Note: Descriptions are shown in the official language in which they were submitted.
CA 02960933 2017-03-10
- 1 -
SULPHUR-CONTAINING POLYOL COMPOUND
I. FIELD OF THE INVENTION
The present invention relates to monomers capable of being used for the
synthesis of
polymers having urethane units (or polyurethanes), especially intended for
adhesive systems
for the adhesive bonding of glass or metal to rubber.
It more particularly relates to the above monomers of the sulphur-containing
polyol type
intended in particular for the synthesis of polyurethanes used in composites
of the
metal/rubber type for articles made of rubber, such as tyres.
2. PRIOR ART
Composites of the metal/rubber type, in particular for tyres, are well known.
They are
generally composed of a matrix made of unsaturated rubber, generally diene
rubber, which
can be crosslinked with sulphur, comprising metal reinforcing elements (or
"reinforcers")
such as wires, films or cords made of carbon steel.
As they are subjected to very high stresses during the rolling of the tyres,
in particular to
repeated actions of compression, bending or variation in curvature, these
composites must, in
a known way, satisfy a large number of sometimes contradictory technical
criteria, such as
uniformity, flexibility, flexural strength and compressive strength, tensile
strength, wear
resistance and corrosion resistance, and must maintain this performance at a
very high level
for as long as possible.
It is easily understood that the adhesive interphase between rubber and
reinforcers plays a
predominant role in the endurance of this performance. The conventional
process for
connecting the rubber compositions to carbon steel consists in coating the
surface of the steel
with brass (copper/zinc alloy), the bonding between the steel and the rubber
matrix being
provided by sulphurization of the brass during the vulcanization or curing of
the rubber. In
order to improve the adhesion, use is generally made, in addition, in these
rubber
compositions, of organic metal salts or metal complexes, such as cobalt salts,
as adhesion-
promoting additives.
In point of fact, it is known that the adhesion between the carbon steel and
the rubber matrix
is capable of weakening over time as a result of the gradual development of
sulphides formed
under the effect of the various stresses encountered, especially mechanical
and/or thermal
P10-3421
2
stresses, it being possible for the above decomposition process to be
accelerated in the
presence of moisture.
Moreover, the use of cobalt salts renders the rubber compositions more
sensitive to
oxidation and to ageing, and significantly increases the cost thereof, not to
mention that
it is desirable to eliminate, in the long run, the use of such cobalt salts in
rubber
compositions due to recent developments in European regulations relating to
metal
salts of this type.
For all the reasons set out above, manufacturers of metal/rubber composites,
in
particular tyre manufacturers, are seeking novel adhesive solutions in order
to
adhesively bond metal reinforcers to rubber compositions, while overcoming, at
least in
part, the above-mentioned disadvantages.
3. BRIEF DESCRIPTION OF THE INVENTION
In point of fact, during their research studies, the Applicants have found a
novel polyol
compound of sulphur-containing type which enables the synthesis of a
polyurethane
which meets such an objective.
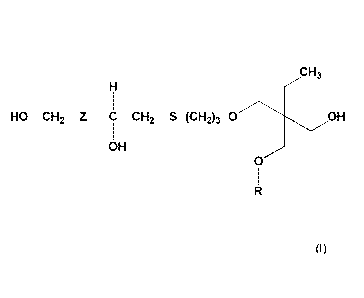
According to the invention, said sulphur-containing polyol compound
corresponds to the
formula (I) below:
CH3
H /
1
HO¨CH2¨Z¨C¨CH2¨S¨(CH2)3-0 OH
1
OH
./
0
1
R
in which:
- Z represents an optional, at least divalent, bonding group, comprising at
least
one carbon atom and possibly comprising a heteroatom;
- R represents hydrogen or a saturated or unsaturated hydrocarbon group,
possibly comprising a heteroatom.
Date Recue/Date Received 2020-12-17
2a
Another embodiment of the invention relates to a sulfur-containing polyol
compound,
preferably useful for the synthesis of a polyurethane by polycondensation with
a
polyisocyanate, said compound corresponding to the formula (I):
CH3
H /
1
HO¨CH2¨Z¨C¨CH2¨S¨(CH2)3-0 OH
1
OH
./
0
1
R
in which:
- Z represents an optional, at least divalent, bonding group selected from
the group
consisting of aliphatic groups comprising 1 to 20 carbon atoms, aliphatic
groups
comprising 1 to 20 carbon atoms and a heteroatom, cycloaliphatic group
comprising
from 3 to 20 carbon atoms, and cycloaliphatic groups comprising for 3 to 20
carbon
atoms and a heteroatom; and
- R represents a hydrogen atom, a saturated hydrocarbon group, a saturated
hydrocarbon group comprising a heteroatom, an unsaturated hydrocarbon group,
or
an unsaturated hydrocarbon group comprising a heteroatom.
By virtue of this sulphur-containing polyol (primary diol) compound in
accordance with
the invention comprising in particular, in addition to its two primary alcohol
functional
groups, on the one hand, at least one secondary alcohol functional group and,
on the
other hand, a thioether functional group in the alpha position with regard to
this
secondary alcohol functional group, it has proved to be possible to prepare a
polyurethane which, used as adhesion primer on metal reinforcers, gives these
reinforcers the major and unexpected
Date Recue/Date Received 2020-12-17
CA 02960933 2017-03-10
- 3 -
advantage of being able to adhere to unsaturated rubber matrices by using
simple textile
adhesives, such as "RFL" (resorcinol-formaldehyde-latex) adhesives, or other
equivalent
adhesive compositions, or else directly (that is to say, without use of such
adhesives) to these
unsaturated rubber matrices when the latter comprise, for example, appropriate
functionalized
unsaturated elastomers, such as, for example, epoxidized elastomers.
The invention also relates to the use of a polyol compound in accordance with
the invention
in the manufacture of a polyurethane (polymer having urethane units) and also
to any
polyurethane resulting from at least one polyol compound in accordance with
the invention.
The invention also relates to any process for the synthesis of a polyurethane
by
polycondensation of at least one polyol compound in accordance with the
invention with a
polyisocyanate compound.
The invention and its advantages will be easily understood in the light of the
detailed
description and exemplary embodiments which follow, and also of Figures 1 to 5
relating to
these examples, which represent or diagrammatically represent:
- a possible scheme for the synthesis of a polyol monomer (Monomer Ml, also
denoted Al here) in accordance with the invention from two compounds
(Compound 1 and Compound 2) (Fig.1);
- a possible scheme for the synthesis of a polyurethane polymer (Polymer Pl)
according to the invention, from Monomer Al and from a diisocyanate monomer
MDI (Monomer A2) (Fig. 2);
- a 'H NMR spectrum (500 MHz) of Monomer Al according to the invention and of
its starting Compound 1 respectively, both dissolved in CDC13 (Fig. 3.1 and
Fig.
3.2);
- another possible scheme for the synthesis, starting from Monomer Al
according to
the invention and another diisocyanate monomer (Monomer A3; benzophenone-
blocked MDI), of the same Polymer P1 (Fig. 4);
- a possible scheme for the synthesis, starting from Monomer Al according to
the
invention and from two other monomers (A4 and A3), of another polyurethane
(Polymer P2) according to the invention (Fig. 5).
4. DETAILED DESCRIPTION OF THE INVENTION
It will be recalled first of all here that a polyurethane is a polymer (by
definition any
homopolymer or copolymer, especially block copolymer) comprising a plurality
of urethane
(-0¨CO¨NH¨) bonds resulting, in a known way, from the addition reaction of a
polyol
P10-3421
CA 02960933 2017-03-10
- 4 -
having at least two primary alcohol functional groups with a polyisocyanate
(compound
bearing at least two isocyanate ¨NCO functional groups), especially with a
diisocyanate in
the case of a polyurethane of the linear type.
The sulphur-containing polyol (primary diol) compound of the invention, which
can be used
especially for the synthesis of a polyurethane, thus corresponds to the
formula:
(t)
7.,CH3
HO __ CH, __ Z __ C __ CH2-S- (CH2)T--0,0H
OH
in which:
- Z represents an optional (that is to say present or absent), at least
divalent, bonding
group, comprising at least one carbon atom and possibly comprising a (at least
one)
heteroatom;
- R represents hydrogen or a saturated or unsaturated hydrocarbon group,
possibly
comprising a (at least one) heteroatom.
This sulphur-containing primary diol compound according to the invention thus
has the main
essential characteristic of comprising a secondary alcohol functional group
and a thioether (¨
S ¨) functional group in the a position (alpha position, that is to say, as a
reminder and by
convention, borne by a carbon adjacent to the carbon bearing the secondary
alcohol
functional group) with respect to this alcohol functional group. Stated
otherwise, the polyol
compound of the invention has the essential characteristic of comprising an a-
hydroxy-
th ioether unit.
Another essential characteristic of this polyol according to the invention is
that one of its two
primary diol functional groups is borne by an end group also bearing an -OR
group as
defined above.
Optional Z is a bonding group, spacing unit, of organic type, preferably
hydrocarbon, also
commonly referred to as "separator" or "spacer" by those skilled in the art.
It may be
saturated or unsaturated.
It may be an aliphatic, cycloaliphatic or aromatic, substituted or
unsubstituted hydrocarbon
group, the aliphatic group preferably comprising I to 30 (more preferentially
I to 20) carbon
atoms, the cycloaliphatic group preferably comprising from 3 to 30 (more
preferentially 3 to
P10-3421
CA 02960933 2017-03-10
- 5 -
20) carbon atoms, the aromatic group comprising from 6 to 30 (more
preferentially 6 to 20)
carbon atoms.
Z more particularly represents an aliphatic bonding group having I to 20
atoms, more
preferentially 1 to 12 carbon atoms, or a cycloaliphatic bonding group having
3 to 20 carbon
atoms, more preferentially 3 to 12 carbon atoms. More particularly still, it
is a C1-C10,
especially CI-C:5 alkylene.
Hydrocarbon Z may comprise at least one (that is to say one or more)
heteroatom preferably
selected from 0, S, N or P, especially in the form of an ether (-0-) or
thioether (-S-) bond,
the latter possibly being present on the carbon chain (Z) itself or on a
substituent of one of its
carbon atoms.
According to another particular embodiment of the invention, Z is not present
in formula (1),
that is to say that the primary diol compound of the invention corresponds in
this case to
formula (II) below:
(II)
HO __________________ CH2 __ C ¨CH2¨S¨ (CF12)-00b1
OH
R thus represents hydrogen or an ethylenically saturated or unsaturated
hydrocarbon group,
possibly comprising at least one (that is to say one or more) heteroatom, such
as, preferably,
0, S, N or P.
R preferably represents hydrogen. an unsaturated hydrocarbon group or a
saturated
hydrocarbon group selected from C1-C18 alkyls, Cs-Cis cycloalkyls and C6-C1
aryls, the
latter being more preferentially selected from C1-C6 alkyls, cyclohexyl and
phenyl, in
particular from Ci-C4 alkyls, more particularly methyl or ethyl, all these
groups possibly
comprising at least one (that is to say one or more) heteroatom, such as,
preferably, 0, S, N
or P.
More preferentially still, R is hydrogen or an unsaturated hydrocarbon group,
possibly
comprising at least one (that is to say one or more) heteroatom, such as,
preferably, 0, S, N
or P.
P10-3421
CA 02960933 2017-03-10
- 6 -
The unsaturated hydrocarbon group may be aliphatic, cycloaliphatic or
aromatic. This
unsaturated group, if it is aliphatic, preferably comprises Ito 20, more
preferentially 1 to 10,
carbon atoms; if it is cycloaliphatic, it preferably comprises 3 to 30, more
preferentially 3 to
20, carbon atoms; if it is aromatic, it preferably comprises from 6 to 30
carbon atoms, more
preferentially 6 to 20 carbon atoms.
By way of examples of preferential -OR groups, mention may especially be made
of the
following groups:
0,
/ H
hydroxy vinyloxy ethynyloxy
0
0
0
allyloxy propynyloxy acryloyloxy
0
0
0
methaeryloyloxy 2,3-epoxypropyloxy 2,3-thi
iranepropyloxy
The formula of an example of (aliphatic) sulphur-containing polyol compound in
accordance
with the invention, denoted Monomer MI, has been represented in structural
form, and also a
synthesis scheme which can be used to obtain this compound has been
represented, in the
appended Figure 1.
This polyol compound (Monomer MI, subsequently also denoted Monomer Al) of
Figure 1
is 3-13-(2-allyloxymethyl-2-(hydroxymethyl)butoxy)propylsulphanylipropanc-1,2-
diol. It can
be obtained, for example, by reaction of trimethylolpropane diallyl ether and
thioglycerol, as
represented diagrammatically in Figure 1; this synthesis will be described in
more detail in
the exemplary embodiments which follow (Test 1 of section 5.1).
This sulphur-containing polyol compound of Figure 1 does indeed correspond to
formulae (I)
and (II) in which, for this example, optional Z is not present and R
represents an allyloxy
group.
The sulphur-containing polyol compound in accordance with the invention
described above
can be used for the synthesis of a polyurethane of the linear type, thus
resulting essentially
from the addition of this primary diol polyol and of a diisocyanate compound.
The
P10-3421
CA 02960933 2017-03-10
- 7 -
diisocyanate which can be used may be aromatic, aliphatic or cycloaliphatic;
it may be a
monomer, a prepolyiner or a quasi-prepolymer, indeed even a polymer.
According to a preferential embodiment, the diisocyanate from which the
polymer of the
invention results is selected from the group consisting of the following
aromatic compounds:
diphenylmethane diisocyanate (abbreviated to "MDI"), toluene diisocyanate
("TDI"),
naphthalene diisocyanate ("NDI"), 3,3'-bitoluene diisocyanate ("TODI"), para-
phenylene
diisocyanate ("PPDI"), their various isomers and the mixtures of these
compounds and/or
isomers.
More preferentially, use is made of an MDI or a TDI, more preferentially still
of an MDI.
All the isomers of MDI (in particular 2,2'-MDI, 2,4'-MDI and 4,4'-MDI) and
their mixtures
can be used, as well as what are referred to as polymeric MDIs (or "PMDIs")
comprising
oligomers of following formula (with p equal to or greater than 1):
OCN I I - I NCO
-P
Diisocyanate compounds of the aliphatic type can also be used, such as, for
example, 1.4-
tetramethy lene diisocyanate, 1,6-hexane diisocyanate
("HDI"), 1.4-
bis(isocyanatomethyl)cyclohexane, 1,3-bis(isocyanatomethyl)cyclohexane,
1.3-
bis(isocyanatomethyl)benzene, 1,4-bis(isocyanatomethyl)benzene, isophorone
diisocyanate
("1PDI"), bis(4-isocyanatocyclohexyl)methane diisocyanate ("Hl2MDI") or 4,4'-
dicyclohexylmethane diisocyanate ("Hl3MDI").
According to a particularly preferential embodiment, the diisocyanate used is
4,4'-MDI (4,4'-
diphenylmethane diisocyanate), having the formula:
I yõ
0=C=N-7 NCO
-
or, if several diisocyanates are used, constitutes the predominant
diisocyanate by weight,
preferably representing, in the latter case, more than 50% of the total weight
of the
diisocyanate compounds.
Use may also advantageously be made of a caprolactam-blocked 4,4'-MDI (for
example the
product in the solid form "Grilbond" IL-6 from EMS), of formula:
0
0
NAN2
N
0
P10-3421
CA 02960933 2017-03-10
-8-
As the invention is not, however, limited to a polymer of the linear type (as
a reminder,
resulting from a diisocyanate), it will also be possible to use, especially
with the aim of
increasing the Tg of the polymer of the invention by formation of a three-
dimensional
network, a triisocyanate compound, such as, for example, an MDI trimer having
a triazine
nucleus of the following formula:
ocNINCO
0 N 0
OCN
The polyurethane resulting from the polyol compound of the invention thus has
the
characteristic of comprising, in addition to its repeat base structural units
having urethane (-
0¨CO¨NH¨) units contributed in a well-known way by the starting polyisocyanate
compound, specific repeat additional units contributed by the polyol monomer
according to
the invention, these additional units comprising at least one unit of formula:
(HI)
H CH3
______________ 0 __ CH2 ____ C CH2 __ S (CH2)3 0
OH
or, when optional Z is absent, of the following formula (IV):
(IV)
________________ 0 __ CH2 __ C CH2-S- (CF12)7-0
OH 0
P10-3421
CA 02960933 2017-03-10
- 9 -
in which formulae Z and R have, of course, the main and preferential
definitions given above.
Figures 2, 4 and 5 represent in detail preferential examples of polyurethanes
resulting from
polyols in accordance with the invention and also various possible schemes for
the synthesis
of these polyurethanes from polyols in accordance with the invention.
First of all, Figures 1 and 2 respectively illustrate possible processes for
the synthesis of a
polyol monomer in accordance with the invention (denoted Monomer Al) and then
of a
polymer (denoted Polymer P1) according to the invention of the polyurethane
type starting
from this Monomer Al and from a diisocyanate monomer MDI (Monomer A2), which
processes will be described in detail subsequently.
This example of Polymer P1 does indeed comprise a repeat unit of formula (V):
¨ 0 ¨ C(0) ¨ NH ¨ Z' ¨ NH ¨ C(0) ¨ 0 ¨ A ¨
as defined above, in which Z' corresponds more particularly to the MDI residue
divalent
group and "A" corresponds to the formula (IV) above.
It is clearly visible in Figure 2 that, in accordance with the invention, the
Polymer PI
contains, in addition to its urethane base units, additional repeat units
comprising, on the one
hand, an a-hydroxy-thioether (¨ CH(OH) ¨ CH2 ¨ S ¨) functional group, and on
the other
hand an -OR group with ethylenic unsaturation (here, an allyloxy functional
group ¨ 0 ¨ CH2
¨ CH = CH2).
Figure 4 illustrates another possible process for the synthesis of this same
Polymer P1
according to the invention, this time starting from the preceding Monomer Al
and from
another diisocyanatc monomer (Monomer A3, caprolactam-blocked MDI), which
process
will be described in detail subsequently.
Figure 5 illustrates another process for the synthesis of another polymer
(Polymer P2) in
accordance with the invention starting from Monomer Al, Monomer A3 and another
polyol
monomer (not in accordance with the invention, Monomer A4), which process will
be
described in detail subsequently.
It is clearly visible in Figure 5 that, in accordance with the invention, the
Polymer P2
comprises, in addition to its urethane base units (-0¨CO¨NH¨), additional
repeat units of
P10-3421
CA 02960933 2017-03-10
- 10 -
general formula (II) having, on the one hand, a thioether (¨ S ¨) functional
group in the a
position relative to a secondary alcohol functional group (¨ CII(OII) ¨), and,
on the other
hand, an -OR group with ethylenic unsaturation (here, an allyloxy functional
group - 0 ¨
CH2 ¨ CH = CF17).
The polyurethane which can be synthesized starting from a sulphur-containing
polyol
compound in accordance with the invention may comprise from ten to several
hundred,
preferably from 20 to 200, structural repeat units as described above. Its
glass transition
temperature Tg, measured by DSC (Differential Scanning Calorimetry), for
example
according to Standard ASTM D3418, is preferably greater than 50 C, more
preferentially
greater than 100 C, in particular between 130 C and 250 C.
By virtue of the primary diol compound of the invention, this polyurethane
exhibits a high
flexibility and a high elongation at break and has furthermore displayed
effective
hydrophobic properties and corrosion-resistance properties.
It can advantageously be used as hydrophobic coating on any type of substrate,
especially
made of metal or glass, or else as adhesion primer on any type of metal
reinforcer, such as,
for example, a thread, a film, a plate or a cord made of carbon steel coated
or not coated with
brass, intended in particular to reinforce an unsaturated rubber matrix, such
as natural rubber.
5. EXEMPLARY EMBODIMENTS OF THE INVENTION
In the present application, unless expressly indicated otherwise, all the
percentages (%)
shown are percentages by weight.
Test 1 - Synthesis of the Monomer Al
The monomer Al (or MI) is 343-(2-allyloxymethy1-2-(hydroxymethyl)-
butoxy)propylsulphanyl]propane-1,2-diol, in accordance with the invention.
This monomer
was synthesized according to the procedure represented diagrammatically in
Figure 1, as
described in detail hereinafter: 4.76 g of Compound 1(90% pure
trimethylolpropane diallyl
ether, from Sigma Aldrich), then 2.16 g of Compound 2 (thioglycerol) were
placed in a 50 ml
glass round-bottomed flask provided with a magnetic stirrer, the mixture being
covered with
a glass stopper to avoid any losses by evaporation; the reaction mixture was
stirred at room
temperature (20 C) for 4 hours, then overnight (around 12 hours) at a
temperature of 80 C.
A transparent, viscous liquid is obtained in this way, the NMR spectrum of
which
(reproduced in Fig. 3.1), compared to the NMR spectrum of starting Compound 1
P10-3421
CA 02960933 2017-03-10
- 11 -
(reproduced in Fig. 3.2), taking as reference the transition of the methyl
group at 0.86 ppm (3
protons) and following the reduction in the integrals corresponding to the
protons of the
vinyloxy group, does indeed confirm to those skilled in the art that it is
Monomer Al, of
formula:
HO
CH,
OH
HO 0
The 1H NMR analysis (500 MHz, CDC13) (Fig. 5.1) of the product gave the
following results:
0.85 (m, 3H), 1.39(m, 2H), 11.86 (m, 1H), 2.65 (m, 3H), 3.46(m, 8H), 3. 73-3.
75 (s, 2H), 3.98(d, 2)-i),
5. /6-5. /9 (d, 11-i9, 5.28 (d, 1H), 5.88(m, 1H).
Finally, the molecular weight of the product, as measured by "ES1"
(Electrospray Ionization)
mass spectrometry in a 1/1 water/acetonitrile mixture (with traces of NaC1),
was evaluated in
negative mode ([M + Cl]- anion) at 357.3 (calculated theoretical value equal
to 357.5) and in
positive mode GM + Nal- cation) at 345.2 (calculated theoretical value equal
to 345.5).
5.2. Test 2- Synthesis of Polymer P1 by reacting Monomers Al and A2
This test gives a detailed description of the synthesis of Polymer P1
according to the
invention starting from Monomers Al and A2, according to the procedure
represented
diagrammatically in Figure 2.
3.40 g of Monomer Al were placed into a dry 50 ml round-bottomed flask then,
as
polymerization catalyst, 18.1 mg (0.3% by weight) of bismuth neodecanoate and
100 ml of 7-
butyrolactone solvent, all under an inert atmosphere (nitrogen stream). A
solution (itself
under an inert atmosphere) of 2.64 g of Monomer A2 (solid MDI dissolved in 20
ml y-
butyrolactone) was then added into the 50 ml round-bottomed flask by means of
a dropping
funnel. The transparent reaction mixture was stirred and heated at 80 C for 4
hours.
3 ml of the solution of Polymer P1 thus obtained was then deposited on a glass
sheet (10 x 10
cm); the glass sheet was placed under vacuum at 80 C for 1 hour until the
solvent (y-
butyrolactone) had evaporated. The transparent film of Polymer PI thus
obtained was
analysed by "ATR-FT1R" (Attenuated Total Reflection InfraRed) spectroscopy:
the synthesis
of a polyurethane is indeed confirmed by the appearance of the peak visible at
1700 em-I,
characteristic of the -OCONH- bond.
P10-3421
CA 02960933 2017-03-10
- 12 -
Incidentally, it was noted that Polymer P1 thus obtained exhibited excellent
adhesion to the
glass (impossibility of separating by pulling the polymer from the glass).
In order to measure its molecular weight, the polymer dissolved in a mixture
(1:20) of y-
butyrolactone and THF (tetrahydrofuran) was then subjected to GPC (Gel
Permeation
Chromatography) analysis (C18 column-reversed phase and THF as mobile phase):
a
molecular weight (Mw) of approximately 140 000 was thus determined
(polystyrene controls
with molecular weight between 500 and 500 000). The same synthesis without
polymerization catalyst led to a very broad elution profile with a predominant
distribution
centred at around 17 000.
Finally, the same synthesis carried out with 1% by weight of catalyst and in
DTP solvent
(1,3-dimethy1-3,4,5,6-tetrahydro-2(/H-pyrimidinone ¨ CAS 7226-23-5) led to a
Tg value
equal to approximately 93 C (DSC from -80 C to 200 C (10 C/min), 2nd pass).
5.3. Test 3- Synthesis of Polymer P1 by reacting Monomers Al and A3
Polymer P1 in accordance with the invention was also synthesized from monomers
Al and
A3 (caprolactam-blocked MDI) according to the simple procedure represented
diagrammatically in Figure 4.
226.4 mg of Monomer Al and 334.6 mg of Monomer A3 ("Grilbond" IL-6) were
placed in a
glass container, then 8 ml of y-butyrolactone solvent were added and the
mixture was heated
under a stream of hot air (120 C) until a clear solution was obtained.
3 ml of this solution were then deposited uniformly on a glass sheet (10 x 10
cm) and
everything was then placed in an oven at 190 C for 15 min under vacuum so as
to eliminate
any traces of solvent. The transparent film of Polymer P1 thus obtained was
characterized as
above (ATR-FTIR) and gave virtually the same infrared spectrum. DSC analysis
(second
pass) from -80 C to 200 C (10 C/min) gave a Tg equal to approximately 120 C.
5.4. Test 4 - Test of adhesion of Polymer 1 in a metal/rubber composite
In this test, a new sample of Polymer P1 according to the invention was
synthesized as
indicated in the preceding test, the y-butyrolactone solvent being simply
replaced with DTP.
A thin film of Polymer PI thus obtained was deposited (at room temperature)
uniformly on
the surface of a brass sheet. Everything was then covered with a layer of
conventional textile
P10-3421
CA 02960933 2017-03-10
- 13 -
adhesive of the RFL type (resorcinol-formaldehyde-latex). After a 5 min pre-
drying operation
at 100 C, everything was then treated for 10 min in the oven at 190 C.
The brass sheet thus coated with the film of Polymer P1 and coated with RFL
adhesive was
subsequently placed in a matrix of conventional rubber composition (in the
raw, non-
vulcanized state) for a belt reinforcement of a passenger vehicle tyre, based
on natural rubber,
on carbon black and silica as filler and on a vulcanization system (sulphur
and sulphenamide
accelerator), this composition being devoid of cobalt salt.
The metal/rubber composite test specimen thus prepared was then placed under a
press and
everything was cured (vulcanized) at 165 C for 30 min under a pressure of 20
bar. After
vulcanization of the rubber, excellent adhesive bonding between the rubber
matrix and the
metal sheet was obtained, despite the absence of cobalt salt in the rubber
matrix; this is
because, during peel tests carried out both at room temperature (23 C) and at
high
.. temperature (100 C), it was found that the failure occurred systematically
in the rubber
matrix itself and not at the interphase between metal and rubber.
5.5. Test 5- Synthesis of Polymer P2 by reacting Monomers Al, A3 and A4
This test describes the synthesis of Polymer P3 in accordance with the
invention starting from
Monomers Al, A3 and A4, according to the procedure represented
diagrammatically in
Figure 5.
1.34 g of Monomer Al, 0.61 g of Monomer AS (BHBA, 2,2-
bis(hydroxymethyl)propionic
acid) then 7.90 g of Monomer A3 (caprolactam-blocked MDI "Grilbond" IL-6 50%-
F) were
added successively into a glass flask. The suspension was stirred by
mechanical vibration
(vortex device) while gently increasing the temperature to approximately 50 C
(stream of hot
air). 2.3 g of the suspension obtained were then distributed homogeneously on
a (10 x 10 cm)
glass sheet which was then treated in an oven for 10 min at 190 C until a
yellow-coloured
clear film was obtained; 10 additional minutes of treatment were carried out
under vacuum so
as to eliminate the gaseous components (i.e. total treatment time of 20 min at
190 C). A thin
yellow film of Polymer P2 according to the invention was thus obtained, which
film adheres
very well to the glass (impossibility of separating by pulling the polymer
from the glass).
150 mg of the above suspension were also placed on a brass sheet (3 x 3 cm)
and treatment
was subsequently carried out in an oven at 190 C for 10 min and then for an
additional 10
min under vacuum (i.e., a total treatment at 190 C of 20 min); excellent
adhesion of the
polymer according to the invention to the metal could also be confirmed, with
impossibility
of separating by pulling the polymer from the brass sheet.
P10-3421
CA 02960933 2017-03-10
- 14 -
In conclusion, the sulphur-containing polyol compounds of the invention make
it possible to
synthesize polyurethanes which are characterized by a high glass transition
temperature, a
high thermal and chemical stability and excellent adhesion to glass or metal.
By virtue of these compounds of the invention, these polyurethanes, used as
adhesion primer
on metal in metal/rubber composites, make it possible very advantageously to
subsequently
adhesively bond the metal to the rubber matrices, for example using simple
textile adhesives,
such as "RFL" (resorcinol-formaldehyde-latex) adhesives or other equivalent
adhesive
compositions, or else directly (that is to say, without use of such adhesives)
to these rubber
.. matrices, for example when these rubber matrices comprise, in particular,
appropriate
functionalized unsaturated elastomers, such as epoxidized elastomers.
Thus, cobalt salts (or other metal salts) can especially be dispensed with in
the rubber
compositions intended to be attached to brass-coated metal reinforcers.
P10-3421