Note: Descriptions are shown in the official language in which they were submitted.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
AGENT WITH ANTIVIRAL PROPERTIES FOR PREVENTING OR TREATING
INDIVIDUALS EXPOSED TO A VIRUS OF THE BIRNAVIRIDAE FAMILY
TECHNICAL FIELD
The present invention relates to molecules that can be used as a prophylactic
agent,
therapeutic agent, a prophylactic food supplement with therapeutic potential,
or an agent with
antiviral properties which prevent infections or treat animals or fish that
have been exposed to
or are infected with a virus of the Bimaviridae family. Specifically, the
invention pertains to
peptides that are effective in decreasing viral infection by reducing or
impeding the production
of viral particles, and can therefore prevent the potential mortalities
associated with virus
infection.
BACKGROUND AND PRIOR ART
Bimaviruses corresponds to a group of viruses possessing two segments of
double-stranded
RNA and belongs in group III according to the general classification of
viruses. This group
contain eight families, all of which have icosahedral symmetry, with a
diameter of 60 nm and
are "unwrapped". Present inside the virions are 5 proteins and between 3 and 4
peptides.
The Bimaviridae family consisits of the Aquabimavirus, Avibirnavirus and
Entomobirna virus
genera. The Aquabirnavirus genus consists of birnavirus that infect fish,
molluscs, crustaceans
and rotifers. Species of the Aquabimavirus genus are the causative agents of
infectious
pancreatic necrotic virus (IPNv), as well as the yellow tail ascites virus
(YTAV), marine
birnavirus (MABV). The Blosnavirus family includes the blotched snakehead
virus (BSNV); the
Avibirnavirus is a genus of viruses that affect specifically poultry, and
corresponds to the cause
of the Infectious Bursa! Disease, which has great impact in poultry industry.
In particular, the
virus is Infectious Bursa! Disease virus (IBDv); and finally, the Drosophila X
virus is part of the
Entomobirnavirus family and infects Drosophila melanogaster (fruit fly).
In the present invention, the infectious pancreatic necrosis virus (IPNv) is
used as a prototype
birnavirus to generate a generic alternative prophylactic that can be used
against the whole
Bimaviridae family of viruses. Therefore, the description provided in here
below directed
specifically to IPNv should be understood as an example and not to be used to
limit the scope
of the invention.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
In a particular case, without intending to limit the scope of the present
invention to a particular
virus species, described below is one of many instances where a Birnaviridae
family virus
represents a major problem for a particular industry. This description should
only be interpreted
as an exemplification of one of the problem cases, bearing in mind that there
are other
members of the Birnaviridae family affecting other industries and these
industries could also
benefit from the present invention.
Chile is the second largest global competitor in salmon farming. However, the
industry is
subject to production highs and lows, which usually are caused by pathogens.
Agents that
have generated the greatest losses to salmon aquaculture are IPNv, ISA and the
bacterium P
salmonis to name the most important. In the case of pancreatic necrosis virus
(IPNv) statistics
reveal losses of the order $200 million per year.
This pathogenic agent is spread throughout all salmon producing countries and
has a particular
characteristic: it is latent in bottom sediments infecting different mollusks
and native fish
surrounding salmon farms without causing disease and can be isolated from fish
farms in
lakes. In addition, the largest outbreaks of disease occur during the early
stages of
development, i.e. first feeding stage and in the transfer of the fish smolt to
the ocean producing
outbreaks with up to 70% mortality.
To date there are several therapeutic tools against this infection with the
greatest benefits
coming from vaccines with antigenic action against the IPNv structural
protein; however this
type of protection can only be used in medium or large size fish.
For these reasons the generation of a new therapeutic alternative is needed to
control the virus
in its early stages and which can be used in the early stages of fish
development and should
be a complement to the tools developed up to now.
Infectious Pancreatic Necrosis Virus
The aquatic Bimavirus are those with the greatest range of infection,
infecting numerous fish
species, among which are the Salmonids. The prototype of the aquatic
Birnaviridae family is
the Infectious Pancreatic Necrosis Virus (IPNv), considering members of this
group, all are
bisegmented double-stranded RNA viruses (dsRNA) able to cause clinical
infection in one of
the respective species. The first pathogenic birnavirus was isolated from a
fish found in a brook
trout (Salvelinus fontinalis) in the National Fish Hatchery, Leetown, West
Virginia, USA. The
agent was obtained during an epizootic of trout fingerlings suffering
pancreatic necrotic
infection (IPN). This necrotic pancreatic virus (IPNv) was deposited in the
American Type
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
Culture Center (ATCC) as ATCC VR299 IPNv and has been detected for many years
in various
locations in North America associated with high mortality rates in juvenile
trout.
Most aquatic birnaviruses are antigenically related, representing a major
serogroup (A) with
serotypes and only a few birnavirus not antigenically related forming a second
serogroup
(B). Most of the IPNv isolated in the USA belong to serotype Al (West Buxton);
the Canadian
isolates (Cl, C2, C3, Jasper) to serotypes A6-A9 and the European isolates
(Sp, Ab, I and Te)
to serotypes A2-A5 and serotype A10. Serotypes Al, A2 and A3 have been
detected in Asia..
IPNv genomic organization
The aquatic birnaviruses have similarities in morphology and biochemical and
biophysical
properties. IPNv virions present the typical characteristics of the
Birnaviridaefamily. The IPNv
genome consists of two segments of double stranded RNA (dsRNA) of 3.1 kb
(segment A) and
2.9 kb (segment B) with non-coding regions (UTR) at the 5 'and 3'.
Segment A contains two partially overlapping open reading frames (ORFs). The
first encodes
a non-structural protein VP5 (145 aa, 17 kDa) which is a cytolytic membrane
protein that while
dispensable for virus replication in cell culture, is important in
pathogenesis in vivo as it is
involved in the release and dispersion of the viral progeny. It has been
proposed that this
protein inhibits apoptosis in the early stages of infection. The second ORF
encodes a
polyprotein of 107 kDa (972 aa) which is proteolytically autoprocessed leading
to the pVP2
protein (508 aa, 54 kDa), VP4 (225 aa, 25 kDa) and VP3 (237 aa, 28 kDa) in a
co-translational
process mediated by VP4 itself. pVP2 is the precursor form of the capsid
structural protein,
VP2. The 74 carboxy terminal residues of pVP2 are processed to yield the
mature form VP2
(442 aa, 48kDa). In any case, the maturation process requires assembly of the
viral capsid
and the small polypeptides generated are retained in the viral particles. Such
peptides have
the ability to disrupt membranes. As occurs in morphogenetic processes in
other viral systems,
this proteolytic maturation process could confer irreversibility to the capsid
assembly.
Viral Proteins
As mentioned above, IPNv needs to process its own proteins to be infective.
Besides
possessing five major proteins including VP2, VP3 and VP4 it generates a
number of peptides
resulting from the processing of the polyprotein.
Studies of the morphology of IPNv found two types of viral particles, A
particles or provirion,
which is the first particle detectable post infection simultaneously with the
double-stranded
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
RNA, suggesting that the virus assembly occurs as soon as the double-stranded
RNA is
replicated. It has also been determined that the particle is not found fully
assembled: it follows
that it corresponds to an intermediary in morphogenesis. After maturation of
the provirions,
infective B particles are produced (virions; 3-4 hours post- infection), where
mature and
completely folded proteins were found. Thus it is concluded that the
maturation of IPNv A
particles not only indicates the acquisition of infectivity, but also the
reduction in the diameter
of the particles. This is demonstrated by densitometry analysis of viral
proteins made by the
two types of particles (A and B), wherein it is seen that the type A particles
have mostly VP2
capsid protein in the non- mature pVP2 form, and that type B particles have
only VP2 and not
the intermediary pVP2.
For the pVP2 maturation process three cleavage sites were identified. Two of
these cleavage
sites (486-487, 495-496) were proposed as a target of the VP4 viral protease.
Of these two
sites, the primary cleavage site at the junction pVP2-VP4, is defined as a [S
/ T] XAA motif.
This consensus sequence shows some similarity to the SKAW sequence found in
the 442-443
region, suggesting that the VP4 could be involved in the cleavage to generate
mature VP2.
Furthermore, recombinant expression of truncated VP2 was detected in CHSE-214
cells at
nanogram concentrations of monoclonal antibody CE4 by ELISA and
lmmunoblotting. This
observation suggests that both the folding and glycosylation of truncated VP2
could be
extremely similar to the native viral protein.
It has been shown that the majority of neutralizing monoclonal antibodies
react with epitopes
within the VP2 protein, it has also been suggested for neutralizing epitopes
for VP3. Three
epitopes have also been found, two variable and one conserved, located in the
central third of
VP2.
Apoptosis in CHSE-214 cells infected with IPNv
Apoptosis is a process of carefully regulated cell death that can be triggered
by a variety of
stimuli, one of these being viral infection. Some features of viral infection
are recognized by
cells as harmful, promoting a defensive response that ends with cell death.
Induction of
apoptosis in most cases is a challenge for successful viral replication. But
sometimes
appropriate manipulation of the apoptotic response results in a cell death
mechanism which
increases the development of the virus. IPNv, like many other viruses, induces
apoptosis in
cultured cells. This response is seen during the early stages of IPNv
multiplication involving
severe morphological damage to the infected cells for example, such as the
blebbing or
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
bubbling of plasmatic membrane. The expression of this cell death program can
be interpreted
as a defense mechanism of the cell against IPNv multiplication. However, most
infected cells
lack an apoptotic response, at least half of the infected cells do not express
apoptotic signals
at any time during the viral replication.
It has been shown that the Annexin V assay is sensitive to the earliest
morphological changes
produced in the cell, and can be used as an early marker of apoptosis,
including apoptosis
detected before the activation of the caspases.
Susceptible hosts and geographic distribution
As mentioned above, aquatic birnaviruses are viruses that have a great range
of action, with
representatives infecting numerous fish species, where salmonid fish are
highlighted, for
example rainbow trout (Oncorhynchus mykiss), river trout (Salvelinus
fontinalis), brown trout
(SaImo trutta), lake trout (Salvelinus namaycush), Atlantic salmon (SaImo
salar), coho salmon
(Oncorhynchus kisutch), and numerous invertebrates and marine fish such as
eels (Anguilla
anguiHa, Anguilla japonica) striped fish, tilapia (Tilapia mossambica), marine
molluscs and
crustaceans of European and Japanese coasts. Consequently, according to the
regions or
habitats of species referred to, IPNv is considered to be endemic in many
parts of America,
Europe and Asia, not being exclusive to Chile.
Methods of transmission
In particular, IPNv is transmitted via feces, urine and sexual secretions of
infected fish. For this
reason it can be transmitted vertically via eggs. Studies of factors affecting
the transmission
and outbreaks of IPN indicate that iodophor used as disinfectant during the
artificial fertilization
process does not completely eradicate IPNv infectivity. The virus is also
transmitted
horizontally, surviving fish from IPNv outbreaks are transformed into vectors
and can carry the
virus throughout their lifetimes. IPNv can also be transmitted through the
feces of fish-eating
birds.
IPNv inhibitory peptides
Previous studies have demonstrated the effectiveness of the use of synthetic
peptides in the
control of certain viral diseases. In Chile, the virus of infectious
pancreatic necrosis virus (IPNv)
has become a fastidious pathogen severely affecting the salmon industry every
year. It is
therefore very urgent to find an effective method for its control. The present
invention is based
on the use of chemically synthesized peptides for the purpose of inhibiting
the assembly and /
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
or attenuating the infectivity of IPNv in an in vitro model as well as in
large scale trials.
For the completion of this document, a search for prior art was conducted
during which,
publication of related patents were found. These are briefly described below.
The document W01994004565 discloses synthetic polypeptides with antigenic
properties
useful in treating IPNv infections in fish, however, unlike the present
invention it focuses on
generating an immune response in fish, while in the present invention there is
provided a
prophylactic formulation which seeks to prevent transmission when an animal is
exposed to
the presence of one of the viruses under consideration, in particular IPNv.
US5165925 discloses a vaccine against VR-299 strains and SP of IPNv, where the
vaccine is
a polypeptide segment obtained from a segment of the virus. In particular it
is mentioned that
this segment includes at least VP2.
The documents US5780448 and US6180614 describe DNA vaccines where the vaccine
consists of a DNA construct that encodes at least one peptide of a pathogen
that attacks
aquaculture species. Specific examples which comprise sequences encoding VP2,
VP3, which
could be combined are described.
U56010705 discloses a vaccine based on a live attenuated virus against the
microorganism
EdwardsieHa ictaluri. It additionally discloses that the vaccine may further
comprise a coding
sequence of VP2 of IPNv (claim 5).
U520040047881 describes recombinant microorganisms which express portions of
different
pathogens. In particular one example is described in which the microorganism
expresses part
of segment A of IPNv. Finally, the microorganisms that express pathogen
polypeptides are
used to produce food for aquatic animals.
U520070286871 discloses a composition for preventing viral infection, where
the composition
generally comprises of an antigen derived from a virus and the antigen is from
IPNv.
U520070248623 describes constructs encoding various virus antigens, among
which is
mentioned IPNv and a DNA vaccine comprising IPNv sequences.
U520100316663 describes a vaccine which corresponds to a fusion protein
between the
translocation domain of Pseudomonas aeruginosaexotoxin A and an antigenic
protein of IPNv.
U520120040010 describes a vaccine formulation comprising excipients allowing
the
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
composition to adhere to the mucous membranes of animals, within which fish
are reported. It
is mentioned that the vaccine can be against IPNv.
US8168201 describes a vaccine comprising a truncated VP2 antigen of IPNv and
also
considers the nucleic acid sequence encoding the truncated polypeptide.
EP1975238 discloses a vaccine for aquatic species. The specification mentions
that the
vaccine can be against IPNv, where the antigens may be from VP1, VP2, VP3.
W02002038770 describes vaccines based on polypeptides VP2 and / or VP3 of
IPNv.
W02003015714 describes a composition which blocks viral replication (viral
budding), wherein
the active agent corresponds to a molecule which corresponds to the fusion of
a transporter
and a peptide that is part of a viral structure. It was mentioned that said
viral structure may be
a peptide of at least 6 amino acids, and also indicates that it can, in a
particular instance, come
from the IPNv VP2 protein. Nevertheless, the indication of the fusion peptide
has a completely
different functionality than the present invention, wherein a first peptide of
the ones disclosed
in W02003015714 helps or improves the entry of a second peptide in the cell.
W02003013597 discloses a vaccine consisting of sub-units of the viral capsid
(VP2, VP3) that
form an empty capsid.
W01999050419 discloses a method for producing a vaccine against IPNv. The
vaccine is an
attenuated virus.
W02008140610 describes a vaccine based on the VP2 viral capsid protein. The
method of
administration is by injection, or feeding of recombinant yeast strains that
express the active
agent (VP2) of the vaccine.
W02011138489 describes the use of casein hydrolysates as antiviral agents.
The document US8168201, in particular mentions the use of a truncated antigen
from VP2.
Furthermore, document W01994004565 discloses synthetic peptides, where key
positions are
identified within peptides (R1, R2, R3) which can be changed to different
amino acids options.
There are many patent documents which disclose some uses of nucleotide or
amino acid
sequences of the VP2 protein from IPNv, and although the present invention
pertains to
peptides derived from the sequence of VP2 of IPNv, it also identifies
essential positions within
the peptides developed.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
The difference of the present invention with respect to the prior art is that
the present invention
is used as an antiviral agent, which prevents the infection of fish, where the
molecules of the
present invention can be used as a prophylactic agent, a therapeutic agent, a
prophylactic food
supplement with therapeutic potential, which have antiviral properties, where
its application,
e.g. through a bath, or through a special formulation or through a food matrix
provided to
animals or fish to be treated, either preventively or as an ongoing treatment
of disease, as a
dietary supplement or as a bath, where the peptides may be provided to the
affected fish or
animals during certain periods. Furthermore, the compositions of the present
invention
impedes the increase in viral load in chronically infected fish, or fish that
are normally exposed
to viral particles.
BRIEF DESCRIPTION OF FIGURES
Figure 1: Inhibition of infective units by p20. Semi-quantitative immune
detection of IPNv in de
novo infected CHSE-214 cells shows the inhibitory action of p20. A. Positive
control. Cells
infected in the absence of peptide. B. Cells infected in the presence of 10 uM
of p20. C.
Negative control. Cells infected in the presence of peptide K-1. D. Focal
Fluorescence Units
on chart (left to right, respectively) shows mean and SD values.
Figure 2: Antiviral Activity of peptide p20 A. CHSE-214 cells permanently
infected with IPNv,
the number of copies of mRNA from VP2 of IPNv was measured with RT-qPCR from
supernatant of cells. B. Calibration curve of number of copies of Topo-VP2
IPNv for qPCR.
Figure 3: Relative quantification of VP2 relative to elongation factor ELF.
Treatment with the
peptide before infection with IPNv.
Figure 4: Relative quantification of VP2 relative to elongation factor ELF.
Treatment with the
peptide after infection with IPNv.
Figure 5: Relative quantification of VP2 relative to elongation factor ELF.
Treatment with
peptide with alanine variations before infection with IPNV.
Figure 6: Relative quantification of VP2 relative to elongation factor ELF.
Treatment with
peptide 182 with alanine variations after infection with IPNV.
Figure 7: Change in viral titre of IPNv in CHSE-214 cells determined by focal
fluorescence in
a treatment with the peptide 182 before infection with IPNv.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
Figure 8: Change in viral titre of IPNv in CHSE-214 cells determined by focal
fluorescence in
a treatment with peptide 182 after infection with IPNv.
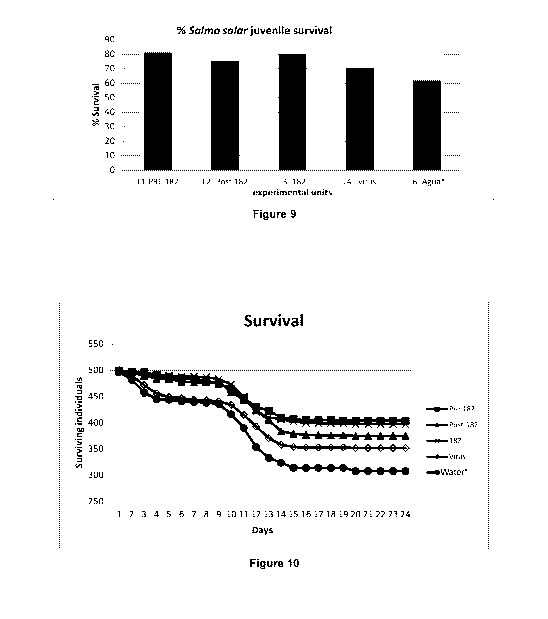
Figure 9: Percentage cumulative survival after experimental trials to test
effectiveness of the
peptide 182 in viva
Figure 10: Trend of mortality shown in trials. No mortality present after day
24 until day 56.
Figure 11: Control versus treatment with peptide; All Results.
Figure 12: despite the heterogeneity of viral load per fish, the presence of
peptides in the food
tends to lower the overall viral load, reflected in the tendency to increase
the relative values of
Ct in time in clear difference with the control formulation without peptide (f-
5) where the trend
is to maintain or even increase the average viral load (higher Ct values).
SUMMARY OF THE INVENTION
The present invention relates to molecules with antiviral properties to
prevent the spread of
infection in animal or fish by a virus of the Bimaviridae family, specifically
the infectious
pancreatic necrosis virus (IPNv). More particularly, the invention pertains to
peptides that are
effective in decreasing viral infection by reducing or impeding the production
of viral particles,
and can therefore prevent the potential mortality resulting from virus
infection and is of
particular use in the prophylaxis or prevention of infections by viruses of
the Bimaviridae family.
In a particular embodiment, we consider the use of peptides applied to the
tanks where the
fish are grown or as part of a feed composition.
DETAILED DESCRIPTION OF THE INVENTION
As mentioned, the present invention pertains to synthetic peptides or
fragments thereof,
specially designed for the prophylactic control of viral diseases of the
Bimaviridae family, more
particularly the infectious pancreatic necrosis virus IPNv which affects many
species of fish.
The present invention, in a first aspect, corresponds to synthetic peptides or
fragments thereof
having the property of reducing the rate of infection in animals exposed to a
particular virus.
In one aspect, the specially processed synthetic peptides were developed based
on a
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
sequence encoding pVP2 of the IPNv capsid.
More particularly, the peptides considered herein correspond to peptides with
an identity of at
least 80%, at least 85%, at least 90%, at least 92%, at least 95%, at least
98%, at least 99%
compared to the amino acid sequences SEQ ID NO:1 (p182), SEQ ID NO:2 (p20), or
fragments
thereof.
In another embodiment, the peptides considered herein correspond to peptides
with an identity
of at least 80%, at least 85%, at least 90%, at least 92%, at least 95%, at
least 98 %, at least
99%, with respect to the nucleotide sequences SEQ ID NO:3 (p182), SEQ ID NO:4
(p20), or
fragments thereof.
In a second aspect, the present invention comprises a prophylactic agent;
therapeutic agent;
prophylactic food supplement with therapeutic potential, wherein said
prophylactic agent;
therapeutic agent; prophylactic food supplement with therapeutic potential
corresponds to at
least one or both peptides described above or fragments thereof, that is,
comprises at least
one, two or three peptides having an identity of at least 80%, at least 85%,
at least 90 %, at
least 92 %, at least 95 %, at least 98 %, at least 99 % compared to the amino
acid sequences
SEQ ID NO:1, SEQ ID NO:2, or fragments thereof, or at least one, two, or three
peptides
having an identity of at least 80%, at least 85 %, at least 90 %, at least 92
%, at least 95 %, to
least 98%, at least 99 %, with respect to the nucleotide sequences SEQ ID
NO:3, SEQ ID
NO:4, or fragments thereof; and one or more pharmaceutically acceptable
excipients.
In a third aspect of the invention, an application of the prophylactic
formulation of the invention
to animals at risk of potential contact with the source of infection of a
virus of the Birnaviridae
family is considered. In a particular embodiment, the animal at risk of
exposure to a virus of
the Birnaviridae family is a fish. In a more specific embodiment, the virus of
the Birnaviridae
family is IPNv.
In yet another aspect, particularly when considering the embodiment of the
invention as a
prophylactic dietary supplement with therapeutic potential, the integration of
the peptides of
the invention in a food matrix in such a way that they can be administered or
provided to
growing animals is considered. According to this aspect of the invention, the
peptides
described above are administered to farmed fish through a composite food.
Since it is not
possible to measure accurately the quantities of peptides that are actually
consumed by each
fish, ranges of amount of peptides administered in a composite food have been
set. Thus, for
example, in the particular embodiment of application through food, the
peptides of the invention
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
according to the amino acid sequences SEQ ID NO:1, SEQ ID NO:2, or the
nucleotide
sequences according to SEQ ID NO:3, SEQ ID NO:4, fragments thereof, and / or
mixtures
thereof, are in concentrations of between 10-19 to 10-5 molar (M), more
preferably between
5*10-9 to 10-6 M, most preferably between 5*10-9 and 10-7 each one.
In a more particular optional embodiment, the peptides of the invention
according to the amino
acid sequences SEQ ID NO:1, SEQ ID NO:2, or the nucleotide sequences according
to SEQ
ID NO:3, SEQ ID NO:4, fragments of them, and / or mixtures thereof, when
included in a food
matrix may be coupled with appropriate molecules, so as to improve its
absorption. Such
molecules can be selected from but not limited to, polymers such as
polyethylene glycol (PEG),
chitosan of various molecular weight.
In an even more particular embodiment of the third aspect of the present
invention, the
application of the prophylactic formulation of the invention is performed in a
culture tank, where
a dip is given to fish fry that could potentially come into contact with IPNv.
In a more specific embodiment, the application of the prophylactic formulation
of the present
invention is applied in concentrations between 10-4 M to 10-19 M, more
preferably between
2 x10-4M to 10-8M, more preferably between 8x10' M to 10-6M, even more
preferably between
10-5 to 5x10-5M.
In a more specific embodiment, the density of fry in the culture tank during
the application of
the prophylactic formulation of the invention is such to allow the proper
development of the fry.
For example, the invention considers densities from 10 kg to 70 kg, more
preferably between
15 kg and 50 kg, more preferably between 20 kg and 40 kg, more preferably
between 25 and
30 kilograms of fry per cubic meter of water in the tank during application of
the prophylactic
formulation of the invention.
In a particular embodiment of the invention, the fry are exposed to the
prophylactic formulation
of the present invention for a period of between 1 and 24 hours, more
preferably between 2
and 15 hours, more preferably between 4 and 10 hours, more preferably for at
least 6 hours.
The invention is illustrated below based on various laboratory tests (in
vitro) and pilot-scale (in
vivo). The examples provided below are not intended to limit the scope of the
invention but to
provide sufficient information so that a person skilled in the art may
understand.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
EXAMPLES
The following examples are provided to illustrate some aspects of the
invention, without
intending to limit its scope, which ultimately is given by the accompanying
claims.
To demonstrate the effective entry of the peptides to the cell line, the
peptides were labelled
with rhodamine-B, and the entry of the peptides into cells evaluated via
fluorescence
microscopy. Subsequently the cytotoxic capacity of the peptides were evaluated
by Trypan
Blue and MIT. Together with this, virus expression was determined by
quantitative analysis
using the technique of "real-time reverse transcription PCR". In order to
obtain quantitative
results on the inhibition of the infectivity and to calculate viral particle
titers in both infection
models semiquantitative immunofluorescence will be performed.
Added to this, using continuous sucrose velocity gradients, the profile or
polysomal distribution
of cultures infected with IPNv subjected to the peptide and without the
peptide was evaluated.
It is expected that some of the designed peptides having interfering activity
against infection
by IPNv, will be used as a basis for the generation of a new prophylactic
product for the salmon
industry.
EXAMPLE 1
in vitro assay 1
Fluorescent Labeling of Peptides and Fluorescence Microscopy
Aliquots of the peptides of the invention were labeled with rhodamine-B (0.2
ml of concentration
2.5mg/m1) and stored at -20 C in the dark until further use. Briefly,
exponentially growing
Chinook salmon embryo cells (CHSE-214) were exposed to 10Ong/well
concentration of
rhodamine-B labeled peptide p20 for 3 hours on 24 well plates. For direct
detection of labeled
peptides, the culture medium was discarded and the cells were washed once with
PBS (pH
7.3), subsequently rinsed three times with PBS-Tween 20 (0.05%) and analyzed
under a Nikon
Eclipse 400 fluorescence microscope and recorded with a Nikon Coolpix 4500
digital camera.
Cells and Cell Culture
CHSE-214 cells and IPNv-persistently infected cells (10, 19) were cultured as
exponentially
growing sub confluent monolayers on 24 well plates in MEM and Leibovitz (L-15)
medium
respectively, supplemented with 10% (v/v) fetal calf serum (FCS) and 2 mM
glutamine and
maintained.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
IPNv growth and titration
The virus growth and titration were evaluated on two models of infection:
fresh CHSE-214 cells
infected de novo and the established CHSE-214-NV1015 cell line persistently
infected with
IPNv . For de novo infections, the virus propagated on CHSE-214 cell
monolayers were
infected at semi confluency at a multiplicity of infection of 0,001 in
Leibovitz's medium (L-15,
Gibco) at 18 C, supplemented with 50 M gentamicyn, 2mM L-glutamine and 10%
fetal bovine
serum (FBS, Gibco). After full cytopathic effect was observed, culture fluids
were harvested
and titered, quantifying the number of infective particles, using a modified
Reed and Muench
protocol (10, 24). The maintenance medium (MM) was identical except that the
serum
concentration was reduced to 2%.
p20 inhibitory potential in de novo infected cells.
The putative antiviral activity of p20 was measured in de novo infected cells
followed by indirect
immuno detection. CHSE-214 cells were grown on 24 well cell culture plates to
confluency and
infected with IPNv at a multiplicity of infection of one (M01= 1). 18 hours
prior to infection (p.i.)
the cells were washed and the peptide added at 10 M, the concentration at
which in our hands
full inhibition was attained for similar peptides (data not shown). The plates
were fixed with
methanol:acetone (3:1) for 30 min at -20 C washed and incubated with a
commercial
polyclonal anti VP2-VP3 (BiosChile, Chile) as the first antibody 1/80 for 1
hour at room
temperature, washed and followed by an anti-rabbit FITC-conjugated antibody
(Fluorotest,
BiosChile). The modified semi quantitative method of Reed and Muench (10, 24),
was used to
determine the focal fluorescent units under a fluorescent microscope. The
distribution of the
fluorescence was analyzed on a Nikon Eclipse 400 fluorescence microscope
equipped with a
100-watt mercury lamp. Color photography was performed with a Nikon Coolpix
4500 digital
camera.
p20 inhibitory potential in persistently infected cells
CHSE-214-NV1015 cells persistently infected with IPNv were grown on 24 well
cell culture
plates until confluence, the peptide was added at a total concentration of 10
M for four hours,
washed and fresh medium added. After 24 hours of peptide treatment total RNA
was extracted
by the Trizol procedure and VP2 mRNAs expression measured by the RT-qPCR real
time
procedure (Stratagene 1 step RT-qPCR Kit) with SNPF primers 5"-CAA CAG GGT TCG
ACA
AAC CAT AC-3" and SNPR primer 5"-TTG ACG ATG TCG GCG TTT C-3". The reaction
was
carried out in 30 I mixture consisting of Brilliant II SYBR Green QRT-PCR
Master Mix Kit,
1-Step (Stratagene, Inc.), primers and template RNA. Samples were amplified
and detected
using a Chromo 4 system (BioRad). The final thermo cycling profile was 50 C
for 55 minutes,
94 C for 10 minutes and 94 C for 30 s, 55 C for 30 s and 72 C for 40 cycles.
800 ng of total
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
RNA per experimental sample were used in each triplicate reaction. In order to
quantify VP2
m RNA copies of IPNv, a standard curve for DNA quantification was established.
To have IPNv
real-time PCR standards, the VP2 region was amplified using published
procedures
standardized in our laboratory. The VP2 amplicon was cloned into PCR 2.1
vector (lnvitrogen
Inc.) and its specificity confirmed by sequencing. Plasmid DNA was isolated
using the Quiaprep
miniprep kit (Quiagen) according to the manufacturer's instructions. The
purified plasmid was
quantified using a Nanodrop device (Nanodrop ND-1000) and serially diluted in
Sigma DNase-
RNase free water to a final concentration ranging from 10 to 1.10 to 10 copies
of genome-
equivalents/24. Two microliters of each dilution were used for real-time PCR
in triplicates to
create a standard curve to be used to quantify putative IPNv DNA amounts in
experimental
samples. (Figures 1 and 2)
Cytotoxicity assay
The putative toxic effect of the synthetic peptide p20 on eukaryotic cells was
measured by
exposing established CHSE-214 cells to the peptide according to standard
procedures
developed in our laboratory (5, 29). Briefly, cell monolayers at 70% semi
confluence were
washed with PBS and then the peptide p20 added at a range of concentrations (1-
100 mM) in
triplicate wells and incubated for the maximum viability time (3 h) without
culture medium.
Samples were then washed three times with excess of PBS before adding 0.1%
trypsin in the
presence of EDTA for 30-60 s to release cells from the monolayer. Individual
cell viability was
determined using the Trypan Blue exclusion technique .
EXAMPLE 2
in vitro assay 2
I. In vitro evaluation of the potential of peptide 182 in the reduction of the
expression of
new IPN virus particles.
To evaluate the effectiveness of peptide 182 in the reduction in the
expression of new viral
particles in CHSE-214 cells infected with IPNv, two experimental situations
designated pre-
infection treatment and post-infection treatment were designed and evaluated.
Both
experimental situations were evaluated by two different processes, which
correspond to
relative quantification of the VP2 gene and determination of viral titer by
counting focal
fluorescence units.
Treatment pre infection
To assess the ability of peptide 182 in reducing the production of new virus
particles in an in
vitro model of infection, CHSE-214 cells were pretreated with decreasing molar
concentration
of peptide 182, from 1*10-4 to 1*10-1 M, determined in the experiments on
entry of rhodamine-
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
labeled peptide and peptide 182 toxicity tests on CHSE-214 cells.
To conduct this test, CHSE-214 cells at 0.8 x 104 cells / ml, were seeded in
two 24 well plates.
24 hours after sowing, the cells were treated for 4 hours with 200uL of
peptide 182 in molar
concentrations. As in the previous cases, the peptide was solubilized in L-15
medium without
serum. After treatment with peptide 182, the cells were washed 2 times with lx
PBS and
subsequently infected with an MOI of lx IPNv which had a titer of 7.6 *107.
Inoculation with the
virus was carried out for 1 hour at 17 Celsius, followed by a gentle washing
with lx PBS.
Maintenance of the infected cells was performed with 1 ml of L-15 medium
supplemented with
2% SFB at 17 C for 16 hours post infection (hpi). (Figure 3)
Treatment Post infection
To determine the effectiveness of the peptide in reducing the number of new
IPN virus particles
CHSE-214 cells were seeded and infected according the previously described
protocols. Post-
infection and after washing with lx PBS, cells were incubated with decreasing
molar
concentrations of peptide 182 from 10-4 to 10-10 M for 4 hours, the peptide
was removed, the
cells were washed with 1 x PBS and kept in 1 ml of L-15 medium with 2% SFB for
16 hpi
(Figure 4).
Evaluation of IPNv titre reduction by focal fluorescence.
To evaluate the effectiveness of peptide 182 in the reduction of viral
particles in pre-infection
and post-infection conditions, the expression of the VP2 protein was measured
by quantifying
relative to elongation factor ELF-1 alpha of the CHSE-214 cells using RNA
extraction and real-
time PCR described in methodology of objective 2. The reduction in viral titre
was evaluated
using the procedure described in the literature and mentioned above,
assessment in this case
only was performed using a commercial monoclonal antibody against VP2 and with
decreasing
concentration of the peptide from 10 -4 to 10 -8M.
RESULTS 1
In vitro evaluation of the potential of peptide 182 in reducing the expression
of new IPN
virus particles.
II. Design of a model of controlled in vivo challenge with IPNv to evaluate
the
effectiveness of peptide 182 in reducing the mortality of infected Salmo salar
fry.
Cultivation system
A unit was designed with 5 culture systems (experimental lines) with
independent recirculating
fresh water. Each culture system is composed of two culture units with a
maximum capacity of
20 liters each, the system consists of a trickling 25L biofilter for the
removal of solids and
nitrogen compounds and a drain tank of 200L for the removal of residual water
and feces. The
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
water removed from the tanks post circulation is treated in the drain with
ozone (03) and UV
light to remove contaminants before returning to the sewer system, ensuring
the removal of
the remnants of the challenge virus.
Individuals and culture conditions.
For the experimental challenge of Atlantic salmon (Salmo salar), fry were
purchased from an
lnvertec fish farm in Curacautin, Araucania region. 5000 individuals with an
average weight of
1.4g were transferred to the premises of the Laboratory of Aquaculture
Engineering, Catholic
University of the Holy Conception (UCSC) where they were acclimated for 11
days prior to the
experiment.
After the acclimatization, fry were separated into groups of 250 individuals
per unit area, there
were a total of 500 fry per culture system (experimental line). The culture
conditions used were
similar to those used in industrial fish farms, ensuring that these said
conditions did not affect
the development of individuals nor influence the results of the challenge. The
general
conditions correspond to a density of 25kg/m3 culture, with cycles of 16 hours
light and 8 hours
dark. Each tank was oxygenated in a ratio of 7 mg / L and the rate of water
exchange in the
system was 250 ml per minute, where the total turnover is within 4 hours.
Table 1: Conditions of culture production used in the in vivo challenge in the
wet lab at UCSC.
Culture conditions Characteristic value Unit of measurement
Water management
Recirculation rate 4 times/hour
Renewal of fresh water 100 % daily
Turnover rate 15 cms/seg
Production
Initial weight of individuals 1.4 grs.
N of individuals per unit 250 individuals
Culture density 25 Kg/m3
Feed (P.C) 2,5 %
Water quality
Ammoia nitrogen < 1 mg/L
Nitrite 0.5 mg/L
pH 6.5 7.5 mg/L
Dissolved oxygen 10 mg/L
Total suspended solids 40 - 80 mg/L
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
Initial evaluation of the Salmo salar fry
Before the challenge, a sample of 50 individuals was analyzed to determine the
status of the
fish and previous infection with IPNv. As described above, the persistence of
the virus is one
of the most common conditions for cultures such as salmon species.
The preliminary screening was performed using VP2SNP-F and VP2SNP-R primer set
using
the protocols for real-time PCR previously described in Methods.
In vivo challenge model on medium scale. Wet Laboratory Model.
The experimental evaluation was conducted for 56 days, after the
acclimatization of the fry.
The application of peptide 182 was performed in a bath by reduction of the
water column. For
the challenge the VR- 299 strain of IPNv obtained from cell cultures of CHSE-
214, was used,
as described previously in Methods. The virus was titered by IFAT at around
7.6 = 107 particles
/ ml. For the challenge, the water column was reduced, leaving the Salmo salar
fry at a density
of 50 kg/m3. Two types of treatment with the peptide described as pre-
challenge treatment
and post-challenge treatment according to the similar condition used in the in
vitro model were
performed. Two culture systems were used as a control, a peptide application
control and the
other an IPNv infection control, a final system was used as control of natural
mortality. Final
concentration of virus used in the challenge was 105 viral particles per ml,
and the peptide
concentration used was 10-5M, the molar concentration was selected based on in
vitro results.
Table 2 summarizes the experimental details.
Table 2: Sample experimental design with peptide 182 (p182). Every situation
has two
separate tanks with 250 Salmo salar fry, average weight 1.4 g.
System Step 1 Step 2 Spet 3
1 hour Step 4
Pre- Bath with p182 for Normal culture Bath with IPNv for Normal
culture
challenge 4 hours. conditions 3 hours. Density conditions.
Density 50kg/m3 Density 25Kg/m3 50kg/m3
Density 25Kg/m3
Post- Bath with IPNv for Normal culture Bath with p182 for Normal
culture
challenge 3 hours. Density conditions 4 hours. Density conditions
50kg/m3 Density 25Kg/m3 50kg/m3
Density 25Kg/m3
Negative Bath with p182 for Normal growing Reduced water bath Normal
growing
Control 4 hours. conditions. conditions for 3 hours conditions
p182 Density 50kg/m3 Density 25Kg/m3 Density 50kg/m3
Density 25Kg/m3
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
Positive Bath with IPNv for Normal growing Reduced water Normal growing
control 3 hours. Density conditions. bath conditions for conditions.
IPNv 50kg/m3 Density 25Kg/m3 4 hours. Density 25Kg/m3
Density 50kg/m3
Water Reduced water Normal growing Reduced Normal growing
Control column for conditions. waterbath conditions.
4 hours. Density 25Kg/m3 conditions for 3 Density 25Kg/m3
Density 50kg/m3 hours.
Density 50kg/m3
RESULTS ll
Designing a challenge model of controlled in vivo infection with IPNv to
evaluate the
effectiveness of peptide 182 in reducing the mortality of infected Salmo salar
fry.
Initial evaluation of Salmo salar fry
The initial state of the Salmo salar fry with respect to preexisting
conditions, was determined
by an initial sampling which indicated that 60% of sampled fish were found to
have IPN viral
load. This is consistent with the literature which explains that the virus
becomes persistent in
fish that survive an outbreak and therefore the offspring may be asymptomatic
carriers of the
virus. Because of this, the untreated tank was considered to represent the
natural mortality
rate which may exist due to the activation of virus in the experimental tanks
and the handling
of the water column in which they were maintained. (Figure 9).
In vivo challenge model on medium scale. Wet laboratory model.
Using the previously detailed conditions, in vivo testing on wet laboratory
scale was performed.
Experimental evidence is presented below as well as graphs showing the daily
mortalities in
the culture systems designed, as well as the accumulated mortality rate
calculated after the
termination of the experiment (Figure 10).
A test was performed in conditions similar to those used in the previous
examples.
The peptide p182 was applied by bath immersion to 300,000 fry with the company
Marine
Harvest. Samples were taken at 0, 30 and 60 days of application of the peptide
and 25
specimens were analyzed.
The results obtained in this study are summarized in the two graphs in Figure
11.
EXAMPLE 4
Evaluation of Peptides interfering with IPNv incorporated into the diet of
Salmo salar fry
persistently infected with the virus
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
OBJECTIVE: To determine whether interfering peptides, already tested for their
inhibitory
effect on viral load "in vivo", are able to induce the same effect via feed.
The evaluation of the interfering IPNv peptides incorporated into food
consisted of two stages.
FIRST STAGE: developed at the experimental station in Quillaipe in
collaboration with
Aquadvise, where five diet formulations previously prepared by oil bath with
interfering
peptides p182; p20 and the mixture of both to use as an unrelated control
(Table 3) were
applied to a total of 1000 specimens of Salmo salar per formulation with an
initial average
weight of 13 grams which increased to an average of 20 grams during the 45
days of the
peptide experiment. There were no significant differences in body weight gain
between the fish
fed with the different formulations (Table 4).
The fish were distributed in 5 tanks of 40 fish each per formulation (Table
5). Environmental
parameters were measured in each tank (temperature in C and % oxygen
saturation) daily
for 45 days of the trial, which remained constant throughout the time.
During the 45 days of the trial there were 5 mortalities, not associated IPNv
infection.
To assess the initial viral load of fish analyzed, 30 were selected to
determine the degree of
infection with the following agents: IPNv (56.6%); ISAv (0%); P salmonis (0%).
For the assessment liver, kidney and heart of each fish was sent to GIM-PUCV
individually
preserved in RNA later 6.
SECOND STAGE: developed at IMT-PUCV. To determine the viral load of fish
analyzed, 180
untreated fish were taken at time "0" and the variation in Ct values was
quantitatively
determined in reference to the ratio of viral VP2 gene / cellular ELF. Four
time points were
analyzed in the experiment (days 10, 20, 30 and 45), removing 25 fish per
formulation for which
the ratio was Ct Vp2/ELF was determined in duplicate except for day 45 when 25
fish per
formulation were tested without duplicates, in a pool of organs per fish of
which 30 mg were
taken to extract RNA with E.Z.N.A. kit for qRT-PCR.
SUMMARY OF RESULTS
The results indicate:
1.- That the average viral load decreases sequentially with time in fish fed
with interfering
peptides
2.- That the range of Ct values becomes smaller over time in fish treated with
interfering
peptides
3.- The above effect is more significant in the synergy between peptides p182
and p20
(Formulation 4)
4.- Although there is a reducing effect of the control peptide, the two
specific peptides exhibit
a higher level of attenuation.
CA 02954876 2017-01-11
WO 2016/005796 PCT/1B2014/063047
Table 3: Formulations of the IPNv interfering peptides incorporated in the
feed
Molarity FORMULATION
Formulation 1 2.10 -7 Unrelated peptide
Formulation 2 2.10 -7 Peptide 182
Formulation 3 2.10 -9 Peptide 19
Formulation 4 2.10 -7+ 2.10 -9 Peptide 182+19
Formulation 5 0 Feed without peptide
Table 4: Variation in the body weight of the fish analyzed
Weight gain per treatment from initial sampling to final sampling (day 45)
weight
gain in
daily 45
weight days
initial average initial final average Final
gain of per
N weight at biomass N weight biomass Growth
SGR a fish tank
Treatment Tanks fish day 0 (g) (g) Fish
day 45 (g) (g) (0/0) (g) (g)
3-8-11-16-
A 23 200 12.9 2580.16 124 20.03 2483.8
55.27 0.98 0.158 7.13
4-9-12-17-
24 200 13.0 2600.66 120 20.18 2421.5
55.18 0.98 0.159 7.18
5-10-13-
C 18-25 200 12.76 2551.90 124 19.31 2394.6
51.35 0.92 0.146 6.55
2-7-14-19-
22 200 12.91 2581.3 125 20.04 2505.2
55.28 0.98 0.159 7.14
1-6-15-20-
21 200 12.99 2597.1 123 19.67 2419.5
51.48 0.92 0.149 6.69
Table 5: Tank distribution for the tests on peptides interfering with IPNv
Formulation Diet Tanks by diets N of fish x N of fish per
tank Diet
T - 1 A 3 - 8 - 11 - 16 - 23 40 200
1-2 B 4 - 9 - 12 - 17 - 24 40 200
1-3 C 5 - 10 - 13 - 18- 25 40 200
1-4 D 2 - 7 - 14 - 19 - 22 40 200
1-5 E 1 - 6 - 15 - 20 - 21 40 200
Table 6: Processing scheme of samples at GIM - PUCV.
Organ Heart + Liver+ Kidney
Step 1 Pool
Step 2 30 mg/fish
Step 3 RNA extraction
Step 4 qRT-PCR (VP2/ELF)
Store at -80 C
CA 02954876 2017-01-11
WO 2016/005796
PCT/1B2014/063047
Table 7: Summary of fish processed (All samples in duplicate)
Formulations Day 0 Day 10 Day 20 Day 30 Day 45
Peptide 25 25 25 25/125
control
Peptide 182 - 25 25 25 25/125
Peptide 19 - 25 25 25 25/125
Peptide 25 25 25 25/125
182+19
5/peptide 25 25 25 25/125
Control 180
Total qRT- 360 250 250 250 125
PCR
reactions
Table 8: Controls. Persistently infected fish, maximum and minimum values of
Ct for the
VP2 gene compared to the cell marker ELF.
Samples Ct minor Ct minor Average Ct major Ct major Average
Day 0 180 (x2) 17.64 17.38 17.5 31.13 30.97 31.05
Initial 30 (x1) 18.00 - 40.00 - 28.32
control
INDUSTRIAL APPLICABILITY
The present invention provides peptides that are suitable for pharmaceutical
or veterinary
compositions, which can help in the prophylaxis of viruses of the Birnaviridae
family, more
particularly, for the prophylaxis of fish that can be exposed to IPNv.