Note: Descriptions are shown in the official language in which they were submitted.
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
1
PERFUSION MEDIA
FIELD OF INVENTION
The present invention pertains to media suitable for a manufacturing a
therapeutic protein
in a perfusion process.
BACKGROUND OF THE INVENTION
Proteins such as those intended for pharmaceutical applications can be
manufactured
using either a batch, fed batch method or a perfusion method. The present
invention is directed
to perfusion processes, including those used for manufacture of therapeutic
proteins.
Perfusion processes for manufacturing therapeutic proteins are sensitive to
changes in the
composition of culture media, temperature, accumulation of metabolic wastes,
and bioreactor
physico-chemical parameters. Inadequate or fluctuating conditions affect
protein
posttranslational modifications such as its glycoprofile, the latter being
known to correlate with
pharmacokinetic properties.
Perfusion processing is desirable over fed-bath processing because it enables
production
of more product in a given period of time with improved cost of goods. Hence
it is desirable to
overcome the challenges of developing suitable feed conditions that will
support a perfusion
process.
U.S. Patent 7,300,773 and EP 1,781,802 disclose production of the fusion
protein TNFR-
IG using a fed batch process in which feed media are prescribed having
specified concentrations
of amino acids and/or inorganic ions.
BRIEF DESCRIPTION OF THE DRAWINGS
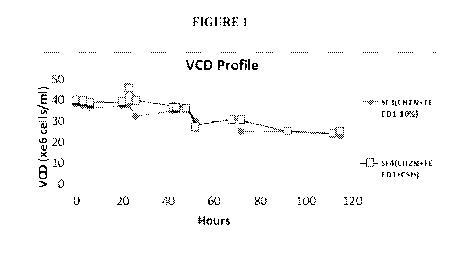
Fig. 1 is a chart of viable cell density (VCD) of cultures containing the
lower
concentrations feeds (SF3 and SF4) in accordance with the present invention.
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
2
Fig. 2 is a chart of VCD of cultures containing more highly enriched nutrient
media.
Fig. 3 is a chart of ammonia levels in the cultures containing more highly
enriched
nutrient media.
Fig. 4 is a bar chart of protein production in the reduced nutrient media and
the more
highly enriched nutrient media.
Fig. 5 is a picture of an isoelectric focusing (IEF) gel that contains
distribution of charged
species of SFM4CHO basal medium supplemented with two different concentrations
of Cell
Boost 5 feed, as well as that of commercial etanercept (Enbre10).
Fig. 6 is a chart of VCD of cultures containing 10% and 20% CHOZN feed.
Fig. 7 is also a chart of VCD of cultures containing 10% and 20% CHOZN feed.
Fig. 8 is a picture of an IEF gel that contains isoform distribution in
product isolated from
cultures containing 10% and 20% feed cultures; as well as that of reference
standard.
Fig. 9 is a bar chart of protein production in the cultures containing leaner
(10%) and
richer (20%) feed concentration.
Fig. 10 is a chart of VCD and viability of culture grown as described in
Example 7.
SUMMARY OF THE INVENTION
We have now discovered that the media used in a perfusion process must be
substantially
less rich in terms of nutrient content, in particular amino acid content, than
media intended for
use in a batch or fed batch process. For purposes hereof, nutrient content
should be understood
as the concentration of given nutrients in the perfusion reactor volume. In
particular, our
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
3
invention concerns a perfusion process in which perfusion is conducted in the
presence of a feed
medium containing a total amino acid concentration less than or equal to 70 mM
and preferably
in the range of about 15 mM to about 65 mM. In various embodiments of the
invention the total
amino acid concentration is in a range selected from: about 15 to 20 mM; about
20 to 25 mM;
about 25 to 30 mM; about 30 to 35 mM; about 35 to 40 mM; about 40 to 45 mM;
about 45 to 50
mM; about 50 to 55 mM; about 55 to 60 mM; about 60 to 65 mM; and between 65
and 70 mM.
The total amino acid concentrations prescribed herein are less than those
recommended
for both fed batch and perfusion processes in U.S Patent 7,300,773. By way of
comparison, US
Patent 7,300,773 requires a total amino acid concentration above 70 mM.
Notwithstanding the
assertion in the '773 patent that such concentrations would be understood by
persons skilled in
the art as capable of being employed in perfusion systems (see column 18,
lines 5 to 11), the
present disclosure is based in part on our finding, to the contrary, that
conducting a perfusion
process for manufacture of a therapeutic protein using feed media satisfying
the high total amino
acid concentrations prescribed in the '773 patent results in production of
substantially reduced
amounts of the desired protein, and therefore, that the amino acid
concentrations in media
intended for perfusion must necessarily be reduced, and preferably
substantially reduced, in
terms of total amino acid concentration. Provided that amino acid
concentration constraints of
the present invention are met, it should be understood by persons skilled in
the art that various
non-amino acid components typically employed in feed media (e.g. vitamins,
hydrolysates, etc.)
may be adjusted empirically in a known manner without departing from the
spirit and scope of
the present teaching.
In further embodiments of the invention, a perfusion process for manufacturing
a
biological protein employs feed media satisfying the reduced total amino acid
concentration
referenced above, and where the feed media is further characterized by one or
more of the
following criteria: a molar glutamine to cumulative asparagine ratio of
greater than 2; a molar
glutamine to total amino acid concentration ratio of greater than 0.2; a molar
inorganic ion to
total amino acid ratio of greater than 1; and a combined amount of glutamine
and asparagine per
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
4
unit volume of less than 16 mM. These criteria should be understood as
denoting steady state
concentrations and amounts in the perfusion reaction vessel.
Our invention further concerns modifying a feed medium that is otherwise
suitable for
fed batch production of a therapeutic protein in order to render such medium
suitable for use in a
perfusion process for manufacturing the protein, where the modification
comprises reducing the
nutrient richness of the fed batch medium such that, when used in the
perfusion reactor, the total
amino acid concentration of the feed media is in the range of about 40 to
about 95 percent, and
preferably about 50 to about 70 percent of the total amino acid concentration
of the fed-batch
feed medium. Preferably, this method achieves either or both of (i) a
production titer
comparable to that achievable with a fed batch process using the higher-
nutrient feed; and/or (ii)
a substantial reduction in the levels of ammonia that would otherwise be
generated in the
perfusion reactor if such perfusion process were conducted using the same
media as that used in
a fed batch process.
In an embodiment of the invention, the perfusion method uses the following
steps: (a)
preparing a mixture comprising cells capable of expressing a desired
therapeutic protein, and a
culture medium suitable for conducting such expression; (b) in a suitable
vessel containing the
mixture, causing the cells to produce the protein; and (c) periodically or
continuously removing
spent culture medium from, and adding fresh culture medium to, the reaction
vessel.
The invention can be applied to the manufacture of any therapeutic protein. In
an
embodiment hereof, the therapeutic protein can be selected from any fusion
protein or any
antibody. Fusion proteins can include TNFR-Fc (sometimes referred to as TNFR-
Ig) fusion
proteins. Antibodies can include anti-TNF antibodies. Further non limiting
examples of proteins
suitable for manufacture in the present invention include etanercept,
rituximab, adalimumab,
trastuzumab, bevacizumab, eculizumab and natalizumab, as well as biosimilar or
bio better
variants thereof. It should be understood that the perfusion process of the
invention is not
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
limited to any specific therapeutic protein. Thus other proteins than those
mentioned here can be
produced, such as, e.g, erythropoetins.
In a further embodiment, the invention is a method for producing a therapeutic
protein
comprising the steps of (a) preparing a mixture comprising CHO cells capable
of expressing the
5
protein, and a culture medium suitable for conducting such expression; (b) in
a suitable vessel
containing the mixture, causing the cells to produce the protein; and (c)
periodically or
continuously removing spent culture medium from, and adding fresh culture
medium to, the
reaction vessel, wherein the culture medium comprises (i) a total amino acid
concentration of
about 15 to about 65 mM; and at least one of: a suitable base media (e.g.,
SFM4CHO, BalanCD
CHO Growth A, HyCell CHO, etc.), a complex chemically-defined feed (e.g.,
BalanCD CHO
Feed 1), dexamethasone, ManNAc, cottonseed hydrolysate and D-(+)-galactose.
In a further embodiment of the perfusion method, prior to step (a), the cells
capable of
expressing etanercept are grown in a growth phase at a temperature selected
from; (i) about 28 to
about 37 C; and (ii) about 35 to about 36 C.
In another embodiment of the perfusion method, during production of the
etanercept
occurring in steps (b) and (c), above, the reaction vessel is maintained at a
temperature selected
from (i) greater than about 32 C; (ii) greater than about 34 C; (iii) greater
than about 35 C; (iv)
the range of about 33 C to about 36 C; (v) the range of about 35 C to about 36
C; (vi) 32.5 C;
(vii) 33.5 C; (viii) 34.5C; and (ix) 35.5 C. The ability to produce excellent
product quality,
properly folded proteins, and excellent titers at these temperatures is
surprising and unexpected
given contrary teachings in the art directed to the use of lower temperatures
during the
production phase (as compared to the growth phase) in a protein manufacturing
process.
As a composition of matter, our invention is directed to a media composition
formulated
to provide a desired total amino acid concentration in a perfusion process for
manufacturing a
therapeutic protein, wherein the desired concentration, based on the volume of
a perfusion
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
6
reactor used in such process, is in a range selected from the group consisting
of about 15 to 20
mM; about 20 to 25 mM; about 25 to 30 mM; about 30 to 35 mM; about 35 to 40
mM; about 40
to 45 mM; about 45 to 50 mM; about 50 to 55 mM; about 55 to 60 mM; about 60 to
65 mM, and
wherein the composition when offered, recommended or advertised for sale is
accompanied by
written or verbal recommendations or instructions supporting or suggesting its
intended use in
such perfusion process.
A further composition embodiment of the invention is a feed media composition
comprising a total amino acid concentration in a range selected from the group
consisting of
about 15 to 20 mM; about 20 to 25 mM; about 25 to 30 mM; about 30 to 35 mM;
about 35 to 40
mM; about 40 to 45 mM; about 45 to 50 mM; about 50 to 55 mM; about 55 to 60
mM; about 60
to 65 mM, and further comprising at least one of, and preferably at least one
of the following:
cottonseed hydrolysate, dexamethasone, ManNAc, and/or D-(+)-Galactose.
In any of the above embodiments, a preferred range of amino acid concentration
is about
to about 30 mM and the media can include defined and non-defined media.
15
Terms such as "culture," "cell density," "cell viability," "titer," "medium"
(or "media")
"seeding," "growth phase," "production phase," may be understood to have the
meanings well
understood in the art. For example, reference may be had to US Patent
7,300,773.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides methods of manufacturing a fusion protein or an
antibody
via the process known as perfusion. The term "perfusion" as used herein is
intended to generally
denote a process in which a suspension cell culture is continuously or
periodically, and most
preferably continuously, supplied with fresh medium to a bioreactor while
spent culture media is
continuously removed, i.e., harvested (preferably with the product) in order
that product
contained therein can be continuously harvested, and the waste and toxic
materials present in the
spent medium can be removed from the bioreactor. Using appropriate filtration
means well
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
7
known in the art, the cells are then continuously filtered from the harvest
stream and returned to
the bioreactor to maintain a constant culture volume. Such a process,
typically carried out
continuously, allows the cells to reach high densities. Accordingly, densities
as high as 10-75
million cells/mL can routinely be reached and maintained for extended periods
of time, e.g. at
least two weeks, and typically 20 to 60 days. This can result in very highly
productive cell
culture process that can produce for a longer period of time as opposed to
batch or fed-batch
cultures. Alternatively, rather than continuously harvesting product from the
removed spent
medium, the product can be maintained and concentrated in the culture, and
then harvested
periodically, or at the end of the culture. As is well known in the art,
utilization of appropriately
sized filters can allow for removal of only waste, with retention of the
recombinant product in
the bioreactor culture. An object of the present invention is to provide an
appropriate feed media
for use in a perfusion process, in particular processes for manufacturing
therapeutic proteins such
as fusion proteins or antibodies.
Cell culture perfusion processes can typically undergo a feed medium exchange
rate of
0.5 to 2 bioreactor volume per day. The present invention is based on our
discovery that such
perfusion processes will not require as high a concentration of nutrients as
that typically
employed in feed media used in fed batch processing, and, in fact, will
require substantially
lesser concentrations. In particular, we have found that the concentration of
nutrients, in
particular the concentration of amino acids, used in fed batch processes for
manufacturing a
therapeutic protein (e.g., a fusion protein or antibody) must be reduced to
levels that, on the one
hand, are still sufficiently nutrient-rich to support the cells' metabolic
needs at steady-state
levels, but on the other hand not so high in nutrients as to cause other
deleterious effects, such as,
among other things, the overproduction of ammonia or lactate, which will
reduce culture
viability, overall titer and product quality. The invention requires reduced
nutrients levels in
terms of reduced total amino acid concentration levels. Additional nutrient
components may be
empirically determined in a well-known manner based on cell density, perfusion
rate, and
specific productivity rate (pcd), provided the resulting reduced nutrient
level falls within the
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
8
overall amino acid concentration levels specified in the present invention.
Our invention is
further premised on the discovery that the consumption of amino acids in
medium formulated for
perfusion (to stimulate production rather than proliferation) is reduced.
Accordingly, such
medium will support desired productivity with significantly lower
concentrations of amino acids.
Reductions in other feed elements such as glucose, vitamins, etc. is also
feasible.
The perfusion process of the present invention is particularly well suited to
the
manufacture of the fusion protein known as etanercept (including biosimilar
and bio better
variants). Etanercept (Enbrel ) is a dimeric fusion polypeptide consisting of
the extracellular
ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor
receptor (TNFR)
linked to the Fc portion of human IgG1 . It consists of 934 amino acids and
has an apparent
molecular weight of approximately 150 kilodaltons (Physicians Desk Reference,
2002, Medical
Economics Company Inc.) The Fc component of etanercept contains the constant
heavy 2 (CH2)
domain, the constant heavy 3 (CH3) domain and hinge region, but not the
constant heavy 1
(CH1) domain of human IgG1 . An Fc domain can contain one or all of the
domains described
above. Etanercept is usually produced by recombinant DNA technology in a
Chinese hamster
ovary (CHO) mammalian cell expression system.
The perfusion process of the present invention is also well suited to the
manufacture of
the anti-TNF antibody known as adalimumab. Adalimumab (Humira0) is a
recombinant human
IgG1 monoclonal antibody specific for human TNF. Adalimumab is also known as
D2E7.
Adalimumab has two light chains, each with a molecular weight of approximately
24 kilodaltons
(kDa) and two IgG1 heavy chains each with a molecular weight of approximately
49 kDa. Each
light chain consists of 214 amino acid residues and each heavy chain consists
of 451 amino acid
residues. Thus, adalimumab consists of 1330 amino acids and has a total
molecular weight of
approximately 148 kDa. The term adalimumab is also intended to encompass so-
called bio-
similar or bio-better variants of the adalimumab protein used in commercially
available
Humira0.
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
9
The feed medium used in the present invention preferably comprises a base
medium such
as BalanCDO, and HyCell , supplemented with dexamethasone. Cells producing a
protein
(e.g., etanercept or biosimilar or bio better variant thereof) are present in
the perfusion vessel at a
density of at least 10,000,000 cells/ml, and preferably at density of at least
5,000,000, and most
preferably at least about 10,000,000 cells/ml. Prior to step (a), during a
growth phase for the
cells capable of expressing the desired protein (before substantial initiation
of production), such
cells capable of expressing the protein can be grown at a temperature selected
from; (i) about 28
to about 37 C; and (ii) preferably about 35 to about 36 C. During a
subsequent production
phase, involving perfusion processing, the etanercept production is carried
out at a temperature
selected from (i) greater than about 32'; (ii) greater than about 34'; (iii)
greater than about 35 C;
(iv) the range of about 33 C to about 36 C; (v) the range of about 35 C to
about 36 C; (vi)
32.5 C; (vii) 33.5 C; (viii) 34.5 C; and (ix) 35.5 C. The method of the
invention preferably
comprises continuously or periodically, but preferably continuously,
harvesting the etanercept
during the production phase of the perfusion process. Moreover, the removal of
spent medium
and replacement with fresh culture medium occurs preferably continuously.
Harvesting of the
desired protein present in the continuously withdrawn culture medium is
preferably carried out
continuously.
The perfusion method of the present invention can be used for any therapeutic
protein,
including, e.g., fusion proteins and monoclonal antibodies. Examples of
proteins suitable for
production in the perfusion process of the invention include etanercept,
adalimumab,
trastuzumab, rituximab, bevacizumab, infliximab, eculizumab, and natalizumab,
as well as
biosimilar or bio better variants of such proteins. It should be understood
however that the
perfusion process of the present invention is not limited to any specific
protein.
The volumetric productivity of the described process and the quality of the
produced
etanercept can be evaluated by using several methods well known to those
skilled in the art.
These methods include but are not limited to assays that quantify total and
active protein (titers),
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
qualify level of protein sialylation such as the isoelectric focusing (IEF)
gels, hydrophobic
Interaction chromatography and others.
The perfusion process of the present invention can produce correctly folded
proteins in
excellent yields, and preferably at production temperatures higher than those
previously thought
5 necessary or desirable in the art.
EXAMPLES
The following materials are used in the Examples.
Vendor
Raw Material Useful
Source Catalog Category Use Notes
Description Range
Number
Ba1anCDTM CHO Base
Irvine Scientific 94120-10L n/a
base medium
Growth A medium
HyCloneTM HyCell Base
Thermo Scientific SH30933 n/a
base medium
CHO medium
HyCloneTM Base
used in seed
Thermo Scientific SH30518.04 n/aSFM4CHO medium train;
used at 10
mM final; to
D-(+)-Galactose SAFC G5388 Glycan feed < 10
optimize
product
quality
used at 0.8 -
1.0 uM; to
Dexamethasone SAFC D4902 Glycan feed < 1 uM
optimize
product
quality
used at 10
ManNAc
mM final; to
(N- SAFC A8176 Glycan feed < 20
optimize
acetylmannosamine)
product
quality
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
11
Vendor
Raw Material Useful
Source Catalog Category Use
Notes
Description Range
Number
Boosts titer
when added
Ba1anCDTM CHO
Irvine Scientific 94119-10L Titer feed 10%
(v/v) alone or with
Feed 1
other titer
feed
Used in
HyCloneTM Cell 10-20%
Thermo Scientific SH30865.04 Titer
feed control
Boost 5 (v/v)
experiments
Cottonseed
increases
FrieslandCampina
Hydrolysate CNE50M-UF Titer feed 15% (v/v)
specific
Domo
("CSH")
productivity
EX-Cell CHOZN 10-20%
complex feed
SAFC 24331C-10L
Titer feed for fed batch
Platform Feed (v/v)
process
Base medium
for initial
Base
Growth Medium SAFC 87509CP n/a
growth in
medium
production
bioreactor
Complex
medium for
Production medium SAFC 87496CP Complex productionn/a
medium phase
in
production
bioreactor
Base medium
for production
Base
Production medium SAFC 87612CP n/a phase
in
medium
production
bioreactor
EXAMPLE 1
In this experiment we used feed media comprised of a 1:1 mixture of Ba1aIICDTM
CHO
Growth A and HyCloneTM HyCell media supplemented with EX-CELL CHOZN and Feed 1
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
12
feeds, cotton seed hydrolysate, galactose, L-glutamine, and glucose. Some
conditions involved
additional supplementation with vitamins, amino acids, and higher
concentrations of CHOZN
and Feed 1, making the medium significantly richer (See SF5, SF6 and SF7,
below). Prior to
addition of the feed media, the seed density was 40 million cells per
milliliter of culture.
Perfusion processing was simulated in that culture received complete exchange
of medium 24
hours after seeding and the culture was then continued for 96 hours (with no
further feeding or
media exchange). Culture performance was monitored with respect to viable cell
density,
viability, metabolic profile (ammonia and lactate production, L-glutamine and
glucose
consumption, pH levels, glutamate production), and productivity. As shown in
the data
presented below, cultures with greater vitamin and amino acid supplementation
(see SF5 and
SF6, below) produced very high levels of ammonia (>40 mM) resulting in
premature decline of
viability. We did not observe any other metabolic changes when compared to
data obtained for
the surviving cells other than the exceptionally high ammonia levels. The
titers obtained on the
higher-nutrient nutrient media were also negatively affected even during the
time when the
viability was comparable between all cultures. The lower nutrient cultures
without the extra
vitamins and amino acids (SF3 and SF4) remained at high viability, had good
productivity, and
yielded material of good product quality (see Data section below).
This Example 1, using a single feed replacement at 24 hr, followed by a 96 hr
production
phase (without further media exchange during the 96 hour period) demonstrates
that a medium
intended for use in perfusion (in which media is continuously or periodically
exchanged) will
necessarily require reduced levels of nutrients, at levels lower than that
used for the feeds in SF3
and SF4. Accordingly, as provided in Examples 2 and 3 below, feed media used
in a perfusion
process should employ a total amino acid content of less than 70 mM and
preferably a total
amino acid content in the range of about 15 to about 30 mM, yet still result
in excellent cell
density, cell viability and production titer. Total amino acid content as used
herein should be
understand to mean the total, steady state, amino acid concentration based on
the volume of the
perfusion reaction vessel.
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
13
RESULTS OF EXAMPLE 1
Table 1, below, summarizes the feed media used in five experiments (SF3
through SF7)
in which, as described above, media is exchanged, after seeding, at 24 hours,
and the process is
__ then allowed to continue (without any further media exchange) for an
additional 96 hours. This
experiment compared lower nutrient feeds (runs SF3, SF4) with higher nutrient
feeds (runs SFS,
SF6, SF6 and SF7). The feeds shown in the Table were used to supplement a base
medium
consisting of BalanCD and Hycell (as identified in the materials listing,
above). Vitamins
(Invitrogen, cat# 11120-052) and amino acids (Invitrogen, cat# 11130-036) were
added as lx
__ concentration from 100x stock.
TABLE 1
PERFUSION FEEDS
SF3 SF4 SFS SF6 SF7
Feeds Lower Nutrient Lower Nutrient Higher Nutrient Higher Nutrient
__ Higher
Feed Feed Feed Feed Nutrient
Feed
CHOZN 10% 10% 10% 10% 20%
CSH 7.5% 7.5% 7.5%
Feed 1 10% 10% 10% 10% 20%
L-Gln 8 mM 8 mM 8 mM 8 mM 8 mM
Vitamins lx lx
AA lx
Gal 10 mM 10 mM 10 mM 10 mM 10 mM
Figure 1 sets forth data on viable cell density (VCD) of cultures containing
the lower
__ concentrations feeds (SF3 and SF4) in accordance with the present
invention. Note that very
high culture densities were obtained. Relatively leaner medium (10% CHOZN
+7.5% cotton
seed hydrolysate (CSH) and 10% Feed 1, and with no additional supplementation
with vitamins
or amino acids) (see SF3 and SF4) provided sufficient nutritional support for
cells to sustain high
viability for at least 96 hours.
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
14
Figure 2 demonstrates (in the case of Feeds SF5, SF6 and SF7) that the more
highly
enriched nutrient media (resulting from supplementation with vitamins and
amino acids or
complex feeds) resulted in reduced culture longevity. In particular, we
discovered that addition
of amino acids at concentrations that appear to exceed the metabolic
requirements of the culture
results in a rapid decline of viability. It was further noted that all
cultures with shorter longevity
(e.g., SF6 and SF7) produced high levels of ammonia (see Figure 3).
Figure 3 shows that cultures (see SF6 and SF7 in Table 1 above) using the
higher nutrient
feed media display unacceptably high production of ammonia which we postulate
may cause or
contribute to premature decline of viability.
Figure 4 shows that protein production in the higher nutrient medium (e.g.,
SF5, SF6 and
SF7) is negatively affected as shown by reduced titers. Compare the production
from cultures
using the reduced nutrient feeds of SF3 and SF4 according to the invention
with the production
achieved from cultures using the more highly enriched feeds of SF5, SF6 and
SF7.
EXAMPLE 2
Based on the data obtained in Example 1, and in accordance with our invention,
we find
that a medium with reduced nutrients, such as that employed in runs SF3 and
SF4 in Example 1
above, can be modified to further substantially reduce total amino acid
concentration when
employed in a perfusion process undergoing continuous or periodic media
replacement. In
particular, in such a perfusion process, a total concentration of amino acids
less than 70 mM, and
preferably in the range of about 15 mM to about 65 mM, and most preferably in
the range of
about 20 mM to about 30 mM will support the metabolic needs of the cell
culture in a perfusion
process for manufacturing therapeutic proteins such as, for example, a TNFR-Fc
fusion protein,
or a anti-TNF monoclonal antibody. Accordingly, in a perfusion process
involving periodic
media replacements, a perfusion process according to the invention is employed
in which feed
media, having total amino acid concentration of less than 70 mM and preferably
in the range of
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
about 15 to about 65 mM, and most preferably about 20 to about 30 mM is
replaced every 24
hours with fresh medium. Such a process which simulates perfusion is
equivalent to a perfusion
rate of 1 bioreactor volume per day. Cells are inoculated at a density of
preferably 50 million
cells per milliliter, and medium is exchanged every 24 hours for a total of 3
exchanges. The
5 culture is terminated on day 4 (total time 96 hours). Viable cell density
and viability is noted
daily. Titer and isoform profile reflecting product quality are determined for
each harvest
sample using appropriate assays known in the art. Cells may require
approximately 3 days (72
h) to gradually switch the metabolism to production mode as reflected by
improved titer and
product quality (96 h samples). The process results in excellent cell density
and cell viability as
10 well as excellent titer, notwithstanding the use of feed media
containing substantially lower total
amino acid concentrations as compared to media typically employed in fed batch
processes, such
as recommended in U.S. patent, 7,300,773 in which the total cumulative amino
acid
concentration is required to exceed 70 mM.
EXAMPLE 3
PERFUSION WITH CONTINUOUS MEDIA REPLACEMENT
Example 2 is repeated, except that a continuous perfusion process is employed
in which
fresh media is continuously introduced to the reactor, and spent media is
continuously removed
in a manner well known in the art, whereby, in accordance with the present
invention, the steady
state total amino acid concentration of the feed media in the reactor is less
than 70 mM, and
preferably in the range of 15 to about 65 mM, and most preferably in the range
of about 20 to
about 55 mM. The continuous perfusion process results in excellent cell
density, cell viability
and titer. In addition to the requirements for total amino acid concentration,
the feed medium is
preferably formulated such that at the steady state media concentration in the
perfusion reactor
one or more of the following criteria are met: (i) a steady state total amino
acid amount per unit
volume less than about 70 mM; (ii) a steady state glutamine to cumulative
asparagine ratio of
greater than about 2; (iii) a steady state glutamine to total amino acid ratio
of greater than about
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
16
0.2; (iv) a steady state molar inorganic ion to total amino acid ration
greater than 1; and (v) a
steady state combined amount of glutamine and asparagine per unit volume of
less than 16 mM.
The term steady state is intended to denote that the concentration and ratio
of feed components,
as expressed above, remain essentially constant at the stated levels in the
perfusion reaction
vessel.
EXAMPLE 4
We evaluated various media compositions allowing for robust culture
performance under
perfusion conditions with respect to growth, productivity, and product
quality. In one of our
experiments achieving cell density of 10 million cells per milliliter we
tested SFM4CHO basal
medium supplemented with two different concentrations of Cell Boost 5 feed and
found that
leaner (10% feed), resulting in a total amino acid concentration of
approximately 50mM, resulted
in better product quality than a culture that was supplemented with 20% feed
resulting in a total
amino acid concentration of approximately 100 mM. The difference in quality is
demonstrated
by the data shown in Figure 5). Lanes 2 and 4 show a distribution of charged
species very
similar to that for commercial etanercept (Enbre10) (lane 6), with a
preponderance of acidic
bands migrating further into the gel. In contrast, samples from the richer
feeds (lanes 3 and 5)
show less of the acidic, rapidly-migrating species and are less similar to
Enbre10.
A subsequent experiment tested higher culture densities (25 million cells per
ml) using
exactly the same feed conditions. We found that the medium formulation with
the lower content
of Cell Boost 5 feed was unable to sustain culture viability, ultimately
resulting in decline of
product quality. However, we also found that while the feed medium containing
the higher total
amino acid concentration (approx. 100 mM) did support a high viability, the
product quality was
not comparable to that observed at the lower cell density. This outcome
necessitated further
investigations to find other media and feeds that could provide good product
quality while
supporting cell densities of 25-50 million cells per ml. We therefore
investigated several based
media and media combinations in batch mode in order to identify media
compositions that were
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
17
able to support the highest longevity of cultures seeded at 30 to 50 million
cells per mL of
culture. This investigation included the experiment and finding set forth in
Example 1 above.
EXAMPLE 5
Media Requirements in the Perfusion Production
of a TNFR-Fc fusion protein
We conducted a variety of medium exchange experiments simulating perfusion
using
high density cultures (25 x 106 cells/m1) in the production of a TNFR-Fc
fusion protein under
development as a biosimilar to etanercept. In these experiments we
consistently found that high
viability can be achieved with feeds that provide reduced concentrations of
nutrients. Our
findings are exemplified by an experiment in which two high density cultures
were established,
each containing 25 million cells per milliliter of culture, both cultures
utilized the same base
medium (BalanCD/Hycell, 1:1) and additional supplementation with 10 mM
galactose and 10
mM ManNAc. The difference between both cultures was in the level of
supplementation with
CHOZN feed (10% versus 20%) and cottonseed hydrolysate (7.5% versus 15%). Both
feeds
provided additional amino acid supplies to the cultures. Viability of the
culture with the lower
concentration of feeds matched that of the culture that received the richer
medium. This result
indicated that reduction of feed concentration from 20% (98 mM total amino
acid content) to
10% (78 mM total amino acid content) did not limit the nutritional
requirements of that culture
and clearly indicated that the total amino acid concentration could be reduced
further to the
levels that we have now prescribed in the present invention. Data is shown in
Figure 6 and
Figure 7.
Spent media analysis with respect to amino acid content showed that culture
with lower
feed content, even after 48 hours of cultivation, did not deplete in a
significant way any of the
amino acid provided. This demonstrates that amino acid content is higher than
the culture's
nutritional requirements and in accordance with the present invention can be
further reduced
below the level of 78 mM. Accordingly, we find that cells perfused with medium
at a constant
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
18
rate will need much lower levels of amino acids, and that the rate of
perfusion equal to 1
bioreactor volume per day should require a steady state total amino acid
concentration in the
range of about 20-50 mM.
One of the primary requirements in product development projects is the high
titer
expression of product with the desired quality. Most proteins require proper
posttranslational
modification for their therapeutic activities. For example, the TNFR-FC fusion
protein produced
in this Example requires a significant degree of sialylation resulting in a
specific distribution of
isoforms based on a molecular charge profile. Isoelectric focusing analysis
shown by IEF gel
(Figure 8) demonstrates that reduction of feed content did not result in
alteration of the product
isoform distribution as compared to commercial reference standard and samples
obtained from
cultures fed with 20% feed.
In Figure 8 isoelectric focusing (IEF) gel shows comparable isoform
distribution in
product isolated from cultures containing lower (10%) feed concentration
(shown in wells 1 and
4, first and second medium exchange, ME), as compared to that isolated from
amino acid rich
(20%) feed cultures (shown in wells 2 and 5, first and second medium exchange,
ME). Profile of
reference standard is shown in well 3.
As seen in Figure 9, titers obtained for cultures fed with leaner (10% feed)
and richer
(20%) show that higher concentration of feeds can result in lower culture
productivity. By
providing cells with the nutrient requirements for perfusion specified in the
present invention,
and thus avoiding unnecessarily rich medium, one can achieve a culture
metabolic state in
perfusion processing that results in better productivity and product quality
compared to media
requirements heretofore recommended in the art (see, e.g., U.S. 7, 300, 773).
EXAMPLE 6
For production of a TNFR-Fc fusion protein under development as a biosimilar
of
etanercept, the seed train is expanded in large-volume shake flasks at 37 C
in SFM4CHO. The
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
19
production bioreactor is inoculated at seeding densities of from 1 to 5 x 106
cells/mL in medium
where the total amino acid concentration is varied in nine separate
repetitions of this experiment
from about 15 to about 70 mM (see the table, below). In each of the runs,
temperature during a
production phase (with continuous perfusion) is from 33.5 C to 35 C. An
ATFTm cell retention
device (Refine Technology) is used to recirculate medium (containing waste
products and
desired product) past a hollow fiber filter, with recirculation rates from
0.05 to 2.0 working
culture volumes per minute. The culture is first expanded in a growth phase
for 0 to 2 days, and
then perfusion is initiated at rates from 0.2 to 2 culture volumes per day to
facilitate a production
phase. New medium is added, as spent medium (containing the product) is
harvested through a
0.2 um pore size hollow fiber filter. Harvested fluid is chilled to 2-8 C,
purified by capture on
protein A resin. Aliquots are analyzed for titer and for product quality
attributes such as N-
glycan distribution and HIC analysis (to evaluate the relative amounts of
properly folded
etanercept, versus improperly folded/aggregated (inactive) material). The
total amino acid
concentrations investigated in these runs is shown below:
Total (Steady State) Amino Acid Concentration (approx.)
RUN # 1 15 to 20 mM
RUN #2 20-25 mM
RUN #3 25-30 mM
RUN #4 30-35 mM
RUN #5 35-40 mM
RUN #6 45-50 mM
RUN #7 55-60 mM
RUN #8 60-65 mM
RUN #9 65-70 mM
Analysis of the viable cell density, viability, product quality and titer for
each of runs 1
through 9 demonstrates excellent results with respect to these characteristics
at the lower end of
SUBSTITUTE SHEET (RULE 26)
CA 02937965 2016-07-26
WO 2015/116820
PCT/US2015/013524
the total amino acid concentration range of 15 to 70 mM, preferably in the
range of about 15 to
about 30 mM, with increasing deterioration in these characteristics as one
progresses to the
higher end of the range.
EXAMPLE 7
5
For production of a TNFR-Fc fusion protein under development as a biosimilar
of
etanercept, the seed train was expanded in large-volume shake flasks at 37 C
in SFM4CHO.
The production bioreactor was inoculated at seeding densities of from 0.3 to
0.75 x 106 cells/mL
in a series of perfusion bioreactors in medium where the total amino acid
concentration was
about 20 to about 56 mM. In each of the runs, temperature during a production
phase (with
10
continuous perfusion) was 35 to 37 C. An ATFTm cell retention device (Refine
Technology)
was used to recirculate medium (containing waste products and desired product)
past a hollow
fiber filter, with recirculation rates from 0.5 to 2.0 working culture volumes
per day. The culture
was first expanded in a growth phase for up to 8 days, with perfusion at a
cell-specific perfusion
rate of 0.05 to 0.1 nL per cell per day. The temperature was reduced to, for
example, 33.5 C to
15
facilitate a production phase. New medium was added, as spent medium
(containing the
product) was harvested through a 0.2 um pore size hollow fiber filter.
Harvested fluid was
chilled to 2-8 C, in preparation for capture on protein A resin. Aliquots
were analyzed for titer
and for product quality attributes such as N-glycan distribution and HIC
analysis (to evaluate the
relative amounts of properly folded etanercept, versus improperly
folded/aggregated (inactive)
20 material).
Analysis of the viable cell density, viability, product quality and titer for
each run
demonstrated excellent results with respect to these characteristics at the
lower end of the total
amino acid concentration range of 20 to 56 mM, preferably in the range of
about 15 to about 30
mM. As shown in Figure 10, a culture grown as described here achieved about 30
million
cells/mL during a nine-day growth phase and maintained that cell concentration
at high viability
during a production phase that extended for an additional 11 to 12 days.
SUBSTITUTE SHEET (RULE 26)