Note: Descriptions are shown in the official language in which they were submitted.
CA 02951542 2016-12-07
WO 2015/195382 1 PCT/US2015/034592
HIGH ENERGY AGGREGATES OF COAL FINES AND
BENEFICIATED ORGANIC-CARBON-CONTAINING FEEDSTOCK
FIELD OF THE INVENTION
The present invention relates generally to the production of biomass and coal
aggregates.
BACKGROUND OF THE INVENTION
The vast majority of fuels are distilled from crude oil or obtained from
natural gas
pumped from limited underground reserves, or mined from coal. As the earth's
crude oil
supplies become more difficult and expensive to collect and there are growing
concerns
about the environmental effects of coal other than clean anthracite coal, the
world-wide
demand for energy is simultaneously growing. Over the next ten years,
depletion of the
remaining world's easily accessible crude oil reserves, natural gas reserves,
and low-sulfur
bituminous coal reserves will lead to a significant increase in cost for fuel
obtained from
crude oil, natural gas, and coal.
The search to find processes that can efficiently convert biomass to fuels and
by-
products suitable for transportation and/or heating is an important factor in
meeting the
ever-increasing demand for energy. In addition, processes that have solid
byproducts that
have improved utility are also increasingly in demand.
Biomass is a renewable organic-carbon-containing feedstock that contains plant
cells
and has shown promise as an economical source of fuel. However, this feedstock
typically
contains too much water and contaminants such as water-soluble salts to make
it an
economical alternative to common sources of fuel such as coal, petroleum, or
natural gas.
Historically, through traditional mechanical/chemical processes, plants would
give
up a little less than 25 weight percent of their moisture. And, even if the
plants were sun
or kiln-dried, the natural and man-made chemicals and water-soluble salts that
remain in
the plant cells combine to create corrosion and disruptive glazes in furnaces.
Also, the
remaining moisture lowers the heat-producing million British thermal units per
ton
(MMBTU per ton) energy density of the feedstock thus limiting a furnace's
efficiency. A
BTU is the amount of heat required to raise the temperature of one pound of
water one
degree Fahrenheit, and 1 MM BTU/ton is equivalent to 1.163 Gigajoules per
metric tonne
CA 02951542 2016-12-07
WO 2015/195382 2 PCT/US2015/034592
(GJ/MT). Centuries of data obtained through experimentation with a multitude
of biomass
materials all support the conclusion that increasingly larger increments of
energy are
required to achieve increasingly smaller increments of bulk density
improvement. Thus,
municipal waste facilities that process organic-carbon-containing feedstock, a
broader class
of feedstock that includes materials that contain plant cells, generally
operate in an energy
deficient manner that costs municipalities money. Similarly, the energy needed
to process
agricultural waste, also included under the general term of organic-carbon-
containing
feedstock, for the waste to be an effective substitute for coal or petroleum
are not commercial
without some sort of governmental subsidies and generally contain
unsatisfactory levels of
either or both water or water-soluble salts. The cost to suitably transport
and/or prepare such
feedstock in a large enough volume to be commercially successful is expensive
and currently
uneconomical. Also, the suitable plant-cell-containing feedstock that is
available in sufficient
volume to be commercially useful generally has water-soluble salt contents
that result in
adverse fouling and contamination scenarios with conventional processes.
Suitable land for
growing a sufficient amount of energy crops to make economic sense typically
are found in
locations that result in high water-soluble salt content in the plant cells,
i.e., often over 4000
mg/kg on a dry basis.
Attempts have been made to prepare organic-carbon-containing feedstock as a
solid renewable fuel, coal substitute, or binders for the making of coal
aggregates from
coal fines, but these have not been economically viable as they generally
contain water-
soluble salts that can contribute to corrosion, fouling, and slagging in
combustion
equipment, and have high water content that reduces the energy density to well
below that
of coal in large part because of the retained moisture. However, there remains
a need for
biomass or biochar as it is a clean renewable source of solid fuel if it could
be made cost-
effectively with a more substantial reduction in its content of water and
water-soluble salt
for use as coal substitutes or as high energy binders with coal fines.
Solid byproducts with improved beneficial properties are an important factor
in
meeting the ever-increasing demand for energy. The present invention fulfills
these needs
and provides various advantages over the prior art.
SUMMARY OF THE INVENTION
CA 02951542 2016-12-07
WO 2015/195382 3 PCT/US2015/034592
Embodiments of the present are directed to a composition aggregate from, in
part,
renewable, unprocessed organic-carbon-containing feedstock and a process. The
composition is a high energy processed biomass / coal blended compact
aggregate that
comprises at least 10 wt% of a coal having an energy density of at least 21
MMBTU/ton
(24 GJ/MT) and at least 10 wt% of a processed biomass comprising a processed
organic-
carbon-containing feedstock with characteristics that include an energy
density of at least
17 MMBTU/ton (20 GJ/MT) and a water-soluble intracellular salt content that is
decreased more than 60 wt% on a dry basis for the processed organic-carbon-
containing
feedstock from that of unprocessed organic-carbon-containing feedstock that
was the
source of the processed organic-carbon-containing feedstock. The high energy
processed
biomass / coal blended compact aggregate is made from unprocessed organic-
carbon-
containing feedstock converted into the processed organic-carbon-containing
feedstock
with a beneficiation sub-system and blended with coal into a blended compact
aggregate
in a blending sub-system.
The process of making high energy processed biomass / coal blended compact
aggregate that comprises at least 10 wt% of a coal having an energy density of
at least 21
MMBTU/ton (24 GJ/MT) and at least 10 wt% of a processed biomass comprises
three
steps. The first step is to input into a system comprising a first, a second,
and a third
subsystem components comprising coal and a renewable, unprocessed organic-
carbon-
containing feedstock that includes free water, intercellular water,
intracellular water,
intracellular water-soluble salts, and at least some plant cells comprising
cell walls that
include lignin, hemicellulose, and microfibrils within fibrils. The second
step is to pass
unprocessed organic-carbon-containing feedstock through a beneficiation sub-
system
process to result in processed biomass having a water content of less than 20
wt% and a
water soluble intracellular salt content that is reduced by more than 60 wt%
on a dry basis
for the processed organic-carbon-containing feedstock from that of the
unprocessed
organic-carbon-containing feedstock. The third step is to pass the processed
biomass
through a blending sub-system process, to be joined with coal to result in a
high energy
processed biomass / coal blended compact aggregate that comprises at least 10
wt% of a
coal having an energy density of at least 21 MMBTU/ton (24 GJ/MT) and at least
10 wt%
of a processed biomass comprising a processed organic-carbon-containing
feedstock with
characteristics that include an energy density of at least 17 MMBTU/ton (20
GJ/MT) and a
CA 02951542 2016-12-07
WO 2015/195382 4 PCT/US2015/034592
water-soluble intracellular salt content that is decreased more than 60 wt% on
a dry basis
for the processed organic-carbon-containing feedstock from that of the
unprocessed
organic-carbon-containing feedstock.
The invention is a high energy processed biomass / coal blended compact
aggregate that that has not been previously possible. Known blends of high
energy coal
and biomass comprise less than 30 wt percent biomass with a significantly
lower energy
density that lowers the energy density of at least 21 MMBTU/ton (24 GJ/MT) and
with
added salt impurities from the biomass. In addition, the invention allows the
productive
use of coal fines that comprise up to 50 wt% of a coal mining output and are
currently
stockpiled with little possibility of transporting the coal fines because of
their potential
explosive nature. The invention now allows aggregates with from 10 wt% to 90
wt% high
energy coal fines with an energy density of at least 21 MMBTU/ton (24 GJ/MT)
and from
10 wt% to 90 wt% processed biomass having an energy density of at least 17
MMBTU/ton
(20 GJ/MT). The low water-soluble intracellular salt content of the processed
biomass
substantially reduces adverse corrosive wear and maintenance cleaning of the
devices that
is typical today. The uniform low water content and uniform, high energy
density of the
beneficiated organic-carbon-containing feedstock used to make the high energy
coal /
processed biomass aggregates allows for a wide variety of renewable organic-
carbon-
containing feedstock to be used in the heating subsystems of the process in a
cost efficient
manner. During the beneficiation section of the process, the substantial
reduction of
water-soluble salts reduces the adverse results that occur with the subsequent
use of the
processed organic-carbon-containing feedstock. In addition, energy needed to
remove
water from unprocessed organic-carbon-containing feedstock described above to
a content
of below 20 wt % and a substantial amount of the water-soluble salt with the
invention is
significantly less than for conventional processes. In some embodiments, the
total cost per
weight of the beneficiated feedstock is reduced by at least 60% of the cost to
perform a
similar task with known mechanical, physiochemical, or thermal processes to
prepare
renewable organic-carbon-containing feedstock for use in subsequent fuel
making
operations such as heating sub-systems such as an oxygen-deficient thermal sub-
system or
an oxygen-deficient microwave sub-system and blending sub-systems to make
aggregates
of high energy coal and processed biomass.
CA 02951542 2016-12-07
WO 2015/195382 5 PCT/US2015/034592
The above summary is not intended to describe each embodiment or every
implementation of the present invention. Advantages and attainments, together
with a
more complete understanding of the invention, will become apparent and
appreciated by
referring to the following detailed description and claims taken in
conjunction with the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a diagram of a typical plant cell with an exploded view of a
region of its
cell wall showing the arrangement of fibrils, microfibrils, and cellulose in
the cell wall.
Figure 2 is a diagram of a perspective side view of a part of two fibrils in a
secondary plant cell wall showing fibrils containing microfibrils and
connected by strands
of hemicellulose and lignin
Figure 3 is a diagram of a cross-sectional view of a section of bagasse fiber
showing where water and water-soluble salts reside inside and outside plant
cells.
Figure 4 is a diagram of a side view of an embodiment of a reaction chamber in
a
beneficiation sub-system.
Figure 5A is a diagram of the front views of various embodiments of pressure
plates in a beneficiation sub-system.
Figure 5B is a perspective view of a close-up of one embodiment of a pressure
plate shown in Figure 5A.
Figure 5C is a diagram showing the cross-sectional view down the center of a
pressure plate with fluid vectors and a particle of pith exposed to the fluid
vectors.
Figure 6A is a graphical illustration of the typical stress-strain curve for
lignocellulosic fibril.
Figure 6B is a graphical illustration of pressure and energy required to
decrease the
water content and increase the bulk density of typical organic-carbon-
containing
feedstock.
Figure 6C is a graphical illustration of the energy demand multiplier needed
to
achieve a bulk density multiplier.
CA 02951542 2016-12-07
WO 2015/195382 6 PCT/US2015/034592
Figure 6D is a graphical illustration of an example of a pressure cycle for
decreasing water content in an organic-carbon-containing feedstock with an
embodiment
of the invention tailored to a specific the organic-carbon-containing
feedstock.
Figure 7 is a table illustrating the estimated energy consumption needed to
remove
at least 75 wt % water-soluble salt from organic-carbon-containing feedstock
and reduce
water content from 50 wt % to 12 wt % with embodiments of the beneficiation
sub-system
of the invention compared with known processes.
Figure 8 is a diagram of a side view of an embodiment of a beneficiation sub-
system having four reaction chambers in parallel, a pretreatment chamber, and
a vapor
condensation chamber.
Figure 9 is a diagram of a side view of an embodiment of a horizontal
sublimation
oxygen-deficient thermal sub-system with a reactor chamber having one pass.
Figure 10 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes, a flexible shaft seal, and a high temperature
adjustable shaft
cover plate.
Figure 11A is a diagram of a side view of an embodiment of the flexible shaft
seal
casing with the rope seals compressed in place.
Figure 11B is a diagram of a view of an element of the embodiment of Fig 11A
showing a back view of the frame holding the double rope seal.
Figure 11C is a diagram of a view of an element of the embodiment of Fig 11A
showing a back view of the frame holding a single rope seal.
Figure 11D is diagram of a front view and side view of an element of the
embodiment of Fig 11A showing a cover that compresses the double rope seal of
Fig. 11B.
Figure 11E is a diagram of a front view and side view of an element of the
embodiment of Fig 11A showing a cover that compresses the single rope seal of
Fig 11C.
Figure 12A is a diagram of the front view and side view of an embodiment of
the
high temperature adjustable cover plate showing a top half
Figure 12B is a diagram of the front view and side view of the embodiment of
the
high temperature adjustable cover plate of Fig. 12A showing a bottom half.
Figure 12C is a diagram of the front view of the embodiment of the high
temperature adjustable cover plate of Fig. 12A showing the top half of Fig.
12A and the
bottom half of Fig. 12B joined.
CA 02951542 2016-12-07
WO 2015/195382 7 PCT/US2015/034592
Figure 12D is a diagram of the front view of the assembled high temperature
adjustable cover plate in the cold temperature position.
Figure 12E is a diagram of the front view of the assembled high temperature
adjustable cover plate in the hot temperature position.
Figure 13 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes, a flexible shaft seal, a high temperature
adjustable shaft cover
plate, and a high temperature vertical support stand.
Figure 14A is a front view of an embodiment of a vertical stand showing a
curved
cradle and a horizontal ring for passing coolant.
Figure 14B is a top view of the embodiment of Fig. 14A showing the cooling
ring.
Figure 14C is a front view of an embodiment of a vertical stand showing a
curved
cradle and a vertical up and down cooling passage within the vertical shaft of
the vertical
stand.
Figure 15 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes and a four-pass bypass manifold attached to the
outside of the
reaction chamber to increase residence time.
Figure 16 is a diagram of a side view of an embodiment of a system with a
substantially horizontal reaction chamber having two passes, a compression
chamber, and
a drying chamber.
Figure 17 is a diagram of a side view of an embodiment of a system with a
substantially vertical reaction chamber.
Figure 17A is a diagram of the reaction tube array of the embodiment shown in
Fig. 17.
Figure 18A and 18B illustrate side and cross sectional views, respectively, of
a
reaction chamber of an embodiment of a microwave sub-system configured to
convert
organic-carbon-containing materials to biochar.
Figure 18C is a diagram of a tilted reaction chamber of an embodiment of the
microwave sub-system.
Figure 18D is a diagram of a side view of an embodiment of the microwave sub-
system.
CA 02951542 2016-12-07
WO 2015/195382 8 PCT/US2015/034592
Figure 19A is a block diagram of an embodiment of the microwave sub-system
that uses the reaction chamber illustrated in Figures 9A and 9B for water/air
extraction and
a reaction process.
Figure 19B illustrates an embodiment of the microwave sub-system that includes
feedback control.
Figure 20A shows a microwave sub-system which includes multiple stationary
magnetrons arranged on a drum that is disposed outside a cylindrical reaction
chamber
having one or more microwave-transparent walls.
Figure 20B illustrates an embodiment of a microwave sub-system having a drum
supporting magnetrons which may be rotated around the longitudinal axis of the
reaction
chamber while the reaction chamber is concurrently rotated around its
longitudinal axis;
Figure 20C shows an embodiment of a microwave-sub system reaction chamber
with a feedstock transport mechanism comprising baffles.
Figure 21 illustrates a system having a rotating magnetron in addition to a
secondary heat source.
Figure 22 depicts a microwave sub-system wherein a magnetron is moved along
the longitudinal axis of the reaction chamber and is rotated around the
longitudinal axis of
the reaction chamber.
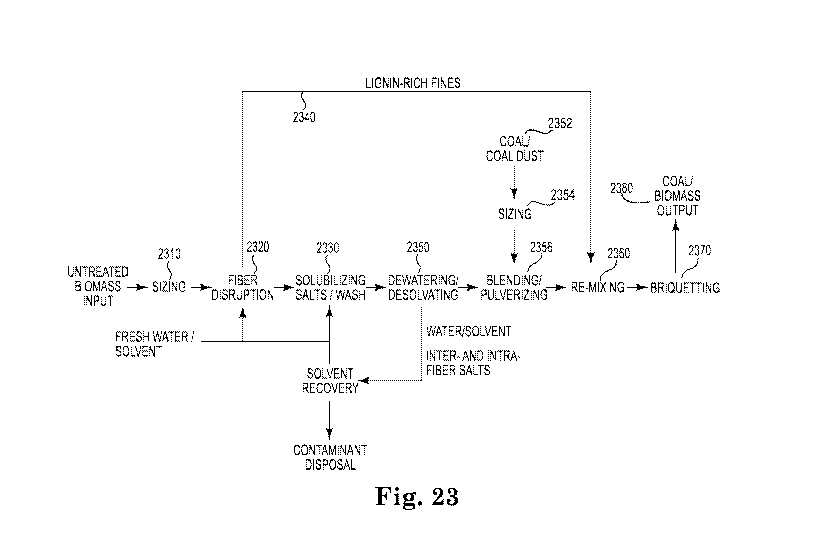
Figure 23 is a diagram of a process to make high energy coal and processed
biomass aggregates from unprocessed organic-carbon-containing feedstock.
Figure 24 is a block diagram of an embodiment of a process for passing
unprocessed organic-carbon-containing feedstock through a beneficiation sub-
system to
create a processed organic-carbon-containing feedstock with a water content of
less than
20 wt% and a water-soluble salt content that is decreased by more than 60 wt%
on a dry
basis for the processed organic-carbon-containing feedstock from that of
unprocessed
organic-carbon-containing feedstock.
Figure 25 is a block diagram of an embodiment of a process for passing
unprocessed organic-carbon-containing feedstock through a beneficiation sub-
system to
create a processed organic-carbon-containing feedstock with a water content of
less than
20 wt%, a water-soluble salt content that is decreased by more than 60 wt% on
a dry basis
for the processed organic-carbon-containing feedstock from that of unprocessed
organic-
carbon-containing feedstock, and an energy cost of removing the water-soluble
salt and
CA 02951542 2016-12-07
WO 2015/195382 9 PCT/US2015/034592
water that is reduced to less than 60 % of the cost per weight of similar
removal from
known mechanical, known physiochemical, or known thermal processes.
Figure 26 is a table showing relative process condition ranges and water and
water-
soluble salt content for three types of organic-carbon-containing feedstock
used in the
beneficiation sub-system.
Figure 27 is a block diagram of an embodiment of a process for passing
processed
organic-carbon-containing feedstock through a horizontal sublimation oxygen-
deprived
thermal sub-system to create a processed biochar having an energy density of
at least 17
MMBTU/ton (20 GJ/MT), a water content of less than 10 wt%, and water-soluble
salt that
is decreased more than 60 wt% on a dry basis for the processed organic-carbon-
containing
feedstock from that of unprocessed organic-carbon-containing feedstock.
Figure 28 is a block diagram of an embodiment of a process for passing
processed
organic-carbon-containing feedstock through a vertical sublimation oxygen-
deprived
thermal sub-system to create a processed biochar having an energy density of
at least 17
MMBTU/ton (20 GJ/MT), a water content of less than 10 wt%, and water-soluble
salt that
is decreased more than 60 wt% on a dry basis for the processed organic-carbon-
containing
feedstock from that of unprocessed organic-carbon-containing feedstock.
Figure 29 is a block diagram of an embodiment of a process for passing
processed
organic-carbon-containing feedstock through a microwave sub-system to create a
solid
renewable fuel processed biochar having an energy density of at least 17
MMBTU/ton (20
GJ/MT), a water content of less than 10 wt%, water-soluble salt that is
decreased more
than 60 wt% on a dry basis for the processed organic-carbon-containing
feedstock from
that of unprocessed organic-carbon-containing feedstock, and pores that have a
variance in
pore size of less than 10 %.
While the invention is amenable to various modifications and alternative
forms,
specifics have been shown by way of example in the drawings and will be
described in
detail below. It is to be understood, however, that the intention is not to
limit the
invention to the particular embodiments described. On the contrary, the
invention is
intended to cover all modifications, equivalents, and alternatives falling
within the scope
of the invention as defined by the appended claims.
DETAILED DESCRIPTION OF VARIOUS EMBODIMENTS
CA 02951542 2016-12-07
WO 2015/195382 10 PCT/US2015/034592
The high energy processed biomass / coal blended compact aggregate that
comprises at least 10 wt% of a coal having an energy density of at least 21
MMBTU/ton
(24 GJ/MT) and at least 10 wt% of a processed biomass comprising a processed
organic-
carbon-containing feedstock with characteristics that include an energy
density of at least
17 MMBTU/ton (20 GJ/MT) and a water-soluble intracellular salt content that is
decreased more than 60 wt% on a dry basis for the processed organic-carbon-
containing
feedstock from that of unprocessed organic-carbon-containing feedstock. The
high energy
processed biomass / coal blended compact aggregate is made from unprocessed
organic-
carbon-containing feedstock converted into the processed organic-carbon-
containing
feedstock with a beneficiation sub-system and blended with coal into a blended
compact
aggregate in a blending sub-system. In some embodiments, the processed biomass
is
processed biochar having an energy density of at least 21 MMBTU/ton (24 GJ/MT)
such
that the high energy aggregates have no loss of energy density over the coal
component.
The processed biomass of the invention has the advantages of being cleaner
than coal and
coming from a renewable source, i.e., agricultural and plant materials,
without the burdens
of current biomass processes that are inefficient and remove less if any of
the salt found in
unprocessed renewable biomass. There are several aspects of the invention that
will be
discussed: coal, processed biomass, processed biochar, blended aggregate,
unprocessed
renewable organic-carbon-containing feedstock, beneficiation sub-system,
heating sub-
system, blending sub-system, beneficiation sub-system process, heating sub-
system
process, and blending sub-system process.
Coal
The term "coal" is used to describe a variety of fossilized plant materials,
but no
two coals are exactly alike. Heating value, ash melting temperature, sulfur
and other
impurities, mechanical strength, and many other chemical and physical
properties must be
considered when matching specific coals to a particular application. Coal is
classified into
four general categories, or "ranks." They range from lignite through sub-
bituminous and
bituminous to anthracite, reflecting the progressive response of individual
deposits of coal
to increasing heat and pressure. The carbon content of coal supplies most of
its heating
value, but other factors also influence the amount of energy it contains per
unit of weight.
The amount of energy in coal is expressed in British thermal units per ton or
2000 pounds.
CA 02951542 2016-12-07
WO 2015/195382 11 PCT/US2015/034592
A BTU is the amount of heat required to raise the temperature of one pound of
water one
degree Fahrenheit. About 90 percent of the coal in this country falls in the
bituminous and
sub-bituminous categories, which rank below anthracite and, for the most part,
contain less
energy per unit of weight. Bituminous coal predominates in the Eastern and Mid-
continent coal fields, while sub-bituminous coal is generally found in the
Western states
and Alaska. Lignite ranks the lowest and is the youngest of the coals. Most
lignite is
mined in Texas, but large deposits also are found in Montana, North Dakota,
and some
Gulf Coast states.
The energy density of coal varies with its type with some overlap. Anthracite
is
coal with the highest carbon content, between 86 and 98 percent, and an energy
density or
heat value of over 30 MMBTU/ton (35 GJ/MT). Most frequently associated with
home
heating, anthracite is a very small segment of the U.S. coal market. There are
7.3 billion
tons of anthracite reserves in the United States, found mostly in 11
northeastern counties
in Pennsylvania. The most plentiful form of coal in the United States,
bituminous coal is
used primarily to generate electricity and make coke for the steel industry.
The fastest
growing market for coal, though still a small one, is supplying heat for
industrial
processes. Bituminous coal has a carbon content ranging from 45 to 86 percent
carbon and
an energy density or heat value of 21 MMBTU/ton to 31 MMBTU/ton (24 GJ/MT to
36
GJ/MT). Ranking below bituminous is sub-bituminous coal with 35-45 percent
carbon
content and an energy density or heat value between 16.6 MMBTU/ton to 26
MMBU/ton
(19 GJ/MT to 30 GJ/MT). Reserves are located mainly in a half-dozen Western
states and
Alaska. Although its heat value is lower, this coal generally has a lower
sulfur content
than other types, which makes it attractive for use because it is cleaner
burning. Lignite is
a geologically young coal which has the lowest carbon content, 25-35 percent,
and an
energy density or heat value ranging between 8 MMBTU/ton to 16.6 MMBTU/ton (9
GJ/MT to 19 GJ/MT). Sometimes called brown coal, it is mainly used for
electric power
generation. As used in this document, coal of any type that has an energy
density of at
least 21 MMBTU/ton (24 GJ/MT) will be called high energy coal and coal of any
type
having an energy density of less than 21 MMBTU/ton (24 GJ/MT) will be called
low
energy coal.
Coal has inorganic impurities associated with its formation underground over
millions of years. The inorganic impurities are not combustible, appear in the
ash after
CA 02951542 2016-12-07
WO 2015/195382 12 PCT/US2015/034592
combustion of coal in such situations as, for example boilers, and contribute
to air
pollution as the fly ash particulate material is ejected into the atmosphere
following
combustion. The inorganic impurities result mainly from clay minerals and
trace
inorganic impurities washed into the rotting biomass prior to its eventual
burial. An
important group of precipitating impurities are carbonate minerals. During the
early
stages of coal formation, carbonate minerals such as iron carbonate are
precipitated either
as concretions (hard oval nodules up to tens of centimeters in size) or as
infillings of
fissures in the coal. Impurities such as sulfur and trace elements (including
mercuiy,
germanium, arsenic, and uranium) are chemically reduced and incorporated
during coal
formation. Most sulfur is present as the mineral pyrite (FeS2), sulfate
minerals (CaSO4
and .FeSO4), or organic complexes, and this may account for up to a few per
cent of the
coal volume. Burning coal oxidizes these compounds, releasing oxides of sulfur
(SO,
SO2, SO/, S707, S601, S102, etc.), notorious contributors to acid. rain. The
trace elements
(including mercury, germanium, arsenic, and uranium) were significantly
enriched in the
coal are also released by burning it, contributing to atmospheric pollution.
Processed Biomass
Biomass made from renewable organic-carbon-containing feedstock by the
beneficiation process is referred to as processed biomass in this document.
The processed
biomass of the invention comprises a solid carbon fuel comprising less than 20
wt% water,
and water-soluble intracellular salt that is less than 60 wt% on a dry basis
that of
unprocessed organic-carbon-containing feedstock. The processed biomass is made
from
unprocessed organic-carbon-containing feedstock that is converted into a
processed
organic-carbon-containing feedstock in a beneficiation sub-system. As used in
this
document, processed biomass pellets are a solid product of beneficiated
organic-carbon-
containing feedstock that is subsequently pelletized. Organic-carbon-
containing feedstock
used to make the processed biomass of the invention can contain mixtures of
more than
one renewable feedstock.
The processed biomass component of high energy aggregates of the invention is
cleaner than coal. The impurities discussed above are not present in any
significant
amount In particular, processed biomass contains substantially no sulfur. Some
embodiments have a sulfur content of less than 1000 mg/kg (0.1 wt%) or less
than 1000
parts per million (ppm), some of less than 100 mg/kg (100 ppm, some of less
than 10
CA 02951542 2016-12-07
WO 2015/195382 13 PCT/US2015/034592
in/kg (10 ppm). in contrast coal has significantly more sulfur. The sulfur
content in coal
ranges of from 4000mgikg (OA wt%) to 40,000 mgkg (4 wt%) and varies with type
of
coal. The typical sulfur content in anthracite coal is from 6000 mg/kg (0.6
wt%) to 7700
mg/kg (0.77 wt%). The typical sulfur content in bituminous coal is from 7000
mg/kg (0.7
wt%) to 40.000 mg/kg (4 wt%). The typical sulfur content in lignite coal is
about 4000
mg/kg (0.4 wt%). Anthracite coal is too expensive for extensive use in
burning. Lignite is
poor quality coal, with a low energy density or BTU/wt.
in addition, processed biomass has substantially no nitrate, arsenic, mercury
or
uranium. Some embodiments have a nitrate content of less than 500 mg/kg (500
ppm),
some of less than 150 mg/kg (150 ppm), versus a nitrate content in coal of
typically over
20,000 mg.kg (2 wt%). Some embodiments have a arsenic content of less than 2
mg/kg (2
ppm), some of less than 1 mg/kg (1 ppm), some less than 0,1 roglkg or 100
parts per
billion (ppb), and some less than 0.01 mg/kg ( 0 ppb) versus a arsenic content
in coal of
from over 1 mg/kg to over 70 mg/kg (1 ppm to 70 ppm). Some embodiments have a
mercury content that is negligible, i. c., less than it microgram/kg (1 ppb),
versus mercury
content in coal of from 0.02 mg/kg (20 ppb) to 0.3 mg/kg (300 ppb). Similarly,
some
embodiments have a uranium content that is also negligible, i. e., less than 1
microgram/kg
(1 ppb), versus a uranium content in coal of from 20 mg/kg (20 ppm) to 315
ing/kg (315
ppm) with an average of about 65 mg/kg (ppm) and. the uranium content in the
ash from
the coal with an average of about 210 mg/kg (210 ppm).
Processed Bio char
Char made from renewable organic-carbon-containing feedstock by the
beneficiation process is referred to as processed biochar in this document.
The processed
biochar of the invention comprises a solid carbon fuel comprising less than 10
wt% water,
and water-soluble salt that is less than 60 wt% on a dry basis for the
processed organic-
carbon-containing feedstock from that of unprocessed organic-carbon-containing
feedstock. The water-soluble intracellular salt content decrease is based on
comparing the
processed organic-carbon-containing feedstock before it is passed through the
heating sub-
system to the unprocessed organic-carbon-containing feedstock because the
heating
process can lower the wt of the solid biomass on a dry basis as some is
converted to
biooils and biogases and removed as discussed below under the heating
subsystem section.
CA 02951542 2016-12-07
WO 2015/195382 14 PCT/US2015/034592
The processed biochar is made from unprocessed organic-carbon-containing
feedstock that
is converted into a processed organic-carbon-containing feedstock in a
beneficiation sub-
system, and that is then passed through an oxygen-deprived thermal sub-system.
As used
in this document, processed biochar is the solid product of the devolatization
of
beneficiated organic-carbon-containing feedstock. Organic-carbon-containing
feedstock
used to make the processed biochar of the invention can contain mixtures of
more than one
renewable feedstock.
Other forms of char are also known. Some of these chars include, for example,
char made by the pyrolysis of biomass, also known as charcoal. Charcoal has an
energy
density of about 26 MMBTU/ton (30 GJ/MT) and contains all of the water-soluble
salt
residues found in the starting biomass used to make the charcoal. Charcoal has
various
uses including, for example, a combustible fuel for generating heat for
cooking and
heating, as well as a soil amendment to supply minerals for fertilizing soils
used for
growing agricultural and horticultural products. Char has also been made by
passing
biomass through an open microwave oven similar to a bacon cooker that is
exposed to the
external atmosphere containing oxygen and contains pores with a variance
similar to that
made by a thermal process that has a liquid phase.
In contrast, the processed biomass component of high energy aggregates of the
invention is cleaner than coal. The impurities discussed above are not present
in any
significant amount. In particular, processed biomass contains substantially no
sulfur.
Some embodiments have a sulfur content of less than 1000 mg/kg (0.1 wt(.!4) or
less than
1000 parts per million (ppm), some of less than 00 inglg (100 ppm, some of
less than 10
mg/kg (10 ppm). In contrast coal has significantly more sulfur. The sulfur
content in coal
ranges of from 4000mg/kg (OA wt%) to 40,000 mg/kg (4 wt%) and varies with type
of
coat The typical sulfur content in anthracite coal is from 6000 mg/kg (0.6
wt/o) to 7700
mg/kg (0,77 wt%), The typical sulfur content in bituminous coal is from 7000
mg/kg (0,7
wt%) to 40.000 mg/kg (4 wt%). The typical sulfur content in lignite coal is
about 4000
mg/kg (0.4 wt%). Anthracite coal is too expensive for extensive use in
burning. Lignite is
poor quality coal, with a low energy density or BTU/wt.
In addition, processed biorna,ss has substantially no nitrate, arsenic,
mercury or
uranium. Some embodiments have a nitrate content of less than 500 mg/kg (500
ppm),
CA 02951542 2016-12-07
WO 2015/195382 15 PCT/US2015/034592
some of less than 150 mg/kg (150 ppm), versus a nitrate content in coal of
typically over
20,000 mg,kg (2 wt%). Some embodiments have a arsenic content of less than 2
m.g/kg (2
ppm), some of less than 1 mg/kg (1 ppm), some less than 0.1 mg/kg or 100 parts
per
billion (ppb), and some less than 0.01 mg/kg (10 ppb) versus a arsenic content
in coal of
from over 1 mg/kg to over 70 mg/kg (1 ppm to 70 ppm). Some embodiments have a
mercury content that is negligible, i. e., less than I micrograinlkg (1 ppb),
versus mercury
content in coal of from 0.02 mg/kg (20 ppb) to 0.3 mg/kg (300 ppb). Similarly,
some
embodiments have a uranium content that is also negligible, i. e., less than 1
microgram/kg
(1 ppb), versus a uranium content in coal of from 20 mg/kg (20 ppm) to 315
mg/kg (315
ppm) with an average of about 65 mg/kg (ppm) and the uranium content in the
ash from
the coal with an average of about 210 mg/kg (210 ppm).
Other forms of char are also known. Some of these chars include, for example,
char made by the pyrolysis of biomass, also known as charcoal. Charcoal has an
energy
density of about 26 MMBTU/ton (30 GJ/MT) and contains all of the water-soluble
salt
residues found in the starting biomass used to make the charcoal. Charcoal has
various
uses including, for example, a combustible fuel for generating heat for
cooking and
heating, as well as a soil amendment to supply minerals for fertilizing soils
used for
growing agricultural and horticultural products. Char has also been made by
passing
biomass through an open microwave oven similar to a bacon cooker that is
exposed to the
external atmosphere containing oxygen and contains pores with a variance
similar to that
made by a thermal process that has a liquid phase.
In biochar made by thermal heat or infrared radiation, the heat is absorbed on
the
surface of any organic-carbon-containing feedstock and then is re-radiated to
the next
level at a lower temperature. This process is repeated over and over again
until the
thermal radiation penetrates to the inner most part of the feedstock. All the
material in the
feedstock absorbs the thermal radiation at its surfaces and different
materials that make up
the feedstock absorb the IR at different rates. A delta temperature of several
orders of
magnitude can exist between the surface and the inner most layers or regions
of the
feedstock. As a result, the solid organic-carbon-containing feedstock locally
passes
through a liquid phase before it is volatilized. This variation in temperature
may appear in
a longitudinal direction as well as radial direction depending on the
characteristics of the
feedstock, the rate of heating, and the localization of the heat source. This
variable heat
CA 02951542 2016-12-07
WO 2015/195382 16 PCT/US2015/034592
transfer from the surface to the interior of the feedstock can cause cold and
hot spots,
thermal shocks, uneven surface and internal expansion cracks, fragmentation,
eject surface
material and create aerosols. All of this can result in microenvironments that
cause side
reactions with the creation of many different end products. These side
reactions are not
only created in the feedstock but also in the volatiles that evaporate from
the feedstock and
occupy the vapor space in the internal reactor environment before being
collected.
A common thermal process, pyrolysis, produces biochar, liquids, and gases from
biomass by heating the biomass in a low/no oxygen environment. The absence of
oxygen
prevents combustion. Typical yields are 60% bio-oil, 20% biochar, and 20%
volatile
organic gases. High temperature pyrolysis in the presence of stoichiometric
oxygen is
known as gasification, and produces primarily syngas. By comparison, slow
pyrolysis can
produce substantially more char, on the order of about 50%.
Another thermal process is a sublimation process that produces biochar and
gases
from biomass in a low/no oxygen environment. The absence of oxygen also
prevents
combustion. Typical yields are 70% fuel gas and 30% biochar. Sublimation can
occur in
a vertical manner that lends itself to heavier/denser biomass feedstock such
as, for
example, wood and a horizontal manner that lends itself to lighter / less
dense biomass
feedstock such as, for example, wheat straw.
An alternative to thermal processes, the process to make char by microwave
radiation uses heat that is absorbed throughout the organic-carbon-containing
feedstock.
The process uses microwave radiation from the oxygen-starved microwave process
system. With microwave radiation, the solid part of the feedstock is nearly
transparent to
the microwave radiation and most of the microwave radiation just passes
through. In
contrast to the small absorption cross section of the solid feedstock, gaseous
and liquid
water strongly absorb the microwave radiation increasing the rotational and
torsional
vibrational energy of the water molecules. Therefore, the gaseous and liquid
water that is
present is heated by the microwaves, and these water molecules subsequently
indirectly
heat the solid feedstock. Thus any feedstock subjected to the microwave
radiation field is
exposed to the radiation evenly, inside to outside, no matter what the
physical dimensions
and content of the feedstock. With microwaves, the radiation is preferentially
absorbed by
water molecules that heat up. This heat is then transferred to the surrounding
environment
resulting in the feedstock being evenly and thoroughly heated.
CA 02951542 2016-12-07
WO 2015/195382 17 PCT/US2015/034592
In all of the above processes, water-soluble salt that is in all renewable
organic-
carbon-containing feedstock is not removed This has the adverse effect of
increasing ash
content in combusted char and increasing wear and maintenance costs from
corrosion and
slagging, a deposition of a viscous residue of impurities during combustion.
In contrast,
the process to make the processed biochar of the invention used a
beneficiation sub-system
to process the unprocessed organic-carbon ¨containing feedstock to remove most
of the
water and water-soluble salts, and an oxygen-deficient thermal sub-system to
convert the
processed organic-carbon-containing feedstock into a processed biochar.
In contrast, the processed biochar of an embodiment of the invention contains
much less water-soluble salt than that of currently known biochar. The oxygen-
deficient
thermal sub-system of the invention are similar to those discussed above but
use a
processed organic-carbon-containing feedstock rather than an unprocessed
organic-
carbon-containing feedstock used by the above oxygen¨deficient thermal
processes
mentioned above.
Blended Aggregates
The blended aggregates offer significantly cicaner fuel fbr use in such
processes as
boilers without a commonly associated reduction in energy density.
The high energy processed biomass / coal blended compact aggregate of the
invention has several improved characteristics when compared to a biomass /
coal blended
compact aggregate made with unprocessed organic-carbon-containing feedstock.
First, the
high energy processed biomass / coal blended compact aggregate contains
significantly
less salt than that produced from current processes that use similar
unprocessed organic-
carbon-containing feedstock. The salt in the processed organic-carbon-
containing
feedstock and thus in the resulting processed biomass is reduced by at least
60 wt% on a
dry basis for the processed organic-carbon-containing feedstock from that of
the salt
content of the unprocessed organic-carbon-containing feedstock. The reduction
in salt
content is based on a comparison between processed organic-carbon-containing
feedstock
on a dry basis and unprocessed organic-carbon-containing feedstock on a dry
basis. After
the subsequent heating step to convert processed organic-carbon-containing
feedstock into
processed biochar, the salt content is not further changed but the total
solids on a dry basis
are reduced by some percentage because some of the solids of the processed
organic-
carbon-containing feedstock are being converted into liquid or gas phases.
Another effect
CA 02951542 2016-12-07
WO 2015/195382 18 PCT/US2015/034592
of the salt reduction between the processed and unprocessed organic-carbon-
containing
feedstock is that the fixed carbon of the resulting processed biochar in some
embodiments
is higher and the ash content is lower because there is less salt that forms
ash during
subsequent combustion of the biochar in a boiler. In addition, the adverse
effect of salt in
the boiler is reduced, wear is slower, and maintenance cleaning of the
equipment is less
often and less arduous.
Second, the high energy processed biomass / coal blended compact aggregate has
a
high energy density, approaching that of the high energy coal component of at
least 21
MMBTU/ton (24 GJ/MT). The energy density of the processed biomass is at least
17
MMBTU/ton (20 GJ/MT) with some embodiments being at least 21 MMBTU/ton (24
GJ/MT). Some embodiments have an energy density of at least 23 MMBTU/ton (27
GJ/MT), some at least 26 MMBTU/ton (30 GJ/MT), some at least 28 MMBTU/ton (33
GJ/MT), some at least 30 MMBTU/ton (35 GJ/MT), and some at least 31 MMBTU/ton
(36 GJ/MT). In contrast, the energy density of unprocessed biochar made with
oxygen
deficient heating of unprocessed organic-carbon-containing feedstock other
than those
discussed in this document is often between 10 MMBTU/ton (12 GJ/MT) and 12
MMBTU/ton (14 GJ/MT) and generally under 26 MMBTU/ton (30 GJ/MT) ¨ and still
have the retained salts from the unprocessed biomass.
Third, the high energy processed biomass / coal blended compact aggregate can
contain significantly less pollutants associated with coal by itself depending
on the content
of the processed biomass used in the aggregate. This processed aggregate
contains little if
any pollutants normally associated with coal. These pollutants associated with
coal
include, for example, mercury (neurotoxin), arsenic (carcinogen), and Sx0y
when the coal
is combusted. Processed biomass contains less than 0.1 wt% of any one of the
above
impurities, some embodiments contain less than 0.01wt%, some less than 0.001
wt %,
some less than 0.0001 wt%.
Fourth, higher levels of processed biomass can be blended with high energy
coal
fines to permit a variety of scenarios depending on what is desired. Because
of the lower
salt content and higher energy density of the processed biomass, coal biomass
blends that
contain at least 10 wt% biomass are now possible with energy densities
approximating or
exceeding that of the coal component and with substantially reduced levels of
intracellular
salt from the biomass component. Ill some embodiments, the biomass content is
at least
CA 02951542 2016-12-07
WO 2015/195382 PCT/US2015/034592
19
20 wt%, in some at least 30 wt%, in some at least 40 wt%, in some at least 50
wt%, in
some at least 60 wt%, in some at least 70 wt%, and in some at least 80 wt%.
Similarly,
because of the low salt content and high energy density of the processed
biomass, it is now
possible to safely transport coal fines in blends that contain at least 10 wt%
coal. In some
embodiments, the coal content is at least 20 wt%, in some at least 30 wt%, in
some at least
40 wt%, in some at least 50 wt%, in some at least 60 wt%, in some at least 70
wt%, and in
some at least 80 wt%. 717his permits coal fines to be safely used in commerce
as a fuel
source for such processes as heating boilers. It also permits a significant
portion of the
blend to be renewable solid fuel without a sacrifice of cleanliness of
materials or energy
density associated with current blends of unprocessed biomass and coal.
In some embodiments of the inventions, organic-carbon-containing feedstock
used
to make the high energy processed biomass / coal blended compact aggregate of
the
invention can contain mixtures of more than one renewable feedstock when the
processed
organic-carbon-containing feedstock is made to have substantially uniform
energy
densities regardless of the type of organic-carbon-containing feedstock used.
Unprocessed Organic-Carbon-Containing Feedstock
Cellulose bundles, interwoven by hemicellulose and lignin polymer strands, are
the
stuff that makes plants strong and proficient in retaining moisture. Cellulose
has evolved
over several billion years to resist being broken down by heat, chemicals, or
microbes. In
a plant cell wall, the bundles of cellulose molecules in the microfibrils
provide the wall
with tensile strength. The tensile strength of cellulose microfibrils is as
high as 110
kg/mm2, or approximately 2.5 times that of the strongest steel in laboratory
conditions.
When cellulose is wetted, as in the cell walls, its tensile strength declines
rapidly,
significantly reducing its ability to provide mechanical support. But in
biological systems,
the cellulose skeleton is embedded in a matrix of pectin, hemicellulose, and
lignin that act
as waterproofing and strengthening material. That makes it difficult to
produce fuels from
renewable cellulose-containing biomass fast enough, cheap enough, or on a
large enough
scale to make economical sense. As used herein, organic-carbon-containing
material
means renewable plant-containing material that can be renewed in less than 50
years and
includes plant material such as, for example herbaceous materials such as
grasses, energy
crops, and agricultural plant waste; woody materials such as tree parts, other
woody waste,
and discarded items made from wood such as broken furniture and railroad ties;
and
CA 02951542 2016-12-07
WO 2015/195382 20 PCT/US2015/034592
animal material containing undigested plant cells such as animal manure.
Organic-carbon-
containing material that is used as a feedstock in a process is called an
organic-carbon-
containing feedstock
Unprocessed organic-carbon-containing material, also referred to as renewable
biomass, encompasses a wide array of organic materials as stated above. It is
estimated
that the U.S. alone generates billions of tons of organic-carbon-containing
material
annually. As used in this document, beneficiated organic-carbon-containing
feedstock is
processed organic-carbon-containing feedstock where the moisture content has
been
reduced, a significant amount of dissolved salts have been removed, and the
energy
density of the material has been increased. This processed feedstock can be
used as input
for processes that make several energy-producing products, including, for
example, liquid
hydrocarbon fuels, solid fuel to supplant coal, and synthetic natural gas.
As everyone in the business of making organic-carbon-containing feedstock is
reminded, the energy balance is the metric that matters most. The amount of
energy used
to beneficiate organic-carbon-containing feedstock and, thus, the cost of that
energy must
be substantially offset by the overall improvement realized by the
beneficiation process in
the first place. For example, committing 1000 BTU to improve the heat content
of the
processed organic-carbon-containing feedstock by 1000 BTU, all other things
being equal,
does not make economic sense unless the concurrent removal of a significant
amount of
the water-soluble salt renders previously unusable organic-carbon-containing
feedstock
usable as a fuel substitute for some processes such as boilers.
As used herein, organic-carbon-containing feedstock comprises free water,
intercellular water, intracellular water, intracellular water-salts, and at
least some plant
cells comprising cell walls that include lignin, hemicellulose, and cellulosic
microfibrils
within fibrils. In some embodiments, the water-soluble salt content of the
unprocessed
organic-carbon-containing feedstock is at least 4000 mg/kg on a dry basis. In
other
embodiments the salt content may be more than 1000 mg/kg, 2000 mg/kg, or 3000
mg/kg.
The content is largely dependent on the soil where the organic-carbon-
containing material
is grown. Regions that are land rich and more able to allow land use for
growing energy
crops in commercial quantities often have alkaline soils that result in
organic-carbon-
containing feedstock with water-soluble salt content of over 4000 mg/kg.
CA 02951542 2016-12-07
WO 2015/195382 21
PCT/US2015/034592
Water-soluble salts are undesirable in processes that use organic-carbon-
containing
feedstock to create fuels. The salt tends to shorten the operating life of
equipment through
corrosion, fouling, or slagging when combusted. Some boilers have standards
that limit
the concentration of salt in fuels to less than 1500 mg/kg. This is to find a
balance
between availability of fuel for the boilers and expense of frequency of
cleaning the
equipment and replacing parts. If economical, less salt would be preferred. In
fact, salt
reduction through beneficiation is an enabling technology for the use of salt-
laden biomass
(e.g. hogged fuels, mesquite, and pinyon-junipers) in boilers. Salt also
frequently poisons
catalysts and inhibits bacteria or enzyme use in processes used for creating
beneficial
fuels. While some salt concentration is tolerated, desirably the salt levels
should be as low
as economically feasible.
The water-soluble salt and various forms of water are located in various
regions in
plant cells. As used herein, plant cells are composed of cell walls that
include microfibril
bundles within fibrils and include intracellular water and intracellular water-
soluble salt.
Figure 1 is a diagram of a typical plant cell with an exploded view of a
region of its cell
wall showing the arrangement of fibrils, microfibrils, and cellulose in the
cell wall. A
plant cell (100) is shown with a section of cell wall (120) magnified to show
a fibril (130).
Each fibril is composed of microfibrils (140) that include strands of
cellulose (150). The
strands of cellulose pose some degree of ordering and hence crystallinity.
Plant cells have a primary cell wall and a secondary cell wall. The secondary
cell
wall varies in thickness with type of plant and provides most of the strength
of plant
material. Figure 2 is a diagram of a perspective side view of a part of two
fibrils bundled
together in a secondary plant cell wall showing the fibrils containing
microfibrils and
connected by strands of hemicellulose, and lignin. The section of plant cell
wall (200) is
composed of many fibrils (210). Each fibril 210 includes a sheath (220)
surrounding an
aggregate of cellulosic microfibrils (230). Fibrils 210 are bound together by
interwoven
strands of hemicellulose (240) and lignin (250). In order to remove the
intracellular water
and intracellular water-soluble salt, sections of cell wall 200 must be
punctured by at least
one of unbundling the fibrils from the network of strands of hemicellulose 240
and lignin
250, decrystallizing part of the strands, or depolymerizing part of the
strands.
The plant cells are separated from each other by intercellular water. An
aggregate
of plant cells are grouped together in plant fibers, each with a wall of
cellulose that is wet
CA 02951542 2016-12-07
WO 2015/195382 22 PCT/US2015/034592
on its outside with free water also known as surface moisture. The amount of
water
distributed within a specific organic-carbon-containing feedstock varies with
the material.
As an example, water is distributed in fresh bagasse from herbaceous plants as
follows:
about 50 wt % intracellular water, about 30 wt % intercellular water, and
about 20 wt %
free water. Bagasse is the fibrous matter that remains after sugarcane or
sorghum stalks
are crushed to extract their juice.
Figure 3 is diagram of a cross-sectional view of a fiber section of bagasse
showing
where water and water-soluble salts reside inside and outside plant cells. A
fiber with an
aggregate of plant cells (300) is shown with surface moisture (310) on the
outer cellulosic
wall (320). Within fiber 300 lays individual plant cells (330) separated by
intercellular
water (340). Within each individual plant cell 330 lays intracellular water
(350) and
intracellular water-soluble salt (360).
Conventional methods to beneficiate organic-carbon-containing feedstock
include
thermal processes, mechanical processes, and physiochemical processes. Thermal
methods include heat treatments that involve pyrolysis and torrefaction. The
thermal
methods do not effectively remove entrained salts and only serve to
concentrate them.
Thus thermal processes are not acceptable for the creation of many energy
creating
products such as organic-carbon-containing feedstock used as a fuel substitute
to the likes
of coal and petroleum. Additionally, all conventional thermal methods are
energy
intensive, leading to an unfavorable overall energy balance, and thus
economically
limiting in the commercial use of organic-carbon-containing feedstock as a
renewable
source of energy.
The mechanical method, also called pressure extrusion or densification, can be
divided into two discrete processes where water and water-soluble salts are
forcibly
extruded from the organic-carbon-containing material. These two processes are
intercellular and intracellular extrusion. The extrusion of intercellular
water and
intercellular water-soluble salt occurs at a moderate pressure, depending upon
the
freshness of the organic-carbon-containing material, particle size, initial
moisture content,
and the variety of organic-carbon-containing material. Appropriately sized
particles of
freshly cut herbaceous organic-carbon-containing feedstock with moisture
content
between 50 wt % and 60 wt % will begin extruding intercellular moisture at
pressures as
CA 02951542 2016-12-07
WO 2015/195382 23 PCT/US2015/034592
low as 1,000 psi and will continue until excessive pressure forces the
moisture into the
plant cells (essentially becoming intracellular moisture).
As the densification proceeds, higher pressures, and hence higher energy
costs, are
required to try to extrude intracellular water and intracellular water-soluble
salt. However,
stiff cell walls provide the biomass material with mechanical strength and are
able to
withstand high pressures without loss of structural integrity. In addition,
the formation of
impermeable felts more prevalent in weaker cell walled herbaceous material has
been
observed during compaction of different herbaceous biomass materials above a
threshold
pressure. This method is energy intensive. In addition, it can only remove up
to 50
percent of the water-soluble salts on a dry basis (the intracellular salt
remains) and is
unable to reduce the remaining total water content to below 30 wt percent.
The felts are formed when long fibers form a weave and are bound together by
very small particles of pith. Pith is a tissue found in plants and is composed
of soft,
spongy parenchyma cells, which store and transport water-soluble nutrients
throughout the
plant. Pith particles can hold 50 times their own weight in water. As the
compression
forces exerted during the compaction force water into the forming felts, the
entrained pith
particles collect moisture up to their capacity. As a result, the moisture
content of any felt
can approach 90%. When felts form during compaction, regardless of the forces
applied,
the overall moisture content of the compacted biomass will be substantially
higher than it
would have been otherwise had the felt not formed. The felt blocks the exit
ports of the
compaction device as well as segments perpendicular to the applied force, and
the water is
blocked from expulsion from the compaction device. The felt also blocks water
passing
through the plant fibers and plant cells resulting in some water passing back
through cell
wall pores into some plant cells. In addition, it can only remove up to 50
percent of the
water-soluble salts on a dry basis and is unable to reduce more than the water
content to
below 30 wt percent.
The physiochemical method involves a chemical pretreatment of organic-carbon-
containing feedstock and a pressure decompression prior to compaction to
substantially
improve the quality of densified biomass while also reducing the amount of
energy
required during compaction to achieve the desired bulk density. Chemically,
biomass
comprises mostly cellulose, hemicellulose, and lignin located in the secondary
cell wall of
relevant plant materials. The strands of cellulose and hemicellulose are cross-
linked by
CA 02951542 2016-12-07
WO 2015/195382 24 PCT/US2015/034592
lignin, forming a lignin-carbohydrate complex (LCC). The LCC produces the
hydrophobic barrier to the elimination of intracellular water. In addition to
the paper
pulping process that solublizes too much of the organic-carbon-containing
material,
conventional pre-treatments include acid hydrolysis, steam explosion, AFEX,
alkaline wet
oxidation, and ozone treatment. All of these processes, if not carefully
engineered, can be
can be expensive on a cost per product weight basis and are not designed to
remove more
than 25% water-soluble salt on a dry weight basis.
In addition, the energy density generally obtainable from an organic-carbon-
containing material is dependent on its type, i.e., herbaceous, soft woody,
and hard woody.
Also mixing types in subsequent uses such as fuel for power plants is
generally
undesirable because the energy density of current processed organic-carbon-
containing
feedstock varies greatly with type of plant material.
As stated above, plant material can be further subdivided in to three sub
classes,
herbaceous, soft woody and hard woody, each with particular water retention
mechanisms.
All plant cells have a primary cell wall and a secondary cell wall. As stated
earlier, the
strength of the material comes mostly from the secondary cell wall, not the
primary one.
The secondary cell wall for even soft woody materials is thicker than for
herbaceous
material.
Herbaceous plants are relatively weak-walled plants, include corn, and have a
maximum height of less than about 10 to 15 feet (about 3 to 5 meters (M)).
While all
plants contain pith particles, herbaceous plants retain most of their moisture
through a high
concentration of pith particles within the plant cells that hold water like
balloons because
these plants have relatively weak cell walls. Pressure merely deforms the
balloons and
does not cause the plant to give up its water. Herbaceous plants have about 50
% of their
water as intracellular water and have an energy density of unprocessed
material at about
5.2 million BTUs per ton (MMBTU/ton) or 6 Gigajoules per metric ton (GEMT).
Soft woody materials are more sturdy plants than herbaceous plants. Soft woody
materials include pines and typically have a maximum height of between 50 and
60 feet
(about 15 and 18 M). Their plant cells have stiffer walls and thus need less
pith particles
to retain moisture. Soft woody materials have about 50 % of their water as
intracellular
water and have an energy density of about 13-14 MMBTU/ton (15-16 GJ/MT).
CA 02951542 2016-12-07
WO 2015/195382 25 PCT/US2015/034592
Hard woody materials are the most sturdy of plants, include oak, and typically
have a maximum height of between 60 and 90 feet (18 and 27 M). They have
cellulosic
plant cells with the thickest secondary cell wall and thus need the least
amount of pith
particles to retain moisture. Hard woody materials have about 50 % of their
water as
intracellular water and have an energy density of about 15 MMBTU/ton (17
GJ/MT).
There is a need in the energy industry for a system and method to allow the
energy
industry to use organic-carbon-containing material as a commercial alternative
or adjunct
fuel source. Much of the land available to grow renewable organic-carbon-
containing
material on a commercial scale also results in organic-carbon-containing
material that has
a higher than desired content of water-soluble salt that typically is at
levels of at least 4000
mg/kg. Forest products in the Pacific Northwest are often transported via
intracoastal
waterways, exposing the biomass to salt from the ocean. Thus such a system and
method
must be able to remove sufficient levels of water-soluble salt to provide a
suitable fuel
substitute. As an example, boilers generally need salt contents of less than
1500 mg/kg to
avoid costly maintenance related to high salt in the fuel. In addition, the
energy and
resulting cost to remove sufficient water to achieve an acceptable energy
density must be
low enough to make the organic-carbon-containing material feedstock a suitable
alternative in processes to make coal or hydrocarbon fuel substitutes.
There is also a need for a process that can handle the various types of plants
and
arrive at processed organic-carbon-containing feedstock with similar energy
densities.
The invention disclosed does allow the energy industry to use processed
organic-
carbon-containing material as a commercial alternative fuel source. Some
embodiments
of the invention remove almost all of the chemical contamination, man-made or
natural,
and lower the total water content to levels in the range of 5 wt % to 15 wt %.
This allows
the industries, such as the electric utility industry to blend the organic-
carbon-containing
feedstock on a ratio of up to 50 wt % processed organic-carbon-containing
feedstock to 50
wt % coal with a substantial reduction in the amount of water-soluble salt and
enjoy the
same MMBTU/ton (GJ/MT) efficiency as coal at coal competitive prices.
Literature has
described organic-carbon-containing feedstock to coal ratios of up to 30%. A
recent
patent application publication, EP2580307 A2, has described a ratio of up to
50% by
mechanical compaction under heat, but there was no explicit reduction in water-
soluble
salt content in the organic-carbon-containing feedstock. The invention
disclosed herein
CA 02951542 2016-12-07
WO 2015/195382 26 PCT/US2015/034592
explicitly comprises substantial water-soluble salt reduction through a
reaction chamber
with conditions tailored to each specific unprocessed organic-carbon-
containing feedstock
used. As discussed below, additional purposed rinse subsections and subsequent
pressing
algorithms in the compaction section of the Reaction Chamber may be beneficial
to
process organic-carbon-containing feedstock that has a particularly high
content of water-
soluble salt so that it may be used in a blend with coal that otherwise would
be unavailable
for burning in a coal boiler. This also includes, for example, hog fuel,
mesquite, and
Eastern red cedar.
In addition, the invention disclosed does permit different types of organic-
carbon-
containing feedstock to be processed, each at tailored conditions, to result
in processed
outputs having preselected energy densities. In some embodiments of the
invention, more
than one type of feedstock with different energy densities that range from 5.2
to 14
MMBTU/ton (6 to 16 GJ/MT) may be fed into the reaction chamber in series or
through
different reaction chambers in parallel. Because each type of organic-carbon-
containing
feedstock is processed under preselected tailored conditions, the resulting
processed
organic-carbon-containing feedstock for some embodiments of the system of the
invention
can have a substantially similar energy density. In some embodiments, the
energy density
is about 17 MMBTU/ton (20GJ/MT). In others it is about 18, 19, or 20 MMBTU/ton
(21,
22, or 23 GJ/MT). This offers a tremendous advantage for down-stream processes
to be
able to work with processed organic-carbon-containing feedstock having similar
energy
density regardless of the type used as well as substantially reduced water-
soluble content.
The process of the invention uses a beneficiation sub-system to create the
processed organic-carbon-containing feedstock that is a clean economical
material to be
used for creating a satisfactory high energy processed biomass / coal blended
compact
aggregate from renewable biomass, an optional heating sub-system, and a
blending
subsystem for converting the processed organic-carbon-containing feedstock and
coal into
the high energy processed biomass / coal blended compact aggregate of the
invention.
The first subsystem will now be discussed.
Beneficiation Sub-System
The beneficiation sub-system is used to make processed organic-carbon-
containing
feedstock comprises at least three elements, a transmission device, at least
one reaction
chamber, and a collection device. As used in this document, the beneficiation
sub-system
CA 02951542 2016-12-07
WO 2015/195382 27 PCT/US2015/034592
refers to the system that is used to convert unprocessed organic-carbon-
containing
feedstock into processed organic-carbon-containing feedstock.
The first element, the transmission device, is configured to convey into a
reaction
chamber unprocessed organic-carbon-containing feedstock comprising free water,
intercellular water, intracellular water, intracellular water-soluble salt,
and at least some
plant cells comprising cell walls that include lignin, hemicellulose, and
cellulosic
microfibrils within fibrils. The transmission device may be any that is
suitable to convey
solid unprocessed organic-carbon-containing feedstock into the reaction
chamber to obtain
a consistent residence time of the feedstock in the reaction chamber. The
transmission
devices include such devices at augers that are well known in the chemical
industry.
Particle size of the unprocessed organic-carbon-containing feedstock should be
sufficiently small to permit a satisfactorily energy balance as the
unprocessed organic-
carbon-containing feedstock is passed through the system to create processed
organic-
carbon-containing feedstock. In some embodiments, the unprocessed organic-
carbon-
containing feedstock arrives at some nominal size. Herbaceous material such
as, for
example, energy crops and agricultural waste, should have a particle size
where the
longest dimension is less than 1 inch (2.5 cm). Preferably, most wood and wood
waste
that is freshly cut should have a longest length of less than 0.5 inches (1.3
cm).
Preferably, old wood waste, especially resinous types of wood such as, for
example pine,
has a particle size with a longest dimension of less than 0.25 inches (about
0.6 cm) to
obtain the optimum economic outcome, where throughput and energy/chemical
consumption are weighed together.
Some embodiments of the system may also include a mastication chamber before
the reaction chamber. This mastication chamber is configured to reduce
particle size of
the organic-carbon-containing feedstock to less than 1 inch (2.5 cm) as the
longest
dimension. This allows the organic-carbon-containing feedstock to arrive with
particle
sized having a longest dimension larger than 1 inch (2.5 cm). In some
embodiments, the
longest dimension is less than 0.75 inches (1.9 cm), and in some less than 0.5
inches (1.3
cm).
Some embodiments of the system may also include a pretreatment chamber to
remove contaminants that hinder creation of the passageways for intracellular
water and
water-soluble salts to pass from the cellulosic-fibril bundles. The chamber is
configured
CA 02951542 2016-12-07
WO 2015/195382 28 PCT/US2015/034592
to use for each organic-carbon-containing feedstock a particular set of
conditions
including time duration, temperature profile, and chemical content of
pretreatment
solution to at least initiate the dissolution of contaminates. The
contaminants include
resins, rosins, glue, and creosote. The solid slurry, including any incipient
felts, may be
collected for use as binders in the processed organic-carbon-containing
feedstock that is
the primary end product. Separated oils may be collected as a stand-alone
product such as,
for example, cedar oil.
The second element, the reaction chamber, includes at least one entrance
passageway, at least one exit passageway, and at least three sections, a wet
fibril
disruption section, a vapor explosion section, and a compaction section. The
first section,
the wet fibril disruption section, is configured to break loose at least some
of the lignin and
hemicellulose between the cellulosic microfibrils in the fibril bundle to make
at least some
regions of cell wall more penetrable. This is accomplished by at least one of
several
means. The organic-carbon-containing feedstock is mixed with appropriate
chemicals to
permeate the plant fibrils and disrupt the lignin, hemicellulose, and LCC
barriers.
Additionally, the chemical treatment may also unbundle a portion of the
cellulose fibrils
and/or microfibrils, de-crystallizing and/or de-polymerizing it. Preferably,
the chemicals
are tailored for the specific organic-carbon-containing feedstock. In some
embodiments,
the chemical treatment comprises an aqueous solution containing a miscible
volatile gas.
The miscible gas may include one or more of ammonia, bicarbonate/carbonate, or
oxygen.
Some embodiments may include aqueous solutions of methanol, ammonium
carbonate, or
carbonic acid. The use of methanol, for example, may be desirable for organic-
carbon-
containing feedstock having a higher woody content to dissolve resins
contained in the
woody organic-carbon-containing feedstock to allow beneficiation chemicals
better
contact with the fibrils. After a predetermined residence time of mixing, the
organic -
carbon-containing feedstock may be steam driven, or conveyer by another means
such as a
piston, into the next section of the reaction chamber. In some embodiments,
process
conditions should be chosen to not dissolve more than 25 wt % of the lignin or
hemicellulose as these are important contributors to the energy density of the
processed
organic-carbon-containing feedstock. Some embodiments of the system, depending
on the
specific organic-carbon-containing feedstock used, may have temperatures of at
least
135 C, at least 165 C, or at least 180 C; pressures of at least 260 psig, at
least 280 psig, at
CA 02951542 2016-12-07
WO 2015/195382 29 PCT/US2015/034592
least 375 psig, or at least 640 psig; and residence times of at least 15
minutes (min), 20
min, or 30 min.
In some embodiments, micro-particles and lignin-rich fragments suspended in
the
effluent is withdrawn from the reactive chamber for subsequent use. The micro
particles
and lignin is cleansed of water-soluble salts and other impurities as needed.
The resulting
slurry, often white, acts as a high energy biomass binder that is then mixed
with the
processed organic-carbon-containing feedstock before the pelletizing step.
This reduces
the need for heat during pelletizing.
The second section, the vapor explosion section, is in communication with the
wet
fibril disruption section. It at least is configured to volatilize plant
fibril permeable fluid
through rapid decompression to penetrate the more susceptible regions of the
cell wall so
as to create a porous organic-carbon-containing feedstock with cellulosic
passageways for
intracellular water and water-soluble salts to pass from the cellulosic-fibril
bundles. The
organic-carbon-containing feedstock is isolated, heated, pressurized with a
volatile fluid
comprising steam. The applied volatile chemicals and steam penetrate into the
plant
fibrils within the vapor explosion section due to the high temperature and
pressure. After
a predetermined residence time dictated by the specific organic-carbon-
containing
feedstock used, pressure is released rapidly from the reaction chamber by
opening a fast-
opening valve into an expansion chamber that may be designed to retain the
gases,
separate them, and reuse at least some of them in the process for increased
energy/chemical efficiency. Some embodiments may have no expansion chamber
where
retention of gasses is not desired. Some embodiments of the system, depending
on the
specific organic-carbon-containing feedstock used, may have a specific
pressure drop in
psig of at least 230, at least 250, at least 345, or at least 600; and
explosive durations of
less than 500 milliseconds (ms), less than 300 ms, less than 200 ms, less than
100 ms, or
less than 50 ms.
Some embodiments may include gas inlets into the wet fibril disruption section
of
the reaction chamber to deliver compressed air or other compressed gas such
as, for
example, oxygen. After delivery to the desired pressure, the inlet port would
be closed
and the heating for the reaction would proceed. Note that this could allow for
at least one
of three things: First, an increase in total pressure would make subsequent
explosion more
powerful. Second, an increase in oxygen content would increase the oxidation
potential of
CA 02951542 2016-12-07
WO 2015/195382 30 PCT/US2015/034592
the processed organic-carbon-containing feedstock where desirable. Third, a
provision
would be provided for mixing of organic-carbon-containing feedstock, water,
and
potentially other chemicals such as, for example, organic solvents, through
bubbling
action of gas through a perforated pipe at bottom of reaction chamber.
The net effect on the organic-carbon-containing feedstock of passing through
the
wet fibril disruption section and the vapor explosion section is the
disruption of fibril cell
walls both physically through pressure bursts and chemically through selective
and
minimal fibril cellulosic delinking, cellulose depolymerization and/or
cellulose
decrystallization. Chemical effects, such as hydrolysis of the cellulose,
lignin, and
hemicellulose also can occur. The resulting organic-carbon-containing
feedstock particles
exhibit an increase in the size and number of micropores in their fibrils and
cell walls, and
thus an increased surface area. The now porous organic-carbon-containing
feedstock is
expelled from the vapor explosion section into the next section.
The third section, the compaction section is in communication with the vapor
explosion section. The compression section at least is configured to compress
the porous
organic-carbon-containing feedstock between pressure plates configured to
minimize
formation of felt that would close the reaction chamber exit passageway made
to permit
escape of intracellular and intercellular water, and intracellular and
intercellular soluble
salts. In this section , the principle process conditions for each organic-
carbon-containing
feedstock is the presence or absence of a raised pattern on the pressure
plate, the starting
water content, the processed water content, and final water content. The
compaction
section of the system of the invention requires a raised patterned surface on
the pressure
plates for feedstock comprising herbaceous plant material feedstock. However,
the section
may or may not require the raised pattern surface for processing soft woody or
hard woody
plant material feedstock depending on the specific material used and its
freshness from
harvest. Some embodiments of the system, depending on the specific organic-
carbon-
containing feedstock used, may have a starting water contents ranging from 70
to 80 wt %,
from 45 to 55 wt % or from 40 to 50 wt %; and processed water content of from
4 to 15 wt
% depending on actual targets desired.
The third element, the collection device, is in communication with the
reaction
chamber. The collection chamber at least is configured to separate non-fuel
components
from fuel components and to create a processed organic-carbon-containing
feedstock.
CA 02951542 2016-12-07
WO 2015/195382 31 PCT/US2015/034592
This feedstock has a water content of less than 20 wt % and a water-soluble
salt content
that is decreased by at least 60 % on a dry basis. Some embodiments have the
water
content less than 20 wt % after allowing for surface moisture to air dry. Some
embodiments have a processed organic-carbon-containing feedstock that has a
water
content of less than 15 wt %. Other embodiments have processed organic-carbon-
containing feedstock that has a water content of less than 12 wt %, less than
10 wt %, less
than 8 wt %, or less than 5 wt %. Some embodiments have a water-soluble salt
content
that is decreased by at least 65 % on a dry basis. Other embodiments have a
water-soluble
salt content that is decreased by at least 70 % on a dry basis, 75 % on a dry
basis, at least
80 % on a dry basis, at least 85 % on a dry basis, at least 90 % on a dry
basis, or at least 95
% on a dry basis.
Some embodiments of the system may further include at least one rinsing
subsection. This subsection is configured to flush at least some of the water-
soluble salt
from the porous organic-carbon-containing feedstock before it is passed to the
compaction
section. In some embodiments where the salt content is particularly high, such
as brine-
soaked hog fuel (wood chips, shavings, or residue from sawmills or grinding
machine used
to create it and also known as "hammer hogs"), the system is configured to
have more than
one rinsing subsection followed by another compaction section. The separated
water,
complete with dissolved water soluble salts, may be collected and treated for
release into
the surrounding environment or even reused in the field that is used to grow
the renewable
organic-carbon-containing feedstock. The salts in this water are likely to
include
constituents purposefully added to the crops such as fertilizer and
pesticides.
The beneficiation sub-system of the invention can better be understood through
depiction of several figures. Figure 4 is a diagram of a side view of an
embodiment of a
reaction chamber in communication with an expansion chamber to retain gasses
emitted
from the decompressed carbon-containing feedstock. A reaction chamber (400) is
shown
with a wet fibril disruption section (410). Solvent (412) and unprocessed
organic-carbon-
containing feedstock (414) that has been chipped to less than 0.5 inches (1.3
cm) are fed in
to wet fibril disruption section 410 through valves (416) and (418),
respectively to become
prepared for the next section. The pretreated organic-carbon-containing
feedstock is then
passed to a vapor explosion section (420) through a valve (422). Valves are
used between
chambers and to input materials to allow for attainment of specified targeted
conditions in
CA 02951542 2016-12-07
WO 2015/195382 32 PCT/US2015/034592
each chamber. Volatile expansion fluid, such as water, or water based volatile
mixtures,
are fed in to vapor expansion chamber 420 through a valve (424). The gas
released from
the porous organic-carbon-containing feedstock created during decompression is
fed
through a fast release valve (428) into an expansion chamber (not shown) to
retain the gas
for possible reuse. The compaction section (430) received the porous organic-
carbon-
containing feedstock through a valve (432) where the water and water-soluble
salt are
substantially removed from porous organic-carbon-containing feedstock and it
is now
processed organic-carbon-containing feedstock.
As stated above, the pressure plates in the compaction section are configured
to
minimize felt formation. Felt is an agglomeration of interwoven fibers that
interweave to
form an impermeable barrier that stops water and water-soluble salts entrained
in that
water from passing through the exit ports of the compaction section.
Additionally, any
pith particles that survived the beneficiation process in the first two
sections of reaction
chamber can be entrained in the felt to absorb water, thereby preventing
expulsion of the
water during pressing. Therefore, felt formation traps a significant fraction
of the water
and salts from being extruded from the interior of biomass being compressed.
Figures 5A,
5B, and 5C show embodiments of pressure plates and how they work to minimize
felt
formation so that water and water-soluble salts are able to flow freely from
the compaction
section. Figure 5A is a diagram of the front views of various embodiments of
pressure
plates. Shown is the surface of the pressure plate that is pressed against the
downstream
flow of porous organic-carbon-containing feedstock. Figure 5B is a perspective
view of a
close-up of one embodiment of a pressure plate shown in Figure 5A. Figure 5C
is a
diagram showing the cross-sectional view down the center of a pressure plate
with force
vectors and felt exposed to the force vectors. The upstream beneficiation
process in the
first two sections of the reaction chamber has severely weakened the fibers in
the biomass,
thereby also contributing to the minimization of felt formation.
Some embodiments achieve the processed organic-carbon-containing feedstock
water content and water-soluble salt reduction over unprocessed organic-carbon-
containing feedstock with a cost that is less than 60 % that of the cost per
weight of
processed organic-carbon-containing feedstock from known mechanical, known
physiochemical, or known thermal processes. In these embodiments, the reaction
chamber
is configured to operate at conditions tailored for each unprocessed organic-
carbon-
CA 02951542 2016-12-07
WO 2015/195382 33 PCT/US2015/034592
containing feedstock and the system is further engineered to re-capture and
reuse heat to
minimize the energy consumed to lead to a particular set of processed organic-
carbon-
containing feedstock properties. The reaction chamber sections are further
configured as
follows. The wet fibril disruption section is further configured to use fibril
disruption
conditions tailored for each organic-carbon-containing feedstock and that
comprise at least
a solvent medium, time duration, temperature profile, and pressure profile for
each
organic-carbon-containing feedstock. The second section, the vapor explosion
section, is
configured to use explosion conditions tailored for each organic-carbon-
containing
feedstock and that comprise at least pressure drop, temperature profile, and
explosion
duration to form volatile plant fibril permeable fluid explosions within the
plant cells. The
third section, the compaction section, is configured to use compaction
conditions tailored
for each organic-carbon-containing feedstock and pressure, pressure plate
configuration,
residence time, and pressure versus time profile.
The importance of tailoring process conditions to each organic-carbon-
containing
feedstock is illustrated by the following discussion on the
viscoelastic/viscoplastic
properties of plant fibrils. Besides the differences among plants in their
cell wall
configuration, depending on whether they are herbaceous, soft woody or hard
woody,
plants demonstrate to a varying degree of some interesting physical
properties. Organic-
carbon-containing material demonstrates both elastic and plastic properties,
with a degree
that depends on both the specific variety of plant and its condition such as,
for example,
whether it is fresh or old. The physics that governs the elastic/plastic
relationship of
viscoelastic/viscoplastic materials is quite complex. Unlike purely elastic
substances, a
viscoelastic substance has an elastic component and a viscous component.
Similarly, a
viscoplastic material has a plastic component and a viscous component. The
speed of
pressing a viscoelastic substance gives the substance a strain rate dependence
on the time
until the material's elastic limit is reached. Once the elastic limit is
exceeded, the fibrils in
the material begin to suffer plastic, i.e., permanent, deformation. Figure 6A
is a graphical
illustration of the typical stress-strain curve for lignocellulosic fibril.
Since viscosity, a
critical aspect of both viscoelasticity and viscoplasticity, is the resistance
to thermally
activated deformation, a viscous material will lose energy throughout a
compaction cycle.
Plastic deformation also results in lost energy as observed by the fibril's
failure to restore
itself to its original shape. Importantly, viscoelasticity/viscoplasticity
results in a
CA 02951542 2016-12-07
WO 2015/195382 34 PCT/US2015/034592
molecular rearrangement. When a stress is applied to a viscoelastic material,
such as a
particular organic-carbon-containing feedstock, some of its constituent
fibrils and
entrained water molecules change position and, while doing so, lose energy in
the form of
heat because of friction. It is important to stress that the energy that the
material loses to
its environment is energy that is received from the compactor and thus energy
that is
expended by the process. When additional stress is applied beyond the
material's elastic
limit, the fibrils themselves change shape and not just position. A "visco"-
substance will,
by definition, lose energy to its environment in the form of heat.
An example of how the compaction cycle is optimized for one organic-carbon-
containing feedstock to minimize energy consumption to achieve targeted
product values
follows. Through experimentation, a balance is made between energy consumed
and
energy density achieved. Figure 6B is a graphical illustration of pressure and
energy
required to decrease the water content and increase the bulk density of
typical organic-
carbon-containing feedstock. Bulk density is related to water content with
higher bulk
density equaling lower water content. The organic-carbon-containing feedstock
compaction process will strike an optimum balance between cycle time affecting
productivity, net moisture extrusion together with associated water-soluble
salts and
minerals, permanent bulk density improvement net of the rebound effect due to
viscoelastic/viscoplastic properties of the feedstock, and energy consumption.
Figure 6C is an experimentally derived graphical illustration of the energy
demand
multiplier needed to achieve a bulk density multiplier. The compaction cycle
can be
further optimized for each variety and condition of organic-carbon-containing
feedstock to
achieve the desired results at lesser pressures, i.e., energy consumption, by
incorporating
brief pauses into the cycle. Figure 6D is a graphical illustration of an
example of a
pressure cycle for decreasing water content in an organic-carbon-containing
feedstock
with an embodiment of the invention tailored to a specific organic-carbon-
containing
feedstock.
In a similar manner, energy consumption can be optimized during the wet fibril
disruption and the vapor explosion parts of the system. Chemical pretreatment
prior to
compaction will further improve the quality of the product and also reduce the
net energy
consumption. For comparison purposes, the pressure applied to achieve a bulk
density
multiplier of "10" in Figure 6C was on the order of 10,000 psi, requiring
uneconomically
CA 02951542 2016-12-07
WO 2015/195382 35 PCT/US2015/034592
high cost of capital equipment and unsatisfactorily high energy costs to
decompress the
organic-carbon-containing feedstock.
Figure 7 is a table illustrating the estimated energy consumption needed to
remove
at least 75 wt % water-soluble salts from organic-carbon-containing feedstock
and reduce
water content from 50 wt % to 12 wt % with embodiments of the invention
compared with
known processes. Waste wood with a starting water content of 50 wt % was used
in the
estimate to illustrate a side-by-side comparison of three embodiments of the
invention
with known mechanical, physiochemical, and thermal processes. The embodiments
of the
system selected use a fibril swelling fluid comprising water, water with
methanol, water
with carbon dioxide bubbled into it produces carbonic acid H2CO3. As seen in
the table,
and discussed above, known mechanical processes are unable to reduce the water
content
to 12 wt %, known physiochemical processes are unable to reduce water-soluble
salt
content by over 25 wt %, and known thermal processes are unable to remove any
water-
soluble salt. The total energy requirement per ton for the three embodiments
of the
invention, that using methanol and water, carbon dioxide and water, and just
water is 0.28
MMBTU/ton (0.31 3, 0.31 MMBTU/ton (0.36 GJ/MT), and 0.42 MMBTU/ton (.49
GJ/MT), respectively. This is compared to 0.41 MMBTU/ton (0.48 GJ/MT), 0.90
MMBTU/ton (1.05 GJ/MT), and 0.78 MMBTU/ton (0.91 GJ/MT) for known mechanical,
known physiochemical, and known thermal processes, respectively. Thus, the
estimated
energy requirements to remove water down to a content of less than 20 wt % and
water-
soluble salt by 75 wt % on a dry basis for embodiments of the system invention
to less
than 60% that of known physiochemical and known thermal processes that are
able to
remove that much water and water-soluble salt. In addition, the system
invention is able
to remove far more water-soluble salt than is possible with known
physiochemical and
known thermal processes that are able to remove that much water.
Multiple reaction chambers may be used in parallel to simulate a continuous
process. Figure 8 is a diagram of a side view of an embodiment of a
beneficiation sub-
system having four reaction chambers in parallel, a pretreatment chamber, and
a vapor
condensation chamber. A system (800) includes an input section (802) that
delivers
organic-carbon-containing feedstock to system 800. Feedstock passes through a
mastication chamber (804) prior to entry into an organic-carbon-containing
feedstock
hopper ((806) from where is passes on to a pretreatment chamber (810).
Contaminants are
CA 02951542 2016-12-07
WO 2015/195382 36 PCT/US2015/034592
removed through a liquid effluent line (812) to a separation device (814) such
as a
centrifuge and having an exit stream (815) for contaminants, a liquid
discharge line (816)
that moves liquid to a filter media tank (818) and beyond for reuse, and a
solid discharge
line (820) that places solids back into the porous organic-carbon-containing
feedstock.
Liquid from the filter medial tank 818 is passed to a remix tank (822) and
then to a heat
exchanger (824) or to a second remix tank (830) and to pretreatment chamber
810. The
organic-carbon-containing feedstock passes onto one of four reaction chambers
(840)
comprising three sections. The first section of each reaction chamber, a wet
fibril
disruption section (842), is followed by the second section, a vapor explosion
section
(844), and a rinsing subsection (846). A high pressure steam boiler (848) is
fed by a
makeup water line (850) and the heat source (not shown) is additionally heated
with fuel
from a combustion air line (852). The main steam line (854) supplies steam to
pretreatment chamber 810 and through high pressure steam lines (856) to
reaction
chambers 840. A vapor expansion chamber (860) containing a vapor condensation
loop is
attached to each vapor explosion sections with vapor explosion manifolds (862)
to
condense the gas. A volatile organic components and solvent vapor line (864)
passes the
vapor back to a combustion air line (852) and the vapors in vapor expansion
chamber 860
are passes through a heat exchanger (870) to capture heat for reuse in
reaction chamber
840. The now porous organic-carbon-containing feedstock now passes through the
third
section of reaction chamber 840, a compaction section (880). Liquid fluid
passes through
the liquid fluid exit passageway (884) back through fluid separation device
(814) and solid
processed organic-carbon-containing feedstock exits at (886).
Heating Sub-System
The optional heating sub-system is used to convert the processed organic-
carbon-
containing feedstock to processed biochar for subsequent blending with coal.
In its
broadest understanding, the heating sub-system comprises a reaction chamber
configured
to heat the processed organic-carbon-containing feedstock in an oxygen
deficient
atmosphere. The heating sub-system comprises at least two forms, one an oxygen-
deficient thermal sub-system and one a microwave sub-system. Others are
possible as
long as they provide heat in an oxygen deficient environment.
Oxygen-Deficient Thermal Sub-System
CA 02951542 2016-12-07
WO 2015/195382 37 PCT/US2015/034592
The oxygen-deficient thermal sub-system is used to convert the processed
organic-
carbon-containing feedstock from the beneficiation sub-system into the clean
porous
processed biochar of the invention. In its broadest understanding, the oxygen-
deficient
thermal sub-system comprises a reaction chamber configured to heat processed
organic-
carbon-containing feedstock in an atmosphere that contains less than 4 percent
oxygen to a
temperature sufficient to convert at least some processed organic-carbon-
containing
feedstock into processed biogas and processed biochar. In some embodiments,
the
atmosphere contains less than 3 percent oxygen and in some less than 2 percent
oxygen.
The sub-system further comprises at least two aspects that are suitable for
the invention ¨
a conventional pyrolysis oxygen-deficient thermal system, and a sublimation
oxygen-
deficient thermal sub-system.
Pyrolysis Oxygen-Deficient Thermal Sub-System
The common pyrolysis oxygen-deficient thermal sub-system produces biochar,
liquids, and gases from biomass by heating the biomass in a low/no oxygen
environment.
The absence of oxygen prevents combustion. The relative yield of products from
pyrolysis varies with temperature. Temperatures of 400-500 C (752-932 F)
produce
more char, while temperatures above 700 C (1,292 F) favor the yield of
liquid and
gaseous fuel components. Pyrolysis occurs more quickly at the higher
temperatures,
typically requiring seconds instead of hours. Typical yields are 60% bio-oil,
20% biochar,
and 20% volatile gases. In the presence of stoichiometric oxygen
concentration, high
temperature pyrolysis is also known as gasification, and produces primarily
syngas. By
comparison, slow pyrolysis can produce substantially more char, on the order
of about
50%. The main benefit from the invention is that the resulting processed
biochar made
with processed organic-carbon-containing feedstock has a water-soluble salt
content that is
reduced by at least 60 wt% from that of processed biochar made with similar
unprocessed
organic-carbon-containing feedstock. In some embodiments, the water-soluble
salt
content is reduced by at least 65 wt%; in some at least 70 wt%; in some at
least 80 wt%; in
some at least 85 wt%; at least some at least 90 wt%.
Sublimation Oxygen-Deficient Thermal Sub-System
Another oxygen-deficient thermal sub-system is sublimation oxygen-deficient
thermal sub-system. Unlike the pyrolysis sub-system, the feedstock in the
sublimation
CA 02951542 2016-12-07
WO 2015/195382 38 PCT/US2015/034592
sub-system does not pass through a liquid phase and the products are only fuel
gases and
processed biochar.
The following description relates to approaches for processing organic-carbon-
containing feedstock into gaseous fuel and a processed biochar fuel by a
sublimation sub-
system. The gaseous fuel is primarily methane but also may include ethane,
propane, and
butane depending on the nature of the organic-carbon-containing feedstock and
the
residence times employed during the sublimation process. Processed biochar
fuel is the
solid carbon-based residue that is unable to be converted into gaseous fuel at
a sublimation
temperature. Alternatively, system conditions may be adjusted to
preferentially create
more than the minimum processed biochar.
The sublimation sub-system is a high temperature sub-system configured to
convert a solid renewable biomass to a gaseous fuel and processed biochar
cleanly without
passing through a liquid state, a passage that can result in many side
reactions discussed
above under gasification. The key to sublimation is to expose the solid to a
high
temperature in the absence of free water and in a substantially oxygen free
atmosphere.
Under sublimation, the methane molecules and higher carbon groups such as
ethane,
propane, and butane, are rejoined after being deconstructed from a carbon
chain in the
feedstock without breaking down to carbon dioxide and water.
The processed biochar made in the sublimation sub-system has several
advantages
over that of the low water-soluble salt content of processed biochar made in
the pyrolysis
sub-system discussed above. First, the processed biochar contains
substantially no
volatiles that may remain in the pyrolysis sub-system. Volatiles present
during
depolymerization temperatures cause adverse reactions with carbons to form
compounds
other than the desired processed biogas fuels and processed biochar of the
invention. In
addition, volatiles in the processed biochar reduce the heating content of the
processed
biochar by reducing the fixed carbon in the resulting biochar. In the
invention, the
processed biochar is substantially devolatilized of condensable and non-
condensable gases
and vapors. In some embodiments, the content of the gases and vapors is
reduced by at
least 95% by weight, in some embodiments it is reduced by at least 97% by
weight, and in
some it is reduced by at least 99% by weight versus char made from unprocessed
organic-
carbon-containing feedstock in the pyrolysis sub-system with a volatile
content of at least
10% by weight. This is desirable in processed biochar applications such as a
coke
CA 02951542 2016-12-07
WO 2015/195382 39 PCT/US2015/034592
alternative for steel making, gasification, and combustion applications such
as boilers
because of the lowered content of adverse corrosive compounds in the processed
biochar
of the invention over that of processed biochar from the same feedstock but
with the
pyrolysis process.
Second, the processed biochar has a higher heating value than that of
processed biochar
made with the same feedstock by the pyrolysis process. Because of better
devolatization, there
are fewer side reactions with the volatiles and carbon during later use
involving
combustion of the processed biochar as fuel. Thus, a greater degree of fixed
carbon can
remain in the processed biochar of the invention than in the processed biochar
from
similar feedstock in a pyrolysis sub-system. The fixed carbon content in some
embodiments is increased by at least 5% by weight, in some embodiments by at
least 10%
by weight, in some embodiments by at least 15% by weight, and in some
embodiments by
at least 20% by weight. This increase in fixed carbon can result in an
increase in the heat
content of some embodiments of the processed biochar of the invention over
that of
processed biochar from the same feedstock in a pyrolysis sub-system. Heat
content is
affected by the type of organic-carbon-containing feedstock used and generally
ranges
from at least 20 MMBTU/ton (23 GJ/MT) to over 28 MMBTU/ton (33 GJ/MT) compared
with less than 12 MMBTU/ton (14 GJ/MT) to less than 20 MMBTU/ton (23 GJ/MT)
for
similar organic-carbon-containing feedstock made in the pyrolysis sub-system.
In some
embodiments, the heat content of the processed biochar is increased by at
least 20% over
processed biochar by the pyrolysis sub-system, in some by at least 30%, in
some by at
least 40%, in some by at least 50%, in some by at least 60%, and by some at
least 70%. In
addition, because of the reduced amount of volatiles, the processed biochar of
the
invention can burn without visible smoke and with a smaller flame than seen
with biochar
made of a similar feedstock in a pyrolysis sub-system. For some embodiments,
the flame
can be at least 5% less, for some embodiments at least 10% less, for some
embodiments at
least 15% less, for some embodiments at least 20% less, for some embodiments
at least
25% less, for some embodiments at least 30% less, for some embodiments at
least 35%
less, and for some embodiments at least 40% less.
The sublimation system can be further illustrated by a horizontal sublimation
system and a vertical sublimation system. Other orientations may be
contemplated.
Horizontal Sublimation Oxygen-Deficient Thermal Sub-System
CA 02951542 2016-12-07
WO 2015/195382 40 PCT/US2015/034592
The horizontal sublimation oxygen-deficient thermal sub-system comprises four
elements. The first is a hot box configured to be able to (1) heat from an
ambient
temperature to an operating sublimation temperature, (2) maintain an initial
operating
sublimation temperature and a final operating sublimation temperature that are
stable
within less than 10 C, and (3) cool from operating sublimation temperatures
to an
ambient temperature. All without leaking any oxygen into the hot box and
having at least
one heat source in communication with the interior of the hot box to supply
heat as
needed. The second element is at least one substantially horizontal reaction
chamber
largely located within the hot box and having a surface. The reaction chamber
is
configured to heat the processed organic-carbon-containing feedstock without
external
catalyst or additional water to an operating sublimation temperature in a time
frame that is
short enough to sublime at least part of the processed organic-carbon-
containing feedstock
without creating substantially any liquid. The reaction chamber is also
configured to heat
from an ambient temperature to an operating sublimation temperature, operate
at a
sublimation temperature, and cool from an operating sublimation temperature to
an
ambient temperature without leaking any product gas fuel into the surrounding
hot box,
and comprising an input end outside the hot box. The reaction chamber is
further
configured to receive compressed feedstock through an input line and an output
end
outside the hot box and configured to discharge product gas fuel through a
discharge line
and solid char fuel through an output line. The third element is a first
powered transport
mechanism that is located within the reaction chamber and is configured to
convey
sublimation products of the processed organic-carbon-containing feedstock
through the
reaction chamber as the processed organic-carbon-containing feedstock is
transformed into
processed biogas and processed biochar. The fourth element is a gas-tight
element on both
the input line and output line and configured to prevent hot biogas from
adversely
escaping from the reaction chamber.
The overall process will now be discussed for using a substantially horizontal
sublimator to efficiently convert processed carbon-containing feedstock to
product biogas
fuel and solid processed biochar fuel. The process will be discussed briefly
for an
embodiment that needs processed organic-carbon-containing feedstock
preparation, drying
and compression, and uses an apparatus with two reaction chambers and burners
to deliver
sublimation heat. In some embodiments, the beneficiation sub-system is
attached directly
CA 02951542 2016-12-07
WO 2015/195382 41 PCT/US2015/034592
to horizontal sublimation oxygen-deficient thermal sub-system and the
processed organic-
carbon-containing feedstock needs little if any preparation, drying, and
compression. In
some embodiments, the desired properties of the processed organic-carbon-
containing
feedstock are such that at least some additional preparation, drying and or
compression are
desirable. Briefly, the sublimation sub-system of this embodiment of the
invention is
configured to be able to perform a preparation step, a drying step, a
compression step, a
sublimation step, and a separation step. Processed organic-carbon-containing
feedstock
preparation will be dictated by the physical characteristics of the processed
organic-
carbon-containing feedstock being considered for processing/conversion such as
its water
content and physical characteristics such as size and thickness. Size
reduction of carbon-
containing feedstock will enhance the compressibility of the processed organic-
carbon-
containing feedstock to allow for maximum throughput in the reaction chamber.
In some
embodiments, sizes are of a volume that is less than the equivalent of a cube
about 2 cm
(about 0.75 in) on a side with a length in any one direction of no more than
about 5 cm
(about 2 in).
After the processed organic-carbon-containing feedstock is properly prepared,
it
will then pass through a gas-tight element on an input line into a
substantially horizontal
drying chamber with an internal auger and be treated with recycled heat from
the
downstream process to drive off as much free water as possible. Reducing the
free water
content will increase the heat absorption by the processed organic-carbon-
containing
feedstock and reduce the amount of oxygen present from the water inside the
sublimation
reaction chamber and the finished product biogas fuel and processed biochar.
Reducing
the free water and the oxygen will result in less carbon dioxide and carbon
monoxide in
the product biogas fuel. Less CO2 and CO byproduct is desirable because it
increases the
energy content of the processed biochar and the produced biogas.
After the drying chamber, the processed organic-carbon-containing feedstock
will
pass into a compression chamber containing a compression screw that is
designed to
compress the carbon-containing feedstock to the desired density. This
compression
further decreases any free water remaining. It also removes entrained air in
the processed
organic-carbon-containing feedstock that also will minimize the oxygen present
in the
sublimation reaction chamber. This dewatered, de-aired, and densified carbon-
containing
feedstock will enter the reaction chamber.
CA 02951542 2016-12-07
WO 2015/195382 42 PCT/US2015/034592
The compression chamber develops a feedstock plug at its exit that enters the
reaction chamber. This plug acts as a partial barrier or seal so a minimal
amount of gases
produced in the reaction chamber backflow and escape. The gas-tight element on
the
input line prevents the rest of the gases from escaping the system.
In the embodiment being discussed, the sublimation sub-system has a physical
plant that is a three dimensional, rectangular box with an internal
substantially horizontal
reaction chamber running along the top, a drop passage, and then a second
substantially
horizontal reaction chamber in the reverse direction of the top reaction
chamber. Each
reaction chamber contains its own auger for transporting the feedstock, is
continuous, and
is completely sealed against the escape of any hot product gas fuel.
In this embodiment, burners are external to the heating box but attached to it
and
will heat the space between the inside wall of the heating box and the outside
walls of the
reaction chamber configuration so that there will be no intermingling of the
heated transfer
air heating the external surface of the reaction chamber and the contents of
the processed
organic-carbon-containing feedstock in the reaction chamber undergoing
sublimation. All
the internal surfaces of the heating box are lined with high thermal
insulating material so
as to minimize heat loss and minimize the internal reactor air space.
After the compression screw, the processed organic-carbon-containing feedstock
plug now enters the reaction chamber. The reaction chamber containing an auger
inside of
it that may be inside of a tube vented to a head space above the tube but
within the
reaction chamber for aggregation of the gases that are generated. The auger
propels and
rotates the processed organic-carbon-containing feedstock so that it is evenly
exposed to
the sidewalls of the tube or reaction chamber for efficient heat exchange and
to 'turn over'
the feedstock for even heating. The reaction chamber is heated from the
outside surface of
the reaction chamber so the transfer heats the air and any combustion products
from the
burners do not get intermingled with the processed organic-carbon-containing
feedstock
and/or product gas fuel or biogas. The reaction chamber is constructed to
prevent the
leaking out of any hot gases.
The processed organic-carbon-containing feedstock is then augured down the
length of the reaction chamber. At the end of the reaction chamber, the carbon-
containing
feedstock drops down into a second augured reaction chamber that is of the
same design as
CA 02951542 2016-12-07
WO 2015/195382 43 PCT/US2015/034592
the first reactor tube. The configuration of the three, chambers including the
connecting
passage looks like a U rotated 90 degrees to the left.
The processed organic-carbon-containing feedstock has now been reduced to
devolatilized carbon and volatile gases. The volatile gases are passing and
mixing with
the hot carbon surfaces and reacting with it to form a dissociated hot product
gas fuel. The
residence time in both of the reaction chambers allows the volatile gases
created during
the sublimation to deconstruct down to several different structures
approaching and
including that of methane, and to move down the reaction chambers as product
biogas
fuel.
At the end of the second reactor chamber are two outlets. One outlet is for
the
devolatized carbon to pass through a gas-tight mechanism and be collected as
solid
biochar fuel and the second outlet is for the product biogas to be captured.
The product
gas is filtered and allowed to cool after it exits the reaction chamber as the
final product
biogas fuel and then stored.
More specifically, the apparatus aspect of the invention comprises a system
that
includes a hot box, at least one reaction chamber, a first powered transport
mechanism,
and gas-tight elements. The hot box is configured to be able to heat from an
ambient
temperature to an operating sublimation temperature, maintain an initial
operating
sublimation temperature and a final operating sublimation temperature that are
stable
within less than 10 C, and cool from operating sublimation temperatures to an
ambient
temperature without leaking any oxygen into the hot box and having at least
one heat
source in communication with the interior of the hot box to supply heat as
needed.
The temperature needed to sublime processed organic-carbon-containing
feedstock
depends on the individual feedstock. If an operating temperature is too low, a
liquid forms
during the phase change from solid to gas with accompanying adverse reactions
discussed
above and associated with gasification processes. If the temperature is too
high, energy is
wasted in an already endothermic reaction. Operating sublimation temperatures
are
typically between 600 C and 850 C. More common low-density, processed, organic-
carbon-containing feedstock have operating sublimation temperatures between
650 C and
750 C.
For the above reasons, the operating temperature in the reaction chamber
should be
reasonably stable during operation of the apparatus. In some embodiments where
the
CA 02951542 2016-12-07
WO 2015/195382 44 PCT/US2015/034592
reaction chamber has a shorter length and the flowrate of the processed
organic-carbon-
containing feedstock is smaller, the operating temperature may be
substantially constant
within less than 10 C. In other embodiments having a longer residence time
and a larger
processed organic-carbon -containing feedstock throughput, the reaction
chamber may not
be constant but rather forms a profile through the reaction chamber drops from
the
beginning to the end. In these embodiments, for energy efficiency reasons, the
individual
temperatures of the temperature profile through the reaction chamber should be
stable
during operation within less than 10 C.
The heat source must be able to heat the inside of the hot box to a stable
operating
sublimation temperature and maintain that temperature during the operation of
the
apparatus. Heat sources may include any that can provide sufficient heat and
include, for
example, infrared sources, laser sources and combustion sources. Embodiments
that use
combustion sources have the additional advantage in that they can be fueled by
some of
the product biogas fuel such that they require no additional energy from
external sources.
Such embodiments may be self sufficient during operation with as little as 10
percent of
the product gas fuel that is created in the apparatus. Some embodiments may be
self-
sufficient with as little as 7 percent and some with as little as 5 percent.
This is due to the
high energy content of the product gas fuel and the variable amounts of energy
needed to
process different feedstock.
The at least one reaction chamber is substantially horizontal, located largely
within
the hot box, has a surface, and is configured to heat the processed organic-
carbon-
containing feedstock without external catalyst or additional water to an
operating
sublimation temperature in a time frame that is short enough to sublime at
least part of the
processed organic-carbon-containing feedstock without creating substantially
any liquid.
Also, it is configured to heat from an ambient temperature to an operating
sublimation
temperature, operate at a sublimation temperature, and cool from an operating
sublimation
temperature to an ambient temperature without leaking any product biogas fuel
into the
surrounding hot box. Further, it comprises an input end outside the hot box
and
configured to receive compressed feedstock through an input line and an output
end
outside the hot box and configured to discharge product biogas fuel through a
pressure-
isolation element and processed biochar fuel through an output line.
CA 02951542 2016-12-07
WO 2015/195382 45 PCT/US2015/034592
Sublimation is a reaction that deconstructs smaller gaseous hydrocarbons from
an
organic-carbon-based feedstock and more particularly in the case of the
invention from a
processed organic-carbon-based feedstock. In the invention, the gas collects
as a
processed biogas fuel as it interacts with processed biochar solid fuel
residue. Thus, there
is no need for expensive external catalysts and subsequent elaborate reforming
operations
to create the processed biogas fuel. In addition, the sublimation of the
invention is
conducted in the presence of minimal oxygen since any oxygen reacts to cause
non-fuel
reaction products such as carbon dioxide and carbon monoxide. Thus it is
desirable to not
use superheated steam in contact with the processed organic-carbon-containing
feedstock
to achieve operating sublimation temperature. Some oxygen that is
interstitially locked in
the cells may be in the processed organic-carbon-containing feedstock. Also,
some
oxygen may enter the reaction chamber because of potentially incomplete drying
when
that drying step is desired. Both of these sources of potential oxygen sources
should be
mitigated by the beneficiation pre-processing: cell walls broken exposing
interstitial water
and oxygen to expulsion and also a good pre-drying process. Thus, these
sources of
oxygen comprise a small portion and contribute to less than 5 percent of the
gaseous
product and often less than 3 percent or 2 percent depending on the particular
processed
organic-carbon-containing feedstock used.
To avoid passing through the liquid phase, the solid surface of the processed
organic-carbon-containing feedstock should reach the sublimation temperature
immediately. In some embodiments, this is within 1 millisecond. In some
embodiments,
the time is within less than 0.1 millisecond. In still others it is within
less than 0.01
millisecond.
Some embodiments have a single reaction chamber. These are constructed to
withstand the temperature changes associated with passing from ambient to
operating
sublimation temperatures during start up operation and the reverse during
shutdown
operations. Features may include thicker walls and/or the use of supporting
elements such
as gussets where the conversion part of the reaction chamber wall is in
communication
with the side of the hot box.
The first powered transport mechanism is located within the reaction chamber
and
is configured to convey sublimation products of the processed organic-carbon-
containing
feedstock through the reaction chamber as the processed organic-carbon-
containing
CA 02951542 2016-12-07
WO 2015/195382 46 PCT/US2015/034592
feedstock is transformed into product gas fuel and solid char fuel. Some
embodiments
have a reaction chamber that comprises a tube containing the first powered
transport
mechanism. The reaction chamber also has a head space in communication with
the tube
for the collection of product biogas fuel as it is created. The first powered
transport
mechanism is configured to advance the solid portions of the processed organic-
carbon-
containing feedstock, particularly the low-density forms of the processed
organic-carbon-
containing feedstock. It is also configured to assist intermixing with the
heat of the
surface of the reaction chamber to assist in maintaining a stable operating
sublimation
temperature in contact with the solid parts of the feedstock as product biogas
fuel
continues to be removed from the solid parts of the feedstock. The first
transport
mechanism is one that is able to effectively operate at a sublimation
temperature and not
be adversely impaired by thermal expansion and contraction during the starting
up and
cooling down phases of operation. One example of an effective first transport
mechanism
is in an augur.
The gas-tight element is on both the input line and output line and configured
to
prevent hot product fuel biogas from adversely escaping from the reaction
chamber.
Leaks that permit product biogas fuel to exit the reaction chamber in an
unregulated
manner can cause a serious safety concern. Combustible product biogas fuel in
the
presence of hot surfaces can cause fires and explosions. Examples of gas-tight
elements
effective for this purpose at the temperatures discussed are a rotary valve, a
rotary vacuum
valve, and actuated double-gate valve. Alternatively, a more expensive
configuration may
include a box surrounding the hot box of the sublimation sub-system with purge
nitrogen
under a positive pressure in the box to keep any escaping gas from becoming
hazardous.
The operating pressure in the reaction chamber may be protected from adverse
instability from the product biogas fuel leaving in its discharge line by
passing the product
gas fuel through a pressure isolation element. This helps maintain the stable
sublimation
conditions within the reaction chamber. Pressure isolation elements include,
for example,
bubblers and cyclones to maintain pressure in the reaction chamber.
Alternatively, the
pressure in the reaction chamber may be controlled through the product biogas
fuel being
discharged into gas tight holding tanks.
Some embodiments of the system have at least two substantially horizontal
reaction chambers that are in communication with each other in series, and the
first
CA 02951542 2016-12-07
WO 2015/195382 47 PCT/US2015/034592
powered transport mechanism has a part of a shaft that extends outside each
reaction
chamber and the hot box. Embodiments with more than one reaction chamber in
series
provide systems able to process higher amounts of carbon-containing feedstock
with
similar footprints to that of some systems having a single reaction chamber.
These
embodiments further comprise an adjustable sealing element located outside the
hot box at
the region of the hot box surrounding a collar about the extended part of the
first powered
transport mechanism. The adjustable sealing element is configured to prevent
the adverse
entry from outside the hot box of external oxygen entering the hot box during
changing
temperatures of startup and shutdown operations, and during steady-state
sublimation
operation. Leaks that permit oxygen to enter the hot box from the outside or
product gas
fuel to enter from the reaction chamber can cause undesirably large
fluctuations in the
operating sublimation temperatures. They represent an additional and
uncontrolled source
of heat when they combust.
Each sealing element comprises an adjustable plate and an adjustable seal to
permit
satisfactory exclusion of additional undesirable oxygen leaking into the hot
box or reaction
chamber through undesirable leaks created during thermal expansion and
contraction of
elements of the system during startup and shutdown operations. The adjustable
plate
comprises a substantially vertical plate that is adjustably attached to the
hot box and
configured to vertically move the collar about the extended part of the shaft
of the first
powered transport mechanism to prevent adverse contact between collar and the
shaft.
The adjustable seal is in communication with the adjusting plate, located
about the
extended portion of the shaft of the first powered transport mechanism and
comprises a
cone and rope configuration designed to maintain a gas-tight seal about the
shaft of the
first powered transport mechanism as it extends from the hot box.
The residence time in the reaction chamber varies with the nature of the
processed
organic-carbon-containing feedstock and the quantity being processed.
Typically,
between at least 50 percent by weight and over 90 percent by weight of
processed organic-
carbon-containing feedstock can be converted into product gas fuel with the
remainder
being solid char fuel having an energy density similar to coal. Longer
residence times
allow more methane units to reassociate from the disassociated gas and may
result in a
higher conversion to product gas fuel approaching over 70 weight percent to
over 90
weight percent. Residence times may range from less than 10 minutes in some
CA 02951542 2016-12-07
WO 2015/195382 48
PCT/US2015/034592
embodiments to less than 5 minutes in some embodiments to less than 2 minutes
in some
embodiments. Excessively long residence times have no adverse effect on the
conversion
once the theoretical conversion is substantially achieved.
In some embodiments the reactor chambers further comprise manifolds attached
to
the outside of the reaction chambers within the hot box. The reaction chamber
surface and
the manifold are configured to allow dissociated gas to pass between the
reaction chamber
and the manifold to increase the time the disassociated gas is exposed to
sublimation
temperatures. In some cases, this additional time may result in dissociating
gases that
have longer carbon ¨carbon structured chains such as, for example, ethane,
propane, and
butane, to further disassociate into methane.
Some embodiments of the system of the invention further comprise a vertical
support within the hot box and further beneath the lower substantially
horizontal reaction
chamber to support its weight during startup, shutdown, and operating
conditions where
thick reaction chamber walls and support elements such as gussets are not
desirable or not
feasible to provide adequate support. Generally, the vertical support is
configured to be
dimensionally stable to within about 2.5 cm (about one inch) in the vertical
direction over
temperature variations between ambient temperature and about 850 C that may
occur
during the startup, operation, and shutdown of the substantially horizontal
reaction
chamber.
Vertical dimensional stability is achieved by the use of insulation in
combination
with the use of cooling material flowing through the support in addition to
the use of
insulation. The cooling material is that commonly associated with cooling and
includes,
for example, water; refrigerants such as halogenated gas, carbon
tetrachloride,
chlorofluorocarbons, hydrochlorofluorocarbons, ammonia, carbon dioxide,
ethane,
propane, ether, and dimethylether; gaseous coolants such as air, hydrogen,
inert gases, and
sulfur hexafluoride; liquid coolants such as water, ethylene glycol,
diethylene glycol,
propylene glycol, and Freon by DuPont; and solid coolants such as dry ice.
Cooling materials may pass through or around a vertical support in any manner
that maintains the desired vertical dimensional stability. When the vertical
support is not
cooled, thermal expansions may result in vertical expansions of several
inches. This is
enough to cause welds in the supported reaction chamber to break and leak
product gas
fuel into the hot box or out into the environment. As discussed above, this
can cause a
CA 02951542 2016-12-07
WO 2015/195382 49 PCT/US2015/034592
safety issue and can adversely destabilize the operating temperature profile
in the reaction
chamber. Some embodiments may have the cooling material pass horizontally
along the
vertical support walls near the hot reaction chamber that is being supported.
Some
embodiments may have cooling material flow vertically up into the shaft of the
vertical
support. Other configurations are also possible as long as they limit vertical
thermal
expansion sufficiently to not cause leaks in welds in the reaction chamber.
Some embodiments of the system may further comprise a preparation chamber that
is outside the hot box. This is useful when carbon-containing feedstock is not
supplied in
a dried and compressed manner. The preparation chamber is in communication
with the
substantially horizontal reaction chamber, is configured to remove some free
water and
oxygen from the processed organic-carbon-containing feedstock, and is
configured to
compress the processed organic-carbon-containing feedstock into a plug before
it enters
the substantially horizontal reaction chamber.
The preparation chamber also comprises a second powered transport mechanism
that is located partly within the preparation chamber and has a part that
extends outside the
preparation chamber. The preparation chamber is configured to perform one or
more of
moving the processed organic-carbon-containing feedstock through the
preparation
chamber and compressing the processed organic-carbon-containing feedstock
within the
preparation chamber as it is dried of more free water.
Heat may be supplied internally for the drying function. In some embodiments,
the
heat used to dry the processed organic-carbon-containing feedstock comes from
the
combustion gasses in the hot box. In some embodiments, the heat may come from
at least
one of the hot product gas fuel and the solid char fuel through heat
conveyance devices
such as, for example, heat exchangers.
In some embodiments, the preparation chamber may be subdivided into a drying
chamber and a compression chamber where additional drying may occur. The
compression chamber may be equipped with its own second powered transport
mechanism. The drying chamber may be equipped with its own third powered
transport
mechanism. In this embodiment, the drying chamber or pre-preparation chamber
is in
communication with the compression chamber or preparation chamber and is
configured
to reduce the particle size of low density processed organic-carbon-containing
feedstock
to a size and remove the bulk of initial water and trapped air to permit the
processed
CA 02951542 2016-12-07
WO 2015/195382 50 PCT/US2015/034592
organic-carbon-containing feedstock to be more easily conveyed through the
preparation
chamber of the system, more easily compressed there without entraining oxygen
or water,
and more easily heated there to a sublimation temperature without permitting
the
formation of a liquid phase. In this embodiment, a third powered transport
mechanism
that precedes and is in communication with the pre-preparation chamber, has a
part that
extends outside the pre-preparation chamber. The mechanism is configured to
perform
one or more of moving the processed organic-carbon-containing feedstock
through the
pre-preparation chamber and compressing the processed organic-carbon-
containing
feedstock within the pre-preparation chamber in more manageable sized
particles.
In both cases, the individual transport mechanisms are to advance processed
organic-carbon-containing feedstock forward into a condition for sublimation.
One
example of a transport mechanism is an augur but others are suitable if they
accomplish
the desired function.
The system of the invention may further comprise various units to prepare the
processed organic-carbon-containing feedstock into a condition to be used by
the system
of the invention. Various feedstock must have their size reduced as discussed
above to
dimensions that can be dried, compressed, and sublimated in a timely manner.
By way of
illustration, tires must be reduced to tire crumbs and straws or stalks must
be reduced to
shapes that are more readily conveyed through the preparation chamber of the
system,
more easily compressed there without entraining oxygen or water, and more
easily heated
there to a sublimation temperature without permitting the formation of a
liquid phase.
Units may include, for example, devices that grind, chop, slice, or cut.
Figures 9 to 17 illustrate various embodiments of the sublimation oxygen
deficient
thermal systems described above. The same numbers are used for similar
functional
elements even if the embodiments are different. Figure 9 is a diagram of a
side view of an
embodiment of a system with a single substantially horizontal reaction chamber
having
one pass. A system (900) is depicted with a hot box (910) containing a vent
(912) that
surrounds a reaction chamber (920). The vent is needed when the hot box is
heated with
burners that create combustion products. When heat is generated by other
sources of heat,
excess gas may not be generated that needs to be vented. Reaction chamber 920
has a
surface (921), and contains a first transport mechanism (922), an augur, with
a shaft (924).
At one end of reaction chamber 920 and extending outside hot box 910 is a
front end (930)
CA 02951542 2016-12-07
WO 2015/195382 51 PCT/US2015/034592
that processed organic-carbon-containing feedstock enters into thorough an
input line
(932) with a rotary vacuum valve (934) to isolate any sublimed gases within
the reaction
chamber. At the other end of the reaction chamber and extending outside hot
box 910 is a
back end (940) where product gas fuel exits from a discharge line (942) with a
pressure
isolation element (944) positioned to isolate any sublimed processed biogas
within the
reaction chamber and solid processed biochar fuel exits from a discharge line
(946) with a
rotary vacuum valve (948) positioned to isolate any sublimed processed biogas
within the
reaction chamber.
Figure 10 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes, a flexible shaft seal, and a high temperature
adjustable shaft
cover plate. System 900 is depicted with hot box 910 containing vent 912 that
surrounds a
reaction chamber 920 and is on a base (914). Reaction chamber 920 with surface
921 is
configured like an open "U" on its side with two horizontal passages connected
with a
vertical passage on the right ends. Each horizontal passage contains first
transport
mechanism 922, an augur, with a shaft 924. Each shaft extends out of the
horizontal
passages of reaction chamber 920 and hot box 910. For each shaft end, a high
temperature
adjustable shaft seal plate (926) encloses each shaft collar (927) and
adjustably fastens to
the hot box. For each shaft end, an adjustable high temperature seal (928) is
fastened on
shaft collar 927 at one end and encompasses both a portion of shaft collar 927
and a
portion of the extended shaft end of shaft 924. At the end of the first
reaction chamber
920 and extending outside hot box 910 is front end 930 into which processed
organic-
carbon-containing feedstock enters thorough input line 932 with rotary vacuum
valve 934
positioned to isolate any sublimed process biogas within the reaction chamber.
At the end
of the second reaction chamber 920 and extending outside hot box 910 is back
end 940
where processed biogas fuel exits from discharge line 942 with cooling element
944
positioned to isolate any sublimed process biogas within the reaction chamber
and solid
char fuel exits from discharge line (946) with rotary vacuum valve (948)
positioned to
isolate any sublimed processed biogas within the reaction chamber.
The high temperature adjustable seal and plate that is shown in Fig 10 may
have
various forms as long as the function is accomplished. One embodiment is
illustrated in
Figures 11A to 11E and Figures 12A to 12E adjustable shaft seal plate 926.
Figure 11A is
a diagram of a side view of an embodiment of the high temperature adjustable
shaft seal
CA 02951542 2016-12-07
WO 2015/195382 52
PCT/US2015/034592
casing with the rope seals compressed in place. Seal 928 comprises a casing
(1000) that
contains a double rope seal base plate (1010) in its front end facing the end
of shaft 1024.
Base 1010 is connected to two rope seals (1020) to form a double rope seal.
This
construction is further illustrated in Fig. 11B. The backend of casing 1000
that faces hot
box 910 contains a boltable collar (1040) that is configured to affix shaft
collar 927 next to
single rope seal 1020 on a single rope seal base plate (1050) that is further
illustrated in
Fig. 11C.
Figure 11B is a diagram of a view of an element of the embodiment of Fig 11A
showing a back view of the frame holding the double rope seal. The view is one
of
looking through casing 1000 from the end of shaft 924. Bolt holes (1060A) are
depicted.
Figure 11C is a diagram of a view of an element of the embodiment of Fig 11A
showing a back view of the frame holding a single rope seal. The view is one
of looking
through casing 1000 from hot box 910. Bolt holes (1060B) are depicted.
Figure 11D is diagram of a front view and side view of an element of the
embodiment of but not shown in Fig 11A showing a cover that compresses the
double
rope seal of Fig. 11B. The front view is of the side that faces the rope seal.
A cover
(1070) comprises a raised inner compression ring (1072) that has a sloping
cross-sectional
edge attached to an outer support ring (1074) with bolt holes 1060A. When
bolted to the
holes of Fig. 11B, raised inner compression ring 1072 pushes the rope seal
inward against
the shaft to eliminate adverse leaks of air containing oxygen from entering
the hot box
during startup and shutdown temperature expansion and contraction cycles.
Figure 11E is a diagram of a front view and side view of an element of the
embodiment of but not shown in Fig 11A showing a cover that compresses the
single rope
seal of Fig 11C. The front view is of the side that faces the rope seal. A
cover (1080)
comprises a raised inner compression ring (1082) that has a square cross-
sectional edge
attached to an outer support ring (1084) with bolt holes 1060B. When bolted to
the holes
of Fig. 11C, raised inner compression ring 1082 pushes the rope seal downward
against
seal collar 1040 to eliminate adverse leaks of air containing oxygen from
entering the hot
box during startup and shutdown temperature expansion and contraction cycles.
Figure 12A is a diagram of the front view and side view of an embodiment of
the
high temperature adjustable cover plate showing a top half. The upper half
(1110) of high
temperature adjustable seal plate 926 comprises two adjustable holes (1112),
connecting
CA 02951542 2016-12-07
WO 2015/195382 53 PCT/US2015/034592
holes (1114), and a semicircular opening (1116) designed to fit around half of
shaft collar
927. The cross-section (1118) is straight.
Figure 12B is a diagram of the front view and side view of the embodiment of
the
high temperature adjustable cover plate of Fig. 12A showing a bottom half. The
lower
half (1120) of high temperature adjustable seal plate 926 comprises two
adjustable holes
(1122), connecting holes (1124), a semicircular opening (1126) designed to fit
around half
of shaft collar 927, and a step plate (1125) that contains connecting holes
1124 to permit a
smooth surface to contact the hot box when assembled. The cross-section (1128)
is
stepped.
Figure 12C is a diagram of the front view of the embodiment of the high
temperature adjustable cover plate of Fig. 12A showing the top half of Fig.
12A and the
bottom half of Fig. 12B joined.
Figure 12D is a diagram of the front view of the assembled high temperature
adjustable cover plate in the cold temperature position. As seen, because hot
box 910 has
not yet experienced thermal expansion, shaft 924 exits hot box 910 through
collar 927 at a
lower position to avoid adversely having collar 927 contact shaft 924 during
operation.
Figure 12E is a diagram of the front view of the assembled high temperature
adjustable cover plate in the hot temperature position. As seen, because hot
box 910 has
thermally expanded in an upward manner during start-up heating operations,
collar 927
must be moved upward to avoid adversely contacting shaft 924 during operation.
Adjustable holes 1112 and 1122 permit such adjustment. Some embodiments use
manual
adjustment. Some embodiments use automated adjustment.
Figure 13 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes, a flexible shaft seal, a high temperature
adjustable shaft cover
plate, and a vertical support stand. This embodiment is similar to the
embodiment shown
in Fig. 10 except a high temperature vertical support (1200) is used to
support reaction
chamber 920 within hot box 910. Vertical support 1200 comprises a vertical
shaft (1210)
and a cradle (1220) to hold reaction chamber 920. The stability of the
vertical shaft and
cradle configuration is reinforced with gussets (1230) attaching shaft 1210 to
cradle 1220
and shaft 1210 to system base 914.
The high temperature vertical support stand that is shown in Fig 13 may have
various forms as long as the function is accomplished. One embodiment is
illustrated in
CA 02951542 2016-12-07
WO 2015/195382 54 PCT/US2015/034592
Figures 14A and 14B. A variation of that embodiment is illustrated in Figure
14C. Figure
14A is a front view of an embodiment of a vertical stand showing a curved
cradle and a
horizontal ring for passing coolant. The cradle is designed to conform to the
bottom of
reaction chamber 920. In embodiments of the system where the bottom of
reaction
chamber 920 is other than curvature, a different conforming shape of the
cradle would be
employed. Shaft 1210 is surrounded with insulation (not shown). However, heat
passing
from reaction chamber 920 through cradle 1220 to stand 1210 can cause
adversely large
vertical thermal expansion of shaft 1210 as discussed above. A cooling ring
(1240)
horizontally displaced within the upper part of shaft 1210 can be used to
minimize thermal
expansion of shaft 1210 to satisfactory lengths over the ranges of
temperatures employed
by the apparatus as discussed above.
Figure 14B is a top view of the embodiment of Fig. 14A showing cooling ring
1240.
Figure 14C is a front view of an embodiment of a vertical stand showing a
curved
cradle and a vertical up and down cooling passage within the vertical shaft of
the vertical
stand.
Figure 15 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes and a four-pass bypass manifold attached to the
outside of the
reaction chamber to increase residence time. The system (1300) comprises a
bypass
manifold (1310 that is in communication with the processed biogas within
reaction
chamber through apertures (not shown) in the surface (921) of the reaction
chamber and
manifold where they connect. This permits the deconstructed gas product to
experience
extended residence times where appropriate for desired conversion of processed
organic-
carbon-containing feedstock into product gas fuel and solid char fuel.
Figure 16 is a diagram of a side view of an embodiment of a system with a
reaction
chamber having two passes, a compression chamber, and a drying chamber. This
system
is similar to that shown in Fig. 14 with additional processing chambers. The
passage of
material as it enters and progresses through the system until it exits as
product fuel is
shown by a heavy line. System 1500 comprises a preparation chamber (1510) for
compressing carbon-containing feedstock into a plug prior to entry into the
front end 930
of the reaction chamber. A second powered transport mechanism (1520) is inside
the
chamber to accomplish the compression. Some additional drying may also occur
here. A
CA 02951542 2016-12-07
WO 2015/195382 55 PCT/US2015/034592
pre-preparation chamber (1530) is in communication with the preparation
chamber 1510
with a third powered transport mechanism (1540) to convey the processed
organic-carbon-
containing feedstock in a heated environment to dry the feedstock. A channel
(1550) is
used to funnel hot combustion gases from the hot box into the pre-preparation
chamber to
assist in part or all of this drying.
Another embodiment of the invention involves a process for converting a carbon-
containing compound to product gas fuel and solid char fuel. The process
comprises at
least four steps. The first step is inputting processed organic-carbon-
containing feedstock
into a substantially horizontal sublimating reaction chamber largely contained
within a hot
box and configured to be able to heat from an ambient temperature to an
operating
sublimation temperature, operate at a sublimation temperature, and cool from
an operating
sublimation temperature to an ambient temperature without leaking any hot
product gas
fuel from the reaction chamber into the hot box or atmosphere, or leaking any
oxygen
from outside the hot box into the hot box. The second step is heating
processed organic-
carbon-containing feedstock to a sublimating temperature before it is able to
form a liquid
phase. The third step is maintaining the temperature at a sublimation
temperature for a
residence time that is as long a time as needed to convert the carbon-
containing feedstock
to product gas fuel and solid char fuel. The fourth step is separating the
product gas fuel
from the solid char fuel.
Heat generated by the process may be used in various ways. Some embodiments
may use direct heated combustion gases from the hot box to a pre-preparation
chamber to
dry the processed organic-carbon-containing feedstock before it enters a
preparation
chamber for compression, if needed, and a sublimation chamber. Some
embodiments may
use the heat for other purposes such as heating buildings.
Heat used to sublimate the feedstock may be supplied by combusting part of the
product fuel gas. Sublimation temperatures can be maintained with a small
fraction of the
product gas fuel being used as fuel for burners as discussed above.
Vertical Sublimation Oxygen-Deficient Thermal Sub-System
The vertical sublimation oxygen-deficient thermal sub-system comprises three
elements, a vertical reaction chamber, a first powered transport mechanism,
and a self-
adjusting seal. The first, at least one substantially vertical reaction
chamber, is configured
to heat the processed organic-carbon-containing feedstock without external
catalyst or
CA 02951542 2016-12-07
WO 2015/195382 56 PCT/US2015/034592
additional water, carbon dioxide, or carbon monoxide, to an operating
sublimation
temperature in a time frame that is short enough to sublime at least part of
the processed
organic-carbon-containing feedstock without creating substantially any liquid.
The
second, the first powered transport mechanism, is located partly within the
reaction
chamber, has an extended part that extends outside the reaction chamber, and
is configured
to convey sublimation products of the processed organic-carbon-containing
feedstock
through the reaction chamber as the processed organic-carbon-containing
feedstock is
transformed into biogas and processed biochar. The third, the self-adjusting
seal, is
configured to continuously contain the processed biogas within the reaction
chamber at the
region surrounding the extended part of the powered transport mechanism during
changing
temperatures of startup and shutdown operations, and during steady-state
sublimation
temperature during operation.
To better understand this sub-system, the vertical sublimation sub-system will
be
discussed with reference at times to a particular embodiment or embodiments.
However,
it is understood that other embodiments may be used as long as they perform
the
sublimation desired.
The vertical sublimation oxygen-deficient thermal sub-system is designed for
processing high-density feedstock, like tires, plastic, wood, and coal. High
density means
that the feedstock has a high weight per unit volume. Feedstock preparation
will be
dictated by characteristics of the processed organic-carbon-containing
feedstock such as
size or thickness, and density of the processed organic-carbon-containing
feedstock from
the beneficiation sub-system. In general the desirable size or thickness is on
the order of
less than 0.5 inch (13 mm) in the longest dimension of the particle. The
particle size is
important in the vertical sub-system because denser materials take more time
to heat
thoroughly from the particle surface to its internal midpoint. Volatile gases
are formed at
the midpoint or center of the particle and have to travel to the surface of
the particle where
they are released into the reaction chamber environment. The sublimed gas
should be
created as quickly as possible and stay in the gas phase at all times for best
conversion of
the processed organic-carbon-containing feedstock into processed biogas and
processed
biochar. A high density feedstock allows the particles to fall through the
reaction chamber
and reach the bottom where they are eventually separated in to the gas and
solid forms. At
times, the processed organic-carbon-containing feedstock may have to be
further
CA 02951542 2016-12-07
WO 2015/195382 57 PCT/US2015/034592
compressed to achieve desired density and further manipulated to achieve
desired particles
sizes.
After the processed organic-carbon-containing feedstock is properly prepared,
it is
conveyer to the top of the vertical sub-system by such as, for example, an
auger or some
other material conveying device. During the transportation of the feedstock,
heat may be
recycled from downstream processes to maximize removal of any free water. Then
the
feedstock is deposited into a hopper of a compression auger. The compression
auger
reduces the free water and entrained air content. This will increase the heat
absorption by
the feedstock and reduce the amount of oxygen present. Reducing the oxygen
content that
comes from the water and air will result in less carbon dioxide and carbon
monoxide in the
produced biogas and less contaminants in the processed biochar. A feedstock
plug or seal
is created at the end of the compression screw at the entrance point to the
reaction
chamber. Thus, when the feedstock enters the reaction chamber, the produces
biogas that
is created does not travel back and escape to create a hazardous situation.
As the feedstock enters the reaction chamber, the feedstock is immediately
subjected to a stream of superheated gas that sublimes the volatiles from the
feedstock. As
the volatilized gas and the devolatized feedstock, now reduced to carbon,
falls the length
of the reaction chamber tube, the volatilized gas and the carbon solid
intermingle, react,
and gain momentum. At the bottom of the shared common reaction chamber tube,
the
devolatilized carbon drops down into a collection hopper and the gas stream is
split into
two streams that move laterally over and up two reaction chamber tubes on
either side of
the common down tube. The reaction chamber looks like two of the letters "0"
that are
connected in the middle. The two up tubes of the reaction chamber now carry
the hot gas
upward and assisted by a turbine fan. Two-thirds of the way up each of the two
up-tubes
is a super-heater that raises the temperature of the gas. It is more
economical to super heat
just the gas than to heat the incoming feedstock. The super heated gas is now
reaching the
top of the up reaction chamber tubes and is directed from the top of each up
tube, laterally,
over to the top of the shared common down tube where the entrance of the
feedstock is
located. The super heated gas then is used to sublime the incoming feedstock
and
everything repeats itself in the down tube in a closed loop cycle. When the
tubes in the
reaction chamber are in equilibrium and balanced, the produced biogas is
pulled through
an outlet at the top of one of the upward reaction tubes, cooled, and stored
as processed
CA 02951542 2016-12-07
WO 2015/195382 58 PCT/US2015/034592
biogas. The carbon exits the bottom of the common middle tube and is
transported by
auger, cooled, and collected for storage as processed biochar.
The reaction chamber in the sub-system is a three dimensional, rectangular box
with the longest side perpendicular to the ground. On the topside is a
compression screw
and feedstock entrance port. On the bottom side is a collection cone with an
exit auger at
the bottom of the cone for produced biochar.
Inside the reaction chamber heater box are three connected and continuous
tubes
with the middle tube shared between the two outside tubes such that the three
tubes act as
one tube. The middle tube acts as a down draft while the two outside tubes act
as updrafts.
In this configuration, the feedstock enters at the top of the middle tube and
free falls as the
feedstock traverses the length of the tube. This is where the sublimation of
the feedstock
occurs. There is a junction at the bottom of the middle tube where the tube
makes two
lateral splits. At the end of each lateral split, a tube continues up on both
sides of the
middle tube. Thus, all three reaction chamber tubes are continuous and sealed
so that the
reaction chamber remains isolated from outside contaminants and only contains
the
feedstock that is to be processed. No external air, steam, or catalyst is
introduced.
On the outside of the reaction chamber, but connected to it, are two burners
that
heat the space between the inside of the outside box wall and the outside of
the inside wall
of the internal tube configuration. This space is heavily insulated and keeps
the reaction
chamber environment at a minimum temperature.
In operation, the sublimated feedstock at the bottom of the middle downdraft
tube
of the reaction chamber has separated into a devolatilized carbon and
processed biogas.
The stream of processed biogas splits and travels laterally to the outside
updraft reaction
chamber tubes. The devolatilized carbon settles into the collection cone and
is removed
by an auger. The devolatilized carbon is still in the heated reaction chamber
environment
so this acts as a polishing step to make sure all of the volatile gases that
can be created will
be captured and continue in the subliming process through the reaction chamber
updraft
tubes. After some residence time, the carbon can be passed through an auger
into a
cooling chamber and then stored as processed biochar. Residence time depends
on the
nature and volume of the processed organic-carbon-containing feedstock. In
some
embodiments, the residence time is less than 10 minutes, in some less than 7
minutes, in
some less than 5 minutes, in some less than 3 minutes, and in some less than 2
minutes.
CA 02951542 2016-12-07
WO 2015/195382 59 PCT/US2015/034592
At the split at the bottom of the middle downdraft tube the carbon drops out
and
only the processed biogas continues to travel laterally to the outside updraft
tubes of the
reaction chamber. The product processed biogas travels up the updraft tubes
carried by
their own inertia from traversing the downdraft tube with some optional
assistance by a
turbine fan placed at the top of the updraft tubes. Attached on the outside
wall of both
updraft tubes but still inside of the external wall of the reaction chamber
box is laced one
super heater on each outside tube. During startup, as the processed biogas
traverses the
updraft tube back to the top of the top part of the reaction chamber tubes, it
passes through
the super heaters and the temperature in the reaction chamber is increased to
a preselected
temperature that is the desired equilibrium temperature. It is more economical
to super
heat just the processed biogas than the input processed organic-carbon-
containing
feedstock. The superheating assists in the further dissociation of the
processed biogas
when it comes in contact with the devolatilized carbon in the downdraft tube.
As the two superheated processed biogas streams reach the top of the two
outside
tubes, they are comingled with the fresh incoming feedstock as it enters the
middle
downdraft tube and sublime that feedstock. The cycle of the fresh feedstock
coming into
the reaction chamber, the fresh feedstock mixing with the superheated
processed biogas
and the mixture entering the reaction chamber tubes completes the reaction
processing
cycle. When the reaction reaches equilibrium and balance, more feedstock is
added and
both processed biochar and processed biogas is removed according to
predetermined
production rates.
Figure 17 is a diagram of a side view of an embodiment of a system with a
substantially vertical reaction chamber. This system (1600) has a compression
feed
system (1610) that is in communication with a compression auger (1620).
Processed
organic-carbon-containing feedstock follows a double circular path (1630) with
circulating
processed biogas (1640) a reaction chamber (1632) that is more clearly
illustrated in Fig.
17A. Reaction chamber 1632 is in a hot box (1665). A bypass (1645) directs
some
overflow heat from hot box 1665 to preheat the incoming processed organic-
carbon-
containing feedstock in compression feed system 1610. Heat exchangers 1650
within hot
box 1665 super heat circulating processed biogas 1640 to achieve and maintain
the target
subliming temperature. A carbon auger (1660) removes the processed biochar.
The rest
CA 02951542 2016-12-07
WO 2015/195382 60 PCT/US2015/034592
of the overflow heated gas leaves hot box 1665 through a heater exhaust exit
(1670) and
processed biogas passes through a processed biogas exhaust exit (1680).
Figure 17A is a diagram of tube array of the embodiment shown in Fig. 17. A
reaction chamber (1632) is depicted with a tube array comprising a middle
downdraft
reaction tube (1634) bracketed by two outer updraft reaction tubes (1636)
within hotbox
1665. Heat exchangers 1650 heat outer updraft reaction tubes 1636 below the
cross tee
and the input downdraft tube 1634 above the cross tee. Excess hot gas leaves
through
exhaust exit 1670 and processed biogas leaves through outlet 1680 in reaction
chamber
1632.
Microwave Sub-System
The microwave sub-system is another form of the heating sub-system that is
used
to convert the processed organic-carbon-containing feedstock from the
beneficiation sub-
system into a processed biochar that is subsequently made into a high energy
processed
biomass / coal blended compact aggregate of the invention. The sub-system
comprises a
processed biochar composition made from a processed organic-carbon-containing
feedstock that passes through a microwave process sub-system. The sub-system
includes
at least one reaction chamber within a microwave reflective enclosure and
comprising at
least one microwave-transparent chamber wall and a reaction cavity configured
to hold the
processed organic-carbon-containing feedstock in an externally supplied oxygen-
free
atmosphere. A microwave sub-system includes at least one device configured to
emit
microwaves when energized. The microwave device is positioned relative to the
reaction
chamber so that the microwaves are directed through the microwave-transparent
chamber
wall and into the reaction cavity. The sub-system also includes a mechanism
that provides
relative motion between the microwave device and the reaction chamber. The
processed
biochar composition includes substantially no free water. Also the processed
biochar
composition includes a number of pores per volume that is at least 10 percent
more than
would have been in a char made with the same feedstock but using a thermal
process that
creates a liquid phase during the process. The characteristics of the
feedstock and
resulting processed biochar have already been discussed above. The microwave
process
used to make the processed biochar of the invention is now discussed.
In the following description of the illustrated embodiments, references are
made to
the accompanying drawings that help to illustrate various embodiments of the
microwave
CA 02951542 2016-12-07
WO 2015/195382 61 PCT/US2015/034592
process used to make the processed biochar of the invention. It is to be
understood that
other embodiments of the process may be utilized and structural and functional
changes
may be made without departing from the scope of the present invention.
The following description relates to approaches for processing solid and/or
liquid
organic-carbon-containing feedstock into fuels, e.g., diesel fuels, gasoline,
kerosene, etc.,
by microwave enhanced reaction deconstruction processes.
Deconstruction, also referred to as "cracking", is a refining process that
uses heat
to break down (or "crack") hydrocarbon molecules into shorter hydrocarbon
chains which
are useful as fuels. Deconstruction may be enhanced by adding a catalyst to
the feedstock
which increases the speed of the reaction and/or reduces the temperature
and/or the
radiation exposure required for the processes. Furthermore, the catalyst, such
as zeolite,
has a nanostructure which allows only molecules of a certain size to enter the
crystalline
grid or activate the surface areas of the catalyst and to interact with the
catalyst. Thus, the
catalyst advantageously is very effective at controlling the product produced
by the
reaction processes because only substances having a specified chain length may
be
produced using the catalytic process. Catalytic deconstruction is particularly
useful for
transforming biomass and other organic-carbon-containing feedstock into fuels
useable as
transportation or heating fuels.
One aspect of efficient deconstruction is the ability to heat and irradiate
the
feedstock substantially uniformly to the temperature that is sufficient to
cause
deconstruction as well as activate the catalyst. Upon deconstruction, long
hydrocarbon
chains "crack" into shorter chains. Microwave heating has been shown to be
particularly
useful in heating systems for thermal deconstruction. Heating systems such as
flame,
steam, and/or electrical resistive heating, heat the feedstock by thermal
conduction through
the reaction chamber wall. These heating systems operate to heat the feedstock
from the
outside of the reaction chamber walls to the inside of the feedstock, whereas
microwaves
heat uniformly throughout the width of the reaction chamber. Using non-
microwave
heating sources, the heat is transferred from the heat source outside wall to
the inside of
the vessel wall that is in direct contact with the feedstock mixture. The heat
is then
transferred to the surfaces of the feedstock and then transferred, again,
through the
feedstock until the internal areas of the feedstock are at a temperature near
the temperature
of the reaction chamber wall.
CA 02951542 2016-12-07
WO 2015/195382 62 PCT/US2015/034592
One problem with this type of external heating is that there are time lags
between
vessel wall temperature transmission and raising the feedstock temperature
that is
contained in the center of the vessel as well as the internal area of the
feedstock matrix.
Mixing the feedstock helps to mitigate these conditions. Still, millions of
microenvironments exist within the reactor vessel environment and the
feedstock particles
themselves. This causes uneven heat distribution within the reaction chamber
of varying
degrees. These variant temperature gradients cause uncontrollable side
reactions to occur
as well as degradation of early conversion products that become over-reacted
because of
the delay in conversion reaction timeliness. It is desirable to produce and
retain consistent
heating throughout the feedstock and the reaction products so that good
conversion
economics are achieved and controllable. Microwave heating is an efficient
heating
method and it also serves to activate catalytic sites.
Embodiments of the invention are directed to a reaction chamber system that
can
be used to process any organic-carbon-containing feedstock, whether solid
and/or liquid,
to extract the volatile organic compounds in the feedstock at a temperature
range that will
produce liquid transportation fuels.
Microwaves are absorbed by the water molecules in the material that is
irradiated
in the microwave. When the water molecules absorb the microwaves, the
molecules
increase their vibrorotational motions, which create heat by friction, and the
heat is
convected to the surrounding material. The reason microwaves are absorbed by
water
molecules is specific to the covalent bonds that attach the hydrogen to the
oxygen in a
water molecule. The oxygen atom in water has a large electronegativity
associated with it.
Electronegativity for an element is its propensity to collect extra electrons,
either
completely in an ionic bond or through skewing the electron cloud of a
covalent bond
toward that element. The driving force is from quantum chemistry, namely the
filling of
the 2p shell of oxygen from the addition of 2 electrons. The electronegativity
scale, driven
by the stability of filling the outer electron shell, starting at the most
electronegative
element, is F>0>N>C1>Br>S>C>H. Therefore, the valence electrons in water are
skewed
toward the oxygen, creating a permanent electric dipole moment with the
negative pole
toward the oxygen and the positive pole between the two hydrogen atoms. The
electrons
from the two hydrogen atoms are drawn closer to the oxygen atom. This gives
this end of
the molecule a slight negative charge and the two hydrogen atoms then have a
slight
CA 02951542 2016-12-07
WO 2015/195382 63 PCT/US2015/034592
positive charge. The consequence of this distortion is that the water molecule
possesses a
permanent electric dipole. The dipole feature of the water molecule allows the
molecule
to absorb the microwave radiation and increases the rotational speed of
gaseous water
molecules and/or increases the low frequency vibrational movements associated
with
frustrated rotations of the extended structure of liquid water. The increased
motion of the
water molecules causes friction that turns to heat and then convects out into
the irradiated
material.
To take advantage of this feature of microwave radiation, a reaction chamber
system described herein takes advantage of microwave irradiation and heating
in
processing feedstock that contains carbon and can be converted to
transportation fuels.
The reactor may be made from a substantially microwave transparent substance
such as
quartz, a crystalline material that is substantially transparent to microwave
radiation.
Because quartz can be manipulated into many shapes, it provides design
discretion for
shaping the reaction chamber, but in one example the reaction chamber is
configured in
the shape of a tube or cylinder. The cylindrical shape allows for the
feedstock to feed in
one end and exit at the opposite end. An example of a suitable reaction
chamber would be
a quartz tube that is about four feet (1.2 meters) long with a wall thickness
of about 3/16
inch (4.8 mm).
The microwave reaction chamber is surrounded by a microwave reflective
enclosure. This causes the microwave radiation to pass repeatedly through the
reaction
chamber and devolatize the organic-carbon-containing feedstock after the
water, if
present, is evaporated and driven off The microwave reflective enclosure is
any that
reflects microwaves. Materials include, for example, sheet metal assembled as
Faraday
cages that are known to the art.
Microwave radiation is generated by a magnetron or other suitable device. One
or
more microwave producing devices, e.g., magnetrons can be mounted external to
the
quartz tube wall. Magnetrons come in different power ranges and can be
controlled by
computers to irradiate the processing feedstock with the proper power to
convert the
feedstock to most desirable fuel products efficiently, given the residence
time in the
reactor. In one application, the magnetron can be mounted on a cage that would
rotate
around the outside of the reactor tube as well as travel the length of the
reactor tube.
Feedstock traveling through the length of the inside of the tube will be
traveling in a plug
CA 02951542 2016-12-07
WO 2015/195382 64 PCT/US2015/034592
flow configuration and can be irradiated by fixed and/or rotating magnetrons.
A computer
may be used to control the power and/or other parameters of the microwave
radiation so
that different feedstock, with different sizes and densities can be irradiated
at different
parameter settings specific to the feedstock and thus convert the feedstock
more
efficiently.
These configurations of a reactor will allow efficient processing of
feedstock, from
relatively pure feedstock streams to mixed feedstock streams that include
feedstock of
different densities, moisture contents, and chemical makeup. Efficiencies can
occur
because the fuel products are extracted from the reactor chamber as they are
vaporized
from the feedstock, but further processing of the remaining feedstock occurs
until different
fuel products are vaporized and extracted. For example, dense feedstock, such
as plastics,
take longer to process into a useable fuel than less dense feedstock, such as
foam or wood
chips. The microwave sub-system described herein continues to process dense
feedstock
without over-processing the earlier converted products from the less dense
feedstock. This
is accomplished by using both stationary and rotating microwave generators.
One example of a mixed feedstock would be unsorted municipal solid waste. In
some implementations, catalyst may be added in the feedstock which helps in
the
conversion of the feedstock as well as the speed at which the conversion can
progress. A
catalyst can be designed to react at the preset processing temperature inside
the reactor or
to react with the impinging microwave radiation. In some embodiments, no
catalyst is
required. In other embodiments, the catalyst may be a rationally designed
catalyst for a
specific feedstock.
The plug flow configuration with the reactors described herein will allow
adjustments to the residence time that the feedstock resides within the
reactor core for
more efficient exposure to the heat and the radiation of the microwaves to
produce the
desired end products.
Inlets and/or outlets, e.g., quartz inlets and/or outlets can be placed along
the walls
of the reaction chamber to allow for pressure and/or vacuum control. The
inlets and
outlets may allow the introduction of inert gases, reactive gases and/or the
extraction of
product gases.
Thus, the design of the microwave-transparent reaction chamber, the use of
microwaves as a heating and radiation source with fixed and/or rotating
magnetrons, plug
CA 02951542 2016-12-07
WO 2015/195382 65 PCT/US2015/034592
flow processing control, with or without the use of catalysts, will allow the
processing of
any organic-carbon-containing feedstock. An advantage to beneficiating the
organic-
carbon-containing feedstock is that it has, to a large extent, already been
brought to an
acceptable moisture level and is already fairly homogeneous. For homogeneity
on the
macro-scale, the output from different organic-carbon-containing feedstock
inputs have
substantially similar characteristics (e.g. energy density, consistency,
moisture content),
and these characteristics extend throughout the material. On, the molecular
scale, with
fewer salts present, there are fewer microenvironments where the microwaves
would
deposit energy differently than in the bulk of the organic-carbon-containing
feedstock.
Therefore, the heating would be more uniform from beneficiated organic-carbon-
containing feedstock than from raw unprocessed organic-carbon-containing
feedstock
inputs.
A microwave sub-system in accordance with embodiments of the invention
includes a reaction chamber having one or more substantially microwave-
transparent walls
and a microwave heating/radiation system. The microwave heating/radiation
system is
arranged so that microwaves generated by the heating/radiation system are
directed
through the substantially microwave-transparent walls of the reaction chamber
and into the
reaction cavity where the feedstock material is reacted without substantially
heating the
walls of the reaction chamber. To enhance the temperature uniformity of the
feedstock,
the reaction chamber and the heating/radiation system may be in relative
motion, e.g.,
relative rotational and/or translational motion. In some implementations, the
heating
system may rotate around a stationary reaction chamber. In some
implementations, the
feedstock within the reaction chamber may rotate by the use of flights with
the
heating/radiation system remaining stationary. In some implementations, the
reaction
chamber may rotate with the heating system remaining stationary. In yet other
implementations, both the reaction chamber and the heating/radiation system
may rotate,
e.g., in countercurrent, opposing directions. To further increase temperature
uniformity,
the system may include a mechanism for stirring and/or mixing the feedstock
material
within the reaction chamber. The reaction chamber may be tilted during
reaction process,
for example, to force the feedstock to go through the catalytic bed.
Figures 18A and 18B illustrate side and cross sectional views, respectively,
of a
microwave sub-system (1800) for converting organic-carbon-containing feedstock
to
CA 02951542 2016-12-07
WO 2015/195382 66 PCT/US2015/034592
liquid fuel and processed biochar fuel in accordance with embodiments of the
invention.
Although a reaction chamber (1810) may be any suitable shape, reaction chamber
1810 is
illustrated in Figures 18A and 18B as a cylinder having a cylindrical wall
(1811) that is
substantially transparent to microwaves in the frequency range and energy used
for the
reaction process. Reaction chamber 1810 includes a reaction cavity (1812)
enclosed by
cylindrical wall 1811. Microwave sub-system 1800 includes a transport
mechanism
(1818) configured to move the feedstock through the reaction chamber. The
operation of
microwave sub-system 900 with regard to the reactions taking place within
reaction
chamber 1810 may be modeled similarly to that of a plug flow reactor.
As illustrated in Figure 18A, a microwave sub-system includes transport
mechanism 1818 for moving the feedstock material through reaction chamber
1810.
Transport mechanism 1818 is illustrated as a screw auger, although other
suitable
mechanisms, e.g., conveyer, may also be used. Transport mechanism 1818 may
further
provide for mixing the feedstock within the reaction chamber. In some
embodiments,
reaction chamber wall 1811 may have a thickness of about 3/16 inch (4.8
millimeters).
The smoothness of reaction chamber wall 1811 facilitates the movement of the
feedstock
through reaction chamber 1810.
A heating/radiation subsystem (1815) may include any type of heating and/or
radiation sources, but preferably includes a microwave generator (1816) such
as a
magnetron which is configured to emit microwaves (1813) having a frequency and
energy
sufficient to heat the organic-carbon-containing feedstock to a temperature
sufficient to
facilitate the desired reaction of the feedstock, for example, for
deconstruction of the
feedstock, microwaves in a frequency range of about 0.3 GHz to about 300 GHz
may be
used. For example, the operating power of the magnetrons may be in the range
of about 1
Watt to 500 kilowatts. Magnetron 1816 is positioned in relation to reaction
chamber 1810
so that microwaves 1813 are directed through wall 1811 of reaction chamber
1810 and
into reaction cavity 1812 to heat and irradiate the material therein. A
mechanism (1817)
provides relative motion between magnetron 1816 and reaction chamber 1810
along
and/or around longitudinal axis 1820 of reaction chamber 1810. In some
embodiments,
mechanism 1817 may facilitate tilting reaction chamber 1810 and/or magnetron
1816 at an
angle 0 (see Figure 18C) to facilitate the reaction of the feedstock and/or
the extraction of
gases, for example. In the embodiment illustrated in Figures 18A-C, magnetron
916 is
CA 02951542 2016-12-07
WO 2015/195382 67 PCT/US2015/034592
positioned on rotational mechanism 1817, such as a rotatable cage or drum that
rotates
magnetron 1816 around stationary reaction chamber 1810. In some
implementations, the
rotation around the chamber may not be complete, but the rotation path may
define an arc
around the circumference of the reaction chamber. The rotation may occur back
and forth
along the path of the arc. As previously mentioned, in some embodiments,
reaction
chamber 1810 may be the rotating component, or both magnetron 1816 (also
called the
heating/radiation subsystem) and reaction chamber 1810 may rotate, e.g., in
opposing,
countercurrent directions. The rotation between the reaction chamber and the
magnetron
provides more even heating and more even microwave exposure of the feedstock
within
reaction cavity 1812, thus enhancing the efficient reaction chemistry of the
feedstock
and/or other processes that are temperature/radiation dependent, such as
removal of water
from the feedstock. The rotation lessens the temperature gradient and/or
maintains a more
constant microwave flux across the plug inside the reaction chamber.
Reaction chamber 1810 may include one or more entry ports (1820), e.g., quartz
entry ports, configured to allow the injection or extraction of substances
into or out of
reaction cavity 1812. Reaction chamber 1810 is also surrounded by a microwave-
reflective enclosure (1822). In one implementation, the quartz ports may be
used to
extract air and/or oxygen from the reaction cavity. Extraction of air and/or
oxygen may be
used to suppress combustion which is desirable for some processes.
For example, in certain embodiments, microwave sub-system 1800 may be used to
preprocess the feedstock through compression and/or removal of air and/or
water. In this
application, gases such as hydrogen and/or nitrogen may be injected through
one or more
ports 1820 to hydrogenate and/or suppress combustion of the feedstock.
Reaction
chamber 1810 may also include one or more exit ports (1821), e.g., quartz exit
ports,
configured to allow passage of water, water vapor, air, oxygen and/or other
substances
and/or by-products from reaction chamber 1810. In other embodiments, the
processed
organic-carbon-containing feedstock is already sufficiently compressed and
reduced in
both air and water to be introduced directly into the reaction chamber.
Figure 18D is a diagram illustrating a microwave-sub-system (1850) for
producing
fuel from organic-carbon-containing feedstock in accordance with embodiments
of the
invention. Microwave sub-system 1850 includes an input hopper (also referred
to as a
load hopper) 18951) configured to allow introduction of the feedstock material
into
CA 02951542 2016-12-07
WO 2015/195382 68
PCT/US2015/034592
microwave sub-system 1850. A gearmotor auger drive (1852) provides a drive
system for
the auger (1853) that transports the feedstock through microwave sub-system
1850. As
the feedstock is compressed in load hopper 951, air is extracted through an
atmosphere
outlet (1854). A seal (1855) isolates load hopper 1851 from a reaction chamber
(1856) to
maintain a level of vacuum. Reaction chamber 1856 includes walls of a
microwave-
transparent material. One or more stationary microwave heads 1857 are
positioned at the
walls of the reaction chamber 1856. In addition, microwave sub-system 1850
includes one
or more rotating microwave heads (1858). In one implementation, each rotating
microwave head is located at a fixed position with respect the longitudinal
axis (1860) of
reaction chamber 1856. The rotating microwave head is mounted on a slipring
bearing
(1859) which allows microwave head 958 to rotate around reaction chamber 1856.
A
microwave reflective enclosure (1862) encompasses reaction chamber 1856. In
some
implementations rotating microwave head(s) 958 may rotate around the
longitudinal axis
1860 of the reaction chamber 1856 as well as moving back and forth along the
longitudinal axis 1860. Microwave sub-system 950 includes a seal at the exit
of reaction
chamber 1856 to maintain the reaction chamber vacuum. In some embodiments, the
organic-carbon-containing feedstock is compressed in the sub-beneficiation
system
discussed earlier before it enters the microwave sub-system and little if any
air extraction
is needed.
Figure 19A is a block diagram of a microwave sub-system 1900 that uses one or
more of the reaction chamber illustrated in Figures 18A and 18B. The reaction
chamber
1920, 1930 may be arranged and/or operated in series or in a parallel
configuration. An
extraction process (1920) and a reaction process (1930) depicted in Figures
19A and 10B
are illustrated as occurring in two separate reaction chambers, e.g., that
operate at different
temperatures. Alternatively, the extraction process and the reaction process
may be
implemented in a single reaction chamber with two separate zones, e.g., two
separate
temperature zones.
In microwave sub-system 1900 of Figure 19, one or both of water/air extraction
section 1920 and reaction section 1930 may be similar to the reaction chamber
in
microwave sub-system 1800 of Figures 18A and 18B. Organic-carbon-containing
feedstock, such as, for example, one or more of manure containing plant cells,
wood chips,
and plant-based cellulose, enters the microwave sub-system through a hopper
(1911), and
CA 02951542 2016-12-07
WO 2015/195382 69
PCT/US2015/034592
traverses an airlock (1912) to enter a feedstock preparation module (1913). If
needed, a
catalyst, such as zeolite, and/or other additives that enhance the reaction
process, for
example to adjust the pH, may be introduced into microwave sub-system 1000
through
input hopper 1911 and/or the entry ports (shown in Figure 18B). In the
feedstock
preparation module 1913, the feedstock material may be shredded to a
predetermined
particle size that may be dependent on the properties of the feedstock, such
as the purity,
density, and/or chemical composition of the feedstock. If used, the catalyst
may be added
at the time that the feedstock is being prepared so that the catalyst is
evenly dispersed
within the feedstock material before entering a reaction chamber (1931). In
general, the
less uniform the feedstock, the smaller the particle size needed to provide
efficient
reaction.
After the initial feedstock preparation stage, the shredded and mixed
feedstock is
transported by a transport mechanism 1915 into the extraction chamber 1921 of
the next
stage of the process. An air/water extraction subsystem (1920), which performs
the
optional processes of water and/or air extraction prior to the reaction
process, includes a
heating/radiation module (1922) comprising at least a magnetron (1923)
configured to
generate microwaves (1926) that may be mounted on a rotational or stationary
mechanism
(1927). If mounted on a rotational mechanism, the mechanism rotates magnetron
1923
either partially or fully around extraction chamber 1921 as microwaves 1926
are directed
through a wall (1924) of extraction chamber 1921 and into an extraction cavity
(1925)
impinging on and heating the feedstock therein. In some embodiments, heating
module
1922 may utilize only one magnetron 1923 or only two or more magnetrons
without using
other heat/radiation sources.
In some embodiments, heating/radiation module 1922 may utilize magnetron 1923
in addition to other heat sources, such as heat sources that rely on thermal
conduction
through the wall of the extraction chamber, e.g., flame, steam, electrical
resistive heating,
recycled heat from the process, and/or other heat sources. During the air
and/or water
extraction process, the feedstock may be heated to at least 100 C, the boiling
point of
water, to remove excess water from the feedstock. The excess water (e.g., in
the form of
steam) and/or other substances may exit extraction chamber 1921 via one or
more exit
ports. Additives to the feedstock, such as inert and/or reactive gases
including hydrogen
and/or nitrogen, may be introduced via one or more input ports into extraction
chamber
CA 02951542 2016-12-07
WO 2015/195382 70 PCT/US2015/034592
1921 of the water/air extraction process. In addition to being heated and
irradiated by
microwaves, the feedstock may also be subjected to a pressurized atmosphere
and/or a
vacuum atmosphere and/or may be mechanically compressed to remove air from
extraction chamber 1921.
After the optional air and/or water extraction process, transport mechanism
1915
moves the feedstock to the next processing stage, a reaction section (1930)
which involves
the reaction process, e.g., thermal deconstruction, of the feedstock. After
the
feedstock/catalyst mixture enters a reaction chamber (1931) surrounded by the
microwave
reflecting enclosure (1938), the mixture is heated to a temperature that is
sufficient to
facilitate the desired reaction. For example a temperature of in a range of
about 200 C to
about 350 C is used to crack the hydrocarbons in the feedstock into shorter
chains to
produce liquid fuel through deconstruction. In addition to being heated, the
feedstock may
also be subjected to a pressurized atmosphere or a vacuum atmosphere, and/or
may be
mechanically compressed in reaction chamber 1931.
In some embodiments, heating/radiation in the reaction chamber 1031 is
accomplished using a magnetron (1933) emitting microwaves (1936). Magnetron
1033
may rotate relative to reaction chamber 1031. As previously described in
connection with
the water extraction section 1920, a rotating magnetron (1933) may be
supported by
rotational mechanism (1937), such as a cage or drum. Rotational mechanism 1937
allows
relative rotational motion between magnetron 1933 and reaction chamber 1931.
For
example, magnetron 1933 may rotate completely around reaction chamber 1931 or
the
rotation of magnetron 1933 may proceed back and forth along an arc that
follows the
circumference of reaction chamber 1931. The rotating magnetron heating system
1933
may be supplemented using a stationary magnetron, and/or other conventional
heat
sources such as a flame or electrical resistive heating. Rotating magnetron
1933 provides
more even heating/radiation of the feedstock material and catalyst within a
reaction cavity
(1935) and enhances the heating properties over that of stationary heat
sources.
The cracked hydrocarbons vaporize and are collected in a condenser (1041) and
liquefy and then are sent to a distiller (1940) to produce the diesel fuel,
while heavier,
longer chain hydrocarbon molecules may be recycled back to the reaction
chamber. In
some implementations, distillation may not be necessary, and the fuel product
only needs
to be filtered.
CA 02951542 2016-12-07
WO 2015/195382 71 PCT/US2015/034592
In some configurations, it is desirable to control the processes of the
reaction to
allow a higher efficiency of fuel extraction from the feedstock. Figure 19B is
a block
diagram of a microwave sub-system (1905) that includes the sub-system
components
described in connection with Figure 19A along with a feedback control system
(1950).
The illustrated feedback control system 1950 includes a controller (1951) and
one or more
sensors (1952), (1953), (1954) which may be configured to sense parameters at
various
stages during the process. Feedback control system 1950 may include sensors
1952 at the
feedstock preparation stage which are configured to sense parameters of the
feedstock
and/or feedstock preparation process. For example, sensors 1952, may sense the
chemical
composition of the feedstock, density, moisture content, particle size, energy
content or
other feedstock parameters. Sensors 1952 may additionally or alternatively
sense the
conditions within the feedstock preparation chamber, e.g., flow, pressure,
temperature,
humidity, composition of the gases present in the chamber, etc. Sensors 1952
develop
signals (1955a) which are input to controller electronics 1951 where they are
analyzed to
determine the condition of the feedstock and/or the feedstock preparation
process. In
response to sensed signals 1955a, controller 1951 develops feedback signals
(1955b)
which control the operation of the feedstock preparation module (1913). For
example, in
some implementations, the controller 1951 may control feedstock preparation
module
1913 to continue to shred and/or grind the feedstock material until a
predetermined
particle size and/or a predetermined particle size variation is detected. In
another example,
based on the sensed chemical composition of the feedstock, controller 1951 may
cause a
greater or lesser amount of catalyst to be mixed with the feedstock or may
cause different
types of catalyst to be mixed with the feedstock.
A control system (1950) may also develop feedback signals (1956b), (1957b) to
control the operation of water extraction module 1920 and/or the reaction
module 1930,
respectively, based on sensed signals 1956a, 1957a. For example, the sensors
(1953),
(1954) may sense the temperature of the water extraction and/or reaction
processes and
controller 1951 may develop feedback signals 1956b, 1957b to control the
operation of
heating/radiation systems 1922, 1932, e.g., power, frequency, pulse width,
rotational or
translational velocity, etc. of one or both of magnetrons 1923, 1933.
Controller 1051 may
develop feedback signals to the magnetrons to control the amount of radiation
impinging
on the feedstock so that the feedstock will not be over-cooked or under-cooked
and
CA 02951542 2016-12-07
WO 2015/195382 72
PCT/US2015/034592
development of hot spots will be avoided. Controller system 1950 may control
the
injection of various substances into one or both of the extraction chamber
and/or the
reaction chamber 1921, 1931 through the entry ports to control the processes
taking place
within the chambers 1921, 1931. Biochar, the residue of the depleted
feedstock, is sent to
a storage unit. In some embodiments, controller system 1950 may be used to
control
conditions that beneficially affect the properties of the processed biochar
where specific
properties are desired beyond that resulting just from the feedstock choice.
After the
distillation stage, the heavy hydrocarbons may be recycled back into the
reaction chamber
and the lighter hydrocarbons may be sent on to a polymerization stage.
The reaction chambers may be made of quartz, glass, ceramic, plastic, and/or
any
other suitable material that is substantially transparent to microwaves in the
frequency and
energy range of the reaction processes. In some configurations, the
heating/radiation
systems described herein may include one or more magnetrons that rotate
relative to the
reaction chamber. In some embodiments, the magnetrons may be multiple and/or
may be
stationary. Figure 20A illustrates a reaction system (2000) which includes
multiple
stationary magnetrons (2011) arranged on a drum (2012) that acts as a Faraday
cage and is
disposed outside a cylindrical reaction chamber (2013) having one or more
microwave -
transparent walls. In reaction system 2000, the drums made of a material that
is
microwave opaque, such as, for example, metal, so as to cause the microwaves
in reaction
chamber 2013 to reflect back and forth through the feedstock, thus more
efficiently being
used to convert the feedstock into liquid renewable fuel and solid renewable
fuel biochar.
The operation of the magnetrons may be continuous, or may be pulsed, e.g., in
a
multiplexed pattern. In some embodiments (Figure20B), drum 2013 supporting
magnetrons 2011 may be rotated (2030) around the longitudinal axis (2050) of
reaction
chamber 2012 and/or reaction chamber 2012 may be rotated (2020) around its
longitudinal
axis 2050.
A feedstock transport mechanism may be disposed within a reaction chamber. For
example, as illustrated in Figure 20C, the feedstock transport mechanism may
comprise
one or more baffles (2061) that are configured to move the feedstock through a
reaction
chamber (2060) as the reaction chamber rotates. The baffles 361 may be mounted
to the
walls of reaction chamber 2060 and/or may be otherwise installed within the
reaction
CA 02951542 2016-12-07
WO 2015/195382 73 PCT/US2015/034592
chamber to provide movement of feedstock within and through reaction chamber
2060,
e.g., longitudinally through the reaction chamber.
In some embodiments, illustrated in Figure 21, one or more secondary heat
sources
(2150), such as a flame, steam, and/or electric resistive heating, or recycled
heat, may be
used in addition to magnetrons (2116) which are stationary, or are supported
on a
mechanism (2117) that rotates around the circumference of the reaction chamber
(2120)
enclosed in a microwave-reflecting Faraday cage (2121). In some
configurations,
magnetrons 2116 may not make a complete revolution around reaction chamber
2120, but
may rotate back and forth (2119) along an arc that follows the circumference
of reaction
chamber 2120. Various configurations are possible as long as the feedstock is
exposed to
substantially uniform heat throughout the mass of the feedstock particles to
form
processed biochar having pore density, distribution, and variance in size and
distribution
as described above for processed biochar of the invention.
Movement of the one or more magnetrons relative to the reaction chamber may
also include motion that moves the magnetron along the longitudinal axis of
the reaction
chamber, as illustrated in Figure 22. A reaction chamber (2210) and a cage
(2220) are
illustrated that support a magnetron (2230). Cage 2220 and magnetron 1330 may
be
moved (2240) back and forth along the longitudinal axis (2250) of reaction
chamber 2210
and over a metal microwave-reflecting Faraday cage (2215) enclosing reaction
chamber
2210. In some implementations, in addition to and/or concurrent with the
motion (2240)
of cage 2220 and magnetron 2230 along longitudinal axis 2250, cage 22320, and
magnetron 2230 may be rotated (2260) around the longitudinal axis 2250.
Blending Sub-System
The blending sub-system is used to size the coal particles and processed
biomass,
mix the coal particles with the processed biomass, pulverize the blend, and
compact the
blend into a high energy processed biomass / coal blended compact aggregate
that, for
example, is suitable for use in a coal combustion apparatus such as many
electricity-
producing power plants that combust coal. The blending sub-system first
comprises one
or more sizing chambers to separately or together size coal and processed
biomass into
suitable sized particles for subsequent blending. Because coal and biomass
powder have a
potential to be explosive, chambers that handle them may have oxygen-deficient
atmospheres. Any chunks of coal are reduced to the size of fines in an oxygen-
deficient
CA 02951542 2016-12-07
WO 2015/195382 74 PCT/US2015/034592
atmosphere, if necessary to prevent any danger of explosions. Coal particles
and biomass
particles may be sized to the proper dimensions and mixed at the same time as,
for
instance, in a high speed vortex. Similar sized particles of coal and
processed biomass are
easier to mix into subsequent aggregates that are substantially uniform. In
some
embodiments a suitable size is on the order of particles being able to pass
through an 8
mesh size with square holes of 0.097 inches (2.380 mm). Some embodiments have
particles able to pass through a finer screen such as a 16 mesh screen with
square holes of
0.0469 inches (1.190 mm) on a side. Similarly, oxygen-deficient atmospheres
may be
used in the blending system as needed to prevent explosions from high
concentration s of
processed biomass and coal dust or fines. Next the sized particles of coal and
processed
biomass is combined in a blending chamber that is configured to combine
properly sized
particles of high energy coal with processed biomass into a blended powder of
a
predetermined ratio of high energy coal to processed biomass. Then the blend
passes to a
compacting chamber that is configured to compress the blended powder into high
energy
blended compact aggregates. Finally, the high energy processed biomass / coal
blended
compact aggregate is collected in a collection chamber. In some embodiments,
the
processed biomass and coal are mixed and simultaneously sized and blended in a
device,
such as for example, a high speed vortex in the blending and pulverizing unit
followed by
remixing as necessary with high energy biomass binder.
In some embodiments, the pelletizing sub-system further comprises a heating
chamber configured to apply sufficient heat to the processed organic-carbon-
containing
feedstock to reduce its water content to less than 10% by weight and to form
the high
energy processed biomass / coal blended compact aggregates. In some
embodiments, the
compression chamber and the heating chamber are the same chamber.
In some embodiments, the vapor explosion section of the beneficiation sub-
system
further comprises a wash element that is configured to remove and clean micro
particles of
unprocessed organic-carbon-containing feedstock, lignin fragments, and
hemicellulosic
fragments from the vapor explosion section into a fine, sticky mass of biomass
with high
lignin content that is a high energy processed biomass binder. In this
embodiment, the
blending chamber of the blending subsection is further configured to receive
the high
energy binder to permit at least one of lower temperatures or less if any
additional high
energy processed biomass binder content in a compaction chamber formation
during
CA 02951542 2016-12-07
WO 2015/195382 75
PCT/US2015/034592
formation of blended compact aggregates. In another embodiment, another binder
such as
corn starch may be added in lieu of or in combination with the high lignin
micro particles
from the vapor explosion section to the compression chamber to lower the
temperature
needed to produce viable pellets.
Figure 23 is a diagram of a system to make high energy processed biomass /
coal
blended compact aggregate from unprocessed organic-carbon-containing feedstock
with
the addition of micro particle and lignin slurry that is optional. In this
embodiment, the
unprocessed organic-carbon-containing feedstock, untreated biomass input, is
sized
(2310), then passed through beneficiation reaction chamber where the fibers
are disrupted
(2320), the salt is solubilized and the feedstock is then washed (2330).
During this step,
the effluent containing micro particles and lignin is removed (2340), washed
and
introduced to the processed organic-carbon-containing feedstock in a remixing
step (2360)
after it has gone through a dewatering and desolvating step (2350). Coal and
coal dust or
fines (2352) is similarly sized (2354), blended with processed biomass in a
blending and
pulverizing unit (2356), and remixed (2360) as necessary with high energy
biomass
binder. The mixture is then compacted in to aggregates or briquettes (2370)
and collected
(2380). The use of the washed effluent stream of high energy biomass may also
serve to
reduce the need for heat to form the blended aggregates although heat still
may be
advantageous to remove additional water.
Processes
The invention also comprises a process for making a high energy processed
biomass / coal blended compact aggregate that comprises at least 10 wt% of a
coal having
an energy density of at least 21 MMBTU/ton (24 GJ/MT) and at least 10 wt% of a
processed biomass comprises three steps. The first step is to input into a
system
comprising a first, a second, and a third subsystem components comprising coal
and a
renewable, unprocessed organic-carbon-containing feedstock that includes free
water,
intercellular water, intracellular water, intracellular water-soluble salts,
and at least some
plant cells comprising cell walls that include lignin, hemicellulose, and
microfibrils within
fibrils. The second step is to pass unprocessed organic-carbon-containing
feedstock
through a beneficiation sub-system process to result in processed biomass
having a water
content of less than 20 wt% and a water soluble intracellular salt content
that is reduced by
at least 60 wt% on a dry basis for the processed organic-carbon-containing
feedstock from
CA 02951542 2016-12-07
WO 2015/195382 76 PCT/US2015/034592
that of the unprocessed organic-carbon-containing feedstock. The third step is
to pass the
processed biomass through a blending sub-system process, to be joined with
coal to result
in a high energy processed biomass / coal blended compact aggregate that
comprises at
least 10 wt% of a coal having an energy density of at least 21 MMBTU/ton (24
GJ/MT)
and at least 10 wt% of a processed biomass comprising a processed organic-
carbon-
containing feedstock with characteristics that include an energy density of at
least 17
MMBTU/ton (20 GJ/MT) and a water-soluble intracellular salt content that is
decreased
more than 60 wt% on a dry basis for the processed organic-carbon-containing
feedstock
from that of unprocessed organic-carbon-containing feedstock. Some embodiments
augment the process by using a heating sub-system process to make a processed
biomass
that is a processed biochar having an energy density of at least 21 MMBTU/ton
(24
GJ/MT).
The process includes two aspects of the beneficiation process for making
processed
carbon-containing feedstock with the beneficiation sub-system discussed above
and four
aspects of the heating sub-system, three aspects of the oxygen-deficient
thermal process
for converting the processed carbon-containing feedstock into processed
biochar and one
aspect of a microwave process for converting the processed carbon-containing
feedstock
into processed biochar.
Beneficiation Sub-System Process
The beneficiation process step comprises the step of passing unprocessed
organic-
carbon-containing feedstock through a beneficiation sub-system process to
result in
processed organic-carbon-containing feedstock having a water content of less
than 20 wt%
and a salt content that is reduced by at least 60 wt% on a dry basis for the
processed
organic-carbon-containing feedstock from that of the unprocessed organic-
carbon-
containing feedstock. There are two aspects of the beneficiation sub-system
process. The
first focuses on the properties of the processed organic-carbon-containing
feedstock and
the second focuses on the energy efficiency of the process of the invention
over that of
currently known processes for converting unprocessed organic-carbon-containing
feedstock into processed organic-carbon-containing feedstock suitable for use
with
downstream fuel producing systems. Both use the beneficiation sub-system
disclosed
above.
First Aspect
CA 02951542 2016-12-07
WO 2015/195382 77 PCT/US2015/034592
The first aspect of the beneficiation process step of the invention comprises
four
steps. The first step is inputting into a reaction chamber unprocessed organic-
carbon-
containing feedstock comprising free water, intercellular water, intracellular
water,
intracellular water-soluble salts, and at least some plant cells comprising
cell walls that
include lignin, hemicellulose, and microfibrils within fibrils. Some
embodiments have
unprocessed organic-carbon-containing feedstock that comprises water-soluble
salts
having a content of at least 4000 mg/kg on a dry basis.
The second step is exposing the feedstock to hot solvent under pressure for a
time
at conditions specific to the feedstock to make at least some regions of the
cell walls
comprising crystallized cellulosic fibrils, lignin, and hemicellulose more
able to be
penetrable by water-soluble salts without dissolving more than 25 percent of
the lignin and
hemicellulose. As mentioned above, this is accomplished by one or more of
unbundling
regions of at least some fibrils, depolymerizing at least some strands of
lignin and/or
hemicellulose, or detaching them from the cellulose fibrils, thereby
disrupting their
interweaving of the fibrils. In addition, the cellulose fibrils and
microfibrils can be
partially depolymerized and/or decrystallized.
The third step is rapidly removing the elevated pressure so as to penetrate
the more
penetrable regions with intracellular escaping gases to create porous
feedstock with open
pores in at least some plant cell walls. In some embodiments the pressure is
removed to
about atmospheric pressure in less than 500 milliseconds (ms), less than 300
ms, less than
200 ms, less than 100 ms, or less than 50 ms.
The fourth step is pressing the porous feedstock with conditions that include
an
adjustable compaction pressure versus time profile and compaction time
duration, and
between pressure plates configured to prevent felt from forming and blocking
escape from
the reaction chamber of intracellular and intercellular water and
intracellular water-soluble
salts, and to create processed organic-carbon-containing feedstock that has a
water content
of less than 20 wt % and a water-soluble salt content that is decreased by at
least 60 % on
a dry basis that of the unprocessed organic-carbon-containing feedstock. In
some
embodiments, the water content is measured after subsequent air-drying to
remove
remaining surface water. In some embodiments, the pressure plate has a pattern
that is
adapted to particular organic-carbon-containing feedstock based on its
predilection to form
felts and pith content as discussed above. In some embodiments, the pressure
amount and
CA 02951542 2016-12-07
WO 2015/195382 78 PCT/US2015/034592
pressure plate configuration is chosen to meet targeted processed organic-
carbon-
containing feedstock goals for particular unprocessed organic-carbon-
containing
feedstock. In some embodiments, the pressure is applied in steps of increasing
pressure,
with time increments of various lengths depending on biomass input to allow
the fibers to
relax and more water-soluble salt to be squeezed out in a more energy
efficient manner. In
some embodiments, clean water is reintroduced into the biomass as a rinse and
to
solubilize the water-soluble slats before the fourth step begins.
The process may further comprise a fifth step, prewashing the unprocessed
organic-carbon-containing feedstock before it enters the reaction chamber with
a particular
set of conditions for each organic-carbon-containing feedstock that includes
time duration,
temperature profile, and chemical content of pretreatment solution to at least
initiate the
dissolution of contaminates that hinder creation of the cell wall passageways
for
intracellular water and intracellular water-soluble salts to pass outward from
the interior of
the plant cells.
The process may further comprise a sixth step, masticating. The unprocessed
organic-carbon-containing feedstock is masticated into particles having a
longest
dimension of less than 1 inch (2.5 centimeters) before it enters the reaction
chamber.
The process may further comprise a seventh step, separating out the
contaminants.
This step involves the separating out of at least oils, waxes, and volatile
organic
compounds from the porous feedstock with solvents less polar than water.
As with the system aspect, the unprocessed organic-carbon-containing feedstock
may comprise at least two from a group consisting of an herbaceous plant
material, a soft
woody plant material, and a hard woody plant material that are processed in
series or in
separate parallel reaction chambers. In addition, in some embodiments, the
energy density
of each plant material in the processed organic-carbon-containing feedstock
may be
substantially the same. In some embodiments, the organic-carbon-containing
feedstock
comprises at least two from the group consisting of an herbaceous plant
material, a soft
woody plant material, and a hard woody plant material, and wherein the energy
density of
each plant material in the processed organic-carbon-containing feedstock is at
least 17
MMBTU/ton (20 GJ/MT).
Figure 24 is a block diagram of a process for making processed organic-carbon-
containing feedstock with less than 60 percent water-soluble salt on a dry
basis over that
CA 02951542 2016-12-07
WO 2015/195382 79 PCT/US2015/034592
of its unprocessed form and with less than 20 wt % water. Step 2410 involves
inputting
unprocessed organic-carbon-containing feedstock that has at least some plant
cells that
include intracellular water-soluble salt and cell walls comprising lignin into
a reaction
chamber. Step 2420 involves exposing the feedstock to hot solvent under
pressure for a
time to make some regions of the cell walls comprising crystallized cellulosic
fibrils,
lignin, and hemicellulose more able to be penetrable by water-soluble salts
without
dissolving more than 25 percent of the lignin and hemicelluloses. Step 2430
involves
removing the pressure so as to penetrate at least some o f the cell walls so
as to create
porous feedstock with open pores in its plant cell walls. Step 2440 involves
pressing the
porous feedstock with a plate configured to prevent felt from blocking escape
of
intracellular water and intracellular water-soluble salts from the reaction
chamber so as to
create processed organic-carbon-containing feedstock that has a water content
of less than
wt% and a water-soluble salt content that is decreased by at least 60 wt% on a
dry basis
for the processed organic-carbon-containing feedstock from that of unprocessed
organic-
15 carbon-containing feedstock.
Second Aspect
The second aspect is similar to the first except steps have an efficiency
feature and
the resulting processed organic-carbon-containing feedstock has a cost
feature. The
second aspect also comprises four steps. The first step is inputting into a
reaction chamber
20 organic-carbon-containing feedstock comprising free water, intercellular
water,
intracellular water, intracellular water-salts, and at least some plant cells
comprising
lignin, hemicellulose, and fibrils within fibril bundles. Each step emphasizes
more
specific conditions aimed at energy and material conservation. The second step
is
exposing the feedstock to hot solvent under pressure for a time at conditions
specific to the
feedstock to swell and unbundle the cellular chambers comprising partially
crystallized
cellulosic fibril bundles, lignin, hemicellulose, and water-soluble salts
without dissolving
more than 25 percent of the lignin and to decrystallize at least some of the
cellulosic
bundles. The third step is removing the pressure to create porous feedstock
with open
pores in its cellulosic chambers. After possibly mixing with fresh water to
rinse the
material and solubilize the water-soluble salts, the fourth step is pressing
the porous
feedstock with an adjustable compaction pressure versus time profile and
compaction
duration between pressure plates configured to prevent felt from forming and
blocking
CA 02951542 2016-12-07
WO 2015/195382 80 PCT/US2015/034592
escape from the reaction chamber of intracellular and intercellular water and
intracellular
water-soluble salts, and to create a processed organic-carbon-containing
feedstock that has
a water content of less than 20 wt %, a water-soluble salt content that is
decreased by at
least 60 wt % on a dry basis, and a cost per weight of removing the water and
the water-
soluble salt is reduced to less than 60 % of the cost per weight of similar
water removal
from known mechanical, known physiochemical, or known thermal processes.
Figure 25 is a block diagram of a process for making processed organic-carbon-
containing feedstock with less than 50 wt % water-soluble salt of a dry basis
than that of
unprocessed organic-carbon-containing feedstock and less than 20 wt % water,
and at a
cost per weight of less than 60 % that of similar water removal from known
mechanical,
known physiochemical, or known thermal processes that can remove similar
amounts of
water and water-soluble salt. Step 2510 involves inputting unprocessed organic-
carbon-
containing feedstock that has at least plant cells comprising intracellular
water-soluble
salts and plant cell walls that include lignin into a reaction chamber. Step
2520 involves
exposing the feedstock to hot solvent under pressure for a time to make some
regions of
the cell walls comprising of crystallized cellulosic fibrils, lignin, and
hemicellulose more
able to be penetrable by water-soluble salts without dissolving more than 25
percent of the
lignin and hemicelluloses. Step 2530 involves removing the pressure so as to
penetrate at
least some of the cell walls to create porous feedstock with open pores in its
plant cell
walls. Step 2540 involves pressing the porous feedstock with a plate
configured to prevent
felt from blocking escape of intracellular water and intracellular water-
soluble salts from
the reaction chamber so as to create processed organic-carbon-containing
feedstock that
has a water content of less than 20 wt%, a water-soluble salt content that is
decreased by at
least 60 wt% on a dry basis over that of unprocessed organic-carbon-containing
feedstock,
and a cost per weight of removing the water and water-soluble salt that is
reduced to less
than 60 % of the cost per weight of similar water removal from known
mechanical,
physiochemical, or thermal processes.
Energy efficiencies are achieved in part by tailoring process conditions to
specific
organic-carbon-containing feedstock as discussed above. Some embodiments use
systems
engineered to re-capture and reuse heat to further reduce the cost per ton of
the processed
organic-carbon-containing feedstock. Some embodiments remove surface or free
water
left from the processing of the organic-carbon-containing feedstock with air
drying, a
CA 02951542 2016-12-07
WO 2015/195382 81 PCT/US2015/034592
process that takes time but has no additional energy cost. Figure 26 is a
table that shows
some process variations used for three types of organic-carbon-containing
feedstock
together with the resulting water content and water-soluble salt content
achieved. It is
understood that variations in process conditions and processing steps may be
used to raise
or lower the values achieved in water content and water-soluble salt content
and energy
cost to achieve targeted product values. Some embodiments have achieved water
contents
as low as less than 5 wt % and water-soluble salt contents reduced by as much
as over 95
wt % on a dry basis from its unprocessed feedstock form.
Oxygen-Deficient Thermal Sub-system Process
The oxygen-deficient thermal sub-system process step comprises passing the
processed organic-carbon-containing feedstock through an oxygen-deficient sub-
system
process to result in a solid fuel composition having an energy density of at
least 17
MMBTU/ton (20 GJ/MT) a water content of less than 10 wt%, and a water-soluble
salt
that is decreased by at least 60 wt% on a dry basis for the processed organic-
carbon-
containing feedstock from that of the unprocessed organic-carbon-containing
feedstock.
In the broadest perspective the process comprises three steps. The first step
is to
input into a system, comprising a first and a second subsystem, an unprocessed
organic-
carbon-containing feedstock that includes free water, intercellular water,
intracellular
water, intracellular water-soluble salts, and at least some plant cells
comprising cell walls
that include lignin, hemicellulose, and microfibrils within fibrils. The
second step is to
pass the unprocessed organic-carbon-containing feedstock through the first sub-
system, a
beneficiation sub-system process, to result in processed organic-carbon-
containing
feedstock having a water content of less than 20 wt% and a salt content that
is reduced by
at least 60 wt% on a dry basis for the processed organic-carbon-containing
feedstock from
that of the unprocessed organic-carbon-containing feedstock, The third step is
to pass the
processed organic-carbon-containing feedstock through the second sub-system,
an
oxygen-deficient thermal sub-system process, to result in a solid fuel
composition having
an energy density of at least 17 MMBTU/ton (20 GJ/MT) a water content of less
than 10
wt%, and water-soluble salt that is decreased by at least 60 wt% on a dry
basis for the
processed organic-carbon-containing feedstock from that of the unprocessed
organic-
carbon-containing feedstock.
CA 02951542 2016-12-07
WO 2015/195382 82 PCT/US2015/034592
The process that uses the horizontal oxygen-deficient thermal sub-system
involves
four steps. The first step is to input processed organic-carbon-containing
feedstock into a
substantially horizontal sublimating reaction chamber largely contained within
a hot box.
The reaction chamber is configured to be able to (1) heat from an ambient
temperature to
an operating sublimation temperature, (2) operate at a sublimation
temperature, and (3)
cool from an operating sublimation temperature to an ambient temperature. This
is done
without leaking any hot product gas fuel from the reaction chamber into the
hot box or
atmosphere, or leaking any oxygen from outside the hot box into the hot box.
The second
step is to heat the processed organic-carbon-containing feedstock to a
sublimating
temperature before it is able to form a liquid phase. The third step is to
maintain the
temperature at a sublimation temperature for a residence time that is as long
a time as
needed to convert the processed organic-carbon-containing feedstock to
processed biogas
and processed biochar. The fourth step is to separate the processed biogas
from the
processed biochar.
These steps are depicted in Figure 27, a flow diagram of the process for
generating
processed biochar from processed organic-carbon-containing feedstock in
accordance with
embodiments of the invention. In 2710 the processed organic-carbon-containing
feedstock
is inputted into a horizontal sublimating reaction chamber contained within a
hot box
without leaking any hot product gas fuel from the reaction chamber into the
hot box or
atmosphere, or leaking any oxygen from outside the hot box into the hot box.
Next, in
step 2720, the processed organic-carbon-containing feedstock is heated to a
sublimating
temperature before it is able to form a liquid phase. In step 2730, the
temperature is
maintained at a sublimation temperature for a residence time that is as long a
time as is
needed to convert the processed organic-carbon-containing feedstock to
processed biogas
fuel and processed biochar fuel. Finally, in step 2740 the product gas fuel
and the
processed biochar fuel are separated from each other.
In some embodiments the process may use a horizontal sublimation sub-system,
depending on its size, wherein the substantially horizontal sublimating
reaction chamber is
supported by a vertical support. It is beneath the substantially horizontal
reaction
chamber. It is also configures to be dimensionally stable in the vertical
direction over
temperature variations of from ambient temperature to about 850 C that may
occur during
CA 02951542 2016-12-07
WO 2015/195382 83 PCT/US2015/034592
the startup, operating, and shutdown operations of the substantially
horizontal reaction
chamber.
The process that uses the vertical oxygen-deficient thermal sub-system
involves
four steps. This is depicted in Figure 28. In 2810, the first step is to input
processed
organic-carbon-containing feedstock into a substantially vertical sublimating
reaction
chamber. In 2820, the second step is to heat the processed organic-carbon-
containing
feedstock to a sublimating temperature before it is able to form a liquid
phase. In 2830,
the third step is to maintain the temperature at a sublimation temperature for
a residence
time that is as long a time as is needed to convert the processed organic-
carbon-containing
feedstock to processed biogas and processed biochar. In 2840, the fourth step
is to
separate the processed biogas from the processed biochar.
Microwave Sub-system Process
The microwave sub-system process step comprises passing the processed organic-
carbon-containing feedstock through a microwave sub-system process to result
in a solid
renewable fuel composition having an energy density of at least 17 MMBTU/ton
(20
GJ/MT) a water content of less than 10 wt%, water-soluble salt that is
decreased by at
least 60 wt% on a dry basis for the processed organic-carbon-containing
feedstock from
that of the unprocessed organic-carbon-containing feedstock, and pores that
have a
variance in pore size of less than 10%.
Figure 29 is a block diagram of an embodiment of a process for passing
processed
organic-carbon-containing feedstock through a microwave sub-system to create a
solid
renewable fuel processed biochar of the invention. The processed organic-
carbon-
containing feedstock is input (2910) to a reaction chamber having walls that
are
substantially transparent to microwaves used to heat and/or irradiate the
feedstock. The
heating and/or radiation occur by directing (2920) the microwave energy
through the walls
of the reaction chamber so that it impinges on the feedstock disposed within
the reaction
chamber. The feedstock is heated/irradiated (2930) by the microwaves,
optionally in the
presence of a catalyst, until reaction of the organic-carbon-containing
molecules occurs to
produce the desirable end fuel product. The fuel product created by the
reaction processes
are collected (2940).
Blending Sub-system Process
CA 02951542 2016-12-07
WO 2015/195382 84 PCT/US2015/034592
The blending sub-system process step comprises three steps. When handling coal
dust, fines, or powder, an oxygen-deficient atmosphere may be employed to
minimize the
occurrence of explosions.
The first step is a sizing step to reduce the size of the particles of coal
and
processed biomass to one that permits easy subsequent mixing. Any coal chunks
present
are reduced to the size of coal dust or coal fines for easy transport into the
blending
process. In some embodiments, the processed biomass and coal are mixed and
simultaneously sized and blended in a high speed vortex in the blending and
pulverizing
unit. In some embodiments a suitable size is on the order of particles being
able to pass
through an 8 mesh size with square holes of 0.097 inches (2.380 mm). Some
embodiments have particles able to pass through a finer screen such as a 16
mesh screen
with square holes of 0.0469 inches (1.190 mm) on a side. With some studies
using
magnetic fields, half of the impurities were be removed from coal with the
removal of less
than 5 wt% of the carbon in the coal for coal sized to pass through an 16 mesh
screen.
The second step is a combining step to combine both the coal and the processed
biomass into a blended powder of a predetermined ratio of coal to processed
biomass. In
some embodiments, a high energy biomass binder is added. The high energy
biomass
binder is formed in the beneficiation sub-system process, discussed above,
with the
removing and cleaning of micro particles of unprocessed organic-carbon-
containing
feedstock, lignin fragments, and hemicellulosic fragments from the vapor
explosion
section. The high energy biomass binder, a fine, sticky mass of biomass with
high lignin
content, is then added to the blended powder to permit at least one of lower
temperatures
or more cohesiveness in the compressing step during formation of blended
compact
aggregates.
The third step is compressing the blended powder into a multitude of blended
compact aggregates that are safe for transport. In some embodiments the
compression is
done under heat to at least assist the formation of aggregates or reduce the
content of water
to less than 10% by weight. In some embodiments, the steps of compression and
heating
are done at the same time.
Various modifications and additions can be made to the preferred embodiments
discussed hereinabove without departing from the scope of the present
invention.
Accordingly, the scope of the present invention should not be limited by the
particular
CA 02951542 2016-12-07
WO 2015/195382 85
PCT/US2015/034592
embodiments described in this document, but should be defined only by the
claims set
forth below and equivalents thereof