Note: Descriptions are shown in the official language in which they were submitted.
APPARATUS, SYSTEM, AND METHOD FOR CONVERTING VARIED SOURCE
INDUSTRY WASTE INTO ENERGY
CROSS-REFERENCE TO RELATED APPLICATION DATA
[0001] This application claims priority to U.S. Provisional Patent Application
Serial No.
62/040,943.
FIELD OF TECHNOLOGY
[0002] The present application relates generally to waste and waste stream
processing,
and more particularly to converting varied source industry wastes into energy.
BACKGROUND
[0003] Presently, manufacturing processes produce wastes that are harmful to
the
environment. These wastes manifest in the form of solids, liquids, and gases
that many
times find their way deep into the ground to pollute underground water
resources. The
wastes also find their way into water streams that eventually meet rivers
thereby polluting
the rivers. If/when these wastes are sent to a landfill, they tend to
disintegrate into most
potent greenhouse gases such as methane.
[0004] Sometimes the wastes have been incinerated in an attempt to reduce the
amount of
waste generated by manufacturing processes. Incineration of the wastes has
been used to
recover energy from the wastes. However, incineration processes has various
drawbacks.
For example, incineration of these wastes causes emissions of more toxic gases
such as
dioxins and furans into the air. Furthermore, incineration completely rules
out production of
gaseous fuel from the wastes as an energy source because during incineration
none of the
hydrocarbons of the wastes survive in a combustible form.
[0005] Energy recovery by means of gasification has also been used. However,
because
industry and manufacture processing generates wastes from varied sources, many
of which
are not similar in terms of physical and chemical properties, it has posed a
considerable
challenge to well established methods of gasification such as fluidized bed
gasification,
moving bed gasification, and entrained bed gasification. In order to work
efficiently,
each of these methods require strict adherence to physical and chemical
properties for the
waste being processed. This is a problem because industry and manufacturing
processes
often to produce wastes at different stages of
1
Date Recue/Date Received 2021-10-05
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
manufacturing that are not uniform with respect to physical and chemical
properties.
SUMMARY
[0006] The present disclosure generally provides an improved gasification
apparatus, system,
and method for processing varied source wastes containing hydrocarbons. This
results in a
single apparatus, system, or method to process hydrocarbon-containing
materials produced from
various stages of manufacturing processes, thereby increasing environmental
stewardship.
[0007] The system and method include the use of a gasification apparatus
comprising a rotary
kiln reactor and a gas distributor. The rotary kiln reactor and gas
distributor are configured to
generate multiple reaction environments within the gasification apparatus.
Each of the reaction
environments has unique temperature and pressure conditions that process
various components
of the hydrocarbon-containing wastes.
[0008] Gasification is a process by which hydrocarbon-containing
materials/wastes are
converted into a combustible mixture of gases including carbon monoxide,
hydrogen, methane,
water vapor, and carbon dioxide. This combustible mixture of gases has the
potential of
providing a direct source of energy to industry and to manufacturing
processing, or it can be used
as fuel for generating steam and or electricity for manufacture processing.
According to the
present disclosure, the conversion of the combustible mixture of gases into
energy uses an
improved gasification method, as described herein, which is amenable to
complete utilization of
all hydrocarbon-containing wastes generated by manufacturing and industry
processes. This
results in less environmental pollution and considerable savings for the
manufacturing industry.
[0009] Additional features of the present disclosure will become apparent to
those skilled in the
art upon consideration of the following detailed description exemplifying the
best mode for
carrying out the disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Embodiments of devices, systems, and methods are illustrated in the
figures of the
accompanying drawings which are meant to be exemplary and not limiting, in
which like
references are intended to refer to like or corresponding parts, and in which:
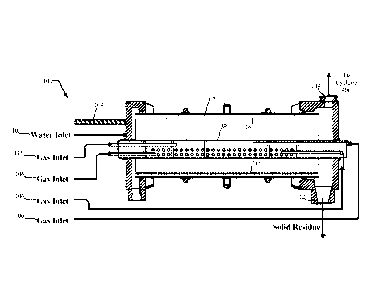
[0011] FIG. 1 is a side cross-section view of an apparatus/gasifier for
processing hydrocarbon-
containing wastes according to the present disclosure;
2
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
[0012] FIG. 2A is a perspective view of a gas distributor of the
apparatus/gasifier according to
the present disclosure;
[0013] FIG. 2B is an end view of the gas distributor of the apparatus/gasifier
according to the
present disclosure;
[0014] FIGS. 3A through 3D are end cross-section views of the
apparatus/gasifier depicting
effects of varying rotation speeds of a rotary kiln reactor of the
apparatus/gasifier according to
the present disclosure;
[0015] FIG. 4 is a system block flow diagram illustrating system components
for processing
hydrocarbon-containing wastes according to the present disclosure;
[0016] FIG. 5 is a process flow diagram illustrating methods for processing
hydrocarbon-
containing wastes according to the present disclosure;
[0017] FIG. 6 is a system block flow diagram illustrating an exemplary use of
the present
disclosure in converting industry wastes into gaseous fuel;
[0018] FIG 7 is an illustration of use of the present invention in converting
another type of
industry waste into gaseous fuel; and
[0019] FIG 8 is an illustration of the present invention in converting yet
another type of industry
waste into gaseous fuel.
DETAILED DESCRIPTION
[0020] The detailed description of aspects of the present disclosure set forth
herein makes
reference to the accompanying figures, which show various embodiments by way
of illustration.
While these various embodiments arc described in sufficient detail to enable
those skilled in the
art to practice the disclosure, it should be understood that other embodiments
may be realized
and that logical and mechanical changes may be made without departing from the
spirit and
scope of the disclosure. Thus, the detailed description herein is presented
for purposes of
illustration only and not of limitation. For example, the steps recited in any
of the method or
process descriptions may be executed in any order and are not limited to the
order presented.
Moreover, references to a singular embodiment may include plural embodiments,
and references
to more than one component may include a singular embodiment.
[0021] The present disclosure generally relates to an improved gasification
apparatus, system,
and method for processing hydrocarbon-containing wastes. The system and method
include the
3
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
use of a gasification apparatus comprising a rotary kiln reactor and a gas
distributor. The rotary
kiln reactor and gas distributor are configured to generate multiple reaction
environments within
the gasification apparatus. Each of the reaction environments has unique
temperature and
pressure conditions that process various components of the hydrocarbon-
containing wastes. This
is beneficial because it enables processing of varied-source hydrocarbon-
containing wastes with
widely varying physical and chemical properties.
[0022] Referring to FIG. 1, an apparatus (i.e., gasifier) 100 for performing
physical and chemical
gas-solid reactions is described. The apparatus 100 includes a rotary kiln
reactor 102 that
provides a first stage of gas-solid reactions in the apparatus 100. The rotary
kiln reactor 102 is
designed to produce optimum gas-solid interaction of hydrocarbon and non-
hydrocarbon-
containing wastes with gases. As such, the rotary kiln reactor 102 may include
a means 104
(such as a conveyor or screw feeder) for introducing hydrocarbon solids and
liquids into the
rotary kiln reactor 102, gas inlets 106 for introducing gas into the rotary
kiln reactor 102 using a
gas distributor 108, a means 110 for introducing water into the rotary kiln
reactor 102, a means
112 for removing solids from the rotary kiln reactor 102, and a means 114 for
removing gas from
the rotary kiln reactor 102. Solids/ash are removed from the apparatus 100 at
a rate
commensurate with the presence of noncombustible fraction present in the
incoming waste. An
inner surface of the rotary kiln reactor 102 may be lined with refractory 116
so that the rotary
kiln reactor 102 may be operated at a temperature up to about 2200 F.
[0023] The gas distributor 108 (an expanded view being provided in FIGS. 2A
and 2B) may be
divided into four or more zones. The number of zones the gas distributor 108
has may depend
upon the length of the apparatus 100 and/or the rotary kiln reactor 102.
According to non-
limiting, illustrative examples, the gas distributor 108 may have as little as
2 zones and as many
as 8 zones. However, one skilled in the art should appreciate the gas
distributor having other
numbers of zones without departing from the scope of the present disclosure.
In an example,
each respective zone of the gas distributor 108 receives gas from a single gas
inlet 106.
However, one skilled in the art should appreciate that each zone may receive
gas from more than
one gas inlet 106. Each zone may receive a unique composition and quantity of
gas from one or
more gas inlets 106 that are distinct from gas compositions received by other
zones of the gas
distributor 108.
[0024] The gas distributor 108 may be a tubular structure having a circular or
nearly circular
4
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
cross-section (as depicted in FIG. 2B). Moreover, the gas distributor 108 may
be a stationary
structure supported at or proximate ends 202 thereof by the rotary kiln
reactor 102. Support by
the rotary kiln reactor 102 may occur through the use of stationary hoods.
Each zone of the gas
distributor 108 includes gas outlet ports 204 through which gas from the gas
inlets 106 are
introduced into the apparatus 100. Each zone of the gas distributor 108 may
contain equal
numbers of gas ports or each zone may contain a unique number of gas ports
different from those
of other zones. The number of gas ports each zone has may depend upon the
maximum amount
of gas to be introduced at that zone and the pressure at which the gases are
available. The gas
ports are located long about 180 degrees of the circumference of a tubular
portion 206 of the gas
distributor.
[0025] A movable overlay 210 is fitted to the tubular portion 206 of the gas
distributor 108. As
illustrated, the movable overlay 208 is a half-spherical structure that covers
about 180 degrees of
the circumference of the tubular portion 206. As such, the overlay 208 may be
capable of
covering all or substantially all of the gas outlet ports 204 while in a
single orientation.
However, one skilled in the art should appreciate the overlay 208 covering
more or less than 180
degrees of the tubular portion 206, less than substantially all of the gas
outlet ports 204, and
having any shape that allows it to mate with the tubular portion 206 without
departing from the
scope of the present disclosure. The movable overlay 208 is configured to
rotate about the
tubular portion 206 to direct the flow of gas through the gas outlet ports 204
within desired
ranges. For example, the movable overlay 208 may be moved to cover less gas
outlet ports 204
when less pressure is desired and more gas outlet ports 204 when more pressure
is desired. The
composition of the gas distributor 108 and overlay pipe 208 may be selected to
withstand
temperature of up to about 2200 F.
[0026] The hydrocarbon-containing wastes may be conveyed into the rotary kiln
reactor 102
using a solid conveyer 104 such as a screw feeder, for example. The screw
feeder 104 uses a
rotating screw blade to move the hydrocarbon-containing wastes into the rotary
kiln reactor 102.
[0027] Water may be introduced into the rotary kiln reactor 102 via a water
inlet 110. The
water may be introduced at rate of about 25% to about 30% by weight of that of
the varied-
source hydrocarbon-containing waste on dry basis. Moreover, the hydrocarbon-
containing
wastes may be in gas, solid and/or liquid states. The gases introduced into
the gas distributor
108 may be oxygen and/or non-oxygen bearing. The gases may be delivered into
the rotary kiln
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
reactor 102 in varying quantities and compositions along the length of the
rotary kiln reactor 102
in a manner that allows the gases to contact the hydrocarbon-containing wastes
along a wall of
the rotary kiln reactor 102.
[0028] Distribution of the gases along the length of the rotary kiln reactor
102 may be achieved
by varying the lengths of the gas inlets 106 within the rotary kiln reactor
102. In an example, as
depicted in FIG. 1, each gas inlet 106 may have a length different from
lengths of the other gas
inlets 106. However, one skilled in the art should appreciate two or more of
the gas inlets 106
having equal or substantially equal lengths without departing from the spirit
and scope of the
present disclosure.
[0029] Contact of the solid and liquid hydrocarbon-containing wastes with the
gases may result
in physical interaction and chemical reactions that reshape the chemical
composition of the
gaseous fuel generated from the said wastes. Moreover, contacts of the solid
and liquid
hydrocarbon-containing wastes with the gases may also result in thermochemical
transformation
that transforms the solids into a gaseous state. These interactions and
transformations produce
gaseous fuels. The apparatus 100 of the present disclosure, while being
configured to perform
the above identified interactions and transformations, may also be configured
to dry and
devolatilize hydrocarbon-containing wastes, to remove and destroy agents of
organic
contamination in inorganic materials including soils, as well as to produce
bio-chars from
biomasses without necessitating physical alternation of the apparatus 100. The
apparatus 100
may be configured to perform only a portion of the above identified operations
at a time, in
which case switching between configurations to perform the different
operations may be
automatic and instantaneous.
[0030] The apparatus 100 operates independent of the type of hydrocarbon-
containing wastes,
thereby enabling hydrocarbon-containing wastes with varying compositions and
physical
properties to be processed by the apparatus 100 without needing to alter the
apparatus 100 in any
material way. The apparatus 100 also operates independent of the size of the
hydrocarbon-
containing wastes introduced therein, thereby allowing hydrocarbon-containing
wastes of
varying sizes to be processed by the apparatus 100 without needing to alter
the apparatus 100 in
any material way. For example, the apparatus 100 may process hydrocarbon-
containing wastes
ranging from about 0.1 inches to about 6 inches, and preferably from about 0.1
inches to about 2
inches. In an example, the apparatus 100 may be configured to permit passage
of gas through
6
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
the apparatus 100 that is about 40 times greater in weight than that of the
hydrocarbon-
containing wastes introduced into and processed by the rotary kiln reactor
102. In another
example, the apparatus 100 may be configured to permit passage of gas through
apparatus 100
that is about 20 times greater in weight than that of the hydrocarbon-
containing wastes
introduced into and processed by the rotary kiln reactor 102. The apparatus
100 may perform
processing operations at a range of temperatures including about 100 F to
about 3000 F, and
preferably from about 100 F to about 2200 F. The apparatus 100 may also
perform processing
operations at a range of pressures, including pressure within the apparatus
100 being about minus
1 inch of water column to about 100 inches of water column.
[0031] Referring to FIGS. 3A through 3D, operational conditions within the
rotary kiln reactor
102 are described. While the rotary kiln reactor 102 is exemplarily
illustrated as rotating counter
clockwise, the rotary kiln reactor 102 is not limited to merely counter
clockwise rotation. Upon
hydrocarbon-containing wastes 302 being introduced into the rotary kiln
reactor 102 via the inlet
means 104 (e.g., described above with reference to FIG. 1), inertial forces
caused by rotation of
the rotary kiln reactor 102 cause solids within the hydrocarbon-containing
wastes 302 to
gravitate to an outer wall 304 of the rotary kiln reactor 102. The rotary kiln
reactor 102 may
rotate prior to introduction of the hydrocarbon-containing wastes 302 or may
not start rotating
until after the hydrocarbon-containing wastes 302 are introduced therein. The
amount of surface
area coverage of the hydrocarbon-containing wastes 302 along the surface area
of the outer wall
304 of the rotary kiln reactor 102 depends on the speed of rotation of the
rotary kiln reactor 102.
In a stationary mode (illustrated in FIG. 3A), solids of the hydrocarbon-
containing wastes 302
settle at the bottom of the rotary kiln reactor 102. As the speed of rotation
of the rotary kiln
reactor 102 increases (FIG. 3B illustrates low rotation speed, FIG. 3C
illustrates medium rotation
speed, and FIG. 3D illustrates high rotation speed), the solids of the
hydrocarbon-containing
wastes 302 become more disbursed along the outer wall 304, thereby covering a
larger surface
area of the outer wall 304. Relative positions of the gas distributor 108 and
the overlay pipe 208
may be altered to adjust or modify trajectories of the gas outlet ports 204
with respect to the
interior of the rotary kiln reactor 102 (demonstrated, e.g., by a comparison
of the gas distributor
108 and the overlay pipe 208 within FIGS. 3, 4 through 3D). The gas
distributor 108 and the
overlay pipe 208 may be configured to direct a maximum amount of a contact
between gases
dispersed from the gas outlet ports 204 and the hydrocarbon-containing wastes
302.
7
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
[0032] As mentioned herein, wet hydrocarbon-containing wastes may be dried
within the
apparatus 100, namely within the rotary kiln reactor 102. While the wet
hydrocarbon-containing
wastes are in the rotary kiln reactor 102, hot gases are entered into the
rotary kiln reactor 102 via
the gas outlet ports 204. The hot gases may have a temperature of about 300 F
to about 1000 F.
Prior to introduction into the rotary kiln reactor 102, the wet hydrocarbon-
containing wastes may
be at room temperature. Preferable drying of wet hydrocarbon-containing wastes
is attained
when the hot gases are uniformly distributed within the four zones of the gas
distributor 108.
During contact between the wet hydrocarbon-containing wastes with the hot
gases, heat is
transferred from the gases to the hydrocarbon-containing wastes (e.g.,
solids), causing the
hydrocarbon-containing wastes to heat up to a range of about 150 F to about
250 F, at which
time moisture in the hydrocarbon-containing wastes (e.g., solids) evaporates
and converts into
steam. The steam is discharged from the rotary kiln reactor 102 with the other
hot gases and
directed to a cyclone 400 (described in detail below).
[0033] The general reactions or schematic reactions involved during drying of
the wet
hydrocarbon-containing wastes are as follows:
Hydrocarbon-Containing Material + Water + Hot Gas 4 Hydrocarbon-Containing
Material + Steam + Cooled Gas
[0034] The dried hydrocarbon-containing wastes are discharged from the rotary
kiln reactor 102
as ash via the means 112 for removing solids from the rotary kiln reactor 102.
[0035] The apparatus 100 may perform pyrolysis of the hydrocarbon-containing
wastes by
heating hydrocarbon solids to a temperature in the range of about 800 F to
about 1000 F, at
which time volatile matter present in the hydrocarbon-containing wastes is
vaporized. The
volatile matter comprises mainly large molecule hydrocarbons, small molecule
hydrocarbons,
combustible gases including carbon monoxide and hydrogen, and non-combustible
gases
including carbon dioxide, nitrogen and water. In utilizing the apparatus 100
for the pyrolysis of
the hydrocarbon-containing wastes, the hydrocarbon-containing wastes are
introduced into the
rotary kiln reactor 102 where they are contacted with hot gases introduced
into the rotary kiln
reactor 102 through the gas distributor 108.
[0036] The general reactions or schematic reactions involved during pyrolysis
of the
hydrocarbon-containing wastes are as follows:
Hydrocarbon-Containing Wastes + Hot Gases ¨4 Hydrocarbons + CO + H2 CO2 +
8
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
H20
Hydrocarbons Liquid Hydrocarbons + Gaseous Hydrocarbons
[0037] Vaporization of the hydrocarbon-containing wastes may also occur using
the following
methodology. The hydrocarbon-containing wastes are partially combusted to
generate adequate
heat to raise the temperature of the hydrocarbon-containing wastes to about
800 F to about
1000 F. Prior to introduction of hydrocarbon-containing wastes, the rotary
kiln reactor 102 is
heated to a temperature above an ignition temperature of the hydrocarbon-
containing wastes.
Oxygen-bearing gases used to ignite the hydrocarbon-containing wastes are
introduced into the
rotary kiln reactor 102 through the gas distributor 108. Room temperature
hydrocarbon-
containing wastes are introduced into the preheated rotary kiln reactor 102.
Introduction of the
room temperature hydrocarbon-containing wastes into the rotary kiln reactor
102 may occur
prior, during, or after the oxygen-bearing gases are introduced into the
rotary kiln reactor 102.
Beneficial results for the pyrolysis of the hydrocarbon-containing wastes
using this method are
attained when the oxygen-bearing gases are uniformly distributed throughout
the four zones of
the gas distributor 108. During the contact of solid hydrocarbon-containing
wastes with the
oxygen-bearing gases in the rotary kiln reactor 102, the hydrocarbon-
containing wastes are
partially combusted. The heat of combustion causes the temperature of the
hydrocarbon-
containing wastes to rise to about 800 F to about 1000 F, at which time
volatiles contained in the
hydrocarbon-containing wastes evaporate into a gaseous phase. The general
reactions or
schematic reactions involved in this pyrolysis methodology include the
following:
Hydrocarbon-Containing Wastes + Air Hydrocarbons + CO + H2 + CO2 + H20 + N2
Hydrocarbons Liquid Hydrocarbons + Gaseous Hydrocarbons
[0038] According to the aforementioned methodology, the solid residue
discharged from the
rotary kiln reactor 102 contains inorganic components of the hydrocarbon-
containing wastes as
well as fixed carbon present in the hydrocarbon-containing wastes. This solid
residue has clean
burning properties and is therefore considered high-grade solid fuel. When the
hydrocarbon-
containing wastes employed during this pyrolysis methodology is biomass, the
solid residue
discharged from the rotary kiln reactor 102 constitutes bio-char.
[0039] When the intended use of the apparatus 100 is to carry out gasification
of the
hydrocarbon-containing wastes to produce clean gaseous fuel for utility use,
the hydrocarbon-
containing wastes are reacted with oxygen bearing gases (i.e., air) and water
(i.e., vapor) at an
9
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
elevated temperature to convert hydrocarbon-containing material into a mixture
of combustible
and non-combustible gases. The fuel gas mixture may include carbon monoxide,
hydrogen,
methane, ethane, carbon dioxide, water vapour, and nitrogen. Additionally, the
fuel gas mixture
may have a caloric value in the range of about 80 to about 320 BTU per cubic
foot irrespective
of a composition of the varied-source hydrocarbon-containing waste
processed/gasified. In this
instance, room temperature hydrocarbon-containing wastes are introduced into
the kiln reactor
102, which is preheated to a temperature above an ignition temperature of the
hydrocarbon-
containing wastes. Oxygen-bearing gases are used to ignite the hydrocarbon-
containing wastes
and are introduced into the rotary kiln reactor 102 through the gas
distributor 108. For
gasification to occur beneficially, the hydrocarbon-containing wastes may have
about 20% to
about 50% water content. If the hydrocarbon-containing wastes do not contain a
sufficient water
content prior to introduction into the rotary kiln reactor 102, water is
introduced to the
hydrocarbon-containing wastes while in the rotary kiln reactor 102.
Alternatively, instead of
water, steam may be introduced to the hydrocarbon-containing wastes while in
the rotary kiln
reactor 102.
[0040] Upon entering the preheated rotary kiln reactor 102, a small amount of
volatile matter
from the hydrocarbon-containing wastes is instantly vaporized. Due to the
rotary kiln reactor
102 being preheated to the volatile matter's flash point of combustion, the
volatile material is
instantaneously ignited when contacted with air or some other oxygen-bearing
gas. For the
present gasification methodology, the quantity of oxygen-bearing gases
introduced along the
length of the rotary kiln reactor 102 is far below that required for complete
combustion of the
hydrocarbon-containing wastes. The quantity of oxygen-bearing gases may be in
the range of
about 30% to about 70% percent by volume of that required for complete
combustion of the
hydrocarbon-containing wastes. The chemical composition of the hydrocarbon-
containing
wastes, the amount of moisture contained therein, and the intended temperature
of the
gasification reaction dictate the quantity of the oxygen bearing gases.
[0041] During gasification, four distinct zones of gas-sold reactions are
created along a length of
the rotary kiln reactor 102 and the corresponding temperature in each of the
zones results from
partial combustion of vaporized volatile matter of the hydrocarbon-containing
wastes and the
gasification reactions between the vapors of water and hydrocarbon-containing
wastes. The four
zones result from controlling the fraction of total oxygen bearing gases
allowed to enter the
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
rotary kiln reactor 102.
[0042] The wastes generated by industry and manufacturing processes vary
significantly in
terms of their physical and chemical properties. In order to be able to
process each of these
wastes separately or in combination, suitable reaction conditions within the
rotary kiln reactor
102 to align with the requirements of the wastes should be provided. The
physical properties of
hydrocarbon-containing wastes generally relate to size, density, and their
moisture content. The
physical properties require that the wastes are allotted certain residence
time within the rotary
kiln reactor 102 in order for the wastes to fully react with gaseous reactants
within the bounds of
the rotary kiln reactor 102. The ability of the present disclosure to increase
localized
temperatures within zones of the rotary kiln reactor 102 speeds up reactions
within the rotary
kiln reactor 102. In this manner, the apparatus 100 of the present disclosure
is able to
accommodate variations in the physical properties of received hydrocarbon-
containing wastes.
[0043] In contrast, the chemical properties of the hydrocarbon-containing
wastes are
characterized by their elemental compositions and their volatility as
determined by amount of
volatile carbon content and fixed carbon contained within the wastes. The
elemental
composition determines the amount of oxygen-bearing gases as well as amount of
water required
to fully gasify the waste. The volatility dictates where the reaction gases
are introduced for
effective gasification of the waste. For example, a mixture of plastic waste
and char includes
almost 50% volatile carbon and 50% fixed carbon whereas textile waste includes
of mostly
volatile carbon. For gasification of plastics and char mixture, a gradual
introduction of oxygen-
bearing gases along the length of the rotary kiln reactor 102 is an effective
mode for gasification.
The reason for gradual introduction of reactant gases is that the volatile
carbon has a tendency to
instantly react with the reactant gases whereas the fixed carbon requires
longer contact time with
reactant gases for gasification reactions to take place. The rotary kiln
reactor 102 of the present
disclosure has the ability to introduce the reactant gases according to the
dictate of the waste
along the length of the rotary kiln reactor 102 through the zoned gas
distributor 108. For textile
waste, an effective mode for gasification includes introduction of most of the
requisite oxygen-
bearing gases and water in the zone close to where the waste is introduced
into the rotary kiln
reactor 102. Thus, in this case all oxygen-bearing gases may enter in the
first zone of the gas
distributor 108.
[0044] The following paragraphs describe an exemplary application of the
present disclosure for
11
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
gasification using hydrocarbon-containing wastes containing almost equal parts
of volatile
carbon and fixed carbon. As the below is merely an exemplary application, it
is not meant to be
limiting. One skilled in the art should appreciate the present disclosure
providing an endless
number of reaction conditions within the bounds of the rotary kiln reactor 102
in order to
accommodate all types of gas/solid reactions required for efficient
gasification of varied-source
hydrocarbon-containing wastes.
[0045] In the following example, waste containing about equal parts of
volatile carbon and fixed
carbon is processed in a first zone, which may be closest to the entry of the
hydrocarbon-
containing material into the rotary kiln reactor 102, the temperature is
maintained below about
800 F so the moisture contained in the hydrocarbon-containing material is
evolved first,
followed by partial evaporation of the volatile matter. In the first zone,
about 10% to about 25%
of the oxygen-bearing gases are introduced. In the first zone, the following
reactions represent
the interactions between the gas(es) and solid hydrocarbon-containing wastes:
Hydrocarbon-Containing Material + Hot Gases Volatile Matter + Steam
Volatile Matter + Air CO2 + CO + H2 + H20 Hydrocarbons
[0046] In the second zone, another about 10% to about 25% of the oxygen-
bearing gases are
introduced to further combust the volatile matter, which continues to
vaporize. In the second
zone, the temperature is allowed to rise to about 1000 F to about 1200 F. The
objective of the
second zones configuration is to completely vaporize the volatile matter from
the hydrocarbon-
containing material.
[0047] In the third zone, another about 25% to about 40% oxygen-bearing gases
are introduced
and directed towards the hydrocarbon-containing material, which should now be
devoid of
volatile matter but including fixed carbon and inorganic components of the
hydrocarbon-
containing wastes. The configuration of the third zone allows for full
combustion of the fixed
carbon. In the third zone, the temperature is allowed to rise to a range of
about 1800 F to about
2000 F in order to accelerate combustion of the fixed carbon. The heavy
hydrocarbons and the
combustible gases present inside of the rotary kiln reactor 102 at the third
zone also partially
combust with the oxygen-bearing gas. The vapors of water present in the gas
inside the rotary
kiln reactor 102 at the third zone also react with the fixed carbon as well as
with the heavy
hydrocarbon molecules present in the vaporized volatile matter, thereby
causing these molecules
to break down into smaller hydrocarbon molecules and combustible gases
comprising mainly
12
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
carbon monoxide and hydrogen. In the third zone, the main reactions are as
follows:
C +02 CO2
CO + H2 + 02 + Heavy Hydrocarbons CO2 + H20 + CH4 + C2H6 + CO + H2
[0048] In the fourth zone and subsequent zones (if any), conditions similar to
those in the third
zone are maintained with respect to the temperature and the amount of oxygen-
bearing gases
introduced therein.
[0049] For example, if the hydrocarbon-containing waste was replaced by waste
containing
almost all volatile carbon, 100% of the oxygen-bearing gases would enter in
the first zone of the
gas distributor 108 and all of the gasification reactions would take place
within the first zone.
[0050] Not all components of the apparatus 100 are necessary for all
processing functions and
therefore only pertinent components of the apparatus 100 may be utilized
depending upon the
processing function carried out by the apparatus 100. Idle components of the
apparatus 100, not
utilized during particular processing functions, may simply be bypassed,
thereby not impacting
the efficiency of the specific processing function in any way.
[0051] Referring to FIG. 4, a system 400 for processing hydrocarbon-containing
wastes is
described. Hydrocarbon-containing wastes are directed from a storage 402
(e.g., a hopper) a
gasifier (such as the apparatus 100) using a conveyor means (e.g., screw
feeder) 404. The
hydrocarbon-containing wastes may be processed by the gasifier/apparatus 100
using the
functionality described herein.
[0052] Gases introduced into the gasifer/apparatus 100, gases generated by
reaction of the
introduced gases with the hydrocarbon-containing wastes, and reacted ash
generated in but not
otherwise disposed of by the gasifer/apparatus 100 are directed to a cyclone
406. Additionally,
solids/ash enter the cyclone 406 at a rate commensurate with the presence of a
non-combustible
fraction present in the hydrocarbon-containing waste. These gases and ash may
have a
temperature of about 1800 F. At the cyclone 406, at least a portion of the
received ash is
separated from the gases, and the ash is dispelled from the system. The gases
remaining in the
cyclone 406 may be cooled by two methods before they are utilized as source of
energy. One
method of cooling occurs by means of direct contact with water in a quencher
408. An
alternative method for cooling the gas is by using an indirect means of
contacting the gas with
water in a Waste Heat Exchanger ("WHE") 414.
[0053] Upon exiting the quencher 408 or upon exiting the WHE 414, the gas is
further purified
13
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
to remove additional ash using either a cyclone 410 or filter 416 before it is
utilized by, for
example, a burner 412. By way of example, a surge tank 418 is included in the
system 400 to
mitigate surges in production of fuel gas from the gasification of hydrocarbon-
containing wastes
because of its variability with respect to physical and chemical properties.
[0054] For example, the gases may have a temperature of about 1800 F upon
entering the
quencher 408 and a temperature of about 350 F upon exiting the quencher 408.
The gases may
have a substantially constant temperature during transfer between the quencher
408 and burner
412, and between the WHE 414 and the burner 412. For example, the constant
temperature may
be about 350 F.
[0055] The gases may be about 1800 F while entering the WHE 414 and may be
about 350 F
while leaving the WHE 414. Lime may be introduced into the filter 416 to
remove contaminants
therein.
[0056] Attention should now be given to FIG. 5, which illustrates a method 500
for processing
hydrocarbon-containing wastes according to the present disclosure. At block
502 hydrocarbon-
containing wastes are reacted with oxygen-bearing gases and water under at
least three different
reaction environments. This may be performed using the apparatus/gasifier 100.
The
hydrocarbon-containing wastes may have about 20% to about 50% water content.
Oxygen-
bearing gases are used in all or almost all of the reaction environments
described below. The
overall reaction may involve gasifying the hydrocarbon-containing wastes. A
first reaction
environment involves room temperature hydrocarbon-containing wastes entering
the apparatus at
which time at least a portion of the volatile matter of the hydrocarbon-
containing wastes is
instantly vaporized due to the apparatus being preheated to a temperature
above the ignition
temperature/flash point of combustion of the hydrocarbon-containing wastes. In
a second
reaction environment, temperature of the apparatus is maintained below about
800 F, causing
moisture contained in the hydrocarbon-containing wastes to evolve first,
following by partial
evaporation of the volatile matter. In a third reaction environment,
temperature of the apparatus
is maintained between about 1000 F and about 1200 F, causing complete
vaporization of the
volatile matter from the hydrocarbon-containing wastes. This results in fixed
carbon and
inorganic components remaining in the hydrocarbon-containing wastes. In a
fourth reaction
environment, the apparatus is maintained in a range of about 1800 F to about
2000 F, causing
combustion of the fixed carbon of the hydrocarbon-containing wastes. The
conditions of the
14
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
fourth reaction environment also results in heavy hydrocarbons combustible
gases being partially
combusted, as well as vapors of water reacting with the hydrocarbons to create
smaller
hydrocarbon molecules and combustible gases (e.g., carbon monoxide and
hydrogen). A fifth
reaction environment and subsequent reaction environments (if any) have
similar conditions to
those in the fourth reaction environment.
[0057] At block 504 solid residues resulting from the gasification of
hydrocarbon-containing
wastes are separated from the gases generated from the gasification of the
hydrocarbon-
containing wastes. This may be performed using the cyclone 406. At block 506
gaseous
hydrocarbon-containing wastes are quenched using direct contact with water.
Quenching of the
gaseous hydrocarbon-containing wastes may be performed using the quencher 408.
At block
508 additional solid residues are separated from the quenched gases generated
from the
gasification of the hydrocarbon-containing wastes. This may be performed using
the quencher
cyclone 410. At block 510 the separated gases are combusted. Combustion of the
said gases
may be performed using the burner 412.
[0058] At block 512 thermal energy is captured by indirect means from hot
gases generated from
the gasification of hydrocarbon-containing wastes. This may be performed using
the WHE 414.
At block 514 additional solid ash is separated from the hydrocarbon-containing
wastes. This
may be performed using the filters 416. At block 516 the gases generated by
the gasification of
hydrocarbon-containing wastes are combusted as illustration of one utility of
fuel gases
generated by gasification of the hydrocarbon-containing wastes. This may be
performed using
the burner 412. Alternative options for utilization of the gases are direct
replacement of fuels in
industry and manufacturing processing, direct replacement of fuels in boilers
for generating
steam, and direct replacement of fuels in gas engines for generating
electricity.
[0059] Referring now to FIG. 6, an exemplary system 600 for converting
industry wastes into
gaseous fuel is described. The varied-source industry waste source utilized
may be a
combination of various wasted generated by a typical chemical processing
plant. Exemplary
components of the varied-industry wastes that may be processed by the system
600 are depicted
in Table 1. The system 600 illustrates how the present disclosure may be
utilized for recovering
energy using 15 tons/day of varied-source waste in the form of steam and in
the form of usable
fuel gas.
CA 02959017 2017-02-22
WO 2016/029093
PCT/US2015/046257
Approx.
Source of Waste Generation Physical state of Composition f Waste
(Processing Train) Quantity (Tons waste o
per Year).
Soaked with lube oil,
Organic Residue 200 Semi Solid grease, HC in varying
range of 10 to 100%
Solid granular &
Bio Sludge 800
powder
VCM Coke 25 Solid granular/ easily C 80 %,H 1.4 %, 0
12.96
crushable lumps %,P 0.03 %,S 0.17 %
Cracker Coke
Solid granular/ easily
crushable lumps
LOI at 800 C including
LOD 91.4 % to 99.8 %
Spent Resin 100 Solid granular Moisture at 105 C 19 % to
47.7 %, Ash Content 8.6
%to 0.2 %
Spent carbon 40 Solid granular
Charred Polymer, PSF
Solid, Semi solid
effluent pit mucks, PP skim 35 100 % combustible
mud, pellets, fines
pond pellets & fines
Rhypox froth 180 Semi Solid 100 % combustible
¨ %
TerpthalicAcid , 100
PTWM (Optional) 2000 Solid
%78 e combustibl
TI02 Slurry 3 Semi Solid ¨80 % Glycol
C 56 %, H3.9 %,N0.08
Degraded Dowtherm 20 Liquid/ thick liquid
%,037 %,PNil,S0 12 %
Sticky balls
Additional Upcoming plants
SBR PBR (Rubber barring 65 (Scparatcd and in
lumps of different
waste)
.............................. size)
Scrap filter, bag filters & Solid tubular, square,
cartridge filter 4 rectangle etc. shapes
Old records 4 Paper A4 to A3 sizes
Thermocole Waste 2 Different shapes
Non recyclable Office
garbage 10 Paper, used cups etc
Solid (shape of cover
FRP waste 4 of motor etc.)
PET Dust 20 100 % combustible
Leaves, Branches, trunk,
Garden Waste 1560 Grass etc.
Total Waste Pcr Year
(Tons) 5112
Generation Rate Tons/Day
(Based on 90% availability) 15
Table 1. Potential composition of waste generated by typical chemical plant.
16
CA 02959017 2017-02-22
WO 2016/029093 PCT/US2015/046257
[0060] FIG. 7 illustrates how in practice the present invention would be
utilized for recovering
energy using 15 tons per day of another type of varied source waste in the
form of steam and in
the form of usable fuel gas.
[0061] The distinguishing characteristics between the waste types in FIG. 6,
FIG. 7, and FIG. 8
are their inherent calorific values which makes them different from the
perspective of their
chemical properties. The gases may enter the WHE 414 at a rate of about 2,110
kg/h, about
2,756 kg,/h, or about 3,212 kg/h, for example. The gases may enter the
quencher 408 at a rate of
about 1,385 kg/h or about 1,400 kg/h, for example. The gases may enter the
cyclone 410 at a rate
of about 1,720 kg/h, about 1,820 kg/h, or about 1,920 kg/h, for example. The
gases may enter the
burner 412 at a rate of about 1,720 kg/h, about 1,820 kg/h, or about 1,920
kg/h, for example.
The gases may enter the cyclone 406 at a rate of about 2,100 kg/h, about 2,756
kg/h, or about
3,212 kg,/h, for example. The ash may be dispelled from the system at a rate
of about 9.65 kg/h,
about 6.80 kg/h, or about 3.88 kg/h, for example. Despite their differences in
chemical
properties, the heating value of the fuel generated as a result of the
thermochemical
transformation by the apparatus of the present invention remains almost the
same.
[0062] The above teachings of the present disclosure are meant to be
illustrative. They were
chosen to explain the principles and application of the disclosure and are not
intended to be
exhaustive or to limit the disclosure. Many modifications and variations of
the disclosed
embodiments may be apparent to those of skill in the art. Moreover, it should
be apparent to one
skilled in the art, that the disclosure may be practiced without some or all
of the specific details
and steps disclosed herein.
[0063] The specification and drawings are, accordingly, to be regarded in an
illustrative rather
than a restrictive sense. It should, however, be evident that various
modifications and changes
may be made thereunto without departing from the broader spirit and scope of
the disclosure as
set forth in the claims.
17