Note: Descriptions are shown in the official language in which they were submitted.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
1
LOW DUCTILITY ALLOY
The present invention relates to a low ductility steel tube for use in
chemical
engineering applications. In particular, the invention relates to a high
strength steel
tube which has low ductility at elevated temperatures. Such tubes are
typically used in
chemical plants for transporting reactants and products. One such application
includes
the use in plants for producing hydrogen and methanol. The tubes could also be
used
when producing ethylene and other hydrocarbons.
Steam reforming is the most widespread process for the generation of hydrogen-
rich
synthesis gas from light carbohydrates. The feed material is natural gas,
mostly in the
form of methane, which is ultimately converted into methanol and hydrogen
using
water. This is an endothermic reaction of the gas with water in the form of
steam which
takes place at high temperatures in catalytic tube reactors. The natural gas
feed is
mixed with superheated steam with the appropriate ratio of steam/carbon to
allow
efficient conduct of the reforming process. The mixture then is distributed
via manifold
in vertical rows of catalyst-filled reformer tubes. The mixture flows from top
to bottom of
the tubes and is heated from the outside through the catalyst and reacts
endothermically to produce hydrogen and carbon monoxide which are collected by
outlet manifold.
It is necessary to heat the exposed the tubes to very high temperatures (above
900 C)
to allow the endothermic reaction to take place continuously. This places
stringent
design requirements on the reactor. In addition, the reaction generally takes
place at
elevated pressures and since the reaction occurs at relatively high pressures
(above
20kg/cm2 and up to 40kg/cm2) in the tube, creep damage of the tube is the
usual
parameter limits the working lifetime of the tube.
The increase in the availability of shale gas has driven the development of
the use of
reforming reactors, particularly in the USA and as China. In addition, there
is an
increasing demand for the production of methanol from a combination of
substoichiometric combustion and catalytic steam reforming. It is recognised
that the
annual production of methanol exceeds 40 million tons and continues to grow by
4%
per year. Methanol has traditionally been used as feed for production of a
range of
chemicals including acetic acid and formaldehyde. In recent years methanol has
also
been used for production of dimethylether, and olefins by so called methanol-
to olefins
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
2
process or as blendstock for motor fuel. Consequently, the limited lifespan of
conventional tubes used in reforming reactors represents a problem for the
industry.
Another area in which the tubes could be of potential value is in ethylene
production
since this process is also conducted at elevated temperatures and pressures.
Ethylene
is a basic chemical which is widely used in the production of a number of
common
items such as plastic packaging that are prepared by polymerisation of
ethylene and
ethylene derivatives. The feedstock for an ethylene plant is usually ethane or
other
natural gases and gas liquids derived from traditional oil and gas sources.
The gas,
principally or entirely ethane, is heated in a steam mix in order to break
down the
ethane into ethylene, hydrogen and other by-products. This process is known as
cracking.
Steam cracking is a petro-chemical process which, in general terms, saturated
hydrocarbons are broken down into smaller, frequently unsaturated,
hydrocarbons and
hydrogen. This process represents the principal industrial method for
producing lighter
alkenes for subsequent use in a variety of chemical processes. Naphtha
cracking
represents the dominant source of ethylene globally, although gas cracking has
recently become more important. Irrespective of the exact nature of the
feedstock and
of the chemical process used to produce ethylene and other olefins, the
cracking
process is invariably conducted at elevated temperatures and these are
typically in the
region of 800 to 1100 C.
Sudden cooling then stops the reaction and the subsequent mixture of gaseous
products can be compressed, chilled and then separated in a serious of
distillations
towers. Product such as ethene and other alkenes are individually recovered at
this
stage. Any un-cracked ethane or other feedstock material is recycled and the
remaining by-product gases are further processed for other uses.
Pipes of this type are generally prepared by a centrifugal casting process.
Centrifugal
casting is a well-established process that is used to cast thin-walled
cylinders, pipes
and other axially symmetric objects. One benefit of this process is that it
allows precise
control of the metallurgy and crystal structure of the alloy product. It is
generally used
for casting iron, steel, stainless steels and alloys of aluminium, copper and
nickel. The
centrifugal casting process employs a permanent mould which is rotated about
its axis
at high speeds of typically 300 to 3000 rpm as the molten metal is poured. The
molten
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
3
metal is centrifugally thrown towards the inside mould wall where it is able
to solidify
after cooling. The resulting cast cylinder i.e. tube, has a fine grain and the
surface
roughness of the outer surface of the cylinder is relatively low. The internal
surface has
slightly more impurities and inclusions but may be subject to machining to
modify the
surface roughness and / or geometry.
JP64-031931 describes the production of a curved tube made of heat-resistant
alloy.
The tube is prepared by centrifugal casting and the alloy of JP64-031931 is
made from
high strength and heat-resistant cast steel containing 15 to 30% chromium, 20
to 40%
nickel as well as the optional inclusion of smaller quantities of manganese
and
molybdenum. Small quantities of niobium and titanium are also added to the
alloy.
The cast tube is then subjected to the further step of an aging treatment at a
temperature of from 700 to 1100 C to deposit secondary carbide within the
grain
structure. This patent does not attempt to control the primary carbide
formation or to
control the relative amounts of niobium and titanium, or carbon and nitrogen.
Subsequently it is subjected to another processing step involving high
frequency
bending or die-bending at a temperature in the range of 550 to 1100 C.
W02012/121389 discloses an alloy intended for use in nuclear applications such
as in
heat exchanges in pressurised water reactors. The material is said to have
excellent
thin workability and corrosion resistance. This material is based on a nickel-
chromium-
iron alloy and contains small amounts of manganese, titanium, and optionally
aluminium as alloying elements.
EP1679387 discloses a heat-resistant cast steel which has good high-
temperature
strength, aged ductility and creep rupture strength for use as a material in
steam
reforming reaction tubes in fuel cell hydrogen generation systems. The cast
steel
contains chromium and nickel, together with manganese, niobium, titanium and
cerium
as alloying elements.
In addition to all of the usual technical issues associated with preparing a
steel pipe for
use in chemical plant, there are two particular problems which need to be
addressed
when fabricating pipes for this type of application. These issues arise
because of the
harsh working environment that the steel tubes will be exposed to and the fact
that any
'downtime' in plant operation is very costly in terms of lost production. The
pipes need
to be both strong enough to withstand the condition and be resistant to creep
i.e.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
4
deformation over time when exposed to elevated temperature. With conventional
pipes, there is an issue with pipe creep and pipe sag due to the high
temperatures
used during the cracking process and these effects become more problematic
over
time. In the case of reforming reactors, the possible failure of such pipes
could have
catastrophic effects. Equally, the necessary maintenance schedule for reactors
of this
type means that the "down-time" can present a significant economic burden.
Another problem resides in the generation of carbon as a by-product of the
cracking
reaction which can contaminate the product and also build up on the inside of
the
pipework. This causes constrictions and necessitates more frequent maintenance
and
consequent plant downtime.
The present invention aims to provide pipes which are creep-resistant i.e.
pipes which
have a low ductility compared to other steel alloys at elevated temperatures.
A further
aim is to provide low ductility alloy steels which can be produced in normal
atmospheric
conditions i.e. in air and to be prepared either in a reduced pressure
atmosphere or in
an inert atmosphere. This represents a considerable processing and economic
advantage.
It is also an aim of the present invention to prepare a pipe which can be
produced in a
process which is convenient to run, so that the manufacturing process is
relatively
straight forward. It is also an aim to provide a process which is applicable
to the large
scale production of steel alloy pipes. The invention aims to provide a more
economic
production method and/or which is also more economic when the whole of life
use and
maintenance interruptions are considered. It is also an aim of the present
invention to
provide a steel alloy which is economical to manufacture and which avoids or
reduces
the need for expensive alloying components.
It is also an aim to have pipes which can be prepared without the need for
further
subsequent processing steps.
It is a further aim to provide pipes which have low internal and external
surface
roughness.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
It is another aim to produce pipes who metallurgical properties mean that they
tend to
be less prone to carbon accumulation. This is particularly important for use
in cracking
reactors.
5 A further aim is to produce pipes which have high strength and / or are
high in
toughness. Another aim is that the pipes should have a good "shelf-life". Long
term
exposure under high temperature conditions can be quite detrimental to
conventional
steel alloys used in such applications. A further aim is to produce steel
alloys which
have good high-temperature strength over an extended period of time. A further
aim is
to provide steel alloys that have good corrosion resistance, particularly at
elevated
temperatures such as those found in a chemical plant. Another aim is to
produce pipes
in which the corrosion resistance is maintained over an extended period of
time in use.
The invention satisfies some or all of the above aims.
According to the present invention, there is provided a steel pipe comprising:
from 20.0 to 40.0 atomic % nickel,
from 20.0 to 40.0 atomic % chromium,
from 1.0 to 3.0 atomic % silicon,
from 0.5 to 2.5 atomic % carbon,
from 0.01 to 1.0 atomic % nitrogen
from 0.01 to 0.90 atomic % niobium,
from 1.0 to 3.0 atomic % manganese, and
from 0.01 to 0.90 atomic % of one or more of: titanium, hafnium, zirconium,
vanadium,
tungsten, and molybdenum,
wherein:
(a) the total amount of niobium and one or more of a second carbide forming
element
selected from: titanium, hafnium, zirconium, vanadium, tungsten and molybdenum
is
from 0.50 to 0.91 atomic %, preferably 0.60 to 0.75;
(b) the total amount of carbon plus nitrogen is in the range of 1.2 to 3.0
atomic %,
preferably in the range 1.5 to 2.5 atomic %;
(c) the amount (nitrogen / carbon) is in the range 0.20 to 1.0, and
(d) the amount [nitrogen / (the second carbide forming element(s) plus
niobium)] is in
the range 0.2 to 1.1, preferably 0.4 to 1.0;
with the balance of the composition being iron and incidental impurities.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
6
The alloy compositions of the present invention have a reduced propensity to
suffer
from stress fractures. The occurrence of stress fractures and the strength and
ductility
of an alloy composition are generally dictated by the occurrence of
dislocations and
their distribution throughout the bulk material. Good high-temperature
strength and
creep-resistance are properties which are mainly due to the precipitation
strengthening
of the grain interiors by alloy carbides. Precipitation strengthening is
governed by the
precipitate size, shape, distribution and crystallographic orientation within
the
surrounding matrix. The steel alloys of the present invention have excellent
mechanical properties and show enhanced creep resistance as well as improved
strength.
Metal carbides that normally provide the strengthening effect in steels are
derived from
niobium, vanadium, molybdenum and tungsten. Hafnium, zirconium and titanium
are
also known carbide formers. All of these elements can be classified as carbide-
forming
elements.
The principal carbide forming component in the alloys of the invention is
usually
niobium and the remainder of the abovementioned carbide-forming elements may
be
used (alone or in a combination of one or more of them) as a second carbide-
forming
component. One important feature resides in the careful control of the total
amount of
the niobium and the one or more second carbide-forming elements. This total of
the
carbide-forming elements mentioned above is deliberately controlled to a
maximum of
from 0.5 to 0.91, preferably from 0.60 to 0.91 atomic weight %. Thus the total
amount
of niobium together with one or more of titanium, hafnium, zirconium,
vanadium,
tungsten and molybdenum is never greater than 0.91 atomic weight %. Usually,
the
niobium will be the principal part (in terms of the number of elemental atoms)
of this
total. Thus niobium will account for 50 atomic % of this total of the carbide-
forming
elements and is more usually at least 80 atomic %, and may be at least 90
atomic % or
even at least 95 atomic % of the total. In some circumstances, however, the
niobium
may be present in an amount of less than 50 atomic % of the total.
The steel alloy of the invention consequently has a relatively small and
dispersed
carbide formation compared with known steels for similar applications. It is
this feature,
arising from a careful control of the metallurgical composition, which gives
contributes
to the improved mechanical properties. A further benefit of the steel pipes of
the
invention is that they require no subsequent treating.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
7
Classical precipitation strengthening of alloys due to carbide formation
varies as a
function of time at a given temperature. Initially, clusters of solute atoms
form and
then, eventually, precipitate forms which is largely coherent with the matrix.
The
precipitates strengthen the matrix because they prevent dislocation movement
which in
turn inhibits plastic deformation. The steel alloy composition of the
invention is
designed to control primary carbide formation and ensure a substantially
homogeneous
distribution of carbide throughout the matrix. It is also designed to ensure
smaller,
more regular, carbide growth.
Each of the elemental components described in the above composition plays an
important role in the creep resistant steel of the present invention. The
combination of
elements gives rise to the very low ductility i.e. high creep resistance that
is observed
in the case of the present invention. Furthermore, the combination of elements
also
contributes to the high-temperature strength of the steel tube. This high
strength and
high creep resistance is manifested over an extended period of time relative
to
conventional alloys.
Carbon is an important component of the steel for providing tensile strength
and
resistance to creep rupture. Carbon is an essential component in the carbide
formation
which provides the steel of the present invention with its unique properties.
The carbon
improves the strengthening of the alloy by precipitation of the primary and
secondary
carbides as follows: chromium based carbides (M7C3) and niobium carbides
during
solidification (primary carbides), and chromium based carbides (M23C6) and
niobium
carbides, niobium carbido-nitrides, niobium nitrides during ageing (secondary
carbides). However, too high a quantity of carbon can result in grain boundary
corrosion resistance due to excessive carbide formation and can also result in
reduced
strength to excessive carbide formation. Consequently, carbon must be present
in an
amount in the range of from 1.2 to 2.5 atomic %. Preferably, it is in the
range of
from1.5 to 2.5 atomic %, and more preferably it is present in an amount from
1.75 to
2.25 atomic %.
Not only is it important to control the absolute amount of carbon in the alloy
composition but it is also important to control the amount of carbon relative
to the
amount of nitrogen.The total of (carbon + nitrogen) needs to be above a
minimum level
to allow the precipitation of minimum quantity of fine primary chromium
carbides and
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
8
fine secondary carbides/carbo-nitrides and also needs to be below a maximum
level to
avoid over saturation of the austenitic matrix, and as a consequence, loss of
the
beneficial effect of fast solidification and loss of the control of
precipitation before and
during solidification. Hence, the total amount of carbon plus nitrogen is in
the range of
from 1.2 to 3.0 atomic%,
Nitrogen is required because it forms austenite together with carbon and it
contributes
to high-temperature strength. Nitrogen allows the dilution, dispersion, and
the
homogenisation of the carbon. The control of the amount of nitrogen is
important
because it slows the precipitation of primary chromium carbides when it is
added in a
suitable quantity. In effect, the nitrogen helps to control the 'behaviour' of
the carbon
so to control its several precipitations. The nitrogen participates in the
precipitation of
secondary niobium carbides, niobium carbido-nitrides, and niobium nitrides
during
ageing. However, if the quantity of nitrogen is too large then an excessive
amount of
nitrides are produced which reduces the toughness of the alloy over an
extended
period of time. Both the absolute quantity of nitrogen, and the quantity
relative to
carbon are important to ensure high strength. The nitrogen allows control of
the carbide
precipitations and hence nitrogen must be added in a quantity that is
controlled relative
to that of carbon. Therefore, as more carbon is added so more N is needed;
similarly
as less carbon is added then less nitrogen is needed. The nitrogen disperses
the
carbon in the austenitic matrix which, with fast solidification, slows down
the
precipitation of primary chromium carbides (M7C3) and limits the segregation
of the
carbon close to the primary carbides. Accordingly, in addition, the ratio
(nitrogen/carbon) must be in the range of from 0.20 to 1.00, and preferably is
in the
range 0.20 to 0.50 atomic %.
Consequently, nitrogen must be present in an amount in the range of from 0.01
to 1.0
atomic %. Preferably, it is in the range of from 0.20 to 0.70 atomic %, and
more
preferably it is present in an amount from 0.30 to 0.50 atomic %.
Silicon provides the function of a deoxidiser and is usually an essential
component in
an austenite stainless steel. Silicon may also contribute to increasing the
stability of
any surface oxide film. On the other hand, if the content of silicon is too
high the
workability of the steel is reduced. A high Si content can also cause the
formation of a
detrimental phase known as the G phase which is composed of nickel, silicon
and
niobium (Ni16Nb6Si7). Consequently, silicon must be present in an amount in
the
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
9
range of from 1.0 to 3.0 atomic%.Preferably, it is in the range of from1.45 to
1.75
atomic%, and more preferably it is present in an amount from 1.65 to 1.75
atomic %.
Nickel is an element which is essential in order to obtain a stable austenite
structure
and improves the stability of austenite and supresses the generation of the
sigma
phase. Nickel is the austenitic stabiliser element, allowing the alloy to be
generally
strong at above 8000. Therefore it forms a stable matrix with the iron which
allows the
possible precipitation of the carbides/nitrides. The lower limit of the nickel
content is
chosen simply for the reason that this is a sufficient amount for improving
the stability
of austenite with respect to the lower limits of the other elements. The lower
limit is
20.0 atomic %. The upper limit is chosen on the grounds of economy and also
with
respect to the upper limits of the other alloy components. Furthermore, nickel
when
present in conjunction with chromium forms a stabilised austenitic structure
which
imparts additional strength and resistance to oxidation at elevated
temperatures.
There are diminishing returns as the content of nickel rises hence the
practical upper
limit is around 40.0 atomic%.Preferably, nickel is present is in the range of
from 25.0 to
35.0 atomic %, and more preferably it is present in an amount from 30.0 to
33.0 atomic
%.
Chromium provides a well-documented and effective corrosion resistance and
oxidation resistance effect. Chromium also acts as a carbide-former, ensuring
the
creep strengthening precipitations in the alloy. Chromium-based carbide of
general
formula M7C3 is formed during solidification (primary carbide formation) and
chromium-based carbide of general formula M23C6 is formed during ageing
(secondary carbide formation).The lower limit of 20.0 atomic weight % of
chromium is
required in order to ensure sufficient oxidation resistance and the upper
limit of 40.0
atomic weight% is determined by the fact that above this level it is difficult
to obtain a
stable austenite phase. In addition, a high level of chromium renders the
steel
unworkable. Preferably, chromium is present in the range of from 20.0 to 30.0
atomic
%, and more preferably it is present in an amount from 22.5 to 27.5 atomic %.
The principal function of niobium in the alloy is to act as a carbide forming
element.
Niobium allows formation of stable carbides, and even more stable carbo-
nitride and
nitrides in the alloy. Niobium carbides form during solidification (primary
carbides), and
niobium carbides, niobium carbido-nitrides, and niobium nitrides form during
ageing
(secondary carbides). Similarly, the presence of titanium, or one or more of
the second
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
carbide-forming elements, is to form carbides. Although titanium is mainly
intended for
the formation of carbides it is also engaged in the formation of nitrides and
carbo-
nitrides to some degree. The addition of niobium needs to be carefully
controlled in
order to ensure sufficient, but not too much, carbide formation. The primary
carbides
5 are of the form M73C. The niobium carbide which is formed gives an
enhanced creep
rupture strength and also contributes to maintenance of the properties of the
high
strength and high creep resistance steel alloy over an extended period of
time.
Consequently, niobium must be present in an amount in the range of from 0.01
to 0.90
atomic %. Preferably, it is in the range of from 0.60 to 0.80 atomic %, and
more
10 preferably it is present in an amount from 0.65 to 0.75 atomic %. The
ratio N/(Nb+
second carbide forming element) is also important. The quantity needs to be
such that
it allows the beneficial precipitation of very small secondary niobium
nitrides (MN) (less
than 50nm) during ageing of the alloy. Hence the amount the amount [nitrogen /
(the
second carbide forming element(s) plus niobium)] is in the range 0.20 to 1.10.
In addition to controlling the upper limit in order to avoid excessive carbide
formation,
the present of excess niobium may also reduce corrosion resistance and / or
oxidation
resistance. Hence the total amount of niobium and one or more of: titanium,
hafnium,
zirconium, vanadium, tungsten and molybdenum is from 0.50 to 0.91 atomic
weight %,
preferably 0.60 to 0.91 atomic weight %, and is more preferably from 0.65 to
0.80
atomic %, and most preferably is from 0.70 to 0.80 atomic %.
Titanium is added to the alloy as a deoxidiser. Furthermore, titanium as a
carbide
forming element not only forms titanium carbides but is also able to form a
titanium-
niobium double carbide precipitate which improves creep strength. The addition
of too
high an amount of titanium can lead to undesirable oxide formation thereby
reducing
strength. Consequently, titaniumwhen present must be present in an amount in
the
range of from 0.01 to 0.90 atomic %. Preferably, it is in the range of from
0.01 to 0.20
atomic %, and more preferably it is present in an amount from 0.01 to 0.10
atomic %.
Similar restrictions apply to the other carbide forming elements: hafnium,
zirconium,
vanadium, tungsten and molybdenum which taken individually and independently
when
present must be present in an amount in the range of from 0.01 to 0.90 atomic
%.
Preferably, any of those elements when present is present in an amount in the
range of
from 0.01 to 0.20 atomic %, and more preferably is present in an amount from
0.01 to
0.10 atomic %. Only titanium need be present as the second carbide forming
element.
Thus in one embodiment the alloy contains onlynickel, chromium, silicon,
carbon,
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
11
nitrogen, niobium, manganese, and titanium with the balance being iron and
incidental
impurities. Equally, any one of those other elements may be present as the
sole
second carbide-forming element and the composition would then contain only
nickel,
chromium, silicon, carbon, nitrogen, niobium, manganese, and one of the other
second
carbide-forming elements described above with the balance being iron and
incidental
impurities.
The double carbide formation of niobium and titanium is the reason for the
careful
control of the total amount of niobium and titanium and / or one of the other
carbide-
forming elements hafnium, zirconium, vanadium, tungsten and molybdenum. Each
of
those other carbide-forming elements is able to function in a similar way to
titanium in
forming carbides which contribute to enhanced creep rupture strength. Similar
considerations apply, in terms of the need to avoid excess carbide formation,
when
using these elements hence the requirement that the upper limit of these
elements is
controlled to 0.90 atomic weight % either when present alone as a sole
component
(other than niobium) or when present in combination with one another.
Manganese is a required component of the steels of the present invention
because it
can improve the workability of the alloy. It is also an effective de-oxidant
and
contributes to austenite formation in the steel. The addition of too much
manganese
can result in a reduction in high-temperature strength and also toughness over
an
extended period of time. Consequently, manganese must be present in an amount
in
the range of from 1.0 to 3.0 atomic %. Preferably, it is in the range of from
1.0 to 2.0
atomic %.
Alloys according to the present invention are produced in a conventional
furnace and
without the need for a special atmosphere. The first stage of preparing the
alloy
involves working out the relative proportions by weight of the various
component
minerals (which are the source of the various elements required in the final
alloy) in
order to achieve the desired amounts of the various elements which are
required in the
final alloy. The solid minerals are added to the hot furnace. Heating is
continued in
order to melt all of the mineral components together and ensure a thorough
mixing of
the minerals in the furnace so that the elements are properly distributed
within the
matrix.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
12
Once melting and mixing has been achieved, any slag is decanted from the
furnace in
order to remove impurities and clean the bath of liquid alloy in the furnace.
A sample of
the molten alloy is then removed from the furnace, allowed to cool and
analysed by x-
ray fluorescence in order to determine its elemental composition. An
adjustment to the
composition may or may not be required at this stage to accommodate for any
elemental mass loss due to volatility. The composition is adjusted by the
addition of
further minerals as necessary, and optionally re-analysed to ensure that the
desired
composition has been achieved.
After the desired composition has been achieved, the temperature is further
raised
above the melting temperature to a tapping temperature in order to ensure easy
pouring of the melt. At the same time, the mould is prepared for centrifugal
casting.
The mould is a conventional centrifugal casting mould and this type of mould
is well
known to the skilled person. The process of preparing the mould involves
washing the
mould with water/steam to clean it and to remove any old mould wash or coating
that
might have been used in a previous casting process. The washed mould is then
coated
with an insulating/release agent which is required to prevent the alloy from
sticking to
the mould after casting. A typical insulating/release agent is silica.
A disc of ceramic is then added to the centrifugal casting mould in the manner
known in
the art in order to ensure that the mould is liquid tight and ready for
casting. This
prevents any alloy leakage during the casting process. The mould temperature
is
adjusted in preparation for the casting and may be in the range of 200 to 300
C. The
mould is then rotated at high speed to obtain usually the range of 80g to
120g, with a
rotation providing 100g being typical for a centrifugal casting speed.
A ladle is then brought to the furnace and a desired weight of alloy is tapped
off for the
purposes of casting. The ladle itself is preheated to a temperature in the
region of 800
to 1000 C in order to minimise cooling of the alloy after pouring. Alloy is
then
transferred to the hot ladle. At this stage, a further analysis of the alloy
may be
performed and any microaddition of elemental components may also, optionally,
be
performed in order to adjust the final chemistry of the alloy if this is
necessary.
The molten alloy in the ladle is then transferred to a pouring cup. The nose
of the
pouring cup has previously been adjusted to ensure that it mates with and
properly fits
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
13
the size of the input tube for the centrifugal casting mould. The level of
molten alloy in
the pouring cup is maintained in order to maintain adequate flow of alloy into
the mould
which is in effect fed by gravity. This provides a continuous flow of alloy
into the mould
until all of the weight of the alloy has been poured into the mould. The mould
is rotated
at high speed i.e. maintained at the centrifugal casting speed during the
process and
whilst the alloy is molten. The length of time the casting process takes
depends
ultimately on the desired thickness of the tube required and the skilled
person is able to
determine a suitable rotation time for a particular thickness of tube and
weight of alloy.
The mould is gradually slowed down as the alloy cools from its solidification
point.
Generally speaking, a "fast" solidification process is one in which the alloy
is cast and
then cools at a rate of more than about 100 C per minute and a "slow"
solidification
process is one in which the alloy is cast and then cools at a rate of about 50
C or
greater per minute. The casting process is usually completed in less than
about 10
minutes. The tube is extracted after the mould stops and the process may be
repeated
again.
Alloys of the invention can be assessed by the Larson-Miller relation. The
Larson-
Miller relation, also widely known as the Larson-Miller Parameter is a
parametric
relation used to extrapolate experimental data on creep and rupture life of
engineering
materials. Larson and Miller (Larson, Frank R. and Miller, James: A Time-
Temperature
Relationship for Rupture and Creep Stresses. Trans. ASME, vol. 74, pp. 765-
775)
proposed that creep rate could adequately be described by an Arrhenius-type
rate
equation which correlates the creep process rate with the absolute
temperature. They
established also that creep rate is inversely proportional to time.
Using the assumption that activation energy for the creep process is
independent of
applied stress, it is possible to relate the difference in rupture life to
differences in
temperature for a given stress. The Larson-Miller model is used for
experimental tests
so that results at certain temperatures and stresses can predict rupture lives
of time
spans that would be impractical to reproduce in the laboratory. In our
invention we use
a time span of 100,000 hours.
In an embodiment, the alloy of the present invention has a minimum stress
value of
40MPa when measured at 850 C for 100,000 hours rupture time. In other words,
following the Larson Miller model in our predictive test for an extended
rupture time of
100,000 hours, the alloy of the present invention has a minimum stress value
of 40
CA 02953240 2016-12-02
WO 2016/005724 PCT/GB2015/051740
14
MPa at 850 C In a further embodiment, the minimum stress value is 30 MPa at
900
C. In another embodiment, the minimum stress value is 20 MPa at 950 C. In yet
another embodiment, the minimum stress value is 15 MPa at 1000 C. In a still
further
embodiment, the minimum stress value is 10 MPa 1050 C. In further
embodiments,
the alloy will exhibit creep properties such that it satisfies to or more of
the above
minimum stress values taken in any combination. In a particularly preferred
embodiment, the alloy of the present invention has creep properties in which
the
minimum stress is the same as or higher in the range from 900 C to 1050 C
than the
line represented by H39WM in Figure 3.
Creep strength can be measured in accordance with the standard industrial test
ASTM
E139-1.
Alloys having the following compositions were produced in accordance with the
invention.
H39WM+ at% - general requirements
Ni at% Cr at% Nb at% Si at% WTI N/(M+Ti) N/C N+C at%
N at% N at% C at% C at% Fe
30 min 25.5min 0.78 2 0.7 0.5 0.2 2.25 2.45
0.350 0.550 1.900 2.100
balance
max Max Optimum Min Min Min Max Min Max Min Max
CA 02953240 2016-12-02
WO 2016/005724 PCT/GB2015/051740
H39WM++ at% - general requirements
Ni at% Cr at% Nb at.% Si at% M+Ti N/(M+Ti) N/C N+C
at% N at% N at% C at% C at% Fe
0.75 0.4 0.22 1.9 2
30 min 25.5 min 0.7 1
0.300 0.400 1.600 1.700
32.5 26.0 max Max Optimum Min Min Min Max Min Max Min Max balance
max max
H39WM+ (Trial A)
Fe Ni Cr Si C Nb Mn N Ti C+N N/C Nb+Ti N/(Ti+Nb)
A wt% 37.18 34.94 24.53 0.88 0.44 1.12 0.78 0.10
0.04
at% 36.28 32.44 25.72 1.71 2.00 0.66 0.77 0.38 0.04 2.37 0.19 0.70
0.539
5
H39WM++ (Trial B)
Trial C Si Mn Ni Cr Mo Nb W Ti Zr N Fe
Bwt% 0.35 0.76 0.72 36.07 24.36 0.01 1.24 0.04 0.04 0.01 0.08 36.32
at% 1.61 1.48 0.71 33.71 25.69 0.01 0.73 0.01 0.05 0.01 0.31 35.67
Trial C+N N/C Nb+Ti N/(Ti+Nb)
B wt%
at% 1.92 0.20 0.79 0.401
H39WM+ (further production examples)
Tubes Fe Ni Cr Si C Nb Mn N Ti C+N N/C Nb+Ti N/(Ti+Nb)
production
1 wt% 37.51 34.71 24.19 0.96 0.44 1.22 0.82 0.10 0.05
at% 36.61 32.23 25.36 1.86 2.00 0.72 0.81 0.40 0.05 2.39 0.20 077 0.517
2 wt% 37.59 34.95 24.03 0.88 0.43 1.22 0.76 0.11 0.03
at% 36.68 32.45 25.19 1.71 1.95 0.72 0.75 0.44 0.04 2.40 0.23 0.75 0.591
3 wt% 37.61 34.85 24.08 0.88 0.43 1.23 0.77 0.11
0.03
at% 36.71 32.36 25.25 1.71 1.95 0.72 0.76 0.44 0.04 2.39 0.22 0.76 0.573
4 wt% 37.66 34.79 24.02 0.97 0.43 1.19 0.81 0.10 0.04
at% 36.75 32.30 25.18 1.88 1.95 0.70 0.80 0.38 0.04 2.33 0.20 0.74 0.516
5 wt% 37.46 35.15 23.99 0.87 0.43 1.19 0.76 0.11
0.04
at% 36.56 32.64 25.15 1.69 1.95 0.70 0.75 0.42 0.05 2.37 0.22 0.74 0.565
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
16
The steel tubes of the present invention show excelled high-temperature
strength and
low ductility i.e. high creep resistance. The tubes also display excellent
corrosion
resistance at elevated temperatures over an extended period of time.
Consequently,
these steels are particularly suited to use in chemical plant under demanding
environments such as a reformer. In addition, it is expected that steel tubes
according
to the invention may be used in other applications such as ethylene crackers
and in
nuclear applications in heat exchanges and the like, such as those found in
pressurised
water reactors.
Without wishing to be bound by theory, it is believed that the beneficial
properties of the
steel alloys of the present invention arise due to the improved primary
carbide
precipitation and subsequent secondary carbide formation that occurs due to
the
carefully controlled relationships between the carbon, nitrogen and carbide
forming
elements in the alloys of the present invention. The alloys of the present
invention
benefit from particularly small carbide formation and the carbides formed in
the steels
of the present invention are longer and thinner than those in comparable
nickel
chromium steels.
We consider that careful control of the niobium carbide formation relative to
other
carbides so that relatively a greater proportion of niobium carbide is formed
in the
alloys according to the invention. For example, the standard H39WM alloy
contains
25% by weight chromium, 35% by weight nickel, 1% by weight niobium and 0.4% by
weight carbon together with micro additions of other alloying elements and
this alloy
has a chromium carbide (present as Cr3C7) present in an amount of 74%, based
on a
fraction analysis of a photo at 200 times magnification. In the alloys of the
present
invention this is found at levels around 61%.
Similarly, the niobium carbide content in the traditional H39WM alloy is
typically about
26% whereas in the alloys according to the present invention it is around 39%,
based
on a fraction analysis of a photo at 200 times magnification. An important
feature of
the alloys of the present invention is that they have a more homogeneous
carbide
formation. In other words, the carbides that are formed are more similar in
size to one
another and are smaller than in the conventional alloys. Thus, not only do the
alloys of
the present invention contain smaller carbides in otherwise apparently similar
alloy
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
17
compositions but also contain a greater proportion of niobium carbide. A lower
limit of
30%, and more preferably 35% of niobium carbide as a proportion of the total
amount
of carbide present is preferred. Similarly, a maximum proportion of 70%, and
more
preferably a maximum of 65% of the total carbides present is represented by
chromium
carbide. Again, these figures refer to a fraction analysis of a photo at 200
times
magnification. The presence of smaller and more dispersed carbides in the
present
invention improve the creep resistance of the steel because the growth of
secondary
carbides over time reduces the ability to stop movement of dislocations. This
in turn
means that the steel would become weakened over time.
The fast precipitation of niobium from the melt during the centrifugal casting
process
allows the alloy compositions of the present invention to be cast with the
homogeneous
carbide formation and relatively larger proportion of niobium carbides to
chromium
carbides as compared with convention steel alloys.
A further important feature of the alloys of the present invention relates to
the amount
of secondary chromium carbides on the surface. In the conventional alloys, the
surface
fraction of Cr307 is about 4% whereas in the alloys according to the invention
it is at
least 6% and more preferably 8% based on a fraction analysis of a photo at 200
times
magnification.
The properties of a steel according to the invention having the composition
H39WM+
(Trial A) and H39WM++ (Trial B) were investigated and the results are shown in
the
following tables.
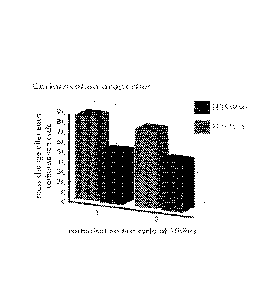
Figures 1 to 4 show the properties of the steels H39WM (a conventional steel
alloy)
and H39WM+ (a steel alloy according to the invention). Figure 1 shows the
carburisation properties over a carburisation test cycle of 100 hours, Figure
2 shows
the improvements in MSW thickness, Figure 3 shows the creep properties and
rupture
strength after 1000 hours at a variety of temperatures, and Figure 4 shows a
constant
stress creep test. The superior properties of the steels of the present
invention are also
evident in each case relative to the conventional steel from the following
data for
H39WM+.
CA 02953240 2016-12-02
WO 2016/005724 PCT/GB2015/051740
18
Room temperature tensile properties (Minimum values) N/mm2)
UTS 460 (66.7 ksi)
0.2% PS 225 (32.6 ksi)
Elongation 10%
Coefficient of linear expansion mm/mm C (1/K)
20-100 C 14.4 x 10-6
20-800 C 16.8 x 10-6
20-1000 C 17.6x 10-6
20-1100 C 18.1 x 10-6
Density
7.97 Gm/cc (0.288 lb/in3)
Hot tensile properties N/mm2 (Typical value)
800 C 900 C 1000 C 1100 C
Uts 250 (36.3 ksi) 160 (23.2 ksi) 98 (14.2 ksi)
74 (10.7 ksi)
0.2% PS 162 (23.5 ksi) 107 (15.5 ksi) 72 (10.4 ksi)
59 (8.6 ksi)
Elongation 26% 28.5% 32.5% 21%
Thermal conductivity (w/mK)
100 C 13.0 (0.031 cal/cm sec C)
800 C 24.3 (0.058 cal/cm sec C)
1000 C 27.7 (0.066 cal/cm sec C)
1100 C 29.7 (0.071 cal/cm sec C)
Figure 5 shows the constant stress creep test at 950 C.
The following tables show the creep rupture test results and the composition
of the
tube is shown below (H39WM+ (Trial A)). The carbon content was greater than 1
at%.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
19
Fe Ni Cr Si C Nb Mn N Ti
A wt% 37.18 34.94 24.53 0.88 0.44 1.12 0.78
0.10 0.04
at% 36.28 32.44 25.72 1.71 2.00 0.66 0.77 0.38 0.04
Temp ( C) Stress (Mpa) Creep test life (hrs)
1050 30 240 &289
1075 30 108 & 130
1100 30 33 & 34
Temperature Minimum Strain rate
( 0C) ( %/hr)
1050 1.10E-03 & 1.10E-03
1075 1.44E-03 & 1.04E-03
1100 1.88E-04 & 4.14E-04
Further steel alloys having the following compositions were produced.
Elements
(wt%)
TUBE Fe Ni Cr Si C Nb Mn N
1 34.9 24.0 0.88 0.43 1.16 0.76 0.11
37.9
2 35.0 24.0 0.88 0.43 1.22 0.76 0.11
37.7
3
37.1 35.2 24.5 0.85 0.42 1.10 0.78 0.10
4
37.8 34.9 24.1 0.88 0.43 1.23 0.77 0.11
5
37.7 34.7 24.2 0.96 0.43 1.22 0.82 0.10
6 37.8 34.8
24.0 0.97 0.43 1.19 0.81 0.10
The creep test results for these production examples are shown in the table
below.
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
Tubes 986C / 42.3Mpa
Creep test life
(hrs)
1 217
2 220
3 232
4 242
5 264
6 267
The tables below show a comparison between the creep test results for the
conventional alloy H39WM, and alloys of the invention H39WM+ and H39WM++. All
of
the alloys were produced under identical casting conditions which were as
follows:
5
Mould Mould wash (Silica based) 1.0-1.3mm
thickness
Mould temperature 240-2500 (stick)
Tapping Temperature 17100-17200
Casting Temperature 1615C-16200
weight 225kg / 278kg
Tube Outside diameter 127.4mm
Minimum Sound Wall 13.5mm / 16.7mm
CA 02953240 2016-12-02
WO 2016/005724
PCT/GB2015/051740
21
Creep Test Results for H39WM, H39WM+ and H39WM++
Temp Stress Life (hrs)
H39WM H39WM+ H39WM++
900 58.88 415 701 717
950 42.17 546 734 863
980 35.42 487 802
1000 31.24 494 813
1050 22.05 520 683
1100 16 230 379 329
* data not available
It is evident that both H39WM+ and H39WM++ exhibited superior lifetimes
relative to
H39WM across the temperature range tested. The temperatures at which the
alloys of
the invention and the conventional alloy was tested are representative of
temperatures
which might typically be experienced by the tubes in use. Thus, it can be seen
that the
tubes produced from alloys according to the invention exhibited extended,
lifetimes
both at low temperatures and also at high temperatures, relative to tubes made
of the
known H39MW alloy using the same process. Indeed, across all the temperature
ranges where data was available, it can be seen that the lifetimes have been
extended
by about 50% or more relative to tubes produced from conventional alloys. This
represents a significant advantage in engineering applications.