Note: Descriptions are shown in the official language in which they were submitted.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
1
Description
Method and System for recovery of methane from hydrocarbon streams
The invention relates to a method for recovery of methane from hydrocarbon
streams
and a system for recovery of methane from hydrocarbon streams.
Methane is a very important natural gas which is used in a huge variety of
different
applications. One important use of methane is as a fuel, since burning methane
produces less carbon dioxide for each unit of heat released in comparison with
other
hydrocarbon fuels. Usually methane is supplied in the form of a liquefied
natural gas
(LNG) for storage and transportation purposes. Another very important use of
methane
is the application of methane as a reactant in a technical synthesis. Methane
is an
important starting material, for example, for the technical synthesis of
hydrogen,
methanol, ethylene, hydrogen cyanide, methyl halogenides, or organic
compounds.
In general, such technical grade syntheses yield a synthetic gas mixture
(reaction
mixture) comprising different reaction products, unreacted starting materials
and,
optionally, other compounds which were introduced during the reaction process,
but did
not participate in the reaction itself. Different methods have been developed
in order to
separate the target product, or the target products, from the reaction
mixture.
Commonly a demethaniser is applied in order to separate methane and other
hydrocarbon-free compounds (e.g. hydrogen or nitrogen) from the remaining
hydrocarbon compounds in the reaction mixture, which comprise a carbon content
of
C2 or higher.
The use of a demethaniser system on a synthetic gas mixture aims at the
separation of
methane and other hydrocarbon-free gases from a reaction mixture in order to
facilitate
a further subsequent separation step of the now methane-free hydrocarbon
fraction
comprising hydrocarbons with a carbon content of C2 or higher. The thus
separated
methane content is generally discharged or has to be further processed in
order to be
used for any further process or synthesis.
In this regard, US 2013/225884A1 discloses processes for producing and
separating
ethane and ethylene, wherein an oxidative coupling of methane (OCM) product
gas
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
2
comprising ethane and ethylene is introduced to a separation unit comprising
two
separators. Within the separation unit, the OCM product gas is separated to
provide a
C2-rich effluent, a methane-rich effluent, and a nitrogen-rich effluent.
Given that the availability of methane (and other natural resources) is
limited and the
worldwide demand is increasing it is problematic that the unreacted reactant
methane
cannot be reused, in particular for further synthesis purposes, without an
extensive
treatment prior of said reuse.
This problem is solved by a method comprising the features of the independent
claim 1
and an integrated system comprising the features of the independent claim 13
which
allow a recovery of methane from hydrocarbon streams, wherein the recovery of
methane particularly allows the facile recycling and reuse of the methane
content for
further synthesis purposes.
The method of the invention for the recovery of methane from hydrocarbon
streams
comprises the following steps:
a. introducing a feed fluid stream, which comprises a methane fluid, at least
one
hydrocarbon-free fluid, wherein in particular said at least one hydrocarbon-
free
fluid is nitrogen, and at least one hydrocarbon fluid, into a demethaniser
system;
b. separating said feed fluid stream in said demethaniser system into
- a carbon-rich fraction, which comprise hydrocarbons with a carbon
content of C2 and higher, and
- a separation stream, which comprises methane fluid and at least one
hydrocarbon-free fluid;
c. introducing said separation stream into a hydrocarbon-free fluid separation
system, in particular in a cryogenic hydrocarbon-free fluid separation system,
more particularly into a cryogenic nitrogen rejection system; wherein
preferably
said separation stream is compressed by a compressor system before said
separation stream is introduced in said hydrocarbon-free fluid separation
system, wherein preferably said separation stream is compressed to a pressure
of 25 bar to 80 bar;
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
3
d. Separating said separation stream in said free fluid separation system into
a
methane stream and a hydrocarbon-free fluid stream.
The method of the invention allows the provision of an essentially pure
methane stream
and a good separation of said methane stream from the feed fluid stream, which
may
be used in a reaction process for further products.
According to the invention, the term "feed fluid stream" is to be understood
as a liquid
and/or a gas stream comprising liquid or gaseous methane, liquid or gaseous
hydrocarbon compounds, and/or a hydrocarbon-free fluid in liquid and/or
gaseous form.
According to the invention the term "hydrocarbon compounds" is to be
understood as
compounds with a carbon content of C2 or higher which comprise at least one
hydrogen-carbon bond. Such hydrocarbon compounds are particularly alkane or
alkene
compounds like ethane, ethane (ethylene), propane or propene (propylene) and
the
like.
According to the invention the term "hydrocarbon-free fluid" is to be
understood as a
compound in a liquid or a gaseous form which comprises no hydrogen-carbon
bond,
such as hydrogen, nobel gases, CO, CO2, or nitrogen. A hydrocarbon-free fluid
is
particularly argon, CO, hydrogen or nitrogen, more particularly nitrogen.
In some embodiments said feed fluid stream derives from a synthesis system
which
uses methane as a reactant. Such synthesis systems may be a system designated
for
the oxidative coupling of methane (OCM) or a methane pyrolysis. In some
embodiments the synthesis system is a system designated for the oxidative
coupling of
methane (OCM).
The oxidative coupling of methane is a known chemical reaction (OCM reaction)
applied to the conversion of methane into further chemicals, in particular
into ethan,
ethylene, C3- hydrocarbons or C4- hydrocarbons, more particularly ethylene.
The
reaction is generally carried out in the presence of a catalyst and comprises
several
reaction and separation steps for producing ethylene from a methane feed. The
methane feed is generally mixed with compressed air and comprises after the
reaction
with the catalyst nitrogen, methane, CO, CO2, hydrocarbons with a carbon
content of
C2 or higher (e.g. ethan, ethylene, C3- hydrocarbons or C4- hydrocarbons), and
water.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
4
The principle product of OCM is ethylene, the world's largest commodity
chemical, and
the fundamental building block of the chemical industry. However, methane
activation
is difficult owing to its thermodynamic properties. This limits the efficient
utilisation of
methane, an important petrochemical resource. The application of a catalyst in
the
reaction system and the adjustment of the reaction conditions have improved
the
conversion of methane in an OCM reaction. However, the products of OCM
reactions
¨ depending on the reaction conditions ¨ may react to undesired by-products.
In order
to improve the selectivity of the products, such as ethylene, a low conversion
of
methane is used. Thus, a significant amount of unreacted methane is left in
the
reaction mixture.
In some embodiments, said methane stream is recycled and reused as a reaction
product in a technical synthesis.
In some embodiments, the feed fluid stream is derived from a synthesis system,
which
uses methane as a reactant and said feed fluid stream is separated in said
demethaniser system into said carbon-rich fraction and said separation stream,
wherein said separation stream is introduced into said hydrocarbon-free fluid
separation system, in particular in said cryogenic hydrocarbon-free fluid
separation
system, more particularly into said cryogenic nitrogen rejection system, and
wherein
said separation stream is separated in said hydrocarbon-free fluid separation
system
into a methane stream and a hydrocarbon-free stream.
In some embodiments, the feed fluid stream is derived from a synthesis system
designated for an OCM reaction and said feed fluid stream is separated in said
demethaniser system into said carbon-rich fraction and said separation stream,
wherein said separation stream is introduced into said nitrogen rejection
system, in
which said separation stream is separated into a methane stream and a nitrogen
stream.
In some embodiments, said separation stream is compressed by said compressor
system before said separation stream is introduced in said hydrocarbon-free
fluid
separation system, wherein in particular said separation stream is compressed
to a
pressure of 25 bar to 75 bar, preferably to a pressure of 25 bar to 60 bar,
more
preferably to a pressure of 25 to 40 bar, more preferably to a pressure of 30
to 40 bar,
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
particularly to a pressure of 30 bar. The boundaries of the above pressure
ranges may
also be combined in an arbitrary fashion. Furthermore, in some embodiments,
the
lower pressure boundary of these pressure ranges may also be one of: 12 bar,
13 bar,
14 bar, 15 bar, 16 bar, 17 bar, 18 bar, 19 bar, 20 bar, 21 bar, 22 bar, 23
bar, 24 bar, 25
5 bar, 26 bar, 27 bar, 28 bar, 29 bar.
The compression of the separation stream after leaving this demethaniser
system and
before the introduction into the hydrocarbon-free fluid separation system
allows for a
better separation and isolation of methane and hydrocarbon-free gas, in
particular
nitrogen, from the separation stream as in comparison to a direct introduction
of the
separation stream from the demethanizer system into the hydrocarbon-free fluid
separation system.
As discussed previously, a higher pressure in the hydrocarbon-free fluid
separation
system allows for a better separation of methane and the hydrocarbon-free gas.
However, a lower pressure is preferred in the demethaniser system, since the
increase
of pressure in the demethaniser would lead to higher hydrocarbon product
losses. For
example, if the feed fluid stream is derived from an OCM separation system,
too high a
pressure would lead to ethylene product losses. The use of a compressor
system, in
particular of a 3-step compressor system (e.g. a compressor system described
in the
document W002/088612A1) allows for a compensation of the preferably lower
pressure in the demethaniser system and allows the separation of the
separation
stream in the hydrocarbon-free fluid separation system at a higher pressure.
In some embodiments the separation stream derived from the compressor is
cooled
down, in particular the separation stream is cooled down in a plate fin heat
exchanger,
and expanded to a lower pressure, before said separation stream is introduced
into the
hydrocarbon-free fluid separation system.
In some embodiments, said carbon-rich fraction from the demethaniser system is
transferred to a C2-splitter in which hydrocarbons with different carbon
contents of said
carbon-rich fraction are separated from each other. The use of a C2-splitter
allows
separation and isolation of target products from the carbon-rich fraction. C2-
splitters
are known in the art, and the dimensions and separation conditions depend on
the
target compound, which depends in itself on the previously-applied synthesis
system,
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
6
which provides the feed fluid stream. For example, if an OCM reaction system
is used,
which provides the feed fluid stream, the C2-splitter is designed and operated
in such a
way that the target compound ethylene can be separated in high purity. If the
separated methane stream (as described previously) is recycled and
reintroduced into
the OCM reaction system (synthesis system), the target compound ethylene could
be
achieved in a more cost-effective way, since the (nowadays restricted) natural
resource
methane is used more efficiently.
In some embodiments, said carbon rich fraction of the demethanizer system is
reboiled
in a reboiler, in particular said carbon rich fraction of the demethanizer
system is
reboiled before said carbon rich fraction is transferred to said C2-splitter.
In some embodiments, at least parts of the feed fluid stream are liquidized in
a cooling
system before the introduction into a demethaniser unit of said demethaniser
system.
The demethaniser unit is designed to separate methane and the hydrocarbon-free
fluid,
in particular nitrogen, from the hydrocarbons with a carbon content of C2 or
higher from
the reaction mixture derived from the reaction system. A demethaniser unit may
be for
example a distillation column.
In some embodiments, said feed fluid stream is separated in said cooling
system into a
liquid feed fluid stream and a gaseous feed fluid stream, wherein said liquid
feed fluid
stream is transferred to said demethaniser unit and said gaseous feed fluid
stream is
transferred to a (first) expander booster system, in which said gaseous feed
fluid
stream is expanded to a lower pressure before introducing into said
demethaniser unit.
The expansion of the gaseous feed fluid stream in the expander booster system
allows
recovery of work power, which might be used in a subsequent compression step
in the
demethaniser system.
In some embodiments, the liquid feed fluid stream from the cooling system and
the
gaseous feed fluid stream from the (first) expander booster system are
combined in
said demethaniser unit and separated in the demethaniser unit into a carbon-
rich
fraction and separation stream. After the separation the separation stream is
introduced into a second expander booster system, in which it is expanded, in
particular expanded to approximately 4 bar, before said separation stream is
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
7
introduced in said hydrocarbon-free fluid separation system. The expansion of
the
separation stream provides the chilling duty used in the demethaniser system.
In some embodiments, said gases feed fluid stream is introduced into a first
expander
booster system in which said gases feed fluid stream is expanded to a lower
pressure
before introducing into said demethaniser unit. Furthermore, said separation
stream
from said demethaniser unit is introduced in the second expander booster
system in
which it is expanded to provide said chilling duty. Additionally, the work
power of both
expanders is recovered to recompress the separation stream, in particular to
recompress the separation stream to approximately 6 bar, before said
separation
stream is introduced into said hydrocarbon-free fluid separation system.
In some embodiments, the demethaniser system is operated at a pressure of 6 to
40
bar.
In some embodiments, the demethaniser unit of said demethaniser system is
operated
at a pressure of 9 to 25 bar, in particular at a pressure of approximately 13
bar. In
some embodiments the demethaniser unit is operated at a temperature range of -
20 to
-170 C. In some embodiments, the demethaniser unit comprises a degrading
temperature range along its longitudinal axis, wherein in particular the
demethaniser
unit comprises a temperature of - 30 C at the bottom of the demethaniser
unit, and a
temperature of approximately - 150 C at the top of the demethaniser unit.
In some embodiments, said separation stream is introduced in at least one high-
pressure column arranged in said hydrocarbon-free fluid separation system, in
which
said separation stream is separated in a methane-rich bottom liquid and an
essentially
pure gaseous hydrocarbon-free overhead, wherein said methane-rich bottom
liquid is
transferred into at least one low-pressure column arranged in said hydrocarbon-
free
fluid separation system, in which said methane-rich bottom liquid is separated
in
hydrocarbon-free gas and a methane-rich liquid fraction. The methane-rich
liquid
fraction is at least partially vaporized ¨ providing a liquid fraction and a
methane gas
fraction ¨ wherein the cold derived from said liquid methane gasification is
used for the
separation process. Thus, allowing for the separation of a hydrocarbon-free
gas
fraction and a methane gas fraction, wherein both fractions are discharged
from the
low-pressure column.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
8
In some embodiments, the low-pressure hydrocarbon-free gas fraction and the
liquid
fraction ¨ derived from the partial vaporization of said methane-rich bottom
liquid ¨ are
used to cool the inlet streams of both columns.
In some embodiments, said methane-rich bottom liquid is transferred to the mid
part of
said low-pressure column.
In some embodiments, said methane-rich bottom liquid and said gaseous
hydrocarbon-
free overhead are sub cooled in a cooler, particularly a reflux cooler, to
approximately -
160 C before they are transferred to said low-pressure column.
In some embodiments, said hydrocarbon-free overhead from the high-pressure
column
is at least partially condensed and said bottom liquid from the low-pressure
column is
at least partially vaporised on a heat exchanger, in particular on a heat
exchanger
which is arranged between said high-pressure column and said low-pressure
column.
In some embodiments, said at least one high-pressure column and said at least
one
low-pressure column are integrated in one unit, wherein said at least one high-
pressure column and said at least one low-pressure column are interconnected
with a
heat exchanger situated between both columns.
The use of a high-pressure column and a low-pressure column, particularly
connected
with a heat exchanger situated between both columns, allows for the separation
of the
separation stream into an essentially pure hydrocarbon-free gas and an
essentially
pure methane gas. In a preferred embodiment the feed fluid stream is provided
from an
OCM reaction system, thus comprising a very high content of nitrogen and a
substantial amount of methane. The method of the invention allows firstly the
separation and isolation of the methane and nitrogen mixture (separation
stream) from
the reaction mixture of the OCM reaction (the feed fluid stream) in said
demethaniser
system, and secondly the separation from each other in said cryogenic nitrogen
rejection system in a very high purity. Thus, the gaseous methane can be
recycled and
reused in the OCM reaction system.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
9
In some embodiments said high-pressure column is operated at a pressure of 6
to 40
bar, in particular at a pressure of approximately 20 bar, and a temperature of
-160 to
-90 C, in particular at a temperature of approximately -140 C, and wherein
said low-
pressure column is operated at a pressure of 1 to 5 bar, in particular at a
pressure of
approximately 2 bar, and a temperature of -220 to -180 C, in particular at a
temperature of approximately - 190 C.
The use of the aforementioned separation conditions, in particular use of such
a high
pressure in the hydrocarbon-free fluid separation system, allows for a good
separation
of methane from a hydrocarbon-free gas.
According to another aspect of the invention the invention comprises a system
for
recovery of methane from hydrocarbon streams comprising:
a. a demethaniser system, which is designated to separate a feed fluid stream,
which comprises methane fluid, at least one hydrocarbon-free fluid, wherein in
particular said at least one hydrocarbon-free fluid is nitrogen, and at least
one
hydrocarbon fluid, into
- a carbon-rich fraction, which comprises hydrocarbons with
carbon
content of C2 and higher, and
- a separation stream, which comprises methane fluid and at least one
hydrocarbon-free fluid,
b. a hydrocarbon-free fluid separation system, in particular a cryogenic
hydrocarbon-free fluid separation system, more particularly a cryogenic
nitrogen
rejection system, which is designated to separate said separation stream into
a
methane stream and a hydrocarbon-free stream; and
c. preferably a compressor system that is configured to compress said
separation
stream to a pressure of 12 bar to 80 bar upstream said hydrocarbon-free fluid
separation system.
In some embodiments, the compressor system is configured to compress said
separation stream to a pressure of 15 bar to 75 bar, preferably to a pressure
of 20 bar
to 60 bar, more preferably to a pressure of 25 bar to 40 bar, more preferably
to a
pressure of 30 bar to 40 bar, particularly to a pressure of 30 bar, before
said separation
stream is introduced in said hydrocarbon-free fluid separation system.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
The boundaries of these pressure ranges may also be combined in an arbitrary
fashion. Furthermore, in some embodiments, the lower pressure boundary of
these
pressure ranges may also be one of: 12 bar, 13 bar, 14 bar, 15 bar, 16 bar, 17
bar, 18
bar, 19 bar, 20 bar, 21 bar, 22 bar, 23 bar, 24 bar, 25 bar, 26 bar, 27 bar,
28 bar, 29
5 bar.
In some embodiments the system of the invention comprises a synthesis system,
which uses methane as a reactant and provides said feed fluid stream, wherein
in
particular said synthesis system is a system for oxidated coupling of methane
(OCM),
10 wherein in particular the system comprises means to transfer the
recovered and
isolated methane from the hydrocarbon-free fluid separation system to the
synthesis
system.
Concerning further embodiments, reference is made to the detailed description
of the
method of the invention and the figures.
Further details and features of the invention are described in the following
figures of
two embodiments of the invention.
Figure 1 shows a first embodiment of the invention comprising a
demethaniser
system 1 and a hydrocarbon-free fluid separation system 2; and
Figure 2 shows a second embodiment of the invention comprising a
demethaniser system 1, a cryogenic nitrogen rejection system 2",
and an OCM synthesis system 3.
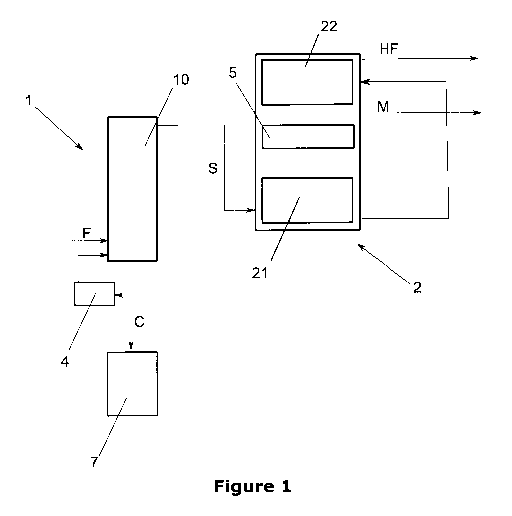
Figure 1 shows a system for recovery of methane from hydrocarbon streams
comprising a demethaniser system 1 and a hydrocarbon-free fluid separation
system 2.
A feed fluid stream F, comprising methane fluid, at least one hydrocarbon-free
fluid,
and at least one hydrocarbon fluid is introduced into a demethaniser unit 10
of the
demethaniser system 1. The demethaniser unit 10 is operated at a pressure of
13 bar.
Different pressures may be applied as necessary.
The demethaniser unit 10 comprises a temperature gradient with a temperature
of - 30
C at the bottom of the demethaniser unit 10 and a temperature of approximately
- 150
C at the top of the demethaniser unit 10. Thus the demethaniser unit 10 allows
for a
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
11
separation of the feed fluid stream F into a carbon-rich fraction C at the
bottom of the
demethaniser unit 10, and a separation stream S, which comprises methane fluid
and
at least one hydrocarbon-free fluid, in particular nitrogen, at the top of the
demethaniser
unit 10.
Optionally the feed fluid stream F may be cooled down with at least one
cooling system
(not depicted in the figure), wherein each separated liquid of each cooling
step (liquid
feed stream) is introduced into the demethaniser 10. A remaining gaseous feed
stream
may be transferred from the cooling systems into an expander boost system (not
depicted in the figure), in which it is expanded to a lower pressure and
subsequently
introduced into the demethaniser unit 10.
The carbon-rich fraction C from the bottom of the demethaniser 10 is reboiled
in a
reboiler 4 in order to provide a carbon-rich fraction C, which is free of
methane and
hydrocarbon-free fluids like nitrogen. The carbon-rich fraction C is then
transferred to a
C2 splitter 7 for further separation in order to isolate the target product
from the carbon-
rich fraction C. For example, the target product is ethylene if the feed fluid
stream F is
derived from a synthesis system 3 (see figure 2), which applies the oxidative
methane-
coupling reaction (OCM).
The separation stream S is then transferred from the top of the demethaniser
unit 10 to
the hydrocarbon-free fluid separation unit 2. Optionally the separation stream
S may be
transferred - prior to the introduction to the hydrocarbon-free fluid
separation system 2 -
into a second expander (not depicted), where it is expanded to approximately 4
bar,
providing the chilling duty used in the demethaniser system. The work power of
the first
and the second expander can be recovered in order to recompress the separation
stream S to approximately 6 bar, before it is introduced into the hydrocarbon-
free fluid
separation system 2.
The hydrocarbon-free fluid separation system 2 comprises a high-pressure
column 21
and a low-pressure column 22, which are interconnected with a heat exchanger 5
situated between the high-pressure column 21 and the low-pressure column 22.
Before the separation stream S is introduced into the bottom of the high-
pressure
column 21 it may be cooled down by, for example, a plate fin heat exchanger.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
12
Alternatively, the high-pressure column 21 and the low-pressure column 22 can
be
constructed as separate columns.
In the high-pressure column 21 the separation stream S is separated into a
methane-
rich bottom liquid at the bottom of the high pressure column 21 and a gaseous
stream,
comprising essentially pure hydrocarbon-free overhead product, in particular
an
essentially pure nitrogen overhead product. The pressure at the bottom of the
high-
pressure column 21 is approximately 20 bar, and the temperature is
approximately -
140 C. The bottom liquid from the bottom of the high pressure column 21 is
transferred to the mid-section of the upper low-pressure column 22.
Optionally the bottom liquid may be sub-cooled in a reflex cooler to
approximately - 160
C before it is transported to the mid-section of the low-pressure column 22.
The low-
pressure column 22 operates at a pressure of 2 bar, which allows for a further
separation of hydrocarbon-free gas, in particular nitrogen, and methane, due
to their
physical properties.
Columns 21 and 22 are connected by an integrated heat exchanger 5. In this
heat
exchanger 5 the overhead vapour from the high-pressure column 22 will be
condensed
while simultaneously the bottom liquids from the lower-pressure column 22 will
be
partially vaporised. The low-pressure hydrocarbon-free gas, in particular
nitrogen, and
the methane can be used to cool the inlet streams of both columns. The use of
the
high-pressure column 21, the low-pressure column 22 and the integrated
exchanger 5
allows for the separation and isolation of a hydrocarbon-free gas HF, in
particular
nitrogen, and methane M in a high purity. Alternatively, the high-pressure
column 21,
the low-pressure column 22 and the integrated exchanger 5 may be separate
units.
The hydrocarbon-free product HF, in particular nitrogen, can be sent to the
atmosphere, while the isolated methane M can be recycled and introduced into a
reaction process, which uses methane as a reactant. Alternatively, hydrocarbon-
free
product HF and the isolated may be further processed before being sent to the
atmosphere or being recycled and introduced into a reaction process.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
13
Figure 2 shows a system for recovery of methane from hydrocarbon streams
comprising a demethaniser system 1, a cryogenic nitrogen rejection system
2"and a
synthesis system 3, which uses methane in an OCM reaction.
Concerning the description and the features of functions or applications with
the same
numbering or letter, reference is made to the description of Figure 1. The
system for
recovery of methane from hydrocarbon streams is essentially the same as in
Figure 1.
The two main differences are that the feed fluid stream F derives from a
synthesis
system 3 which uses methane as a reactant in an OCM reaction. Thus, the
separation
stream S comprises essentially methane and nitrogen. Another difference is
that,
before the separation stream S is transferred from the demethaniser system 1
and
introduced into the cryogenic nitrogen rejection system 2", the separation
stream S is
compressed with a compression system 6 to approximately 25 bar to 80 bar,
preferably
to a pressure of 25 bar to 75 bar, preferably to a pressure of 25 bar to 60
bar, more
preferably to a pressure of 25 to 40 bar, in particular to 30 bar. The other
pressure
ranges stated above may also be used.
As discussed previously, a cryogenic nitrogen rejection system 2" provides a
very good
separation and isolation of nitrogen and methane, if it is operated with a
high pressure.
Conversely the demethaniser system 1 is preferably operated at a lower
pressure in
order to minimise product losses concerning the main product ethylene (derived
from
the OCM reaction). Thus, the use of a compressor system 6, in order to provide
a
separation stream S with a higher pressure compared to the situation in the
dennethanizer system 1, compensates for these deficiencies.
The use of the feed fluid stream F derived from an OCM reaction, the
separation of the
feed fluid stream F in a demethaniser unit 10 into a carbon-rich fraction C
and a
separation stream S, the compression of said separation stream S, the
subsequent
separation of the compressed separation stream S in a cryogenic nitrogen
rejection
system 2" into essentially pure nitrogen and essentially pure methane, and the
recycling and re-use of the thus separated methane in the aforementioned OCM
reaction allows for an efficient and economically effective use of the
important reactant
methane.
CA 02954549 2017-01-09
WO 2016/015849
PCT/EP2015/001518
14
List of references
demethaniser system 1
demethaniser unit 10
hydrocarbon-free fluid separation system 2
cryogenic hydrocarbon-free fluid separation system 2'
cryogenic nitrogen rejection system 2"
high-pressure column 21
low-pressure column 22
synthesis system 3
Reboiler 4
heat exchanger 5
compression system 6
C2 splitter 7
hydrocarbon-free fluid stream HF
methane stream M
feed fluid stream F
separation stream S
carbon rich fraction C