass through an air filter
2406.
The air filter 2406 can prevent particles or particulate from entering further
into the housing
2402. In some examples, the air filter 2406 can be the same in structure and
function as the
air filter 2236 (FIGS. 22 and 23).
Air passing through the air filter 2406 can enter a volatilizing or entraining
chamber
2408. In some examples, the volatilizing chamber 2408 can accommodate delivery
cartridges, such as 1802 (FIGS. 17 and 18), 2102 (FIG. 21), or 2202 (FIGS. 22
and 23). An
interior of the volatilizing or entraining chamber 2408 can include electrodes
that connect to
corresponding electrodes on a rolled sheet during use. Air leaving the
volatilizing or
entraining chamber 2408 can include a prescribed dose of the herbal
extract(s), which is
volatilized or entrained from the cartridge during use (2409).
A vortex chamber 2410 can reduce a cross-section surface area of gas passing
there
through. The reduced surface area can increase the velocity of gas passing
there through,
which can be desirable.
Gas or vapor leaving the vortex chamber 2410 can optionally pass through a
misting
ring 2412, which can optionally inject mist from a misting reservoir 2422 into
the gas. In
some examples, the mist can include water. In some examples, the mist can
include one or
more flavorings or scents. The misting reservoir can alternatively be filled
with an emulsion
of water and squalene or mineral oil or paraffin oil. The emulsion can act as
an adjuvant in
substitution for the adjuvant layer described above.
In some examples, the misting ring 2412 can be activated by a controller, such
as
2244 (FIGS. 22 and 23). In some examples, the misting reservoir 2422 is
refillable. In some
of these examples, the housing 2402 can define a port 2424, through which the
misting
CA 02934983 2016-06-30
reservoir 2422 can be refilled. In some of these examples, the material to
refill the misting
reservoir 2422 can be poured through the port 2424 in the housing 2402. In
some examples,
the material to refill the misting reservoir 2422 can be inserted via a
cartridge, or other
container, through the port 2424 in the housing 2402. As described further
below in
reference to FIG. 25, a pump can be used with the reservoir 2422 to deliver
the solution from
the reservoir 2422 to the misting ring 2412. As shown in FIG. 24, in an
example, the misting
reservoir 2422 can be located within the vortex chamber 2410. In other
examples, the
misting reservoir 2422 can be located in an alternative location within the
housing 2402 or
external to the housing 2402.
Gas or vapor leaving the misting ring 2412 can enter a mixing chamber 2414.
The
gas or vapor, moving with an increased velocity from the vortex chamber 2410,
can expand
within the mixing chamber 2414. This expansion can form a vortex, which can
improve
mixing of the mist with the gas or vapor (2415). The inclusion of a misting
ring in the
delivery system 2400 can be used to moisturize and cool the air leaving the
volatilizing
chamber 2408 and can improve inhalation of the vapors or aerosol from the
delivery
cartridge. The mist can be added to the vapors using additional or alternative
features to the
misting ring 2412. In an example, a misting solution can be packaged
separately or together
with a delivery cartridge. The misting solution can be available in different
flavors to
accommodate user preferences. It is recognized that the misting ring 2412 or
comparable
misting feature can be used in the other delivery systems described above. The
misting ring
2412 can be used independently of the housing electrode design of FIG. 24. The
delivery
system 2400 of FIG. 24 can alternatively exclude the misting ring 2412.
Vapor or aerosol from the mixing chamber 2414 can exit the housing 2402
through a
mouthpiece 2416. In some examples, the mouthpiece 2416 is removable from the
housing
2402. A removable mouthpiece 2416 can help ensure sterility for the user. In
other
examples, the mouthpiece 2416 can be attached to and non-removable from the
housing
2402.
The housing 2402 can include an optional status indicator, which can display
visual
indicia that indicate a status of the housing during use. In the example of
FIG. 24, the status
indicator can include three light emitting diodes (LEDs, 2418) radiating
outward from the
housing 2402. This is but one example of a status indicator; other suitable
examples can also
be used.
41
CA 02934983 2016-06-30
In the specific example of FIG. 24, each LED 2418 corresponds to a housing
electrode and a corresponding electrode on the rolled sheet. In the specific
example of FIG.
24, when the cartridge is inserted into the volatilizing or entraining chamber
2408, the
controller can sense a voltage drop across adjacent pairs of electrodes, and
can direct
corresponding LEDs 2418 to glow red. In this example, a red color indicates
that a
corresponding dose on the rolled sheet is ready to be volatilized. In this
example, a user can
depress a button 2420 on the housing 2402, which can instruct the housing to
direct current
through a corresponding portion of the substrate. The button 2420 can operate
as a 'go
button'. In other examples, the button 2420 can include additional
functionality with regards
to operating the delivery system 2400. In the specific example of FIG. 24,
when the user
depressed the button for the first time, for a particular rolled sheet,
corresponding LEDs can
alternately blink red and green. In a specific example, blinking red and green
can indicate
that the controller is heating a selected dose on the rolled sheet. In some
examples, the
heating can take a relatively short period of time, such as two seconds. In
some examples,
when a dose is ready to be volatilized or entrained, a corresponding LED can
turn solid green.
In some examples, when a user depresses the button 2420 for a second time, the
controller
can monitor an internal pressure, such as in the volatilizing or entraining
chamber 2408 or the
mixing chamber 2414. In some examples, the controller can include a pressure
sensor that
detects a drop in pressure. When the pressure drops, corresponding to an
inhalation by the
user, the controller can volatilize or entrain the corresponding herbal
extract dose on the
rolled sheet. In some examples, the pressure sensor can provide a rate at
which the herbal
extract is being depleted to the controller. In some examples, one or more
LEDs can blink at
a rate indicative of the rate at which the herbal extract is depleted. In some
examples, when
the controller determines that a dose of the herbal extract is fully
dispensed, one of more
LEDs can turn off.
In other examples, more or less than the three LEDs 2418 can be used in the
housing
2402. The LEDs as described above are but one specific example of a status
indicator; other
status indicators can also be used.
As shown in FIG. 24, the delivery system 2400 can optionally include a dose
selection
switch 2426 as part of the electronic controller module 2502 (schematically
shown in FIG.
25) for selecting how many dosages are dispensed at one time from a delivery
cartridge
inserted in the chamber 2408. In some examples, the dose selection switch 2426
can include
settings labeled as "1", "2", "3", up to the number of doses capable of being
delivered from
42
CA 02934983 2016-06-30
the cartridge. For example, if the dose selection switch 2426 is set to "3",
then the delivery
system 2400 can dispense three doses from the cartridge at one time. In
operation, the
controller module 2502 is removably and electronically connected with delivery
system 2400
through the interface connectors 2500. The schematic representation of the
connection and
electronic control circuits of module 2502 is shown in FIG. 25.
FIG. 25 is a schematic drawing of an example of an interface connector 2500.
The
interface connector 2500 can form various connections, including electrical,
hydraulic, and
gaseous connections, between a controller 2502 for a vaporizing or entraining
delivery
system such as nebulizer 2400 (FIG. 24), which is the vaporizing or entraining
nebulizer
shown in outline form in FIG 25 (2504). The interface connector 2500 is but
one example of
a connector; other suitable connectors can also be used. The vaporizing or
entraining
nebulizer 2504 is an outline of for example the nebulizer shown in FIG. 24 and
can
alternatively be other nebulizer shapes having similar functions. The
controller 2502 can be
external to the nebulizer 2504, attachable thereto, or integrally formed
therewith. The
interface connector 2500, the controller 2502 and the vaporizing or entraining
nebulizer 2504
can be part of the delivery system.
A controllable switching matrix 2506 can control voltages directed to each
electrode
2508 on a delivery cartridge usable in the vaporizing or entraining nebulizer
2504. The
controller 2502 can include a controllable current source 2510 to generate the
current, and a
voltage detector 2512 to monitor the voltage across the leads of the current
source 2510. The
controllable switching matrix 2506 can controllably switch the electrical
connection of each
electrode between the two sides of the current source 2510, thus switching or
alternating a
voltage applied to each electrode between a relatively low value and a
relatively high value.
When the relative voltages between a pair of adjacent electrodes 2508 are
equal (e.g., both
relatively low or both relatively high), then no current flows between the
electrodes 2508.
When the voltages between the pair of adjacent electrodes 2508 are different
(e.g., one
relatively low and one relatively high), then current flows from the electrode
having the
relatively high voltage to the electrode having the relatively low voltage.
The current
generates heat, and the heat volatilizes the desired dose of the herbal
extract, which is
disposed between the electrodes 2508 in the pair, as described above. The
controller 2502
can track which doses have been volatilized or entrained, so that current is
directed through
each adjacent pair of electrodes 2508 only a single time during use of a
particular delivery
cartridge.
43
CA 02934983 2016-06-30
As shown in FIG. 25, a misting reservoir and pump 2514 can be included in the
same
mechanical housing as the controllable switching matrix 2506 and, in an
example, can be
housed within the controller 2502. The interface connector 2500 can
hydraulically connect
the controller 2502 to the vaporizing or entraining nebulizer 2504 such that
the misting
reservoir and pump 2514 can controllably direct a specified volume of mist,
through the
interface connector 2500, to a mister 2516, such as a misting ring 2412 (FIG.
24). In some
examples, the controller 2502 supplies a fixed volume of mist for each dose of
the herbal
extract. In some examples, the controller 2502 allows a user to select the
volume of mist for
each dose of the herbal extract. For instance, the mist volume can be selected
mechanically,
such as with a knob, level, or button on the housing. Alternatively, the mist
volume can be
selected electronically, such as by one or more buttons on the housing of the
vaporizing or
entraining nebulizer 2504 or the controller 2502.
A pressure sensor 2518 can be included in the controller 2502. The pressure
sensor
2518 can measure one or more pressures in the delivery system 2504, such as at
an orifice
2520, which can be located, for example, proximate to the mouth of the user.
In some
examples, the controller 2502 can use the pressure sensor 2518 as a trigger
switch, which can
trigger additional actions from the controller 2502. When the user inhales
from the
vaporizing or entraining nebulizer 2504, the pressure at a particular
location, such as at the
orifice 2520, drops. The pressure sensor 2518 can detect the drop in pressure,
and the
controller 2502 can take a suitable action, such as directing suitable
voltages to the electrodes
2508 to initiate delivery of an herbal extract dose, and/or directing the
misting reservoir 2514
to dispense mist. In other examples, the controller 2502 can connect to a Get
Ready/Go
button on the housing, similar to the button 2420 shown in FIG. 24, to trigger
suitable
actions.
The interface connector 2500 can optionally include additional electrical
connections
between the controller 2502 and the vaporizing or entraining nebulizer 2504.
For instance, an
optional LED controller 2522 can electrically connect, through the interface
connector 2500,
to one or more LEDs 2524 on or in the housing. In some examples, the
controller 2502 can
additionally connect to a dose selection switch disposed on the housing. In
some examples,
the controller can electrically connect to a power source disposed on or in
the housing.
Although several features, for example, the misting reservoir and pump 2514,
are
described above as being part of the controller 2502, it is recognized that
some or all of these
44
CA 02934983 2016-06-30
features do not have to be physically contained within the same housing as the
controller
2502 but can still be controlled by the controller 2502.
It is recognized that a delivery system, like the system 2400 of FIG. 24, can
exclude a
controller, or a controller could be used having more or less features as the
controller 2502
shown in FIG. 25. In a delivery system that excludes a controller, a user can
manually
control operation of the electrodes (or other means of volatilizing or
entraining the one or
more herbal extracts), or similarly, the user can manually deliver a misting
solution to a
mixing chamber by manually activating the pump for the mist reservoir.
FIG. 26A is a perspective representation and schematic of an example of a wet
extraction apparatus with casting chamber for formation of a substrate coated
with an herbal
extract. A large container for conducting the wet extraction of herbal extract
from the
herbaceous plant material is the operational device for performing this
process. Typically,
the large container is a five portal vessel 2601 that can be any size chemical
operation vessel
ranging from 1 L to 10,000 L or larger. The five portals of the vessel (2601A,
B, C, D and E)
provide access, delivery and inlet openings to the interior of the vessel.
Door 2601XT in
extraction vessel 2601 allows large scale access to the interior of the vessel
and enables
removal of residual herbaceous plant material after the extraction process.
Although any
arrangement of inlet portals is within the scope of the invention, this
example provide
separate inlets for the various substances and solvents as well as operational
devices. Inlet
2601A is removably connected to a container (solvent container 2602),
typically an addition
funnel, with valve for variable rate introduction of solvent into the
extraction vessel 2601.
Inlet 2601B is removably connected to a stirring rod and paddle 2603. The rod
and paddle
extend into the interior region of the vessel, are powered by a variable speed
electric motor
(not shown) and provide distribution and mixing of the plant material and
solvent. Inlet
2601C is removably connected to a hopper with slide valve for delivery of the
herbaceous
plant material to the vessel 2601 (delivery hopper 2604). Preferably the
herbaceous plant
material is cleaned, dried and comminuted into very small particles. Transport
of the
particles from the delivery hopper into the extraction vessel may be
accomplished by gravity
flow, pressurized delivery or mechanical transport through the delivery chute.
Inlet 2601D is
removably connected to a tank of inert gas (gas tube 2605). If the herbal
extract is oxidation
sensitive, a cloud or atmosphere of inert gas such as nitrogen or argon may be
introduced
over the solution of solvent and plant material. Inlet 2601E is removably
connected to an
outlet tube capped at its distal end with a glass frit or other screen
material (outlet tube 2606).
CA 02934983 2016-06-30
The outlet tube extends into the interior of the vessel and the frit or screen
resides at the
bottom of the extraction vessel. Outlet tube 2606 is optionally, removably
connected to a
trap having an inlet and outlet (trap 2607). The outlet tube 2606 will carry
solution and
residue of plant material into the trap 2607. The trap allows the residue to
settle and enables
further transport of clean solution to a concentrator to remove substantially
most of the
solvent from the solution, provide a concentrate and the concentrate delivered
to distribution
tube 2608a. If trap 2607 is not needed, it can be bypassed and solution
delivered to a
concentrator to remove substantially most of the solvent from the solution,
provide a
concentrate and the concentrate delivered to distribution tube 2608A.
Distribution tube 2608A is removably and variably connected through a Y tube
with
valve 2608B to a chromatographic column 2609A on one side of the Y tube and a
fine flow
spray tube 2609B on the other side of the Y tube through a transport tube
2609C. The valve
of Y tube 2608B directs solution flow either to cylinder 2609A or to spray
tube 2609B
through transport tube 2609C. The outlet of cylinder 2609A connects to a valve
arrangement
and hence to spray tube 2609B. Spray tube 2609B is movably positioned over
casting basin
2610 and is designed to deliver a fine sheet of concentrate onto the casting
basin. Spray tube
2609B can be transported along the length of the casting basin so as to
deliver a layer of
concentrate into the casting basin. Also movably positioned over casting basin
2610 is
evaporator tube 2611. Evaporator tube 2611 is positioned at an appropriate
distance behind
spray tube 2609B. Evaporator tube 2611 is connected to a tank of dry inert gas
and is
equipped with an exhaust hood 2612. The combination of the evaporator tube,
gas and
exhaust hood enable evaporation of solvent from the cast solution in the
casting basin so as to
provide a dried layer of herbal extract.
In operation, finely divided herbaceous plant material that preferably has
been cleaned
and dried to remove water is placed into delivery hopper 2604. The valve or
other transport
mechanism for delivery of the material to the vessel 2601 is actuated and an
appropriate
amount of material is delivered to the vessel. Solvent container 2602 is
filled with an
appropriate solvent in which it is known that the herbal extract is soluble.
The solvent
container valve is actuated and an appropriate amount of solvent added to the
vessel 2601.
The stirring rod/paddle 2603 is actuated and the solution of solvent and plant
material stirred.
Heat may be applied to the solution through a heating mantle around the vessel
(not shown).
The temperature of the heating process can be appropriately controlled so as
to increase the
46
CA 02934983 2016-06-30
rate of extraction of the herbal extract from the material but not to
volatilize the solvent to a
great extent. Additional solvent may be added from time to time to replenish
solvent loss.
After an appropriate time for achievement of extraction of the herbal extract
from the
material, gas flow from gas tube 2605 is initiated. At the same time, the
valve of the solvent
container 2602 is checked to assure it is closed and the gas tight seal around
the stirring
rod/paddle is checked to assure sealing. The flow of inert gas into flask 2601
increases the
internal pressure and causes the solution to flow out through the outlet tube
2606. The glass
frit or screen at the distal end of the outlet tube 2606 provides separation
of solution from
residual plant material. The increased pressure inside vessel 2601 causes the
solution to
transport optionally into trap 2607 as the trap fills with solution, any
residual plant material
carried by the solution settles to the bottom of the trap. Clean solution
passes from the trap
outlet tube into distribution tube 2608a. If the solution from tube 2606 is
clean, trap 2607 can
be bypassed and the solution delivered directly to tube 2608A. Distribution
tube 2608a
connects with Y tube and valve 2608B. The Y tube valve can control delivery of
the solution
either to a chromatography column 2609A or to spray tube 2609B.
Delivery to chromatography column 2609A is an option for further purification
of the
herbal extract in the solution. The column may be a silica gel, diatomaceous
earth, cellulose,
cellulose derivatives, alumina, polystyrene microparticles and similar
chromatographic
materials. Passing the solution through the column will separate a mixture of
substances in
the herbal extract such that each substance will exit the column at a
different time owing to
its retention factor in the column material. In this fashion, a gross herbal
extract can be
further refined to a particular substantially purified substance.
Identification of the various
fractions can be made by UV and/or IR identification of the fraction at the
outflow end of the
column (not shown). If the column 2609A is chosen for passage of the solution,
the
chromatographed, desired herbal extract exiting the column may be directed to
the next
appropriate step.
With the passage of solution through either column 2609A or by bypassing
column
2609A, the solution can be optionally and preferably concentrated by vacuum
evaporation of
a significant portion of solvent. This may be accomplished for example by roto-
vacuum
evaporation on a rotostripper (not shown). The solution is concentrated into a
concentrate
with a minimum amount of solvent present to enable the concentrate to flow.
The concentrate from vacuum evaporation may be transported to spray tube
2609B.
The spray tube is utilized to lay down a broad flat sheet of concentrate into
casting basin
47
CA 02934983 2016-06-30
2610. Spray tube 2609B is dimensioned so that its length spans the width of
the casting
basin. Spray tube 1609B has a slit running its entire length so that the spray
of concentrate
out of the spray tube constitutes a continuous sheet of spray. Into casting
basin 2610 is
prepositioned substrate 2012. The dimensions of the casting basin and the
substrate are
coordinated so that the substrate fits tightly into the casting basin. The
edges of the substrate
seal against the sides of the casting basin so that concentrate is unable to
seep past the edges
and down underneath the substrate. The edges of the casting basin are higher
than the
thickness of the substrate and the sheet of concentrate to be laid down so
that the sheet of
concentrate is held in a stationary configuration on top of the substrate. As
concentrate flows
out through the slit of spray tube 2608B, the tube may be moved along the
length of casting
basin 2610 or spray tube 2608B may be remain stationary above casting basin
2610. Because
the concentrate is liquid, it will seek its own uniform level on the substrate
2612 in casting
basin 2610 when spray tube 2608B is stationary. Nevertheless, transport of
spray tube
2608B along the length of casting pan 2610 is preferred. At an appropriate
distance and time
following the operation of spray tube 2608B, evaporator tube 2611 is actuated
to emit a sheet
of inert gas to evaporate the solvent from the layer of concentrate laid down
on the substrate
2612. Evaporator tube 2611 spans the width of the casting basin and is
configured with a thin
slit or with a line of holes along its long axis. The slit or holes are
arranged to direct a stream
of inert gas substantially parallel and at some distance above the casting
pan. Preferably,
evaporator tube 2611 can be rotated around its long axis so as to direct to
flow of inert gas
upward and perpendicular to the substrate, or at any rotary angle form upward
and
perpendicular to downward and perpendicular. Evaporator tube 2611 also can be
moved
along the length of the casting basin, i.e., is translatable along the length
of the casting basin
so that it will translate from one end to the other of the casting basin. In
operation, the
evaporator tube is actuated after completion of the deposit of concentrate on
the substrate.
The flow of inert gas is first directed upward so as to evaporate a
substantial portion of the
solvent from the deposited concentrate. As the deposited concentrate become
sticky to tacky
and tends toward dryness, the flow of inert gas is directed more and more
downward so as to
continue drying and finally leaving a dried layer of herbal extract on the
substrate.
This process can be repeated to deposit multiple layers of herbal extract. For
subsequent layers, the evaporator tube preferably is operated substantially
soon after the
spray tube has deposited a sheet of concentrate. In this fashion, a subsequent
sheet of
48
CA 02934983 2016-06-30
concentrate is dried fairly rapidly as it is laid down so that the underlying
layer of herbal
extract is not re-dissolved.
It is preferred to include an exhaust hood above the casting basin, spray tube
and
evaporator tube so that evaporated solvent can be captured and disposed of
without
exhausting solvent into the atmosphere.
Solvents useful for wet extraction include water, chloroform, ether, ethyl
alcohol,
glyme, hexane, and similar polar protic or polar aprotic or nonpolar organic
solvents having
boiling point below 100 C.
As an alternative, as described and depicted in FIG 26B, a wet extraction
process can
be employed to produce a series of solutions of herbal extracts held in remote
storage vessels
2950A, B and C. On the other side of the process, individual hoppers 2604 A, B
and C are
used to deliver different herbaceous plant material to the extraction vessel
2601. Each hopper
can contain a different individual herbaceous plant material. The chutes of
the individual
hoppers can be actuated to deliver the herbaceous material to extraction
vessel 2601 through
inlet 2601C. Following the wet extraction, the solution can be transported to
an individual
storage vessel, one of vessels 2650 A, B and C. The residual herbaceous
material remaining
in extraction vessel 2601 can be removed through door 2601XT.
The storage vessels 2651 A, B and C will have inlets connected by a multi
directional
flow controller (a multiple position valve assembly 2630) to delivery tube
2609D. The
controller 2630 can direct the flow of different solutions of herbal extract
to individual
storage vessels 2651 A, B and C. When needed, the multi-positional valve 2952
connected to
the outlets 2951A, B and C of the storage vessels can be actuated and the
appropriate solution
from a chosen storage vessel delivered to a concentrator (vacuum evaporator)
and the
concentrate delivered to slit tube 2609b. The concentrate from storage vessel
2950 A, B or C
may be cast as an individual herbal extract layer on a substrate or as one of
a multiple number
of layers on a substrate as described above.
There can be potential advantages to delivering the herbal extract(s) using
the delivery
cartridges described herein. For instance, the herbal extract(s) dosage and
purity can be
accurately controlled during the manufacturing process. In some examples, an
advantage can
include allowing a user to ingest herbal extract(s) in a safe, repeatable
accurate dose suitable
for research and clinical trials. In some examples, an advantage can include
forming the
cartridge from recyclable aluminum. In some examples, an advantage can include
depositing
the herbal extract(s) onto the aluminum substrate in a carefully controlled
and regulated
49
CA 02934983 2016-06-30
process, transported to the user. In some examples, an advantage can include
removing the
toxins during factory processing and disposing of the toxins properly. In some
examples, an
advantage can include recycling the cartridge, with no waste. In some
examples, an
advantage can include convenience for the user, and lack of smoke when used.
In some
examples, an advantage can include disposing multiple doses on a single
cartridge, which
further enhances convenience, functionality as well as lowering shipping cost.
In some
examples, an advantage can include the flexibility in accurately setting a
dose level, which
can provide functionality to both users and researchers alike. In some
examples, an advantage
can include optionally adding a moisturizing mist, and perhaps a pleasant
flavor, which
improves the overall experience and comfort for the user.
EMBODIMENTS
The present application provides for the following exemplary embodiments, the
numbering of which is not to be construed as designating levels of importance:
Embodiment 1 provides a method of purifying an herbal extract from an
herbaceous
plant composition and the method can comprise heating the composition to a
first
temperature to volatilize the herbal extract into a first vapor, and
condensing the first vapor
onto a substrate to form a first coating, the first coating comprising the
herbal extract
Embodiment 2 provides the method of Embodiment 1 optionally configured to
provide multiple layers of herbal extracts, each layer being a different
herbal extract. The
method comprises practice of embodiment 1 to provide a first coating and
optionally further
comprising, after forming the first coating, heating a second herbaceous plant
composition to
a second temperature to volatilize a second herbal extract into a second
vapor, and
condensing the second vapor onto the substrate to form a second coating over
the first
coating, the second coating comprising the second herbal extract. As an option
of
embodiment 2, one of the herbal extracts may be a cannabis extract.
Embodiment 3 provides the method of Embodiment 1 optionally configured such
that
the substrate includes a first side and a second side and the first coating is
formed on the first
side of the substrate and comprises the first herbal extract. The method
optionally further
comprises heating the second herbaceous plant composition to volatilize the
second herbal
extract into a second vapor and condensing the second vapor onto the second
side of the
substrate to form a second coating, the second coating comprising the second
herbal extract.
As an option of embodiment 3, one of the herbal extracts may be a cannabis
extract.
CA 02934983 2016-06-30
Embodiment 4 provides the method of Embodiment 1 optionally configured such
that
the first coating comprises the first herbal extract and the method optionally
further
comprising, after forming the first coating, heating the second herbaceous
plant composition
to volatilize the second herbal extract into a second vapor, and condensing
the second vapor
onto a second substrate to form a coating comprising the second herbal
extract. As an option
of embodiment 4, one of the herbal extracts may be a cannabis extract.
Embodiment 5 provides the method of Embodiment 1 optionally configured such
that
the first temperature is equal to or greater than a temperature sufficient to
volatilize the first
herbal extract.
Embodiment 6 provides the method of any of Embodiments 1-5 optionally
configured
such that condensing the first vapor onto a substrate includes placing the
substrate on or near
a cooling bar.
Embodiment 7 provides the method of any of Embodiments 1-6 optionally
configured
such that the herbaceous plant composition is one or more of the flowers,
seeds buds, leaves,
stems, branches, bark and/or roots of the herbaceous plant.
Embodiment 8 provides the method of Embodiment 7 optionally further comprising
processing the herbaceous plant composition by comminuting the flowers, buds,
seeds,
leaves, stems, branches, bark and/or roots into very small pieces prior to
heating the raw
herbal extracts.
Embodiment 9 provides a method of pre-treating the very small pieces of
herbaceous
plant material of Embodiment 8 by drying the very small pieces in air at
ambient temperature
to slightly above ambient temperature to remove water, and optionally
collecting the removed
water and separating any herbal extract present in the removed water.
Embodiment 10 provides the method of Embodiment 9 optionally further
comprising
vacuum drying the very small pieces of herbaceous plant material to produce
vapors
composed of water and herbal extracts, collecting the vapors by condensation
and separating
the herbal extracts from the water by dissolution of the herbal extracts with
a water
immiscible solvent.
Embodiment 11 provides a method of making a delivery cartridge and can
comprise
practice of Embodiments 1-10 followed by converting the coated substrate into
a three-
dimensional structure configured for use as a delivery cartridge.
51
CA 02934983 2016-06-30
Embodiment 12 provides the method of Embodiment 11 optionally configured such
that converting the coated substrate includes rolling the coating substrate to
form a spirally-
wound cylindrical shape.
Embodiment 13 provides the method of Embodiment 12 optionally configured such
that a plurality of spacers is placed along the coated substrate prior to
converting. The
plurality of spacers can be configured to allow for airflow through the
spirally-wound
cylindrical shape.
Embodiment 14 provides the method of Embodiment 11 optionally configured such
that the coated substrate comprises a first end and a second end opposite to
the first end and
the method can further comprise creating a plurality of notches at multiple
locations on the
coated substrate between the first and second ends. The notches can create an
interface and
an interval between adjacent notches defines a segment of coated substrate.
The method can
further comprise bending the segments relative to one another at the
interfaces so as to form a
saw-tooth pattern.
Embodiment 15 provides the method of Embodiment 14 optionally further
comprising
connecting the first end to the second end to form a closed polygonal shape.
Embodiment 16 provides the method of any of Embodiments 11-15 optionally
further
comprising ascertaining an average amount of the herbal extract(s) in the
coating per unit
area of the coated substrate.
Embodiment 17 provides the method of any of Embodiments 11-16 optionally
configured such that converting the coated substrate into a three-dimensional
structure
includes determining a total area of the coated substrate to use for the three-
dimensional
structure based on a predetermined amount of the herbal extract(s) in the
delivery cartridge.
Embodiment 18 provides the method of any of Embodiments 11-17 optionally
further
comprising attaching one or more layers to the coated substrate prior to
converting the coated
substrate into a three-dimensional structure, the one or more layers
configured to provide at
least one of flavor or adjuvant substance with the herbal extract(s).
Embodiment 19 provides the method of any of Embodiments 11-18 optionally
further
comprising depositing multiple overlapping or sequential layers of herbal
extract(s) on one or
both sides of the substrate, the multiple layers each being a different herbal
extract.
Embodiment 20 provides the method of any of Embodiments 11-19 optionally
configured such that the vaporization temperatures for producing the herbal
extract vapors are
selected according to the known vaporization temperatures of the individual
herbal extract(s).
52
CA 02934983 2016-06-30
Embodiment 21 provides a delivery product comprising a coated substrate with
one or
more coating layers, the one or more coating layers including one or more
herbal extracts
Embodiment 22 provides the delivery product of Embodiment 21 optionally
configured such that the coated substrate is converted into a three-
dimensional structure
configured to maximize surface area of the three-dimensional structure and
allow for passage
of air through the three-dimensional structure, in order to volatize the
herbal extract(s)for
inhalation by a user when heat is applied to at least one of the three-
dimensional structure or
the air passing through the three-dimensional structure.
Embodiment 23 provides the delivery product of Embodiment 22 optionally
configured such that the three-dimensional structure is a cylindrical shape
having multiple
layers of the coated substrate, and the three-dimensional structure is formed
by rolling the
coated substrate into a spiral.
Embodiment 24 provides the acts delivery product of Embodiment 22 optionally
configured such that the three-dimensional structure is tubular and includes a
longitudinal
opening extending from a first end to a second end of the three-dimensional
structure, and a
cross-section of the three-dimensional structure is a polygon.
Embodiment 25 provides the delivery product of Embodiment 22 optionally
configured such that the three-dimensional structure is rectangular and
includes multiple
layers of the coated substrate folded in a saw-tooth pattern and compressed
together to form
the rectangular shape.
Embodiment 26 provides the delivery product of any of Embodiments 22-25
optionally in combination with a delivery device configured to receive the
three-dimensional
structure and comprising a heating element for heating the three-dimensional
structure to
volatilize or entrain the herbal extract(s) in the three-dimensional structure
into a vapor or
aerosol.
Embodiment 27 provides the delivery product of Embodiment 26 optionally
configured to have multiple layers of herbal extracts, each layer being a
different herbal
extract and the volatilization or entrainment of the herbal extract(s) is
simultaneous or
sequential and is controlled by management of the heating temperature produced
by the
delivery device.
Embodiment 28 provides the delivery product of Embodiment 27 optionally
configured to include misting of water such that a hot aerosol is passed over
the delivery
53
CA 02934983 2016-06-30
product and the delivery device further comprises a misting reservoir
hydraulically connected
to the mister.
Embodiment 29 provides the delivery product of any of Embodiments 22-28
optionally further comprising one or more additional layers attached to the
coated substrate
and configured to provide at least one of flavor or adjuvant of the one or
more herbal
extract(s).
Embodiment 30 provides the delivery product of any of Embodiments 22-29
optionally configured such that the coated substrate includes first and second
electrodes
extending laterally on the coated substrate at first and second longitudinal
locations, the first
and second electrodes each having an electrical resistance sufficient to
conduct current
laterally such that at least a portion of the coated substrate can be
resistively heated, and the
herbal extract or extracts volatilize or entrain in response to the resistive
heating.
Embodiment 31 provides a delivery system comprising a coated substrate with
one or
more coating layers, the one or more coating layers including herbal
extract(s), and a heating
element for heating the coated substrate to a temperature to volatize or
entrain the herbal
extract(s) in the one or more coating layers into a vapor or aerosol inhalable
by a user.
Embodiment 32 provides the delivery system of Embodiment 31 optionally
configured such that the coated substrate is converted into a delivery
cartridge configured to
maximize surface area of the delivery cartridge and allow for passage of air
through delivery
cartridge, in order to volatize or entrain herbal extract(s) for inhalation by
a user when heat is
applied to delivery cartridge or the air passing through the delivery
cartridge.
Embodiment 33 provides the delivery system of Embodiment 31 or 32 optionally
configured such that the heating element is contained within the delivery
device and the
delivery cartridge is receivable within a receptacle of the delivery device to
heat the delivery
cartridge.
Embodiment 34 provides the delivery system of any of Embodiments 31-33
optionally configured such that the heating element is part of a vaporizer or
a nebulizer.
Embodiment 35 provides the delivery system of any of Embodiments 31-34
optionally further comprising a mister configured to add a mist to the vapor.
Embodiment 36 provides the delivery system of Embodiment 35 optionally further
comprising a misting reservoir hydraulically connected to the mister.
Embodiment 37 provides the delivery system of any of Embodiments 31-36
optionally configured such that the coated substrate includes first and second
electrodes
54
CA 02934983 2016-06-30
extending laterally on the coated substrate at first and second longitudinal
locations, the first
and second electrodes each having an electrical resistance sufficient to
conduct current
laterally along the substrate, the substrate having an electrical resistance
high enough to
conduct current longitudinally between the first and second electrodes and
resistively heat at
least a portion of the coated substrate in response to the current conducted
there through, and
the herbal extract(s) volatilizes into a gas or vapor or entrains as an
aerosol in response to the
resistive heating.
Embodiment 38 provides the delivery system of Embodiment 37 optionally
configured such that the heating element includes first and second housing
electrodes to
deliver current between the first and second electrodes on the substrate to
resistively heat at
least a portion of the coated substrate.
Embodiment 39 provides a delivery product including a cylindrical structure
extending in a longitudinal direction and formed from a substrate of an
electrically
conductive material. The cylindrical structure can include first and second
electrodes
extending laterally on the substrate at respective first and second
longitudinal locations, the
first and second electrodes each having an electrical resistance sufficient to
conduct current
laterally along the substrate, and a first substrate portion extending
longitudinally between the
first and second electrodes, the first substrate portion having an electrical
resistance high
enough to conduct current longitudinally between the first and second
electrodes and
resistively heat the first substrate portion in response to the current
conducted there through.
The cylindrical structure can also include a first dose of an herbal extract
disposed on the first
substrate portion and configured to volatilize or entrain into a gas or vapor
or aerosol in
response to the resistive heating of the first substrate portion.
Embodiment 40 provides the delivery product of Embodiment 39 optionally
configured such that the substrate is rolled to form the cylindrical structure
having a spiral
cross-section, when viewed from a longitudinal end of the rolled sheet, and
can optionally
further comprise a plurality of electrically insulating spacers positioned to
space apart
adjacent layers of the substrate.
Embodiment 41 provides the delivery product of Embodiment 40 optionally
configured such that the first and second electrodes are attached to the
substrate prior to
rolling the substrate to form the cylindrical structure.
Embodiment 42 provides the delivery product of any of Embodiments 39-41
optionally further comprising a housing configured to receive the cylindrical
structure within
CA 02934983 2016-06-30
a cavity in the housing, the cavity sized and shaped to correspond to the
cylindrical structure,
the housing having first and second housing electrodes around a circumference
of the cavity
and facing inward toward the cavity. The first and second housing electrodes
can be
positioned longitudinally to respectively contact the first and second
electrodes of the
cylindrical structure when the cylindrical structure is inserted into the
housing, and the first
and second housing electrodes can be configured to deliver current between the
first and
second electrodes of the cylindrical structure.
Embodiment 43 provides the delivery product of any of Embodiments 39-42
optionally configured such that the cylindrical structure further includes a
third electrode
extending laterally across the cylindrical structure at a third longitudinal
location, so that the
second electrode is positioned longitudinally between the first and third
electrodes; and the
third electrode has an electrical resistance small enough to conduct current
laterally along the
cylindrical structure. The cylindrical structure further includes a second
substrate portion
extending longitudinally between the second and third electrodes; and the
second substrate
portion has an electrical resistance sufficient to conduct current
longitudinally between the
second and third electrodes and resistively heat the second substrate portion
in response to the
current conducted there through. A second dose of the herbal extract which is
the same as or
different from the first dose can be disposed on the second substrate portion
and configured
to volatilize into a gas or vapor or entrain into an aerosol in response to
the resistive heating
of the second substrate portion.
Embodiment 44 provides the delivery product of Embodiment 43 optionally
further
comprising a housing configured to receive the cylindrical structure within a
cavity in the
housing, the cavity sized and shaped to correspond to the cylindrical
structure, the housing
having first, second, and third housing electrodes around a circumference of
the cavity and
facing inward toward the cavity, the first, second, and third housing
electrodes being
positioned longitudinally to respectively contact the first, second, and third
electrodes of the
cylindrical structure when the cylindrical structure is inserted into the
housing, the first and
second housing electrodes configured to deliver current between the first and
second
electrodes of the cylindrical structure, and the second and third housing
electrodes configured
to deliver current between the second and third electrodes of the cylindrical
structure.
Embodiment 45 provides the delivery product of Embodiment 44 optionally
further
comprising a controller positioned in the housing and configured to deliver
current between
the first and second housing electrodes to provide the first dose of the
herbal extract to a
56
CA 02934983 2016-06-30
patient, and further configured to deliver current between the second and
third housing
electrodes to provide second and third doses of the herbal extract to the
patient, the herbal
extract of the second and third doses being the same as or different from the
herbal extract of
the first dose and the herbal extracts of the second and third doses being the
same or being
different.
Embodiment 46 provides the delivery product of Embodiment 45 optionally
configured such that the controller delivers current between the first and
second housing
electrodes at a first time to provide the first dose of the herbal extract to
a user and delivers
current between the second and third housing electrodes at a second time and
third, different
from the first time, to provide the second dose and third dose of herbal
extract to the user.
Embodiment 47 provides the delivery product of Embodiment 45 optionally
configured such that the controller delivers current between the first and
second housing
electrodes and simultaneously delivers current between the second and third
housing
electrodes to provide the first, second and doses of herbal extracts to the
user at the same
time.
Embodiment 48 provides the delivery product of any of Embodiments 44-47
optionally configured such that the housing is elongated and includes a first
longitudinal end
configured to deliver the vapor or aerosol into a user's mouth.
Embodiment 49 provides the delivery product of any of Embodiments 39-48
optionally configured such that the herbal extract(s) include at least one of
a coniferous
extract, menthol, nutmeg oil, digitalis, methyl salicylate, acetyl salicylic
acid or the methyl
ester thereof, tetrahydrocannabinol, cannabidiol, arachidonic acid, a steroid
such as
budesonide, mometasone or fluticasone, niacin, caffeine, cacao extract, or
coca leaf extract.
The purified forms of some of these extracts may be purchased as substances
previously
obtained from herbaceous plants and/or optionally synthetically modified. The
non-salt
forms, e.g., free bases, free acids and non-complexed neutral forms are
preferred for
volatilization and/or entrainment as vapors and/or aerosols.
Embodiment 50 provides the delivery product of any of Embodiments 39-49
optionally configured such that the first and second electrodes are formed
integrally with the
substrate and are thicker than the first substrate portion.
Embodiment 51 provides the delivery product of any of Embodiments 39-50
optionally configured such that the housing further comprises a mister
configured to add a
mist to the volatized first dose of the one or more herbal extracts.
57
CA 02934983 2016-06-30
Embodiment 52 provides the delivery product of Embodiment 51 optionally
configured such that the housing further comprises a misting reservoir
hydraulically
connected to the mister.
Embodiment 53 provides an apparatus including a cylindrical structure
extending in a
longitudinal direction and formed from a substrate of an electrically
conductive material. The
cylindrical structure can include a plurality of electrodes extending
laterally on the substrate
at respective longitudinal locations, each electrode in the plurality having
an electrical
resistance sufficient to conduct current laterally along the substrate. The
cylindrical structure
can include at least one substrate portion extending longitudinally between
the adjacent
electrodes in the plurality, each substrate portion having an electrical
resistance sufficient to
conduct current longitudinally between the adjacent electrodes and resistively
heat the
substrate portion in response to the current conducted there through. The
cylindrical structure
can include herbal extract(s) disposed on each substrate portion and
configured to volatilize
or entrain into a vapor or aerosol in response to the resistive heating of the
substrate portion
Embodiment 54 provides the apparatus of Embodiment 53 optionally configured
such
that the substrate is rolled to form the cylindrical structure having a spiral
cross-section, when
viewed from a longitudinal end of the rolled sheet, and optionally further
comprising a
plurality of electrically insulating spacers positioned to space apart
adjacent layers of the
substrate.
Embodiment 55 provides the apparatus of Embodiment 54 optionally configured
such
that the first and second electrodes are attached to the substrate prior to
rolling the substrate
to form the cylindrical structure.
Embodiment 56 provides the apparatus of any of Embodiments 53-55 optionally
configured such that a first lateral end of the substrate is connected to a
second lateral end of
the substrate to form the cylindrical structure having a tubular shape, and
each of the plurality
of electrodes extend around an exterior circumference of the tubular shape.
Embodiment 57 provides the apparatus of any of Embodiments 53-56 optionally
further comprising a housing configured to receive the cylindrical structure
within a cavity
sized and shaped to receive the cylindrical structure, the housing having a
plurality of
housing electrodes around a circumference of the cavity and facing inward
toward the cavity,
each housing electrode being positioned longitudinally to respectively contact
a respective
electrode of the cylindrical structure when the cylindrical structure is
inserted into the
58
CA 02934983 2016-06-30
housing. Each pair of adjacent housing electrodes can be configured to deliver
current
between a corresponding pair of adjacent electrodes of the cylindrical
structure.
Embodiment 58 provides the apparatus of Embodiment 57 optionally further
comprising a controller positioned in the housing and configured to deliver
current between
adjacent pairs of housing electrodes at sequential times to provide a dose of
the
corresponding herbal extract to a user at each sequential time, or deliver
current between
adjacent pairs of housing electrodes simultaneously to provide more than one
dose of the
corresponding herbal extract(s) to the user at one time.
Embodiment 59 provides the apparatus of any of Embodiments 53-58 optionally
configured such that the herbal extract or extracts includes at least one of a
coniferous extract,
menthol, nutmeg oil, digitalis, methyl salicylate, acetyl salicylic acid or
the methyl ester
thereof, tetrahydrocannabinol, cannabidiol, arachidonic acid, a steroid such
as budesonide,
mometasone or fluticasone, niacin, caffeine, cacao extract, or coca leaf
extract. The purified
forms of some of these extracts may be purchased as substances previously
obtained from
herbaceous plants and/or optionally synthetically modified. The non-salt
forms, e.g., free
bases, free acids and non-complexed neutral forms are preferred for
volatilization and/or
entrainment as vapors and/or aerosols.
Embodiment 60 provides the apparatus of any of Embodiments 53-59 optionally
configured such that the housing further comprises a mister configured to add
a mist to the
volatilized herbal extract or extracts.
Embodiment 61 provides the apparatus of Embodiment 60 optionally configured
such
that the housing further comprises a misting reservoir hydraulically connected
to the mister.
Embodiment 62 provides a method including forming or providing a sheet of
conductive material, the sheet extending in longitudinal and lateral
dimensions, the sheet
having a plurality of contact portions spaced apart longitudinally and
extending laterally
across the sheet, the sheet having at least one substrate portion extending
longitudinally
between a pair of adjacent contact portions, the contact portions having a
thickness greater
than a thickness of the at least one substrate portion. The method including
depositing an
herbal extract or multiple herbal extracts on the corresponding substrate
portion or portions,
the herbal extract(s) being configured to volatilize into a vapor or entrain
as an aerosol in
response to resistive heating of the respective substrate portion, and
converting the sheet into
a cylindrical structure.
59
CA 02934983 2016-06-30
Embodiment 63 provides the method of Embodiment 62 optionally configured such
that converting the sheet into a cylindrical structure includes rolling the
sheet such that the
cylindrical structure has a spiral cross-section, when viewed from a
longitudinal end of the
rolled sheet. The method can optionally further comprise, as the sheet is
rolled, placing a
plurality of electrically insulating spacers between adjacent layers of the
sheet, the spacers
being spaced apart to allow a flow of gas or aerosol there-around.
Embodiment 64 provides the method of Embodiment 62 or 63 optionally configured
such that converting the sheet into a cylindrical structure includes
connecting a first lateral
end of the sheet to a second lateral end of the sheet to form the cylindrical
structure having a
tubular shape, and each of the plurality of contact portions extends around a
circumference of
the tubular shape.
Embodiment 65 provides the method of any of Embodiments 62-64 optionally
configured such that the cylindrical structure is configured for use as a
delivery cartridge.
Embodiment 66 provides the method of any of Embodiments 62-65 wherein the
herbal extract(s)includes at least one of a coniferous extract, menthol,
nutmeg oil, digitalis,
methyl salicylate, acetyl salicylic acid or the methyl ester thereof,
tetrahydrocannabinol,
cannabidiol, arachidonic acid, a steroid such as budesonide, mometasone or
fluticasone,
niacin, caffeine, cacao extract, or coca leaf extract. The purified forms of
some of these
extracts may be purchased as substances previously obtained from herbaceous
plants and/or
optionally synthetically modified. The non-salt forms, e.g., free bases, free
acids and non-
complexed neutral forms are preferred for volatilization and/or entrainment as
vapors and/or
aerosols.
Embodiment 67 provides a method, system, product or apparatus of any one or
any
combination of Embodiments 1-66, which can be optionally configured such that
all steps or
elements recited are available to use or select from.
Embodiment 68 provides a method, system, product and/or apparatus for
practicing
wet extraction and isolation of herbal extract(s)from one or more comminuted
herbaceous
plant compositions to produce one or more purified herbal extracts and
deposition of one or
more herbal extract on corresponding portions of a substrate to provide a
substrate coated
with one or more herbal extracts, the herbal extract(s) being present as
overlapping layers on
the substrate or as segregated layers on the substrate, the coated substrate
being preferably
configured to provide a delivery cartridge corresponding to a delivery system
wherein the
CA 02934983 2016-06-30
delivery system is configured to volatilize the herbal extract(s) or entrain
the herbal extract(s)
so as to produce a vapor or aerosol of the herbal extract(s) for
administrative delivery by
inhalation to a patient.
Embodiment 69 provides a method and product of Embodiment 68 in which the wet
extraction and isolation includes formation of a concentrate of the one or
more comminuted
herbaceous plant compositions in one or more appropriate solvents so as to
extract the herbal
extract(s) from the composition or compositions and provide one or more
solutions of herbal
extract(s), concentrating the one or more solutions to form concentrates or
optionally
crystallizing the herbal extract(s)from the solutions or optionally forming
fleet oils of the one
or more concentrated solutions and depositing and/or casting the one or more
crystallized
herbal extract(s) and/or one or more oils of herbal extracts and/or one or
more concentrates
onto the one or more corresponding portions of the substrate.
Embodiment 70 provides a method and product of Embodiments 68 and 69 in which
the concentration of the one or more solutions is accomplished by stirred
vacuum removal of
solvent and the crystallization is accomplished by addition of a non-solvent
for the herbal
extract(s) to the concentrated solution of herbal extract(s).
Embodiment 71 provides a method, system, product and/or apparatus of
Embodiments 68, 69 and /or 70 wherein the herbaceous plant material is pre-
treated as
described in Embodiments 7-10.
Embodiment 72 provides a coated substrate, a delivery cartridge and/or a
delivery
system of any of Embodiments 11-66 wherein the substrate coated with one or
more
overlapping or segregated layer is herbal extract(s) is produced according to
the wet
extraction methods of Embodiments 68, 69 and/or 70 and optionally by
incorporating the pre-
treatment of herbaceous plant material according to Embodiments 7-10.
Embodiment 73 provides an apparatus including a cylindrical structure
extending in a
longitudinal direction and formed from a substrate of an electrically
conductive material. The
cylindrical structure can include a plurality of electrodes extending
laterally on the substrate
at respective longitudinal locations, each electrode in the plurality having
an electrical
resistance sufficient to conduct current laterally along the substrate. The
cylindrical structure
can include at least one substrate portion extending longitudinally between
the adjacent
electrodes in the plurality, each substrate portion having an electrical
resistance sufficient to
conduct current longitudinally between the adjacent electrodes and resistively
heat the
substrate portion in response to the current conducted there-through. The
cylindrical
61
CA 02934983 2016-06-30
structure can include herbal extract(s) of Embodiments 68-70 disposed on each
substrate
portion and configured to volatilize into a gas or vapor or to become
entrained as an aerosol
in air in response to the resistive heating of the substrate portion and an
optional flow of air
through the cylindrical structure.
62