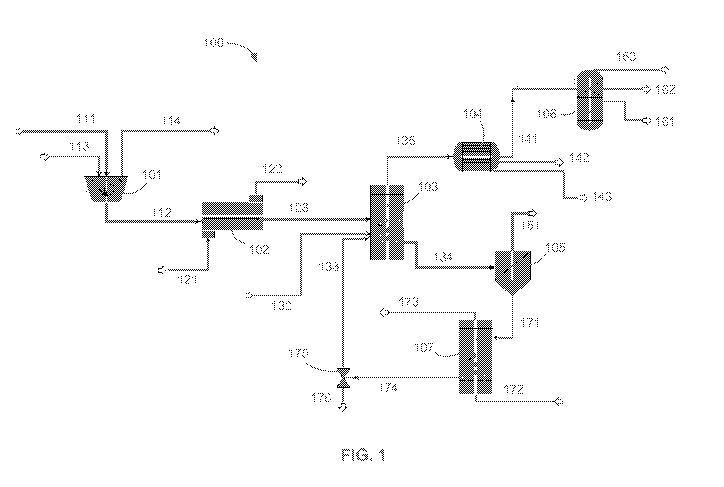Note: Descriptions are shown in the official language in which they were submitted.
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
SYSTEM AND PROCESS FOR THE MANUFACTURE OF
HYDROCARBONS AND UPGRADED COAL BY CATALYTIC MILD
TEMPERATURE PYROLYSIS OF COAL
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of and priority to U.S. Provisional
Patent
Application No. 62/002,674, filed on May 23, 2014, which is incorporated by
reference herein in
its entirety.
FIELD
[0002] The present technology relates generally to a system and process for
the conversion of
solid carbonaceous materials to a set of usable products. More specifically,
the technology
relates to a system and process that utilizes a catalyst to convert the solid
carbonaceous materials
into a gaseous product, a liquid product, and/or an upgraded solid product
(e.g., an upgraded
solid carbonaceous product).
BACKGROUND
[0003] Given the uneven global distribution of crude oil reserves and finite
nature of crude oil
reserves in the spotlight, there is an ever-increasing need to develop
alternative production
technologies based on alternative feedstocks (e.g., coal, biomass, etc.). In
the past decades, coal
to liquid (CTL) technologies have achieved some progress. The most technically
defined route
for producing hydrocarbon liquids involves gasification, generally involving
relatively high
temperature steam and oxygen co-feeds to produce syngas. Significant cleaning
of the resulting
syngas is required prior to further conversion to a methanol intermediate, or
for direct synthesis,
and thus, generally involves an integrated, multistep approach, gasification-
based facility, which
is typically costly to build and operate. Another route for producing
hydrocarbon liquids from
coal is the so-called direct coal liquefaction (DCL) route which involves
direct liquefaction via
-1-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
high pressure treatment of coal solids with pure hydrogen. Even though the DCL
process
typically utilizes catalysts, the desired hydrocarbon product selectivity out
of the catalytic reactor
is low and further processing is required. In other words, the DCL process
cannot be tailored to
produce specific hydrocarbon products, and in particular, lower molecular
weight hydrocarbons.
Therefore, the DCL product stream requires significant additional chemical
upgrading steps
resulting in facilities which are also cost intensive to build and operate.
Another conventional
route for producing hydrocarbon liquids from coal is through mild temperature
gasification or
pyrolysis. The resulting liquid product stream from conventional pyrolysis
contains relatively
high concentrations of high molecular weight tars that require considerable
upgrading, typically
via catalytic hydrogenation. The overall pyrolysis product selectivity to non-
hetero-atom
containing Ci¨C12 hydrocarbon products is relatively low.
SUMMARY
[0004] In one aspect, a process for upgrading a solid carbonaceous material is
provided. The
process includes heating the solid carbonaceous material in the presence of a
catalyst under
partial pyrolysis conditions, and obtaining an upgraded solid carbonaceous
product, a gaseous
product, and a spent catalyst. One non-limiting example of solid carbonaceous
material is coal
and, therefore, one non-limiting example of upgraded solid carbonaceous
product is an upgraded
coal product.
[0005] In another aspect, a process for converting a solid carbonaceous
material in a
beneficiation system into an upgraded solid carbonaceous product is provided.
The process
includes introducing the solid carbonaceous material and a catalyst into a
pyrolysis reactor to
produce a gaseous product stream and a solid product stream, where the solid
product stream
includes the upgraded solid carbonaceous product. The process further includes
recovering the
gaseous product stream from the reactor, and recovering the solid product
stream from the
reactor. One non-limiting example of solid carbonaceous material is coal and,
therefore, one
non-limiting example of upgraded solid carbonaceous product is an upgraded
coal product.
-2-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0006] In another aspect, a process for converting a biomass in a
beneficiation system into an
upgraded solid product is provided. The process includes introducing the
biomass and a catalyst
into a pyrolysis reactor to produce a gaseous product stream and an upgraded
solid product
stream, where the solid product stream includes spent catalyst and the
upgraded solid product.
The process further includes separating the upgraded solid product and the
spent catalyst, and
transferring the separated spent catalyst to a regenerator that removes at
least a portion of any
unpyrolyzed coal, coke, and other carbonaceous material from the spent
catalyst. A weight of
ash retained in the upgraded solid product is at least 60 weight percent of
ash in the biomass
introduced into the pyrolysis reactor. The process further includes
transferring the gaseous
product stream to a separator that produces a liquid product and a gaseous
product.
[0007] The pyrolysis reactor may operate from about 300 C to about 1,100 C.
In some
embodiments, this may include from about 350 C to about 850 C, or from about
400 C to
about 700 C. The carbonaceous material may have a residence time of about
0.01 second to
about 5 hours. In some embodiments, the residence time is from about 0.1
second to about 1
minute. The catalyst loading for the pyrolysis reactor may be from about 0
(zero) to about 100 g
of catalyst/g of carbonaceous feed material. In some embodiments the catalyst
loading may be
from about 0.05 g catalyst/g feed material to about 10 g catalyst/g feed
material. The heating
rate of the carbonaceous material in the reactor may be from about 0.1 C/sec
to about 1000
C/sec.
[0008] In one embodiment, the starting carbonaceous material enters directly
into the pyrolysis
reactor. In other embodiments, prior to entering the pyrolysis reactor, the
carbonaceous material
may be pre-processed, such as, for example, via a pulverizer, a dryer, and/or
any other suitable
pre-processer or pre-process discussed below.
[0009] In one embodiment, the starting carbonaceous material is introduced
into a pyrolysis
reactor with a catalyst, which can be mixed with the carbonaceous material
before entering the
reactor, or within the reactor. The solid stream exiting the reactor includes
spent catalyst and the
upgraded solid product. The solid stream is then separated into a first solid
stream containing
predominantly the upgraded solid product, which may be sent for further
processing or
-3-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
combustion as an upgraded solids fuel, and a second solid stream containing
predominantly spent
catalyst, which may be sent to a regeneration reactor before being recycled
back into the
pyrolysis reactor. For example, the particle size distributions of upgraded
carbonaceous material
and the catalyst can be intentionally different, allowing for appropriate
classification technology
to separate the two solids by differences in particle size, weight and/or
density. Other
technologies may be used as an alternative or in conjunction with the
classifier, which include
but are not limited to other classifier technologies, electrostatic
separation, electrodynamic
separation, triboelectric separation and/or magnetic separation. High gradient
magnetic
separators, which use high magnetic gradients to attract weakly paramagnetic,
or very fine
ferromagnetic, particles, may be utilized with the systems of this
application. Open gradient
magnetic separators, which segregate falling particles in a falling stream
according to their
magnetic characteristics, may be utilized with the systems described herein.
Electrodynamic
separators, in which feed particles become charged from ion bombardment and
are pinned to a
rotating drum, may be utilized with the systems described herein. In such
electrodynamic
separators, particles with higher conductivity tend to lose their charge
faster while those particles
with less conductivity (i.e., more insulated particles) tend to stay attached,
leading to separation
of the particles. Triboelectric separators, which charge particles through
friction, then pass the
particles through an electric field to be deflected according to the sign and
magnitude of their
charge.
[0010] According to another embodiment, the solids (e.g., the catalyst and
carbonaceous
material) may remain separated within the pyrolysis reactor. One example is
where the catalyst
is immobilized in the reactor, such as where the catalyst is immobilized by
plating on the walls
of the reactor, while the carbonaceous material enters and exits the pyrolysis
reactor. In such a
system, the need for solid-solid separation of catalyst from the upgraded
solid product outside
the reactor is essentially eliminated, since the solids remain separated
within the reactor.
[0011] According to another embodiment, the solids are commingled and then
separated within
the reactor. One such example is where the reactor is in the form a fluidized
bed, in which the
catalyst and starting carbonaceous material are mixed (e.g., commingled)
inside (or before
-4-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
entering) the reactor. After the carbonaceous material has intimately
interacted with the catalyst,
the mixed solid product stream is passed through a solid-solid separator
located inside the reactor
(e.g., an internal classifier) to separate the catalyst and carbonaceous
material. In such a system,
the need for solid-solid separation of catalyst from product solids outside
the reactor is
essentially eliminated, since separation occurs within the reactor.
[0012] The gaseous product stream may be transferred to one or more separation
units, such as
to condense a liquid product stream and separate the products into desired
fractions. One such
example of a separation unit is an acid gas removal system, wherein sulfur-
containing chemicals
and most of the carbon dioxide are removed, each into high purity (e.g.,
concentrated) streams.
Other separators may be used, and other compounds may be removed, such as
hydrogen cyanide
(HCN) or ammonia (NH3). The highly concentrated stream of carbon dioxide can
be captured
for sequestration or used in the plant or pipelined for use externally, such
as for enhanced oil
recovery. The highly concentrated stream of sulfur containing compounds can be
processed for
landfilling, to produce useable solid sulfur, or to produce a useable sulfur-
containing compound,
such as sulfuric acid.
[0013] One gaseous product stream may include one or more non-condensable
gases or
chemicals, such as, for example, methane, ethane, ethylene, carbon monoxide,
carbon dioxide,
and/or hydrogen. The non-condensable stream may also be processed further to
produce syngas
for production of other chemical products, such as, but not limited to,
methanol, mixed alcohols
and/or Fischer Tropsch products. This non-condensable stream may be used as
fuel in the
process, such as to provide heat for the process or other unit operations in
the plant. For
example, it may be beneficial to pass a gaseous product (e.g., methane,
ethylene, ethane,
hydrogen, etc.) through the pyrolyzer as a recycle gas or a second pass
stream. The recycled
gaseous product may provide additional heat and/or hydrogen (since coal is
generally low in
hydrogen) into the reactor. The liquid stream can be captured as synthetic
crude oil, or separated
further to extract useable hydrocarbon chemicals into two or more chemical
streams.
[0014] In addition, it may be advantageous to introduce fuel gases into the
pyrolyzer from an
external source. For example, natural gas and/or natural gas liquids (e.g.,
propane, propene,
-5-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
butane, butene, isobutane, isobutene, etc.) may be added from external
sources. These added
fuel gases would serve the dual purposes of providing fluidization gases and
increasing the yield
of useful fuels. This would be particularly beneficial if the facility (e.g.,
plant) were near so-
called "stranded gas" reserves where such components are often disposed of by
flare. This
mixture of natural gas, natural gas liquids, and other condensable
hydrocarbons are often referred
to as "wet gas". For purposes of this patent, "wet gas" will be understood to
be a mixture of
natural gas, natural gas liquids, and other condensable hydrocarbons.
[0015] Such co-production may advantageously provide synergistic benefits. The
presence of
free radicals in the coal and higher hydrocarbons in the process will catalyze
the pyrolysis of
these fuel gases effectively carrying out a gas-to-liquids conversion in
parallel with the coal
beneficiation. This is of particular benefit to methane pyrolysis, which is
extremely difficult to
carry out without the presence of free radicals. Free radicals increase the
per-pass pyrolysis
conversion of methane to higher fuels from <1% to about 10%.
[0016] Another embodiment relates to a system and a process for converting a
carbonaceous
material into multiple usable products utilizing a catalyst. The carbonaceous
material may
include a coal (e.g., a low-grade coal), a biomass, a waste, bitumen, or a
combination of any two
or more carbonaceous materials. The carbonaceous raw material may be pre-
processed prior to
entering the pyrolysis reactor, such as by pulverization to resize (e.g.,
grind, reduce, etc.) the
particles of raw, carbonaceous material and/or drying to reduce the moisture
content in the raw,
carbonaceous material.
[0017] The pyrolysis reactor may be configured as a moving bed, an entrained
flow bed, a
fluidized bed, or any suitable reactor where all solid material (e.g.,
carbonaceous material,
catalyst, etc.) moves through the reactor. Alternatively, the pyrolysis
reactor may be configured
as a fixed bed or any suitable system where the catalyst remains stationary
during the reaction
with the carbonaceous material that enters and exits the reactor.
[0018] The solid material exiting the pyrolysis reactor, including the
upgraded solid product
and the spent catalyst, are separated into at least two solid streams (i.e. a
solid-solid separation)
including a first stream of predominantly upgraded solid product and a second
stream of
-6-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
predominantly spent catalyst (e.g., a catalyst stream), except for the
immobilized catalyst reactor
where only a single solid stream of upgraded solid product exits the reactor.
The solid-solid
separation of the solid material may be performed partially or fully in situ
within the pyrolysis
reactor and/or after exiting the reactor. In other words, the solid material
exiting the pyrolysis
reactor may be separated internally to the reactor or externally. The solid-
solid separation of the
first and second streams can be performed using a classifier, or similar
technology, such as
where the solids are separated based on particle size, mass, and/or density,
electrostatic
separation techniques, or magnetic separation techniques.
[0019] The catalyst stream including spent catalyst may be sent to a
regenerator (e.g., a
regeneration unit) where the spent catalyst is regenerated by combusting any
residual (e.g.,
remaining, left-over, etc.) carbonaceous material to produce mainly
regenerated catalyst and ash
residue of the combusted carbonaceous material. A portion of the spent
catalyst may be sent
back to the reactor without being regenerated, or may be discarded. An oxygen-
carrying gas,
such as air, may be introduced into the regeneration reactor to regenerate the
spent catalyst and
combust the remaining carbonaceous material in the regeneration reactor. A gas
stream exiting
from the regenerator may include resultant flue gas. Part, or all, of the flue
gas exiting the
regenerator may be utilized to provide heat directly or indirectly to the
pyrolysis reactor, the
dryer, and/or another element of the system. Some, or all, of the gas exiting
the regenerator may
be used to transport the regenerated catalyst to the pyrolysis reactor.
[0020] The non-solid product exiting from the pyrolysis reactor may be
separated into at least
two streams (e.g., a gaseous product stream and a liquid product stream). The
liquid product
stream may be processed further through chemical upgrading, by separation
processes, or
collected as synthetic crude product stream, which can be refined into
constituents (e.g., C5-C12)
or hydrocarbons, including aromatics. The gaseous product stream may be
processed further
through chemical upgrading, by separation processes into multiple useable
process streams
including a non-condensable stream, or used within the plant, such as gaseous
fuel or collected
as another product stream.
-7-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0021] Some, or all, of the non-condensable gas product stream may be burned
for heating
value. The heat may be used in the pre-processing of the carbonaceous raw
material, in the
pyrolysis reactor, or elsewhere in the plant.
[0022] Some, or all, of the gaseous product stream may be introduced into an
acid gas removal
system, wherein the sulfur-carrying compounds and/or nitrogen-containing
compounds (e.g.,
ammonia and hydrogen cyanide) and/or the carbon dioxide are/is separated. The
sulfur-carrying
compounds can be further processed and sold as useable sulfur-containing
compounds, such as,
but not limited to, elemental sulfur and/or sulfuric acid. The carbon dioxide
stream can be
sequestered, or used as a useable product such as, but not limited to, the
purpose of enhanced oil
recovery.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] FIG. 1 is a schematic diagram of an illustrative embodiment of a system
and process
flow for converting a carbonaceous material into one or more usable products.
[0024] FIG. 2 is a schematic diagram of another illustrative embodiment of a
system and
process flow for converting a carbonaceous material into one or more usable
products.
[0025] FIG. 3 is a schematic diagram of another illustrative embodiment of a
system and
process flow for converting a carbonaceous material into one or more usable
products.
[0026] FIG. 4 is a schematic diagram of yet another illustrative embodiment of
a system and
process flow for converting a carbonaceous material into one or more usable
products.
[0027] FIG. 5 is a graph comparing the yield of various products produced
through an
experimental system using a catalyst vs. sand.
[0028] FIG. 6 is another graph comparing the yield of various products
produced through an
experimental system using a catalyst vs. sand.
[0029] FIG. 7 is yet another graph comparing the yield of various products
produced through
an experimental system using a catalyst vs. sand.
-8-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0030] FIG. 8 is a cross-sectional view of an exemplary embodiment of a
pyrolysis reactor
configured to provide solid-solid separation.
[0031] FIG. 9 is a perspective view of a portion of the pyrolysis reactor of
FIG. 8.
[0032] FIG. 10 is another perspective view of a portion of the pyrolysis
reactor of FIG. 8.
[0033] FIG. 11 is a partial cross-sectional/perspective view of another
exemplary embodiment
of a pyrolysis reactor configured to provide solid-solid separation.
[0034] FIG. 12 is a schematic view of another exemplary embodiment of a
pyrolysis reactor
configured to provide solid-solid separation.
DETAILED DESCRIPTION
[0035] Prior to discussing the various embodiments disclosed in this
application, several terms
used in this application will be defined and discussed in detail for
clarification. The term "solid
carbonaceous material" (e.g., SCM, carbonaceous material or CM) is a material
that is solid at
standard temperature and pressure (25 C, 1 bar absolute pressure) that
includes and/or yields
carbon and/or a hydrocarbon. Non-limiting examples of solid carbonaceous
material include
coal, peat, lignite, biomass, coke, semi-coke, petroleum coke, tars, or
asphalt. The term
"carbonaceous material" refers to "solid carbonaceous material," unless stated
otherwise. The
term "volatile matter," the term "moisture" (e.g., water), the term "fixed
carbon," and the term
"ash content" of solid carbonaceous material shall mean the values that are
determined by
proximate analysis as defined in ASTM 3172. The term "upgraded solid
carbonaceous product"
(e.g., upgraded solid carbonaceous material) is any material having one or
more of the following
nine characteristics relative to the starting carbonaceous material. First, a
higher heating value
(abbreviated in this application as "HHV")_as defined by ASTM D5865 method (as
received
basis, e.g., including moisture, ash, and other non-combustible material).
Second, a higher
heating value by ASTM D5865 method (dry basis, where moisture is determined by
ASTM 3172
method). Third, a higher heating value by ASTM D5865 method (dry, ash-free
basis where
moisture and ash are determined by ASTM 3172 method). Fourth, a lower volatile
matter by
ASTM D3172 method. Fifth, a lower overall sulfur content by ASTM D4239 method.
Sixth, a
-9-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
lower organic sulfur content by ASTM D2492 method. Seventh, a lower sulfate
content by
ASTM D2492 method. Eighth, a lower pyritic sulfur content by ASTM D2492
method. Ninth, a
lower moisture by ASTM D3172 method. Further, the term "upgraded" used with
any specific
material (e.g., coal, biomass, etc.) shall also be defined as provided above.
It shall be understood
that the term "upgraded carbonaceous product" implies "upgraded solid
carbonaceous product,"
unless stated otherwise.
[0036] When referring to "retention" and other comparable terms (e.g.,
retained, retaining), it
is noted that the processes, as disclosed in this application, fundamentally
transform a feedstock
material including a solid carbonaceous material into a solid carbonaceous
product and,
therefore, it is often more accurate to consider how much of each component in
the solid
carbonaceous feed material is retained in the solid carbonaceous product,
rather than the absolute
weight fraction of the components in the solid carbonaceous product.
Accordingly, the term
"retention" denotes a portion of a given component in the solid carbonaceous
feedstock that is
contained in the upgraded carbonaceous product. For example, the retention of
component X,
may be discussed herein (e.g., in a table) as kg of component X contained in
upgraded
carbonaceous product divided by 100 kg of component X contained in solid
carbonaceous feed
material. Thus, it may be considered as an unconverted weight fraction. For
example, if 100 kg
of coal containing 3% by weight sulfur is converted to 60 kg of upgraded coal
containing 3%
weight sulfur, the retention of sulfur is 60 kg per 100 kg of sulfur feed,
because the coal feed
contained 3 kg of sulfur whereas the upgraded coal product contained 1.8 kg of
sulfur. The term
"de-asher" is any device which reduces the ash content in a solid carbonaceous
material.
[0037] One objective of the pyrolysis of solid carbonaceous materials is to
form higher value
fuels and organic chemicals. As such, it is desirable to maximize the
conversion of organic
components in the feed solid carbonaceous materials to the desired end states.
However, as a
practical matter, nearly all naturally occurring (e.g., biomass and fossil-
based) solid
carbonaceous feedstocks contain ash ¨ inorganic material bound into the solid
carbonaceous
material. This ash is extremely stable and in most chemical transformations of
solid
carbonaceous material (e.g., combustion, gasification, pyrolysis), as the
solid carbonaceous
-10-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
material is converted ash becomes liberated from the solid carbonaceous
material. For example,
in a coal-fired steam generation station, this ash can be manifested as a slag
or fly-ash co-
product.
[0038] This liberated ash (e.g., "free ash") has been particularly problematic
in catalytic
transformations. Catalytic materials are often highly designed for a
particular and/or controlled
selectivity and activity. This is done by designing the catalyst's surface
chemistry and
morphology, including its porosity and structure. Ash interferes with all of
these critical
features. Inorganic ions in the ash can manipulate the surface chemistry of
active sites, and ash
fines can clog pores and change morphology in general. This leads to a number
of adverse
effects, several of which are discussed herein. First, reduced activity due to
reduction in active
and/or available surface area of the catalyst can lead to lower productivity
of the reactor and
lower yields of products. Second, poisoning can occur, requiring more frequent
regeneration of
the catalyst or in even worse, irreversible deactivation of the catalyst
requiring replenishment of
spent catalyst with new catalyst, leading to excessive waste and an
uneconomical process. Third,
reduced selectivity to desired products can occur. For example, selectivity to
the desired light
hydrocarbons and BTEX (defined below) is determined by the shape and surface
activity of the
catalyst. Changes in pore size will reduce the selectivity to these materials
resulting in
uncontrolled thermal pyrolysis. Fourth, an increase in undesired by-products
can occur.
Uncontrolled thermal pyrolysis, in the absence of a catalyst, results in many
undesired materials,
such as tars and heteroatom containing organics (such as phenol, which is
toxic to humans and it
is also a poison in many downstream refining operations).
[0039] Various tar fractions are known. For example, these include ammoniacal
liquor
(boiling range of about 100 C), light oil (boiling range from 100 C to about
170 C) and
potentially containing materials such as benzene, toluene, and xylenes, middle
oil (boiling range
from 170 C to about 230 C and potentially containing lower naphthalene
fractions, heavy oil
(boiling range from 230 C to about 270 C and potentially containing higher
naphthalene
fractions, green oil (boiling range from 270 C to about 360 C and
potentially containing
anthracene fractions, and the residual matter or "pitch" boiling at greater
than 360 C.
-11-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0040] Likewise Goodman et al. in their 1953 report entitled "Low Temperature
Carbonization
Assay of Coal in a Precision Laboratory Apparatus" used the Fischer Assay
method to determine
the main pyrolysis products from various types of coal in terms of product
yield to char, tar,
water, light oil and gas components. The light oil components are described as
comprising
varying amounts of benzene, toluene, xylenes and aromatic naphthas as well as
small amounts of
carbon disulfide, naphthalene, unsaturated hydrocarbons and saturated paraffin
hydrocarbons.
Based on the Fischer Assay method, the tars contain higher hydrocarbon
constituents than light
oil and the higher hydrocarbons are comprised mainly of hydrocarbon molecules
with carbon
atoms greater than about C10, and boiling range above about 270 C. For
purposes of this
application, "tars" or "tar products" will be understood to be defined by this
reference (i.e.
hydrocarbons containing greater than 10 carbon atoms, and "non-tar light oils"
or "non-tar light
oil products" or simply "light oils" or "light oil products" will be
understood to mean any
hydrocarbon containing 10 or less than carbons. Typical uncatalyzed coal
pyrolysis processes
tend to yield a relatively low product weight ratio of light oil products to
tar products, of much
less than 1 in the range of about 0.08 - 0.25; whereas the processes of this
application tend to
yield a relatively high product weight ratio of light oil products to tar
products, much greater
than 1 in the range of about 5 - 1000 or greater. Also, a lower level of
phenol (and other
heteroatom-containing organics) is produced in the processes of this
application. Without
wishing to be bound by any particular theory or explanation, it appears that
the presence of an
active catalyst reduces the amount of phenol produced. For example, in Figure
7, we can see that
phenol levels are higher when sand (with little or no catalytic activity is
used) compared to when
a catalyst is used. Even more advantageous, the toluene levels produced
increase when a catalyst
is used (compared to when sand with little or no catalytic activity is used),
such that an amount
of toluene produced is greater than an amount of phenol produced on a weight
basis (i.e., an
amount of phenol produced is less than an amount of toluene produced on a
weight basis). Also,
in Table 2 (below) we can see that no phenol, or any other heteroatoms are
observed in either
example embodiment. In summary, without wishing to be bound by any theory or
explanation,
we believe that two principle factors contribute to this superior performance:
1) Presence of an
-12-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
active catalyst, and 2) sequestration (e.g., absorption and/or adsorption) of
any tars produced in
the upgraded CM product, which is most often subsequently and harmlessly
burned as a fuel.
[0041] By our working explanation, presence of an active catalyst is important
to the superior
performance of this process. It is further believed that the processes of this
application have
superior operability and selectivity to desired products compared to previous
processes, because
much less ash is liberated compared to the previous processes. The reduction
in ash liberation is
believed to be in part due to intentionally limiting conversion levels of the
feed solid
carbonaceous material to retain a majority of ash in the product carbonaceous
material. Thus, the
catalyst is exposed to much less "free ash" than in the previous processes. It
is a unique aspect
(and somewhat counterintuitive) to intentionally limit the conversion of the
solid carbonaceous
feedstock. While the economics and productivity of converting as much of the
solid
carbonaceous feedstock may be compelling, it is believed that controlling
conversion such that
free ash production is minimized has a greater benefit than maximizing
conversion of solid
carbonaceous feedstock.
[0042] The term "non-condensable (fuel) gas" is any material, which cannot be
condensed by
pressurization into a liquid at 40 C (i.e., a gas possessing a critical
temperature below 40 C).
Further, a non-condensable gas that liberates heat upon reaction with air or
oxygen is referred to
herein as a "non-condensable fuel gas."
[0043] The term "light condensable fuels," such as liquefiable petroleum gases
(LPGs), are
gases that liberate heat upon reaction with air or oxygen and are condensable
at 40 C by
pressurization, but do not bear more than 7 carbon atoms and are not aromatic.
[0044] The term "light hydrocarbons" is any material that is either a non-
condensable fuel gas
or a light condensable fuel, as defined herein.
[0045] The term "BTEX" denotes Benzene, toluene, ethylbenzene, m-xylene, p-
xylene, or o-
xylene.
[0046] The term "heteroatom containing organics" is any organic molecule
bearing sulfur,
oxygen, and/or nitrogen.
-13-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0047] The term "higher hydrocarbons" is any hydrocarbon that is not a light
hydrocarbon or
BTEX, as defined herein.
[0048] The term "weighted hour space velocity" (WHSV) denotes the feed rate of
substrate in
weight per hour divided by weight of catalyst contained in the reactor. WHSV
has the units of
hr-1. As used herein, the weight hourly space velocity will be based on the
hourly feed of coal
by weight on a dry basis, and the weight of catalyst contained in the reactor.
The weight of the
catalyst will be determined in one of two ways. For reactors where no catalyst
is removed or
added (e.g., batch or fixed-bed reactors), the catalyst weight will be the
initial weight of catalyst
charged to the reactor. For reactors where some or all of the catalyst is
elutriated from the
reactor continuously (e.g., fluid-bed, hybrid, HERB, and riser reactors), the
catalyst weight will
be the steady state weight of the catalyst in the reactor, where steady state
is defined by the input
of fresh catalyst equaling the amount leaving (weight basis). This is often
referred to as the
catalyst "hold-up" and can be measured or estimated from known correlations
for fluid-bed or
riser reactors.
[0049] The term "catalyst activity" denotes the weight of volatile matter
converted per catalyst
weight over a given amount of time. The catalyst activity can be calculated by
multiplying the
WHSV by the fractional conversion of volatile matter in the reactor, or
equivalently the WHSV *
(1-the retention of volatile matter).
[0050] The term "fresh catalyst" denotes a catalyst that has never been
exposed to reactants at
reaction conditions, such as new catalyst received from a vendor.
[0051] The term "spent catalyst" denotes any catalyst that has less activity
at the same reaction
conditions (e.g., temperature, pressure, inlet flows) than the catalyst had
when it was originally
exposed to the process. This can be due to a number of reasons, several non-
limiting examples
of causes of catalyst deactivation are coking or carbonaceous material
sorption or accumulation,
metals (and ash) sorption or accumulation, attrition, morphological changes
including changes in
pore sizes, cation or anion substitution, and/or chemical or compositional
changes.
[0052] The term "regenerated catalyst" denotes a catalyst that had become
spent, as defined
above, and was then subjected to a process that increased its activity, as
defined above, to a level
- 14-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
greater than it had as a spent catalyst. This may involve, for example,
reversing transformations
or removing contaminants outlined above as possible causes of reduced
activity. The
regenerated catalyst may have an activity greater than or equal to the fresh
catalyst, but typically,
regenerated catalyst has an activity that is between the spent and fresh
catalyst.
[0053] The term "HERB" is an acronym that stands for a Hybrid, Elutriating,
Riser-Bed. Any
reactor that has two solids with dissimilar densities or particle sizes,
operated in such a way that
one of the solids is substantially elutriated from the reactor (i.e., the
majority of particles are
entrained in a fluid and carried out in a riser mode), whereas the other solid
is substantially
fluidized, but not elutriated in the reactor, as in a non-limiting example of
a bubbling-bed reactor.
FIGS. 9-13 are non-limiting examples of HERB pyrolysis reactors.
[0054] The term "pyrolysis" refers to the thermochemical decomposition of
organic substances
at elevated temperatures in the absence of oxygen. In general, pyrolysis of
organic substances
produces a gas (and liquid products when the gas product temperatures are
reduced), and leaves
behind a solid residue richer in carbon content, a char. Pyrolysis differs
from other high-
temperature processes like combustion and hydrolysis in that it usually does
not involve
reactions with oxygen, water, or any other reagents. As used herein, pyrolysis
shall be further
stipulated to exclude the on-purpose addition of high-pressure (in excess of 4
bar) steam
(typically referred to as "reforming"), and the on-purpose addition of high-
pressure (in excess of
4 bar) hydrogen (typically referred to as "hydrotreating"), although these
techniques may be used
post-pyrolysis on the pyrolysis products for further upgrading. In addition,
pyrolysis, as used
herein, shall not exclude water or hydrogen added with reagents at lower
pressure or as a part of
a mixture with reagents, including but not limited to, water added into the
gaseous fluidization
media, or moisture carried into the pyrolysis reaction sorbed on the solid
carbonaceous material
or catalyst.
[0055] The so-called proximate analysis of coal is an assay of the moisture,
ash, volatile
matter, and fixed carbon as determined by a series of prescribed or standard
test methods. It
serves as a simple means of determining the distribution of products obtained
when a coal
sample is heated under specific conditions. By definition, the proximate
analysis of coal
-15-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
separates the products into four groups, which are (1) moisture; (2) volatile
matter, consisting of
gases and vapors driven off during pyrolysis; (3) fixed carbon, the
nonvolatile fraction of coal;
and (4) ash, the inorganic residue remaining after combustion. The standard
test method for
proximate analysis (ASTM D-3172) covers the methods of analysis associated
with the
proximate analysis of coal and coke and is, in fact, a combination of the
determination of each of
three of the properties and calculation of a fourth. Moisture, volatile
matter, and ash are all
determined by subjecting the coal to prescribed temperature levels for
prescribed time intervals.
The losses of weight are, by stipulation, due to loss of moisture (at about
107 C for 1 hour) and
loss of volatile matter (at about 950 C for 7 minutes). The residue remaining
after ignition at
the final temperature is called ash. Fixed carbon is the difference of these
three values summed
and subtracted from 100. In low-volatile materials, such as coke and
anthracite coal, the fixed-
carbon value equates approximately to the elemental carbon content of the
sample. Although
these procedures were initially developed for coal, the same ASTM methods have
been widely
used for biomass and other organic substances. In this application, any
references to volatile
matter, fixed carbon, ash, and moisture on any solid carbonaceous material,
both received or
synthesized, will be understood to be measured by this method.
[0056] Because the test method for volatile matter, as described above, is
designed in such a
way to drive off substantially all the volatile matter contained in the
starting material, this
process could be described as afull pyrolysis; whereas partial pyrolysis may
be characterized by
partial liberation of volatile matter and achieved by adjusting pyrolysis
conditions to milder
conditions (e.g., lower pyrolysis temperature < 950 C and/or lower pyrolysis
time < 7 minutes).
[0057] Volatile matter values of coal are important in choosing the best match
between a
specific type of coal-burning equipment and the coal to use with the
equipment. Such values are
valuable to fuel engineers in setting up and maintaining proper burning rates.
As a general
observation, low volatile anthracite coal will not burn as fast as a high
volatile bituminous coal
and, therefore, these two fuel types are not necessarily interchangeable on a
given boiler
configuration. Thus, the amount of retained volatile matter in a pyrolyzed
coal is an important
quality factor, among others, determining it's suitability as a boiler fuel.
For example, if the
-16-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
product coal is over-pyrolyzed into a semi-coke or coke like product, then
this may limit its off-
take option as a boiler fuel although it may be used for metallurgical
applications, such as
steelmaking.
[0058] The solid carbonaceous material may be pre-treated before entering the
catalytic
pyrolyzer. Pretreatment steps may be performed in such a way as to improve the
reactivity of the
starting carbonaceous material in the catalytic pyrolyzer and/or to improve
the overall the quality
of the products being produced in the catalytic pyrolyzer. One such
pretreatment example may
include comminution (e.g., pulverizing) and classification of the starting
carbonaceous material
in order to enhance the starting carbonaceous material's heat and mass
transfer
characteristics. Other examples may include removal of moisture (e.g., drying)
and/or removal
of ash and mineral components (e.g., washing) from the starting carbonaceous
material in order
to promote high reactivity of the pretreated carbonaceous material in the
catalytic pyrolyzer (e.g.,
enhanced release and reactivity of volatile matter in the catalytic
pyrolyzer).
[0059] The term "immobilized" (e.g., "immobilization," etc.) when referring to
catalyst in a
reactor, means that the catalyst is prevented from exiting the reactor, not
necessarily that the
catalyst remains stationary or fixed in place within the reactor.
Immobilization of the catalyst
can be accomplished by a number of methods including, but not limited to, the
following: First,
the catalyst may be fixed in place via deposition, plating, or adhesion to a
packing, monolith, or
wall of the reactor. Second, the use of extrudates or large catalyst particles
or grains of catalyst
may be employed, such that the catalyst cannot be fluidized or entrained by
the gas flow. Third,
a size or mass exclusion where the catalyst may be fluidized, but is not
carried or entrained out
by the fluidization gas but the carbonaceous reactant is carried by the
fluidization gas (i.e.,
"elutriation") may be employed. Fourth, a size exclusion where the catalyst is
prevented from
exiting the reactor by sieve size allowing smaller sized carbonaceous reactant
to pass the sieve
but not the catalyst may be employed. For the third and fourth methods, the
catalyst is not fixed
in place and can move around within the reactor, but is confined to the
reactor.
-17-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0060] The term "predominantly" when referring to upgraded solid product or
catalyst, such as
in a stream of predominantly upgraded solid product/catalyst, means more than
50%. Thus, a
stream of predominantly upgraded coal product includes more than 50% upgraded
coal product.
[0061] The abbreviation HC is used to refer to hydrocarbons to generically
define any
molecule containing hydrogen and carbon atoms. We also will often denote
hydrocarbons by the
number of carbon atoms contained in the molecule by the formal Cx where x is
the number of
carbon atoms contained in the hydrocarbon. For example, C1, C2, and C3 will be
understood to
mean any hydrocarbon containing 1, 2, or 3 carbons, respectively.
[0062] The term "separating" when referring to the separation of solids,
liquids, gases, or any
combination thereof does not necessarily mean 100% separation occurs. Even
when pure
separation may be desirable, it should be understood that 100% separation is
generally not
obtainable, so the term "separation" means as close to 100% separation as is
practicable.
[0063] Referring generally to the Figures, disclosed are systems and processes
for an integrated
thermal pyrolysis and catalytic conversion of coal to obtain a beneficiated
coal product stream
which is substantially reduced in moisture, sulfur, mercury, nitrogen, and
oxygen content; and to
obtain a hydrocarbon-rich product stream which is substantially free of high
molecular weight
tars and hetero-atom containing compounds. The process combines a set of unit
operations
including a catalytic pyrolysis reactor, a catalyst regeneration unit, and at
least partial product
separation into gaseous, liquid, and solid product streams. A solid-solid
separation step is
included in the process to separate upgraded (e.g., beneficiated) coal product
from the spent
catalyst. The process may also include a gaseous separator, such as, for
example, an acid gas
removal (AGR) system, which removes or separates undesirable compounds and/or
elements
from the gaseous product stream. An AGR system may be utilized to remove or
separate any
one or combination of, for example, carbon dioxide (CO2), which has no heating
value, hydrogen
sulfide (H2S), ammonia (NH3), hydrogen cyanide (HCN), as well as any other
polluting and/or
sulfur carrying compounds. Products separated by the gaseous separate (e.g.,
the AGR) may
include a sulfur containing compound stream and/or a high concentration CO2
stream, which can
be used for enhanced oil recovery, CO2 sequestration, or other suitable
purposes.
-18-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0064] The systems and processes, as disclosed herein, may convert
carbonaceous materials,
such as low-grade coals, biomass, bitumen, solid waste, or any suitable carbon-
carrying material
into a set of usable products including an upgraded carbonaceous product, as
well as gas and
liquid products. A carbonaceous raw material releases volatile matter when
heated to pyrolysis
temperatures. Less suitable carbonaceous materials would include those such as
coke, which has
been substantially depleted of its volatile matter content. The catalytic
pyrolysis reactor converts
some or all of the volatile matter into a gaseous product and some portion of
the volatile matter
may also be converted into a solid char or coke residue. The remaining solid
material is an
upgraded solid stream with higher heating value (higher energy density) and
lower polluting
elements, such as sulfur, mercury, and/or nitrogen, compared to the starting
carbonaceous
material. Some light hydrocarbon compounds (e.g., Ci to C3) may be co-fed to
the pyrolyzer
and/or recovered from the hydrocarbon product stream and recycled back to the
pyrolyzer.
[0065] The systems and processes, as disclosed herein, may utilize any
combination of, for
example, a pyrolysis reactor (e.g., system, unit, etc.), a catalyst
regeneration reactor (e.g., system,
unit, etc.), and/or a solid-solid separation system (e.g., unit). A catalyst
may be utilized in the
systems and processes to convert the solid carbonaceous materials into a
usable gaseous product,
a usable liquid product, a usable solid product, or any combination thereof.
[0066] The pyrolysis reactors, as disclosed herein, may utilize a process that
is a relatively mild
in temperature and has a short duration in time, thereby promoting partial
catalytic pyrolysis, as
opposed to a full removal of volatile matter, of the solid carbonaceous
material. The mild
temperature pyrolysis reactors may operate from about 300 C about 1100 C. In
some
embodiments, the pyrolysis reactors may operate from about 350 C to about 850
C. In other
embodiments, the pyrolysis reactors may operate from about 400 C to about 700
C. The
pyrolysis reactors utilizing mild temperature conditions and partial pyrolysis
processes provide
many advantages compared to other technologies, several of which are discussed
herein. First,
the milder operating conditions (e.g., temperature) are less energy intensive.
Second, the gas
product stream recovered contains less tars. For example, an amount of light
oils (e.g., non-tars,
which may include LPGs) produced is greater than an amount of tars produced by
the processes
-19-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
of this application. According to some embodiments, a ratio of the weight of
light oils produced
in the processes (of this application) to the weight of tars produced in the
processes is greater
than 0.3. According to other embodiments, the ratio is greater than 1. The
pyrolysis reactors, as
disclosed herein, may yield a relatively high product weight ratio of light
oil products to tar
products, much greater than 1 (e.g., 5 ¨ 1000, or greater). This simplifies
the product handling
and/or eliminates tar production, which is encountered in other coal pyrolysis
processes. Third,
the systems may produce three different phases of usable products, each of
which can be used for
a wide variety of purposes including, but not limited to, a fuel or a chemical
precursor. For
example, the systems may produce a usable solid product (e.g., a solid
stream), a usable liquid
product (e.g., a liquid stream), a usable gaseous product (e.g., a gaseous
stream), or any
combination thereof The solid stream contains a significantly upgraded quality
of solid
carbonaceous matter, which can be used as fuel or processed further, such as
for full gasification.
The physical states of matter for the fluid streams may be liquid or gaseous
depending on the
temperature and pressure of the particular stream. The fluid streams may
contain a fraction of
high value olefins and aromatics that can be separated or sold in bulk as
synthetic crude oil to be
processed in existing refineries. The fluid streams may also contain a variety
of chemicals that
can be used as fuels within the plant or separated for saleable fuels (e.g.,
hydrogen, carbon
monoxide, LPGs, natural gas liquids (NGL), etc.), as monomers, and/or as
intermediates for
subsequent chemical processes. Fourth, by running the pyrolysis reactors at
mild temperature
conditions only a portion of volatile matter will be liberated from the
starting solid carbonaceous
material, the remainder of which will be retained in the upgraded solid
product, such as upgraded
solid coal product so that the upgraded solid coal product is more suitable
for downstream
combustion operations.
[0067] FIG. 1 illustrates an illustrative embodiment of a system 100
configured to use a solid
carbonaceous material, such as, for example low-grade coal. As shown, the
system includes a
pulverizer 101, a dryer 102, an assembly for performing the pyrolysis (e.g., a
pyrolysis reactor
103, a pyrolyzer, etc.), a separator (e.g., a condenser 104, a classifier 105,
a product separation
unit 106, etc.), and a regenerator 107 (e.g., regeneration assembly,
regenerator unit, regeneration
reactor). The coal is introduced into the pulverizer 101 via a pipe 111 (e.g.,
conveyor, tube, etc.)
-20-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
for pre-processing to reduce the size of the coal into appropriate sized
particles, which are then
passed into the dryer 102, such as through a pipe 112. The pulverizer 101 may
include an inlet
113 configured to introduce air (e.g., relative dry air) into the pulverizer
to help dry the coal
during pulverization and an outlet 114 configured to remove relatively wet air
from the
pulverizer 101. In the dryer 102, the coal particles are subjected to a drying
gas (e.g., air) to
reduce the moisture content in the coal particles. The dryer 102 includes an
inlet 121 for
introducing relatively dry air and an outlet 122 configured to exit the
relatively wet air from the
dryer 102. The dried coal is then passed from the dryer 102 to the pyrolysis
reactor 103, such as
through an inlet pipe 123 (e.g., a first inlet) that fluidly connects an
outlet of the dryer 102 to an
inlet of the pyrolysis reactor 103.
[0068] In the pyrolysis reactor 103, the coal and the catalyst come into
contact. The catalyst
may be introduced via a second inlet. According to one non-limiting example,
the reactor 103
includes a second inlet 132 configured to introduce fresh catalyst (e.g.,
previously non-reacted
catalyst) and a third inlet 133 configured to introduce regenerated catalyst
into the reactor 103.
The catalyst may be an acid catalyst, a fluid cracking catalyst, a
hydrocracking catalyst, and the
like. One or more catalyst types may be used at the same time. Such catalysts
and supports for
such catalysts may include, but are not limited to metals such as Mo, Zn, Ga,
Pt, W, Ni, V, Co,
Mn, or Cu; metal oxides; carbon-based materials; and mixtures of any two or
more thereof
Illustrative examples of such catalysts and catalyst supports may include, but
are not limited to,
platinum, palladium, ruthenium, osmium, nickel, cobalt, rhenium, molybdenum,
zinc, gallium,
tungsten, vanadium, manganese, copper, or a mixture or alloy of any two or
more such metals,
natural zeolites, synthetic zeolites, carbon nanotubes, graphene, graphite,
alumina, and silica.
The catalysts may be microporous (pore size up to 2 nanometers) in some
embodiments. In
other embodiments, the catalyst may be mesoporous (pore size from 2 to 50
nanometer) or
macroporous (pore size greater than 50 nanometers). And in other embodiments
the catalyst
material may be a hybrid containing any combination of micrporous, mesoporous
and
macroporous structures.
-21-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0069] Some zeolites, although not necessarily all, may be of the formula
Mx/nRA102)x(5i02)A=n1H20, where M is an alkaline or alkaline earth metal, x
and y are the
total number of tetrahedra per unit cell where the ratio of y/x is from about
1 to about 5 for an
alumina-based zeolite, or y/x is from about 10 to about 100 for a silica
zeolite. With M as an
alkaline or alkaline earth metal, n is 1 or 2. The ratio of Si/Alin the
formula may range from
12:1 to 1000:1. In some embodiments, the ratio of Si/A1 in the formula is from
14:1 to 500:1. In
some embodiments, the ratio of Si/Alin the formula is from 15:1 to 250:1. In
the general
formula m is the number of water molecules of crystallization. Other synthetic
zeolites are
generally known and may be used as well. Illustrative zeolites include, but
are not limited to,
those with topologies AEL, BEA, CHA, EUO, FAO, FAU, FER, KFI, LTA, LTL, MAZ,
MOR,
MEL, MTW, LEV, OFF, TON, MWW, MCM and MFI. Zeolites may also include those
such
as, but not limited to, ZSM-5, PSH-3, ITQ-2, ZSM-12, MCM-22, MCM-36, MCM-49,
MCM-
56, MCM-58, MCM-68, H-Beta, H-Y, H-USY, H-MOR and HZSM-5. Illustrative unit
cell
compositions of zeolites include, but are not limited to,
Nai2RA102)12(Si02)12]=27H20;
Na6RA102)6(Si02)10]=12H20; (Na,TPA)3[(A102)3(SiO2)93]=16H20;
Na86[(A102)86(5i02)106]=264H20; Na56[(A102)56(5i02)136]=250H20; and
Na8RA102)8(5i02)40]=24H20, where TPA is tetrapropylammonium. Other frameworks
as
described by the Famework Type Code (FTC) may also be used.
[0070] Some zeolites, although not necessarily all, may be of the formula
IMthi(F120)yl[AlxSi(t_x)02d4ZA, where the guest species are listed between the
braces ("1...1") and
the host framework is listed between the brackets ("[...]"). M represents a
charge-balancing
cation, x is the number of framework Al atoms in the unit cell, n is the
cation charge, y is the
number of adsorbed water molecules, t is the total number of framework
tetrahedral atoms in the
unit cell (Al+Si), and IZA is the code for the framework type assigned by the
Structure
Commission of the International Zeolite Association.
[0071] The zeolites for use in the systems and processes, as disclosed herein,
can be post-
treated (e.g., by de-alumination or by ion exchange, such as is required to
convert, for example,
the sodium form to H-form (e.g., H-Beta, H-Y, H-USY, H-MOR and HZSM-5). Such
de-
-22-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
aluminated zeolites may, for example, help promote ethylene oligomerization.
Also, for
example, the zeolite particles may be densifled (e.g., post-processed to
increase the bulk density
of the particles).
[0072] The catalysts may have a wide range of pore sizes (e.g., average pore
size). For
example, the catalyst may have a pore size from about 0.26 to about 0.74 nm.
This includes
catalysts with a pore size from about 0.26 to about 0.57 nm, about 0.28 to
about 0.48 nm, about
0.31 to about 0.45 nm, as well as from about 0.51 to about 0.55 nm, about 0.53
to about 0.56 nm,
and about 0.65 to about 0.70 nm. For example, an A zeolite (e.g., having an
LTA structure) may
have a pore size of about 0.41 nm. Also, for example, a P zeolite (e.g.,
having a GIS structure)
may have a pore size of about 0.31 x 0.45 nm. Also, for example, a ZSM-5
zeolite (e.g., having
an MFI structure) may have a pore size of about 0.53 x 0.56 nm. Also, for
example, a ZSM-5
zeolite (e.g., having an MFI structure) may have a pore size of about 0.53 x
0.56 nm or about
0.51 x 0.55 nm. Also, for example, an X zeolite (e.g., having an FAU
structure) may have a pore
size of about 0.74 nm. Also, for example, a Y zeolite (e.g., having an FAU
structure) may have a
pore size of about 0.74 nm. Also, for example, a Mordenite zeolite (e.g.,
having an MOR
structure) may have a pore size of about 0.65 x 0.70 nm or about 0.26 x 0.57
nm. The pore sizes
provided in this application are examples, and are not limiting in nature.
[0073] The pyrolysis reactors may operate from about 300 C to about 1100 C.
The
carbonaceous material may have a residence time of 0.01 seconds to about 5
hours. In some
embodiments, the residence time is from about 0.1 second to about 1 minute.
The catalyst
loading for the pyrolysis reactor may be from about 0.01 g catalyst/g
carbonaceous feed material
to about 100 g catalyst/g carbonaceous feed material. In some embodiments, the
catalyst
loading is from about 0.05 g catalyst/ g fee to about 20 g catalyst/g feed. In
yet other
embodiments, the catalyst loading is from about 0.1 g catalyst/g feed to about
10 g catalyst/g
feed. The heating rate of the carbonaceous material being introduced into the
pyrolysis reactor
may be from about 0.1 C/second to about 1000 C/second. However, it is noted
that flash
pyrolysis can involve a heating rate in the reactor in excess of 1000
C/second.
-23-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0074] Also shown in FIG. 1, the solids are passed from an outlet of the
pyrolysis reactor 103
to the classifier 105 via a pipe 134. The pyrolysis reactor 103 may include
other outlets. For
example, gas products may be removed from the pyrolysis reactor 103 by way of
a second outlet
and passed through a line 135 (e.g., pipe) to, for example, the condenser 104
for further
processing (e.g., separation). The classifier 105 is configured to separate
the upgraded coal
product, which is recovered via a first outlet 151 of the classifier, and the
spent catalyst, which is
recovered via a second outlet 171 of the classifier.
[0075] According to one illustrative embodiment, the pyrolysis reactor is a
fluidized bed where
the coal and catalyst are suspended in a gaseous phase and mixed for a desired
reaction residence
time. The solids may be separated in the reactor to produce a first solid
stream containing
predominantly upgraded coal and a second solid stream containing predominantly
spent catalyst.
Upgraded coal generally refers to low ranked coal (e.g., sub-bituminous,
lignite, etc.) that has
been altered (e.g., improved), such as by removing moisture and/or pollutants,
to increase the
efficiency and/or reduce the emissions of the coal when burned (e.g.,
combusted). As an
example, catalytic pyrolysis of the coal may take place at a temperature of
about 350 C to about
850 C, and may have a residence time from 0.1 second to about 1 minute during
the catalytic
process.
[0076] The first solid stream of upgraded coal can be removed from the reactor
for further
processing or use. For example, the upgraded coal can be pelletized or
briquetted for purposes of
transporting the upgraded coal elsewhere. Also, for example, the upgraded coal
can be used
within the plant as a solid fuel. The stream of solid product may have a
certain amount of spent
catalyst carried over with it. The second solid stream (e.g., a used catalyst
stream), which may
contain some upgraded coal, is transferred (e.g., transported), such as
through a pipe or
conveyor, to the regenerator where it is mixed with air or any suitable oxygen-
carrying gas to
burn off any coke and/or residual coal on the spent catalyst to regenerate the
catalyst.
[0077] As shown in FIG. 1, the spent catalyst is separated by the classifier
105 and sent to the
regenerator 107 via a line 171 (e.g., pipe) for regeneration (e.g.,
rejuvenation, etc.) in the form of
a spent catalyst stream. The spent catalyst stream may come directly from the
pyrolysis reactor,
-24-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
such as for pyrolysis reactors that perform solid-solid separation internally.
An oxygen-
containing gas stream may be introduced into the regenerator 107, which may be
used to
combust all or nearly all of the combustible matter, such as coke, coal, etc.,
that is carried within
the spent catalyst stream into the regenerator 107. The regenerator 107
includes an inlet through
which the gas stream enters the regenerator. The gas inlet may be the same as
or different than
the spent catalyst stream inlet. For example, gas (e.g., air) may be
introduced through a gas inlet
172. The regenerator also includes an outlet line 174 (e.g., pipe) through
which the regenerated
catalyst stream exits, such as to enter the pyrolysis reactor. In the
regenerator, air or another
suitable oxygen-carrying gas is introduced to burn at least a portion of the
coke/coal off of the
spent catalyst to regenerate the spent catalyst. The regenerator 107 may
include an outlet 173
through which regenerator exit gas, such as flue gas, exits the regenerator.
Optionally, at least a
portion of the regenerator exit gas may be used to carry the regenerated
catalyst to the pyrolysis
reactor. A small purge of the regenerated catalyst may be used to prevent the
accumulation of
the ash or other impurities. For example, a purge valve 175 may be provided in-
line between the
outlet line 174 and a pipe connecting a first outlet of the purge valve 175 to
the third inlet 133 of
the pyrolysis reactor 103. A second outlet 176 of the purge valve 175 is
configured to pass the
purged catalyst from the system.
[0078] Once regenerated, the catalyst may be returned to the pyrolysis reactor
103 for further
catalytic pyrolysis of coal. For example, the regenerated catalyst may be
reintroduced into the
pyrolysis reactor 103 via the third inlet 133. The pyrolysis reactor 103 may
include additional or
fewer inlets and/or outlets. For example, the reactor may include first and
second outlets, where
the solid stream is configured to exit the reactor through the first outlet
and the fluid stream is
configured to exit the reactor through the second outlet.
[0079] A portion of the gas exiting the regenerator may include a portion of
catalyst (e.g.,
fines), which may be recovered and recombined with a portion of regenerated
catalyst in the
regenerated catalyst stream or introduced directly into the pyrolysis reactor
to maintain a desired
catalyst-to-coal ratio. The catalyst-to-coal ratio may be, for example, from
about 0.001 g
catalyst/g carbonaceous feed to about 100 g catalyst/g carbonaceous feed. In
some
-25-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
embodiments, the catalyst-to-coal ratio is from about 0.01 g catalyst/g
carbonaceous feed to
about 100 g catalyst/g carbonaceous feed. In other embodiments, the catalyst-
to-coal ratio is
from about 0.05 g catalyst/g carbonaceous feed to about 20 g catalyst/g
carbonaceous feed. In
yet other embodiments, the catalyst-to-coal ratio is from about 0.1 g
catalyst/g carbonaceous
feed to about 10 g catalyst/g carbonaceous feed. Other reactive or nonreactive
solids may be
included in the catalyst regeneration cycle as a heat source. For example,
sand can be
recirculated with the catalyst, where the sand absorbs excess heat in the
regenerator. The hot
sand is carried into the pyrolysis reactor where its heat is absorbed by the
incoming
carbonaceous solid material.
[0080] The fluid product stream out of the pyrolysis reactor may be
transferred downstream for
further processing. For example, the fluid product stream may be transferred
to a set of unit
operations where the fluid product stream is separated into one or more liquid
hydrocarbon
product streams, one or more gaseous product streams, one or more aqueous
streams, and/or a
combination of any two or more such streams. As shown in FIG. 1, the fluid
product stream is
passed from the pyrolysis reactor 103 to a system (e.g., a condenser 104)
configured to act as a
partial condenser affecting a gas-liquid separation. The condenser 104 may
also serve the
function of a liquid-liquid decanter allowing for separation of an aqueous
liquid phase from a
hydrocarbon liquid phase due to immiscibility. As shown, a gas stream is
passed, such as
through a line 141 (e.g., an outlet pipe), from an outlet of the condenser 104
to a product
separation unit 106 for further processing. According to an exemplary
embodiment, organic
liquids are separated from aqueous liquids by the condenser 104, where the
organic liquids are
removed from the condenser 104 via a second outlet line 142 (e.g., pipe) and
the aqueous liquids
are removed from the condenser 104 via a third outlet line 143 (e.g., pipe).
Part, or all, of each
aqueous and/or hydrocarbon liquid stream may be used downstream in, for
example, other
systems or processes in the plant (e.g., facility). In one such example, at
least a portion of an
aqueous liquid stream and/or a portion of a hydrocarbon stream may be used for
briquetting the
coal product stream. The liquid hydrocarbon stream may be packaged as
synthetic crude oil, or
further separated into specific product streams, such as, for example, a BTEX
and/or a BTX
(e.g., mixtures of aromatic hydrocarbons such as, but not limited to, benzene,
toluene, and the
-26-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
three xylene isomers) rich stream. The liquid hydrocarbon stream may also be
processed further
by chemical upgrading in other chemical reactors, such as for the purpose of
de-oxygenation of
any oxygen carrying products. The gaseous product stream may be used in the
plant as a fuel or
separated into one or more than one useable product stream.
[0081] As part of the separation units of the gas and liquid products, the
product stream may be
processed through an acid gas removal system to capture sulfur-carrying
compounds, nitrogen-
containing compounds (e.g., ammonia or hydrogen cyanide), and/or carbon
dioxide. The carbon
dioxide-rich stream can be sequestered, sold and transported, or used for
enhanced oil recovery.
As shown in FIG. 1, the CO2 stream can be separated by the product separation
unit 106 and
removed via a first outlet line 161 (e.g., pipe). The sulfur-carrying
compounds may be processed
further before being packaged as a sulfur-carrying product (e.g., as elemental
solid sulfur, as
sulfuric acid, etc.), or sent to a landfill. Also shown in FIG. 1, the sulfur-
carrying compounds
can be separated by the product separation unit 106 and removed via a second
outlet line 162
(e.g., pipe). The acid gas removal system may be one of the first separation
processes of the
fluid product stream exiting the pyrolysis reactor, or may be further
downstream in the
separation process. Other compounds/products may be recovered as well. For
example, the
product separation unit 106 may be configured to separate hydrocarbons, such
as from the gas
stream, and pass the recovered hydrocarbons via a third outlet line 163 (e.g.,
pipe).
[0082] Heat from other processes in the system may be used as input heat into
the pyrolysis
reactor, such as to heat the carbonaceous material. For example, heat from the
hot regenerated
catalyst may be used to provide heat and to maintain a desired temperature in
the pyrolysis
reactor. Also, for example, the flue gas exiting the catalyst regenerator may
be used to provide
heat either directly, or indirectly, to the dryer and/or pyrolysis reactor.
The flue gas exiting the
regeneration unit may be mixed with some of the gas product stream and
combusted to generate
heat for the dryer, the pyrolysis reactor, and/or other devices, processes, or
units in the plant.
The mixing of the flue gas with some of the combustible gas may advantageously
further reduce
the oxygen content in the flue gas. The non-condensable gas stream may be
combusted with
plant air, fresh air, and/or air from within the process to provide heat to
the dryer, pyrolysis
-27-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
reactor, regeneration reactor, and/or other devices, processes, or units
within the plant. This
combusted gas may also be used to generate steam, such as for use within the
facility.
[0083] According to one embodiment, the regeneration reactor uses a pure
oxygen stream or an
oxygen rich stream to combust all or most combustible matter on the spent
catalyst or the
carbonaceous matter that is carried over into the regeneration reactor.
According to another
embodiment, an oxygen lean (e.g., where the oxygen content is diluted to less
than 2%) stream is
utilized with the regenerator. According to other embodiments, hydrogen,
steam, CO2, CO, or
any combination thereof is used to remove the carbon off the spent catalyst.
In yet another
embodiment, oxygen is mixed with the previously mentioned chemicals to remove
the coke on
the spent catalyst. In all these embodiments, the exiting gas stream may be
rich in CO2 which
can be separated for sequestration, enhanced oil recovery, or other suitable
purposes. According
to one example, at least a portion of any unpyrolyzed coal, coke, and
carbonaceous material is
removed from the catalyst by at least one of combustion, steam, and a reducing
gas.
[0084] According to one embodiment, the solids of the pyrolysis reactor are
removed from the
reactor and separated ex situ into a predominantly solid stream and a
predominantly spent
catalyst stream (e.g., a stream that has more than 50% catalyst). Since the
cost of the catalyst is
significantly higher than the carbonaceous material, it is desirable to keep
and recycle as much
catalyst as possible. Thus, the solid-solid separation may be tailored to
minimize the amount of
catalyst that is comingled with the solid stream, even at the expense of
increasing the amount of
carbonaceous material that is comingled with the spent catalyst stream.
According to an
exemplary embodiment, eighty percent (80%) by weight or more of the spent
catalyst is captured
during the solid-solid separation. Preferably, ninety percent (90%) by weight
or more of the
spent catalyst is captured during the solid-solid separation.
[0085] This solid-solid separation can be performed by any number of
separation processes
including, but not limited to, classifiers, magnetic separation, electro-
static separation, or a
combination of any two or more such separation processes. For example, the
particle size
distributions of carbonaceous material and the catalyst are intentionally
different, allowing for
appropriate classification technology to separate the two solids by
differences in particle size,
-28-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
weight and/or density. In one such example, the carbonaceous material enters
the pyrolysis
reactor with average size particles from about 100 [tm to about 300 um. In
another such
example, the carbonaceous material has average size particles from about 10
[tm to about 100
um. In one example, the catalyst enters the pyrolysis reactor with average
size particles of about
300 [tm to about 500 pm. In another such example, the catalyst has average
size particles from
about 5001..tm to about 1000 pm. A classifier is used to effectively separate
the upgraded solid
product from the catalyst. In another embodiment, the solid stream exiting the
regenerator may
also be separated to remove all or some of the ash and/or impurities before
the regenerated
catalyst stream is returned to the pyrolysis reactor. For example, the solid
stream may be
demineralized and/or demetalized to remove impurities. A wet process, solid-
liquid process, or
any other suitable process may be used to rejuvenate the spent catalyst.
"Fresh" catalyst as
make-up catalyst could be supplied by a vendor, whom has rejuvenated and/or
demetalized spent
catalyst from other sources, such as, for example, reusing catalyst from a
pyrolysis process
discussed in this application or reusing a catalyst from a conventional
fluidized catalytic cracking
(FCC) process. Stated differently, the "fresh" catalyst does not have to
consist of only "virgin"
(e.g., unreacted) catalyst, but may be a mixture of "virgin" catalyst and
rejuvenated catalyst.
[0086] Some, or all, of the air streams may be used within the system (e.g.,
the process of the
system) or elsewhere in the facility. For example, the air exiting the
pulverizer may be used as
over-fire air in the boiler to reduce NO (e.g., nitrous oxides, nitric oxides,
etc.) production in the
furnace. Similarly, the flue gas from the regenerator and light gas products
from the pyrolysis
reactor (CO, H2, methane, ethane and ethylene) may be used in the furnace and
mixed with other
chemicals for its heating value and as a reburn stream or chemical injection
for de-NO x purposes.
[0087] In any of the above embodiments, the pyrolysis reactor may be
configured such that
both catalyst and carbonaceous solid materials enter and exit the pyrolysis
reactor at controlled
rate(s) (e.g., flow rate, movement rate, etc.). In another embodiment, the
pyrolysis reactor may
be configured such that the catalyst is immobilized within the pyrolysis
reactor while the solid
carbonaceous material enters and exits the reactor. In either embodiment, a
carrier gas may, or
may not, be utilized to pneumatically transport the movable solids through the
reactor and to
-29-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
hydrodynamically enhance mixing and chemical conversion in the reactor. With
an immobilized
catalyst in the reactor, the need for ex-situ solid-solid separation may be
eliminated.
[0088] FIGS. 2 and 3 illustrate other illustrative embodiments of systems
configured to
produce a usable solid stream, a usable liquid stream, and/or a usable gaseous
stream from a
feedstock (e.g., a carbonaceous feedstock, such as coal). The systems shown in
FIGS. 2 and 3
are similar to the system of FIG. 1, except each system is configured to
introduce a recycled gas
(that is taken from another process in the system) into the pyrolyzer. Thus,
common reference
numerals have been used in FIGS. 1-3 to identify common elements (e.g.,
components,
assemblies etc.).
[0089] The system of FIG. 2 utilizes a recycled gas that has been separated
from other gases
via a gas-gas separator (e.g., the product separation unit 106, an AGR). The
gas stream that is
separated from the usable liquid(s) in a gas-liquid separator (e.g., a
condenser) is then separated
into two or more usable gas streams, and at least a portion of one or more of
the usable gas
streams is then routed back to the pyrolyzer as recycled gas. As shown in FIG.
2, the gas stream
from the gas-liquid separator is separated by the product separation unit 106
into three usable gas
streams, where the first is a CO2 stream 161, the second is a sulfur-
containing gas stream 162
(e.g., H25), and the third is a hydrocarbon gas stream 163. At least a portion
of the hydrocarbon
gas stream 163 is routed, such as through a line 265 (e.g., pipe), to the
pyrolyzer 203 to be used
therein. For example, the gas-gas separator may isolate methane, a portion of
which is directed
to the pyrolyzer 203 and the remainder is recovered for use elsewhere. The
system 200 may
optionally include a valve or other suitable device that separates, for
example, a usable gas
stream (e.g., the hydrocarbon gas stream) into two separate streams, such that
a portion is used as
recycled gas and the other is used elsewhere. The system 200 may optionally
include a valve to
purge a portion of the recycled gas stream between the product separation unit
and the pyrolyzer.
As shown in FIG. 2, a first valve 264 is configured to control a flow of the
hydrocarbon gas
stream 163 through the line 265 toward the pyrolyzer 203 and a flow of the
hydrocarbon gas to
be recovered for other purposes, and a second valve 266 is provided downstream
of the line 265
-30-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
to control the flow of the gas stream to be purged via a purge outlet 268 and
the gas stream that
is recycled back to the pyrolyzer 203 via the recycle line 267 (e.g., pipe).
[0090] Also referring to FIG. 2, and according to one example, the gas stream
exiting the
pyrolysis reactor 203 through a gas outlet contains a non-condensable fuel
gas. The gas stream
may enter, for example, an AGR system where all sulfur compounds and at least
the bulk of the
carbon dioxide gas are removed. The sulfur compounds can be disposed of (e.g.
landfilled) or
converted to a usable product, such as solid elemental sulfur or sulfuric
acid. The CO2 stream
can be further purified or transferred elsewhere for other purposes, such as
enhanced oil
recovery. The cleaned gas stream may then be further processed to recover the
light molecular
weight gases (mainly methane, ethane, ethylene, hydrogen, CO) and used to
fluidize the
pyrolysis reactor. One advantage of recycling the light molecular weight gases
back to in the
pyrolyzer is that these gases have a second interaction in the pyrolyzer,
prompting an increased
yield of the liquid hydrocarbon fraction. Another advantage of recycling these
gases back to the
pyrolyzer is that it obviates the need for introducing another feed to the
process (e.g., nitrogen
for fluidization and as carrier gas) which lowers operating and equipment
costs. The non-
condensable gas fraction not recirculated in the pyrolysis reactor can also be
used in the
regeneration reactor, dryer, pyrolysis reactor, or burned in the boiler for
added heat and/or de-
NO x technology. The non-condensable gas may also be fractionated by cryogenic
distillation or
other suitable means into individual components (e.g., methane, ethane,
ethylene, hydrogen,
carbon monoxide) and further processed, recycled or sold.
[0091] The system of FIG. 3 utilizes a recycled gas that has not been
separated via a gas-gas
separator (e.g., a product separation unit, an AGR). In other words, at least
a portion of the gas
stream that is separated from the usable liquid(s) in a gas-liquid separator
(e.g., a condenser) is
sent directly (prior to gas-gas separation) to the pyrolyzer 303 as recycled
gas. The remainder of
the gas stream may be sent downstream to fractionation units, such as
cryogenic distillation or
other suitable separation devices, to further separate the gas stream into
more than one usable gas
product streams. As shown in FIG. 3, the gas stream exiting the condenser 104
is piped via a
line 341 (e.g., pipe) to a valve 364, which controls the flow of the gas
stream to the product
-31-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
separation unit 106 via a line 366 (e.g., pipe) and the flow of the gas stream
to the pyrolyzer 303
via a line 365 (e.g., pipe). Thus, the valve 364 controls how much of the gas
exiting the
condenser 104 is distributed as recycled gas to the pyrolyzer 303 and how much
of the gas is
distributed further processing downstream by the product separation unit 106.
[0092] Also referring to FIG. 3, according to an illustrative embodiment, the
recirculated gas
comes out of the first condenser 104 prior to entering the product separation
unit 106. The main
advantage of recovering the recirculation gas before the separation units is
that the separation
units can be sized smaller because they do not process the recirculating gas.
The smaller size
reduces equipment size and cost, as well as reduces the energy requirement for
the separation.
As with the embodiment in FIG. 2, the light molecular weight gases entering
the pyrolysis
reactor promote an increased pyrolysis yield of the liquid hydrocarbon
fraction.
[0093] In yet other embodiments, the pyrolysis reaction is staged into more
than one reactor
system. FIG. 4 illustrates an example of such a staged reactor system. As
shown, the system
includes a first pyrolysis assembly 403 (e.g., a first pyrolyzer, first
reactor, etc.), in which a
carbonaceous feedstock material, such as coal, is pyrolyzed to produce a
gaseous pyrolysis
product stream and a solid product. The gas product stream is transferred from
the first pyrolysis
assembly 403 to a second pyrolysis assembly 408 via a first outlet pipe 435.
The solid product is
transferred from the first pyrolysis assembly 403 to a classifier 105 via a
second outlet pipe 434.
[0094] As shown in FIG. 4, the pyrolysis reaction in the first pyrolysis
assembly 403 is a
catalytic reaction. In the catalytic first pyrolysis assembly, the catalyst
may be fresh (i.e., new)
catalyst, regenerated catalyst (e.g., from the regenerator), or any
combination thereof. The solid
product produced by the first pyrolysis assembly 403 is delivered to a
downstream solid-solid
separator (e.g., a classifier 105) via a line 434 (e.g., pipe) to separate the
spent catalyst and the
upgraded solid product (e.g., upgraded coal). The gaseous pyrolysis product
stream produced by
the first pyrolysis assembly 403 is delivered to the downstream second
pyrolysis assembly 408
(e.g., a second pyrolyzer, second reactor, etc.) via a line 435 (e.g., pipe).
Like with FIGS. 2
and 3, FIG. 4 also includes common reference numerals with numerals used in
FIGS. 1-3, which
-32-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
are meant to identify similar or common elements (e.g., components, assemblies
etc.). Hence,
the description of the common reference numerals is not duplicated here.
[0095] As shown in FIG. 4, the second pyrolysis assembly 408 includes an inlet
configured to
receive the gaseous product stream from the first pyrolysis assembly, such as
through the inlet
line 435, and therein catalytically processes the gaseous product stream into
a gaseous product
and a liquid product. The second pyrolysis assembly 408 may include additional
inlets. For
example, the second pyrolysis assembly 408 may include a second inlet that is
configured to
receive fresh catalyst via a line 481 (e.g., pipe) and a third inlet that is
configured to receive
regenerated catalyst via a line 482 (e.g., pipe). The second pyrolysis
assembly 408 may include
one or more outlets. For example, the second pyrolysis assembly 408 may
include a first outlet
through which the gas product is transferred to the condenser 104 via a line
484 (e.g., pipe) and a
second outlet through which the spent catalyst is transferred to a regenerator
via a line 483 (e.g.,
pipe). Thus, the spent catalyst may be delivered to a regenerator to
regenerate the spent catalyst.
FIG. 4 illustrates a first catalyst regenerator 107 configured to regenerate
the spent catalyst used
in the first pyrolysis assembly 403 and a second catalyst regenerator 409,
which is separate from
the first catalyst regenerator 107, and is configured to regenerate the spent
catalyst used in the
second pyrolysis assembly 408. Having two separate regenerators may be
advantageous for
systems in which different catalysts are used in the two pyrolysis assemblies.
[0096] The second catalyst regenerator 409 receives the spent catalyst via an
inlet line 483
(e.g., pipe) from the second pyrolysis assembly 408. An oxygen-containing gas
stream may be
introduced into the regenerator 409 by way of inlet line 492 and may be used
to combust all or
nearly all of the combustible matter carried within the spent catalyst from
the second pyrolysis
assembly 408. The regenerator 409 also includes an outlet line 494 (e.g.,
pipe) through which
the regenerated catalyst exits the regenerator 409. At least a portion of the
regenerated catalyst
may be routed to the second pyrolysis assembly 408. The regenerator 409 may
include a second
outlet 491 through which regenerator exit gas, such as flue gas, exits the
regenerator. Optionally,
at least a portion of the regenerator exit gas may be used to carry the
regenerated catalyst to the
pyrolysis reactor. A small purge of the regenerated catalyst may be used to
prevent the
-33-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
accumulation of the ash or other impurities. For example, a purge valve 495
may be provided in-
line of the outlet line 494 to pass the purged catalyst from the system via
line 496. Line 482 may
be configured to introduce regenerated catalyst back to the second pyrolysis
assembly 408. Line
482 may be downstream from the purge valve 495.
[0097] Although FIG. 4 illustrates two separate regenerators, the system may
be configured
having a single regenerator, such as where the same catalyst is being used in
both pyrolyzers.
The solid product may be sent to a first downstream process, the liquid
product may be sent to a
second downstream process, and the gaseous product may be sent to a third
downstream process.
For example, the systems may include product separation units, such as
condensers, AGRs, or
any other suitable separators to further refine the product output of the
system.
[0098] In the pyrolysis reactor/step, a portion of the volatile matter of a
solid carbonaceous
feedstock is generated in the presence of solid catalyst. This feedstock can
be coal of any raffl(
(including lignite), biomass, or peat, as examples. In the reactor, it is
desirable to maximize the
contact between the catalyst and the solid feedstock in order to control yield
and selectivity to the
greatest extent possible. From this standpoint, a fluidized-bed or riser
reactor would be
desirable. The design of such a bed would be done in order to maximize the
mixing and avoid
spontaneous separation of the two solid substrates (catalyst and feedstock).
Given that mixing in
fluid bed and riser reactors is largely dictated by each solid's fluidization
characteristics, which
in turn is driven by the particle size and shape, it would be generally
desirable to match size and
shape of both the catalyst and the solid feedstock. On the other hand, it is
desirable to separate
the spent catalyst from the feedstock after the pyrolysis step, because the
spent catalyst needs to
be regenerated and re-used (e.g., due to its relative high cost compared to
the feedstock), and the
remaining solid from the carbonaceous feedstock needs to be removed and
processed as a
saleable co-product (along with the pyrolysis gas). Most standard,
industrially available solid-
solid separators typically rely on differences in size, density, and shape
either by using size
exclusion, fluidization, or classification in order to accomplish separation.
Thus, there is an
inherent trade-off in the combination of these two unit-operations. Either
make the morphology
of the two solids similar, thereby providing good reactive contact in the
pyrolyzer while
-34-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
sacrificing efficient separation leading to product loss, catalyst loss, or
expensive and/or exotic
separation schemes, or having poor mixing in the reactor leading to low per-
pass yields and/or
poor selectivity of the desired products. Due to these tradeoffs, typically
two separate unit
operations are utilized to accomplish reaction and product separation.
[0099] One way of eliminating the downstream solid-solid separator (e.g.,
classifier) is to use a
pyrolysis reactor configured to utilize an immobilized catalyst while the
carbonaceous material
enters and exits the reactor, such as via an inlet and an outlet.
Immobilization may
advantageously allow an intrinsic separation of the carbonaceous reactant from
the catalyst
without requiring an additional separation unit operation provided downstream
from the reactor.
However, one must also address the need to regenerate the catalyst because the
catalyst cokes as
a normal consequence of carrying out the pyrolysis reaction.
[0100] According to one illustrative method, three cycles are used. In the
first cycle, the
pyrolysis reactor is charged with catalyst, which may be preheated to reactor
temperature. The
reactor is heated to the desired pyrolysis temperature, and coal is introduced
along with a
fluidization gas. The fluidization gas may be any non-oxidizing gas,
including, but not limited
to, nitrogen, helium, neon, argon, hydrocarbon gases, recycled or fresh fuel
gas, recycled or fresh
liquefiable petroleum gases, carbon dioxide, or hydrogen. The fluidization
provides mixing
between the carbonaceous reactant and the catalyst solid. The reactor may be
run in (a) a true
batch mode, where a defined amount of carbonaceous reactant is introduced
initially and kept in
the pyrolysis reactor for a defined time, or (b) semi-batch, where the
reactant is continuously fed
into the pyrolysis reactor and continually removed via entrainment out of the
pyrolysis reactor
with fluidization and product gases. In case (a), residence time is controlled
by fixing the dwell
time of the charge, and then increasing the fluidization gas velocity to
entrain the solid products
and pneumatically convey the solid products out of the reactor, whereby it is
recharged again
with new carbonaceous reactant feedstock. In case (b), the gas velocity of the
fluidization gas
must exceed the entrainment velocity of the solid carbonaceous reactant and
the residence time is
controlled by adjusting the gas velocity to the desired amount (but should
always be greater than
the entrainment velocity of the solid carbonaceous feed). In both true batch
and semi-batch
-35-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
mode, the catalyst will eventually coke. This will be apparent as the product
selectivity will
change. Production of hydrocarbon products will drop, and usually, selectivity
will change as
well. When this happens, fresh feedstock is stopped (or no more batches are
added), and the
reactor is put into the second cycle.
[0101] In the second cycle, the spent catalyst and upgraded carbonaceous
product are
separated. If it is being run as a true batch, the fluidization velocity is
increased such that the
upgraded carbonaceous product is entrained out of the reactor. If it is being
run as in semi-batch
mode, the flow of carbonaceous material feedstock into the reactor is stopped
and fluidization
velocity (i.e., superficial gas velocity) may be increased. In either case,
once the upgraded
carbonaceous product flow stops or an acceptable portion of the upgraded
product has exited the
reactor, then the reactor is ready for the third cycle.
[0102] In the third cycle, the reactor is then put into regeneration mode. The
reactor
fluidization gas is changed from the non-oxidizing gas, to an oxidizing gas,
including, but not
limited to, air, oxygen, nitrous, or other nitrogen oxides. The oxidizing gas
may be further
diluted by any inert gas. The coke is then burned off the spent catalyst in an
exothermic reaction.
This will create hot flue gas and the reactor may need to be cooled. This heat
and hot flue gas
may be captured and utilized elsewhere in the process, including but not
limited to, drying of
coal, raising steam for downstream acid-gas removal, pre-heating of reactants
for the pyrolysis
cycle, or direct flue gas injection into the coal-fired boiler or into a heat
recovery steam
generator.
[0103] Once the catalyst is regenerated, the reactor could then be returned to
the first cycle,
and pyrolysis would be started again. It is noted that by using multiple
reactors and valve
switching, this process could be configured in such a way that production
could advantageously
be run in a continuous or near continuous manner (in the case of batch-wise
pyrolysis). For
example, three reactors could be used in a cyclic-swing configuration, with
one of the three
reactors operating in each cycle at all times (i.e., one reactor in the first
cycle of pyrolysis, one
reactor in the second cycle of separation, and one reactor in the third cycle
of regeneration).
-36-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0104] FIGS. 8-12 illustrate exemplary embodiments of pyrolysis reactors that
are configured
to provide solid-solid separation in the pyrolysis reactors, which may
eliminate the need for
providing a downstream solid-solid separator. The pyrolysis reactors are
designed to allow for
maximum contact of dissimilar (e.g., size, density, shape) catalyst(s) and
feedstock(s) to solve
many of the problems noted above. For example, the pyrolysis reactors of FIGS.
8-12 are
configured to take advantage of dissimilar size, density, and shape to
maximize contacting; allow
for independent control of reactor residence time of the solid feedstock and
catalyst, which could
substantially reduce the amount of catalyst recirculation by enabling the
catalyst to absorb more
coke and by-products rather than being forced out of the reactor before
needed; and provide
separation within the reactor, possibly eliminating the need for a separate
unit operation to affect
the solid/solid separation. The reactors may be configured to use flow-fields,
sieves, plates,
tilting, and/or solids transfer valves to continuously contact and separate
the solids. Each reactor
may include two solids with substantially different particle size diameter
distributions (PSD),
such that a properly-sized sieve will not pass most of the solids with the
larger PSD and pass
most of the solids with the smaller PSD. The two solids may have substantially
different
fluidization characteristics, such that a gas fluidization velocity exists
where one solid rises (the
more buoyant solid), and one solid falls (the less buoyant solid). A gas flow
field may be
configured to encourage the less buoyant solid downward, and the more buoyant
solid upward.
Each reactor may include one or more obstacles configured to encourage
contacting the two
dissimilar solids on their respective journeys through the reactor. Contacting
may be further
increased by feeding counter-currently (i.e., the less buoyant solid at the
top, and taking it from
the bottom, while the more buoyant solid is fed to the bottom reactor and
removed from the top).
Each reactor has the ability to adapt the flow field and path of the particles
to provide
independent control of residence time in the reactor of the solid feedstock
and catalyst. The
catalyst may be larger and less buoyant than the solid feedstock, and it may
be desirable to keep
the catalyst residence time substantially greater than that of the solid
feedstock. However, each
reactor may be adjustable to accommodate a number of possibilities including,
but not limited to,
providing longer residence for solid feedstock than the catalyst, or equal
residence time for the
-37-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
solid feedstock and the catalyst, as well as providing solid feedstock that is
less buoyant than that
of the catalyst.
[0105] FIGS. 8-10 illustrate a pyrolysis reactor 503 configured to provide
solid-solid
separation of a feedstock and a catalyst. The reactor 503 includes a housing
530 having a
generally elongated tubular shape that defines an internal core chamber 532
that can be divided
into a plurality of sub-chambers 532a-532g with a varying flow field to
provide mixing and
disengagement in each chamber. The housing 530 has an inlet end 533 configured
to introduce a
feedstock, such as low grade pulverized coal, into the housing. The housing
530 has an outlet
534 at an opposite end from the inlet end 533. The upgraded feedstock, such as
upgraded coal
product, exits via entrainment with any gases exiting the reactor via the
outlet end. Optionally,
the reactor may include one or more fixed plates in the core chamber. As shown
in FIG. 8, the
reactor 503 includes a first fixed plate 536 that is provided between the
inlet end 533 and a first
plate assembly 541, and also includes a second fixed plate 537 provided
between the outlet end
534 and a sixth plate assembly 546. Each fixed plate 536, 537 may be
configured having a
plurality of holes (e.g., apertures, openings, orifices, etc.), similar to or
the same as the sieve
plates discussed below.
[0106] The core chamber 532 can be divided into the sub-chambers 532a-532g by
way of one
or more movable plate assemblies. As shown in FIG. 8, the reactor includes six
plate assemblies
541-546. However, other examples of reactors may be configured having a
greater or fewer
number of plate assemblies.
[0107] Each plate assembly 541-546 may include at least one plate. As shown in
FIGS. 9 and
10, each plate assembly includes a solid plate 548 (e.g., a plate having no
orifices, holes, or
apertures) and a sieve plate 549 (e.g., a plate having at least one orifice,
hole, or aperture, and
according to an exemplary embodiment a plate having a plurality of apertures)
where each plate
(e.g., solid, sieve) can be moved independently relative to the housing 530
(and core chamber) to
control the flow between a pair of adjacent sub-chambers of the reactor 503.
For example, each
plate 548, 549 may be configured to slide between a fully open position, in
which the entire plate
is positioned outside the core chamber 532 (e.g., outside the housing), and a
fully closed
-38-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
position, in which the plate covers the entire cross-sectional area of the
core chamber. Each plate
may be positioned at a plurality of intermediate positions between the fully
open and fully closed
positions. When a solid plate 548 is in the fully closed position, flow
through the core chamber
is completely blocked by the solid plate. When a sieve plate 549 is in the
fully closed position,
flow around the plate is prohibited, but flow through the one or more
apertures of the sieve plate
can occur. At the various intermediate positions, the plates influence the
flow (e.g., increase the
flow, restrict the flow). The sieve plates 549 are configured to pass a
feedstock (e.g., a
carbonaceous material, such as low grade coal) while preventing catalyst from
passing. Each
plate assembly may be configured to be moved from the same side (e.g., a top
side) of the
housing, such that a gap between a distal end of the plate and the housing
forms on the same
opposing side (e.g., a bottom side) of the housing 530 when the plates are at
least partially open.
Alternatively, the reactors having two or more plate assemblies may be
configured having plate
assemblies moving from different sides of the housing, such as to provide gaps
on alternating
sides of the reactor.
[0108] The alignment of the reactor 503, such as relative to vertical and
horizontal, may be
varied to change the relative angle of the reactor. For example, the angle of
the reactor may be
adjusted to be any angle from 0 (zero) degrees to 90 (ninety) degrees. The
reactor may be
configured to form catalyst beds (e.g., an agglomeration of catalyst
particles) having different
sizes by adjusting the plates and the angle of the reactor. The angle of the
reactor is used to
create varying cross-sectional area in each of the chambers. This changes the
effective
fluidization velocity throughout the chamber. The velocity flow-field can be
further manipulated
by varying the sieve plate and/or solid plate position as well. In other
words, the size of the
catalyst beds may be tailored by adjusting the plates and angle of the
reactor. This arrangement
provides a very flexible geometry which advantageously helps manipulate the
solids and
fluidization medium (usually a gas, but possibly a liquid), to create maximum
mixing while
avoiding plugging and bridging in the solids flow. FIG. 9 shows a portion of
the housing 530
and core chamber 532 having a single plate assembly including a sieve plate
549 and a solid
plate 548, which are able to move relative to the housing 530 and independent
of one another.
As shown, both the sieve plate 549 and the solid plate 548 are in intermediate
positions.
-39-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0109] FIG. 10 shows some of the features of the geometry of a mixing chamber
(e.g., the
mixing within the sub-chamber 532b). It is readily apparent that the available
cross sectional
area for flow varies markedly in the chamber. For the sake of understanding
the flow, but not
limiting the reactor to any particular theory or explanation, four
aspects/features to controlling
the flows and mixing are further described. First, the line A1 denotes the
cross sectional area
available for the less buoyant solid to fall into the lower chamber. Second,
the line A2 denotes
the cross sectional area available for the more buoyant solid and the
fluidization medium to rise
from the lower chamber. Third, the line A3 denotes the widest cross sectional
area available for
transport in the chamber. Fourth, the arrow Qfluid denotes the flow of
fluidization medium in the
sub-chamber. The velocity (u) of the fluidization gas or of either solid can
be roughly estimated
by dividing its volumetric flow by the cross sectional area available for
flow. Therefore, the
downward flow of the less buoyant solids at its most choked point would be
given by position 1
and can be calculated using calculation (1) below.
uless buoyant solid Q less buoyant solid
(1) 1 /A1
[0110] The importance of this point is that solids can become plugged due to
bridging during
in solids transfer and storage. However, the upward fluidization velocity can
assure that the
solids do not stagnate in the sub-chamber. The fluidization velocity is
determined by the cross
sectional area at position 2 and can be calculated using calculation (2)
below.
fluid Q fluid > ,more buoyant solid rõ,
less buoyant solid
(2)
U2 ¨ A an
emu Umf
2
[0111] Preferably, this fluidization velocity is greater than, or equal to,
the critical entrainment
velocity of the more buoyant solid. In some embodiments, the fluidization
velocity is greater
than or equal to the minimum fluidization velocity of the less buoyant solid.
This will create a
churning, turbulent zone as the solids are forced upward by the fluidization
media, preventing
solids plugging as the less buoyant particle falls out of the chamber, and
mixing the two
dissimilar solids through the turbulence.
-40-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0112] Position 3 (e.g., line A3) in FIG. 10 is the point where the maximum
cross sectional area
is available for flow. At point 3, the fluidization velocity is the lowest,
which can be calculated
using calculation (3) below. This allows a significant amount of independent
tuning to allow for
separation and increase contact time. If a degree of separation and good
contacting is desired,
this velocity can be tuned to be less than the fluidization velocity of the
less buoyant solid,
creating a quiescent zone of lightly bubbling less buoyant particles and
forcing the more buoyant
particles to pass through.
fluid Qfluid < uless buoyant solid
U3
(3) ¨
A3 mf
[0113] One advantage of a tilted reactor (e.g., relative to vertical) is that
the tilt can be used to
adjust these various velocities to maximize mixing and separation. Also, for
the example of
pyrolysis, pyrolysis gas is evolved during the reaction and flows together in
the same direction
with the fluidization gas, increasing the overall amount of gas flow along the
length of the
reactor, leading to increased superficial gas velocity along the length of the
reactor. This can be
accounted for by varying the tilt or the volume in each chamber (e.g., by
adjusting the plate
spacing). Varying tilt can also be accomplished by series reactors, or by
bending the pipe.
Additionally, the configuration of the reactor of FIG. 8-10 allows the more
buoyant and less
buoyant solids residence times to be controlled independently, as their paths
are different through
the vessel. In a co-current, entrained riser, all solids and fluidization
media residence times are
roughly the same and dictated by the fluidization characteristics. For the
case where one of the
solids is a catalyst, and the other solid carbonaceous material is a feedstock
to be reactive, it is
desirable to independently control these residence times. Finally, in vessels
with multiple
staging, extra coal separation could be accomplished by feeding the more
buoyant material at a
higher stage than the bottommost stage, giving it more chance to disengage
from the less buoyant
material. In this case, this lower stage (below the injection of solid
carbonaceous feed coal)
could be used to regenerate spent catalyst using an oxidizing gas.
[0114] The reactor 503 may include a second outlet 538 that is configured to
pass spent
catalyst, such as to a regenerator. Also shown in FIG. 8, the second outlet
538 is provided near
the inlet end 533 of the housing 530. For example, the second outlet 538 may
be provided in the
-41-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
bottom side of the first sub-chamber between the first fixed plate and the
first plate assembly.
The reactor 503 may include a second inlet 539 that is configured to introduce
catalyst (e.g., new
catalyst, regenerated catalyst, a combination thereof). Also shown in FIG. 8,
the second inlet
539 may be provided near the outlet end 534 of the housing 530. For example,
the second inlet
539 may be provided in a top side of the seventh sub-chamber between the
second fixed plate
and the sixth plate assembly. This arrangement may advantageously utilize
gravity in bringing
the catalyst from the second inlet toward the second outlet.
[0115] FIG. 11 illustrates another illustrative embodiment of a pyrolysis
reactor 603 configured
to provide solid-solid separation of a feedstock and a catalyst. The reactor
603 includes a
housing 630 having a generally elongated tubular shape extending from a first
end 631 to a
second end 632. According to one example, the reactor 603 is aligned
substantially vertically,
with the first end 631 at a bottom side and the second end 632 at a top side.
According to other
examples, the reactor 603 can be tilted, such as to be aligned at an oblique
angle relative to
vertical. The reactor 603 may include one or more plate assemblies that are
configured to divide
an internal core chamber 640 into a plurality of sub-chambers (e.g., the sub-
chambers 641-646).
[0116] The reactor 603 may include one or more inlets. As shown in FIG. 11,
disposed at the
first end 631 is a first inlet 633 that is configured to receive a fluidizing
gas. Also disposed near
the first end 631 is a second inlet 634 that is configured to introduce a
feedstock, such as low
grade coal, into the reactor 603. For example, the second inlet 634 may be
configured to
introduce feedstock into a second sub-chamber 642 of the reactor 603. Disposed
near the second
end 632 is a third inlet 635 that is configured to introduce catalyst (e.g.,
new catalyst, regenerated
catalyst, a combination thereof) into the reactor 603. For example, the third
inlet 635 may be
configured to introduce catalyst into the sixth sub-chamber 646.
[0117] The reactor 603 may include one or more outlets. As shown in FIG. 11,
the reactor 603
includes a first outlet 636, which is configured to remove spent catalyst from
the reactor, and a
second outlet 637, through which upgraded feedstock, such as an upgraded coal
product, is
recovered. The first outlet 636 may be disposed near the first end 631. For
example, the first
outlet 636 may be configured to remove spent catalyst from the first sub-
chamber 641. The
-42-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
second outlet 637 may be disposed near the second end 632. For example, the
second outlet 637
may be configured to remove upgraded coal product and to remove off gases
(e.g., pyrolysis
product gas and/or fluidization gas) from an outlet sub-chamber that is
downstream from the
sixth sub-chamber 646. According to another example, the off gas may be outlet
via the second
outlet 637, such that the upgraded feedstock and off gases exit the reactor
603 together. Thus,
the off gases and upgraded feedstock may be separated inside or outside the
reactor 603.
[0118] As noted above, the reactor 603 may include one or more plate
assemblies configured
to define the reactor into sub-chambers. As shown in FIG. 11, the reactor 603
includes seven
plate assemblies 651-657, which divide the reactor 603 into sub-chambers 641-
646, along with
an inlet sub-chamber and an outlet sub-chamber. Each plate assembly includes
one or more
plates. As shown, each of the first and seventh plate assemblies 651, 657
include a single plate
configured as a sieve plate with a plurality of apertures in the plate. The
size of the apertures
may be tailored. According to an exemplary embodiment, the size of the
apertures are
configured to allow the particles of the feedstock to pass through the
apertures, while preventing
the particles of catalyst from passing through the apertures. Thus, the sieve
plates may separate
catalyst and feedstock while the feedstock flows through the reactor from sub-
chamber to sub-
chamber. The plates of the first and seventh plate assemblies 651, 657 are
fixed relative to the
housing 630. The second thru sixth plate assemblies 652-656 may be configured
to include a
sieve plate and a second plate disposed adjacent the sieve plate, where the
second plate has an
opening disposed in a solid portion. The feedstock may pass through the sieve
plate and the
opening in the second plate. According to another example, each of the second
thru sixth plate
assemblies 652-656 include a single plate having a solid portion and a sieve
portion, where the
sieve portion is configured to restrict the flow of catalyst but allows
feedstock to flow through
the apertures of the sieve portion. The sieve portion of the second thru sixth
plate assemblies
652-656 may be offset from a longitudinal axis 658, such as, for example, in
an alternating
manner as shown in FIG. 11, where the second, fourth, and sixth sieve plates
are on a similar
side of the longitudinal axis and the third and fifth sieve plates are on a
similar side that is
opposite to the side of the second, fourth and sixth sieve plates. This offset
arrangement of the
sieve portions may advantageously induce an alternating flow of feedstock
through the reactor
-43-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
603, which may create more exposure of the feedstock to the catalyst and
increase residence
time.
[0119] As shown, each of the second thru sixth sub-chambers 642-646 acts as a
staged reaction
zone, where the feedstock is exposed to catalyst to form an upgraded feedstock
as a result of the
reaction. The first sub-chamber 641 serves as a disengagement zone, such that
the spent catalyst
passing through the second plate assembly 652 can be recaptured for
regeneration via the
outlet 636.
[0120] The reactor 603 may be configured to include one or more transfer
valves (e.g., bypass
valve, slide valve, gate valve, etc.) configured to control the flow through
reactor 603. For
example, a valve 660 may be provided to control the flow of catalyst between
each pair of
adjacent sub-chambers. A first valve 660 fluidly connects the first and second
sub-chambers 641,
642 by way of a first pipe extending from the first sub-chamber 641 to the
valve 660 and a
second pipe extending from the second sub-chamber 642 to the valve. Similarly,
second, third,
fourth, and fifth valves 660 fluidly connect the second and third sub-chambers
642, 643, the third
and fourth sub-chambers 643, 644, the fourth and fifth sub-chambers 644, 645,
and the fifth and
sixth sub-chambers 645, 646 sub-chambers, respectively. Each of the second,
third, fourth, and
fifth valves 660 include a pipe connecting each sub-chamber with the valve.
Each valve 660 is
adjustable to change (e.g., increase, decrease) the flow rate through the
valve.
[0121] A valve may be provided to control the flow through each inlet and/or
outlet of the
reactor 603. For example, a valve may be provided to control the flow of
feedstock through the
first inlet 634 and/or the catalyst through the second inlet 635. Also, for
example, a valve may
be provided to control the flow of spent catalyst through the first outlet 636
and/or the flow of
upgraded feedstock through the second outlet 637.
[0122] The reactor 603 of FIG. 11 accomplishes many of the desired effects
disclosed in this
application by manually controlling the solids flow of the more buoyant
particles including
particles of a solid carbonaceous material feedstock, using transfer valves
for controlling the
downward flow of the less buoyant particles including catalyst, and preventing
upward flow of
catalyst by entirely dividing the core chamber into sub-chambers with plate
assemblies including
-44-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
plates (e.g., sieve plate, block plate, etc.). In this configuration, there is
no open cross-section
area allowing complete free flow of solids, since the flow of catalyst
particles is restricted. As
with a diagonal fluid bed, this reactor is designed to force the more buoyant
particle upward, and
uses sieves to trap the less buoyant particle in each sub-chamber.
[0123] The reactor 603 of FIG. 11 may avoid packing of the catalyst at the
bottom by
partitioning the reactor into zones (e.g., sub-chambers). The zones may be of
variable volume to
account for increased gas flow as pyrolysis gas is evolved during the reaction
it commingles and
flows together and in the same direction with the fluidization gas, increasing
the overall amount
of gas flow along the length of the reactor. By having plates that are mostly
solid, but include
sieves in part of the plate designed to let only the feedstock (e.g., coal)
pass, the reactor separates
the solids (e.g., feedstock, catalyst), which may advantageously eliminate the
need for solid-solid
separation outside the reactor 603. Preferably, the cross sectional area of
the partial sieves
creates a local velocity higher than the critical entrainment velocity of the
more buoyant
particles. More preferably, the cross sectional area of partial sieves can be
designed to create
local velocities greater than the minimum fluidization velocity of the less
buoyant particles,
forcing a churning motion. The location of the sieve openings/apertures can be
varied from sieve
to sieve to force the feedstock through a tortuous path in the reactor. The
slide or gate valves
may occasionally be opened to allow catalyst to pass downward in the reactor.
The frequency
may be determined by a coking rate. These could also simply be discharges to a
common
catalyst collection receptacle. This could also be metered continuously by
typical solids
metering valves, such as a rotary valve or auger. Advantageously, the lowest
sub-chamber (e.g.,
the first sub-chamber 641) is provided below the coal feed point (e.g., the
inlet 634) to allow any
entrained coal time to disengage. Advantageously, the disengagement zone may
become the
regeneration zone, allowing hot flue gas to pass into the pyrolyzer. In this
case the fluidizing gas
could contain an oxidizing component, such as oxygen. Also, the oxidizing gas
may, preferably,
be enriched air or oxygen, such as to avoid the system becoming overloaded
with nitrogen.
[0124] FIG. 12 illustrates another illustrative embodiment of a pyrolysis
reactor 703 configured
to provide solid-solid reactor of a solid carbonaceous material feedstock and
a catalyst. The
-45-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
pyrolysis reactor 703 also provides solid-solid separation of an upgraded
solid carbonaceous
product and the spent catalyst. In particular, the reactor 703 is configured
to utilize a fluidized
catalyst (e.g., bubbling bed of catalyst) that is not entrained, as discussed
above.
[0125] The reactor 703 includes a housing 730 having a first (e.g., lower)
section 731 and a
second (e.g., upper) section 732. The first section 731 has a generally
tubular shape defining an
internal lower chamber, and the second section 732 has a generally conical
shape defining an
internal upper chamber. A frusto-conical intermediate portion may interconnect
the first section
731 and the second section 732. The reactor 703 may include one or more than
one inlets. A
first feed pipe 741 is disposed at an end of the second section 732 and is
fluidly connected to a
first dipleg 751 extending into a fluidized regime 735. A second feed pipe 742
is disposed at the
end of the second section 732 and is fluidly connected to a second dipleg 752
extending into the
fluidized regime 735. A feedstock may be introduced into the internal chamber
of the reactor
703 through one of the first and second feed pipes 741, 742, while a catalyst
may be introduced
into the internal chamber via the other of the first and second feed pipes. As
shown, the reactor
703 includes a third inlet that is disposed at an end of the first section 731
(e.g., the end opposite
the end of the second section 732) and configured to receive fluidization gas
from pipe 743. The
third inlet is configured to introduce fluidization gas into the internal
chamber via a fluidization
gas distributer 745. Reference numeral "739" denotes a fluidized bed level
(e.g., an adjustable
interface of dense bed below with dilute phase solids above).
[0126] The reactor 703 may include one or more than one outlet. Also shown in
FIG. 12, a
first outlet pipe 761 is disposed at the second section 732 (e.g., the end
thereof). The first outlet
pipe 761 is configured to discharge fluidization gas, pyrolysis product gases
and the upgraded
carbonaceous product. A second outlet pipe 762 is disposed at the first
section 731 (e.g., the end
thereof) and configured to discharge the spent catalyst. For example, the
second outlet pipe 762
may surround the third inlet and pipe 743. Thus, the upgraded carbonaceous
product exits the
reactor 703 via the first outlet 761 and the spent catalyst exits the reactor
703 via second outlet
pipe 762. In experimental studies of this reactor, we have demonstrated that
the reactor can be
-46-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
run such that solids exiting the reactor through exit port 761 contain a
weight ratio less than 1
weight part catalyst per 100 parts of carbonaceous product.
[0127] Now, a comparison including actual data recovered from a reactor
similar to the reactor
703, which was configured to run first as a riser reactor (i.e., Example 1)
and then second as a
hybrid elutriating riser bed (HERB) reactor (i.e., Example 2), will be
discussed. In the first run,
the riser reactor was configured to operate providing a partial catalytic
pyrolysis of coal. The
reactor was configured having a 3/4 inch diameter pyrolysis reactor with 8
feet of heated height,
which was fed with a coal and catalyst mixture. The reactor also had an
unheated disengagement
zone, which was approximately 7 feet high x 3/4 inch diameter, provided above
the heated zone.
Catalyst and coal entered the reactor from the bottom. Additionally, nitrogen
was provided as a
fluidization gas via a sparger at the bottom of the reactor. Gases and solids
were taken off the
top of the reactor where they entered a cyclone separator where solids and
gases were separated
at the effluent temperature of the pyrolysis reactor. Product gases were then
sampled with a gas
chromatograph (GC) to determine light components (e.g., components lower than
Benzene), and
the heavier components were condensed in a liquid trap and then injected into
a GC column for
quantitative analysis. The spent catalyst and upgraded coal product were
weighed to determine
catalyst recovery efficiency and coal conversion. The upgraded coal product
was analyzed for
carbon content, volatile matter, ash, and sulfur. In the embodiments described
in this
application, we have generally described the catalyst as being larger, denser,
or less mobile than
the carbonaceous material. This choice is driven by economic rather than
technical
considerations. The economic drivers favor configuring the catalyst as the
larger, denser, or less
mobile material because the larger, denser, or less mobile material tends to
be handled less and
tends to be transported less. Because of the reduced handling, losses will be
minimized and as
the catalyst is usually more expensive than the carbonaceous material, it is
generally better to
minimize the catalyst losses. However, it is just as valid to reverse the
roles of the catalyst and
the carbonaceous material. What is required is differences in densities,
sizes, or mobility; which
material is larger, denser, or less mobile has no impact on the operability or
effectiveness of the
process. Therefore, it should be understood that any embodiments specifically
referring to the
catalyst being larger, less mobile, or denser than the CM, also implicitly
disclose configurations
-47-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
where the CM is larger, less mobile, or denser than the catalyst.
Commensurately, all locations
of the CM and catalyst in process schematics would be inverted as well.
[0128] During start-up, the reactor was heated by supplying hot catalyst from
an attached
heated regenerator vessel which served as the catalyst reservoir during the
studies. The catalyst
was recirculated between the regenerator vessel and the pyrolysis reactor
until a steady state
temperature was attained. Catalyst flow was controlled by a gate valve between
the regenerator
vessel and the pyrolysis reactor. Flow rate was calibrated by gate valve
position and weight
beforehand, and validated by total weights before and after the run. Once the
temperature was
attained, coal feed was introduced at the bottom of the pyrolysis reactor via
an auger. Feed rate
of coal was controlled by the speed of the auger. At the same time, the
combined flow of
upgraded coal product and spent catalyst from the riser was diverted to a
product collection
vessel. The run was carried out until the catalyst in the regenerator vessel
(reservoir) was
depleted.
[0129] Process conditions and results are contained in Table 1 (below).
Although the
experiment ran steady and in control, a number of shortfalls of this
configuration were identified.
First, as a practical matter, it was difficult to run for extended periods of
time, because the
catalyst consumption was high. The catalyst came out with the product and had
to be separated
in another step. Second, it was clear from the results that an optimal contact
time for the
catalytic pyrolysis reactions was not provided. When compared to results in
the smaller lab
apparatus (Figures 5-7), conversion of coal was low, and yields of all
hydrocarbon and fuel
products were low.
[0130] In the configuration of Example 1, increasing residence time was
difficult because it
required a decrease in fluidization velocity, which was discovered to result
in a loss of
entrainment of the catalyst. However, it was determined that upgraded coal
product could be
successfully separated from catalyst by adjusting fluidization velocity to
thereby enable
elutriation of the particles of coal to pass through a bubbling bed of
catalyst. In the vessel, there
was adequate mixing of the coal with catalyst in the lower bubbling bed zone,
and because the
coal was less dense and smaller in average particle size, the coal would
elutriate out of the bed
-48-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
while the catalyst stayed behind. It is postulated that for this to occur, the
fluidization velocity
was adjusted to an intermediate velocity provided between the two entrainment
velocities
associated with each of the two particle types, coal and catalyst. This vessel
is considered a
hybrid reactor, because it acts as a riser with respect to coal flow and a
bubbling, fluidized bed
with respect to catalyst, while accomplishing an elutriation separation. Thus,
this reactor is
described as a HERB reactor in this application.
[0131] In Example 2, the same reactor configuration as Example 1 was used, but
the procedure
was modified to enable it to run as a HERB reactor, to carry out partial
pyrolysis of coal. First,
an 8 feet heated section of the riser was filled with fluidized catalyst. Then
the coal was fed into
the base of the pyrolyzer. During the run, no fresh catalyst was added.
[0132] Despite the differences between Example 1 and Example 2, Example 2
provides a
number of advantages over Example 1. First, Example 2 is able to run for much
longer extended
periods of time, compared to Example 1, while using much less catalyst.
Second, Example 2
serves the dual purpose of separating the upgraded coal product from the
catalyst in addition to
carrying out the catalytic pyrolysis reaction. The results show that very
little catalyst escaped the
pyrolysis reactor during the run. Without being bound by theory or
explanation, it is believed
that the most relevant factor in achieving good selectivity and conversion in
Example 2, the
HERB pyrolysis reactor configuration, is having a high concentration of
catalyst in the coal, to
promote more favorable conditions for controlled selectivity and conversion.
This is supported
by the results in Table 1, in which fuels yield increases from 8.43% in
Example 1 to 22.80% in
Example 2, more valuable fuels (e.g., condensable fuels) increases with the
lighter components
rising from 3.74% in Example 1 to 7.42% in Example 2, and the most valuable
BTEX
components rise from 0.29% in Example 1 to 2.18% in Example 2. The results are
indicative of
effective contacting of the solid carbonaceous feedstock (e.g., coal) with a
selective and active
catalyst.
[0133] It is noted that although on a superficial basis, the residence (i.e.,
contact) time of the
solid carbonaceous material and catalyst in Example 2 is longer than in
Example 1 (see line 14 of
Table 1), if one corrects for the percent volume of the reactor having
catalyst, then a calculated
-49-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
effective contact time of the coal with catalyst is actually lower in Example
2. For example,
making such a correction assuming that approximately 76% of the reactor volume
is catalyst, the
effective contact time of the coal in Example 2 is 0.65 seconds compared to
3.77 seconds in
Example 1. Therefore, while we set out to increase contact time with this
reactor with the desire
of increasing extent of reaction, we in fact, decreased the residence time of
coal, and yet
surprisingly, the extent of reaction substantially increased. This can be
explained in part by the
theory that the effective residence time during which the feed coal was in
active contact with
catalyst was actually higher in Example 2.
[0134] Table 1: Working parameters of the reactors of Examples 1 and 2
discussed above,
along with recovered products according to these two examples.
Example 1: Example 2:
Riser HERB
1. Run
duration, minutes 8.9 70.0
2. Raw coal
feed (dry basis), kg 0.269 0.531
3. Upgraded
coal product out (dry basis), kg 0.201 0.344
4. Coal feed
rate, kg/hr 1.809 0.455
5. Fresh
catalyst feed rate, kg/hr (for HERB: initial charge/run time) 11.158
0.325
6. Riser lift
gas velocity (in riser, corrected for T P), m/s 1.476 0.895
7. Riser lift
gas volumetric flow, m3/s 4.21E-04 2.55E-04
8. Density of
coal in reactor, kg/m3 1.226 0.509
9. Catalyst
fluidization state (riser, fluid) riser fluid
10. If fluid,
kg of catalyst in reactor N/A 3.81E-01
11. Density of
catalyst in reactor, kg/m3 4.62 548
12. Bulk
density of catalyst, kg/m3 720 720
13. Available void volume % not occupied by catalyst (1-Bulk/Bed 99%
24%
density)
14. Coal
Residence time, seconds 1.65 2.72
15. Corrected
coal residence time based on available void volume 1.64 0.65
16.
Catalyst/coal ratio (based on fresh catalyst and coal feeds) 3.77 0.41
17. Catalyst
to coal ratio based on bed densities 3.77 1077.46
18. Reactor
Average temperature, C 491.61 494.09
PROCESS PERFORMANCE, ALL VALUES IN KG PRODUCED PER 100
KG OF DRY COAL FEED
19. Raw coal
conversion 25.2 35.1
20. Total
fuels yield (ex. H25), kg per 100 kg 6.89 17.67
-50-
CA 02950138 2016-11-23
WO 2015/179806
PCT/US2015/032252
21. Total non-
condensable fuel gases 3.51 10.38
22. CO
1.80 4.80
23. H2
0.02 0.20
24. METHANE 0.61
2.53
25. ETHYLENE 0.85
2.05
26. ETHANE 0.23
0.79
27. ACETYLENE 0.00
0.01
28. LPG:
light condensable fuels (ex. BTX and higher 3.19 5.85
hydrocarbons)
29. PROPYLENE 1.66
2.72
30. N-PROPANE 0.18
0.28
31. PROPADIENE 0.00
0.02
32. CYCLOPROPANE 0.00
0.00
33. METHYLACETYLENE 0.00
0.00
34. ISOBUTANE 0.11
0.08
35. ISOBUTYLENE 0.37
0.62
36. 1-
BUTENE 0.16 0.28
37. 1,3-
BUTADIENE 0.05 0.54
38. N-BUTANE 0.05
0.05
39. TRANS-2-BUTENE 0.16
0.20
40. CIS-2-BUTENE 0.16
0.21
41. CYCLOBUTANE 0.00
0.00
42. ISOPENTANE 0.03
0.03
43. 1-
PENTENE 0.09 0.07
44. N-PENTANE 0.03
0.03
45. TRANS-2-PENTENE 0.00
0.07
46. CIS-2-PENTENE 0.06
0.03
47. 2-METHYL-2-BUTENE 0.00
0.09
48. 3-METHYLPENTANE 0.00
0.00
49. 2-METHYLPENTANE 0.00
0.00
50. 1-
HEXENE 0.00 0.08
51. N-HEXANE 0.08
0.45
52. 2,3-DIMETHYLPENTANE 0.00
0.00
53. 2-METHYL-1-BUTENE 0.00
0.00
54. BTEX
total 0.19 1.43
55. BENZENE 0.11
0.80
56.
Toluene 0.08 0.56
57. Xylene
0.00 0.07
-51-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
58. HIGHER
AND HETEROATOM CONTAINING 0.00 0.00
HYDROCARBONS
59. SULFUR GASES
60. H2S
1.37 2.71
61. SO2
0.00 0.00
62. Other sulfur gases 0.18 0.24
FEED COAL ANALYSIS, WT% DRY BASIS
63. Fixed
Carbon 30.9 30.9
64. Volatile
Matter 34.4 34.4
65. Ash
34.75 34.75
66. Total
sulfur 3.9 3.9
67. Pyrite
0.546 0.546
68. Sulfate
0.026 0.026
69. Organic
3.315 3.315
[0135] It should be appreciated that this process can be used on a variety of
carbonaceous
materials with varying amounts of volatile matter, ash, fixed carbon, sulfur,
and heating values
(often referred to as rank). However, through testing various coals, we have
found that even
disparate ranks of coal and carbonaceous materials can be compared by looking
at conversion
and yields based on the feed carbonaceous material.
[0136] To better elucidate this point, the following table contains yield
calculations and others
figures of merit based on the above data. Additionally, we have summarized the
ranges observed
in all of our continuous reactor runs in the following table, as well as our
projected ranges based
on our empirical knowledge and based on simulations using mass and energy
balances. It should
be stressed that these are non-limiting ranges for the practice of the
processes/systems of this
application.
[0137] Table 2: Figures of merit in process performance calculated from data
in Table 1.
Example 1: Example 2:
-------------------------------------------------------- Riser HERB
1. kg of
upgraded coal per 100 kg of dry coal feed 74.7 64.8
2. kg of
sulfur in upgraded coal per 100 kg of sulfur in coal feed 41.4 35.9
,
-52-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
3. kg of ash in upgraded coal per 100 kg of ash in coal feed 97.4
kg of fixed carbon in upgraded coal per 100 kg of fixed carbon in
4. coal feed 68.1
kg of volatile matter in upgraded coal per 100 kg of volatile matter in
5. coal feed
26.9 28.7
Hydrocarbon yields based on volatile matter content
kg of non-condensable fuels produced per 100 kg of volatile matter in
6. coal feed
10.2 30.2
7. kg of
LPGproduced per 100 kg of volatile matter in coal feed 9.3 17.0
8. kg of BTEX
per kg of volatile matter in coal feed 0.6 4.2
9. kg of all other hydrocarbons per kg of volatile matter in coal feed
10. total kg of
all hydrocarbons per kg of volatile matter in coal feed 20.0 51.3
Hydrocarbon selectivities based on volatile matter converted
kg of non-condensable fuels produced per 100 kg of volatile matter
11. converted in reaction 14.0 42.3
kg of LPG produced per 100 kg of volatile matter converted in
12. reaction 12.7 _________________________________________________ 23.9
kg of BTEX produced per 100 kg of volatile matter converted in
13. reaction 0.8 5.8
kg of all other hydrocarbons and heteroatoms produced per 100 kg of
14. volatile matter converted in reaction
total kg of all hydrocarbons per kg of volatile matter converted in
15. reactor 27.4
72.0
16. HHV fuel value of input coal, as received, MJ/kg 12.8
12.8
17. HHV fuel value of output coal, as received, MJ/kg 13.7
14.7
Upgrade factor: HHV of output coal/HHV input coal (as received
18. basis) 1.07
1.15
19. Weight hour
space velocity, (kg/hr dry coal feed) / (kg catalyst) 3.93 1.07
[0138] Table 3: Ranges in figures of merit in process performance observed in
continuous runs
(both HERB and riser configurations), and projected based on lab data,
continuous runs, and
simulations based on mass and energy balances.
-53-
CA 02950138 2016-11-23
WO 2015/179806
PCT/US2015/032252
Range in Expected
continuous coal ranges
feed reactors
(inclusive)
1. kg of
upg_r_aded CM__per 100 k_g_of dry CM feed 55 - 100 40 - 100
2. kg of
sulfur in upgraded CM per 100 kg of sulfur in CM feed 25 - 79 0 - 80
(overall)
0 - 50
(organic)
50 - 100
(pyritic)
0 - 50
__________________________________________________________________ (sulfate)
3. kg of ash in ripg_r_aded CM ______ 100 kg_of ash in CM feed 93 -
100 60 - 100
kg of fixed carbon in upgraded CM per 100 kg of fixed carbon
4. in CM feed 61- 100 50 - 100
kg of volatile matter in upgraded CM per 100 kg of volatile
5. matter in
CM feed 23 - 73 0 - 90
Hydrocarbon yields based on volatile matter content
kg of non-condensable fuels produced per 100 kg of volatile
6. matter in CM feed 1-39 0-40
7. _______________________________________________________________ kg of LPG
produced per 100 kg of volatile matter in CM feed 1 - 21 0 - 40
8. kg_of BTEX_p_e_ r_k_g_of volatile matter in CM feed 0 - 9 0 -
40
kg of all other hydrocarbons per kg of volatile matter in CM
9.feed 0 - 0 0-20
10. total kg of all hydrocarbons per kg of volatile matter in CM feed 2 -
62 10 - 90
... Hydrocarbon selectivities based on volatile matter converted
kg of non-condensable fuels produced per 100 kg of volatile
11.matter converted in reaction 1 - 42 0 - 60
_
kg of LPG produced per 100 kg of volatile matter converted in
12.reaction 1 - 23 0 - 60
kg of BTEX produced per 100 kg of volatile matter converted in
13. reaction 0 - 9
0 - 60
kg of all other hydrocarbons and heteroatoms produced per 100
14. kg of volatile matter converted in reaction 0 - 0
0 - 40
total kg of all hydrocarbons per kg of volatile matter converted
15. in reactor 2-71 2- 100
Upgrade factor: HHV of output solid material/HHV input solid
16. material
(as received basis) 1.04 - 1.62 0.9 - 1.80
17. Weight hour space velocity, (kg/hr dry coal feed) / (kg catalyst)
0.31-12.5 0.2-20
Catalyst to coal feed ratio, (kg/hr dry catalyst feed) / (kg/hr coal
18. feed)
0.08 - 72 0 - 100
19. kg of
CO2produced per 100 kg of dry coal feed 1-20 1-25
20. kg of CO2
produced per 100 kg of volatile matter feed 2-57 2-65
-54-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0139] These figures of merit are illustrative of the unique ability of this
processes/systems of
this application to carry out upgrading of carbonaceous materials while
providing beneficial
materials and in process that has superior operability to prior methods.
Further explanation of
the significance in reference to row numbers is as follows:
[0140] Row 2 is a measure of the weight fraction of sulfur retained in
upgraded CM. This
process reduces the sulfur in upgraded CM. Without wishing to be bound by any
theory or
explanation, our results suggest that organic sulfur and sulfates in the
carbonaceous material is
liberated as hydrogen sulfides, and only pyritic sulfur is retained. We expect
the pyritic sulfur to
stay with the ash, so the retention of pyritic sulfur to be similar to the ash
retention in the
upgraded CM.
[0141] Row 3 is a measure of the weight fraction of ash retained in the
upgraded carbonaceous
material. This process is unique in that most of the ash in the carbonaceous
material is retained in
the carbonaceous material. This is a key advantage of this process: very
little ash is free to sorb
on the catalyst and clog the catalyst pores. Ash accumulation on catalyst is a
known irreversible
deactivation mechanism of most pyrolysis catalysts, so our process will suffer
less from this
problem.
[0142] Row 4 is a measure of the weight fraction of fixed carbon retained in
the upgraded
carbonaceous material. Most, and in many cases, all, of the fixed carbon is
retained in the
upgraded carbonaceous product. Fixed carbon is known to be difficult to
pyrolyze, particularly at
the temperatures of this process, so without being bound to any particular
theory or explanation,
we believe that the fixed carbon not retained in the upgraded carbonaceous
material is either
oxidized by oxygen in the CM, or becomes coke on the catalyst. In other words,
no fixed carbon
is transformed into hydrocarbon products.
[0143] Row 5 is a measure of the weight fraction of volatile matter retained
in the upgraded
carbonaceous material. Without being bound to any particular theory or
explanation, we believe
that the volatile matter is the major source for all hydrocarbons liberated in
this process because
the fixed carbon is much more difficult to pyrolyze. As can be seen from our
experimentally
observed ranges, we typically retain some portion of the volatile matter.
However, we chose to
-55-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
keep some volatile matter in the product coal because our desired end product
in these
experiments was a coal that can be efficiently burned in a boiler. (A coal
that is efficiently
burned in a boiler is often referred to as a "steam coal"). Steam coals
require some volatile
matter so that they may be easily ignited. Without volatile matter, a coal
will not burn efficiently.
However, coals with low volatile matter are often useful as coking coals, used
in steelmaking.
Our process may be run at more aggressive conditions (e.g., higher
temperature, residence time)
to convert most or all of the volatile matter giving a coking coal, suitable
for use in steelmaking.
[0144] In rows 6 ¨ 10, we recalculate fractional yields by weight based on
volatile matter in the
CM rather than the total CM weight. Based on our non-limiting working theory,
we believe that
as most of the hydrocarbons come from the volatile matter, it makes sense to
base yields and
selectivities on volatile matter content. We have seen that this allows us to
predictably compare
performance across various ranks of carbonaceous feedstocks.
[0145] In rows 11 ¨ 15, we calculate fractional selectivities by weight based
on volatile matter
converted in the process. The total selectivity to hydrocarbons (Row #15) is a
good measure of
how effective the process is at utilize the converted volatile matter. A
perfect process would
approach 100%. It can readily be seen the effectiveness of running the reactor
in the HERB
configuration, as over 70% of the converted volatile matter yields
hydrocarbons.
[0146] In row 16, we calculate an upgrade index. This upgrade index is defined
as the ratio of
the heating value of the upgraded carbonaceous product and the heating value
of the feed
carbonaceous material (on an as received basis). A number of factors determine
this index, and
although we often increase the heating value, it is not necessarily a higher
value because a
number of factors move the value in opposite directions. For example, nearly
all of the moisture
is removed from the feed material. This will increase the heating value and
increase the
efficiency in a boiler. Also, for example, much of the volatile matter is
removed from the feed
material. Depending on the relative heating value of the volatile matter to
the remainder of the
components in the CM, this can either increase or decrease the product's
heating value. For
example, if the ash content is high in the feed material, the non-volatile
contents in the CM
(Fixed Carbon + Ash) will be low relative to the volatile matter, so reduction
in volatile matter
-56-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
will reduce heating value. Also, for example, most of the oxygen is removed
from the feed
material. This has the effect of increasing the heating value of the product
relative to the feed
material. Based on the above factors, we would expect a higher upgrade index
for carbonaceous
feedstocks with high oxygen, high moisture, and low ash, and vice versa.
[0147] In rows 19 and 20 (of Table 3), it should be noted that the CO2
production may vary
depending on the type of carbonaceous matter used.
[0148] Now, an illustrative product composition of a mild catalytic pyrolysis
reactor will be
described. An illustrative result of the pyrolysis reactor is presented in
Table 4 (below). The
product composition is based on an analysis of experimental laboratory results
of a low-grade
coal catalytically pyrolyzed at 400 C using a zeolite catalyst. Approximately
45% of the
volatile matter was converted to gaseous, liquid, and upgraded solid product,
such as upgraded
coal product. The level of conversion may be tailored (e.g., increased,
reduced), such as by
increasing or reducing the reaction temperature and/or reactor residence time.
The usable
products contain valuable olefins and aromatics. The results of the catalytic
pyrolysis showed no
compounds larger than C12, indicating that little or no tar or other highly
viscous material
handling would be necessary if this catalytic pyrolysis reaction was scaled-up
to a larger
commercial size and practiced precisely as it was in the small-scale lab
experiment.
[0149] Table 4: Estimated chemical composition of product (hydrocarbons only)
for low-grade
coal sample catalytically pyrolyzed with a zeolite catalyst.
-57-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
Carbon # Weight % Category Weight %
C1 8.55 Paraffins 25.73
C2 12.19 Olefins 42.59
C3 27.97 Aromatics 26.80
C4 14.56 Oxygenated 4.88
hydrocarbons
C5 1.70
C6 3.45
C7 10.47
C8 14.30
C9 1.01
C10 2.98
C11 0.59
C12 2.23
[0150] Now, the results of experiments using an experimental test setup are
shown,
graphically, in FIGS. 5-7. The experimental system included a fluidized bed
reactor. FIG. 5
compares the yields of various compounds including lower molecular weight
hydrocarbons of
the system using a catalyst and sand at 400 C. FIG. 6 compares the yields of
various
compounds including lower molecular weight hydrocarbons from the system using
a catalyst at
400 C, sand at 400 C, and the catalyst at 600 C. FIG. 7 compares the yields
of various
compounds from the system using a catalyst at 400 C, sand at 400 C, and the
catalyst at
600 C.
[0151] The systems and processes, as disclosed herein, may be integrated with
other industrial
applications. Examples of such industrial applications include, but are not
limited to, coal-fired
power generation facilities (e.g., plants), gas to liquid (GTL) facilities,
coal/coke/biomass
gasification (CCBG) facilities, blast furnace (BF) facilities, and oil
refining and/or steam
cracking facilities. Coal-fired power generation facilities may serve as an
outlet for waste heat
-58-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
from the systems, excess steam from the systems, fuel gas for a co-firing
boiler, fuel gas use as
staged NO reduction (e.g., rich reagent injection, as a so-called "reburn"
stream, etc.), and/or
upgraded coal to a boiler. GTL facilities may be used with the systems to
upgrade lower value
fuel gas and/or other hydrocarbons, especially light hydrocarbons (e.g.,
Ci¨C4), into other higher
value useful heavier liquid hydrocarbons (e.g., liquid transportation fuels)
by any suitable
method. For example, GTL facilities may be used with the systems to convert
the hydrocarbons
into syngas and syngas into Fischer-Tropsch liquids, to convert syngas into
methanol and
methanol into gasoline, as an oligomerizer of light olefins, such as ethylene-
propylene-butylene,
into gasoline range hydrocarbons, for the alkylation of iso-butylene with
butane to form iso-
octane fuel additive, as well as for other conversions. CCBG facilities may
serve as an outlet for
waste heat from the systems, excess steam from the systems, fuel gas for co-
feeding to a gasifier,
upgraded coal as feed to a gasifier, fuel gas as co-feed to raw synthesis gas
(e.g., supply of H2
and CO), and/or fuel gas and hydrocarbons to steam reforming to supply
additional H2/CO. BF
facilities could use upgraded coal from the systems as a substitute for
metallurgical coke feed or
as a pulverized coal injection (PCI) feed in the facility. Oil refining and/or
steam cracking
facilities may serve as an outlet for waste heat and/or excess steam from the
systems, as well as
with hydrocarbon and fuel gas feeds for production of hydrogen, CO, methane,
ethane, ethylene,
propane, propylene, butanes, butenes, pentanes, pentenes, and all of their
derivatives including
fuels, solvents, monomers, polymers, specialty chemicals and large arrays of
refined products.
[0152] Now, a calculated example of a process according to one embodiment will
be
described. The following embodiment includes a coal beneficiation plant
integrated at a
pulverized coal-fired power plant. For this example, the coal beneficiation
plant is designed to
use a North Dakota lignite coal for a nominal 50 tons/hour raw coal capacity.
The ultimate and
proximate analysis for the North Dakota Lignite coal is presented in Table 5
(Kitto & Stultz,
2005).
[0153] Table 5: Coal analysis for North Dakota Lignite coal (Kitto & Stultz,
2005):
Original North Dakota Lignite
-59-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
Proximate (Wt N Ultimate (Dry Wt N
Moisture 33.3 C 63.3
VM (Dry) 43.6 H 4.5
FC (Dry) 45.3 N 1
Ash (Dry) 11.1 S 1.1
Heating Value (Btu/lb) 0 19
As Received 7,090 Ash 11.1
[0154] For the calculated example, a system as shown in FIG. 2 utilizing
recirculated gas (e.g.,
methane ) into the pyrolysis reactor, was used in the calculations. The
beneficiation system was
calculated using a low-ranked coal as the solid carbonaceous material to
produce one or more
usable products. The low-ranked coal enters the pulverizer, where its particle
size is reduced to
an appropriate size distribution. Air is introduced into the pulverizer to
move the coal within the
pulverizer and to remove some of the moisture from the coal. As an example,
the pulverized
coal exiting the pulverizer may have a moisture content of about 29%. To
further reduce the
moisture content of the coal, the coal is separated from the pulverizer air
and then transferred to
the dryer, such as through a pipe, where the coal is dried by hot air until
its moisture content is
about 3%. Thus, air may be passed through the dryer to dry the coal. The
system may be
configured to utilize flue gas from the catalyst regenerator to either
directly or indirectly dry the
pulverized coal. The system may be configured to utilize hot flue gas that is
generated by
burning fuel to either directly or indirectly dry the pulverized coal. The
coal may be separated
from the gas prior to the coal being transferred to the pyrolyzer (e.g., the
catalytic pyrolysis
reactor).
[0155] The coal enters the pyrolyzer, wherein regenerated catalyst and fresh
catalyst are
fluidized with the coal with sufficient residence time for the pyrolysis to
reach the desired extent
of the reaction at about 400 C. In one example, 45% of the volatile matter in
the coal is
converted to the gaseous product composition similar to the results in the
experimental example
discussed above. All, or nearly all, of the remaining moisture in the coal is
removed in the
pyrolysis reactor. All, or nearly all, of the sulfur in the coal is converted
to H25, COS, and SO2,
-60-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
resulting in the upgraded coal having a significantly lower sulfur content. A
methane loop may
be utilized to fluidize the particles inside the pyrolysis reactor and carry
the solids and gas
products out of the reactor. The solid stream is first separated from the gas
stream, and then is
further separated into a predominantly upgraded coal stream and a
predominantly spent (e.g.,
deactivated) catalyst stream. The catalyst in the reactor is deactivated,
mainly through coke
deposition on the catalyst. A classifier may be utilized to separate the solid
products into an
upgraded coal stream and a stream of spent catalyst.
[0156] The upgraded coal product, which generally is in powder form, may be
transported for
further processing to convert the coal powder into an easily transportable
product (such as pellets
or briquettes). The upgraded coal may also be injected into the boiler for
combustion and steam
generation purposes. Because of the quality of the upgraded coal, the upgraded
coal burns
cleaner and more efficiently than the original raw coal introduced into the
pulverizer (e.g., the
low-ranked coal). The upgraded coal has a higher heating value, which
according to one
example is about 11,760 Btu/lb (as compared to 7,090 Btu/lb) and a production
rate of 24.2
tons/hour. The coal properties (which have been calculated) are presented in
Table 6, provided
below. In this example, the upgraded coal retains over 80% of its original
heating value while
the majority of the remaining heating value is in the gaseous and liquid
product.
[0157] Table 6: Expected coal analysis for the upgraded coal.
Upgraded North Dakota Lignite
Proximate (Wt %) Ultimate (Dry Wt N
Moisture 0.0 C 65.7
VM (Dry) 27.0 H 4.53
FC (Dry) 56.5 N 1.24
Ash (Dry) 16.5 S 0.62
Heating Value (Btu/lb) 0 11.4
As Received 11,760 Ash 16.5
-61-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0158] The predominantly spent catalyst stream is transferred to the
regenerator through an
inlet, while air is introduced into the regenerator through a second inlet. In
this calculated
example, the spent catalyst has about 5% carbon by weight from the coking.
Also, the solid-
solid separation results in the spent catalyst stream having less than about
3% of the upgraded
coal in the spent catalyst. The air burns the coke off of the spent catalyst
at about 600 C in the
regenerator. It is expected that any upgraded coal entering the regenerator
with the spent catalyst
will be fully combusted, leaving only the coal ash and the regenerated
catalyst. The exiting gas
stream from the regenerator is a flue gas with approximately 4% oxygen, a
level which promotes
nearly complete combustion of all carbonaceous material entering the
regenerator, including the
coke on the spent catalyst. The flue gas has approximately 20% CO2 by volume.
The gas and
solids exit separately from the regeneration reactor. A small amount of the
regenerated or
recycled catalyst stream (e.g., about 3% or less) may be purged through a
purging device that is
fluidly connected to an outlet of the regenerator. The purged catalyst stream
prevents
accumulation of coal ash in the catalyst recirculation loop. The remaining
recycled or
regenerated catalyst stream may be transferred to other elements in the
system, such as, for
example, the pyrolyzer through a recycled catalyst stream input.
[0159] The pyrolyzer may also be configured to utilize a fresh amount of
catalyst (i.e., non-
recycled or regenerated catalyst) to maintain the desired catalyst-to-coal
ratio. The fresh catalyst
is introduced into the pyrolyzer through an inlet, and the amount of fresh
catalyst may be
metered or controlled to maintain the catalyst-to-coal ratio in the pyrolyzer.
[0160] Based on the calculated data presented in FIG. 5, at 400 C, the
expected final gaseous
and liquid product streams out of the pyrolysis reactor and downstream
separation units for this
50 ton/hr coal input are:
Non-condensable Fuel Gas 1,100 lb/hr
Butane/LPG 580 lb/hr
BTEX 940 lb/hr
Higher Hydrocarbons 2,800 lb/hr
-62-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
Solid sulfur 390 lb/hr
CO2 (CCS quality) 9,400 lb/hr
Non-condensable gas 800 lb/hr
[0161] Another system may involve flue gas pyrolysis fluidization, in which
all or a portion of
the flue gas from the regeneration reactor may be used to fluidize the
pyrolysis reactor.
Preferably, enough flue gas will be used to provide any necessary heat for the
pyrolysis reactor
and to help fluidize the coal and catalyst, as well as the carrier gas for the
gaseous product out of
the pyrolysis reactor. With air fed into the regeneration reactor, the flue
gas will consist mainly
of a combination of N2, H205 CO25 CO, and SO2. Any one or more of 02,
hydrogen, CO2, CO
and/or steam could be used in the regeneration of the catalyst. Alternatively,
flue gas from the
regenerator may be kept separate from the pyrolysis reactor.
[0162] In general, CO2 capture and purification is more difficult if CO2 is
present in dilute
quantities, such as in the presence of nitrogen gas, and/or in the presence of
trace amounts of
oxygen, such as in a flue gas. As such, the required process equipment for CO2
capture and
purification is larger when CO2 is contaminated with nitrogen and oxygen
gases. And many
processing systems, (e.g., acid gas recovery systems) cannot recover CO2 when
it is too dilute.
In the systems and processes, as disclosed herein, a high concentration of
substantially nitrogen-
free and oxygen-free CO2 is produced in the pyrolysis reactor. Therefore, an
acid gas removal
system dedicated only to capturing the CO2 from the pyrolysis reactor will
have relatively
smaller equipment, and more recovery technology options may be employed by
this system, than
would otherwise be the case if the regenerator was air fired and if the
resultant nitrogen and
oxygen laden flue gases from the regenerator were commingled with pyrolysis
gases.
[0163] It should be noted that although coal has been discussed as an example
of a
carbonaceous material for use as a feedstock in the systems and the processes
described in this
application, other suitable materials can be used in the systems and
processes. For example,
other types of coals that may be used as feedstock in the systems and
processes described in this
application include but are not limited to lignite or brown coals, sub-
bituminous, bituminous,
anthracite, peat, or any combination thereof Coal derived liquids or oils
including but not
-63-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
limited to pyrolysis derived oils, oily coal slurry, coking derived oils,
gasification derived oils,
hydrogenation derived oils, or any combination thereof may be used as
feedstock. Tar sands
including but not limited to raw tar sands, tar sand derived liquids, asphalt,
bitumen, or any
combination thereof may be used as feedstock. Oil shale including but not
limited to raw oil
shale, oil shale derived liquids, kerogen, or any combination thereof may be
used as feedstock.
Waste oils including but not limited to cooking oils, motor oils, etc., as
well as combinations
thereof may be used as feedstock. Municipal waste, such as solid waste or
waste water treatment
sludge, may be used as feedstock. Waste plastics, such as recycled plastics,
may be used as
feedstock. Biomass including but not limited to lignocellulosic biomass (e.g.,
various
agricultural residues, wheat and rice straw, corn stover, forestry residues,
saw dust, wood chips
and bark, etc.), lignocellulosic biomass derived oils (e.g., pyrolysis derived
oils, hydropyrolysis
oils, biocrude, etc.), various lipid containing oils (e.g., plant derived
lipid oils, jatropha, palm,
algal derived lipid oils, etc.), and combinations thereof may be used as
feedstock. Petroleum
including but not limited to various petroleum derived oils, crude oil,
refinery derived oil,
asphalt, synthetic crude oil, bottom oils, residual oils, heavy oils, and
combinations thereof may
be used as feedstock. Other suitable materials may be used as feedstock as
well. Preferably, the
carbonaceous raw material releases volatile matter when exposed to thermal
pyrolyzation
conditions (e.g., heated to pyrolysis temperatures). Less suitable
carbonaceous materials would
include those such as coke, which has been substantially depleted of volatile
matter content.
Moreover, any of the feedstocks mentioned above may be used independently or
as a co-feed
(e.g., co-fed feedstock) with one or more other feedstocks, which may be taken
from the
feedstocks mentioned above.
[0164] Para. A. A process for upgrading a solid carbonaceous material,
comprising: heating
the solid carbonaceous material in the presence of a catalyst under partial
pyrolysis conditions,
and obtaining an upgraded solid carbonaceous product, a gaseous product, and a
spent catalyst.
[0165] Para. B. The process of Para. A, wherein the solid carbonaceous
material is coal and
the upgraded solid carbonaceous product is an upgraded coal product.
-64-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0166] Para. C. The process of Para. A or B, wherein a weight of fixed carbon
retained in the
upgraded solid carbonaceous product is at least 50 weight percent of fixed
carbon in the solid
carbonaceous material.
[0167] Para. D. The process of any one of Paras. A-C, wherein a weight of ash
retained in the
upgraded solid carbonaceous product is at least 60 weight percent of ash in
the solid
carbonaceous material.
[0168] Para. E. The process of any one of Para. A-D, wherein a weight of
volatile matter
retained in the upgraded solid carbonaceous product is from about 10 to about
90 weight percent
of volatile matter in the solid carbonaceous material.
[0169] Para. F. The process of any one of Para. A-E, wherein a weight of
volatile matter
retained in the upgraded coal product is from about 10 to about 90 weight
percent of volatile
matter in the coal.
[0170] Para. G. The process of any one of Para. A-F, further comprising
pretreating the
starting solid carbonaceous material prior to heating under partial pyrolysis
conditions using at
least one of a dryer, a de-asher, and a washer.
[0171] Para. H. The process of any one of Para. A-G, further comprising
obtaining an amount
of CO2 greater than about 10 weight % of the volatile matter in the starting
solid carbonaceous
material.
[0172] Para. I. The process of any one of Para. A-H, further comprising
separating the gaseous
product from the upgraded solid carbonaceous product.
[0173] Para. J. The process of any one of Para. I, further comprising
condensing the separated
gaseous product into a gaseous stream and a liquid stream.
[0174] Para. K, The process of any one of Para. A-J, further comprising
obtaining an amount
of a non-condensable fuel gas from about 1 to about 40 weight % of the
volatile matter in the
starting solid carbonaceous material.
-65-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0175] Para. L. The process of any one of Para. A-K, further comprising
obtaining an amount
of a non-condensable fuel gas from about 1 to about 40 weight % of the
volatile matter in the
starting coal.
[0176] Para. M. The process of any one of Para. A-L, further comprising
obtaining an amount
of LPG greater than from about 1 to about 40 weight % of the volatile matter
in the starting solid
carbonaceous material.
[0177] Para. N. The process of any one of Para. A-M, further comprising
obtaining an amount
of BTEX from about 0.5 to about 40 weight % of the volatile matter in the
starting solid
carbonaceous material.
[0178] Para. 0. The process of any one of Para. A-N, further comprising
obtaining an amount
of Higher Hydrocarbons from about 0.3 to about 20 weight % of the volatile
matter in the
starting solid carbonaceous material.
[0179] Para. P. The process of any one of Para. A-0, further comprising
obtaining an amount
of heteroatom-containing organics that is no greater than 5 weight % of the
volatile matter in the
starting solid carbonaceous material.
[0180] Para. Q. The process of any one of Para. A-P, wherein the spent
catalyst and the
upgraded solid carbonaceous product are recovered as a mixture.
[0181] Para. R. The process of any one of Para. A-Q, wherein the spent
catalyst and the
upgraded solid carbonaceous product are recovered separately.
[0182] Para. S. The process of any one of Para. A-R, further comprising
regenerating the spent
catalyst by contacting the spent catalyst with a mixture of gases containing
at least one oxidizing
gas to form a regenerated catalyst.
[0183] Para. T. The process of any one of Para. S, wherein at least a portion
of the regenerated
catalyst is heated in the presence of additional solid carbonaceous material
in a subsequent
partial pyrolysis reaction.
-66-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0184] Para. U. The process of any one of Para. A-T, further comprising
regenerating the
spent catalyst by acid washing the spent catalyst with an acidic solution to
form a regenerated
catalyst.
[0185] Para. V. The process of any one of Para. S-U, wherein at least a
portion of the
regenerated catalyst is heated in the presence of additional solid
carbonaceous material in a
subsequent partial pyrolysis reaction.
[0186] Para. W. The process of Para. A-V, wherein a weight of total sulfur
retained in the
upgraded solid carbonaceous product is no more than 80 weight percent of the
total sulfur in the
starting solid carbonaceous material.
[0187] Para. X. The process of any one of Para. A-V, wherein a weight of
organic sulfur
retained in the upgraded solid carbonaceous product is no more than 50 weight
percent of the
organic sulfur in the starting solid carbonaceous material.
[0188] Para. AA. A process for converting a solid carbonaceous material in a
beneficiation
system into a upgraded solid carbonaceous product, the process comprising:
introducing the solid carbonaceous material and a catalyst into a pyrolysis
reactor to
produce a gaseous product stream and a solid product stream, wherein the solid
product stream comprises the upgraded solid carbonaceous product;
recovering the gaseous product stream from the reactor; and
recovering the solid product stream from the reactor.
[0189] Para. AB. The process of Para. AA, wherein the solid carbonaceous
material is coal
and the upgraded solid carbonaceous product is an upgraded coal product.
[0190] Para. AC. The process of any one of Para. AA-AB, wherein the catalyst
is immobilized
in the pyrolysis reactor; and the process further comprises separating the
upgraded solid
carbonaceous product from the catalyst inside the pyrolysis reactor.
[0191] Para. AD. The process of any one of Para. AA-AC, further comprising:
recovering a separated spent catalyst from the pyrolysis reactor;
-67-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
transferring the spent catalyst to a regenerator; and
regenerating the spent catalyst in the regenerator, in which unpyrolyzed coal,
coke, and carbonaceous material are removed from the spent catalyst.
[0192] Para. AE. The process of any one of Para. AA-AD, further comprising:
transferring the gaseous product stream to a separator; and
at least partially condensing the gaseous product stream in the separator
producing a refined gas stream, a hydrocarbon liquid stream, and an aqueous
liquid phase stream.
[0193] Para. AF. The process of any one of Para. AA-AE, wherein the solid
product stream
further comprises a spent catalyst, the process further comprising:
separating the solid product stream into the upgraded solid carbonaceous
product
and the spent catalyst after recovering the solid product stream from the
pyrolysis reactor, wherein the separated spent catalyst comprises the catalyst
and at least one of unpyrolyzed coal, coke, and carbonaceous material.
[0194] Para. AG. The process of Para. AF, further comprising:
transferring the separated catalyst to a regenerator in which at least a
portion of
the at least one of the unpyrolyzed coal, coke, and carbonaceous material is
removed from the catalyst; and
transferring the gaseous product stream to a separator in which the gaseous
product stream is at least partially condensed in the separator producing a
refined gas stream, a hydrocarbon liquid stream, and an aqueous liquid phase
stream.
[0195] Para. AH. The process of any one of Para. AA-AG, wherein at least a
portion of the at
least one of the unpyrolyzed coal, coke, and carbonaceous material is removed
from the catalyst
by at least one of combustion, steam, and a reducing gas.
-68-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0196] Para. Al. The process of any one of Para. AA-AH, wherein the pyrolysis
reactor is
configured as one of a HERB, a fluidized bed, a moving bed, or an entrained
flow bed, and
wherein the coal and the catalyst move through the pyrolysis reactor.
[0197] Para. AJ. The process of any one of Para. AA-AI, wherein the solid
product stream is
transferred outside the pyrolysis reactor to a solid-solid separator that
separates the upgraded
solid carbonaceous product and the spent catalyst.
[0198] Para. AK. The process of any one of Para. AA-AJ, wherein the solid-
solid separator
includes a classifier that separates the upgraded solid carbonaceous product
from the spent
catalyst based on one of particle size, mass, or density.
[0199] Para. AL. The process of any one of Para. AA-AK, wherein at least one
of a size and a
density of the spent catalyst is different than at least one of a size and a
density of the upgraded
solid carbonaceous product, and wherein the classifier of the solid-solid
separator separates the
upgraded solid carbonaceous product and the spent catalyst based on at least
one of size and
density.
[0200] Para. AM. The process of any one of Para. AA-AL, further comprising:
reducing a size of the particles of the solid carbonaceous material in a
pulverizer
prior to being introduced into the pyrolysis reactor; and
pretreating the solid carbonaceous material in a pretreating device that
includes at
least one of a dryer configured to dry the coal from the pulverizer utilizing
a
stream of heated fluid, a washer configured to wash the coal from the
pulverizer, and a de-asher configured to remove ash from the coal, wherein
the pretreating device is provided between the pulverizer and the pyrolysis
reactor.
[0201] Para. AN. The process of any one of Para. AM, wherein the stream of
heated fluid is
hot flue gas produced by a regenerator during removal of at least a portion of
any unpyrolyzed
coal, coke, and carbonaceous material from the spent catalyst utilizing an
oxygen-carrying gas.
-69-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0202] Para. AO. The process of any one of Para. AE-AN, wherein the separator
further
includes an acid gas removal system that separates at least one of a sulfur-
carrying compound, a
nitrogen-carrying compound, and carbon dioxide from the gaseous product
stream.
[0203] Para. AP. The process of any one of Para. AA-A0, wherein the catalyst
introduced into
the pyrolysis reactor includes a first portion comprising regenerated catalyst
received from a
regenerator and a second portion comprising new catalyst that has not been
regenerated, and
wherein the first portion of regenerated catalyst has a higher relative
temperature than the new
catalyst and the coal, such that the regenerated catalyst is a heating medium
to heat the coal
introduced into the pyrolysis reactor.
[0204] Para. AQ. The process of any one of Para. AA-AP, wherein the catalytic
pyrolysis of
the solid carbonaceous material takes place at a temperature from about 350 C
to about 850 C.
[0205] Para. AR. The process of any one of Para. AA-AQ, wherein the solid
carbonaceous
material introduced into the pyrolysis reactor has a weighted hour space
velocity from about 0.2
to about 25 kg/hr per kg of catalyst.
[0206] Para. AS. The process of any one of Para. AA-AR, wherein the solid
carbonaceous
material has a residence time during the catalytic process from about 0.1
second to about 1
minute.
[0207] Para. AT. The process of any one of Para. AA-AS, wherein a weight ratio
of the
catalyst to solid carbonaceous material introduced into the pyrolysis reactor
is from about 0 to
about 100.
[0208] Para. AU. The process of any one of Para. AA-AS, further comprising:
providing an acid gas removal system that is configured to capture and
isolating
CO2 from at least one of the gaseous product from the pyrolysis reactor and a
gas from a regenerator configured to regenerate spent catalyst from the
pyrolysis reactor; and
-70-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
obtaining an amount of CO2 greater than about 4 weight % of the dry ash free
coal.
[0209] Para. AV. The process of any one of Para. AA-AU, further comprising
obtaining an
amount of CO2 greater than about 10 weight % of the volatile matter in the
starting solid
carbonaceous material.
[0210] Para. AW. The process of any one of Para. AA-AV, further comprising
obtaining an
amount of CO2 greater than about 4 weight % of the dry ash free coal.
[0211] Para. AX. The process of any one of Para. AA-AW, further comprising:
regenerating a spent catalyst in a regenerator configured to produce a hot
flue gas
during regeneration; and
transferring at least a portion of the hot flue gas to the pyrolysis reactor
to fluidize
the pyrolysis reactor.
[0212] Para. AY. The process of any one of Para. AX, wherein a gaseous fluid
comprising at
least one of CO, CO2, water, hydrogen, and oxygen is introduced into the
regenerator to facilitate
removal of unpyrolyzed coal, coke, and carbonaceous material from the spent
catalyst.
[0213] Para. AZ. The process of any one of Para. AY, further comprising
collecting the hot
flue gas that includes CO2 for one of carbon sequestration or enhanced oil
recovery.
[0214] Para. BA. The process of any one of Para. AX-AZ, further comprising
passing the hot
flue gas through a heat exchanger to produce heat that is used to heat the
solid carbonaceous
material in the pyrolysis reactor.
[0215] Para. BB. The process of any one of Para. AX-BA, wherein the
regenerator uses steam
in addition to, or instead of, air to remove the coal, coke, and carbonaceous
material from the
spent catalyst by at least one of hydrolysis and steam gasification.
-71-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
[0216] Para. BC. The process of any one of Para. AX-BB, wherein the
regenerator uses
hydrogen or at least one other hydrogen-containing chemical, including
hydrocarbons, to
reductively remove the coal, coke, and carbonaceous material from the spent
catalyst.
[0217] Para. BD. The process of any one of Para. AA-BC, wherein a gas is co-
fed into the
pyrolysis reactor, wherein the gas comprises at least one light hydrocarbon
compound that is
recovered from the gaseous product stream.
[0218] Para. BE. The process of any one of Para. AA-BD, wherein the at least
one light
hydrocarbon compound is recycled back to the pyrolysis reactor.
[0219] Para. BF. The process of any one of Para. AA-BE, further comprising
obtaining an
amount of BTEX from about 0.5 to about 80 weight % of the volatile matter in
the starting solid
carbonaceous material.
[0220] Para. BG. The process of any one of Para. AA-BF, wherein a biomass is
co-fed into the
pyrolysis reactor.
[0221] Para. BH. The process of any one of Para. AA-BG, wherein at least one
of an oil shale,
a coal derived liquid, a tar sand, and a petroleum is co-fed into the
pyrolysis reactor.
[0222] Para. BI. The process of any one of Para. AA-BH, wherein at least one
of a wet gas and
a natural gas is co-fed into the pyrolysis reactor.
[0223] Para. BJ. The process of any one of Para. AA-BI, wherein the pyrolysis
reactor
includes a stationary catalyst, such that the solid carbonaceous material
moves relative to the
catalyst through the reactor, to produce the gaseous product stream and the
solid product stream,
the process further comprising:
transferring the gaseous product stream to a separator to at least partially
condense at least a portion of the gas product stream into a liquid product
and
a gaseous product; and
-72-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
wherein the solid product stream contains less than 1 weight part catalyst per
100
parts upgraded carbonaceous product.
[0224] Para. CA. A process for converting a biomass in a beneficiation system
into an
upgraded solid product, the process comprising:
introducing the biomass and a catalyst into a pyrolysis reactor to produce a
gaseous product stream and an upgraded solid product stream, the solid
product stream comprising spent catalyst and the upgraded solid product;
separating the upgraded solid product and the spent catalyst;
transferring the separated spent catalyst to a regenerator that removes at
least a
portion of any unpyrolyzed coal, coke, and other carbonaceous material from
the spent catalyst; and
transferring the gaseous product stream to a separator that produces a liquid
product and a gaseous product;
wherein a weight of ash retained in the upgraded solid product is at least 60
weight percent of ash in the biomass introduced into the pyrolysis reactor.
[0225] Para. CB. The process of Para. CA, wherein an amount of phenol produced
is less than
an amount of toluene produced on a weight basis.
[0226] Para. CC. The process of Para. CA or CB, wherein an amount of tars
produced is less
than an amount of light oils produced on a weight basis.
[0227] As utilized herein, the terms "approximately," "about,"
"substantially", and similar
terms are intended to have a broad meaning in harmony with the common and
accepted usage by
those of ordinary skill in the art to which the subject matter of this
disclosure pertains. It should
be understood by those of skill in the art who review this disclosure that
these terms are intended
to allow a description of certain features described and claimed without
restricting the scope of
these features to the precise numerical ranges provided. Accordingly, these
terms should be
interpreted as indicating that insubstantial or inconsequential modifications
or alterations of the
-73-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
subject matter described and claimed are considered to be within the scope of
the invention as
recited in the appended claims.
[0228] The terms "coupled," "connected," and the like, as used herein, mean
the joining of two
members directly or indirectly to one another. Such joining may be stationary
(e.g., permanent)
or moveable (e.g., removable or releasable). Such joining may be achieved with
the two
members or the two members and any additional intermediate members being
integrally formed
as a single unitary body with one another or with the two members or the two
members and any
additional intermediate members being attached to one another.
[0229] References herein to the positions of elements (e.g., "top," "bottom,"
"above," "below,"
etc.) are merely used to describe the orientation of various elements in the
FIGURES. It should
be noted that the orientation of various elements may differ according to
other illustrative
embodiments, and that such variations are intended to be encompassed by the
present disclosure.
[0230] The construction and arrangement of the elements of the systems (e.g.,
beneficiation
systems) as shown in the illustrative embodiments are illustrative only.
Although only a few
embodiments of the present disclosure have been described in detail, those
skilled in the art who
review this disclosure will readily appreciate that many modifications are
possible (e.g.,
variations in sizes, dimensions, structures, shapes and proportions of the
various elements, values
of parameters, mounting arrangements, use of materials, colors, orientations,
etc.) without
materially departing from the novel teachings and advantages of the subject
matter recited. For
example, elements shown as integrally formed may be constructed of multiple
parts or elements,
the position of elements may be reversed or otherwise varied, and the nature
or number of
discrete elements or positions may be altered or varied.
[0231] Additionally, the word "illustrative" is used to mean serving as an
example, instance, or
illustration. Any embodiment or design described herein as "illustrative" is
not necessarily to be
construed as preferred or advantageous over other embodiments or designs (and
such term is not
intended to connote that such embodiments are necessarily extraordinary or
superlative
examples). Rather, use of the word "illustrative" is intended to present
concepts in a concrete
manner. Accordingly, all such modifications are intended to be included within
the scope of the
-74-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
present disclosure. Other substitutions, modifications, changes, and omissions
may be made in
the design, operating conditions, and arrangement of the preferred and other
illustrative
embodiments without departing from the scope of the appended claims.
[0232] As used herein, "about" will be understood by persons of ordinary skill
in the art and
will vary to some extent depending upon the context in which it is used. If
there are uses of the
term which are not clear to persons of ordinary skill in the art, given the
context in which it is
used, "about" will mean up to plus or minus 10% of the particular term.
[0233] The use of the terms "a" and "an" and "the" and similar referents in
the context of
describing the elements (especially in the context of the following claims)
are to be construed to
cover both the singular and the plural, unless otherwise indicated herein or
clearly contradicted
by context. Recitation of ranges of values herein are merely intended to serve
as a shorthand
method of referring individually to each separate value falling within the
range, unless otherwise
indicated herein, and each separate value is incorporated into the
specification as if it were
individually recited herein. All methods described herein can be performed in
any suitable order
unless otherwise indicated herein or otherwise clearly contradicted by
context. The use of any
and all examples, or exemplary language (e.g., "such as") provided herein, is
intended merely to
better illuminate the embodiments and does not pose a limitation on the scope
of the claims
unless otherwise stated. No language in the specification should be construed
as indicating any
non-claimed element as essential.
[0234] While certain embodiments have been illustrated and described, it
should be understood
that changes and modifications can be made therein in accordance with ordinary
skill in the art
without departing from the technology in its broader aspects as defined in the
following claims.
[0235] The embodiments, illustratively described herein may suitably be
practiced in the
absence of any element or elements, limitation or limitations, not
specifically disclosed herein.
Thus, for example, the terms "comprising," "including," "containing," etc.
shall be read
expansively and without limitation. Additionally, the terms and expressions
employed herein
have been used as terms of description and not of limitation, and there is no
intention in the use
of such terms and expressions of excluding any equivalents of the features
shown and described
-75-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
or portions thereof, but it is recognized that various modifications are
possible within the scope
of the claimed technology. Additionally, the phrase "consisting essentially
of' will be
understood to include those elements specifically recited and those additional
elements that do
not materially affect the basic and novel characteristics of the claimed
technology. The phrase
"consisting of' excludes any element not specified.
[0236] The present disclosure is not to be limited in terms of the particular
embodiments
described in this application. Many modifications and variations can be made
without departing
from its spirit and scope, as will be apparent to those skilled in the art.
Functionally equivalent
methods and compositions within the scope of the disclosure, in addition to
those enumerated
herein, will be apparent to those skilled in the art from the foregoing
descriptions. Such
modifications and variations are intended to fall within the scope of the
appended claims. The
present disclosure is to be limited only by the terms of the appended claims,
along with the full
scope of equivalents to which such claims are entitled. It is to be understood
that this disclosure
is not limited to particular methods, reagents, compounds compositions or
biological systems,
which can of course vary. It is also to be understood that the terminology
used herein is for the
purpose of describing particular embodiments only, and is not intended to be
limiting.
[0237] As will be understood by one skilled in the art, for any and all
purposes, particularly in
terms of providing a written description, all ranges disclosed herein also
encompass any and all
possible subranges and combinations of subranges thereof Any listed range can
be easily
recognized as sufficiently describing and enabling the same range being broken
down into at
least equal halves, thirds, quarters, fifths, tenths, etc. As a non-limiting
example, each range
discussed herein can be readily broken down into a lower third, middle third
and upper third, etc.
As will also be understood by one skilled in the art all language such as "up
to," "at least,"
"greater than," "less than," and the like, include the number recited and
refer to ranges which can
be subsequently broken down into subranges as discussed above. Finally, as
will be understood
by one skilled in the art, a range includes each individual member.
[0238] All publications, patent applications, issued patents, and other
documents referred to in
this specification are herein incorporated by reference as if each individual
publication, patent
-76-
CA 02950138 2016-11-23
WO 2015/179806 PCT/US2015/032252
application, issued patent, or other document was specifically and
individually indicated to be
incorporated by reference in its entirety. Definitions that are contained in
text incorporated by
reference are excluded to the extent that they contradict definitions in this
disclosure.
[0239] Other substitutions, modifications, changes and omissions may also be
made in the
design, operating conditions and arrangement of the various illustrative
embodiments without
departing from the scope of the present invention. For example, any element
disclosed in one
embodiment may be incorporated or utilized with any other embodiment disclosed
herein. Also,
for example, the order or sequence of any process or method steps may be
varied or re-
sequenced according to alternative embodiments. Any means-plus-function clause
is intended to
cover the structures described herein as performing the recited function and
not only structural
equivalents but also equivalent structures. Other substitutions,
modifications, changes and
omissions may be made in the design, operating configuration, and arrangement
of the preferred
and other illustrative embodiments without departing from the scope of the
appended claims.
-77-