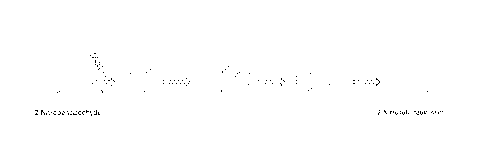Note: Descriptions are shown in the official language in which they were submitted.
NITROBENZALDEHYDE PROTON RELEASE FOR
MANIPULATION OF CELLULAR ACIDOSIS
BACKGROUND
[001] The present invention relates generally to manipulation of pH, and
more
specifically to the use of nitrobenzaldehyde or its analogs as a treatment for
a disease state.
[002] Cancer is defined as a malignant disease involving unregulated cell
growth,
metastasis throughout the body, and evasion of apoptosis. Due to its rapidly
evolving nature,
few similarities have been found across different cancers. Present research is
currently aimed
at the detection and distinction of specific traits, behaviors, and mutations
unique to cancers.
Current cancer treatments include chemotherapy, radiation, and biological
therapy. Classic
cancer therapies often require surgery or a combination of therapies for
effective treatment.
Most treatments that are in use today rely on inhibition of cell division from
radiation damage
or activation of apoptosis pathways. Newer treatment strategies focus on
targeting
unregulated proteins, such as HER-2 in breast cancer cells. A limitation to
current methods of
cancer treatment includes damage to healthy tissue, which in some cases can
result in DNA
damage, a known cause of cancer. Despite new treatment methodologies,
significant changes
in immediate cell death have yet to be reported. Cancerous cells that survive
treatment,
which are responsible for reoccurrence of cancer, are generally more
aggressive and resistant
to treatment (McCarty and Whitaker, Alternative Medicine Review, 2010,
15(3):264-72).
Despite advances in the field, cancer mortality rates remain high (American
Cancer Society,
Cancer Treatment and Survivorship Facts Et Figures 2012-2013, 2012). There
remains a need
for additional cancer therapies.
SUMMARY
[003] Certain embodiments are directed to photo-activated compounds for the
manipulation of pH in a cell. In certain aspects the photo-activated compound
is 2-
nitrobenzaldehyde (NBA). NBA can be used to induce apoptosis in a target cell,
a targeted
apoptosis-based therapy. The targeted apoptosis therapy provides for (i)
diffusion and toxicity
of a photo-activated compound (e.g., NBA) in a biological system, (ii)
induction of focal
acidosis, (iii) induction of cellular damage and death in response to a sudden
acidosis, and (iv)
targeting of cells or tissues of biological systems.
1
Date Recue/Date Received 2021-07-09
[004] Certain embodiments are directed to a method of treatment for certain
diseases
through inducing intracellular acidosis. In certain aspects the methods
comprise administering
NBA to a patient, tissue, or cell; exposing a target tissue or cells to
certain wavelengths of
light to photo-activate a compound resulting in the uncaging of hydrogen ions
and induction of
acidosis, apoptosis, and/or cell death. In certain aspects exposure to a light
is provided using
a "flash paradigm." Light can be provided pulsed or flashed. In certain
aspects light is
provided in 1, 2, 3, 4, 5, 6, or more flashes. A flash can be for 10, 20, 30,
40, 50, 60, 70, 80,
90, 100 seconds or more, including all values and ranges there between. In
certain aspects a
flash regime is provided having a first flash of 20, 40, 60, 80, or 100
seconds; a second flash of
10, 20, 30, 40, 50, or 60 seconds; and a third flash of 20, 40, 60, 80, 100
seconds. The
intervals between flashes can be 1, 1.5, 2, 2.5, 3, 3.5, 4 or more minutes,
including all values
and ranges there between. In certain embodiments there are about 2-minute
intervals
between each flash. In further aspects the flash regime can be a first 60
second flash followed
by a 2-minute interval, a second 30 second flash followed by a 2-minute
interval, and a third
60 second flash (60-30-60). Ranges of exposure time as well as power of
various light sources
may be utilized under any combination of effective time intervals and exposure
to induce
various magnitudes of intracellular acidification.
[005] In certain aspects the light is ultraviolet (UV) light. In a further
aspect the UV light
will have a wavelength in the range of 200 to 400 nm. In certain aspects the
light will have a
wavelength of 350 nm. The light source can be a laser or non-laser light
source.
[006] In certain embodiments the photo-activated compound is administered
systemically in combination with a targeted light exposure. In other aspects
the photo-
activated compound is administered locally. In certain aspects the photo-
activated compound
is administered using a needle, endoscope, cannula, catheter or other
appropriate medical
device for the target site. In a further aspect the photo-activated compound
is administered
topically if the target tissue is the skin. In certain aspects the photo-
activated compound is
NBA or a functional analog thereof.
[007] NBA is a small molecule that has a high likelihood of crossing
cellular and bacterial
membranes. Uncaging hydrogen ions by UV activated NBA causes damage by
inducing cell wide
acidosis in eukaryotic cells. The ability to damage a wide range of proteins
and enzymatic
processes inside a pathogen, e.g., bacterium, reduces the chances of the
evolution of
resistance compared to traditional antibiotics. In some embodiments NBA is
administered and
2
Date Recue/Date Received 2021-07-09
photo-uncaged to manipulate the activity of intracellular processes by the
activation or
deactivation of enzymes and proteins through modulation of pH,, and subsequent
cellular
function, which does not necessarily entail the death of the cell or tissue
into which NBA is
administered and photo-caged or photo-activated. In some embodiments, uncaged
Fr from
NBA or similar compound will be utilized in order to attain a desired
intracellular pH for the
activation of cellular dedifferentiation of stem cell lines (Gao et al.,
Cellular Physiology and
Biochemistry, 2014, 33(1):185-94).
[008] In some embodiments for the local treatment of internal tissues and
cancers, the
light may be targeted to specific tissues with the use of an external light
source, or an
internal light source. In certain aspects the light can be focused or targeted
to a defined
location. Internal light sources may include an endoscope, a fiber-optic light
source, an
implantable illuminator, or a source of chemiluminescence such as luminol.
[009] In other embodiments a photo-activated compound can be used to kill
or impede
the growth of microorganisms, including, but not limited to bacteria, fungi,
and amoebas. The
ability to kill microorganisms with the compounds and methods described herein
has
applications as a cleaning or sterilization agent, particularly in
applications such as point of
injury care, mass casualty management, and natural disaster situations. A
surface or object to
be sterilized can be contacted with the photo-activated compound. The
contacted surface or
object is then expose to an appropriate light source comprising a wavelength
that activates or
uncages the compound.
[010] Other embodiments of the invention involve the use of nanoparticles.
Such
nanoparticles may vary in rare earth metal-doped upconverting cores,
hydrophilic
biocompatible polymers, compound (e.g., NBA) loading concentrations and silica
loading
times. In certain aspects a nanoparticle comprises a compound (e.g., NBA)
coupled (directly
or via linker) to a particle (e.g., a KYb2F7 core). In certain aspects
particles with a KYb2F7 core
showed very high efficacy in vitro with minimal peripheral damage.
Nanoparticles can be
constructed of various cores ranging in diameter from approximately 50 - 100
nm.
Nanoparticles may be selected based upon upconversion spectra that are
collected for each
core to test the best ultraviolet emission revealing maximum uncaging. Photon
upconversion
or upconversion (UC) is a process in which the sequential absorption of two or
more photons
leads to the emission of light at shorter wavelength than the excitation
wavelength. It is an
anti-Stokes type emission. An example is the conversion of infrared light to
visible light.
3
Date Recue/Date Received 2021-07-09
Materials by which upconversion can take place often contain ions of d-block
and f-block
elements (e.g., Ti2+, Ni2+, Mo3+, Re4+, and 0s4+). In certain aspects a
nanoparticle is imaged
using a transmission electron microscope (TEM) and tested for its zeta
potential.
[011] Various biocompatible polymers, such as PEG, polyvinylpyrrolidone,
and photo
linkers, may be conjugated to the nanoparticle in order to allow for the most
efficient loading
and cellular uptake of NBA and/or other uncaging compounds, such as calcium.
Silica may be
used to protect the polymers from extra- or intracellular interactions that
may cause cell
toxicity and/or degradation. The use of silica may also be employed to further
reduce the
zeta potential on the nanoparticle for the increase of cellular uptake.
Various surfactants,
antibodies, or ligands can be applied to the nanoparticles for targeted
therapies. All
combinations of the previously mentioned may be tested in vitro and in vivo
for the overall
efficacy of the nanoparticle in a plated condition and in a living animal for
long-term effects.
[012] Other methods of targeting nanoparticles to specific tissues or
cancer may also be
applied. Targeting may be through passive targeting based phenomenon such as
the enhanced
permeation and retention effect, caused by leaky angiogenic vessels and poor
lymphatic
drainage that has been used to explain why macromolecules and nanoparticles
are found at
higher ratios in tumors compared to normal tissues. Targeting may also be
accomplished by
active targeting in which ligands are coupled to the nanoparticle that
correspond to molecules
found exclusively in cancer cells, or which are found in greater abundance in
cancer cells.
[013] The ability to effectively target specific cells in a focal manner is
a promising
therapeutic delivery mechanism. Cancer cells can exhibit a variety of unique
characteristics
that can be targeted for the focal delivery of a therapeutic. Delivery
mechanisms designed to
target specific cancer cells offer the ability to reduce toxic side effects
usually associated
with chemotherapeutic treatment (Gabizon et al., Advanced drug delivery
reviews 2004,
56:1177-92). The wide array of possible targets for cancer includes surface
receptors such as
human epidermal growth factor receptor 2 (Emde et al., Crit Rev Oncol Hematol.
2012, 84
Suppl 1:e49-57; Colombo et al., Pharmacol Res 2010, 62(2):150-65), folate
receptors (Salazar
et at., Cancer metastasis reviews, 2007, 26:141-52), and estrogen receptors
(Kleinsmith et al.,
"Cancer screening, diagnosis, and treatment", in Principles of Cancer Biology.
Pearson
Education, Inc., 2006; 218-224). Other possible targets for therapeutic
delivery mechanisms
include cytokines (Przepiorka et al., Blood. 2002, 95(1):83-89; Kleinsmith et
al., "Cancer
screening, diagnosis, and treatment", in Principles of Cancer Biology. Pearson
Education, Inc.,
4
Date Recue/Date Received 2021-07-09
2006; 218-224) and growth factors (de Bruin et al., Cancer Discov. 2014; Wang
et al.,
Cardiovasc Res 2013, 98(1):56-63). A more comprehensive list of possible
targets can be
found on Table 1.
Table 1 - Potential targets for cancer therapy
Target Cancer(s) Citations
Epidermal Growth Factor Lung cancer de Bruin et al., Cancer
Discov.
(EGF) 2014
Fibroblast Growth Factor Non-small-cell lung cancer Wang et al.,
Cardiovasc. Res.
(FGF) 98(1):56-63
Platelet-derived Growth Non-small-cell lung cancer Tian et al., Zhongguo
Fei Al Za
Factor (PDGF) Zhi. 2014, 17(1):42-48
Vascular Endothelial Growth Breast, ovaria, lung, Kleinsmith et al., in
Principles of
Factor (VEGF) gastric, colorectal Cancer Biology. Pearson
Education, Inc., 2006:218-24
TNF-a Hairy cell leukemia Kleinsmith et al., Id.
INF-13 Kaposi's sarcoma Kleinsmith et al., Id.
CD20 Non-Hodgkin's B cell Kleinsmith et al., Id.
lymphoma
Estrogen Receptor Breast cancer Kleinsmith et al., Id.
human epidermal growth Breast cancer Emde et al., Crit Rev.
Oncol.
factor receptor 2 (HER2) Hematol. 2012, 84 Suppl.
1:e49-
57; Colombo et al., Pharmacol
Res 2010, 62(2):150-65
Folate Receptor-a Epithelial cancer (non- Kularatne et al.,
Angwewandte
mucinous adenocarcinomas Chemie International Edition,
of ovary, uterus and cervix, 2013, 52:12100-103; Salazar et
ependymal brain) al., Cancer metastasis
reviews,
2007, 26:141-52; van Dam et al.,
Nature medicine 2011, 17:1315-
19
Folate Receptor-B Chronic and acute Kularatne et al.,
Angwewandte
myelogenous leukemia; Chemie International
Edition,
pancreatic cancer- 2013, 52:12100-103; Salazar
et
associated macrophages al,. Cancer metastasis
reviews,
2007, 26:141-52; Kurahara et al.,
Annals of surgical oncology
2012, 19:2264-71
Interleukin-2 Kidney cancer and Przepiorka et al., Blood.
2002,
melanoma 95(1):83-89
BCR-ABL* Chronic myelogenous Kleinsmith et al., in
Principles of
leukemias Cancer Biology. Pearson
Education, Inc., 2006:218-24
[014] Other embodiments of the invention are discussed throughout this
application. Any
embodiment discussed with respect to one aspect applies to other aspects as
well and vice
versa. Each embodiment described herein is understood to be embodiments that
are
Date Recue/Date Received 2021-07-09
applicable to all aspects of the invention. It is contemplated that any
embodiment discussed
herein can be implemented with respect to any device, method, or composition,
and vice
versa. Furthermore, systems, compositions, and kits of the invention can be
used to achieve
methods of the invention.
[015] The use of the word "a" or "an" when used in conjunction with the
term
"comprising" in the claims and/or the specification may mean "one," but it is
also consistent
with the meaning of "one or more," "at least one," and "one or more than one."
[016] Throughout this application, the term "about" is used to indicate
that a value
includes the standard deviation of error for the device or method being
employed to
determine the value.
[017] The use of the term "or" in the claims is used to mean "and/or"
unless explicitly
indicated to refer to alternatives only or the alternatives are mutually
exclusive, although the
disclosure supports a definition that refers to only alternatives and
"and/or."
[018] As used in this specification and claim(s), the words "comprising"
(and any form of
comprising, such as "comprise" and "comprises"), "having" (and any form of
having, such as
"have" and "has"), "including' (and any form of including, such as "includes"
and "include'') or
"containing" (and any form of containing, such as "contains" and "contain")
are inclusive or
open-ended and do not exclude additional, unrecited elements or method steps.
[019] Other objects, features and advantages of the present invention will
become
apparent from the following detailed description. It should be understood,
however, that the
detailed description and the specific examples, while indicating specific
embodiments of the
invention, are given by way of illustration only, since various changes and
modifications within
the spirit and scope of the invention will become apparent to those skilled in
the art from this
detailed description.
DESCRIPTION OF THE DRAWINGS
[020] The following drawings form part of the present specification and are
included to
further demonstrate certain aspects of the present invention. The invention
may be better
understood by reference to one or more of these drawings in combination with
the detailed
description of the specification embodiments presented herein.
6
Date Recue/Date Received 2021-07-09
[021] FIG. 1 is a diagram illustrating the conversion of 2-
nitrobenzaldehyde (NBA) to 2-
nitrosobenzoic acid. The diagram also illustrates the reaction mechanism that
ensues with
exposure of NBA to ultraviolet (UV) light at a wavelength of 350nm. NBA
exposure to 350nm
UV light results in the release of protons and the formation of 2-
nitrosobenzoic acid.
[022] FIG. 2 is a diagram of the proposed positive feedback pathway in MCF-
7 cells,
which occurs following significant increases in intracellular 1-1+ from NBA in
response to a UV
flash. 2.1 illustrates the exposure of intracellular NBA to 350 nm wavelength
UV flash
paradigm, causing a sudden increase in proton concentration in the
intracellular space. This
intracellular acidification results in the concomitant osmotic water loss or
change in cell
volume. The increase in intracellular H activates NHE1 to extrude protons in
exchange for
sodium ions (2.2a). Sodium ions act as osmolytes, drawing water to the area of
high sodium
concentration inside the cell. The movement of water to the area of high Na,
or the extrusion
of water, leads to a deformation of the cell wall and/or cell shrinkage. The
deformation of
the cell wall results in phosphorylation and activation of the tyrosine kinase
Janus kinase II
(Jak-2) (Garnovskaya et al., J Blot Chem. 2003, 278(19): 16908-15) (2.2b). The
continued
activation of NHE1 in response to the intracellular H+ creates increases in
intracellular osmotic
pressure, resulting in an outward protrusion on the cellular membrane, or a
bleb (2.3). The
activity of NHE1 attempts to compensate for the focal acidosis inside the
cell, causing
intracellular Na + levels to rise, further promoting the hydrostatic pressure
within, and
subsequent size of the bleb. In response to the local increase in
intracellular Na+
concentration, the sodium calcium exchanger 1 (NCX1) reverses exchange
activity (Yi et al., J.
Biol Chem. 2012, 287(13):10316-24), leading to the efflux of Na + and influx
of Ca' (2.4a).
Complexation of phosphorylated Jak-2 and calmodulin (CaM; 2.4b) increases the
Jak-2-
dependent tyrosine phosphorylation of CaM. The increase in intracellular Ca2+
via the reversal
of NCX1 facilitates the binding of Ca2+ to calmodulin, independent of the
phosphorylation
state of CaM. While both phosphorylated CaM (Garnovskaya et al., J Blot Chem.
2003,
278(19): 16908-15) and calcified CaM (Koster et al., J Blot Chem. 2011,
286(47): 40954-61)
increase the activity of NHE1, the combination of phosphorylation and
calcification of CaM
(2.5) imparts the highest affinity of CaM to NHE1 (Koster et al., J Blot Chem.
2011, 286(47):
40954-61) (2.6a), and thereby promotes the greatest activation of NHE1 (Koster
et al., J Blot
Chem. 2011, 286(47): 40954-61). In spite of Na + extrusion via NCX1,
maintained activation of
NHE1 is facilitated by the phosphorylated-calcified CaM, leading to further
Ca2+ entry (2.6b
and 2.6c). The supraphysiological increases in intracellular 1-1+ induced by
flash photolysis of
7
Date Recue/Date Received 2021-07-09
NBA leads to cell shrinkage followed by subsequent activation of NHE1, Jak-2,
and NCX1;
creating a novel pathway of positive feedback. This ultimately leads to
increased intracellular
osmotic pressure, cellular blebs, and potential rupture of the cell membrane.
[023] FIG. 3 illustrates the ratio of DCFDA fluorescence (RFL) of PC12
cells in response to
bath application of high potassium/nigericin solutions titrated to pH values
of 2.0, 4.0, 5.0,
6.0, 7.0, and 8Ø This DCFDA calibration curve permits the optical conversion
of DCFDA
emitted fluorescence ratios to intracellular pH.
[024] FIG. 4 illustrates the DCFDA (10 pM) emitted fluorescence of PC12
cells (40x).
These cells were also loaded with NBA (1 mM).
[025] FIG. 5 illustrates the percentage of apoptosis in PC12 cells (n =
362) over time in
response to focal decreases in pH, following the uncaging of H+ from NBA (1
mM). Each set of
symbols represents a separate experiment after exposure to various UV flash
paradigms. These
data demonstrate our ability to induce apoptosis in 76 to 100% of cells
exposed to pH,-induced
apoptosis within 125 minutes.
[026] FIG. 6 illustrates the percentage of necrosis in PC12 cells (n = 118)
over time in
response to focal decreases in pH, following the uncaging of 1-1+ from NBA (1
mM). PC12 cells (n
= 68) were exposed to a 60-30-60 UV flash paradigm alone, without being loaded
with NBA.
PC12 cells (n = 68) were exposed to UV flash paradigm a without being loaded
with NBA. PC12
cells (n = 75) were also exposed to time alone with no UV or NBA.
[027] FIG. 7 illustrates a summary of the percentage of apoptosis in PC12
cells (n = 362)
over time in response to focal decreases in pH, following the uncaging of 1-1+
from NBA (1 mM)
and our control conditions of UV alone (n = 76), NBA alone (n = 47), and time
alone (n = 71).
There were significant differences between the NBA-UV treated cells and the
three control
conditions. There were no significant differences between the UV alone, NBA
alone, and time
alone.
[028] FIG. 8 illustrates mean optical recordings of pH, from PC12 cells (n
= 421) loaded
with 10 pM DCFDA and 1 mM NBA before (7.55 pH,) and after (6.37 pH,) flash
photolysis. We
were able to induce a significant acidosis (P < 0.01) in response to flash
photolysis of NBA. The
mean change in pH, (A pH,) was 1.18 pH units. These data support our abilities
to 1) optically
8
Date Recue/Date Received 2021-07-09
record valid pH, measurements, and 2) quantify the pH, decreases associated
with the
uncaging of 1-1+ from NBA.
[029] FIG. 9 illustrates representative pictures of differential
interference contrast (DIC)
microscopy of PC12 cells (n = 68) at 40x magnification over the course of an
experiment to
induce acidosis with a 60-30-60 UV flash paradigm. (A) PC12 cells prior to
flash photolysis. (B)
PC12 cells following flash photolysis, showing reduction of cell size and
changes in cell
morphology. (C) PC12 cells prior to UV flash with an overlay depicting the
outlines of cell
shape. (D) PC12 cells following UV flash and uncaging of 1-1+, with an overlay
of the pre-
treatment cell outlines shown in (C). (E) A DIC image PC12 cells approximately
2 hours
following flash photolysis of NBA with an overlay of the fluorescent apoptosis
marker Annexin
V.
[030] FIG. 10 illustrates representative pictures of ratiometric
fluorescence microscopy
of MCF-7 cells at 40x magnification over the course of an experiment to induce
acidosis with a
60-30-60 UV flash paradigm. (A) MCF-7 cells loaded with the 10 pM DCFDA prior
to flash
photolysis. (B) MCF-7 cells following flash photolysis, showing reduction of
cell size and
changes in cell morphology. (C) MCF-7 cells prior to UV flash with an overlay
depicting the
outlines of cell shape. (D) MCF-7 cells following UV flash (t = 79 min) with
an overlay of the
pre-treatment cell outlines shown in (C). (E) DIC of MCF-7 cells approximately
2 hours
following flash photolysis of NBA. The scale bar indicates fluorescence ratio
measurements
below 2.00.
[031] FIG. 11 illustrates emitted fluorescence of DCFDA-loaded MCF-7 cells
following
flash photolysis of NBA with a 60-30-60 UV flash paradigm. Images were
acquired at t = 50
minutes. Arrows show the appearance of some somatic blebs, signifying cell
death. The scale
bar indicates fluorescence ratio measurements below 2.00.
[032] FIG. 12 illustrates mean optical recordings of pH, from MCF-7 cells
(n = 76) loaded
with 10 pM DCFDA and 1 nnM NBA before (7.38 pH,) and after (6.22 pH,) flash
photolysis. We
were able to induce a significant acidosis (P < 0.01) in response to flash
photolysis of NBA. The
mean change in pH, (A pH,) was 0.97 pH units. The initial pH, of MCF-7 cells
was not
significantly different from the pH, of PC12 cells (see FIG. 8). In addition,
the magnitude of
acidosis attained in MCF-7 cells (0.97 pH units) was not significantly
different from the
magnitude of pH, attained in PC12 cells (1.18 pH units).
9
Date Recue/Date Received 2021-07-09
[033] FIG. 13 illustrates the mean percentage of MCF-7 cell death,
indicated by blebbing
or Annexin V fluorescence, by condition. MCF-7 cells (n = 262) were loaded
with 1 mM NBA and
subsequently exposed to a 60-30-60 UV flash paradigm. As separate controls MCF-
7 cells were
exposed to a 60-30-60 UV flash paradigm alone (n = 112), NBA alone (n = 47),
or time alone (n
= 20). Linear regression analyses indicate apoptosis and cell death with
treatment of MCF-7
cells with NBA and UV light was significantly greater than our controls
[034] FIG. 14 illustrates a schematic representation of the KYb2F7 and
NaYF4 core
nanoparticles PEGylated, doped with NBA, then coated in mesoporous-silica (A);
and a
schematic representation of the KYb2F7 and NaYF4 core nanoparticles PEGylated
then doped
with NBA (B).
[035] FIG. 15 illustrates optical recordings of the average pH, of PC12
cells (n = 22) over
time in response to flash photolysis of NBA. The cells received a 60-30-60 UV
flash paradigm at
each of the downward arrows. The average pH, drop for each flash was 0.72,
0.36, 0.36,
respectively.
[036] FIG. 16 illustrates optical recordings of the average pH, of MCF-7
cells (n = 13)
over time in response to flash photolysis of NBA. The cells received a 60-30-
60 UV flash
paradigm at each of the downward arrows. The average pH, drop for each flash
was 0.68,
0.35, 0.59, respectively.
[037] FIG. 17 illustrates the percentage of MCF-7 breast cancer cell death
over time in
response to the activation of a KYb2F7 upconverting nanoparticle in vitro.
Cells were loaded
with nanoparticles prior to photoactivation by 980 nm light.
[038] FIG. 18 illustrates MDA-MB-231 breast cancer cells in response to
excitation of the
pH sensitive fluorophore DCFDA (A) and the apoptotic marker, Annexin-V (B)
prior to
photoactivation of NBA (magnification = 40x). One hour after NBA
photoactivation, these
same cells demonstrated a lower DCFDA emitted fluorescence (C), which
coincides with a
lower pHi, and significant expression of apoptosis (D). (images C a D
magnification = 40x).
[039] FIG. 19 illustrates the triple negative breast cancer MDA-MB-231
tumors in a
control mouse 5 days after a 0.1 ml aCSF injection (A) and an NBA-phototherapy
mouse 3 days
after 0.1 ml 1 mM NBA and phototherapy. The margins of the tumor are outlined
with a
yellow dashed line.
Date Recue/Date Received 2021-07-09
[040] FIG. 20 illustrates the significant decreases in the percent change
in tumor volume
in NBA-phototherapy (NBA) treated mice when compared to control aCSF (CON)
treated mice.
The percent change in tumor volume was normalized in each animal to the tumor
volume on
the day of treatment. The one-time treatment of NBA induced significant
decreases in the
percent change in tumor volume for up to 9 continuous days.
[041] FIG. 21 illustrates the significant decreases in tumor volume (mm3)
in NBA-
phototherapy (NBA) treated mice when compared to control aCSF (CON) treated
mice. The
one-time treatment of NBA induced significant decreases in the tumor volume
from the
second day post-treatment for up to 13 days post treatment. The significant
reduction in
tumor volume in NBA treated animals compared to control ended at day 14
because day 14
represented the beginning of the control animals being euthanized in response
to reaching
humane endpoints.
[042] FIG. 22 illustrates the significant increase in survival in vivo of
our novel NBA
photodynamic therapy. Aggressive triple negative MDA-MB-231 human breast
cancer tumors in
nude mice were give a one-time treatment of either control aCSF (CON) or NBA
photodynamic
therapy (NBA). The mean days survived ( 1 SEM) were significantly larger in
NBA treated
mice when compared to control mice.
DESCRIPTION
[043] Most cancers can be characterized by an excessive production and
extrusion of
lactic acid via aerobic glycolysis known as the Warburg Effect. The excessive
production of
intracellular acid is effectively extruded by cancer cells resulting in an
acidic extracellular
environment, which has been directly linked to metastatic potential (McCarty
and Whitaker,
Alternative Medicine Review, 2010, 15(3):264-72). Extracellular acidosis
results in an
increased rate of blood-vessel formation, or angiogenesis, at the site of a
tumor, as well as
decreased effectiveness of most chemotherapeutic drugs, which are alkaline in
nature. In
order to survive in this acidic environment, cancer cells express or up-
regulate proton
exchangers, most notably sodium/hydrogen exchanger 1 (NHE1), which extrude
protons and
maintain a slightly alkaline intracellular pH (pH,).
[044] While an excessive amount of acid production and upregulation of NHE1
contributes to tumor aggressiveness, that same excessive extrusion could also
be turned
against cancer cells as a form of treatment. Due to the inherent challenges of
selectively
11
Date Recue/Date Received 2021-07-09
treating only NHE1 in cancer cells, direct disruption of pH, has been the
elusive target of
cancer therapies since the discovery of the Warburg Effect in 1931. NHE1
blockers, such as
amiloride-based drugs, act to kill cancer by trapping protons inside and
subsequently dropping
pH, to induce apoptosis. Other experimental techniques that implement pH
manipulation
include alkalization of the extracellular space with sodium bicarbonate-laced
drinking water,
or increasing oxygen perfusion to the tumor mass itself (Raghunand and
Gillies, British Journal
of Cancer, 1999, 80(7):1005-11).
[045] As with many chemotherapeutic approaches, NHE1 blockers are not
focally
restricted to the tumor site, causing systemic damage to peripheral tissue due
to the
ubiquitous distribution of NHE1 on all living cells. Alternate forms of
intracellular
acidification, such as NH4C1 prepulse (Zamora et al., The Open Zoology
Journal, 2013, 6:8-17)
are not focal, difficult to implement, cytotoxic, and time consuming.
[046] 2-nitrobenzaldehyde ("NBA") is a photoactivated molecule capable of
immediately
releasing a proton upon exposure to 350 nm ultraviolet (UV) light (Ravindran
et al., Cellular
Signaling 2012, 24(5):981-90). NBA passively diffuses through the cellular
membrane and
becomes trapped due to the slight positive charge on the molecule. Once inside
the cell, NBA
is capable of remaining inactive and intact until excited by UV light (200 nm
to 410 nm,
Diaspro et al., Q Rev Biophys. 2005, 38(2):97-166). NBA has been used to
induce a focal, rapid
intracellular acidosis, resulting in intrinsic apoptosis of the target cell.
Due to the ease of
entry into the cell, the tendency of the molecule to remain inside of the
cell, the nontoxic
nature of the molecule, and the instant acidification upon exposure to UV
light, NBA is an
ideal mechanism for disruption of pH,. The fast, focal disruption of pH,
regulation in cancer
cells is an untapped method of cancer treatment that holds great potential as
an alternate to
chemotherapy and radiation. In addition, treatment is not "cancer-specific"
and can be used
in treating a myriad of cancers, such as multi-drug resistant (MDR) cancers.
I. NANOPARTICLES
[047] Photodynamic therapy (PDT) is one of the fastest growing modalities
for treating
many types of cancers. This technology is in its infancy and currently is not
effective enough
to nullify the need for more effective treatments such as surgery or
chemotherapy. PDT has
been utilized as a treatment for other diseases such as vein graphs to limit
intimal hyperplasia
post-surgery (Lamuraglia et al., Journal of Vascular Surgery, 1995, 21(6):882-
90), psoriasis
(Almutawa et al., Photodermatol. Photoimmunol. Photomed. 2013), and polypoidal
choroidal
12
Date Recue/Date Received 2021-07-09
vasculopathy (Sliwinska et al., Prog Retin Eye Res. 2013, 37:182-99). However,
the most
promising application for PDT is its use in oncology. PDT applied to cancer
cells in vitro and in
vivo has shown cell death as high as 60% (Idris et al., Nature Medicine, 2012,
18(10):1580-85).
Currently, one of the most effective uses of PDT employs the photoactivation
of a
nanoparticle to cause intracellular release of reactive oxygen species (ROS)
for cancer cell
therapy (Idris et al., Nature Medicine, 2012, 18(10):1580-85).
[048] Unlike their more stable elemental oxygen counterpart, ROS consist of
radical and
non-radical oxygen species that are capable of intracellular protein
regulation through redox
reactions (Bartosz et al., Biochemical Pharmacology, 2009, 77(8):1303-15;
Circu et al., Free
Radical Biology Et Medicine, 2010, 48(6):749-62). Endogenously produced by the
mitochondria,
ROS are involved in a wide array of intracellular signal regulation, from cell
proliferation and
gene regulation to mitochondrial oxidative stress and apoptosis (Ray et al.,
Cellular Signaling
2012, 24(5):981-90). ROS can contribute to the activation of an apoptotic
death cascade by
opening a permeability transition pore complex to allow for the activation of
apoptosis-
inducing factors such as cytochrome c and caspase (Martindale et al., Journal
of Cellular
Physiology, 2002, 192(1):1-15; Gupta et al., Antioxidants Et Redox Signaling,
2012,
16(11):1295-1322).
[049] A nanoparticle was constructed to improve PDT in B16-0 melanoma
cancer cells by
utilizing a dual-photosensitizing nanoparticle to enhance cell death from the
photoactivation
of ROS (Idris et al., Nature Medicine, 2012, 18(10):1580-85). This particular
nanoparticle had a
NaYF4 crystal core uniformly coated in mesoporous silica for the in vitro
experiments. Each
nanoparticle was loaded with Merocyanine 540 (MC540) and Zinc (II)
Phthalocyanine (ZnPc),
both of which are photosensitizing drugs that are only activated when
introduced to visible
light. Upon the upconversion of 980 nm near infrared light (NIR) to visible
light, MC540 and
ZnPc release ROS and induce subsequent cellular damage (Idris et al., Nature
Medicine, 2012,
18(10):1580-85). To test the efficacy of the ROS treatment, the coated
nanoparticles were
conjugated with folic acid and polyethylene glycol (PEG) to facilitate
movement across the
cell membrane. The nanoparticles were combined with cultured melanoma cancer
cells to
facilitate endocytosis, which were then injected into C57BL/6 mice. The PDT
and subsequent
release of ROS was successful in reducing tumor size and increasing apoptosis
in vivo.
[050] Cancer cell death (63%) and tumor size reduction reported by Idris et
al. (2012) are
insufficient to be considered as a successful clinical treatment. This
percentage of cell death
13
Date Recue/Date Received 2021-07-09
is not high enough to stop uncontrollable cell proliferation and avoid
additional treatments,
such as surgery and chemotherapy, to remove remaining cancer cells. This
particular
nanoparticle also was not loaded into the tumor after a mass had formed.
Rather, the cancer
cells were grown in vitro and loaded with the nanoparticles before being
introduced to the
mouse. Culturing cancer cells with a PDT nanoparticle prior to introducing
them in vivo,
although moderately successful, is not a realistic approach for treatment
analogous to
treatment in humans.
[051] The proton donor NBA will diffuse across the cell membrane and remain
in the
intracellular space. Facilitation of the cellular entry of NBA is based upon
the creation of an
extracellular gradient, thereby allowing NBA to passively diffuse through the
cell membrane
into the intracellular space. The greater electronegativity of oxygen creates
a polar carbonyl
group and a relatively large molecular dipole moment. The non-bonding electron
pairs of
oxygen make aldehydes hydrogen-bond acceptors, thereby increasing their water
solubility.
Removal of NBA from the extracellular space does not result in an efflux of
NBA, due primarily
to NBA's increased solubility inside the cell.
[052] Effective concentrations of intracellular NBA are not cytotoxic and
do not disrupt
cellular function. Kohse et al., (J Am Chem Soc. 2013, 135(25):9407-11) photo
activated the
enzyme acid phosphatase from pH 8.0 to 6.0 by the activation of a pH jump
using flash
photolysis of NBA and did not report any degradation of enzyme function as
result of exposure
to NBA. Flash photolysis of NBA to reduce pH, of rat ventricular myocytes
(Swietach et al.,
Biophys J, 2007, 92(2):641-53) did not alter the H buffer capacity of these
cells. More
importantly, in the absence of UV exposure, NBA (1 mM) did not alter diastolic
Ca', cellular
contraction, or "the mechanisms of spatial pH, regulation". The inventors
recently loaded the
entire in vitro tadpole brainstem with 10 pM NBA and observed no disruption in
the
spontaneous, fictive respiratory motor output or central respiratory
chemoreceptor responses,
indicating that at this concentration, NBA does not exert cytotoxic effects on
cells which
compose the respiratory neural circuits (Ravindran et al., Journal of Health
Care for the Poor
and Underserved, 2011 22(4):174-86).
II. CELLULAR PATHWAYS AND APOPTOSIS MECHANISMS
[053] The ability to effectively target specific cells in a focal manner is
a promising
therapeutic delivery mechanism. Cancer cells can exhibit a variety of unique
characteristics,
which can be targeted for the focal delivery of a therapeutic. Delivery
mechanisms designed
14
Date Recue/Date Received 2021-07-09
to target specific cancer cells offer the ability to reduce toxic side effects
usually associated
with chemotherapeutic treatment (Gabizon et al., Cancer Chemother Pharmacol.
2010,
66(1):43-52). The wide array of possible targets for cancer includes surface
receptors such as
human epidermal growth factor receptor 2 (Emde et al., Critical reviews in
oncology/ hematology 2012, 84:49-57; Colombo et al., Pharmacol Res 2010,
62(2):150-65),
folate receptors (Salazar et al., Cancer metastasis reviews, 2007, 26:141-52),
and estrogen
receptors (Kleinsmith et al., in Principles of Cancer Biology. Pearson
Education, Inc., 2006,
218-24). Other possible targets for therapeutic delivery mechanisms include
cytokines
(Przepiorka et at., Blood. 2002, 95(1):83-89; Kleinsmith et al., in Principles
of Cancer Biology.
Pearson Education, Inc., 2006, 218-24), growth factors (de Bruin et al.,
Cancer Discov. 2014;
Wang et al., Cardiovasc Res 2013, 98(1):56-63), and other cellular pathways
and mechanisms.
A. Sodium Hydrogen Exchanger 1 (NHE1)
[054] The sodium hydrogen exchanger 1 (NHE1) is a ubiquitously expressed
ion
transporter that is highly conserved genetically. NHE1 facilitates the
exchange of extracellular
Na + for intracellular Fl+, promotes hypotonicity, and increases cell volume.
NHE1 is
characterized by a highly variable intracellular C-terminus, which can be
modulated to
mediate cellular behaviors including adhesion, morphology, migration, and
proliferation
(Putney et al., Annual Reviews. 2002, (42):527-52). NHE1 is a vital
transporter for cell
survival.
[055] NHE1 is modulated by many mediators in the cell. Calmodulin (CaM) is
a messenger
protein that, once bound by calcium or phosphorylated, activates NHE1 via the
intracellular
regulatory domain (Koster et al., J Biol Chem. 2011, 286(47):40954-961). Once
bound, NHE1
will be constitutively activated until CaM is unbound. Upon cell volume loss,
phosphorylated
Janus kinase II will phosphorylate CaM, which then activates NHE1. Activation
of NHE1 will
cause sodium influx and a concomitant water entry into the cell in an effort
to reestablish cell
volume. NHE1 is often dimerized with the sodium calcium exchanger 1 (NCX1),
which
normally transports Na + into the cell and Ca2+ out of the cell. When
intracellular Na + levels
rise, the NCX1 reverses the direction of ion transport to prevent further
increases in
intracellular Na. The dimerization of NHE1 and NCX1 is normally utilized to
modulate osmotic
pressure. NHE1 is upregulated in cancer cells, contributing to the
effectiveness of pH,
regulation, tumor invasiveness, and avoidance of pH,-induced apoptosis
(Cardone et al.,
Nature Review Cancer. 2005, 5(10):786-95).
Date Recue/Date Received 2021-07-09
B. Phosphatidylserine
[056] Phosphatidylserine (PS) is a membrane phospholipid that is normally
sequestered
to the interior leaflet of a cell's phospholipid bilayer. The expression of PS
on the outer
surface of the cell membrane has two causes, the lack of membrane asymmetry
maintenance
by adenosine triphosphate-dependent aminophospholipid translocase, and the
activation of
lipid scramblases that quickly flip PS to either membrane surface (Verhoven et
al., Journal of
Experimental Medicine, 1995, 182(5): 1597-601).
[057] Scramblases are not yet identified and may be one or several proteins
that can be
activated by at least two pathways. All nucleated human cells have an
apoptotic pathway that
lead to the expression of PS on the outer membrane surface. Hematopoietic
cells also have a
reversible, calcium-dependent pathway for expressing PS; non-nucleated
erythrocytes have
only this calcium-dependent path (Bevers & Williamson, Federation of European
Biochemical
Societies Letters, 2010, 584(13):2724-30). The apoptotic pathway utilizes the
collapse of lipid
asymmetry to expose PS to the extracellular space as a signal for non-
inflammatory
phagocytotic removal.
[058] Early in the apoptotic pathway, aminophospholipid translocase is
downregulated
(Verhoven et al., Journal of Experimental Medicine, 1995, 182(5):1597-601).
Then, once
mitochondrial outer membrane permeabilization (MOMP) occurs, scramblase will
proceed to
transport PS to the outer membrane leaflet. Apoptosis inhibitors acting on the
pathway before
MOMP occurs can prevent PS expression. Conversely, inhibitors acting after
MOMP will block
downstream apoptotic events, but PS expression will still occur (Bevers Ft
Williamson,
Federation of European Biochemical Societies Letters, 2010, 584(13):2724-30).
[059] PS expression in non-hematopoietic cells is a definitive indicator of
cell death and
can be shown by labeling with fluorescently conjugated Annexin V, a naturally
occurring
protein with a high affinity for PS. Because PS can also be exposed when a
cell membrane
loses integrity, a secondary dye that becomes trapped in the membrane can be
used to verify
that PS exposure is due only to the apoptotic route (Schutters &
Reutelingsperger, Springer,
2010, (15):1072-82).
C. P53 and Bc1-2
[060] TP53 is a multifaceted gene involved in a complicated, yet common,
pathway. The
regulatory network of P53 is studied for its most common occurrences in
pathological
16
Date Recue/Date Received 2021-07-09
conditions, such as neurological degeneration, atherosclerosis and cancer
(Amaral et al.,
Discovery Medicine, 2010, 9(45):145-52). The most commonly seen causes of
cancer are due to
damage or deletion of the TP53 gene (Olivier et al., Cold Spring Harbor
Perspectives in
Biology, 2010, 2:1-17). Mutations to this gene are the cause of over 50% of
cancers seen today
(Kleinsmith, "Cancer screening, diagnosis, and treatment", in Principles of
Cancer Biology.
Pearson Education, Inc., 2006, 218-24).
[061] The TP53 gene, located on chromosome 17 in humans, is critical for
the regulation
of the cell cycle. TP53 has been described as a tumor suppressing gene for its
role in
preventing damaged or mutated DNA from replicating out of control, causing
cancer. P53 is
upregulated when the cell detects DNA damage, hypoxic conditions, or
irregularities in the
cell cycle. Under normal conditions P53, can pause the cell cycle and allow
for DNA repair
mechanisms to fix the damage and resume the cell cycle when fixed, or cause
the cell to go
immediately into apoptosis. There are many pathways for P53 to induce
mitochondrial
apoptosis, but one of the most direct routes is the induction of Bax, a
proapoptotic protein.
Bax binds directly to the mitochondria, causing the release of cytochrome c.
When P53 is
mutated or deleted, the anti-apoptotic protein Bcl-2 can sequester and further
block the
functionality of P53. This blockage of the actions of P53 allows for cancer
progression
regardless of DNA damage or cell cycle malfunction. Another route for cancer
progression is
the over expression of Bcl-2, leading to the sequestration of all P53 being
transcribed and
ultimately blocking the apoptotic cascade (Olivier et al., Cold Spring Harbor
Perspectives in
Biology, 2010, 2:1-17).
D. Jak-2 and CaM
[062] When MCF-7 cells are introduced to our unique treatment, the loss of
cell volume
is observed as early as 3 minutes into treatment. Cell volume decrease has
been observed in
the first stages of apoptosis (Orlov et al., Am J. Physiol. Cell Physiol.
2013, 305(4):C361-372),
and has been attributed to the enhanced activation of water permeable channels
(Remillard
et al., Am J Physiol. Lung Cell Mol Physiol. 2004, 286(1):L49-67). In
addition, when Fl+ is
uncaged during our novel treatment, NBA undergoes photochemical rearrangement
into 2-
nitrosobenzoic acid (Diaspro et al., Q Rev Biophys. 2005, 38(2):97-166). The
increase in Fl+
combined with the decrease in intracellular Fl+ buffering constituents would
also contribute to
decreases in cell volume (Fraser et al., Journal of Physiology, 2005,
563(3):745-64). This
intracellular water shedding causes many intracellular regulatory proteins to
respond and
activate mechanisms to counter the loss of water. One regulatory pathway that
is rapidly
17
Date Recue/Date Received 2021-07-09
activated by the water loss and subsequent decrease in cell volume is the
JAK/STAT pathway,
specifically Janus kinase II (Jak2).
[063] In response to cellular volume loss, Jak2 is activated through
phosphorylation. Jak-
2 activation can cause phosphorylation of calmodulin (CaM) (Benaim and
Villalobo, Eur J
Biochem. 2002, 269(15):3619-31), which binds to the intracellular C-terminus
of NHE1 to
constitutively activate it. This activation of NHE1 rapidly increases the
intracellular
concentration of Na, causing cell volume recovery though the recruitment of
water to the
cytosol.
[064] Calmodulin is a second messenger protein found in eukaryotic cells.
CaM is
involved in a variety of process, including the mechanical increase in
activation of NHE1
(Garnovskaya et al., J Blot Chem. 2003, 278(19):16908-15). The affinity of CaM
to the C-
terminus of NHE1 can be increased by either the phosphorylation or
calcification of CaM
(Garnovskaya et al., J Blot Chem. 2003, 278(19):1 6908-15; Koster et al., J
Blot Chem. 2011,
286(47):40954-61).
E. Microorganisms
[065] Bacterial resistance to antibiotics presents an ongoing challenge to
health care.
The majority of resistance is due to enzymatic adaptations that allow bacteria
to degrade
antibiotics before affecting their target (Benveniste et al., Annual Reviews,
1973, 42:471-
506). Once this enzymatic action is developed, it can be quickly transferred
from one
organism to another through transformation, defeating the effectiveness of
drugs. Other
methods of resistance include the bacteria's ability to hinder the uptake of
antibiotics, as
seen with tetracycline and sulfonamide, or a change in the target as with
Staphylococcus
aureus adapting ribosomes to confer resistance to erythromycin (Davies and
Davies,
Microbiology and Molecular Biology Reviews, 2010, 74(3):417-33). Simple,
single-target and
single-structure advances in antibiotics require similarly simple defensive
adaptations to be
performed in these organisms to exhibit a resistance.
[066] Bacteria are a diverse group of organisms that can live in a wide
range of
environments including extreme ranges of pH. Alkaliphiles may thrive in a
range of
extracellular pH from 7.5-10.6, and acidophiles may grow easily at pH 1.0-8.0;
however, the
internal pH of even these extremophiles is much closer to neutral, with an
internal pH of 7.5-
8.3 and 6.0-7.0 respectively (Slonczewski et al., Advances in Microbial
Physiology, 2009, 55:1-
18
Date Recue/Date Received 2021-07-09
79). To maintain these exceptional differences in pH, membrane lipids allow
bacterial species
to be varyingly impermeable to proton movements. Other methods of controlling
internal pH
include coupling down-gradient ion exchange with up-gradient proton exchange
such as the
Na/H+ antiporter of Escherichia coil; metabolic switching which makes neutral
or acidic
products; and various buffering systems (Slonczewski et al., Advances in
Microbial Physiology,
2009, 55:1-79). For example, Listeria monocytogenes, a bacterium responsible
for fatal
infections associated with processed foods (Shabala et al., International
Journal of Food
Microbiology, 2002, 75:89-97), encephalitis (Armstrong and Fung, Clin. Infect.
Dis. 1993,
16(5):689-702), pneumonia (Whitelock-Jones et al., South African Medical
Journal, 1989,
75(4):188-89), septicemia (Gray and Killinger, Bacteriol. Rev. 1966, 30:309-
82), meningitis
(Gray and Killinger, Bacteriol. Rev. 1966, 30:309-82), intrauterine, and
cervical infection in
pregnant women which may lead to spontaneous abortions, maintains its pHi
between 7.6 to
8.0, and "failure to maintain pHi homeostasis leads to loss of cell viability
and, therefore, may
be used as a sensitive indicator of the bacterial death at the cellular level"
(Shabala et al.,
International Journal of Food Microbiology, 2002, 75:89-97). Vacuolar-type H -
ATPase (V-
ATPase) in bacteria and fungi maintain important intracellular proton
gradients in organelles
such as endosomes and lysosomes that are important in driving calcium uptake
via the H+/Ca2+
antiporter. It is possible that significant intracellular and/or intra-
organelle acidosis via our
NBA treatment would severely disrupt not only acidity, but also important
processes necessary
for pathogenesis.
F. Enzymatic Activity
[067]
Metabolic disorders have plagued society for centuries. Recent advances in
science
have shown over-active enzymes are one of many causes of these diseases;
however, the
pathophysiology of metabolic disorders is poorly understood (Mairet-Coello et
al., Neuron,
2013, 78(1):94-108). Alzheimer's disease is one of the most prevalent forms of
dementia,
affecting over 5 million people in the United States, and is caused by
hyperactivity of cellular
enzymes (Mairet-Coello et al., Neuron, 2013, 78(1):94-108). In vivo studies
have shown that
hyperphosphorylation of the microtubule-associated protein Tau by the AMP-
activated kinase
(AMPK) leads to loss of neuronal spines used for excitatory synaptic
transmission (Mairet-
Coello et al., Neuron, 2013, 78(1):94-108).
Reducing the activity of this kinase,
pharmacologically or through genetic deletion, showed neuronal spines were not
subjected to
the synaptotoxic over-phosphorylation of Tau and had protective effect on
hippocampal
neurons (Mairet-Coello et al., Neuron, 2013, 78(1):94-108).
19
Date Recue/Date Received 2021-07-09
[068] Enzyme kinetics is a well-studied area of science and has developed
many
empirical models regarding enzyme functionality in various conditions. One of
these models is
used to describe and predict the activity of enzymes as a function of pH
(Tijskens et al.,
Biotechnol. Bioeng., 2001, 72(3):323-30). Most enzymes function within the
biological range, a
pH between 6 and 8, and have an optimal pH in which their functions are
greatest. However,
these functional interactions reduce when pH is altered. With significant
decreases in pH,,
enzymes begin to unfold due to protonation of functional groups and the
dissociation of
hydrogen bonds, which reduces their activity (Tijskens et al., Biotechnol
Bioeng, 2001,
72(3):323-30. Donten and Hamm (Chemical Physics, 2013, 422:124-30) applied pH
"jumps" by
administering femtosecond pulses of UV light to NBA to alter the rate and
magnitude of
folding of poly-L-glutamic acid. While Donten and Hamm (2013) were successful
in inducing
protein folding, they produced acidic solutions in the test cuvette as low as
pH 4.0, which
would cause intracellular damage and/or apoptosis. Using the uncaging
technique described
herein to reduce the pH, of cells will permit the alteration of the activity
of many proteins,
such as AMPK. This reduction in AMPK activity will reduce the synaptotoxic
effects of
hyperphosphorylated Tau and stave off the progression of Alzheimer's and other
metabolic
diseases due to hyperactive enzymes. The use of an NBA nanoparticle will allow
for very focal
and graded decreases in pH, in vivo.
[069] Histone deacetylases (HDAC) are a family of enzymes that remove
acetyl groups
from the amino acid lysine on a histone, thereby increasing the charge on the
histone tail.
This increase in charge on the histone tail promotes the high-affinity binding
between the
histones and DNA, leading to a more condensed DNA and prevention of
transcription.
Currently, 11 HDAC genes have been described (Shultz et al., Biochemistry,
2004, 43:11083-
91) and their activity has been linked to conditions such as Amyotrophic
Lateral Sclerosis (ALS)
(Miskiewicz et al., Cell Reports, 2014, 8:94-102), gastric, prostate, colon,
breast, cervical and
gastric cancer (Ropero and Estellar, Molecular Oncology, 2007, 19-25). In
contrast, histone
acetylation neutralizes histone charges and decreases histone's ability to
bind to DNA. This
decrease in histone binding promotes chromatin expansion and gene
transcription, such as the
activation of the tumor suppressor gene P53 (Phiel et al., Journal of
Biological Chemistry,
2001, 276(39):36734-41). Although the inhibition of HDACs by valproic acid and
trichostatin A
(TSA) have been effective in the treatment of epilepsy, the precise mechanisms
of action are
unknown (Phiel et al., Journal of Biological Chemistry, 2001, 276(39):36734-
41). Shultz et
al., (2004) reported the optimal pHi range for maximum catalytic efficiency of
HDACs 1, 2, 3,
Date Recue/Date Received 2021-07-09
6, 8, and 10. Each of these HDACs demonstrated a pHi dependence for the
maximal rate of
deacetylation reactions. In addition, the pH profiles for HDAC isozymes
maximum catalytic
efficiency "were bell-shaped, with maxima in the range of pH 7.6-8.3" (Shultz
et al., 2004).
Flash photolysis of NBA as described herein is capable of inducing discrete
reductions in pHi
that could be maintained for prolonged periods of time (FIGs. 15-16). Lowering
the pHi moves
the catalytic rate away from the maximum catalytic efficiency; significant
reductions in pHi
may serve as a novel inhibitor of HDACs and reductions in the pathogenesis of
a myriad of
diseases implicated with over activity of HDACs.
G. Stem cell pluripotency
[070] Stem cells are a highly studied area of biology; however, the process
to create
stem cells yields very few viable dedifferentiated cells. Current techniques
aim to reprogram
terminally differentiated cells by reverting them back, or dedifferentiating,
into an embryonic
state. Many cells in the body remain in a controlled dividing state and are
commonly located
in regions of the body that are in need of constant source of cell replacement
or cell division,
such as skin cells and bone marrow cells. Gao et al., (Cellular Physiology and
Biochemistry,
2014, 33(1):185-94) recently reported that human-derived mesenchymal umbilical
cord cells
(hUC-MSCs) differentiated into osteoblast cells in response to sustained
decreases in pH,
alone. Gao et al., (Cellular Physiology and Biochemistry, 2014, 33(1):185-94)
optically
measured pH, while inducing decreases in pH, by exposing the hUC-MSCs to the
ammonium
chloride prepulse technique, while simultaneously blocking NHE1 with
cariporide. The
methods described herein allow the induction of precise, incremental decreases
in only pH, for
longer, sustained intervals to achieve differentiation from certain stem cell
lines (Gao et al.,
Cellular Physiology and Biochemistry, 2014, 33(1):185-94).
H. Neuronal activities
[071] Protein structure and function can be altered by ionization by free
H. Several
studies support the important role of pH on proteins involved in central
nervous system
signaling. Acid-activated currents described in hippocampal neurons by Wemmie
et al.,
(Neuron, 2002, 34(3):463-77), were necessary for long-term potentiation, as
the loss of these
acid sensing ion channels (ASIC) in null mice resulted in impaired hippocampal
long-term
potentiation and defective eyeblink conditioning and spatial learning. The
ASIC la serves as a
proton receptor on dendritic spines of hippocampal neurons and influences
intracellular
calcium signaling (Zha et al., Proc. Natl. Acad. Sci USA, 2006, 103(44):16556-
61). Increases in
the concentration of extracellular free 1-1+ affects many ligand-gated ion
channels;
21
Date Recue/Date Received 2021-07-09
acetylcholine and NMDA receptors are inhibited by acidic extracellular pH and
inhibited by
alkaline extracellular pH (Del Castillo et al., 1962, J. Cell Comp Physiol.
59:35-44; Giffard et
al., Brain Res, 1990, 506:339-42; Palma et al., 1991, J Membr. Biol, 120:67-
73; Traynelis and
Cull-Candy, 1990, Nature, 345:347-50). GABA receptor activities are enhanced
by acidic
extracellular pH and inhibited by alkaline extracellular pH (Kaila and Ransom,
1994, pH and
brain function (New York: Wiley-Liss); Smart and Constanti, 1982, Proc R Soc
Lond B Biol
Sci,215:327-41; Takeuchi and Takeuchi, 1967, J Physiol., 1967, 191:575-90. The
ability of our
technique to cause focal acidosis in the extracellular space adjacent to the
above listed
ligand-gated ion channels, and virtually all ligand-gated ion channels that
are affected by
protonation, will provide a unique mechanism to alter neuronal input and
functional output of
neural circuits.
III. TARGETING AGENTS
[072] Targeting agents can be attached to the compounds or particles
described herein
to guide or target the conjugates to the target area in vivo. The targeted
delivery in vivo
enhances the cellular uptake of these compounds or particles to enhance
therapeutic
efficacies. In certain aspects, antibodies or cell penetrating peptides can be
for targeted
delivery to the tumor site.
[073] In one aspect of the invention, the targeting moiety is a single
chain antibody
(SCA) or single-chain antigen-binding antibody, monoclonal antibody, cell
adhesion peptides
such as RGD peptides and Selectin, cell penetrating peptides (CPPs) such as
TAT, Penetratin
and (Arg)9, receptor ligands, targeting carbohydrate molecules or lectins,
oligonucleotide,
oligonucleotide derivatives such as locked nucleic acid (LNA) and aptamers, or
the like.
[074] The targeting moieties can be labeled such as biotinylated compounds,
fluorescent
compounds, radiolabeled compounds. A suitable tag is prepared by linking any
suitable
moiety, e.g., an amino acid residue, to any art-standard emitting isotope,
radio-opaque label,
magnetic resonance label, or other non-radioactive isotopic labels suitable
for magnetic
resonance imaging, fluorescence-type labels, labels exhibiting visible colors
and/or capable of
fluorescing under ultraviolet, infrared or electrochemical stimulation, to
allow for imaging or
detection during administration, treatment, and so forth. Optionally, the
diagnostic tag is
incorporated into and/or linked to a conjugated photo-activated moiety,
allowing for
monitoring of the distribution of a therapeutic biologically active material
within an animal or
human patient.
22
Date Recue/Date Received 2021-07-09
[075] The terms "single chain antibody" (SCA), "single-chain antigen-
binding molecule or
antibody" or "single-chain Fv" (sFv) are used interchangeably. The single
chain antibody has
binding affinity for the antigen. Single chain antibody (SCA) or single-chain
Fvs can and have
been constructed in several ways. A description of the theory and production
of single-chain
antigen-binding proteins is found in commonly assigned U.S. patent application
Ser. No.
10/915,069 and U.S. Pat. No. 6,824,782.
IV. PERMANENT AND RELEASABLE LINKERS
[076] Linkers used in context of the current invention can include
bifunctional linkers.
The bifunctional can be permanent or releasable linkers. The bifunctional
linkers include
amino acids, amino acid derivatives, or chemical linkers. Amino acids can be
among naturally
occurring and non-naturally occurring amino acids. Derivatives and analogs of
the naturally
occurring amino acids, as well as various art-known non-naturally occurring
amino acids (D or
L), hydrophobic or non-hydrophobic, are also contemplated to be within the
scope of the
invention. A suitable non-limiting list of the non-naturally occurring amino
acids includes 2-
aminoadipic acid, 3-aminoadipic acid, beta-alanine, beta-aminopropionic acid,
2-aminobutyric
acid, 4-aminobutyric acid, piperidinic acid, 6-aminocaproic acid, 2-
aminoheptanoic acid, 2-
aminoisobutyric acid, 3-aminoisobutyric acid, 2-aminopimelic acid, 2,4-
aminobutyric acid,
desmosine, 2,2-diaminopimelic acid, 2,3-diaminopropionic acid, N-ethylglycine,
N-
ethylasparagine, 3-hydroxyproline, 4-hydroxyproline, isodesmosine, allo-
isoleucine, N-
methylglycine, sarcosine, N-methyl-isoleucine, 6-N-methyl-lysine, N-
methylvaline, norvaline,
norleucine, and ornithine. Some preferred amino acid residues are selected
from glycine,
alanine, methionine and sarcosine, and more preferably, glycine.
A. Releasable Linkers
[077] In certain aspects the compounds or particles described herein
contain a photo-
activated moiety attached to a releasable linker. The photo-activated moiety
can be released
in a controlled manner.
[078] Among the releasable linkers can be benzyl elimination-based linkers,
trialkyl lock-
based linkers (or trialkyl lock lactonization based), bicine-based linkers,
acid labile linkers,
lysosomally cleavable peptides and capthepsin B cleavable peptides. Among the
acid labile
linkers can be disulfide bond, hydrazone-containing linkers and thiopropionate-
containing
linkers.
23
Date Recue/Date Received 2021-07-09
[079]
Alternatively, the releasable linkers are intracellular labile linkers,
extracellular
linkers and acidic labile linkers. The acidic labile linkers, such as
hydrazone linkages, can be
hydrolyzed in the acidic lysosome environment. Some suitable releasable
linkers are
oligopeptides including such as Val-Cit, Ala-Leu-Ala-Leu, Gly-Phe-Leu-Gly and
Phe-Lys. One
preferred releasable linker is a peptidyl linker (Val-Cit) which can be
specifically degraded by
capthepsin B.
[080]
Various releasable linkers, benzyl elimination based or trialkyl lock based,
are
described, for example, in commonly assigned U.S. Pat. Nos. 6,180,095,
6,720,306, 5,965,119,
6,624,142 and 6,303,569. The bicine-based linkers are also described in
commonly assigned
U.S. Pat. Nos. 7,122,189 and 7,087,229 and U.S. patent application Ser. Nos.
10/557,522,
11/502,108, and 11/011,818.
B. Permanent Linkers
[081]
In certain aspects, the targeting moiety such as the SCA is attached to the
multifunctional linker through a permanent linker. The permanent linkers are
capable of
conjugating the targeting moiety and the multifunctional linker. One preferred
permanent
linker can be a molecule like a maleimidyl-containing molecule which can
provide a thio-ether
bond.
C. Pharmaceutical Formulations and Administration
[082]
In certain embodiments, compositions comprise 1, 2, 3 or more therapeutic
agents
with one or more of the following: a pharmaceutically acceptable diluent; a
carrier; a
solubilizer; an emulsifier; a preservative; and/or an adjuvant. Such
compositions may contain
an effective amount of at least one anti-cancer agent. Thus, the use of one or
more anti-
cancer agents that are provided herein in the preparation of a pharmaceutical
composition of
a medicament is also included. Such compositions can be used in the treatment
of a variety
of cancers or other diseases or conditions.
[083]
The agents described herein may be formulated into therapeutic compositions in
a
variety of dosage forms such as, but not limited to, liquid solutions or
suspensions, tablets,
pills, powders, suppositories, polymeric microcapsules or microvesicles,
liposomes, and
injectable or infusible solutions.
The preferred form depends upon the mode of
administration and the particular disease targeted. The compositions also
preferably include
pharmaceutically acceptable vehicles, carriers, or adjuvants, well known in
the art.
24
Date Recue/Date Received 2021-07-09
[084] Acceptable formulation components for pharmaceutical preparations are
nontoxic
to recipients at the dosages and concentrations employed. In addition to the
therapeutics
agents that are provided, compositions may contain components for modifying,
maintaining,
or preserving, for example, the pH, osmolarity, viscosity, clarity, color,
isotonicity, odor,
sterility, stability, rate of dissolution or release, adsorption, or
penetration of the
composition.
[085] Formulation components are present in concentrations that are
acceptable to the
site of administration. Buffers are advantageously used to maintain the
composition at
physiological pH or at a slightly lower pH, typically within a pH range of
from about 4.0 to
about 8.5, or alternatively, between about 5.0 to 8Ø Pharmaceutical
compositions can
comprise TRIS buffer of about pH 6.5-8.5, or acetate buffer of about pH 4.0-
5.5, which may
further include sorbitol or a suitable substitute therefor.
[086] The pharmaceutical composition to be used for in vivo administration
is typically
sterile. Sterilization may be accomplished by filtration through sterile
filtration membranes.
If the composition is lyophilized, sterilization may be conducted either prior
to or following
lyophilization and reconstitution. The composition for parenteral
administration may be
stored in lyophilized form or in a solution. In certain embodiments,
parenteral compositions
are placed into a container having a sterile access port, for example, an
intravenous solution
bag or vial having a stopper pierceable by a hypodermic injection needle, or a
sterile pre-
filled syringe ready to use for injection.
[087] The above compositions can be administered using conventional modes
of delivery
including, but not limited to, intravenous, intraperitoneal, oral,
intralymphatic, subcutaneous
administration, intraarterial, intramuscular, intrapleural, intrathecal, and
by perfusion
through a regional catheter. Local administration to a tumor is also
contemplated by the
present invention. When administering the compositions by injection, the
administration may
be by continuous infusion or by single or multiple boluses. For parenteral
administration, the
therapeutic agents may be administered in a pyrogen-free, parenterally
acceptable aqueous
solution comprising the desired anti-cancer agents in a pharmaceutically
acceptable vehicle.
A particularly suitable vehicle for parenteral injection is sterile distilled
water in which one or
more therapeutic agents are formulated as a sterile, isotonic solution,
properly preserved.
[088] Once the pharmaceutical composition of the invention has been
formulated, it may
be stored in sterile vials as a solution, suspension, gel, emulsion, solid, or
as a dehydrated or
Date Recue/Date Received 2021-07-09
lyophilized powder. Such formulations may be stored either in a ready-to-use
form or in a
form (e.g., lyophilized) that is reconstituted prior to administration.
[089] For the compounds of the present invention, alone or as part of a
pharmaceutical
composition, such doses are between about 0.001 mg/kg and 1 mg/kg body weight,
preferably
between about 1 and 100 pg/kg body weight, most preferably between 1 and 10
pg/kg body
weight. Therapeutic compositions or regimens may be administered 1, 2, 3, 4,
5, 6, 7, 8, 9,
10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 or more times, and they may be
administered every
1, 2, 3, 4, 5, 6, 7, 8,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22,
23, 24 hours, or 1,2,
3,4, 5, 6,7 days, or 1,2, 3,4, 5 weeks, or 1,2, 3, 4, 5, 6, 7, 8, 9, 10, 11,
12 months.
[090] Therapeutically effective doses will be easily determined by one of
skill in the art
and will depend on the severity and course of the disease, the patient's
health and response to
treatment, the patient's age, weight, height, sex, previous medical history
and the judgment
of the treating physician.
[091] In some methods of the invention, the target tissue or cell is a
cancer or cancer
cell. The cancer cell may be in a patient. The patient may have a solid tumor.
In such cases,
embodiments may further involve performing surgery on the patient, such as by
resecting all
or part of the tumor. Compositions may be administered to the patient before,
after, or at
the same time as surgery. In additional embodiments, patients may also be
administered
directly, endoscopically, intratracheally, intratumorally, intravenously,
intralesionally,
intramuscularly, intraperitoneally, intracranially, regionally,
percutaneously, topically,
intraarterially, intravesically, or subcutaneously.
[092] Methods of treating cancer or other diseases or conditions may
further include
administering to the patient chemotherapy or radiotherapy, which may be
administered more
than one time. Chemotherapy includes, but is not limited to, cisplatin (CDDP),
carboplatin,
procarbazine, mechlorethamine, cyclophosphamide, camptothecin, ifosfamide,
melphalan,
chlorambucil, bisulfan, nitrosurea, dactinomycin, daunorubicin, doxorubicin,
bleomycin,
plicomycin, mitomycin, etoposide (VP16), tamoxifen, taxotere, taxol,
transplatinum, 5-
fluorouracil, vincristin, vinblastin, methotrexate, gemcitabine, oxaliplatin,
irinotecan,
topotecan, or any analog or derivative variant thereof. Radiation therapy
includes, but is not
limited to, X-ray irradiation, UV-irradiation, y-irradiation, electron-beam
radiation, or
microwaves. In addition, a cell or patient may be administered a protease or
peptidase to
increase the production of infectious EEV form of the virus from cells.
Moreover, a cell or a
26
Date Recue/Date Received 2021-07-09
patient may be administered a microtubule stabilizing agent, including, but
not limited to,
taxane, as part of methods of the invention. It is specifically contemplated
that any of the
compounds or derivatives or analogs, can be used with these combination
therapies.
EXAMPLES
[093] The following examples as well as the figures are included to
demonstrate
preferred embodiments of the invention. It should be appreciated by those of
skill in the art
that the techniques disclosed in the examples or figures represent techniques
discovered by
the inventors to function well in the practice of the invention, and thus can
be considered to
constitute preferred modes for its practice. However, those of skill in the
art should, in light
of the present disclosure, appreciate that many changes can be made in the
specific
embodiments which are disclosed and still obtain a like or similar result
without departing
from the spirit and scope of the invention.
EXAMPLE 1
[094] Materials. Dulbecco's modified Eagle's medium (DMEM), RPM! 1640
(Roswell Park
Memorial Institute 1640), fetal bovine serum (FBS), horse serum, gentamicin,
trypsin-EDTA,
Carboxy-DCFDA (5-(and-6)-Carboxy-2',7'-Dichlorofluorescein Diacetate) and
Annexin V Alexa
Fluor 568 were bought from Life Technologies. NaCl, KCl, MgCl2, CaCl2, HEPES,
and glucose
were all purchased from Fisher Scientific. 2-Nitrobenzaldehyde (NBA),
Amiloride, Nigericin,
NGF-7S, Corning() 75cm2 Rectangular Canted Neck Cell Culture Flask with Vent
Cap were all
purchased from Sigma Aldrich. Rat pheochromocytoma (PC12) cells and human
breast
adenocarcinoma (MCF-7) cells were purchased from the American Type Culture
Collection.
Thirty-five mm culture dishes were purchased from Santa Cruz Biotechnology.
Nanoparticles
were synthesized by Dr. Brian Yust and Francisco Pedraza of the University of
Texas at San
Antonio. Cells and Cell Culture PC12 cells were cultured in RPM! 1640 (1X)
supplemented with
5% FBS, 10% Horse Serum and 500 pL of gentannicin at 37 C under an atmosphere
of 5% CO2
and 95% air. The cells were plated on 35 mm dishes at an appropriate range and
cultured until
80-90% confluent. MCF-7 cells were cultured in DMEM (1X) supplemented with 5%
FBS and 500
pL of gentannicin at 37 C under an atmosphere of 5% CO2 and 95% air. The cells
were plated in
a 75 cm2 Corning flask allowing for cell growth to appropriate density and
passed to 35 mm
dishes, then grown until 80-90% confluent for experimentation. When PC12 or
MCF-7 cells
were passed, 1mL of 0.25% Trypsin-EDTA (1X) was used to suspend cells for
passage. DCFDA
Stock Solution Carboxy-DCFDA (5-(and-6)-Carboxy-2',7'-Dichlorofluorescein
Diacetate; 1 mg)
27
Date Recue/Date Received 2021-07-09
was dissolved in 201 pL of dinnethyl sulfoxide (DMSO) to create a stock
solution of 9.4 mM. The
stock solution was stored in a dark biosafety cabinet to protect the light
sensitive compound.
[095] Calibrating and Measuring pH with DCFDA. For ratiometric pH,
measurements,
PC12 cells were loaded with the pH-sensitive dye Carboxy-DCFDA (10pM in rat
aCSF). Nigericin
was titrated to a pH of 2.0, 4.0, 5.0, 6.0, 7.0 or 8Ø The emitted
fluorescence of DCFDA was
recorded from cells at each of the titrated pH solutions. The ratiometric
emitted fluorescence
intensity was observed at an excitation of 495 nm/440 nm on 30 second
intervals until emitted
fluorescence (504/530nm) reach a steady state. Each ratio from individual
cells (n = 421) was
recorded for each pH. A curve of best fit was created (R2 = 93.02) and used
for the conversion
of fluorescence to pH,.
[096] NBA Loading. NBA was (3.0224 grams) dissolved in 500 mL of methanol
to make a
stock solution of 40 mM. For each experiment, 75 pL of stock was combined with
3 mL of rat
aCSF for cell bath application. The final dilution of 1 mM NBA-rat aCSF is
well below the
allowable 5 mM limit of cytotoxicity (Sweitach et al., Biophys J, 2007,
92(2):641-53). The NBA
loading procedure for PC12 cells was the same procedure for the MCF-7 cells.
[097] Apoptosis Indicator. The apoptosis indicator Annexin V Alexa Fluor
568 was
stored in a solution containing 25 mM HEPES, 140 mM NaCl, 1 mM EDTA, pH 7.4,
plus 0.1%
bovine serum albumin (BSA). A 10 pL of stock Annexin V Alexa Fluor 568 was
diluted in 2 mL of
binding buffer. The binding buffer consisted of: 10 mM HEPES, 140 mM NaCl, and
2.5 mM
CaCl2, at a pH of 7.4.
[098] Amiloride
Procedure. Amiloride (N-Amidino-3,5-diamino-6-
chloropyrazinecarboxamide hydrochloride hydrate) (266.09 mg) was added to 20
mL of DMSO
to make a final stock solution of 50 mM. This stock was further diluted to 1
mM prior to bath
application to cultured cells.
[099] Rat aCSF stock solution. PC12 and MCF-7 cells were perfused in 2 mL
of rat aCSF
solution (150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM HEPES, 5 mM glucose, pH 7.4)
during
experimental procedure.
[0100] Optic Recording of pH; PC12.
DCFDA stock solution was diluted to a
concentration of 10 pM in a 3 mL rat aCSF solution. PC12 cells were flashed
every 30 second at
495/440nm for a duration of 900/700 ms, respectively, at 40x magnification and
2 seconds at
28
Date Recue/Date Received 2021-07-09
each wavelength under the 10x magnification. Cells were washed three times
with rat aCSF
after 10 minutes and prior to recording fluorescence to monitor steady state.
[0101] Optical Recording of pH i MCF-7. DCFDA stock solution was diluted to
a
concentration of 10 pM in a 3 mL rat aCSF solution. MCF-7 cells were flashed
every 30 seconds
at 495/440nm for a duration of 900/700 ms, respectively, at 40x magnification
and 2 seconds
at each wavelength under the 10x magnification. Cells were washed three times
with rat
aCSF after a steady state of fluorescence was observed, indicating that the
fluorescence
reached its max fluorescence since cancer cells are more alkaline. Optical
recording of pH,
was monitored for a minimum of 10 minutes to represent a steady state.
[0102] Optical Recording of pHi MDA-MB-231. DCFDA stock solution was
diluted to a
concentration of 10 pM in a 3 mL rat aCSF solution. MBA-MD-231 cells were
flashed every 30
seconds at 495/440nm for a duration of 900/700 ms, respectively, at 40x
magnification and 2
seconds at each wavelength under the 10x magnification. Cells were washed
three times with
rat aCSF after a steady state of fluorescence was observed, indicating that
the fluorescence
reached its max fluorescence since cancer cells are more alkaline. Optical
recording of pH,
was monitored for a minimum of 10 minutes to represent a steady state.
[0103] NBA Loading for Focal Acidification of PC12. Seventy-five pL of NBA
stock
solution was mixed in 3 mL of rat aCSF and bath applied to PC12 cells for 10
minutes. After
bath application, cells were washed a minimum of three times with rat aCSF.
PC12 cells were
exposed to UV light using various flash paradigms. After each exposure, pH was
monitored for
2 minutes to quantify pH, acidification.
[0104] NBA Load for Focal Acidification of MCF-7. Seventy-five pL of NBA
stock
solution was mixed in 3 mL of rat aCSF and bath applied to MCF-7 cells for 10
minutes. After
bath application, cells were washed a minimum of three times with rat aCSF.
MCF-7 cells were
exposed to 60-30-60 UV light paradigm. After each exposure, pH was monitored
for 2 minutes
to quantify pH, acidification.
[0105] NBA Load for Focal Acidification of MDA-MB231. Seventy-five pL of
NBA stock
solution was mixed in 3 mL of rat aCSF and bath applied to MDA-MB-231 cells
for 10 minutes.
After bath application, cells were washed a minimum of three times with rat
aCSF. MDA-MB-
231 cells were exposed to 60-30-60 UV light paradigm. After each exposure, pH
was monitored
for 2 minutes to quantify pH, acidification.
29
Date Recue/Date Received 2021-07-09
[0106] Cell Death Quantification PC12. One hour after the UV flash paradigm
to induce
intracellular acidosis, the cells were lightly washed and the media was
replaced with the
phosphatidylserine indicator Annexin V Alexa Fluor 568. This solution was made
using 10 pL of
the stock solution and diluted in 2 ml of rat aCSF. Fifteen minutes was
allowed for membrane
binding, then fluorescence was monitored for the next hour or until all cells
were expressing
apoptosis.
[0107] Cell Death Quantification MCF-7. After UV flash paradigm, the
presences of
blebs were observed and recordings of the pH in the intrablebular inflationary
space (pHb)
were acquired using the DCFDA fluorescence as they expanded. The blebs seen
with DCFDA
fluorescence showed continuous expansion and never reduced in size. We
quantified blebs as
cellular death based on previously published findings which described blebbing
as an
irreversible death process (Andrade et al., Biology of the Cell, 2010,
102(1):25-35).
[0108] Cell Death Quantification MDA-MB-231. One hour after the UV flash
paradigm to
induce intracellular acidosis, the cells were lightly washed and the media was
replaced with
the phosphatidylserine indicator Annexin V Alexa Fluor 568. This solution was
made using 10
pL of the stock solution and diluted in 2 ml of rat aCSF. Fifteen minutes was
allowed for
membrane binding, then fluorescence was monitored for the next hour or until
all cells were
expressing apoptosis. As with MCF-7 cells, we quantified blebs as cellular
death based on
previously published findings which described blebbing as an irreversible
death process
(Andrade et al., Biology of the Cell, 2010, 102(1):25-35).
[0109] Amiloride Application. The NHE1 blocker, Amiloride, was bath applied
at a pH of
7.4 to MCF-7 cells at concentrations ranging from 1 pM to 1 nnM. After the
cells were loaded
with DCFDA, NBA, respectively washed, and exposed to the UV paradigm, pHb was
recorded.
[0110] Nanoparticle Application. Nanoparticles of various rare earth
doped
upconverting cores coated with various hydrophilic biocompatible polymer, such
as
polyethylene glycol (PEG), cell membrane mediated polymers were synthesized
and
introduced to MCF-7 cancer cells. The two particles tested consisted of
KYb2F7or NaYF4. These
cancer cells were incubated in growth media with nanoparticles ranging from 10
minutes to
one week. When preparing for experimentation, the cancer cells were washed
with rat aCSF
five times and placed under the microscope, then loaded with DCFDA under the
same
procedure as previously mentioned. A continuous wave of 980 nm diode laser was
aligned to
Date Recue/Date Received 2021-07-09
the region of cells recording pH, and set to 400 or 700 mW. The laser shutter
was manually
controlled for exposure of 10 or 20 minutes.
[0111] Control Experiments. Controls in each experiment were performed by
moving 10
mm or more away from the UV or 980nm exposed region. Recordings of pH, were
performed to
detect if NBA or the nanoparticle affected the cell or inadvertently uncaged
hydrogens in the
periphery of the plate. Detection of apoptosis and/or blebs was performed
using Annexin V
Alexa Fluor 568 and DCFDA optical recording. Control experiments were
performed in the
following conditions: UV alone, time alone, and NBA alone. Each one of the
control
experiments was performed for a minimum of 3 hours.
RESULTS
[0112] DCFDA Calibration. The emitted fluorescence of cells exposed to pH
specific-
nigericin solution was used for the construction of the DCFDA calibration
curve. Ratiometric
fluorescence of individual cells was recorded at pH of 2.0, 4.0, 5.0, 6.0,
7.0, or 8Ø Each pH
unit represents a separate experiment at which several cells were optically
recorded and
monitored for a steady state when exposed to the pH specific-nigericin
solution (FIG. 3a).
Data were graphed and revealed a line of best fit with an R-squared value (y =
-0.1356x2 +
2.62x - 3.6204) (R2 = 0.93), permitting the future conversion of emitted
fluorescence to pH,.
After the calibration was completed, the formula applied to each experiment
showed the
average starting pH, was 7.7.
[0113] Induced Acidosis in PC12 Cells. We assessed the ability of
photoactivated NBA to
focally induce significant intracellular acidosis by optically recording pH,
from individual PC12
cells in vitro (n = 423) prior to and after proton release from NBA. After
loading with DCFDA,
emitted fluorescence ratios were recorded before exposing the cells to varying
flash-time
paradigms of 350nm wavelength UV light. Mean pH, of the PC12 cells after
application of NBA
and prior to photolysis (7.55 0.025) was significantly different from mean
pH, after
photolysis of NBA with UV exposure (6.37 0.03; P < 0.001). Changes in pH, (A
pH,) with
treatment were normalized as a change from pH, prior to treatment (A pH, =
1.18).
[0114] Quantification of PC12 Cell Death. After focal acidification was
achieved in
PC12 cells, Annexin V Alexa Fluor 568 was bath applied to mark for
phosphatidylserine
membrane inversion resulting from and indicative of apoptosis. The cells were
monitored for
at least an hour after flash photolysis in the presence of NBA for
fluorescence which would
31
Date Recue/Date Received 2021-07-09
indicate apoptosis. We observed a range of apoptosis spanning from 76 to 100%
(n = 362; R2 =
0.98) with an average apoptosis of 84.0 1.33%. Linear regression analysis of
these data over
time indicated significant apoptosis was achieved within 2 hours in response
to acidosis from
the flash photolysis of NBA (P < 0.01). The mean percent apoptosis with NBA
treatment (84.0
1.33%) was significantly greater from control experiments in which PC12 cells
were exposed to
UV light in the absence of NBA (n = 76; R2 = -0.54; P < 0.001). The percentage
of cells
exhibiting apoptosis in response to UV exposure alone ranged from 2.3 to
10.3%, with a mean
percentage of 6.4 1.0%. The percentage of apoptosis with NBA and UV
treatment was also
significantly greater (P < 0.01) than control experiments in which cells were
exposed to
neither NBA nor UV light (i.e., the effects of time alone; n = 71; R2 = 0.92).
The percentage of
cells exhibiting apoptosis in response to time alone ranged from 3.1 to 4.5%,
with a mean
percentage of 3.8 0.4%. The percentage of cells exhibiting apoptosis in
response to NBA
alone was 2.1% (n = 47). Linear regression analyses indicated that the
percentage of apoptosis
in response to UV exposure alone, time alone, or NBA alone were significantly
less than the
percentage of apoptosis in response to NBA and UV treatment (P < 0.01).
Control experiments
were not significantly different from each other (P < 0.01).
[0115] Induced Acidosis in MCF-7 Cells. After assessing the ability of NBA
to focally
decrease the pH, in PC12 cells, the same experimental procedure was performed
on an MCF-7
breast cancer cells. Emitted fluorescence ratios were taken before and after
NBA flash
photolysis to monitor pH,. Mean pH, of MCF-7 cells after the cells after
loading with NBA and
before the 60-30-60 UV flash paradigm (7.38 0.13; n = 76) was significantly
different from
the mean pH, of cells after the flash paradigm in the presence of NBA (6.22
0.15). Changes
in pH, with treatment were normalized as a change from pH, prior to treatment.
The post
treatment mean L pH, (1.16 pH units) was significantly different from the mean
pH, prior to
treatment (P < 0.001).
[0116] Quantification of MCF-7 Cell Death. After focal acidification was
achieved in
MCF-7 cells, Annexin V Alexa Fluor 568 was bath applied to mark for
phosphatidylserine
membrane inversion resulting from and indicative of apoptosis. The cells were
monitored for
at least 1 hour after flash photolysis in the presence of NBA for fluorescence
which would
indicate apoptosis. MCF-7 cells were also observed to exhibit cellular
blebbing upon focal
intracellular acidification and did not exhibit fluorescence in the presence
of Annexin V Alexa
Fluor 568 until the bleb completely separated from the cell or after several
hours (1-6 hrs)
post treatment. As previously discussed, research indicated that blebbing was
indicative of
32
Date Recue/Date Received 2021-07-09
apoptosis. The number of blebs were counted upon formation as a function of
time as another
indication of cell death. While previous research indicates that upon
activation of NCX1 there
is often a reduction in the size of the bleb (Yi et al., 2012), we did not
observe any reductions
in the occurrence or size of blebs over time in response to our NBA-UV
treatment. In response
to flash photolysis of NBA, we observed a range of apoptosis, as indicated by
cellular blebbing
and/or apoptosis via fluorescence of Annexin V, spanning from 94.9 to 100.0%
(n = 262; R2 =
0.92) with an average apoptosis over time of 98.3 0.3%. Linear regression
analysis revealed a
significant decrease (P < 0.01) the percentage of apoptosis for MCF-7 cells
exposed to only UV
light (7.1%) over 2 hours. The percentage of apoptosis observed in MCF-7 cells
exposed only to
NBA (2.3%, n = 236, R2 = 0.87) was significantly less (P < 0.0001) than the
percentage of
apoptosis in MCF-7 cell treated with NBA and UV light. Our control experiment
to evaluate the
percentage of apoptosis in response to time alone (n = 20) revealed no
apoptosis over 2 hours.
As our results produced no cell death what so ever, linear regression was not
performed.
[0117] Induced Acidosis in MDA-MB-231 Cells. After assessing the ability of
NBA to
focally decrease the pH, in both PC12 and MCF-7 cells, the same experimental
procedure was
performed on the highly aggressive, triple negative MDA-MB-231 breast cancer
cells. Emitted
fluorescence ratios were taken before and after NBA flash photolysis to
monitor pH,. Mean pH,
of MDA-MB-231 cells after the cells after loading with NBA and before the 60-
30-60 UV flash
paradigm (6.47 0.06; n = 38) was significantly different (P < 0.001) from
the mean pH, of
cells after the flash paradigm in the presence of NBA (2.78 0.23). The post
treatment mean
pH, (3.68 0.18 pH units) was significantly different from the mean pH, prior
to treatment
(P <0.001).
[0118] Quantification of MDA-MB-231 Cell Death. After focal acidification
was achieved
in MDA-MB-231 cells, Annexin V Alexa Fluor 568 was bath applied to mark for
phosphatidylserine membrane inversion resulting from and indicative of
apoptosis. The cells
were monitored for at least 1 hour after flash photolysis in the presence of
NBA for
fluorescence which would indicate apoptosis. MDA-MB-231 cells were also
observed to exhibit
cellular blebbing upon focal intracellular acidification and did not exhibit
fluorescence in the
presence of Annexin V Alexa Fluor 568 until the bleb completely separated from
the cell or
after several hours (1-6 hrs.) post treatment. As previously discussed,
research indicated that
blebbing was indicative of apoptosis. Similar to our MCF-7 results, MBA-MB-231
cells
demonstrated a range of apoptosis, as indicated by cellular blebbing and/or
apoptosis via
33
Date Recue/Date Received 2021-07-09
fluorescence of Annexin V in response to flash photolysis of NBA (FIG. 18). We
observed 55.3%
MDA-MB-231 cell death in less than three hours following NBA flash photolysis.
EXAMPLE 2
[0119] Nanoparticle Induced Acidosis in MCF-7 Cell Death. Several
upconversion
nanoparticles were synthesized and loaded with NBA to be delivered to MCF-7
cancer cells.
Key properties of the particles illustrated in FIG. 14A and 14B are
illustrated in Tables 2 and 3.
[0120] Table 2: Key properties of the particle illustrated in FIG. 14A.
Si-NBA-PEG-Core NaYF4 KYb2F7
NBA Concentration 1, 4, 20 mM 1, 4, 20 mM
NBA Load Time 3 hours 3 hours
Estimated Size (diameter) 50-100 nm 10 nm
980 nm Exposure Time 10 min 10 min
Acidify Water (pH units) -4 -2
Acidity Intracellular Space - -1.5
Cytotoxicity - None
Notes: - Mostly Clumpy
[0121] Table 3: Key properties of the particle illustrated in FIG. 14B.
PEGylated-Particle NaYF4 KYb2F7
NBA Concentration 40 mM 40 mM
NBA Load Time 12 hours
Estimated Size (diameter) 50-100 nm 10 nm
980 nm Exposure Time 10 min 10 min
Acidify Water (pH units) 3.5 3.5
Acidity Intracellular Space - -1
Cytotoxicity - Yes
Notes: - Clumpy
[0122] The nanoparticle chosen consisted of KYb2F7 core coated with PEG for
NBA load
(FIG. 14A). After the MCF-7 cells were loaded with DCFDA, the nanoparticles
were
ultrasonicated and bath applied to the cells (n = 618) for 10 minutes. A 980nm
diode laser was
34
Date Recue/Date Received 2021-07-09
then aligned to the cells within the field of view of the microscope.
Recordings of pH, were
collected for 30 minutes to monitor if the nanoparticle affected the pH or was
cytotoxic; the
results yielded no cytotoxicity within the timeframe. The cells were then
exposed to the
980nm wavelength for 10 minutes at 400 mW for fluorescence resonance energy
transfer to
NBA, well below the tolerable intensity for human cells (Idris et al., Nature
Medicine, 2012,
18(10):1580-85). MCF-7 cells were then monitored for changes in pH, and for
the formation of
cellular blebbing indicating cell death over the next 172 minutes. After
recording and
application of Annexin V Alexa Fluor 568, cell death was recorded at 95.1%.
Quantification of
the percentage of MCF-7 breast cancer cell death in response to photo-
upconversion of the
KYb2F7 nanoparticle is illustrated in FIG. 17.
[0123] A second nanoparticle tested used the KYb2F7 core with a mesoporous
silica
coating. These nanoparticles were added to MCF-7 cells and monitored for
cytotoxicity over a
four-day period. The cells proliferated very well and showed minimal cell
death. MCF-7 cells
that were exposed to the NBA nanoparticle were then loaded with DCFDA and
monitored for
minutes. The cells were exposed to the 980nm laser for 10 minutes at a 700 mW
setting.
We then recorded pH, for a 10-minute period without the laser exposure. We
then exposed the
MCF-7 cells to a second 10-minute 980nm exposure. Cells were then monitored
for 3 hours
following the last laser excitation, recording the DCFDA fluorescence every 30
seconds. Photo
upconversion of NBA using this nanoparticle resulted in 98.7% cell death (n =
326) by loss in
membrane integrity (Annexin V fluorescence) or blebbing. The control region in
this
experiment was approximately 10 mm from the laser exposed cells. MCF-7 cells
in this control
region were exposed to the nanoparticle for 4 days but were not exposed to the
980nm laser.
Annexin V expression or cellular blebbing indicated a 5.78% cell death (n =
190) in these
control cells.
EXAMPLE 3
[0124] In this embodiment of the invention, 2-nitrobenzaldehyde is used to
focally induce
acidosis in a system. It is necessary to prove the ability of NBA to release
protons for
acidification of a system, for example the intracellular space. Proof of
concept experiments
were performed which showed that:
[0125] We can prove, with the use of ratiometric pH-sensitive fluorescent
dyes, that NBA
can release a proton and induce acidosis inside of a cell. In our experiments
PC12 cells were
loaded with the ratiometric fluorescent dye DCFDA. DCFDA was utilized for
measuring changes
Date Recue/Date Received 2021-07-09
in the intracellular pH (pH,) of a cell over a broad pH range from 2.0 to 7.0
(FIG. 3A, 3B). In
this embodiment of the invention, NBA (1 mM) was bath applied at room
temperature to PC12
cells and allowed to passively diffuse across the cell membrane. Emitted
fluorescence ratios
were taken prior to photoactivation of NBA with a flash photolysis paradigm
(FIG. 15). Emitted
fluorescence ratios were also recorded after NBA was exposed to a wavelength
of 350nm UV
light. Using fluorescence microscopy, we can optically record and quantify
changes in pH,.
[0126] NBA was bath applied to PC12 cells for 10 minutes in order to allow
for adequate
diffusion across the cell membrane, as previously described. The cells were
then exposed to
different time paradigms of 350 nm UV light. Flash photolysis of NBA was
observed to induce a
mean acidosis of 1.18 pH units (n = 421; FIG. 8). The magnitude of
intracellular acidification
can be correlated to the amount of time NBA is exposed to UV light, giving us
the ability to
control the extent of the induced acidosis (Kohse, J. Am. Chem. Soc. 2013,
135(25):9407-11)
(FIG 15). A single preparation of NBA can induce acidosis multiple times after
UV flash
photolysis. Experiments were performed in which PC12 cells were observed to
undergo
changes in pH, from a physiological range of 7.5 to 3.5 (FIG. 15). The
magnitude of
intracellular acidification was correlated to the flash photolysis paradigm.
We have the ability
to induce intracellular acidosis far outside the biological range of cellular
activity and optimal
intracellular protein function. NBA proton release with UV light is a
reproducible and
consistent method of inducing acidosis in a system.
EXAMPLE 4
[0127] In this embodiment of the invention, we prove that intracellular NBA
exposed to
flash photolysis results in focal cellular damage and death. NBA was allowed
to passively
diffuse into PC12 cells and exposed to a flash-photolysis paradigm which
resulted in the focal
release of protons within the cells. DCFDA was used to optically record
ratiometric
fluorescence in response to induced NBA proton release within the cells. Mean
pH, from before
flash photolysis to after flash photolysis was significantly reduced by 1.18
pH units (FIG. 8).
Cell death resulting from the significant decreases in pH, was quantified by
fluorescent
labeling with Annexin V Alexa Fluor 568 as well as Ethidium Homodimer III, two
dyes which
discernibly fluoresce in response to apoptosis and necrosis, respectively. The
amount of
fluorescence due to apoptosis was recorded as a function of time after an
acidosis challenge
(HG. 5). Similarly, fluorescence due to necrosis was recorded as a function of
time after an
acidosis challenge (FIG. 6). The average percent cell death due to apoptosis
after flash
photolysis of NBA was significantly different from the average percent cell
death due to
36
Date Recue/Date Received 2021-07-09
apoptosis in control experiments in which cells were exposed to DCFDA alone,
NBA alone, or
time alone (FIG. 7).
[0128] Resulting directly from a focal decrease in pH, to the point outside
of optimum
biological range of functionality, PC12 cells exhibited cellular damage and
death within 1 to 4
hours of flash photolysis (FIG. 7). This cellular death and damage may be the
result of pH-
induced damage to cellular organelles and/or via the pH-induced alteration of
enzyme
function or protein interactions. PC12 cells in control regions not exposed to
flash photolysis
did not exhibit a significant amount of cellular death (FIG. 7). These data
suggest that NBA
proton release resulting from UV light exposure is a rapid and focal method of
terminating the
functional mechanisms of a cell.
EXAMPLE 5
[0129] Our inventive treatment with NBA causes a drop in intracellular pH
(pH,) (FIG. 12)
which causes focal and significant cell death in MCF-7 breast cancer cells.
This change in pH,
is determined by the amount of time the cells are exposed to UV light once
they are treated
with NBA, and can produce mild acidosis within biological pH range. Acidosis
can also be
induced so that the intracellular pH is no longer within a normal biological
range (Ravindran,
Journal of Health Care for the Poor and Underserved, 2011. 22(4):174-186).
Control
experiments were performed in order to test the toxicity and diffusion of NBA
in MCF-7 cells.
The pH, of the cells was monitored with the fluorescent dye DCFDA, with
control experiments
testing the toxicity of DCFDA alone, NBA alone, UV flash alone. A time control
experiment was
also conducted in order to determine if the death seen in other controls was
due to the time
the cultured cells were not in the incubator. After exposing the cells to the
experimental
conditions and taking recordings for approximately 2 hours, Annexin V Alexa
Fluor 568 and
optical bleb monitoring were used in order to observe apoptosis. In cells
exposed to only one
of the conditions, observed apoptosis was minimal compared to the percentages
of cells dead
or undergoing apoptosis in the cells treated with both NBA and UV (FIG. 13).
[0130] Our novel technique will cause rapid, focal decreases in pH, to
induce cell death in
cells that excel at evading normal apoptotic mechanisms, such as cancer.
Experiments were
conducted in order to show that focal intracellular acidification would be
sufficient to induce
cell death, a feat that has to date evaded cancer researchers. In MCF-7 cells
that were
exposed to treatment, their cellular membranes did not go through normal
apoptosis
mechanisms that would have resulted in membrane inversion, allowing apoptosis
to be
37
Date Recue/Date Received 2021-07-09
monitored with Annexin V. In the case of the cancer cells, the membranes
swelled in various
areas and showed cellular blebbing, a phenomenon that was not seen in the PC12
experiments. The literature surrounding the mechanism of bleb formation is
limited, however
it has been reported that cellular blebbing was a sign of irreversible
processes that resulted in
cell death (Andrade et al., Biology of the Cell, 2010, 102(1):25-35).
Therefore. in many of
the MCF-7 experiments, the appearance of blebs was used in conjunction with
Annexin V in
order to determine the percentage of cell death.
[0131] Our results showed a focal, controlled, and extremely quick death
process (FIG.
13). Once the cells incubated in NBA were exposed to UV, significant drops in
pH, outside of
physiological range, cellular blebbing, and morphological changes were seen in
minutes, with
percentages of death above 90% within 2 hours. Cells that were exposed to NBA
but not the
UV flash (control regions) had pH, values in the normal physiological range,
while neighboring
cells exposed to UV underwent cellular blebbing and showed apoptosis when
imaged with
Annexin V. This cellular death and damage may be the result of pH-induced
damage to
cellular organelles and/or via the pH-induced alteration of enzyme function or
protein
interactions. Control regions were evaluated in the same 35 mm dish as the
treated cells,
approximately 10 mm away from the area of treatment. Despite the proximity to
the treated
area, control regions did not show the levels of apoptosis seen in the treated
areas (FIG. 13),
exhibiting the focal nature of these treatments.
EXAMPLE 6
[0132] Our inventive treatment with NBA causes a drop in intracellular pH
(pH,) which
causes focal and significant cell death in the highly aggressive triple
negative MDA-MB-231
breast cancer cells. This change in pH, is determined by the amount of time
the cells are
exposed to UV light once they are treated with NBA, and can produce mild
acidosis within
biological pH range, or severe acidosis which is outside the physiological
range. We
demonstrate the ability to induce apoptosis and cell death in 55.3% the
aggressive triple
negative breast cancer exposed to an NBA and flash photolysis paradigm which
caused a
significant decrease in pHi of 3.68 0.18 pH units (P < 0.001).
EXAMPLE 7
[0133] Our described inventive treatment of cells invokes a novel, positive
feedback
system which facilitates cell death, and that this positive feedback pathway
could serve as an
38
Date Recue/Date Received 2021-07-09
advantageous mechanism induce cell death in any cell demonstrating a
resistance to common
therapies, such as multidrug resistant cancers and antibiotic resistant
bacteria.
[0134]
The described positive feedback pathway in MCF-7 cells, which occurs following
significant increases in intracellular H+ from NBA in response to a UV flash.
FIG. 2.1 illustrates
the exposure of intracellular NBA to 350 nm wavelength UV flash paradigm,
causing a sudden
increase in proton concentration in the intracellular space and concomitant
osmotic water loss
or change in cell volume. The increase in intracellular H+ activates NHE1 to
extrude protons in
exchange for sodium ions (FIG. 2.2a). Sodium ions act as osmolytes, drawing
water to the
area of high sodium concentration inside the cell. The movement of water to
the area of high
Na, or the extrusion of water leads to a deformation of the cell wall and/or
cell shrinkage.
The deformation of the cell wall results in phosphorylation and activation of
the tyrosine
kinase Jak-2 (Garnovskaya et al., J Blot Chem. 2003, 278(19):16908-15) (FIG.
2.2b). The
continued activation of NHE1 in response to the intracellular H creates
increases in
intracellular osmotic pressure, resulting in an outward protrusion on the
cellular membrane,
or a bleb (FIG. 2.3). The activity of NHE1 attempts to compensate for the
focal acidosis inside
the cell, causing intracellular Na + levels to rise, further promoting the
hydrostatic pressure
within, and subsequent size of the bleb. In response to the local increase in
intracellular Na+
concentration, the sodium calcium exchanger 1 (NCX1) reverses exchange
activity (Yi et al., J.
Biol. Chem. 2012, 287(13):10316-24), leading to the efflux of Na + and influx
of Ca' (FIG.
2.4a). Complexation of phosphorylated Jak-2 and calmodulin (CaM; FIG. 2.4b)
increases the
Jak-2-dependent tyrosine phosphorylation of CaM. Phosphorylated CaM binds to
the C..
terminus of NHE1, constitutively activating NHE1 (Garnovskaya et al., J Biol
Chem. 2003,
278(19):16908-15). The increase in intracellular Ca' via the reversal of NCX1
facilitates the
binding of Ca" to calmodulin, independent of the phosphorylation state of CaM.
While both
phosphorylated CaM (Garnovskaya et al., J Biol Chem. 2003, 278(19):16908-15)
and calcified
CaM (Koster et al., J Blot Chem. 2011, 286(47):40954-61) increase the activity
of NHE1, the
combination of phosphorylation and calcification of CaM (FIG. 2.5) imparts the
highest affinity
of CaM to NHE1 (Koster et at., J Blot Chem. 2011, 286(47):40954-61; (FIG.
2.6a), and thereby
promotes the greatest activation of NHE1 (Koster et al., J Blot Chem. 2011,
286(47):40954-
61). In spite of Na + extrusion via NCX1, maintained activation of NHE1 is
facilitated by the
phosphorylated-calcified CaM, leading to further Ca" entry (FIG. 2.6b and
2.6c). We propose
that the supraphysiological increases in intracellular H induced by flash
photolysis of NBA
leads to cell shrinkage followed by subsequent activation of NHE1, Jak-2, and
NCX1; creating a
39
Date Recue/Date Received 2021-07-09
novel pathway of positive feedback, this ultimately leads to increased
intracellular osmotic
pressure, cellular blebs, and potential rupture of the cell membrane. We
propose this
positive feedback pathway will also be effective in killing multidrug
resistant cancer,
especially given the increased expression of NHE1 in multidrug resistant
cancer (Hoffman and
Lambert, Philosophical Transactions of the Royal Society London Biological
Sciences, 2014,
369(1638):20130109).
EXAMPLE 8
[0135] Our described inventive treatment will allow us to produce modest,
incremental,
and precise decreases in pH, alone and correlate the magnitude of change in
pH, to the
percent yield of reprogramming mouse splenic CD45+ cells to a pluripotent
state.
EXAMPLE 9
[0136] Our inventive technique is effective in significantly decreasing the
growth of
cancerous tumors in vivo. We injected the aggressive triple negative breast
cancer MDA-MB-
231-GFP (2 x 106 cells) into the mammary fat pad of 5-week old female nude
mice. Tumors
appeared after approximately one week following injection. Treatment was
initiated once
the tumors reached and maintained a length of 5 mm for two consecutive days.
The mice
received a one-time treatment of either a control injection if 0.1 ml of aCSF
to the tumor or
0.1 ml of NBA (1mM) followed by photoactivation. The NBA/photoactivation
treatment was
conducted as follows: one hour after NBA injection, the animal was
anesthetized (1.5 - 4%
isoflurane) prior to the insertion of a 200 pm fiber optic cannula into the
tumor mass. The
interior of the tumor was illuminated with 405 nm light (80-85 mW) via a
ceramic cannula
using the same illumination paradigm which was effective in vitro,
specifically 60 seconds of
illumination, 60 seconds of no illumination, 30 seconds of illumination, 60
seconds of no
illumination, and 60 seconds of illumination. This paradigm is referred to as
a "60-30-60"
illumination paradigm.
[0137] Tumors were measured daily in order to calculate changes in tumor
volume using
the formula:
[0138] Tumor Volume (TV) = Length x width' / 2
[0139] The percent change in tumor volume was calculated by normalizing the
percent
change to the tumor volume recorded on the day of treatment. Digital Vernier
calipers were
used to perform daily tumor measurements from control and NBA photo-treated
mice. We
Date Recue/Date Received 2021-07-09
observed significant reductions in the growth of tumors in MBA treated mice
(FIG. 19).
Statistical analyses indicate that the one-time treatment of the tumors by NBA
phototherapy
produced significant reductions the percent change in tumor growth (FIG. 20)
and in tumor
volume (FIG. 21). The one-time NBA phototherapy produced significant
reductions in percent
change in tumor growth from the treatment day when compared to the mean
percent change
in tumor growth in control aCSF treated mice (FIG. 20). This "complete
response" of the
reduction in the mean percent change in tumor volume was significantly reduced
(P <0.05) for
up to nine days.
[0140] The one-time NBA phototherapy produced significant reductions in
tumor volume
from the treatment day when compared to the tumor volume in control aCSF
treated mice
(FIG. 21). This "complete response" of the reduction in the mean tumor volume
was
significantly reduced (P <0.05) for up to 13 days.
EXAMPLE 10
[0141] The described inventive technique is effective in increasing the
duration of
survival in animals with cancer in vivo. In this embodiment of the invention,
we prove that
employing the technique to photoactivate NBA in cancer tumors leads to
significant increases
in animal survival. NBA was injected into the established triple negative
breast cancer MDA-
MB-231 tumors of 6 week old nude mice. One hour was permitted to allow
adequate diffusion
of the NBA into the intracellular space of the cancer cells. Following this
one-hour diffusion
period, the tumors were exposed to 405 nm photo-activating light (85 mW) for a
60-30-60
excitation paradigm (described previously).
[0142] Following their one-time treatment of either NBA with photo-
activation or control
aCSF, mice were monitored and euthanized in accordance with the Humane
Endpoints
approved by the University of Texas at San Antonio Institutional Animal Care
and Use
Committee (IACUC). The control aCSF treated mice (n=4) survived for an average
of 16.5
1.66 days from the day of treatment, with survival days from treatment ranging
from 14 to 19
days. In contrast, the one-time NBA treated mice survived for 35.2 5.70 days
from the day
of treatment (FIG. 22). The NBA treated mice survival ranged from 18 to 50
days from
treatment. This increase in survival represents a significant (P = 0.012)
113.3% increase in
survivability as a result of the inventive NBA treatment.
41
Date Recue/Date Received 2021-07-09