Note: Descriptions are shown in the official language in which they were submitted.
CA 02959946 2017-03-01
WO 2016/022504
PCT/1JS2015/043510
A COHERENT BLOOD COAGULATION STRUCTURE OF WATER-INSOLUBLE
CHITOSAN AND WATER-DISPERSIBLE STARCH COATING
BACKGROUND OF THE ART
1. Field of the Invention
The present invention relates to the field of blood control at wound sites and
coagulation of
blood within a structure provided at a wound site.
2. Background of the Art
It has been recognized in the prior art that it is desirable to stop bleeding
by applying
materials to the wound or tissue which initiate or enhance blood coagulation.
Such materials
have included collagen, gelatin, oxidized regenerated cellulose, kaolin,
polysaccharides such
as starch or chitosan, and liquid glues (cyanoacrylate adhesives, gelatinous
glues, UV curable
polymers, etc.) to name a few. Other materials which are made from human or
animal blood
components such as thrombin, albumin and fibrinogen have been used but carry
the risk of
virus infection and are expensive to manufacture.
One method used to reduce bleeding involves initiating or accelerate blood
clotting by
applying hygroscopic porous particles directly to a wound. In this method the
porous particles
absorb the water from blood allowing the natural fibrinogens within the blood
to coagulate,
which results in a blood clot. The pore size of such particles should be such
that water is able
to be readily absorbed by the particles, but the clot forming blood components
(thrombin,
fibrinogen, fibrin, platelets, etc.) are not. The size of the pores,
therefore, should be less than
1 micrometer (1,000 nm) and preferably less than 0.1 micrometer (100 nm). The
particles
may be made of many different materials, although it is preferable that the
materials be
biocompatible and eventually absorbed by the body. Another method in U.S.
Patent No.
4,822,349 (Hursey) describes the use of zeolites, or molecular sieves, for
accelerating
clotting. The zeolites are used in a particle form either as a powder poured
onto or into a
wound, or embedded in a wound dressing. However, while effective at adsorbing
water from
the blood and stopping bleeding, this method suffers from several problems.
Zeolites are
inorganic and are not readily absorbed by the body. This creates significant
difficulties in
caring for the wound once the bleeding is stopped. The zeolite particles,
which have been
placed in the wound, must be debrided or scraped out of the wound once the
bleeding has
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
2
stopped. This can be painful for the trauma victim and require multiple
surgical
debridements. There is also an exothermic reaction when water is adsorbed into
zeolites that
can cause the temperatures at the wound site to reach 40 C to 50 C or higher
which can
damage tissue and irritate the patient. Also a significant number of people
can have allergic
reactions to the zeolites. Another major concern is that the loose zeolite
particles can become
entrained in a blood vessel where they will continue to promote formation of
clots. These
small clots, which can then circulate in the blood system, can potentially
cause embolisms,
strokes, or other clot related problems. U.S. Patent No. 6,060,461 (Drake)
describes the use
of particles made of porous materials from within the classes of
polysaccharides, cellulosics,
polymers (natural and synthetic), inorganic oxides, ceramics, zeolites,
glasses, metals and
composites.
Polysaccharides are preferred because of their ready availability and modest
cost.
They are widely known to be biocompatible and are readily absorbed by the body
over time.
Polysaccharides can be provided as starch, cellulose, and even chitosan.
Chitosan based
wound dressings provided under the trade names SoftSeal -STF chitosan, CeloxTM
chitosan,
"Hem ' chitosan are all products based upon chitosan chemistry. The chitosan
is
derived from chitin particles obtained from crustaceans such as crab or
shrimp. The particles
can be applied in a powder form directly to the wound, or held in place on the
wound.
However, powders are difficult to apply, especially to wounds in which blood
is flowing
since the powders can be washed away with the flowing blood before clotting
can be
initiated.
A solution to the problem of the powders washing away is described in the
Hursey
and Drake patents wherein they embed or attach the powders to a wound
dressing. The
wound dressing can take the form of a sheet or film in which the particles are
adhered to or to
the surface of fibers which make up woven or non-woven gauze-like fabric or
sheet. The
particles can also be interspersed with fibers, filaments or other particles
in a self-supporting
structure, entangled within the fibrous elements of a net, web, fabric or
sheet. However, both
the biocompatible particles and the zeolite particles suffer the same problem
in that they can
become entrained in the blood vessels and cause clotting related problems in
the blood
vessels. While both of the Hursey and Drake patents describe the use of a
dressing with the
particles embedded or attached to a dressing for ease of application, there
still exists the
danger of the particles shedding from dressing and becoming entrained in the
blood vessel
and causing clotting within a blood vessel. In addition, the use of a dressing
made of one
material combined with the particles made of a different material increases
problems of
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
3
biocompatibility and absorption. It also increases the complexity of
manufacturing and
consequently manufacturing costs. U.S. Patent No. 3,620,218 (Schmitt)
discloses a felt made
of polyglycolic acid fibers which may be used as a hemostat. However, the
felted fibers can
float from a bleeding surface and are generally too porous.
U.S. Patent No. 3,937,223 (Roth) discloses an asserted improvement upon US
Patent
No. 3,620,218 (Schmitt) by compaction of the felt on at least one side to
provide strength and
rigidity to the felt as well as providing a smoother surface which can be
drawn into close
conformity to the wound and thus reduce pockets in the felt where blood or
other fluids can
accumulate. Roth uses filaments of about 0.5 to 12 deniers per filament
(approximately 7 urn
to 34 urn) and, conveniently, 2 to 6 deniers (approximately 14 urn to 24 um)
per filament.
These fibers are quite large and stiff which creates large pores when made
into a felt. To
reduce the large pores one compresses the felt to smoothen the surface of the
felt and to press
the filaments closer together to create smaller pores between the fibers and
thereby enhance
hemostatic properties of the felt. However, even after compaction this
technique suffers from
the large open regions or void volume. When the filaments are compressed
together, the void
volume, or amount of open area between fibers, is greatly reduced. The amount
of open area
between fibers is important as the open area allows water to be wicked between
the fibers,
leaving behind platelets and other clotting agents, thus initiating the
clotting process. The
void volumes in compressed and calendared felts are typically less than 70%
and usually less
than 50%. The low void volumes in the felt reduces the hemostatic
effectiveness of the
compressed felts since the wicking of the water from the blood is a function
of the surface
area of fibers in contact with the blood and the capillary effect created by
the pore size as
well as the number of pores in the surface of the media. In addition, to the
less than optimal
hemostatic properties, the fibers in the felt which have not been compacted or
embossed are
not bonded to the other fibers.
U.S. Patent No. 8,063,264 (Spearman) discloses a wound dressing and a method
for
enhancing the clotting comprising a plurality of hydrophilic microfibers
bonded to each other
to form a mat with the plurality of microfibers having a pore size
sufficiently small to inhibit
wicking platelets from a wound into the microfibers so that when applied to a
wound the
blood coagulates and the microfibers remain external to the wound. Another
example of
fiber matrix wound dressing for hemostasis is U.S. Patent No.8,703,176 (Zhu).
4
U.S. Patent No. 7,101,862 (Cochrum) provides hemostatic compositions
useful to promote hemostasis at active bleeding wound sites. The hemostatic
compositions typically include an article containing cellulose, e.g., cotton
gauze, and
a polysaccharide covalently linked to the cellulose, or a polysaccharide
ionically
cross-linked and in association with the article. Methods of making and using
the
hemostatic compositions are also provided.
U.S. Patent 8,575,132 (Ji) describes a modified starch material for
biocompatible hemostasis, biocompatible adhesion prevention, tissue healing
promotion, absorbable surgical wound sealing and tissue bonding, when applied
as a
biocompatible modified starch to the tissue of animals. The modified starch
material
produces hemostasis, reduces bleeding of the wound, extravasation of blood and
tissue exudation, preserves the wound surface or the wound in relative wetness
or
dryness, inhibits the growth of bacteria and inflammatory response, minimizes
tissue
inflammation, and relieves patient pain. Any excess modified starch not
involved in
hemostatic activity is readily dissolved and rinsed away through saline
irrigation
during operation. After treatment of surgical wounds, combat wounds, trauma
and
emergency wounds, the modified starch hemostatic material is rapidly absorbed
by
the body without the complications associated with gauze and bandage removal.
SUMMARY OF THE INVENTION
An absorbent layer for controlling or moderating blood flow from a wound
may include a non-woven fabric layer of water-insoluble chitosan fibers having
a
coating of water-absorbent starch on at least one face of the fabric layer.
The coating
of water-absorbent starch may penetrate into the fabric layer from a first
surface over
the chitosan fibers to a depth of at least 25% of the fabric layer of chitosan
fibers. The
chitosan fibers may have average diameters of from 5 to 30 micrometers. The
average
weight of starch/chitosan may decrease from the first surface from which the
starch
has penetrated into the fabric to the depth of at least 50% of the fabric
layer. The
starch may be modified to include hydrophilic groups into or onto molecular
chains of
the starch.
In yet another aspect, the present invention provides an absorbent layer
comprising a non-woven fabric layer of water-insoluble chitosan fibers having
a
continuous coating of water-absorbent starch on at least one face of the
fabric layer,
CA 2959946 2018-08-23
4a
wherein the starch has been modified to include hydrophilic groups into or
onto
molecular chains of the starch.
BRIEF DESCRIPTION OF THE FIGURES
Figure lA is a scanning electron micrograph (SEM) of a nonwoven chitosan fiber
matrix without any added starch. The view is a top view of the fiber mat. The
magnification is 20X.
CA 2959946 2018-08-23
CA 02959946 2017-03-01
W02016/022504
PCT/US2015/043510
Figure 1B is an electron micrograph (SEM) of a nonwoven chitosan fiber matrix
without any
added starch. The view is a top view of the fiber mat. The magnification is
20X.
Figure 2A is a light micrograph (LM) of nonwoven chitosan fiber matrix without
any added
starch. The view is an edge view of the fiber mat. The magnification is 25X.
5 Figure 2B is a light micrograph (LM) of nonwoven chitosan fiber matrix
without any added
starch. The view is a top view of the fiber mat. The magnification is 25X.
Figure 3A is a scanning electron micrograph (SEM) of a nonwoven chitosan fiber
matrix with
an additional coating of starch. The view is a top view. The magnification is
20X.
Figure 3B is a scanning electron micrograph (SEM) of a nonwoven chitosan fiber
matrix with
an additional coating of starch. The view is a top view. The magnification is
100X.
Figure 4A is a light micrograph (LM) of nonwoven chitosan fiber matrix with an
additional
coating of starch. The view is a top view of the fiber mat. The magnification
is 25X.
Figure 4B is a light micrograph (LM) of nonwoven chitosan fiber matrix with an
additional
coating of starch. The view is a top view of the fiber mat. The magnification
is 67X. The
image shows the transparency/translucency to white light of the modified
starch coating and
transparency/translucency to white light of the underlying chitosan fabric
substrate.
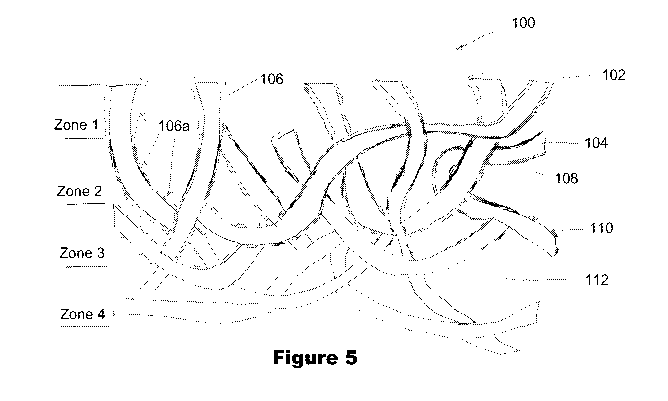
Figure 5 is a schematic representation of a cutaway side view of the chitosan
fiber matrix
similar to that of Figures 3A and 3B emphasizing distribution of the starch
layer 106a from
one surface 102 into the non-woven chitosan 104. Remembering that this Figure
5 is an
illustrative rendition and is not intended to be a limiting description of
distribution of
materials, thicknesses and rheology of layers, it can be seen that in Zone 1,
there is a heavier
thickness 106 of the starch coating 106a. In Zone 2, there is a thinner
coating 108 of the
starch coating 106a. In Zone 3, there tends to be a more discontinuous coating
110 of the
starch coating 106a. In Zone 4, there is essentially no starch coating 106a in
this rendition.
There are open volumes 112 between the coated 106a chitosan fibrous elements
104 within
the matrix 100. The distribution is illustrative of how materials are likely
to be distributed
from a single side (through surface 102) application of the starch coating
106a. Different
methods of application (e.g., two-side application, dipping, pressure-coating,
spray coating,
meniscus coating and the like) will create varying patterns if starch
distribution within the
matrix 100. For example, with two side coating application of the starch, a
complete cross-
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
6
section distribution might look more like a mirror image of Zone 1, Zone 2,
Zone 2 again and
Zone 1 again (in order) so that there is a relatively continuous, though
varying in thickness,
coating of starch across the entire thickness of the matrix. It might also be
possible to have
mirror cross-sections of a) Zone 1, Zone 2, Zone 3m Zone 2, Zone 1, orb) Zone
1, Zone 2,
Zone 3, Zone 4, Zone 3, Zone 2 and Zone 1, Not only the thickness of the
starch coating
106 may vary across the thickness of the fabric, but also the coating weight
per volume will
vary and the coating weight variations (in a two-side coated matrix) may be
different from
one surface versus the other surface.
Figure 5A is an SEM of Chitosan-STF fibers machine coated with starch. The
magnification
is 100X.
Figure 5B is a LM of Chitosan-STF fibers machine coated with starch. The
magnification is
67X.
Figure 6A is an SEM of Chitosan-STF fibers machine coated with starch. The
magnification
is 50X.
Figure 6B is an LM of Chitosan-STF fibers machine coated with starch.
Magnification is
33X.
DETAILED DESCRIPTION OF THE INVENTION
An absorbent layer has a non-woven fabric layer of water-insoluble chitosan
fibers
having a coating of water-absorbent starch on at least one face of the fabric
layer. The
coating of water-absorbent starch may penetrate into the fabric layer from a
first surface over
the chitosan fibers to a depth of at least 25% of the fabric layer of chitosan
fibers towards a
second surface. The chitosan fibers may have average diameters (not lengths)
of from 5 to 30
micrometers, 8 to 25 micrometers or 10 to 20 micrometers. The fibers may have
aspect ratios
of from 1.5 to 50 (or higher if continuous fibers are used). The average
weight of
starch/chitosan decreases from the first surface from which the starch has
penetrated into the
fabric to the depth of at least 50% of the fabric layer. The decrease may be
graded or gradual
(e.g., nominally from a 60/40 starch to chitosan at the surface region (e.g.,
the first 10% of
depth) to a nominal 10/90 ratio). The gradation may be straight-line linear or
slower drop-off
or faster drop-off depending upon methodology of applying the starch and/or
intentional
design. The coating may be on only one side of the layer or may be applied
from both sides
to provide an asymmetric distribution or symmetric distribution, respectively.
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
7
The starch component of the layer, as described in greater detail herein may
be a
starch that has been modified to include hydrophilic groups attached onto
molecular chains of
the starch. The layer may be carried on a structural support secured to the
second surface of
the layer.
A method is provided herein of mediating blood flow from a wound by applying
the
first surface of a layer according to the present technology against the wound
from which
blood is flowing and clotting blood from the wound.
Chitin is recovered from crab shells by treatment with acid to remove the
minerals
and with alkali to remove protein. After these treatments, purified chitin
remains as thin,
white sheets that are further processed into chitosan. Chitosan may be made by
heating
purified chitin with strong alkali to remove some of the acetyl groups from
the polymer
chains. These exposed amino groups have a positive (also known as cationic)
charge in water
or dilute acid. When 50% or more of the amino groups have been exposed the
material
becomes soluble in water or dilute acid due to the repulsion of the charged
groups along the
polymer chain. Thus, chitosan is defined as a derivative of chitin in which
50% or more of
the amino groups are exposed or the chemical term is deacetlylated.
Starch is produced by all green plants and is stored for future energy use by
the plant
in their leaves, roots, and seeds. Commercial raw starch is obtained from
corn, potato, rice,
wheat and other seed or tuber crops. The raw starch is insoluble in water and
is not
efficacious as a hemostatic agent. Raw starch must be modified to increase its
hydrophilicity.
The modification process may be completely physical, chemical or a combination
of the two.
In general, raw starch is treated with water and heat to swell the raw starch
granules and
convert the material to a gelatinous mass. Further physical treatment such as
extrusion or roll
processing can be used to increase the hydrophilicity. After heating the raw
starch with a
measured amount of water, starch granules swell to a pasty substance,
regularly arranged
micelles of starch are broken, crystallites disappear, and the resulting
composition is easily
degraded by amylase. The pre-gelatinized starch is able to swell and/or
dissolve in cold or
room temperature water and form an adhesive paste whose tendency for
retrogradation is
lower than that of raw starch, affording easier handling during the production
process.
The present technology uses water dispersible or water-soluble starch. Where
the
term "starch" is used with reference to the starch coatings according to the
present technology
the term starch is defined and limited to water-soluble, water-dispersible
and/or gelatinized
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
8
starches as known in the art. The modified starch may be either physically
and/or chemically
modified as described herein.
GELATINIZED STARCH FURTHER MODIFIED BY CHEMICAL TREATMENT.
Physically modified starch, for example, a pre-gelatinized starch treated
solely with
spray drying or irradiation process, is remarkably safe as a bio-absorbable,
hemostatic
material since it is not treated with any chemical agents and is readily
degraded by enzymes
present in the tissues.
Chemical modification of the starch polymer chains can further enhance the
hydrophilicity of the gelatinized starch. The hydroxyl groups on the glucose
monomers of
starch can be reacted with a wide variety of chemical agents in order to
introduce chemical
functionality that can significantly increase the attraction of the starch for
water. It has been
found that introduction of carboxymethyl, hydroxyethyl or hydroxypropyl groups
are
particularly useful. Other useful modifications include phosphate
esterification, and cross-
linking using bifunctional agents such as epichlorohydrin. A complete
discussion of the many
.. possible modified starches useful as hemostatic agents is found in US
Patent 8,575,132 (Ji).
When applied to a bleeding wound, the hemostatic efficacy of a particular
starch
composition is affected by both the water absorption characteristics
(hydrophilicity) and the
viscosity of the resulting starch-blood composition. The hemostatic properties
of a particular
modified starch depend upon, first, the characteristics of the raw starch,
such as molecular
weight of the starch polymers, and the relative amounts of amylose and
amylopectin in the
raw starch; and, second, modifications made to the particular starch by
chemical treatments
such as carboxymethylation or hydroxymethylation. US patent 8,575,132 provides
a useful
discussion of the parameters found useful in preparing hemostatic starch
compositions. In
particular, a molecular weight range of 15,000 to 2,000,000 Daltons, a water
absorption
capacity ranging from greater than one gram of water per gram of modified
starch to 500
grams of water per gram of modified starch, and inclusion of at least one
carboxymethyl
starch or one hydroxymethyl starch were found useful in preparing efficacious
hemostatic
compositions. Daltons are international atomic mass units. One Dalton is
equivalent to
lgram per mole of substance, so the units are metric. The water absorption
capacities are
given in metric units of grams of water absorbed by one gram of starch. These
compositions,
when contacting blood, produce a "starch-blood coagulation matrix" that has
strong adhesive
characteristics which can seal wounded tissue and stop bleeding. In addition,
the interaction
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
9
between the formed blood coagulation matrix and the functional groups of
tissue proteins
causes the "starch-blood coagulation matrix'' to adhere to and seal the
wounded tissue,
resulting in hemostasis.
Other biocompatible hemostatic materials that may be added to the modified
starches
can comprise one or more of the groups of gelatin, collagen, carboxymethyl
cellulose,
oxidized cellulose, oxidized regenerated cellulose, and chitosan. The weight
ratio between
the modified starch and any other biocompatible hemostatic materials
preferably is: 95:5,
90:10, 85:15, 80:20, 75:25, 70:30, 65:35, 60:40, 55:45, 50:50, 45:55, 40:60,
35:65, 30:70.
This additional coagulant material may be added to the described modified
starch hemostatic
material before or during the vacuum freeze drying production process to
produce a
composite hemostatic composite. The production process may involve, but is not
limited to,
pre-mixing the coagulant material with the modified starch directly before any
vacuum freeze
drying process. The coagulant of the present invention comprises one or more
combinations
of the following group of blood coagulation factors: thrombin, fibrinogen, or
calcium salts.
The topical application of the layer of chitosan and modified starch can be
used as a
hemostatic agent to manage and control bleeding wound surfaces in humans,
mammals,
birds, or reptiles. Another advantage of the class of modified starch
hemostatic material
described herein is the rapid particle dispersion/dissolution in water,
facilitating both the easy
deposition of the starch onto the chitosan fabric material and the easy
removal of excess
modified starch particles from the wound by simple saline irrigation. The
residual modified
starch not actively involved in hemostasis can be rinsed away by irrigation.
In the treatment
of battle wounds, self rescue, or first aid, the hemostatic material remaining
in small amounts
will be absorbed by the body and the irritation of wound debridement or gauze
removal is
avoided.
'The chitosan/modified starch hemostatic material has properties of stability,
extended
shelf life, resistance to high and low pressure, resistance to high
temperatures up to 60 C and
low temperatures down to -40 C, convenient storage, and physical stability.
Therefore, it may
also be employed as a hemostatic material for the military, emergency, and
first-aid uses.
Particularly, it can be adapted for extreme environmental conditions such as
desert areas,
polar regions, alpine areas, outer space, and underwater probes.
The chitosan/modified starch compositions are pliable and flexible. Therefore,
they
can be conformed to r wound surfaces with various shapes, sizes, and features,
such as deep
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
and irregular traumatic wounds. The chitosan/modified starch compositions
contemplated
here are easily sterilized using gamma irradiation, ultraviolet radiation,
oxirane or ozone
sterilization. Chemical treatments and addition of chemicals and elements
(e.g., organic
antimicrobials iodine, cupric ion, silver particles, etc.) may further
sterilize or may retain
5 antimicrobial activity of the coated material. Such additions may be made
during or after
manufacture of the structure.
One example of a production process for a biocompatible modified starch
material
useful in the present invention, comprising the steps of: First, providing a
modified
hygroscopic biocompatible starch material and loading it into an agglomerating
apparatus
10 under 40-50 C; and second, adding distilled water and producing a
modified starch finished
product material by particle agglomerating and pellet processing. The modified
starch
finished product material has a molecular weight over 15,000 Daltons (for
instance, 15,000 to
about 2,000,000 Daltons) and a grain diameter of 10-1000 m, wherein starch
grains with
diameters of 30-500 m represent no less than 95% of the total amount of starch
grains. The
modified starch material according to the present invention can he applied as
a suspension in
water or other solvents or as a dry powder to a preformed layer of chitosan,
which may or
may not already be self-supporting. The layer may be made self-supporting by
the coalescing
of the aqueous (or organic solution such as alcoholic) solution or dispersion
of the starch onto
at least one surface of the chitosan layer. The chitosan/modified starch layer
according to the
present invention can be used on soft tissue and organs to rapidly and
effectively control
bleeding.
As noted herein, the chitosan/modified starch layer may be provided in a
structure
having additional layers associated into a final structure. For example, one
multi-layer
structure contemplated is:
1) A multilayer pad;
2) An exterior layer has fabric of chitosan/modified starch fabric;
3) The exterior layer is optionally separated from another layer by a freeze
dried starch
sheet;
4) At least one exterior chitosan fabric layer (as many as three) and separate
internal
starch layers may be used as carrier layers bound to chitosan layers.
5) An optional support layer comprised of a polymeric or similarly elastic
material. An
example is polypropylene, polyurethane, polytetrafluoroethylene, or flexible
silicone.
CA 02959946 2017-03-01
WO 2016/022504
PCT/1JS2015/043510
11
A preferred structure as enabled above would have the fiber layer composition
formed from
chitosan fibers which are insoluble in water and starch particles which are
soluble, or mostly
soluble in water. The chitosan fibers are preferably 10 to 20 micrometers in
diameter. The
starch material (which is commercially available from Starch Medical, Inc.) is
comprised
mostly of carboxymethyl starch and is soluble and/or swellable in water. There
is preferably
no intentional cross-linking of either chitosan or starch and at least no
significant crosslinking
(e.g., >5%).
An underlying concept of one aspect of the present technology is to combine
the
chitosan fibers, which exert a hemostatic effect by virtue of a positive
charge on the fiber
surface interacting with the negative charge on the surface of red blood cells
and platelets,
with a starch-based hemostat to get a synergistic effect. The combination
products show
better results than either product alone.
The chitosan fibers and the starch powder could be combined as a dry mix (and
preferably exposed to moisture to assure securing the modified starch to the
chitosan fibers)
and some tests show that this works. It is preferred to make a stable,
reproducible
formulation by coating the chitosan fibers with a solution/suspension of
starch and drying the
composite.
The chitosan layer (having the fibers bound or loosely associated thereto)
then has the
at least one-side coating of the modified starch applied as by dip-coating,
roller coating, spray
coating, meniscus coating, slot coating, brush coating or the like. The
gradation and depth of
penetration of the applied modified starch liquid composition is controlled by
viscosity,
density, concentration, pressure and properties of the solution in combination
with the
particular coating techniques and rate and volume of application of the
composition, as well
as drying and pressure application parameters.
Water-soluble starches may be provided according to numerous technologies and
sources, including at least U.S. Patents Nos. 4,076,663 (Masuda) in which a
highly water-
absorbent resin is produced by polymerizing (A) starch or cellulose, (B) at
least one
monomer having a polyinerizable double bond which is water-soluble or becomes
water-
soluble by hydrolysis and (C) a crosslinking agent, and subjecting, if
necessary, the resulting
product to hydrolysis; U.S. Patent No. 6,833,488 describing a bio-compatible,
biodegradable
macromolecular water-absorbent polymeric material having a three-dimensional
configuration with intermolecular covalent bonds and containing free
functional groups
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
12
selected from OH, SH, NI-12, and COOH. The polymer is formed by polymer-
polymer inter-
coupling interaction between a natural water-soluble polymer A or its
derivatives having a
molecular weight between 20,000 and 500,000 Da, and a synthetic polymer B in a
ratio of
A:B of 15:85 to 85:15; U.S. Patent No. 8,710,21.2 (Thibodeau) describing an
absorbent.
material consisting of a molecular network of starch molecules, the starch
molecules
comprising an amylopectin content of at least 90% (w/w). The molecular network
can either
be comprised of self-entangled starches or cross-linked starches;
DESCRIPTION OF CHITOSAN AND CHITOSAN-STF
US Patent 8,703,176 (Zhu) describes a unique and proprietary chitosan
structure and
formulation. The structure is a nonwoven fleece made from high molecular
weight chitosan
fibers that offers a significant improvement in hemostasis performance and
reliability. This
material provides a strong technology platform that can be used to create a
family of products
each with its own indications for use. Commercial hemostatic products using
this technology
are produced by Chitogen Inc.
Chitosan is a polymer, soluble in water or dilute acid, made from chitin by
chemical
treatment Chitin is an abundant natural product that is the primary structural
material in the
shells of shrimp, lobsters and other crustaceans. Chitin's structural role in
shells is similar to
CHEMICAL STRUCTURES OF CELLULOSE (Image a) AND CHITIN AND CHITOSAN (Image b)
cs= H3
"
0
= 1
3 NI j
, = 0
OH -OH
...n
(b)
the role of cellulose in plants. Both chitin and cellulose are high molecular
weight polymers
containing glucose molecules linked together to form long, linear
polysaccharide chains.
CA 02959946 2017-03-01
= WO
2016/022504 PCT/US2015/043510
13
Chitin is recovered from the crab shell by treatment with acid to remove the
minerals
followed by treatment with alkali to remove protein. After these treatments,
purified chitin
remains as thin, white sheets that are further processed into chitosan.
Chitosan is made by
heating purified chitin with strong alkali (40% sodium hydroxide) to remove
some of the
acetyl groups from the polymer chains. These exposed amino groups have a
positive (also
known as cationic) charge in water or dilute acid. When 50% or more of the
amino groups
have been exposed the material becomes soluble in water or dilute acid due to
the repulsion
of the charged groups along the polymer chain. Thus, chitosan is defined as a
derivative of
chitin in which 50% or more of the amino groups are exposed or the chemical
term is
deacetlylated.
Hemostatic products made from chitosan (SoftSeal -STF, Chitogen Inc.) are non-
woven pads composed of chitosan fibers attached to a thin polypropylene
backing material.
The pad is intended to be used as an aid in the management of topical bleeding
wounds such
as vascular access sites and topical lacerations.
The principle of operation of chitosan¨based products is believed to result
from
bioadhcsion between the chitosan polymer chains (positive charge) and blood
and tissue
components (negative charge) as well as pressure related tamponade. The charge
density and
uniformity of the positive charge is enhanced by the surface treated fiber
(STF) process as
described by US Patent 8,575,132.
EXAMPLE, DESCRIPTION OF DUPLEX FORMULATION
Chitosan fibers (Soft-Seal-STF(TM), Chitogen Inc.) were coated with
carboxymethyl starch
(AMP-66, Starch Medical) using an airless spray technology and the resulting
duplex
structures were evaluated using a recognized animal bleeding model.
Chitosan fibers were spray-coated with two levels of modified starch.
Carboxymethyl
starch, 5.0 grams or 10 grams was dissolved in 600 ml of 5% acetic acid. The
spray coating
was done by hand control using a spray painter (home use) held approximately
12 inches
(30.5 cm) from the fabric which was placed on a paper background. The sprayer
was
activated for a total of three passes and total exposure time was
approximately 2 to 3 seconds.
The sprayed chitosan fibers were air-dried overnight and placed into a zip-
lock bag. No
evidence of leakage from the sprayed material was observed.
CA 02959946 2017-03-01
= WO
2016/022504 PCT/US2015/043510
14
The ability of the two prototypes to control bleeding and their adherence to
the
bleeding surface was assessed. These studies investigated the rapid control of
bleeding using
a hold or compression times of 1 minute or 2 minutes used to determine
hemostatic
performance from the bleeding soft organ.
In both the 5 and 10 gram starch solutions, coated fibers were observed on the
surface
of the fiber mat with uncoated fibers in the depths of the mat.
Figure 5 is a schematic representation a cutaway side view of the chitosan
fiber
matrix 100 similar to that seen in Figures 3A and 3B emphasizing distribution
of the AMP
layer 106a from one surface 102 into the non-woven chitosan 104. Remembering
that this
Figure 5 is an illustrative rendition and is not intended to be a limiting
description of
distribution of materials, thicknesses and rheology of layers, it can be seen
that in Zone 1,
there is a heavier thickness 106 of the starch coating 106a. In Zone 2, there
is a thinner
coating 108 of the starch coating 106a. In Zone 3, there tends to be a more
discontinuous
coating 110 of the starch coating 106a. In Zone 4, there is essentially no
starch coating 106a
in this rendition. There are open volumes 112 between the coated 106a chitosan
fibrous
elements 104 within the matrix 100. The distribution is illustrative of how
materials are
likely to be distributed from a single side (through surface 102) application
of the starch
coating 106a. Different methods of application (e.g., two-side application,
dipping, pressure-
coating, spray coating, meniscus coating and the like) will create varying
patterns if starch
distribution within the matrix 100. For example, with two side coating
application of the
starch, a complete cross-section distribution might look more like a mirror
image of Zone 1,
Zone 2, Zone 2 again and Zone 1 again (in order) so that there is a relatively
continuous,
though varying in thickness, coating of starch across the entire thickness of
the matrix. It
might also be possible to have mirror cross-sections of a) Zone 1, Zone 2,
Zone 3, Zone 2,
Zone 1, orb) Zone 1, Zone 2, Zone 3, Zone 4, Zone 3, Zone 2 and Zone 1, Not
only the
thickness of the starch coating 106 may vary across the thickness of the
fabric, but also the
coating weight per volume will vary and the coating weight variations (in a
two-side coated
matrix) may be different from one surface versus the other surface,
ANIMAL TEST RESULTS
Experiment 1
CA 02959946 2017-03-01
= WO
2016/022504 PCT/US2015/043510
The soft organs of a live pig were selected for a bleeding model to test the
efficacy of
the preparations. A 6 mm biopsy punch that was inserted to a depth of
approximately 6 mm
to create circular incisions in both the liver and spleen of the pig. The test
material was
applied and held for one minute for liver incisions and two minutes for
spleenic incisions.
5 The bleeding model is described in detail in the protocol is XVE004, from
American
Preclinical Services, Minneapolis, MN. The duplex formulations of chitosan
fiber and
modified starch at the 5 gram and lOgram level showed superior performance to
the chitosan
fiber control. There was less bleed through at the 1 minute hold for the liver
and 2 minute
hold for the spleen. It was determined that the 1 minute hold although
adequate for the liver
10 was insufficient for the spleen.
This bleeding model was a very severe test for hemostatic pads. A hold time of
one
or two minutes, although useful for the animal model, is not specifically
intended to predict
use in clinical situations.
In this particular study, we observed an improvement in hemostatic performance
for
15 the 10 gram material when compared to the lower concentration, 5 grams.
However both
were an improvement compared to the plain STF and thus the three
concentrations (zero,
0.8% and 1.7% grams/ml) provide a clear dose response trend.
The chitosan fiber/modified starch composition was folded over to provide a
double
thickness layer. This configuration was also tested in the more demanding
spleen bleeding
sites and was found to be very effective. In contrast, the control chitosan
fiber pad alone
when doubled up did not achieve an improved hemostatic control.
These experiments show that a single layer of chitosan fibers, coated with
modified
starch provide an improved hemostatic pad for topical, percutaneous injury.
For more severe
bleeding, the use of multiple layers is preferred.
Experiment 2
Using the same materials and method as used for Experiment 1, a new set of
starch
fiber pads coated with modified starch was prepared. The pads were tested in
the same
animal bleeding model.
Light microscope and scanning electron microscope images are observed. Wounds
treated with pads of chitosan fibers coated with modified starch oozed less
than the chitosan
CA 02959946 2017-03-01
WO 2016/022504
PCT/US2015/043510
16
fiber pads alone. Oozing is defined as blood leaking from the edge of the
wound after the pad
is applied and held in place for one or two minutes. Such oozing is considered
a failure to
control the bleeding from the wound.
Using the liver and the spleen, we tested 19 chitosan fiber pads (control) and
18 pads
of chitosan fiber coated with modified starch. Both groups exhibited good
hemostatic
performance but for the control pads, where 4 pads oozed (failed). That is
4/19 = 21%
showed some blood leakage. For the chitosan fibers coated with modified starch
there were
no failures. Using Fisher's Exact Test to compare these results we calculate
that the
probability of these results being due to chance is .056.