Note: Descriptions are shown in the official language in which they were submitted.
COPPER REMOVAL METHOD FOR AQUEOUS NICKEL CHLORIDE SOLUTION
Technical Field
[0001]
The present disclosure relates to a
hydrometallurgical process of nickel and cobalt including
separating and recovering cobalt from an aqueous nickel
chloride solution containing cobalt by solvent extraction
using an organic solvent formed by diluting a tertiary
amine as an extractant with an aromatic hydrocarbon as a
diluent.
[0002]
More specifically, the present disclosure relates to
a method for removing copper from an aqueous nickel
chloride solution in which an aqueous cobalt chloride
solution having a low copper concentration is obtained as
a stripped aqueous phase from an aqueous nickel chloride
solution containing cobalt and having a high copper
concentration by removing copper accumulated in an organic
phase after stripping obtained by eliminating cobalt from
an organic phase containing extracted cobalt to thereby
reduce the copper concentration in an organic phase.
Background Art
[0003]
In smelting of nickel and cobalt, there has been
produced nickel matte containing, as a main component,
nickel sulfide such as Ni3S2 obtained by a so-called
1
CA 2943483 2017-12-05
pyrometallurgical process, for example, nickel sulfide
obtained by melting nickel sulfide ore in a blast furnace,
nickel sulfide obtained by adding sulfur to nickel oxide
ore and melting the mixture in an electric furnace, and
the like.
[0004]
On the other hand, there has also been produced a
mixed sulfide containing nickel and cobalt which contains
a sulfide such as NiS as a main component (hereinafter,
referred to as a mixed sulfide) obtained by subjecting
nickel oxide ore having a low nickel grade to High
Pressure Acid Leaching (HPAL for short) and removing
impurities including iron from the HPAL solution, followed
by wet sulfidation, for example, by blowing hydrogen
sulfide gas into the leaching solution containing nickel
ions and cobalt ions.
[0005]
Examples of practically used methods of refining
nickel and cobalt using the above nickel matte and mixed
sulfide as a raw material include a method of leaching the
nickel matte and mixed sulfide with chlorine gas and
subjecting the leached nickel ions and cobalt ions to
electrowinning to be produced as electric nickel and
electric cobalt, as described in Patent Literature I.
[0006]
In the method described above, the mixed sulfide is
repulped in a chloride aqueous solution, and then chlorine
gas is blown into the resulting slurry to thereby
2
CA 2943483 2017-12-05
chlorine-leach nickel and cobalt into the chloride aqueous
solution.
A ground nickel matte is brought into contact with
the resulting chlorine leaching solution containing
divalent copper-chloro complex ions as an oxidizing agent
to perform displacement reaction between copper and nickel
to thereby displace/leach nickel from the nickel matte
into the solution.
[0007]
Subsequently, impurities such as iron, lead, copper,
and zinc are removed from the resulting leachate after
displacement leaching; cobalt in the leachate after
displacement leaching is separated by a method such as
solvent extraction; and then nickel is subjected to
electrowinning to produce electric nickel.
Cobalt separated here is subjected to further removal
of impurities in a processing route different from nickel
and then subjected to electrowinning to be produced as
electric cobalt.
[0008]
This method is simple and has achieved efficient and
economical production; for example, chlorine gas generated
in electrowinning is reused in leaching.
Further, in the technique of Patent Literature 1,
copper contained in a very small amount in the raw
material such as the nickel matte and mixed sulfide is an
impurity for refining nickel and cobalt, but it is
utilized as an oxidizing agent in the chlorine leaching
3
CA 2943483 2017-12-05
step and the displacement leaching step and circulated
between the chlorine leaching step and the displacement
leaching step.
[0009]
In the displacement leaching step, nickel in the
nickel matte is displaced/leached into the solution by
performing displacement reaction between a divalent
copper-chloro complex ion and Ni3S2 and Ni (metallic
nickel) in the nickel matte; on the other hand, the
copper-chloro complex ion becomes a solid in the form of
Cu2S or Cu (metallic copper).
[0010]
That is, in the displacement leaching step, the
removal of copper from a chlorine leaching solution is
also performed at the same time, and the copper
concentration in the leachate after displacement leaching
will be 0.02 g/L or less. The copper carried in from the
raw material is gradually accumulated in the chlorine
leaching step and the displacement leaching step while it
is circulated between the chlorine leaching step and the
displacement leaching step. Therefore, in order to
maintain the copper concentration in the chlorine leaching
solution at an appropriate level in the range of 10 to 60
g/L, the copper balance is maintained by taking copper out
of the system as copper powder, for example, by subjecting
the chlorine leaching solution to copper removal
electrolysis.
4
CA 2943483 2017-12-05
I
[0011]
Incidentally, in the hydrometallurgical process of
nickel, the separation of nickel and cobalt which are
contained in an acidic aqueous solution is the most
important technical element.
For example, there has also been performed a method
of adding chlorine gas as an oxidizing agent and a nickel
carbonate slurry as a neutralizing agent to a nickel
aqueous solution containing cobalt to separate cobalt as a
trivalent hydroxide. However, in order to completely
remove cobalt in the aqueous solution as a solid, nickel
in an amount of about 3 times the weight of cobalt has
also produced a trivalent hydroxide. Thus, since the
separability of nickel and cobalt is poor, the above
method cannot be said to be an efficient and economical
method.
[0012]
So, a solvent extraction method with various organic
extractants is mainly used at present for the separation
of nickel and cobalt contained in an acidic aqueous
solution.
In the solvent extraction method for separating
nickel and cobalt, an acidic phosphate extractant such as
D2EHPA (Di-(2-ethylhexyl)phosphoric acid) and an amine
extractant such as TNOA (Tri-n-octylamine) are used as an
organic extractant.
CA 2943483 2017-12-05
[0013]
Both the acidic phosphate extractant and the amine
extractant which are used have excellent separation
performance of nickel and cobalt. However, generally, the
acidic phosphate extractant is used when an anion is a
sulfate ion, and the amine extractant is used when an
anion is a chloride ion.
[0014]
When a chloride ion concentration in a chloride
aqueous solution of 200 g/L or more which is the chloride
aqueous solution has a sufficiently high chloride ion
concentration, cobalt forms a chloro complex ion, but
nickel does not form a chloro complex ion. Therefore, the
amine extractant has a higher separation factor of cobalt
and nickel than the acidic phosphate extractant.
[0015]
Further, in the case of the acidic phosphate
extractant, the cost of a neutralizing agent is required
because protons are released from the extractant by the
extraction of metal ions, and also a clad is often
produced by the variation of pH.
The clad is a solid such as a metal hydroxide. Since
the clad is stagnated and accumulated intermediate between
an organic phase and an aqueous phase in an oil separator,
it greatly inhibits the separation of oil and water which
is an important technical element of solvent extraction.
6
CA 2943483 2017-12-05
[0016]
A method for separating cobalt with an amine
extractant from an aqueous nickel chloride solution
containing cobalt and other impurity elements is based on
a technique as will be described below in a solvent
extraction step comprising an extraction stage, a washing
stage, and a stripping stage.
[0017]
As the extractant, it is more preferred to use a
tertiary amine (R3N) than to use a primary amine (RNH2) or
a secondary amine (R2NH) (wherein R represents an optional
saturated or unsaturated hydrocarbon group).
The reason is that a tertiary amine is more polar, is
highly reactive, and has a lower solubility in water.
When the tertiary amine is activated by adding
hydrochloric acid thereto, it holds the extraction
capability of metal-chloro complex ions and also has
excellent separation characteristics of nickel and cobalt.
[0018]
In the above extraction stage, a metal species which
forms a chloro complex ion, such as Co, Cu, Zn, and Fe, is
extracted into an organic phase to produce an amine
carrying a chloro complex ion of a metal element. Note
that since nickel does not form a chloro complex ion, it
remains in the extraction residual solution and is
separated.
Therefore, when an aqueous nickel chloride solution
contains a chloro complex ion of a metal which more easily
7
CA 2943483 2017-12-05
forms a chloro complex ion than cobalt, that is, has a
higher stability of a chloro complex ion than cobalt, for
example, a chloro complex ion of copper, zinc, or iron,
such a metal will also be extracted.
[0019]
A washing stage is optionally provided. In the
washing stage, when a large number of entrainment in an
organic phase after extraction, that is, impurities
contained in fine waterdrops suspended in the organic
phase, are present, the impurities are removed by dilution
and removal treatment with washing water.
[0020]
Next, in the stripping stage, cobalt can be
eliminated and transferred into an aqueous phase by
bringing the washed organic phase, that is, the amine
carrying the chloro complex ion of cobalt, into contact
with a weakly acidic aqueous solution.
Here, the organic phase from which cobalt has been
stripped, that is, the regenerated extractant, is returned
again to the extraction stage and cyclically used. Thus,
extraction, washing, and stripping will be repeated.
[0021]
However a metal which is more easily carried by amine
as a chloro complex ion than cobalt, such as copper, zinc,
and iron, is hardly eliminated under relatively weak
stripping conditions for eliminating cobalt. Therefore,
when the amine extractant is cyclically used in the
8
CA 2943483 2017-12-05
solvent extraction step, copper, zinc, iron, and the like
are gradually accumulated in the extractant.
If such accumulation of metal advances, an amino
group which should contribute to extractive reaction will
be occupied by the accumulated metal. Therefore, this will
cause a sharp reduction in the extraction capability of an
extractant. Further, since the viscosity of extractant
increases, the increase will cause a reduction in oil-
water separability.
[0022]
In order to cope with the above problem, a scrubbing
stage has been provided to regenerate the extractant in
order to separate and remove, for example, copper, zinc,
iron, and the like from the amine extractant carrying a
chloro complex ion of these metals.
For example, Patent Literature 2 describes a method
including taking out a part of an organic phase after
stripping, removing zinc contained in the organic phase as
an impurity by neutralization treatment, then activating
the extractant, and bringing the activated organic phase
into contact with a stripped aqueous phase which is an
aqueous cobalt chloride solution.
[0023]
The method of removing copper, zinc, iron, and the
like contained in the amine extractant after stripping by
neutralization treatment is based on a reaction
accompanied by precipitate formation. Therefore, it is
necessary to subject a mixture containing an organic phase,
9
CA 2943483 2017-12-05
an aqueous phase, and a precipitate to solid-liquid
separation with filtration equipment such as a filter
press. The method is not only poor in filterability,
handleability, and workability, but also has a safety
problem in that a hazardous material is filtered with
machinery and equipment. Therefore, the method is hardly
said to be a preferred method.
Further, since the organic phase adheres to the
precipitate, this problem leads to the loss of expensive
extractant, causing an increase in the extractant cost.
And further, in the disposal of the mixture of the
taken-out oil and the heavy metal, considerable technique
and cost have been required in order to prevent an
environmental problem from occurring.
[0024]
Therefore, an improved method shown in Patent
Literature 3 is proposed and performed as a means to solve
these problems.
This method includes subjecting an organic phase
after stripping to alkali neutralization, taking out an
organic phase which does not contain a precipitate by
sedimentation from a mixture containing an organic phase,
an aqueous phase, and a precipitate after alkali
neutralization, and subjecting a mixture containing an
aqueous phase, a precipitate, and the balance of the
organic phase to acid dissolution.
CA 2943483 2017-12-05
[0025]
An aqueous phase containing copper, zinc, iron, and
the like after acid dissolution is sent to a next
treatment step such as a waste water treatment step, and
an organic phase containing copper, zinc, iron, and the
like is returned to the scrubbing stage.
This method has eliminated the handling of the
precipitate mixed with the organic phase and the aqueous
phase.
[0026]
In the scrubbing methods of the above Patent
Literature 2 and Patent Literature 3, an alkali for
neutralization and hydrochloric acid for activation are
required. Therefore, a method which does not use these
chemicals is also proposed, for example, in Patent
Literature 4.
This is a method including washing an organic phase
after stripping with water or an aqueous solution having a
chloride ion concentration of 0 to 5 g/L so that a ratio
(organic phase/aqueous phase) may be set to 1 to 10. This
method is a method in which equipment is simple and which
is advantageous in cost, as compared with the methods of
Patent Literature 2 and Patent Literature 3.
[0027]
However, this method is a technique that is realized
only under the conditions within a limited narrow range in
which the solution to be extracted contains 25 mg/L or
11
CA 2943483 2017-12-05
less of iron and 0.1 mg/L or less of zinc. Further, the
target impurity metal is iron or zinc.
[0028]
Incidentally, in the process of leaching a nickel
matte and a mixed sulfide with chlorine gas and subjecting
leached nickel ions and cobalt ions to electrowinning to
be produced as electric nickel and electric cobalt as
described in Patent Literature 1, copper contained in a
very small amount in the raw material such as the nickel
matte and the mixed sulfide is utilized as an oxidizing
agent in the chlorine leaching step and the displacement
leaching step and circulated between the chlorine leaching
step and the displacement leaching step.
Therefore, it is necessary to maintain the copper
concentration in the chlorine leaching solution at an
appropriate level in the range of 10 to 60 g/L.
[0029]
On the other hand, since copper is an impurity in the
refining of nickel and cobalt, the copper concentration in
the displacement leaching solution needs to be 0.02 g/L or
less.
In order to reduce the copper concentration in the
displacement leaching soluLion to a level that is lower
than the copper concentration in the chlorine leaching
solution, there is required a nickel matte containing Ni
and Ni3S2 which have a higher reducing power than that of
NiS contained in the mixed sulfide.
12
CA 2943483 2017-12-05
[0030]
However, if the amount of the mixed sulfide treated
is increased for increasing the production volume of
nickel and cobalt, a situation of relative shortage of the
nickel matte will also occur. Therefore, a technique that
can flexibly respond to the change of the raw material
composition ratio as much as possible has been required.
Specifically, it has been required to establish a
technique of removing copper that can respond even if the
copper concentration in a leachate after displacement
leaching, that is, the copper concentration in a solution
to be extracted in the solvent extraction step, has
increased from 0.02 g/L to 0.2 g/L.
[0031]
Although copper accumulated in the extractant can be
removed by a method of alkali neutralization in the above
scrubbing stage as a countermeasure to the above problem,
an alkali for neutralization and hydrochloric acid for
activation are required, resulting in not only an increase
in cost but also an increase in a scrubbing ratio of the
stripped organic phase.
[0032]
Further, the alkali neutralization causes various
problems: for example, an organic solvent is degraded and
decomposed with a strong alkali to reduce the extraction
capability of amine, and the concentration of the
decomposed organic substance in the aqueous phase after
scrubbing increases to increase the COD load to wastewater.
13
CA 2943483 2017-12-05
[0033]
Furthermore, since the above alkali neutralization
process is a method in which zinc is the main target of
removal, it has been required to establish a technique of
selectively removing copper accumulated in the amine
extractant independently from the method.
Citation List
Patent Literature
[0034]
Patent Literature 1:
Japanese Patent Laid-Open No. 2012-026027
Patent Literature 2:
Japanese Patent Laid-Open No. S60-121236
Patent Literature 3:
Japanese Patent Laid-Open No. 2010-196162
Patent Literature 4:
Japanese Patent Laid-Open No. 2010-196122
Summary
Technical Problem
[0035]
An object of selected embodiments is to provide
method for removing copper from an aqueous nickel chloride
solution including separating and recovering cobalt and
removing copper, zinc, and iron, from an aqueous nickel
chloride solution, by solvent extraction with an organic
solvent formed by using a tertiary amine as an extractant
14
CA 2943483 2017-12-05
and using an aromatic hydrocarbon as a diluent of the
amine, the method being capable of preventing reduction in
the extraction capability and oil-water separability of
the organic solvent by selectively removing copper
accumulated in the extractant, and, as compared with prior
art, the method being capable of treating an aqueous
nickel chloride solution having a high copper
concentration without increasing chemical cost and without
degrading and decomposing the extractant to increase the
COD load of wastewater.
Solution to Problem
[0035a]
Certain exemplary embodiments provide a method for
removing copper from an aqueous nickel chloride solution
including separating and recovering cobalt and removing
copper, zinc, and iron, from the aqueous nickel chloride
solution containing cobalt, copper, zinc, and iron, by
solvent extraction using, as an organic phase, an organic
solvent containing a tertiary amine as an extractant and
an aromatic hydrocarbon as a diluent, the method
sequentially comprising the following steps (1) to (3):
(1) an extraction step of extracting cobalt, copper, zinc,
and iron from the aqueous nickel chloride solution that
contains cobalt, copper, zinc, and iron and has a nickel
concentration of 170 to 210 g/L, a cobalt concentration of
2 to 10 g/L, and a copper concentration of 0.01 to 0.2 g/L,
into the organic phase to form a first organic phase
CA 2943483 2017-12-05
containing cobalt, copper, zinc, and iron and obtain an
aqueous nickel chloride solution from which cobalt, copper,
zinc, and iron are removed; (2) a stripping step of
bringing a weakly acidic aqueous solution into contact
with the first organic phase containing cobalt, copper,
zinc, and iron obtained in the step (1) to thereby
eliminate cobalt from the first organic phase to obtain a
second aqueous phase of an aqueous cobalt chloride
solution and a second organic phase after the stripping,
the second organic phase containing copper, zinc, and
iron; and (3) a copper recovery step of mixing and
contacting water or dilute hydrochloric acid having a pH
of 1 or more with the second organic phase after stripping
to selectively strip copper in the second organic phase
into a third copper-stripped aqueous phase to thereby
recover copper from the second organic phase, wherein the
extraction of step (1) is carried out using the third
organic phase obtained after removal of copper in the
step (3), a copper concentration in the second aqueous
cobalt chloride solution obtained in the stripping
step (2) is reduced to 0.3 g/L or less by the stripping of
the first organic phase with the weakly acidic aqueous
solution, thereby forming the second organic phase having
copper therein and the second aqueous solution, whereby a
copper concentration of 0.4 g/L or less in the third
organic phase is achieved by producing the second aqueous
solution having the copper concentration reduced to
0.3 g/L or less.
16
CA 2943483 2018-09-24
h
[0036]
In order to achieve the above object, the present
inventors have paid attention to the point that when the
concentration of chloride ions in an aqueous phase is low,
copper accumulated in an extractant after stripping easily
transfers to the aqueous phase as compared with zinc and
iron, and have made intensive studies on extraction
conditions such as the concentration of chloride ions in
the aqueous phase, that is, the pH of dilute hydrochloric
acid, to be mixed and brought into contact with the
stripped organic phase and the 0/A ratio. As a result, the
present inventors have found that, copper accumulated in
the extractant after stripping can be selectively removed
by mixing and contacting water or dilute hydrochloric acid
having a pH of 1 or more with the stripped organic phase
so that the 0/A ratio may be 1.5 or less, thereby
completing the method described herein.
[0037]
Specifically, the first aspect of selected
embodiments is a method for removing copper from an
aqueous nickel chloride solution including separating and
recovering cobalt and removing copper, zinc, and iron,
from the aqueous nickel chloride solution containing
cobalt, copper, zinc, and iron, by solvent extraction
using, as an organic phase, an organic solvent containing
a tertiary amine as an extractant and an aromatic
hydrocarbon as a diluent, the method sequentially
including the following steps (1) to (3).
17
CA 2943483 2017-12-05
[0038]
(1) An extraction step of extracting cobalt, copper, zinc,
and iron from the aqueous nickel chloride solution
containing cobalt, copper, zinc, and iron into the organic
phase to form an organic phase containing cobalt, copper,
zinc, and iron and obtain an aqueous nickel chloride
solution from which cobalt, copper, zinc, and iron are
removed;
(2) a stripping step of bringing a weakly acidic aqueous
solution into contact with the organic phase containing
cobalt, copper, zinc, and iron obtained in the step (1) to
thereby eliminate cobalt therein from the organic phase to
obtain an aqueous phase of an aqueous cobalt chloride
solution and an organic phase after stripping containing
copper, zinc, and iron; and
(3) a copper recovery step of mixing and contacting water
or dilute hydrochloric acid having a pH of 1 or more with
the stripped organic phase to strip copper in the organic
phase into the aqueous phase to thereby recover copper,
and using the organic phase containing zinc and iron from
which copper has been removed as an organic solvent in the
step (1).
[0039]
Next, the second aspect of the present invention is
the method for removing copper from an aqueous nickel
chloride solution according to the first aspect, wherein,
in the extraction step (1), the aqueous nickel chloride
solution containing cobalt, copper, zinc, and iron has a
18
CA 2943483 2017-12-05
nickel concentration of 170 to 210 g/L, a cobalt
concentration of 2 to 10 g/L, and a copper concentration
of 0.01 to 0.2 g/L; and the copper concentration in the
aqueous cobalt chloride solution obtained in the stripping
step (2) is reduced to 0.3 g/L or less by removing copper
in the organic phase containing cobalt, copper, zinc, and
iron obtained in the step (1) until the copper
concentration reaches 0.4 g/L or less.
[0040]
The third aspect of the present invention is the
method for removing copper from an aqueous nickel chloride
solution according to the first and second aspects,
wherein, in the copper recovery step (3), the volume ratio
of the organic phase to the aqueous phase is 1.5 or less.
[0041]
The fourth aspect of the present invention is the
method for removing copper from an aqueous nickel chloride
solution according to the first to third aspects, wherein
the tertiary amine is tri-normal-octylamine (TNOA) or tri-
iso-octylamine (TIOA).
Advantageous Effects of Invention
[0042]
According to the present invention, the reduction in
the extraction capability and oil-water separability of
the organic solvent can be prevented by selectively
removing copper accumulated in the extractant; and even if
the copper concentration in the solution to be extracted
19
CA 2943483 2017-12-05
which is an aqueous nickel chloride solution increases
from 0.02 g/L to 0.2 g/L, the copper concentration in an
aqueous cobalt chloride solution obtained in the stripping
step can be reduced to 0.3 g/L or less by removing copper
in the organic phase until the copper concentration
reaches 0.4 g/L or less.
[0043]
Further, as compared with the conventional art, since
an alkali for neutralization and hydrochloric acid for
activation are not required, the method of the present
invention does not increase chemical cost and does not
degrade and decompose the extractant to increase the COD
load of wastewater.
[0044]
Since the present invention is a simple method of
mixing and contacting water or dilute hydrochloric acid
having a pH of 1 or more with the stripped organic phase,
a simple equipment modification is enough, and efficient
operation can be achieved at a low cost and a low
environmental load.
In addition to the above effects, the present
invention can increase the raw material treatment ratio of
a mixed sulfide to a nickel matte, and can increase the
production volume of electric nickel and electric cobalt
by increasing the treatment of the mixed sulfide.
CA 2943483 2017-12-05
Brief Description of Drawings
[0045]
Figure 1 is a schematic flow sheet of a nickel and cobalt
smelting process including the present invention.
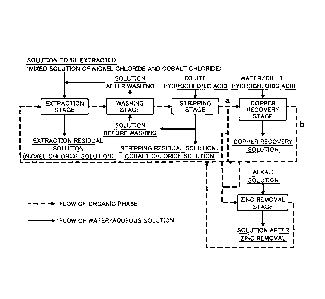
Figure 2 is a schematic flow sheet of the solvent
extraction step in the copper removal method of the
present invention.
Figure 3 is a view showing the relationship between the
copper concentration in an organic phase and the copper
concentration in an aqueous phase.
Figure 4 shows the relationship between the chloride ion
concentration in an aqueous phase and the distribution
ratio into an organic phase, with respect to copper, zinc,
and iron.
Description of Embodiments
[0046]
Hereinafter, the method for removing copper from an
aqueous nickel chloride solution of the present invention
will be described in detail.
The method for removing copper from an aqueous nickel
chloride solution of the present invention includes
separating and recovering cobalt and removing copper, zinc,
and iron, from an aqueous nickel chloride solution
containing cobalt, copper, zinc, and iron, by solvent
extraction using an organic solvent formed by using a
tertiary amine as an extractant and an aromatic
21
CA 2943483 2017-12-05
hydrocarbon as a diluent for diluting the extractant, the
method including the following steps (1) to (3).
[0047]
(1) Extraction step
An extraction step of extracting cobalt, copper, zinc,
and iron from the aqueous nickel chloride solution
containing cobalt, copper, zinc, and iron used as an
aqueous phase to form an organic phase containing cobalt,
copper, zinc, and iron and obtain an aqueous nickel
chloride solution (aqueous phase) from which cobalt,
copper, zinc, and iron are removed;
(2) Stripping step
A stripping step of eliminating cobalt from the
organic phase containing cobalt, copper, zinc, and iron
with a weakly acidic aqueous solution used as an aqueous
phase to obtain an aqueous cobalt chloride solution; and
(3) Copper recovery step
A copper recovery step of mixing and contacting water
or dilute hydrochloric acid having a pH of 1 or more used
as an aqueous phase with the stripped organic phase from
which cobalt has been eliminated to strip copper from the
organic phase into the aqueous phase and returning the
organic phase from which copper has been removed to the
extraction step (1).
[0048]
1. Nickel and Cobalt Smelting Process
Figure 1 shows a schematic flow sheet of a nickel and
cobalt smelting process including the present invention.
22
CA 2943483 2017-12-05
The present invention is a technique related to the
scrubbing of the stripped organic phase in the solvent
extraction step (the step shown in A in Figure 1) in all
the steps of the nickel and cobalt smelting process.
However, the present invention is also a technique for
achieving the total optimization with respect to the main
elements of the nickel and cobalt smelting process, such
as a raw material treatment ratio, chlorine leaching,
displacement leaching (cementation), solution purification
up to solvent extraction, and solvent extraction.
Therefore, each of the main elements will be described in
detail with reference to Figure 1.
[0049]
(1) Raw Material
There are two types of main raw materials, a nickel
matte and a mixed sulfide.
The nickel matte refers to nickel sulfide obtained by
a so-called pyrometallurgical process, such as nickel
sulfide obtained by melting nickel sulfide ore in a blast
furnace and nickel sulfide obtained by adding sulfur to
nickel oxide ore and melting the mixture in an electric
furnace.
[0050]
The nickel matte contains Ni3S2 and Ni (metallic
nickel) as a main component, and the approximate chemical
composition thereof is 65 to 80% by weight of Ni, about 1%
by weight of Co, 0.1 to 4% by weight of Cu, 0.1 to 5% by
weight of Fe, and 20 to 25% by weight of S.
23
CA 2943483 2017-12-05
[0051]
A nickel matte using nickel sulfide ore as a raw
material is characterized by higher impurity content as
compared with a nickel matte using nickel oxide ore as a
raw material, and the main input source of copper to the
nickel and cobalt smelting process is the nickel matte
using nickel sulfide ore as a raw material.
Therefore, the input amount of copper greatly varies
with the treatment amount of the nickel matte using nickel
sulfide ore as a raw material.
[0052]
On the other hand, the mixed sulfide refers to a
mixed sulfide containing nickel and cobalt obtained by
subjecting nickel oxide ore having a low nickel grade to
high pressure acid leaching and removing impurities
including iron from the high pressure acid leaching
solution, followed by wet sulfidation, for example, by
blowing hydrogen sulfide gas into the leaching solution
containing nickel ions and cobalt ions.
[0053]
The mixed sulfide contains NiS and CoS as main
components, and the approximate chemical composition
thereof is 55 to 60% by weight of Ni, 3 to 6% by weight of
Co, less than 0.1 by weight of Cu, 0.1 to 1% by weight of
Fe, and 30 to 35% by weight of S.
24
CA 2943483 2017-12-05
[0054]
(2) Chlorine Leaching
The mixed sulfide and a cementation residue to be
described below is repulped in a chloride aqueous solution,
and then chlorine gas is blown into the resulting slurry,
thereby chlorine-leaching nickel and cobalt in the mixed
sulfide and nickel and copper in the cementation residue
into the chloride aqueous solution.
[0055]
In this step, chloro complex ions of divalent copper
act as a direct leaching agent for dissolving a metal in
the mixed sulfide and the cementation residue, and the
chlorine gas indirectly participates in the leaching
reaction by oxidizing monovalent ions of copper to
divalent ions of copper.
Therefore, a certain amount of copper is
indispensable in the chlorine leaching reactions, and it
is important to maintain the copper concentration in the
chlorine leaching solution at an appropriate level in the
range of 10 to 60 g/L.
Main chlorine leaching reaction formulas are shown in
the following formulas (1) to (4).
[0056]
[Formula 1]
NiS + 2CuC142- -+ Ni2+ + S + 2C1- + 2CuC132- ¨(1)
Cu2S + 2CuC142- + 4C1- ¨> 4CuC132- + Su ¨(2)
Cu + CuC142- ¨> 2CuC132- -= ( 3 )
2CuC132 + C12 --> 2CuC142- -= (4)
CA 2943483 2017-12-05
[0057]
The chlorine leaching reaction conditions include an
oxidation-reduction potential of the aqueous nickel
chloride solution during the reactions of 480 to 550 mV
(based on Ag/AgC1 electrode) and a temperature of 105 to
115 C.
[0058]
(3) Displacement Leaching (Cementation)
The displacement leaching step comprises two stages,
a first displacement leaching step ("displacement leaching
1" in Figure 1) and a second displacement leaching step
("displacement leaching 2" in Figure 1).
In the first displacement leaching step, nickel and
cobalt in the mixed sulfide is leached using the oxidizing
power of chloro complex ions of divalent copper contained
in the chlorine leaching solution.
In the displacement leaching solution obtained in the
first displacement leaching step, the chloro complex ion
of divalent copper is reduced to the chloro complex ion of
monovalent copper.
[0059]
Next, in the second displacement leaching step, the
displacement leaching solution obtained in the first
displacement leaching step is brought into contact with a
nickel matte to thereby perform the cementation reaction
between copper ions in the displacement leaching solution
and nickel in the nickel matte.
26
CA 2943483 2017-12-05
In the cementation reaction, solid nickel is eluted
to form nickel ions, and copper ions in the solution which
are electrochemically equivalent to the eluted nickel form
a solid. Therefore, it can be said that the displacement
leaching step is a copper removal step of removing copper
contained in the chlorine leaching solution as a solid.
[0060]
Since the copper ions in the displacement leaching
solution form a solid in the form of Cu2S or Cu metal, the
copper concentration in the leachate after displacement
leaching obtained in the second displacement leaching step
is 0.02 g/L or less.
[0061]
In order to reduce the copper concentration in the
leachate after displacement leaching, there is required a
nickel matte containing Ni (metallic nickel) and Ni3S2
which have a higher reducing power than that of NiS
contained in the mixed sulfide.
[0062]
A cementation residue including insoluble residues of
the mixed sulfide and the nickel matte and a solid
containing copper obtained from the cementation reaction
is subjected to solid-liquid separation from the leachate
after displacement leaching obtained in the second
displacement leaching sLep and sent to the chlorine
leaching step.
Main displacement leaching reaction formulas are
shown in following formula (5) to (7).
27
CA 2943483 2017-12-05
[0063]
[Formula 2]
Ni3S4 + 6CuC142- ¨> 3Ni2+ + 4S + 6C1- + 6CuC132- === (5)
4CuC132- + S ¨> Cu2S + 2CuC142- 4C1- ( 6)
Ni + 2CuC132- -4 Ni2+
+ 20u + 6C1- (7)
[0064]
The displacement leaching reaction conditions include
an oxidation-reduction potential of the aqueous nickel
chloride solution during the reactions of 50 to 300 mV
(based on Ag/AgC1 electrode) and a temperature of 70 to
100 C.
[0065]
In the second displacement leaching step, the amount
of copper to be removed from the solution is determined by
the product of the amount of the chlorine leaching
solution and the copper concentration in the chlorine
leaching solution.
The amount of the chlorine leaching solution is
determined by the amount of the mixed sulfide treated in
the chlorine leaching step and the first displacement
leaching step.
The copper concentration in the chlorine leaching
solution will be a constant value because the
concentration is maintained at an appropriate level in the
range of 10 to 60 g/L in order to continue the optimum
chlorine leaching operation.
28
CA 2943483 2017-12-05
[0066]
Therefore, when the treatment ratio of the nickel
matte to the mixed sulfide is reduced, the nickel matte
may be insufficient for the amount of copper to be removed.
In this case, the copper concentration in the leachate
after displacement leaching obtained in the second
displacement leaching step will increase.
[0067]
(4) Solution Purification
A leachate after displacement leaching (solution
after cementation) is sent to a solution purification step.
The solution purification step comprises an iron removal
step, a solvent extraction step, a lead removal step, and
a zinc removal step.
[0068]
In the iron removal step, the leachate after
displacement leaching is subjected to a treatment in which
chlorine gas as an oxidizing agent and a nickel carbonate
slurry as a neutralizing agent are added to the leachate
after displacement leaching to produce a precipitate
containing ferric hydroxide as a main component to thereby
reduce the iron concentration in the leachate after
displacement leaching from 1 to 2 g/L to 15 mg/L or less.
Since the pH of the aqueous solution in the iron
removal step is about 2.0 to 2.5, copper hydroxide is not
produced in this step.
Further, since copper is not removed in the iron
removal step, when the copper concentration in the
29
CA 2943483 2017-12-05
leachate after displacement leaching increases, the copper
concentration in the solution to be extracted to be
supplied to the next solution purification step (solvent
extraction) will increase.
[0069]
Although details will be described below, in the
solvent extraction step, TNOA which is an amine extractant
is mixed and brought into contact with an iron-removed
solution having a nickel concentration of 170 to 210 g/L,
a cobalt concentration of 2 to 10 g/L, a copper
concentration of 0.02 g/L or less, a zinc concentration of
0.01 to 0.03 g/L, and an iron concentration of 15 mg/L or
less, thereby transferring cobalt, copper, zinc, and iron
from the aqueous phase to the organic phase.
[0070]
In the lead removal step, chlorine gas is added as an
oxidizing agent and a nickel carbonate slurry is added as
a neutralizing agent, thereby removing lead in the nickel
chloride solution after solvent extraction as lead oxide,
in the same manner as in the iron removal step.
Since the pH of the extraction residual solution in
the lead removal step is 4 to 5, a part of nickel also
forms a precipitate as a trivalent hydroxide.
[0071]
In the zinc removal step, the chloro complex ion of
zinc remaining in a very small amount of about 0.1 mg/L in
a de-leaded solution after lead removal is removed by
being adsorbed onto a weakly basic anion exchange resin.
CA 2943483 2017-12-05
[0072]
2. Solvent Extraction Step
Next, the solvent extraction step constituting the
copper removal method according to the present invention
and constituting a part of the above solution purification
step (4) will be described in detail.
[0073]
(1) Constitution of Solvent Extraction Step
Figure 2 shows a schematic flow sheet of the solvent
extraction step according to the present invention. The
flow sheet also shows the flow of the copper removal
method according to the present invention.
A multistage countercurrent system is employed in the
solvent extraction, and the system comprises an extraction
stage, a washing stage, a stripping stage, and a copper
recovery stage.
An extraction apparatus of a mixer settler system is
used, and in Examples used for describing the present
invention, the extraction stage is constituted by three
stages, the washing stage is constituted by three stages,
the stripping stage is also constituted by three stages,
and the copper recovery stage is constituted by one stage.
Note that, in order to remove zinc and iron in the
stripped organic phase in the stripping stage, a zinc
removal stage is preferably provided in parallel or in
series with the copper recovery stage.
31
CA 2943483 2017-12-05
[0074]
That is, when the copper recovery stage and the zinc
removal stage are independently operated, copper, zinc,
and iron in the organic phase can be efficiently removed:
the copper recovery stage including taking out a
predetermined amount of the stripped organic phase
depending on the copper concentration in the solution to
be extracted, and mixing and bringing the taken-out
organic phase into contact with water or dilute
hydrochloric acid having a pH of 1 or more to strip copper
in the organic phase into the aqueous phase; and the zinc
removal stage including a predetermined amount of the
stripped organic phase depending on the degree of
enrichment of zinc or iron in the stripped organic phase,
and neutralizing the taken-out organic phase with an
alkali to remove zinc and iron in the organic phase.
[0075]
Since zinc and iron are input from a mixed sulfide,
the zinc concentration in the solution to be extracted is
influenced by the zinc content in the mixed sulfide and
the treatment amount of the mixed sulfide.
Since iron is removed in the iron removal step, the
iron concentration of the solution to be extracted is
always constant.
On the other hand, the copper concentration in the
solution to be extracted is determined by the treatment
ratio of the nickel matte to the mixed sulfide.
32
CA 2943483 2017-12-05
[0076]
That is, since the zinc concentration and the copper
concentration in the solution to be extracted
independently vary, and particularly the width of
variation of the copper concentration is large, it is more
efficient to independently operate the removal of zinc and
the removal of copper in the organic phase.
[0077]
Further, the cost can be reduced also by a serial
arrangement, that is, by a method including mixing and
contacting water or dilute hydrochloric acid haying a pH
of 1 or more as an aqueous phase with an organic phase a
stripped to strip copper from the organic phase a into the
aqueous phase and then neutralizing an organic phase b
after copper recovery with an alkali to remove zinc and
iron in the organic phase, because the amount of the
alkali used in the zinc removal stage can be reduced
consistent with the amount of copper.
[0078]
(2) Extractant and Reaction
A tertiary amine is used as an extractant, and tri-
normal-octylamine (TNCA) or tri-iso-octylamine (TIOA) is
preferably used.
An aromatic hydrocarbon is used as a diluent of the
extractant.
In order to adjust the viscosity of an organic phase,
the extractant concentration in the organic phase
(extractant and diluent) is set to 20 to 40% by volume.
33
CA 2943483 2017-12-05
I 1
[0079]
The tertiary amine is activated by the addition of
hydrochloric acid according to the following formula (8).
Thereby, the tertiary amine holds the extraction
capability of metal-chloro complex ions as shown in
formula (9) and formula (9') and has excellent separation
characteristics of nickel and cobalt.
[0080]
[Formula 3]
R3N: + HC1 -* R3N:HC1 (8)
2R3N:HC1 + MC142 -* (R3N:H)2MC14 + 2C1- -.(9)
[0081]
M in the above formula (9) represents a metal species
which forms a chloro complex ion, such as Co, Cu, and Zn.
Since the form of the chloro complex ion is different
depending on the valence of a metal ion, the following
formula (9') is valid, for example, in the case of Fe
(trivalent).
Note that ":" in formula (8), formula (9), and
formula (9') represents the unshared electron pair of a
nitrogen atom.
[0082]
[Formula 4]
R3N:HC1 + FeC14 R3N:HFeC14 + Cl-
[0083]
In the extraction stage, a metal species which forms
a chloro complex ion, such as Co, Cu, Zn and Fe, is
extracted into an organic phase by the reaction shown by
34
CA 2943483 2017-12-05
formula (9) or formula (9') to produce an amine carrying a
chloro complex ion of the metal element. In this regard,
since nickel does not form a chloro complex ion, it
remains in the extraction residual solution and is
separated.
Therefore, when an aqueous nickel chloride solution
contains a chloro complex ion of a metal which more easily
forms a chloro complex ion than cobalt, that is, has a
higher stability of a chloro complex ion than cobalt, for
example, copper, zinc, or iron, such a metal will also be
extracted.
[0084]
On the other hand, in the stripping stage, cobalt can
be eliminated and transferred into an aqueous phase
according to the following formula (10) which is a reverse
reaction of formula (9) by bringing the organic phase
after washing, that is, the amine carrying the chloro
complex ion of cobalt, into contact with a weakly acidic
aqueous solution.
[0085]
[Formula 5]
(R3N:H)2CoC14 -* 2R3N:HC1 + CoC12 -(10)
[0086]
3. Copper Removal by Solvent Extraction
The main function of solvent extraction is to extract
and separate cobalt from a solution to be extracted (mixed
solution of nickel chloride and cobalt chloride) in the
first place. However, if copper can be selectively
CA 2943483 2017-12-05
eliminated from the stripped organic phase as further
function, the role of a step of removing copper in the
solution to be extracted can also be played.
[0087]
Therefore, although the removal of copper from the
chlorine leaching solution in the nickel and cobalt
smelting process is basically performed in the
displacement leaching (cementation) step, the removal of
copper by solvent extraction will exhibit an effect when
the copper concentration in the leachate after
displacement leaching greatly varies or increases due to
the shortage of a nickel matte and the like.
[0088]
It is the first feature of the present invention to
use the solvent extraction as a copper removal step.
In the extraction stage, even if the copper
concentration in the solution to be extracted is as high
as 0.2 g/L, almost the whole amount of copper ions can be
extracted into the organic phase.
This is because the chloride ion concentration in the
solution to be extracted is as high as 200 to 250 g/L, and
copper forms a stable chloro complex ion.
[0089]
On the other hand, in the stripping stage for
recovering cobalt as an aqueous cobalt chloride solution,
the chloride ion concentration is 70 to 100 g/L, which is
lower than that in the extraction stage.
36
CA 2943483 2017-12-05
Therefore, when the chloride ion concentration is 70
to 100 g/L, the copper-chloro complex ion in the organic
phase will be unstable, and a part of copper is stripped
into the aqueous phase.
Since the copper concentration in the aqueous phase
is proportional to the copper concentration in the organic
phase, the copper concentration in the aqueous cobalt
chloride solution will increase when the copper
concentration in the organic phase increases.
When the copper concentration in the aqueous cobalt
chloride solution increases, the solution purification
(copper removal) load of a subsequent aqueous cobalt
chloride solution will increase, and the copper content in
electric cobalt as a product may be increased.
[0090]
Figure 3 shows the relationship between the copper
concentration in an organic phase and the copper
concentration in an aqueous phase in the stripping stage.
The data in Figure 3 is the operation data when an
organic solvent, containing 20% by volume or 30% by volume
of tri-normal-octylamine (TNOA) as an extractant and 80%
by volume or 70% by volume of an aromatic hydrocarbon as a
diluent, is used in the solvent extraction step of a
multistage countercurrent system, comprising three
extraction stages, three washing stages, and three
stripping stages.
37
CA 2943483 2017-12-05
[0091]
The solution to be extracted (mixed aqueous solution
of nickel chloride and cobalt chloride) has a nickel
concentration of 170 to 210 g/L, a cobalt concentration of
2 to 10 g/L, and a copper concentration of 0.02 to
0.2 g/L; and the stripping residual solution (aqueous
cobalt chloride solution) has a cobalt concentration of 50
to 70 g/L.
[0092]
Figure 3 shows that although the inclination of a
regression line, that is, the distribution ratio, changes
with the concentration of the extractant, the copper
concentration in the aqueous cobalt chloride solution can
be reduced to 0.3 g/L or less by reducing the copper in
the organic phase down to a copper concentration of
0.4 g/L or less.
It is the second feature of the present invention to
produce the aqueous cobalt chloride solution having a low
copper concentration.
[0093]
The copper recovery stage of the present invention is
a step of selectively separating and removing copper from
copper, zinc, and iron concentrated in the organic phase
into the aqueous phase.
The stability of copper, zinc, and iron in the
organic phase is the stability of the chloro complex ion
of a metal, and the stability is positively correlated
with the chloride ion concentration in the aqueous phase.
38
CA 2943483 2017-12-05
[0094]
Figure 4 shows the relationship between the chloride
ion concentration in the aqueous phase and the
distribution ratio into the organic phase, with respect to
copper, zinc, and iron.
Here, among copper, zinc, and iron, copper has the
lowest stability in the organic phase and tends to be
distributed into the aqueous phase.
Therefore, if an aqueous phase having a low chloride
ion concentration is mixed and brought into contact with
the stripped organic phase, copper in the organic phase
can be selectively eliminated and transferred into the
aqueous phase.
[0095]
As a result of intensive studies on the stripping
behavior of the chloride ion concentration and copper, the
present inventors have been able to find that water or
dilute hydrochloric acid having a pH of 1 or more is
suitable as a solution before stripping.
Among these, copper is easily eliminated from the
organic phase when water is used, but the use of
hydrochloric acid having a pH of 1 is favorable because
the elimination of hydrochloric acid from the organic
phase can be prevented, and a subsequent activation
treatment of the extractant with hydrochloric acid is not
required.
39
CA 2943483 2017-12-05
[0096]
According to the present invention, a solvent
extraction step as shown in Figure 2 is constituted to
adjust the copper concentration in the organic phase to
0.4 g/L or less. Thereby, a solution to be extracted
having a copper concentration of up to 0.2 g/L can be
treated, and the copper concentration in the aqueous
cobalt chloride solution after stripping can be reduced to
0.3 g/L or less.
In other words, it is possible to cope with the
copper load 10 times the conventional art (zinc removal
stage by alkali neutralization).
[0097]
In the present invention, since copper is recovered
without using an alkali, it is possible to achieve cost
reduction as compared with a zinc removal stage of
conventional art in which zinc and iron in the organic
phase are removed, for example, by alkali neutralization.
[0098]
Further, since the organic phase is not brought into
contact with strong alkali, the COD components produced by
the decomposition of the organic phase are present in the
copper recovery solution only in a low concentration, and
the COD load to a post step can be reduced.
That is, various problems of conventional art (zinc
removal stage by alkali neutralization) can be solved by
performing weak elimination using water having a pH of
CA 2943483 2017-12-05
from 1 to a neutral region instead of or in combination
with conventional strong elimination using an alkali.
Note that the elimination of hydrochloric acid from
the organic phase can also be suppressed by using dilute
hydrochloric acid having a pH of about 1 for a solution
before copper recovery.
Examples
[0099]
Next, the present invention will be further described
using Examples.
Example 1
[0100]
In a solvent extractor using a mixer settler of a
multistage countercurrent system comprising three
extraction stages, three washing stages, and three
stripping stages, solvent extraction operation was
performed using an organic solvent containing 20% by
volume of tri-normal-octylamine (TN0A) which is a tertiary
amine as an extractant and 80% by volume of an aromatic
hydrocarbon as a diluent.
[0101]
The solution to be extracted (mixed aqueous solution
of nickel chloride and cobalt chloride) has a nickel
concentration of 170 to 210 g/L, a cobalt concentration of
2 to 10 g/L, a copper concentration of 0.01 to 0.02 g/L, a
zinc concentration of 0.02 to 0.03 g/L, and an iron
concentration of 10 to 14 mg/L; and the stripping residual
41
CA 2943483 2017-12-05
solution (aqueous cobalt chloride solution) has a cobalt
concentration of 50 to 70 g/L and a copper concentration
of 0.1 to 0.2 g/L.
With respect to each flow rate, the flow rate of the
organics is 1000 to 1300 L/min; the flow rate of the
solution to be extracted is 1300 to 1600 L/min; and the
flow rate of the solution before stripping is 140 to
170 L/min.
[0102]
Subsequently, a part of the stripped organic phase
was sent to the copper recovery stage (one stage) using a
mixer settler as in the extraction stage and the like, and
mixed with neutral water.
The flow rate of the organic phase was set to 48 to
54 L/min; the flow rate of the aqueous phase was set to 48
to 54 L/min; and the 0/A ratio was set to 0.9 to 1.1.
[0103]
The results are shown in Table 1, which reveals that
the copper in the organic phase is selectively stripped
into the aqueous phase.
In this regard, the COD in the aqueous phase after
copper recovery was 20 mg/L.
In Tables 1 and 2, "Stripping organic" represents the
organic phase "a" after the stripping in Figure 2, and
"Copper recovery organic" represents the organic phase "b"
after the copper recovery in Figure 2.
42
CA 2943483 2017-12-05
[0104]
[Table 1]
Stripping organic Copper recovery organic
Cu (g/L) Fe (g/L) Zn (g/L) Cu (g/L) Fe (g/L) Zn (g/L)
0.29 0.43 0.87 0.07 0.24 0.82
Example 2
[0105]
Solvent extraction operation was performed under the
same conditions as in Example 1 except that an organic
solvent containing 30% by volume of TNOA and 70% by volume
of an aromatic hydrocarbon was used.
Subsequently, the organic phase and the aqueous phase
were mixed at an 0/A ratio of 0.9 to 1.1 in the same
manner as in Example 1 except that a part of the stripped
organic phase was sent to the copper recovery stage, and
dilute hydrochloric acid having a pH of 1 was used as the
aqueous phase.
[0106]
The results are shown in Table 2, which revealed that
copper in the organic phase was selectively stripped into
the aqueous phase even with hydrochloric acid having a pH
of 1 in a manner similar to neutral water.
[0107]
[Table 2]
Stripping organic Copper recovery organic
Cu (g/L) Fe (g/L) Zn (g/L) Cu (g/L) Fe (g/L) Zn (g/L)
0.14 0.93 2.00 0.01 0.61 1.70
43
CA 2943483 2017-12-05
[0108]
(Comparative Example 1)
Solvent extraction was performed under the same
conditions as in Example 1, and a part of the stripped
organic phase was then sent to the zinc removal stage (one
stage) comprising three 3-m3 FRP tanks and mixed with
dilute caustic soda in a neutralization tank which is the
first tank. Here, the dilute caustic soda used has a
caustic soda concentration of 118 g/L.
Subsequently, from a mixture containing an organic
phase, an aqueous phase, and a precipitate after alkali
neutralization, the organic phase which does not contain
the precipitate was taken out by overflow, and a mixture
containing the aqueous phase, the precipitate, and the
balance of the organic phase was sent to an acid dissolver
which is the second tank to be subjected to acid
dissolution with 35% hydrochloric acid.
[0109]
Note that the flow rate of taking out the organic
phase was set to 48 to 54 L/min, and the flow rate of the
dilute caustic soda was set to 48 to 54 L/min. The amount
of the 35% hydrochloric acid used was set to 15 L/min.
[0110]
The resulting COD concentration in the aqueous phase
after zinc removal by alkali neutralization was 230 mg/L.
[0111]
Thus, copper in the organic phase can be selectively
removed to reduce copper to a target concentration by
44
CA 2943483 2017-12-05
treating the stripping organic in the copper recovery
stage, and the copper concentration in the stripped
organic phase is adjusted to 0.4 g/L or less by adjusting
the amount of the organic solvent taken out, that is, the
treatment amount in the copper recovery stage.
[0112]
Further, as compared with Comparative Example
(conventional method), neither caustic soda for
neutralization nor hydrochloric acid for acid dissolution
is required, and the COD concentration in the aqueous
phase after treatment can be reduced to about 1/10.
Note that since when the copper concentration in the
organic phase is further increased, the copper
concentration in the aqueous phase will increase with the
increase, the amount that is removed from the organic
solvent will increase.
Reference Signs List
[0113]
a Organic phase after stripping
Organic phase after copper recovery
CA 2943483 2017-12-05