Note: Descriptions are shown in the official language in which they were submitted.
CA 02945955 2016-10-14
WO 2015/160358
PCT/US2014/034601
TITLE: MULTIPLE SPEED PROCESS FOR PRESERVING HEAT SENSITIVE
PORTIONS OF A THERMOKINETICALLY MELT BLENDED BATCH
INVENTOR: Chris Brough
TITLE OF INVENTION
Multiple Speed Process for Preserving Heat Sensitive Portions of a
Thermokinetically Melt Blended Batch
RELATED APPLICATIONS
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present disclosure relates in general to the field of pharmaceutical
manufacturing, and more particularly, to thermokinetic mixing of active
pharmaceutical ingredients (APIs) to produce novel dosage forms.
2. DESCRIPTION OF RELATED ART
Current high-throughput molecular screening methods used by the
pharmaceutical industry have resulted in a vast increase in the proportion of
newly
discovered molecular entities which are poorly water-soluble. The therapeutic
potential of many of these molecules is often not fully realized either
because the
molecule is abandoned during development due to poor pharmacokinetic profiles,
or
because of suboptimal product performance.
Also, in recent years the
pharmaceutical industry has begun to rely more heavily on formulational
methods for
improving drug solubility owing to practical limitations of salt formation and
chemical
modifications of neutral or weakly acidic/basic drugs. Consequently, advanced
formulation technologies aimed at the enhancement of the dissolution
properties of
poorly water-soluble drugs are becoming increasingly more important to modern
drug delivery.
U.S. Pat. No. 4,789,597, issued to Gupta, is directed to the incorporation of
chemically reactive agents on resin particles. Briefly, chemically reactive
agents are
1
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
locked to particles of suitable synthetic resins without wholly fluxing the
resins. A
high quality intermediate product is obtained having no premature reaction
taking
place, suitable for further techniques. The process includes the steps of
intensively
mixing and thermokinetically heating a batch of finely divided resin
particles, with a
chemically reactive agent, in an enclosed mixing chamber with a plurality of
blades
attached to arms rotating about a central axis within the chamber, and having
a
blade tip speed of at least about 18 meters per second, mixing the batch until
the
chemically reactive agent is locked to the resin particles, ensuring that
temperature
of the batch stays well below decomposition temperature of the reactive agent
and
below fluxing temperature of the resin particles, discharging the batch from
the
mixing chamber and cooling the discharged batch to avoid agglomeration of the
resin
particles.
U.S. Pat. No. 5,895,790, issued to Good, is directed to thermosetting a wide
range of polymer blends. Briefly, a wide range of polymer blends and waste
thermoset material can be recovered. One method of thermosetting a wide range
polymer blends forms a homogenous and adaptable material. This material has a
melt index of zero and a relatively predictable density. Very high levels of
fibrous
non-polymers may be added to the first material.
U.S. Pat. No. 6,709,146, issued to Little, is directed to a thermokinetic
mixer
and method of using the mixer. Briefly, a thermokinetic mixer has a mixing
chamber
with shaft projections removable at least in part and replaceable without
cutting the
projections from the shaft. In one embodiment, only a tip portion of such
projections
are removable and replaceable without such cutting. In another embodiment,
shaft
projections into the mixing chamber include a tooth having a substantially
reticulated
face forming a deflecting surface such that substantially all mixing chamber
particles
encountering the tooth strike are deflected at an incident substantially
lateral angle
from the deflecting surface.
U.S. Pat. No. 4,764,412, issued to Burns, discloses the use of a high speed
mixer with a heated jacket about its vertical mixing chamber to first mix a
set of
components at 1700 rpm. The high speed mixer is stopped and after additional
components are added, the rotational speed of the mixer is increased to 3400
rpm.
2
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
Operation of the high speed mixer at a rotation speed of 3400 rpm generates
heat
which is advantageous in further processing of the mixture.
U.S. Patent Appl. No. 12/196,154, filed by the same inventor as this
application and additional co-inventors, is directed to the application of
thermokinetic
compounding in the field of pharmaceutical manufacturing.
Thermokinetic
compounding is a method of thermokinetic mixing until melt blended.
A
pharmaceutical composition or composite made by thermokinetic compounding may
be further processed according to methods well known to those of skill in the
field,
including but not limited to hot melt extrusion, melt granulation, compression
molding, tablet compression, capsule filling, film-coating, or injection
molding into a
final product. One embodiment is directed to a method of making a
pharmaceutical
composition that includes one or more active pharmaceutical ingredients with
one or
more pharmaceutically acceptable excipients by the thermokinetic compounding
process. Another embodiment is directed to the composite comprising one or
more
APIs with one or more pharmaceutically acceptable excipients made by
thermokinetic compounding is the final product.
Although the application of thermokinetic compounding in the field of
pharmaceutical manufacturing offers significant advantages over other
methodologies known in the pharmaceutical arts, it is possible that issues can
arise
in continuously melt blending certain heat sensitive or thermolabile
components with
certain non-thermolabile components using a thermokinetic mixer. Blending such
a
combination of components often requires using an elevated shaft speed or a
reduced shaft speed for an extended processing time sufficient to impart
complete
amorphosity on the fully processed batch. In certain cases, this results in an
exceedance of a limit temperature or heat input for an unacceptable duration.
The
batch thus experiences unacceptable degradation of the thermolabile
components,
as the substantial amount of heat absorbed by the entire batch results in
thermal
degradation of thermolabile components instead of increasing overall batch
temperature. Substantially complete amorphosity is a measure well-known in the
art
of pharmaceutical preparation and processing; bioavailability may be
significantly
impaired in compositions lacking substantially complete amorphosity.
3
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
BRIEF SUMMARY OF THE INVENTION
The present disclosure unexpectedly solves the issues associated with
blending certain heat sensitive or thermolabile components in a thermokinetic
mixer
by using multiple speeds during a single, rotationally continuous operation on
a batch
containing thermolabile components. Identified herein is a novel thermokinetic
mixer
and mixing process that can blend heat sensitive or thermolabile components
while
minimizing any substantial thermal degradation. In particular, the disclosure
is useful
in processing mixtures that include thermolabile components whose exposure to
a
melt temperature or a cumulative heat input over a defined time period results
in
substantial degradation. The resulting pharmaceutical compositions have
increased
bioavailability and stability. In addition, the methods disclosed herein are
easily
scalable to commercial production of pharmaceutical compositions.
One embodiment of the present disclosure is a method for continuous
blending and melting of an autoheated mixture in the mixing chamber of a high
speed mixer, where a first speed is changed mid-processing to a second speed
upon
achieving a first desired process parameter. In another embodiment, the second
speed may be maintained until a final process parameter is achieved, whereupon
shaft rotation is stopped and a melt blended batch is withdrawn or ejected
from the
mixing chamber for further processing. In another embodiment, one or more
intermediate speed changes may be made to the shaft rotational speed between
the
second speed and stopping the shaft rotation. Process parameters which
determine
shaft speed changes are predetermined and may be sensed and displayed,
calculated, inferred, or otherwise established with reasonable certainty so
that the
speed change(s) are made during a single, rotationally continuous processing
of a
batch in a mixing chamber of the high speed mixer. Another embodiment is the
use
of variations in the shape, width and angle of the facial portions of the
shaft
extensions or projections that intrude into the main processing volume to
control
translation of rotational shaft energy delivered to the extensions or
projections into
heating energy within particles impacting the portions of the extensions or
projections.
4
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
The present inventor investigated the melt blending of various mixtures
including thermolabile components in a thermokinetic mixing chamber. The
present
inventor unexpectedly found that using multiple speeds during a single,
rotationally
continuous operation on certain batches containing thermolabile components
solved
the problem of exceeding a limit temperature or excessive heat input for the
batch.
The present inventor also surprisingly found that varying the shape, width and
angle
away from a shaft axis plane of a shaft extension or projection provided a
method of
controlling the shear delivered to a particle, which in turn provided control
over shaft
energy translated into heat energy available for softening or melting a
polymer part
of a particle in a thermokinetic mixing chamber.
An embodiment of the present disclosure is a method of blending a
composition of two or more ingredients, wherein the ingredients comprise one
or
more heat sensitive or thermolabile components, wherein the resulting
composition
is amorphous, homogenous, heterogenous, or heterogeneously homogenous, the
method comprising mixing the ingredients in a thermokinetic mixing chamber,
wherein a thermokinetic mixer shaft is operated at a first speed until
achieving a
predetermined parameter, at which time the shaft speed is adjusted to a second
speed for a second time period, wherein the mixing process is substantially
uninterrupted between the first and second time periods. In another embodiment
of
the present disclosure, the thermokinetic mixer shaft is operated at one or
more
speeds until achieving a predetermined parameter, at which time the shaft
speed is
adjusted to a different speed for a different time period, wherein the mixing
process
is substantially uninterrupted between the two or more time periods. An
example of
such an embodiment is a method of blending a composition of two or more
ingredients, wherein a thermokinetic mixer shaft is operated at a first speed
until
achieving a predetermined parameter, at which time the shaft speed is adjusted
to a
second speed for a second time period, wherein the mixing process is
substantially
uninterrupted between the first and second time periods, and wherein at the
end of
the second time period a rotational speed of the shaft is changed from the
second
speed to a third speed for a third time period upon achieving a predetermined
parameter. In one embodiment, the mixing process is substantially
uninterrupted
between the second and third time periods.
5
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
In certain embodiments, the heat sensitive or thermolabile components may
comprise one or more active pharmaceutical ingredients, one or more
pharmaceutically acceptable excipients, or one or more pharmaceutically
acceptable
heat sensitive polymers. In other embodiments, the heat sensitive or
thermolabile
components may comprise one or more active pharmaceutical ingredients and one
or more pharmaceutically acceptable excipients or heat sensitive polymers. In
other
embodiments, the active pharmaceutical ingredients and one or more
pharmaceutically acceptable excipients are added in a ratio of from about 1:2
to 1:9,
respectively. In still other embodiments, the active pharmaceutical
ingredients and
one or more pharmaceutically acceptable heat sensitive polymers are added in a
ratio of from about 1:2 to 1:9, respectively. In certain embodiments, the
second time
period may be at least about five percent, 10 percent, 15 percent, 20 percent,
25
percent or more of the first time period. In other embodiments, the speed
during the
second time period is increased by about 100 revolutions per minute ("RPM"),
200
RPM, 300 RPM, 400 RPM, 500 RPM, 600 RPM, 700 RPM, 800 RPM, 900 RPM,
1000 RPM, 1100 RPM, 1200 RPM, 1300 RPM, 1400 RPM, 1500 RPM, 1600 RPM,
1700 RPM, 1800 RPM, 1900 RPM, 2000 RPM, 2100 RPM, 2200 RPM, 2300 RPM,
2400 RPM, 2500 RPM, or more as compared to the speed during the first time
period. For example, in one embodiment the first speed is greater than 1000
RPM
and the second speed is 200 to 400 RPM greater than the first speed. In
another
embodiment, the first speed is greater than 1000 RPM and the second speed is
200
to 1000 RPM greater than the first speed. In still another embodiment, the
first
speed is greater than 1000 RPM and the second speed is 200 to 2500 RPM greater
than the first speed.
In one embodiment, the end of the first time period is substantially before
the
mixing chamber temperature reaches the shear transition temperature or melting
point of any substantial component of the ingredients. In another embodiment,
the
end of the first time period is a predetermined time period and a change to
the
second speed is made automatically by the thermokinetic mixer at the end of
the first
time period. In yet another embodiment, the end of the first time period is
substantially before the mixing chamber temperature reaches the shear
transition
temperature of an active pharmaceutical ingredient in the ingredients. In
still another
6
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
embodiment, the end of the first time period is substantially before mixing
chamber
temperature reaches the shear transition temperature of an excipient in the
ingredients. In another embodiment, the end of the first time period is
substantially
before mixing chamber temperature reaches the shear transition temperature of
a
heat sensitive polymer in the ingredients.
In one embodiment, the end of the second or any subsequent time period is
substantially before an active pharmaceutical ingredient experiences
substantial
thermal degradation. In another embodiment, the end of the second or any
subsequent time period is substantially before an excipient ingredient
experiences
substantial thermal degradation. In yet another embodiment, the end of the
second
or any subsequent time period is substantially before a heat sensitive polymer
ingredient experiences substantial thermal degradation. In one embodiment, at
the
end of the second or any subsequent time period the active pharmaceutical
ingredient and an excipient of the ingredients are substantially amorphous. In
another embodiment, at the end of the second or any subsequent time period the
active pharmaceutical ingredient and a heat sensitive polymer of the
ingredients are
substantially amorphous. In other embodiments, upon achieving a final process
parameter, the shaft rotation is stopped and a batch or composite is withdrawn
or
ejected from the mixing chamber for further processing. In certain
embodiments, the
batch or composite is withdrawn or ejected at or below the glass transition
temperature of at least one of the components of the batch or composite. In
other
embodiments, the batch or composite is further processed by hot melt
extrusion,
melt granulation, compression molding, tablet compression, capsule filling,
film-
coating, or injection molding. In other embodiments, the batch or composite is
withdrawn or ejected at the beginning of a RPM plateau, for example before
degradation occurs in the batch or composite. In other embodiments, the RPM
deceleration prior to withdrawal or ejection of the batch or composite is
modulated to
produce a more uniform batch or composite.
Another embodiment of the present disclosure is directed to a method of
compounding one or more active pharmaceutical ingredients and at least one
polymeric pharmaceutically acceptable excipient to produce an amorphous,
7
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
homogenous, heterogenous, or heterogeneously homogenous composition, the
method comprising thermokinetic mixing of the active pharmaceutical
ingredient(s)
and at least one polymeric pharmaceutically acceptable excipient in a chamber
at a
first speed effective to increase the temperature of the mixture, and at a
time point at
which the temperature is below the shear transition temperature of any active
pharmaceutical ingredient or polymeric pharmaceutically acceptable excipient
in the
mixture, increasing the mixer rotation to a second speed to produce an
amorphous,
homogenous, heterogenous, or heterogeneously homogenous composition, wherein
the increase is accomplished without stopping the mixing or opening the
chamber.
In another embodiment of the present disclosure, the method comprises
thermokinetic mixing in a chamber at one or more speeds effective to increase
the
temperature of the mixture, at which time the shaft speed is adjusted to a
different
speed for a different time period, and at a time point at which the
temperature is
below the shear transition temperature of any active pharmaceutical ingredient
or
polymeric pharmaceutically acceptable excipient in the mixture, and increasing
the
mixer rotation to one or more different speeds, wherein the increase is
accomplished
without stopping the mixing or opening the chamber.
Certain embodiments of the present disclosure are directed to thermokinetic
mixers used to produce a pharmaceutical composition comprising one or more
heat
sensitive or thermolabile components. Various embodiments of the mixer may
comprise one or more and any combination of the following: (1) a mixing
chamber,
for example a substantially cylindrical mixing chamber; (2) a shaft disposed
through
the center axis of the mixing chamber; (3) an electric motor connected to the
shaft,
for example which is effective to impart rotational motion to the shaft; (4)
one or more
projections or extensions from the shaft and perpendicular to the long axis of
the
shaft; (5) one more heat sensors, for example attached to a wall of the mixing
chamber and operative to detect heat or temperature of at least a portion of
the
interior of the mixing chamber; (6) a variable frequency device, for example
connected to the motor; (7) a door disposed in a wall of the mixing chamber,
for
example which is effective when opened during a process run to allow the
contents
of the mixing chamber to pass out of the mixing chamber; and (8) an electronic
controller. In certain embodiments, a hygroscopic condition is maintained
within the
8
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
thermokinetic mixer. In other embodiments, the thermokinetic mixers are
designed
to maximize shear during batch processing.
In certain embodiments, the electronic controller is in communication with the
temperature sensors, the door and the variable frequency device. In some
embodiments, the electronic controller comprises a user input device, a timer,
an
electronic memory device configured to accept user input of process parameters
or
predetermined parameters for two or more stages of a thermokinetic mixing
processing, and a display.
In an embodiment, the process parameters or
predetermined parameters are saved in the memory device and displayed on the
monitor for one or more stages of a process run. In certain embodiments, when
one
of the predetermined parameters is met during a stage of a processing run, the
electronic controller automatically moves the process run to the subsequent
stage.
In other embodiments, the mixing chamber is interiorly lined by interior liner
pieces.
The liner pieces may be made of material that minimizes any stickiness of the
batch
during processing, for example stainless steel and other such steel alloys,
titanium
alloys (such as nitrided or nitride-containing titanium), and wear and heat
resistant
polymers (such as Teflon ).
In one embodiment of the present disclosure, at least one of the temperature
sensors detects infrared radiation, for example wherein the radiation level is
output
as temperature on the display.
In other embodiments, the predetermined
parameters may be any one or a combination of the following: temperature, rate
of
temperature change, shaft rotational speed (e.g., rate of acceleration and
deceleration), amperage draw of the electric motor, time of stage, or rate of
withdrawal or exit of the batch or composite. One of skill in the art will be
able to
change each of the following parameters to obtain a batch or composite with
the
desired characteristics through routine experimentation. In another
embodiment, the
output display may be any one or a combination of the following: chamber
temperature, motor revolutions per minute, amperage draw of the motor, or
cycle
elapsed time.
In certain embodiments of the present disclosure, the one or more projections
or extensions from the shaft comprise a base and an end portion, and, for
example,
9
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
the end portion may be removable from the base portion and the base portion
may
be removable from the shaft. In other embodiments, the projections or
extensions
are replaceable in the thermokinetic mixer, for example based on wear and tear
or
different batch parameters. In one embodiment, the one or more projections or
extensions from the shaft comprise one or more main facial portions having a
width
of at least about 0.75 inches, at an angle of between 15 to 80 degrees from a
shaft
axis plane. In other embodiments, the one or more projections or extensions
from
the shaft comprise one or more main facial portions having a width of at least
about
0.80 inches, 0.85 inches, 0.90 inches, 0.95 inches, 1.0 inches, 1.1 inches,
1.2
inches, 1.3 inches, 1.4 inches, 1.5 inches, 1.6 inches, 1.7 inches, 1.8
inches, 1.9
inches, 2.0 inches, 2.1 inches, 2.2 inches, 2.3 inches, 2.4 inches, 2.5
inches, 2.6
inches, 2.7 inches, 2.8 inches, 2.9 inches, 3.0 inches, 3.1 inches, 3.2
inches, 3.3
inches, 3.4 inches, 3.5 inches, 3.6 inches, 3.7 inches, 3.8 inches, 3.9
inches, 4.0
inches, 4.1 inches, 4.2 inches, 4.3 inches, 4.4 inches, 4.5 inches, 4.6
inches, 4.7
inches, 4.8 inches, 4.9 inches, 5.0 inches, or greater, at an angle of about
15, 20, 25,
30, 35, 40, 45, 50, 55, 60, 65, 70, 75, or 80 degrees from a shaft axis plane.
In
certain embodiment, the one or more projections or extensions from the shaft
control
translation of rotational shaft energy delivered to the projections or
extensions into
heating energy within particles impacting the projections.
In other embodiments, these dimensions of the one or more projections or
extensions from the shaft are designed to increase the shear profile of the
population
of shear-resistant particles in the batch, for example to produce
substantially
amorphous composites. In certain embodiments, the dimensions of the one or
more
projections or extensions from the shaft are designed to produce composites
that are
at least about 60, 65, 70, 75, 80, 85, 90, 95, or 99 percent amorphous.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
The following drawings form part of the present specification and are included
to further demonstrate certain aspects of the present disclosure. The
disclosure may
be better understood by reference to one or more of these drawings in
combination
with the detailed description of specific embodiments presented herein.
FIG. 1. A view of the thermokinetic mixer assembly.
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
FIG. 2. An exploded view of the thermokinetic mixer.
FIG. 3. A shaft-radial cutaway view of a thermokinetic mixing chamber.
FIG. 4. An exploded view of the thermokinetic mixing chamber.
FIG. 5. Analysis of batch sensed temperature, shaft rotational speeding in
RPMs, and amperage draw on the motor as a directly proportional measure of
energy input into the batch at any moment with one rotational shaft speed.
FIG. 6. Analysis of batch sensed temperature, shaft rotational speeding in
RPMs, and amperage draw on the motor as a directly proportional measure of
energy input into the batch at any moment with two rotational shaft speeds.
FIG. 7. A graph block diagram of a thermokinetic mixer process at two or
more rotational shaft speeds.
FIG. 8. A cross section of a main facial portion of a prior art shaft
extension.
FIG. 9. A cross section of a main facial portion of a shaft extension with a
shaft axial plane at an angle of about 15 degrees.
FIG. 10. A cross section of a main facial portion of a shaft extension with a
shaft axial plane at an angle of about 30 degrees.
FIG. 11. A cross section of a main facial portion of a shaft extension with a
shaft axial plane at an angle of about 45 degrees.
FIG. 12. A cross section of a main facial portion of a shaft extension with a
shaft axial plane at an angle of about 60 degrees.
FIG. 13. An alternative design of a cross section of a main facial portion of
a
shaft extension.
FIG. 14. An alternative design of a cross section of a main facial portion of
a
shaft extension.
FIG. 15. An alternative design of a cross section of a main facial portion of
a
shaft extension.
FIG. 16. An alternative design of a cross section of a main facial portion of
a
shaft extension.
11
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
FIG. 17. An alternative design of a cross section of a main facial portion of
a
shaft extension.
FIG. 18. An alternative design of a cross section of a main facial portion of
a
shaft extension.
FIG. 19. An exploded view of the thermokinetic mixer showing internal liner
pieces.
FIG. 20. A generalized side view of a shaft extension's top face interaction
with an inside surface of a mixing chamber.
FIG. 21. A perspective view of a shaft extension with variable top face path
lengths.
FIG. 22. An alternative design of a front face of a shaft extension.
DETAILED DESCRIPTION OF THE INVENTION
Although making and using various embodiments of the present disclosure
are discussed in detail below, it should be appreciated that the present
disclosure
provides many inventive concepts that may be embodied in a wide variety of
contexts. The specific aspects and embodiments discussed herein are merely
illustrative of ways to make and use the disclosure, and do not limit the
scope of the
disclosure.
To facilitate the understanding of this disclosure, a number of terms are
defined below. Terms defined herein have meanings as commonly understood by a
person of ordinary skill in the areas relevant to the present disclosure.
Terms such
as "a", "an" and "the" are not intended to refer to only a singular entity,
but include
the general class of which a specific example may be used for illustration.
With
regard to the values or ranges recited herein, the term "about" is intended to
to
capture variations above and below the stated number that may achieve
substantially the same results as the stated number. In the present
disclosure, each
of the variously stated ranges is intended to be continuous so as to include
each
numerical parameter between the stated minimum and maximum value of each
range. For Example, a range of about 1 to about 4 includes about 1, 1, about
2, 2,
about 3, 3, about 4, and 4. The terminology herein is used to describe
specific
12
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
embodiments of the disclosure, but their usage does not delimit the
disclosure,
except as outlined in the claims.
As used herein, the term "thermokinetic compounding" or "TKC" refers to a
method of thermokinetic mixing until melt blended. TKC may also be described
as a
thermokinetic mixing process in which processing ends at a point sometime
prior to
agglomeration.
As used herein, the term "main facial portion" refers to the "top face" of a
shaft
extension. The top face of a shaft extension is the face facing the inside
wall of the
mixing chamber of a thermokinetic mixer.
As used herein, the term "shear transition temperature" refers to the point at
which further energy input does not result in an immediate rise in
temperature.
As used herein, the phrase "a homogenous, heterogenous, or
heterogeneously homogenous composite or an amorphous composite" refers to the
various compositions that can be made using the TKC method.
As used herein, the term "heterogeneously homogeneous composition" refers
to a material composition having at least two different materials that are
evenly and
uniformly distributed throughout the volume.
As used herein, "bioavailability" is a term meaning the degree to which a drug
becomes available to the target tissue after being administered to the body.
Poor
bioavailability is a significant problem encountered in the development of
pharmaceutical compositions, particularly those containing an active
ingredient that
is not highly soluble. In certain embodiments such as formulations of
proteins, the
proteins may be water soluble, poorly soluble, not highly soluble, or not
soluble. The
skilled artisan will recognize that various methodologies may be used to
increase the
solubility of proteins, e.g., use of different solvents, excipients, carriers,
formation of
fusion proteins, targeted manipulation of the amino acid sequence,
glycosylation,
lipidation, degradation, combination with one or more salts and the addition
of
various salts.
As used herein, the phrase "pharmaceutically acceptable" refers to molecular
entities, compositions, materials, excipients, carriers, and the like that do
not
13
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
produce an allergic or similar untoward reaction when administered to humans
in
general.
As used herein, the term "active pharmaceutical ingredient" or "API" is
interchangeable with the terms "drug," "drug product," "medication," "liquid,"
"biologic," or "active ingredient." As used herein, an "API" is any component
intended to furnish pharmacological activity or other direct effect in the
diagnosis,
cure, mitigation, treatment, or prevention of disease, or to affect the
structure or any
function of the body of humans or other animals. In certain embodiments, the
aqueous solubility of the API may be poorly soluble.
Examples of APIs that may be utilized in the present disclosure include, but
are not limited to, antibiotics, analgesics, vaccines, anticonvulsants, anti-
diabetic
agents, anti-fungal agents, anti-neoplastic agents, anti-parkinsonian agents,
anti-
rheumatic agents, appetite suppressants, biological response modifiers,
cardiovascular agents, central nervous system stimulants, contraceptive
agents,
dietary supplements, vitamins, minerals, lipids, saccharides, metals, amino
acids
(and precursors), nucleic acids and precursors, contrast agents, diagnostic
agents,
dopamine receptor agonists, erectile dysfunction agents, fertility agents,
gastrointestinal agents, hormones, immunomodulators, anti-hypercalcemia
agents,
mast cell stabilizers, muscle relaxants, nutritional agents, ophthalmic
agents,
osteoporosis agents, psychotherapeutic agents, parasympathomimetic agents,
parasympatholytic agents, respiratory agents, sedative hypnotic agents, skin
and
mucous membrane agents, smoking cessation agents, steroids, sympatholytic
agents, urinary tract agents, uterine relaxants, vaginal agents, vasodilator,
anti-
hypertensive, hyperthyroids, anti-hyperthyroids, anti-asthmatics and vertigo
agents.
In certain embodiments, the API is a poorly water-soluble drug or a drug with
a high
melting point.
The API may be found in the form of one or more pharmaceutically
acceptable salts, esters, derivatives, analogs, prodrugs, and solvates
thereof. As
used herein, a "pharmaceutically acceptable salt" is understood to mean a
compound formed by the interaction of an acid and a base, the hydrogen atoms
of
the acid being replaced by the positive ion of the base. Non-limiting examples
of
14
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
pharmaceutically acceptable salts include sulfate, citrate, acetate, oxalate,
chloride,
bromide, iodide, nitrate, bisulfate, phosphate, acid phosphate, isonicotinate,
lactate,
salicylate, acid citrate, tartrate, oleate, tannate, pantothenate, bitartrate,
ascorbate,
succinate, maleate, gentisinate, fumarate, gluconate, glucaronate, saccharate,
formate, benzoate, glutamate, methanesulfonate, ethanesulfonate,
benzenesulfonate, p-toluenesulfonate, and pamoate. Another method for defining
the ionic salts may be as an acidic functional group, such as a carboxylic
acid
functional group, and a pharmaceutically acceptable inorganic or organic base.
Non-
limiting examples of bases include, but are not limited to, hydroxides of
alkali metals
such as sodium, potassium and lithium; hydroxides of calcium and magnesium;
hydroxides of other metals, such as aluminum and zinc; ammonia; and organic
amines, such as unsubstituted or hydroxy substituted mono-, di-, or
trialkylamines;
dicyclohexylamine; tributylamine; pyridine; N-methyl-N-ethylamine;
diethylamine;
triethylamine; mono-, bis- or tris-(2-hydroxy-lower alkyl amines), such as
mono- bis-
or tris-(2-hydroxyethyl)amine, 2-hydroxy-tert-butylamine, or tris-
(hydroxymethyl)methylamine, N,N-di-lower alkyl-N-(hydroxy lower alkyl)-amines,
such as N,N-dimethyl-N-(2-hydroxyethyl)amine, or tri-(2-hydroxyethyl)amine; N-
methyl-D-glucamine; and amino acids such as arginine, lysine, and the like.
A variety of administration routes are available for delivering the APIs to a
patient in need. The particular route selected will depend upon the particular
drug
selected, the weight and age of the patient, and the dosage required for
therapeutic
effect. The pharmaceutical compositions may conveniently be presented in unit
dosage form. The APIs suitable for use in accordance with the present
disclosure,
and their pharmaceutically acceptable salts, derivatives, analogs, prodrugs,
and
solvates thereof, can be administered alone, but will generally be
administered in
admixture with a suitable pharmaceutical excipient, diluent, or carrier
selected with
regard to the intended route of administration and standard pharmaceutical
practice.
The APIs may be used in a variety of application modalities, including oral
delivery as tablets, capsules or suspensions; pulmonary and nasal delivery;
topical
delivery as emulsions, ointments or creams; transdermal delivery; and
parenteral
delivery as suspensions, microemulsions or depot. As used herein, the term
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
"parenteral" includes subcutaneous, intravenous, intramuscular, or infusion
routes of
administration.
The excipients and adjuvants that may be used in the presently disclosed
compositions and composites, while potentially having some activity in their
own
right, for example, antioxidants, are generally defined for this application
as
compounds that enhance the efficiency and/or efficacy of the active
ingredients. It is
also possible to have more than one active ingredient in a given solution, so
that the
particles formed contain more than one active ingredient.
As stated, excipients and adjuvants may be used to enhance the efficacy and
efficiency of the APIs. Non-limiting examples of compounds that can be
included are
binders, cryoprotectants, lyoprotectants, surfactants, fillers, stabilizers,
polymers,
protease inhibitors, antioxidants and absorption enhancers. The excipients may
be
chosen to modify the intended function of the active ingredient by improving
flow, or
bio-availability, or to control or delay the release of the API. Specific
nonlimiting
examples include: sucrose, trehaolose, Span 80, Tween 80, Brij 35, Brij 98,
Pluronic,
sucroester 7, sucroester 11, sucroester 15, sodium lauryl sulfate, oleic acid,
laureth-
9, laureth-8, lauric acid, vitamin E TPGS, Gelucire 50/13, Gelucire 53/10,
Labrafil,
dipalmitoyl phosphadityl choline, glycolic acid and salts, deoxycholic acid
and salts,
sodium fusidate, cyclodextrins, polyethylene glycols, labrasol, polyvinyl
alcohols,
polyvinyl pyrrolidones and tyloxapol. Using the process of the present
disclosure,
the morphology of the active ingredients can be modified, resulting in highly
porous
microparticles and nanoparticles.
Exemplary thermal binders that may be used in the presently disclosed
compositions and composites include but are not limited to polyethylene oxide;
polypropylene oxide; polyvinylpyrrolidone; polyvinylpyrrolidone-co-
vinylacetate;
acrylate and methacrylate copolymers; polyethylene; polycaprolactone;
polyethylene-
co-polypropylene; alkylcelluloses such as methylcellulose;
hydroxyalkylcelluloses
such as hydroxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose,
and
hydroxybutylcellulose; hydroxyalkyl alkylcelluloses such as hydroxyethyl
methylcellulose and hydroxypropyl methylcellulose; starches, pectins;
polysaccharides such as tragacanth, gum arabic, guar gum, and xanthan gum. One
16
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
embodiment of the binder is poly(ethylene oxide) (PEO), which can be purchased
commercially from companies such as the Dow Chemical Company, which markets
PEO under the POLY OX.TM. trademark exemplary grades of which can include
WSR N80 having an average molecular weight of about 200,000; 1,000,000; and
2,000,000.
Suitable grades of PEO can also be characterized by viscosity of solutions
containing fixed concentrations of PEO, such as for example:
POLYOX Viscosity Range
Aqueous Solution
Water-Soluble Resin NF
at 25 C, mPa.s
POLYOX Water-Soluble Resin NF WSR N-10 30-50 (5% solution)
POLYOX Water-Soluble Resin NF WSR N-80 55-90 (5% solution)
POLYOX Water-Soluble Resin NF WSR N-
600-1,200 (5% solution)
750
POLYOX Water-Soluble Resin NF WSR-205 4,500-8,800 (5% solution)
POLYOX Water-Soluble Resin NF WSR-1105 8,800-17,600 (5% solution)
POLYOX Water-Soluble Resin NF WSR N-
12K 400-800(2% solution)
POLYOX Water-Soluble Resin NF WSR N-
60K 2,000-4,000 (2% solution)
POLYOX Water-Soluble Resin NF WSR-301 1,650-5,500 (1`)/0
solution)
POLYOX Water-Soluble Resin NF WSR
5,500-7,500 (1`)/0 solution)
Coagulant
POLYOX Water-Soluble Resin NF WSR-303 7,500-10,000 (1`)/0
solution)
Suitable thermal binders that may or may not require a plasticizer include,
for
example, Eudragit.TM. RS PO, Eudragit.TM. S100, Kollidon SR (poly(vinyl
acetate)-
co-poly(vinylpyrrolidone) copolymer), Ethocel.TM. (ethylcellulose), HPC
(hydroxypropylcellulose), cellulose acetate butyrate, poly(vinylpyrrolidone)
(PVP),
poly(ethylene glycol) (PEG), poly(ethylene oxide) (PEO), poly(vinyl alcohol)
(PVA),
hydroxypropyl methylcellulose (HPMC), ethylcellulose (EC),
hydroxyethylcellulose
(HEC), sodium carboxymethyl-cellulose (CMC), dimethylaminoethyl methacrylate--
17
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
methacrylic acid ester copolymer, ethylacrylate--methylmethacrylate copolymer
(GA-
MMA), 0-5 or 60 SH-50 (Shin-Etsu Chemical Corp.), cellulose acetate phthalate
(CAP), cellulose acetate trimelletate (CAT), poly(vinyl acetate) phthalate
(PVAP),
hydroxypropylmethylcellulose phthalate (HPMCP), poly(methacrylate
ethylacrylate)
(1:1) copolymer (MA-EA), poly(methacrylate methylmethacrylate) (1:1) copolymer
(MA-MMA), poly(methacrylate methylmethacrylate) (1:2) copolymer, Eudragit L-30-
D.TM. (MA-EA, 1:1), Eudragit L-100-55.TM. (MA-EA, 1:1),
hydroxypropylmethylcellulose acetate succinate (HPMCAS), Coateric.TM. (PVAP),
Aquateric.TM. (CAP), and AQUACOAT.TM. (HPMCAS), polycaprolactone, starches,
pectins; polysaccharides such as tragacanth, gum arabic, guar gum, and xanthan
gum.
The stabilizing and non-solubilizing carrier may also contain various
functional
excipients, such as: hydrophilic polymer, antioxidant, super-disintegrant,
surfactant
including amphiphillic molecules, wetting agent, stabilizing agent, retardant,
similar
functional excipient, or combination thereof, and plasticizers including
citrate esters,
polyethylene glycols, PG, triacetin, diethylphthalate, castor oil, and others
known to
those or ordinary skill in the art. Extruded material may also include an
acidifying
agent, adsorbent, alkalizing agent, buffering agent, colorant, flavorant,
sweetening
agent, diluent, opaquant, complexing agent, fragrance, preservative or a
combination
thereof.
Exemplary hydrophilic polymers which can be a primary or secondary
polymeric carrier that can be included in the composites or composition
disclosed
herein include poly(vinyl alcohol) (PVA), polyethylene-polypropylene glycol
(e.g.
POLO)(AMER.TM.), carbomer, polycarbophil, or chitosan. Hydrophilic polymers
for
use with the present disclosure may also include one or more of hydroxypropyl
methylcellulose, carboxymethylcellulose, hydroxypropyl cellulose, hydroxyethyl
cellulose, methylcellulose, natural gums such as gum guar, gum acacia, gum
tragacanth, or gum xanthan, and povidone. Hydrophilic polymers also include
polyethylene oxide, sodium carboxymethycellulose, hydroxyethyl methyl
cellulose,
hydroxymethyl cellulose, carboxypolymethylene, polyethylene glycol, alginic
acid,
gelatin, polyvinyl alcohol, polyvinylpyrrolidones, polyacrylamides,
18
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
polymethacrylamides, polyphosphazines, polyoxazolidines,
poly(hydroxyalkylcarboxylic acids), carrageenate alginates, carbomer, ammonium
alginate, sodium alginate, or mixtures thereof.
By "immediate release" is meant a release of an active agent to an
environment over a period of seconds to no more than about 30 minutes once
release has begun and release begins within no more than about 2 minutes after
administration. An immediate release does not exhibit a significant delay in
the
release of drug.
By "rapid release" is meant a release of an active agent to an environment
over a period of 1-59 minutes or 0.1 minute to three hours once release has
begun
and release can begin within a few minutes after administration or after
expiration of
a delay period (lag time) after administration.
As used herein, the term "extended release" profile assumes the definition as
widely recognized in the art of pharmaceutical sciences. An extended release
dosage form will release the drug (i.e., the active agent or API) at a
substantially
constant rate over an extended period of time or a substantially constant
amount of
drug will be released incrementally over an extended period of time. An
extended
release tablet generally effects at least a two-fold reduction in dosing
frequency as
compared to the drug presented in a conventional dosage form (e.g., a solution
or
rapid releasing conventional solid dosage forms).
By "controlled release" is meant a release of an active agent to an
environment over a period of about eight hours up to about 12 hours, 16 hours,
18
hours, 20 hours, a day, or more than a day. By "sustained release" is meant an
extended release of an active agent to maintain a constant drug level in the
blood or
target tissue of a subject to which the device is administered.
The term "controlled release", as regards to drug release, includes the terms
"extended release", "prolonged release", "sustained release", or "slow
release", as
these terms are used in the pharmaceutical sciences. A controlled release can
begin within a few minutes after administration or after expiration of a delay
period
(lag time) after administration.
19
CA 02945955 2016-10-14
WO 2015/160358
PCT/US2014/034601
A slow release dosage form is one that provides a slow rate of release of drug
so that drug is released slowly and approximately continuously over a period
of 3 hr,
6 hr, 12 hr, 18 hr, a day, 2 or more days, a week, or 2 or more weeks, for
example.
The term "mixed release" as used herein refers to a pharmaceutical agent that
includes two or more release profiles for one or more active pharmaceutical
ingredients. For example, the mixed release may include an immediate release
and
an extended release portion, each of which may be the same API or each may be
a
different API.
A timed release dosage form is one that begins to release drug after a
predetermined period of time as measured from the moment of initial exposure
to the
environment of use.
A targeted release dosage form generally refers to an oral dosage form that is
designed to deliver drug to a particular portion of the gastrointestinal tract
of a
subject. An exemplary targeted dosage form is an enteric dosage form that
delivers
a drug into the middle to lower intestinal tract but not into the stomach or
mouth of
the subject. Other targeted dosage forms can deliver to other sections of the
gastrointestinal tract such as the stomach, jejunum, ileum, duodenum, cecum,
large
intestine, small intestine, colon, or rectum.
By "delayed release" is meant that initial release of drug occurs after
expiration of an approximate delay (or lag) period. For example, if release of
drug
from an extended release composition is delayed two hours, then release of the
drug
begins at about two hours after administration of the composition, or dosage
form, to
a subject. In general, a delayed release is opposite of an immediate release,
wherein release of drug begins after no more than a few minutes after
administration. Accordingly, the drug release profile from a particular
composition
can be a delayed-extended release or a delayed-rapid release. A "delayed-
extended" release profile is one wherein extended release of drug begins after
expiration of an initial delay period. A "delayed-rapid" release profile is
one wherein
rapid release of drug begins after expiration of an initial delay period.
A pulsatile release dosage form is one that provides pulses of high active
ingredient concentration, interspersed with low concentration troughs. A
pulsatile
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
profile containing two peaks may be described as "bimodal." A pulsatile
profile of
more than two peaks may be described as multi-modal.
A pseudo-first order release profile is one that approximates a first order
release profile. A first order release profile characterizes the release
profile of a
dosage form that releases a constant percentage of an initial drug charge per
unit
time.
A pseudo-zero order release profile is one that approximates a zero-order
release profile. A zero-order release profile characterizes the release
profile of a
dosage form that releases a constant amount of drug per unit time.
The resulting composites or compositions disclosed herein may also be
formulated to exhibit enhanced dissolution rate of a formulated poorly water
soluble
drug.
An example of a composition or formulation having a stable release profile
follows. Two tablets having the same formulation are made. The first tablet is
stored
for one day under a first set of conditions, and the second tablet is stored
for four
months under the same first set of conditions. The release profile of the
first tablet is
determined after the single day of storage and the release profile of the
second
tablet is determined after the four months of storage. If the release profile
of the first
tablet is approximately the same as the release profile of the second tablet,
then the
tablet/film formulation is considered to have a stable release profile.
Another example of a composition or formulation having a stable release
profile follows. Tablets A and B, each comprising a composition according to
the
present disclosure, are made, and Tablets C and D, each comprising a
composition
not according to the present disclosure, are made. Tablets A and C are each
stored
for one day under a first set of conditions, and tablets B and D are each
stored for
three months under the same first set of conditions. The release profile for
each of
tablets A and C is determined after the single day of storage and designated
release
profiles A and C, respectively. The release profile for each of tablet B and D
is
determined after the three months of storage and designated release profiles B
and
D, respectively. The differences between release profiles A and B are
quantified as
are the differences between release profiles C and D. If the difference
between the
21
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
release profiles A and B is less than the difference between release profiles
C and D,
tablets A and B are understood to provide a stable or more stable release
profile.
Specifically, the TKC process can be used for one or more of the following
pharmaceutical applications.
Dispersion of one or more APIs, wherein the API is a small organic molecule,
protein, peptide, or polynucleic acid; in polymeric and/or non-polymeric
pharmaceutically acceptable materials for the purpose of delivering the API to
a
patient via oral, pulmonary, parenteral, vaginal, rectal, urethral,
transdermal, or
topical routes of delivery.
Dispersion of one or more APIs, wherein the API is a small organic molecule,
protein, peptide, or polynucleic acid; in polymeric and/or non-polymeric
pharmaceutically acceptable materials for the purpose of improving the oral
delivery
of the API by improving the bioavailability of the API, extending the release
of the
API, targeting the release of the API to specific sites of the
gastrointestinal tract,
delaying the release of the API, or producing pulsatile release systems for
the API.
Dispersion of one or more APIs, wherein the API is a small organic molecule,
protein, peptide, or polynucleic acid; in polymeric and/or non-polymeric
pharmaceutically acceptable materials for the purpose of creating bioerodable,
biodegradable, or controlled release implant delivery devices.
Producing solid dispersions of thermolabile APIs by processing at low
temperatures for very brief durations.
Producing solid dispersions of APIs in thermolabile polymers and excipients
by processing at low temperatures for very brief durations.
Rendering a small organic API amorphous while dispersing in a polymeric,
non-polymeric, or combination excipient carrier system.
Dry milling of crystalline API to reduce the particle size of the bulk
material.
Wet milling of crystalline API with a pharmaceutically acceptable solvent to
reduce the particle size of the bulk material.
22
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
Melt milling of a crystalline API with one or more molten pharmaceutical
excipients having limited miscibility with the crystalline API to reduce the
particle size
of the bulk material.
Milling crystalline API in the presence of polymeric or non-polymeric
excipient
to create ordered mixtures where fine drug particles adhere to the surface of
excipient particles and/or excipient particles adhere to the surface of fine
drug
particles.
Producing heterogeneously homogenous composites or amorphous
composites of two or more pharmaceutical excipients for post-processing, e.g.,
milling and sieving, which are subsequently utilized in secondary
pharmaceutical
operations well known to those of skill in the art, e.g., film coating,
tableting, wet
granulation and dry granulation, roller compaction, hot melt extrusion, melt
granulation, compression molding, capsule filling, and injection molding.
Producing single phase, miscible composites of two or more pharmaceutical
materials previously considered to be immiscible for utilization in a
secondary
processing step, e.g. melt extrusion, film coating, tableting and granulation.
Pre-plasticizing polymeric materials for subsequent use in film coating or
melt
extrusion operations.
Rendering a crystalline or semi-crystalline pharmaceutical polymer
amorphous, which can be used as a carrier for an API in which the amorphous
character improves the dissolution rate of the API-polymer composite, the
stability of
the API-polymer composite, and/or the miscibility of the API and the polymer.
Deaggregate and disperse engineered particles in a polymeric carrier without
altering the properties of the engineered particles.
Simple blending of an API in powder form with one or more pharmaceutical
excipients.
Producing composites comprising one or more high melting point APIs and
one or more thermolabile polymers without the use of processing agents.
23
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
Homogenously dispersing a coloring agent or opacifying agent within a
polymer carrier or excipient blend.
In the following detailed description of preferred embodiments of the present
disclosure, reference is made to the figures in the drawings, in which the
same
numeral refers to an identical or similar part in different figures.
The present disclosure is directed to a novel thermokinetic mixer and mixing
process that can blend heat sensitive or thermolabile components without
substantial
thermal degradation. In particular, the disclosure is useful in processing
mixtures
that include thermolabile components whose exposure to a melt temperature or a
cumulative heat input over a defined time period results in degradation. One
embodiment of present disclosure is directed to a method for a continuous melt
blend of an autoheated mixture in the mixing chamber of a high speed
thermokinetic
mixer, where a first speed is changed mid-process to a second speed upon
achieving a first desired or predetermined process parameter. In other
embodiments, the second speed is changed mid-process to a third speed upon
achieving a second desired or predetermined process parameter. Additional
speed
changes are also within the scope of the present disclosure, as dictated by
the
number of desired or predetermined processing parameters needed to produce the
desired composition or composite.
This process is especially applicable for producing solid dispersions of
thermolabile APIs by processing at low temperatures for very brief durations
at
multiple speeds, producing solid dispersions of APIs in thermolabile polymers
and
excipients by processing at low temperatures for very brief durations at
multiple
speeds, producing solid dispersions of APIs in thermolabile excipients by
processing
at low temperatures for very brief durations at multiple speeds, and producing
solid
dispersions of heat sensitive polymers by processing at low temperatures for
relatively brief durations at multiple speeds.
One embodiment is to use two or more different speeds during thermokinetic
processing of a batch to reduce required processing time after a shear
transition
temperature of a portion of the batch is reached. Another embodiment is to use
two
or more different speeds during thermokinetic processing of a batch to reduce
24
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
required processing time where the batch reaches a temperature whereafter a
substantial amount of heat generated by frictional contact with shaft
extensions
and/or an inside surface of the mixing chamber produces thermal degradation of
one
or more components of the batch, and reducing the speed. Yet a further
embodiment is to use two or more different speeds during thermokinetic
processing
of a batch to reduce required processing time where the batch reaches a
temperature whereafter a substantial amount of heat generated by frictional
contact
with shaft extensions and/or an inside surface of the mixing chamber does not
result
in an overall temperature increase for the batch. Yet a further embodiment is
to
provide a thermokinetic processing method using two speeds to reduce thermal
degradation of thermolabile or heat sensitive polymers or components of a
batch
processed thereby.
In one embodiment, at least a portion of a batch in the mixing chamber of the
high speed mixer comprises heat sensitive or thermolabile components whose
exposure to a limit temperature or limit of cumulative heat input over a
defined time
period must be substantially prevented or limited to obtain a melt blended
batch with
acceptable degradation of the heat sensitive or thermolabile components. In
this
embodiment, at least one of the speed changes between a start and end of the
process is made so that the limit temperature or limit of heat input is not
exceeded,
thereby preserving the heat sensitive or thermolabile components in the
composition
or composite.
Thermolabile components include, but are not limited to, thermolabile APIs,
excipients or polymers. Heat sensitive polymers include, but are not limited
to,
nylon, polytrimethylene terephthalate, polybutene-1, polybutylene
terephthalate,
polyethylene terephthalate, polyolefins such as polypropylene and high-density
or
low- density polyethylene, and mixtures or copolymers thereof, which polymers
can
be subject to surface and bulk polymer deficiencies as well as extrusion
limitations.
Other heat sensitive polymers include poly (methylmethacrylate), polyacetal,
polyionomer, EVA copolymer, cellulose acetate, hard polyvinylchloride and
polystyrene or copolymers thereof. A limit temperature in the disclosed
process for
such heat sensitive polymers may be chosen by maintaining sensed temperature
of
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
a batch within an acceptable range from the well known degradation temperature
for
that polymer, such as about 5, 10, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70,
75, 80,
85, 90, 95, or 100 degrees Celsius from a temperature at which it is known in
the art
that heat sensitive polymers begin to undergo degradation of a desired process
parameter.
One embodiment of the present disclosure is a method for continuous
blending and melting of an autoheated mixture in the mixing chamber of a high
speed mixer, where a first speed is changed mid-processing to a second speed
upon
achieving a first desired or predetermined process parameter. In one
embodiment,
the second speed is maintained until a final desired or predetermined process
parameter is achieved, whereupon shaft rotation is stopped and a melt blended
batch is withdrawn or ejected from the mixing chamber for further processing.
The
shaft operates at one or more intermediate rotational speeds between changing
to
the second speed and stopping the shaft rotation. Process parameters which
determine shaft speed changes are predetermined and may be sensed and
displayed, calculated, inferred, or otherwise established with reasonable
certainty so
that the speed change(s) is made during a single, rotationally continuous
processing
of a batch in a mixing chamber of the high speed mixer. Process parameters
include
without limitation temperature, motor RPM, amperage draw, and time.
This disclosure is also directed to a thermokinetic mixer that can blend heat
sensitive or thermolabile components without substantial thermal degradation.
One
embodiment of the thermokinetic mixer has a high horsepower motor driving the
rotation of a horizontal shaft with teeth-like protrusions that extend outward
normal to
the rotational axis of the shaft. The shaft is connected to a drive motor. The
portion
of the shaft containing the protrusions is contained within an enclosed vessel
where
the compounding operation takes place, i.e., a thermokinetic mixing chamber.
The
high rotational velocity of the shaft coupled with the design of the shaft
protrusions
imparts kinetic energy onto the materials being processed. A temperature
sensor
senses the temperature within the thermokinetic mixing chamber. Once a set
temperature is sensed, a first speed is changed to a second speed.
26
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
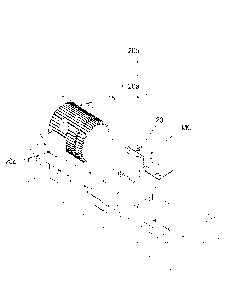
FIG. 1 shows a view of one embodiment of the disclosed thermokinetic mixer
assembly. A temperature sensor 20 is connected to a thermokinetic mixing
chamber
MC. The temperature sensor 20 provides information to a programmable logic
controller 20a which appears on a programmable logic controller display 20b. A
drive motor 15 controls the speed of the shaft which rotates through the
mixing
chamber MC. The drive motor 15 is controlled by a variable frequency drive
20c.
The variable frequency drive 20c also provides information to the programmable
logic controller 20a which appears on the programmable logic controller
display 20b.
When a desired process parameter is met, the programmable logic controller 20a
signals the variable frequency drive 20c to change the frequency of the
electrical
power supplied to the drive motor 15. The drive motor 15 changes the shaft
speed
of the shaft. The temperature sensor 20 can be a sensor to radiation emitted
from
batch components.
FIG. 2 shows an exploded view of one embodiment of the thermokinetic
mixer. A frame 1 supports associated components such that a shaft assembly 2
is
inserted in an axis of a shaft hole through end plate 3 and a feed screw hole
through
end plate 4, the two end plates defining enclosing ends of a mixing chamber
cylinder,
the bottom portion of the cylinder defined by the inside surface of the lower
housing
5. Lower housing 5 comprises a dropout opening closed off during operation
with
discharge door 6. The upper housing 7 comprises an upper part of the cylinder
of
the inside surface of the mixing chamber. The feed housing 8 is adapted to
permit
feeding of material to the feed screw of the shaft assembly so that such
material is,
in combination with the feed screw rotation, compressingly forced into mixing
chamber from an external feed. Door 6 rotatably closes about discharge door
pivot
pin 9. End plate 3 has attached to it a rack & pinion cylinder 18 with spacer
10
interposed. At the top of housing 7 is mounted a bracket 11 with which to
support an
infrared temperature sensor 20 for the mixing chamber. Door guard 12 protects
the
sometimes high temperature door 6 from accidental human contact with dropout
material. Rotary guard 13 and drive coupling guard 14 guard human operators
from
contact with rotating components during operation. Drive motor 15 is
preferably an
electric motor with sufficient power to accomplish the disclosed operation.
The pillow
blocks 16 and 17 support the shaft assembly 2.
27
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
In an example of a system in which the process parameters that determine
shaft speed changes are measured in the mixing chamber and/or drive motor,
FIG. 7
shows a block flow diagram of the disclosed process where a mixing chamber MC
is
connected by a shaft to a drive motor 42, where a variable frequency drive 41
controls the rotational speed of drive motor 42. In certain embodiments, shaft
speed
can be from 0 through 5000 RPM. Further, a programmable logic controller 40
determines and carries out a change in rotational shaft speed using a variable
frequency drive 41 according to the disclosed process. The programmable logic
controller 40 comprises setpoints entered by a user for determination of a
need for
changing a rotational shaft speed in drive motor 42 and to transmit to the
variable
frequency drive 41 a command to change such speed after rotational processing
of
the batch load has been added to the mixing chamber. The programmable logic
controller may incorporate a microprocessor comprising memory incorporating a
control program adapted to act upon achievement of setpoints entered by a user
relying on sensor data transmitted from drive motor 42 and/or mixing chamber
MC,
and include a user interface such as a programmable logic controller display
for a
user to observe operating time and/or sensor data transmitted from drive motor
42
and/or mixing chamber MC. The programmable logic controller optionally
comprises
a method for a user to directly change motor shaft speeds upon consideration
of
predetermined process parameters (such as operation time) or upon comparison
of
predetermined process parameters with sensor data transmitted from drive motor
42
and/or mixing chamber MC (such as batch temperature, amperage draw, and shaft
speed). The programmable logic controller optionally comprises an automated
control method to change motor shaft speeds upon microprocessor operation at
predetermined, stored process parameters (such as operation time) or upon
comparison of predetermined, stored process parameters with sensor data
transmitted from drive motor 42 and/or mixing chamber MC (such as batch
temperature, amperage draw and shaft speed).
A description of components of one embodiment of a thermokinetic mixer for
the disclosed process is shown in FIGS. 3 and 4. Figure 3 shows a shaft-radial
cutaway view of a mixing chamber MC for a thermokinetic mixer of the
disclosure
with halves 5 and 7 joined to form a cylindrical mixing cavity having shaft 23
rotating
28
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
in rotation direction 24 in an axial length of the chamber. Shaft extensions
30 extend
from their releasable connection on shaft 23 to a position near an inside
surface 19.
Shaft extension 30 comprises top face 22 and front face 21. Particles 26a-26e
show
impingement of such particles on shaft extension 30 and on inside surface 27,
which
impingement causes comminution and/or frictional heating of the particles by
the
shear generated by such impingement. Further, FIG. 4 is an exploded view of
the
extensions and mixing chamber shown in FIG. 3, where shaft extensions 30a,
30b,
and 30c each having a top face 22 and a front face 21 defined upon a
replaceable
tooth which is adapted to be secured to foot section 31 by bolt 33. Section 31
is
adapted to be replaceably fixed to shaft 23 (continued from motor shaft 37) at
slot 35
by way of bottom section 32 of section 31. FIG. 4 shows that particles are
generally
moving in direction 38 when they encounter shaft extensions 30a to 30c. Shaft
extension 30a is shown having its front face 21 aligned effectively opposing
those of
shaft extensions 30b and 30c.
With a typical batch process, a user will first select two components, which
could include, for example, a thermolabile API and a polymer excipient. The
user will
then empirically determine the shear transition temperatures of the two
components.
The user will then set the process parameters (temperature, RPM, amperage
draw,
and time) in the programmable logic controller to change from the first speed
to the
second speed as is suitable for the shear transition temperatures of the
components.
Any of the setpoints entered by the user can be used as a stop point following
the
period of the second speed.
FIG. 5 shows certain potential differences between the methods of the
present disclosure, and that of a thermokinetic mixing method using a
substantially
single shaft speed. FIG. 5 shows a graph of batch sensed temperature, shaft
rotational speed in RPMs, and amperage draw on the motor as a directly
proportional measure of energy input into the batch at any moment in the
processing. As a specific example the following composition was
thermokinetically
processed to form a batch of Griseofulvin : PVP (1 : 2 ratio) at a batch size
of 60
grams. Griseofulvin represents a thermolabile API. PVP represents an
excipient. A
series of three tests is represented in FIG. 5 and was conducted in a
thermokinetic
29
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
mixer similar in construction to that shown in FIGS. 3 and 4, where front
faces 21
project in a forward rotation direction with a side to side width of about 1.0
inches
and are maintained at about 30 degrees away from a plane extending from an
axis
of the shaft 23 through a leading edge of the front faces 21 with a height of
about 2.5
inches. The batch in FIG. 5 was processed under thermokinetic, autoheating
conditions in which a substantially single shaft speed was used. The y-axis is
applicable to temperature (values times 10) and shaft speed in RPM (value
times
30). Time on the x-axis is in increments of 0.10 seconds. If the composition
of this
batch were thermokinetically mixed at rotational shaft speeds substantially
higher
than that shown in FIG. 5, i.e., at 2500 RPM and higher, inspection of the
final
product showed that it was unacceptably crystalline and insufficiently
amorphous.
This result would be unexpected to one of skill in the art. Higher shaft
speeds are
taught in the thermokinetic mixing art to assure better mixing, which did not
occur at
higher shaft speeds with these materials. When the example batch composition
was
processed as shown in FIG. 5, at lower rotational shaft speed, inspection of
the final
product showed that it was sufficiently amorphous and adequate for
bioavailability.
However, unacceptable thermal degradation of the thermolabile API occurred,
which
rendered the batch unacceptable.
In FIG. 5, at time zero, amperage draw immediately increased to 35 amps
(1050 on the graph). Ejection of the batch was at about 17.6 seconds or where
RPMs are shown to dramatically decline. The rotational shaft speed was set for
1800 RPMs and reached that speed within about 2 seconds from start. Within
about
7 seconds, the batch temperature reached 260 F, the shear transition
temperature
for the excipient. Above the shear transition temperature, the excipient's
resistance
to shear dramatically decreased and energy delivered to the batch by
impingement
of particles and molten material on the extension surfaces and inside surface
of the
mixing chamber consequently also dramatically decreased (the amperage draw
dropped to about one half when the shear transition temperature was reached in
the
batch temperature). From about 7 seconds to 16 seconds, the batch temperature
of
the composition was not rising while substantial energy continued to be
absorbed by
the batch. Such energy that did not result in increased temperature translated
to
thermal degradation of the thermolabile or heat sensitive components. This
test
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
confirms in general that once a significant amount of a component, i.e.,
greater than
weight percent, 10 weight percent, 20 weight percent, or 30 weight percent, in
a
thermokinetically melt blended batch reaches its shear transition temperature
or
melting point, a substantial amount of heat absorbed by the entire batch
results in
5 thermal degradation of thermolabile or heat sensitive components instead
of
increasing overall batch temperature. This is clearly shown in the time range
from 7
through 16 seconds in FIG. 5, where batch temperature actually decreased with
continuous energy input to the batch.
The same batch and thermokinetic mixer in FIG. 5 were used in FIG. 6, but
two speeds were implemented through the continuous rotational batch
processing.
In FIG. 6, a programmable logic controller connected to an infrared sensor and
a
variable frequency drive was used for detecting a batch temperature, comparing
the
batch temperature to a predetermined setpoint, and automatically changing
rotational shaft speed of the thermokinetic mixer to another speed for the
duration of
the process until the batch was released by way of opening a bottom dropout
door.
A first speed was set for 1800 RPM and a second speed was set for 2600 RPM.
The predetermined setpoint for the batch temperature was chosen to be 200 F as
a
substantial level below the excipient shear transition temperature. It is
critical to
effect a speed change before a substantial component's shear transition
temperature
is reached, and the system requires response time between the moment a sensed
batch temperature is transmitted to the programmable logic controller and the
shaft
speed actually is changed. As shown in FIG. 6, no substantial energy input to
the
batch was diverted from overall batch temperature increase. The processed
batch
showed substantially complete amorphosity and no detectable thermal
degradation
of the API with an overall processing time of about 6.5 seconds. This time
stands in
dramatic contrast to that of the processing time of that in FIG. 5 at 17.6
seconds.
FIG. 6 indicates that shaft rotational speed for certain thermolabile
components should be substantially increased at or before a substantial
component
or portion of a thermokinetically batch reaches a shear transition temperature
or
melting point, whereafter processing time should be minimized. In certain
embodiments, a first speed should be increased by about 100 RPM, 200 RPM, 300
31
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
RPM, 400 RPM, 500 RPM, 600 RPM, 700 RPM, 800 RPM, 900 RPM, 1000 RPM, or
more to a second speed. In other embodiments, a processing time after the
second
speed starts until the batch is released from the mixing chamber should be
about 5
percent, 10 percent, 15 percent, 20 percent, 25 percent or more of the total
time the
batch was processed at the first speed.
It is well known in the art that impact of a particle on a surface imparts
energy
to the particle. It is a feature of thermokinetic, auto-heating mixers to
provide impact
on a particle containing polymers whereby imparted energy is translated partly
into
heat energy to soften and/or melt those polymers. However, the thermokinetic
mixing art generally directs those skilled in the art to provide impact for
particles in
thermokinetic mixers in a manner that lacks fine control of translation of
impact
energy into heat energy. The present disclosure provides for and describes
methods
for such control. Highly cross-linked polymers and thermoset compounds are
highly
refractory to softening and melting for the same reason they are preferred,
i.e., they
resist breaking down. Yet, they are shown to be of value in some combinations
of
components processed with thermokinetic mixing. Indeed, thermokinetic mixing
is
essentially the only way to process highly cross-linked polymers and
thermosets due
to their resistance to melting and blending in any other manner. In the
thermokinetic
mixing art, increasing rotational shaft speed and/or processing time were
understood
to be the method by which melt-resistant polymers could be induced to
translate
sufficient impact energy to heat energy to effect a softened or molten state
for further
processing. The present embodiment discloses an apparatus and methods by which
impact energy translation to heat energy can be effectively controlled.
Two primary impact surfaces, the front face and the top face of a shaft,
control
impact translation to heat energy in a thermokinetic mixer. Those two surfaces
are
the facial portions of the shaft extensions that intrude into the outer 30
percent or
less of volume of the mixing chamber (the volume is referred to hereafter as
the
"main processing volume"; it includes a most restricted zone of about one inch
inward radius from the inside cylindrical wall of the mixing chamber) and the
inside
cylindrical surface of the mixing chamber itself. Changing the inside
cylindrical
surface of the mixing chamber is not a practical option ¨ that surface, being
32
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
stationary, must remain smooth and cylindrically uniform to resist buildup of
molten
materials and to allow for skidding and sliding autoheating contact with
particles
being moved through the mixing chamber.
The present disclosure uses variations in the top face of the shaft extensions
that intrude into the main processing volume to control translation of
rotational shaft
energy delivered to the extensions into heating energy within particles
impacting the
portions. It has been found that varying the width and angle away from a shaft
axis
plane for the main facial portion provides a controllable variation in shear
delivered to
a particle impacting the portion, which in turn provides control over shaft
energy
translated into heat energy available for softening or melting a polymer part
of a
particle in a thermokinetic mixing chamber.
Referring again to FIGS. 3 and 4, it has been found that providing particles
within the mixing chamber a cumulative experienced shear which is determined
by
the shape and dimensions of a rotation-directed facial surface of extensions
from the
shaft and the inside surfaces of the mixing chamber results in the autoheating
phenomena of thermokinetic mixing. Substantially all the particles within a
mixing
chamber during shaft rotation inhabit the outer 30 percent of the volume of
the
internal space, i.e., the centrifugal force of the rotation of the extensions
maintains
the particulates and molten materials away from a central volume of the mixing
chamber. Thus, the effective thermokinetic mixer must be designed so that
distal
end parts of the shaft extensions are formed to accomplish the three functions
of
direct high shear (on the end part front face of the extension), indirect high
shear (on
the inside surfaces of the mixing chamber), and centrifugal maintenance of
material
in the outer volume of the mixing chamber. The top faces of shaft extensions
30a to
30c form a substantially vertical rectangle arranged at an angle away from a
plane
passing through an axis of shaft 23. It has been found that changing the
width,
angle, or varying the shape of the simple rectangle or arcuate paddle of the
shaft
provides an unexpected improvement and control over cumulative shear delivered
to
particles within a mixing chamber of a thermokinetic mixer, which, in turn,
provides
control over imparted heat energy and desired heat input to heat sensitive or
thermolabile components in a processed batch.
33
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
For these specific comparisons of the operation of thermokinetic mixers with
several configurations of a main facial portion, it is assumed that energy
input
through the shaft and the shaft rotational speed is about the same and that
the
number of shaft extensions and their spacing along the length of the shaft
within the
mixing chamber is substantially the same. Thus, the comparisons will show the
effect of changing the shapes of the main facial portion.
In general, decreasing the width relative to the length of the main facial
portion increases shaft energy translated into heat energy available for
softening or
melting a polymer part of a particle in a thermokinetic mixing chamber. The
width
must be above a minimum contact width so that a particle experiences a sliding
impact along the width, the particle is induced into a "skid" or energy
imparting
frictional contact, rolling and sliding at the period of time for impact on
the portion.
Mere normal glancing impact of a particle on a surface is relatively
ineffective in
imparting thermokinetic, autoheating energy for softening or melting. Yet,
easily
melted and heat-labile or heat sensitive polymers in some cases are sometimes
processed with a main facial portion providing just such glancing impact to
provide
more control over heat application to such components. Consistent with this
teaching, polymers refractory or resistant to softening or melting by
application of
heat are often processed with a main facial portion of minimum width (at least
0.25
inches) aligned at a minimum angle back from a shaft axial plane (for example,
at
least 10 degrees or at least 15 degrees) providing a contact time for
essentially the
same energy input, whereby distribution of that energy into skidding and
rotational
motion improves autoheating of the particle's polymer content.
A design of a shaft extension currently found in the Draiswerke Gelimat
thermokinetic mixer has the cross section 50 shown in FIG. 8, having a rounded
main facial portion 51 and an overall substantially spiral shape with a width
of about
2 inches. Relative shear 52 shown in a number of shortened arrows directed at
the
main facial portion 51 is not substantial for this design. Thus, this device
has been
relatively costly in terms of increased processing time and shaft power to
generate
sufficient thermokinetic heating to melt-blend polymers with substantial
resistance to
softening or melting. As such, it is relatively inadequate for processing heat
labile or
34
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
heat sensitive polymers having such resistance. There has been no suggestion
in
the thermokinetic mixing art that changing the width or angle of the main
facial
portion relative to a shaft axial plane would have any affect on thermokinetic
processing of polymers. The present disclosure discloses such embodiments in
FIGS. 9 through 12.
FIGS. 9 through 12 respectively show main facial portion cross sections 53
through 56 having main facial portions 57 through 60 with identical widths at
angles
of about 15 degrees, 30 degrees, 45 degrees and 60 degrees back from a shaft
axial
plane for the extensions which they represent. The projected widths on that
shaft
axial plane of main facial portions 57 through 60 are shown respectively in
lengths 65
through 68 and are directly related to relative shears 61 through 64, where an
increasing angle of a main facial portion relative to a shaft axial plane with
identical
width decreases the projected width onto the plane and unexpectedly increases
relative shear for the same shaft power input, rotational shaft speed and
extension
spacing and arrangement on the shaft. With this disclosure, it is now possible
to
control autoheating by delivered shear in the extensions of a thermokinetic
mixer.
Decreasing the widths of main facial portions while maintaining the angle
relative to a
shaft axial plane maintains total heat input into a thermokinetically
processed batch
in the mixers but increases shear upon any individual particle by reducing
projected
length along the shaft axial plane.
Thus, the shear strength of polymers processed by way of thermokinetic,
autoheating mixing and blending can now be matched to the relative shear
energy
imparted by the shaft extensions in the mixing chamber. A further design
refinement
is desirable where, as is quite common, polymer components in a batch comprise
both high shear and low shear polymers. Providing a main facial portion suited
for a
high shear component imparts shear energy which may deliver too much heat
energy to low shear components. In such a case, the low shear component tends
to
soften and roll along the width of the main facial portion, further increasing
the heat
generated, while the high shear components tend to leave that surface more
readily.
Such a circumstance could tend to cause incomplete mixing with the high shear
components insufficiently melted or overheating of low shear components. There
is
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
yet a further need for designs of a main facial portion that achieve an
optimal shear
delivery to high and low shear components in a thermokinetic batch.
It has been found that increasing the width of the main facial portion
achieves
this optimization. At an angle of between 15 to 80 degrees from a shaft axis
plane,
and the main facial portion having a width of at least 0.75 inches, provides
sufficient
path travel for both high and low shear polymer components in a batch so that
the
high shear components remain in sliding and skidding contact with the main
facial
portion long enough to generate heat and absorb heat from lower shear
components
to become softened and thereby blend with the low shear components.
Alternate designs for the main facial portion are shown in FIGS. 13 through
17, respectively, showing main facial portion cross sections 69, 72, 76, 80,
84, and
87. FIG. 13 shows cross section 69 comprising a leading acute surface 70
extending rearward to obtuse surface 71, providing a first low shear surface
followed
by a higher shear surface. FIG. 14 shows cross section 72 comprising a leading
acute surface 73 extending rearward to 90 degree surface 74, which in turn
extends
rearward to tailing acute surface 75, providing a first low shear surface
followed by a
higher shear surface and a lower shear surface. FIG. 15 shows cross section 76
comprising a leading acute surface 77 extending rearward to obtuse surface 78,
which in turn extends rearward to tailing acute surface 79, providing a first
low shear
surface followed by a higher shear surface and a lower shear surface. FIG. 16
shows cross section 80 comprising a leading obtuse surface 73 extending
rearward
to acute surface 74, which in turn extends rearward to tailing obtuse surface
75,
providing a first high shear surface followed by a lower shear surface and a
high
shear surface. FIG. 17 shows cross section 84 comprising a leading and rising
arcuate surface 85 extending rearward to a tailing and reducing arcuate
surface 86
degree surface 74, which in turn extends rearward to tailing acute surface 75,
providing a first low shear surface followed by a higher shear surface and a
lower
shear surface. FIG. 18 shows cross section 87 comprising a leading acute
surface
88 and a tailing acute surface 89, providing a first low shear surface
followed by a
higher or lower shear surface, depending on the shear of the batch components.
36
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
In light of the above teaching of these embodiments, the top face 22 of FIG. 4
is a significant element in providing thermokinetic contact with particles in
the mixing
chamber and causing them to impact the inside cylindrical surface of the
mixer.
FIG. 19 shows another significant embodiment of the thermokinetic mixer of
the present disclosure, in that halves 5 and 7 and door 6 are respectively
interiorly
lined by interior liner pieces 5a, 7a and 6a. The liner pieces are adapted to
intimately
lie adjacent to inside surfaces of halves 5 and 7 and door 6 during operation
of the
mixer, thereby providing any of a diverse set of thermokinetic frictional
contact
surfaces desired for accelerated particles, such desired surfaces selected
from
among any appropriate or optimized materials for liner pieces 5a, 7a and 6a.
FIG.
19 shows in exploded view the liner pieces 5a, 7a and 6a separated from their
adjacent (as installed) parts. Bolting the halves 5 and 7 together cause liner
pieces
5a and 7a to secure to line the inside surfaces of those halves 5 and 7. Holes
in end
sections of liner piece 6a allows for bolted connection of it to door 6. In
thermokinetic mixers known to those of skill in the art, the inside surfaces
of the
mixing chamber were limited to those steel alloys with sufficient mechanical
and
thermal strength required for encasing and enclosing the thermokinetic
operation of
such mixers. Therefore, known thermokinetic mixers were limited in their
processing
capabilities to only those mixtures which would not excessively adhere to a
smooth
inside surface of steel alloy of the mixing chamber and which, at the same
time,
would impinge beneficially on those surfaces to provide frictional heating of
particles
in the mixture. Further, even relatively slight wear on the inside surfaces of
the
mixing chambers of thermokinetic mixers can dramatically alter the efficacy of
the
generation of thermokinetic heating of chambered particles, in that the
distance
between the shaft extensions and the inside surface of the mixing chamber is
specifically designed to optimize thermokinetic heating by the interaction of
particles
moving between the inside surface of the mixing chamber and the shaft
extensions.
Thus, such slight wear can require that the entire, relatively expensive set
of halves
5 and 7 to be replaced in such thermokinetic mixers. The present embodiment
eliminates such excessive cost. Liner pieces 5a, 7a and 6a are relatively much
less
in cost to replace than halves 5 and 7 and door 6. Replacement of the liner
pieces is
quite simple and fast. Preferred liner piece composition includes stainless
steel
37
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
(alloys with greater than 12 weight percent chrome) and other such steel
alloys,
titanium alloys (such as nitrided or nitride-containing titanium), and wear
and heat
resistant polymers (such as Teflon ). It is another embodiment of the present
disclosure to provide non-smooth inside surfaces for liner pieces 5a, 7a and
6a, such
as parallel or spiral grooving about the inside cylindrical surfaces of liner
pieces 5a,
7a and 6a, surface texturing, and/or electropolishing. Such materials and
texturing
for liner pieces 5a, 7a and 6a are intended to obtain an optimum or desirable
balance of characteristics which will reduce undesirable adhesion of
thermokinetically melted particles and/or promote thermokinetic frictional
contact of
mixing chamber particles in their travel among the shaft extensions and the
inside
surfaces of the liner pieces 5a, 7a and 6a.
In a further embodiment of the present disclosure whereby materials or
texturing of liner pieces 5a, 7a and 6a are selected to obtain the objects of
thermokinetic mixing, shaft extension portions comprising the front and top
impact
faces of the shaft extensions are adapted by way of material composition
and/or
texturing similar to those changes just disclosed for the inside surfaces of
liner
pieces 5a, 7a and 6a.
Another feature of the present disclosure is that the top face of the shaft
extensions, i.e., those which extend at least with a slight elevation rearward
above
the height of the front face of the shaft extension to form a ramp structure
upon
which chambered particles impinge (faces 22 of FIGS. 3 and 4), are the primary
location of wear among the inside surfaces of the mixing chamber. The
consequences of this discovery are considerable with respect to the design of
shaft
extensions in thermokinetic mixers. It has been found that such a top face has
a
function very different than that of the front face. A front face of a shaft
extension
drags a particle along its rearward directed width, causing the particle to be
driven
substantially in a direction of an axis of the drive shaft. Such an axis-
driven particle
will then tend to engage yet another front face of a shaft extension of a
rearward and
next line of shaft extensions. The motion of particles in contact with a top
face of a
shaft extension driven by shaft rotation is very different, imparting in such
motion a
38
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
substantially greater frictional, thermokinetic energy to a particle than the
front face
of the shaft extension.
FIG. 20 shows a side view (a view in the direction of the axis of a shaft to
which it is mounted) of a removable portion of a shaft extension 30 showing a
front
face 21 and top face 22. Reference elevations 30b to 30d are measured from a
base
level 30a. Neither front face 21 or top face 22 are shown in plan view but
rather are
shown with their projections upon the side shaft-axial view. Top face 22
comprises a
front edge rising from elevation 30c to 30b and thereafter sweeping rearward
and
upward to similarly inclined rear edge with a highest elevation 30d. Only a
part of
the inside surface of half 7 is shown as separated from top face 22 and
portions P1
to P4 represent the path of a particle impinging first upon top face 22 and
then upon
the inside surface of half 7. It has been found that the area of greatest wear
on any
inside surface of the mixing chamber is along the rearward area from the front
edge
represented by the line from elevations 30c to 30b, i.e., the impact point of
a particle
at portion P1. A major portion of kinetic energy is clearly translated to
frictional
heating to the particle in that area as evidenced by the substantial wear on
such
hardened surfaces. Top face 22 rises more rapidly at its far edge along
elevations
30b to 30d than along the near edge starting at elevation 30c, resulting in a
relatively
long frictional travel path of the particle along portion P2 and being ramp-
launched
from elevation 30d toward the inside surface of half 7. Upon frictional,
spinning, and
dragging contact with the inside surface of half 7 at portion P3, the
extensively
heated particle rebounds from the inside surface of half 7 to again contact a
top face
of another shaft extension. The length of portion P2 substantially controls
required
frictional heating time for thermokinetic mixing and melting for a batch of
particles
within the mixing chamber of the present disclosure. The present disclosure
comprises selecting a shaft extension which provides to an impinging particle
in
thermokinetic mixing a top face contact path of longer or shorter length and
angle of
deflection to thereby control a substantial or majority of frictional heating
contact of
chambered particles to a desired batch temperature.
FIG. 21 shows a perspective view of a specific embodiment of the shaft
extension of FIG. 20 having a concave front face 21 and a top face 22 capable
of
39
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
producing variable lengths of portions P2' (longer) and P2" (shorter)
respectively for
portions P3' and P3". In certain embodiments, the top face 22 comprise a
convex
surface with a radius of about 4.5 inches extending from its front, leading
edge to its
rearmost edge.
In certain embodiments, a shaft extension providing a relatively long
frictional
contact path for particles being processed by the mixer of the present
disclosure are
preferred for providing shortened processing times, i.e., to heat a batch to a
desired
temperature as quickly as possible. Such control of heating and processing
times is
directly applicable to the disclosed process of two step continuous
thermokinetic
mixing, whereby increasing rotational shaft speed will more swiftly impart
frictional
heating for melting energy to the particles more refractory or resistant to
lower speed
heating. It has been found that non-uniformity of materials in a batch
processed
thermokinetically, i.e., either by composition or particle size, results in
greater or
lesser frictional path contact with the insides of the mixing chamber.
Particles more
resistant to melting, either by way of higher melting temperatures or
hardness, will
rebound more quickly from frictional contact with the inside surfaces of a
thermokinetic mixer and thereby require more processing time than less
refractory
particles. Thermokinetic mixing to a final, desired processing consistency for
heat
labile or heat damageable components generally favors reaching a target batch
temperature as quickly as possible. Certain embodiments of the present
disclosure
provide short, medium, long or mixed lengths of particle frictional contact
paths along
a top face of a shaft extension, either by way of a single or multiple
processing shaft
speeds, to achieve the more effective mixing of certain thermolabile
components.
It is well known to those in the art that the topmost surfaces of shaft
extensions in the Draiswerke mixers are merely arcuately tapered and smoothed
ends of a generally sinous shaft extension. As such, the ability of such
mixers to
provide substantial top face shearing, frictional heating to thermokinetic
mixing
chamber particles is essentially minimized. To accomplish additional top face-
like
frictional paths for particles in the mixing chamber and to accomplish other
objects of
the present disclosure, FIG. 22 discloses a frontal view of an OPEN 30 shaft
extension having a central OPENING so that particles can pass through it
during
CA 02945955 2016-10-14
WO 2015/160358 PCT/US2014/034601
processing and impinging on identically rearwardly angled pairs of surfaces
A1/A2,
B1/B2 and 01/02. It will be appreciated that surfaces A1/A2 together act upon
particles as a top face and that surfaces B1/B2 and 01/02 act upon particles
as front
faces. FIG. 22 more generally discloses that shaft extensions may be formed in
a
donut or toroid shape or in the shape of a diamond with a central opening to
accomplish the more effective mixing of certain thermolabile components.
All of the compositions and/or methods disclosed and claimed herein can be
made and executed without undue experimentation in light of the present
disclosure.
While the compositions and methods of this disclosure have been described in
terms of preferred embodiments, it will be apparent to those of skill in the
art that
variations may be applied to the compositions and/or methods and in the steps
or in
the sequence of steps of the method described herein without departing from
the
concept, spirit and scope of the disclosure. All such similar substitutes and
modifications apparent to those skilled in the art are deemed to be within the
spirit,
scope and concept of the disclosure as defined by the appended claims.
41