Note: Descriptions are shown in the official language in which they were submitted.
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
METHODS FOR MONITORING CD4+ T-HELPER TYPE 1 RESPONSE
IN CANCER AND IMMUNE RESTORATION
[0001] This application claims priority and benefit from U. S.
Provisional Patent Application Serial No. 61/953,726 filed on March 14,
2014.
ACKNOWLEDGMENT
[0002] The present invention was developed in part with government
support under grant number RO1 CA096997 awarded by the National
Institutes of Health. The government has certain rights in this invention.
FIELD
[0003] The present embodiments are directed to progressive loss of
immune response in cancer, in particular the loss of anti-HER2/neu CD4+ T-
helper type 1 ("Thl") response in HER2-driven breast cancer and the
restoration thereof, and diagnostic monitoring methods, treatment methods
and tools based thereon.
BACKGROUND
[0004] Breast cancer ("BC") is a leading cause of cancer-related
mortality worldwide. See, Jemal, A., et al., Global Cancer Statistics. CA: A
Cancer Journal for Clinicians 61:69-90 (2011). Through the development
of gene expression signatures, at least four broad phenotypes of breast
1
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
neoplasms are now recognized: luminal A and B, basal-like, and human
epidermal growth factor receptor-2/neu ("HER2P0s"). See, Perou, C.M., et
al., Nature 406:747-52 (2000). HER2 overexpression, a molecular
oncodriver in several tumor types including about 20-25% of BCs (Meric,
F., et al., .1 Am Coll. Surg. 194:488-501 (2002)), is associated with an
aggressive clinical course, resistance to chemotherapy, and a poor overall
prognosis in BC. See, Henson, E.S., Clin. Can. Res. 12:845-53 (2006)
("Henson, et al.") and Wang, G.S., MoL Med. Rep. 6:779-82 (2012). In
incipient BC, HER2 overexpression is associated with enhanced
invasiveness (Roses, R.E., et al., Cancer EpidemioL Biomarkers & Prey.
18(5):1386-9 (2009)), tumor cell migration (Wolf-Yadlin, A., et al.,
Molecular Systems Biology 2:54 (2006)), and the expression of
proangiogenic factors (Wen, X.F., et al., Oncogene 25:6986-96 (2006)),
suggesting a critical role for HER2 in promoting a tumorigenic environment.
Although HER2-targeted therapies (i.e., Herceptie/trastuzumab), in
combination with chemotherapy, have significantly improved survival in
HER2P s BC patients (Piccart-Gebhart., M.J., et al., N. Eng. J. Med.
353:1659-72 (2005)), a substantial proportion of patients become resistant to
such therapies (Pohlmann, P.R., et al., Clin. Can. Res. 15:7479-91(2009)
("Pohlman, et al.")). Strategies to identify patient subgroups at high risk of
2
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
treatment failure, as well as novel approaches to improve response rates to
HER2-targeted therapies, are needed.
[0005] Growing evidence indicates that robust cellular immune
responses in the tumor microenvironment are associated with improved
outcomes in BC, particularly in the HER2P' subtype. See, Alexe.,G., et al.,
Can. Res. 67:10669-76 (2007). To that end, progress has been made in
deciphering the individual immune mediators of these antitumor effects.
Although cytotoxic CD8+ T lymphocytes ("CTL") were historically
considered the primary effectors of antitumor immunity (Mahmoud, S. M.,
et al., J. Clin. Oncol. 29:1949-55 (2011)), boosting CTL responses with
peptide vaccines in HER2-driven BC has yielded minimal clinical impact
(Amin., A., et al., Cancer Immunol. Immunother. 57(12): 1817-25 (2008)),
possibly because CTLs function suboptimally without adequate CD4+ T-
lymphoCyte help as reported by Bos, R., et al., Cancer Res. 70:8368-77
(2010). In addition to being critical for the generation and persistence of
CTLs, CD4 T-helper ("Th") cells mediate antitumor effects through other
mechanisms, including direct cytotoxic tumoricidal activity, modulation of
antitumor cytokine responses, and potentiation of long-term immunologic
memory (Cintolo, J. A., et al., Future Oncol. 8:1273-99 (2012)). By
facilitating immunoglobulin class switching, Th cells also contribute to
3
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
antitumor humoral immunity and effector B-cell responses. See, Parker,
D.C., et al., Ann. Rev. Immunol. 11:331-60(1993) ("Parker, et al.") . Indeed,
the infiltration of interferon ("IFN")-7 producing CD4+ T-helper type 1
("Thl") cells in the tumor microenvironment is associated with improved
prognosis in BC. See, Gu-Trantien, C., et al., J. Clin. Inv. 123:2873-92
(2013).
[0006] The role of systemic anti-HER2 ON+ Thl responses in HER2-
driven breast tumorigenesis, however, remains unclear. There remains an
unmet need for strategies to predict patient subgroups at high risk of
treatment failure, as well as approaches to improve response rates to HER2-
targeted therapy with trastuzumab and chemotherapy. Thus, one or more
present embodiments are directed to addressing one or more of the problems
identified herein.
BRIEF SUMMARY
[0007] In one broad aspect, there is provided a method for diagnosing
or treating a mammalian subject having, or at risk of developing cancer,
comprising: generating a circulating anti-cancer CD4+ Thl response from
antigen-presenting cells ("APCs") or their precursors and CD4 T-cells from
a sample of the subject's blood which causes secretion of interferon-gamma
4
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
("IFN-y"); and detecting the anti-cancer CD4 Thl response to determine if
the response is depressed.
[0008] In another aspect, the generating step further comprises:
isolating unexpanded peripheral blood mononuclear cells ("PBMCs") from
the blood sample; and pulsing the PBMCs and APC-precursor monocytes
therein with a composition comprising immunogenic MHC class II binding
peptides based on the type of cancer that afflicts the subject, thereby
activating CD4+ Thl cells in the PBMC's to secrete IFN-y; and the detection
step comprises detecting the secreted IFN-y.
[0009] In an alternative aspect, the generation step further comprises:
co-culturing purified CD4+ T-cells from the subject sample with APC
immature or mature dendritic cells ("DCs") from the subject sample pulsed
with a composition comprising immunogenic MHC class II binding peptides
based on the type of cancer that afflicts the subject, thereby activating the
CD4+ T-cells to secrete IFN-y; and the detection step comprises detecting the
secreted IFN-y.
100101 In another aspect, the cancer is selected from the group
consisting of breast, brain, bladder, esophagus, lung, pancreas, liver,
prostate, ovarian, colorectal, and gastric cancer or any combination thereof..
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0011] In another aspect, the cancer is HER2-expressing.
[0012] In a further aspect, the cancer is HER2-positive breast cancer,
the subject is a human female, and the immunogenic MHC class II binding
peptides are based on the HER2 molecule
[0013] In preferred embodiments, the composition further comprises
HER2 MHC class II antigen binding peptides which comprise:
Peptide 42-56 (SEQ ID NO: 1); Peptide 98-114 (SEQ ID NO: 2);
Peptide 328-345 (SEQ ID NO: 3); Peptide776-790 (SEQ ID NO: 4);
Peptide 927-941 (SEQ ID NO: 5); and Peptide 1166-1180 (SEQ ID
NO: 6).
[0014] In preferred embodiments the IFN-y production is measured by
IFN-y enzyme-linked immunospot assay ("ELISPOT").
[0015] In another aspect there is a method for restoring HER2-specific
CD4+ Thl immune response in a HER2-positive breast cancer patient in
need thereof, comprising: administering to the patient a therapeutically
effective amount of a DC vaccine comprising autologous DCs pulsed with
immunogenic HER2 MHC class II binding peptides ("DC vaccination") to
elevate the patient's anti-HER2 CD4+ Thl response; and measuring the anti-
HER2 CD4+Th1 response of the patient pre- and post-DC vaccination
6
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
according to the generating and detecting steps of the above aspects to
determine the amount of increase in the response, wherein the method for
restoring further comprises: measuring the status of the anti-HER2 CD4+Th1
response restoration of the patient post-DC vaccination by conducting the
generating and detecting steps of the above aspects at one or more additional
time intervals to monitor said response restoration.
[0016] In another aspect there is a method for screening individuals for
breast or other cancer, comprising: detecting anti-HER2 CD4+ Thl
responses of the individuals according to the method of the generating and
detecting steps of the above aspects to determine if the responses are
depressed as compared to healthy individuals.
[0017] In another aspect there is a method for screening individuals at
risk for developing breast or other cancer, comprising: detecting anti-HER2
CD4+ Thl responses of the individuals according to the method of the
generating and detecting steps of the above aspects to determine if the
responses are depressed as compared to healthy individuals.
[0018] In another aspect there is a method for predicting whether a
patient with HER-positive breast cancer will respond well to standard non-
immune therapy such as chemotherapy and trastuzumab, comprising:
7
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
detecting the anti-HER2 CD4 Th1 response of the patient according to the
method of the generating and detecting steps of the above aspects.
[0019] In another aspect there is a method of predicting new breast
events in HER2-positive-invasive breast cancer ("HER2P"-IBC") patients
treated with trastuzumab and chemotherapy, comprising: measuring the anti-
HER2 CD4+Th1 response of the patient according to the method of the
generating and detecting steps of the above aspects to determine if said
response is depressed.
[0020] In another aspect there is a method of predicting pathologic
response of HER2-positive breast cancer following neoadjuvant trastuzumab
and chemotherapy ("TIC") therapy in a HER2-positive breast cancer patient,
comprising: measuring the degree of anti-HER2 CD4+ Th 1 responsiveness in
said patient post-T/C treatment according to the method of the generating
and detecting steps of the above aspects to determine if said response is a
significantly higher anti-HER2 CD4- Thl response associated with
neoadjuvant pathological complete response (no residual invasive breast
cancer on postoperative pathology) or a lower response associated with non-
pathological complete response and further, wherein in the case of a non-
8
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
pathological complete response in said patient, the anti-HER2 CD4+ Th 1
response of said patient is restored by DC vaccination.
[0021] In another broad aspect there is a method for diagnosing or
treating a mammalian subject having, or at risk of developing cancer,
comprising: obtaining blood from the subject; performing a blood test
thereon which measures suppression in anti-cancer CD4+ Thl response, and
in the case of suppression; administering to the subject a cancer medicament
in an effective amount selected from the group consisting of DC vaccine,
targeted cancer therapy such as trastuzumab, conventional cancer therapy
such as chemotherapy, surgery, and radiation.
[0022] For a better understanding of exemplary embodiments, together
with other and further features and advantages thereof, reference is made to
the following description, taken in conjunction with the accompanying
drawings, and the scope of the claimed embodiments will be pointed out in
the appended claims.
9
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] The
following detailed description of preferred embodiments
will be better understood when read in conjunction with the appended
drawings. For the purpose of illustrating the embodiments, there are shown
in the drawings embodiments which are presently preferred. It should be
understood, however, that the preferred embodiments are not limited to the
precise arrangements and instrumentalities of the embodiments shown in the
drawings.
[0024] Figure 1 is a hierarchy diagram representing patient/donor
groups included in the study described herein. Cohorts are labeled A¨H.
Treatment schedules in cohorts G and H, as well as time-points at which
blood was drawn are indicated in red callout boxes. Specifically, in the T/C-
treated HER2P's-IBC cohort (G), patients received either neoadjuvant T/C,
followed by surgery and completion of adjuvant trastuzumab; patients
selected for a surgery-first approach completed adjuvant T/C. Blood was
drawn either <6 months or >6 months from completion of adjuvant
trastuzumab.
[0025] Figure 2 shows dendritic cell ("DC") vaccination strategy.
Patients' monocytes are first separated from other white blood cells by
leukapheresis and elutriation. These monocytes are then cultured in serum-
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
free medium ("SFM") with granulocyte-macrophage colony-stimulating
factor ("GM-CSF") and interleukin ("IL")-4 to become immature dendritic
cells ("IDCs" or "iDCs"). These cells are then pulsed with six HER2 MHC
class II binding peptides, and interferon ("IFN")- y and lipopolysaccharide
("LPS") are added to complete the maturing and activation process to
achieve full DC activation to DC is before injecting back into the patient.
See, Fracol, M., et al., Ann. Surg. Oncol. 20(10):3233 (2013). In the case of
HLA-A2P0s patients, half of the cells were pulsed with a MHC class I
binding peptide and the other half with a different MHC class 1 binding
peptide.
[0026] Figures 3A
and 3B are graphs showing inter-assay precision of
ELISPOT. For the Figure 3A studies, three parallel replicates over three
days were run for samples from five donors (represented by different
symbols) with known varying anti-HER2 reactivity in ELISPOT assays.
The mean coefficient of variance ("Mean CV") was plotted against
cumulative anti-HER2 Thl response ("Mean SFC ("spot forming
cells")/2x105 cells") for donors stimulated ex vivo with a HER2 extracellular
domain ("ECD") peptide mix (peptide 42-56 (SEQ ID NO: 1), peptide 98-
114 (SEQ ID NO: 2), and peptide 328-345 (SEQ ID NO: 3)). Error bars
represent standard deviation ("SD") of the replicates. Figure 3B shows the
11
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
standard deviation ("SD") of three assays on separate days plotted against
cumulative Thl response ("Mean SFC/2x105 cells") for each donor
(represented by different symbols) as a measure of inter-assay variability.
The connecting line represenst linear regression of the SD generated, with
95% confidence intervals of the regression shown with parallel dotted lines.
[0027] Figure 4 shows graphs which show the linearity of ELISPOT.
Triplicate samples of peripheral blood mononuclear cells ("PBMCs") from
two high-responding HER2-reactive donors (DONOR #1, (triangles) and
DONOR #2, (circles)) were serially diluted into PBMCs from a known
allogeneic non-HER2 responder (same PBMC donor for all assays), and
stimulated ex vivo with a HER2 ECD peptide mix (peptide 42-56 (SEQ ID
NO: 1), peptide 98-114 (SEQ ID NO: 2), and peptide 328-345 (SEQ ID
NO: 3)). Unstimulated background was subtracted for each dilution point in
the ELISPOT assays.
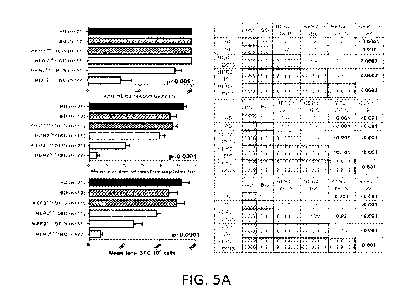
[0028] Figures 5A-5D show anti-HER2 CD4- Thl response and
IgGl/IgG4 reactivity are progressively lost in HER2P" breast tumorignesis.
Figure 5A shows histograms (left panels) of IFNI, ELISPOT analysis of
systemic CDµ T-cells and anti-HER2 CD4+ Thl response; corresponding
post-hoc Scheffe p-value comparisons between patient groups are shown
12
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
alongside the histograms (right panels). The patient groups studied were:
HD (healthy donors); BD (benign breast biopsy); HER2"g DCIS; HER2"g
IBC (non-equivocal HER2ne5 (HER2 0 and 1+) invasive breast cancer);
HER2P" DCIS (HER2P's ductal carcinoma in situ); and HER2P s IBC (Stage
I/II HER2P" invasive breast cancer). The top histogram shows overall anti-
HER2 responsivity (%100) (percentage of patients responding to >1 reactive
peptide) (also referred to as "anti-HER2 responsivity"); the middle
histogram shows mean number of reactive peptides (n) (the mean number of
reactive peptides ("n") the patients in the group reacted to as a whole) (also
referred to as "response repertoire"); and the bottom histogram shows mean
total SFC/106 cells (total sum of reactive spots (spot-forming cells "SFC"
per 106 cells from IFN-y ELISPOT analysis) from all 6 MHC Class II
binding peptides from each subject group) (also referred to as "cumulative
response") (all ANOVA p<0.001). A progressive loss of CD4+ Thl
response in HER2P" breast tumorigenesis is shown (i.e. HD/BD HER2P 9-
DCIS--> HER2P"-IBC) when assessed by anti-HER2 responsivity, response
repertoire, and cumulative response. No differences in Thl responses were
found between HER2"eg-DCIS and HER2"eg-IBC (IHC 0/1+) and HD/BD
subjects. Figure 5B shows IFN-y production by ELISPOT (cumulative
response (mean total SFC/2x105 cells)) in the same respective patient groups
13
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
as in Figure 5A, with the addition of the T/C-treated HER2P s-IBC patient
group ("T/C" means trastuzumab and chemotherapy). Results are presented
as median interquartile range ("IQR") IFN-y SFC per 2x105 cells in box-
and whiskers plots. Figure 5C shows histograms for variations in anti-HER2
Thl cumulative responses in HD/BDs stratified by donor age (<50 years v.
?_50 years) (upper left panel), menopausal status (pre-menopausal v. post-
menopausal) (upper right panel), race (white v. other) (lower left panel) and
gravidity (zero v. >1 pregnancies) (lower right panel) Within each Thl
metric, results are expressed as proportion or mean ( SEM). Figure 5D
shows ELISA results of serum reactivity against recombinant HER2 ECD
peptides. ELISA measurements are shown as optical density ("OD") at
1:100 sera dilutions (grouped scatter plot, with horizontal lines indicating
mean OD). Anti-HER2 IgG1 antibody levels (top panel) and anti-HER2
IgG4 antibody levels (bottom panel) were measured in HD (circles/left),
HER2P"-DCIS (squares/middle), and HER2P'6-IBC (triangles/right) patients
(***p<0.001 by unpaired t-test or ANOVA with post-hoc Scheffe testing, as
applicable). Significantly elevated anti-HER2 IgG1 and IgG4 antibody
levels were present in HER2P 9-DCIS patients compared with HDs, that
decayed in HER2P"-IBC patients.
14
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0029] Figure 6 shows individual HER2 peptide contributions to
cumulative CD4+ Thl immunity in HER2P" breast tumorigenesis for HD
(healthy donors); BD (benign breast biopsy); HER21 DCIS; HER2 "g IBC
(non-equivocal HER2" g (HER2 0 and 1+) invasive breast cancer); HER2P s
DCIS (HER2P' ductal carcinoma in situ); and HER2' IBC (Stage I/II
HER2P" invasive breast cancer) patients do not reflect immune sculpting.
HER2 extracellular domain ("ECD")-restricted peptides and intracellular
domain ("ICD")-restricted peptides were used. Thl reactivity profiles are
shown for ECD peptide 42-56 ("ECD p42-56") (SEQ ID NO: 1) (top left);
ECD peptide 98-114 ("ECD p98-114") (SEQ ID NO: 2) (middle left) and
ECD peptide 328-345 ("ECD p328-345") (SEQ ID NO: 3) (bottom left) and
for ICD peptide 776-790 ("ICD p7'76-'790") (SEQ ID NO: 4) (top right);
ICD peptide 927-941 ("ICD p927-941") (SEQ ID NO: 5) (middle right); and
ICD peptide 1166-1180 ("ICD p1166-1180") (SEQ ID NO: 6) (bottom
right). Individual peptide-specific responses are depicted as mean IFN-y
SFC per 2x105 PBMCs by ELISPOT. Thl reactivity profiles show a
significant stepwise decline in anti-HER2 Thl immunity across a continuum
(HD4BD-->HER2"g-DCIS4HER2"eg-IBC4HER2P"-DCIS->HER2P's-
IBC) in HER2P" breast tumorigenesis (all p<0.005 by ANOVA). Results
are expressed as mean SEM.
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0030] Figure 7 shows minimal temporal variability in donor anti-
HER2 Thl responses. Donor-matched anti-HER2 Thl cumulative response
(left panel) and response repertoire (right panel), generated from blood
samples obtained at least 6 months apart, are plotted for paired HD (green
triangles; n=4) and treatment-naïve HER2P"-IBC subjects (blue squares;
n=4). Minimal within-donor Thl response variability was observed in both
HD and treatment-naïve HER2P"-IBC subjects over time (all p=NS).
[0031] Figures 8A-8E show anti-HER2 Thl deficit in HER2P"-IBC is
not attributable to lack of immunocompetence or increase in
immunosuppressive phenotypes, but is associated with a functional shift in
IFN-y:IL-10-producing phenotypes. Figure 8A shows IFN-y production by
measuring cumulative Thl response (mean total SFC/105 cells) to recall
stimuli tetanus toxoid or Candida albicans in IFN-y ELISPOT. Results are
presented as median interquartile range (IQR) IFN-y SFC per 2x105 cells
in box-and-whiskers plots. PBMCs from HER2P'9-IBC patients, both
treatment-naïve and TIC treated, did not differ significantly from those of
HDs. Figure 8B, top panels, show representative flow cytometry stainings
using PBMCs from HD, HER2P"-IBC (Stage I/II) and HER2P"-IBC s/p TIC
(patient TIC-treated) patients to determine their immunophenotype. Relative
proportions of CD4+ (CD3 CD4) (top stainings) or CD8+ (CD3+CD8 ) T-
16
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
cells (bottom stainings) are shown and are represented in the bottom
histograms which show respectively, relative proportions of CD4+
(CD3'CD4+) T-cells (left) and CD8+ (CD3+CD8 ) T-cells (right) for the
patient groups: HD (dark bars), Stage I/II HER"-IBC (medium bars) and
HER2P"-IBC s/p TIC (light bars). PBMCs from HER2P06-IBC patients, both
treatment-naïve and TIC treated, did not differ significantly from those of
HDs. Figure 8C, top panels, show representative flow cytometry stainings
using PBMCs from HD, HERP s-IBC (Stage I/II) and HER2P"-IBC s/p TIC
to determine their immunophenotype. Relative proportions of regulatory T-
cells ("Treg"; CD4 CD25+FoxP3 ) (top stainings) and myeloid-derived
suppressor cells ("MD SC"; CD1113 CD33 TILA-DR-CD83-) (bottom
stainings) are shown and are represented in the lower histograms which
show respectively relative proportions of, regulatory T-cells (Treg;
CD4+CD25 FoxP3+) (left) or myeloid-derived suppressor cells ("MDSC";
CD11b CD33+HLA-DR-CD83-) (right) for the patient groups: HD (dark
bars), Stage VII HER"-IBC (medium bars) and HER2P"-IBC s/p TIC (light
bars). PBMCs from HER2P"-IBC patients, both treatment-naïve and TIC
treated, did not differ significantly from those of HDs. Figure 8D shows
circulating HER2-specific IL-10 production does not vary between patient
groups. PBMCs from HER2P s-IBC patients, both treatment-naïve (HER2P"-
17
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
IBC) and those receiving TIC (HER2P s-IBC s/p TIC), did not differ
significantly from HDs in anti-HER2 IL-10 production via ELISPOT,
assessed by overall anti-HER2 responsivity (top), repertoire (middle), and
cumulative response. (bottom). Results are expressed as proportion or mean
SEM. Figure 8E shows relative HER2-specific IFN-y and IL-10
production in HER2P s breast tumorigenesis. Donor-matched cumulative
IFN-y production and IL-10 production (SFC/106 cells) across six HER2
HER2 Class II peptides in HD, HER2"-IBC (treatment-naïve), and
HER2P 9-IBC s/p TIC (T/C-treated) patients were compared. The bar graphs
show the relative HER2-specific IFN-y to IL-10 proportions via percentage
of SFC contribution (% depicted in graphs) across the patient groups for
HER2 antigen-specific reaction (top panel) and positive control (CD3 or
CD8/28) (bottom panel) ;(IFN-y production (green) ; IL-10 production
(red)). Relative HER2-specific IFN-y to IL-10 proportions decreased
significantly from HDs to HER2P 9-IBC patients with or without TIC-
treatment. Absolute IFN-y:IL-10 production ratio changed from 6.6:1 (HDs)
to 0.97:1 (TIC-treated) and 0.74:1 (HER2P 9-IBC), respectively (top panel).
No significant relative shifts in IFN-y:IL-10 production were observed to
positive controls (anti-CD3/anti-CD3/CD28) (bottom panel).
18
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0032] Figures 9A-9B show systemic loss in anti-HER2 CD4+ TH1
subsets is not related to disproportionate peritumoral T lymphocyte
trafficking in HER2P s breast lesions. Figure 9A shows two photographs of
representative hematoxylin and eosin ("H&E") stainings of tissue samples
from HERT" -DCIS lesions (top) and HER2P"-IBC tumors (bottom)
(magnification bars 25 pm). The arrows point to a relative paucity of
lymphocytic infiltrate observed in the peritumoral Aroma of HER2P"-IBC
tumors (bottom) as compared with HER2P"-DCIS lesions (top) by
immunohistochemical staining. Stromal lymphocyte infiltration in
evaluable HER2P"-DCIS (n=14) and HER2P's-IBC (n=8) is quantified as
low (<15% involvement), moderate (15-24%) and high (>25%) in the
adjoining table. Figure 9B shows four photographs of the results of
multiplex-labeled immunofluorescence in representative HER2P"-DCIS
(left) and HER2P's -IBC (right) lesions. A striking paucity of CD4+ T-cells
(green signal) was observed in 5/5 (100%) HER2P"-IBC tumors, where the
predominant infiltrating and stromal lymphocytic infiltrate is CD8- (yellow
signal). By comparison, a predominantly CD4+ T-cell infiltrate was seen in
DCIS-containing ducts (4/4 tumors). Representative HER2P'6-DCIS and
IBC lesions are depicted; multiplexed-labeled images are shown above
corresponding H&E sections (magnification bar 25p.m).
19
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0033] Figures 10A-10D show CD4+ Thl induces apoptosis of
HER2"', but not HER210, human and murine breast cancer cells. Figure
10A shows (top panels) photographic results of western blot analysis for
detection of cleaved caspace-3. SK-BR-3 cells were co-cultured with: Lane
1) - complete medium alone (complete medium); Lane 2) - 106 CD4+ T-cells
alone (CD4+ only); Lanes 3 and 4) - 106 CD4+ T-cells plus 105 HER2 Class
II peptide ("iDC H") - or irrelevant Class II BRAF peptide ("iDC B") -
pulsed immature DCs ("iDCs"), respectively; Lanes 5 and 6) - 106 CD4+ T-
cells plus 105 each HER2 ("DC1 H") - or BRAF ("DC1 B") -pulsed DC is,
respectively; Lane 7) - CD4- 106 DC1 H 105+ IFN-y & TNF- a neutr Ab
and Lane 8) - ON+ 106 DC1 H 105+ IgG isotype control Ab. Increased
caspase-3 cleavage indicated dose-dependent apoptosis of SK-BR-3 cells
when co-cultured with DC1 H:CD4+ T-cells, but not DC1 B, iDC H, or iDC
B groups. Vinculin was used as a loading control. The displayed western
blot is representative of three experiments. The middle panel bar graph (red
bars) shows results expressed as mean caspace-3/vinculin ratios SEM
indicating fold induction of apoptosis (quantified using ImageJ software)
that corresponds to western blot Lanes 1-6 in the top panel. In the bar graph
to the right (black bars) (corresponding to western blot Lanes 7-8 in the top
panel) the bars represent % rescue of apoptosis/mean caspase-3/vinculin
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
ratio SEM (31.4+5.3% IFN-y/TNF-a neutralization vs. control) over three
experiments. Compared with IgG isotype control, CD4-:DC1 H-induced
SK-BR-3 apoptosis was significantly rescued by neutralizing IFN-y and
TNF-a. The bottom panel shows corresponding production of IFN-y (left y-
axis) (solid bars) and TNF-a (right y-axis) (lined bars) in respective co-
cultures by ELISA. Results are expressed in pg/mL, and are representative
of three experiments. Figure 10B shows photographs of the cells of the
"CD4 only," "CD4+ + DC1 B", and "CD4+ + DC1 H," cell groups.
Apoptotic cells were revealed by DAPI staining. In the CD4+ + DC1 H
group, a greater number of apoptotic cells (asterisks) were observed when
compared with CD4+ + DC1 B or CD4+ only groups. The bar graph (right)
shows % apoptotic cells (fold induction) of apoptotic cells for the three cell
groups pictured, with a 25-fold increase in apoptosis for the CD4+ + DC H
group that correlates with the visual results. Results are representative of
three experiments, and expressed as mean % apoptotic cells SEM. Figure
10C shows photographs of the results of western blot analysis in which
HER2high SK-BR-3, HER intermediate MC
F-7, / and HER21 w MDA-MB-231
human BC cells uniformly maintained expression of IFN-y-Ra and TNF-a-
R1 receptors. Vinculin was used as a loading control. Figure 10D shows
that in transgenic murine HER2high mammary carcinoma TUBO (top graph)
21
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
and MMC15 (HER21ligh) cells (middle graph), combination treatment with
recombinant murine ("rm") Thl cytokines rmIFN-y and rmTNF-a resulted
in significantly greater apoptosis compared with untreated controls (no Rx)
or treatment with either cytokine alone. This effect was not reproduced with
dual rmIFN-y + rmTNF-a treatment in murine HER21 willeg cells 4T1 (bottom
graph). Results are representative of three experiments, and expressed as
mean % apoptotic cells SEM, detected by proportion of PIP s/Annexin VP s
cells by flow cytomety. (* p50.05, **p<0.01, *** p<0.001).
[0034] Figures 11A-11C show HER2high, but not HER210w, human BC
cells are sensitive to CD4+ Thl-mediated apoptosis, by virtue of Thl-
elaborated cytokines IFN-y and TNF-a. Figure 11A shows (top panels)
photographic results of western blot analysis for detection of cleaved
caspace-3. Using a transwell system, 50x103 MCF-7 (HER2intennediate) and
50x103MDA-MB-231 (HER21 w) cells were co-cultured with medium alone
(complete medium), 10 CD4+ T-cells alone (CD4+ only), and 10 CD4+ T-
cells + 105 each HER2 (DC1 H)- or BRAF control (DC1 B)-pulsed DC1s.
Caspase-3 cleavage shown in the western blots and represented in the
corresponding bar graphs below (lower panel) indicated increased apoptosis
of MCF-7 (left panels), but not MDA-MB-231 cells (right panels) when co-
cultured with DC1 H:CD4+ T-cells. Vinculin was used as loading control.
22
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
The displayed western blots are representative of three experiments, and
results are expressed as mean caspase-3/vinculin ratios SEM (indicating
fold induction of apoptosis Figure 11B shows photographs of western blot
results of co-culturing SK-BR-3 cells with the supernatants from the
following treatment conditions in Figure 10A [complete medium alone; 106
CD4+ T-cells alone (CD4+ only); CD4+ T-cells + HER2-pulsed iDC ("iDC
H"); CD4+ T-cells + BRAF-pulsed iDC ("iDC B"); CD4+ T-cells + 105
HER2-pulsed DC! ("DC1 H"); and CD4+ T-cells + 105 BRAF-pulsed DC1
("DC1 B")] were co-cultured with 50x103 SK-BR-3 cells. Relatively higher
cleaved caspase-3 levels were detected in the DC1 H:CD4+ group compared
with DC1 B, iDC H, iDC B, or CD4- only groups. Results are
representative of three experiments. Figure 11C shows photographs of
western blot results (top panels) of culturing SK-BR-3 (left), MCF-7
(center), and MDA-MB-231 cells (right) with indicated amounts of TNF-a
and IFN-y for detection of cleaved caspace-3. The bars of the lower panel
bar graph correspond to the lanes of the western blot displayed in the top
panels. Combination treatment with Thl cytokines IFN-y and TNF-a
resulted in greater apoptosis in SK-BR-3 (HER2high; 10 ng/mL TNF-a+100
U/mL IFN-y) and MCF-7 (HER2inteh1ediate; 100 ng/mL TNF-a+1000 U/mL
IFN-y) cells, compared with untreated controls. MDA-MB-231 cells
23
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
(HER210w; 200 ng/mL TNF-u+2000 U/mL IFN-y) remained largely
unaffected by dual IFN-y + TNF-a treatment. Results are representative of
three experiments. ( *p<0.05, **p<0.001).
[0035] Figures 12A-12E show anti-HER2 CD4- Thl immunity is
differentially restored following HER2-pulsed DC1 immunization, but not
after HER2-targeted therapies Figure 12A is a graph of CD4+ Thl responses
in treatment-naive HER2P"-IBC patients ("HER2P"-IBC no tx") (black) and
HER2P"-IBC patients receiving trastuzumab and chemotherapy ("t/C-treated
HER2P s-IBC") (red), assessed by overall anti-HER2 responsivity (top),
response repertoire (middle), and cumulative response (bottom). Compared
with treatment-naïve Stage I/II HER2P"-IBC patients (no tx), anti-HER2
Thl responses were not globally augmented following TIC treatment in
stage I-III HER2P"-IBC patients (TIC-treated), illustrated by anti-HER2
responsivity (top), repertoire (middle), or cumulative response (bottom).
The relative proportion of IFN-y:IL-1 0 reactive cells (% depicted in lower
panel histograms; IFN-y: solid; IL-10: diagonal lines) following HER2-
specific and tetanus (positive control) stimuli did not improve in T/C-treated
(n=5) compared with no tx (n=5). Figure 12B is a graph of CD4+ Thl
responses in HER2P'9-IBC patients immediately prior to and following
HER2 pulsed-DC1 immunization ("HER2P 8-IBC PRE vax") (black) and
24
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
("HER2P s-IBC POST vax") (green) respectively, assessed by overall anti-
HER2 responsivity (top), response repertoire (middle), and cumulative
response (bottom). Significant improvements in all anti-HER2 Thl immune
metrics were observed in 11 Stage I HER2P"-IBC (PRE vax) patients
immediately following HER2 pulsed-DC1 immunization (POST vax).
While relative proportion of IFN-y to IL:10 reactive cells (% depicted in
lower panel histograms ; IFN-y: solid; IL-10: diagonal lines) did not change
appreciably following tetanus stimulation, HER2-pulsed vaccination
significantly increased the relative proportion of IFN-y to IL:10 reactive
cells in POST vax (n=5) compared with PRE vax (n=5) patients. Figure 12C
shows stage-matched effects of DC vaccination and
trastuzumab/chemotherapy on anti-HER2 Till immunity. Matched
comparison between AJCC Stage I treatment-naïve ("No tx"), T/C-treated
("TIC-treated"), and HER2-pulsed DC1 immunization ("POST-vax")
HER2P"-IBC patients were assessed by overall anti-HER2 responsivity
(top), response repertoire (middle), and cumulative response (bottom). The
differential Till restoration following HER2-pulsed DC1 immunization, but
not TIC treatment, persisted on stage-matched comparisons in Stage I
HER2P"-IBC patients. Results are expressed as proportion or mean I SEM;
("p<0.01, ***p<0.001). Figures 12D and 12E show the durability of CD4+
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Thl immune response after DC vaccination. Immune responses in were
compared in Stage I/II HER2P"-IBC patients pre-DC vaccination ("PRE
VACCINE"), immediately after DC vaccination("IMMEDIATE POST
VACCINE") and? 6 months after vaccination (">6 MO POST
VACCINE"). Beyond the immediate post-vaccination period, anti-HER2
CD4+ Thl immunity remained durably augmented in 9 of 11 evaluable
patients >6 months following vaccination, despite initiation/completion of
systemic chemotherapy in all patients by this time-point (broken arrows).
Scatter plots demonstrate CD4+ Thl reactivity profiles by response
repertoire (Figure 12D) and cumulative response (Figure 12E) for individual
vaccinated subjects.
[0036] Figures 13A-13E show depressed anti-HER2 Thl responses
following T/C treatment correlate with adverse clinical and pathologic
outcomes. The graphs of Figures 13A-13D show subgroup analysis of TIC-
treated HER2P"-IBC patients demonstrated no appreciable differences in
anti-HER2 responsivity (top graphs), repertoire (middle graphs), or
cumulative response (bottom graphs) when stratified by Figure 13A-
sequencing of chemotherapy (neoadjuvant vs. adjuvant); Figure 13B- time
from completion of trastuzumab to enrollment in study (<6 vs. >6 months);
Figure 13C- estrogen-receptor status (ERP" vs. ER") and Figure 13D-
26
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
pathologic stage (I vs. II vs. III). Figure 13E shows that compared with
HER2P"-IBC patients who did not incur breast events ("No BE") following
completion of T/C, patients incurring BEs ("+BE") had significantly
depressed anti-HER2 responsivity (left top graph) and cumulative Thl
responses (bottom left graph). In HER2P"-IBC patients achieving
pathologic complete response (pCR) following neoadjuvant TIC, anti-HER2
Thl response repertoire (right middle graph) and cumulative response (right
bottom graph) was significantly greater compared to non-pCR patients.
DETAILED DESCRIPTION
[0037] It is to be understood that the figures, images and descriptions
of the present embodiments have been simplified to illustrate elements that
are relevant for a clear understanding, while eliminating, for the purposes of
clarity, many other elements which may be found in the present
embodiments. Those of ordinary skill in the pertinent art will recognize that
other elements are desirable and/or required in order to implement the
present embodiments. However, because such elements are well known in
the art, and because such elements do not facilitate a better understanding of
the present embodiments, a discussion of such elements is not provided
herein.
27
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0038] Reference throughout this specification of "one embodiment"
or "an embodiment" or the like means that a particular feature, structure, or
characteristic described in connection with the embodiment is included in at
least one embodiment. Thus appearances of the phrases "in one
embodiment" or "in an embodiment" or the like in various places throughout
this specification are not necessarily all referring to the same embodiment.
[0039] In addition, for the purpose of promoting an understanding of
the principles of the present disclosure, reference will now be made to the
embodiments shown and described herein, and specific language will be
used to describe the same. It will, nevertheless, be understood that no
limitation of the scope of the disclosure is thereby intended; any alterations
and further modifications of the described or illustrated embodiments and
any further applications of the principles of the disclosure as illustrated
herein are contemplated as would normally occur to one skilled in the art to
which the disclosure relates. All limitations of scope should be determined
in accordance with and as expressed in the eventual claims of one or more
issued patents.
28
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Definitions
[0040] Unless defined otherwise, all technical and scientific terms used
herein have the same meaning as commonly understood by one of ordinary
skill in the art to which the inventive subject matter of this disclosure
belongs. Although any methods and materials similar or equivalent to those
described herein can be used in the practice or testing of the present
embodiments, the preferred methods and materials are described.
[0041] Generally, the nomenclature used herein and the laboratory
procedures in cell culture, molecular genetics, organic chemistry, and
nucleic acid chemistry and hybridization are those well-known and
commonly employed in the art.
[0042] Standard techniques are used for nucleic acid and peptide
synthesis. The techniques and procedures are generally performed according
to conventional methods in the art and various general references (e.g.,
Sambrook and Russell, 2012, Molecular Cloning, A Laboratory Approach,
Cold Spring Harbor Press, Cold Spring Harbor, NY, and Ausubel et al.,
2012, Current Protocols in Molecular Biology, John Wiley & Sons, NY),
which are provided throughout this document.
29
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0043] The nomenclature used herein and the laboratory procedures
used in analytical chemistry and organic syntheses described below are those
well-known and commonly employed in the art. Standard techniques or
modifications thereof are used for chemical syntheses and chemical
analyses.
[0044] As used herein, each of the following terms has the meaning
=
associated with it in this section.
[0045] The articles "a" and "an" are used herein to refer to one or to
more than one (i.e., to at least one) of the grammatical object of the
article.
By way of example, "an element" means one element or more than one
element.
[0046] "About" as used herein when referring to a measurable value
such as an amount, a temporal duration, and the like, is meant to encompass
variations of 20%, or 10%, or 5%, or 1%, or +0.1% from the specified
value, as such variations are appropriate to perform the disclosed methods.
[0047] "Adjuvant therapy" for breast cancer as used herein refers to
any treatment given after primary therapy (i.e., surgery) to increase the
chance of long-term survival. "Neoadjuvant therapy" is treatment given
before primary therapy.
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0048] The term "antigen" or "ag" as used herein is defined as a
molecule that provokes an immune response. This immune response may
involve either antibody production, or the activation of specific
immunologically-competent cells, or both. One of ordinary skill in the art
will understand that any macromolecule, including virtually all proteins or
peptides, can serve as an antigen. Furthermore, antigens can be derived
from recombinant or genomic DNA. A skilled artisan will understand that
any DNA, which comprises a nucleotide sequences or a partial nucleotide
sequence encoding a protein that elicits an immune response therefore
encodes an "antigen" as that term is used herein. Furthermore, one skilled in
the art will understand that an antigen need not be encoded solely by a full
length nucleotide sequence of a gene. It is readily apparent that the present
embodiments include, but are not limited to, the use of partial nucleotide
sequences of more than one gene and that these nucleotide sequences are
arranged in various combinations to elicit the desired immune response.
Moreover, a skilled artisan will understand that an antigen need not be
encoded by a "gene" at all. It is readily apparent that an antigen can be
generated or synthesized or can be derived from a biological sample. Such a
biological sample can include, but is not limited to a tissue sample, a tumor
sample, a cell or a biological fluid.
31
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0049] An "antigen presenting cell" or "APC" is a cell that is capable
of activating T cells, and includes, but is not limited to,
monocytes/macrophages, B cells and dendritic cells ("DCs").
[0050] "Antigen-pulsed APC" or an "antigen-loaded APC" includes an
APC which has been exposed to an antigen and activated by the antigen.
For example, an APC may become Ag-loaded in vitro, e.g., during culture in
the presence of an antigen. An APC may also be loaded in vivo by exposure
to an antigen. An "antigen-loaded APC" is traditionally prepared in one of
two ways: (I) small peptide fragments, known as antigenic peptides, are
"pulsed" directly onto the outside of the APCs; or (2) the APC is incubated
with whole proteins or protein particles which are then ingested by the APC.
These proteins are digested into small peptide fragments by the APC and are
eventually transported to and presented on the APC surface. In addition, an
antigen-loaded APC can also be generated by introducing a polynucleotide
encoding an antigen into the cell.
[0051] "Anti-HER2 response" is the immune response specifically
against HER2 protein.
[0052] The term "anti-tumor effect" as used herein, refers to a
biological effect which can be manifested by a decrease in tumor volume, a
32
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
decrease in the number of tumor cells, a decrease in the number of
metastases, an increase in life expectancy, or amelioration of various
physiological symptoms associated with the cancerous condition. An "anti-
tumor effect" can also be manifested by the ability of binding peptides,
polynucleotides, cells and antibodies in prevention of the occurrence of
tumor in the first place.
[0053] "Apoptosis" is the process of programmed cell death. Caspase-
3 is a frequently activated death protease.
[0054] As used herein, the term "autologous" refers to any material
derived from the same individual to which it is later to be introduced.
[0055] The term "B cell" as used herein is defined as a cell derived
from the bone marrow and/or spleen. B cells can develop into plasma cells
which produce antibodies.
[0056] "Binding peptides." See, "11ER2 binding peptides."
[0057] The term "cancer" as used herein is defined as a
hyperproliferation of cells whose unique trait--loss of normal control--
results
in unregulated growth, lack of differentiation, local tissue invasion, and/or
metastasis. Examples include but are not limited to, breast cancer, prostate
33
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
cancer, ovarian cancer, cervical cancer, skin cancer, bladder cancer,
esophageal cancer, pancreatic cancer, colorectal cancer, gastric cancer, renal
cancer, liver cancer, brain cancer, lymphoma, leukemia, lung cancer, germ-
cell tumors, and the like.
[0058] "CD4+ Thl cells," "Thl cells," "CD4+ T-helper type lcells,"
"CD4 T cells," and the like are defined as a subtype of T-helper cells that
express the surface protein CD4 and produce high levels of the cytokine
IFN-y. See also, "T-helper cells."
[0059] "Cumulative response" means the combined immune response
of a patient group expressed as the total sum of reactive spots (spot-forming
cells "SFC" per 106 cells from IFN-y ELISPOT analysis) from all 6 MHC
class II binding peptides from a given patient group.
[0060] "DC vaccination," "DC immunization," "DC1 immunization,"
and the like refer to a strategy using autologous dendritic cells to harness
the
immune system to recognize specific molecules and mount specific
responses against them.
[0061] The term "dendritic cell" or "DC" is an antigen presenting cell
existing in vivo, in vitro, ex vivo, or in a host or subject, or which can be
derived from a hematopoietic stem cell or a monocyte. Dendritic cells and
34
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
their precursors can be isolated from a variety of lymphoid organs, e.g.,
spleen, lymph nodes, as well as from bone marrow and peripheral blood.
DCs have a characteristic morphology with thin sheets (lamellipodia)
extending in multiple directions away from the dendritic cell body.
Typically, dendritic cells express high levels of MHC and costimulatory
(e.g., B7-1 and B7-2) molecules. Dendritic cells can induce antigen specific
differentiation of T cells in vitro, and are able to initiate primary T cell
responses in vitro and in vivo. In the context of vaccine production, an
"activated DC" is a DC that has been exposed to a Toll-like receptor agonist
such as lipopolysaccharide "LPS." An activated DC may or may not be
loaded with an antigen. See also, "mature DC."
[0062] "DC-1
polarized dendritic cells," "DC1s" and "type-1 polarized
DCs" refer to mature DCs that secreteThl-driving cytokines, such as IL-12,
IL-18, and IL-23. DC1s are fully capable of promoting cell-mediated
immunity. DC1s are pulsed with HERZ MHC class II-binding peptides in
preferred embodiments herein.
[0063] "Estrogen
receptor ("ER") positive" or "ERP's" cancer is cancer
which tests positive for expression of estrogen. Conversely, "ER negative"
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
cancer tests negative for such expression. Analysis of ER status can be
performed by any method known in the art.
[0064] "HER2" is a member of the human epidermal growth factor
receptor ("EGFR") family. HER2 is overexpressed in approximately 20-
25% of human breast cancer and is expressed in many other cancers.
[0065] "HER2 binding peptides," "HER2 MHC class II binding
peptides," "binding peptides," "HER2 peptides," "immunogenic MHC class
II binding peptides," "antigen binding peptides," "HER2 epitopes," "reactive
peptides," and the like as used herein refer to MHC Class IT peptides derived
from or based on the sequence of the HER2/neu protein, a target found on
approximately 20-25% of all human breast cancers and their equivalents.
HER2 extracellular domain "ECD" refers to a domain of HER2 that is
outside of a cell, either anchored to a cell membrane, or in circulation,
including fragments thereof. HER2 intracellular domain "ICD" refers to a
domain of the HER2/neu protein within the cytoplasm of a cell. According
to a preferred embodiment HER2 epitopes or otherwise binding peptides
comprise 6 HER2 binding peptides which include 3 HER2 ECD peptides
and 3 HER2 ICD peptides.
Preferred HER2 ECD peptides comprise:
Peptide 42-56: HLDMLRHLYQGCQVV (SEQ ID NO: );
36
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Peptide 98-114: RLRIVRGTQLFEDNYAL (SEQ ID NO: 2); and
Peptide 328-345: TQRCEKCSKPCARVCYGL (SEQ ID NO: 3);
Preferred HER2 ICD peptides comprise:
Peptide 776-790: GVGSPYVSRLLGICL (SEQ ID NO: 4);
Peptide 927-941: PAREIPDLLEKGERL (SEQ ID NO: 5); and
Peptide 1166-1180: TLERPKTLSPGKNGV (SEQ ID NO: 6).
[0066] "HER2P "' is the classification or molecular subtype of a type
of breast cancer as well as numerous other types of cancer. HER2 positivity
is currently defined by gene amplification by FISH (fluorescent in situ
hybridization) assay and 2+ or 3+ on intensity of pathological staining.
[0067] "HER2"eg" is defined by the lack of gene amplification by
FISH, and can encompass a range of pathologic staining from 0 to 2+ in
most cases.
[0068] "Isolated" means altered or removed from the natural state. For
example, a nucleic acid or a peptide naturally present in a living animal is
not "isolated," but the same nucleic acid or peptide partially or completely
separated from the coexisting materials of its natural state is "isolated." An
isolated nucleic acid or protein can exist in substantially purified form, or
can exist in a non-native environment such as, for example, a host cell.
37
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[0069] The term "major histocompatibility complex" or "MHC" as
used herein is defined as a specific cluster of genes, many of which encode
evolutionary related surface proteins involved in antigen presentation, which
are among the most important determinants of histocompatibility. Class I
MHC, or MHC class I, function mainly in antigen presentation to CD8 T
lymphocytes. Class II MHC, or MHC class II, function mainly in antigen
presentation to CD4+ T lymphocytes (T-helper cells).
[0070] "Mature DC" as used herein means a dendritic cell that
expresses molecules, including high levels of MHC class II, CD80 (B7.1)
and CD86 (B7.2) molecules. In contrast, immature DCs ("iDCs" or "IDCs")
express low levels of MHC class II, CD80 (B7.1) and CD86 (B7.2)
molecules, yet can still take up an antigen. "Mature DC" also refers to an
antigen presenting cell existing in vivo, in vitro, ex vivo, or in a host or
subject that may also be DC1-polarized (i.e., fully capable of promoting cell-
mediated immunity.)
[0071] "Metrics" of CD4+ Thl responses (or Thl responses) are
defined for each subject group analyzed for anti-HER2 CD4+ Thl immune
response: (a) overall anti-HER2 responsivity (expressed as percent of
subjects responding to >1 reactive peptide); (b) response repertoire
38
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
(expressed as mean number of reactive peptides (n) recognized by each
subject group); and (c) cumulative response (expressed as total sum of
reactive spots (spot-forming cells "SFC" per 106 cells from IFN-y ELISPOT
analysis) from 6 MHC Class II binding peptides from each subject group.
[0072] "Non-equivocal HER2neg is defined as non-gene amplified and
0 or 1+ on pathologic staining. "Equivocal HER2"g" is defined as non-gene
amplified but 2+ on pathologic staining.
[0073] "Responsivity" or "anti-HER2 responsivity" are used
interchangeably herein to mean the percentage of subjects responding to at
least 1 of 6 binding peptides.
[0074] "Response repertoire" is defined as the mean number ("n") of
reactive peptides recognized by each subject group.
[0075] "Sample" or "biological sample" as used herein means a
biological material from a subject, including but is not limited to blood,
organ, tissue, exosome, plasma, saliva, urine and other body fluid. A sample
can be any source of material obtained from a subject.
[0076] The terms "subject," "patient," "individual," and the like are
used interchangeably herein, and refer to any animal, or cells thereof
39
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
whether in vitro or in situ, amenable to the methods described herein. In
certain non-limiting embodiments, the patient, subject or individual is a
human.
[0077] The term "targeted therapies" as used herein refers to cancer
treatments that use drugs or other substances that interfere with specific
target molecules involved in cancer cell growth usually while doing little
damage to normal cells to achieve an anti-tumor effect. Traditional
cytotoxic chemotherapy drugs, by contrast, act against all actively dividing
cells. In breast cancer treatment monoclonal antibodies, specifically
trastuzumab/Herceptin, targets the HER2/neu receptor.
[0078] "T/C" is defined as trastuzumab and chemotherapy. This refers
to patients that receive both trastuzumab and chemotherapy before/after
surgery for breast cancer.
[0079] The terms "T-cell" or "T cell" as used herein are defined as a
thymus-derived cell that participates in a variety of cell-mediated immune
reactions.
[0080] The terms "T-helper cells," "helper T cells," "Th cells," and the
like are used herein with reference to cells indicates a sub-group of
lymphocytes (a type of white blood cell or leukocyte) including different cell
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
types identifiable by a skilled person in the art. In particular, T-helper
cells
are effector T-cells whose primary function is to promote the activation and
functions of other B and T lymphocytes and/or macrophages. Helper T cells
differentiate into two major subtypes of cells known as "Thl" or "Type 1"
and "Th2" or "Type 2" phenotypes. These Th cells secrete cytokines,
proteins, or peptides that stimulate or interact with other leukocytes. "Thl
cell," "CD4+ Th 1 cell," "CD4+ T-helper typel cell," "CD4+ T cell" and the
like as used herein refer to a mature T-cell that has expressed the surface
glycoprotein CD4. CD4+ T-helper cells become activated when they are
presented with peptide antigens by MHC class II molecules which are
expressed on the surface of antigen-presenting peptides ("APCs") such as
dendritic cells. Upon activation of a CD4+ T helper cell by the MHC-
antigen complex, it secretes high levels of cytokines such as interferon-y
("IFN-y"). Such cells are thought to be highly effective against certain
disease-causing microbes that live inside host cells, and are critical in
antitumor response in human cancer.
[0081] "Treg" "Treg" and "regulatory T-cells" are used herein to refer
to cells which are the policemen of the immune system, and which act to
regulate the anti-cancer activities of the immune system. They are increased
41
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
in some cancers, and are mediators in resistance to immunotherapy in these
cancer types.
[0082] "Therapeutically effective amount" or "effective amount" are
used interchangeably herein, and refer to an amount of a compound,
formulation, material, or composition, as described herein, that when
administered to a patient, is effective to achieve a particular biological
result.
The amount of a compound, formulation, material, or composition described
herein, which constitutes a "therapeutically effective amount" will vary
depending on the compound, formulation, material, or composition, the
disease state and its severity, the age of the patient to be treated, and the
like.
The therapeutically effective amount can be determined routinely by one of
ordinary skill in the art having regard to his/her own knowledge and to this
disclosure.
[0083] The terms "treat," "treating," and "treatment," refer to
therapeutic or preventative measures described herein. The methods of
"treatment" employ administration to a subject, in need of such treatment, a
composition or method of the present embodiments, for example, a subject
afflicted with a disease or disorder, or a subject who ultimately may acquire
such a disease or disorder, in order to prevent, cure, delay, reduce the
42
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
severity of, or ameliorate one or more symptoms of the disorder or recurring
disorder, or in order to prolong the survival of a subject beyond that
expected in the absence of such treatment.
[0084] The term "vaccine" as used herein is defined as a material used
to provoke an immune response after administration of the material to an
animal, preferably a mammal, and more preferably a human. Upon
introduction into a subject, the vaccine is able to provoke an immune
response including, but not limited to, the production of antibodies,
cytokines and/or other cellular responses.
[0085] Ranges: throughout this disclosure, various aspects of the
embodiments can be presented in a range format. It should be understood
that the description in range format is merely for convenience and brevity
and should not be construed as an inflexible limitation on the scope of the
embodiments. Accordingly, the description of a range should be considered
to have specifically disclosed all the possible subranges as well as
individual
numerical values within that range. For example, description of a range
such as from 1 to 6 should be considered to have specifically disclosed
subranges such as from 1 to 3, from 1 to 4, from 1 to 5, from 2 to 4, from 2
to 6, from 3 to 6 etc., as well as individual numbers within that range, for
43
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
example, 1, 2, 2.7, 3, 4, 5, 5.3, and 6. This applies regardless of the
breadth
of the range.
[0086] Reference will now be made in detail to several embodiments,
examples of which are also illustrated in the accompanying drawings,
photographs, and/or illustrations.
DESCRIPTION
[0087] The lifetime risk of breast cancer development is nearly one in
eight. The erb-B2 oncogene (HER-2/neu) is a molecular driver that is
overexpressed in a significant number of breast, ovarian, gastric esophageal,
lung, pancreatic, prostate and other solid tumors. HER2 overexpression
("HER2P s"), a molecular oncodriver in several tumor types including
approximately 20-25% of breast cancers, is associated with a more clinically
aggressive disease, resistance to chemotherapy, higher rates of recurrence
and metastasis, and worse overall prognosis. In incipient breast cancer,
HER2 overexpression is associated with enhanced invasiveness, tumor cell
migration, and the expression of proangiogenic factors, suggesting a critical
role for HER2 in promoting a tumorigenic environment. In a retrospective
analysis of ductal carcinoma in situ ("DCIS") patients, DCIS lesions
overexpressing HER2 were over six times as likely to be associated with
44
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
invasive breast cancer than were DCIS lesions without HER2
overexpression.
[0088] Although molecular targeting therapies targeting HER2, i.e.,
trastuzumab, has resulted in tremendous positive clinical effect in this type
of breast cancer, the almost universal resistance to the existing HER2
therapies in advanced disease states, plus disease relapse in a sizeable
proportion of women who receive the targeted therapy prove the need for
additional strategies targeting HER2. The promise of vaccines that activate
the immune system against HER2 which seek to mitigate tumor progression
and preventing recurrence while encouraging, is yet to be fully realized.
Therefore there remains a need for additional tests and therapies to diagnose
and treat HER2 breast cancer. The present embodiments described herein
address these issues.
[0089] The role of systemic anti-HER2 CD4+ Thl responses in HER2-
driven breast tumorigenesis, however, remains unclear. The embodiments
described herein are based on the identification of a progressive loss of anti-
HER2 CD4+ Thl response across a tumorigenic continuum in HER2" -
breast cancer, which appears to be HER2-specific and regulatory T-cell
(Treg)-independent. Specifically, there is an inverse correlation of anti-HER2
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
CD4+ Th1 responses with HER2 expression and disease progression.
Additionally, the depressed anti-HER2 Thl responses in HER2P"-invasive
breast cancer were differentially restored after HER2-pulsed type-1
polarized dendritic cell ("DC1") vaccinations, but the depressed responses
were not restored following HER2-targeted therapy with trastuzumab and
chemotherapy ("TIC") as will be detailed herein or by other standard
therapies such as surgical resection or radiation. The restored anti-HER2
Thl responses also appear to be durable for at least about six months or
longer.
[0090] = Preferred embodiments described herein provide materials and
methods for generating, and detecting the circulating anti-cancer CD4+ Thl
response in mammalian subjects. Blood tests/assays are provided which
generate a circulating anti- cancer CD4+ Thl response (i.e., IFN-y-secreting)
and the resulting IFN- y production is detected and measured. In other
preferred embodiments, subject blood samples containing CD4+ Th I cells
and antigen-presenting cells or precursors thereof are pulsed with MHC
class II immunogenic peptides based on the type of cancer the subject is
afflicted with and which are capable of inducing an immune response in said
subject. Preferably the antigen-presenting cells or precursors thereof are
mature or immature dendritic cells or monocyte precursors thereof. In
46
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
particularly preferred embodiments, the cancer is preferably HERZ-
expressing and the mammalian subject is preferably a human, and more
preferably the cancer is HER2P ' breast cancer and the human subject is a
female.
[0091] The herein
identified anti-HER2 CD4+ Thl response decrement
allows the detected immune response generated in such blood tests to be
used as a cancer diagnostic/response predictor alone or in tandem with the
use of specialized vaccines to restore a patient's immune response. The
preferred embodiments described herein thus shift the focus of cancer
diagnosis and therapy to patient immunity and use of blood tests to
determine and/or predict the immune response against a cancer, including
patients at risk for recurrence, as opposed to diagnosis and treatment
methods that rely on identification of tumor cells.
[0092] A preferred
embodiment is provided for generating a circulating
anti-HER2 CD4 Thl response in a mammalian subject by isolating
unexpanded peripheral blood mononuclear cells ("PBMCs") from a subject
and pulsing the PBMCs with a composition comprising HER2-derived MHC
class II antigenic binding peptides capable of generating an immune
response in the subject. Without wishing to be bound by any particular
47
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
theory, when the binding peptides are presented to CD4+ Thl cells that are
present in the PBMC sample they activate the CD4+ Thl cells and the
activated CD4+ Thl cells produce interferon-7 ("IFN-7"). DC1s (type-1
polarized dendritic cells) derived from precursor pluripotent monocytes
contained in the subject's PBMC sample are antigen-presenting cells
("APCs") which upon exposure to the binding peptides become antigen-
loaded APCs which present the MHC class II antigen binding peptides to the
subject's CD4+.Th1 cells in the sample thereby activating the CD4+ Thl cells
to produce/secrete IFN-7. The IFN-7 thereby produced is subsequently
measured for analysis.
[0093] In an alternate preferred embodiment, a circulating anti HER2
CD4+ Thl response is generated in a mammalian subject by co-culturing
previously unstimulated purified CD4+ T-cells from a subject blood sample
with autologous immature or mature dendritic cells ("iDCs" or "mature
DCs", collectively, "DCs") pulsed with a composition comprising HER2-
derived MHC class II antigenic binding peptides capable of generating an
immune response in the subject. Without wishing to be bound by any
particular theory, when the binding peptides are presented to CD4+ Th 1 cells
present in the T-cell sample they activate the CD4+ Thl cells and the
activated CD4+ Thl cells produce/secrete IFN-7. The immature DCs are
48
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
matured to DC l's, which present the MHC class II binding peptides to the
subject's CD4+ Thl cells that are present in the sample thereby activating the
CD4+ Thl cells to produce IFN-7, which is subsequently measured for
analysis.
[0094] In both alternate preferred embodiments for generating anti-
HER2 immune response in a subject, IFN- y produced by anti-HER2 CD4-
Thl cells is detected and measured via IFN- 7 enzyme-linked immunospot
("ELISPOT") assay, although it should be understood by one skilled in the
art that other detection methods may be used. For example, flow cytometry,
enzyme-linked immunosorbent assay ("ELISA"), and immunofluorescence
("IF") can be used for monitoring immune response. Alternatively, in
instances of immune monitoring of patients, it can be advantageous to
measure the ratio of IFN-7 to IL-10 (as was done in the Reference Example
and shown in Figure 8E) as opposed to, or in addition to, a straight IFN-7
test such as ELISPOT which shows total CD4+ cell spots. Such testing
would be particularly advantageous for patients at risk. Further, the use of
immunofluorescence provides other ways to measure and visualize immune
response via use of ELISPOT readers that read results by fluorescence. In
such instances the results can be arranged to show 2, 3, or more
49
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
cytokines/other secreted immune molecules, each showed in a different
color, in the same patient sample.
[0095] Those skilled in the art can readily appreciate, other suitable
APC's may be used in addition to dendritic cells and monocytes, such as, for
example, macrophages, and B cells.
[0096] In preferred embodiments IFN-y ELISPOT assays are
performed to detect IFG-y production (positive peptide response: threshold
minimum 20 SFC/2x105 and 2x greater than unstimulated control). Results
are preferably expressed as three metrics of Thl response: (a) overall anti-
HER2 responsivity (expressed as percent of subjects responding to >1
reactive peptide); (b) response repertoire (expressed as mean number of
reactive peptides (n) recognized by each subject group); and (c) cumulative
response (expressed as total sum of reactive spots (spot-forming cells "SFC"
per 106 cells from IFN-y ELISPOT analysis) from all 6 MHC class II
binding peptides from each subject group.
[0097] In preferred embodiments for HER2I's cancers, DCs, immature
or type-1 polarized DC is, are pulsed with a composition comprising 6 MHC
class II binding peptides derived from or based on HER2 that are capable of
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
generating an immune response in a patient. HER2 MHC class II binding
peptides or epitopes include:
Peptide 42-56: HLDMLRHLYQGCQVV (SEQ ID NO: 1);
Peptide 98-114: RLRIVRGTQLFEDNYAL (SEQ ID NO: 2);
Peptide 328-345: TQRCEKCSKPCARVCYGL (SEQ ID NO: 3);
Peptide 776-790: GVGSPYVSRLLGICL (SEQ ID NO: 4);
Peptide 927-941: PAREIPDLLEKGERL (SEQ ID NO: 5); and
Peptide 1166-1180: TLERPKTLSPGKNGV (SEQ ID NO: 6).
In embodiments where donors have A2.1 blood type HER2 MHC class I
peptides or epitopes include:
Peptide 369-377: KIFGSLAFL (SEQ ID NO: 7); and
Peptide 689-697: RLLQETELV (SEQ ID NO: 8).
[0098] As described further herein, the HER2 binding
peptides/epitopes of the preferred embodiments are not limited to the six
above-referenced peptides and also include peptides that are functional
equivalents or alternatives of the binding peptides identified by SEQ ID
NOS: 1-6 as will be discussed in more detail herein. There are additional
class I peptides that may be used for subjects with A2.1 and A3.1 blood
types as well as other blood types (e.g., AS, A6) which comprise class I
peptides that bind any phenotype.
51
CA 02942721 2016-09-13
WO 2015/139003
PCT/U52015/020613
[0099] There are many other HER2P" solid cancers in addition to
breast cancer, such as, for example, brain, bladder, esophagus, lung,
pancreas, liver, prostate, ovarian, colorectal, and gastric, and others, for
which the materials and methods of the embodiments described herein can
be used for diagnosis and treatment. Therefore the six anti-HER2 binding
peptides described above may be used in accordance with the herein
embodiments to generate immune responses capable of detection and useful
for diagnostics for these and other HER2-expressing cancers.
[00100] Vaccines can be developed to target HER2-expressing tumors
using the same anti-HER2 binding peptides described above or may employ
any composition of HER2 that is immunogenic such as, for example, DNA,
RNA, peptides, or proteins or components thereof such as the ICD and ECD
domains. For example, subjects can be vaccinated against the whole HER2
protein and the six above-referenced binding peptides can be used to monitor
the patient's immune response. Similarly vaccines can be developed for
other types of cancer such as other members of the HER2 family which
includes HER1, HER3, and c-MET.
[00101] Although the present preferred embodiments are directed to
treating and diagnosing HER2P s breast cancer in women it should be readily
52
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
appreciated by the skilled artisan that the present embodiments are not
limited to female humans. The presently preferred embodiments includes
male humans, for example, HER2-expressing prostate cancer, as well as
other mammalian subjects
Compositions
[00102] The preferred embodiments include use of isolated peptides
derived from or otherwise based on the HER2 protein. The binding peptides
of the preferred embodiments represent epitopes of the corresponding HER2
protein. Although a presently preferred embodiment features six HER2
MHC class II binding peptides/epitopes, other possible MHC class II HER2
peptides can be used in the present embodiments in that any components of
the entire HER2 molecule can be used as a source for other binding peptides
so long as they are sufficiently immunologically active in patients.
[00103] In preferred embodiments the HER2 binding peptides comprise
six HER2 MHC class II binding peptides, having the sequences:
Peptide 42-56: HLDMLRHLYQGCQVV (SEQ ID NO: 1);
Peptide 98-114: RLRIVRGTQLFEDNYAL (SEQ ID NO: 2);
Peptide 328-345: TQRCEKCSKPCARVCYGL (SEQ ID NO: 3);
Peptide 776-790: GVGSPYVSRLLGICL (SEQ ID NO: 4);
Peptide 927-941: PAREIPDLLEKGERL (SEQ ID NO: 5); and
53
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Peptide 1166-1180: TLERPKTLSPGKNGV (SEQ ID NO: 6).
[00104] The HER2 epitope identified by SEQ ID NO: 1 represents
positions 42-56 of the HER2 protein. The HER2 epitope identified by SEQ
ID NO: 2 represents positions 98-114 of the HER2 protein. The HER2
epitope identified by SEQ ID NO: 3 represents positions 328-345 of the
HER2 protein. The HER2 epitope identified by SEQ ID NO: 4 represents
positions 776-790 of the HER2 protein. The HER2 epitope identified by
SEQ ID NO: 5 represents positions 927-941 of the HER2 protein. The
HER2 epitope identified by SEQ ID NO: 6 represents positions 1166-1180
of the HER2 protein.
[00105] Further, the skilled artisan can further appreciate that
embodiments described herein are not limited to the use of all 6 of the
binding peptides described in connection with preferred embodiments
herein. Any number of the described binding peptides may be employed in
patient blood tests, with the lower range being about two or three, with the
caveat that there must be sufficient immunological activity with the patient's
CD4+ t-cells so as to cause production of IFN-y. Therefore in instances
where HER-derived Class II biding peptides are used which are fewer
than/different than those of the set of six described in connection with the
preferred embodiments herein, the number of binding peptides may well be
54
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
substantially less than or greater than six depending on the immune
responses generated in subjects.
[00106] As described herein, the HER2 binding peptides of the
preferred embodiments also encompass peptides that are functional
equivalents of the peptides identified by SEQ ID NOS: 1-6. Such functional
equivalents may have an altered sequence in which one or more of the amino
acids in the corresponding HER2 peptide sequence are substituted or in
which one or more amino acids are deleted from or added to the
corresponding reference sequence. For example, 1 to 3 amino acids may be
added to the amino terminus, carboxy terminus, or both. In some examples,
the HER2 peptides can be glycosylated.
[00107] The HER2 binding peptides or any peptide in accordance with
the present embodiments may be cyclized or linear. When cyclized, the
epitopes may be cyclized in any suitable manner. For example, disulfide
bonds may be formed between selected cysteine ("Cys") pairs in order to
provide a desired confirmation. It is believed that the formation of cyclized
epitopes may provide conformations that improve the immune response.
[00108] In other instances, the HER2 binding peptides may be the retro-
inverso isomers of the HER2 binding peptides. The retro-inverso
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
modification comprises the reversal of all amide bonds within the peptide
backbone. This reversal may be achieved by reversing the direction of the
sequence and inverting the chirality of each amino acid residue by using D-
amino acids instead of the L-amino acids. This retro-inverso isomer form
may retain planarity and conformation restriction of at least some of the
peptide bonds.
[00109] Non-conservative amino acid substitutions and/or conservative
substitutions may also be made. Substitutions are conservative amino acid
substitutions when the substituted amino acid has similar structural or
chemical properties with the corresponding amino acid in the reference
sequence. By way of example, conservative amino acid substitutions
involve substitution of one aliphatic or hydrophobic amino acid, e.g.,
alanine, valine, leucine and isoleucine, with another; substitution of one
hydroxyl-containing amino acid, e.g., serine and threonine, with another;
substitution of one acidic residue, e.g., glutamic acid or aspartic acid, with
another; replacement of one amide-containing residue, e.g., asparagine and
glutamine, with another; replacement of one aromatic residue, e.g.,
phenylalanine and tyrosine, with another; replacement of one basic residue,
e.g., lysine, arginine and histidine, with another; and replacement of one
56
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
small amino acid, e.g., alanine, serine, threonine, methionine, and glycine,
with another.
[00110] In some instances, the deletions and additions are located at the
amino terminus, the carboxy terminus, or both, of one of the sequences of
the binding peptides of the preferred embodiments. For example, a HER2
binding peptide equivalent has an amino acid sequence which is at least 70%
identical, at least 80% identical, at least 85% identical, at least 90%
identical, at least 91%, at least 92%, at least 93%, at least 94%, at least
95%,
at least 96%, at least 97%, at least 98%, or at least 99% identical to the
corresponding HER2 binding peptide sequences. Sequences which are at
least 90% identical have no more than 1 alteration, i.e., any combination of
deletions, additions or substitutions, per 10 amino acids of the reference
sequence. Percent identity is determined by comparing the amino acid
sequence of the variant with the reference sequence using known or to be
developed programs in the art.
[00111] For functional equivalents that are longer than a corresponding
HER2 binding peptide sequence, the functional equivalent may have a
sequence which is at least 90% identical to the HER2 peptide sequence and
57
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
the sequences which flank the HER2 peptide sequences in the wild-type
HER2 protein.
[00112] Functional equivalents of the HER2 binding peptides may be
identified by modifying the sequence of the peptide and then assaying the
resulting polypeptide for the ability to stimulate a subject's monocytes, DC's
or other antigen-presenting cells that present the binding peptides/epitopes
to
CD4+ Thl cells.
[00113] In accordance with other embodiments, chimeric peptides and
compositions comprising one or more chimeric peptides are provided.
According to various embodiments, the chimeric peptides comprise a HER2
peptide, another peptide, and a linker joining the HER2 peptide to the other
peptide. It will be further understood that any suitable linker may be used.
For example, depending upon the peptide used, the HER2 binding peptide
may be linked to either the amino or the carboxy terminus of the other.
binding peptide. The location and selection of the other peptide depends on
the structural characteristics of the HER2 peptide, whether alpha helical or
beta-turn or strand.
[00114] In another embodiment, the linker may be a peptide of from
about 2 to about 15 amino acids, about 2 to about 10 amino acids, or from
58
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
about 2 to about 6 amino acids in length. The chimeric peptides may be
linear or cyclized. Additionally, the HER2 peptides, the other peptides,
and/or the linker may be in retro-inverso form. Thus the HER2 peptide
along could be in retro inverso form. Alternatively, the HER2 peptide and
the other peptide could be in retro inverso form. In another example, the
HER2 peptide, the other epitope, and the linker could be in retro inverso
form.
[00115] Peptides, including chimeric peptides can be prepared using
well known techniques. For example, the peptides can be prepared
synthetically, using either recombinant DNA technology or chemical
synthesis. Peptides of the present embodiments may be synthesized
individually or as longer polypeptides composed of two or more peptides.
The peptides of the presently preferred embodiments are preferably isolated,
i.e., substantially free of other naturally occurring host cell proteins and
fragments thereof.
[00116] The peptides and chimeric peptides of the present embodiments
may be synthesized using commercially available peptide synthesizers. For
example, the chemical methods described in Kaumaya, P.T.P., et al., "De
Novo" Engineering of Peptide Immunogenic and Antigenic Determinants as
59
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Potential Vaccines, in Peptides, Design, Synthesis and Biological Activity,
pp 133-164 (1994), may be used. For example, HER2 binding peptides may
be synthesized co-linearly with another binding peptide to form a chimeric
peptide. Peptide synthesis may be performed using Fmoc/t-But chemistry.
The peptides and chimeric peptides may be cyclized in any suitable manner.
For example, disulfide bonds may be achieved using differentially protected
cysteine residues, iodine oxidation, the addition of water to boost removal of
Acm group and the concomitant formation of a disulfide bond, and/or the
silyl chloride-sulfoxide method.
[00117] The peptides
and chimeric peptides may also be produced using
cell-free translation systems and RNA molecules derived from DNA
constructs that encode the epitope or binding peptide. Alternatively, the
epitopes or chimeric peptides may be made by transfecting host cells with
expression vectors that comprise a DNA sequence that encodes the
respective epitope or chimeric peptide and then inducing expression of the
polypeptide in the host cells. For recombinant production, recombinant
constructs comprising one or more of the sequences which encode the
binding peptide epitope, chimeric peptide, or a variant thereof are introduced
into host cells by conventional methods such as calcium phosphate
transfection, DEAE-dextran mediated transfection, microinjection, cationic
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
lipid-mediated transfection, electroporation, transduction, scrape lading,
ballistic introduction or infection.
[00118] The binding peptides of the present embodiments may contain
modifications, such as glycosylation, side chain oxidation, or
phosphorylation; so long as the modifications do not destroy the biological
activity of the binding peptides. Other modifications include incorporation
of D-amino acids or other amino acid mimetics.
[00119] The binding peptides of the embodiments can be prepared as a
combination, which includes two or more peptides. The peptides may be in
a cocktail or may be conjugated to each other using standard techniques.
For example, the peptides can be expressed as a single polypeptide
sequence. The peptides in the combination may be the same or different.
[00120] The present embodiments should also be construed to
encompass "mutants," "derivatives," and "variants" of the peptides of the
embodiments (or of the DNA encoding the same) which mutants, derivatives
and variants are peptides which are altered in one or more amino acids (or,
when referring to the nucleotide sequence encoding the same, are altered in
one or more base pairs) such that the resulting peptide (or DNA) is not
61
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
identical to the sequences recited herein, but has the same biological
property as the peptides disclosed herein.
[00121] Some embodiments also provide a polynucleotide encoding at
least one peptide selected from a peptide having the sequence of any one or
more of SEQ ID NOS: 1-6. The nucleic acid sequences include both the
DNA sequence that is transcribed into RNA and the RNA sequence that is
translated into a peptide. According to other embodiments, the
polynucleotides are inferred from the amino acid sequence of the peptides of
the preferred embodiments. As is known in the art several alternative
polynucleotides are possible due to redundant codons, while retaining the
biological activity of the translated peptides.
[00122] Further, preferred embodiments encompass an isolated nucleic
acid encoding a peptide having substantial homology to the binding peptides
disclosed herein. Preferably, the nucleotide sequence of an isolated nucleic
acid encoding a peptide of the invention is "substantially homologous", that
is, is about 60% homologous, more preferably about 70% homologous, even
more preferably about 80% homologous, more preferably about 90%
homologous, even more preferably, about 95% homologous, and even more
62
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
preferably about 99% homologous to a nucleotide sequence of an isolated
nucleic acid encoding a binding peptide of preferred embodiments.
[00123] It is to be understood explicitly that the scope of the preferred
embodiments encompasses homologs, analogs, variants, derivatives and
salts, including shorter and longer peptides and polynucleotides, as well as
peptide and polynucleotide analogs with one or more amino acid or nucleic
acid substitution, as well as amino acid or nucleic acid derivatives, non-
natural amino or nucleic acids and synthetic amino or nucleic acids as are
known in the art, with the stipulation that these modifications must preserve
the immunological activity of the original binding peptide. Specifically any
active fragments of the active binding peptides as well as extensions,
conjugates and mixtures are encompassed according to the principles
described herein.
[00124] The preferred embodiments should be construed to include any
and all isolated nucleic acids which are homologous to the nucleic acids
described and referenced herein, provided these homologous DNAs have the
biological activity of the binding peptides disclosed herein.
[00125] The skilled artisan will understand that the nucleic acids of the
preferred embodiments encompass an RNA or a DNA sequence encoding a
63
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
peptide of a preferred embodiment, and any modified forms thereof,
including chemical modifications of the DNA or RNA which render the
nucleotide sequence more stable when it is cell free or when it is associated
with a cell. Chemical modifications of nucleotides may also be used to
enhance the efficiency with which a nucleotide sequence is taken up by a
cell or the efficiency with which it is expressed in a cell. Any and all
combinations of modifications of the nucleotide sequences are contemplated
in the preferred embodiments.
[00126] Further, any number of procedures may be used for the
generation of mutant, derivative or variant forms of a peptide of the
preferred embodiments using recombinant DNA methodology well known
in the art such as, for example, that described in Sambrook and Russell,
supra, and Ausubel et at., supra. Procedures for the introduction of amino
acid changes in a peptide or polypeptide by altering the DNA sequence
encoding the polypeptide are well known in the art and are also described in
these, and other, treatises.
[00127] The nucleic acids encoding the binding peptides of the
preferred embodiments can be incorporated into suitable vectors e.g.,
retroviral vectors. These vectors are well known in the art. The nucleic
64
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
acids or the vectors containing them usefully can be transferred into a
desired cell, which cell is preferably from a patient.
Cryopreservation
[00128] After PBMCs are obtained from subjects and separated, they
can be cryopreserved before performing any blood tests/assays described
herein using methods well known to the skilled artisan.
Use As Diagnostic/Prognostic/Treatment Monitoring Tool
[00129] As described herein, it has been found that loss of circulating
HER2-reactive IFN-y 6 CD4+ Thl cells begins as early as DCIS breast
cancer and substantially declines in early invasive Stage I HER2P ' tumors.
More specifically, there is a stepwise anti-HER2 CD4+ Thl response
decrement across the continuum in breast tumorigenesis from healthy donors
to HER2P ' DCIS (ductal carcinoma in situ) to HER2P ' IBC (invasive breast
cancer). There are reasons for what this loss of immune response is not due
to, namely, it is not due to cancer-related immuno suppression, it is not
likely
related to an increase in translocation to invasive lesions, and it is
independent of regulatory T cells.
[00130] An embodiment based on this finding of loss of anti-HER2
CD4+ Thl immune response provides a method for screening apparently
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
healthy individuals for breast and other cancers that might not be detected
via mammography or other screening approaches, comprising performing
rapid immune tests/assays, the blood tests of the preferred embodiments, for
detecting anti-HER2 CD4- Thl responsiveness. Test results for such
individuals that are lower than those for healthy individuals, would allow for
more definitive testing and quicker exercising of therapeutic options. For
example, the blood tests herein can be advantageously used to identify
patients at risk in whom vaccination may be considered to reduce risk of
HER-2 expressing breast cancer. For instance, patients at risk may be those
following completion of lactation, pregnancies, and other life stressing
events that may reduce the response.
[00131] Such a screening method can also be beneficial for patients at
high risk for developing breast cancer, due to factors such as genetic
disposition or lifestyle factors. From a diagnostic perspective, an immune
biomarker can be developed to screen such high-risk patients for fluctuations
in their anti-HER2 Thl immunity. While IHC staining or FISH profiling of
breast biopsy specimens offer only an isolated snapshot of a tumor's
evolution, immune profiling (such as with this potential biomarker) may
provide a glimpse into the natural history and immune repercussions of a
tumor. It can be used to predict patients diagnosed with any breast cancer
66
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
whether they may be at risk for a HER-2 expressing new breast event or
recurrence.
[00132] In another embodiment, diagnostic or monitoring tests based on
loss of anti-HER2 Thl response may be used to predict whether a patient
with HER2P" breast cancer will respond well to standard non-immune
therapy such as chemotherapy plus trastuzumab.
[00133] According to a further embodiment, as will be detailed herein,
CD4+ Thl responses are capable of being preferentially restored via
autologous DC1 vaccination with HER2-derived Class II peptides (DC1
immunization) as compared with targeted (e.g., trastuzumab) or
conventional (i.e., chemotherapy) breast cancer therapies. As such, in
HER2Pc's-IBC patients, CD4+ Thl responses were effectively restored after
HER2-pulsed DC vaccination, but not following trastuzumab/cytotoxic
chemotherapy ("T/C") treatment. The blood tests of the preferred
embodiments are therefore performed pre-vaccination and post-vaccination
to determine the extent of restoration, or non-restoration of Thl immune
response. Thus a patient can have their CD4+ Thl response easily
reevaluated after breast cancer therapy via the blood tests herein to
67
CA 02942721 2016-09-13
WO 2015/139003
PCT/U52015/020613
determine if any previously found CD4 Th1 immune loss has been restored
by vaccination.
[00134] In another embodiment, a blood test relying on the anti HER2
CD4+ Thl response decrement may be used to determine whether DC1
vaccination has adequately restored or increased anti-HER2 immunity to
levels capable of providing protection against further incursions of cancer.
Post-DC1 vaccination, the blood tests of the preferred embodiments can be
performed numerous times, preferably on a schedule as recommended by the
patient's physician, so as to track the patient's CD4+ Thl immune status.
These additional tests may take place many months, e.g., at least up to about
60 months or more, after vaccination due to the durability of the vaccine-
induced sensitization to the HER2 tumor target and the likeliness of
protection over long periods of time.
[00135] Use of the blood tests herein may be used to show the degree of
HER2-responsiveness post-chemo/trastuzumab treatment for HER2-
expressing invasive breast cancer patients. A correlation is shown herein
with how well such patients will respond to therapy, and thereby are
predictive of outcome. For example, depressed anti-HER2 thl responses
68
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
predict an increased risk of subsequent recurrence in adjuvant-T/C-treated
patients.
[00136] Other embodiments provide methods for an immune strategy,
i.e., DC vaccination, to enhance or restore the anti-HER2 CD4+ Thl loss
found in HER2P' invasive breast cancer patients. This capacity for
"immunorestoration" can be exploited for therapy in combination with
current trastuzumab regimens. It would also provide a rationale for
combining vaccination with standard therapies including chemotherapy plus
trastuzumab.
[00137] Another embodiment suggests an immune correlate for
predicting risk of new breast events. In HER2P"-IBC patients treated with
chemotherapy/trastuzumab, it was shown that response variations, and more
particularly, depressed anti-HER2 CD4+ Thl responses, are associated with
an increased risk of new breast cancer events. Thus such depressed
responses can be used to predict outcomes as to whether a patient will likely
endure a new breast event and if so, will likely require additional therapy. A
biomarker can thus be developed based thereon.
[00138] Further, the observation described herein that HER2P" breast
cancer cells, expressing IFN-y and TNF-a receptors, undergo apoptosis upon
69
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
exposure to Thl-derived cytokines (including IFN-y and TNF-a, the
archetypical cytokines produced by Thl cells) suggests that anti-HER2 Thl
cells may be instrumental in controlling or eliminating HER2-expressing
cells during physiologic processes such as breast involution. IFN-y and
TNF-u receptor expression was found on all HERD' breast cancer cell lines
tested as described herein, and it was seen that these anti-HER2 CD4+ Thl
cells produce soluble factors that cause apoptosis of HER2-expressing breast
cancer cell lines. This suggests that anti-HER2 Till may be instrumental in
controlling or eliminating HER2-expressing cells during physiologic
processes such as breast involution and may explain how CD4+ Thl cells,
which cannot recognize Class II"g Class P" tumor cells, can nonetheless
mediate tumor cell destruction.
[00139] As described
in detail herein, a further embodiment provides an
immune correlate for predicting pathologic responsiveness to standard
neoadjuvant therapy in HER2P's breast cancer. Experiments were designed
to study how the degree of HER2 responsiveness post-chemo/trastuzumab
treatment for HER2-expressing invasive breast cancer patients correlates
with how well they will respond to therapy. Such experiments revealed an
immune correlate for predicting pathologic responsiveness to standard
neoadjuvant therapy. An association was found between neoadjuvant
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
complete responders and significantly higher anti-HER2 CD4+ Thl
responses, compared with patients who did not have pathologic complete
responses.
[00140] While the magnitude of HER2-specific Thl depression for TIC-
treated HER2P s-IBC patients correlates with an increased risk of subsequent
recurrence of new breast events, in contrast, the above-described
preservation of anti-HER2 CD4' Thl immunity is associated with complete
pathologic response to neoadjuvant chemotherapy. Taken together, these
data suggest that anti-HER2 Thl immune reactivity may be used as a
biomarker to help identify vulnerable patient subgroups at risk of clinical or
pathologic failure.
[00141] Although the present embodiments as described herein may
include specific reference to HER2-expressing breast tumors, it should be
understood by those skilled in the art that other types of HER2-expressing
tumors such as, for example only, ovarian, gastric esophageal, lung,
pancreatic, liver, prostate and other solid tumors, may benefit from the
teachings of the present embodiments. Similarly, those skilled in the art can
appreciate that the teachings herein can extend to non-HER2-expressing
71
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
breast cancer, including triple-negative and ER-positive as well as other
tumors.
[00142] Additionally, there are other HER family targets from the
receptor tyrosine kinase family that can be used in accordance with the
preferred embodiments. The HER family consists of four related signaling
molecules: HER1, HER2, HER3, and HER4 that are involved in a variety of
cancers. While it is known that over-expression of HER-2 is found in about
20% to 25% of breast cancers, it has been found that other HER family
members are involved in both early and invasive breast cancer, as well as
other cancers. For example, HER1 is expressed on a small number of breast
cancers, generally those that are triple negative. C-Met is a growth factor
receptor involved in recurrence of many cancers that activates HER3. HER3
is over-expressed in colon, prostate, breast and melanoma. HER3 is
expressed in a large number of DCIS lesions and breast cancers. HER3 can
be detected in the residual DCIS at the time of surgery in some patients who
received the DC1 HER2 vaccination. Thus, other HER family targets such
as HER3, HER1 and c-Met that cause breast cancer and other solid cancers
may be beneficially targeted and peptide vaccines against these other targets
developed as was done for HER2 described herein. Accordingly, a breast
cancer panel containing oncodrivers/proposed oncodrivers such as HER2,
72
CA 02942721 2016-09-13
WO 2(115/139003
PCT/US2015/020613
HER3, HER1 and C-Met for identifying which molecules are expressed in a
patient's breast tumor can be developed as a therapy aid and used as vaccine
target molecules. Thus it is contemplated that in addition to the DC1
vaccine described herein for HER2 similar vaccines can be developed for the
non-HER2-expressing breast cancer types.
EXAMPLES
[00143] The preferred embodiments are further described in detail by
reference to the following experimental examples. These examples are
provided for purposes of illustration only, and are not intended to be
limiting
unless otherwise specified. Thus, the preferred embodiments should in no
way be construed as being limited to the following examples, but rather,
should be construed to encompass any and all variations which become
evident as a result of the teaching provided herein.
[00144] Without further description, it is believed that one of ordinary
skill in the art can, using the preceding description and the following
illustrative examples, make and utilize the present embodiments and practice
the claimed methods. The following working examples therefore,
specifically point out the preferred embodiments, and are not to be construed
as limiting in any way the remainder of the disclosure.
73
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00145] The following Reference Example includes Methods, Results,
and Discussion sections.
[00146] REFERENCE EXAMPLE
METHODS
Patient Selection and Study Design
[00147] After approval by the Institutional Review Board of the
University of Pennsylvania, 143 patients were consecutively recruited to
participate in the presently described study and informed consent was
obtained. Anti-HER2 CD4+ Thl ("Thl")responses were examined in
healthy donors ("HD") (n=21), and patients with benign breast disease
("BD") ( n=10), HER2Ileg-DCIS (n=11), HER2"g (0/1+) IBC (n=11),
HER2P"-DCIS (n=31), and HER2P'9-IBC (n=22) patients. Th 1 responses of
patients enrolled in neoadjuvant DC1 immunization trials for HER2P 6-
DCIS and found to have Stage I HER2P 6-IBC at surgery (n=11), were
analyzed pre- and post-immunization (immediately and >6 months after).
Thl responses in treatment-naive HER2P 9-IBC patients were compared with
responses in T/C-treated Stage I-III HER2P"-IBC patients (n=37). Figure 1
shows study-eligible patient and donor cohorts. TIC-treated HER2P`9-IBC
patients were surveilled for development of BEs, defined as any locoregional
74
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
or distant recurrence. Table 1 below shows the demographic and tumor-
related characteristics of the present study populations (age, race, AJCC
pathological stage, hormone receptor status, timing of chemotherapy, and
time from completion of trastuzumab (when applicable) for individual
patient subgroups) ("IBC":invasive breast cancer; "DCIS": ductal carcinoma
in situ; "T/C": trastuzumab/chemotherapy). Following TIC treatment,
HER2P"-IBC patients were observed for development of subsequent breast
events ("BEs"), defined as any locoregional or distant recurrence. Thl
immune responses of all subjects were generated and analyzed
prospectively.
Table 1
o
k.4
=
Characteristic Healthy Donor Benign Breast HER2neg-DCIS
HER2neg-IBC HER2pos HER2pos IBC TIC-treated .
cn
n=21 Disease n=10 n=11
(0 or 1+) n=11 DCIS n=31 n=22 HER2pos-IBC ---
...,,
n=37
ca
_
o
Age
(..)
Mean + SE 45.1 + 2.7 .42.3 + 4.6 53.3 + 2.4 58.5 5.7
54.3 1.8 56.8+ 3.1 53.0 + 2.2
Range 28 - 63 22 - 66 , 21 -54 28 - 83
35 - 83 36 - 88 28 - 85
_
# % # % # I % # %
# %
Race/Ethnicity
Caucasian ., 15 71.4 8 _ 80.0 8 _ 72.7
, 10 90.9 26 83.9 16 72.7 30 81.1
African-American _ 1 4.8 220 0
_ . 1 _ 9.1 ,_ 1 9.1 4_ 12.9 _ 3 13.6 5
13.5
Asian _ 5 23.8 0 0 1 9.1 0 0
1 3.2 2 9.1 , 0 0.0 Q
_
.
Hispanic 0 0 0 0 1 9.1 0 00
_
0 1 4.5 2 _ 5.4
r.,
AJCC Stage ..
.
r.,
.
-]
Stage 1 MOMEMORAMONWEIMASOMMORMENOM 9 81.8 ilinglipailipii] 16 72.7
8 21.6 N,
1--
,Stage 2 ill!'.1.,MONEWNEMKNOWNMOVOIMM:mgiail:Ma 1 9.1 MiEggiNi 6
27.3 20 54.1 N,
.--1
1--
c,
Stage 3 .:lei'.i.ailVilinaMMUMBENNOMES0..::MMENNiggi:!.: 1 9.1 MailBERN 0
0 9 _ 24.3 .
,
_
.
Hormone receptor status
.
,
.
_
-
ERpos gingannagningaigannigiilign 11 100 9 81.8
19 61.3 12 54.5 21 56.8 1--
,..
-
,
PRI:vs li!iiifilMgi;]':gaiMMUNNininMnifiMilL 11 100 9 81.8
17 54.8 11 50.0 19 _ 51.4
_
Chemotherapy sequence._ ..
_
Nenadjuvant ANIMENNINVEIBMOVEMMORRINIONEROMI 0 0
a:iiigineiMiiiiiiEiiid!imiii]aiiiiiiimiNgtiF 12 _ 32.4
_
Adjuvant aring.gnage.n.nangagannargningategn 5 45.5 igannegailegianMaima
25 67.6
None ang..MENRIBROMMONENINNON.EN 6
54.5 MIEMMEMINEWNOMMIL 0 , 0
_ Time from completion of -trastuzumab to study enrollment
_õ .õ .., .. ...,.....
<6 months
EMONSMOMR:njlingninEinineng.'NO.ORKiMMEMMAME=MOMMR::::MW:li.:f:MTM.:.;=i?..mi:t
i:iqggpi*i_ 16 43.2
.
>6 months
MansisystmemilaSSIBMONSEENNEENIENEMINESEMEREMBESEINSEMEMMES 21 56.8
_
r)
,-i
ct
k..0
=
u,
=
= t..3
=
c,
,-,
(..4
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Vaccine Trial Design and Immunization Procedure
[00148] Two neoadjuvant trials of HER2-pulsed, type 1-polarized DC
vaccination "DC1 vaccination" for patients with HER2Ns-DCIS were
conducted. DC vaccines were prepared as described previously. See, Koski,
G. K., et al., J. Immonother. 35(1): 54 (2012) ("Koski, et al."); Sharma, A.,
et al., Cancer 118(17):4354 (2012) ("Sharma, etal."); Fracol, M., et al., Ann.
Surg. Oncol. 20(10):3233 (2013); Lee, M. K. 4th, et al., Expert Rev.
8(11):e74698 (2013); Czemiecki, B.J., etal., Cancer Res. 67(4):1842
(2007); Czemiecki, B. J., et al., Cancer Res. 67(14):6531 (2007); and U.S.
Published Application US 2013/0183343 Al.
[00149] DC vaccination strategy used in the present studies is shown in
Figure 2. As shown therein, monocytic DC precursors (CD14+ peripheral
blood monocytes) were obtained from subjects via tandem
leukapheresis/countercunent centrifugal elutriation. DCs were cultured
overnight in macrophage serum-free medium ("SFM") (Cellgro/Mediatech,
Manassas, VA) with granulocyte macrophage colony stimulating factor
("GM-CSF") (250 IU/mL; Berlex, Wayne, NJ) and IL-4 (1000u/mL; R&D
Systems, Minneapolis, MN)-these are considered immature DCs ("iDC").
The following day iDCs were pulsed with six HER2 MHC class II binding
peptides (42-56 (SEQ ID NO: 1); 98-114 (SEQ ID NO: 2); and 328-345
77
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
(SEQ ID NO: 3) (extracellular domain of HER2), and 776-790 (SEQ ID
NO: 4); 927-941 (SEQ ID NO: 5); and 1166-1180 (SEQ ID NO:6)
(intracellular domain of HER2)) (see, Disis, M. L., et al., Clin. Can. Res.
5:1289 (1999)) (United Biochemical Research, Seattle, WA; peptides stored
lyophilized and reconstituted in sterile PBS for use). After 8-12 hours of
incubation, IFN-y (1,000 U/mL) was added. The following day, NIH
reference standard lipopolysaccharide ("LPS") was added (10 ng/mL) to
achieve full DC activation to a type 1-polarized phenotype ("DC1") 6 hours
before harvest. For HLA-A2.1P" patients, DC1s were pulsed with two
additional MHC class I binding peptides (peptide 369-377 (SEQ ID NO: 7)
and peptide 689-697 (SEQ ID NO: 8). Harvested cells were washed and lot
release criteria of >70% viability, negative Gram stain, and endotoxin <5
EU/kg were confirmed.
1001501 Intra-nodal and/or intra-lesion vaccine injection was performed
as described by Koski, et al. Briefly, immunizations were administered in
the National Institutes of Health-designated General Clinical Research
Center at the Hospital of the University of Pennsylvania. Injections
comprised 10-20 million HER2-pulsed DC 1s suspended in lml sterile
saline, and administered by ultrasound guidance into groin lymph nodes,
breast, or both. Immunizations were administered once weekly for 6 weeks,
78
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
and all patients completed 6 immunizations. Immunization-related safety
and toxicity data has been reported previously by Sharma, et al.
Immune Response Detection
[00151] Circulating anti-HER2 CD4+ Thl responses were generated
from patient unexpanded peripheral blood mononuclear cells ("PBMCs")
pulsed with the six above-referenced HER2 class II binding peptides, by
measuring IFNI/ production via enzyme-linked immunosorbent spot
("ELISPOT") assays. ELISPOT was performed according to methods
described by Koski, et al. Briefly, PVDF membrane plates (Mabtech Inc.,
Cincinnati, OH) were coated overnight with anti-IFN-y capture antibody (1-
D1K (Mabtech)). Cryopreserved PBMCs that were isolated using density
gradient centrifugation, were thawed into pre-warmed DMEM medium
supplemented with 5% human serum. After plates were washed and
blocked, PBMCs were plated in triplicate (2x105 cells/well), and the plates
were incubated at 37 C for 24-36 hours with either HER2-derived Class IT
binding peptides (41.1g) (peptide 42-56 (SEQ ID NO:1); peptide 98-114 (SEQ
ID NO:2); peptide 328-345 (SEQ ID NO:3); peptide 776-790 (SEQ ID
NO:4); peptide 927-941 (SEQ ID NO:5); and peptide 1166-1180 (SEQ ID
NO:6), media alone (unstimulated control), or positive control (anti-human
CD3 and anti-CD28antibodies (0.51.1g/mL each), both BD Pharmingen, San
79
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Diego, CA). After washing, detection antibody (7 B6-1-biotin (Mabtech);
100 lig/mL;) was added to each well, and the plates were incubated at 37 C
for 2 hours. Next, 1:1000 diluted streptavidin-horseradish peroxidase in
PBS + 0.5% FCS was added before incubation for an additional 1 hour at
37 C. TMB substrate solution (Kirkegaard & Perry Laboratories,
Gaithersburg, MD) was then added to reveal spot formation. After color
development, wells were washed with tap water. Spot forming cells
("SFC") were counted using an automated ELISPOT reader (ImmunoSpot
CTL, Cleveland, OH).
[00152] Additionally, recall Thl responses were examined by
stimulating evaluable PBMCs from specific patient subsets with 1:100-
diluted recall stimuli Candida albicans (Allen-ned Laboratories, San Diego,
CA) and tetanus toxoid (Santa Cruz Biotechnology, Dallas, TX). In order to
determine the relative functional activity of Trcg and/or Th2 phenotypes, IL-
production was measured by ELISPOT, as described by Guerkov, R.E.,
et al., J. Immunol. Meth. 279:111(2003), with 2.5 ig/m1 of anti-CD3
antibody used as a positive control.
[00153] Since inter-replicate variability in ELISPOT assays was low
(data not shown), an empiric method of determining antigen-specific
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
response was carried out. A positive response to an individual HER2 Class
II peptide was defined as: (1) threshold minimum of 20 SFC/2x105 cells in
experimental wells after subtracting unstimulated background; and (2) at
least a two-fold increase of antigen-specific SFCs over the background.
Three separate metrics of CD4+ Thl responses were defined for each patient
group: (a) overall anti-HER2 responsivity (i.e,. proportion of patients
responding to >1 peptide) ("responsivity"), (b) mean number of reactive
peptides ("response repertoire"), and (c) cumulative response across 6
peptides (reported as SFC/106 cells) ("cumulative response").
Inter-Assay Precision of ELISPOT
1001541 Inter-assay precision of ELISPOT was performed as described
previously by Maecker, H.T., et al. BMC Immunology 9:9 (2008) ("Maecker,
et al.") When the mean coefficient of variance ("CV") (three parallel
replicates over three days) was plotted against cumulative Thl response for
five donors stimulated ex vivo with a HER2 extracellular domain ("ECD")
peptide mix (peptide 42-56 (SEQ ID NO: 1); peptide 98-114(SEQ ID NO:
2); and peptide 328-345 (SEQ ID NO: 3)) a characteristic non-linear
relationship was observed. Mean CV increased dramatically as cumulative
response approached zero as shown in Figure 3A. Due to the non-linear
relationship between CV and cumulative response level, standard deviation
81
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
("SD") of three assays on 3 separate days was plotted against cumulative
Thlresponse as a measure of inter-assay variability. Id. As shown in Figure
3B, SD was found to be linearly related with the cumulative response
(connecting line represents linear regression of the SD generated, with 95%
confidence intervals of the regression shown with parallel dotted lines)
(R2=0.96. p<0.0001).
[00155] Linearity
studies were conducted in which triplicate samples of
PBMCs donated from two high-responding HER2-reactive responders were
serially diluted into PBMCs from a known allogenic non-HER2 responder,
and stimulated ex vivo with a HER2 ECD peptide mix (peptide 42-56 (SEQ
ID NO: 1); peptide 98-114(SEQ ID NO: 2); and peptide 328-345 (SEQ ID
NO: 3)). The same non-responding donor was used for all assays.
Unstimulated background was subtracted for each dilution point. A
significant linear relationship between Thl response and dilution
concentration was observed in both donors (#1: triangles; #2: circles).
Collectively, these data suggest that EISPOT assays are precise, reliable, and
reproducible.
82
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
HER2 Antibody Detection
[00156] ELISA was performed to test patient sera for endogenous IgG1
and IgG4 anti-HER2 antibodies. EIA/RIA plates were coated with HER2
ECD peptides (5 [2g/m1; Speed Biosystems, Rockville, MD) in bicarbonate
buffer, and incubated overnight at room temperature ("RT"). The following
day, plates were blocked with 1% casein in PBS, sera (1:100 dilution) added
in quadruplicate in blocking buffer, incubated for 2hours, and washed three
times before the addition of 1:500-diluted HRP-conjugated anti-human
secondary antibody specific for either IgG1 or IgG4 (Life Technologies,
Grand Island, NY). After incubation for lhour, plates were washed and
developed with TMB substrate solution (Kirkegaard & Perry Laboratories)..
Flow Cvtometry
[00157] PBMC suspensions were prepared in FACS buffer (PBS+1%
FCS+0.01% azide), and anti-human -CD3, -CD4, -CD8, -CD 83, -HLA-DR,
-CD11b, -CD33, -CD19, -CD56, -CDI 6 (all BD Biosciences, San Jose, CA),
-CD4, and -CD25 (both Biolegend, San Diego, CA) were used to determine
relative PBMC immunophenotype. After washing, cells were incubated for
30 minutes at RT with antibody mixtures. Following incubation, cells were
washed three times with FACS buffer and fixed with 2% paraformaldehyde.
Stained samples were analyzed within 24 hours. Intracellular staining of
83
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
PBMCs with anti-FoxP3 (eBioscience, San Diego, CA) using a FoxP3
fixation/permeabilization kit (Biolegend) was performed according to the
manufacturer's instructions. Flow cytometric analysis was performed using
a BD LSR-II cytometer, and datasets were analyzed using CellQuest Pr0TM
software (BD Biosciences).
Pathologic Staining
[00158] Formalin-fixed, paraffin-embedded tissue blocks from
HER2P's-DCIS and ¨IBC tumors were sectioned and stained with
hematoxylin and eosin ("H&E") to assess peritumoral lymphocytic
infiltrates. Multiplex-labeled IF (PerkinElmer, Waltham, MA) was used to
examine lymphocyte subpopulations in sample cases from HER2P s-DCIS
and -IBC tumors (see, Wang, C., et al., Journal for Immunotherapy of
Cancer 118:1(Suppl. 1) 54 (2013) ("Wang, C., et al."). Tumors were stained
for CD4, CD8, CD20, and 4',6'-diamino-2-phenylindole ("DAPI") with
same-species fluorescence labeling using tyramide signal amplification.
Images were analyzed using a Vectra multispectral microscope with pattern
recognition software to identify tumor, stroma, and T-/B- lymphocytes.
84
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Apoptosis Assays
[00159] BC cell lines with a spectrum of HER2 expression (Ithimakin,
S., et al., Can. Res. 73:1635-46 (2013)) ¨ HER2high SK-BR-3, HER2intem'ediate
MCF-7, HER210w MDA-MB-231 (American Type Culture Collection) ¨
were cultured in RPMI-1640 + 10% FBS (Cellgro/Mediatech, Manassas,
VA). 50x103 BC cells were plated in a transwell system (BD Biosciences),
and co-cultured with 106 CD4+ T-cells and 105 DC1s (mature DCs) or iDCs
(immature DCs). DC1s, iDCs and CD4+ T-cells were obtained from select
post-vaccinated patients as described by Sharma, et al. DC1s/iDCs were
pulsed with Class II HER2 or irrelevant control BRAF peptides (20 g/m1)
for 24 hours at 37 C. Specifically, as shown in Figurel0A, 50x103 SK-BR-
3 cells were co-cultured with medium alone ("complete medium"), 106
human CD4+ T-cells alone ("CD4+ only"), 106 CD4+ T-cells + 105 each of
HER2 Class II peptide ("iDC H")- or irrelevant Class II BRAF peptide
("iDC B")-pulsed iDCs, and 106 CD4+ T-cells + 105 each HER2 ("DC1 H")-
or BRAF ("DC1 B")-pulsed DC1s. DC1s/iDCs were pulsed with Class II
HER2 or irrelevant control BRAF peptides (20 g/ml) for 24hours at 37 C.
Control wells contained culture medium or CD4+ T-cells only.
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
1001601 Polyclonal goat IgG anti-human TNF-ct (0.06 g/mL per
0.75ng/mL TNF-a) and IFN-y (0.3ug/mL per 5ng/mL IFN-y) antibodies
(R&D Systems, Minneapolis, MN) were used to neutralize Thl cytokines,
with goat IgG isotype as control. Following treatments, BC cells were lysed
and subjected to western blot analysis for cleaved caspase-3 detection.
Degree of nuclear fragmentation was assessed by DAPI staining.
Additionally, apoptosis in 50x103 BC cells incubated with (i) supernatants
from iDC:CD4+ or DC1:CD4+ T-cell co-cultures, or (ii) TNF-a (10-200
ng/mL as indicated) + IFN-y (100-2000 U/mL as indicated) (R&D Systems)
was examined by cleaved caspase-3 detection.
[001611 Transgenic murine mammary carcinoma lines expressing high
levels of rodent HER2/ErbB2 (HER21igh TUBO and MMC15 [the latter a
generous gift of Li-Xin Wang, Cleveland Clinic]) and HER21"'g (4T1)
were incubated with medium (RPMI-1640+10%FCS) alone, recombinant
mouse rmTNF-a (Ing/m1; Peprotech) alone, rmIFN-y (12.5ng/m1;
Peprotech) alone, or combination rmTNF-a + rmIFN-y for 72 hours at 37 C.
Following trypsinization, harvested cells were washed and resuspended in
FACS buffer, and FITC-Annexin V (4 1) and PI (41) added. Cells were
incubated at 4 C for 20 minutes, washed twice, and subjected to flow
cytometry. Apoptotic cells were defined as those staining positive for both
86
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
markers. Vinculin was used as a loading control. Corresponding mean
caspase-3/vinculin ratios SEM , indicating fold induction of apoptosis,
were quantified using ImageJ software.
ELISA
[00162] Capture and
biotinylated detection antibodies and standards for
IFN-y and TNF-a (BD Pharmingen) were used according to the
manufacturer's protocols.
Statistical Analysis
100163] Descriptive
statistics were employed to summarize distributions
of patient characteristics and immune response variables. Continuous
variables were summarized by mean, SEM, and range and categorical
variables by frequency and percentage. Data transformation (natural log or
square root) was applied, when necessary, to meet assumptions of
parametric testing. ANOVA with post-hoc Scheffe paired testing
(parametric) or Kruskal-Wallis testing (non-parametric) were employed to
compare continuous variables for >3 groups. Student's t-test was used for 2-
group comparisons. Fisher's exact test was employed to compare
categorical variables in multi-level tables. Student's paired t-test and
McNemar's exact test were used to evaluate within-patient paired changes
87
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
(e.g., pre-vaccination vs. post-vaccination) in Thl response variables. A p-
value p<00.05 was considered statistically significant. All tests were two-
sided. Statistical analyses were performed in either SPSS (IBM Corp.). or
StatXact (Cytel Corp. San Diego, CA).
RESULTS
Patient Characteristics
[00164] After random consecutive enrollment, 143 subjects met study
inclusion criteria. Mean age of participants was 53.111.4 (range, 21-88)
years and a majority (79.0%) were Caucasian. Patient/donor cohorts, with
time-points at which blood was drawn, are indicated in Figure 1 and
Methods section above. Donors' demographic and tumor-related
characteristics of study participants are detailed in Table 1 above. Twenty-
six (83.9%) and 11(50.0%) patients in the HER2P 9-DCIS and ¨IBC cohorts,
respectively, were previously enrolled in neoadjuvant type 1-polarized
("DC1") vaccination trials for HER2P"-DCIS; their patient/turnor
characteristics have been reported by Sharma, et al. .
88
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
Loss of Systemic Anti-HER2 Thl Immunity Correlates with Progression of
Breast Tumorigenesis
[00165] Using peripheral blood mononuclear cells ("PBMCs"),
variations in systemic anti-HER2 CD4+ Thl response across a tumorigenesis
continuum were examined prospectively by ex vivo HER2 peptide-
stimulated IFN-y ELISPOT assays. Three Thl response metrics were
compared between groups: (a) overall anti-HER2 responsivity (proportion of
patients responding to >1 peptide), (b) mean number of reactive peptides
(repertoire), and (c) cumulative response across 6 class 11 peptides described
above. When compared with healthy donors ("HD") or patients with benign
breast disease ("BD") (Figure 1, cohort A), a significant stepwise decline in
Thl response was observed in HER217 breast cancer patients. Beginning in
treatment-naïve HER2P"-DCIS (Figure 1, cohort C) and reaching a low
point in treatment-naïve Stage I/II HER2P"-IBC (Figure 1, cohort F), this
progressive loss of Thl immunity was observed uniformly across all Thl
response metrics. For instance, the overall anti-HER2 responsivity
decreased from 100% in HD/BD to 84% in HER2P"-DCIS to 32% in
HER2P"-IBC patients (p<0.0001). Similar significant stepwise decrements
response repertoire (5.210.2 vs. 4.510.4 vs. 2.010.3 vs. 0.4102; p<0.0001),
and cumulative response (259.9123.5 vs. 225.1125.5 vs. 126.1124.4 vs.
89
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
32.3 5.4 spot-forming cells ("SFC")/106 cells, p<0.0001) were observed
across HD, BD, HER2P's-DCIS, and Stage I/II HER2P s-IBC patients,
respectively, as shown in Figure 5A. On post-hoc comparison, Thl
responses in HER2Pes-DCIS patients were significantly lower than in HDs
when assessed by response repertoire (p<0.001) and cumulative response
(p=0.001) but not overall responsivity (p=0.07). Thl responses in HER2P s-
IBC patients were further suppressed in that these patients had significantly
lower overall responsivity (p=0.0003), repertoire (p=0.001), and cumulative
response (p<0.001) compared with HER2P s-DCIS patients. The percentage
of reactive cells per 106 PBMCs ranged from 0.03% in HD to 0.003% in
HER2P 9-IBC patients.
[00166] It is to be
noted that Thl responses in treatment-naive HER2"eg-
DCIS (Figure 1 cohort B) or HER2"g-IBC (Figure 1 cohort D) patients and
HD/BD patients did not vary appreciably. Compared with HER2"eg-DCIS
patients, however, HER2P"-DCIS patients demonstrated significantly lower
anti-HER2 Thl repertoire (p<0.001) and cumulative response (p=0.02).
Similarly, compared with HER2"eg-IBC patients, HER2P 9-IBC patients had
lower responsivity (p=0.0003), repertoire (p<0.001), and cumulative
response (p<0.001) as seen in Figure 5A.
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00167] Individual HER2 peptide-specific contributions to cumulative
Thl responses across patient groups demonstrated similar stepwise Thl
decrements from HD/BD to HER2P"-IBC patients, across all HER2
extracellular domain ("ECD") and intracellular domain ("ICD") peptide
reactivity profiles (p<0.0050) as shown in Figure 6. Disproportionate
focusing of Thl immune responses towards a selective HER2 epitope(s) in
DCIS/IBC patients may not explain the progressive Thl loss in HER2P"
tumorigenesis.
[00168] In order to investigate if Thl responses in HD/BD donors were
disproportionately higher in certain subgroups, responses were compared by
age (<50 yr (n=16), 250 yr (n=15)), menopausal status (pre-menopausal
(n=16), post-menopausal (n=15)), race (White (n=23), other
(Black/Asian/etc.; n=8)), or gravidity (zero (n=12), >1 (n=19) pregnancies).
No significant differences in anti-HER2 Thl repertoire or cumulative
response were observed in HD subgroups stratified by age, race, or
menopausal status; however, gravid donors (i.e. 21 pregnancies) had a
significantly higher anti-HER2 Th 1 repertoire (5.310.2 vs. 4.610.2, p=0.01)
and cumulative response (293.1121.2 vs. 178.2119.0, p=0.0008) compared
with non-gravid donors (Figure. 5C). Temporal variability in Thl responses
was examined in HD/BDs and HER2"-IBC donors (n=4 each); in blood
91
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
drawn from the same patients at >6 month intervals, relatively unchanged
Thl repertoires and cumulative responses were observed over time as seen
in Figure 7.
Anti-HER2 IgG1 and IgG4 Antibody Responses Are Lost In HER2P's-IBC
[00169] After noting pre-existing anti-HER2 Thl responses in HDs that
decay in HER2P" breast tumorigenesis, serum reactivity was examined
against recombinant HER2 ECD peptides using available sera from HDs,
HER2P"-DCIS and HER2P"-IBC patients. Both IgGl, associated with Thl
immunity, and IgG4, associated with chronic antigen exposure, were
evaluated. Compared with HDs (n=12) and treatment-naive HER2P"-IBC
patients (n=-7), a relative increase in both anti-HER2 IgG1 and IgG4 (both
p<0.0001) levels was observed in HER2P'6-DCIS patients (n=10 IgGl, n=11
IgG4) by ELISA as shown in Figure 5D. Comparatively lower anti-HER2
antibody levels in HER2P"-IBC patients suggest that endogenous anti-HER2
response is lost upon disease progression.
CD4+ Thl Response in Equivocal HER2-Expressing IBC Differs
Significantly from Non-Equivocal HER2neg-IBC
[00170] Thl profiles in HER2lleg4BC patients were examined in order
to identify subgroups with a relative decline in anti-HER2 Thl immunity.
92
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
When compared with non-equivocal HER2"eg-IBC (IHC 0/1+) patients
(n-11), equivocal HER2-expressing (IHC 2+/FISH negative) IBC patients
(n=7) demonstrated significantly lower overall responsivity (28.6% [IHC
2+] vs. 100% in [IHC 0/1+], p=0.002), repertoire (0.3+0.2 vs. 3.9+0.3,
p<0.0001), and cumulative response (21.4+6.5 vs. 191.2+11.7 SFC/106 cells,
p=0.002). Thl responses in equivocal HER2-expressing IBC patients
resembled those seen in HER2Pes-IBC patients represented in Figure 5A. IL-
production measured via ELISPOT and the relative proportion of Treg
(CD4+CD25 FoxP3+) cells by flow cytometry did not differ significantly
between equivocal and non-equivocalHER2"eg-IBC patients (data not
shown).
Thl Response Loss is Not Related to Host-Level T-Cell Anergy or
Increasingly Immunosuppressive Circulating Immune Phenotype
[00171]
Immunocompetence in evaluable donor subgroups was assessed
by measuring Thl response to anti-CD3/anti-CD28 via IFN-y ELISPOT,
these responses also served as donor-specific positive controls in all
ELISPOT assays. Median anti-CD3/CD28 responses did not differ (1098
vs. 1104 vs. 1032 vs. 1099 vs. 1318 vs. 1032 SFC/2x105 cells, p=0.22)
between HD/BD (n=31), HER2"g-DCIS (n=11), HERreg-IBC (n=11),
93
CA 02942721 2016-09-13
WO 2015/139003
PCMS2015/020613
HER2P s-DCIS (n=5) HER2P"-IBC (n=11), and T/C-treated HER2P"-IBC
(n=37) cohorts, respectively as seen in Figure 5B. Moreover, Thl
responses to recall stimuli [tetanus toxoid (105117.0 vs. 96115.6 vs.
101111.3 SFC/2x105), and Candida albicans (185110.2 vs. 199115.3 vs.
181+14.6 SFC/2x105)] were similar between evaluated HD (n=10),
HER2P s-IBC (n=11), and T/C-treated IBC (n=10) cohorts, respectively as
seen in Figure 8A. Collectively, these data suggest that the progressive anti-
HER2 Thl response loss in HER2-driven BC is not attributable to host-level
T-cell anergy or impaired antigen-presenting capacity in IBC patients'
PBMCs.
1001721 Using flow cytometry, the mean proportion of CD3+CD4+
(72.812.3% vs. 62.613.2% vs. 63.316.9%, p=0.26) and CD3+CD8+
(25.112.9% vs. 37.914.7% vs. 38.216.6%, p=0.15) cells did not differ
significantly between PBMCs from HDs, HER2pos-IBC, and T/C-treated
HER2pos-IBC cohorts, respectively as is shown in Figure 8B. No
differences in proportions of B-cells (CD19+) or natural killer (NK) cells
(CD3-CD16+) were observed between groups (data not shown). Systemic
immunosuppressive phenotypes were then compared between the following
groups. As shown in Figure 8C mean proportions of CD4+CD25+FoxP3+
cells (Treg) (1.810.3% vs. 1.510.2% vs. 1.710.3%, p=0.78), and
94
CA 02942721 2016-09-13
WO 2015/139003 PCT/US2015/020613 .
CD11b+CD33+HLA-DR-CD83- cells (Myeloid-derived Suppressor Cells
"MDSCs") (0.6+0.1% vs. 1.0 0.3% vs. 0.9+0.1%, p=0.33) did not differ
significantly between HD, HER2pos-IBC, and T/C-treated HER2pos-IBC
subgroups, respectively.
[00173] HER2-specific IL-10 production, a surrogate for T-helper type
2 ("Th2") and/or Tõg function, was also examined across patient subgroups
via ELISPOT. Figure 8D shows anti-HER2 responsivity (all 100%),
repertoire (1.810.4 vs. 1.8 0.2 vs. 2.0A.3), and cumulative response
(77.4+15.2 vs. 66.6 8.2 vs. 92.84.7) did not differ significantly between
HD, HER2P's-IBC, and T/C-treated IBC cohorts, respectively. Figure 8E
shows IL-10 production to anti-CD3 stimulus was similar across all
evaluated groups. While overall IL-10 production did not differ between
subgroups, donor-matched HER2-specific IFN-'y:IL-10 production ratios
dramatically shifted from 6.6:1 (relative Thl-favoring phenotype) in HDs to
0.74:1 and 0.97:1 (relative Tõg/Th2-favoring phenotype) in untreated and
T/C-treated HER2P 9-IBC patients, respectively (p=0.009) (top panel).
Systemic Thl Response Loss is Unrelated to Disproportionate T-
Lymphocyte Trafficking to HER2P'9-IBC Lesions
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00174] Immunohistochemical ("IHC") analysis of 14 HER2P s-DCIS
and 8 HER2P s-IBC lesions, available for pathologic review, was performed
to determine if the systemic IFN-yP ' CD4+ response loss was related to
disproportionate lymphocyte trafficking to IBC lesions. The results are
shown in Figure 9A. Whereas moderate (215% stromal involvement) to
high (225%) lymphocyte levels were observed aggregating in stromal
regions outside DCIS-containing ducts in a majority (12/14; 85.7%) of
evaluable patients (top) (shown by arrow), a relative paucity of lymphocytes
(arrow) was seen around invasive foci in all 8 IBC patients 98/8; 100%)
(bottom).
[00175] Lymphocytic phenotypes were analyzed by a novel multiplex-
labeled irnmunofluorescence ("IF") imaging technique which discriminates
tumor and stromal regions, and reliably detects relative CD4+ (green signal),
CD8+ (yellow), and CD20+ (red) subpopulations as described by Wang, C.,
et al. The results are shown in Figure 9B. A majority of stromal ("StL")
and tumor-infiltrating lymphocytes ("TIL") in HER2P 0-IBC tumors
comprised CD8+ cells (upper right panel). Moreover, a relative paucity of
CD4+ TIL/StL was observed in HER2P"-IBC tumors compared with DCIS
lesions (upper left panel). Disproportionate peritumoral CD4+ T-cell
96
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
trafficking to HER2P"-IBC lesions may not explain the systemic depletion
of IFN-7P" CD4+ T-cell subsets.
High/Intermediate HER2-Expressing, But Not Low HER2-Expressing, BC
Cells Are Susceptible to CD4+ Thl-Mediated Apoptosis
[00176] Thl -mediated effects on HER2high SK-BR-3, HER2intennediate
MCF-7, and HER210' MDA-MB-231 BC cell lines in vitro were also
evaluated. Co-culture of increasing proportions of HER2 Class II peptide-
specific CD4+ Thl cells, sensitized with HER2-pulsed DC1, with the above
types of HER-expressing BC cells using a transwell culture system resulted
in striking dose-dependent apoptosis of SK-BR-3 evidenced by increased
caspace-3 detection by western blot analysis shown in Figure 10A and MCF-
7, but not MDA-MB-231, BC cells as seen in Figure 11A. In contrast,
apoptosis was relatively insignificant in BC cells co-cultured with CD4+ T-
cells sensitized by immature DCs (iDC H and iDC B) or control Class II
peptide (BRAF)-pulsed DC1s (DC! B's) as seen in Figures 10A and 11A.
Quantification of Thl cytokines elaborated in these co-culture supernatants
by ELISA indicated significantly increased IFN-y and TNF-ct production
from CD4+ T-cell:HER2-pulsed DC1, compared with CD4+:BRAF control-
97
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
DC1, co-cultures as shown in Figure 10A, corresponding with the degree of
apoptosis observed.
[00177] A similarly specific apoptosis was observed in SK-BR-3 cells
when incubated with supernatants from CD4+ T-cell:HER2-pulsed DC1 co-
cultures, but not CD4+:HER2-iDC or CD4+:BRAF control-DC1 co-cultures
as shown in Figure 11B. Compared with controls, HER2-specific Thl cells
resulted in a 25-fold increase in SK-BR-3 apoptosis as evidenced by DAPI
staining as seen in Figure 10B, right photograph and bar graph. Taken
together, these data suggest that anti-HER2 CD4+ Thl cells produce soluble
factors that mediate apoptosis of high/intermediate HER2-expressing, but
not low HER2-expressing, breast cancer cells.
[00178] Importantly, HER21ligh SK-BR-3 apoptosis could be
significantly rescued by neutralizing IFN-y and TNF-a, as seen in Figure
10A, suggesting a critical role for pleiotropic Thl cytokines in mediating
HER2-specific cellular apoptosis. To explore these observations further, the
impact of IFN-y and TNF-a treatment on BC cells were examined.
Regardless of HER2 expression, human BC cells uniformly maintained IFN-
y and TNF-a receptor expression as seen in Figure 10C. IFN-y and TNF-a
treatment resulted in significant apoptosis of HER2high SK-BR-3 and
98
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
HER2inter1ediate MCF-7, but not HER210w MDA-MB-231, cells as seen in
Figure 11C. Next, to assess if reinstatement of HER2 expression in MDA-
MB-231 cells restored susceptibility to Thl cytokine-mediated apoptosis,
MDA-MB-231 cells were stably transfected with a wild-type HER2 plasmid
(pcDNA-HER2) or with control empty vector (pcDNA3; kind gifts of Mark
I. Greene, University of Pennsylvania) and treated with IFN-y and TNF-a
(2000 U/ml and 200 ng/ml, respectively; doses equivalent to those used
against MDA-MB-231 cells in Figure 11C). Significant IFN-y/TNF-a-
induced apoptosis was observed in HER2-transfected, but not vector-
transfected, MDA-MB-231 cells (data not shown).
[00179] Finally, this Thl cytokine-mediated HER2-specific apoptosis
was corroborated in transgenic murine mammary carcinoma cells. Dual
treatment with recombinant mouse 1FN-y and TNF-a, but not with either
cytokine alone, resulted in significant apoptosis of HER2'" TUBO and
MMC15, but not HER2I0/h1Cs 4T1, cells as seen in Figure 10D.
Thl Response Loss in HER2P'9-IBC is Restored After HER2-Pulsed DC
Vaccination, but not Following HER2-Targeting or Conventional Therapies
[00180] Differential effects following TIC treatment and HER2-pulsed
DC1 immunization on Thl responses in HER2Ns-IBC patients were
99
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
analyzed and the results are shown in Figure 12A, top panels. Treatment-
naïve stage I/II HER2Ns-IBC patients (n=22; Figure 1 cohort F) and T/C-
treated stage I-III HER2P's-IBC patients (n=37; Figure 1 cohort G) did not
differ significantly in anti-HER2 responsivity (31.6% untreated vs. 45.9%
T/C-treated, p=0.39), repertoire (0.4 0.2 vs. 0.8 0.2, p=0.24), or cumulative
response (32.3 5.4 vs. 54.5 12.0 SFC/106, p=0.97). As shown in Figure
12B, top panels, following HER2-pulsed DC1 vaccination in 11 Stage I
HER2P s-IBC patients (Figure 1 cohort H), however, significant
improvements were observed in anti-HER2 responsivity (18.2% pre-vaccine
vs. 90.9% post-vaccine, p=0.0035), repertoire (0.34.2 vs. 3.7 0.5,
p<0.0001), and cumulative response (29.7 7.9 vs. 162.8 33.7 SFC/106,
p<0.0001). The striking Thl restoration effect following DC1 vaccination,
but not after T/C receipt, persisted on stage-matched comparison between
Stage I treatment-naïve (n=11), T/C-treated (n=8), and vaccinated (n=11)
HER2P 9-IBC patients as seen in Figure 12C.
[00181] Differences in relative proportions of IFN-yP'9:IL-10N9 reactive
T-cells were examined following DC1 vaccination compared with T/C
treatment. In concurrently performed donor-matched comparisons, while
both HER2-specific IFN-y (196.8 56.8 post-vaccine vs. 32.1 6.1 pre-
vaccine SFC/106, p=0.02) and IL-10 (79.0 7.4 vs. 33.8 5.1 SFC/106,
100
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
p=0.001) responses were augmented following HER2-pulsed DC1
vaccination, relative IFN-y:IL-10 response ratios shifted from 0.95:1
(relative Treg/Th2-favoring) pre-vaccination to 2.5:1 (Thl-favoring) post-
vaccination (p=0.008). However, relative IFN-y:IL-10 response ratios did
not indicate a significant shift toward a Thl-favoring phenotype following
TIC treatment (0.97:1) compared with treatment-naïve (0.74:1, p=0.78)
HER2P"-IBC patients See, Figures 12A and 12B, lower horizontal bar
graphs.
[00182] Longitudinal Thl immune evaluation >6 months' post-
vaccination was possible for nine (81.8%) patients. As shown in Figures
12D and 12 E, despite completion of postoperative chemotherapy following
vaccination in all patients, durable anti-HER2 Thl reactivity was observed at
a median duration of 16 (range 6-60) months vs. pre-vaccination baseline:
anti-HER2 responsivity (100% >6 mo post-vaccine vs. 22.2% pre-vaccine,
p=0.008), repertoire (4.0 0.4 vs. 0.3 0.2, p<0.0001), cumulative response
(255.1 49.2 vs. 33.8 9.2 SFC/106, p=0.006).
[00183] Subgroup analysis of the TIC-treated cohort was performed in
order to investigate variations in Thl reactivity by sequence of
chemotherapy (neoadjuvant or adjuvant); time from completion of
101
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
prescribed trastuzumab to study enrollment (< or >6 months); estrogen-
receptor status (ERP" or ERneg); and pathologic stage (I-III). Figure 13A
shows chemotherapy sequence (neoadjuvant [n=12] vs. adjuvant [n=25];),
Figure 13B shows time from trastuzumab completion (<6 [n=16] vs. >6
months [n=21];), or Figure 13C shows ER status (ERP" [n=21] vs. ER"g
[n=16];) did not impact anti-HER2 Till responsivity, repertoire, or
cumulative response (all p=NS). Importantly, Figure 13D shows AJCC
stage I (n=8), stage II (n=20), or stage III (n=9) TIC-treated patients did
not
differ by any Thl metric, suggesting that the observed anti-HER2 Thl
deficit in HER2P 5-IBC was independent of disease burden. Moreover, these
data collectively suggest that dominant Thl reactivity profiles of particular
subgroups are not responsible for the lack of immune restoration observed
globally in TIC-treated HER2P"-IBC patients.
Depressed anti-HER2 Thl Responses Correlate with Adverse
Clinicopathologic Outcomes
[00184] To assess the translational relevance of these findings, an
evaluation was made to determine if Thl response variations in TIC-treated
HER2Pes-IBC patients were associated with the development of subsequent
breast events ("BE;" defined as any locoregional/distant recurrence).
102
CA 02942721 2016-09-13
WO 2015/139003 PCT/US2015/020613
Median follow-up was 33.5 (interquartile range "IQR" 25.5-45.8) months.
As shown in Table 2 below (showing demographic and clinical
characteristics of HER2P"-IBC patients incurring subsequent breast cancer
events (defined as any locoregional or systemic recurrence) following
trastuzumab and chemotherapy treatments), eight patients (21.6%) suffered
BEs following TIC treatment at a median duration of 29 (IQR 16.2-36)
months. Figure 13E, left panels, show that compared with patients without
BEs, BE-incurring patients had significantly depressed anti-HER2
responsivity (top) (12.5% +BE vs. 55.2% no BE; p=0.048) and cumulative
responses (bottom) (9.413.6 vs. 66.9114.5 SFC/106; p=0.046), but not
response repertoire (middle) (1.0310.3 vs. 0.1310.1; p=0.11).
TABLE 2
Pt Age at Type of Location, if Stage at Timing of
Time to
no. study recurrence distant initial TIC recurrence
entry recurrence diagnosis receipt (months)
(yrs)
1 64 Systemic Bone, brain 3 Adjuvant 26
2 63 Locoregion 3 Adjuvant 31
al
3 53 Locoregion 2 Adjuvant 21
al
4 43 Locoregion 2 Adjuvant 12
al
49 Locoregion Bone 2 Adjuvant 31
al,
Systemic
103
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
6 67 Locoregion 1 Adjuvant 102
al
7 85 Locoregion 3 Adjuvant 36
al
8 34 Locoregion 3 Neoadjuva 14
al nt
[00185] In 12 (32.4%) T/C-treated HER2Ns-IBC patients receiving
neoadjuyant TIC, anti-HER-2 Thl responses were compared between
pathologic complete responders ("pCR"; defined as no evidence of residual
invasive BC on postoperative pathology) and non-pCR patients. The results
in Figure 13E, right panels, show pCR, achieved in 4 patients (33.3%), was
associated with significantly higher anti-HER2 repertoire (3.3 1.1 vs.
0.13 0.13, p=0.002)(middle) and cumulative response (193.1 64.9 vs.
13.6 4.6, p=0.002) (bottom) compared with non-pCR patients; anti-HER2
responsivity (100% vs. 25%, p=0.06) (top) did not reach statistical
significance.
DISCUSSION
[001861 The advent of checkpoint inhibitors (Topalian, S.L., et al., N.
Eng. J. Med. 366:2443-54 (2012)), and use of immune-modulating strategies
such as vaccines (Kantoff, P.W., et al., N. Eng. J. Med. 363:411-22 (2010)),
toll-like receptor agonists, or adoptive T-cell therapies against tissue-
specific
104
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
epitopes (Kalos, M., et al., Sci. Transl. Med. 3(95):95ra 73 (2011) and
Rosenberg, S.A., Nature Reviews Clinical Oncology 8:577-85 (2011)) have
set the stage for more effective cancer imrnunotherapies. Most of these
therapies are geared toward broad-based immune modulation. In parallel
with these discoveries, genomic profiling has identified specific molecular
drivers of tumorigenesis, including v-raf murine sarcoma viral oncogene
homolog-Bl ("BRAF"), epidermal growth factor receptor ("EGFR"),
hepatocyte growth factor receptor ("c-MET"), and HER2. While therapies
targeting such "oncodrivers" achieve encouraging response rates, their
success is relatively short-lived because most tumors ultimately recur or
become therapy-resistant (Pohlmann, et al. and Flaherty, K.T., et al., N. Eng.
J. Med. 363:809-19 (2010)). Identifying onco driver-specific immune
deficits during tumor development may provide therapeutic opportunities
tailored to specific cancer subtypes. Herein described is believed to be the
first study that identifies a CD4+ Thl immune deficit in tumorigenesis
specific to the molecular oncodriver of a defined BC phenotype, namely
HER2/neu.
[00187] The decay in anti-HER2 CD4+ Thl immunity commences in
the premalignant DCIS phase, and becomes progressively lost in early
invasive disease states. Moreover, Thl immunity appears to be lost
105
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
specifically in HER2-overexpressing phenotypes. Utilizing a broad
tumorigenic continuum, it has been demonstrated herein that anti-HER2 Thl
responses in HER2neg-DCIS and HER2neg-IBC patients (IHC 0/1+) closely
resembled those seen in HD/BD donors, and were significantly higher than
Thl responses seen in HER2P" (IHC 3+ or 2+/FISH positive) DCIS and IBC
patients, respectively; additionally, Thl immunity appears to be lost in
equivocal HER2-expressing (IHC 2+/FISH negative) individuals and
resembled those seen in HER2P's-IBC patients. Particularly, the
maintenance of HER2-specific CD4+ immunity in HER2"g-IBC patients
may, in part, explain their improved clinical outcome after vaccination with
HER2 peptides aimed at activating CD8+ T-cells. See, Benavides, L.C., et
al., Clin. Can. Res. 15:2895-904 (2009).
1001881 It is somewhat surprising that HD/BDs maintained a readily
identifiable population of circulating anti-HER2 Thl cells. Since HER2 is
normally a membrane constituent in branching breast ductal cells during
pregnancy and lactation (Press, M,F., et al., Oncogene 5:953-62 (1990)), it is
plausible that pre-existing CD4+ T-cell responses in HD/BDs are generated
as a result of HER2-epitope presentation by antigen-presenting cells
("APCs") within the breast. Indeed, although independent of age, race, or
menopausal status, pre-existing anti-HER2 Thl immunity in HD/BDs was
106
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
higher in gravid compared with non-gravid donors; notably, the latter is a
population at increased risk for BC development. Furthermore, the striking
pro-apoptotic effect of HER2-specific Thl¨via cytokines IFN-y and TNF-a¨
in HER2h1gh, but not HER210\', BC cell lines expressing IFN-y/TNF-a
receptors in vitro, imply that anti-HER2 Th I may be instrumental in
controlling or eliminating HER2-overexpressing cells during physiologic
processes such as breast involution. Thus, a pre-existing anti-HER2 Thl
immunity in HDs may confer protection against tumorigenic events, while
abrogation of anti-HER2 Thl function may represent a tumor-driven
mechanism to evade immune surveillance during HER2P" tumorigenesis.
Interestingly, recent evidence suggests that preferential death programming
of circulating tumor-associated antigen (e.g., MAGE6, EphA2)-specific
CD4+ Thl may contribute to the immune dysfunction observed in melanoma
patients with active disease (Wesa, A.K., et al., Front. Oncol. 4:266 (2014).
Similar mechanisms may be involved in the loss of anti-HER2 CD4 Thl
immunity observed in the present study¨deciphering, and targeting, such
mechanisms may be critical for the development of immune interventions
aimed at primary BC prevention. These mechanisms, as well as the
functional significance of anti-HER2 Thl cells in breast homeostasis,
warrant further investigation.
107
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00189] Although antecedent HER2-Thl immunity was maintained in
HD/BDs, HER2-reactive humoral responses were not. In the healthy breast,
priming of CD4+ Thl cells by APCs in a non-inflammatory setting, while
contributing to homeostasis of HER2-expressing cells via IFN-y/TNF-a
secretion, may not drive antibody production. In HER2P'-DCIS, however, a
relative increase in HER2-reactive IgGl/IgG4 was associated with
intermediate, but not absent, Thl responses. Appearance of HER2 antigenic
stimulus on evolving tumors, and its subsequent presentation by APCs to
remaining Thl cells in an inflammatory environment, may allow for
transient antibody production. Ultimately, in HER2P"-IBC, waning of CD4+
T-cell help may erode the continued production of antibodies, resulting in
their eventual disappearance. This dissipation of both arms of adaptive
immunity could render these patients incapable of primary tumor prevention
and control.
[00190] In addition to those discussed above, the loss of anti-HER2 Thl
immunity may reflect other mechanisms ¨ for instance, chronic T-cell
exhaustion or peripheral tolerance with a contributory role for co-inhibitory
signals (e.g., TIMs, PD-L1, CTLA-4, etc.), or alterations in HER2-reactive
immune phenotypes. Indeed, although overall IL-10 responses are
maintained across the tumorigenic continuum, HER2-specific responses
108
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
functionally shift from strongly Thl-favoring (in HD/BDs) toward a
relatively Th2/Treg-favoring (in HER2P s-IBC) phenotype when evaluated by
antigen-specific IFN-7:IL-10 ratios. The intact, albeit muted, Thl
responsivity in 7/22 (32%) HER2P"-IBC patients, therefore, may reflect an
ongoing balance between Thl antitumor immune defense and tolerogenic
Treg/Th2 contributions28 (Levings, M,K, et al., Blood 105:1162-9(2005)
during tumorigenesis.
1001911 Nonetheless, the loss of anti-HER2 Thl immunity was not
attributable to absolute increases in circulating immunosuppressive
populations in HER2P"-IBC patients. Although previous studies have
reported higher levels of circulating Treg and/or MDSCs in advanced (Stage
III/IV) BC (Liyanage, U.K., et al., J. Inununol. 169:2756-61 (2002)) and
other solid tumors (Zhang, B., et al., PLOS ONE 8(2):e57114 (2013), in this
study, early-stage (Stage I/II) IBC patients appear to have comparable
immunosuppressive profiles to HDs. The dramatic decline in anti-HER2
Thl responses in these patients, therefore, is even more compelling.
Furthermore, this decline in peripheral blood anti-HER2 IFN-y 6CD4+ T-
cell subsets was unrelated to (i) immune sculpting, since a bias was not
observed towards selective HER2 peptide reactivity with progressive
tumorigenesis; or (ii) discrepantly greater CD4+ T-cell trafficking to
invasive
109
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
tumors. The latter finding should be interpreted with caution, however,
since these data do not address sequestration or depletion of HER2-specific
CD4+ TILs in the tumor microenvironment. Finally, the anti-HER2 Thl
immune depression could not be explained by generalized host-level T-cell
anergy in IBC patients; however, the present study cannot completely
exclude antigen-specific cellular-level anergy as a possible explanation for
this phenomenon.
[00192] Importantly, this anti-HER2 Thl depression was associated
with an increased risk of locoregional or distant recurrence in T/C-treated
HER2P"-IBC patients. In contrast, anti-HER2 Thl preservation correlated
with pCR following neoadjuvant TIC. Taken together, these data suggest
that monitoring anti-HER2 Thl immune reactivity following HER2-directed
therapies may identify vulnerable subgroups at risk of clinical or pathologic
failure. Moreover, the association of an anti-HER2 Thl deficit with
unfavorable clinicopathologic outcomes warrants a search for therapeutic
strategies that might reverse such an immune deficit.
[00193] Even after controlling for disease burden (i.e. pathologic stage),
the depressed anti-HER2 Thl responses in HER2P'9-IBC patients remained
globally unaffected by surgery, radiation, chemotherapy, or HER2-targeted
110
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
trastuzumab. Several studies have demonstrated the ability of trastuzumab
to reduce growth and induce apoptosis in HER2P s tumors (Dogan, I., et al.,
MoL Cell. Biochem. 347:41-51 (2011), as well as to sensitize HER2P s cells
to the tumoricidal effects of cytotoxic chemotherapy (Henson, E.S., et al.,
Clin. Cancer Res.12:645-53 (2006)). Despite these benefits, the use of
trastuzumab did not appreciably restore HER2-specific Thl immunity in a
majority of patients, including those with Stage I disease. In addition, an
almost universal resistance to these HER2-targeted therapies is observed in
advanced disease states. Pohlman, et al. Additional strategies targeting
HER2, therefore, are required.
[00194] One such strategy, described herein, may be autologous DC1
immunization with HER2-derived Class II peptides. Following neoadjuvant
HER2-pulsed DC1 vaccination in HER2P's-IBC patients (followed by
surgery), durable restoration of anti-HER2 Thl immunity was observed up
to 60 months post-vaccination. Altogether, these data suggest that (i) this
HER2-specific CD4 Thl immune deficit is not immunologically "fixed,"
since it can be corrected with appropriate immunologic interventions; and
(ii) combination of vaccination (or other immune-modulating strategies)
with existing humoral-based HER2-targeted therapies may improve long-
term outcomes in this disease. Indeed, in murine models, the collaboration
111
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
of cellular (IFN-y-producing CD4+, but not CD8+, T-cells (Sakai, Y., et al.,
Cancer Res. 64:8022-8 (2004)) and humoral HER2-directed immunity is
essential for eradication of HER2P" tumors (Reilly, R.T., et al., Cancer Res.
61:880-3 (2001)).
[00195] Collectively, the present findings have implications for immune
monitoring and therapy selection in HER2P"-BC patients. As discussed,
they justify addition of anti-HER2 immunizations to standard HER2-
targeted therapies in high-risk populations with HER2-driven BC; indeed,
trials have been initiated testing such combinations in HER2P 9-IBC patients
with residual disease after neoadjuvant TIC, and those with advanced disease
following adjuvant therapy. Moreover, while conventional surveillance
strategies (radiographic imaging, IHC/FISH profiling of breast biopsy
specimens, etc.) offer only an isolated snapshot of a tumor's evolution,
monitoring high-risk patients for real-time fluctuations in their anti-HER2
Thl immunity may provide a glimpse into the natural history and immune
repercussions of a tumor. Judicious incorporation of CD4+ Thl immune
detection protocols into future BC clinical trial design appears justified.
[00196] In summary, it is believed that herein is the first description, to
our knowledge, of the progressive and specific loss of CD4- Thl immunity
112
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
to a molecular oncodriver during breast tumorigenesis. Glimpses into the
unfavorable clinical and pathologic outcomes associated with depressed
anti-HER2 Thl immunity imply that immune restoration with vaccination or
other immune modulating strategies may be worth pursuing in these high-
risk patients to mitigate tumor progression or prevent recurrence. Additional
studies are warranted to determine whether anti-HER2 CD4+ responses are
lost in other HER2Ns cancers (i.e. ovarian, gastric, etc.), and if there is a
generalized loss in Thl immunity to other molecular oncodrivers during
tumorigenesis.
EXPERIMENTAL EXAMPLE
Anti-HER2 CD4+ Thl Response Is a Novel Immune Correlate
to Pathologic Response Following Neoadjuvant Therapy in
HER2-Positive Breast Cancer
1001971 In
contemporary practice, patients with larger resectable tumors
often benefit from neoadjuvant administration of trastuzumab and
chemotherapy (TIC), with nearly 40%-60% achieving pathologic complete
response ("pCR"). See, Gianni, L., et al., Lancet 375:377-84 (2010); Untch,
M., et al., J. Clin. Oncol. 28:2024-31 (2010); Untch, M., et al., J. Clin.
Oncol. 29:3351-7 (2011). Compared with evidence of residual disease at
surgery ("<pCR"), attainment of pCR following neoadjuvant TIC is an
113
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
established surrogate for decreased recurrence and improved long-term
survival.
[00198] , The above Reference Example demonstrated a progressive loss
in anti-HER2 CD4+ T-helper type-1 ("Thl") immunity across a tumorigenic
continuum in HER2P"-breast cancer. Of particular interest, this HER2-
specific Thl response is preserved in healthy volunteers as well as patients
harboring HER2lieg (0-1+) invasive breast cancer ("IBC"). In HER2P'6-IBC
patients, this anti-HER2 Thl deficit does not appear to be impacted by
standard therapies ¨ surgical resection, radiation, or TIC treatment ¨ but
instead can be "restored" following HER2-pulsed type-l-polarized dendritic
cell (DC1) vaccinations. Moreover, also shown was that depressed anti-
HER2 Thl responses predict an increased risk of subsequent recurrence in
adjuvant T/C-treated patients. These observations prompted a study of
whether similar depressed anti-HER2 Thl responses are observed in another
known harbinger of recurrence, namely, <pCR status following neoadjuvant
T/C (Kim, M.M., et al., Ann. Oncol. 24:1999-2004 (2013)); conversely, it
was hypothesized that preservation/restoration of anti-HER2 Thl responses
may be associated with pCR. Therefore differences in anti-HER2 Thl
responses between pCR and <pCR patients were examined to identify
modifiable immune correlates to pathologic response.
114
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00199] Anti-HER2 CD4+ Thl responses were analyzed prospectively
for 87 HER2P"-IBC patients (3+ or 27FISH-positive) and responses were
compared between stage I/II HER2P"-IBC (n=22) and stage I-III T/C-treated
HER2P s-IBC patients (n=65). In the T/C-treated cohort ¨ anti-HER2 Thl
responses were generated following completion of adjuvant trastuzumab ¨
responses were stratified by timing of chemotherapy (i.e., neoadjuvant vs.
adjuvant), and further sub-stratified by pCR and <pCR status within the
neoadjuvant cohort. pCR was defined as absence of residual invasive cancer
on pathologic examination of the resected breast specimen and sampled
lymph nodes (i.e., ypTO/Tis ypN0).
[00200] Four patients in the <pCR cohort were recruited to join an
adjuvant HER2-pulsed type-l-polarized DC (DC!) vaccination trial
(NCT02061423); anti-HER2 Thl responses in these patients were analyzed
pre- and post-immunization.
[00201] Methods
[00202] As described in the Reference Example, circulating anti-HER2
CD4+ Thl responses were examined in unexpanded PBMCs pulsed ex vivo
with six HER2-derived class II peptides (peptide 42-56, peptide 98-114,
peptide 328-345, peptide 776-790, peptide 927-941, and peptide 1166-
115
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
1180) (SEQ ID NOS: 1-6), by measuring IFN-y production via enzyme-
linked immunosorbent spot (ELISPOT) assays. ELISPOT was performed as
described in the Reference Example. PBMCs from HLA-A2.11's donors
were stimulated with two HER2-derived class I peptides: peptide 369-377
(SEQ ID NO: 7) and peptide 689-697 (SEQ ID NO: 8) with PMA (50ng/m1)
and ionomycin (1 g/m1; Sigma-Aldrich) serving as positive control.
1002031 An empiric method of determining antigen-specific response
was employed. A positive response to an individual HER2 peptide was
defined as: (1) threshold minimum of 20 SFC/2x105 cells in experimental
wells after subtracting unstimulated background; and (2) >two-fold increase
of antigen-specific SFCs over background. Thl response metrics were anti-
HER2 responsivity, number of reactive peptides (repertoire), and cumulative
response across 6 peptides (SFC/106 cells) as described in the Reference
Example. Thl responses of <pCR patients (n=4) receiving adjuvant HER2-
pulsed type-1- polarized dendritic cell (DC1) vaccination were analyzed pre-
/post-immunization.
1002041 Results
[00205] The study comprised 87 patients. Depressed anti-HER2 Till
responses in treatment-naïve HER2P 8-IBC patients (n=22) did not improve
116
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
globally after TIC treatment (n=65). Compared with adjuvant-T/C,
neoadjuvant-T/C (61.5%) was associated with higher Thl repertoire (1.5 vs.
0.8, p=0.048). While pCR (n=16) and <pCR (n=24) patients did not differ
in demographic/clinical characteristics, pCR patients were more likely to
have ER" tumors. pCR patients demonstrated dramatically higher anti-
HER2 responsivity (94% vs. 33%, p=0.0002), repertoire (3.3 vs. 0.3,
p<0.0001), and cumulative response (148.2 vs. 22.4, p<0.0001) compared
with <pCR patients. This disparity was mediated by CD4 T-bet+IFN-7+
phenotypes, and not attributable to <pCR patients' immune incompetence,
host-level T-cell anergy, or increased immunosuppressive populations. In
four <pCR patients, Thl repertoire (3.7 vs. 0.5, p=0.014) and cumulative
responses (192.3 vs. 33.9, p=0.014) improved significantly following HER2-
pulsed DC1 vaccination.
[00206] Conclusion
[00207] Anti-HER2 Thl response is a novel immune correlate to
pathologic response following neoadjuvant-T/C. In <pCR patientsHER-2
expressing patients receiving neaodjuvant therapy, depressed Thl responses
can be restored with HER2-Thl immune interventions and may improve
pCR or recurrence rates.
117
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[00208] Thus addition of HER2-targeted Thl immune interventions to
neoadjuvant T/C regimens and/or in the adjuvant setting for high-risk <pCR
subgroups may be justified. Moreover, in light of the demonstration in the
Reference Example that depressed anti-HER2 Thl immunity correlates with
subsequent recurrence in adjuvant T/C-treated patients, monitoring high-risk
<pCR patients for real-time fluctuations in anti-HER2 Thl immunity may
complement existing radiographic surveillance, and help identify critical
windows in which to intervene therapeutically.
[00209] In summary, this believed to be the first description of a
critical
association between anti-HER2 CD4:' Th I immunity and pCR following
neoadjuvant T/C in HER2P`9-IBC patients. Although causality cannot be
confirmed, the dramatic IFNI, anti-HER2 Thl deficit observed in <pCR
patients following neoadjuvant T/C raises the possibility that immune rescue
= with HER2-Th I interventions may complement standard HER2-targeted
strategies in improving outcomes in these high-risk patients.
[00210] The disclosures of each and every patent, patent application,
and publication cited herein are hereby incorporated herein by reference in
their entirety.
118
CA 02942721 2016-09-13
WO 2015/139003
PCT/US2015/020613
[002111 This disclosure has been presented for purposes of illustration
and description but is not intended to be exhausting or limiting. Many
modifications and variations will be apparent to those of ordinary skill in
the
art. The embodiments were chosen and described in order to explain
principles and practical application, and to enable others of ordinary skill
in
the art to understand the disclosure for various embodiments with various
modifications as are suited to the particular use contemplated.
[00212] Although illustrative embodiments have been described herein
with reference to the accompanying drawings, it is to be understood that the
embodiments are not limited to those particular descriptions, and that
various other changes and modifications may be devised therein by one
skilled in the art without departing for the scope or spirit of the
disclosure.
The appended claims are intended to be construed to include all such
embodiments and equivalent variations.
119