Note: Descriptions are shown in the official language in which they were submitted.
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
COMBINATION IMMUNO THERAPY AND RADIOTHERAPY FOR THE
TREATMENT OF HER-2-POSITIVE CANCERS
FIELD OF INVENTION
[001] This invention provides methods for inducing an immune response against
a Her-
2/neu antigen-expressing tumor and for treating the same and vaccinating
against the same in
human and canine subjects using a combination of radiation therapy and a
recombinant
attenuated Listeria strain vaccine.
BACKGROUND OF THE INVENTION
[002] Her-2/neu (referred to henceforth as "Her-2") is a 185 kDa glycoprotein
that is a
member of the epidermal growth factor receptor (EGFR) family of tyrosine
kinases, and is
overexpressed in 25 to 40% of all breast cancers and in many cancers of the
bone
(osteosarcoma ¨ OSA), ovaries, lung, pancreas, brain, and gastrointestinal
tract. Patients with
cancers that overexpress Her-2 exhibit tolerance even with detectable humoral,
CD8+ T cell,
and CD4+ T cell responses directed against Her-2.
[003] Large breed dogs spontaneously develop OSA that recapitulates many
aspects of
human pediatric OSA including histologic heterogeneity, aggressive local
disease and early
metastases. At diagnosis, 95% of dogs have micrometastatic disease and despite
amputation
and chemotherapy, the median survival time is 10 months with most dogs
euthanized due to
progressive metastatic disease. The overall survival of human patients with
metastatic
osteosarcoma ranges from 10-50%, depending on the location and the number of
metastatic
foci.
[004] Radiation therapy (RT), which is used to destroy tumor cells or to alter
tumor/stroma
architecture, is an integral part of treatment of many types of cancer.
However, because OSA
is radioresistant to standard dose of radiotherapy, it is not used for
treating OSA.
[005] Recently there has been evidence that RT may synergize with targeted
immune
therapy. For example, RT induces immunogenic cell death wherein tumor cells
die slowly
over time from apoptosis, necrosis and/or mitotic catastrophe, leading to the
clearance of the
dying cells by the immune system. This in turn serves as a potential source of
tumor antigens
for immune therapy. RT also modulates tumor cell surface expression of cell
death receptors,
tumor-associated antigens and adhesion molecules, which render the tumor cells
more
susceptible to immune-mediated killing.
[006] The present invention meets the needs of subjects suffering from OSA
with
surprising findings that radiation therapy when combined with a recombinant
Listeria-Her-
2/neu vaccine (ADXS31-164) that was generated using the LmddA vaccine vector
which has
1
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
a well-defined attenuation mechanism and is devoid of antibiotic selection
markers is
particularly effective against osteosarcoma and pulmonary metastasis.
SUMMARY OF THE INVENTION
[007] In one embodiment, the present invention provides a method of treating a
Her-2/neu-
expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain, and wherein the administration of said radiation therapy comprises at
least two
administrations of said radiation therapy.
[008] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
subject comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[009] In another embodiment, the present invention provides a method of
prolonging
survival in a subject suffering from a Her-2/neu-expressing tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide, and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0010] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a subject suffering from a Her-2/neu-expressing tumor
growth or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
2
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0011] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a subject suffering from a Her-2/neu-expressing
tumor growth or
cancer comprising the step of administering a combination of radiation therapy
and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional adjuvant and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0012] In one embodiment, the subject is a human. In one embodiment, the human
subject is
a child. In another embodiment, the human subject is an adult. In another
embodiment, the
subject is a canine.
[0013] In another embodiment, administering said fusion polypeptide to said
subject
prevents escape mutations within said tumor.
[0014] In another embodiment, said Her-2/neu chimeric antigen comprises at
least 5, 9, 13,
14, or 17 of the mapped human MHC-class I epitopes. In another embodiment,
said Her-
2/neu chimeric antigen comprises at least 5, 9, 13, 14, or 17 of the canine
MHC-class I
epitopes.
[0015] In one embodiment, the nucleic acid molecule is integrated into the
Listeria genome.
In another embodiment, the nucleic acid molecule is in a plasmid in said
recombinant
Listeria vaccine strain and the plasmid is stably maintained in the
recombinant Listeria
vaccine strain in the absence of antibiotic selection.
[0016] In one embodiment, the recombinant Listeria lacks the actA virulence
gene. In one
embodiment, the additional polypeptide is selected from the group consisting
of: a) non-
hemolytic LLO protein or N-terminal fragment, b) a PEST sequence, or c) an
ActA
fragment. In one embodiment, the metabolic enzyme encoded by said second open
reading
frame is an alanine racemase enzyme or a D-amino acid transferase enzyme. In
some
embodiments of this invention, a recombinant attenuated Listeria strain is
ADXS31-164.
[0017] In one embodiment, the recombinant attenuated Listeria strain is
administered with
3
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
an independent adjuvant, which, in one embodiment, comprises a
granulocyte/macrophage
colony-stimulating factor (GM-CSF) protein, a nucleotide molecule encoding a
GM-CSF
protein, saponin QS21, monophosphoryl lipid A, or an unmethylated CpG-
containing
oligonucleotide.
[0018] In one embodiment, the cancer is osteosarcoma (OSA). In another
embodiment, the
cancer or tumor is pulmonary metastatic disease. In one embodiment,
administration
comprises at least two administrations of said recombinant attenuated Listeria
strain. In one
embodiment, the administration of said radiation therapy comprises at least
two
administrations of said radiation therapy. In one embodiment, provided herein
is a
combination therapy comprising a radiation therapy and administration of
ADXS31-164
provided herein. In one embodiment, the radiation therapy is administered
prior to
administration of the recombinant attenuated Listeria strain.
[0019] In another embodiment, the subject does not undergo amputation prior to
administration of said radiation therapy and said recombinant attenuated
Listeria strain. In
another embodiment, the method further comprises administering said radiation
therapy and
said recombinant attenuated Listeria strain following a relapse or metastasis
in said subject,
which in one embodiment, is pulmonary metastatic disease.
[0020] In one embodiment, the method results in increased overall survival of
said subject.
In another embodiment, the method results in a delay of metastatic disease in
a subject. In
another embodiment, the method results in an increased Her-2/neu specific T
cell response.
In another embodiment, said elicitation of an enhanced immune response results
in increased
overall survival of said subject. In another embodiment, said elicitation of
an enhanced
immune response results in a delay of metastatic disease in a subject. In one
embodiment, the
metastatic disease is pulmonary metastatic disease. In another embodiment,
said elicitation of
an enhanced immune response results in an increased Her-2/neu specific T cell
response.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The subject matter regarded as the invention is particularly pointed
out and distinctly
claimed in the concluding portion of the specification. The invention,
however, both as to
organization and method of operation, together with objects, features, and
advantages
thereof, may best be understood by reference to the following detailed
description when read
with the accompanying drawings in which:
[0022]Figure 1. Construction of ADXS31-164. (A) Plasmid map of pAdv164, which
harbors bacillus subtilis dal gene under the control of constitutive Listeria
p60 promoter for
complementation of the chromosomal dal-dat deletion in LmddA strain. It also
contains the
4
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
fusion of truncated LL0(l_441) to the chimeric human HER2/neu gene, which was
constructed
by the direct fusion of 3 fragments the HER2/neu: EC1 (aa 40-170), EC2 (aa 359-
518) and
ICI (aa 679-808). The vector schematic on the right shows details pAdv164
expressing a
chimeric HER2/neu fusion protein consisting of 2 extracellular domains and one
intracellular
domain of human HER2/neu fused to truncated LLO. The plasmid is maintained
within the
recombinant dal/dat/ actA- listeria strain
(LmddA) by means of auxotrophic
complementation of the dal gene (See Examples). (B) Expression and secretion
of tLLO-
ChHer2 was detected in Lm-LLO-ChHer2 (Lm-LLO-138) and LmddA-LLO-ChHer2
(ADXS31-164) by western blot analysis of the TCA precipitated cell culture
supernatants
blotted with anti-LLO antibody. A differential band of ¨104 KD corresponds to
tLLO-
ChHer2. The endogenous LLO is detected as a 58 KD band. Listeria control
lacked ChHer2
expression.
100231Figure 2. Immunogenic properties of ADXS31-164 (A) Cytotoxic T cell
responses
elicited by HER2/neu Listeria-based vaccines in splenocytes from immunized
mice were
tested using NT-2 cells as stimulators and 3T3/neu cells as targets. Lm-
control was based on
the LmddA background that was identical in all ways but expressed an
irrelevant antigen
(HPV16-E7). (B) IFN-y secreted by the splenocytes from immunized FVB/N mice
into the
cell culture medium, measured by ELISA, after 24 hours of in vitro stimulation
with
mitomycin C treated NT-2 cells. (C) IFN-y secretion by splenocytes from HLA-A2
transgenic mice immunized with the chimeric vaccine, in response to in vitro
incubation with
peptides from different regions of the protein. A recombinant ChHer2 protein
was used as
positive control and an irrelevant peptide or no peptide groups constituted
the negative
controls as listed in the figure legend. IFN-y secretion was detected by an
ELISA assay using
cell culture supernatants harvested after 72 hours of co-incubation. Each data
point was an
average of triplicate data +/- standard error. * P value < 0.001.
100241Figure 3. Tumor Prevention Studies for Listeria-ChHER2/neu Vaccines
HER2/neu transgenic mice were injected six times with each recombinant
Listeria-ChHer2
or a control Listeria vaccine. Immunizations started at 6 weeks of age and
continued every
three weeks until week 21. Appearance of tumors was monitored on a weekly
basis and
expressed as percentage of tumor free mice. *p<0.05, N = 9 per group.
100251Figure 4. Effect of immunization with ADXS31-164 on the % of Tregs in
Spleens. FVB/N mice were inoculated s.c. with 1 x 106 NT-2 cells and immunized
three
times with each vaccine at one week intervals. Spleens were harvested 7 days
after the
second immunization. After isolation of the immune cells, they were stained
for detection of
Tregs by anti CD3, CD4, CD25 and FoxP3 antibodies. dot-plots of the Tregs from
a
5
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
representative experiment showing the frequency of CD25 /FoxP3+ T cells,
expressed as
percentages of the total CD3+ or CD3+CD4+ T cells across the different
treatment groups.
[0026]Figure 5. Effect of immunization with ADXS31-164 on the % of tumor
infiltrating Tregs in NT-2 tumors. FVB/N mice were inoculated s.c. with 1 x
106 NT-2
cells and immunized three times with each vaccine at one week intervals.
Tumors were
harvested 7 days after the second immunization. After isolation of the immune
cells, they
were stained for detection of Tregs by anti CD3, CD4, CD25 and FoxP3
antibodies. (A). dot-
plots of the Tregs from a representative experiment. (B). Frequency of CD25
/FoxP3+ T
cells, expressed as percentages of the total CD3+ or CD3+CD4+ T cells (left
panel) and
intratumoral CD8/Tregs ratio (right panel) across the different treatment
groups. Data is
shown as mean SEM obtained from 2 independent experiments.
100271Figure 6. Vaccination with ADXS31-164 can delay the growth of a breast
cancer
cell line in the brain. Balb/c mice were immunized thrice with ADXS31-164 or a
control
Listeria vaccine. EMT6-Luc cells (5,000) were injected intracranially in
anesthetized mice.
(A) Ex vivo imaging of the mice was performed on the indicated days using a
Xenogen X-
100 CCD camera. (B) Pixel intensity was graphed as number of photons per
second per cm2
of surface area; this is shown as average radiance. (C) Expression of HER2/neu
by EMT6-
Luc cells, 4T1-Luc and NT-2 cell lines was detected by Western blots, using an
anti-
HER2/neu antibody. J774.A2 cells, a murine macrophage like cell line was used
as a
negative control.
100281Figure 7. Shows the first 18 canine osteosarcoma patients vaccinated
with ADXS31-
164, following amputation and chemotherapy.
100291Figure 8. Shows that ADXS31-164 administration does not cause A) early
evidence
of dilated cardiomyopathy. B) Sequential cardiac troponin I levels evaluated
over the course
of the study showing that the levels stay within the normal range throughout
the study period
for the majority of dogs. It should be noted that the one dog with a
temporarily increased
cardiac troponin I level had unremarkable echocardiograms at the time these
values were
mildly increased (see also Fig 26D).
100301Figure 9. Shows ADXS31-164 associated changes in A) body temperature and
B)
systolic blood pressure. Body temperature and systolic blood pressure were
recorded at
baseline and every 2 hours post ADXS31-164 administration. Parameters for each
dog at
each vaccination are displayed. Horizontal bars represent median values for
all dogs in each
dose group at each time point. *p<0.05, **p<0.005
6
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
100311Figure 10. Shows treatment schedule of combination ADXS31-164 and
palliative
radiation therapy (RT) in the context of primary appendicular osteosarcoma
without
amputation and chemotherapy.
100321Figure 11. Top panel: Radiographs showing the presence of a fracture of
the proximal
humerus associated with osteosarcoma and those taken after fracture fixation
using two bone
plates and an intramedullary pin. Bottom panel: A CT scan of the chest shoing
no evidence
of metastatic disease at enrollment. Radiographs were also taken at baseline
and after 8
ADXS31-164 administrations. These radiographs show no evidence of pulmonary
metastatic
disease and the presence of boney callus surrounding the fracture site
indicating fracture
healing despite the presence of osteosarcoma.
100331Figure 12. Timeline of a pilot phase I clinical trial to evaluate the
safety and efficacy
of a L. monocytogenes recombinant expressing ADXS31-164 to elicit
therapeutically
effective anti-tumor immunity in dogs with appendicular osteosarcoma, that
undergo limb
amputation and follow up chemotherapy.
[0034]Figure 13 A-B. Treatment-related adverse events and survival curves
following
ADXS-31-164 administration. Figure 13A shows treatment-related adverse events.
Figure
13B shows all dogs without metastatic disease at the time of trial enrollment.
Dogs in the
control group underwent limb amputation followed by either carboplatin alone
or carboplatin
plus Adriamycin. 2 dogs have been censored from the vaccine arm as they died
of unrelated
causes (1 dog died from aspiration pneumonia, the other died from
nephroblastoma).
Vaccinated group Red line; Control group Black line.
100351Figure 14. Radiographic images of primary and metastatic osteosarcoma
(OSA) in a
human (A) and canine (B) patient. In both species, primary lesions are
characterized by areas
of proliferation and lysis in the bone metaphysis (arrows in A).
[0036]Figure 15. Schematic of the phase I, 3+3 clinical trial to evaluate the
safety and
efficacy of ADXS31-164 in dogs with HER2+ osteosarcoma (OSA). Privately owned
dogs
with spontaneous HER2+ appendicular OSA underwent standard of care amputation
and
follow up carboplatin chemotherapy. Three weeks after the last carboplatin
dose, dogs were
vaccinated with either 2x108, 5x108, 1x109 or 3.3x109 CFU of ADXS31-164
intravenously
(three vaccinations given three weeks apart). Dogs were re-staged every 2
months until death
to determine vaccine efficacy in preventing metastatic disease.
100371Figure 16. HER2/neu expression in canine primary osteosarcoma. (A) H&E
stain of
primary OSA from a dog showing nests of malignant osteoblasts and osteoid
deposition. (B)
Immunohistochemical evaluation of canine primary OSA showing HER2/neu
expression
within malignant osteoblasts. (C) Western blot of primary OSA samples from 5
privately
7
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
owned dogs showing variable expression of HER2/neu. Positive controls are: MCF-
7 human
mammary carcinoma cell line andCAMAC2 a canine mammary carcinoma cell line.
100381Figure 17. Hematological values at baseline (Pre) and at 24 hours after
(Post)
ADXS31-164 administration. Pre and Post values from all dogs within each dose
group at
each vaccination were averaged. *p<0.05, ** p<0.005. Shows a transient, but
statistically
significant increase in white blood cell and neutrophil counts (A-B) that
occurred 24 hours
after ADXS31-164 administration and that were accompanied by a transient
decrease in
platelets and lymphocytes (C-D).
100391Figure 18. ADXS31-164 induced increases in white blood cells (WBC),
neutrophil
and monocyte counts correlate with survival. WBC, neutrophil and monocyte
counts were
measured at baseline and 24 hours after vaccination. The percent increase was
calculated
following each vaccination and averaged for each dog. (A) Results are
displayed according
to survival (dead or alive). (B) Results are displayed according to ADXS31-164
dose
received. Horizontal bars represent median values of the group.
100401Figure 19. Shows the results of evaluation of Her-2 specific T cell
responses induced
by ADXS31-164 by IFN-7 ELISpot.
100411Figure 20. Shows repeat "booster" vaccinations Stimulate Her-2 specific
immunity.
(A) Shows the results for patient 289-003. (B) Shows the results for patient
289-004. EC1,
EC2 and IC1 represent the peptide fragments of the HER2/neu polypeptide.
100421Figure 21. Kaplan Meier estimates for (A) Time To Metastasis (TTM) and
(B) OSA
Specific Survival.
100431Figure 22. Shows that ADXS31-164 prevents development of metastatic
disease. (A
and B) Thoracic radiographs taken 3 weeks after carboplatin therapy (A) and 3
weeks after
the third ADXS31-164 vaccine (B) showing an increase in size of the pre-
existing metastatic
nodule in the right cranial lung lobe but lack of further metastatic disease
development in
remaining lung lobes. (C and D) Pulmonary nodule identified on thoracoscopy
that
fluoresces under near infra-red light following administration of ICG (C).
Grossly normal
appearing pulmonary tissue removed at the time of metastatectomy showing
fluorescence
under near infra-red light (inset) (D). (E and F) H&E stained histopathology
of (E)
pulmonary nodule and (F) fluorescing normal pulmonary tissue showing
significant
hemorrhage and necrosis of encapsulated pulmonary nodule (E) and focal area of
inflammation in grossly normal appearing pulmonary tissue (F). (G and H)
Immunohistochemistry of pulmonary nodule at low (G) and high (H) magnification
showing
CD3+ T cells surrounding the pulmonary nodule with minimal CD3+ T cells within
the
neoplastic tissue. (I and J) Immunohistochemistry of normal appearing
pulmonary tissue at
8
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
low (I) and high (J) magnification showing focal accumulations of CD3+ T
cells. (K) High
magnification H&E stain of focal pneumonia showing large abnormal cells with
mitotic
figures surrounded by lymphocytes. (L) Vimentin stain of pneumonic region
showing a large
vimentin positive cell, with prominent mitotic figures surrounded by
mononuclear cells.
100441Figure 23. ADXS31-164 delays/prevents metastatic disease and prolongs
overall
survival in dogs with spontaneous HER2+ osteosarcoma. Shown is a Kaplan-Meier
survival
curve of vaccinated dogs compared with a historical control group. The control
group
consisted of dogs with HER2+ appendicular OSA, treated with amputation and
follow-up
chemotherapy but who did not receive ADXS31-164. P<0.0001. Vaccinated group
Red line;
Control group Black line.
100451Figure 24. Shows that ADXS31-164 breaks tolerance to HER2/neu. PBMCs
were
collected at baseline, 3 weeks after the 3rd vaccine (9 weeks) and 2 months
later (17 weeks)
and analyzed by IFN-7 ELISpot for responses to the highly conserved IC1 domain
of
HER2/neu. Results presented divided dogs into early responders, late
responders and
apparent non-responders. NA indicates that the 17 week sample for these dogs
was not yet
evaluated.
100461Figure 25A-D. Shows that ADXS31-164 does not adversely affect cardiac
function.
Cardiac parameters LVID (diastole) (Figure 25A), LVID (systole) (Figure 25B)
and
fractional shortening (Figure 25C) were evaluated for each dog at baseline,
the time of
vaccination and every 2 months thereafter. Cardiac troponin I levels were
evaluated at the
same time points (Figure 25D).
[0047]Figure 26. Shows that ADXS31-164 breaks immune tolerance to the highly
conserved intracellular domain of HER2/neu.
[0048]Figure 27A-D. Shows that radiation therapy in conjunction with ADXS31-
164
therapy delays progression of primary osteosarcoma (OSA) in subject 386-002
(Figure 27A),
subject 385-005 (Figure 27B), subject 386-003 (Figure 27C), and subject 386-
007 (Figure
27D).
100491Figure 28 A-C. Shows the results of a pain questionnaire in subjects
with pain
interfering with general activity (Figure 28A), in subjects with pain
interfering with the
ability to walk (Figure 28B), and in subjects with pain interfering with the
enjoyment of life
(Figure 28C).
100501Figure 29A-D. Shows that palliative radiation therapy in conjunction
with ADXS31-
164 therapy reduces lysis, promotes tumor consolidation, and prolongs survival
of subjects
(Figure 29 A-D). Lateral (Figure 29A) and AP (Figure 29C) radiographic views
of a distal
tibial osteosarcoma lesion demonstrating marked cortical bone remodeling, and
reduction in
9
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
lysis, following 16Gy radiation (given as 8Gy on 2 consecutive days starting
on 9.13.2014)
and 3 doses of ADXS31-164 (11.25.2014). Lateral (Figure 29B) and AP (Figure
29D)
radiographic views of a distal radial osteosarcoma lesion treated with 16Gy
radiation (given
as 8Gy on 2 consecutive days starting on 7.16.2014) and 3 doses of ADXS31-164
(10.13.2014). Note the significant reduction in swelling and bony lysis within
the distal
portion of the radius (compare radiographs dated 10.13.2014 with 7.16.2014 in
Figure 29B).
There is increased bone density on the medial aspect of the distal tibia
(compare radiographs
dated 10.13.2014 with 7.16.2014 in Figure 29D). There is a small minimally
displaced bone
fracture of the medial aspect of the distal radius seen on the 10.13.2014
radiographs in Figure
to 29D.
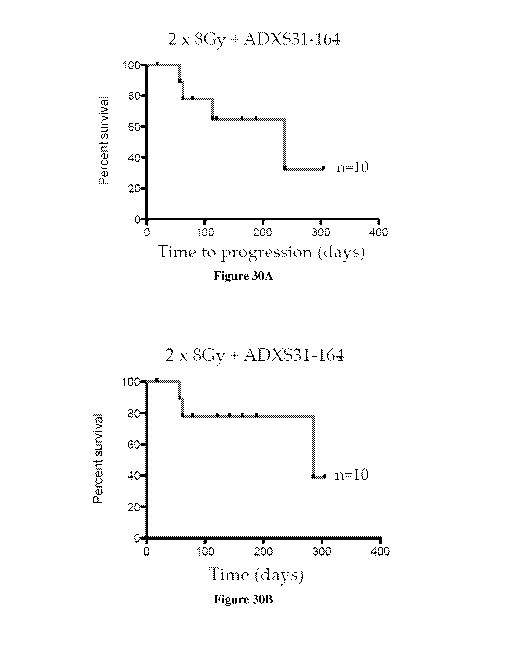
[0051]Figure 30A-B. Shows that radiation therapy in conjunction with ADXS31-
164
prolongs survival in dogs with osteosarcoma (OSA).
[0052]It will be appreciated that for simplicity and clarity of illustration,
elements shown in
the figures have not necessarily been drawn to scale. For example, the
dimensions of some of
the elements may be exaggerated relative to other elements for clarity.
Further, where
considered appropriate, reference numerals may be repeated among the figures
to indicate
corresponding or analogous elements.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
[0053] In the following detailed description, numerous specific details are
set forth in order
to provide a thorough understanding of the invention. However, it will be
understood by
those skilled in the art that the present invention may be practiced without
these specific
details. In other instances, well-known methods, procedures, and components
have not been
described in detail so as not to obscure the present invention.
[0054] In one embodiment, the present invention provides a method of treating
a tumor
growth or cancer in a subject comprising the step of administering a
combination of radiation
therapy and a recombinant attenuated Listeria strain comprising a nucleic acid
comprising a
first open reading frame encoding a fusion polypeptide comprising a tumor
specific antigen
fused to an additional polypeptide.
[0055] In one embodiment, the present invention provides a method of
preventing a tumor
growth or cancer in a subject comprising the step of administering a
combination of radiation
therapy and a recombinant attenuated Listeria strain comprising a nucleic acid
comprising a
first open reading frame encoding a fusion polypeptide comprising a tumor
specific antigen
fused to an additional polypeptide.
[0056] In one embodiment, the present invention provides a method of eliciting
an enhanced
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
immune response against a tumor growth or cancer in a subject comprising the
step of
administering a combination of radiation therapy and a recombinant attenuated
Listeria strain
comprising a nucleic acid comprising a first open reading frame encoding a
fusion
polypeptide comprising a tumor specific antigen fused to an additional
polypeptide.
[0057] In one embodiment, the present invention provides a method of
prolonging survival
in a subject suffering from a tumor growth or cancer comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a tumor specific antigen fused to an additional polypeptide.
[0058] In one embodiment, the present invention provides a method of delaying
metastatic
disease in a subject suffering from a tumor growth or cancer comprising the
step of
administering a combination of radiation therapy and a recombinant attenuated
Listeria strain
comprising a nucleic acid comprising a first open reading frame encoding a
fusion
polypeptide comprising a tumor specific antigen fused to an additional
polypeptide.
[0059] In one embodiment, the present invention provides a method of breaking
tolerance to
a tumor specific antigen in a subject suffering from a tumor growth or cancer
expressing said
tumor specific antigen comprising the step of administering a combination of
radiation
therapy and a recombinant attenuated Listeria strain comprising a nucleic acid
comprising a
first open reading frame encoding a fusion polypeptide comprising a tumor
specific antigen
fused to an additional polypeptide.
[0060] In one embodiment, the tumor specific antigen is Her-2/neu.
[0061] In one embodiment, the present invention provides a method of treating
a Her-2/neu-
expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide.
[0062] In one embodiment, the present invention provides a method of
preventing a Her-
2/neu-expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide.
[0063] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
subject comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
11
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide.
[0064] In another embodiment, the present invention provides a method of
prolonging
survival in a subject suffering from a Her-2/neu-expressing tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide.
[0065] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a subject suffering from a Her-2/neu-expressing tumor
growth or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide.
[0066] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a subject suffering from a Her-2/neu-expressing
tumor growth or
cancer comprising the step of administering a combination of radiation therapy
and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional adjuvant.
[0067] In one embodiment, the recombinant attenuated Listeria strain further
comprises a
second open reading frame encoding a metabolic enzyme, wherein said metabolic
enzyme
complements an endogenous gene that is mutated in the chromosome of said
recombinant
attenuated Listeria strain.
[0068] In one embodiment, the present invention provides a method of treating
a Her-2/neu-
expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain.
[0069] In one embodiment, the present invention provides a method of
preventing a Her-
2/neu-expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
12
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain.
[0070] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
subject comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0071] In another embodiment, the present invention provides a method of
prolonging
survival in a subject suffering from a Her-2/neu-expressing tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide, and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0072] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a subject suffering from a Her-2/neu-expressing tumor
growth or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0073] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a subject suffering from a Her-2/neu-expressing
tumor growth or
cancer comprising the step of administering a combination of radiation therapy
and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional adjuvant and a second open reading frame encoding a metabolic
enzyme,
13
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0074] In one embodiment, the administration of said radiation therapy
comprises at least
two administrations of said radiation therapy.
[0075] In one embodiment, the present invention provides a method of treating
a Her-2/neu-
expressing tumor growth or cancer in a subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain, and wherein the administration of said radiation therapy comprises at
least two
administrations of said radiation therapy.
[0076] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
subject comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0077] In another embodiment, the present invention provides a method of
prolonging
survival in a subject suffering from a Her-2/neu-expressing tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide, and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0078] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a subject suffering from a Her-2/neu-expressing tumor
growth or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
14
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0079] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a subject suffering from a Her-2/neu-expressing
tumor growth or
cancer comprising the step of administering a combination of radiation therapy
and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional adjuvant and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain, and wherein the
administration
of said radiation therapy comprises at least two administrations of said
radiation therapy.
[0080] In one embodiment, the subject is a human. In one embodiment, the human
subject is
a child. In another embodiment, the human subject is an adult.
[0081] In one embodiment, the present invention provides a method of treating
a Her-2/neu-
expressing tumor growth or cancer in a human subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain.
[0082] In one embodiment, the present invention provides a method of
preventing a Her-
2/neu-expressing tumor growth or cancer in a human subject comprising the step
of
administering a combination of radiation therapy and a recombinant attenuated
Listeria strain
comprising a nucleic acid comprising a first open reading frame encoding a
fusion
polypeptide comprising a Her-2/neu chimeric antigen fused to an additional
polypeptide, and
a second open reading frame encoding a metabolic enzyme, wherein said
metabolic enzyme
complements an endogenous gene that is mutated in the chromosome of said
recombinant
attenuated Listeria strain.
[0083] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
human subject comprising the step of administering a combination of radiation
therapy and a
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0084] In another embodiment, the present invention provides a method of
prolonging
survival in a human subject suffering from a Her-2/neu-expressing tumor growth
or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide, and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0085] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a human subject suffering from a Her-2/neu-expressing
tumor growth
or cancer comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0086] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a human subject suffering from a Her-2/neu-
expressing tumor
growth or cancer comprising the step of administering a combination of
radiation therapy
and a recombinant attenuated Listeria strain comprising a nucleic acid
comprising a first
open reading frame encoding a fusion polypeptide comprising a Her-2/neu
chimeric antigen
fused to an additional adjuvant and a second open reading frame encoding a
metabolic
enzyme, wherein said metabolic enzyme complements an endogenous gene that is
mutated in
the chromosome of said recombinant attenuated Listeria strain.
[0087] In another embodiment, the subject is a canine. In one embodiment, the
canine is a
dog.
[0088] In one embodiment, the present invention provides a method of treating
a Her-2/neu-
expressing tumor growth or cancer in a canine subject comprising the step of
administering a
combination of radiation therapy and a recombinant attenuated Listeria strain
comprising a
nucleic acid comprising a first open reading frame encoding a fusion
polypeptide comprising
16
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
a Her-2/neu chimeric antigen fused to an additional polypeptide, and a second
open reading
frame encoding a metabolic enzyme, wherein said metabolic enzyme complements
an
endogenous gene that is mutated in the chromosome of said recombinant
attenuated Listeria
strain.
[0089] In one embodiment, the present invention provides a method of
preventing a Her-
2/neu-expressing tumor growth or cancer in a canine subject comprising the
step of
administering a combination of radiation therapy and a recombinant attenuated
Listeria strain
comprising a nucleic acid comprising a first open reading frame encoding a
fusion
polypeptide comprising a Her-2/neu chimeric antigen fused to an additional
polypeptide, and
a second open reading frame encoding a metabolic enzyme, wherein said
metabolic enzyme
complements an endogenous gene that is mutated in the chromosome of said
recombinant
attenuated Listeria strain.
[0090] In another embodiment, the present invention provides a method of
eliciting an
enhanced immune response against a Her-2/neu-expressing tumor growth or cancer
in a
canine subject comprising the step of administering a combination of radiation
therapy and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0091] In another embodiment, the present invention provides a method of
prolonging
survival in a canine subject suffering from a Her-2/neu-expressing tumor
growth or cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a Her-2/neu chimeric antigen fused to
an
additional polypeptide, and a second open reading frame encoding a metabolic
enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
chromosome of said recombinant attenuated Listeria strain.
[0092] In another embodiment, the present invention provides a method of
delaying
metastatic disease in a canine subject suffering from a Her-2/neu-expressing
tumor growth or
cancer comprising the step of administering a combination of radiation therapy
and a
recombinant attenuated Listeria strain comprising a nucleic acid comprising a
first open
reading frame encoding a fusion polypeptide comprising a Her-2/neu chimeric
antigen fused
to an additional polypeptide and a second open reading frame encoding a
metabolic enzyme,
wherein said metabolic enzyme complements an endogenous gene that is mutated
in the
17
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
chromosome of said recombinant attenuated Listeria strain.
[0093] In another embodiment, the present invention provides a method of
breaking
tolerance to Her-2/neu in a canine subject suffering from a Her-2/neu-
expressing tumor
growth or cancer comprising the step of administering a combination of
radiation therapy
and a recombinant attenuated Listeria strain comprising a nucleic acid
comprising a first
open reading frame encoding a fusion polypeptide comprising a Her-2/neu
chimeric antigen
fused to an additional adjuvant and a second open reading frame encoding a
metabolic
enzyme, wherein said metabolic enzyme complements an endogenous gene that is
mutated in
the chromosome of said recombinant attenuated Listeria strain.
[0094] In one embodiment, the present invention provides a method of delaying
metastatic
disease or treating metastatic disease in a subject. In one embodiment, the
metastatic disease
is pulmonary metastatic disease.
[0095] Thus, in one embodiment, the present invention provides a method of
delaying
pulmonary metastatic disease in a subject suffering from a tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a tumor specific antigen fused to an
additional
polypeptide.
[0096] In another embodiment, the present invention provides a method of
treating
pulmonary metastatic disease in a subject suffering from a tumor growth or
cancer
comprising the step of administering a combination of radiation therapy and a
recombinant
attenuated Listeria strain comprising a nucleic acid comprising a first open
reading frame
encoding a fusion polypeptide comprising a tumor specific antigen fused to an
additional
polypeptide.
[0097] In one embodiment, provided herein are methods for preventing,
treating,
prolonging survival, delaying metastatic disease, breaking tolerance to Her-
2/neu,
vaccinating against a Her2-neu antigen-expressing tumor, inducing an immune
response,
eliciting an enhanced immune response against sub-dominant epitopes of the
Her2-neu
antigen, while circumventing mutation avoidance. In another embodiment, the
administration
of the fusion polypeptide of the present invention to the subject prevents
escape mutations
within said tumor. In another embodiment, circumventing mutation avoidance is
due to
epitope spreading. In yet another embodiment, mutation avoidance is due to the
chimeric
nature of the antigen.
[0098] In another embodiment, provided herein is an immunogenic composition
for use in
the claimed methods comprising a fusion polypeptide, wherein said fusion
polypeptide
18
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
comprises a Her-2/neu chimeric antigen fused to an additional polypeptide, and
wherein
administering the fusion protein to a subject having an Her-2/neu-expressing
tumor prevents
escape mutations within said tumor. In another embodiment, provided herein is
a
recombinant Listeria vaccine strain for use in the claimed methods comprising
the
immunogenic composition.
[0099] In one embodiment, the recombinant attenuated Listeria strain is a
vaccine strain. In
one embodiment, the nucleic acid referred to herein is a nucleic acid
molecule.
[00100] In one embodiment, the recombinant attenuated Listeria strain for use
in the
methods of the present invention further comprises a nucleic acid molecule
comprising a
third open reading frame encoding a metabolic enzyme, and wherein the
metabolic enzyme
complements an endogenous gene that is mutated in the chromosome of the
recombinant
Listeria strain.
[00101] In another embodiment, provided herein is a recombinant attenuated
Listeria strain
comprising a nucleic acid molecule, wherein the nucleic acid molecule
comprises a first open
reading frame encoding a polypeptide, wherein the polypeptide comprises a Her-
2/neu
chimeric antigen, wherein the nucleic acid molecule further comprises a second
and a third
open reading frame, each encoding a metabolic enzyme, and wherein the
metabolic enzyme
complements an endogenous gene that is mutated in the chromosome of said
recombinant
Listeria strain.
[00102] In one embodiment, the nucleic acid molecule is integrated into the
Listeria
genome. In another embodiment, the nucleic acid molecule is in a plasmid in
the
recombinant Listeria vaccine strain. In yet another embodiment, the plasmid is
stably
maintained in the recombinant Listeria vaccine strain in the absence of
antibiotic selection. In
another embodiment, the plasmid does not confer antibiotic resistance upon the
recombinant
Listeria. In another embodiment, the recombinant Listeria strain is
attenuated. In another
embodiment, the recombinant Listeria is an attenuated auxotrophic strain. In
another
embodiment, the high metabolic burden that the expression of a foreign antigen
exerts on a
bacterium such as one of the present invention is also an important mechanism
of
attenuation.
[00103] In one embodiment the attenuated strain is LmddA. In another
embodiment, this
strain exerts a strong adjuvant effect, which is an inherent property of
Listeria-based
vaccines. One manifestation of this adjuvant effect is the 5-fold decrease in
the number of the
intratumoral Tregs caused by either Listeria expressing an antigen other than
a human
chimeric Her-2/neu or the ADXS-31-164 (expressing a human chimeric Her-2/neu)
vaccines
(see Figure 5 herein). In another embodiment, the LmddA vector expressing a
different
19
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
antigen (HPV16 E7) is also associated with a significant decrease in the
frequency of Tregs
in the tumors, likely as a consequence of innate immunity responses. In
another embodiment,
the LmddA vector expresses a prostate-specific antigen (PSA), a human
papilloma virus
(HPV) antigen (E6, E7). In another embodiment, the HPV strain is HPV16, HPV18,
or any
strain known in the art.
[00104] In one embodiment, the attenuated auxotrophic Listeria vaccine strain
is the ADXS-
31-164 strain. ADXS-31-164 is based on a Listeria vaccine vector which is
attenuated due to
the deletion of virulence gene actA and retains the plasmid for Her-2/neu
expression in vivo
and in vitro by complementation of dal gene. In one embodiment, ADXS31-164
expresses
and secretes the chimeric Her-2/neu protein fused to the first 441 amino acids
of listeriolysin
0 (LLO). In another embodiment, ADXS31-164 exerts strong and antigen specific
anti-
tumor responses with ability to break tolerance toward Her-2/neu in transgenic
animals (see
Examples). In another embodiment, the ADXS31-164 strain is highly attenuated
and has a
better safety profile than previous Listeria vaccine generations, as it is
more rapidly cleared
from the spleens of the immunized mice. In another embodiment, the ADXS31-164
results in
a longer delay of tumor onset in transgenic animals than Lm-LLO-ChHer2, the
antibiotic
resistant and more virulent version of this vaccine (see Figure 3). In one
embodiment, the
Lin-LLO-ChHer2 strain is Lm-LLO-138.
[00105] In another embodiment, ADXS31-164 strain is highly immunogenic, able
to break
tolerance toward the Her-2/neu self-antigen and prevent tumor formation in Her-
2/neu
transgenic animals. In another embodiment, ADXS31-164 causes a significant
decrease in
intra-tumoral T regulatory cells (Tregs). In another embodiment, the lower
frequency of
Tregs in tumors treated with LmddA vaccines resulted in an increased
intratumoral
CD8/Tregs ratio, suggesting that a more favorable tumor microenvironment can
be obtained
after immunization with LmddA vaccines. In another embodiment, the use of this
chimeric
antigen does not result in escape mutations indicating that tumors do not
mutate away from a
therapeutic efficacious response to treatment with this novel antigen (see
Example 6). In
another embodiment, peripheral immunization with ADXS31-164 delays the growth
of a
metastatic breast cancer cell line in the brain (see Example 7).
[00106] In another embodiment, canine subjects suffering from osteosarcoma and
provided
treatment including amputation, chemotherapy, and vaccination with ADXS31-164,
have
prolonged survival compared with control subjects not receiving the
vaccination with
ADXS31-164 (see Examples 9 and 10). In another embodiment, canine subjects
suffering
from osteosarcoma and provided treatment including amputation, chemotherapy,
and
vaccination with ADXS31-164, show reduced metastasis compared with control
subjects not
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
receiving the vaccination with ADXS31-164 (see Example 10). In another
embodiment,
canine subjects suffering from osteosarcoma and provided treatment including
amputation,
chemotherapy, and vaccination with ADXS31-164, show increased specific T cell
response
induced compared with control subjects not receiving the vaccination with
ADXS31-164
(see Example 10). In another embodiment, canine subjects suffering from
osteosarcoma and
provided radiation therapy prior to vaccination with ADXS31-164, have
prolonged survival
compared with control subjects receiving either only radiation therapy or only
vaccination
with ADXS31-164 (see Example 11). In another embodiment, canine subjects
suffering from
osteosarcoma and provided radiation therapy prior to vaccination with ADXS31-
164 show
reduced metastasis compared with control subjects receiving either only
radiation therapy or
only vaccination with ADXS31-164 (see Example 11).
[00107] In another embodiment, the terms "ADXS31-164," "Lm-human chimeric Her-
2/neu," "Lm-huHer2-neu," and "Lm-hucHer-2/neu," are used interchangeably
herein.
[00108] In one embodiment, osteosarcoma cells are not easily killed by
radiation, so
radiation therapy is rarely used to treat osteosarcoma. In one embodiment,
recombinant
attenuated, antibiotic-free Listeria-expressing chimeric antigens are useful
for preventing,
and treating a cancer or solid tumors, as exemplified herein. In another
embodiment, the
tumor is a Her-2/neu positive tumor. In another embodiment, the cancer is a
Her-2/neu-
expressing cancer. In another embodiment, the cancer is breast cancer, a
central nervous
system (CNS) cancer, a head and neck cancer, an osteosarcoma (OSA), a canine
OSA,
Ewing's sarcoma (ES), or any Her-2/neu-expressing cancer known in the art. In
another
embodiment, a canine osteosarcoma is an appendicular osteosarcoma. In another
embodiment, the tumor is an osteosarcoma tumor, a breast tumor, a head and
neck tumor, or
any other antigen-expressing tumor known in the art. In another embodiment,
said cancer or
solid tumor is a result of relapse or metastatic disease. In one embodiment,
the metastatic
disease is pulmonary metastatic disease.
[00109] In one embodiment, the present invention provides methods of treating,
preventing,
or delaying metastases. In one embodiment, the present invention provides
methods of
treating, preventing, or delaying metastases of OSA. In one embodiment, the
metastases are
in the lung. In another embodiment, the metastases are in another tissue. In
another
embodiment, the metastases are in bone, which in one embodiment is proximal to
the site of
the initial OSA, and in another embodiment, is distal to the site of the
initial OSA. In another
embodiment, the metastases are in the kidney. In another embodiment, the
metastases are in
the heart. In another embodiment, the metastases are isolated. In another
embodiment, the
metastases are an isolated local recurrence. In another embodiment, the
metastases are multi-
21
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
site metastases.
[00110] In another embodiment, recombinant Listeria expressing a chimeric Her-
2/neu are
useful as a therapeutic vaccine for the treatment of Her-2/neu overexpressing
solid tumors. In
another embodiment, the Her-2/neu chimeric antigen provided herein is useful
for treating
Her-2/neu-expressing tumors and preventing escape mutations of the same. In
another
embodiment, the term "escape mutation" refers to a tumor mutating away from a
therapeutic
efficacious response to treatment.
[00111] In one embodiment, provided herein is a nucleic acid molecule
comprising a first
open reading frame encoding a recombinant polypeptide provided herein, wherein
the
nucleic acid molecule resides within the recombinant Listeria vaccine strain.
In another
embodiment, the nucleic acid molecule provided herein is used to transform the
Listeria in
order to arrive at a recombinant Listeria. In another embodiment, the nucleic
acid provided
herein lacks a virulence gene. In another embodiment, the nucleic acid
molecule integrated
into the Listeria genome carries a non-functional virulence gene. In another
embodiment, the
virulence gene is mutated in the genome of the recombinant Listeria. In yet
another
embodiment, the nucleic acid molecule is used to inactivate the endogenous
gene present in
the Listeria genome. In yet another embodiment, the virulence gene is an actA
gene. In
another embodiment, the virulence gene is a prfA gene. In another embodiment,
the virulence
gene is an in1B gene. As will be understood by a skilled artisan, the
virulence gene can be
any gene known in the art to be associated with virulence in the recombinant
Listeria.
[00112] In one embodiment, the metabolic gene, the virulence gene, or both is
lacking in a
chromosome of the Listeria strain. In another embodiment, the metabolic gene,
the virulence
gene, or both is lacking in the chromosome and in any episomal genetic element
of the
Listeria strain. It will be appreciated by a skilled artisan that the term
"episome," "episomal,"
etc. refer to a plasmid vector or use thereof that does not integrate into the
chromosome of
the Listeria provided herein. In another embodiment, the term refers to
plasmid vectors that
integrate into the chromosome of the Listeria provided herein. In another
embodiment, the
metabolic gene, the virulence gene, or both is lacking in the genome of the
Listeria strain. In
one embodiment, the metabolic gene, the virulence gene, or both is mutated in
the
chromosome. In another embodiment, the metabolic gene, the virulence gene, or
both is
deleted from the chromosome. In another embodiment, the metabolic gene, the
virulence
gene, or both is inactivated in the chromosome.
[00113] In another embodiment, the nucleic acids and plasmids provided herein
do not
confer antibiotic resistance upon the recombinant Listeria.
[00114] "Nucleic acid molecule" refers, in one embodiment, to a plasmid. In
another
22
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
embodiment, the term refers to an integration vector. In another embodiment,
the term refers
to a non-integration vector. In another embodiment, the term refers to a
plasmid comprising
an integration vector. In another embodiment, the integration vector is a site-
specific
integration vector. In another embodiment, a nucleic acid molecule of methods
and
compositions of the present invention are composed of any type of nucleotide
known in the
art. Each possibility represents a separate embodiment of the present
invention.
[00115] "Metabolic enzyme" refers, in another embodiment, to an enzyme
involved in
synthesis of a nutrient required by the host bacteria. In another embodiment,
the term refers
to an enzyme required for synthesis of a nutrient required by the host
bacteria. In another
embodiment, the term refers to an enzyme involved in synthesis of a nutrient
utilized by the
host bacteria. In another embodiment, the term refers to an enzyme involved in
synthesis of a
nutrient required for sustained growth of the host bacteria. In another
embodiment, the
enzyme is required for synthesis of the nutrient. Each possibility represents
a separate
embodiment of the present invention.
[00116] "Stably maintained" refers, in another embodiment, to maintenance of a
nucleic
acid molecule or plasmid in the absence of selection (e.g. antibiotic
selection) for 10
generations, without detectable loss. In another embodiment, the period is 15
generations. In
another embodiment, the period is 20 generations. In another embodiment, the
period is 25
generations. In another embodiment, the period is 30 generations. In another
embodiment,
the period is 40 generations. In another embodiment, the period is 50
generations. In another
embodiment, the period is 60 generations. In another embodiment, the period is
80
generations. In another embodiment, the period is 100 generations. In another
embodiment,
the period is 150 generations. In another embodiment, the period is 200
generations. In
another embodiment, the period is 300 generations. In another embodiment, the
period is 500
generations. In another embodiment, the period is more than 500 generations.
In another
embodiment, the nucleic acid molecule or plasmid is maintained stably in vitro
(e.g. in
culture). In another embodiment, the nucleic acid molecule or plasmid is
maintained stably in
vivo. In another embodiment, the nucleic acid molecule or plasmid is
maintained stably both
in vitro and in vitro. Each possibility represents a separate embodiment of
the present
invention.
[00117] In one embodiment, the present invention provides a recombinant
Listeria strain
expressing the antigen. The present invention also provides recombinant
polypeptides
comprising a listeriolysin (LLO) protein fragment fused to a Her-2 chimeric
protein or
fragment thereof, vaccines and immunogenic compositions comprising same, and
methods of
inducing an anti-Her-2 immune response and treating and vaccinating against a
Her-2-
23
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
expressing tumor, comprising the same.
[00118] In another embodiment, a recombinant Listeria strain of the present
invention has
been passaged through an animal host. In another embodiment, the passaging
maximizes
efficacy of the strain as a vaccine vector. In another embodiment, the
passaging stabilizes the
immunogenicity of the Listeria strain. In another embodiment, the passaging
stabilizes the
virulence of the Listeria strain. In another embodiment, the passaging
increases the
immunogenicity of the Listeria strain. In another embodiment, the passaging
increases the
virulence of the Listeria strain. In another embodiment, the passaging removes
unstable sub-
strains of the Listeria strain. In another embodiment, the passaging reduces
the prevalence of
unstable sub-strains of the Listeria strain. In another embodiment, the
Listeria strain contains
a genomic insertion of the gene encoding the antigen-containing recombinant
peptide. In
another embodiment, the Listeria strain carries a plasmid comprising the gene
encoding the
antigen-containing recombinant peptide. In another embodiment, the passaging
is performed
by any other method known in the art.
[00119] In one embodiment, the polypeptide provided herein is a fusion protein
comprising
an additional polypeptide selected from the group consisting of: a) non-
hemolytic LLO
protein or N-terminal fragment, b) a PEST sequence, or c) an ActA fragment,
and further
wherein said additional polypeptide is fused to the Her-2/neu chimeric
antigen. In another
embodiment, the additional polypeptide is functional. In another embodiment, a
fragment of
the additional polypeptide is immunogenic. In another embodiment, the
additional
polypeptide is immunogenic.
[00120] In another embodiment, the polypeptide provided herein is a fusion
protein
comprising a non-hemolytic LLO protein or N-temanal fragment fused to the Her-
2/neu
chimeric antigen. In another embodiment, a fusion protein of methods and
compositions of
the present invention comprises an ActA sequence from a Listeria organism. In
one
embodiment, ActA proteins and fragments thereof augment antigen presentation
and
immunity in a similar fashion to LLO.
[00121] In one embodiment of methods and compositions of the present
invention, the
fusion protein comprises the Her-2/neu antigen and an additional polypeptide.
In another
embodiment, the additional polypeptide fused to Her-2/neu antigen is referred
to as an
additional adjuvant polypeptide. In one embodiment, the additional polypeptide
is a non-
hemolytic LLO protein or fragment thereof (Examples herein). In another
embodiment, the
additional polypeptide is a PEST sequence. In another embodiment, the
additional
polypeptide is an ActA protein or a fragment thereof.
[00122] The additional polypeptide of methods and compositions of the present
invention is,
24
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
in another embodiment, a listeriolysin (LLO) peptide. In another embodiment,
the additional
polypeptide is an ActA peptide. In another embodiment, the additional
polypeptide is a PEST
sequence peptide. In another embodiment, the additional polypeptide is any
other peptide
capable of enhancing the immunogenicity of an antigen peptide. Each
possibility represents a
separate embodiment of the present invention.
[00123] Fusion proteins comprising the Her-2/neu chimeric antigen may be
prepared by any
suitable method, including, for example, cloning and restriction of
appropriate sequences or
direct chemical synthesis by methods discussed below. Alternatively,
subsequences may be
cloned and the appropriate subsequences cleaved using appropriate restriction
enzymes. The
fragments may then be ligated to produce the desired DNA sequence. In one
embodiment,
DNA encoding the antigen can be produced using DNA amplification methods, for
example
polymerase chain reaction (PCR). First, the segments of the native DNA on
either side of the
new terminus are amplified separately. The 5 end of the one amplified sequence
encodes the
peptide linker, while the 3' end of the other amplified sequence also encodes
the peptide
linker. Since the 5' end of the first fragment is complementary to the 3' end
of the second
fragment, the two fragments (after partial purification, e.g. on LMP agarose)
can be used as
an overlapping template in a third PCR reaction. The amplified sequence will
contain
codons, the segment on the carboxy side of the opening site (now forming the
amino
sequence), the linker, and the sequence on the amino side of the opening site
(now forming
the carboxyl sequence). The antigen is ligated into a plasmid. Each method
represents a
separate embodiment of the present invention.
[00124] The results of the present invention demonstrate that administration
of compositions
of the present invention has utility for inducing formation of antigen-
specific T cells (e.g.
cytotoxic T cells) that recognize and kill tumor cells (Examples herein).
[00125] In one embodiment, the present invention provides a recombinant
polypeptide
comprising an N-temanal fragment of an LLO protein fused to a Her-2 chimeric
protein or
fused to a fragment thereof. In one embodiment, the present invention provides
a
recombinant polypeptide consisting of an N-terminal fragment of an LLO protein
fused to a
Her-2 chimeric protein or fused to a fragment thereof.
[00126] In another embodiment, the Her-2 chimeric protein of the methods and
compositions of the present invention is a human Her-2 chimeric protein. In
another
embodiment, the Her-2 protein is a mouse Her-2 chimeric protein. In another
embodiment,
the Her-2 protein is a rat Her-2 chimeric protein. In another embodiment, the
Her-2 protein is
a primate Her-2 chimeric protein. In another embodiment, the Her-2 protein is
a canine Her-2
chimeric protein. In another embodiment, the Her-2 protein is a Her-2 chimeric
protein of
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
human or any other animal species or combinations thereof known in the art.
Each possibility
represents a separate embodiment of the present invention.
[00127] In another embodiment, a Her-2 protein is a protein referred to as
"Her-2/neu,"
"Erbb2," "v-erb-b2," "c-erb-b2," "neu," or "cNeu." In another embodiment, the
term
Her2/neu, or grammatical equivalents thereof, is also referred to herein as
"Her-2," "Her-2
protein," "HER2 protein," or "HER2"). Each possibility represents a separate
embodiment of
the present invention.
[00128] In one embodiment, the Her2-neu chimeric protein, harbors two of the
extracellular
and one intracellular fragments of Her-2/neu antigen showing clusters of MHC-
class I
epitopes of the oncogene, where, in another embodiment, the chimeric protein,
harbors 3
H2Dq and at least 17 of the mapped human MHC-class I epitopes of the Her-2/neu
antigen
(fragments EC1, EC2, and IC1) (See Figure 1A). In another embodiment, the
chimeric
protein harbors at least 13 of the mapped human MHC-class I epitopes
(fragments EC2 and
IC1). In another embodiment, the chimeric protein harbors at least 14 of the
mapped human
MHC-class I epitopes (fragments EC1 and IC1). In another embodiment, the
chimeric
protein harbors at least 9 of the mapped human MHC-class I epitopes (fragments
EC1 and
IC2). In another embodiment, the Her2-neu chimeric protein is fused to a non-
hemolytic
listeriolysin 0 (LLO). In another embodiment, the Her2-neu chimeric protein is
fused to
truncated listeriolysin 0 (tLL0). In another embodiment, the Her2-neu chimeric
protein is
fused to the first 441 amino acids of the Listeria-monocytogenes listeriolysin
0 (LLO)
protein and expressed and secreted by the Listeria monocytogenes attenuated
auxotrophic
strain LmddA. In another embodiment, the expression and secretion of the
fusion protein
tLLO-ChHer2 from the attenuated auxotrophic strain provided herein that
expresses a
chimeric Her-2/neu antigen/LLO fusion protein is comparable to that of the Lm-
LLO-
ChHer2 in TCA precipitated cell culture supernatants after 8 hours of in vitro
growth (See
Figure 1B).
[00129] In one embodiment, no CTL activity is detected in naïve animals or
mice injected
with an irrelevant Listeria vaccine (See Figure 2A). While in another
embodiment, the
attenuated auxotrophic strain (ADXS31-164) provided herein is able to
stimulate the
secretion of IFN-y by the splenocytes from wild type FVB/N mice (Figure 2B).
[00130] In another embodiment, the metabolic enzyme of the methods and
compositions
provided herein is an amino acid metabolism enzyme, where, in another
embodiment, the
metabolic enzyme is an alanine racemase enzyme. In another embodiment, the
metabolic
enzyme is a D-amino acid transferase enzyme. In another embodiment, the
metabolic
enzyme catalyzes a formation of an amino acid used for a cell wall synthesis
in the
26
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
recombinant Listeria strain, where in another embodiment, the metabolic enzyme
is an
alanine racemase enzyme.
[00131] In another embodiment, the gene encoding the metabolic enzyme is
expressed
under the control of the Listeria p60 promoter. In another embodiment, the
inlA (encodes
intemalin) promoter is used. In another embodiment, the hly promoter is used.
In another
embodiment, the ActA promoter is used. In another embodiment, the integrase
gene is
expressed under the control of any other gram positive promoter. In another
embodiment, the
gene encoding the metabolic enzyme is expressed under the control of any other
promoter
that functions in Listeria. The skilled artisan will appreciate that other
promoters or
polycistronic expression cassettes may be used to drive the expression of the
gene. Each
possibility represents a separate embodiment of the present invention.
[00132] In another embodiment, the Her-2 chimeric protein is encoded by the
following
nucleic acid sequence set forth in SEQ ID NO:1
[00133]
gagacccacctggacatgctccgccacctctaccagggctgccaggtggtgcagggaaacctggaactcacctacct
gccc accaatgccagcctgtccttcctgc aggatatcc
aggaggtgcagggctacgtgctcatcgctcacaaccaagtgaggcaggt
cccactgcagaggctgcggattgtgcgaggcacccagctattgaggacaactatgccctggccgtgctagacaatggag
acccgc
tgaacaataccacccctgtcacaggggcctccccaggaggcctgcgggagctgcagcttcgaagcctcacagagatctt
gaaagga
ggggtcttgatcc agcggaacccccagctctgctaccaggac
acgattngtggaagaatatccaggagtttgctggctgc aagaaga
tattgggagcctggcatttctgccggagagattgatggggacccagcctccaacactgccccgctccagccagagcagc
tccaag
tgtttgagactctggaagagatcacaggnacctatacatctcagcatggccggacagcctgcctgacctcagcgtcttc
cagaacctg
caagtaatccggggacgaattctgcacaatggcgcctactcgctgaccctgcaagggctgggcatcagctggctggggc
tgcgctc
actgagggaactgggcagtggactggccctcatccaccataacacccacctctgatcgtgcacacggtgccctgggacc
agctatt
cggaacccgcaccaagctctgctccacactgccaaccggccagaggacgagtgtgtgggcgagggcctggcctgccacc
agctg
tgcgcccgagggcagcagaagatccggaagtacacgatgcggagactgctgcaggaaacggagctggtggagccgctga
cacc
tagcggagcgatgcccaaccaggcgcagatgcggatcctgaaagagacggagctgaggaaggtgaaggtgatggatctg
gcgc
ttttggcacagtctacaagggcatctggatccctgatggggagaatgtgaaaattccagtggccatcaaagtgttgagg
gaaaacaca
tcccccaaagccaacaaagaaatcttagacgaagcatacgtgatggctggtgtgggctccccatatgtctcccgccttc
tgggcatctg
cctgacatccacggtgcagctggtgacacagcttatgccctatggctgcctcttagactaa (SEQ ID NO: 1).
[00134] In another embodiment, the Her-2 chimeric protein has the sequence:
[00135[ETHLDMLRHLYQGCQVVQGNLELTYLPTN
ASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRI
VRGTQLFEDNYALAVLDNGDPLNNTTPVTGAS
PGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTI
LWKNIQEFAGCKKIFGSLAFLPESFDGDPASNT
APLQPEQLQVFETLEEITGYLYISAWPDSLPDL
27
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
SVFQNLQVIRGRILHNGAYSLTLQGLGISWLGL
RSLRELGSGLALIHHNTHLCFVHTVPWDQLFRN
PHQALLHTANRPEDECVGEGLACHQLCARGQQ
KIRKYTMRRLLQETELVEPLTPSGAMPNQAQM
RILKETELRKVKVLGSGAFGTVYKGIWIPDGEN
VKIPVAIKVLRENTSPKANKEILDEAYVMAGVGS
PYVSRLLGICLTSTVQLVTQLMPYGCLLD(SEQID
NO: 2).
[00136] Table 1 below shows the percent (%) identity between the amino acid
sequences of
human and canine Her-2 EC and IC fragments, respectively.
28
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Table 1
Rvaim SW.$5,1,01.0VW:nlaA5,LOMMV.M1W1:;55MALMIXM:5MMIVIV
SWW*1:0M*Z.V5I4M5WOQMOIA1V.W0005MAIANWWWW.4M
x-**-xx.A,*.:s,444,*.
WMOVKaa:M0 .,SSM ZZIOat'SVZ?:Z.V=M WWSV s*Mtn.:MX:KZZ,MSIM MOM t
t5aM.**: , :VW:t=VM.A0,06M.MI:M.W.,WOOV.:0MW3Mwavownwe.V.M.,:tt)M0
emuaa :mvocaawa. 5E0 NG: V .4,3' .?40
,õ õ.õ 66
limwnw...vnItxtmnyziw,ww:tpm:vm:::nanumcwm,m4,4
5:A514CMAVMUggITMV::::5;m*MK:5:PWAV5VMAVNIOM4 Mffigti.
Mazw0. OZSVI...t.W3M0144 3EQ V:V.V z
<zok*.t.:.ao :EMUgAMAItt .4 M NO: =?0 *A:m EC2 '
.444 *Se.* :4e, 4:
gkiA*M.
.ntYys0:U:MN. M...W.:5:÷WV3;:anoM,NM
,s,k-s, c 6;6 *:Sc- Se.* Se¨$: = c it4 = 9:k. 44:4 =-
ic:a x# x+
i*otka-a
.
CifsnLM.: . MftWANWNOVM:ZW;.4.g:MM*V4WOMMI:5KLW*Wnl :Kgi-14K*51,WWV ;#10
M****6***%**-:,:**.st,.6M6*6**********,,,,6-****6***:****..6.6.# 96 st,c,:
ftV:5, OZ .A. EAVMAM ,t.CQ Ntk n CM.W.
74
PO 137] In another embodiment, an amino acid sequence encoding a human Her-
2/neu EC1
fragment is set forth in (SEQ ID NO: 69):
[00138] SLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDN
GDPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKN
NQLALTLIDTNRSRACHPCSPMCK (SEQ ID NO: 69).
[00139] In another embodiment, an amino acid sequence encoding a canine Her-
2/neu EC1
fragment is set forth in (SEQ ID NO: 70):
[00140] SLSFLQDIQEVQGYVLIAHS QVRQIPLQRLRIVRGTQLFEDNYALAVLDNG
DPLEGGIPAPGAAPGGLRELQLRSLTEILKGGVLIQRSPQLCHQDTILWKDVFHKNN
QLALTLIDTNRSRACPPCSPACK (SEQ ID NO: 70).
[00141] In another embodiment, an amino acid sequence encoding a human Her-
2/neu EC2
fragment is set forth in (SEQ ID NO: 71):
[00142] TAPLQPEQLQVFETLEEITGYLYIS AWPDS LPDLS VFQNLQVIRGRILHNGA
YSLTLQGLGISWLGLRSLRELGS (SEQ ID NO: 71).
[00143] In another embodiment, an amino acid sequence encoding a canine Her-
2/neu EC2
fragment is set forth in (SEQ ID NO: 72):
ILOO1 441 TAPLQPEQLRVFEALEEITGYLYISAWPDSLPNLSVFQNLRVIRGRVLHDGA
YSLTLQGLGISWLGLRSLRELGS (SEQ ID NO: 72).
29
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[001451 In another embodiment, an amino acid sequence encoding a human Her-
2/neu IC1
fragment is set forth in (SEQ ID NO: 73):
[00146] NQAQMRILKETELRKVKVLGS GAFGTVYKGIWIPDGENVKIPVAIKVLREN
TSPKANKEILDEAYVMAGVGSPYVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENR
GRLGSQDLLNWCMQIAKGMSYLED(SEQ ID NO: 73).
[00147] In another embodiment, an amino acid sequence encoding a canine Her-
2/neu IC1
fragment is set forth in (SEQ ID NO: 74):
[00148] NQAQMRILKETELRKVKVLGS GAFGTVYKGIWIPDGENVKIPVAIKVLREN
TSPKANKEILDEAYVMAGVGSPYVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENR
GRLGSQDLLNWCMQIAKGMSYLED(SEQ ID NO: 74).
[00149] In one embodiment, the human amino acid sequence of Her-2 EC1 fragment
(SEQ
ID NO: 69) has 89% identity with that of a canine Her-2 EC1 fragment (SEQ ID
NO: 70). In
another embodiment, the human amino acid sequence of Her-2 EC2 fragment (SEQ
ID NO:
71) has 93% identity with that of a canine Her-2 EC2 fragment (SEQ ID NO: 72).
In another
embodiment, the human amino acid sequence of Her-2 IC1 fragment (SEQ ID NO:
73) has
98% identity with that of a canine Her-2 IC1 fragment (SEQ ID NO: 74).
[00150] In one embodiment, the Her2 chimeric protein or fragment thereof of
the methods
and compositions provided herein does not include a signal sequence thereof.
In another
embodiment, omission of the signal sequence enables the Her2 fragment to be
successfully
expressed in Listeria, due the high hydrophobicity of the signal sequence.
Each possibility
represents a separate embodiment of the present invention.
[00151] In another embodiment, the fragment of a Her2 chimeric protein of
methods and
compositions of the present invention does not include a transmembrane domain
(TM)
thereof. In one embodiment, omission of the TM enables the Her-2 fragment to
be
successfully expressed in Listeria, due the high hydrophobicity of the TM.
Each possibility
represents a separate embodiment of the present invention.
[00152] In one embodiment, the nucleic acid sequence of rat-Her-2/neu gene is
CCGGAATCGCGGGCACCCAAGTGTGTACCGGCACAGACATGAAGTTGCGGCTC
CCTGCCAGTCCTGAGACCCACCTGGACATGCTCCGCCACCTGTACCAGGGCTGT
CAGGTAGTGCAGGGCAACTTGGAGCTTACCTACGTGCCTGCCAATGCCAGC CTC
TCATTCCTGCAGGACATCCAGGAAGTTCAGGGTTACATGCTCATCGCTCACAAC
CAGGTGAAGC GC GTCC CACTGCAAAGGCTGC GCATCGTGAGAGGGACCCAGCT
CTTTGAGGACAAGTATGCCCTGGCTGTGCTAGACAACCGAGATCCTCAGGACA
ATGTCGCCGCCTCCACCCCAGGCAGAACCCCAGAGGGGCTGCGGGAGCTGCAG
CTTCGAAGTCTCACAGAGATCCTGAAGGGAGGAGTTTTGATCCGTGGGAACCCT
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
CAGCTCTGCTACCAGGACATGGTTTTGTGGAAGGACGTCTTCCGCAAGAATAAC
CAACTGGCTCCTGTCGATATAGACACCAATCGTTCCCGGGCCTGTCCACCTTGT
GCCCCCGCCTGCAAAGACAATCACTGTTGGGGTGAGAGTCCGGAAGACTGTCA
GATCTTGACTGGCACCATCTGTACCAGTGGTTGTGCCCGGTGCAAGGGCCGGCT
GCCCACTGACTGCTGCCATGAGCAGTGTGCCGCAGGCTGCACGGGCCCCAAGC
ATTCTGACTGCCTGGCCTGCCTCCACTTCAATCATAGTGGTATCTGTGAGCTGCA
CTGCCCAGCCCTCGTCACCTACAACACAGACACCTTTGAGTCCATGCACAACCC
TGAGGGTCGCTACACCTTTGGTGCCAGCTGCGTGACCACCTGCCCCTACAACTA
CCTGTCTACGGAAGTGGGATCCTGCACTCTGGTGTGTCCCCCGAATAACCAAGA
GGTCACAGCTGAGGACGGAACACAGCGTTGTGAGAAATGCAGCAAGCCCTGTG
CTCGAGTGTGCTATGGTCTGGGCATGGAGCACCTTCGAGGGGCGAGGGCCATC
ACCAGTGACAATGTCCAGGAGTTTGATGGCTGCAAGAAGATCTTTGGGAGC CT
GGCATTTTTGCCGGAGAGCTTTGATGGGGACCCCTCCTCCGGCATTGCTCCGCT
GAGGCCTGAGCAGCTCCAAGTGTTCGAAACCCTGGAGGAGATCACAGGTTACC
TGTACATCTCAGCATGGCCAGACAGTCTCCGTGACCTCAGTGTCTTCCAGAACC
TTCGAATCATTCGGGGACGGATTCTCCACGATGGCGCGTACTCATTGACACTGC
AAGGCCTGGGGATCCACTCGCTGGGGCTGCGCTCACTGCGGGAGCTGGGCAGT
GGATTGGCTCTGATTCACCGCAACGCCCATCTCTGCTTTGTACACACTGTACCTT
GGGACCAGCTCTTCCGGAACCCACATCAGGCCCTGCTCCACAGTGGGAACCGG
CCGGAAGAGGATTGTGGTCTCGAGGGCTTGGTCTGTAACTCACTGTGTGCCCAC
GGGCACTGCTGGGGGCCAGGGCCCACCCAGTGTGTCAACTGCAGTCATTTCCTT
CGGGGCCAGGAGTGTGTGGAGGAGTGCCGAGTATGGAAGGGGCTCCCCCGGGA
GTATGTGAGTGACAAGCGCTGTCTGCCGTGTCACCCCGAGTGTCAGCCTCAAAA
CAGCTCAGAGACCTGCTTTGGATCGGAGGCTGATCAGTGTGCAGCCTGCGCCCA
CTACAAGGACTCGTCCTCCTGTGTGGCTCGCTGCCCCAGTGGTGTGAAACCGGA
CCTCTCCTACATGCCCATCTGGAAGTACCCGGATGAGGAGGGCATATGCCAGCC
GTGCCCCATCAACTGCACCCACTCCTGTGTGGATCTGGATGAACGAGGCTGCCC
AGCAGAGCAGAGAGCCAGCCCGGTGACATTC ATCATTGCAACTGTAGTGGGCG
TCCTGCTGTTCCTGATCTTAGTGGTGGTCGTTGGAATCCTAATCAAACGAAGGA
GACAGAAGATCCGGAAGTATACGATGCGTAGGCTGCTGCAGGAAACTGAGTTA
GTGGAGCCGCTGACGCCCAGCGGAGCAATGCCCAACCAGGCTCAGATGCGGAT
CCTAAAAGAGACGGAGCTAAGGAAGGTGAAGGTGCTTGGATCAGGAGCTTTTG
GCACTGTCTACAAGGGCATCTGGATCCCAGATGGGGAGAATGTGAAAATCCCC
GTGGCTATCAAGGTGTTGAGAGAAAACACATCTCCTAAAGCCAACAAAGAAAT
TCTAGATGAAGCGTATGTGATGGCTGGTGTGGGTTCTCCGTATGTGTCCCGCCT
31
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
CCTGGGCATCTGCCTGACATCCACAGTACAGCTGGTGACACAGCTTATGCCCTA
CGGCTGCCTTCTGGACCATGTCCGAGAACACCGAGGTCGCCTAGGCTCCCAGGA
CCTGCTCAACTGGTGTGTTCAGATTGCCAAGGGGATGAGCTACCTGGAGGACGT
GCGGCTTGTACACAGGGACCTGGCTGCCCGGAATGTGCTAGTCAAGAGTCCCA
ACCACGTCAAGATTACAGATTTCGGGCTGGCTCGGCTGCTGGACATTGATGAGA
CAGAGTACCATGCAGATGGGGGCAAGGTGCCCATCAAATGGATGGCATTGGAA
TCTATTCTCAGACGCCGGTTCACCCATCAGAGTGATGTGTGGAGCTATGGAGTG
ACTGTGTGGGAGCTGATGACTTTTGGGGCCAAACCTTACGATGGAATCCCAGCC
CGGGAGATCCCTGATTTGCTGGAGAAGGGAGAACGCCTACCTCAGCCTC CAAT
CTGCACCATTGATGTCTACATGATTATGGTCAAATGTTGGATGATTGACTCTGA
ATGTCGCCCGAGATTCCGGGAGTTGGTGTCAGAATTTTCACGTATGGCGAGGGA
CCCCCAGCGTTTTGTGGTCATCCAGAACGAGGACTTGGGCCCATCCAGCCCCAT
GGACAGTACCTTCTACCGTTCACTGCTGGAAGATGATGACATGGGTGACCTGGT
AGACGCTGAAGAGTATCTGGTGCCCCAGCAGGGATTCTTCTCCCCGGACCCTAC
CCCAGGCACTGGGAGCACAGCCCATAGAAGGCACCGCAGCTCGTCCACCAGGA
GTGGAGGTGGTGAGCTGACACTGGGCCTGGAGCCCTCGGAAGAAGGGCCCCCC
AGATCTCCACTGGCTCCCTCGGAAGGGGCTGGCTCCGATGTGTTTGATGGTGAC
CTGGCAATGGGGGTAACCAAAGGGCTGCAGAGCCTCTCTCCACATGACCTC AG
CCCTCTACAGCGGTACAGCGAGGACCCCACATTACCTCTGCCCCCCGAGACTGA
TGGCTATGTTGCTCCCCTGGCCTGCAGCCCCCAGCCCGAGTATGTGAACCAATC
AGAGGTTCAGCCTCAGCCTCCTTTAACCCCAGAGGGTCCTCTGCCTCCTGTCCG
GCCTGCTGGTGCTACTCTAGAAAGACCCAAGACTCTCTCTCCTGGGAAGAATGG
GGTTGTCAAAGACGTITTTGCCTTCGGGGGTGCTGTGGAGAACCCTGAATACTT
AGTACCGAGAGAAGGCACTGCCTCTCCGCCCCACCCTTCTCCTGCCTTCAGCCC
AGCCTTTGACAACCTCTATTACTGGGACCAGAACTCATCGGAGCAGGGGCCTCC
ACCAAGTAACTTTGAAGGGACCCCCACTGCAGAGAACCCTGAGTACCTAGGCC
TGGATGTACCTGTA (SEQ ID NO: 45).
[00153] In one embodiment, the nucleic acid sequence encoding the rat/Her-
2/neu EC1
fragment is
CCCAGGCAGAACCCCAGAGGGGCTGCGGGAGCTGCAGCTTCGAAGTCTCACAG
AGATCCTGAAGGGAGGAGTTTTGATCCGTGGGAACCCTCAGCTCTGCTACCAGG
ACATGGTTTTGTGGAAGGACGTCTTCCGCAAGAATAACCAACTGGCTCCTGTCG
ATATAGACACCAATCGTTCCCGGGCCTGTCCACCTTGTGCCCCCGCCTGCAAAG
ACAATCACTGTTGGGGTGAGAGTCCGGAAGACTGTCAGATCTTGACTGGCACC
ATCTGTACCAGTGGTIGTGCCCGGTGCAAGGGCCGGCTGCCCACTGACTGCTGC
32
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
CATGAGCAGTGTGCCGCAGGCTGCACGGGCCCCAAGCA (SEQ ID NO: 46).
[00154] In another embodiment, the nucleic acid sequence encoding the rat Her-
2/neu EC2
fragment is:
GGTCACAGCTGAGGACGGAACACAGCGTTGTGAGAAATGCAGCAAGCCCTGTG
CTCGAGTGTGCTATGGTCTGGGCATGGAGCACCTTCGAGGGGCGAGGGCCATC
ACCAGTGACAATGTCCAGGAGTTTGATGGCTGCAAGAAGATCTTTGGGAGC CT
GGCATTTTTGCCGGAGAGCTTTGATGGGGACCCCTCCTCCGGCATTGCTCCGCT
GAGGCCTGAGCAGCTCCAAGTGTTCGAAACCCTGGAGGAGATCACAGGTTACC
TGTACATCTCAGCATGGCCAGACAGTCTCCGTGACCTCAGTGTCTTCCAGAACC
TT CGAATCATTCGGGGACGGATTCTCCACGATGGCGCGTACTCATTGACACTGC
AAGGCCTGGGGATCCACTCGCTGGGGCTGCGCTCACTGCGGGAGCTGGGCAGT
GGATTGGCTCTGATTCACCGCAACGCCCATCTCTGCTTTGTACACACTGTACCTT
GGGACCAGCTCTTCCGGAACCCACATCAGGCCCTGCTCCACAGTGGGAACCGG
CCGGAAGAGGATTGTGGTCTCGAGGGCTTGGTCTGTAACTCACTGTGTGCCCAC
GGGCACTGCTGGGGGCCAGGGCCCACCCA (SEQ ID NO: 47).
[00155] In another embodiment, the nucleic acid sequence encoding the rat Her-
2/neu IC1
fragment is:
[00156] CGCCCAGCGGAGCAATGCCCAACCAGGCTCAGATGCGGATCCTAAAAG
AGACGGAGCTAAGGAAGGTGAAGGTGCTTGGATCAGGAGCTTTTGGCACTGTC
TACAAGGGCATCTGGATCCCAGATGGGGAGAATGTGAAAATCCCCGTGGCTAT
CAAGGTGTTGAGAGAAAACACATCTCCTAAAGCCAACAAAGAAATTCTAGATG
AAGCGTATGTGATGGCTGGTGTGGGTTCTCCGTATGTGTCCCGCCTCCTGGGCA
TCTGCCTGACATCCACAGTACAGCTGGTGACACAGCTTATGCCCTACGGCTGCC
TICTGGACCATGTCCGAGAACACCGAGGTCGCCTAGGCTCCCAGGACCTGCTCA
ACTGGTGTGTTCAGATTGCCAAGGGGATGAGCTACCTGGAGGACGTGCGGC TT
GTACACAGGGACCTGGCTGCCCGGAATGTGCTAGTCAAGAGTCCCAACCAC GT
CAAGATTACAGATTTCGGGCTGGCTCGGCTGCTGGACATTGATGAGACAGAGT
ACCATGCAGATGGGGGCAAGGTGCCCATCAAATGGATGGCATTGGAATCTATT
CTCAGACGCCGGTTCACCCATCAGAGTGATGTGTGGAGCTATGGAGTGACTGTG
TGGGAGCTGATGACTITTGGGGCCAAACCTTACGATGGAATCCCAGCCCGGGA
GATCCCTGATTTGCTGGAGAAGGGAGAACGC CTACCTCAGCCTCCAATCTGCAC
CATTGATGTCTACATGATTATGGTCAAATGTTGGATGATTGACTCTGAATGTCG
CCCGAGATTCCGGGAGTTGGTGTCAGAATTITCACGTATGGCGAGGGACCCCCA
GCGTTTTGTGGTCATCCAGAACGAGGACTTGGGCCCATCCAGCCCCATGGACAG
TACCTTCTACCGTTCACTGCTGGAA (SEQ ID NO: 48).
33
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00157] In one embodiment, the nucleic acid sequence of human-Her-2/neu gene
is:
[00158] ATGGAGCTGGC GGCCTTGTGC C GCTGGGGGCTCCTC CTC GC C CTCTTGC
CCCCCGGAGCCGCGAGCACCCAAGTGTGCACCGGCACAGACATGAAGCTGCGG
CTCCCTGCCAGTCCCGAGACCCACCTGGACATGCTCCGCCACCTCTACCAGGGC
TGCCAGGTGGTGCAGGGAAACCTGGAACTCACCTACCTGCCCACCAATGCCAG
CCTGTCCTTCCTGCAGGATATCCAGGAGGTGCAGGGCTACGTGCTCATCGCTCA
CAACCAAGTGAGGCAGGTCCCACTGCAGAGGCTGCGGATTGTGCGAGGCACCC
AGCTCTTTGAGGACAACTATGCCCTGGCCGTGCTAGACAATGGAGACCCGCTGA
ACAATACCACCCCTGTCACAGGGGCCTCCCCAGGAGGCCTGCGGGAGCTGCAG
CTTCGAAGCCTCACAGAGATCTTGAAAGGAGGGGTCTTGATCCAGCGGAACCC
CCAGCTCTGCTACCAGGACACGATTTTGTGGAAGGACATCTTCCACAAGAACAA
CCAGCTGGCTCTCACACTGATAGACACCAACCGCTCTCGGGCCTGCCACCCCTG
TICTCCGATGTGTAAGGGCTCCCGCTGCTGGGGAGAGAGTTCTGAGGATTGTCA
GAGCCTGACGCGCACTGTCTGTGCCGGTGGCTGTGCCCGCTGCAAGGGGCCACT
GCCCACTGACTGCTGCCATGAGCAGTGTGCTGCCGGCTGCACGGGCCCCAAGC
ACTCTGACTGCCTGGCCTGCCTCCACTTCAACCACAGTGGCATCTGTGAGCTGC
ACTGCCCAGCCCTGGTCACCTACAACACAGACACGTTTGAGTCCATGCCCAATC
CC GAGGGC C GGTATACATTC GGC GC CAGCTGTGTGACTGC CTGTCC CTACAACT
AC CTTTCTAC GGAC GTGGGATC CTGCAC C CTC GTCTGC C CC CTGCACAAC CAAG
AGGTGACAGCAGAGGATGGAACACAGCGGTGTGAGAAGTGCAGCAAGCCCTGT
GCCCGAGTGTGCTATGGTCTGGGCATGGAGCACTTGCGAGAGGTGAGGGCAGT
TAC CAGTGC CAATATC CAGGAGTTTGC TGGCTGCAAGAAGATCTTTGGGAGC CT
GGCATTTCTGCC GGAGAGCTTTGATGGGGAC C CAGC CTC CAACACTGC CC C GCT
CCAGCCAGAGCAGCTCCAAGTGTTTGAGACTCTGGAAGAGATCACAGGTTACC
TATACATCTCAGCATGGCCGGACAGCCTGCCTGACCTCAGCGTCTTCCAGAACC
TGCAAGTAATC C GGGGAC GAATTCTGC ACAATGGC GC CTACTC GCTGAC C CTGC
AAGGGCTGGGCATCAGCTGGCTGGGGCTGCGCTCACTGAGGGAACTGGGCAGT
GGACTGGCCCTCATCCACCATAACACCCACCTCTGCTTCGTGCACACGGTGCCC
TGGGACCAGCTCTTTCGGAACCCGCACCAAGCTCTGCTCCACACTGCCAACCGG
CCAGAGGAC GAGTGTGTGGGC GAGGGC CTGGC CTGC CACC AGCTGTGC GC C C G
AGGGCACTGCTGGGGTCCAGGGCCCACCCAGTGTGTCAACTGCAGCCAGTTCCT
TCGGGGCCAGGAGTGCGTGGAGGAATGCCGAGTACTGCAGGGGCTCCCCAGGG
AGTATGTGAATGCCAGGCACTGTTTGCCGTGCCACCCTGAGTGTCAGCCCCAGA
ATGGCTCAGTGACCTGTTTTGGACCGGAGGCTGACCAGTGTGTGGCCTGTGCCC
ACTATAAGGACCCTCCCTTCTGCGTGGCCCGCTGCCCCAGCGGTGTGAAACCTG
34
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
ACCTCTCCTACATGCCCATCTGGAAGTTICCAGATGAGGAGGGCGCATGCCAGC
CTTGCCCCATCAACTGCACCCACTCCTGTGTGGACCTGGATGACAAGGGCTGCC
CCGCCGAGCAGAGAGCCAGCCCTCTGACGTCCATCGTCTCTGCGGTGGTTGGCA
TICTGCTGGTCGTGGTCTTGGGGGTGGTCTTTGGGATCCTCATCAAGCGACGGC
AGCAGAAGATCCGGAAGTACACGATGCGGAGACTGCTGCAGGAAACGGAGCT
GGTGGAGCCGCTGACACCTAGCGGAGCGATGCCCAACCAGGCGCAGATGCGGA
TCCTGAAAGAGACGGAGCTGAGGAAGGTGAAGGTGCTTGGATCTGGCGCTTTT
GGCACAGTCTACAAGGGCATCTGGATCCCTGATGGGGAGAATGTGAAAATTCC
AGTGGCCATCAAAGTGTTGAGGGAAAACACATCCCCCAAAGCCAACAAAGAAA
TCTTAGACGAAGCATACGTGATGGCTGGTGTGGGCTCCCCATATGTCTCCCGCC
TICTGGGCATCTGCCTGACATCCACGGTGCAGCTGGTGACACAGCTTATGCCCT
ATGGCTGCCTCTTAGACCATGTCCGGGAAAACCGCGGACGCCTGGGCTCCCAG
GACCTGCTGAACTGGTGTATGCAGATTGCCAAGGGGATGAGCTACCTGGAGGA
TGTGCGGCTCGTACACAGGGACTTGGCCGCTCGGAACGTGCTGGTCAAGAGTCC
CAACCATGTCAAAATTACAGACTTCGGGCTGGCTCGGCTGCTGGACATTGACGA
GACAGAGTACCATGCAGATGGGGGCAAGGTGCCCATCAAGTGGATGGCGCTGG
AGTCCATTCTCCGCCGGCGGTTCACCCACCAGAGTGATGTGTGGAGTTATGGTG
TGACTGTGTGGGAGCTGATGACTTTIGGGGCCAAACCTTACGATGGGATCCCAG
CCCGGGAGATCCCTGACCTGCTGGAAAAGGGGGAGCGGCTGCCCCAGCCCCCC
ATCTGCACCATTGATGTCTACATGATCATGGTCAAATGTTGGATGATTGACTCT
GAATGTCGGCCAAGATTCCGGGAGTTGGTGTCTGAATTCTCCCGCATGGCCAGG
GACCCCCAGCGCTTTGTGGTCATCCAGAATGAGGACTTGGGCCCAGCCAGTCCC
TIGGACAGCACCTTCTACCGCTCACTGCTGGAGGACGATGACATGGGGGACCTG
GTGGATGCTGAGGAGTATCTGGTACCCCAGCAGGGCTTCTTCTGTCCAGACCCT
GCCCCGGGCGCTGGGGGCATGGTCCACCACAGGCACCGCAGCTCATCTACCAG
GAGTGGCGGTGGGGACCTGACACTAGGGCTGGAGCCCTCTGAAGAGGAGGCCC
CCAGGTCTCCACTGGCACCCTCCGAAGGGGC TGGCTCCGATGTATTTGATGGTG
ACCTGGGAATGGGGGCAGCCAAGGGGCTGCAAAGCCTCCCCACACATGACCCC
AGCCCTCTACAGCGGTACAGTGAGGACCCCACAGTACCCCTGCCCTCTGAGACT
GATGGCTACGTTGCCCCCCTGACCTGCAGCCCCCAGCCTGAATATGTGAACCAG
CCAGATGTTCGGCCCCAGCCCCCTTCGCCCCGAGAGGGCCCTCTGCCTGCTGCC
CGACCTGCTGGTGCCACTCTGGAAAGGGCCAAGACTCTCTCCCCAGGGAAGAA
TGGGGTCGTCAAAGACGTTTTTGCCTTTGGGGGTGCCGTGGAGAACCCCGAGTA
CTTGACACCCCAGGGAGGAGCTGCCCCTCAGCCCCACCCTCCTCCTGCCTICAG
CCCAGCCTTCGACAACCTCTATTACTGGGACCAGGACCCACCAGAGCGGGGGG
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
CTCCACCCAGCACCTTCAAAGGGACACCTACGGCAGAGAACCCAGAGTACCTG
GGTCTGGACGTGCCAGTGTGAACCAGAAGGCCAAGTCCGCAGAAGCCCTGA
(SEQ ID NO: 49).
[00159] In another embodiment, the nucleic acid sequence encoding the human
Her-2/neu
EC1 fragment implemented into the chimera spans from 120-510 bp of the human
EC1
region and is set forth in (SEQ ID NO: 50).
[00160] GAGACCCACCTGGACATGCTCCGCCACCTCTACCAGGGCTGCCAGGTG
GTGCAGGGAAACCTGGAACTCACCTACCTGCCCACCAATGCCAGCCTGTCCTTC
CTGCAGGATATCCAGGAGGTGCAGGGCTACGTGCTCATCGCTCACAACCAAGT
GAGGCAGGTCC CACTGCAGAGGCTGC GGATTGTGC GAGGC ACC CAGCTCTTTG
AGGACAACTATGCCCTGGCCGTGCTAGACAATGGAGACCCGCTGAACAATACC
ACCCCTGTCACAGGGGCCTCCCCAGGAGGCCTGCGGGAGCTGCAGCTTCGAAG
CCTCACAGAGATCTTGAAAGGAGGGGTCTTGATC CAGC GGAAC CC C CAGCTCT
GCTACCAGGACACGATTTTGTGGAAG (SEQ ID NO: 50).
[00161] In one embodiment, the complete EC1 human Her-2/neu fragment spans
from (58-
979 bp of the human Her-2/neu gene and is set forth in (SEQ ID NO: 54).
[00162] GCCGCGAGCACCCAAGTGTGCACCGGCACAGACATGAAGCTGCGGCTC
CCTGCCAGTCCCGAGACCCACCTGGACATGCTCCGCCACCTCTACCAGGGCTGC
CAGGTGGTGCAGGGAAACCTGGAACTCACCTACCTGCCCACCAATGCCAGC CT
GTC CTTCCTGCAGGATATC CAGGAGGTGCAGGGCTAC GTGCTCATC GCTCACAA
CCAAGTGAGGCAGGTCCCACTGCAGAGGCTGCGGATTGTGCGAGGCACCCAGC
TCTTTGAGGACAACTATGC C CTGGC C GTGCTAGACAATGGAGAC C C GC TGAACA
ATAC CAC CC CTGTCACAGGGGCCTC CC CAGGAGGCCTGCGGGAGCTGCAGCTTC
GAAGCCTCACAGAGATCTTGAAAGGAGGGGTCTTGATCCAGCGGAACCCCCAG
CTCTGCTACCAGGACACGATTTTGTGGAAGGACATCTTCCACAAGAACAACCAG
CTGGCTCTCACACTGATAGACACCAACCGCTCTCGGGCCTGCCACCCCTGTTCT
CCGATGTGTAAGGGCTCCCGCTGCTGGGGAGAGAGTTCTGAGGATTGTCAGAG
CCTGACGCGCACTGTCTGTGCCGGTGGCTGTGCCCGCTGCAAGGGGCCACTGCC
CACTGACTGCTGCCATGAGCAGTGTGCTGCCGGCTGCACGGGCCCCAAGCACTC
TGACTGCCTGGCCTGCCTCCACTTCAACCACAGTGGCATCTGTGAGCTGCACTG
CCCAGCCCTGGTCACCTACAACACAGACACGTTTGAGTCCATGCCCAATCCCGA
GGGCCGGTATACATTCGGCGCCAGCTGTGTGACTGCCTGTCCCTACAACTACCT
TTCTACGGACGTGGGATC CTGCACCCTCGTCTGCCCCCTGCACAACCAAGAGGT
GACAGCAGAGGAT (SEQ ID NO: 54).
[00163] In another embodiment, the nucleic acid sequence encoding the human
Her-2/neu
36
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
EC2 fragment implemented into the chimera spans from 1077-1554 bp of the human
Her-
2/neu EC2 fragment and includes a 50 bp extension, and is set forth in (SEQ ID
NO: 51).
[00164] AATATCCAGGAGTTTGCTGGCTGCAAGAAGATCTTTGGGAGCCTGGCA
TITCTGCCGGAGAGCTTTGATGGGGACCCAGCCTCCAACACTGCCCCGCTCCAG
CCAGAGCAGCTCCAAGTGTTTGAGACTCTGGAAGAGATCACAGGTTACCTATAC
ATCTCAGCATGGCCGGACAGCCTGCCTGACCTCAGCGTCTTCCAGAACCTGCAA
GTAATC CGGGGAC GAATTCTGCACAATGGC GC CTACTC GCTGACC CTGCAAGG
GCTGGGCATCAGCTGGCTGGGGCTGCGCTCACTGAGGGAACTGGGCAGTGGAC
TGGCCCTCATCCACCATAACACCCACCTCTGCTTCGTGCACACGGTGCCCTGGG
ACCAGCTCTTTCGGAACCCGCACCAAGCTCTGCTCCACACTGCCAACCGGCCAG
AGGACGAGTGTGTGGGCGAGGGCCTGGCCTGCCACCAGCTGTGCGCCCGAGGG
(SEQ ID NO:51).
[00165] In one embodiment, complete EC2 human Her-2/neu fragment spans from
907-
1504 bp of the human Her-2/neu gene and is set forth in (SEQ ID NO: 55).
[00166] TACCTTTCTACGGACGTGGGATCCTGCACCCTCGTCTGCCCCCTGCACA
ACCAAGAGGTGACAGCAGAGGATGGAACACAGCGGTGTGAGAAGTGCAGCAA
GCCCTGTGCCCGAGTGTGCTATGGTCTGGGCATGGAGCACTTGCGAGAGGTGA
GGGCAGTTAC CAGTGC CAATATC CAGGAGTTTGCTGGCTGCAAGAAGATCTTTG
GGAGCCTGGCATTTCTGCCGGAGAGCTTTGATGGGGACCCAGCCTCCAACACTG
CCCCGCTCCAGCCAGAGCAGCTCCAAGTGTTTGAGACTCTGGAAGAGATCACA
GGTTACCTATACATCTCAGCATGGCCGGACAGCCTGCCTGACCTCAGCGTC TTC
CAGAACCTGCAAGTAATCCGGGGACGAATTCTGCACAATGGCGCCTACTCGCT
GACCCTGCAAGGGCTGGGCATCAGCTGGCTGGGGCTGCGCTCACTGAGGGAAC
TGGGCAGTGGACTGGCCCTCATCCACCATAACACCCACCTCTGCTTCGTGCACA
CGGTGCCCTGGGACCAGCTCTTTCGGAACCCGCACCAAGCTCTGCTCCACACTG
CCAACCGGCCAGAG (SEQ ID NO: 55).
[00167] In another embodiment, the nucleic acid sequence encoding the human
Her-2/neu
IC1 fragment implemented into the chimera is set forth in (SEQ ID NO: 52).
[00168] CAGCAGAAGATCCGGAAGTACACGATGCGGAGACTGCTGCAGGAAAC
GGAGCTGGTGGAGCCGCTGACACCTAGCGGAGCGATGCCCAACCAGGCGCAGA
TGCGGATCCTGAAAGAGACGGAGCTGAGGAAGGTGAAGGTGCTTGGATCTGGC
GCTTTTGGCACAGTCTACAAGGGCATCTGGATCCCTGATGGGGAGAATGTGAA
AATTCCAGTGGCCATCAAAGTGTTGAGGGAAAACACATCC CC CAAAGC CAACA
AAGAAATCTTAGACGAAGCATACGTGATGGCTGGTGTGGGCTCCCCATATGTCT
CC C GC CTTCTGGGCATCTGCCTGACATC CAC GGTGCAGCTGGTGACACAGC TTA
37
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
TGCCCTATGGCTGCCTCTTAGACT (SEQ ID NO:52).
[00169] In another embodiment, the nucleic acid sequence encoding the complete
human
Her-2/neu IC1 fragment spans from 2034-3243 of the human Her-2/neu gene and is
set forth
in (SEQ ID NO: 56).
[00170] CAGCAGAAGATCCGGAAGTACACGATGCGGAGACTGCTGCAGGAAAC
GGAGCTGGTGGAGCCGCTGACACCTAGCGGAGCGATGCCCAACCAGGCGCAGA
TGCGGATCCTGAAAGAGACGGAGCTGAGGAAGGTGAAGGTGCTTGGATCTGGC
GCTTTTGGCACAGTCTACAAGGGCATCTGGATCCCTGATGGGGAGAATGTGAA
AATTCCAGTGGCCATCAAAGTGTTGAGGGAAAACACATCC CC CAAAGC CAACA
AAGAAATCTTAGACGAAGCATACGTGATGGCTGGTGTGGGCTCCCCATATGTCT
CC C GC CTTCTGGGCATCTGCCTGACATC CAC GGTGCAGCTGGTGACACAGC TTA
TGCCCTATGGCTGCCTCTTAGACCATGTCCGGGAAAACCGCGGACGCCTGGGCT
CC CAGGAC CTGCTGAACTGGTGTATGCAGATTGC CAAGGGGATGAGCTACC TG
GAGGATGTGC GGCTCGTACACAGGGACTTGGC C GCTC GGAAC GTGCTGGTC AA
GAGTCCCAACCATGTCAAAATTACAGACTTCGGGCTGGCTCGGCTGCTGGACAT
TGACGAGACAGAGTACCATGCAGATGGGGGCAAGGTGCCCATCAAGTGGATGG
CGCTGGAGTCCATTCTC C GC C GGC GGTTCAC C CAC CAGAGTGATGTGTGGAGTT
ATGGTGTGACTGTGTGGGAGCTGATGACTTTTGGGGCCAAACCTTACGATGGGA
TCCCAGCCCGGGAGATCCCTGACCTGCTGGAAAAGGGGGAGCGGCTGCCCCAG
CCCCCCATCTGCACCATTGATGTCTACATGATCATGGTCAAATGTTGGATGATT
GACTCTGAATGTCGGCCAAGATTCCGGGAGTTGGTGTCTGAATTCTCCCGCATG
GCCAGGGACCCCCAGCGCTTTGTGGTCATCCAGAATGAGGACTTGGGCCCAGC
CAGTCCCTTGGACAGCACCTTCTACCGCTCACTGCTGGAGGACGATGACATGGG
GGACCTGGTGGATGCTGAGGAGTATCTGGTACCCCAGCAGGGCTTCTTCTGTCC
AGAC C CTGC CC C GGGC GC TGGGGGCATGGTC CAC CACAGGCAC C GCAGCTCAT
CTACCAGGAGTGGCGGTGGGGACCTGACACTAGGGCTGGAGCCCTCTGAAGAG
GAGGCCCCCAGGTCTCCACTGGCACCCTCCGAAGGGGCT (SEQ ID NO: 56).
[00171] The LLO utilized in the methods and compositions provided herein is,
in one
embodiment, a Listeria LLO. In one embodiment, the Listeria from which the LLO
is
derived is Listeria monocytogenes (LM). In another embodiment, the Listeria is
Listeria
ivanovii. In another embodiment, the Listeria is Listeria welshimeri. In
another embodiment,
the Listeria is Listeria seeligeri. In another embodiment, the LLO protein is
a non-Listerial
LLO protein. In another embodiment, the LLO protein is a synthetic LLO
protein. In another
embodiment it is a recombinant LLO protein.
[00172] In one embodiment, the LLO protein is encoded by the following nucleic
acid
38
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
sequence set forth in (SEQ ID NO: 3)
[00173]
atgaaaaaaataatgctagtttttattacacttatattagttagtctaccaattgcgcaacaaactgaagcaaaggatg
catct
gcattcaataaagaaaattcaatttcatccatggcaccaccagcatctccgcctgcaagtcctaagacgccaatcgaaa
agaaacacg
cggatgaaatcgataagtatatacaaggattggattacaataaaaacaatgtattagtataccacggagatgcagtgac
aaatgtgccg
ccaagaaaaggttacaaagatggaaatgaatatattgttgtggagaaaaagaagaaatccatcaatcaaaataatgcag
acattcaagt
tgtgaatgcaatttcgagcctaacctatccaggtgctctcgtaaaagcgaattcggaattagtagaaaatcaaccagat
gnctccctgta
aaacgtgattcattaacactcagcattgatttgccaggtatgactaatcaagacaataaaatagttgtaaaaaatgcca
ctaaatcaaacg
ttaacaacgcagtaaatacattagtggaaagatggaatgaaaaatatgctcaagcttatccaaatgtaagtgcaaaaat
tgattatgatga
cgaaatggcttacagtgaatcacaattaattgcgaaatttggtacagcatttaaagctgtaaataatagcttgaatgta
aacttcggcgca
atcagtgaagggaaaatgcaagaagaagtcattagnttaaacaaatttactataacgtgaatgttaatgaacctacaag
accttccagat
ttttcggcaaagctgttactaaagagc agttgcaagcgcttggagtgaatgcagaaaatcctcctgc
atatatctcaagtgtggcgtatg
gccgtcaagtttatttgaaattatcaactaattcccatagtactaaagtaaaagctgatttgatgctgccgtaagcgga
aaatctgtctcag
gtgatgtagaactaacaaatatcatcaaaaancttccttcaaagccgtaatttacggaggttccgcaaaagatgaagtt
caaatcatcga
cggcaacctcggagacttacgcgatanttgaaaaaaggcgctacttnaatcgagaaacaccaggagttcccattgatat
acaacaaa
cttcctaaaagacaatgaattagctgttattaaaaacaactcagaatatattgaaacaacttcaaaagcttatacagat
ggaaaaattaaca
tcgatcactctggaggatacgttgctcaattcaacatttcttgggatgaagtaaattatgat (SEQ ID NO: 3).
[00174] In another embodiment, the LLO protein comprises the sequence SEQ ID
NO: 4
[00175] MKKIMLVFITLILVSLPIAQQTEAKDAS AF
NKENSISSMAPPASPPASPKTPIEKKHADEIDK
YIQGLDYNKNNVLVYHGDAVTNVPPRKGYKD
GNEYIVVEKKKKSINQNNADIQVVNAISSLTYP
GALVKANSELVENQPDVLPVKRDSLTLSIDLPG
MTNQDNKIVVKNATKSNVNNAVNTLVERWNE
KYAQAYPNVSAKIDYDDEMAYSESQLIAKFGT
AFKAVNNSLNVNFGAISEGKMQEEVISFKQIYY
NVNVNEPTRPSRFFGKAVTKEQLQALGVNAEN
PPAYISSVAYGRQVYLKLSTNSHSTKVKAAFD
AAVS GKSVS GDVELTNIIKNSSFKAVIYGGS AK
DEVQIIDGNLGDLRDILKKGATFNRETPGVPIA
YTTNFLKDNELAVIKNNSEYIETTSKAYTDGKI
NIDHSGGYVAQFNISWDEVNYD(SEQIDNO:4)
[00176] The first 25 amino acids of the proprotein corresponding to this
sequence are the
signal sequence and are cleaved from LLO when it is secreted by the bacterium.
Thus, in this
embodiment, the full length active LLO protein is 504 residues long. In
another embodiment,
the LLO protein has a sequence set forth in GenBank Accession No. DQ054588,
DQ054589,
39
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
AY878649, U25452, or U25452. In another embodiment, the LLO protein is a
variant of an
LLO protein. In another embodiment, the LLO protein is a homologue of an LLO
protein.
Each possibility represents a separate embodiment of the present invention.
[00177] In another embodiment, "truncated LLO" or "tLLO" refers to a fragment
of LLO
that comprises the PEST domain. In another embodiment, the terms refer to an
LLO
fragment that does not contain the activation domain at the amino terminus and
does not
include cystine 484. In another embodiment, the LLO fragment consists of a
PEST sequence.
In another embodiment, the LLO fragment comprises a PEST sequence. In another
embodiment, the LLO fragment consists of about the first 400 to 441 amino
acids of the 529
amino acid full-length LLO protein. In another embodiment, the LLO fragment is
a non-
hemolytic form of the LLO protein.
[00178] In another embodiment of methods and compositions of the present
invention, a
polypeptide encoded by a nucleic acid sequence of methods and compositions of
the present
invention is a fusion protein comprising the chimeric Her-2/neu antigen and an
additional
polypeptide, where in another embodiment, the fusion protein comprises, inter
alia, a Listeria
Monocytogenes non-hemolytic LLO protein (Examples herein).
[00179] In one embodiment, the LLO fragment consists of about residues 1-25.
In another
embodiment, the LLO fragment consists of about residues 1-50. In another
embodiment, the
LLO fragment consists of about residues 1-75. In another embodiment, the LLO
fragment
consists of about residues 1-100. In another embodiment, the LLO fragment
consists of about
residues 1-125. In another embodiment, the LLO fragment consists of about
residues 1-150.
In another embodiment, the LLO fragment consists of about residues 1175. In
another
embodiment, the LLO fragment consists of about residues 1-200. In another
embodiment,
the LLO fragment consists of about residues 1-225. In another embodiment, the
LLO
fragment consists of about residues 1-250. In another embodiment, the LLO
fragment
consists of about residues 1-275. In another embodiment, the LLO fragment
consists of about
residues 1-300. In another embodiment, the LLO fragment consists of about
residues 1-325.
In another embodiment, the LLO fragment consists of about residues 1-350. In
another
embodiment, the LLO fragment consists of about residues 1-375. In another
embodiment,
the LLO fragment consists of about residues 1-400. In another embodiment, the
LLO
fragment consists of about residues 1-425. Each possibility represents a
separate embodiment
of the present invention.
[00180] In another embodiment, a fusion protein of methods and compositions of
the
present invention comprises a PEST sequence, either from an LLO protein or
from another
organism, e.g. a prokaryotic organism.
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00181] The PEST amino acid sequence has, in another embodiment, a sequence
selected
from SEQ ID NO: 5-9. In another embodiment, the PEST sequence is a PEST
sequence from
the Listeria Monocytogenes ActA protein. In another embodiment, the PEST
sequence is
KTEEQPSEVNTGPR (SEQ ID NO: 5), KASVTDTSEGDLDSSMQSADESTPQPLK (SEQ
ID NO: 6), KNEEVNASDFPPPPTDEELR (SEQ ID NO: 7), or
RGGIPTSEEFSSLNSGDFTDDENSETTEEEIDR (SEQ ID NO: 8). In another embodiment,
the PEST sequence is from Streptolysin 0 protein of Streptococcus sp. In
another
embodiment, the PEST sequence is from Streptococcus pyogenes Streptolysin 0,
e.g.
KQNTASTETTTTNEQPK (SEQ ID NO: 9) at amino acids 35-51. In another embodiment,
the PEST sequence is from Streptococcus equisimilis Streptolysin 0, e.g.
KQNTANTETTTTNEQPK (SEQ ID NO: 10) at amino acids 38-54. In another embodiment,
the PEST sequence is another PEST amino acid sequence derived from a
prokaryotic
organism. In another embodiment, the PEST sequence is any other PEST sequence
known in
the art. Each possibility represents a separate embodiment of the present
invention.
[00182] In one embodiment, fusion of an antigen to the PEST sequence of
Listeria
Monocytogenes enhanced cell mediated and anti-tumor immunity of the antigen.
Thus,
fusion of an antigen to other PEST sequences derived from other prokaryotic
organisms will
also enhance immunogenicity of the antigen. PEST sequence of other prokaryotic
organism
can be identified in accordance with methods such as described by, for example
Rechsteiner
and Rogers (1996, Trends Biochem. Sci. 21:267-271) for Listeria Monocytogenes.
Alternatively, PEST amino acid sequences from other prokaryotic organisms can
also be
identified based by this method. Other prokaryotic organisms wherein PEST
amino acid
sequences would be expected to include, but are not limited to, other Listeria
species. In
another embodiment, the PEST sequence is embedded within the antigenic
protein. Thus, in
another embodiment, "fusion" refers to an antigenic protein comprising both
the antigen and
the PEST amino acid sequence either linked at one end of the antigen or
embedded within
the antigen.
[00183] In another embodiment, provided herein is a vaccine comprising a
recombinant
polypeptide of the present invention. In another embodiment, provided herein
is a vaccine
consisting of a recombinant polypeptide of the present invention.
[00184] In another embodiment, provided herein is a nucleotide molecule
encoding a
recombinant polypeptide of the present invention. In another embodiment,
provided herein is
a vaccine comprising the nucleotide molecule.
[00185] In another embodiment, provided herein is a nucleotide molecule
encoding a
recombinant polypeptide of the present invention.
41
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00186] In another embodiment, provided herein is a recombinant polypeptide
encoded by
the nucleotide molecule of the present invention.
[00187] In another embodiment, provided herein is a vaccine comprising a
nucleotide
molecule or recombinant polypeptide of the present invention.
[00188] In another embodiment, provided herein is an immunogenic composition
comprising a nucleotide molecule or recombinant polypeptide of the present
invention.
[00189] In another embodiment, provided herein is a vector comprising a
nucleotide
molecule or recombinant polypeptide of the present invention.
[00190] In another embodiment, provided herein is a recombinant form of
Listeria
comprising a nucleotide molecule of the present invention.
[00191] In another embodiment, provided herein is a vaccine comprising a
recombinant
form of Listeria of the present invention.
[00192] In another embodiment, provided herein is a culture of a recombinant
form of
Listeria of the present invention.
[00193] In one embodiment, a vaccine or composition for use in the methods of
the present
invention comprises a recombinant Listeria monocytogenes, in any form or
embodiment as
described herein. In one embodiment, the vaccine or composition for use in the
present
invention consists of a recombinant Listeria monocytogenes of the present
invention, in any
form or embodiment as described herein. In another embodiment, the vaccine or
composition
for use in the methods of the present invention consists essentially of a
recombinant Listeria
monocytogenes of the present invention, in any form or embodiment as described
herein. In
one embodiment, the term "comprise" refers to the inclusion of a recombinant
Listeria
monocytogenes in the vaccine or composition, as well as inclusion of other
vaccines,
compositions or treatments that may be known in the art. In another
embodiment, the term
"consisting essentially of' refers to a vaccine, whose functional component is
the
recombinant Listeria monocytogenes, however, other components of the vaccine
may be
included that are not involved directly in the therapeutic effect of the
vaccine and may, for
example, refer to components which facilitate the effect of the recombinant
Listeria
monocytogenes (e.g. stabilizing, preserving, etc.). In another embodiment, the
term
"consisting" refers to a vaccine, which contains the recombinant Listeria
monocytogenes.
[00194] In
another embodiment, provided herein is a method of impeding or delaying
metastatic disease origination from a HER2-expressing tumor in a subject,
wherein and in
another embodiment, the method comprises the step of administering to the
subject a
composition comprising the recombinant Listeria vaccine strain described
herein.
[00195] In another embodiment, the methods of the present invention comprise
the step of
42
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
administering a recombinant Listeria monocytogenes, in any form or embodiment
as
described herein. In one embodiment, the methods of the present invention
consist of the step
of administering a recombinant Listeria monocytogenes of the present
invention, in any form
or embodiment as described herein. In another embodiment, the methods of the
present
invention consist essentially of the step of administering a recombinant
Listeria
monocytogenes of the present invention, in any form or embodiment as described
herein. In
one embodiment, the term "comprise" refers to the inclusion of the step of
administering a
recombinant Listeria monocytogenes in the methods, as well as inclusion of
other methods or
treatments that may be known in the art. In another embodiment, the term
"consisting
to essentially of' refers to a methods, whose functional component is the
administration of
recombinant Listeria monocytogenes, however, other steps of the methods may be
included
that are not involved directly in the therapeutic effect of the methods and
may, for example,
refer to steps which facilitate the effect of the administration of
recombinant Listeria
monocytogenes. In one embodiment, the term "consisting" refers to a method of
administering recombinant Listeria monocytogenes with no additional steps.
[00196] In another embodiment, the Listeria of methods and compositions of the
present
invention is Listeria monocytogenes. In another embodiment, the Listeria is
Listeria ivanovii.
In another embodiment, the Listeria is Listeria welshimeri. In another
embodiment, the
Listeria is Listeria seeligeri. Each type of Listeria represents a separate
embodiment of the
present invention.
[00197] In one embodiment, the Listeria strain of the methods and compositions
of the
present invention is the ADXS31-164 strain. In another embodiment, ADXS31-164
stimulates the secretion of IFN-y by the splenocytes from wild type FVB/N
mice. Further,
the data presented herein show that ADXS31-164 is able to elicit anti-Her-
2/neu specific
immune responses to human epitopes that are located at different domains of
the targeted
antigen.
[00198] In another embodiment, the present invention provides a recombinant
form of
Listeria comprising a nucleotide molecule encoding a Her-2 chimeric protein or
a fragment
thereof.
[00199] In one embodiment, the two molecules of the fusion protein (the LLO,
ActA
fragment or PEST sequence and the antigen) are joined directly. In another
embodiment, the
two molecules are joined by a short spacer peptide, consisting of one or more
amino acids. In
one embodiment, the spacer has no specific biological activity other than to
join the proteins
or to preserve some minimum distance or other spatial relationship between
them. In another
embodiment, the constituent amino acids of the spacer are selected to
influence some
43
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
property of the molecule such as the folding, net charge, or hydrophobicity.
In another
embodiment, the two molecules of the protein (the LLO fragment and the
antigen) are
synthesized separately or unfused. In another embodiment, the two molecules of
the protein
are synthesized separately from the same nucleic acid. In yet another
embodiment, the two
molecules are individually synthesized from separate nucleic acids. Each
possibility
represents a separate embodiment of the present invention.
[00200] In one embodiment, nucleic acids encoding the recombinant polypeptides
provided
herein also encode a signal peptide or sequence. In another embodiment, the
fusion protein of
methods and compositions of the present invention comprises an LLO signal
sequence from
LLO. In one embodiment, a heterologous antigen may be expressed through the
use of a
signal sequence, such as a Listerial signal sequence, for example, the
hemolysin signal
sequence or the actA signal sequence. Alternatively, for example, foreign
genes can be
expressed downstream from a L. monocytogenes promoter without creating a
fusion protein.
In another embodiment, the signal peptide is bacterial (Listerial or non-
Listerial). In one
embodiment, the signal peptide is native to the bacterium. In another
embodiment, the signal
peptide is foreign to the bacterium. In another embodiment, the signal peptide
is a signal
peptide from Listeria monocytogenes, such as a secA 1 signal peptide. In
another
embodiment, the signal peptide is a Usp45 signal peptide from Lactococcus
lactis, or a
Protective Antigen signal peptide from Bacillus anthracis. In another
embodiment, the signal
peptide is a secA2 signal peptide, such the p60 signal peptide from Listeria
monocytogenes.
In addition, the recombinant nucleic acid molecule optionally comprises a
third
polynucleotide sequence encoding p60, or a fragment thereof. In another
embodiment, the
signal peptide is a Tat signal peptide, such as a B. subtilis Tat signal
peptide (e.g., PhoD). In
one embodiment, the signal peptide is in the same translational reading frame
encoding the
recombinant polypeptide.
[00201] In another embodiment, provided herein is a method of inducing an anti-
Her-2
immune response in a subject, comprising administering to the subject a
recombinant
nucleotide encoding a recombinant polypeptide comprising an N-temanal fragment
of a
LLO protein fused to a Her-2 chimeric protein or fused to a fragment thereof,
thereby
inducing an anti-Her-2 immune response in a subject.
[00202] In one embodiment, provided herein is a method of eliciting an
enhanced immune
response to a Her-2/neu-expressing tumor in a subject, where in another
embodiment, the
method comprises administering to the subject a composition comprising the
recombinant
Listeria vaccine strain provided herein. In another embodiment, the immune
response against
the Her-2-expressing tumor comprises an immune response to a subdominant
epitope of the
44
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Her-2 protein. In another embodiment, the immune response against the Her-2-
expressing
tumor comprises an immune response to several subdominant epitopes of the Her-
2 protein.
In another embodiment, the immune response against the Her-2-expressing tumor
comprises
an immune response to at least 1-5 subdominant epitopes of the Her-2 protein.
In another
embodiment, the immune response against the Her-2-expressing tumor comprises
an
immune response to at least 1-10 subdominant epitopes of the Her-2 protein. In
another
embodiment, the immune response against the Her-2-expressing tumor comprises
an
immune response to at least 1-17 subdominant epitopes of the Her-2 protein. In
another
embodiment, the immune response against the Her-2-expressing tumor comprises
an
immune response to at least 17 subdominant epitopes of the Her-2 protein.
[00203] Point mutations or amino-acid deletions in the oncogenic protein Her-
2/neu, have
been reported to mediate treatment of resistant tumor cells, when these tumors
have been
targeted by small fragment Listeria-based vaccines or trastuzumab (a
monoclonal antibody
against an epitope located at the extracellular domain of the Her-2/neu
antigen). Described
herein is a chimeric Her-2/neu based composition which harbors two of the
extracellular and
one intracellular fragments of Her-2/neu antigen showing clusters of MHC-class
I epitopes of
the oncogene. This chimeric protein, which harbors 3 H2Dq and at least 17 of
the mapped
human MHC-class I epitopes of the Her-2/neu antigen was fused to the first 441
amino acids
of the Listeria-monocytogenes listeriolysin 0 protein and expressed and
secreted by the
Listeria monocytogenes attenuated strain LmddA.
[00204] Previous reports have shown that when Her-2/neu transgenic mice were
immunized
with Listeria-based vaccines expressing and secreting small fragments of the
Her-2/neu
antigen separately (each of which harbored only one H2Dq epitope of the Her-
2/neu
oncogene), Her-2/neu over-expressing tumors could escape due to mutations in
those
epitopes of the Her-2/neu antigen targeted by each vaccine (see Singh R,
Paterson Y.
Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res 2007;67:
1887-
92). Demonstrated herein is the unexpected result that when three or more
epitopes of the
Her-2/neu protein are incorporated in a chimeric vaccine, it can eliminate the
selection and
escape of these tumors by escape mutations. Immunization with the novel Her-
2/neu
chimeric Listeria vaccines did not result in any escape mutations that could
be associated
with point mutations or amino acid deletions in the Her-2/neu antigen (see
Example 4
herein).
[00205] In one embodiment, provided herein is a method of engineering a
Listeria vaccine
strain to express a Her-2 chimeric protein or recombinant polypeptide
expressing the
chimeric protein, the method comprising transforming a Listeria strain with a
nucleic acid
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
molecule. In another embodiment, the nucleic acid molecule comprises a first
open reading
frame encoding a polypeptide, wherein the polypeptide comprises a Her-2/neu
chimeric
antigen. In another embodiment, the nucleic acid molecule further comprises a
second open
reading frame encoding a metabolic enzyme, and wherein said metabolic enzyme
complements an endogenous gene that is mutated in the chromosome of the
recombinant
Listeria strain, thereby engineering a Listeria vaccine strain to express a
Her-2 chimeric
protein.
[00206] In one embodiment, the methods and compositions provided herein
further
comprise an adjuvant, which in one embodiment, is an independent adjuvant,
where in
another embodiment, the adjuvant or independent adjuvant comprises a
granulocyte/macrophage colony-stimulating factor (GM-CSF) protein, a
nucleotide molecule
encoding a GM-CSF protein, saponin QS21, monophosphoryl lipid A, or an
unmethylated
CpG-containing oligonucleotide. In another embodiment, the adjuvant is an
aluminum
adjuvant, Freund's adjuvant, MPL, emulsion, SBAS2, a nucleotide molecule
encoding an
immune-stimulating cytokine, a bacterial mitogen, or a bacterial toxin.
[00207] In one embodiment, an "adjuvant" is a component that potentiates the
immune
responses to an antigen and/or modulates it towards the desired immune
responses. In one
embodiment, the adjuvant is an immunologic adjuvant which in one embodiment is
a
substance that acts to accelerate, prolong, or enhance antigen-specific immune
responses
when used in combination with specific vaccine antigens.
[00208] In one embodiment, an "independent" adjuvant is an adjuvant that is
independent,
which in one embodiment, is not identical to the "additional adjuvant
polypeptide" of the
present invention which is present in a fusion polypeptide with a tumor
specific antigen,
which in one embodiment, is Her-2/neu.
[00209] In one embodiment, attenuated Listeria strains, such as Listeria
Monocytogenes
delta-actA mutant (Brundage et al, 1993, Proc. Natl. Acad. Sci., USA, 90:11890-
11894), L.
monocytogenes delta-plcA (Camilli et al, 1991, J. Exp. Med., 173:751-754), or
delta-ActA,
delta INL-b (Brockstedt et 5 al, 2004, PNAS, 101:13832-13837) are used in the
present
invention. In another embodiment, attenuated Listeria strains are constructed
by introducing
one or more attenuating mutations, as will be understood by one of ordinary
skill in the art
when equipped with the disclosure herein. Examples of such strains include,
but are not
limited to Listeria strains auxotrophic for aromatic amino acids (Alexander et
al, 1993,
Infection and Immunity 10 61:2245-2248) and mutant for the formation of
lipoteichoic acids
(Abachin et al, 2002, Mol. Microbiol. 43:1-14) and those attenuated by a lack
of a virulence
gene (see examples herein).
46
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00210] In another embodiment, the nucleic acid molecule of methods and
compositions of
the present invention is operably linked to a promoter/regulatory sequence. In
another
embodiment, the first open reading frame of methods and compositions of the
present
invention is operably linked to a promoter/regulatory sequence. In another
embodiment, the
second open reading frame of methods and compositions of the present invention
is operably
linked to a promoter/regulatory sequence. In another embodiment, the third
open reading
frame of methods and compositions of the present invention is operably linked
to a
promoter/regulatory sequence. In another embodiment, each of the open reading
frames are
operably linked to a promoter/regulatory sequence. Each possibility represents
a separate
embodiment of the present invention.
[00211] The skilled artisan, when equipped with the present disclosure and the
methods
provided herein, will readily understand that different transcriptional
promoters, terminators,
carrier vectors or specific gene sequences (e.g. those in commercially
available cloning
vectors) can be used successfully in methods and compositions of the present
invention. As
is contemplated in the present invention, these functionalities are provided
in, for example,
the commercially available vectors known as the pUC series. In another
embodiment, non-
essential DNA sequences (e.g. antibiotic resistance genes) are removed. Each
possibility
represents a separate embodiment of the present invention. In another
embodiment, a
commercially available plasmid is used in the present invention. Such plasmids
are available
from a variety of sources, for example, Invitrogen (La Jolla, CA), Stratagene
(La Jolla, CA),
Clontech (Palo Alto, CA), or can be constructed using methods well known in
the art.
[00212] In another embodiment, a plasmid such as pCR2.1 (Invitrogen, La Jolla,
CA),
which is a prokaryotic expression vector with a prokaryotic origin of
replication and
promoter/regulatory elements is used to facilitate expression of a polypeptide
of the present
invention in a prokaryotic organism. In another embodiment, extraneous
nucleotide
sequences are removed to decrease the size of the plasmid and increase the
size of the
cassette that can be placed therein.
[00213] Such methods are well known in the art, and are described in, for
example,
Sambrook et al. (1989, Molecular Cloning: A Laboratory Manual, Cold Spring
Harbor
Laboratory Press, New York) and Ausubei et al. (1997, Current Protocols in
Molecular
Biology, Green & Wiley, New York).
[00214] Antibiotic resistance genes are used in the conventional selection and
cloning
processes commonly employed in molecular biology and vaccine preparation.
Antibiotic
resistance genes contemplated in the present invention include, but are not
limited to, gene
products that confer resistance to ampicillin, penicillin, methicillin,
streptomycin,
47
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
erythromycin, kanamycin, tetracycline, cloramphenicol (CAT), neomycin,
hygromycin,
gentamicin and others well known in the art. Each gene represents a separate
embodiment of
the present invention.
[00215] Methods for transforming bacteria are well known in the art, and
include calcium-
chloride competent cell-based methods, electroporation methods, bacteriophage-
mediated
transduction, chemical, and physical transformation techniques (de Boer et al,
1989, Cell
56:641-649; Miller et al, 1995, FAS EB J., 9:190-199; Sambrook et al. 1989,
Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York; Ausubel
et al.,
1997, Current Protocols in Molecular Biology, John Wiley & Sons, New York;
Gerhardt et
al., eds., 1994, Methods for General and Molecular Bacteriology, American
Society for
Microbiology, Washington, DC; Miller, 1992, A Short Course in Bacterial
Genetics, Cold
Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.) In another
embodiment, the
Listeria vaccine strain of the present invention is transformed by
electroporation. Each
method represents a separate embodiment of the present invention.
[00216] In another embodiment, conjugation is used to introduce genetic
material and/or
plasmids into bacteria. Methods for conjugation are well known in the art, and
are described,
for example, in Nikodinovic J et al. (A second generation snp-derived
Escherichia coli-
Streptomyces shuttle expression vector that is generally transferable by
conjugation. Plasmid.
2006 Nov;56(3):223-7) and Auchtung JM et al (Regulation of a Bacillus subtilis
mobile
genetic element by intercellular signaling and the global DNA damage response.
Proc Natl
Acad Sci U S A. 2005 Aug 30;102 (35):12554-9). Each method represents a
separate
embodiment of the present invention.
[00217] "Transforming," in one embodiment, is used identically with the term
"transfecting," and refers to engineering a bacterial cell to take up a
plasmid or other
heterologous DNA molecule. In another embodiment, "transforming" refers to
engineering a
bacterial cell to express a gene of a plasmid or other heterologous DNA
molecule. Each
possibility represents a separate embodiment of the present invention.
[00218] Plasmids and other expression vectors useful in the present invention
are described
elsewhere herein, and can include such features as a promoter/regulatory
sequence, an origin
of replication for gram negative and gram positive bacteria, an isolated
nucleic acid encoding
a fusion protein and an isolated nucleic acid encoding an amino acid
metabolism gene.
Further, an isolated nucleic acid encoding a fusion protein and an amino acid
metabolism
gene will have a promoter suitable for driving expression of such an isolated
nucleic acid.
Promoters useful for driving expression in a bacterial system are well known
in the art, and
include bacteriophage lambda, the bla promoter of the beta-lactamase gene of
pBR322, and
48
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
the CAT promoter of the chloramphenicol acetyl transferase gene of pBR325.
Further
examples of prokaryotic promoters include the major right and left promoters
of 5
bacteriophage lambda (PL and PR), the trp, recA, lacZ, lad, and gal promoters
of E. coli, the
alpha-amylase (Ulmanen et al, 1985. J. Bacteriol. 162:176-182) and the S28-
specific
promoters of B. subtilis (Gilman et al, 1984 Gene 32:11- 20), the promoters of
the
bacteriophages of Bacillus (Gryczan, 1982, In: The Molecular Biology of the
Bacilli,
Academic Press, Inc., New York), and Streptomyces promoters (Ward et al, 1986,
Mol. Gen.
Genet. 203:468-478). Additional prokaryotic promoters contemplated in the
present
invention are reviewed in, for example, Glick (1987, J. Ind. Microbiol. 1:277-
282);
Cenatiempo, (1986, Biochimie, 68:505-516); and Gottesman, (1984, Ann. Rev.
Genet.
18:415-442). Further examples of promoter/regulatory elements contemplated in
the present
invention include, but are not limited to the Listerial prfA promoter, the
Listerial hly
promoter, the Listerial p60 promoter and the Listerial ActA promoter (GenBank
Acc. No.
NC_003210) or fragments thereof.
[00219] In another embodiment, a plasmid of methods and compositions of the
present
invention comprises a gene encoding a fusion protein. In another embodiment,
subsequences
are cloned and the appropriate subsequences cleaved using appropriate
restriction enzymes.
The fragments are then, in another embodiment, ligated to produce the desired
DNA
sequence. In another embodiment, DNA encoding the antigen is produced using
DNA
amplification methods, for example polymerase chain reaction (PCR). First, the
segments of
the native DNA on either side of the new terminus are amplified separately.
The 5 end of the
one amplified sequence encodes the peptide linker, while the 3' end of the
other amplified
sequence also encodes the peptide linker. Since the 5' end of the first
fragment is
complementary to the 3' end of the second fragment, the two fragments (after
partial
purification, e.g. on LMP agarose) can be used as an overlapping template in a
third PCR
reaction. The amplified sequence will contain codons, the segment on the
carboxy side of the
opening site (now forming the amino sequence), the linker, and the sequence on
the amino
side of the opening site (now forming the carboxyl sequence). The antigen is
ligated into a
plasmid. Each method represents a separate embodiment of the present
invention.
[00220] In another embodiment, the present invention further comprises a phage
based
chromosomal integration system for clinical applications. A host strain that
is auxotrophic for
essential enzymes, including, but not limited to, d-alanine racemase will be
used, for example
Lmdal(-)dat(-). In another embodiment, in order to avoid a "phage curing
step," a phage
integration system based on PSA is used (Lauer, et al., 2002 J Bacteriol,
184:4177-4186).
This requires, in another embodiment, continuous selection by antibiotics to
maintain the
49
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
integrated gene. Thus, in another embodiment, the current invention enables
the
establishment of a phage based chromosomal integration system that does not
require
selection with antibiotics. Instead, an auxotrophic host strain will be
complemented.
[00221] The recombinant proteins of the present invention are synthesized, in
another
embodiment, using recombinant DNA methodology. This involves, in one
embodiment,
creating a DNA sequence that encodes the fusion protein, placing the DNA in an
expression
cassette, such as the plasmid of the present invention, under the control of a
particular
promoter/regulatory element, and expressing the protein. DNA encoding the
fusion protein
(e.g. non-hemolytic LLO/antigen) of the present invention is prepared, in
another
embodiment, by any suitable method, including, for example, cloning and
restriction of
appropriate sequences or direct chemical synthesis by methods such as the
phosphotriester
method of Narang et al. (1979, Meth. Enzymol. 68: 90-99); the phosphodiester
method of
Brown et al. (1979, Meth. Enzymol 68: 109-151); the diethylphosphoramidite
method of
Beaucage et al. (1981, Tetra. Lett., 22: 15 1859-1862); and the solid support
method of U.S.
Pat. No. 4,458,066.
[00222] In another embodiment, chemical synthesis is used to produce a single
stranded
oligonucleotide. This single stranded oligonucleotide is converted, in various
embodiments,
into double stranded DNA by hybridization with a complementary sequence, or by
polymerization with a DNA polymerase using the single strand as a template.
One of skill in
the art would recognize that while chemical synthesis of DNA is limited to
sequences of
about 100 bases, longer sequences can be obtained by the ligation of shorter
sequences. In
another embodiment, subsequences are cloned and the appropriate subsequences
cleaved
using appropriate restriction enzymes. The fragments are then ligated to
produce the desired
DNA sequence.
[00223] In another embodiment, DNA encoding the fusion protein or the
recombinant
protein of the present invention is cloned using DNA amplification methods
such as
polymerase chain reaction (PCR). Thus, the gene for non-hemolytic LLO is PCR
amplified,
using a sense primer comprising a suitable restriction site and an antisense
primer comprising
another restriction site, e.g. a non-identical restriction site to facilitate
cloning. The same is
repeated for the isolated nucleic acid encoding an antigen. Ligation of the
non-hemolytic
LLO and antigen sequences and insertion into a plasmid or vector produces a
vector
encoding non-hemolytic LLO joined to a terminus of the antigen. The two
molecules are
joined either directly or by a short spacer introduced by the restriction
site.
[00224] In another embodiment, the molecules are separated by a peptide spacer
consisting
of one or more amino acids, generally the spacer will have no specific
biological activity
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
other than to join the proteins or to preserve some minimum distance or other
spatial
relationship between them. In another embodiment, the constituent amino acids
of the spacer
are selected to influence some property of the molecule such as the folding,
net charge, or
hydrophobicity. In another embodiment, the nucleic acid sequences encoding the
fusion or
recombinant proteins are transformed into a variety of host cells, including
E. coli, other
bacterial hosts, such as Listeria, yeast, and various higher eukaryotic cells
such as the COS,
CHO and HeLa cells lines and myeloma cell lines. The recombinant fusion
protein gene will
be operably linked to appropriate expression control sequences for each host.
Promoter/
regulatory sequences are described in detail elsewhere herein. In another
embodiment, the
plasmid further comprises additional promoter regulatory elements, as well as
a ribosome
binding site and a transcription temanation signal. For eukaryotic cells, the
control sequences
will include a promoter and an enhancer derived from e.g. immunoglobulin
genes, SV40,
cytomegalovirus, etc., and a polyadenylation sequence. In another embodiment,
the
sequences include splice donor and acceptor sequences.
[00225] In one embodiment, the term "operably linked" refers to a
juxtaposition wherein the
components so described are in a relationship permitting them to function in
their intended
manner. A control sequence "operably linked" to a coding sequence is ligated
in such a way
that expression of the coding sequence is achieved under conditions compatible
with the
control sequences.
[00226] In another embodiment, in order to select for an auxotrophic bacterium
comprising
the plasmid, transformed auxotrophic bacteria are grown on a media that will
select for
expression of the amino acid metabolism gene. In another embodiment, a
bacteria
auxotrophic for D-glutamic acid synthesis is transformed with a plasmid
comprising a gene
for D-glutamic acid synthesis, and the auxotrophic bacteria will grow in the
absence of D-
glutamic acid, whereas auxotrophic bacteria that have not been transformed
with the plasmid,
or are not expressing the plasmid encoding a protein for D-glutamic acid
synthesis, will not
grow. In another embodiment, a bacterium auxotrophic for D-alanine synthesis
will grow in
the absence of D-alanine when transformed and expressing the plasmid of the
present
invention if the plasmid comprises an isolated nucleic acid encoding an amino
acid
metabolism enzyme for D-alanine synthesis. Such methods for making appropriate
media
comprising or lacking necessary growth factors, supplements, amino acids,
vitamins,
antibiotics, and the like are well known in the art, and are available
commercially (Becton-
Dickinson, Franldin Lakes, NJ). Each method represents a separate embodiment
of the
present invention.
[00227] In another embodiment, once the auxotrophic bacteria comprising the
plasmid of
51
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
the present invention have been selected on appropriate media, the bacteria
are propagated in
the presence of a selective pressure. Such propagation comprises growing the
bacteria in
media without the auxotrophic factor. The presence of the plasmid expressing
an amino acid
metabolism enzyme in the auxotrophic bacteria ensures that the plasmid will
replicate along
with the bacteria, thus continually selecting for bacteria harboring the
plasmid. The skilled
artisan, when equipped with the present disclosure and methods herein will be
readily able to
scale-up the production of the Listeria vaccine vector by adjusting the volume
of the media
in which the auxotrophic bacteria comprising the plasmid are growing.
[00228] The skilled artisan will appreciate that, in another embodiment, other
auxotroph
strains and complementation systems are adopted for the use with this
invention.
[00229] In one embodiment, provided herein is a method of impeding the growth
of a Her-
2-expressing tumor in a subject, wherein and in another embodiment, the method
comprises
the step of administering to the subject a composition comprising the
recombinant Listeria
vaccine strain described herein.
[00230] In another embodiment, provided herein is a method of eliciting an
enhanced
immune response to a Her-2/neu-expressing tumor in a subject, wherein and in
another
embodiment, the method comprises the step of administering to the subject a
composition
comprising the recombinant Listeria vaccine strain described herein. In yet
another
embodiment, the immune response against the Her-2/neu-expressing tumor
comprises an
immune response to at least one subdominant epitope of the Her-2/neu protein.
[00231] In one embodiment, provided herein is a method of preventing an escape
mutation
in the treatment of Her-2/neu over-expressing tumors, wherein and in another
embodiment,
the method comprises the step of administering to said subject a composition
comprising the
recombinant Listeria vaccine strain provided herein.
[00232] In another embodiment, provided herein is a method of preventing the
onset of a
Her-2/neu antigen-expressing tumor in a subject, wherein and in another
embodiment, the
method comprises the step of administering to the subject a composition
comprising the
recombinant Listeria vaccine strain provided herein.
[00233] In one embodiment, provided herein is a method of decreasing the
frequency of
intra-tumoral T regulatory cells, wherein and in another embodiment, the
method comprises
the step of administering to the subject a composition comprising the
recombinant Listeria
vaccine strain provided herein.
[00234] In one embodiment, provided herein is a method of decreasing the
frequency of
intra-tumoral myeloid derived suppressor cells, wherein and in another
embodiment, the
method comprises the step of administering to the subject a composition
comprising the
52
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
recombinant Listeria vaccine strain provided herein.
[00235] In another embodiment, provided herein is a method of decreasing the
frequency of
myeloid derived suppressor cells, wherein and in another embodiment, the
method comprises
the step of administering to the subject a composition comprising the
recombinant Listeria
vaccine strain provided herein.
[00236] In one embodiment, provided herein a method of preventing the
development of a
Her-2/neu-expressing tumor in a subject, wherein and in another embodiment,
the method
comprises the step of administering to the subject a composition comprising
the recombinant
Listeria vaccine strain provided herein.
[00237] In another embodiment, provided herein is a method of preventing the
formation of
a metastatic disease coming from an Her-2/neu-expressing tumor in a subject,
wherein and in
another embodiment, the method comprises the step of administering to the
subject a
composition comprising the recombinant Listeria vaccine strain the provided
herein.
[00238] In another embodiment, provided herein is a method of treating a
metastatic disease
originating from a Her-2/neu-expressing tumor in a subject, wherein and in
another
embodiment, the method comprises the step of administering to the subject a
composition
comprising the recombinant Listeria vaccine strain provided herein.
[00239] In one embodiment, provided herein is a method of administering the
composition
of the present invention. In another embodiment, provided herein is a method
of
administering the vaccine of the present invention. In another embodiment,
provided herein
is a method of administering the recombinant polypeptide or recombinant
nucleotide of the
present invention. In another embodiment, the step of administering the
composition,
vaccine, recombinant polypeptide or recombinant nucleotide of the present
invention is
performed with an attenuated recombinant form of Listeria comprising the
composition,
vaccine, recombinant nucleotide or expressing the recombinant polypeptide,
each in its own
discrete embodiment. In another embodiment, the administering is performed
with a different
attenuated bacterial vector. In another embodiment, the administering is
performed with a
DNA vaccine (e.g. a naked DNA vaccine). In another embodiment, administration
of a
recombinant polypeptide of the present invention is performed by producing the
protein
recombinantly, then administering the recombinant protein to a subject. Each
possibility
represents a separate embodiment of the present invention.
[00240] In another embodiment, the immune response elicited by methods and
compositions of the present invention comprises a CD8+ T cell-mediated
response. In
another embodiment, the immune response consists primarily of a CD8+ T cell-
mediated
response. In another embodiment, the only detectable component of the immune
response is
53
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
a CD8+ T cell-mediated response.
[00241] In another embodiment, the immune response elicited by methods and
compositions provided herein comprises a CD4+ T cell-mediated response. In
another
embodiment, the immune response consists primarily of a CD4+ T cell-mediated
response. In
another embodiment, the only detectable component of the immune response is a
CD4+ T
cell-mediated response. In another embodiment, the CD4+ T cell-mediated
response is
accompanied by a measurable antibody response against the antigen. In another
embodiment,
the CD4+ T cell-mediated response is not accompanied by a measurable antibody
response
against the antigen.
to [00242] In another embodiment, the present invention provides a method
of inducing a
CD8+ T cell-mediated immune response in a subject against a subdominant CD8+ T
cell
epitope of an antigen, comprising the steps of (a) fusing a nucleotide
molecule encoding the
Her2-neu chimeric antigen or a fragment thereof to a nucleotide molecule
encoding an N-
terminal fragment of a LLO protein, thereby creating a recombinant nucleotide
encoding an
LLO-antigen fusion protein; and (b) administering the recombinant nucleotide
or the LLO-
antigen fusion to the subject; thereby inducing a CD8+ T cell-mediated immune
response
against a subdominant CD8+ T cell epitope of an antigen.
[00243] In one embodiment, provided herein is a method of increasing
intratumoral ratio of
CD8+/T regulatory cells, wherein and in another embodiment, the method
comprises the step
of administering to the subject a composition comprising the recombinant
polypeptide,
recombinant Listeria, or recombinant vector of the present invention.
[00244] In another embodiment, provided herein is a method of decreasing the
frequency
of intra-tumoral T regulatory cells, wherein and in another embodiment, the
method
comprises the step of administering to the subject a composition comprising
the recombinant
Listeria vaccine strain provided herein.
[00245] In another embodiment, the immune response elicited by the methods and
compositions provided herein comprises an immune response to at least one
subdominant
epitope of the antigen. In another embodiment, the immune response does not
comprise an
immune response to a subdominant epitope. In another embodiment, the immune
response
consists primarily of an immune response to at least one subdominant epitope.
In another
embodiment, the only measurable component of the immune response is an immune
response to at least one subdominant epitope. Each type of immune response
represents a
separate embodiment of the present invention.
[00246] In one embodiment, methods of this invention break tolerance in a
subject to a Her-
2/neu expressing tumor or cancer in said subject, wherein and in another
embodiment, the
54
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
method comprises the step of administering to the subject a composition
comprising the
recombinant Listeria vaccine strain provided herein.
[00247] Methods of measuring immune responses are well known in the art, and
include,
e.g. measuring suppression of tumor growth, flow cytometry, target cell lysis
assays (e.g.
chromium release assay), the use of tetramers, and others. Each method
represents a separate
embodiment of the present invention.
[00248] In another embodiment, the present invention provides a method of
impeding the
growth of a Her-2-expressing tumor in a subject, wherein and in another
embodiment, the
method comprises administering to the subject a combination of radiation
therapy and a
recombinant polypeptide comprising an N-terminal fragment of a LLO protein
fused to the
Her-2 chimeric protein or a fragment thereof or a recombinant nucleotide
encoding the
recombinant polypeptide, wherein the subject mounts an immune response against
the Her-2-
expressing tumor, thereby impeding the growth of a Her-2-expressing tumor in a
subject.
[00249] In another embodiment, the present invention provides a method of
delaying or
inhibiting a metastatic disease emanating from a Her-2-expressing tumor in a
subject,
wherein and in another embodiment, the method comprises administering to the
subject a
combination of radiation therapy and a recombinant polypeptide comprising an N-
terminal
fragment of a LLO protein fused to the Her-2 chimeric protein or a fragment
thereof or a
recombinant nucleotide encoding the recombinant polypeptide, wherein the
subject mounts
an immune response against the Her-2-expressing tumor, thereby delaying or
inhibiting the
metastatic disease emanating from a Her-2-expressing tumor in a subject.
[00250] In another embodiment, the present invention provides a method of
improving the
antigenicity of a Her-2 chimeric protein, wherein and in another embodiment,
the method
comprises the step of fusing a nucleotide encoding an N-terminal fragment of a
LLO protein
to a nucleotide encoding the Her-2 protein or a fragment thereof to create a
recombinant
nucleotide, thereby improving the antigenicity of a Her-2 chimeric protein.
[00251] In another embodiment, provided herein is a method of improving the
antigenicity
of a Her-2 chimeric protein, wherein and in another embodiment, the method
comprises
engineering a Listeria strain to express the recombinant nucleotide. In
another embodiment, a
different bacterial vector is used to express the recombinant nucleotide. In
another
embodiment, the bacterial vector is attenuated. In another embodiment, a DNA
vaccine (e.g.
a naked DNA vaccine) is used to express the recombinant nucleotide. In another
embodiment, administration of the LLO-Her-2 chimera fusion peptide encoded by
the
nucleotide is performed by producing the protein recombinantly, then
administering the
recombinant protein to a subject. Each possibility represents a separate
embodiment of the
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
present invention.
[00252] In one embodiment, the present invention provides a method that
induces "epitope
spreading" of a tumor. In another embodiment, the immunization using the
compositions and
methods provided herein induce epitope spreading onto other tumors bearing
antigens other
than the antigen carried in the vaccine of the present invention.
[00253] In another embodiment, the dominant epitope or subdominant epitope is
dominant
or subdominant, respectively, in the subject being treated. In another
embodiment, the
dominant epitope or subdominant epitope is dominant or subdominant in a
population being
treated.
[00254] In one embodiment, provided herein is a method of preventing,
treating,
suppressing, inhibiting, inducing an immune response against, or eliciting an
enhanced
immune response against sub-dominant epitopes against a cancer or a tumor
growth in a
subject by epitope spreading wherein and in another embodiment, said cancer is
associated
with expression of an antigen or fragment thereof comprised in the composition
of the
present invention. In another embodiment, the method comprises administering
to said
subject a composition comprising the recombinant polypeptide, recombinant
Listeria, or
recombinant vector of the present invention. In yet another embodiment, the
subject mounts
an immune response against the antigen-expressing cancer or the antigen-
expressing tumor,
thereby treating, suppressing, or inhibiting a cancer or a tumor growth in a
subject.
[00255] "Dominant CD8+ T cell epitope," in one embodiment, refers to an
epitope that is
recognized by over 30% of the antigen-specific CD8+ T cells that are elicited
by vaccination,
infection, or a malignant growth with a protein or a pathogen or cancer cell
containing the
protein. In another embodiment, the term refers to an epitope recognized by
over 35% of the
antigen-specific CD8+ T cells that are elicited thereby. In another
embodiment, the term
refers to an epitope recognized by over 40% of the antigen-specific CD8+ T
cells. In another
embodiment, the term refers to an epitope recognized by over 45% of the
antigen-specific
CD8+ T cells. In another embodiment, the term refers to an epitope recognized
by over 50%
of the antigen-specific CD8+ T cells. In another embodiment, the term refers
to an epitope
recognized by over 55% of the antigen-specific CD8+ T cells. In another
embodiment, the
term refers to an epitope recognized by over 60% of the antigen-specific CD8+
T cells. In
another embodiment, the term refers to an epitope recognized by over 65% of
the antigen-
specific CD8+ T cells. In another embodiment, the term refers to an epitope
recognized by
over 70% of the antigen-specific CD8+ T cells. In another embodiment, the term
refers to an
epitope recognized by over 75% of the antigen-specific CD8+ T cells. In
another
embodiment, the term refers to an epitope recognized by over 80% of the
antigen-specific
56
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
CD8+ T cells. In another embodiment, the term refers to an epitope recognized
by over 85%
of the antigen-specific CD8+ T cells. In another embodiment, the term refers
to an epitope
recognized by over 90% of the antigen-specific CD8+ T cells. In another
embodiment, the
term refers to an epitope recognized by over 95% of the antigen-specific CD8+
T cells. In
another embodiment, the term refers to an epitope recognized by over 96% of
the antigen-
specific CD8+ T cells. In another embodiment, the term refers to an epitope
recognized by
over 97% of the antigen-specific CD8+ T cells. In another embodiment, the term
refers to an
epitope recognized by over 98% of the antigen-specific CD8+ T cells.
[00256] "Subdominant CD8+ T cell epitope," in one embodiment, refers to an
epitope
recognized by fewer than 30% of the antigen-specific CD8+ T cells that are
elicited by
vaccination, infection, or a malignant growth with a protein or a pathogen or
cancer cell
containing the protein. In another embodiment, the term refers to an epitope
recognized by
fewer than 28% of the antigen-specific CD8+ T cells. In another embodiment,
the term refers
to an epitope recognized by over 26% of the antigen-specific CD8+ T cells. In
another
embodiment, the term refers to an epitope recognized by fewer than 24% of the
antigen-
specific CD8+ T cells. In another embodiment, the term refers to an epitope
recognized by
over 22% of the antigen-specific CD8+ T cells. In another embodiment, the term
refers to an
epitope recognized by fewer than 20% of the antigen-specific CD8+ T cells. In
another
embodiment, the term refers to an epitope recognized by over 18% of the
antigen-specific
CD8+ T cells. In another embodiment, the term refers to an epitope recognized
by fewer than
16% of the antigen-specific CD8+ T cells. In another embodiment, the term
refers to an
epitope recognized by over 14% of the antigen-specific CD8+ T cells. In
another
embodiment, the term refers to an epitope recognized by over 12% of the
antigen-specific
CD8+ T cells. In another embodiment, the term refers to an epitope recognized
by fewer than
10% of the antigen-specific CD8+ T cells. In another embodiment, the term
refers to an
epitope recognized by over 8% of the antigen-specific CD8+ T cells. In another
embodiment,
the term refers to an epitope recognized by fewer than 6% of the antigen-
specific CD8+ T
cells. In another embodiment, the term refers to an epitope recognized by
fewer than 5% of
the antigen-specific CD8+ T cells. In another embodiment, the term refers to
an epitope
recognized by over 4% of the antigen-specific CD8+ T cells. In another
embodiment, the
term refers to an epitope recognized by fewer than 3% of the antigen-specific
CD8+ T cells.
In another embodiment, the term refers to an epitope recognized by fewer than
2% of the
antigen-specific CD8+ T cells. In another embodiment, the term refers to an
epitope
recognized by fewer than 1% of the antigen-specific CD8+ T cells. In another
embodiment,
the term refers to an epitope recognized by fewer than 0.5% of the antigen-
specific CD8+ T
57
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
cells.
[00257] Each type of the dominant epitope and subdominant epitope represents a
separate
embodiment of the present invention.
[00258] The antigen in methods and compositions of the present invention is,
in one
embodiment, expressed at a detectable level on a non-tumor cell of the
subject. In another
embodiment, the antigen is expressed at a detectable level on at least a
certain percentage
(e.g. 0.01%, 0.03%, 0.1%, 0.3%, 1%, 2%, 3%, or 5%) of non-tumor cells of the
subject. In
one embodiment, "non-tumor cell" refers to a cell outside the body of the
tumor. In another
embodiment, "non-tumor cell" refers to a non-malignant cell. In another
embodiment, "non-
tumor cell" refers to a non-transformed cell. In another embodiment, the non-
tumor cell is a
somatic cell. In another embodiment, the non-tumor cell is a germ cell. Each
possibility
represents a separate embodiment of the present invention.
[00259] "Detectable level" refers, in one embodiment, to a level that is
detectable when
using a standard assay. In one embodiment, the assay is an immunological
assay. In one
embodiment, the assay is enzyme-linked immunoassay (ELISA). In another
embodiment, the
assay is Western blot. In another embodiment, the assay is FACS. It is to be
understood by a
skilled artisan that any other assay available in the art can be used in the
methods provided
herein. In another embodiment, a detectable level is determined relative to
the background
level of a particular assay. Methods for performing each of these techniques
are well known
to those skilled in the art, and each technique represents a separate
embodiment of the
present invention.
[00260] In one embodiment, vaccination with recombinant antigen-expressing
Listeria
Monocytogenes induces epitope spreading. In another embodiment, vaccination
with LLO-
antigen fusions, even outside the context of Her2, induces epitope spreading
as well. Each
possibility represents a separate embodiment of the present invention.
[00261] In another embodiment, the present invention provides a method of
impeding the
growth of an Her-2-expressing tumor in a subject, comprising administering to
the subject a
recombinant polypeptide comprising an N-terminal fragment of a LLO protein
fused to a
Her-2 chimeric antigen, wherein the antigen has one or more subdominant CD8+ T
cell
epitopes, wherein the subject mounts an immune response against the antigen-
expressing
tumor, thereby impeding the growth of an Her-2-expressing tumor in a subject.
In another
embodiment, the antigen does not contain any of the dominant CD8+ T cell
epitopes. In
another embodiment, provided herein is a method of impeding the growth on a
Her-2-
expressing tumor in a subject, comprising administering to the subject a
recombinant form of
Listeria comprising a recombinant nucleotide encoding the recombinant
polypeptide
58
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
provided herein.
[00262] In another embodiment, the present invention provides a method for
inducing
formation of cytotoxic T cells in a host having cancer, comprising
administering to the host a
composition of the present invention, thereby inducing formation of cytotoxic
T cells in a
host having cancer.
[00263] In another embodiment, the present invention provides a method of
reducing an
incidence of cancer, comprising administering a composition of the present
invention. In
another embodiment, the present invention provides a method of ameliorating
cancer,
comprising administering a composition of the present invention. Each
possibility represents
a separate embodiment of the present invention.
[00264] In one embodiment, the composition is administered to the cells of the
subject ex
vivo; in another embodiment, the composition is administered to the cells of a
donor ex vivo;
in another embodiment, the composition is administered to the cells of a donor
in vivo, then
is transferred to the subject. Each possibility represents a separate
embodiment of the present
invention.
[00265] In one embodiment, the cancer treated by a method of the present
invention is
breast cancer. In another embodiment, the cancer is a Her2 containing cancer.
In another
embodiment, the cancer is a melanoma. In another embodiment, the cancer is
pancreatic
cancer. In another embodiment, the cancer is ovarian cancer. In another
embodiment, the
cancer is gastric cancer. In another embodiment, the cancer is a carcinomatous
lesion of the
pancreas. In another embodiment, the cancer is pulmonary adenocarcinoma. In
another
embodiment, the cancer is colorectal adenocarcinoma. In another embodiment,
the cancer is
pulmonary squamous adenocarcinoma. In another embodiment, the cancer is
gastric
adenocarcinoma. In another embodiment, the cancer is an ovarian surface
epithelial
neoplasm (e.g. a benign, proliferative or malignant variety thereof). In
another embodiment,
the cancer is an oral squamous cell carcinoma. In another embodiment, the
cancer is non
small-cell lung carcinoma. In another embodiment, the cancer is a CNS
carcinoma. In
another embodiment, the cancer is an endometrial carcinoma. In another
embodiment, the
cancer is a bladder cancer. In another embodiment, the cancer is mesothelioma.
In another
embodiment, the cancer is malignant mesothelioma (MM). In another embodiment,
the
cancer is a head and neck cancer. In another embodiment, the cancer is a
prostate carcinoma.
[00266] In one embodiment, the cancer is an osteosarcoma, which in one
embodiment is a
cancerous bone tumor. In one embodiment, the osteosarcoma is any one of the
following
subtypes: osteoblastic, chondroblastic, fibroblastic OSA, telangiectatic OSA,
small cell OSA,
low-grade central OSA, periosteal OSA, paraosteal OSA, secondary OSA, high-
grade
59
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
periosteal OSA, or extraskeletal OSA.
[00267] In another embodiment, the cancer is a Her-2/neu expressing
osteosarcoma. In one
embodiment, the osteosarcoma is canine osteosarcoma. In another embodiment,
the
osteosarcoma is localized osteosarcoma. In another embodiment, the
osteosarcoma is
metastatic osteosarcoma. In another embodiment, the osteosarcoma is high grade
osteosarcoma. In another embodiment, the osteosarcoma is canine appendicular
osteosarcoma. In another embodiment, the cancer is pulmonary metastatic
disease. Each
possibility represents a separate embodiment of the present invention.
[00268] In another embodiment of the methods of the present invention, the
subject mounts
an immune response against the antigen-expressing tumor or target antigen,
thereby
mediating the anti-tumor effects.
[00269] In another embodiment, the present invention provides an immunogenic
composition for treating cancer, the composition comprising a fusion of a
truncated LLO to a
Her-2 chimeric protein. In another embodiment, the immunogenic composition
further
comprises a Listeria strain expressing the fusion. Each possibility represents
a separate
embodiment of the present invention. In another embodiment, the present
invention provides
an immunogenic composition for treating cancer, the composition comprising a
Listeria
strain expressing a Her-2 chimeric protein.
[00270] In another embodiment, provided herein is an immunogenic composition
comprising a recombinant form of Listeria of the present invention.
[00271] In one embodiment, a treatment protocol of the present invention is
therapeutic. In
another embodiment, the protocol is prophylactic. In another embodiment, the
vaccines of
the present invention are used to protect people at risk for cancer such as
breast cancer or
other types of Her2-containing tumors because of familial genetics or other
circumstances
that predispose them to these types of ailments as will be understood by a
skilled artisan. In
another embodiment, the vaccines are used as a cancer immunotherapy after
debulking of
tumor growth by surgery, conventional chemotherapy or radiation treatment. In
another
embodiment, the vaccines are combined with radiation treatment and either
surgery,
conventional chemotherapy or both. Following such treatments, the vaccines of
the present
invention are administered so that the CTL response to the tumor antigen of
the vaccine
destroys remaining metastases and prolongs remission from the cancer. In
another
embodiment, vaccines are used as a cancer immunotherapy in combination with
surgery,
conventional chemotherapy, radiation treatment, or any combination thereof. In
another
embodiment, such combination treatment is used in subjects that cannot undergo
amputation.
In another embodiment, such combination treatment is used in subjects with
primary
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
osteosarcoma that cannot undergo amputation. In another embodiment, vaccines
of the
present invention are used to affect the growth of previously established
tumors and to kill
existing tumor cells. Each possibility represents a separate embodiment of the
present
invention.
[00272] In another embodiment, the vaccines and immunogenic compositions
utilized in
any of the methods described above have any of the characteristics of vaccines
and
immunogenic compositions of the present invention. Each characteristic
represents a separate
embodiment of the present invention. It is to be understood that compositions
described in
the context of the compositions and uses of the present invention may be
referred to as
immunogenic compositions and vice versa.
[00273] Various embodiments of dosage ranges are contemplated by this
invention. In one
embodiment, in the case of vaccine vectors, the dosage is in the range of 0.4
LD50/dose. In
another embodiment, the dosage is from about 0.4-4.9 LD50/dose. In another
embodiment the
dosage is from about 0.5-0.59 LD50/dose. In another embodiment the dosage is
from about
0.6-0.69 LD50/dose. In another embodiment the dosage is from about 0.7-0.79
LD50/dose. In
another embodiment the dosage is about 0.8 LD50/dose. In another embodiment,
the dosage
is 0.4 LD50/dose to 0.8 of the LD50/dose.
[00274] In another embodiment, the dosage is 107 bacteria/dose. In another
embodiment, the
dosage is 1.5 x 107 bacteria/dose. In another embodiment, the dosage is 2 x
107 bacteria/dose.
In another embodiment, the dosage is 3 x 107 bacteria/dose. In another
embodiment, the
dosage is 4 x 107 bacteria/dose. In another embodiment, the dosage is 6 x 107
bacteria/dose.
In another embodiment, the dosage is 8 x 107 bacteria/dose. In another
embodiment, the
dosage is 1 x 108 bacteria/dose. In another embodiment, the dosage is 1.5 x
108 bacteria/dose.
In another embodiment, the dosage is 2 x 108 bacteria/dose. In another
embodiment, the
dosage is 3 x 108 bacteria/dose. In another embodiment, the dosage is 4 x 108
bacteria/dose.
In another embodiment, the dosage is 6 x 108 bacteria/dose. In another
embodiment, the
dosage is 8 x 108 bacteria/dose. In another embodiment, the dosage is 1 x 109
bacteria/dose.
In another embodiment, the dosage is 1.5 x 109 bacteria/dose. In another
embodiment, the
dosage is 2 x 109 bacteria/dose. In another embodiment, the dosage is 3 x 109
bacteria/dose.
In another embodiment, the dosage is 5 x 109 bacteria/dose. In another
embodiment, the
dosage is 6 x 109 bacteria/dose. In another embodiment, the dosage is 8 x 109
bacteria/dose.
In another embodiment, the dosage is 1 x 1010 bacteria/dose. In another
embodiment, the
dosage is 1.5 x 10m bacteria/dose. In another embodiment, the dosage is 2 x
1010
bacteria/dose. In another embodiment, the dosage is 3 x 1010 bacteria/dose. In
another
embodiment, the dosage is 5 x 1010 bacteria/dose. In another embodiment, the
dosage is 6 x
61
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
1010 bacteria/dose. In another embodiment, the dosage is 8 x 1010
bacteria/dose. In another
embodiment, the dosage is 8 x 109 bacteria/dose. In another embodiment, the
dosage is 1 x
1011 bacteria/dose. In another embodiment, the dosage is 1.5 x 1 01 1
bacteria/dose. In another
embodiment, the dosage is 2 x 1011 bacteria/dose. In another embodiment, the
dosage is 3 x
1011 bacteria/dose. In another embodiment, the dosage is 5 x 1011
bacteria/dose. In another
embodiment, the dosage is 6 x 1011 bacteria/dose. In another embodiment, the
dosage is 8 x
1011 bacteria/dose. In another embodiment, the dosage is 5.0 x 108
bacteria/dose. In another
embodiment, the dosage is 3.3 x 109 bacteria/dose. In another embodiment, a
composition for
the use in the methods provided herein comprises 3.3 x 109 Listeria/dose. Each
possibility
represents a separate embodiment of the present invention.
[00275] In one embodiment, a vaccine or immunogenic composition of the present
invention is administered alone to a subject. In another embodiment, the
vaccine or
immunogenic composition is administered together with another cancer therapy,
which in
one embodiment is radiation therapy. Each possibility represents a separate
embodiment of
the present invention.
[00276] The recombinant Listeria of methods and compositions of the present
invention is,
in one embodiment, stably transformed with a construct encoding a Her-2
chimeric antigen
or an LLO-Her-2 chimeric antigen fusion. In one embodiment, the construct
contains a
polylinker to facilitate further subcloning. Several techniques for producing
recombinant
Listeria are known.
[00277] In one embodiment, the construct or nucleic acid molecule is
integrated into the
Listerial chromosome using homologous recombination. Techniques for homologous
recombination are well known in the art, and are described, for example, in
Baloglu S, Boyle
SM, et al. (Immune responses of mice to vaccinia virus recombinants expressing
either
Listeria monocytogenes partial listeriolysin or Brucella abortus ribosomal
L7/L12 protein.
Vet Microbiol 2005, 109(1-2): 11-7); and Jiang LL, Song HH, et al.,
(Characterization of a
mutant Listeria monocytogenes strain expressing green fluorescent protein.
Acta Biochim
Biophys Sin (Shanghai) 2005, 37(1): 19-24). In another embodiment, homologous
recombination is performed as described in United States Patent No. 6,855,320.
In this case,
a recombinant Listeria Monocytogenes strain that expresses E7 was made by
chromosomal
integration of the E7 gene under the control of the hly promoter and with the
inclusion of the
hly signal sequence to ensure secretion of the gene product, yielding the
recombinant
referred to as Lm-AZ/E7. In another embodiment, a temperature sensitive
plasmid is used to
select the recombinants. Each technique represents a separate embodiment of
the present
invention.
62
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00278] In another embodiment, the construct or nucleic acid molecule is
integrated into the
Listerial chromosome using transposon insertion. Techniques for transposon
insertion are
well known in the art, and are described, inter alia, by Sun et al. (Infection
and Immunity
1990, 58: 3770-3778) in the construction of DP-L967. Transposon mutagenesis
has the
advantage, in another embodiment, that a stable genomic insertion mutant can
be formed but
the disadvantage that the position in the genome where the foreign gene has
been inserted is
unknown.
[00279] In another embodiment, the construct or nucleic acid molecule is
integrated into the
Listerial chromosome using phage integration sites (Lauer P, Chow MY et al,
Construction,
characterization, and use of two Listeria monocytogenes site-specific phage
integration
vectors. J Bacteriol 2002;184(15): 4177-86). In certain embodiments of this
method, an
integrase gene and attachment site of a bacteriophage (e.g. U153 or PSA
listeriophage) is
used to insert the heterologous gene into the corresponding attachment site,
which may be
any appropriate site in the genome (e.g. comK or the 3' end of the arg tRNA
gene). In
another embodiment, endogenous prophages are cured from the attachment site
utilized prior
to integration of the construct or heterologous gene. In another embodiment,
this method
results in single-copy integrants. Each possibility represents a separate
embodiment of the
present invention.
[00280] In another embodiment, one of various promoters is used to express the
antigen or
fusion protein containing same. In one embodiment, a Listeria monocytogenes
promoter is
used, e.g. promoters for the genes hly, actA, plea, plcB and mpl, which encode
the Listerial
proteins hemolysin, actA, phosphotidylinositol-specific phospholipase,
phospholipase C, and
metalloprotease, respectively. Each possibility represents a separate
embodiment of the
present invention.
[00281] In another embodiment, methods and compositions of the present
invention utilize a
homologue of a Her-2 chimeric protein or LLO sequence of the present
invention. In another
embodiment, the methods and compositions of the present invention utilize a
Her-2 chimeric
protein from a non-human mammal. The terms "homology," "homologous," etc, when
in
reference to any protein or peptide, refer in one embodiment, to a percentage
of amino acid
residues in the candidate sequence that are identical with the residues of a
corresponding
native polypeptide, after aligning the sequences and introducing gaps, if
necessary, to
achieve the maximum percent homology, and not considering any conservative
substitutions
as part of the sequence identity. Methods and computer programs for the
alignment are well
known in the art.
[00282] In another embodiment, the term "homology," when in reference to any
nucleic
63
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
acid sequence similarly indicates a percentage of nucleotides in a candidate
sequence that are
identical with the nucleotides of a corresponding native nucleic acid
sequence.
[00283] In another embodiment, the present invention provides an isolated
nucleic acid
encoding a signal peptide or a recombinant polypeptide or fusion protein of
the present
invention. In one embodiment, the isolated nucleic acid comprises a sequence
sharing at least
65% homology with a nucleic acid encoding the signal peptide or the
recombinant
polypeptide or the fusion protein of the present invention. In another
embodiment, the
isolated nucleic acid comprises a sequence sharing at least 75% homology with
a nucleic
acid encoding the signal peptide or the recombinant polypeptide or the fusion
protein of the
present invention. In another embodiment, the isolated nucleic acid comprises
a sequence
sharing at least 85% homology with a nucleic acid encoding the signal peptide
or the
recombinant polypeptide or the fusion protein of the present invention. In
another
embodiment, the isolated nucleic acid comprises a sequence sharing at least
90% homology
with a nucleic acid encoding the signal peptide or the recombinant polypeptide
or the fusion
protein of the present invention. In another embodiment, the isolated nucleic
acid comprises
a sequence sharing at least 95% homology with a nucleic acid encoding the
signal peptide or
the recombinant polypeptide or the fusion protein of the present invention. In
another
embodiment, the isolated nucleic acid comprises a sequence sharing at least
97% homology
with a nucleic acid encoding the signal peptide or the recombinant polypeptide
or the fusion
protein of the present invention. In another embodiment, the isolated nucleic
acid comprises
a sequence sharing at least 99% homology with a nucleic acid encoding the
signal peptide or
the recombinant polypeptide or the fusion protein of the present invention.
[00284] Homology is, in one embodiment, detemaned by computer algorithm for
sequence
alignment, by methods well described in the art. For example, computer
algorithm analysis
of nucleic acid sequence homology may include the utilization of any number of
software
packages available, such as, for example, the BLAST, DOMAIN, BEAUTY (BLAST
Enhanced Alignment Utility), GENPEPT and TREMBL packages.
[00285] In another embodiment, "homology" refers to identity to a sequence
selected from a
sequence (nucleic acid or amino acid sequence) provided herein of greater than
65%. In
another embodiment, "homology" refers to identity to a sequence selected from
a sequence
provided herein of greater than 70%. In another embodiment, the identity is
greater than
75%. In another embodiment, the identity is greater than 78%. In another
embodiment, the
identity is greater than 80%. In another embodiment, the identity is greater
than 82%. In
another embodiment, the identity is greater than 83%. In another embodiment,
the identity is
greater than 85%. In another embodiment, the identity is greater than 87%. In
another
64
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
embodiment, the identity is greater than 88%. In another embodiment, the
identity is greater
than 90%. In another embodiment, the identity is greater than 92%. In another
embodiment,
the identity is greater than 93%. In another embodiment, the identity is
greater than 95%. In
another embodiment, the identity is greater than 96%. In another embodiment,
the identity is
greater than 97%. In another embodiment, the identity is greater than 98%. In
another
embodiment, the identity is greater than 99%. In another embodiment, the
identity is 100%.
Each possibility represents a separate embodiment of the present invention.
[00286] In another embodiment, homology is determined via detemanation of
candidate
sequence hybridization, methods of which are well described in the art (See,
for example,
"Nucleic Acid Hybridization" Hames, B. D., and Higgins S. J., Eds. (1985);
Sambrook et al.,
2001, Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Press, N.Y.;
and
Ausubel et al., 1989, Current Protocols in Molecular Biology, Green Publishing
Associates
and Wiley Interscience, N.Y). For example, methods of hybridization may be
carried out
under moderate to stringent conditions, to the complement of a DNA encoding a
native
caspase peptide. Hybridization conditions being, for example, overnight
incubation at 42 C
in a solution comprising: 10-20 % formamide, 5 X SSC (150 mM NaC1, 15 mM
trisodium
citrate), 50 mM sodium phosphate (pH 7. 6), 5 X Denhardt's solution, 10 %
dextran sulfate,
and 20 ug/m1 denatured, sheared salmon sperm DNA.
[00287] In one embodiment of the present invention, "nucleic acids" refers to
a string of at
least two base-sugar-phosphate combinations. The term includes, in one
embodiment, DNA
and RNA. "Nucleotides" refers, in one embodiment, to the monomeric units of
nucleic acid
polymers. RNA may be, in one embodiment, in the form of a tRNA (transfer RNA),
snRNA
(small nuclear RNA), rRNA (ribosomal RNA), mRNA (messenger RNA), anti-sense
RNA,
small inhibitory RNA (siRNA), micro RNA (miRNA) and ribozymes. The use of
siRNA and
miRNA has been described (Caudy AA et al, Genes & Devel 16: 2491-96 and
references
cited therein). DNA may be in form of plasmid DNA, viral DNA, linear DNA, or
chromosomal DNA or derivatives of these groups. In addition, these forms of
DNA and
RNA may be single, double, triple, or quadruple stranded. The term also
includes, in another
embodiment, artificial nucleic acids that may contain other types of backbones
but the same
bases. In one embodiment, the artificial nucleic acid is a PNA (peptide
nucleic acid). PNA
contain peptide backbones and nucleotide bases and are able to bind, in one
embodiment, to
both DNA and RNA molecules. In another embodiment, the nucleotide is oxetane
modified.
In another embodiment, the nucleotide is modified by replacement of one or
more
phosphodiester bonds with a phosphorothioate bond. In another embodiment, the
artificial
nucleic acid contains any other variant of the phosphate backbone of native
nucleic acids
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
known in the art. The use of phosphothiorate nucleic acids and PNA are known
to those
skilled in the art, and are described in, for example, Neilsen PE, Curr Opin
Struct Biol 9:353-
57; and Raz NK et al Biochem Biophys Res Commun. 297:1075-84. The production
and use
of nucleic acids is known to those skilled in art and is described, for
example, in Molecular
Cloning, (2001), Sambrook and Russell, eds. and Methods in Enzymology: Methods
for
molecular cloning in eukaryotic cells (2003) Purchio and G. C. Fareed. Each
nucleic acid
derivative represents a separate embodiment of the present invention.
[00288] Protein and/or peptide homology for any amino acid sequence listed
herein is
determined, in one embodiment, by methods well described in the art, including
immunoblot
analysis, or via computer algorithm analysis of amino acid sequences,
utilizing any of a
number of software packages available, via established methods. Some of these
packages
may include the FASTA, BLAST, MPsrch or Scanps packages, and may employ the
use of
the Smith and Waterman algorithms, and/or global/local or BLOCKS alignments
for
analysis, for example. Each method of determining homology represents a
separate
embodiment of the present invention.
[00289] In another embodiment, the present invention provides a kit comprising
a reagent
utilized in performing a method of the present invention. In another
embodiment, the present
invention provides a kit comprising a composition, tool, or instrument of the
present
invention.
[00290] The terms "contacting" or "administering," in one embodiment, refer to
directly
contacting the cancer cell or tumor with a composition of the present
invention. In another
embodiment, the terms refer to indirectly contacting the cancer cell or tumor
with a
composition of the present invention. In another embodiment, methods of the
present
invention include methods in which the subject is contacted with a composition
of the
present invention after which the composition is brought in contact with the
cancer cell or
tumor by diffusion or any other active transport or passive transport process
known in the art
by which compounds circulate within the body.
[00291] In another embodiment, methods of this invention may include at least
a single
administration of a composition of this invention, wherein in another
embodiment, methods
of this invention may include multiple administrations of a composition of
this invention.
Each possibility represents a separate embodiment of the present invention.
[00292] In one embodiment, the present invention provides methods in which
recombinant
Listeria is administered only once. In another embodiment, Listeria is
administered twice. In
another embodiment, Listeria is administered three times. In another
embodiment, Listeria is
administered four times. In another embodiment, Listeria is administered more
than four
66
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
times. In another embodiment, Listeria is administered multiple times. In
another
embodiment, Listeria is administered at regular intervals, which in one
embodiment, may be
daily, weekly, every two weeks, every three weeks, or every month. Each
possibility
represents a separate embodiment of the present invention.
[00293] In one embodiment, the present invention provides methods in which
radiation
therapy is administered only once. In another embodiment, radiation therapy is
administered
twice. In another embodiment, radiation therapy is administered three times.
In another
embodiment, radiation therapy is administered four times. In another
embodiment, radiation
therapy is administered more than four times. In another embodiment, radiation
therapy is
administered multiple times. In another embodiment, radiation therapy is
administered at
regular intervals, which in one embodiment, may be daily, weekly, every two
weeks, every
three weeks, or every month. Each possibility represents a separate embodiment
of the
present invention.
[00294] In one embodiment, the radiation therapy is administered prior to the
administration
of the recombinant attenuated Listeria. In another embodiment, the radiation
therapy is
administered twice prior to the first administration of the recombinant
attenuated Listeria. In
another embodiment, the radiation therapy is administered three times prior to
the first
administration of the recombinant attenuated Listeria.
[00295] In another embodiment, the recombinant attenuated Listeria is
administered prior to
the administration of the radiation therapy. In another embodiment, the
recombinant
attenuated Listeria is administered twice prior to the first administration of
the radiation
therapy. In another embodiment, the recombinant attenuated Listeria is
administered three
times prior to the first administration of the radiation therapy.
[00296] In another embodiment, the terms "gene" and "recombinant gene" refer
to nucleic
acid molecules comprising an open reading frame encoding a polypeptide of the
invention.
Such natural allelic variations can typically result in 1-5% variance in the
nucleotide
sequence of a given gene. Alternative alleles can be identified by sequencing
the gene of
interest in a number of different individuals or organisms. This can be
readily carried out by
using hybridization probes to identify the same genetic locus in a variety of
individuals or
organisms. Any and all such nucleotide variations and resulting amino acid
polymorphisms
or variations that are the result of natural allelic variation and that do not
alter the functional
activity are intended to be within the scope of the invention.
Pharmaceutical Compositions
[00297] It
will be appreciated by a skilled artisan that the terms "immunogenic
composition", "composition" and "pharmaceutical composition" may be used
67
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
interchangeably. It is also to be understood that administration of such
compositions
enhances an immune response, or increase a T effector cell to regulatory T
cell ratio or elicit
an anti-tumor immune response, as further provided herein.
[00298] In
one embodiment, the immunogenic composition provided herein
comprises a recombinant Listeria provided herein.
[00299] In
one embodiment, a "combination therapy" refers to the combination
of radiation therapy described herein administered in conjunction with, or
prior to
administration of a composition comprising the recombinant Listeria provided
herein.
[00300] The pharmaceutical compositions containing vaccines and compositions
of the
present invention are, in another embodiment, administered to a subject by any
method
known to a person skilled in the art, such as parenterally, paracancerally,
transmucosally,
transdermally, intramuscularly, intravenously, intra-dermally, subcutaneously,
intra-
peritonealy, intra-ventricularly, intra-cranially, intra-vaginally or intra-
tumorally.
[00301] In another embodiment of the methods and compositions provided herein,
the
vaccines or compositions are administered orally, and are thus formulated in a
form suitable
for oral administration, i.e. as a solid or a liquid preparation. Suitable
solid oral formulations
include tablets, capsules, pills, granules, pellets and the like. Suitable
liquid oral formulations
include solutions, suspensions, dispersions, emulsions, oils and the like. In
another
embodiment of the present invention, the active ingredient is formulated in a
capsule. In
accordance with this embodiment, the compositions of the present invention
comprise, in
addition to the active compound and the inert carrier or diluent, a hard
gelating capsule.
[00302] In another embodiment, the vaccines or compositions are administered
by
intravenous, intra-arterial, or intra-muscular injection of a liquid
preparation. Suitable liquid
formulations include solutions, suspensions, dispersions, emulsions, oils and
the like.
[00303] In one embodiment, the pharmaceutical compositions are administered
intravenously and are thus formulated in a form suitable for intravenous
administration. In
another embodiment, the pharmaceutical compositions are administered intra-
arterially and
are thus formulated in a form suitable for intra-arterial administration. In
another
embodiment, the pharmaceutical compositions are administered intra-muscularly
and are
thus formulated in a form suitable for intra-muscular administration.
[00304] In one embodiment, repeat administrations (booster doses) of
compositions of this
invention may be undertaken immediately following the first course of
treatment or after an
interval of days, weeks or months to achieve tumor regression. In another
embodiment,
repeat doses may be undertaken immediately following the first course of
treatment or after
an interval of days, weeks or months to achieve suppression of tumor growth.
Assessment
68
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
may be detemaned by any of the techniques known in the art, including
diagnostic methods
such as imaging techniques, analysis of serum tumor markers, biopsy, or the
presence,
absence or amelioration of tumor associated symptoms.
[00305] In one embodiment, a subject is administered a booster dose every 1-2
weeks, every
2-3 weeks, every 3-4 weeks, every 4-5 weeks, every 6-7 weeks, every 7-8 weeks,
or every 9-
weeks in order to achieve the intended anti-tumor response. In one embodiment,
a subject
is administered a booster dose every 1-2 months, every 2-3 months, every 3-4
months, every
4-5 months, every 6-7 months, every 7-8 months, or every 9-10 months in order
to achieve
the intended anti-tumor response.
10 [00306]
In one embodiment, the term "treating" refers to curing a disease. In another
embodiment, "treating" refers to preventing a disease. In another embodiment,
"treating"
refers to reducing the incidence of a disease. In another embodiment,
"treating" refers to
ameliorating symptoms of a disease. In another embodiment, "treating" refers
to increasing
performance free survival or overall survival of a patient. In another
embodiment, "treating"
refers to stabilizing the progression of a disease. In another embodiment,
"treating" refers to
inducing remission. In another embodiment, "treating" refers to slowing the
progression of a
disease. The terms "reducing", "suppressing" and "inhibiting" refer in another
embodiment
to lessening or decreasing. Each possibility represents a separate embodiment
of the present
invention.
[00307] The term "about" as used herein means in quantitative terms plus or
minus 5%, or
in another embodiment plus or minus 10%, or in another embodiment plus or
minus 15%, or
in another embodiment plus or minus 20%.
[00308] It is to be understood by the skilled artisan that the term "subject"
can encompass a
mammal including an adult human or a human child, teenager or adolescent in
need of
therapy for, or susceptible to, a condition or its sequelae, and also may
include non-human
mammals such as dogs, cats, pigs, cows, sheep, goats, horses, rats, and mice.
It will also be
appreciated that the term may encompass livestock. The term "subject" does not
exclude an
individual that is normal in all respects.
[00309] In one embodiment, the term "subject" also encompasses dogs that
cannot undergo
amputation. In another embodiment, the term "subject" also encompasses humans
that
cannot undergo surgery. In another embodiment, the term "subject" also
encompasses
humans that cannot undergo amputation.
[00310] It will be appreciated by the skilled artisan that the term "mammal"
for purposes of
treatment refers to any animal classified as a mammal, including, but not
limited to, humans,
domestic and farm animals, and zoo, sports, or pet animals, such as canines,
including dogs,
69
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
and horses, cats, cattle, pigs, sheep, etc.
[00311] A "therapeutically effective amount", in reference to the treatment of
tumor, refers
to an amount capable of invoking one or more of the following effects: (1)
inhibition, to
some extent, of tumor growth, including, slowing down and complete growth
arrest; (2)
reduction in the number of tumor cells; (3) reduction in tumor size; (4)
inhibition (i.e.,
reduction, slowing down or complete stopping) of tumor cell infiltration into
peripheral
organs; (5) inhibition (i.e., reduction, slowing down or complete stopping) of
metastasis; (6)
enhancement of anti-tumor immune response, which may, but does not have to,
result in the
regression or rejection of the tumor; and/or (7) relief, to some extent, of
one or more
symptoms associated with the disorder. A "therapeutically effective amount" of
a vaccine
provided herein for purposes of treatment of tumor may be determined
empirically and in a
routine manner.
[00312] In one embodiment, compositions for use in the methods of the present
invention
comprise a second open reading frame encoding a metabolic enzyme, wherein said
metabolic
enzyme complements an endogenous gene that is mutated in the chromosome of
said
recombinant attenuated Listeria strain. In another embodiment, the metabolic
enzyme
complements an endogenous gene that is lacking in the chromosome of said
recombinant
attenuated Listeria strain.
[00313] In one embodiment, "mutated" or "mutant" describes a deletion. In
another
embodiment, "mutated" or "mutant" describes an inactivation. In another
embodiment,
"mutated" or "mutant" describes a truncation. In another embodiment, "mutated"
or
"mutant" describes an addition. In another embodiment, "mutated" or "mutant"
describes a
substitution. In another embodiment, "mutated" or "mutant" describes insertion
of a
premature stop codon. In another embodiment, "mutated" or "mutant" describes a
change to
one or more nucleic acids within a gene which disrupts expression of the gene.
[00314] In one embodiment, "radiation therapy" or "radiotherapy" refers to the
medical use
of ionizing radiation as part of cancer treatment to control or eradicate
malignant cells.
Radiotherapy may be used for curative, adjuvant, or palliative treatment.
Suitable types of
radiotherapy include conventional external beam radiotherapy, stereotactic
radiation therapy
(e.g., Axesse, Cyberknife, Gamma Knife, Novalis, Primatom, Synergy, X-Knife,
TomoTherapy or Trilogy), Intensity-Modulated Radiation Therapy, particle
therapy (e.g.,
proton therapy), brachytherapy, delivery of radioisotopes, intraoperative
radiotherapy, Auger
therapy, Volumetric modulated arc therapy (VMAT), Virtual simulation, 3-
dimensional
conformal radiation therapy, and intensity-modulated radiation therapy, etc.
It is to be
understood that this list is not meant to be limiting.
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00315] In one embodiment, radiation therapy uses high-energy radiation to
shrink tumors
and kill cancer cells. In one embodiment, X-rays, gamma rays, and charged
particles are
types of radiation that may be used for cancer treatment. In one embodiment,
radiation
therapy kills cancer cells by damaging their DNA either directly or by
creating free radicals
within the cells that can in turn damage the DNA.
[00316] In one embodiment, the radiation may be delivered by a machine outside
the body
(external-beam radiation therapy), or in another embodiment, it may come from
radioactive
material placed in the body near cancer cells (internal radiation therapy,
also called
brachytherapy).
[00317] In one embodiment, systemic radiation therapy uses radioactive
substances, such as
radioactive iodine, that travel in the blood to kill cancer cells.
[00318] In one embodiment, the present invention provides a method for
concomitantly
treating radiation insensitive cancers such as osteosarcomas with standard
radiation in
combination with immunotherapy, such as administration of recombinant Listeria
in a
regimen which requires shorter radiation treatment times, thus ameliorating
side effects
ordinarily associated with radiation treatment.
[00319] In one embodiment, the radiation is administered according to this
invention by
standard techniques with standard megavoltage equipment, such as AECL
Theratron 80,
Varian Clinac 4 or Varian Clinac. In one embodiment, the maximum size of the
radiation
portal should be no greater than 300 cm2. In one embodiment, a suitable does
is between
about 15 Gy and 35 Gy, with the specific dose dependent on the area of the
body treated.
Thus, a dose to the spinal cord would be about 35 Gy, whereas a dose to the
bilateral kidneys
would be about 15 Gy and to the whole liver 20 Gy. Breaks in the therapy are
at the
discretion of the clinician taking into consideration the patients tolerance
for radiation
therapy.
[00320] In another embodiment, radiation dosages in the combination therapy
are
administered in sequence. In another embodiment, radiation dosages in the
combination
therapy are administered in on consecutive days as exemplified herein (see
Example 11). In
another embodiment, radiation doses of 8Gy in the combination therapy are on
consecutive
days for a total dose of 16 Gy prior to administration of Multiple doses (up
to 8) of ADXS31-
164 were given once every 3 weeks as demonstrated in Examples herein (see
Example 11).
In another embodiment, radiation doses of 8Gy in the combination therapy are
on
consecutive days for a total dose of 16 Gy prior to administration of Multiple
doses (up to 8)
of up to 3.3x109 CFU ADXS31-164 were given once every 3 weeks as demonstrated
in
Examples herein (see Example 11). In another embodiment, radiation doses of
8Gy in the
71
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
combination therapy are on consecutive days for a total dose of 16 Gy prior to
administration
of Multiple doses (up to 8) of 3.3x109 CFU ADXS31-164 were given once every 3
weeks,
as demonstrated in Examples herein (see Example 11). In one embodiment, the
multiple
doses range of ADXS31-164 provided herein is from 1-8, 8-15, 15-25, or as many
as
required to achieve an intended therapeutic goal.
[00321] In one embodiment, total radiation doses administered for a
combination therapy
provide herein range from 70-80 Gy. In another embodiment, total radiation
doses
administered for a combination therapy provided herein range from 10-26 GY. In
another
embodiment, radiation doses ranging from 10-26 GY are administered in
sequence. In one
embodiment, the sequence may be hourly, daily, weekly or bi-weekly. In one
embodiment,
radiation doses ranging from 70-80 Gy are administered in sequence. In another
embodiment, radiation doses ranging from 10-26 GY are administered. In another
embodiment, radiation doses are approximately a/3=5.4 Gy and =1.73 Gy-1 for
an adult
male.
[00322] In one embodiment, the radiation therapy described in the present
invention as part
of a combination therapy is palliative radiation therapy. In one embodiment,
radiation
therapy may be given with palliative intent. In one embodiment, palliative
treatments are
intended to relieve symptoms and reduce the suffering caused by cancer or a
tumor. In
another embodiment, the radiation therapy described herein as part of a
combination therapy
is meant to cure the cancer or tumor.
[00323] The following examples are presented in order to more fully illustrate
the preferred
embodiments of the invention. They should in no way be construed, however, as
limiting the
broad scope of the invention.
EXAMPLES
[00324] Materials and Methods
[00325] Oligonucleotides were synthesized by Invitrogen (Carlsbad, CA) and DNA
sequencing was done by Genewiz Inc, South Plainfield, NJ. Flow cytometry
reagents were
purchased from Becton Dickinson Biosciences (BD, San Diego, CA). Cell culture
media,
supplements and all other reagents, unless indicated, were from Sigma (St.
Louise, MO).
Her-2/neu HLA-A2 peptides were synthesized by EZbiolabs (Westfield, IN).
Complete
RPMI 1640 (C-RPMI) medium contained 2mM glutamine, 0.1 mM non-essential amino
acids, and 1mM sodium pyruvate, 10% fetal bovine serum,
penicillin/streptomycin, Hepes
(25mM). The polyclonal anti-LLO antibody was described previously and anti-Her-
2/neu
antibody was purchased from Sigma.
[00326] Mice and Cell Lines
72
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00327] All animal experiments were performed according to approved protocols
by
IACUC at the University of Pennsylvania or Rutgers University. FVB/N mice were
purchased from Jackson laboratories (Bar Harbor, ME). The FVB/N Her-2/neu
transgenic
mice, which overexpress the rat Her-2/neu onco-protein were housed and bred at
the animal
core facility at the University of Pennsylvania. The NT-2 tumor cell line
expresses high
levels of rat Her-2/neu protein, was derived from a spontaneous mammary tumor
in these
mice and grown as described previously. DIJI-R-G8 (3T3/neu) cells were
obtained from
ATCC and were grown according to the ATCC recommendations. The EMT6-Luc cell
line
was a generous gift from Dr. John Ohlfest (University of Minnesota, MN) and
was grown in
complete C-RPMI medium. Bioluminescent work was conducted under guidance by
the
Small Animal Imaging Facility (SAIF) at the University of Pennsylvania
(Philadelphia, PA).
[00328] Listeria constructs and antigen expression
[00329] Her-2/neu-pGEM7Z was kindly provided by Dr. Mark Greene at the
University of
Pennsylvania and contained the full-length human Her-2/neu (hHer2) gene cloned
into the
pGEM7Z plasmid (Promega, Madison WI). This plasmid was used as a template to
amplify
three segments of hHer-2/neu, namely, EC1, EC2, and IC1, by PCR using pfx DNA
polymerase (Invitrogen) and the oligos indicated in Table 1.
[00330] Table 2: Primers for cloning of Human her-2-Chimera
Table 2
DNA sequence Base pair
Amino acid
region region or
junctions
Her-2- TGATCTCGAGACCCACCTGGACATGCTC (SEQ ID NO: 57) 120-510 40-170
Chimera (F)
HerEC1- CTACCAGGACACGATTTTGTGGAAG-AATATCCA
EC2F GGAGTTTGCTGGCTGC (SEQ ID NO: 58)
(Junction) 510/1077 170/359
HerEC1- GCAGCCAGCAAACTCCTGGATATT-CTTCCACAA
EC2R AATCGTGTCCTGGTAG (SEQ ID NO: 59)
(Junction)
HerEC2- CTGCCACCAGCTGTGCGCCCGAGGG-
ICIF CAGCAGAAGATCCGGAAGTACACGA (SEQ ID NO: 60)
(Junction) 1554/2034 518/679
HerEC2- TCGTGTACTTCCGGATCTTCTGCTG
ICIR CCCTCGGGC GCACAGCTGGTGGCAG (SEQ ID NO: 61)
(Junction)
73
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Her-2- GTGGCCCGGGTCTAGATTAGTCTAAGAGGCAGCCATAGG 2034-2424 679-808
Chimera (R) (SEQ ID NO: 62)
[00331] The Her-2/neu chimera construct was generated by direct fusion by the
SOEing
PCR method and each separate hHer-2/neu segment as templates. Primers are
shown in
Table 3.
[00332] Sequence of primers for amplification of different segments human Her2
regions.
Table 3
DNA sequence Base pair region Amino
acid
region
Her-2 -EC1 (F) CCGCCTCGAGGCCGCGAGCACCCAAGTG 58-979 20-326
(SEQ ID NO: 63)
Her-2 -EC1 (R) CGCGACTAGTTTAATCCTCTGCTGTCACCTC
(SEQ ID NO: 64)
Her-2-EC2(F) CCGCCTCGAGTACCTTTCTACGGACGTG (SEQ 907-1504 303-501
ID NO: 65)
Her- 2- EC2(R) CGCGACTAGTTTACTCTGGCCGGTTGGCAG
(SEQ ID NO: 66)
Her-2-Her-2- CCGCCTCGAGCAGCAGAAGATCCGGAAGTAC 2034-3243 679-1081
IC1 (F) (SEQ ID NO: 67)
Her-2 -IC1 (R) CGCGACTAGTTTAAGCCCCTTCGGAGGGTG
(SEQ ID NO: 68)
[00333] ChHer2 gene was excised from pAdv138 using XhoI and SpeI restriction
enzymes,
and cloned in frame with a truncated, non-hemolytic fragment of LLO in the
Lmdd shuttle
vector, pAdv 134. The sequences of the insert, LLO and hly promoter were
confirmed by
DNA sequencing analysis. This plasmid was electroporated into electro-
competent actA, dal,
dat mutant Listeria monocytogenes strain, LmddA and positive clones were
selected on Brain
Heart infusion (BHI) agar plates containing streptomycin (250m/m1). In some
experiments
similar Listeria strains expressing hHer-2/neu (Lm-hHer2) fragments were used
for
comparative purposes. These have been previously described. In all studies, an
irrelevant
Listeria construct (Lm-control) was included to account for the antigen
independent effects
of Listeria on the immune system. Lm-controls were based on the same Listeria
platform as
ADXS31-164, but expressed a different antigen such as HPV16-E7 or NY-ESO-1.
74
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Expression and secretion of fusion proteins from Listeria were tested. Each
construct was
passaged twice in vivo.
[00334] Cytotoxicity assay
[00335] Groups of 3-5 FVB/N mice were immunized three times with one week
intervals
with 1 x 108 colony forming units (CFU) of Lm-LLO-ChHer2, ADXS31-164, Lm-hHer2
ICI
or Lm-control (expressing an irrelevant antigen) or were left naive. NT-2
cells were grown in
vitro, detached by trypsin and treated with mitomycin C (250 1..tg/m1 in serum
free C-RPMI
medium) at 37 C for 45 minutes. After 5 washes, they were co-incubated with
splenocytes
harvested from immunized or naive animals at a ratio of 1:5 (Stimulator:
Responder) for 5
days at 37 C and 5% CO2. A standard cytotoxicity assay was performed using
europium
labeled 3T3/neu (DHER-G8) cells as targets according to the method previously
described.
Released europium from killed target cells was measured after 4 hour
incubation using a
spectrophotometer (Perkin Elmer, Victor2) at 590 nm. Percent specific lysis
was defined as
(lysis in experimental group-spontaneous lysis)/(Maximum lysis-spontaneous
lysis).
[00336] Interferon-7 secretion by splenocytes from immunized mice
[00337] Groups of 3-5 FVB/N or HLA-A2 transgenic mice were immunized three
times
with one week intervals with 1 x 108 CFU of ADXS31-164, a negative Listeria
control
(expressing an irrelevant antigen) or were left naive. Splenocytes from FVB/N
mice were
isolated one week after the last immunization and co-cultured in 24 well
plates at 5 x 106
cells/well in the presence of mitomycin C treated NT-2 cells in C-RPMI medium.
Splenocytes from the HLA-A2 transgenic mice were incubated in the presence of
1 1..tM of
HLA-A2 specific peptides or 1iag/m1 of a recombinant His-tagged ChHer2
protein, produced
in E. coli and purified by a nickel based affinity chromatography system.
Samples from
supernatants were obtained 24 or 72 hours later and tested for the presence of
interferon-y
(IFN-y) using mouse IFN-y Enzyme-linked immunosorbent assay (ELISA) kit
according to
manufacturer' s recommendations.
[00338] INF-7 ELISpot Assay
[00339] Cryopreserved PBMC from each indicated time point were thawed, rested
overnight at 37 C and then counted. Cells were stimulated with 2.5 uM pools of
overlapping
human Her-2/neu peptides (11mers overlapping by 5 amino acids) that represent
the EC1,
EC2 and IC1 domains of Her-2/neu present in the chimeric vaccine, and
recombinant human
IL-2 (Invitrogen, Fredrick, MD) for 5 days. Cells were harvested, washed twice
in 1 x PBS
and counted. IFN-y ELISpot assays were performed according to the
manufacturer's protocol
using a commercial canine IFN-y ELISpot assay kit (R&D Systems, Minneapolis,
MN).
Briefly, 0.8 - 2 x 105 stimulated cells were incubated with 2.5 uM of EC1, EC2
or IC1
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
peptide pools plus IL-2 or IL-2 alone (to determine background counts). All
assays were
performed in duplicates. Plates were developed according to the manufacturer's
instructions.
Spots were counted using a CTL-Immunospot analyzer (C.T.L, Shaker Heights,
OH).
Number of spots were normalized by subtracting twice the number of spots
counted in non-
stimulated wells.
[00340] Tumor studies in Her2 transgenic animals
[00341] Six weeks old FVB/N rat Her-2/neu transgenic mice (9-14/group) were
immunized
6 times with 5 x 108 CFU of Lm-LLO-ChHer2, ADXS31-164 or Lm-control. They were
observed twice a week for the emergence of spontaneous mammary tumors, which
were
measured using an electronic caliper, for up to 52 weeks. Escaped tumors were
excised when
they reached a size lcm2 in average diameter and preserved in RNAlater at -20
C. In order to
determine the effect of mutations in the Her-2/neu protein on the escape of
these tumors,
genomic DNA was extracted using a genomic DNA isolation kit, and sequenced.
[00342] Effect of ADXS31-164 on regulatory T cells in spleens and tumors
[00343] Mice were implanted subcutaneously (s.c.) with 1 x 106 NT-2 cells. On
days 7, 14
and 21, they were immunized with 1 x 108 CFUs of ADXS31-164, LmddA-control or
left
naive. Tumors and spleens were extracted on day 28 and tested for the presence
of
CD3 /CD4E/FoxP3+ Tregs by FACS analysis. Briefly, splenocytes were isolated by
homogenizing the spleens between two glass slides in C-RPMI medium. Tumors
were
minced using a sterile razor blade and digested with a buffer containing DNase
(12U/m1),
and collagenase (2mg/m1) in PBS. After 60 min incubation at RT with agitation,
cells were
separated by vigorous pipetting. Red blood cells were lysed by RBC lysis
buffer followed by
several washes with complete RPMI-1640 medium containing 10% FBS. After
filtration
through a nylon mesh, tumor cells and splenocytes were resuspended in FACS
buffer (2%
FIN/PBS) and stained with anti-CD3-PerCP-Cy5.5, CD4-FITC, CD25-APC antibodies
followed by permeabilization and staining with anti-Foxp3-PE. Flow cytometry
analysis was
performed using 4-color FACS calibur (BD) and data were analyzed using cell
quest
software (BD).
[00344] Statistical analysis
[00345] The log-rank Chi-Squared test was used for survival data and student's
t-test for the
CTL and ELISA assays, which were done in triplicates. A p-value of less than
0.05 (marked
as *) was considered statistically significant in these analyzes. All
statistical analysis was
done with either Prism software, V.4.0a (2006) or SPSS software, V.15.0
(2006). For all
FVB/N rat Her-2/neu transgenic studies we used 8-14 mice per group, for all
wild-type
FVB/N studies we used at least 8 mice per group unless otherwise stated. All
studies were
76
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
repeated at least once except for the long term tumor study in Her-2/neu
transgenic mouse
model.
EXAMPLE 1:
GENERATION OF L. MONOCYTOGENES STRAINS THAT SECRETE LLO
FRAGMENTS FUSED TO Her-2 FRAGMENTS: CONSTRUCTION OF
ADXS31-164.
[00346] Construction of the chimeric Her-2/neu gene (ChHer2) was described
previously.
Briefly, ChHer2 gene was generated by direct fusion of two extracellular (aa
40-170 and aa
359-433) and one intracellular fragment (aa 678-808) of the Her-2/neu protein
by SOEing
PCR method. The chimeric protein harbors most of the known human MHC class I
epitopes
of the protein. ChHer2 gene was excised from the plasmid, pAdv138 (which was
used to
construct Lm-LLO-ChHer2) and cloned into LmddA shuttle plasmid, resulting in
the plasmid
pAdv164 (Figure 1A). There are two major differences between these two plasmid
backbones. 1) Whereas pAdv138 uses the chloramphenicol resistance marker (cat)
for in
vitro selection of recombinant bacteria, pAdv164 harbors the D-alanine
racemase gene (dal)
from bacillus subtilis, which uses a metabolic complementation pathway for in
vitro
selection and in vivo plasmid retention in LmddA strain which lacks the dal-
dat genes. This
vaccine platform was designed and developed to address FDA concerns about the
antibiotic
resistance of the engineered Listeria vaccine strains. 2) Unlike pAdv138,
pAdv164 does not
harbor a copy of the prfA gene in the plasmid (see sequence below and Figure
1A), as this is
not necessary for in vivo complementation of the Lmdd strain. The LmddA
vaccine strain also
lacks the actA gene (responsible for the intracellular movement and cell-to-
cell spread of
Listeria) so the recombinant vaccine strains derived from this backbone are
100 times less
virulent than those derived from the Lmdd, its parent strain. LmddA-based
vaccines are also
cleared much faster (in less than 48 hours) than the Lmdd-based vaccines from
the spleens of
the immunized mice. The expression and secretion of the fusion protein tLLO-
ChHer2 from
this strain was comparable to that of the Lm-LLO-ChHer2 in TCA precipitated
cell culture
supernatants after 8 hours of in vitro growth (Figure 1B) as a band of ¨104 KD
was detected
by an anti-LLO antibody using Western Blot analysis. The Listeria backbone
strain
expressing only tLLO was used as negative control.
[00347] pAdv164 sequence (7075 base pairs) (see Figure 1):
[00348] cggagtgtatactggettactatgttggc actgatgagggtgtc agtgaagtgatc atgtggc
aggagaaaaaaggctg
caccggtgcgtcagcagaatatgtgatacaggatatattccgcttcctcgctcactgactcgctacgcteggtcgttcg
actgeggcga
geggaaatggettacgaacggggcggagatttcctggaagatgccaggaagatacttaacagggaagtgagagggccgc
ggcaa
agccgtttttccataggctccgcccccctgacaagcatcacgaaatctgacgctcaaatcagtggtggcgaaacccgac
aggactata
77
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
aagataccaggcgatccccctggcggctccctcgtgcgctctcctgttcctgccatcggataccggtgtcattccgctg
ttatggccg
cgtagtctcattccacgcctgacactcagaccgggtaggcagttcgctccaagctggactgtatgcacgaaccccccgt
tcagtccg
accgctgcgccttatccggtaactatcgtcttgagtccaacccggaaagacatgcaaaagcaccactggcagcagccac
tggtaattg
atttagaggagttagtcttgaagtcatgcgccggttaaggctaaactgaaaggacaagtatggtgactgcgctcctcca
agccagttac
ctcggttcaaagagaggtagctcagagaaccttcgaaaaaccgccctgcaaggcggattacgattcagagcaagagatt
acgcgc
agaccaaaacgatctcaagaagatcatcttattaatcagataaaatatactagccctcctagattagtatattcctatc
ttaaagttactata
tgtggaggcattaacatttgttaatgacgtcaaaaggatagcaagactagaataaagctataaagcaagcatataatat
tgcgtttcatctt
tagaagcgaatttcgccaatattataattatcaaaagagaggggtggcaaacggtatttggcattattaggttaaaaaa
tgtagaaggag
agtgaaacccatgaaaaaaataatgctagtattattacacttatattagttagtctaccaattgcgcaacaaactgaag
caaaggatgcat
ctgcattcaataaagaaaattcaatttcatccatggcaccaccagcatctccgcctgcaagtcctaagacgccaatcga
aaagaaacac
gcggatgaaatcgataagtatatacaaggattggattacaataaaaacaatgtattagtataccacggagatgcagtga
caaatgtgcc
gccaagaaaaggttacaaagatggaaatgaatatattgagtggagaaaaagaagaaatccatcaatcaaaataatgcag
acattcaa
gttgtgaatgcaatttcgagcctaacctatccaggtgctctcgtaaaagcgaattcggaattagtagaaaatcaaccag
atgactccctg
taaaacgtgattcattaacactcagcattgatttgccaggtatgactaatcaagacaataaaatagagtaaaaaatgcc
actaaatcaaa
cgttaacaacgcagtaaatacattagtggaaagatggaatgaaaaatatgctcaagcttatccaaatgtaagtgcaaaa
attgattatgat
gacgaaatggcttacagtgaatcacaattaattgcgaaataggtacagcatttaaagctgtaaataatagcttgaatgt
aaacttcggcg
caatcagtgaagggaaaatgcaagaagaagtcattagattaaacaaatttactataacgtgaatgttaatgaacctaca
agaccacca
gatattcggcaaagctgttactaaagagcagttgcaagcgcaggagtgaatgcagaaaatcctcctgcatatatctcaa
gtgtggcgt
atggccgtcaagatatttgaaattatcaactaattcccatagtactaaagtaaaagctgcttagatgctgccgtaagcg
gaaaatctgtct
caggtgatgtagaactaacaaatatcatcaaaaattcaccttcaaagccgtaatttacggaggaccgcaaaagatgaag
ttcaaatcat
cgacggcaacctcggagacttacgcgatattagaaaaaaggcgctacattaatcgagaaacaccaggagacccattgct
tatacaa
caaacttcctaaaagacaatgaattagctgttattaaaaacaactcagaatatattgaaacaacttcaaaagcttatac
agatggaaaaatt
aacatcgatcactctggaggatacgagctcaattcaacatttcagggatgaagtaaattatgatctcgagacccacctg
gacatgctcc
gccacctctaccagggctgccaggtggtgcagggaaacctggaactcacctacctgcccaccaatgccagcctgtcctt
cctgcagg
atatccaggaggtgcagggctacgtgctcatcgctcacaaccaagtgaggcaggtcccactgcagaggctgcggattgt
gcgaggc
acccagctctagaggacaactatgccctggccgtgctagacaatggagacccgctgaacaataccacccctgtcacagg
ggcctcc
ccaggaggcctgcgggagctgcagcttcgaagcctcacagagatcttgaaaggaggggtcttgatccagcggaaccccc
agctct
gctaccaggacacgattagtggaagaatatccaggagatgctggctgcaagaagatctagggagcctggcatactgccg
gagag
attgatggggacccagcctccaacactgccccgctccagccagagcagctccaagtgtagagactctggaagagatcac
aggtta
cctatacatctcagcatggccggacagcctgcctgacctcagcgtcaccagaacctgcaagtaatccggggacgaattc
tgcacaat
ggcgcctactcgctgaccctgcaagggctgggcatcagctggctggggctgcgctcactgagggaactgggcagtggac
tggccc
tcatccaccataacacccacctctgcttcgtgcacacggtgccctgggaccagctcatcggaacccgcaccaagctctg
ctccacact
gccaaccggccagaggacgagtgtgtgggcgagggcctggcctgccaccagctgtgcgcccgagggcagcagaagatcc
gga
agtacacgatgcggagactgctgcaggaaacggagctggtggagccgctgacacctagcggagcgatgcccaaccaggc
gcag
atgcggatcctgaaagagacggagctgaggaaggtgaaggtgcaggatctggcgcattggcacagtctacaagggcatc
tggatc
78
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
cctgatggggagaatgtgaaaattccagtggccatcaaagtgagagggaaaacacatcccccaaagccaacaaagaaat
cttagac
gaagcatacgtgatggctggtgtgggctccccatatgtctcccgccactgggcatctgcctgacatccacggtgcagct
ggtgacac
agcttatgccctatggctgcctcttagactaatctagacccgggccactaactcaacgctagtagtggatttaatccca
aatgagccaac
agaaccagaaccagaaacagaacaagtaacattggagttagaaatggaagaagaaaaaagcaatgatttcgtgtgaata
atgcacg
aaatcattgcttattatttaaaaagcgatatactagatataacgaaacaacgaactgaataaagaatacaaaaaaagag
ccacgaccag
ttaaagcctgagaaactttaactgcgagccttaattgattaccaccaatcaattaaagaagtcgagacccaaaatttgg
taaagtatttaat
tactttattaatcagatacttaaatatctgtaaacccattatatcgggtattgaggggatttcaagtcataagaagata
ccaggcaatcaatt
aagaaaaacttagttgattgccattttgttgtgattcaactagatcgtagcttctaactaattaattacgtaagaaagg
agaacagctgaat
gaatatccctatgttgtagaaactgtgcttcatgacggcttgttaaagtacaaatttaaaaatagtaaaattcgctcaa
tcactaccaagcc
aggtaaaagtaaaggggctatattgcgtatcgctcaaaaaaaagcatgattggcggacgtggcgttgactgacttccga
agaagcga
ttcacgaaaatcaagatacatttacgcattggacaccaaacgatatcgttatggtacgtatgcagacgaaaaccgttca
tacactaaag
gacattctgaaaacaatttaagacaaatcaataccttattattgatatgatattcacacggaaaaagaaactatttcag
caagcgatatat
aacaacagctattgatttaggattatgcctacgttaattatcaaatctgataaaggttatcaagcatattagattagaa
acgccagtctatgt
gacttcaaaatcagaatttaaatctgtcaaagcagccaaaataatctcgcaaaatatccgagaatattttggaaagtca
tgccagttgatc
taacgtgcaatcattttgggattgctcgtataccaagaacggacaatgtagaattattgatcccaattaccgttattca
tcaaagaatggc
aagattggtctttcaaacaaacagataataagggctttactcgttcaagtctaacggattaagcggtacagaaggcaaa
aaacaagtag
atgaaccctggataatctcttattgcacgaaacgaaattttcaggagaaaagggatagtagggcgcaatagcgttatgt
ttaccctctctt
tagcctactttagttcaggctattcaatcgaaacgtgcgaatataatatgatgagtttaataatcgattagatcaaccc
ttagaagaaaaag
aagtaatcaaaattgttagaagtgcctattcagaaaactatcaaggggctaatagggaatacattaccattctagcaaa
gcagggtatc
aagtgatttaaccagtaaagatttatttgtccgtcaagggtggataaattcaagaaaaaaagaagcgaacgtcaacgtg
acatttgtca
gaatggaaagaagatttaatggcttatattagcgaaaaaagcgatgtatacaagccttatttagcgacgaccaaaaaag
agattagaga
agtgctaggcattcctgaacggacattagataaattgctgaaggtactgaaggcgaatcaggaaattactttaagatta
aaccaggaag
aaatggtggcattcaacttgctagtgttaaatcattgttgctatcgatcattaaattaaaaaaagaagaacgagaaagc
tatataaaggcg
ctgacagcttcgataatttagaacgtacatttattcaagaaactctaaacaaattggcagaacgccccaaaacggaccc
acaactcgat
ttgatagctacgatacaggctgaaaataaaacccgcactatgccattacatttatatctatgatacgtgtagtattcta
gctggctagctta
attgcttatatttacctgcaataaaggatacttacttccattatactcccattaccaaaaacatacggggaacacggga
acttattgtacag
gccacctcatagttaatggtacgagccttcctgcaatctcatccatggaaatatattcatccccctgccggcctattaa
tgtgactatgtg
cccggcggatattcctgatccagctccaccataaattggtccatgcaaattcggccggcaattttcaggcgattcccac
acaaggatgt
cggtccctacaattttcggagccagccgtccgcatagcctacaggcaccgtcccgatccatgtgtcatttccgctgtgt
actcggctcc
gtagctgacgctctcgccattctgatcagatgacatgtgacagtgtcgaatgcagggtaaatgccggacgcagctgaaa
cggtatctc
gtccgac
atgtcagcagacgggcgaaggccatacatgccgatgccgaatctgactgcattaaaaaagcctatttcagccggagtcc
a
gcggcgctgttcgcgcagtggaccattagattcataacggcagcggagcaatcagctattaaagcgctcaaactgcatt
aagaaata
gcctcatcatttcatccgctgtcgcaaaatgggtaaataccccatgcactttaaacgagggttgcggtcaagaattgcc
atcacgactg
aacttcacctctgtattacaccaagtctgacatccccgtatcgaccttcagatgaaaatgaagagaacctatttcgtgt
ggcgggctgc
ctcctgaagccattcaacagaataacctgttaaggtc acgtcatactcagcagcgattgccac
atactccgggggaaccgcgccaag
79
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
caccaatataggcgccttcaatccattttgcgcagtgaaatcgcttcatccaaaatggccacggccaagcatgaagcac
ctgcgtcaa
gage agcctttgctgtttctgc atc
accatgcccgtaggcgtttgetttcacaactgccatcaagtggacatgttcaccgatatgttttttc a
tattgctgacattttcattatcgcggacaagtcaatttccgcccacgtatctctgtaaaaaggttngtgctcatggaaa
actectctctnnt
cagaaaatcccagtacgtaattaagtatttgagaattaattttatattgattaatactaagtttacccagttttcacct
aaaaaacaaatgatga
gataatagctccaaaggctaaagaggactataccaactatttgttaattaa (SED ID NO: 53)
EXAMPLE 2:
ADXS31-164 IS AS IMMUNOGENIC AS LM-LLO-ChHER2.
[00349] Immunogenic properties of ADXS31-164 in generating anti-Her-2/neu
specific
cytotoxic T cells were compared to those of the Lin-LLO-ChHer2 vaccine in a
standard CTL
assay. Both vaccines elicited strong but comparable cytotoxic T cell responses
toward Her-
2/neu antigen expressed by 3T3/neu target cells. Accordingly, mice immunized
with a
Listeria expressing only an intracellular fragment of Her2-fused to LLO showed
lower lytic
activity than the chimeras which contain more MHC class I epitopes. No CTL
activity was
detected in naïve animals or mice injected with the irrelevant Listeria
vaccine (Figure 2A).
ADXS31-164 was also able to stimulate the secretion of IFN-y by the
splenocytes from wild
type FVB/N mice (Figure 2B). This was detected in the culture supernatants of
these cells
that were co-cultured with mitomycin C treated NT-2 cells, which express high
levels of
Her-2/neu antigen (Figure 5C).
[00350] Proper processing and presentation of the human MHC class I epitopes
after
immunizations with ADXS31-164 was tested in HLA-A2 mice. Splenocytes from
immunized HLA-A2 transgenics were co-incubated for 72 hours with peptides
corresponding to mapped HLA-A2 restricted epitopes located at the
extracellular
(HLYQGCQVV SEQ ID NO: 11 or KIFGSLAFL SEQ ID NO: 12) or intracellular
(RLLQETELV SEQ ID NO: 13) domains of the Her-2/neu molecule (Figure 2C). A
recombinant ChHer2 protein was used as positive control and an irrelevant
peptide or no
peptide as negative controls. The data from this experiment show that ADXS31-
164 is able
to elicit anti-Her-2/neu specific immune responses to human epitopes that are
located at
different domains of the targeted antigen.
EXAMPLE 3:
ADXS31-164 WAS MORE EFFICACIOUS THAN LM-LLO-ChHER2 IN
PREVENTING THE ONSET OF SPONTANEOUS MAMMARY TUMORS.
[00351] Anti-tumor effects of ADXS31-164 were compared to those of Lm-LLO-
ChHer2 in
Her-2/neu transgenic animals which develop slow growing, spontaneous mammary
tumors at
20-25 weeks of age. All animals immunized with the irrelevant Listeria-control
vaccine
developed breast tumors within weeks 21-25 and were sacrificed before week 33.
In contrast,
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Listeria-Her-2Ineu recombinant vaccines caused a significant delay in the
formation of the
mammary tumors. On week 45, more than 50% of ADXS31-164 vaccinated mice (5 out
of
9) were still tumor free, as compared to 25% of mice immunized with Lm-LLO-
ChHer2. At
week 52, 2 out of 8 mice immunized with ADXS31-164 still remained tumor free,
whereas
all mice from other experimental groups had already succumbed to their disease
(Figure 3).
These results indicate that despite being more attenuated, ADXS31-164 is more
efficacious
than Lm-LLO-ChHer2 in preventing the onset of spontaneous mammary tumors in
Her-2/neu
transgenic animals.
EXAMPLE 4:
MUTATIONS IN HER-2/NEU GENE UPON IMMUNIZATION WITH ADXS31-164.
[00352] Mutations in the MHC class I epitopes of Her-2/neu have been
considered
responsible for tumor escape upon immunization with small fragment vaccines or
trastuzumab (Herceptin), a monoclonal antibody that targets an epitope in the
extracellular
domain of Her-2/neu. To assess this, genomic material was extracted from the
escaped
tumors in the transgenic animals and sequenced the corresponding fragments of
the neu gene
in tumors immunized with the chimeric or control vaccines. Mutations were not
observed
within the Her-2/neu gene of any vaccinated tumor samples suggesting
alternative escape
mechanisms (data not shown).
EXAMPLE 5:
ADXS31-164 CAUSES A SIGNIFICANT DECREASE IN INTRA-TUMORAL T
REGULATORY CELLS.
[00353] To elucidate the effect of ADXS31-164 on the frequency of regulatory T
cells in
spleens and tumors, mice were implanted with NT-2 tumor cells. Splenocytes and
intra-
tumoral lymphocytes were isolated after three immunizations and stained for
Tregs, which
were defined as CD3 /CD4/CD25 /FoxP3+ cells, although comparable results were
obtained with either FoxP3 or CD25 markers when analyzed separately. The
results indicated
that immunization with ADXS31-164 had no effect on the frequency of Tregs in
the spleens,
as compared to an irrelevant Listeria vaccine or the naïve animals (See Figure
4). In contrast,
immunization with the Listeria vaccines caused a considerable impact on the
presence of
Tregs in the tumors (Figure 5A). Whereas in average 19.0% of all CD3+ T cells
in untreated
tumors were Tregs, this frequency was reduced to 4.2% for the irrelevant
vaccine and 3.4%
for ADXS31-164, a 5-fold reduction in the frequency of intra-tumoral Tregs
(Figure 5B).
The decrease in the frequency of intra-tumoral Tregs in mice treated with
either of the
LmddA vaccines could not be attributed to differences in the sizes of the
tumors. In a
representative experiment, the tumors from mice immunized with ADXS31-164 were
81
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
significantly smaller [mean diameter (mm) SD, 6.71 0.43, n=51 than the tumors
from
untreated mice (8.69 0.98, n=5, p<0.01) or treated with the irrelevant vaccine
(8.41 1.47,
n=5, p=0.04), whereas comparison of these last two groups showed no
statistically
significant difference in tumor size (p=0.73). The lower frequency of Tregs in
tumors treated
with LmddA vaccines resulted in an increased intratumoral CD8/Tregs ratio,
suggesting that
a more favorable tumor microenvironment can be obtained after immunization
with LmddA
vaccines. However, only the vaccine expressing the target antigen Her-2/neu
(ADXS31-164)
was able to reduce tumor growth, indicating that the decrease in Tregs has an
effect only in
the presence on antigen-specific responses in the tumor.
EXAMPLE 6:
NO ESCAPE MUTATIONS WERE INTRODUCED BY LISTERIA VACCINE
EXPRESSING HER-2 CHIMERA.
[00354] Tumor samples of the mice immunized with different vaccines such as Lm-
LLO-
138, LmddA164 and irrelevant vaccine Lm-LLO-NY were harvested. The DNA was
purified from these samples and the DNA fragments corresponding to Her-2/neu
regions
IC1, EC1 and EC2 were amplified and were sequenced to determine if there were
any
immune escape mutations. The alignment of sequence from each DNA was performed
using
CLUSTALW. The results of the analysis indicated that there were no mutations
in the DNA
sequences harvested from tumors. The reference sequences are listed below:
[00355] Alignment of EC2 (975 -1029 bp of Her-2-neu)
[00356] GGTCACAGCTGAGGACGGAACACAGCGTTGTGAGAAATGCAGCAAGC
CCTGTGCT (SEQ ID NO:14)
[00357] CGAGTGTGCTATGGTCTGGGCATGGAGCACCTTCGAGGGGCGAGGGCC
ATCACCAGTGAC (SEQ ID NO:15)
[00358] AATGTCCAGGAGTTTGATGGCTGCAAGAAGATCTTTGGGAGCCTGGCA
TTTTTGCCGGAG (SEQ ID No:16)
[00359] AGCTTTGATGGGGACCCCTCCTCCGGCATTGCTCCGCTGAGGCCTGAGC
AGCTCCAAGTG (SEQ ID No:17)
[00360] TTCGAAACCCTGGAGGAGATCACAGGTTACCTGTACATCTCAGCATGG
CCAGACAGTCTC (SEQ ID NO: 18)
[00361] CGTGACCTCAGTGTCTTCCAGAACCTTCGAATCATTCGGGGACGGATTC
TCCACGATGGC (SEQ ID NO: 19)
[00362] GCGTACTCATTGACACTGCAAGGCCTGGGGATCCACTCGCTGGGGCTG
CGCTCACTGCGG (SEQ ID NO: 20)
[00363] GAGCTGGGCAGTGGATTGGCTCTGATTCACCGCAACGCCCATCTCTGCT
82
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
TTGTACACACT (SEQ ID NO: 21)
[00364] GTACCTTGGGACCAGCTCTTCCGGAACCCACATCAGGCCCTGCTCCAC
AGTGGGAACCGG (SEQ ID NO: 22)
[00365] CC GGAAGAGGATTGTGGTCTC GAGGGCTTGGTCTGTAACTCACTGTGT
GCCCACGGGCAC (SEQ ID NO: 23)
[00366] TGCTGGGGGCCAGGGCCCACCCAGTGTGTCAACTGCAGTCATTTCCTTC
GGGGCCAGGAG (SEQ ID NO: 24)
[00367] Alignment of IC1 (2114-3042 bp of Her-2-neu)
[00368] CGCCCAGC GGAGCAATGCCCAACCAGGCTCAGATGCGGATCCTAAAAG
AGACGGAGC (SEQ ID NO: 25)
[00369] TAAGGAAGGTGAAGGTGCTTGGATCAGGAGCTTTTGGCACTGTCTACA
AGGGCATCTGGA (SEQ ID NO: 26)
[00370] TCCCAGATGGGGAGAATGTGAAAATCCCCGTGGCTATCAAGGTGTTGA
GAGAAAACACAT (SEQ ID NO: 27)
[00371] CTCCTAAAGCCAACAAAGAAATTCTAGATGAAGCGTATGTGATGGCTG
GTGTGGGTTCTC (SEQ ID NO: 28)
[00372] CGTATGTGTCCCGCCTCCTGGGCATCTGCCTGACATCCACAGTACAGCT
GGTGACACAGC (SEQ ID NO: 29)
[00373]
TTATGCCCTACGGCTGC CTTCTGGACCATGTCCGAGAACACCGAGGTCGCCTAG
GCTCCC (SEQ ID NO: 30)
[00374] AGGACCTGCTCAACTGGTGTGTTCAGATTGCCAAGGGGATGAGCTACC
TGGAGGACGTGC(SEQ ID NO: 31)
[00375] GGCTTGTACACAGGGACCTGGCTGCCCGGAATGTGCTAGTCAAGAGTC
CCAACCACGTCA(SEQ ID NO: 32)
[00376] AGATTACAGATTTCGGGCTGGCTCGGCTGCTGGACATTGATGAGACAG
AGTACCATGCAG (SEQ ID NO: 33)
[00377] ATGGGGGCAAGGTGCCCATCAAATGGATGGCATTGGAATCTATTCTCA
GACGCCGGTTCA(SEQ ID NO: 34)
[00378] CCCATCAGAGTGATGTGTGGAGCTATGGAGTGACTGTGTGGGAGCTGA
TGACTTTTGGGG (SEQ ID NO: 35)
[00379] CCAAACCTTACGATGGAATCCCAGCCCGGGAGATCCCTGATTTGCTGG
AGAAGGGAGAA (SEQ ID NO: 36)
[00380] CGCCTACCTCAGCCTCCAATCTGCACCATTGATGTCTACATGATTATGG
TCAAATGTT (SEQ ID NO: 37)
83
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00381] GGATGATTGACTCTGAATGTCGCCCGAGATTCCGGGAGTTGGTGTCAG
AATTTT (SEQ ID NO: 38)
[00382] CACGTATGGCGAGGGACCCCCAGCGTTTTGTGGTCATCCAGAACGAGG
ACTT (SEQ ID NO: 39)
[00383] Alignment of EC1 (399-758 bp of Her-2-neu)
[00384] CCCAGGCAGAACCCCAGAGGGGCTGCGGGAGCTGCAGCTTCGAAGTCT
CACAGAGATCCT (SEQ ID NO: 40)
[00385] GAAGGGAGGAGTTTTGATCCGTGGGAACCCTCAGCTCTGCTACCAGGA
CATGGTTTTGTG (SEQ ID NO: 41)
[00386] CCGGGCCTGTCCACCTTGTGCCCCCGCCTGCAAAGACAATCACTGTTGG
GGTGAGAGTCC (SEQ ID NO: 42)
[00387] GGAAGACTGTCAGATCTTGACTGGCACCATCTGTACCAGTGGTTGTGC
CCGGTGCAAGGG (SEQ ID NO: 43)
[00388] CCGGCTGCCCACTGACTGCTGCCATGAGCAGTGTGCCGCAGGCTGCAC
GGGCCCCAAGCA (SEQ ID NO: 44)
EXAMPLE 7:
PERIPHERAL IMMUNIZATION WITH ADXS31-164 CAN DELAY THE
GROWTH OF A METASTATIC BREAST CANCER CELL LINE IN THE
BRAIN.
[00389] Mice were immunized IP with ADXS31-164 or irrelevant Lm-control
vaccines and
then implanted intra-cranially with 5,000 EMT6-Luc tumor cells, expressing
luciferase and
low levels of Her-2/neu (Figure 6C). Tumors were monitored at different times
post-
inoculation by ex vivo imaging of anesthetized mice. On day 8 post-tumor
inoculation,
tumors were detected in all control animals, but none of the mice in ADXS31-
164 group
showed any detectable tumors (Figure 6A and B). ADXS31-164 could clearly delay
the
onset of these tumors, as on day 11 post-tumor inoculation, all mice in the
negative control
group had already succumbed to their tumors, but all mice in ADXS31-164 group
were still
alive and only showed small signs of tumor growth. These results strongly
suggest that the
immune responses obtained with the peripheral administration of ADXS31-164
could
possibly reach the central nervous system and that LmddA-based vaccines might
have a
potential use for treatment of CNS tumors.
EXAMPLE 8:
TREATMENT OF CANINE OSTEASARCOMA BY IMMUNIZATION WITH ADXS31-
164.
84
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00390]
Canine Osteosarcoma is a cancer of long (leg) bones that is a leading killer
of
large dogs over the age of 10 years. Standard treatment is amputation
immediately after
diagnosis, followed by chemotherapy. Invariably, however, the cancer
metastasizes to the
lungs. With chemotherapy, dogs survive about 18 months compared to 6-12
months, without
treatment. The HER2 antigen is believed to be present in up to 50% of
osteosarcoma.
ADXS31-164 creates an immune attack on cells expressing this antigen and has
been
developed to treat human breast cancer.
[00391]
Dogs with a histological diagnosis of osteosarcoma and evidence of
expression of HER2/neu by malignant cells are eligible for enrollment.
Canine Osteosarcoma Trial
[00392] In
the first regiment the limbs are amputated, followed by round of
chemotherapy treatment. 3 doses of Her-2 vaccine are subsequently administered
with or
without a 6 month interval booster.
[00393] All
dogs are to receive 4 weeks of carboplatin therapy. Four weeks after the
last carboplatin dose, dogs are to receive ADXS-HER2 once every three weeks
for a total of
3 doses. Group 1 (3 dogs) receive 1x108 CFU per dose, Group 2 (3 dogs) each
receive 5x108
CFU per dose and Group 3 (3 dogs) receives 1x109 CFU per dose. Additional dogs
are added
to a Group to gather more data should if a potentially dose limiting
toxicities, be observed.
Therefore 9-18 dogs may be treated in the initial study.
[00394] In the second
regiment, the same as the first regiment is repeated with the
exception that only a single dose of vaccine is administered before
chemotherapy (1 month
before)for a total of 4 doses.
[00395]
Further, in both regiments a single dose is administered a month after
chemotherapy.
EXAMPLE 9:
PHASE 1 DOSE ESCALATION STUDY EVALUATING THE SAFETY OF ADXS-
cHER2 IN COMPANION DOGS WITH HER-2/NEU OVEREXPRESSING CANINE
OSTEOSARCOMA.
[00396] A pilot phase I dose escalation study was performed to determine the
dose of a L.
monocytogenes expressing human Her-2/neu recombinant vaccine that can safely
and
effectively stimulate tumor-specific immunity in dogs with osteosarcoma. The
tumors of all
dogs presenting to PennVet for limb amputation due to suspected or confirmed
OSA were
routinely harvested and evaluated histopathologically to confirm the diagnosis
of OSA. In
addition, tumor sections from all dogs were evaluated by IHC and Western blot
analysis to
determine whether the tumor expresses Her-2/neu. Only dogs with a histological
diagnosis of
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
OSA and evidence of expression of Her-2/neu by malignant cells were eligible
for
enrollment. Single cell suspensions of tumor tissue taken at surgery were
cryopreserved and
used as autologous tumor targets in chromium release assays to determine anti-
tumor
immunity.
[00397] Up to 18 privately owned dogs with appendicular OSA and confirmed
expression
of Her2-neu were enrolled (Figure 7). At enrollment (3 weeks post last
carboplatin
treatment), all dogs received basic clinical laboratory tests including a
Complete Blood
Count (CBC), Chemistry Screen (CS) and urinalysis (UA) and a baseline
evaluation of
cardiac function by echocardiography and measurement of cardiac-specific
Troponin I
(cTnI) levels. Thoracic radiographs were taken to determine whether pulmonary
metastases
are present. Only dogs with no evidence of pulmonary metastases were eligible
for inclusion
in the study. At the time of enrollment, peripheral blood mononuclear cells
(PBMCs) are
collected to assess baseline levels of anti-tumor immunity (see Assessment of
anti-tumor
immunity). Furthermore, blood was taken to evaluate baseline immune function
to ensure
they were no longer immune suppressed by carboplatin. Only dogs with
functionally intact
immune systems were eligible to receive the Listeria vaccine.
[00398] Lm recombinant dosing and data capture
[00399] All dogs were vaccinated using a single Lm-huHer-2/neu recombinant
vaccine. The
first Lm-huHer2-neu vaccine were given three weeks after the last carboplatin
dose and were
given once every three weeks after this for a total of 3 doses (Figure 7).
[00400] Group 1 (3 dogs) received the ADXS31-164 (Lm-hucHer-2/neu) vaccine at
1 x108
CFU per dose, Group 2 (3 dogs) each received 5x108 CFU per dose, Group 3 (3
dogs)
receive 1x109 CFU per dose, and 3.3 x 109 CFU per dose (1 dog). Recombinant Lm
was
administered as a slow intravenous infusion over 30 minutes. The dose chosen
for Group 1 is
the established safe dose for the chimeric huHer-2/neu recombinant in mice. In
humans, the
non-toxic dose for Lm-LLO-E7 is only one log higher than that established in
mice, and this
dose is the dose evaluated in Group 3 in this pilot trial.
[00401] At the time of Lm administration, dogs were monitored for evidence of
systemic
adverse effects. During infusion, heart rate and rhythm was monitored by ECG
and
respiratory rate were recorded. Further, heart damage was monitored using
ultrasound and by
measuring Troponin I levels (Figure 8). Following infusion, dogs were
monitored closely for
48 hours. Core body temperature was monitored continuously for <12 hours post
infusion
using the Vital Sense continuous body temperature monitoring system by
MiniMitter
Respironics (routinely used in our Veterinary Clinical Trials Center, VCIC).
Pulse rate,
rhythm and quality, respiratory rate and effort, were monitored and recorded
every hour for
86
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
the first 6 hours then every 4 hours thereafter, as well as blood pressure and
temperature
(Figure 9). All symptoms consistent with immune stimulation are noted and
fluids,
analgesics, anti-emetics and anti-histamines are used as necessary to control
severe reactions.
All dogs were observed six times a day and any signs of toxicological effects
of the
recombinants including discomfort, lethargy, nausea, vomiting and diarrhea
were recorded.
Blood samples were taken at 24, 48 and 72 hours after the first ADXS31-164
vaccine for
cultures to assess the clearance of Lm after systemic administration.
[00402] Assessment of anti-tumor immunity
[00403] Three weeks following the last carboplatin dose, dogs receive a
routine clinical
examination and baseline blood work including CBC, CS, UA and cTnI levels.
PBMCs are
taken at this time for baseline evaluation of anti-tumor immunity. Repeat
immune assessment
is performed at the time of each vaccination and three weeks after the last
vaccination.
PBMCs are analyzed for Her-2/neu specific T cell responses by CFSE
proliferation, cytokine
production (ELISpot and qRT-PCR) and CTL assay against autologous tumor
targets as
outlined below (Figure 12).
Results
[00404] To date, we have performed a total of 41 infusions of ADXS31-164 in 16
dogs.
Number of Number of Rationale
dogs infusions
1 5 Two additional infusions post priming series to
treat metastatic disease
4 4 One additional infusion post priming series to
maintain tumor free status
4 3 Finished scheduled priming series
1 2 Succumbed to metastatic disease prior to finish of
priming course
2 1 Succumbed to metastatic disease prior to finish of
priming course
4 1 Priming course of vaccinations underway
[00405] ADXS31-164 dose has ranged from 1 x 108, 5 x 108, 1 x 109 and 3.3 x
109 CFU.
Dose Total number of doses Number of Reported side effects
received administered dogs
1 x 108 9 3 Fever,
nausea, vomiting, elevated liver
87
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
enzymes
x 108 9 3
Fever, nausea, vomiting, elevated liver
enzymes
1 x 109 17 10
Fever, nausea, vomiting, elevated liver
enzymes, thrombocytopenia
3.3 x 109 1 1 Nausea, vomiting,
[00406] Standard operating procedure for vaccine administration
[00407] A standard operating procedure was developed for the administration of
ADXS31-
164. One hour prior to vaccination, patients receive 2 mg/kg diphenhydramine
via
5
intramuscular injection and 0.2mg/kg ondansetron as a slow intravenous push.
The vaccine
was kept at -80 C and thawed patient-side. It was administered in 200mls of
0.9% NaC1 over
30 mins. The infusion line is then flushed with 30 mls of Plasmalyte. Dogs are
sent home
with a three day course of amoxicillin (to start 72 hours post vaccination)
and a 7 day course
of liver supplement (S-adenosyl-methionine) that aids in cellular growth and
repair.
[00408] The primary endpoint of the study was to deteimine the maximum
tolerated dose of
ADXS31-164.
[00409] Doses up to 3.3 x 109 were well tolerated in dogs ranging in body
weight from 25kg
to 67kg. All side effects reported were grade I toxicities and the maximum
tolerated dose has
yet to be reached. Side effects routinely occurred within 2-4 hours of vaccine
administration.
High fevers usually resolved with intravenous isotonic fluids delivered at
maintenance rate
(4m1s/kg/hour) for 2-4 hours. In two cases where fevers reached 104.7 and
above, a single
subcutaneous injection of carprofen induced normothermia within 1-2 hours.
Nausea and
vomiting was usually self-limiting but in cases where several episodes are
noted, 1 mg/kg
cerenia is administered and this was very effective at preventing further
nausea and vomiting.
A total of 5 dogs developed mild, grade I elevations in liver enzymes within
48 hours of
vaccine administration ¨ these resolved by one week post vaccination.
[00410] Clearance of Listeria
[00411] After performing blood cultures on all 16 dogs vaccinated to date
there was no
detectable Listeria in the peripheral circulation of any of the dogs at 24
hours post
vaccination. Shedding of Listeria in the urine and feces of vaccinated dogs
was not assessed.
[00412] Secondary endpoints for the study are progression-free survival and
overall
survival. A statistically significant overall survival advantage in dogs with
osteosarcoma has
been observed when ADXS31-164 is administered after limb amputation and 4
doses of
carboplatin. Early results from the first two dose groups (6 dogs) show a
significant survival
88
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
advantage in dogs that received ADXS31-164 compared to 6 dogs whose owners
elected not
to participate in the trial but who were followed for survival (p=0.003)
(Figure 13). The mean
survival time for unvaccinated dogs is 239.5 days. The mean survival time for
vaccinated
dogs has not yet been reached. This remains true when all dogs within the
intent to treat
group are included in analysis.
[00413] In conclusion, there was no evidence of significant short or long-term
side effects
on the cardiovascular, hematopoietic, hepatic, or renal systems. Moreover,
administration of
ADXS31-164 in the presence of minimal residual disease can delay/prevent
metastatic
disease and prolong overall survival of dogs with Her-2/neu positive
osteosarcoma.
EXAMPLE 10:
PHASE 1 CLINICAL TRIAL EVALUATING ADXS31-164 IN THE SPONTANEOUS
CANINE MODEL OF OSTEROSARCOMA (OSA).
[00414] Vaccine manufacture
[00415] Design and generation of ADXS31-164. Briefly, the dal dat actA mutant
strain of
Listeria monocytogenes (Lm) was transfected with the pADV plasmid carrying a
chimeric
human HER2/neu construct. The construct contains 2 extracellular domains (EC1
and EC2)
and one intracellular domain (IC1) of the human HER2/neu molecule that contain
the
majority of HLA-A2 restricted immunodominant epitopes, fused to a truncated
listeriolysin
0 construct. The transfer plasmid also contains the bacillus p60 dal gene and
is maintained
within the mutant Lm via auxotrophic complementation. There is no bacterial
resistance
cassette. Vaccines were manufactured by Vibalogics GmbH (Cuxhaven, Germany)
and
stored at -80 C prior to use.
[00416] Histopathology, Staging and Immunohistochemistry
[00417] Histopathological assessment of all primary appendicular osteosarcoma
tumors was
performed by a board certified veterinary pathologist (J.E.). Tumors were
described as
osteoblastic, chondroblastic, fibroblastic and telangiectatic based on
histological features.
Primary tumors were scored based on mitotic index, nuclear pleomorphism and
the amount
of matrix and necrosis present. Histological scores were converted into a
grade (I, II or III).
[00418] For HER2/neu staining, 5 micron thick serial sections of formalin
fixed, decalcified,
paraffin embedded tissues were mounted on negatively charged glass slides.
Sections were
heated at 80 C for 20 minutes, immersed in Pro Par (clearant) and rehydrated
in ethanol.
Antigen retrieval was performed by boiling sections in sodium citrate buffer
(pH ¨9.0).
Endogenous peroxidase was blocked using 3% hydrogen peroxide. Staining was
performed
with a rabbit anti-human HER2/neu antibody (Neu(c-18):sc-284, Santa Cruz
Biotecnology)
or a rabbit IgG isotype (Universal Negative Control serum, NC498, Biocare
Medical).
89
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
Bound antibody was detected using the Universal Streptavadin-Biotin2 System
(DAKO/LSAB2, HRP). Tissues were stained with 3,3'-diaminobenzidine solution
(DAKO)
and counterstained with hematoxylin. Slides were viewed using a Nikon E600
infinity
corrected upright microscope. Bright field images were acquired using a Nikon
Digital Sight
DS-Fi 1 color camera and a NIS-Element BR3.0 for image analysis. Tissue
sections were
evaluated and scored for HER2/neu positivity by a board certified pathologist
(J.E.) based on
the percentage of neoplastic cells staining for HER2/neu (<10% = 1, 10%-50% =
2, >50% =
3) and the intensity of HER2/neu staining (weak = 1, moderate = 2, strong =
3). Scores were
based on cells analyzed within 10 hpf for each tissue section. A combined
HER2/neu score
was obtained by multiplying the two separate scores given for percentage of
tumor cells
positive for HER2/neu staining and HER2/neu staining intensity. Only dogs with
greater than
10% of their tumor cells staining positive for HER2/neu were eligible for
trial enrollment.
[00419] Eligibility Criteria and Clinical Trial Design
[00420] Dogs with a histopathological and immunohistochemical diagnosis of
HER2/neu
positive OSA that had undergone primary tumor removal either by limb
amputation or limb-
sparing surgery and had received 4 doses of 300mg/m2 carboplatin given once
every 3 weeks
(or once every 4 weeks if myelosuppression occurred) as adjuvant chemotherapy
were
eligible for screening. Dogs were screened three weeks after their last
carboplatin treatment.
A thorough physical examination, Complete Blood Count (CBC), Chemistry Screen
(CS)
and Urinalysis (UA) were performed to determine general health status. Basic
innate and
adaptive immune function was tested using a flow cytometric neutrophil
oxidative burst
assay and mitogen-induced lymphocyte proliferation assay respectively.
Baseline cardiac
status was evaluated by electrocardiography, echocardiography and serum
cardiac troponin I
levels. Thoracic radiographs were performed to determine the presence of
pulmonary
metastatic disease (see Figure 14B). Only those dogs found to be systemically
healthy with
intact innate and adaptive immune function, no evidence of underlying cardiac
disease and
no evidence of pulmonary metastatic disease were eligible for enrollment. Dogs
that died
during the course of the study underwent necropsy. The presence and location
of metastatic
disease was recorded and histopathology and immunohistochemistry to evaluate
HER2/neu
expression in metastatic lesions were performed.
[00421] Immune analysis
[00422] Neutrophil oxidative burst assay. Red blood cells in sodium heparin
anti-coagulated
blood were lysed using 0.83% NH4C1 and the remaining white blood cells were
washed
twice in 1 x PBS. Cells were labeled with 15ug/m1 of dihydrorhodamine 123 (DHR-
123;
Molecular Probes, Grand Island, NY) and activated with 3 nM phorbol 12-
myristate 13-
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
acetate (PMA, Sigma, St. Louis, MO) for 30 minutes at 37 C. Cells were placed
on ice for 15
minutes prior to flow cytometric analysis. Cells were acquired on a FACS Canto
cytometer
(BD Biosciences, San Jose, CA) and analyzed using FloJo software (Treestar,
San Carlos,
CA).
[00423] Lymphocyte proliferation assay. Peripheral Blood Mononuclear Cells
(PBMCs)
were isolated from sodium heparin anti-coagulated whole blood by density
centrifugation.
PBMCs were washed twice in 1 x PBS and counted. Cells were labeled with 5uM
CFSE and
stimulated with 1.25uM Concanavalin A at 37 C for 5 days. Cells were
harvested, washed
twice in FACS buffer, labeled with APC-conjugated rat anti-canine CD4 and PE
conjugated
rat anti-canine CD8 antibodies (Serotec, Raleigh, NC) and analyzed by flow
cytometry. For
immune function analysis, peripheral blood taken from healthy colony dogs
(IACUC
#804197) was used as a positive control.
[00424] T cell subset analysis. PBMCs taken at baseline, prior to each
vaccination, at re-
stage and at every 2 months thereafter were analyzed for CD4 and CD8 T cell
subsets.
Briefly, cryopreserved cells were thawed and washed twice in FACS buffer (lx
PBS, 0.2%
BSA fraction V, and 4 mM sodium azide) prior to surface staining with mouse
anti-canine
CD3, PE-labeled rat anti-dog CD8 or Alexa-labeled rat anti-dog CD4 (Serotec,
Raleigh,
NC). Cells were incubated with the vital dye 7-ADD immediately prior to flow
cytometric
acquisition. Total CD4 + and CD8 + T cell numbers were calculated from the
flow cytometric
percentages and total lymphocyte counts determined using a Cell Dyn 370005
Hematology
analyzer.
[00425] Vaccine Administration
[00426] Prior to vaccination, dogs received the 5HT3 antagonist ondansetron
(0.2mg/kg)
intravenously and the H1 receptor blocker, diphenhydramine (2mg/kg)
intramuscularly to
prevent nausea and anaphylaxis respectively. A standard 3+3 clinical trial
design was
employed. ADXS31-164 was administered at the following doses; Group 1 (2 x 108
CFU) ,
Group 2 (5 x 108 CFU), Group 3 (1 x 109 CFU) and Group 4 (3.3 x 109 CFU).
ADXS31-164
was diluted in 100mls 0.9% NaC1 (Groups 1 and 2) and 200mls 0.9% NaC1 (Groups
3 and 4)
and administered intravenously over 30 minutes. Temperature, pulse,
respiratory rate, heart
rate and rhythm (by EKG) and blood pressure were monitored every hour
following infusion.
In cases where body temperature exceeded 103 F, dogs were placed on
intravenous
Plasmalyte at 4m1s/kg/hr until their temperature fell below 103 F. Dogs were
monitored
every hour for signs of lethargy, nausea or vomiting. Blood samples were drawn
24 hours
and one week post vaccination to assess for any changes in hematological or
biochemical
parameters and blood cultures were performed at 24 hours post vaccination to
determine
91
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
persistance of live bacteria in the blood stream. All dogs received a short
course of
amoxycillin and S-Adenosylmethionine (SAMe) 72 hours after vaccination to kill
any
remaining listeria and provide anti-oxidant support to the liver.
[00427] Owners with dogs that were free of metastatic disease at least 5
months after
receiving the last vaccine in the initial series were offered the option to
receive a booster
vaccine at a standard dose of 1 x 109 CFU. Booster vaccines were administered
as described
and dogs were monitored after infusion as described above.
[00428] Toxicity
[00429] Toxicity was graded according to the Veterinary Co-operative Oncology
Group-
Common Terminology Criteria for Adverse Events (VCOG-CTCAE). Assessment of
cardiac
toxicity was performed through serial electrocardiograms, echocardiograms and
serum
cardiac troponin I levels at baseline, at the time of each vaccination, 3
weeks after the last
vaccination and every 2 months thereafter until death. Parameters assessed
included Left
Ventricular Fractional Shortening (LVFS) and Left Ventricular Internal
Dimension in
diastole (LVIDd) and Left Ventricular Internal Dimension in systole (LVIDs).
LVIDd and
LVIDs were normalized to body weight to account for the wide range of body
size amongst
dogs.
[00430] ELISpot analysis
[00431] Cryopreserved PBMC from each indicated time point were thawed, rested
overnight at 37 C and then counted. Cells were stimulated with 2.5 uM pools
of overlapping
human HER2/Neu peptides (11mers overlapping by 5 amino acids) that represent
the EC1,
EC2 and IC1 domains of HER2/Neu present in the chimeric vaccine, and
recombinant
human IL-2 (Invitrogen, Fredrick, MD) for 5 days. Cells were harvested, washed
twice in 1 x
PBS and counted. IFN-y ELISpot assays were performed according to the
manufacturer' s
protocol using a commercial canine IFN-y ELISpot assay kit (R&D Systems,
Minneapolis,
MN). Briefly, 0.8 - 2 x 105 stimulated cells were incubated with 2.5 uM of
EC1, EC2 or IC1
peptide pools plus IL-2 or IL-2 alone (to determine background counts). All
assays were
performed in duplicates. Plates were developed according to the manufacturer's
instructions.
Spots were counted using a CTL-Immunospot analyzer (C.T.L, Shaker Heights,
OH).
[00432] Primary and Secondary Outcome Measures
[00433] Time To Metastasis (TTM) was calculated as the time between amputation
and
development of metastatic disease. OSA Specific Survival was calculated as the
time
between amputation and death. Patients that died of unrelated causes were
censored at the
time of their death.
RESULTS
92
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00434] Eighteen dogs that fulfilled the eligibility criteria were enrolled in
this phase I
clinical trial. The age, breed, sex, tumor location, subtype, grade and
HER2/neu status were
recorded (Table 4). A standard 3+3 clinical trial design was employed. ADXS31-
164 was
administered at the following doses; Group 1: 2 x 108 CFU (n=3), Group 2: 5 x
108 CFU
(n=3), Group 3: 1 x 109 CFU (n=9), and Group 4: 3 x 109 CFU (n=3). Five
additional dogs
with pre-existing pulmonary metastatic disease, identified at the time of
screening also
received ADXS31-164 on a compassionate care basis (Table 4). Four of these
dogs had
strong HER2/neu staining in >50% of neoplastic cells from their primary tumor.
Three of
these dogs had multiple pulmonary metastatic nodules and two dogs had a single
metastatic
nodule at screening. Dogs with multiple pulmonary nodules received one vaccine
each
before disease progression and withdrawal from the study for alternative
treatments. The two
dogs with single nodules received the full course of three vaccines each. Dogs
with pre-
exisiting metastatic disease received either 1 x 109 CFU (n=3) or 3 x 109 CFU
(n=2)
ADXS31-164 (Table 5).
[00435] Figure 15 shows a schematic of the time-line of the phase 1 clinical
trial, wherein
three vaccinations were administered following amputation and follow-up
chemotherapy.
[00436] Table 4: Signalment and tumor characteristics of enrolled dogs
OVERALL
HER2 SURVIVAL
AGE BREED SEX TUMOR LOCATION SUBTYPE GRADE SCORE
DOSE (days)
12.5 American Pit Bull FS Proximal humerus Osteoblastic E
2 2 x 10,8 738
11.5 Mixbreed FS Distal radius
OsteoblasticI 5 2 x 10^8 267
9 Labrador MC Proximal humerus Fibroblastic
II 7.5 2 x 10^8 977+
...............................................................................
...............................................................................
...............................................................................
...............................................................................
...............................................................................
...........................................................,
6 Mixbreed FS Distal tibia Osteoblastic I
4.5 5 x 10^8 943+
7 Rottwe i ler MC Distal ulna r Osteoblastic III
2.25 5 x 10^8 925+
4.5 English Bulldog MC Proximal humerus
OsteoblasticI 4 5 x 10^8 346
6 OES MC Distal femur Osteoblastic II
1.5 1 x 10^9 744+
9 Greyhound MC Proximal humerus Osteoblastic II
5 1 x 10^9 444
8 Golden Retriever MC Distal ulna r
FibroblasticI 3 1 x 10^9 488+
2 Labrador FS Proximal tibia
FibroblasticI 4.5 1 x 10^9 438+
7.5 Cavalier King Charles FS Proximal tibia
Osteoblastic II 7.5 1 x 10^9 439+
6.5 Golden Retriever FS Distal radius
OsteoblasticI 4.5 1 x 10^9 430+
10 Greyhound MC Distal femur
OsteoblasticII 2 1 x 10^9 276
5.5 Labrador MC Distal femur Osteoblastic I 9
1 x 10^9 312+
9 Golden Retriever FS Distal femur
OsteoblasticI 6 1 x 10^9 336+
6.6 Great Dane MC Distal radius Osteoblastic II
7.5 3 x 10^9 259
7 Mixbreed MC Proximal humerus Osteoblastic II
9 3 x 10^9 345+
6.5 Rottweiler FS Proximal humerus Osteoblastic II
6 3 x 10^9 332+
[00437] Table 5: Signalment and tumor
characteristics
of dogs with pre-existing metastatic disease treated on a compassionate care
basis.
93
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
OVERALL
HER2 SURVIVAL
AGE BREED SEX TUMOR LOCATION SUBTYPE GRADE SCORE
DOSE (days)
Neopolitan Mastiff MC Distal radius Fibroblastic I 7.50 1
x 10^9 233
6.5 Great Dane FS Distal radius 6 1 x 10"9 256
2 Labrador F Proximal fibula Osteoblastic III 7.5
1 x 10^9 153
6.5 Bernese Mountain Dog FS Distal ulnar Osteoblastic
III 8.25 3 x 10^9 336
7 Rottweiler MC Distal radius Osteoblastic II 4.00
3 x 10^9 231
RESULTS
[00438] Safety and Toxicity=Safety was evaluated for all 23 vaccinated dogs.
All dogs
tolerated ADXS31-164 administration well with only transient, low grade
toxicities observed
5 on the
day of vaccination (Table 6). A statistically significant increase in body
temperature
occurred 4 hours after ADXS31-164 administration in all groups irrespective of
dose (Fig
9A). Hypotension was not observed at any time point or at any dose (Fig. 9B).
8/18 dogs
(without pre-existing metastatic disease) and 3/5 dogs (with pre-existing
metastatic disease)
developed fevers of >103 F within 4 hours of vaccination and were given
intravenous fluids
to at that
time. Three dogs received a single dose of a non-steroid anti-inflammatory
drug to
reduce body temperature. In all cases, fevers resolved without further
intervention. Transient
lethargy, nausea and vomiting that did not require therapeutic intervention
occurred within 4
hours of vaccination regardless of dose. In two dogs transient single or
bigeminal ventricular
premature contractions were identified shortly after vaccination. One dog with
pre-existing
metastatic disease developed ventricular tachycardia within 2 hours of
vaccination. Treatment
with lidocaine, procainamide, sotalol and corticosteroids had little effect
however, the
arrhythmia resolved within 72 hours. Transient, but statistically significant
increases in white
blood cell and neutrophil counts occurred 24 hours after ADXS31-164 and were
accompanied
by a transient decrease in platelets and lymphocytes (Fig. 17). Although there
was no
correlation between ADXS31-164 dose and magnitude of hematological change,
there was a
significant difference in the magnitude of white blood cell, neutrophil and
monocyte
responses between dogs that survived and those that died (Fig. 18A-F). Mild,
transient
increases in the serum concentrations of liver enzymes occurred in
approximately half of the
dogs, consistent with mild inflammation caused by the hepatotropic Listeria
(Table 6). All
changes identified in the peripheral blood were asymptomatic and resolved
within one week
of ADXS31-164 administration. No significant changes in renal function were
documented in
any dog. 19/23 dogs had blood cultures performed 24 hours after ADXS31-164
administration and all were negative, consistent with rapid clearance of the
highly attenuated
LmddA strain.
[00439] Given that HER2/neu targeted monoclonal antibodies cause cardio
toxicity we
evaluated biomarkers of cardiac damage and echocardiographic measures of
dysfunction
including cardiac troponin I, fractional shortening (%), LVIDd and LVIDs at
baseline, prior to
94
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
each vaccination and every 2 months thereafter. No significant, sustained
changes in cardiac
troponin I, fractional shortening, LVIDd or LVIDs were identified in any of
the vaccinated
dogs (Fig. 26A-D). One dog in Group 3 showed a stepwise increase in serum
cardiac troponin
I at the time of each vaccination however, this was not accompanied by
echocardiographic
signs of dysfunction. Values returned to baseline following the last
vaccination and were not
elevated on repeat assessments.
[00440] Throughout the clinical trial cardiac troponin I levels were measured
along with
fractional shortening, Left Ventricular Internal Diameter in systole (LVIDs)
and LVID in
diastole (LVIDd) as shown in Figure 25 (A-D), there was no evidence of long or
short-term
cardio toxicity following administration of ADXS31-164.
[00441] Table 6 below presents data showing minimal treatment related adverse
events were
reported during the clinical trial.
[00442] Table 6: Treatment Related Adverse Events occurring at or within 48
hours of
ADXS31 - 164 vaccination.
CA 02947677 2016-11-01
WO 2015/167748 PCT/US2015/024048
Number of Dogs with Treatment Related Adverse Events
ADXS31-164 dose 2x108 5x108 1x109 3x109 Total
Number of dogs recruited 3 3 11 6 23
Pyrexia (T>103) Grade 1 2 1 5 5 13
Fatigue Grade 1 1 0 7 2 10
Grade 1 1 2 10 2 15
Nausea
Grade 2 1 0 0 0 1
Grade 1 1 2 9 3 15
Vomiting
Grade 2 2 0 0 3 5
Grade 1 0 1 0 0 1
Arrhythmias
Grade 2 0 0 0 1 1
Grade 1 0 0 2 1 3
Tachycardia
Grade 2 0 0 0 1 1
Hyoptension 0 0 0 0 0
Hypertension Grade 1 2 3 8 5 18
Grade 1 2 2 6 3 13
Thrombocytopenia
Grade 2 0 0 2 1 3
y-GT Grade 1 0 2 1 0 3
Grade 1 0 1 6 1 8
ALKP Grade 2 0 0 0 1 1
Grade 3 1 0 0 0 1
Grade 1 1 1 3 0 5
ALT Grade 2 0 0 0 1 1
Grade 3 1 0 0 0 1
Grade 1 1 1 4 2 8
AST Grade 2 0 0 2 0 2
Grade 3 0 0 1 0 1
BUN 0 0 0 0 0
CREA 0 0 0 0 0
Cardiac Troponin I Grade 1 0 0 1 1 2
[00443] Conclusion: ADSX31-164 toxicities were low grade and transient
[00444] Immune Response to ADXS31-164
[00445] The results
presented in Figure 18 demonstrate that an early immune
response to ADXS31-164 in dogs receiving the vaccines predicted survival of
the dogs.
Figure 18 shows that ADXS31-164 induced increases in WBC, neutrophil and
monocytes
counts, which correlated with survival and were accompanied by a transient
decrease in
platelets and lymphocytes (Figure 17).
[00446] The ability
of ADXS31-164 to induce and maintain an immune response, and
in particular to induce HER2/Neu specific T cell immunity was assessed during
the clinical
trial. In order to evaluate the immune response and to determine if a HER2/Neu
specific T
96
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
cell response was induced by ADXS31-164, HER2/Neu specific T cell numbers were
assessed by IFN-y ELISpot. Samples were taken at baseline (3 weeks post
carboplatin), at
every vaccination and every 2 months thereafter. Figure 19 shows the results
of the ELISpot
assay.
[00447] HER2/neu
Specific Immune Responses. Immunological responses against
the human EC1, EC2 and IC1 domains of HER2/neu (sharing 89%, 93% and 98%
identity
with canine HER2/neu respectively) were detected at baseline in 4/18, 6/18 and
1/18 dogs
respectively. Induced IFN-y responses against one or more of the HER2/neu
domains were
detected in 7 dogs 3 weeks after the third ADXS31-164 vaccination (Table 7).
Five of these
dogs developed immune responses against the highly conserved IC1 domain. Five
additional
dogs developed IFN-y responses against the IC1 domain 2 months later. Three
additional
dogs developed IFN-y responses against either EC2 alone, EC2 and IC1 or EC1,
EC2 and
EC3 at the time of relapse (dogs 001, 002 and 017). 3 dogs that developed
immunological
responses against HER2/neu during their initial vaccination series were
evaluated by IFN-y
ELISpot over 15 to 17 months. HER2/Neu specific IFN-y responses were not
maintained
however, the dogs remained free of metastatic disease during this time. 10
dogs received
additional booster vaccinations, of the 6 evaluable, 2 dogs had detectable
increases in
HER2/neu specific IFN-y responses 2 months after booster vaccination. Of the 8
dogs that
relapsed, 5 had no increase in HER2/neu specific IFN-y responses 3 weeks after
ADXS31-
164.
97
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00448] Table 7
3 WEEKS POST TIME
TO OVERALL
DOG BASELINE ADXS31-164 2
MONTH RE-CHECK 4 MONTH RE-CHECK RELAPSE SURVIVAL
EC1 EC2 IC1 EC1 EC2 IC1 EC1 EC2 IC1 EC1 EC2 IC1
= 001 - - - - - - - - - -R +R -
R 350R 738
= 002 - + - - - - :# DEAD
185L 267
1000
^ 004 + - - + + + - - - - - - 966+
:
's 007 + + - + - - ND ND ND 869B
948+
= 008 - - - * I.
ND ND ND ND ND ND 318B" 346
= 013 - - - - - - - - + - -
+ 767+
= 017 - - - - - - - - - 322B
444
511+
025 - - - + - - - 461+
= 026 - + - + + - - 462--
= 027 - - - - - - 1. - - -
453--
= 036 - - - :0* *t DEAD 251L
= 038 - - - - - - - + - ND
ND ND 335+
039 - - - - + - -R -R +R 315L
359+
^ 030 + + + - + + ND ND ND
DEAD 226' 259
= 037 - - - ND
ND ND ND ND ND 190L :40+
= 040 - - - - - - - - -
ND ND ND 355+
[00449] Booster vaccinations. Ten of the 18 dogs without metastatic
disease at
enrollment were administered a single booster vaccine between 5 and 10 months
after the
initial vaccine series. Four of these dogs received additional booster
vaccines given between
4 and 15 months after the first booster vaccine. Similar low grade, transient
side effects
were noted at the time of booster vaccination as with the initial vaccination
series.
[00450] Figure 20 (A and B) show that repeat booster vaccinations also
stimulated
HER2 specific immunity. Repeat booster vaccinations were administered at 6 and
10
to months for animal 289-003, and at 8 months for animal 289-004.
[00451] Clinical Outcomes. 8/18 dogs in the vaccinated group relapsed,
4 with
pulmonary metastatic disease and 4 with bone metastases. Two dogs with bone
metastases
progressed to pulmonary metastases. One dog with a bone lesion in her sacrum
died from
aspiration pneumonia and one dog with a solitary pulmonary nodule died of
nephroblastoma however, necropsy specimens from bone and lung lesions
respectively
were not available for histopathological confirmation of metastatic
osteosarcoma. These
two dogs were censored from OSA specific survival analysis. Dogs that relapsed
received
different rescue chemo- and radiation therapies at the discretion of the
primary clinician.
The 4 dogs with bone metastases were treated with analgesics only (1 dog),
palliative
radiation alone (1 dog) or in combination with chemotherapy (2 dogs). Two dogs
received
Adriamycin and 1 dog received palladia for the treatment of pulmonary
metastatic disease.
Median OSA specific survival for vaccinated dogs has not yet been reached.
Kaplan-Meier
98
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
survival curves for TTM and OSA Specific Survival are shown in Fig. 21.
Overall survival
rates at 1 and 2 years for vaccinated dogs are 71.4% and 57% respectively. Of
the 12 dogs
that developed HER2/neu specific IFN-y responses within 2 months of
vaccination, 9 are
still alive (3 dogs > 900 days, 1 dog>700 days, 3 dogs > 400 days and 2 dogs >
300 days
and 7 remain tumor free to date (Table 7)). The results presented in Figure 24
demonstrate
that ADXS31-164 breaks the tolerance to HER2/Neu. This may be significant for
the
treatment of OSA as well as other HER2/Neu tumors and/or cancers.
[00452] Necropsy findings. 6/18 dogs died during the study period
and necropsies
were performed on 4 of these dogs. Three dogs were found to have multifocal
grade II and
III metastatic osteosarcoma involving the lungs (3 dogs), bone (2 dogs),
mediastinum (1
dog) and kidney (1 dog). One dog, euthanized on account of a large progressive
renal mass
was found to have nephroblastoma. This dog also had a single pulmonary nodule
but this
was unfortunately not evaluated by histopathology.
[00453] Survival, Prolonged Survival, Tumor Progression following
Administration of ADXS31-164
[00454] Three dogs with multiple metastatic pulmonary nodules at
screening and
treated on a compassionate care basis received one vaccine each before disease
progression
and removal from the study. The two dogs presenting with solitary metastatic
pulmonary
nodules at the time of screening received all three vaccines (see Table 5 for
signalment and
tumor characteristics). Progressive pulmonary metastatic disease occurred in
one of these
dogs despite vaccination. No additional pulmonary lesions developed in the
second dog
despite the pre-exisiting pulmonary nodule doubling in size every 3 weeks
(Fig. 22A and
B). CT scan one week after the last vaccination, confirmed the absence of
additional
metastatic lesions and the dog underwent metastatectomy. Prior to surgery, the
dye
indocyanine green (ICG), used to detect tumor margins and areas of
inflammation, was
administered intravenously and at surgery, fluorescence under near infra-red
light was seen
in the pulmonary nodule and several other areas of healthy appearing pulmonary
parenchyma near the solitary nodule (Fig. 22C and D). Histopathology of the
pulmonary
nodule revealed metastatic OSA with large areas of hemorrhage and necrosis,
surrounded
by a thick fibrous capsule (Fig. 22E). IHC showed an accumulation of CD3+ T
cells around
the fibrous capsule with very few T cells within the nodule itself (Fig. 22G
and H). Other
areas identified by near infra-red fluorescence showed focal areas of T cell
infiltrates (Fig.
22F, 221 and 22J). T cells were seen surrounding abnormally large, vimentin
positive cells
with prominent mitotic figures (Fig. 22K and 22L). These findings suggest that
single
metastatic sarcoma cells may be effectively targeted by tumor specific T cells
within the
99
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
lung and provide a possible mechanism by which ADXS31-164 prevents metastatic
pulmonary disease. The dog recovered well from surgery and remained free of
pulmonary
metastatic disease for 5 months before developing widespread aggressive,
HER2/neu+
metastatic disease in the subcutaneous tissue (osteoblastic, grade II and
chondroblastic,
grade III), mediastinum (osteoblastic, grade II) and diaphragm (osteoblastic,
grade III).
Results show that despite induction of HER2/neu specific T cell responses, off-
tumor side
effects were not identified, hence induction of HER2/neu specific T cells is
responsible for
elimination of HER2/neu positive metastatic cells and long term protection
from disease
recurrence. This is supported by the timing of HER2/Neu-specific T cell
expansion which in
5 dogs occurred approximately 8 months post diagnosis, when many dogs will
develop
metastatic disease and by the histopathological findings of focal T cell
responses within the
pulmonary parenchyma of one dog following vaccination and metastatectomy.
[00455] The results presented in Figure 22 and Figure 23
demonstrate that
administration of ADXS31-164 delays and/or prevents metastatic disease and
prolongs the
overall survival in dogs with spontaneous HER2+ osteosarcoma. As can be seen
in both
figures, dogs receiving vaccine had significantly extended survival time,
while the median
survival for those dogs receiving vaccine has not yet been reached.
[00456] While our study demonstrates the effectiveness of this
approach in
preventing metastatic disease, vaccination with ADXS31-164 was unable to
induce
regression of pre-existing gross, pulmonary metastatic disease in 5 dogs
treated on a
compassionate care basis. In one dog this appeared to be associated with a
failure of T cells
to penetrate the fibrous capsule surrounding the metastatic lesion or for
those cells to
survive within the established tumor microenvironment (Fig. 22C). However, in
the same
dog, focal areas of T cell infiltrates surrounding large, actively dividing
mesenchymal cells,
purported to be metastatic OSA cells were identified in grossly normal lung
parenchyma
and unexpectedly, following metastatectomy this dog did not develop further
pulmonary
metastatic disease. Taken together, these data suggest that ADXS31-164
prevents
pulmonary metastatic disease through its ability to induce potent innate
immune responses
that may sensitize metastatic OSA cells to FAS/FASL mediated apoptosis and
adaptive
immune responses in the form of HER2/Neu specific T cells that eliminate
micrometastatic
pulmonary disease.
[00457] Conclusions:
[00458] At the time of filing this application 12/18 dogs have not
developed
pulmonary metastatic disease, demonstrating that ADXS31-164 prevents
metastatic
disease in a subject suffering from spontaneous HER2+ osteosarcoma when
administered
100
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
in the setting of minimal residual disease. Vaccinated dogs showed a
statistically
significant increase in overall survival compared to a historical HER2/Neu+
control
group. Median survival in the HER2/Neu+ control dogs (n=11) was 316 days
(p=0.032)
wherein the median survival in ADSX31-164 treated dogs has not been reached.
Further,
the results indicate that ADXS31-164 breaks peripheral tolerance to the highly
conserved
IC1 domain of HER2/Neu (Figure 26). The magnitude of the increase in
leucocytes
within 24 hours of ADXS31-164 administration (Figure 18) correlates with
survival,
suggesting that outcome depends in part upon the ability of the dog' s immune
system to
respond to the vaccine. Importantly, this study showed that administration of
up to 3 x
109 CFU of ADXS31-164 to dogs with spontaneous OSA is safe and causes only
transient, low grade side effects at the time of administration. Moreover,
prevention of
pulmonary metastatic disease maybe in part associated with CD3+ T cell
mediated
elimination of microscopic metastatic disease in the lung. This work has
important
implications for pediatric OSA and other human cancers that express HER2/Neu.
[00459] Moreover, here
we show that administration of ADXS31-164 in doses
up to 3.3 x 101\9 CFU are safe in the dog and despite inducing HER2/neu
specific
immunity, do not lead to short or long term cardio toxicity. On target, off
tumor side
effects including cardio toxicity has been associated with the administration
of large
numbers of HER2/neu specific T cells or when trastuzumab has been used
concurrently
with anthracyclines. We employed a standard chemotherapy protocol without
doxorubicin to reduce any potential risk of cardio toxicity.
[00460] Our
study demonstrates that ADXS31-164 can prevent pulmonary
metastatic disease in dogs with OSA. These results demonstrate safety and
unprecedented survival times in dogs with OSA and pave the way to investigate
the
ability of ADXS31-164 to prevent metastatic disease in patients with HER2/neu
expressing tumors including pediatric osteosarcoma and mammary carcinoma.
EXAMPLE 11:
COMBINATION ADXS31-164 AND RADIATION THERAPY FOR THE
TREATMENT OF CANINE AND HUMAN OSTEOSARCOMA (OSA) and
PULMONARY METASTATIC DISEASE.
[00461] A recombinant Listeria monocytogenes expressing a human chimeric Her-
2/neu
construct (ADXS31-164) used in combination with palliative radiation to
prevent pulmonary
metastatic disease and prolong overall survival in dogs with spontaneous
appendicular
osteosarcoma is described. Given the similarities between canine and human
osteosarcoma,
we believe that this combination will be effective therapy for human disease.
101
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00462] Materials and Methods
[00463] Vaccine preparation
[00464] The details of the construction of ADXS31-164 vaccine have been
described above.
The ADXS31-164 vaccine stocks were prepared and stored as 1 ml aliquots in
freezer at -70
C. Before injection, vaccine stocks were thawed at 37 C for 2-3 min and then
washed
twice with phosphate-buffer saline (PBS) and resuspended in PBS at a final
concentration of
5x108 colony forming units (CFU)/ml. Each dog was immunized intraperitoneally
with 200
ul of this suspension.
[00465] RT and immunotherapy
[00466] Ten systemically healthy dogs with histopathologically confirmed,
treatment naïve,
HER2+ appendicular OSA, and no evidence of cardiac or metastatic disease were
enrolled.
All dogs received 16Gy of RT in two fractions on consecutive days, followed by
the first of 8
intravenous doses of ADXS31-164 (3.3 x 101\9 CFU per dose) given once every 3
weeks.
Immunization with the Listeria- based vaccine was performed every 3 weeks
(e.g., on days
7, 28, 49, 70, 91, 112, 133 and 154) (Figure 10). Immune analysis was also
performed on the
day of immunization.
[00467] On days 4 and 5, external beam RT of 8 Gy was delivered using a
Siemens 6 MV
linear accelerator. The RT was given under general anesthesia. Tumors were
evaluated
clinically every three weeks and radiographically at baseline, at the fourth
vaccine
administration (day 70) and at the eighth vaccine administration (day 154). At
these time
points, thoracic radiographs were performed to determine the presence of
pulmonary
metastatic disease.
[00468] A bone biopsy to confirm the diagnosis of osteosarcoma was performed
at the time
of enrollment. Complete Blood Count (CBC), Chemistry Screen (CS), Urinary
Analysis
(UA), electrocardiogram (EKG)/Echocardiogram/ serum concentration of cardiac
troponin I
(cTn1) and radiographs of the affected limb and the thorax were performed on
Days 0, 70,
and 133 and every 2 months thereafter until euthanasia. On the day of
euthanasia Complete
Blood Count (CBC), Chemistry Screen (CS), Urinary Analysis (UA), Immune
analysis,
electrocardiogram (EKG)/Echocardiogram/ serum concentration of cardiac
troponin I (cTn1);
and necropsy were performed.
[00469] ELISpot assayCryopreserved PBMC from each indicated time point were
thawed,
rested overnight at 37 C and then counted. Cells were stimulated with 2.0 uM
pools of
overlapping human HER2/Neu peptides (1 lmers overlapping by 5 amino acids)
that
represent the EC1, EC2 and IC1 domains of HER2/Neu present in the chimeric
vaccine, and
recombinant human IL-2 (Invitrogen, Fredrick, MD) for 5 days. Cells were
harvested,
102
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
washed twice in 1 x PBS and counted. IFN-y ELISpot assays were performed
according to
the manufacturer's protocol using a commercial canine IFN-y ELISpot assay kit
(R&D
Systems, Minneapolis, MN). Briefly, 0.1 - 3 x 105 stimulated cells were
incubated with 2.5
uM of EC1, EC2 or IC1 peptide pools or none (to determine background counts).
All assays
were performed in duplicates. Plates were developed according to the
manufacturer's
instructions. Spots were counted using a CTL-Immunospot analyzer (C.T.L,
Shaker Heights,
OH).
Results
[00470] We evaluated the use of ADXS31-164 as adjuvant therapy in dogs with
spontaneous osteosarcoma as described herein above. ADXS31-164 was
administered to
dogs with spontaneous appendicular OSA following 16 Gy RT administered on two
consecutive days. Up to 8 doses of ADXS31-164 were administered. This work
showed
repeat administrations of 3.3 x 109 CFU of ADXS31-164 to be safe.
[00471] The potential synergy between radiation therapy and ADXS31-164 to
promote
antitumor immunity (in particular the generation of Her-2/neu specific T
cells), retard the
progression of the primary tumor and prevent/delay pulmonary metastatic
disease was then
explored.
[00472] Figure 10 describes the treatment protocol. Dogs were screened on day
0 for
enrollment in the trial. Screening included evaluation of baseline blood
tests, urinalysis,
cardiac evaluation, thoracic and affected limb radiographs and bone biopsy to
confirm the
diagnosis of osteosarcoma. Palliative radiation was given on 2 consecutive
days following
enrollment. Multiple doses (up to 8) of ADXS31-164 were given once every 3
weeks
following palliative radiation therapy with only transient, low-grade, side
effects (data not
shown). Thoracic and limb radiographs were repeated at day 70 and 154.
Lameness scores
were assigned by two board certified veterinary orthopedic surgeons to each
dog at each time
point based on their evaluation of videos taken of each dog at each time
point. Owners also
filled in a validated pain questionnaire that documented the owners perception
of quality of
life (Figure 28). 5/10 dogs are still alive, three without evidence of tumor
progression or
pulmonary metastatic disease. At the time of writing, median survival is 285
days which
compares favorably with a historical median survival of 136 days achieved with
RT alone. In
one patient, presenting for trial enrollment with a pathological fracture of
the proximal
humerus that was stabilized by 2 bone plates and an intramedullary pin,
radiographs show
evidence of the fracture healing and no evidence of pulmonary metastatic
disease three
months after radiation therapy and ADXS31-164 administration, (Figure 11).
103
CA 02947677 2016-11-01
WO 2015/167748
PCT/US2015/024048
[00473] Moreover, results show that palliative radiation therapy in
conjunction with
ADXS31-164 therapy reduces lysis, promotes tumor consolidation, and prolongs
survival of
subjects (Figure 29 A-Dand Figure 30-A-B). Lateral (Figure 29A) and AP (Figure
29C)
radiographic views of a distal tibial osteosarcoma lesion demonstrating marked
cortical bone
remodeling, and reduction in lysis, following 16Gy radiation (given as 8Gy on
2 consecutive
days starting on 9.13.2014) and 3 doses of ADXS31-164 (11.25.2014). Lateral
(Figure 29B)
and AP (Figure 29D) radiographic views of a distal radial osteosarcoma lesion
treated with
16Gy radiation (given as 8Gy on 2 consecutive days starting on 7.16.2014) and
3 doses of
ADXS31-164 (10.13.2014). Note the significant reduction in swelling and bony
lysis within
the distal portion of the radius (compare radiographs dated 10.13.2014 with
7.16.2014 in
Figure 29B). There is increased bone density on the medial aspect of the
distal tibia (compare
radiographs dated 10.13.2014 with 7.16.2014 in Figure 29D). There is a small
minimally
displaced bone fracture of the medial aspect of the distal radius seen on the
10.13.2014
radiographs in Figure 29D. Therefore, ADXS31-164 may be used without
chemotherapy; in
combination with radiation and potentially in the neo-adjuvant setting, prior
to amputation
and chemotherapy to prevent metastatic disease.
[00474] While certain features of the invention have been illustrated and
described herein,
many modifications, substitutions, changes, and equivalents will now occur to
those of
ordinary skill in the art. It is, therefore, to be understood that the
appended claims are
intended to cover all such modifications and changes as fall within the true
spirit of the
invention.
104