Note: Descriptions are shown in the official language in which they were submitted.
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Streptococcus pneumoniae capsular polysaccharides and conjugates thereof
Field of the invention
The invention relates to isolated Streptococcus pneumoniae serotype 15B
capsular
polysaccharide and processes for their preparation. The invention also relates
to
immunogenic conjugates comprising Streptococcus pneumoniae serotype 15B
capsular
polysaccharide covalently linked to a carrier protein, processes for their
preparation and
immunogenic compositions and vaccines comprising them.
Background
Streptococcus pneumoniae are Gram-positive, lancet shaped cocci that are
usually seen in
pairs (diplococci), but also in short chains or as single cells. They grow
readily on blood agar
plates with glistening colonies and display alpha hemolysis unless grown
anaerobically
where they show beta hemolysis. The cells of most pneumococcal serotypes have
a capsule
which is a polysaccharide coating surrounding each cell. This capsule is a
determinant of
virulence in humans, as it interferes with phagocytosis by preventing
antibodies from
attaching to the bacterial cells. Currently there are more than 90 known
pneumococcal
capsular serotypes identified, with the 23 most common serotypes accounting
for
approximately 90% of invasive disease worldwide. As a vaccine, the
pneumococcal
polysaccharide coat can confer a reasonable degree of immunity to
Streptococcus
pneumoniae in individuals with developed or unimpaired immune systems, but the
capsular
polysaccharide conjugated to a suitable carrier protein allows for an immune
response in
infants and elderly who are also at most risk for pneumococcal infections.
Since the introduction of the first 7-valent pneumococcal conjugate vaccine
(PCV7 or
Prevnar) in 2000, invasive disease from those seven serotypes (4, 6B, 9V, 14,
18C, 19F, and
23F) has nearly disappeared. The addition of serotypes 1, 3, 5, 6A, 7F and 19A
in Prevnar
13 further decreased the numbers of invasive pneumococcal disease.
However, the incidence of invasive pneumococcal diseases caused by non-vaccine
serotypes such as Streptococcus pneumoniae serotypes 15A, 15B and 15C has
recently
increased (see for example Beall B. et al, Journal of Clinical Microbiology.
44(3):999-1017,
2006, or Jacobs et Al, Clin Infect Dis. (2008) 47 (11): 1388-1395). None of
the currently
marketed pneumococcal vaccine provides an appropriate protection against
serotype 15B
Streptococcus pneumoniae in human and in particular in children less than 2
years old.
Therefore, there is a need for immunogenic compositions that can be used to
induce an
1
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
immune response against serotype 15B Streptococcus pneumoniae. It would also
be an
additional benefit if such immunogenic composition could be used to protect
subjects against
serotype 15C and/or 15A Streptococcus pneumoniae.
Summary of the invention
In one aspect the present disclosure provides an isolated Streptococcus
pneumoniae
serotype 15B capsular polysaccharide having a molecular weight between 5kDa
and
500kDa.
In a further aspect, the present disclosure provides an isolated Streptococcus
pneumoniae
serotype 15B capsular polysaccharide comprising at least 0.1, 0.2, 0.3, 0.4,
0.5, 0.6, 0.7 or
0.8, preferably at least 0.6 mM, acetate per mM of said Streptococcus
pneumoniae serotype
15B capsular polysaccharide
In a further aspect, the present disclosure provides an isolated Streptococcus
pneumoniae
serotype 15B capsular polysaccharide comprising at least 0.1, 0.2, 0.3, 0.4,
0.5, 0.6, 0.7 or
0.8, preferably at least 0.6 mM glycerol per mM of said Streptococcus
pneumoniae serotype
15B capsular polysaccharide.
In a further aspect, the present disclosure provides an immunogenic conjugate
comprising an
isolated Streptococcus pneumoniae serotype 15B capsular polysaccharide
disclosed herein
covalently linked to a carrier protein. In one aspect, said carrier protein is
CRM197.
In a further aspect, the present disclosure provides an immunogenic
composition comprising
an immunogenic conjugate disclosed herein and a physiologically acceptable
vehicle. In one
aspect, said immunogenic composition further comprises at least one additional
antigen. In
one aspect, said immunogenic composition further comprises an adjuvant.
In a further aspect, the present disclosure provides a vaccine comprising an
immunogenic
composition as disclosed herein.
In a further aspect, the present disclosure provides a process for producing
an isolated
serotype 15B polysaccharide as disclosed herein, the process comprising the
steps of:
(a) preparing a fermentation culture of Streptococcus pneumonia serotype 15B
bacterial
cells;
(b) lysing the bacterial cells in said fermentation culture;
(c) purifying Streptococcus pneumoniae serotype 15B capsular polysaccharide
from the
fermentation culture; and,
(d) sizing the purified Streptococcus pneumoniae serotype 15B capsular
polysaccharide by
high pressure homogenization.
2
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a further aspect, the present disclosure provides a process for producing
an activated
Streptococcus pneumoniae serotype 15B capsular polysaccharide, said process
comprising
the step of reacting an isolated Streptococcus pneumoniae serotype 15B
capsular
polysaccharide as disclosed herein with an oxidizing agent. In one aspect, the
present
disclosure provides an activated serotype 15B capsular polysaccharide obtained
or
obtainable by the above process.
In a further aspect, the present disclosure provides a process for the
preparation of an
immunogenic conjugate comprising Streptococcus pneumoniae serotype 15B
capsular
polysaccharide covalently linked to a carrier protein, the process comprising
the steps of:
(a) compounding an activated polysaccharide as disclosed herein with a carrier
protein;
(b) reacting the compounded, activated polysaccharide and carrier protein with
a reducing
agent to form a serotype 15B capsular polysaccharide-carrier protein
conjugate. In one
aspect, the present disclosure provides an immunogenic conjugate obtained or
obtainable by
the above process.
In a further aspect, the present disclosure provides a method of protecting a
subject against
an infection with serotype 15B Streptococcus pneumoniae, the method comprising
administering to a subject an immunogenic amount of the immunogenic
composition or the
vaccine disclosed herein.
In a further aspect, the present disclosure provides a method of treating or
preventing a
Streptococcus pneumoniae infection, disease or condition associated with
serotype 15A, 15B
and/or 150 Streptococcus pneumoniae in a subject, the method comprising the
step of
administering a therapeutically or prophylactically effective amount of an
immunogenic
composition or a vaccine disclosed herein.
Brief description of the drawinds
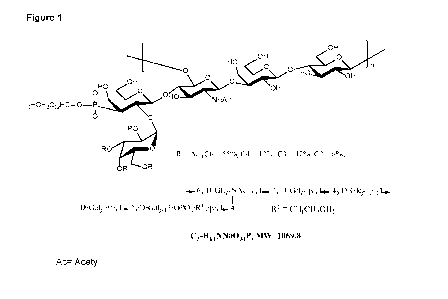
Figure 1 - Structure of Pneumococcal Capsular polysaccharide Serotype 15B
Repeat Unit
Detailed description of the invention
The present invention may be understood more readily by reference to the
following detailed
description of the preferred embodiments of the invention and the Examples
included herein.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
pertains. Although any methods and materials similar or equivalent to those
described
herein can be used in the practice or testing of the present invention,
certain preferred
3
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
methods and materials are described herein. In describing the embodiments and
claiming
the invention, certain terminology will be used in accordance with the
definitions set out
below.
Definitions
As used herein, the "molecular weight" of a polysaccharide or of a
polysaccharide-carrier
protein conjugate refers to molecular weight calculated by size exclusion
chromatography
(SEC) combined with multiangle laser light scattering detector (MALLS).
As used herein, the term "free polysaccharide" means a serotype 15B capsular
polysaccharide that is not covalently conjugated to the carrier protein, but
is nevertheless
present in the serotype 15B capsular polysaccharide-carrier protein conjugate
composition.
The free polysaccharide may be non-covalently associated with (i.e., non-
covalently bound
to, adsorbed to, or entrapped in or with) the polysaccharide-carrier protein
conjugate.
The percentage of free polysaccharide is measured after the final purification
of the serotype
15B capsular polysaccharide-carrier protein conjugate. Preferably it is
measured within 4
weeks after the final purification. It is expressed as a percentage of the
total polysaccharide
in the sample.
As used herein, the term "serotype 15B polysaccharide" or "serotype 15B
capsular
polysaccharide" refers to a Streptococcus pneumoniae serotype 15B capsular
polysaccharide.
As used herein, the term "serotype 15B glycoconjugate" or "serotype 15B
conjugate" refers
to an isolated serotype 15B polysaccharide covalently conjugated to a carrier
protein.
As used herein, the term "degree of oxidation" (DO) refers to the number of
sugar repeat
units per aldehyde group generated when the isolated polysaccharide is
activated with an
oxidizing agent. The degree of oxidation of a polysaccharide can be determined
using routine
methods known to the man skilled in the art.
As used herein, the term "subject" refers to a mammal, including a human, or
to a bird, fish,
reptile, amphibian or any other animal. The term "subject" also includes
household pets or
research animals. Non-limiting examples of household pets and research animals
include:
dogs, cats, pigs, rabbits, rats, mice, gerbils, hamsters, guinea pigs,
ferrets, monkeys, birds,
snakes, lizards, fish, turtles, and frogs. The term "subject" also includes
livestock animals.
4
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Non-limiting examples of livestock animals include: alpaca, bison, camel,
cattle, deer, pigs,
horses, llamas, mules, donkeys, sheep, goats, rabbits, reindeer, yak,
chickens, geese, and
turkeys.
Isolated serotype 15B capsular polysaccharide
As shown in figure 1, the polysaccharide repeating unit of serotype 15B
consists of a
branched trisaccharide backbone (one N-acetylglucosamine (GIcpNAc), one
galactopyranose
(Gal) and one glucopyranose (Glcp)) with an aGalp-r3Galp disaccharide branch
linked to the
04 hydroxyl group of GlcpNAc. The phosphoglycerol is linked to the 03 hydroxyl
group of the
r3Galp residue in the disaccharide branch. Serotype 15B capsular
polysaccharide is 0-
acetylated and the total amount of 0-acetylation is approximately 0.8 to 0.9 0-
acetyl groups
per polysaccharide repeating unit (see for example C. Jones et Al,
Carbohydrate Research,
340 (2005) 403-409). Capsular polysaccharide from serotype 15C serotype has
the identical
backbone structure as serotype 15B but lacks the 0-acetylation.
The isolated serotype 15B polysaccharide of the invention can be obtained by a
process
comprising the steps of:
(a) preparing a fermentation culture of serotype 15B Streptococcus pneumonia
bacterial
cells;
(b) lysing the bacterial cells in said fermentation culture;
(c) purifying serotype 15B polysaccharide from the fermentation culture; and,
(d) sizing the purified serotype 15B polysaccharide by high pressure
homogenization.
Serotype 15B polysaccharides can be obtained directly from bacteria using
isolation
procedures known to one of ordinary skill in the art (see for example methods
disclosed U.S.
Patent App. Pub. Nos. 20060228380, 20060228381, 20070184071, 20070184072,
20070231340, and 20080102498 or W02008118752). In addition, they can be
produced
using synthetic protocols.
Serotype 15B Streptococcus pneumoniae strains may be obtained from established
culture
collections (such as for example ATCC deposit strain No ATCC10354 or strain
available from
the Streptococcal Reference Laboratory of the Center for disease control and
prevention,
Atlanta, GA)) or clinical specimens.
5
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
The bacterial cells are preferably grown in a soy based medium. Following
fermentation of
bacterial cells that produce Streptococcus pneumoniae serotype 15B capsular
polysaccharides, the bacterial cells are lysed to produce a cell lysate. The
bacterial cells may
be lysed using any lytic agent. A "lytic agent" is any agent that aids in cell
wall breakdown
and release of autolysin which causes cellular lysis including, for example,
detergents. As
used herein, the term "detergent" refers to any anionic or cationic detergent
capable of
inducing lysis of bacterial cells. Representative examples of such detergents
for use within
the methods of the present invention include deoxycholate sodium (DOC), N-
lauroyl
sarcosine, chenodeoxycholic acid sodium, and saponins.
In one embodiment of the present invention, the lytic agent used for lysing
bacterial cells is
DOC. DOC is the sodium salt of the bile acid deoxycholic acid, which is
commonly derived
from biological sources such as cows or oxen. DOC activates the LytA protein,
which is an
autolysin that is involved in cell wall growth and division in Streptococcus
pneumoniae. The
LytA protein has choline binding domains in its C-terminal portion, and
mutations of the lytA
gene are known to produce LytA mutants that are resistant to lysis with DOC.
In one embodiment of the present invention, the lytic agent used for lysing
bacterial cells is a
non-animal derived lytic agent. Non-animal derived lytic agents for use within
the methods of
the present invention include agents from non-animal sources with modes of
action similar to
that of DOC (i. e., that affect LytA function and result in lysis of
Streptococcus pneumoniae
cells). Such non-animal derived lytic agents include, but are not limited to,
analogs of DOC,
surfactants, detergents, and structural analogs of choline. In one embodiment,
the non-
animal derived lytic agent is selected from the group consisting of
decanesulfonic acid, tert-
octylphenoxy poly(oxyethylene)ethanols (e.g. Igepale CA-630, CAS #: 9002-93-1,
available
from Sigma Aldrich, St. Louis, MO), octylphenol ethylene oxide condensates
(e.g. Triton X-
100, available from Sigma Aldrich, St. Louis, MO), N-lauroyl sarcosine, N-
lauroyl sarcosine
sodium, lauryl iminodipropionate, sodium dodecyl sulfate, chenodeoxycholate,
hyodeoxycholate, glycodeoxycholate, taurodeoxycholate, taurochenodeoxycholate,
and
cholate. In another embodiment, the non-animal derived lytic agent is N-
lauroyl sarcosine. In
another embodiment, the lytic agent is N-lauroyl sarcosine sodium.
The serotype 15B polysaccharide may then be isolated from the cell lysate
using purification
techniques known in the art, including the use of centrifugation, depth
filtration, precipitation,
ultra-filtration, treatment with activate carbon, diafiltration and/or column
chromatography
(See, for example, U.S. Patent App. Pub. Nos. 20060228380, 20060228381,
20070184071,
20070184072, 20070231340, and 20080102498 or W02008118752). The purified
serotype
15B capsular polysaccharide can then be used for the preparation of
immunogenic
conjugates.
6
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Preferably, in order to generate conjugates with advantageous filterability
characteristics
and/or yields, sizing of the polysaccharide to a lower molecular weight (MVV)
range is
performed prior to the conjugation to a carrier protein. Advantageously, the
size of the
purified serotype 15B polysaccharide is reduced while preserving critical
features of the
structure of the polysaccharide such as for example the presence of 0-acetyl
groups.
Preferably, the size of the purified serotype 15B polysaccharide is reduced by
mechanical
homogenization.
In a preferred embodiment, the size of the purified serotype 15B
polysaccharide is reduced
by high pressure homogenization. High pressure homogenization achieves high
shear rates
by pumping the process stream through a flow path with sufficiently small
dimensions. The
shear rate is increased by using a larger applied homogenization pressure and
exposure
time can be increased by recirculating the feed stream through the
homogenizer.
The high pressure homogenization process is particularly appropriate for
reducing the size of
the purified serotype 15B polysaccharide while preserving the structural
features of the
polysaccharide such as the presence of 0-acetyl groups.
The isolated serotype 15B capsular polysaccharide obtained by purification of
serotype 15B
polysaccharide from the Streptococcus pneumoniae lysate and optionally sizing
of the
purified polysaccharide can be characterized by different parameters including
for example
the molecular weight, the mM of glycerol per mM of said serotype 15B capsular
polysaccharide or the mM of acetate per mM of said serotype 15B capsular
polysaccharide.
The degree of 0-acetylation of the polysaccharide can be determined by any
method known
in the art, for example, by proton NMR (see for example Lemercinier and Jones
(1996)
Carbohydrate Research 296; 83-96, Jones and Lemercinier (2002) J.
Pharmaceutical and
Biomedical Analysis 30; 1233-1247, WO 05/033148 or W000/56357). Another
commonly
used method is described in Hestrin (1949) J. Biol. Chem. 180; 249-261.
Preferably, the
presence of 0-acetyl groups is determined by ion-HPLC analysis.
The presence of 0-acetyl in a purified, isolated or activated serotype 15B
capsular
polysaccharide or in a serotype 15B polysaccharide-carrier protein conjugate
is expressed as
the number of mM of acetate per mM of said polysaccharide or as the number of
0-acetyl
group per polysaccharide repeating unit.
7
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
The presence of glycerolphosphate side chains can be determined by measurement
of
glycerol using high performance anion exchange chromatography with pulsed
amperometric
detection (HPAEC-PAD) after its release by treatment of the polysaccharide
with hydrofluoric
acid (HF). The presence of glycerol in a purified, isolated or activated
serotype 15B
polysaccharide or in a serotype 15B polysaccharide-carrier protein conjugate
is expressed as
the number of mM of glycerol per mM of serotype 15B polysaccharide.
The isolated serotype 15B capsular polysaccharide can also be produced
synthetically using
methods known to the man skilled in the art.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
has a
molecular weight between 5 and 500kDa, 50 and 500 kDa, 50 and 450kDa, 100 and
400kDa,
100 and 350 kDa. In a preferred embodiment, the isolated serotype 15B capsular
polysaccharide has a molecular weight between 100 and 350kDa. In a preferred
embodiment, the isolated serotype 15B capsular polysaccharide has a molecular
weight
between 100 and 300kDa. In a preferred embodiment, the isolated serotype 15B
capsular
polysaccharide has a molecular weight between 150 and 300kDa.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
comprises at
least 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 or 0.8 mM acetate per mM of said
serotype 15B capsular
polysaccharide. In a preferred embodiment, the isolated serotype 15B capsular
polysaccharide comprises at least 0.5, 0.6 or 0.7 mM acetate per mM of said
serotype 15B
capsular polysaccharide. In a preferred embodiment, the isolated serotype 15B
capsular
polysaccharide comprises at least 0.6 mM acetate per mM of said serotype 15B
capsular
polysaccharide. In a preferred embodiment, the isolated serotype 15B capsular
polysaccharide comprises at least 0.7 mM acetate per mM of said serotype 15B
capsular
polysaccharide. In a preferred embodiment, the presence of 0-acetyl groups is
determined
by ion-HPLC analysis.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
comprises at
least 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 or 0.8 mM glycerol per mM of said
serotype 15B capsular
polysaccharide. In a preferred embodiment, the isolated serotype 15B capsular
polysaccharide comprises at least 0.5, 0.6 or 0.7 mM glycerol per mM of said
serotype 15B
capsular polysaccharide. In a preferred embodiment, the isolated serotype 15B
capsular
polysaccharide comprises at least 0.6 mM glycerol per mM of said serotype 15B
capsular
8
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
polysaccharide. In a preferred embodiment, the isolated serotype 15B capsular
polysaccharide comprises at least 0.7 mM glycerol per mM of said serotype 15B
capsular
polysaccharide.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 350 kDa, preferably 150 and 350kDa, and
comprises at
least 0.6 mM acetate per mM of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 350 kDa, preferably 150 and 350kDa, and
comprises at
least 0.6 mM glycerol per mM of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
comprises at
least 0.6 mM acetate per mM of said serotype 15B capsular polysaccharide and
at least 0.6
mM glycerol per mM of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 350 kDa, preferably 150 and 350kDa, and
comprises at
least 0.6 mM acetate per mM of said serotype 15B capsular polysaccharide and
at least 0.6
mM glycerol per mM of said serotype 15B capsular polysaccharide.
Serotype 15B capsular polysaccharide-carrier protein conjugate
The isolated serotype 15B capsular polysaccharide may be conjugated to a
carrier protein to
obtain an immunogenic conjugate. The isolated polysaccharide can be conjugated
to the
carrier protein by methods known to the skilled person (See, for example, U.S.
Patent App.
Pub. Nos. 20060228380, 20070184071, 20070184072, 20070231340 or
W02011/100151).
In an embodiment, the polysaccharide may be activated with 1-cyano-4-
dimethylamino
pyridinium tetrafluoroborate (CDAP) to form a cyanate ester. The activated
polysaccharide
may be coupled directly or via a spacer (linker) group to an amino group on
the carrier
protein. For example, the spacer could be cystamine or cysteamine to give a
thiolated
polysaccharide which could be coupled to the carrier via a thioether linkage
obtained after
reaction with a maleimide-activated carrier protein (for example using GMBS)
or a
haloacetylated carrier protein (for example using iodoacetimide or N-
succinimidyl
bromoacetate or SIAB, or SIA, or SBAP). Preferably, the cyanate ester
(optionally made by
CDAP chemistry) is coupled with hexane diamine or adipic acid dihydrazide
(ADH) and the
9
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
amino-derivatised saccharide is conjugated to the carrier protein using
carbodiimide (e.g.
EDAC or EDC) chemistry via a carboxyl group on the protein carrier. Such
conjugates are
described for example in W093/15760, WO 95/08348 and WO 96129094.
Other suitable techniques use carbodiimides, hydrazides, active esters,
norborane, p-
nitrobenzoic acid, N-hydroxysuccinimide, S--NHS, EDC, TSTU. Many are described
in
International Patent Application Publication No. WO 98/42721. Conjugation may
involve a
carbonyl linker which may be formed by reaction of a free hydroxyl group of
the saccharide
with CD! (See Bethell et al., 1979,1. Biol. Chern. 254:2572-4; Hearn et al.,
1981, J.
Chromatogr.218:509-18) followed by reaction with a protein to form a carbamate
linkage.
This may involve reduction of the anomeric terminus to a primary hydroxyl
group, optional
protection/deprotection of the primary hydroxyl group, reaction of the primary
hydroxyl group
with CD! to form a CD! carbamate intermediate and coupling the CD! carbamate
intermediate
with an amino group on a protein.
In a preferred embodiment, the isolated serotype 15B capsular polysaccharide
is conjugated
to the carrier protein by reductive amination. Reductive amination involves
activation of the
polysaccharide by oxidation and conjugation of the activated polysaccharide to
a protein
carrier by reduction.
Activation of serotype 15B capsular polysaccharide
An activated serotype 15B capsular polysaccharide is obtained by reacting an
isolated
serotype 15B capsular polysaccharide with an oxidizing agent. For example,
said activated
serotype 15B capsular polysaccharide can be obtained by a process comprising
the following
steps:
(a) preparing a fermentation culture of serotype 15B Streptococcus pneumonia
bacterial
cells;
(b) lysing the bacterial cells in said fermentation culture;
(C) purifying serotype 15B polysaccharide from the fermentation culture;
(d) sizing the purified serotype 15B polysaccharide by high pressure
homogenization.
(e) reacting the sized serotype 15B polysaccharide with an oxidizing agent.
In a preferred embodiment, the concentration of isolated serotype 15B capsular
polysaccharide which is reacted with an oxidizing agent is between 0.1 and 10
mg/mL, 0.5
and 5 mg/mL, 1 and 3 mg/mL, or about 2 mg/mL.
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a preferred embodiment, the oxidizing agent is periodate. The periodate
oxidises vicinal
hydroxyl groups to form carbonyl or aldehyde groups and causes cleavage of a C-
C bond.
The term 'periodate' includes both periodate and periodic acid. This term also
includes both
metaperiodate (104) and orthoperiodate (1065). The term 'periodate' also
includes the various
salts of periodate including sodium periodate and potassium periodate. In a
preferred
embodiment, the oxidizing agent is sodium periodate. In a preferred embodiment
the
periodate used for the oxidation of serotype 15B capsular polysaccharide is
metaperiodate.
In a preferred embodiment the periodate used for the oxidation of serotype 15B
capsular
polysaccharide is sodium metaperiodate.
In a preferred embodiment, the polysaccharide is reacted with 0.01 to 10, 0.05
to 5, 0.1 to 1,
0.5 to 1, 0.7 to 0.8, 0.05 to 0.5, 0.1 to 0.3 molar equivalent of oxidizing
agent. In a preferred
embodiment, the polysaccharide is reacted with about 0.1, 0.15, 0.2, 0.25,
0.3, 0.35, 0.4,
0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95 molar equivalent
of oxidizing agent.
In a preferred embodiment, the polysaccharide is reacted with about 0.15 molar
equivalent of
oxidizing agent. In a preferred embodiment, the polysaccharide is reacted with
about 0.25
molar equivalent of oxidizing agent. In a preferred embodiment, the
polysaccharide is
reacted with about 0.5 molar equivalent of oxidizing agent. In a preferred
embodiment, the
polysaccharide is reacted with about 0.6 molar equivalent of oxidizing agent.
In a preferred
embodiment, the polysaccharide is reacted with about 0.7 molar equivalent of
oxidizing
agent.
In a preferred embodiment, the duration of the reaction is between 1 and 50,
10 and 30, 15
and 20, 15 and 17 hours or about 16 hours.
In a preferred embodiment, the temperature of the reaction is maintained
between 15 and
45 C, 15 and 30 C, 20 and 25 C. In a preferred embodiment, the temperature of
the reaction
is maintained at about 23 C.
In a preferred embodiment, the oxidation reaction is carried out in a buffer
selected from
sodium phosphate, potassium phosphate, 2-(N-morpholino)ethanesulfonic acid
(MES) or Bis-
Tris. In a preferred embodiment, the buffer is potassium phosphate.
In a preferred embodiment, the buffer has a concentration of between 1 and 500
mM, 1 and
300mM, 50 and 200mM. In a preferred embodiment the buffer has a concentration
of about
100mM.
11
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a preferred embodiment, the oxidation reaction is carried out at a pH
between 4 and 8, 5
and 7, 5.5 and 6.5. In a preferred embodiment, the pH is about 6.
In preferred embodiment, the activated serotype 15B capsular polysaccharide is
obtained by
reacting 0.5 to 5 mg/mL of isolated serotype 15B capsular polysaccharide with
0.2 to 0.3
molar equivalent of periodate at a temperature between 20 and 25 C.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
is purified.
The activated serotype 15B capsular polysaccharide is purified according to
methods known
to the man skilled in the art such as gel permeation chromatography (GPC),
dialysis or
ultrafiltration/diafiltration. For example, the activated capsular
polysaccharide is purified by
concentration and diafiltration using an ultrafiltration device.
In a preferred embodiment, the invention relates to an activated serotype 15B
capsular
polysaccharide obtained or obtainable by the above disclosed process.
In a preferred embodiment, the degree of oxidation of the activated serotype
15B capsular
polysaccharide is between 2 and 20, 2 and 15, 2 and 10, 2 and 5, 5 and 20, 5
and 15, 5 and
10, 10 and 20, 10 and 15, 15 and 20. In a preferred embodiment the degree of
oxidation of
the activated serotype 15B capsular polysaccharide is between 2 and 10, 4 and
8, 4 and 6, 6
and 8, 6 and 12, 8 and 12, 9 and 11, 10 and 16, 12 and 16, 14 and 18, 16 and
20, 16 and 18,
or 18 and 20.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
has a
molecular weight between 5 and 500kDa, 50 and 500 kDa, 50 and 450kDa, 100 and
400kDa,
100 and 350 kDa. In a preferred embodiment, the activated serotype 15B
capsular
polysaccharide has a molecular weight between 100 and 350kDa. In a preferred
embodiment, the activated serotype 15B capsular polysaccharide has a molecular
weight
between 100 and 300kDa. In a preferred embodiment, the activated serotype 15B
capsular
polysaccharide has a molecular weight between 150 and 300kDa. In a preferred
embodiment, the activated serotype 15B capsular polysaccharide has a molecular
weight
between 100 and 250kDa.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
comprises at
least 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 or 0.8 mM acetate per mM of said
serotype 15B capsular
polysaccharide. In a preferred embodiment, the activated serotype 15B capsular
polysaccharide comprises at least 0.5, 0.6 or 0.7 mM acetate per mM of said
serotype 15B
12
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
capsular polysaccharide. In a preferred embodiment, the activated serotype 15B
capsular
polysaccharide comprises at least 0.6 mM acetate per mM of said serotype 15B
capsular
polysaccharide. In a preferred embodiment, the activated serotype 15B capsular
polysaccharide comprises at least 0.7 mM acetate per mM of said serotype 15B
capsular
polysaccharide. In a preferred embodiment, the presence of 0-acetyl groups is
determined
by ion-HPLC analysis.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
comprises at
least 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 or 0.8 mM glycerol per mM of said
serotype 15B capsular
polysaccharide. In a preferred embodiment, the activated serotype 15B capsular
polysaccharide comprises at least 0.5, 0.6 or 0.7 mM glycerol per mM of said
serotype 15B
capsular polysaccharide. In a preferred embodiment, the activated serotype 15B
capsular
polysaccharide comprises at least 0.6 mM glycerol per mM of said serotype 15B
capsular
polysaccharide. In a preferred embodiment, the activated serotype 15B capsular
polysaccharide comprises at least 0.7 mM glycerol per mM of said serotype 15B
capsular
polysaccharide.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 250 kDa and comprises at least 0.6 mM acetate
per mM
of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 250 kDa and comprises at least 0.6 mM
glycerol per mM
of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
comprises at
least 0.6 mM acetate per mM of said serotype 15B capsular polysaccharide and
at least 0.6
mM glycerol per mM of said serotype 15B capsular polysaccharide.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
has a
molecular weight between 100 and 250 kDa and comprises at least 0.6 mM acetate
per mM
of said serotype 15B capsular polysaccharide and at least 0.6 mM glycerol per
mM of said
serotype 15B capsular polysaccharide.
In an embodiment, the activated serotype 15B capsular polysaccharide is
lyophilized,
optionally in the presence of cryoprotectant/Iyoprotectant. In an embodiment,
said
cryoprotectant/Iyoprotectant is a saccharide. In a preferred embodiment, the
saccharide is
13
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
selected from sucrose, trehalose, raffinose, stachyose, melezitose, dextran,
mannitol, lactitol
and palatinit. In a preferred embodiment, the saccharide is sucrose. The
lyophilized activated
capsular polysaccharide can then be compounded with a solution comprising the
carrier
protein.
In another embodiment, the activated serotype 15B capsular polysaccharide is
compounded
with the carrier protein and lyophilized optionally in the presence of
cryoprotectant/Iyoprotectant. In an embodiment, said
cryoprotectant/Iyoprotectant is a
saccharide. In a preferred embodiment, the saccharide is selected from
sucrose, trehalose,
raffinose, stachyose, melezitose, dextran, mannitol, lactitol and palatinit.
In a preferred
embodiment, the saccharide is sucrose. The co-lyophilized polysaccharide and
carrier
protein can then be resuspended in solution and reacted with a reducing agent.
In an embodiment, the invention relates to a lyophilized activated serotype
15B capsular
polysaccharide.
In an embodiment the invention relates to the co-lyophilized activated
serotype 15B capsular
polysaccharide and protein carrier. In a preferred embodiment, the protein
carrier is CRM197.
Coniuqation of activated serotype 15B capsular polysaccharide with a carrier
protein
The activated serotype 15B capsular polysaccharide can be conjugated to a
carrier protein
by a process comprising the step of:
(a) compounding the activated serotype 15B capsular polysaccharide with a
carrier protein,
and,
(b) reacting the compounded activated serotype 15B capsular polysaccharide and
carrier
protein with a reducing agent to form a serotype 15B capsular polysaccharide-
carrier protein
conjugate.
The conjugation of activated serotype 15B capsular polysaccharide with a
protein carrier by
reductive amination in dimethylsulfoxide (DMSO) is suitable to preserve the 0-
acetyl content
of the polysaccharide as compared for example to reductive amination in
aqueous solution
where the level of 0-acetylation of the polysaccharide is significantly
reduced. In a preferred
embodiment, step (a) and step (b) are carried out in DMSO.
In a preferred embodiment, step (a) comprises dissolving lyophilized serotype
15B capsular
polysaccharide in a solution comprising a carrier protein and DMSO. In a
preferred
14
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
embodiment, step (a) comprises dissolving co-lyophilized serotype 15B capsular
polysaccharide and carrier protein in DMSO.
When steps (a) and (b) are carried out in aqueous solution, steps (a) and (b)
are carried out
in a buffer, preferably selected from PBS, MES, HEPES, Bis-tris, ADA, PIPES.
MOPSO,
BES, MOPS, DIPSO, MOBS, HEPPSO, POPSO, TEA, EPPS, Bicine or HEPB, at a pH
between 6.0 and 8.5, 7 and 8 or 7 and 7.5. In a preferred embodiment the
buffer is PBS. In a
preferred embodiment the pH is about 7.3.
In a preferred embodiment, the concentration of activated serotype 15B
capsular
polysaccharide in step (b) is between 0.1 and 10 mg/mL, 0.5 and 5 mg/mL, 0.5
and 2 mg/mL.
In a preferred embodiment, the concentration of activated serotype 15B
capsular
polysaccharide in step (b) is about 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8,
0.9, 1, 1.1, 1.2, 1.3,
1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9
or 3 mg/mL.
In a preferred embodiment the initial input ratio (weight by weight) of
activated serotype 15B
capsular polysaccharide to carrier protein is between 5:1 and 0.1:1, 2:1 and
0.1:1, 2:1 and
1:1, 1.5:1 and 1:1, 0.1:1 and 1:1, 0.3:1 and 1:1, 0.6:1 and 1:1.
In a preferred embodiment the initial input ratio of activated serotype 15B
capsular
polysaccharide to carrier protein is about 0.6:1 to 1.5:1, preferably 0.6:1 to
1:1. Such initial
input ratio is particularly suitable to obtain low levels of free
polysaccharide in the
immunogenic conjugate.
In a preferred embodiment the initial input ratio of activated serotype 15B
capsular
polysaccharide to carrier protein is about 0.4:1, 0.5:1, 0.6:1, 0.7:1, 0.8:1,
0.9:1, 1:1, 1.1:1,
1.2:1, 1.3:1, 1.4:1, 1.5:1, 1.6:1, 1.7:1, 1.8:1, 1.9:1 or 2:1.
In an embodiment, the reducing agent is sodium cyanoborohydride, sodium
triacetoxyborohydride, sodium or zinc borohydride in the presence of Bronsted
or Lewis
acids, amine boranes such as pyridine borane, 2-Picoline Borane, 2,6-diborane-
methanol,
dimethylamine-borane, t-BuMe'PrN-BH3,
benzylamine-BH3 or 5-ethyl-2-methylpyridine
borane (PEMB). In a preferred embodiment, the reducing agent is sodium
cyanoborohydride.
In a preferred embodiment, the reducing agent is sodium 2-Picoline Borane.
In a preferred embodiment, the quantity of reducing agent used in step (b) is
between about
0.1 and 10 molar equivalents, 0.5 and 5 molar equivalents, 1 and 2 molar
equivalents. In a
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
preferred embodiment, the quantity of reducing agent used in step (b) is about
1, 1.1, 1.2,
1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9 or 2 molar equivalents.
In a preferred embodiment, the duration of step (b) is between 1 and 60 hours,
10 and 50
hours, 40 and 50 hours; 42 and 46 hours. In a preferred embodiment, the
duration of step (b)
is about 44 hours.
In a preferred embodiment, the temperature of the reaction in step (b) is
maintained between
and 40 C, 15 and 30 C or 20 and 26 C. In a preferred embodiment, the
temperature of
10 the reaction in step (b) is maintained at about 23 C.
In a preferred embodiment, the process for the preparation of an immunogenic
conjugate
comprising Streptococcus pneumoniae serotype 15B capsular polysaccharide
covalently
linked to a carrier protein further comprises a step (step (c)) of capping
unreacted aldehyde
(quenching) by addition of NaBH4.
In a preferred embodiment, the quantity of NaBH4 used in step (c) is between
0.1 and 10
molar equivalents, 0.5 and 5 molar equivalent 1 and 3 molar equivalents. In a
preferred
embodiment, the quantity of NaBH4 used in step (c) is about 2 molar
equivalents.
In a preferred embodiment, the duration of step (c) is between 0.1 and 10
hours, 0.5 and 5
hours, 2 and 4 hours. In a preferred embodiment, the duration of step (c) is
about 3 hours.
In a preferred embodiment, the temperature of the reaction in step (c) is
maintained between
15 and 45 C, 15 and 30 C or 20 and 26 C. In a preferred embodiment, the
temperature of
the reaction in step (c) is maintained at about 23 C.
In a preferred embodiment the yield of the conjugation step (step b) is
greater than 50%,
55%, 60%, 65%, 70%, 75%, 80%, 85% or 90%. In a preferred embodiment the yield
of the
conjugation step (step b) is greater than 60%. In a preferred embodiment the
yield of the
conjugation step (step b) is greater than 70%. The yield is the amount of
serotype 15B
polysaccharide in the conjugate x100 / amount of activated polysaccharide used
in the
conjugation step.
In a preferred embodiment, the process for the preparation of an immunogenic
conjugate
comprising Streptococcus pneumoniae serotype 15B capsular polysaccharide
covalently
linked to a carrier protein comprises the steps of:
16
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
(a) preparing a fermentation culture of serotype 15B Streptococcus pneumonia
bacterial
cells;
(b) lysing the bacterial cells in said fermentation culture;
(c) purifying serotype 15B polysaccharide from the fermentation culture;
(d) sizing the purified serotype 15B polysaccharide by high pressure
homogenization;
(e) reacting the sized serotype 15B polysaccharide with an oxidizing agent;
(f) compounding the activated serotype 15B polysaccharide with a carrier
protein, and,
(g) reacting the compounded activated serotype 15B polysaccharide and carrier
protein with
a reducing agent to form a serotype 15B polysaccharide-carrier protein
conjugate; and,
(h) capping unreacted aldehyde (quenching) by addition of NaBH4.
In a preferred embodiment the yield of the conjugation step (step g) of the
above process is
greater than 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85% or 90%. In a preferred
embodiment the yield of the conjugation step (step g) is greater than 60%. In
a preferred
embodiment the yield of the conjugation step (step g) is greater than 70%. The
yield is the
amount of serotype 15B polysaccharide in the conjugate x100) / amount of
activated
polysaccharide used in the conjugation step.
After conjugation of the serotype 15B capsular polysaccharide to the carrier
protein, the
polysaccharide-protein conjugate can be purified (enriched with respect to the
amount of
polysaccharide-protein conjugate) by a variety of techniques known to the
skilled person.
These techniques include dialysis, concentration/diafiltration operations,
tangential flow
filtration, precipitation/elution, column chromatography (DEAE or hydrophobic
interaction
chromatography), and depth filtration.
In a preferred embodiment the carrier protein is non-toxic and non-reactogenic
and
obtainable in sufficient amount and purity. Carrier proteins should be
amenable to standard
conjugation procedures.
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
is
conjugated to a carrier protein which is selected in the group consisiting of:
DT (Diphtheria
toxin), TT (tetanus toxid) or fragment C of TT, CRM197 (a nontoxic but
antigenically identical
variant of diphtheria toxin) other DT point mutants, such as CRM176, CRM228,
CRM 45
(Uchida et al J. Biol. Chem. 218; 3838-3844, 1973); CRM 9, CRM102, CRM 103 and
CRM107 and other mutations described by Nicholls and Youle in Genetically
Engineered
Toxins, Ed: Frankel, Maecel Dekker Inc, 1992; deletion or mutation of Glu-148
to Asp, Gln or
Ser and/or Ala 158 to Gly and other mutations disclosed in US 4709017 or US
4950740;
17
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
mutation of at least one or more residues Lys 516, Lys 526, Phe 530 and/or Lys
534 and
other mutations disclosed in US 5917017 or US 6455673; or fragment disclosed
in US
5843711, pneumococcal pneumolysin (Kuo et al (1995) Infect lmmun 63; 2706-13)
including
ply detoxified in some fashion for example dPLY-GMBS (WO 04081515,
PCT/EP2005/010258) or dPLY-formol, PhtX, including PhtA, PhtB, PhtD, PhtE
(sequences
of PhtA, PhtB, PhtD or PhtE are disclosed in WO 00/37105 or WO 00/39299) and
fusions of
Pht proteins for example PhtDE fusions, PhtBE fusions, Pht A-E (WO 01/98334,
WO
03/54007, W02009/000826), OMPC (meningococcal outer membrane protein - usually
extracted from N.meningitidis serogroup B - EP0372501 ), PorB (from N.
meningitidis), PD
(Haemophilus influenza protein D - see, e.g., EP 0 594 610 B), or
immunologically functional
equivalents thereof, synthetic peptides (EP0378881 , EP0427347), heat shock
proteins (WO
93/17712, WO 94/03208), pertussis proteins (WO 98/58668, EP0471 177),
cytokines,
lymphokines, growth factors or hormones (W010 91/01146), artificial proteins
comprising
multiple human CD4+ T cell epitopes from various pathogen derived antigens
(Falugi et al
(2001 ) Eur J Immunol 31 ; 3816-3824) such as N19 protein (Baraldoi et al
(2004) Infect
lmmun 72; 4884-7) pneumococcal surface protein PspA (WO 02/091998), iron
uptake
proteins (WO 01/72337), toxin A or B of C. difficile (WO 00/61761 ). In an
embodiment, the
activated serotype 15B capsular polysaccharide is conjugated to DT (Diphtheria
toxoid). In
another embodiment, the activated serotype 15B capsular polysaccharide is
conjugated to
TT (tetanus toxid). In another embodiment, the activated serotype 15B capsular
polysaccharide is conjugated to fragment C of TT. In another embodiment, the
activated
serotype 15B capsular polysaccharide is conjugated to PD (Haemophilus
influenza protein D
- see, e.g., EP 0 594 610 B).
In a preferred embodiment, the activated serotype 15B capsular polysaccharide
of the
invention is conjugated to CRM197 protein. The CRM197 protein is a nontoxic
form of
diphtheria toxin but is immunologically indistinguishable from the diphtheria
toxin. CRM197 is
produced by C. diphtheriae infected by the nontoxigenic phage 13197t0- created
by
nitrosoguanidine mutagenesis of the toxigenic corynephage beta (Uchida, T. et
al. 1971,
Nature New Biology 233:8-11). CRM197 is purified through ultrafiltration,
ammonium sulfate
precipitation, and ion-exchange chromatography. The CRM197 protein has the
same
molecular weight as the diphtheria toxin but differs therefrom by a single
base change
(guanine to adenine) in the structural gene. This single base change causes an
amino acid
substitution glutamic acid for glycine) in the mature protein and eliminates
the toxic
properties of diphtheria toxin. The CRM197 protein is a safe and effective T-
cell dependent
18
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
carrier for saccharides. Further details about CRM197 and production thereof
can be found
e.g. in US 5,614,382.
In an embodiment, the invention relate to an immunogenic conjugate comprising
Streptococcus pneumoniae serotype 15B capsular polysaccharide covalently
linked to a
carrier protein. In an embodiment, the invention relate to an immunogenic
conjugate
comprising Streptococcus pneumoniae serotype 15B capsular polysaccharide
covalently
linked to a carrier protein by reductive amination. In an embodiment, the
invention relate to
an immunogenic conjugate comprising Streptococcus pneumoniae serotype 15B
capsular
polysaccharide covalently linked to a carrier protein by reductive amination
in DMSO. In a
preferred embodiment, the carrier protein is CRM197. In a preferred
embodiment, the
polysaccharide is an isolated serotype 15B capsular polysaccharide as defined
herein. In a
preferred embodiment, the polysaccharide is an isolated serotype 15B capsular
polysaccharide as defined herein which has been sized by high pressure
homogenization.
In a preferred embodiment, the immunogenic conjugate comprises less than about
50, 45,
40, 35, 30, 25, 20 or 15% of free serotype 15B capsular polysaccharide
compared to the total
amount of serotype 15B capsular polysaccharide. In a preferred embodiment the
immunogenic conjugate comprises less than about 25% of free serotype 15B
capsular
polysaccharide compared to the total amount of serotype 15B capsular
polysaccharide. In a
preferred embodiment the immunogenic conjugate comprises less than about 20%
of free
serotype 15B capsular polysaccharide compared to the total amount of serotype
15B
capsular polysaccharide. In a preferred embodiment the immunogenic conjugate
comprises
less than about 15% of free serotype 15B capsular polysaccharide compared to
the total
amount of serotype 15B capsular polysaccharide.
In a preferred embodiment, the immunogenic conjugate has a molecular weight
between
3000 and 20000kDa; 5000 and 10000kDa; 5000 and 20000kDa; 8000 and 20000kDa;
8000
and 16000 KDa; or 10000 and 16000 KDa. The molecular weight of the immunogenic
conjugate is measured by SEC-MALLS.
In a preferred embodiment, the ratio (weight by weight) of serotype 15B
capsular
polysaccharide to carrier protein in the conjugate is between 0.5 and 3. In a
preferred
embodiment, the ratio of serotype 15B capsular polysaccharide to carrier
protein in the
conjugate is between 0.4 and 2, 0.5 and 2, 0.5 and 1.5, 0.5 and 1, 1 and 1.5,
1 and 2. In a
19
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
preferred embodiment, the ratio of serotype 15B capsular polysaccharide to
carrier protein in
the conjugate is between 0.7 and 0.9.
Size exclusion chromatography media (CL-4B) can be used to determine the
relative
molecular size distribution of the conjugate. Size Exclusion Chromatography
(SEC) is used in
gravity fed columns to profile the molecular size distribution of conjugates.
Large molecules
excluded from the pores in the media elute more quickly than small molecules.
Fraction
collectors are used to collect the column eluate. The fractions are tested
colorimetrically by
saccharide assay. For the determination of Kd, columns are calibrated to
establish the
fraction at which molecules are fully excluded (V0), (Kd=0), and the fraction
representing the
maximum retention (V,), (Kd=1). The fraction at which a specified sample
attribute is
reached (V,), is related to Kd by the expression, Kd = (V, - V0)/ - Vo).
In a preferred embodiment, at least 20% of the immunogenic conjugate has a Kd
below or
equal to 0.3 in a CL-4B column. In a preferred embodiment, at least 30% of the
immunogenic
conjugate has a Kd below or equal to 0.3 in a CL-4B column. In a preferred
embodiment, at
least 40% of the immunogenic conjugate has a Kd below or equal to 0.3 in a CL-
4B column.
In a preferred embodiment, at least 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%,
80%, or
85% of the immunogenic conjugate has a Kd below or equal to 0.3 in a CL-4B
column. In a
preferred embodiment, at least 60% of the immunogenic conjugate has a Kd below
or equal
to 0.3 in a CL-4B column. In a preferred embodiment, at least 70% of the
immunogenic
conjugate has a Kd below or equal to 0.3 in a CL-4B column.
In a preferred embodiment, between 40% and 90% of the serotype 15B immunogenic
conjugate has a Kd below or equal to 0.3 in a CL-4B column. In a preferred
embodiment,
between 50% and 90% of the serotype 15B immunogenic conjugate has a Kd below
or equal
to 0.3 in a CL-4B column. In a preferred embodiment, between 65% and 80% of
the serotype
15B immunogenic conjugate has a Kd below or equal to 0.3 in a CL-4B column.
In a preferred embodiment, the immunogenic conjugate comprises at least 0.1,
0.2, 0.3, 0.4,
0.5, 0.6, 0.7 or 0.8 mM acetate per mM serotype 15B capsular polysaccharide.
In a preferred
embodiment, the immunogenic conjugate comprises at least 0.5, 0.6 or 0.7 mM
acetate per
mM serotype 15B capsular polysaccharide. In a preferred embodiment, the
immunogenic
conjugate comprises at least 0.6 mM acetate per mM serotype 15B capsular
polysaccharide.
In a preferred embodiment, the immunogenic conjugate comprises at least 0.7 mM
acetate
per mM serotype 15B capsular polysaccharide. In a preferred embodiment, the
presence of
0-acetyl groups is determined by ion-HPLC analysis.
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a preferred embodiment, the ratio of mM acetate per mM serotype 15B
capsular
polysaccharide in the immunogenic conjugate to mM acetate per mM serotype 15B
capsular
polysaccharide in the isolated polysaccharide is at least 0.6, 0.65, 0.7,
0.75, 0.8, 0.85, 0.9, or
0.95. In a preferred embodiment, the ratio of mM acetate per mM serotype 15B
capsular
polysaccharide in the immunogenic conjugate to mM acetate per mM serotype 15B
capsular
polysaccharide in the isolated polysaccharide is at least 0.7. In a preferred
embodiment, the
ratio of mM acetate per mM serotype 15B capsular polysaccharide in the
immunogenic
conjugate to mM acetate per mM serotype 15B capsular polysaccharide in the
isolated
polysaccharide is at least 0.9. In a preferred embodiment, the presence of 0-
acetyl groups is
determined by ion-HPLC analysis.
In a preferred embodiment, the ratio of mM acetate per mM serotype 15B
capsular
polysaccharide in the immunogenic conjugate to mM acetate per mM serotype 15B
capsular
polysaccharide in the activated polysaccharide is at least 0.6, 0.65, 0.7,
0.75, 0.8, 0.85, 0.9,
or 0.95. In a preferred embodiment, the ratio of mM acetate per mM serotype
15B capsular
polysaccharide in the immunogenic conjugate to mM acetate per mM serotype 15B
capsular
polysaccharide in the activated polysaccharide is at least 0.7. In a preferred
embodiment, the
ratio of mM acetate per mM serotype 15B capsular polysaccharide in the
immunogenic
conjugate to mM acetate per mM serotype 15B capsular polysaccharide in the
activated
polysaccharide is at least 0.9. In a preferred embodiment, the presence of 0-
acetyl groups is
determined by ion-HPLC analysis.
In a preferred embodiment, the immunogenic conjugate comprises at least 0.1,
0.2, 0.3, 0.4,
0.5, 0.6, 0.7 or 0.8 mM glycerol per mM serotype 15B capsular polysaccharide.
In a preferred
embodiment, the immunogenic conjugate comprises at least 0.5, 0.6 or 0.7 mM
glycerol per
mM serotype 15B capsular polysaccharide. In a preferred embodiment, the
immunogenic
conjugate comprises at least 0.6 mM glycerol per mM serotype 15B capsular
polysaccharide.
In a preferred embodiment, the immunogenic conjugate comprises at least 0.7 mM
glycerol
per mM serotype 15B capsular polysaccharide.
The degree of conjugation is the number of lysine residues in the carrier
protein that are
conjugated to serotype 15B capsular polysaccharide. The evidence for lysine
modification of
the carrier protein, due to covalent linkages to the polysaccharides, is
obtained by amino acid
analysis using routine methods known to those of skill in the art. Conjugation
results in a
reduction in the number of lysine residues recovered, compared to the CRM197
protein
starting material used to generate the conjugate materials.
21
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a preferred embodiment, the degree of conjugation of the immunogenic
conjugate is
between 2 and 15, 2 and 13, 2 and 10, 2 and 8, 2 and 6, 2 and 5, 2 and 4, 3
and 15, 3 and
13, 3 and 10, 3 and 8, 3 and 6, 3 and 5, 3 and 4, 5 and 15, 5 an 10, 8 and 15,
8 and 12, 10
and 15 or 10 and 12. In a preferred embodiment, the degree of conjugation of
the
immunogenic conjugate is between 2 and 5.
Immunogenic composition
The term "immunogenic composition" relates to any pharmaceutical composition
containing
an antigen, e.g., a microorganism or a component thereof, which composition
can be used to
elicit an immune response in a subject.
As used herein, "immunogenic" means an ability of an antigen (or an epitope of
the antigen),
such as a bacterial capsular polysaccharide, or an immunogenic conjugate or
immunogenic
composition comprising an antigen, to elicit an immune response in a host such
as a
mammal, either humorally or cellularly mediated, or both.
In an embodiment, the disclosure relate to an immunogenic composition
comprising an
immunogenic serotype 15B capsular polysaccharide-carrier protein conjugate
disclosed
herein.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of binding to serotype
15B
Streptococcus pneumonia. In an embodiment, the immunogenic composition
disclosed
herein, when administered to a subject, induces the formation of antibodies
capable of
binding to serotype 15B Streptococcus pneumonia as measured by a standard
ELISA assay.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of binding to serotype
15B and 15A
and/or 150 Streptococcus pneumonia. In an embodiment, the immunogenic
composition
disclosed herein, when administered to a subject, induces the formation of
antibodies
capable of binding to serotype 15B and 15A and/or 150 Streptococcus pneumonia
as
measured by a standard ELISA assay.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of binding to serotype
15B and 150
Streptococcus pneumonia. In an embodiment, the immunogenic composition
disclosed
22
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
herein, when administered to a subject, induces the formation of antibodies
capable of
binding to serotype 15B and 150 Streptococcus pneumonia as measured by a
standard
ELISA assay.
In the ELISA (Enzyme-linked lmmunosorbent Assay) method, antibodies from the
sera of
vaccinated subjects are incubated with polysaccharides which have been
adsorbed to a solid
support. The bound antibodies are detected using enzyme-conjugated secondary
detection
antibodies.
In an embodiment said ELISA assay is the standardized (WHO) ELISA assay as
defined by
the WHO in the 'Training manual for Enzyme linked immunosorbent assay for the
quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA).'
(accessible
at http://www.vaccine.uab.edulELISA%20protocol.pdf ; accessed on March 31st,
2014).
The ELISA measures type specific IgG anti-S. pneumoniae capsular
polysaccharide (PS)
antibodies present in human serum. When dilutions of human sera are added to
type-specific
capsular PS-coated microtiter plates, antibodies specific for that capsular PS
bind to the
microtiter plates. The antibodies bound to the plates are detected using a
goat anti-human
IgG alkaline phosphatase-labeled antibody followed by a p-nitrophenyl
phosphate substrate.
The optical density of the colored end product is proportional to the amount
of anticapsular
PS antibody present in the serum.
In an embodiment, the immunogenic composition of the invention is able to
elicit IgG
antibodies in human which are capable of binding S. pneumoniae serotypes 15B
polysaccharide at a concentration of at least 0.05, 0.1, 0.2, 0.3, 0.35, 0.4
or 0.5 pg/ml as
determined by ELISA assay.
In an embodiment, the immunogenic composition of the invention is able to
elicit IgG
antibodies in human which are capable of binding S. pneumoniae serotypes 150
polysaccharide at a concentration of at least 0.05, 0.1, 0.2, 0.3, 0.35, 0.4
or 0.5 pg/ml as
determined by ELISA assay.
In an embodiment, the immunogenic composition of the invention is able to
elicit IgG
antibodies in human which are capable of binding S. pneumoniae serotypes 15B
and 150
polysaccharide at a concentration of at least 0.05, 0.1, 0.2, 0.3, 0.35, 0.4
or 0.5 pg/ml as
determined by ELISA assay.
23
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of killing serotype 15B
Streptococcus
pneumonia in an opsonophagocytosis assay (OPA) as disclosed herein. In an
embodiment,
the immunogenic composition disclosed herein, when tested in an OPA assay as
disclosed
herein, has an OPA titer greater than the OPA titer obtained with an
unconjugated native
Streptococcus pneumonia serotype 15B capsular polysaccharide.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of killing serotype 150
Streptococcus
pneumonia in an opsonophagocytosis assay as disclosed herein. In an
embodiment, the
immunogenic composition disclosed herein, when tested in an OPA assay as
disclosed
herein, has an OPA titer greater than the OPA titer obtained with an
unconjugated native
Streptococcus pneumonia serotype 15C capsular polysaccharide.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of killing serotype 15B
and 150 and/or
15A Streptococcus pneumonia in an opsonophagocytosis assay as disclosed
herein.
In an embodiment, the immunogenic composition disclosed herein, when
administered to a
subject, induces the formation of antibodies capable of killing serotype 15B
and 150.
The pneumococcal opsonophagocytic assay (OPA), which measures killing of S.
pneumoniae cells by phagocytic effector cells in the presence of functional
antibody and
complement, is considered to be an important surrogate for evaluating the
effectiveness of
pneumococcal vaccines.
Opsonophagocytic assay (OPA) can be conducted by incubating together a mixture
of
Streptococcus pneumoniae cells, a heat inactivated human serum to be tested,
differentiated
HL-60 cells (phagocytes) and an exogenous complement source (e.g. baby rabbit
complement). Opsonophagocytosis proceeds during incubation and bacterial cells
that are
coated with antibody and complement are killed upon opsonophagocytosis. Colony
forming
units (cfu) of surviving bacteria that escape from opsonophagocytosis are
determined by
plating the assay mixture. The OPA titer is defined as the reciprocal dilution
that results in a
50% reduction in bacterial count over control wells without test serum. The
OPA titer is
interpolated from the two dilutions that encompass this 50% killing cut-off.
An endpoint titer of 1:8 or greater is considered a positive result in these
killing type OPA.
24
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In an embodiment, the immunogenic composition of the invention is able to
elicit a titer of at
least 1:8 against S. pneumoniae serotype 15B in at least 50% of the subjects
as determined
by opsonophagocytic killing assay (OPA). In an embodiment, the immunogenic
composition
of the invention is able to elicit a titer of at least 1:8 against S.
pneumoniae serotype 15B in
at least 60%; 70%, 80%, 90%, or at least 93% of the subjects as determined by
opsonophagocytic killing assay (OPA).
In an embodiment, the immunogenic composition of the invention is able to
elicit a titer of at
least 1:8 against S. pneumoniae serotype 150 in at least 50% of the subjects
as determined
by opsonophagocytic killing assay (OPA). In an embodiment, the immunogenic
composition
of the invention is able to elicit a titer of at least 1:8 against S.
pneumoniae serotype 150 in
at least 60%; 70%, 80%, 90%, or at least 95% of the subjects as determined by
opsonophagocytic killing assay (OPA).
Formulation of the immunogenic composition of the present invention can be
accomplished
using art-recognized methods. For instance, the immunogenic conjugates of the
invention
can be formulated with a physiologically acceptable vehicle to prepare the
composition.
Examples of such vehicles include, but are not limited to, water, buffered
saline, polyols (e.g.,
glycerol, propylene glycol, liquid polyethylene glycol) and dextrose
solutions.
In a preferred embodiment, the immunogenic composition may comprise at least
one
additional antigen. In a preferred embodiments, the immunogenic composition
may
comprises at least one additional Streptococcus pneumoniae capsular
polysaccharide.
In a preferred embodiment, the immunogenic composition may comprise at least
one
additional Streptococcus pneumoniae capsular polysaccharide conjugated to a
carrier
protein. In a preferred embodiment, said carrier protein is 0RM197.
In certain embodiments, the immunogenic composition comprises one or more
adjuvants. As
defined herein, an "adjuvant" is a substance that serves to enhance the
immunogenicity of
an immunogenic composition of this invention. Thus, adjuvants are often given
to boost the
immune response and are well known to the skilled artisan. Suitable adjuvants
to enhance
effectiveness of the composition include, but are not limited to:
(1) aluminum salts (alum), such as aluminum hydroxide, aluminum phosphate,
aluminum
sulfate, etc.;
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
(2) oil-in-water emulsion formulations (with or without other specific
immunostimulating
agents such as muramyl peptides (defined below) or bacterial cell wall
components), such
as, for example,
(a) MF59 (PCT Pub. No. WO 90/14837), containing 5% Squalene, 0.5%5 Tween 80,
and
0.5% Span 85 (optionally containing various amounts of MTP-PE (see below,
although not
required)) formulated into submicron particles using a microfluidizer such as
Model 110Y
microfluidizer (Microfluidics, Newton, MA),
(b) SAF, containing 10% Squalene, 0.4% Tween 80, 5% pluronic-blocked polymer
L121, and
thr-MDP (see below) either microfluidized into a submicron emulsion or
vortexed to generate
a larger particle size emulsion, and
(c) RibiTM adjuvant system (RAS), (Corixa, Hamilton, MT) containing 2%
Squalene, 0.2%
Tween 80, and one or more bacterial cell wall components from the group
consisting of 3-0-
deaylated monophosphorylipid A (MPLTm) described in U.S. Patent No. 4,912,094
(Corixa),
trehalose dimycolate (TDM), and cell wall skeleton (CWS), preferably MPL + CWS
(De1OXTm);
(3) saponin adjuvants, such as Quil A or STIMULONTm QS-21 (Antigenics,
Framingham, MA)
(U.S. Patent No. 5,057,540) may be used or particles generated therefrom such
as ISCOMs
(immunostimulating complexes);
(4) bacteriallipopolysaccharides, synthetic lipid A analogs such as aminoalkyl
glucosamine
phosphate compounds (AGP), or derivatives or analogs thereof, which are
available from
Corixa, and which are described in U.S. Patent No.6, 113,918; one such AGP is
2-[(R)-3-
Tetradecanoyloxytetradecanoylamino]ethyl
2-Deoxy-4-Ophosphono-3-0-[(R)-3-
tetradecanoyloxytetradecanoy1]-2-[(R)-3tetradecanoyloxytetradecanoylamino]-b-D-
glucopyranoside, which is also know as 529 (formerly known as RC529), which is
formulated
as an aqueous form or as a stable emulsion, synthetic polynucleotides such as
oligonucleotides containing CpG motif(s) (U.S. Patent No. 6,207,646);
(5) cytokines, such as interleukins (e.g., IL-1, IL-2, IL-4, IL-5, IL-6, IL-7,
IL-12, IL-15, IL-18,
etc.), interferons (e.g., gamma interferon), granulocyte macrophage colony
stimulating factor
(GM-CSF), macrophage colony stimulating factor (M-CSF), tumor necrosis factor
(TNF),
costimulatory molecules 87-1 and 87-2, etc.;
(6) detoxified mutants of a bacterial ADP-ribosylating toxin such as a cholera
toxin (CT)
either in a wild-type or mutant form, for example, where the glutamic acid at
amino acid
position 29 is replaced by another amino acid, preferably a histidine, in
accordance with
published international patent application number WO 00/18434 (see also WO
02/098368
and WO 02/098369), a pertussis toxin (PT), or an E. coli heat-labile toxin
(LT), particularly
LT-K63, LT-R72, CT-5109, PT-K9/G129 (see, e.g.,WO 93/13302 and WO 92/19265);
and
(7) other substances that act as immunostimulating agents to enhance the
effectiveness of
the composition.
26
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Muramyl peptides include, but are not limited to, N-acetyl-muramyl-L-threonyl-
D-isoglutamine
(thr-MDP), N-acetyl-normuramyl-L-alanine-2-(1'-2'-dipalmitoyl-
sn-glycero-3-
hydroxyphosphoryloxy)-ethylamine (MTP-PE), etc.
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise a CpG Oligonucleotide as adjuvant. A CpG oligonucleotide as
used herein
refers to an immunostimulatory CpG oligodeoxynucleotide (CpG ODN), and
accordingly
these terms are used interchangeably unless otherwise indicated.
lmmunostimulatory CpG
oligodeoxynucleotides contain one or more immunostimulatory CpG motifs that
are
unmethylated cytosine-guanine dinucleotides, optionally within certain
preferred base
contexts. The methylation status of the CpG immunostimulatory motif generally
refers to the
cytosine residue in the dinucleotide. An immunostimulatory oligonucleotide
containing at
least one unmethylated CpG dinucleotide is an oligonucleotide which contains a
5'
unmethylated cytosine linked by a phosphate bond to a 3' guanine, and which
activates the
immune system through binding to Toll-like receptor 9 (TLR-9). In another
embodiment the
immunostimulatory oligonucleotide may contain one or more methylated CpG
dinucleotides,
which will activate the immune system through TLR9 but not as strongly as if
the CpG
motif(s) was/were unmethylated. CpG immunostimulatory oligonucleotides may
comprise
one or more palindromes that in turn may encompass the CpG dinucleotide. CpG
oligonucleotides have been described in a number of issued patents, published
patent
applications, and other publications, including U.S. Patent Nos. 6,194,388;
6,207,646;
6,214,806; 6,218,371; 6,239,116; and 6,339,068.
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise any of the CpG Oligonucleotide described at pages 3 lines 22
to page 12
line 36 of W02010/125480.
Different classes of CpG immunostimulatory oligonucleotides have been
identified. These
are referred to as A, B, C and P class, and are described in greater detail at
pages 3 lines 22
to page 12 line 36 of W02010/125480. Methods of the invention embrace the use
of these
different classes of CpG immunostimulatory oligonucleotides.
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise an A class CpG Oligonucleotide. Preferably, the "A class" CpG
oligonucleotide of the invention has the following nucleic acid sequence: 5'
GGGGACGACGTCGTGGGGGGG 3' (SEQ ID NO: 1). Some non-limiting examples of A-
Class oligonucleotides include: 5' G*G*G GACGACGTCGTG G*G*G*G*G*G
3' (SEQ ID NO: 2) ; wherein * refers to a phosphorothioate bond and _ refers
to a
phosphodiester bond.
27
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise a B class CpG Oligonucleotide. In one embodiment, the CpG
oligonucleotide for use in the present invention is a B class CpG
oligonucleotide represented
by at least the formula:
5' X1X2CGX3X4 3', wherein X1, X2, X3, and X4 are nucleotides. In one
embodiment, X2 is
adenine, guanine, or thymine. In another embodiment, X3 is cytosine, adenine,
or thymine.
The B class CpG oligonucleotide sequences of the invention are those broadly
described
above in US patents Ps 6,194,388, 6,207,646, 6,214,806, 6,218,371, 6,239,116
and
6,339,068. Exemplary sequences include but are not limited to those disclosed
in these latter
applications and patents.
In an embodiment, the "B class" CpG oligonucleotide of the invention has the
following
nucleic acid sequence:
5' TCGTCGTTTTTCGGTGCTTTT 3' (SEQ ID NO: 3), or
5' TCGTCGTTTTTCGGTCGTTTT 3' (SEQ ID NO: 4), or
5' TCGTCGTTTTGTCGTTTTGTCGTT 3' (SEQ ID NO: 5), or
5' TCGTCGTTTCGTCGTTTTGTCGTT 3' (SEQ ID NO: 6), or
5' TCGTCGTTTTGTCGTTTTTTTCGA 3' (SEQ ID NO: 7).
In any of these sequences, all of the linkages may be all phosphorothioate
bonds. In another
embodiment, in any of these sequences, one or more of the linkages may be
phosphodiester, preferably between the "C" and the "G" of the CpG motif making
a semi-soft
CpG oligonucleotide. In any of these sequences, an ethyl-uridine or a halogen
may substitute
for the 5' T; examples of halogen substitutions include but are not limited to
bromo-uridine or
iodo-uridine substitutions.
Some non-limiting examples of B-Class oligonucleotides include:
5' T*C*G*T*C*G*T*T*T*T*T*C*G*G*T*G*C*T*T*T*T 3' (SEQ ID NO: 8), or
5' T*C*G*T*C*G*T*T*T*T*T*C*G*G*T*C*G*T*T*T*T 3' (SEQ ID NO: 9), or
5' T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T*T*T*G*T*C*G*T*T 3' (SEQ ID NO: 10), or
5' T*C*G*T*C*G*T*T*T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T 3' (SEQ ID NO: 11), or
5' T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T*T*T*T*T*T*C*G*A 3' (SEQ ID NO: 12).
wherein* refers to a phosphorothioate bond.
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise a C class CpG Oligonucleotide. In an embodiment, the "C class"
CpG
oligonucleotides of the invention has the following nucleic acid sequence:
5' TCGCGTCGTTCGGCGCGCGCCG 3' (SEQ ID NO: 13), or
5' TCGTCGACGTTCGGCGCGCGCCG 3' (SEQ ID NO: 14), or
5' TCGGACGTTCGGCGCGCGCCG 3' (SEQ ID NO: 15), or
5' TCGGACGTTCGGCGCGCCG 3' (SEQ ID NO: 16), or
28
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
5' TCGCGTCGTTCGGCGCGCCG 3' (SEQ ID NO: 17), or
5' TCGACGTTCGGCGCGCGCCG 3' (SEQ ID NO: 18), or
5' TCGACGTTCGGCGCGCCG 3' (SEQ ID NO: 19), or
5' TCGCGTCGTTCGGCGCCG 3' (SEQ ID NO: 20), or
5' TCGCGACGTTCGGCGCGCGCCG 3' (SEQ ID NO: 21), or
5' TCGTCGTTTTCGGCGCGCGCCG 3' (SEQ ID NO: 22), or
5' TCGTCGTTTTCGGCGGCCGCCG 3' (SEQ ID NO: 23), or
5' TCGTCGTTTTACGGCGCCGTGCCG 3' (SEQ ID NO: 24), or
5' TCGTCGTTTTCGGCGCGCGCCGT 3' (SEQ ID NO: 25).
In any of these sequences, all of the linkages may be all phosphorothioate
bonds. In another
embodiment, in any of these sequences, one or more of the linkages may be
phosphodiester, preferably between the "C" and the "G" of the CpG motif making
a semi-soft
CpG oligonucleotide.
Some non-limiting examples of C-Class oligonucleotides include:
5' T*C_G*C_G*T*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 26), or
5' T*C_G*T*C_G*A*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 27), or
5' T*C_G*G*A*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 28), or
5' T*C_G*G*A*C_G*T*T*C_G*G*C*G*C*G*C*C*G 3' (SEQ ID NO: 29), or
5' T*C_G*C_G*T*C_G*T*T*C_G*G*C*G*C*G*C*C*G 3' (SEQ ID NO: 30), or
5' T*C_G*A*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 31), or
5' T*C_G*A*C_G*T*T*C_G*G*C*G*C*G*C*C*G 3' (SEQ ID NO: 32), or
5' T*C_G*C_G*T*C_G*T*T*C_G*G*C*G*C*C*G 3' (SEQ ID NO: 33), or
5' T*C_G*C_G*A*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 34), or
5' T*C*G*T*C*G*T*T*T*T*C*G*G*C*G*C*G*C*G*C*C*G 3' (SEQ ID NO: 35), or
5' T*C*G*T*C*G*T*T*T*T*C*G*G*C*G*G*C*C*G*C*C*G 3' (SEQ ID NO: 36), or
5' T*C*G*T*C_G*T*T*T*T*A*C_G*G*C*G*C*C_G*T*G*C*C*G 3' (SEQ ID NO: 37), or
5' T*C_G*T*C*G*T*T*T*T*C*G*G*C*G*C*G*C*G*C*C*G*T 3' (SEQ ID NO: 38)
wherein * refers to a phosphorothioate bond and _ refers to a phosphodiester
bond.
In any of these sequences, an ethyl-uridine or a halogen may substitute for
the 5' T;
examples of halogen substitutions include but are not limited to bromo-uridine
or iodo-uridine
substitutions.
In an embodiment of the present invention, the immunogenic compositions as
disclosed
herein comprise a P class CpG Oligonucleotide. In an embodiment, the CpG
oligonucleotide
for use in the present invention is a P class CpG oligonucleotide containing a
5' TLR
activation domain and at least two palindromic regions, one palindromic region
being a 5'
palindromic region of at least 6 nucleotides in length and connected to a 3'
palindromic
29
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
region of at least 8 nucleotides in length either directly or through a
spacer, wherein the
oligonucleotide includes at least one YpR dinucleotide. In an embodiment, said
oligoonucleotide is not T*C_G*T*C_G*A*C_G*T*T*C_G*G*C*G*C_G*C*G*C*C*G (SEQ ID
NO: 27). In one embodiment the a P class CpG oligonucleotide includes at least
one
unmethylated CpG dinucleotide. In another embodiment the TLR activation domain
is TCG,
TTCG, TTTCG, TYpR, TTYpR, TTTYpR, UCG, UUCG, UUUCG, TTT, or TTTT. In yet
another embodiment the TLR activation domain is within the 5' palindromic
region. In another
embodiment the TLR activation domain is immediately 5' to the 5' palindromic
region.
In an embodiment, the "P class" CpG oligonucleotides of the invention has the
following
nucleic acid sequence: 5' TCGTCGACGATCGGCGCGCGCCG 3' (SEQ ID NO: 39).
In said sequences, all of the linkages may be all phosphorothioate bonds. In
another
embodiment, one or more of the linkages may be phosphodiester, preferably
between the
"C" and the "G" of the CpG motif making a semi-soft CpG oligonucleotide. In
any of these
sequences, an ethyl-uridine or a halogen may substitute for the 5' T; examples
of halogen
substitutions include but are not limited to bromo-uridine or iodo-uridine
substitutions.
A non-limiting example of P-Class oligonucleotides include:
5' T*C_G*T*C_G*A*C_G*A*T*C_G*G*C*G*C_G*C*G*C*C*G 3' (SEQ ID NO: 40)
wherein * refers to a phosphorothioate bond and _ refers to a phosphodiester
bond.
In one embodiment the oligonucleotide includes at least one phosphorothioate
linkage. In
another embodiment all internucleotide linkages of the oligonucleotide are
phosphorothioate
linkages. In another embodiment the oligonucleotide includes at least one
phosphodiester-
like linkage. In another embodiment the phosphodiester-like linkage is a
phosphodiester
linkage. In another embodiment a lipophilic group is conjugated to the
oligonucleotide. In one
embodiment the lipophilic group is cholesterol.
In an embodiment, all the internucleotide linkage of the CpG oligonucleotides
disclosed
herein are phosphodiester bonds ("soft" oligonucleotides, as described in the
PCT
application W02007/026190). In another embodiment, CpG oligonucleotides of the
invention
are rendered resistant to degradation (e.g., are stabilized). A "stabilized
oligonucleotide "
refers to an oligonucleotide that is relatively resistant to in vivo
degradation (e.g. via an exo-
or endo-nuclease). Nucleic acid stabilization can be accomplished via backbone
modifications. Oligonucleotides having phosphorothioate linkages provide
maximal activity
and protect the oligonucleotide from degradation by intracellular exo- and
endo-nucleases.
The immunostimulatory oligonucleotides may have a chimeric backbone, which
have
combinations of phosphodiester and phosphorothioate linkages. For purposes of
the instant
invention, a chimeric backbone refers to a partially stabilized backbone,
wherein at least one
internucleotide linkage is phosphodiester or phosphodiester-like, and wherein
at least one
other internucleotide linkage is a stabilized internucleotide linkage, wherein
the at least one
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
phosphodiester or phosphodiester-like linkage and the at least one stabilized
linkage are
different. When the phosphodiester linkage is preferentially located within
the CpG motif such
molecules are called "semi-soft" as described in the PCT application
W02007/026190.
The size of the CpG oligonucleotide (i.e., the number of nucleotide residues
along the length
of the oligonucleotide) also may contribute to the stimulatory activity of the
oligonucleotide.
For facilitating uptake into cells, CpG oligonucleotide of the invention
preferably have a
minimum length of 6 nucleotide residues. Oligonucleotides of any size greater
than 6
nucleotides (even many kb long) are capable of inducing an immune response if
sufficient
immunostimulatory motifs are present, because larger oligonucleotides are
degraded inside
cells. In certain embodiments, the CpG oligonucleotides are 6 to 100
nucleotides long,
preferentially 8 to 30 nucleotides long. In important embodiments, nucleic
acids and
oligonucleotides of the invention are not plasmids or expression vectors.
In an embodiment, the CpG oligonucleotides disclosed herein comprise
substitutions or
modifications, such as in the bases and/or sugars as described at paragraph
134 to 147 of
W02007/026190.
In an embodiment, the CpG oligonucleotide of the present invention is
chemically modified.
Examples of chemical modifications are known to the skilled person and are
described, for
example in Uhlmann E. et al. (1990), Chem. Rev. 90:543, S. Agrawal, Ed.,
Humana Press,
Totowa, USA 1993; Crooke, S.T. et al. (1996) Annu. Rev. Pharmacol. Toxicol.
36:107-129;
and Hunziker J. et al., (1995), Mod. Synth. Methods 7:331-417. An
oligonucleotide
according to the invention may have one or more modifications, wherein each
modification is
located at a particular phosphodiester internucleoside bridge and/or at a
particular [3-D-ribose
unit and/or at a particular natural nucleoside base position in comparison to
an
oligonucleotide of the same sequence which is composed of natural DNA or RNA.
In some embodiments of the invention, CpG-containing nucleic acids might be
simply mixed
with immunogenic carriers according to methods known to those skilled in the
art (see, e.g.
W003/024480).
In a particular embodiment of the present invention, any of the immunogenic
composition
disclosed herein comprises from 2pg to 100mg of CpG oligonucleotide,
preferably from
0.1 mg to 50 mg CpG oligonucleotide, preferably from 0.2mg to 10 mg CpG
oligonucleotide,
preferably from 0.3 mg to 5 mg CpG oligonucleotide, even preferably from 0.5
to 2 mg CpG
oligonucleotide, even preferably from 0.75 to 1.5 mg CpG oligonucleotide. In a
preferred
embodiment, the immunogenic composition disclosed herein comprises
approximately 1mg
CpG oligonucleotide.
In a preferred embodiment, the adjuvant is an aluminum-based adjuvant selected
from the
group consisting of aluminum phosphate, aluminum sulfate and aluminum
hydroxide. In one
31
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
embodiment, the immunogenic compositions described herein comprise the
adjuvant
aluminum phosphate.
In a preferred embodiments, the immunogenic compositions of the invention
further comprise
at least one of a buffer, a cryoprotectant, a salt, a divalent cation, a non-
ionic detergent, an
inhibitor of free radical oxidation, a diluent or a carrier.
The immunogenic composition optionally can comprise one or more
physiologically
acceptable buffers selected from, but not limited to Tris (trimethamine),
phosphate, acetate,
borate, citrate, glycine, histidine and succinate. In certain embodiments, the
formulation is
buffered to within a pH range of about 5.0 to about 7.0, preferably from about
5.5 to about
6.5.
The immunogenic composition optionally can comprise one or more non-ionic
surfactants,
including but not limited to polyoxyethylene sorbitan fatty acid esters,
Polysorbate-80 (Tween
80), Polysorbate-60 (Tween 60), Polysorbate-40 (Tween 40) and Polysorbate-20
(Tween
20), polyoxyethylene alkyl ethers, including but not limited to Brij 58, Brij
35, as well as others
such as Triton X-100; Triton X- 114, NP40, Span 85 and the Pluronic series of
non-ionic
surfactants (e. g. , Pluronic 121). In a preferred embodiment, the immunogenic
composition
comprises Polysorbate-80 or Polysorbate-40, preferably Polysorbate-80. In a
preferred
embodiment, the immunogenic composition comprises Polysorbate-80 at a
concentration
from about 0.001% to about 2% (with up to about 0.25% being preferred) or
Polysorbate-40
at a concentration from about 0.001% to 1% (with up to about 0.5% being
preferred).
The invention further relates to vaccines comprising the immunogenic
composition of the
invention.
Methods for inducing an immune response and protecting against infection
The present disclosure also includes methods of use for immunogenic
compositions
described herein. For example, one embodiment of the disclosure provides a
method of
inducing an immune response against Streptococcus pneumoniae, comprising
administering
to a subject an immunogenic amount of any of the immunogenic compositions
described
herein.
One embodiment of the disclosure provides a method of protecting a subject
against
an infection with Streptococcus pneumoniae, or a method of preventing
infection with
32
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Streptococcus pneumoniae, or a method of reducing the severity of or delaying
the onset of
at least one symptom associated with an infection caused by Streptococcus
pneumoniae, the
methods comprising administering to a subject an immunogenic amount of any of
the
immunogenic compositions described herein.
One embodiment of the disclosure provides a method of protecting a subject
against
an infection with serotype 15B Streptococcus pneumoniae, or a method of
preventing
infection with serotype 15B Streptococcus pneumoniae, or a method of reducing
the severity
of or delaying the onset of at least one symptom associated with an infection
caused by
serotype 15B Streptococcus pneumoniae, the methods comprising administering to
a subject
an immunogenic amount of any of the immunogenic compositions described herein.
One embodiment of the disclosure provides a method of protecting a subject
against
an infection with serotype 150 Streptococcus pneumoniae, or a method of
preventing
infection with serotype 150 Streptococcus pneumoniae, or a method of reducing
the severity
of or delaying the onset of at least one symptom associated with an infection
caused by
serotype 15C Streptococcus pneumoniae, the methods comprising administering to
a subject
an immunogenic amount of any of the immunogenic compositions described herein.
One embodiment of the disclosure provides a method of protecting a subject
against
an infection with serotype 15A Streptococcus pneumoniae, or a method of
preventing
infection with serotype 15A Streptococcus pneumoniae, or a method of reducing
the severity
of or delaying the onset of at least one symptom associated with an infection
caused by
serotype 15A Streptococcus pneumoniae, the methods comprising administering to
a subject
an immunogenic amount of any of the immunogenic compositions described herein.
One embodiment of the disclosure provides a method of treating or preventing a
Streptococcus pneumoniae infection, disease or condition associated with
serotype 15A, 15B
and/or 150 (preferably 15B and/or 150, more preferably 15B) Streptococcus
pneumoniae in
a subject, the method comprising the step of administering a therapeutically
or
prophylactically effective amount of an immunogenic composition described
herein to the
subject. Another embodiment provides a method of treating or preventing a
Streptococcus
pneumoniae infection, disease or condition associated with a serotype 15A, 15B
and/or 150
(preferably 15B and/or 150, more preferably 15B) Streptococcus pneumoniae in a
subject,
the method comprising generating a polyclonal or monoclonal antibody
preparation from the
immunogenic composition described herein, and using said antibody preparation
to confer
passive immunity to the subject.
33
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In one embodiment, the disclosure relates to the use of the immunogenic
conjugate
or immunogenic composition disclosed herein for the manufacture of a
medicament for
protecting a subject against an infection with Streptococcus pneumoniae,
and/or preventing
infection with Streptococcus pneumoniae, and/or reducing the severity of or
delaying the
onset of at least one symptom associated with an infection caused by
Streptococcus
pneumoniae, and/or protecting a subject against an infection with serotype
15A, 15B and/or
150 (preferably 15B and/or 150, more preferably 15B) Streptococcus pneumoniae
and/or
preventing infection with serotype 15A, 15B and/or 150 (preferably 15B and/or
150, more
preferably 15B) Streptococcus pneumoniae, and/or reducing the severity of or
delaying the
onset of at least one symptom associated with an infection caused by serotype
15A, 15B
and/or 150 (preferably 15B and/or 150, more preferably 15B) Streptococcus
pneumoniae.
In one embodiment, the disclosure relates to the use of the immunogenic
conjugate
or immunogenic composition disclosed herein for protecting a subject against
an infection
with Streptococcus pneumoniae, and/or preventing infection with Streptococcus
pneumoniae, and/or reducing the severity of or delaying the onset of at least
one symptom
associated with an infection caused by Streptococcus pneumoniae, and/or
protecting a
subject against an infection with serotype 15A, 15B and/or 150 (preferably 15B
and/or 150,
more preferably 15B) Streptococcus pneumoniae and/or preventing infection with
serotype
15A, 15B and/or 150 (preferably 15B and/or 150, more preferably 15B)
Streptococcus
pneumoniae, and/or reducing the severity of or delaying the onset of at least
one symptom
associated with an infection caused by serotype 15A, 15B and/or 150
(preferably 15B and/or
15C, more preferably 15B) Streptococcus pneumoniae.
An "immune response" to an immunogenic composition is the development in a
subject of a humoral and/or a cell-mediated immune response to molecules
present in the
immunogenic composition or vaccine composition of interest. For purposes of
the present
disclosure, a "humoral immune response" is an antibody-mediated immune
response and
involves the induction and generation of antibodies that recognize and bind
with some affinity
for the antigen in the immunogenic composition or vaccine of the disclosure,
while a
"cell-mediated immune response" is one mediated by T-cells and/or other white
blood cells.
A "cell-mediated immune response" is elicited by the presentation of antigenic
epitopes in
association with Class I or Class II molecules of the major histocompatibility
complex (MHC),
CD1 or other non-classical MHC-like molecules. This activates antigen-specific
CD4+ T
helper cells or CD8+ cytotoxic T lymphocyte cells ("CTLs"). CTLs have
specificity for peptide
antigens that are presented in association with proteins encoded by classical
or non-classical
34
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
MHCs and expressed on the surfaces of cells. CTLs help induce and promote the
intracellular destruction of intracellular microbes, or the lysis of cells
infected with such
microbes. Another aspect of cellular immunity involves an antigen-specific
response by
helper T-cells. Helper T-cells act to help stimulate the function, and focus
the activity of,
nonspecific effector cells against cells displaying peptide or other antigens
in association with
classical or non-classical MHC molecules on their surface. A "cell-mediated
immune
response" also refers to the production of cytokines, chemokines and other
such molecules
produced by activated T-cells and/or other white blood cells, including those
derived from
CD4+ and CD8+ T-cells. The ability of a particular antigen or composition to
stimulate a
cell-mediated immunological response may be determined by a number of assays,
such as
by lymphoproliferation (lymphocyte activation) assays, CTL cytotoxic cell
assays, by
assaying for T-lymphocytes specific for the antigen in a sensitized subject,
or by
measurement of cytokine production by T cells in response to re-stimulation
with antigen.
Such assays are well known in the art. See, e.g., Erickson et al. (1993) J.
lmmunol.
151:4189-4199; and Doe et al. (1994) Eur. J. lmmunol. 24:2369-2376.
As used herein, "treatment" (including variations thereof, e.g., "treat" or
"treated")
means any one or more of the following: (i) the prevention of infection or re-
infection, as in a
traditional vaccine, (ii) the reduction in the severity of, or, in the
elimination of symptoms, and
(iii) the substantial or complete elimination of the pathogen or disorder in
question. Hence,
treatment may be effected prophylactically (prior to infection) or
therapeutically (following
infection).
In the present disclosure, prophylactic treatment is the preferred mode.
According to a particular embodiment of the present disclosure, compositions
and methods
are provided that treat, including prophylactically and/or therapeutically
immunize, a host
animal against a serotype 15A, 15B and/or 15C (preferably 15B and/or 15C, more
preferably
15B) Streptococcus pneumoniae infection. The methods of the present disclosure
are useful
for conferring prophylactic and/or therapeutic immunity to a subject. The
methods of the
present disclosure can also be practiced on subjects for biomedical research
applications.
An "immunogenic amount", and "immunologically effective amount," both of which
are used interchangeably herein, refers to the amount of antigen or
immunogenic
composition sufficient to elicit an immune response, either a cellular (T-
cell) or humoral
(B-cell or antibody) response, or both, as measured by standard assays known
to one skilled
in the art.
35
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
In a preferred embodiment, said subject is a human. In a most preferred
embodiment, said
subject is a newborn (i.e. under three months of age), an infant (from 3
months to one year of
age) or a toddler (i.e. from one year to four years of age).
In an embodiment, the immunogenic compositions disclosed herein are for use as
a vaccine.
In such embodiment, the subject to be vaccinated may be less than 1 year of
age. For
example, the subject to be vaccinated can be about 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11 or 12
months of age. In an embodiment, the subject to be vaccinated is about 2, 4 or
6 months of
age. In another embodiment, the subject to be vaccinated is less than 2 years
of age. For
example the subject to be vaccinated can be about 12-15 months of age. In some
cases, as
little as one dose of the immunogenic composition according to the invention
is needed, but
under some circumstances, a second, third or fourth dose may be given (see
regimen
section).
In an embodiment of the present invention, the subject to be vaccinated is a
human adult 50
years of age or older, more preferably a human adult 55 years of age or older.
In an
embodiment, the subject to be vaccinated is a human adult 65 years of age or
older, 70
years of age or older, 75 years of age or older or 80 years of age or older.
In an embodiment the subject to be vaccinated is an immunocompromised
individual, in
particular a human. An immunocompromised individual is generally defined as a
person who
exhibits an attenuated or reduced ability to mount a normal humoral or
cellular defense to
challenge by infectious agents.
In an embodiment of the present invention, the immunocompromised subject to be
vaccinated suffers from a disease or condition that impairs the immune system
and results in
an antibody response that is insufficient to protect against or treat
pneumococcal disease.
In an embodiment, said disease is a primary immunodeficiency disorder.
Preferably, said
primary immunodeficiency disorder is selected from the group consisting of:
combined T- and
B-cell immunodeficiencies, antibody deficiencies, well-defined syndromes,
immune
dysregulation diseases, phagocyte disorders, innate immunity deficiencies,
autoinflammatory
disorders, and complement deficiencies. In an embodiment, said primary
immunodeficiency
disorder is selected from the one disclosed on page 24 line 11 to page 25 line
19 of the PCT
application W02010/125480.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated suffers from a disease selected from the groups consisting of: HIV-
infection,
acquired immunodeficiency syndrome (AIDS), cancer, chronic heart or lung
disorders,
36
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
congestive heart failure, diabetes mellitus, chronic liver disease,
alcoholism, cirrhosis, spinal
fluid leaks, cardiomyopathy, chronic bronchitis, emphysema, Chronic
obstructive pulmonary
disease (COPD), spleen dysfunction (such as sickle cell disease), lack of
spleen function
(asplenia), blood malignancy, leukemia, multiple myeloma, Hodgkin's disease,
lymphoma,
kidney failure, nephrotic syndrome and asthma.
In an embodiment of the present invention, the immunocompromised subject to be
vaccinated suffers from malnutrition.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated is taking a drug or treatment that lowers the body's resistance to
infection. In an
embodiment, said drug is selected from the one disclosed on page 26 line 33 to
page 26 line
40 of the PCT application W02010/125480.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated is a smoker.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated has a white blood cell count (leukocyte count) below 5 x 109 cells
per liter, or
below 4 x 109 cells per liter, or below 3 x 109 cells per liter, or below 2 x
109 cells per liter, or
below 1 x 109 cells per liter, or below 0.5 x 109 cells per liter, or below
0.3 x 109 cells per liter,
or below 0.1 x 109 cells per liter.
White blood cell count (leukocyte count): The number of white blood cells
(WBCs) in the
blood. The WBC is usually measured as part of the CBC (complete blood count).
White
blood cells are the infection-fighting cells in the blood and are distinct
from the red (oxygen-
carrying) blood cells known as erythrocytes. There are different types of
white blood cells,
including neutrophils (polymorphonuclear leukocytes; PMNs), band cells
(slightly immature
neutrophils), T-type lymphocytes (T cells), B-type lymphocytes (B cells),
monocytes,
eosinophils, and basophils. All the types of white blood cells are reflected
in the white blood
cell count. The normal range for the white blood cell count is usually between
4,300 and
10,800 cells per cubic millimeter of blood. This can also be referred to as
the leukocyte count
and can be expressed in international units as 4.3 - 10.8 x 109 cells per
liter.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated suffers from neutropenia. In a particular embodiment of the present
invention, the
immunocompromised subject to be vaccinated has a neutrophil count below 2 x
109 cells per
liter, or below 1 x 109 cells per liter, or below 0.5 x 109 cells per liter,
or below 0.1 x 109 cells
per liter, or below 0.05 x 109 cells per liter.
37
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
A low white blood cell count or "neutropenia" is a condition characterized by
abnormally low
levels of neutrophils in the circulating blood. Neutrophils are a specific
kind of white blood cell
that help prevent and fight infections. The most common reason that cancer
patients
experience neutropenia is as a side effect of chemotherapy. Chemotherapy-
induced
neutropenia increases a patient's risk of infection and disrupts cancer
treatment.
In a particular embodiment of the present invention, the immunocompromised
subject to be
vaccinated has a CD4+ cell count below 500/mm3, or CD4+ cell count below
300/mm3, or
CD4+ cell count below 200/mm3, CD4+ cell count below 100/mm3, CD4+ cell count
below
75/mm3, or CD4+ cell count below 50/mm3.
CD4 cell tests are normally reported as the number of cells in mm3. Normal CD4
counts are
between 500 and 1600, and CD8 counts are between 375 and 1100. CD4 counts drop
dramatically in people with HIV.
In an embodiment of the invention, any of the immunocompromised subject
disclosed herein
is a human male or a human female.
The amount of a conjugate in a composition is generally calculated based on
total
polysaccharide, conjugated and non-conjugated for that conjugate.
For example, a
conjugate with 20% free polysaccharide will have about 80 pg of conjugated
polysaccharide
and about 20 pg of non-conjugated polysaccharide in a 100 pg polysaccharide
dose. The
protein contribution to the conjugate is usually not considered when
calculating the dose of a
conjugate. Generally, each dose will comprise 0.1 to 100 pg of polysaccharide,
particularly
0.1 to 10 pg, and more particularly 1 to 10 pg and more particularly 1 to 5
lig. Preferably
each dose will comprise about 1.1, 2, 2.2, 3, 3.3, 4, 4.4 lig of
polysaccharide.
Optimal amounts of components for a particular immunogenic composition or
vaccine can be
ascertained by standard studies involving observation of appropriate immune
responses in
subjects. Following an initial vaccination, subjects can receive one or
several booster
immunizations adequately spaced.
The effectiveness of an antigen as an immunogen, can be measured either by
proliferation assays, by cytolytic assays, such as chromium release assays to
measure the
ability of a T-cell to lyse its specific target cell, or by measuring the
levels of B-cell activity by
measuring the levels of circulating antibodies specific for the antigen in
serum. An immune
response may also be detected by measuring the serum levels of antigen
specific antibody
induced following administration of the antigen, and more specifically, by
measuring the
38
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
ability of the antibodies so induced to enhance the opsonophagocytic ability
of particular
white blood cells, as described herein. The level of protection of the immune
response may
be measured by challenging the immunized host with the antigen that has been
administered. For example, if the antigen to which an immune response is
desired is a
bacterium, the level of protection induced by the immunogenic amount of the
antigen is
measured by detecting the percent survival or the percent mortality after
challenge of the
animals with the bacterial cells. In one embodiment, the amount of protection
may be
measured by measuring at least one symptom associated with the bacterial
infection, e.g., a
fever associated with the infection. The amount of each of the antigens in the
multi-antigen
or multi-component vaccine or immunogenic compositions will vary with respect
to each of
the other components and can be determined by methods known to the skilled
artisan. Such
methods would include procedures for measuring immunogenicity and/ or in vivo
efficacy.
The disclosure further provides antibodies and antibody compositions which
bind specifically
and selectively to the capsular polysaccharides or immunogenic conjugates of
the present
disclosure. In some embodiments, antibodies are generated upon administration
to a subject
of the capsular polysaccharides or immunogenic conjugates of the present
disclosure. In
some embodiments, the disclosure provides purified or isolated antibodies
directed against
one or more of the capsular polysaccharides or immunogenic conjugates of the
present
disclosure. In some embodiments, the antibodies of the present disclosure are
functional as
measured by killing bacteria in either an animal efficacy model or via an
opsonophagocytic
killing assay. In some embodiments, the antibodies of the disclosure confer
passive
immunity to a subject. The present disclosure further provides polynucleotide
molecules
encoding an antibody or antibody fragment of the disclosure, and a cell, cell
line (such as
hybridoma cells or other engineered cell lines for recombinant production of
antibodies) or a
transgenic animal that produces an antibody or antibody composition of the
disclosure, using
techniques well-known to those of skill in the art.
Examples
Example 1: Preparation of isolated Streptococcus pneumoniae serotype 15B
capsular
polysaccharide
1.1 Fermentation and Purification
Serotype 15B capsular polysaccharides can be obtained directly from bacteria
using isolation
procedures known to one of ordinary skill in the art (see for example methods
disclosed U.S.
Patent App. Pub. Nos. 20060228380, 20060228381, 20070184071, 20070184072,
39
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
20070231340, and 20080102498 or W02008118752). The serotype 15B Streptococcus
pneumonia were grown in a seed bottle and then transferred to a seed
fermentor. Once the
targeted optical density was reached, the cells were transferred to a
production fermentor.
The fermentation was broth was inactivated by the addition of N-lauroyl
sarcosine and
purified by ultrafiltration and diafiltration.
The purified Streptococcus pneumoniae serotype 15B polysaccharide was then
sized by high
pressure homogenization using a PANDA 2K homogenizer 0 (GEA Niro Soavi) to
produce
the isolated Streptococcus pneumoniae serotype 15B polysaccharide.
Preferably, the isolated Streptococcus pneumoniae serotype 15B capsular
polysaccharide
obtained by the above process comprises at least 0.6 mM acetate per mM of
serotype 15B
capsular polysaccharide and has a molecular weight between 50kDa and 500kDa,
preferably
150 to 350kDa.
1.2 Oxidation of Isolated Streptococcus pneumoniae serotype 15B capsular
polysaccharide
Polysaccharide oxidation was carried out in 100 mM potassium phosphate buffer
(pH 6.0
0.2) by sequential addition of calculated amount of 500 mM potassium phosphate
buffer (pH
6.0) and WFI to give final polysaccharide concentration of 2.0 g/L. If
required, the reaction pH
was adjusted to pH 6.0, approximately. After pH adjustment, the reaction
temperature was
adjusted to 23 2 C. Oxidation was initiated by the addition of
approximately 0.25 molar
equivalents of sodium periodate. The oxidation reaction was performed at 23
2 C during
16 hrs, approximately.
Concentration and diafiltration of the activated polysaccharide was carried
out using 10K
MWCO ultrafiltration cassettes. Diafiltration was performed against 20-fold
diavolumes of
WFI. The purified activated polysaccharide was then stored at 5 3 C. The
purified activated
saccharide was characterized inter alia by (i) saccharide concentration by
colorimetric assay;
(ii) aldehyde concentration by colorimetric assay; (iii) Degree of Oxidation
(iv) Molecular
Weight by SEC-MALLS and (v) presence of 0-acetyl and glycerol.
SEC-MALLS is used for the determination of the molecular weight of
polysaccharides and
polysaccharide-protein conjugates. SEC is used to separate the polysaccharides
by
hydrodynamic volume. Refractive index (RI) and multi-angle laser light
scattering (MALLS)
detectors are used for the determination of the molecular weight. When light
interacts with
matter, it scatters and the amount of scattered light is related to the
concentration, the square
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
of the dn/dc (the specific refractive index increments), and the molar mass of
the matter. The
molecular weight measurement is calculated based on the readings from the
scattered light
signal from the MALLS detector and the concentration signal from the RI
detector.
The degree of oxidation (DO = moles of sugar repeat unit / moles of aldehyde)
of the
activated polysaccharide was determined as follows:
The moles of sugar repeat unit is determined by various colorimetric methods,
example by
using Anthrone method. By the Anthrone mthod, the polysaccharide is first
broken down to
monosaccharides by the action of sulfuric acid and heat. The Anthrone reagent
reacts with
the hexoses to form a yellow-green colored complex whose absorbance is read
spectrophotometrically at 625nm. Within the range of the assay, the absorbance
is directly
proportional to the amount of hexose present.
The moles of aldehyde also is determined simultaneously, using MBTH
colorimetric method.
The MBTH assay involves the formation of an azine compound by reacting
aldehyde groups
(from a given sample) with a 3-methyl-2-benzothiazolone hydrazone (MBTH assay
reagent).
The excess 3-methyl-2-benzothiazolone hydrazone oxidizes to form a reactive
cation. The
reactive cation and the azine react to form a blue chromophore. The formed
chromophore is
then read spectroscopically at 650 nm.
Preferably, the activated Streptococcus pneumoniae serotype 15B capsular
polysaccharide
obtained by the above process comprises at least 0.6 mM acetate per mM of
serotype 15B
capsular polysaccharide and has a molecular weight between 50kDa and 500kDa,
preferably
100 to 250kDa.
1.3 Conjugation of activated Streptococcus pneumoniae serotype 15B capsular
polysaccharide with CRM197
The conjugation process consists of the following steps:
a) Compounding with sucrose excipient and lyophilization
b) Reconstitution of the lyophilized activated polysaccharide and CRM197
c) Conjugation of activated polysaccharide to CRM197 and capping
d) Purification of the conjugate
a) Compounding with Sucrose excipient, and Lyophilization
The activated polysaccharide was compounded with sucrose to a ratio of 25
grams of
sucrose per gram of activated polysaccharide. The bottle of compounded mixture
was then
lyophilized. Following lyophilization, bottles containing lyophilized
activated polysaccharide
41
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
were stored at -20 5 C. Calculated amount of CRM197 protein was shell-frozen
and
lyophilized separately. Lyophilized CRM197 was stored at -20 5 C.
b) Reconstitution of Lyophilized Activated Polysaccharide and CRM197 Protein
Lyophilized activated polysaccharide was reconstituted in anhydrous dimethyl
sulfoxide
(DMSO). Upon complete dissolution of polysaccharide, an equal amount of
anhydrous
DMSO was added to lyophilized CRM197 for reconstitution.
c) Conjugation and Capping
Reconstituted activated polysaccharide was combined with reconstituted CRM197
in the
reaction vessel (input ratio: 0.8:1), followed by mixing thoroughly to obtain
a clear solution
before initiating the conjugation with sodium cyanoborohydride. The final
polysaccharide
concentration in reaction solution is approximately 1 g/L. Conjugation was
initiated by adding
1.0 ¨ 1.5 MEq of sodium cyanoborohydride to the reaction mixture and was
incubated at 23
2 C for 40-48 hrs. Conjugation reaction was terminated by adding 2 MEq of
sodium
borohydride (NaBH4) to cap unreacted aldehydes. This capping reaction
continued at 23
2 C for 3 1 hrs.
d) Purification of the conjugate
The conjugate solution was diluted 1:10 with chilled 5 mM succinate-0.9%
saline (pH 6.0) in
preparation for purification by tangential flow filtration using 100-300K MWCO
membranes.
The diluted conjugate solution was passed through a 5 pm filter and
diafiltration was
performed using 5 mM succinate-0.9% saline (pH 6.0) as the medium. After the
diafiltration
was completed, the conjugate retentate was transferred through a 0.22pm
filter.
The conjugate was diluted further with 5 mM succinate / 0.9% saline (pH 6), to
a target
saccharide concentration of approximately 0.5 mg/mL. Final 0.22pm filtration
step was
completed to obtain the immunogenic conjugate.
Preferably, the conjugate obtained by the above process comprises at least 0.6
MM
acetate per mM of serotype 15B capsular polysaccharide, has a molecular weight
between 3000 and 20000kDa and has a degree of conjugation between 2 and 6.
Example 2: Characterization of immunogenic conjugate comprising Streptococcus
pneumoniae serotype 15B capsular polysaccharide covalently linked to a CRM197
42
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Conjugate 1 was prepared by the process disclosed in example 1. Conjugates 2
and 3 were
prepared by a similar process using different amount of oxidizing agent.
Conjugate 4 was
prepared by a similar process except that the purified serotype 15B capsular
polysaccharide
was not sized and was activated to a lower DO (higher oxidation level) and the
conjugation
was performed in aqueous medium. Conjugate 5 was prepared by a similar process
except
that the purified serotype 15B capsular polysaccharide was sized by chemical
hydrolysis and
the conjugation was performed in aqueous medium. Conjugates 6 and 7 were
prepared by a
similar process except that the purified serotype 15B capsular polysaccharide
was not sized.
The obtained conjugates were characterized and the results are summarized in
Table 1.
Table 1: Streptococcus pneumoniae serotype 15B capsular polysaccharide-CW/1197
conjugates
Conjugate 1 2 3 4 5 6
7
Polysaccharide Sized Sized Sized Native Hydrolyzed Native Native
O-Acetylation; 0.69 0.69 0.69 1.01 0.66 0.76
NA
Polysaccharide
(pmol acetate/pmol
poly)
Solvent medium DMSO DMSO DMSO Aqueous Aqueous DMSO DMSO
Activated 11.4 5.8 9.7 4.8 8.8 5
12
Polysaccharide DO
Activated 196KDa 218KDa 235KDa 435 KDa 270KDa 431KDa 460KDa
Polysaccharide MW
Yield (%) 87.2 64 63.7 96.2 78.8 24.2
26.2
Saccharide Protein 0.68 0.65 0.71 1.22 1.29 0.9
1.5
Ratio
Free Saccharide (%) < 5 < 5 6.1 18.1 14.2 8.8
18
Conjugate MW, 6190KDa 7090KDa 7937KDa 1766KDa 1029KDa 6293KDa 4466KDa
SEC-MALLS
O-Acetylation, 0.68 0.7 0.68 0.61 0.44 0.85
NA
Conjugate (pmol
acetate/pmol poly)
< 0.3 Kd (%), SEC NA 73 NA NA 62 NA
NA
Degree of Conj 3.7 3.9 4.1 NA 3.4 NA
NA
(AAA); Modified Lys
% 0-Acetyl Retained 99% 100% 99.5% 60% 67% 100% NA
in Conjugate
The percentage of free polysaccharide is measured by a procedure utilizing
aluminium
hydroxide gel to bind protein and covalently bound saccharide for removal by
centrifugation.
Samples are mixed with phosphate buffered aluminium hydroxide gel and
centrifuged.
Bound saccharide is pelleted with the gel and free saccharide remains in the
supernatant.
The resulting supernatant and controls samples are quantitated by appropriate
colorimetric
43
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
assays to determine the percentage of free saccharide and to confirm
sufficient removal of
protein and recovery of saccharide.
For the Amino Acid analysis the polysaccharide-protein sample is first
hydrolyzed into its
individual components as free amino acids, using 6N hydrochloric acid (HCI)
hydrolysis
under vacuum and heat (160 C for 15 minutes). After hydrolysis, the samples
are analyzed
using Amino Acid Analyzer. The individual amino acids are separated through
ion exchange
chromatography using a step gradient of sodium citrate buffer with temperature
and flow rate
changes. After separation, the amount of each amino acid residual is
quantitatively
determined using a postcolumn ninhydrin coupling detection system. In this
system, the
ninhydrin is mixed with the column eluate in the postcolumn reactor system and
the mixture
passed into the photometer. The reaction of ninhydrin with eluated amino acids
yields a
purple compound that absorbs maximally at 570 nm. This absorbance is a linear
response
(function) of the amount of a -amino groups present and this reaction provides
quantitative
colorimetric assay for all organic compounds with a -amino groups. In the
reaction with imino
acids such as proline and hydroxylproline, which do not have free amino group,
a bright
yellow compound is generated and monitored at 440 nm. The peak areas for each
amino acid are calculated using both 570 and 440 nm wavelength outputs.
The yield is calculated as follows: (amount of polysaccharide in the conjugate
x100) / amount
of activated polysaccharide.
Conjugates (4 and 5) generated using in aqueous medium demonstrated
significant loss in
0-acetyl levels. Conjugates generated in DMSO solvent, using native
polysaccharide
without MW sizing (6 and 7) did not demonstrate loss in 0-acetyl levels.
However, the
conjugate yields were very poor in addition to poor filterability
characteristics. Conjugates
generated in DMSO using polysaccharides that were sized by high pressure
homogenization
(1, 2 and 3) had high yield and better filterability characteristics with
significant preservation
of 0-acetyl levels. These conjugates also had very low levels of free
polysaccharides.
Example 3: Opsonophagocytic activity (OPA) assay
The immunogenicity of the conjugates of the invention can be assessed using
the
opsonophagocytic assay (OPA) described below.
44
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Groups of 30 6-7 week old female Swiss Webster mice were immunized with 0.001
pg, 0.01
pg, or 0.1 pg of test conjugates via the subcutaneous route on week 0. The
mice were
boosted with the same dose of conjugate on week 3 and then bled at week 4.
Serotype-
specific OPAs were performed on week 4 sera samples.
OPAs are used to measure functional antibodies in murine sera specific for S.
pneumoniae
serotype 15B. Test serum is set up in assay reactions that measure the ability
of capsular
polysaccharide specific immunoglobulin to opsonize bacteria, trigger
complement deposition,
thereby facilitating phagocytosis and killing of bacteria by phagocytes. The
OPA titer is
defined as the reciprocal dilution that results in a 50% reduction in
bacterial count over
control wells without test serum. The OPA titer is interpolated from the two
dilutions that
encompass this 50% killing cut-off.
OPA procedures were based on methods described in Hu et al., Clin Diagn Lab
Immunol
2005;12(February (2)):287-95 with the following modifications. Test serum was
serially
diluted 2.5-fold and added to microtiter assay plates. Live serotype 15B
target bacteria were
added to the wells and the plates were shaken at 37 C for 30 minutes.
Differentiated HL-60
cells (phagocytes) and baby rabbit serum (3- to 4-week old, Pel-Freez0, 6.25%
final
concentration) were added to the wells, and the plates were shaken at 37 C for
45 minutes.
To terminate the reaction, 80 pL of 0.9% NaCI was added to all wells, mixed,
and a 10pL
aliquot were transferred to the wells of MultiScreen HTS HV filter plates
(Millipore )
containing 200 pL of water. Liquid was filtered through the plates under
vacuum, and 150 pL
of HySoy medium was added to each well and filtered through. The filter plates
were then
incubated at 37 C, 5% CO2 overnight and were then fixed with Destain Solution
(Bio-Rad).
The plates were then stained with Coomassie Blue and destained once. Colonies
were
imaged and enumerated on a Cellular Technology Limited (CTL) ImmunoSpot
Analyzer .
Raw colony counts were used to plot kill curves and calculate OPA titers.
The immunogenicity of conjugates 1 and 2 has been tested according to the
above
mentioned assay. One additional conjugate and an unconjugated native S.
pneumoniae
serotype 15B capsular polysaccharide (unconjugated PS) were also tested in the
same
assay:
Conjugate 9 was prepared by conjugation of native (i.e not sized) serotype 15B
capsular
polysaccharide to CRM197 by reductive amination in aqueous solution.
The results are shown in table 2.
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
Table 2: OPA Titers of Animal Testing
OPA GMT (Geometric Mean antibody Titer) (95% CI)
0.001 pg 0.01 pg 0.1 pg
Conjugate 1 485 (413, 569) 804 (565, 1145) 1563 (1048, 2330)
Conjugate 2 556 (438, 707) 871 (609, 1247) 1672 (1054, 2651)
Conjugate 9 395 (329, 475) 856 (627, 1168) 1802 (1108, 2930)
Unconjugated PS - 698 (466, 1045)
As shown in the above table, conjugates 1 and 2, when administered to mice,
generate
antibodies capable of opsonizing serotype 15B S. pneumoniae, triggering
complement
deposition, thereby facilitating phagocytosis and killing of bacteria by
phagocytes. In addition,
despite their lower molecular weight, they also exhibited similar level of
immunogenicity as
compared to conjugate 9 which has not been sized.
Example 4: Cross-functional OPA responses between serotype 15B and serotype
15C
Pneumococcal serogroup 15 includes four structurally-related serotypes: 15A,
15B, 15C, and
15F. Serotypes 15B and 15C are undistinguishable by genetic typing techniques
and have
similar capsular polysaccharide (PS) composition, except that the 15B-PS is
the 0-
acetylated variant of 15C-PS. To understand whether anti-capsular PS
antibodies for
serotype 15B are functionally cross-reacting with serotype 15C, 10 rabbits
were immunized
with PCV16v and PCV20v vaccines both containing an immunogenic conjugate
comprising
Streptococcus pneumoniae serotype 15B capsular polysaccharide covalently
linked to
CRM197 as disclosed herein as part of their formulation. Sera from pre- and
post-vaccination
were tested in OPA assays against serotypes 15B and 15C target pneumococcal
strains.
Of the 10 rabbits from each group, 100% had OPA response to serotype 15B
following
immunization with a serotype 15B conjugate. Of these same samples, 100% had
OPA
response to serotype 15C as well (Table 1 and Table 2). Low OPA titers were
observed in
prevaccination sera in 15C OPA. However, over 10-fold GMT OPA titer increase
with post
vaccination sera compared to pre vaccination demonstrated that the immunogenic
conjugates of the invention induces the formation of antibodies capable of
killing serotype
15B and 15C Streptococcus pneumonia in an OPA.
PCV16v is a 16-valent conjugates composition comprising glycoconjugates from
S. pneumoniae
serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 15B, 18C, 19A, 19F, 22F, 23F and 33F
(16vPnC) all
individualy conjugated to CRM197.
46
CA 02937190 2016-07-18
WO 2015/110942
PCT/1B2015/050316
PCV20v is a 20 valent conjugates composition comprising glycoconjugates from
S. pneumoniae
serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A,
19F, 22F, 23F and 33F
(20vPnC) all individualy conjugated to CRM197.
Table 1. OPA Titers Against serotypes 15B and 15C strains in Rabbit Sera Pre
and Post
vaccination with PCV16v
15B OPA 15C OPA
Animal wk0 wk4 wk0 wk4
1 4 4129 50 2524
2 4 1645 182 472
3 4 1131 126 818
4 4 3199 50 1189
5 4 2664 36 727
6 4 4589 68 2492
7 11 3601 169 1137
8 4 1838 165 672
9 4 1334 98 528
4 1108 204 2425
GMT 4 2222 98 1075
Table 2. OPA Titers Against serotypes 15B and 15C strains in Rabbit Sera Pre
and Post
vaccination with PCV20v
15B OPA 15C OPA
Animal wk0 wk4 wk0 wk4
1 4 3784 indeterminable* 2353
2 4 862 480 938
3 4 3056 69 1497
4 4 1948 indeterminable* 1316
5 4 2360 4 4665
6 4 1594 indeterminable* 1835
7 4 4943 172 4085
8 4 2419 117 1458
9 4 1245 indeterminable* 527
10 4 616 indeterminable* 545
GMT 4 1917 77 1515
10 * Titer cannot be determined due to bad killing curves
47