Note: Descriptions are shown in the official language in which they were submitted.
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
1
PACKAGE FOR CONSUMER CARE PRODUCTS
FIELD
This disclosure relates to packages for consumer care products and methods of
manufacturing the same. The packages are particularly suited for
antiperspirant and/or deodorant
products, but can equally be employed for other types of consumer care
products.
BACKGROUND
Traditionally, consumer care products such as antiperspirants and/or deodorant
products are
packaged in an oval or round plastic barrel component. The top of the barrel
is open to allow the
product to be exposed and dispensed for use, while the opposite, i.e. bottom,
end of the barrel
contains a mechanism (e.g., a product support elevator coupled with a hand-
rotatable screw) to assist
in the dispensing of the product.
Antiperspirant and deodorant compositions are offered by manufacturers in a
variety of sizes
and product forms such as liquids, creams, gels, semi-solids, and solid
sticks. These products have
different ingredients, active levels, solvents, viscosities, shapes, sizes,
and fill volumes to address a
variety of consumer preferences and needs. In this regard, manufacturers
desire a more efficient
way of producing these numerous product offerings, especially under a single
brand.
Currently, manufacturers may use different size barrels to accommodate
different fill
volumes. Alternatively, manufacturers may accommodate different fill volumes
by changing the
spindle and/or the elevator designs. A change in one molded component of the
packaging requires
adaptations of the other components. Each packaging design must be adapted to
avoid
manufacturing, shipping, storage, and dispensing problems that are associated
with these different
product offerings. For example, different fill volumes for compositions may
exhibit different
stability profiles, may apply different internal pressures on the package, may
require air-tight seals,
may cause different degrees of solvent syneresis or weeping, and may require
different package
designs for ease of and consistent dosing of the composition.
In addition, manufactures have historically used a large number of injection
molding parts to
make different packaging components for the various product offerings. As a
result, sometimes as
many as 50-75 or more different molds must be developed, used, and maintained
in the injection
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
2
molding process. Thus, multiple product offerings to consumers present a major
challenge to
manufacturers.
Thus, a need exists for interchangeable package components to accommodate
different fill
volumes within a single package and/or product chamber configuration. The use
of the same mold
parts to manufacture packages that accommodate different fill volumes reduces
manufacturing cost
and complexity since fewer injection molds are needed. Also, manufacturing may
be consolidated to
fewer manufacturing lines. These advantages are provided while still providing
a dispensing
packaging demonstrating adequate strength, flexibility, aesthetic appearance,
stability, and
dispensing consistency for a variety of product offerings.
SUMMARY
The present disclosure is directed to consumer care products and/or packages.
In accordance
with one of the embodiments, a package for consumer care products and methods
of manufacturing
the same are provided. The packages are particularly suited for antiperspirant
and/or deodorant
products, but can equally be employed for other types of consumer care
products.
In one embodiment, a movable elevator platform for use in a dispensing package
includes a
coupling sleeve capable of engaging a screw assembly and a rim surrounding the
coupling sleeve.
The rim is non-removably attached to the coupling sleeve and includes a
flange. The movable
elevator platform has a vertical contact surface area and a horizontal contact
surface area. About
20% to about 90% of the horizontal contact surface area is located on the
flange. The flange has a
width that does not exceed about 8.5 mm.
In another embodiment, a movable elevator platform for use in a dispensing
package includes
a coupling sleeve capable of engaging a screw assembly and a rim surrounding
the coupling sleeve.
The rim is non-removably attached to the coupling sleeve and includes a
flange. The movable
elevator platform has a vertical contact surface area and a horizontal contact
surface area. The
flange has a width that does not exceed 8.5 mm. The ratio of the vertical
contact surface area to the
horizontal contact surface area is from 1:10 to 10:1.
BRIEF DESCRIPTION OF THE DRAWINGS
While the specification concludes with claims that particularly point out and
distinctly claim
the invention, it is believed that the present invention will be better
understood from the following
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
3
description of embodiments, taken in conjunction with the accompanying
drawings in which:
FIG. 1 is a front view of an illustrative consumer care product and dispensing
package
according to one or more embodiments shown and described herein.
FIG. 2 is an exploded perspective view of an illustrative dispensing package
for a consumer
care product, illustrating some of the individual components and having a form
suitable for bottom
filling according to one or more embodiments shown and described herein;
FIG. 3 is cross-sectional front view taken along a major axis of an
illustrative dispensing
packaging with a movable elevator platform at a first fill volume position
according to one or more
embodiments shown and described herein;
FIG. 4 is cross-sectional front view taken along a major axis of an
illustrative dispensing
packaging with a movable elevator platform at a second fill volume position
according to one or
more embodiments shown and described herein;
FIG. 5 is an example of a front view of an illustrative movable elevator
platform according to
one or more embodiments shown and described herein;
FIG. 6 is a side view of the movable elevator platform of FIG. 5;
FIG. 7 is a top view of the movable elevator platform of FIG. 5;
FIG. 8 is a perspective, bottom view of the movable elevator platform of FIG.
5;
FIG. 9 is a bottom view of the movable elevator platform of FIG. 5;
FIG. 10 is a perspective, bottom view of the movable elevator platform of FIG.
5;
FIG. 11 is a cross-sectional view of the front view of the movable elevator
platform of FIG.
5;
FIG. 12A is a perspective, bottom view of the movable elevator platform of
FIG. 5 and
illustrating a plane for the horizontal cross section according to one or more
embodiments shown
and described herein;
FIG. 12B is a perspective, bottom view of the movable elevator platform of
FIG. 5 after
horizontal cross sectional cut according to one or more embodiments shown and
described herein;
FIG. 12C is a perspective, bottom view of the movable elevator platform of
FIG. 5 filled with
a composition according to one or more embodiments shown and described herein;
FIG. 13A is a perspective, top view of a prior art elevator;
FIG. 13B is a perspective, bottom view the elevator of FIG. 13A; and
FIG. 13C is a perspective, bottom view of the elevator of FIG. 13A after a
horizontal cross
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
4
sectional cut.
DETAILED DESCRIPTION
While the specification concludes with the claims particularly pointing out
and distinctly
claiming the invention, it is believed that the present invention will be
better understood from the
following description.
As used herein, "consumer care product", which may also be referred to as the
"product",
refers to any consumer care product, including, but not limited to, beauty
care products, household
care products, health care products, pet care products, and the like.
"Antiperspirants", as used herein, includes
antiperspirants, deodorants,
deodorant/antiperspirants and body sprays, and may also be considered as
beauty care products.
The term "translucent", as used herein may include "frosted", "glittered",
"pearlescence" and
the like and is defined herein as the practice of inducing a low level of
light scattering into an
otherwise "clear" material causing the material to become matted in
appearance.
As used herein, "substantially opaque" refers to the ability to sufficiently
block the
transmission of light so that bodies lying behind are not easily perceivable.
Substantially opaque
includes "tinted" and is defined herein as the practice of adding a low level
of pigment or dye into a
material for the purpose of imparting a color into the material.
As used herein, "identifier" relates to a means for communicating between the
consumer and
the consumer care product such that the consumer may readily identify the
consumer care product
and its associated traits, including, but not limited to product form, product
performance, scents and
the like. Identifiers of the present invention may include, but are not
limited to, pressure sensitive
labels; shrink wrap labels; indicia; colors or other visually detectable or
discernable aspects (e.g.,
"sparkles" or "glitter" via incorporation of interference pigments) that are
part of the material from
which the packaging components are made or that is subsequently added to the
manufactured
components; defined relief, indentation, windows and/or gaps formed in the
components during or
after their manufacture; cast designs, including but not limited to novelty
casting to identify
characters, paraphernalia, animals, and the like; particular shapes or other
means of decoration
and/or information sharing used to identify and distinguish the product. The
identifiers may be
formed concurrently with the manufacture of the components with which they are
associated, may
be introduced during the manufacture of the components, and/or may be formed
or applied to the
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
components after the components are manufactured. The identifiers of the
present invention may be
the same or different from one another.
As used herein, "novelty cast" may include, but is not limited to,
casts/shapes that replicate
cars, sport balls, animals or people figures, characters, logos, sport
paraphernalia (e.g., helmets, bats,
5 jerseys, shoes and the like), fashion accessories and the like.
By "brand sub line" it is meant a line of products that are targeted to a
particular consumer
sub-group, provides a real or perceived distinctive benefit, and/or manifests
a real or perceived
distinctive attribute.
By way of example, a consumer care product may be an
antiperspirant/deodorant product with the sub lines including, a sensitive
skin line, a botanical line, a
high performance / high efficacy line, and a no fragrance line. Another
example of sub lines may
include a "treatment" line that comprises treatments to address extreme
personal care conditions
(e.g., malodor, excessive perspiration (hyperhidrosis), excessive dandruff,
excessive dryness, or
oiliness), a "high performance" line that targets superior performance as
compared to other offered
products, an "essentials" line that provides value-added, trusted or reliable
performance, and an
"expressives" line that provides sensorial experiences with reliable
performance. There may be a
single product form or multiple product forms within a given sub line. For
example, antiperspirant
and deodorant products can come in a variety of forms, including solids, soft
solids, gels, and roll-
ons. Various sub lines may include the same or different product forms and may
include the same
number or a different number of product forms. The consumer care product may
include a single
source identifier (e.g. single brand name) for the multiple sub lines.
FIG. 1 is a front elevation of one embodiment of a dispensing package 100 of a
consumer
care product as fully assembled. The dispensing package 100 comprises an outer
cap 300, an outer
jacket 200, a source identifier 192, and an identifier 191.
FIG. 2 is an exploded perspective view of FIG. 1 of a dispensing package 100
for a consumer
care product shown and described herein, illustrating some of the individual
components. FIG. 2
shows generally one embodiment where the dispensing package 100 may comprise
at least one
product chamber 110 and an outer jacket 200 for dispensing a consumer care
composition. The
dispensing package 100 further comprises an outer cap 300, optionally a seal
component 310, a
movable elevator platform 320, and a screw assembly 330.
As shown in FIG. 2 an illustrative dispensing package 100 has a product
chamber 110 that at
least partially surrounds and supports a consumer care composition, and an
outer surface 130, an
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
6
upper dispensing end 140, a lower end 150, top opening 160 that allows the
consumer care
composition to move up and outward, and a top ridged opening 161.
The consumer care composition may be in the form of a solid, semi-solid,
liquid, gel, mousse
or the like. Held within the surrounding walls, particularly an inner surface
(not shown) of the
product chamber 110, the composition may be dispensed from the top opening 160
of the product
chamber 110 and from the top ridged opening 161, both located at the upper
dispensing end 140 of
the product chamber 110.
The sidewall of the product chamber 110 may terminate in an upper dispensing
end 140.
With respect to the product chamber 110 and outer surface 130, the distance
from the upper
dispensing end 140 to the lower end 150 of the sialewall may optionally vary,
moving around the
circumference of such component (not shown), giving rise to an upper end or
top opening that is
higher in some spots than others, for example, the product chamber 110 may be
higher along the
sides of the dispenser than at the back or front of the dispensing package
100. This allows for
flexibility in body design and can allow the upper dispensing end of the
product chamber 110 to be
configured such that, toward the end of the dispenser life, it is less likely
to come into contact with
the surface to which the product is applied. Optionally, the upper dispensing
end of the product
chamber 110 may be beveled or chamfered.
FIGS. 3 and 4 are cross-sectional front views taken along the major axis of
one embodiment
of the dispensing packaging. FIG. 3 shows the movable elevator platform 320 at
a first fill volume
position 560. FM. 4 shows the movable elevator platform 320 at a second fill
volume position 570.
As shown in FIGS. 3 and 4, a movable elevator platform 320 comprises a
coupling sleeve
325 having a non-threaded section 530 and a threaded section 540 along an
inner surface 550 of the
coupling sleeve 325. The dispensing packaging further comprises a screw
assembly 330 comprising
a spindle 332 that supports threads 333, a seal 334 extending around the
circumference of the spindle
332, a threaded first portion 335 coupled to the threaded section 540 along
the inner surface of the
coupling sleeve 325 of the movable elevator platform 320. The screw assembly
330 further
comprises a non-threaded second portion 336. In this embodiment, the seal 334
frictionally engages
with the non-threaded section 530 of the coupling sleeve 325, providing a seal
that otherwise is
maintained during the advancement of the movable elevator platform 320 along
an axis from a first
fill volume position 560 to a second fill volume position 570. In one
embodiment the seal 334 that
frictionally engages with the non-threaded section 530 of the coupling sleeve
325 provides a seal
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
7
that substantially prevents air and/or liquid from passing between the seal
334 and the non-threaded
section 530 of the coupling sleeve 325. In another embodiment, the frictional
engagement of the
seal 334 (or the seal 334) is maintained for a distance corresponding to the
distance that the movable
elevator platform 320 moves along an axis from a first fill volume position
560 to a second fill
volume position 570, the distance being from about 0.1 inch to about 1.0 inch,
and/or from about 0.2
inch to about 0.6 inch.
Also as shown in FIGS. 3 and 4, the non-threaded section 530 of the movable
elevator
platform 320 is at the lower end of the inner surface 550 of the coupling
sleeve 325 and the threaded
section 540 is at the upper end of the inner surface 550 of the coupling
sleeve 325. The movable
elevator platform 320 further comprises a rim 400 that is in frictional
contact with the inner surface
120 of the product chamber 110 along the product chamber major axis 180 and
minor axis 190. In
an embodiment, the seal 334 extends beyond the outer surface 361 of the
spindle 332. The seal 334
may have a first diameter and the inner surface 550 of the non-threaded
section 530 of the coupling
sleeve 325 has a second diameter, wherein the first diameter is greater than
the second diameter.
In some embodiments, the seal 334 may comprise a continuous bead around the
circumference of the outer surface of the spindle 332, as shown in FIGS. 3 and
4. Alternatively, the
seal 334 may be a thread that is dimensioned to frictionally engage with the
inner surface 550 of the
non-threaded section 530 of the coupling sleeve 325, thereby providing a seal
and allowing the
movable elevator platform 320 to advance along an axis from a first fill
volume position 560 to a
second fill volume position 570.
In some embodiments the dispensing packaging 100 further comprises a ratchet
platform 380
where the non-threaded second portion 336 of the spindle 332 extends from the
ratchet platform 380
to the seal 334 for a distance of about 5 mm to about 45mm or from about 8 mm
to about 35 mm or
from about 10 mm to about 30 mm.
The spindle 332 may be separately molded and attached to the screw base or the
spindle 332
may be molded integrally with the screw base.
In one embodiment, the fill volume provides a composition volume of from about
5 ml to
about 200 ml and/or from about 25 ml to about 150 ml and/or from about 40 ml
to about 100 ml
and/or from about 50 ml to about 80 ml. In one embodiment, the second fill
volume position 570 is
about 1% to about 30% greater and/or about 5% to about 25% greater, and/or
about 10% to about
20% greater, than the first fill volume position 560 of the same size package.
In one embodiment,
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
8
the first fill volume position 560 provides a composition volume from about 15
ml to about 60 ml, or
from about 25 ml to about 50 and the second fill volume position 570 provides
a composition
volume from about 70 ml to about 200 ml or from about 75 ml to about 100 ml.
The size of the package depends, in part, upon the composition to be
dispensed, the dose at
which it is applied, the dispenser's intended life, and the intended use
(e.g., value size, samples,
travel size, and the like). The volume of the product chamber 110 will
typically be larger than the
volume of consumer care composition to accommodate component features and
production
requirements.
In one embodiment, the consumer care product is a top fill product, e.g.
wherein the
composition is filled into the product chamber 110 from the top of the
package, comprising an
antiperspirant or deodorant composition.
In addition, minimizing the amount of plastic used in the dispensing package
100 is also
advantageous in terms of cost. However, thin plastic walls are difficult to
make in the injection
molding processes. In order to house compositions with different rheologies,
in the same or similar
packaging, manufacturers using interchangeable molds must make sure that the
package has enough
strength to work for all product sizes, shapes, and composition rheologies.
For example, more
torque is usually required to move a solid deodorant composition through the
dispensing opening of
the package compared to liquid compositions. For liquid compositions, more
frictional engagement
may be needed to ensure that the liquid composition does not leak around the
circumference of the
platform and/or the screw assembly 330. Thus, it may be necessary to provide
the packaging with
more frictional contact between the outside surface of the movable elevator
platform 320 and the
inner surface of the product chamber 110. This may result in more force placed
on the walls of the
product chamber 110 and consequently the outer jacket 200.
In certain embodiments, the product chamber 110 can be molded of a more rigid,
more
expensive plastic to hold the consumer care composition with adequate strength
while the outer
jacket 200 may be molded of a less expensive material. The opposite may also
be employed. Also,
the same or similar materials of equal thickness may be utilized for both the
product chamber 110
and the outer jacket 200 of the dispensing package 100. Product sold under the
same branding may
be manufactured where the outer jacket 200 varies as to size, color, shape,
etc. to identify the
composition while the product chamber 110 is kept constant regardless of the
product features.
Likewise, the design of the outer jacket 200 could be kept constant, while the
outer surface 130 of
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
9
the product chamber 110 may vary in terms of color, surface features, etc.
In some embodiments, the dispensing package 100 is made of less material, with
adequate
versatility and strength, whereby the product chamber 110 is in frictional
contact with the inside
surface of the outer jacket 200, where the product chamber 110 may remain
constant as the shape,
color, size, etc. of outer jacket 200 is varied.
In some embodiments, the design of the movable elevator platforms 320
described herein
may reduce the amount of plastic required, as compared to conventional movable
elevator platforms.
The movable elevator platforms 320 described herein also reduce the amount of
unusable
composition required within the dispensing package 100, as compared to
conventional elevator
platforms. Often because of the inherent design of the movable elevator
platforms, the
manufacturers of such dispensing packages will have to overfill the dispensing
package. The
overfilled amount may be unusable to the user of the dispensing package 100,
as it is required to
bind the composition to the movable elevator platform so that the composition
may be capable of
being moved within the dispensing package.
In some embodiments, the movable elevator platforms 320 described herein also
allow for
the dispensing package 100 to be bottom-filled. Bottom-filling is a well-known
method of
manufacturing antiperspirants and is typically characterized by filling a
dispensing package 100 with
a composition in its molten state from the bottom of the dispensing package
100 and allowing the
composition to cool. It is not uncommon to bottom-fill a dispensing package
100 while the movable
elevator platform 320 and screw assembly 330 are present within the dispensing
package 100. When
the dispensing package 100 is bottom-filled while the movable elevator
platform 320 and screw
assembly 330 are present, the design of the movable elevator platform 320
should be one that does
not significantly restrict the flow of the composition through the movable
elevator platform 320. If
too much flow is restricted because of the design of the movable elevator
platforni 320, then the
dispensing package 100 may not be suitable for bottom-filling. Suitable
movable elevator platforms
for use in bottom-filled dispensing packages typically have a number of
apertures to permit the flow
of the composition through the movable elevator platform 320 while the
composition is in a molten
state. If the movable elevator does not have enough apertures and/or too many
components that
restrict flow, the movable elevator platform 320 may be unsuitable for use in
a bottom-filled
dispensing package 100 or may retard the filling of each dispensing package
100, resulting in
inefficiencies during the manufacturing process.
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
The movable elevator platforms described herein have a horizontal contact
surface area on
the bottom face located on plane X and a vertical contact surface area located
on plane Y (as
illustrated in FIG. 5). The vertical and horizontal contact surface areas are
determined as follows. A
three-dimensional (3D) computer-aided design (CAD) software program, such as,
for example,
5 SolidWorks (Dassault Systemes SOLIDWORKS Corp., Waltham, MA), may be
used to calculate
the vertical and horizontal contact surface areas. First, a horizontal cross
section 580 is made at the
highest point of the shortest partition inside the rim (as shown in FIGS. 12A
& 12B), which typically
represents the lowest fill point of the composition within the elevator (as
shown in FIG. 12C). All
horizontal areas such as those located on a flange and a partition that are
below the horizontal cross
10 section 580 (as shown in FIG. 12B) and inside the rim of the elevator
when viewed from the bottom
of the elevator are counted. All horizontal areas visible from the top view
are not included in the
calculation. The vertical contact surface area includes any vertical partition
area, vertical flange
area, vertical tab area, and any other vertical area which is below the
horizontal cross section 580,
including the inside and outside of the rim. However, the vertical area
located within the port 520
for a screw is not included in the vertical contact surface area, while the
vertical area of the
perimeter of the exterior wall 600 of the port 520 for a screw is included.
The ratio of the vertical contact surface area (VCSA) to the horizontal
contact surface area
(HCSA) may be from about 1:10 to about 10:1, alternatively from about 1:5 to
about 8:1, or
alternatively from about 1:2 to about 7:1. In some embodiments, the VSCA to
HCSA ratio may be
about 6:1. As a non-limiting example, the vertical contact surface area may be
about 20.53 cm2 and
the horizontal surface area is about 3.51 cm2. The movable elevator platforms
320 described herein
may have about 20% to about 90%, or alternatively from about 45% to about 70%,
of the horizontal
contact surface area located on the flange. In some examples, about 50% to
about 60% of the HCSA
is located on the flange. In some examples, less than about 58% of the HCSA is
located on the
flange.
The movable elevator platforms 320 described herein may also include one or
more
partitions. In some embodiments, about 10% to about 60% of the HCSA is located
on the one or
more partitions. In some embodiments, about 20% to 40%, or alternatively from
about 25% to about
35%, of the horizontal contact surface area is located on the one or more
partitions. In some
embodiments, greater than about 26% of the horizontal contact surface area is
located on the
partitions.
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
11
The movable elevator platforms 320 described herein preferentially locate a
significant
percentage of the contact surface area to the horizontal contact surfaces in
part by locating a
significant portion of the contact surface to the outer portions of the
movable elevator platforms 320
while still having a flange 470 and other components that do not significantly
impact the flow of the
compositions during bottom-filling. Such a design reduces the amount of VSCA
required to adhere
to and move the composition within the dispensing package 100. In this regard,
reducing the amount
of vertical contact surface area required may also reduce the amount of
overfill required. Thus, the
movable elevator platforms 320 described herein may decrease the amount of
overfilling necessary
and the amount of plastic required to manufacture the movable elevator
platform 320, while
allowing the dispensing package to be bottom-filled through the movable
elevator platform 320.
The movable elevator platforms 320 described herein include a rim, a coupling
sleeve, and
partitions that form apertures. The movable elevator platforms 320 described
herein also have a top
face and a bottom face. The top face in some examples may be flat, concave, or
convex. In some
examples, the movable elevator platform 320 may have a flange 470 located on
or about the rim.
As shown in FIG. 5, the movable elevator platform 320 may include a rim 400, a
coupling
sleeve 325 attached to the rim 400, and one or more partitions 410 that are
attached to the rim 400
and form apertures 420. In some embodiments, the coupling sleeve 325 may be
attached to the rim
400 via one or more partitions 410, and in some cases, may be further
supported using one or more
tapered partitions 440. The movable elevator platform 320 may have a top face
450 and a bottom
face 460. In some embodiments, the top face 450 may be concave, convex, or
flat. In some
embodiments, the rim 400 may include a flange 470 located on or about the top
end 480 of the rim
400.
As shown in FIG. 6, the coupling sleeve 325 may include a port 520 for
engaging the screw
assembly 330. Also as shown in FIG. 6, the tapered partitions 440 may be
located on opposites sides
of the coupling sleeve 325. As shown in FIG. 7, the movable elevator platform
320 may include
numerous partitions 410 that altogether form numerous apertures 420. In some
embodiments, the
movable elevator platform 320 includes more than 4 apertures, and in some
embodiments, has at
least 12 apertures. In some embodiments, the movable elevator platform 320 may
include a tab 495
for removing the movable elevator platform 320 from the mold during
manufacture.
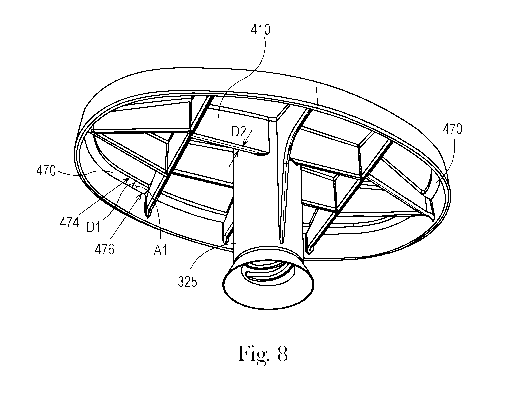
As shown in FIG. 8, the flange 470 of the movable elevator platform 320 may
have a width
D1 from the proximal end 474 of the flange 470 to the distal end 476 of the
flange 470, when viewed
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
12
from the bottom view as shown, of about 1 mm to about 5 mm, alternatively from
about 1.5 mm to
about 4 mm, or alternatively from about 1.8 mm to about 3.6 mm. The width of
D1 may vary
around the flange 470 when the flange 470 is tapered. In some examples, when
the width of D1
varies around the movable elevator platform 320 so as to be a tapered flange
470, the width of D1,
while varying, never exceeds about 8.5 mm, about 8 mm, about 7 mm, about 6 mm,
about 5 mm,
about 4 mm, or even about 3 mm.
In some embodiments, the partition 410 may have a width D2 from the proximal
end 414 of
the partition 410 to the distal end 416 of the partition 410. In some
embodiments, all of the
partitions 410 have a similar or the same D2. In some embodiments, D2 is about
0.69 mm. In some
examples, the length of D2 varies among the partitions 410. In some
embodiments, the width D1 (at
any point around the flange 470) is greater than the width of D2 (at any one
point along the partition)
for at least one partition. In some embodiments, D1 is greater than D2 for
more than one partition.
In some embodiments, at least one D2 is about 0.69 mm, alternatively less than
about 1 mm,
alternatively less than about 2 mm, or alternatively less than about 5 mm. In
some embodiments,
none of the partitions have a D2 that exceeds about 5 mm, about 2 mm, or about
1.2 mm. In some
embodiments, the ratio of D1 to D2 is always at least greater than about 2, in
some embodiments,
even greater than about 3, and in some embodiments, greater than about 5, even
though the width of
D1 may vary around the flange 470 and the width of D2 may also vary.
As shown in FIG. 9, the flange 470 may overhang from the rim 400 when viewed
from the
bottom of the movable elevator platform 320. In some embodiments, the coupling
sleeve 325 has a
width of D3 as measured at the bottom face 460 of the movable elevator
platform 320 from the outer
most ends, as illustrated in FIG. 10. As shown in FIG. 10, the rim 400 may
have a height of D4 as
measured from the junction 485 (of the flange 470 and the rim 400) to the
bottom end 490 of the rim
400. In some embodiments, the partition 410 may include a top end 500 and
bottom end 510 and
may have a height of D5 as measured from the top end 500 to the bottom end
510. In some
embodiments, the height D5 is always greater than the height D4. In some
embodiments, D5 is more
than about 10% greater than D4, alternatively about 20% greater than D4, or
alternatively about 30%
greater than D4. In some embodiments, D4 is about 3.53 mm and D5 is about 5.18
mm. As
illustrated in FIGS. 8 and 10, the line formed to calculate the width of D1,
D2, D3, D4, and D5
always has an angle Al that is 90 degrees with respect to the midpoint of the
line and the start and
end of the line.
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
13
As shown in FIG. 11, the coupling sleeve 325 may include a non-threaded
section 530
located in closer proximity to the top face 450 and a threaded section 540 for
engaging the screw
assembly 330 located at the entrance of the port 520 (FIG. 10).
As illustrated in Table A below, the movable elevator platform 320 is a
significant
improvement over the prior art elevator 620 shown in FIGS. 13A-13C, as the
movable elevator
platform 320 is still capable of being bottom filled. While the prior art
elevator 620 contains a
flange, the flange's dimensions may impact the bottom-filling process such
that the elevator is
incapable of being bottom-filled. In this regard, the prior art elevator 620
has a D1 that is too long in
certain areas of the flange which may impede the fill of the composition
through the elevator. In
contrast, the movable elevator 320, while having a relatively low VCSA: HSCA
ratio, is capable of
being bottom filled.
TABLE A
Movable Prior Art
Elevator Elevator 620
Platform 320
Horizontal Area of Flange 1.78 cm2 2.66 cm2
Longest D1 of Flange 3.0 mm 8.99mm
Vertical Contact Surface Area 20.53 cm2 16.12 cm2
(VCSA)
Horizontal Contact Surface Area 3.51 cm2 4.52 cm2
(HCSA)
VCSA:HSCA Ratio 5.85:1 3.56:1
% of HCSA Located on Flange 50.7 58.8
% of HCSA Located on Partitions 26.15 16.72
% of HCSA Located on Bottom End 21.36 24.49
of Rim
% of HCSA Located on Tab 1.79 NA
Bottom-Fill Capable Yes No
Illustrative Packaging Materials and Manufacturing
A variety of thermoplastic materials or rigid and semi-rigid materials can be
used for the
product chamber, outer jacket, and other components of the package described
herein, such as, for
example, the movable elevator platform 320 (FIG. 5). For example, rigid and
semi-rigid materials
may include, but are not limited to, metals, including, but not limited to,
aluminum, magnesium
alloy, steel; glass, including, but not limited to, laminates; and polymeric
materials such as
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
14
polypropylene (PP), polyethylene (PE), polystyrene (PS), polyethylene-
terepthalate (PET), styrene-
acrylonitrile copolymer (SAN), polyethylene-terepthalate copolymers,
polycarbonate (PC),
polyamides, acrylonitrile-butadiene- styrene (ABS), thermoplastic elastomers,
polyoxymethylene
copolymer and mixtures thereof.
In one embodiment, the molten thermoplastic material has a viscosity, as
defined by the melt
flow index (MFI) of about 0.1 g/10 min to about 500 g/10 min, as measured by
ASTM D1238
performed at temperature of about 23 C with about a 2.16 kg weight. For
example, for
polypropylene, the melt flow index can be in a range of about 0.5 g/10 min to
about 200 g/10 min.
Other suitable melt flow indexes include about 1 g/10 min to about 400 g/10
min, about 10 g/10 min
to about 300 g/10 min, about 20 to about 200 g/10 min, about 30 g/10 min to
about 100 g/10 min,
and about 50 g/10 min to about 75 g/10 min. The MFI of the material is
selected based on the
application and use of the molded package. For example, thermoplastic
materials with an MFI of
about 5 g/10 min to about 50 g/10 min may be suitable for use as caps and
closures for dispensing
packaging.
In one embodiment, the thermoplastic material can be, for example, a
polyolefin. Illustrative
polyolefins include, but are not limited to, polypropylene, polyethylene,
polymethylpentene, and
polybutene-1. Any of the aforementioned polyolefins could be sourced from bio-
based feedstocks,
such as sugarcane or other agricultural products, to produce a bio-
polypropylene or bio-
polyethylene.
Polyolefins advantageously demonstrate shear thinning when in a molten state.
Shear
thinning is a reduction in viscosity when the fluid is placed under
compressive stress. Shear thinning
can beneficially allow for the flow of the thermoplastic material to be
maintained throughout the
injection molding process. Without intending to be bound by theory, it is
believed that the shear
thinning properties of a thermoplastic material, and in particular
polyolefins, results in less variation
of the materials viscosity when the material is processed at lower pressures.
Other suitable thermoplastic materials include renewable polymers such as, for
example,
polymers produced directly from organisms, such as polyhydroxyalkanoates
(e.g., poly(beta-
hydroxyalkanoate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate, NODAX , and
bacterial
cellulose; polymers extracted from plants, agricultural and forest, and
biomass, such as
polysaccharides and derivatives thereof (e.g., gums, cellulose, cellulose
esters, chitin, chitosan,
starch, chemically modified starch, and particles of cellulose acetate),
proteins (e.g., zein, whey,
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
gluten, and collagen), lipids, lignins, and natural rubber; thermoplastic
starch produced from starch
or chemically modified starch and polymers derived from naturally sourced
monomers and
derivatives, such as bio-polyethylene, bio-polypropylene, polytrimethylene
terephthalate, polylactic
acid, NYLON 11, alkyd resins, succinic acid-based polyesters, and bio-
polyethylene terephthalate.
5
The suitable thermoplastic materials may include a blend or blends of
different thermoplastic
materials. For example, the blend may be a combination of materials derived
from virgin bio-
derived or petroleum-derived materials, or recycled materials of bio-derived
or petroleum-derived
materials. One or more of the thermoplastic materials in a blend may be
biodegradable.
Thermoplastic materials may be biodegradable.
10
The thermoplastic material can also be, for example, a polyester. Illustrative
polyesters
include, but are not limited to, polyethylene terphthalate (PET). The PET
polymer could be sourced
from bio-based feedstocks, such as sugarcane or other agricultural products,
to produce a partially or
fully bio-PET polymer. Other suitable thermoplastic materials include
copolymers of polypropylene
and polyethylene, and polymers and copolymers of thermoplastic elastomers,
polyester, polystyrene,
15
polycarbonate, poly(acrylonitrile-butadiene-styrene), poly(lactic acid), bio-
based polyesters such as
poly(ethylene furanate) polyhydroxyalkanoate, poly(ethylene furanoate),
(considered to be an
alternative to, or drop-in replacement for, PET), polyhydroxyalkanoate,
polyamides, polyacetals,
ethylene-alpha olefin rubbers, and styrene-butadiene- styrene block
copolymers. The thermoplastic
material can also be a blend of multiple polymeric and non-polymeric
materials. The thermoplastic
material can be, for example, a blend of high, medium, and low molecular
polymers yielding a
multi-modal or bi-modal blend. The multi-modal material can be designed in a
way that results in a
thermoplastic material that has superior flow properties yet has satisfactory
chemo/physical
properties. The thermoplastic material can also be a blend of a polymer with
one or more small
molecule additives. The small molecule could be, for example, a siloxane or
other lubricating
molecule that, when added to the thermoplastic material, improves the
flowability of the polymeric
material.
Polymeric materials may also include various fillers known to the skilled
artisan, such as, for
example, mica, interference pigments, wood flour; or materials that are
capable of "blooming" to the
surface of a molded component. Other additives may include inorganic fillers
such calcium
carbonate, calcium sulfate, talcs, clays (e.g., nanoclays), aluminum
hydroxide, calcium silicate
(CaSiO3), glass formed into fibers or microspheres, crystalline silicas (e.g.,
quartz, novacite,
CA 02969753 2017-06-02
WO 2016/106041
PCT/US2015/066038
16
crystallobite), magnesium hydroxide, mica, sodium sulfate, lithopone,
magnesium carbonate, iron
oxide; organic fillers such as rice husks, straw, hemp fiber, wood flour; or
wood, bamboo, or
sugarcane fiber.
The dimensions and values disclosed herein are not to be understood as being
strictly limited
to the exact numerical values recited. Instead, unless otherwise specified,
each such dimension is
intended to mean both the recited value and a functionally equivalent range
surrounding that value.
For example, a dimension disclosed as "40 mm" is intended to mean "about 40
mm."
Every document cited herein, including any cross referenced or related patent
or application
and any patent application or patent to which this application claims priority
or benefit thereof, is
hereby incorporated herein by reference in its entirety unless expressly
excluded or otherwise
limited. The citation of any document is not an admission that it is prior art
with respect to any
invention disclosed or claimed herein or that it alone, or in any combination
with any other reference
or references, teaches, suggests or discloses any such invention. Further, to
the extent that any
meaning or definition of a term in this document conflicts with any meaning or
definition of the
same term in a document incorporated by reference, the meaning or definition
assigned to that term
in this document shall govern.
While particular embodiments of the present invention have been illustrated
and described, it
would be obvious to those skilled in the art that various other changes and
modifications can be
made without departing from the spirit and scope of the invention. It is
therefore intended to cover
in the appended claims all such changes and modifications that are within the
scope of this
invention.