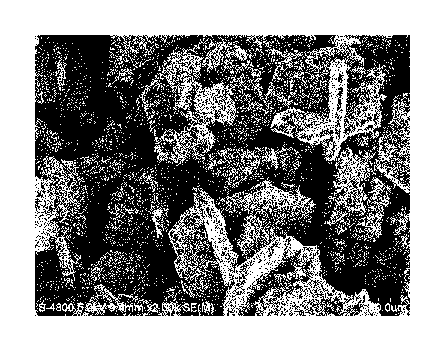Note: Descriptions are shown in the official language in which they were submitted.
CA 02959294 2017-02-24 W7336
1
DESCRIPTION
A
METALLIC COPPER PARTICLES, AND PRODUCTION METHOD THEREFOR
Technical Field
[0001]
The present invention relates to a metallic copper particle and a process for
producing the metallic copper particle. The present invention also relates to
a dispersion in
which the metallic copper particle is blended and a process for producing the
dispersion. The
present invention further relates to an electrode, a wiring pattern, and a
film coating formed by
using the metallic copper dispersion, and still further relates to a
decorative article with the film
coating formed thereon, an antimicrobial article with the film coating formed
thereon, and a
process for producing a metallic copper-containing film for use in them.
Background Art
[0002]
Metallic copper particle is an inexpensive material having a good electrical
conductivity, and has been widely used as a material for securing electrical
conduction, such as a
member for forming a circuit of a printed wiring board, various electrical
contact members, an
external electrode member for a capacitor or the like, and the metallic copper
particle has been
also used in an internal electrode for a multilayer ceramic capacitor in
recent years.
[0003]
Dispersion blending metallic copper particles is a general term that usually
includes compositions, such as coating materials, paints, pastes, and inks,
which is obtained by
dispersing a metallic copper particle in a solvent, and further blending one
or more additives
such as a binder, a dispersant, and a viscosity modifier therein when
necessary. Such a
dispersion is used, by taking advantage of characteristics of the metallic
copper particles, in
various uses such as uses to secure electrical conduction, antistatic uses,
uses to shield
electromagnetic waves, and uses to give metallic luster or antibacterial
properties. Specifically,
the metallic copper particles are used, by taking advantage of characteristics
thereof, for
shielding electromagnetic wave in transparent members of a liquid crystal
display or the like.
Moreover, the technique for forming a fine electrode or a fine circuit-wiring
pattern has been
proposed. This technique is as follows: a dispersion blending metallic copper
particles is
applied on a substrate to form an electrode pattern or circuit-wiring pattern
by a coating method
CA 02959294 2017-02-24
W7336
2
such as screen,printing or inkjet printing, and thereafter the metallic copper
particles are fused by
heating at a relatively low temperature. This has been being applied
particularly to the
production of printed wiring boards. Furthermore, the fusion between the
metallic copper
particles easily progresses even under a mild heating condition to exhibit
metallic luster, and thus
such a simple technique for preparing a mirror surface has been attracting
attention in design and
decoration uses. In recent years, its applications as a joining material in a
device that is used at
high temperatures, such as a power semiconductor, has been also studied.
[0004]
As for metallic copper particle and dispersion dispersing the metallic copper
particles, for example, Patent Literature 1 discloses that a metallic copper
particle is generated by
mixing a divalent copper oxide and a reducing agent in a solvent in the
presence of a complexing
agent and protective colloid to reduce the divalent copper oxide, and that the
metallic copper
particles obtained there are dispersed in a dispersion medium to prepare a
fluid composition.
Further, in Patent Literature 1, as the protective colloid, a gelatin is
illustrated, and as the
reducing agent, hydrazine reducing agents such as hydrazine and hydrazine
compounds like
hydrazine hydrochloride, hydrazine sulfate, and hydrazine hydrate; sodium
borohydride, sodium
sulfite, sodium hydrogen sulfite, sodium thiosulfate, sodium nitrite, and
sodium hyponitrite;
phosphorous acid and salts thereof such as sodium phosphite; and
hypophosphorous acid and
salts thereof such as sodium hypophosphite are listed.
Moreover, Patent Literature 2 discloses a dispersion including: a metallic
copper
particle having a gelatin on the surface of the particle; a polymeric
dispersant; and an organic
solvent in which the gelatin has a difference between an amine value and an
acid value (amine
value - acid value) of 0 or less, and the polymeric dispersant has a
difference between an amine
value and an acid value (amine value - acid value) of 0 to 50.
Furthermore, Patent Literature 3 discloses that a nano size metallic particle
is
mixed with a micron size metallic particle while performing the treatment to
adsorb it on the
surface of the micron size metallic particle, thereby forming a fine particle
adsorbed mixed body
in which the nano size metallic particle is adsorbed on the surface of the
micron size metallic
particle.
Citation List
Patent Literature
[0005]
Patent Literature 1: WO 2006/019144 Al
CA 02959294 2017-02-24
W7336
3
Patent Literature 2: WO 2010/024385 Al
Patent Literature 3: JP 4848674 B
4
Summary of Invention
Technical Problem
[0006]
Patent Literatures 1 and 2 disclose the following: the metallic copper
particle is
obtained by reducing copper oxide with hydrazine in the presence of a gelatin
are excellent in the
dispersion stability and are heat-meltable at a relatively low temperature,
and thus the metallic
copper particles are fired under a reducing atmosphere and are suitably used
in various uses such
as uses to secure electrical conduction, antistatic uses, uses to shield
electromagnetic waves, and
uses to give metallic luster or antibacterial properties. However, in the
methods disclosed in
these Patent Literatures, there is the problem that the metallic copper
particle cannot be easily
produced for the reasons such as that a closed firing facility is required to
perform a firing under
a reducing atmosphere. Moreover, Patent Literature 3 discloses that a high
electrical
conductivity is exhibited through the heat treatment at a low temperature. In
this case,
however, it is considered to be difficult to sufficiently reduce the
resistance of an easily oxidized
metal like copper. Therefore, a metallic copper particle which can be fired
under a nonreducing
atmosphere such as nitrogen and which can provide an excellent sinterability
at a lower
temperature and a sufficiently low volume resistance value has been desired.
Solution Problem
[0007]
In order to solve the above problems, the present inventors have searched for
a
metallic copper particle having a volume resistance value of 1 x 10-2 II=cm or
less after heating
the metallic copper particle at a temperature of 300 C under a nitrogen
atmosphere. As a result,
the inventors have found, for example, that the above problems can be solved
by a metallic
copper particle in which at least one fine metallic copper particle is adhered
on the surface of a
large diameter metallic copper particle, and that when copper oxide and
hypophosphorous acid
and/or a salt thereof are mixed in a solvent in the presence of a gelatin
and/or a collagen peptide
to reduce the copper oxide, the desired metallic copper particle having a
volume resistance value
of 1 x 10-2 sy cm or less after the metallic copper particle is heated at a
temperature of 300 C
under a nitrogen atmosphere can be unexpectedly obtained, and thus have
completed the present
invention. In the present invention, the "metallic copper particle" is a
superordinate concept
CA 02959294 2017-02-24
W7336
4
including a large diameter metallic copper particle; at least one fine
metallic copper particle and
an aggregate thereof; and further includes the case where a small metallic
copper particle is
mixed therewith.
[0008]
Namely, one embodiment according to the present invention relates to (1) a
metallic copper particle including at least one fine metallic copper particle
and a large diameter
metallic copper particle wherein the at least one fine metallic copper
particle are adhered on the
surface of the large diameter metallic copper particle, and another embodiment
according to the
present invention relates to (2) a process for producing a metallic copper
particle having a
volume resistance value of 1 x 10-2 n- cm or less after heating the metallic
copper particle at a
temperature of 300 C under a nitrogen atmosphere, the process comprising
mixing copper oxide
and hypophosphorous acid and/or a salt thereof in a solvent in the presence of
a gelatin and/or a
collagen peptide, thereby reducing the copper oxide.
Specifically, the present invention is as follows.
(1) A metallic copper particle including at least one fine metallic copper
particle
and a large diameter metallic copper particle wherein the at least one fine
metallic copper
particle is adhered on a surface of the large diameter metallic copper
particle.
(2) The metallic copper particle according to (1), wherein an aggregate of the
fine
metallic copper particles is adhered on the surface of the large diameter
metallic copper particle.
(Hereinafter, the metallic copper particle defined in each of (1) and (2) is
sometimes referred to
as a "composite particle")
(3) The metallic copper particle according to (1) or (2), further including a
small
metallic copper particle in a mixed state. (Hereinafter, the metallic copper
particle defined in
(3) is sometimes referred to as a "mixed particle" in contrast with the -
composite particle".)
(4) The metallic copper particle according to (1) or (2), wherein a gelatin
and/or a
collagen peptide exist on at least one selected from the group consisting of
the metallic copper
particle, the large diameter metallic copper particle, and the at least one
fine metallic copper
particle.
(5) The metallic copper particle according to (3), wherein a gelatin and/or a
collagen peptide exist on at least one selected from the group consisting of
the metallic copper
particle, the large diameter metallic copper particle, the at least one fine
metallic copper particle,
and the small metallic copper particle.
(6) The metallic copper particle according to (1), (2), or (4), wherein at
least one
selected from the group consisting of the metallic copper particle, the large
diameter metallic
CA 02959294 2017-02-24
W7336
copper particle, and the at least one fine metallic copper particle includes
an organic acid and/or
a salt thereof.
(7) The metallic copper particle according to (3) or (5), wherein at least one
selected from the group consisting of the metallic copper particle, the large
diameter metallic
5 copper particle, the at least one fine metallic copper particle, and the
small metallic copper
particle includes an organic acid and/or a salt thereof.
(8) The metallic copper particle according to any one of (1) to (7), having a
specific surface area of 0.1 to 10 m2/g.
(9) A process for producing a metallic copper particle having a volume
resistance
value of 1 x 10-2 }cm or less after heating the metallic copper particle at a
temperature of
300 C under a nitrogen atmosphere, the process comprising mixing a copper
oxide and
hypophosphorous acid and/or a salt thereof in a solvent in the presence of a
gelatin and/or a
collagen peptide, thereby reducing the copper oxide.
(10) The process for producing a metallic copper particle according to (9),
wherein the gelatin and/or the collagen peptide exist in 1 to 500 parts by
mass with regard to 100
parts by mass of the metallic copper particle.
(11) The process for producing a metallic copper particle according to (9) or
(10),
wherein the reduction reaction is performed in a temperature range of 40 to 95
C.
(12) The process for producing a metallic copper particle according to any one
of
(9) to (11) comprising mixing the copper oxide and the hypophosphorous acid
and/or the salt
thereof in the solvent in the presence of the gelatin and/or the collagen
peptide, and an amine
complexing agent, thereby reducing the copper oxide.
(13) The process for producing a metallic copper particle according to any one
of
(9) to (12) comprising mixing the copper oxide and the hypophosphorous acid
and/or the salt
thereof in the solvent in the presence of the gelatin and/or the collagen
peptide, and an organic
acid, thereby reducing the copper oxide.
(14) The process for producing a metallic copper particle according to any one
of
(9) to (13) comprising mixing the copper oxide and the hypophosphorous acid
and/or the salt
thereof in the solvent in the presence of the gelatin and/or the collagen
peptide, an amine
complexing agent, and an organic acid, thereby reducing the copper oxide.
(15) The process for producing a metallic copper particle according to any one
of
(9) to (14), wherein the reduction reaction is performed at a pH of 3 or
lower.
(16) A metallic copper dispersion including the metallic copper particle
according
to any one of (1) to (8).
CA 02959294 2017-02-24
W7336
6
. .
t
Advantageous Effects of Invention
...
[0009]
The metallic copper particle according to the present invention can be fired
under
a nonreducing atmosphere such as nitrogen, is excellent in sinterability at
lower temperatures,
and exhibits a sufficiently low volume resistance value even in the case of a
low temperature
heating. Moreover, by using the process for producing a metallic copper
particle according to
the present invention, the metallic copper particle that is excellent in
sinterability at low
temperatures and that exhibits a sufficiently low volume resistance value even
when heated
under a nonreducing atmosphere can simply be produced. Therefore, the metallic
copper-
containing film that is excellent in electrical conductivity and metallic
color tone can be simply
produced by applying a dispersion including the metallic copper particles
according to the
present invention on the surface of a base material or by heating the
dispersion under a
nonreducing atmosphere after the application. Moreover, the dispersion can be
also used for
joining members. Furthermore, the metallic copper-containing film can be also
produced by
performing heating, light irradiation, plasma irradiation, or the like under a
reducing atmosphere
in place of or together with heating under a nonreducing atmosphere.
For these reasons, in the present invention, metallic copper particle(s) and
the
dispersion including them can be used in materials for securing electrical
conduction, materials
for antistatic, materials for shielding electromagnetic waves, materials for
giving metallic luster
or antibacterial properties, and the like, and can be used particularly in
uses for forming a fine
electrode and a fine circuit-wiring pattern, such as a print wiring board
making use of the
electrical conductivity of the metallic copper-containing film, in uses for
joining chips and
substrates, in design and decoration uses making use of metallic color tone of
the metallic
copper-containing film, and the like.
Brief Description of Drawings
[0010]
[Fig. 1] Fig. 1 shows an X-ray diffraction chart for sample A produced in
Example 1.
[Fig. 2] Fig. 2 shows an electron micrograph for sample A produced in Example
1.
[Fig. 3] Fig. 3 shows an electron micrograph (enlarged photograph) for sample
A produced in
Example 1.
[Fig. 4] Fig. 4 shows an electron micrograph for sample B produced in Example
2.
[Fig. 5] Fig. 5 shows an electron micrograph (enlarged photograph) for sample
B produced in
CA 02959294 2017-02-24
W7336
7
Example 2.
[Fig. 6] Fig. 6 shows an electron micrograph for sample C produced in Example
3.
[Fig. 7] Fig. 7 shows an electron micrograph (enlarged photograph) for sample
C produced in
Example 3.
[Fig. 8] Fig. 8 shows an electron micrograph for sample D produced in Example
4.
[Fig. 9] Fig. 9 shows an electron micrograph (enlarged photograph) for sample
D produced in
Example 4.
[Fig. 10] Fig. 10 shows an electron micrograph for sample E produced in
Example 5.
[Fig. 11] Fig. 11 shows an electron micrograph (enlarged photograph) for
sample E produced in
Example 5.
[Fig. 12] Fig. 12 shows an electron micrograph for sample F produced in
Example 6.
[Fig. 13] Fig. 13 shows an electron micrograph (enlarged photograph) for
sample F produced in
Example 6.
[Fig. 14] Fig. 14 shows an electron micrograph for sample G produced in
Example 7.
[Fig. 15] Fig. 15 shows an electron micrograph (enlarged photograph) for
sample G produced in
Example 7.
[Fig. 16] Fig. 16 shows an electron micrograph for sample H produced in
Example 8.
[Fig. 17] Fig. 17 shows an electron micrograph (enlarged photograph) for
sample H produced in
Example 8.
[Fig. 18] Fig. 18 shows an electron micrograph for sample I produced in
Example 9.
[Fig. 19] Fig. 19 shows an electron micrograph (enlarged photograph) for
sample I produced in
Example 9.
[Fig. 20] Fig. 20 shows an electron micrograph for sample J produced in
Example 10.
[Fig. 21] Fig. 21 shows an electron micrograph (enlarged photograph) for
sample J produced in
Example 10.
[Fig. 22] Fig. 22 shows an electron micrograph for sample K produced in
Example 11.
[Fig. 23] Fig. 23 shows an electron micrograph (enlarged photograph) for
sample K produced in
Example 11.
[Fig. 24] Fig. 24 shows an electron micrograph for sample L produced in
Example 12.
[Fig. 25] Fig. 25 shows an electron micrograph (enlarged photograph) for
sample L produced in
Example 12.
[Fig. 26] Fig. 26 shows an electron micrograph for sample M produced in
Example 13.
[Fig. 27] Fig. 27 shows an electron micrograph (enlarged photograph) for
sample M produced in
Example 13.
CA 02959294 2017-02-24
W7336
8
[Fig. 28] Fig. 28 shows an electron micrograph for sample Z produced in
Example 26.
[Fig. 29] Fig. 29 shows an electron micrograph (enlarged photograph) for
sample Z produced in
Example 26.
[Fig. 30] Fig. 30 shows an electron micrograph for sample AE produced in
Comparative
Example 1.
[Fig. 311 Fig. 31 shows an electron micrograph (enlarged photograph) for
sample AE produced
in Comparative Example 1.
[Fig. 32] Fig. 32 shows an electron micrograph for sample AF produced in
Comparative
Example 2.
[Fig. 33] Fig. 33 shows an electron micrograph (enlarged photograph) for
sample AF produced in
Comparative Example 2.
[Fig. 34] Fig. 34 shows an electron micrograph for sample AG produced in
Comparative
Example 3.
[Fig. 35] Fig. 35 shows an electron micrograph (enlarged photograph) for
sample AG produced
in Comparative Example 3.
[Fig. 36] Fig. 36 shows an electron micrograph of a cross section of a
metallic copper-containing
film produced by heating, at 120 C in the air, sample Q produced in Example
17.
[Fig. 37] Fig. 37 shows an electron micrograph (enlarged photograph) of a
cross section of a
metallic copper-containing film produced by heating, at 120 C in the air,
sample Q produced in
Example 17.
Description of Embodiments
[0011]
In the present invention, the "metallic copper particle" is a composite
particle in
which two kinds of particles each having a relatively different particle
diameter are composited.
Herein, the particle having a larger particle diameter is referred to as the
"large diameter metallic
copper particle", and the particle having a smaller particle diameter are
referred to as the "fine
metallic copper particle". The "particle diameter" does not necessarily mean
the average
primary particle diameter, and is appropriately defined in consideration of
the shape, distribution,
and the like. Specifically, the metallic copper particle according to the
present invention is a
metallic copper particle which includes a large diameter metallic copper
particle and at least one
fine metallic copper particle adhered on the surface of the large diameter
metallic copper
particle, and is a particle in which the at least one fine metallic copper
particle and the large
diameter metallic copper particle are composited and not merely mixed. It is
preferable that an
CA 02959294 2017-02-24
W7336
9
aggregate of the fine metallic copper particles is adhered on the surface of
the large diameter
metallic copper particle. The metallic copper particle according to the
present invention also
includes one in which a "small metallic copper particle" of a different type
from the above
composite particle is mixed in addition to the above composite particle.
[0012]
The "metallic copper" in the present invention is a substance having metallic
property, the substance including at least metal copper, metal copper alloy,
or copper hydride,
and the "metallic copper" may be metal copper or an alloy including metal
copper as the main
component, such as a copper-tin, copper-zinc, copper-bismuth, copper-nickel,
copper-lead, or
copper-phosphorus alloy. Copper hydride is classified as a copper compound,
but is converted
to metal copper by heating, and thus is defined herein as included in the
metallic copper.
Moreover, the metallic copper particle may be a particle of which the surface
is coated with a
metal such as silver or tin, a metallic copper alloy, or a metal oxide such as
silica or alumina, and
may also include an impurity, a copper compound, a copper alloy, a stabilizer
against oxidation,
or the like on the surface or inside of the metallic copper particle as long
as the inclusion of them
does not affect its uses. For example, phosphorus of a component of a reducing
agent is liable
to remain in the metallic copper particle. The content of phosphorus can be
adjusted by the
amount of phosphorus to be used during the reduction reaction, washing after
the reduction
reaction, or the like, and is preferably about 0 to about 5 parts by mass with
regard to 100 parts
by mass of the metallic copper particle, more preferably 0 to 2 parts by mass,
still more
preferably 0 to 1 part by mass. The gelatin and/or the collagen peptide which
act as a protective
colloid also remain on the surface or the like of the metallic copper
particle, but the content
thereof can be adjusted by the amount of the gelatin and/or the collagen
peptide to be used or by
removing the gelatin and/or the collagen peptide after the reduction reaction.
Moreover, in the
case where a complexing agent is used, the complexing agent is included in the
metallic copper
particle according to the amount of the complexing agent to be used.
[0013]
In the present invention, the "large diameter metallic copper particle" refers
to a
coarser particle compared with the fine metallic copper particle described
below. The shape of
the large diameter metallic copper particle is not particularly limited, and
the large diameter
metallic copper particle having an arbitrary shape can be used. For example, a
particle having a
shape constituted by a curved surface such as a spherical shape or an
elliptical shape, a particle
having a shape constituted by a polyhedron such as a flat shape, a granular
shape, a rectangular
parallelepiped shape, a cubic shape, a rod shape, a needle-shaped particle, or
a wire shape, a
CA 02959294 2017-02-24
W7336
particle having such a shape as flat plates are combined, and a particle
having an irregular shape
which cannot be specified in shape can be used, and these particles may be
mixed. With respect
to the particle having such a shape as flat plates are combined, it can be
confirmed that such a
particle partially exists in the electron micrographs shown in, for example,
Figs. 2, 4, and 6. In
5 the present invention, the shape of the metallic copper particle, the
large diameter metallic
copper particle, the fine metallic copper particles and an aggregate thereof
can be observed with
a scanning electron microscope (which is sometimes written as "SEM"
hereinafter). The
particle having a flat shape refers to a particle of which the thickness is
thinner with regard to the
flat surfaces of the particle. It is preferable that in view of the volume
resistivity after heating,
10 the large diameter metallic copper particle is a particle having such a
shape as flat plates are
combined, a particle having a flat shape, or a particle having a granular
shape, or the like.
[0014]
The particle diameter of the large diameter metallic copper particle can be
appropriately selected according to its uses (e.g. film thickness, film width,
and the like). In the
case where the particle diameter can be specified by the average primary
particle diameter, it is
preferable that the average primary particle diameter is generally 0.1 to 100
gm, and the range of
1.0 to 30 gm is more preferable. The average primary particle diameter is
preferably at least 5
times as large as that of the fine metallic copper particles described below.
The average
primary particle diameter is determined by measuring the particle diameters of
100 or more
particles that are randomly selected from an SEM image and calculating the
number average of
the measured particle diameters. In the case of a highly anisotropic particle,
the maximum
diameter of the particle is defined as a particle diameter of the particle.
For example, in the
case of particles having a flat shape, the average width of the flat surfaces
of the particles
(specifically, average value of maximum diameters of the flat surfaces of the
particles) is defined
as the average primary particle diameter, and the average primary particle
diameter thereof is
preferably in a range of 0.1 to 100 gm, more preferably in a range of 0.5 to
50 gm, and still more
preferably in a range of 1.0 to 30 gm. The average thickness of the particles
having a flat shape
can be appropriately set, and the average thickness thereof is preferably
0.005 to 10 gm, more
preferably 0.01 to 10 vim, and still more preferably 0.05 to 5 gm. In the case
of particles having
a granular shape or the like, the average primary particle diameter of the
particles (specifically,
average value of maximum diameters of the particles) is preferably in a range
of 0.1 to 100 gm,
more preferably in a range of 0.5 to 50 pm, and still more preferably in a
range of 1.0 to 30 um.
[0015]
The "fine metallic copper particle" in the present invention refer to a finer
particle
CA 02959294 2017-02-24
W7336
11
than the large diameter metallic copper particle ('namely, a particle having
smaller particle
diameter than the large diameter metallic copper particle), and the component
composition
thereof may be the above metallic copper having the same quality as the large
diameter metallic
copper particle, or may be the above metallic copper having the different
quality from the large
diameter metallic copper particle. The shape of the fine metallic copper
particle is not
particularly limited, and the fine metallic copper particle having an
arbitrary shape can be used.
In the case where the particle diameter is specified by the average primary
particle diameter, the
average primary particle diameter of the fine metallic copper particles is
preferably in a range of
2 to 500 nm, more preferably 5 to 300 nm, and still more preferably in a range
of 10 to 250 nm.
The average primary particle diameter of the fine metallic copper particles is
also determined by
measuring each maximum particle diameter of the 100 or more fine metallic
copper particles that
are randomly selected from an SEM image and calculating the number average of
the measured
maximum particle diameters. In the case where the particle shape in the
aggregated interface
between the adjacent particles may be unclear because at least part of the
fine metallic copper
particles in the present invention form an aggregate as described below. In
this case, however,
the particle shape may be estimated from its outline.
In the case where the particle diameter can be specified by the average
primary
particle diameter, when the average primary particle diameter of the fine
metallic copper
particles is, for example, 400 nm within the preferable range of 2 to 500 nm
described above, the
range of 0.1 to 100 p.m which is described above as a generally preferable
range of the average
primary particle diameter of the large diameter metallic copper particle means
a range larger than
0.4 gm to 100 um or smaller (a range of 2 pm to 100 pm when it is further
required considering
that the average primary particle diameter of the large diameter metallic
copper particles is
preferably at least 5 times as large as that of the fine metallic copper
particles) within the range.
When the average primary particle diameter of the fine metallic copper
particles is, for example,
10 nm, it is meant that the range of 0.1 to 100 gm which is described above as
a generally
preferable range of the average primary particle diameter of the large
diameter metallic copper
particles is preferable. The average primary particle diameter of the fine
metallic copper
particles is preferably 1/5 or smaller of the average particle diameter of the
large diameter
metallic copper particles, more preferably 1/7 or smaller, and still more
preferably 1/10 or
smaller.
[0016]
Regarding the fine metallic copper particle, it is preferable that an
aggregate is
formed by the aggregation of a plurality of the fine metallic copper
particles. The
CA 02959294 2017-02-24
W7336
12
"aggregation" herein is distinguished from "agglomeration" referring to a
state where adjacent
particles are in contact with each other at a point(s), and refers to a state
where adjacent particles
are bound each other through necking or fusion or a state where adjacent
particles share a face(s)
each other. It can be confirmed through the observation of a SEM image whether
the aggregate
is formed by the aggregation of a plurality of the fine metallic copper
particles. In the case
where the particles share a face(s) each other even when an interface is
observed between the
particles, the particles are considered as forming an aggregate. A plurality
of the fine metallic
copper particles may aggregate to form a particle having an irregular shape.
The aggregate is
formed by the aggregation of the two or more fine metallic copper particles,
preferably the three
or more fine metallic copper particles, and more preferably the four or more
fine metallic copper
particles.
[0017]
The metallic copper particle of the present invention is a composite particle
in
which at least one fine metallic copper particle is adhered on the surface of
the large diameter
metallic copper particle, and preferably, an aggregate formed by the
aggregation of the fine
metallic copper particles are adhered on the surface of the large diameter
metallic copper
particle. The adhesion may be performed by aggregation, adsorption, or
combination thereof.
It can be confirmed through the observation of a SEM image whether at least
one fine metallic
copper particle and/or the aggregate of the fine metallic copper particles is
adhered on the
surface of the large diameter metallic copper particle. When the large
diameter metallic copper
particle and at least one fine metallic copper particle are merely mixed, the
adhesion state of
them cannot be obtained, and the state where a plurality of the fine metallic
copper particles
aggregate cannot be also obtained. Additionally, in this case, the large
diameter metallic copper
particle and the fine metallic copper particle(s) exist individually. Thus,
the large diameter
metallic copper particle and the fine metallic copper particle(s) can be
clearly distinguished from
the metallic copper particle according to the present invention. Also, all of
the fine metallic
copper particles do not necessarily form the aggregate, and some of the fine
metallic copper
particles may be adhered as an agglomerate or single particle on the surface
of the large diameter
metallic copper particle. It is preferable that the aggregation between the
large diameter
metallic copper particles less frequently occurs. As described below, the
particle which
constitutes the above metallic copper particle, namely, the particle having a
flat shape; the
particle having a granular shape or the like; the particle having such a shape
as flat plates are
combined; or the particle having an irregular shape can be produced by
adjusting the amount of
the gelatin and/or the collagen peptide, or the complexing agent to be used at
the time of the
CA 02959294 2017-02-24
W7336
13
reduction reaction and the condition of the reduction reaction, and further
the metallic copper
particles in the states where these particles are mixed can be also produced
thereby.
[0018]
It is preferable that one embodiment of the metallic copper particle according
to
the present invention is a mixed particle including the above metallic copper
particle (i.e.
composite particle) in a mixed state with a small metallic copper particle of
a different type from
it.
The "the small metallic copper particle" refer to, in the state of being mixed
with the above
metallic copper particle (i.e. the composite particle in which the at least
one fine metallic copper
particle and/or an aggregate thereof are adhered on the surface of the large
diameter metallic
copper particle), a particle(s) other than the above composite particle. In
this case, the
component composition thereof may be the above metallic copper having the same
quality as the
composite particle or may be the above metallic copper having the different
quality from it. It
is preferable that the particle diameter of the small metallic copper particle
is smaller than that of
the above large diameter metallic copper particle (in other words, the
particle diameter of the
above large diameter metallic copper particle is larger than that of the small
metallic copper
particle). In the case where the particle diameter can be specified by the
average primary
particle diameter, the average primary particle diameter of the small metallic
copper particles is,
for example, preferably in a range of 2 to 1000 nm, more preferably in a range
of 5 to 500 nm,
and still more preferably 10 to 400 nm. The average primary particle diameter
of the small
metallic copper particles is also determined by measuring the maximum particle
diameter of each
of 100 or more particles that are randomly selected from an SEM image and
calculating the
number average of the measured maximum particle diameters. The shape of the
small metallic
copper particle is not particularly limited, and the small metallic copper
particle having an
arbitrary shape can be used.
The state where the composite particle and the small metallic copper particle
are
mixed is obtained by simultaneously producing the composite particle and the
small metallic
copper particle as well as by separately adding the small metallic copper
particle to the produced
composite particle. By employing such a state, sinterability thereof at a
further lower
temperature becomes excellent, and a much lower volume resistance value is
provided even in
the case of a low temperature heating, compared with the case where the
metallic copper particle
(i.e. composite particle) is singularly used. The reason is not necessarily
clear, but it is
considered that a large number of the small metallic copper particles exist in
spaces between the
metallic copper particles (i.e. composite particles) during the film
formation, thereby enhancing
the conduction among the metallic copper particles. It is favorable that the
small metallic
CA 02959294 2017-02-24
W7336
14
copper particle exists independently from the metallic copper particle,
namely, a state where the
small metallic copper particle is not adhered on the surface of the large
diameter metallic copper
particle but exist individually from the metallic copper particle is
preferable. The state of the
small metallic copper particle is not particularly limited, and the small
metallic copper particle
may exist in the state of a single particle, may exist in the state of an
agglomerate formed by
gathering a plurality of the small metallic copper particles, may exist in the
state of an aggregate
of the small metallic copper particles as in the case of the fine metallic
copper particles, or may
exist in the state of the mixture of them. The mixing ratio of the metallic
copper particle (i.e.
composite particle) and the small metallic copper particle can be
appropriately set, but the
amount of the small metallic copper particle is preferably in a range of 1 to
50% by mass with
regard to the metallic copper particle (i.e. composite particle), more
preferably in a range of 2 to
30% by mass, and still more preferably in a range of 3 to 20% by mass.
[0019]
As an index of the volume resistance value of the metallic copper particle
according to the present invention, the volume resistance value of a metallic
copper-containing
film prepared by heating and firing the metallic copper particles at a
temperature of 300 C under
a nitrogen atmosphere is used. Specifically, the volume resistance value
measured according to
the "< Method 1 for Measuring Volume Resistance Value >" described below is 1
x 10-2 C2-cm or
less, preferably 1 x 10-3 CY cm or less, and more preferably 1 x 10-4 CY cm or
less. In this way,
when using the metallic copper particle according to the present invention,
its sintering occurs
even when being heated at a temperature of 300 C under a nitrogen atmosphere,
and thus has a
low volume resistance value and a high electrical conductivity.
< Method 1 for Measuring Volume Resistance Value >
A copper paste is prepared by: mixing 10 g of a metallic copper powder, 3.5 g
of a
vehicle (resin: 20% by mass of ethyl cellulose N200 and solvent: terpineol),
and 6.5 g of
terpineol; and then kneading the mixture with a three-roll mill. The prepared
copper paste is
applied to an alumina substrate and fired, using an atmosphere tube furnace,
at 300 C for one
hour under a nitrogen atmosphere to prepare a metallic copper-containing film.
The specific
resistance value of the prepared metallic copper-containing film is measured
using MCP-T610
Loresta GP manufactured by Mitsubishi Chemical Analytech Co., Ltd. by a direct
current four-
terminal method. Thereafter, the cross section is observed with a scanning
electron microscope
to measure the film thickness, and the volume resistance value is calculated
with regard to the
specific resistance value.
[0020]
CA 02959294 2017-02-24
W7336
The metallic copper particle according to the present invention has a low
volume
resistance value after heating it at a temperature of 300 C under a nitrogen
atmosphere.
=
Therefore, a copper-containing film or joined body having a low volume
resistance value can be
produced even at a temperature of 300 C or lower under a nonreducing
atmosphere (namely,
5 under an inert atmosphere) such as nitrogen or argon, and a copper-
containing film or joined
body having a low volume resistance value can be also produced even at a
temperature of 300 C
or lower under a reducing atmosphere such as hydrogen. It is preferable that
the heating
temperature of the metallic copper particle according to the present invention
is a lower
temperature in the case where plastic is used as a base material. For example,
a temperature of
10 200 C or lower is more preferable, and a temperature of 150 C or lower
is still more preferable.
Furthermore, a copper-containing film having a low volume resistance value can
be also
produced by performing light irradiation, plasma irradiation, or the like in
place of or together
with the heating under the above non-reducing atmosphere (i.e. under an inert
atmosphere) or
under the above reducing atmosphere.
15 [0021]
In this way, the metallic copper particle according to the present invention
can be
fired under a nonreducing atmosphere such as nitrogen, is excellent in
sinterability at a lower
temperature, and exhibits a sufficiently low volume resistance value even in
the case of a low
temperature heating. The reason is not necessarily clear, but it is considered
that the sinterablity
at a low temperature and the reduction in the volume resistivity are provided,
for example, by the
following: the melting point of the fine metallic copper particle is
dominantly low; the increase
in the melting point due to aggregation when the fine metallic copper
particles form an aggregate
thereof is unexpectedly small; it is presumed that the contact area with the
air outside becomes
small to suppress the oxidation of the fine metallic copper particles by
forming the aggregate;
and further, the gaps between the large diameter metallic copper particles
having a volume
resistance value comparable to that of the bulk are efficiently connected by
the fine metallic
copper particle(s) (and/or an aggregate thereof) or the small metallic copper
particle mixed
therewith during heating.
[0022]
The metallic copper particle according to the present invention is, as
described
above, the following: a composite particle in which at least one fine metallic
copper particle
and/or an aggregate thereof are adhered on the surface of a large diameter
metallic copper
particle; or a mixed particle including the composite particle in which the at
least one fine
metallic copper particle and/or the aggregate thereof are adhered on the
surface of the large
CA 02959294 2017-02-24
W7336
16
cliameter metallic popper particle, in a mixed state with a small metallic
copper particle.
In the metallic copper particle according to the present invention, it is
preferable
that at least one selected from the group consisting of the metallic copper
particle, the large
diameter metallic copper particle, the at least one fine metallic copper
particle, and the small
metallic copper particle has a gelatin and/or a collagen peptide, and it is
more preferable that the
surface of the metallic copper particle and/or the surface of the at least one
fine metallic copper
particle have a gelatin and/or a collagen peptide. In addition, the meaning of
"the at least one
fine metallic copper particle has a gelatin and/or a collagen peptide" is not
only that non-
aggregated fine metallic copper particles have the gelatin or the like but
also that at least one fine
metallic copper particle constituting an aggregate has the gelatin or the
like.
Namely, in the metallic copper particle according to the present invention, it
is
preferable that the gelatin and/or the collagen peptide exist on at least one
selected from the
group consisting of the metallic copper particle (i.e. the composite particle
in which the at least
one fine metallic copper particle and/or an aggregate thereof are adhered on
the surface of the
large diameter metallic copper particle), the large diameter metallic copper
particle and the at
least one fine metallic copper particle which constitute the above composite
particle. In the
case of the mixed particle in which the small metallic copper particle is
mixed with the above
composite particle, it is preferable that the gelatin and/or the collagen
peptide exist on at least
one selected from the group consisting of the metallic copper particle (i.e.
composite particle),
the large diameter metallic copper particle, the at least one fine metallic
copper particle, and the
small metallic copper particle. Among others, it is more preferable that the
gelatin and/or the
collagen peptide exist on the surface of the composite particle and/or the
surface of the at least
one fine metallic copper particle constituting the composite particle. As a
result, the oxidation
of the metallic copper particle in the presence of oxygen can be suppressed,
and thus the volume
resistivity after heating can be further reduced. Moreover, the gelatin and
the collagen peptide
serve as protective colloid, and can suppress the agglomeration of the
metallic copper particles in
an aqueous solvent. It is preferable that the gelatin and/or the collagen
peptide exist in a range
of about 0.1 to about 15 parts by mass with regard to 100 parts by mass of the
metallic copper
particle and the like (namely, at least one particle selected from the above
group wherein the at
least one particle has the gelatin and/or the collagen peptide) because
desired effects are
obtained, and the more preferable range is about 0.1 to about 10 parts by
mass. The details
about the gelatin and/or the collagen peptide that can be used will be
described in the paragraphs
related to the production process. The content of the gelatin is determined by
performing CHN
analysis of the metallic copper particle based on the assumption that the
total amounts of C, H,
CA 02959294 2017-02-24
W7336
17
and N in % by mass satisfying the ratio of C, H, and N in the used gelatin
originates in the
gelatin.
[0023]
In the metallic copper particle according to the present invention, it is
preferable
that at least one selected from the group consisting of the metallic copper
particle, the large
diameter metallic copper particle, the at least one fine metallic copper
particle, and the small
metallic copper particle includes an organic acid and/or a salt thereof. In
addition, the meaning
of "the at least one fine metallic copper particle includes an organic acid
and/or a salt thereof" is
not only that non-aggregated fine metallic copper particles include an organic
acid or the like but
also that the at least one fine metallic copper particle constituting an
aggregate includes an
organic acid or the like.
Namely, in the metallic copper particle according to the present invention, it
is
preferable that the organic acid and/or a salt thereof exist on at least one
selected from the group
consisting of the metallic copper particle (i.e. the composite particle in
which the at least one fine
metallic copper particle and/or an aggregate thereof are adhered on the
surface of the large
diameter metallic copper particle), the large diameter metallic copper
particle and the at least one
fine metallic copper particle which constitute the above composite particle.
In the case of the
mixed particle in which the small metallic copper particle is mixed with the
above composite
particle, it is preferable that the organic acid and/or a salt thereof exist
on at least one selected
from the group consisting of the metallic copper particle (i.e. composite
particle), the large
diameter metallic copper particle, the at least one fine metallic copper
particle, and the small
metallic copper particle. The organic acid and/or a salt thereof may exist in
a mixed state with
the metallic copper particle, or may be adsorbed on the surface of the
metallic copper particle.
In particular, it is preferable that the organic acid and/or a salt thereof
are adsorbed on the surface
of the metallic copper particle. It is considered that the organic acid and/or
a salt thereof
facilitate the sintering between the metallic copper particles at a low
temperature during heating,
and the volume resistivity after heating the metallic copper particle at a low
temperature can be
much more reduced. Specifically, the volume resistance value of a metallic
copper-containing
film prepared by heating and firing the metallic copper particles at a
temperature of 120 C under
an air atmosphere is used as an index, and a volume resistance value of 1 x
101 cm or less can
be achieved in terms of the volume resistance value measured according to the
"< Method 2 for
Measuring Volume Resistance Value >" described below, and further a metallic
copper-
containing film having a volume resistance value in the order of 1 x 10-3 n=cm
can be also
obtained. Examples of the organic acid and/or a salt thereof include
carboxylic acids, amino
CA 02959294 2017-02-24
W7336
18
acids, aminocarboxylic acids and salts thereof. Among them, carboxylic acids
are preferable,
and formic acid is more preferable. In the case where the metallic copper
particle and the like
include the organic acid and/or a salt thereof, the content thereof can be
appropriately set, but is
preferably set to 0.01 to 1% by mass in the metallic copper particle and the
like.
[0024]
The specific surface area of the metallic copper particle according to the
present
invention, which is measured by a nitrogen adsorption BET method is preferably
about 0.1 to
about 10 m2/g, more preferably about 0.2 to about 8 m2/g, still more
preferably about 0.3 to
about 7 m2/g, and much more preferably about 1 to about 6 m2/g. It is
considered that the
specific surface area of the metallic copper particle reflects an abundance
ratio of the large
diameter metallic copper particle and the at least one fine metallic copper
particle (In this regard,
in the case where the small metallic copper particle is mixed therewith, the
above abundance
ratio further includes them.). In the case where the BET specific surface area
is within the
above range, the metallic copper particle is excellent in sinterability at a
further lower
temperature and exhibits a much lower volume resistance value even in the case
of a low
temperature heating.
[0025]
Among others, the metallic copper particle having a specific surface area in a
range of 1 to 5 m2/g provides a metallic copper-containing film exhibiting
electrical conductivity
when being heated at a temperature of 120 C under an air atmosphere.
Specifically, the volume
resistance value is used as an index, and a volume resistance value in the
order of 1 x 10+1Q=cm
can be achieved in terms of the volume resistance value measured according to
the "< Method 2
for Measuring Volume Resistance Value >" described below, and further a
metallic copper-
containing film that exhibits a volume resistance value of 1 x 10-10:cm or
less is obtained. In
this way, the metallic copper particle according to the present invention has
a low volume
resistance value and a high electrical conductivity because the sinterability
or the contact
property between the particles are improved even when heating the metallic
copper particle at a
temperature of 120 C under an air atmosphere. Therefore, the metallic copper
particle
according to the present invention can be used with a base material having a
low heat resistance
temperature, and can be employed in a wide range of uses. Moreover, the firing
in the air can
be performed, and thus constraints on facilities such as control of an
atmosphere can be avoided.
< Method 2 for Measuring Volume Resistance Value >
A copper paste is prepared by: mixing 5 g of a metallic copper powder, a
phenol
resin (0.62 g of Resitop: PL-5208 (containing 59% by weight of phenol resin as
active
CA 02959294 2017-02-24
W7336
19
ingredient)), and 0..26 g of ethylene glycol monobutyl ether acetate using a
deaerating stirrer; and
then kneading the mixture with a three-roll mill. The prepared copper paste is
applied to an
alumina substrate and fired at 120 C for 10 minutes in a natural convection
type drier to prepare
a metallic copper-containing film. The specific resistance value of the
obtained metallic
copper-containing film is measured using MCP-T610 Loresta GP manufactured by
Mitsubishi
Chemical Analytech Co., Ltd. by a direct current four-terminal method.
Thereafter, the cross
section is observed with a scanning electron microscope to measure the film
thickness, and the
volume resistance value is calculated based on the above specific resistance
value.
[0026]
The metallic copper particle according to the present invention, when being
blended with a solvent, a resin, and the like to prepare a dispersion,
exhibits a high fluidity even
in the case where the concentration is high. Dispersion including metallic
particles in a nano
order is generally liable to lose fluidity when the concentration thereof
becomes high.
Micronization of a metallic copper particle, which is a general method for
improving the
sinterability at a low temperature, is in a trade-off relation with fluidity
of the dispersion, and
thus it is difficult to make the concentration of the dispersion high. To the
contrary, when using
the metallic copper particle according to the present invention, the
dispersion which has such an
excellent sinterability at a low temperature that the sintering can be
performed even by heating at
120 C in the air and which maintains a sufficient fluidity even when the
concentration of the
metallic copper particle is made to be 50% by mass or more can be prepared.
The reason is not
clear, but it is considered that the capture of a solvent and the like can be
reduced because the at
least one fine metallic copper particle is adhered on the large diameter
metallic copper particle or
because the fine metallic copper particles in a state of aggregation thereof
are adhered on the
large diameter metallic copper particle. Due to this characteristic, the
metallic copper
dispersion according to the present invention can be suitably used in joining
materials and the
like for which a high concentration dispersion is required.
[0027]
The metallic copper particle according to the present invention can be applied
to
various kinds of dispersions (coating materials, paints, metallic pastes,
inks, and the like), and is
suitable for the application in metallic pastes among others. The metallic
paste herein is a paste
including a metallic copper particle, a binder resin, a solvent, and the like
as main components,
in which a surfactant, a crosslinking agent, a polymeric dispersant, or the
like are appropriately
blended, thereby providing suitable fluidity and viscosity. The metallic paste
can be used for
various kinds of printing, and can be suitably used for printing by a
platemaking, particularly.
CA 02959294 2017-02-24
W7336
xamples .9f the printing by a platemaking include screen printing, offset
printing, and gravure
printing, and the screen printing is preferable in view of thick-film
formation. The screen
printing is a method of placing a paste on a screen in which holes
corresponding to a wiring or
electrode pattern are formed, followed by rubbing off the paste with a
squeegee to print the
5 wiring or electrode pattern on a substrate. By the screen printing,
printing to form a thick film
having a thickness of several gm to several tens I.tm can be easily performed,
and thus the screen
printing is often utilized in a production process of printed wiring boards,
electronic parts, or flat
panel displays. It is desirable that the metallic paste has a certain degree
of viscosity in view of
thick-film formation, and a metallic paste having a viscosity of 2000 rnPa-s
or higher is generally
10 used.
[0028]
The metallic copper particle according to the present invention has a
characteristic
that the thixotropy index value is relatively high when a metallic paste is
prepared by blending
the metallic copper particle with a solvent, a resin, and the like. The
thixotropy index (which is
15 referred to as TI value, hereinafter) herein is a value calculated from
a viscosity ratio of the
viscosity (na) of the metallic paste when the metallic paste is stirred at a
predetermined low shear
rate to the viscosity (rib) of the metallic paste when the metallic paste is
stirred at a
predetermined high shear rate, and is specifically calculated from the
following expression.
TI = na/nib
20 The measurement of the viscosity na and the viscosity 1b that are
needed for
calculation of the TI value is performed under the following condition.
<Method for Preparing Metallic Paste>
A metallic paste (Cu solid content of 75% by mass) is prepared by: mixing 9 g
of
a metallic copper powder, 1 g of a vehicle (resin: 20% by mass of ethyl
cellulose N200, and
solvent: terpineol), and 2 g of terpineol; and kneading the mixture with a
three-roll mill.
<Method for Measuring Viscosity of Metallic Paste>
The viscosity of the metallic paste is measured using a B type viscometer
(model
I-1B DV-I+) manufactured by Brookfield AMETEK. The measurement temperature is
set at
20 C, and CPE-52 is used as a corn spindle. The viscosity (p) at a shear rate
of 10 [1/sec] and
the viscosity (rib) at a shear rate of 100 [1/sec] are measured, and the TI
value is calculated by
applying the measured na and rib to the above expression.
[0029]
The fact that the TI value is high means that although the viscosity of a
paste is
suitably maintained in a normal state, when a high shear force is applied to
the paste, the
CA 02959294 2017-02-24
W7336
21
yiscosity thereof i easily lowered. In a metallic paste using the metallic
copper particle
according to the present invention, the TI value can be set to be dominantly
high value, and
specifically, the TI value can be set to be 3.0 or more, preferably 3.5 or
more, and more
preferably 4.0 or more. Therefore, for example, in the screen printing, the
fluidity of the
metallic paste during continuous printing becomes favorable, and a thick film
can be obtained
after completion of patterning on a substrate. Moreover, cracks,
disconnection, short-circuits,
bleeding, and the like are suppressed, and thus the thick film can be
reproducibly obtained during
continuous printing. Furthermore, in printing such as inkjet printing in which
a high shear force
is applied to the metallic paste, ejection of the metallic paste from holes
can be made smooth,
and fixing of the metallic paste to a printing medium can be made favorable.
The reason that the TI value of the metallic copper dispersion (i.e. metallic
paste)
as an embodiment according to the present invention is high is not necessarily
clear, but it is
considered that the at least one fine metallic copper particle (and/or an
aggregate of the fine
metallic copper particles) adhered on the large diameter metallic copper
particle or (in the case
where the small metallic copper particle is mixed therewith,) the small
metallic copper particle
serves such a function as a lubricant so as to contribute to the improvement
of the TI value.
[0030]
Next, the present invention relates to a method for producing the metallic
copper
particle, and in the method, a copper compound and hypophosphorous acid and/or
a salt thereof
are mixed in a solvent in the presence of a gelatin and/or a collagen peptide
to reduce the copper
compound, thereby producing the metallic copper particle. In the present
invention, it is
preferable to use a gelatin and/or a collagen peptide, copper oxide, and
hypophosphorous acid
and/or a salt thereof. By using these three compounds, the metallic copper
particle having a
volume resistance value of 1 x 10-2 f2-cm or less after the metallic copper
particle is heated at a
temperature of 300 C under a nitrogen atmosphere can be easily produced.
Particularly,
according to this method, the large diameter metallic copper particle and the
at least one fine
metallic copper particle each of which has a different average particle
diameter can be produced
by a single reduction operation, and thus there is no need to perform a
complicated treatment that
powders each of which has a different average particle diameter are mixed.
Moreover, a
metallic copper particle including a large diameter metallic copper particle
and at least one fine
metallic copper particle wherein the at least one fine metallic copper
particle is adhered on the
surface of the large diameter metallic copper particle can be also prepared.
Further, the metallic
copper particle in which an aggregate of a plurality of the fine metallic
copper particles is
adhered on the surface of the large diameter metallic copper particle can be
also prepared.
CA 02959294 2017-02-24
W7336
22
Furthermore, whep a mixed particle in which the small metallic copper particle
is mixed with the
composite particle adhering the at least one fine metallic copper particle
and/or an aggregate
thereof on the surface of the large diameter metallic copper particle, the
metallic copper particle
(i.e. composite particle) and the small metallic copper particle each of which
has a different
shape and particle diameter can be easily produced by a single reduction
operation.
[0031]
The gelatin includes the following: a gelatin in a state as extracted; a
gelatin
obtained by hydrolyzing the above gelatin in a state as extracted so as to
become lower the
molecular weight (, which is sometimes referred to as a "collagen peptide",
hereinafter); and a
gelatin obtained by chemically modifying these gelatins ('which is sometimes
referred to as a
"modified gelatin", hereinafter). In general, a gelatin is an animal protein
obtained from a
collagen as a parental material. In the production process of a gelatin, a
pretreatment of raw
materials is performed with an inorganic acid such as hydrochloric acid or
sulfuric acid, or lime
in order to efficiently extract a high-quality gelatin from raw materials such
as cattle bones, cattle
hides, and pig hides. The gelatin obtained through the pretreatment with the
inorganic acid is
called an "acid-treated gelatin" and the gelatin obtained from the
pretreatment with the lime is
called an "alkali-treated gelatin" (or "lime-treated gelatin"). During the
process of extracting a
gelatin, an acid amide in a collagen is hydrolyzed, and releases ammonia to
change into a
carboxyl group, and thus the isoionic point of the gelatin is lowered. Because
particularly the
alkali-treated gelatin is deamidized to nearly 100% in a liming process, the
isoionic point is in an
acidic region, and the pH thereof is nearly 5. On the other hand, the acid-
treated gelatin has a
low deamidization ratio because of a short raw material treatment period, and
thus has an
isoionic point in an alkaline region, and the pH thereof is about 8 to about 9
near the isoionic
point of a collagen. For these reasons, a gelatin has an amine value because
of having a basic
group and a hydroxy group, and further has an acid value because of having an
acidic group. In
the present invention, it is preferable that the gelatin exists on the surface
of the metallic copper.
More preferably, the gelatin is the alkali-treated gelatin. Also, the gelatin
having a difference
between the amine value and the acid value measured according to the method
described below,
namely "(amine value - acid value)", of 0 or less is preferable. More
preferably, the difference
between the amine value and the acid value is in a range of -50 to 0. Compared
with the acid-
treated gelatin, the alkali-treated gelatin exhibits excellent effects as a
protective colloid of the
metallic copper particle, and thus is preferable.
[0032]
Moreover, the collagen peptide (i.e. hydrolyzed gelatin) is, directly or
through a
CA 02959294 2017-02-24
W7336
23
gelatin, obtained by hydrolyzing a collagen (specifically, collagen protein)
included in animal
bones and hides by means of an enzyme, acid, alkali, or the like. As a
hydrolysis method for
=
obtaining the collagen peptide (i.e. hydrolyzed gelatin), conventionally known
methods can be
used. For example, the hydrolysis can be performed according to a method of
using an enzyme,
a method of using chemical treatment with an acid or alkali, or the like. As
the enzyme, any
enzyme may be used as long as the enzyme has a function of cleaving a peptide
bond of a
gelatin. The enzyme is usually called a proteolytic enzyme or protease.
Specific examples of
the enzyme include a collagenase, a thiol protease, a serine protease, an
acidic protease, an
alkaline protease, a metal protease, or the like, and one of them may be
singularly used, or two or
more thereof may be used in combination. Examples of the thiol protease
include plant-derived
thiol proteases such as a chymopapain, papain, a promelain, and a ficin and
animal-derived thiol
proteases such as a cathepsin, and calcium-dependent proteases. Examples of
the serine
proteases include trypsin, cathepsin D, or the like. Examples of the acid
protease include
hepsin, chymosin, or the like. When the enzyme is used, it is preferable to
use 0.01 to 5 parts
by mass of the enzyme with regard to 100 parts by mass of the gelatin before
the hydrolysis
treatment, and it is preferable that the temperature condition of the
hydrolysis is 30 to 70 C and
the treatment time is 0.5 to 24 hours. When the hydrolysis is performed using
the enzyme,
deactivation of the enzyme is performed after the treatment. The deactivation
of enzyme is
performed by heating, and the heating temperature is, for example, 70 to 100
C.
[0033]
When the acid or alkali is used, it is preferable to set the pH of the gelatin
solution
to be 3 or less, or 10 or more, and it is preferable that the temperature
condition of the hydrolysis
is 50 to 90 C and the treatment time is 1 to 8 hours. Examples of the acid
include hydrochloric
acid, sulfuric acid, and nitric acid. Examples of the alkali include sodium
hydroxide and
calcium hydroxide. When the hydrolysis is performed with the acid or alkali,
desalting is
performed by neutralization with a neutralizing agent or by an ion exchange
resin. At the time
when the hydrolysis treatment is completed, the hydrolyzed gelatin is
dissolved or dispersed in
the hydrolysis treatment liquid. Various purification treatments which are
usually used can be
applied to this solution. The purification treatment is not particularly
limited. For example,
activated carbon can be added to improve tone of color or textures, or remove
impurities, or
conventionally known solid-liquid separation treatment such as filtration or
centrifugal
separation can be applied to remove impurities.
[0034]
The modified gelatin may be obtained by chemically modifying gelatin, namely,
CA 02959294 2017-02-24
VV7336
24
by chemicajly modifying a side chain of each amino acid residue, a terminal
amino group, a
terminal carboxyl group, or the like, included in a gelatin. For example, by
chemically
,
modifying the side chain of amino acid residues included in the gelatin to
introduce the
following: a functional group including a nitrogen element, such as an amino
group, an imino
group, a cyano group, an azo group, an azi group, a nitrile group an
isonitrile group, a diimide
group, a cyano group, an isocyanate group, and a nitro group; a functional
group including a
sulfur element, such as a thiol group, sulfone group, a sulfide group, and a
disulfide group; and a
functional group including both the nitrogen element and the sulfur element,
such as a
thioisocyanate group and a thioamide group, the average particle diameter of
the metallic copper
particle to be obtained can be controlled to various levels according to the
kind and amount of
the above functional groups.
[0035]
As a general chemical modification method, for example, the method having the
steps of: adding a water-soluble carbodiimide to a gelatin solution so as to
activate a carboxyl
group included in a gelatin; and then reacting an arbitrary amino compound
with the activated
carboxyl group to amidate the gelatin can be used. According to this method,
for example, an
amino acid such as methionine, which includes a sulfur element or an amino
acid such as lysine,
which includes a nitrogen element can be simply introduced. Examples of the
water-soluble
carbodiimide include 1-ethy1-3-(3-dimethylaminopropyl)carbodiimide (EDC), 1-
cyclohexy1-3-
(2-morpholiny1-4-ethyl)carbodiimide -p-toluenesulfonic acid salt (CMC), N,N'-
dicyclohexylcarbodiimide (DCC), and the like. The gelatin that is applicable
to the present
invention may be gelatin obtained by performing the hydrolysis treatment and
the chemical
modification. In this case, the chemical modification may be performed after
the hydrolysis
treatment, or the hydrolysis treatment may be performed after the chemical
modification.
[0036]
In the present invention, the average particle diameter of the metallic copper
particle can be controlled by selecting whether the size of the average
molecular weight of the
gelatin is large or small. In this case, regardless of the measurement methods
of the average
molecular weight such as the mass average molecular weight and the number
average molecular
weight, any measurement methods can be used as the judgment standard on
whether the size of
the average molecular weight of the gelatin is large or small. Specifically,
taking the mass
average molecular weight as an example, the mass average molecular weight of
the gelatin to be
used is preferably 2000 to 300000. Moreover, it is preferable that the number
average
molecular weight of the gelatin is 200 to 60000. When the average molecular
weight is too
CA 02959294 2017-02-24
W7336
small, there is a risk that the gelatin does not sufficiently function as
protective colloid. Also,
= when the average molecular weight is too large, there is a risk that it
becomes difficult to control
the average particle diameter, and there is also a risk that the content of
organic components in
the protective colloid becomes too large. The mass average molecular weight of
the gelatin is
5 more preferably 250000 or less, still more preferably 200000 or less, and
particularly preferably
2000 to 200000. Moreover, the number average molecular weight of the gelatin
is more
preferably 50000 or less, still more preferably 30000 or less, and
particularly preferably 500 to
20000. In this way, the reason why the hydrolyzed gelatin of which the
molecular weight is
lowered by the hydrolysis is preferable is because by using such a gelatin,
the variation of the
10 particle diameter distribution of the metallic copper particles to be
obtained becomes small, and
is also because the sintering at a lower temperature in preparing a metallic
copper-containing
film becomes possible.
[0037]
(Measurement of Molecular Weight of Gelatin)
15 The "average molecular weight" in the present invention is a value
measured by a
"PAGI method". The "PAGI method" herein is a method for estimating the
molecular weight
distribution by determining a chromatogram of a sample solution by a gel
filtration technique
using a high-performance liquid chromatography. Specifically, the average
molecular weight
was measured according to the following method. In a 100 mL measuring flask,
2.0 g of a
20 sample was placed, an eluent consisting of an equal amount mixed
solution of 0.1 mol/L
potassium dihydrogen phosphate, and 0.1 mol/L disodium hydrogen phosphate was
added
thereto, and then the sample was expanded for 1 hour, the resultant was heated
at 40 C for 60
minutes to dissolve the sample, then the resulting eluent was diluted
accurately 10 times after
cooling to room temperature, and the resultant solution was used as a test
liquid. The
25 chromatogram of the test liquid was determined by the following gel
filtration method.
Columns: Shodex Asahipak GS 620 7G installed in tandem with another one was
used. By
using flow rate: 1.0 mL/min, column temperature: 50 C., measurement
wavelength: 230 nm,
and pullulan (P-82, manufactured by SHOWA DENKO K.K.) of which the molecular
weight is
known, the elution time was determined, and thereby a calibration curve was
made. Thereafter,
the gelatin was analyzed, and the mass average molecular weight and number
average molecular
weight of this specimen were determined using the following equation. In the
following
equation, Si represents absorbance at each point, and Mi represents a
molecular weight at elution
time Ti.
Mass average molecular weight = (Si x Mi)/ ESi
CA 02959294 2017-02-24
W7336
26
, Number average molecular weight = ESi/(ESi/Mi)
[0038]
It is preferable that the amount of the gelatin and/or the collagen peptide to
be
used is 1 to 500 parts by mass with regard to 100 parts by mass of the
metallic copper particle to
be produced, more preferably 5 to 500 parts by mass, still more preferably 5
to 300 parts by
mass, most preferably 5 to 200 parts by mass. The reason why the above range
is preferable is
because in the case where the amount of the gelatin and/or the collagen
peptide to be used is in
the above range, a metallic copper particle having a desired volume resistance
value after the
metallic copper particle is heated at a temperature of 300 C under a nitrogen
atmosphere can be
produced. The other protective colloids may be used in addition to the gelatin
and/or the
collagen peptide as long as the volume resistance value of the metallic copper
particle is not
impaired. The degree of aggregation of the fine metallic copper particles can
be controlled
according to the amount of the gelatin and/or the collagen peptide to be used,
and the fine
metallic copper particles more easily aggregate when the amount of the gelatin
and/or the
collagen peptide to be used is smaller.
[0039]
Next, a copper compound is used as a raw material for a metallic copper
particle.
As the copper compound, the following: hardly soluble (or insoluble) copper
compounds such as
copper oxides; water soluble copper compounds such as copper sulfate, copper
nitrate, copper
formate, copper acetate, copper chloride, copper bromide, and copper iodide;
and one or more
copper compounds selected from these compounds can be used. Particularly,
copper oxides,
copper sulfate, copper nitrate, and copper formate are preferable, and copper
oxides are more
preferable among them. With respect to copper oxides, when a divalent copper
oxide such as
copper oxide (copper (II) oxide) or copper hydroxide (copper (II) hydroxide)
or a monovalent
copper oxide such as cuprous oxide (copper (I) oxide) or copper hydroxide
(copper (I)
hydroxide) is used, a metallic copper particle having a desired volume
resistance value can be
produced. Among them, the "divalent copper oxides" are more preferable than
the "monovalent
copper oxides". In the "divalent copper oxide", the atomic valence of the
copper oxide is
divalent (Cu2+), and includes copper (II) oxide, copper (II) hydroxide, and a
mixture thereof.
The divalent copper oxide may appropriately include an impurity such as
another metal, a metal
compound, or a nonmetallic compound, but it is preferable that the monovalent
copper oxide is
substantially free of impurities except for those in an inevitable amount.
Moreover, a divalent
copper oxide having X-ray diffraction peaks assigned to copper (II) oxide is
preferably used. It
is preferable to use the copper (II) oxide having an average crystallite
diameter in a range of 20
CA 02959294 2017-02-24
W7336
27
to 500 nm, the average crystallite diameter calculated from the following
expression 1 based on
an X-ray diffraction peak of (110) plane of the copper (II) oxide, and a range
of 50 to 200 nm is
still more preferable. When the average crystallite diameter of the divalent
copper oxide is at
least in the range, a desired metallic copper particle can be produced. When
the average
crystallite diameter is smaller than the above range, the copper (II) oxide
has a small particle
diameter and low crystallinity. As a result, a dissolution rate of the copper
(II) oxide
accelerates, and thus the reduction reaction rate is difficult to be
controlled unless a large amount
of a complexing agent is used. On the other hand, when the average crystallite
diameter is
larger than the above range, the particle diameter is large and its
crystallinity is favorable. As a
result, a dissolution rate decelerates, and thus unreacted copper (II) oxide
is liable to remain in
the metallic copper particle unless the reduction reaction time is long.
Therefore, the above
range is preferable. The process for producing the copper oxide is not
limited, and a copper
oxide industrially produced, for example, through an electrolytic process, a
chemical conversion
process, an oxidation-by-heating process, a thermal decomposition process, an
indirect wet
process, or the like can be used. Another copper compound may be used in
addition to the
copper oxide as long as the volume resistance value of the metallic copper
particle is not
impaired.
Expression 1: DHKL = K*X/13cos0
DHKL: average crystallite diameter (A)
X,: wavelength of X-ray
13: half-width value of diffraction peak
0: Bragg's angle
K: constant (= 0.9)
[0040]
Next, when hypophosphorous acid (i.e. phosphinic acid) and/or a salt thereof
are
used as a reducing agent, a metallic copper particle having a desired volume
resistance value
after the metallic copper particle is heated at a temperature of 300 C under a
nitrogen
atmosphere can be produced compared with the case where a reducing agent such
as hydrazine is
used. Examples of the hypophosphite include salts such as a sodium salt and a
potassium salt,
and when the hypophosphite is used, the reaction easily progresses by
adjusting on an acidic side
the pH in the reduction reaction. The amount of the reducing agent to be used
can be
appropriately set as long as it is an amount capable of reducing a copper
compound to a metallic
copper particle, and the range of 0.33 to 5 mol with regard to 1 mol of copper
included in the
copper compound is preferable. When the amount of the reducing agent is
smaller than the
CA 02959294 2017-02-24
W7336
28
above range, the reaction is hard to progress, so that the metallic copper
particle is not
. sufficiently produced. Also, when the amount of the reducing agent is
larger than the above
range, the reaction excessively progresses, so that the desired metallic
copper particle is hard to
obtain. Therefore, the above range is preferable. The amount of the reducing
agent to be used
is more preferably in a range of 0.4 to 4 mol, and still more preferably 0.5
to 4 mol. Also,
another reducing agent may be used in addition hypophosphorous acid and/or a
salt thereof in a
range where there is no problem in terms of the volume resistance value of the
metallic copper
particle.
[0041]
It is preferable that the reduction reaction is performed at a pH of 3 or
lower.
The above reduction reaction performed at a pH of 3 or lower does not mean
that the reaction is
constantly performed at a pH of 3 or lower but that the reduction reaction has
only to pass
through a state where the pH is 3 or lower. In particular, it is preferable
that aging at the time of
and after the completion of the reduction reaction is performed at a pH of 3
or lower. It is
considered that a balance among the elution of a copper ion, the nuclear
generation rate of
copper, and the nuclear growth rate of copper can be kept by using, as a raw
material, a copper
compound including at least copper oxide which is hardly soluble and reducing
it in a liquid
medium having a pH of 3 or lower by using hypophosphorous acid and/or a salt
thereof as a
reducing agent in the presence of gelatin and/or a collagen peptide.
Therefore, a metallic
copper particle including a large diameter metallic copper particle and at
least one fine metallic
copper particle wherein the at least one fine metallic copper particle is
adhered on the large
diameter metallic copper particle can be produced, and further the metallic
copper particle
specifically characterized as one embodiment of the present invention, in
which an aggregate of
a plurality of the fine metallic copper particles are adhered on the surface
of the large diameter
metallic copper particle can be also produced. Moreover, a mixed particle in
which the metallic
copper particle (i.e. composite particle) and a small metallic copper particle
are mixed can be
also produced by selecting a reduction condition.
[0042]
Next, in the present invention, a complexing agent may be added at the time of
the reduction reaction when necessary, and it is preferable to use the amine
complexing agent
such as the amines or the alkanol amines, described below. It is considered
that the
"complexing agent" in the present invention acts in a process eluting copper
ions from the copper
compound or in a process reducing the copper compound to produce metallic
copper. The
"complexing agent" in the present invention means a compound capable of
forming a copper
CA 02959294 2017-02-24
W7336
29
complex c9mpound by binding of donor atoms in a ligand included in the
complexing agent with
copper ions or metallic copper, and examples of the donor atom include
nitrogen, oxygen, and
sulfur. Specifically, as examples thereof, the complexing agents described in
the following (1)
to (5) are included.
(1) The complexing agents having nitrogen as the donor atom include, for
example, (a) amines (for example, primary amines such as butylamine,
ethylamine, propylamine,
and ethylenediamine; secondary amines such as dibutylamine, diethylamine,
dipropylamine and
imines such as piperidine and pyrrolidine; tertiary amines such as
tributylamine, triethylamine,
and tripropylamine; and those having two or more kinds of the primary to
tertiary amines in one
molecule of diethylenetriamine or triethylenetetramine), (b) nitrogen-
containing heterocyclic
compounds (for example, imidazole, pyridine, and bipyridine), (c) nitriles
(for example,
acetonitrile, and benzonitrile) and cyanides, (d) ammonia and ammonium
compounds (for
example, ammonium chloride, and ammonium sulfate), and (e) oximes.
(2) The complexing agents having oxygen as the donor atom include, for
example, (a) carboxylic acids (for example, oxycarboxylic acids such as citric
acid, malic acid,
tartaric acid, and lactic acid; monocarboxylic acids such as acetic acid and
formic acid;
dicarboxylic acids such as oxalic acid and malonic acid; aromatic carboxylic
acids such as
benzoic acid), (b) ketones (for example, monoketones such as acetone, and
diketones such as
acetylacetone and benzoylacetone), (c) aldehydes, (d) alcohols (for example,
monohydric
alcohols, glycols, and glycerols), (e) quinones, (f) ethers, (g) phosphoric
acid (for example,
orthophosphoric acid) and phosphoric acid compounds (for example,
hexametaphosphoric acid,
pyrophosphoric acid, and phosphorous acid), and (h) sulfonic acid or sulfonic
acid compounds.
(3) The complexing agents having sulfur as the donor atom include, for
example, (a) aliphatic thiols (for example, methyl mercaptan, ethyl mercaptan,
propyl mercaptan,
isopropyl mercaptan, n-butyl mercaptan, allyl mercaptan, and dimethyl
mercaptan), (b) alicyclic
thiols (such as cyclohexyl thiol), (c) aromatic thiols (for example,
thiophenol), (d) thioketones,
(e) thioethers, (f) polythiols, (g) thiocarbonic acids (for example,
trithiocarbonic acids), (h)
sulfur-containing heterocyclic compounds (for example, dithiol, thiophene, and
thiopyran), (i)
thiocyanates and isothiocyanates, and (j) inorganic sulfur compounds (for
example, sodium
sulfide, potassium sulfide, and hydrogen sulfide).
(4) The complexing agents having two or more kinds of donor atoms include,
for example, (a) amino acids (where the donor atoms are nitrogen and oxygen:
for example,
neutral amino acids such as glycine and alanine; basic amino acids such as
histidine and
arginine; and acidic amino acids such as aspartic acid and glutamic acid), (b)
amino
CA 02959294 2017-02-24
W7336
,polycarbw,cylic acids (where the donor atoms are nitrogen and oxygen: for
example,
ethylenediaminetetraacetate (EDTA), nitrilotriacetate (NTA), iminodiacetate
(IDA),
ethylenediaminediacetate (EDDA), ethyleneglycoldiethyletherdiaminetetraacetate
(GEDA)), (c)
alkanolamines (where the donor atoms are nitrogen and oxygen: for example,
ethanolamine,
5 diethanolamine, and triethanolamine), (d) nitroso compounds and nitrosyl
compounds (where
donor atoms are nitrogen and oxygen), (e) mercaptocarboxylic acids (where
donors are sulfur
and oxygen: for example, mercaptopropionic acid, mercaptoacetic acid,
thiodipropionic acid,
mercaptosuccinic acid, dimercaptosuccinic acid, thioacetic acid, and
thiodiglycolic acid), (f)
thioglycols (donors are sulfur and oxygen: for example, mercaptoethanol, and
thiodiethylene
10 glycol), (g) thionic acids (where the donors are sulfur and oxygen), (h)
thiocarbonic acids (where
the donor atoms are sulfur and oxygen: for example, monothiocarbonic acid,
dithiocarbonic acid,
and thione carbonic acid), (i) aminothiols (where the donors are sulfur and
nitrogen: for example,
aminoethylmercaptan and thiodiethylamine), (j) thioamides (where the donor
atoms are sulfur
and nitrogen: for example, thioformamide), (k) thioureas (where the donor
atoms are sulfur and
15 nitrogen), (1) thiazoles (where the donor atoms are sulfur and nitrogen:
for example, thiazole, and
benzothiazole), (m) sulfur-containing amino acids (where the donors are
sulfur, nitrogen and
oxygen: for example, cysteine, methionine).
(5) Examples of salts of the above compounds and derivatives
thereof include
alkali metal salts such as trisodium citrate, potassium sodium tartrate,
sodium hypophosphite,
20 and disodium ethylenediaminetetraacetate; and esters of carboxylic acid,
phosphoric acid, and
sulfonic acid.
[0043]
Among these complexing agents, at least one thereof can be used. The amount
of the complexing agent to be used can be appropriately set, but it is
preferable to set the amount
25 of the complexing agent to be used in a range of 0.01 to 500 parts by
mass with regard to 1000
parts by mass of the copper compound because the effects of the present
invention are easily
obtained. By reducing the amount of the complexing agent to be used within the
above range,
primary particles of the metallic copper particle can be made smaller, and by
increasing the
amount of the complexing agent to be used, primary particles thereof can be
made larger. The
30 amount of the complexing agent to be used is preferably in a range of
0.1 to 500 parts by mass,
still more preferably in a range of 0.5 to 250 parts by mass.
[0044]
In the present invention, the complexing agent including at least one selected
from nitrogen and oxygen as the donor atom is preferable because the effects
of the present
CA 02959294 2017-02-24
W7336
31
invention are easily obtained. Specifically, at least one complexing agent
selected from amines,
nitrogen-containing heterocyclic compounds, nitriles, cyanides, carboxylic
acids, ketones,
phosphoric acid and phosphoric acid compounds, amino acids,
aminopolycarboxylic acids,
alkanolamines, salts thereof, or derivatives thereof is more preferable. Among
the carboxylic
acids, oxycarboxylic acids are preferable; among the ketones, diketones are
preferable; and
among the amino acids, basic or acidic amino acids are preferable. Further, it
is preferable that
the complexing agent is at least one complexing agent selected from
butylamine, ethylamine,
propylamine, dibutylamine, diethylamine, dipropylamine, tributylamine,
triethylamine,
tripropylamine, imidazole, citric acid or alkali metal salts thereof,
acetylacetone,
hypophosphorous acid or alkali metal salts thereof, histidine, arginine,
ethylenediaminetetraacetate or alkali metal salts thereof, ethanolamine, and
acetonitrile. As
described above, the amount of the oxygen or nitrogen complexing agent to be
used is preferably
in a range of 0.01 to 500 parts by mass with regard to 1000 parts by mass of
the copper
compound, more preferably in a range of 0.1 to 500 parts by mass, and still
more preferably in a
range of 0.5 to 250 parts by mass.
[0045]
In the present invention, it is preferable to use the complexing agent
including
oxygen as the donor atom, and among others, it is more preferable to use the
complexing agent
corresponding to an organic acid. As described above, it is preferable to
perform the reduction
reaction in a region of a pH of 3 or lower. In this regard, it is considered
that the use of the
organic acid as the complexing agent can be lower the initial pH of the
reaction liquid and have,
in the reduction reaction, some effect on the elution of copper ions and the
nuclear generation
and nuclear growth rate of copper. Therefore, the metallic copper particle
including a large
diameter metallic copper particle and at least one fine metallic copper
particle wherein the at
least one fine metallic copper particle is adhered on the large diameter
metallic copper particle
can be produced more effectively, and the metallic copper particle
specifically characterized as
one embodiment of the present invention, in which an aggregate of a plurality
of the fine metallic
copper particles is adhered on the surface of the large diameter metallic
copper particle can be
also produced more effectively. Moreover, by selecting a reduction condition,
the mixed
particle in which the composite particle and the small metallic copper
particle are mixed can be
also produced, and thus a mixture (i.e. mixed particle) of the large diameter
metallic copper
particle on which the partially aggregated fine metallic copper particles are
adhered and the
small metallic copper particle is easily obtained. Further, in the state where
the metallic copper
particle (i.e. composite particle) and the small metallic copper particle are
mixed, the specific
CA 02959294 2017-02-24
W7336
32
,surface area easily falls within the range of 1 to 6 m2/g, and in this case,
the ratio of both
particles (the metallic copper particle (i.e. composite particle) and the
small metallic copper
particle) is a ratio in the case of exhibiting particularly excellent
sinterability at low temperature.
Furthermore, it is presumed that an organic acid remains adsorbed on the
surface of the produced
metallic copper particle, and the organic acid disappears even at a relatively
low temperature
during heating to make it easy to sinter the metallic copper particles, so
that the volume
resistance value at the time of a low temperature heating can remarkably be
reduced. Examples
of the organic acid include carboxylic acids, amino acids, and aminocarboxylic
acids. Among
these organic acids, carboxylic acids are more preferable, and formic acid is
most preferable
therein.
[0046]
In the production methods according to the present invention, in the case of
using
the complexing agent corresponding to the organic acid, the volume resistance
value of a
metallic copper-containing film prepared by performing heating and firing at a
temperature of
120 C under an air atmosphere is used as an index, and a volume resistance
value of 1 x 10-1
Q-cm or less can be achieved in terms of the volume resistance value measured
according to the
< Method 2 for Measuring Volume Resistance Value > described above, and
further a metallic
copper-containing film that exhibits a volume resistance value of 1 x 02 cm or
less can be
obtained. In this way, the metallic copper particle according to the present
invention has a low
volume resistance value and a high electrical conductivity because the
sinterability or the contact
property between the particles are improved even when heating the metallic
copper particle at a
temperature of 120 C under an air atmosphere. Therefore, the metallic copper
particle
according to the present invention can be used with a base material having a
low heat resistance
temperature, and can be employed in a wide range of uses. Moreover, the firing
in the air can
be performed, and thus constraints on facilities such as control of an
atmosphere can be avoided.
[0047]
In the present invention, in the case where the gelatin and/or the collagen
peptide
and, when necessary, the complexing agent exist at the time of mixing the
copper oxide and the
reducing agent, the order to add each raw material is not limited. Examples of
adding each raw
material include (1) a method for performing the concurrent addition of the
copper oxide and the
reducing agent to a solvent including the gelatin and/or the collagen peptide,
and, when
necessary, the complexing agent, (2) a method for adding the reducing agent to
a solvent
including the gelatin and/or the collagen peptide, the copper compound, and,
when necessary, the
complexing agent, (3) a method for performing the concurrent addition of the
reducing agent and
CA 02959294 2017-02-24
W7336
33
the compleNing agent to a solvent including the gelatin and/or the collagen
peptide, and the
copper compound, and (4) a method for adding a mixed solution of the reducing
agent and the
complexing agent to a solvent including the gelatin and/or the collagen
peptide, and the copper
compound. Among these, methods (3) and (4) are preferable because the reaction
is easily
controlled, and method (4) is particularly preferable. The copper compound,
the reducing
agent, the complexing agent, and the gelatin and/or the collagen peptide may
be suspended or
dissolved in a solvent in advance before these are used in the reduction
reaction. In addition,
the "concurrent addition" means a method for separately adding the copper
compound and the
reducing agent, or the complexing agent and the reducing agent at the same
time during the
reaction or at the same time period during the reaction, and includes not only
continuous addition
of both materials during the reaction but also intermittent addition of one or
both materials.
[0048]
As a solvent, for example, an aqueous solvent or an organic solvent such as an
alcohol is used, and the aqueous solvent is preferably used. It is preferable
that the reaction
temperature is in a range of 10 C to a boiling point of the used solvent
because the reaction
easily progresses, more preferably in a range of 20 to 100 C because a fine
metallic copper
particle is obtained, still more preferably in a range of 30 to 95 C,
particularly preferably 40 to
95 C. As described above, the pH of the reaction liquid may be 3 or less
during the reduction
reaction. In the case where hypophosphorous acid is used as a reducing agent,
the initial pH of
the reaction liquid is not particularly limited and may be appropriately set
because the pH can be
lowered by the addition of hypophosphorous acid. In the case where the
hypophosphite is used
as a reducing agent, it is preferable to adjust the initial pH of the reaction
liquid to 3 or lower by
adding an arbitrary acid in advance. It is preferable to add an organic acid
to the reaction liquid
in advance as described above irrespective of whichever reducing agent is
used. In addition,
the pH may be lowered with only an organic acid, and the pH may be also set at
3 or lower using
an organic acid in combination with hypophosphorous acid as a reducing agent.
An inorganic
acid other than hypophosphorous acid, such as phosphoric acid, a phosphate,
pyrophosphoric
acid, or pyrophosphate, may be used for the pH adjustment. In particular, by
the use of
pyrophosphoric acid for the pH adjustment, the reduction reaction of copper
oxide using
hypophosphorous acid (i.e. phosphinic acid) and/or a salt thereof can be
softly progressed, and
thus the heat generation at the time of the reaction can be suppressed.
Furthermore, by making
it easy to control the reaction rate, the adjustment of the particle size
becomes easy. Moreover,
a defoaming agent may be used in order to suppress foaming during reaction.
The reaction time
can be controlled and set by the time for adding raw materials such as a
reducing agent, and
CA 02959294 2017-02-24
W7336
34
about 10 minutes tp six hours is appropriate, for example. After the
completion of adding raw
materials such as a reducing agent, the reaction liquid may be subjected to
aging as it is. The
aging temperature or time can be appropriately set. The aging temperature at
the same level as
the above reaction temperature is appropriate, and the aging time of about 10
minutes to about
six hours is appropriate.
[0049]
The production of a particle having a flat shape becomes easy by adding 10% by
mass or more of polymer gelatin with regard to the produced metallic copper
particle. Also, the
above production becomes easy when the temperature of the reduction reaction
is 50 C or
higher. The production of a particle having a granular shape or the like
becomes easy by
adding 10% by mass or more of the collagen peptide with regard to the produced
metallic copper
particle. Moreover, the production of a particle having a granular shape or
the like becomes
easy when the temperature of the reduction reaction is set at 20 to 90 C in
the presence of the
gelatin and/or the collagen peptide. The production of the particle having an
irregular shape
becomes easy when the temperature of the reduction reaction is set at 90 C or
higher. The
average particle diameter of the large diameter metallic copper particle and
the abundance ratio
of the large diameter metallic copper particle and the at least one fine
metallic copper particle
can be adjusted according to the reaction temperature or aging temperature,
and the average
particle diameter and the abundance ratio of the large diameter metallic
copper particle become
larger as the temperature becomes higher. The average particle diameter and
shape of the large
diameter metallic copper particle and the abundance ratio of the large
diameter metallic copper
particle and the at least one fine metallic copper particle can be also
adjusted according to the
reaction time or aging time. Moreover, the average particle diameter and shape
of the small
metallic copper particle, and the abundance ratio of the metallic copper
particle (i.e. composite
particle) and the small metallic copper particle can be also adjusted
according to the reaction
time or aging time.
[0050]
In the present invention, the mixed particle including the metallic copper
particle
(specifically, the composite particle in which the at least one fine metallic
copper particle and/or
an aggregate thereof are adhered on the surface of the large diameter metallic
copper particle) in
a mixed state with the small metallic copper particle can be produced at once
without undergoing
a mixing process of particles, according to the above production methods.
According to the
above production methods, the metallic copper particle in which the size or
particle shape of the
large diameter metallic copper particle, the size or particle shape of the at
least one fine metallic
CA 02959294 2017-02-24
W7336
,eopper particle, or,the size or particle shape of the small metallic copper
particle is different, and
the metallic copper particle in which the abundance ratio of them is different
can be produced.
Moreover, the above mixed particle including the small metallic copper
particle in the mixed
state can be also obtained by mixing the above metallic copper particle
(specifically, the
5 composite particle in which the at least one fine metallic copper
particle and/or an aggregate
thereof are adhered on the surface of the large diameter metallic copper
particle) and the small
metallic copper particle separately prepared.
[0051]
According to the above methods, the metallic copper particles, in the presence
of
10 the gelatin and/or the collagen peptide and the complexing agent when
necessary, are produced,
and then fractionation and washing are performed when necessary. Moreover, the
gelatin
and/or the collagen peptide, adhered on the surface of the metallic copper
particles are
decomposed by adding a protective colloid remover to the solvent after the
reaction, thereby
agglomerating the metallic copper particles, and subsequently, the resultant
can be fractionated.
15 The "protective colloid remover" is a compound that decomposes or
dissolves protective colloid
to suppress the action of the protective colloid, and when part, if not all,
of the protective colloid
can be removed from the solvent, the effects according to the present
invention are obtained.
The kind of protective colloid remover is appropriately selected according to
the protective
colloid to be used. Specifically, for removing the protein protective colloid,
proteases such as
20 serine proteases (for example, trypsin and chymotrypsin), thiol
proteases (for example, papain),
acid proteases (for example, pepsin), and metalloproteases can be used. The
additive amount of
the protective colloid remover may be an amount which protective colloid can
be removed to
such an extent that the metallic copper particles can be agglomerated and
fractionated.
Although the additive amount of the protective colloid remover is different
depending on the
25 kind thereof, in the case of a protease, with regard to 1000 parts by
mass of protein protective
colloid, a range of 0.001 to 1000 parts by mass is preferable, 0.01 to 200
parts by mass is more
preferable, and 0.01 to 100 parts by mass is still more preferable. The
temperature of the
solvent at the time of adding the protective colloid remover can be
appropriately set, and may be
in the state where the temperature of the reduction reaction is retained, or a
range of 10 C to the
30 boiling point of the used solvent is preferable because the removal of
the protective colloid easily
progresses, and a range of 40 to 95 C is more preferable. When the protective
colloid remover
is added and then the resultant state is appropriately retained, the
protective colloid can be
decomposed, and for example, about 10 minutes to about 10 hours of the
retention time is
appropriate. After removing the protective colloid to agglomerate the metallic
copper particles,
CA 02959294 2017-02-24
W7336
36
fractionation is performed by an ordinary method. The method for performing
the fractionation
is not particularly limited, and methods such as gravity filtration, pressure
filtration, vacuum
filtration, suction filtration, centrifugal filtration, and natural
sedimentation can be used.
However, from the industrial viewpoint, the pressure filtration, the vacuum
filtration, and the
suction filtration are preferable, and it is preferable to use a filter
machine such as a filter press
and a roll press because the dehydration ability is high and the treatment of
a large amount is
possible.
[0052]
As an embodiment of the above method, it is preferable to further add a
flocculant
agent after adding the protective colloid remover because the yield is much
more improved.
The publicly known flocculants can be used, and specific examples thereof
include anionic
flocculants (for example, partially hydrolyzed products of polyacrylamide,
acrylamide-sodium
acrylate copolymers, and sodium alginate), cationic flocculants (for example,
polyacrylamide,
dimethylaminoethyl methacrylate, dimethylaminoethyl acrylate, polyamidine, and
chitosan), and
amphoteric flocculants (for example, acrylamide-dimethylaminoethyl acrylate-
acrylic acid
copolymers). The additive amount of the flocculants can be appropriately set
according to a
required amount, and is preferably in a range of 0.5 to 100 parts by mass with
regard to 1000
parts by mass of the metallic copper particle, and more preferably in a range
of 1 to 50 parts by
mass.
[0053]
Alternatively, a similar effect of improving the yield is also obtained by
adding a
protective colloid remover after adjusting the pH of the solvent in a range of
1 to 8 using an
alkali in place of the use of the flocculant. When the pH is lower than 1, the
metallic copper
particle corrodes or dissolves, and thus a range of 1 to 7 is a preferable pH
region. It is more
preferable that the pH be in a range of 1 to 6 because the amount of the
alkali to be used is
reduced.
[0054]
After the metallic copper particles are subjected to a solid-liquid separation
and
washed when necessary, the solid of the metallic copper particles obtained
thereby can be used
by dispersing it in an aqueous solvent or an organic solvent such as an
alcohol, preferably in the
aqueous solvent. Alternatively, the solid of the metallic copper particles may
be dried by an
ordinary method, and further the solid can be used by dispersing it in an
aqueous solvent or an
organic solvent such as an alcohol, preferably in the aqueous solvent after
drying. The metallic
copper particle is easily oxidized, and thus it is preferable that drying is
performed under an
CA 02959294 2017-02-24
W7336
37
,atmospherc of an inert gas such as nitrogen or argon in order to suppress
oxidization. After
, drying, grinding may be performed when necessary.
[0055]
Next, the present invention relates to a metallic copper dispersion including
the
above metallic copper particle. Any aqueous solvent and/or any organic solvent
can be used as
a dispersion medium, and a polymeric dispersant may be used when necessary.
Moreover,
another metallic particle such as a silver, nickel, copper, or tin particle or
an alloy particle such as
a copper-tin alloy particle may be mixed with the metallic copper particle
when necessary. The
mixing ratio of the metallic particle or the alloy particle can be
appropriately set.
[0056]
In the present invention, the gelatin and/or the collagen peptide suitably
exist on
the surface of the metallic copper particle. However, the gelatin and/or the
collagen peptide
have a high acid value, and thus the metallic copper particle having the
gelatin and/or the
collagen peptide on the surface thereof dissociates in a solvent to be
electrically negative and is
easy to agglomerate in an organic solvent. Thus, it is preferable to mix a
polymeric dispersant
in order to neutralize acid sites which are the cause of an acid value of the
gelatin and/or the
collagen peptide. The polymeric dispersant as well as the gelatin and/or the
collagen peptide
includes a hydroxyl group, an acidic group, a basic group, and the like, and
thus has an amine
value and an acid value, and the polymeric dispersant having an amine value of
10 to 150
mgKOH/g is preferable, more preferably 10 to 130 mgKOH/g, still more
preferably 10 to 90
mgKOH/g, particularly preferably 15 to 80 mgKOH/g, and most preferably 15 to
50 mgKOH/g.
The amine value in the above range can contribute to the dispersion stability
of the metallic
copper particle in an organic solvent, and thus is preferable. Moreover, with
respect to the
amine value and acid value of the polymeric compound, it is preferable that
the polymeric
compound has an amine value (i.e. base site) and an acid value (i.e. acid
site) in an amount
which is almost equal to or more than the amount to compensate (i.e.
neutralize) the amine value
and acid value of the gelatin and/or the collagen peptide that exist on the
surface of the metallic
copper particle, and it is preferable that the difference between the amine
value and the acid
value, namely (i.e. "(amine value - acid value)") is in a range of 0 to 50,
and more preferably in a
range of 1 to 30. The polymeric dispersant may be electrostatically bound to
the acid sites or
base sites of the gelatin and/or the collagen peptide through the base sites
or acid sites thereof.
For these reasons, it is considered that (amine value of polymeric dispersant
x mass of polymeric
dispersant) - (acid value of gelatin x mass of gelatin) is preferably 0 or
more.
[0057]
CA 02959294 2017-02-24
W7336
38
It i preferable that the polymeric dispersant has a specific heat capacity of
1.0 to
2.0 J/(g-K) at the glass transition point. This is because the heat
accumulation amount of the
polymeric dispersant is so small that the heat amount necessary for raising a
temperature by 1 K
can be small and the heat amount added for the purpose of decomposition can be
small. The
specific heat capacity is more preferably in a range of 1.2 to 1.9 J/(g-K),
and still more
preferably in a range of 1.3 to 1.8 J/(g=K). Moreover, it is preferable that
the polymeric
dispersant has a glass transition point in a range of -70 to 10 C because the
glass transition
occurs at a low temperature to make the heat amount added for the purpose of
decomposition
small. The glass transition point is preferably in a range of -70 to 7 C,
still more preferably in a
range of -70 to 5 C, and still more preferably in a range of -70 to 0 C. For
these reasons, in the
present invention, a more preferable polymeric dispersant has an amine value
of 10 to 90
mgKOH/g and a glass transition point in a range of -70 to 10 C, and a still
more preferable
polymeric dispersant has an amine value of 10 to 90 mgKOH/g, a glass
transition point in a
range of -70 to 10 C, and a specific heat capacity of 1.0 to 2.0 J/(g-K) at
the glass transition
point.
(Measurement of Specific Heat Capacity at Glass Transition Point)
According to JIS K 7123-1987 "Testing Methods for Specific Heat Capacity of
Plastics", the specific heat capacity was measured with DSC Q 100 Type
manufactured by TA
Instruments. With respect to a temperature-raising pattern, the temperature
was held at -90 C
for 5 minutes, then raised to 40 C at 5 C/min, and held at 40 C for 5 minutes.
As analytical
software, option software "Thermal Specialty Library" manufactured by TA
Instruments was
used.
(Measurement of Glass Transition Point)
According to JIS K 7121-1987 "Testing Methods for Transition Temperatures of
Plastics", the glass transition point was measured with DSC Q 100 Type
manufactured by TA
Instruments. With respect to a temperature-raising pattern, the temperature
was held at -90 C
for 5 minutes, then raised to 40 C at 5 C/min, and held at 40 C for 5 minutes.
[0058]
The polymeric dispersant is, for example, a polymer or copolymer having a
tertiary amino group, quaternary ammonium group, heterocyclic group having a
basic nitrogen
atom, or a basic group such as a hydroxyl group, and may have an acidic group
such as a
carboxyl group, and thus the amine value and acid value of the polymeric
dispersant are
compensated, so that (amine value - acid value) may be 0. The polymeric
dispersant having an
amine value higher than the acid value is preferable, and (amine value - acid
value) is in a range
CA 02959294 2017-02-24
W7336
39
of 0 to 50, And moye preferably in a range of 1 to 30. Because the basic group
or acidic group
of the polymeric dispersant is a functional group affinitive to the metallic
copper particle covered
with the gelatin, the polymeric dispersant having one or more basic or acidic
groups in the main
chain and/or the side chain is preferable, and the polymeric dispersant having
several basic or
acidic groups in the main chain and/or the side chain is more preferable. The
basic or acidic
groups may be included at one terminal of the main chain of the polymer and/or
one terminal of
the side chain of the polymer. The straight-chain polymers such as A-B block
type polymers;
polymers having a comb-shaped structure with a plurality of side chains; and
the like can be used
as the polymeric dispersant. The mass average molecular weight of the
polymeric dispersant is
not limited, but it is preferable that the mass average molecular weight
measured by a gel
permeation chromatography method is in a range of 2000 to 1000000 g/mol. When
the mass
average molecular weight is less than 2000 g/mol, the dispersion stability is
not sufficient, and
when the mass average molecular weight exceeds 1000000 g/mol, the viscosity is
too high and
the handling is likely to be difficult. Thus, the mass average molecular
weight is more
preferably in a range of 4000 to 1000000 g/mol, still more preferably in a
range of 10000 to
1000000 g/mol, and further more preferably in a range of 1000 to 100000 g/mol.
Moreover, the
polymeric dispersant including a small amount of elements of phosphorus,
sodium, and
potassium is preferable, and the polymeric dispersant not including these
elements is more
preferable. When the elements of phosphorus, sodium, and potassium are
included in the
polymeric dispersant, the elements remain as ash in producing an electrode, a
wiring pattern, or
the like by heating and firing, and thus the polymeric dispersant not
including these elements is
preferable. One or more of such polymeric dispersants can be appropriately
selected and used.
[0059]
Specifically, the polymeric dispersant includes polymers having a basic group
such as salts of long-chain polyaminoamides and polar acid esters, unsaturated
polycarboxylic
acid polyaminoamides, polycarboxylic acid salts of polyaminoamides, and salts
of long-chain
polyaminoamides and acid polymers. Moreover, the polymeric dispersant includes
alkylammonium salts, amine salts, and amide amine salts of polymers such as
acrylic polymers,
acrylic copolymers, modified polyester acids, polyether ester acids, polyether
carboxylic acids,
and polycarboxylic acids, and straight-chain type acrylic polymers or straight-
chain type acrylic
copolymers are preferable. Commercially available polymeric dispersants can be
also used as
such a polymeric dispersant. Examples of the commercially available polymeric
dispersant
include DISPERBYK (which is a registered trade-mark)-106, DISPERBYK-109,
DISPERBYK-
110, DISPERBYK-111, DISPERBYK-130, DISPERBYK-161, DISPERBYK-162,
CA 02959294 2017-02-24
W7336
,DISPERByK-163õ DISPERBYK-167, DISPERBYK-168, DISPERBYK-180, DISPERBYK-
182, DISPERBYK-183, DISPERBYK-184, DISPERBYK-185, DISPERBYK-2000,
,
DISPERBYK-2001, DISPERBYK-2013, DISPERBYK-2163, DISPERBYK-2164, BYK-4512,
BYK-P105, LPN-21854, and LPC-22124 (, all of which are manufactured by BYK-
Chemie
5 GmbH), FLOWLEN DOPA-15B, FLOWLEN DOPA-15BHFS, FLOWLEN 17HF, FLOWLEN
DOPA-22, FLOWLEN DOPA-33, and FLOWLEN DOPA-44 (, all of which are manufactured
by
Kyoeisha Chemical Co., Ltd.), and ED-212 and ED-213 ('all of which are
manufactured by
Kusumoto Chemicals, Ltd.).
[0060]
10 The amine values of the gelatin and/or the collagen peptide, and
the polymeric
dispersant denote the total amount of free bases and bases, and expressed by
an equivalent
amount of potassium hydroxide in mg to the amount of hydrochloric acid needed
to neutralize 1
g of a sample. Moreover, the acid value denotes the total amount of free fatty
acids and fatty
acids, and expressed by an amount of potassium hydroxide in mg needed to
neutralize 1 g of a
15 sample. Specifically, the amine value and the acid value are measured by
the following method
according to JIS K7700 or ASTM D2074 below.
(Method for Measuring Amine Value)
In 300 mL of a mixed solvent of ethanol and pure water, 5 g of the gelatin
and/or
the collagen peptide, or the polymeric dispersant, and several drops of a
bromocresol green
20 ethanol solution are dissolved. Then, to the resultant mixed solution a
0.1 M HC1 ethanol
solution whose factor (correction coefficient) has been calculated is added,
and the amine value
is calculated from the titer of the 0.1M HC1 ethanol solution when yellow of a
bromocresol green
indicator continues for 30 seconds.
(Method for Measuring Acid Value)
25 In 300 mL of pure water, 5 g of the gelatin and/or the collagen
peptide, or the
polymeric dispersant, and several drops of a phenolphthalein solution are
dissolved. Then, to
the resultant mixed solution a 0.1M KOH ethanol solution whose factor
(correction coefficient)
has been calculated is added. The acid value is calculated from the titer of
the 0.1M KOH
ethanol solution when light red of a phenolphthalein indicator continues for
30 seconds.
30 [0061]
The organic solvent can appropriately be selected, and specifically, at least
one
selected from hydrocarbon solvents such as toluene, xylene, solvent naphtha,
normal hexane,
isohexane, cyclohexane, methylcyclohexane, normal heptane, tridecane,
tetradecane, and
pentadecane; alcoholic solvents such as methanol, ethanol, butanol, IPA
(isopropyl alcohol),
CA 02959294 2017-02-24
W7336
41
normal propyl alcohol, 2-butanol, TBA (tertiary butanol), butanediol,
ethylhexanol, benzyl
- alcohol, and terpineol; ketone solvents such as acetone, methyl ethyl
ketone, methyl isobutyl
ketone, DIBK (diisobutyl ketone), cyclohexanone, and DAA (diacetone alcohol);
ester solvents
such as ethyl acetate, butyl acetate, methoxybutyl acetate, cellosolve
acetate, amyl acetate,
normal propyl acetate, isopropyl acetate, methyl lactate, ethyl lactate, and
butyl lactate; ether
solvents such as methyl cellosolve, cellosolve, butyl cellosolve, dioxane,
MTBE (methyl tertiary
butyl ether), and butyl carbitol; glycol solvents such as ethylene glycol,
diethylene glycol,
triethylene glycol, and propylene glycol; glycol ether solvents such as
diethylene glycol
monomethyl ether, triethylene glycol monomethyl ether, propylene glycol
monomethyl ether,
and 3-methoxy-3-methyl-l-butanol; and glycol ester solvents such as ethylene
glycol
monomethyl ether acetate, PMA (propylene glycol monomethyl ether acetate),
diethylene glycol
monobutyl ether acetate, and diethylene glycol monoethyl ether acetate can be
used for the
organic solvent. The organic solvent having a low viscosity is preferable for
adaptation
reduction in viscosity of the metallic copper dispersion, and the organic
solvent having a
viscosity in a range of 1 to 20 mPa-s is preferable. As such an organic
solvent, toluene, butyl
carbitol, butanol, propylene glycol-l-monomethyl ether-2-acetate, butyl
cellosolve, tetradecane,
and the like are suitably used. Also, the aqueous solvent can be appropriately
selected, and
specifically, water, and water and water-soluble solvents can be used.
[0062]
It is preferable that the gelatin and/or the collagen peptide exist in an
amount
within a range of about 0.1 to about 15 parts by mass with regard to 100 parts
by mass of the
metallic copper particle because a desired effect is obtained. The more
preferable range is
about 0.1 to about 10 parts by mass. It is preferable that the polymeric
dispersant be in a range
of 0.1 to 20 parts by mass with regard to 100 parts by mass of the metallic
copper particle
because a desired effect is obtained. The above range is preferable because
when the amount of
the polymeric dispersant is too much smaller than the above range, it is
difficult to obtain the
effects of the present invention, and when the amount of the polymeric
dispersant is too much
larger than the above range, the electrical conductivity may be obstructed in
electrode material
uses and cloudiness or the like may occur to deteriorate an appearance in
decorative article uses.
The more preferable range is 0.1 to 10 parts by mass. The concentration of the
metallic copper
particle in the dispersion can be appropriately adjusted, and specifically,
the concentration of the
metallic copper particle can be adjusted to 10 % by mass or more, preferably
10 to 99% by mass,
and more preferably about 20 to about 95% by mass.
[0063]
CA 02959294 2017-02-24
W7336
42
, The metallic copper dispersion according to the present
invention can maintain a
= sufficient fluidity even when the concentration of the metallic copper
particle is made 50% by
mass or more. Therefore, the metallic copper dispersion according to the
present invention can
be suitably used for joining materials and the like for which a high-
concentration paste is
required. Moreover, in the metallic copper dispersion according to the present
invention, the
metallic copper particle is sufficiently dispersed, and thus even if the
metallic copper particle is
in a high concentration, viscosity of the dispersion can be adjusted to be
relatively low. For
example, the viscosity of the dispersion can be set to preferably 100 mPa-s or
less, more
preferably 1 to 30 mPa.s, and still more preferably 1 to 20 mPa.s.
Furthermore, the dispersion
according to the present invention can be suitably used for ink jet printing,
spray coating, and the
like by setting the concentration of the metallic copper particle to an
appropriate concentration of
15% by mass or more.
[0064]
In the metallic copper dispersion according to the present invention, in
addition to
the above metallic copper particle, the above aqueous solvent and/or the above
organic solvent,
and the above polymeric dispersant when necessary, a curable resin, a
thickener, a plasticizer, an
antifungal agent, a surfactant, a non-surfactant type dispersant, a surface
control agent (leveling
agent), and the like can be appropriately blended when necessary. The curable
resin can further
improve adhesion of a coating product to a base material. As the curable
resin, resins of a
dissolved type in a low-polar and non-aqueous solvent, an emulsion type, a
colloidal dispersion
type, and the like can be used without limitation. Moreover, as for the kind
of the curable resin,
known resins such as protein polymers, acrylic resins, polyester resins,
urethane resins, phenol
resins, epoxy resins, and cellulose can be used without limitation. It is
preferable that the
blending amount of the curable resin component is 10 parts by mass or less
with regard to 100
parts by mass of the metallic copper particle, the more preferable range is 8
parts by mass or less,
and a range of 5 parts by mass or less is still more preferable. As the
surfactant, a cationic
surfactant is preferable, and is a compound having surface activity in a
portion showing positive
electric charge by the dissociation in an aqueous solvent. Examples thereof
include (1)
quaternary ammonium salts ((a) aliphatic quaternary ammonium salts (such as
[RN(CH3)3] X",
[RWN(CH3)2]+X-, [RR'R"N(CH3)]+X-, and [RR'R"R"'N]+X- wherein R, R', R", and
R'" represent
a same or different alkyl group and X represents a halogen atom such as Cl,
Br, and I, and the
same shall apply hereinafter), and (b) aromatic quaternary ammonium salts
(such as
[R3N(CH2Ar)]+X- and [RR'N(CH2Ar)2] X- wherein Ar represents an aryl group),
and (c)
heterocyclic quaternary ammonium salts (such as pyridinium salts ([C6H5N-R1+X-
) and
CA 02959294 2017-02-24
W7336
43
imidazolinium salts ([R-CN(CNR'R")C2f14]+X")), and (2) alkylamine salts (such
as RH2NY,
RR'HNY, and RR'R'`NY wherein Y represents an organic acid, an inorganic acid,
or the like), and
one of these may be used, or two or more thereof may be used. Specifically,
the aliphatic
quaternary ammonium salts include octyltrimethylammonium chloride,
stearyltrimethylammonium chloride, cetyltrimethylammonium chloride,
cetyltrimethylammonium bromide, lauryltrimethylammonium chloride,
dioctyldimethylammonium chloride, distearyldimethylammonium chloride,
trioctylmethylammonium chloride, tristearylmethylammonium chloride,
tetraoctylammonium
chloride, and the like. The aromatic quaternary ammonium salts include
decyldimethylbenzylammonium chloride, lauryldimethylbenzylammonium chloride,
stearyldimethylbenzylammonium chloride, benzethonium chloride, and the like.
The
heterocyclic quaternary ammonium salts include cetylpyridinium chloride, an
alkyl
isoquinolinium bromide, and the like. The alkylamine salts include neutralized
products of
octylamine, decylamine, laurylamine, stearylamine, coconut oil amine,
dioctylamine,
distearylamine, trioctylamine, tristearylamine, and dioctylmethylamine
neutralized with an
inorganic acid such as hydrochloric acid, nitric acid, and sulfuric acid, or a
carboxylic acid such
as acetic acid. Alternatively, a neutralized product obtained by reacting a
mercapto carboxylic
acid on the surface of the metallic copper particle and/or a salt thereof with
alkylamine may be
used as the alkylamine salt. Among the quaternary ammonium salts, those having
at least one
alkyl group with a number of carbon atoms of 8 or more or benzyl group are
particularly
preferable, and such quaternary ammonium salts include
stearyltrimethylammonium chloride
(number of carbon atoms of alkyl group: 18), octyltrimethylammonium chloride
(number of
carbon atoms of alkyl group: 8), lauryltrimethylammonium chloride (number of
carbon atoms of
alkyl group: 12), cetyltrimethylammonium chloride (number of carbon atoms of
alkyl group:
16), cetyltrimethylammonium bromide (number of carbon atoms of alkyl group:
16),
tetraoctylammonium bromide (number of carbon atoms of alkyl group: 8),
dimethyltetradecylbenzylammonium chloride (number of carbon atoms of alkyl
group: 14),
distearyldimethylbenzylammonium chloride (number of carbon atoms of alkyl
group: 18),
stearyldimethylbenzylammonium chloride (number of carbon atoms of alkyl group:
18), and
benzalkonium chloride (number of carbon atoms of alkyl group: 12 to 18).
Moreover, among
the alkylamines of the alkylamine salts, those having at least one alkyl group
with a number of
carbon atoms of 8 or more are preferable, and such alkylamines include
octylamine (number of
carbon atoms of alkyl group: 8), laurylamine (number of carbon atoms of alkyl
group: 12),
stearylamine (number of carbon atoms of alkyl group: 18), dioctylamine (number
of carbon
CA 02959294 2017-02-24
W7336
44
atoms of altcyl group: 8), dilaurylamine (number of carbon atoms of alkyl
group: 12),
distearylamine (number of carbon atoms of alkyl group: 18), trioctylamine
(number of carbon
atoms of alkyl group: 8), and trilaurylamine (number of carbon atoms of alkyl
group: 12).
Moreover, the surface control agent controls surface tension of an organic
solvent dispersion
prevent defects such as cissing and craters, and the surface control agents
include acrylic surface
control agents, vinyl surface control agents, silicone surface control agents,
fluorine surface
control agents, and the like. The additive amounts of the surfactant and the
surface control
agent can be appropriately adjusted, and it is preferable that the amount is,
for example, 2.0 parts
by mass or less with regard to 100 parts by mass of the metallic copper
particle, and more
preferably 0.2 parts by mass or less.
Furthermore, in the metallic copper dispersion according to the present
invention,
a fine metal particle other than the metallic copper may be appropriately
blended according to
the purpose of use in a range where the characteristics of the metallic copper
of the present
invention are not obstructed. For example, a fine metal particle such as gold,
silver, nickel, or
tin may be blended in the metallic copper dispersion.
[0065]
The metallic paste according to the present invention includes a metallic
copper
particle, a binder resin, a solvent, and the like as main components, and
appropriately including a
surfactant, a crosslinking agent, a polymer dispersant, and the like blended
therein. The
metallic paste has a characteristic of having a relatively high thixotropy
index (TI) value
measured by the method described above, and specifically, the TI value can be
set to 3.0 or more,
preferably 3.5 or more, and more preferably 4.0 or more. The metallic paste
actually used is
prepared by appropriately blending the metallic copper particle and the like,
and is desirable to
have a certain degree of viscosity in view of a thick film formation, and
generally, a metallic
paste having a viscosity of 2000 mPa-s or higher is preferable.
[0066]
Next, one embodiment according to the present invention is a process for
producing a metallic copper dispersion including: mixing a copper compound and
hypophosphorous acid and/or a salt thereof in a solvent in the presence of
gelatin and/or a
collagen peptide to reduce the copper compound; thereafter performing solid-
liquid separation;
and subsequently mixing and dispersing an obtained metallic copper particle in
an aqueous
solvent and/or an organic solvent. Moreover, preferably, one embodiment
according to the
present invention is a process for producing a metallic copper dispersion
including: reducing a
copper compound in the presence of gelatin and/or a collagen peptide in an
aqueous solvent;
CA 02959294 2017-02-24
W7336
thereafter performing solid-liquid separation; and subsequently mixing and
dispersing: an
= obtained metallic copper particle having the gelatin and/or the collagen
peptide on a surface of
the particle; and a polymeric dispersant in an organic solvent.
[0067]
5 A wet mixer is used for mixing the metallic copper particle and the
aqueous
solvent and/or the organic solvent, and, for example, fixed mixers such as
stirrers, spiral mixers,
ribbon mixers, and fluidizing mixers, rotary mixers such as cylindrical mixers
and twin
cylindrical mixers, wet grinding mills such as sand mills, ball mills, bead
mills, colloid mills, and
sand grinder mills, shakers such as paint shakers, and dispersion machines
such as ultrasonic
10 dispersion machines can be used. After appropriately selecting the mixer
and the like from
among those described above, mixing conditions thereof, mixing time thereof,
and a dispersion
media thereof are appropriately set. In this way, a metallic copper dispersion
including the
metallic copper particle dispersed in the organic solvent is obtained.
Moreover, the metallic
copper particle may be ground before mixing when necessary using a grinding
mill such as a
15 compression grinding type mill, an impact compression grinding type
mill, a shearing grinding
type mill, and a friction grinding type mill. Also, the metallic copper
particle may be mixed at
the same time when the metallic copper particle is ground.
[0068]
Next, a metallic copper-containing film for an electrode, a wiring pattern, a
design
20 or decorative film coating, and the like, which use the metallic copper
dispersion as one
embodiment according to the present invention will be described. The metallic
copper-
containing film is a film in which a metallic copper is fixed on a base
material. In addition, a
metallic copper-containing film in which the metallic copper particle is more
firmly fixed can be
obtained by adding a curable resin to the dispersion. Moreover, by applying
heat to the film
25 coating or irradiating the film coating with light or plasma, the
metallic copper particle is molten
and bonded, and can be fixed still more firmly. In such a metallic copper-
containing film, the
thickness, size, shape, and the like are not limited, the film thickness may
be thin or thick, and
the whole surface of the base material or part thereof may be covered with the
metallic copper-
containing film. Alternatively, the metallic copper-containing film may have a
fine line shape
30 formed on part of the base material, a broad line shape, or a fine dot
shape. As to the specific
uses, the metallic copper-containing film can be used for an electrode and a
wiring pattern by
making use of conductivity of metallic copper, and can be also used for
decoration uses and
antimicrobial uses by making use of tone of color and antimicrobial action of
metallic copper.
Moreover, the metallic dispersion can be used for joining uses.
CA 02959294 2017-02-24
W7336
46
[0069]
A decorative article or an antimicrobial article which are one embodiment
according to the present invention is obtained by forming the metallic copper-
containing film on
at least part of the surface of a base material, and a metal color tone or
antibacterial properties of
the metallic copper particle are given on the surface of the base material of
the decorative article
or the antimicrobial article. The whole surface of the base material can be
colored to give a
metal color tone or antibacterial properties, and additionally, design, a
mark, and a logo mark can
be formed on part of the surface of the base material, or other characters,
figures, and symbols
can be also formed. As the base material, an inorganic material such as metal,
glass, ceramics,
rock, and concrete, an organic material such as rubber, plastics, paper, wood,
leather, fabric, and
fiber, and a material in which the inorganic material and the organic material
are used in
combination or are compounded can be used. The metallic copper-containing film
can be
formed on a raw material before processing the base material having such a
material quality into
an article to be used to give a decoration or antibacterial properties, or in
all articles after
processing the base material, a decoration or antibacterial properties can be
given. In this case,
the case where a decoration or antibacterial properties is given on the
surfaces of articles coated
in advance on the surfaces of these base materials is also included.
Specific examples of articles giving the decoration or antibacterial
properties
include the following:
(1) exterior and interior of transportation such as automobiles, tracks, and
buses, a
bumper, a doorknob, a rearview mirror, a front grille, a reflecting plate of a
lamp, a display
instrument, and the like;
(2) exterior of electric appliances such as a television set, a refrigerator,
a
microwave oven, a personal computer, a mobile phone, and a camera, a remote
control, a touch
panel, a front panel, and the like;
(3) exterior of buildings such as houses, buildings, department stores,
stores,
shopping malls, pachinko parlors, wedding halls, funeral halls, shrines, and
temples, window
glass, an entrance, a doorplate, a gate, a door, a doorknob, a show window,
interior, and the like;
(4) house facilities such as lighting equipment, furniture, furnishings,
toilet
equipment, Buddhist altars and fittings, a Buddha statue, and the like;
(5) utensils such as hardware and tableware;
(6) vending machines of beverage, tobacco, and the like;
(7) containers for synthetic detergents, skin care products, soft drinks,
alcoholic
beverages, confectionery, food products, tobacco, and pharmaceuticals;
CA 02959294 2017-02-24
W7336
47
. , (8) packing materials such as wrapping paper and a carton
box;
. (9) outfits and accessories such as clothes, shoes, bags, glasses,
artificial nails,
artificial hair, and jewels;
(10) sporting goods such as a baseball bat, and a golf club, and products for
hobbies such as fishing tools;
(11) stationery such as pencils, colored paper, notebooks, and postcards for
New
Year's greetings, and business equipment such as desks and chairs; and
(12) covers and bands for books, toys such as dolls and small toy cars, cards
such
as a commuter pass, and recording media such as CDs and DVDs. Moreover, human
nails,
skin, eyebrows, hair, and the like can be used as a base material.
[0070]
Next, one embodiment according to the present invention is a process for
producing a metallic copper-containing film wherein the above metallic copper
dispersion is
used. A step (a) in the production process according to the present invention
is a step of
adhering the metallic copper dispersion on the surface of the base material. A
step (b) is a step
of heating the metallic copper-containing film produced in the above step (a)
under a
nonreducing gas atmosphere or under a reducing gas atmosphere. A step (c) is a
step of
irradiating the whole or partial region of the metallic copper-containing film
with light after the
above step (a). Moreover, a step (d) is a step of irradiating the whole or
partial region of the
metallic copper-containing film with plasma after the step (a). Further, a
step (e) is a step of
removing the metallic copper-containing film in the region not irradiated
after the above step (c)
or (d). Furthermore, a step (f) is a step of transferring the metallic copper-
containing film
obtained through the above steps (a) to (d) on another base material. The
metallic copper-
containing film can be also produced in the above step (a), and the subsequent
steps (b) to (f) are
a step performed when necessary. A firm metallic copper-containing film can be
produced by
performing any one of the steps (b) to (e), and moreover, by performing the
step (f), a metallic
copper-containing film can be simply produced on a base material which is
difficult to directly
form the metallic copper-containing film. Moreover, when an electrode and a
wiring pattern
are produced, it is also possible to perform any combination of steps (b) to
(f) after the step (a).
[0071]
Step (a)
The metallic copper dispersion according to the present invention is adhered
(which is typically expressed by "applied" hereinafter) on the base material.
As for application
of the metallic copper dispersion, a general printing method or transfer
method, such as screen
CA 02959294 2017-02-24
W7336
48
printing, grpvure printing, flexographic printing, ink jet printing, or offset
printing, or a general
application method using a spray, a slit coater, a curtain coater, a bar
coater, a brush, a pen brush,
or a spin coater can be used. The thickness of the coated layer is not
particularly limited, and
can be appropriately selected according to the purpose of use and uses,
however, a thickness of
0.001 to 100 gm is preferable, and a thickness of 0.005 to 70 gm is more
preferable. An
application pattern at this time can be applied on the whole surface of the
base material, and can
be also applied in pattern or figuratus form. According to the application
method, the purpose
of use, and uses, the particle diameter of the metallic copper particle, the
kind of the polymeric
dispersant, the organic solvent, and other compounds can be appropriately
selected. Similarly,
viscosity of the dispersion and the concentration of metallic copper can be
appropriately
selected.
[0072]
As the base material, glasses such as alkali-free glass, quartz glass,
crystallized
transparent glass, Pyrex (which is a registered trade-mark) glass, and
sapphire glass; inorganic
materials such as A1203, MgO, Be0, Zr02, Y203, CaO, and GGG (gadolinium-
gallium-garnet);
acrylic resins such as PET (polyethylene terephthalate), PEN (polyethylene
naphthalate),
polypropylene, polycarbonate, and polymethyl methacrylate; vinyl chloride
resins such as
polyvinyl chloride and vinyl chloride copolymers; organic materials such as
epoxy resins,
polyarylates, polysulfones, polyethersulfones, polyimides, fluororesins,
phenoxy resins,
polyolefin resins, nylons, styrene resins, and ABS resins; and a substrate
formed by using a
composite material in which inorganic particles having a diameter of several
nanometers are
dispersed in the organic material; a silicon wafer; and a metal plate, and the
like can be used.
The base material can be appropriately selected from these materials according
to uses, and used
as a flexible base material in a film form and the like or a rigid base
material. In addition, the
size of the base material is not limited, the shape of the base material may
be any shape such as a
disc shape, a card shape, and a sheet-like shape, and the surface of the base
material does not
need to be planar, and may have depressions and projections, or may have a
curved surface.
[0073]
On the base material, a foundation layer may be provided in order to improve
planarity of the surface of the base material and adhesive strength and in
order to prevent
deterioration of the metallic copper-containing film. Examples of materials
for the foundation
layer include polymer materials such as polymethyl methacrylate, acrylic acid-
methacrylic acid
copolymers, styrene-maleic anhydride copolymers, polyvinyl alcohols, N-
methylolacrylamide,
styrene-vinyltoluene copolymers, chlorosulfonated polyethylenes,
nitrocellulose, polyvinyl
CA 02959294 2017-02-24
W7336
49
chloride, po,lyvinytidene chloride, chlorinated polyolefins, polyesters,
polyimides, vinyl acetate-
= vinyl chloride copolymers, ethylene-vinyl acetate copolymers,
polyethylenes, polypropylenes,
and polycarbonates; thermosetting resins, photocurable or electron beam
curable resins; and
surface modifiers such as coupling agents. As the material of the foundation
layer, materials
having high adhesion between the base material and the metallic copper-
containing film are
preferable. Specifically, thermosetting, photocurable or electron beam curable
resins, and
surface modifiers such as coupling agents (for example, silane coupling
agents, titanate coupling
agents, germanium coupling agents, and aluminum coupling agents), colloidal
silica, and the like
are preferable.
[0074]
The foundation layer can be formed by dissolving or dispersing the above
material in an appropriate solvent to prepare a coating liquid, applying the
coating liquid on the
surface of the base material using a coating method such as spin coating, dip
coating, extrusion
coating, and bar coating. It is preferable that the layer thickness (at the
drying) of the
foundation layer is generally 0.001 to 20 m, and more preferably 0.005 to 10
pm.
[0075]
When necessary, a film after the metallic copper dispersion is applied thereon
may be heated at an appropriate temperature to evaporate and remove (which is
described as
"drying by heating" hereinafter) the organic solvent or the aqueous solvent
(in this case,
depending on the kind thereof, other compounds having low-boiling point are
included) in the
metallic copper-containing film. The temperature for drying by heating can be
appropriately
set, but in order to suppress oxidization of metallic copper, the temperature
of 150 C or less is
preferable, and the temperature of 120 C or less is more preferable. The
heating time can also
be set appropriately. Also, an atmosphere can be appropriately set, and
further, heating can be
performed under a nonreducing gas atmosphere (i.e. inert gas atmosphere (for
example, nitrogen
or argon) or oxygen gas-containing atmosphere (for example, in the air)) or a
reducing gas
atmosphere. Nitrogen gas, argon gas, helium gas, and the like can be used as
an inert gas. In
addition, evaporation and removal of the organic solvent or the like is not
limited to drying by
heating, and a natural drying method or a reduced pressure drying method may
be used. In the
case of reduced pressure drying, it is performed under pressure lower than
atmospheric pressure,
and specifically, the reduced pressure drying may be performed under vacuum
pressure and
ultra-vacuum pressure.
[0076]
Step (Preliminary Step for Step (b))
CA 02959294 2017-02-24
W7336
After the step (a), it is preferable to heat the metallic copper-containing
film at an
appropriate temperature when necessary. By heating, organic compounds included
in the
metallic copper-containing film, such as the gelatin and/or the collagen
peptide, and the
polymeric dispersant can be decomposed and/or vaporized (which is described as
"oxidization
5 firing by heating" hereinafter). It is preferable that the heating is
performed under an oxygen-
containing atmosphere in order to accelerate decomposition and/or vaporization
of the organic
compounds, and more preferably in an oxygen-containing gas stream. It is
preferable that the
concentration of oxygen in the atmosphere is 10 to 10000 ppm because
oxidization of the
metallic copper particle does not progress so fast. The temperature for
oxidization firing by
10 heating can be appropriately set according to the kind of the base
material or the like, and the
temperature of 100 to 500 C is preferable, and the temperature of 120 to 300 C
is more
preferable. The heating time can be also set appropriately, and can be set to,
for example, about
one minute to about 48 hours, and the heating time of about 10 minutes to
about 48 hours is
preferable.
15 [0077]
Step (b)
A copper-containing film is heated at an appropriate temperature under a
nonreducing gas atmosphere (i.e. under inert gas atmosphere or oxygen gas-
containing
atmosphere (for example, in the air)) or under a reducing gas atmosphere
(which is described as
20 "firing by heating" hereinafter). The inert gas atmosphere is
preferable, and nitrogen gas, argon
gas, helium gas, and the like can be used as an inert gas. In the present
step, fusion between the
metallic copper particles formed in the previous step such as "Preliminary
Step for Step (b)" is
made to occur, and, when necessary, a reduction reaction of the copper
compound or the like to
metallic copper is made to occur. This is because the melting point of the
nano-size particle
25 (i.e. the fine metallic copper particle or the small metallic copper
particle) included in the
metallic copper particle according to the present invention becomes lower than
that of a bulk due
to a size effect thereof, and thus this nano size particle is molten even in a
relatively low
temperature range. As a result, electric resistance can be remarkably reduced
and a metal color
tone can be improved through the step in a short time. For example, hydrogen
gas, carbon
30 monoxide gas, and the like can be used as a reducing gas, and the
nitrogen gas including about
0.1 to about 5% of hydrogen gas is preferable in view of safety and
availability. The heating
temperature can be appropriately set according to the kind of the base
material or the like, and
the heating temperature of 50 to 500 C is preferable, the heating temperature
of 80 to 300 C is
more preferable, and a temperature from the heating temperature in the step
(i.e. "Preliminary
CA 02959294 2017-02-24
W7336
51
=Step for Step (b)"),to 300 C is still more preferable. The heating time can
be also set
= appropriately, and can be set to, for example, about one minute to about
48 hours, and the
heating time of about 10 minutes to about 48 hours is preferable. By this
heating step, a
volume resistance value of the obtained metallic copper-containing film can be
made at 1 x 10-2
0-cm or less, preferably 1 x 10-30-cm or less, more preferably 1 x 104 Q=cm or
less, and still
more preferably 1 x i0 cmor less.
[0078]
The step for evaporation and removal of the organic solvent, which is
performed
when necessary, the step for the oxidization firing by heating (i.e.
"Preliminary Step for Step
(b)"), and the step for the firing by heating (i.e. "Step (b)") may be
performed separately, or may
be performed continuously. Moreover, these steps are not limited to the case
of performing the
step for the oxidization firing by heating after the drying by heating, and
the step for the
oxidization firing by heating can be performed after a natural drying or
reduced pressure drying
is performed without performing the drying by heating, or the organic solvent
can be evaporated
and removed in the step for the oxidization firing by heating, which also
serves as the step for the
drying by heating, and these steps do not need to be clearly distinguished.
[0079]
Step (c)
The whole or partial region of the metallic copper-containing film produced in
the
step (a) is irradiated with light. The light may be infrared rays, visible
rays, ultraviolet rays, X-
rays (soft X-rays to hard X-rays), a laser beam that radiates by amplifying
light, or sunlight. A
pattern is drawn on the base material by moving a light source or the base
material while
irradiating the metallic copper-containing film with the light. A pattern can
be also drawn on
the base material by converging a laser beam oscillated with a laser
oscillator, setting a diameter
of irradiation appropriately, and moving a laser mount section or the base
material while
irradiating the metallic copper-containing film with the laser beam. The light
is absorbed by the
metallic copper-containing film, and along with the decomposition and/or
vaporization of the
organic compounds such as the gelatin and/or the collagen peptide and the
polymeric dispersant
by the heat generated thereby, the fusion between the metallic copper
particles occurs, and thus
the reduction of electric resistance of an irradiated portion of the metallic
copper-containing film
and the improvement of a metal color tone thereof can be provided. The nano
size particle (the
fine metallic copper particle or the small metallic copper particle) according
to the present
invention has the melting point lower than the melting point of a bulk due to
a size effect thereof,
and thus the pattern can be drawn with a relatively low energy and at a high
speed.
CA 02959294 2017-02-24
W7336
52
,[0080] ,
According to kinds and blending amounts of the gelatin and/or the collagen
peptide, the polymeric dispersant, the complexing agent and the like which are
used, a
wavelength of the light can be arbitrarily selected in a range where the
metallic copper-
containing film can absorb the light, and the light with a wavelength in an
ultraviolet region, a
visible light region, an infrared region, or the like is preferable because it
is easy to use. Light
sources that emit incandescent light, discharge light, electroluminescence, or
the like can be used
as the light source, and an incandescent lamp, light sources that make use of
luminescence by
discharge such as an infrared lamp, a visible light lamp, an ultraviolet lamp,
a mercury lamp, and
xenon lamp, semiconductor devices (e.g. light emitting diodes) and the like
that emit light when
a voltage is applied, such as LED can be used as the light source. Typical
lasers include:
semiconductor lasers using GaN, GaAsAl, InGaAsP, or the like; excimer lasers
using ArF, KrF,
XeCl, or the like; dye lasers using rhodamine, or the like; gas lasers using
He-Ne, He-Cd, CO2,
Ar ion, or the like; free electron lasers; solid state lasers such as ruby
lasers and Nd: YAG lasers;
and so on. Moreover, a higher order harmonic wave such as a second harmonic
wave and third
harmonic wave of these lasers may be also used, and a laser beam at any
wavelength in the
ultraviolet region, the visible light region, and the infrared region can be
used. Further,
irradiation of a continuous wave or irradiation of a pulse wave may be used.
Conditions on
applied energy such as a diameter of irradiation of the light, a scan speed,
and an output can be
appropriately set in a range in which oxidization of metallic copper, and
ablation and peening of
the metallic copper-containing film do not occur. The diameter of irradiation
can be
appropriately set in accordance with a pattern or figure to be drawn, and the
diameter of
irradiation of 10 lam to 5 mm is suitable. The scan speed can be also set
appropriately
according to other parameters, required accuracy, manufacturing capacity, and
the like.
[0081]
An atmosphere performing light irradiation such as an inert gas atmosphere, a
reducing gas atmosphere, and an oxygen gas-containing atmosphere (e.g. air
atmosphere) can be
appropriately set. By using the metallic copper dispersion according to the
present invention, a
metallic copper-containing film having a low resistance and a good metal color
tone can be
formed without causing the oxidation of copper in the metallic copper-
containing film even
under the oxygen gas-containing atmosphere (e.g. air atmosphere), which is
expected to be
attributed to the presence of the gelatin. Specifically, this can be achieved
by irradiation with a
continuous wave laser beam having a wavelength in the infrared region at a
scan speed of 1 to
500 mm/s and at an output range of 1 to 140 W under the oxygen gas-containing
atmosphere
CA 02959294 2017-02-24
W7336
53
(e.g. air atmosphere). At this time, conditions on laser irradiation are
adjusted so that main
= peak strength in a Cu20 (111) plane may be 20 or less when main peak
strength in a metallic
copper (111) plane is assumed to be 100 in X-ray diffraction of the metallic
copper-containing
film at a portion irradiated with the laser beam. It is more preferable to set
an output of the
laser beam to be 10 to 100 W, and an output of the laser beam in a range of 20
to 50W is still
more preferable. The semiconductor lasers are preferable because the
semiconductor lasers are
generally suitable for irradiation with a continuous laser beam having a
wavelength in the
infrared region.
[0082]
Step (d)
Next, the whole or partial region of the metallic copper-containing film
produced
in the step (a) is irradiated with plasma to produce a metallic copper-
containing film. In this
step, organic compounds included in the metallic copper-containing film, such
as the gelatin
and/or the collagen peptide, and the polymeric dispersant are decomposed or
vaporized, and
fusion of metallic copper particles is made to occur. Plasma irradiation can
be appropriately
selected from among publicly known methods. For example, a metallic copper-
containing film
is placed in a plasma treatment apparatus, a gas is introduced, and energy is
applied to ionize the
gas to be in a plasma state. Excitation energy that is supplied to the gas is,
for example, electric
discharge, direct current, radio frequency, microwave, or electromagnetic
radiation. Moreover,
in general, plasma can be also generated by applying voltage between two
electrodes to form an
electric field. Gases suitable for plasma treatment include helium, argon,
hydrogen, nitrogen,
air, nitrous oxide, ammonia, carbon dioxide, oxygen, and the like, and the
oxygen gas, the
hydrogen gas, a mixed gas of oxygen and helium or argon, and a mixed gas of
hydrogen and
helium or argon are more preferable. The plasma treatment can be performed
under
atmospheric conditions, or the plasma treatment may be performed in an
apparatus capable of
retaining plasma under a reduced pressure or a vacuum condition. It is
preferable that the
pressure is in a range of about 10 mTorr to about 760 Torr (about 1.333 to
about 101325 Pa).
[0083]
Specifically, the plasma treatment can be performed as described in the
following
example. First of all, the metallic copper-containing film is placed in a
plasma treatment
apparatus, and the base material is heated in the atmospheric air when
necessary. The heating
temperature can be set according to the material quality of the base material,
and the heating
temperature is preferably 180 C or less when a plastic having a low heat
resistance is used, and
more preferably 120 C or less. As the lower limit value of the heating
temperature, a
CA 02959294 2017-02-24
V1/477336
54
temperature, of about 20 C is practical. Next, it is preferable that heating
be performed under a
= reduced pressure or a vacuum condition, and the heating temperature is
preferably 180 C or less,
and still more preferably 120 C or less. The heating time can be appropriately
set. And a gas
is introduced in the plasma treatment apparatus to generate plasma while
heating is continuously
performed, and the whole or partial region of the metallic copper-containing
film is irradiated
with its plasma. It is preferable that microwave energy having a frequency of
2450 MHz is
supplied to generate microwave surface wave plasma. When a partial region is
irradiated with
plasma, the other region can be protected so as not to be irradiated with
plasma by putting a
mask pattern on the metallic copper-containing film. The plasma irradiation
time can be
appropriately set, and is, for example, about 0.01 to about 30 minutes, and a
plasma irradiation
time of about 0.01 to about 10 minutes is suitable. The plasma irradiation can
be also
performed in two stages. In the first step thereof, the metallic copper-
containing film is
irradiated with plasma in the presence of oxygen gas to decompose an organic
compound such as
the gelatin, and thereafter in the second step thereof, the metallic copper-
containing film is
irradiated in the presence of a reducing gas, thereby making it possible to
sinter the metallic
copper particle.
[0084]
Step (e)
Further, an unnecessary portion of the metallic copper-containing film, a
portion
of the metallic copper-containing film, not irradiated with the light in the
above step (c), or a
portion of the metallic copper-containing film, not irradiated with the plasma
in the above step
(d) may be removed using an appropriate solvent when necessary. As the
solvent, various
solvents such as alcohol solvents, glycol ether solvents, and aromatic
solvents can be used. The
unnecessary portion or the like can be removed by immersing the base material
in such a solvent
or wiping off the portion with fabric or paper dipped in the solvent.
[0085]
Step (f)
Next, the whole or partial region of the metallic copper-containing film
produced
on the base material can be also transferred on another base material after
the step (a), the step
(b), the step (c), the step (d), or the step (e).
[0086]
In addition, the steps (b) to (e) after the step (a) can be arbitrarily
combined and
performed. For example, the step (b) can be performed after the step (a), and
the step (c) can
further be performed. Also, the step (c), the step (d), or the step (e) can be
performed after the
CA 02959294 2017-02-24
W7336
step (a), and the step (b) can further be performed. Moreover, in the step
(b), only the
"Preliminary Step for Step (b)" of the step (b) or only the step (b) can be
combined and
performed. For example, the step (c) can be performed after the step (a), and
the step (b) can
further be performed.
5 [0087]
It is preferable that the whole of the metallic copper-containing film
produced by
any one of (a) to (f) in the present invention is sintered because the
resistance value is low.
Thus, it is preferable to perform heating, light irradiation, or plasma
irradiation with sufficient
time and strength for sintering the whole of the metallic copper-containing
film. However, only
10 the surface portion of the metallic copper-containing film may be
sintered and the inside thereof
may not be sintered, and there is no problem even when only part of the
surface is sintered, as
long as the performance of the resistance value or the like, necessary for
uses can be obtained.
The volume resistance value of the metallic copper-containing film is
preferably 50 Ø- cm or
less, more preferably 20 pf/-cm or less, and still more preferably 10 p_Q=cm
or less. The
15 thickness, size, shape, and the like of such a metallic copper-
containing film are not limited, and
the metallic copper-containing film may be a thin film or a thick film, and
the film may cover the
whole or part of the base material. Alternatively, the metallic copper-
containing film may have
a fine wire-like shape or wide wire-like shape formed on part of the base
material, or may have a
fine dotted shape. It is preferable that the thickness be, for example, 1 pm
or less, more
20 preferably 0.5 pm or less. As specific uses, the metallic copper-
containing film can be used for
an electrode and a wiring pattern, for joining chips and substrates, and for
other uses making use
of the electrical conductivity of metallic copper, and can be also used for
decoration uses and
antibacterial uses making use of color tone or antibacterial properties of
metallic copper.
25 Examples
[0088]
Hereinafter, the present invention will be described in more detail giving
Examples, however the present invention is not limited to these Examples.
[0089]
30 Example 1
To 150 ml of pure water, 24 g of industrial copper(II) oxide (N-120
manufactured
by NC-Tech Co., Ltd.) and 9.55 g of gelatin (amine value of 23, acid value of
29, amine value -
acid value = -6, and mass average molecular weight of 200000) as protective
colloid were added
and mixed, and the temperature of the mixed solution was raised to 80 C. After
the
CA 02959294 2017-02-24
W7336
56
temperature, was ra,ised, a solution prepared by mixing 1.2 g of aminoethanol
as a complexing
- agent and 99 g of 50% hypophosphorous acid in 150 ml of pure water was
added to the mixed
solution under stirring, the resultant mixture was reacted with copper oxide
for one hour, and
then the reaction solution was subjected to aging for two hours to produce a
copper particle
coated with the gelatin. Thereafter, the copper particle was subjected to
filtration and washing
until a specific conductivity of a filtrate reached 100 ttS/cm or less, and
dried for 10 hours at a
temperature of 60 C under an atmosphere of nitrogen gas to obtain a metallic
copper particle
coated with the gelatin (sample A).
[0090]
Examples 2 to 5
Metallic copper particles (samples B to E) according to the present invention
were
obtained in the same manner as in Example 1 except that the amount of the
gelatin in Example 1
was changed to the amounts described in Table 1.
[0091]
Examples 6 to 7
Metallic copper particles (samples F to G) according to the present invention
were
obtained in the same manner as in Example 1 except that the reaction
temperature set at 80 C in
Example 1 was changed to 60 C or 70 C.
[0092]
Example 8
A metallic copper particle (sample H) according to the present invention was
obtained in the same manner as in Example 1 except that the aminoethanol in
Example 1 was not
added.
[0093]
Example 9
A metallic copper particle (sample I) according to the present invention was
obtained in the same manner as in Example 1 except that the aminoethanol in
Example 1 was
added in an amount of 4.86 g.
[0094]
Example 10
A metallic copper particle (sample J) according to the present invention was
obtained in the same manner as in Example 1 except that the gelatin in Example
1 had a mass
average molecular weight of 10000.
[0095]
CA 02959294 2017-02-24
W7336
57
,Example 11,
= A metallic copper particle (sample K) according to the present invention
was
obtained in the same manner as in Example 1 except that the gelatin in Example
1 was 19.11 g of
gelatin having a mass average molecular weight of 10000.
[0096]
Example 12
A metallic copper particle (sample L) according to the present invention was
obtained in the same manner as in Example 1 except that a collagen peptide
having a mass
average molecular weight of 5000 was further used.
[0097]
Example 13
A metallic copper particle (sample M) according to the present invention was
obtained in the same manner as in Example 1 except that 19.11 g of a collagen
peptide having a
mass average molecular weight of 5000 was further used.
[0098]
Examples 14 to 15
Metallic copper particles (samples N to 0) according to the present invention
were obtained in the same manner as in Example 7 except that the aging time in
Example 7 was
changed to one hour or three hours.
[0099]
Examples 16 to 18
Metallic copper particles (samples P to R) according to the present invention
were
obtained in the same manner as in Example 7 except that to the mixed solution
of industrial
copper (II) oxide, gelatin, and pure water in Example 7, citric acid, formic
acid, or lactic acid
was further added as an organic acid.
[0100]
Examples 19 to 20
Metallic copper particles (sample S to T) according to the present invention
were
obtained in the same manner as in Example 1 except that the time for adding
hypophosphorous
acid in Example 1 was changed to two hours or three hours.
[0101]
Examples 21 to 24
Metallic copper particles (samples U to X) according to the present invention
were obtained in the same manner as in Example 17 except that the amount of
the gelatin in
CA 02959294 2017-02-24
W7336
58
Example 17, was clanged.
[0102]
Examples 25 to 27
Metallic copper particles (samples Y to AA) according to the present invention
were obtained in the same manner as in Example 17 except that the amount of
the organic acid in
Example 17 was changed to the amounts described in Table 1.
[0103]
Example 28
A metallic copper particle (sample AB) according to the present invention was
obtained in the same manner as in Example 17 except that the reaction
temperature in Example
17 was changed to 40 C.
[0104]
Example 29
A metallic copper particle (sample AC) according to the present invention was
obtained in the same manner as in Example 17 except that the aminoethanol in
Example 17 was
not added.
[0105]
Example 30
A metallic copper particle (sample AD) according to the present invention was
obtained in the same manner as in Example 29 except that 9.62 g of
pyrophosphoric acid was
added as a pH-adjusting agent to the mixed solution of industrial copper (II)
oxide, gelatin, and
pure water in Example 29.
[0106]
Comparative Example 1
A metallic copper particle (sample AE) was obtained in the same manner as in
Example 1 except that the gelatin in Example 1 was not used.
[0107]
Comparative Example 2
To 350 ml of pure water, 24 g of industrial copper (II) oxide (N-120:
manufactured by NC-Tech Co., Ltd.) and 9.55 g of gelatin (amine value of 23,
acid value of 29,
amine value - acid value ---- -6, and mass average molecular weight of
200,000) as protective
colloid were added and mixed, and after the pH of the mixed solution was
adjusted at 9 using
15% ammonia water, the temperature of the mixed solution was raised from room
temperature to
90 C in 30 minutes. After the temperature was raised, a solution prepared by
mixing 1.2 g of
CA 02959294 2017-02-24
W7336
59
an aminoethanol solution and 38 g of 80% hydrazine monohydrate to 15 ml of
pure water was
added to the mixed solution in 60 minutes under stirring, and the resultant
mixture was reacted
with the copper (II) oxide for one hour to produce a copper particle. After
producing the fine
copper particle, 5 mL of a serine protease (Ptoteinase K: manufactured by
Worthington
Biochemical Corporation) was added as a protective colloid remover, and the
resultant mixture
was held for one hour. Thereafter, the mixture was subjected to filtration and
washing until a
specific conductivity of a filtrate reached 100 laS/cm or less, and dried for
10 hours at a
temperature of 60 C under an atmosphere of nitrogen gas to obtain a metallic
copper particle
(sample AF).
[0108]
Comparative Example 3
A metallic copper particle having a flat shape (sample AG) was obtained by
mixing and suspending 10 g of the copper particle which is coated with the
gelatin and which has
an average particle diameter of 500 nm, synthesized in Comparative Example 2,
30 g of ethanol,
and 50 g of zircon beads; shaking the suspension with a paint shaker for three
hours;
subsequently separating and removing the beads; and then filtrating the
resultant.
[0109]
Comparative Example 4
A metallic copper particle (sample AH) was obtained in the same manner as in
Example 1 except that the copper oxide in Example 1 was changed to copper
sulfate.
[0110] Production conditions described above are listed together in
Table 1. Moreover,
the pH before adding a reducing agent and the pH after aging are shown in
Table 2 for some
samples.
[0111]
CA 02959294 2017-02-24
W7336
[Table 1]
,
. Amount of
Amount
Mass average
reducing agent Reaction
of gelatin Kind of Amount of Kind of Amount
molecular (g)
Sample weight of
complexing completing
organic of organic (50 temperature
gelatin (g) agent agent acid
and
Hypophos phorous [C]
(8)
(g) acid)
Example 1 A 200000 9.55 Aminoethanol 1.2 Not added 0 99
80
Example 2 B 200000 4.78 Aminoethanol 1.2 Not added 0 99
80
Example 3 C 200000 5.73 Aminoethanol 1.2 Not added 0 99
80
Example 4 D 200000 14.33 Aminoethanol 1.2 Not added 0 99
80
Example 5 E 200000 19.11 Aminoethanol 1.2 Not added 0 99
80
Example 6 F 200000 9.55 Aminoethanol 1.2 Not added 0 99
60
Example 7 G 200000 9.55 Aminoethanol 1.2 Not added 0 99
70
Example 8 H 200000 9.55 Not added 0 ,Not added 0 99
80
Example 9 I 200000 9.55 Aminoethanol 4.86 Not added 0 99
80 ,
Example 10 J 10000 9.55 Aminoethanol 1.2 Not added 0 99
80 ,
_
Example 11 K 10000 19.11 Aminoethanol 1.2 Not added 0 99
80
Example 12 L 5000 9.55 Aminoethanol 1.2 Not added 0 99
80
Example 13 M 5000 19.11 Aminoethanol 1.2 Not added 0 99
80
Example 14 N 200000 9.55 Aminoethanol 1.2 Not added 0 , 99
70
Example 15 0 200000 9.55 Aminoethanol 1,2 Not added 0 99
70
Example 16 P 200000 9.55 Aminoethanol 1.2 Citric acid 11.5 ,
99 70
Example 17 Q 200000 9.55 Aminoethanol 1.2 Formic acid 3.1
99 70
Example 18 R 200000 9.55 Aminoethanol 1.2 ,Lactic acid 5.4
99 70
Example 19 S 200000 9.55 Aminoethanol 1.2 Not added 0 99
80 .
Example 20 T 200000 9.55 Aminoethanol 1.2 Not added 0 99
80
,
Example 21 U 200000 1.91 Aminoethanol 1.2 Formic acid 3.1
99 70
Example 22 V 200000 3.82 Aminoethanol 1.2 Formic acid 3.1
99 70
Example 23 W 200000 5.73 Aminoethanol 1.2 Formic acid 3.1
99 70
Example 24 X 200000 7.64 , Aminoethanol 1.2 Formic acid 3.1
99 70
Example 25 Y 200000 9.55 Aminoethanol 1.2 Formic acid 1.6
99 70
Example 26 Z 200000 9.55 Aminoethanol 1.2 Formic acid 4.8
99 70
Example 27 AA 200000 9.55 Aminoethanol 1.2 Formic acid 6.2
99 70
Example 28 AB 200000 9.55 Aminoethanol 1.2 Formic acid 3.1
99 40
Example 29, AC 200000 9.55 Not added 0 Formic acid 3.1 99
70
Example 30 AD 200000 9.55 Not added 0 Formic acid 3.1 99
70
, .
Comparative ,, .,..,
Example 1 11-c' - 0 Aminoethanol 1.2 Not added 0 99
80
Comparative 38g of
Example 2 AF 200000 9.55 Aminoethanol 1.2 Not added 0 Hydrazine
90
Comparative
AH 200000 9.55 Aminoethanol 1.2 Not added 0 99
80
Example 4
[0112]
CA 02959294 2017-02-24
W7336
61
[Table 2] ,
Sample Initial pH pH after aging
Example 1 A 8.4 0.8
Example 16 F 3.5 0.7
Example 17 Q 3.4 0.7
Example 18 R 3.5 0.7
Example 25 Y 3.7 0.7
Example 26 Z 3.0 0.6
Example 27 AA 2.8 0.6
Example 30 AD 1.4 0.6
Comparative
AE 8.6 0.8
Example 1
Comparative
AF 9.0 9.8
Example 2
[0113]
As a result of X-ray diffraction of the samples (A to AH) obtained in the
Examples and the Comparative Examples, peaks of metallic copper were confirmed
for all the
samples, and thus it was found that all the samples were metallic copper. Fig.
1 shows an X-ray
diffraction chart of sample A. Moreover, the specific surface areas (according
to nitrogen
adsorption BET method) and the amounts of phosphorus (according to XRF
analysis) included in
these samples are shown in Table 3. It was found that the samples of the
Examples include
phosphorus in an amount of about 0.2 to about 0.4% by mass. Further, it was
found from these
electron micrographs that in the samples of the Examples, fine metallic copper
particles were
adhered on the surface of a large diameter metallic copper particle, and
partially aggregated fine
metallic copper particles were adhered on the surface of the large diameter
metallic copper
particle. Furthermore, it was also found that the metallic copper particles
(i.e. composite
particles) and the small metallic copper particles coexisted. On the other
hand, it was found
that in the samples of the Comparative Examples, particles having one kind of
shape and almost
uniform size existed. As one example, in Fig. 2 to Fig. 35, electron
micrograph (SEM
photograph) of each of the samples (A to M, Z, AE, AF, AND AG) is shown.
Moreover, the
primary particle diameters of the samples (A to AH) are shown in Table 3.
[0114]
CA 02959294 2017-02-24
W7336
62
[Table 3]
, ' .
.
Specific surface area Amount of P Average primary particle Average primary
particle
Sample
diameter of fine particles diameter of large diameter
[m2/g] [% by mass] [nm] particles [it
m]
Example 1 A 1.9 0.26 128 15.9
Example 2 8 1.9 0.27 47 13.2
Example 3 C 1.1 0.26 103 13.5
Example 4 D 1.7 0.25 154 15.1
Example 5 E 1.9 0.26 70 12.3
Example 6 F 5.3 0.32 88 4.1
Example 7 G 2.7 0.26 114 9.8
Example 8 H 1.8 0.24 111 9.8
Example 9 1 1.8 0.25 121 9.8
Example 10 3 1.5 0.25 123 2.3
Example 11 K 1.5 0.25 148 2.3
Example 12 L 1.2 0.25 161 6.3
Example 13 M 2.0 0.25 93 12.1
Example 14 N 4.2 0.21 179 6.26
Example 15 0 3.8 0.21 134 11.02
Example 16 P 3.2 0.25 45 3.7
Example 17 Q 3.3 0.24 186 4.5
Example 18 R 3.4 0.23 134 5.5
Example 19 S 3.2 0.25 125 5.5
Example 20 T 3.1 0.25 143 6.2
Example 21 U 2.9 0.21 164 16.9
Example 22 V 2.7 0.25 173 14.5
Example 23 W 3.8 0.25 202 9.1
Example 24 X 3.7 0.26 211 10.4
Example 25 Y 3.1 0.28 173 10.0
Example 26 Z 3.0 0.26 203 9.4
Example 27 AA 3.2 0.24 155 18.2
Example 28 AB 5.9 0.25 149 2.2
Example 29 AC 3.5 , 0.25 140 15.2
Example 30 AD 3.5 0.30 60 10.2
Comparative
AE 0.5 0.24 Not exist 3.8
Example 1
Comparative
Example 2 AF 1.5 - Not exist 0.5
Comparative
Example 3 AG 2.5 - Not exist 2.4
Comparative
AH 3.1 0.25 129 Not exist
Example 4
[0115]
The CHN analysis was performed for the metallic copper powders of samples N
and Q to estimate the amounts of gelatin and of formic acid. Specifically,
the amount of gelatin
was calculated from the ratio of CHIN components in the gelatin, and the
residual organic content
CA 02959294 2017-02-24
W7336
63
was estimated as the amount of formic acid and the like. The results are shown
in Table 4. In
. the sample Q in which formic acid was added, the organic content
originating in formic acid and
the like was large, and it is suggested that formic acid is adsorbed on the
surface. The CHN
analysis was performed using Vario III CHN Elemental Analyzer manufactured by
Elementar
Analysensysteme GmbH, capable of analyzing the amount of C, H, and N with a
TCD (Thermal
conductivity detector) by burning and gasifying an organic component on the
surface of each
powder and separating the gas with a column.
[0116]
[Table 4]
Gelatin [wt%] Formic acid and the like [vt%] Total amount
of CHN [wt%]
Sample
C H N C H N C H N
N 0.50 0.08 0.18 0.01 0.00 0.00 0.51 0.08
0.18
Q 0.37 0.06 0.13 0.14 0.02 0.00 0.51 0.08
0.13
[0117]
Production 1 of Metallic Copper-Containing Film (Heating at 300 C under
Nitrogen
Atmosphere)
Copper pastes were prepared by mixing 10 g of each of the samples (A to AG)
obtained in the Examples and the Comparative Examples, 3.5 g of a vehicle
(resin: 20% by mass
of ethyl cellulose N200 and solvent: terpineol), and 6.5 g of terpineol, and
then kneading the
resultant mixture with a three-roll mill. Each of the prepared copper pastes
was applied on an
alumina substrate with an applicator and fired using an atmosphere tube
furnace at 300 C for one
hour under a nitrogen atmosphere to prepare metallic copper-containing films.
The specific
resistance values of the obtained metallic copper-containing films were
measured using MCP-
T610 Loresta GP manufactured by Mitsubishi Chemical Analytech Co., Ltd. by a
direct current
four-terminal method. Thereafter, the cross sections were observed with a
scanning electron
microscope to measure the film thicknesses, and the volume resistance values
were calculated.
The results are shown in Table 5. The volume resistance values are 1 x 10-2
SYcm or less in all
the samples of the Examples. And, it is presumed that the existence state, the
ratio, the particle
diameter, the aggregation state, and the like of the fine metallic copper
particles and the large
diameter metallic copper particle give an influence on the results. Moreover,
it is presumed that
the existence state, the ratio, the particle diameter, the aggregation state,
and the like of the
metallic copper particle (i.e. composite particle) and the small metallic
copper particles give an
influence on the results. Alternatively, it is presumed that because formic
acid existing on the
surface is easy to disappear at low temperatures, the sintering was
facilitated. On the other
CA 02959294 2017-02-24
W7336
64
hand, in all the samples of the Comparative Examples, the volume resistance
values were 1 x 102
, 12=cm or more.
[0118]
[Table 5]
Volume resistance value Film thickness
Sample
[CI =cm] , [ ii In]
Example 1 A 9.20E-05 10.1
Example 2 B 2.40E-03 11,7
Example 3 C 5.70E-04 11.1
Example 4 D 4.40E-04 17.1
Example 5 E 1.00E-04 7.4
Example 6 F 2.10E-04 11.8
Example 7 G 1.50E-04 9.5
Example 8 H 6.60E-03 10.1
Example 9 I 1.60E-03 15.5
Example 10 J 6.10E-03 9.9
Example 11 K 7.40E-03 10.5
Example 12 L 3.70E-03 , 12.4
Example 13 M 3.90E-03 11.2
Example 14 N 4.50E-05 12.0
Example 15 0 6.52E-05 11.1
Example 16 P 1.10E-04 10.5
Example 17 Q 5.00E-05 11.3
Example 18 R 5.80E-05 11.8
Example 19 S 1.90E-03 10.5
Example 20 T 1.10E-04 , 8.5
,
Example 21 U 2.20E-05 9.8
Example 22 V 3.50E-05 11.6
Example 23 W 4.20E-05 11.4
Example 24 X 2.30E-05 11.5
Example 25 Y 1.10E-04 10.8
Example 26 Z 3.60E-05 10.2
Example 27 AA 5.10E-05 11.2
Example 28 AB 1.20E-04 11.0
Example 29 AC 8.00E-05 10.9
Example 30 AD 6.55E-05 11.2
Comparative
Example 1 AE 8.60E+04 14.6
Comparative
Example 2 AF 1.10E+04 15.1
Comparative
Example 3 AG 5.50E+02 11.5
[0119]
Next, metallic copper-containing films were prepared in the same manner as
CA 02959294 2017-02-24
W7336
',Production .1 of Metallic Copper-Containing Film" described above, except
that the metallic
. copper particles each of which was prepared by mixing the sample W and
the sample X in a ratio
as shown in Table 6 were used, and the volume resistance values were measured
for the metallic
copper-containing films. The results are shown in Table 6. The volume
resistance values can
5 be further reduced by mixing the sample W and the sample X in a manner as
described in the
Table and preparing pastes thereof. The similar effect can be also expected by
mixing the
metallic copper particle according to the present invention and a commercially
available copper
powder.
[0120]
10 [Table 6]
Sample WEgi Sample X[g] Volume resistance value [L1 -cm]
Example 31 5 0 5.10E-05
Example 32 4 1 2.15E-05
Example 33 3 2 2.20E-05
Example 34 2 3 5.30E-05
Example 35 1 4 6.40E-05
Example 36 0 5 1.20E-04
[0121]
Production 2 of Metallic Copper-Containing Film (Sintering with Plasma)
Copper pastes were prepared using sample A obtained in the Examples and
15 sample AF obtained in the Comparative Examples according to the above
method. Each copper
paste was applied on a PET film with an applicator to prepare each metallic
copper-containing
film. Thereafter, plasma treatment was performed using Micro Labo-PS
manufactured by
Nissin Inc. under the following condition, and thereby each metallic copper-
sintered film was
obtained.
20 First of all, the metallic copper-containing film was placed on a
stage heated at
100 C in the plasma apparatus to perform heating at a predetermined time of
180 seconds or 30
seconds. Thereafter, the pressure inside the apparatus was reduced for 60
seconds, 3% H2-He
gas was introduced to the apparatus for 30 seconds, and plasma irradiation was
performed for
180 seconds. After performing the plasma treatment, cooling was performed by
purging N2 gas
25 for 90 seconds to obtain a metallic copper-sintered film (film thickness
of 10 um). The results
are shown in Table 7. It has been found that a metallic copper-containing film
having a low
resistance can be produced, even through plasma treatment is performed, by the
use of the
metallic copper particle according to the present invention.
[0122]
CA 02959294 2017-02-24
W7336
66
s[Table 7]
Sample Treated base material Heater temperature Volume resistance value [EI
.cm]
Example 1 A PET 100 C 2.26E-04
Comparative
PET 100 C 0.L.
Example 2
* In the table, 0.L. represents a value equal to or more than the upper limit
of measurement
of the measurement apparatus. The value is roughly 1 X 10+4 -cm or more, while
it depends
on the film thickness.
[0123]
Production 3 of Metallic Copper-Containing Film (Heating at 120 C in the Air)
Copper pastes were prepared by mixing 5 g of each of the samples (A to AH)
obtained in the Examples and the Comparative Examples, a phenol resin (0.62 g
of Resitop: PL-
5208 (containing 59% by weight of phenol resin as an active ingredient)), and
0.26 g of ethylene
glycol monobutyl ether acetate using a deaerating stirrer, and then kneading
the resultant mixture
with a three-roll mill. Each of the prepared copper pastes was applied on an
alumina substrate
with an applicator, and fired at 120 C for 10 minutes in a natural convection
type drier to prepare
metallic copper-containing films each of which has a film thickness of about
25 gm. The
specific resistance values of the obtained metallic copper-containing films
were measured using
MCP-T610 Loresta GP manufactured by Mitsubishi Chemical Analytech Co., Ltd. by
a direct
current four-terminal method. Thereafter, the cross sections were observed
with a scanning
electron microscope to measure the film thicknesses, and the volume resistance
values were
calculated based on the specific resistance values. The results are shown in
Table 8. From the
fact that low volume resistance values are obtained by firing at a low
temperature of 120 C, it
can be said that the material according to the present invention is excellent
in sinterability at a
low temperature. Moreover, Figs. 36 and 37 show an SEM image of the cross
section in the
metallic copper-containing film of the sample Q produced in Example 17. It was
confirmed
from these SEM images that the metallic copper particles according to the
present invention are
sintered by firing at a low temperature of 120 C in the air.
[0124]
CA 02959294 2017-02-24
W7336
67
[Table 8] =
Volume resistance value
Sample
[ = cm]
Example 1 A 1.90E-02
Example 2 B 5.00E-02
Example 3 C 8.20E-02 ,
Example 4 D 7.50E-02
Example 5 E 9.80E-02
Example 7 G 1.30E-01
Example 8 H 2.50E-01
Example 9 I 2.50E-01
Example 10 J 3.10E-01
Example 11 K 9.50E-02
Example 12 L 9.20E-02
Example 13 M 8.30E-02
Example 14 N 4.40E+01
Example 15 0 5.90E-02
Example 16 P 4.80E-02
Example 17 Q 2.10E-03
Example 18 R 3.70E-01
Example 19 S 1.60E-01
Example 20 T 2.80E+00
Example 21 U 6.70E-02
Example 22 V 7.40E-03
Example 23 W 5.10E-03
Example 24 X 2,00E-03
Example 25 Y 2.00E-03
Example 26 Z 3.10E-03
Example 27 AA 5.00E-03
Example 29 AC 8.05E-03
Example 30 AD 1.20E-02
Comparative
AE 0.L.
Example 1
Comparative
Example 2
Comparative
AG 0.L.
Example 3
Comparative
AH 0.L.
Example 4
* In the table, O.L. represents a value equal to or more than the upper limit
of
measurement of the measurement apparatus. The value is roughly 1 X 10'4 -cm
or more, while it depends on the film thickness.
[0125]
Production of Metallic Paste
Metallic pastes (Cu solid content of 75% by mass) were prepared by mixing 9
g
of each of the samples (A, C, E, J, N, Q, AB, AF, AG) obtained in the Examples
and the
CA 02959294 2017-02-24
W7336
68
Comparative Examples, 1 g of a vehicle (resin: 20% by mass of ethyl cellulose
N200 and
solvent: terpineol), and 2 g of terpineol and kneading the resultant mixture
with a three-roll mill.
The viscosity of the metallic paste was measured for each paste produced with
a B type
viscometer (model HB DV-I+) manufactured by Brookfield AMETEK setting the
measurement
temperature at 20 C and using CPE-52 as a corn spindle. The viscosity (ria) at
a low shear rate
(10 [1/sec]) and the viscosity (rib) at a high shear rate (100 [1/sec]) were
measured, and the value
of the viscosity (rla) was divided by the value of the viscosity (rib) to
calculate a thixotropy index
(TI) value. These results are shown in Table 9.
In the pastes (i.e. metallic pastes) using the metallic copper particles of
the
Examples according to the present invention, the TI values are dominantly
higher (specifically,
TI values are 3.0 or more) than those in the Comparative Examples. For this
reason, for
example, in the screen printing, the fluidity of the metallic paste during
continuous printing
becomes favorable, and a thick film can be obtained after completion of
patterning on a
substrate. Moreover, cracks, disconnection, short-circuits, bleeding, and the
like are
suppressed, and thick films are reproducibly obtained during continuous
printing. Furthermore,
in printing, such as inkjet printing, during which a high shear force is
applied to the metallic
paste, ejection of the metallic paste from holes can be made smooth, and
fixing of the metallic
paste to a printing medium becomes favorable.
[0126]
[Table 9]
Viscosity Dews] TI
Sample
n a b (77 a/ n b)
Example 1 A 31500 6415 4.91
Example 3 C 26500 6000 4.42
Example 5 E 28500 5500 5.18
Example 10 35146 5015 7.01
Example 14 N 25152 7055 3.57
Example 17 Q 22700 5159 4.40
Example 28 AB 58260 10820 5.38
Comparative
AF 23300 8200 2.84
Example 2
Comparative
Example 3 AG 35688 12000 2.97
Industrial Applicability
[0127]
According to the present invention, a metallic copper particle which can be
fired
under a nonreducing atmosphere such as nitrogen and which is excellent in
sinterability at a
lower temperature can be simply produced. The metallic copper particle can be
used in
CA 02959294 2017-02-24
W7336
69
materials fol.. securing electrical conduction, materials for antistatic,
materials for shielding
electromagnetic waves, materials for giving metallic luster or antibacterial
properties, and other
s
materials, and can be used particularly in uses for forming a fine electrode
and a fine circuit-
wiring pattern such as a printed wiring board, making use of the electrical
conductivity of the
metallic copper-containing film, in uses for joining chips and substrates, and
in design and
decoration uses making use of metallic color tone of the metallic copper-
containing film.