Note: Descriptions are shown in the official language in which they were submitted.
WOOD PRODUCTS WITH ENHANCED RESISTANCE TO GRAYING
AND WATER INFILTRATION AND RELATED TECHNOLOGY
TECHNICAL FIELD
[0001] The present technology is related to wood products, such as
engineered and non-
engineered lumber used in the construction of residential and commercial
buildings.
BACKGROUND
[0002] Many wood products used in construction are intended to be
protected from
outdoor elements, such as by paint, siding, roofing, or other covering. In
practice, however,
these wood products are often exposed to outdoor elements for significant
periods of time. For
example, wood products are often stored outdoors at a lumber yard or at a
construction site.
Alternatively or in addition, wood products may be installed at a construction
site and then left
uncovered for weeks or months during the course of construction. Exposure to
outdoor
elements can cause undesirable changes in wood products. For example, exposure
to
precipitation can cause some wood products to expand non-uniformly. Wood
products that
have expanded due to exposure to precipitation often must be reshaped (e.g.,
by sanding or
planing) to fit properly with other building components. As another example of
an undesirable
change, wood products exposed to sunlight often gray due to photo-degradation
of flavonoids
and/or other colored wood constituents. Many consumers consider wood products
that are gray
to be weathered, old, and undesirable, even if there is no measurable loss of
strength associated
with the gray color. For these and/or other reasons, there is a need for
innovation in this field.
SUMMARY
[0002a] Accordingly, there is described a composite wood product,
comprising: a
composite substrate including wood and a binder, wherein a CIELab b* value of
the substrate
decreases by a first amount to a first value in response to a 120-day exposure
at 7 inches
separation distance to a UV lamp with a UVA (315-400nm) output of 13.6 W and a
UVB (280-
3I5nm) output of 3.0 W; and a sealant disposed within a surface portion of the
substrate,
-1-
Date recue/Date received 2023-06-05
wherein the sealant includes substituted aniline photoresponsive molecules at
an average
concentration greater than 1000 parts per million in an outermost one
millimeter of the
substrate, wherein a CIELab b* value of the sealant increases by a second
amount to a second
value in response to the 120-day exposure at 7 inches separation distance to
the UV lamp, and
wherein a CIELab b* value of the wood product in response to the 120-day
exposure at 7
inches separation distance to the UV lamp is greater than the first value.
[0002b] There is also described a method of making a composite wood
product, the
method comprising: applying liquid water to a composite substrate, wherein the
substrate
includes wood and a binder; applying a penetrating sealant to the substrate
after applying the
liquid water to the substrate, wherein the sealant includes at least 50% by
weight aromatic
isocyanate molecules having the same or different respective molecular weights
less than 300
daltons; and exposing the substrate and the sealant to an average temperature
less than 120 C
over a 24-hour period immediately after applying the sealant to the substrate,
wherein at least
some of the aromatic isocyanate molecules form photoresponsive molecules
during the 24-hour
period, and wherein an average concentration of the photoresponsive molecules
after the 24-
hour period is greater than 1000 parts per million in an outermost one
millimeter of the
substrate.
BR 11-,F DESCRIPTION OF THE DRAWINGS
[0003] Many aspects of the present technology can be better understood
with reference to
the following drawings. The components in the drawings are not necessarily to
scale. Instead,
emphasis is placed on illustrating clearly the principles of the present
technology. For ease of
reference, throughout this disclosure the same reference numbers may be used
to identify
-1a-
Date recue/Date received 2023-06-05
identical, similar, or analogous components or features of more than one
embodiment of the
present technology.
[0004] Figure 1 is a transverse cross-sectional end view of a composite
wood product in
accordance with an embodiment of the present technology.
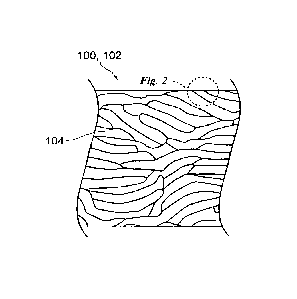
[0005] Figure 2 is an enlarged view of a portion of Figure 1.
[0006] Figure 3 is a plot of yellowness relative to ultraviolet light
exposure for a wood
product and components thereof in accordance with an embodiment of the present
technology.
[0007] Figure 4 is a diagram of chemical reactions that may occur in
connection with at
least some embodiments of the present technology.
[0008] Figure 5 is a flow chart illustrating a method for making a
composite wood
product in accordance with an embodiment of the present technology.
[0009] Figure 6 is a flow chart illustrating a method for using a composite
wood product
in accordance with an embodiment of the present technology.
[0010] Figure 7 is a photograph of a sample of Parallam parallel strand
lumber
("Parallam") labeled with reference axes.
[0011] Figure 8 is a photograph of a sample of untreated Parallam (left)
and a sample of
Parallam treated with a sealant in accordance with an embodiment of the
present technology
(right) after a three day one-sided wetting test.
[0012] Figure 9 is a photograph of a sample of untreated Parallam (left), a
sample of
Parallam treated with a sealant in accordance with an embodiment of the
present technology
(middle), and a sample of Parallam treated with a conventional sealant (right)
after a three day
one-sided wetting test.
[0013] Figure 10 is a photograph of three samples of Parallam treated with
a sealant in
accordance with an embodiment of the present technology after exposure to a
three day one-
sided wetting test.
-2-
CA 2972163 2017-06-27
[0014] Figure 11 is a photograph of three samples of untreated ParaIlam
after exposure to
a three day one-sided wetting test.
DETAILED DESCRIPTION
[0015] Wood products and related devices, systems, and methods in
accordance with
embodiments of the present technology can at least partially address one or
more problems
associated with conventional technologies whether or not such problems are
stated herein. For
example, wood products in accordance with at least some embodiments of the
present
technology include innovative sealants that reduce water infiltration and
graying. A wood
product in accordance with a particular embodiment includes a composite
substrate and a
sealant disposed within a surface portion of the substrate. The substrate can
include wood and a
binder. The sealant can include a crosslinked urethane resin containing
photoresponsive
molecules. When the wood product is exposed to precipitation, the sealant can
reduce or
eliminate infiltration of water into an interior of the wood product, thereby
preventing or
eliminating swelling associated with such water infiltration. When the wood
product is
exposed to sunlight, the sealant can gradually yellow, thereby at least
partially counteracting a
simultaneous gradual graying of the substrate. Accordingly, the wood product
can remain
relatively stable in dimension and color even after long periods of exposure
to outdoor
elements. For example, treated wood products in accordance with at least some
embodiments
of the present technology are able to maintain dimensional stability and a
desirable tan/brown
coloration for 2-3 months longer than corresponding untreated wood products.
[0016] Specific details of wood products and related products and methods
in accordance
with several embodiments of the present technology are described herein with
reference to
Figures 1-11. Although these embodiments may be disclosed herein primarily or
entirely in the
context of composite wood products for structural applications, other contexts
in addition to
those disclosed herein are within the scope of the present technology. For
example, at least
some features of composite wood products described herein may be implemented
in the context
of non-composite wood products. As another example, at least some features of
wood products
for structural applications described herein may be implemented in the context
of wood
-3-
CA 2972163 2017-06-27
products for non-structural applications. Furthermore, it should be
understood, in general, that
other products and methods in addition to those disclosed herein are within
the scope of the
present technology. For example, products and methods in accordance with
embodiments of
the present technology can have different and/or additional configurations,
components, and/or
procedures than those disclosed herein. Moreover, a person of ordinary skill
in the art will
understand that products and methods in accordance with embodiments of the
present
technology can be without one or more of the configurations, components,
and/or procedures
disclosed herein without deviating from the present technology.
[0017] As used herein, the term "wood product" refers to a product
manufactured from
logs, such as lumber (e.g., boards, dimensional lumber, solid-sawn lumber,
joists, headers,
beams, timbers, moldings, laminated lumber, finger-jointed lumber, and semi-
finished lumber),
composite wood products, and components of any of the aforementioned examples.
The term
"composite wood product" refers to a range of derivative wood products
manufactured by
binding together strands, particles, fibers, veneers, and/or other wood pieces
together with
adhesive. Examples of composite wood products include glulam, plywood,
ParaIlam, oriented
strand board, oriented strand lumber, laminated veneer lumber, laminated
strand lumber,
particleboard, medium density fiberboard, cross-laminated timber, and
hardboard, among
others.
[0018] Figure 1 is a transverse cross-sectional end view of a composite
wood product 100
in accordance with an embodiment of the present technology. Figure 2 is an
enlarged view of a
portion of Figure 1. With reference to Figures 1 and 2 together, the wood
product 100 can
include a composite substrate 102 including pieces (e.g., chips, flakes,
strands, etc.) of wood
104 and a binder 106 (Figure 2) that holds together the wood 104. The wood
product 100 can
further include a sealant 108 disposed within a surface portion of the
substrate 102. In at least
some cases, the binder 106 and the sealant 108 are polymeric materials
dispersed within
networks of cellulose fibers of the wood 104. In Figure 2, the binder 106 and
the sealant 108
are shown only at surface portions of the substrate 102 and the wood 104,
respectively. It
should be understood, however, that the binder 106 and the sealant 108 may be
distributed more
widely, such as throughout the substrate 102 and the wood 104, respectively.
-4-
CA 2972163 2017-06-27
100191 The sealant 108 can include photoresponsive molecules that gradually
react to
form colored molecules during exposure of the wood product 100 to outdoor
elements, such as
light or other radiation. In this way, the photoresponsive molecules can at
least partially mask
or otherwise compensate for gradual graying of the substrate 102. The
photoresponsive
molecules can be present within the surface portion of the substrate 102 at a
relatively high
concentration. For example, the photoresponsive molecules can be present
within an outermost
one millimeter of the substrate 102 at an average concentration greater than
1000 parts per
million. Alternatively, the surface portion of the substrate 102 in which the
photoresponsive
molecules are present at an average concentration greater than 1000 parts per
million can be an
outermost 0.025 millimeter of the substrate 102, an outermost 0.10 millimeter
of the substrate,
an outermost 0.50 millimeter of the substrate, or an outermost 0.75 millimeter
of the substrate.
The surface portion of the substrate 102 in which the photoresponsive
molecules are present at
an average concentration greater than 1000 parts per million can also be
greater than 1
millimeter, such as any depth of 1-6 millimeters.
100201 The sealant 108 can further include colorizing molecules that cause
the wood
product 100 to have a desirable starting color before the wood product 100 is
exposed to
outdoor elements. The photoresponsive molecules and the colorizing molecules
can be derived
from the same or similar precursors. The colorizing molecules can include
covalently bound
chromophores that give these molecules a strong initial color. For example,
covalently bound
chromophores can be responsible for most or all of the color of the colorizing
molecules.
Unlike the photoresponsive molecules, the colorizing molecules are not
expected to increase in
yellowness in response to exposure to ultraviolet light. Both the
photoresponsive molecules
and the colorizing molecules can be well suited for protecting the wood
product 100 from water
infiltration.
100211 In several embodiments, the photoresponsive molecules and the
colorizing
molecules are part of a crosslinked resin. The resin can be present within an
outermost one
millimeter of the substrate 102 at an average concentration from 0.05% to
15.0% by weight. In
at least some cases, the resin is a urethane resin. The photoresponsive
molecules can be
substituted aniline compounds. In several embodiments, the photoresponsive
molecules are
-5-
CA 2972163 2017-06-27
formed from the reaction of 4,4'-MDI (4,4'-methylene diphenyl diisocyanate),
2,4'-MDI (2,4'-
methylene diphenyl diisocyanate), pMDI (polymeric methylene diphenyl
diisocyanate), TDI
(toluene diisocyanate), or another suitable aromatic multifunctional
isocyanate and water. The
photoresponsive molecules can be formed in a sufficient concentration in the
wood product
100, for example, by applying water and low molecular weight multifunctional
isocyanates to a
surface of the substrate 102 and maintaining the substrate 102 at a relatively
low temperature
(e.g., less than about 160 F). Once formed, the photoresponsive molecules can
react with
oxygen when exposed to ultraviolet light to form colored compounds that
closely resemble the
color of natural wood.
[0022] The colorizing molecules can be formed from the reaction of 4-4'-
MDI, 2-4'-MDI,
pMDI, TDI, or another suitable aromatic multifunctional isocyanate and a
polyol colorant.
Examples of suitable polyol colorants include those in the REACTINT product
line
manufactured and distributed by Milliken and Company of Spartanburg, SC.
Additional
examples of polyol colorants are described in U.S. Patent No. 4,912,203.
[0023] Figure 3 is a plot of change in yellowness relative to ultraviolet
light exposure
time for the wood product 100, the substrate 102, and the sealant 108. It
should be understood
that Figure 3 is an approximation based on the results of several experiments.
Figure 3
indicates how yellowness changes in response to ultraviolet light exposure
time for the wood
product 100, the substrate 102, and the sealant 108. In practice, different
untreated wood
substrates are expected to turn gray at different rates. For example, OSB
having a surface layer
with pMDI binder is expected to turn gray faster than OSB having a surface
layer with phenol-
formaldehyde binder. Both of these product types are expected to experience an
extended
period of non-graying when treated with sealants in accordance with
embodiments of the
present technology. Likewise, the change in yellowness associated with the
sealant 108 is
expected to be proportional to the amount of the sealant 108 applied to the
substrate 102.
[0024] In a particular example, the time for the wood product 100 to turn
gray under field
exposure conditions may be extended by 2-3 months given an application of
isocyanate of 4-6
g/ft2, a total application of water of 4-8 g/ft2, and a curing temperature of
less than 120 F. In
Figure 3, yellowness is expressed as b* on a CIELab scale from 0-60, and time
is expressed as
-6-
CA 2972163 2017-06-27
days of exposure to a UV lamp (Ultra Vitalux by Osram) with a UVA (315-400nm)
output of
13.6 W and a UVB (280-315nm) output of 3.0 W. The test specimens (2" x 6")
were
positioned 7" from the lamp and maintained at a temperature of 108 F and a
relative humidity
of 30-50%. On the CIELab scale, there is a lower threshold for the b* value of
about 12 below
which most consumers perceive a wood product to be gray. For some
applications, there might
also be an upper threshold above which most consumers perceive a wood product
to be
unnatural. It can be desirable to maintain the yellowness of the wood product
100 between the
upper and lower thresholds for as long as possible.
[0025] As shown in Figure 3, the yellowness of the substrate 102 (without
sealant) may
actually increase for a period of time when it is initially exposed to
ultraviolet light. However,
with continued exposure, the yellowness will decrease and eventually the
substrate 102 will
have a gray appearance. This can occur, for example, due to ultraviolet light
degrading
naturally occurring yellow and red-colored flavonoids and/or other colored
compounds in the
wood 104. For some wood products the initial yellowness is about 22 on the
CIELab b* scale
and the gray threshold is about 15. The yellowness of the sealant 108 may
increase by about 18
on the CIELab b* scale during the first 60-day exposure period. This can
occur, for example,
due to the gradual formation of quinone-like molecules in a urethane matrix of
the sealant 108.
With contributions from both the substrate 102 and the sealant 108, the
yellowness of the
treated wood product 100 may decrease to about 18 by the end of the 120-day
exposure period.
100261 In the absence of the sealant 108, the yellowness of the wood
product 100 over the
course of the 120-day exposure may be the same as the yellowness of the
substrate 102. As
shown in Figure 3, the yellowness of the substrate 102 may fall below the
lower threshold of 15
at about 62 days of exposure, whereas the yellowness of the wood product 100
may remain
above the lower threshold of 15 for the full 120-day exposure and beyond. This
can be at least
partially due to the sealant 108 replenishing the substrate 102 with new
colored compounds as
the naturally occurring colored compounds in the wood 104 are depleted. In
this way, the
sealant 108 can significantly delay the onset of an undesirable level of
graying in the wood
product 100 in response to sunlight exposure while still allowing the wood
product 100 to
remain below the upper threshold before the ultraviolet light exposure period
begins. The
-7-
CA 2972163 2017-06-27
change in yellowness of the sealant 108 can be at least primarily due to the
photoresponsive
molecules. The initial yellowness of the sealant 108 can be at least primarily
due to the
colorizing molecules.
[0027] Eventually, sunlight exposure is expected to break down the colored
molecules
derived from the photoresponsive molecules, leading to a decrease in
yellowness of the sealant
108. This can be seen in Figure 3 as a decrease in the yellowness of the
sealant 108 beginning
after about 60 days of ultraviolet light exposure. This decrease may continue
until the sealant
108 returns to its starting color. The peak yellowness of the sealant 108 is
expected to occur
after at least 5 days of ultraviolet light exposure. Over the course of the
increasing and
decreasing yellowness of the sealant 108, the onset of an undesirable level of
graying in the
wood product 100 can be significantly delayed. In at least some cases, this
delay is at least 2-3
months, which is longer than typical periods of sunlight exposure in most
construction and
lumberyard settings.
[0028] In the embodiment illustrated in Figure 3, the yellowness of the
sealant 108
increases steadily over the course of the 60-day ultraviolet light exposure
period, and the
yellowness of the substrate 102 and the wood product 100 decrease over the
period of about 35
days to about 75 days of exposure. In other embodiments, the changes in the
yellowness of the
sealant 108, the substrate 102, and/or the wood product 100 can be more
variable. For
example, although the period of ultraviolet light exposure over which the
yellowness of the
substrate 102 first increases by about 5.5 and then decreases by about 15.5,
and the yellowness
of the sealant 108 increases by about 18, is about 60-62 days in the
illustrated embodiment, in
other embodiments, the period can be less than 60-62 days or greater than 60-
62 days. For
example, the period can be 30 days, 45 days, 75 days, 90 days, 105 days, 120
days, or another
period within a range from 15 days to 150 days of ultraviolet light exposure.
[0029] Conventionally, a sealant that protects a wood product from water
infiltration and
significantly delays the onset of an undesirable level of graying has not been
available. By way
of theory and without wishing to be bound to theory, these properties of the
sealant 108 may be
at least partially due to constituent low molecular weight precursors of
quinone-like
-8-
CA 2972163 2017-06-27
compounds. For example, the photoresponsive molecules of the sealant 108 may
form colored
quinone-like molecules in response to all or a portion of the 120-day
ultraviolet light exposure.
[0030] The photoresponsive molecules can be substituted aniline compounds
that do not
initially absorb visible light to a significant extent, but have the ability
to gradually transform
into quinone-like molecules when exposed to oxygen and ultraviolet light over
time. The
resulting quinone-like molecules may absorb visible light and consequently
impart yellowness
to the wood product 100. The quinone-like molecules can include six-membered
carbon rings
that contain two double bonds, which are conjugated. These quinone-like
molecules can further
contain two carbonyl groups. In addition to increasing the resistance of the
wood product 100
to graying, the photoresponsive molecules and the quinone-like molecules may
provide the
wood product 100 with durable resistance to water infiltration. In at least
some cases, the
photoresponsive molecules within the outermost one millimeter of the substrate
102 have the
same or different respective molecular weights less than 500 daltons.
[0031] The formulation of the sealant 108 can be selected to promote the
formation of
low-molecular weight precursors of desirable quinone-like molecules. For
example, when first
applied to the substrate 102, the sealant 108 can include at least 5% (e.g.,
at least 25%, 50%,
70%, 80%, or 90%) by mass of aromatic multifunctional isocyanates with at
least 55% (e.g., at
least 70%, 80%, or 90%) by mass of the aromatic multifunctional isocyanates
having the same
or different molecular weights less than 300 daltons. The relatively small
size of these aromatic
multifunctional isocyanates may allow for faster and more complete penetration
into the surface
of the substrate 102, thereby reducing the concentration of the isocyanate in
any region of the
wood which helps to retard polymerization of the isocyanate.
[0032] Achieving a high concentration (e.g., 1000 parts per million or
more) of low-
molecular weight precursors of desirable quinone-like molecules in the wood
product 100 is
also dependent on process conditions before, after, and/or while the sealant
108 is applied to the
substrate 102. In at least some embodiments, the surface portion of the
substrate 102 is wetted
with liquid water before and/or after application of the sealant 108.
Furthermore, the substrate
102 can be maintained at low temperatures during and after application of the
sealant 108. For
example, the substrate 102 can be exposed to an average temperature less than
160 F over a 24-
-9-
CA 2972163 2017-06-27
hour period immediately after the substrate 102 is treated with the low
molecular weight
isocyanate. In at least some cases, maintaining the substrate 102 at high
moisture content and a
low temperature can enhance the formation and persistence of desirable
photoresponsive
molecules.
[0033] Figure 4 is a diagram of chemical reactions that may occur in
connection with at
least some embodiments of the present technology. With reference to Figure 4,
and with the
understanding that Figure 4 is described solely by way of non-limiting theory,
an ultraviolet-
light-reactive urethane with quinone-like precursors can be prepared by
applying an aromatic
isocyanate to moist wood and subjecting the applied aromatic isocyanate to low
temperatures
for a period of at least 24 hours. Water can be added to the wood before and
after application
of the aromatic isocyanate. The low temperatures help to retard polymerization
reactions and
allow a relatively high percentage of the isocyanate groups to be converted
into amines.
Because the resulting amines are diluted within the wood tissue and are
maintained at relatively
low temperatures, they are more likely to be preserved instead of quickly
reacting with
remaining isocyanate functional groups to form polyureas. Eventually, when the
amine groups
on aromatic rings of the aromatic isocyanates are exposed to ultraviolet light
and oxygen, these
aromatic isocyanates may be converted to quinone-like compounds having an
intense yellow
color.
[0034] Figure 5 is a flow chart illustrating a method 200 for making the
wood product
100 in accordance with an embodiment of the present technology. With reference
to Figures 1-
together, the method 200 can include preparing the sealant 108 (block 202). In
one example,
the method 200 includes reacting a minor portion of the aromatic isocyanate
molecules within
the sealant 108 with molecules of a polyol colorant to form oligomer molecules
having
covalently bound chromophores. The chromophores are selected to give the
substrate 102 an
initial color similar to the natural color of the wood 104. In a particular
example, a
combination of yellow, red, and blue polyol dyes is added to the aromatic
isocyanate to produce
a color that is initially brown or tan. The polyol colorant can be combined
with the aromatic
isocyanate in a tank at a molar ratio that involves a substantial excess of
the aromatic isocyanate
before the sealant 108 is applied to the substrate 102. Alternatively, the
polyol colorant can be
-10-
CA 2972163 2017-06-27
applied to the substrate 102 separately. In at least some cases, the polyol
colorant promotes
reaction of the aromatic isocyanate with water, thereby reducing manufacturing
times.
[0035] The method 200 can further include applying liquid water to the
substrate 102
(block 204). For example, liquid water can be applied to a surface of the
substrate 102 at a rate
of at least one gram of liquid water per square foot of the surface, such as a
rate within a range
from 1 to 15 grams of liquid water per square foot of the surface or a rate
within a range from 2
to 6 grams of liquid water per square foot of the surface. The liquid water
can be applied to
one, some, or all sides of the substrate 102, such as using sprayers or
rollers. Alternatively, the
substrate 102 can be dipped in liquid water. In at least some cases, the
substrate 102 is at an
elevated temperature (e.g., 65 C) when the liquid water is applied. This can
be due to the
substrate 102 being recently formed in a high-temperature process. The liquid
water can be at
20 C and can cool the substrate 102 by 10 C or more.
[0036] Next, the method 200 can include applying the sealant 108 to the
substrate 102
(block 206). For example, the sealant 108 can be applied to a surface of the
substrate 102 at a
rate of at least one gram of the sealant 108 per square foot of the surface,
such as a rate within a
range from 1 to 10 grams of the sealant 108 per square foot of the surface or
a rate within a
range from 2 to 6 grams of the sealant 108 per square foot of the surface. The
sealant 108 can
be applied to one, some, or all sides of the substrate 102, such as using
sprayers or rollers.
Alternatively, the substrate 102 can be dipped in the sealant 108. When
contacting the substrate
102, the sealant 108 can include at least 50% by weight aromatic isocyanate
molecules having
the same or different respective molecular weights less than 300 daltons. In
at least some cases,
the sealant 108 includes at least 80% by weight MDI isomers. In several
embodiments, the
sealant 108 includes a first isomer of MDI (e.g., 2,4'-MDI) at a concentration
of at least 20% by
weight, and a second isomer of MDI (e.g., 4,4'-MDI) different than the first
isomer of MDI at a
concentration of at least 20% by weight.
[0037] After the substrate 102 is treated with the sealant 108, the method
200 can include
reapplying liquid water to the substrate 102 (block 208). For example, liquid
water can be
reapplied to a surface of the substrate 102 at a rate of at least one gram of
liquid water per
square foot of the surface, such as a rate within a range from 1 to 15 grams
of liquid water per
-I -
CA 2972163 2017-06-27
square foot of the surface or a rate within a range from 2 to 6 grams of
liquid water per square
foot of the surface. The liquid water can be applied to one, some, or all
sides of the substrate
102, such as using sprayers or rollers. Alternatively, the substrate 102 can
be dipped in liquid
water. In some embodiments, the liquid water is at 20 C. In these and other
embodiments, the
liquid water can further cool the substrate 102, which can be useful, for
example, to inhibit
emission of a volatile component of the sealant 108 in a finishing line
workplace. In addition
or alternatively, the liquid water can be useful to wash away any excess
sealant 108 that, if left
in place, would form a film at the surface of the substrate 102. In yet
another alternative, excess
sealant 108 can be mechanically removed from the substrate 102 by scraping,
doctoring, etc. in
order to avoid film-formation on the surface of substrate 102. If allowed to
form, such a film
may have an undesirable effect on the surface characteristics of the substrate
102. For example,
when the substrate 102 is to be used as decking and in other cases, a film
formed from excess
sealant 108 may cause the surface of the substrate 102 to be excessively
smooth.
[0038] The method 200 can further include maintaining the wood product 100
at low
temperature (block 210) after reapplying liquid water to the substrate 102.
For example, the
method 200 can include exposing the substrate 102 and the sealant 108 to an
average
temperature less than 160 F (e.g., within a range from 50 F to 150 F) over a
24-hour period
immediately after applying the sealant 108 to the substrate 102. At least some
of the aromatic
isocyanate molecules in the sealant 108 can form photoresponsive molecules
during the 24-hour
period such that an average concentration of the photoresponsive molecules
after the 24-hour
period is greater than 1000 parts per million in an outermost one millimeter
of the substrate
102.
[0039] Figure 6 is a flow chart illustrating a method 300 for using the
wood product 100
in accordance with an embodiment of the present technology. With reference to
Figures 1-4
and 6 together, the method 300 can include installing or storing the wood
product 100 at a
building site (block 302). The method 300 can further include photochemically
graying the
substrate 102 (block 304) by exposing the surface portion of the substrate 102
to sunlight. In at
least some cases, this includes decreasing a b* value of the substrate 102 by
at least 7 on a
CIELab scale. While photochemically graying the substrate 102, the method 300
can include
-12-
CA 2972163 2017-06-27
photochemically yellowing the sealant 108 (block 306) by exposing
photoresponsive molecules
within the sealant 108 to the sunlight. For example, the method 300 can
include
photochemically forming quinone-like molecules from the photoresponsive
molecules. In at
least some cases, photochemically yellowing the sealant 108 includes
increasing a b* value of
the sealant 108 by about 18 on the CIELab scale.
EXAMPLES
[0040] The following examples are provided to illustrate certain particular
embodiments
of the disclosure. It should be understood that additional embodiments not
limited to the
particular features described are consistent with the following examples.
Figure 7 is a
photograph of a block of Parallam labeled with reference axes that may be
referenced in the
following examples.
Example 1. First Parallel Strand Lumber Three-Day One-Sided Wetting Test
[0041] A comparative coating known as ISO-5 was applied to two opposing
major faces
(2.0" x 2.0") of blocks (2.0" x 2.0" x 1.5") of Parallam at a spread rate of
about 15 g/ft2 using an
air-pressurized paint gun. The ISO-5 contained approximately 40% isocyanate by
mass with
48% of the isocyanate component being multifunctional isocyanates with a
molecular weight
under 300 daltons (in the form of mixed isomers of MDI), and the remaining 52%
being higher
molecular weight oligomers of MDI. A coating known as W-15 in accordance with
an
embodiment of the present technology was applied to two opposing major faces
(2.0" x 2.0")
of blocks (2.0" x 2.0" x 1.75") of Parallam at a spread rate of approximately
15 g/ft2. The W-15
contained approximately 93% by mass multifunctional aromatic isocyanates with
a molecular
weight below 300 daltons, and the remaining 7% being higher molecular weight
oligomers of
MDI. The moisture absorptions of the ISO-5 and W-15 coated blocks were
compared to
uncoated blocks of Parallam in a three-day one-sided wetting test (with
wetting occurring on
one of the 2" x 2" major surfaces of each block). The average amount of
moisture absorbed by
the blocks is provided in Table 1.
-13-
CA 2972163 2017-06-27
Table 1. Moisture Absorption Test Data
Percent of
Average Average Multifunctional
Average Percentage of
Mass of Mass of Aromatic
Mass of Multifunctional
Water Water
Isocyanates Under
Formulation Water Aromatic
Absorbed Absorbed 300 Daltons in
Absorbed Isocyanates in the
After 2 After 3 Isocyanate
After 1 Day Total Formulation
Days Days
Component of the
Formulation
None 44.6 g 47.8 g 53.2 g NA NA
ISO-5 6.5 g 17.9 g 32.5 g 44% 48%
W-15 3.0 g 9.3 g 13.5 g 100% 93%
[0042] Figure 8 is a photograph of cross-sectional cuts of an untreated
Parallam sample
and a Parallam sample treated with W-15 at the end of a three day one-sided
wetting test. The
major face pictured for each sample is perpendicular to the major face
directly exposed to
water. This view illustrates the impact of water absorption on the inner
region of the wood
product.
Example 2. Solid Sawn Lumber Fourteen-Day Submersion Test
[0043] Ten sections of southern yellow pine lumber were treated with
different
penetrating liquid formulations and then subjected to a post curing step. The
specimens were
approximately 1.5" thick x 3.5" wide x 6.25" long. The specimens were cut to
minimize any
defects and were sorted into similar density groups. Another ten sections of
southern yellow
pine were treated with a phenol formaldehyde resin formulation known as Apinee
80R using a
double vacuum treatment cycle in a pressure treating vessel. The Apinee 80R
did not include
any isocyanates. The treated specimens were then cured for 24 hours at 55 C.
Another ten
sections were dip treated for 20 seconds with a penetrating liquid formulation
that consisted of
50% vinyltrimethoxy silane and 50%, by mass, aromatic isocyanates. The
isocyanate
component consisted of approximately 93% multifunctional aromatic isocyanates
(MDI) with a
molecular weight below 300 daltons, and the remaining 7% being higher
molecular weight
-14-
CA 2972163 2017-06-27
oligomers of MDI. This formulation is known as SIS-2. The dip treated
specimens were then
stored in a 90% humidity room for seven days to facilitate curing. All of the
specimens were
then subjected to a 14-day submersion test. The average amount of formulation
that was
absorbed into the specimens and the average amount of water absorbed after 7
days and 14 days
of submersion are provided in Table 2.
Table 2. Moisture Absorption Test Data
Percent of
Multifunctional
Average Average Average Percentage of
Aromatic
Mass of Mass of Mass of Multifunctional
Isocyanates
Formulation Water Water Aromatic
Under 300
Formulation Absorbed Absorbed Absorbed Isocyanates in the
Daltons in
into After 7 After 14 Total
Isocyanate
Specimens Days Days Formulation
Component of
the Formulation
None NA 141 g 177g NA NA
Apinee 80-R 36g 78 g 123 g 0% 0%
SIS-2 3 g 30 g 54 g 50% 93%
Example 3. Second Parallel Strand Lumber Three-Day One-Sided Wetting Test
[0044] Six blocks (2.0" x 2.0" x 1.75") of Parallam were coated on two
opposing major
faces (2.0" x 2.0") with W-15 at a spread rate of approximately 8.0 g/ft2. Six
more blocks (2.0"
x 2.0" x 1.75") were coated on two opposing major faces (2.0" x 2.0") with a
pMDI formulation
known as Rubinate 1840 supplied by Huntsman Polyurethanes, which contains less
than 50%
multifunctional aromatic isocyanates of molecular weight below 300 daltons.
The same
8.0 g/ft2 spread rate was used for the pMDI coated blocks. These coated blocks
of Parallam
were compared to uncoated blocks of Parallam in a three day one-sided wetting
test. The
average amount of moisture gained by the blocks is provided in Table 3 and the
average
thickness increase is provided in Table 4.
-15-
CA 2972163 2017-06-27
Table 3. Average Mass Gain Due to Water Absorption.
Percent of
Multifunctional
Average Percentage of
A
Aromatic
Average Average
Mass of Multifunctional
Mass of Mass of Isocyanates
Water Aromatic
Formulation Water Water
Under 300
Absorbed Isocyanates in
Absorbed Absorbed
Daltons in
After 1 Day the Total
After 2 Days After 3 Days
Isocyanate
Formulation
Component of
the Formulation
None 33.8 g 42.8 g 48.0 g NA NA
pMDI 21.6g 31.5g 39.0 g 100% 48%
W-15 4.3 g 6.5 g 8.0 g 100% 93%
Table 4. Average Thickness Increase Due to Water Absorption.
Percent of
Average Average Average Percentage of
Multifunctional
Thickness Thickness Thickness Multifunctional Aromatic
Increase Increase Increase Aromatic Isocyanates Under
Formulation
After 1 After 2 After 3 Isocyanates in the
300 Daltons in
Day Days Days Total Isocyanate
(Inches) (Inches) (Inches) Formulation
Component of the
Formulation
None 0.205" 0.255" 0.268" NA NA
,
pMDI 0.142" 0.201" 0.241" 100% 48%
W-15 0.039" 0.062" 0.074" 100% 93%
100451 Figure 9 is a photograph of cross-sectional cuts of a side-by-side
comparison
between Parallam samples exposed to a three day one-sided wetting test. The
major face
pictured for each sample is not the face directly exposed to water, but is
instead a face on the
side of the block. Figure 9 illustrates the impact of water absorption on the
inner region of the
wood product. The W-15 treated block remains dry and is not warped. The
control and
Rubinate samples are wet and warped. Figure 10 is a photograph of faces on the
sides of blocks
-16-
CA 2972163 2017-06-27
of Parallam treated with a W-15 after exposure to a three day one-sided
wetting test. Figure 11
is a photograph of faces on the sides of blocks of untreated Parallam after
exposure to a three
day one-sided wetting test.
Example 4. Production-Scale Treatment of Parallam
[0046] This example describes a process for production-scale treatment of
Parallam in
accordance with an embodiment of the present technology. Parallam in this
example is made
by: (1) treating long wooden strands with phenol/formaldehyde bonding resin
and emulsified
wax, (2) forming a mat from the treated strands, and (3) consolidating the mat
under conditions
of heat and pressure to form a billet. The billets are cut into smaller
sections (beams, headers,
or columns) that are used as framing members. The treatment described in this
example
includes three main operations that occur sequentially beginning about 1.5
hours after the billets
are formed. The operations include a preliminary water treatment, application
of a formulation
known as "W18.G11," and a final water treatment, each occurring in a different
booth.
Parallam is transported through the booths on conveyors at 60-150 ft/min.
[0047] The W18.G11 is prepared in an on-site, temperature-controlled mix
tank plumbed
to a first storage tank and a second storage tank. The first storage tank
holds Lupranate 280,
which is manufactured by the BASF Corporation of Wyandotte, MI. The second
storage tank
holds a mixture of Reactint Yellow X15, Reactint Red X64, and Reactint Blue
X17AB, all
manufactured by Milliken Chemical of Spartanburg, SC. The two components are
loaded into
the mix tank at a ratio of 99 parts by mass Lupranate 280 and 1 part by mass
of the total
reactive dye mixture. Once loaded, the components are stirred at a temperature
of about 15-
27 C within the mix tank and allowed to react for a period of at least 15
minutes. The resulting
batch of finished W18.G11 includes about 60% 4,4'-MDI, 31% of a mixture of
2,2'-MDI and
2,4'-MDI, 8% oligomeric pMDI, 0.9% polyol dyes, and 0.1% optical brightener.
[0048] The preliminary water treatment includes applying liquid water via
low-pressure
sprayers to the top, bottom, left, and right surfaces of the Parallam at a
target application rate of
2-6 g/ft2 depending on the temperature of the Parallam, which may be about 60
F to 160 F. A
portion of the applied water is absorbed into the Parallam and the rest of the
water evaporates.
-17-
CA 2972163 2017-06-27
The preliminary water treatment may serve to cool the surfaces of the Parallam
and to reduce
emission of the subsequently applied W18.G11. After the preliminary water
treatment,
W18.G11 is applied to the top, bottom, left and right surfaces of the Parallam
at a target
application rate of 6 g/ft2. The booth in which the W18.G11 is applied is
coupled to a
ventilated enclosure. Within the booth, the conveyor system contacts the
Parallam at only a
limited number of points in order to avoid transferring large amounts of
W18.G11 onto the
conveyor. Once applied, the W18.G1 I rapidly absorbs into the Parallam. The
final water
treatment is similar to the preliminary water treatment. Within the third
booth, liquid water is
applied via low-pressure sprayers to the top, bottom, left, and right surfaces
of the Parallam at a
target application rate of 2-6 g/ft2 depending on the temperature of the
Parallam, which may be
less than 13T F.
[0049] After the preliminary water treatment, the application of W18.G11,
and the final
water treatment, the treated Parallam is stacked, packaged, and moved to a
storage location
outdoors. MDI emissions from the treated Parallam in the finishing line
workplace may be
sufficiently low to yield an air-borne MDI concentration of less than 1 ppb.
The treated
Parallam is allowed to cure for a period of time sufficient to convert low
molecular weight
isocyanate molecules to reaction products. The resulting modified Parallam has
enhanced
resistance to water infiltration and graying relative to untreated Parallam.
Conclusion
[0050] This disclosure is not intended to be exhaustive or to limit the
present technology
to the precise forms disclosed herein. Although specific embodiments are
disclosed herein for
illustrative purposes, various equivalent modifications are possible without
deviating from the
present technology, as those of ordinary skill in the relevant art will
recognize. In some cases,
well-known structures and functions have not been shown and/or described in
detail to avoid
unnecessarily obscuring the description of the embodiments of the present
technology.
Although steps of methods may be presented herein in a particular order, in
alternative
embodiments the steps may have another suitable order. Similarly, certain
aspects of the
present technology disclosed in the context of particular embodiments can be
combined or
eliminated in other embodiments. Furthermore, while advantages associated with
certain
-18-
CA 2972163 2017-06-27
embodiments may have been disclosed in the context of those embodiments, other
embodiments may also exhibit such advantages, and not all embodiments need
necessarily
exhibit such advantages or other advantages disclosed herein to fall within
the scope of the
present technology.
[0051]
Throughout this disclosure, the singular terms "a," "an," and "the" include
plural
referents unless the context clearly indicates otherwise. Similarly, unless
the word "or" is
expressly limited to mean only a single item exclusive from the other items in
reference to a list
of two or more items, then the use of "or" in such a list is to be interpreted
as including (a) any
single item in the list, (b) all of the items in the list, or (c) any
combination of the items in the
list. Additionally, the terms "comprising" and the like are used throughout
this disclosure to
mean including at least the recited feature(s) such that any greater number of
the same
feature(s) and/or one or more additional types of features are not precluded.
Directional terms,
such as "upper," "lower," "front," "back," "vertical," and "horizontal," may
be used herein to
express and clarify the relationship between various elements. It should be
understood that
such terms do not denote absolute orientation. Reference herein to "one
embodiment," "an
embodiment," or similar formulations means that a particular feature,
structure, operation, or
characteristic described in connection with the embodiment can be included in
at least one
embodiment of the present technology. Thus, the appearances of such phrases or
formulations
herein are not necessarily all referring to the same embodiment. Furthermore,
various particular
features, structures, operations, or characteristics may be combined in any
suitable manner in
one or more embodiments of the present technology.
-19-
CA 2972163 2017-06-27