Note: Descriptions are shown in the official language in which they were submitted.
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
SYSTEMS AND METHODS FOR TRACKING INVENTORY AND
DISTRIBUTION OF MEDICATIONS IN A HEALTHCARE FACILITY
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application No.
62/018,711, filed on June 30, 2014. The entire disclosure of the application
referenced above is incorporated herein by reference.
FIELD
[0002] The present disclosure relates to systems and methods for managing
the storage and distribution of pharmaceuticals and medical supplies in a
healthcare facility.
BACKGROUND
[0003] The background description provided here is for the purpose of
generally
presenting the context of the disclosure. Work of the presently named
inventors,
to the extent it is described in this background section, as well as aspects
of the
description that may not otherwise qualify as prior art at the time of filing,
are
neither expressly nor impliedly admitted as prior art against the present
disclosure.
[0004] In a healthcare facility, pharmaceuticals (i.e., medications) and other
medical supplies are distributed from a central distribution location (e.g., a
central pharmacy) using a medication management system. Medication
management systems may be classified as centralized medication management
systems or decentralized medication management systems. For example, in a
centralized medication management system, medications may be provided from
the central pharmacy directly to a healthcare professional (e.g., a nurse)
that will
be administering the medications to respective patients.
[0005] Conversely, in a decentralized medication management system, multiple
medication dispensing sites are located remotely from a centralized
distribution
location, such as a facility's pharmacy. The remote dispensing sites, such as
a
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
nurses' station in a hospital ward, serve as base stations from which
healthcare
professionals can readily access medications or other medical supplies to be
administered to the patients under their care.
[0006] A decentralized medication management system may implement a
decentralized medication dispensing system (MDS). An MDS can comprise a
cabinet having a plurality of storage compartments, such as drawers, shelves,
or
bins, for example. The storage compartments are stocked with individual
medications and/or medication doses or other medical supplies by the
pharmacy. The contents of the base stations are thoroughly inventoried and the
distribution of medications and medical supplies is carefully controlled.
Access
to the MDS (and to the individual storage compartments in the MDS) is limited
and can be gained only by healthcare professionals with the appropriate
credentials. A user interface controls access to and records the inventory,
and
distribution of the medications and medical supplies from the MDS can be
computer controlled.
[0007] In some implementations, the MDS may correspond to an automated
dispensing machine (ADM) that stores medications in secure transportable
compartments. The compartments may be loaded (i.e., stocked with
medications) in the pharmacy and then transported to the ADM. A nurse
removes medications from the compartment at the ADM and transports the
medications (e.g., in a pocket) to the patient and administers the medication.
SUMMARY
[0008] Systems and methods according to the principles of the present
disclosure relate to tracking medications in a healthcare facility from the
central
pharmacy to the patient. For example, after medications are removed from a
medication base station (e.g., an ADM) and transferred to and/or stored in a
transport apparatus (e.g., a patient specific drawer, portable container, bin,
compartment and/or mobile point-of-care (POC) workstation) and/or are
otherwise under the control of a healthcare professional, and prior to the
medications being administered to the patient, inventory data, including the
in-
stock quantities and locations of the medications can be tracked and/or
2
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
monitored and recorded by a medication system. A central inventory database
storing comprehensive inventory data, for the stock of medications throughout
the healthcare facility, is not updated to reflect any changes to the in-stock
quantities of medications in inventory until the medication system
communicates
to the central inventory database that the medication has been administered to
the patient or otherwise disposed of by the healthcare professional.
[0009] The medication system includes a control module that determines when
a medication is removed from a central pharmacy and/or a medication base
station for administration to a patient, such as by a healthcare professional
according to a healthcare facility's medication management system protocols.
Upon removal from of the medication from the central pharmacy and/or the
medication base station, the medication is transferred to and/or stored in a
transport apparatus under the control of the healthcare professional for
securely
transporting medications to a patient care area (e.g., the patient's bedside)
and
administering the medications to the patient as prescribed. The transport
apparatus can include, for example, a patient specific drawer, portable
container,
or mobile POC workstation, and/or are otherwise under the control of a
healthcare professional. A remote, patient-specific medication database,
separate from the central inventory database associated with the healthcare
facility, stores or has access to inventory data for the medications in the
healthcare facility, including, for example, the quantities of the medications
located in the central pharmacy and/or in the medication base station. In
addition, the local medication inventory database records and/or stores
inventory
data including the quantities and locations of the medications in the
transport
apparatus and not yet administered to a patient.
[0010] The control module can track and/or monitor when medications are
removed from the central pharmacy and/or the medication base station and
placed in the transport apparatus and update the inventory data in the remote
medication database accordingly, including the in-stock quantities of the
medications in the transport apparatus. The control module can also track
and/or monitor when medications are administered by the healthcare
professional to the patient and update the inventory data in the remote
3
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
medication database accordingly. The control module can then update the
inventory data in the central inventory database based on the updated
inventory
data of the remote medication database of the medication system.
[0011] Further areas of applicability of the present disclosure will become
apparent from the detailed description, the claims and the drawings. The
detailed
description and specific examples are intended for purposes of illustration
only
and are not intended to limit the scope of the disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The present disclosure will become more fully understood from the
detailed description and the accompanying drawings, wherein:
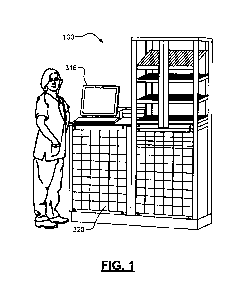
[0013] FIG. 1 is an example ADM;
[0014] FIG. 2 is an example mobile POC workstation;
[0015] FIG. 3 is an example medication management system including a
medication system according to the principles of the present disclosure;
[0016] FIG. 4 is an example medication system according to the principles of
the present disclosure;
[0017] FIG. 5 illustrates example information stored by a remote medication
database according to the principles of the present disclosure; and
[0018] FIG. 6 illustrates and example medication method according to the
principles of the present disclosure.
[0019] In the drawings, reference numbers may be reused to identify similar
and/or identical elements.
DETAILED DESCRIPTION
[0020] In a medication management system (e.g., a centralized, decentralized,
and/or hybrid medication management system), inventory data for the
medications maintained within a healthcare facility may be stored in a
medication
inventory database. Inventory data can include information about the in-stock
quantities and storage locations of the stock of medications on-hand in the
4
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
healthcare facility. Typically, the inventory data corresponds to medications
stored in a central pharmacy and, in a decentralized medication management
system, medications stored in medication base stations (e.g., ADMs)
distributed
throughout the healthcare facility. For example, the central inventory
database
may store inventory data including information about, inter alia, the stock on-
hand of unit dose packages of medications and their locations in the facility.
The
inventory data can be indexed and/or tallied by medication type and/or
location
(e.g., whether the medication is in the central pharmacy or in a particular
medication base station).
[0021] The medication management system generally updates the inventory
data for the medications when a healthcare professional (e.g., a nurse)
removes
a unit (e.g., a dose) of the medication from any of the medication base
stations
for administering the medication to a patient according to facility protocols.
For
example, the total stock for the medication may be reduced by the number of
units of the medication that are removed from the medication base station. In
other words, the medication management system may consider that the
medication is removed from inventory even before the medication has been
administered to a patient, or otherwise disposed of by the healthcare
professional (e.g., "wasted"). As such, there can exist a discrepancy between
the inventory data reflected in the central inventory database and the actual
stock on-hand of the medication in the healthcare facility. Typically, then,
it is
after the medication is administered to the patient by the healthcare
professional
according to facility protocols that an electronic Medical Administration
Record
(eMAR) is generated and the discrepancy in the inventory data of the central
inventory database can be resolved.
[0022] Accordingly, the central inventory database generally may not track
and/or monitor the inventory data for medications that are in transit between
a
medication base station and a patient care area.
[0023] Systems and methods according to the principles of the present
disclosure relate to tracking and/or monitoring the medications in a
healthcare
facility from the central pharmacy all the way to the patient (e.g., the
patient
bedside or other patient care area). For example, when a medication is removed
5
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
from a medication base station and placed in a transport apparatus, the in-
stock
quantity and location of the medication can be tracked and/or monitored (e.g.,
at
predefined intervals or in real-time) through the administering of the
medication
to the patient. Although examples of the systems and methods are described
herein with respect to decentralized medication management systems, the
principles of the present disclosure may be implemented in centralized
medication management systems and/or hybrid medication management
systems, as well.
[0024] For example only, an update to the inventory data of the central
inventory database may not be made in response to the healthcare professional
removing a medication from the pharmacy or medication base station. Instead,
the corresponding inventory data reflected in the central inventory database,
including the stock on-hand, may not be updated until the medication is
actually
administered to the patient. For example, a separate medication system may
continue to monitor the quantities and locations of the medication and
generate
and/or store that inventory data in a remote, patient-specific medication
database. The inventory data may include, but is not limited to, information
about the identity of the healthcare professional that removed the medication
from the medication base station, the identity of the transport mechanism
(e.g., a
POC workstation) to which the medication was transferred, a location within
the
healthcare facility of the medication (e.g., where it is and/or where it is
being
transported), etc. The medication system may be in continuous, conditional,
and/or periodic communication with the medication inventory database.
[0025] For example only, the medication system may record inventory data
reflecting that the medication was removed from the medication base station
and
transferred to and/or stored in a transport apparatus for subsequent
administration to a patient without also updating the total quantity inventory
data
in the central inventory database. Accordingly, the inventory data in the
central
inventory database may reflect the medication withdrawn from the medication
base station that is in transit for administration to a patient as still
included within
the stock on-hand in the medical base station. Or, the inventory data of
central
inventory database may still include the withdrawn medication as part of the
6
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
stock on-hand in the healthcare facility, while also indicating that the
medication
was removed from the medication base station. The medication system may
subsequently inform the central inventory database (and/or the medication base
station) when the medication is administered or otherwise disposed of (i.e.,
"wasted") by the healthcare professional. The central inventory database may
then update its inventory data, including the stock quantities of the
medication
throughout the facility and/or the status of the medication (e.g.,
administered to a
patient or wasted).
[0026] In this manner, the medication systems and methods according to the
principles of the present disclosure track and/or monitor, separately from and
supplemental to a central inventory database, the stock quantities, locations,
and
statuses of medications, and particularly medications removed from medication
base stations and transferred to transport apparatus for subsequent
administering to patients. Accordingly, current information is maintained
about
medications removed from medication base stations but not yet administered to
respective patients.
[0027] Referring now to FIGS. 1-3, FIGS. 1 and 2 show an example medication
base station 100 and mobile POC workstation 200, respectively. FIG. 3 shows
an example medication system 300 according to the principles of the present
disclosure operating within a medication management system 304. While the
medication management system 304 is described as a decentralized medication
management system, the medication system 300 may also be implemented in a
centralized medication management system or hybrid medication management
system. Accordingly, as described, the example medication management
system 304 includes the medication base station 100 and the mobile workstation
200.
[0028] In an example implementation, medications are provided from a central
pharmacy 308 to one or more medication base stations 100. A central inventory
database 312 stores inventory data about the medications, such as stock
quantities of each medication available in the healthcare facility, locations
of the
medications (e.g., stock quantities of each medication in the central pharmacy
308 and/or in respective medication base stations 100, etc.). At the
medication
7
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
base station 100, the healthcare professional accesses either the mobile
workstation 200 or the medication base station 100 according to facility
protocols
(e.g., by utilizing a user access control module 316 on one of the workstation
200 or base station 100). The healthcare professional then obtains information
related to one or more medications prescribed for a particular patient. The
information about patient specific medication is placed in a queue that can be
accessed by the control module 316, as appropriate.
[0029] In one example implementation, as the healthcare professional
approaches the base station 100 with the mobile workstation 200, the base
station 100 and the workstation 200 may negotiate a communication link. After
the communication link is secured, the base station 100 receives or reads the
information in the queue containing the information about patient-specific
medication and prescription information for a given patient. The base station
100 then enables access by the healthcare professional to respective storage
locations (e.g., drawers 320) containing the particular medications for that
patient. At the same time the mobile workstation 200 enables access by the
healthcare professional to the patient specific drawer 324 for that patient on
the
mobile workstation 200.
[0030] The healthcare professional then retrieves the medications from the
drawers 320 of the base station 100 and may record the retrieval activity
according to facility protocols. The healthcare professional then places those
medications in the patient-specific drawer 324 on the mobile workstation 200
and
may record that activity according to facility protocols. These steps are
repeated
for each of the medications for the patient that are retrieved from the base
station 100 and placed in the patient specific drawer 324 on the mobile
workstation 200. Then the steps may also be repeated for any number of
patients under the care of the healthcare professional.
[0031] The healthcare professional can thereafter administer the medications
to
the patient at the patient's bedside 328. For
example, the healthcare
professional transports the mobile workstation 200 to the patient. At that
time,
the healthcare professional can access the mobile workstation 200 according to
facility protocols utilizing the control module 316 on the workstation 200.
The
8
CA 02954045 2016-12-30
WO 2016/003855 PCT/1JS2015/038229
healthcare professional then selects the patient for administration of
medications. The control module 316 then enables access by the healthcare
professional to the patient-specific drawer 324 containing the medications for
that patient. The healthcare professional then removes the medications from
the
patient-specific drawer 324 and administers the medications to the patient
according to facility protocols (e.g., according to the well-known "five
rights"
protocol). This may include using the control module 316 to record that the
medications have been administered. Once the medications are administered to
the first patient, the healthcare professional can then proceed to successive
patients whose medications are contained in the mobile workstation 200, if
any.
[0032] Either or both of the medication base station 100 and the mobile
workstation 200 may be configured to communicate with peripheral devices,
such as bar code readers, PDAs, biometric security devices (e.g., a
fingerprint
scanner), scanners, card readers, keyboards, RFID systems, and the like. The
medication base station 100 and/or the mobile workstation 200 (e.g., via
respective control modules 316) may implement the operating protocols of the
healthcare facility for managing the distribution of medications from a
pharmacy
to a patient.
[0033] While the central inventory database 312 stores, for example, the
inventory data including stock quantities for medications in the central
pharmacy
308 and/or in the respective medication base stations 100, the medication
system 300 separately monitors, tracks, generates and/or stores inventory data
about medications removed from the medication base station 100 and
transferred to and/or stored in the mobile workstation 200 (or another
transport
apparatus under the control of a healthcare professional). For example, the
stock quantities of the medications in the healthcare facility that are
maintained
by the central inventory database 312 may typically be updated (e.g.,
diminished
to reflect spent inventory) when the healthcare provider removes the
medications
from the medication base station 100. However, in the medication management
system 304, the stock quantities of the medications are also maintained in the
inventory data of a remote, patient-specific medication database 408 (shown in
FIG. 4). The inventory data of the remote medication database 408 are not
9
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
diminished merely in response to the medication being removed from the
medication base station 100. Instead, the remote medication database 408
continues to reflect thatstock associated with the corresponding medication
base
station 100 still remains, and therefore is included within a total stock
quantity of
the facility, even while the central inventory database 312 may reflect a
different
(diminished) stock quantity.
[0034] The inventory data for the medications located in the mobile
workstation
200 may also associate the medications with a particular healthcare provider
(e.g., the healthcare provider that removed the medication from the medication
base station 100), a particular patient, and/or a location of the mobile
workstation
200. For example only, the location of the mobile workstation 200 may be
determined based on a predetermined portion of the healthcare facility
assigned
to the mobile workstation 200, by RFID or another real time location system
(RTLS), WLAN communication, etc.
[0035] The medication system 300 is in communication with one or more of the
central inventory database 312, the medication base station 100, and/or the
mobile workstation 200 to maintain and selectively update the stock quantities
of
the medications in the healthcare facility recorded in the central inventory
database 312. For example, the medication system 300 may inform the central
inventory database 312 to update the inventory data, including the stock
quantities of a medication, only after receiving confirmation from a
healthcare
professional that the medication was administered to the patient or otherwise
disposed (i.e., "wasted"). The central inventory database 312 may then update
the stock quantities and/or status of the medication.
[0036] Although schematically shown separate from the medication base
station 100 and the mobile workstation 200, the medication system 300 may be
a separate device or module (e.g., implemented within a handheld device), or
may be implemented within the medication base station 100 and/or the mobile
workstation 200 (e.g., within respective control modules 216).
[0037] Referring now to FIG. 4, a schematic representation of an example
medication system 400 includes a control module 404, a remote, patient-
specific
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
medication database 408, communication interfaces 412 and 416, a user
interface 420 (e.g., a graphical user interface), and an optional tracking
module
424. The control module 404 controls and coordinates communication and
processing of information between the remote medication database 408, the
communication interfaces 412 and 416, the user interface 420, and the tracking
module 424. For example, the control module 404 receives information about
various medications removed from medication base stations 100 and transferred
and/or stored in mobile workstations 200 via the communication interface 416
and/or the user interface 420, receives information about locations of a
mobile
workstation 200, medications removed from medication base stations 100,
healthcare professionals, and/or various devices implemented the medication
system 400 from the tracking module 424, and stores the information to and
retrieves the information from the remote medication database 408. The control
module 404 also provides information to be communicated via the
communication interfaces 412 and 416 and the user interface 420.
[0038] The user interface 420 allows a user (e.g., a healthcare professional)
to
interact with the medication system 400 and may include, for example only, a
display(e.g., for a graphical user interface), user input controls, etc. In
implementations where the medication system 400 is incorporated in the
medication base station 100 or the mobile workstation 200, the user interface
420 may correspond to a user interface of the control modules 316. The
healthcare professional may input information according to facility protocols,
such as, for example, identity authentication and/or security credentials,
medication removed from the medication base station 100, medication
administered to respective patients, etc.
[0039] The communication interface 412 exchanges information with the central
inventory database 412, and the communication interface 416 exchanges
information with the medication base station 100 and/or the mobile workstation
200. For example only, the communication interfaces 412 and 416 may
communicate using WiFi or other WLAN signals, Bluetooth, various wired
communication protocols, or any other suitable long or short range
communication protocols.
11
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
[0040] The tracking module 424 may implement RFID or another RTLS to
determine a location of a device implementing the medication system 400. The
location of the device (which may be associated with a particular medication
removed from the medication base station 100) may be recorded in the inventory
data generated and/or stored in the remote medication database, communicated
to the central inventory database 312 via the communication interface 412,
and/or communicated to the medication base station 100 and/or the mobile
workstation 200 via the communication interface 416. In some implementations,
the device implementing the medication system 400 corresponds to the mobile
workstation 200.
[0041] The control module 404 may receive information related to removal of a
medication from the medication base station 100 (and, in some implementations,
therefore stored in the mobile workstation 200). For example, the control
module
404 may learn that the medication is removed from the medication base station
100 via automatic communication transmitted from the medication base station
100 (via the communication interface 416) that can be selectively triggered by
the a drawer 320 being opened, a medication being removed from the drawer
320, a healthcare professional inputting information (via user interface 420,
one
of the control modules 316, etc.) indicating that the medication was removed
from the medication base station 100 or that the medication was placed in a
patient specific drawer 324, the patient specific drawer being opened and/or
the
medication being placed therein, etc. It can be appreciated that the above are
only several examples describing how the control module 404 may determine
when a medication is removed from the medication base station 100 and that
other implementations are anticipated.
[0042] The control module 404 records information about the medication in the
remote medication database 408. For example only, the remote medication
database 408 may be implemented using memory located on a handheld device
including the medication system 400. For example, the remote medication
database 408 may store information about stock quantities of medications in
the
healthcare facility consistent with the stock quantities stored in the central
inventory database 312, and/or stock quantities of the medications in
respective
12
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
medication base stations 100. The remote medication database 408 may store
further information about medication removed from the pharmacy and/or the
base station 100 and placed in the mobile workstation 200. This further
information may indicate various stock quantities (and locations) of
medications
that still reside in the healthcare facility, but not in the pharmacy 308 or a
base
station 100. In other words, the remote medication database 408 records
further
information indicating an amount of a medication that has been removed from
respective base stations 100 and has not yet been administered to a patient or
otherwise disposed. Accordingly, the further information indicates an amount
(and a location) of the medication that is located in various mobile
workstations
200, that is being carried by a healthcare professional, etc.
[0043] The central inventory database 312 may store information about the
stock quantities of the medication, but may not reflect the amount of the
medication still in the healthcare facility but not yet administered to a
patient. For
example, in a typical medication management system the central inventory
database may reduce the stock quantities of the medication when the medication
is removed from the base station 100 even though the medication has not been
administered or otherwise disposed (e.g., is in a mobile workstation 200).
Conversely, according to the principles of the present disclosure, the central
inventory database 312 maintains (i.e., does not reduce) the stock quantities
of
the medication upon removal of the medication from the base station 100.
Instead, the medication system 400 stores, in the remote medication database
408, information indicating that the stock quantities of the medication in the
healthcare facility is the same, but some portion of the medication is now in
transport between a respective base station 100 and a patient's bedside 328.
Accordingly, the remote medication database 408, and consequently the central
inventory database 312, has more robust and accurate information about the
total inventories of various medications within the healthcare facility.
[0044] Once the healthcare professional has administered the medication, the
remote medication database 408 can be updated, accordingly, to reflect the
actual reduction in the stock quantities of the medication in the healthcare
facility
(i.e., to reflect an actual inventory changing event). For example, the stock
13
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
quantities of the medication, as recorded in the inventory data of the remote
medication database 408, is reduced by an amount of the medication
administered. A corresponding stock quantity of the medication in the mobile
workstation 200 is also reduced. The remote medication database 408 may be
updated according to user input at the user interface 420, automatically in
response to a predetermined protocol being followed (e.g., bar codes
associated
with the patient, medication, healthcare professional, etc. being scanned as
part
of a medication administration protocol), and/or another suitable condition.
[0045] The medication system 400 may then update the central inventory
database 312 (via the communication interface 412) to adjust the stock
quantities of the medication in the healthcare facility. For example, the
medication system 400 may update the central inventory database 312
automatically upon confirmation that the medication was administered or
disposed of, on a periodic (e.g., real time) basis, and/or in response to some
other condition (e.g., in response to a prompt from a healthcare professional
at
the base station 100 and/or the mobile workstation 200).
[0046] In embodiments, the medication base station 100, the mobile
workstation 200, and/or another storage or transport item may include a
compartment (e.g., the drawer 324, a removable module or cassette, etc.)
movable between an open configuration and a closed configuration. Contents
(e.g., medication and/or other items) may be transferred to and from the
compartment in the open configuration. Conversely, the contents of the
compartment are not accessible in the closed configuration.
[0047] The control module 404 may communicate with the compartment (e.g.,
electronically, wirelessly, etc.). When a medication prescribed to a certain
patient is to be loaded into the compartment, the control module may receive
an
association signal that identifies the patient and store (e.g., a patient-
specific
database within the remote medication database 408 and/or the medication
inventory database 312), based on the association signal, an association
between the patient and the compartment. The association signal may be
generated upon access to the medication base station 100, the medication
system 300, etc. by a healthcare professional.
14
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
[0048] The control module 404 may then receive a load signal identifying the
medication prescribed to the patient and, upon verification that the
medication is
indeed prescribed to the patient associated with the compartment, (e.g., via
the
patient-specific database), cause the compartment to move to the open
configuration to allow the healthcare professional to load the medication. The
control module 404 stores (e.g., in the patient-specific database) information
indicating that the prescribed medication is now stored in the compartment. In
embodiments, the control module 404 may also inform the medication inventory
database 312 that the medication is stored within the compartment in the
medication management system 304. The compartment may then be
transitioned to the closed configuration after the medication is placed
therein.
Subsequently, the control module 404 may receive a dispense signal (prompted
by the healthcare professional attempting to access the compartment to
administer the medication to the patient). The control module 404 changes the
compartment to the open configuration in response to the dispense signal.
[0049] The healthcare professional can then access the compartment to
remove the medication and administer the medication to the patient. The
healthcare professional may provide some indication that the medication was
administered to the patient (e.g., via the user interface 420), causing an
administration signal to be provided to the control module 404. The control
module 404 can then update the patient-specific database (and/or the
medication inventory database) accordingly.
[0050] Referring now to FIG. 5, example information 500 stored by the remote
medication database 408 includes a medication type 504, a total stock quantity
508 of the medication in the healthcare facility, an amount 512 of the of the
medication in the pharmacy, amounts 516 and 520 of the medication in
respective base stations (1 and 2), and respective amounts 524, 528, and 532
of
the medication in respective mobile workstations (WS 1, 2, and 3).
Accordingly,
the total stock quantity 508 corresponds to a tally or sum of the stock
quantities
512, 516, 520, 524, 528, and 532. In contrast, the central inventory database
312 may only store information about the total stock quantity 508, the amount
512 of the medication in the pharmacy, and the amounts 516 and 520 of the
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
medication in the respective base stations 100. When a particular medication
is
administered as described above, the remote medication database 408 is
updated accordingly. For example, as medication is administered, the amount of
the medication in a particular one of the mobile workstations 200 is updated,
and
the total stock quantity 508 is reduced accordingly. The medication system 400
then updates the central inventory database 312 with the updated total stock
quantity 508 stored in the remote medication database 408.
[0051] Referring now to FIG. 6, an example medication method 600 begins at
604. At 608, a healthcare professional removes a medication from a central
pharmacy or a medication base station 100 and transfers the medication to a
transport apparatus, e.g., according to a facility-approved protocol. At 612,
the
method 600 updates a remote medication database 408 (separate from a central
inventory database 312 associated with the medication management system of
the healthcare facility) according to the medication removed from the
pharmacy/base station. At 616, the method 600 determines whether the
medication has been administered to a patient or otherwise disposed. If true,
the
method 600 continues to 620. If false, the method 600 continues to 616. At
620,
the method 600 updates the remote medication database 408 to reflect that the
medication was administered.
[0052] In some implementations, at 624, 628, 632, and 636 the method 600
may optionally generate and transmit a medication restock request, e.g., to
the
central pharmacy, and/or a medication reorder request, e.g., to the central
pharmacy and/or a third party pharmaceuticals supplier, based on the updated
remote medication database 408. For example, at 624, the method 600
determines whether to generate a restock request to the central pharmacy to
restock the medication base station 100. For example only, the method 600 may
generate the restock request if the administration of the medication, as
confirmed at 616, results in the stock quantity of the medication located in
the
medication base station 100 to decrease below a predetermined threshold. If
the result of 624 is true, the method 600 transmits the restock request to the
central pharmacy 308 to cause the central pharmacy 308 to have the medication
base station 100 restocked. If false, the method 600 continues to 632.
16
CA 02954045 2016-12-30
WO 2016/003855 PCT/US2015/038229
[0053] As a further example, at 632, the method 600 determines whether to
generate a reorder request to obtain new stock of the medication for the
healthcare facility. For example only, the method 600 may generate the reorder
request if the administration of the medication, as confirmed at 616, results
in the
total stock of the medication in the healthcare facility to decrease below a
predetermined threshold. If the result of 632 is true, the method 600
transmits
the reorder request to a third party pharmaceuticals supplier outside of the
healthcare facility and/or to the central pharmacy. If false, the method 600
continues to 640.
[0054] At 640, the method 600 updates the central inventory database 312
according to the information stored in the remote medication database 408. The
method 600 ends at 644.
[0055] The foregoing description of the embodiments has been provided for
purposes of illustration and description. It is not intended to be exhaustive
or to
limit the invention. Individual elements or features of a particular
embodiment
are generally not limited to that particular embodiment, but, where
applicable,
are interchangeable and can be used in a selected embodiment, even if not
specifically shown or described. The same may also be varied in many ways.
Such variations are not to be regarded as a departure from the invention, and
all
such modifications are intended to be included within the scope of the
invention.
[0056] Example embodiments are provided so that this disclosure will be
thorough, and will fully convey the scope to those who are skilled in the art.
Numerous specific details are set forth such as examples of specific
components, devices, and methods, to provide a thorough understanding of
embodiments of the present disclosure. It will be apparent to those skilled in
the
art that specific details need not be employed, that example embodiments may
be embodied in many different forms and that neither should be construed to
limit the scope of the disclosure. In some example embodiments, well-known
processes, well-known device structures, and well-known technologies are not
described in detail.
17