Note: Descriptions are shown in the official language in which they were submitted.
I
METHOD FOR REMOVING SODIUM FROM CHLORIDE SOLUTIONS
FIELD OF THE INVENTION
The present invention relates to a method for removing sodium from
chloride containing solutions in a hydrometallurgical metal recovery
processes.
More specifically the invention relates to removal of sodium from hydrometal-
lurgical process solutions resulting from chloride based leaching of nickel
con-
taining ores and/or concentrates.
BACKGROUND OF THE INVENTION
In chloride based leaching of ores and/or concentrates, in particular
of nickel containing ores and/or concentrates, the leaching solution is
typically
recirculated within the process. This leads to build-up of elements such as so-
dium and potassium. Sodium is typically present in the solution as sodium
chloride. In low concentrations, i.e. less than about 10 g/L, this is not a
prob-
lem. However, the sodium will make the solution more concentrated and even-
tually more solution is needed to leach same amount of metals if sodium is not
removed.
Traditional way to remove impurities such as sodium is to take out a
small bleed stream from the process. In concentrated chloride solutions this
is
not an economical option since the solution needs to be treated and the chlo-
rides need to be replaced.
The invention is based on the fact that the sodium chloride crystal-
lizes before the other salts present in the solution for example calcium
chloride
or ammonium chloride.
BRIEF DESCRIPTION OF THE INVENTION
An object of the present invention is thus to provide a method so as
to alleviate the above disadvantages. The objects of the invention are
achieved by a method, which is characterized by what is stated in the inde-
pendent claims.
The invention based on the realization that sodium can be removed
from the hydrometallurgical process streams simultaneously to adjusting the wa-
ter content of the process stream. Accordingly taking out only the impurity
that
needs to be removed the volume of the bleed stream can be minimized and the
process can be made more economical and usually more environmental friendly.
Date Recue/Date Received 2021-12-16
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
2
Since the sodium input to the process is usually quite small and
process can tolerate some sodium, all sodium is not needed to be removed
from the process stream. This enables crystallization from the bleed stream
instead of the main process stream. This means smaller equipment and lower
investment cost. Since the water is needed to be evaporated due to the closed
solution circulation in the process the saturated solution can be produced for
the crystallization when the evaporation is also put to the bleed stream. Crys-
tallization from the saturated solution is easier since it starts immediately
when
more water is evaporated or temperature is lowered.
Sodium chloride can be taken with this invention as almost pure
product since some sodium is left to the depleted sodium solution. Sodium
chloride is the first salt that crystallizes from the solution and therefore
amount
of solid impurities in the sodium chloride product is small. To further purify
so-
dium chloride product it can be washed.
BRIEF DESCRIPTION OF THE DRAWINGS
In the following the invention will be described in greater detail by
means of preferred embodiments with reference to the attached drawings, in
which
Figure 1 is a flow diagram illustrating an embodiment of the method
of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a method for removing sodium from
a chloride-based hydrometallurgical process stream comprising the steps of:
(a) withdrawing a bleed stream of hydrometallurgical process liquors
and/or slurries from the chloride-based hydrometallurgical process stream;
(b) evaporating water from the bleed stream to obtain a saturated
sodium containing solution;
(c) crystallizing a part of the sodium chloride comprised in the satu-
rated sodium containing solution from the said solution to obtain a sodium de-
pleted solution; and
(d) returning the sodium depleted solution to the process stream.
The method of the present invention is suitable for treating any hy-
drometallurgical process streams resulting from leaching of metal containing
ores and/or concentrates with chloride based leaching solutions, such as a
concentrated CaCl2 based solution. The said hydrometallurgical process
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
3
stream further comprises ammonium chloride, nickel chloride, copper chloride,
cobalt chloride, and/or magnesium chloride. Typically the said hydrometallurgi-
cal process stream comprises ammonium chloride. The hydrometallurgical
process stream typically comprises significant amounts of sodium. In
particular
the hydrometallurgical process stream comprises 1 to 50 g/L, more particularly
5 to 25 g/L, sodium. Further preferably the hydrometallurgical process stream
comprises 10 to 100 g/L Ca and/or 0.1 to 10 g/L NH4.
The method of the present invention is particularly suitable for
treatment of leaching solution resulting from leaching of nickel containing
ore
and/or concentrate. The term "concentrate" as used herein refers to any prod-
uct generally produced from metal containing ore for concentrating the desired
metal(s) in the product and/or removing impurities from the mined ore for fur-
ther treatment of the product for the recovery of one or more of said
metal(s).
Such concentrate can be produced by any suitable methods known to a per-
son skilled in the art.
Preferably the method of the present invention provides for removal
of at least 30%, more preferably at least 50%, typically 30 to 90%, more typi-
cally 60 to 80%, of sodium comprised in the hydrometallurgical process stream
before the withdrawal of the bleed stream. Typically the sodium depleted solu-
tion returned into the main process stream, i.e. the hydrometallurgical
process
stream, comprises less than 4 wt%, preferably less than 2 wt%, sodium. In
some cases where evaporation need of the process is big the sodium concen-
tration is higher in the main process stream after the evaporation and sodium
removal than before those process steps due to the decreased total amount of
the process stream.
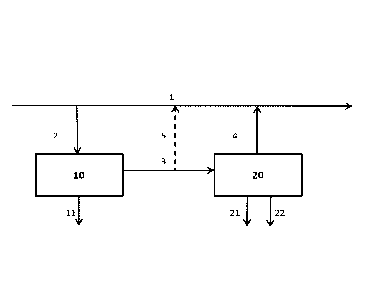
Figure 1 shows an example of a process flow of the method of the
present invention. Referring to Figure 1, a bleed stream 2 is withdrawn from a
hydrometallurgical process stream 1. The bleed stream can be withdrawn at
any process stage after leaching of the ore and/or concentrate. Preferably the
bleed stream is withdrawn at a process stage where the concentration of any
valuable metals in the said process stream is as low as possible to prevent
loss said valuable metals during the removal of sodium. Advantageously the
bleed stream is withdrawn after a process stage whereby the acid concentra-
tion of the said process stream is under 5 g/L. Such process stages include
precipitation, solvent extraction and neutralization stages. In nickel
leaching
such process stages are in particularly iron precipitation, solvent extraction
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
4
and/or ammonia regeneration. Suitable locations for the withdrawal include
after iron precipitation, after one of the solvent extraction stages, and/or
after
regeneration of ammonia. In a preferred aspect of the present invention the
bleed stream is withdrawn after regeneration of ammonia.
The volume of the bleed stream is dependent of the amount of water
that needs to be removed from the process stream to maintain water balance. If
water balance is taken care in other parts of the process the bleed stream is
dependent of the amount of sodium needed to be removed, but typically the wa-
ter balance determine the bleed stream amount. The amount of bleed stream
can be calculated from the amount of water needed to be removed from the
process at this stage, from the salt concentration of the process stream and
the
salt concentration of the saturated sodium containing solution, e.g. a
solution
containing 50 g/L CaCl2 and 5 g/L NaCI has a salt concentration of 55 g/L. Un-
less otherwise denoted, the term "salt concentration" as used herein and
hereaf-
ter refers to the amount of all dissolved salts in the particular stream. The
salt
concentration of a solution is dependent on temperature and the nature of
salts
in the said solution. The process stream typically comprises 5 to 35 wt%
salts,
more typically 10 to 30 wt% of the total weight of the said stream. The
saturated
sodium containing solution typically contains 35 to 70 wt% of salts more
typically
40 to 65 wt% of the total weight of the saturated solution. Typically a
variation of
10%, more typically 5%, is tolerated for the amount of required bleed stream.
If the salt concentration in the process solution, evaporation need or salt
con-
centration the saturated sodium containing solution changes, also the amount
of
bleed stream will change. Examples how the amount of the bleed stream can be
calculated are presented in examples 1 and 2.
The bleed stream is preferably withdrawn continuously from the hy-
dronnetallurgical process stream i.e. as a continuous bleed stream of the hy-
drometallurgical process stream. The continuous operation of sodium chloride
crystallization enables smaller equipment and more even sodium concentration
in the process. The other option is to withdraw a continuous bleed stream for
evaporation of water in step (b) and operate the crystallization of sodium
chlo-
ride in step (c) as a batch process.
The bleed stream 2 is then subjected to evaporation 10 in the evap-
oration step (b) to remove water 11 from the bleed stream and to concentrate
sodium in the bleed stream. This results in a saturated or nearly saturated so-
dium containing solution 3 which is subjected to crystallization 20 in the
crystal-
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
lization step (c). The term "saturated or nearly saturated" as used in context
of
the saturated sodium containing solution refers to the fact that further
evapora-
tion of water from the solution would cause crystallization of sodium chloride
thus enabling the crystallization of the same in the next step.
5 The evaporation step (b) 10 is typically performed in a multi stage
evaporation equipment. In a multi stage evaporation the first evaporation step
is typically performed atmospherically or near atmospherically. Further evapo-
ration step(s) are typically performed under reduced pressure. Advantageously
steam formed in the first evaporation stage is utilized for indirect heating
of the
further evaporation step(s).
Depending on the process sodium balance, a part of the saturated
or nearly saturated sodium containing solution 3 can be fed back to the main
process between evaporation of the water and crystallization of the sodium
chloride as a concentrated stream 5. Thus in an embodiment of the present
invention a part of the saturated sodium containing solution (3) obtained in
step (b) is returned to the hydrometallurgical process stream before
crystalliza-
tion step (c). Alternatively or additionally part of a partly concentrated
sodium
containing solution i.e. part of the bleed stream being concentrated in step
(b)
can be returned to the hydrometallurgical process stream before it is
saturated
or nearly saturated. This is possible when a multi stage evaporation equipment
is utilized and/or the evaporation step is otherwise performed in multiple sub-
steps. In the above mentioned embodiments the bleed stream of hydrometal-
lurgical process liquors and/or slurries withdrawn from the chloride-based hy-
drometallurgical process stream may be 100% of the hydrometallurgical pro-
cess stream.
The concentrated stream 5 is preferably returned continuously to
the hydrometallurgical process stream 1. The amount of such concentrated
stream is dependent on the sodium amount in the stream, sodium removal
need in the process and the amount of sodium at the concentrated stream af-
ter crystallization, stream 4. For example if the sodium amount in the stream
3
is 100 kg/h and sodium is needed to be removed from the process 50 kg/h and
the stream 4 after crystallization contains 10 kg/h sodium, 40% of the stream
3
is fed back to the main process stream in stream 5. Typically the variation of
stream split percentage value is 10%, more typically 5%.
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
6
The saturated sodium containing solution 3 is then fed to crystalliza-
tion step (c) 20 to remove sodium as sodium chloride 22 from the saturated
sodium containing solution. Crystallization can be achieved by evaporation of
water thus causing the sodium chloride to crystallize from the saturated solu-
tion or decreasing the temperature to cause the sodium chloride to
crystallize.
In evaporation crystallization option the evaporated water is removed from the
process with stream 21. Typically crystallization in step (c) 20 is achieved
in a
separate crystallization device. Alternatively crystallization step (c) 20 can
be
achieved in the same equipment as the evaporation step (b) 10. Further, part
of the evaporation step can be achieved in a first evaporation equipment to
concentrate the stream and the final saturation and the crystallization step
is
achieved in an another equipment.
Typically a slurry comprising sodium chloride crystals is obtained
from the crystallization step. The slurry is then mostly dried in a centrifuge
to
obtain mostly dry or dry crystals. The dried crystals may then be washed with
water to remove remaining mother liquor and to further purify the crystals.
The
washing solution is preferably recirculated to the crystallization step (c) to
im-
prove the recovery of sodium chloride.
Preferably at least 30 wt%, preferably from 30 to 100 wt%, more
preferably from 50 to 95 wt%, of the sodium chloride contained in the
originally
withdrawn bleed stream is crystallized. Removal of sodium chloride in step (c)
results in a sodium depleted solution 4 that is returned to the process stream
to
keep the salts in the process in a closed circulation.
The sodium depleted solution 4 is returned to the hydrometallurgical
process stream 1 typically at the same process stage where the bleed stream
2 was withdrawn from the process stream 1. This enables continuation of the
following process steps in a conventional manner.
The sodium depleted solution 4 is preferably returned to the hydro-
metallurgical process stream i.e. as a continuous sodium depleted stream. This
enables a steady salt concentration in the main process stream. Even if the
crystallization is operated as a batch process a continuous stream of sodium
depleted solution to the process stream is needed since big differences in the
salt concentrations in other parts of the process make it difficult to operate
the
process. The hydronnetallurgical process stream 1 typically contains from 1 to
50 g/L, more typically 5 to 25 g/L sodium after the sodium depleted solution
is
mixed with the said process stream.
CA 02959113 2017-02-23
WO 2016/034769 PCT/F12015/050569
7
EXAMPLES
Example 1
50 t/h water is removed from a 100 t/h process solution. The flow of
the needed bleed stream amount is calculated followingly: The process solu-
tion comprises 20 wt% of salts. 60 wt% of salts in the saturated solution is
used. The 100 t/h process solution flow then contains 20 t/h salts, 80 t/h
water
and produced saturated solution contains 20 t/h salts and 13.3 t/h water.
Therefore in the bleed stream is 50 t/h + 13.3 t/h = 63.3 t/h water. This
means
that water needed to be put to the bleed stream is 63.3 t/h / 80 t/h = 0.791
times the water in the initial process stream.
Example 2
When process stream is 100 t/h, water evaporation need is 10 t/h,
salt concentration of the process solution is 25 wt% and saturated solution
salt
concentration is 60 wt%, the amount of bleed stream is 33.3%.
It will be obvious to a person skilled in the art that, as the technology
advances, the inventive concept can be implemented in various ways. The in-
vention and its embodiments are not limited to the examples described above
but may vary within the scope of the claims.