Note: Descriptions are shown in the official language in which they were submitted.
CA 02968346 2017-05-18
PROCEDURE FOR OBTAINING INSTANTANEOUS QUINOA
FIELD OF THE INVENTION
This invention relates to a process for the production of
instantaneous quinoa flour which comprises cooking, milling
the quinoa and a subsequent enzymatic treatment with amylases
and proteases.
STATE OF THE ART
Quinoa is a pseudo-cereal that is nutritionally
characterized by high nutritional value and because the
quality and balance of its proteins are superior to the same
characteristics of cereals. 37% of the proteins that quinoa
has are formed by essential amino acids that are those that
are not produced in the human body and therefore need to be
ingested through the diet.
In the last decades, we have studied the different
functionalities of this seed, such as the production of
bioactive peptides. Quinoa is relevant especially because
opens the possibility of obtaining food of high added value
directed to athletes, seniors, children or those people who
require special diet regimens.
1
. .
CA 02968346 2017-05-18
In the state of the art, WO 2013 026785 discloses how to
carry out an enzymatic hydrolysis from a ground carbohydrate-
rich feed mixture by the use of at least one amylase enzyme
in an extruder. Said document describes that the milled
mixture has at least 50% carbohydrates. As an example of raw
materials, different cereals and pseudo-cereals are
disclosed, among which is quinoa.
The enzymes used in the processes of this previous enzymatic
hydrolysis are a -amylases, 13-amylases (EC 3.2.1.1),
pullulanases (EC: 3.2.1.41) and glucoamylases.
Further, this prior discloses an enzyme /feed mix ratio (w/w)
of 0.05 to 0.00005. That is, an enzyme /substrate ratio (by
weight) of between 5 g enzyme per 100 g of food mix and 0.005
g of enzyme per 100 g of ground mix.
The conditions reported by WO 2013 026785 for performing an
enzymatic hydrolysis in an extruder comprises a temperature
between 60 C and 80 C for less than 30 minutes and a pH for
the aqueous solution to be added to the extruder between 5
and 8.
Another document reported in the state of the art is patent
application W02012CL73 which teaches an example of enzymatic
hydrolysis from a solution based on previously ground quinoa
2
, .
CA 02968346 2017-05-18
flour having a particle size of less than 100 pm and water,
wherein the pH of the solution is adjusted to 6 and then
added to amylase, increasing the temperature to 55 C for 1
h with constant stirring. After which, the temperature is
increased to 85 C, resulting in an increase of reducing
sugars.
The above suggests that extending the enzymatic hydrolysis
for times greater than 20 minutes does not achieve an effect
on the content of such reducing sugars.
The Peruvian application PE20130604 teaches to produce a
hydrolyzed quinoa beverage, the raw material of which are
milled and ground quinoa grains, from which a suspension is
created. This suspension is thermally treated prior to
commencing the enzymatic hydrolysis as such and then after
conditioning and enzymatic supplementation, the hydrolyzate
is centrifuged, the insoluble fraction is subjected to
leaching and, finally, the steps of concentration, thermal
treatment and packaging.
Quinoa contains 13 g of protein, 45% more than corn, 27%
more than oats, almost twice as much as rice (95%) and
similar to wheat. A portion of about 50 g of quinoa flour
covers more than 10% of the daily requirement of an adult
protein (56 g). A ration of 10 to 15 g of quinoa flour (1.3
3
CA 02968346 2017-05-18
to 2 g of protein) covers more than 10% of the daily
requirement of protein in children with 1 to 8 years. The
quality of the quinoa protein is good, since it has all the
essential amino acids in sufficient quantity to be considered
as a good source of these.
Unlike grains of mass consumption, quinoa is not deficient
in lysine. For example, when compared to wheat, quinoa
contains 50% more of this amino acid. In animal and human
studies it has been shown that the quality of the quinoa
protein is similar to that of casein (milk protein).
The absorption of nutrients (digestibility) of ground quinoa
(quinoa flour) is better than the absorption of quinoa in
grain. Quinoa is gluten-free, so this food could be promoted
to increase food choices within the timid diet of celiac
patients.
Quinoa can be used for the production of breads, noodles,
flakes and cookies that increase the supply of these foods
for celiacs, who represent approximately 1% of the
population.
However, there is still an unmet need related to the
processes for obtaining quinoa flours with improved
solubilities, especially for instantaneous quinoa flours.
4
CA 02968346 2017-05-18
In this regard, it has been found that the quinoa flours
obtained according to the process of this invention have
advantages over conventional quinoa flour, mainly as regards
the solubility of the product in water given the combination
of the cooking step of quinoa in the extrusion and the
subsequent hydrolysis step with the enzymes in the process
of the invention.
This improvement in the solubility of the final product
will then translate into an improvement in the
digestibility in the consumers.
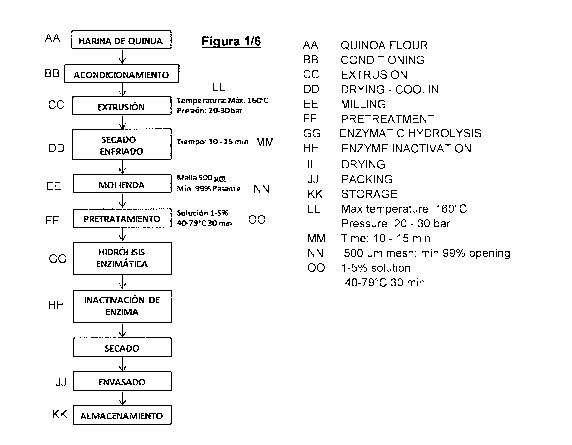
DETAILED DESCRIPTION OF THE FIGURES
Figure 1 shows a general outline of the process according to
the invention.
Figure 2 shows a graph which reports the amounts of
hydrolyzed starch, residual starch and glucose released for
different enzymes used in the process of the invention at
the same concentrations and over a 5 hour incubation period.
Figure 3 shows the result of the incubation for 5 hours in
accordance with the process of the invention using the enzyme
a-amylase by varying its concentration.
CA 02968346 2017-05-18
Figure 4 shows the result of the incubation for 5 hours in
accordance with the process of the invention using the
Kleistase SD80 enzyme varying its concentration.
Figure 5 illustrates the comparison of the results of
hydrolyzed starch, residual starch and released glucose
obtained for two quinoa flours, one by conventional methods
and the other by the process of this invention, using a-
amylase in step of hydrolyzate.
Figure 6 illustrates the comparison of the results of
hydrolyzed starch, residual starch and released glucose
obtained for two quinoa flours, one by conventional methods
and the other by the process of this invention, using
Kleistaase SD800 in the step of hydrolyzed.
DETAILED DESCRIPTION OF THE INVENTION
The subject invention relates to the development of
instantaneous Quinoa flour obtained by a process of cooking
and milling quinoa flour from which a product is obtained
which is subsequently subjected to an enzymatic hydrolysis
using enzymes but not limited to amylases and proteases.
6
. .
CA 02968346 2017-05-18
The stages prior to the process of the invention comprise
the reception of the input (quinoa flour), where the
inspection and verification of the sanitary conditions of
the means of transport and the quality characteristics of
the quinoa flour and the containers which contain them.
Then, the quinoa flour is weighed according to the batch to
be prepared, followed by the conditioning consisting of
emptying the flour into a vibrating screen with a selector
screen and a protective magnet. By means of pneumatic
transport, it is carried to a tank which has a conductor at
the outlet to the extruder.
The process of the invention, which may comprise the
aforementioned previous steps, further comprises the step of
firing by high temperature effect and pressure in an
extruder. The temperature is increased thanks to the
transformation of the mechanical energy into heat in the
canon of the extruder and by its configuration ensures the
proper friction and shear conditions.
In this stage, temperatures up to 170 C, preferably between
110 C and 140 C and pressures between 15-40 bar (1.5-4 MPa)
are reached thanks to the mechanical action of the elements
with which each one is composed of the two existing screws,
added to the addition of water to the extruder that is vital,
7
CA 02968346 2017-05-18
ranging from 4 liters /hour to 90 liters /hour. The average
time of stay of the material in process within the equipment
varies between 1 to 3 minutes. As the product exits through
the nozzle of the extruder, the water evaporates due to the
change in temperature and pressure and the quinoa undergoes
an expansion, increasing its surface and with humidity
between 0 and 8%. This process of extrusion allows obtaining
unit mass particles of a given size (called pellets).
Subsequently, the pellets are transported by a pneumatic
system to a three zone dryer oven. The first two zones are
of application of heat to temperatures that reach 120-160 C,
while the Ciltima zone is of cooling by circulation of air.
The residence time of the product in process at this stage
is approximately 10 to 15 minutes.
In the next step, the dry pellets are transported by a bucket
elevator and conveyor belt to the mill of interchangeable
mesh hammers, proceeding to their final grinding by reducing
the particle size between 200 and 600 microns.
The product obtained in the previous step is dissolved in
water at a concentration between 1 and 10% (w/v), preferably
5% (w/v). The solution is then subjected to a temperature of
between 40 and 70 C, preferably 65 C, for 30 minutes under
stirring.
8
CA 02968346 2017-05-18
After 30 minutes, the enzyme is added to perform the
enzymatic hydrolysis in a range of enzyme concentration of
0.01 to 0.5% (grams of enzyme /100 grams of dry flour),
wherein the enzymes may be selected from the group consisting
of amylases and proteases. The amylases may be selected from
the group consisting of Fungamil 800L, Bacterial a-amylasa,
Kleistasa SD800, Amyloglucosidase, Amylase AG300L Saczyme
and mixtures thereof. The proteases may be selected from the
group consisting of Alcalase, Neutrase, Flavourzyme,
NovoPro, Protamex and mixtures thereof. In one embodiment of
the invention, the enzyme may comprise the mixture of
amylaceas and proteases, in proportions of 0.02 amylase (gr
enzyme /100 g extruded quinoa flour) and 2% protease (gr
enzyme /100 g Extruded quinoa flour).
It is then allowed to incubate for 5 hours at a pH selected
from 4 to 8 at 40-60 C.
At the end of the incubation time, the inactivation of the
enzymes is carried out by thermal treatment at a temperature
of 90-110 C for a time between 5 and 20 minutes.
9
CA 02968346 2017-05-18
Once the enzymes are inactivated, the product passes into
the final lyophilization step. At this stage the product is
subjected to a vacuum pressure of 0.25 mbar (2.5X10-5 MPa)
and is brought to a freezing state at -40 C and then the
temperature is increased by 5 C each time, until reaching
25 C, holding each temperature for a time of 4 hours. The
total process takes 48 hours, in which the vacuum pressure
is maintained.
Optionally, the product may be spray-dried at an inlet
temperature of 100 C to 320 C and an outlet temperature of
80 C to 130 C or by any other drying process employing
pressures below 1 atm (0.1 MPa) and temperatures below 100 C,
which does not generate a denaturation of the proteins and
peptides.
The powdered product obtained is subsequently packaged and
stored.
EXAMPLES
Different tests were carried out with different enzymes in
order to obtain a hydrolyzate with low glucose content
released and high solubility.
CA 02968346 2017-05-18
Amylases and proteases were separately assessed to determine
the dose, temperature, pH and incubation time for each
enzyme.
The optimum parameters for the amylases were determined by
evaluating the content of hydrolyzed starch (g/L) and
released glucose (g/L) from the hydrolysis.
Optimal parameters for proteases were determined by
evaluating the peptide concentration (mg/ml) in the
hydrolyzed product.
Optimization of Temperature and pH:
Temperature and pH optimum values for proteases were
established based on previous experiments with these
enzymes:
Protease Temperatura pH
Alcalase 60 C 8
Neutrase 45 C 6.5
Flavourzyme 45 C 6.5
Novo Pro 60 C 6.5
11
CA 02968346 2017-05-18
Protamex 45 C 6.5
For the case of the amylases, tests were performed to
determine the optimum temperature and pH, considering the
following fixed parameters:
- Reaction volume: 1.5 ml.
- Incubation time: 30 minutes
- Substrate: Quinoa flour at 5% (w/v) pre-incubated for
30 minutes at 65 C with shaking.
- Enzyme dose: 0.5% (g of enzyme/100g of substrate),
except for Bacterial a-amylase and Kleistada SD80, for
which the dose was reduced to 0.1%.
For the optimization of temperature, pH 6 was used; while
for the optimization of pH a temperature of 60 C was used.
Temperature Optimization for Amylases:
Temperature Residual Glucose Hydrolyzed
( C) starch produced starch
(g/L) (g/L) (g/L)
12
CA 02968346 2017-05-18
Fungamil 800L 0.5%
14.11 0.59
40 9.81 0.59 1.26 0.00
(58.57 %)
13.89 0.31
50 8.85 0.31 .1.45 0.04
(62.61 %)
16.86 0.90
60 7.52 0.90 1.79 007
(68.24 %)
Bacterial a-
0.1%
amylase
19.69 0.31
40 4.23 0.31 1.65 0.02
(82.13 %)
19.33 0.06
50 3.42 0.06 1.85 0.01
(85.57 %)
21.84 0.17
60 2.54 0.17 2.36 0.00
(89.26 %)
Kleistasa SDSO 0.1%
21.69 0.11
40 2.22 0.11 1.66 0.05
(90.60 %)
20.98 0.25
50 1.77 0.25 1.92 0.03
(92.53 %)
1.29 23.09 0.03
60 2.29 0.01
0Ø3 (94.55 %)
Amylasa AG 300L 0.5%
1.85 0.03
40 22.07 0.03 2.51 0.19
(6.81 %)
13
CA 02968346 2017-05-18
0.76 1.88
50 21.99 1.88 3.30 0.01
(7.15 %)
4.92 1.63
60 19.46 1.63 4.34 0.26
(17.82 %)
Antiloglucosidasa 0.5%
3.14 1.69
40 20.78 1.69. 5.30 0.25
(12.26 %)
2.62 1.38
50 20.12 1.38 6.65 0.02
(15.02 96)
8.96 0.06
60 15.41 0.06 8.01 0.09
(34.91 %)
Saczyme 0.5%
1.85 0.08
40 22.0 0.08 4.24 0.04
(6.81 %)
1.21+0.11
50 21.53 0.11 5.36 0.09
(9.08 96)
4.90 0.42
60 19.48 0.42 6.91 0.25
(17.74 %)
Optimization of pH for amylases:
Residual Glucose Hydrolyzed
PH starch produced starch
(g/L) (g/L) (g/L)
14
. .
CA 02968346 2017-05-18
Fungamil 800L 0.5%
18.24 0.44
4 5.17 0.44 4.79 0.13
(78.19 %)
15.85 0.09
5 5.26 0.09 3.92 0.19
(75.40 %)
16.11 0.35
6 7.17 0.35 5.12 0.78
(69.37 %)
12.39 0.87
7 12.10 0.87 0.31 0.12
(51.63 %)
Bacterial a-
0.1%
amylase
10.53 0.53
4 12.88 0.53 4.67 1.27
(44.22 %)
16.53 0.23
5 4.58 0.23 3.35 1.49
(78.03 %)
20.86 0.14
6 2.41 0.14 2.59 0.25
(89.60 %)
22.85 0.28
7 1.63 0.28 1.13 0.27
(93.19 %)
Kleistasa SDSO 0.1%
12.74 1.35
4 10.67 1.35 4.43 0.43
(53.79 %)
17.68 0.23
5 3.42 0.23 3.41 0.05
(83.57 %)
CA 02968346 2017-05-18
21.76 0.17
6 1.51 0.17 2.86 0.04
(93.47 %)
23.33 0.06
7 1.15 0.06 1.05 0.03
(95.18 %)
Amylase AG 300L 0.5%
7.72 1.54
4 15.69 1.54 9.60 0.27
(33.89 %)
6.22 1.45
14.89 1.45 5.87 0.91
(30.35 %)
3.41 0.29
6 19.86 0.29 5.34 0.64
(15.12 %)
2.31 0.78
7 22.18 0.78 1.87 0.12
(11.30 %)
Amyloglucosidase 0.5%
16.85 0.59
4 6.56 0.59 17.29 0.12
(72.36 %)
9.52 0.03
5 11.59 0.03 12.90 0.75
(45.79 %)
6.12 0.34
6 17.15 0.34 8.40 0.05
(26.66 %)
3.91 0.00
7 20.58 0.00 2.75 0.00
(17.68 %)
Saczyme 0.5%
13.14 0.73
4 10.27 0.73 16.61 0.43
(56.73 %)
16
CA 02968346 2017-05-18
7.92+0.15
13.18 0.15 11.42 0.17
(38.31 %)
6.15 0.67
6 17.13 0.67 7.69 0.11
(26.81 %)
6.04 0.26
7 18.44 0.26 4.17 0.08
(26.22 %)
In accordance with the above results, the following values
of temperature and pH were selected for the amylases:
Amylase Temperatura pH
Fungannil 800L 60 C 4
Bacterial a-
60 C 7
amylase
Kieistasa SD80 60 C 7
Amylase AG 300L 60 C 4
Amyloglucosidase 60 C 4
Saczyme 60 C 4
2 Optimization of enzyme dose:
To determine the best dose of enzyme, an incubation time of
30 minutes was used.
Optimization of enzyme doses for proteases:
Dose Peptide concentration (mg/mL)
17
CA 02968346 2017-05-18
Protease 0.02% 0.2% 2% 10% 20%
Alcalase 0.38+0.00 0.61 0.01 0.98 0.07 1.12 0.0 1.13 0.0
pH 8, 60 C 3 2
Neutrase
0.92 0.0 0.82 0.0
pH 6.5, 0.39 0.02 0.58 0.01 0.81
0.01
2 3
45 C
Flavourzyme
1.58 0.0 1.84 0.0
pH 6.5, 0.39 0.01 0.54 0.01 1.17
0.08
6 5
45 C
Novo Pro
1.04 0.0 1.00 0.0
pH 6.5, 0.39 0.01 0.61 0.02 0.95
0.03
6 4
60 C
Protamex
1.04 0.0 1.04 0.0
pH 6.5, 0.44 0.02 0.67 0.02 0.94
0.06
6 6
45 C
Enzyme dosage optimization for amylases:
Enzyme dosage Residual Glucose Hydrolyzed
(%) starch produced starch
(g/L) (g/L) (g/L)
Fungamil 800L pH 4 60 C
17.11 0.26
0.5 5.36 0.26 5.20 0.18
(75.91 %)
18
CA 02968346 2017-05-18
10.33 0.72
0.1 12.14 0.72 3.91 0.14
(45.41 %)
6.32 0.11
0.05 16.14 0.11 2.49 0.29
(27.40 %)
2.55 0.72
0.02 19.91 0.72 1.66 0.07
(10.42 %)
1.35 0.92
0.01 21.11 0.92 0.91 0.10
(5.04. %)
Bacterial a-
pH 7 60 C
amylase
19.85 0.09
0.1 1.69 0.09 1.32 0.09
(92.34 %)
12.83 0.49
0.01 8.72 0.49 0.71 0.06
(60.59 %)
4.45 1.14
0.002 17.09 1.14 0.58 0.06
(22.72 %)
2.96 0.97
0.001 18.59 0.97 0.43 0.01
(15.97 %)
2.41 0.66
0.0005 19.13 0.66 0.28 0.03
(13.50 %)
Kleistasa SDSO pH 7 60 C
16.43 0.84
0.1 6.03 0.84 21.00 0.26
(72.27 %)
11.21 0.63
0.01 11.25 0.63 14.14 1.58
(48.26 %)
19
CA 02968346 2017-05-18
6.46 0.44
0.002 16.00 0.44 9.15 0.44
(26.43 %)
3.04 0.46
0.001 19.42 0.46 5.53 0.14
(10.69 %)
0.71 0.06
0.0005 21.63 0.06 6.32 0.36
(0.55 %)
Amylase AG 300L pH 4 60 C
16.43 0.84
2 6.03 0.84 21.00 0.26
(72.27 %)
11.21 0.63
1 11.25 0.63 14.14 1.58
(48.26 %)
6.46 0.44
0.5 16.00 0.44 9.15 0.44
(26.43 %)
3.04 0.46
0.3 19.42 0.46 5.53 0.14
(10.69 %)
0.71 0.06
0.1 21.63 0.06 6.32 0.36
(0.55 %)
Amyloglucosidase pH 4 60 C
15.16 0.36
0.5 6.31 0.36 19.87 0.35
(71.01 %)
13.82 0.38
0.4 8.65 0.38 16.61 0.05
(60.24 %)
10.2.6 0.67
0.3 12.21 0.67 14.63 0.43
(43.88 %)
CA 02968346 2017-05-18
8.28 0.41
0.2. 14.19 0.41 10.56 1.20
(34.76 %)
3.96 0.84
0.1 19.12 0.84 2.14 0.03
(12.10 %)
Saczyme pH 4 60 C
16.06 0.88
0.8 6.41 0.38 19.15 0.26
(71.18 %)
10.11 0.14
0.4 12.35 0.14 13.83 0.36
(44.43 %)
5.20 0.53
0.2 17.26 0.53 8.30 0.21
(22.35 %)
3.01 1.63
0.1 19.45 1.63 4.79 0.17
(12.49 %)
1.94 0.42
0.05 20.52 0.42 2.60 0.07
(7.69 %)
Optimal dose per enzyme:
Enzyme Activity Dose
Alcalase Protease 2%
Neutrase Protease 2%
Flavourzyme Protease 2%
Novo Pro Protease 2%
Promatex Protease 2%
21
CA 02968346 2017-05-18
Fungamil 800L a-amylase 0,5%
Bacterial a- a-amylase
0,02%
amylase
Kleistasa SD80 a-amylase 0,02%
Amylase AG 300L Glucoamylase 0,3%
Amyloglucosidase Glucoamylase 0,1%
Saczyme Glucoamylase 0,4%
30 Optimization of incubation time:
Using the doses selected in the previous step, we tried to
optimize the incubation time for each enzyme.
Optimization of incubation time for proteases:
Time Peptide concentration (mg/mL)
Protease 30 min 2 horas 5 horas 24 horas
Alcalase 2% 0.93 0.08 1.17 0.06 1.33 0 04
2.49 0.00
pH 8, 60 C
Neutrase 2% 0.68 0.01 0.97 0.01 0.89 0.05
1.29 0.09
pH 6.5, 45 C
Flavourzyme 0.99 0Ø 1.65 0.02 2.03 0.01 2.35 0.01
2% 2
pH 6.5, 45 C
Novo Pro 2% 0.86 0.06 1.01 0.04 1.02 0.05
1.66 0.00
pH 6.5, 60 C
22
CA 02968346 2017-05-18
Protamex 2% 0.84+0.00 0.86 0.01 1.04 0.01
1.72 0.06
pH 6.5, 45 C
Time Yield of hydrolysis (%)*
Protease 30 min 2 horas 5 horas 24 horas
Alcalase 2% 15.22 1.0 18.77 0.9 21.10 0.8
37.06 1.90
pH 8, 60 C 0 5 0
Neutrase 2% 10.52 0.1 14.92 0.3 13.58 0.7
19.82 1.27
pH 6.5, 45 C 8 0 2
Flavourzyme 15.20 0.9
25.34 1.3 31.23 1.4
2% 5 36.18 0.52
6 4
pH 6.5, 45 C
Novo Pro 2% 13.2 + 15.47 0.6
15.7 0.71 25.46 0.44
pH 6.5, 60 C 0.76 3
Protamex 2% 12.88 0.1 13.24 0.3 15.96 0.3
26.51 1.02
pH 6.5, 45 C 1 0 1
* Yield calculated by taking into account an initial protein
value of 13% in the substrate.
Optimization of incubation time for amylases:
23
CA 02968346 2017-05-18
Incubation Time Residual Glucose Hydrolyzed
starch produced starch
(g/L) (g/L) (g/L)
Fungamil 800L 0.5 % pH 4 60 C
19.51 0.44
30 minutes 5.54 0.43 7.50 0.17
(77.88 %)
20.44 0.09
1 hour 4.60 0.09 9.82 0.23
(81.62 %)
20.50 0.12
2 hours 4.54 0.12 12.90 1.46
(81.87 %)
20.77 0.49
3 hours 4.28 0.49 16.08 0.53
(82.92 %)
21.03 0.29
4 hours 4.01 0.29 17.18 0.17
(83.98 %)
21.76 0.18
hours 3.29 0.18 18.87 0.04
(87.21%)
Bacterial a-
0.02 % pH 7 60 C
amylase
17.14 0.29
30 minutes 7.35 0.29 0.94 0.05
(70.65 %)
17.82 0.21
1 hour 6.68 0.21 1.29 0.02
(72.74 %)
19.21 0.21
2 hours 5.29 0.21 2.53 0.04
(7.8.42 %)
24
CA 02968346 2017-05-18
19.7110.03
3 hours 4.78 0.03 3.73 0.53
(80.48 %)
20.32 0.12
4 hours 4.17 0.12 3.74 0.10
(82.98 %)
22.00 0.06
hours 2.50 0.06 4.14 0.48
(89.26 %)
Kleistasa SDSO 0.02 % pH 7 60 C
19.14 0.12
30 minutes 5.36 0.12 0.82 0.22
(78.61 %)
20.49 0.30
1 hour 4,00 0.30 1.54 0.01
(83.66 %)
21.69 0.09
2 hours 2.80 0.09 2.68 0.15
(88.56 %)
22.33 0.09
3 hours 2.17 0.09 3.99 0.17
(91.14 %)
22.66 0.21
4 hours 1.83 0.21 4.19 0.14
(92.52 %)
23.22 0,23
5 hours 1.28 0.23 4.22 0.19
(94.50%)
Amylase AG 300L 0.3 % pH 4 60 C
3.04 0.46
30 minutes 19.42 0.46 6.32 0.14
(25.30'%)
8.91 1.11
1 hour 17.09 1.11 8.12 1.28
(34.28 %)
CA 02968346 2017-05-18
12.24 1.54
2 hours 13.77 1.54 14.48 0.35
(47.07 %)
17.48 0.76
3 hours 8.53 0.76 18.84 1.35
(67.21 %)
19.81 1.50
4 hours 6.20 1.50 20.99 0.98
(76.17 %)
24.61 0.32
hours 2.42 0.32 26.78 0.89
(90.71 %)
Amyloglucosidase 0.1 % pH 4 60 C
3.96 0.84
30 minutes 18.50 0.84 2.14 0.03
(28.84 %)
8.24 0.45
1 hour 17.77 0.45 9.04 0.20
(31.68 %)
15.27 2.09
2 hours 10;73 2.09 16.10 0.49
(58.73 %)
19.51 0.13
3 hours 6.49 0.13 20.09 2.00
(75.03 %)
20.09 0.41
4 hours 5.92 0.41 21.25 0.89
(77.24 %)
26.48 0.21
5 hours 0.54 0.21 22.33 1.45
(97.92 %)
Saczyme 0.1 % pH 4 60 C
3.01 1.63
30 minutes 19.45 1.63 4.79 0.17
(13.38 %)
26
CA 02968346 2017-05-18
6.63 0.01
1 hour 19.37 0.01 7.76 0.01
(25.50 %)
10.96 1.21
2 hours 15.04 1.21 13.21 0.23
(42.15 %)
15.51 1.06
3 hours 10.49 1.06 17.02 0.09
(59.65 %)
16.32 0.72
4 hours 9.68 0.72 17.61 0.45
(62.76 %)
23.19 0.50
hours 3.83 0.50 24.89 0.47
(85.27 %)
Summary of optimum conditions:
ENZYME ACTIVITY PH T Dose
( C) (%) incubation
(h)
Fungamil 800L a-amylase 4 60 0.5 5
Bacterial a-
a-amylase 7 60 0.02 5
amylase
Kleistasa SD80 a-amylase 7 60 0.02 5
Amyloglucosidase Glucoamylase 4 60 0.3 5
Amylase AG 300L Glucoamylase 4 60 0.1 5
Saczyme Glucoamylase 4 60 0.1 5
Alcalase Protease 8 60 2.0 5
27
CA 02968346 2017-05-18
Neutrase Protease 6.5 45 2.0 5
Flavourzyme Protease 6.5 45 2.0 5
Novo Pro Protease 6.5 60 2.0 5
Protamex Protease 6.5 45 2.0 5
EXAMPLE 1
Two amylases were selected for the hydrolysis: Bacterial a-
amylase and Kleistasa.
A further hydrolysis was carried out with the selected
amylases using as substrate extruded quinoa flour obtained
according to the process of the invention and an unexpected
effect was found, since the glucose released during the
hydrolysis was less with quinoa flour extruded than with
conventional quinoa flour under the same incubation
conditions (5 h, 60 C)
28
Incubation
Incubation with bacterial a- Incubation with
kleistasa
SUBSTRATE SOLUTION without enzyme amylase
SD80
5%
Initial Initial Residual Hydrolyzed Free
'Residual Hydrolyzed Free
starch glucose starch starch glucose
starch starch glucose
(g/L) (g/L) (g/L) (g/L)
(g/L) (g/L) (g/L) (g/L)
Quinoa flour 24.50 1.62 2.50 22.00
4.14 1.28+ 23.22 4.22 P
0.43 0.10 0.06 0.06 0.47
0.23 0.23 0.19
1.0
Instantaneous 26.72 0.02 2.99 23.73
1.01 2.28 24.44 1.63+
quinoa flour 2.94 0.01 0.03 0.03
0.15 0.06 0.06 0.10
(According to the
process of the
invention)
CA 02968346 2017-05-18
When analyzing the hydrolysis of the extruded quinoa flour
(made by the process of this invention) with the chosen
enzymes, an unexpected effect was surprisingly encountered,
since less glucose was released compared to the hydrolysis
of the flour of quinoa obtained by other conventional
processes, in which the extrusion step is not included.
EXAMPLE 2
Two proteases and one amylase were selected for hydrolysis
with a combination of enzymes.
A hydrolysis was carried out with the inoculated amylase
(Bacterial a-amylase), which was called hydrolyzed 1,
another (hydrolyzed 2) with the mixture of Bacterial a-
amylase + NovoPro and another one called hydrolyzate 3 with
the mixture of Bacterial a-amylase + Protamex.
The following table shows the characterization of the
products in terms of starch, glucose and peptides
CA 02968346 2017-05-18
Sample Starch
Glucose Peptides
(g/100g) (g/100g) Peptides
Instantaneous quinoa flour 2.99
0.07
(according to the process of 55.92 1.09 1.18 0.04
the invention)
Hydrolyzed 1 with Bacterial 5.24 0.24 3.56 0.01
5.88 0.20
a-amylase
Hydrolyzed 2 with Bacterial 7.40 0.61 2.62 0.01
8.94 0.38
a-amylase
Hydrolyzed 3 with Bacterial 6.58 0.06 2.25 0.06
7.72 0.18
a-amylase
Then, in a solubility test, the three hydrolyzed products
and the untreated quinoa flour in 5% solution were placed in
50 milliliter specimens and allowed to settle for 24 hours.
After this time, it was observed that the flour precipitate
was 19 ml for the untreated quinoa, 4 ml for the hydrolyzate
1, 6 ml for the hydrolyzate 2 and 5 ml for the hydrolyzate
3.
These data show that the products according to the process
of the invention reduce the volume of the precipitated
material by 79%, 68% and 74% respectively for the hydrolyzate
1, 2 and 3, so that the process of this invention
substantially improves the solubility of the product
obtained with respect to the original untreated flour.
31